Abstract
Background
Globally, 11% of babies are born preterm each year. Preterm birth (PTB) is a leading cause of neonatal death and under-five mortality and morbidity, with lifelong sequelae in those who survive. PTB disproportionately impacts low/middle-income countries (LMICs) where the burden is highest.
Objectives
This scoping review sought to the evidence for interventions that reduce the risk of PTB, focusing on the evidence from LMICs and describing how context is considered in evidence synthesis.
Design
We conducted a scoping review, to describe this wide topic area. We searched five electronic databases (2009–2020) and contacted experts to identify relevant systematic reviews of interventions to reduce the risk of PTB. We included published systematic reviews that examined the effectiveness of interventions and their effect on reducing the risk of PTB. Data were extracted and is described narratively.
Results
139 published systematic reviews were included in the review. Interventions were categorised as primary or secondary. The interventions where the results showed a greater effect size and consistency across review findings included treatment of syphilis and vaginal candidiasis, vitamin D supplementation and cervical cerclage. Included in the 139 reviews were 1372 unique primary source studies. 28% primary studies were undertaken in LMIC contexts and only 4.5% undertaken in a low-income country (LIC) Only 10.8% of the reviews sought to explore the impact of context on findings, and 19.4% reviews did not report the settings or the primary studies.
Conclusion
This scoping review highlights the lack of research evidence derived from contexts where the burden of PTB globally is greatest. The lack of rigour in addressing contextual applicability within systematic review methods is also highlighted. This presents a risk of inappropriate and unsafe recommendations for practice within these contexts. It also highlights a need for primary research, developing and testing interventions in LIC settings.
Keywords: Maternal medicine, International health services, Public health, Health policy
Strengths and limitations of this study.
Scoping review methodology enabled us to look at a broad topic area and analyse how context is taken into account in the included systematic reviews. Primary studies not reported in systematic reviews will therefore have not been included in our analysis.
We were not able to identify the setting of all primary studies where this was not reported and there is a risk that some studies, which have multiple publications may have been double counted.
We only included systematic reviews published in English.
Background
Preterm birth (PTB) is a global and public health priority. It is defined by the WHO as delivery before 37 completed weeks of gestation, with extremely preterm delivery defined as occurring at less than 28 weeks, very preterm delivery occurring between 28 and 32 weeks, and moderate to late preterm delivery occurring from 32 through 36 weeks.1 It is one of the leading causes of neonatal death and under-five mortality and morbidity, with lifelong sequelae.2 Children born prematurely have increased risks of cognitive problems, such as academic underachievement, behavioural problems and cerebral palsy than those born at full term.3 They are more likely to experience hospital admission due to infection, particularly during infancy.4 For parents, the financial, social and emotional effects are devastating.3
The global burden of preterm birth (PTB) is falling more heavily on countries with fewer resources to manage the medical, social and economic complexities of caring for premature infants. Globally, there are approximately 15 million live PTBs each year, which is estimated to be about 11% of all deliveries each year, ranging from about 8.7% in northern Europe to 13.4% in North Africa.5 6 The majority of PTBs occur in low/middle-income countries (LMICs).6 The highest PTB rates in 2014 occurred in Southeast Asia, South Asia and sub-Saharan Africa. Nine of the 11 countries with the highest rates were in Africa. Furthermore, 60% of all PTBs were estimated to have occurred in sub-Saharan Africa and South Asia accounting for just over 9 million of the almost 15 million PTBs that occurred worldwide in 2010 resulting in a PTB rate of 12.8% in those settings.
Patterns of PTB differ between high-income countries (HICs) and LMICs. However, the differences in these patterns, causes and distribution of PTB is unclear and have not been fully explored. PTB is multifactorial in its aetiology and has distinct biological pathways. The aetiologies differ according to gestational age, ethnicity and characteristics unique to each population. In order to redress the burden of PTB in LMICs, additional insight into the causative and associated factors in these settings is required.
While a number of reviews and overviews of reviews of interventions to reduce the risk of PTB have been undertaken,7–10 none have explored how many of the primary studies included in these reviews were undertaken in LMIC contexts. It is clear that some interventions that are effective in HIC contexts but may be harmful in LMIC settings, such as the use of antenatal corticosteroids11 and cerclage.12 It is also possible that treatments effective in HIC contexts may be even more beneficial or appropriate in LMIC contexts, such as nutritional supplements, interventions to increase birth spacing or interventions to improve the accuracy of measuring gestational age.
We have undertaken a broad scoping review of systematic reviews on interventions to reduce the risk of PTB identifying primary studies undertaken in LMICs. This will allow us to identify potential areas for further synthesis of the evidence and also to identify gaps in the research in order to direct future primary research.
Review objectives
To identify systematic reviews that have sought to explore the effectiveness, safety and acceptability of interventions to prevent PTB.
To map research evidence to global settings to identify the geographical and economic contexts in which evidence is derived.
To identify where gaps in the research base exist (for real world, effectiveness, pragmatic studies) in LMIC contexts to inform future research and to generate research priorities.
To describe the methods used in meta-analysis to take into account geographical and regional differences in PTB.
Methods
We used a scoping review methodology13 to describe the existing evidence (systematic reviews) available across primary and secondary interventions to prevent PTB, published between 2009 and 2020. Systematic scoping draws on methods described by Arksey and O’Malley14 for scoping reviews: ‘[…] a form of knowledge synthesis that addresses an exploratory research question aimed at scoping key concepts, types of evidence, and gaps in research related to a defined area or field by systematically searching, selecting, and synthesizing existing knowledge’.14 The approach enabled us to highlight the evidence gap and to assist with simultaneously undertaking a research prioritisation exercise and guideline development, as well as to inform a broader programme of research that aimed to develop effective postnatal interventions to mitigate PTB in LMIC settings. It also enabled us to generate a mega-map, an interactive table supported on our project website and designed as a visual tool to identify research gaps and facilitate ready access to relevant evidence (https://www.primeglobalhealth.co.uk/evidence-map-2-7-2020.html).
Identifying relevant studies
Relevant systematic reviews were identified by systematic searches in the following electronic databases: Ovid MEDLINE, Cochrane Database of Systematic Reviews, PsycINFO via Ovid, EMBASE via Ovid and CINAHL via EBSCO. Each database was searched using the database thesaurus and the key word/free text method with terms relating to PTB combined with a systematic reviews filter. The search strategy incorporated the following limitations: articles written in English, and Human studies only from April 2009 to July 2020. Relevant systematic reviews were identified by systematic searches in the following electronic databases: MEDLINE, The Cochrane Library, PsycINFO, EMBASE and CINAHL. Each database was searched using the database thesaurus and the key word/free text method. The search strategy incorporated the following limitations: articles written in English, and Human studies only from April 2009 to July 2020. The date limit was selected due to the existence of a previous review for which the studies were conducted in April 2009.15 Full search strategies have been described and published.16
We began with a framework of interventions identified by two existing reviews7 8 as these were broad in their focus and encompassed a range of interventions. Any new intervention types identified during the screening process were then added to the map.
The process of study selection was based on inclusion and exclusion criteria as described in box 1. After removal of duplicates and irrelevant studies, based on the titles and abstracts, all potentially relevant reviews were read in full. Citations were screened by two reviewers (FC and one of the following team members SS, SMJ, EA, JB, BMG, BN, KP) independently and differences were resolved by discussion.
Box 1. Inclusion/exclusion criteria based on PICOS.
Population
Pregnant women at less than 37 completed weeks gestation without signs of threatened preterm labour or premature rupture of membranes.
Excluded reviews where the study population was defined by comorbidities.
Intervention
All interventions deliverable during pregnancy to prevent spontaneous preterm birth (PTB) (these included clinical, behavioural and nutritional interventions and health systems and policy interventions).
All interventions assessed the risk of PTB.
Excluded interventions given to pregnant women to improve neonatal outcomes.
Comparators
We included any comparator, including placebo or alternative treatments.
Outcomes
We included reviews which focused on PTB as an outcome.
Where it is reported, we state how many of the primary studies measured PTB as an outcome and the resulting data used in the synthesis.
Study design
Systematic reviews published between April 2009 and July 2020, of studies that have evaluated interventions to prevent PTB, or that measured PTB as a relevant outcome.
Outcomes
PTB (<28, <34, <37 weeks gestation).
We recorded neonatal outcomes and adverse outcomes if reported within the review.
Data extraction and coding
Data were extracted using an agreed and piloted template and coded in Excel by two reviewers working independently (FC and one of the following team members SS, SMJ, EA, JB, BMG, BN, KP) differences were resolved by discussion. The following data categories were extracted: number of included studies, review PICO, setting of primary studies and any analysis that took into account study setting or population characteristics, PTB outcomes, assessment of adverse effects and recommendations for practice and research. PTB rates in low-income countries (LICs), lower middle-income countries (LMCs), upper middle-income countries (UMCs) and HICs settings were drawn from data published in a rigorous review of national civil registration and vital statistics to determine global, regional and national estimates of levels of PTB.6
Where reported information allowed, we used the World Bank categories to identify the categories of all country settings identified in the reviews.17
The population, interventions, comparators, outcomes and reviewer conclusions for future research were tabulated and described narratively. The country or countries of the included primary studies were noted, and the methods used in the review for analyses of data from different settings was also recorded and described. We did not contact review authors for missing data.
Patient and public involvement
This review was undertaken as part of a larger programme of research in PTB (NIHR Global Health under grant (17/63/26)). The programme iPatient and public involvements informed by key stakeholders and a patient and public involvement (PPI) advisory group comprising representatives from Sheffield, Bangladesh, and South Africa. The design and questions for the review were informed by consultation with these groups.
Results
Our search identified 3133 citations which were screened by two reviewers. A third reviewer was also involved where there was a lack of consensus or uncertainty regarding inclusion. Following screening, 424 full text papers were retrieved for data extraction. At data extraction a further 285 were excluded. The process of identifying the included reviews is summarised in figure 1.
Figure 1.
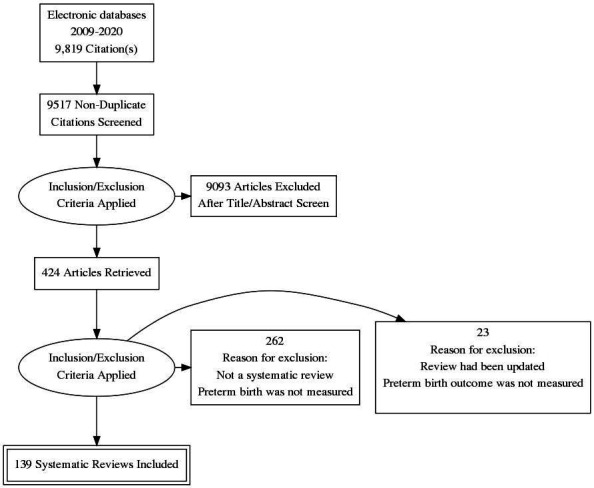
Flow of studies through review process.
We included 139 reviews which addressed a range of primary and secondary interventions and measured the effectiveness of the intervention in reducing the risk of PTB. These are summarised in table 1. There was a considerable variation in the number of included studies in the reviews for each intervention, reflecting differing research questions objectives (therefore different PICOs) and search strategies.
Table 1.
Summary of included systematic reviews and settings of primary studies included in the review
| Interventions | Number of reviews | Number of primary studies | Country NR | Country of primary study | Studies where setting NK | ||||
| LI | LM | UM | HI | Mixed | |||||
| Primary prevention interventions | |||||||||
| Health systems | |||||||||
| Models of antenatal care delivery (group/specialised)61–71 | 11 | 68 | 2 | 0 | 2 | 2 | 64 | 0 | 0 |
| Midwifery led care72 | 1 | 15 | 0 | 0 | 0 | 0 | 15 | 0 | 0 |
| Improving ANC coverage28 | 1 | 34 | 0 | 10 | 15 | 5 | 0 | 0 | 0 |
| Health behaviours | |||||||||
| Smoking cessation35 73 | 2 | 111 | 0 | 0 | 0 | 1 | 110 | 0 | 0 |
| Weight management21 74–78 | 6 | 70 | 1 | 0 | 2 | 8 | 60 | 0 | 0 |
| Nutritional interventions | |||||||||
| Macronutrient supplements29 30 | 2 | 34 | 0 | 3 | 9 | 10 | 8 | 4 | 0 |
| Micronutrient supplements21 27–31 35–40 67–86 | 33 | 481 | 2 | 29 | 82 | 122 | 214 | 6 | 9 |
| Vitamin D27 31 36 79–81 | 6 | 75 | |||||||
| Vitamin A37 82 | 2 | 24 | |||||||
| Vitamin E, C, E and C38 39 83 | 3 | 67 | |||||||
| Iron, folic acid, iron and folic acid40 84–90 | 8 | 182 | |||||||
| Fish oil91–95 | 5 | 38 | |||||||
| Zinc32 96 | 2 | 25 | |||||||
| Calcium41 42 | 2 | 27 | |||||||
| Iodine97 | 2 | 14 | |||||||
| Multiple micronutrients43 44 98 | 3 | 29 | |||||||
| Screening and treatment of periodontal disease 99–110 | 12 | 46 | 0 | 0 | 3 | 7 | 36 | 0 | 0 |
| Screening and prevention/treatment of infection | 14 | 91 | 2 | 2 | 2 | 6 | 79 | 0 | 2 |
| Asymptomatic bacteriuria23 111–113 | 4 | ||||||||
| Screening and antibiotics for syphilis24 | 1 | ||||||||
| Influenza vaccine114 115 | 2 | ||||||||
| Lower genital tract infection26 | 1 | ||||||||
| UTI116 117 | 2 | ||||||||
| Vaginal candidiasis25 | 1 | ||||||||
| Non-specific infection118 119 | 2 | ||||||||
| Malaria33 120 121 | 3 | 17 | 0 | 8 | 7 | 2 | 2 | 0 | 0 |
| Secondary prevention interventions | |||||||||
| Cerclage18 22 45 122–136 | 18 | 123 | 10 | 0 | 7 | 11 | 42 | 51 | |
| Bed rest137–139 | 3 | 40 | 1 | 4 | 0 | 0 | 36 | 0 | 0 |
| Cervical pessary140–145 | 6 | 16 | 0 | 0 | 0 | 1 | 14 | 1 | 0 |
| Progesterone19 20 146–159 | 16 | 59 | 5 | 1 | 7 | 8 | 28 | 4 | 11 |
| Tocolytics160–172 | 11 | 167 | 3 | 1 | 0 | 13 | 68 | 0 | 84 |
ANC, antenatal care; HI, high income; LI, low income; LM, low middle; NK, not known; NR, not reported; UM, upper middle; UTI, urinary tract infection.
Context of primary studies
A total of 1372 primary studies were included across all of the 139 reviews Not all of these studies will have been measuring PTB as an outcome but were included within the review which may have been measuring a range of maternal outcomes including PTB. The largest number of primary studies were those evaluating micronutrient supplements (n=481) and tocolytics (n=167). A total of 113 of the reviews described the country in which the primary studies were undertaken and so these data were known for 1288 (93.9%) of 1372 included primary studies. Of these, 390 (30.3%) were undertaken in LMICs, 15 primary studies were multicentre and included data gathered from LMIC and HIC settings, though only 3 of these studies included LICs. Of the studies undertaken in LMICs, a majority (n=255) examined the effects of nutritional supplements. Excluding nutritional intervention studies, the proportion of LMIC-based primary studies of interventions to reduce PTB accounts for only (n=135) 10.5% of the included studies where settings are known.
Of the total number of primary studies undertaken in LMIC contexts, those studies undertaken in LIC settings represented a very small proportion of included studies. Participants from LICs were represented in only 4.5% (n=58) of the total number of studies, and if the nutritional intervention studies are excluded, they account for only 2.5% (n=32) of the studies evaluating interventions. Of those primary studies that were undertaken in LMIC settings the numbers within each country category differed significantly. The proportion of the studies that are undertaken in LIC, LMC and UMC were 14.9% (n=58), 34.8% (n=136) and 50.2% (n=196), respectively. There are only single trials that have evaluated the impact of progesterone, tocolytics and interventions to increase calorie intake in LIC settings. There are no trials that have evaluated smoking cessation, preventing excessive weight gain, prevention and treatment of periodontal disease, influenza vaccine and cervical pessaries. The number of trials in each of the country categories within each intervention type are shown in table 1.
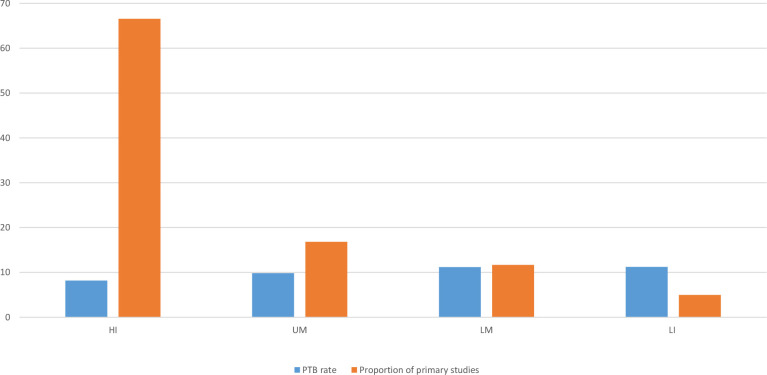
When these data are compared alongside data that shows the prevalence of PTB globally it is clear that there is an inverse pattern in the distribution of the data (figure 2).
Figure 2.
Rates of PTB and proportion of primary studies undertaken in each setting. HI, high income; LI, low income; LM, low middle; PTB, preterm birth; UM, upper middle.
Effectiveness of interventions
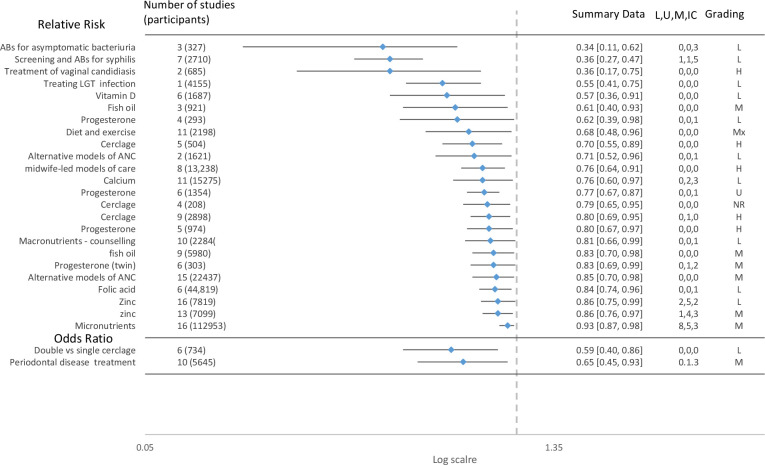
The effectiveness of interventions in reducing the risk of PTB was variable with no intervention showing consistent effectiveness across the included reviews. Although interpretation of these data is limited by the lack of quality appraisal of the included reviews, and therefore should be viewed with caution. Overall, the scoping review demonstrates considerable inconsistency of results of interventions. Of the 139 reviews, 28 reported a reduction in PTB in intervention versus control, 80% (n=111) of the reviews found that the intervention had no impact in reducing the risk of PTB. The summary result (relative risk (RR) and OR are shown in figure 3). The results show the reduction in PTB less than 37 weeks gestation. In three reviews the intervention was not statistically significant at 37 weeks but was reported as statistically significant at 34 weeks,18 35 weeks19 and 36 weeks20. Two reviews reported a positive effect of the intervention in reducing risk of PTB but reported the outcome on a continuous measure. These included the effectiveness of macronutrient supplements21 (SMD −0.19 (95% CI −0.34 to −0.04)) and cerclage (mean difference 95% CI 33.98 days (17.88 to 50.08)).22 The interventions reporting binary outcomes which appear to have the greatest effect (RR=0.2–0.4) in reducing PTB are: antibiotics for asymptomatic bacteriuria23 (RR=0.34 (95% CI 0.11 to 0.62), the screening and treatment of syphilis24 (RR=0.36 (95% CI 0.27 to 0.47), and treatment of vaginal candidiasis25 (RR=0.36, (95% CI 0.17 to 0.75). Interventions with moderate effects (RR=0.4–0.6) included treating lower genital tract infection26 and vitamin D supplements.27 Four of the reviews (figure 2) with a positive effect of the intervention considered that the strength of evidence supporting the finding could be considered high and the finding reliable. None of these reviews included studies conducted in LIC settings, and only one included one study in an LMIC.
Figure 3.
Summary results of systematic reviews of interventions showing reduction in risk of preterm birth. ANC, antenatal care; L, U, M, IC, low, low middle, upper middle-income countries; LGT, lower genital tract; RR, relative risk.
Dealing with context and generalisability within evidence synthesis
The authors of the included reviews used different approaches to dealing with the contextual variation when pooling data from primary studies, which was either to ignore, document, explore or control for differences. Twenty-seven reviews (23.8%) did not describe the setting of the primary study, ignoring variation in outcomes that may arise as a result of these differences. This occurred most frequently in reviews of cervical cerclage (see table 1). The majority of the included reviews 86 (76.1%) documented the country in which the primary study was carried out either within the text, tables of study characteristics or in accompanying appendices, but this was not considered further in terms of its implications for the findings, or application for future practice or research.
Eight reviews27–34 sought to explore the impact of geographical and economic context by undertaking a subgroup analysis comparing trials conducted in low income settings with those in high income settings or regression analysis with geographical regions as covariates (Africa, Americas, Southeast Asia, Europe, Eastern Mediterranean, Western Pacific). In addition, one study34 listed the country instead of the author name on the forest plot allowing ready visualisation of differences across settings. Nine reviews35–43 undertook subgroup analysis based on features of the population that might vary across settings and influence the effectiveness of the intervention, such as baseline nutritional status of the mother. One review44 exploring multiple micronutrient supplementation controlled for settings by limiting the review to include only those studies undertaken in LMIC contexts. Four reviews19 20 44 45 undertook an IPD (individual patient data) analysis, allowing subgroup analyses about differences in effect more easily than with aggregate data. This approach allowed comparison between effects for women recruited and receiving the intervention in different settings, effect sizes in each country could also be shown in the analyses.
Discussion
This scoping review has revealed an inverse pattern of research, with only 30.3% of published research included in systematic reviews of interventions reporting PTB outcomes carried out in LMIC settings, and only 4.5% was conducted in the poorest countries in the world where the burden of PTB is greatest. The distribution of types of intervention tested and evaluated in these settings is not even across interventions, but is largely focused on very context specific interventions (prevention of malarial infection) and nutritional supplementation. Similar patterns of a mismatch between research effort and health needs in non-high income regions have been identified across a broad range of diseases.46 47 It has also been previously reported that primary research often fails to capture those with the greatest healthcare needs such as vulnerable populations.48 49
This review has also revealed a limited approach in evidence synthesis to explore the applicability of findings across geographical settings and to draw attention to these gaps with a resultant risk that interventions shown to be effective in HI settings may not translate to LIC settings and may indeed have adverse effects when applied to LIC settings. Likewise, the focus of research in HIC settings means that interventions that may have greater benefit in LIC settings—where the problem is greatest—remain untested or replicated with larger numbers of participants. Adolescent pregnancy and short inter pregnancy intervals, both of which are more common in LMICs, have been highlighted as important risk factors for PTB50 yet there is a lack of data on interventions to address these and their effectiveness in reducing the risk of PTB.
The lack of robust evidence to inform both the primary and secondary prevention of PTB in LIC settings, where the prevalence of PTB is highest presents challenges for developing appropriate and contextually relevant clinical guidance. The factors that mean findings cannot be generalised from high resource settings to low and middle resource settings are multiple and will differ across interventions. Ethnicity, poverty, gender dynamics, pollution, temperature, climate, diet, access to healthcare, educational status, employment conditions are all examples of factors that might play a role in these differences. Improved understanding of the aetiopathogenesis of PTB is also necessary for defining an accurate model of risk prediction and would help in understanding what factors in local settings increase risk and facilitate the development of an accurate model of risk prediction.51
Two recent overviews of reviews9 10 also found that few interventions are effective in PTB prevention. The following interventions were identified in these reviews as showing positive or possible benefit: lifestyle and behavioural changes (including diet and exercise); nutritional supplements (including calcium, zinc and vitamin D supplementation); nutritional education; and screening for lower genital tract infections. Positive effects of secondary interventions were found for low dose aspirin among women at risk of pre-eclampsia; clindamycin for treatment of bacterial vaginosis; treatment of vaginal candidiasis; progesterone in women with prior spontaneous PTB and in those with short mid-trimester cervical length; L-arginine in women at risk for pre-eclampsia; levothyroxine among women with thyroid disease; calcium supplementation in women at risk of hypertensive disorders; smoking cessation; cervical length screening in women with history of PTB with placement of cerclage in those with short cervix; cervical pessary in singleton gestations with short cervix; and treatment of periodontal disease. Our review findings were in concordance, although, in addition, we identified screening and antibiotic treatment for syphilis, and positive effects of fish oil supplements. In most instances the trials were small and authors recommended larger well-designed randomised controlled trials (RCTs). The lack of consistency across review findings for interventions also merits more exploration. Compromised methodological rigour can inflate trial findings by 30%–50%.52 53 Some of the differences in our review findings reflect some differences in the included reviews.
The interventions identified in this review, and those of Matei et al9 and Medley et al10 informing guideline development, clinical practice and policy decision making have been little tested in LMIC settings. In those interventions where there is more consistency in review findings such as cervical cerclage, there are no studies that have been conducted in low-income settings and over half of the reviews did not report or consider settings in their analyses.
This scoping review has shown that many authors of systematic reviews fail to use design and statistical approaches that adequately address contextual variations between the included source studies and imperfectly represent ‘real world’ conditions within the target context. While those reviews that sought to take into account LMIC contexts were unable to conduct the analyses due to a lack of data, they nonetheless were able to highlight the gaps in research, for example the lack of studies in vitamin D undertaken in Africa.31
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting standards reference ‘context’ in terms of the circumstances requiring the review itself, rather than referencing the contexts of studies included in the review.54 The PRISMA extension for Complex Interventions includes the elements of ‘time’ and ‘setting’.55 However, grouping LMIC data, or even LI data may still be too broad. Even within the categories of LIC there is considerable diversity that may impact on how an intervention works and within countries there may also be considerable diversity between the wealthiest and poorest groups. For example, the time taken to reach comprehensive emergency obstetric care facilities in low resource settings is often underestimated and for most women is likely to be 120 min of travel time.56 Context cannot be standardised, it will vary from review to review, as different interventions and different populations are considered. ‘Context’ and the factors that might influence the efficacy, uptake, acceptability, appropriateness, accessibility and availability of an intervention requires a good understanding of the aetiology and mechanisms by which risk factors interact with environmental, microbial, socio-political and health system variations across settings.57
It must be acknowledged that there are significant barriers to undertaking research in many settings across the globe. These include very practical challenges such as a lack of access to high-quality data and the challenges of estimating gestational age.58 Recent changes to global health funding arena include a very large proportion being spent on the pandemic as well as government reductions, for example, in the UK.59 These reductions in funding will undermine what has been a growth in research in LMIC settings and will impede efforts to address the imbalances highlighted in this scoping review.
A number of limitations exist in this scoping review. We have not sought to identify the setting of primary studies where this is not reported in the systematic review. We have also not limited our analysis to studies within the reviews that only contributed findings to the risk of PTB. Most reviews explored several maternal and infant outcomes. Therefore, in this scoping review, included primary studies may not have contained PTB outcome data. We limited our scoping review to exploring evidence within systematic reviews as these are key sources of evidence to inform guideline development and policy decision making. It is possible that further primary studies have been published but are not included in this analysis. Nevertheless, it gives an indication of the distribution of research being undertaken in the poorest regions of the world that address PTB.
Conclusion
Only 4.5% of primary research to examine the effectiveness of interventions to reduce the risk of PTB is carried out in settings where the burden is greatest. No interventions which reduce the risk of PTB, judged to be supported by strong evidence, include studies undertaken in low resource settings. In the synthesis of studies, current methods often fail to address the contextual variation and consider the applicability of findings in low resource, high burden settings. This has implications for supporting policy making, and development of contextually relevant clinical guidelines. While methods can be undertaken to improve approaches to evidence synthesis, they cannot compensate for the lack of primary research in low resource settings. This is critical if global health inequalities are to be addressed and millennium development goals60 to reduce under-five mortality are to be achieved. Funding and supporting research in LMICs would have a threefold benefit; first, if the prevalence of the disease is higher it is easier to reach statistical significance for efficacy or inefficacy of each tested intervention. Second, it would address the knowledge gap highlighted in this review and finally—and most importantly—the implementation of effective interventions would have the potential for greater public health impact where the risks are greater, more prevalent and outcomes more severe.
Supplementary Material
Footnotes
Twitter: @dclovesGP, @KerryParris
Contributors: FC, PS-P, LC prepared the protocol, AS designed search strategies, SS, SMJ, CM, EA, JB, BMG, KP, BN collected and analysed or interpreted data. FC prepared manuscript. SS, AS, SMJ, CM, EA, JB, BMG, KP, PS-P, LC, BN, DOA edited or read and approved the final manuscript. FC is the guarantor and accepts full responsibility for the conduct of this study.
Funding: NIHR provided funding for researcher support.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data extracted from the included reviews is available on request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.WHO . Who: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand 1977;56:247–53. [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 3.Brydges CR, Landes JK, Reid CL, et al. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Dev Med Child Neurol 2018;60:452–68. 10.1111/dmcn.13685 [DOI] [PubMed] [Google Scholar]
- 4.Coathup V, Boyle E, Carson C, et al. Gestational age and hospital admissions during childhood: population based, record linkage study in England (TIGAR study). BMJ 2020;371:m4075. 10.1136/bmj.m4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howson CP, Kinney MV, McDougall L, et al. Born too soon: preterm birth matters. Reprod Health 2013;10:1–9. 10.1186/1742-4755-10-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barros FC, Bhutta ZA, Batra M, et al. Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy Childbirth 2010;10:1–36. 10.1186/1471-2393-10-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhutta ZA, Das JK, Bahl R, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet 2014;384:347–70. 10.1016/S0140-6736(14)60792-3 [DOI] [PubMed] [Google Scholar]
- 9.Matei A, Saccone G, Vogel JP, et al. Primary and secondary prevention of preterm birth: a review of systematic reviews and ongoing randomized controlled trials. Eur J Obstet Gynecol Reprod Biol 2019;236:224–39. 10.1016/j.ejogrb.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 10.Medley N, Vogel JP, Care A, et al. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2018;11:CD012505. 10.1002/14651858.CD012505.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opiyo N, Stones W. Corticosteroids for preterm deliveries: missing evidence. Cochrane Database Syst Rev 2017;5:ED000121. 10.1002/14651858.ED000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egwuatu VE. Complications of cervical cerclage in Igbo women. J Natl Med Assoc 1986;78:245. [PMC free article] [PubMed] [Google Scholar]
- 13.White H, Albers B, Gaarder M, et al. Guidance for producing a Campbell evidence and gap MAP. Campbell Syst Rev 2020;16:e1125. 10.1002/cl2.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 15.Allen F, Gray R, Oakley L. Technical guide to the infant mortality evidence map: systematic reviews of interventions targeting major potentially modifiable risk factors for infant mortality, 2009. [Google Scholar]
- 16.Sutton A, Campbell F. The ScHARR LMIC filter: adapting a low- and middle-income countries geographic search filter to identify studies on preterm birth prevention and management. Res Synth Methods 2022. 10.1002/jrsm.1552. [Epub ahead of print: 10 Feb 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bank TW . World bank country and lending groups, 2021. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 [Accessed 18 Mar 2021].
- 18.Pergialiotis V, Vlachos DG, Prodromidou A, et al. Double versus single cervical cerclage for the prevention of preterm births. J Matern Fetal Neonatal Med 2015;28:379–85. 10.3109/14767058.2014.921676 [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol 2017;49:303–14. 10.1002/uog.17397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol 2018;218:161–80. 10.1016/j.ajog.2017.11.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gresham E, Bisquera A, Byles JE, et al. Effects of dietary interventions on pregnancy outcomes: a systematic review and meta-analysis. Matern Child Nutr 2016;12:5–23. 10.1111/mcn.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehsanipoor RM, Seligman NS, Saccone G, et al. Physical Examination-Indicated Cerclage: a systematic review and meta-analysis. Obstet Gynecol 2015;126:125–35. 10.1097/AOG.0000000000000850 [DOI] [PubMed] [Google Scholar]
- 23.Smaill FM, Vazquez JC, Cochrane Pregnancy and Childbirth Group . Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2015;8:CD000490. 10.1002/14651858.CD000490.pub3 [DOI] [PubMed] [Google Scholar]
- 24.Blencowe H, Cousens S, Kamb M, et al. Lives saved tool supplement detection and treatment of syphilis in pregnancy to reduce syphilis related stillbirths and neonatal mortality. BMC Public Health 2011;11 Suppl 3:S9. 10.1186/1471-2458-11-S3-S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts CL, Algert CS, Rickard KL, et al. Treatment of vaginal candidiasis for the prevention of preterm birth: a systematic review and meta-analysis. Syst Rev 2015;4:31. 10.1186/s13643-015-0018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, et al. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database Syst Rev 2015:CD006178. 10.1002/14651858.CD006178.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S-S, Tao Y-H, Huang K, et al. Vitamin D and risk of preterm birth: up-to-date meta-analysis of randomized controlled trials and observational studies. J Obstet Gynaecol Res 2017;43:247–56. 10.1111/jog.13239 [DOI] [PubMed] [Google Scholar]
- 28.Mbuagbaw L, Medley N, Darzi AJ, et al. Health system and community level interventions for improving antenatal care coverage and health outcomes. Cochrane Database Syst Rev 2015:CD010994. 10.1002/14651858.CD010994.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girard AW, Olude O. Nutrition education and counselling provided during pregnancy: effects on maternal, neonatal and child health outcomes. Paediatr Perinat Epidemiol 2012;26 Suppl 1:191–204. 10.1111/j.1365-3016.2012.01278.x [DOI] [PubMed] [Google Scholar]
- 30.Ota E, Hori H, Mori R, et al. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev 2015;6:CD000032. 10.1002/14651858.CD000032.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth DE, Leung M, Mesfin E, et al. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ 2017;359:j5237. 10.1136/bmj.j5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaffee BW, King JC. Effect of zinc supplementation on pregnancy and infant outcomes: a systematic review. Paediatr Perinat Epidemiol 2012;26 Suppl 1:118–37. 10.1111/j.1365-3016.2012.01289.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radeva-Petrova D, Kayentao K, ter Kuile FO, et al. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst Rev 2014;9:CD000169. 10.1002/14651858.CD000169.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota E, Mori R, Middleton P, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev 2015;31. 10.1002/14651858.CD000230.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamberlain C, O'Mara-Eves A, Oliver S, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2013;10:CD001055. 10.1002/14651858.CD001055.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol 2016;164:148–55. 10.1016/j.jsbmb.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCauley ME, van den Broek N, Dou L, et al. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev 2015;10:CD008666. 10.1002/14651858.CD008666.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rumbold A, Ota E, Hori H, et al. Vitamin E supplementation in pregnancy. Cochrane Database Syst Rev 2015:CD004069. 10.1002/14651858.CD004069.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rumbold A, Ota E, Nagata C, et al. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev 2015:CD004072. 10.1002/14651858.CD004072.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, et al. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015;2015. 10.1002/14651858.CD004736.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmeyr GJ, Lawrie TA, Atallah Álvaro N, et al. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev 2018;10:CD001059. 10.1002/14651858.CD001059.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmeyr GJ, Manyame S, Medley N, et al. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst Rev 2019;393. 10.1002/14651858.CD011192.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keats EC, Haider BA, Tam E, et al. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2019;145. 10.1002/14651858.CD004905.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith ER, Shankar AH, Wu LS-F, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health 2017;5:e1090–100. 10.1016/S2214-109X(17)30371-6 [DOI] [PubMed] [Google Scholar]
- 45.Berghella V, Ciardulli A, Rust OA, et al. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: systematic review and meta-analysis of randomized controlled trials using individual patient-level data. Ultrasound Obstet Gynecol 2017;50:569–77. 10.1002/uog.17457 [DOI] [PubMed] [Google Scholar]
- 46.Bellows BW, Conlon CM, Higgs ES, et al. A taxonomy and results from a comprehensive review of 28 maternal health voucher programmes. J Health Popul Nutr 2013;31:S106. [PubMed] [Google Scholar]
- 47.Atal I, Trinquart L, Ravaud P, et al. A mapping of 115,000 randomized trials revealed a mismatch between research effort and health needs in non-high-income regions. J Clin Epidemiol 2018;98:123–32. 10.1016/j.jclinepi.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 48.Dab W. Commentary on sphere (strengthening public health research in Europe) literature reviews. Eur J Public Health 2007;17 Suppl 1:8–9. 10.1093/eurpub/ckm060 [DOI] [PubMed] [Google Scholar]
- 49.Shepherd V. Research involving adults lacking capacity to consent: the impact of research regulation on ‘evidence biased’ medicine. BMC Med Ethics 2016;17:1–8. 10.1186/s12910-016-0138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization . Born too soon: the global action report on preterm birth, 2012. [Google Scholar]
- 51.Della Rosa PA, Miglioli C, Caglioni M, et al. A hierarchical procedure to select intrauterine and extrauterine factors for methodological validation of preterm birth risk estimation. BMC Pregnancy Childbirth 2021;21:1–17. 10.1186/s12884-021-03654-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linde K, Scholz M, Ramirez G, et al. Impact of study quality on outcome in placebo-controlled trials of homeopathy. J Clin Epidemiol 1999;52:631–6. 10.1016/s0895-4356(99)00048-7 [DOI] [PubMed] [Google Scholar]
- 53.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–12. 10.1001/jama.273.5.408 [DOI] [PubMed] [Google Scholar]
- 54.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guise J-M, Butler ME, Chang C, et al. AHRQ series on complex intervention systematic reviews-paper 6: PRISMA-CI extension statement and checklist. J Clin Epidemiol 2017;90:43–50. 10.1016/j.jclinepi.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 56.Banke-Thomas A, Wong KLM, Ayomoh FI, et al. "In cities, it's not far, but it takes long": comparing estimated and replicated travel times to reach life-saving obstetric care in Lagos, Nigeria. BMJ Glob Health 2021;6:e004318. 10.1136/bmjgh-2020-004318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers L, De Brún A, McAuliffe E. Defining and assessing context in healthcare implementation studies: a systematic review. BMC Health Serv Res 2020;20:591. 10.1186/s12913-020-05212-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel JP, Chawanpaiboon S, Moller A-B, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 2018;52:3–12. 10.1016/j.bpobgyn.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 59.C S. UKRI official development assistance letter 11 March 2021, 2021. Available: https://www.ukri.org/our-work/ukri-oda-letter-11-march-2021/ [Accessed 23 Mar 2021].
- 60.World Health Organization . Millennium development goals, 2008. [Google Scholar]
- 61.Allen J, Gamble J, Stapleton H, et al. Does the way maternity care is provided affect maternal and neonatal outcomes for young women? A review of the research literature. Women Birth 2012;25:54–63. 10.1016/j.wombi.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 62.Catling CJ, Medley N, Foureur M. Group versus conventional antenatal care for women. Cochrane Database Syst Rev 2015;2:CD007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dodd JM, Dowswell T, Crowther CA. Specialised antenatal clinics for women with a multiple pregnancy for improving maternal and infant outcomes. Cochrane Database Syst Rev 2015:CD005300. 10.1002/14651858.CD005300.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev 2015;7:CD000934. 10.1002/14651858.CD000934.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dowswell T, Middleton P, Weeks A. Antenatal day care units versus hospital admission for women with complicated pregnancy. Cochrane Database Syst Rev 2009:CD001803. 10.1002/14651858.CD001803.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez Turienzo C, Sandall J, Peacock JL. Models of antenatal care to reduce and prevent preterm birth: a systematic review and meta-analysis. BMJ Open 2016;6:e009044. 10.1136/bmjopen-2015-009044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lathrop B. A systematic review comparing group prenatal care to traditional prenatal care. Nurs Womens Health 2013;17:118–30. 10.1111/1751-486X.12020 [DOI] [PubMed] [Google Scholar]
- 68.Malouf R, Redshaw M. Specialist antenatal clinics for women at high risk of preterm birth: a systematic review of qualitative and quantitative research. BMC Pregnancy Childbirth 2017;17:51. 10.1186/s12884-017-1232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruiz-Mirazo E, Lopez-Yarto M, McDonald SD. Group prenatal care versus individual prenatal care: a systematic review and meta-analyses. J Obstet Gynaecol Can 2012;34:223–9. 10.1016/S1701-2163(16)35182-9 [DOI] [PubMed] [Google Scholar]
- 70.Sheeder J, Weber Yorga K, Kabir-Greher K. A review of prenatal group care literature: the need for a structured theoretical framework and systematic evaluation. Matern Child Health J 2012;16:177–87. 10.1007/s10995-010-0709-1 [DOI] [PubMed] [Google Scholar]
- 71.Whitworth M, Quenby S, Cockerill RO, et al. Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes. Cochrane Database Syst Rev 2011;9:CD006760. 10.1002/14651858.CD006760.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandall J, Soltani H, Gates S, et al. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database Syst Rev 2013:CD004667. 10.1002/14651858.CD004667.pub3 [DOI] [PubMed] [Google Scholar]
- 73.Coleman T, Chamberlain C, Davey M-A, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2012;9:CD010078. 10.1002/14651858.CD010078 [DOI] [PubMed] [Google Scholar]
- 74.Dodd JM, Grivell RM, Crowther CA, et al. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010;117:1316–26. 10.1111/j.1471-0528.2010.02540.x [DOI] [PubMed] [Google Scholar]
- 75.Muktabhant B, Lawrie TA, Lumbiganon P, et al. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;6:CD007145. 10.1002/14651858.CD007145.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shepherd E, Gomersall JC, Tieu J, et al. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev 2017;2017. 10.1002/14651858.CD010443.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanentsapf I, Heitmann BL, Adegboye ARA. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth 2011;11:81. 10.1186/1471-2393-11-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thangaratinam S, Rogozińska E, Jolly K, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess 2012;16. 10.3310/hta16310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr 2018;172:635–45. 10.1001/jamapediatrics.2018.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pérez-López FR, Pasupuleti V, Mezones-Holguin E, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2015;103:1278-88.e4. 10.1016/j.fertnstert.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 81.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 2012;26 Suppl 1:75–90. 10.1111/j.1365-3016.2012.01283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 2012;26 Suppl 1:36–54. 10.1111/j.1365-3016.2012.01284.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahimi R, Nikfar S, Rezaie A, et al. A meta-analysis on the efficacy and safety of combined vitamin C and E supplementation in preeclamptic women. Hypertens Pregnancy 2009;28:417–34. 10.3109/10641950802629667 [DOI] [PubMed] [Google Scholar]
- 84.Lassi ZS, Salam RA, Haider BA, et al. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst Rev 2013:CD006896. 10.1002/14651858.CD006896.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mantovani E, Filippini F, Bortolus R, et al. Folic acid supplementation and preterm birth: results from observational studies. Biomed Res Int 2014;2014:481914. 10.1155/2014/481914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peña-Rosas JP, De-Regil LM, Dowswell T, et al. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2012;7:CD009997. 10.1002/14651858.CD009997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peña-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev 2009:CD004736. 10.1002/14651858.CD004736.pub3 [DOI] [PubMed] [Google Scholar]
- 88.Saccone G, Berghella V. Folic acid supplementation in pregnancy to prevent preterm birth: a systematic review and meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol 2016;199:76–81. 10.1016/j.ejogrb.2016.01.042 [DOI] [PubMed] [Google Scholar]
- 89.Zhang Q, Wang Y, Xin X, et al. Effect of folic acid supplementation on preterm delivery and small for gestational age births: a systematic review and meta-analysis. Reprod Toxicol 2017;67:35–41. 10.1016/j.reprotox.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 90.Imdad A, Bhutta ZA. Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatr Perinat Epidemiol 2012;26 Suppl 1:168–77. 10.1111/j.1365-3016.2012.01312.x [DOI] [PubMed] [Google Scholar]
- 91.Chen B, Ji X, Zhang L, et al. Fish oil supplementation improves pregnancy outcomes and size of the newborn: a meta-analysis of 21 randomized controlled trials. J Matern Fetal Neonatal Med 2016;29:2017–27. 10.3109/14767058.2015.1072163 [DOI] [PubMed] [Google Scholar]
- 92.Kar S, Wong M, Rogozinska E, et al. Effects of omega-3 fatty acids in prevention of early preterm delivery: a systematic review and meta-analysis of randomized studies. Eur J Obstet Gynecol Reprod Biol 2016;198:40–6. 10.1016/j.ejogrb.2015.11.033 [DOI] [PubMed] [Google Scholar]
- 93.Saccone G, Berghella V. Omega-3 supplementation to prevent recurrent preterm birth: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol 2015;213:135–40. 10.1016/j.ajog.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 94.Salvig JD, Lamont RF. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2011;90:825–38. 10.1111/j.1600-0412.2011.01171.x [DOI] [PubMed] [Google Scholar]
- 95.Saccone G, Saccone I, Berghella V. Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: which evidence? J Matern Fetal Neonatal Med 2016;29:2389–97. 10.3109/14767058.2015.1086742 [DOI] [PubMed] [Google Scholar]
- 96.Mori R, Ota E, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev 2012;3:CD000230. [DOI] [PubMed] [Google Scholar]
- 97.Harding KB, Peña-Rosas JP, Webster AC, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev 2017;3:CD011761. 10.1002/14651858.CD011761.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fall CHD, Fisher DJ, Osmond C, et al. Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull 2009;30:S533–46. 10.1177/15648265090304S408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwendicke F, Karimbux N, Allareddy V, et al. Periodontal treatment for preventing adverse pregnancy outcomes: a meta- and trial sequential analysis. PLoS One 2015;10:e0129060. 10.1371/journal.pone.0129060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corbella S, Del Fabbro M, Taschieri S, et al. Periodontal disease and adverse pregnancy outcomes: a systematic review. Italian Oral Surgery 2012;11:132–46. 10.1016/j.ios.2011.04.002 [DOI] [Google Scholar]
- 101.Fogacci MF, Vettore MV, Thomé Leão AT, Leao AT. The effect of periodontal therapy on preterm low birth weight: a meta-analysis. Obstet Gynecol 2011;117:153–65. 10.1097/AOG.0b013e3181fdebc0 [DOI] [PubMed] [Google Scholar]
- 102.George A, Shamim S, Johnson M, et al. Periodontal treatment during pregnancy and birth outcomes: a meta-analysis of randomised trials. Int J Evid Based Healthc 2011;9:122–47. 10.1111/j.1744-1609.2011.00210.x [DOI] [PubMed] [Google Scholar]
- 103.Pimentel Lopes De Oliveira GJ, Amaral Fontanari L, Chaves De Souza JA, et al. Effect of periodontal treatment on the incidence of preterm delivery: a systematic review. Minerva Stomatol 2010;59:543–50. [PubMed] [Google Scholar]
- 104.Polyzos NP, Polyzos IP, Zavos A, et al. Obstetric outcomes after treatment of periodontal disease during pregnancy: systematic review and meta-analysis. BMJ 2010;341:c7017. 10.1136/bmj.c7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosa MIda, Pires PDS, Medeiros LR, et al. Periodontal disease treatment and risk of preterm birth: a systematic review and meta-analysis. Cad Saude Publica 2012;28:1823–33. 10.1590/s0102-311x2012001000002 [DOI] [PubMed] [Google Scholar]
- 106.Shah M, Muley A, Muley P. Effect of nonsurgical periodontal therapy during gestation period on adverse pregnancy outcome: a systematic review. J Matern Fetal Neonatal Med 2013;26:1691–5. 10.3109/14767058.2013.799662 [DOI] [PubMed] [Google Scholar]
- 107.Uppal A, Uppal S, Pinto A, et al. The effectiveness of periodontal disease treatment during pregnancy in reducing the risk of experiencing preterm birth and low birth weight: a meta-analysis. J Am Dent Assoc 2010;141:1423–34. 10.14219/jada.archive.2010.0104 [DOI] [PubMed] [Google Scholar]
- 108.Kim AJ, Lo AJ, Pullin DA, et al. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol 2012;83:1508–19. 10.1902/jop.2012.110636 [DOI] [PubMed] [Google Scholar]
- 109.da Silva HEC, Stefani CM, de Santos Melo N, et al. Effect of intra-pregnancy nonsurgical periodontal therapy on inflammatory biomarkers and adverse pregnancy outcomes: a systematic review with meta-analysis. Syst Rev 2017;6:197. 10.1186/s13643-017-0587-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iheozor-Ejiofor Z, Middleton P, Esposito M, et al. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst Rev 2017;6:CD005297. 10.1002/14651858.CD005297.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guinto VT, De Guia B, Festin MR, et al. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010:CD007855. 10.1002/14651858.CD007855.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Widmer M, Lopez I, Gülmezoglu AM, et al. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev 2015;11:CD000491. 10.1002/14651858.CD000491.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Angelescu K, Nussbaumer-Streit B, Sieben W, et al. Benefits and harms of screening for and treatment of asymptomatic bacteriuria in pregnancy: a systematic review. BMC Pregnancy Childbirth 2016;16:336. 10.1186/s12884-016-1128-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fell DB, Platt RW, Lanes A, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG 2015;122:17–26. 10.1111/1471-0528.12977 [DOI] [PubMed] [Google Scholar]
- 115.Zhang C, Wang X, Liu D, et al. A systematic review and meta-analysis of fetal outcomes following the administration of influenza A/H1N1 vaccination during pregnancy. Int J Gynaecol Obstet 2018;141:141–50. 10.1002/ijgo.12394 [DOI] [PubMed] [Google Scholar]
- 116.Schneeberger C, Geerlings SE, Middleton P, et al. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst Rev 2015:CD009279. 10.1002/14651858.CD009279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vazquez JC, Abalos E. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database Syst Rev 2011;1:CD002256. 10.1002/14651858.CD002256.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flenady V, Hawley G, Stock OM, et al. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev 2013;12:CD000246. 10.1002/14651858.CD000246.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thinkhamrop J, Hofmeyr GJ, Adetoro O. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database of Systematic Reviews 2015:CD002250. 10.1002/14651858.CD002250.pub2 [DOI] [PubMed] [Google Scholar]
- 120.Manyando C, Kayentao K, D'Alessandro U, et al. A systematic review of the safety and efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria during pregnancy. Malar J 2012;11:141. 10.1186/1475-2875-11-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gamble C, Ekwaru PJ, Garner P, et al. Insecticide-Treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med 2007;4:e107. 10.1371/journal.pmed.0040107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev 2017;6:CD008991. 10.1002/14651858.CD008991.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berghella V, Keeler SM, To MS, et al. Effectiveness of cerclage according to severity of cervical length shortening: a meta-analysis. Ultrasound Obstet Gynecol 2010;35:468–73. 10.1002/uog.7547 [DOI] [PubMed] [Google Scholar]
- 124.Berghella V, Rafael TJ, Szychowski JM, et al. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol 2011;117:663–71. 10.1097/AOG.0b013e31820ca847 [DOI] [PubMed] [Google Scholar]
- 125.Defranco EA, Valent AM, Newman T, et al. Adjunctive therapies to cerclage for the prevention of preterm birth: a systematic review. Obstet Gynecol Int 2013;2013:528158. 10.1155/2013/528158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal Cerclage placed via laparoscopy for the prevention of preterm birth. J Minim Invasive Gynecol 2018;25:277–86. 10.1016/j.jmig.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 127.Namouz S, Porat S, Okun N. Emergency cerclage: literature review (provisional Abstract). Obstetrical and Gynecological Survey 2013;68:379–88. [DOI] [PubMed] [Google Scholar]
- 128.Rafael TJ, Berghella V, Alfirevic Z. Cervical stitch (cerclage) for preventing preterm birth in multiple pregnancy. Cochrane Database Syst Rev 2014;9:CD009166. 10.1002/14651858.CD009166.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saccone G, Rust O, Althuisius S, et al. Cerclage for short cervix in twin pregnancies: systematic review and meta-analysis of randomized trials using individual patient-level data. Acta Obstet Gynecol Scand 2015;94:352–8. 10.1111/aogs.12600 [DOI] [PubMed] [Google Scholar]
- 130.Smith J, DeFranco EA. Tocolytics used as adjunctive therapy at the time of cerclage placement: a systematic review. J Perinatol 2015;35:561–5. 10.1038/jp.2015.38 [DOI] [PubMed] [Google Scholar]
- 131.Zeybek B, Hill A, Menderes G, et al. Robot-Assisted abdominal Cerclage during pregnancy. JSLS 2016;20. 10.4293/JSLS.2016.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X, Luo X, Xiao X. Cervical cerclage for preventing preterm birth in twin pregnancies. A systematic review and meta-analysis (provisional Abstract). Database of Abstracts of Reviews of Effects 2013;4:632–8. [PubMed] [Google Scholar]
- 133.Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219:10–25. 10.1016/j.ajog.2018.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conde-Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol 2013;208:42.e1-42.e18. 10.1016/j.ajog.2012.10.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jarde A, Lutsiv O, Park CK, et al. Preterm birth prevention in twin pregnancies with progesterone, pessary, or cerclage: a systematic review and meta-analysis. BJOG 2017;124:1163–73. 10.1111/1471-0528.14513 [DOI] [PubMed] [Google Scholar]
- 136.Jarde A, Lutsiv O, Park CK, et al. Effectiveness of progesterone, cerclage and pessary for preventing preterm birth in singleton pregnancies: a systematic review and network meta-analysis. BJOG 2017;124:1176–89. 10.1111/1471-0528.14624 [DOI] [PubMed] [Google Scholar]
- 137.Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst Rev 2010;7:CD000110. 10.1002/14651858.CD000110.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maloni JA. Antepartum bed rest for pregnancy complications: efficacy and safety for preventing preterm birth. Biol Res Nurs 2010;12:106–24. 10.1177/1099800410375978 [DOI] [PubMed] [Google Scholar]
- 139.Sosa CG, Althabe F, Belizán JM, et al. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database Syst Rev 2015;3:CD003581. 10.1002/14651858.CD003581.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin Z, Chen L, Qiao D. Cervical pessary for preventing preterm birth: a meta-analysis. J Matern Fetal Neonatal Med 2017. [DOI] [PubMed] [Google Scholar]
- 141.Liem SMS, van Pampus MG, Mol BWJ, et al. Cervical pessaries for the prevention of preterm birth: a systematic review. Obstet Gynecol Int 2013;2013:1–10. 10.1155/2013/576723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in twin pregnancies with short cervical length: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2017;30:2918–25. 10.1080/14767058.2016.1268595 [DOI] [PubMed] [Google Scholar]
- 143.Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analysis. Obstet Gynecol Surv 2018;73:13–14. 10.1097/01.ogx.0000528014.01777.83 [DOI] [PubMed] [Google Scholar]
- 144.Thangatorai R, Lim FC, Nalliah S. Cervical pessary in the prevention of preterm births in multiple pregnancies with a short cervix: PRISMA compliant systematic review and meta-analysis. J Matern Fetal Neonatal Med 2018;31:1638–45. 10.1080/14767058.2017.1319930 [DOI] [PubMed] [Google Scholar]
- 145.Zheng L, Dong J, Dai Y, et al. Cervical pessaries for the prevention of preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019;32:1654–63. 10.1080/14767058.2017.1414795 [DOI] [PubMed] [Google Scholar]
- 146.Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol 2016;48:308–17. 10.1002/uog.15953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saccone G, Khalifeh A, Elimian A, et al. Vaginal progesterone vs intramuscular 17α-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2017;49:315–21. 10.1002/uog.17245 [DOI] [PubMed] [Google Scholar]
- 148.Samuel M, Chong YS. Progestational agents for treating threatened or established preterm labour. Cochrane Database of Systematic Reviews 2010:CD006770. [DOI] [PubMed] [Google Scholar]
- 149.Suhag A, Saccone G, Berghella V. Vaginal progesterone for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol 2015;213:479–87. 10.1016/j.ajog.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 150.Dodd JM, Grivell RM, OBrien CM, et al. Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy. Cochrane Database Syst Rev 2019;2019. 10.1002/14651858.CD012024.pub3. [Epub ahead of print: 20 11 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sotiriadis A, Papatheodorou S, Makrydimas G. Perinatal outcome in women treated with progesterone for the prevention of preterm birth: a meta-analysis. Ultrasound Obstet Gynecol 2012;40:257–66. 10.1002/uog.11178 [DOI] [PubMed] [Google Scholar]
- 152.Lim CED, Ho KKW, Cheng NCL, et al. Combined oestrogen and progesterone for preventing miscarriage. Cochrane Database Syst Rev 2013;9:CD009278. 10.1002/14651858.CD009278.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet 2009;373:646–8. 10.1016/S0140-6736(09)60947-8 [DOI] [PubMed] [Google Scholar]
- 154.Likis FE, Edwards DRV, Andrews JC, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis. Obstet Gynecol 2012;120:897–907. 10.1097/AOG.0b013e3182699a15 [DOI] [PubMed] [Google Scholar]
- 155.Palacio M, Ronzoni S, Sánchez-Ramos L, et al. Progestogens as maintenance treatment in arrested preterm labor: a systematic review and meta-analysis. Obstet Gynecol 2016;128:989–1000. 10.1097/AOG.0000000000001676 [DOI] [PubMed] [Google Scholar]
- 156.Prior M, Hibberd R, Asemota N, et al. Inadvertent P-hacking among trials and systematic reviews of the effect of progestogens in pregnancy? A systematic review and meta-analysis. BJOG 2017;124:1008–15. 10.1111/1471-0528.14506 [DOI] [PubMed] [Google Scholar]
- 157.Rode L, Langhoff-Roos J, Andersson C, et al. Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies. Acta Obstet Gynecol Scand 2009;88:1180–9. 10.3109/00016340903280982 [DOI] [PubMed] [Google Scholar]
- 158.Schmouder VM, Prescott GM, Franco A, et al. The rebirth of progesterone in the prevention of preterm labor. Ann Pharmacother 2013;47:527–36. 10.1345/aph.1R281 [DOI] [PubMed] [Google Scholar]
- 159.Velez Edwards DR, Likis FE, Andrews JC, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis by drug route. Arch Gynecol Obstet 2013;287:1059–66. 10.1007/s00404-013-2789-9 [DOI] [PubMed] [Google Scholar]
- 160.Chawanpaiboon S, Laopaiboon M, Lumbiganon P, et al. Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes. Cochrane Database Syst Rev 2014;3:CD010800. 10.1002/14651858.CD010800.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crowther CA, Brown J, McKinlay CJD, et al. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev 2014;8:CD001060. 10.1002/14651858.CD001060.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gaudet LM, Singh K, Weeks L, et al. Effectiveness of terbutaline pump for the prevention of preterm birth. A systematic review and meta-analysis. PLoS One 2012;7:e31679. 10.1371/journal.pone.0031679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haas DM, Caldwell DM, Kirkpatrick P, et al. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ 2012;345:e6226. 10.1136/bmj.e6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McNamara HC, Crowther CA, Brown J. Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour. Cochrane Database Syst Rev 2015;12:CD011200. 10.1002/14651858.CD011200.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vogel JP, Nardin JM, Dowswell T, et al. Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database Syst Rev 2014;7:CD006169. 10.1002/14651858.CD006169.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dodd JM, Crowther CA, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database Syst Rev 2012;12:CD003927. 10.1002/14651858.CD003927.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Flenady V, Reinebrant HE, Liley HG, et al. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev 2014;30:CD004452. 10.1002/14651858.CD004452.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Giorgino FL, Egan CG. Use of isoxsuprine hydrochloride as a tocolytic agent in the treatment of preterm labour: a systematic review of previous literature. Arzneimittelforschung 2010;60:415–20. 10.1055/s-0031-1296305 [DOI] [PubMed] [Google Scholar]
- 169.Mackeen AD, Seibel-Seamon J, Grimes-Dennis J, et al. Tocolytics for preterm premature rupture of membranes. Cochrane Database Syst Rev 2011:CD007062. 10.1002/14651858.CD007062.pub2 [DOI] [PubMed] [Google Scholar]
- 170.Saccone G, Suhag A, Berghella V. 17-alpha-hydroxyprogesterone caproate for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol 2015;213:16–22. 10.1016/j.ajog.2015.01.054 [DOI] [PubMed] [Google Scholar]
- 171.van Vliet E, Dijkema GH, Schuit E, et al. Nifedipine maintenance tocolysis and perinatal outcome: an individual participant data meta-analysis. BJOG 2016;123:1753–60. 10.1111/1471-0528.14249 [DOI] [PubMed] [Google Scholar]
- 172.Yamasmit W, Chaithongwongwatthana S, Tolosa JE, et al. Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy. Cochrane Database Syst Rev 2012;9:CD004733. 10.1002/14651858.CD004733.pub3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data extracted from the included reviews is available on request from the corresponding author.