Abstract
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), a highly infectious agent associated with unprecedented morbidity and mortality. A failure to stop growth of COVID‐19‐linked morbidity rates is caused by SARS‐CoV‐2 mutations and the emergence of new highly virulent SARS‐CoV‐2 strains. Several acquired SARS‐CoV‐2 mutations reflect viral adaptations to host immune defence. Mutations in the virus Spike‐protein were associated with the lowered effectiveness of current preventive therapies, including vaccines. Recent in vitro studies detected diminished neutralisation capacity of vaccine‐induced antibodies, which are targeted to bind Spike receptor‐binding and N‐terminal domains in the emerging strains. Lower than expected inhibitory activity of antibodies was reported against viruses with E484K Spike mutation, including B.1.1.7 (UK), P.1 (Brazil), B.1.351 (South African), and new Omicron variant (B.1.1.529) with E484A mutation. The vaccine effectiveness is yet to be examined against new mutant strains of SARS‐CoV‐2 originating in Europe, Nigeria, Brazil, South Africa, and India. To prevent the loss of anti‐viral protection in vivo, often defined as antibody resistance, it is required to target highly conserved viral sequences (including Spike protein) and enhance the potency of antibody cocktails. In this review, we assess the reported mutation‐acquiring potential of coronaviruses and compare efficacies of current COVID‐19 vaccines against ‘parent’ and ‘mutant’ strains of SARS‐CoV‐2 (Kappa (B.1.617.1), Delta (B.1.617.2), and Omicron (B.1.1.529)).
Keywords: antibody resistance, COVID‐19, mutant variants of concerns, SARS‐CoV‐2 strains, vaccine
Abbreviations
- ExoN
exoribonuclease
- NTD
N‐terminal domain
- ORF
open reading frame
- RBD
receptor binding domain
- TMPRSS2
Transmembrane protease serine 2 precursor
- VOCs
variants of concerns
1. INTRODUCTION
The causative agent of 21st century pandemic (2019–2021 ongoing), severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), belongs to Coronaviridae family in Nidovirales order of enveloped viruses [mad1]. There are four genera of coronaviruses in the family (sub‐family of Orthocoronavirinae):(a) alpha‐coronavirus (alpha‐CoV or Alpha) (alternative names:20I/501Y.V1, VOC‐202012/01), (b) beta‐coronavirus (also known as Sarbecovirus, 1 20H/501Y.V2, VOC‐202012/02)(beta‐CoV or Beta), (c) gamma‐coronavirus (gamma‐CoV or Gamma) (alternative names: 20J/501Y.V3, VOC‐202101/02), and (d) delta‐coronavirus (delta‐CoV or Delta) (alternative names: VOC‐21APR‐03, G/452R.V3, 21A/S:478K). 2 Alpha‐ and beta‐CoVs can infect bats, pigs, cats, mice, and humans, 3 , 4 , 5 , 6 , 7 , 8 , 9 whereas the gamma‐ and delta‐CoVs were shown to infect birds and mammals 10 , 11 , 12 , 13 (Table 1). Recently classified coronaviruses (the family Coronaviridae) are represented by 45species (the latest confirmed count, 4 August 2021 15 ) that are grouped in 25 subgenera and 4 genera. 16 The significant changes in CoV taxonomy happened during 2021 years and 39 species (27 subgenera and five genera) were reported in 2020. 15 The list of species is most likely will be extended and adjusted, considering that during 15‐year period (2003–2018) 339 SARS‐CoV genomes were identified, including 274 CoV genomes from humans. 1 The most recent addition is Omicron variant 17 which is required to be classified by International Committee on Taxonomy of Viruses. For some CoVs discovered in metagenomics studies, host and virus pathogenicity remains unknown, 18 while the genome sequence is the only known characteristic. 15 Currently, 58 complete genome sequences have been reported for Orthocoronavirinae subfamily including 24 genomes for alpha‐CoVs, 18 for beta‐CoVs, 10 for delta‐CoVs, 5 for gamma‐CoVs, and yet‐to‐be‐identified number of subfamilies for Omicron (Table 1). 19 , 22 Increasing population immunity, a product of natural infections and immunizations, is predicted to amplify the selection pressure on the mutating virus and increase the evolution of mutants towards emergence of antibody resistant strains.
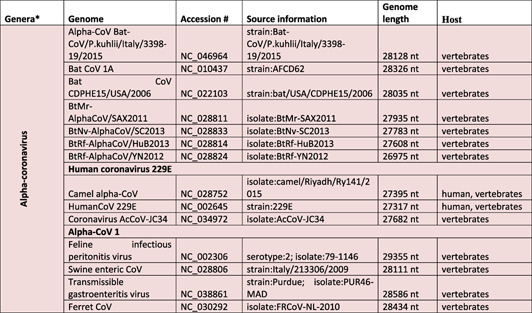
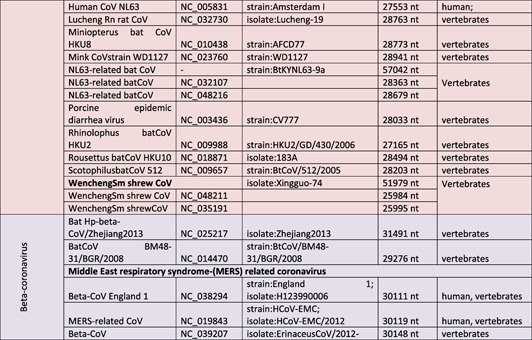
TABLE 1.
Taxonomic classification of pathogenic coronaviruses and their specific host system
|
|

|

|
Note: The table was adjusted according to the current classification reported at https://talk.ictvonline.org/taxonomy/.
aGenera of Orthocoronavirinae(taxid 10239). The table reflects the total number of currently identified Orthocoronavirinae species. Order: Nidovirales; Sub‐order: Cornidovirineae; Family: Coronaviridae; Subfamily: Orthocoronavirinae. 14
Human coronavirus NL63 (HCoV‐NL63) and 229E (HCoV‐229E) were identified as alpha‐CoVs. 23 , 24 Human coronavirus HKU1 (HCoV‐HKU1), OC43 (HCoV‐OC43, SARS‐CoV, MERS‐CoV), and SARS‐CoV‐2 (the causative agent of coronavirus disease 2019 (COVID‐19)) were grouped as beta‐CoVs. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 32 The phylogenetic analysis of CoVs revealed that SARS‐CoV‐2 is more closely related to bat‐SL‐CoV ZXC21 and bat‐SL‐CoV ZC45, and more distantly related to SARS‐CoV. 33 Beta‐ (HCoV‐OC43 and HCoV‐HKU1) and alpha‐CoVs (HCoV‐229E and HCoVNL63) were shown to infect the upper respiratory tract and cause mild respiratory diseases in humans and animals. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Similar infection‐related symptoms were recorded for the most recent variant (Omicron). 17 The lower respiratory tract infection with SARS‐CoV, SARS‐CoV‐2, and MERS‐CoV provoked various degrees of respiratory syndromes and extra‐respiratory complications in humans. 21 , 34
According to the World Health Organization (WHO) statistical report, 35 nearly 350 million COVID‐19 infection cases have been reported on 24 January 2022. The number of SARS‐CoV‐2‐infected patients and related death is constantly growing. 36 The pandemic resulted in nearly 6 million deaths worldwide. 35 COVID‐19 infection rate increased globally due to the emergence of mutant SARS‐CoV‐2 strains and lack of efficient anti‐viral agents. In this review, we discuss the integrated genome and emergence of mutant variants of SARS‐CoV‐2. The efficacy of existing SARS‐CoV‐2 vaccines is assessed to accentuate the urgent need for the vaccine amendment and design of complex approaches in vaccination schemes. The study also evaluates the progress of SARS‐CoV‐2 mutations towards development of antibody resistance in mutant strains of this virus. As predicted, emergence of variants of concern (VOCs), including highly infectious delta and omicron strains, indicates direction of viral mutations towards less severe, but more spreadable VOCs. Higher transmissibility was reported for the most recent VOC Omicron (B.1.1.529). 37 , 39
2. CORONAVIRUSES (CoVs): STRUCTURAL CHARACTERIZATION
Human‐infecting coronaviruses (CoVs) are single positive‐stranded RNA viruses (+ssRNA). The presence of 5′ cap structure and 3′‐poly‐A tail was detected in CoVs along with 27–32 kbp length of the CoV genome. 40 , 41 Approximately 2/3rd of SARS‐CoV‐2 genome is considered as conserved sequence, starting with the replication/transcription‐related genes encoded by two large overlapping open reading frames (ORF), such as ORF1a and ORF1b, at 5′ terminus (Graphical abstract). ORFs are translated into nonstructural proteins by ribosomal frame shifting. 42 The other 1/3rd of SARS‐CoV‐2 genome (at 3′ terminus) encodes four important structural components, including spike (S), envelop (E), membrane (M), and nucleocapsid (N) proteins (Figure 1). 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 The virus ORF1a and ORF1b codes contain information about polyprotein 1a and 1b that can be consequently cleaved into 16 non‐structural proteins (NSP) by specific proteases (chymotrypsin like protease (3CLpro), main protease, and papain like protease. 33 , 52 , 53 Genes coding for accessory proteins were also reported in 3′ region. 33 HCoV‐OC43 and HCoV‐HKU1 were shown to encode haemagglutinin esterase (HE) gene. 54 , 55 , 56 CoVs from different viral groups express various numbers of accessory proteins that are not necessary for virus replication, but impact SARS pathogenicity. Notably, six additional ORFs (ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10) that are located between structural genes, may code more accessory proteins. 57 SARS‐CoV genome contains codes for eight accessory proteins (3a, 3b, 6, 7a, 7b, 8a, 8b, and 9b). 58 , 59 Genome of MERS‐CoV(≈30 kb) encodes ORF1a (NSP1‐11), ORF1b (NSP12‐16), four structural proteins (S, E, M, and N), and five accessory proteins (3, 4a, 4b, 5, and 8b). 60 SARS‐CoV and MERS‐CoV genomes are packed in a capsid structure by N protein, while the other proteins (S, M, and E) form the envelope around the capsid. 61 Debates about the number of accessory proteins in SARS‐CoV‐2 still continue. For instance, one study reported successful identification of eight predicted accessory protein ORFs (3a, 6, 7a, 7b, 8, 9b, 9c, 10), 62 while the other indicated expression of only five canonical accessory ORFs (3a, 6, 7a, 7b, 8). 63
FIGURE 1.
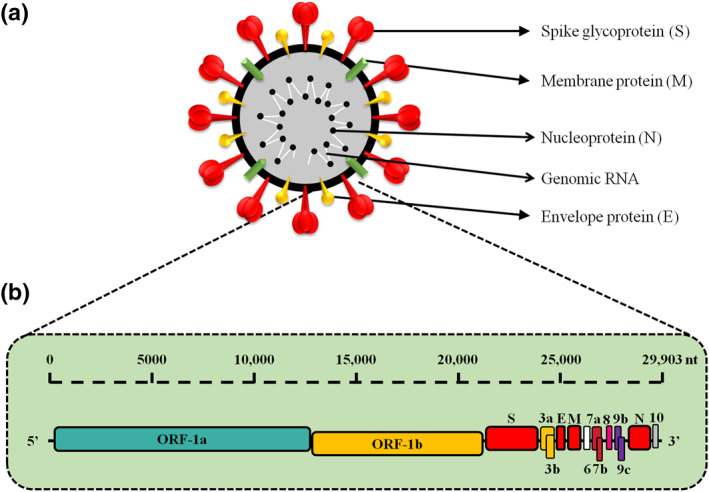
Characteristic structure of SARS‐CoV‐2 and its genomic organization. (a) SARS‐CoV‐2 is composed of single‐stranded RNA, spike (S)‐glycoprotein, and structural N, M, and E proteins. (b) Schematic organization of SARS‐CoV‐2 genome
The trimeric, cell‐surface glycoprotein spike (S‐protein) was identified as a potential SARS‐CoV‐2 therapeutic target responsible for the binding and penetration of the virus to the host cells. However, S‐proteins are required to be primed (cleaved) by the host serine protease TMPRSS2. 64 The S‐protein priming allows successful virus binding the angiotensin‐converting enzyme 2 (ACE2), a receptor for the entry of both SARS‐CoV and SARS‐CoV‐2 viruses. Cell entrance of MERS‐CoV is mediated by a different receptor, dipeptidyl peptidase 4 (DPP4). 65 Variations (mutations) in the S‐protein are associated with the host tissue tropism and severity response range to CoVs. 65 Genomic sequencing analysis of S‐protein structure in SARS‐CoV and SARS‐CoV‐2 delineated 76% sequence homology. Both SARS‐CoV and SARS‐CoV‐2 share eight conserved binding positions and six semi‐conserved positions in S‐protein domains. 66 , 67 Aside from ACE2, SARS‐CoV‐2 S‐protein was shown to interact with neuropilin 1 (NRP1) 68 and Basigin2/EMMPRIN/CD147 (CD147). 69 The involvement of CD147 in pathogenesis of other widely spread and highly harmful viruses was demonstrated. 70
3. CORONAVIRUSES AND INFLAMMATION
Several biological functions of SARS‐CoV‐2 accessory proteins have been described. For instance, 3a and 3b proteins were shown to activate apoptosis and stimulate release of proinflammatory cytokines. 71 Alternatively, protein 6 blocked pro‐inflammatory interferon (INF) signalling, but stimulated DNA synthesis. Activation of nuclear factor kappa B (NF‐κB)by ORF3a, M, ORF7a, and N proteins was reported. 72 Accordingly, ORF7a was indicated as a promising anti‐inflammatory therapeutic target in COVID‐19 patients. 72 Previously, SARS‐CoV protein 7a was also shown to trigger NF‐κB and mitogen‐activated protein kinase p38 (MAPK) signalling pathways. 73 , 74 Alternatively, it was reported that SARS‐CoV structural M protein can block NF‐κB activity in cell models in vitro, 75 suggesting complex involvement of viral proteins in the regulation of pro‐inflammatory pathways. The functional role of protein 7b remains unclear. ORF3b, ORF6, ORF7a and ORF8 may interfere with IFN type I pathway and damage host immunity. 76 Moreover, protein 8 (ORF8a and ORF8b) induces endoplasmic reticulum (ER) stress and triggers the activating transcription factor 6 (ATF6), responsible for the regulation of unfolded protein responses. 77 Interestingly, it seems that signalling roles of protein 8a and 8b are different. Protein 8a was detected to activate caspase‐dependent apoptosis, whereas protein 8b may impact host DNA synthesis. Intracellular roles and structure of SARS‐CoV‐2 accessory proteins was recently reviewed. 76
4. MUTATIONS IN SARS‐CoV‐2 VARIANTS
Since the beginning of COVID‐19 outbreak in Wuhan (China), over 12,000 mutations have been observed in the reference SARS‐CoV‐2 sequence (hCoV‐19/Wuhan/WIV04/2019). 78 According to phylogenetic, variant, and microsatellite analysis, coronaviruses acquire genomic mutations during the course of transmission and replication in the host cells, with approximately one nucleotide substitution every ≈11 days. 79 Consequently, over 1.4 million SARS‐CoV‐2 sequences, including 3913 major representative variants genomes, have been detected worldwide and stored in the global SARS‐CoV‐2 sequence database (Global Initiative on Sharing Avian Influenza Data (GISAID)). 80 Notably, functional changes in major SARS proteins associated with virus infectivity were preceded by limited numbers of genetic mutations that will be discussed in this review.
Specific mutations in SARS‐CoV‐2 trigger divergent phenotypic alterations that may enhance virus adaptation to the host environment. Mutational study of codon bias revealed host‐virus interactions and linked specific mutational changes to the severity of SARS‐CoV‐2 pathogenesis. 81 Mutational hotspots inflicted in SARS‐CoV‐2 NSPs were shown to promote virulence of the pathogen. 82 NSP2 and NSP3, S‐protein, and NSP12 RNA‐dependent RNA polymerase (RdRp/NSP12) are major SARS components associated with enhanced infectivity. Accordingly, specific mutations in NSP2, NSP3, and S‐proteins were linked to the increased virulence of SARS‐CoV‐2. 83 The genomic analysis demonstrated that mutations in SARS‐CoV‐2 can accumulate at a slower rate than mutation rates observed in other RNA viruses, including flu virus, and HIV. 84 S‐protein located mutations were identified in SARS‐CoV‐2 VOCs with higher virus infectivity and disease severity, including Delta (B.1.617.1 and B.1.617.2) 85 and Omicron (BA.1/B.1.1.529) 39 variants that caused global health crisis. 86 Presence of mutations facilitated development of resistance to vaccine‐generated immunity. While vaccinated (2 doses of BNT162b2 or ChAdOx1 nCoV‐19) patients infected with Alpha variant indicated good level of immune protection, 85 patients infected with mutated S‐protein variants were less protected and indicated lower level of anti‐viral antibodies. 85 Mutations in other regions, including modifications within SARS ORF1a genome, a key region for NSP mutations, have largely unclear consequences for human health 86 and require thorough investigation.
The main function of S‐protein of SARS‐CoV‐2 is associated with the targeted binding to ACE2 receptors and receptor‐facilitated intracellular transmission. 66 Gussow et al., assessed the role of S‐protein and other components and characteristics of SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV. 87 Enhanced virulence of SARS variants was linked to the mutations pertaining to S‐protein mutations that allowed a better cell binding and promoted higher fatality rates. 88 Multiple SARS‐CoV‐2 mutant variants with high virulence were reported. 89 For instance, African and Asian countries were marked by the highest percentages of unique mutations in S‐proteins. 90 European and North American S‐protein variants were marked by a larger number of diverse haplotype blocks that contained nonsynonymous variants. 84 , 88 Among all the variants, mutations in S‐protein were associated with higher virulence and, therefore, S‐protein is considered to be the most significant target for neutralising antibodies and vaccine development. 84 , 88 However, recently reports delineated the emergence of more virulent double mutant strains of SARS‐CoV‐2 64 which suggests that several targets (including, but not limited to S‐protein) should be considered during the development of more effective vaccines.
Mutations in NSP1 of ORF1a/ORF1b were directly associated with abnormal levels of virulence and transmissibility. Accordingly, antibodies against NSP1 were shown to regulate and antagonise viral replication. 91 , 92 Mutations in ORF allow the generation viral proteins which can induce MHC‐I expression in the infected hosts and promote viral escape from immune surveillance. 93 , 94 It has been shown that Alpha variant contains ORF with a premature stop codon at 27th position. 95 Interestingly, partial deletions of NSP1 and ORF8 (Δ382 variant in Singapore) were observed in VOCs detected in Sichuan, China (NSP1: Δ500‐532 variant). 92 , 93 , 94 A side from NSP1, non‐RBD regions mutation D614G is one of the most widely occurring mutations reported in 99% of current variants. 96 , 97 The mutation helps to enhance S‐protein density, prevent S2 shedding, and facilitate a higher infection rate. 98 , 99 Higher rate of deletions was also detected in antibodies‐recognising domain (N‐terminus region of S1 subunit. 100 For instance, Alpha and Beta variants were shown to contain numerous mutations in S1 subunit, including ΔRDR1 (recurrent deletion region (RDR)), ΔHV 69–70, ΔRDR2, ΔY144, ΔRDR4, and ΔLAL 242–244; whereas ΔRDR3 and ΔI210 were reported in B.1.36 (Indian Delta variant). 100
4.1. SARS‐CoV‐2 mutations in Indian isolates
Growing number of SARS cases and deaths in India was associated with the emergence of a novel variant, B.1.617 (defined as Delta variant). This variant gained eight mutations in RBD of the S‐protein. Among those, the mutations such as L452R and E484Q were shown to facilitate binding of the RBD to ACE2. 101 The introduced mutation‐linked changes were suggested to modulate antibody‐mediated neutralisation. Supporting this, B.1.617 was predominantly reported to be resistant to the treatment with bamlanivimab and escaped the antibody‐dependent eradication, activated by infection or vaccination. 64
Comparative whole genome sequencing analysis of SARS isolates in the Indian population delineated the occurrence of different mutations, including nonsynonymous, synonymous, and nonsense mutations in SARS‐CoV‐2. Nonsynonymous mutations that alter the protein sequences were 3.07 times more prevalent than synonymous mutations in Indian isolates. 81 The isolates were segregated into 22 groups according to the phylogenetic clade analysis which depicted various sub‐clades categorisation of the variants with unique‐coexisting mutations. The mutant variant dominance in the Indian subcontinent was distributed as follows: 73.34% of A2a clade, 23.29% of A3 clade, and 5.36% of B clade variants. Furthermore, 33 mutations were reported in 9 different protein coding genes (NSP3 (7 mutations), NSP12 (5 mutations), NSP2 (4 mutations), N (3 mutations), NSP4 (2 mutations), NSP6 (1 mutation), ORF3a (1 mutation), ORF8 (1 mutation)), and one mutation in 5′‐UTR region of the isolated SARS‐CoV‐2 genome. C241T, C3037T, A23403G and C14408T mutations were observed at a higher frequency (<50%) in Indian isolates. 102 Alternatively, G25563T (in ORF3a), C26735T (in NSP14), and C18877T (in M protein) mutations were observed less frequently (15%) in the Indian genome. 102
Further analysis demonstrated the presence of 4 silent mutations (in D294D/S, F106F/NSP3, S76S/NSP4 and Y789Y/S) without an apparent effect in the protein structure modulation. However, it was suggested that these mutations may influence the codon usage and modulate the translation process. 103 Mutation observed in the 5′‐UTR region could impact protein folding and transcription of SARS‐CoV‐2 genome. 103 Specific mutations in S‐protein (L54F, K77M, R78M, D294D, E583D, Q677H), 103 , 104 NSP3 (G716I, T749I, A994D, D1121G, S1197R), RdRp/NSP12 (A97V, L329I, G571S, V880I), NSP2 (S301F, G339S), and N‐protein (S194L) coding genes were unique to Indian isolates. 27 , 28 L54F, K77M, and R88M mutations were identified in the N‐terminal domain (NTD) domain of S1 subunit. NTD mutations were suggested to impact the virus binding ability in host cells. 105 Mutations of E583D and Q677H in the linker domain located in between S1 and S2 subunits could also influence the virus entry via modulating the serine proteases. 105 Q57H (in ORF3a), T265I (NSP3,T85I) and L3606F (NSP6, L37F)(in ORF1ab), L84S (ORF8), and N203 (204del‐insKR) mutations were also reported in Indian isolates. 105
SARS‐CoV‐2 genomic integrity predominantly depends on the functional efficacy of RdRp/NSP12. The functional alterations in this region, including A97V and L329I (NiRaN domain), V880I (thumb domain), and G571S (finger domain)mutations, were suggested to change the viral susceptibility to the anti‐viral drugs remdesivir, ribavirin, and favipiravir. 106 S194L mutation in the central region of N‐protein was associated with alterations in oligomerisation during viral assembly and replication. 107 , 108 Dominant presence of four specific mutations (C241T/5′‐UTR, D614G/S, F106F/NSP3 and P323L/RdRp) has been observed in the isolates from several geographic regions of the Indian subcontinent. A side from these 4 mutations, a group of 5 co‐dominant mutations (L37F/NSP6, T1198K/NSP3, A97V/RdRp, Y789Y/S, and P13L/N) was detected in South India and North India. 109 D614G, NSP12 (P323L) and NSP12 (A97V) dominant mutations were found in the isolates collected in Maharashtra, Tamil Nadu, and New Delhi. D614G (75% dominant) mutation had high prevalence in Maharashtra, although other mutations (NSP12 (P323L), G204R, R203K) in NSPs indicated prevalent rates. The variant strains with NSP12 (A97V), N (S202N), and NSP2 (G339S) mutations were reported highly prevalent in West Bengal 110 :
A computational study by Das et al. (2021) 81 delineated the characteristic mutations in the Indian SARS‐CoV‐2 genome. The study reported a total of 536 position‐specific mutations in SARS‐CoV‐2 protein coding regions. Most susceptible six protein codes (ORF1ab, S‐ and N‐proteins, ORF3a, ORF7a, and ORF8) were found mutated in Indian isolates. ORF3a exhibited ≈4% of its total length mutated. Substantial % of mutation rate was also observed in ORF1ab and S‐protein. 81 Deleterious substitutions in the SARS genome were suggested to facilitate decline in the stability of second codon and other putative functional domains. A substantial quantity of single point mutation was reported, including G > T (at first and third positions of codon) and C > T (at second codon) substitutions. Mutations (57 deleterious amino acid substitutions) in these codons were suggested to impact virus protein functions, disease pathogenesis, and vaccine efficacy against mutant strains. 81
4.2. Emerging and spreading of SARS COV‐2 variants
Whole genome sequencing technique was used to analyse thousands of SARS‐CoV‐2 isolates around the world. SARS‐CoV‐2 genome sequences were organised into haplotype groups 111 , 112 , 113 that were later extended and adjusted using data from 56 countries/territories. The large collection of data is stored at the GISAID database and contains information about 66 common haplotypes. 114 Information about SARS‐CoV‐2 genome mutations (global data from 48 countries) was also collected and stored in the Children's Hospital of Los Angeles (CHLA) COVID‐19 Analysis Research Database (CARD). The largest SARS‐CoV‐2 haplotypes in Europe and USA are presented in Figure 2. 114 The haplotypes were grouped in 13 major clusters (often defined as clades or lineages) (H1–H13). Groups H1–H3 contain the largest collection of variants. 114 Further analysis of 74,992 sequences (collected during the period from 1 June 2020 to 15 November 2020) led to the discovery of new sub‐haplotypes (H1a, H1b, H1r), the currently dominating haplotypes at GISAID. 114 , 115 Many countries attempt to record new rapidly mutating variants using different abbreviations. Consequently, the existing haplotype naming requires unification. For instance, haplotypes H1, H1a, H1b, and H1r are identified in GISAID as clades G, GR, GH, and GV, and in Next strain classification ‐ as 20A, 20B, 20C, and 20E. 114 , 116
FIGURE 2.
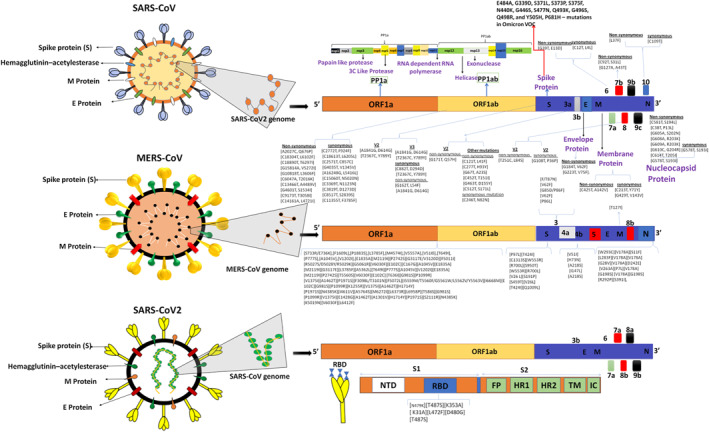
Phylogenetic and mutational analyses of lethal (or associated with the disease severity) coronavirus mutations in the reference genome. Upper panel – SARS‐CoV‐2: Approximately 4000 mutations were observed in S‐protein of SARS‐CoV‐2. Majority of these mutations do not influence COVID‐19 disease severity. Synonymous & nonsynonymous SARS‐CoV‐2 mutations in ORF1ab, S‐protein (v2, v3), E/M/N proteins, and amino acid substitutions (E484K) can enhance virus transmissibility via more effective S‐protein and ACE2 receptor binding. 81 Middle panel: MERS CoV: The Saudi Arabian human isolates contained a number of unique amino acid substitutions in ORF1ab (41 mutations), N‐protein (10 mutations mutations), S‐protein (9), and ORF4b (5 mutations) which could enhance virulence of this strain in human body. Lower panel: SARS‐CoV: Naturally selected mutations were observed in RBD region (7 mutations). These mutations can modulate RBD/hACE2 interactions of evolving SARS‐CoV variants
The co‐evolving mutations were registered in the clades G (H1), GH (H1a), and GR (H1b) from isolates collected in developing countries. The commonly mutated variant D614G (in S‐protein) was also found in EU and USA patients. The variant was also reported in India as described above, indicating a global spread and high virulence of this variant. Other commonly observed mutations include C241T/5UTR (in ORF1ab), C3037T (in ORF1ab), and mutations in NPS12/RdRp. 117 A novel variant of SARS‐CoV‐2, named VOC 202012/01 (or B.1.1.7, Alpha variant), was originally observed in UK and later identified in several other nations. 118 This variant exhibited 17 mutations mostly in S‐protein. 119 Aside from D614G substitution, S‐protein code of this variant contains additional 8 mutations. Two deletions (ΔH69/ΔV70 and ΔY144) were detected in the NTD.B.1.1.7 S‐protein RBD contains one substitution (N501Y) and one substitution (P681H) is near its Furin cleavage site. According to the UK COVID‐19 genomics consortium, B.1.1.7 variants exhibit 70% more transmissible efficacy compared to the reference strain. 120
Mutations in S‐protein coding genes were observed in the majority of VOCs. Compared with the Wuhan reference sequence, 10 mutations were found in B.1.351 (β variant, South Africa) and 12 in P.1 (γ variant, Brazil) with three RBD‐located changes. 78 B.1.351 (also named N501Y.V2) has some minor mutational similarities to the UK variant B.1.1.7. 118 Notably, B.1.1.7 and B.1.351 demonstrated resistance to the monoclonal antibodies that target NTD. 64 B.1.351 variant contains 9 mutations in the S‐protein gene, including a cluster of mutations in the NTD, substitutions (K417N, E484K and N501Y) in the RBD and near the Furin cleavage site. The substitution at position 484 (E484K; RBD of the S‐protein) was found in both B.1.351 and P.1 lineages. However, B.1.351 is resistant to major antibodies that target the RBD.
RBD locus of S‐protein mediates SARS‐CoV‐2 binding to ACE2 22 , 64 , 121 and consequent viral transport into intracellular space. Notably, S‐protein's RBD is the target for many potent neutralising antibodies. 122 The S‐targeting antibodies were suggested to block RBD‐ACE2 interaction and provide natural and vaccine‐induced protection from SARS‐CoV‐2 infection. Recently discovered ε variant B.1.427/429 (reported in the USA along another new variant B.1.526 123 , 124 and B.1.617.1 (κ variant, reported in India also contain mutations in the RBD of the S‐protein, including L452R. The mutations can enhance virus binding efficacy to ACE2 and protect those variants from anti‐viral immunity associated with human leucocyte antigen (HLA)‐A24 defence mechanisms. 125 It has been reported that L452R mutation could promote replication‐promoting change which consequently increases s‐protein stability, viral infectivity (fusogenicity), and promotes viral replication. 125 Enhanced transmissibility was observed for the B.1.351 (N501Y.V2) variant which substantially increased the binding capacity to the host cells. 118 Thus, similar to the other RNA viruses, SARS‐CoV‐2 has been evolving with divergent mutations and novel variants are expected. 118
WHO registered the emergence of Lambda variant (also named as the C.37 lineage, designated on 14 June 2021) in many South American countries (“Tracking SARS‐CoV‐2 variants” 126 ). Despite being a very recent addition to the VOC list, presence of Lambda variant has been observed in 26 countries 127 (GISAID database; https://www.gisaid.org). The variant contains three notable mutations (G75V, T76I, and RSYLTPGD246‐253N) in the NTD. Two other important mutations, L452Q and F490S, were detected in the RBD region. However, the S‐protein NTD‐located unique mutation (RSYLTPGD246‐ 253N) in the Lambda variant was suggested responsible for the virulence of this strain. 128 There is a possibility that RSYLTPGD246‐253N, L452Q and F490S mutations may facilitate strong resistance of this strain to the ‘natural and vaccine‐related immune defence mechanisms’. 129 Recent study reported detection of antibodies to S‐protein NTD and to the Nucleocapsid (N) protein. 130
Omicron variant (BA.1/B.1.1.529) genome contains unprecedented number of mutations (over 18,261) with majority of them (97%) detected in the coding region, and only 3% located in the extragenic region. 131 Omicron genetic signature was reported by GISAID and demonstrated 11 mutations (6 deletions and one insertion) in NTD, with unique N211 and in s214EPE mutations. 131 Several mutations (N501Y, D614G, K417N, T478K) were suggested to amplify the reinfection risk and facilitate vaccine resistance. 132 Omicron and Delta contain two similar RBD mutations that lead to S‐protein modifications, increased ACE2 binding affinity, and ability to evade immune surveillance. 133 , 134 Notably, S‐protein is the target of T‐cell neutralising antibodies during clearance of the viral infection. Comparing the variant infection rate, it was found that omicron is four times more infectious than the wild type and twice more than the Delta variant. 36 However, it was recently demonstrated that additional (booster) mRNA vaccine doses may help to improve anti‐viral antibody responses against new SARS‐CoV‐2 variants. 135
It was also noted that Omicron is rapidly spreading, 133 , 136 escaping from the convalescent sera. 131 , 137 , 138 Two‐dose course with existing vaccine indicated escape of omicron from immune cross‐neutralisation. 37 Minimal neutralisation efficacy observed against Omicron variants with the convalescent sera (from vaccinated individuals) could be associated with presence of more than 30 amino acid mutations across S‐protein in RBD. 38 Other RBD‐located mutation (G339D, S371L, S373P, S375F, N440K, G446S, S477N, Q493K, G496S, Q498R, and Y505H) may also contribute fast spreading of Omicron variant, 37 although further investigations are required. Recent in vitro study demonstrated that geometric mean neutralisation titres (GMT) against Omicron (used as pseudovirus variant) after two doses of BNT162b2 were 22.8‐fold lower compared to the original Wuhan variant. 137 Surprisingly, the booster (third dose) of BNT162b2 provided higher neutralising GMT levels against the Omicron variant (23.4‐fold increase) compared to the effect observed after second vaccine dose. 137 Supporting these findings, another recent study indicated significant protective effects of 3rd dose (≥6 months after second injection) of mRNA vaccine (BNT162b2 or mRNA‐1273) against Omicron and Delta variants. The adjusted odds ratio (OR) for Omicron cases (with third mRNA vaccine dose) versus unvaccinated controls was 0.33 (95% CI, 0.31–0.35). The adjusted OR among Delta cases versus unvaccinated was 0.065 (95% CI, 0.059–0.071), indicating a better protection against Delta. 138 The data may be called preliminary as the study included only 244 Omicron and 679 Delta cases injected with third dose of vaccine. 138 Notably, extended intervals (>42 days) between second and 3rd vaccine doses provided better immunogenicity. 38 , 39 , 139 A significant gain in the neutralisation activity against Omicron has been observed in the individuals who received vaccination of third dose (booster) of mRNA vaccine (6 months after second dose). 140 , 141 , 142 BNT162b2 third dose was also more effective against Beta and Delta variants. 143 , 144 Future studies should evaluate Omicron variant resistance to the other vaccine‐generated responses.
Despite mutations across the S‐protein's RBD region could support a strain resistance against host immunity, 74 , 75 , 76 NTD‐located mutations may provide an additional pathway for the viral escape from antibody‐based neutralisation. 145 , 146 , 147 Antibody‐linked sensitivity and infection spread were recently associated with the natural mutations in NTD. 148 Polyclonal antibodies generated by the human immune system were shown to bind to the several RBD sites that are undergoing intense evolutionary changes. 149 Therefore, current RBD‐targeting antibodies alone may not provide an efficient protection from the virus. 150 It was suggested to search for the non‐RBD‐targeting antibodies and design antibody cocktails. This suggestion requires experimental confirmation.
4.2.1. RBD mutations and SARS‐CoV‐2 virulence
Evolutionary selection pushes SARS viruses to adapt towards better penetration into host cells via receptor (ACE2) binding and higher resistance to the host anti‐viral immunity. Both adaptations can facilitate the increased virulence of COVID‐19 strains. Mutations in the RBD region were observed in recently identified variants, including mutations K417N/E484K (in Beta‐variant), E484A and N501Y (in Omicron), which enhanced the mutant virulence. Moreover, the increased mortality was also linked to the N501Y in the B.1.1.7 variant. 151 Two mutations, N479K and T487S located on S‐protein can modulate the RBD/ACE2 binding affinity. 152 , 153 Furthermore, N479K fosters the formation of energetically unfavourable positive charge at RBD/ACE2 interface, while T487S can eliminate an energetically favourable hydrophobic interaction when RBD interact with Lys‐353 in human ACE2 (hACE2). 121 , 154 Similar effect was observed in the strains with D480G mutation which can foster Tyr‐436 interactions and enhance binding efficacy of RBD with chimeric ACE2 (Figure 2). Asp‐480 and Gly‐480 can also confer viral adaptation in order to bind A to CE2. 155 Another mutation L472F can reinforce RBD/hACE2 interactions. However, L472P can weaken the interactions of RBD with ACE2 due to steric clash of Phe‐472 with Thr‐82 in chimeric ACE2. 155 Exact molecular and structural mechanisms underlying the mutant‐host interactions are not completely clarified and require further investigation. It is also necessary to elucidate the mechanism of natural selection of RBD‐located mutation.
5. MUTATIONS AND MERS‐CoV VIRULENCE
Similarly, the enhancement in virulence was observed in the mutated strains of MERS‐CoV. Unique amino acid substitutions of eight MERS‐CoV isolates were observed in ORF1ab (41 mutations), N‐protein (10 mutations), S‐protein (9 mutations), and ORF4b (5 mutations). 156 , 157 Distinct to SARS, a low mutation rate was observed in MERS‐CoV strains isolated from the humans. It was attributed to the decline in immunological pressure against MERS‐CoV strains in humans. 158 Furthermore, random mutations were also observed in nsp1‐nsp3, nsp12, nsp13, and nsp16 (ORF1ab replicase) (Figure 2). 156 S1 and S2 subunits of S‐protein in MERS‐CoV were shown to gain substitutions (such as T424I, S459T, W553R) in RBD. 159 The S2 subunit‐located substitutions S950T, Q1009L, and C1313S were also observed in the fusion peptide, heptad repeat region‐1, and transmembrane region, respectively. 156 In addition, MERS‐CoV key‐components for regulation of viral genomic RNA were also shown to contain substitutions, including V178A (in NTD), A300 (in C‐terminal domain (CTD)) G198S, D242E, S11F, P7L, and G28V (in N‐arm). 83 Several substitutions (L293F, V263A, R292P, and W293C) were detected at the MERS‐CoV CTD region. 156 Similar to other Beta‐coronaviruses, the MERS‐CoV encodes 5 accessory proteins (ORF3, ORF4a/4b, ORF5, and ORF8b). 159 Mutations were detected in ORF3 (G85D/P86F, V62F, and T87N), ORF4a (E102Q), ORF4b (H73N, A218S, V51I, I147L, H243Q), ORF5 (198M), and of M‐protein (T127I). The indicated mutations were suggested to enhance MERS‐CoV virulence (Figure 2).
6. SARS MUTATIONS AND MICROSATELLITE ANALYSIS
The adapted phylogenetic strategies including WGS, analysis of mutational variations in nucleotide sequences, and microsatellite analysis were used to understand, compare, and predict evolutionary development of coronaviruses. 160 For instance, it has been shown that the average MERS‐CoV and BAT‐CoV genomes may differ at 134.21 and 136.72 sites, respectively. Microsatellite comparative analysis of viral strains also indicated differences between MERS‐CoV and SARS‐CoV‐2 (106.8 and 107 sites, respectively). The microsatellite difference was higher than the difference between SARS‐CoV and BAT‐CoV (95.8 and 98.5, respectively). However, SARS‐CoV genome is significantly less distinct from SARS‐CoV‐2 reference genome (only 26.64 site differences). Interestingly, novel SARS‐CoV‐2 strains may be related to BAT‐CoV (88% genome identity), while MERS‐CoV is more distinct from SARS‐CoV‐2 reference genome. 160 The comparison helped to identify a unique PRPA peptide in the mutated SARS‐CoV‐2 genomes.
The ratio of non‐synonymous to synonymous mutations was found to be different in all strains. It was the lowest (0.29) in BAT‐CoV. Other strains had higher ratios as follows: 0.31 for SARS‐CoV, 1.46 for MERS‐CoV, and 1.57 for SARS‐CoV‐2 genomes. MERS‐CoV and SARS‐CoV‐2 were shown to acquire mutations across missense regions. 160 MERS‐CoV, SARS‐CoV‐2, BAT‐CoV, and SARS‐CoV were screened for highly recurrent (dominant) mutations. SARS‐CoV‐2 exhibited changes at four major mutation sites (nucleotide positions) as follows: 241 (C/T, upstream), 3037 (C/T), 14408 (C/T), and 23403 (A/G) (Figure 2 ). BAT‐CoV, SARS‐CoV, and MERS‐CoV exhibited 1690, 2178, and 4390 mutations, respectively. 160 Potentially, SARS‐CoV‐2 genome exhibited the largest occurrence of microsatellites compared to the other strains. This information about highly mutated regions in new strains is valuable for the design of novel vaccines.
7. CURRENTLY EFFECTIVE COVID‐19 VACCINES
During the development of COVID‐19 vaccines, the key‐role of S‐protein in the interaction of the virus with human ACE2 receptors was considered as the leading factor for the onset of infection. Therefore, current vaccines and available antibodies were designed to target the S‐protein of the virus. The S‐protein RBD and NTD are the current targets of most vaccine‐generated neutralising antibodies. Notably, anti‐RBD antibodies are 10‐ to 100‐fold more powerful than anti‐NTD antibodies. 161 Anti‐RBD antibody pool in convalescent patients represents 90% of the serum‐circulating neutralising activity, 162 thus confirming the importance of this region. Variations in RBD mutations are limited as virus has to maintain its ACE2‐binding capacity. Alternatively, NTD encoding sequence is more open for mutational variability. 163 However, the receptor binding motif of S‐protein has operational plasticity and excessive mutations in RBD, which may abrogate the antibody efficacy after vaccination. 164 The currently available anti‐COVID‐19 vaccines and their targets are discussed below.
Comirnaty ( also known as Tozinameran or BNT162b2) vaccine has been developed by Pfizer‐BioNTech and approved in 85 countries. It is mRNA‐based vaccine with a high level of safety 165 and act against the full‐length Spike gene. Vaccination with this agent resulted in the formation of persistent germinal centres and B cell‐based responses associated with the generation of strong humoral immunity. 166 As a promising sign of long lasting immunity, the presence of plasmablast responses was also reported. 167 It has been previously shown that antigen‐specific germinal B cells may remain functional for a year. 168 , 169 However, the vaccine induced production of fewer antibodies targeting NTD of the S‐protein, 166 indicating a need to introduce a wide range of vaccine‐related targets. Recently, 18 clinical trials of this vaccine were reported in 12 countries. Total of 43, 548 individuals (aged ≥16 years) participated in one of the clinical trials and the vaccine showed 95% efficacy (30 μg per dose, two doses) and exhibited an efficient level of protection against the symptomatic COVID‐19. 170 BNT162b2 can also neutralise SARS‐CoV‐2 variants and deliver combined adaptive humoral & cellular immune responses through the activation of antigen‐specific CD8+ and Th1‐type CD4+ T‐cell (anti‐Spike) responses. 171
mRNA‐1273 vaccine (developed by Moderna and often named as Spikevax ) trials were approved in 46 countries and 16 clinical trials have been initiated in 3 countries. 156 , 172 Approximately 30,420 individuals (≥18 years old) participated in one of the clinical trials. 173 , 174 The vaccine showed 90% efficacy (100 μg per dose, two doses). The vaccine targets mRNA‐1273 which encodes the S‐2P antigen and induces CD4+ T‐cell responses and activation of Th1 cytokine production. 173 , 174 Spikevax is often offered as booster dose after two previous doses of other mRNA vaccines, including BNT162b2. However, it remains to determine the efficacy of Spikevax against Omicron variant.
Both BNT162b2 and mRNA‐1273 vaccines were shown to generate IgM/IgG responses and provide good humoral anti‐viral immunity. 171 , 173 , 174 , 175 , 176 , 177 However, these vaccines were only partially protective against VOCs with RBD mutations. 171 , 173 , 174 , 177 Vaccine‐induced neutralisation efficacy of 14 out of 17 detected antibody types declined against variants with K417N, or E484K, or N501Y mutations. 178 , 179 , 180 , 181 , 182 VOCs with E484K and Q493R mutations exhibited resistance to class 2 antibodies; whereas variants with R346S, N439K, and N440K mutations resisted class 3 antibodies. 178 , 179 , 180 , 181 , 182
EpiVacCorona (developed by the Vektor State Research Centre of Virology and Biotechnology in Russia) has just been approved in 3 countries. It is a multi‐epitope vaccine which targets several regions, including the RBD region in the S2 protein, M, and N proteins. 174 , 183
Sputnik V ( also known as Gam‐Covid‐Vac ) vaccine is an adenovirus viral vector‐based vaccine which was developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is approved in 65 countries. Currently, 19 clinical trials of this vaccine are reported to be ongoing in 6 countries. Among these, 6 trials are in phase 3 and ongoing in Venezuela (Bolivarian Republic) (NCT04642339), United Arab Emirates (NCT04656613), Russian Federation (NCT04741061; NCT04530396), Belarus (NCT04564716), and India (NCT04640233 – Phase 2 & 3). Total 21,977 adult participants were tested in the trial and indicated promising results with 91.6% efficacy. The vaccination provided robust humoral and cellular immunity that was marked by the presence of activating RBD‐specific IgG, virus neutralising antibodies, and IFN‐γ responses. 184
Convidecia or Ad5‐nCoV vaccine (developed by CanSino Biologics Inc. (“CanSinoBIO”) (SHSE: 688185, HKEX: 06185) in China) is one of the most recently approved COVID‐19 vaccines. A single dose administration of this vaccine contains 5 × 1010 viral particles (vp) and was shown to be safe in healthy adults. The vaccine induces efficient immune responses in healthy adults, although the older people exhibited significantly lower anti‐viral immune responses. Suggestively, the older people may require additional doses of this vaccine. 185
Vaxzevria (also known as AZD1222 , Covishield , or Oxford‐AstraZeneca COVID‐19 vaccine) was developed by AstraZeneca. The vaccine has been approved in 40 countries. Currently, clinical trials of Vaxzevria are in Phase 2/3 in India, UK, Brazil, and South Africa with a total of 20,000 participants. 186 The significant problem associated with this vaccine is unexpected, although very rare pro‐thrombotic side effects. 187 Covishield (Indian‐made version of AstraZeneca's Vaxzevria) is administered in two doses. Each dose contains 5 × 1010 vp in 0.5 ml. The agent is a recombinant, replication‐deficient chimpanzee adenovirus vector encoding S‐glycoprotein. This vaccine was found to be effective in generating neutralising antibodies against the S‐protein in adults from all age groups. The vaccination with this agent resulted in development of immunocompetency on 14th day after booster 2nddose. 188 , 189
Ad26.COV2.S (also known as JNJ‐78436735 or Ad26.COV2.S ) was developed by Johnson & Johnson. Vaccine is a recombinant S‐protein attached to replication‐incompetent human adenovirus type 26 vector. The vaccine was approved in 41 countries. The administration of this vaccine can lower viral titre substantially within 14 days. A single dose of Ad26.COV2.S vaccine exerted effective protective humoral and cellular immune responses. 190 , 191
NVX‐CoV2373 (Novavax), recombinant S‐protein/nanoparticle (Matrix‐M) adjuvant vaccine, was found highly immunogenic. 192 , 193 The efficacy of this vaccine against new variants was not reported.
Covaxin (also known as BBV152 A, B, C ) was developed by Bharat Biotechin collaboration with the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV) in India. The vaccine has been approved in 9 countries. The vaccine was 78% efficient against mild, moderate, and severe COVID‐19. Initial Phase 1 clinical study has shown that the vaccine provokes a prolonged humoral and cell‐mediated immunity after second vaccine dose within 3 months. 194
CoronaVac (also known as Sinovac ) was developed by the Chinese company Sinovac Biotech (Sinovac Life Sciences, Beijing, China). This vaccine was approved in 2 countries. The vaccinated subjects indicated the presence of vaccine‐induced antibodies against the S‐protein, although the effectiveness was not very high. 195 The vaccine contains the inactivated SARS‐CoV‐2particles. 196 , 197
BBIBP‐CorV (also known as the Sinopharm or BIBP ) is one of two inactivated virus COVID‐19 vaccines developed by Sinopharm's Beijing Institute of Biological Products. The vaccine contains inactivated SARS‐CoV‐2 particles and was approved in 40 countries. The vaccine provoked humoral responses generating neutralising antibodies after the second dose of the vaccination 198 (Table 2).
TABLE 2.
Comparative clinical efficacy of mRNA vaccine, viral‐vector vaccine, inactivated pathogen vaccine, protein subunit vaccine, virus‐like particle vaccine among several participants, and their individual efficacy rate by the assessment of Nab (neutralizing antibody) responses, and Immune response rates
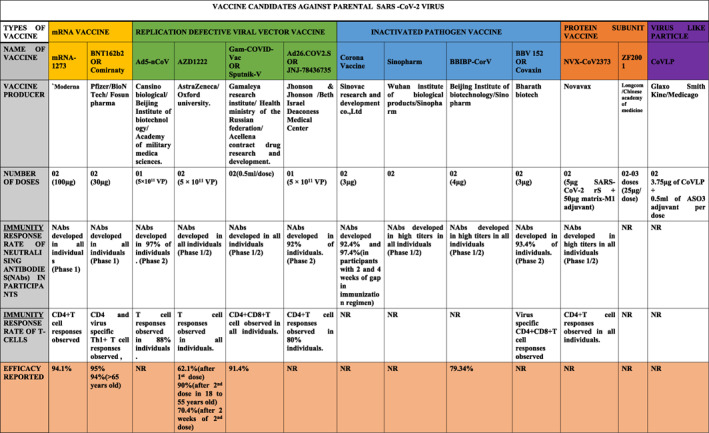
|
Abbreviations: NAbs, Neutralizing Antibodies; NR, Not Reported.
8. DEVELOPMENT OF ANTIBODY‐RESISTANCE: COMPARATIVE VACCINE EFFICACY FOR REFERENCE AND MUTANT VARIANTS
The successful generation of anti‐COVID‐19 immunity by the current vaccines is determined by the virological diversity and epidemiological spreading of variants. The large variety of emerging SARS‐CoV‐2 strains undermined vaccine efficiency and long‐term protection against the virus. Pandemic spread of SARS‐CoV‐2 is marked by emergence of new mutated strains. Some of those mutations were shown to assist viral escape from antibody‐dependent therapies. Approximately, one to two single nucleotide mutations may accumulate in SARS‐CoV‐2 genome within 30 days (½ the rate of influenza and ¼ the rate of HIV). The slower mutating ability of SARS‐CoV2 is associated with expression of a novel exoribonuclease (ExoN), the correcting enzyme for coding errors generated during viral replication. Accordingly, the genetic inactivation of ExoN in SARS‐CoV and murine CoV enhanced the mutation rate by 15 to 20 folds. 199
S‐protein is composed of 1273 amino acids and most of the successful vaccines were developed to target Spike (S) gene sequence. The reported S gene mutations and conformational changes in S‐protein were associated with severity of viral pathogenesis. If these mutation‐induced ‘RBD of S‐protein’ with conformational changes are not recognized by the initial antibody response, the virus can escape clearance by immune mechanisms in these circumstances. Consequently, the vaccines that were designed to target the reference SARS‐CoV‐2 or similar variants may be inefficient against later SARS mutants. 200
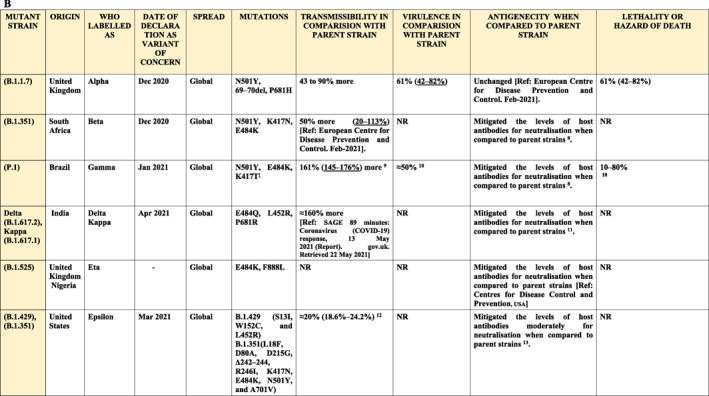
Newly identified SARS‐CoV‐2 variants in the UK exhibited higher evolutionary advantages in transmission and virulence. To investigate the mutation‐associated changes, three mutant strains of SARS‐CoV‐2 were engineered and included (a) N501Y (found in UK and SA), (b) 69/70‐deletion + N501Y + D614G (found in UK); and (c) E484K + N501Y + D614G (found in South Africa). All these mutants were neutralised using the sera of 20 BTN162b2‐vaccinated recipients. 200 The neutralisation geometric mean titres (GMTs) of the collected human sera against the engineered viruses was not much different from parental GMTs (0.81–1.46‐fold). This finding indicates that presence of mutation did not influence the neutralisation process by the sera from BTN162b2‐vaccinated subjects, compared to the vaccine effect against the parental strains. However, it does not eliminate a possibility that other mutants will be neutralised effectively. Clinical characteristics of some variants were shown to foster biological advantages of the virus in host systems, indicating a higher virulence, lower efficacy of vaccines and targeted therapies. 201
Epidemiological reports indicated that SARS variants with the emerging D614G amino acid (AC) mutation in S‐protein RBD demonstrated a higher virulence and more severely affected the exposed population, compared to the reference strain. 202 The presence of G614 form was also associated with the substantial rise in viral load and infectivity as the conformation delivers a greater capability for binding to the human ACE2 receptor. 202 The G614 variant exhibited a greater replication rate than the ancestral D614 strain in both cell lines and primary airway human epithelial cells. G614 variants were more resistant to the vaccine administration. 202 , 203
Majority of vaccines have been designed to target S‐protein to block its binding to ACE2 receptors. However, the vaccine‐induced antibodies may not effectively recognise mutant variants of SARS‐CoV‐2 for neutralisation. 204 The current vaccines can trigger specific antibody responses to specific immunogenic regions of the virus. However, these antibodies may not neutralise mutant S‐protein strains. This suggestion was unfortunately supported by the study of vaccine efficacy against the South African 1.351 mutant variant, which has mutations in the immunogenic region of S‐protein. This variant managed to escape vaccine‐induced antibody attack generated by previous vaccination. 205 Another clinical study reported the low efficacy of Moderna (mRNA1273‐based) and Pfizer‐BioNTech (BNT162b2) vaccines against mutant variants in 20 patients. 109
Significantly higher levels of anti‐SARS‐CoV‐2 IgM and IgG antibodies were detected 8 weeks after second dose vaccination with mRNA vaccines (BNT162b2 or mRNA‐1273). 171 , 173 , 174 , 177 However, 14 extracted antibodies (out of the detected 17) were inefficient in the neutralisation of mutant variants with K417N, or E484K, and N501Y mutations. 206 Effects of neutralising antibodies were also moderately decreased against UK B1.1.7 mutant strain. 171 , 173 , 174 , 177 Notably, a single dose of BNT162b2 was sufficient to generate some neutralising antibody responses against UK B.1.17 which contains 23 mutations (17 amino acid (AC) substitutions with 8 of them in S‐protein). 207 A significant decrease was observed for antibodies that targeted NTD and receptor‐binding motifs, but not the RBD part that is responsible for ACE2 interaction in B.1.1.7. Furthermore, recognition of NTD by antibodies can be also influenced by presence of deletion, substitutions, and insertions in mutated variants, 164 , 208 although some antibodies are still able to bind RBD and neutralise the virus. 209 The vaccine sera was shown to contain a wide range of neutralising titres of <1:4 and predominantly mitigated the mutant UK B.1.117 viral titres by 3.85‐fold. 207 Further decline in the neutralisation was observed after introduction of E484K substitution (observed in VOC 202102/02), suggesting an inefficiency of BNT162b2 against this mutant. 171 , 173 , 174 , 177 Current and future studies should delineate the comparative efficacy of vaccines against co‐evolving mutant variants B.1.17 (Tables 2 and 3).
TABLE 3.
Comparative clinical efficacy of mRNA vaccine, viral‐vector vaccine, inactivated pathogen vaccine, protein subunit vaccine, virus‐like particle vaccines against ‘various mutant variants’ of SARS CoV‐2 in UK, USA, South Africa, Brazil, India, and Nigeria compared to their efficacy against ‘parental strain’ and their individual Neutralization antibody responses, and Immune response rates 163 , 173 , 215 , 221 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236
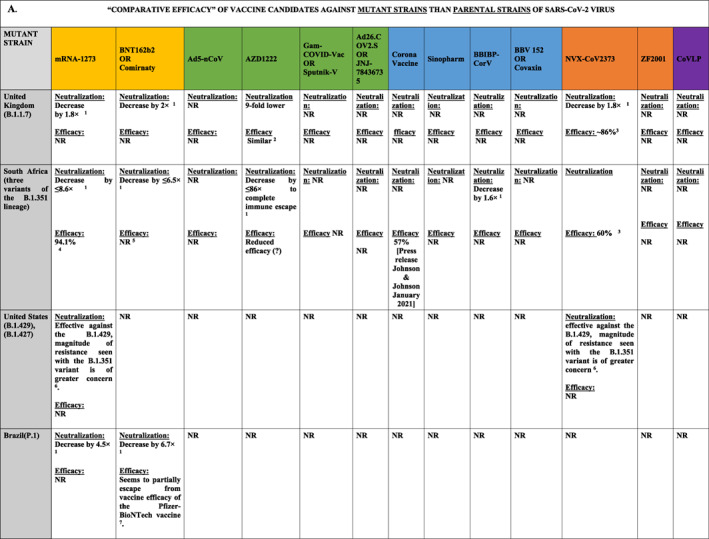
|
|
Abbreviation: NR, Not Reported.
BNT162b2 and other mRNA‐based vaccines are delivered using lipid nanoparticles. The vaccines demonstrated a very high neutralisation efficiency (>94%) and disease prevention in clinical studies. However, the emergence of SARS mutant variants raised several concerns about the future efficacy of current vaccines. Other inactivated protein vaccines, including AZD1222, JNJ‐78436735, NVX‐CoV2373, and CoronaVac, indicated good clinical efficacy. However, the emerging data from South Africa and Brazil where pandemics were widely dominated by novel variant strains, suggests that the neutralisation‐resistant variants may have contributed to the decline in vaccine efficacy. 221 , 223 Another study which tested the cross‐neutralisation of B.1.1.7 mutant variants by convalescent and vaccine sera indicated a minimal efficacy of BNT162b2 or mRNA‐1273vaccinations. 222 It was concluded that P.1 (35 mutations, 17 AC substitutions) and B.1.351 variants (N501Y.V2), both with K417N/T, E484K, and N501Y RBD‐located mutations, exhibit the minimal neutralisation titres with of BNT162b2/mRNA‐1273 compared to the parental strain titres. 164 , 222 , 223 Positive charge at E484 and shortened side chain at the residue 417 were suggested to prevent the interaction of virus with antibodies and associated defects in immune responses. 209 The resistance to vaccine‐generated antibodies was also associated with NTD‐located deletions (Y144del and 242‐244del). 223
BNT162b2 (Pfizer‐BioNTech) first dose has not generated the neutralising antibodies against B.1.351 for 2–3 weeks period, although only a small cohort of 15 people was tested. 163 The second dose generated low titres of neutralising antibodies in 60% (1 week after the vaccination) to 77% (3 weeks after the vaccination) of the tested subjects. Presence of E484K mutation could be considered as the main cause for the mitigation in overall vaccine potency. 163
B.1.351 (N501Y.V2) variant contains 23 mutations and 15 AC substitutions. 224 The strain is marked by the presence of N501Y mutation (asparagine (N) replaced by tyrosine (Y) at 501 position in RBD) in S‐protein. 210 B.1.351 strain also contains deletions in the NTD that prevents proper binding of neutralising antibodies. 209 The variant escaped elimination by host immune mechanisms in 48% of convalescent serum samples. 225 Aside from the neutralisation escape, 226 the strain has comparatively a higher viral transmissibility and reinfection capacity than the parental strain. Accordingly, it could induce severe COVID‐19 outcomes than parental strains. 210 It seems that current vaccines cannot guarantee reliable protection against 501Y.V2 strain (Table 3). 209
Low clinical efficacy against B.1.351 mutant was also observed after administration of two doses of ChAdOx1 nCoV‐19 vaccine [AZD1222]. 227 It has been concluded that the neutralising‐antibody responses failed to protect from B.1.351 strain and the vaccine‐primed T‐cell responses cannot confer protection from severe COVID‐19. Another vaccine trial indicated that 2 doses of NVX‐CoV2373 vaccine administration resulted in 49.4% protection against B.1.351 variant. 228 Ad26.COV2.S vaccine exhibited a better protection against B.1.351 in South African subjects. 190 , 229 However, Ad26.COV2.S vaccination also induced thrombotic thrombocytopaenia. 230 This vaccine is composed of human Ad26‐based vector and encodes S‐protein which does not shed S1 subunit due to the knockout of furin cleavage site. 230 The promising data were observed with NVX‐CoV2373 and JNJ‐78436735 vaccines that demonstrated a high level of protection against the B.1.351 variant and prevented the development of severe disease symptoms. 231
P.2 variants were also shown to escape neutralisation due to the presence of E484K mutation in the RBD domain. Similarly, P.1 strain (with 3 RBD‐located mutations), 224 B.1.1.298, and B.1.429 also exhibited a significant potential to avoid neutralisation by vaccine‐induced antibodies. 222 This data indicates an urgent need for the development of complex vaccines capable of generating a wide range of neutralising polyclonal antibodies. The more efficient vaccines should be designed to neutralise multiple antigenic epitopes.
Recent studies indicated a significant drop in the vaccine efficiency in neutralisation of several emerging variants, including B.1.351. For instance, Novavax (NVX‐CoV2373) efficiency was decreased from 96% to 48% 231 and Oxford–AstraZeneca (ChAdOx1) neutralisation effect collapsed from 62% to 10%. 233 Significant fall in efficiency was demonstrated with JNJ‐78436735 (Johnson & Johnson). 233 However, the neutralisation titre was not changed for the B.1.1.7 strain. 208 Targeted clinical trials are currently underway to test the efficacy of newly developed vaccines and (vaccine‐generated antibodies) against emerging variants. Considering the mutations impact on the vaccine efficiency, continuous monitoring of the viral change and vaccine adjustment are required.
9. CONCLUSIONS
Emergence of SARS‐CoV‐2 mutants indicates that COVID‐19 pandemic is transforming into an ongoing wave‐like SARS epidemic. Various SARS strains are detected nearly every day all over the world. It is predictable that new highly virulent strains will appear in the near future in different parts of the world. 20 , 51 Unfortunately, there is currently no guarantee that existing vaccines can provide required protection against the emerging strains. 209 However, vaccines that target S‐protein domains demonstrated encouraging data and lead to the generation of broadly neutralising antibodies. Promising results were also demonstrated after introduction of mRNA vaccine boosts that lead to 1000‐fold increase in neutralising titres. 234 , 236 The data indicated that first‐generation vaccines can prime the immune system and, after introduction of a booster, it is possible to provide protection against emerging strains. Another possibility is to use antibody cocktails and generate immunity to a large set of SARS variants. Application of antibody‐based therapy should be used cautiously, aiming to avoid the emergence of antibody‐resistance mutations. 209 Comparison of S‐protein and other virulence‐related mutations in common variants (Beta, Delta, and Omicron) of SARS CoV‐2 with global transmissibility may deliver essential information for the development of global anti‐COVID preventive therapies. Monitoring and reporting of susceptibility to new variants, disease‐related complications associated with new mutations, and the rate of re‐infection in previously infected and/or vaccinated individuals is required as a global effort to reduce the number of COVD‐19 hospitalisation and death. Effects of booster doses look promising at this stage, although development of new vaccines is also warranted. Current data indicates that not all antibodies from the vaccinated individuals were able to neutralise the new strains completely, although there is an indication that booster vaccination will decrease COVID‐19 severity.
Frequency of recurrent vaccination and type of booster dose vaccination may be adjusted to enhance protection against the evolving mutant strains. Moderna (USA) manufacturers reported booster dose availability and efficiency against mutant strains of SARS‐CoV‐2. Following this, it is required to confirm the efficacy of other approved vaccines against strains with the mutated S‐protein sites. Meanwhile, it is important to initiate viral genomic surveillance, timely vaccine adjustment, and expedite development of next‐generation antibody‐based therapies. Using available prediction technologies, it is possible to design second and third generation vaccines against emerging variant viruses. It is also necessary to develop immunogens for several viral genome domains, including more conservative domains that are less likely to be changed.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
All authors were involved in the design, conceptualisation, and writing of this review. NMB, OS, JL, EL, and RF wrote different subsections of the manuscript. NMB, JL, RF, and OS proofread and edited the final manuscript version. All authors reviewed and approved the final version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
The manuscript preparation was financially supported by National Natural Science Foundation of China (No. 81700729).
Beeraka NM, Sukocheva OA, Lukina E, Liu J, Fan R. Development of antibody resistance in emerging mutant strains of SARS CoV‐2: impediment for COVID‐19 vaccines. Rev Med Virol. 2022;32(5):e2346. 10.1002/rmv.2346
Narasimha M. Beeraka and Olga A. Sukocheva contributed equally.
Contributor Information
Junqi Liu, Email: fccliujq@zzu.edu.cn.
Ruitai Fan, Email: fccfanrt@zzu.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Luk HK, Li X, Fung J, Lau SK, Woo PC. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol. 2019;71:21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. King AM, Lefkowitz EJ, Mushegian AR, et al. Changes to taxonomy and the international code of virus classification and nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Arch Virol. 2018;163:2601‐2631. [DOI] [PubMed] [Google Scholar]
- 3. Kusanagi K‐i, Kuwahara H, Katoh T, et al. Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate. J Veter Med Sci. 1992;54:313‐318. [DOI] [PubMed] [Google Scholar]
- 4. Li W, Shi Z, Yu M, et al. Bats are natural reservoirs of SARS‐like coronaviruses. Science. 2005;310:676‐679. [DOI] [PubMed] [Google Scholar]
- 5. Poon LL, Chu DK, Chan K‐H, et al. Identification of a novel coronavirus in bats. J Virol. 2005;79:2001‐2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drexler JF, Corman VM, Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antivir Res. 2014;101:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedersen NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Veter J. 2014;201:123‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kúdelová M, Belvončíková P, Vrbová M, et al. Detection of murine herpesvirus 68 (MHV‐68) in dermacentor reticulatus ticks. Microb Ecol. 2015;70:785‐794. [DOI] [PubMed] [Google Scholar]
- 9. Cui J, Li F, Shi Z‐L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woo PC, Lau SK, Lam CS, et al. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J Virol. 2009;83:908‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woo PC, Lau SK, Lam CS, et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995‐4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo PC, Lau SK, Lam CS, et al. Discovery of a novel bottlenose dolphin coronavirus reveals a distinct species of marine mammal coronavirus in gammacoronavirus. J Virol. 2014;88:1318‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Ma Y, Zhang Y, Liang X, et al. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio. 2015;6:e00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. NIH.Gov . Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) sequences in NCBI cirus. Available online: https://www.ncbi.nlm.nih.gov/genomes/GenomesGroup.cgi?taxid=2501931
- 15. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2019;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siddell SG, Walker PJ, Lefkowitz EJ, et al. Additional changes to taxonomy ratified in a special vote by the international committee on taxonomy of viruses (October 2018). Arch Virol. 2019;164:943‐946. [DOI] [PubMed] [Google Scholar]
- 17. Araf Y, Akter F, Tang Yd, et al. Omicron variant of SARS‐CoV‐2: genomics, transmissibility, and responses to current COVID‐19 vaccines. J Med Virol. 2022;94(5):1825‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y‐Z, Chen Y‐M, Wang W, Qin X‐C, Holmes EC. Expanding the RNA virosphere by unbiased metagenomics. Annual Review of Virology. 2019;6:119‐139. [DOI] [PubMed] [Google Scholar]
- 19. NIH.Gov . Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) sequences in NCBI virus. Accessed May 8, 2021. [Google Scholar]
- 20. Beeraka NM, Tulimilli SV, Karnik M, et al. The current status and challenges in the development of vaccines and drugs against Severe Acute Respiratory Syndrome‐Corona Virus‐2 (SARS‐CoV‐2). BioMed Res Int. 2021;2021:8160860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herrera AS, Beeraka NM, Sinelnikov MY, et al. The beneficial effects of QIAPI 1® against pentavalent arsenic‐induced lung toxicity a hypothetical model for SARS CoV2‐induced lung toxicity. Curr Pharm Biotechnol. 2021. [DOI] [PubMed] [Google Scholar]
- 22. Beeraka NM, Sadhu SP, Madhunapantula SV, et al. Strategies for targeting SARS CoV‐2: small molecule inhibitors—the current status. Front Immunol. 2020;11:552925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190‐193. [DOI] [PubMed] [Google Scholar]
- 24. Chiu SS, Hung Chan K, Wing Chu K, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967‐1976. [DOI] [PubMed] [Google Scholar]
- 26. Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953‐1966. [DOI] [PubMed] [Google Scholar]
- 27. Vabret A, Mourez T, Gouarin S, Petitjean J, Freymuth F. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis. 2003;36:985‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woo PC, Lau SK, Chu C‐m, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zakivan ABS, Bestebroer TM, Osterhaus AD, Fouchier RA, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814‐1820. [DOI] [PubMed] [Google Scholar]
- 30. Du L, Tai W, Zhou Y, Jiang S. Vaccines for the prevention against the threat of MERS‐CoV. Expet Rev Vaccine. 2016;15:1123‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92:408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID‐19). StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 35. NIH.Gov . WHO Coronavirus (COVID‐19) Dashboard. 2021. [Google Scholar]
- 36. Beeraka NM, Tulimilli SV, Greeshma MV, et al. COVID‐19 Effects on Geriatric Population and Failures of Aminoquinoline Therapy: Compilation of Studies from EU, USA, and China; Safety and Efficacy of Vaccines in the Prevention & Treatment of COVID-19. Curr Med Chem. 2022. https://public.tableau.com/app/profile/covid.19.data.resource.hub/viz/COVID‐19Cases_15840488375320/COVID‐19GlobalView [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Wu S, Wu B, et al. SARS‐CoV‐2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal transduction and targeted therapy. 2021;6:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saxena SK, Kumar S, Ansari S, et al. Characterization of the novel SARS‐CoV‐2 omicron (B. 1.1. 529) variant of concern and its global perspective. J Med Virol. 2021;94(4):1738‐1744 [DOI] [PubMed] [Google Scholar]
- 39. Collie S, Champion J, Moultrie H, Bekker L‐G, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181‐192. 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Wit E, Van Doremalen N, Falzarano D, Munster VJ. SARS, MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193‐292. 10.1016/S0065-3527(06)66005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demogines A, Farzan M, Sawyer SL. Evidence for ACE2‐utilizing coronaviruses (CoVs) related to severe acute respiratory syndrome CoV in bats. J Virol. 2012;86:6350‐6353. 10.1128/JVI.00311-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neuman BW, Kiss G, Kunding AH, et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11‐22. 10.1016/j.jsb.2010.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeDiego ML, Alvarez E, Almazan F, et al. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J Virol. 2007;81:1701‐1713. 10.1128/JVI.01467-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Method Mol Biol. 2015;1282:1‐23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cui L, Wang H, Ji Y, et al. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J Virol. 2015;89:9029‐9043. 10.1128/JVI.01331-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3. 10.1128/mBio.00473-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Czub M, Weingartl H, Czub S, He R, Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23:2273‐2279. 10.1016/j.vaccine.2005.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karnik M, Beeraka NM, Uthaiah CA, et al. A review on SARS‐CoV‐2‐induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Mol Neurobiol. 2021:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ziebuhr J, Snijder EJ, Gorbalenya AE. Virus‐encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol. 2000;81:853‐879. [DOI] [PubMed] [Google Scholar]
- 54. de Groot R, Baker S, Baric R, Ziebuhr J. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, eds. Coronaviridae. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. 2011;87(14):7790–7792. [Google Scholar]
- 55. Desforges M, Desjardins J, Zhang C, Talbot PJ. The acetyl‐esterase activity of the hemagglutinin‐esterase protein of human coronavirus OC43 strongly enhances the production of infectious virus. J Virol. 2013;87:3097‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang X, Dong W, Milewska A, et al. Human coronavirus HKU1 spike protein uses O‐acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin‐esterase protein as a receptor‐destroying enzyme. J Virol. 2015;89:7202‐7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khailany R, Safdar M, Ozaslan M. Genomic characterization of a novel SARS‐CoV‐2. Gene Rep. 2020;19:100682‐102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marra MA, Jones S, Astell C, et al. The genome sequence of the SARS‐associated coronavirus. Science. 2003;300:1399‐1404. [DOI] [PubMed] [Google Scholar]
- 59. Snijder EJ, Bredenbeek PJ, Dobbe JC, et al. Unique and conserved features of genome and proteome of SARS‐coronavirus, an early split‐off from the coronavirus group 2 lineage. J Mol Biol. 2003;331:991‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alanagreh La, Alzoughool F, Atoum M. The human coronavirus disease COVID‐19: its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens. 2020;9:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS‐CoV‐2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim D, Lee J‐Y, Yang J‐S, Kim JW, Kim VN, Chang H. The architecture of SARS‐CoV‐2 transcriptome. Cell. 2020;181:914‐921.e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fenizia C, Galbiati S, Vanetti C, et al. SARS‐CoV‐2 entry: at the crossroads of CD147 and ACE2. Cells. 2021;10:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181:281‐292.e286. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elbe S, Buckland‐Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall. 2017;1:33‐46. 10.1002/gch2.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Daly JL, Simonetti B, Klein K, et al. Neuropilin‐1 is a host factor for SARS‐CoV‐2 infection. Science. 2020;370:861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang K, Chen W, Zhang Z, et al. CD147‐spike protein is a novel route for SARS‐CoV‐2 infection to host cells. Signal transduction and targeted therapy. 2020;5:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xiong L, Edwards CK, Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int J Mol Sci. 2014;15:17411‐17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu DX, Fung TS, Chong KK‐L, Shukla A, Hilgenfeld R. Accessory proteins of SARS‐CoV and other coronaviruses. Antivir Res. 2014;109:97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Su C‐M, Wang L, Yoo D. Activation of NF‐κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS‐CoV‐2. Sci Rep. 2021;11:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kanzawa N, Nishigaki K, Hayashi T, et al. Augmentation of chemokine production by severe acute respiratory syndrome coronavirus 3a/X1 and 7a/X4 proteins through NF‐κB activation. FEBS Lett. 2006;580:6807‐6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kopecky‐Bromberg SA, Martinez‐Sobrido L, Palese P. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen‐activated protein kinase. J Virol. 2006;80:785‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fang X, Gao J, Zheng H, et al. The membrane protein of SARS‐CoV suppresses NF‐κB activation. J Med virology. 2007;79:1431‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Redondo N, Zaldívar‐López S, Garrido JJ, Montoya M. SARS‐CoV‐2 accessory proteins in viral pathogenesis: knowns and unknowns. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rashid F, Dzakah EE, Wang H, Tang S. The ORF8 protein of SARS‐CoV‐2 induced endoplasmic reticulum stress and mediated immune evasion by antagonizing production of interferon beta. Virus Res. 2021;296:198350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the P. 1 strain of SARS‐CoV‐2. Cell. 2021;184:2939‐2954.e2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kannan S, Ali PSS, Sheeza A. Evolving biothreat of variant SARS‐CoV‐2–molecular properties, virulence and epidemiology. Eur Rev Med Pharmacol Sci. 2021;25:4405‐4412. [DOI] [PubMed] [Google Scholar]
- 80. GISAID . Global initiative on sharing avian influenza data (GISAID). Available online: https://www.gisaid.org/
- 81. Das JK, Sengupta A, Choudhury PP, Roy S. Characterizing genomic variants and mutations in SARS‐CoV‐2 proteins from Indian isolates. Gene Rep. 2021;25:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Beeraka NM, Tulimilli SV, Karnik M, et al. The current status and challenges in the development of vaccines and drugs against severe acute respiratory syndrome‐ corona virus‐2 (SARS‐CoV‐2). BioMed Res Int; 2021;2021:8160860. 10.1155/2021/8160860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Angeletti S, Benvenuto D, Bianchi M, Giovanetti M, Pascarella S, Ciccozzi M. COVID‐2019: the role of the nsp2 and nsp3 in its pathogenesis. J Med Virol. 2020;92:584‐588. 10.1002/jmv.25719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Callaway E. The coronavirus is mutating ‐ does it matter? Nature. 2020;585:174‐177. 10.1038/d41586-020-02544-6 [DOI] [PubMed] [Google Scholar]
- 85. Eyre DW, Taylor D, Purver M, et al. Effect of covid‐19 vaccination on transmission of alpha and delta variants. N Engl J Med. 2022;386(8):744‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Khateeb J, Li Y, Zhang H. Emerging SARS‐CoV‐2 variants of concern and potential intervention approaches. Crit Care. 2021;25:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gussow AB, Auslander N, Faure G, Wolf YI, Zhang F, Koonin EV. Genomic determinants of pathogenicity in SARS‐CoV‐2 and other human coronaviruses. Proc Natl Acad Sci USA. 2020;117:15193‐15199. 10.1073/pnas.2008176117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell. 2020;182:812‐827. 10.1016/j.cell.2020.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koyama T, Platt D, Parida L. Variant analysis of SARS‐CoV‐2 genomes. Bull World Health Organ. 2020;98:495‐504. 10.2471/BLT.20.253591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Justo Arevalo S, Zapata Sifuentes D, Huallpa CJ, et al. Global geographic and temporal analysis of SARS‐CoV‐2 haplotypes normalized by COVID‐19 cases during the pandemic. Front Microbiol. 2021;12:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xia H, Cao Z, Xie X, et al. Evasion of type I interferon by SARS‐CoV‐2. Cell Rep. 2020;33:108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin J‐w, Tang C, Wei H‐c, et al. Genomic monitoring of SARS‐CoV‐2 uncovers an Nsp1 deletion variant that modulates type I interferon response. Cell host microbe. 2021;29:489‐502.e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Flower TG, Buffalo CZ, Hooy RM, Allaire M, Ren X, Hurley JH. Structure of SARS‐CoV‐2 ORF8, a rapidly evolving immune evasion protein. Proc Natl Acad Sci. 2021;118:e2021785118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Young BE, Fong S‐W, Chan Y‐H, et al. Effects of a major deletion in the SARS‐CoV‐2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020;396:603‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Touret F, Luciani L, Baronti C, et al. Replicative fitness of a SARS‐CoV‐2 20I/501Y. V1 variant from lineage B. 1.1. 7 in human reconstituted bronchial epithelium. mBio. 2021;12:e00850‐00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Starr TN, Greaney AJ, Hilton SK, et al. Deep mutational scanning of SARS‐CoV‐2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295‐1310.e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS‐CoV‐2 fitness. Nature. 2021;592:116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang L, Jackson CB, Mou H, et al. SARS‐CoV‐2 spike‐protein D614G mutation increases virion spike density and infectivity. Nat Commun. 2020;11:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hou YJ, Chiba S, Halfmann P, et al. SARS‐CoV‐2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McCarthy KR, Rennick LJ, Nambulli S, et al. Recurrent deletions in the SARS‐CoV‐2 spike glycoprotein drive antibody escape. Science. 2021;371:1139‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li Q, Nie J, Wu J, et al. SARS‐CoV‐2 501Y. V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184:2362‐2371.e2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang H‐C, Chen C‐h, Wang J‐H, et al. Analysis of genomic distributions of SARS‐CoV‐2 reveals a dominant strain type with strong allelic associations. Proc Natl Acad Sci. 2020;117:30679‐30686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mercatelli D, Giorgi FM. Geographic and genomic distribution of SARS‐CoV‐2 mutations. Front Microbiol. 2020;11:1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guan Q, Sadykov M, Mfarrej S, et al. A genetic barcode of SARS‐CoV‐2 for monitoring global distribution of different clades during the COVID‐19 pandemic. Int J Infect Dis. 2020;100:216‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS‐CoV‐2 entry by using human ACE2. Cell. 2020;181:894‐904.e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kirchdoerfer RN, Ward AB. Structure of the SARS‐CoV nsp12 polymerase bound to nsp7 and nsp8 co‐factors. Nat Commun. 2019;10:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yu I‐M, Oldham ML, Zhang J, Chen J. Crystal structure of the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein dimerization domain reveals evolutionary linkage between corona‐and arteriviridae. J Biol Chem. 2006;281:17134‐17139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao P, Cao J, Zhao L‐J, et al. Immune responses against SARS‐coronavirus nucleocapsid protein induced by DNA vaccine. Virology. 2005;331:128‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sarkar R, Mitra S, Chandra P, et al. Comprehensive analysis of genomic diversity of SARS‐CoV‐2 in different geographic regions of India: an endeavour to classify Indian SARS‐CoV‐2 strains on the basis of co‐existing mutations. Archives Virology. 2021;166:801‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Alai S, Gujar N, Joshi M, Gautam M, Gairola S. Pan‐India novel coronavirus SARS‐CoV‐2 genomics and global diversity analysis in spike protein. Heliyon. 2021;7:e06564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS‐CoV‐2 genomes. Proc Natl Acad Sci. 2020;117:9241‐9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pachetti M, Marini B, Benedetti F, et al. Emerging SARS‐CoV‐2 mutation hot spots include a novel RNA‐dependent‐RNA polymerase variant. J Transl Med. 2020;18:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS‐CoV‐2. Natl Sci Rev. 2020;7:1012‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brufsky A. Distinct viral clades of SARS‐CoV‐2: implications for modeling of viral spread. J Med Virol. 2020;92(9):1386‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. GISAID . Nextstrain. 2021. [Google Scholar]
- 116. Liu S, Shen J, Fang S, et al. Genetic spectrum and distinct evolution patterns of SARS‐CoV‐2. Front Microbiol. 2020;11:2390 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS‐CoV‐2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Triggle CR, Bansal D, Ding H, et al. A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS‐CoV‐2 and COVID‐19 as a basis for controlling the pandemic. Front Immunol. 2021;12:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wise J. Covid‐19: new coronavirus variant is identified in UK. BMJ. 2020;371:m4857. 10.1136/bmj.m4857 [DOI] [PubMed] [Google Scholar]
- 120. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science. 2005;309:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 122. Cerutti G, Rapp M, Guo Y, et al. Structural basis for accommodation of emerging B. 1.351 and B. 1.1. 7 variants by two potent SARS‐CoV‐2 neutralizing antibodies. Structure. 2021;29(7):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hossain MK, Hassanzadeganroudsari M, Apostolopoulos V. The emergence of new strains of SARS‐CoV‐2. What does it mean for COVID‐19 vaccines? Expet Rev Vaccine. 2021:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. Review of COVID‐19 variants and COVID‐19 vaccine efficacy: what the clinician should know? J Clin Med Res. 2021;13:317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Motozono C, Toyoda M, Zahradnik J, et al. SARS‐CoV‐2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29:1124‐1136.e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. WHO . Tracking SARS‐CoV‐2 variants. 2021. [Google Scholar]
- 127. (GISAID) . G.G.I.o.S.A.I.D.
- 128. Baj A, Novazzi F, Ferrante FD, et al. Introduction of SARS‐COV‐2 C. 37 (WHO VOI lambda) from Peru to Italy. J Med Virol. 2021;93(12):6460‐6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Agwa SH, Kamel MM, Elghazaly H, et al. Association between interferon‐lambda‐3 rs12979860, TLL1 rs17047200 and DDR1 rs4618569 variant polymorphisms with the course and outcome of SARS‐CoV‐2 patients. Genes. 2021;12:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N‐terminal domain of the Spike protein of SARS‐CoV‐2. Science. 2020;369:650‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hoffmann M, Krüger N, Schulz S, et al. The omicron variant is highly resistant against antibody‐mediated neutralization–implications for control of the COVID‐19 pandemic. Cell. 2021;185(3):447‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and delta variant of SARS‐CoV‐2: a comparative computational study of spike protein. J Med Virol. 2021;94(4):1641‐1649. [DOI] [PubMed] [Google Scholar]
- 133. Kandeel M, Mohamed MEM, Abd El‐Lateef HM, Venugopala KN, El‐Beltagi HS. Omicron variant genome evolution and phylogenetics. J Med Virol. 2021;94(4):1627‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang L, Cheng G. Sequence analysis of the emerging sars‐CoV‐2 variant omicron in South Africa. J Med Virol. 2021;94(4):1728‐1733. [DOI] [PubMed] [Google Scholar]
- 135. Hossain G, Tang Yd, Akter S, Zheng C. Roles of the polybasic furin cleavage site of spike protein in SARS‐CoV‐2 replication, pathogenesis, and host immune responses and vaccination. J Med Virol. 2021;94(5):1815‐1820. [DOI] [PubMed] [Google Scholar]
- 136. He X, Hong W, Pan X, Lu G, Wei X. SARS‐CoV‐2 omicron variant: characteristics and prevention. MedComm. 2021;2(4):838‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Muik A, Lui BG, Wallisch A‐K, et al. Neutralization of SARS‐CoV‐2 Omicron by BNT162b2 mRNA vaccine–elicited human sera. Science. 2022;375(6581):678‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Accorsi EK, Britton A, Fleming‐Dutra KE, et al. Association between 3 doses of mRNA COVID‐19 vaccine and symptomatic infection caused by the SARS‐CoV‐2 omicron and delta variants. JAMA. 2022;327(7):639‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Grunau B, Goldfarb DM, Asamoah‐Boaheng M, et al. Immunogenicity of extended mRNA SARS‐CoV‐2 vaccine dosing intervals. JAMA. 2021;327(3):279‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gaebler C, Wang Z, Lorenzi JC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591:639‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang Z, Muecksch F, Schaefer‐Babajew D, et al. Naturally enhanced neutralizing breadth against SARS‐CoV‐2 one year after infection. Nature. 2021:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS‐CoV‐2 omicron variant. N Engl J Med. 2021;386(6):599‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS‐CoV‐2 Omicron infection. N Engl J Med. 2021;386(5):492‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Falsey AR, Frenck RW, Jr , Walsh EE, et al. SARS‐CoV‐2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. McCallum M, De Marco A, Lempp FA, et al. N‐terminal domain antigenic mapping reveals a site of vulnerability for SARS‐CoV‐2. Cell. 2021;184:2332‐2347.e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Li D, Edwards RJ, Manne K, et al. In vitro and in vivo functions of SARS‐CoV‐2 infection‐enhancing and neutralizing antibodies. Cell. 2021;184(16):4203‐4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liu Y, Soh WT, Kishikawa J‐i, et al. An infectivity‐enhancing site on the SARS‐CoV‐2 spike protein targeted by antibodies. Cell. 2021;184(13):3452‐3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rahman MS, Islam MR, Alam ARU, et al. Evolutionary dynamics of SARS‐CoV‐2 nucleocapsid protein and its consequences. J Med virology. 2021;93:2177‐2195. [DOI] [PubMed] [Google Scholar]
- 149. Greaney AJ, Starr TN, Barnes CO, et al. Mapping mutations to the SARS‐CoV‐2 RBD that escape binding by different classes of antibodies. Nat Commun. 2021;12:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhou H, Chen Y, Zhang S, et al. Structural definition of a neutralization epitope on the N‐terminal domain of MERS‐CoV spike glycoprotein. Nat Commun. 2019;10:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Widera M, Mühlemann B, Corman VM, et al. Surveillance of SARS‐CoV‐2 in Frankfurt am main from October to december 2020 reveals high viral diversity including spike mutation N501Y in B. 1.1. 70 and B. 1.1. 7. Microorganisms. 2021;9:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Li W, Zhang C, Sui J, et al. Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Qu X‐X, Hao P, Song X‐J, et al. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J Biol Chem. 2005;280:29588‐29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Li F. Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections. J Virol. 2008;82:6984‐6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wu K, Peng G, Wilken M, Geraghty RJ, Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J Biol Chem. 2012;287:8904‐8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. AlBalwi MA, Khan A, AlDrees M, et al. Evolving sequence mutations in the Middle East respiratory syndrome coronavirus (MERS‐CoV). J Infect Public Health. 2020;13:1544‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ba Abduallah MM, Hemida MG. Comparative analysis of the genome structure and organization of the Middle East respiratory syndrome coronavirus (MERS‐CoV) 2012 to 2019 revealing evidence for virus strain barcoding, zoonotic transmission, and selection pressure. Rev Med Virol. 2021;31:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Alnazawi M, Altaher A, Kandeel M. Comparative genomic analysis MERS CoV isolated from humans and camels with special reference to virus encoded helicase. Biol Pharm Bull. 2017;40:1289‐1298. [DOI] [PubMed] [Google Scholar]
- 159. McBride R, Fielding BC. The role of severe acute respiratory syndrome (SARS)‐coronavirus accessory proteins in virus pathogenesis. Viruses. 2012;4:2902‐2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Rehman HA, Ramzan F, Basharat Z, Shakeel M, Khan MUG, Khan IA. Comprehensive omparative genomic and microsatellite analysis of SARS, MERS, BAT‐SARS, and COVID‐19 coronaviruses. J Med Virol. 2021;93(7):4382‐4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Andreano E, Nicastri E, Paciello I, et al. Extremely potent human monoclonal antibodies from COVID‐19 convalescent patients. Cell. 2021;184:1821‐1835.e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Andreano E, Rappuoli R. SARS‐CoV‐2 escaped natural immunity, raising questions about vaccines and therapies. Nat Med. 2021;27:759‐761. [DOI] [PubMed] [Google Scholar]
- 163. Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS‐CoV‐2 B. 1.1. 7 and B. 1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917‐924. [DOI] [PubMed] [Google Scholar]
- 164. Chen RE, Zhang X, Case JB, et al. Resistance of SARS‐CoV‐2 variants to neutralization by monoclonal and serum‐derived polyclonal antibodies. Nat Med. 2021;131(14):717‐726.e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS‐CoV2 BNT162b2 (Tozinameran) prime‐boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Turner JS, O’Halloran JA, Kalaidina E, et al. SARS‐CoV‐2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Weisel FJ, Zuccarino‐Catania GV, Chikina M, Shlomchik MJ. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity. 2016;44:116‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292‐1299. [DOI] [PubMed] [Google Scholar]
- 169. Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Marco JJG, Pasquín MJÁ, Martín SM. Efectividad y seguridad de las vacunas para el SARS‐CoV‐2 actualmente disponibles. FMC‐Formación Médica Continuada en Atención Primaria. 2021;28(8):442‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Pegu A, O’Connell S, Schmidt SD, et al. Durability of mRNA‐1273 vaccine–induced antibodies against SARS‐CoV‐2 variants. Science. 2021;373(6561):1372‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2—preliminary report. N Engl J Med. 2020;383(20):1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Formica N, Mallory R, Albert G, et al. Different dose regimens of a SARS‐CoV‐2 recombinant spike protein vaccine (NVX‐CoV2373) in younger and older adults: a phase 2 randomized placebo‐controlled trial. PLoS Med. 2021;18:e1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gorman MJ, Patel N, Guebre‐Xabier M, et al. Fab and Fc contribute to maximal protection against SARS‐CoV‐2 following NVX‐CoV2373 subunit vaccine with Matrix‐M vaccination. Cell Rep Med. 2021;2:100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly‐specific T cells in humans. Nature. 2021;595(7868):572-577. [DOI] [PubMed] [Google Scholar]
- 178. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. N Engl J Med. 2020;383:1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature. 2020;584:437‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Weisblum Y, Schmidt F, Zhang F. Fuggite da anticorpi neutralizanti da varianti di proteine spike SARS‐CoV‐2. Elife. 2020;9:e61312.33112236 [Google Scholar]
- 181. Barnes CCAJee, Abernathy ME, Ma Dam K, et al. SARS‐CoV‐2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Starr TN, Greaney AJ, Addetia A, et al. Prospective mapping of viral mutations that escape antibodies used to treat COVID‐19. Science. 2021;371:850‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pollet J, Chen W‐H, Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv Drug Deliv Rev. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Zhu F‐C, Guan X‐H, Li Y‐H, et al. Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18 years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2020;396:479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kiem CT, Andronico A, Bosetti P, et al. Benefits and risks associated with different uses of the COVID‐19 vaccine Vaxzevria: a modelling study, France, May to September 2021. Euro Surveill. 2021;26:2100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID‐19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26. COV2. S vaccine against Covid‐19. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26. COV2. S covid‐19 vaccine. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX‐CoV2373 covid‐19 vaccine against the B. 1.351 variant. N Engl J Med. 2021;384:1899‐1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX‐CoV2373 Covid‐19 vaccine. N Engl J Med. 2021;385:1172‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: a double‐blind, randomised, phase 1 trial. Lancet Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hitchings MDT, Ranzani OT, Torres MSS, et al Effectiveness of CoronaVac among healthcare workers in the setting of high SARS‐CoV‐2 Gamma variant transmission in Manaus, Brazil: a test‐negative case‐control study. Lancet Reg Health Am. 2021. 10.1016/j.lana.2021.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kaur SP, Gupta V. COVID‐19 Vaccine: a comprehensive status report. Virus Res. 2020;288:198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;1(2):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ogando NS, Zevenhoven‐Dobbe JC, van der Meer Y, Bredenbeek PJ, Posthuma CC, Snijder EJ. The enzymatic activity of the nsp14 exoribonuclease is critical for replication of MERS‐CoV and SARS‐CoV‐2. J Virol. 2020;94:e01246‐01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Xie X, Liu Y, Liu J, et al. Neutralization of SARS‐CoV‐2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine‐elicited sera. Nat Med. 2021;27:620‐621 10.1038/s41591-021-01270-4 [DOI] [PubMed] [Google Scholar]
- 201. Triggle CR, Bansal D, Ding H, et al. A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS‐CoV‐2 and COVID‐19 as a basis for controlling the pandemic. Front Immunol. 2021;12:631139. 10.3389/fimmu.2021.631139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 Spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell. 2020;182:812‐827.e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Baric RS. Emergence of a highly fit SARS‐CoV‐2 variant. N Engl J Med. 2020;383(27):2684‐2686. [DOI] [PubMed] [Google Scholar]
- 204. Li D‐D, Li Q‐H. SARS‐CoV‐2: vaccines in the pandemic era. Military Med Res. 2021;8:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Wibmer CK, Ayres F, Hermanus T, et al. SARS‐CoV‐2 501Y. V2 escapes neutralization by South African COVID‐19 donor plasma. Nat Med. 2021;27:622‐625. [DOI] [PubMed] [Google Scholar]
- 206. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592:616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Collier DA, De Marco A, Ferreira IA. et al. Sensitivity of SARS‐CoV‐2 B. 1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593(7857):136‐141. [DOI] [PubMed] [Google Scholar]
- 208. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS‐CoV‐2 variants B. 1.351 and B. 1.1. 7. Nature. 2021;593:130‐135. [DOI] [PubMed] [Google Scholar]
- 209. Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS‐CoV‐2 variant B. 1.351 from natural and vaccine‐induced sera. Cell. 2021;184:2348‐2361.e2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Abdool Karim SS, de Oliveira T. New SARS‐CoV‐2 variants—clinical, public health, and vaccine implications. N Engl J Med. 2021;384:1866‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Emary KRW, Tanya G, Parvinder KA, et al. Efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine against SARS‐CoV‐2 variant of concern 202012/01 (B. 1.1. 7): an exploratory analysis of a randomised controlled trial. The Lancet. 2021;397(10282):1351‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Mahase E. British Medical Journal Publishing Group; 2021.
- 213. Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2‐elicited serum. N Engl J Med. 2021;384:1466‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Shen X, Tang H, Pajon R, et al. Neutralization of SARS‐CoV‐2 variants B. 1.429 and B. 1.351. N Engl J Med. 2021;384(24):2352‐2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Hoffmann M, Arora P, Groß R, et al. SARS‐CoV‐2 variants B. 1.351 and P. 1 escape from neutralizing antibodies. Cell. 2021;184:2384‐2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Coutinho RM, Marquitti FM, Ferreira LS, et al. Model-based estimation of transmissibility and reinfection of SARS‐CoV‐2 P. 1 variant. Commun Med. 2021;1(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P. 1 SARS‐CoV‐2 lineage in Manaus, Brazil. Science. 2021;372(6544):815‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yadav PD, Sapkal GN, Abraham P, et al. Neutralization of variant under investigation B. 1.617. 1 with sera of BBV152 vaccinees. Clin Infect Dis. 2022;74(2):366‐368. [DOI] [PubMed] [Google Scholar]
- 219. Deng X, Garcia‐Knight MA, Khalid MM, et al. Transmission, infectivity, and neutralization of a spike L452R SARS‐CoV‐2 variant. Cell. 2021;184(13):3426‐3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Wadman M. California coronavirus strain may be more infectious—and lethal. Science. 2021. [Google Scholar]
- 221. Cohen J. South Africa suspends use of AstraZeneca’s COVID‐19 vaccine after it fails to clearly stop virus variant. Science. 2021;10. [Google Scholar]
- 222. Garcia‐Beltran WF, Lam EC, Denis KS, et al. Multiple SARS‐CoV‐2 variants escape neutralization by vaccine‐induced humoral immunity. Cell. 2021;184:2372‐2383.e2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wang R, Zhang Q, Ge J, et al. Analysis of SARS‐CoV‐2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity. 2021;54:1611‐1621.e1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Novelli G, Biancolella M, Mehrian‐Shai R, et al. COVID‐19 one year into the pandemic: from genetics and genomics to therapy, vaccination, and policy. Hum Genomics. 2021;15:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bignami E, Manca D, Bellini V. Riding the waves of COVID‐19 pandemics–a call for a multiobjective compromise. Trend Anaesth Crit Care. 2021;38:13‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Schmidt F, Weisblum Y, Rutkowska M, et al. High genetic barrier to SARS‐CoV‐2 polyclonal neutralizing antibody escape. Nature. 2021;592:616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV‐19 Covid‐19 vaccine against the B. 1.351 variant. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX‐CoV2373 covid‐19 vaccine against the B. 1.351 variant. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Neuzil KM. Interplay between Emerging SARS‐CoV‐2 Variants and Pandemic Control. 2021. [DOI] [PubMed] [Google Scholar]
- 230. Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26. COV2. S vaccination—response from the manufacturer. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Novavax. 11 March 2021. https://ir.novavax.com/node/15661/pdf
- 232. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV‐19 Covid‐19 vaccine against the B. 1.351 variant. N Engl J Med. 2021;384:1885‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 2021;6(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384:1372‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins In Evolving Genes and Proteins. Elsevier; 1965:97‐166. [Google Scholar]
- 236. Liu Z, VanBlargan LA, Bloyet L‐M, et al. Identification of SARS‐CoV‐2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell host microbe. 2021;29:477‐488.e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


