Abstract
Background
Determining how prior immune checkpoint inhibitor (ICI) therapy influences outcomes in cancer patients presenting with COVID‐19 is essential for patient management but must account for confounding variables.
Methods
We performed a systematic review and meta‐analysis of studies reporting adjusted effects of ICIs on survival, severe events, or hospitalisation in cancer patients with COVID‐19 based on variables including age, gender, diabetes mellitus, hypertension (HTN), chronic obstructive pulmonary disease, and other comorbidities. When adjusted effects were unavailable, unadjusted data were analysed.
Results
Of 42 observational studies (38 retrospective), 7 reported adjusted outcomes for ICIs and 2 provided sufficient individual patient data to calculate adjusted outcomes. In eight studies, adjusted outcomes were based on ≤7 variables. Over all studies, only one included >100 ICI patients while 26 included <10. ICIs did not alter the odds ratio (95%CI) (OR) of death significantly (random effects model), across adjusted (n = 8) [1.31 (0.58–2.95) p = 0.46; I 2 = 42%, p = 0.10], unadjusted (n = 30) [1.06 (0.85–1.32) p = 0.58; I 2 = 0%, p = 0.76] or combined [1.09 (0.88;1.36) p = 0.41; I 2 = 0%, p = 0.5)] studies. Similarly, ICIs did not alter severe events significantly across adjusted (n = 5) [1.20 (0.30–4.74) p = 0.73; I 2 = 52%, p = 0.08], unadjusted (n = 19) [(1.23 (0.87–1.75) p = 0.23; I 2 = 16%, p = 0.26] or combined [1.26 (0.90–1.77) p = 0.16; I 2 = 25%, p = 0.14] studies. Two studies provided adjusted hospitalisation data and when combined with 13 unadjusted studies, ICIs did not alter hospitalisation significantly [1.19 (0.85–1.68) p = 029; I 2 = 5%, p = 0.40]. Results of sensitivity analyses examining ICI effects based on 5 variables were inconclusive. Certainty of evidence was very low.
Conclusions
Across studies with adjusted and unadjusted results, ICIs did not alter outcomes significantly. But studies with comprehensive adjusted outcome data controlling for confounding variables are necessary to determine whether ICIs impact COVID‐19 outcomes in cancer patients.
Keywords: cancer, COVID‐19, immune checkpoint inhibitors, immunotherapy, SARS‐COV2d
Abbreviations
- COVID‐19
coronavirus disease 2019
- COPD
chronic obstructive pulmonary disease
- DM
diabetes
- HR
Hazard ratio
- HTN
hypertension
- ICI
immune checkpoint inhibitor
- ICI patients
patients that had previously received ICI therapy
- IT
immunotherapies
- non‐ICI patients
patients that had not previously received ICI therapy
- OR
odds ratio
1. BACKGROUND
The incidence of severe disease and mortality with COVID‐19 is higher in patients with cancer. 1 , 2 , 3 An unanswered question is whether prior immune checkpoint inhibitor (ICI) therapy, while highly effective for certain cancers, contributes to these worsened outcomes. 4 , 5 , 6 , 7 , 8 ICIs counter the immunosuppressive effects their targeted checkpoint molecules exert on innate and adaptive immune responses resulting in enhanced anti‐tumour responses. 9 , 10 ICIs may also affect anti‐viral responses. 11 , 12 , 13 , 14 However, the immune‐related adverse events, including pneumonitis, that ICIs can produce and that occur days to months after treatment ends, could be precipitated by or complicate the intense inflammatory response COVID‐19 produces in some patients. 6 , 15
Determining whether prior ICI therapy has beneficial host defence or harmful inflammatory effects in cancer patients presenting with COVID‐19 is critical for patient management. 4 , 5 , 6 , 7 , 8 There is an increasing number of published reports examining the impact of anti‐cancer therapies, including ICIs, on outcomes for cancer patients with COVID‐19. However, both cancer and non‐cancer factors influence COVID‐19 outcomes and confound assessment of ICI effects. 16 , 17 , 18 , 19 While an ideal study examining the impact of ICIs on COVID‐19 outcomes would adjust for these variables and a systematic review addressing this question would focus on such adjusted studies, this has generally not been the case. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Among eight published systematic reviews of studies investigating cancer patients presenting with COVID‐19 that previously received immunotherapies (IT) including ICIs, only 3 provided analyses of adjusted outcomes with IT, and each of these was based on five or fewer published studies. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Furthermore, only two of these systematic reviews specifically differentiated between ICIs and non‐ICI ITs. 26 , 27 This distinction is essential since ICIs have different mechanisms of action and biologic effects than non‐ICI ITs and in two recent studies, <10% of patients reportedly receiving ITs had received ICIs. 28 , 29 Of note, despite the rapidly increasing number of reports providing data on prior ICI treatment in cancer patients presenting with COVID‐19, the two systematic reviews available so far examining this question included only 10 and 13 published reports respectively. 26 , 27 Therefore, the primary purpose of our systematic review was to analyse studies presenting the adjusted effects specifically of ICI therapy on either survival, a severe event, or need for hospitalisation in cancer patients presenting with COVID‐19.
2. METHODS
This systematic review was prepared using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement on guidance for literature review and data extraction (Supplementary‐File A) and registered with the International Prospective Register of Systematic Reviews on 01/20/2021 (CRD42021222708). Additional methodologic details can be found in the Supplementary‐Methods.
2.1. Literature search and study inclusion
Published studies were retrieved and analysed that provided data on patients with cancer presenting with COVID‐19 and that allowed a within study comparison of patients that had previously received ICI therapy (ICI patients) versus those who had not (non‐ICI patients) regarding survival, severe events, or need for hospitalisation related to COVID‐19. Severe events included either a composite severe event (i.e., any one of several outcomes such as respiratory failure, sepsis, or intensive care unit (ICU) admission defined and reported together as a severe event), development of respiratory failure (including Acute Respiratory Distress Syndrome or need for intubation/mechanical ventilation or non‐mechanical ventilatory support), non‐pulmonary organ failure or sepsis, or need for ICU admission. Using search terms and strategies listed in Supplementary‐File B published studies were identified in the following databases from inception through 5/1/21 without language restrictions: PubMed, EMBASE, Scopus, and Web of Science. Title and abstract followed by full text reviews were conducted by two authors (S.J.M. and P.Q.E.) and disagreements resolved by a third author (P.T.P.). Recovered reports were hand searched for additional studies. Studies were included only when it could be confirmed from the publication or by correspondence with study authors, what the number and outcomes were of patients that had received ICI therapy as opposed to other ITs. Abstracts were not included.
2.2. Data extraction
Two investigators (S.J.M. and P.Q.E.) independently extracted data from reports using a standardised extraction form (Supplementary‐File C). These data, detailed fully in the Supplemental‐Methods, included among others: numbers of patients that had or had not previously received an ICI agent; whether ICIs had been administered alone or with another anti‐cancer therapy; time from last ICI treatment to COVID‐19 diagnosis; patient outcomes comparing ICI versus non‐ICI patients including mortality, severe events and need for hospitalisation; duration and completeness of follow‐up (i.e., proportion of patients follow‐up was available for); whether a study's patient enrolment potentially overlapped with another study; and the methods (model and effect type and variables adjusted for) and results of adjusted analyses performed for the effects of ICI on the outcomes of interest. If more than one type of severe event type was reported in a study, only one was selected for analysis in the following hierarchical order; a composite severe event, development of respiratory failure, non‐pulmonary organ failure or sepsis, or need for ICU admission. When sufficient individual patient data were available in reports for adjusted analysis, these were recorded.
2.3. Quality of evidence and GRADE assessment
Two authors (S.J.M and P.Q.E) independently assessed included studies for quality of evidence using the nine‐point Newcastle‐Ottawa Scale tool (Supplementary‐File D). 30 Disagreements were resolved by a third author (P.T.P.). GRADE analysis was performed to assess certainty of data. 31 Publication bias was assessed by funnel plot and Egger's regression.
2.4. Statistical methods
Results from multivariable analyses were used when presented. Hazard ratio (HR) and relative risk (RR) were converted to odds ratio (95% CIs) (OR) (proportional hazard was assumed for HR). If multivariable analysis was not reported but individual patient data were provided, we performed multivariable logistic regression with these data if they included at least 10 subjects with the less frequent outcome and allowed adjustment for all of the following: age, gender and the presence or absence of hypertension (HTN), diabetes (DM), heart disease and chronic obstructive pulmonary disease (COPD). In one study that reported results from multivariable analyses for both ICI alone and ICI plus another anti‐cancer agent, the results were combined for a single analysis. 32 If neither multivariable analysis or sufficient individual patient data for analysis were provided, unadjusted analysis was performed, and the ORs are presented and analysed. Heterogeneity among studies was assessed using the Q statistic and I2 value. A random effects model was used to estimate the overall effects of ICI therapy in all analyses. Conventional forest plots were prepared, with the size of point estimates proportional to the inverse variance of each estimate. Five subgroup analyses were done using random‐effects meta‐analysis and adjusted and unadjusted results combined that compared studies with: <10 versus ≥10 ICI patients; patients receiving ICI therapy alone versus studies with patients receiving ICIs and another anti‐cancer therapy; either only patients that had received an ICI <60 days before COVID‐19 diagnosis or provided a mean or median time from last treatment to diagnosis of <60 days versus studies that included some or all patients who had received their last ICI within ≥60 days before the COVID‐19 diagnosis; possible overlapping patient enrolments versus without overlap; and Newcastle‐Ottawa scores ≥7 versus <7. 30 These subgroup analyses were based on the following rationales: small studies are inherently at risk for imprecision and prior systematic reviews have excluded studies with <10 patients receiving IT; patients requiring ICIs with other anti‐cancer therapies might have advanced cancer and worsened outcomes; more recent versus more remote exposure to ICIs might affect the risk of immune‐related adverse events; patients included repetitively in more than one study might influence analysed outcomes; risk of bias and study quality might impact study results. Studies not reporting data for these subgroups were not included in the respective sensitivity analysis. We used SAS version 9.4 for the multivariable analysis of individual patient data. Meta‐analyses were conducted using R (version 4.0.3) 33 and packages meta (version 4.16–2) 34 and metaphor (version 2.4–0). 35
3. RESULTS
3.1. Summary of retrieved studies
Of 20,980 retrieved reports, 42 met study inclusion criteria 28 , 29 , 32 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 (Supplementary‐Figure 1). Study authors responded to requests to clarify the numbers and outcomes of IT patients receiving ICI therapy for 13 included reports 28 , 29 , 42 , 43 , 45 , 48 , 49 , 50 , 57 , 61 , 62 , 66 , 67 but not for 13 other reports which were excluded. Published data allowed determination of ICI patient numbers and outcomes in all other included studies.
Table 1 summarises study characteristics including country, centre number, cancer type, COVID‐19 diagnosis‐method, patient location, data source and enrolment dates. Total length of follow‐up ranged from 12 to 218d in studies, but follow‐up duration and completeness were unclear in 18 studies. All studies were observational, 34 were solely retrospective, and 4 included retrospective and prospective patients. Seven studies provided adjusted results for one or more outcome and two studies provided sufficient individual patient data for adjusted analyses. Only four studies specifically focussed on prior ICI therapy alone and two of these reported adjusted outcomes.
TABLE 1.
Study characteristics
| Study [Author(y)ref] | Country | Number of centres | Patient location | Study designdata source | Cancer types studied | COVID‐19 diagnosis criteria | Dates of enrolment | Reported length of follow‐up period (d) | Stated purpose of the study is to examine the effect of ICIs on outcomes | Study provided adjusted or patient level data |
|---|---|---|---|---|---|---|---|---|---|---|
| Albiges (’20) 36 | France | 1 | In/Out | Retro a | Mixed | PCR+/Clin | 3/24/20–4/29/20 | 23 (13, 33) b | No | NR |
| Angelis (’20) 37 | UK | 4 | In/Out | Pro a | Mixed | PCR+ | 3/1/20–4/30/20 | UC | No | NR |
| Assad (’20) 38 | France | 1 | In/Out | Retro a | Mixed | PCR+ | 3/1/20–4/15/20 | 25 c | No | NR |
| Calles (’20) 39 | Spain | 1 | In/Out | Retro a | Lung | PCR+ | 2/24/20–5/12/20 | 30 | No | NR |
| Crolley (’20) 40 | UK | 2 | In/Out | Retro a | Mixed | PCR+/Clin | 3/2/20–5/31/20 | UC | No | NR |
| Dai (’20) 41 | China | 14 | In | Retro a | Mixed | PCR+/Clin | 1/1/20–2/24/20 | 0 d | No | Pt level |
| de Joode (’20) 42 | Netherlands | 45 | In | Retro | Mixed | PCR+/Clin | 3/2/20–5/4/20 | UC | No | NR |
| Di Cosimo (’21) 43 | Italy | 26 | In/Out | Retro a | Mixed | PCR+ | 3/1/20–9/30/20 | 138 (12–218) e | No | NR |
| Fillmore (’21) 44 | US | US VAs | In/Out | Retro | Mixed | PCR+ | 1/1/20–5/4/20 | UC | No | NR |
| Fuentes‐Antras (’20) 45 | Spain | 1 | In | Pro a | Solid | PCR+ | 2/21/20–5/8/20 | UC | No | NR |
| Garassino (’20) 32 | Internat | 87 | In/Out | Pro | Thoracic | PCR+/Clin | 3‐26‐20–4/12/20 | 15 (8–24) f | No | NR |
| Gonzalez‐Cao (’21) 46 | Spain | 26 | In/Out | Retro | Melanoma | PCR+/Clin | 4/1/20–5/17/20 | UC | Yes | NR |
| Goudsmit (’21) 47 | Belgium | 1 | In/Out | Retro a | Mixed | PCR+/Clin | 3/10/20–5/18/20 | UC | No | NR |
| Grivas (’21) 48 | US, Canada | 79 | In/Out | Retro | Mixed | PCR+ | 3/17/20–11/18/20 | 42 (22, 90) b | No | NR |
| Hanna (’21) 49 | US | 2 | In/Out | Retro a | Head/neck | PCR+ | 3/11/20–6/1/20 | UC | No | NR |
| Jee (’21) 50 | US | 1 | In/Out | Retro a | Mixed | PCR+ | 3/8/20–6/2/20 | 28 g | No | NR |
| Kalinsky (’20) 51 | US | 1 | In/Out | Retro a | Breast | PCR+/Clin | 3/10/20–4/29/20 | 26 (1, 38) e | No | NR |
| Klebanov (’21) 52 | US | 1 | In/Out | Retro | Mixed | PCR+ | 3/1/20–6/19/20 | UC | Yes | Adj |
| Lara (’20) 53 | US | 6 | In/Out | Retro a | Gyn | PCR+/Clin | 3/1/20–4/22/20 | UC | No | Adj |
| Lee (’20) 54 | UK | 55 | In/Out | Pro | Mixed | PCR+ | 3/18/20–4/26/20 | 5 (0–38) h | Anti‐cancer Rxs | Adj |
| Luo (’20) 54 | US | 1 | In/Out | Retro a | Lung | PCR+/Clin | 3/12/20–4/13/20 | 14 (7, 23) b | Yes | Adj |
| Mandala (’21) 56 | Italy | 1 | In/Out | Pro/Retro a | Mixed | PCR+ | 3/5/20–5/18/20 | UC | Yes | NR |
| Mehta A. (’21) 28 | India | 1 | In | Retro a | Mixed | PCR+ | 6/8/20–8/20/20 | 63 | No | NR |
| Mehta V. (’20) 57 | US | 1 | In | Retro a | Mixed | PCR+ | 3/18/20–4/8/20 | UC | No | NR |
| Nakamura (’20) 58 | Japan | 1 | In | Retro a | Mixed | PCR+ | 1/31/20–5/25/20 | UC | No | NR |
| Nichetti (’20) 59 | Italy | 1 | In/Out | Pro a | Solid | PCR+/Clin | 2/16/20–4/10/20 | UC | Anti‐cancer Rxs | NR |
| Nie (’20) 60 | China | 12 | In | Retro a | Lung | PCR+ | 1/3/20–5/6/20 | UC | No | NR |
| Pinato (’20) 61 | Germany, Italy, Spain, UK | 19 | In/Out | Retro i | Mixed | PCR+ | 2/26/20–4/1/20 | 19 ± 16 j | No | NR |
| Pinto (’20) 62 | Italy | 4 | In | Retro | Mixed | PCR+ | 2/1/20–4/3/20 | 87 k | No | NR |
| Robilotti (’20) 63 | US | 1 | In/Out | Retro a | Mixed | PCR+ | 3/10/20–5/7/20 | ≥30 | No | Adj |
| Rogado (’20) 64 | Spain | 1 | In/Out | Retro a | Mixed | PCR+/Clin | 2/1/20–4/7/20 | UC | No | NR |
| Russell (’20) 65 | UK | 1 | In/Out | Pro/Retro a | Mixed | PCR+ | 2/29/20–5/12/20 | 37 (18, 49) b | No | NR |
| Singh (’20) 66 | US | 1 | In | Retro a | Mixed | PCR+ | 3/10/20–4/17/20 | 16 k | No | NR |
| Sng (’20) 67 | UK | 1 | In | Retro a | Solid | PCR+ | 3/1/20–5/31/20 | 18 (8–44) e | Anti‐cancer Rxs | Adj |
| Stroppa (’20) 68 | Italy | 1 | In | Retro a | Mixed | PCR+ | 2/21/20–3/18/20 | 15d after DC | No | NR |
| Trapani (’20) 69 | Italy | 1 | In/Out | Retro a | Mixed | PCR+ | 2/1/20–4/2/20 | UC | No | NR |
| Wang (’20) 70 | China | 1 | In | Retro a | Mixed | PCR+ | 12/30/19–3/10/20 | UC | No | NR |
| Wood (’20) 29 | Internat | Multi | In/Out | Retro | Heme | PCR+/Clin | 4/1/20–7/8/20 | UC | No | NR |
| Yarza (’20) 71 | Spain | 1 | In | Pro/Retro a | Solid | PCR+/Clin | 3/9/20–4/19/20 | UC | Anti‐cancer Rxs | Adj |
| Yu (’20) 72 | China | 1 | In | Retro a | Solid | PCR+/Clin | 12/30/19‐2/17/20 | UC | No | NR |
| Zhang, H (’20) 73 | China | 5 | In | Retro a | Mixed | PCR+/Clin | 1/5/20–3/18/20 | UC | No | Pt level |
| Zhang, L (’20) 74 | China | 3 | In | Retro a | Solid | PCR+ | 1/13/20–2/26/20 | UC | No | NR |
Abbreviations: Adj, Adjusted; ASH, American Society of Haematology; CCC19 Reg, COVID‐19 and Cancer Consortium; Clin, patient met clinical criteria for COVID‐19; d, days; DC, discharge; DOCC, Dutch Oncology COVID‐19 Consortium; GEM, Spanish melanoma registry; Heme, haematological malignancies; In, in patient; Internat, international; MGH, Massachusetts General Hospital registry; Mixed, solid and haematological cancers; Multi, multiple centres; Out, out patient; PCR+, patients included based on positive SARS‐CoV‐2 PCR test; PCR+/Clin, patients included based on PCR + test and/or clinical criteria; Pro, prospective; Pt, patient; Ref, reference number; RET, Reggio Emilia Tumour; Retro, retrospective; Rx, therapy; Solid, solid tumours; TERAVOLT Reg, Thoracic Cancers International COVID‐19 Collaboration registry; UC, Unclear; UKCCMP, UK Coronavirus Cancer Monitoring Project; VA CDW, Veterans Administration Corporate Data Warehouse; VA, Veterans Administration.
Chart review.
median and interquartile range.
median.
follow‐up ended on the last day of enrolment.
median and range.
median (and interquartile range) follow‐up since COVID‐19 diagnosis.
follow‐up time period of all survivors.
median time (range) from COVID‐19 diagnosis to the time endpoints met.
OnCOVID Reg.
mean and standard deviation.
follow‐up past final enrolment.
3.2. Quality of evidence
Of the 42 studies analysed, 19 had Newcastle‐Ottawa scale scores of ≥7 and 23 had scores of <7 (Supplementary‐Table 1). Among the 19 studies with higher scores, only two received a score of 9, while 14 did not appear to provide a 30d follow‐up or were not clear whether follow‐up of all patients was complete, and 4 studies did not control for cancer type or other factors potentially influencing comparisons of ICI versus non‐ICI patients. All 9 studies that provided adjusted outcomes or individual patient data for adjusted analysis had scores ≥7. Scores <7 in studies were due to both inadequate comparability of study groups and unclear or insufficient follow up.
3.3. Mortality
Thirty‐eight studies provided data allowing comparison of mortality in ICI versus non‐ICI patients (Figure 1, Supplementary‐Table 2). Six of these included adjusted outcomes, and two reported sufficient individual patient data to perform adjusted analyses (Tables 1 and 2). Three of these eight studies included >10 ICI patients. Studies adjusted for seven or fewer variables (Table 2). The most common variables included age, gender, HTN, COPD, DM, and heart disease.
FIGURE 1.
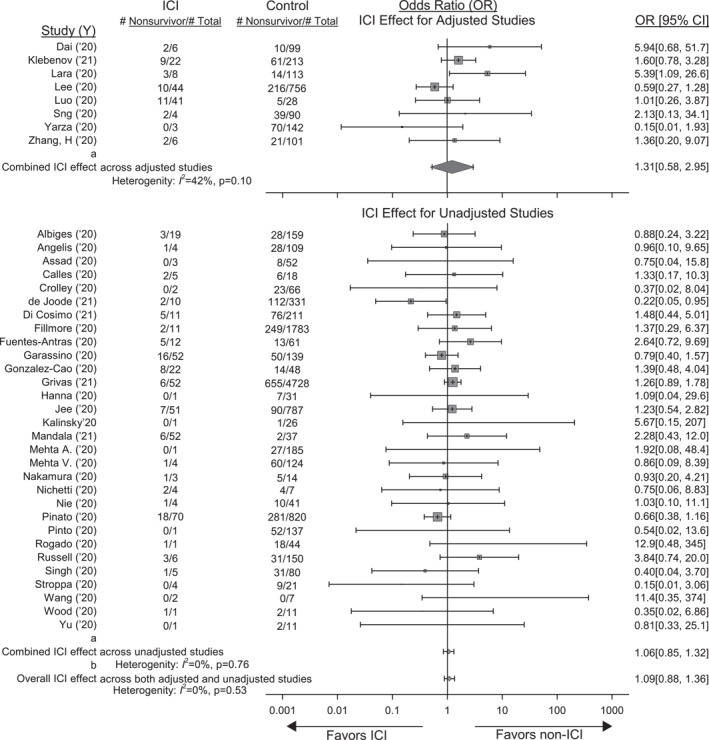
Forrest plot showing the effect of prior immune checkpoint inhibitor (ICI) therapy on the odds ratio [OR (95% CI)] of death in studies providing data on cancer patients presenting with COVID‐19 that had or had not previously received ICIs. Also shown are the total numbers of patients (Total) and the number of non‐survivors (NS) in patients that had or not previously received ICI. Shown at the top are the adjusted effects of ICIs on the OR of death in the eight studies reporting these data and the combined OR and I2 value (random effects model). See Table 2 for the models, variables and effect types reported in these studies. Effects were converted to the OR of death for all studies as described in the methods. Shown at the bottom are the unadjusted effects of ICIs on the OR of death from 30 studies that provided data allowing this calculation and the combined OR and I2 value. The effects of ICIs did not differ significantly (p = 0.56) comparing studies with adjusted and unadjusted results and the overall OR of death and I2 value for all 38 studies is shown at the very bottom of the figure
TABLE 2.
Summary of effect type, model, ICI patient numbers and variables included in studies presenting adjusted outcome analysis data or used in analysis of individual patient data from studies for either survival, severe events, or need for hospitalisation
| Author(y)Ref | Effect type | Model | ICI patient number | Variables included in multivariate analysis |
|---|---|---|---|---|
| Survival | ||||
| Studies providing adjusted outcome data | ||||
| Klebanov (’21) 52 | OR | Logistic regression | 22 | Age, gender, race, cancer category, CCI severity grade, median income, local infection |
| Lara (’20) 53 | RR | Poisson regression | 8 | Age, race, number of comorbidities, performance status, smoking history |
| Lee (’20) 54 | OR | Logistic regression | 44 | Age, gender, DM, HTN, COPD, other comorbidities |
| Luo (’20) 55 | OR | Logistic regression | 40 | Gender, smoking history |
| Sng (’20) 67 | HR | Cox proportional hazards | 4 | Age, HTN, CVD |
| Yarza (’20) 71 | OR | Logistic regression | 8 | Age, HTN, CVD |
| Studies providing individual patient data | ||||
| Dai (’20) 41 | OR | Logistic regression | 6 | Age, gender, DM, HTN, COPD, other comorbidities |
| Zhang, H (’20) 73 | OR | Logistic regression | 6 | Age, gender, DM, HTN, COPD, other comorbidities |
| Severe events | ||||
| Studies providing adjusted outcome data | ||||
| Luo (’20) 55 , a | OR | Logistic regression | 40 | Gender, smoking history |
| Robilotti (’20) 63 , b | HR | Logistic regression | 31 | Age, sex, race, DM, HTN, CVD, COPD/asthma, and 9 others c |
| Yarza (’20) 71 , b | OR | Logistic regression | 8 | Age, HTN, CVD |
| Studies providing individual patient data | ||||
| Dai (’20) 41 , a | OR | Logistic regression | 6 | Age, gender, DM, HTN, COPD, other comorbidities |
| Zhang, H (’20) 73 , a | OR | Logistic regression | 6 | Age, gender, DM, HTN, COPD, other comorbidities |
| Need for hospitalisation | ||||
| Studies providing adjusted outcome data | ||||
| Luo (’20) 55 | OR | Logistic regression | 40 | Gender, smoking history |
| Robilotti (’20) 63 | OR | Logistic regression | 31 | Age, sex, race, DM, HTN, CVD, COPD/asthma, and 9 others c |
Abbreviations: CCI, Charlson Comorbidity Index; CVD, cardiovascular disease; HR, hazards ratio; OR, odds ratio; RR, relative risk.
Composite severe event.
Respiratory failure severe event.
Chronic kidney disease, BMI, smoking status, cancer type and metastases, major surgery ≤30d, systemic chemo ≤30d, chronic lymphopenia, chronic steroids, ICI ≤ 90d.
The effects of ICI therapy on the odds ratios (95% CI) (OR) of death across the eight studies with adjusted results were not significant [combined OR 1.31 (0.58; 2.95), p = 0.46] although there was moderate but non‐significant heterogeneity (I 2 = 42%, p = 0.10) (Figure 1). ICI's effects on the OR of death across the 30 studies with unadjusted outcome data were also not significant [1.06 (0.85; 1.32), p = 0.58] but were consistent (I 2 = 0%; p = 0.76)]. Adjusted versus unadjusted effects did not differ (p = 0.56) and over the 38 studies were not significant [1.09 (0.88;1.36) p = 0.41; I 2 = 0%, p = 0.53)].
Characteristics from individual studies employed in four of the five sensitivity analyses are shown in Supplementary‐Table 3. Data on the Newcastle Ottawa score is noted above. As shown in Table 3, the OR of death was increased in studies where some or all patients received ICI therapy within ≥60d before COVID‐19 diagnosis versus patients only receiving ICI therapy <60d or a mean or median time to treatment <60d before COVID‐19 diagnosis (p = 0.04). Study subgroups did not differ significantly in the other four sensitivity analyses (p = 0.29–0.46).
TABLE 3.
Results of sensitivity analyses
| Mortality | ||
| Studies (n) with <10 patients versus ≥10 patients | ||
| <10 patients (n = 24) | ≥10 patients (n = 14) | p = 0.29 |
| 1.36 (0.85, 2.18) (I 2 = 0%) | 1.03 (0.78, 1.36) (I 2 = 18%) | |
| Studies (n) with ICI given within <60d versus ≥60d a | ||
| <60d (n = 18) b | ≥60d (n = 12) b | p = 0.04 |
| 0.86 (0.60, 1.23) (I 2 = 18%) | 1.26 (1.05, 1.53) (I 2 = 0%) | |
| Studies (n) with ICI alone versus ICI + other a | ||
| ICI alone (n = 9) c | ICI + other (n = 17) c | p = 0.39 |
| 1.58 (0.82, 3.04) (I 2 = 0%) | 1.18 (0.80, 1.76) (I 2 = 7%) | |
| Studies (n) with potential overlap versus no potential overlap | ||
| No overlap (n = 27) | Overlap (n = 11) | p = 0.42 |
| 1.23 (0.98, 1.53) (I 2 = 0%) | 1.01 (0.62, 1.64) (I 2 = 29%) | |
| Studies (n) with NOS ≥ 7 versus < 7 | ||
| ≥7 (n = 15) | <7 (n = 23) | p = 0.46 |
| 1.25 (0.78, 1.99) (I 2 = 11%) | 1.04 (0.81, 1.33) (I 2 = 0%) | |
| Severe events | ||
| Studies (n) with <10 patients versus ≥10 patients | ||
| <10 patients (n = 14) | ≥10 patients (n = 10) | p = 0.83 |
| OR 1.35 (0.72, 2.55) (I 2 = 0%) | OR 1.25 (0.77, 2.03) (I 2 = 53%) | |
| Studies (n) with ICI given within <60d versus ≥60d a | ||
| <60d (n = 15) d | ≥60d (n = 8) d | p = 0.61 |
| 1.19 (0.81, 1.75) (I 2 = 0%) | 1.42 (0.72, 2.82) (I 2 = 62%) | |
| Studies (n) with ICI alone versus ICI + other a | ||
| ICI alone (n = 7) e | ICI + other (n = 12) e | p = 0.83 |
| 1.89 (0.95, 3.76) (I 2 = 0%) | 1.76 (1.18, 2.63) (I 2 = 0%) | |
| Studies (n) with potential overlap versus no potential overlap | ||
| No overlap (n = 16) | Overlap (n = 8) | p = 0.02 |
| 0.88 (0.55, 1.41) (I 2 = 2%) | 1.66 (1.11, 2.47) (I 2 = 0%) | |
| Studies (n) with NOS ≥ 7 versus < 7 | ||
| ≥7 (n = 13) | <7 (n = 11) | p = 0.33 |
| 1.54 (0.80, 2.96) (I 2 = 19%) | 1.10 (0.75, 1.62) (I 2 = 22%) | |
| Need for hospitalisation | ||
| Studies (n) with < 10 patients versus ≥10 patients | ||
| <10 patients (n = 8) | ≥10 patients (n = 7) | p = 0.91 |
| 1.17 (0.54, 2.53) (I 2 = 0%) | 1.23 (0.71, 2.11) (I 2 = 47%) | |
| Studies (n) with ICI given within <60d versus ≥60d a | ||
| <60d (n = 5) d | ≥60d (n = 9) d | p = 0.22 |
| 1.67 (0.64, 4.38) (I 2 = 60%) | 1.05 (0.74, 1.50) (I 2 = 0%) | |
| Studies (n) with ICI alone versus ICI + other a | ||
| ICI alone (n = 7) f | ICI + other (n = 6) f | p = 0.02 |
| 2.22 (1.08, 4.55) (I 2 = 0%) | 1.06 (0.77, 1.45) (I 2 = 0%) | |
| Studies (n) with potential overlap versus no potential overlap | ||
| No overlap (n = 10) | Overlap (n = 5) | p = 0.03 |
| 0.88 (0.68, 1.14) (I 2 = 58%) | 1.50 (0.82, 2.75) (I 2 = 0%) | |
| Studies (n) with NOS ≥ 7 versus < 7 | ||
| ≥7 (n = 8) | 7 (n = 7) | p = 0.85 |
| 1.20 (0.62, 2.31) (I 2 = 4%) | 1.12 (0.70, 1.81) (I 2 = 0%) | |
Abbreviations: NA, not applicable; NOS, Newcastle Ottawa score; NR, not reported.
See text for criteria subgroups were based on.
data not reported in 8 studies with this outcome.
data not reported in 12 studies with this outcome.
data not reported in 1 study with this outcome.
data not reported 5 studies with this outcome.
data not reported in 2 studies with this outcome.
3.4. Severe events
Twenty‐four studies provided data comparing severe events in ICI versus non‐ICI patients including a composite severe event (see methods) in 17 studies, respiratory failure in 4 studies, and ICU admission for 3 studies (Figure 2, Supplementary‐Table 2). Three studies reported adjusted outcomes, and two included sufficient individual patient data to perform adjusted analyses. Two of these studies had ≥10 ICI patients. While one study adjusted for up to 16 variables, the other 4 studies included the number and types of variables as in the survival analysis (Table 2).
FIGURE 2.
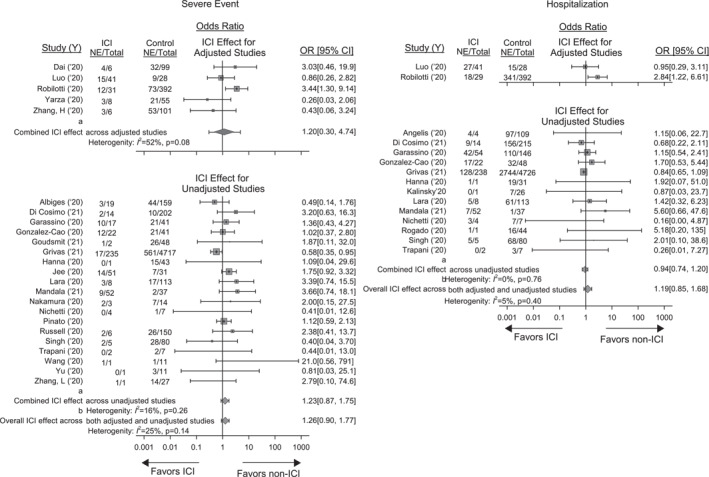
Forrest plots showing the effect of prior immune checkpoint inhibitor (ICI) therapy on the odds ratio [OR (95% CI)] of severe events and need for hospitalisation in studies providing data on cancer patients presenting with COVID‐19 that had or had not previously received ICIs. Also shown for each outcome are the total numbers of patients (Total) and the number of patients with either event (NE) that had or not previously received ICI. For severe events, shown at the top are the adjusted effects of ICIs on the OR of a severe event in five the studies reporting these data and the combined adjusted OR and I2 value (random effects model). See Table 2 for the models, variables and effect types reported in these studies. Effects were converted to the OR of a severe event for all studies as described in the methods. Shown at the bottom are the unadjusted effects of ICIs on the OR of a severe event from 19 studies that provided data allowing this calculation and the combined OR and I2 value. The effects of ICIs did not differ significantly (p = 0.96) comparing studies with adjusted and unadjusted results and the overall OR and I2 value of a severe event for all 24 studies is shown at the very bottom of the panel. For hospitalisation, only 2 studies reported the adjusted effects of ICIs on the need for this and no combined OR was calculated for these two. Thirteen studies provided unadjusted OR for hospitalisation and the combined OR and I2 value for these studies are shown. The adjusted effects of ICs in the two studies did not differ significantly (p = 0.25) from the unadjusted effects from the 13 studies that allowed this calculation and the overall effect of ICIs on the OR (95%CI) and I2 value of hospitalisation for all 15 studies is shown at the very bottom of the panel
The effects of ICI therapy on OR of severe events across the five studies with adjusted outcome results were not significant but had moderate heterogeneity that did not reach significance [1.20 (0.30; 4.74), p = 0.73; I 2 = 52%; p = 0.08)]. Across the 19 studies with unadjusted outcome data, ICI effects on the OR of severe events were also not significant [(OR 1.23 (0.87; 1.75), p = 0.23; I 2 = 16%, p = 0.26). ICI effects did not differ comparing adjusted versus unadjusted studies (p = 0.96) and, when combined across all 24 studies, the OR of severe events with ICIs was still not significant although there was moderate heterogeneity [(1.26 (0.90, 1.77), p = 0.16; I 2 = 25%, p = 0.14]. Sensitivity analyses were performed on the same subgroups as for mortality (Table 3, Supplementary‐Table 3). In studies with possible overlapping patent enrolments, the OR of severe events was increased (p = 0.02) in ICI patients. Study subgroups did not differ significantly in the remaining four sensitivity analyses (p = 0.33–0.83).
3.5. Hospitalisation
Fifteen studies provided data comparing need for hospitalisation in ICI versus non‐ICI patients (Figure 2, Supplementary‐Table 2). Only two studies included adjusted outcome results, and none provided individual patient data for adjusted analysis. Therefore, adjusted and unadjusted results were analysed together. Across the 15 studies, ICI therapy was not associated with a significant effect on the OR of hospitalisation [1.19 (0.85; 1.68), p = 0.29; I 2 = 5%, p = 0.40]. In sensitivity analyses, ICI therapy had an increased effect on the OR of hospitalisation in studies with ICI therapy alone versus studies with ICI therapy with another anti‐cancer treatment (p = 0.02) and studies with versus without possible overlapping patient enrolment (p = 0.03) but not the other 3 analyses (p = 0.22–0.91) (Table 3, Supplementary‐Table 3).
3.6. Certainty of evidence (GRADE) and publication bias
Because all studies analysed here were observational and most were retrospective, the overall certainty of evidence presented starts at a low level. 75 Certainty of evidence was further downgraded to very low, based on risk of bias, inconsistency, indirectness, and/or imprecision, for four of the five GRADE criteria for each of the three outcomes assessed (Supplementary‐Table 4). Overall, a high proportion of studies (23 of 42%, 55%) had Newcastle‐Ottawa scale scores <7 and a potential for risk of bias (Supplementary‐Table 1). There was moderate or greater heterogeneity (I 2 ≥ 42%) among adjusted studies for survival and severe events. Overall, results were indirect since only 4 studies (10%) specifically examined the impact of ICI therapy on outcomes, 46 , 52 , 55 , 56 and 13 studies required clarification from authors regarding the number and outcomes of patients receiving ICIs among all IT patients. 28 , 29 , 42 , 43 , 45 , 48 , 49 , 50 , 57 , 61 , 62 , 66 , 67 Finally, these findings are imprecise since only 1 study included >100 ICI patients while 37 had <50 and 26 had <10 ICI patients. Based on funnel plots and Egger's regression analysis (p = 0.66, 0.31 and 0.19 for mortality, severe events and hospitalisation respectively) the outcome results did not appear subject to publication bias (Supplementary‐Figure 2).
4. DISCUSSION
In this systematic review and meta‐analysis, across 9 studies presenting adjusted outcome data and 33 studies with unadjusted results, there was no clear evidence that prior ICI therapy altered survival, the incidence of severe events or need for hospitalisation in cancer patients presenting with COVID‐19. Sensitivity analyses examining ICI effects based on 5 variables were inconclusive. While eight other published systematic reviews have examined IT in this patient group, the present one has notable strengths: it is the most recent study; it included three to four times the number of reports in the final analysis of mortality and severe events; it examined two times the number of published studies with adjusted outcome data and almost five times the number of studies with ICI patients alone (Supplementary‐Table 5). 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Unfortunately, GRADE analysis demonstrated that even with this increasing body of data the certainty of evidence regarding the effects of ICIs in cancer patients with COVID‐19 is very low.
The literature has repeatedly emphasised the need to determine whether prior ICI therapy impacts outcomes in cancer patients with COVID‐19. 4 , 5 , 6 , 7 , 8 When treating patients with COVID‐19 pneumonia, any theoretical anti‐viral effect that ICIs exert must be weighed against the well documented immune‐related adverse events, including pneumonitis, that ICIs can produce. 6 , 11 , 12 , 13 , 14 , 15 There is growing concern of probable synergy, or an inability to distinguish between COVID‐19 pneumonitis and pneumonitis as an adverse event ICI therapy. 76 Additionally, as a result of reinvigorated T‐cells, patients on ICIs might have an increased risk of cytokine release syndrome, an important cause of mortality in COVID‐19. 77 If harmful, besides implications for discontinuation of ICI therapy, this determination would influence the management of worsening organ injury in infected patients, such as possibly supporting earlier corticosteroid use for pneumonitis. 78 Alternatively, if ICIs exert a beneficial host defence effect as proposed for other viral infections, continued or even initiation of ICIs might be considered. 11 , 12 , 13 , 14
The present study highlights the weaknesses in currently available data to assess the impact of ICI therapy on COVID‐19 outcomes. Thirty‐eight of the studies analysed were solely or partially retrospective ones. Only four studies specifically examined the effects of ICIs on the outcomes of cancer patients with COVID‐19. Only one study included more than 100 ICI patients and 26 (62%) had <10 patients. Despite the complexity of cancer patients with COVID‐19, eight of the nine studies providing adjusted outcome data controlled for seven or fewer variables. There were not sufficient data to examine the effects of specific ICI regimens. While sensitivity analysis suggested some associations with timing of ICI treatment, treatment regimen, and studies with potential overlapping patient enrolment, these findings were not consistent across all three outcomes and are difficult to interpret.
Overall, the present analysis indicates that more comprehensive observational studies will be required to determine how ICIs impact COVID‐19 outcomes in cancer patients. Such databases would need to contain detailed patient‐level data and clearly differentiate ICI from non‐ICI IT‐treated patients. The databases would have to reliably assess the impact of a range of covariates confounding interpretation of the effects of ICIs including among others: type and stage of cancer, presence of other anti‐cancer therapies, and patient performance status; the certainty, duration and severity of COVID‐19 itself; the specific type, mechanism of action, and regimen of the ICIs being used; information on a range of non‐cancer variables like those in the Charlson co‐morbidity index 79 ; and comprehensive assessment of outcomes and patient follow‐up. Power analysis, possibly based on the known ICI adverse event rate in uninfected cancer patients, would be necessary.
There are growing databases and registries that could provide stronger estimates of the effects of prior ICI therapy in cancer patients with COVID‐19. Both national and international registries focussed primarily on cancer patients such as The Thoracic Cancers International COVID‐19 Collaboration might provide the most informative results. 80 More general registries like the US Veterans Administration's National Database could also be used. 81 Early data from several of these sources were included in the present analysis. 29 , 32 , 42 , 44 , 46 , 48 , 52 , 54 , 61
On the one hand, the persistence of SARS‐CoV‐2 variants and continued high infection rates coupled with the widespread use of ICIs for cancer emphasises the importance of understanding how prior ICI treatment alters COVID‐19 outcomes in cancer patients. But these circumstances may provide a unique opportunity to understand whether ICIs should be considered for the treatment of other types of acute or chronic infections. Therapy with ICIs has been proposed for infections as varied as malaria, HIV and HBV. 82 While several trials directly assessing the effects ICIs in COVID‐19 have been planned (NCT04413838, NCT04356508, NCT04268537, NCT04268537, NCT04333914), results are not available. But a comprehensive assessment of the effects of ICI therapy in COVID‐19 cancer patients might prove informative regarding their therapeutic value for this and other types of viral infection.
There are several limitations to this study. First, although our search criteria were broad and resulted in over 20,000 reports, in this rapidly evolving field it is possible that very recent studies were overlooked. Second, our search did not include pre‐print literature which had not yet undergone peer‐review. Third we restricted our review to English publications. Finally, despite attempts to contact authors to clarify patient numbers, outcomes and types of IT therapy investigated, 13 studies had to be excluded from analysis as there was no response to these communications.
In conclusion, while vaccination has reduced COVID‐19 in some counties, the infection remains a pressing health problem in large parts of the world. Even with more widespread vaccination, SARS‐CoV‐2 and its variants will remain a health threat well into the future, especially for patients with cancer. Given the effectiveness and need for ICI therapy in many cancer patients, it is essential to understand with the most credible evidence how ICIs impact outcome when these patients develop COVID‐19.
CONFLICTS OF INTEREST
The authors declare no competing interests.
ETHICS STATEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
Parizad Torabi‐Parizi and Samuel J. Minkove conceived and designed the study with contributions from Junfeng Sun and Peter Q. Eichacker. Diane Cooper conducted the initial search and contributed to the manuscript. Parizad Torabi‐Parizi, Samuel J. Minkove and Peter Q. Eichacker reviewed search results and Samuel J. Minkove, Xizhong Cui, Yan Li and Peter Q. Eichacker extracted data. Parizad Torabi‐Parizi, Junfeng Sun, Samuel J. Minkove, and Peter Q. Eichacker wrote and edited the manuscript. All authors reviewed and approved the final version of this manuscript for submission.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Supplementary Material S1
Supplementary Material S2
Supplementary Material S3
Supplementary Material S4
Supplementary Material S5
Supplementary Material S6
Supplementary Material S7
Supplementary Material S8
Supplementary Material S9
Supplementary Material S10
Supplementary Material S11
Supplementary Material S12
ACKNOWLEDGEMENTS
The National Institutes of Health (NIH) had no role in the design of the study or the collection, analysis, and interpretation of the data. The opinions expressed in this manuscript reflect those of the authors and do not reflect the views of the NIH, the Department of Health and Human Services, or of the United States Government. The authors would like to acknowledge key feedback and suggestions provided by Karen Goldstein, M.D., and Andrzej Kosinski, Ph.D. of the Duke University Department of Medicine and Department of Biostatistics and Bioinformatics. We thank the following authors for correspondence and clarification of the primary data: Karlijn de Joode, M.D.; Serena Di Cosimo, MD; Jesus Fuentes Antrás, MD; Anouk Goudsmit M.D.; Petros Grivas M.D. PhD.; Glenn Hanna, M.D.; Anurag Mehta M.B.B.S, M.D; Vikas Mehta MD MPH; David Pinato, M.D. PhD.; Carmine Pinto, M.D.; Mieke Van Hemelrijck, PhD; Sunny R K Singh, MD; Christopher CT Sng MRCP PGCert (Ed.) FHEA; William Wood, M.D. MPH. Intra‐mural funding from the NIH supported this work.
Minkove SJ, Sun J, Li Y, et al. Comprehensive adjusted outcome data are needed to assess the impact of immune checkpoint inhibitors in cancer patients with COVID‐19: results of a systematic review and meta‐analysis. Rev Med Virol. 2022;32(5):e2352. 10.1002/rmv.2352
Contributor Information
Samuel J. Minkove, Email: samuel.minkove@nih.gov.
Parizad Torabi‐Parizi, Email: torabiparizip@cc.nih.gov.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee AJX, Purshouse K. COVID‐19 and cancer registries: learning from the first peak of the SARS‐CoV‐2 pandemic. Br J Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meng Y, Lu W, Guo E, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID‐19 patients: a propensity score‐matched analysis. J Hematol Oncol. 2020;13(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bersanelli M. Controversies about COVID‐19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12(5):269‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garassino MC, Ribas A. At the crossroads: COVID‐19 and immune‐checkpoint blockade for cancer. Cancer Immunol Res. 2021;9(3):261‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossi E, Schinzari G, Tortora G. Pneumonitis from immune checkpoint inhibitors and COVID‐19: current concern in cancer treatment. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan RJ, Johnson DB, Rini BI, et al. COVID‐19 and immune checkpoint inhibitors: initial considerations. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kattan J, Kattan C, Assi T. Do checkpoint inhibitors compromise the cancer patients' immunity and increase the vulnerability to COVID‐19 infection? Immunotherapy. 2020;12(6):351‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li B, Chan HL, Chen P. Immune checkpoint inhibitors: basics and challenges. Curr Med Chem. 2019;26(17):3009‐3025. [DOI] [PubMed] [Google Scholar]
- 10. Robert C. A decade of immune‐checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao P, Lazare C, Cao C, et al. Immune checkpoint inhibitors in the treatment of virus‐associated cancers. J Hematol Oncol. 2019;12(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gay CL, Bosch RJ, Ritz J, et al. Clinical trial of the anti‐PD‐L1 antibody BMS‐936559 in HIV‐1 infected participants on suppressive antiretroviral therapy. J Infect Dis. 2017;215(11):1725‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frebel H, Nindl V, Schuepbach RA, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209(13):2485‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID‐19 infections. JCI Insight. 2020;5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dipasquale A, Persico P, Lorenzi E, Rahal D, Santoro A, Simonelli M. COVID‐19 lung injury as a primer for immune checkpoint inhibitors (ICIs)‐related pneumonia in a patient affected by squamous head and neck carcinoma treated with PD‐L1 blockade: a case report. J Immunother Cancer. 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID‐19: a systematic review and meta‐analysis. J Infect. 2020;81(2):e93‐e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID‐19‐related hospitalization, intensive Care unit admission, invasive mechanical ventilation, and death ‐ United States, march‐december 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age‐specific mortality and immunity patterns of SARS‐CoV‐2. Nature. 2021;590(7844):140‐145. [DOI] [PubMed] [Google Scholar]
- 19. Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID‐19. SN Compr Clin Med. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li P, Li L, Wang S, Liu Y, Li Z, Xia S. Effect of antitumor therapy on cancer patients infected by SARS‐CoV‐2: a systematic review and meta‐analysis. Cancer Med. 2021;10(5):1644‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Lu H, Wang W, Liu Q, Zhu C. Clinical risk factors for mortality in patients with cancer and COVID‐19: a systematic review and meta‐analysis of recent observational studies. Expert Rev Anticancer Ther. 2021;21(1):107‐119. [DOI] [PubMed] [Google Scholar]
- 22. Park R, Lee SA, Kim SY, de Melo AC, Kasi A. Association of active oncologic treatment and risk of death in cancer patients with COVID‐19: a systematic review and meta‐analysis of patient data. Acta Oncol. 2021;60(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 23. Wang B, Huang Y. Immunotherapy or other anti‐cancer treatments and risk of exacerbation and mortality in cancer patients with COVID‐19: a systematic review and meta‐analysis. OncoImmunology. 2020;9(1):1824646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yekeduz E, Utkan G, Urun Y. A systematic review and meta‐analysis: the effect of active cancer treatment on severity of COVID‐19. Eur J Cancer. 2020;141:92‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID‐19‐infected cancer patients: a systematic review and meta‐analysis. J Natl Cancer Inst. 2021;113(4):371‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazarus G, Budiman RA, Rinaldi I. Does immune checkpoint inhibitor increase the risks of poor outcomes in COVID‐19‐infected cancer patients? A systematic review and meta‐analysis. Cancer Immunol Immunother. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian W, Ye Y, Zuo L, et al. Immune checkpoint inhibitors use and effects on prognosis of COVID‐19 infection: a systematic review and meta‐analysis. Immunotherapy. 2021;13(15):1271‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta A, Vasudevan S, Parkash A, Sharma A, Vashist T, Krishna V. COVID‐19 mortality in cancer patients: a report from a tertiary cancer centre in India. PeerJ. 2021;9:e10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood WA, Neuberg DS, Thompson JC, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a report from the ASH research collaborative data hub. Blood Adv. 2020;4(23):5966‐5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. 2013,. 2013. [Google Scholar]
- 31. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garassino MC, Whisenant JG, Huang LC, et al. COVID‐19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry‐based, cohort study. Lancet Oncol. 2020;21(7):914‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. R Core Team . (2020). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing V. https://www.R‐project.org/ [Google Scholar]
- 34. Balduzzi SRG, Schwarzer G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evidence‐Based Mental Health. 2019;22:153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viechtbauer WCm‐aiRwtmpJoSS, 36(3), 1‐48. https://www.jstatsoft.org/v36/i03/ [Google Scholar]
- 36. Albiges L, Foulon S, Bayle A, et al. Determinants of the outcomes of patients with cancer infected with SARS‐CoV‐2: results from the Gustave Roussy cohort. Nature Cancer. 2020;1:965‐975. [DOI] [PubMed] [Google Scholar]
- 37. Angelis V, Tippu Z, Joshi K, et al. Defining the true impact of coronavirus disease 2019 in the at‐risk population of patients with cancer. Eur J Cancer. 2020;136:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Assaad S, Avrillon V, Fournier ML, et al. High mortality rate in cancer patients with symptoms of COVID‐19 with or without detectable SARS‐COV‐2 on RT‐PCR. Eur J Cancer. 2020;135:251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calles A, Aparicio MI, Alva M, et al. Outcomes of COVID‐19 in patients with lung cancer treated in a tertiary hospital in madrid. Front Oncol. 2020;10:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crolley VE, Hanna D, Joharatnam‐Hogan N, et al. COVID‐19 in cancer patients on systemic anti‐cancer therapies: outcomes from the CAPITOL (COVID‐19 Cancer PatIenT Outcomes in North London) cohort study. Ther Adv Med Oncol. 2020;12:1758835920971147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS‐CoV‐2: a multicenter study during the COVID‐19 outbreak. Cancer Discov. 2020;10(6):783‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Joode K, Dumoulin DW, Tol J, et al. Dutch Oncology COVID‐19 consortium: outcome of COVID‐19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Di Cosimo S, Tagliaferri B, Generali D, et al. Baseline characteristics and outcomes of cancer patients infected with SARS‐CoV‐2 in the Lombardy region, Italy (AIOM‐L CORONA): a multicenter, observational, ambispective, cohort study. Cancers (Basel). 2021;13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fillmore NR, La J, Szalat RE, et al. Prevalence and outcome of COVID‐19 infection in cancer patients: a national Veterans affairs study. J Natl Cancer Inst. 2021;113(6):691‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fuentes‐Antras J, Manzano A, Marquina G, et al. A snapshot of COVID‐19 infection in patients with solid tumors. Int J Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gonzalez‐Cao M, Carrera C, Rodriguez Moreno JF, et al. COVID‐19 in melanoma patients: results of the Spanish melanoma group registry, GRAVID study. J Am Acad Dermatol. 2021;84(5):1412‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goudsmit A, Cubilier E, Meert AP, et al. Factors associated with SARS‐CoV‐2 infection and outcome in patients with solid tumors or hematological malignancies: a single‐center study. Support Care Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grivas P, Khaki AR, Wise‐Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID‐19 severity among patients with cancer: a report from the COVID‐19 and Cancer Consortium. Ann Oncol. 2021;32(6):787‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanna GJ, Rettig EM, Park JC, et al. Hospitalization rates and 30‐day all‐cause mortality among head and neck cancer patients and survivors with COVID‐19. Oral Oncol. 2021;112:105087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jee J, Stonestrom AJ, Devlin S, et al. Oncologic immunomodulatory agents in patients with cancer and COVID‐19. Sci Rep. 2021;11(1):4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kalinsky K, Accordino MK, Hosi K, et al. Characteristics and outcomes of patients with breast cancer diagnosed with SARS‐Cov‐2 infection at an academic center in New York City. Breast Cancer Res Treat. 2020;182(1):239‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klebanov N, Pahalyants V, Murphy WS, et al. Risk of COVID‐19 in patients with cancer receiving immune checkpoint inhibitors. Oncologist. 2021;26(5):e898‐e901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lara OD, O'Cearbhaill RE, Smith MJ, et al. COVID‐19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126(19):4294‐4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee LY, Cazier JB, Angelis V, et al. COVID‐19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD‐1 blockade on severity of COVID‐19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mandala M, Lorigan P, De Luca M, et al. SARS‐CoV‐2 infection and adverse events in patients with cancer receiving immune checkpoint inhibitors: an observational prospective study. J Immunother Cancer. 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID‐19 in a New York hospital system. Cancer Discov. 2020;10(7):935‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura S, Kanemasa Y, Atsuta Y, et al. Characteristics and outcomes of coronavirus disease 2019 (COVID‐19) patients with cancer: a single‐center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. 2021;26(3):485‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nichetti F, Bini M, Ambrosini M, et al. COVID‐19 risk for patients undergoing anticancer treatment at the outpatient clinic of the National Cancer Institute of Milan: the COVINT study. ESMO Open. 2020;5(Suppl 3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nie L, Dai K, Wu J, et al. Clinical characteristics and risk factors for in‐hospital mortality of lung cancer patients with COVID‐19: a multicenter, retrospective, cohort study. Thorac Cancer. 2021;12(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pinato DJ, Zambelli A, Aguilar‐Company J, et al. Clinical portrait of the SARS‐CoV‐2 epidemic in European cancer patients. Cancer Discov. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pinto C, Berselli A, Mangone L, et al. SARS‐CoV‐2 positive hospitalized cancer patients during the Italian outbreak: the cohort study in Reggio Emilia. Biology (Basel). 2020;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID‐19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rogado J, Obispo B, Pangua C, et al. Covid‐19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;22(12):2364‐2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Russell B, Moss C, Papa S, et al. Factors affecting COVID‐19 outcomes in cancer patients: a first report from guy's cancer center in London. Front Oncol. 2020;10:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh SRK, Thanikachalam K, Jabbour‐Aida H, Poisson LM, Khan G. COVID‐19 and cancer: lessons learnt from a Michigan hotspot. Cancers (Basel). 2020;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sng CCT, Wong YNS, Wu A, et al. Cancer history and systemic anti‐cancer therapy independently predict COVID‐19 mortality: a UK tertiary hospital experience. Front Oncol. 2020;10:595804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stroppa EM, Toscani I, Citterio C, et al. Coronavirus disease‐2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol. 2020;16(20):1425‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Trapani D, Marra A, Curigliano G. The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region. Eur J Cancer. 2020;132:199‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang J, Zhang J, Tu Y, et al. Cancer patients in SARS‐CoV‐2 infection: a single‐center experience from Wuhan. J Cancer. 2020;11(21):6243‐6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yarza R, Bover M, Paredes D, et al. SARS‐CoV‐2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020;135:242‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu J, Ouyang W, Chua MLK, Xie C. SARS‐CoV‐2 transmission in patients with cancer at a tertiary Care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang H, Wang L, Chen Y, et al. Outcomes of novel coronavirus disease 2019 (COVID‐19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;126(17):4023‐4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). In: www.training.cochrane.org/handbook Cochrane, 2021.
- 76. Pezeshki PS, Rezaei N. Immune checkpoint inhibition in COVID‐19: risks and benefits. Expert Opin Biol Ther. 2021;21(9):1173‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor‐related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune‐related adverse events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep. 2020;22(4):39. [DOI] [PubMed] [Google Scholar]
- 79. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 80. Whisenant JG, Trama A, Torri V, et al. TERAVOLT: thoracic cancers international COVID‐19 collaboration. Cancer Cell. 2020;37(6):742‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. US Veterans Administration’s National Database In:2021. [Google Scholar]
- 82. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018;18(2):91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Supplementary Material S2
Supplementary Material S3
Supplementary Material S4
Supplementary Material S5
Supplementary Material S6
Supplementary Material S7
Supplementary Material S8
Supplementary Material S9
Supplementary Material S10
Supplementary Material S11
Supplementary Material S12
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


