SARS‐CoV‐2 vaccines administered in the UK are highly effective in preventing hospitalisation and death from COVID‐19 [1]. Patients with immunocompromise are less likely to be able to mount a satisfactory immunological response to the vaccine and therefore may remain at higher risk of moderate‐to‐severe COVID‐19 [2]. Understanding the reasons and risk‐factors for admission will provide insight into strategies for future vaccination. This study aimed to characterise the hospitalised vaccinated population and identify the effect of the relationship between vaccination status and immunocompetence on hospital mortality using the prospective observational cohort recruited from the UK Coronavirus Clinical Information Network (CO‐CIN).
ISARIC4C/CO‐CIN collected data on hospitalised patients with COVID‐19 in the UK since February 2020 [3]. The National Immunisation Management Service contains vaccine type and date of first and/or second vaccination since the COVID‐19 vaccination programme started in the UK on 8 December 2020. We linked data in CO‐CIN and the National Immunisation Management Service and restricted our population to adults admitted to hospital with symptomatic polymerase chain reaction (PCR)‐positive SARS‐CoV‐2 infection with at least 28 days of follow‐up. This is a complete case analysis. Patients with re‐infection were removed from this analysis. We categorised patients into the following three groups: no virus immunity – unvaccinated patients and patients experiencing symptoms ≤ 20 days after first vaccination dose [4]; first dose failure – patients experiencing symptoms ≥ 21 days after first vaccination dose or patients experiencing symptoms ≤ 13 days after second vaccination dose; and second dose failure – patients experiencing symptoms ≥ 14 days after second vaccination dose. Immunocompromise was defined as pre‐existing immunological or metabolic disorder (e.g. severe combined immunodeficiency or common variable immunodeficiency); solid organ transplant; HIV/AIDS; cancer on active treatment with chemotherapy or immune modifying drugs; or receipt of immunosuppressing drugs. We assessed the association between immunocompromise, vaccine failure status and 28‐day mortality, adjusting for age, sex, ethnicity, socio‐economic status and comorbidity using logistic regression with an interaction between immunocompromise and vaccine failure status.
There were 40,870 patients recruited to ISARIC4C/CO‐CIN between 8 December 2020 and 15 August 2021 with symptomatic PCR‐positive COVID‐19. At the time of admission, 33,856 (82.8%) patients were unvaccinated; 5332 (13.0%) had received their first vaccination; and 1682 (4.1%) had received their second vaccination. Of the 7014 patients who had received a vaccination, 3606 (51.4%) had no virus immunity; 1941 (27.7%) had first dose failure; and 1467 (20.9%) had second dose failure (see online Supporting Information Figure S1), proportions which persisted when restricting to patients with at least 60 days of follow‐up (see online Supporting Information Figure S2). Despite lower absolute values, the relative proportion of immunocompromised patients increased from no virus immunity (12.4%) to first dose failure (17.5%) to second dose failure (20.6%) (Table 1).
Table 1.
Patient characteristics stratified by immunocompetency. Values are number (proportion) or number
|
Immunocompetent n = 35,581 |
Immunocompromised n = 5289 |
Overall n = 40,870 | |
|---|---|---|---|
| Sex | |||
| Female | 15,662 (86.4%) | 2456 (13.6%) | 18,118 |
| Male | 19,885 (87.6%) | 2823 (12.4%) | 22,708 |
| Missing | 34 (77.3%) | 10 (22.7%) | 44 |
| Ethnicity | |||
| White | 24,414 (86.1%) | 3934 (13.9%) | 28,348 |
| South Asian | 2220 (88.6%) | 285 (11.4%) | 2505 |
| Black | 909 (88.9%) | 114 (11.1%) | 1023 |
| East Asian | 181 (91.9%) | 16 (8.1%) | 197 |
| Other | 2380 (88%) | 324 (12%) | 2704 |
| Missing | 5477 (89.9%) | 616 (10.1%) | 6093 |
| Vaccination tier | |||
| Tier 2 | 9274 (88.5%) | 1209 (11.5%) | 10,483 |
| Tier 3 | 3454 (82.7%) | 725 (17.3%) | 4179 |
| Tier 4 | 3655 (66.4%) | 1848 (33.6%) | 5503 |
| Tier 5 | 2850 (90.7%) | 291 (9.3%) | 3141 |
| Tier 6 | 6241 (83.7%) | 1216 (16.3%) | 7457 |
| Tier 7 | 1632 (100%) | 0 | 1632 |
| Tier 8 | 1850 (100%) | 0 | 1850 |
| Tier 9 | 1711 (100%) | 0 | 1711 |
| Tier 10 | 4914 (100%) | 0 | 4914 |
| IMD quintile | |||
| 1 (most deprived) | 9438 (88.5%) | 1231 (11.5%) | 10,669 |
| 2 | 7353 (86.9%) | 1110 (13.1%) | 8463 |
| 3 | 6528 (87.2%) | 958 (12.8%) | 7486 |
| 4 | 6008 (86.3%) | 953 (13.7%) | 6961 |
| 5 (least deprived) | 5472 (85.6%) | 920 (14.4%) | 6392 |
| Missing | 782 (87%) | 117 (13%) | 899 |
| Comorbidities | |||
| Chronic kidney disease | 4176 (81.7%) | 937 (18.3%) | 5113 |
| Solid organ transplant | 0 | 324 (100%) | 324 |
| Chronic cardiac disease | 7905 (84.1%) | 1491 (15.9%) | 9396 |
| Chronic pulmonary disease | 4187 (75.3%) | 1374 (24.7%) | 5561 |
| Diabetes | 4692 (85.6%) | 789 (14.4%) | 5481 |
| Obesity | 5101 (86.2%) | 820 (13.8%) | 5921 |
| Chronic neurological disorder | 3106 (87.2%) | 457 (12.8%) | 3563 |
| Dementia | 2922 (89.8%) | 331 (10.2%) | 3253 |
| Vaccine failure | |||
| No information on the date of symptom onset | 75 (92.6%) | 6 (7.4%) | 81 |
| No virus immunity | 32,740 (87.6%) | 4641 (12.4%) | 37,381 |
| First dose failure | 1601 (82.5%) | 340 (17.5%) | 1941 |
| Second dose failure | 1165 (79.4%) | 302 (20.6%) | 1467 |
IMD, index of multiple deprivation.
After adjustment, vaccination reduced the odds of mortality in patients admitted to hospital (Fig. 1 and online Supporting Information Figure S3). Immunocompromised patients had consistently higher odds of mortality compared with immunocompetent patients (Fig. 1), and there was a significant interaction between vaccination status and immunocompromise (p = 0.001).
Figure 1.
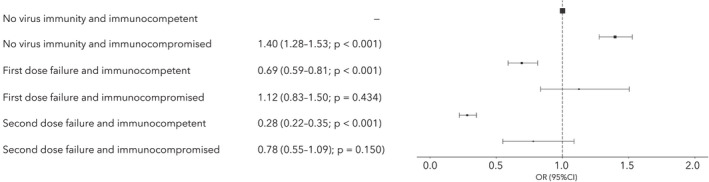
Logistic regression odds of 28‐day mortality for immunocompetent vs. immunocompromised patients, stratified by vaccine group: no virus immunity; first dose failure; and second dose failure. Odds adjusted for age; sex; ethnicity; socio‐economic status; and comorbidity
Most patients hospitalised with symptomatic COVID‐19 since the vaccination programme began in the UK have not been vaccinated, and for those who have received a vaccine, most admissions occurred within 3 weeks of the first dose before the vaccine would be expected to be effective (see online Supporting Information Figure S1). It is important to highlight to the general population that there is a lag between receiving a vaccination and developing the immunity required to prevent hospitalisation or death, as awareness may alter post‐vaccination behaviour. We found that vaccination generally reduced the odds of in‐hospital mortality in both immunocompetent and immunocompromised patients; however, this effect was reduced in immunocompromised patients. This is consistent with previous study findings that although patients with weakened immune systems mount a response to COVID‐19 vaccines, the rates of seroconversion and antibody generation are lower [2, 5, 6, Kearns et al., preprint, https://doi.org/10.2139/ssrn.3910058].
This analysis was undertaken before the emergence of the omicron variant and before third booster doses were available to all adults, and should be repeated in the context of omicron to examine the effect of third doses on outcomes for patients admitted to hospital with COVID‐19. Public health messaging regarding booster vaccine doses and non‐pharmaceutical interventions should target this vulnerable immunocompromised group. Alternative strategies such as prophylactic or therapeutic administration of high potency monoclonal antibodies should also be considered.
Supporting information
Figure S1. Time between first and second vaccination and onset of symptoms, up to 150 days.
Figure S2. Time between first and second vaccination and onset of symptoms, up to 60 days.
Figure S3. Full model for 28‐day in‐hospital mortality.
Acknowledgements
The authors acknowledge the data linkages undertaken by N. Tarbatt and N. Abbotts (NHS England). This work is supported by grants from the NIHR, the MRC and by the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at the University of Liverpool in partnership with Public Health England, National Institute for Health Research Health Protection Research Unit in Respiratory Infections at Imperial College London with Public Health England, NIHR Biomedical Research Centre at Imperial College London, EU Platform for European Preparedness Against (Re‐)emerging Epidemics and NIHR Clinical Research Network for providing infrastructure support for this research. The views expressed are those of the authors and not necessarily those of the Department for Health and Social Care, Department for International Development, NIHR, MRC, Wellcome or Public Health England. LT reports grants from MRC during the conduct of the study, and grants from Wellcome Trust, Innovate UK, NIHR, MRC and EU Horizon 2020 outside the submitted work. MS reports grants from Department for Health and Social Care NIHR UK, grants from MRC UK, Health Protection Research Unit in Emerging and Zoonotic Infections at the University of Liverpool, during the conduct of the study, and from Integrum Scientific LLC outside the submitted work. AD reports a grant from Wellcome Trust outside the submitted work. No other competing interests declared.
References
- 1. Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS‐CoV‐2 infections in the United Kingdom. Nature Medicine 2021; 27: 1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. Journal of the American Medical Association 2021; 325: 1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Docherty BB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. British Medical Journal 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall VJ, Foulkes S, Saei A, et al. COVID‐19 vaccine coverage in health‐care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021; 397: 1725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carr EJ, Harvey R, Wall EC. Neutralising antibodies after COVID‐19 vaccination in UK haemodialysis patients. Lancet 2021; 398: 1038–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakkar A, Gonzalex‐Lugo JD, Goradia N, et al. Seroconversion rates following COVID‐19 vaccination among patients with cancer. Cancer Cell 2021; 39: 1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Time between first and second vaccination and onset of symptoms, up to 150 days.
Figure S2. Time between first and second vaccination and onset of symptoms, up to 60 days.
Figure S3. Full model for 28‐day in‐hospital mortality.


