Abstract
Aims
The COVID‐19 pandemic is a global public health emergency and patients with diabetes mellitus (DM) are disproportionately affected, exhibiting more severe outcomes. Recent studies have shown that metformin is associated with improved outcomes in patients with COVID‐19 and DM and may be a potential candidate for drug repurposing. We aimed to investigate the effects of metformin on outcomes in patients with COVID‐19 and DM.
Methods
Databases (PubMed, Scopus, Web of Science, EMBASE, Clinicaltrials.gov and Cochrane library) were searched up to 10 April 2021 for studies reporting data on metformin use in COVID‐19 patients with DM. The risk of bias was assessed using the Newcastle–Ottawa scale. Certainty of evidence was rated using the GRADE approach. The primary outcome was mortality reported as odds ratio (OR). A random‐effects meta‐analysis was carried out on both unadjusted and adjusted ORs. This study is registered with PROSPERO, CRD42020221842.
Results
In total, 2 916 231 patients from 32 cohort studies were included in the quantitative and qualitative synthesis. The meta‐analysis showed that metformin was significantly associated with lower mortality in COVID‐19 patients with DM in both unadjusted (OR 0.61 [95% confidence interval: 0.53–0.71], P < .00001, I 2 = 70%) and adjusted (OR 0.78 [95% confidence interval: 0.69–0.88], P < .00001, I 2 = 67%) models.
Conclusion
Poor outcomes in COVID‐19 patients with DM can be attributed to inadequate glycaemic control and weakened immune responses. Metformin has multiple effects that can improve outcomes in patients with DM and our findings highlight a possible role of its use. However, robust randomised trials are needed to thoroughly assess its use.
Keywords: biguanides, coronavirus disease 2019, COVID‐19, diabetes mellitus, metformin, SARS‐CoV‐2, treatment, type 2 diabetes
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), the causative agent of coronavirus disease 2019 (COVID‐19), has been associated with 141 400 973 total cases worldwide with 3 026 206 deaths and 18 283 297 active cases as of 18 April 2021. 1 While it is estimated that the overall case fatality ratio of the disease is 1.38% (95% confidence interval [Cl], 1.23–1.53), which is much lower than that of SARS‐CoV and MERS‐CoV with 15% and 35% respectively, the absolute number of deaths have been remarkably high due to its high transmission rate and increased risk of death with age and comorbidities. 2
Diabetes mellitus (DM) and hyperglycaemia have been identified as poor prognostic markers for pneumonia and observations from previous outbreaks have elucidated the damaging role of hyperglycaemia in infection. 3 It has been reported that a known history of diabetes (odds ratio [OR] 3.0; 95% CI, 1.4–6.3) and elevated fasting plasma glucose levels (OR 3.3; 95% CI, 1.4–7.7) are independent predictors of mortality in patients infected with SARS‐CoV. 4 A retrospective cohort study of 144 patients admitted for SARS‐CoV also showed that the DM as a comorbidity was independently associated with a 3‐fold increase in poor outcomes (relative risk, 3.1; 95% CI, 1.4–7.2). 5 With regards to COVID‐19, a retrospective, multicentre study of 7377 COVID‐19 patients in China demonstrated that those with new or pre‐existing diabetes and poor glycaemic control had higher all‐cause mortality, higher progression to multiorgan failure and required more medical interventions (such as systemic corticosteroids, antibiotics, noninvasive and invasive ventilation) than patients without type 2 diabetes (T2D) or with well‐controlled blood glucose. 6 Another report from China that analysed 174 COVID‐19 patients demonstrated that patients with DM had a higher prevalence of CVD and were also at a higher risk of pneumonia, uncontrolled inflammatory responses and hypercoagulable states due to dysfunctional glucose metabolism. 7 In addition, they presented with increased lactate dehydrogenase, C‐reactive protein and D‐dimer, and more pronounced lung pathologies on CT scans, supporting the notion that diabetes is a risk factor for rapid progression of COVID‐19. Lastly, a recent meta‐analysis that included 16 003 patients found diabetes to be significantly associated with mortality from COVID‐19 with an OR of 1.90 (95% Cl, 1.37–2.64) and a combined pooled OR of mortality or severity to be 2.16 (95% Cl, 1.74–2.68). 8
Acute and chronic hyperglycaemia can significantly impact the body's innate and adaptive immune responses towards infection and is associated with dysfunctional neutrophil chemotaxis, impaired macrophage function (via decreased superoxide formation) and dampened T‐cell mediated responses. 9 In an in vivo mouse model investigating the effects of DM following MERS‐CoV infection, more severe disease was observed in diabetic male mice, which was characterised by fewer inflammatory macrophages, CD4+ T cells and higher levels of interleukin (IL)‐17a. 10 In another model, hyperglycaemic mice demonstrated higher rates of influenza infection and bacterial pneumonia due to decreased alveolar macrophage activation as well as lower levels of chemokines. 11 This dysfunctional immune system has been mirrored in patients with COVID‐19 and poor glycaemic control as they exhibited lower peripheral counts of CD4+ and CD8+ T cells, NK cells, impaired macrophage activation and phagocytosis as well as higher levels of proinflammatory markers. 12 Lower serum levels of IL‐10, an anti‐inflammatory cytokine that inhibits the synthesis and release of proinflammatory cytokines such as IL‐6, interferon‐γ and tumour necrosis factor (TNF)‐α, which are characteristic of the cytokine storm seen in COVID‐19, have also been observed in patients with DM. 13 In terms of localised defence, insulin resistance and hyperglycaemia can lower levels of surfactant protein‐D, a lung derived innate immune protein, as well as increase glucose concentrations in the airway surface liquid which consequently promotes lung pathogen overgrowth. 14 Therefore, patients with DM already exhibit a baseline level of low‐grade inflammation due to hyperglycaemia and insulin resistance which predisposes them to an exaggerated immune response upon SARS‐CoV‐2 infection. This leads to a characteristic hyperinflammatory syndrome or cytokine storm responsible for the progression to severe disease (Figure 1).
FIGURE 1.
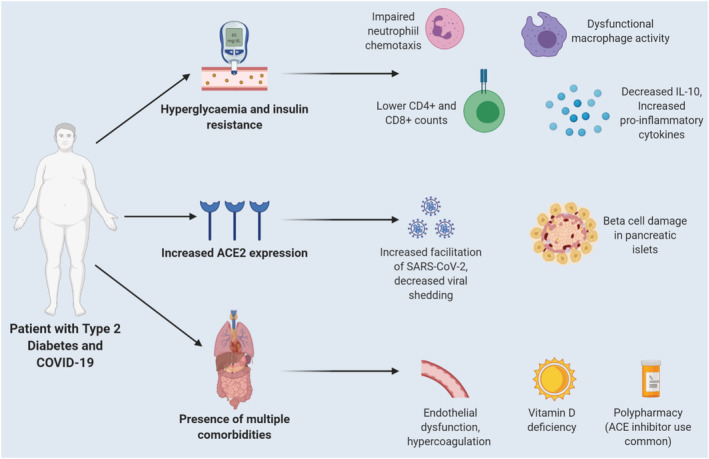
Mechanisms contributing to worsened outcomes in patients with COVID‐19 and diabetes mellitus (DM). Acute and chronic hyperglycaemia compromises the innate immune system through impaired neutrophil chemotaxis, decreased macrophage activity, dampened T‐cell responses, decreased levels of anti‐inflammatory cytokines (IL‐10) and increased proinflammatory cytokines. The increased angiotensin‐converting enzyme 2 (ACE2) expression in these patients favours more efficient SARS‐CoV‐2 entry into cells and potential damage to β islet cells of the pancreas. The presence of multiple comorbidities contributes to endothelial dysfunction and hypercoagulation. Lastly, vitamin D deficiency is a common finding which can further compromise the immune system
Recent evidence has shown that metformin, an oral biguanide, is associated with reduced mortality and improved outcomes in patients with COVID‐19 and DM with an OR of 0.77 (95% CI 0.73–0·81) reported in 1 study. 15 , 16 , 17 , 18 Alongside its glucose‐lowering effects, metformin has immunomodulatory, anti‐inflammatory, antitumour, antiageing and antiviral activity, which suggests the possibility of it to affect outcomes in critical illness. 19 Indeed, several studies have demonstrated the use of metformin being associated with reduced complications such as diabetic cardiomyopathy, myocardial infarction and hypertrophy. 20 Moreover, metformin has been found to be linked with lower mortality in other lung pathologies such as chronic obstructive pulmonary disorder and tuberculosis. 21 , 22 It is thus important to examine whether the pleiotropic effects of metformin could be beneficial in COVID‐19 patients with DM.
1.1. Objectives
The aim of this study was to perform a systematic review and meta‐analysis to investigate whether the use of metformin in COVID‐19 patients with new or pre‐existing diabetes mellitus affects outcomes such as mortality and severity of the disease.
2. METHODS
This systematic review and meta‐analysis are reported based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) protocol. 23 This study was also registered in the PROSPERO, international prospective register of systematic reviews (ID: CRD42020221842) on 20 November 2020.
2.1. Search strategy and study selection
A comprehensive search strategy with the relevant key terms were designed for use in PubMed, Scopus, Web of Science, EMBASE, Cochrane library and Clinicaltrials.gov to retrieve all relevant studies published in English language between 1 December 2019 and 10 April 2021. The search strategy used for the PubMed database was:
(“COVID‐19”[All Fields] OR “COVID‐19”[MeSH Terms] OR “COVID‐19 Vaccines”[All Fields] OR “COVID‐19 Vaccines”[MeSH Terms] OR “COVID‐19 serotherapy”[All Fields] OR “COVID‐19 Nucleic Acid Testing”[All Fields] OR “covid‐19 nucleic acid testing”[MeSH Terms] OR “COVID‐19 Serological Testing”[All Fields] OR “covid‐19 serological testing”[MeSH Terms] OR “COVID‐19 Testing”[All Fields] OR “covid‐19 testing”[MeSH Terms] OR “SARS‐CoV‐2”[All Fields] OR “sars‐cov‐2”[MeSH Terms] OR “Severe Acute Respiratory Syndrome Coronavirus 2”[All Fields] OR “NCOV”[All Fields] OR “2019 NCOV”[All Fields] OR ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields] OR “COV”[All Fields]) AND 2019/11/01[PubDate]: 3000/12/31[PubDate])) AND (“metformin”[MeSH Terms] OR metformin[title] OR biguanide[title] OR insulin[title]) AND (“2019/12/01”[PubDate]: “2021/04/10”[PubDate])
The inclusion and exclusion criteria for the studies were as follows:
The studies should be published in English language in all databases.
All participants should be adult patients (>18 y) diagnosed with COVID‐19 and diabetes mellitus.
Study design should be randomised controlled trials (RCTs) or observational studies (retrospective/prospective cohort, cross‐sectional and case–control) that looked at patients taking metformin vs. patients not taking metformin which reported at least 1 outcome (mortality or composite endpoint) and an OR, risk ratio or hazards ratio in either adjusted and/or nonadjusted forms.
The studies should have at least 70 patients with COVID‐19 and diabetes mellitus.
Case reports, conference abstracts, review articles, commentaries, editorials and nonhumane studies were excluded.
The authors searched each database and the titles and abstracts were reviewed to identify any potentially relevant articles. After identification of articles, the full texts were individually analysed according to the inclusion and exclusion criteria. Additionally, hand searches were performed to include any relevant studies that were not shown in the initial database search. The reference lists of included articles, review articles and meta‐analyses were also screened to identify any other potential studies that could be utilised.
2.2. Data extraction and quality assessment
The following data were extracted from each study and transferred to a spreadsheet: author(s), date of online publication, country, study design, number of patients with COVID‐19 and diabetes mellitus, sex, patient age‐range, primary outcomes (mainly mortality), and OR (unadjusted and/or adjusted forms). In studies that reported sufficient data on metformin use vs. nonmetformin use but did not report an OR, the OR was calculated separately on statistical software and put in the nonadjusted OR column.
The quality of the included studies and their risk of bias was assessed using the Newcastle–Ottawa Scale (NOS). The NOS has been developed to assess the quality of cohort studies used for a systematic review by using a star system where each study is judged on 3 broad domains: the selection of study groups; the comparison of cohorts and the establishment of the outcomes of interest in the cohort. 24 Each study was rated a maximum of 4 stars for selection, 2 stars for comparability and 3 stars for the outcome, with a total score of 9 and higher scores indicating better quality.
The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach 25 was used to evaluate the quality of evidence for outcomes. All included studies only reported mortality as an outcome, and thus the effect of metformin on mortality in COVID‐19 patients with DM was tested. Only the studies that reported an adjusted OR was included in the analysis. The certainty of evidence was classified as very low, low, moderate or high using the GRADE criteria. RCTs start as high‐quality evidence and observational studies as low‐quality evidence, which can subsequently be rated down based on 5 factors (risk of bias, inconsistency, indirectness, imprecision and publication bias), or rated up based on 3 factors (large magnitude of effect, dose–response gradient and plausible biases reducing an apparent treatment effect). The GRADEpro software was used to rate evidence and present it in GRADE evidence profiles and summary of findings tables. 26
2.3. Data synthesis and statistical analysis
To evaluate the association between metformin use and mortality, the ORs in nonadjusted and adjusted forms (with 95% CI) were taken as the effect size. A meta‐analysis was then performed with the generic inverse variance method using a random‐effects model to account for any significant heterogeneity between studies. To avoid any confounding effects, separate meta‐analyses were carried out for unadjusted ORs and adjusted ORs that were reported/calculated from each study. Data including mortality in the metformin group, mortality in the nonmetformin group and total number of patients in each group were entered when calculating the nonadjusted ORs. Adjusted ORs were taken directly from the studies. Sensitivity analyses were carried out by excluding studies that could significantly influence the overall analysis, including those with large sample sizes or anomalous ORs. A P‐value of <.05 was set to show a statistically significant association. The heterogeneity of the studies was assessed using the inconsistency index (I 2), with values of 25, 50 and 75% representing low, moderate and high heterogeneity, respectively. The possibility of publication bias was assessed through visual inspection of a funnel plot and results from the Eggers's and Begg's test. All analyses were performed on Microsoft Excel, MedCalc (Version 20.013) and Review Manager software 5.4.1 (Cochrane collaboration).
3. RESULTS
3.1. Study selection and characteristics
The initial literature search from all databases (PubMed, Scopus, Web of Science, EMBASE, Clinicaltrials.gov, Cochrane library) and other sources yielded 1349 articles. After excluding duplicates, 753 articles were retained for title and abstract screening. Of these, 708 articles were excluded with the remaining 45 articles fully assessed for eligibility. Finally, 32 studies with 2 916 231 patients were included in the qualitative and quantitative synthesis 15 , 16 , 17 , 18 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 (Figure 2). Out of the 32 studies, 11 were from China; 6, USA; 6, UK; 4, South Korea; 2, France; 1, Spain; 1, Italy; and 1, Belgium. No RCTs or cross‐sectional studies were identified from the search. All of the 32 studies identified were observational cohort studies (31 retrospective and 1 prospective) and 5 were preprint studies. The included studies scored between 7–9 on the NOS with a mean score of 8.59 ± 0.61, signifying a high overall quality of studies used for the analysis. The characteristics of the included studies are shown in Table 1.
FIGURE 2.
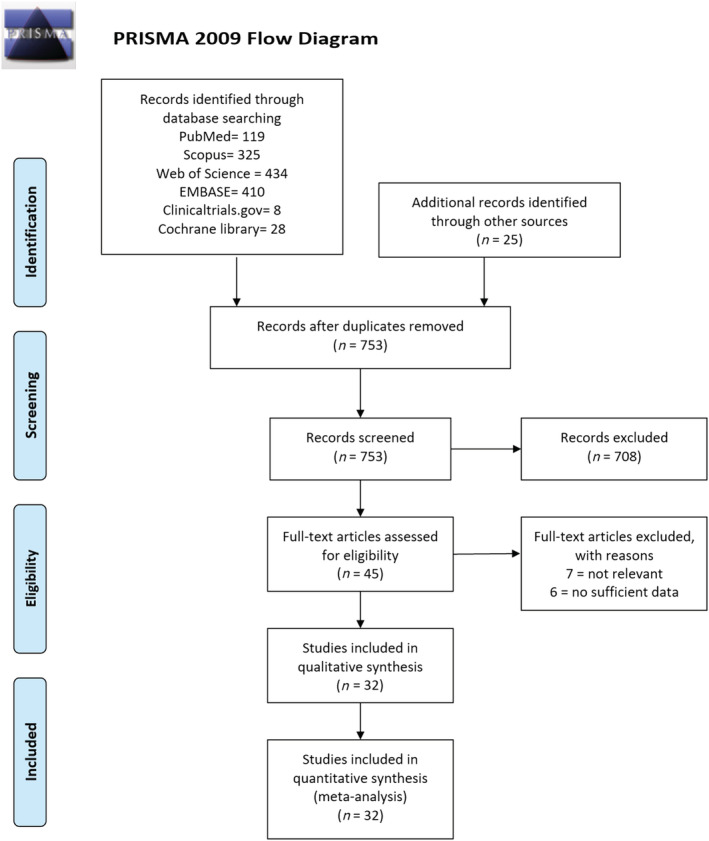
PRISMA flow diagram for study selection
TABLE 1.
Characteristics of the included studies
| Author | Study design | Country | Total participants | % male | Mean/median age (y) | Mortality—metformin users | Mortality—nonmetformin users | Non adjusted OR (95% CI) | Adjusted OR (95% CI) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Crouse et al., 2020 15 | Retrospective cohort | USA | 239 | 50.6% | 50–70 (40.6%) | 8/76 | 34/144 | 0.38 (0.17–0.87) | 0.33 (0.13–0.84) | 8 |
| Bramante et al., 2020 16 | Retrospective cohort | USA | 6256 | 51.6% vs. 44.6% | 73.0 (66.0–80.0) vs. 76.0 (67.0–84.0) | 394/2333 | 791/3923 | 0.802 (0.701–0.917) | 0.911 (0.784–1.060) | 9 |
| Cariou et al., 2020 17 | Prospective cohort | France | 1317 | 64.9% | 69.8 ± 13 | 63/746 | 77/571 | 0.59 (0.42–0.84) | 0.80 (0.45–1.43) | 8 |
| Khunti et al., 2021 18 | Retrospective cohort | UK | 2 851 456 | 55.9% | 67 (IQR 57–77) | 6295/1800005 | 7184/1355765 | 0.6588 (0.6368–0.6815) | 0.77 (0.73–0.81) | 9 |
| Perez‐Belmonte et al., 2020 27 | Retrospective cohort | Spain | 2666 | 61.9% | 74.9 ± 8.4 | 244/825 | 244/663 | 0.73 (0.58–0.90) | 1.0 (0.6856–1.4585) | 9 |
| Do et al., 2020 28 | Retrospective cohort | South Korea | 1865 | 51.8% vs. 42.1% | (64.8 ± 11.4) vs. (67.4 ± 12.1) | N/A | N/A | 0.62 (0.36–1.10) | 0.77 (0.44–1.35) | 9 |
| Chen et al., 2020 29 | Retrospective cohort | China | 904 | 46.6% | 56.0 (IQR 39.0–67.0) | 4/43 | 15/77 | 0.4239 (0.1311–1.3706) | 0.62 (0.17–2.20) | 8 |
| Lally et al., 2020 30 | Retrospective cohort | USA | 775 | 97.3% | 75.6 ± 10.8 | 16/127 | 144/555 | 0.42 (0.26–0.69) | 0.48 (0.28–0.84) | 9 |
| Gao et al., 2020 31 | Retrospective cohort | China | 110 | 41.8% | 65 (IQR 58–74) | 16/56 | 4/54 | 5·0 (1.5489–16·1408) a | 3.964, (1.034–15.194) a | 9 |
| Kim et al., 2020 32 | Retrospective cohort | South Korea | 1082 | 45·1% | 68.3 ± 11.9 vs. 56.5 ± 18.0 | N/A | N/A | N/A | 0·36 (0.10–1.23) | 9 |
| Luo et al., 2020 33 | Retrospective cohort | China | 283 | 53% vs. 57.5% | 63.0 (55.8–68.3) vs, 65.0 (57.5–71.0) | 3/104 | 22/179 | 0·21 (0.0618–0.7266) | 0.23 (0.06–0.82) | 9 |
| Philipose et al., 2020 34 | Retrospective cohort | UK | 466 | 59.2% | 45/199 | 55/267 | 1.1263 (0.7216–1.7581) | 1.39 (0.84–2.16) | 8 | |
| Chung et al., 2020 35 | Retrospective cohort | South Korea | 110 | 43·6% | 56.9 ± 17·0 | N/A | N/A | N/A | 0.417 (0.050–3.469) a | 8 |
| Abu‐Jamous et al., 2020 36 | Retrospective cohort | UK | 411 | N/A | N/A | 4/23 | 94/169 | 0.1680 (0.0548–0.5149) | 0.19 (0.05–0.70) | 9 |
| Liu et al., 2020 37 | Retrospective cohort | China | 192 | 54.7% | 66.0 (IQR 59.0–71.0) | N/A | N/A | 0.14 (0.01–1.50) | 0.18 (0.02–1.84) | 8 |
| Q. Zhang et al., 2020 38 | Retrospective cohort | China | 74 | 48.6% | 62 (IQR 58–81) | 4/25 | 23/49 | 0.2153 (0.0644–0·7203) a | N/A | 8 |
| Silverii et al., 2020 39 | Retrospective cohort | Italy | 159 | 54.1% | 73.31 ± 12.66 | 21/76 | 38/83 | 0.441 (0.2291–0.8608) | 0.6 (0.39–0.93) | 9 |
| J. Zhang et al., 2020 40 | Retrospective cohort | China | 312 | 45% | 57 (IQR 38–66) | 1/20 | 25/64 | 0.0821 (0.0103–0.6524) a | N/A | 7 |
| Li et al., 2020 41 | Retrospective cohort | China | 131 | N/A | N/A | 2/37 | 21/94 | N/A | 0.1986 (0.0441–0.8950) | 9 |
| Cheng et al., 2021 42 | Retrospective cohort | China | 407 | 47.9% | 48 (IQR 36–58) | N/A | N/A | 0.34 (0.07–1.62) a | N/A | 9 |
| Bramante et al., 2021 43 | Retrospective cohort | USA | 9555 | 47% | 60.4 (51.7–69.0) vs. 54.2 (42.4–65.3) | N/A | N/A | 0.32 (0.15–0.66) | 0.38 (0.16–0.91) | 9 |
| Oh and Song, 2021 44 | Retrospective cohort | South Korea | 5752 | 44.9% | (70.5 ± 12.3) vs. 71.0 ± (13.4) | N/A | N/A | N/A | 1.26 (0.81–1.95) | 9 |
| Ghany et al., 2021 45 | Retrospective cohort | USA | 1139 | 39% vs. 41% | (70.9 ± 8.9) vs. (71.2 ± 8.9) | 10/243 | 49/350 | 0.2619 (0.1299–0.5281) | 0.34 (0.19–0.59) | 9 |
| Li et al., 2021 46 | Retrospective cohort | China | 131 | 56.5% | 66.8 ± 11.6 | 2/37 | 21/94 | 0.1986 (0.0441–0.8950) | N/A | 9 |
| Goodall et al., 2020 47 | Retrospective cohort | UK | 981 | 64.3% | 69 (IQR 56–80) | N/A | N/A | 0.97 (0.75–1.25) | 0.99 (0.78, 1.25) | 9 |
| Izzi‐Engbeaya et al., 2020 48 | Retrospective cohort | UK | 889 | 60% | 65.8 ± 17.5 | N/A | N/A | 1.14 (0.74–1.76) | N/A | 7 |
| Dashti et al., 2020 49 | Retrospective cohort | USA | 4140 | 45% | 52 (IQR 36–65) | 42/529 | 145/1435 | 0.7673 (0.5359–1.0986) | N/A | 8 |
| Wang et al., 2021 50 | Retrospective cohort | UK | 20 366 | 52.0% vs. 52.3% | (67.8 ± 13.1) vs. (67.2 ± 11.8) | N/A | N/A | 0.76 (0.29–1.97) | 0.87 (0.34–2.20) | 9 |
| Orioli et al., 2021 51 | Retrospective cohort | Belgium | 73 | 48% | 69 ± 14 | 4/45 | 7/28 | 0.2927 ± 0.0769–1.1137 | N/A | 8 |
| Lalau et al., 2021 52 | Retrospective cohort | France | 2449 | 64% | 70.9 ± 12.5 | 122/1496 | 153/953 | 0.4643 (0.3604–0.5981) | 0.710 (0.537–0.938) | 9 |
| Jiang et al., 2021 53 | Retrospective cohort | China | 328 | 49% vs. 54.8% | 64.0 (56.5–70.0) vs. 67.0 (60.0–76.0) | 3/100 | 25/228 | 0.48 (0.13–1.74) | 0.54 (0.13–2.26) | 9 |
| Cheng et al., 2020 54 | Retrospective cohort | China | 1213 | 53.8% vs. 49.9% | 63.0 (IQR 56.0–69.0) | N/A | N/A | 0.87 (0.36–2.12) | 0.90 (0.85–0.95) | 9 |
OR = odds ratio; CI = confidence interval; NOS = Newcastle–Ottawa Scale; IQR = interquartile range.
outcomes were reported as composite endpoints.
3.2. Association between metformin use and mortality
Forest plots for the effects of metformin on mortality in COVID‐19 patients with diabetes mellitus are shown in Figures 3 and 4. Pooled analysis showed a statistically significant association between metformin use and reduced mortality in both unadjusted (OR 0.61 [95% CI: 0.53–0.71]), P < .00001, I 2 = 70%) and adjusted (OR 0.78 [95% CI: 0.69–0.88], P < .00001, I 2 = 67%) models. A sensitivity analysis was performed excluding the study by Gao et al., 31 which then yielded an overall OR 0.60 (95% CI: 0.53–0.69), P < 00001, I 2 67% in the unadjusted analysis and an OR 0.77 (95% CI: 0.69–0.87), P < .00001, I 2 66% in the adjusted analysis. Furthermore, given that the study by Khunti et al. 18 contributed to 2 851 456 out of 2 916 231 patients in this meta‐analysis, another sensitivity analysis was performed excluding this study which yielded an overall OR 0.58 (95% CI: 0.48–0.70), P < .0001, I 2 70% in the unadjusted analysis and an OR 0.75 (95% CI: 0.65–0.88), P < .00001, I 2 61% in the adjusted analysis.
FIGURE 3.
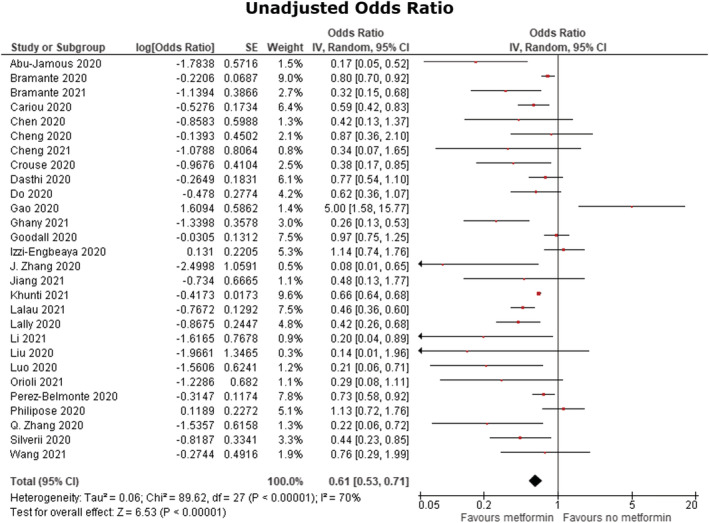
Unadjusted odds ratio. Forest plot of meta‐analysis of the effect of metformin consumption on mortality in COVID‐19 patients with diabetes mellitus, random‐effects model
FIGURE 4.
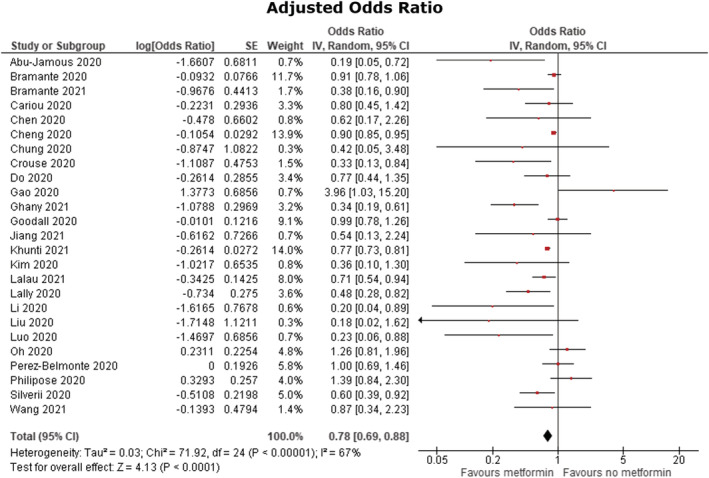
Adjusted odds ratio. Forest plot of meta‐analysis of the effect of metformin consumption on mortality in COVID‐19 patients with diabetes mellitus, random‐effects model
3.3. Publication bias
In this meta‐analysis the inverted funnel plot analysis from the adjusted studies showed a symmetrical distribution on the funnel plot, highlighted by the contour lines, indicating a low risk of publication bias (Figure 5). The P‐values for the Egger's and Begg's test were .061 and .064, respectively.
FIGURE 5.
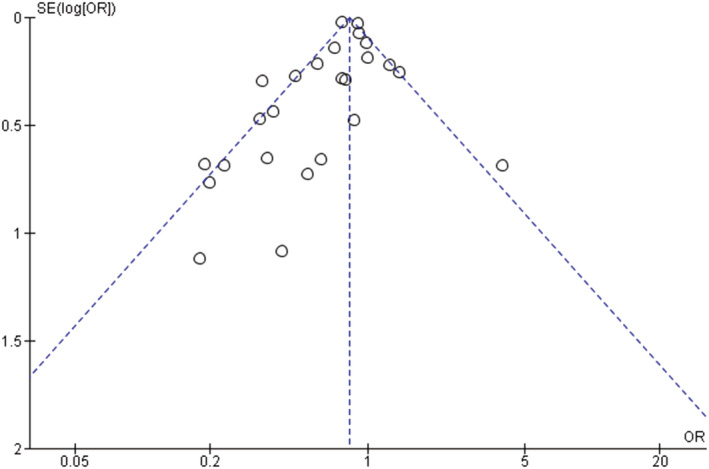
Funnel plot for the assessment of publication bias, adjusted studies
3.4. Certainty measure—GRADE approach
Table 2 shows the GRADE evidence profile and summary of findings for the adjusted studies. Population comprised of COVID‐19 patients with DM taking or not taking metformin. The outcome was mortality (reported in adjusted OR). The risk difference (95% CI) with metformin use was based on the assumed risk (i.e. risk with no metformin use, which was the mean control group risk across included studies) and the relative effect (95% CI) of the intervention. Overall certainty of evidence was rated as low quality, with the assessment of criteria shown in the table and the associated footnotes.
TABLE 2.
GRADE evidence profile and summary of findings: metformin for patients with COVID‐19 and diabetes mellitus
| Certainty assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants (studies, type) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence* | Relative effect (95% CI) | Anticipated absolute effects (95% CI) | |
| Risk with no metformin use a | Risk difference with metformin use | ||||||||
| 2 910 211 (25 observational studies, adjusted) | Not serious b | P value on test for heterogeneity <.00001 I 2 = 67% c | Not serious | Not serious d | None e | ⨁⨁◯◯ low | aOR 0.78 (0.69 to 0.88) | 232 per 1000 | 41 fewer per 1000 (from 60 fewer to 22 fewer) |
CI: confidence interval; aOR: adjusted odds ratio.
The basis for the control risk is the mean control group risk across studies. Risk difference (and its 95% CI) is based on control risk and relative effect (converted to risk ratio) of the intervention (and its 95% CI).
All studies were nonrandomised and assessed using the Newcastle–Ottawa Scale; while some studies had a higher risk of bias than others, no important difference was noted in sensitivity analyses excluding studies at higher risk of bias; hence we did not rate down for risk of bias.
Although there was a high I 2 value (which can be exaggerated in observational studies), 25 most of the studies, especially those with larger sample sizes, reported a benefit with metformin use. Final decision not to rate down on inconsistency.
Although there were some studies with high confidence intervals, these studies contributed to a small percentage of the weight of the meta‐analysis. Furthermore, the study by Khunti et al., 18 which composed a significant proportion of our meta‐analysis, showed a considerable beneficial effect, with a small confidence interval. Thus, the final decision was not to rate down.
Visual inspection of the funnel plot as well as empirical examination of results showed no publication bias.
GRADE category of evidence: high certainty (we are very confident that the true effect lies close to that of the estimate of the effect); moderate certainty (we are moderately confident in the effect estimate; the true effect is probably close to the estimate, but it is possibly substantially different); low certainty (our confidence in the effect estimate is limited; the true effect could be substantially different from the estimate of the effect); very low certainty (we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect).
4. DISCUSSION
The findings of this extensive meta‐analysis showed that the use of metformin in patients with COVID‐19 and diabetes was significantly associated with lower mortality with a pooled unadjusted OR of 0.61 (95% CI, 0.53–0.71) and a pooled adjusted OR of 0.78 (95% CI, 0.69–0.88), both with a moderate heterogeneity of I 2 = 70% and I 2 = 67% respectively. To our knowledge, this meta‐analysis has used the largest number of studies and sample size so far with regards to metformin use in COVID‐19 patients with DM across multiple regions, reporting both adjusted and nonadjusted ORs, and provides valuable insight into the potential preventative and treatment strategies to minimise the progression and fatality of the disease in this subset of patients. This analysis builds on the findings by Lukito et al., 55 which analysed 10 233 patients and also found metformin use to be associated with lower mortality (adjusted OR 0.64 [0.43, 0.97], P = .035).
The presence of multiple comorbidities such as obesity, cardiovascular disease, hypertension and kidney damage in patients with DM is a common finding when admitted for COVID‐19 and studies have confirmed that they are at an even higher risk of severe outcomes such as mortality. 56 Both hyperglycaemia and insulin resistance are associated with endothelial dysfunction and prothrombotic activity. 57 With COVID‐19 also being linked to coagulation abnormalities in the latter stages of the disease, the presence of both hyper‐coagulation states puts these patients at an even higher risk of life‐threatening thrombotic complications. 12 Additionally, the prevalence of obesity in patients with T2D is widely observed and a body mass index of >35 kg/m2 is considered a risk factor for poor prognosis and associated with an increased requirement for invasive mechanical ventilation. 58 This is largely due to the conflicting comorbidities that compromise cardiometabolic health and obese patients being more prone to obstructive sleep apnoea and decreased pulmonary ventilation which predisposes them to poor outcomes when faced with respiratory tract infections. 59
Increased levels of angiotensin‐converting enzyme 2 (ACE2) have been noted in the kidneys, lungs, heart and pancreas in murine models of DM and ACE2 is also overexpressed in chronic diseases in humans, which is thought to facilitate higher levels of SARS‐CoV‐2 entry into various organs and thus contribute to the poorer prognoses. A recent study showed that patients with DM or hypertension had reduced clearance and shedding of the SARS‐CoV‐2 virus which could be explained by the increased ACE2 expression. 60 Within the pancreas, ACE2 expression has been seen specifically in the β cells of the islets of Langerhans which can have implications during a SARS‐CoV‐2 infection. Indeed, it has been shown that SARS‐CoV could potentially enter the pancreas using ACE2 and subsequently damage the islets causing an acute insulin deficiency and hyperglycaemia and, while this is not confirmed with SARS‐CoV‐2, it may be likely that not only is diabetes correlated with a poor prognosis, but infection itself could trigger new‐onset diabetes in patients with COVID‐19. 61 This notion has been supported by the increased plasma blood glucose seen in COVID‐19 patients without diabetes, increased reports of acute crises such as diabetic ketoacidosis and hyperosmolar hyperglycaemic state in COVID‐19 patients with diabetes, newly diagnosed diabetes in admitted COVID‐19 patients and the unusually increased insulin requirements in patients. 62 , 63 , 64 Taken together, the association between DM and severe outcomes in COVID‐19 can be attributed to an array of mechanisms not limited to poor baseline glycaemic control, diminished T‐cell and macrophage function, increased susceptibility to SARS‐CoV‐2 entry and the presence of other comorbidities, summarised in Figure 1. It is therefore essential that patients with DM receive optimal glycaemic control and tailored treatment to both minimise the risk of infection and severe clinical outcomes.
The results from this meta‐analysis suggest that metformin could be effective in not only maintaining adequate glycaemic control but directly enhancing immune responses to the SARS‐CoV‐2, improving outcomes in patients with DM. The study by Khunti et al., 18 a nationwide observational cohort study assessing 2 851 465 patients, which provided a significant percentage to our meta‐analysis, found metformin, SGLT2 inhibitors and sulfonylureas to be associated with lower mortality, with metformin being most associated with decreased mortality. This study included all people diagnosed with T2D in England and comprehensively assessed the risk across all classes of glucose‐lowering drugs. Moreover, the cohort included all patients with T2D regardless of their admission status and the outcome of all deaths due to COVID‐19 included both in‐hospital and outside hospital, hence providing a robust outcome without influence of hospital bias and resource availability.
Metformin acts via activation of 5‐adenosine monophosphate‐activated protein kinase (AMPK). 20 AMPK has been shown to modify ACE2 by phosphorylating ACE2 Ser680 in human umbilical vein endothelial cells and human embryonic kidney 293 (HEK293T) cells. 65 Therefore, it could be proposed that AMPK brings about a conformational change in the ACE2 receptor which leads to decreased binding within the SARS‐CoV‐2 receptor and hence reduced infectivity and a reduced risk of severe disease. 66 Another study in human umbilical vein endothelial cells showed that AMPK activation was able to inhibit the replication of Zika virus in endothelial cells as well as upregulate the expression of genes with antiviral properties (such as interferons, OAS2 and MX1) while downregulating inflammatory mediators such as TNF‐α. 67
Other mechanisms that metformin uses to improve outcomes in COVID‐19 is through its ability to reduce the levels of proinflammatory cytokines such as IL‐6 and TNF‐α, which are characteristically seen in the systemic cytokine storm while boosting the levels of anti‐inflammatory cytokines, namely IL‐10. 68 One study used in this meta‐analysis found that metformin had sex‐specific immunomodulatory actions in that the reduction in cytokines were more prominent in females than males. 16 Metformin also strengthens both the adaptive and innate immune system by promoting autophagy, which helps contain and destroy pathogens, alters the composition of gastrointestinal microbiota, stimulates the formation of M2 macrophages and CD8+ memory T cells, increases the neutrophil to lymphocyte ratio, stabilises mast cells and improves protection against reactive oxygen species. 69 , 70 , 71 Lastly, it has also shown to improve endothelial function, which can thus limit vascular damage and the risk of thrombotic events, which are life‐threatening complications of COVID‐19. 72
Previous studies on humans have shown consistent benefits—a recent meta‐analysis of 5 cohort studies showed that the use of metformin in patients with diabetes before admission resulted in significantly less mortality during sepsis compared to nonusers (OR 0.59 95% CI 0.43–0.79). 73 In a follow‐up study of 5266 patients, metformin use was associated with a significantly decreased risk of mortality in patients with DM and chronic obstructive pulmonary disease (OR 0.30, 95% CI 0.10–0.93) after adjustment of multiple confounders. 74 However, while metformin does have a good safety profile, some patients may develop gastrointestinal side effects including nausea, vomiting, diarrhoea and bloating. These may be eliminated in over 80% of patients with the administration of newer extended‐release formulations and use after a meal. 75 Additionally, the use of metformin has been challenged due to its risk of lactic acidosis especially in patients with renal and liver dysfunction. The study by Gao et al. 31 with a relatively small sample size of 110 patients highlighted a potential safety concern for metformin; however, it was noted that both blood glucose (8.17 vs. 6.39 mmol/L, P = .013) and lactate dehydrogenase levels (212.00 vs. 178.50 U/L, P = .024) upon admission were statistically significantly higher in the metformin group vs. nonmetformin group, which are both predictors of poor outcomes. 6 , 76 It should also be noted that as metformin is an oral drug, orotracheal intubated patients cannot receive this treatment and hence are more likely to exhibit worse outcomes. Thus, with hyperglycaemia being an independent risk factor for severe outcomes in COVID‐19 patients, metformin's ability to improve insulin sensitivity and modify glycogen synthesis taken together with its immunomodulatory, anti‐inflammatory effects, antiviral effects (Figure 6) as well as the results from this meta‐analysis, suggests that metformin may be highly beneficial in attenuating the cytokine storm seen in patients with DM and COVID‐19 while maintaining optimal glycaemic control and hence improve outcomes.
FIGURE 6.
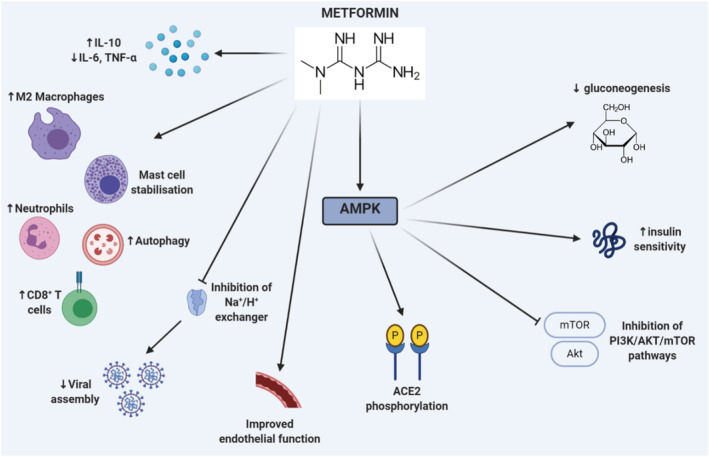
Potential mechanisms through which metformin can improve outcomes in COVID‐19 patients with diabetes mellitus (DM). Metformin dampens levels of proinflammatory cytokines (interleukin [IL]‐6 and tumour necrosis factor [TNF]‐α) while boosting anti‐inflammatory cytokines (namely IL‐10). It increases levels of M2 macrophages, neutrophils, CD8+ T cells, stabilises mast cells, promotes autophagy and improves endothelial function. It can also inhibit the Na+/H+ exchanger on cells, thereby increasing cellular pH and impairing viral replication. Metformin also activates 5‐adenosine monophosphate‐activated protein kinase (AMPK) which leads to decreased gluconeogenesis, increased insulin sensitivity, inhibition of PI3K/AKT/mTOR pathways and phosphorylation of angiotensin‐converting enzyme 2 (ACE2) receptors, which impairs binding and subsequent entry of SARS‐CoV‐2 into cells
This systematic review has several limitations that need to be addressed in order to ascertain a clear picture with regards to metformin use in COVID‐19 patients with DM. Firstly, most of the studies were retrospective in nature and are therefore prone to selection bias, recall bias and misclassification bias, especially in large sample sizes and hence randomised prospective studies are needed in geographically diverse cohorts to solidify the association between metformin use and improved outcomes in patients. Although most of the studies included did adjust for major confounding factors, the residual confounding effect cannot be eliminated. These include the use of treatments such as ACE inhibitors/angiotensin receptor blockers, which are commonly prescribed in patients with DM for nephroprotection and could have played a role in either improving or worsening outcomes. Additionally, the prevalence of obesity in some studies was missed and a large body of evidence has suggested the association between obesity and severe outcomes in COVID‐19. In some studies, the proportion of metformin users vs. nonusers was uneven and the presence of multiple comorbidities was fewer in the metformin users vs. nonusers which could indeed alter the strength and validity of the results. Moreover, there were a few studies that contained a small sample size in the metformin group and some that reported a composite outcome rather than plain mortality. Strategies for future studies to tackle these biases could be through restriction and matching of individuals when selected for the study as well as stratification and propensity score matching when undergoing the data analysis phase to strengthen the comparability of the treatment vs. the control group. 77 While we tried to extract all relevant information from each study, most studies failed to report the mean duration of diabetes, dosage regimens and duration of treatment, which probably contributed to the heterogeneity seen in our meta‐analysis (alongside differences in mean/median ages, percentage of male patients used and differences in healthcare delivery and patient characteristics from different countries). More patient‐specific data on this could have provided more detailed information regarding metformin's pharmacokinetics, interactions and potential use in clinical practice.
The use of the GRADE approach provides valuable information regarding the certainty of evidence and sheds light into further research that may be warranted. Our review included the GRADE approach and used mortality as an outcome; however, it is likely that future studies, alongside RCTs will provide more data regarding metformin use in this population and we call for future reviews to use the GRADE approach to assess multiple outcomes (such as hospital admission, risk of deterioration, intubation and recovery) to thoroughly assess the potential clinical implications of metformin use. With this in mind, more research is needed to confirm the role of metformin in COVID‐19 through blinded RCTs. Given its promising nature, multiple trials are currently underway including the MET‐Covid trial (ClinicalTrials.gov identifier: NCT04510194) and the DMMETCOVI9 trial (ClinicalTrials.gov identifier: NCT04626089), which are estimated to be completed by late 2021 to early 2022.
5. CONCLUSION
This systematic review evaluated whether the use of metformin affected outcomes in COVID‐19 patients with DM and the results from this extensive meta‐analysis showed that it was associated with lower mortality in both nonadjusted and adjusted ORs. Beyond its effects on glycaemic control, metformin could improve outcomes in these patients through multiple mechanisms, some of which lead to an attenuated cytokine storm, improved adaptive and innate immune system, and decreased viral entry. The findings in this review provide valuable insight into the impact of DM on COVID‐19 and the possible preventative and protective role of metformin in these patients. Given that metformin is an inexpensive drug with a good safety profile, it should be thoroughly assessed for its use in not only COVID‐19 patients with DM but all patients. Indeed, RCTs are the next steps in thoroughly investigating the effectiveness of metformin in the treatment of COVID‐19 and with time may prove to be a wonder drug.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 78
COMPETING INTERESTS
None.
ACKNOWLEDGEMENTS
The creation of Figures 1 and 6 were done through BioRender (©BioRender – biorender.com), which provides excellent tools for scientific drawing. We thank the library staff at the University of Nottingham for their assistance and input in devising the search strategies as well as the data extraction, quality assessment and analyses stages.
This study was funded by the University of Nottingham.
Ganesh A, Randall MD. Does metformin affect outcomes in COVID‐19 patients with new or pre‐existing diabetes mellitus? A systematic review and meta‐analysis. Br J Clin Pharmacol. 2022;88(6):2642-2656. doi: 10.1111/bcp.15258
Funding information University of Nottingham
DATA AVAILABILITY STATEMENT
All data used in this manuscript including the search strategies, list of included and excluded studies, data extracted, quality assessment and assessment of publication bias are available upon reasonable request.
REFERENCES
- 1. COVID Live Update: 141400793 Cases and 3 026 206 Deaths from the Coronavirus ‐ Worldometer n.d. https://www.worldometers.info/coronavirus/ (accessed April 18, 2021).
- 2. Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis. 2020;20:630‐631. doi: 10.1016/S1473-3099(20)30257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care. 2018;41(10):2127‐2135. doi: 10.2337/dc18-0287 [DOI] [PubMed] [Google Scholar]
- 4. Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623‐628. doi: 10.1111/j.1464-5491.2006.01861.x [DOI] [PubMed] [Google Scholar]
- 5. Booth CM. Clinical Features and Short‐term Outcomes of 144 Patients With SARS in the Greater Toronto Area. JAMA. 2003;289(21):2801‐2809. doi: 10.1001/jama.289.21.JOC30885 [DOI] [PubMed] [Google Scholar]
- 6. Zhu L, She Z‐G, Cheng X, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID‐19 and Pre‐existing Type 2 Diabetes. Cell Metab. 2020;31(6):1068‐1077.e3. doi: 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of <scp > COVID</scp > −19. Diabetes Metab Res Rev. 2020;36(7):e3319. doi: 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID‐19? A meta‐analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:535‐545. doi: 10.1016/j.dsx.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic Activity Is Impaired in Type 2 Diabetes Mellitus and Increases after Metabolic Improvement. PLoS ONE. 2011;6(8):e23366. doi: 10.1371/journal.pone.0023366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS‐CoV infection. JCI Insight. 2019;4(20):e131774. doi: 10.1172/jci.insight.131774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwabara WMT, Yokota CNF, Curi R, Alba‐Loureiro TC. Obesity and Type 2 Diabetes mellitus induce lipopolysaccharide tolerance in rat neutrophils. Sci Rep. 2018;8(1):17534. doi: 10.1038/s41598-018-35809-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of <scp > COVID</scp > −19. Am J Hematol. 2020;95:834‐847. doi: 10.1002/ajh.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kartika R, Purnamasari D, Pradipta S, Larasati RA, Wibowo H. Impact of low interferon‐γ and il‐10 levels on tnf‐α and il‐6 production by pha‐induced pbmcs in type 2 diabetes mellitus. J Inflamm Res. 2020;13:187. doi: 10.2147/JIR.S245064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandez‐Real JM, Valdes S, Manco M, et al. Surfactant Protein D, a Marker of Lung Innate Immunity, Is Positively Associated With Insulin Sensitivity. Diabetes Care. 2010;33(4):847‐853. doi: 10.2337/dc09-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID‐19 and diabetes. MedRxiv 2020;Preprint. [DOI] [PMC free article] [PubMed]
- 16. Bramante CT, Ingraham NE, Murray TA, et al. Metformin and risk of mortality in patients hospitalised with COVID‐19: a retrospective cohort analysis. Lancet Healthy Longevity. 2020;2(1):e34‐e41. doi: 10.1016/S2666-7568(20)30033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. doi: 10.1007/s00125-020-05180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khunti K, Knighton P, Zaccardi F, et al. Prescription of glucose‐lowering therapies and risk of COVID‐19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endo. 2021;9(5):293‐303. doi: 10.1016/S2213-8587(21)00050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheen AJ. Metformin and COVID‐19: From cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46(6):423‐426. doi: 10.1016/j.diabet.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014;20(6):953‐966. doi: 10.1016/j.cmet.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 21. Ho T‐W, Huang C‐T, Tsai Y‐J, Lien AS‐Y, Lai F, Yu C‐J. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir Res. 2019;20(1):69. doi: 10.1186/s12931-019-1035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang M, He J. Impacts of metformin on tuberculosis incidence and clinical outcomes in patients with diabetes: a systematic review and meta‐analysis. Eur J Clin Pharmacol. 2020;76(2):149‐159. doi: 10.1007/s00228-019-02786-y [DOI] [PubMed] [Google Scholar]
- 23. Joober R, Schmitz N, Annable L, Boksa P. Publication bias: What are the challenges and can they be overcome? J Psychiatry Neurosci. 2012;37(3):149‐152. doi: 10.1503/jpn.120065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 25. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing Summary of Findings tables—binary outcomes. J Clin Epidemiol. 2013;66:158‐172. doi: 10.1016/j.jclinepi.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 27. Pérez‐Belmonte LM, Torres‐Peña JD, López‐Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID‐19 in association with glucose‐lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1):359. doi: 10.1186/s12916-020-01832-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Do JY, Kim SW, Park JW, Cho KH, Kang SH. Is there an association between metformin use and clinical outcomes in diabetes patients with COVID‐19? Diabetes Metab. 2020;47(4):101208. doi: 10.1016/j.diabet.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Yang D, Cheng B, et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID‐19 in Association With Glucose‐Lowering Medication. Diabetes Care. 2020;43(7):1399‐1407. doi: 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 30. Lally M, Tsoukas P, Halladay C, O'Neill E, Gravenstein S, Rudolph JL. Metformin Is Associated With Decreased 30‐Day Mortality Among Nursing Home Residents Infected With SARS‐CoV2. J Am Med Dir Assoc. 2020;22(1):193‐198. doi: 10.1016/j.jamda.2020.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao Y, Liu T, Zhong W, et al. Risk of Metformin in Patients With Type 2 Diabetes With COVID‐19: A Preliminary Retrospective Report. Clin Transl Sci. 2020;13(6):1055‐1059. doi: 10.1111/cts.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim MK, Jeon JH, Kim SW, et al. The clinical characteristics and outcomes of patients with moderate‐to‐severe coronavirus disease 2019 infection and diabetes in Daegu. South Korea Diab Metab J. 2020;44(4):602‐613. doi: 10.4093/dmj.2020.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo P, Qiu L, Liu Y, et al. Metformin Treatment Was Associated with Decreased Mortality in COVID‐19 Patients with Diabetes in a Retrospective Analysis. Am J Trop Med Hyg. 2020;103(1):69‐72. doi: 10.4269/ajtmh.20-0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Philipose Z, Smati N, Wong CS, Aspey K, Mendall M. Obesity, old age, and frailty are the true risk factors for COVID‐19 mortality and not chronic disease or ethnicity. MedRxiv 2020;Preprint.
- 35. Chung SM, Lee YY, Ha E, et al. The Risk of Diabetes on Clinical Outcomes in Patients with Coronavirus Disease 2019: A Retrospective Cohort Study. Diabetes Metab J. 2020;44(4):621‐622. doi: 10.4093/dmj.2020.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abu‐Jamous B, Anisimovich A, Baxter J, et al. Associations of comorbidities and medications with COVID‐19 outcome: A retrospective analysis of real‐world evidence data. MedRxiv 2020.
- 37. Liu Z, Bai X, Han X, et al. The association of diabetes and the prognosis of COVID‐19 patients: A retrospective study. Diabetes Res Clin Pract. 2020;169:108386. doi: 10.1016/j.diabres.2020.108386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Q, Wei Y, Chen M, Wan Q, Chen X. Clinical analysis of risk factors for severe COVID‐19 patients with type 2 diabetes. J Diabetes Complications. 2020;34(10):107666. doi: 10.1016/j.jdiacomp.2020.107666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silverii GA, Monami M, Cernigliaro A, et al. Are diabetes and its medications risk factors for the development of COVID‐19? Data from a population‐based study in Sicily. Nutr Metab Cardiovasc Dis. 2020;31(2):396‐398. doi: 10.1016/j.numecd.2020.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang J, Kong W, Xia P, et al. Impaired Fasting Glucose and Diabetes Are Related to Higher Risks of Complications and Mortality Among Patients With Coronavirus Disease 2019. Front Endocrinol. 2020;11:525. doi: 10.3389/fendo.2020.00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Wei Q, Li WX, et al. METFORMIN USE IN DIABETES PRIOR TO HOSPITALIZATION: EFFECTS ON MORTALITY IN COVID‐19. Endocr Pract. 2020;26(10):1166‐1172. doi: 10.4158/EP-2020-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng X, Xin S, Chen Y, et al. Effects of metformin, insulin on COVID‐19 patients with pre‐existed type 2 diabetes: A multicentral retrospective study. Life Sci. 2021;275:119371. doi: 10.1016/j.lfs.2021.119371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bramante CT, Buse J, Tamaritz L, et al. Outpatient metformin use is associated with reduced severity of COVID‐19 disease in adults with overweight or obesity. J Med Virol. 2021;93(7):4273‐4279. doi: 10.1002/jmv.26873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oh TK, Song I‐A. Metformin use and risk of COVID‐19 among patients with type II diabetes mellitus: an NHIS‐COVID‐19 database cohort study. Acta Diabetol. 2021;58(6):771‐778. doi: 10.1007/s00592-020-01666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghany R, Palacio A, Dawkins E, et al. Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab Syndr Clin Res Rev. 2021;15(2):513‐518. doi: 10.1016/j.dsx.2021.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Wei Q, McCowen K, et al. Inpatient Use of Metformin and Acarbose Is Associated with Reduced Mortality of COVID‐19 Patients with Type 2 Diabetes Mellitus. Research Square. 2021;e00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodall JW, Reed TAN, Ardissino M, et al. Risk factors for severe disease in patients admitted with COVID‐19 to a hospital in London, England: a retrospective cohort study. Epidemiol Infect. 2020;148:e251. doi: 10.1017/S0950268820002472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Izzi‐Engbeaya C, Distaso W, Amin A, et al. Severe COVID‐19 and Diabetes ‐ A Retrospective Cohort Study from Three London Teaching Hospitals. MedRxiv 2021. [DOI] [PMC free article] [PubMed]
- 49. Dashti H, Bates DW, Roche E, Fiskio J, Mora S, Demler OV. Clinical Characteristics and Severity of COVID‐19 Disease in Patients from Boston Area Hospitals. MedRxiv 2020.
- 50. Wang J, Cooper JM, Gokhale K, et al. Association of Metformin with Susceptibility to COVID‐19 in People with Type 2 Diabetes. J Clin Endocrinol Metabol. 2021;106(5):1255‐1268. doi: 10.1210/clinem/dgab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orioli L, Servais T, Belkhir L, et al. Clinical characteristics and short‐term prognosis of in‐patients with diabetes and COVID‐19: A retrospective study from an academic center in Belgium. Diabetes Metab Syndr Clin Res Rev. 2021;15(1):149‐157. doi: 10.1016/j.dsx.2020.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lalau J‐D, Al‐Salameh A, Hadjadj S, et al. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID‐19. Diabetes Metab. 2021;47(5):101216. doi: 10.1016/j.diabet.2020.101216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jiang N, Chen Z, Liu L, et al. Association of metformin with mortality or ARDS in patients with COVID‐19 and type 2 diabetes: A retrospective cohort study. Diabetes Res Clin Pract. 2021;173:108619. doi: 10.1016/j.diabres.2020.108619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheng X, Liu Y‐M, Li H, et al. Metformin Is Associated with Higher Incidence of Acidosis, but Not Mortality, in Individuals with COVID‐19 and Pre‐existing Type 2 Diabetes. Cell Metab. 2020;32(4):537‐547.e3. doi: 10.1016/j.cmet.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lukito AA, Pranata R, Henrina J, Lim MA, Lawrensia S, Suastika K. The Effect of Metformin Consumption on Mortality in Hospitalized COVID‐19 patients: a systematic review and meta‐analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(6):2177‐2183. doi: 10.1016/j.dsx.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lemkes BA, Hermanides J, DeVries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. 2010;8(8):1663‐1669. doi: 10.1111/j.1538-7836.2010.03910.x [DOI] [PubMed] [Google Scholar]
- 58. Simonnet A, Chetboun M, Poissy J, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) Requiring Invasive Mechanical Ventilation. Obesity. 2020;28(7):1195‐1199. doi: 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID‐19. Nature Reviews. Endocrinology. 2020;16(7):341‐342. doi: 10.1038/s41574-020-0364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pal R, Bhansali A. COVID‐19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang J‐K, Lin S‐S, Ji X‐J, Guo L‐M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193‐199. doi: 10.1007/s00592-009-0109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with <scp > COVID</scp > −19. Diabetes Obes Metab. 2020;22(10):1897‐1906. doi: 10.1111/dom.14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jornayvaz FR, Assouline B, Pugin J, Gariani K. Extremely high‐dose insulin requirement in a diabetic patient with COVID‐19: a case report. BMC Endocr Disord. 2020;20(1):155. doi: 10.1186/s12902-020-00632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rayman G, Lumb A, Kennon B, et al. Guidance on the management of Diabetic Ketoacidosis in the exceptional circumstances of the COVID‐19 pandemic. Diabet Med. 2020;37(7):1214‐1216. doi: 10.1111/dme.14328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu J, Li X, Lu Q, et al. AMPK: a balancer of the renin–angiotensin system. Biosci Rep. 2019;39(9):BSR20181994. doi: 10.1042/BSR20181994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ursini F, Ciaffi J, Landini MP, Meliconi R. COVID‐19 and diabetes: Is metformin a friend or foe? Diabetes Res Clin Pract. 2020;164:108167. doi: 10.1016/j.diabres.2020.108167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singh S, Singh PK, Suhail H, et al. AMP‐Activated Protein Kinase Restricts Zika Virus Replication in Endothelial Cells by Potentiating Innate Antiviral Responses and Inhibiting Glycolysis. J Immunol. 2020;204(7):1810‐1824. doi: 10.4049/jimmunol.1901310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cameron AR, Morrison VL, Levin D, et al. Anti‐Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119(5):652‐665. doi: 10.1161/CIRCRESAHA.116.308445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schuiveling M, Vazirpanah N, Radstake TRDJ, Zimmermann M, Broen JCA. Metformin, A New Era for an Old Drug in the Treatment of Immune Mediated Disease? Curr Drug Targets. 2018;19(8):945‐959. doi: 10.2174/1389450118666170613081730 [DOI] [PubMed] [Google Scholar]
- 70. Menendez JA. Metformin and SARS‐CoV‐2: mechanistic lessons on air pollution to weather the cytokine/thrombotic storm in COVID‐19. Aging. 2020;12(10):8760‐8765. doi: 10.18632/aging.103347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ouyang J, Isnard S, Lin J, et al. Metformin effect on gut microbiota: insights for HIV‐related inflammation. AIDS Res Therapy. 2020;17(1):10. doi: 10.1186/s12981-020-00267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Matsiukevich D, Piraino G, Lahni P, et al. Metformin ameliorates gender‐and age‐dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochimica et Biophysica Acta (BBA) ‐ Mol Basis Dis. 2017;1863(10):2680‐2691. doi: 10.1016/j.bbadis.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liang H, Ding X, Li L, et al. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta‐analysis of cohort studies. Crit Care. 2019;23(1):50. doi: 10.1186/s13054-019-2346-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mendy A, Gopal R, Alcorn JF, Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology. 2019;24(7):646‐651. doi: 10.1111/resp.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Henry RR, Frias JP, Walsh B, et al. Improved glycemic control with minimal systemic metformin exposure: Effects of Metformin Delayed‐Release (Metformin DR) targeting the lower bowel over 16 weeks in a randomized trial in subjects with type 2 diabetes. PLOS One. 2018;13(9):e0203946. doi: 10.1371/journal.pone.0203946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054‐1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Skelly A, Dettori J, Brodt E. Assessing bias: the importance of considering confounding. Evidence‐Based Spine‐Care J. 2012;3(01):9‐12. doi: 10.1055/s-0031-1298595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alexander SPH, Christopoulos A, Davenport AP, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176(S1). doi: 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this manuscript including the search strategies, list of included and excluded studies, data extracted, quality assessment and assessment of publication bias are available upon reasonable request.


