Abstract
Alzheimer’s disease (AD) is the most common form of dementia. AD brain pathology starts decades before the onset of clinical symptoms. One early pathological hallmark is blood-brain barrier dysfunction characterized by barrier leakage and associated with cognitive decline. In this review, we summarize the existing literature on the extent and clinical relevance of barrier leakage in AD. First, we focus on AD animal models and their susceptibility to barrier leakage based on age and genetic background. Second, we re-examine barrier dysfunction in clinical and postmortem studies, summarize changes that lead to barrier leakage in patients and highlight the clinical relevance of barrier leakage in AD. Third, we summarize signaling mechanisms that link barrier leakage to neurodegeneration and cognitive decline in AD. Finally, we discuss clinical relevance and potential therapeutic strategies and provide future perspectives on investigating barrier leakage in AD. Identifying mechanistic steps underlying barrier leakage has the potential to unravel new targets that can be used to develop novel therapeutic strategies to repair barrier leakage and slow cognitive decline in AD and AD-related dementias.
Keywords: Alzheimer’s Disease, Blood-brain Barrier, Barrier Dysfunction, Barrier Leakage, Neurovasculature, Cerebrovasculature
1. INTRODUCTION
1.1. Alzheimer’s Disease: Background, Diagnosis, Pathophysiology, and Current Challenges
Background.
More than 100 years ago, German physician Dr. Alois Alzheimer described “a peculiar disease” that was later named after him (Alzheimer’s disease, AD). Alzheimer defined the disease as presenile dementia with plaques and neurofibrillary tangles (NFTs) as the characteristic pathological hallmarks (Alzheimer, 1906, 1907; Fischer, 1907, 1910). “Dementia” is a general term that refers to loss of cognitive function such as attention, memory, communication, problem-solving, visual perception, and self-management (“Dementia Fact Sheet,”). AD is the most common type of dementia and an irreversible, progressive disease that turns patients into helpless individuals depending on others for performing everyday activities (“Alzheimer’s Disease Fact Sheet,”). In the initial stages, AD patients experience agitation, anxiety, loss of appetite, mood swings, sleep disturbances, and, importantly, a decline in their cognitive abilities (“Alzheimer’s Disease Fact Sheet,”; Ehrenberg, et al., 2018). With the progression of the disease, agitation in patients increases, and episodes of memory loss become more frequent. These episodes come with reduced executive function, deficits in visuospatial skills, as well as speech problems. Anxiety, loss of appetite, mood swings, and sleep disturbances tend to subside at this advanced AD stage. In the late and more severe stages of AD, delusions and hallucinations set in, and patients become vegetative with minimal activity. Based on the age at diagnosis, AD is classified as early-onset or late-onset AD. Specifically, early-onset AD starts between the ages of 30 – 60 years and is due to familial genetic mutations. In contrast, late-onset AD begins after the age of 60 due to various unknown environmental and lifestyle factors.
Diagnosis.
A proper AD diagnosis involves a series of cognitive, imaging, and laboratory tests (Sabbagh, Lue, Fayard, & Shi, 2017). Usually, a clinician recommends cognitive evaluation if the patient or caregiver reports memory-related concerns. In this evaluation, the patient is assessed through one or more standardized tests such as the Mini-Cognitive Assessment, the General Practitioner Assessment of Cognition, Saint Louis University Mental Status Examination (SLUMS), a Clinical Dementia Rating, or the Mini-Mental State Examination (MMSE). The clinician may also perform neurological exams and metabolic tests to exclude other causes that could impair cognition. Further assessment involves looking for amyloid and tau deposits in the brain with cerebrospinal fluid (CSF) biomarker analysis, structural magnetic resonance imaging (MRI), or positron emission tomography (PET). Despite all these state-of-the-art diagnostic methods, a final, definitive diagnosis of AD is based on clinical symptoms, neuropathological markers, and the distribution of plaques and tangles and can only be made postmortem by a team of neuropathologists, neuropsychologists, and neurologists (Braak & Braak, 1991; Ehrenberg, et al., 2018; Nelson, et al., 2012).
Pathophysiology.
Historically, plaques and tangles have been observed in and around degenerating neurons and blood vessels in brain autopsy samples from AD patients (Divry, 1927; Morel, 1954; Scholz, 1938). Electron microscopy and diffraction studies in the 1960s demonstrated that plaques and NFTs are large, organized structures of β-pleated and paired helical filaments (Eanes & Glenner, 1968; Terry, 1963; Terry, Gonatas, & Weiss, 1964). By the 1980s, multiple biochemical studies found that amyloid-beta (Aβ, 4 kDa) accumulates in these plaques as oligomers, protofibrils, and fibrillar sheets (Figure 1A) (Glenner & Wong, 1984; Masters, 1984; Masters, et al., 1985; Roher, et al., 1996). Aβ is a 36–43 amino acid peptide that forms when amyloid precursor protein (APP) is cleaved by alpha- and gamma-secretases within the transmembrane region (Esch, et al., 1990; Sisodia, Koo, Beyreuther, Unterbeck, & Price, 1990). In AD, altered APP processing and impaired Aβ clearance result in 4- to 6-fold higher Aβ brain levels compared to Aβ brain levels typically found in individuals that age (De Strooper, et al., 1998; Funato, et al., 1998; Paresce, Chung, & Maxfield, 1997; Roher, et al., 2009; Scheuner, et al., 1996). Data from several studies showed that NFTs contain the microtubule-associated protein tau (~50 kDa) that is abnormally hyperphosphorylated and forms tau oligomers, granular tau aggregates, and paired helical filaments that accumulate in the brain of AD patients (Figure 1B; Grundke-Iqbal et al. 1986a; Grundke-Iqbal et al. 1986b; Grundke-Iqbal et al. 1979; Köpke et al. 1993; Maeda et al. 2006; Maeda et al. 2007(Grundke-Iqbal, Iqbal, Quinlan, et al., 1986; Grundke-Iqbal, Iqbal, Tung, et al., 1986; Grundke-Iqbal, Johnson, Terry, Wisniewski, & Iqbal, 1979; Kopke, et al., 1993; Maeda, et al., 2007). Tau peptides are 31–32 amino acids long with 2–3 phosphate molecules per peptide that are maintained by tau-specific kinases and phosphatases in the brain (Goedert & Crowther, 1989; Kopke, et al., 1993). In AD, the levels of total tau protein and abnormally hyperphosphorylated tau proteins can be 4- to 8-fold higher than tau protein levels found in the brain of healthy control individuals (Iqbal, Liu, & Gong, 2016). Brain accumulation of both Aβ and tau causes several pathophysiological changes that affect cognitive function in AD patients (Figure 1C-G). One of these pathophysiological changes involves intracellular calcium dysregulation in neurons and astrocytes, leading to excitotoxicity and astrogliosis (Figure 1C) (Abdul, et al., 2009; Bales, et al., 1998; Mattson, et al., 1992). Another pathophysiological change involves astrogliosis and microgliosis, where reactive astrocytes and microglia affect synaptic transmission, which contributes to cognitive impairment (Figure 1D; (DeKosky & Scheff, 1990; Hong, et al., 2016; Itagaki, McGeer, Akiyama, Zhu, & Selkoe, 1989; Terry, et al., 1991; Vogels, Murgoci, & Hromadka, 2019). A third pathophysiological change is a reduced lysosomal degradation capacity of neurons that leads to autophagy and neuronal loss (Figure 1E) (Cataldo, Barnett, Mann, & Nixon, 1996; Nixon, et al., 2005)). The fourth change is development of cerebral amyloid angiopathy (CAA), where Aβ builds up in cerebral blood vessels resulting in ruptured vessel walls over time (Figure 1F) (E. E. Smith & Greenberg, 2009; Vinters, 1987). While plaques and tangles directly affect neurons in the brain, CAA disrupts synaptic and neurovascular networks by causing microhemorrhages, infarcts, and white matter lesions in the brain (E. E. Smith & Greenberg, 2009; Vinters, 1987). Fifth, CAA causes neurovascular dysfunction, which worsens cognitive function in AD patients (Brenowitz, Nelson, Besser, Heller, & Kukull, 2015; K. Jellinger, 2002). Besides these changes, myelin breakdown also affects synaptic connectivity in AD (Figure 1G) (Bartzokis, et al., 2003). Collectively, these pathophysiological changes make AD a multifactorial disease and add to the challenges in finding an effective treatment for patients.
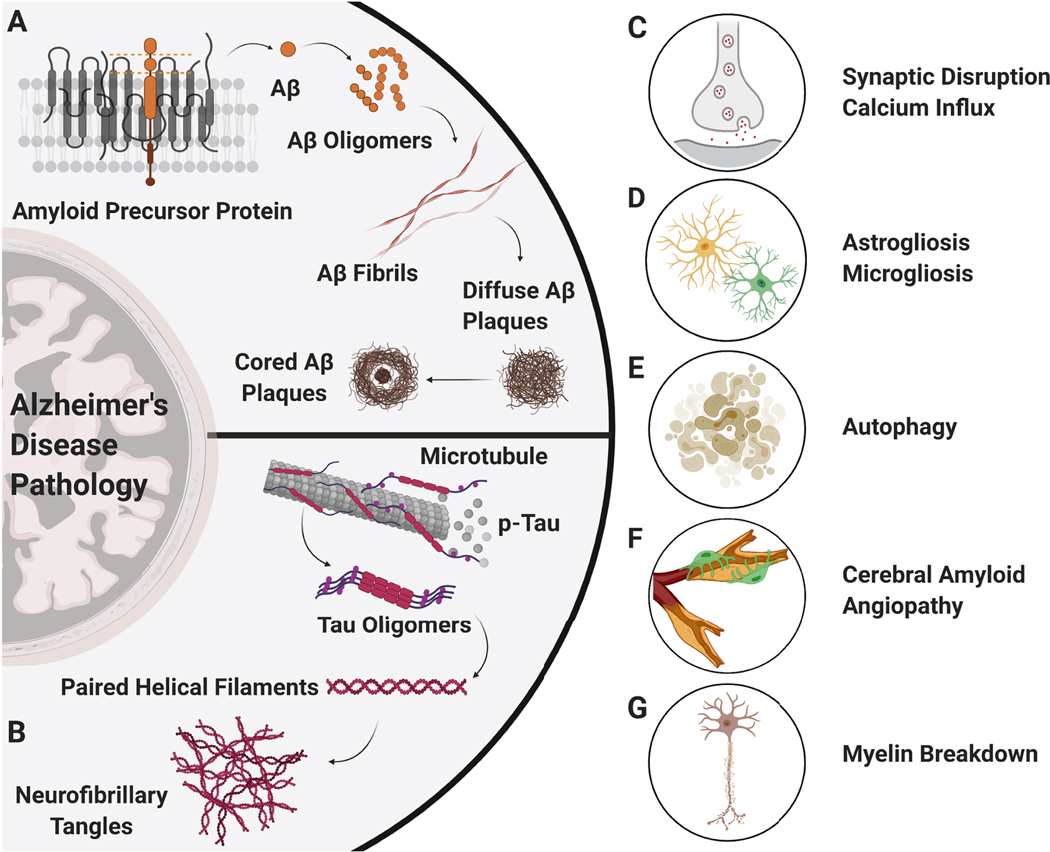
Figure 1. Pathological Changes in the Central Nervous System in Alzheimer’s Disease (AD).
Amyloid-beta (Aβ) and tau have been proposed to be the two key players that drive pathological progression in AD. A) Aβ, formed by the abnormal and sequential cleavage of amyloid precursor protein (APP), binds to other Aβ subunits to form oligomers, protofibrils, fibrils, diffused Aβ plaques (surrounded by glial inflammation), and Aβ plaques with a neuritic core causing neurodegeneration. B) In contrast, microtubule-associated tau proteins get abnormally hyperphosphorylated to form tau oligomers, paired helical filaments (PHF), and neurofibrillary tangles that drive AD pathology. Together, Aβ, tau, and their protein aggregates affect both neuronal and glial cells in the brain leading to C) elevated calcium influx and disruption in synaptic transmission, D) astrocytic and microglial inflammation, E) autophagy, F) cerebral amyloid angiopathy, and G) breakdown of the myelin sheath around neurons.
Current Challenges.
AD drug development is focused on 5 areas: 1) neurotransmitter systems (38%), 2) Aβ pathology (33%), 3) neuroinflammation (17%), 4) tau pathology (10%), and 5) cholesterol metabolism (2%; (“Therapeutics”)). Despite all efforts, only 5 FDA-approved drugs are currently available, and they all target the neurotransmitter system (Di Santo, Prinelli, Adorni, Caltagirone, & Musicco, 2013; Molino, Colucci, Fasanaro, Traini, & Amenta, 2013). Three of these drugs, donepezil, galantamine, and rivastigmine, are cholinesterase inhibitors that increase acetylcholine levels in the synaptic cleft, thereby improving cholinergic neurotransmission and counteracting cognitive decline in AD. The fourth drug, memantine, is an N-methyl-D-aspartate receptor antagonist that blocks the effect of excess glutamate, thereby preventing glutamate-mediated calcium excitotoxicity in the brain. The fifth drug, aducanumab, targets parenchymal Aβ aggregates in the brain (Salloway, et al., 2009). All these drugs have a modest effect on cognition at best and lower dementia-related symptoms in only 40–70% of AD patients (Di Santo, et al., 2013; “Effects of Alzheimer’s disease drugs,”). Moreover, these improvements tend to subside after 6–12 months of pharmacotherapy as symptoms worsen, and none of these drugs influence the underlying pathophysiological processes (“Effects of Alzheimer’s disease drugs,”). To date, experimental drugs developed to have a disease-modifying effect show no significant improvement in cognitive performance in clinical studies (Cummings, Lee, Ritter, Sabbagh, & Zhong, 2019). Despite tremendous research efforts, none of these experimental drugs has made it to the market. Thus, there is a critical need for effective disease-modifying strategies that slow the progression of AD pathology and cognitive decline in AD patients.
1.2. Anatomy and Physiology of the Blood-Brain Barrier
Anatomy.
The cerebrovascular network originates from large pial arteries (diameter: 200–1,000 μm) that are surrounded by the CSF and the three meningeal layers at the brain surface (Bevan, Dodge, Walters, Wellman, & Bevan, 1999). Branches from the large pial arteries form penetrating arteries (70–200 μm) that dive deep into the brain parenchyma. These descending arteries feed parenchymal arterioles (20–70 μm) that subdivide into smaller precapillary arterioles (< 35 μm) and eventually form capillaries (3–7 μm). These capillaries are a unique part of the cerebrovascular network. With a diameter of 3–7 μm and a thickness of 0.1 μm, brain capillaries are the smallest vessels of the cerebrovascular system and form the structural basis of the blood-brain barrier (Figure 2) (Rodriguez-Baeza, Reina-de la Torre, Poca, Marti, & Garnacho, 2003). Structurally, brain capillary endothelial cells are covered by a 0.2–2 μm thick glycocalyx layer on the inner luminal side and a 50–100 nm vascular basement membrane on the outer abluminal side (Figure 2A, 2B) (Chappell, Westphal, & Jacob, 2009; L. Xu, Nirwane, & Yao, 2019). The glycocalyx layer on the luminal side is a scaffold of short membrane-bound glycoproteins as well as long, sulfated, and non-sulfated glycosaminoglycan chains that adsorb other proteins, water molecules, enzymes, and cells to nourish and protect the endothelial cell from injury, blood flow changes, and toxic substances (Chappell, et al., 2009; Schaefer & Schaefer, 2010). On the abluminal side, the basement membrane is composed of fibrous proteoglycans like collagen, laminin, nidogen, and fibronectin. Here, collagen and laminin isoforms self-assemble to create primary scaffolds held together by nidogen, fibronectin, thrombospondin, and other proteins and enzymes (Figure 2B) (Thomsen, Routhe, & Moos, 2017; L. Xu, et al., 2019)). Proteoglycans such as perlecan and agrin further stabilize this network, modulate signaling mechanisms, and regulate cell-matrix interactions (Barber & Lieth, 1997; Liebner, Czupalla, & Wolburg, 2011; Martin & Sanes, 1997; Osada, et al., 2011; Thomsen, et al., 2017). Proteoglycans in the basement membrane also recruit, anchor, and embed pericytes for each capillary to regulate capillary blood flow and neurovascular responses. They also adhere to and anchor astrocytes to coordinate oxygen and glucose delivery (Attwell, et al., 2010; Hirschi & D’Amore, 1996). Adhesion molecules that help proteoglycans mediate these interactions include immunoglobulin-cell adhesion molecules (IgCAMs), junctional adhesion molecules (JAMs), integrins, as well as cadherins and selectins (Hynes, 1986; Kramer & Marks, 1989; Pytela, Pierschbacher, Ginsberg, Plow, & Ruoslahti, 1986; Sarelius & Glading, 2015). Connexins and other junctional proteins further seal the junctions between astrocytes and pericytes (Figure 2C-G) (Zhao & Gong, 2015). Together, endothelial cells, pericytes, astrocytes, and neurons form the “Neurovascular Unit” (NVU) that regulates barrier function and controls what goes in and out of the brain (Figure 2C-G) (Iadecola, 2004; McConnell, Kersch, Woltjer, & Neuwelt, 2017).
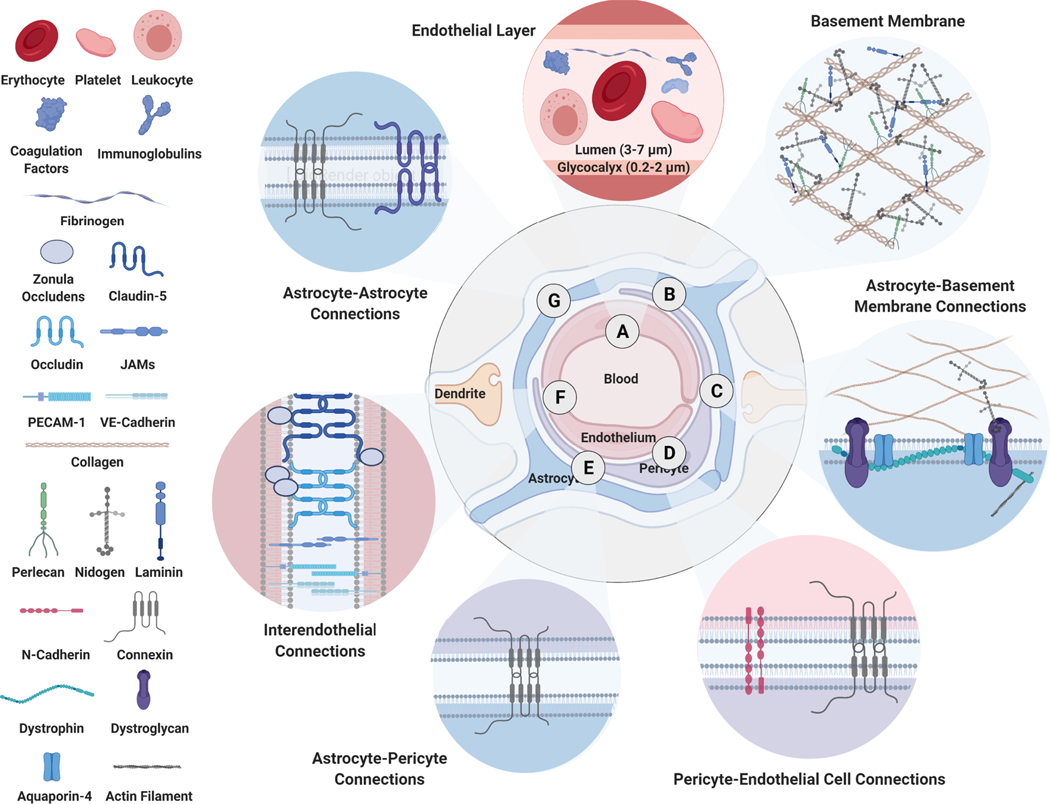
Figure 2. Blood-Brain Barrier in Health.
A healthy neurovascular unit at the capillary endothelium forms the structural basis of the blood-brain barrier. A) Brain capillary endothelial cells are covered with a 200–2,000 nm thick glycoprotein-rich glycocalyx layer on the luminal side and B) a 50–100 nm thick proteoglycan-rich vascular basement membrane on the abluminal side. Proteoglycans in the vascular basement membrane anchor C) astrocytes and D) pericytes around capillary endothelial cells. E) Adhesion molecules further seal the connections among and between astrocytes and pericytes. F-G) At the endothelial interface, interendothelial junctions are further sealed by proteins such as claudin-5, occludin, zonula occludens-1, 2, 3, platelet endothelial cell adhesion molecule-1 (PECAM-1), vascular cell adhesion molecule-1 (VCAM-1), vascular endothelial (VE) – cadherins and form an intact blood-brain barrier.
Physiology.
The blood-brain barrier represents 1) a physical barrier that prevents passive brain uptake of endogenous and exogenous compounds, 2) a biochemical barrier that actively restricts brain uptake of xenobiotics and regulates nutrient supply, 3) a metabolic barrier that degrades neurotoxins, drugs, and other compounds at the blood-brain interface, and 4) an immunological barrier that limits immune cell entry into the brain. By forming a tight seal, tight junctions create a physical barrier between adjacent endothelial cells. This physical barrier lacks intercellular clefts and has low pinocytotic activity making it extremely difficult for compounds to cross it (Figure 2F). A myriad of influx and efflux transporters at the luminal and abluminal membrane of the endothelial cells constitute a biochemical barrier (Abbott, 2013; Daneman & Prat, 2015; Hartz & Bauer, 2011). The transporters at the barrier rigorously regulate what goes in and out of the brain. Phase I and phase II metabolic enzymes in endothelial cells comprise the metabolic barrier (Agundez, Jimenez-Jimenez, Alonso-Navarro, & Garcia-Martin, 2014). Initially, it was thought that there was an immunological barrier that prevents immune cells from entering the brain, and thus, the brain is an “immune-privileged organ”. This concept was proven incorrect; instead, the immunological barrier tightly regulates the migration of immune cells into and out of the brain (Greenwood, et al., 2011).
In a healthy brain, the blood-brain barrier is an active and dynamic barrier that separates the periphery from the CNS, prevents entry of a broad spectrum of compounds into the brain, maintains brain homeostasis, supplies the brain with oxygen and nutrients, and takes care of waste removal. In disease, however, this barrier is often damaged and can no longer maintain the functions required to maintain brain homeostasis and proper brain function.
1.3. Blood-Brain Barrier Leakage in Alzheimer’s Disease
In this review, we define barrier dysfunction as an umbrella term for pathophysiological changes that occur at the blood-brain barrier in AD. These changes include structural alterations at the NVU, changes in Aβ and glucose influx/efflux transport mechanisms and leakage of bloodborne proteins for more detail we refer the reader to existing reviews (Kyrtata, Emslet, Sparasci, Parkes, & Dickie, 2021; Kirabali, et al., 2020; Kadry, Noorani, & Cucullo, 2020; Jia, et al., 2020; Chagnot, Barnes, & Montagne, 2021; Wang, et al., 2021). In the remainder of this article, we specifically focus on barrier leakage.
Blood-brain barrier leakage in AD was first observed more than 40 years ago, but it was not established until recently that a leaky barrier contributes to AD pathology (Glenner, 1979; Iadecola, 2013). A series of pathophysiological changes in AD affect the NVU and alter blood-brain barrier properties, thus leading to barrier dysfunction and ultimately leakage (Figure 3) (Kalaria & Harik, 1992; Montagne, et al., 2020; Richard, et al., 2010). Over the past decade, numerous studies have indicated a link between barrier leakage and cognitive decline in AD (de la Torre, 2017; Di Marco, Farkas, Martin, Venneri, & Frangi, 2015; Love & Miners, 2016; Montagne, et al., 2020; Nation, et al., 2019; Zhao & Gong, 2015). In this section, we will outline the most prominent changes that occur in each of these NVU components during AD.
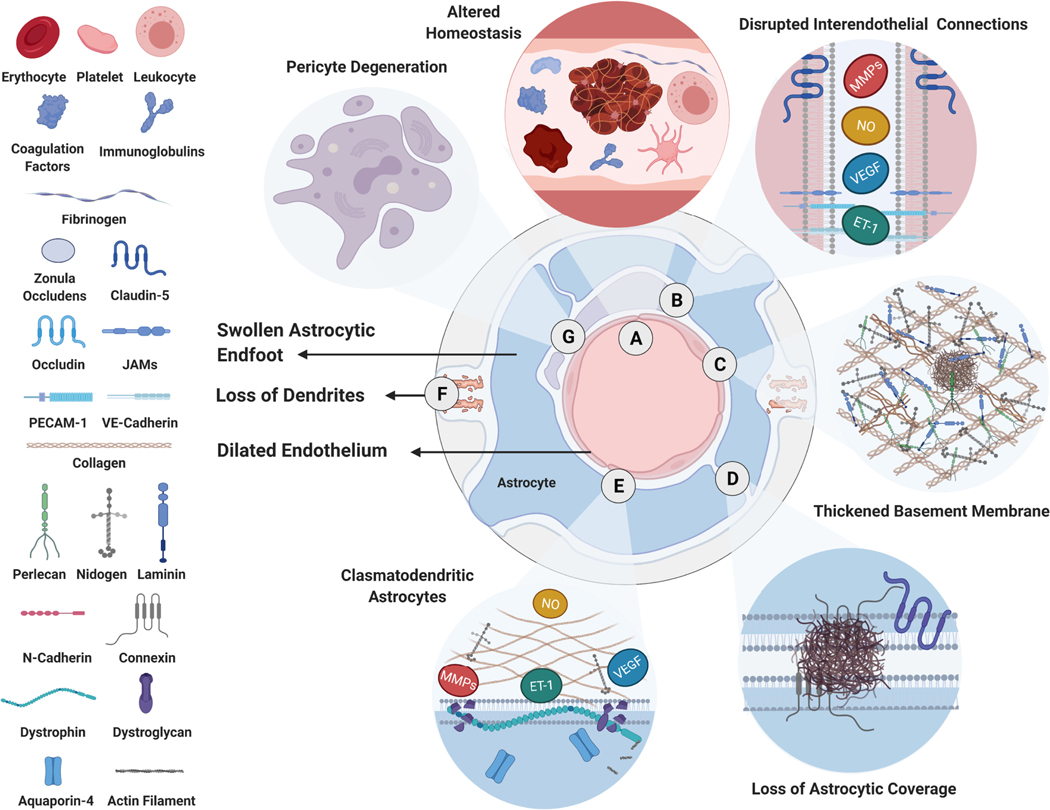
Figure 3. Blood-Brain Barrier in Alzheimer’s Disease.
Pathological changes at the neurovascular unit in AD include A) increased coagulation and thrombosis, B) structural changes in endothelial cells and disruption of interendothelial junctions, C) altered proteoglycan and glycoprotein levels in the basement membrane, D) structural changes in astrocytes such as swollen astrocytic end-feet, loss of astrocytic coverage around endothelial cells, E) clasmatodendrosis, F) in addition to a loss of dendritic connections and G) pericytes around endothelial cells.
Endothelial Cells.
In AD, endothelial cells undergo structural changes that disrupt interendothelial junctions and thus limit their connections and interactions with other NVU components (Figure 3A, 3B) (Oikari, et al., 2020). For example, data from several studies show that in conditions like CAA, shedding of platelet endothelial cell adhesion molecule (PECAM-1/CD31) and apoptosis occur in endothelial cells (Magaki, et al., 2018; Nielsen, Londos, Minthon, & Janciauskiene, 2007; Xue, et al., 2012). In addition, other adhesion molecules such as the intercellular adhesion molecule 1 (ICAM-1) and the vascular cell adhesion molecule 1 (VCAM-1) contribute to the disruption of tight-junction proteins, increased actin stress fiber formation, and altered endothelial cell (Clark, Manes, Pober, & Kluger, 2007; Haarmann, et al., 2015). These changes disrupt paracellular transport at the blood brain barrier. In addition, endothelial cells also show increased transcellular movement of bloodborne substances via macropinocytosis, clathrin-dependent endocytosis, caveolae-mediated transcytosis, or vesicular trafficking (Zhou et al., 2021; Pandit et al., 2020; De Bock et al., 2016; Gurnik et al., 2016). Increased paracellular and transcellular pathways contribute to a leaky NVU in AD. Altered Glycocalyx and Basement Membrane. Structural and biochemical alterations in the glycocalyx and basement membrane change cell-cell and cell-matrix adhesion at the entire NVU in AD (Figure 3B, C). Data from multiple studies indicate that these alterations are due to elevated proteoglycan levels in the brain. Fillit et al. (Fillit, Kemeny, Luine, Weksler, & Zabriskie, 1987) and Jenkins and Bachelard (Jenkins & Bachelard, 1988) showed that total proteoglycan levels are elevated in brain samples of AD patients compared to levels in samples of non-demented subjects. For instance, small cell-surface proteoglycans such as glypican (60–70 kDa) and syndecan (20–30 kDa) are located more frequently around capillaries that display CAA pathology (Lashley, et al., 2006; Lorente-Gea, et al., 2020; van Horssen, et al., 2001; Watanabe, et al., 2004). Hyaluronic acid (HA) is another glycocalyx and capillary basement membrane proteoglycan that is elevated in CSF samples from AD patients (Nagga, Hansson, van Westen, Minthon, & Wennstrom, 2014; Nielsen, Palmqvist, Minthon, Londos, & Wennstrom, 2012). In this regard, it is noteworthy that elevated HA protein levels are associated with increased Braak staging and a greater extent of cognitive decline in AD patients (Reed, et al., 2019). Collagen and agrin are two other proteoglycans that contribute to basement membrane thickening and fragmentation in AD (Christov, Ottman, Hamdheydari, & Grammas, 2008; Keable, et al., 2016; Magaki, et al., 2018; Merlini, Wanner, & Nitsch, 2016; Szpak, et al., 2007). Agrin shows “ragged and irregular” immunoreactivity within the cerebral microvasculature of AD patients compared to a uniform immunoreactivity along the cerebral microvasculature of cognitively normal subjects (Donahue, et al., 1999; van Horssen, et al., 2001). Agrin fragmentation also increases with AD progression, leading to higher soluble/fragmented agrin levels and reduced concentrations of insoluble/full-length agrin in brain samples (Berzin, et al., 2000; Salloway, et al., 2002). Collectively, altered proteoglycan composition in the capillary basement membrane affects barrier integrity in AD.
Astrocytes.
In AD, astrocytes undergo structural changes that lead to “clasmatodendrosis”, i.e., beading and disintegration of astrocyte projections along with cytoplasmic vacuolization, atrophy, and swelling of astrocytic end-feet (Figure 3D, 3E) (Boespflug, et al., 2018; Cullen, 1997; Higuchi, Miyakawa, Shimoji, & Katsuragi, 1987; Mancardi, Perdelli, Rivano, Leonardi, & Bugiani, 1980; Montagne, Zhao, & Zlokovic, 2017; Sweeney, Sagare, & Zlokovic, 2018; Yamashita, Miyakawa, & Katsuragi, 1991). At the end feet, reduced levels of anchoring proteins such as aquaporin-4 (AQP4), inwardly rectifying potassium channel (Kir4.1), and dystrophin 1 further limit astrocyte-endothelial connections (Boespflug, et al., 2018; Wilcock, Vitek, & Colton, 2009; Zeppenfeld, et al., 2017). These structural changes reduce astrocyte anchoring at the NVU, decreasing cerebral blood flow and neurovascular interactions (Iadecola, 2013).
Neurons.
Neurons regulate cerebrovascular organization and cerebral blood flow by secreting neurotransmitters such as glutamate, acetylcholine, or γ-aminobutyric acid at the neuronal-glial interfaces near synapses (Girouard & Iadecola, 2006; Hendrikx, et al., 2019; Lacoste, et al., 2014; Muoio, Persson, & Sendeski, 2014; Petzold & Murthy, 2011). Extensive synaptic dysfunction and neuronal loss in AD can thus affect cerebrovascular tone and cerebral blood flow (Figure 3F) (Babic, 1999; Hamel, 2004; Kirkwood, et al., 2016; Mesulam, et al., 2019; Oh, et al., 2019; Van Beek & Claassen, 2011). Initial postmortem studies also showed that cortical neurons in brain tissue slices from AD patients are immunoreactive for proteins like agrin and occludin at the NVU (Berzin, et al., 2000; Romanitan, Popescu, Winblad, Bajenaru, & Bogdanovic, 2007). In contrast, CSF analysis does not indicate a strong association between markers of neuronal injury and blood-brain barrier dysfunction in AD (Muszynski, et al., 2017). Thus, the relationship between neurodegeneration and barrier dysfunction requires more research.
Pericytes.
In AD, pericytes undergo aggressive degradation (Halliday, et al., 2016; Wilhelmus, et al., 2007). In addition, cell surface receptors such as neural/glial antigen-2 (NG2) expressed along pericytes undergo extensive shedding, rendering these receptors unfunctional (Figure 3G) (Halliday, et al., 2016; Wilhelmus, et al., 2007). Data from postmortem studies using human tissue samples indicate that pericytes along degenerated vessels accumulate lipofuscin-like material and Aβ fibrils in AD (Baloyannis & Baloyannis, 2012; Raha, et al., 2017; Szpak, et al., 2007). As Aβ pathology progresses, these pericytes do not fully adhere to the vascular basement membrane loaded with Aβ clusters and collagen fibrils (Szpak, et al., 2007). In vitro, Aβ fibrils reduce soluble NG2 levels, a proteoglycan that supports pericyte-basement membrane interactions (Schultz, Nielsen, Minthon, & Wennstrom, 2014). Hippocampal slices from AD patients (n=13) have 1.5-fold lower (p < 0.05) NG2-positive pericytes per capillary compared to hippocampal slices from non-demented subjects (n=9) (Schultz, et al., 2018). Moreover, low levels of pericyte-specific soluble proteins are directly associated with lower cognitive scores for episodic memory, semantic memory, perceptual speed, visuospatial ability, and global cognition in AD patients (Bourassa, Tremblay, Schneider, Bennett, & Calon, 2020).
Blood-Brain Barrier Dysfunction Leads to Barrier Leakage.
While we defined barrier dysfunction as an umbrella term for all pathophysiological changes at the NVU, barrier leakage specifically refers to increased transcellular and paracellular movement of bloodborne substances into the brain. Persistent stressors such as impaired perivascular Aβ clearance, oxidative stress, and reduced proteasomal degradation can cause sustained injuries and degeneration at the NVU, causing blood-brain barrier leakage. Meta-analyses indicate that barrier leakage is a common clinical and postmortem observation in AD that worsens cognitive decline and affects outcomes of experimental therapeutic strategies in enrolled AD patients (Farrall & Wardlaw, 2009; Janelidze, et al., 2017).
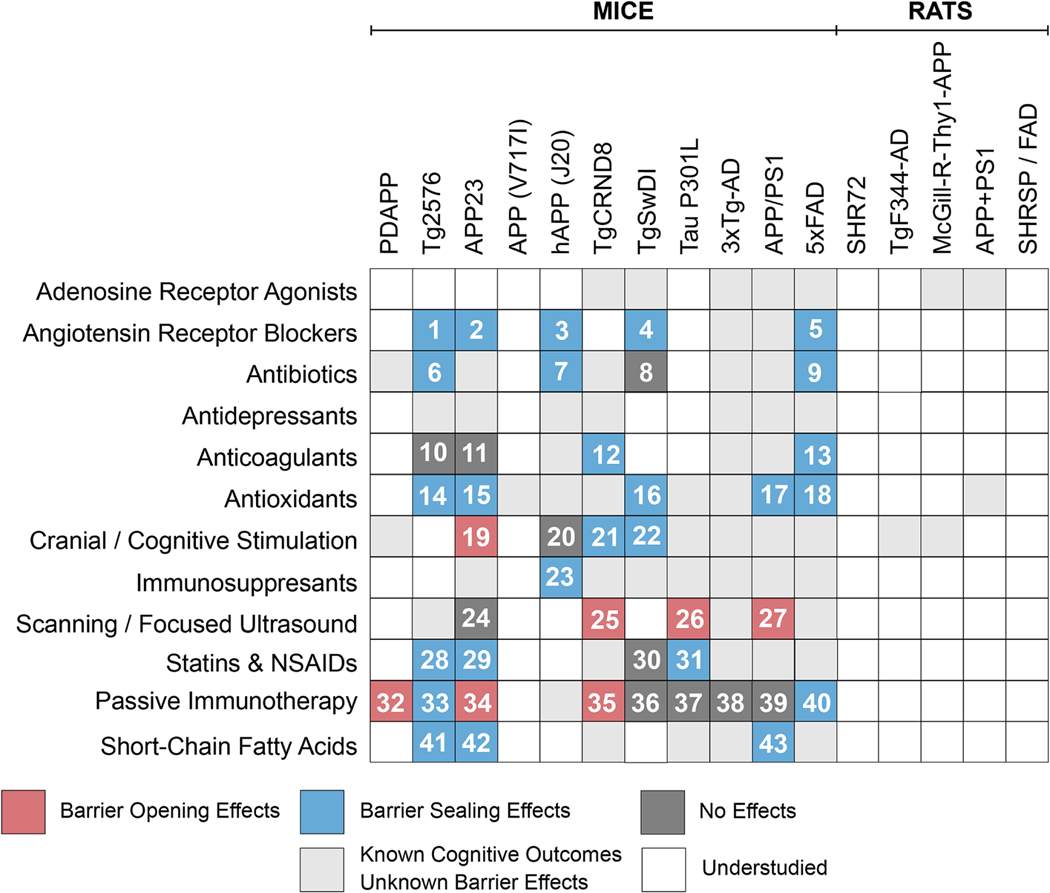
2. Preclinical Evidence of Barrier Leakage in Animal Models of Alzheimer’s Disease
Animal models are essential in preclinical AD research to study mechanisms underlying pathological processes and test novel therapeutic strategies to facilitate their translation into the clinic. In this section, we first introduce transgenic mouse AD models and describe their usefulness to study blood-brain barrier leakage. We then shift the focus to other animal models, including transgenic rats, dogs, and macaques that develop Aβ pathology and describe how these models could be used to investigate barrier leakage in AD.
2.1. Barrier Leakage in Mouse Models of Alzheimer’s Disease
Transgenic mouse models are specifically designed for AD research to mimic certain aspects of disease pathology. Transgenic AD mice aggressively accrue neuropathological changes and cognitive decline over their lifetime. Recent studies found that transgenic AD mice also develop blood-brain barrier dysfunction that causes varying degrees of barrier leakage at different ages depending on the genetic make-up of the respective model (Figure 4). These pathological changes make transgenic mouse models suitable for 1) studying underlying mechanism(s) that link barrier leakage to cognition and 2) testing mechanism-based therapeutic strategies to repair barrier leakage and slow cognitive decline in AD. In the following paragraphs, we summarize characteristic features of commonly used mouse AD models, outline the extent and timeline of barrier leakage, and highlight potential mechanisms that lead to barrier leakage in these mice.
Figure 4. Blood-Brain Barrier Leakage & Pathological Changes in Transgenic Mouse Models of AD.
Overview of eleven of the most frequently used transgenic mouse models of AD and the onset (in months) of different pathological changes in each model. Pathological changes include cognitive dysfunction (beige), Aβ pathology (grey), cerebral amyloid angiopathy (tan), cerebral blood flow reduction (light red), and microhemorrhages (light blue). An exhaustive literature search (1970 – 2020) revealed that barrier leakage has been previously characterized in these mice using tracers like albumin (dark red, 70 kDa), dextrans (4–150 kDa), IgG (150 kDa), Evans Blue dye (0.96 kDa) and Gadolinium (0.33 kDa). The extent of leakage for these tracers is depicted as log (fold-change) of the leakage value for transgenic mice compared to that of age-matched non-transgenic mice.
2.1.1. PDAPP Model.
In 1995, Games et al. (Games, et al., 1995) generated PDAPP mice, the first transgenic mouse AD model (background strain: C57B6 x DBA2). The model is called PDAPP (short for “PDGF promoter plus APP”) because PDAPP mice harbor a human amyloid precursor protein (hAPP) gene segment containing the Indiana mutation V717F and the neuronal platelet-derived growth factor β (PDGF-β) promoter that drives hAPP overexpression in the brain, heart, and lungs (Caroni, 1997; Games, et al., 1995). With increasing age, PDAPP mice develop spatial working memory deficits (4 months) followed by recognition memory deficits (6 months), Aβ deposits (6–9 months), and neuronal dystrophy (14 months) (Dodart, Mathis, Bales, Paul, & Ungerer, 1999; Hartman, et al., 2005; Masliah, Sisk, Mallory, & Games, 2001). Seubert et al. (Seubert, et al., 2008) showed that insoluble Aβ plaques drive cerebral Aβ deposition in PDAPP mice, and Kim et al. (Kim, Basak, & Holtzman, 2009) showed that PDAPP mice do not develop tauopathies or have neuronal loss and cognitive changes in young (< 6-months) PDAPP mice precede Aβ deposition. Thus, interpretation of the nature of the cognitive impairment in this model requires careful consideration. Neurovascular pathology in PDAPP mice, specifically CAA and microhemorrhages, occurs at 15 months but is not as widespread as in other mouse AD models such as the Tg2576 or APP23 models described below (Racke, et al., 2005; Schroeter, et al., 2008). Fryer and co-workers showed that knocking out apolipoprotein-E (APOE) prevents development of CAA and microhemorrhages in 15- and 24-month-old PDAPP mice (Fryer, et al., 2005). Paul et al. (J. Paul, Strickland, & Melchor, 2007) showed that Evans blue (0.96 kDa) extravasation in 6-month-old PDAPP mice is 10-fold higher than in age-matched wild type mice. Since Evans blue binds to large endogenous molecules like IgG and albumin among other proteins, it is unclear if these changes reflect extravasation of free dye or protein-bound dye (Saunders, Dziegielewska, Mollgard, & Habgood, 2015). In contrast, Blockx et al. (Blockx, et al., 2016) used dynamic contrast-enhanced MRI (DCE-MRI) and detected trace amounts of gadolinium leakage in the brain parenchyma of 12-to-16-month-old PDAPP and wild type mice that received the contrast agent gadolinium by intravenous bolus injection. In summary, the mouse PDAPP model is suitable for studying the link between neurovascular Aβ pathology, CAA, and blood-brain barrier leakage in AD.
2.1.2. Tg2576 Model.
In 1996, Hsiao et al. (Hsiao, et al., 1996) described the Tg2576 mouse AD model (background strain: Swiss-Webster x C57B6/DBA2). To date, this model is one of the most commonly used and best-characterized mouse models in preclinical AD research. Tg2576 mice harbor the Swedish double-mutation KM670/671NL on the hAPP gene. In patients, this double-mutation causes early-onset familial AD. The mouse-hamster prion protein promoter non-specifically drives hAPP overexpression in Tg2576 mice in the brain and several peripheral tissues such as the heart, kidney, and lungs (Boy, et al., 2006). Unlike PDAPP mice that overexpress neuronal hAPP, non-specific hAPP overexpression in Tg2576 mice causes brain-wide and early Aβ pathology. Specifically, Tg2576 mice develop soluble Aβ pools (< 3 months) followed by spatial working memory deficits (5 months), reduced cerebral blood flow (8 months), Aβ plaques (11–13 months), and tau oligomers associated with cerebrovascular Aβ deposits (22 months) (Castillo-Carranza, et al., 2017; Irizarry, McNamara, Fedorchak, Hsiao, & Hyman, 1997; Kawarabayashi, et al., 2001). Eventually, these soluble Aβ pools drive cognitive decline and CAA in Tg2576 mice by 9–10 months (S. W. Park, et al., 2014; Robbins, et al., 2006; Shin, et al., 2007; Westerman, et al., 2002). Using Tg2576 mice, Gregory et al. (Gregory, et al., 2012) showed intravenous administration of the γ-secretase inhibitor LY411575 and the high-affinity Aβ-binding protein gelsolin prevented CAA formation but did not clear pre-existing CAA deposits. This finding suggests that CAA spreads in the anterior brain regions of these mice and propagates laterally between 10–16 months of age along leptomeningeal vessels and develops globally by 23 months of age (Domnitz, et al., 2005). Further, CAA develops along existing networks of affected vessels instead of forming new foci in unaffected vessels (Robbins, et al., 2006). Microhemorrhages start to appear in Tg2576 mice at about 15 months of age, and the number of microhemorrhages increases with age (Fisher, et al., 2011). Fryer et al. (Fryer, et al., 2005) showed that knocking in the APOE4 allele in Tg2576 mice exacerbates CAA pathology but not parenchymal Aβ plaque deposition, which indicates that APOE4 expression triggers CAA and microhemorrhages in this mouse model.
Paul et al. (J. Paul, et al., 2007) showed that in 6-month-old Tg2576 mice, Evans blue leakage is 10-fold higher compared to that in age-matched wild type mice. Likewise, Goldman et al. (Elhaik Goldman, et al., 2018) reported a 2-fold higher leakage for gadolinium-based tracers in 8-month-old Tg2576 mice compared to age-matched wild type mice. Data from both studies also show that leakage of the respective vascular marker is 2-fold higher in 12-month-old Tg2576 mice than in wild type mice (Elhaik Goldman, et al., 2018; J. Paul, et al., 2007). Over time, older (10–25 months) Tg2576 mice show dense vascular Aβ deposits that are immunoreactive for IgG, plasminogen (84–90 kDa), tissue plasminogen activator (tPA; 70 kDa), and albumin (70 kDa), indicating that barrier leakage contributes to extravasation of blood-borne substances in the brain (Kumar-Singh, et al., 2005; J. W. Lee, et al., 2007). Together, these studies show that in the microvasculature of Tg2576 mice, barrier leakage follows CAA.
Despite ample evidence showing barrier leakage in Tg2576 mice, a leaky barrier does not necessarily imply easier brain access for intravenously injected tracers or therapeutic drugs. Thakker et al. (Thakker, et al., 2009) first observed this phenomenon for the anti-Aβ antibody 6E10, which was more effective in resolving CAA after intracerebroventricular infusion using osmotic minipumps compared to intravenous injection. Data from a study by Hawkes et al. (Hawkes, et al., 2011) support this finding: fluorescent-labeled dextran (10 kDa) was intravenously injected into 22-month-old Tg2576 and wild type mice, but the marker reached fewer capillaries in Tg2576 mice than in wild type mice (~1 vs. 12%). The biochemical changes underlying barrier leakage in capillaries of Tg2576 mice include reduced protein expression levels and disrupted expression patterns of tight junctions, including zonula occludens (ZO-1) and occludin (Biron, Dickstein, Gopaul, & Jefferies, 2011; Hartz & Bauer, 2011). Consistent with this, Keaney et al. (Keaney, et al., 2015) found that treating Tg2576 mice with claudin-5 and occludin siRNAs increased paracellular barrier permeability for smaller (3 kDa) but not larger (10 kDa) dextrans.
In summary, soluble Aβ drives blood-brain barrier leakage and vascular injury in Tg2576 mice. Hence, the Tg2576 mouse model is useful for studying soluble Aβ changes across the blood-brain interfaces, the impact of CAA, barrier leakage, and cognitive impairment in AD.
2.1.3. APP23 Model.
In 1997, Sturchler-Pierrat et al. (Sturchler-Pierrat, et al., 1997) described the APP23 mouse AD model (background strain: C57BL/6). Like the Tg2576 mouse model, APP23 mice harbor the Swedish double-mutation on the hAPP gene, but the neuronal Thy1 promoter drives hAPP overexpression (Caroni, 1997; Sturchler-Pierrat, et al., 1997). Differences in the promoter between the APP23 (Thy1) and Tg2576 (PrP) models reflect several differences in the onset and severity of Aβ pathology in these mice. First, in contrast to Tg2576 mice, soluble Aβ blood levels do not increase in APP23 mice over time (Calhoun, et al., 1999; Kuo, et al., 2001; Thal, Griffin, & Braak, 2008). Second, APP23 mice develop spatial memory deficits 3 months earlier and Aβ plaques and tauopathy 6 months earlier than Tg2576 mice. Thus, the timeline and extent of Aβ pathology differ between these two models. (Calhoun, et al., 1999; Kelly, et al., 2003; Reuter, Venus, Heiler, et al., 2016; Sturchler-Pierrat, et al., 1997; Van Dam, et al., 2003). Third, knocking out the APOE allele leads to vascular damage and oxidative stress in APP23 mice (Tibolla, et al., 2010). In contrast, overexpressing APOE4 leads to vascular injury and oxidative stress in Tg2576 mice (Fryer, et al., 2005).
Vascular abnormalities are evident in APP23 mice at 6 months of age. One of these abnormalities is activated platelets that overexpress integrin on their surface, which facilitates the adhesion of activated platelets with fibrinogen and collagen, leading to vessel occlusion (Jarre, et al., 2014). In addition, IgG levels are elevated (1.2-fold) in neurons, glial cells, and blood vessels of 6-month-old APP23 mice compared to age-matched wild type mice, suggesting that IgG synthesis occurs in various brain cells in these mice (Beckmann, et al., 2003; J. Shang, et al., 2016). By 11 months of age, reduction in cerebral blood flow is evident, whereas CAA and microhemorrhages occur around 18 months (Beckmann, et al., 2003; Kuo, et al., 2001; Winkler, et al., 2001). Around the same time, APP23 mice also have other vascular irregularities such as kinked, twisted, and/or studded blood vessels with knobs and protrusions and missing vascular networks in the frontal and temporal cortices (Meyer, Ulmann-Schuler, Staufenbiel, & Krucker, 2008; Winkler, et al., 2001). Additionally, multiple studies indicate that in APP23 mice, cerebral blood flow changes and CAA precede vascular injury (Luth, Holzer, Gartner, Staufenbiel, & Arendt, 2001; Mueggler, Baumann, Rausch, Staufenbiel, & Rudin, 2003; Mueggler, et al., 2002; Thal, et al., 2008). Hence, APP23 mice are a suitable AD model to study cerebrovascular changes and the mechanism leading to barrier leakage in AD.
2.1.4. APP(V717I) Model.
In 1999, Moechars et al. (Moechars, et al., 1999) described the APP(V717I) mouse AD model (background strain: C57BL/6 x FVB/N). These mice harbor the hAPP gene with the APP London (V717I) mutation, one of the earliest known and most common familial AD mutations worldwide (Cruts, Theuns, & Van Broeckhoven, 2012; Goate, et al., 1991). The neuronal Thy1 promoter drives hAPP overexpression leading to spatial memory deficits (6 months), which is followed by the formation of Aβ plaques (10 months), CAA (15 months), and microhemorrhages (25–30 months) (Caroni, 1997; Van Dorpe, et al., 2000). However, the onset and extent of barrier leakage in APP(V717I) mice is not well-understood. A recent clinical report by Lloyd et al. (Lloyd, et al., 2020) highlighted the role of the hAPP (V7171) mutation in leptomeningeal CAA, which sparked interest in this mutation because this may affect leakage at the blood-CSF interface. Hence, APP(V717I) mice are suitable for exploring how the hAPP (V717I) mutation contributes to CAA at the blood-CSF barrier in AD.
2.1.5. J20 Model.
In 2000, Mucke et al. (Mucke, et al., 2000) described the J20 mouse AD model (background strain: C57BL6). This model harbors the Indiana (V717F) mutation and the Swedish double-mutation in the hAPP gene with the PDGF-β promoter driving neuronal hAPP overexpression (Mucke, et al., 2000; Sasahara, et al., 1991). J20 mice develop high soluble Aβ brain levels (1–2 months), neuronal loss (3 months), and spatial memory impairment (4 months) before Aβ plaques occur (5–7 months) (I. H. Cheng, et al., 2007; Hong, et al., 2016; Shabir, et al., 2020; Wright, et al., 2013). At about 11 months of age, J20 mice have reduced cerebral blood flow, CAA, and microhemorrhages (Lin, et al., 2013), which is followed by barrier leakage (12 months), as is evident from 2-fold higher extravasation of fluorescein isothiocyanate (FITC)-labeled dextran (150 kDa) compared to age-matched wild type mice (Van Skike, et al., 2018). Van Skike et al. ((Van Skike, et al., 2018)) also observed that junctional adhesion molecule-A protein levels are reduced by 50% in brain capillaries of 10-month-old J20 mice compared to age-matched wild type mice. By 15–19 months of age, J20 mice display a 20% reduced pericyte function and 20% lower AQP-4 immunoreactivity compared to age-matched wild type mice (Kimbrough, Robel, Roberson, & Sontheimer, 2015). These studies indicate that Aβ pathology precedes barrier dysfunction and leakage. Thus, the J20 mouse model is ideal for evaluating how Aβ pathology causes barrier leakage in AD.
2.1.6. TgCRND8 Model.
In 2001, Chishti et al. (Chishti, et al., 2001) described the TgCRND8 mouse AD model (background strain: C3H/He-C57Bl/6). This model harbors the hAPP gene with the Swedish and Indiana mutations, and the hamster PrP promoter drives hAPP overexpression in neurons and non-neuronal tissue (Boy, et al., 2006). TgCRND8 mice develop spatial deficits (3 months) before the development of Aβ plaques (5 months), neuronal loss (6 months), and tauopathies (7–12 months) (Bellucci, et al., 2007; Brautigam, et al., 2012; Chishti, et al., 2001). CAA and reduced cerebral blood flow develop earlier in TgCRND8 mice than in J20 mice (6 months vs. 11 months) due to impaired perivascular Aβ clearance (Cortes-Canteli, et al., 2019; Lin, et al., 2013). By 12 months, TgCRND8 mice have developed capillary CAA, a 2-fold increase in hypertrophied pericytes, and a 20% reduction in perivascular AQP-4 immunoreactivity compared to age-matched wild type mice (Cortes-Canteli, Mattei, Richards, Norris, & Strickland, 2015). Permeability studies also indicate that Evans blue has 12-fold higher intensity levels in 6-month-old TgCRND8 mice than in age-matched wild type mice (J. Paul, et al., 2007). Evans blue permeability values were 7.5-fold higher in 12-month-old TgCRND8 mice than in age-matched wild type mice. At 12 months, brain capillaries from TgCRND8 mice also become tortuous and develop fragmented perivascular fibrin deposits (Durrant, Ruscher, Sheppard, Coleman, & Ozen, 2020; J. Paul, et al., 2007). More recently, Yuan et al. (Yuan, et al., 2020) have reported that intrathecally infused tracers colocalize with CAA sites in TgCRND8 mice. It remains to be seen if CAA contributes to barrier leakage in TgCRN8 mice. In summary, TgCRND8 mice are a suitable model to study the link between CAA and barrier leakage in AD.
2.1.7. 3xTg-AD Model.
In 2003, Oddo et al. (Oddo, et al., 2003) developed the triple transgenic mouse model 3xTg-AD (background strain: C57BL/6). This model harbors the human APP gene segment with the 1) Swedish mutation, 2) the human MAPT gene segment with the P301L mutation, and 3) the human presenilin-1 gene segment bearing the M146V mutation. All three genes are under the control of the neuronal Thy1.2 promoter, and 3xTg-AD mice develop long-term memory retention deficits (4 months) before Aβ deposition (6 months) and tauopathy (12 months) occur (Billings, Oddo, Green, McGaugh, & LaFerla, 2005; Oddo, et al., 2003). Zerano et al. (Zenaro, et al., 2015) further showed that neutrophils infiltrate the brain in 4–6-month-old 3xTg-AD mice, causing early cognitive decline. The age at CAA onset and the extent of microhemorrhages is not known for 3xTg-AD mice. However, multiple studies indicate that barrier leakage in 3xTg-AD mice is subtle. First, data from a study by Do et al. (Do, et al., 2014) show that the diffusion rate of the highly lipophilic drug diazepam (0.3 kDa) is unchanged in 3–18-month-old 3xTg-AD mice. Second, St-Amour et al. (St-Amour, et al., 2013) found that intravenously infused IgGs remain in the capillary lumen of 3xTg-AD mice at 10–11 months of age. Third, Bourassa et al. (Bourassa, Alata, Tremblay, Paris-Robidas, & Calon, 2019) observed that antibody internalization by transferrin receptors is not significantly different between 12, 18, and 22-month-old 3xTg-AD mice. In contrast, with DCE-MRI, Chiquita et al. (Chiquita, et al., 2019) found a 2-fold higher leakage value in 3xTg-AD mice compared to wild type mice. This study, however, had a small sample size, and mice were pooled across ages (4–12 months). One potential explanation for the minimal barrier leakage observed in 3xTg-AD could be the 20% increase in collagen protein levels and the 32% increase in basement membrane thickness (Bourasset, et al., 2009). Hence, 3xTg-AD mice are a suitable model for evaluating the effect of basement membrane changes on barrier leakage in AD.
2.1.8. TgSwDI Model.
In 2004, Davis et al. (Davis, et al., 2004) described the TgSwDI mouse AD model (background strain: C57BL/6). This model harbors an hAPP gene segment with three mutations (Swedish, Dutch (E693Q), and Iowa (D694N)), and hAPP overexpression is driven by the neuronal Thy1 promoter (Davis, et al., 2004). TgSwDI mice simultaneously develop Aβ pathology, memory deficits, and reduced cerebral blood flow as early as 3 months of age, but it is unknown if TgSwDI mice develop tauopathies or neuronal loss (Davis, et al., 2004; Searcy, Le Bihan, Salvadores, McCulloch, & Horsburgh, 2014; F. Xu, et al., 2007). Data from several studies show that the Dutch and Iowa mutations drive Aβ deposition in the cerebrovasculature, which leads to CAA and microhemorrhages in TgSwDI mice at age 6 months (Davis, et al., 2004; Davis, et al., 2006; Van Vickle, et al., 2008). Xu et al. (F. Xu, et al., 2007) demonstrated that knocking out the APOE allele causes a shift from microvascular to parenchymal Aβ deposits without changing the overall Aβ burden indicating that APOE is the driving force for CAA and microhemorrhages in this AD model. Searcy et al. (Searcy, et al., 2014) conducted a longitudinal study and showed that APOE protein levels increased 3-fold in 9-month-old TgSwDI mice compared to 3-month-old wild type mice, indicating that CAA may also increase with age in these mice. Choi et al. (S. Choi, Singh, Singh, Khan, & Won, 2020) recently found that inhibiting endothelial nitric oxide synthase with dimethylarginine (50 mg/kg/day, 56 days) in 8-month-old TgSwDI mice reduces cerebral blood flow, increases Evans blue brain levels, causes loss of tight junction proteins, leads to stress fiber formation, and reduces capillary density compared to age-matched wild type mice. Capillaries from 12-month-old TgSwDI mice display reduced pericyte coverage and decreased perivascular AQP-4 expression (Duncombe, et al., 2017). These changes occur before any changes in basement-membrane protein levels (e.g., nidogen-1, laminin, collagen) (Searcy, et al., 2014). Hence, TgSwDI mice are ideal to evaluate the mechanism underlying barrier leakage in AD.
2.1.9. Tau P301L Model.
In 2005, Terwel et al. (Terwel, et al., 2005) described the Tau P301L mouse AD model that harbors the human tau gene with the P301L mutation. In this model, tau overexpression is driven by the neuronal Thy1 promoter (background strain: FVB/N). As a result, Tau P301L mice develop sensorimotor and learning deficits (5 months) before displaying tangle-like pathology in the hindbrain and cortex (8 months). Further, Bennet et al. (Bennett, et al., 2017) demonstrated that Tau P301L mice have decreased cerebral blood flow by age 12 months, which is accompanied by obstructed capillaries, a reduced capillary diameter, and increased vascular density. The authors also showed in 2–18-month-old mice that angiogenic markers such as vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9) are upregulated, suggesting that increased vascular density is linked to angiogenesis (Bennett, et al., 2017). In addition, MMPs degrade the basement membrane and tight junction proteins and contribute to barrier leakage in other mouse AD models (Hartz, et al., 2012). In summary, Tau P301L mice are suitable for evaluating tau-mediated changes of barrier dysfunction in AD.
2.1.10. APP/PS1 Model.
In 2004, Jankowsky et al. (Jankowsky, et al., 2004) described the APP/PS1 mouse AD model (background strain: C57BL/6). This model harbors the hAPP gene with the Swedish mutation and the human presenilin-1 (PSEN-1) gene segment without exon 9; the overexpression of both hAPP and PSEN-1 is driven by the mouse PrP promoter (Jankowsky, et al., 2004). APP/PS1 mice develop Aβ pathology and CAA (6 months) before neuronal loss (8–10 months) and spatial memory deficits and microhemorrhages (12 months) (Jackson, et al., 2016; Jankowsky, et al., 2004; Lefterov, et al., 2010; Liao, et al., 2014; Volianskis, Kostner, Molgaard, Hass, & Jensen, 2010). In addition, Garcia-Alloza et al. (Garcia-Alloza, et al., 2009) and other groups have shown that neuroinflammation plays a role in barrier dysfunction in APP/PS1 mice (Harcha, et al., 2015; Xie, et al., 2020). Specifically, these groups showed microglial activation, reduced microvascular blood flow, and decreased ZO-1 protein expression levels in 6 to 11-month-old APP/PS1 mice before parenchymal Aβ deposition and CAA occurred (Garcia-Alloza, et al., 2009; Harcha, et al., 2015; Xie, et al., 2020). Other researchers conducted gadolinium-based DCE-MRI studies in 14- and 24-month-old APP/PS1 mice and showed that interferon-gamma (IFNγ)-producing cells shift APP/PS1 microglia to an activated state that results in more cytokine production leading to barrier leakage with aging (Minogue, et al., 2014). In summary, APP/PS1 mice are appropriate for investigating the effect of neuroinflammatory changes on barrier leakage in AD.
2.1.11. 5xFAD Model.
In 2005, Oakley and colleagues (Oakley, et al., 2006) developed the 5xFAD mouse AD model (background strain: C57BL6). This model is named after the five familial AD mutations it harbors: 1) human APP gene with the Swedish mutation, 2) human APP gene with the Florida (I716V) mutation, 3) human APP gene with the London (V717I) mutation, 4) human PSEN1 gene with the M146L mutation, and 5) the human PSEN1 gene with the L286 mutation. The neuronal Thy1 promoter drives overexpression of hAPP and human PSEN1, and consequently, 5xFAD mice develop aggressive amyloid pathology (1.5–2 months) before spatial memory deficits (4–5 months) and neuronal loss (6–12 months) (Devi & Ohno, 2010; Jawhar, Trawicka, Jenneckens, Bayer, & Wirths, 2012; Oakley, et al., 2006; Xiao, et al., 2015). In addition, 5xFAD mice also develop meningeal CAA, and the deletion of endothelial nitric oxide synthase can further enhance this pathology (Hu, Kotarba, & Van Nostrand, 2013). Reduced cerebral blood flow occurs at 5–6 months of age and develops simultaneously with stalled brain capillaries, increased adhesion of neutrophils, and neuroinflammation (Cruz Hernandez, et al., 2019). Hence, data from barrier leakage studies in 5xFAD mice present a rather complex picture. For example, some studies report that tracers like thioflavin-S (318 Da) and sodium fluorescein (332 Da) were not detectable in the brain of 5xFAD mice (Barton, et al., 2019; Marottoli, et al., 2017). In contrast, other studies using FITC-labeled albumin (70 kDa) showed an age-dependent increase in microvascular leakage and reduced capillary length across multiple brain regions in 4 to 12-month-old 5xFAD mice (Giannoni, et al., 2016).
Microglia regulate many of the changes observed at the blood-brain barrier in 5xFAD mice. In a recent study, Spangenberg et al. (Spangenberg, et al., 2019) used clodronate to deplete microglia in 5xFAD mice. As a result, they found reduced claudin-5 protein levels in endothelial cells, increased number of microhemorrhages, and a shift from parenchymal to vascular Aβ deposition. Hence, 5xFAD mice are suitable for evaluating the effects of vascular Aβ depositions on barrier function.
2.1.12. SAMP8 Mice.
In 1988, Yagi and colleagues (1988) developed the Senescence-Accelerated Mouse (SAMP8) model (background strain: AKR/J). SAMP8 mice develop oxidative stress (2 months) before tau hyperphosphorylation (3 months), learning and memory deficits (4 months), inflammation (5 months), Aβ deposition (6 months), synaptic degeneration (8 months) and neuronal loss (10 months; Liu and Shi, 2020). Studies by Ueno et al. show a 1.6-fold increase in hippocampal permeability for 125I-human serum albumin and horseradish peroxidase in 13-month-old mice compared to 3-month-old mice (Ueno et al., 1993; Ueno et al., 1997). Ultrastructural studies by Ueno et al. (2001) also show extravasated horseradish peroxidase near endothelial cells with thickened cytoplasm, collagen depositions, and damaged pericytes. These findings were observed in 12-month-old SAMP8 mice but not in 3- or 6-month-old SAMP8 mice, indicating that barrier leakage in SAMP8 mice was associated with aging instead of learning and memory deficits (Ueno et al., 2001). Confocal imaging studies by Pelegri et al. also show significant IgG extravasation in the hippocampi in 6-to-15-month-old SAMP8 mice (Pelegri et al., 2007; del Valle et al., 2009). In contrast, Banks and colleagues did not find age-related differences in the brain uptake of various bloodborne molecules such as albumin, insulin, interleukin, tumor necrosis factor and IgM antibodies (Banks et al., 2000; Moinuddin et al., 2000; Banks et al., 2001, Banks et al. 2007). Nonaka et al. (2002) observed differences in brain uptake values of 131I-labeled pituitary adenylate cyclase-activating polypeptide between 2-month-old and 12-month-old SAMP8 mice; these differences were attributed to differences in Aβ pathology between the two groups. Taken together, these studies indicate a lack of consensus on blood-brain barrier intactness and the role of Aβ pathology on barrier leakage in SAMP8 mice.
2.2. Barrier Leakage in Other Models of Alzheimer’s Disease
Barrier dysfunction and cerebrovascular abnormalities are also present in aged and cognitively impaired human APP-overexpressing transgenic rats, aged dogs, and aged non-human primates. In aged macaques, capillary CAA is more commonly observed near mature Aβ plaques and collapsing or degenerating capillaries (D’Angelo, Dooyema, Courtney, Walker, & Heuer, 2013; Latimer, et al., 2019; Nakamura, et al., 1998; Ndung’u, et al., 2012). Aged dogs with cognitive impairment develop CAA, and collagen fibrils build up between astrocyte endfeet and capillary basement membranes leading to cerebral hemorrhages (Kimotsuki, et al., 2005; Morita, Mizutani, Sawada, & Shimada, 2005; Rodrigues, et al., 2018). With age, transgenic AD rats develop a thickened capillary basement membrane, disrupted tight junction proteins, detached astrocytes, and capillary endothelial cell deformation, which is why transgenic AD rats represent a suitable model to investigate barrier leakage (Bors, et al., 2018; Mooradian, 1988). Data from several transgenic rat AD models that overexpress hAPP (TgF344, McGill-R-Thy1-APP, APP+PS1) and/or human tau (SHR72, SHRSP/FAD) indicate that microvascular abnormalities are directly associated with AD pathology. For example, SHR72 rats develop cerebral blood flow impairment before Aβ accumulation, vascular changes, or cognitive deficits (Koson, et al., 2008). In these rats, gene expression levels of MMP-9, coagulation factors, angiogenic, and adhesion molecules (selectins, ICAM-1, VCAM-1) increase in brainstem capillaries with aging (Grammas & Ovase, 2002; Majerova, et al., 2019). Another example is the APP+PS1 model, where rats develop abluminal Aβ and collagen deposits within enlarged perivascular spaces in the brain (Klakotskaia, et al., 2018). A third example is the SHRSP/FAD rat model where AQP-4 shifts from a perivascular to a neuronal expression pattern, PECAM-1 protein levels are elevated around tight junction proteins, and collagen thickness is increased compared to wild type rats (Denver, et al., 2019). A fourth example is the McGill-R-Thy1-APP rat that displays increased vessel branching, collagen thickness, and angiogenesis when the Notch signaling pathway is genetically activated (Galeano, et al., 2018). Another example is the TgF344-AD rat that has reduced mural cell repairing capacity in arterioles compared to wild type rats (Bazzigaluppi, et al., 2019). Due to the limited number of studies in non-murine AD models, it is unclear when and how blood-brain barrier leakage develops in these species. In summary, these studies show that blood-brain barrier dysfunction is associated with pathological changes in AD across multiple animal models.
Although transgenic models have been widely used to study pathological processes associated with barrier leakage in AD, certain challenges limit further investigation of the mechanisms underlying barrier leakage in AD. First, transgenic models are a tool for studying pathological changes that follow protein expression but not the events preceding protein expression. Second, transgenic models that overexpress human APP and/or PS proteins develop widespread Aβ pathology but may not develop aggressive tangle pathology, neuronal loss, and cognitive deficits (Sabbagh et al., 2013). In contrast, transgenic models that overexpress human tau proteins may not develop Aβ pathology and fail to capture the entire spectrum of pathological changes in AD. Transgenic models that overexpress human Aβ and tau pathology do not inform us about pathological changes linked to sporadic or late onset AD. These models provide very different mechanistic information about barrier dysfunction and leakage that can be relevant but difficult to compare across studies and provide translational value.
3. Clinical Evidence of Barrier Leakage in Alzheimer’s Disease Patients
Detection of blood-brain barrier leakage in AD patients goes back to the 1980s when researchers discovered serum-borne proteins such as IgG and albumin in the brain parenchyma, indicating a compromised blood-brain barrier in AD patients (Alafuzoff, Adolfsson, Bucht, & Winblad, 1983; Alafuzoff, Adolfsson, Grundke-Iqbal, & Winblad, 1987). Although the idea that barrier leakage could play a role in AD pathology was controversial for decades, recent clinical data emphasizes a link between barrier leakage and cognitive decline in AD patients. In the following section, we will summarize the studies that identified barrier leakage in AD.
3.1. Cerebrospinal Fluid-to-Plasma Ratio
The CSF-serum ratio, also referred to as Q value, is one of the first and most commonly used clinical parameters to characterize barrier leakage. In a healthy individual, protein levels of blood-borne proteins such as IgG (150 kDa) and albumin (70 kDa) are 100- to 200-fold higher in the blood (IgG: 7–16 g/L; Albumin: 35–50 g/L) compared to that in the CSF (IgG: 0–0.08 g/L; Albumin: 0–0.03 g/L) (Mayo Clinic Family Health Book 5th Edition). Thus, an elevated Q value for albumin (Qalb) reflects extravasated albumin in the CSF (Rothschild, Oratz, & Schreiber, 1980). The QIgG reflects both extravasated and intrathecally synthesized IgG in the central nervous system (CNS). Except for one study by Elovaara et al. (Elovaara, Seppala, Palo, Sulkava, & Erkinjuntti, 1988), QIgG values are not significantly different between AD patients and age-matched non-demented individuals (Table 1). A 100- to 200-fold difference in these concentrations (QIgG value < 0.007) is likely due to intact CNS barriers that restrict blood-borne proteins from entering the CSF (Garton, Keir, Lakshmi, & Thompson, 1991). Depending on the severity of the pathology, QIgG values indicate slight to moderate barrier leakage (0.01–0.03), severe barrier leakage (0.03–0.10), or even complete barrier breakdown (QIgG > 0.1) (Schliep & Felgenhauer, 1978). Over the past 50 years, multiple research groups have estimated that Qalb and QIgG are slightly elevated (> 0.007) in AD patients compared to non-demented individuals (Tables 1 and 2). More systematic meta-analyses have identified that increased Qalb and QIgG values are 0.65 to 1.3-fold-higher (p < 0.05) in AD patients compared to non-demented individuals (Farrall & Wardlaw, 2009; Jankowsky, et al., 2004; Musaeus, et al., 2020; Muszynski, et al., 2017; Olsson, et al., 2016; Skillback, et al., 2017). Collectively, these studies indicate that CSF-to-serum ratios are a surrogate marker for barrier leakage in AD. Careful interpretation is required when using CSF-to-serum ratio since the rate of CSF turnover is reduced in aging which causes IgG and albumin accumulation in the CSF and, in turn, a higher-than-expected CSF-to-plasma ratio (Sakka, Coll, & Chazal, 2011).
Table 1. IgG Quotient (QIgG) values for Alzheimer’s Disease (AD) and Nondemented (ND) Subjects.
Table 1 lists historical studies and more recent meta-analyses that indicate minimal to no barrier impairment for IgG (i.e., QIgG < 0.01) in AD and ND subjects. References: (Elovaara, Icen, Palo, & Erkinjuntti, 1985; Elovaara, et al., 1983; Frolich, et al., 1991; Hampel, Kotter, Padberg, Korschenhausen, & Moller, 1999; Kay, et al., 1987; Leonardi, Gandolfo, Caponnetto, Arata, & Vecchia, 1985; Muszynski, et al., 2017; Sjögren, et al., 2001).
| Study | Age (ND) | Age (AD) | N (ND) | N (AD) | QIgG (ND) | QIgG (AD) | Fold-change (AD / ND) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Elovaara et al. (1983) | 67.4 | 1.3 | 67.1 | 1.3 | 22 | 22 | 0.002 | 0.001 | 0.003 | 0.001 | 1.42 | < 0.01 |
| Elovaara et al. (1985) | 71.5 | 1.4 | 73 | 2.2 | 10 | 10 | 0.003 | 0.001 | 0.002 | 0 | 0.63 | < 0.01 |
| Leonardi et al. (1985) | 43.1 | 17.4 | 64.6 | 7.9 | 64 | 52 | 0.002 | 0.001 | 0.002 | 0.001 | 1.14 | NS |
| Kay et al. (1987) | 71.5 | 12 | 66.4 | 8.4 | 14 | 31 | 0.003 | 0.001 | 0.003 | 0.002 | 1.16 | NS |
| Frolich et al. (1991) | 68.8 | 5.7 | 69.1 | 8.3 | 25 | 25 | 0.002 | 0.001 | 0.003 | 0.002 | 1.23 | NS |
| Hampel et al. (1999) | 72.8 | 6.9 | 67.8 | 12 | 11 | 51 | 0.002 | 0.001 | 0.003 | 0.002 | 1.22 | NS |
| Sjögren et al. (2001) | 73.8 | - | 71.8 | - | 17 | 41 | 0.005 | 0.002 | 0.005 | 0.002 | 1.02 | NS |
| Muszynski et al. (2017) | - | - | - | - | 45 | 23 | 0.002 | - | 0.003 | - | 1.11 | 0.121 |
| No Impairment (0 < QIgG < 0.01) | Moderate Impairment (0.01 < QIgG < 0.03) | Severe Impairment (0.03 < QIgG < 0.1) | Breakdown (QIgG > 0.1) | |||||||||
Table 2. Albumin Quotient (QAlb) values for Alzheimer’s Disease (AD) and Nondemented (ND) Subjects.
Table 2 lists historical studies and more recent meta-analyses that indicate minimal to moderate barrier impairment for albumin (i.e., 0.01 < Qalb < 0.03) in AD and ND subjects. References: (Alafuzoff, et al., 1983; Bjerke, et al., 2011; Blennow, Wallin, & Chong, 1995; Blennow, Wallin, Uhlemann, & Gottfries, 1991; Chalbot, et al., 2011; Elovaara, et al., 1985; Frolich, et al., 1991; Hampel, et al., 1999; Janelidze, et al., 2017; Kay, et al., 1987; Kim, et al., 2009; Leonardi, et al., 1985; Mecocci, et al., 1991; Musaeus, et al., 2020; Rösler, Wichart, & Jellinger, 2001; Sjögren, et al., 2001; Sjögren, et al., 2002; Sjogren, Gustafson, Wikkelso, & Wallin, 2000; Skillback, et al., 2017; Skoog, et al., 1998; Sun, Liu, Nguyen, & Bing, 2003; Sundelöf, et al., 2010; Wada, 1998).
| Study | Age (ND) | Age (AD) | N (ND) | N (AD) | QIgG (ND) | QIgG (AD) | Fold-change (AD / ND) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Kim et al. (2015) | 60.6 | 2.3 | 60.4 | 6.2 | 25 | 32 | 0.005 | 0.002 | 0.005 | 0.003 | 1.18 | NS |
| Sjögren et al. (2000) | 71.1 | 7.3 | 62 | 5.6 | 18 | 21 | 0.005 | 0.002 | 0.006 | 0.003 | 1.21 | NS |
| Leonardi et al. (1985) | 43.1 | 17.4 | 64.6 | 7.9 | 64 | 52 | 0.005 | 0.002 | 0.005 | 0.002 | 1.1 | NS |
| Alafuzoff et al. (1983) | 69 | - | 65 | - | 16 | 20 | 0.008 | 0.003 | 0.01 | 0.004 | 1.27 | < 0.05 |
| Sjögren et al. (2000) | 71.5 | 5.2 | 66 | 7.8 | 32 | 60 | 0.006 | 0.002 | 0.006 | 0 | 0.97 | NS |
| Kay et al. (1987) | 65.4 | 12 | 66.4 | 8.4 | 14 | 31 | 0.006 | 0.001 | 0.006 | 0.003 | 1.08 | NS |
| Elovaara et al. (1985) | 67.4 | 1.3 | 67.1 | 1.3 | 22 | 22 | 0.004 | 0.001 | 0.005 | 0.002 | 1.39 | <0.01 |
| Hampel et al. (1999) | 72.8 | 6.9 | 67.8 | 12 | 11 | 51 | 0.006 | 0.002 | 0.006 | 0.003 | 1.02 | - |
| Bjerke M et al. (2011) | 68 | - | 68 | - | 30 | 30 | 0.006 | 0.001 | 0.006 | 0.002 | 1.01 | NS |
| Rösler et al. (2001) | 45.2 | 2.1 | 68.7 | 2.1 | 49 | 27 | 0.005 | 0 | 0.007 | 0.001 | 1.27 | NS |
| Rösler et al. (2001) | 45.2 | 2.1 | 68.7 | 2.1 | 49 | 27 | 0.003 | 0 | 0.004 | 0 | 1.4 | NS |
| Frolich et al. (1991) | 68.8 | 5.7 | 69.1 | 8.3 | 25 | 25 | 0.005 | 0.001 | 0.006 | 0.003 | 1.23 | NS |
| Chalbot et al. (2011) | 59.4 | 9.2 | 69.3 | 4.3 | 21 | 54 | 0.005 | 0.002 | 0.005 | 0.002 | 0.92 | NS |
| Sjögren et al. (2002) | 68.6 | 7.5 | 70.6 | 5.3 | 17 | 19 | 0.005 | 0.002 | 0.006 | 0.002 | 1.12 | NS |
| Blennow et al. (1991) | 71.5 | 11 | 71.8 | 7.3 | 50 | 118 | 0.006 | 0.002 | 0.007 | 0.002 | 1.2 | - |
| Sjögren et al. (2001) | 78.9 | - | 71.8 | - | 17 | 19 | 0.005 | 0.002 | 0.008 | 0.003 | 1.49 | NS |
| Rosengren et al. (1999) | 66 | 6.4 | 71.9 | 5 | 39 | 37 | 0.006 | 0.002 | 0.006 | 0.002 | 1.11 | NS |
| Blennow et al. (1995) | 64.6 | 6.2 | 72 | 7.5 | 32 | 45 | 0.006 | 0.002 | 0.007 | 0.002 | 1.31 | < 0.01 |
| Elovaara et al. (1985) | 71.5 | 1.4 | 73 | 2.2 | 10 | 10 | 0.004 | 0.001 | 0.006 | 0.002 | 1.67 | <0.01 |
| Mecocci et al. (1991) | 60 | 4.1 | 73.3 | 1.3 | 17 | 46 | 0.007 | 0.001 | 0.006 | 0.001 | 0.86 | - |
| Sjögren et al. (2000) | 71.1 | 7.3 | 73.4 | 3 | 18 | 21 | 0.005 | 0.002 | 0.007 | 0.002 | 1.25 | NS |
| Janelidze et al. (2017) | 75 | 6 | 76 | 7 | 65 | 75 | 0.006 | 0.003 | 0.008 | 0.003 | 1.21 | <0.001 |
| Wada (1998) | 78.6 | 2.4 | 77.6 | 0.3 | 7 | 14 | 0.007 | 0.001 | 0.01 | 0.001 | 1.38 | <0.001 |
| Sundelöf et al. (2010) | 70.1 | 9.7 | 77.7 | 9.2 | 28 | 101 | 0.005 | 0.002 | 0.006 | 0.004 | 1.12 | NS |
| Chalbot et al. (2011) | 59.4 | 9.2 | 80.4 | 3.3 | 21 | 54 | 0.005 | 0.002 | 0.005 | 0.002 | 1.02 | NS |
| Skoog et al. (1998) | 85 | - | 85 | - | 29 | 13 | 0.007 | 0.002 | 0.009 | 0.005 | 1.37 | 0.046 |
| Sun et al. (2003) | 61 – 77 | - | 52 – 85 | - | 11 | 141 | 0.006 | 0.001 | 0.007 | 0 | 1.23 | - |
| Musaeus et al. (2020) | 65.9 | 7.5 | 72.6 | 8.9 | 34 | 289 | 0.005 | 0.002 | 0.007 | 0.004 | 1.33 | 0.004 |
| Skillback et al. (2017) | 73 | 5 | 75 | 6 | 292 | 666 | 0.006 | 0.002 | 0.007 | 0.003 | 1.15 | <0.001 |
| Skillback et al. (2017) | 73 | 5 | 75 | 6 | 292 | 130 | 0.006 | 0.002 | 0.007 | 0.008 | 1.15 | NS |
| No Impairment (0 < Qalb < 0.01) | Moderate Impairment (0.01 < Qalb < 0.03) | Severe Impairment (0.03 < Qalb < 0.1) | Breakdown (Qalb > 0.1) | |||||||||
3.2. Permeability Coefficients
Perfusion-based neuroimaging provides another alternative for measuring barrier leakage in AD patients. The rationale for perfusion-based imaging is that intravenously infused contrast agents (i.e., water-soluble paramagnetic compounds) alter the properties of water as it reaches different parts of the brain, resulting in an increased contrast during brain scans. Before the 2000s, barrier leakage was assessed by X-ray or CT imaging using iodinated contrast agents such as Hypaque-76 (1.45 kDa) or Conray-60 (0.81 kDa), or by PET imaging using 68Ga-EDTA (1.23 kDa) to obtain blood-to-brain transfer coefficients. In 1987, Schlageter et al. (Schlageter, Carson, & Rapoport, 1987) took the first PET-based measurements of barrier leakage in AD patients (n=5) and non-demented individuals (n=5) following an infusion of 2.5–5 mCi 68Ga-EDTA. In their study, Schlageter et al. (Schlageter, et al., 1987) acquired radioactivity-based PET scans over 90 minutes and found that k1 values (1.2 × 10−3 min−1) were similar for both cohorts. In 1990, Dysken et al. (Dysken, Nelson, Hoover, Kuskowski, & McGeachie, 1990) used a dynamic computed tomography (CT) scan setup with enough sensitivity to be able to capture Hypaque-76 (45 mL, 76% solution) leakage into the brain tissues of AD patients (n=26) and non-demented individuals (n=26). Dysken et al. (Dysken, et al., 1990) observed that the washout rate for Hypaque-76 was 3.7-fold lower (p > 0.05) in AD patients compared to that in non-demented individuals when brains were scanned at a higher spatial resolution (0.3 cm compared to 2.89 cm in Schlageter et al. (Schlageter, et al., 1987)) and over multiple regions of interest (16 compared to 2 in (Schlageter, et al., 1987). Under the current AD diagnostic criteria, the AD patients studied by Schlageter and co-workers would fall under the mixed dementia category. In 1998, Caserta et al. (Caserta, Caccioppo, Lapin, Ragin, & Groothuis, 1998) further improved the spatial resolution of dynamic CT scans and found in AD patients (n=14) a higher leakage rate for Conray-60 (2 mL/kg; 60% solution; ~ 0.005 min−1) compared to 68Ga-EDTA (~ 0.0012 min−1 in (Schlageter, et al., 1987)). However, k values for AD patients (n=14) were not significantly different from those of non-demented individuals (n=9). Despite improved imaging methods, PET and dynamic CT imaging are limited by long scan times (Raja, Rosenberg, & Caprihan, 2018) that can be overcome by DCE-MRI-based imaging methods (Raja, et al., 2018). While CT or MRI-based methods detect tracer concentrations in the sub-millimolar range, PET-based methods can detect tracer concentrations in the picomolar range. T1 signal intensities can then be used to obtain permeability coefficient (Ktrans) using comprehensive pharmacokinetic models such as the Tofts standard model, Tofts extended models, and the Patlak model (Patlak, Blasberg, & Fenstermacher, 1983; Tofts, et al., 1999). In the next two paragraphs, we will briefly discuss advances in DCE-MRI studies in 1) patients with mild cognitive impairment (MCI) and in 2) AD patients.
MCI Patients.
Early DCE-MRI analyses by Wang et al. (H. Wang, Golob, & Su, 2006) show that Ktrans values were similar in the cerebelli and hippocampi of MCI patients (7 males, 4 females) and non-demented individuals (5 males, 6 females). In this study (H. Wang, et al., 2006), both treatment groups received Omniscan (Gd-DTPA-BMA; 0.57 kDa; 0.11mmol/kg i.v.) prior to a 5-minute T1-weighted DCE-MRI scan (1.5 T). More recent DCE-MRI studies revealed significant differences in the hippocampal Ktrans values between MCI patients and non-demented individuals at a higher magnetic field strength (3 T) (Barnes, et al., 2016; Montagne, et al., 2015; Montagne, et al., 2020; Nation, et al., 2019). In these studies, subjects were infused with gadobentate dimeglumine (1.06 kDa; 0.05 mmol/kg), and scans were acquired over 10–30 minutes at a temporal resolution of 15.4 s per scan (Barnes, et al., 2016). At these settings, mean Ktrans values were in the range of 0.5 – 2 × 10−3 min-1. Further, mean Ktrans values in the hippocampus and parahippocampal gyrus were 1.2-fold higher in MCI patients (20 females, 19 males) compared to mean Ktrans values in non-demented individuals (130 females, 76 males; (Montagne, et al., 2020)). In contrast, in other brain regions, Ktrans values were not significantly different. This pattern was consistent even after adjusting for several cofactors such as age, APOE status, Aβ and tau pathology, vascular risk factors, tissue volume, and cognitive scores. Taken together, in MCI patients, barrier leakage occurs in the hippocampus and is subtle (~ 1 kDa; ~10−3 min−1).
AD Patients.
A DCE-MRI analysis by Starr et al. (Starr, Farrall, Armitage, McGurn, & Wardlaw, 2009) shows that gadobentate dimeglumine (1.06 kDa; 20 ml administered intravenously at a rate of 5 ml/s) distributes differently in the CNS of AD patients (5 males, 10 females) compared to non-demented individuals (10 males, 5 females). Specifically, T1 signal intensities in the gray matter reached a local maximum value 15 minutes post-injection in AD patients compared to 26 minutes in non-demented individuals. A similar trend was observed in the CSF of both groups. Data from recent DCE-MRI studies show that low molecular weight gadobutrol (0.6 kDa; 0.1 mmol/kg) provided detectable Ktrans values for AD patients and non-demented individuals under a dual-time resolution MRI sequence scanning protocol (Freeze, et al., 2020; van de Haar, Burgmans, et al., 2016; van de Haar, et al., 2017; van de Haar, Jansen, et al., 2016; Verheggen, et al., 2020). This approach provides temporal resolution at the expense of reduced spatial resolution for the first 2 minutes of scanning. Consequently, Ktrans values across different brain regions were 10-fold lower in AD patients (~10−4 min−1; n=7) than in MCI patients (~10−3 min−1; n=9) (van de Haar, Burgmans, et al., 2016). After noise correction, Ktrans values were 13-fold higher (p=0.014) in AD patients (n=16; 9 males, 7 females) compared to non-demented subjects (n=17; 11 males, 6 females) (van de Haar, et al., 2017). This difference was significant for longer scan times (>14 minutes), indicating that noise can lead to an underestimation of Ktrans values for shorter scan times (< 15 min). Taken together, these studies show that barrier leakage is subtle (< 1 kDa) but significantly higher in AD patients compared to MCI patients or non-demented individuals.
3.3. Cerebral Microbleeds
Non-perfusion-based neuroimaging allows detection of cerebral microbleeds (CMBs) as indirect evidence for barrier leakage in patients and postmortem brain tissue. CMBs are 2–10 mm round, hemosiderin-rich deposits that appear as dark spots under a T2-weighted gradient echo or susceptibility-weighted MRI scan (Greenberg, et al., 2009; Nakata, et al., 2002). Anatomically, CMBs are associated with arteries, arterioles, and capillaries, but the association between CMBs and each vascular segment is unclear (Fisher, French, Ji, & Kim, 2010). Multiple studies indicate that CMBs are common in AD and CAA patients and are significantly (p < 0.05) more prevalent in AD patients compared to non-demented control individuals (Table 3) (K. Jellinger, 2002; K. A. Jellinger, 1997; Kalaria, 1997; Nakata, et al., 2002). Among AD patients, the number of CMBs increase with age (> 75+ years), severity of CAA pathology, cerebrovascular burden, and the presence of the APOE e4 allele (Benedictus, et al., 2013; Graff-Radford, et al., 2017; Kester, et al., 2014; Kimberly, et al., 2009; S. Lee, et al., 2018; Nakata-Kudo, et al., 2006; Pettersen, et al., 2008; Sparacia, et al., 2017; Vernooij, et al., 2008; Yates, et al., 2014). Regionally, CMBs are more prevalent in the lobar region compared to deep or infratentorial brain regions. Between the different lobes, CMBs are more prevalent in the occipital lobe, followed by the temporal lobe, frontal lobe, basal ganglia, parietal lobe, and cerebellum in AD patients (Banerjee, et al., 2017; Goos, et al., 2009; Nagasawa, Kiyozaka, & Ikeda, 2014; Olazaran, et al., 2014; Pettersen, et al., 2008; Shams, et al., 2015; Vernooij, et al., 2008). The prevalence of lobar CMBs is directly associated with severe white matter changes, low Aβ42 levels in the plasma, and high tau levels (p < 0.05) in the CSF of AD patients (Goos, et al., 2012; S. Lee, et al., 2018; Noguchi-Shinohara, et al., 2017; Sparacia, et al., 2017). Further, lobar CMBs are specific to AD and have not been observed in other dementias (Gungor, et al., 2015; Uetani, et al., 2013). CMBs are also closely related to barrier leakage in MCI. Specifically, Poliakova et al. (Poliakova, Levin, Arablinskiy, Vasenina, & Zerr, 2016) observed that MCI patients who develop CMBs (n=13) had 1.5-fold higher Qalb values (p < 0.05) compared to MCI patients without CMBs (n=12). In another study, Goos et al. (Goos, et al., 2012) found that AD patients with lobar CMBs (n=20) and non-lobar CMBs had similar Qalb values (n=15).
Table 3. Cerebral Microbleeds (CMBs) in Alzheimer’s Disease (AD) and Nondemented (ND) Subjects.
Table 3 lists selected studies that show CMB incidences in AD and ND subjects. References: (Brundel, et al., 2012; Doi, et al., 2014; Duits, et al., 2015; Goos, et al., 2012; Hanyu, Tanaka, Shimizu, Takasaki, & Abe, 2003; Kester, et al., 2014; Nagasawa, et al., 2014; Pettersen, et al., 2008; Uetani, et al., 2013).
| Study | Age (ND) | Age (AD) | N (ND) | N (AD) | MRI Settings | CMBs (ND, %) | CMBs (AD, %) | Fold-change (AD / ND) | p value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||||
| Hanyu et al. (2003) | 75.6 | 6.6 | 77.3 | 6.5 | 55 | 59 | T2* GRE (1.5 T) | 7% | 68% | 9.32 | < 0.01 |
| Pettersen et al. (2008) | - | - | - | - | 25 | 80 | T2* GRE (1.5 T) | 12% | 29% | 2.4 | - |
| Brundel et al. (2012) | 72 | 4.5 | 74.3 | 8.6 | 18 | 18 | T2*GRE (7.0 T) | 57% | 78% | 1.36 | < 0.05 |
| Goos et al. (2012) | 65 | 6 | 67 | 6 | 22 | 52 | T2* GRE (3.0 T) | 0% | 50% | - | - |
| Uetani et al. (2013) | 54 | 108 | 6 | 24 | 75 | 71 | SWI (3.0 T) | 48% | 22% | 0.46 | < 0.001 |
| Kester et al. (2014) | 254 | 293 | 194 | 143 | 67 | 59 | T2* GRE (1.0, 1.5, 3.0 T) | 21% | 14% | 0.67 | < 0.05 |
| Yates et al. (2014) | 74.2 | 7.3 | 74.6 | 8.4 | 97 | 40 | SWI (3.0 T) | 22% | 43% | 1.96 | < 0.05 |
| Nasagawa et al. (2014) | 59 | 9 | 67 | 8 | 337 | 547 | T2* GRE (1.5 T) | 14% | 21% | 1.53 | - |
| Doi et al. (2015) | - | - | - | - | 18 | 72 | SWI (3.0 T) | 13% | 33% | 2.67 | < 0.05 |
| Duits et al. (2015) | 65 | 6 | 67 | 6 | 26 | 52 | T2* GRE (3.0 T) | 0% | 50% | - | - |
| GRE: Gradient Echo MRI | SWI : Susceptibility-weighted Imaging | ||||||||||
Improving the clinical detection of CMBs can potentially increase its utility as a diagnostic marker for barrier leakage in AD. For instance, clinical detection of CMBs suffers from reproducibility issues due to varying imaging protocols, equipment, and assessment criteria like every other imaging method (De Guio, et al., 2016; Rabelo, et al., 2017). For instance, MRI scans at ultra-high magnetic field strengths (e.g., 7 T) detect more CMBs in patients than MRI scans with conventional magnetic field strengths (e.g., 1.5 T, 3 T) (McKiernan & O’Brien, 2017; Reuck, Caparros-Lefebvre, Deramecourt, & Maurage, 2011). A meta-analysis by Sephery et al. (Sepehry, Rauscher, Hsiung, & Lang, 2016) also shows that susceptibility-weighted MRI scans identify twice as many CMBs compared to gradient-echo MRI scans. Standardized acquisition parameters and MRI scans at ultra-high magnetic field strengths can thus facilitate a more uniform assessment of CMBs in the clinical setting.
Although we extensively discussed CMBs as a postmortem evidence of barrier leakage in AD, CMBs cover a subset of pathological changes that affects small arteries, arterioles, capillaries, and small veins in the brain (Cuadrado-Godia et al. 2018). Other changes such as subcortical infarcts, lacunae of vascular origin, white matter hyperintensities, and widened perivascular spaces are also recognized in MRI and CT scans and are covered under the umbrella term of cerebral small vessel disease (cSVD, see review by Chojdak-Lukasiewicz, Dziadkowiak, Zimny, & Paradowski, 2021). In addition to CMBs, white matter hyperintensities are typically observed in the MRI scans of AD patients are negatively correlate with the cognitive status of AD patients (Birdsill et al. 2014). Findings by Freeze et al. further show a positive association between white matter hyperintensity volume and gadolinium leakage rates in the gray and white matter MRI scans of 80 patients (32 CNI, 34 MCI, 14 AD; Freeze et al. 2020). Further investigation is needed to identify the association between barrier leakage rates and other forms of cSVDs in patients.
4. Postmortem Evidence of Barrier Leakage in Alzheimer’s Disease Patients
In this section, we focus on studies that describe an association between pathological changes, markers of barrier dysfunction, and barrier leakage in AD. Specifically, we discuss structural changes at the blood-brain barrier that correlate with barrier leakage. We then highlight studies that found IgG, prothrombin, and fibrinogen as surrogate markers for barrier leakage.
4.1. Altered Tight Junction Proteins
Tight junction (TJ) proteins such as ZO-1, occludin, and claudin-5 tightly seal endothelial cells to form a barrier against paracellular transport of solutes into or out of the brain. In AD, TJ proteins are disassembled, delocalized, disrupted, or lost, which contributes to barrier leakage and cognitive decline (Carrano, et al., 2011; Debette, et al., 2015; S. Lee, et al., 2018). Fiala et al. (Fiala, et al., 2002) showed a distorted staining pattern for ZO-1 in brain capillaries of tissue slices from AD patients (n=8) compared to a linear and continuous pattern for ZO-1 in capillaries of tissue slices from non-demented subjects (n=5). Romanitan et al. (Romanitan, et al., 2007) reported increased occludin expression in capillaries of brain tissue slices from AD patients (n=4) compared to non-demented individuals (n=5). In another study, Viggars et al. (Viggars, et al., 2011) reported that 76–80% of examined capillaries in tissue slices from patients with mild (Braak I/II; n=28), moderate (Braak III/IV; n=47), and severe (Braak V/VI; n=17) AD pathology showed a disrupted staining pattern for ZO-1 and a diffuse staining pattern for claudin-5. In a fourth study, Yamazaki et al. (Yamazaki, et al., 2019) found that claudin-5 protein levels (p < 0.05) were reduced in the cortex, whereas occludin protein levels were reduced in the temporal, entorhinal, and cingulate cortices from AD patients (n=19; p < 0.05) compared to cognitively normal individuals with no Aβ pathology (n=10).
In CAA, the expression pattern of TJ proteins is disrupted. For example, Carrano et al. (Carrano, et al., 2011) observed that claudin-5, occludin, and ZO-1 immunoreactivities are reduced by 60–80% around Aβ-covered capillaries compared to Aβ-free capillaries in occipital cortical slices from AD patients with severe AD (Braak III-VI) and mild-to-severe capillary CAA pathology. Collectively, current data suggest that disrupted TJ protein expression is an underlying mechanistic sign of barrier dysfunction and might be an indicator for barrier leakage in postmortem brain tissue samples of AD patients.
4.2. Altered Basement Membrane
An altered or ‘thickened’ basement membrane along the capillary bed is another indicator of barrier leakage in AD (Mancardi, et al., 1980; Scheibel & Duong, 1988; Scheibel, Duong, & Tomiyasu, 1987; Yamashita, et al., 1991). Scheibel et al. (Scheibel & Duong, 1988; Scheibel, et al., 1987) found that basement membrane thickening was “irregular” and “perforated” at multiple spots along the capillaries in brain tissue slices from AD patients but that the endothelial cell lining beneath these perforations was not damaged. Further, Kalaria et al. (Kalaria & Pax, 1995) observed thickened basement membranes immunoreactive for collagen-IV around collapsed or absent capillaries in cortical tissue slices from AD patients (n=18), whereas these envelopes were not present in slices from non-demented individuals (n=9). Other researchers found that AD patients (n=9) have higher levels of collagen I (8-to-9-fold), collagen III (8-to-9-fold), and collagen IV (5-fold) in their brain capillary basement membranes compared to non-demented individuals (n=9) (Christov, et al., 2008).
Elevated collagen levels are indicative of a thickened basement membrane and CAA pathology. In a study by Vinters et al. (Vinters, 1987) the authors show that collagen IV deposits are frequently present around intact capillaries with severe CAA pathology. In contrast, van Horssen et al. (van Horssen, et al., 2001) found that collagen IV deposits were associated with CAA pathology in 50 out of 73 examined frontal cortices compared to 37 out of 73 occipital cortices obtained from AD patients. In addition, collagen IV deposits correlate with higher incidences of microhemorrhages in AD. Specifically, Cullen et al. (Cullen, Kocsi, & Stone, 2005, 2006) found that these collagen IV deposits exist as ‘halos’ around heme and fibrinogen in cortical brain tissue slices of AD patients (n=11). Taken together, these studies suggest that collagen deposition and/or thickening of the basement membrane could be an indicator for barrier leakage in AD.
4.3. Astrocytic Abnormalities
In AD, swollen and reactive perivascular astrocytes often co-localize with barrier leakage markers such as fibrinogen and IgG and release permeability enhancers such as endothelin-1 (ET-1) and VEGF that disrupt TJ proteins and induce barrier leakage (Fouda, Fagan, & Ergul, 2019; Michinaga & Koyama, 2019; Tomimoto, et al., 1996). Levels of these enhancers are elevated in brain tissue samples from AD patients compared to samples from non-demented individuals. For example, data from several studies show elevated ET-1 levels in cortical brain slices from AD patients compared to those from non-demented individuals (Minami, Kimura, Iwamoto, & Arai, 1995; Palmer, Barker, Kehoe, & Love, 2012; W. W. Zhang, et al., 1994). Data from other studies show enhanced VEGF immunoreactivity along arterioles, venules, and capillaries in cortical tissue slices from AD patients compared to those from non-demented individuals (Provias & Jeynes, 2008, 2011, 2014; Thirumangalakudi, Samany, Owoso, Wiskar, & Grammas, 2006; Thomas, Miners, & Love, 2015). Some studies also highlight that ET-1 and VEGF levels correlate with markers of cerebrovascular dysfunction and barrier leakage. In this regard, Thomas et al. (Thomas, et al., 2015) demonstrated that ET-1 protein levels in brain homogenate from AD patients (n=20) correlate with a low myelin-associated glycoprotein to proteolipid protein ratio, an indicator for cerebral hypoperfusion. Likewise, Zhang et al. (J. B. Zhang, et al., 2016) reported that VEGF serum levels were 2-fold higher in AD patients that developed CMBs (n=47) compared to AD patients without CMBs (n=99). Janelidze et al. (Janelidze, et al., 2017) demonstrated that VEGF protein levels in the CSF correlate with Qalb values in MCI and AD patients.
In conclusion, these studies indicate that levels of permeability modulators in the brain, such as VEGF and ET-1, correlate with indicators of cerebrovascular dysfunction and barrier leakage in AD.
4.4. Altered Pericyte Coverage
In AD, pericytes often display structural and functional abnormalities (Miners, Kehoe, Love, Zetterberg, & Blennow, 2019). In 1987, Scheibel et al. (Scheibel, et al., 1987) reported that irregularly shaped capillaries found in brain tissue slices from deceased AD patients (n=15) displayed rounded and conical extrusions resembling pericyte cell bodies. However, in brain tissue from non-demented individuals (n=10), these extrusions along capillary beds occur only occasionally (Scheibel, 1987; Scheibel, et al., 1987). Additionally, Stewart et al. (Stewart, Hayakawa, Akers, & Vinters, 1992) found more pericytes around capillaries as well as reduced wall thickness, reduced mean luminal diameter, increased cleft length, increased number of inter-endothelial junctions per unit vessel length, and increased mean junction length in AD patients. In contrast, more recent analyses of postmortem AD brain tissue show that a reduced number of pericytes indicates barrier leakage (Halliday, et al., 2016; Miners, Schulz, & Love, 2018; Sengillo, et al., 2013). In 2013, Sengillo et al. ((Sengillo, et al., 2013)) reported that pericyte numbers in hippocampal and cortical brain tissue slices from AD patients (n=6) are 0.4-fold and 0.6-fold lower than pericyte numbers in non-demented individuals (n=6), respectively. In this study, pericyte numbers were inversely correlated with extravasated IgG and fibrin levels in cortical and hippocampal slices of AD patients. In another study, the same group showed that the number of pericytes was inversely correlated with IgG and fibrin immunoreactivity and the APOE e4 status of AD patients (Halliday, et al., 2016). In another study, Miners et al. (Miners, et al., 2018) showed that platelet-derived growth factor receptor-beta (PDGFRβ) immunoreactivity is inversely correlated with fibrinogen immunoreactivity and low brain oxygenation levels in precuneus tissue slices from AD patients (n=49). In contrast, these correlations were not observed in non-demented individuals (n=37). Miners et al. (Miners, et al., 2019) also demonstrated that elevated CSF levels of soluble PDGFRβ correlate with elevated Qalb and hippocampal Ktrans values in samples from MCI and AD patients. Taken together, these studies show that structural changes in pericytes and reduced pericyte numbers correlate with cerebrovascular dysfunction and barrier leakage in AD.
4.5. IgG Immunoreactivity
The presence of IgG in postmortem brain tissue samples is one of the earliest published findings considered to be an indicator of barrier leakage in AD. In the 1970s, Ishii et al. (Ishii & Haga, 1975, 1976) showed IgG extravasation in brain tissue slices of deceased AD patients, but the pathological mechanisms behind this finding were unclear at the time. Other research groups made similar observations and hypothesized that an activated complement pathway was causing IgG accumulation in the brain parenchyma of AD patients (Alafuzoff, et al., 1987; Eikelenboom & Stam, 1982; Mann, Francis, Hoffman, & Montes, 1982; Powers, Sullivan, & Rosenthal, 1982). Based on data from elution experiments, Goust et al. (Goust, Mangum, & Powers, 1984) suggested that IgG in the brain likely comes from extracellular fluidic compartments due to a compromised barrier rather than from an intraparenchymal immune response. In 1989, Liu et al. ((H. M. Liu, Atack, & Rapoport, 1989) presented another alternative by demonstrating that continuous brain accumulation of IgG occurred through retrograde neuronal transport or via circumventricular organs that lack a blood-brain barrier. This form of IgG transport is unlikely due to the presence of specialized ependymal cells called ‘tanycytes’ that form a blood-CSF barrier at the circumventricular organs in the brain (Langlet, Mullier, Bouret, Prevot, & Dehouck, 2013). Taken together, these studies show that IgG immunoreactivity in brain parenchyma is associated with several pathological processes occurring in AD (e.g., intraparenchymal synthesis or microglial response), and therefore, may not be a specific marker for barrier leakage.
4.6. Prothrombin Immunoreactivity
Prothrombin is another bloodborne protein that is often used as a surrogate marker for barrier leakage in AD. Prothrombin (72–74 kDa) is synthesized in the liver and is converted into thrombin (37 kDa) at the onset of coagulation. Lewczuk et al. (Lewczuk, Reiber, & Ehrenreich, 1998) showed that 95% of prothrombin resides in the blood (95–150 mg/L), and less than 1% (0.1–1 mg/L) is present in the CSF in healthy individuals (n=18) (Smirnova, et al., 1997). In contrast, multiple studies using postmortem tissue highlight that prothrombin accumulates in the brain parenchyma of AD patients (Akiyama, Ikeda, Kondo, & McGeer, 1992; Berzin, et al., 2000; Borroni, et al., 2002; Lewczuk, et al., 1999; Mari, et al., 1996; Zipser, et al., 2007). Some of these studies demonstrated that prothrombin accumulation in the brain parenchyma is associated with barrier leakage (Berzin, et al., 2000; Lewczuk, et al., 1999; Zipser, et al., 2007). Specifically, Berzin et al. (Berzin, et al., 2000) evaluated prothrombin immunoreactivity in brain tissue slices obtained from AD patients at different Braak stages (Braak I/II, n=9; Braak III/IV, n=7; Braak V/VI, n=7) and compared them to non-demented subjects (n=12). The authors showed that prothrombin immunoreactivity was elevated around thin, tortuous capillaries in brain tissue slices from AD patients but not in brain slices from non-demented subjects. Zipser et al. (Zipser, et al., 2007) examined a larger cohort of postmortem brain tissue (Braak I/II, n=27; Braak III/IV, n=29; Braak V/VI, n=27; non-demented cases, n=10) and observed that prothrombin immunoreactivity was more frequently associated with shrunken endothelial cells in samples from AD patients at Braak stages V/VI compared to non-demented subjects. In contrast, prothrombin immunoreactivity was restricted to the capillary lumen in brain slices from AD patients at Braak stages I/II and non-demented subjects. Collectively, these studies suggest that prothrombin and thrombin immunoreactivity in the brain are potential indicators of barrier leakage in AD. Careful interpretation is required when using prothrombin as a leakage index as prothrombin can be synthesized in the brain, especially in an injured state (Arai, Miklossy, Klegeris, Guo, & McGeer, 2006; Weinstein, Gold, Cunningham, & Gall, 1995).
4.7. Fibrinogen Immunoreactivity
Fibrinogen (340 kDa) is a glycoprotein frequently used to identify barrier leakage in postmortem brain tissue. Fibrinogen is synthesized in the liver and circulates in the blood. In a vascular injury, fibrinogen is converted to fibrin (0.15 kDa), which seals the sites of vascular injury by forming blood clots. In AD patients, elevated blood levels of fibrinogen form large, unregulated clots that induce endothelial dysfunction (E. B. Smith, 1986; van Oijen, Witteman, Hofman, Koudstaal, & Breteler, 2005). Data from in vitro studies show that fibrinogen initiates the formation of F-actin fibers, which leads to widening of interendothelial junctions and albumin leakage ((Patibandla, et al., 2009; Tyagi, Roberts, Dean, Tyagi, & Lominadze, 2008). In the brain parenchyma, fibrinogen forms an insoluble complex with albumin that is resistant to degradation and can be detected immunochemically (Adams, Passino, Sachs, Nuriel, & Akassoglou, 2004; Chung, et al., 2016; Cortes-Canteli, et al., 2015; Cullen, et al., 2005, 2006; Lipinski & Sajdel-Sulkowska, 2006; Viggars, et al., 2011). Fiala et al. (Fiala, et al., 2002) reported that fibrinogen immunoreactivity was 7-fold higher (p < 0.05) in temporal cortical slices from AD patients (n=3) compared to those from non-demented individuals (n=4). Ryu and McLarnon (Ryu & McLarnon, 2009) reported that fibrinogen immunoreactivity was 4.5-fold higher (p=0.003) in entorhinal cortical slices from AD patients (n=8) compared to those from non-demented individuals (n=7). In white matter tissue from AD patients with capillary CAA (n = 8), Magaki et al. (Magaki, et al., 2018) found 2-fold increased fibrinogen immunoreactivity (p < 0.05) compared to tissue from non-demented individuals (n=10). Other researchers found that fibrinogen protein levels were 1.2-fold higher (p = 0.0026; n=49) in precuneus samples from AD patients compared to those in non-demented individuals (n=37) (Miners, et al., 2018).
Extravasated fibrinogen correlates with the severity of cerebrovascular injury. Cullen et al. (Cullen, et al., 2005, 2006) found fibrinogen immunoreactivity adjacent to Aβ deposits and heme-rich deposits, which are often at branch points of arterioles, venules, and capillaries in brain slices from AD patients (n=11) compared to non-demented individuals (n=10). Miners et al. (Miners, et al., 2018) found that fibrinogen protein levels were 1.2-fold higher (p < 0.01) in brain tissue samples from AD patients with severe CAA pathology compared to samples from AD patients without CAA pathology, indicating an association between fibrinogen levels and vascular Aβ deposits. The authors also found that extravasated fibrinogen directly correlated with severe cerebral hypoperfusion. Careful interpretation is required when using fibrinogen as a leakage index as fibrinogen synthesis is known to occur in the injured brain (Golanov, et al., 2019).
In addition to fibrinogen, fibrin also accumulates in the brain, primarily in the frontal cortex, hippocampus, entorhinal cortex, hippocampal formation, and the parahippocampal gyrus. Cortes-Canteli et al. (Cortes-Canteli, et al., 2015) showed that fibrin protein levels are 100-fold higher (p < 0.05) in cortical brain tissue samples from AD patients (n=29) compared to levels detected in samples from non-demented individuals (n=8). In hippocampus samples from AD patients, fibrin levels were 20-fold higher (p < 0.01) compared to those from non-demented individuals. Extravasated fibrin deposits were also present as ‘perivascular clouds’ around capillaries.
Together, these studies confirm that extravasated fibrinogen and fibrin are surrogate markers for barrier leakage in postmortem brain tissue samples from AD patients.
5. Molecular Mechanisms Involved in Barrier Leakage in Alzheimer’s Disease
Molecular mechanisms underlying barrier leakage in AD are not fully understood yet. In this section, we summarize the most prominent mechanisms that have been identified thus far in brain endothelial cell lines in vitro, in postmortem brain tissue samples from AD patients, or in vivo in animal models (Figure 5).
Figure 5. Mechanisms leading to Blood-Brain Barrier Leakage in Alzheimer’s Disease.
A) Schematic overview of transmembrane receptors at the neurovascular unit and their ligands that have been previously shown to induce blood-brain barrier leakage in vitro or in vivo. B) Many of these ligand-receptor pairs lead to one or more downstream signaling pathways such as PI3K/Akt, MAPK, or RhoA/ROCK that cause physiological changes in and around capillary endothelial cells. C) Some of these changes include downregulation of tight junction protein expression, delocalization of tight junction proteins from the interendothelial junctions, degradation of tight junction proteins, or changes in the cytoskeletal architecture of endothelial cells.
Hypoxia-Induced Barrier Leakage.
Hypoxia triggers barrier leakage in endothelial cells (Brown & Davis, 2005; Lochhead, et al., 2010). One signaling pathway in hypoxia involves activating the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2). In this pathway, hypoxia activates Nrf2, which moves to the nucleus where it binds to antioxidant response elements (AREs) in the promoter region of target genes such as heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase (NQO1) and initiates the transcription and translation of antioxidants to inhibit the production of reactive oxygen species (ROS) (Kensler, Wakabayashi, & Biswal, 2007; McSweeney, Warabi, & Siow, 2016; Tebay, et al., 2015). This pathway, however, is dysregulated in AD due to Aβ-mediated increase in ROS levels (Butterfield, 2018; Fao, Mota, & Rego, 2019). ROS alter ZO-1, claudin-5, and occludin at the blood-brain barrier, which disassembles the TJ complex (Carrano, et al., 2011; Hyeon, Lee, Yang, & Jeong, 2013; Wan, Chen, & Li, 2014). Another signaling pathway that leads to barrier leakage via ROS generation is bradykinin signaling. In AD, the bradykinin pathway is upregulated due to increased tPA activity (Medina, et al., 2005; Singh, Chen, Ghosh, Strickland, & Norris, 2020) (Figure 5A). Mechanistically, tPA converts plasminogen to plasmin generating bradykinin, which activates bradykinin receptors on endothelial cells (Gauberti, Potzeha, Vivien, & Martinez de Lizarrondo, 2018; Marcos-Contreras, et al., 2016; Medina, et al., 2005; Singh, et al., 2020). Activating bradykinin receptors dilates arteries/arterioles and constricts venules/veins resulting in cerebral hypoperfusion, vascular injury, and barrier leakage (Marcos-Contreras, et al., 2016). Cerebral hypoperfusion further generates ROS, disrupts TJ proteins, and increases IgG extravasation, which are all phenomena observed in APP23 mice (Shang, et al., 2019; S. Shang, et al., 2016; Shi, et al., 2019). A third signaling pathway involves angiotensins that are upregulated in AD, leading to increased ROS and IgG brain levels in 5xFAD mice (Takane, et al., 2017). In addition to ROS, nitric oxide is a key player involved in inducing barrier leakage. A recent study by Logsdon et al. identified that nitric oxide synthase can transiently disrupt Claudin-5 at the blood-brain barrier in mice up to 72 hours following traumatic brain injury (Logsdon et al., 2018). These effects were alleviated in mice treated with N(G)-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor. The effect of nitric oxide synthase on barrier leakage in AD is yet to be studied in AD animal models.
Cytoskeletal Changes and Barrier Leakage.
Cytoskeletal proteins regulate barrier integrity by stabilizing TJ protein complexes (Gonzalez-Mariscal, Betanzos, Nava, & Jaramillo, 2003). In this regard, cytoskeletal changes lead to actin polymerization, actin-myosin stress fiber formation, and calcium dysregulation, ultimately destabilizing TJ protein complexes (Carman, Mills, Krenz, Kim, & Bynoe, 2011; M. K. Choi, Liu, Wu, Yang, & Zhang, 2020) (Figure 5C). One of the pathways destabilizing TJ protein complexes is adenosine signaling. Briefly, brain damage activates adenosine signaling that induces phosphorylation of actin-binding proteins, formation of stress fibers, and detachment of TJ proteins from the interendothelial junctions (Figure 5A) (Carman, et al., 2011; Garcia-Ponce, Citalan-Madrid, Velazquez-Avila, Vargas-Robles, & Schnoor, 2015; C. Guo, et al., 2020; Hicks, O’Neil, Dubinsky, & Brown, 2010; Koss, et al., 2006; Kuhlmann, et al., 2007). Data from in vivo studies show that damage increases adenosine levels which results in increased blood-brain barrier permeability of 10 and 70 kDa dextrans in APP/PS1 mice (Carman, et al., 2011). Another signaling pathway is driven by calcium that, under normal conditions, binds to E-cadherin and occludin to stabilize cell-cell adhesion in brain endothelial cell lines. However, calcium in excess dissociates the tight junctional complex from the cytoskeleton at the interendothelial junctions (Pokutta, Herrenknecht, Kemler, & Engel, 1994; Ye, Tsukamoto, Sun, & Nigam, 1999). In vitro studies revealed that Aβ42 activation of the receptor for advanced glycation end products (RAGE) dysregulates calcium homeostasis in bEnd.3 cells (Ahn, et al., 2018; Kook, et al., 2012; Kook, et al., 2014) (Figure 5A). Aβ42 binding to RAGE also disrupts adherens junction proteins by activating the mammalian target of rapamycin (mTOR) or calmodulin-dependent protein kinase-β/AMP-activated protein kinase signaling (Chen, Li, Wang, & Liu, 2020; Van Skike, et al., 2018) (Figure 5A, 5B). Another mechanism that drives barrier leakage involves upregulation of several mitogen-activated protein (MAP) kinases in AD (Rodriguez-Perdigon, Solas, & Ramirez, 2016; S. Shang, et al., 2016; Zhu, et al., 2001) (Figure 5B). Cytoskeletal reorganization in pericytes also affects barrier integrity, and these changes are due to RhoA/Rho-associated protein kinase (RhoA/ROCK) signaling. In this regard, Park et al. (J. C. Park, et al., 2017) demonstrated that in 5xFAD mice, pericytes secrete low levels of Annexin A1 compared to wild type mice. Annexin A1 is an endogenous RhoA/ROCK signaling inhibitor and anti-inflammatory protein, and Park et al. (J. C. Park, et al., 2017) showed that low levels of Annexin A1 levels initiate barrier leakage (Figure 5C).
Growth Factor-Mediated Barrier Leakage.
Three growth factors – VEGF, transforming growth factor β (TGFβ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) – have been shown to affect barrier leakage. While VEGF and TGFβ promote neuronal survival and neuronal/glial cell proliferation by various signaling pathways, they also induce barrier leakage by activating Src-kinase that phosphorylates adhesion proteins and disassembles TJ proteins in endothelial cells (Croll & Wiegand, 2001; Garcia-Barroso, et al., 2013; R. Paul, et al., 2001; Ruiz de Almodovar, Lambrechts, Mazzone, & Carmeliet, 2009; Weis & Cheresh, 2005). At the brain capillary endothelium, VEGF activates PI3 kinase/Akt signaling that upregulates MMP-9 protein levels, redistributes ZO-1, and recruits claudin-5 and occludin away from the tight junctional complex (Kilic, et al., 2006; Miao, et al., 2014; Valable, et al., 2005). TGFβ, on the other hand, induces leakage either by activating the RGMa-CDC42-PAK-1 signaling pathway in astrocytes and causing glial scar formation or by reducing pericyte and mural cell coverage around capillaries, which then leads to small vessel disease (Kato, et al., 2020; Satoh, Tabunoki, Ishida, Saito, & Arima, 2013). GM-CSF induces downregulation of ZO-1 and claudin-5 protein expression via the ubiquitin-proteasome pathway endothelial cells (Boyd, et al., 2010; Li, et al., 2018; Murphy, Yang, & Cordell, 1998; Ridwan, Bauer, Frauenknecht, von Pein, & Sommer, 2012; Satoh, et al., 2013; J. Shang, et al., 2016).
Cytokine-Induced Barrier Leakage.
Proinflammatory cytokines such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha subunit (TNFα) induce barrier leakage through more than one signaling pathway. For example, IL-1β suppresses sonic hedgehog signaling in astrocytes, which in turn downregulates TJs in endothelial cells in vitro ((Y. Wang, et al., 2014)). IL-1β also activates extracellular signal-regulated kinase (ERK) signaling, which upregulates MMP-9 and disrupts TJ proteins (Gu & Wiley, 2006; Mori, Kondo, Ohshima, Ishida, & Mukaida, 2002; Ralay Ranaivo, Zunich, Choi, Hodge, & Wainwright, 2011; Yang, Zhao, Zhang, Zhang, & Xu, 2016). TNFα activates c-Jun N-terminal kinases (JNK) that upregulate MMPs, which degrade TJ proteins (Machida, et al., 2017; Zhan, et al., 2015). Further, Guglielmotto et al. (Guglielmotto, et al., 2019) demonstrated that TNFα and IL-6 both activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and mitogen-activated protein kinases (MAPK) that disrupt claudin-5 expression in 5xFAD mice. Another pro-inflammatory cytokine, interferon-γ (IFNγ), decreases pericyte coverage of endothelial cells by initiating PDGFRβ internalization, which is a critical signaling step that leads to pericyte detachment and degradation. This loss of pericytes ultimately contributes to barrier leakage (Heldin & Westermark, 1999; Jansson, et al., 2016; Lindahl, Johansson, Leveen, & Betsholtz, 1997).
Taken together, these studies highlight that several signaling pathways are involved in the disruption of TJ proteins and loss of pericyte coverage, contributing to barrier leakage in AD models. However, further studies are needed to confirm these signaling pathways in AD patients.
6. Clinical Relevance of Barrier Leakage in Alzheimer’s Disease.
Emerging evidence indicates that barrier leakage in AD contributes to cognitive decline in patients and therefore is of clinical relevance. In this section, we will summarize the literature that defines the relationship between barrier leakage and cognitive impairment, we will highlight the importance of comorbidities, and we will point out opportunities for therapeutic intervention to repair barrier leakage in AD. Barrier leakage generally increases with aging (Montagne et al. 2015; Verheggen et al., 2020) but the age effect on leakage index is highly variable among AD and nondemented patients. These variations are partly due to the age of the patient, genetic factors and lifestyle choices which are not discussed in this section.
6.1. Association Between Barrier Leakage and Cognitive Impairment
Barrier leakage in AD was first observed over 40 years ago, but the concept that a leaky barrier contributes to cognitive impairment is relatively novel (Glenner, 1979; Iadecola, 2013). Early findings were based on the detection of extravasated plasma proteins in the brain of deceased AD patients, suggesting a leaky barrier (Blennow, et al., 1990; Wada, 1998). However, at the time, not all researchers detected barrier leakage, leaving the field with a controversy for decades (Alafuzoff, et al., 1987; Munoz, Erkinjuntti, Gaytan-Garcia, & Hachinski, 1997). Moreover, other researchers did not observe an association between barrier leakage markers (Qalb values) and cognition scores (MMSE) in AD patients (Janelidze, et al., 2017; Matsumoto, et al., 2007, 2008; Skillback, et al., 2017). Recent advances in neuroimaging enabled researchers to carefully address the discrepancy between these studies by using novel MRI techniques combined with capillary markers that allow reliable detection of barrier leakage. In 2015, Montagne and co-workers (Montagne et al., 2015) provided in vivo evidence in MCI patients that barrier leakage in the aging hippocampus is associated with cognitive impairment. Further, van de Haar et al. (van de Haar, Burgmans, et al., 2016) showed a 5-fold increase of gadobutrol leakage in the brain of AD patients compared to control individuals (p < 0.05). This data also showed that barrier leakage is global and correlates with cognitive decline in patients with early-stage AD. Based on the data, the authors hypothesized that “a compromised BBB may be part of a cascade of pathologic events that eventually lead to cognitive decline and dementia” (van de Haar, Burgmans, et al., 2016). Similar results have recently been obtained by other groups as well (Montagne, et al., 2016; Shams, et al., 2015). Specifically, Montagne et al. (Montagne, et al., 2016) demonstrated that individuals with MCI (n = 20) had 1.5-fold higher Ktrans values (p < 0.001) compared to age-matched non-demented individuals (n = 18); both male and female subjects included. Additionally, Ktrans values were directly associated (p < 0.01) with 1.5-fold higher Qalb values in MCI individuals (n = 17) compared to Qalb values in non-demented individuals (n = 14). Likewise, Shams et al. (Shams, et al., 2015) showed that the prevalence of cerebral microbleeds is associated with lower cognitive scores (p < 0.006) in elderly, demented patients (n = 1,504). In the case of severe cerebrovascular injury (CMBs > 8), CMBs were found to correlate with poor MMSE scores of AD patients (n = 21) (Goos, et al., 2009). Barrier leakage and cognitive decline also occur in individuals with MCI who do not have extensive AD pathology (p < 0.001) (Nation, et al., 2019). Together, these studies provide evidence of a correlation between barrier leakage and cognitive decline. Thus, emerging evidence indicates that, in AD patients, barrier leakage and memory decline are potentially mechanistically linked, but more studies are needed to define this link.
6.2. Effect of Comorbidities on Barrier Leakage in Alzheimer’s Disease
Nearly 61% of AD patients examined across various clinical care settings have 3 or more comorbidities that can lead to increased AD severity (Doraiswamy, Leon, Cummings, Marin, & Neumann, 2002). AD patients are at a significantly higher risk to develop epilepsy, renal dysfunction, diabetes and/or cardiovascular disease (Ford et al., 2018; Rojas et al., 2021; Song et al., 2021). In this section, we will focus on these comorbidities and discuss how each of them contributes to barrier leakage in AD.
Epilepsy.
Epilepsy is a well-known co-morbidity in AD (Forstl, et al., 1992; Volicer, Smith, & Volicer, 1995). Indeed, more than 25% of the general AD patient population develop seizures (Palop & Mucke, 2016; Vossel, et al., 2013; Vossel, et al., 2016). New evidence shows that early-stage AD patients experience seizures, and data from the most recent clinical studies demonstrate that as many as 65% of AD patients have seizures (Horvath, Kiss, Szucs, & Kamondi, 2019; Lam, et al., 2020; Vossel, Tartaglia, Nygaard, Zeman, & Miller, 2017). These data indicate that epilepsy incidence in AD has long been underestimated and is much greater than initially reported. Blood-brain barrier leakage is one common pathological hallmark both diseases share (Vossel, et al., 2016). Patients with epilepsy develop barrier dysfunction, neurovascular inflammation, and leukocyte accumulation that can lead to barrier leakage (Rempe, Hartz, & Bauer, 2016; Rempe, et al., 2018; van Vliet, et al., 2016; Weissberg, et al., 2015). Barrier leakage itself contributes to seizure genesis in these patients through a pernicious positive feedback loop that promotes epilepsy progression (Weissberg, Reichert, Heinemann, & Friedman, 2011). In this feedback loop, seizures drive barrier leakage that exacerbates neuroinflammation and subsequent seizure burden (Vezzani & Janigro, 2009; Weissberg, et al., 2015). In AD patients, data from clinical studies best highlight the impact of epilepsy: in AD patients with epilepsy, cognitive decline starts 5–7 years earlier, and life expectancy is further reduced compared to seizure-free AD patients (Amatniek, et al., 2006; Cretin, et al., 2016; Forstl, et al., 1992; Irizarry, et al., 2012; McAreavey, Ballinger, & Fenton, 1992; M. Mendez & Lim, 2003; M. F. Mendez, Catanzaro, Doss, R, & Frey, 1994; Picco, et al., 2011; Rao, Dove, Cascino, & Petersen, 2009; Romanelli, Morris, Ashkin, & Coben, 1990; Sarkis, et al., 2015; Volicer, et al., 1995; Vossel, et al., 2013).
Despite high clinical relevance, researchers have mostly ignored the role seizures play in AD, leaving clinicians with limited therapeutic options for these patients. Barrier leakage in AD with epilepsy is poorly understood, and effective treatment options are not available. Thus, more research is needed to identify the underlying mechanism that exacerbates barrier leakage and establish an intervention strategy to help repair barrier dysfunction in AD with epilepsy.
Renal Dysfunction.
Chronic kidney disease (CKD) is a gradual decline of renal function that is characterized by reduced creatinine serum levels and high urine albumin levels indicating poor glomerular filtration, and thus, kidney damage (Deckers, et al., 2017). Mechanistically, CKD causes the release of cytokines into the blood followed by cytokine infiltration of the CNS, worsening existing blood-brain barrier leakage (Helmer, et al., 2011; Miranda, Cordeiro, Dos Santos Lacerda Soares, Ferreira, & Simoes, 2017; Miwa, et al., 2014; Zammit, Katz, Bitzer, & Lipton, 2016). Mild CKD is associated with endothelial dysfunction and AD, whereas severe CKD is associated with a high grade of endothelial dysfunction that causes lacunar infarcts, white matter hyperintensities, and ultimately vascular dementia (Helmer et al. 2011; Miwa et al. 2014; Zammit et al. 2016).
Diabetes.
Diabetes is a metabolic disorder that leads to persistently high blood glucose levels in the body. Data from case-controlled DCE-MRI studies by Starr et al. (Starr, et al., 2003) show that diabetic patients are more likely to have increased gadolinium brain uptake compared to non-diabetic individuals, especially in deep brain regions compared to cortical regions, which indicates barrier leakage. In addition, Janelidze et al. (Janelidze, et al., 2017) recently confirmed that diabetic patients have elevated Qalb values compared to non-diabetic individuals. Although the relative contribution of diabetes-induced barrier leakage in AD is yet to be determined, in vivo studies by Salameh et al. show that oxidative stress can trigger pericyte loss and barrier leakage in diabetic mice (Salameh et al., 2016). Further investigation is required to validate the causative role of oxidative stress on barrier leakage in diabetic AD versus diabetic nondemented patients.
Cardiovascular Comorbidities.
Cardiovascular comorbidities such as hypertension, atrial fibrillation, and transient ischemic attacks correlate with high Ktrans values for gadobentate dimeglumine in AD patients, MCI patients, and non-demented individuals (Blennow, et al., 1990; Bowman, et al., 2007; Nation, et al., 2019). Other studies also highlight that CMBs correlate with high blood pressure in AD patients (Benedictus, et al., 2013; Nagasawa, et al., 2014; Olazaran, et al., 2014). In addition, Bridges et al. (Bridges, et al., 2014) showed elevated fibrinogen levels in the subcortical brain regions of hypertensive patients compared to non-hypertensive patients (n = 84; Braak stages: 0-II). Together, these studies suggest a correlation between barrier leakage and cardiovascular conditions in AD patients.
In summary, comorbidities such as epilepsy, renal dysfunction, diabetes, and cardiovascular disease can increase the onset and extent of barrier leakage in AD patients irrespective of the severity of pathology or cognitive impairment induced by these conditions.
6.3. Therapeutic Strategies for Barrier Leakage in AD: Opportunities and Challenges
Current FDA-approved drugs for AD therapy have been designed to treat clinical dementia symptoms by slowing memory loss. However, these drugs have a modest effect on cognition, do not stop AD progression, are not effective in all patients, and lose efficacy after ~6–12 months. Thus, there is an urgent need for new, disease-modifying approaches to treat AD patients successfully. In this regard, therapeutic strategies to repair barrier leakage open up new opportunities for treatment. The following section highlights current approaches that alter barrier properties and contrast the success obtained in preclinical studies with challenges faced in the clinic (Figure 6).
Figure 6. Therapeutic Strategies for Blood-Brain Barrier Leakage in Alzheimer’s Disease.
Summary of selected studies that show the relevance of therapeutic strategies towards barrier leakage in transgenic AD mice and rats. The therapeutic strategies depicted here have either blood-brain barrier opening effects (red), blood-brain barrier sealing effects (blue), or no effect on the blood-brain barrier (dark gray). The numbers in each square refer to published studies shown in Table 4 for each strategy/model combination. Note that the number of white spaces indicates unstudied/unknown strategy/model combinations.
Anticoagulants, Antiplatelet and Fibrinolytic Agents.
Anticoagulants, antiplatelet and fibrinolytic agents regulate blood flow by reducing blood clot formation (Hogg & Weitz, 2017). These agents have differential effects on the blood-brain barrier in AD. Among anticoagulants, vitamin K antagonists such as heparin and warfarin reduce vitamin-K-dependent clotting factors but induce barrier leakage and microhemorrhages in AD patients (W. Cheng, Liu, Li, & Li, 2018; Harter, Levine, & Henderson, 2015). Another anticoagulant category is thrombin inhibitors that reduce the number of hypertrophied pericytes and perivascular astrocytes in 7-to-14-month-old TgCRND8 mice compared to age-matched untreated TgCRND8 mice (Cortes-Canteli, et al., 2019). Antiplatelet drugs such as dipyridamole, cilostazol, and tadalafil prevent microhemorrhages from worsening in Tg2576, TgSwDI, and J20 mice, respectively (Fisher, et al., 2011; Garcia-Barroso, et al., 2013; Hattori, et al., 2016; Maki, et al., 2014). Recombinant activated protein C (3K3-APC) is another therapeutic agent with anticoagulant and antiplatelet properties that delays coagulation and reduces IgG leakage and microbleeds in hypertensive rats (Lazic, et al., 2019; Marottoli, et al., 2017). Fibrinolytic agents, such as spinosyn and recombinant tPA, degrade fibrin in blood clots but are known to induce microhemorrhages in 5xFAD mice and APP23 mice (Cai, et al., 2020). In summary, non-Vitamin-K antagonists, oral anticoagulants, antiplatelet agents, and 3K3-APC have barrier-sealing effects, whereas vitamin K antagonists and fibrinolytic agents increase microhemorrhages in preclinical AD models.
Angiotensin Receptor Blockers.
Angiotensin receptor blockers are prescribed for hypertension, a comorbidity that correlates with an increased risk for developing AD in the elderly population (Kivipelto, et al., 2001). Angiotensin receptor blockers are prescribed in response to elevated angiotensin-II levels in the blood that increases cerebral blood flow (Fleegal-DeMotta, Doghu, & Banks, 2009). Conversely, angiotensin-II receptor blockers such as olmesartan prevent barrier leakage by upregulating tight junction mRNA levels or limiting oxidative stress in APP23 and 5xFAD mice (Nakagawa, Hasegawa, Uekawa, & Kim-Mitsuyama, 2017; Nakagawa, Hasegawa, Uekawa, Senju, et al., 2017; Pelisch, et al., 2011; Takeda, et al., 2009). This effect was not observed for non-angiotensin receptor blockers such as hydralazine and nifedipine (Takeda, et al., 2009). Together, these preclinical studies show that angiotensin-II receptor blockers reduce barrier leakage in preclinical AD models, but more studies are needed to confirm a therapeutic benefit in patients.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
NSAIDs inhibit the production of proinflammatory cytokines by blocking the cyclooxygenase enzymes COX-1 and COX-2 and prevent barrier disruption in vivo (Banks, et al., 2015; Candelario-Jalil, et al., 2007; Grosser, Ricciotti, & FitzGerald, 2017). In a recent study, Elfakhri et al. (Elfakhri, Abdallah, Brannen, & Kaddoumi, 2019) showed that the NSAID etodolac combined with the antioxidant a-tocopherol reduced IgG extravasation in 5xFAD mice. This combination therapy also shifted APP processing towards the non-amyloidogenic pathway in this study. Qosa et al. (Qosa, Batarseh, et al., 2015; Qosa, Mohamed, et al., 2015) and Al Rihani et al. (Al Rihani, Darakjian, & Kaddoumi, 2019; Al Rihani, Lan, & Kaddoumi, 2019) also reported that a combination of oleocanthal and extra virgin olive oil reduced IgG extravasation in TgSwDI mice. Although these preclinical studies indicate that NSAIDs reduce barrier leakage in AD, results from the “AD Anti-inflammatory Prevention Trial” show that NSAIDs increase the severity of AD pathology (Breitner, et al., 2011). Thus, the clinical benefit of NSAIDs is questionable and requires further studies.
Passive Aβ Immunotherapy.
Passive Aβ immunotherapy involves monoclonal antibodies raised against different Aβ conformations that are thought to enter the brain parenchyma via non-specific transcytosis. Over the past few decades, passive Aβ immunotherapy therapies have been extensively investigated in clinical trials (van Dyck, 2018). However, no cognitive improvements have been observed in AD patients enrolled in the antibody treatment arm compared to AD patients in the placebo arm in these clinical trials (van Dyck, 2018). In this regard, all clinical trials using passive Aβ immunotherapy have failed to improve cognition in AD patients. Poor cognitive outcomes in AD patients in the passive Aβ immunotherapy arm have been attributed to 1) poor antibody brain penetration (< 0.1%) and 2) testing the wrong patient population by focusing on patients with advanced AD or including patients with unconfirmed Aβ deposition (Jack, et al., 2013; van Dyck, 2018). Brain MRI scans of patients who received monoclonal antibodies show that passive Aβ immunotherapy causes Aβ-related imaging abnormalities (ARIA) such as cerebral edema (ARIA-E) and microhemorrhages (ARIA-H), indicating barrier leakage in these patients (van Dyck et al. 2018). The severity of ARIA-E or ARIA-H incidences in AD patients varied depending on the monoclonal Aβ antibody tested. IgG1 antibodies like bapineuzumab (IgG1: against Aβ1–5 residues), gantenerumab (IgG1: against Aβ13–24 and Aβ18–27), and aducanumab (IgG1: against Aβ36) resulted in high (> 3%) ARIA incidences in the enrolled patient population (Rinne & Nagren, 2010; Salloway, et al., 2014; Salloway, et al., 2009; Sevigny, et al., 2016; van Dyck, 2018). In contrast, humanized antibodies against mid-Aβ residues such as solanezumab (IgG1: against Aβ16–26) and crenezumab (IgG4: against Aβ13–24) recognize all forms of Aβ species and result in low (< 1%) ARIA incidences (Cummings, et al., 2019; Doody, et al., 2014; Farlow, et al., 2012; van Dyck, 2018). In some cases, antibodies do not cause ARIA incidences. One such example is ponezumab, an IgG2 raised against Aβ30–40 residues, that recognizes only monomeric species and did not cause ARIA incidences in patients (Landen, et al., 2013; van Dyck, 2018). Studies on BAN2401, an IgG1 raised against Aβ protofibrils, are ongoing, but previous reports indicate that the number of ARIA incidences are similar between patients receiving BAN2401 and placebo (Landen, et al., 2013; Logovinsky, et al., 2016; van Dyck, 2018).
Aducanumab, a high-affinity, fully human monoclonal IgG1 antibody with specificity to Aβ (N-terminus 3–6) soluble oligomers and insoluble fibers, revived the hope to find a therapeutic drug for AD. The antibody was specifically designed to bind aggregated forms of Aβ in the parenchyma, but not vascular Aβ. A single-dose study led by Ferrero and colleagues (Ferrero, et al., 2016) showed that aducanumab at doses ≤ 30 mg/kg follows linear pharmacokinetics and has an acceptable safety and tolerability profile in humans. Subsequently to this report, Sevigny et al. (Sevigny, et al., 2016) showed that aducanumab given monthly for one year reduces Aβ brain levels in a dose- and time-dependent manner in patients with prodromal or mild AD. Importantly, data from this study indicated that aducanumab treatment also slowed cognitive decline as measured by Clinical Dementia Rating-Sum of Boxes and Mini-Mental State Examination scores. In June 2021, aducanumab (Aduhelm™) received FDA approval under the agency’s accelerated approval pathway for AD treatment. Thus, aducanumab is the first FDA-approved drug to reduce Aβ levels. However, the accelerated approval pathway comes with the requirement to verify the clinical benefit of the drug in a post-approval trial. Should aducanumab not show a clinical benefit in this post-approval trial, the FDA approval may be withdrawn. Recently published reports question the benefits of aducanumab treatment. For example, no significant changes in the primary endpoints were observed between the aducanumab-treated and placebo-treated groups in patients with early-stage dementia (Angelo & Ward, 2021). Furthermore, patients that received aducanumab and patients with genetic variants of APOE4 have reported higher and chronic incidences of ARIA-E and ARIA-H than patients receiving placebo (Salloway & Cummings, 2021; VandeVrede, et al., 2020). In addition to the side effects and the relatively low efficacy profile, high patient-associated costs may limit the usage of aducanumab in patients (Anderson, Ayanian, Souza, & Landon, 2021).
In summary, passive immunotherapies are a recent promising advancement that also has challenges. For example, it remains unclear how antibodies enter the brain from the CSF and resolve Aβ species in vivo. In addition, with limited information on a ‘therapeutic window’ for antibodies to be effective, passive immunotherapy poses a risk of causing barrier leakage in AD patients, which could counteract their potential therapeutic benefit.
mTOR inhibitors.
mTOR inhibition is a recently developed strategy to reduce barrier leakage in preclinical AD models (Van Skike & Galvan, 2019; Van Skike, et al., 2018). Several studies have shown that mTOR inhibition reduces barrier leakage in preclinical models for AD, cerebral ischemia, and epilepsy (W. Guo, Feng, Miao, Liu, & Xu, 2014; Van Skike, et al., 2018; van Vliet, et al., 2012). Lin et al. (Lin, Parikh, Hoffman, & Ma, 2017; Lin, et al., 2020) presented data indicating that rapamycin (mTOR inhibitor) prevents barrier leakage in APOE4 transgenic mice by inhibiting cyclophilin-A-dependent proinflammatory pathways. More research is needed to evaluate the potential of mTOR inhibitors to reduce barrier leakage in AD patients.
Taken together, these studies outline the effect of potential pharmacological interventions to repair barrier leakage in preclinical AD models and highlight the challenge of translating these strategies to treat AD patients.
7. FUTURE PERSPECTIVES
The studies summarized in this review highlight barrier leakage as an essential part of AD pathology and suggest that repairing barrier leakage could help slow cognitive decline. However, an effective, disease-modifying therapy is currently not available. More research is needed to identify triggers inducing barrier leakage, unravel mechanistic steps, and map signaling pathways underlying barrier leakage in AD. At the preclinical and clinical level, longitudinal studies using novel imaging techniques combined with biochemical methods could help detect and visualize the onset and extent of barrier leakage. Animal models could be used to identify underlying mechanisms of barrier leakage and test novel therapeutic approaches to repair this leakage in AD. In addition, the link between barrier leakage and cognitive decline is poorly understood, and more studies are needed to define this link further.
Moreover, barrier leakage could serve as an early biomarker for vascular contributions to cognitive impairment (VCID) in AD and AD-related dementias. In this regard, large-scale longitudinal neuroimaging clinical studies combined with cognitive tests could provide critical insights into this link. Recognizing that barrier leakage may be a key feature in some patients, but maybe not in others, could further help to shape the definition of VCID, AD, and AD-related dementias and open a new area for therapeutic intervention. Barrier leakage could also serve as a critical “checkpoint” in clinical trials to help with patient profiling based on their susceptibility to developing leakage for better, targeted intervention strategies.
In summary, barrier dysfunction as a whole and barrier leakage in particular are yet understudied areas that hold the promise for early detection of AD and for the development of new therapeutic strategies that, for the first time, would have a disease-modifying effect in AD patients.
Table 4. Effect of Therapeutic Strategies on the Blood-Brain Barrier in AD Models.
Table 4 lists studies indicated in Figure 6 that show the effects of therapeutics strategies in transgenic AD mice and rats. References: (Batarseh, et al., 2017; Batarseh & Kaddoumi, 2018; Beckmann, Gerard, Abramowski, Cannet, & Staufenbiel, 2011; Bien-Ly, et al., 2015; Blockx, et al., 2016; Burbach, et al., 2007; Burgess, Dubey, et al., 2014; Burgess, Nhan, Moffatt, Klibanov, & Hynynen, 2014; Chauhan & Sandoval, 2007; Chauhan, Siegel, & Lichtor, 2004; J. J. Choi, et al., 2008; Cortes-Canteli, et al., 2019; Dubey, et al., 2020; Elfakhri, et al., 2019; Fan, DeFilippis, & Van Nostrand, 2007; Faraco, et al., 2016; Fitz, et al., 2014; Hattori, et al., 2016; Herring, et al., 2010; Hur, et al., 2018; Jordao, et al., 2010; Jordao, et al., 2013; Kook, et al., 2014; Lazic, et al., 2019; W. J. Lee, et al., 2020; Leinenga & Gotz, 2018; Leinenga, Koh, & Gotz, 2019; M. Liu, et al., 2018; X. Liu, et al., 2019a, 2019b; Lynch, et al., 2021; Marinescu, et al., 2017; Michael, et al., 2019; Mueggler, et al., 2003; Nyul-Toth, et al., 2020; Ongali, et al., 2014; Pandit & Mukhopadhyay, 2004; Y. H. Park, et al., 2020; Passos, Kilday, Gillen, Cribbs, & Vasilevko, 2016; Poduslo, Curran, Wengenack, Malester, & Duff, 2001; Poon, Pellow, & Hynynen, 2021; Reuter, Venus, Grudzenski, et al., 2016; Robison, et al., 2020; Royea, Martinot, & Hamel, 2020; Saito, Buciak, Yang, & Pardridge, 1995; Schultheiss, et al., 2006; Shabir, et al., 2020; Sharp, et al., 2019; J. Song, Choi, Whitcomb, & Kim, 2017; St-Amour, et al., 2013; Takeda, et al., 2009; Thakker, et al., 2009; Uekawa, et al., 2016; Van Skike, et al., 2018; Vasilevko, Xu, Previti, Van Nostrand, & Cribbs, 2007; Weber-Adrian, et al., 2019; Xhima, et al., 2020; Xie, et al., 2020; Xiong, et al., 2021; M. Xu, et al., 2020; J. Yan, et al., 2015; P. Yan, et al., 2015).
| # In Fig. 6 | Therapeutic Strategy | Model | Reference |
|---|---|---|---|
| 1 | Angiotensin Receptor Blockers | Tg2576 Mice | Nyül-Töth et al. 2020; Passos et al. 2016; Faraco et al. 2016 |
| 2 | Angiotensin Receptor Blockers | APP23 Mice | Takeda et al. 2009 |
| 3 | Angiotensin Receptor Blockers | hAPP (J20) Mice | Ongali et al. 2014; Royea et al. 2020 |
| 4 | Angiotensin Receptor Blockers | TgSwDI Mice | Faraco et al. 2016 |
| 5 | Angiotensin Receptor Blockers | 5xFAD Mice | Uekawa et al. 2016 |
| 6 | Antibiotics | Tg2576 Mice hAPP (J20) Mice | Yan et al. 2015 |
| 7 | Antibiotics | Tg2576 Mice hAPP (J20) Mice | Van Skike et al. 2018 |
| 8 | Antibiotics | TgSwDI Mice | Fan et al. 2007 |
| 9 | Antibiotics | 5xFAD Mice | Yan et al. 2015 |
| 10 | Anticoagulants | Tg2576 Mice | Micheal et al. 2019 |
| 11 | Anticoagulants | APP23 Mice | Marinescu et al. 2017 |
| 12 | Anticoagulants | TgCRND8 Mice | Cortes-Canteli et al. 2019 |
| 13 | Anticoagulants | 5xFAD Mice | Lazic et al. 2019 |
| 14 | Antioxidants | Tg2576 Mice | Park et al. 2020; Han et al. 2015 |
| 15 | Antioxidants | APP23 Mice | Liu et al. 2019a; Liu et al. 2019b |
| 16 | Antioxidants | TgSwDI Mice | Saito et al. 2017 |
| 17 | Antioxidants | APP/PS1 Mice | Xu et al 2020 |
| 18 | Antioxidants | 5xFAD Mice | Elfakhri et al 2019; Batarseh & Kaddoumi, 2018; Song et al. 2017; Batarseh et al. 2017; Kook et al. 2014 |
| 19 | Cranial / Cognitive Stimulations | APP23 Mice | Mueggler et al. 2003 |
| 20 | Cranial / Cognitive Stimulations | hAPP (J20) Mice | Shabir et al. 2020; Sharp et al. 2019 |
| 21 | Cranial / Cognitive Stimulations | TgCRND8 Mice | Herring et al. 2010 |
| 22 | Cranial / Cognitive Stimulations | TgSwDI Mice | Robison et al. 2020 |
| 23 | Immunosuppressants | hAPP (J20) Mice | Van Skike et al. 2018 |
| 24 | Passive Immunotherapies | PDAPP Mice | Blockx et al. 2016 |
| 25 | Passive Immunotherapies | Tg2576 Mice | Thakker et al. 2009 |
| 26 | Passive Immunotherapies | APP23 Mice | Burbach et al. 2007; Fitz et al. 2014; Beckmann et al. 2011 |
| 27 | Passive Immunotherapies | TgCRND8 Mice | Dubey et al. 2020; Jordäo et al. 2010; Chauhan, 2007; Chauhan et al. 2004 |
| 28 | Passive Immunotherapies | TgSwDI Mice | Vasilevko et al. 2007 |
| 29 | Passive Immunotherapies | 3xTg-AD | St-Amour et al. 2013 |
| 30 | Passive Immunotherapies | Tau P301L Mice | Bien-Ly et al. 2015 |
| 31 | Passive Immunotherapies | APP/PS1 Mice | Podulso et al. 2001 |
| 32 | Passive Immunotherapies | 5xFAD Mice | Xiong et al. 2021 |
| 33 | Scanning / Focused Ultrasound | APP23 Mice | Leinenga et al. 2019; Leinenga & Götz, 2018 Poon et al. 2021; Lynch et al. 2021; Dubey et al. 2020 |
| 34 | Scanning / Focused Ultrasound | TgCRND8 Mice | Liu et al. 2018; Burgess et al. 2014a; Burgess et al. 2014b; Jordäo et al. 2013; Jordäo et al. 2010; Xhima et al. 2020; Weber-Adrian et al. 2019; |
| 35 | Scanning / Focused Ultrasound | Tau P301L Mice | Pandit et al. 2019 |
| 36 | Scanning / Focused Ultrasound | APP/PS1 Mice | Choi et al 2008 |
| 37 | Scanning / Focused Ultrasound | 5xFAD Mice | Lee et al. 2020 |
| 38 | Short-Chain Fatty Acids | Tg2576 Mice | Hur et al. 2018 |
| 39 | Short-Chain Fatty Acids | APP23 Mice | Reuter et al 2016 |
| 40 | Short-Chain Fatty Acids | APP/PS1 Mice | Xie et al. 2020 |
| 41 | Statins & NSAIDs | Tg2576 Mice | Garcia-Alloza et al. 2009 |
| 42 | Statins & NSAIDs | APP23 Mice | Reuter et al. 2016; Schultheiss et al. 2006 |
| 43 | Statins & NSAIDs | TgSwDI Mice | Hattori et al. 2016 |
ACKNOWLEDGEMENTS
We thank the members of the Hartz and Bauer laboratories for proofreading the manuscript.
FUNDING SOURCES
This project was supported by grant number 2R01AG039621 from the National Institute on Aging (to A.M.S.H.). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- APOE
Apolipoprotein E
- APP
Amyloid Precursor Protein
- AQP-4
Aquaporin-4
- ARIA
Amyloid Related Imaging Abnormalities
- Aβ
Amyloid-Beta
- CAA
Cerebral Amyloid Angiopathy
- CAM
Cell Adhesion Molecule
- CKD
Chronic Kidney Disease
- CMBs
Cerebral Microbleeds
- CNS
Central Nervous System
- COX
Cyclooxygenase
- CSF
Cerebrospinal Fluid
- CT
Computed Tomography
- DCE-MRI
Dynamic Contrast-Enhanced Magnetic Resonance Imaging
- ERK
Extracellular Signal Regulated Kinase
- ET-1
Endothelin-1
- FDA
Food and Drug Administration
- GM-CSF
Granulocyte-Macrophage Colony-Stimulating Factor
- HA
Hyaluronic Acid
- hAPP
Human Amyloid Precursor Protein
- HO-1
Heme Oxygenase-1
- ICAM-1
Intercellular Adhesion Molecule-1
- IFNγ
Interferon gamma
- IgG
Immunoglobulin G
- IL
Interleukin
- JAM
Junctional Adhesion Molecule
- JNK
c-Jun N-terminal Kinase
- Kir4.1
Inwardly Rectifying Potassium Channel 4.1
- Ktrans
Permeability Coefficient
- MAPK
Mitogen Associated Protein Kinase
- MCI
Mild Cognitive Impairment
- MMP
Matrix Metalloproteinase
- MMSE
Mini-Mental State Examination
- MRI
Magnetic Resonance Imaging
- mTOR
Mammalian Target of Rapamycin
- NFkB
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
- NFT
Neurofibrillary Tangles
- NG2
Neural/glia Antigen-2
- NQO-1
NADPH Dehydrogenase Quinone-1
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NSAID
Non-Steroidal Anti-Inflammatory Drug
- NVU
Neurovascular Unit
- PDGF-β
Platelet-Derived Growth Factor - Beta Subunit
- PECAM-1
Platelet-Endothelial Cell Adhesion Molecule-1
- PET
Positron Emission Tomography
- PrP
Prion Protein
- PS/PSEN
Presenilin
- Qalb
Albumin Quotient
- QIgG
IgG Quotient
- RAGE
Receptor for Advanced Glycation End Products
- ROCK
Rho-Associated Protein Kinase
- ROS
Reactive Oxygen Species
- siRNA
Small Interfering Ribonucleic Acid
- SAMP8
Senescence-Accelerated Prone 8
- SLUMS
Saint Louis University Mental Status Examination (SLUMS)
- TEER
Trans-Endothelial Electrical Resistance
- TGFβ
Transforming Growth Factor Beta
- TJ
Tight Junction
- TNFα
Tumor Necrosis Factor Alpha
- tPA
Tissue Plasminogen Activator
- VCAM-1
Vascular Adhesion Molecule-1
- VCID
Vascular Contributions to Cognitive Impairment
- VEGF
Vascular Endothelial Growth Factor
- ZO-1
Zonula Occludens-1
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ (2013). Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis, 36, 437–449. [DOI] [PubMed] [Google Scholar]
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H 3rd, Kraner SD, & Norris CM (2009). Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci, 29, 12957–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RA, Passino M, Sachs BD, Nuriel T, & Akassoglou K (2004). Fibrin mechanisms and functions in nervous system pathology. Mol Interv, 4, 163–176. [DOI] [PubMed] [Google Scholar]
- Agundez JA, Jimenez-Jimenez FJ, Alonso-Navarro H, & Garcia-Martin E (2014). Drug and xenobiotic biotransformation in the blood-brain barrier: a neglected issue. Front Cell Neurosci, 8, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KC, Learman CR, Dunbar GL, Maiti P, Jang WC, Cha HC, & Song MS (2018). Characterization of Impaired Cerebrovascular Structure in APP/PS1 Mouse Brains. Neuroscience, 385, 246–254. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Ikeda K, Kondo H, & McGeer PL (1992). Thrombin accumulation in brains of patients with Alzheimer’s disease. Neurosci Lett, 146, 152–154. [DOI] [PubMed] [Google Scholar]
- Al Rihani SB, Darakjian LI, & Kaddoumi A (2019). Oleocanthal-Rich Extra-Virgin Olive Oil Restores the Blood-Brain Barrier Function through NLRP3 Inflammasome Inhibition Simultaneously with Autophagy Induction in TgSwDI Mice. ACS Chem Neurosci, 10, 3543–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rihani SB, Lan RS, & Kaddoumi A (2019). Granisetron Alleviates Alzheimer’s Disease Pathology in TgSwDI Mice Through Calmodulin-Dependent Protein Kinase II/cAMP-Response Element Binding Protein Pathway. J Alzheimers Dis, 72, 1097–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Adolfsson R, Bucht G, & Winblad B (1983). Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci, 60, 465–472. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Adolfsson R, Grundke-Iqbal I, & Winblad B (1987). Blood-brain barrier in Alzheimer dementia and in non-demented elderly. An immunocytochemical study. Acta Neuropathol, 73, 160–166. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease Fact Sheet. In. (Vol. 2021): National Institute of Aging. [Google Scholar]
- Alzheimer A (1906). Über einen eigenartigen schweren ErkrankungsprozeB der Hirnrinde. Neurologisches Centralblatt., 23, 1129–1136. [Google Scholar]
- Alzheimer A (1907). Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie und Psychisch-gerichtliche Medizin, 64, 146–148. [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, & Stern Y (2006). Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia, 47, 867–872. [DOI] [PubMed] [Google Scholar]
- Anderson TS, Ayanian JZ, Souza J, & Landon BE (2021). Representativeness of Participants Eligible to Be Enrolled in Clinical Trials of Aducanumab for Alzheimer Disease Compared With Medicare Beneficiaries With Alzheimer Disease and Mild Cognitive Impairment. JAMA, 326, 1627–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo M, & Ward L (2021). Aducanumab Fails to Produce Efficacy Results Yet Obtains US Food and Drug Administration Approval. Popul Health Manag. [DOI] [PubMed] [Google Scholar]
- Arai T, Miklossy J, Klegeris A, Guo JP, & McGeer PL (2006). Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J Neuropathol Exp Neurol, 65, 19–25. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, & Newman EA (2010). Glial and neuronal control of brain blood flow. Nature, 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic T (1999). The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry, 67, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Du Y, Dodel RC, Yan GM, Hamilton-Byrd E, & Paul SM (1998). The NF-kappaB/Rel family of proteins mediates Abeta-induced neurotoxicity and glial activation. Brain Res Mol Brain Res, 57, 63–72. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, & Baloyannis IS (2012). The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci, 322, 117–121. [DOI] [PubMed] [Google Scholar]
- Banerjee G, Kim HJ, Fox Z, Jäger HR, Wilson D, Charidimou A, Na HK, Na DL, Seo SW, & Werring DJ (2017). MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain, 140, 1107–1116. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, & Morley JE (2000). Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. J Gerontol A Biol Sci Med Sci, 55, B601–B606. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, & Steinitz M (2007). Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer’s disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol, 206, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y, Cook DG, & Reed MJ (2015). Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation, 12, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Moinuddin A, & Morley JE (2001). Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging, 22, 671–676. [DOI] [PubMed] [Google Scholar]
- Barber AJ, & Lieth E (1997). Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev Dyn, 208, 62–74. [DOI] [PubMed] [Google Scholar]
- Barnes SR, Ng TS, Montagne A, Law M, Zlokovic BV, & Jacobs RE (2016). Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med, 75, 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton SM, Janve VA, McClure R, Anderson A, Matsubara JA, Gore JC, & Pham W (2019). Lipopolysaccharide Induced Opening of the Blood Brain Barrier on Aging 5XFAD Mouse Model. J Alzheimers Dis, 67, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, & Mintz J (2003). White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol, 60, 393–398. [DOI] [PubMed] [Google Scholar]
- Batarseh YS, Bharate SS, Kumar V, Kumar A, Vishwakarma RA, Bharate SB, & Kaddoumi A (2017). Crocus sativus Extract Tightens the Blood-Brain Barrier, Reduces Amyloid beta Load and Related Toxicity in 5XFAD Mice. ACS Chem Neurosci, 8, 1756–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh YS, & Kaddoumi A (2018). Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-beta load and related toxicity in a mouse model of Alzheimer’s disease. J Nutr Biochem, 55, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzigaluppi P, Beckett TL, Koletar MM, Hill ME, Lai A, Trivedi A, Thomason L, Dorr A, Gallagher D, Librach CL, Joo IL, McLaurin J, & Stefanovic B (2019). Combinatorial Treatment Using Umbilical Cord Perivascular Cells and Abeta Clearance Rescues Vascular Function Following Transient Hypertension in a Rat Model of Alzheimer Disease. Hypertension, 74, 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Gerard C, Abramowski D, Cannet C, & Staufenbiel M (2011). Noninvasive magnetic resonance imaging detection of cerebral amyloid angiopathy-related microvascular alterations using superparamagnetic iron oxide particles in APP transgenic mouse models of Alzheimer’s disease: application to passive Abeta immunotherapy. J Neurosci, 31, 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Schuler A, Mueggler T, Meyer EP, Wiederhold KH, Staufenbiel M, & Krucker T (2003). Age-dependent cerebrovascular abnormalities and blood flow disturbances in APP23 mice modeling Alzheimer’s disease. J Neurosci, 23, 8453–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci C, Lilli C, Baroni T, Parnetti L, Sorbi S, Emiliani C, Lumare E, Calabresi P, Balloni S, & Bodo M (2007). Differences in extracellular matrix production and basic fibroblast growth factor response in skin fibroblasts from sporadic and familial Alzheimer’s disease. Mol Med, 13, 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, & van der Flier WM (2013). Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer’s disease. Neurobiol Aging, 34, 2488–2494. [DOI] [PubMed] [Google Scholar]
- Bennett RE, DeVos SL, Dujardin S, Corjuc B, Gor R, Gonzalez J, Roe AD, Frosch MP, Pitstick R, Carlson GA, & Hyman BT (2017). Enhanced Tau Aggregation in the Presence of Amyloid beta. Am J Pathol, 187, 1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, & Stopa EG (2000). Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging, 21, 349–355. [DOI] [PubMed] [Google Scholar]
- Bevan JA, Dodge J, Walters CL, Wellman T, & Bevan RD (1999). As human pial arteries (internal diameter 200–1000 microm) get smaller, their wall thickness and capacity to develop tension relative to their diameter increase. Life Sci, 65, 1153–1161. [DOI] [PubMed] [Google Scholar]
- Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W, Shihadeh V, Ulufatu S, Foreman O, Lu Y, DeVoss J, van der Brug M, & Watts RJ (2015). Lack of Widespread BBB Disruption in Alzheimer’s Disease Models: Focus on Therapeutic Antibodies. Neuron, 88, 289–297. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, & LaFerla FM (2005). Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron, 45, 675–688. [DOI] [PubMed] [Google Scholar]
- Biron KE, Dickstein DL, Gopaul R, & Jefferies WA (2011). Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS One, 6, e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, Hermann BP, Larue A, Sager MA, & Bendlin BB (2014). Regional white matter hyperintensities: aging, Alzheimer’s disease risk, and cognitive function. Neurobiol Aging, 35, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke M, Zetterberg H, Edman Å, Blennow K, Wallin A, & Andreasson U (2011). Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimers Dis, 27, 665–676. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, & Chong JK (1995). Cerebrospinal fluid ‘neuronal thread protein’ comes from serum by passage over the blood-brain barrier. Neurodegeneration, 4, 187–193. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Davidsson P, Fredman P, Gottfries CG, & Svennerholm L (1990). Intra-blood-brain-barrier synthesis of immunoglobulins in patients with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord, 4, 79–86. [PubMed] [Google Scholar]
- Blennow K, Wallin A, Uhlemann C, & Gottfries CG (1991). White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol Scand, 83, 187–193. [DOI] [PubMed] [Google Scholar]
- Blockx I, Einstein S, Guns PJ, Van Audekerke J, Guglielmetti C, Zago W, Roose D, Verhoye M, Van der Linden A, & Bard F (2016). Monitoring Blood-Brain Barrier Integrity Following Amyloid-beta Immunotherapy Using Gadolinium-Enhanced MRI in a PDAPP Mouse Model. J Alzheimers Dis, 54, 723–735. [DOI] [PubMed] [Google Scholar]
- Boespflug EL, Simon MJ, Leonard E, Grafe M, Woltjer R, Silbert LC, Kaye JA, & Iliff JJ (2018). Targeted Assessment of Enlargement of the Perivascular Space in Alzheimer’s Disease and Vascular Dementia Subtypes Implicates Astroglial Involvement Specific to Alzheimer’s Disease. J Alzheimers Dis, 66, 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Akkawi N, Martini G, Colciaghi F, Prometti P, Rozzini L, Di Luca M, Lenzi GL, Romanelli G, Caimi L, & Padovani A (2002). Microvascular damage and platelet abnormalities in early Alzheimer’s disease. J Neurol Sci, 203–204, 189–193. [DOI] [PubMed] [Google Scholar]
- Bors L, Toth K, Toth EZ, Bajza A, Csorba A, Szigeti K, Mathe D, Perlaki G, Orsi G, Toth GK, & Erdo F (2018). Age-dependent changes at the blood-brain barrier. A Comparative structural and functional study in young adult and middle aged rats. Brain Res Bull, 139, 269–277. [DOI] [PubMed] [Google Scholar]
- Bourassa P, Alata W, Tremblay C, Paris-Robidas S, & Calon F (2019). Transferrin Receptor-Mediated Uptake at the Blood-Brain Barrier Is Not Impaired by Alzheimer’s Disease Neuropathology. Mol Pharm, 16, 583–594. [DOI] [PubMed] [Google Scholar]
- Bourassa P, Tremblay C, Schneider JA, Bennett DA, & Calon F (2020). Brain mural cell loss in the parietal cortex in Alzheimer’s disease correlates with cognitive decline and TDP-43 pathology. Neuropathol Appl Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourasset F, Ouellet M, Tremblay C, Julien C, Do TM, Oddo S, LaFerla F, & Calon F (2009). Reduction of the cerebrovascular volume in a transgenic mouse model of Alzheimer’s disease. Neuropharmacology, 56, 808–813. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, & Quinn JF (2007). Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology, 68, 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy J, Leergaard TB, Schmidt T, Odeh F, Bichelmeier U, Nuber S, Holzmann C, Wree A, Prusiner SB, Bujard H, Riess O, & Bjaalie JG (2006). Expression mapping of tetracycline-responsive prion protein promoter: digital atlasing for generating cell-specific disease models. Neuroimage, 33, 449–462. [DOI] [PubMed] [Google Scholar]
- Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, & Potter H (2010). GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis, 21, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol, 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Brautigam H, Steele JW, Westaway D, Fraser PE, St George-Hyslop PH, Gandy S, Hof PR, & Dickstein DL (2012). The isotropic fractionator provides evidence for differential loss of hippocampal neurons in two mouse models of Alzheimer’s disease. Mol Neurodegener, 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN, & Group AR (2011). Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement, 7, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz WD, Nelson PT, Besser LM, Heller KB, & Kukull WA (2015). Cerebral amyloid angiopathy and its co-occurrence with Alzheimer’s disease and other cerebrovascular neuropathologic changes. Neurobiol Aging, 36, 2702–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges LR, Andoh J, Lawrence AJ, Khoong CHL, Poon W, Esiri MM, Markus HS, & Hainsworth AH (2014). Blood-brain barrier dysfunction and cerebral small vessel disease (arteriolosclerosis) in brains of older people. J Neuropathol Exp Neurol, 73, 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, & Davis TP (2005). Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun, 327, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Brundel M, Heringa SM, de Bresser J, Koek HL, Zwanenburg JJ, Jaap Kappelle L, Luijten PR, & Biessels GJ (2012). High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J Alzheimers Dis, 31, 259–263. [DOI] [PubMed] [Google Scholar]
- Burbach GJ, Vlachos A, Ghebremedhin E, Del Turco D, Coomaraswamy J, Staufenbiel M, Jucker M, & Deller T (2007). Vessel ultrastructure in APP23 transgenic mice after passive anti-Abeta immunotherapy and subsequent intracerebral hemorrhage. Neurobiol Aging, 28, 202–212. [DOI] [PubMed] [Google Scholar]
- Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, & Hynynen K (2014). Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology, 273, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Nhan T, Moffatt C, Klibanov AL, & Hynynen K (2014). Analysis of focused ultrasound-induced blood-brain barrier permeability in a mouse model of Alzheimer’s disease using two-photon microscopy. J Control Release, 192, 243–248. [DOI] [PubMed] [Google Scholar]
- Butterfield DA (2018). Perspectives on Oxidative Stress in Alzheimer’s Disease and Predictions of Future Research Emphases. J Alzheimers Dis, 64, S469–S479. [DOI] [PubMed] [Google Scholar]
- Cai M, Jung I, Kwon H, Cho E, Jeon J, Yun J, Lee YC, Kim DH, & Ryu JH (2020). Spinosin Attenuates Alzheimer’s Disease-Associated Synaptic Dysfunction via Regulation of Plasmin Activity. Biomol Ther (Seoul), 28, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, & Jucker M (1999). Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci U S A, 96, 14088–14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, & Rosenberg GA (2007). Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther, 323, 488–498. [DOI] [PubMed] [Google Scholar]
- Carman AJ, Mills JH, Krenz A, Kim DG, & Bynoe MS (2011). Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci, 31, 13272–13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P (1997). Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods, 71, 3–9. [DOI] [PubMed] [Google Scholar]
- Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, & de Vries HE (2011). Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal, 15, 1167–1178. [DOI] [PubMed] [Google Scholar]
- Caserta MT, Caccioppo D, Lapin GD, Ragin A, & Groothuis DR (1998). Blood-brain barrier integrity in Alzheimer’s disease patients and elderly control subjects. J Neuropsychiatry Clin Neurosci, 10, 78–84. [DOI] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Nilson AN, Van Skike CE, Jahrling JB, Patel K, Garach P, Gerson JE, Sengupta U, Abisambra J, Nelson P, Troncoso J, Ungvari Z, Galvan V, & Kayed R (2017). Cerebral Microvascular Accumulation of Tau Oligomers in Alzheimer’s Disease and Related Tauopathies. Aging Dis, 8, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Barnett JL, Mann DM, & Nixon RA (1996). Colocalization of lysosomal hydrolase and beta-amyloid in diffuse plaques of the cerebellum and striatum in Alzheimer’s disease and Down’s syndrome. J Neuropathol Exp Neurol, 55, 704–715. [DOI] [PubMed] [Google Scholar]
- Chagnot A, Barnes SR, & Montagne A (2021). Magnetic Resonance Imaging of Blood-Brain Barrier permeability in Dementia. Neuroscience, 474, 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalbot S, Zetterberg H, Blennow K, Fladby T, Andreasen N, Grundke-Iqbal I, & Iqbal K (2011). Blood-cerebrospinal fluid barrier permeability in Alzheimer’s disease. J Alzheimers Dis, 25, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell D, Westphal M, & Jacob M (2009). The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol, 22, 155–162. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, & Sandoval J (2007). Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother Res, 21, 629–640. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, & Lichtor T (2004). Effect of age on the duration and extent of amyloid plaque reduction and microglial activation after injection of anti-Abeta antibody into the third ventricle of TgCRND8 mice. J Neurosci Res, 78, 732–741. [DOI] [PubMed] [Google Scholar]
- Chen G, Li J, Wang Z, & Liu W (2020). Ezetimibe protects against spinal cord injury by regulating autophagy and apoptosis through inactivation of PI3K/AKT/mTOR signaling. Am J Transl Res, 12, 2685–2694. [PMC free article] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, & Mucke L (2007). Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem, 282, 23818–23828. [DOI] [PubMed] [Google Scholar]
- Cheng W, Liu W, Li B, & Li D (2018). Relationship of Anticoagulant Therapy With Cognitive Impairment Among Patients With Atrial Fibrillation: A Meta-Analysis and Systematic Review. J Cardiovasc Pharmacol, 71, 380–387. [DOI] [PubMed] [Google Scholar]
- Chiquita S, Ribeiro M, Castelhano J, Oliveira F, Sereno J, Batista M, Abrunhosa A, Rodrigues-Neves AC, Carecho R, Baptista F, Gomes C, Moreira PI, Ambrosio AF, & Castelo-Branco M (2019). A longitudinal multimodal in vivo molecular imaging study of the 3xTg-AD mouse model shows progressive early hippocampal and taurine loss. Hum Mol Genet, 28, 2174–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, & Westaway D (2001). Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem, 276, 21562–21570. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Wang S, Brown TR, Small SA, Duff KE, & Konofagou EE (2008). Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound. Ultrason Imaging, 30, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MK, Liu H, Wu T, Yang W, & Zhang Y (2020). NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Nat Commun, 11, 3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Singh I, Singh AK, Khan M, & Won J (2020). Asymmetric dimethylarginine exacerbates cognitive dysfunction associated with cerebrovascular pathology. FASEB J, 34, 6808–6823. [DOI] [PubMed] [Google Scholar]
- Chojdak-Lukasiewicz J, Dziadkowiak E, Zimny A & Paradowski B (2021). Cerebral small vessel disease: A review. Adv Clin Exp Med, 30, 349–356. [DOI] [PubMed] [Google Scholar]
- Christov A, Ottman J, Hamdheydari L, & Grammas P (2008). Structural changes in Alzheimer’s disease brain microvessels. Curr Alzheimer Res, 5, 392–395. [DOI] [PubMed] [Google Scholar]
- Chung YC, Kruyer A, Yao Y, Feierman E, Richards A, Strickland S, & Norris EH (2016). Hyperhomocysteinemia exacerbates Alzheimer’s disease pathology by way of the beta-amyloid fibrinogen interaction. J Thromb Haemost, 14, 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PR, Manes TD, Pober JS, & Kluger MS (2007). Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol, 127, 762–774. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Kruyer A, Fernandez-Nueda I, Marcos-Diaz A, Ceron C, Richards AT, Jno-Charles OC, Rodriguez I, Callejas S, Norris EH, Sanchez-Gonzalez J, Ruiz-Cabello J, Ibanez B, Strickland S, & Fuster V (2019). Long-Term Dabigatran Treatment Delays Alzheimer’s Disease Pathogenesis in the TgCRND8 Mouse Model. J Am Coll Cardiol, 74, 1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M, Mattei L, Richards AT, Norris EH, & Strickland S (2015). Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration. Neurobiol Aging, 36, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretin B, Sellal F, Philippi N, Bousiges O, Di Bitonto L, Martin-Hunyadi C, & Blanc F (2016). Epileptic Prodromal Alzheimer’s Disease, a Retrospective Study of 13 New Cases: Expanding the Spectrum of Alzheimer’s Disease to an Epileptic Variant? J Alzheimers Dis, 52, 1125–1133. [DOI] [PubMed] [Google Scholar]
- Croll SD, & Wiegand SJ (2001). Vascular growth factors in cerebral ischemia. Mol Neurobiol, 23, 121–135. [DOI] [PubMed] [Google Scholar]
- Cruts M, Theuns J, & Van Broeckhoven C (2012). Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat, 33, 1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Hernandez JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, Park L, Vinarcsik LK, Ivasyk I, Rivera DA, Kang Y, Cortes-Canteli M, Peyrounette M, Doyeux V, Smith A, Zhou J, Otte G, Beverly JD, Davenport E, Davit Y, Lin CP, Strickland S, Iadecola C, Lorthois S, Nishimura N, & Schaffer CB (2019). Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci, 22, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Godia E, Dwivedi P, Sharma S, Ois Santiago A, Roquer Gonzalez J, Balcells M, Laird J, Turk M, Suri HS, Nicolaides A, Saba L, Khanna NN, & Suri JS (2018). Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J Stroke, 20, 302–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KM (1997). Perivascular astrocytes within Alzheimer’s disease plaques. Neuroreport, 8, 1961–1966. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Kocsi Z, & Stone J (2005). Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab, 25, 1656–1667. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Kocsi Z, & Stone J (2006). Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging, 27, 1786–1796. [DOI] [PubMed] [Google Scholar]
- Cummings J, Lee G, Ritter A, Sabbagh M, & Zhong K (2019). Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y), 5, 272–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo OM, Dooyema J, Courtney C, Walker LC, & Heuer E (2013). Cerebral amyloid angiopathy in an aged sooty mangabey (Cercocebus atys). Comp Med, 63, 515–520. [PMC free article] [PubMed] [Google Scholar]
- Daneman R, & Prat A (2015). The blood-brain barrier. Cold Spring Harb Perspect Biol, 7, a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, & Van Nostrand WE (2004). Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem, 279, 20296–20306. [DOI] [PubMed] [Google Scholar]
- Davis J, Xu F, Miao J, Previti ML, Romanov G, Ziegler K, & Van Nostrand WE (2006). Deficient cerebral clearance of vasculotropic mutant Dutch/Iowa Double A beta in human A betaPP transgenic mice. Neurobiol Aging, 27, 946–954. [DOI] [PubMed] [Google Scholar]
- De Bock M, Van Haver V, Vanderroucke RE, Decrock E, Wang N, & Leybaert L (2016) Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia, 64, 1097–1123. [DOI] [PubMed] [Google Scholar]
- De Guio F, Jouvent E, Biessels GJ, Black SE, Brayne C, Chen C, Cordonnier C, De Leeuw FE, Dichgans M, Doubal F, Duering M, Dufouil C, Duzel E, Fazekas F, Hachinski V, Ikram MA, Linn J, Matthews PM, Mazoyer B, Mok V, Norrving B, O’Brien JT, Pantoni L, Ropele S, Sachdev P, Schmidt R, Seshadri S, Smith EE, Sposato LA, Stephan B, Swartz RH, Tzourio C, van Buchem M, van der Lugt A, van Oostenbrugge R, Vernooij MW, Viswanathan A, Werring D, Wollenweber F, Wardlaw JM, & Chabriat H (2016). Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood Flow Metab, 36, 1319–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC (2017). Are Major Dementias Triggered by Poor Blood Flow to the Brain? Theoretical Considerations. J Alzheimers Dis, 57, 353–371. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, & Van Leuven F (1998). Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature, 391, 387–390. [DOI] [PubMed] [Google Scholar]
- Debette S, Ibrahim Verbaas CA, Bressler J, Schuur M, Smith A, Bis JC, Davies G, Wolf C, Gudnason V, Chibnik LB, Yang Q, deStefano AL, de Quervain DJ, Srikanth V, Lahti J, Grabe HJ, Smith JA, Priebe L, Yu L, Karbalai N, Hayward C, Wilson JF, Campbell H, Petrovic K, Fornage M, Chauhan G, Yeo R, Boxall R, Becker J, Stegle O, Mather KA, Chouraki V, Sun Q, Rose LM, Resnick S, Oldmeadow C, Kirin M, Wright AF, Jonsdottir MK, Au R, Becker A, Amin N, Nalls MA, Turner ST, Kardia SL, Oostra B, Windham G, Coker LH, Zhao W, Knopman DS, Heiss G, Griswold ME, Gottesman RF, Vitart V, Hastie ND, Zgaga L, Rudan I, Polasek O, Holliday EG, Schofield P, Choi SH, Tanaka T, An Y, Perry RT, Kennedy RE, Sale MM, Wang J, Wadley VG, Liewald DC, Ridker PM, Gow AJ, Pattie A, Starr JM, Porteous D, Liu X, Thomson R, Armstrong NJ, Eiriksdottir G, Assareh AA, Kochan NA, Widen E, Palotie A, Hsieh YC, Eriksson JG, Vogler C, van Swieten JC, Shulman JM, Beiser A, Rotter J, Schmidt CO, Hoffmann W, Nothen MM, Ferrucci L, Attia J, Uitterlinden AG, Amouyel P, Dartigues JF, Amieva H, Raikkonen K, Garcia M, Wolf PA, Hofman A, Longstreth WT Jr., Psaty BM, Boerwinkle E, DeJager PL, Sachdev PS, Schmidt R, Breteler MM, Teumer A, Lopez OL, Cichon S, Chasman DI, Grodstein F, Muller-Myhsok B, Tzourio C, Papassotiropoulos A, Bennett DA, Ikram MA, Deary IJ, van Duijn CM, Launer L, Fitzpatrick AL, Seshadri S, Mosley TH Jr., Cohorts for H, & Aging Research in Genomic Epidemiology, C. (2015). Genome-wide studies of verbal declarative memory in nondemented older people: the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium. Biol Psychiatry, 77, 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers K, Camerino I, van Boxtel MP, Verhey FR, Irving K, Brayne C, Kivipelto M, Starr JM, Yaffe K, de Leeuw PW, & Kohler S (2017). Dementia risk in renal dysfunction: A systematic review and meta-analysis of prospective studies. Neurology, 88, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, & Scheff SW (1990). Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol, 27, 457–464. [DOI] [PubMed] [Google Scholar]
- del Valle J, Duran-Vilaregut J, Manich G, Camins A, Pallas M, Vilaplana J, & Pelegri C (2009). Time-course of blood-brain barrier disruption in senescence-accelerated mouse prone 8 (SAMP8) mice. Int J Dev Neurosci, 27, 47–52. [DOI] [PubMed] [Google Scholar]
- del Valle J, Duran-Vilaregut J, Manich G, Camins A, Pallas M, Vilaplana J, & Pelegri C (2009). Cerebral amyloid angiopathy, blood-brain barrier disruption and amyloid accumulation in SAMP8 mice. Neurodegener Dis, 421–429. [DOI] [PubMed] [Google Scholar]
- Dementia Fact Sheet. In. (Vol. 2021): World Health Organization. [Google Scholar]
- Denver P, D’Adamo H, Hu S, Zuo X, Zhu C, Okuma C, Kim P, Castro D, Jones MR, Leal C, Mekkittikul M, Ghadishah E, Teter B, Vinters HV, Cole GM, & Frautschy SA (2019). A Novel Model of Mixed Vascular Dementia Incorporating Hypertension in a Rat Model of Alzheimer’s Disease. Front Physiol, 10, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, & Ohno M (2010). Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer’s disease model mice. Eur J Neurosci, 31, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco LY, Farkas E, Martin C, Venneri A, & Frangi AF (2015). Is Vasomotion in Cerebral Arteries Impaired in Alzheimer’s Disease? J Alzheimers Dis, 46, 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo SG, Prinelli F, Adorni F, Caltagirone C, & Musicco M (2013). A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimers Dis, 35, 349–361. [DOI] [PubMed] [Google Scholar]
- Divry P (1927). Etude histochimique des plaques seniles J Belge de Neurologie et de Psychiatrie, 27, 643–657. [Google Scholar]
- Do TM, Alata W, Dodacki A, Traversy MT, Chacun H, Pradier L, Scherrmann JM, Farinotti R, Calon F, & Bourasset F (2014). Altered cerebral vascular volumes and solute transport at the blood-brain barriers of two transgenic mouse models of Alzheimer’s disease. Neuropharmacology, 81, 311–317. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Bales KR, Paul SM, & Ungerer A (1999). Early regional cerebral glucose hypometabolism in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Neurosci Lett, 277, 49–52. [DOI] [PubMed] [Google Scholar]
- Doi Y, Takeuchi H, Mizoguchi H, Fukumoto K, Horiuchi H, Jin S, Kawanokuchi J, Parajuli B, Sonobe Y, Mizuno T, & Suzumura A (2014). Granulocyte-colony stimulating factor attenuates oligomeric amyloid beta neurotoxicity by activation of neprilysin. PLoS One, 9, e103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domnitz SB, Robbins EM, Hoang AW, Garcia-Alloza M, Hyman BT, Rebeck GW, Greenberg SM, Bacskai BJ, & Frosch MP (2005). Progression of cerebral amyloid angiopathy in transgenic mouse models of Alzheimer disease. J Neuropathol Exp Neurol, 64, 588–594. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Berzin TM, Rafii MS, Glass DJ, Yancopoulos GD, Fallon JR, & Stopa EG (1999). Agrin in Alzheimer’s disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc Natl Acad Sci U S A, 96, 6468–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R, Alzheimer’s Disease Cooperative Study Steering, C., & Solanezumab Study, G. (2014). Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med, 370, 311–321. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Leon J, Cummings JL, Marin D, & Neumann PJ (2002). Prevalence and impact of medical comorbidity in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci, 57, M173–177. [DOI] [PubMed] [Google Scholar]
- Dubey S, Heinen S, Krantic S, McLaurin J, Branch DR, Hynynen K, & Aubert I (2020). Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc Natl Acad Sci U S A, 117, 32691–32700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits FH, Hernandez-Guillamon M, Montaner J, Goos JD, Montañola A, Wattjes MP, Barkhof F, Scheltens P, Teunissen CE, & van der Flier WM (2015). Matrix Metalloproteinases in Alzheimer’s Disease and Concurrent Cerebral Microbleeds. J Alzheimers Dis, 48, 711–720. [DOI] [PubMed] [Google Scholar]
- Duncombe J, Lennen RJ, Jansen MA, Marshall I, Wardlaw JM, & Horsburgh K (2017). Ageing causes prominent neurovascular dysfunction associated with loss of astrocytic contacts and gliosis. Neuropathol Appl Neurobiol, 43, 477–491. [DOI] [PubMed] [Google Scholar]
- Durrant CS, Ruscher K, Sheppard O, Coleman MP, & Ozen I (2020). Beta secretase 1-dependent amyloid precursor protein processing promotes excessive vascular sprouting through NOTCH3 signalling. Cell Death Dis, 11, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dysken MW, Nelson MJ, Hoover KM, Kuskowski M, & McGeachie R (1990). Rapid dynamic CT scanning in primary degenerative dementia and age-matched controls. Biol Psychiatry, 28, 425–434. [DOI] [PubMed] [Google Scholar]
- Eanes ED, & Glenner GG (1968). X-ray diffraction studies on amyloid filaments. J Histochem Cytochem, 16, 673–677. [DOI] [PubMed] [Google Scholar]
- Effects of Alzheimer’s disease drugs. In. (Vol. 2021). Alzheimer’s Society. [Google Scholar]
- Ehrenberg AJ, Suemoto CK, França Resende EP, Petersen C, Leite REP, Rodriguez RD, Ferretti-Rebustini REL, You M, Oh J, Nitrini R, Pasqualucci CA, Jacob-Filho W, Kramer JH, Gatchel JR, & Grinberg LT (2018). Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis, 66, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, & Stam FC (1982). Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol, 57, 239–242. [DOI] [PubMed] [Google Scholar]
- Elfakhri KH, Abdallah IM, Brannen AD, & Kaddoumi A (2019). Multi-faceted therapeutic strategy for treatment of Alzheimer’s disease by concurrent administration of etodolac and alpha-tocopherol. Neurobiol Dis, 125, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhaik Goldman S, Goez D, Last D, Naor S, Liraz Zaltsman S, Sharvit-Ginon I, Atrakchi-Baranes D, Shemesh C, Twitto-Greenberg R, Tsach S, Lotan R, Leikin-Frenkel A, Shish A, Mardor Y, Schnaider Beeri M, & Cooper I (2018). High-fat diet protects the blood-brain barrier in an Alzheimer’s disease mouse model. Aging Cell, 17, e12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara I, Icen A, Palo J, & Erkinjuntti T (1985). CSF in Alzheimer’s disease. Studies on blood-brain barrier function and intrathecal protein synthesis. J Neurol Sci, 70, 73–80. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Paetau A, Lehto VP, Dahl D, Virtanen I, & Palo J (1983). Immunocytochemical studies of Alzheimer neuronal perikarya with intermediate filament antisera. J Neurol Sci, 62, 315–326. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Seppala I, Palo J, Sulkava R, & Erkinjuntti T (1988). Oligoclonal immunoglobulin bands in cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Acta Neurol Scand, 77, 397–401. [DOI] [PubMed] [Google Scholar]
- Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, & Ward PJ (1990). Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science, 248, 1122–1124. [DOI] [PubMed] [Google Scholar]
- Fan R, DeFilippis K, & Van Nostrand WE (2007). Induction of complement proteins in a mouse model for cerebral microvascular A beta deposition. J Neuroinflammation, 4, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fao L, Mota SI, & Rego AC (2019). Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res Rev, 54, 100942. [DOI] [PubMed] [Google Scholar]
- Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, & Iadecola C (2016). Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab, 36, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, Sethuraman G, DeMattos RB, Mohs R, Paul SM, & Siemers ER (2012). Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement, 8, 261–271. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, & Wardlaw JM (2009). Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging, 30, 337–352. [DOI] [PubMed] [Google Scholar]
- Ferrero J, Williams L, Stella H, Leitermann K, Mikulskis A, O’Gorman J, & Sevigny J (2016). First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement (N Y), 2, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, & Vinters HV (2002). Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest, 32, 360–371. [DOI] [PubMed] [Google Scholar]
- Fillit HM, Kemeny E, Luine V, Weksler ME, & Zabriskie JB (1987). Antivascular antibodies in the sera of patients with senile dementia of the Alzheimer’s type. J Gerontol, 42, 180–184. [DOI] [PubMed] [Google Scholar]
- Fischer O (1907). Miliaere Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmassige Veraenderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiat Neurol, 22, 361–372. [Google Scholar]
- Fischer O (1910). Die presbyophrene Demenz, deren anatomische Grundlage und klinische Abgrenzung, Z ges Neurol Psychiat, 3, 371–471. [Google Scholar]
- Fisher M, French S, Ji P, & Kim RC (2010). Cerebral microbleeds in the elderly: a pathological analysis. Stroke, 41, 2782–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Vasilevko V, Passos GF, Ventura C, Quiring D, & Cribbs DH (2011). Therapeutic modulation of cerebral microhemorrhage in a mouse model of cerebral amyloid angiopathy. Stroke, 42, 3300–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Castranio EL, Carter AY, Kodali R, Lefterov I, & Koldamova R (2014). Improvement of memory deficits and amyloid-beta clearance in aged APP23 mice treated with a combination of anti-amyloid-beta antibody and LXR agonist. J Alzheimers Dis, 41, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleegal-DeMotta MA, Doghu S, & Banks WA (2009). Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab, 29, 640–647. [DOI] [PubMed] [Google Scholar]
- Ford E, Greenslade N, Paudyal P, Bremner S, Smith HE, Banerjee S, Sadhwani S, Rooney P, Oliver S, & Cassell J (2018). Predicting dementia from primary care records: A systematic review and meta-analysis. PLoS One, 13, e0194735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstl H, Burns A, Levy R, Cairns N, Luthert P, & Lantos P (1992). Neurologic signs in Alzheimer’s disease. Results of a prospective clinical and neuropathologic study. Arch Neurol, 49, 1038–1042. [DOI] [PubMed] [Google Scholar]
- Fouda AY, Fagan SC, & Ergul A (2019). Brain Vasculature and Cognition. Arterioscler Thromb Vasc Biol, 39, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze WM, Jacobs HIL, de Jong JJ, Verheggen ICM, Gronenschild E, Palm WM, Hoff EI, Wardlaw JM, Jansen JFA, Verhey FR, & Backes WH (2020). White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging, 85, 113–122. [DOI] [PubMed] [Google Scholar]
- Frolich L, Kornhuber J, Ihl R, Fritze J, Maurer K, & Riederer P (1991). Integrity of the blood-CSF barrier in dementia of Alzheimer type: CSF/serum ratios of albumin and IgG. Eur Arch Psychiatry Clin Neurosci, 240, 363–366. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, & Holtzman DM (2005). Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci, 25, 2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R, & Ihara Y (1998). Quantitation of amyloid beta-protein (A beta) in the cortex during aging and in Alzheimer’s disease. Am J Pathol, 152, 1633–1640. [PMC free article] [PubMed] [Google Scholar]
- Galeano P, Leal MC, Ferrari CC, Dalmasso MC, Martino Adami PV, Farias MI, Casabona JC, Puntel M, Do Carmo S, Smal C, Aran M, Castano EM, Pitossi FJ, Cuello AC, & Morelli L (2018). Chronic Hippocampal Expression of Notch Intracellular Domain Induces Vascular Thickening, Reduces Glucose Availability, and Exacerbates Spatial Memory Deficits in a Rat Model of Early Alzheimer. Mol Neurobiol, 55, 8637–8650. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, & et al. (1995). Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature, 373, 523–527. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Prada C, Lattarulo C, Fine S, Borrelli LA, Betensky R, Greenberg SM, Frosch MP, & Bacskai BJ (2009). Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J Neurochem, 109, 1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barroso C, Ricobaraza A, Pascual-Lucas M, Unceta N, Rico AJ, Goicolea MA, Salles J, Lanciego JL, Oyarzabal J, Franco R, Cuadrado-Tejedor M, & Garcia-Osta A (2013). Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology, 64, 114–123. [DOI] [PubMed] [Google Scholar]
- Garcia-Ponce A, Citalan-Madrid AF, Velazquez-Avila M, Vargas-Robles H, & Schnoor M (2015). The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb Haemost, 113, 20–36. [DOI] [PubMed] [Google Scholar]
- Garton MJ, Keir G, Lakshmi MV, & Thompson EJ (1991). Age-related changes in cerebrospinal fluid protein concentrations. J Neurol Sci, 104, 74–80. [DOI] [PubMed] [Google Scholar]
- Gauberti M, Potzeha F, Vivien D, & Martinez de Lizarrondo S (2018). Impact of Bradykinin Generation During Thrombolysis in Ischemic Stroke. Front Med (Lausanne), 5, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni P, Arango-Lievano M, Neves ID, Rousset MC, Baranger K, Rivera S, Jeanneteau F, Claeysen S, & Marchi N (2016). Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol Dis, 88, 107–117. [DOI] [PubMed] [Google Scholar]
- Girouard H, & Iadecola C (2006). Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985), 100, 328–335. [DOI] [PubMed] [Google Scholar]
- Glenner GG (1979). Congophilic microangiopathy in the pathogenesis of Alzheimer’s syndrome (presenile dementia). Med Hypotheses, 5, 1231–1236. [DOI] [PubMed] [Google Scholar]
- Glenner GG, & Wong CW (1984). Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun, 120, 885–890. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, & et al. (1991). Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature, 349, 704–706. [DOI] [PubMed] [Google Scholar]
- Goedert M, & Crowther RA (1989). Amyloid plaques, neurofibrillary tangles and their relevance for the study of Alzheimer’s disease. Neurobiol Aging, 10, 405–406; discussion 412–404. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Sharpe MA, Regnier-Golanov AS, Del Zoppo GJ, Baskin DS, & Britz GW (2019). Fibrinogen Chains Intrinsic to the Brain. Front Neurosci, 13, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, & Jaramillo BE (2003). Tight junction proteins. Prog Biophys Mol Biol, 81, 1–44. [DOI] [PubMed] [Google Scholar]
- Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, & van der Flier WM (2009). Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke, 40, 3455–3460. [DOI] [PubMed] [Google Scholar]
- Goos JD, Teunissen CE, Veerhuis R, Verwey NA, Barkhof F, Blankenstein MA, Scheltens P, & van der Flier WM (2012). Microbleeds relate to altered amyloid-beta metabolism in Alzheimer’s disease. Neurobiol Aging, 33, 1011 e1011–1019. [DOI] [PubMed] [Google Scholar]
- Goust JM, Mangum M, & Powers JM (1984). An immunologic assessment of brain-associated IgG in senile cerebral amyloidosis. J Neuropathol Exp Neurol, 43, 481–488. [DOI] [PubMed] [Google Scholar]
- Graff-Radford J, Simino J, Kantarci K, Mosley TH Jr., Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR Jr., Vemuri P, & Knopman DS (2017). Neuroimaging Correlates of Cerebral Microbleeds: The ARIC Study (Atherosclerosis Risk in Communities). Stroke, 48, 2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, & Ovase R (2002). Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer’s disease brain. Am J Pathol, 160, 1583–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, & Breteler MMB (2009). Cerebral microbleeds: a guide to detection and interpretation. The Lancet Neurology, 8, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, & Engelhardt B (2011). Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol, 37, 24–39. [DOI] [PubMed] [Google Scholar]
- Gregory JL, Prada CM, Fine SJ, Garcia-Alloza M, Betensky RA, Arbel-Ornath M, Greenberg SM, Bacskai BJ, & Frosch MP (2012). Reducing available soluble beta-amyloid prevents progression of cerebral amyloid angiopathy in transgenic mice. J Neuropathol Exp Neurol, 71, 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Ricciotti E, & FitzGerald GA (2017). The Cardiovascular Pharmacology of Nonsteroidal Anti-Inflammatory Drugs. Trends Pharmacol Sci, 38, 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, & Wisniewski HM (1986). Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem, 261, 6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, & Binder LI (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A, 83, 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Johnson AB, Terry RD, Wisniewski HM, & Iqbal K (1979). Alzheimer neurofibrillary tangles: antiserum and immunohistological staining. Ann Neurol, 6, 532–537. [DOI] [PubMed] [Google Scholar]
- Gu BJ, & Wiley JS (2006). Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood, 107, 4946–4953. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Repetto IE, Monteleone D, Vasciaveo V, Franchino C, Rinaldi S, Tabaton M, & Tamagno E (2019). Stroke and Amyloid-beta Downregulate TREM-2 and Uch-L1 Expression that Synergistically Promote the Inflammatory Response. J Alzheimers Dis, 71, 907–920. [DOI] [PubMed] [Google Scholar]
- Gungor I, Sarro L, Graff-Radford J, Zuk SM, Tosakulwong N, Przybelski SA, Lesnick T, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Filippi M, Petersen RC, Jack CR Jr., & Kantarci K (2015). Frequency and topography of cerebral microbleeds in dementia with Lewy bodies compared to Alzheimer’s disease. Parkinsonism Relat Disord, 21, 1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnik S, Devraj K, Macas J, Yamaji M, Starke J, Scholz A, Sommer K, Di Tacchio M, Vutuuri R, Beck H, Mittelbronn M, Foer C, Pfeilschifter W, Liebner S, Peters KG, Plate KH, & Reiss Y (2016). Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol, 131, 753–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Wang H, Liang W, Xu W, Li Y, Song L, Zhang D, Hu Y, Han B, Wang W, Yang Y, Bei W, & Guo J (2020). Bilobalide reversibly modulates blood-brain barrier permeability through promoting adenosine A1 receptor-mediated phosphorylation of actin-binding proteins. Biochem Biophys Res Commun, 526, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Guo W, Feng G, Miao Y, Liu G, & Xu C (2014). Rapamycin alleviates brain edema after focal cerebral ischemia reperfusion in rats. Immunopharmacol Immunotoxicol, 36, 211–223. [DOI] [PubMed] [Google Scholar]
- Haarmann A, Nowak E, Deiss A, van der Pol S, Monoranu CM, Kooij G, Muller N, van der Valk P, Stoll G, de Vries HE, Berberich-Siebelt F, & Buttmann M (2015). Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin alpha-4-transduced outside-in signalling. Acta Neuropathol, 129, 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, & Zlokovic BV (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab, 36, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E (2004). Cholinergic modulation of the cortical microvascular bed. Prog Brain Res, 145, 171–178. [DOI] [PubMed] [Google Scholar]
- Hampel H, Kotter HU, Padberg F, Korschenhausen DA, & Moller HJ (1999). Oligoclonal bands and blood-cerebrospinal-fluid barrier dysfunction in a subset of patients with Alzheimer disease: comparison with vascular dementia, major depression, and multiple sclerosis. Alzheimer Dis Assoc Disord, 13, 9–19. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Tanaka Y, Shimizu S, Takasaki M, & Abe K (2003). Cerebral microbleeds in Alzheimer’s disease. J Neurol, 250, 1496–1497. [DOI] [PubMed] [Google Scholar]
- Harcha PA, Vargas A, Yi C, Koulakoff AA, Giaume C, & Saez JC (2015). Hemichannels Are Required for Amyloid beta-Peptide-Induced Degranulation and Are Activated in Brain Mast Cells of APPswe/PS1dE9 Mice. J Neurosci, 35, 9526–9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter K, Levine M, & Henderson SO (2015). Anticoagulation drug therapy: a review. West J Emerg Med, 16, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, & Holtzman DM (2005). Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci, 25, 6213–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, & Bauer B (2011). ABC transporters in the CNS - an inventory. Curr Pharm Biotechnol, 12, 656–673. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klunemann HH, Schuierer G, & Schlachetzki F (2012). Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke, 43, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Maki T, Saito S, Yamamoto Y, Nagatsuka K, & Ihara M (2016). Influence of Low-Dose Aspirin on Cerebral Amyloid Angiopathy in Mice. J Alzheimers Dis, 52, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, & Carare RO (2011). Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol, 121, 431–443. [DOI] [PubMed] [Google Scholar]
- Heldin CH, & Westermark B (1999). Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev, 79, 1283–1316. [DOI] [PubMed] [Google Scholar]
- Helmer C, Stengel B, Metzger M, Froissart M, Massy ZA, Tzourio C, Berr C, & Dartigues JF (2011). Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology, 77, 2043–2051. [DOI] [PubMed] [Google Scholar]
- Hendrikx D, Smits A, Lavanga M, De Wel O, Thewissen L, Jansen K, Caicedo A, Van Huffel S, & Naulaers G (2019). Measurement of Neurovascular Coupling in Neonates. Front Physiol, 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A, Blome M, Ambree O, Sachser N, Paulus W, & Keyvani K (2010). Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol, 20, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks K, O’Neil RG, Dubinsky WS, & Brown RC (2010). TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol Cell Physiol, 298, C1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y, Miyakawa T, Shimoji A, & Katsuragi S (1987). Ultrastructural changes of blood vessels in the cerebral cortex in Alzheimer’s disease. Jpn J Psychiatry Neurol, 41, 283–290. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, & D’Amore PA (1996). Pericytes in the microvasculature. Cardiovasc Res, 32, 687–698. [PubMed] [Google Scholar]
- Hogg K, & Weitz JI (2017). Blood Coagulation and Anticoagulant, Fibrinolytic, and Antiplatelet Drugs. In Brunton LL, Hilal-Dandan R & Knollmann BC (Eds.), Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. New York, NY: McGraw-Hill Education. [Google Scholar]
- Hong JH, Kang JW, Kim DK, Baik SH, Kim KH, Shanta SR, Jung JH, Mook-Jung I, & Kim KP (2016). Global changes of phospholipids identified by MALDI imaging mass spectrometry in a mouse model of Alzheimer’s disease. J Lipid Res, 57, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Kiss M, Szucs A, & Kamondi A (2019). Precuneus-Dominant Degeneration of Parietal Lobe Is at Risk of Epilepsy in Mild Alzheimer’s Disease. Front Neurol, 10, 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, & Cole G (1996). Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science, 274, 99–102. [DOI] [PubMed] [Google Scholar]
- Hu ZI, Kotarba AM, & Van Nostrand WE (2013). Absence of Nitric Oxide Synthase 3 Increases Amyloid beta-Protein Pathology in Tg-5xFAD Mice. Neurosci Med, 4, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Mateo V, Amalric N, Babiak M, Bereziat G, Kanony-Truc C, Clerc T, Blaise R, & Limon I (2018). Cerebrovascular beta-amyloid deposition and associated microhemorrhages in a Tg2576 Alzheimer mouse model are reduced with a DHA-enriched diet. FASEB J, 32, 4972–4983. [DOI] [PubMed] [Google Scholar]
- Hyeon S, Lee H, Yang Y, & Jeong W (2013). Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med, 65, 789–799. [DOI] [PubMed] [Google Scholar]
- Hynes RO (1986). Fibronectins. Sci Am, 254, 42–51. [DOI] [PubMed] [Google Scholar]
- Iadecola C (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci, 5, 347–360. [DOI] [PubMed] [Google Scholar]
- Iadecola C (2013). The pathobiology of vascular dementia. Neuron, 80, 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, & Gong CX (2016). Tau and neurodegenerative disease: the story so far. Nat Rev Neurol, 12, 15–27. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Jin S, He F, Emond JA, Raman R, Thomas RG, Sano M, Quinn JF, Tariot PN, Galasko DR, Ishihara LS, Weil JG, & Aisen PS (2012). Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol, 69, 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, & Hyman BT (1997). APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol, 56, 965–973. [DOI] [PubMed] [Google Scholar]
- Ishii T, & Haga S (1975). Identification of components of immunoglobulins in senile plaques by means of fluorescent antibody technique. Acta Neuropathol, 32, 157–162. [DOI] [PubMed] [Google Scholar]
- Ishii T, & Haga S (1976). Immuno-electron microscopic localization of immunoglobulins in amyloid fibrils of senile plaques. Acta Neuropathol, 36, 243–249. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, & Selkoe D (1989). Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol, 24, 173–182. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, & Petersen RC (2013). Brain beta-amyloid load approaches a plateau. Neurology, 80, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Rudinskiy N, Herrmann AG, Croft S, Kim JM, Petrova V, Ramos-Rodriguez JJ, Pitstick R, Wegmann S, Garcia-Alloza M, Carlson GA, Hyman BT, & Spires-Jones TL (2016). Human tau increases amyloid beta plaque size but not amyloid beta-mediated synapse loss in a novel mouse model of Alzheimer’s disease. Eur J Neurosci, 44, 3056–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Hertze J, Nagga K, Nilsson K, Nilsson C, Swedish Bio FSG, Wennstrom M, van Westen D, Blennow K, Zetterberg H, & Hansson O (2017). Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging, 51, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, & Borchelt DR (2004). Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet, 13, 159–170. [DOI] [PubMed] [Google Scholar]
- Jansson D, Scotter EL, Rustenhoven J, Coppieters N, Smyth LC, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, & Dragunow M (2016). Interferon-gamma blocks signalling through PDGFRbeta in human brain pericytes. J Neuroinflammation, 13, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarre A, Gowert NS, Donner L, Munzer P, Klier M, Borst O, Schaller M, Lang F, Korth C, & Elvers M (2014). Pre-activated blood platelets and a pro-thrombotic phenotype in APP23 mice modeling Alzheimer’s disease. Cell Signal, 26, 2040–2050. [DOI] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, Bayer TA, & Wirths O (2012). Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging, 33, 196 e129–140. [DOI] [PubMed] [Google Scholar]
- Jellinger K (2002). Prevalence of Alzheimer’s disease in very elderly people: a prospective neuropathological study. Neurology, 58, 671–672; author reply 671–672. [DOI] [PubMed] [Google Scholar]
- Jellinger KA (1997). Neuropathological staging of Alzheimer-related lesions: the challenge of establishing relations to age. Neurobiol Aging, 18, 369–375; discussion 389–392. [DOI] [PubMed] [Google Scholar]
- Jenkins HG, & Bachelard HS (1988). Glycosaminoglycans in cortical autopsy samples from Alzheimer brain. J Neurochem, 51, 1641–1645. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wang N, Zhang Y, Xue D, Lou H, & Liu X (2020). Alteration in the Function and Expression of SLC and ABC Transporters in the Neurovascular Unit in Alzheimer’s Disease and the Clinical Significance. Aging Dis. 11, 390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordao JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, & Aubert I (2010). Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One, 5, e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordao JF, Thevenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O’Reilly M, Huang Y, McLaurin J, Hynynen K, & Aubert I (2013). Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol, 248, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadry H, Noorani B, & Cucullo L (2020). A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 17. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN (1997). Cerebrovascular degeneration is related to amyloid-beta protein deposition in Alzheimer’s disease. Ann N Y Acad Sci, 826, 263–271. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, & Harik SI (1992). Carnitine acetyltransferase activity in the human brain and its microvessels is decreased in Alzheimer’s disease. Ann Neurol, 32, 583–586. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, & Pax AB (1995). Increased collagen content of cerebral microvessels in Alzheimer’s disease. Brain Res, 705, 349–352. [DOI] [PubMed] [Google Scholar]
- Kato T, Sekine Y, Nozaki H, Uemura M, Ando S, Hirokawa S, & Onodera O (2020). Excessive Production of Transforming Growth Factor beta1 Causes Mural Cell Depletion From Cerebral Small Vessels. Front Aging Neurosci, 12, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, & Younkin SG (2001). Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci, 21, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AD, May C, Papadopoulos NM, Costello R, Atack JR, Luxenberg JS, Cutler NR, & Rapoport SI (1987). CSF and serum concentrations of albumin and IgG in Alzheimer’s disease. Neurobiol Aging, 8, 21–25. [DOI] [PubMed] [Google Scholar]
- Keable A, Fenna K, Yuen HM, Johnston DA, Smyth NR, Smith C, Al-Shahi Salman R, Samarasekera N, Nicoll JA, Attems J, Kalaria RN, Weller RO, & Carare RO (2016). Deposition of amyloid beta in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta, 1862, 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney J, Walsh DM, O’Malley T, Hudson N, Crosbie DE, Loftus T, Sheehan F, McDaid J, Humphries MM, Callanan JJ, Brett FM, Farrell MA, Humphries P, & Campbell M (2015). Autoregulated paracellular clearance of amyloid-beta across the blood-brain barrier. Sci Adv, 1, e1500472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Bondolfi L, Hunziker D, Schlecht HP, Carver K, Maguire E, Abramowski D, Wiederhold KH, Sturchler-Pierrat C, Jucker M, Bergmann R, Staufenbiel M, & Sommer B (2003). Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol Aging, 24, 365–378. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, & Biswal S (2007). Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol, 47, 89–116. [DOI] [PubMed] [Google Scholar]
- Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, Barkhof F, Scheltens P, & van der Flier WM (2014). Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol, 71, 855–862. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, & Hermann DM (2006). The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF’s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J, 20, 1185–1187. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, & Holtzman DM (2009). The role of apolipoprotein E in Alzheimer’s disease. Neuron, 63, 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, & Greenberg SM (2009). Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology, 72, 1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough IF, Robel S, Roberson ED, & Sontheimer H (2015). Vascular amyloidosis impairs the gliovascular unit in a mouse model of Alzheimer’s disease. Brain, 138, 3716–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimotsuki T, Nagaoka T, Yasuda M, Tamahara S, Matsuki N, & Ono K (2005). Changes of magnetic resonance imaging on the brain in beagle dogs with aging. J Vet Med Sci, 67, 961–967. [DOI] [PubMed] [Google Scholar]
- Kirabali T, Rust R, Rigotti S, Siccoli A, Nitsch RM, & Kulic L (2020). Distinct changes in all major components of the neurovascular unit across different neuropathological stages of Alzheimer’s disease. Brain Pathol. 30. 1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood CM, MacDonald ML, Schempf TA, Vatsavayi AV, Ikonomovic MD, Koppel JL, Ding Y, Sun M, Kofler JK, Lopez OL, Yates NA, & Sweet RA (2016). Altered Levels of Visinin-Like Protein 1 Correspond to Regional Neuronal Loss in Alzheimer Disease and Frontotemporal Lobar Degeneration. J Neuropathol Exp Neurol, 75, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, & Nissinen A (2001). Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ, 322, 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klakotskaia D, Agca C, Richardson RA, Stopa EG, Schachtman TR, & Agca Y (2018). Memory deficiency, cerebral amyloid angiopathy, and amyloid-beta plaques in APP+PS1 double transgenic rat model of Alzheimer’s disease. PLoS One, 13, e0195469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook SY, Hong HS, Moon M, Ha CM, Chang S, & Mook-Jung I (2012). Abeta(1)(−)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci, 32, 8845–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook SY, Lee KM, Kim Y, Cha MY, Kang S, Baik SH, Lee H, Park R, & Mook-Jung I (2014). High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis, 5, e1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, & Grundke-Iqbal I (1993). Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem, 268, 24374–24384. [PubMed] [Google Scholar]
- Koson P, Zilka N, Kovac A, Kovacech B, Korenova M, Filipcik P, & Novak M (2008). Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. Eur J Neurosci, 28, 239–246. [DOI] [PubMed] [Google Scholar]
- Koss M, Pfeiffer GR 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, & Wang Q (2006). Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol, 176, 1218–1227. [DOI] [PubMed] [Google Scholar]
- Kramer RH, & Marks N (1989). Identification of integrin collagen receptors on human melanoma cells. J Biol Chem, 264, 4684–4688. [PubMed] [Google Scholar]
- Kuhlmann CR, Tamaki R, Gamerdinger M, Lessmann V, Behl C, Kempski OS, & Luhmann HJ (2007). Inhibition of the myosin light chain kinase prevents hypoxia-induced blood-brain barrier disruption. J Neurochem, 102, 501–507. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, & Van Broeckhoven C (2005). Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol, 167, 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Beach TG, Sue LI, Scott S, Layne KJ, Kokjohn TA, Kalback WM, Luehrs DC, Vishnivetskaya TA, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Weller RO, & Roher AE (2001). The evolution of A beta peptide burden in the APP23 transgenic mice: implications for A beta deposition in Alzheimer disease. Mol Med, 7, 609–618. [PMC free article] [PubMed] [Google Scholar]
- Kyrtata N, Emsley HCA, Sparasci O, Parkes LM, & Dickie BR (2021). A Systematic Review of Glucose Transport Alterations in Alzheimer’s Disease. Front Neurosci. 15. 626636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, Costa Lda F, & Gu C (2014). Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron, 83, 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AD, Sarkis RA, Pellerin KR, Jing J, Dworetzky BA, Hoch DB, Jacobs CS, Lee JW, Weisholtz DS, Zepeda R, Westover MB, Cole AJ, & Cash SS (2020). Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology, 95, e2259–e2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CB Jr., Bales K, Alvey C, McCush F, Yang J, Kupiec JW, & Bednar MM (2013). Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol, 36, 14–23. [DOI] [PubMed] [Google Scholar]
- Langlet F, Mullier A, Bouret SG, Prevot V, & Dehouck B (2013). Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol, 521, 3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T, Holton JL, Verbeek MM, Rostagno A, Bojsen-Moller M, David G, van Horssen J, Braendgaard H, Plant G, Frangione B, Ghiso J, & Revesz T (2006). Molecular chaperons, amyloid and preamyloid lesions in the BRI2 gene-related dementias: a morphological study. Neuropathol Appl Neurobiol, 32, 492–504. [DOI] [PubMed] [Google Scholar]
- Latimer CS, Burke BT, Liachko NF, Currey HN, Kilgore MD, Gibbons LE, Henriksen J, Darvas M, Domoto-Reilly K, Jayadev S, Grabowski TJ, Crane PK, Larson EB, Kraemer BC, Bird TD, & Keene CD (2019). Resistance and resilience to Alzheimer’s disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol Commun, 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic D, Sagare AP, Nikolakopoulou AM, Griffin JH, Vassar R, & Zlokovic BV (2019). 3K3A-activated protein C blocks amyloidogenic BACE1 pathway and improves functional outcome in mice. J Exp Med, 216, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Namkoong H, Kim HK, Kim S, Hwang DW, Na HR, Ha SA, Kim JR, & Kim JW (2007). Fibrinogen gamma-A chain precursor in CSF: a candidate biomarker for Alzheimer’s disease. BMC Neurol, 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zimmerman ME, Narkhede A, Nasrabady SE, Tosto G, Meier IB, Benzinger TLS, Marcus DS, Fagan AM, Fox NC, Cairns NJ, Holtzman DM, Buckles V, Ghetti B, McDade E, Martins RN, Saykin AJ, Masters CL, Ringman JM, Frster S, Schofield PR, Sperling RA, Johnson KA, Chhatwal JP, Salloway S, Correia S, Jack CR Jr., Weiner M, Bateman RJ, Morris JC, Mayeux R, Brickman AM, & Dominantly Inherited Alzheimer N (2018). White matter hyperintensities and the mediating role of cerebral amyloid angiopathy in dominantly-inherited Alzheimer’s disease. PLoS One, 13, e0195838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Liao YC, Wang YF, Lin YS, Wang SJ, & Fuh JL (2020). Summative Effects of Vascular Risk Factors on the Progression of Alzheimer Disease. J Am Geriatr Soc, 68, 129–136. [DOI] [PubMed] [Google Scholar]
- Lefterov I, Fitz NF, Cronican AA, Fogg A, Lefterov P, Kodali R, Wetzel R, & Koldamova R (2010). Apolipoprotein A-I deficiency increases cerebral amyloid angiopathy and cognitive deficits in APP/PS1DeltaE9 mice. J Biol Chem, 285, 36945–36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinenga G, & Gotz J (2018). Safety and Efficacy of Scanning Ultrasound Treatment of Aged APP23 Mice. Front Neurosci, 12, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinenga G, Koh WK, & Gotz J (2019). Scanning ultrasound in the absence of blood-brain barrier opening is not sufficient to clear beta-amyloid plaques in the APP23 mouse model of Alzheimer’s disease. Brain Res Bull, 153, 8–14. [DOI] [PubMed] [Google Scholar]
- Leonardi A, Gandolfo C, Caponnetto C, Arata L, & Vecchia R (1985). The integrity of the blood-brain barrier in Alzheimer’s type and multi-infarct dementia evaluated by the study of albumin and IgG in serum and cerebrospinal fluid. J Neurol Sci, 67, 253–261. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Reiber H, & Ehrenreich H (1998). Prothrombin in normal human cerebrospinal fluid originates from the blood. Neurochem Res, 23, 1027–1030. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Wiltfang J, Lange M, Jahn H, Reiber H, & Ehrenreich H (1999). Prothrombin concentration in the cerebrospinal fluid is not altered in Alzheimer’s disease. Neurochem Res, 24, 1531–1534. [DOI] [PubMed] [Google Scholar]
- Li M, Wen Y, Zhang R, Xie F, Zhang G, & Qin X (2018). Adenoviral vector-induced silencing of RGMa attenuates blood-brain barrier dysfunction in a rat model of MCAO/reperfusion. Brain Res Bull, 142, 54–62. [DOI] [PubMed] [Google Scholar]
- Liao F, Hori Y, Hudry E, Bauer AQ, Jiang H, Mahan TE, Lefton KB, Zhang TJ, Dearborn JT, Kim J, Culver JP, Betensky R, Wozniak DF, Hyman BT, & Holtzman DM (2014). Anti-ApoE antibody given after plaque onset decreases Abeta accumulation and improves brain function in a mouse model of Abeta amyloidosis. J Neurosci, 34, 7281–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Czupalla CJ, & Wolburg H (2011). Current concepts of blood-brain barrier development. Int J Dev Biol, 55, 467–476. [DOI] [PubMed] [Google Scholar]
- Lin AL, Parikh I, Hoffman JD, & Ma D (2017). Neuroimaging Biomarkers of Caloric Restriction on Brain Metabolic and Vascular Functions. Curr Nutr Rep, 6, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Parikh I, Yanckello LM, White RS, Hartz AMS, Taylor CE, McCulloch SD, Thalman SW, Xia M, McCarty K, Ubele M, Head E, Hyder F, & Sanganahalli BG (2020). APOE genotype-dependent pharmacogenetic responses to rapamycin for preventing Alzheimer’s disease. Neurobiol Dis, 139, 104834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, & Galvan V (2013). Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab, 33, 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, & Betsholtz C (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science, 277, 242–245. [DOI] [PubMed] [Google Scholar]
- Lipinski B, & Sajdel-Sulkowska EM (2006). New insight into Alzheimer disease: demonstration of fibrin(ogen)-serum albumin insoluble deposits in brain tissue. Alzheimer Dis Assoc Disord, 20, 323–326. [DOI] [PubMed] [Google Scholar]
- Liu HM, Atack JR, & Rapoport SI (1989). Immunohistochemical localization of intracellular plasma proteins in the human central nervous system. Acta Neuropathol, 78, 16–21. [DOI] [PubMed] [Google Scholar]
- Liu M, Jevtic S, Markham-Coultes K, Ellens NPK, O’Reilly MA, Hynynen K, Aubert I, & McLaurin J (2018). Investigating the efficacy of a combination Abeta-targeted treatment in a mouse model of Alzheimer’s disease. Brain Res, 1678, 138–145. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu J, & Shi JS SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer’s Disease. J Alzheimers Dis, 75, 385–395. [DOI] [PubMed] [Google Scholar]
- Liu X, Yamashita T, Shang J, Shi X, Morihara R, Huang Y, Sato K, Takemoto M, Hishikawa N, Ohta Y, & Abe K (2019a). Clinical and Pathological Benefit of Twendee X in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J Stroke Cerebrovasc Dis, 28, 1993–2002. [DOI] [PubMed] [Google Scholar]
- Liu X, Yamashita T, Shang J, Shi X, Morihara R, Huang Y, Sato K, Takemoto M, Hishikawa N, Ohta Y, & Abe K (2019b). Twendee X Ameliorates Phosphorylated Tau, alpha-Synuclein and Neurovascular Dysfunction in Alzheimer’s Disease Transgenic Mice With Chronic Cerebral Hypoperfusion. J Stroke Cerebrovasc Dis, 28, 104310. [DOI] [PubMed] [Google Scholar]
- Lloyd GM, Trejo-Lopez JA, Xia Y, McFarland KN, Lincoln SJ, Ertekin-Taner N, Giasson BI, Yachnis AT, & Prokop S (2020). Prominent amyloid plaque pathology and cerebral amyloid angiopathy in APP V717I (London) carrier - phenotypic variability in autosomal dominant Alzheimer’s disease. Acta Neuropathol Commun, 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead J, Movaffaghy A, Falsini B, Harding S, Riva C, & Molyneux M (2010). The effects of hypoxia on the ERG in paediatric cerebral malaria. Eye (Lond), 24, 259–264. [DOI] [PubMed] [Google Scholar]
- Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, Basun H, & Lannfelt L (2016). Safety and tolerability of BAN2401--a clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimers Res Ther, 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon AF, Meabon JS, Cline MM, Bullock KM, Raskind MA, Peskind ER, Banks WA, & Cook DG (2018). Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci Rep, 8, 11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente-Gea L, Garcia B, Martin C, Ordiales H, Garcia-Suarez O, Pina-Batista KM, Merayo-Lloves J, Quiros LM, & Fernandez-Vega I (2020). Heparan Sulfate Proteoglycans Undergo Differential Expression Alterations in Alzheimer Disease Brains. J Neuropathol Exp Neurol, 79, 474–483. [DOI] [PubMed] [Google Scholar]
- Love S, & Miners JS (2016). Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol, 131, 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth HJ, Holzer M, Gartner U, Staufenbiel M, & Arendt T (2001). Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer’s disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res, 913, 57–67. [DOI] [PubMed] [Google Scholar]
- Lynch M, Heinen S, Markham-Coultes K, O’Reilly M, Van Slyke P, Dumont DJ, Hynynen K, & Aubert I (2021). Vasculotide restores the blood-brain barrier after focused ultrasound-induced permeability in a mouse model of Alzheimer’s disease. Int J Med Sci, 18, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida T, Takata F, Matsumoto J, Miyamura T, Hirata R, Kimura I, Kataoka Y, Dohgu S, & Yamauchi A (2017). Contribution of thrombin-reactive brain pericytes to blood-brain barrier dysfunction in an in vivo mouse model of obesity-associated diabetes and an in vitro rat model. PLoS One, 12, e0177447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Sahara N, Saito Y, Murayama M, Yoshiike Y, Kim H, Miyasaka T, Murayama S, Ikai A, & Takashima A (2007). Granular tau oligomers as intermediates of tau filaments. Biochemistry, 46, 3856–3861. [DOI] [PubMed] [Google Scholar]
- Magaki S, Tang Z, Tung S, Williams CK, Lo D, Yong WH, Khanlou N, & Vinters HV (2018). The effects of cerebral amyloid angiopathy on integrity of the blood-brain barrier. Neurobiol Aging, 70, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerova P, Michalicova A, Cente M, Hanes J, Vegh J, Kittel A, Kosikova N, Cigankova V, Mihaljevic S, Jadhav S, & Kovac A (2019). Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS One, 14, e0217216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki KC, Ridker PM, Brown WV, Grundy SM, Sattar N, & The Diabetes Subpanel of the National Lipid Association Expert, P. (2014). An assessment by the Statin Diabetes Safety Task Force: 2014 update. J Clin Lipidol, 8, S17–29. [DOI] [PubMed] [Google Scholar]
- Mancardi GL, Perdelli F, Rivano C, Leonardi A, & Bugiani O (1980). Thickening of the basement membrane of cortical capillaries in Alzheimer’s disease. Acta Neuropathol, 49, 79–83. [DOI] [PubMed] [Google Scholar]
- Mann JM, Francis DP, Hoffman RE, & Montes J (1982). Assessment of immunoglobulin use for hepatitis A control in New Mexico. Public Health Rep, 97, 516–520. [PMC free article] [PubMed] [Google Scholar]
- Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, Orset C, Pruvost M, Anfray A, Frigout Y, Hommet Y, Lebouvier L, Montaner J, Vivien D, & Gauberti M (2016). Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood, 128, 2423–2434. [DOI] [PubMed] [Google Scholar]
- Mari D, Parnetti L, Coppola R, Bottasso B, Reboldi GP, Senin U, & Mannucci PM (1996). Hemostasis abnormalities in patients with vascular dementia and Alzheimer’s disease. Thromb Haemost, 75, 216–218. [PubMed] [Google Scholar]
- Marinescu M, Sun L, Fatar M, Neubauer A, Schad L, van Ryn J, Lehmann L, & Veltkamp R (2017). Cerebral Microbleeds in Murine Amyloid Angiopathy: Natural Course and Anticoagulant Effects. Stroke, 48, 2248–2254. [DOI] [PubMed] [Google Scholar]
- Marottoli FM, Katsumata Y, Koster KP, Thomas R, Fardo DW, & Tai LM (2017). Peripheral Inflammation, Apolipoprotein E4, and Amyloid-beta Interact to Induce Cognitive and Cerebrovascular Dysfunction. ASN Neuro, 9, 1759091417719201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PT, & Sanes JR (1997). Integrins mediate adhesion to agrin and modulate agrin signaling. Development, 124, 3909–3917. [DOI] [PubMed] [Google Scholar]
- Masliah E, Sisk A, Mallory M, & Games D (2001). Neurofibrillary pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. J Neuropathol Exp Neurol, 60, 357–368. [DOI] [PubMed] [Google Scholar]
- Masters CL (1984). Etiology and pathogenesis of Alzheimer’s disease. Pathology, 16, 233–234. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, & Beyreuther K (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A, 82, 4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Yanase D, Noguchi-Shinohara M, Ono K, Yoshita M, & Yamada M (2007). Blood-brain barrier permeability correlates with medial temporal lobe atrophy but not with amyloid-beta protein transport across the blood-brain barrier in Alzheimer’s disease. Dement Geriatr Cogn Disord, 23, 241–245. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yanase D, Noguchi-Shinohara M, Ono K, Yoshita M, & Yamada M (2008). Cerebrospinal fluid/serum IgG index is correlated with medial temporal lobe atrophy in Alzheimer’s disease. Dement Geriatr Cogn Disord, 25, 144–147. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, & Rydel RE (1992). beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci, 12, 376–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic Family Health Book 5th Edition. Mayo Clinic Press. [Google Scholar]
- McAreavey MJ, Ballinger BR, & Fenton GW (1992). Epileptic seizures in elderly patients with dementia. Epilepsia, 33, 657–660. [DOI] [PubMed] [Google Scholar]
- McConnell HL, Kersch CN, Woltjer RL, & Neuwelt EA (2017). The Translational Significance of the Neurovascular Unit. J Biol Chem, 292, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan EF, & O’Brien JT (2017). 7T MRI for neurodegenerative dementias in vivo: a systematic review of the literature. J Neurol Neurosurg Psychiatry, 88, 564–574. [DOI] [PubMed] [Google Scholar]
- McSweeney SR, Warabi E, & Siow RC (2016). Nrf2 as an Endothelial Mechanosensitive Transcription Factor: Going With the Flow. Hypertension, 67, 20–29. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Parnetti L, Reboldi GP, Santucci C, Gaiti A, Ferri C, Gernini I, Romagnoli M, Cadini D, & Senin U (1991). Blood-brain-barrier in a geriatric population: barrier function in degenerative and vascular dementias. Acta Neurol Scand, 84, 210–213. [DOI] [PubMed] [Google Scholar]
- Medina MG, Ledesma MD, Dominguez JE, Medina M, Zafra D, Alameda F, Dotti CG, & Navarro P (2005). Tissue plasminogen activator mediates amyloid-induced neurotoxicity via Erk1/2 activation. EMBO J, 24, 1706–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, & Lim G (2003). Seizures in elderly patients with dementia: epidemiology and management. Drugs Aging, 20, 791–803. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Catanzaro P, Doss RC, R AR, & Frey WH 2nd. (1994). Seizures in Alzheimer’s disease: clinicopathologic study. J Geriatr Psychiatry Neurol, 7, 230–233. [DOI] [PubMed] [Google Scholar]
- Merlini M, Wanner D, & Nitsch RM (2016). Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol, 131, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Lalehzari N, Rahmani F, Ohm D, Shahidehpour R, Kim G, Gefen T, Weintraub S, Bigio E, & Geula C (2019). Cortical cholinergic denervation in primary progressive aphasia with Alzheimer pathology. Neurology, 92, e1580–e1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EP, Ulmann-Schuler A, Staufenbiel M, & Krucker T (2008). Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc Natl Acad Sci U S A, 105, 3587–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Dong Y, Fang W, Shang D, Liu D, Zhang K, Li B, & Chen YH (2014). VEGF increases paracellular permeability in brain endothelial cells via upregulation of EphA2. Anat Rec (Hoboken), 297, 964–972. [DOI] [PubMed] [Google Scholar]
- Michael N, Grigoryan MM, Kilday K, Sumbria RK, Vasilevko V, van Ryn J, Cribbs DH, Paganini-Hill A, & Fisher MJ (2019). Effects of Dabigatran in Mouse Models of Aging and Cerebral Amyloid Angiopathy. Front Neurol, 10, 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michinaga S, & Koyama Y (2019). Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Kimura M, Iwamoto N, & Arai H (1995). Endothelin-1-like immunoreactivity in cerebral cortex of Alzheimer-type dementia. Prog Neuropsychopharmacol Biol Psychiatry, 19, 509–513. [DOI] [PubMed] [Google Scholar]
- Miners JS, Kehoe PG, Love S, Zetterberg H, & Blennow K (2019). CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimers Res Ther, 11, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JS, Schulz I, & Love S (2018). Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J Cereb Blood Flow Metab, 38, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue AM, Jones RS, Kelly RJ, McDonald CL, Connor TJ, & Lynch MA (2014). Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol Aging, 35, 1442–1452. [DOI] [PubMed] [Google Scholar]
- Miranda AS, Cordeiro TM, os Santos Lacerda Soares TM, Ferreira RN, & Simoes ESAC (2017). Kidney-brain axis inflammatory cross-talk: from bench to bedside. Clin Sci (Lond), 131, 1093–1105. [DOI] [PubMed] [Google Scholar]
- Miwa K, Tanaka M, Okazaki S, Furukado S, Yagita Y, Sakaguchi M, Mochizuki H, & Kitagawa K (2014). Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology, 82, 1051–1057. [DOI] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, & Van Leuven F (1999). Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem, 274, 6483–6492. [DOI] [PubMed] [Google Scholar]
- Moiuddin A, Morley JE, & Banks WA (2000). Regional variations in the transport of interleukin-1alpha across the blood-brain barrier in ICR and aging SAMP8 mice. Neuroimmunomodulaton, 8, 165–170. [DOI] [PubMed] [Google Scholar]
- Molino I, Colucci L, Fasanaro AM, Traini E, & Amenta F (2013). Efficacy of memantine, donepezil, or their association in moderate-severe Alzheimer’s disease: a review of clinical trials. ScientificWorldJournal, 2013, 925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, & Zlokovic BV (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron, 85, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, & Zlokovic BV (2016). Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol, 131, 687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D’Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, & Zlokovic BV (2020). APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature, 581, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Zhao Z, & Zlokovic BV (2017). Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med, 214, 3151–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD (1988). Effect of aging on the blood-brain barrier. Neurobiol Aging, 9, 31–39. [DOI] [PubMed] [Google Scholar]
- Morel FW, E. (1954). General and cellular pathochemistry of senile and presenile alterations of the brain. In Proceedings of the 1st International Congress on Neuropathology, Rome (pp. 347–374). Torino. [Google Scholar]
- Mori R, Kondo T, Ohshima T, Ishida Y, & Mukaida N (2002). Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB J, 16, 963–974. [DOI] [PubMed] [Google Scholar]
- Morita T, Mizutani Y, Sawada M, & Shimada A (2005). Immunohistochemical and ultrastructural findings related to the blood--brain barrier in the blood vessels of the cerebral white matter in aged dogs. J Comp Pathol, 133, 14–22. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, & McConlogue L (2000). High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci, 20, 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueggler T, Baumann D, Rausch M, Staufenbiel M, & Rudin M (2003). Age-dependent impairment of somatosensory response in the amyloid precursor protein 23 transgenic mouse model of Alzheimer’s disease. J Neurosci, 23, 8231–8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueggler T, Sturchler-Pierrat C, Baumann D, Rausch M, Staufenbiel M, & Rudin M (2002). Compromised hemodynamic response in amyloid precursor protein transgenic mice. J Neurosci, 22, 7218–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DG, Erkinjuntti T, Gaytan-Garcia S, & Hachinski V (1997). Serum protein leakage in Alzheimer’s disease revisited. Ann N Y Acad Sci, 826, 173–189. [DOI] [PubMed] [Google Scholar]
- Muoio V, Persson PB, & Sendeski MM (2014). The neurovascular unit - concept review. Acta Physiol (Oxf), 210, 790–798. [DOI] [PubMed] [Google Scholar]
- Murphy GM Jr., Yang L, & Cordell B (1998). Macrophage colony-stimulating factor augments beta-amyloidinduced interleukin-1, interleukin-6, and nitric oxide production by microglial cells. J Biol Chem, 273, 20967–20971. [DOI] [PubMed] [Google Scholar]
- Musaeus CS, Gleerup HS, Hogh P, Waldemar G, Hasselbalch SG, & Simonsen AH (2020). Cerebrospinal Fluid/Plasma Albumin Ratio as a Biomarker for Blood-Brain Barrier Impairment Across Neurodegenerative Dementias. J Alzheimers Dis, 75, 429–436. [DOI] [PubMed] [Google Scholar]
- Muszynski P, Kulczynska-Przybik A, Borawska R, Litman-Zawadzka A, Slowik A, Klimkowicz-Mrowiec A, Pera J, Dziedzic T, & Mroczko B (2017). The Relationship between Markers of Inflammation and Degeneration in the Central Nervous System and the Blood-Brain Barrier Impairment in Alzheimer’s Disease. J Alzheimers Dis, 59, 903–912. [DOI] [PubMed] [Google Scholar]
- Nagasawa J, Kiyozaka T, & Ikeda K (2014). Prevalence and clinicoradiological analyses of patients with Alzheimer disease coexisting multiple microbleeds. J Stroke Cerebrovasc Dis, 23, 2444–2449. [DOI] [PubMed] [Google Scholar]
- Nagga K, Hansson O, van Westen D, Minthon L, & Wennstrom M (2014). Increased levels of hyaluronic acid in cerebrospinal fluid in patients with vascular dementia. J Alzheimers Dis, 42, 1435–1441. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Hasegawa Y, Uekawa K, & Kim-Mitsuyama S (2017). Chronic kidney disease accelerates cognitive impairment in a mouse model of Alzheimer’s disease, through angiotensin II. Exp Gerontol, 87, 108–112. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Hasegawa Y, Uekawa K, Senju S, Nakagata N, Matsui K, & Kim-Mitsuyama S (2017). Transient Mild Cerebral Ischemia Significantly Deteriorated Cognitive Impairment in a Mouse Model of Alzheimer’s Disease via Angiotensin AT1 Receptor. Am J Hypertens, 30, 141–150. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Nakayama H, Goto N, Ono F, Sakakibara I, & Yoshikawa Y (1998). Histopathological studies of senile plaques and cerebral amyloidosis in cynomolgus monkeys. J Med Primatol, 27, 244–252. [DOI] [PubMed] [Google Scholar]
- Nakata-Kudo Y, Mizuno T, Yamada K, Shiga K, Yoshikawa K, Mori S, Nishimura T, Nakajima K, & Nakagawa M (2006). Microbleeds in Alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular disease. Dement Geriatr Cogn Disord, 22, 8–14. [DOI] [PubMed] [Google Scholar]
- Nakata Y, Shiga K, Yoshikawa K, Mizuno T, Mori S, Yamada K, & Nakajima K (2002). Subclinical brain hemorrhages in Alzheimer’s disease: evaluation by magnetic resonance T2*-weighted images. Ann N Y Acad Sci, 977, 169–172. [DOI] [PubMed] [Google Scholar]
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, & Zlokovic BV (2019). Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med, 25, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndung’u M, Hartig W, Wegner F, Mwenda JM, Low RW, Akinyemi RO, & Kalaria RN (2012). Cerebral amyloid beta(42) deposits and microvascular pathology in ageing baboons. Neuropathol Appl Neurobiol, 38, 487–499. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, & Beach TG (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol, 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HM, Londos E, Minthon L, & Janciauskiene SM (2007). Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol Dis, 26, 27–35. [DOI] [PubMed] [Google Scholar]
- Nielsen HM, Palmqvist S, Minthon L, Londos E, & Wennstrom M (2012). Gender-dependent levels of hyaluronic acid in cerebrospinal fluid of patients with neurodegenerative dementia. Curr Alzheimer Res, 9, 257–266. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, & Cuervo AM (2005). Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol, 64, 113–122. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Banks WA, Mizushima H, Shioda S & Morley JE (2002). Regional differences in PACAP transport across the blood-brain barrier in mice: a possible influence of strain, amyloid beta protein, and age. Peptides, 23, 2197–2202. [DOI] [PubMed] [Google Scholar]
- Noguchi-Shinohara M, Komatsu J, Samuraki M, Matsunari I, Ikeda T, Sakai K, Hamaguchi T, Ono K, Nakamura H, & Yamada M (2017). Cerebral Amyloid Angiopathy-Related Microbleeds and Cerebrospinal Fluid Biomarkers in Alzheimer’s Disease. J Alzheimers Dis, 55, 905–913. [DOI] [PubMed] [Google Scholar]
- Nyul-Toth A, Tarantini S, Kiss T, Toth P, Galvan V, Tarantini A, Yabluchanskiy A, Csiszar A, & Ungvari Z (2020). Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer’s disease. Geroscience, 42, 1685–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, & Vassar R (2006). Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci, 26, 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, & LaFerla FM (2003). Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron, 39, 409–421. [DOI] [PubMed] [Google Scholar]
- Oh SB, Kim MS, Park S, Son H, Kim SY, Kim MS, Jo DG, Tak E, & Lee JY (2019). Clusterin contributes to early stage of Alzheimer’s disease pathogenesis. Brain Pathol, 29, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikari LE, Pandit R, Stewart R, Cuni-Lopez C, Quek H, Sutharsan R, Rantanen LM, Oksanen M, Lehtonen S, de Boer CM, Polo JM, Gotz J, Koistinaho J, & White AR (2020). Altered Brain Endothelial Cell Phenotype from a Familial Alzheimer Mutation and Its Potential Implications for Amyloid Clearance and Drug Delivery. Stem Cell Reports, 14, 924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazaran J, Ramos A, Boyano I, Alfayate E, Valenti M, Rabano A, & Alvarez-Linera J (2014). Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am J Alzheimers Dis Other Demen, 29, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, & Zetterberg H (2016). CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol, 15, 673–684. [DOI] [PubMed] [Google Scholar]
- Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, Lecrux C, Imboden H, & Hamel E (2014). Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol Dis, 68, 126–136. [DOI] [PubMed] [Google Scholar]
- Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, & del Zoppo GJ (2011). Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab, 31, 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JC, Barker R, Kehoe PG, & Love S (2012). Endothelin-1 is elevated in Alzheimer’s disease and upregulated by amyloid-beta. J Alzheimers Dis, 29, 853–861. [DOI] [PubMed] [Google Scholar]
- Palop JJ, & Mucke L (2016). Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci, 17, 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, Koh WK, Sullivan RKP, Palliyaguru T, Patron RG, & Gotz J (2020) Role for caveolin-mediated transcytosis in facilitating transport of large cargoes into the brain via ultrasound. J Control Release, 327, 667–675. [DOI] [PubMed] [Google Scholar]
- Pandit K, & Mukhopadhyay P (2004). Insulin therapy--role beyond glucose control. J Indian Med Assoc, 102, 572, 574, 576 passim. [PubMed] [Google Scholar]
- Paresce DM, Chung H, & Maxfield FR (1997). Slow degradation of aggregates of the Alzheimer’s disease amyloid beta-protein by microglial cells. J Biol Chem, 272, 29390–29397. [DOI] [PubMed] [Google Scholar]
- Park JC, Baik SH, Han SH, Cho HJ, Choi H, Kim HJ, Choi H, Lee W, Kim DK, & Mook-Jung I (2017). Annexin A1 restores Abeta1–42 -induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway. Aging Cell, 16, 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kim JH, Mook-Jung I, Kim KW, Park WJ, Park KH, & Kim JH (2014). Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice. Neurobiol Aging, 35, 2013–2020. [DOI] [PubMed] [Google Scholar]
- Park YH, Shin SJ, Kim HS, Hong SB, Kim S, Nam Y, Kim JJ, Lim K, Kim JS, Kim JI, Jeon SG, & Moon M (2020). Omega-3 Fatty Acid-Type Docosahexaenoic Acid Protects against Abeta-Mediated Mitochondrial Deficits and Pathomechanisms in Alzheimer’s Disease-Related Animal Model. Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos GF, Kilday K, Gillen DL, Cribbs DH, & Vasilevko V (2016). Experimental hypertension increases spontaneous intracerebral hemorrhages in a mouse model of cerebral amyloidosis. J Cereb Blood Flow Metab, 36, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patibandla PK, Tyagi N, Dean WL, Tyagi SC, Roberts AM, & Lominadze D (2009). Fibrinogen induces alterations of endothelial cell tight junction proteins. J Cell Physiol, 221, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, & Fenstermacher JD (1983). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab, 3, 1–7. [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, & Melchor JP (2007). Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med, 204, 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, & Cheresh DA (2001). Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med, 7, 222–227. [DOI] [PubMed] [Google Scholar]
- Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, Matsumoto M, Kohno M, & Nishiyama A (2011). Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens, 24, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegri C, Canudas AM, del Valle J, Casadesus G, Smith MA, Camins A, Pallas M & Vilaplana J (2007). Increased permeability of blood-brain barrier on the hippocampus of a murine model of senescence. Mech Ageing Dev. 128, 522–528. [DOI] [PubMed] [Google Scholar]
- Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, Rogaeva E, & Black SE (2008). Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol, 65, 790–795. [DOI] [PubMed] [Google Scholar]
- Petzold GC, & Murthy VN (2011). Role of astrocytes in neurovascular coupling. Neuron, 71, 782–797. [DOI] [PubMed] [Google Scholar]
- Picco A, Archetti S, Ferrara M, Arnaldi D, Piccini A, Serrati C, di Lorenzo D, Morbelli S, & Nobili F (2011). Seizures can precede cognitive symptoms in late-onset Alzheimer’s disease. J Alzheimers Dis, 27, 737–742. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Wengenack TM, Malester B, & Duff K (2001). Permeability of proteins at the blood-brain barrier in the normal adult mouse and double transgenic mouse model of Alzheimer’s disease. Neurobiol Dis, 8, 555–567. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Herrenknecht K, Kemler R, & Engel J (1994). Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem, 223, 1019–1026. [DOI] [PubMed] [Google Scholar]
- Poliakova T, Levin O, Arablinskiy A, Vasenina E, & Zerr I (2016). Cerebral microbleeds in early Alzheimer’s disease. J Neurol, 263, 1961–1968. [DOI] [PubMed] [Google Scholar]
- Poon C, Pellow C, & Hynynen K (2021). Neutrophil recruitment and leukocyte response following focused ultrasound and microbubble mediated blood-brain barrier treatments. Theranostics, 11, 1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JM, Sullivan L, & Rosenthal CJ (1982). Permanganate oxidation of senile cerebral amyloid and its relationship to AA protein. Acta Neuropathol, 58, 275–278. [DOI] [PubMed] [Google Scholar]
- Provias J, & Jeynes B (2008). Neurofibrillary tangles and senile plaques in Alzheimer’s brains are associated with reduced capillary expression of vascular endothelial growth factor and endothelial nitric oxide synthase. Curr Neurovasc Res, 5, 199–205. [DOI] [PubMed] [Google Scholar]
- Provias J, & Jeynes B (2011). Correlation analysis of capillary APOE, VEGF and eNOS expression in Alzheimer brains. Curr Alzheimer Res, 8, 197–202. [DOI] [PubMed] [Google Scholar]
- Provias J, & Jeynes B (2014). The role of the blood-brain barrier in the pathogenesis of senile plaques in Alzheimer’s disease. Int J Alzheimers Dis, 2014, 191863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ginsberg MH, Plow EF, & Ruoslahti E (1986). Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science, 231, 1559–1562. [DOI] [PubMed] [Google Scholar]
- Qosa H, Batarseh YS, Mohyeldin MM, El Sayed KA, Keller JN, & Kaddoumi A (2015). Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem Neurosci, 6, 1849–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Mohamed LA, Batarseh YS, Alqahtani S, Ibrahim B, LeVine H 3rd, Keller JN, & Kaddoumi A (2015). Extra-virgin olive oil attenuates amyloid-beta and tau pathologies in the brains of TgSwDI mice. J Nutr Biochem, 26, 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo AG, Teixeira CV, Magalhaes TN, Carletti-Cassani AFM, Amato Filho AC, Joaquim HP, Talib LL, Forlenza O, Ribeiro PA, Secolin R, Lopes-Cendes I, Cendes F, & Balthazar ML (2017). Is cerebral microbleed prevalence relevant as a biomarker in amnestic mild cognitive impairment and mild Alzheimer’s disease? Neuroradiol J, 30, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, & DeMattos RB (2005). Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci, 25, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha AA, Henderson JW, Stott SR, Vuono R, Foscarin S, Friedland RP, Zaman SH, & Raha-Chowdhury R (2017). Neuroprotective Effect of TREM-2 in Aging and Alzheimer’s Disease Model. J Alzheimers Dis, 55, 199–217. [DOI] [PubMed] [Google Scholar]
- Raja R, Rosenberg GA, & Caprihan A (2018). MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies. Neuropharmacology, 134, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralay Ranaivo H, Zunich SM, Choi N, Hodge JN, & Wainwright MS (2011). Mild stretch-induced injury increases susceptibility to interleukin-1beta-induced release of matrix metalloproteinase-9 from astrocytes. J Neurotrauma, 28, 1757–1766. [DOI] [PubMed] [Google Scholar]
- Rao SC, Dove G, Cascino GD, & Petersen RC (2009). Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy Behav, 14, 118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Damodarasamy M, Pathan JL, Chan CK, Spiekerman C, Wight TN, Banks WA, Day AJ, Vernon RB, & Keene CD (2019). Increased Hyaluronan and TSG-6 in Association with Neuropathologic Changes of Alzheimer’s Disease. J Alzheimers Dis, 67, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempe RG, Hartz AMS, & Bauer B (2016). Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab, 36, 1481–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempe RG, Hartz AMS, Soldner ELB, Sokola BS, Alluri SR, Abner EL, Kryscio RJ, Pekcec A, Schlichtiger J, & Bauer B (2018). Matrix Metalloproteinase-Mediated Blood-Brain Barrier Dysfunction in Epilepsy. J Neurosci, 38, 4301–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuck JD, Caparros-Lefebvre D, Deramecourt V, & Maurage CA (2011). Hippocampal microbleed on a post-mortem t(2) *-weighted gradient-echo 7.0-tesla magnetic resonance imaging? Case Rep Neurol, 3, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter B, Venus A, Grudzenski S, Heiler P, Schad L, Staufenbiel M, Hennerici MG, & Fatar M (2016). Statin Therapy and the Development of Cerebral Amyloid Angiopathy--A Rodent in Vivo Approach. Int J Mol Sci, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter B, Venus A, Heiler P, Schad L, Ebert A, Hennerici MG, Grudzenski S, & Fatar M (2016). Development of Cerebral Microbleeds in the APP23-Transgenic Mouse Model of Cerebral Amyloid Angiopathy-A 9.4 Tesla MRI Study. Front Aging Neurosci, 8, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E, van Gool WA, Hoozemans JJ, van Haastert ES, Eikelenboom P, Rozemuller AJ, & van de Berg WD (2010). Morphometric changes in the cortical microvascular network in Alzheimer’s disease. J Alzheimers Dis, 22, 811–818. [DOI] [PubMed] [Google Scholar]
- Ridwan S, Bauer H, Frauenknecht K, von Pein H, & Sommer CJ (2012). Distribution of granulocyte-monocyte colony-stimulating factor and its receptor alpha-subunit in the adult human brain with specific reference to Alzheimer’s disease. J Neural Transm (Vienna), 119, 1389–1406. [DOI] [PubMed] [Google Scholar]
- Rinne JO, & Nagren K (2010). Positron emission tomography in at risk patients and in the progression of mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis, 19, 291–300. [DOI] [PubMed] [Google Scholar]
- Robbins EM, Betensky RA, Domnitz SB, Purcell SM, Garcia-Alloza M, Greenberg C, Rebeck GW, Hyman BT, Greenberg SM, Frosch MP, & Bacskai BJ (2006). Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer disease. J Neurosci, 26, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LS, Gannon OJ, Thomas MA, Salinero AE, Abi-Ghanem C, Poitelon Y, Belin S, & Zuloaga KL (2020). Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer’s disease. J Neuroinflammation, 17, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues LL, Mesquita LP, Costa RC, Gomes RG, Biihrer DA, & Maiorka PC (2018). Multiple infarcts and hemorrhages in the central nervous system of a dog with cerebral amyloid angiopathy: a case report. BMC Vet Res, 14, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Baeza A, Reina-de la Torre F, Poca A, Marti M, & Garnacho A (2003). Morphological features in human cortical brain microvessels after head injury: a three-dimensional and immunocytochemical study. Anat Rec A Discov Mol Cell Evol Biol, 273, 583–593. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Perdigon M, Solas M, & Ramirez MJ (2016). JNK: A Putative Link Between Insulin Signaling and VGLUT1 in Alzheimer’s Disease. J Alzheimers Dis, 50, 963–967. [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, & Emmerling MR (1996). Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem, 271, 20631–20635. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, & Sabbagh MN (2009). Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement, 5, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Chavez-Castillo M, Bautista J, Ortega A, Nava M, Salazar J, Diaz-Camargo E, Medina O, Rojas-Quintero J, & Bermudez V (2021). Alzheimer’s disease and type 2 diabetes mellitus: Pathophysiologic and pharmacotherapeutics links. World J Diabetes, 12, 745–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli MF, Morris JC, Ashkin K, & Coben LA (1990). Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Arch Neurol, 47, 847–850. [DOI] [PubMed] [Google Scholar]
- Romanitan MO, Popescu BO, Winblad B, Bajenaru OA, & Bogdanovic N (2007). Occludin is overexpressed in Alzheimer’s disease and vascular dementia. J Cell Mol Med, 11, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler N, Wichart I, & Jellinger KA (2001). Clinical significance of neurobiochemical profiles in the lumbar cerebrospinal fluid of Alzheimer’s disease patients. J Neural Transm (Vienna), 108, 231–246. [DOI] [PubMed] [Google Scholar]
- Rothschild MA, Oratz M, & Schreiber SS (1980). Albumin synthesis. Int Rev Physiol, 21, 249–274. [PubMed] [Google Scholar]
- Royea J, Martinot P, & Hamel E (2020). Memory and cerebrovascular deficits recovered following angiotensin IV intervention in a mouse model of Alzheimer’s disease. Neurobiol Dis, 134, 104644. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, & Carmeliet P (2009). Role and therapeutic potential of VEGF in the nervous system. Physiol Rev, 89, 607–648. [DOI] [PubMed] [Google Scholar]
- Ryu JK, & McLarnon JG (2009). A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med, 13, 2911–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN, Lue LF, Fayard D, & Shi J (2017). Increasing Precision of Clinical Diagnosis of Alzheimer’s Disease Using a Combined Algorithm Incorporating Clinical and Novel Biomarker Data. Neurol Ther, 6, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Buciak J, Yang J, & Pardridge WM (1995). Vector-mediated delivery of 125I-labeled beta-amyloid peptide A beta 1–40 through the blood-brain barrier and binding to Alzheimer disease amyloid of the A beta 1–40/vector complex. Proc Natl Acad Sci U S A, 92, 10227–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, & Cummings J (2021). Aducanumab, Amyloid Lowering, and Slowing of Alzheimer Disease. Neurology, 97, 543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Gur T, Berzin T, Tavares R, Zipser B, Correia S, Hovanesian V, Fallon J, Kuo-Leblanc V, Glass D, Hulette C, Rosenberg C, Vitek M, & Stopa E (2002). Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J Neurol Sci, 203–204, 183–187. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab, & Clinical Trial, I. (2014). Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med, 370, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M, & Bapineuzumab 201 Clinical Trial, I. (2009). A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology, 73, 2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarelius IH, & Glading AJ (2015). Control of vascular permeability by adhesion molecules. Tissue Barriers, 3, e985954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis RA, Thome-Souza S, Poh MZ, Llewellyn N, Klehm J, Madsen JR, Picard R, Pennell PB, Dworetzky BA, Loddenkemper T, & Reinsberger C (2015). Autonomic changes following generalized tonic clonic seizures: An analysis of adult and pediatric patients with epilepsy. Epilepsy Res, 115, 113–118. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Fries JW, Raines EW, Gown AM, Westrum LE, Frosch MP, Bonthron DT, Ross R, & Collins T (1991). PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell, 64, 217–227. [DOI] [PubMed] [Google Scholar]
- Sakka L, Coll G, & Chazal J (2011). Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolrygol Head Neck Dis, 128, 308–316. [DOI] [PubMed] [Google Scholar]
- Satoh J, Tabunoki H, Ishida T, Saito Y, & Arima K (2013). Accumulation of a repulsive axonal guidance molecule RGMa in amyloid plaques: a possible hallmark of regenerative failure in Alzheimer’s disease brains. Neuropathol Appl Neurobiol, 39, 109–120. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Dziegielewska KM, Mollgard K, & Habgood MD (2015). Markers for blood-brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci, 9, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, & Schaefer RM (2010). Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res, 339, 237–246. [DOI] [PubMed] [Google Scholar]
- Scheibel AB (1987). Alterations of the cerebral capillary bed in the senile dementia of Alzheimer. Ital J Neurol Sci, 8, 457–463. [DOI] [PubMed] [Google Scholar]
- Scheibel AB, & Duong T (1988). On the possible relationship of cortical microvascular pathology to blood brain barrier changes in Alzheimer’s disease. Neurobiol Aging, 9, 41–42. [DOI] [PubMed] [Google Scholar]
- Scheibel AB, Duong TH, & Tomiyasu U (1987). Denervation microangiopathy in senile dementia, Alzheimer type. Alzheimer Dis Assoc Disord, 1, 19–37. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, & Younkin S (1996). Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med, 2, 864–870. [DOI] [PubMed] [Google Scholar]
- Schlageter NL, Carson RE, & Rapoport SI (1987). Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab, 7, 1–8. [DOI] [PubMed] [Google Scholar]
- Schliep G, & Felgenhauer K (1978). Serum-CSF protein gradients, the blood-GSF barrier and the local immune response. J Neurol, 218, 77–96. [DOI] [PubMed] [Google Scholar]
- Scholz W (1938). Studien zur Pathologie der Hirngefäße. II. Die drusige Entartung der Hirnarterien und Capillaren (eine Form seniler Gefäßerkrankung). Z Gesamte Neurol Psychiatr, 162, 694–715. [Google Scholar]
- Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, & Games D (2008). Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J Neurosci, 28, 6787–6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss C, Blechert B, Gaertner FC, Drecoll E, Mueller J, Weber GF, Drzezga A, & Essler M (2006). In vivo characterization of endothelial cell activation in a transgenic mouse model of Alzheimer’s disease. Angiogenesis, 9, 59–65. [DOI] [PubMed] [Google Scholar]
- Schultz N, Brannstrom K, Byman E, Moussaud S, Nielsen HM, Netherlands Brain B, Olofsson A, & Wennstrom M (2018). Amyloid-beta 1–40 is associated with alterations in NG2+ pericyte population ex vivo and in vitro. Aging Cell, 17, e12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz N, Nielsen HM, Minthon L, & Wennstrom M (2014). Involvement of matrix metalloproteinase-9 in amyloid-beta 1–42-induced shedding of the pericyte proteoglycan NG2. J Neuropathol Exp Neurol, 73, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy JL, Le Bihan T, Salvadores N, McCulloch J, & Horsburgh K (2014). Impact of age on the cerebrovascular proteomes of wild-type and Tg-SwDI mice. PLoS One, 9, e89970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, & Zlokovic BV (2013). Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol, 23, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehry AA, Rauscher A, Hsiung GY, & Lang DJ (2016). Microbleeds in Alzheimer’s Disease: A Neuropsychological Overview and Meta-Analysis. Can J Neurol Sci, 43, 753–759. [DOI] [PubMed] [Google Scholar]
- Seubert P, Barbour R, Khan K, Motter R, Tang P, Kholodenko D, Kling K, Schenk D, Johnson-Wood K, Schroeter S, Gill D, Jacobsen JS, Pangalos M, Basi G, & Games D (2008). Antibody capture of soluble Abeta does not reduce cortical Abeta amyloidosis in the PDAPP mouse. Neurodegener Dis, 5, 65–71. [DOI] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, & Sandrock A (2016). The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature, 537, 50–56. [DOI] [PubMed] [Google Scholar]
- Shabir O, Sharp P, Rebollar MA, Boorman L, Howarth C, Wharton SB, Francis SE, & Berwick J (2020). Enhanced Cerebral Blood Volume under Normobaric Hyperoxia in the J20-hAPP Mouse Model of Alzheimer’s Disease. Sci Rep, 10, 7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, Cavallin L, Aspelin P, Kristoffersen-Wiberg M, & Wahlund LO (2015). Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis-the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol, 36, 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Yamashita T, Tian F, Li X, Liu X, Shi X, Nakano Y, Tsunoda K, Nomura E, Sasaki R, Tadokoro K, Sato K, Takemoto M, Hishikawa N, Ohta Y, & Abe K (2019). Chronic cerebral hypoperfusion alters amyloid-beta transport related proteins in the cortical blood vessels of Alzheimer’s disease model mouse. Brain Res, 1723, 146379. [DOI] [PubMed] [Google Scholar]
- Shang J, Yamashita T, Zhai Y, Nakano Y, Morihara R, Fukui Y, Hishikawa N, Ohta Y, & Abe K (2016). Strong Impact of Chronic Cerebral Hypoperfusion on Neurovascular Unit, Cerebrovascular Remodeling, and Neurovascular Trophic Coupling in Alzheimer’s Disease Model Mouse. J Alzheimers Dis, 52, 113–126. [DOI] [PubMed] [Google Scholar]
- Shang S, Yang YM, Zhang H, Tian L, Jiang JS, Dong YB, Zhang K, Li B, Zhao WD, Fang WG, & Chen YH (2016). Intracerebral GM-CSF contributes to transendothelial monocyte migration in APP/PS1 Alzheimer’s disease mice. J Cereb Blood Flow Metab, 36, 1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PS, Ameen-Ali KE, Boorman L, Harris S, Wharton S, Howarth C, Shabir O, Redgrave P, & Berwick J (2019). Neurovascular coupling preserved in a chronic mouse model of Alzheimer’s disease: Methodology is critical. J Cereb Blood Flow Metab, 271678X19890830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Ohta Y, Liu X, Shang J, Morihara R, Nakano Y, Feng T, Huang Y, Sato K, Takemoto M, Hishikawa N, Yamashita T, & Abe K (2019). Chronic Cerebral Hypoperfusion Activates the Coagulation and Complement Cascades in Alzheimer’s Disease Mice. Neuroscience, 416, 126–136. [DOI] [PubMed] [Google Scholar]
- Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, & Ayata C (2007). Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain, 130, 2310–2319. [DOI] [PubMed] [Google Scholar]
- Singh PK, Chen ZL, Ghosh D, Strickland S, & Norris EH (2020). Increased plasma bradykinin level is associated with cognitive impairment in Alzheimer’s patients. Neurobiol Dis, 139, 104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, & Price DL (1990). Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science, 248, 492–495. [DOI] [PubMed] [Google Scholar]
- Sjögren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, Granérus AK, Vanderstichele H, Vanmechelen E, & Blennow K (2001). Both total and phosphorylated tau are increased in Alzheimer’s disease. J Neurol Neurosurg Psychiatry, 70, 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren M, Davidsson P, Wallin A, Granérus AK, Grundström E, Askmark H, Vanmechelen E, & Blennow K (2002). Decreased CSF-beta-amyloid 42 in Alzheimer’s disease and amyotrophic lateral sclerosis may reflect mismetabolism of beta-amyloid induced by disparate mechanisms. Dement Geriatr Cogn Disord, 13, 112–118. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Gustafson L, Wikkelso C, & Wallin A (2000). Frontotemporal dementia can be distinguished from Alzheimer’s disease and subcortical white matter dementia by an anterior-to-posterior rCBF-SPET ratio. Dement Geriatr Cogn Disord, 11, 275–285. [DOI] [PubMed] [Google Scholar]
- Skillback T, Delsing L, Synnergren J, Mattsson N, Janelidze S, Nagga K, Kilander L, Hicks R, Wimo A, Winblad B, Hansson O, Blennow K, Eriksdotter M, & Zetterberg H (2017). CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol Aging, 59, 1–9. [DOI] [PubMed] [Google Scholar]
- Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, & Blennow K (1998). A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology, 50, 966–971. [DOI] [PubMed] [Google Scholar]
- Smirnova IV, Salazar A, Arnold PM, Glatt S, Handler M, & Festoff BW (1997). Thrombin and its precursor in human cerebrospinal fluid. Thromb Haemost, 78, 1473–1479. [PubMed] [Google Scholar]
- Smith EB (1986). Fibrinogen, fibrin and fibrin degradation products in relation to atherosclerosis. Clin Haematol, 15, 355–370. [PubMed] [Google Scholar]
- Smith EE, & Greenberg SM (2009). Beta-amyloid, blood vessels, and brain function. Stroke, 40, 2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Choi SM, Whitcomb DJ, & Kim BC (2017). Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis, 8, e3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Pan KY, Xu H, Qi X, Buchman AS, Bennett DA, & Xu W (2021). Association of cardiovascular risk burden with risk of dementia and brain pathologies: A population-based cohort study. Alzheimers Dement, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, Zhang Y, Spevak W, Lin J, Phan NY, Habets G, Rymar A, Tsang G, Walters J, Nespi M, Singh P, Broome S, Ibrahim P, Zhang C, Bollag G, West BL, & Green KN (2019). Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat Commun, 10, 3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparacia G, Agnello F, La Tona G, Iaia A, Midiri F, & Sparacia B (2017). Assessment of cerebral microbleeds by susceptibility-weighted imaging in Alzheimer’s disease patients: A neuroimaging biomarker of the disease. Neuroradiol J, 30, 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Amour I, Pare I, Alata W, Coulombe K, Ringuette-Goulet C, Drouin-Ouellet J, Vandal M, Soulet D, Bazin R, & Calon F (2013). Brain bioavailability of human intravenous immunoglobulin and its transport through the murine blood-brain barrier. J Cereb Blood Flow Metab, 33, 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, Farrall AJ, Armitage P, McGurn B, & Wardlaw J (2009). Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res, 171, 232–241. [DOI] [PubMed] [Google Scholar]
- Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, & Marshall I (2003). Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry, 74, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA, Hayakawa K, Akers MA, & Vinters HV (1992). A morphometric study of the blood-brain barrier in Alzheimer’s disease. Lab Invest, 67, 734–742. [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, & Sommer B (1997). Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A, 94, 13287–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Liu M, Nguyen XV, & Bing G (2003). P38 MAP kinase is activated at early stages in Alzheimer’s disease brain. Exp Neurol, 183, 394–405. [DOI] [PubMed] [Google Scholar]
- Sundelöf J, Sundström J, Hansson O, Eriksdotter-Jönhagen M, Giedraitis V, Larsson A, Degerman-Gunnarsson M, Ingelsson M, Minthon L, Blennow K, Kilander L, Basun H, & Lannfelt L (2010). Higher cathepsin B levels in plasma in Alzheimer’s disease compared to healthy controls. J Alzheimers Dis, 22, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, & Zlokovic BV (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol, 14, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, Bertrand E, Pasennik E, Mendel T, Stepien T, Leszczynska A, & Rafalowska J (2007). Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL. Immunohistochemical and ultrastructural studies. Folia Neuropathol, 45, 192–204. [PubMed] [Google Scholar]
- Takane K, Hasegawa Y, Lin B, Koibuchi N, Cao C, Yokoo T, & Kim-Mitsuyama S (2017). Detrimental Effects of Centrally Administered Angiotensin II are Enhanced in a Mouse Model of Alzheimer Disease Independently of Blood Pressure. J Am Heart Assoc, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, & Morishita R (2009). Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension, 54, 1345–1352. [DOI] [PubMed] [Google Scholar]
- Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, & Hayes JD (2015). Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med, 88, 108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD (1963). The Fine Structure of Neurofibrillary Tangles in Alzheimer’s Disease. J Neuropathol Exp Neurol, 22, 629–642. [DOI] [PubMed] [Google Scholar]
- Terry RD, Gonatas NK, & Weiss M (1964). Ultrastructural Studies in Alzheimer’s Presenile Dementia. Am J Pathol, 44, 269–297. [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, & Katzman R (1991). Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol, 30, 572–580. [DOI] [PubMed] [Google Scholar]
- Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Van Haesendonck C, Borghgraef P, & Van Leuven F (2005). Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem, 280, 3963–3973. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Weatherspoon MR, Harrison J, Keene TE, Lane DS, Kaemmerer WF, Stewart GR, & Shafer LL (2009). Intracerebroventricular amyloid-beta antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proc Natl Acad Sci U S A, 106, 4501–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, & Braak H (2008). Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J Cell Mol Med, 12, 1848–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therapeutics. In. (Vol. 2021): ALZFORUM Database. [Google Scholar]
- Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, & Grammas P (2006). Angiogenic proteins are expressed by brain blood vessels in Alzheimer’s disease. J Alzheimers Dis, 10, 111–118. [DOI] [PubMed] [Google Scholar]
- Thomas T, Miners S, & Love S (2015). Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer’s disease and vascular dementia. Brain, 138, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Routhe LJ, & Moos T (2017). The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab, 37, 3300–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibolla G, Norata GD, Meda C, Arnaboldi L, Uboldi P, Piazza F, Ferrarese C, Corsini A, Maggi A, Vegeto E, & Catapano AL (2010). Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis, 210, 78–87. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, & Weisskoff RM (1999). Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging, 10, 223–232. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, & Kimura J (1996). Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke, 27, 2069–2074. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Roberts AM, Dean WL, Tyagi SC, & Lominadze D (2008). Fibrinogen induces endothelial cell permeability. Mol Cell Biochem, 307, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekawa K, Hasegawa Y, Senju S, Nakagata N, Ma M, Nakagawa T, Koibuchi N, & Kim-Mitsuyama S (2016). Intracerebroventricular Infusion of Angiotensin-(1–7) Ameliorates Cognitive Impairment and Memory Dysfunction in a Mouse Model of Alzheimer’s Disease. J Alzheimers Dis, 53, 127–133. [DOI] [PubMed] [Google Scholar]
- Ueno M, Akiguchi I, Hosokawa M, Shinnou M, Sakamoto H, Takemura M, & Higuchi K (1997). Age-related changes in the brain transfer of blood-borne horseradish peroxidase in the hippocampus of senescence-accelerated mouse. Acta Neuropathol, 93, 233–240. [DOI] [PubMed] [Google Scholar]
- Ueno M, Akiguchi I, Tagi H, Naiki H, Fujiayashi Y, Kimura J, & Takeda T (1993). Age-related changes in barrier function in mouse brain I. Accelerated age-related increase of brain transfer of serum albumin in accelerated senescence prone SAM-P/8 mice with deficits in learning and memory. Arch Gerontol Geriatr, 16, 233–248. [DOI] [PubMed] [Google Scholar]
- Ueno M, Sakamoto H, Kanenishi K, Onodera M, Akiguchi I, & Hosokawa M (2001). Ultrastructural and permeability features of microvessels in the periventricular area of senescence-accelerated mice (SAM). Microsc Res Tech, 53, 232–238. [DOI] [PubMed] [Google Scholar]
- Uetani H, Hirai T, Hashimoto M, Ikeda M, Kitajima M, Sakamoto F, Utsunomiya D, Oda S, Sugiyama S, Matsubara J, & Yamashita Y (2013). Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am J Neuroradiol, 34, 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, & Petit E (2005). VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab, 25, 1491–1504. [DOI] [PubMed] [Google Scholar]
- Van Beek AH, & Claassen JA (2011). The cerebrovascular role of the cholinergic neural system in Alzheimer’s disease. Behav Brain Res, 221, 537–542. [DOI] [PubMed] [Google Scholar]
- Van Dam D, D’Hooge R, Staufenbiel M, Van Ginneken C, Van Meir F, & De Deyn PP (2003). Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci, 17, 388–396. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Burgmans S, Jansen JF, van Osch MJ, van Buchem MA, Muller M, Hofman PA, Verhey FR, & Backes WH (2016). Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology, 281, 527–535. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Jansen JFA, Jeukens C, Burgmans S, van Buchem MA, Muller M, Hofman PAM, Verhey FRJ, van Osch MJP, & Backes WH (2017). Subtle blood-brain barrier leakage rate and spatial extent: Considerations for dynamic contrast-enhanced MRI. Med Phys, 44, 4112–4125. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Wong SM, Hofman PAM, Burgmans S, Verhey FRJ, & Backes WH (2016). Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging, 45, 190–196. [DOI] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, & Van Leuven F (2000). Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. Am J Pathol, 157, 1283–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck CH (2018). Anti-Amyloid-beta Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol Psychiatry, 83, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen J, Otte-Holler I, David G, Maat-Schieman ML, van den Heuvel LP, Wesseling P, de Waal RM, & Verbeek MM (2001). Heparan sulfate proteoglycan expression in cerebrovascular amyloid beta deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol, 102, 604–614. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, & Breteler MM (2005). Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke, 36, 2637–2641. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, & Galvan V (2019). mTOR in cerebrovascular disease. Aging (Albany NY), 11, 1331–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, & Galvan V (2018). Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol, 314, H693–H703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vickle GD, Esh CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, Walker DG, Lue LF, Beach TG, Davis J, Van Nostrand WE, Castano EM, & Roher AE (2008). Tg-SwDI transgenic mice exhibit novel alterations in AbetaPP processing, Abeta degradation, and resilient amyloid angiopathy. Am J Pathol, 173, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, de Vries HE, Aronica E, & Gorter JA (2012). Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia, 53, 1254–1263. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, de Vries HE, Dijkhuizen RM, & Gorter JA (2016). Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats II: Potential mechanisms. Epilepsia, 57, 70–78. [DOI] [PubMed] [Google Scholar]
- VandeVrede L, Gibbs DM, Koestler M, La Joie R, Ljubenkov PA, Provost K, Soleimani-Meigooni D, Strom A, Tsoy E, Rabinovici GD, & Boxer AL (2020). Symptomatic amyloid-related imaging abnormalities in an APOE epsilon4/epsilon4 patient treated with aducanumab. Alzheimers Dement (Amst), 12, e12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevko V, Xu F, Previti ML, Van Nostrand WE, & Cribbs DH (2007). Experimental investigation of antibody-mediated clearance mechanisms of amyloid-beta in CNS of Tg-SwDI transgenic mice. J Neurosci, 27, 13376–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild E, Palm WM, Postma AA, Jansen JFA, Verhey FRJ, & Backes WH (2020). Increase in blood-brain barrier leakage in healthy, older adults. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, & Breteler MM (2008). Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology, 70, 1208–1214. [DOI] [PubMed] [Google Scholar]
- Vezzani A, & Janigro D (2009). Leukocyte-endothelial adhesion mechanisms in epilepsy: cheers and jeers. Epilepsy Curr, 9, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggars AP, Wharton SB, Simpson JE, Matthews FE, Brayne C, Savva GM, Garwood C, Drew D, Shaw PJ, & Ince PG (2011). Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer-type pathology: a study in the MRC-CFAS population neuropathology cohort. Neurosci Lett, 505, 2530. [DOI] [PubMed] [Google Scholar]
- Vinters HV (1987). Cerebral amyloid angiopathy. A critical review. Stroke, 18, 311–324. [DOI] [PubMed] [Google Scholar]
- Vogels T, Murgoci AN, & Hromadka T (2019). Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Commun, 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, Kostner R, Molgaard M, Hass S, & Jensen MS (2010). Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1deltaE9deleted transgenic mice model of ss-amyloidosis. Neurobiol Aging, 31, 1173–1187. [DOI] [PubMed] [Google Scholar]
- Volicer L, Smith S, & Volicer BJ (1995). Effect of seizures on progression of dementia of the Alzheimer type. Dementia, 6, 258–263. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, & Mucke L (2013). Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol, 70, 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF, Darwish SM, Van Berlo V, Barnes DE, Mantle M, Karydas AM, Coppola G, Roberson ED, Miller BL, Garcia PA, Kirsch HE, Mucke L, & Nagarajan SS (2016). Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol, 80, 858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, & Miller BL (2017). Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol, 16, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H (1998). Blood-brain barrier permeability of the demented elderly as studied by cerebrospinal fluid-serum albumin ratio. Intern Med, 37, 509–513. [DOI] [PubMed] [Google Scholar]
- Wan W, Chen H, & Li Y (2014). The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer’s disease. Int J Neurosci, 124, 75–81. [DOI] [PubMed] [Google Scholar]
- Wang D, Chen F, Han Z, Yin Z, Ge X, & Lei P (2021). Relationship Between Amyloid-beta Deposition and Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Front Cell Neurosci. 15. 695479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Golob EJ, & Su MY (2006). Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging, 24, 695–700. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, & Suzumura A (2014). Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One, 9, e110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Araki W, Chui DH, Makifuchi T, Ihara Y, & Tabira T (2004). Glypican-1 as an Abeta binding HSPG in the human brain: its localization in DIG domains and possible roles in the pathogenesis of Alzheimer’s disease. FASEB J, 18, 1013–1015. [DOI] [PubMed] [Google Scholar]
- Weber-Adrian D, Kofoed RH, Chan JWY, Silburt J, Noroozian Z, Kugler S, Hynynen K, & Aubert I (2019). Strategy to enhance transgene expression in proximity of amyloid plaques in a mouse model of Alzheimer’s disease. Theranostics, 9, 8127–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, & Gall CM (1995). Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J Neurosci, 15, 2906–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, & Cheresh DA (2005). Pathophysiological consequences of VEGF-induced vascular permeability. Nature, 437, 497–504. [DOI] [PubMed] [Google Scholar]
- Weissberg I, Reichert A, Heinemann U, & Friedman A (2011). Blood-brain barrier dysfunction in epileptogenesis of the temporal lobe. Epilepsy Res Treat, 2011, 143908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, Oppenheim H, Ardizzone C, Becker A, Frigerio F, Vezzani A, Buckwalter MS, Huguenard JR, Friedman A, & Kaufer D (2015). Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis, 78, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, & Ashe KH (2002). The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci, 22, 1858–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Vitek MP, & Colton CA (2009). Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience, 159, 1055–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, Otte-Holler I, van Triel JJ, Veerhuis R, Maat-Schieman ML, Bu G, de Waal RM, & Verbeek MM (2007). Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol, 171, 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, Tolnay M, Staufenbiel M, & Jucker M (2001). Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci, 21, 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, Clark IA, Abdipranoto A, & Vissel B (2013). Neuroinflammation and neuronal loss precede Abeta plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS One, 8, e59586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhima K, Markham-Coultes K, Nedev H, Heinen S, Saragovi HU, Hynynen K, & Aubert I (2020). Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer’s disease. Sci Adv, 6, eaax6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao NA, Zhang J, Zhou M, Wei Z, Wu XL, Dai XM, Zhu YG, & Chen XC (2015). Reduction of Glucose Metabolism in Olfactory Bulb is an Earlier Alzheimer’s Disease-related Biomarker in 5XFAD Mice. Chin Med J (Engl), 128, 2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yan L, Zeng H, Chen W, Lu JH, Wan JB, Su H, & Yao X (2020). Fish oil protects the blood-brain barrier integrity in a mouse model of Alzheimer’s disease. Chin Med, 15, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R, Zhou XG, Tang Y, Wu JM, Sun YS, Teng JF, Pan R, Law BY, Zhao Y, Qiu WQ, Wang XL, Liu S, Wang YL, Yu L, Yu CL, Mei QB, Qin DL, & Wu AG (2021). Lychee seed polyphenol protects the blood-brain barrier through inhibiting Abeta(25–35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice. Phytother Res, 35, 954–973. [DOI] [PubMed] [Google Scholar]
- Xu F, Grande AM, Robinson JK, Previti ML, Vasek M, Davis J, & Van Nostrand WE (2007). Early-onset subicular microvascular amyloid and neuroinflammation correlate with behavioral deficits in vasculotropic mutant amyloid beta-protein precursor transgenic mice. Neuroscience, 146, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nirwane A, & Yao Y (2019). Basement membrane and blood-brain barrier. Stroke Vasc Neurol, 4, 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Feng J, Tang M, Guo Q, Zhan J, Zhu F, Lei H, & Kang Q (2020). Blocking retrograde axonal transport of autophagosomes contributes to sevoflurane-induced neuron apoptosis in APP/PS1 mice. Acta Neurol Belg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Cai X, Li W, Zhang Z, Dong W, & Hui G (2012). Elevated plasma endothelial microparticles in Alzheimer’s disease. Dement Geriatr Cogn Disord, 34, 174–180. [DOI] [PubMed] [Google Scholar]
- Yagi H, Katoh S, Akiguchi I, & Takeda T (1988). Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res, 474, 86–93. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Miyakawa T, & Katsuragi S (1991). Vascular changes in the brains with Alzheimer’s disease. Jpn J Psychiatry Neurol, 45, 79–84. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Shinohara M, Shinohara M, Yamazaki A, Murray ME, Liesinger AM, Heckman MG, Lesser ER, Parisi JE, Petersen RC, Dickson DW, Kanekiyo T, & Bu G (2019). Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain, 142, 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Liu XH, Han MZ, Wang YM, Sun XL, Yu N, Li T, Su B, & Chen ZY (2015). Blockage of GSK3beta-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer’s disease. Neurobiol Aging, 36, 211–227. [DOI] [PubMed] [Google Scholar]
- Yan P, Zhu A, Liao F, Xiao Q, Kraft A, Gonzales E, Perez R, Greenberg SM, Holtzman D, & Lee JM (2015). Minocycline reduces spontaneous hemorrhage in mouse models of cerebral amyloid angiopathy. Stroke, 46, 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zhao K, Zhang X, Zhang J, & Xu B (2016). ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro. Neural Plast, 2016, 8928530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, & Rowe CC (2014). Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology, 82, 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Tsukamoto T, Sun A, & Nigam SK (1999). A role for intracellular calcium in tight junction reassembly after ATP depletion-repletion. Am J Physiol, 277, F524–532. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Liu X, Xian YF, Tang Y, Zou J, Zhang X, Huang P, Wu W, Song YQ, & Lin ZX (2020). Origins of Beta Amyloid Differ Between Vascular Amyloid Deposition and Parenchymal Amyloid Plaques in the Spinal Cord of a Mouse Model of Alzheimer’s Disease. Mol Neurobiol, 57, 278–289. [DOI] [PubMed] [Google Scholar]
- Zammit AR, Katz MJ, Bitzer M, & Lipton RB (2016). Cognitive Impairment and Dementia in Older Adults With Chronic Kidney Disease: A Review. Alzheimer Dis Assoc Disord, 30, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nani S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, & Constantin G (2015). Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA1 integrin. Nat Med, 21, 880–886. [DOI] [PubMed] [Google Scholar]
- Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, & Iliff JJ (2017). Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol, 74, 91–99. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kook S, Kaoud TS, Dalby KN, Gurevich EV, & Gurevich VV (2015). Arrestin-3-Dependent Activation of c-Jun N-Terminal Kinases (JNKs). Curr Protoc Pharmacol, 68, 2 12 11–12 12 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JB, Li MF, Zhang HX, Li ZG, Sun HR, Zhang JS, & Wang PF (2016). Association of serum vascular endothelial growth factor levels and cerebral microbleeds in patients with Alzheimer’s disease. Eur J Neurol, 23, 1337–1342. [DOI] [PubMed] [Google Scholar]
- Zhang WW, Badonic T, Hoog A, Jiang MH, Ma KC, Nie XJ, & Olsson Y (1994). Astrocytes in Alzheimer’s disease express immunoreactivity to the vaso-constrictor endothelin-1. J Neurol Sci, 122, 90–96. [DOI] [PubMed] [Google Scholar]
- Zhao Y, & Gong CX (2015). From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration. Cell Mol Neurobiol, 35, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Shi SX, Liu N, Jiang Y, Karim MS, Vodovoz SJ, Wang X, Zhang B, & Dumont AS (2021). Caveolae-Mediated Endothelial Transcytosis across the Blood-Brain Barrier in Acute Ischemic Stroke. J Clin Med, 10, 3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, & Smith MA (2001). Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem, 76, 435–441. [DOI] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, & Stopa EG (2007). Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging, 28, 977–986. [DOI] [PubMed] [Google Scholar]