Abstract
Background
Biomarker assessments for nivolumab monotherapy efficacy in previously treated patients with non‐small cell lung cancer (NSCLC) remain unclear. We evaluated whether body mass index (BMI) and Glasgow prognostic score (GPS) are useful for assessing the efficacy of nivolumab alone as a second‐line treatment in patients with pretreated NSCLC.
Methods
Data of 99 patients treated with second‐line nivolumab monotherapy for NSCLC between January 2016 and December 2019 were evaluated for prognostic values of BMI and GPS to assess their usefulness in predicting progression‐free survival (PFS) and overall survival (OS).
Results
The Eastern Cooperative Oncology Group‐performance status (PS) independently predicted the second‐line nivolumab monotherapeutic effect; good PS (0–1) correlated with significantly longer PFS (4.3 vs. 1.9 months, log‐rank; p = 0.0004) and OS (17.7 vs. 4.6 months, log‐rank; p < 0.0001) than poor PS. BMI independently predicted survival, with high BMI (≥22.1 kg/m2) associated with significantly longer OS (19.1 vs. 8.5 months, log‐rank; p = 0.0023) than low BMI (<22.1 kg/m2). However, GPS showed no significant difference for PFS or OS.
Conclusion
Among patients with NSCLC treated with nivolumab monotherapy as second‐line treatment, PS was significantly correlated with both PFS and OS and BMI with OS. Thus, BMI could be a useful predictor of survival in these patients.
Keywords: body mass index, carcinoma, glasgow prognostic score, nivolumab, non‐small‐cell lung, performance status, survival
BMI independently predicted survival, with high BMI (≥22.1 kg/m2) associated with significantly longer OS (19.1 vs. 8.5 months, log‐rank; p = 0.0023) than low BMI (<22.1 kg/m2). Our findings demonstrate that BMI could be a useful predictor of survival in NSCLC patients treated with nivolumab monotherapy as second‐line treatment.

INTRODUCTION
Lung cancer has the highest mortality rate worldwide, and non‐small cell lung cancer (NSCLC) accounts for the majority (approximately 85%–90%) of all lung cancers. 1 Several randomized phase III trials revealed that in patients with NSCLC whose disease has progressed while on platinum‐based chemotherapy, treatment with programmed cell death‐1 (PD‐1), or programmed cell death‐ligand‐1 (PD‐L1) blockade improved overall survival (OS) compared with standard chemotherapy. 2 , 3 , 4 , 5 Thus, nivolumab monotherapy is regarded as a standard second‐line treatment for patients with NSCLC with disease progression after first‐line chemotherapy.
In previous reports, body mass index (BMI) has been used as a prognostic indicator for various cancers. 6 , 7 , 8 , 9 In patients with NSCLC treated with immune checkpoint inhibitors (ICIs), the presence of sarcopenia has reportedly had a negative prognostic effect. 6 In addition, BMI has reportedly been associated with ICI‐related therapy outcomes in solid malignancies such as melanoma, renal cell carcinoma, and NSCLC. 7 To date, reports on the association between BMI and Glasgow prognostic score (GPS) and the therapeutic efficacy of nivolumab monotherapy as a second‐line treatment for NSCLC are limited. A relationship between BMI and the effect of ICIs in NSCLC has recently been reported. 8 When the BMI cutoff was set at 22 kg/m2 (categorized as high and low BMI groups), there was no statistically significant difference in measures of treatment efficacy, such as progression‐free survival (PFS) or OS, between the high and low BMI cohorts of NSCLC having a high PD‐L1 expression (≥50%) who received first‐line pembrolizumab monotherapy. However, when patients with NSCLC received ICI monotherapy (nivolumab/pembrolizumab/atezolizumab) as a second‐ or subsequent‐line therapy, treatment efficacy was significantly better in patients with a higher BMI than in patients with a lower BMI. Furthermore, using a BMI cutoff value of 21.4 kg/m2, BMI independently predicted survival because patients with high BMIs (≥21.4 kg/m2) had significantly longer OS (not reached vs. 14.1 months, p = 0.006) than patients with low BMIs (<21.4 kg/m2) and among patients with NSCLC and high PD‐L1 expression (≥50%) who were administered first‐line pembrolizumab monotherapy. 9 Thus, the association between BMI and the therapeutic effect of ICIs in NSCLC is not fully understood.
Additionally, many patients with NSCLC are diagnosed at an advanced, inoperable disease stage, and they continually experience systemic inflammatory reactions (SIR) and weight loss, which reportedly have a significant impact on cancer cachexia. 10 , 11 Thus, prognosis attributable to cancer is assessed using an SIR‐based scoring system such as the GPS, which comprises albumin concentrations and serum C‐reactive protein (CRP) levels 10 and is an independent prognostic factor for NSCLC. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Although the relationship between the GPS and the therapeutic effects of various types of ICIs with various lines of treatment for NSCLC has been reported in several studies, 9 , 21 , 22 few reports have evaluated the relationship between GPS and effect of nivolumab monotherapy as a second‐line treatment of NSCLC. In addition, we recently reported that in patients with high PD‐L1 expression who received pembrolizumab monotherapy as a first‐line treatment for NSCLC, GPS was significantly correlated with both PFS and OS and BMI was significantly correlated with OS, which could be used to predict outcomes in these patients. 9 We sought to determine whether these results hold true for patients with NSCLC who received nivolumab monotherapy as a second‐line therapy. Therefore, we evaluated whether BMI and GPS could predict survival for nivolumab alone as a second‐line treatment in patients with pretreated NSCLC.
METHODS
Patients
In the current study, we retrospectively evaluated 99 consecutive patients with advanced or metastatic NSCLC without driver gene mutation/translocation who received platinum‐based combination chemotherapy as the first‐line treatment followed by nivolumab monotherapy as a second‐line treatment after disease progression between January 2016 and December 2019. The pathological diagnosis of NSCLC was classified according to the 2015 World Health Organization Classification of Tumors, and the disease stage was assessed using version eight of the Tumor‐Node‐Metastasis (TNM) staging classification system. The inclusion criteria for enrolled patients were as follows: (1) histologically‐ or cytologically‐confirmed NSCLC, (2) platinum‐based combination chemotherapy treated with first‐line chemotherapy, and (3) nivolumab treatment as second‐line chemotherapy. The included patients did not receive any ICIs prior to nivolumab monotherapy as the second‐line treatment. Nivolumab, intravenously administered at 3 mg/kg or 240 mg/day every 2 weeks, was repeated until the disease progressed, unacceptable toxicity was observed, or the patient refused treatment. For first‐line and subsequent‐line therapeutic regimens, the regimen was chosen at the discretion of the attending physician. Patients' electronic medical record data were viewed and data on patient backgrounds and treatment responses to second‐line nivolumab were collected retrospectively. The study design was approved by the Institutional Ethics Committee of the International Medical Center, Saitama Medical University (approval number: 20–247). The protocol was performed in accordance with the 1964 Declaration of Helsinki (revised in 2008). The requirement for written informed consent was waived by the Ethics Committee because of the retrospective nature of the study; however, the patients were provided with an opt‐out opportunity.
Evaluation of treatment efficacy
Serum CRP and albumin concentration levels were measured on the day of or day before initiation of nivolumab monotherapy. The GPS was classified into three groups according to the combination of CRP and albumin as follows: GPS 0, 1, and 2 included patients with a CRP <1.0 mg/dl and albumin >3.5 mg/dl; CRP only was increased or albumin only was decreased; and CRP was ≥1.0 mg/dl and albumin level <3.5 mg/dl, respectively. BMI calculated before the start of nivolumab treatment was defined as weight (kg) divided by height (m) squared. Patients were classified into high and low BMI groups, as defined by the receiver operating characteristic (ROC) curve: low (<22.1 kg/m2) and high (≥22.1 kg/m2) BMI. The appropriate cutoff value differentiating the high and low BMI groups, based on the ROC curve analysis for OS, was 22.1 kg/m2 (area under the curve [AUC]: 0.582; sensitivity: 60.7%; specificity: 41.6%).
Tumor response was evaluated as the best overall response and maximum tumor shrinkage. Radiographic tumor responses were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. 23 PFS was defined as the time from the initial nivolumab monotherapy to disease progression or death. OS was defined as the time from the initial nivolumab monotherapy to death or was censored on the date of the last follow‐up.
Statistical analysis
For statistical analysis, we used Fisher's exact test and Welch's t‐test for categorical and continuous variables, respectively. Cox proportional hazards models with stepwise regression were applied to identify factors predicting PFS and OS, and the results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). The Kaplan–Meier method was used to estimate survival as a function of time, and survival differences were analyzed using the log‐rank test. We performed univariate and multivariate logistic regression analyses according to the different outcome variables. The statistical significance level was set at a p‐value ≤0.05. All statistical analyses were performed using the JMP software for Windows, version 11.0 (SAS Institute).
RESULTS
Patient backgrounds and therapeutic efficacy
Table 1 presents the characteristics of the 99 patients including 77 men (77.8%) and 22 women (22.2%), with a median age of 69 years (range, 31–80 years). The Eastern Cooperative Oncology Group (ECOG)‐performance status (PS) was 0–1 in 77 patients (77.8%) and 2–3 in 22 patients (22.2%). Adenocarcinoma accounted for 56 of the 99 patients (56.6%). Eighty‐two patients (82.8%) had stage III–IV disease. The number of patients not evaluated for PD‐L1 expression was 82 (82.8%). All cases were wild‐type, negative, or unknown for driver gene mutation/translocation status. The median BMI was 22.0 (range, 14.3–33.7 kg/m2). The median number of nivolumab administration cycles was four (range, 1–97). The responses to nivolumab monotherapy among all patients were identified as follows: complete response (n = 1), partial response (n = 18), stable disease (n = 30), and progressive disease (n = 40). Thus, the overall response rate was 19.2% (95% CI: 11.4–26.9), and the disease control rate was 49.5% (95% CI: 39.6–59.3).
TABLE 1.
Patient baseline characteristics
| Characteristics | Patients (n) |
|---|---|
| Total number of patients (n) | 99 |
| Sex | |
| Men/women | 77/22 |
| Median age at initiation of nivolumab (years) [range] | 69 (31–80) |
| Performance status (PS) | |
| 0/1/2/3/4 | 36/41/15/7/0 |
| Clinical disease stage at diagnosis | |
| III/IV/postoperative recurrence | 16/66/17 |
| Smoking history | |
| Current or former/never | 84/15 |
| Histological classification | |
| Adenocarcinoma/squamous cell carcinoma/others | 56/25/18 |
| PD‐L1 tumor proportion score (%) | |
| ≥1/0/untested | 3/14/82 |
| Driver gene mutation/translocation | |
| EGFR/ALK/wild‐type, negative, or unknown | 0/0/99 |
| BMI (kg/m2) | |
| Median [range] | 22.0 (14.3–33.7) |
| Prior radiation therapy | |
| Yes/no | 36/63 |
| Administration cycles of nivolumab | |
| Median (range) | 4 (1–97) |
| Treatment response | |
| CR | 1 |
| PR | 18 |
| SD | 30 |
| PD | 40 |
| NE | 10 |
| Response rate (%) (95% CI) | 19.2 (11.4–26.9) |
| Disease control rate (%) (95% CI) | 49.5 (39.6–59.3) |
| Laboratory data (median) [range] | |
| Albumin (g/dl) | 3.7 (2.3–4.7) |
| CRP (mg/dl) | 0.773 (0.01–25.983) |
Abbreviations: ALK, anaplastic lymphoma kinase; BMI, body mass index; CI, confidence interval; CR, complete response; CRP, C‐reactive protein.; EGFR, epidermal growth factor receptor; NE, not evaluated; PD, progressive disease; PD‐L1, programmed death‐ligand 1; PR, partial response; PS, performance status; SD, stable disease.
Comparative analysis of the GPS and BMI
The patient characteristics according to GPS and BMI are shown in Table 2. The GPS values at the initiation of nivolumab administration revealed a GPS of 0–1 in 72 patients and a GPS of 2 in 27 patients. There were significant differences (p < 0.05) in ECOG‐PS, clinical stage at diagnosis, prior radiotherapy, albumin levels, and CRP levels between GPS categories (0–1 vs. 2). At the start of nivolumab administration, low and high BMI were reported in 48 and 51 patients, respectively. The administration cycles of nivolumab were significantly different (p < 0.05) between the BMI categories.
TABLE 2.
Results of patient baseline characteristics according to the Glasgow prognostic score and body mass index
| GPS | BMI | |||||
|---|---|---|---|---|---|---|
| Variables | 0–1 | 2 | p‐value | Low (<22.1) | High (≥22.1) | p‐value |
| Patients (n) | 72 | 27 | 48 | 51 | ||
| Baseline characteristics | ||||||
| Sex | ||||||
| Men/women | 55/17 | 22/5 | 0.78 | 38/10 | 39/12 | 0.81 |
| Median age at treatment (years) (range) | 69 (31–80) | 69 (50–79) | 0.46 a | 67.5 (50–79) | 70 (31–80) | 0.25 a |
| Performance status (PS) | ||||||
| 0–1/3–4 | 63/9 | 14/13 | 0.0003 | 34/14 | 43/8 | 0.14 |
| Smoking history | ||||||
| Yes/no | 62/10 | 22/5 | 0.54 | 42/7 | 42/9 | 0.78 |
| Histological classification | ||||||
| Adenocarcinoma/nonadenocarcinoma | 43/29 | 13/14 | 0.36 | 26/22 | 30/21 | 0.68 |
| Clinical stage at diagnosis | ||||||
| III–IV/postoperative recurrence | 55/17 | 27/0 | 0.0051 | 42/6 | 40/11 | 0.29 |
| BMI (kg/m2) | ||||||
| Median (range) | 22.3 (14.3–33.7) | 21.7 (15.4–27.7) | 0.06 a | 20.1 (14.3–22.0) | 24.0 (22.1–33.7) | ‐ |
| Prior radiation therapy | ||||||
| Yes/no | 19/53 | 17/10 | 0.0011 | 20/28 | 16/35 | 0.30 |
| Administration cycles of nivolumab | ||||||
| Median (range) | 5 (1–97) | 4 (1–36) | 0.05 a | 4 (1–69) | 5 (1–97) | 0.04 a |
| Treatment response | ||||||
| CR | 1 | 0 | 0 | 1 | ||
| PR | 11 | 7 | 9 | 9 | ||
| SD | 26 | 4 | 12 | 18 | ||
| PD | 27 | 13 | 22 | 18 | ||
| NE | 7 | 3 | 5 | 5 | ||
| Response rate (%) (95% CI) | 16.6 (8.0–25.2) | 25.9 (9.3–42.4) | 0.39 | 18.3 (7.7–29.7) | 20.0 (8.7–30.5) | >0.99 |
| Disease control rate (%) (95% CI) | 52.7 (41.2–64.3) | 40.7 (22.2–59.2) | 0.36 | 44.8 (29.7–57.7) | 54.0 (41.7–68.5) | 0.31 |
| Laboratory data | ||||||
| Median (range) | ||||||
| Albumin (g/dl) | 3.9 (3.2–4.7) | 2.9 (2.3–3.4) | <0.0001 a | 3.6 (2.3–4.7) | 3.8 (2.7–4.7) | 0.05 a |
| CRP (mg/dl) | 0.411 (0.01–7.787) | 5.162 (1.029–25.983) | <0.0001 a | 1.17 (0.01–23.789) | 0.754 (0.028–25.983) | 0.49 a |
Note: p‐values in bold are statistically significant (p < 0.05).
Abbreviations: BMI, body mass index; CI, confidence interval; CR, complete response; CRP, C‐reactive protein; GPS, Glasgow Prognostic Score; NE, not evaluated; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
Welch's t‐test.
Analysis of survival
The median PFS interval was 3.4 months (95% CI: 2.1–4.7) (Figure 1a), the median OS interval was 13.4 months (95% CI: 9.5–17.7) (Figure 1b), and the median follow‐up duration was 12.7 (range, 0.3–63.7) months. Of the 99 patients included in the analysis, 72 died while 27 were still alive as of September 30, 2021, the data cutoff date. As shown in Table 3, the results of the univariate and multivariate analyses of PFS and OS are demonstrated. Univariate analyses of PFS demonstrated significant associations with ECOG‐PS. Multivariate analyses demonstrated that PFS was associated with PS (0–1 and 2–3) (HR: 0.44, p = 0.0027). Furthermore, significant correlations were found between ECOG‐PS and BMI in the univariate analysis of OS. Multivariate analyses revealed that OS was significantly correlated with PS (0–1 and 2–3) (HR: 0.25, p < 0.0001) and BMI (low/high) (HR 1.79, p = 0.0184). Figure 2 demonstrates the survival curves for PFS and OS, according to ECOG‐PS, GPS, and BMI; an ECOG‐PS of 0–1 correlated significantly with longer PFS and OS than an ECOG‐PS of 2–3 (both, p < 0.05; Figure 2a, b). There was no statistically significant difference in PFS and OS between a GPS of 0–1 and a GPS of 2 (Figure 2c,d). Although BMI was not associated with PFS (p = 0.12, Figure 2e), BMI was significantly associated with OS (p = 0.0023, Figure 2f). Furthermore, in the forest plot analysis, key clinical factors of survival (PFS or OS) by BMI subgroup (high or low) were evaluated (Figure 3a,b). As a result, men aged <75 years or ≥ 75 years, ECOG‐PS of 0–1, smokers, adenocarcinoma, stage III–IV disease, prior radiotherapy, and GPS of 0–1 were identified as significant factors of OS according to the BMI subgroups, although none were factors of PFS except for current or former smoker (Figure 3a,b).
FIGURE 1.

Kaplan–Meier curves for progression‐free survival (PFS) and overall survival (OS) among 99 patients who received nivolumab monotherapy as a second‐line treatment. (a) The median PFS was 3.4 months (95% confidence interval [CI] 2.1–4.7). (b) The median OS was 13.4 months (95% CI: 9.5–17.7)
TABLE 3.
Univariate and multivariate analyses of progression‐free survival and overall survival
| Median PFS | Univariate analysis | Multivariate analysis | Median OS | Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | (months) | HR | 95% CI | p‐value | HR | 95% CI | p‐value | (months) | HR | 95% CI | p‐value | HR | 95% CI | p‐value |
| Sex | ||||||||||||||
| Men/women | 3.0/3.4 | 0.97 | 0.60–1.65 | 0.92 | 14.3/11.9 | 0.94 | 0.55–1.71 | 0.84 | ||||||
| Age (years) | ||||||||||||||
| <75/≥75 | 3.0/7.7 | 1.28 | 0.74–2.37 | 0.37 | 13.3/17.4 | 1.33 | 0.71–2.78 | 0.37 | ||||||
| Performance status (PS) | ||||||||||||||
| 0–1/2–3 | 4.3/1.9 | 0.42 | 0.26–0.71 | 0.0016 | 0.44 | 0.27–0.74 | 0.0027 | 17.7/4.6 | 0.22 | 0.13–0.39 | <0.0001 | 0.25 | 0.14–0.44 | <0.0001 |
| Smoking history | ||||||||||||||
| Yes/No | 3.5/2.8 | 0.90 | 0.52–1.66 | 0.72 | 13.4/19.2 | 1.24 | 0.66–2.58 | 0.50 | ||||||
| Histological classification | ||||||||||||||
| Adenocarcinoma/nonadenocarcinoma | 3.1/3.5 | 1.03 | 0.68–1.58 | 0.86 | 15.5/11.5 | 0.85 | 0.53–1.36 | 0.49 | ||||||
| Clinical stage at diagnosis | ||||||||||||||
| III–IV/postoperative recurrence | 2.4/6.2 | 1.69 | 0.98–3.12 | 0.05 | 11.5/23.6 | 1.38 | 0.78–2.65 | 0.26 | ||||||
| Prior radiotherapy | ||||||||||||||
| Yes/No | 5.3/2.4 | 0.67 | 0.42–1.04 | 0.07 | 15.7/13.4 | 0.88 | 0.53–1.43 | 0.63 | ||||||
| GPS | ||||||||||||||
| 0, 1/2 | 3.7/2.3 | 0.79 | 0.50–1.27 | 0.32 | 16.1/5.7 | 0.61 | 0.37–1.02 | 0.06 | ||||||
| BMI (kg/m2) | ||||||||||||||
| Low (<22.1)/high (≥22.1) | 2.4/3.8 | 1.38 | 0.91–2.11 | 0.12 | 1.29 | 0.84–1.97 | 0.22 | 8.5/19.1 | 2.05 | 1.28–3.32 | 0.0027 | 1.79 | 1.10–2.93 | 0.0184 |
Note: The reference arms are the variables shown in the right‐sided arms. p‐values in bold are statistically significant (p < 0.05).
Abbreviations: BMI, body mass index; CI, confidence interval; GPS, Glasgow prognostic score; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; PS, performance status.
FIGURE 2.
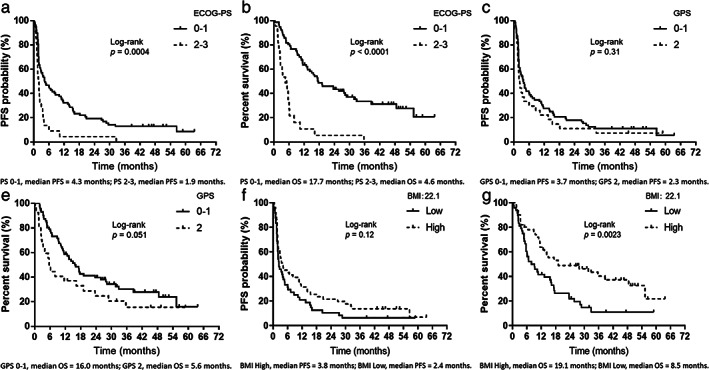
Kaplan–Meier curves for progression‐free survival (PFS) and overall survival (OS) according to the Eastern Cooperative Oncology Group (ECOG) performance status (PS), Glasgow prognostic score, and body mass index. (a) PFS according to ECOG‐PS at the initiation of nivolumab administration (Glasgow prognostic score [GPS] 0–1, median PFS = 4.3 months; GPS 2, median PFS = 1.9 months). (b) Overall survival (OS) according to ECOG‐PS at the initiation of nivolumab monotherapy (GPS 0–1, median OS = 17.7 months; GPS 2, median OS = 4.6 months). (c) PFS according to GPS at the initiation of nivolumab administration (GPS 0–1, median PFS = 3.7 months; GPS 2, median PFS = 2.3 months). (d) OS according to GPS at the initiation of nivolumab administration (GPS 0–1, median OS = 16.0 months; GPS 2, median OS = 5.6 months). (e) PFS according to BMI at the initiation of nivolumab administration (body mass index [BMI] high, median PFS = 3.8 months; BMI low, median PFS = 2.4 months). (f) OS according to BMI at the initiation of nivolumab administration (BMI high, median OS = 19.1 months; BMI low, median OS = 8.5 months)
FIGURE 3.
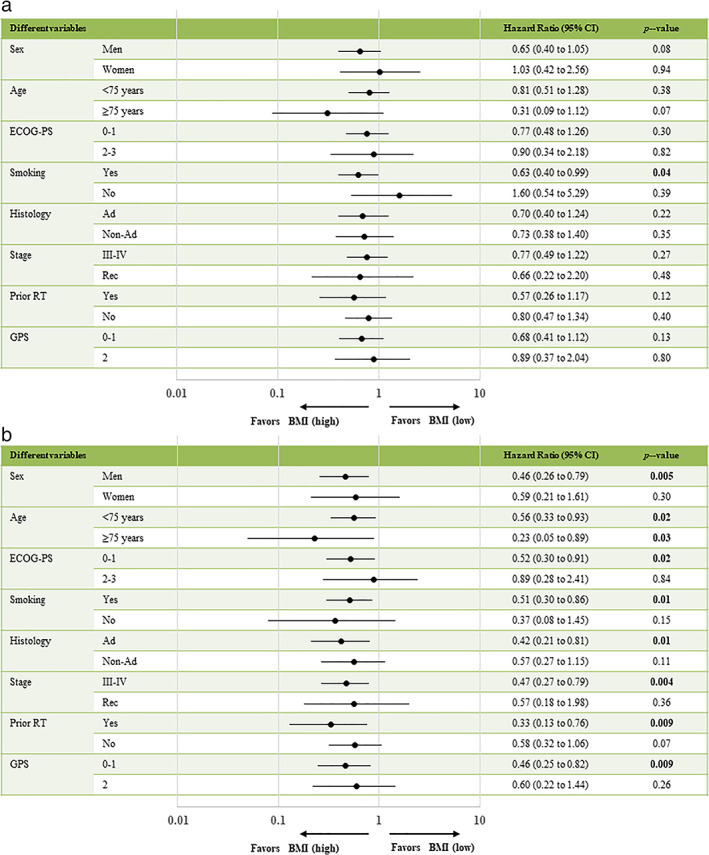
(a) Analysis of PFS and (b) OS by key clinical factors of body mass index. Ad, adenocarcinoma; BMI, body mass index; CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group‐performance status; GPS, Glasgow prognostic score; PFS, progression‐free survival; rec, recurrence; RT, radiotherapy
DISCUSSION
This study evaluated the relationship between BMI and GPS and treatment survival efficacy in patients treated with nivolumab monotherapy as a second‐line treatment for NSCLC. In multivariate analyses, ECOG‐PS and BMI were independent prognostic factors for OS, suggesting that ECOG‐PS and BMI are clinically useful factors for predicting OS in patients who were treated with nivolumab monotherapy as a second‐line treatment for NSCLC. In addition, high BMI was identified as an independent predictor for OS, particularly in men aged <75 years or ≥ 75 years with ECOG‐PS of 0–1 who are smokers with adenocarcinoma, stage III–IV disease, prior radiotherapy, and a GPS of 0–1. Additionally, a high BMI was an independent predictor of OS irrespective of age.
The GPS was derived from serum CRP and albumin concentrations that can be easily measured in routine clinical practice. 10 Although several reports demonstrate associations between the GPS and therapeutic effects of ICIs in patients with NSCLC with different treatment lines and various ICIs and levels of PD‐L1 expression, 9 , 20 , 21 no significant differences between GPSs of 0–1 and 2 were observed in terms of PFS and OS in the current study. Reasons for this may include the relatively small number of patients and the influence of various factors from previous treatments since this was a study of second‐line treatments. However, the cause is still unclear, and further investigations are needed in the future. Furthermore, as shown in Figure 3b, the high BMI group had better OS than the low BMI group with GPSs of 0–1, suggesting that the high BMI group had a better prognosis with a GPS of 0–1.
In this study, ECOG‐PS and BMI were independent prognostic factors for OS in multivariate analyses. In particular, the ECOG‐PS, which is a subjective scoring system that evaluates the overall general condition of patients with cancer and is correlated independently with PFS and OS, has been a demonstrated potent prognostic factor. 24 , 25 The present study reaffirms PS as a strong prognostic factor, as reported in previous studies, 24 , 25 and suggests that our study population likely reflects the general patient population. Regarding BMI, a retrospective study of a large number of patients reported that a high BMI was associated with prolonged PFS and OS after ICI treatment in patients with advanced melanoma. 26 Other retrospective studies on solid malignancies such as melanoma, renal cell carcinoma, and NSCLC, reported that BMI is associated with ICI efficacy. 7 In addition, a relationship between BMI in patients with NSCLC treated with ICI and survival, such as PFS and OS, has been oberved. 8 BMI is significantly related with the survival efficacy of ICI therapy in patients with NSCLC treated with second‐line or subsequent‐line PD‐1/PD‐L1 blockade therapy, and is reported to have better outcomes for patients with a high BMI. Table 4 summarizes the results of our study and those of previous studies that have evaluated BMI in patients with advanced NSCLC previously treated with ICIs. 8 , 27 , 28 , 29 , 30 , 31
TABLE 4.
Reports of the body mass index on immune checkpoint inhibitor therapy for pretreated advanced non‐small cell lung cancer
| Report | Year | Region | Study type | Sample size | Stage | Treatment | BMI cutoff | Treatment line | Outcome according to BMI HR (95% CI), p‐value | |
|---|---|---|---|---|---|---|---|---|---|---|
| PFS | OS | |||||||||
| Popinat et al. 27 | 2019 | France | Retrospective | 55 | IV | Nivolumab monotherapy | 24.7 | Pretreated (≥second line) | NR |
(High/Low): NR, p = 0.082 OS tends to be longer |
| Ichihara et al. 22 | 2020 | Japan | Retrospective | 429 | Advanced, recurrence | Pembrolizumab, nivolumab, or atezolizumab monotherapy | 22.0 | Pretreated (≥second line) |
(High/Low): 0.79 (0.64–0.98), p = 0.036 |
(High/Low): 0.73 (0.57–0.95), p = 0.021 |
| Kichenadasse et al. 28 | 2020 | Global | Prospective | 1434 | IV, recurrence | Atezolizumab monotherapy | 25.0 | Untreated and pretreated |
(High/Low): overweight, 0.89 (0.78–1.01), p = 0.09, obese, 0.86 (0.73–1.01), p = 0.09 |
(High/Low): overweight, 0.81 (0.68–0.95), p < 0.001, obese, 0.64 (0.51–0.81), p < 0.001 |
| Dimitrakopoulos 29 | 2020 | Greece | Retrospective and prospective | 112 | III–IV | Pembrolizumab or nivolumab monotherapy | 26.26 | Untreated and pretreated (≥second‐line) |
(High/Low): 0.738 (0.471–1.156), p = 0.160 |
(High/Low): 0.853 (0.507–1.436), p = 0.542 |
| Takada et al. 30 | 2020 | Japan | Retrospective | 226 | IIIB–IV, recurrence | Pembrolizumab or nivolumab monotherapy | 19.1 | Untreated and pretreated (≥second‐ line) |
(Low/High): 1.47 (1.04–2.05), p = 0.0269 |
(Low/High): 1.29 (1.10–2.30), p = 0.0138 |
| Dragomir et al. 31 | 2021 | Romania | Retrospective | 80 | I–IV | Nivolumab monotherapy | 25.0 | Pretreated |
(High/Low): 0.96 (0.96–1.91), p = 0.001 |
NR |
| Current study | Japan | Retrospective | 99 | III–IV, recurrence | Nivolumab monotherapy | 22.1 | Pretreated (second‐line only) |
(Low/High): 1.29 (0.84–1.97), p = 0.22 |
(Low/High): 1.79 (1.10–2.93), p = 0.0184 |
|
Note: p‐values in bold are statistically significant (p < 0.05).
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; NR, not reported; OS, overall survival; PFS, progression‐free survival.
When comparing the high and low BMI cohorts, the number of nivolumab administration cycles was statistically significantly higher in the high BMI cohort. However, the OS was significantly better in the high BMI cohort, but not the PFS. This may enhance the prognostic benefit of nivolumab monotherapy in patients with a high BMI and may also provide patients with the opportunity to receive more nivolumab cycles and an improved course of treatment after nivolumab. A study examining the association between BMI at the start of treatment and OS in 703 patients with metastatic NSCLC treated with nivolumab and pembrolizumab found that low BMI was associated with shorter OS (HR: 1.66, p = 0.002) and a high BMI with a longer OS (HR: 0.75, p = 0.039), similar to the findings of our study. 32 Another report examined the correlation of BMI with PFS and OS in patients with melanoma using ipilimumab and showed a nonsignificant trend toward longer OS in overweight patients, (p = 0.056, log‐rank test; HR: 1.81, Cl: 95%: 0.98–3.33). Moreover, there was no significant difference in PFS (p = 0.924, log‐rank test; HR: 1.03, CI: 95%: 0.62–1.70). 33 In patients with previously treated NSCLC, no significant difference was observed in PFS or OS between the low and high BMI groups (Table 4), while other studies reported both PFS and OS as being better in the high BMI group. 22 , 30 However, some reports indicate better OS in the high BMI group only, consistent with our study. 27 , 28 This discrepancy between PFS and OS is a common observation when assessing the efficacy of immunotherapy. Reasons for this include the difficulty in determining reliable PFS, and the possibility of residual effects of immunotherapy. Our findings are consistent with those of previous studies suggesting that high BMI is associated with improved survival with ICI therapy in various cancer types (melanoma, renal cell carcinoma, and NSCLC). Although previous reports included second‐line nivolumab monotherapy, none focused exclusively on second‐line nivolumab monotherapy. Furthermore, BMI can be easily used in daily clinical practice. BMI was an independent prognostic factor for OS as well as ECOG‐PS (Table 3) in our analysis. BMI is an objective parameter that allows for a more accurate and objective classification of patients. Therefore, BMI can be easily measured prior to the start of treatment in the clinical setting. In clinical practice, medical oncologists do not usually hesitate to initiate nivolumab monotherapy simply because of a low BMI, although they are hesitant in patients with poor PS. Therefore, these two indices (ECOG‐PS and BMI) of different dimensions need to complement each other in clinical practice, and it is reasonable to take BMI into account in clinical practice when administrating ICI therapy. In addition, the results showed that a high BMI is associated with a good prognosis, although this does not mean that obesity is acceptable for improving the prognosis of patients with NSCLC, and identification of the optimal BMI should be investigated in the future.
The pathophysiology of the positive correlation between overweight and survival after ICI treatment is currently unclear, although leptin‐mediated T cell dysfunction has been implicated. 34 Based on this hypothesis, nivolumab, which acts as an anti‐PD‐1 antibody and inhibits the binding between PD‐L1/PD‐1 molecules, may induce better efficacy in patients with high BMI and manifest PD‐1 T cell exhaustion. However, the rationale for this remains unclear and there is a need to clarify the pathophysiology of how immunotherapy offers better prognostic effects in patients who are overweight.
There are several limitations to this study. First, this was a retrospective study that relied on subjective efficacy judgments by the treating physicians, which undeniably led to treatment response and PFS data variabilities. Second, there is no absolutely established cutoff value for BMI or laboratory data, and various cutoff values have been used in previous studies. We used ROC curves to determine the BMI cutoff value and those of previous studies for GPS. Furthermore, the BMI cutoff value for Westerners may be different from that of Japanese people, who are mostly small‐statured. Therefore, it is important to validate the clinical relevance of these cutoff values in a larger patient cohort in future. Another prognostic factor that affected survival in patients with NSCLC is weight loss before treatment, a known indicator for cachexia. 35 , 36 , 37 Weight loss before or during treatment may have various effects in the therapeutic response to ICI administration. In our study, BMI was calculated using a single measurement of height and weight prior to nivolumab administration, although the role of weight loss prior to or during treatment was difficult to analyze or evaluate because of inconsistent records of weight loss prior to and during nivolumab administration. Third, the nivolumab dose was adjusted to 3 mg/kg bodyweight on some occasions, and at other times to a fixed dose of 240 mg/day. In this regard, in Japan, the dosage of nivolumab was approved to change from 3 mg/kg to 240 mg/bodyweight by the Japanese regulatory authority in August 2018, and since then the dosage of 3 mg/kg cannot be used. Therefore, patients who earlier received the 3 mg/kg dose also switched to 240 mg/bodyweight dosage during the course of therapy. A previous report has shown the equivalence of nivolumab administration at 3 mg/kg and 240 mg/bodyweight, 38 and we do not believe that this has any impact on the current study.
In conclusion, this study confirmed ECOG‐PS as an independent prognostic factor for PFS and OS. Furthermore, BMI was an independent prognostic factor for OS. Although further studies are needed to validate these findings, the results suggest that BMI assessment may be useful in predicting the OS of patients who received nivolumab monotherapy as a second‐line treatment. Therefore, a larger study is needed to evaluate whether our results can be generalized to other second‐line ICI‐treated patient populations. Furthermore, it is unclear from the findings of the current study whether BMI can be considered a treatment effect modifier, although future large‐scale analyses of the effects of BMI subgroups in clinical trials of ICI treatments may answer this question.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Ms Kyoko Nakagawa for her assistance in preparing the manuscript and Editage (www.editage.jp) for English language editing.
Imai H, Naito E, Yamaguchi O, Hashimoto K, Iemura H, Miura Y, et al. Pretreatment body mass index predicts survival among patients administered nivolumab monotherapy for pretreated non‐small cell lung cancer. Thorac Cancer. 2022;13:1479–1489. 10.1111/1759-7714.14417
Hisao Imai and Erika Naito contributed equally to this work.
Funding informationThis research received no external funding.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, HI, upon reasonable request.
REFERENCES
- 1. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 5. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non‐small cell lung cancer treated with PD‐1 inhibitors: a preliminary retrospective study. Sci Rep. 2019;9:2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti‐PD‐1/PD‐L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichihara E, Harada D, Inoue K, Sato K, Hosokawa S, Kishino D, et al. The impact of body mass index on the efficacy of anti‐PD‐1/PD‐L1 antibodies in patients with non‐small cell lung cancer. Lung Cancer. 2020;139:140–5. [DOI] [PubMed] [Google Scholar]
- 9. Imai H, Kishikawa T, Minemura H, Yamada Y, Ibe T, Yamaguchi O, et al. Pretreatment Glasgow prognostic score predicts survival among patients with high PD‐L1 expression administered first‐line pembrolizumab monotherapy for non‐small cell lung cancer. Cancer Med. 2021;10:6971–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMillan DC. An inflammation‐based prognostic score and its role in the nutrition‐based management of patients with cancer. Proc Nutr Soc. 2008;67:257–62. [DOI] [PubMed] [Google Scholar]
- 11. Proctor MJ, Talwar D, Balmar SM, O'Reilly DSJ, Foulis AK, Horgan PG, et al. The relationship between the presence and site of cancer, an inflammation‐based prognostic score and biochemical parameters. Initial results of the Glasgow inflammation outcome study. Br J Cancer. 2010;103:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation‐based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum‐based chemotherapy for inoperable non‐small‐cell lung cancer. Br J Cancer. 2004;90:1704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gioulbasanis I, Pallis A, Vlachostergios PJ, Xyrafas A, Giannousi Z, Perdikouri IE, et al. The Glasgow prognostic score (GPS) predicts toxicity and efficacy in platinum‐based treated patients with metastatic lung cancer. Lung Cancer. 2012;77:383–8. [DOI] [PubMed] [Google Scholar]
- 14. Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment Glasgow prognostic score in patients with advanced inoperable non‐small cell lung cancer. J Thorac Oncol. 2012;7:655–62. [DOI] [PubMed] [Google Scholar]
- 15. Umihanic S, Umihanic S, Jamakosmanovic S, Brkic S, Osmic M, Dedic S, et al. Glasgow prognostic score in patients receiving chemotherapy for non‐small‐cell lung cancer in stages IIIb and IV. Med Arch. 2014;68:83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang AG, Chen HL, Lu HY. Comparison of Glasgow prognostic score and prognostic index in patients with advanced non‐small cell lung cancer. J Cancer Res Clin Oncol. 2015;141:563–8. [DOI] [PubMed] [Google Scholar]
- 17. Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, et al. Prognosis in advanced lung cancer ‐ a prospective study examining key clinicopathological factors. Lung Cancer. 2015;88:304–9. [DOI] [PubMed] [Google Scholar]
- 18. Zhu L, Li X, Shen Y, Cao Y, Fang X, Chen J, et al. A new prognostic score based on the systemic inflammatory response in patients with inoperable non‐small‐cell lung cancer. Onco Targets Ther. 2016;9:4879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minami S, Ihara S, Kim SH, Yamamoto S, Komuta K. Lymphocyte to monocyte ratio and modified Glasgow prognostic score predict prognosis of lung adenocarcinoma without driver mutation. World J Oncol. 2018;9:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasahara N, Sunaga N, Tsukagoshi Y, et al. Post‐treatment Glasgow prognostic score predicts efficacy in advanced non‐small‐cell lung cancer treated with anti‐PD1. Anticancer Res. 2019;39:1455–61. [DOI] [PubMed] [Google Scholar]
- 21. Takamori S, Takada K, Shimokawa M, Matsubara T, Fujishita T, Ito K, et al. Clinical utility of pretreatment Glasgow prognostic score in non‐small‐cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2021;152:27–33. [DOI] [PubMed] [Google Scholar]
- 22. Araki T, Tateishi K, Sonehara K, Hirota S, Komatsu M, Yamamoto M, et al. Clinical utility of the C‐reactive protein:albumin ratio in non‐small cell lung cancer patients treated with nivolumab. Thorac Cancer. 2021;12:603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 24. Capewell S, Sudlow MF. Performance and prognosis in patients with lung cancer. The Edinburgh Lung Cancer Group. Thorax. 1990;45:951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non‐small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620–30. [DOI] [PubMed] [Google Scholar]
- 26. McQuade JL, Daniel CR, Hess KR, et al. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Popinat G, Cousse S, Goldfarb L, Becker S, Gardin I, Salaün M, et al. Sub‐cutaneous fat mass measured on multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non‐small cell lung cancer treated by nivolumab. Onco Targets Ther. 2019;8:e1580128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non‐small cell lung cancer. JAMA Oncol. 2020;6:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dimitrakopoulos FI, Nikolakopoulos A, Kottorou A, Kalofonou F, Liolis E, Frantzi T, et al. PIOS (Patras immunotherapy score) score is associated with best overall response, progression‐free survival, and post‐immunotherapy overall survival in patients with advanced non‐small‐cell lung cancer (NSCLC) treated with anti‐program cell death‐1 (PD‐1) inhibitors. Cancers (Basel). 2020;12:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takada K, Takamori S, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, et al. Serum markers associated with treatment response and survival in non‐small cell lung cancer patients treated with anti‐PD‐1 therapy. Lung Cancer. 2020;145:18–26. [DOI] [PubMed] [Google Scholar]
- 31. Dragomir R, Dragomir AS, Negru A, Săftescu S, Popovici D, Schenker M, et al. Role of combining neutrophil‐to‐lymphocyte ratio and pretreatment body mass index in predicting progression‐free survival in patients with non‐small cell lung cancer treated with nivolumab. Exp Ther Med. 2021;21:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhi J, Khozin S, Kuk D, Torres AZ, Sorg R, Lee SE, et al. Association of baseline body mass index (BMI) with overall survival (OS) in patients (PTS) with metastatic non‐small cell lung cancer (mNSCLC) treated with nivolumab (N) and pembrolizumab (P). J Clin Oncol. 2018;36:6553. [Google Scholar]
- 33. Richtig G, Hoeller C, Wolf M, Wolf I, Rainer BM, Schulter G, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi‐Centre study. PLOS ONE. 2018;13:e0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD‐1 checkpoint blockade. Nat Med. 2019;25:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buccheri G, Ferrigno D. Prognostic factors in lung cancer: tables and comments. Eur Respir J. 1994;7:1350–64. [DOI] [PubMed] [Google Scholar]
- 36. Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170:e75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morel H, Raynard B, d'Arlhac M, et al. Prediagnosis weight loss, a stronger factor than BMI, to predict survival in patients with lung cancer. Lung Cancer. 2018;126:55–63. [DOI] [PubMed] [Google Scholar]
- 38. Zhao X, Suryawanshi S, Hruska M, Feng Y, Wang X, Shen J, et al. Assessment of nivolumab benefit‐risk profile of a 240‐mg flat dose relative to a 3‐mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, HI, upon reasonable request.


