Abstract
The current COVID-19 pandemic has demonstrated that we are not prepared to deal with food security amid unexpected situations; the FAO (Food and Agriculture Organization) has stipulated that the future of our food & agriculture looks challenging toward the year 2050; primarily in response to the fact that global population is expected to increase by 9 billion people by 2050. Although entomophagy has been practiced by humans for thousands of years, until recently, edible insects have gained special attention due to their high nutritional value (particularly their high protein and essential amino acid content) and lower environmental impact that could help alleviate the global food demand. Edible insects are classified into eight main orders belonging to Blattodea (cockroaches and termites), Coleoptera (beetles), Diptera (flies), Hemiptera (cicadas, stink bugs), Hymenoptera (bees, wasps, ants), Lepidoptera (butterflies, moths), Odonata (dragonflies), and Orthoptera (crickets, grasshoppers, locusts). Several traditional cooking (e.g., boiling, roasting, sun-drying) and processing technologies (e.g., pasteurization, enzymatic proteolysis, high pressure processing) have shown that it is feasible to prepare safe and nutritious insects and/or foods with insects. Nevertheless, challenges associated with consumers acceptance to eat insects, as well as potential presence of anti-nutritive factors and allergens, need to be carefully evaluated as the industry grows in the coming years. Foreseeing such food shortages during pandemics and future food security concerns, consumers, scientists, and the food industry need to consider the value of farming insects as promising protein sources.
Keywords: Entomophagy, Insects, Insect protein, Insect allergens, Insect processing, Microbial safety, Sustainability
1. Humand entomophagy: Historical, nutritional and sustainability perspective
Historical evidence shows that insects have been used as a food source, a term known as entomophagy. Although entomophagy is defined as the dietary consumption of insects by any organism, it is most likely used to refer to the human consumption of insects (Costa-Neto & Dunkel, 2016). The fact is that entomophagy has been practiced since early hominids like Paranthropus (or Australopithecus) robustus (Late Pliocene and Early Pleistocene) in South Africa, who used bone tools do dig into termite mounds. In Northern Spain, dental plaque from an early hominid revealed microfossils of insect fragments, while coprolites found in the Lakeside cave (Utah, USA), suggest that migratory grasshoppers (Melanoplus sanguinipes) were consumed by humans; other prehistoric human coprolites containing chitinous exoskeletons from insects were also found in different States of the USA as well as in Mexico and Peru (Van Huis, 2017). Based on these findings, paleoanthropologists believe that insects could have indeed played an important role in the diet of early humans.
As we go through historical records, we have evidence from Aristotle in the Historia Animalium that cicadas were harvested and considered a delicacy in ancient Greece, while Pliny the Elder (AD 23/24-79) wrote that Romans consumed “cossus,” a highly coveted dish consisting of larvae from the longhorn beetle (Van Huis et al., 2013). The Old Testament in the Bible describes the four kinds of locusts which the Hebrews were allowed to eat (Leviticus, XI: 21–22), and the New Testament (Mark 1:6) describes John the Baptist's food as consisting of “locusts and wild honey” (Costa-Neto & Dunkel, 2016). Moving into our modern-day cultures, we know that pre-Hispanic Mesoamerican cultures also practiced entomophagy. For example, documents from the New Spain (Nueva España) describe that the Aztecs consumed a variety of insects, including winged ants, grasshoppers, mosquito eggs, and worms from the maguey plant (Novo, 1997). Interestingly, many of these insects are still consumed today in public markets in Mexico City (Fig. 1 ) (Liceaga, 2021). Indigenous peoples from the United States and Canada were also known to eat insects such as grasshoppers, crickets, caterpillars, flies, cicadas, beetles, ants, bees and yellowjackets (Capinera, 2008). For example, the Tlicho from the Northwest Territories of Canada ate warble fly larvae (Oedemagena or Hypoderma) collected during the butchering of caribou carcasses (Lesnik, 2018). Some cultures also give spiritual attributes to certain insect species, such as consuming wasps for bringing prosperity, protection and abundance (Ramos-Elorduy, 2009).
Fig. 1.
Degustation plate of insects traditionally eaten by the Aztecs and still available in pubic markets in Mexico City. Insects, clockwise from the top, are grasshoppers (plain), chicatana ants, jumiles (stink bugs), chinicuiles (red maguey worms), cocopache (leaf-footed bug), grasshoppers (salted), grasshoppers (adobo), ahuautles or “mosco de río” (water fly), and acociles (crayfish). Scorpions are in the center of the plate.
Photo by Andrea M. Liceaga.
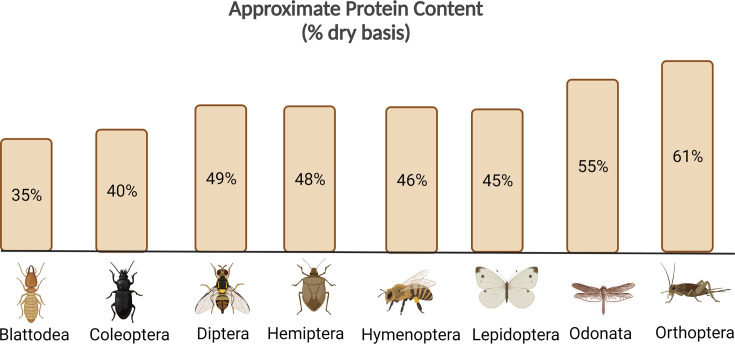
Although through history several plant and animal species have been domesticated and become part of our modern-day staple diet, we know that nearly 2.5 million people worldwide who continue to eat insects and have remained as an important aspect of their culture (van Huis, Dicke, & Van Loon, 2015). In countries such as Thailand, China, Africa, Mexico and Colombia, insects are well known for their nutritional benefits and considered dietary staples (Pal & Roy, 2014), while in southern Ghana, palm weevil larvae (locally known as “Gbamedo”) are one of the most widely consumed insects considered a delicacy (Agbemafle, Hadzi, Amagloh, Zotor, & Reddy, 2020). There are close to 2000 edible insect species catalogued to this day, classified within 8 main orders belonging to Blattodea (cockroaches and termites), Coleoptera (beetles), Diptera (flies), Hemiptera (cicadas, stink bugs), Hymenoptera (bees, wasps, ants), Lepidoptera (butterflies, moths), Odonata (dragonflies), and Orthoptera (crickets, grasshoppers, locusts) (Liceaga, Aguilar-Toalá, Vallejo-Cordoba, González-Córdova, & Hernández-Mendoza, 2021). Worldwide, more families of Lepidoptera are reared by humans than any other insect. In the United States, the waxworm (G. mellonella) is a lepidopteran commonly mass-reared for the animal feed industry as well as for fish bait (Dossey, Tatum, & McGill, 2016). However, worldwide the house cricket (order: Orthoptera) and yellow mealworm (order: Coleoptera) are the most popular farmed insects exclusively for human consumption (Melgar-Lalanne, Hernández-Álvarez, & Salinas-Castro, 2019). Most edible insect species have nutritional yields comparable to conventional meat on a per-gram basis (Table 1 ). A compilation of the nutrient compositions of over 200 edible insect species showed that these insects are composed primarily of protein and fat, followed by fiber, nitrogen free-extract, non-fiber carbohydrates and ash (Rumpold & Schlüter, 2013). Insects can primarily be an excellent source of protein, as they contain all essential amino acids. The protein content will vary by insect species and their life-cycle stage, with crickets, grasshoppers and locusts (order: Orthoptera) having overall the highest protein content (61% dry basis), followed by dragonflies and damselflies (order: Odonata) with 55% protein (dry basis); cockroaches and termites (order: Blattodea) have the lowest overall protein content (35% dry basis) (Fig. 2 ) (Liceaga et al., 2021).
Table 1.
Comparison of nutritional composition found on traditional (raw) protein sources and two domesticated insects (crickets and yellow mealworms) used for human consumption.
| Nutrien | Salmona | Chickenb | Beefc | Porkd | Cricketse | Yellow mealwormse |
|---|---|---|---|---|---|---|
| Protein | 22.2% | 22.2% | 22.5% | 21.0% | 21.3%% | 20.3% |
| Fat | 4.7% | 2.6% | 8.7% | 2.2% | 7.3% | 13.8% |
| Carbohydrates | 0.0% | 0.0% | 0.0% | 0.0% | 4.1% | 3.1% |
| Fiber | 0.0% | 0.0% | 0.0% | 0.0% | 3.2% | 1.7% |
National Nutrient Database for Standard reference (ndb.nal.usda.gov): report 173691 Salmon, sockeye, raw.
National Nutrient Database for Standard reference (ndb.nal.usda.gov): report 05062 Chicken, broiler or fryers, breast, skinless, boneless, meat only, raw.
National Nutrient Database for Standard reference (ndb.nal.usda.gov): report 1390 Beef Round, prime, raw.
National Nutrient Database for Standard reference (ndb.nal.usda.gov): report 10060, Pork, fresh, lion, tenderloin, separable lean only, raw.
Whole, raw crickets and mealworms (wet basis with 69.07% and 62.44% moisture content, respectively).
Data adapted from Finke, M. D. (2004). Nutrient content of insects: Springer.; Liceaga, A. M., Aguilar-Toalá, J. E., Vallejo-Cordoba, B., González-Córdova, A. F., & Hernández-Mendoza, A. (2021). Insects as an Alternative Protein Source. Annual review of food science and technology, 13, 19–34; Rumpold, B. A., & Schlüter, O. K. (2013). Nutritional composition and safety aspects of edible insects. Molecular nutrition & food research, 57(5), 802-823.
Fig. 2.
Approximate protein content for the eight most common edible insect orders.
Figure created with BioRender.com
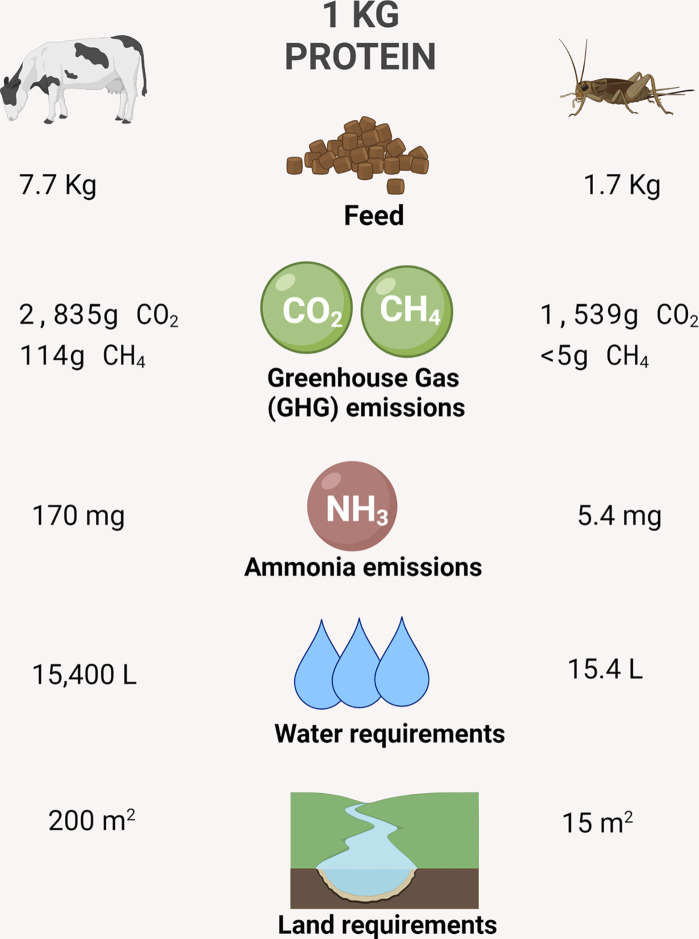
In most western cultures, particularly North American and European, entomophagy is exercised sparingly and insects are mainly considered a food novelty rather than a source of nutrients (Raubenheimer & Rothman, 2013). In fact, in several western countries, human consumption of insects is regarded as a cultural taboo and opinions on eating insects are associated with feelings of disgust and reluctance (Pal & Roy, 2014). However, this perception has slowly begun to change as reports from the Food and Agriculture Organization (FAO) for the United Nations indicate that the global population is likely to increase to 9 billion by 2050 (Van Huis et al., 2013). Currently, nearly 1 billion people go hungry; therefore, as the population continues to rise, so will the amount people that go without food. It is well documented that agricultural land is already pressured by the food demand of the current population. Thus, by the year 2050, world food demand will need to increase by at least 50% with farmers producing 60% more crop calories (7400 trillion calories), and increasing land use by 593 million hectares (twice the size of India) (Searchinger, Walte, Hanson, & Ranganathan, 2019). Exploring other sources of protein that are also more sustainable should alleviate pressures on current food sources such as livestock. Domesticated (farmed) insects are highlighted among such alternatives because they already are integrated in many food cultures around the globe. At present, the FAO encourages entomophagy due to the high economic opportunity that insect farming represents and lower environmental impact (Hall, Jones, O'Haire, & Liceaga, 2017). Compared to other domesticated animals, farm-raised insects require less resources to produce the same amount of protein. Overall, insect breeding requires much less food and land relative to livestock production. Furthermore, reared (farmed) insects inflict a smaller climate impact in terms of greenhouse gases (GHG) and ammonia emissions. Insects can be reared on less land for short periods, due to their short life cycle, requiring significantly less water and energy with a higher intrinsic growth rate than traditional livestock (Fig. 3 ).
Fig. 3.
Comparison of estimated resources needed to produce 1 kg of protein from livestock and farmed (domesticated) insects, respectively.
Figure created using data from Goodland, R., & Anhang, J. (2009). Livestock and climate change: What if the key actors in climate change are... cows, pigs, and chickens? World Watch; Van Huis, A., Van Itterbeeck, J., Klunder, H., Mertens, E., Halloran, A., & Vantomme, P. (2013). Edible Insects: Future Prospects for Food and Feed Security. Food and Agriculture Organization of the United Nations. FAO Forestry Paper, FAO, Rome (187 pp). Figure created with BioRender.com.
For example, reared crickets use 15 m2 of land to produce 1 kg of protein, while cattle use 200 m2, they emit lower GHG emissions and ammonia per unit of protein, use less water and feed. In perspective, livestock can produce up to 100 times more GHG than mealworms (Tenebrio molitor) and emit 8–12 times more ammonia compared to house crickets (Acheta domesticus) (Goodland & Anhang, 2009; Oonincx et al., 2010). Another important consideration when selecting sustainable protein sources relates to the efficiency conversion of ingested food (ECI), which estimates the ability of the animal to convert feed into body mass. In some insects, the ECI can be up to 44%; crickets in particular are twice as efficient as pigs and broiler chickens, four times greater than sheep, and six times higher than a steer (Nakagaki & Defoliart, 1991). These data confirm the need to develop an alternative agriculture system beyond conventional food sources that considers the rising global population. It has been proposed that substituting at least 25% of protein from livestock with other more sustainable proteins, would allow the reforestation of agrarian land and reduce 4% or more of agricultural greenhouse gases (GHG), equivalent to 23 million metric tons per year (EPA, U. S. E. P. A, 2017; Searchinger et al., 2019; Steinfeld, Gerber, Wassenaar, Castel, & de Haan, 2006). In this sense, insect farming (rearing) has gained attention as a nutritional and sustainable approach to this problem. In fact, insect farming has been labeled as an emerging “mini-livestock production system” (Abbasi & Abbasi, 2016). Currently, the majority of industrial-scale edible insect farms are located in Europe (e.g., France and the Netherlands) and North America (e.g., Canada and USA) (Fig. 4 ). These vertical, sustainable farms rear mainly crickets (e.g., Acheta domesticus) and yellow mealworms (Tenebrio molitor) and rely on their own core breeding stock to ensure a great production of insect biomass, thus limiting the possibility of introducing diseases into the system (Baiano, 2020). Another major advantage of farmed insects is that current farming practices do not utilize chemical agents, for example, antibiotics, steroids, hormones, pesticides, or other synthetic chemical components that are often used in vertebrate livestock operations (Dossey et al., 2016).
Fig. 4.
(A) Aspire Food Group's 100,000 sq ft fully-automated cricket production and processing facility located in London, Ontario, Canada. Once operational, this landmark plant is expected to produce 10,000 tons of crickets/year. (B) An early iteration of robotic watering technology at Aspire Food Group Research & Development facility in Austin, Texas, USA.
Photographs reprinted with permission of Aspire Food Group.
2. Traditional methods and commercial processing technologies used for insects
Edible insects are customarily prepared using traditional methods such as sun-drying, roasting, boiling, steaming, baking, frying, and stewing, among others (Table 2 ). Nowadays, they are typically consumed as whole insects (raw or cooked), processed (non-recognizable form), and in the form of extracts (Liceaga et al., 2021). The food industry is showing interest in this novel protein source, as evidenced by several startup companies and number of scientific publications in the last decade, with market trends leading toward a global edible insect market of approximately USD 8 billion in the next 10 years (Liceaga et al., 2021). As a result of this market increase aimed toward Western cultures, other approaches to preparing insects must rely in processing methods that render insects into non-recognizable forms, like flours or powders, protein hydrolysates, fermentable substrates, etc. (Table 2) (Liceaga, 2021; Melgar-Lalanne et al., 2019). The use of different drying technologies seems to be the most commonly used approach for preserving and processing edible insects. However, each drying method used will have different effects on the insects’ nutritional composition and stability. For example, (Kröncke et al., 2018) reported that drying techniques caused minor changes in protein, fat, and fiber content of yellow mealworms (Tenebrio molitor). However, oven drying, microwave drying, fluidized bed drying, and drying with a vacuum decreased (P < 0.05) the protein solubility, while freeze dried mealworms exhibited the highest lipid oxidation compared to the other drying methods. Overall, vacuum oven and microwave drying technologies were reported to be an alternative to conventional oven drying and freeze drying. In contrast, Lenaerts, Van Der Borght, Callens, and Van Campenhout (2018) showed that freeze drying Tenebrio molitor increased lipid oxidation compared to microwave drying, which displayed minor changes in protein, fat, and ash content of the mealworms; the application of a vacuum during the microwave drying process did not add an advantage, since the proximate and fatty acid composition of the mealworms were not significantly affected. Strategies for process-optimized drying of edible insects are still needed to ensure nutrient quality and product functionality.
Table 2.
List of some traditional cooking and commercial processing methods reported in literature for edible insects.
There are clear indications that Western consumers are more inclined to eat insect protein when insects are in non-visible or unrecognizable form in the food or masked by a familiar flavor (e.g., chocolate covered). Studies report that showing a full image of the insect as a marketing strategy for insect-based food products in a retail setting, significantly decreased consumers’ willingness to buy that particular food product (Baker, Shin, & Kim, 2016). This demonstrates the frail acceptance by consumers toward edible insects and/or entomophagy. Additionally, consumers have shown a more positive emotional response to food products that were formulated with the incorporation of non-recognizable insects (i.e., in the form of a flour or powder) compared to those foods that were formulated with insects that remained in a recognizable (i.e., visible) form (Gmuer, Nuessli Guth, Hartmann, & Siegrist, 2016). Sensory evaluation studies also indicate that meals containing visible insects were rated much more negatively in terms of attractiveness and likelihood of eating it, compared to foods formulated with insect protein or insect flours (i.e., pulverized insects into a fine powder) (Caparros Megido et al., 2014; Schösler, De Boer, & Boersema, 2012; Tranter, 2013; Tucker, 2014). Dossey et al. (2016) indicates that caution should be used when using terms such as “insect flour” as this may cause confusion in consumers who might think that the insect flour will have the same properties for cooking and baking as those found in products like grain flours. The authors explain that while insects are composed primarily of protein, followed by fat and fiber (chitin), true flours (e.g., wheat) are made primarily of starches and fiber, followed by protein. Nevertheless, food extrusion, typically used in the production of cereal-based foods using flours, is a good example of a processing method that has allowed for the incorporation of insects as enrichment ingredients, into baked goods and pasta (Carcea, 2020). Other methods reported in literature for processing edible insects rely on separating the protein from the insect exoskeleton (high in chitin) by means of controlled, enzymatic proteolysis with commercial food-grade proteases such as alcalase (Liceaga, 2019). The resulting protein hydrolysates or protein powders tend to have an overall improvement on the protein's functional properties (e.g., solubility, emulsifications, foaming) by effectively separating the insoluble chitin from the protein; these highly-soluble protein hydrolysates can be used in food formulation as protein supplements, emulsifiers and stabilizers, and flavor enhancers, among others (Liceaga, 2019). In one study, corn tortillas were successfully formulated with 20% cricket protein powder, which increased the limiting amino acid lysine from 0.2 g to 1.0 g/100g and also received positive acceptability scores (degree of liking > 6.5) for aroma and flavor, despite panelists (n = 112) knowing that the tortillas contained cricket protein (Calzada-Luna, Martin-Gonzalez, Mauer, & Liceaga, 2021). This demonstrates the potential to develop familiar or staple food products that have some of their traditional protein replaced by insect protein derived from a processing method that transforms the insect into a non-recognizable form.
As with other traditional protein sources (e.g., dairy, meat, etc.), processing methods that involve heat treatments like pasteurization and commercial sterilization, are known to effectively decrease microbial loads, inactivate enzymes as well as increase the nutritional quality, and digestibility of insects (Agbemafle et al., 2020; Liceaga, 2021). There is limited scientific literature available on commercial thermal processing methods applied to edible insects and their effect on the safety, nutritional quality, and protein functionality. This is because the insects-as-food industry remains in the early stages of production, processing, and commercialization in comparison to the other long-established food industries (e.g., dairy, meat, poultry) (Cho, Zhao, Kim, Kim, & Chung, 2018). Meyer-Rochow, Gahukar, Ghosh, and Jung (2021) provide a detailed description on the effects of different traditional cooking and processing methods, grouped by insect order, on final product quality.
In terms of microbial safety, studies on raw and heat-treated yellow mealworm (Tenebrio molitor) and house crickets (Acheta domesticus) indicate that thermal processing was effective at eliminating pathogenic bacteria (Klunder, Wolkers-Rooijackers, Korpela, & Nout, 2012). Grabowski and Klein (2017) evaluated the microbial quality of a variety of processed edible insect species (e.g., deep fried, seasoned, cooked, dried, powdered, and frozen). Their results showed that dried and seasoned insects contained higher microbial counts than those that were cooked or deep fried. All samples tested negative for Salmonella, L. monocytogenes, E. coli and Stapyhlococcus aureus; however, dried and powdered insects contained B. cereus, coliforms, Listeria ivanovii, Aspergillus spp., Penicillium spp., and Cryptococcus neoformans (Grabowski & Klein, 2017). In another study, Nyangena et al. (2020) examined the effects of different traditional processing techniques (i.e., boiling, toasting, solar-drying, and sun-drying, etc.) on the proximate composition and microbiological quality of different edible insect species (Acheta domesticus, Ruspolia differens, Hermetia illucens and Spodoptera littoralis) relative to the raw and/or un-processed insects. Authors reported that boiling and roasting or toasting were the most effective methods for increasing the protein content and decreasing or eliminating aerobic mesophilic bacteria, Staphylococcus aureus, Salmonella, yeasts, and molds. Bußler et al. also reported a 3-log microbial reduction was achieved for Tenebrio molitor flour following exposure to cold atmospheric pressure plasma for 15 min; whereas equally long thermal treatments at 120°C and 140°C were found to completely inactivate the native microorganism flora (Bußler et al., 2016). Lastly, other studies have reported that fermentation of soy sauce-analog using Tenebrio molitor, Bacillus licheniformis, and Aspergillus oryzaep, resulted in the amino-nitrogen and aromatic compound content increasing indicating protein degradation that did not affect the nutritional and sensory quality of the fermented sauce (Cho et al., 2018; Mouritsen, Duelund, Calleja, & Frøst, 2017; Yi, Van Boekel, Boeren, & Lakemond, 2016).
Another important consideration is that applying processing technologies insects can lead to development of insect-based food ingredients beyond proteins, including fiber, lipids, and other insect components (e.g., polyphenols) that could serve multiple functions in the food and beverage industries. In addition to fermentation, methods like enzymatic proteolysis, microwave-extraction, ultrasonication, and high-pressure processing are capable of releasing bioactive compounds such as peptides, phenolic compounds, and chitin that can have potential benefits to human health in the prevention or control of diseases such as hypertension, inflammation, and type-II diabetes (Hall & Liceaga, 2020). For example, edible cricket chitosan obtained from the chitin, a by-product of the protein extraction using microwave-assisted enzymatic proteolysis, was shown to have antimicrobial and hypolipidemic activity comparable to that of commercial shellfish chitosan (Malm & Liceaga, 2021); whereas some phenolic compounds with antioxidant and antimicrobial activity were successfully extracted and characterized from farmed edible crickets (Nino, Reddivari, Ferruzzi, & Liceaga, 2021).
3. Applications of insect protein in food and beverage formulations
Several applications using edible insects have been considered for the food and beverage industries. The most common application of edible insects has been in bakery and cereal-based products such as cookies, bread, tortillas, and pasta. For example, roasted speckled cockroach (Nauphoeta cinerea) powder ranging from 5% to 15% (w/w) was used as protein enrichment of wheat flour to formulate bread. Results showed that the 10% enrichment formulation was the most similar to the whole what bread control, and also presented the best nutritional characteristics including higher protein (22.6% vs 9.7%, dry basis) and fiber (2.3% vs 2.0%, dry basis) and an acceptability index above 75% (de Oliveira et al., 2017).
Replacing wheat flour with 5% insect flour from house cricket (A. domesticus), mealworm, and black soldier fly (Hermetia illucens), resulted in bread loafs with decreased water absorption and increased dough formation and stability (González, Garzón, & Rosell, 2018). Cricket powder (10–30%) was also used to enrich wheat bread. Compared to control breads (using only wheat flour), breads containing cricket powder showed a higher nutritional profile in terms of fatty acid composition, high protein content and also showed a significant enrichment in the essential amino acids lysine, tyrosine, valine, and methionine (Osimani et al., 2018). In another study, microwave-dried yellow mealworm powder (20%) was used to enrich wheat flour and produce different cereal-based snacks using 3D printing technology (Severini, Azzollini, Albenzio, & Derossi, 2018). Corn tortillas is another example of a baked cereal product fortified with edible insects. In this study, 20% cricket (Acheta domesticus) protein powders were used to formulate corn tortillas. The improved nutritional quality (including essential amino acids) as well as comparable physico-chemical and sensory acceptability scores of the fortified products, compared to control tortillas, demonstrates the high potential of using edible insects to fortify staple food products that have limiting essential amino acids without compromising the palatability (Calzada-Luna et al., 2021).
Reports indicate that companies are currently working on extraction and restructuration of insect proteins into versatile food ingredients, like soluble protein powders for beverages and textured insect proteins for meat analogues, and egg or dairy replacements in baking and food processing applications (Shockley, Lesnik, Allen, & Muñoz, 2018). Minced cooked insects are also being used to formulate meat analogue foods like hamburgers, meatballs, and sausages (Elhassan, Wendin, Olsson, & Langton, 2019; Fraqueza & Patarata, 2017). Efforts to produce protein supplements, beverages and energy bars based on insect powder are also documented (Mutungi et al., 2019). One study looked at the application of insects to formulate animal-sourced foods as a strategy to achieve protein and micronutrient density of infants and young children in developing countries. In this study, crickets and palm weevil larvae were blended with a sweet potato porridge and compared to the maize-peanut-soybean blend (Weanimix). The results showed that the edible insect foods had several advantages over the mainstream blend, including ease of preparation, improved nutritional composition (e.g., meeting protein requirements), and lower risk of aflatoxin contamination (Agbemafle et al., 2020). Other recent applications include the incorporation of edible insects for space food applications such as the Mars mission. In this context, the required food to support life in a space mission and/or closed ecological environments could be harvested from enclosed agriculture systems. Because protein from animal origin will be difficult to produce due to its constraints related to the extraterrestrial environment, efficiency in the use of biomass energy, such as reared edible insects, should be considered (Katayama, Yamashita, Wada, & Mitsuhashi, 2005).
In addition to the use of insects and their protein to formulate foods, some studies have investigated the application of other insect components for food formulation. For example, lipids derived from insect biomass of two species (Hermetia illucens and Tenebrio molitor) were applied as an alternative for plant and animal lipids in spreadable products like margarine or butter (Smetana, Leonhardt, Kauppi, Pajic, & Heinz, 2020). Authors reported that it was possible to substitute up to 75% of the lipids with insect-derived fats without negative effects on the spreading ability or color of the products. In another study, cookies prepared with insect oils had higher omega-3 fatty acids, flavonoids, and vitamin E than the cookies formulated with plant oils. Consumers’ acceptance was also high for those cookies prepared with Ruspolia differens and sesame oils, respectively, compared to those formulated with olive and Schistocerca gregaria oils (Cheseto, Baleba, Tanga, Kelemu, & Torto, 2020).
Finally, the effort to develop novel processing methods and foods that incorporate edible insects can also be evidenced by the vast number of patent applications. Baiano (2020) lists the most recent patents containing edible insects or insect powders. These include cookies, rice cakes, energy bars, soup, tea, rice noodles, jerky, coffee, salad dressing, and tofu, among others. These commercial patent applications highlight the broad interest by scientists and the private sector to include edible insects in food and beverage formulations.
4. Challenges and future prospects of insect protein
One of the major challenges facing edible insects is the acceptance by consumers particularly in Western society, where food neophobic factors have resulted in edible insects being regarded with a feeling of disgust or viewed as a cultural taboo (Gmuer et al., 2016). Food neophobia is regarded as the fear to eating new foods and can occur in all type of consumers; however, the level of neophobia response will vary amongst consumers depending on their age, gender, education, social status, among others (Tuorila, Lähteenmäki, Pohjalainen, & Lotti, 2001). In this sense, studies have shown that consumers categorized as neophobics have a low willingness to purchase food products containing edible insects, compared to consumers who have a positive disposition to eat new foods (neophilics) (Lombardi, Vecchio, Borrello, Caracciolo, & Cembalo, 2019). However, there is no evidence confirming that neophobics will not accept edible insects. As previously discussed in this chapter, research suggests that incorporating insects as part of an ingredient within a familiar food will help alleviate some of those neophobic, psychological constrains. Moving forward, education and industry efforts will need to find pathways to promote edible insects in order to eventually normalize their consumption just as it has been done over decades with other “novel” foods like sushi, plant-based meat-analogues, etc.
Although insects are consumed by many people all over the world, safety aspects remain important challenges. This chapter has already discussed some of the microbial safety concerns associated with edible insects and the processing methods that have shown to decrease their microbial load. Other safety concerns are related to the anti-nutrient content in some edible insect species. Due to insects’ herbivore feeding behavior, farmed insects are primarily fed plant-based diets rich in allelochemicals such as phenolic compounds (Nino et al., 2021). These allelochemicals can be a good source of antioxidants, but some can also have anti-nutritive effects. For example, crickets are reported to have 3159 mg/100 g and 900 mg/100 g of phytate and tannins, respectively; while grasshoppers have 1100 mg/100 g and 1050 mg/100 g of phytate and tannins, respectively (Meyer-Rochow et al., 2021). Nevertheless, similar to antinutritive compounds found in other foods, most processing methods (e.g., boiling, drying) can decrease their content (Liceaga et al., 2021). Furthermore, the advantage of farming edible insects will allow for selecting carefully-designed plant diets that can minimize the concentration of these compounds.
Other insect compounds, like chitin and allergens, can also present challenges. Excessive consumption of chitin, found primarily in the insect's exoskeleton, is speculated to increase risks of urinary stone formation and chronic degenerative disease (Yhoung-aree, 2008). Potential allergic responses from insect chitin ingestion have also been reported (Bush, 2008). However, there is no clear link to these effects with chitin consumption. The presence of protein allergens, on the other hand, remains a major safety concern surrounding edible insects. Ongoing studies have identified antigens and IgE-binding proteins from several insect species that are correlated to an allergic reaction upon exposure or consumption (Feng et al., 2018; Pali-Schöll et al., 2019; Ribeiro, Cunha, Sousa-Pinto, & Fonseca, 2018). For instance, immunoreactions have been associated with silkworm (Bombyx mori) (Liu, Tian, & Chen, 2001), teak caterpillar cocoons (Hyblaea puera) (Lukiwati, 2010), grasshoppers (Srivastava, Babu, & Pandey, 2009; Vetter, 1995), house crickets (Acheta domesticus) (Abdelmoteleb et al., 2018) and farmed tropical banded crickets (Gryllodes sigillatus) (Hall, Johnson, & Liceaga, 2018). The major shrimp allergen, tropomyosin, is known to be a cross-sensitizing allergen in several edible insects due to its reported immunological relationships between crustaceans and arthropods (which include insects) (Abdelmoteleb et al., 2018; Ayuso, Reese, Leong-Kee, Plante, & Lehrer, 2002; Hall & Liceaga, 2021; Wong, Huang, & Lee, 2016). This is because tropomyosin, a highly-conserved protein that can exist in different isoforms, is present in all vertebrate species as well as among invertebrate's (e.g., insects) muscle and non-muscle cells (Leung et al., 1996). Therefore, there is a high degree of cross-reactivity between homologous proteins found in crustaceans (shellfish) and other arthropods (Leoni, Volpicella, Dileo, Gattulli, & Ceci, 2019; Pali-Schöll et al., 2019; Volpicella, Leoni, Dileo, & Ceci, 2019), suggesting that individuals with a shellfish allergy should avoid eating insects (MacEvilly, 2000). Table 3 shows the immunoinformatics results of shared sequence homology (> 60% identity) for cricket tropomyosin and allergens from various species of shellfish, insects, and nematodes. It can be observed that the top matches were for tropomyosin from Lep s 1 silverfish (Lepisma saccharina) and Pan b 1.0101 northern shrimp (Pandalus borealis). Matches were also obtained with other insect allergens such as house dust mite (Dermatophagoides farinae) paramyosin (Der f 11), American cockroach (Periplaneta americana) tropomyosin (Per a 7), and German cockroach (Blattella germanica) tropomyosin (Bla g 7) (Hall & Liceaga, 2021).
Table 3.
Cricket tropomyosin predicted sequence homology with reported allergens derived from insects, shellfish and nematodes.
| Species | Allergen | Sequence link in SwissProt/NCBI | Full alignmenta |
|
|---|---|---|---|---|
| E-val | %ID | |||
| Lepisma saccharina | Leps 1 | CAC84590 | 1.7e-050 | 81.50% |
| Pandalus borealis | Pan b 1.0101 | CBY17558 | 4.8e-049 | 78.50% |
| Penaeus monodon | Pen m 1 | AAX37288 | 2.2e-040 | 67.30% |
| Penaeus aztecus | Pen a 1 | 11893851 | 1.2e-039 | 65.40% |
| Homarus americanus | Hom a 1.0102 | AAC48288 | 9.5e-042 | 69.30% |
| Litopenaeus vannamei | Lit v 1.0101 | EU410072 | 1.1e-040 | 67.30% |
| Homarus americanus | Hom a 1.0101 | O44119 | 3.3e-041 | 67.80% |
| Periplaneta americana | Per a 7.0102 | AAD19606 | 2.9e-041 | 68.30% |
| Blattella germanica | Bla g 7.0101 | AAF72534 | 4.5e-041 | 68.30% |
| Dermatophagoides farinae | Der f 10.0101 | BAA04557 | 3.7e-041 | 67.80% |
| Chironomus kiiensis | Chi k 10 | CAA09938 | 7.4e-042 | 68.80% |
| Tyrophagus putrescentiae | Tyr p 10.0101 | AAT40866 | 9.8e-038 | 65.60% |
| Blomia tropicalis | Blot 10.0101 | ABU97466 | 1.4e-041 | 68.30% |
| Metapenaeus ensis | Met e 1 | Q25456 | 3.6e-039 | 66.80% |
| Panulirus stimpsoni | Pan s 1 | O61379 | 4.7e-041 | 67.30% |
| Lepidoglyphus destructor | Lep d 10 | Q9NFZ4 | 5.3e-038 | 66.20% |
| Dermatophagoides farinae | Der p 10 | O18416 | 6.5e-042 | 68.30% |
| Charybdis feriatus | Cha f 1 | Q9N2R3 | 2.1e-040 | 67.30% |
| Ascaris lumbricoides | Asc l 3.0101 | ACN32322 | 3.5e-042 | 68.80% |
| Anisakis simplex | Ani s 3 | Q9NAS5 | 5.8e-042 | 69.30% |
| Helix aspersa | Hel as 1 | CAB38044 | 3.7e-041 | 67.80% |
| Haliotis diversicolor | Hal d 1 | AAG08987 | 1e-039 | 65.40% |
| Mimachlamys nobilis | Mim n 1 | AAG08989 | 6e-041 | 67.80% |
| Perna viridis | Per v 1 | AAG08988 | 2e-041 | 68.30% |
| Crassostrea gigas | Cra g 1 | AAK96889 | 1.1e-040 | 67.30% |
| Dermatophagoides farinae | Der p 11 | AAO73464 | 3.7e-041 | 67.80% |
| Blomia tropicalis | Blo t 11 | AAM83103 | 3.5e-042 | 69.30% |
| Dermatophagoides farina | Der f 11.0101 | AAK39511 | 4.2e-041 | 68.30% |
Parameters assessed are % Identity and E-score. Significance is assumed when the expected score is below 1.0 or a > 50% identity match (www.allermatch.org). Duplicates were not included on the list.
Table reprinted from Hall, F., & Liceaga, A. (2021). Isolation and proteomic characterization of tropomyosin extracted from edible insect protein. Food Chemistry: Molecular Sciences, 100049, with permission from Elsevier.
Further research is needed in order to establish the effect of the different food processing technologies on edible insect allergens. For example, studies report that treating insects with heat (e.g., baking, boiling), frying, and high-pressure processing did not have much effect on lowering an immuno-response (i.e., IgE-binding) (Broekman et al., 2015; Jeong et al., 2016; Mills & Mackie, 2008; Phiriyangkul, Srinroch, Srisomsap, Chokchaichamnankit, & Punyarit, 2015; Van Broekhoven, Bastiaan-Net, de Jong, & Wichers, 2016). Conversely, Hall and Liceaga (2021) demonstrated that microwave-heated and protease-treated cricket tropomyosin had lower IgE and IgG reactivity compared to cricket tropomyosin treated by convection heating. Based on immunoinformatics and proteomics analyses, the decreased allergenicity was associated with increased cleavage of the epitope region of the tropomyosin that had been protease-treated with microwave heating. This agrees with other reports by El Mecherfi et al. (2011) and Ketnawa and Liceaga (2017) that microwave heating can increase the rate of an allergenic protein's unfolding, enhancing the exposure of its epitope region, to proteases that would have not been otherwise accessible under convection heating. As more edible insects become part of formulated foods and beverages, it will be crucial to continue the assessment for the presence of potential allergens, toxicants, and anti-nutritive factors.
5. Conclusion
Edible insects are gaining attention as potential protein sources that could help alleviate the predicted protein demand by the year 2050. The lower environmental impact of insect farming places them as leaders in the future development of more sustainable foods worldwide. The incorporation of these novel protein sources as viable ingredients will largely depend on consumers’ perception and acceptance of products containing edible insects. Decades of research on processing technologies and product development for the plant, meat and dairy industries have created the necessary knowledge to overcome food processing and safety challenges to produce safe and palatable foods for consumers. Like with traditional protein sources, the incorporation of insect protein into food and beverage formulations will present its challenges and limitations that will require extensive research to ensure that processing technologies and formulation strategies work in the same form as they have done for traditional proteins. Overall, edible insects are a highly nutritious source of protein, fiber, and lipids. Early education efforts and mechanisms to process them into non-recognizable forms, will allow for the normalized attitude towards eating insects by modern-day society. Studies show that insects can be processed using similar technologies to those applied for traditional proteins; therefore, the possibilities of developing convenient, safe, palatable, or even shelf-stable, insect-based food products is vast. Foreseeing food shortages during pandemics like the 2020 COVID-19 pandemic, and food security concerns towards the year 2050, consumers, scientists, and the food industry need to consider the value of farming insects as promising protein sources.
References
- Abbasi T., Abbasi S. Reducing the global environmental impact of livestock production: the minilivestock option. Journal of Cleaner Production. 2016;112:1754–1766. [Google Scholar]
- Abdelmoteleb M., Palmer L.K., Pavlovikj N., Marsh J.T., Johnson P.E., Goodman R.E. Bioinformatics and Proteomics Evaluations to Consider IgE Binding Assays for Potential Cross-Reactivity Between House-Cricket (Aceta domesticus) Used in Food, Crustaceans and Cockroaches. Journal of Allergy and Clinical Immunology. 2018;141(2):AB263. [Google Scholar]
- Agbemafle I., Hadzi D., Amagloh F.K., Zotor F.B., Reddy M.B. Nutritional, microbial, and sensory evaluation of complementary foods made from blends of orange-fleshed sweet potato and edible insects. Food. 2020;9(9):1225. doi: 10.3390/foods9091225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves A.V., Sanjinez-Argandoña E.J., Linzmeier A.M., Cardoso C.A.L., Macedo M.L.R. Food value of mealworm grown on Acrocomia aculeata pulp flour. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso R., Reese G., Leong-Kee S., Plante M., Lehrer S.B. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. International Archives of Allergy and Immunology. 2002;129(1):38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- Baiano A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends in Food Science & Technology. 2020;100:35–50. [Google Scholar]
- Baker M.A., Shin J.T., Kim Y.W. An exploration and investigation of edible insect consumption: the impacts of image and description on risk perceptions and purchase intent. Psychology & Marketing. 2016;33(2):94–112. [Google Scholar]
- Broekman H., Knulst A., den Hartog Jager S., Monteleone F., Gaspari M., De Jong G., et al. Effect of thermal processing on mealworm allergenicity. Molecular Nutrition & Food Research. 2015;59(9):1855–1864. doi: 10.1002/mnfr.201500138. [DOI] [PubMed] [Google Scholar]
- Bush R.K. Indoor allergens, environmental avoidance, and allergic respiratory disease. Paper presented at the Allergy and Asthma Proceedings; 2008. [DOI] [PubMed] [Google Scholar]
- Bußler S., Rumpold B.A., Fröhling A., Jander E., Rawel H.M., Schlüter O.K. Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: Impact on microbial load and quality attributes in comparison to dry heat treatment. Innovative Food Science & Emerging Technologies. 2016;36:277–286. [Google Scholar]
- Calzada-Luna G., Martin-Gonzalez F.S., Mauer L., Liceaga A. Cricket (Acheta domesticus) protein hydrolysates’ impact on the physicochemical, structural and sensory properties of tortillas and tortilla chips. Journal of Insects as Food and Feed. 2021;7(1):109–120. [Google Scholar]
- Caparros Megido R., Sablon L., Geuens M., Brostaux Y., Alabi T., Blecker C., et al. Edible insects acceptance by Belgian consumers: Promising attitude for entomophagy development. Journal of Sensory Studies. 2014;29(1):14–20. [Google Scholar]
- Capinera J.L. In: Encyclopedia of Entomology. Capinera J.L., editor. Springer; Dordrecht, The Netherlands: 2008. Native American Culture and Insects; pp. 2546–2550. [Google Scholar]
- Carcea M. Multidisciplinary Digital Publishing Institute; 2020. Quality and Nutritional/Textural Properties of Durum Wheat Pasta Enriched with Cricket Powder. [Google Scholar]
- Cheseto X., Baleba S., Tanga C.M., Kelemu S., Torto B. Chemistry and sensory characterization of a bakery product prepared with oils from African edible insects. Food. 2020;9(6):800. doi: 10.3390/foods9060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.-H., Zhao H.-L., Kim J.-S., Kim S.-H., Chung C.-H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Innovative Food Science & Emerging Technologies. 2018;45:186–195. [Google Scholar]
- Costa-Neto E.M., Dunkel F. Insects as sustainable food ingredients. Elsevier; 2016. Insects as food: history, culture, and modern use around the world; pp. 29–60. [Google Scholar]
- de Oliveira L.M., da Silva Lucas A.J., Cadaval C.L., Mellado M.S. Bread enriched with flour from cinereous cockroach (Nauphoeta cinerea) Innovative Food Science & Emerging Technologies. 2017;44:30–35. [Google Scholar]
- Del Hierro J.N., Gutiérrez-Docio A., Otero P., Reglero G., Martin D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chemistry. 2020;309 doi: 10.1016/j.foodchem.2019.125742. [DOI] [PubMed] [Google Scholar]
- Dossey A., Tatum J., McGill W. Insects as sustainable food ingredients. Elsevier; 2016. Modern insect-based food industry: current status, insect processing technology, and recommendations moving forward; pp. 113–152. [Google Scholar]
- El Mecherfi K.E., Saidi D., Kheroua O., Boudraa G., Touhami M., Rouaud O., et al. Combined microwave and enzymatic treatments for β-lactoglobulin and bovine whey proteins and their effect on the IgE immunoreactivity. European Food Research and Technology. 2011;233(5):859–867. [Google Scholar]
- Elhassan M., Wendin K., Olsson V., Langton M. Quality aspects of insects as food—nutritional, sensory, and related concepts. Food. 2019;8(3):95. doi: 10.3390/foods8030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, U. S. E. P. A. (2017). Sources of Greenhouse Gas Emissions. Retrieved from https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions.
- Feng Y., Chen X.M., Zhao M., He Z., Sun L., Wang C.Y., et al. Edible insects in China: Utilization and prospects. Insect Science. 2018;25(2):184–198. doi: 10.1111/1744-7917.12449. [DOI] [PubMed] [Google Scholar]
- Fombong F.T., Van Der Borght M., Vanden Broeck J. Influence of freeze-drying and oven-drying post blanching on the nutrient composition of the edible insect Ruspolia differens. Insects. 2017;8(3):102. doi: 10.3390/insects8030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraqueza M., Patarata L. Future Foods. InTech; London: 2017. Constraints of HACCP application on edible insect for food and feed; pp. 89–113. [Google Scholar]
- Gmuer A., Nuessli Guth J., Hartmann C., Siegrist M. Effects of the degree of processing of insect ingredients in snacks on expected emotional experiences and willingness to eat. Food Quality and Preference. 2016;54:117–127. doi: 10.1016/j.foodqual.2016.07.003. [DOI] [Google Scholar]
- González C.M., Garzón R., Rosell C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innovative Food Science & Emerging Technologies. 2018 [Google Scholar]
- Goodland R., Anhang J. World Watch; 2009. Livestock and climate change: What if the key actors in climate change are... cows, pigs, and chickens? [Google Scholar]
- Grabowski N.T., Klein G. Microbiology of cooked and dried edible Mediterranean field crickets (Gryllus bimaculatus) and superworms (Zophobas atratus) submitted to four different heating treatments. Food Science and Technology International. 2017;23(1):17–23. doi: 10.1177/1082013216652994. [DOI] [PubMed] [Google Scholar]
- Hall F., Johnson P.E., Liceaga A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chemistry. 2018;262:39–47. doi: 10.1016/j.foodchem.2018.04.058. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Hall F., Jones O.G., O'Haire M.E., Liceaga A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chemistry. 2017;224:414–422. doi: 10.1016/j.foodchem.2016.11.138. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Hall F., Liceaga A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. Journal of Functional Foods. 2020;64:103634. doi: 10.1016/j.jff.2019.103634. Retrieved from. [DOI] [Google Scholar]
- Hall F., Liceaga A. Isolation and proteomic characterization of tropomyosin extracted from edible insect protein. Food Chemistry: Molecular Sciences. 2021;100049 doi: 10.1016/j.fochms.2021.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Álvarez A.-J., Mondor M., Piña-Domínguez I.-A., Sánchez-Velázquez O.-A., Melgar Lalanne G. Drying technologies for edible insects and their derived ingredients. Drying Technology. 2021;39(13):1991–2009. [Google Scholar]
- Jeong K.Y., Son M., Lee J.Y., Park K.H., Lee J.-H., Park J.-W. Allergenic characterization of 27-kDa glycoprotein, a novel heat stable allergen, from the pupa of silkworm, Bombyx mori. Journal of Korean Medical Science. 2016;31(1):18–24. doi: 10.3346/jkms.2016.31.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau E., Mutungi C., Kinyuru J., Imathiu S., Tanga C., Affognon H., et al. Moisture adsorption properties and shelf-life estimation of dried and pulverised edible house cricket Acheta domesticus (L.) and black soldier fly larvae Hermetia illucens (L.) Food Research International. 2018;106:420–427. doi: 10.1016/j.foodres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Katayama N., Yamashita M., Wada H., Mitsuhashi J. Entomophagy as part of a space diet for habitation on Mars. The Journal of Space Technology and Science. 2005;21(2):2_27-22_38. [Google Scholar]
- Ketnawa S., Liceaga A.M. Effect of Microwave Treatments on Antioxidant Activity and Antigenicity of Fish Frame Protein Hydrolysates. Food and Bioprocess Technology. 2017;10:582–591. [Google Scholar]
- Klunder H., Wolkers-Rooijackers J., Korpela J., Nout M. Microbiological aspects of processing and storage of edible insects. Food Control. 2012;26(2):628–631. [Google Scholar]
- Kröncke N., Böschen V., Woyzichovski J., Demtröder S., Benning R. Comparison of suitable drying processes for mealworms (Tenebrio molitor) Innovative Food Science & Emerging Technologies. 2018;50:20–25. [Google Scholar]
- Lenaerts S., Van Der Borght M., Callens A., Van Campenhout L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chemistry. 2018;254:129–136. doi: 10.1016/j.foodchem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Leoni C., Volpicella M., Dileo M.C., Gattulli B.A., Ceci L.R. Chitinases as food allergens. Molecules. 2019;24(11):2087. doi: 10.3390/molecules24112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik J.J. University Press of Florida; 2018. Edible insects and human evolution. [Google Scholar]
- Leung P.S., Chow W.K., Duffey S., Kwan H.S., Gershwin M.E., Chu K.H. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergen. Journal of Allergy and Clinical Immunology. 1996;98(5):954–961. doi: 10.1016/s0091-6749(96)80012-1. [DOI] [PubMed] [Google Scholar]
- Liceaga A.M. Approaches for Utilizing Insect Protein for Human Consumption: Effect of Enzymatic Hydrolysis on Protein Quality and Functionality. Annals of the Entomological Society of America. 2019;112(6):529–532. [Google Scholar]
- Liceaga A.M. Processing insects for use in the food and feed industry. Current opinion in insect science. 2021;48:32–36. doi: 10.1016/j.cois.2021.08.002. [DOI] [PubMed] [Google Scholar]
- Liceaga A.M., Aguilar-Toalá J.E., Vallejo-Cordoba B., González-Córdova A.F., Hernández-Mendoza A. Insects as an Alternative Protein Source. Annual Review of Food Science and Technology. 2021;13:19–34. doi: 10.1146/annurev-food-052720-112443. [DOI] [PubMed] [Google Scholar]
- Liu X., Tian J., Chen D. An allergic shock case that resulted from consuming silkworm pupae. Chinese Recuperative Medicine. 2001;10(2):80. [Google Scholar]
- Lombardi A., Vecchio R., Borrello M., Caracciolo F., Cembalo L. Willingness to pay for insect-based food: The role of information and carrier. Food Quality and Preference. 2019;72:177–187. [Google Scholar]
- Lukiwati D.R. Teak caterpillars and other edible insects in Java. Forest Insects as Food: Humans Bite Back. 2010;99 [Google Scholar]
- MacEvilly C. Bugs in the system. Nutrition Bulletin. 2000;25(4):267–268. [Google Scholar]
- Malm M., Liceaga A.M. Physicochemical Properties of Chitosan from Two Commonly Reared Edible Cricket Species, and Its Application as a Hypolipidemic and Antimicrobial Agent. Polysaccharides. 2021;2(2):339–353. [Google Scholar]
- Melgar-Lalanne G., Hernández-Álvarez A.J., Salinas-Castro A. Edible insects processing: Traditional and innovative technologies. Comprehensive Reviews in Food Science and Food Safety. 2019;18(4):1166–1191. doi: 10.1111/1541-4337.12463. [DOI] [PubMed] [Google Scholar]
- Mendoza-Salazar A., Santiago-López L., Torres-Llanez M.J., Hernández-Mendoza A., Vallejo-Cordoba B., Liceaga A.M., et al. In Vitro Antioxidant and Antihypertensive Activity of Edible Insects Flours (Mealworm and Grasshopper) Fermented with Lactococcus lactis Strains. Fermentation. 2021;7(3):153. [Google Scholar]
- Meyer-Rochow V.B., Gahukar R.T., Ghosh S., Jung C. Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Food. 2021;10(5):1036. doi: 10.3390/foods10051036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.C., Mackie A.R. The impact of processing on allergenicity of food. Current Opinion in Allergy and Clinical Immunology. 2008;8(3):249–253. doi: 10.1097/ACI.0b013e3282ffb123. [DOI] [PubMed] [Google Scholar]
- Mishyna M., Martinez J.-J.I., Chen J., Benjamin O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera) Food Research International. 2019;116:697–706. doi: 10.1016/j.foodres.2018.08.098. [DOI] [PubMed] [Google Scholar]
- Mouritsen O.G., Duelund L., Calleja G., Frøst M.B. Flavour of fermented fish, insect, game, and pea sauces: Garum revisited. International Journal of Gastronomy and Food Science. 2017;9:16–28. [Google Scholar]
- Mutungi C., Irungu F., Nduko J., Mutua F., Affognon H., Nakimbugwe D., et al. Postharvest processes of edible insects in Africa: A review of processing methods, and the implications for nutrition, safety and new products development. Critical Reviews in Food Science and Nutrition. 2019;59(2):276–298. doi: 10.1080/10408398.2017.1365330. [DOI] [PubMed] [Google Scholar]
- Nakagaki B.J., Defoliart G.R. Comparison of diets for mass-rearing Acheta domesticus (Orthoptera: Gryllidae) as a novelty food, and comparison of food conversion efficiency with values reported for livestock. Journal of Economic Entomology. 1991;84(3):891–896. [Google Scholar]
- Nino M.C., Reddivari L., Ferruzzi M.G., Liceaga A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta domesticus. Food. 2021;10(10):2295. doi: 10.3390/foods10102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo S. Porrua Hnos; 1997. Cocina mexicana o historia gastronómica de la ciudad de México. [Google Scholar]
- Nyangena D.N., Mutungi C., Imathiu S., Kinyuru J., Affognon H., Ekesi S., et al. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Food. 2020;9(5):574. doi: 10.3390/foods9050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonincx D.G., van Itterbeeck J., Heetkamp M.J., van den Brand H., van Loon J.J., van Huis A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimani A., Milanović V., Cardinali F., Roncolini A., Garofalo C., Clementi F., et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innovative Food Science & Emerging Technologies. 2018;48:150–163. [Google Scholar]
- Otero P., Gutierrez-Docio A., Del Hierro J.N., Reglero G., Martin D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chemistry. 2020;314 doi: 10.1016/j.foodchem.2020.126200. [DOI] [PubMed] [Google Scholar]
- Pal P., Roy S. Edible insects: future of human food–a review. International Letters of Natural Sciences. 2014;21 [Google Scholar]
- Pali-Schöll I., Meinlschmidt P., Larenas-Linnemann D., Purschke B., Hofstetter G., Rodríguez-Monroy F.A., et al. Edible insects: Cross-recognition of IgE from crustacean-and house dust mite allergic patients, and reduction of allergenicity by food processing. WAO Journal. 2019;12 doi: 10.1016/j.waojou.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiriyangkul P., Srinroch C., Srisomsap C., Chokchaichamnankit D., Punyarit P. Effect of food thermal processing on allergenicity proteins in Bombay locust (Patanga succincta) International Journal of Food Engineering. 2015;1(1):23–28. [Google Scholar]
- Purschke B., Brüggen H., Scheibelberger R., Jäger H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.) European Food Research and Technology. 2018;244(2):269–280. [Google Scholar]
- Ramos-Elorduy J. Anthropo-entomophagy: Cultures, evolution and sustainability. Entomological Research. 2009;39(5):271–288. [Google Scholar]
- Raubenheimer D., Rothman J.M. Nutritional ecology of entomophagy in humans and other primates. Annual Review of Entomology. 2013;58:141–160. doi: 10.1146/annurev-ento-120710-100713. [DOI] [PubMed] [Google Scholar]
- Ribeiro J.C., Cunha L.M., Sousa-Pinto B., Fonseca J. Allergic risks of consuming edible insects: A systematic review. Molecular Nutrition & Food Research. 2018;62(1):1700030. doi: 10.1002/mnfr.201700030. [DOI] [PubMed] [Google Scholar]
- Rumpold B.A., Schlüter O.K. Nutritional composition and safety aspects of edible insects. Molecular Nutrition & Food Research. 2013;57(5):802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- Schösler H., De Boer J., Boersema J.J. Can we cut out the meat of the dish? Constructing consumer-oriented pathways towards meat substitution. Appetite. 2012;58(1):39–47. doi: 10.1016/j.appet.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Searchinger T., Walte R., Hanson C., Ranganathan J. World Resources Institute; 2019. World Resources Report: Creating a Sustainable Food Future. A Menu of Solutions to Feed Nearly 10 Billion People by 2050; p. 564. [Google Scholar]
- Severini C., Azzollini D., Albenzio M., Derossi A. On printability, quality and nutritional properties of 3D printed cereal based snacks enriched with edible insects. Food Research International. 2018;106:666–676. doi: 10.1016/j.foodres.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Shockley M., Lesnik J., Allen R.N., Muñoz A.F. Edible insects in sustainable food systems. Springer; 2018. Edible insects and their uses in North America; past, present and future; pp. 55–79. [Google Scholar]
- Smetana S., Leonhardt L., Kauppi S.-M., Pajic A., Heinz V. Insect margarine: Processing, sustainability and design. Journal of Cleaner Production. 2020;264 [Google Scholar]
- Srivastava S., Babu N., Pandey H. Traditional insect bioprospecting-As human food and medicine. Indian Journal of Traditional Knowledge. 2009;8(4):485–494. [Google Scholar]
- Ssepuuya G., Aringo R., Mukisa I., Nakimbugwe D. Effect of processing, packaging and storage-temperature based hurdles on the shelf stability of sautéed ready-to-eat Ruspolia nitidula. Journal of Insects as Food and Feed. 2016;2(4):245–253. [Google Scholar]
- Steinfeld H., Gerber P., Wassenaar T., Castel V., de Haan C. Food & Agriculture Org; 2006. Livestock's long shadow: environmental issues and options. [Google Scholar]
- Tiencheu B., Womeni H.M., Linder M., Mbiapo F.T., Villeneuve P., Fanni J., et al. Changes of lipids in insect (Rhynchophorus phoenicis) during cooking and storage. European Journal of Lipid Science and Technology. 2013;115(2):186–195. [Google Scholar]
- Tranter H. University of East Anglia; Norwich: 2013. Insects Creeping into English Diets: Introducing Entomophagy to School Children in a Provincial Town. [Google Scholar]
- Tucker C.A. The significance of sensory appeal for reduced meat consumption. Appetite. 2014;81:168–179. doi: 10.1016/j.appet.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Tuorila H., Lähteenmäki L., Pohjalainen L., Lotti L. Food neophobia among the Finns and related responses to familiar and unfamiliar foods. Food Quality and Preference. 2001;12(1):29–37. [Google Scholar]
- Van Broekhoven S., Bastiaan-Net S., de Jong N.W., Wichers H.J. Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species. Food Chemistry. 2016;196:1075–1083. doi: 10.1016/j.foodchem.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Van Huis A. Did early humans consume insects. Journal of Insects as Food and Feed. 2017;3(3):161–163. [Google Scholar]
- van Huis A., Dicke M., Van Loon J. Wageningen Academic Publishers; 2015. Insects to feed the world. [Google Scholar]
- Van Huis A., Van Itterbeeck J., Klunder H., Mertens E., Halloran A., Vantomme P. Food and Agriculture Organization of the United Nations; 2013. Edible Insects: Future Prospects for Food and Feed Security. FAO Forestry Paper, FAO, Rome (187 pp) [Google Scholar]
- Vandeweyer D., Lenaerts S., Callens A., Van Campenhout L. Effect of blanching followed by refrigerated storage or industrial microwave drying on the microbial load of yellow mealworm larvae (Tenebrio molitor) Food Control. 2017;71:311–314. [Google Scholar]
- Vetter R. A case of ingestant allergy from eating a grasshopper. The Food Insects Newsletter. 1995;8(5) [Google Scholar]
- Volpicella M., Leoni C., Dileo M.C., Ceci L.R. Progress in the Analysis of Food Allergens through Molecular Biology Approaches. Cell. 2019;8(9):1073. doi: 10.3390/cells8091073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L., Huang C.H., Lee B.W. Shellfish and house dust mite allergies: is the link tropomyosin? Allergy, Asthma & Immunology Research. 2016;8(2):101–106. doi: 10.4168/aair.2016.8.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynants E., Crauwels S., Verreth C., Gianotten N., Lievens B., Claes J., et al. Microbial dynamics during production of lesser mealworms (Alphitobius diaperinus) for human consumption at industrial scale. Food Microbiology. 2018;70:181–191. doi: 10.1016/j.fm.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Yhoung-aree J. Edible insects in Thailand: nutritional values and health concerns. Forest insects as food: humans bite back. Proceedings of a workshop on Asia-Pacific resources and their potential for development, Chiang Mai, Thailand, 19-21; 2008. [Google Scholar]
- Yi L., Van Boekel M.A., Boeren S., Lakemond C.M. Protein identification and in vitro digestion of fractions from Tenebrio molitor. European Food Research and Technology. 2016;242(8):1285–1297. [Google Scholar]
- Zhao X., Vázquez-Gutiérrez J.L., Johansson D.P., Landberg R., Langton M. Yellow mealworm protein for food purposes-extraction and functional properties. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0147791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska E., Karaś M., Baraniak B. Comparison of functional properties of edible insects and protein preparations thereof. LWT. 2018;91:168–174. [Google Scholar]
Further reading
- Finke M.D. Springer; 2004. Nutrient content of insects. [Google Scholar]