Summary
Background
Current data suggest that dietary fibre (DF) interaction with the gut microbiota largely contributes to their physiological effects. The bacterial fermentation of DF leads to the production of metabolites, most of them are volatile. This study analyzed the breath volatile metabolites (BVM) profile in healthy individuals (n=15) prior and after a 3-week intervention with chitin-glucan (CG, 4.5 g/day), an insoluble fermentable DF.
Methods
The present exploratory study presents the original data related to the secondary outcomes, notably the analysis of BVM. BVM were analyzed throughout the test days -in fasting state and after standardized meals - using selected ion flow tube mass spectrometry (SIFT-MS). BVM production was correlated to the gut microbiota composition (Illumina sequencing, primary outcome), analyzed before and after the intervention.
Findings
The data reveal that the post-prandial state versus fasting state is a key determinant of BVM fingerprint. Correlation analyses with fecal microbiota spotlighted butyrate-producing bacteria, notably Faecalibacterium, as dominant bacteria involved in butyrate and other BVM expiration. CG intervention promotes interindividual variations of fasting BVM, and decreases or delays the expiration of most exhaled BVM in favor of H2 expiration, without any consequence on gastrointestinal tolerance.
Interpretation
Assessing BVM is a non-invasive methodology allowing to analyze the influence of DF intervention on the gut microbiota.
Funding
FiberTAG project was initiated from a European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL) and was supported by the Service Public de Wallonie (SPW-EER, convention 1610365, Belgium).
Keywords: Gut microbiota, Fibre, Short-chain fatty acids, Chitin-glucan, Fermentation, Breath volatile metabolome
Research in context.
Evidence before this study
The health effect of dietary fibres (DF) is of utmost importance considering the high capacity of the gut bacteria to produce bioactive metabolites upon DF fermentation. The identification of new biomarkers of DF intake through non-invasive analysis remains challenging. Breath analysis is a real-time, non-invasive and innovative method for detecting a wide range of volatile metabolites in expired breath. It is proposed as useful in disease diagnosis and as an indicator of metabolic disorders. However, the breath signature reflecting the interaction between DF intake and the gut microbiota has never been investigated until now.
Added value of this study
In the present exploratory study performed in healthy volunteers, we show that the nutritional status of the subjects is determinant to record the breath fingerprints, evaluated through selected ion flow tube mass spectrometry (SIFT-MS). The analysis reveals that several breath volatile metabolites (BVM) such as butyric acid, caproic acid, butanol, ethanol, phenol, methane, pentane, triethylamine, 3-hydroxybutanone and 2,3 butanedione can be exhaled in response to a standardized breakfast rich in DF. Most of those BVM are correlated with butyrate-producing bacteria, notably Faecalibacterium, a dominant gut bacteria, and may thus be proposed as biomarkers of the post-prandial gut bacterial metabolism. This study also shows that gut microbiota and exhaled BVM profile were modified after a chronic intake of chitin-glucan (CG), an insoluble DF whereas H2 expiration increased.
Implications of all the available evidence
Our results validate the hypothesis that the non-invasive breath analysis is an interesting tool to assess DF intake - gut microbiota interaction. It can be applicable for the follow up of most intervention studies targeting the gut microbiota for the improvement of health.
Alt-text: Unlabelled box
Introduction
The gut microbiota composition can be highly variable between individuals and the dietary habits explain around 20% of the microbial structural variations in humans. This highlights the potential for dietary strategies in disease management through gut microbiota modulation.1, 2, 3 Knowing that diet and microbiome are the strongest determinants of the human serum metabolome,4 the identification of bioactive metabolites issued from the interaction of food components and microbiota could be helpful in proposed biomarkers of health improvement based on nutritional strategies. Breath Volatile Metabolites (BVM) have been proposed as potential surrogate markers of gut dysbiosis in gastrointestinal diseases, as well as microbial signature of the fermentation of dietary fibre (DF).5,6 Indeed, the gut bacteria are a major source of volatile metabolites such as methane, H2, short chain fatty acids (SCFA) or alkanes. Such metabolites are partly exhaled in breath. There are almost 900 volatile compounds in the exhaled breath of healthy humans.7 This suggests that BVM analysis could be an indicator of metabolic disorders, of specific dietary habits, or both.6,8 We have previously shown that a single intake of a DF (chitin glucan) supplementation in the morning, creates a specific BVM profile throughout the day in healthy volunteers.9
The nutritional interest of DF comes from the recognition of their benefits for health based on an extensive literature. On the basis of studies in animals and humans, it has been proposed that DF might increase satiety, improve metabolic disorders, and modulate gut-related immunity through mechanisms related to SCFA production, thereby influencing endocrine and metabolic functions and intestinal epithelial integrity.10, 11, 12, 13, 14 Most DF are fermentable, entirely or at some extend and with different kinetics.15
In the context of the FiberTAG project (Joint Programming Initiative “A Healthy Diet for a Healthy Life” 2017-2020 https://www.fibertag.eu/), we aimed at establishing a set of bioactive bacterial metabolites linking DF intake and gut microbiota-related health effect through non-invasive and innovative methods (therefore excluding blood sampling).16 A monocentric longitudinal intervention study intervention has been performed in healthy volunteers consisting in the daily administration of 4,5 g of chitin-glucan (CG), a novel insoluble DF considered as safe food ingredient by the European Food Safety Authority,17 for 3 weeks. We have demonstrated that after 3 weeks, such a supplementation with CG induces specific changes in the gut microbiota composition, promoting mainly Eubacterium, Dorea and Roseburia genera, and increased butyric, iso-valeric and caproic acids level in the faeces.18 Following those data, we hypothesized that BVM measurement after 3 weeks-CG intervention could reflect the interaction between DF intake and the gut microbiota. In the present article, we present data of BVM measurement performed by using selected ion flow tube mass spectrometry (SIFT-MS), in fasting state and in response to meals, the data being correlated to the fecal microbiome sequencing.
Methods
Participants and study design
The FiberTAG study is an interventional monocentric study including two test days aiming to characterize gut microbiota composition and activity after a daily supplementation of 4.5 g of chitin-glucan (Kiotransine® from KitoZyme, Belgium) during 3 weeks, by analyzing volatile metabolites released in the breath and gut microbiota in stool samples. It is a longitudinal protocol, where the data were compared after 21 days of treatment versus time 0 in the same volunteers. Healthy young subjects were chosen to investigate the relevance of supplementing CG in the context of a healthy lifestyle. Sample size was estimated based on the primary outcome and using the software PASS 14.0.7 and the paired mean power analysis test. Fifteen subjects are sufficient to detect with 94% of power a mean difference of 2% with an estimated standard deviation of difference of 2% (two-sided) in specific gut bacteria level (primary outcome). Of note, this number is in line with other studies focusing on breath metabolites analyses.19, 20, 21
Healthy subjects were recruited by the Center of Investigation in Clinical Nutrition (CICN-UCLouvain) by displaying posters on the university site and by mails, by social networks, local newspapers and local flyers disposed in shops and doctor's offices in nearby cities. Thirty-four healthy males and females were recruited and underwent the screening test. Among them, sixteen subjects were included in the study based on the predefined inclusion and exclusion criteria and therefore received the allocated intervention. Fifteen completed the study.
Subjects were pre-screened by phone or mail using a questionnaire to test if they met the inclusion criteria (men or women, aged 18–40 years, body mass index (BMI) between 18 and 25 kg/m2, Caucasian, non-smoker, in good general health as evidenced by medical history and physical examination, the use of effective contraception for women, and need to be H2-producer. The exclusion criteria were: gastro-intestinal disorders, chronic or intestinal diseases (such as ulcers, diverticulitis or inflammatory bowel diseases), smoking, current or recent use of antibiotics, pro/prebiotics (as a dietary supplement) or any products affecting the gut transit (such as laxatives) within 4 weeks before starting the study, use of drugs that modify the composition of gut microbiota (antidiabetic drugs, cholesterol-lowering drugs, and proton pump inhibitors), pregnant or lactating woman (and woman who did not use highly effective contraception), psychiatric problems (and/or using antipsychotics), chronic intake of drugs, following a particular diet (e.g., vegetarian, high-fibre, or high-protein diets), allergy or food intolerance (e.g., lactose or gluten), presence of allergy or intolerance to one component of the tested product, excessive alcohol consumption (more than 3 units/day) and participation in another clinical trial 1 month before the screening visit. The recruitment was conducted from February to March 2018. The study was conducted from April to May 2018.
Thirty-four healthy males and females were recruited and underwent the screening test using lactulose to select H2-producers as previously describes and to exclude subjects with small intestinal bacteria overgrowth (SIBO).9 They were then invited to perform a screening test to assess if they are H2-producers and stool were collected. Screening lactulose test was performed at least 4 weeks before the intervention in order to assess if they are producer of H2. Briefly, fasted subjects (at least 10 h) received on oral load of 10 g of lactulose; then, exhaled H2 were measured every 30 min during 4 h (using Lactotest 202, Medical Electronic Construction, MEC). A minimal increase of 10 ppm of H2 during three successive measurements is needed to select the H2-producers. No increase should be observed in the first 30 min to avoid subjects with SIBO. Participants provided informed consent as well as stated willingness to comply with all study procedures and availability for the duration of the study.
Following the screening test, selected subjects performed the medical examination with the physician investigator to verify that they were healthy. After this medical examination, subjects were advised by a dietician to avoid diets too rich in DF, from at least two weeks before the intervention. Food products consumption containing high amounts of dietary fibers (including whole grains, artichokes, Jerusalem artichokes, salsifies, leeks, onions) was limited to once a week.
Two test days were organized before (day 0) and after (day 21) the nutritional intervention with CG to evaluate the kinetic of BVM production after the ingestion of a test meal rich in fibre. Subjects had to complete of 3 days-food diaries (two days in the week and one day in the weekend) one week before the two test visits to report all food and beverages consumed during the day. The Nubel Pro program and the table of composition from Nubel 2010 were used to assess macronutrient and total fiber intake. Within 4 days before day 0 and day 21, all subjects were asked to provide fresh stool samples. During the intervention, subjects were contacted frequently by mail or phone to verify that they still met the inclusion/exclusion criteria, that they followed the dietary advices and for the stool collection. Moreover, subjects were asked to complete an electronic questionnaire (via a software called REDCap, Research Electronic Data Capture) every day during the intervention, which allowed monitoring subjects’ side effects and compliance. Empty and unused packets were returned to measure compliance.
Ethics
This study was approved by the local ethical committee (Comité d'Ethique Hospitalo-Facultaire UCLouvain/Cliniques Universitaires Saint-Luc; reference 2017/11SEP/437) and written informed consent was obtained from all subjects before inclusion in the study. The trial was carried out in accordance with the Good Clinical Practice (GCP) as required by the following regulations: the Belgian law of 7 May 2004 regarding experiments on the human persons and the EU Directive 2001/20/EC on Clinical Trials. The trial was registered at ClinicalTrials.gov under identification number NCT03505177; Registered 23 April 2018).
Study outcomes
The primary outcome of the study was the evaluation of 3-weeks of CG supplementation on the gut microbiota composition and results were already published by our team.18 The present exploratory study presents the original data related to the secondary outcomes, notably the analysis of BVM upon a test day with highly controlled meal intake. Those data were obtained from the same subjects that were considered for the study of the primary outcome and previously presented.18
Test days
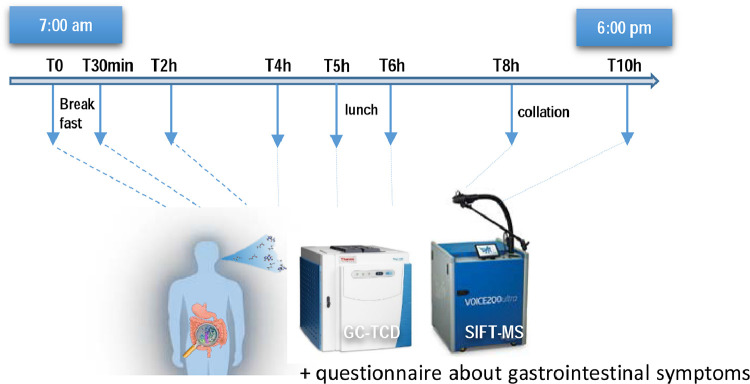
Both test days (day 0 and day 21) were organized in the same way. An overview of the study design is shown in Figure 1.
Figure 1.
Overview of the study design during the test days.
The evening before the two intervention days, subjects were asked to eat rice and meat, without vegetables and to avoid alcohol. Fasted subjects (from 9 pm the day before) arrived at 7 am at the investigation center (CICN). The study coordinator reviewed the inclusion and exclusion criteria and verified if instructions were adequately followed. After mouth wash (with Perio Aid Intensive Care), breath samples were taken to analyze BVM at baseline. Then, subject received a standardized breakfast rich in fibres (15 g in total) composed of 2 slices of rye bread coated with 25 g carob spread, and 200 ml orange juice. The breakfast has been eaten by all the participants within 15 min. Breath samples were collected at different times during 10 h after the breakfast ingestion to measure BVM. In the meantime, every hour, subjects were asked to a 100-mm VAS questionnaire at baseline and every hour for 10 h after the ingestion of the breakfast to investigate the 8 gastrointestinal symptoms (cramps, bloating, rumbling, discomfort, flatulence, burps, gastrointestinal reflux and nausea) usually described after the consumption of fibre. The scales were scored by measuring the distance (in mm) from 0 with a ruler. Scores were expressed as changes from baseline in cm. Of note, 2 h after the breakfast ingestion, subjects were free to drink water. Five hours after the start, the subjects received a standardized lunch composed of white bread, ham and cheese. The lunch has been eaten by all the participants within 15 min. At 4 pm (8 h after the start) subjects received a collation (sweet waffle). The test day ended at 6 pm.
Breath volatile metabolites (BVM) analysis
Breath samples (200 mL) were taken using specific sampling bags (FMONP.SBAG1.01 from MEC R&D SPRL, Belgium). The subjects were trained to blow deeply and slowly through the sampler after normal breathing. The bags were directly transported to Interscience (Louvain-La-Neuve, Belgium) and then connected first to the SIFT-MS (Voice 200ultra, Syft Technologies, New Zealand) for BVM measurement, then to the GC using a TCD detector (Trace 1310 GC, Thermo Scientific, USA) for H2 analysis; all analyses being performed within 2h after the harvest of the exhaled air as previously described.9 Three data sets of MS-fragments were obtained from H3O+, NO+ and O2+ ionizations (untargeted analysis). In addition, we targeted volatile metabolites known to study energy metabolism and to study the gut-related metabolic effects of DF, in particular those known to be a reflect of gut fermentation (coming from bacterial metabolism).8,21 Concentrations in ppm, determined by using an equation as previously described.9 Of note, we did not perform any data normalization step before identification of targeted BVM analyzed in the present study. Indeed, it was shown that several normalization approaches including probabilistic quotient normalization (PQN), were prone to introducing artefactual differences, in particular false positive biomarker identification and was not recommended for biomarkers identification through SIFT-MS technology.22,23 However, although it is known that the bags can have some inter-variations, we have considered BVM concentrations determined in bags filled with an inert gaz at each time point of the study, i.e. 0h-30min-2h-4h-5h-6h-8h-10h for both test days in order to remove a background bias that can appear due to environment and atmospheric conditions (such as temperature). Therefore, identified BVM (including H2) in breath samples were first corrected with the values obtained at each time point from those bags filled with helium (background removal). Then, we expressed data as absolute values in ppm or as changes from baseline in ppm.
Data integration and statistical analysis
A per protocol analysis was completed for this study. Data are expressed as medians ± interquartile ranges. However, since the median values are 0 for most of the gastrointestinal symptoms whatever the time considered, data are expressed also as means ± SD for Table S3 and Figure S5. Net area under the curve (AUC) were calculated for each symptom recorded every hour and for each identified BVM recorded during 10 h. Wilcoxon matched-pairs signed rank test were used to compare net AUC. In addition, mixed model followed by Sidak post-test were performed to compare evolution BVM over time (Figure S2). PCA model (presented in the Figure S1 and the supplementary video) was built on raw data without any normalization step by using R (v3.5.1, package “ade4”). Correlation analysis was performed between BVM and genera, with a mean of relative abundance ≥ 1% at day 0 or day 21, by Spearman's correlation test using the “corrplot” package in R. A significance level of p < 0.05 was adopted for all analyses.
Role of the funding source
The funding source had no role in study design, data collection, data analysis, data interpretation, writing of the report and in the decision to submit the paper for publication. The corresponding author confirms that she had full access to all the data of the study and had final responsibility for the decision to submit for publication.
Results
Subjects
Seven men and eight women were selected as H2-producers based on the lactulose test (data not shown) and completed the study respecting inclusion and exclusion criteria; only one volunteer dropped out on the last test day (no reason was given). There was 96.5% compliance in the participants. Baseline characteristics of participants are shown in Table S1. Carbohydrate, lipid, protein, fibre and alcohol intakes were not significantly affected whereas energy intake decreased by approximately 15% after 3 weeks of CG supplementation.18
Higher H2 was expired in response to the ingestion of a breakfast rich in DF after CG treatment
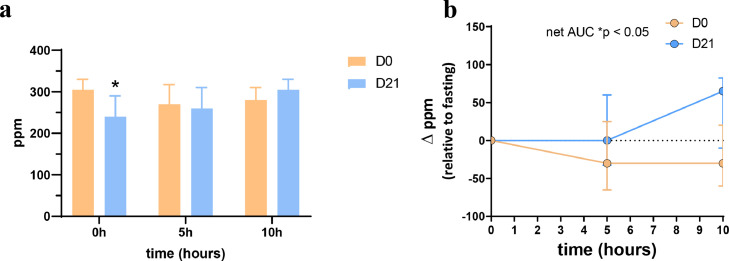
The Figure 2 presents H2 exhaled in the breath and measured with GC-TCD at time 0h, 5h, and 10h. H2 was exhaled with concentrations higher than 200 ppm. Basal levels obtained at fasting state (time 0h) were significantly different between both test days (p = 0.0186). Interestingly, exhaled H2 decreased 5h and 10h (-20 ppm) after the breakfast intake at day 0 whereas it increased (+ 20 ppm) after CG intervention (p = 0.0001).
Figure 2.
Hydrogen exhaled in the breath of healthy subjects (n=15) in response to a standardized breakfast intake prior (day 0) and after (day 21) chitin-glucan intake. Data present absolute values (a) and changes from baseline (b). Data are medians ± interquartile ranges (*p < 0.05; matched-pairs Wilcoxon signed-rank test).
BVM profile in the breath was modified in response to the ingestion of a breakfast rich in DF after CG treatment
Untargeted analysis using SIFT-MS was performed to record the breath fingerprints at different times during the test days. The dynamic evolution of MS-fragments obtained by ionisation with three precursor ions (H3O+, NO+, and O2+) was shown through principal component analysis (PCA) analysis (Figure S1 and supplementary video). We observed that separate clusters were formed depending on the time of measurement for the 15 subjects at both test days. The most discriminant time was the overnight-fasted state (time 0h) versus fed state (from time 30 min), pre-prandial state (meaning before lunch, time 4h and 5h) or post-prandial-post absorptive state (from time 6h). Furthermore, we observed that the individual variability was greater at overnight-fasted state, an effect promoted after CG treatment during 3 weeks.
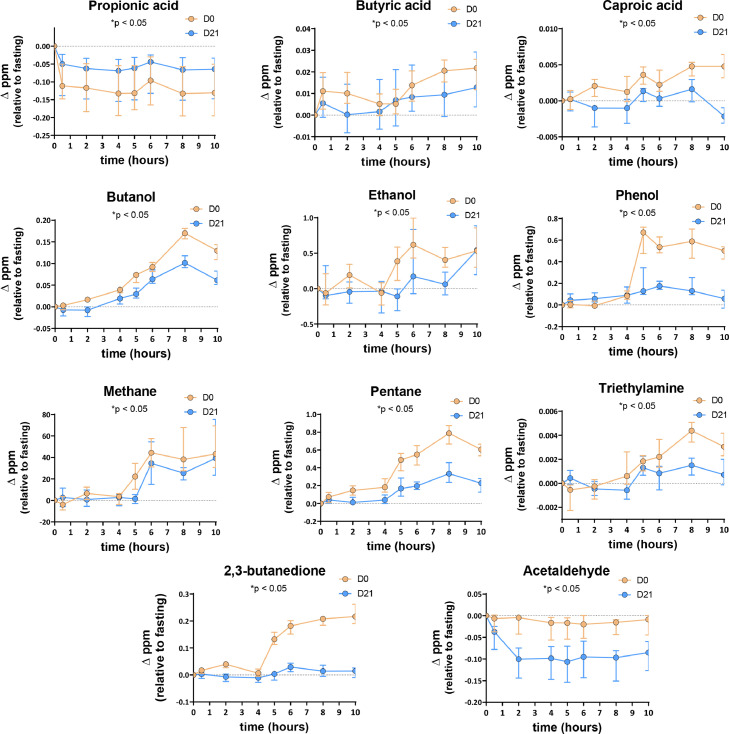
A targeted analysis was conducted to identify 24 BVM. namely issued from bacterial metabolism.8,9,21 The evolution of all BVM exhaled in the breath over time prior and after 3 weeks of CG intake are presented in Figure S2 in absolute values (ppm) whereas Figures 3 and S3 presented the concentrations in ppm corrected by the baseline values obtained at time 0h. We observed a large variation in concentration from one BVM exhaled in the breath to another from 0.001 to 100 ppm (Figure S3). We found that only two BVM -methane and acetone- were detected at levels higher than 1 ppm and both were not affected by CG intake. Among BVM detected in the breath at lower levels (between 0.01 and 1 ppm), acetaldehyde and phenol expired at fasted state (time 0h) were significantly higher at day 21 versus day 0 whereas fasting exhaled pentane, butyric acid and 3-hydroxybutanone were lower after the intervention (mixed model: p < 0.0001 for treatment). Exhaled H2S, triethylamine and caproic acid detected with levels below 0.01 ppm were also different at time 0h between both test days.
Figure 3.
Targeted BVM concentrations (changes from baseline) exhaled in breath of healthy subjects (n=15) in response to a standardized breakfast intake prior (day 0) and after (day 21) chitin-glucan intake (and significantly affected by the chitin-glucan intake during 3 weeks). Data are medians ± interquartile ranges (*p < 0.05; matched-pairs Wilcoxon signed-rank test on net area under the curve).
We focused our study on the evolution of exhaled BVM produced -expressed as change versus the baseline value in fasted state- after ingestion of a breakfast rich in DF (Figures 3 and S3). Mixed model revealed a significant effect of time for all targeted BVM (p < 0.05). A significant effect of CG treatment was obtained for butyric acid, caproic acid, ethanol, phenol, methane, pentane, triethylamine, 2,3-butanedione, and acetaldehyde (mixed model performed on all BVM evolution: p < 0.05). Among 6 SCFA identified, the net AUC of the evolution of butyric and caproic acid concentrations after the DF-rich breakfast was significantly decreased by the CG treatment (p = 0.0353 and p = 0.0004, respectively) (Figure 3). Besides changes in SCFA exhaled in the breath, Figure 3 revealed 8 other BVM with a net AUC significantly lower after the nutritional intervention with CG, that are butanol (p = 0.0084), ethanol (p = 0.0034), phenol (p < 0.0001), methane (p = 0.0151), pentane (p = 0.0001), triethylamine (p = 0.0215), 2,3-butanedione (p < 0.0001) and acetaldehyde (p = 0.0003). The power calculation and the effect size on those secondary outcomes were presented in the Table S2. We observed that the power and the size effect are excellent (>80% and >0.8, respectively) for 6 of them. Propionic acid and acetaldehyde were the sole BVM that decreased in response to the breakfast without any other variation observed throughout the day. The decrease of acetaldehyde was more pronounced after CG treatment whereas the level of exhaled propionic acid remained higher all day long after CG intake.
Overall, it is important to note that for some BVM, their level was higher in the fasting than in all measurement performed later on, in the post-prandial or post-absorptive state. It is the case for propionic acid, propanol and benzene that drastically dropped (by more than 40-fold for propionic acid, and around 10-fold for propanol) after the breakfast (Figures 3, S3). Acetic acid and acetaldehyde, also dropped down at a lesser extend (by about 2-fold) upon feeding. All the other BVM remained stable or increased at different time points during the test days.
Concerning exhaled SCFA, butyric, caproic and (iso)valeric acids increased just after the breakfast (mostly at time 2h) before CG intervention with a return to basal values only for (iso)valeric acids, whereas butyric and caproic acids continued to rise (Figures 3, S3). Furthermore, the highest level of SCFA exhaled in the breath (except for acetic and propionic acids that decreased) were obtained much later, i.e. at least 8h after the standard breakfast when CG was consumed during 3 weeks.
As observed for butyric acid, caproic acid, butanol, ethanol, phenol, methane, pentane, triethylamine, pentane, and 2,3-butanedione increased in response to fibre-rich breakfast at day 0 before CG intervention, with the highest levels obtained mainly 8 h after breakfast intake (Figure 3). Surprisingly, CG consumption during 3 weeks reduced the magnitude of the response to the DF-enriched breakfast for those microbial metabolites at time 8h (*p < 0.05; matched-pairs Wilcoxon signed-rank test performed at time 8h). Although similar evolution of BVM were observed for other targeted BVM (e.g. hydroxybutanone followed the same trend than butyric acid), matched-pairs Wilcoxon signed-rank test performed on total net AUC did not reveal any significant effect of CG intervention (Figure S3).
Importantly, BVM were differently affected considering the post-prandial post-breakfast or post-lunch states (Figure S4). Most significant changes occurred in the post-lunch state upon the CG supplementation: among BVM that increased after the lunch versus the fasted state for both days, higher levels of 2-methylbutyric acid, isovaleric acid, acetone exhaled in the breath whereas lower levels of triethylamine, caproic acid, butyric acid, butanol, ethanol, phenol, pentane and methane were observed after CG treatment (Figure S4b).
Gastrointestinal tolerance after ingestion of a breakfast rich in DF was not modified after the CG treatment
Knowing that hydrogen production after feeding was higher after CG treatment, and that this DF is largely fermented, the question of the potential effect of CG treatment on gastrointestinal symptoms merited to be evaluated. We show that the baseline scores of gastrointestinal parameters before and after CG treatment were not significantly different (p>0.05) (Table S3). Gastrointestinal tolerance was investigated after ingesting of the standardised breakfast rich in fibre. The evolution of the different scores throughout the day was not significantly affected by CG intake (matched-pairs Wilcoxon signed-rank test on net AUC: p>0.05) (Figure S5).
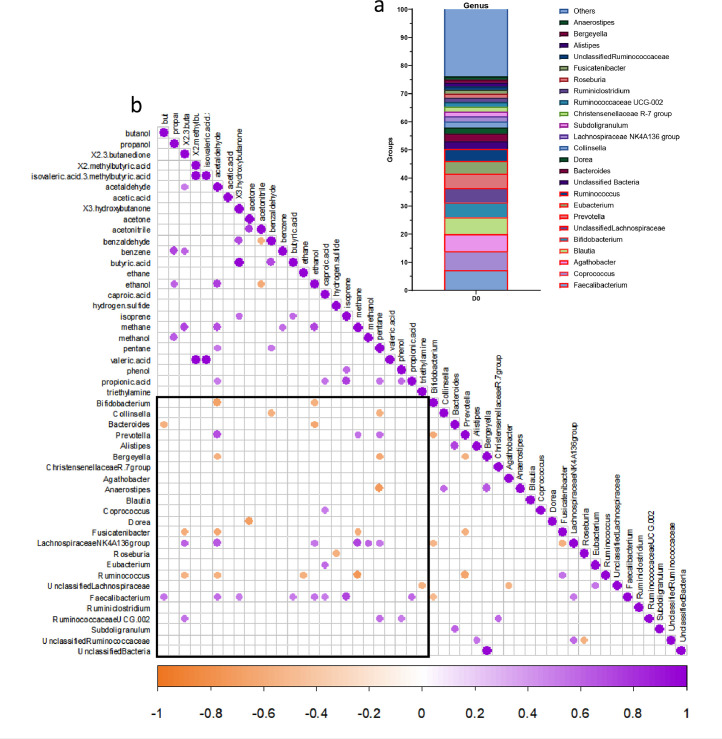
Correlation analyses between exhaled BVM and gut microbiota composition
The gut microbiota of participants was analyzed before and after 3 weeks of CG intervention.18 Figure 4a shows the relative abundance at the genus level at baseline prior any DF intervention. We observed that 50% of the fecal bacteria of healthy subjects were dominated by 9 genera, most of them being butyrate-producing bacteria (Faecalibacterium, Ruminococcus, Eubacterium, unclassified Lachnospiraceae, Coprococcus). Correlation analysis was performed between gut microbiota composition and BVM prior CG intervention (day 0) considering the time where the highest levels of BVM were obtained, i.e 8h after the breakfast. Interestingly, Faecalibacterium, the most abundant one (7 % in relative abundance) was correlated (p = 0.0498) with exhaled butyric acid 8h after the breakfast intake (Figure 4b). Moreover, this genus was also positively correlated with other BVM, most of them being bacterial metabolites9: butanol (p = 0.0351), acetaldehyde (p = 0.0260), ethanol (p = 0.0351), isoprene (p = 0.0019), propionic acid (0.0189), caproic acid (0.0498) and 3-hydroxybutanone (p = 0.0498). At that time, other exhaled bacterial metabolites (propionic acid, 2,3 butanedione, phenol, pentane, methane) were positively correlated with abundant genera (> 1%), mainly Prevotella or genera from Ruminococcaceae and Lachnospiraceae families (p < 0.05).
Figure 4.
Correlation analysis between breath volatile metabolites (ppm, exhaled at time 8h) and gut microbiota composition of 15 healthy subjects (n=15) prior chitin-glucan intervention (day 0). Barplots of relative abundance of genus levels accounting for more than 1% for each subject (a). Heatmap of correlations between genera accounting for more than 1% for each subject and BVM (at time 8h, in ppm, corrected by the baseline value at fasted state) (b). The presence of a circle indicates that the correlation is significant, p < 0.05 (Spearman's correlation test).
The impact of CG intake on gut microbial changes after 3 weeks of intervention was previously described.18 We concluded that a 3-week CG supplementation did not induce major changes in the overall gut microbiota composition (no change in α-diversity and β-diversity indexes). We have previously shown that CG consumption for 3 weeks resulted in specific changes to bacterial genera and species.18 Indeed, we found that 3 weeks of CG intake significantly increased Lachnospiraceae UCG-004, Roseburia, Eubacterium, Ruminococcaceae UCG-003 and UCG-005, whereas it decreased Subdoligranulum, Bergeyella, Blautia and Dorea. However, among bacteria that increased due to CG intervention during 3 weeks (and accounting for more than 1% for each subject), none of those were positively related to expiration of bacterial metabolites (see correlation analyses between BVM at time 8h and genera at D21 in Supplementary data).
Discussion
In general, metabolites measured in biological fluids are considered as interesting biomarkers of human clinical and nutritional status and can therefore be proposed as a useful diagnostic aid for monitoring metabolic diseases.6 Breath analysis is a non-invasive and sensitive medical diagnostic tool that offers the possibility of rapid measurements.8 For example, BVM analysis hence serve as attractive, non-invasive biomarkers for diagnosing and monitoring irritable bowel syndrome and inflammatory bowel disease.24 However, the origin of volatile metabolites is not yet fully understood. Analytical methods are continually developed to allow for better detection and quantification of volatile metabolites, thus enabling the detection of a wider range of biomarkers of pathophysiology and nutritional status. Direct MS techniques such as SIFT-MS have gained increasing interest because they offer the promise of selectivity, speed and sensitivity (low parts per billion by volume levels), and SIFT-MS is particularly well suited for the purpose of exhaled breath analysis.25,26 This technique can be used in untargeted scan mode, where it provides pattern-based classification capacity but usually, it is applied in targeted mode, which means that only a limited set of components are measured simultaneously.26,27 The contribution of nutrition in the profile of BVM remains poorly evaluated.
Some studies monitored exhaled breath upon changes in dietary habits (high versus low fat dairy drink, high versus low fibre diets, gluten-free diet).20,21,28, 29, 30 Recently, we have analysed the impact of a single intake of an insoluble DF (4.5 g CG) on breath volatile metabolome in healthy subjects. The targeted approach obtained with SIFT-MS analysis and correlation analyses with gut microbiota composition suggested that exhaled butyric acid, triethylamine, pentane, 3-hydroxybutanone and 2,3-butanedione were identified as microbial signature of CG intake with the highest levels observed 6h after its ingestion.9 In a recent study, it was shown that no significant changes in volatile compound profiles (using thermal desorption gas chromatography coupled to time-of-flight mass spectrometry) were found after 4 weeks of a prebiotic dietary fibre supplementation (galacto-oligosaccharides, 15g/day) both in elderly as well as in young adults after an overnight fast, indicating relatively small differences in exhaled breath profiles, which is in line with microbiota composition and fecal metabolite data.19 The objective of the present exploratory study was to investigate the complex interaction between DF and gut microbiota by assessing BVM before and after 3 weeks of CG consumption in a representative homogeneous population of healthy young gender-mixed volunteers with the same level of education. The recruitment of subjects performed on a university site allowed to reach this objective since 7 men and 8 women with a mean age of 21 in good general health, as evidenced by medical history and physical examination, were included in the study. We have applied the SIFT-MS methodology in order to follow exhaled BVM in response to a standardized breakfast rich in fibre prior and after chronic consumption of CG (4.5g per day during 21 days). There was an excellent compliance of the participants reaching 96.5%. Thus, this parameter does not impact on study inferences. We selected rye bread and carob spread to design the test meal because of their DF richness and fermentation capacity was already demonstrated. Indeed, carob spread is a high DF and polyphenol containing product with 17% of total DF (in which 10% are soluble DF).31 Rye bread contains 11% of total DF mainly fructans and water-extractable arabinoxylans which explained its high fermentation rate and extent capacity in the in vitro colon model.32 Of particular interest, rye fractions (bran or aleurone) were shown to produce high relative proportions of butyric acid and phenolic microbial metabolites were also produced in this model. The high proportion of DF present in this standardized breakfast (15g) can be associated with gastrointestinal symptoms,18,33 partly due to a rapid fermentation causing gas production or bloating. However, this breakfast seemed to be well tolerated regarding analysis of 8 gastrointestinal symptoms and we observed that 3 weeks of CG intake did not influence the gastrointestinal tolerance towards a breakfast rich in fibre in healthy subjects.
Untargeted analysis revealed that breath volatile metabolome was highly influenced by the nutritional status of the subjects. The most important inter-subject variability was observed when the measurement was performed after an overnight-fasting (time 0h versus other times). A shift in BVM clearly occurred after feeding suggesting that the nutritional status is important to consider for breath analysis interpretation. We highlighted that the dietary intake of CG during 3 weeks emphasized the inter-individual variability whatever the nutritional status, i.e. independently of the time of the day or a meal ingestion. It has already observed that effects of the fasted or fed state on breath constituents are complex.8 In the present study, we observed that the highest levels of acetic acid, propionic acid, acetaldehyde, propanol, benzene were found at fasted state in the morning. Among them, only exhaled propionic acid and acetaldehyde were influenced by the chronic DF supplementation: exhaled propionic acid increased whereas acetaldehyde decreased after CG intake in post-prandial state. Correlation analysis revealed that breath propionic acid is mainly related to the presence of Faecalibacterium at day 0 and Ruminococcaceae at day 21. Breath acetaldehyde, in the absence of exogenous alcohol administration, may be considered as a bacterial metabolite since.34 However, since exhaled propionic acid and acetaldehyde did not increase in response to a meal rich in DF (versus fasted state), those both BVM cannot be proposed as key biomarkers of DF intake.
In the first 4 h of the test days, only few BVM were significantly affected. Before CG intervention, SCFA with 4 carbon atoms rapidly increased in the breath after breakfast ingestion. Despite the rinsing of the mouth with an antiseptic solution before breath sampling at time 0, we cannot exclude that the bacterial fermentation occurring in the mouth or throat could participate to the early increase (at time 30 min) of exhaled breath BVM, as already hypothesized by others.21,35 Importantly, both butyric and caproic acids were the sole SCFA which level in the breath increased from time 4h. They could be related to gut fermentation of the DF present at high concentration in the breakfast. Butyrate is produced by bacteria through butyrate kinase or via butyryl CoA:acetate CoA transferase whereas caproic acid has been recently shown to be produced from lactate oxidation.36,37 In bacteria, lactate oxidation provides acetyl-CoA which enters elongation process to form butyrylCoA and hexanoylCoA (also called caproylCoA).
Besides SCFA, a rise in butanol, ethanol, phenol, methane, pentane, triethylamine, 2,3-butanedione, acetone, acetonitrile, benzaldehyde, 3-hydroxybutanone, H2S or methanol also occurred after lunch before CG intervention (at day 0) and often peaked after 8 h. For those BVM, it seems relevant to propose the gut fermentation of nutrients reaching the colon as the driver of their production and release in the breath. Therefore, the correlation analyses were performed between BVM (in ppm, corrected by the baseline value at fasted state) at the time where most of breath BVM were present in the breath at the highest levels, i.e. 8h after the breakfast intake and genera (with relative abundance > 1%). Butyric and caproic acids were positively correlated to Faecalibacterium, the most abundant genus detected in healthy subjects at baseline. This genus was also correlated with high levels (obtained at time 8h) of other exhaled BVM recognized as bacterial metabolites such as butanol, ethanol and 3-hydroxybutanone.9,38,39 Furthermore, 2,3 butanedione, phenol, pentane and methane are other bacterial metabolites exhaled in the breath and were positively correlated with other interesting butyrate-producing bacteria, mainly from Ruminococcaceae and Lachnospiraceae families. Bacterial production of 3-hydroxybutanone and 2,3-butanedione is related to citrate metabolism through the citrate–oxaloacetate–pyruvate–acetolactate–acetoin/diacetyl pathway, where pyruvate is considered as the precursor.39 Alcohol/aldehyde dehydrogenase found in some Clostridia converts butyryl-CoA to n-butanol.40 While the origin of breath methane is undoubtedly linked to gut fermentation, intestinal bacteria are also considered as a major source of breath pentane versus the contribution of endogenous membrane lipid peroxidation.8,41,42 It should be interesting to have data supporting the relative contribution of bacterial versus host metabolism in the production of the metabolites such as pentane. Indeed, it was already shown that exhaled pentane was dependent on dietary linoleate intake, and was blunted upon antibiotic treatment.8,41 Altogether, our data suggest that SCFA such as butyric and caproic acids but also other metabolites such as ethanol, butanol, phenol, 3-hydroxybutanone, 2,3 butanedione, pentane and methane exhaled in the breath are issued from bacterial metabolism in the lower part of the gut, depending mostly on bacteria known to be butyrate-producers. It should be interesting to know if the metabolic pathways present the identified bacteria are able to produce such metabolites. Undeniably, further studies are required to study the origin and the fate of most BVM metabolites.
We have demonstrated that the shift in the gut microbiota composition occurring upon CG intervention during 3 weeks (mainly increase of Roseburia and Eubacterium genera) is not due to intra-individual variations of microbiome with time and could clearly be attributed to CG treatment, making those bacteria privileged targets of CG intake.18 In fact, Roseburia is known for their important ability to utilize diet-derived polysaccharides and in turn produce butyrate.36 Consistently, we highlighted higher fecal butyric acid concentration after CG supplementation.18 However, this effect was not accompanied by higher expiration of this SCFA in the breath in response to a meal rich in DF. We observed that C5 and C6 SCFA were also affected by the dietary intervention but at much lesser extent (delta < 0.01 ppm). Besides SCFA exhaled in the breath, 7 BVM reflecting bacterial metabolism in the colon were significantly affected by the nutritional intervention with CG. Indeed, butanol, ethanol, phenol, methane, pentane, triethylamine and 2,3-butanedione were exhaled with highest levels obtained at least 6h after the DF-enriched breakfast but the amplitude of the response was much lower after the CG supplementation. Of particular interest, analysis focusing on post-lunch state revealed that exhaled butyric acid, caproic acid, butanol, ethanol, phenol, triethylamine, pentane and methane decreased after CG intervention. In contrast, breath H2 increased after CG intervention in response to the DF-enriched breakfast. We observed that the basal level of H2 release in the fasting state after the treatment were significantly different between both test days. Knowing that the evening meal – and its content in DF- may influence the H2 production in the next morning,43 we have standardized the evening meal (that consisted in eating meat, rice without vegetables) in order to minimize DF intake. Therefore, the different levels in fasting state at day 0 and day 21, could be due to the modifications of the gut microbiota composition (and function) linked to chronic CG intake and the potential fermentation of nutrients in the large intestine during the night. In fact, microbial H2 cycling is central to metabolic homeostasis and microbial composition in the human gastrointestinal tract. Molecular H2 is produced as an end product of carbohydrate fermentation, and may be subsequently utilized by cross-feeding microbes for growth and in the production of larger molecules (H2 is reoxidised primarily by sulfate-reduction, acetogenesis, and methanogenesis).42,44 Wolf et al. has demonstrated that 70% of gastrointestinal microbial species encode the genetic capacity to metabolise H2 and its relative proportion compared to the other bacterial products depend on the environmental conditions (e.g. pH).44 Furthermore, the presence of H2 also produces a thermodynamic environment favouring SCFA production.42 We have also to consider that more than 90% of the colon bacteria are butyrate-producers represented by Eubacterium/Roseburia spp., which belong to clostridial cluster XIVa (24% of the total colon microbiota), and Faecalibacterium prausnitzii, which belongs to the clostridial cluster IV (7% of the total colon microbiota).45 A quantitative link or stoichiometrically balanced pathway between bacterial substrate metabolism and H2 gas formation have already been proposed in cluster XIVa butyrate-producing colon bacteria.45,46 Considering the higher proportion of two important butyrate-producers (Eubacterium and Roseburia) observed after chronic consumption of CG, those links were probably involved in mechanisms explaining the higher production of breath H2.
We have identified several limitations to our study. First, it is a longitudinal protocol, where the data were compared after 21 days of treatment versus time 0 in the same subjects. This kind of strategy has already been used in order to highlight microbial signature of a prebiotic DF.47 We cannot rule out that some changes in BVM may have occurred with time independent of the treatment. Secondly, this study is focused on a small cohort investigated in a single center. Thirdly, it potentially included some bias by self-reporting of food diaries. Finally, hydrogen-producers only (and excluding subjects with SIBO) were considered.
In conclusion, this study showed that several bacterial metabolites can be exhaled in the breath in response to a meal rich in DF. Although we cannot strictly exclude the presence of an altered metabolic homeostasis and/or an altered gut microbiota, it is important to note that medical history, drug history, inclusion and exclusion criteria and some clinical parameters obtained at the baseline led us to exclude metabolic diseases known to be link with gut dysbiosis such as obesity, diabetes or hypertension, and to suppose this young population as “healthy”. This exploratory study identified exhaled butyric acid, caproic acid, butanol, ethanol, phenol, methane, pentane, triethylamine, 3-hydroxybutanone and 2,3 butanedione as biomarkers of the gut bacterial metabolism after a breakfast rich in DF. We have to note that the power is less than 80% only for 4 out of those 10 BVM. Our data suggested that the chronic intake of CG, an insoluble DF, decreased or delayed the expiration of most exhaled BVM in favor of H2 expiration in response to a meal rich in DF, without inducing uncomfortable gastrointestinal symptoms. The lower exhalation of BVM such as triethylamine, acetaldehyde and pentane could be interesting to explore in the context of inflammatory bowel disease (Crohn's disease, ulcerative colitis) or nonalcoholic fatty liver disease (NAFLD) since their level can be found significantly elevated in the breath of those patients.48,49 Altogether with our previous study devoted to study breath volatile metabolome after a single intake of CG,9 we confirmed that analysis of BVM in exhaled air can be used as a dynamic approach to noninvasively assess the complex interaction between DF and gut microbiota. Our data also support the interest of focusing on the role of nutrition on exhaled BVM profiling at a time where this non-invasive procedure is proposed as an interesting tool to unravel metabolic and infectious diseases, like recently shown for Covid 19 infection in human.50 This study supports the relevance of breath volatile metabolomic approach to unravel nutrients-gut microbiota-host interactions. Further experiments with different types of DF would allow to unravel the metabolomic signature of their interaction with the gut microbiome, knowing that the concepts of selective fermentation of DF is largely studied in vitro, but poorly assessed in vivo. The non-invasive sampling required for this type of analysis also appears as a positive outcome of our data.
Contributors
Conceptualization: A.M.N., N.M.D.; Data curation: A.M.N., J.R.; Formal analysis: A.M.N., J.R., Z.Z.; Funding acquisition: N.M.D., V.M., M.L., S.C.B., J.W.; Investigation: A.M.N., J.R. Methodology: A.N.M., J.R., Z.Z., L.B.B.; Project administration: N.M.D., A.N.M.; Resources: P.D.C., N.M.D., V.M.; Software: J.R.; Supervision: N.M.D.; Validation with verification of the underlying data: N.M.D., A.M.N., J.R.; Visualization: A.N.M., J.R.; Writing—original draft: A.N.M.; Writing—review and editing: J.R., Z.Z., J-A.N., P.D.C., L.B.B., N.M.D. All authors read and approved the final version of the manuscript.
Declaration of interests
P.D.C. is cofounder of A-Mansia Biotech SA and owner of patents on Akkermansia muciniphila, gut microbes and metabolic diseases. The other authors declare no conflict of interest.
Acknowledgments
Acknowledgments
We are very grateful to Barbara Pachikian from the UCLouvain platform CICN (Center of Investigation in Clinical Nutrition) for her helpful support and criticism during the preparation of the study and the investigation with volunteers. We thank Remi Selleslagh and Véronique Allaeys for the excellent technical assistance. We thank the data analysis consulting service of UCLouvain (platform “Support en méthodologie et calcul statistique”) and more specifically Céline Bugli for their advice and the validation of statistical analyses. All cited individuals provided written consent. We also thank the volunteers who participated in this study. NMD is a recipient of grants from the Fonds de la Recherche Scientifique (FRS-FNRS) [PINT-MULTI R.8013.19 (NEURON-ERANET, call 2019) and PDR T.0068.19], and from the Fédération Wallonie-Bruxelles (Action de Recherche Concertée ARC18- 23/092). PDC is a research director at FRS-FNRS and is supported by Fonds de la Recherche Scientifique (FRFS-WELBIO: WELBIO-CR-2022 A–02, and EOS program no.40007505).
Data sharing statement
The study protocol and the datasets generated during and/or analyzed during the current study, including deidentified participant data will be available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104051.
Appendix. Supplementary materials
References
- 1.Zhang C., Zhang M., Wang S., et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4(2):232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 2.Rothschild D., Weissbrod O., Barkan E., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 3.Klingbeil E., de La, Serre C.B. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):R1254–R1R60. doi: 10.1152/ajpregu.00037.2018. [DOI] [PubMed] [Google Scholar]
- 4.Bar N., Korem T., Weissbrod O., et al. A reference map of potential determinants for the human serum metabolome. Nature. 2020;588(7836):135–140. doi: 10.1038/s41586-020-2896-2. [DOI] [PubMed] [Google Scholar]
- 5.Rieder F., Kurada S., Grove D., et al. A distinct colon-derived breath metabolome is associated with inflammatory bowel disease, but not its complications. Clin Transl Gastroenterol. 2016;7(11):e201. doi: 10.1038/ctg.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rondanelli M., Perdoni F., Infantino V., et al. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J Anal Methods Chem. 2019;2019 doi: 10.1155/2019/7247802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lacy Costello B., Amann A., Al-Kateb H., et al. A review of the volatiles from the healthy human body. J Breath Res. 2014;8(1) doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- 8.Ajibola O.A., Smith D., Spanel P., Ferns G.A. Effects of dietary nutrients on volatile breath metabolites. J Nutr Sci. 2013;2:e34. doi: 10.1017/jns.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neyrinck A.M., Rodriguez J., Zhang Z., et al. Noninvasive monitoring of fibre fermentation in healthy volunteers by analyzing breath volatile metabolites: lessons from the FiberTAG intervention study. Gut Microbes. 2021;13(1):1–16. doi: 10.1080/19490976.2020.1862028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delzenne N.M., Olivares M., Neyrinck A.M., et al. Nutritional interest of dietary fiber and prebiotics in obesity: lessons from the MyNewGut consortium. Clin Nutr. 2020;39(2):414–424. doi: 10.1016/j.clnu.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Delzenne N.M., Neyrinck A.M., Cani P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Factories. 2011;10(Suppl 1):S10. doi: 10.1186/1475-2859-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delzenne N.M., Neyrinck A.M., Backhed F., Cani P.D. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7(11):639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 13.Lordan C., Thapa D., Ross R.P., Cotter P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11(1):1–20. doi: 10.1080/19490976.2019.1613124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephen A.M., Champ M.M., Cloran S.J., et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30(2):149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 16.Neyrinck A.M., Rodriguez J., Vinoy S., et al. The FiberTAG project: tagging dietary fibre intake by measuring biomarkers related to the gut microbiota and their interest for health. Nutr Bull. 2020;45(1):59–65. doi: 10.1111/nbu.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EFSA Panel on Dietetic Products NaAN Scientific opinion on the safety of ‘Chitin-glucan’ as a novel food ingredient. EFSA J. 2010;8:1687. [Google Scholar]
- 18.Rodriguez J., Neyrinck A.M., Zhang Z., et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes. 2020;12(1) doi: 10.1080/19490976.2020.1810530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilms E., An R., Smolinska A., et al. Galacto-oligosaccharides supplementation in prefrail older and healthy adults increased faecal bifidobacteria, but did not impact immune function and oxidative stress. Clin Nutr. 2021;40(5):3019–3031. doi: 10.1016/j.clnu.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Hageman J.H.J., Nieuwenhuizen A.G., van Ruth S.M., Hageman J.A., Keijer J. Application of volatile organic compound analysis in a nutritional intervention study: differential responses during five hours following consumption of a high- and a low-fat dairy drink. Mol Nutr Food Res. 2019;63(20) doi: 10.1002/mnfr.201900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raninen K.J., Lappi J.E., Mukkala M.L., et al. Fiber content of diet affects exhaled breath volatiles in fasting and postprandial state in a pilot crossover study. Nutr Res. 2016;36(6):612–619. doi: 10.1016/j.nutres.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Filzmoser P., Walczak B. What can go wrong at the data normalization step for identification of biomarkers? J Chromatogr A. 2014;1362:194–205. doi: 10.1016/j.chroma.2014.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Noonan M.J., Tinnesand H.V., Buesching C.D. Normalizing gas-chromatography-mass spectrometry data: method choice can alter biological inference. BioEssays. 2018;40(6) doi: 10.1002/bies.201700210. [DOI] [PubMed] [Google Scholar]
- 24.Van Malderen K., De Winter B.Y., De Man J.G., De Schepper H.U., Lamote K. Volatomics in inflammatory bowel disease and irritable bowel syndrome. EBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruderer T., Gaisl T., Gaugg M.T., et al. On-line analysis of exhaled breath focus review. Chem Rev. 2019;119(19):10803–10828. doi: 10.1021/acs.chemrev.9b00005. [DOI] [PubMed] [Google Scholar]
- 26.Dryahina K., Spanel P., Pospisilova V., et al. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2013;27(17):1983–1992. doi: 10.1002/rcm.6660. [DOI] [PubMed] [Google Scholar]
- 27.Stefanuto P.H., Zanella D., Vercammen J., et al. Multimodal combination of GC x GC-HRTOFMS and SIFT-MS for asthma phenotyping using exhaled breath. Sci Rep. 2020;10(1):16159. doi: 10.1038/s41598-020-73408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kistler M., Szymczak W., Fedrigo M., et al. Effects of diet-matrix on volatile organic compounds in breath in diet-induced obese mice. J Breath Res. 2014;8(1) doi: 10.1088/1752-7155/8/1/016004. [DOI] [PubMed] [Google Scholar]
- 29.Baranska A., Tigchelaar E., Smolinska A., et al. Profile of volatile organic compounds in exhaled breath changes as a result of gluten-free diet. J Breath Res. 2013;7(3) doi: 10.1088/1752-7155/7/3/037104. [DOI] [PubMed] [Google Scholar]
- 30.Barros R., Moreira A., Fonseca J., et al. Dietary intake of alpha-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br J Nutr. 2011;106(3):441–450. doi: 10.1017/S0007114511000328. [DOI] [PubMed] [Google Scholar]
- 31.Nasar-Abbas S.M., Z E.H., Vu T.H., Khan M.K., Esbenshade H., Jayasena V. Carob kibble: a bioactive-rich food ingredient. Compr Rev Food Sci Food Saf. 2016;15(1):63–72. doi: 10.1111/1541-4337.12177. [DOI] [PubMed] [Google Scholar]
- 32.Nordlund E., Aura A.M., Mattila I., Kosso T., Rouau X., Poutanen K. Formation of phenolic microbial metabolites and short-chain fatty acids from rye, wheat, and oat bran and their fractions in the metabolical in vitro colon model. J Agric Food Chem. 2012;60(33):8134–8145. doi: 10.1021/jf3008037. [DOI] [PubMed] [Google Scholar]
- 33.Eswaran S., Muir J., Chey W.D. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):718–727. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- 34.Salaspuro M.P. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci. 2003;40(2):183–208. doi: 10.1080/713609333. [DOI] [PubMed] [Google Scholar]
- 35.Turner C., Parekh B., Walton C., Spanel P., Smith D., Evans M. An exploratory comparative study of volatile compounds in exhaled breath and emitted by skin using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(4):526–532. doi: 10.1002/rcm.3402. [DOI] [PubMed] [Google Scholar]
- 36.Louis P., Flint H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X., Tao Y., Liang C., et al. The synthesis of n-caproate from lactate: a new efficient process for medium-chain carboxylates production. Sci Rep. 2015;5:14360. doi: 10.1038/srep14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J., Ngo J., Blake D., et al. Improved predictive models for plasma glucose estimation from multi-linear regression analysis of exhaled volatile organic compounds. J Appl Physiol (1985) 2009;107(1):155–160. doi: 10.1152/japplphysiol.91657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zalan Z., Hudacek J., Toth-Markus M., et al. Sensorically and antimicrobially active metabolite production of Lactobacillus strains on Jerusalem artichoke juice. J Sci Food Agric. 2011;91(4):672–679. doi: 10.1002/jsfa.4232. [DOI] [PubMed] [Google Scholar]
- 40.Bao T., Feng J., Jiang W., Fu H., Wang J., Yang S.T. Recent advances in n-butanol and butyrate production using engineered Clostridium tyrobutyricum. World J Microbiol Biotechnol. 2020;36(9):138. doi: 10.1007/s11274-020-02914-2. [DOI] [PubMed] [Google Scholar]
- 41.Gelmont D., Stein R.A., Mead J.F. The bacterial origin of rat breath pentane. Biochem Biophys Res Commun. 1981;102(3):932–936. doi: 10.1016/0006-291x(81)91627-2. [DOI] [PubMed] [Google Scholar]
- 42.Smith N.W., Shorten P.R., Altermann E.H., Roy N.C., McNabb W.C. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes. 2019;10(3):270–288. doi: 10.1080/19490976.2018.1546522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandberg J.C., Bjorck I.M.E., Nilsson A.C. Effects of whole grain rye, with and without resistant starch type 2 supplementation, on glucose tolerance, gut hormones, inflammation and appetite regulation in an 11-14.5 hour perspective; a randomized controlled study in healthy subjects. Nutr J. 2017;16(1):25. doi: 10.1186/s12937-017-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf P.G., Biswas A., Morales S.E., Greening C., Gaskins H.R. H2 metabolism is widespread and diverse among human colonic microbes. Gut Microbes. 2016;7(3):235–245. doi: 10.1080/19490976.2016.1182288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Vuyst L., Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149(1):73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68(10):5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiel S., Bindels L.B., Pachikian B.D., et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr. 2019;109(6):1683–1695. doi: 10.1093/ajcn/nqz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dryahina K., Smith D., Bortlik M., Machkova N., Lukas M., Spanel P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn's disease and ulcerative colitis. J Breath Res. 2017;12(1) doi: 10.1088/1752-7163/aa8468. [DOI] [PubMed] [Google Scholar]
- 49.Alkhouri N., Cikach F., Eng K., et al. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur J Gastroenterol Hepatol. 2014;26(1):82–87. doi: 10.1097/MEG.0b013e3283650669. [DOI] [PubMed] [Google Scholar]
- 50.Lichtenstein M., Turjerman S., Pinto J.M., Barash O., Koren O. Pathophysiology of SARS-CoV-2 infection in the upper respiratory tract and its relation to breath volatile organic compounds. mSystems. 2021 doi: 10.1128/mSystems.00104-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.