Abstract
Background
In 2019, a viral and respiratory pathology called COVID-19 emerged in Wuhan, China, and spread to other continents. Its main symptoms include fever, cough, dyspnea, myalgia, anorexia and respiratory distress in the most severe cases, which can lead to death. Furthermore, manifestations in the oral cavity such as ageusia and dysgeusia, as well as lesions in other regions of the oral cavity, can be observed.
Main body
This systematic review and meta-analysis aimed to critically assess the clinical evidence on the use of photobiomodulation (PBMT) and antimicrobial photodynamic therapy (aPDT) for the treatment of oral lesions in patients infected with Sars-Cov-2. The literature extracted from electronic databases such as PubMed, Medline, CINAHL, and Google Scholar was screened for eligibility, and relevant articles were included. The review is limited to manuscripts published in English, Spanish and Portuguese language between December 2019 and October 2021. A total of 5 articles with 11 cases retracting PBMT and aPDT as therapeutic strategies for the regression of oral lesions and painful symptoms. The results show favoring the associated use of PBMT with aPDT (P = 0.004), and the isolated use of PBMT with the result of significant “P = 0.005” and good confidence interval (7.18, 39.20) in ulcerative lesions, herpetic, aphthous, erythematous, petechiae and necrotic areas.
Conclusions
PBMT and aPDT could be effective in the treatment of oral lesions of patients infected with Sars-Cov-2 in a short period of time; however, more long-term randomized clinical trials studies are needed to define the therapeutic strategy.
Keywords: Photodynamic therapy, Low level laser therapy, LLLT, Oral manifestation, Coronavirus, Sars-CoV-2, COVID-19
Background
COVID-19 is an acute respiratory pathology and the set of signs and symptoms develop rapidly in a synchronous and varied manner, leading to a global characterization of the name “Severe Acute Respiratory Syndrome Coronavirus 2” (Sars-CoV-2) (Singh et al. 2021; Pacheco et al. 2021). The most common symptoms are fever, dyspnea, cough, myalgia and anorexia (Sousa and Paradella 2021; Jiang et al. 2020; Huang et al. 2020). In severe cases, pulmonary impairment occurs with increased respiratory rate (> 30 times/min.), decreased O2 saturation (< 93% in room air) and PaO2/FiO2 (< 300 mm Hg) are observed (Sousa and Paradella 2021; Berlin et al. 2020). Critical cases include severe acute respiratory syndrome, acute cardiac injury, and multiple organ failure (Sousa and Paradella 2021; Huang et al. 2020; Pacheco et al. 2021).
Due to this new pandemic context, and the growing expansion of the number of cases, it is possible to identify and relate a wide variety of signs and symptoms in these patients contaminated by the coronavirus with the appearance of secondary lesions in the oral cavity (Pacheco et al. 2021).
Many patients infected with Sars-CoV-2 present oral lesions, and as it is a new systemic disease, the database of these lesions is being published in case reports (Pacheco et al. 2021). However, there are reports of oral manifestations by clinicians and researchers related to various comorbidities such as ulcer, erosion, blister, vesicle, pustule, fissured or depapillary tongue, macula, papule, plaque, pigmentation, halitosis, whitish areas, hemorrhagic crust, necrosis, petechiae, swelling, erythema and spontaneous bleeding (Iranmanesh et al. 2021; Pacheco et al. 2021). The most affected sites were tongue (38%), lip mucosa (26%) and palate (22%) (Iranmanesh et al. 2021; Soares et al. 2020). Suggested diagnoses of lesions are diverse, including aphthous stomatitis, herpetic lesions, candidiasis, vasculitis, Kawasaki type, eruption by drug, periodontal necrotizing disease, bullous angina type, angular cheilitis, atypical Sweet's syndrome and Melkersson–Rosenthal syndrome (Iranmanesh et al. 2021; Pacheco et al. 2021). Oral lesions are characterized as symptomatic in 68% of cases and similar in both sexes (49% female and 51% male) (Tamang et al. 2021). And it should be noted that the lack of oral cavity hygiene, opportunistic infections, stress, immunosuppression, vasculitis and inflammatory response secondary to Sars-CoV-2 are being characterized as the most important causal factors for the appearance of these lesions in patients with Sars-CoV-2 (Tsuchiya 2021; Iranmanesh et al. 2021).
Taste disorder (dysgeusia) is the most common oral manifestation found in this niche of patients with Sars-CoV-2 (Jakubovics 2021; Pacheco et al. 2021). In mild cases, oral mucosal lesions evolve before or simultaneously with the initial respiratory symptoms; however, in patients who worsened and required medication and hospitalization, oral lesions had an evolution process approximately between 7 and 24 days after the onset of symptoms (Dos Santos et al. 2021; Pacheco et al. 2021).
There are physiological structures in the oral cavity available through cell receptors called Angiotensin-Converting Enzyme 2 (ACE2) (Jia et al. 2005). The coronavirus has in its structure the glycoprotein S, which is the key factor for the entry of Sars-CoV-2 into the cell, which contains two functional domains: S1 (Zhong et al. 2020) which is an angiotensin converting enzyme receptor binding domain 2 (ACE2) and the S2 which is required for coronavirus fusion and cell membranes. There is a resonance in global studies that ACE2 is likely the receptor for Sars-CoV-2 (Zhong et al. 2020; Cassol-Spanemberg et al. 2016) which could induce an increase in the susceptibility and/or progression of COVID-19 disease, and this severity would be closely linked to increased viral load in the oropharyngeal region with eventual systemic repercussions for respiratory epithelial cells (Zhong et al. 2020).
In case of minimizing these secondary manifestations caused by the Sars-CoV-2 infection, low intensity laser therapy (LLLT) emerges as a practical tool and is characterized by its non-invasiveness to biological tissues, which through a wavelength (red or infrared) stimulates responses to injured tissue by promoting anti-inflammatory, analgesic and healing effects. LLLT promotes the effect of photobiomodulation (PBMT) which is able to regulate antioxidant defenses and reduce oxidative stress (Cassol-Spanemberg et al. 2016).
PBMT is based on the interaction of light with biological tissues, stimulating photo-physical, chemical and biological events in the cell, in the search for better therapeutic responses, consequently altering cell metabolism. It is known that this therapy can currently bio-stimulate or bio-inhibit cells depending on different variables relevant to light (Cassol-Spanemberg et al. 2016). The therapeutic effects of low-power laser irradiation have been investigated in several health areas (Bensadoun et al. 2020). Some beneficial effects such as improved immunosuppression-immunostimulation, stabilization of autoimmune disease and nerve regeneration have been reported in the literature (Zecha et al. 2016; Cassol-Spanemberg et al. 2016).
The basic physiological effects and mechanisms of PBMT are widely discussed in the world literature and have excellent results in healing processes, decontamination, biomodulation of the inflammatory process, and pain control. Indications for clinical applications of PBMT at the cellular level are broad within the healthcare field, and include interdisciplinary therapies that involve the treatment of different pathologies, as well as pain control in general (Pacheco et al. 2021).
It should be noted that the imbalance of the oral cavity microbiota may provide the functional instability of the oropharynx with the development of numerous secondary pathogens related to systemic diseases. This instability open space for colonization by microorganisms in other organs that may evolve into an undesirable effect as they produce potent toxic agents (Pacheco et al. 2021). The oral microbiome in patients has a greater bacterial sensitivity and specificity due to the vulnerability of the immune system. In this context, PBMT proposes another interesting aspect for the decontamination of the oral microbiota in these patients, given that the biosensors contained in the oral region through photodynamic therapy will activate a photosensitizing substance by a red laser with a high efficiency in the decontamination of microorganisms, such as bacteria, viruses and fungi. We call this process antimicrobial photodynamic therapy (aPDT) (Carrera et al. 2016; Pacheco et al. 2021).
The aPDT has photosensitization as a mechanism, which consists of the direct action of light with a photosensitizing agent together with oxygen. This process induces the production of free radicals that promote microbial lysis (Pacheco et al. 2021), promoting decontamination (Issa and Manela-Azulay 2010). This therapy is of low cost, basically without side effects, and the possibility of controlling the oral microbiota (Pacheco et al. 2021).
When a photosensitizing substance, such as methylene blue, for example, is photoactivated in the presence of oxygen, it leads to the generation of reactive oxygen species (Vittar et al. 2008), causing irreversible damage to target structures such as proteins and lipids, leading to death of microorganisms by apoptosis or necrosis (Conrado et al. 2021; Castano et al. 2005). In viral infectious agents, aPDT can affect the lipid or protein structures of the viral envelope membrane, even the nucleic acid, regardless of the specific interaction with a receptor, indicating that it is an efficient tool to eliminate these infectious agents (Conrado et al. 2021; Castano et al. 2005; Wiehe et al. 2019; Monjo et al. 2018; Pacheco et al. 2021).
The aim of this manuscript is to present a systematic review of the literature on the use of PBMT and aPDT in oral lesions of patients affected by the Sars-CoV-2 infection, reviewing all relevant observational studies to answer the following question: photobiomodulation (PBMT) and antimicrobial photodynamic therapy (aPDT) are effective to treat oral manifestations of patients infected with Sars-CoV-2?
Methods
The methodology was defined following the PRISMA guidelines (Preferred Systematic Reviews and Meta-Analysis Report) (Moher et al. 2015), and registered in the database in the International Prospective Registry of Systematic Reviews (PROSPERO) under registration CRD42021250106.
The literature search was conducted in 4 databases and in gray literature, whose question focused in the review was “Photobiomodulation (PBMT) and antimicrobial Photodynamic Therapy (aPDT) are effective to treat oral manifestations of patients infected by Sars-CoV-2?”. The flow diagram was used as the strategy design in this study.
Literature research
A search was performed in the PubMed, Medline, CINAHL, Google Scholar databases, as well as a manual search of the reference lists of the included studies, from January 1, 2019, to October 16, 2021. The first phase established an investigation for define MeSH (Medical Subject Headings) terms to ensure high sensitivity and accuracy, and researchers tracked abstract and publication titles (Conrado et al. 2021). The MESH terms used in searches across all databases were: “COVID 19” OR “Sars-Cov-2” OR “Coronavirus infections” OR coronavirus AND “Photodynamic therapies” OR “Photodynamic therapy” OR “PDT methylene blue” OR “Cold laser decontamination” OR “Photodynamic LLLT” OR Photobiomodulation AND “Oral manifestations” OR “Oral injury” AND “Angiotensin II” OR “ACE2”.
Election criteria
Articles describing photobiomodulation therapy and antimicrobial photodynamic therapy as treatment for oral manifestations in patients infected by the Sars-CoV-2 infection, based on titles and abstract, were included in this review (Conrado et al. 2021). Original articles in English, Spanish and Portuguese within the defined period (2019–2021) with abstract available in the database were evaluated and validated within the inclusion criteria. Inclusion criteria were defined using the PICO strategy (Population, Intervention, Comparison and Outcome) (Table 1) and consisted of "in vivo" studies and case reports, excluding works published as letters to the editor, systematic reviews and articles not available in full.
Table 1.
PICO Strategy with inclusion criteria using on research
| PICO strategy | |
|---|---|
| Population | Patients infected by the Sars-CoV-2 infection who had oral lesions and were treated with PBMT or aPDT laser therapy |
| Intervention | Patients were exposed to photobiomodulation therapy (PBMT) and/or antimicrobial photodynamic therapy (aPDT) |
| Comparison | Comparison between patients who did not use PBMT and/or aPDT |
| Outcome | Observe whether or not there was improvement in the lesions after using PBMT and aPDT |
Selection and quality assessment of relevant studies
Articles selected according to the selection criteria were retrieved in PDF format, numbered and randomly distributed among three researchers. The reference list was manually checked by the researchers in order to retrieve publications that were not previously found in the database search, to increase the sensitivity and quality of the review (Contado et al. 2021). A consensus meeting was held to discuss divergences after evaluating the quality of publications and guaranteeing the validation of the articles selected by the researchers.
Data extraction
Articles included in the study were randomly distributed among researchers to collect relevant data (Conrado et al. 2021).
Through a table, important data were selected, including: author and year of publication, local and type of lesion, applied technique and parameter of laser and outcome, whether there was improvement of the lesion, in how many days there was improvement, whether the type of light source interfered and to observe whether these complementary therapies would be effective or not in the treatment of oral lesions in patients affected by Sars-CoV-2. With the results collected, the data were evaluated one by one, each case addressed in the literature was presented in each row of the table.
Bias risk
Quality and risk of bias in selected articles were independently performed by two authors, using the Joanna Briggs Institute's Critical Assessment Checklist for systematic reviews and research summaries (Moola et al. 2015). The third author was consulted in case of disagreement. Scoring decisions were discussed by all reviewers prior to assessments. Checklists allow for the quantification of scores, where high scores represent a low risk of bias; low scores indicate a high risk of bias or "unclear" domains in publications (Conrado et al. 2021).
Results
Selection and heterogeneity of studies
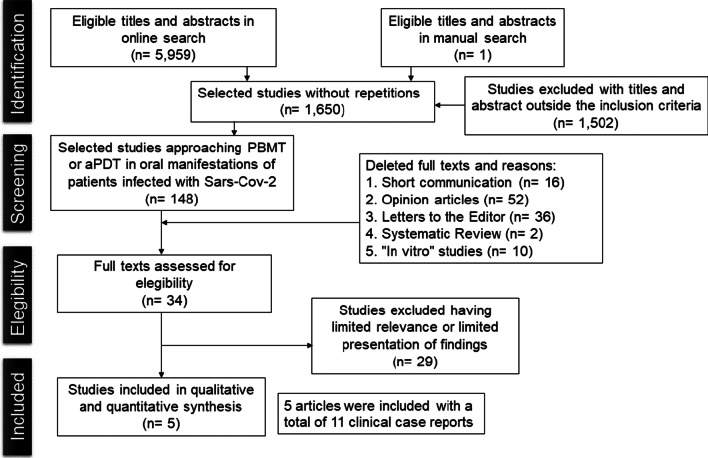
In the first phase, 5,959 articles were identified in the databases and, after removing the duplicates, 1,650 articles remained for reading the title and abstract. After evaluating all records, 32 articles were selected where they were read in full and following the inclusion and exclusion criteria, 5 articles were finally included, as instructed in the Infographic (Fig. 1).
Fig. 1.
Literature search flow diagram and selection criteria adapted from PRISMA (Preferred report items for systematic reviews and meta-analysis)
All studies were carried out in Brazil; they were case reports in the literature and were published between January and October 2021, in English, where patients were admitted to hospitals with symptoms characteristic of COVID-19 and later confirmed through the examination RT-PCR. However, they used different therapeutic approaches for lesions in the oral cavity, including PBMT (Brandão et al. 2021; Baeder et al. 2021), PBMT associated with aPDT (Teixeira et al. 2021; Ramires et al. 2021; Garcez et al. 2021) and awaiting spontaneous regression of the lesions without any laser intervention (Brandão et al. 2021; Baeder et al. 2021). Thus, 5 articles found in the literature presented a total of 11 cases using PBMT and aPDT as therapeutic strategies for the regression of oral lesions and painful symptoms in patients with COVID-19 (Table 2).
Table 2.
Characteristics of the included studies, according to Author and year of publication; Place and type of injury, Technique and Parameters in the use of Laser and outcome
| Author and Year | Local and type of lesion | Technique applied and Laser parameter | Outcome |
|---|---|---|---|
| Brandão et. al. (2021) | Upper lip, lower lip and anterior dorsum of tongue. Necrotic and aphthous ulcers | PBMT. 40 mW, beam area 0.04 cm2, 1 W/cm 2 irradiance, energy 0.4 J and 10 J/cm2 creep and 660 nm | Symptom relief within 2 days and complete resolution of lesions within 11 days |
| Brandão et. al. (2021) | Upper lip, lower lip and anterior dorsum of tongue. Necrotic areas and aphthous ulcers | PBMT. 40 mW, beam area 0.04 cm2, 1 W/cm2 irradiance, energy 0.4 J and 10 J/cm2 creep and 660 nm | Improvement of intraoral lesions in 10 days and lip ulcerations there was no improvement until the publication of the case, as well as the clinical case of the patient |
| Brandão et. al. (2021) | Lateral edge of tongue and palate. Petechiae and necrotic areas | PBMT. 40 mW, beam area 0.04 cm2, 1 W/cm 2 irradiance, energy 0.4 J and 10 J/cm2 creep and 660 nm | Total pain control in 5 days |
| Brandão et. al. (2021) | Upper and lower lip mucosa. Necrotic and hemorrhagic ulcers | PBMT. 40 mW, beam area 0.04 cm2, 1 W/cm 2 irradiance, energy 0.4 J and 10 J/cm2 creep and 660 nm | Pain regression and clinical improvement in 7 days |
| Teixeira et. al. (2021) | Upper and lower lip injury. Hemorrhagic and necrotic ulcers | PBMT and aPDT. 100 mW, 33 J/cm2, 0.5 J and 5 s per point. A total of 6 points were distributed for the injuries. Soon after, an aPDT technique was performed, with 0.01% methylene blue applied to all lesions, and after 3 min (pre-irradiation time), the same laser parameters were used, but providing 40 s (4 J) per lesion, 660 nm | Total improvement of the lesion in 3 days and healing within the first 24 h |
| Teixeira et. al. (2021) | Upper and lower lip injury. Erythematous lesions | PBMT and aPDT. 100 mW, 33 J/cm2, 0.5 J and 5 s per point. A total of 6 points were distributed for the injuries. Soon after, an aPDT technique was performed, with 0.01% methylene blue applied to all lesions, and after 3 min (pre-irradiation time), the same laser parameters were used, but providing 40 s (4 J) per lesion, 660 nm | Total improvement of the lesion after 24 h |
| Teixeira et. al. (2021) | Upper and lower lip injury. Painful scaly lip lesions | PBMT and aPDT. 100 mW, 33 J/cm2, 0.5 J and 5 s per point. A total of 6 points were distributed for the injuries. Soon after, an aPDT technique was performed, with 0.01% methylene blue applied to all lesions, and after 3 min (pre-irradiation time), the same laser parameters were used, but providing 40 s (4 J) per lesion, 660 nm | Total improvement of lesions in 3 days |
| Teixeira et. al. (2021) | Upper and lower lip injury. Painful scaly lip lesions | PBMT and aPDT. 100 mW, 33 J/cm2, 0.5 J and 5 s per point. A total of 6 points were distributed for the injuries. Soon after, an aPDT technique was performed, with 0.01% methylene blue applied to all lesions, and after 3 min (pre-irradiation time), the same laser parameters were used, but providing 40 s (4 J) per lesion, 660 nm | Complete resolution of lesions within 4 days |
| Ramires et. al. (2021) | Upper and lower lip injury. Extensive necrotic ulcers | PBMT and aPDT. aPDT was performed for 2 days. For this, 0.01% methylene blue was applied to all lesions after 5 min (pre-irradiation time), 100 mW, 32.14 J/cm2, 9 J and 9 s per point and PBMT 100 mW, 17.8 J/cm2, 1 J and 10 s of irradiation per spot at 660 and 808 nm, using a laser device programming tool that changes the wavelength periodically (every 5 s) | Wound healing in 4 days |
| Baeder et. al. (2021) | Region between attached gingiva and palate. Ulcers, erythema and vesicles | PBMT. 3 J every 2 days for 1 week, 660 nm | Burning ceased in 7 days and after 14 days, the lesions disappeared completely |
| Garcez et. al. (2021) | Upper and lower lips and inner labial mucosa of the gingiva. Edema with mucosal desquamation, ulceration and blood crusts on the inner surface of the labial mucosa, gingival petechiae and erythematous/pseudomembranous lesions on the dorsum of the tongue, suggestive of candidiasis | PBMT and aPDT. aPDT was performed using a low power laser and methylene blue as a photosensitizer. 300 μM aqueous methylene blue solution was applied to the lips, palate and tongue with a cotton swab for 1 min, followed by irradiation of a laser light source operating at 100 mW and 660 nm with the following protocol: 90 s, resulting in an energy of 9 J per irradiation point and an energy density of 300 J/cm2, total of 6 points—including lips (4 points), palate (2 points) and tongue (4 points), the total irradiation time was 15 min. After the aPDT sessions, oral lesions were irradiated with 2 J of energy per point to cover the oral mucosa surface bilaterally (5 points on each side) using the same equipment, resulting in 20 s of irradiation per point and energy density per stitch of 66 J/cm2 | Oral lesions improved after 3 days of aPDT after which treatment was followed with PBM for 4 days. The patient did not complain of discomfort in the tongue and lips after starting the photodynamic treatment with an antimicrobial |
Of these, 3 were male (27%) and 8 female (73%), where 8 patients were aged over 60 years (mean 75 years) and 3 were younger than 60 years (mean 47 years).
The place most affected by the lesions were upper and lower lip (33.3%), followed by tongue (12.5%), hard palate (8.3%), buccal mucosa (4.1%) and attached gingiva (4,1%).
Among the most frequent types of lesions are ulcers (50%), herpetic lesions (40%), in addition to the rest being expressed by necrotic, erythematous, vesicular and petechiae areas.
The risk of bias assessment was established using the Joanna Briggs Institute (Moola et al. 2015) Critical Assessment List for case series, and the articles analyzed here had a low risk of bias, between 20 and 40% (Table 3).
Table 3.
Risk of bias assessed by the Joanna Briggs Institute Critical appraisal checklist for case series
| Authors | Q.1 | Q.2 | Q.3 | Q.4 | Q.5 | Q.6 | Q.7 | Q.8 | Q.9 | Q.10 | %yes/risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baeder et al. (2021) | U | √ | √ | √ | √ | √ | √ | √ | – | N/A | 70%/30% |
| Brandão et al. (2021) | U | √ | √ | √ | √ | √ | √ | √ | – | N/A | 70%/30% |
| Ramires et al. (2021) | √ | √ | √ | N/A | N/A | √ | √ | √ | – | N/A | 60%/40% |
| Teixeira et al. (2021) | √ | √ | √ | √ | √ | √ | √ | √ | – | N/A | 80%/20% |
| Garcez et al. (2021) | √ | √ | √ | N/A | √ | √ | √ | √ | – | N/A | 70%/30% |
√-yes; –-No; U-Unclear; N/A-not applicable
Photosensitizer and light sources
Brandão et al. (2021), after not observing improvement in vesicular, erythematous and vesicle lesions with the use of antiviral therapy with intravenous Acyclovir (250 mg), 3 times a day for 7 days, used PBMT (Twin Flex, MMOptics, São Carlos, Brazil) with the therapeutic protocol for oral mucositis associated with cancer therapy, in patients with COVID-19, where the laser device was positioned perpendicular to the surface of the oral ulcers, for 10 s per site, operating at a wavelength of 660 nm, 40 mW average power, 0.04 cm2 beam area, 1 W/cm2 irradiance, 0.4 J energy and 10 J/cm2 creep.
Baeder et al. (2021) proposed the treatment of herpetic lesions that did not spontaneously regress in patients infected with COVID-19 through the use of PBMT with a wavelength of 660 nm, with a dose of 3 J every 48 h, in addition to the mouthwash with 0.12% chlorhexidine twice daily for 7 days.
Teixeira et al. (2021) and Ramires et al. (2021) used similar protocols, the first one using an association of aPDT and PBMT for two days. For PBMT, a DUO® Laser device (MMOptics, São Carlos, SP, Brazil) was used at 660 nm, contact mode, point to point, with 100 mW, 33 J/cm2, 0.5 J and 5 s per point. A total of 6 points were distributed for necrotic lesions, bleeding ulcers and erythematous lesions in patients with COVID-19. Soon after, an aPDT technique was performed, with 0.01% methylene blue applied to all lesions, and after 3 min (pre-irradiation time), the same laser parameters were used, but providing 40 s (4 J) for injury. Ramires et al. (2021) differed in the pre-irradiation time, which waited 5 min, and the laser device used was the Therapy EC® (DMC, São Carlos, SP, Brazil) at 660 nm, contact mode, point to point, with 100 mW, 32.14 J/cm2, 9 J and 9 s per stitch. In this case, a total of 30 points were distributed over the affected areas: 20 points on the upper lip and 10 on the lower lip. In addition, on the second day, a PBMT session was held. The same areas described above were irradiated with the same equipment, but using 100 mW, 17.8 J/cm2, 1 J and 10 s of irradiation per point at 660 and 808 nm, using a programming tool that triggers the two lengths simultaneously (every 5 s).
Garcez et al. (2021) used a protocol of 3 sessions of aPDT (660 nm diode laser + methylene blue) on the lips and tongue, every 24 h to control contamination, followed by PBMT (low power laser, 100 mW, 2 J/point) for the lips, tongue and oral mucosa for four additional sessions every 24 h, in ulcerative lesions, hemorrhagic crusts, petechiae and erythematous lesions in patients with COVID-19.
Synthesis of meta-analysis
The synthesis of statistical data was performed through meta-analysis, which consists of the statistical combination of results from two or more separate studies. Potential advantages of meta-analysis include an improvement in accuracy, the ability to answer questions not posed by individual studies, and the opportunity to resolve disputes arising from conflicting claims (Deeks et al. 2021). Meta-analysis was performed on groups of studies that were homogeneous in terms of similarity in population, interventions, and outcomes to provide a meaningful summary (Higgins et al. 2021). Most of the scientific articles selected in this study presented a low risk of bias and corroborated the final production of the analysis.
It should be noted that the results of the 11 clinical cases referring to the 5 articles of the study were evaluated through meta-analysis, considering that these studies presented homogeneous data quantitatively evaluating the use of PBMT and aPDT for the reduction of oral lesions in patients affected by COVID-19.
Repair of oral lesion by PBMT and aPDT/PBMT
The results show favoring the associated use of PBMT with aPDT (P = 0.004) (Pacheco et al. 2021), considering that there was a greater reduction in ulcerative, erythematous, necrotic and petechiae oral lesions in these patients positive by COVID-19, which is demonstrated in the “Forest plot” confidence interval (Fig. 2). The fixed model was applied in this meta-analysis and heterogeneity is not a considerable factor for the analysis.
Fig. 2.
Forest plot demonstrating meta-analysis for oral manifestations for COVID-19 patients facing the treatment of PBMT associated with aPDT
Heterogeneity was analyzed through the analysis of the approximate guide for interpretation in the context of meta-analysis of randomized clinical trials, which is made available by the Cochrane Group of Statistical Methods (Higgins et al. 2021), according to the source of the Review Manager 5.4.1 program and Deeks et al. (2021); as described below:
0% to 40%: may not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
The isolated use of PBMT, according to the meta-analysis, was also effective in the treatment of oral lesions caused by COVID-19 (Santos et al. 2021), with the result of a significant “P = 0.005” and a good confidence interval (7.18, 39.20) (Pacheco et al. 2021) (Fig. 3).
Fig. 3.
Forest plot demonstrating meta-analysis for oral manifestations for COVID-19 patients facing PBMT treatment
Analysis of the main results
PBMT without association with other laser therapy (Brandão et al. 2021; Baeder et al. 2021), the relief of painful symptoms occurred between 2 and 14 days with total healing of the lesion, excluding one case (Case 2) (Brandão et al. 2021), where until the date of publication of the article there was no response to laser therapy or regression of the COVID-19 infection picture.
PBMT associated with aPDT showed the best results with relief of painful symptoms in the first 24 h and healing of the lesion within 4 days (Teixeira et al. 2021; Ramires et al. 2021; Garcez et al. 2021).
Cases where laser therapy was not used as adjuvant, were young patients, mean age of 38.2 years and the lesions had their spontaneous regression within a considerable time, between 4 and 16 days.
Effects of PBMT and aPDT on Sars-CoV-2 contaminated oral tissues
PBMT allowed relief of painful symptoms and complete resolution of lesions in the clinical cases presented in this review. When associated with aPDT, this improvement occurred in a shorter time span, thus suggesting that it is an effective therapy to treat oral lesions in patients affected by COVID-19 (Teixeira et al. 2021).
Discussion
The pandemic process related to COVID-19 in the last two years, current studies related to morbid changes in the oropharyngeal region are in the process of evolution, as therapies for the repair of secondary lesions promoted by Sars-CoV-2 are ongoing. In an incipient way in the health-disease process, as well as the impossibility of clinicians and patients to evaluate a reduced amount of case studies (Pacheco et al. 2021) or long-term research.
In this view, systematic reviews aim to ensure that clinical decisions are made with an up-to-date and complete understanding of the relevant scientific evidence (Lasserson et al. 2021). Judiciously evaluating the scientific literature, systematic reviews provide an up-to-date, qualitative summary of the state of research knowledge about an intervention, address the main research problem, namely, that of bias; as well as direct new research projects, indicating specific gaps in knowledge or whether evidence is lacking (Chalmers et al. 2014; Lasserson et al. 2021).
The present study verified in the available scientific literature whether there was evidence regarding the efficacy and safety of the clinical applicability of PBMT and aPDT as effective options to treat oral manifestations of patients infected by COVID-19 (Chalmers et al. 2014; Pacheco et al. 2021), through a review of narrative case reports from current literature. The studies included in this research evaluated 5 scientific articles, where 11 clinical cases had similar interventions. All clinical cases evaluated in this systematic review demonstrated positive therapeutic effects that promoted a global impact on patients' health.
The focus of the systematic review was to address the use of PBMT, and/or aPDT/PBMT in the cytotoxic reduction of secondary manifestations promoted by COVID-19 (Pacheco et al. 2021; Conrado et al. 2021) that manifest themselves in the oral mucosa, through ulcers, erosions, aphthous lesions, plaques, hemorrhagic crusts among other alterations, being the tongue, labial mucosa and palate the most affected places (Iranmanesh et al. 2021).
Lesions of the stomatognathic system that evolve negatively during the therapeutic process have a refractory potential to compromise and exacerbate immunosuppression, which adds to the appearance of opportunistic infections arising from atypical changes in oral mucosa cells, such as aphthous stomatitis (Pacheco et al. 2021), herpetic lesions and candidiasis.
It is extensive in the current literature that the main receptor for Sars-CoV-2 is ACE2, which would be closely linked to the imbalance of the oral microbiome that could induce an increase and sharp progression of the viral load in the oropharyngeal region with an eventual undesirable systemic repercussion (Zhong et al. 2020).
Furthermore, PBMT and aPDT could become associative or complementary therapeutic strategies, as they present themselves as an auxiliary tool with a positive multi and interdisciplinary nature in the focal control of pathologies of the oropharyngeal tract, in addition to being a low-cost, painless and non-invasive technique (Hennessy and Hamblin 2017). Furthermore, techniques related to low intensity laser therapy constitute, in the contemporary context, a form of treatment of painful symptoms and clinical picture of these diseases in the oral cavity by stimulating analgesic, anti-inflammatory and anti-edematous effects (Zecha et al. 2016; Carrera et al. 2016; Castano et al. 2005; Wiehe et al. 2019; Monjo et al. 2018).
This systematic review contributes to the amount of 11 clinical cases that used PBMT and/or aPDT as therapeutic resources for lesions in the oral cavity of patients affected by COVID-19, or awaiting natural regression of the lesions with topical medication in some cases (Brandão et al. 2021; Baeder et al. 2021; Teixeira et al. 2021; Ramires et al. 2021). The cases were patients admitted to public and private hospitals with symptoms characteristic of COVID-19 and confirmed through the RT-PCR (Corman et al. 2020). Most were female (73%), where 8 patients were aged over 60 years (average 75 years old) and 3 were younger than 60 years old (average 47 years old). The studies were conducted in Brazil, which creates the need for manuscripts in other regions of the world.
In the observational criterion for the incidental evaluation of oral lesions, in ascending order of nature of these manifestations, there was an involvement of ulcerative lesions, necrotic areas, herpetic lesions, erythematous and petechiae (Iranmanesh et al. 2021). Regarding the nature of the oral lesion, in ascending order: upper and lower lips, tongue, hard palate, buccal mucosa and attached gums, which is in agreement with the findings in the literature (Soares et al. 2020; Iranmanesh et al. 2021; Amorim Dos Santos et al 2021).
Brandão et al. (2021) and Baeder et al. (2021) using protocols pertinent to the case, did not observe apparent regression of herpetic lesions on the lip, and used PBMT with clinical improvement of the lesions total tissue repair between 5 and 14 days. When they awaited spontaneous regression of the lesions, medications such as Acyclovir, mouthwash with 0.12% chlorhexidine, ascorbic acid (Brandão et al. 2021; Baeder et al. 2021), were used, in addition to individual medication protocols for each patient due to COVID-19 infection (Lofti et al. 2020; Carlotti et al. 2020; Pacheco et al. 2021).
Teixeira et al. (2021), Ramires et al. (2021) and Garcez et al. (2021) implemented associative protocols between the PBMT and aPDT techniques, and observed relief of painful symptoms in the first 24 h and healing of the lesion within 4 days, in accordance with the findings of Maya et al. (2020) for the treatment of palatal ulcers.
In the observational evaluation criteria of the 5 articles in question, it is important to emphasize that oral lesions appeared in these patients positive by COVID-19 (Cruz Tapia et al. 2020), concomitantly with loss of taste and smell (Mutiawati et al. 2021), worsening of COVID-19 symptoms and were more severe in people older and systemically compromised (Brandão et al. 2021).
Another considerable score is that these lesions were characterized by necrotic ulcers and with great painful symptoms. In young patients, with mild COVID-19, they presented as aphthous ulcers.
The risks of bias were analyzed following the Joanna Briggs Institute's Critical Assessment Checklist for systematic reviews and research summaries (Moola et al. 2015), and was between 60 and 80% in the studies, that is, the closer to 100%, the more reliable the model contextualizes the variability of the response data around the mean, and the risk of bias between 20 and 40% and the lower this risk, the greater the methodological quality (Pacheco et al. 2021).
And it is worth noting the inclusion of the meta-analysis in this systematic review that highlights the intervention with PBMT and aPDT/PBMT with evidence of efficacy corresponding to the 5 studies included, as well as the action of the therapeutic theme in the reduction of secondary lesions in the oropharyngeal region caused by COVID-19, who showed a statistically significant improvement in tissue repair and consequent quality of life. This also alerts about the importance of the dental surgeon in an intensive care unit, as part of the multi-disciplinary team, for the recovery of patients infected with COVID-19, in the improvement of the clinical and symptomatological condition (Amorim et al. 2020). However, it is noteworthy to report that the small number of cases in the literature opens the need for the publication of more articles using these therapeutic protocols, especially including randomized clinical trials.
Therefore, a total of 11 cases were presented here; in this way, we can suggest that PBMT and aPDT could be effective therapies, both isolated, but mainly when associated, as they are relatively low-cost therapies, of practical employability in hospital or outpatient settings, and that they proved to be effective in repairing the clinical picture of oral lesions of different nature and locations, in these patients affected by COVID-19 in a short period of time. However, as it is a recent disease on the world stage and non-random publications, it is necessary that further research on the benefits and harms of the use of low-intensity laser is carried out so that it is really effective in reducing and neutralization of diseases of the oropharyngeal tract in detriment of the probable high viral load of Sars-CoV-2 (Pacheco et al. 2021), for example, investigating the immunoexpression of ACE2 in oral lesions or saliva, and evaluating the possibility of these therapies inhibiting or altering this receptor at these sites.
Therefore, based on current findings, we suggest conducting new randomized clinical trials, which contain a well-developed methodology and include case descriptions with specific protocols for each lesion, in order to enrich the findings in these patients, and also define therapeutic protocols concrete for patients with COVID-19 who present lesions in the oral cavity (Sousa and Paradella 2021).
Conclusions
Based on the findings of this systematic review and literature meta-analysis, PBMT and aPDT were effective in the treatment of oral lesions in patients infected with COVID-19 in a short period of time.
The simultaneous use of PBMT/aPDT techniques, with different wavelengths, and the use of the photosensitive substance of methylene blue enhance the decontamination action of the oral cavity with eventual repair of the affected cells.
It is important to emphasize how meager the results' literature analysis were and that based on current literature no recommendation can be given, except that properly controlled long-term studies are needed with enough patient material.
Acknowledgements
Not applicable
Abbreviations
- PBMT
Photobiomodulation
- aPDT
Antimicrobial photodynamic therapy
- Sars-CoV-2
Severe acute respiratory syndrome Coronavirus 2
- PRISMA
Preferred report on systematic reviews and meta-analysis
- PROSPERO
International prospective registry of systematic reviews
- LLLT
Low intensity laser therapy
- COVID-19
Coronavirus disease 2019
- ACE2
Angiotensin-converting enzyme 2
- MeSH
Medical subject headings
- PICO
Population, intervention, comparison, outcome
- RT-PCR
Reverse transcription polymerase chain reaction
Author contributions
JAP contributed to the idea of the study. SAMC and MCB contributed to study design. JAP, KFM and CROGM established the search strategy and carried out the data extraction. JAP performed the statistical analysis. JAP, KFM and CROGM revised the draft manuscript for important intellectual content. All authors reviewed, edited drafts and approved the final manuscript for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baeder FM, Albuquerque ACL, Corazza PFL, et al. Oral lesions in patients infected with SARS-CoV-2: a case series. Res Soc Dev. 2021;10(4):e45410414349. doi: 10.33448/rsd-v10i4.14349. [DOI] [Google Scholar]
- Bensadoun RJ, Epstein JB, Nair RG, Barasch A, Raber-Durlacher JE, Migliorati C, Genot-Klastersky MT, Treister N, Arany P, Lodewijckx J, Robijns J, World Association for Laser Therapy (WALT) Safety and efficacy of photobiomodulation therapy in oncology: a systematic review. Cancer Med. 2020;9(22):8279–8300. doi: 10.1002/cam4.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- Brandão TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(2):e45–e51. doi: 10.1016/j.oooo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotti APCP, Carvalho WB, Johnston C, Rodriguez IS, Delgado AF. COVID-19 diagnostic and management protocol for pediatric patients. Clinics (sao Paulo) 2020;75:e1894. doi: 10.6061/clinics/2020/e1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera ET, Dias HB, Corbi SCT, et al. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: a critical review. Laser Phys. 2016;26(12):123001. doi: 10.1088/1054-660X/26/12/123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol-Spanemberg J, Figueiredo MA, Cherubini K, Salum FG. Low-level laser therapy: a review of its applications in the management of oral mucosal disorders. Altern Ther Health Med. 2016;22(6):24–31. [PubMed] [Google Scholar]
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn Ther. 2005;2(1):1–23. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers I, Bracken MB, Djulbegovic B, et al. How to increase value and reduce waste when research priorities are set. Lancet (london, England) 2014;383(9912):156–165. doi: 10.1016/s0140-6736(13)62229-1. [DOI] [PubMed] [Google Scholar]
- Conrado P, Sakita KM, Arita GS, Galinari CB, Gonçalves RS, Lopes L, Lonardoni M, Teixeira J, Bonfim-Mendonça PS, Kioshima ES. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagn Photodyn Ther. 2021;34:102221. doi: 10.1016/j.pdpdt.2021.102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz TRO, Peraza LAJ, Guimaraes DM, Matos VLH. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Special Care Dent. 2020;40(6):555–560. doi: 10.1111/scd.12520. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (eds) (2021) Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for systematic reviews of interventions version 6.2, Cochrane. www.training.cochrane.org/handbook
- Dos Amorim SJ, Normando AGC, da Carvalho SRL, et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis. 2020;97:326–328. doi: 10.1016/j.ijid.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Amorim SJ, Normando AGC, da Carvalho SRL, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2021;100(2):141–154. doi: 10.1177/0022034520957289. [DOI] [PubMed] [Google Scholar]
- Garcez AS, Delgado MGT, Sperandio M, et al. Photobiomodulation, photomedicine and laser. Surgery. 2021;39(6):386–389. doi: 10.1089/photob.2020.4977. [DOI] [PubMed] [Google Scholar]
- Hennessy M, Hamblin MR. Photobiomodulation and the brain: a new paradigm. J Opt. 2017;19(1):013003. doi: 10.1088/2040-8986/19/1/013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane. www.training.cochrane.org/handbook [DOI] [PMC free article] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranmanesh B, Khalili M, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID-19 disease: a review article. Dermatol Ther. 2021;34(1):e14578. doi: 10.1111/dth.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa MC, Manela-Azulay M. Photodynamic therapy: a review of the literature and image documentation. An Bras Dermatol. 2010;85(4):501–511. doi: 10.1590/s0365-05962010000400011. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS. Taking stock of COVID-19. J Dent Res. 2021;100(2):113–114. doi: 10.1177/0022034520984713. [DOI] [PubMed] [Google Scholar]
- Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Shi Z, Shu Y, et al. A distinct name is needed for the new coronavirus. Lancet. 2020;395(10228):949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasserson TJ, Thomas J, Higgins JPT (2021) Chapter 1: starting a review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. www.training.cochrane.org/handbook
- Lotfi M, Hamblin MR, Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya R, Ladeira LLC, Maya JEP, Mail LMG, Bussadori SK, Paschoal MAB. The combination of antimicrobial photodynamic therapy and photobiomodulation therapy for the treatment of palatal ulcers: a case report. J Lasers Med Sci. 2020;11(2):228–233. doi: 10.34172/jlms.2020.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjo AL, Pringle ES, Thornbury M, et al. Photodynamic inactivation of herpes simplex viruses. Viruses. 2018;10(10):532. doi: 10.3390/v10100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moola S, Munn Z, Sears K, et al. Conducting systematic reviews of association (etiology): the Joanna Briggs Institute's approach. Int J Evid Based Healthc. 2015;13(3):163–169. doi: 10.1097/XEB.0000000000000064. [DOI] [PubMed] [Google Scholar]
- Mutiawati E, Fahriani M, Mamada SS, Fajar JK, Frediansyah A, Maliga HA, Ilmawan M, Emran TB, Ophinni Y, Ichsan I, Musadir N, Rabaan AA, Dhama K, Syahrul S, Nainu F, Harapan H. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms-a systematic review and meta-analysis. F1000research. 2021;10:40. doi: 10.12688/f1000research.28393.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco JA, Molena KF, Martin CROG, Corona SAM, Borsatto MC (2021) Photobiomodulation (PBMT) and antimicrobial photodynamic therapy (aPDT) in oral manifestations of patients infected by SARS-COV-2: systematic review and meta-analysis, PREPRINT (Version 1) available at Research Square 10.21203/rs.3.rs-664972/v1 [DOI] [PMC free article] [PubMed]
- Ramires MCCH, Mattia MB, Tateno RY, Palma LF, Campos L. A combination of phototherapy modalities for extensive lip lesions in a patient with SARS-CoV-2 infection. Photodiagnosis Photodyn Ther. 2021;33:102196. doi: 10.1016/j.pdpdt.2021.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, Galvão GS, Ribas PF, Peres M, Franco JB. Photodynamic therapy in the treatment of oral lesions caused by paracoccidiomycosis. Photodiagnosis Photodyn Ther. 2021 doi: 10.1016/j.pdpdt.2021.102648. [DOI] [PubMed] [Google Scholar]
- Singh DK, Singh B, Ganatra SR, Gazi M, Cole J, Thippeshappa R, Alfson KJ, Clemmons E, Gonzalez O, Escobedo R, Lee TH, Chatterjee A, Goez-Gazi Y, Sharan R, Gough M, Alvarez C, Blakley A, Ferdin J, Bartley C, Staples H, Kaushal D. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat Microbiol. 2021;6(1):73–86. doi: 10.1038/s41564-020-00841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CD, Carvalho RA, Carvalho KA, Carvalho MG, Almeida OP. Letter to Editor: oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. 2020;25(4):e563–e564. doi: 10.4317/medoral.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa F, Paradella TC. Considerations on oral manifestations of COVID-19. J Med Virol. 2021;93(2):667–668. doi: 10.1002/jmv.26451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang PS, Bajgai GP, Phurpa D, Ngedup S, Pokhrel HP. Knowledge, attitude and practice towards COVID-19 among dental practitioners in Bhutan. IHRJ. 2021;5(2):OR1–OR6. doi: 10.26440/IHRJ/0502.05414. [DOI] [Google Scholar]
- Teixeira IS, Leal FS, Tateno RY, Palma LF, Campos L. Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagnosis Photodyn Ther. 2021;34:102281. doi: 10.1016/j.pdpdt.2021.102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H. Oral symptoms associated with COVID-19 and their pathogenic mechanisms: a literature review. Dent J. 2021;9(3):32. doi: 10.3390/dj9030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittar NB, Prucca CG, Strassert C, Awruch J, Rivarola VA. Cellular inactivation and antitumor efficacy of a new zinc phthalocyanine with potential use in photodynamic therapy. Int J Biochem Cell Biol. 2008;40(10):2192–2205. doi: 10.1016/j.biocel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Wiehe A, O'Brien JM, Senge MO. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci. 2019;18(11):2565–2612. doi: 10.1039/c9pp00211a. [DOI] [PubMed] [Google Scholar]
- Zecha JA, Raber-Durlacher JE, Nair RG, et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24(6):2781–2792. doi: 10.1007/s00520-016-3152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Lin B, Pathak JL, et al. ACE2 and furin expressions in oral epithelial cells possibly facilitate COVID-19 infection via respiratory and fecal-oral routes. Front Med (lausanne). 2020;7:580796. doi: 10.3389/fmed.2020.580796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.