Abstract
Strain selection and improvement in the baker’s yeast industry have aimed to increase the speed of maltose fermentation in order to increase the leavening activity of industrial baking yeast. We identified two groups of baker’s strains of Saccharomyces cerevisiae that can be distinguished by the mode of regulation of maltose utilization. One group (nonlagging strains), characterized by rapid maltose fermentation, had at least 12-fold more maltase and 130-fold-higher maltose permease activities than maltose-lagging strains in the absence of inducing sugar (maltose) and repressing sugar (glucose). Increasing the noninduced maltase activity of a lagging strain 13-fold led to an increase in CO2 production in unsugared dough. This increase in CO2 production also was seen when the maltose permease activity was increased 55-fold. Only when maltase and maltose permease activities were increased in concert was CO2 production by a lagging strain similar to that of a nonlagging strain. The noninduced activities of maltase and maltose permease constitute the largest determinant of whether a strain displays a nonlagging or a lagging phenotype and are dependent upon the MALx3 allele. Previous strategies for strain improvement have targeted glucose derepression of maltase and maltose permease expression. Our results suggest that increasing noninduced maltase and maltose permease levels is an important target for improved maltose metabolism in unsugared dough.
Yeast cells need any one of five unlinked maltose (MAL) loci (MAL1 through MAL4 and MAL6) (8, 27) in order to utilize maltose. Each locus consists of a MALx1 (MALxT) (where x is the locus) gene, encoding maltose permease (9), a MALx2 (MALxS) gene, coding for α-glucosidase (maltase) (10), and a MALx3 (MALxR) gene, encoding a positive regulatory protein (28). The MALx1 and MALx2 genes are divergently transcribed from a bidirectional promoter (MAL intergenic region), and the MALx3 regulatory protein interacts with upstream activating sequences in the MAL intergenic region, inducing transcription in the presence of maltose (21). Expression from native MAL loci is maltose induced (inducing conditions), is glucose repressed (repressing conditions), and has a low basal level of expression in the presence of galactose or ethanol (noninducing conditions) (28).
Industrial yeasts are usually polyploid strains of Saccharomyces cerevisiae or closely related species (13). The ability to utilize maltose, and therefore the regulation of the MAL system, is a key factor in many commercial applications, such as baking, brewing, and distilling (4, 6, 31). In a dough consisting of flour, water, yeast, and salt (unsugared or plain dough), the most abundant available sugar is maltose, produced by the action of amylases on damaged starch (4, 33). Some industrial strains are inoculated into unsugared (plain) dough with low MAL activity (maltase and maltose permease enzymes) and have an undesirable decrease in the 2nd-h gassing rate (30). These strains are termed lagging strains. Others, known as nonlagging strains, maintain a high gassing rate in the 2nd h of leavening. Often, lagging strains have other phenotypes that are desirable, such as good gassing ability in high-sugar dough or stability upon storage. Sexual crosses of nonlagging and lagging strains may not yield progeny with all of the desirable traits.
Before recombinant DNA techniques can be used to alter the lagging phenotype of industrial yeast, detailed genetic and biochemical analyses of industrial strains are needed. The MAL loci, maltose utilization phenotypes, and expression of maltase and maltose permease proteins in laboratory strains of yeast (7, 11, 26, 41) have been characterized extensively; however, little is known about the genetic basis of the nonlagging phenotype in industrial strains. Much remains to be learned about the genetic makeup of industrial strains in order to take full advantage of recombinant DNA techniques for strain improvement (1, 36).
Our working hypothesis is that CO2 production in unsugared dough correlates with MAL activity in industrial strains used for commercial applications. Our objectives were (i) to determine if there are any major differences in regulation of the MAL system between lagging and nonlagging strains and (ii) to identify differences in genetic background that are involved in the nonlagging phenotype of industrial strains.
MATERIALS AND METHODS
Strains and plasmids.
Industrial baker’s strains NL67, NL25, NL89, L38, L83, and L05 were obtained from Burns Philp & Co. Ltd., North Ryde, Sydney, Australia. Strain RMS-14A (MATa trp1 his4 mal0 suc0) (37), which lacks a functional MALx3 gene, was transformed with a construct that has MALx2 and MALx1 structural genes replaced by the marker genes MEL1 and lacZ, respectively, producing strain PB1 (2). Escherichia coli XL1 Blue recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIq ZΔM15 Tn10 (Tetr)] from Stratagene (La Jolla, Calif.) was used for cloning and plasmid propagation. The shuttle vector pBEJ17 (16), with G418 resistance as a dominant marker, was used to introduce MALx3 genes into the baker’s yeast strains. YRp7 (39) was used for the tryptophan auxotrophic strain PB1.
Media and culture conditions.
Bacteria were grown on Luria-Bertani medium (1% peptone, 0.5% yeast extract, 1% NaCl) in the presence of ampicillin (50 μg ml−1) for selection. PB1 Trp+ transformants were grown on minimal medium (0.67% yeast nitrogen base without amino acids, supplemented with all auxotrophic requirements except tryptophan) to maintain YRp7 plasmids. For Northern analysis and enzyme assays, industrial baker’s yeast strains were grown on YP-based medium (0.5% yeast extract, 1% peptone, 0.3% KH2PO4) with or without 220 μg of Geneticin (G418 sulfate; Gibco BRL) ml−1. Fermentable sugars and ethanol were added to a concentration of 2% (wt/vol). Cultures were incubated at 30°C on a platform shaker (200 rpm) and harvested at an optical density at 640 nm of 0.3 to 0.35. Yeast cells tested for gas production were grown in a 100-ml seed culture of YP-based medium which contained 1% sucrose with or without 220 μg of G418 sulfate ml−1. This seed culture was used to inoculate 1 liter of the same media in baffled 2-liter Erlenmeyer flasks. Both cultures were incubated at 30°C on a platform shaker (200 rpm) until late-respiratory phase, when the ethanol level in the medium was between 0.10 and 0.02% (wt/vol). Cultured cells were harvested, washed twice with 0.35 M NaCl, and collected on Whatman paper (no. 3 chromatography) for 30 min. Previously described methods were used to measure gas production in bread dough (25) or alcohol production in synthetic liquid dough (24). Bread dough activities given in the tables are averages of three cultures, each tested in duplicate. Standard errors were less than 10%.
Preparation of MALx3 genes for transformation.
We isolated MALx3 genes by colony hybridization from a YRp7-based minilibrary created from BglII-SalI-digested NL67 chromosomal DNA (2). We subcloned MALx3 genes into other vectors after changing the unique PmlI site of YRp7 to a BamHI site. The BamHI-SalI fragments encoding the positive activator genes were inserted into the BamHI-SalI sites of pBEJ17. Yeast transformants were performed by using a Bio 101 (Vista, Calif.) kit according to the manufacturer’s instructions.
Northern hybridization.
RNA for Northern hybridization was isolated from yeast cells by using TRIZOL reagent (Life Technologies, Inc., Gaithersburg, Md.) according to the manufacturer’s instructions. Thirty micrograms of total RNA was resolved on a 1% agarose gel in 1× morpholinepropanesulfonic acid (MOPS) buffer (20 mM MOPS [sodium salt], 5 mM sodium acetate, 1 mM disodium EDTA [pH 7]) containing 6% (wt/vol) formaldehyde. Nonradioactive digoxigenin hybridization was performed according to the manufacturer’s instructions (Boehringer Mannheim Australia, Sydney, New South Wales, Australia). The 1-kb HindII fragment containing part of the maltose permease gene (MAL6-1), the 1.5-kb BglII fragment containing part of the maltase gene (MAL6-2), the 0.9-kb EcoRI fragment of the transcriptional-activator gene (MAL6-3), and the 2-kb EcoRI-HindIII fragment containing the yeast actin gene (ACT1) were used as hybridization probes.
Production of cell extracts and determination of protein concentration.
Harvested cells were resuspended in breakage buffer (0.1 M citrate buffer [pH 6.5], 0.1 M EDTA, 1 mM dithiothreitol, 0.17 mg of phenylmethylsulfonyl fluoride/ml, 0.7 μM pepstatin) and homogenized for 5 min in the presence of glass beads. The extracts were centrifuged at 4°C for 10 min at 11,000 × g, and the supernatant was used as a cell extract. The protein concentration was determined by the method of Bradford (5).
Enzyme assays.
For the determination of maltase activity, cell extract and 50 mM potassium phosphate buffer (pH 6.8) were added to a total volume of 200 μl, followed by the addition of 1 ml of p-nitrophenyl-glucopyranoside (1 mg ml−1). The specific activity of maltase was defined as nanomoles of p-nitrophenol released per minute per milligram of protein at 28°C and pH 6.8. The β-galactosidase activity of cell extracts was assayed as described by Miller (23). Specific activity was determined as nanomoles of o-nitrophenol released per minute per milligram of protein at 28°C and pH 7. For α-galactosidase activity determination, cell extracts were added to a total volume of 400 μl with assay buffer (39 mM potassium phosphate, 31 mM citric acid [pH 4]), followed by the addition of 100 μl of p-nitrophenol-galactopyranoside (15 mg ml−1). Specific activity was defined as nanomoles of p-nitrophenol released per minute per milligram of protein at 28°C and pH 4. Enzyme activities given are averages of three cultures tested in triplicate. Standard errors for maltase, β-galactosidase, and α-galactosidase activities were less than 10%.
Transport assays.
Maltose transport activity was assayed according to the method of Serrano (38). Yeast cells were suspended in 0.2 M potassium phosphate buffer (pH 6) at a concentration of 90 mg ml−1. The reaction was carried out at 30°C and started by the addition of 30 μl of 10 μCi of α-d-[U-14C]maltose (ICN Biochemicals Australasia Pty. Ltd., Sydney, New South Wales, Australia). Samples were taken at 30-s intervals and stopped by dilution in 4 ml of ice-cold water plus 3.7 mg of iodoacetamide ml−1. The cells were filtered onto Whatman GF/C glass microfiber filters and washed twice with 4 ml of ice-cold water plus 3.7 mg of iodoacetamide ml−1. The radioactivity of filters was determined by liquid scintillation counting using aqueous scintillant; boiled cells were used as a control. Transport activities were defined as picomoles of maltose transported per minute per milligram (dry weight) of yeast cells. Activities given are averages of three cultures tested in triplicate. Standard errors for transport activities were less than 15%.
RESULTS
Positive correlation between yeast maltase and maltose permease activities and 2nd-h CO2 production in unsugared dough.
The abilities of six industrial strains of S. cerevisiae to produce CO2 gas in the 2nd h of a rapid unsugared dough fermentation were strongly correlated to maltase (r = 0.995) and maltose permease (r = 0.963) activities at the time of inoculation in the dough (Table 1). Strains NL67, NL25, and NL89 displayed the gassing characteristics of nonlagging strains (an equal or greater volume of gas was produced in the 2nd h than in the 1st h). By contrast, the volumes of gas produced by L38, L83, and L05 decreased by 60% in the 2nd h, indicating that these are lagging strains. The maltase and maltose permease activities of the three nonlagging strains were at least 7- and 120-fold higher, respectively, than those of the lagging strains (Table 1). These high MAL activities correlated with the 2nd-h gas volumes of the nonlagging strains, which were at least three times higher than those of the lagging strains (Table 1). When inoculated into synthetic dough medium consisting of glucose as the sole carbon source, all strains showed similar levels of gas production over 2 h (data not shown). These findings suggest that differences in maltose utilization affect 2nd-h gassing of lagging and nonlagging baker’s yeast.
TABLE 1.
Fermentation activity in relation to maltase and maltose permease activities in industrial strains of baker’s yeasta
| Strain | Gassingb in:
|
Maltase activityc | Permease activityd | |
|---|---|---|---|---|
| 1st h | 2nd h | |||
| NL67 | 270 | 320 | 360 | 1,700 |
| NL25 | 260 | 240 | 210 | 1,500 |
| NL89 | 270 | 260 | 270 | 2,000 |
| L38 | 190 | 76 | 28 | 12 |
| L83 | 200 | 80 | 29 | 12 |
| L05 | 180 | 72 | 23 | 11 |
Values shown are means of data derived from three experiments. Fermentation activity determinations were carried out in duplicate with standard errors of less than 10%. Assays were carried out in triplicate with standard errors of less than 10% for maltase results and less than 15% for maltose permease results.
Milliliters of CO2 produced per gram (dry weight) of yeast.
In nanomoles of p-nitrophenol released per minute per milligram of protein.
In picomoles of [14C]maltose taken up per minute per milligram (dry weight) of yeast cells.
Nonlagging strains have higher MAL activity under noninduced and induced conditions, but this is highly repressed by glucose.
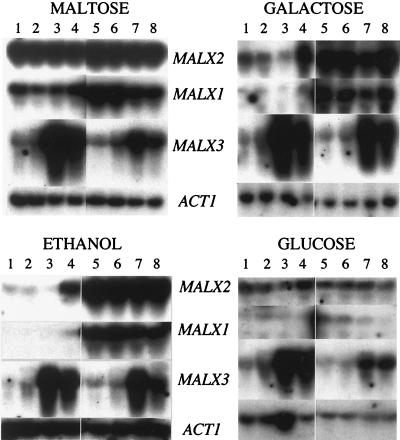
We tested two strains, NL67 (nonlagging) and L38 (lagging), for their maltase and maltose permease activities in mid-log phase in the presence of maltose (inducing), galactose (noninducing), ethanol (noninducing), and glucose (repressing). The major difference between the two strains was seen under noninducing conditions, in which the nonlagging strain produced much higher levels of both activities than the lagging strain (Table 2). Under inducing conditions (maltose), the difference was less marked. Northern analyses of MAL mRNA species indicated that these differences were due to increased transcriptional activity of the MALx2 and MALx1 genes (Fig. 1).
TABLE 2.
Maltase and maltose permease activities of baker’s yeast strains L38 and NL67 and their transformantsa
| Strain | Maltase activityb
|
Permease activityc
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mal | Gal | Eth | Glu | Mal | Gal | Eth | Glu | |
| L38 | 2,000 | 60 | 140 | 2.1 | 2,100 | 32 | 21 | 0.3 |
| NL67 | 3,000 | 1,000 | 1,700 | 14 | 5,100 | 1,100 | 2,800 | 2.2 |
| L38 + BEJ17 | 1,800 | 40 | 130 | 1.4 | 2,000 | 26 | 23 | 0.5 |
| L38 + MALx3–VH1 | 1,800 | 49 | 73 | 1.2 | 2,000 | 20 | 19 | 0.3 |
| L38 + MALx3–VH7 | 1,900 | 540 | 630 | 32 | 2,100 | 370 | 610 | 1.3 |
| L38 + MALx3–VH50 | 1,800 | 860 | 1,200 | 80 | 2,000 | 680 | 920 | 1.6 |
| L38 + MALx3–VH23 | 2,900 | 60 | 130 | 1.3 | 3,800 | 20 | 20 | 0.2 |
| NL67 + BEJ17 | 3,100 | 980 | 1,600 | 6.0 | 5,200 | 1,100 | 2,800 | 1.9 |
| NL67 + MALx3–VH1 | 3,200 | 600 | 1,200 | 5.0 | 5,200 | 640 | 2,000 | 1.9 |
| NL67 + MALx3–VH7 | 3,000 | 1,500 | 1,900 | 57 | 5,200 | 2,200 | 3,100 | 3.8 |
Strains and transformants were grown to mid-log phase in maltose (Mal), galactose (Gal), ethanol (Eth), or glucose (Glu). Values shown are means of data derived from three experiments. Assays were carried out in triplicate with standard errors of less than 10% for maltase results and less than 15% for maltose permease results.
In nanomoles of p-nitrophenol released per minute per milligram of protein.
In picomoles of [14C]maltose taken up per minute per milligram (dry weight) of yeast cells.
FIG. 1.
Northern (RNA) analysis of MALx2, MALx1, and MALx3 mRNA levels in baker’s yeast strains L38 and NL67 and their transformants. Cells were growing exponentially under inducing (maltose), noninducing (galactose or ethanol), and repressing (glucose) conditions. Total RNA was loaded in each lane, and the filters were hybridized with labelled probes (MAL6-2, MAL6-1, MAL6-3, and ACT1). All carbon sources were used at a concentration of 2% (wt/wt). Lanes: 1, L38; 2, L38 + BEJ17; 3, L38 + MALx3–VH1; 4, L38 + MALx3–VH7; 5, NL67; 6, NL67 + BEJ17; 7, NL67 + MALx3–VH1; 8, NL67 + MALx3–VH7.
In the presence of glucose, the maltase and maltose permease activities of both strains were reduced to very low levels (Table 2). It appears, therefore, that neither the glucose repression characteristics of maltase and maltose permease activities nor the level of glucose present in unsugared dough prior to fermentation is important in determining the nonlagging phenotype.
Cloned MALx3 gene from a nonlagging strain results in high noninduced expression of MALx1 and MALx2 genes in a MALx3-negative background.
MALx2 and MALx1 gene expression is regulated at transcription by the MALx3 protein (12, 28). We cloned a series of MALx3 genes from the nonlagging NL67 strain. Three polymorphic MALx3 genes, two with novel restriction maps (MALx3–VH7 and MALx3–VH9) and one (MALx3–VH1) corresponding to the published MAL6-3 (MAL6R) gene (18) were isolated. These genes could complement the malx3-negative phenotype of laboratory strain PB1. The MALx3–VH1 and MALx3–VH9 genes were subject to strong maltose induction, with MEL1 expression (MALx2) increased between 90- and 300-fold and lacZ expression (MALx1) increased as much as 440-fold under induced conditions compared with noninduced conditions (Table 3). The PB1 + MALx3–VH7 strain, however, had much higher levels of MALx2 and MALx1 expression under noninduced conditions but could be induced by maltose to the same final levels as the other transformants (Table 3). Induction levels from the MALx3–VH7 gene were only 5- to 13-fold. The MALx3–VH7 gene, therefore, conferred on a malx3 strain a regulation of MALx1 and MALx2 that was qualitatively similar to that seen in nonlagging strains.
TABLE 3.
Melibiase (MEL1) and β-galactosidase (LacZ) activities of PB1 transformants grown in maltose, galactose, or glucosea
| Strain | MEL1 activityb (MALx2)
|
LacZ activityc (MALx1)
|
||||
|---|---|---|---|---|---|---|
| Mal | Gal | Glu | Mal | Gal | Glu | |
| PB1 + YRp7 | 2.0 | 0.8 | ND | 7.0 | 0.1 | ND |
| PB1 + MALx3–VH1 | 350 | 1.1 | ND | 88 | 0.2 | ND |
| PB1 + MALx3–VH7 | 340 | 26 | 7.4 | 86 | 17 | 0.9 |
| PB1 + MALx3–VH9 | 360 | 4.0 | ND | 93 | 1.0 | ND |
| PB1 + MALx3–VH23 | 510 | 1.4 | ND | 140 | 0.2 | ND |
| PB1 + MALx3–VH50 | 330 | 61 | 22 | 81 | 35 | 4.4 |
Values shown are means of data derived from three experiments. Assays were carried out in triplicate. Standard errors were less than 10%.
In nanomoles of p-nitrophenyl released per minute per milligram of protein. Mal, maltose; Gal, galactose; Glu, glucose; ND, not detected.
In nanomoles of o-nitrophenyl released per minute per milligram of protein.
The MALx3–VH7 gene was mutated to produce MALx3–VH50 (Lys364Glu, Lys371Gly, Phe375Leu), which showed higher noninduced levels in PB1 (Table 3). We also fused the promoter and the 1st 954 bp of the MALx3–VH7 structural gene with the carboxyl terminus of the MALx3–VH1 gene. This construct (MALx3–VH23) regulated the marker genes in strain PB1 with strong maltose induction, but the fully induced activities of melibiase and β-galactosidase were approximately 45 and 60% higher than those seen with all other MALx3 genes (Table 3). These results suggest that NL67 contains a novel MALx3 gene that leads to significantly higher MAL activity under noninduced conditions and that this gene may be responsible for the nonlagging phenotype.
The MALx3–VH7 gene product significantly increases the noninduced MAL activity of a lagging strain.
We subcloned MALx3–VH7, MALx3–VH50, MALx3–VH23, and MALx3–VH1 into pBEJ17, a 2 μm DNA-based high-copy-number plasmid, to provide enough copies of cloned MALx3 genes to override interference that might arise from the original MALx3 genes in strain L38. The genes that previously gave high noninduced levels of expression (MALx3–VH7 and MALx3–VH50) in PB1 also led to very high noninduced levels in the lagging strain (L38). This was not the case for MALx3–VH1-, MALx3–VH23-, and vector only-transformed L38 (Table 2). These results (for MALx3–VH7) could be attributed to increased transcription of MALx1 and MALx2, (Fig. 1). It is unlikely that this increased transcription is due to the presence of multiple copies of the MALx3 genes, since both MALx3–VH7 and MALx3–VH1 constructs are present at similar copy numbers (see, e.g., the MALx3 transcript levels in Fig. 1), and in the presence of multiple copies of MALx3–VH1, the MALx1 and MALx2 genes retained strong inducibility. The differences observed may be due to differences in the structure or regulation of the transcription factors they encode. This is consistent with the effect of mutations in the MALx3–VH50 gene, which increase noninduced maltase and maltose permease levels beyond those seen in strains with the MALx3–VH7 gene.
In the presence of glucose, the activities of maltase and maltose permease in all strains were very low. However, in strains carrying the MALx3–VH7 and MALx3–VH50 genes, there was at least a 10-fold increase in the expression of the MALx2 gene (Table 2).
Higher noninduced levels of maltase and maltose permease increase the ability of a yeast strain to produce CO2 in unsugared dough.
We tested the effect that cloned MALx3 genes have on fermentation ability in unsugared dough by growing transformed strains in YP with 1% sucrose plus 220 μg of Geneticin/ml. Strains were harvested in late-respiratory phase, maltase and maltose permease activities were assayed, and amounts of gas produced in unsugared dough were measured. The maltase and maltose permease activities of L38 + MALx3–VH7 were approximately 9- and 23-fold higher than those of the control strain, L38 + BEJ17, and resulted in a 2.4-fold increase in 2nd-h gassing (Table 4). Similar effects, but with higher enzyme activities and higher levels of gassing, were seen with L38 + MALx3–VH50.
TABLE 4.
Fermentation activity in relation to maltase and maltose permease activities in recombinant strains of baker’s yeasta
| Strain | Gassingb in:
|
Maltase activityc | Permease activityd | |
|---|---|---|---|---|
| 1st h | 2nd h | |||
| L38 + BEJ17 | 180 | 52 | 30 | 12 |
| L38 + MALx3–VH1 | 180 | 51 | 25 | 10 |
| L38 + MALx3–VH7 | 200 | 130 | 270 | 280 |
| L38 + MALx3–VH23 | 180 | 69 | 19 | 10 |
| NL67 + BEJ17 | 220 | 210 | 380 | 1,600 |
| NL67 + MALx3–VH1 | 210 | 210 | 270 | 1,300 |
| NL67 + MALx3–VH7 | 220 | 220 | 400 | 1,700 |
| L38 + PDC1 | 200 | 120 | 31 | 1,800 |
| L38 + PDC1–VH7 | 210 | 180 | 270 | 1,900 |
| L38 + MALx3–VH50 | 220 | 170 | 390 | 530 |
Values shown are means of data received from three experiments. Fermentation activity determinations were carried out in duplicate with standard errors of less than 10%. Assays were carried out in triplicate with standard errors of less than 10% for maltase results and less than 15% for maltose permease results.
Milliliters of CO2 produced per gram (dry weight) of yeast.
In nanomoles of p-nitrophenol released per minute per milligram of protein.
In picomoles of [14C]maltose taken up per minute per milligram (dry weight) of yeast cells.
Strain L38 + PDC1 was developed by integrating a MAL6-1 structural gene fused to the PDC1 promoter at the TRP1 locus of strain L38. This strain showed a maltose permease activity 150-fold higher than that of the control strain but showed no increase in maltase activity. The higher maltose permease activity resulted in a 2.4-fold increase in 2nd-h gas production in unsugared dough (Table 4).
Even though both L38 + MALx3–VH7 and L38 + PDC1 had higher 2nd-h gas production, these levels were still lower than those of the nonlagging strain NL67 (Table 4). Transforming L38 + PDC1 with the BEJ17 + MALx3–VH7 plasmid (L38 + PDC1 + VH7) increased the maltase activity ninefold (Table 4). This combination of maltase and maltose permease increases led to a 3.5-fold increase in 2nd-h gas production, which approaches the activity of the transformed nonlagging control (Table 4). Thus, all strains with significant increases in noninduced maltase and maltose permease activities had corresponding increases in 2nd-h gas production. L38 + MALx3–VH23 had higher maltase and maltose permease activities in the presence of maltose (Table 2), but no significant increase in 2nd-h gas production was evident in unsugared dough (Table 4).
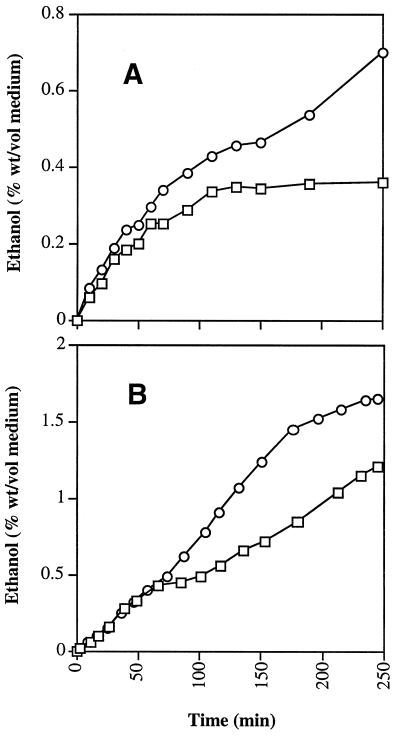
We added 150 μg of cycloheximide ml−1 to unsugared synthetic dough before the addition of yeast. When cycloheximide was added at the start of the fermentation, the nonlagging strain, NL67, was still producing gas 250 min into the fermentation, whereas the lagging strain (L38) was unable to produce gas beyond 110 min (Fig. 2). This result suggests that the nonlagging strain entered the synthetic dough with sufficient levels of maltase and maltose permease proteins to utilize maltose without the need for further protein synthesis.
FIG. 2.
Fermentation by nonlagging and lagging strains of S. cerevisiae in unsugared synthetic dough medium. Yeast cells were inoculated into unsugared synthetic dough containing 1% sucrose and 5% maltose. Samples were withdrawn at intervals and centrifuged, and supernatants were assayed for ethanol by gas chromatography. Values shown are means of data derived from two experiments. Assays were carried out in triplicate with standard errors of less than 10%. (A) Cycloheximide was added at 0 min; (B) no cycloheximide was added. □, L38; ○, L67.
DISCUSSION
We found a strong correlation between a yeast strain’s maltase and maltose permease activities and its ability to leaven unsugared dough. These results support the conclusions of Oda and Ouchi (30) that constitutive expression of maltose-utilization genes is crucial to prolonged leavening of unsugared dough. Here we have shown that the nonlagging phenotype is due to the presence in strains of a MALx3 gene activator that allows constitutive expression of the other MAL genes.
There have been several earlier reports that the nonlagging phenotype is dependent on a high level of maltase expression in the presence of glucose (32, 35), which is a feature of the constitutive expression in the system used by Oda and Ouchi (3, 17, 29, 30). We showed that glucose repression characteristics are not important in the nonlagging industrial strain but that a MALx3 gene capable of conferring high constitutive basal levels of expression of the MAL system under noninduced conditions (i.e., on substrates containing neither glucose nor maltose) is the most relevant feature. The MALx3 regulators we identified could still respond to maltose and lead to further increases in MAL gene expression. This ability to undergo maltose induction above the high basal level may also be required in a good baking strain but is not important to the nonlagging phenotype.
These characteristics are consistent with what is required of a strain used commercially in terms of yield and activity. In industrial practice, cane or beet molasses is used for fed-batch growth of yeast. To obtain a high yeast biomass yield, molasses is added incrementally, and biomass increases under conditions supporting respiration. During growth on sucrose or glucose as substrates in high levels of expression of the MAL genes are undesirable because they lead to a selective disadvantage (20). While glucose repression may be partially relieved due to the batch-fed mode of growth, there may be sufficient repression to prevent high levels of expression of the MAL genes. To finish the fermentation, the molasses feed is cut and the yeast is aerated in order to respire the remaining ethanol before the yeast is harvested and packaged (36). These conditions, with neither maltose nor glucose present, are very similar to the noninduced conditions used in our experiments. We suggest that, like the lagging strain, the nonlagging strain, when placed into unsugared dough, can readily ferment the available sugars (glucose, fructose, and sucrose) and produce CO2 (34). However, due to the MALx3 background of the nonlagging strain, it enters the unsugared dough with significant activities of maltase and maltose permease, thus allowing it to utilize maltose simultaneously. At this stage the concentrations of glucose or fructose present in unsugared dough are not high enough to completely repress expression of the maltase and maltose permease genes or to catabolite inactivate the maltose permease of the nonlagging strain. In support of this, inhibition of protein synthesis at the start of fermentation in unsugared synthetic dough did not prevent a nonlagging strain from fermenting maltose, but it did inhibit the lagging strain.
For a yeast to be useful in leavening unsugared dough, it must contain a MALx3 genetic background that provides for high levels of maltase and maltose permease activities when the yeast enters the dough. These levels enable the strain to continue to produce CO2 at the same rate even after all the easily assimilated sugars are depleted. Thus, CO2 is produced at a constant rate typical of the nonlagging phenotype.
REFERENCES
- 1.Attfield P V. Stress tolerance: the key to effective strains of industrial baker’s yeast. Nat Biotechnol. 1997;15:1351–1357. doi: 10.1038/nbt1297-1351. [DOI] [PubMed] [Google Scholar]
- 2.Bell P J L, Bissinger P H, Evans R J, Dawes I W. A two-reporter gene system for the analysis of bi-directional transcription from the divergent MAL6T-MAL6S promoter in Saccharomyces cerevisiae. Curr Genet. 1995;28:441–446. doi: 10.1007/BF00310813. [DOI] [PubMed] [Google Scholar]
- 3.Bell P J L, Higgins V J, Dawes I W, Bissinger P H. Tandemly repeated 147 bp elements cause structural and functional variation in divergent MAL promoters of Saccharomyces cerevisiae. Yeast. 1997;13:1135–1144. doi: 10.1002/(SICI)1097-0061(19970930)13:12<1135::AID-YEA162>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Beudeker R F, Van Dam H W, Van Der Plaat J B, Vellenga K. Developments in baker’s yeast production. In: Varachtert H, De Mot R, editors. Yeast biotechnology and biocatalysis. New York, N.Y: Marcel Dekker, Inc.; 1990. pp. 103–146. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Burrows S. Baker’s yeast. Econ Microbiol. 1979;4:31–64. [Google Scholar]
- 7.Charron M J, Michels C A. The constitutive, glucose-repression-insensitive mutation of the yeast MAL4 locus is an alteration of the MAL43 gene. Genetics. 1987;115:23–31. doi: 10.1093/genetics/116.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charron M J, Read E, Haut S R, Michels C A. Molecular evolution of the telomere-associated MAL loci of Saccharomyces. Genetics. 1989;122:307–316. doi: 10.1093/genetics/122.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J D, Goldenthal M J, Buchferer B, Marmur J. Mutational analysis of the MAL1 locus of Saccharomyces: identification and functional characterization of three genes. Mol Gen Genet. 1984;196:208–216. doi: 10.1007/BF00328052. [DOI] [PubMed] [Google Scholar]
- 10.Dubin R A, Needleman R B, Gossett D, Michels C A. Identification of the structural gene encoding maltase within the MAL6 locus of Saccharomyces cerevisiae. J Bacteriol. 1985;164:605–610. doi: 10.1128/jb.164.2.605-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubin R A, Charron M J, Haut S R, Needleman R B, Michels C A. Constitutive expression of the maltose fermentative enzymes in Saccharomyces carlsbergensis is dependent upon the mutational activation of a nonessential homolog of MAL63. Mol Cell Biol. 1988;8:1027–1035. doi: 10.1128/mcb.8.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federoff H J, Eccleshall T R, Marmur J. Regulation of maltose synthesis in Saccharomyces carlsbergensis. J Bacteriol. 1983;154:1301–1308. doi: 10.1128/jb.154.3.1301-1308.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gjermansen C, Sigsgaard P. Construction of a hybrid brewing strain of Saccharomyces carlsbergensis by mating of meiotic segregants. Carlsberg Res Commun. 1981;46:1–11. [Google Scholar]
- 14.Goldenthal M J, Vanoni M, Buchferer B, Marmur J. Regulation of MAL gene expression in yeast: gene dosage effect. Mol Gen Genet. 1987;209:508–517. doi: 10.1007/BF00331157. [DOI] [PubMed] [Google Scholar]
- 15.Grylls F S M, Harrison J S. Adaptation of yeast to maltose fermentation. Nature. 1956;178:1471–1472. doi: 10.1038/1781471a0. [DOI] [PubMed] [Google Scholar]
- 16.Hadfield C, Jordan B E, Mount R C, Pretorius G H, Burak E. G418-resistance as a dominant marker and reporter for gene expression in Saccharomyces cerevisiae. Curr Genet. 1990;18:303–313. doi: 10.1007/BF00318211. [DOI] [PubMed] [Google Scholar]
- 17.Kahn N A, Eaton N R. Genetic control of maltase formation in yeast. I. Strains producing high and low basal levels of enzyme. Mol Gen Genet. 1971;112:317–322. doi: 10.1007/BF00334433. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Michels C A. The MAL63 gene of Saccharomyces encodes a cysteine-zinc finger protein. Curr Genet. 1988;14:319–323. doi: 10.1007/BF00419988. [DOI] [PubMed] [Google Scholar]
- 19.Kodama Y, Fukui N, Ashikari T, Shibano Y. Improvement of maltose fermentation efficiency: constitutive expression of MAL genes in brewing yeasts. J Am Soc Brew Chem. 1995;53:24–29. [Google Scholar]
- 20.Langel P, Wohrmann K. The selective advantage of inducible maltase in yeast (Saccharomyces cerevisiae) Genetica. 1981;57:105–111. [Google Scholar]
- 21.Levine J, Tanouye L, Michels C A. The UAS(MAL) is a bidirectional promoter element required for the expression of both the MAL61 and MAL62 genes of the Saccharomyces MAL6 locus. Curr Genet. 1992;22:181–189. doi: 10.1007/BF00351724. [DOI] [PubMed] [Google Scholar]
- 22.Lucero P, Herweijer M, Lagunas R. Catabolite inactivation of the yeast maltose transporter is due to proteolysis. FEBS Lett. 1993;333:165–168. doi: 10.1016/0014-5793(93)80397-d. [DOI] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 24.Myers D K, Lawler D T M, Attfield P V. Influence of invertase activity and glycerol synthesis and retention on fermentation of media with a high sugar concentration by Saccharomyces cerevisiae. Appl Environ Microbiol. 1997;63:145–150. doi: 10.1128/aem.63.1.145-150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers D K, Joseph V M, Pehm S, Galvagno M, Attfield P V. Loading of Saccharomyces cerevisiae with glycerol leads to enhanced fermentation in sweet bread doughs. Food Microbiol. 1998;15:51–58. [Google Scholar]
- 26.Naumov G I, Naumova E S, Michels C A. Genetic variation of the repeated MAL loci in natural populations of Saccharomyces cerevisiae and Saccharomyces paradoxus. Genetics. 1994;136:803–812. doi: 10.1093/genetics/136.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Needleman R B, Michels C A. Repeated family of genes controlling maltose fermentation in Saccharomyces carlsbergensis. Mol Cell Biol. 1983;3:796–802. doi: 10.1128/mcb.3.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Needleman R B, Kaback D B, Dubin R A, Perkins E L, Rosenberg N G, Sutherland K A, Forrest D B, Michels C A. MAL6 of Saccharomyces: a complex genetic locus containing three genes required for maltose fermentation. Proc Natl Acad Sci USA. 1984;81:2811–2815. doi: 10.1073/pnas.81.9.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oda Y, Ouchi K. Maltase genes and α-glucosidase activities: their effects on the dough-leavening. Yeast. 1989;5:S125–S139. [Google Scholar]
- 30.Oda Y, Ouchi K. Role of the yeast maltose fermentation genes in CO2 production rate from sponge dough. Food Microbiol. 1990;7:43–47. [Google Scholar]
- 31.Oliver S. Classical yeast biotechnology. In: Tuite M F, Oliver S G, editors. Saccharomyces. Biotechnology handbooks vol 4. London: Plenum Press; 1991. pp. 213–248. [Google Scholar]
- 32.Osinga K A, Renniers A C H M, Welbergen J W, Roobol R H, van der Wilden W. Maltose fermentation in Saccharomyces cerevisiae. Yeast. 1989;5:S207–S212. . (Special issue. April Seventh International Symposium on Yeasts.) [Google Scholar]
- 33.Ponte J G, Reed G. Bakery foods. In: Reed G, editor. Prescott and Dunns industrial microbiology. 4th ed. Westport, Conn: AVI Publishing Co., Inc.; 1982. pp. 246–292. [Google Scholar]
- 34.Potus J, Poiffait A, Drapron R. Influence of dough-making conditions on the concentration of individual sugars and their utilisation during fermentation. Cereal Chem. 1994;71:505–508. [Google Scholar]
- 35.Randez-Gil F, Sanz P. Construction of industrial baker’s yeast strains able to assimilate maltose under catabolite repression conditions. Appl Microbiol Biotechnol. 1994;42:581–586. [Google Scholar]
- 36.Reed G, Nagodawithana T W. Yeast technology. 2nd ed. New York, N.Y: Van Nostrand Reinhold; 1991. [Google Scholar]
- 37.Rodicio R, Zimmermann F K. Cloning of maltase regulatory genes in Saccharomyces cerevisiae. Curr Genet. 1985;9:539–545. [Google Scholar]
- 38.Serrano R. Energy requirements for maltose transport in yeast. Eur J Biochem. 1977;80:97–102. doi: 10.1111/j.1432-1033.1977.tb11861.x. [DOI] [PubMed] [Google Scholar]
- 39.Struhl K, Stinchcomb D T, Scherer S, Davis R W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci USA. 1979;76:1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trivedi N B, Jacobson G K, Tesch W. Baker’s yeast. Crit Rev Biotechnol. 1986;4:75–110. [Google Scholar]
- 41.Zimmermann F K, Eaton N R. Genetics of induction and catabolite repression of maltase synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1974;134:261–272. doi: 10.1007/BF00267720. [DOI] [PubMed] [Google Scholar]