Summary
Self-grooming is a stereotyped behavior displayed by nearly all animals. Among other established functions, self-grooming is implicated in social communication. However, whether self-grooming specifically influences behaviors of nearby individuals has not been directly tested, partly because of the technical challenge of inducing self-grooming in a reliable and temporally controllable manner. We recently found that optogenetic activation of dopamine D3 receptor expressing neurons in the ventral striatal islands of Calleja robustly induces orofacial grooming in mice. Using this optogenetic manipulation, here we demonstrate that observer mice exhibit social preference for mice that groom more regardless of biological sex. Moreover, grooming-induced social attraction depends on volatile chemosensory cues broadcasted from grooming mice. Collectively, our study establishes self-grooming as a means of promoting social attraction among mice via volatile cues, suggesting an additional benefit for animals to allocate a significant amount of time to this behavior.
Subject areas: Biological sciences, Neuroscience, Sensory neuroscience
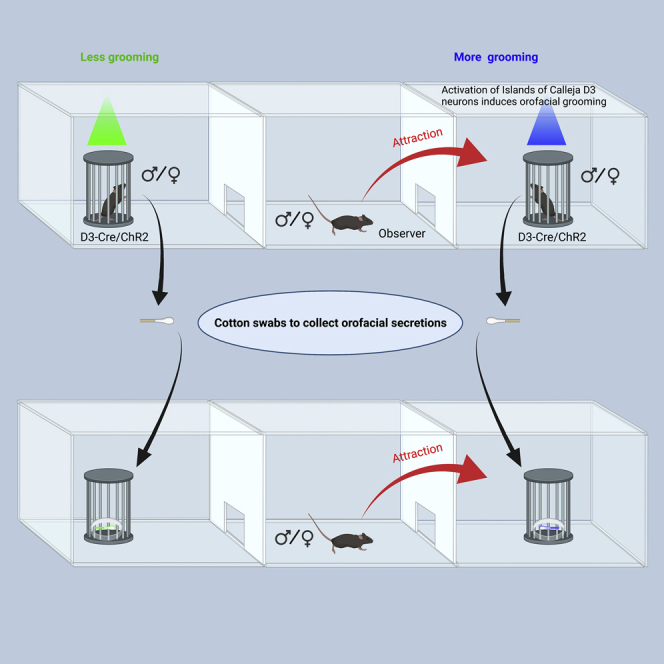
Graphical abstract
Highlights
-
•
An optogenetic approach induces orofacial grooming with temporal precision in mice
-
•
Observer mice show social preference toward mice that groom more regardless of sex
-
•
Preference toward grooming mice requires main olfactory epithelia of observer mice
-
•
Grooming-induced attraction depends on orofacial secretions from grooming mice
Biological sciences; Neuroscience; Sensory neuroscience
Introduction
Self-directed grooming is essential for hygiene maintenance, thermoregulation, de-arousal, and stress reduction; besides, not surprisingly, animals allocate significant time to this behavior (Kalueff et al., 2016; Spruijt et al., 1992). Although self-grooming is often conceptualized as a solitary or asocial behavior, it is implicated in social communication among conspecifics (Bursten et al., 2000; Ferkin and Leonard, 2010; Ferkin et al., 1996, 2001; Wiepkema, 1979). For instance, ground squirrels engage in self-grooming to deescalate agonistic encounters during territorial disputes (Bursten et al., 2000), and female meadow voles appear to be more attracted to males who groom (Ferkin and Leonard, 2010; Ferkin et al., 1996). These lines of evidence suggest that grooming broadcasts sensory cues that can influence behaviors among other nearby animals. However, direct evidence supporting this notion is still lacking, partly because of unpredictability of spontaneous grooming in experimental animals.
Recent advances in the understanding of neurobiological control of self-grooming make it possible to induce this behavior in laboratory mice via optogenetic manipulations of neuronal activity of specific cell types (Hong et al., 2014; Mangieri et al., 2018; Zhang et al., 2021). Notably, the islands of Calleja (IC), clusters of densely packed granule cells situated mostly in the olfactory tubercle (OT; also called tubular striatum (Wesson, 2020)), contribute to a ventral striatal circuit that is involved in grooming control. The IC granule cells are characterized by expression of the dopamine D3 receptor, and optogenetic activation of these neurons reliably induces orofacial grooming (i.e., Phase I to III nose-face-head grooming without Phase IV body licking (Berridge and Fentress, 1987; Kalueff et al., 2007)) in a temporally controllable manner (Zhang et al., 2021).
Using this optogenetic manipulation, we can induce orofacial grooming in mice with temporal precision and directly address previously unanswered questions: 1) does orofacial grooming help mice attract conspecifics, 2) whether such attraction shows sexual dimorphism, and 3) what sensory channel conveys the grooming signal from sender to receiver. Our results demonstrate that observer mice spend more time investigating mice that groom more regardless of biological sex, and that such attraction is mediated by orofacial secretions from grooming mice and requires functional main olfactory epithelia of recipient mice. Overall, this study establishes self-grooming as a means of promoting social attraction via chemosensory communication.
Results
Mice showing more grooming attract nearby individuals regardless of biological sex
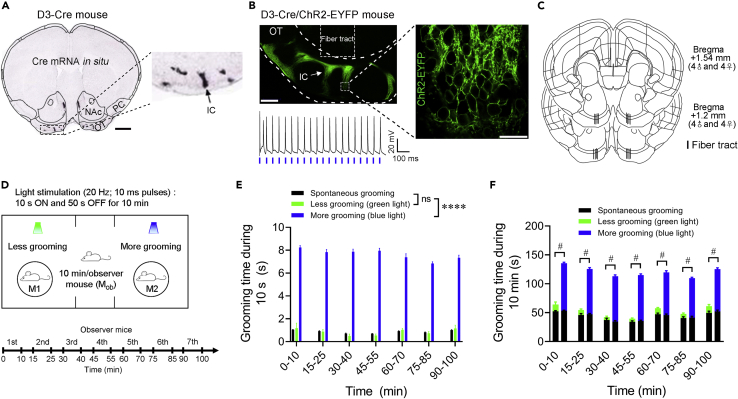
To investigate the potential role of self-grooming in social behaviors, we took advantage of an experimental approach which induces grooming with both reliability and temporal precision (Zhang et al., 2021). The ICs in the ventral striatum (mainly in the OT) are clusters of densely packed, granule cells expressing the dopamine D3 receptor (Figures 1A and 1B). Optogenetic activation of these D3 neurons via an optical fiber implanted in the OT of double transgenic D3-Cre/ChR2 mice robustly induces orofacial grooming (Figures 1B and 1C, and Video S1 (Zhang et al., 2021)). Because most OT/IC D3-ChR2 neurons are fired at a maximum rate of 20 Hz upon current injection and faithfully followed 20 Hz blue light stimulation in brain slices (Figure 1B (Zhang et al., 2021)), we used 20 Hz stimulation for all behavioral experiments. A three-chamber apparatus was used to assess social preference (Figure 1D).
Figure 1.
Paradigm for monitoring social preference during reliable induction of self-grooming
(A) A coronal section across the ventral striatum showing in situ hybridization of Cre mRNA in a D3-Cre mouse. Image credit: Allen Institute (Allen Mouse Brain Connectivity Atlas: http://connectivity.brain-map.org/transgenic/experiment/304166273). Scale bar = 1 mm. Inset: an enlarged view of dotted rectangle area in the left panel. Arrow denotes the IC. PC, piriform cortex. NAc, nucleus accumbens. OT, olfactory tubercle. IC, islands of Calleja.
(B) D3-ChR2 neurons are densely packed in the IC. Left, a representative image (coronal section) showing the IC and the optical fiber tract (upper), as well as the firing of an IC D3-ChR2 neuron upon laser stimulation at 20 Hz (lower). Scale bar = 200 μm. Right, an enlarged image of the IC (dotted rectangle area in the left panel). Scale bar = 20 μm.
(C) Coronal brain panels showing optical fiber placements in D3-Cre/ChR2 mice.
(D) Upper, schematic showing the behavioral strategy in the three-chamber apparatus. Blue light stimulation of OT D3-ChR2 neurons induced robust self-grooming (More grooming) while green light with the same stimulation parameters produced less (Less grooming). Lower, schematic depicting experimental timeline of the test. Ten min test for each observer mouse (Mob) with a 5 min interval between two consecutive mice.
(E and F) Comparison of total grooming time in 10 s (E) and 10 min (F) between spontaneous and light-stimulation conditions in an entire session. Data are shown as the mean ± SEM. One-way ANOVA test with post hoc Tukey’s multiple comparison test (E) and two-tailed unpaired Student’s t test in (F). ∗∗∗∗ or #, p < 0.0001. Raw data are included in Table S1 and results of statistical analyses are included in Table S2.
Two D3-Cre/ChR2 mice (same-sex littermates) with an optical fiber implanted in the OT were placed in each of the two side chambers under a cup (M1 and M2 in Figure 1D). These two mice served as counterparts that groomed more or less, stimulated by blue or green light, respectively, whereas each observer mouse (Mob; unoperated) was placed in the center of the middle chamber and its behavior was recorded for 10 min (Figure 1D). To prevent exhaustion of firing or neurotransmitter release of D3-Cre/ChR2 neurons, the light alternated between ON (10 s) and OFF (50 s) during the 10 min test. Although the grooming time during green light stimulation was not significantly different from spontaneous grooming when light was off, blue light reliably elicited more grooming (7-8 s/10 s stimulation) than green light during the entire session (Figure 1E). During a 10 min test period for each observer mouse, blue light-stimulated mice exhibited a total of 120-140 s orofacial grooming (light-induced plus spontaneous), which more than doubled the grooming time in green light-stimulated counterparts (Figure 1F). Table 1 summarizes the eight pairs of mice (both male and female) used in each of the following experiments.
Table 1.
Light stimulated D3-Cre/ChR2 mouse pairs used in each experiment
| Figure # | Mouse pairs |
|---|---|
| 1E, 1F | Female pairs: 1-4; Male pairs: 1-4 |
| 2A | Female pair 1 |
| 2B | Female pairs: 1, 2 |
| 2C | Male pairs: 1, 2 |
| 3 | Female pairs: 3, 4; Male pairs: 3, 4 |
| 4A, 4B | Female pair 4 |
| 4C, 4D | Male pair 4 |
| S1A | Female pair 1 |
| S1B | Female pairs: 1, 2 |
| S1C | Male pairs: 1, 2 |
| S1D | Female pairs: 1, 2; Male pairs: 1, 2 |
| S3A | Female pairs: 1-4 |
| S3B | Male pairs: 1-4 |
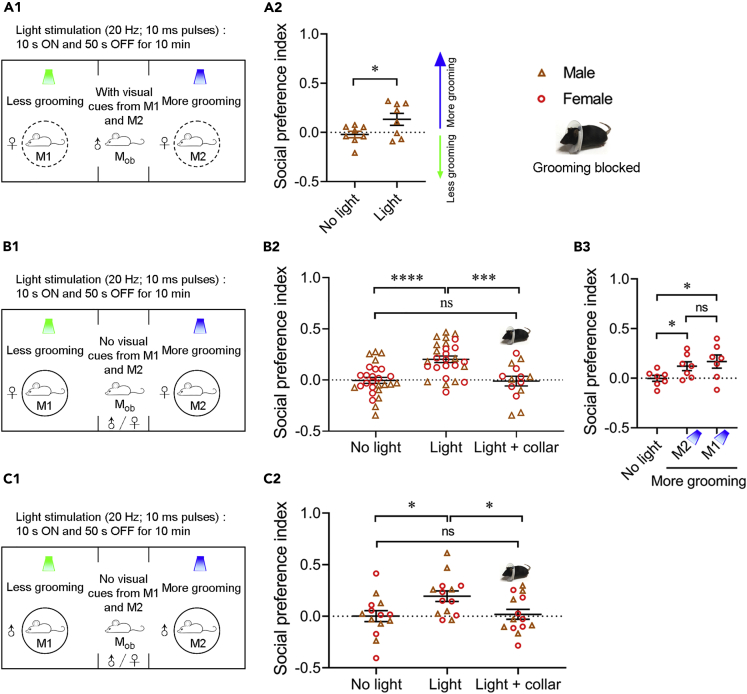
We performed the initial social preference tests with two female mice stimulated by blue (more grooming) or green light (less grooming) under customized cups in the two side chambers, allowing emission of several possibly salient sensory cues (visual, auditory, and olfactory) (Figure 2A1). Each observer mouse (male) was placed in the middle chamber and the time it spent in each of the side chambers was recorded during 10 min, which was used to calculate the social preference index (see STAR Methods for details; the total duration of stay for each observer mouse for all figures was included in Table S1). An index value of 0 indicates no preference and a positive or negative value indicates preference of an observer mouse toward one mouse or the other (Figure 2A2). A control session (no light stimulation) with the same mice was performed 24 h before the light stimulation session. Compared to the no light control, in which the observer mice did not show a preference, they exhibited social preference toward the mouse that received blue light stimulation (more grooming) (Figure 2A2). Such preference was also reflected in an increase in the total investigation time and number of investigation bouts (Figure S1A). To test whether blue light-stimulated mice (more grooming) attract conspecifics via visual communication, we covered the cups with paper towels with numerous tiny holes (Video S2). Observer mice showed similar preference toward mice showing more grooming even in the absence of visual cues from light-stimulated mice (Figures 2B1, 2B2, and S1B), suggesting that visual cues are dispensable for grooming-induced social attraction.
Figure 2.
Observer mice show social preference for mice that groom more regardless of biological sex
(A) Observer mice (n = 8) spent more time on the side of the mouse that groomed more (with visual cues).
(A1) Schematic showing the behavioral strategy.
(A2) Social preference index under no light and light stimulation conditions.
(B) Both male and female observer mice were attracted to the female mouse that groomed more (without visual cues).
(B1) Schematic showing the behavioral strategy.
(B2) Social preference index of observer mice under no light and light stimulation (with or without collar) conditions.
(B3) Observer mice were always attracted to mice that groomed more. Light-stimulated mice were swapped by switching the blue/green light stimulation. For B2, n = 27 observer mice (13 males and 14 females). The observer mice in the light + collar group were a subcohort of the mice tested. For B3, n = 7 observer mice (a subcohort of B2).
(C) Both male and female observer mice were attracted to male mice that groomed more.
(C1) Schematic showing the behavioral strategy.
(C2) Social preference index of observer mice under no light and light stimulation (with or without collar) conditions. For (C2), n = 14 observer mice (7 males and 7 females). Data are shown as the mean ± SEM. Two-tailed paired Student’s t test in (A2, B3) and one-way ANOVA test with post hoc Tukey’s multiple comparison test in (B2, C2). ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, and ns, not significant. Raw data for calculating the social preference index are included in Table S1 and results of statistical analyses in Table S2. See also Figures S1–S3.
We also examined whether grooming mice make audible calls and/or ultrasonic vocalizations by recording sounds up to 96 kHz. Grooming mice did not produce robust vocalizations, even though we were able to record spontaneous audible calls (Figure S2A and Audio S1). For some grooming strokes, we did record associated sounds, which appeared to result from physical contacts of the optical fiber tether with the recording chamber rather than grooming per se (Figure S2B and Audio S2), as no reliable sounds were associated with spontaneous orofacial grooming in mice without a tether (not shown). We did not further investigate whether these sounds could attract other mice, given the sufficiency of chemosensory communication in grooming-induced social attraction as we uncovered by the following experiments.
Across the animal kingdom, many displays of communication are presented uniquely by each sex. Therefore, we investigated whether grooming-induced social attraction is sexually dimorphic. For either male or female light-stimulated mouse pairs, the observer mice (both male and female) exhibited a significantly higher social preference index toward blue light-stimulated mice (more grooming) compared to no light control (Figures 2B1, 2B2, 2C1, 2C2, S1B, and S1C). These data indicate that mice displaying more orofacial grooming attract other mice regardless of their biological sex.
Control experiments were conducted to ensure that the observed social preference was indeed attributed to orofacial grooming. First, as previously described, in each set of experiments, the same observer mice did not show preference when no light was delivered to the two mice in the side chambers. Second, when the two light-stimulated mice wore a collar, which prevented orofacial grooming, social preference toward blue light-stimulated mice (more grooming) was eliminated (Figures 2B2 and 2C2). Upon blue light stimulation, collar-wearing mice still attempted grooming motions, but their forepaws failed to reach the orofacial parts (Video S1). Note that during the experiments, the observer mice could not see light-stimulated mice under the covered cups. This experiment also ruled out the possibility that optogenetic activation of OT D3 neurons directly increases the secretion of volatile chemosensory cues (see below). Third, when the mice receiving blue or green light were swapped or when the chamber assignment was switched, the observer mice always showed preference toward blue light-stimulated mice (more grooming) (Figure 2B3). Fourth, the time that the observer mice spent on the side of blue light-stimulated mice was independent of the testing order (Figure S3), suggesting that orofacial grooming of blue light-stimulated mice was effective in attracting conspecifics throughout the entire session. Furthermore, we quantified the self-grooming behavior conducted by the observer mice when they were near light-stimulated mice and did not find a significant difference between the two sides (Figure S1D), suggesting that grooming in one mouse does not promote self-grooming in nearby mice.
Grooming-induced social attraction depends on chemosensory communication
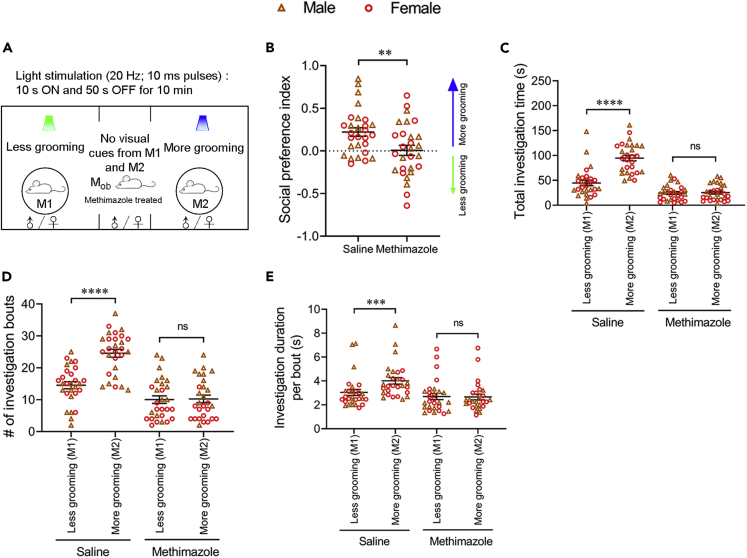
Because olfactory cues are pivotal for social communication in mice, we sought to test the role of the olfactory system in orofacial grooming-induced social attraction by rendering the observer mice hyposmic. We intraperitoneally injected saline (as control) or methimazole in the observer mice and performed behavioral tests four days later (Figure 3A). Methimazole treatment ablated the main olfactory epithelium (MOE) but left the vomeronasal epithelium intact (Figure S4). Consistent with reduced perception of volatile odors (Suzukawa et al., 2011), methimazole-treated observer mice decreased both the total time and number of bouts investigating light-stimulated mice compared to the saline controls (Figures 3C–3E). Nevertheless, methimazole-injected observer mice still moved around in the three-chamber arena, but they did not show social preference toward mice that groomed more as the saline-injected controls (Figure 3B). This result suggests an essential role played by the MOE of the observer mice in this process; however, because of potential off-target effects of methimazole and reduced social investigation, it is unclear whether there is a direct causal link between ablation of the MOE and lack of social preference.
Figure 3.
Social preference toward mice showing more grooming depends on functional main olfactory system of observer mice
(A and B) Ablation of the main olfactory epithelia of observer mice via methimazole treatment abolished their social preference toward blue light-stimulated mice (more grooming). (A) Schematic showing the behavioral strategy. (B) Social preference index of observer mice treated with saline (as control) or methimazole (hyposmic group).
(C–E) Methimazole treatment in observer mice reduced their investigation behavior. Quantification of total investigation time (C), number of investigation bouts (D), and investigation duration per bout (E), under the saline and methimazole treatment condition. n = 28 observer mice (14 males and 14 females) in each group. Data are shown as the mean ± SEM. Two-tailed paired Student’s t test: ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, and ns, not significant. Raw data for calculating the social preference index are included in Table S1 and results of statistical analyses in Table S2. See also Figure S4.
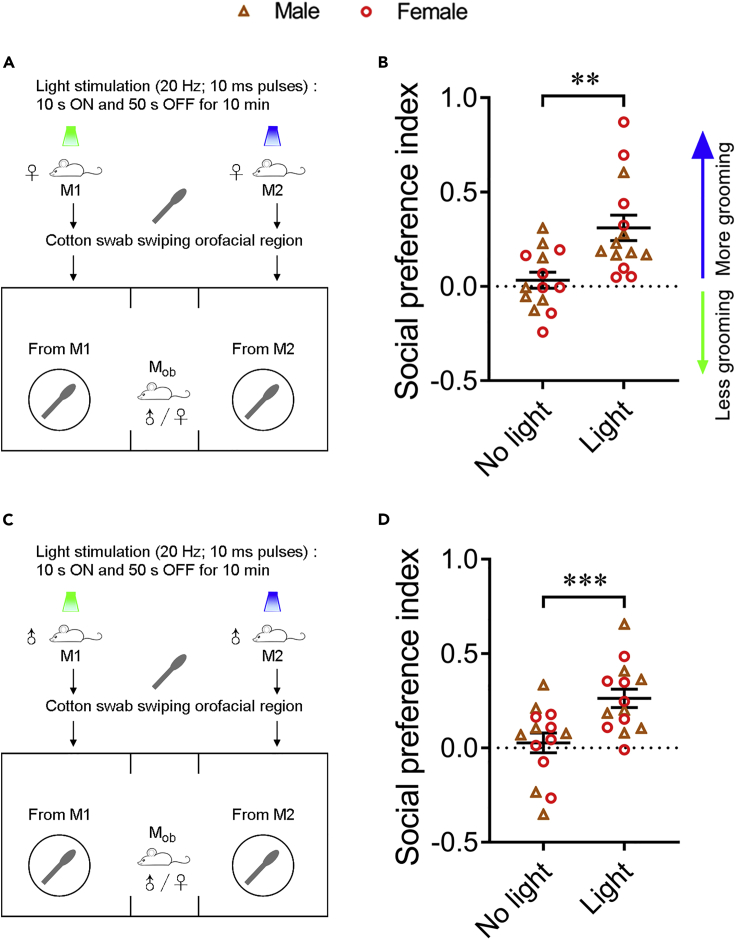
To provide direct evidence that self-grooming induces social attraction via orofacial chemical cues, we used mineral oil-moistened cotton swabs to sample the orofacial region (mouth, nose, cheek, and area surrounding eyes) of female mice that groomed more or less immediately after blue or green light stimulation (Figure 4A). The cotton swabs (each in a petri dish) were then placed under the cups to replace the light-stimulated mice in the previous experiments. Both male and female observer mice showed preference for the cotton swab from blue light-stimulated mice (more grooming) over the green light-stimulated mice (less grooming), in contrast to the control condition in which observer mice did not show preference for orofacial secretions of the same mice before they received light stimulation (Figure 4B). Similar findings were observed when the cotton swab experiments were conducted using orofacial secretions from male light-stimulated mice (Figures 4C and 4D). These results suggest that orofacial secretions from mice are broadcasted during grooming and are sufficient to attract conspecifics.
Figure 4.
Observer mice spend more time investigating orofacial secretions from blue light-stimulated mice
(A and B) Orofacial secretions from female blue light-stimulated mice attracted both male and female conspecifics. (A) Schematic showing the behavioral strategy. (B) Social preference index of observer mice under no light and light condition.
(C and D) Orofacial secretions from male blue light-stimulated mice attracted both female and male conspecifics. (C) Schematic showing the behavioral strategy. (D) Social preference index of observer mice under no light and light condition. n = 14 observer mice (7 males and seven females). In B and D, orofacial secretions collected under the light condition were from the same mice under the no light condition. Data are shown as the mean ± SEM. Wilcoxon matched-pairs signed rank test in (B) and two-tailed paired Student’s t test in (D): ∗∗p < 0.01 and ∗∗∗p < 0.001. Raw data for calculating the preference index are included in Table S1 and results of statistical analyses in Table S2.
Discussion
In the present study, using an optogenetic approach to induce orofacial grooming in a reliable and controllable manner, we demonstrate in mice that self-grooming promotes social attraction—regardless of biological sex—and observer mice are attracted to mice that groom more. This effect is predominantly mediated by orofacial secretions emitted as volatiles during self-grooming and perceived via the recipient’s main olfactory system. This work complements observational studies among other types of animals in the field and extends those observations by providing insights into a causal sensory channel involved in intraspecific communication via self-grooming.
Self-grooming allows an animal to emit rich sensory cues which potentially affect the behaviors of nearby recipients. Grooming without doubt is visually observable and thus a likely channel whereby it is perceived by an observer would be visual. In contrast, in the present experiments, we uncovered in mice that visual cues may be dispensable for grooming-induced social attraction because the effect persists without visual cues (Figure 2). Likewise, auditory cues may not be required because robust audible calls are not emitted during spontaneous grooming in mice without the fiber optic tether (data not shown). Even though we recorded grooming associated sounds, presumably because of physical contacts of the tether with the chamber (Figure S2), these sounds were not sufficient to attract observer mice because the grooming mice in the methimazole experiments (Figure 3) or with the collar (Figure 2) also had the tether. Compared to visual and auditory signals, chemosensory cues offer the unique ethological advantage of communication over time and space. In fact, rodents heavily rely on chemosensory cues for their social behaviors (e.g., Brennan and Kendrick, 2006; Kelliher, 2007; Spehr et al., 2006; Suarez et al., 2012; Wyatt, 2003). Through self-grooming, individuals can enhance the volatility of their bodily scents, potentially attracting conspecifics (Ferkin and Leonard, 2010; Ferkin et al., 1996, 2001; Wiepkema, 1979). Rodents use multiple chemosensory organs including the MOE and vomeronasal organ to detect social cues (Brennan and Kendrick, 2006; Kelliher, 2007; Spehr et al., 2006; Suarez et al., 2012; Wyatt, 2003). Although the vomeronasal organ is involved in communication via pheromones which are largely nonvolatile, the MOE receives and supports the processing of mainly volatile odors (Ache and Young, 2005; Li and Dulac, 2018). Consistent with this notion, methimazole treatment, which ablated the main olfactory epithelium but not the vomeronasal organ in the recipients (Figure S4), abolished their social preference for mice showing more grooming (Figure 3). Although methimazole reduced social investigation in general and might have off-target effects, our findings support that the main olfactory system of the recipient is required for social preference toward mice showing more grooming. We cannot completely rule out the possibility that spontaneous grooming may not increase the secretion of volatile chemosensory cues. But we think this scenario is unlikely because the orofacial grooming induced by optogenetic activation of OT D3 neurons is likely within the physiological range of natural grooming in terms of both duration and stroke frequency. In blue light-stimulated mice, the orofacial grooming duration increased roughly from 10% to 20% within 10 min (Figures 1E and 1F). We previously showed that spontaneous orofacial grooming in these mice ranged from 5% (91 s) to 27% (488 s) within 30 min in their home cage (Zhang et al., 2021). We also compared the stroke frequency between optogenetically induced and spontaneous grooming. As previously reported, the stroke frequency upon 20 Hz, 10 s blue light stimulation was 1.62 ± 0.22 Hz (mean ± SEM; 35 trials from 7 mice) (Zhang et al., 2021). As a comparison, the stroke frequency of spontaneous grooming was 2.83 ± 0.22 Hz (from randomly sampled 16 bouts from five D3-Cre/ChR2 mice). For optogenetically induced grooming, the stroke frequency was averaged for the entire 10 s, but the mice groomed for ∼7-8 s during the 10 s stimulation (Figure 1E). Consequently, the stroke frequency was underestimated compared to spontaneous grooming, in which the stroke frequency was calculated based on individual grooming bouts. Overall, optogenetically induced orofacial grooming does not seem to be artificially stronger than natural grooming.
This finding is not without precedent. It has been proposed that grooming animals may emit volatile cues to communicate their sex, identity, and reproductive status and thereby attract nearby opposite-sex conspecifics (Ferkin and Leonard, 2010; Ferkin et al., 1996, 2001; Wiepkema, 1979). However, to what extent self-grooming serves as a sexually dimorphic communication signal remains to be addressed. Male voles spend more time self-grooming when they are exposed to odors of females and are more responsive to grooming females (Ferkin and Leonard, 2010; Ferkin et al., 1996, 2001). However, the frequency and duration of self-grooming of male voles do not predict mating success (Wolff et al., 2002). Here we demonstrate that mice that groom more attract conspecifics regardless of sex (Figure 2), suggesting that, self-grooming provides broadly appealing social communicative signals in mice. The discrepancies between these studies may mainly result from two reasons. First, secretions released from orofacial glands (e.g., salivary and Harderian glands) and their effects on the recipients may vary in different species. Even in the same species, the secretions may vary under different contexts, hormonal statuses, and experimental conditions. For example, in Mongolian gerbils, Harderian secretions released during self-grooming are enhanced when the animals are exposed to cold temperatures (Thiessen, 1988). Second, in rodents, a complete grooming bout consists of a syntactic chain that progresses sequentially from nose-face-head (phase I-III) grooming to body licking (phase IV) (Berridge and Fentress, 1987; Kalueff et al., 2007). Bodily secretions induced by distinct grooming phases probably vary, which may lead to different behavioral effects in the recipients even in the same species. The optogenetic approach used in our study induces orofacial grooming but not body licking (Zhang et al., 2021). Body licking involves licking of the genital area, which is correlated with sexual behavior in rats (Hernandez-Gonzalez, 2000), probably because of grooming-induced release of sex-specific materials from genital glands.
We found that cotton swabs rubbed against the orofacial parts from mice showing more grooming are sufficient to attract the recipient mice (Figure 4), suggesting that self-grooming may lead to release of compounds from specifically the orofacial glands. Consistent with this finding, grooming-induced social attraction was absent when direct contacts of the forepaws with orofacial parts were prevented by a collar (Figure 2). Orofacial grooming releases and spreads compounds from various glands such as salivary and Harderian (Ritter and Epstein, 1974; Santillo et al., 2020; Yanase et al., 1991). These compounds can serve as social communicative signals in rodents, including mouse (Lee and Ingersoll, 1979), Mongolian gerbil (Harriman and Thiessen, 1985; Pendergrass and Thiessen, 1981; Thiessen et al., 1976), and golden hamster (Seyama and Uchijima, 2007). Our study reveals that orofacial grooming attracts other mice regardless of sex, but it does not rule out the possibility that orofacial grooming also releases sex-specific signals. In fact, the Harderian gland in golden hamster exhibits pronounced sexual dimorphism in histology and products, and males are more attracted to fresh smears of Harderian gland from females than males (Payne, 1979). In addition, Harderian gland secretions from male Mongolian gerbils contribute to the proceptive behaviors of estrous females (Harriman and Thiessen, 1985). Self-grooming released Harderian gland secretions from male mole rats are attractive to both sexes but female secretions are attractive only to males (Shanas and Terkel, 1997). Future studies are warranted to identify the specific types or combinations of orofacial secretions involved in grooming-induced general social attraction versus sexual attraction.
Limitations of the study
We have not directly tested whether spontaneous orofacial grooming in wild-type mice can promote social attraction because of its unpredictability in timing, duration, and intensity. More importantly, it is unclear to what extent our findings in lab-reared mice can be extrapolated to wild mice. Mice living in their natural habitats can form large, complex social groups (Phifer-Rixey and Nachman, 2015; Singleton and Krebs, 2007). Considering that the house mouse mating system has been described as both polygynous and promiscuous and that males are generally territorial, it would be interesting to determine how self-directed orofacial grooming influences social behaviors in natural settings.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-OMP antibody produced in rabbit | Sigma-Aldrich | (Cat# O7889; RRID:AB_796160) |
| Donkey anti-Rabbit Secondary Antibody, Alexa Fluor 488 | Invitrogen | (Cat# A21206) |
| Experimental models: Organisms/strains | ||
| Mouse: Tg(Drd3-cre)KI198Gsat/Mmucd | Mutant Mouse Resource and Research Center (MMRRC) | (RRID:MMRRC_031741-UCD) |
| Mouse: Ai32 | Jackson Laboratory | (RRID:IMSR_JAX:024109) |
| Software and algorithms | ||
| Raven Pro software | Cornell Lab of Ornithology | (Version 1.4) |
| Prism | GraphPad | (GraphPad Prism; RRID:SCR_002798) |
| Photoshop | Adobe | (RRID:SCR_014199) |
| ANY-maze | ANY-maze | (RRID:SCR_014289) |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Minghong Ma (minghong@pennmedicine.upenn.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
The transgenic D3-Cre line (STOCK Tg(Drd3-cre)KI198Gsat/Mmucd, RRID:MMRRC_031741-UCD) was obtained from the Mutant Mouse Resource and Research Center (MMRRC) at University of California at Davis, an NIH-funded strain repository, and was donated to the MMRRC by Nathaniel Heintz, Ph.D., The Rockefeller University, GENSAT and Charles Gerfen, Ph.D., National Institutes of Health, National Institute of Mental Health. The D3-Cre line was crossed with the Cre-dependent channelrhodopsin 2 (ChR2) line (JAX Stock No: 024109 or Ai32 line: Rosa26-CAG-LSL-ChR2(H134R)-EYFP-WPRE) (Madisen et al., 2010) to generate D3-Cre/ChR2-EYFP (or D3-Cre/ChR2) mice. Mice were maintained in temperature- and humidity-controlled animal facilities under a 12 h light/dark cycle with food and water available ad libitum. Both male and female mice (2-3 months old) were used. Mice were group-housed until the surgery of intra-cranial implantation and singly-housed afterwards. All experimental procedures were performed in accordance with the guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Method details
Stereotaxic surgery and optical fiber implantation
Mice were anesthetized with isoflurane (∼3% in oxygen) and secured in a stereotaxic system (Model 940, David Kopf Instruments). Isoflurane levels were maintained at 1.5-2% during the surgery. Body temperature was monitored and maintained at 37°C with a heating pad connected to a temperature control system (TC-1000, CWE Inc.). Local anesthetic (bupivacaine, 2 mg/kg, s.c.) was applied before skin incision and hole drilling on the dorsal skull. In order to target the IC in the OT, which is a large structure, two sets of coordinates from bregma were used: anteroposterior (AP) 1.2 (or 1.54) mm; mediolateral (ML) ±1.1 (or 1.15) mm; dorsoventral (DV), -5.5 (or -5.0) mm. The results were combined since we did not observe significant differences. A cannula (CFMC14L10-Fiber Optic Cannula, Ø2.5 mm Ceramic Ferrule, Ø400 μm Core, 0.39 NA; Thorlabs, Newton, NJ), customized to 6 mm length, was placed in the OT at the same coordinates as described above and fixed on the skull with dental cement. D3-Cre/ChR2 mice were returned to home cage for recovery for one week before behavioral tests. Only mice showed robust grooming behavior upon blue light stimulation were used in behavioral tests and optical fiber placements near the IC were verified post-mortem for all operated mice.
Optical stimulation and behavioral assays
Behavioral tests were performed during the light cycle (9:00 am - 12:00 pm). Mice were acclimated to the testing room at least 1 h before the tests. In experiments using collars, mice were habituated with wearing the collar 2 h per day for 3 consecutive days. Before each test, mice were briefly anesthetized via isoflurane and a flexible optic tether was coupled to the implanted fiber stud with a mating sleeve (Thorlabs Inc.).
A pair of D3-Cre/ChR2 mice (same-sex littermates) with optical fiber implanted in the OT were placed in each of the two side chambers in a three-chamber apparatus (30 cm × 15 cm × 20 cm) and covered under plastic cups. The wall of the cups had parallel vertical cuts (∼10 mm in width with ∼15 mm between two cuts) so that the observer mice could visualize the mice in the side chambers. In experiments without visual cues, the cups were wrapped in paper towels punched with numerous tiny holes (∼1 mm in diameter with ∼5 mm between two holes) for ventilation. Each observer mouse (typically 7 in an entire session) was placed in the center of the middle chamber of the three-chamber apparatus at the beginning of each test. Immediately after the test of one observer mouse, the chamber was quickly wiped using tissues moistened with 75% ethanol. The locations of blue and green light-stimulated mice were counterbalanced across different tests to avoid any side bias. Each observer mouse was subjected to two tests with an interval of 24 h, in which the pair of D3-Cre/ChR2 mice received no light (control condition) or blue/green light stimulation (experimental condition). For light stimulations, one mouse was stimulated with blue laser (473 nm) and the other one with green laser (532 nm) using the same parameters (10-15 mW; 20 Hz with 10 ms pulses). During the 10 min test for each observer mouse, the light stimulation was delivered in a protocol of 10 s ON and 50 s OFF. In experiments where self-grooming was blocked, light-stimulated mice wore a collar to prevent their forepaws from touching orofacial parts. In a subset of experiments, 24 h after the initial test, the location as well as the light pairing were switched for the two mice in the side chambers to exclude potential biases of the side and mouse. For methimazole treatment, observer mice were intraperitoneally injected with either saline (as control) or methimazole (75 mg/kg), and behavioral tests were conducted four days post injection. The behavioral tests were Video-taped via a webcam (30 frames/sec) and analyzed post hoc using the ANY-maze software and verified via visual scoring by experimenters who were blinded to the experimental conditions. According to a standardized protocol (Rein et al., 2020), the social preference index was calculated as (T2-T1)/(T2+T1), where T2 and T1 were the time of an observer mouse spent (i.e., total duration of stay) in each side chamber with the blue (more grooming) or green (less grooming) light-stimulated mouse, respectively. An investigation bout was defined from the time when an observer mouse started to sniff the cup covering a light-stimulated mouse (the nose was within ∼0.5 cm from the cup) to the time when the observer mouse turned away. The total investigation time was the sum of the time the observer mouse spending in all investigation bouts toward one cup during a 10 min test.
Collection of orofacial secretions from light-stimulated mice
Mice were first subjected to either blue or green light stimulations as aforementioned for 10 min. Secretions were immediately collected from the orofacial region (including the mouth, nose, cheek, and area surrounding the eye) using Q-tip cotton swabs that were moistened by mineral oil. For each mouse, a cotton swab was swiped against the orofacial region a total of 15-20 times. The cotton swabs were then placed in petri dishes (diameter: 6 cm), covered by the two cups in the two side compartments of the three-chamber apparatus. Observer mice were then placed at the center of the middle chamber at the beginning of each test, and their activities were Videotaped for 10 min for post hoc calculation of the social preference index. The placement of the petri dish was counterbalanced between different tests to avoid potential side bias.
Audio recording
Audio recording of blue laser induced self-grooming was performed in a sound-attenuated chamber. Within this chamber, a condenser ultrasound microphone (CM16/CMPA, Avisoft Bioacoustics) was fixed above a clean cage. Mice were habituated to the sound attenuated chamber and underwent 4 laser stimulation trials using parameters described above (20 Hz 10 ms pulses for 10 s) with a 5 min interval between stimulations. Acoustic data were acquired at 192 kHz to capture potential audible and ultrasonic sounds related to self-grooming. Data were acquired, visualized, and quantified using Raven Pro v1.4 software (the Cornell Lab of Ornithology).
Ex vivo electrophysiological recording
Mice were deeply anesthetized with ketamine-xylazine (200 and 20 mg/kg body weight, respectively) and decapitated. The brain was dissected out and immediately placed in ice-cold cutting solution containing (in mM) 92 N-Methyl D-glucamine, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 Sodium L-ascorbate, 2 Thiourea, 3 Sodium Pyruvate, 10 MgSO4, and 0.5 CaCl2; osmolality ∼300 mOsm and pH ∼7.3, bubbled with 95% O2-5% CO2. Coronal sections (250 μm thick) containing the OT were cut using a Leica VT 1200S vibratome. Brain slices were incubated in oxygenated artificial cerebrospinal fluid (ACSF in mM: 124 NaCl, 3 KCl, 1.3 MgSO4, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 5.5 glucose, and 4.47 sucrose; osmolality ∼305 mOsm and pH ∼7.3, bubbled with 95% O2-5% CO2) for ∼30 min at 31°C and at least 30 min at room temperature before use. For recordings, slices were transferred to a recording chamber and continuously perfused with oxygenated ACSF. D3-Cre/ChR2-EYFP cells were visualized through a 40× water-immersion objective on an Olympus B×61WI upright microscope equipped with epifluorescence.
Whole-cell patch-clamp recordings were controlled by an EPC-10 amplifier combined with Pulse Software (HEKA Electronik) and analyzed using Igor Pro 6 (Wavematrics). Recording pipettes were made from borosilicate glass with a Flaming-Brown puller (P-97, Sutter Instruments; tip resistance 5-10 MΩ). The pipette solution contained (in mM) 120 K-gluconate, 10 NaCl, 1 CaCl2, 10 EGTA, 10 HEPES, 5 Mg-ATP, 0.5 Na-GTP, and 10 phosphocreatine. Light stimulation was delivered through the same objective via pulses of blue laser (473 nm, FTEC2473-V65YF0, Blue Sky Research, Milpitas, USA) at 20 Hz with 10 ms pulses.
Immunostaining and confocal imaging
Mice were transcardially perfused with 4% paraformaldehyde (PFA) in fresh phosphate buffered saline (PBS). For post-mortem verification of optical fiber placement, brains were post fixed in 4% PFA overnight at 4°C, then transferred into PBS. Coronal slices at 100 μm thick were prepared using a Leica VT 1200S vibratome. The slices were treated with glycerol in PBS (volume ratio 1:1) for 30 min followed by glycerol in PBS (volume ratio 7:3) for 30 min before being mounted onto superfrost slides (Fisher Scientific) for imaging. For immunostaining of the nasal tissues, the heads were post fixed 4% PFA overnight at 4°C, and then decalcified in 0.5 M EDTA (pH 8.0, ethylenediaminetetraacetic acid) for four days and infiltrated in a series of sucrose solutions before being embedded in OCT. The frozen tissues were cut into 20 μm coronal sections on a cryostat. After antigen retrieval in a 95 °C water bath for 10 min, the tissue sections were blocked for 30 min in 0.3% Triton X-100 in PBS with 3% bovine serum albumin, and then incubated at 4°C overnight in the same solution with the primary rabbit anti-OMP (olfactory marker protein; 1:500, O7889 from Sigma). Immunofluorescence was achieved by reaction with the secondary antibody donkey anti-rabbit-488 (A21206 from Molecular Probes, Invitrogen) at 1:200 for one hour. Tissues were washed in 0.3% Triton X-100 in PBS and mounted in Vectashield (Vector Laboratories). Fluorescent images were taken under a SP5/Leica confocal microscope with LAS AF Lite software.
Quantification and statistical analysis
The data from male and female observer mice were initially separated and two-way ANOVA tests were used to assess the effect of sex and treatment (no light, light, and light with collar). In all cases, we found no sex effect and no sex x treatment interaction. We therefore grouped data from male and female observer mice, indicated by different symbols in the figures. Shapiro-Wilk tests were used to verify normal distribution. For normally distributed datasets, parametric statistical tests (student’s t test and one-way ANOVA test) were used; otherwise, non-parametric tests (Wilcoxon matched pairs signed rank tests) were applied. Statistical analysis was performed in GraphPad Prism and figures were assembled in Adobe Photoshop. The source data and statistical analysis outcome are included in Tables S1 and S2, respectively.
Acknowledgments
We thank Drs. Steven Simmons and Amelia Eisch for their help in setting up the sound recording system, Usuy David Leon Tolosa for his assistance in analyzing some of the behavioral videos, and Suna Cranfill for her help in making the Graphical Abstract. This work was supported by the National Institutes of Health R01NS117061, R01DA049545, and R01DA049449 to M.M. and D.W.W., R01DC006213 to M.M., R21DC019193 to J.P.B., and F31MH124372 to E.J.
Author contributions
Conceptualization, Y-F.Z., D.W.W., and M.M.; Methodology, Y-F.Z., E.J., J.P.B., and M.M.; Investigation, Y-F.Z., E.J., and J.P.B.; Formal Analysis, Data Curation, and Visualization, all authors; Writing – Original Draft, Y-F.Z., D.W.W., and M.M; Writing – Review & Editing, all authors; Supervision and Funding Acquisition, D.W.W. and M.M.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects.
Published: May 20, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104284.
Contributor Information
Yun-Feng Zhang, Email: zhang18@pennmedicine.upenn.edu.
Minghong Ma, Email: minghong@pennmedicine.upenn.edu.
Supplemental information
Data and code availability
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ache B.W., Young J.M. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Fentress J.C. Deafferentation does not disrupt natural rules of action syntax. Behav. Brain Res. 1987;23:69–76. doi: 10.1016/0166-4328(87)90243-9. [DOI] [PubMed] [Google Scholar]
- Brennan P.A., Kendrick K.M. Mammalian social odours: attraction and individual recognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursten S.N., Berridge K.C., Owings D.H. Do California ground squirrels (Spermophilus beecheyi) use ritualized syntactic cephalocaudal grooming as an agonistic signal? J. Comp. Psychol. 2000;114:281–290. doi: 10.1037/0735-7036.114.3.281. [DOI] [PubMed] [Google Scholar]
- Ferkin M.H., Leonard S. Neurobiology of Grooming Behavior. Cambridge University Press; 2010. Self-grooming as a form of olfactory communication in meadow voles and prairie voles (Microtus spp.) pp. 19–47. [Google Scholar]
- Ferkin M.H., Leonard S.T., Heath L.A., Paz-y-Mino G. Self-grooming as a tactic used by prairie voles Microtus ochrogaster to enhance sexual communication. Ethology. 2001;107:939–949. doi: 10.1046/j.1439-0310.2001.00725.x. [DOI] [Google Scholar]
- Ferkin M.H., Sorokin E.S., Johnston R.E. Self-grooming as a sexually dimorphic communicative behaviour in meadow voles, Microtus pennsylvanicus. Anim. Behav. 1996;51:801–810. doi: 10.1006/anbe.1996.0084. [DOI] [Google Scholar]
- Harriman A.E., Thiessen D.D. Harderian letdown in male Mongolian gerbils (Meriones unguiculatus) contributes to proceptive behavior. Horm. Behav. 1985;19:213–219. doi: 10.1016/0018-506x(85)90020-0. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M. Prepubertal genital grooming and penile erections in relation to sexual behavior of rats. Physiol. Behav. 2000;71:51–56. doi: 10.1016/s0031-9384(00)00320-6. [DOI] [PubMed] [Google Scholar]
- Hong W., Kim D.W., Anderson D.J. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A.V., Aldridge J.W., LaPorte J.L., Murphy D.L., Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Stewart A.M., Song C., Berridge K.C., Graybiel A.M., Fentress J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher K.R. The combined role of the main olfactory and vomeronasal systems in social communication in mammals. Horm. Behav. 2007;52:561–570. doi: 10.1016/j.yhbeh.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.t., Ingersoll D.W. Salivary cues in the mouse: a preliminary study. Horm. Behav. 1979;12:20–29. doi: 10.1016/0018-506x(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Li Y., Dulac C. Neural coding of sex-specific social information in the mouse brain. Curr. Opin. Neurobiol. 2018;53:120–130. doi: 10.1016/j.conb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri L.R., Lu Y., Xu Y., Cassidy R.M., Arenkiel B.R., Tong Q. A neural basis for antagonistic control of feeding and compulsive behaviors. Nat. Commun. 2018;9:52. doi: 10.1038/s41467-017-02534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A.P. The attractiveness of Harderian gland smears to sexually naive and experienced male golden hamsters. Anim. Behav. 1979;27:897–904. doi: 10.1016/0003-3472(79)90027-7. [DOI] [PubMed] [Google Scholar]
- Pendergrass M.L., Thiessen D.D. Body temperature and autogrooming in the Mongolian gerbil, Meriones unguiculatus. Behav. Neural Biol. 1981;33:524–528. doi: 10.1016/s0163-1047(81)91977-4. [DOI] [PubMed] [Google Scholar]
- Phifer-Rixey M., Nachman M.W. Insights into mammalian biology from the wild house mouse Mus musculus. Elife. 2015;4 doi: 10.7554/eLife.05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein B., Ma K., Yan Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020;15:3464–3477. doi: 10.1038/s41596-020-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter R.C., Epstein A.N. Saliva lost by grooming: a major item in the rat's water economy. Behav. Biol. 1974;11:581–585. doi: 10.1016/s0091-6773(74)90935-3. [DOI] [PubMed] [Google Scholar]
- Santillo A., Chieffi Baccari G., Minucci S., Falvo S., Venditti M., Di Matteo L. The Harderian gland: endocrine function and hormonal control. Gen. Comp. Endocrinol. 2020;297:113548. doi: 10.1016/j.ygcen.2020.113548. [DOI] [PubMed] [Google Scholar]
- Seyama Y., Uchijima Y. Novel function of lipids as a pheromone from the Harderian gland of golden hamster. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007;83:77–96. doi: 10.2183/pjab.83.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanas U., Terkel J. Mole-rat harderian gland secretions inhibit aggression. Anim. Behav. 1997;54:1255–1263. doi: 10.1006/anbe.1997.0533. [DOI] [PubMed] [Google Scholar]
- Singleton G.R., Krebs C.J. The secret world of wild mice. Mouse Biomed. Res. 2007;1:25–51. doi: 10.1016/b978-012369454-6/50015-7. [DOI] [Google Scholar]
- Spehr M., Spehr J., Ukhanov K., Kelliher K.R., Leinders-Zufall T., Zufall F. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell. Mol. Life Sci. 2006;63:1476–1484. doi: 10.1007/s00018-006-6109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt B.M., van Hooff J.A., Gispen W.H. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Suarez R., Garcia-Gonzalez D., de Castro F. Mutual influences between the main olfactory and vomeronasal systems in development and evolution. Front. Neuroanat. 2012;6:50. doi: 10.3389/fnana.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K., Kondo K., Kanaya K., Sakamoto T., Watanabe K., Ushio M., Kaga K., Yamasoba T. Age-related changes of the regeneration mode in the mouse peripheral olfactory system following olfactotoxic drug methimazole-induced damage. J. Comp. Neurol. 2011;519:2154–2174. doi: 10.1002/cne.22611. [DOI] [PubMed] [Google Scholar]
- Thiessen D.D. Body temperature and grooming in the Mongolian gerbil. Ann. N Y Acad. Sci. 1988;525:27–39. doi: 10.1111/j.1749-6632.1988.tb38593.x. [DOI] [PubMed] [Google Scholar]
- Thiessen D.D., Clency A., Goodwin M. Harderian gland pheromone in the Mongolian gerbil Meriones unguiculatus. J. Chem. Ecol. 1976;2:231–238. doi: 10.1007/Bf00987746. [DOI] [Google Scholar]
- Wesson D.W. The tubular striatum. J. Neurosci. 2020;40:7379–7386. doi: 10.1523/JNEUROSCI.1109-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiepkema P.R. The social significance of self-grooming in rats. Neth. J. Zool. 1979;29:622–623. [Google Scholar]
- Wolff J.O., Watson M.H., Thomas S.A. Is self-grooming by male prairie voles a predictor of mate choice? Ethology. 2002;108:169–179. doi: 10.1046/j.1439-0310.2002.00763.x. [DOI] [Google Scholar]
- Wyatt T.D. Cambridge University Press; 2003. Pheromones and Animal Behaviour: Communication by Smell and Taste. [Google Scholar]
- Yanase M., Kanosue K., Yasuda H., Tanaka H. Salivary secretion and grooming behaviour during heat exposure in freely moving rats. J. Physiol. 1991;432:585–592. doi: 10.1113/jphysiol.1991.sp018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.F., Vargas Cifuentes L., Wright K.N., Bhattarai J.P., Mohrhardt J., Fleck D., Janke E., Jiang C., Cranfill S.L., Goldstein N., et al. Ventral striatal islands of Calleja neurons control grooming in mice. Nat. Neurosci. 2021;24:1699–1710. doi: 10.1038/s41593-021-00952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.