Abstract
Introduction
A few studies have evaluated the efficacy and safety of noninsulated radiofrequency microneedling (RFMN) to treat periorbital wrinkles in Asian patients. Recently, wound healing accelerators, polynucleotides (PNs), have received attention in wound management. However, their efficacy and safety have not been fully elucidated following RFNM. This study aimed to evaluate the efficacy and safety of noninsulated RFMN for periorbital wrinkles and the synergistic effect of PNs after RFMN.
Methods
Thirty subjects with Fitzpatrick skin types III to V and facial wrinkles (Fitzpatrick grades I and II) were enrolled. All volunteers were treated over the entire face with noninsulated RFMN for three sessions at 2-week intervals. The left and right sides of each patient’s face were randomly assigned to receive PNs (treatment group) or normal saline solution (control group). The indentation and maximum depth of wrinkles were objectively measured using Antera 3D. Subjective self-evaluations were obtained at baseline, 2 weeks after the third treatment, and at 1, 2, 3, and 6 months after the final treatment. In addition, pain scores, immediate reactions, and other adverse effects were evaluated.
Results
Twenty-nine subjects completed the treatment protocol. Most presented with grade II wrinkling (69%). At 2-month follow-up, Antera 3D image analysis revealed faster improvement for the treatment group. At 6-month follow-up, the majority of subjects reported an improvement of 25–75% in their periorbital wrinkles. The average pain score was 2.2 out of 10. No serious adverse events (infection, pigmentary alteration, persistent erythema, or scarring) were observed.
Conclusions
Noninsulated RFMN is safe and effective for treating periorbital wrinkles and can be used as a modality for transdermal drug delivery. Topical polynucleotides as an adjunctive treatment provide additional benefits for periorbital wrinkle treatment.
Trial Registration Number
TCTR20201105007.
Keywords: Periorbital wrinkles, Polynucleotides, Radiofrequency, Microneedling, Transdermal drug delivery
Key Summary Points
| Why carry out this study? |
| Radiofrequency microneedling (RFNM) is a safe and effective, minimally invasive therapy option for all skin types with various dermatological conditions. Combining RFNM with other treatments can improve its efficacy. |
| The current study evaluated the efficacy and safety of noninsulated RFMN for periorbital wrinkles and the synergistic effect of polynucleotides after RFMN. |
| What was learned from the study? |
| Image analysis using Antera 3D showed faster improvement in the treatment group 2 months posttreatment. Most of the patients reported an improvement of 25–75% at 6 months after treatment. |
| These study results indicate that RFMN is safe and effective in treating periorbital wrinkles and can be used as a modality for transdermal drug delivery. Topical polynucleotides provide additional benefits following RFNM. |
Introduction
The human face has played a significant role throughout history. It defines youthfulness and attractiveness and conveys emotions and recognition [1]. The periorbital region of the face is one of the first areas to show signs of aging because it is not shaded by the contours of the face or hair [2]. This wrinkle-prone area requires rejuvenation treatment [3]. Treatment of periorbital wrinkles is challenging due to their anatomical significance [4]. Several therapeutic modalities have been established to treat periorbital wrinkles while avoiding permanent scarring or eye injuries. They include topical tretinoin, chemical peels, botulinum toxin, dermal filler injections, and energy-based devices. However, no standard treatment for periorbital wrinkles has yet been established [5].
In 1920, Bovie developed a high-frequency alternating-current device that could coagulate or destroy tissue [6]. The first radiofrequency (RF)-based device was approved for cosmetic use in 2002. In 2004, cosmetic approval for the treatment of facial wrinkles and rhytids was granted [7]. The initial RF devices conveyed monopolar energy through the epidermis to the deeper layers of the skin [8]. Transepidermal RF treatments have limitations because the energy delivered through the skin surface can induce epidermal damage, and the depth of heating is not accurate. RF microneedling (RFMN) was developed to improve dermal heating [7]. Hantash and colleagues published the first RFMN study in 2009. Their research found neocollagenesis and neoelastogenesis after RFMN treatment on human skin [9].
Various RF systems have been used successfully and safely for periorbital rejuvenation [10–12]. Recently, invasive RF with a noninsulated microneedle has been shown to safely deliver RF energy to the skin while preserving the epidermis [13]. In addition, RFMN has the potential to provide a direct route for transdermal drug delivery (TDD) of large hydrophilic molecules [14].
This study compared the efficacy and safety of noninsulated bipolar pulsed-type microneedle radiofrequency alone versus its use in combination with polynucleotides to treat periorbital wrinkles. Subjective and objective clinical evaluations were conducted.
Methods
The study enrolled 30 Thai women who had Fitzpatrick skin types III to V, had facial wrinkles (Fitzpatrick grades I and II), and had not received treatment during the previous 12 months. The exclusion criteria were subjects with history of hypertrophic or keloid scarring, who showed signs of active infection or inflammation on the face, were pregnant, or used oral contraceptive pills, anticoagulants, or antiplatelets.
This prospective, double-blind, randomized, controlled, split-face study was conducted at a tertiary university hospital. Before the research began, its protocol was approved by the Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (SI 603/2020). All patients were informed of the therapeutic benefits, risks, and possible complications before enrollment. They also provided written informed consent before study-related procedures. This study was performed in accordance with the Helsinki Declaration of 1964 and its subsequent amendments.
An invasive, bipolar, pulsed-type, alternating-current, radiofrequency device with noninsulated needles (Sylfirm; Viol, Gyeonggi, Korea) was used. It has a handpiece equipped with a disposable single-use tip consisting of 25 minimally invasive noninsulated microneedle electrodes or pins. The pins are arranged in a 5 × 5 pattern (diameter 0.3 mm, distance between electrodes 2 mm). They have a tip length of 3.5 mm, and their penetration depth can be adjusted from 0.5 to 3.5 mm. The amount of RF emission depends on the energy level, which can be set from 1 to 10. Needle insertion and retraction are automatic and controlled by a footswitch.
A transparent liquid containing 0.3% polynucleotides (PNs) as the primary component (REVS NCFS 140HPn; Reanzen Co, Anyang, Korea) was used as a topical product for TDD. The PNs (65–130 kDa) used were in the form of serum contained in vials. Each 3 ml vial contained 9 mg PNs. The other main components were 0.3% hyaluronic acid (1.4 million Da), amino acids, minerals, vitamins, and peptides.
Preoperatively, the treatment areas were cleaned with a mild cleanser and disinfected with 70% isopropyl alcohol. Before each treatment, a eutectic mixture of anesthetic cream (lidocaine 2.5% and prilocaine 2.5%; AstraZeneca LP, Wilmington, DE, USA) was applied topically under occlusion to the treatment area for 1 h.
The patients received three treatments on their entire face at 2-week intervals. The following settings were used: energy level, 2 or 3; penetration depth, 0.8–1.5 mm; three passes with 10–30% overlap. The endpoint was erythema. The left and right sides of each patient’s face were randomly assigned to receive PNs (treatment side) or normal saline solution (control side). The patients were unaware of which intervention was applied to which side. Immediately after the procedure, the treatment and control sides were occluded with a transparent dressing (Tegaderm; 3 M Healthcare, St. Paul, MN, USA) for 1 h. The patients were instructed to apply a UVA/B sunscreen (SPF at least 30) daily and avoid direct sun exposure. No other products or special instructions on skincare were given.
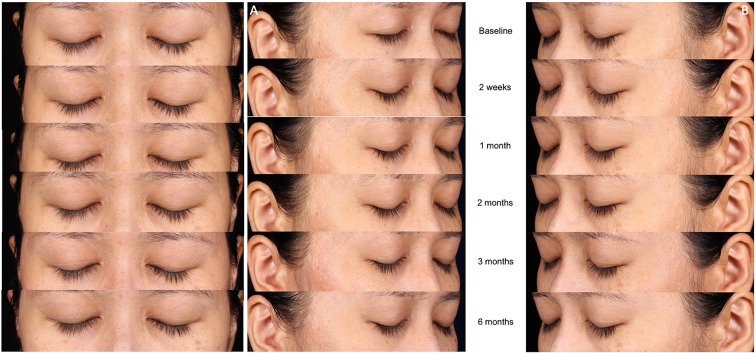
Photographic documentation using identical camera settings, lighting, and patient positioning was obtained at baseline and again following the final treatment (2 weeks and 1, 2, 3, and 6 months after). All digital photographs were acquired with a facial photo fixture using a Canon PowerShot G9 standoff camera (OMNIA Imaging System; Canfield Scientific, Inc., Fairfield, NJ, USA). The fixture ensured a set distance and fixed angles between the patient and the camera. The imaging station provided preset camera angles for the frontal region through the full profile and a matching position image overlay for quick and accurate patient positioning. Flashlamps placed in fixed positions on the camera ensured even illumination of all parts of the face and the ability to examine subjects under controlled lighting.
Objective and subjective evaluations were obtained at baseline and at 2 weeks, 1 month, 2 months, 3 months, and 6 months after the final treatment. The objective evaluations were measurements of the indentations and the maximum depths of wrinkles (using Antera 3D; Miravex Ltd., Dublin, Ireland) and the mean gross elasticity (R2; using a Cutometer; Courage–Khazaka Electronic GmbH, Cologne, Germany). Patients rated the clinical improvement in their wrinkles using a quartile classification scale. The grades were 0 = no improvement, 1–25% improvement, 26–50% improvement, 51–75% improvement, and 76–100% improvement.
Descriptive analysis was used for demographic data. Assessment data for indentation, maximum depth of wrinkles, and patient evaluation scores were subjected to statistical analyses between baseline and the various assessment time points for the control and treatment sides, with a paired-sample t-test and repeated-measures analysis of variance (ANOVA) using PASW Statistics for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Significance was set at a value of P ≤ 0.05.
Results
All patients were women (100%; n = 29), with a mean age of 42.9 ± 6.7 years. Of the 30 subjects enrolled, 29 (96.6%) completed the study and attended the 6-month follow-up. One subject was excluded because she could not attend the follow-up visits. Type IV skin (69%) and grade II wrinkling (69%) predominated. Demographic characteristics are summarized in Table 1.
Table 1.
Patient demographics (n = 29)
| Characteristic | Value |
|---|---|
| Age (years), mean ± SD | 42.9 ± 6.7 |
| (min–max) | (24–54) |
| Sex, n (%) | |
| Female | 29 (100%) |
| Male | 0 (0%) |
| Skin type, n (%) | |
| III | 5 (17.2%) |
| IV | 20 (69.0%) |
| V | 4 (13.8%) |
| Fitzpatrick wrinkle and elastosis scale | |
| I | 9 (31.0%) |
| II | 20 (69.0%) |
| III | 0 (0%) |
| Pain score, mean ± SD | 2.1 ± 1.5 |
| (min–max) | (0–6) |
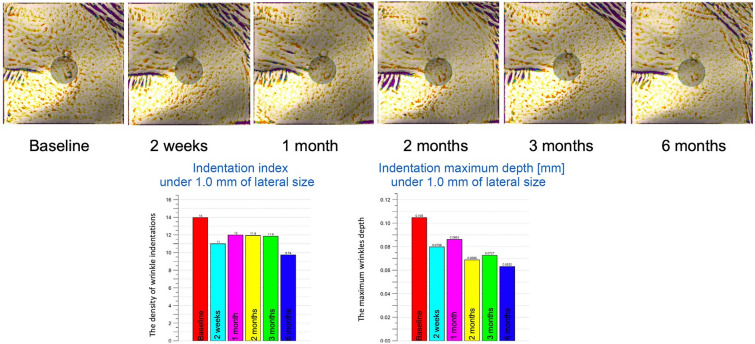
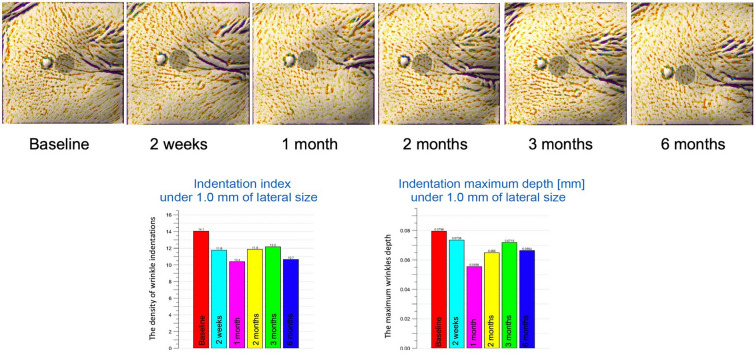
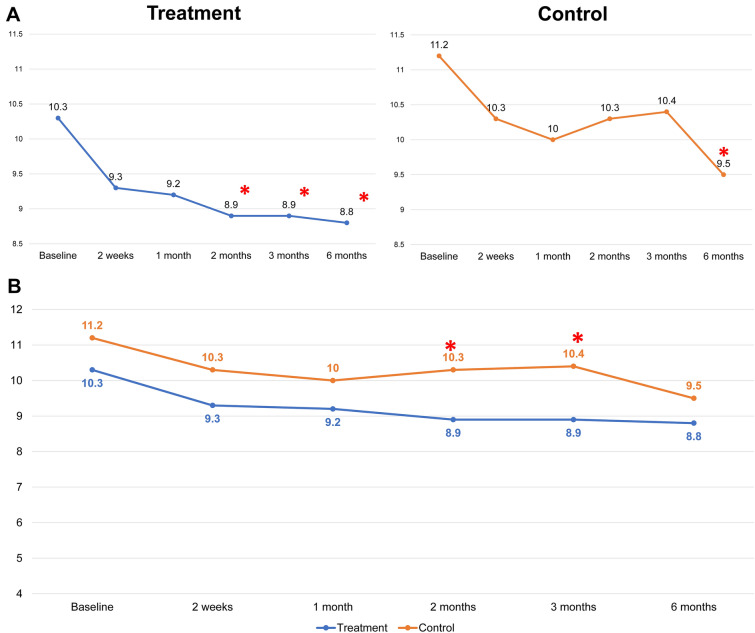
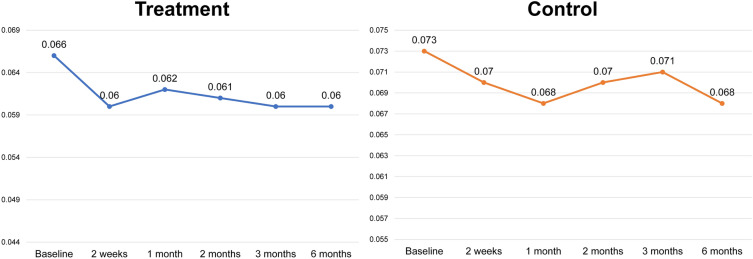
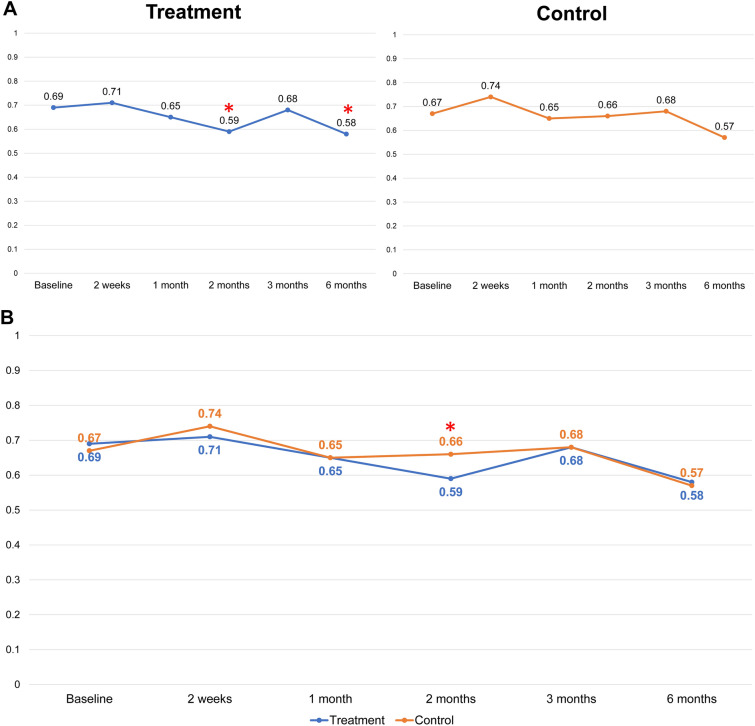
There were no statistically significant differences in the baseline values of the wrinkle indentations (P > 0.05), the maximum wrinkle depth (P > 0.05), or the mean gross elasticity (P > 0.05) of the control and treatment sides. Improvements in the indentation and the maximum depth were observed using Antera 3D (Figs. 1 and 2). Figure 3 illustrates the data for indentation at baseline and at the five follow-up evaluation time points after the final treatment. The indentation of the wrinkles on the treatment side began to improve at the 2-month follow-up (Fig. 3A), and a better improvement was observed on the treatment side than on the control side (P < 0.05 and P = 0.006, respectively; Fig. 3B). A steady improvement was noted on the treatment side (P < 0.05, P = 0.008) and on the control side (P < 0.05, P = 0.041) until the 6-month follow-up (Fig. 3A). No statistically significant differences were observed in the maximum depth of wrinkles on the treatment and control sides at any follow‐up visit (P > 0.05; Fig. 4).
Fig. 1.
Image analysis using Antera 3D at treatment side. Improvement was seen at the 2-month follow-up, and steady improvement was seen until the 6-month follow-up
Fig. 2.
Image analysis using Antera 3D at the control side. Significant improvement was seen at the 6-month follow-up
Fig. 3.
A Intragroup comparison of scores for indentation of wrinkles at the treatment and control sides at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). (*P < 0.05 set as significance). B Comparison between groups of the scores for indentation of wrinkles at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). (*P < 0.05 set as significance)
Fig. 4.
Mean of scores for maximum depth of wrinkles of the treatment and control sides at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). (*P < 0.05 set as significance)
Figure 5 depicts the mean gross elasticity of the control and treatment sides from baseline to the 6-month follow-up. At 2 weeks after the final treatment, there was an improving trend on the treatment side (P > 0.05) and on the control side (P > 0.05). The skin had lower elasticity on the treatment side (P < 0.05 versus P = 0.001) at the 6-month follow-up.
Fig. 5.
A Scores for mean gross elasticity (R2) of the treatment and control sides at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). (*P < 0.05 set as significance). B Comparison of the mean gross elasticity (R2) scores of the treatment and control sides at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). (*P < 0.05 set as significance)
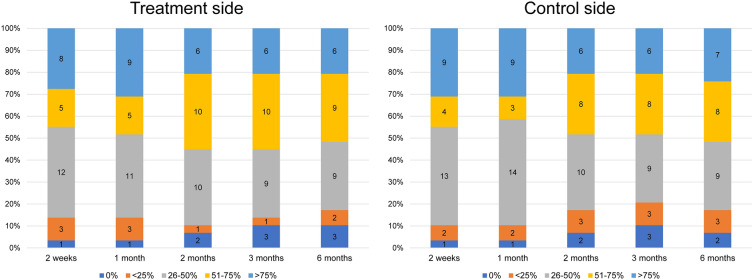
Patient evaluations of their degree of wrinkling (Fig. 6) showed an improvement 2 weeks after the first treatment. On the treatment side, 41% of the patients reported an improvement of 26–50%, 17% reported 51–75%, and 27% reported > 75%. The corresponding values for the control side were 44%, 13%, and 31%. A noticeable improvement was found at the 2-month follow-up. A 51–75% improvement was reported by 34% of cases for the treatment side and by 27% for the control side. Consistent improvement occurred until the 6-month follow-up, with > 25% improvement on both sides for 82% of cases and > 50% improvement for 51% on both sides (Fig. 7). A similar pattern was found with wrinkle indentation on the treatment and control sides at the 2- and 6-month follow-ups.
Fig. 6.
Patient evaluations of the degree of improvement in their wrinkles on the treatment and control sides at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). Patient evaluations of improvement (< 50% vs. ≥ 50%) were not significant different between groups (P = 0.189). (*P < 0.05 set as significance)
Fig. 7.
Representative clinical photographs. A Control side at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after). B Treatment side at baseline and at the five assessment time points following the final treatment (2 weeks and 1, 2, 3, and 6 months after)
Regarding safety, all patients tolerated the procedure well. The topical medication was well tolerated, and there were no reports of infection, hyperpigmentation, hypopigmentation, persistent erythema, or scarring. No or minimal pain was reported, with the mean pain score for the procedure being 2.2 ± 1.9.
Discussion
The face is the region in which patients and other people first notice signs of aging [15]. One of these is wrinkling in the area of the lateral eyebrow [16]. The periorbital area is delicate and anatomically vulnerable. The wrinkling of the overlying skin is due to muscle contractions of the orbicularis oculi associated with various facial expressions, and it is aggravated by ultraviolet-induced photodamage [13]. Generally, Asian skin has a mild to moderate degree of wrinkling [17]. Treatments for the periorbital area include topical medications and various injectable, energy-based, and surgical procedures [5]. Among the treatment modalities that aim to improve photoaged skin, ablative resurfacing is one of the most effective. However, the demand for a procedure with minimal downtime and few adverse events has increased [12]. The approach of using a less invasive and less aggressive skin resurfacing technique than ablative resurfacing on Asians may be more beneficial and may outweigh the risks. The present work is the first to compare the efficacy and safety of using noninsulated microneedle radiofrequency alone versus using it in combination with polynucleotides to treat periorbital wrinkles.
In terms of energy delivery, fractional RF technology is classified into two types. One is RFMN, a system that uses microneedles. The benefits of RFMN include the delivery of uniform heat to dermal layers at controlled depth using microneedles with protection of the skin surface [18–20]. Our previous study found that the thermal response to RFMN treatment induced a wound healing cascade leading to long-term dermal remodeling. Histological examination revealed that newly formed collagen fibers had replaced denatured collagen zones 3 months after a single treatment with RFMN. In addition, neoelastogenesis was demonstrated by Verhoeff–Van Gieson staining at the 1- and 3-month follow-up visits. An immunohistochemical study also showed positive staining for heat shock protein (HSP)70 [21]. Hantash and colleagues discovered that, by 10 weeks after treatment, the RF thermal zone in the reticular dermis had been replaced by new dermal tissue, with consistent increases in HSP47 expression. Regulation of the tissue response to thermal damage is aided by HSPs, which activate transient amplifying epidermal stem cells to replace newly damaged skin [22]. Microneedling itself also causes physical stimulation. It induces a wound healing process that, in turn, stimulates dermal collagen regeneration and remodeling. The result is skin tightening and improved skin laxity and wrinkling [19].
The observations of the current study are consistent with the findings of recent research by Kim et al. [11, 12] and Kwon et al. [5]. Their studies reported 25–43% improvements in periorbital rhytides after three treatment sessions, with effects lasting up to 6 months. Similarly, Cho et al. [13] reported three cases with acceptable improvements to periorbital wrinkling using invasive, bipolar, pulsed-type, alternating-current radiofrequency technology. At the 6-month follow-up to the three treatment sessions of the present investigation, 82% of subjects rated themselves as having more than 25% improvement on both sides. However, 51% reported an improvement exceeding 50% on both sides.
Although the effects of RFMN are slow and progressive, the procedure can be used repeatedly and safely in combination with other treatment modalities to augment its efficacy [20]. Recently, various wound healing accelerators, such as polynucleotides (PNs), stem cell culture media, and platelet-rich plasma, have received attention in wound and scar management. However, their efficacy and safety have not been fully elucidated. PNs are a broad group of molecules, and DNA and RNA are examples. PNs have a molecular weight of 8000 kDa and a viscoelastic texture [23]. They are widely distributed in the extracellular environment of the human body and are used to mediate the remodeling phase of wound healing [24]. The present study enabled us to further investigate the indentation of wrinkles visualized by Antera 3D. After the RFMN treatment, we expected the PNs to directly affect the dermal extracellular matrix and enhance the wound healing process. We found that the treatment side improved better than the control side after the 2-month follow-up, and improvement continued to the 6-month follow-up. However, there was no significant improvement in the Cutometer measurements of the skin elasticity of the control and treatment sides before and after treatment. It could be that the Cutometer measures too superficially to detect dermal changes [10].
Many techniques have been used to increase the penetration of drugs through TDD. They include lasers, dermabrasion, microneedling, and radiofrequency [25]. An intact stratum corneum is nearly impenetrable to most hydrophilic and lipophilic molecules larger than 500 Da [26]. However, quantitative structure–activity measurements show that skin permeation depends not only on molecular weight but also on other physicochemical properties [27]. The enhancement strategies for TDD in this study, which used technology that combines physical enhancement and thermal treatment, showed promise for macromolecules. Even though PNs are large molecules, significantly faster improvement was seen on the treatment side than on the control side. Research by An et al. [28] reported that RFMN enables TDD of polylactic acid, which does not pass through the skin barrier in normal skin. Polylactic acid has a relatively high molecular weight (140 kDa).
In the case of RFMN, the TDD may be due to the combination of mechanical and thermal effects. Ionic vibration and local heating of the skin may be caused by mechanical effects (the piercing of the stratum corneum by the microneedles), the creation of reversible microchannels [29], and the application of high-frequency alternating current. The result is improved drug delivery across the skin. Invasive bipolar devices with noninsulated penetrating pins, pulsed high-frequency delivery, and a short high-frequency conduction time maximize electromagnetic-selective tissue reactions [13]. Pulsed electric fields increase the permeability of the cell membrane (termed electroporation) [30]. Electroporation and heat-based ablation are promising alternative approaches to increasing skin permeability [31]. This technology enables the TDD of many drugs [32]. RFMN devices that use invasive, bipolar, pulsed-type, alternating-current radiofrequency technology show promise for TDD.
Other factors that may contribute to enhancing the absorption of drugs are occlusion and formulation (liquid and gel). The topical PNs were applied under occlusion for 1 h in our study. Considering that water acts as a skin penetration enhancer, occlusion increases the water content of the skin and improves the percutaneous absorption of drugs [33]. The duration of application is critical for TDD, which is most successful during the first 30 min. It is important to remember that microscopic channels close with time due to the accumulation of debris such as fibrin, inflammatory mediators, and keratinocytes [34]. The hydrophobic nature of the stratum corneum allows the penetration of lipid-soluble molecules more readily than water-soluble compounds [25]. Liquid and gel formulations cross the channels produced by ablative fractional lasers with greater affinity than more oily cream and ointment formulations [27]. RFMN may allow the skin to be permeated by water-soluble compounds, allowing hydrophilic substances to cross and fill the channels.
RFMN devices do not rely on chromophores for efficacy or safety and are considered safe for all skin phototypes [5]. In Fitzpatrick skin phototype IV, postinflammatory hyperpigmentation occurs in up to 80% of patients and can last for 3–9 months [35]. The possibility of postinflammatory hyperpigmentation should be considered when treating dark-skinned individuals [17]. Another advantage is the control of bleeding during surgery [14]. The absence of serious adverse events and patient discomfort in this study indicates that RFMN devices are a good option for treating periorbital wrinkles in Asian skin.
The main limitation of this study is the small number of patients. However, the randomized, split-face study protocol and assessments with objective instruments enhanced the reliability of the data. This approach enabled us to achieve significant results with a relatively small group of patients.
Conclusions
Noninsulated RFMN devices are safe and effective in treating periorbital wrinkles in Asians. Combining treatment with PNs is safe and provides a synergistic effect on periorbital wrinkles.
Acknowledgements
Funding
This research project was supported by the Faculty of Medicine Siriraj Hospital, Mahidol University. The device was provided by IDS Medical Systems Thailand Company Ltd, Thailand, and the medications were provided by GFN Group Company Ltd, Thailand. The journal’s Rapid Service Fee was funded by DermAesthetic Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yanisorn Nanchaipruek, Chadakan Yan, Supisara Wongdama, and Sarawalai Rakchart. Rungsima Wanitphakdeedecha had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. The first draft of the manuscript was written by Yuri Yogya, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Medical Writing, Editorial, and Other Assistance
The authors thank Ms. Phonsuk Yamlexnoi, Ms. Chutikan Kiatphansodsai, Ms. Apichaya Jutaphonrakul, and Dr. Surachet Sirisuthivoranunt for their assistance in recruiting subjects and managing the database. The authors are also indebted to Mr. David Park for the English-language editing of this paper, funded by DermAesthetic Co., Ltd.
Disclosures
Yuri Yogya, Rungsima Wanitphakdeedecha, Supisara Wongdama, Yanisorn Nanchaipruek, Chadakan Yan, and Sarawalai Rakchart have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the Ethics Committee of the Siriraj Institutional Review Board (SI 603/2020). Written informed consent was obtained for the publication and use of all patients’ images prior to their enrollment in the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its subsequent amendments. This study was registered on ClinicalTrials.gov and assigned NCT number as TCTR20201105007.
Data Availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Thanking Patient Participant(s)
We thank the patients who participated in the study.
References
- 1.Volpe CR, Ramirez OM. The beautiful eye. Facial Plast Surg Clin. 2005;13(4):493–504. doi: 10.1016/j.fsc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers A, Carruthers J, Hardas B, Kaur M, Goertelmeyer R, Jones D, et al. A validated grading scale for crow’s feet. Dermatol Surg. 2008;34:S173–S178. doi: 10.1111/j.1524-4725.2008.34367.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaczvinsky JR, Griffiths CE, Schnicker MS, Li J. Efficacy of anti-aging products for periorbital wrinkles as measured by 3-D imaging. J Cosmet Dermatol. 2009;8(3):228–233. doi: 10.1111/j.1473-2165.2009.00444.x. [DOI] [PubMed] [Google Scholar]
- 4.Manaloto RMP, Alster TS. Periorbital rejuvenation: a review of dermatologic treatments. Dermatol Surg. 1999;25(1):1–9. doi: 10.1046/j.1524-4725.1999.08049.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwon S-H, Choi J-Y, Ahn GY, Jang WS, Shin J-W, Na J-I, et al. The efficacy and safety of microneedle monopolar radiofrequency for the treatment of periorbital wrinkles. J Dermatol Treat. 2021;32(4):460–464. doi: 10.1080/09546634.2019.1662880. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor JL, Bloom DA, William T. Bovie and electrosurgery. Surgery. 1996;119(4):390–396. doi: 10.1016/S0039-6060(96)80137-1. [DOI] [PubMed] [Google Scholar]
- 7.Weiner SF. Radiofrequency microneedling: overview of technology, advantages, differences in devices, studies, and indications. Facial Plast Surg Clin. 2019;27(3):291–303. doi: 10.1016/j.fsc.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32(1):79–90. doi: 10.1016/j.det.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Hantash BM, Ubeid AA, Chang H, Kafi R, Renton B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41(1):1–9. doi: 10.1002/lsm.20731. [DOI] [PubMed] [Google Scholar]
- 10.Jeon IK, Chang SE, Park G-H, Roh MR. Comparison of microneedle fractional radiofrequency therapy with intradermal botulinum toxin a injection for periorbital rejuvenation. Dermatology. 2013;227(4):367–372. doi: 10.1159/000356162. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Kim J-I, Yang YJ, Nam JH, Kim W-S. Treatment of periorbital wrinkles with a novel fractional radiofrequency microneedle system in dark-skinned patients. Dermatol Surg. 2015;41(5):615–622. doi: 10.1097/DSS.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 12.Kim JK, Roh MR, Park GH, Kim YJ, Jeon IK, Chang SE. Fractionated microneedle radiofrequency for the treatment of periorbital wrinkles. J Dermatol. 2013;40(3):172–176. doi: 10.1111/1346-8138.12046. [DOI] [PubMed] [Google Scholar]
- 13.Cho S, Choi YJ, Kang J-S. Improvement of periorbital wrinkles treated with an invasive non-insulated microneedle pulsed electric signal device. Med Lasers. 2016;5(1):34–38. doi: 10.25289/ML.2016.5.1.34. [DOI] [Google Scholar]
- 14.Seo KY, Yoon MS, Kim DH, Lee HJ. Skin rejuvenation by microneedle fractional radiofrequency treatment in Asian skin; clinical and histological analysis. Lasers Surg Med. 2012;44(8):631–636. doi: 10.1002/lsm.22071. [DOI] [PubMed] [Google Scholar]
- 15.De Biasio F, Miotti G, Zingaretti N, Castriotta L, Parodi PC. Study on the aging dynamics of the periorbital region: from observation to knowledge of physiopathology. Ophthalmic Plast Reconstr Surg. 2019;35(4):333–341. doi: 10.1097/IOP.0000000000001247. [DOI] [PubMed] [Google Scholar]
- 16.Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, Sianati H, Sharepour M, Hadi Y. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29(3):154–168. doi: 10.1016/j.joco.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manuskiatti W, Siriphukpong S, Varothai S, Wanitphakdeedecha R, Fitzpatrick RE. Effect of pulse width of a variable square pulse (VSP) erbium: YAG laser on the treatment outcome of periorbital wrinkles in Asians. Int J Dermatol. 2010;49(2):200–206. doi: 10.1111/j.1365-4632.2009.04292.x. [DOI] [PubMed] [Google Scholar]
- 18.Shin JM, Kim JE. Radiofrequency in clinical dermatology. Med Lasers. 2013;2(2):49–57. doi: 10.25289/ML.2013.2.2.49. [DOI] [Google Scholar]
- 19.Elsaie ML, Choudhary S, Leiva A, Nouri K. Nonablative radiofrequency for skin rejuvenation. Dermatol Surg. 2010;36(5):577–589. doi: 10.1111/j.1524-4725.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 20.Tan MG, Jo CE, Chapas A, Khetarpal S, Dover JS. Radiofrequency microneedling: a comprehensive and critical review. Dermatol Surg. 2021;47(6):755–761. doi: 10.1097/DSS.0000000000002972. [DOI] [PubMed] [Google Scholar]
- 21.Manuskiatti W, Pattanaprichakul P, Inthasotti S, Sitthinamsuwan P, Hanamornroongruang S, Wanitphakdeedecha R, et al. Thermal response of in vivo human skin to fractional radiofrequency microneedle device. BioMed Res Int. 2016 doi: 10.1155/2016/6939018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waibel J, Beer K. Ablative fractional laser resurfacing for the treatment of a third-degree burn. J Drugs Dermatol. 2009;8(3):294–297. [PubMed] [Google Scholar]
- 23.Kim JH, Jeong JJ, Lee YI, Lee WJ, Lee C, Chung WY, et al. Preventive effect of polynucleotide on post-thyroidectomy scars: a randomized, double-blinded, controlled trial. Lasers Surg Med. 2018;50(7):755–762. doi: 10.1002/lsm.22812. [DOI] [PubMed] [Google Scholar]
- 24.Bloemen MC, van der Veer WM, Ulrich MM, van Zuijlen PP, Niessen FB, Middelkoop E. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35(4):463–475. doi: 10.1016/j.burns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Alegre-Sánchez A, Jiménez-Gómez N, Boixeda P. Laser-assisted drug delivery. Actas Dermo-Sifiliograficas (English Edition) 2018;109(10):858–867. doi: 10.1016/j.adengl.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Junsuwan N, Manuskiatti W, Phothong W, Wanitphakdeedecha R. Fractional CO2 laser-assisted Botulinum toxin type A delivery for the treatment of primary palmar hyperhidrosis. Lasers Med Sci. 2021;36(1):233–236. doi: 10.1007/s10103-020-03064-5. [DOI] [PubMed] [Google Scholar]
- 27.Olesen UH, Mogensen M, Haedersdal M. Vehicle type affects filling of fractional laser-ablated channels imaged by optical coherence tomography. Lasers Med Sci. 2017;32(3):679–684. doi: 10.1007/s10103-017-2168-z. [DOI] [PubMed] [Google Scholar]
- 28.An MK, Hong EH, Suh SB, Park EJ, Kim KH. Combination therapy of microneedle fractional radiofrequency and topical poly-lactic acid for acne scars: a randomized controlled split-face study. Dermatol Surg. 2020;46(6):796–802. doi: 10.1097/DSS.0000000000002175. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Chen M, Sun Y, Jin Y, Lu C, Pan X, et al. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021;121:119–133. doi: 10.1016/j.actbio.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Golberg A, Bruinsma BG, Uygun BE, Yarmush ML. Tissue heterogeneity in structure and conductivity contribute to cell survival during irreversible electroporation ablation by “electric field sinks”. Sci Rep. 2015;5(1):1–7. doi: 10.1038/srep08485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 32.Szunerits S, Boukherroub R. Heat: a highly efficient skin enhancer for transdermal drug delivery. Front Bioeng Biotechnol. 2018;6:15. doi: 10.3389/fbioe.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Maghraby GM. Occlusive versus nonocclusive application in transdermal drug delivery. Percutaneous penetration enhancers drug penetration into/through the skin. Springer; 2017. pp. 27–33. [Google Scholar]
- 34.Banzhaf CA, Thaysen-Petersen D, Bay C, Philipsen PA, Mogensen M, Prow T, et al. Fractional laser-assisted drug uptake: impact of time-related topical application to achieve enhanced delivery. Lasers Surg Med. 2017;49(4):348–354. doi: 10.1002/lsm.22610. [DOI] [PubMed] [Google Scholar]
- 35.Sriprachya-anunt S, Marchell NL, Fitzpatrick RE, Goldman MP, Rostan EF. Facial resurfacing in patients with Fitzpatrick skin type IV. Lasers Surg Med. 2002;30(2):86–92. doi: 10.1002/lsm.10012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.