Abstract
The recent FDA approval of the mRNA vaccine for severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) emphasizes the importance of mRNA as a powerful tool for therapeutic applications. The chemically modified mRNA cap analogs contain a unique cap structure, m7G[5′]ppp[5′]N (where N=G, A, C or U), present at the 5′‐end of many eukaryotic cellular and viral RNAs and several non‐coding RNAs. The chemical modifications on cap analog influence orientation's nature, translational efficiency, nuclear stability, and binding affinity. The recent invention of a trinucleotide cap analog provides groundbreaking research in the area of mRNA analogs. Notably, trinucleotide cap analogs outweigh dinucleotide cap analogs in terms of capping efficiency and translational properties. This review focuses on the recent development in the synthesis of various dinucleotide cap analogs such as dinucleotide containing a triazole moiety, phosphorothiolate cap, biotinylated cap, cap analog containing N1 modification, cap analog containing N2 modification, dinucleotide containing fluorescence probe and TAT, bacterial caps, and trinucleotide cap analogs. In addition, the biological applications of these novel cap analogs are delineated.
Keywords: Cap analogs, dinucleotide, trinucleotide, mRNA, ARCA, translation, mRNA vaccination, anti-cancer
Trinucleotide cap analogs outperform dinucleotide cap analogs in terms of capping efficiency and translational properties. mRNA vaccines contain the presence of mRNA cap 1 structure. mRNA cap analogs can be used in the area of anticancer immunization, protein production, and gene therapy.
1. Introduction
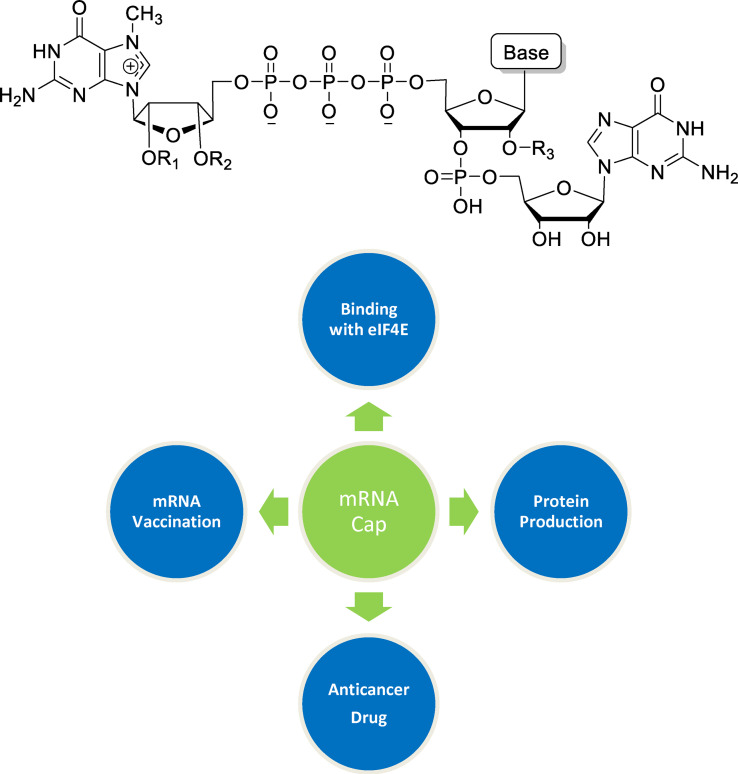
The 5′‐end of cellular and eukaryotic viral RNAs exhibits a matchless cap structure (m7G[5′]ppp[5′]N, where N is any nucleotide) that are synthesized by various RNA polymerases. [1] The N7 guanosine (m7G) residue in the messenger RNA (mRNA) is connected to the first nucleotide of the mRNA transcript via a triphosphate linkage between the two 5′‐hydroxyl groups as shown in Figure 1. The distinct cap structure plays a vital role in providing resistance to 5′‐exonuclease that helps to protect the mRNA from speedy degradation by 5′‐exonuclease activity. It has participated in numerous aspects of mRNA metabolism such as intracellular transport, splicing, subcellular localization, initiation of RNA, translation, and mRNA turnover.[ 2 , 3 ] The most appealing feature of the cap is its strong ability to recognize eukaryotic initiation factor 4E (eIF4E) during the initiation of translation that has been overexpressed in several categories of tumors. [4] The presence of both the delocalized positive charge of the m7guanosine base moiety and the triphosphate bridge in the cap structure contributes to the specific recognition to eIF4E. [5] It has been acknowledged in the literature that eIF4E is a striking target for anticancer directed therapy. [4] The modified cap analog serves as a potential inhibitor to target eIF4E in which the cap analogs may neutralize the overexpressed levels of eIF4E that is highly depend on its ability to bind with eIF4E. In addition to eIF4E protein, the cap structure recognizes other cap‐binding proteins such as CBC80/20 nuclear cap‐binding complex and Vaccinia virus methyltransferase VP39.[ 6 , 7 ]
Figure 1.
The chemical structure of 5′‐capped mRNA.
The most common strategy for the in vitro synthesis of 5′‐capped mRNAs comprises the use of synthetic dinucleotide analog, m7G[5′]ppp[5′]G (standard cap), as an initiation of transcription. However, the existence of two free 3′‐OH groups on both guanosine moieties promotes as the initiating nucleophile for transcription elongation that results in both the forward (m7G[5′]ppp[5′]G[pN]n) and the reverse orientation (G[5′]ppp[5′]m7G[pN]n) products. [8] The mRNA transcripts with forward orientation product is recognized, whereas transcripts with reverse orientation product is not properly recognized during the translational process that decreases the overall translational properties. The breakthrough in the field of mRNA cap is the innovation of an anti‐reverse cap analog (ARCA) that circumvents the problems encountered in the standard cap.[ 9 , 10 ] The ARCA involves 3′‐OH modification on m7G moiety in which the modified cap analog incorporates exclusively in the forward orientation due to the absence of the initiating nucleophile on m7G moiety. Moreover, the exclusive 2′‐OH modification on m7G moiety also serves as the anti‐reverse cap analog even when there is a free 3′‐OH group on m7G moiety. [11] In general, the use of ARCA provides more than two fold translational properties as compared to a standard cap.[ 12 , 13 ] The sugar modifications on m7G moiety not only influences the nature of the orientation but also promotes the translational efficiency, binding affinity, and nuclease stability. Furthermore, the modification of triphosphate chain (i. e.) thiophosphoralate cap analogs display higher translational properties than their parent triphosphate analogs. [14]
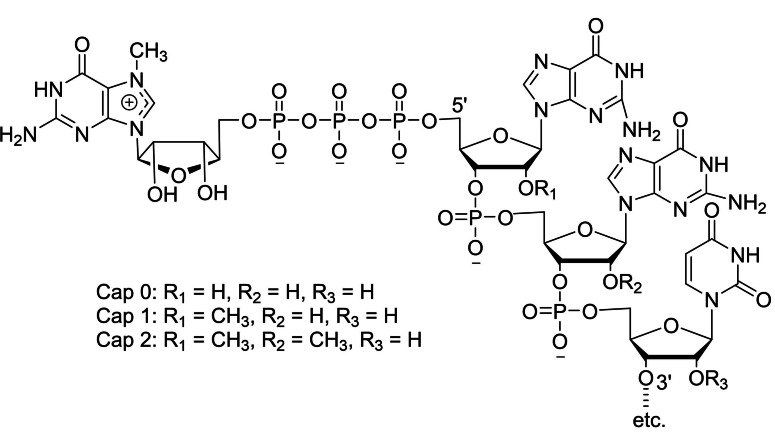
The formation of IVT mRNA transcripts from the use of dinucleotide cap analog (m7GpppN) results in cap 0 structure that does not contain the 2′‐OMe group in the first transcribed nucleotide. The cap 0 structure can also be generated by the use of the Vaccinia virus complex that is accomplished through post‐transcriptional modifications. [15] In the case of higher mammalian cells, the IVT mRNA transcripts with cap 0 structure can further undergo post‐transcriptional modifications to cap 1 structure (m7GpppNm wherein, Nm is a 2′‐O‐methylated nucleotide) and occasionally to cap 2 structure (m7GpppNmNm) even though cap 0 structure has the ability to initiate mRNA translation. [16] The cap 0 structure has been converted into cap 1 structure that involves the transfer of a methyl group from S‐adenosylmethionine by the use of an enzyme, 2′‐O‐methyltransferase. [17] However, this method is not practically feasible for large scale preparations. During viral replication, it is important for the immune system to differentiate between non‐self and self RNA. The presence of the 2′‐O‐methyl group in the first transcribed nucleotide plays a vital role to identify self RNA in the innate immune system and discriminate non‐self RNA that helps to suppress viral replication and pathogenesis.[ 18 , 19 ] The limitations associated with cap 0 structure has been effectively addressed by the use of a trinucleotide cap analog. [20] Cotranscriptional capping with m7GpppNmN‐derived trinucleotide generates the cap 1 structure, in which the first transcribed nucleotide has the 2′‐O‐methyl group. The synthetic trinucleotide cap analog is used to make the IVT mRNA transcripts of Pfizer‐BioNTech mRNA vaccine for severe acute respiratory syndrome coronavirus (SARS‐CoV‐2). [21a] In the case of Moderna mRNA vaccine, IVT mRNA transcript containing cap 1 structure has been obtained by the enzymatic mRNA capping involving Vaccinia capping enzyme and Vaccinia 2′‐O‐methyltransferase. [21b]
The importance and biological application of mRNA cap analog are well recognized through the publications of several review articles.[ 22 , 23 ] Since our previous review in 2017, [24] an array of breathtaking research has emerged in the area of cap analogs, which warrants a new review article to deliver an update and guidance in this research topic. In this review, we summarize an overview of the literature for the chemical synthesis of several modified cap analogs and their biochemical properties, concentrating on advances in the last five years. To facilitate discussion, the chemical synthesis of cap analog has been classified into three different groups such as dinucleotide cap analogs, bacterial caps, and trinucleotide cap analogs. The biochemical properties such as capping efficiency, binding affinity, translational efficiency, and resistant to decapping enzyme for these three groups have been discussed in the biological application part.
2. Chemical Synthesis of Cap Analogs
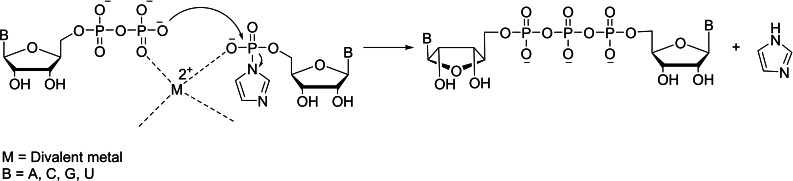
The multiple functional groups such as primary and secondary hydroxyl groups in sugar and amino group in base moiety of the nucleoside/nucleotide make the chemical synthesis of cap analogs extremely challenging. Moreover, these nucleosides/nucleotides are known to cleave under acidic/basic conditions. The coupling reaction between activated nucleotide and non‐activated nucleotide is the most commonly used strategy for the synthesis of cap analogs. [23] The activated nucleotide acts as the electrophile, whereas the non‐activated nucleotide acts as the nucleophile. While there are numerous activating groups such as phenylthio group,[ 25 , 26 ] methoxyphenylthio group,[ 27 , 28 ] 5‐chloro‐8‐quinolyl group, [29] imidazolide,[ 30 , 31 ] and morpholidate [9] have been utilized for the coupling reaction, the most practical and feasible activating group utilizes the use of imidazolide group. Although several types of a divalent metal catalyst such as MgCl2, MnCl2, CaCl2, CdCl2, and ZnCl2 have been employed in both aqueous and anhydrous conditions, [32] the use of zinc chloride under anhydrous conditions involving polar aprotic solvent, DMF provides high yields of product with shortening of reaction time. [33] The involvement of lewis acid catalyst is crucial for the successful coupling reaction that brings both activated and non‐activated nucleotide into close vicinity by divalent metal coordination (Scheme 1). [32] Although these two reactants as such are poorly soluble in DMF, the lewis acid catalyst acts as a template that makes the two reactants solubilize under DMF conditions. The pre‐requisite for the non‐activated nucleotide is the presence of hydrophobic counter ion and the most commonly used one is triethylammonium ion. In contrast to the silica gel purification for the organic compounds, the purification for a cap analog employs the use of anion exchange column chromatogrphy. The most frequently used anion exchange resin is diethylamino ethyl (DEAE) Sepharose or Sephadex and eluting buffer is 1.0 M triethylammonium bicarbonate buffer (TEAB). The total number of phosphate groups present in the mixture dictates the order of elution during the purification process. In general, the monophophate elutes first, then diphosphate and finally cap analog during elution. The chemical synthesis of triphosphate non‐bridging cap analog results in a mixture of two diastereomers. These two diasteromers are separated and isolated as a single isomer by anion exchange followed by reverse phase chromatography using Supelcosil LC‐18‐T column.
Scheme 1.
Mechanistic approach for the metal‐catalyzed coupling reaction.
2.1. Dinucleotide Cap Analogs
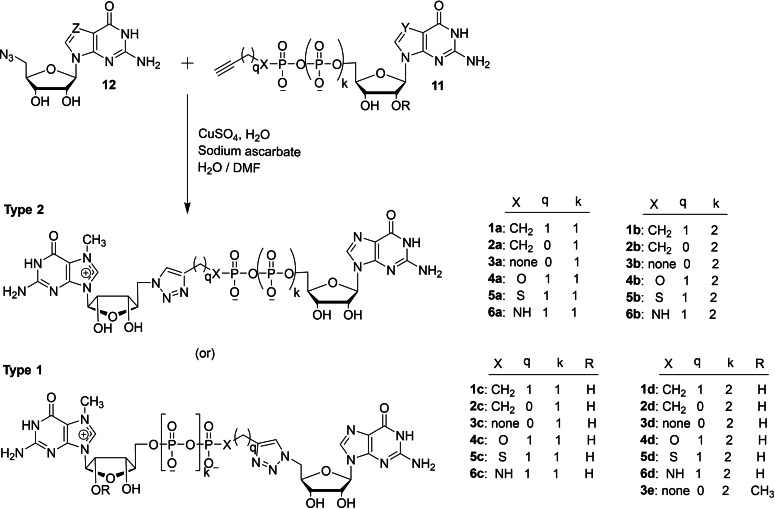
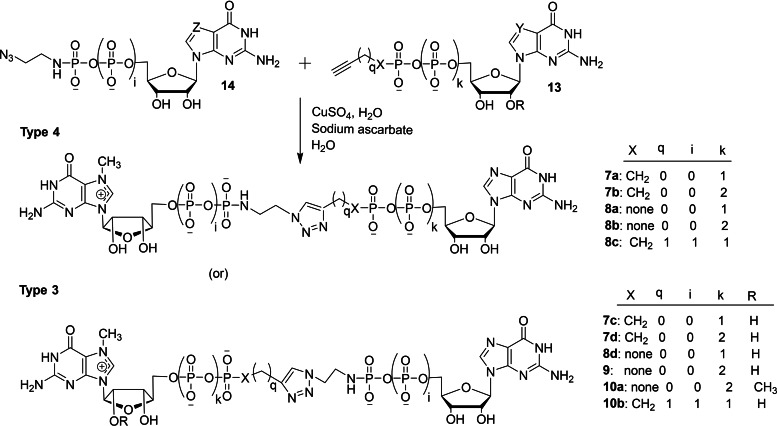
Walczak and co‐workers reported a new route for synthesizing 5′ cap mimics and capped RNAs and studied its biochemical properties. [34] The copper(I)‐catalyzed azide‐alkyne cycloaddition (CuAAC) reaction of nucleotide containing a terminal alkyne within the phosphate chain 11 with azido derivative of nucleoside 12 in several combinations in the presence of copper sulphate and sodium ascorbate under aqueous conditions afforded the corresponding dinucleotide cap analogs containing a triazole moiety 1–6 (Scheme 2). The type 1 class represents a dinucleotide cap containing a triazole moiety directly attached to guanosine moiety, whereas type 2 represents a dinucleotide cap containing a triazole moiety directly attached to m7G moiety. Similarly, the CuAAC reaction of nucleotide containing a terminal alkyne within the phosphate chain 13 with azido derivative of nucleotide 14 in different combinations under standard CuAAC reaction conditions furnished the corresponding dinucleotide cap analog containing a triazole moiety within the phosphate chain 7–10 (Types 3 and 4 – Scheme 3). The crude reaction mixture was quenched with EDTA in order to remove the copper ion and there were 34 dinucleotide cap analogs synthesized and isolated in high purity by using reverse phase HPLC purification (Schemes 2 and 3).
Scheme 2.
Synthesis of dinucleotide cap analogs containing a triazole moiety 1–6.
Scheme 3.
Synthesis of dinucleotide cap analogs containing a triazole moiety attached within the phosphate chain 7–10.
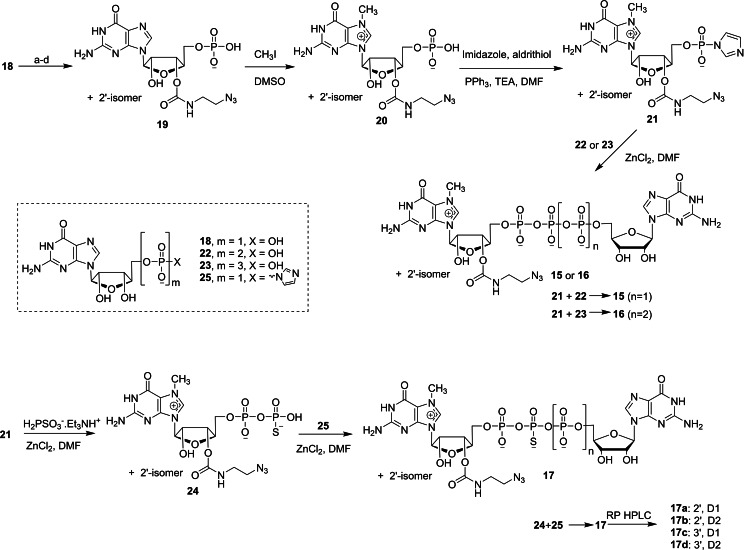
Mamot and co‐workers reported a novel synthesis of dinucleotide cap analogs containing a functionalized azide group at the 2′‐O or 3′‐O position of m7G moiety in order to study the site‐specific labelling of mRNA in living cells (Scheme 4). [35] The key intermediate, Im‐m7GMP containing a functionalized azide group 21 was obtained in three steps, starting from GMP 18. Treatment of GMP 18 with 1,1′‐carbonylimidazole under microwave conditions, followed by opening the resulting 2′,2′‐O,O‐carbonate with excess 2‐azidoethylamine furnished the corresponding carbamate product 19 as a 1 : 2.3 mixture of 2′‐O and 3′‐O regioisomers. The reaction of 19 with methyl iodide in the presence of DMSO as a solvent afforded the corresponding N7‐methylated mononucleotide 20. The imidazolide reaction of 20 with imidazole, triphenylphosphine and aldrithiol using DMF as a solvent afforded the corresponding P‐imidazolide 21. The coupling reaction of GDP 22 or GTP 23 with P‐imidazolide 21 in the presence of zinc chloride as a catalyst and DMF as a solvent afforded the corresponding tri‐ and tetraphosphate cap 15 and 16, respectively. Treatment of triethylammonium salt of thiophosphoric acid with P‐imidazolide 21 in the presence of ZnCl2/DMF system afforded thio diphosphate 24. Finally, the coupling reaction of 24 with Im‐GMP 25 under ZnCl2/DMF system furnished the corresponding β‐phosphorothioate cap analog containing a functionalized azide 17 as a mixture of four isomers. All four isomers such as two P‐diastereoisomeric pairs (ca. 1 : 1) and two 2′‐O/3′‐O regiosomers (ca. 1 : 15) were successfully separated and isolated as a single isomer by reverse phase HPLC. Compounds 15 and 16 were both isolated as a 1 : 1.5 mixture of 2′‐O and 3′‐O regioisomers as these two isomers co‐eluted during HPLC purification.
Scheme 4.
Synthesis of dinucleotide cap analogs containing a functionalized azide group 15–17. Reagents: (a) 1,1′‐carbonyldiimidazole (CDI), μw, DMSO; (b) H2O; (c) NH2(CH2)2N3, 1,8‐diazabicyclo[5.4.0]undec‐7‐ene (26); (d) aq. HCl.
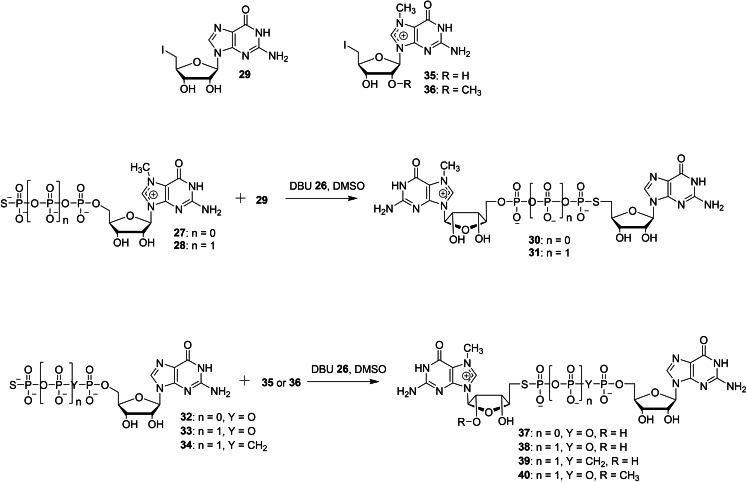
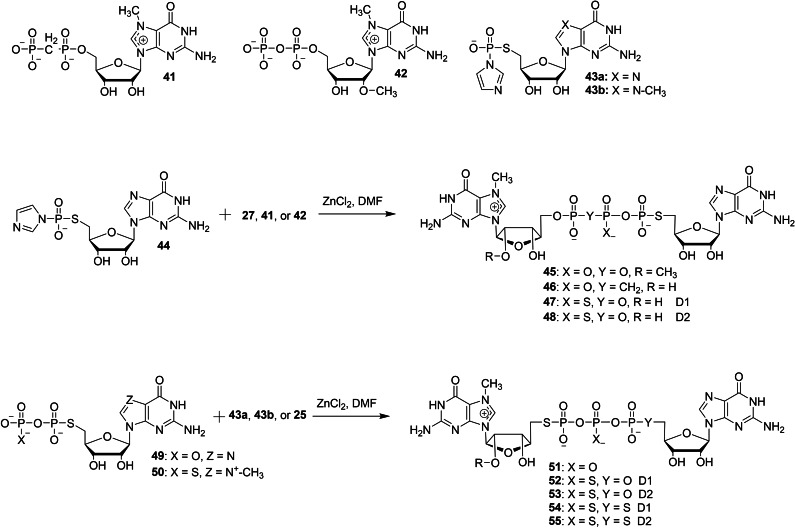
The chemical synthesis of a series of dinucleotide cap analogs containing a 5′‐phosphorothiolate (5′‐PSL) moiety has been reported by Wojtczak et al. in order to study their biochemical properties such as translational efficiency, cap‐binding interactions and decapping assays (Schemes 5 and 6). [36] There are two different types of synthetic approaches that were utilized to make dinucleotide cap analogs containing a 5′‐PSL moiety. First, the synthetic strategy involves the use of SN2 S‐alkylation reaction. The nucleophilic reaction of 7‐methylguanosine containing a 5′‐thiophosphate (27 or 28) with 5′‐iodo guanosine (29) in the presence of DBU 26 as a base, using DMSO as a solvent afforded the corresponding dinucleotide cap analogs containing a α‐PSL modification 30 and 31, respectively in moderate yields (Scheme 5). Similarly, treatment of guanosine containing a 5′‐thiophosphate (32, 33, or 34) with 5′‐iodo 7‐methylguanosine (35 or 36) afforded the corresponding dinucleotide cap analogs containing a γ‐PSL modification 37–40, respectively. Second, the synthetic approach utilized the use of conventional coupling reaction between activated and non‐activated nucleotide. Treatment of modified m7GDP (27, 41, or 42) with 5′‐S‐ImGMP 44 under standard ZnCl2/DMF system afforded the corresponding dinucleotide cap analogs containing a α‐PSL modification 45–48, respectively in moderate yields. The coupling reaction of 5′‐S‐GDP (49 or 50) with modified Im‐GMP (25, 43 a, or 43 b) in the presence of ZnCl2 as a catalyst and DMF as a solvent furnished the corresponding cap analogs containing a γ‐PSL modification 51–55, respectively (Scheme 6). The first approach has been used for the synthesis of dinucleotide cap analogs containing the exclusive α‐PSL or γ‐PSL modifications, whereas the second approach has been utilized for the synthesis of dinucleotide cap containing two or three modifications such as α‐PSL, γ‐PSL, and β‐PS.
Scheme 5.
Synthesis of dinucleotide cap analogs containing a γ‐PSL moiety 37–40.
Scheme 6.
Synthesis of dinucleotide phosphorothiolate cap analogs 45–48 and 51–55.
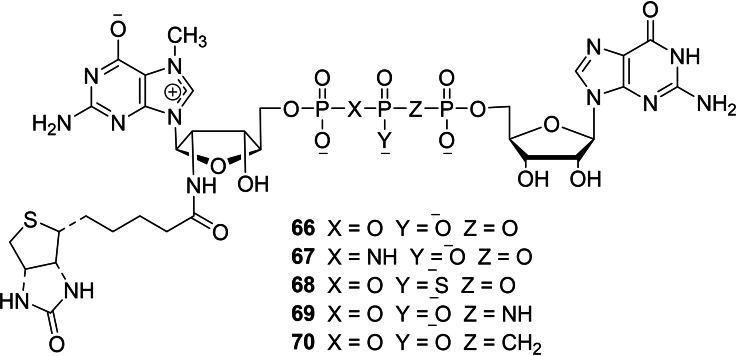
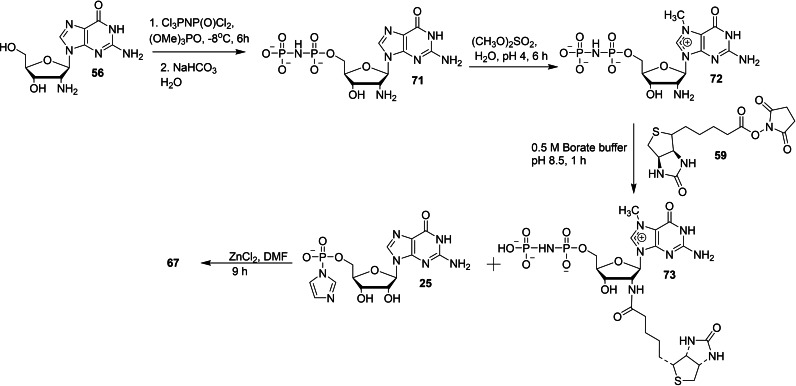
Bednarek et al. reported a series of biotinylated dinucleotide cap analogs with phosphate modification to study the effect of biochemical properties such as capping efficiency, translation efficiency, and hDcp2 decapping susceptibility. [37] The synthetic pathways leading to the formation of biotinylated dinucleotide cap anlogs 66–70 were depicted in Schemes 7 and 8. The key intermediate, 7‐methylguanosine containing biotinylated moiety 60 for the synthesis of 66 and 68–70 was obtained in three steps, starting from 2′‐amino‐2′‐deoxyguanosine (56). The monophosphorylation of 56 using phosphorous oxychloride as a phosphorylating agent, followed by the methylation of the resulting 57 using methyl iodide as a methylating agent, and subsequent biotinylation of the resulting 58 using biotin N‐hydroxysuccinimide ester (59) under aqueous conditions furnished the corresponding 7‐methylguanosine containing a biotinylated moiety 60. The final coupling reaction of 7‐methylguanosine containing a biotinylated moiety 60 with P‐imidazolides of GDP 65 a, or methylenebisphosphonate 65 b in the presence of ZnCl2/DMF system afforded the corresponding biotinylated dinucleotide cap analogs 66 and 70, respectively. Compound 60 was activated into imdazolide salt 61, which was further coupled with guanosine diphosphate containing phosphate modifications 63 or 64 under standard conditions to afford the corresponding cap analogs 69 and 70, respectively. The imidazole activated‐GMP 61 was coupled with thiophosphate and subsequent reaction of the resulting biotinylated thio diphosphate 62 with Im‐GMP 25 afforded the corresponding biotinylated cap analog with β‐S modification 68 (Scheme 7). The key intermediate 73 for the synthesis of 67 was also obtained in three steps, starting from 2′‐amino‐2′‐deoxyguanosine (56). The phosphorylation of 56 with tri‐chloro[(dichlorophosphoryl)imido]phosphorane, followed by the methylation of the resulting 71 and subsequent biotinylation reaction afforded 7‐methylguanosine imidodiphosphate containing biotinylated moiety 73. The final coupling reaction of 73 with Im‐GMP 25 under standard coupling conditions afforded the corresponding biotinylated cap analog 67 (Scheme 8).
Scheme 7.
Synthesis of biotinylated cap analogs 66 and 68–70.
Scheme 8.
Synthesis of biotinylated cap analog 67.
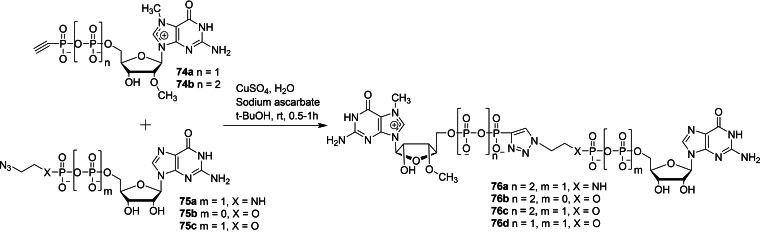
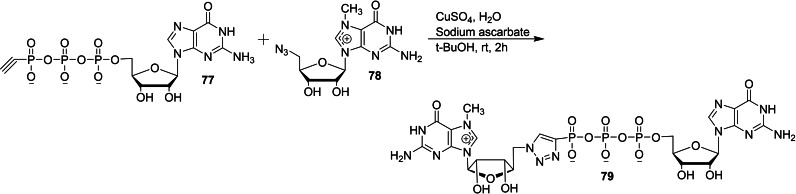
Walczak and co‐workers reported the chemical synthesis of several phosphotriazole cap analogs in order to study its biochemical properties of binding affinity for eIF4E, susceptibility to hDcp2‐catalyzed decapping and translational efficiency. [38] The copper(I)‐catalyzed azide‐alkyne cycloaddition (CuAAC) reaction of phosphonate derivatives of 7,2′‐O‐dimethyl gunaosine 74 with 5′‐[P2‐N‐(2‐azidoethyl)diphosphate 75 a or 5′‐[P−O‐(2‐azidoethyl)phosphate 75 b,c in the presence of copper sulphate and sodium ascorbate under aqueous conditions afforded the corresponding dinucleotide cap analog containing a triazole moiety within the phosphate chain 76 (Scheme 9). Similarly, the CuAAC reaction of phosphonate derivative of guanosine 77 with 5′‐azido‐5′‐deoxy‐7methylguanosine (78) furnished the corresponding dinucleotide cap analog containing a triazole moiety directly attached to m7G 79 (Scheme 10).
Scheme 9.
Synthesis of dinucleotide cap analogs containing a triazole moiety attached within the phosphate chain 76 a–d.
Scheme 10.
Synthesis of dinucleotide cap analog containing a triazole moiety directly attached to m7G 79.
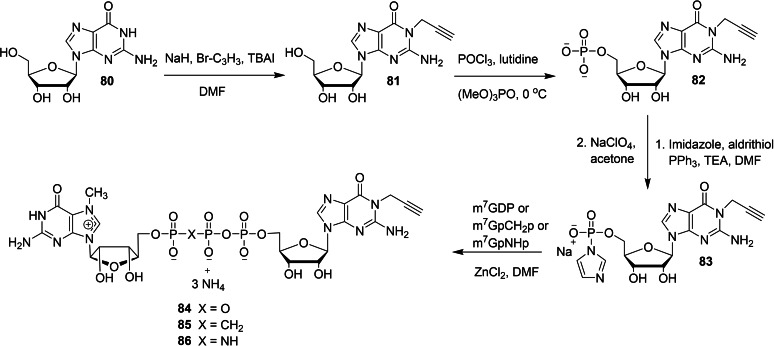
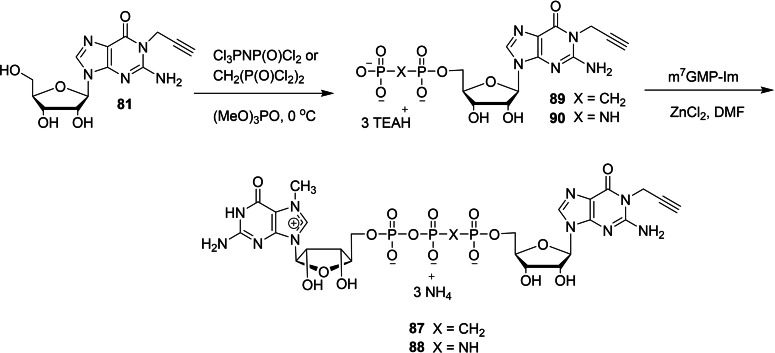
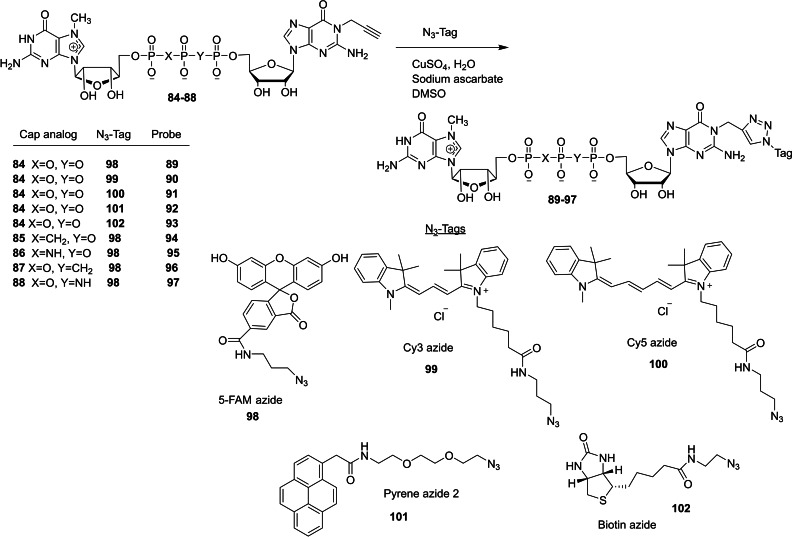
The chemical synthesis of a new series of dinucleotide cap analogs containing an alkyne handle at the N1‐position of guanosine has been reported by Kopcial and co‐workers in order to study the effect of cap‐binding proteins. [39] To begin the synthesis, the key intermediate P‐imidazolide 83 was obtained in three steps starting from guanosine. The reaction between guanosine (80) and propargyl bromide in the presence of sodium hydride and tetrabutylammonium iodide afforded the corresponding N1‐propargylguanosine (81). The monophosphorylation of 81, followed by the activation of the resulting monophosphate 82 by using imidazole/triphenylphosphine/aldrithiol system afforded the desired imidazolide 83. Compounds m7GDP, m7GpCH2P, or m7GpNHp were coupled with P‐imidazole 83 in the presence of zinc chloride as a catalyst and DMF as a solvent afforded the corresponding cap analog with unmodified phosphate bridge 84 and cap analogs modified at the β,γ position 85 and 86, respectively (Scheme 11). Similarly, the coupling reaction of N1‐propargylguanosine 5′‐O‐(1,2‐methylenephosphate) (89) or N1‐propargylguanosine 5′‐O‐(1,2‐imidodiphosphate) (90) with Im‐m7GMP under ZnCl2/DMF system afforded the corresponding cap anlogs modified at the α,β position 87 and 88, respectively (Scheme 12). Finally, the alkyne group present in the cap analogs were functionalized with various probes by a CuAAC reaction. Treatment of various cap analogs containing a propargyl moiety 84–88 with different fluorescent tags azide or biotin azide in the presence of copper sulfate and sodium acetate to afford the corresponding dinucleotide cap analogs containing a labelled probe 89–97 (Scheme 13).
Scheme 11.
Synthesis of cap analogs containing a propargyl moiety at N1 position 84–86.
Scheme 12.
Synthesis of cap analogs containing a propargyl moiety at N1 position 87 and 88.
Scheme 13.
Synthesis of N1 functionalized dinucleotide cap analogs 89–97.
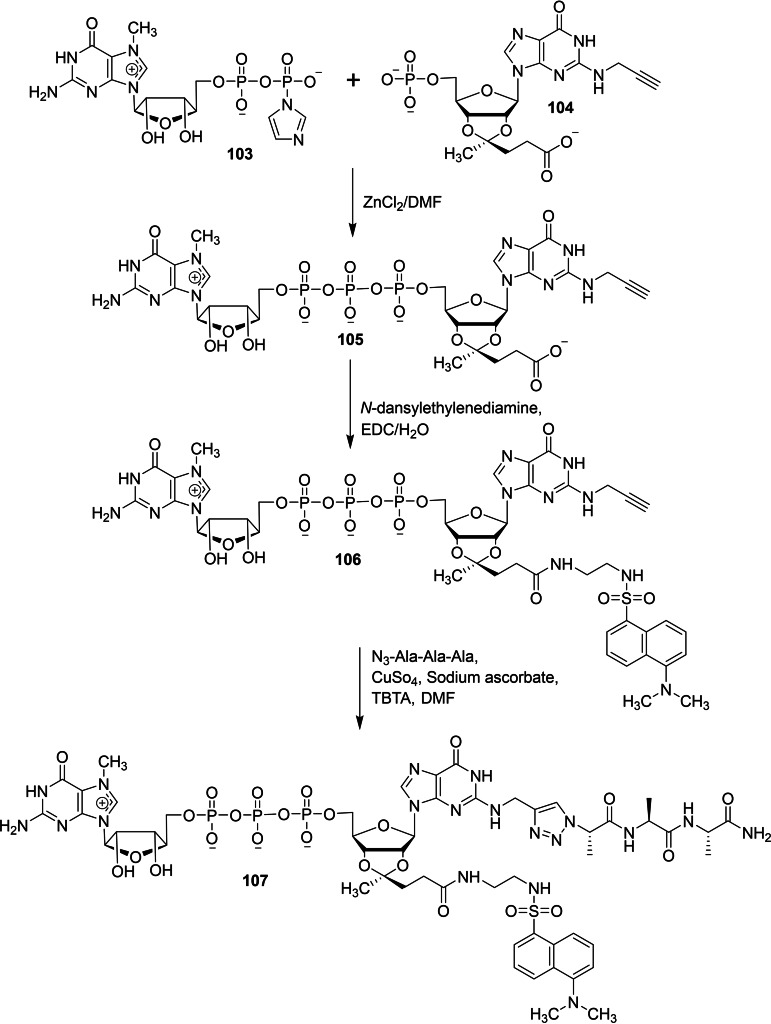
Piecyk and co‐workers reported a chemical synthesis of novel double functionalized dinucleotide cap analog containing fluorescence probe and peptide moiety in order to explore the possibility of utilizing the cap analog for labelling purpose. [40] The coupling reaction of double functionalized mononucleotide 104 with Im‐m7GDP 103 in the presence of zinc chloride as a catalyst afforded the corresponding double functionalized cap analog 105. Next, the peptide coupling reaction between 105 with N‐dansylethylenediamine in the presence of a water‐soluble carbodiimide reagent (1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide, EDC) furnished the corresponding double functionalized cap analog containing fluorescence probe 106. Finally, the click reaction between 106 with N3‐Ala‐Ala‐Ala in the presence of copper sulphate, sodium ascorbate and [(1‐benzyl‐1H‐1,2,3‐triazol‐4‐yl)methyl]amine (TBTA) afforded the corresponding double functionalized dinucleotide cap analog containing fluorescence probe and peptide moiety 107 (Scheme 14).
Scheme 14.
Synthesis of dinucleotide cap analog containing fluorescence probe and peptide 107.
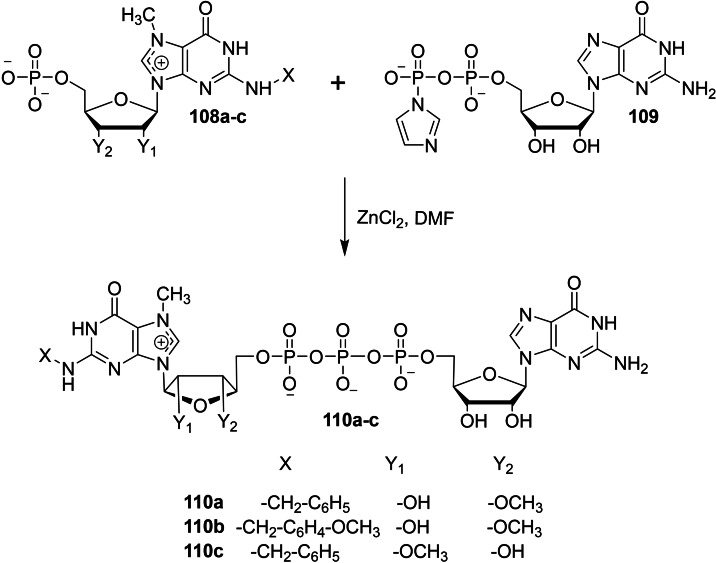
Kocmik et al. reported a chemical synthesis of new dinucleotide cap analogs containing N2 modifications on m7G moiety in order to study its biochemical properties. [41] The coupling reaction of N7‐methyl guanosine monophosphate containing N2 modification 108 with Im‐GDP 109 in the presence of zinc chloride as a catalyst furnished the corresponding dinucleotide cap analog containing N2 modification on m7G moiety 110 (Scheme 15).
Scheme 15.
Synthesis of new dinucleotide cap analog containing N2 modification 110.
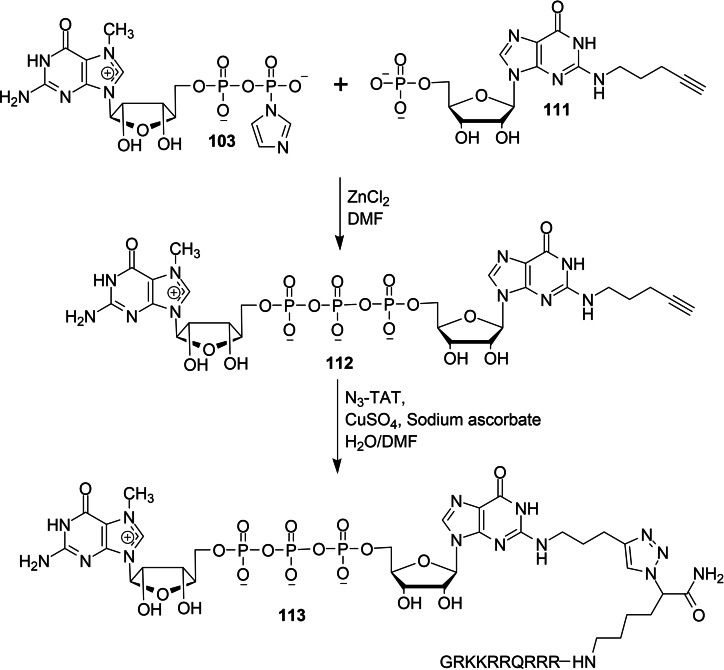
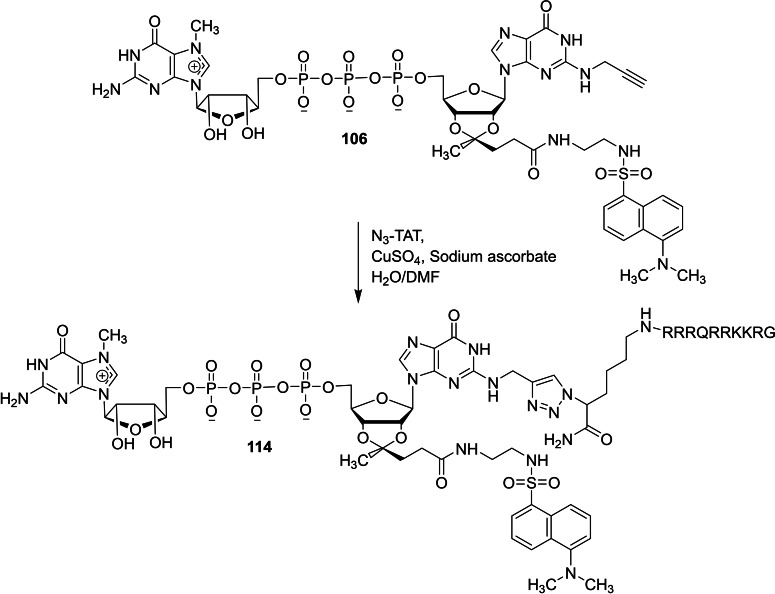
Piecyk and co‐workers reported a chemical synthesis of novel dinucleotide cap analog containing transactivator of transcription (TAT) and dinucleotide cap analog containing TAT and dansyl dye in order to study the effect of human immunodeficiency virus 1 (HIV‐1) TAT derived peptide. [42] Treatment of guanosine monophosphate containing N2 modification 111 with Im‐m7GDP 103 in the presence of zinc chloride as a catalyst afforded the corresponding dinucleotide cap analog containing N2 modification 112. The reaction between 112 and N3‐TAT under standard click chemistry reaction conditions afforded the corresponding dinucleotide cap analog containing TAT 113 (Scheme 16). Treatment of dinucleotide cap analog containing dansyl dye 106 with N3‐TAT under standard click chemistry reaction conditions furnished the corresponding dinucleotide cap analog containing TAT and dansyl dye 114 (Scheme 17).
Scheme 16.
Synthesis of dinucleotide cap analog containing TAT 113.
Scheme 17.
Synthesis of dinucleotide cap analog containing TAT and dansyl dye 114.
2.2. Bacterial Caps
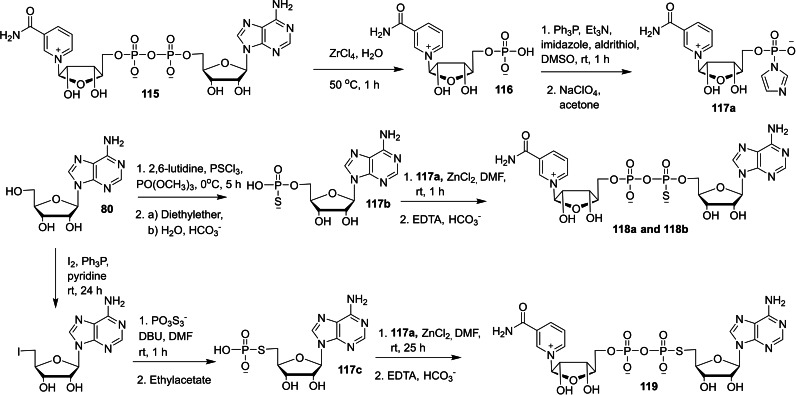
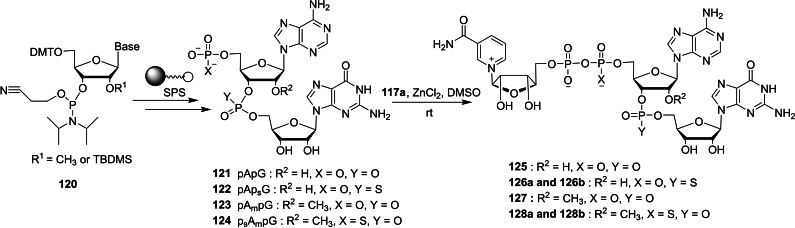
Nicotinamide adenine dinucleotide (NAD) is a coenzyme that consists of two nucleotides joined by pyrophosphate group.[ 43 , 44 ] NAD‐capped RNAs has been termed as bacterial caps that display some structural analogy to the eukaryotic mRNAs containing 7‐methylguanosine cap present at the 5′‐end. [45] Although NAD has been known for over 100 years, the molecular biological applications of NAD‐capped RNAs has been under‐explored area of research. In this context, Mlynarska‐Cieslak and co‐workers reported the chemical synthesis of a series of NAD cap analogs in order to study the susceptibility of NAD‐RNAs with deNADding enzymes. [46] The required intermediate, imidazole‐activated nicotinamide mononucleotide 117 a was obtained in two steps, starting from the commercially available NAD 115. The hydrolysis of 115 in the presence of aqueous ZrCl4 afforded nicotinamide riboside (NR) 5′‐monophosphate 116. Activation of 116 using imidazole/aldrithiol/triphenylphosphine system afforded the corresponding P‐imidazole 117 a. The coupling reaction of adenosine 5′‐phosphorothioate (117 b) with P‐imidazole 117 a in the presence of ZnCl2/DMF system afforded the corresponding two stereoisomers of NAD containing α‐phosphorothioate (α‐PS) moiety 118 a and 118 b. Treatment of adenosine 5′‐phosphorothiolate (117 c) with P‐imidazole 117 a under standard coupling conditions furnished the corresponding NAD containing 5′‐phosphorothiolate (PSL) moiety 119 (Scheme 18). In addition to nicotinamide‐containing dinucleotide cap analogs, the same group reported the synthesis of nicotinamide‐containing trinucleotide cap analogs (Scheme 19). The desired intermediate, dinucleotide precursor 121–124 was obtained by phosphoramidite solid‐phase synthesis using iodine as oxidating reagent and DDTT as sulfurizing reagent. The coupling reaction of 121–124 with P‐imidazole 117 a under standard coupling conditions afforded the corresponding nicotinamide‐containing trinucleotide cap analogs 125–128, respectively.
Scheme 18.
Synthesis of nicotinamide‐containing dinucleotide cap analogs 118 and 119.
Scheme 19.
Synthesis of nicotinamide‐containing trinucleotide cap analogs 125–128.
2.3. Trinucleotide Cap Analogs
Sikorski and co‐workers reported a series of trinucleotide cap analogs and studied protein expression of exogenously delivered mRNA in three different mammalian cell lines and their susceptibility to decapping. [47] The required starting material, dinucleotide 5′‐phosphate 129 (pNpG) was synthesized using solid‐supported standard phosphoramidite chemistry. The coupling reaction of various dinucleotide 5′‐phosphate 121, 123, or 129 with Im‐m7GDP 103 under traditional ZnCl2/DMF system afforded the corresponding trinucleotide cap analog 130 (Scheme 20).
Scheme 20.
Synthesis of trinucleotide cap analogs 130 a–j.
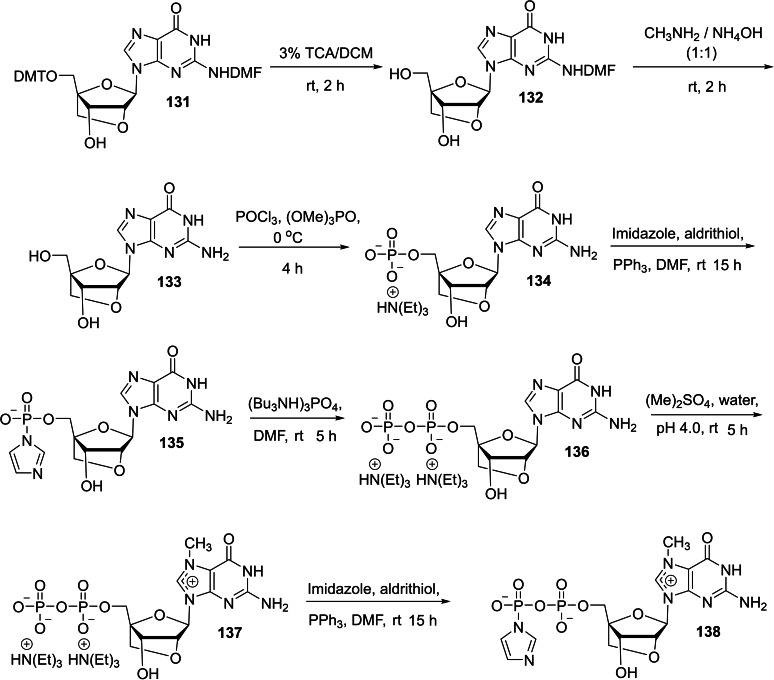
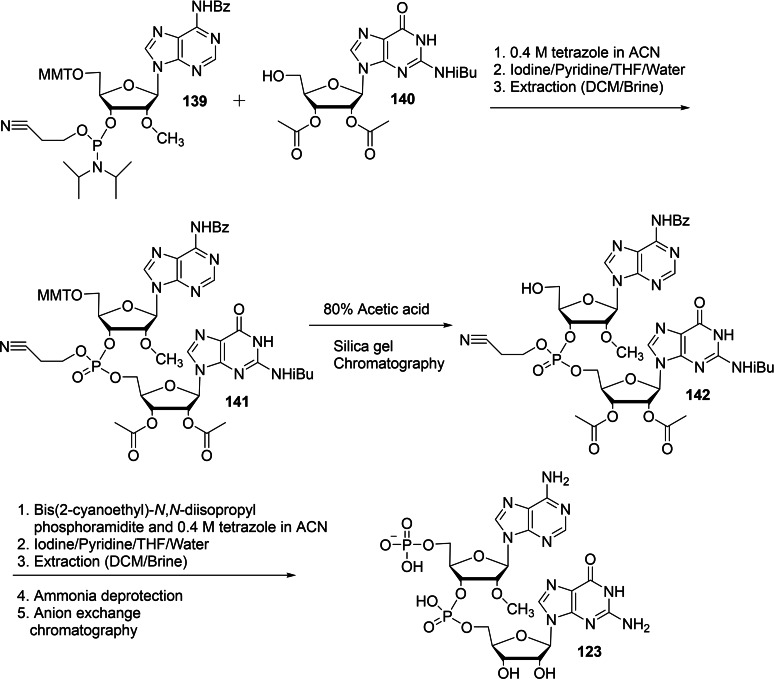
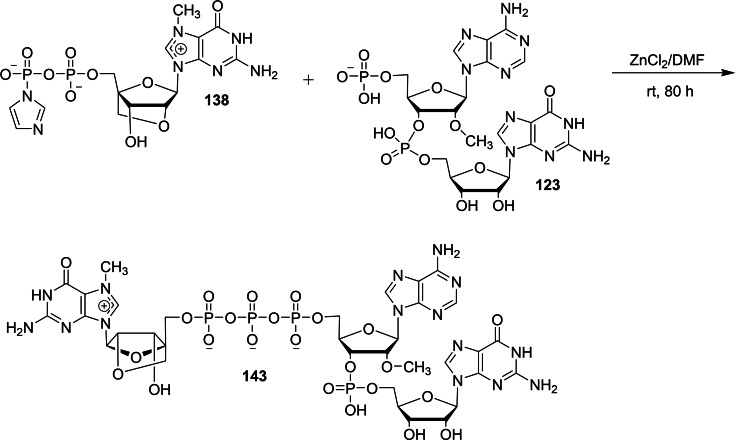
The molecular biological application of locked nucleic acid (LNA) technology serves as a powerful tool in the research areas such as geneotyping and antisense and antigene therapies. [48] The unique conformationally restricted structural feauture provides nuclear resistance, increased thermal stability, hybridization specificity, and sequence stability. [49] We envisaged that the design of a new trinucleotide cap analog bearing an LNA moiety could act as a potential molecular biology tool in the area of mRNA transfection applications such as anticancer immunization, protein production, and gene therapy and mRNA‐based vaccines. In this context, we have reported a synthesis of novel trinucleotide cap analog bearing an LNA moiety and studied its capping efficiency and translational efficiency. [50] The required intermediate, m7(LNA)ImGDP 138 for the synthesis of LNA tricap, m7(LNA)G(5′)ppp(5′)(2′‐OMeA)pG 143 was obtained in seven steps, starting from 5′‐DMT‐N‐DMF LNA guanosine 131 as depicted in Scheme 21. The detritylation of 131 using 3 % trichloroacetic acid in dichloromethane, followed by the deprotection of DMF group using 1 : 1 mixture of aqueous 40 % methylamine and 28 % ammonium hydroxide afforded LNA guanosine 133. The monophosphorylation reaction of LNA guanosine 133 was achieved by using POCl3 as a phosphorylating agent and trimethyl phosphate as a solvent that afforded the corresponding LNA GMP 134. Compound 134 was converted into the corresponding imidazolide 135 using imidazole, triphenylphosphine, and aldrithiol. Next, the resulting imidazolide compound 135 was further phosphorylated using (Bu3NH)3PO4 in the presence of zinc chloride as a catalyst that afforded the corresponding LNA GDP 136. Notably, the methylation of LNA GDP 136 using dimethyl sulfate as a methylating agent under acidic conditions is highly regioselective, affording m7(LNA)GDP 137 as the sole product. The required intermediate, m7(LNA)ImGDP 138 was finally obtained by the imidazolide reaction of 137 with imidazole, triphenylphosphine, and aldrithiol. Another intermediate, p(2′‐OMeA)pG 123 for the synthesis of LNA tricap, m7(LNA)G(5′)ppp(5′)(2′‐OMeA)pG 143 was obtained in three steps as depicted in Scheme 22. Treatment of MMT‐2′‐O‐methyl adenosine (n‐bz) CED phosphoramidite (139) with 2′,3′‐diacetyl guanosine (n‐ibu) (140) in the presence of tetrazole in acetonitrile as an activator, followed by the oxidation using iodine/pyridine mixture afforded the corresponding protected (2′‐OMeA)pG 141. Deprotection of MMT group in 141 was achieved by the use of 80 % acetic acid to afford 142. Finally, the 5′‐hydroxy group of the adenosine group in 142 was phosphorylated by the use of an activated bis(2‐cyanoethyl)‐N,N‐diisopropyl phosphoramidite using tetrazole, followed by the oxidation of trivalent phosphorous using iodine and pyridine and subsequent deprotection using ammonium hydroxide afforded p(2′‐OMeA)pG 123. The reaction pathway leading to the formation of desired 143 for the molecular biological applications is presented in scheme 23. The coupling reaction of p(2′‐OMeA)pG 123 with m7(LNA)ImGDP 138 in the presence of zinc chloride as a catalyst and DMF as a solvent provided m7(LNA)G(5′)ppp(5′)(2′‐OMeA)pG 143.
Scheme 21.
Synthesis of m7(LNA)ImGDP 138.
Scheme 22.
Synthesis of p(2′‐OMeA)pG 123.
Scheme 23.
Synthesis of LNA trinucleotide cap analog 143.
3. Biological Applications of Cap Analogs
The capping efficiency assay provides a powerful tool to determine whether the newly synthesized cap is a substrate for RNA polymerases such as T7, SP3, and SP6 or not. [22f] Furthermore, this assay helps to determine the nature of the orientation as evidenced by the HPLC analysis of the RNA transcription mixture containing short oligonucleotides. [13d] The chemical modifications of cap analogs influence the outcome of the capping efficiency. In addition, factors such as ionic strength and ratio of cap analog to nucleotide mixture determine the capping efficiency of cap analogs.
It has been revealed in the literature that eIF4E protein serves as a powerful weapon for anticancer directed therapy. [4] The cap‐dependent translation is the major contributing causes to tumorigenesis. The binding of the 5′ terminal mRNA cap structure with eIF4E is the rate limiting step of cap‐dependent translation and the concentration of eIF4E influences the regulatory nexus of translation. The proper design of new chemically modified synthetic cap analog can be utilized as an inhibitor that binds with the elevated eIF4E levels in tumor cells and block the function of eIF4E.
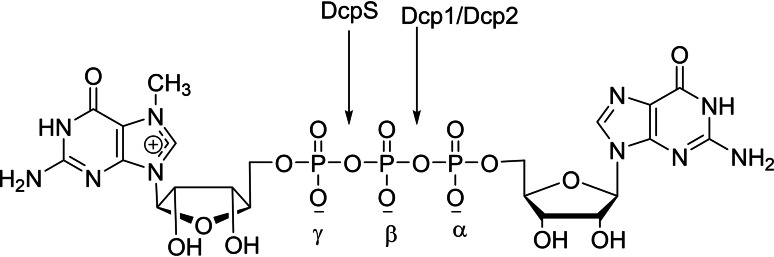
The stability against nuclease plays an important role for the positive therapeutic application based on nucleotide drug. The 5′‐terminal cap structure provides a powerful strategy to enhance nuclease stability and protect cytoplasmic mRNAs. However, several decapping enzymes are involved to remove the cap structure from RNA that results to disease development.[ 51 , 52 ] Consequently, it is vital to selectively regulate the mRNA gene expressions that remain to be challenging clinical problems. There are two major mRNA decay pathways that are involved in eukaryotic cells. [53] First, initiation of shortening of the 3′ poly(A) tail by the PAN2/3 and CCR‐NOT complexes. Second, mRNA undergoes degradation by 5′→3′ hydrolysis due to exonuclease and 3′→5′ hydrolysis due to exosome. In eukaryotic cells, DcpS belongs to the HIT family and Dcp1‐Dcp2 complex belongs to the Nudix family which are the two types of decapping enzymes. The biochemical degradation studies of capped mRNA by scavenger decapping enzyme, DcpS, results in the formation of m7GMP suggesting that the site of cleavage is between the β‐ and γ‐phosphate bond of the cap moiety. In the case of Dcp1‐Dcp2 complex, capped mRNA results in the formation of m7GDP and 5′‐monophosphate RNA that suggests cleavage is happened between the α‐ and β‐phosphate bond of the cap moiety. (Figure 2). The chemically modified mRNA cap analogs play a major role to provide resistance to hydrolysis by decapping enzymes that supports to prevent mRNA against degradation and enhance protein production.
Figure 2.
Cap analog structure showing α/β/γ position and cleavage site.
The biochemical properties such as capping efficiency, translation efficiency, and susceptibility to decapping of 5′‐capped with analogs 15–17 are summarized in Table 1. [35] The capping efficiency for new analogs 15–17 ranges from 72 to 85 %, indicating that these analogs are good substrates for SP6 RNA polymerase (entries 4–9). These analogs display slightly lower efficiency than reference cap analogs (entries 1 and 3). The mRNAs capped with analogs 17 a–d are translated up to 7.5‐fold higher than those for mRNAs capped with standard cap and up to 4‐fold higher than those capped with ARCA (entries 1, 2, and 6–9). The highest translational efficiency is observed for cap analog, 2′‐N3‐m7GppspG D1 17 a (entry 6). The replacement of oxygen to sulphur at β‐position in the triphosphate bridge provides up to 2.3‐fold higher translational efficiency as compared to the parent compound (entries 4–9). These data are in agreement with the previously reported increased translation efficiency provided by the β‐phosphorothioate group. [14] The β‐phosphorothioate modification influences the susceptibility of RNA to decapping that provides stabilization of the transcripts which is comparable to that of m2 7,2′−O GppspG D2‐capped RNA (Table 1, entries 3 and 6–9).
Table 1.
Biochemical properties of RNAs 5′‐capped with cap analogs 15–17.
|
Entry |
Compound |
Capping efficiency (%) |
Translation efficiency |
Susceptibility to decapping |
|---|---|---|---|---|
|
1 |
m7GpppG |
91 |
1.0±0.0 |
74.2±1.9 |
|
2 |
m2 7,3′−O GpppG |
n.d. |
1.8±0.3 |
n.d. |
|
3 |
m2 7,2′−O GppspG D2 |
97 |
5.3±0.2 |
8.6±3.0 |
|
4 |
2′‐N3‐m7GpppG 15 |
85 |
3.7±0.7 |
48.6±1.7 |
|
5 |
2′‐N3‐m7GppppG 16 |
72 |
3.3±0.8 |
44.3±1.9 |
|
6 |
2′‐N3‐m7GppspG D1 17 a |
80 |
7.5±2.0 |
15.1±0.5 |
|
7 |
2′‐N3‐m7GppspG D2 17 b |
77 |
6.9±1.4 |
3.3±1.3 |
|
8 |
3′‐N3‐m7GppspG D1 17 c |
82 |
5.9±0.9 |
13.3±2.6 |
|
9 |
3′‐N3‐m7GppspG D2 17 d |
78 |
6.8±1.3 |
10.7±5.6 |
The susceptibility of the 5′‐phosphorothiolate cap analogs to hydrolysis by the human DcpS enzyme was determined and the results are compiled in Table 2. [36] The cap analogs containing α‐PSL modification undergoes hydrolysis by DcpS (entries 2, 3, 8, 11, and 12). It is noteworthy that the cap analogs containing γ‐PSL modification are resistant to hydrolysis by DcpS (entries 4–7, 9, 10, and 13–16). It appears that the replacement of 5′‐O next to the m7G moiety with 5′‐S protects the cap structure efficiently and prevents from mRNA degradation. These results are in similar agreement with the previously reported for O‐ to CH2 substitution at the β‐γ bridging position and several other modifications within the β‐ or γ‐phosphate.[ 54 , 55 ] It is interesting to compare the results between susceptibility of hDcpS data with translational property data. The mRNAs capped with analogs 40 and 45 in HeLa cells provide 1.1‐fold and 1.4‐fold higher translational efficiency, respectively as compared to ARCA. [36] Although mRNAs with analog 40 at their 5′‐end is more stable against hDcpS than mRNAs with analog 45, the translational efficiency of analog 40 (2.07±0.19) is lower than translational efficiency of 45 (2.75±0.29). These results indicate that susceptibility to hDcpS hydrolysis of 5′‐cap analogs data does not translate into translational efficiency data. Notably, the inhibitory property of cap analog m7GSppspSG D2 55 against endogenous hDcpS activity (EC50=0.70±0.09 nM) is 10‐fold more than clinically tested RG3039 (EC50=6.82±1.08 nM). [36]
Table 2.
Susceptibility to hDcpS hydrolysis of 5′‐phosphorothiolate cap analogs.
|
Entry |
Compound |
Susceptibility to DcpS |
|---|---|---|
|
1 |
m7Gpp |
resistant |
|
2 |
m7GppSG 30 |
hydrolyzed |
|
3 |
m7GpppSG 31 |
hydrolyzed |
|
4 |
m7GSppG 37 |
resistant |
|
5 |
m7GSpppG 38 |
resistant |
|
6 |
m7GSppCH2pG 39 |
resistant |
|
7 |
m2 7,2′−O GSpppG 40 |
resistant |
|
8 |
m2 7,2′−O GpppSG 45 |
hydrolyzed |
|
9 |
m7GpCH2ppSG 46 |
resistant |
|
10 |
m7GSpppSG 47 |
resistant |
|
11 |
m7GppspSG D1 48 |
hydrolyzed |
|
12 |
m7GppspSG D2 51 |
hydrolyzed |
|
13 |
m7GSppspG D1 52 |
resistant |
|
14 |
m7GSppspG D2 53 |
resistant |
|
15 |
m7GSppspSG D1 54 |
resistant |
|
16 |
m7GSppspSG D2 55 |
resistant |
The biochemical properties such as capping efficiency, translation efficiency, and susceptibility to decapping of 5′‐capped with analogs 66–70 are compiled in Table 3. [37] The capping efficiency for new biotinylated cap analogs 66–70 are ranged from 39 to 64 %, indicating that these analogs are substrates for SP6 RNA polymerase (entries 4–8). The mRNAs capped with biotinylated cap analogs 66–70 result in comparable or lower translational efficiency than standard cap analog (entries 1 and 4–8). These data indicate that the presence of 2′‐substituted biotin moiety in cap analog negatively influence the overall translational efficiency of capped mRNA. In order to study the susceptibility of biotin‐labelled cap analogs, short RNAs were subjected to enzymatic degradation by hDcp2. It is noteworthy that phosphate modified cap analogs such as imidodiphosphate 69 and methylenebis(phosphonate) 70 introduced at α,β‐ position of triphosphate provide resistance to mRNA degradation, whereas imidodiphosphate introduced at β,γ‐position of triphosphate 67 undergoes slow mRNA degradation (entries 5, 7 and 8). The β‐S‐modified cap 68 provides partial resistant to mRNA degradation (entry 6). The binding affinity constants (KAS) of biotin‐labelled cap analogs 66–70 for eIF4E are comparable to that of the standard cap analog, indicating that biotin moiety's presence does not impact cap‐eIF4E interaction to a considerable degree and the specificity of binding is maintained.
Table 3.
Biochemical properties of RNAs 5′‐capped with analogs 66–70.
|
Entry |
Compound |
Capping efficiency (%) |
Translation efficiency |
Susceptibility to hDcp2 decapping |
|---|---|---|---|---|
|
1 |
m7GpppG |
76 |
1 |
fast degradation |
|
2 |
m2 7,2′−O GpppG |
69 |
1.56±0.48 |
fast degradation |
|
3 |
ApppG |
43 |
0.03±0.01 |
resistant |
|
4 |
m7 2′‐N‐BiotGpppG 66 |
61 |
0.68±0.16 |
slow degradation |
|
5 |
m7 2′‐N‐BiotGpNHppG 67 |
64 |
0.37±0.12 |
slow degradation |
|
6 |
m7 2′‐N‐BiotGppspG 68 |
61 |
0.93±0.14 |
partially resistant |
|
7 |
m7 2′‐N‐BiotGppNHpG 69 |
39 |
0.11±0.04 |
resistant |
|
8 |
m7 2′‐N‐BiotGppCH2pG 70 |
47 |
0.28±0.08 |
resistant |
The biochemical properties such as capping efficiency, translation efficiency, and binding affinity to eIF4E with analogs 76 and 79 are summarized in Table 4. [38] The capping efficiency for new analogs 76 a–d are ranged from 30 to 42 %, indicating that cap analogs containing a triazole moiety located between phosphate chains are not efficiently recognized by RNA polymerase (entries 4–7). The capping efficiency of compound 79 provides 77 % efficiency (entry 8). These results show that cap analog containing a triazole moiety directly attached to the sugar is more efficiently recognized by RNA polymerase than cap analog containing a triazole moiety located between phosphate chains (entries 4–8). The mRNAs capped with analogs 76 a–d provide higher translational efficiency up to 2.8‐fold as compared to standard cap analog (entries 2 and 4–7), but mRNA capped with analog 79 provide 6.7‐fold lower translational efficiency as compared to standard cap analog (entries 2 and 8). It seems that a triazole moiety directly attached to m7G in cap analog provides unfavorable effect on the overall translational efficiencies (entry 8). In contrast, a triazole moiety located between two phosphate chains does not affect the translational data (entries 4–7). The KAS value for cap analogs containing a triazole moiety located between the phosphate chains 76 a–d provides up to 6.7‐fold higher values as compared to standard cap analog, whereas cap analog containing a triazole moiety attached directly to m7G gives slightly lower than the standard cap analog (entries 2 and 4–8).
Table 4.
Biochemical properties of phosphotriazole cap analogs 76 and 79.
|
Entry |
Compound |
Capping efficiency (%) |
Translation efficiency |
KAS eIF4E [μM−1] |
|---|---|---|---|---|
|
1 |
GpppG |
93 |
0.056 |
n.d |
|
2 |
m7GpppG |
87 |
1.0 |
13.7 |
|
3 |
m2 7,3′−O GpppG |
79 |
1.5 |
10.2 |
|
4 |
76 a |
38 |
2.2 |
82.9 |
|
5 |
76 b |
42 |
2.8 |
49.9 |
|
6 |
76 c |
30 |
1.7 |
87.8 |
|
7 |
76 d |
38 |
1.7 |
24.1 |
|
8 |
79 |
77 |
0.15 |
8.6 |
In order to study the binding effect of substitution at N1 position of cap with eIF4E, the binding constant values (KD) for the cap analog 84–88 – eIF4E complexes were determined. [39] The data show that cap analogs 84–88 have binding affinities comparable to that of the standard cap analog. It seems that the propargyl moiety attached to the N1 position of guanosine does not have any major impact on the interaction with eIF4E. Under standard conditions, cap analogs modified within the phosphate bridge 85–88 are resistant to hDcpS. It is interesting to note that fluorescent probes 94 and 95 display high affinity ligands for DcpS in which probe 95 (KD=14 nM) has 2.5‐fold greater affinity for DcpS than probe 94 (KD=38 nM). [39] The capping efficiency of various cap analogs containing a triazole moiety 1–10 show lower capping efficiency as compared to standard cap analog. [34] The mRNAs capped with cap analogs 1–9 result in lower translational efficiency (0.06 to 0.5) as compared to standard cap (1.0), whereas mRNA capped with cap analog 9 provides comparable translational efficiency (0.89±0.11) to that of standard cap (1.0). [34]
Table 5 summarizes the translational properties of differently capped mRNA in rabbit reticulocyte lysate (RRL) and human embryonic kidney derived (HEK293) cell lines and also decapping by Dcp1/Dcp2 complex. [41] The mRNAs capped with analogs 110 a,110 b in RRL cell line provide lower translational efficiency as compared to ARCA cap analogs (entries 2–5). The highest translational efficiency of 1.72 is observed for cap analog, bn2m2 7,2′−O GpppG 110 c in RRL cell line (entry 6). The mRNAs capped with analogs 110 a–c in HEK293 cell line provide up to 2.2‐fold higher translational efficiency as compared to ARCA cap analog (entries 3–6). The decapping results show that the presence of N2 modification within guanine of 110 a–c decreases stability as compared to ARCA analog (entries 3–6).
Table 5.
Biochemical properties of dinucleotide cap analogs 110 a–c.
|
Entry |
Compound |
Relative translation efficiency (RRL) |
Relative total protein expression (HEK293) |
Decapping % |
|
|---|---|---|---|---|---|
|
5 min. |
30 min. |
||||
|
1 |
m7GpppG |
1 |
1 |
n.d. |
n.d. |
|
2 |
m2 7,2′−O GpppG |
1.59±0.18 |
n.d. |
n.d. |
n.d. |
|
3 |
m2 7,3′−O GpppG |
1.56±0.03 |
1.46±0.50 |
13.7±20.0 |
48.2±7.5 |
|
4 |
110 a |
1.39±0.19 |
3.30±0.86 |
53.4±20.0 |
62.5±1.15 |
|
5 |
110 b |
1.29±0.17 |
2.42±0.30 |
35.4±10.8 |
46.3±18.6 |
|
6 |
110 c |
1.72±0.28 |
2.87±0.50 |
65.8±7.0 |
72.7±4.60 |
The ability of cap analogs containing TAT to inhibit cap‐dependent translation was determined by the in vitro translation of capped‐Renilla luciferase mRNA. Both compounds 113 and 114 inhibit in vitro cap‐dependent translation that strongly indicates the presence of peptide moiety attached to the cap analog does not impact the inhibitory properties. It is noteworthy that the cap analog containing fluorescence probe and TAT 114 translocate into the human breast adenocarcinoma cancer cell line MCF‐7. [42]
In a series of nicotinamide‐containing cap analogs, all cap analogs 117–119 and 125–128 undergo incorporation into RNA to generate the corresponding modified NAD‐capped RNAs. [46] Remarkably, mRNAs capped with nicotinamide‐containing trinucleotide cap analogs 128 a and 128 b are resistant to deNADing enzymes such as NudC, Nudt12, and DXO. [46]
Table 6 summarizes the biochemical properties such as capping efficiency, translational efficiency, binding affinity to eIF4E, and susceptibility to hDcp2 for various trinucleotide cap analogs 130 a–j. [47] The capping efficiency of cap analogs containing a purine nucleotide at the position of the first transcribed nucleotide is higher than that of the standard cap and ARCA (entries 2–5 and 9–12). The highest capping efficiency of 90 % is observed for m7GpppAmpG 130 b (entry 3). The presence of the 2′‐OMe group at the first transcribed nucleotide does not have any influence on the capping efficiency as compared to the parent unmethylated first transcribed nucleotide (entries 2–5, 7, 8, and 11–14). The capping efficiency of cap analogs containing a pyrimidine nucleotide at the position of the first transcribed nucleotide is comparable to that of the standard cap and ARCA (entries 7–10, 13, and 14). The capping efficiency of cap analogs containing a purine nucleotide at the position of the first transcribed nucleotide provides higher capping efficiency than cap analogs containing a pyrimidine nucleotide at the position of the first transcribed nucleotide (entries 2–8 and 11–14). The nature of the base at the first transcribed nucleotide plays an important role on the outcome of the translational efficiency. The presence of adenine at the first transcribed nucleotide provides the highest translational efficiency as compared to other bases such as cytosine, uracil, and guanine (entries 2–5). The presence of guanine at the first transcribed nucleotide provides the lowest translational efficiency (entries 11 and 12). The translational efficiency is in the order of A>C>U>G. It is interesting to compare the effect of 2′‐OMe group of the mRNA transcripts with different cell lines such as JAWS II (mouse immortalized immature dendritic cells), 3T3‐L1 (mouse embryonic cells) and HeLa (cervical cancer). The presence of 2′‐OMe group at the first transcribed mRNA in a dendritic cell provides up to seven‐fold higher translational efficiency as compared to the unmethylated one (entries 2–5, 7, and 8). The presence of the 2′‐OMe group at the first transcribed mRNA in 3T3‐L1 provides comparable translational efficiency to that of the unmethylated ones (entries 2, 3, 7, and 8). The presence of the 2′‐OMe group at the first transcribed mRNA in HeLa cell lines provides slightly higher translational efficiency to that of the unmethylated ones (entries 2, 3, 7, and 8). The highest translational efficiency is observed for m7Gpppm6AmpG 130 d that is 7.39‐fold higher translational efficiency as compared to unmethylated one, m7GpppApG 130 a (entries 2 and 5). Given the binding affinities and susceptibility to decapping by hDcp2 data, it appears that the presence of the 2′‐OMe group at the first transcribed mRNA does not influence cap‐eIF4E interaction and susceptibility to decapping as compared to the unmethylated cap analog (entries 1–14).
Table 6.
Biochemical properties of trinucleotide cap analogs 130 a–j.
|
Entry |
Compound |
Capping (%) |
3T3‐L1 |
HeLa |
JAWS II |
KD eIF4E |
Susceptibility to hDcp2 |
|---|---|---|---|---|---|---|---|
|
1 |
m7GpppA |
n.d. |
n.d. |
n.d. |
n.d |
178 |
n.d. |
|
2 |
m7GpppApG 130 a |
89 |
1.00 |
1.00 |
1.00 |
26 |
0.48 |
|
3 |
m7GpppAmpG 130 b |
90 |
1.01 |
1.21 |
5.01 |
45.6 |
0.45 |
|
4 |
m7Gpppm6ApG 130 c |
78 |
0.45 |
0.90 |
0.90 |
35.2 |
0.46 |
|
5 |
m7Gpppm6AmpG 130 d |
77 |
1.04 |
1.56 |
7.39 |
32.8 |
0.43 |
|
6 |
m7GpppC |
n.d. |
n.d. |
n.d. |
n.d. |
324 |
n.d. |
|
7 |
m7GpppCpG 130 e |
60 |
0.65 |
0.73 |
0.86 |
25.1 |
0.22 |
|
8 |
m7GpppCmpG 130 f |
54 |
0.66 |
0.97 |
2.42 |
29.9 |
0.20 |
|
9 |
m7GpppG |
69 |
0.46 |
n.d. |
0.18 |
80.0 |
n.d. |
|
10 |
ARCA |
56 |
n.d. |
n.d. |
n.d. |
98.0 |
n.d. |
|
11 |
m7GpppGpG 130 g |
80 |
0.33 |
0.34 |
0.05 |
22.7 |
0.10 |
|
12 |
m7GpppGmpG 130 h |
86 |
0.24 |
0.41 |
0.12 |
22.8 |
0.08 |
|
13 |
m7GpppUpG 130 i |
56 |
0.70 |
0.45 |
1.01 |
n.d. |
0.30 |
|
14 |
m7GpppUmpG 130 j |
56 |
0.70 |
0.91 |
3.47 |
n.d. |
0.32 |
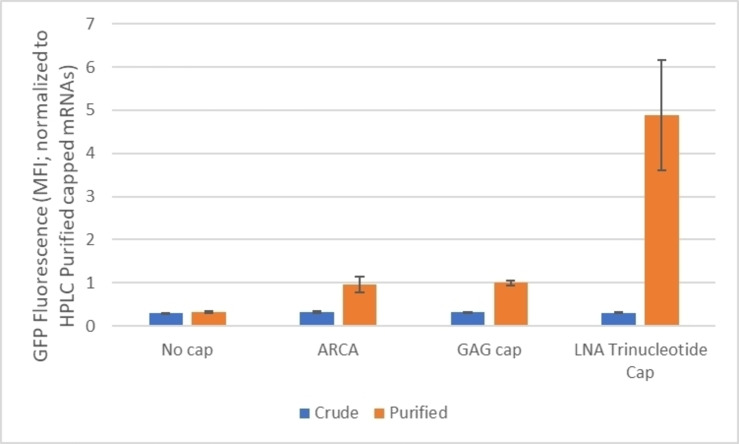
The capping efficiency of new LNA tricap 143 was performed in an in vitro transcription system by using an IVT template encoding GFP to produce transcripts of ∼1000 nucleotides in length with a synthetic 5′‐UTR, a mouse alpha globin 3′‐UTR, and a 120 nucleotide poly(A) tail. [50] The outcome of the assay reveals that LNA cap 143 has a capping efficiency of 53 %, indicating this LNA tricap is a good substrate for T7 RNA polymerase. Next, we determined the translational efficiency of the different caps in JAWS II cells that has been used as a model for antitumor or pathogen‐specific immunity studies. The translational efficiency data of LNA tricap 143, standard trinucleotide cap analogue (GAG), m7G(5′)ppp(5′)AmpG and anti‐reverse cap analogue (ARCA), m2 7,3′−O G(5′)ppp(5′)G are compiled in Figure 3. Notably, the mRNA capped with LNA tricap is translated 5 times higher than GAG trinucleotide or ARCA capped mRNA transcript in the dendritic line.
Figure 3.
Translational efficiency of LNA trinucleotide cap 143, GAG, and ARCA.
4. Conclusion and Future Perspectives
The effect of chemical modification on a cap analog has had a significant impact on the outcome of the biochemical properties such as capping efficiency, translation efficiency, binding affinity to eIF4E, and susceptibility to decapping. [24] The invention of trinucleotide cap analog fostered much research and considerable progress in the area of mRNA cap analogs. The utilization of cotranscriptional m7GpppNmN‐derived trinucleotide in the cotranscriptional reaction produces the corresponding IVT mRNA with cap 1 structure, whereas cotranscriptional capping with m7GpppN‐derived dinucleotide results in the formation of the corresponding IVT mRNA cap 0 structure. The presence of cap 1 structure in mRNA plays an important role to promote translation and also allow cells to differentiate between self and non‐self RNA and suppress viral replication. [56] Remarkably, the trinucleotide cap analog outperforms the dinucleotide cap analog in terms of capping efficiency and translational properties. Based on the remarkable translational properties of m7Gpppm6AmpG 130 c and m7(LNA)G(5′)ppp(5′)(2′‐OMeA)pG 143, it is highly likely that these new trinucleotide cap analogs are able to generate immunogenic protein for a long time span and create a dynamic positive impact on the designing of personalized medicines. There has been growing worldwide concern to prevent infectious diseases due to the recent outbreak of SARS‐CoV‐2, Dengue, and Ebola virus. The mRNA vaccination provides a powerful weapon to control infectious diseases. [57] Given the recent FDA approval of mRNA vaccine for SARS‐CoV‐2, the design and development of novel trinucleotide cap analogs are warranted in the area of mRNA vaccines. One of the biggest challenges in this area is the isolation of 100 % capped mRNA from uncapped mRNA, wherein the uncapped mRNA negatively influences the overall translational efficiency. [13h] ] The development of a practical and feasible method is demanded to isolate the capped mRNA from uncapped mRNA. In addition to the mRNA vaccine, the exploration of mRNA as a vehicle for antigen delivery to dendritic cells is one of the thrust areas of research in view of creating a novel immunotherapeutic approach. [58] Furthermore, considerable efforts should be invested to explore cap analog as a potential inhibitor to target eIF4E protein [59] and antiviral target. [60] The preliminary encouraging results of cell penetrating peptide as a carrier for the internalization of cap analogs open up a new avenue to design novel anticancer drug.[42 Many new therapeutic applications are emerging in the area of mRNA cap analogs and in the next two to five years will see cap analog as a candidate for clinical trials for immunotherapy and anticancer therapy.
Biographical Information
Muthian Shanmugasundaram received his Ph.D. in Organic Chemistry from the University of Madras, Chennai (India). He did three post‐doctoral research at the Tsing Hua University, Hsinchu (Taiwan), University of Arkansas, Fayetteville (USA) and University of Texas at El Paso (USA). His doctoral and post‐doctoral research work involved in the area of heterocyclic chemistry, metal‐mediated organic synthesis, solid‐phase organic synthesis, combinatorial chemistry, supramolecular chemistry, and medicinal chemistry. Currently, he is a Senior Staff Scientist at Thermo Fisher Scientific, Austin, Texas, USA. He has authored in more than 70 peer‐reviewed research papers and has the inventorship of nine patents. His current research focuses in the area of nucleic acid chemistry, mRNA cap analogs for therapeutics, proteinase K inhibitors, and lipid‐based transfection reagent.

Biographical Information
Annamalai Senthilvelan received his Ph.D degree in Organic Chemistry (2002) from University of Madras‐India, under Prof. V. T. Ramakrishnan in the area of Organic photochemistry and heterocyclic chemistry. He carried out two post‐doctoral studies in Taiwan (2002‐2007). One at Academia Sinica in the area of carbohydrate chemistry and another at National Chiao‐Tung University in the area of Organic supramolecular host‐guest chemistry. He joined Prof. Howard E. Zimmerman's group in the department of chemistry, University of Wisconsin‐Madison as research associate (2007‐2010). His research focused in the areas of heterocyclic, photochemistry, and host‐guest chemistry. Currently, since 2010 he is working as Staff scientist at Thermofisher Scientific‐Austin, USA. His research interest focuses on the synthesis of nucleotides, modified nucleotides and nucleotide cap analogs for molecular biology and therapeutic applications, and big dye terminators. He received 2013 Presidential Green Chemistry Challenge Award from the Environmental Protection Agency (EPA)‐USA. His research findings accomplished over 40 publications in peer‐reviewed journals

Biographical Information
In 1996, Dr. Kore received his Ph.D. in Bioorganic Chemistry from Shivaji University, Kolhapur (India), followed by post‐doctoral research at Max‐Planck Institute for Experimental Medicine, Gottingen (Germany) and University of Michigan, Department of Chemistry, Ann Arbor (USA). Dr. Kore currently serves as a Director, Chemistry at Thermo Fisher Scientific, Austin, TX, USA. His research focuses on discovery and development modified nucleoside/nucleotides, Cap analogues for mRNA therapeutics, Modified Oligonucleotides, Modified peptides as a proteinase K inhibitor and lipid‐based transfection reagent for various cell lines. Dr. Kore has authored over 87 scientific publications in leading journals and have inventorship of more than 11 US awarded patents. Throughout his professional career, he has attended and made numerous presentations at local and international scientific meetings and acted as a chair for symposium session. He also serves as an Associate Editor, Current Protocols in Nucleic Acid Chemistry (CPNC), Wiley. He is also recipient of 2013 Presidential Green Chemistry Challenge Award given by the Environmental Protection Agency (EPA) for Greener Synthetic Pathways for manufacturing of various dNTPs for RT/PCR.

M. Shanmugasundaram, A. Senthilvelan, A. R. Kore, Chem. Rec. 2022, 22, e202200005.
References
- 1. Furuichi Y., Shatkin A. J., Adv. Virus Res. 2000, 55, 135–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topisirovic I., Svitkin Y. V., Sonenberg N., Shatkin A. J., Wiley Interdiscip. Rev.: RNA 2011, 2, 277–298. [DOI] [PubMed] [Google Scholar]
- 3. Gu M., Lima C. D., Curr. Opin. Struct. Biol. 2005, 15, 99–106. [DOI] [PubMed] [Google Scholar]
- 4. Jia Y., Polunovsky V., Bitterman P. B., Wagner C. R., Med. Res. Rev. 2012, 32, 786–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niedzwiecka A., Marcotrigiano J., Stepinski J., Jankowska-Anyszka M., Wyslouch-Cieszynska A., Dadlez M., Gingras A. C., Mak P., Darzynkiewicz E., Sonenberg N., Burley S. K., Stolarski R., J. Mol. Biol. 2002, 319, 615–635. [DOI] [PubMed] [Google Scholar]
- 6. Mazza C., Segref A., Mattaj I. W., Cusack S., EMBO J. 2002, 21, 5548–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu G., Gershon P. D., Hodel A. E., Quiocho F. A., Proc. Natl. Acad. Sci. USA 1999, 7149–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasquinelli A. E., Dahlberg J. E., Lund E., RNA 1995, 1, 957–967. [PMC free article] [PubMed] [Google Scholar]
- 9. Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R. E., RNA 2001, 7, 1495. [PMC free article] [PubMed] [Google Scholar]
- 10. Peng Z. H., Sharma V., Singleton S. F., Gershon P. D., Org. Lett. 2002, 4, 161–164. [DOI] [PubMed] [Google Scholar]
- 11. Jemielity J., Fowler T., Zuberek J., Stepinski J., Lewdorowicz M., Niedzwiecka A., Stolarski R., Darzynkiewicz E., Rhoads R. E., RNA 2003, 9, 1108–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grudzien E., Stepinski J., Jankowska-Anyszka M., Stolarski R., Darzynkiewicz E., Rhoads R. E., RNA 2004, 10, 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Kore A. R., Shanmugasundaram M., Charles I., Cheng A. M., Barta T., Bioorg. Med. Chem. Lett. 2007, 17, 5295–5299; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13b. Kore A. R., Shanmugasundaram M., Bioorg. Med. Chem. Lett. 2008, 18, 880–884; [DOI] [PubMed] [Google Scholar]
- 13c. Kore A. R., Shanmugasundaram M., Vlassov A. V., Bioorg. Med. Chem. Lett. 2008, 18, 4828–4832; [DOI] [PubMed] [Google Scholar]
- 13d. Kore A. R., Shanmugasundaram M., Charles I., Vlassov A. V., Barta T. J., J. Am. Chem. Soc. 2009, 131, 6364–6365; [DOI] [PubMed] [Google Scholar]
- 13e. Kore A. R., Shanmugasundaram M., Lett. Org. Chem. 2009, 6, 141–144, Charles; [Google Scholar]
- 13f. Shanmugasundaram M., Chem. Lett. 2009, 38, 652–653; [Google Scholar]
- 13g. Kore A. R., Charles I., Bioorg. Med. Chem. 2010, 18, 8061–806; [DOI] [PubMed] [Google Scholar]
- 13h. Shanmugasundaram M., Charles I., Kore A. R., Bioorg. Med. Chem. 2016, 24, 1204–1208. [DOI] [PubMed] [Google Scholar]
- 14. Kuhn A. N., Diken M., Kreiter S., Selmi A., Kowalska J., Jemielity J., Darzynkiewicz E., Huber C., Türeci Ö., Sahin U., Gene Therapy 2010, 17, 961–971. [DOI] [PubMed] [Google Scholar]
- 15. Shuman S., Surks M., Furneaux H., Hurwitz J., J. Biol. Chem. 1980, 255, 1588–1598. [PubMed] [Google Scholar]
- 16. Ramanathan A., Robb G. B., Chan S. H., Nucleic Acids Research 2016, 44, 7511–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghosh A., Lima C. D., Wiley Interdiscip. Rev. RNA 2010, 1, 152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daffis S., Szretter K. J., Schriewer J., Li J., Youn S., Errett J., Lin T.-Y., Schneller S., Zust R., Dong H., Nature 2010, 468, 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jennifer L. H., Michael S. D., Virology 2015, 479, 480:66–74. [Google Scholar]
- 20. Ishikawa M., Murai R., Hagiwara H., Hoshino T., Suyama K., Nucleic Acids Symp. Ser. (Oxf.) 2009, 53, 129–130. [DOI] [PubMed] [Google Scholar]
- 21.Messengers of hope.
- 21a. Nat. Biotechnol. 2021, 39, 1. https://doi.org/10.1038/s41587-020-00807–1;33376248 [Google Scholar]
- 21b. Ugur S., Alexander M., Evelyna D., Isable V., Lena M. K., Mathias V., Alina B., Kristen P., Jasmin Q., Daniel M., Sebastian B., Verena L., Julian S., Rolf H., Dirk B., Ann-Kathrin E., Jan G., Carsten B., Corinna R., Marie-Cristine K., Ulrich L., Alexandra K.-B., David L., Martin B., Stefanie B., Katalin K., Tania P., Boris F., Armin S., Pei-Yong S., Camila F.-G., John L. P., Kena A. S., Jakob L., Ingrid L. S., Mark C., Warren K., Christos A. K., David C., Philip R. D., Kathrin U. J., Ozlem T., Nature 2020, 586, 594–599.32998157 [Google Scholar]
- 22.
- 22a. Mikkola S., Salomaeki S., Zhang Z., Maeki E., Loennberg H., Curr. Org. Chem. 2005, 9, 999–1022; [Google Scholar]
- 22b. Xiong G., Xiong X., Zhang J., Xibao Shengwuxue Zazhi 2006, 28, 513–517; [Google Scholar]
- 22c. Grudzien-Nogalska E., Stepinski J., Jemielity J., Zuberek J., Stolarski R., Rhoads R. E., Darzynkiewicz E., Methods Enzymol. 2007, 431, 203–227; [DOI] [PubMed] [Google Scholar]
- 22d. Nicolette C. A., Healey D., Tcherepanova I., Whelton P., Monesmith T., Coombs L., Finke L. H., Whiteside T., Miesowicz F., Vaccine 2007, 25, B47–60; [DOI] [PubMed] [Google Scholar]
- 22e. Yamamoto A., Kormann M., Rosenecker J., Rudolph C., Eur. J. Pharm. Biopharm. 2009, 71, 484–489; [DOI] [PubMed] [Google Scholar]
- 22f. Jemielity J., Kowalska J., Rydzik A. M., Darzynkiewicz E., New. J. Chem. 2010, 34, 829–844; [Google Scholar]
- 22g.M. Warminski, P. J. Sikorski, J. Kowalska1, J. Jemielity, Top. Curr. Chem. 2017, 375, 16. [DOI] [PMC free article] [PubMed]
- 23.
- 23a. Kore A. R., Charles I., Shanmugasundaram M., Xiao Z., Conrad R. C., Mini-Rev. Org. Chem. 2008, 5, 179–192; [Google Scholar]
- 23b. Kore A. R., Charles I., Shanmugasundaram M., Current Org.Chem. 2010, 14, 1083–1098. [Google Scholar]
- 24. Shanmugasundaram M., Senthilvelan A., Kore A. R., Current Org. Chem. 2017, 21, 2530–2560. [Google Scholar]
- 25. Nakagawa I., Konya S., Ohtani S., Hata T. A., Synthesis 1980, 556. [Google Scholar]
- 26. Iwase R., Sekine M., Tokumoto Y., Ohshima Y., Hata T., Nucleic Acids Res. 1989, 17, 8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamimura T., Osaki Y., Sekine M., Hata T., Tetrahedron Lett. 1984, 25, 2683–2686. [Google Scholar]
- 28. Sekine M., Nishiyama S., Kamimura T., Osaki Y., Hata T., Bull. Chem. Soc. Jpn. 1985, 850–860. [Google Scholar]
- 29. Fukuoka K., Suda F., Suzuki R., Takaku H., Ishikawa M., Hata T., Tetrahedron Lett. 1994, 35, 1063–1066. [Google Scholar]
- 30. Sawai H., Wakai H., Shimazu M., Tetrahedron Lett. 1991, 32, 6905–6906. [Google Scholar]
- 31. Sawai H., Wakai H., Nakamura-Ozaki A., J. Org.Chem. 1999, 64, 5836–5840. [Google Scholar]
- 32. Sawai H., Shimazu M., Wakai H., Wakabayashi H., Shinozuka K., Nucleosides Nucleotides and Nucleic Acids 1992, 11, 773–785. [Google Scholar]
- 33. Kadokura M., Wada T., Urashima C., Sekine M., Tetrahedron Lett. 1997, 38, 8359–8362. [Google Scholar]
- 34. Walczak S., Nowicka A., Kubacka D., Fac K., Wanat P., Mroczek S., Kowalska J., Jemielity J., Chem. Sci. 2017, 8, 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mamot A., Sikorski P. J., Warminski M., Kowalska J., Jemielity J., Angew. Chem. Int. Ed. 2017, 56, 15628–15632. [DOI] [PubMed] [Google Scholar]
- 36. Wojtczak B. A., Sikorski P. J., Fac-Dabrowska K., Nowicka A., Warminski M., Kubacka D., Nowak E., Nowotny M., Kowalska J., Jemielity J., J. Am. Chem. Soc. 2018, 140, 5987–5999. [DOI] [PubMed] [Google Scholar]
- 37. Bednarek S., Madan V., Sikorski P. J., Bartenschlanger R., Kowalska J., Jemielity J., Phil. Trans. R. Soc. 2018, B 373, 20180167. https://dx.doi.org/10.1098/rstb.2018.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walczak S., Sikorski P. J., Kasprzyk R., Kowalska J., Jemielity J., Org. Biomol. Chem. 2018, 16, 6741–6748. [DOI] [PubMed] [Google Scholar]
- 39. Kopcial M., Wojtczak B. A., Kasprzyk R., Kowalska J., Jemielity J., Molecules 2019, 24, doi:10.3390/molecules24101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piecyk K., Krynska P., Kaluzna J., Jankowska-Anyszka M., Tetrahedron Lett. 2017, 58, 3037–3040. [Google Scholar]
- 41. Kocmik I., Piecyk K., Rudzinska M., Niedzwiecka A., Darzynkiewicz E., Grzela R., Jankowska-Anyszka M., Cell Cycle 2018, 17, 1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piecyk K., Pietrow P., Arnold T., Worch R., Korneeva N. L., Jankowska-Anyszka M. . Bioconjugate Chem. 2020, 31, 1156–1166. [DOI] [PubMed] [Google Scholar]
- 43. Berger F., Ramírez-Hernández M. H., Ziegler M., Trends Biochem. Sci. 2004, 29, 111–118. [DOI] [PubMed] [Google Scholar]
- 44.
- 44a. Jaschke A., Hofer K., Nubel G., Frindert J., Curr. Opin. Microbiol. 2016, 30, 44–49; [DOI] [PubMed] [Google Scholar]
- 44b. Cahova H., Winz M. L., Hofer K., Nubel G., Jaschke A., Nature 2015, 519, 374–377. [DOI] [PubMed] [Google Scholar]
- 45. Jiao X., Doamekpor S. K., Bird J. G., Nickels B. E., Tong L., Hart R. P., Kiledjian M., Cell 2017, 168, 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mlynarska-Cieslak A., Depaix A., Grudzien-Nogalska E., Sikorski P. J., Warminski M., Kiledjian M., Jemielity J., Kowalska J., Org. Lett. 2018, 20, 7650–7655. [DOI] [PubMed] [Google Scholar]
- 47. Sikorski P. J., Warminski M., Kubacka D., Ratajczak T., Nowis D., Kowalska J., Jemielity J., Nucleic. Acids. Res. 2020, 48, 1607–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Veedu R. N., Wengel J., Chem. Biodiversity 2010, 7, 536–542. [DOI] [PubMed] [Google Scholar]
- 49. Kaur H., Babu R. B., Maiti S., Chem. Rev. 2007, 107, 83–112. [DOI] [PubMed] [Google Scholar]
- 50. Senthilvelan A., Vonderfecht T., Shanmugasundaram M., Pal I., Potter J., Kore A. R., Org. Lett. 2021, 23, 4133–4136. [DOI] [PubMed] [Google Scholar]
- 51. Borbolis F., Syntichaki P., FEBS J. 2021, 1–19. [DOI] [PubMed] [Google Scholar]
- 52.
- 52a. Parker R., Song H., Nat Struct Mol Biol. 2004, 11, 121–127; [DOI] [PubMed] [Google Scholar]
- 52b. Meyer S., Temme C., Wahle E., Crit. Rev. Biochem. Mol. Biol. 2004, 39, 197–216. [DOI] [PubMed] [Google Scholar]
- 53. Coller J., Parker R., Annu. Rev. Biochem. 2004, 73, 861–890. [DOI] [PubMed] [Google Scholar]
- 54. Rydzik A. M., Kulis M., Lukaszewicz M., Kowalska J., Zuberek J., Darzynkiewicz Z. E., Jemielity J., Bioorg. Med.Chem. 2012, 20, 1699–1710. [DOI] [PubMed] [Google Scholar]
- 55. Kowalska J., Wypijewska del Nogal A., Darzynkiewicz Z. M., Buck J., Nicola C., Kuhn A. N., Lukaszewicz M., Zuberek J., Strenkowska M., Ziemniak M., Maciejczyk M., Bojarska E., Rhoads R. E., Darzynkiewicz E., Sahin U., Jemielity J., J. Nucleic Acids Res. 2014, 42, 10245–10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Daffis S., Szretter K. J., Schriewer J., Li J., Youn S., Erett J., Lin T. Y., Schneller S., Zust R., Dong H., Nature 2010, 468, 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.
- 57a. Maruggi G., Zhang C., Li J., Ulmer J. B., Yu D., Mol Ther 2019, 10, 757–772.L; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57b. Markiewicz, Drazkowska K., Sikorski P. J., RNA Biology 2021, 18, 669–687; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57c. Chaudhary N., Weissman N., Whitehead K. A., Nat Rev Drug Discov 2021, 20, 817–838; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57d. Eygeris Y., Gupta M., Kim J., Sahay G., Acc. Chem. Res. 2022, 55, 1, 2–12. [DOI] [PubMed] [Google Scholar]
- 58. Tcherepanova I. Y., Adam M. D., Feng X., Hinohara A., Horvatinovich J., Calderhead D., Healey D., Nicolette C. A., BMC Mol. Biol. 2008, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Warminski M., Kowalska J., Nowak E., Kubacka D., Tibble R., Kasprzyk R., Sikorski P. J., Gross J. D., Nowotny M., Jemielity J., ACS Chem. Biol. 2021, 16, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kasprzyk R., Spiewla T. J., Smietanski M., Golojuch S., Vangeel L., De Jonghe S., Jochmans D., Neyts J., Kowalska J., Jemielity J., Antivir. Res. 2021, 193, 105142. [DOI] [PMC free article] [PubMed] [Google Scholar]