Abstract
Coronavirus disease 2019 (COVID‐19) is a novel disease caused by a newly identified virus Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) causing diverse systemic manifestations. The oral cavity too is not spared and the symptoms appear either independently, concurrently, or sequentially. In view of the rising documented cases of oral lesions of COVID‐19, this systematic review aims to assess the prevalence of oral manifestations in COVID‐19 confirmed individuals. An extensive literature search was conducted in databases like Scopus, Pubmed/Medline, Livivo, Lilacs and Google Scholar and varied oral signs and symptoms were reported as per the PRISMA guidelines. Studies published in English language literature only were included and were subjected to the risk of bias using the Joana Briggs Institute Appraisal tools for prevalence studies, case series and case reports. In a two‐phase selection, 34 studies were included: 21 observational, 3 case‐series and 10 case reports. These observational studies included approximately 14,003 patients from 10 countries. In this review, we explored the most commonly encountered oral and dental manifestations in COVID‐19 and identified that loss of taste acuity, xerostomia and anosmia were frequently reported. Elevated incidence of opportunistic infections like mucormycosis and aspergillosis were reported during the treatment due to prolonged intake of steroids. Immunosuppression and poor oral hygiene led to secondary manifestations like enanthematous lesions. However, it is not clear that oral signs and symptoms are due to COVID‐19 infection itself or are the result of extensive treatment regimen followed [PROSPERO CRD42021258264].
Keywords: Covid‐19, oral manifestations, SARS‐CoV‐2 infection
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- AT2
Type II alveolar cells
- COVID‐19
Coronavirus disease 2019
- HSV‐1
Herpes Simplex Virus‐1
- HZ
Herpes Zoster
- MI
myocardial injury
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐analyses
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus 2
- TNF‐α
Tumor necrosis factor‐alpha
- VZV
Varicella Zoster Virus
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by a single‐chain RNA virus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has resulted in global healthcare crisis, with grave health and socioeconomic impact. 1 , 2 The worst affected countries included United States, India and Brazil, as more than 50% of all the cases were reported from these nations. 3 The primary manifestations of COVID‐19 include pneumonia and acute respiratory distress syndrome, although many organ systems have been affected including the gastrointestinal tract, liver, central nervous systems, blood vessels, heart and kidneys. 4 , 5 , 6 Its transmission through respiratory droplets, aerosols, contact and fomites has facilitated the rapid spread worldwide. 5
The most commonly reported manifestations include symptoms common to other viral infections such as fever, cough, sore throat, myalgia, arthralgia, headache, dyspnoea, and excessive sputum production. However, an increasing number of atypical clinical presentations have been reported, such as gastrointestinal symptoms like anorexia, tremors, nausea, vomiting, and diarrhoea, 1 dermatological manifestations, and chemosensory dysfunctions. 2 COVID‐19 also causes direct injury to myocardial cells mediated by angiotensin‐converting enzyme 2 (ACE2) receptors and additionally by systemic inflammation causing indirect myocyte injury. Thus, myocardial injury, arrhythmias, cardiac arrests, heart failure and coagulation abnormality can manifest as cardiovascular abnormalities in COVID‐19 patients, which may cause serious impairment to the patient. 7 There was a positive and moderate correlation between neutrophil lymphocyte ratio values and clinical outcome of acute ischaemic stroke patients with COVID‐19. 8
Noteworthily, persistent long COVID symptoms like anxiety, prolonged depression, chest pain, palpitations, dizziness and hair loss as well as prolonged neuromuscular symptoms (fatigue, anosmia, headache, myalgia and joint pain) are a cause of grave concern in COVID‐19 patients even after two weeks of recovery. 9 , 10 In a systematic review and meta‐analysis, the authors explored the association between delirium and poor prognosis in COVID‐19 patients and suggested that the delirium in older patients can be an important presenting symptom of COVID‐19 implicating poor outcomes and high risk of mortality. 11
Along with these symptoms, this virus can affect other organs including skin, olfactory system and oral cavity. 6 Various manifestations in the oral cavity such as mucosal ulcerative and vesiculobullous lesions, 1 taste changes, gingivitis, 6 inflammation of the papillae of Wharton's duct, plaques on the tongue, 6 xerostomia, halitosis, parotiditis, 5 are reported in the literature. Oral lesions can be an inaugural sign of Covid‐19 or a warning sign of peripheral thrombosis. 6
In Covid‐19 patients, elevated Interleukin‐6 levels and C‐reactive protein implicate worse clinical outcomes as they cause significant cell damage; thus are a critical factor for shock and multiorgan failure. 12 , 13
SARS‐CoV‐2 invades human cells via the receptor angiotensin converting enzyme II (ACE2). Angiotensin‐converting enzyme 2 receptor is highly expressed in the organs at risk, such as lung, heart, oesophagus, kidney, bladder, and ileum, and located specific cell types (i.e., type II alveolar cells (AT2), myocardial cells, proximal tubule cells of the kidney, ileum and oesophagus epithelial cells, and bladder urothelial cells), which are vulnerable to 2019‐nCoV infection. 14 , 15 Besides high expression of ACE2 receptor on the epithelial cells of the tongue and of the salivary glands, could lead to the development of dysgeusia and oral mucosal ulcerations and necrosis in patients with COVID‐19. 6
Though, some of the oral manifestations may be the initial sign of the disease, however, it is still unclear as to whether these oral lesions are due to coronavirus infection or secondary manifestations resulting from local irritants or deterioration of systemic health/immunosuppression or stress or the side effects of treatment, or a combination of these or just a coexisting finding. 16 , 17 , 18 Protean oral presentations have been documented by various authors globally; therefore, a comprehensive overview on the types and prevalence of various oral manifestations is of current interest.
Furthermore, oral healthcare practitioners are expected to have a thorough knowledge of these oral manifestations as they can be referred to in case of identifying COVID‐19 infection. Thus, this systematic review is relevant and aims to identify and bring together all the available evidence on the prevalence of oral manifestations of covid 19.
2. METHODS
2.1. Protocol and registration
The present systematic review protocol was registered at the National Institute of Health Sciences, [PROSPERO, International Prospective Register of Systematic reviews database] under registration CRD42021258264. The data was searched following PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta‐analyses). 19 , 20
2.2. Study design
The present systematic review focussed on the prevalence of oral manifestations of COVID‐19 following the Population, Exposure/Intervention, Control, Outcome, Study type strategy.
2.2.1. Participants/population
COVID‐19 patients of all ages with oro‐dental manifestations.
2.2.2. Intervention, exposure
Not applicable, but exposure is COVID‐19.
2.2.3. Comparator/control
Not applicable.
2.2.4. Outcomes
Main outcome
Oral manifestations in COVID‐19 patients. All types of oral signs and symptoms.
Additional outcomes
Types and prevalence of oral mucosal manifestations such as infections (fungal, viral, and bacterial). Opportunistic lesions; autoimmune and inflammatory lesions (stomatitis); salivary gland disorders; and other oral symptoms such as dysgeusia, dysosmia.
Demographic and epidemiological characteristics of patients (age range, gender, ethnicity, habits).
2.2.5. Inclusion criteria
All the observational and cross‐sectional studies on the prevalence of oral manifestations related to COVID‐19. Due to paucity of literature and continuously evolving information on COVID‐19, case series and case reports were also included. Only English language articles published from March 2020 till February 2022 were included in this systematic review. Oral manifestations included taste disorders (dysgeusia, ageusia and hypogeusia), xerostomia, oral mucosal lesions like aphthous ulcers, geographic tongue, candidiasis, Bell's palsy, trigeminal neuralgia, erythema multiforme like lesions, herpes zoster (HZ), herpes simplex, and opportunistic infections like mucormycosis and aspergillosis.
2.2.6. Exclusion criteria
All narrative reviews, editorial letters and systematic reviews were excluded. Also, the studies where COVID‐19 positivity was not confirmed in the study subjects and where COVID‐19 patients did not report oral signs and symptoms, were not added for analysis.
2.3. Information sources and search strategy
Our electronic search included the PubMed, Scopus, Web of Science and Embase databases using various key words alternately like COVID‐19, humans, mouth diseases, oral manifestations, prevalence, SARS‐CoV‐2 on 4, 5 and 6 June 2021 and further updated our research on 1 February 2022 across these databases (Appendix Table 1). A software reference manager (EndNote X7, Thomson Reuters) was used to collect references and remove duplicate articles.
2.4. Study selection
The article selection was completed by two authors in 2 steps. In step 1, two authors (PS and SM) separately screened the titles and abstracts of all the references through Rayyan software. Only those articles which matched the inclusion criteria were tabbed and the remaining publications were rejected. The concluding decision was taken in consultation with the third author (VW). In step 2, we followed the same selection criteria and only those published full‐text articles were selected which described the prevalence of oral manifestations and oral mucosal lesions in COVID‐19 patients. The same 2 authors were associated independently in step 2. All selected articles were critically and intensely analysed by 3 authors and the new publications were also chosen for selection analysis. If there was any difference of opinion in either of the steps, it was settled by collective consensus among the 3 authors. Eventually, only full‐text articles were preferred and selected for this systematic review.
2.5. Data collection
At the outset, the first (PS) and second (SM) authors extracted the data from the chosen references. An extraction form was developed to list the essential information on the authors, name of the country, year of study, study design, number of subjects, mean age, gender, severity of COVID‐19, clinical features and oral manifestations.
This was followed by the third author (VW) verifying the compiled data and affirmed its accuracy/preciseness/authenticity. If there was disagreement on any issue, it was resolved by discussion and mutual agreement among all the authors. However, final decision was taken by first and second authors. In some articles, if the required information was missing, efforts were made to contact the authors of these publications and the necessary data was filled in the excel sheet.
2.6. Risk of bias within studies
The risk of bias of included studies was assessed by 2 authors (PS and SM) independently using a quality assessment checklist for prevalence studies, case reports and case series adapted by the Joanna Briggs Institute's Critical Appraisal checklist (Munn et al 2015 and Moola et al 2017). In case of difference of opinion, the third author (VW) was consulted. For each article, scoring was concluded only after consultation with all authors, and a study was specified as a high risk of bias when the ‘yes’ score was up to 49%, moderate when 50%–69% and low when >70%. 21 , 22
2.7. Summary measures
In the present systematic review, the chief outcome was the prevalence of all types of oral manifestations in COVID‐19 patients. The additional outcomes included types and prevalence of oral mucosal manifestations, namely, fungal, viral and bacterial infections; autoimmune and inflammatory lesions, salivary gland disorders, neuropathies, taste and smell disturbances, demographic and epidemiological characteristics of patients (age, gender, geographic region, habits).
3. RESULTS
3.1. Study selection and characteristics
Initially, 3643 records were recognized from databases. After removing duplicate publications, 1732 references were left for title and abstract screening. After analysing all the records, finally 72 full‐text articles were shortlisted for the second phase. Both the authors (PS and SM) completed full‐text reading and excluded 30 articles according to the eligibility criteria. Finally, 34 studies were selected for synthesis, of which 21 were prevalence studies, 10 were case‐reports and three were case series on various oral mucosal lesions in COVID‐19 confirmed individuals. A flow‐chart following PRISMA guidelines is presented in Figure 1. The oral presentations were widely distributed globally, as cases were reported from Italy, Germany, Belgium, Spain, Turkey, Portugal, Brazil, Mexico, Egypt, UK, Israel, India and US. Only English language articles were identified which were published from March 2020 till February 2022.
FIGURE 1.
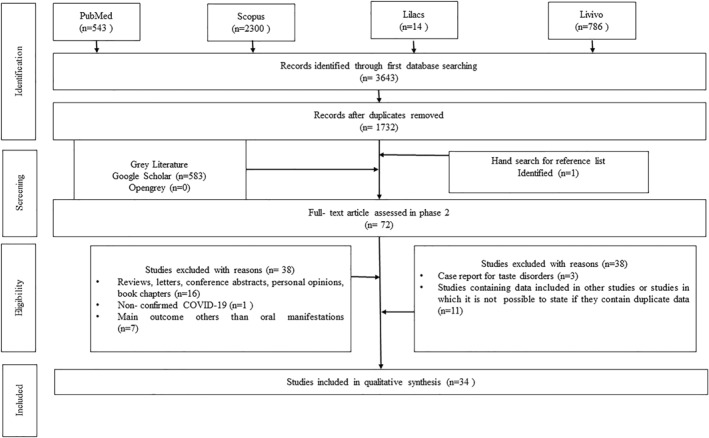
Flow‐diagram depicting selection criteria adapted from Preferred Reporting Items for Systematic Reviews and Meta‐analyses
3.2. Risk of bias within studies
All included studies were assessed for risk of bias following Joana Briggs Institute guidelines and the observations are summarised in Table 1 and Table 2 and details are shown in Appendix Table 3. Prevalence studies, case reports and case series were evaluated with the specified checklist for each study design. 21 , 22
TABLE 1.
Distinctive features of observational studies included in the systematic review (n = 21)
| S. No. | Study Id | Study design | Sample (total no) | Age,Y, mean | Covid‐19 grade/Severity | Clinical signs and symptoms | Oral manifestations | Time of oral presentation | Diagnostic method of oral manifestations | Conclusion | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gherlone EF et al (2021), Milan, Italy | Observational study | 122 (M‐75%, F‐25%) | 62.5 (53.9–74.1 y) for M, 30 (24.6) Y for F | Hospitalised (115), ICU (30 patients out of 122 transferred to ICU | Not reported (confirmed COVID‐19 cases included) | Salivary gland ectasia (46), both dry mouth and salivary gland ectasia (13), masticatory muscle weakness (23), TMJ abnormalities (9), dysgeusia (14), anosmia (12), facial tingling (4), trigeminal neuralgia(4), facial asymmetry (1) | Post‐recovery follow‐up | Extraoral and intraoral physical examination by experienced dental specialist | Oral cavity is a possible target of COVID‐19, with alterations persisting in the vast majority of survivors well after clinical recovery. | Low |
| 2 | Maia CMF et al, 2021, Brazil | Observational study | 8695 (observed during covid period) | Not reported | Hospitalised and non‐hospitalised | Not reported (confirmed COVID‐19 cases included) | Herpes zoster | Before and during COVID‐19 infection | Medical records | There was an increase in HZ cases during the COVID‐19 pandemic, which suggests a correlation between these diseases. | Moderate |
| 3 | Nuno Gonzalez A et al, 2020, Spain | Observational study | 666 | 55.67 years F‐ 58%, M‐42% (not reported) | Hospitalised | Confirmed cases of COVID‐19 | Oral cavity findings were seen in 78 (25.65%) cases, including transient lingual papillitis (11.5%), glossitis with lateral indentations (6.6%), aphthous stomatitis (6.9%), glossitis with patchy depapillation (3.9%) and mucositis (3.9%), enanthema (0.5%), white tongue (1.6%), candidiasis (1%). Burning sensation was reported in 5.3% of patients and taste disturbances (dysgeusia) was commonly associated. | During COVID‐19 infection | Extraoral and intraoral physical examination | Almost half of patients with mild to moderate COVID‐19 admitted in a field‐hospital during a 2‐week period show mucocutaneous findings. Oral cavity is frequently involved and deserves specific examination under the appropriate circumstances to avoid contagion risk. | Low |
| 4 | Biadsee A et al, 2020, Israel | Observational study | 128 (58 M, 70 F) | 36.25 (18–73 Y) | Non‐hospitalised | Cough, weakness, myalgia, fever, headache, impaired sense of smell, sore throat, runny nose, nasal congestion, gastrointestinal symptoms. | Changes in taste sensation, dry mouth, facial pain, masticatory muscle pain, burning sensation, change in tongue sensation, plaque‐like changes in the tongue | During COVID‐19 infection | Web based questionnaire | A considerable number of patients presented with olfactory and oral disorders. Interestingly, women presented with a different cluster of symptoms than men, which may suggest a new clinical approach to diagnosing COVID‐19 disease. | Moderate |
| 5 | Fantozzi PJ et al, 2020, Italy | Observational study | 111, 58 were males, 53 were females | Median age −57 years | Hospitalised, ICU | Fever, cough, dyspnoea, diarrhoea, sore throat, fatigue, myalgia, vomit | Taste dysfunction was the most common reported symptom (59.5%; n = 66), followed by xerostomia (45.9%; n = 51) and olfactory dysfunctions (41.4%; n = 46). | During COVID‐19 infection | Ouestionnaire based survey | Xerostomia, olfactory and gustatory dysfunctions are common symptoms reported as concomitant, and in some cases the sole manifestation of COVID‐19. | Low |
| 6 | Katz J and Yue S, 2021, Florida, USA | Observational study (retrospective cross‐sectional) | RAS and COVID‐19‐ (0.64%) | 18–34 Yr (66%), 10–17 (33%) | Hospitalised and non‐hospitalised | Confirmed cases of COVID‐19 | Prevalence of RAS in covid‐19 patients was 0.64%. | During COVID‐19 infection | Medical records | There is a strong association between COVID‐19 and aphthous ulcers | Low |
| 7 | Giacomelli A et al, 2020, Italy | Cross‐sectional study | 59 | 60 years (50–74) M‐40, F‐19 | Hospitalised | Fever, cough, dyspnoea, sore throat, arthralgia, coryza, headache, asthenia, abdominal symptoms | Olfactory and/or taste disorders (20), taste disorders only (6), olfactory disorders only (3), mixed taste and olfactory disorders (11) | 12 patients (20.3%) presented the symptoms before the hospital admission, whereas 8 (13.5%) experienced the symptoms during the hospital stay. Taste alterations were more frequently (91%) before hospitalisation, whereas after hospitalisation taste and olfactory alteration appeared with equal frequency. | Questionnare based survey | OTDs are fairly frequent in patients with SARS‐CoV‐2 infection and may precede the onset of full‐blown clinical disease. I | Moderate |
| 8 | Sinjari B et al, 2020, Italy | Observational study | 20 | 69.2 Y, 55% M, 45% F | Hospitalised | Confirmed cases of COVID‐19 (clinical manifestations not reported) | Xerostomia (30%), impaired taste (25%), burning sensation (15%), difficulty in swallowing (20%) | During COVID‐19 infection | Questionnaire based survey | This study demonstrates the importance of the close link between SARS‐CoV‐2 and oral manifestations. Dysgeusia may be a warning signal for the patients. | Low |
| 9 | Abubakr N et al, 2021, Egypt | Observational study | 573 | 36.19 ± 9.11 years (range: 19–50 years). | Non‐hospitalised (mild to moderate cases) | Mild to moderate cases | Oral or dental pain (23%), pain in jaw bones or joint (12.0%), halitosis (10.5%), ulcerations (20.4%), and xerostomia (47.6%). Some patients (28.3%) showed 2 or 3 manifestations simultaneously. | During COVID‐19 infection | Mild‐to‐moderate cases of COVID‐19 infection are associated with oral symptoms, and thus the significance of dental examination of patients with communicable diseases should be emphasised. | Low | |
| 10 | El Kady DM et al, 2021, Egypt | Observational study | 58 (53.4% M, 46.6% F) | 18–46 Y | Hospitalised | Fever, cough, shortness of breath, and myalgia or weakness, headache, sputum formation, haemoptysis, diarrhoea | The highest prevalence symptoms were dry mouth 39.7% (n = 23), gustatory dysfunction as 34.5% (n = 20) loss of salt sensation, 29.3% (n = 17) loss of sweet sensation, and 25.9% (n = 15) altered food taste, while the least prevalent symptoms were tongue redness 8.8% (n = 5), and gingival bleeding 7% (n = 4). The most frequently associated symptoms were loss of salt and sweetness, as reported by 27.6% of the participants. | During COVID‐19 infection | Questionnaire based survey (online) | COVID‐19 significantly impacts the oral cavity and salivary glands, as salivary gland‐related symptoms and taste disorders are highly prevalent in COVID‐19 patients. | Low |
| 11 | Moorthy A et al, 2021, Karnataka, India | Retrospective observational study | 18 (15 M, 3 F) | 35–73 years with a mean age of 54.6 years. | Hospitalised | Facial cellulitis, maxillary sinusitis, headache, necrosis of palatal bone/mucosa or acute loss of vision | The fungi noted was mucormycosis in 16 patients, aspergillosis in 1 patient and a mixed fungal infection in 1 patient. | During COVID‐19 infection | Physical extraoral and intraoral examination | There is a significant increase in the incidence of angioinvasive maxillofacial fungal infections in diabetic patients treated for SARS‐CoV‐2 with a strong association with corticosteroid administration. | Low |
| 12 | Bardellini E et al, 2021, Italy | Retrospective cross‐sectional study | 27 children | 4.2 years + 1.7) | Non‐hospitalised | Fever, cough, cutaneous flat papular lesions | Oral pseudomembranous candidiasis (7.4%), geographic tongue (3.7%), coated tongue (7.4%) and hyperaemic pharynx (37%). Taste alteration was reported by 3 patients. | During COVID‐19 infection | Medical records reviewed | As for paediatric sample, COVID‐19 resulted to be associated with non‐specific oral and cutaneous manifestations. There are no specific oral manifestations in children during a COVID‐19 infection. | Moderate |
| 13 | Klein H et al, 2021, Israel | Observational study | 103 (64 M, 39 F) | 35 ± 12 years | Non‐hospitalised | Headache, fever, muscle aches, dry cough, lack of appetite, runny nose, sore throat, productive cough, fatigue, smell change, diarrhoea, vomiting/nausea, breathing difficulty | Taste change. Taste and smell changes were the longest lasting symptoms | During COVID‐19 infection and during recovery period | Questionnaire (phone interview) | Long‐lasting effects of mild COVID‐19 manifested in almost half of the participants reporting at least one unresolved symptom after 6 months. | Moderate |
| 14 | Katz J, 2021, Florida, USA | Cross sectional study | 889 (385 M, 504 F) | Children<18 = 38 (4%) | Hospitalised | Not reported | Dry mouth (Sicca dry mouth and non‐sicca dry mouth) in 9 patients (1.01%) | During COVID‐19 infection | i2b2 data repository platform of university of Florida health centre | Dry mouth related to Sicca and not related to Sicca are strongly associated with COVID‐19. The causes for this are not clear and may include autoimmunity & comorbidities. | Low |
| Adults>18 = 851 (96%) | |||||||||||
| 15 | Villaroel‐Dorrego M et al, 2021, Spain | Observational study | 55 (25 F and 30 M) | 1–89 years, mean 51 ± 23.24 years | Hospitalised (19 in ICU) | Confirmed COVID‐19 cases (clinical manifestations not reported) | Candidiasis and ulcers (7 each), enanthems (2 pts.), geographic tongue and caviar tongue. Altered taste, dry mouth, and painful/burning mouth were noted in 60%, 27.3%, and 36.4% of patients, respectively. Oral mucosal alterations and lesions were prevalent in this series of COVID‐19 patients. Altered taste and a painful/burning mouth were common symptoms. | During COVID‐19 infection | Clinical examination of the lesions with findings recorded in a database | Ulcers were the most commonly observed lesions and included both haemorrhagic and aphthous‐like lesions. Both dysgeusia and oral pain or burning were common in patients with mucosal lesions (68.2% and 77.3%, respectively). In 22 patients (40%) at least one alteration or lesion was observed in the oral mucosa | Moderate |
| 16 | Katz J, 2021, USA | Cross sectional study | 889 | Not reported | Hospitalised | Not reported | Candidiasis only assessed. | During COVID‐19 infection | i2b2 data repository platform was used to analyse the interrelations between COVID‐19, oral candidiasis, and total candidiasis | Total candidiasis was significantly associated with increased risk for COVID‐19, whereas oral candidiasis showed an insignificant trend. COVID‐19 may be a risk factor for total candidiasis. | Low |
| 17 | Subramaniam T et al, 2021, Pune India | Observational study | 713 patients, 416 M, 297 F | 12–80 years | Hospitalised | Not reported | 9 patients reported oral discomfort due to varied forms of oral lesions ranging from herpes simplex ulcers to angular cheilitis (1.26%). | During COVID‐19 infection | Photographs taken of the lesions from a camera, assessed by specialists. | No specific pattern or characteristic oral lesions were noted in a study of 713 COVID‐positive patients in this study to qualify these lesions as oral manifestations of SARS‐CoV‐2 infection. | Low |
| 18 | Freni F et al, 2020, Italy | Observational study | 50 (30 M, 20 F) | 37.7 ± 17.9 | Non‐ hospitalised | Confirmed COVID‐19 cases | Xerostomia, difficult in swallowing food | During COVID‐ 19 infection | Questionnaire based survey | There was an alteration of the sense of taste, of the sense of smell, dry eyes and of the oral cavity and an auditory discomfort, symptoms probably linked to the neurotropism of the virus | Low |
| 19 | Omezli MM and Torul D, 2021, Ordu, Turkey | Observational study | 107 (56 M, 51 F) | 17–65 Y F, 13–70 Y M | Non‐ hospitalised | Confirmed COVID‐19 cases | Xerostomia, burning mouth, pain on chewing | After recovering from COVID‐ 19 infection | Questionnaire based survey | The most frequent finding in patients after the treatment was xerostomia. Taste and smell impairments were more frequently observed in females. | Low |
| 20 | D Giacomo P et al, 2021, Rome, Italy | Observational study | 214 | Not reported | Non hospitalised | Psychological impact of COVID‐19 pandemic on subjects with TMD | Temporomandibular disorders (TMD) | During COVID 19 pandemic | Questionnaire based survey | Awake and sleep bruxism, dental grinding, alteration in the quality and quantity of sleep and fatigue increased | Low |
| 21 | Colonna A et al, 2021, Ferrarra, Italy | Observational study | 506 | Not reported | Non hospitalised | Psychological status, bruxism in TMD reported individuals | Increase in symptoms of TMD | During COVID 19 pandemic | Online survey | 36% and 32.2% of participants reported increased pain in the TMJ and facial muscles, respectively, and almost 50% of the subjects also reported more frequent migraines and/or headaches | Low |
Abbreviation: TMJ, temporomandibular joint.
TABLE 2.
Distinctive features of case series(n = 3) and case reports (n = 10) included in the systematic review
| S. No. | Study Id | Study design | Sample (total no) | Age,Y, mean | Covid‐19 grade/Severity | Clinical signs and symptoms | Oral manifestations | Time of oral presentation | Diagnostic method of oral manifestations | Conclusion | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tapia ROC etal, 2020, Mexico | Case series | 4 (3 F, 1 M) | 41 F, 51 F, 55 F, 42 M (mean age range‐47.2 ± 6.8.) | Non‐hospitalised (Case 1), hospitalised (Case 2), non‐hospitalised (Case 3), non‐hospitalised (Case 4) | Fever, myalgia, headache, dysphagia, hyposmia, nasal congestion | Angina bullosa hemorrhagica, vascular‐like purple macule on palatal mucosa, burning mouth, dysgeusia and erythematous macules | During COVID‐19 infection | Extraoral and intraoral physical examination | It is important to consider that oral mucosal lesions in COVID‐19 individuals could mimic others oral diseases, such as reactive, vascular and immunological disorders, being necessary to differentiate them to establish the correct diagnosis and clinical management in patients with SARS‐CoV‐2 infection. | Moderate |
| 2 | Fisher J et al, 2021, Boston, USA | Case report | 1 | 21 Y, F | Non‐hospitalised | Fever, cough, dyspnoea, left sided facial and neck swelling | acute infectious parotitis, malocclusion due to inflammation surrounding muscles of mastication | During COVID‐19 infection | Physical extraoral and intraoral examination | Atypical presentations of COVID‐19 are being increasingly recognized. Emergency department clinicians must have a high suspicion for COVID‐19 among any patient presenting with infectious symptoms or viral‐associated illnesses and don available PPE accordingly for the initial evaluation. | Moderate |
| 3 | Lima MA et al, 2020, Brazil | Case series | 8 | Females were 7, Male‐1, Mean‐36 years | Non‐hospitalised | Mild respiratory and systemic COVID‐19 SYMPTOMS | Facial palsy | During COVID‐19 infection | Physical extraoral and intraoral examination | Peripheral facial palsy should be added to the spectrum of neurological manifestations associated with COVID‐19. Most patients had an uncomplicated course with good outcome, and SARS‐CoV‐2 RNA could not be detected in Cerebrospinal Fluid (CSF) of any patient. | Low |
| 4 | Ferreira ACAF et al, 2020, Brazil | Case report | 1, M | 39 Y | Hospitalised | Fatigue, diarrhoea, fever | Left facial herpes zoster with intraoral mucosal lesions, hypogeusia | During COVID‐19 infection | Physical extraoral and intraoral examination | The emergence of the latent infection by VZV under a rare presentation might illustrate the impact at least locally of COVID‐19, once retrograde reactivation of VZV was possibly induced in a young immunocompetent patient. | Low |
| 5 | Taslidere B et al, 2021, Turkey | Case report | 1 | 51 Y, F | Hospitalised | Malaise, pneumonia | Hyperaemic, firm oedema in the right lower lip extending towards the jaw, right facial paralysis, fissured tongue, (Melkersson‐Rosenthal syndrome) | During COVID‐19 infection | Physical extraoral and intraoral examination | Activated mast cells also play a part in the pathogenesis of COVID‐19 infection, as they release cytokines in the lungs. COVID‐19 may be associated with which was not previously included in the aetiology of the disease. | Low |
| 6 | Figueiredo R et al, 2020, Portugal | Case report | 1 | 35 years, F | Non‐hospitalised. (Admitted due to 39‐week pregnancy in gynaecology department) | Lagophthalmos, No other symptoms including fever, dyspnoea, cough, anosmia, ageusia | Bell's palsy showing involuntary drooling, left side labial commissure deviation | During COVID‐19 infection | Physical extraoral and intraoral examination | COVID‐19 may be a potential cause of peripheral facial paralysis.Neurological symptoms could be the first and only manifestation of the COVID‐19 | Low |
| Pregnant women have higher susceptibility for peripheral facial palsy and functional prognosis can be worse. | |||||||||||
| 7 | Lechien JR et al, 2020, Belgium | Case series | 3 | 23/F, 31/F, 27/F | Non‐hospitalised | Anorexia, arthralgia, myalgia, fatigue, headache, nasal obstruction, rhinorrhoea, postnasal drip, sore throat, face pain, loss of smell and taste | Parotitis characterised by ear pain, retromandibular oedema, sticky saliva, pain during chewing | During COVID‐19 infection | Physical extraoral and intraoral examination | Parotid inflammation might be encountered in COVID‐19 patients and could be related to intraparotid lymphadenitis. | Low |
| 8 | Caamano DSJ and Beato RA, 2020, Spain | Case report | 1 | 61/M | Non‐hospitalised | Fever, cough | Bilateral facial nerve palsy with unresponsive blink reflex on both eyes, Guillain‐Barré syndrome (GBS) | During COVID‐19 infection | Physical extraoral and intraoral examination | There is a clear emerging group of neurological manifestations during and after SARS‐CoV‐2 infection; some directly linked, others not so much.case is a highly probable GBS DP variant. | Low |
| 9 | Kammerer T et al, 2021, Germany | Case report | 1 (M) | 46Y | Hospitalised (ICU) | Fatigue, dry cough, fever, respiratory distress | Multiple sharply circumscribed ulcerations of the oral mucosa covered by yellow–grey membranes. Secondary herpetic gingivostomatitis | During COVID‐19 infection | Physical extraoral and intraoral examination | COVID‐19 infection and prolonged inpatient care were causal factors of stress induction and immunosuppression, leading to the distinct oral manifestations. | Low |
| 10 | Kitakawa D et al, 2020, Brazil | Case report | 1 | 20 Y/F | Non‐hospitalised | Severe sore throat and headache | Lesions in the median lower lip semimucosa and severe pruritus, with a clinical course of 14 days, clinical diagnosis of herpes simplex infection. | During COVID‐19 infection | Physical extraoral and intraoral examination | Most of these cases could occur in patients who have experienced COVID‐19 infection | Low |
| 11 | Andrews E et al, 2020, USA | Case report | 1 | 40 Y/F | Hospitalised (ICU) | Fevers, shortness of breath and diarrhoea, acute respiratory distress | Severe, persistent macroglossia following prone positioning as part of treatment for COVID‐19. | During COVID‐19 infection | Physical extraoral and intraoral examination | Patient's macroglossia was the result of a prolonged course of prone positioning for treatment of COVID‐19. | Low |
| 12 | Gil JM et al, 2021, Spain | Case report | 1 | 65 Y/M | Non‐hospitalised | General malaise, arthromyalgia, dry cough, and low‐grade fever. | Paroxysmal lancinating pain in the right V1 region that lasted a few seconds and was triggered by a light touch of the skin at a specific point on the scalp. Diagnosed as trigeminal neuralgia. | During COVID‐19 | Physical extraoral and intraoral examination | The new coronavirus SARS‐CoV‐2 is a possible aetiology of secondary Trigeminal neuralgia (TN). Nevertheless, more studies are needed to elucidate the neuropathology of this viral infection. | Low |
| 13 | Soares CD et al, 2020 | Case report | 23 Y/F | 23 Y | Non‐hospitalised | Fever and dry cough 3 days prior to oral presentation | Vesiculobullous lesions in the lips with an erythematous halo | During COVID‐19 | Physical extraoral and intraoral examination | This is the first report showing the SARS‐CoV‐2 spike protein in oral lesions of patients with COVID‐19. | Low |
Abbreviation: PPE, personal protective equipment.
Most prevalence studies (n = 15; 71.4%) presented low risk of bias overall, however, six studies (28.5%) had moderate risk of bias. Similarly low risk of bias was observed in almost all case reports (n = 9; 90%) and case series (n = 2; 66.7%) analysed in this systematic review.
3.3. Synthesis of studies
A total of 14,003 patients from prevalence studies with COVID‐19 were included in this systematic review. For confirmation, Real time reverse transcription polymerase chain reaction test was used for detection of viral RNA.
Olfactory and gustatory disorders were found to be closely associated and were the most commonly reported oral manifestations, followed by xerostomia, anosmia, vesiculobullous lesions and oral ulcers.
Analysis of 21 observational studies on 14,003 patients revealed that there was heterogeneity in the data and it was difficult to draw a conclusive inference of the most prevalent symptom. Nevertheless, 66.7% of the prevalence studies reported primary oral manifestations directly due to COVID‐19 like taste alterations, xerostomia, and salivary gland diseases. One prevalence study conducted showed an increase in the incidence of HZ cases during Covid pandemic times. While four observational studies (19.04%) showed multiple secondary oral manifestations like sialadenitis, dry mouth, mucocutaneous lesions, aphthous ulcers, enanthema, opportunistic infections like mucormycosis, aspergillosis, oral pseudomembranous candidiasis, geographic tongue, coated tongue. Significantly, in at least two questionnaire‐based surveys, Temporomandibular disorders (TMD) were found to be increased, emphasising the psychological impact of COVID‐19.
Additionally, oral mucosal lesions were presented only in case reports and case series. These included primary manifestations probably caused directly by SARS‐CoV‐2 and secondary manifestations caused as a sequelae and treatment of COVID‐19 infection (Table 2). Infection like oral ulcers, angina bullosa hemorrhagica, burning mouth, erythematous macules, plaque‐like changes in the tongue, masticatory muscle pain, acute infectious parotitis and secondary manifestations like facial palsy, enanthema resembling petechiae without erythema, Melkersson‐Rosenthal syndrome, Bell's palsy, parotitis, Guillain‐Barre syndrome, secondary herpetic gingivostomatitis, macroglossia, and trigeminal neuralgia.
While, hospitalised patients were considered as cases of severe COVID‐19, the non‐hospitalised patients were categorised as mild to moderate COVID‐19 cases. Most of the oral presentations were observed during covid‐19 infection period (n = In 61.9% of the observational studies, oral manifestations presented in severe cases of COVID‐19). Taste disorders were more commonly observed in severe cases of COVID‐19 (53.8%). At least three cross‐sectional studies (14.3%) observed taste dysfunction and xerostomia during follow‐up after the recovery of COVID‐19.
In one case series, 4 patients presented with angina bullosa hemorrhagica on palatal mucosa, four patients showed facial paralysis and another one patient presented with acute infectious parotitis and malocclusion. There was one patient showing HZ and two patients showing herpes simplex infection. Melkersson‐Rosenthal syndrome was shown by one patient and trigeminal neuralgia was observed in one Covid‐19 patient. In one case report, macroglossia was seen as a sequelae of severe Covid‐19 patient admitted in ICU due to intubation for a prolonged period.
4. DISCUSSION
Many cases on oral manifestations as primary and secondary presentations in COVID‐19, have been reported and published from many countries as case reports, case series, editorial letters and cross‐sectional studies. Nevertheless, there is a scarcity of studies addressing the incidence of oral manifestations in COVID‐19. Dentists may play a very crucial role in the diagnosis of COVID‐19 infection as some oral presentations are the first sign and symptom of this disease. Loss of smell and taste alterations have been reported to be the initial symptoms even before the common signs and symptoms of this disease like dry cough, dyspnoea, sore throat and fatigue appear. Incidentally, taste and smell dysfunctions are also the longest lasting symptoms of SARS‐CoV‐2 infection. 23 , 24 , 25
4.1. Taste impairment
Dysgeusia (altered taste), hypogeusia (diminished sense of taste) and ageusia (complete loss of taste) were the most commonly reported initial symptoms even before the diagnosis of COVID‐19 was confirmed. 26 , 27 , 28 , 29 , 30 The most probable pathogenesis of taste disturbances in COVID‐19 is due to peripheral neurotropism and direct toxicity to taste buds or olfactory epithelium. Other contributory factors may include inadequate saliva, pro‐inflammatory cytokines, angiotensin II accumulation, systemic diseases, hypozincemia, and excessive use of chemicals. 31 , 32 Also, published studies state that angiotensin‐converting enzyme 2 (ACE 2) cell receptors are expressed in abundance on respiratory epithelium and oral mucosa, mainly dorsal surface of the tongue. Incidentally, SARS‐CoV‐2 has a great affinity for these receptors. 31 , 32 Anosmia or loss of smell sensation is very often observed and reported in association with taste disturbance in COVID‐19 patients. Furthermore, it is not easy for the patient to differentiate between taste and smell disorders. It remains ambiguous whether gustatory dysfunction is seen as a result of olfactory dysfunction or it is the primary manifestation of the SARS‐CoV‐2 virus. 30 , 31 , 32 Experimental studies that document the temporal relationship in the commencement of these two symptoms, need to be conducted.
It was suggested by the American Academy of Otolaryngology that anosmia, hyposmia and dysgeusia should also be combined in the list of screening tools for COVID‐19 in asymptomatic patients. 33 Considering the published data on the most frequently observed and most significant symptoms of SARS‐CoV‐2 infection, the olfactory and taste dysfunctions were formally acknowledged by The US Centres for Disease Control and Prevention. 34
A systematic review conducted on 40 studies (33 cross‐sectional and 7 case‐reports) which included 10,228 patients concluded that gustatory dysfunction may be the most recognized symptom in COVID‐19 patients and should be considered in the scope of the disease's onset and progression. 35 Additionally, oral mucosal lesions have a high probability of manifesting as coinfections and secondary presentations with various clinical aspects such as white and erythematous plaques, irregular ulcers, small blisters, petechiae, and desquamative gingivitis. 18
A Web‐based questionnaire on 128 COVID‐19 patients was used to report findings in an observational study conducted by Biadsee A et al (2020), 36 which revealed that majority of the patients presented with olfactory and oral disorders like dysgeusia, dry mouth, masticatory muscle pain, burning sensation, plaque‐like changes in the tongue. A new clinical approach to diagnosing COVID‐19 disease was suggested as women manifested with different symptoms than men.
Taste dysfunction was the most common reported symptom followed by xerostomia and olfactory dysfunction in a questionnaire‐based survey conducted in an observational study on confirmed COVID‐19 individuals in Italy. 37
In 59 hospitalised COVID‐19 individuals, questionnaire‐based survey revealed that olfactory and taste disorders appeared with equal frequency both before and after the hospital admission. 31 While another observational study, also based on questionnaire‐based survey, emphasised the importance of the close relationship between SARS‐CoV‐2 and oral manifestations and implicated dysgeusia to be the alarming signal for such patients. 38
A systematic review and meta‐analysis found olfactory and gustatory dysfunctions as common symptoms in patients with COVID‐19 and may represent early symptoms in the clinical course of infection. 39 Also, taste and smell dysfunctions were the longest lasting symptoms as they were manifested even after 6 months, as reported in a questionnaire‐based survey conducted in Israel on 103 COVID‐19 patients. 40
Additionally, SARS‐CoV‐2 causes activation of cytokines triggering apoptosis of cells as well as abnormal cell turnover. Consequently, it results in loss of taste buds and failure of differentiation of different types of taste cells manifesting taste dysfunctions in COVID‐19 patients. SARS‐CoV‐2 also affects the peripheral gustatory neurons, causing direct damage of ACE‐2 expressing cells of the peripheral taste neurosensory chemoreceptors and the cranial nerves VII, IX, or X, which are responsible for gustation. It is also hypothesised that zinc imbalance in these patients leads to infection & inflammation of taste buds with SARS‐CoV‐2 causing acute hypozincemia due to zinc chelation and alteration in zinc haemostasis, resulting in taste disturbances. Taste dysfunctions also occur secondary to smell dysfunction, medications and reduced sialic acid concentration. 5
In concordance with other studies, majority of the prevalence studies included in our systematic review observed taste dysfunction and xerostomia as the most common symptom reported early before the manifestation of other symptoms of Covid‐19.
4.2. Oral mucosal lesions
Aphthous ulcers, muco‐cutaneous lesions, erosions, plaque‐like lesions, geographic tongue, candidiasis, mucormycosis, angina bullosa hemorrhagica, herpes zoster ulcers, herpes simplex reactivation (HSV‐1) associated ulcers, sialadenitis have been reported in COVID‐19 suspected individuals as well as confirmed cases. The pathogenesis of occurrence of oral mucosal lesions in COVID‐19 is still not clear. Many published studies report that the oral lesions are manifested as a result of reduced immunity in COVID‐19 patients, or as consequent manifestation of the treatment administered for COVID‐19. 41 , 42 , 43 , 44 , 45 , 46 , 47
It is hypothesised that ulcers and erosions are observed clinically due to direct damage to oral mucosa caused by binding of SARS‐CoV‐2 with oral epithelial cells (keratinocytes and non‐keratinocytes). Therefore, there is increased cell permeability and entry of SARS‐CoV‐2 inside epithelial cells. Also, inflammation may be localised or systemic leading to production of inflammatory cytokines and TNF‐α, causing chemotaxis of neutrophil to inflammatory site of oral mucosa, causing aphthous ulcers. Inflammation in SARS‐CoV‐2 can also cause vasculitis, thrombotic vasculopathy, drug eruption and can also present as non‐specific ulcers, erosions, vesiculobullous lesions and mucositis. 5
Noteworthily, acute infection, medication, neglected oral hygiene, stress and deterioration of systemic health also causes immune system suppression, which leads to manifestation of vesiculobullous lesions, non‐specific oral ulcers, eruptions, recurrent oral herpes simplex virus (HSV‐1) infection (Herpes gingivostomatitis). 5 Different studies have published various oral manifestations which are listed below.
4.3. Muco‐cutaneous manifestations
An observational study carried out on 666 confirmed cases of COVID‐19 patients in Spain, revealed that oral manifestations like transient lingual papillitis, glossitis with lateral indentations, aphthous ulcers, glossitis with patchy depapillation and mucositis, enanthema, candidiasis and burning sensation were observed. Noteworthily, taste disturbances like dysgeusia was frequently associated in this viral infection. The authors concluded that almost half of patients with mild to moderate COVID‐19 infection admitted in a field‐hospital during a 2‐week period show muco‐cutaneous findings. Therefore, oral cavity is frequently involved and must be examined thoroughly under the appropriate conditions to prevent contagion risk. 48
Acute infection, medication, neglected oral hygiene, stress, deterioration of systemic health causes immune system suppression, which leads to manifestation of vesiculobullous lesions, non‐specific oral ulcers, eruptions, recurrent oral herpes simplex virus (herpes simplex reactivation) infection (Herpes gingivostomatitis). 41 , 42
In the present systematic review, almost all these oral lesions were observed during COVID‐19 infection period except the TMD which were the long‐lasting symptoms manifesting even after the recovery of the infection.
4.4. Aphthous ulcers
In a retrospective cross‐sectional study, medical records were reviewed of COVID‐19 patients, and the prevalence of aphthous ulcers was 0.64% compared with 0.148% in the hospital population that served as the control group, implicating a strong association between COVID‐19 and aphthous ulcers. 45
Mild to moderate cases of COVID‐19 infection were associated with oral symptoms like ulcerations, xerostomia, oral pain and pain in the jaw bones in an observational study carried out using online questionnaire on 573 patients. 47
4.5. Herpes zoster
According to an observational study conducted on 8695 patients in Brazil, there was an increase in HZ cases during the COVID‐19 pandemic, which suggests a correlation between these diseases. A case was reported with left facial HZ with intraoral mucosal lesions and hypogeusia in Brazil, implicating the emergence of the latent infection by varicella zoster virus (VZV) under a rare presentation. This illustrates that retrograde reactivation of VZV could be induced in a young immunocompetent COVID‐19 patient. 49
Physical intraoral and extraoral examination of a 39 ‐year old Brazilian COVID‐19 patient showed facial HZ with intraoral mucosal lesions. Thus, the retrograde reactivation of VZV was probably induced in this young immunocompetent patient, indicating the rare possibility of the impact of COVID‐19. 50
4.6. Herpetic gingivostomatitis
COVID‐19 infection and prolonged hospitalisation could cause stress and immunosuppression leading to secondary herpetic gingivostomatitis, as observed in a 46 ‐year old confirmed COVID‐19 patient who was hospitalised in intensive care unit, showing multiple sharply circumscribed ulcerations of the oral mucosa covered by yellow‐grey membranes. Intraoral examination of COVID‐19 patient showed lesions in the median lower lip semimucosa and severe pruritus which persisted clinically for 14 days, and was clinically diagnosed as herpes simplex infection. 51
4.7. Angina bullosa hemorrhagica
Four confirmed cases of COVID‐19 were physically examined where prominent lesions were angina bullosa hemorrhagica, vascular‐like purple macule on palatal mucosa, burning mouth, dysgeusia and erythematous macules. Therefore, it is significant to remember that oral mucosal lesions in COVID‐19 subjects could resemble reactive, vascular and immunologic disorders, making it essential to differentiate them to arrive at a correct diagnosis to effectively manage such patients. 52
4.8. Salivary gland involvement
Patients with COVID‐19 frequently show salivary gland involvement due to ACE‐2 expression in minor salivary glands which is reportedly higher as compared to the lungs, thus making the salivary glands susceptible to SARS‐CoV‐2 viral infection. 28
Extraoral and intraoral physical examination was conducted in an observational study on 122 patients in Italy. The most prevalent oral findings were salivary gland ectasia, dry mouth, masticatory muscle weakness, temporomandibular joint abnormalities, dysgeusia, anosmia, facial tingling, trigeminal neuralgia, facial asymmetry. 53 The authors concluded that oral manifestations persisted in the vast majority of survivors even after clinical recovery and thus oral cavity could be the likely target of COVID‐19. Atypical presentations of COVID‐19 like acute infectious parotitis, malocclusion due to inflammation surrounding muscles of mastication, are being increasingly recognized. Emergency Department clinicians must have a high suspicion for COVID‐19 among any patient presenting with infectious symptoms or viral associated illnesses and don available personal protective equipment accordingly for the initial evaluation. 54
Intraparotid lymphadenitis could lead to parotid inflammation during COVID‐19 as three patients showed parotitis characterised by ear pain, retromandibular oedema, sticky saliva and pain during mastication. 55
A questionnaire‐based survey was carried out on 58 COVID‐19 hospitalised patients, which found xerostomia as the most prevalent symptom, followed by gustatory dysfunction. This suggested that salivary gland‐related symptoms and disorders are highly prevalent in COVID‐19 patients. 56
4.9. Facial palsy
Cases have been observed and published where facial palsy was the first symptom observed in SARS‐CoV‐2 patients and it was suggested that peripheral facial palsy should be added to the spectrum of neurological manifestations associated with COVID‐19. 57 In another 35‐ year old pregnant Portuguese patient, Bell's palsy showed involuntary drooling and there was deviation of the left side labial commissure, suggesting that the neurological symptoms could be the first and only manifestation and, also, pregnancy may illustrate a higher susceptibility for peripheral facial palsy in this viral infection. 58
Another COVID‐19 patient reported with bilateral facial nerve palsy with unresponsive blink reflex involving both eyes and Guillain‐Barre syndrome. This implied that neurological manifestations may be directly or indirectly linked to SARS‐CoV‐2 infection. 59
4.10. Trigeminal neuralgia
Paroxysmal lancinating pain was found in the right VI region that lasted a few seconds and was triggered by a light touch of the skin at a specific point on the scalp in a 65‐year old patient, suggesting SARS‐CoV‐2 as a possible aetiology of secondary trigeminal neuralgia. However, more studies are needed to establish the neuropathology of this viral infection. 60
4.11. Melkersson‐Rosenthal syndrome
In a 51‐year old hospitalised female patient, the right lower lip was hyperaemic and showed firm oedema extending towards the jaw, right facial paralysis and fissured tongue, suggestive of Melkersson‐Rosenthal syndrome. It was concluded that activated mast cells may play a significant role in the pathogenesis of COVID‐19 infection, as they release cytokines in the lungs and may be a probable etiological factor for this presentation. 61
4.12. Fungal infections
There was a significant increase in the incidence of angio‐invasive maxillofacial fungal infections in diabetic patients treated for SARS‐CoV‐2 with a strong association with corticosteroid administration. In a retrospective observational study carried out on 18 COVID‐19 hospitalised patients, mucormycosis was observed in 16 patients while aspergillosis was found in one patient and another one patient revealed a mixed fungal infection. 62 Oral pseudomembranous candidiasis was the most frequently observed oral presentation, followed by geographic tongue and taste alteration, in 27 COVID‐19 positive children in a retrospective study conducted in Italy. 63
Opportunistic fungal infections like candidiasis, mucormycosis and aspergillosis are seen due to immunosuppression caused by the acute infection, heavy medication, poor oral hygiene and debilitated systemic health. 5
4.13. Macroglossia
Severe, persistent macroglossia was observed following a prolonged course of prone positioning for treatment of a 40 year old COVID‐19 patient. 64
4.14. Temporomandibular disorders
Psychological impact of COVID‐19 cannot be neglected as it is leading to aggravation TMD, bruxism and anxiety in COVID‐19 patients even after recovery. 65
5. LIMITATIONS
There are some limitations of this systematic review which must be emphasised. Firstly, there are only few studies on the prevalence of oral findings; most of the oral manifestations are reported as either case reports or letters to editor. Secondly, there is no clear distinction between the primary and secondary manifestations of COVID‐19 and it is still a dilemma that oral presentations are merely incidental findings or caused by the SARS‐CoV‐2 virus itself. Most importantly, in most of the studies, there is no categorisation of the severity of COVID‐19 infection as mild, moderate or severe at the time of investigation, which further despises the causative factors for oral manifestations. Additionally, due to high risk of contamination in such patients, many oral findings may have been under reported. In the present systematic review, many observational studies are based on the Questionnaires or web based questionnaires, which are not reliable for a conclusive diagnosis. Due to deficient high quality prevalence studies on oral manifestations in COVID‐19, meta‐analysis was not conducted for this systematic review, which is a major limitation of this review.
6. CONCLUSION
In this systematic review, the most frequently observed oral presentations were taste alterations, followed by xerostomia and vesiculobullous lesions. Though, increasing number of patients are reporting oral manifestations in COVID‐19, but, it still remains ambiguous whether they are directly due to the deadly infection or are merely seen as secondary presentation during the treatment. In the current scenario of rapidly changing and new mutated strains of COVID‐19 virus, more high‐quality prevalence studies are required to be conducted to find a causal relationship between oral symptoms and this highly contagious virus. Noteworthily, dental professionals can play a key role in the early diagnosis of this viral infection.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Preeti Sharma conducted the literature search and contributed to conception, data acquisition, analysis, interpretation and design of the first draft. Sangeeta Malik conducted the literature search (independent screening and selection of publications) and contributed to conception, data acquisition, analysis. Vijay Wadhwan contributed to conception, data acquisition, analysis and critically revised the manuscript and provided advice to improve the manuscript. Suhasini Gotur Palakshappa and Roli Singh also contributed to the literature search, data acquisition, analysis and critically revised the manuscript. All authors have read the submitted manuscript and agree to be accountable for all aspects of the work.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
None. No funding was received for this study.
Sharma P, Malik S, Wadhwan V, Gotur Palakshappa S, Singh R. Prevalence of oral manifestations in COVID‐19: a systematic review. Rev Med Virol. 2022;e2345. 10.1002/rmv.2345
DATA AVAILABILITY STATEMENT
This is a systematic review and data sharing is not applicable to this article as no new data was created in this study. The data for the included studies is presented in Table 1 and Table 2, while the flow chart for literature search is presented in Figure 1.
REFERENCES
- 1. Dalipi ZS, Dragidella F, Dragidella DK. Oral manifestations of exudative erythema multiforme in a patient with COVID‐19. Case Rep Dent. 2021;2021:1148945. 10.1155/2021/1148945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halboub E, Al‐Maweri SA, Alanazi RH, Qaid NM, Abdulrab S. Orofacial manifestations of COVID‐19: a brief review of the published literature. Braz Oral Res. 2020;34:e124. 10.1590/1807-3107bor-2020.vol34.0124 [DOI] [PubMed] [Google Scholar]
- 3. Franceschi VB, Santos AS, Glaeser AB, et al. Population‐based prevalence surveys during the Covid‐19 pandemic: a systematic review. Rev Med Virol. 2021;31(4):e2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID‐19: a 6‐month update. J Dent Res. 2021;100(12):1321‐1329. 10.1177/00220345211029637 [DOI] [PubMed] [Google Scholar]
- 5. Farid H, Khan M, Jamal S, Ghafoor R. Oral manifestations of Covid‐19‐A literature review. Rev Med Virol. 2021;32(1):e2248. 10.1002/rmv.2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aragoneses J, Suárez A, Algar J, Rodríguez C, López‐Valverde N, Aragoneses JM. Oral manifestations of COVID‐19: updated systematic review with meta‐analysis. Front Med. 2021;8:726753. 10.3389/fmed.2021.726753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwenandar F, Japar KV, Damay V, et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. IJC Heart Vasc. 2020;29:100557. 10.1016/j.ijcha.2020.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarengat RF, Islam MS, Ardhi MS. Correlation of neutrophil‐to‐lymphocyte ratio and clinical outcome of acute thrombotic stroke in patients with COVID‐19. Narrativa J. 2021;1(3):e50. 10.52225/narra.v1i3.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fahriani M, Ilmawan M, Fajar JK, et al. Persistence of long COVID symptoms in COVID‐19 survivors worldwide and its potential pathogenesis‐ A systematic review and meta analysis. Narrativa J. 2021;1(2):e36. 10.52225/narraj.v1i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fajar JK, Ilmawan M, Mamada SS, et al. Global prevalence of persistent neuromuscular symptoms and the possible pathomechanisms in post SARS‐CoV‐2 infection individuals: a systematic review and meta‐analysis. Narrativa J. 2021;1(3):e48. 10.52225/narra.v1i3.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hariyanto TI, Putri C, Hananto JE, Arisa J, Situmeang RFV, Kuniawan A. Delirium is a good predictor for poor outcomes from coronavirus disease 2019 (COVID‐19) pneumonia: a systematic review, meta‐analysis and meta‐regression. J Psychiatr Res. 2021;142:361‐368. 10.1016/j.jpsychires.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sulbaran JAM, Pedreanez A, Carrero Y. C‐reactive protein as an effector molecule in Covid‐19 pathogenesis. Rev Med Virology. 2021;31(6):e2221. 10.1002/rmv.2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coomes EA, Haghbayan H. Interleukin‐6 in covid‐19: a systematic review and meta‐analysis. Rev Med Virology. 2020;30(6):e2141‐9. 10.1002/rmv.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahman MM, Hasan M, Ahmed A. Potential detrimental role of soluble ACE 2 in severe COVID‐19 comorbid patients. Rev Med Virology. 2021;31:e2213‐12. 10.1002/rmv.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandão TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS‐CoV‐2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(2):e45‐e51. 10.1016/j.oooo.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saxena S, Kumar S. Understanding the mechanism of commonly occurring COVID‐19‐associated oral lesions. J Oral Maxillofac Pathol. 2021;25(2):223‐225. 10.4103/0973-029X.325118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID‐19 patient: new signs or secondary manifestations? Int J Infect Dis. 2020;97:326‐328. 10.1016/j.ijid.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Sys Rev. 2015;4(1):1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Base Healthc. 2015;13(3):147‐153. 10.1097/xeb.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 22. Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. Adelaide (Australia). Joanna Briggs Institute; 2017. Chap 7. [Google Scholar]
- 23. Eleizer M, Hautefort C, Hamel A‐L, et al. Sudden and complete olfactory loss function as a possible symptom of COVID‐19. JAMA Otolaryngol Neck Surg. 2020;146(7):674‐675. 10.1001/jamaoto.2020.0832 [DOI] [PubMed] [Google Scholar]
- 24. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection: a novel syndrome. Rhinology. 2020;192(26):E702‐E707. 10.4193/rhin20.114 [DOI] [PubMed] [Google Scholar]
- 25. Levinson R, Elbaz M, Ben‐Ami R, et al. Anosmia and dysgeusia in patients with mild SARS‐CoV‐2 infection. Infectious Diseases (except HIV/AIDS). medRxiv. 2020;52(8):600‐602. [published online ahead of print April 14, 2020]. [DOI] [PubMed] [Google Scholar]
- 26. Mullol J, Alobid I, Sanchez FM, et al. The loss of smell and taste in the COVID‐19 outbreak: a tale of many countries. Curr Allergy Asthma Rep. 2020;20(10):61. 10.1007/s11882-020-00961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahmoud MM, Abuohashish HM, Khairy DA, Bugshan AS, Khan AM, Moothedath MM. Pathogenesis of dysgeusia in COVID‐19 patients: a scoping review. Eur Rev Med Pharmacol Sci. 2021;25(2):1114‐1134. [DOI] [PubMed] [Google Scholar]
- 28. Ku H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1‐5. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lozada‐Nur F, Chainani‐Wu N, Fortuna G, Sroussi H. Dysgeusia in COVID‐19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):344‐346. 10.1016/j.oooo.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132‐134. 10.1177/0194599820922992 [DOI] [PubMed] [Google Scholar]
- 31. Vinayachandran D, Balasubramanian S. Is gustatory impairment the first report of an oral manifestation in COVID‐19? Oral Dis. 2020;27:748‐749. 10.1111/odi.13371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross‐sectional study. Clin Infect Dis. 2020;71(15):889‐890. 10.1093/cid/ciaa330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. American Academy of Otolaryngology‐HeadNeck Surgery . Anosmia, Hyposmia, and Dysgeusia Symptoms of Corona‐virus Disease; 2020. Retrieved from https://www.entnet.org/content/aao‐hns‐anosmia‐hyposmia‐and‐dysgeusia‐symptoms‐coronavirus‐disease [Google Scholar]
- 34. Center for Disease Control and Prevention . Symptoms of Corona‐virus; 2020. Retrieved from https://www.cdc.gov/coronavirus/2019‐ncov/symptoms‐testing/symptoms.html [Google Scholar]
- 35. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID‐19: a living systematic review. J Dent Res. 2021;100(2):141‐154. 10.1177/0022034520957289 [DOI] [PubMed] [Google Scholar]
- 36. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID‐19: sex‐related symptoms‐A potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020 Oct;163(4):722‐728. 10.1177/0194599820934380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fantozzi PJ, Pampena E, Di Vanna D, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID‐19. Am J Otolaryngol. 2020;41(6):102721. 10.1016/j.amjoto.2020.102721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinjari B, D'Ardes D, Santilli M, et al. SARS‐CoV‐2 and oral manifestation: an observational, human study. J Clin Med. 2020;9(10):3218. 10.3390/jcm9103218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020;163(1):3‐11. 10.1177/0194599820926473 [DOI] [PubMed] [Google Scholar]
- 40. Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID‐19 infection: a cohort study in Israeli patients. Clin Microbiol Infect. 2021;27(5):769‐774. 10.1016/j.cmi.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zarch RE, Hosseinzadeh P. COVID‐19 from the perspective of dentists: a case report and brief review of more than 170 cases. Dermatol Ther. 2021;34:1‐6:e14717. 10.1111/dth.14717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS‐CoV‐2 infection: a case series. Oral Dis. 2020;00:1‐3. 10.1111/odi.13635 [DOI] [PubMed] [Google Scholar]
- 43. Favia G, Tempesta A, Barile G, et al. Covid‐19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the university hospital policlinic of bari with a purpose of a new classification. J Clin Med. 2021;10(4):757. 10.3390/jcm10040757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hocková B, Riad A, Valky J, et al. Oral complications of ICU patients with COVID‐19: case‐series and review of two hundred ten cases. J Clin Med. 2021;10(4):581. 10.3390/jcm10040581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Katz J, Yue S. Increased odds ratio for COVID‐19 in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2021;50(1):114‐117. 10.1111/jop.13114 [DOI] [PubMed] [Google Scholar]
- 46. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID‐19). Oral Dis. 2021;27(3):771‐772. 10.1111/odi.13465 [DOI] [PubMed] [Google Scholar]
- 47. Abubakr N, Salem ZA, Kamel AHM. Oral manifestations in mild to moderate cases of COVID‐ 19 viral infection in the adult population. Dent Med Probl. 2021;58(1):7‐15. 10.17219/dmp/130814 [DOI] [PubMed] [Google Scholar]
- 48. Nuno‐Gonzalez A, Martin‐Carrillo P, Magaletsky K, et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID‐19 in a field hospital in Spain: oral and palmoplantar findings. Br J Dermatol. 2021;184(1):184‐185. 10.1111/bjd.19564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maia CMF, Marques NP, de Lucena EHG, de Rezende LF, Martelli DRB, Martelli‐Júnior H. Increased number of Herpes Zoster cases in Brazil related to the COVID‐19 pandemic. Int J Infect Dis. 2021;104:732‐733. 10.1016/j.ijid.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferreira ACAF, Romão TT, Macedo YS, Pupe C, Nascimento OJM, Fellow of the American Academy of Neurology (FAAN) . COVID‐19 and herpes zoster co‐infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27(9):1748‐1750. 10.1111/ene.14361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho LFCS. Short report ‐ herpes simplex lesion in the lip semimucosa in a COVID‐19 patient. Eur Rev Med Pharmacol Sci. 2020;24(17):9151‐9153. [DOI] [PubMed] [Google Scholar]
- 52. Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, Matos Valdez LH. Oral mucosal lesions in patients with SARS‐CoV‐2 infection. Report of four cases. Are they a true sign of COVID‐19 disease? Spec Care Dent. 2020;40(6):555‐560. 10.1111/scd.12520 [DOI] [PubMed] [Google Scholar]
- 53. Gherlone EF, Polizzi E, Tetè G, et al. Frequent and persistent salivary gland ectasia and oral disease after COVID‐19. J Dent Res. 2021;100(5):464‐471. 10.1177/0022034521997112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fisher J, Monette DL, Patel KR, Kelley BP, Kennedy M. COVID‐19 associated parotitis. Am J Emerg Med. 2021;39:254.e1‐254.e3. 10.1016/j.ajem.2020.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lechien JR, Chetrit A, Chekkoury‐Idrissi Y, et al. Parotitis‐like symptoms associated with COVID‐19, France, March‐April 2020. Emerg Infect Dis. 2020;26(9):2270‐2271. 10.3201/eid2609.202059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El Kady DM, Gomaa EA, Abdella WS, Ashraf Hussien R, Abd ElAziz RH, Khater AGA. Oral manifestations of COVID‐19 patients: an online survey of the Egyptian population. Clin Exp Dent Res. 2021;7(5):852‐860. 10.1002/cre2.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lima MA, Silva MTT, Soares CN, et al. Peripheral facial nerve palsy associated with COVID‐19. J Neurovirol. 2020;26(6):941‐944. 10.1007/s13365-020-00912-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Figueiredo R, Falcão V, Pinto MJ, Ramalho C. Peripheral facial paralysis as presenting symptom of COVID‐19 in a pregnant woman. BMJ Case Rep. 2020;13(8):e237146. 10.1136/bcr-2020-237146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain‐Barré Syndrome as a rare neurological complication of SARS‐CoV‐2. J Clin Neurosci. 2020;77:230‐232. 10.1016/j.jocn.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Molina‐Gil J, González‐Fernández L, García‐Cabo C. Trigeminal neuralgia as the sole neurological manifestation of COVID‐19: a case report. Headache. 2021;61(3):560‐562. 10.1111/head.14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taşlıdere B, Mehmetaj L, Özcan AB, Gülen B, Taşlıdere N. Melkersson‐rosenthal syndrome induced by COVID‐19. Am J Emerg Med. 2021;41:262.e5‐262.e7. 10.1016/j.ajem.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moorthy A, Gaikwad R, Krishna S, et al. SARS‐CoV‐2, uncontrolled diabetes and corticosteroids‐an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi‐centric analysis. J Maxillofac Oral Surg. 2021;20(3):1‐8. 10.1007/s12663-021-01532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bardellini E, Bondioni MP, Amadori F, et al. Non‐specific oral and cutaneous manifestations of Coronavirus Disease 2019 in children. Med Oral Patol Oral Cir Bucal. 2021;26(5):e549‐e553. 10.4317/medoral.24461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Andrews E, Lezotte J, Ackerman AM. Lingual compression for acute macroglossia in a COVID‐19 positive patient. BMJ Case Rep. 2020;13(7):e237108. 10.1136/bcr-2020-237108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giacomo Di P, Serritella E, Imondi F, Paolo Di C. Psychological impact of COVID‐19 pandemic on TMD subjects. Eur Rev Med Pharmacol Sci. 2021;25(13):4616‐4626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
This is a systematic review and data sharing is not applicable to this article as no new data was created in this study. The data for the included studies is presented in Table 1 and Table 2, while the flow chart for literature search is presented in Figure 1.


