Key Points
Question
How does the percentage of patients who withdraw from adjuvant trials because of adverse events compare with patients who withdraw from metastatic trials for the same drug?
Findings
In this cross-sectional study, the adjuvant setting was associated with higher rates of discontinuation of drugs because of adverse events or patient withdrawal than in the metastatic setting.
Meaning
These results suggest that adverse effects of treatments in the adjuvant setting must be considered when planning a patient’s course of treatment and reaching the therapeutic window.
This cross-sectional study of clinical trials of cancer drugs compares rates of treatment discontinuation because of adverse events or withdrawal in an adjuvant setting vs a metastatic setting.
Abstract
Importance
Adjuvant drugs are used to reduce the risk of tumor recurrence in patients with cancer who are successfully treated with first-line therapy. The same drugs used in the metastatic or first-line setting are often used in the adjuvant setting, and although the resulting adverse effects may be similar between the 2 settings, tolerability may be different.
Objective
To compare the discontinuation rates of drugs in the adjuvant setting and in the metastatic setting in clinical trials of cancer drugs.
Design, Setting, and Participants
This cross-sectional study examined clinical trials of cancer drugs with results published in major medical and oncology journals between July 2018 through June 2021. Because adjuvant drugs can be used in a metastatic setting, included trials were conducted in an adjuvant setting. Data were analyzed December 2021.
Exposures
Drugs used in the adjuvant setting, which were also used in the metastatic setting for the same tumor indication.
Main Outcomes and Measures
Discontinuation rates in the adjuvant and metastatic settings, which were calculated by dividing the total number of study participants who withdrew or discontinued because of adverse events by the number of participants allocated to the drug arm.
Results
A total of 29 trials with a drug being used in the adjuvant and metastatic setting were found. In the adjuvant setting, the median (IQR) age for study participants was 58.0 (52.0-63.5) years, and the median (IQR) percentage of male participants was 55.5% (0.9%-64.8%). In the metastatic setting, the median (IQR) age for study participants was 61 years, and the median (IQR) percentage of male participants was 55.2% (2.0%-66.0%). Overall, a median (IQR) 21.4% (17.7%-29.4%) of participants discontinued because of adverse events or patient withdrawal in the adjuvant setting compared with a median (IQR) 15.9% (9.7%-21.3%) in the metastatic setting (P = .01). Checkpoint inhibitors (median [IQR] rate of discontinuation, 21.4% [18.6%-31.3%] vs 15.2% [9.9%-19.5%]; P = .01) and targeted drugs (median [IQR] rate of discontinuation, 27.7% vs 14.0%; P < .001) demonstrated a higher rate of discontinuation in the adjuvant setting while cytotoxic drugs (median [IQR] rate of discontinuation, 16.6% [12.2%-23.3%] vs 25.5% [19.8%-28.8%]; P = .07) showed no difference between the 2 settings. The largest differences between adjuvant and metastatic discontinuation rates were for sorafenib (renal cell carcinoma, 43.8% vs 5.5%; difference, 38.2%), imatinib (gastrointestinal stromal tumor, 37.4% vs 6.1%; difference, 31.2%), and erlotinib (non–small cell lung cancer, 37.5% vs 8.4%; difference, 29.0%).
Conclusions and Relevance
In this cross-sectional study of clinical trials that involved novel cancer drugs, drugs used in the adjuvant setting were associated with significantly higher discontinuation rates than in the metastatic setting. This finding suggests that the proposed benefits of adjuvant therapy need to be taken in context of patient’s drug tolerance.
Introduction
Adjuvant therapies are administered after surgical removal of a localized or regional cancer when there is no evidence of distant spread. Metastatic drugs are administered to patients with measurable tumors that have typically spread beyond the primary organ. As such, these drugs have different tasks and are given to different patients. The task of an adjuvant drug is to eradicate or severely delay the growth of micrometastatic disease in a person who has no visible cancer and who is not suffering from symptomatic cancer. The task of a metastatic drug is to eradicate, shrink, or delay growth of visible tumors, which may be causing symptoms or impairing quality of life.
A 2020 empirical comparison1 of adjuvant and metastatic therapies showed that more drugs are approved and used in the metastatic space than the adjuvant space. Approximately 1 in 3 drugs that are approved or recommended for metastatic disease eventually have a role in adjuvant disease.1,2,3 This may be because of the greater biological challenge required for adjuvant drugs or because of differences in tolerability, patient acceptance, or dose delivery.
We set out to assess the differential tolerability of cancer drugs in adjuvant or metastatic space by comparing the rates of dose reduction and discontinuation for the same drug in the same tumor type. This retrospective cross-sectional study examined the discontinuation rates of patients enrolled in clinical trials for drugs offered in both the adjuvant and metastatic settings. We further delineated reasons for discontinuation, route of administration, and drug administered.
Methods
Study Set
We set out to assemble a set of cancer drugs that have been tested in both the adjuvant and metastatic settings for the same tumor type. Given that all adjuvant drugs also have metastatic uses, we sought to isolate these studies by looking at all clinical trials published in the Journal of Clinical Oncology, New England Journal of Medicine, The Lancet, Lancet Oncology, and JAMA Oncology between July 2018 and June 2021. In accordance with 45 CFR §46.102(f), this study was not submitted for institutional review board approval because it involved publicly available data and did not involve individual patient data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
We searched through every issue and identified trials testing novel targeted cancer drug therapies, and specifically searched for the keyword adjuvant to identify trials that tested these therapies in the adjuvant setting. Studies examining the effectiveness of adjuvant therapies in an observational or retrospective design or trials testing duration of adjuvant therapy were excluded. We also excluded clinical trials testing the effectiveness of agents that are not antitumor, radiation therapies, maintenance therapies, and studies that reported on biomarker analysis. We excluded unapproved drugs for any indication in the US and drugs used primarily for central nervous system malignant neoplasms. We further excluded drugs tested in the adjuvant setting but untested or unused in the metastatic setting.
Data Extraction
After identifying relevant trials, we did a web search to find studies in the metastatic setting that used the same drug as in the adjuvant setting for the same tumor indication. For drugs that were US Food and Drug Administration (FDA) approved in the metastatic setting, we searched for published trial data leading to the approval of the drug in the metastatic setting. For drugs that were tested but not approved in the metastatic setting, we searched Google Scholar using keywords for the drug name and metastatic. We prioritized phase 3 over phase 2 studies and studies with larger sample sizes over smaller. Google Scholar was used because previous studies have shown that it returns more relevant results using the same search queries when compared with PubMed.4 For cytotoxic drugs, we searched HemOnc.org to find studies reporting on the use of these drugs and regimens in the metastatic setting.
We made note of the drug name, method of delivery, binding status, total number of participants in the trial, the total number of participants allocated to the intervention arm, the reasons for participant discontinuation, discontinuation numbers for each reason, and the number of participants who had doses reduced. We also abstracted data on dose reductions and whether a trial reported on quality of life (QoL). We tabulated the reasons for discontinuation, including disease progression, protocol violation, and patient missing from follow-up. If a patient withdrew from the study or had an adverse event due to the study drug or otherwise, we included these patients under the adverse events and withdrawal category. Similarly, if a patient died during the study, we included this under the progression and death category. Patients who discontinued because of miscellaneous other reasons were assigned to the other category. We were primarily interested in discontinuation rates due to adverse events and patient withdrawal.
Statistical Analysis
From the number of patients allocated to the intervention arm, we calculated frequencies and percentages for each reason of discontinuation. From these, we calculated medians and ranges for adjuvant and metastatic studies separately. Finally, we performed a Wilcoxon signed-rank test for the statistical significance of differences in medians between adjuvant and metastatic settings. We used Fisher exact test for determining differences in categorical variables. We used a 2-sided P value of .05 for statistical significance. All data were calculated using Microsoft Excel and R Statistical Software version 3.6.1 (R Project for Statistical Computing).
Results
We identified 29 drugs with clinical trials in both the metastatic and adjuvant settings for the same tumor indication (Table 1; eTable in the Supplement). In the adjuvant setting, the median (IQR) age for study participants was 58.0 (52.0-63.5) years, and the median (IQR) percentage of male participants was 55.5% (0.9%-64.8%). In the metastatic setting, the median (IQR) age for study participants was 61.5 (55.8-64.0) years, and the median (IQR) percentage of male participants was 55.2% (2.0%-66.0%). The drugs included 9 (31%) used for breast cancer, 5 (17%) for melanoma, 5 (17%) for non–small cell lung cancer, 3 (10%) for urothelial cancer, 2 (7%) for small cell lung cancer, and 1 (3%) each for head and neck squamous cell carcinoma, renal cell carcinoma, gastric cancer, gastrointestinal stromal tumor cancer, and pancreatic cancer. We found 7 (24%) cytotoxic drugs, 7 (24%) checkpoint inhibitors, and 15 (52%) targeted drug types used in the adjuvant setting.
Table 1. Characteristics of Included Studies Stratified by Mechanism of Action.
| Study characteristic | Studies, No. (%) | |||
|---|---|---|---|---|
| Checkpoint inhibitor (n = 7) | Cytotoxic (n = 7) | Targeted therapy (n = 15) | Overall (n = 29) | |
| Tumor type | ||||
| Breast | 0 | 2 (29) | 7 (47) | 9 (31) |
| Gastric | 1 (14) | 0 | 0 | 1 (3) |
| GIST | 0 | 0 | 1 (7) | 1 (3) |
| HNSCC | 0 | 0 | 1 (7) | 1 (3) |
| Melanoma | 4 (57) | 0 | 1 (7) | 5 (17) |
| NSCLC | 0 | 1 (14) | 4 (27) | 5 (17) |
| Pancreatic | 0 | 1 (14) | 0 | 1 (3) |
| RCC | 0 | 0 | 1 (7) | 1 (3) |
| Urothelial | 2 (29) | 1 (14) | 0 | 3 (10) |
| SCLC | 0 | 2 (29) | 0 | 2 (7) |
| Adjuvant setting | ||||
| Study masking | ||||
| Yes | 5 (71) | 0 | 7 (47) | 12 (41) |
| No. of intervention participants, median (range) | 453 (59-532) | 247 (110-448) | 438 (91-2883) | 406 (59-2883) |
| Intervention participants discontinuing treatment from adverse events or withdrawal, median (range), % | 21.4 (11.7-59.3) | 16.6 (2.2-24.5) | 27.7 (12.6-43.8) | 21.4 (2.2-59.3) |
| Dose reductions | ||||
| Yes | 0 | 3 (43) | 10 (67) | 12 (41) |
| Not allowed | 7 (100) | 0 | 1 (7) | 8 (28) |
| Not reported | 0 | 4 (57) | 4 (27) | 8 (28) |
| Participants with dose reductions, median (range), % | 0 | 36.9 (8.6-78.8) | 40.6 (10.4-87.0) | 40 (8.6-87.0) |
| Disease-free survival | ||||
| Positive | 5 (71) | 3 (43) | 8 (53) | 16 (55) |
| Negative | 2 (29) | 4 (57) | 5 (33) | 11 (38) |
| Inaccessible | 0 | 0 | 2 (13) | 2 (7) |
| Overall survival | ||||
| Positive | 1 (14) | 1 (14) | 0 | 2 (7) |
| Negative | 2 (29) | 6 (86) | 9 (60) | 17 (59) |
| Inaccessible/not reported | 4 (57) | 0 | 6 (40) | 10 (35) |
| Received FDA approval | 4 (57) | 0 | 5 (33) | 9 (31) |
| Metastatic setting | ||||
| Study masking | ||||
| Yes | 3 (42.8) | 0 | 7 (46.7) | 10 (34.5) |
| No. of intervention participants, median (range) | 277 (94-789) | 164 (51-612) | 328 (30-318) | 279 (30-789) |
| Intervention participants discontinuing treatment from adverse events or withdrawal, median (range), % | 15.2 (6.7-56.4) | 25.5 (14.9-32.8) | 14.0 (5.5-28.9) | 16.0 (5.5-56.4) |
| Dose reductions | ||||
| Yes | 0 | 4 (57.1) | 10 (66.7) | 14 (48.3) |
| Not allowed | 7 (100) | 0 | 1 (6.7) | 8 (27.6) |
| Not reported | 0 | 3 (42.9) | 4 (26.7) | 7 (24.1) |
| Participants with dose reductions, median (range), % | 0 | 76.3 (43-86) | 18.6 (4-43) | 24.0 (4-86) |
| Progression-free survival | ||||
| Positive | 5 (71.4) | 1 (14.3) | 12 (80.0) | 18 (62.1) |
| Negative | 1 (14.3) | 2 (28.6) | 2 (13.3) | 5 (17.2) |
| Inaccessible | 1 (14.3) | 4 (57.1) | 1 (6.7) | 6 (20.7) |
| Overall survival | ||||
| Positive | 4 (57.1) | 0 | 6 (40.0) | 10 (34.5) |
| Negative | 2 (28.6) | 3 (42.8) | 8 (53.3) | 13 (44.8) |
| Inaccessible | 1 (14.3) | 4 (57.1) | 1 (6.7) | 6 (20.7) |
| Received FDA approval | 7 (100) | 2 (28.6) | 13 (86.7) | 20 (69.0) |
Abbreviations: FDA, US Food and Drug Administration; HNSCC, head and neck squamous cell carcinoma; NSCLC, non–small cell lung cancer; RCC, renal cell carcinoma; SCLC, small cell lung cancer.
In the adjuvant setting, 12 studies (41%) were masked, and 10 (34%) were masked in the metastatic setting (P = .67). The median (IQR) number of study participants in the adjuvant setting was higher than in the metastatic setting (406 [131-532] vs 279 [164-403] patients; P = .01). When drugs were tested in the adjuvant setting, 16 (55%) demonstrated a disease-free survival (DFS) improvement, but when tested in the metastatic setting, a progression-free survival improvement was demonstrated in 18 studies (62%) (P = .08). In the adjuvant setting, 12 drugs (7%) demonstrated overall survival (OS) improvement, whereas in the metastatic setting, 10 drugs (34%) demonstrated OS improvement (P = .59). For studies that allowed dose reduction (22 studies) and reported percentages (13 studies), the median (IQR) percentage of participants who had doses reduced in the adjuvant setting was 40% (25%-53%), and for the metastatic setting, it was 25% (16%-43%) (P = .27).
Nine of 29 drugs (31%) had already received FDA approval in the adjuvant setting, and 20 of 29 (69%) had already received approval in the metastatic setting. Nine drugs had received approval in both settings. Those drugs (with indications) were pembrolizumab for melanoma, nivolumab for melanoma, nivolumab for urothelial cancer, osimertinib for non–small cell lung cancer, trastuzumab emtansine for breast cancer (stage 1 and residual invasive), pertuzumab for breast cancer, ipilimumab for melanoma, and dabrafenib plus trametinib for melanoma.
The median (IQR) percentage of participants who discontinued because of adverse events or withdrawal was higher in the adjuvant setting compared with the metastatic setting (21.4% [17.7%-29.4%] vs 15.9% [9.7%-21.3%]; P = .01) (Figure 1). When stratifying by mechanism of drug (cytotoxic, checkpoint inhibitor, and targeted), the median (IQR) percentages of participants who discontinued because of adverse events or withdrawal among checkpoint inhibitor drugs were 21.4% (18.6%-31.3%) and 15.2% (9.9%-19.5%) in the adjuvant and metastatic settings, respectively (P = .01) (Table 2; eFigure 1 in the Supplement). Among cytotoxic regimens, the median (IQR) percentages of participants who discontinued because of adverse events and withdrawal were 16.6% (12.2%-23.3%) and 25.5% (19.8%-28.8%) in the adjuvant and metastatic settings, respectively (P = .07). Among targeted drugs, the median percentages of participants who discontinued because of adverse events and withdrawal were 27.7% and 14.0% in the adjuvant and metastatic settings, respectively (P < .001). Discontinuation percentages for drugs in the metastatic vs adjuvant settings by route of administration are shown in eFigure 2 in the Supplement.
Figure 1. Reasons Trial Participants Discontinued Therapy in Drugs Tested in Both Adjuvant and Metastatic Settings.
Table 2. Percentages of Discontinuation by Reason.
| Setting | Treatment discontinuation, median (IQR), % | ||||
|---|---|---|---|---|---|
| Progression and death | P value | Adverse events and withdrawal | P value | Other | |
| Overall | |||||
| Adjuvant | 7.5 (2.9-21.2) | <.001 | 21.4 (17.7-29.4) | .01 | 2.3 (0.7-4.2) |
| Metastatic | 46.5 (30.4-54.5) | 15.9 (9.7-21.3) | 1.7 (0.5-3.4) | ||
| Checkpoint inhibitors | |||||
| Adjuvant | 25.5 (21.7-27.3) | .01 | 21.4 (18.6-31.3) | .01 | 1.7 (0.9-2.3) |
| Metastatic | 45.7 (30.3-59.3) | 15.2 (9.9-19.5) | 2.0 (0.6-3.2) | ||
| Cytotoxic | |||||
| Adjuvant | 1.8 (1.3-5.1) | .03 | 16.6 (12.2-23.3) | .07 | 1.5 (0-6.1) |
| Metastatic | 27.6 (13.2-47.4) | 25.5 (19.8-28.8) | 1.2 (0-3.0) | ||
| Targeted | |||||
| Adjuvant | 7.1 (3.5-15.0) | <.001 | 27.7 (19.6-32.3) | <.001 | 3.3 (0.9-5.2) |
| Metastatic | 48.3 (45.5-54.0) | 14.0 (9.0-16.5) | 2.3 (1.0-3.4) | ||
The drugs with the largest median differences in the percentages of adverse events/withdrawal discontinuation between the metastatic and adjuvant setting (Figure 2) were sorafenib for renal cell carcinoma (43.8% in the adjuvant and 5.5% in the metastatic; difference of 38.2%), imatinib for gastrointestinal stromal tumor cancer (37.4% in the adjuvant and 6.1% in the metastatic; difference of 31.2%), and ipilimumab for melanoma (37.5% in the adjuvant and 8.4% in the metastatic; difference of 29.0%).
Figure 2. Percentage of Individuals Who Discontinued Oncology Drugs Because of Adverse Events and Withdrawal in the Adjuvant vs Metastatic Settings by Drug and Indication.
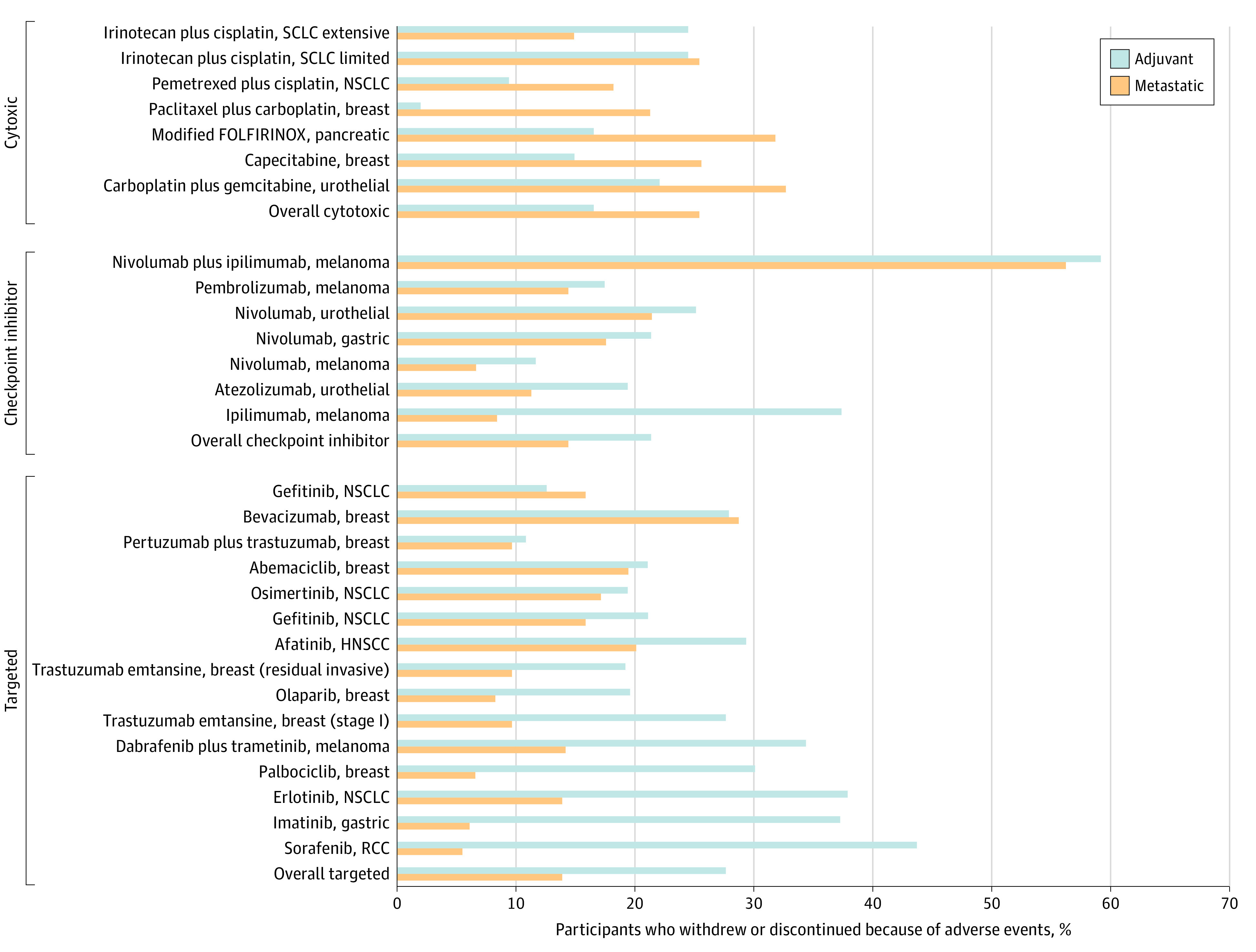
HNSCC indicates head and neck squamous cell carcinoma; NSCLC, non–small cell lung cancer; RCC, renal cell carcinoma; SCLC, small cell lung cancer.
Discussion
We hypothesized that for drugs used in both the adjuvant and metastatic settings, tolerance may differ in the adjuvant setting, in which drugs are administered for primarily noncurative purposes, from the metastatic, where the intent of treatment is to prevent the spread of the tumor. Our findings appeared to support this hypothesis, as we found that discontinuation due to withdrawal or adverse events was significantly higher among patients in the adjuvant setting than in the metastatic setting. Since adjuvant therapies are employed to reduce tumor recurrence and not treat the tumor, our findings are notable as patients may be less willing to tolerate the adverse effects of adjuvant treatment when they have already received what they regard as the main course of treatment for their cancer. However, it is possible that an intolerable drug, because of its severe adverse effects, may have similar discontinuation rates across the metastatic and adjuvant settings. In light of our findings that higher rates of discontinuation because of adverse events and patient withdrawals were associated with the adjuvant setting compared with the metastatic setting, physicians who prescribe these adjuvant medications need to consider the patients’ reduced tolerance to adverse effects in the adjuvant setting and should engage in discussion with patients about the benefits and adverse effects when planning their treatment.
Specifically, we found that discontinuation because of adverse events and patient withdrawal was greater among targeted drugs. Recently, these drugs have been used with greater frequency and for longer duration than cytotoxic drugs, in part because of better patient tolerability.5 Our study challenges the widely held belief that targeted drugs have more favorable tolerability profiles than cytotoxic drugs. At the very least, our results suggest that the methods we use to evaluate tolerability for cytotoxic therapeutic agents may not be appropriate for the new generation of immunologically inspired agents. Furthermore, drugs used in the adjuvant setting often fail to improve OS because they delay tumor growth rather than kill cancer cells.6 Finally, there may be a different risk profile of drugs that are used daily vs cyclically or even orally vs intravenously.
The prophylactic importance of adjuvant therapy for various tumor types has been an ongoing discussion.7 Even for those tumor indications for which adjuvant therapy may be deemed the most beneficial, such as lung, breast, or colorectal cancers, the standard of care in metastatic and adjuvant settings is rapidly evolving.8 About 35% of all oncology drugs recommended and approved by the National Comprehensive Cancer Network in the metastatic setting received approval for adjuvant treatment, with an average of 10 years difference between the 2 approvals.1 However, in light of the approval of drugs based on end points, such as DFS, that may not necessarily correlate with OS, FDA approval for a given drug does not guarantee benefit to the patient.1 By noting the markedly higher rates of patient withdrawal and adverse events and discontinuation rates for novel therapies associated with the adjuvant setting vs the metastatic setting, we elucidated that patient tolerability is a key factor when determining the course of treatment in the adjuvant setting.
Patient tolerability may be less of a concern if these drugs are able to improve other meaningful outcomes for the patient, such as OS. However, adjuvant therapies have only primarily shown improvements in DFS. We found that only 2 of 29 adjuvant drugs we analyzed demonstrated an improvement in OS. The lack of OS benefit for drugs used in the adjuvant setting must be acknowledged when discussing patient tolerability.
Ipilimumab has notable adverse effects, especially when combined with nivolumab.9 We found that ipilimumab had one of the highest differences in the percentage of discontinuation for patients with melanoma, even though the dose was lower than previously approved adjuvant ipilimumab dosages. Tarhini et al10 failed to find any improvement in relapse-free survival in either the 3 or 10 mg dose when compared with high-dose interferon α-2b, although 6-year OS improved slightly (75% vs 80%) with the 3 mg dose but not the 10 mg dose.
QoL is another way to gauge a patient’s acceptability of treatment. Comparing QoL between studies and indications can be challenging because not all drugs are evaluated for QoL. Furthermore, differences in reported QoL metrics, evaluation frequencies, and effects from other treatments place additional hurdles in the way of evaluating the acceptability of treatment by QoL assessment. While it was beyond the scope of our analysis to evaluate differences in QoL, we could only find studies reporting QoL or patient-reported outcomes for 9 of the drugs and indications (31%), and only 1 adjuvant study had QoL results that could be compared with study results of the same drug being tested in the metastatic setting. When this drug (pembrolizumab) was tested in patients with adjuvant melanoma, there was a decline of 1.61 points in QoL.11 This decline in QoL was greater than the same metric when evaluated in the metastatic setting, where there was a decline of 0.3 points.12
Dose reductions may also be another way to gauge patients’ acceptability of treatment. We found that about one-third of studies did not allow dose reductions in the study. For studies that did allow dose reduction and reported the frequency of dose reduction, the percentage of patients who had dose reductions was numerically higher in the adjuvant setting, although the difference was not significant. This finding, in addition to higher discontinuation rates, suggests less patient tolerability and acceptability of drugs used in the adjuvant setting. Tolerability among the general population may be even lower than in clinical trials, as dose reductions have been found to be even higher in the clinical practice setting.13
Limitations
Our study had several limitations. While our study looked at novel cancer therapies published in top medical and oncology journals, it is possible that certain drugs that were tested in the metastatic and adjuvant setting were not featured in 1 of the 6 journals we reviewed. However, given the importance of these journals to the field, most publications of trial data leading to the drug’s approval can be found in one of these journals. Our classification of reasons for discontinuation were dependent on what the authors of the studies reported, and it is possible that patients who dropped out for unspecified reasons may indeed have had adverse events or disease progression without communicating with the trial coordinators, therefore leading to classification bias. Additionally, we only reviewed drugs reported during the last 3-year window, so our findings were far more reflective of the general trend of discontinuation rates in the adjuvant setting. Further investigation may be required to address adjuvant drugs at-large.
Additionally, comparisons made between individual drugs have limited external validity as inclusion criteria and other biases introduce variability that we were not able to adjust for given limited access to patient data. Specifically, in the context of cancer therapies, where benefits in OS treatments have been shown to have 16% reduced benefit in real-world settings than those depicted in clinical trials, it is possible that discontinuations may be underestimated in a clinical trial environment, and the disparity in rates may be greater than reported.14 The reduced benefit in OS between the clinical and real-world setting has been shown for drugs like durvalumab and combination immunotherapies.15,16 This may be partly caused by the selection bias introduced when patients are deemed ineligible for a clinical trial because of health concerns, skewing trial populations toward healthier demographics.17 For example, a 2015 study18 found that 39% of registry patients with metastatic renal cell cancer being treated with tyrosine kinase inhibitors treated at Memorial Sloan Kettering Cancer Center would be deemed ineligible to enroll in a phase III trial of the same drug they received. Finally, our findings of the number of drugs that are FDA approved may not reflect the number of drugs that are used in practice or off-label, which may be more common with cytotoxic drugs than other drug types.
Conclusions
This cross-sectional study found that drugs approved for treatment in both the metastatic and adjuvant settings were associated with higher discontinuation rates in the adjuvant setting, with a median discontinuation rate of 25% and 14% in the adjuvant and metastatic settings, respectively. These results suggest that the adverse effects from treatments in the adjuvant setting must be considered when planning a patient’s course of treatment and reaching the therapeutic window. This is especially important considering that approval based on DFS may not translate to benefits in OS.
eTable. Study Trials
eFigure 1. Discontinuation Percentages (Adverse Events and Withdrawal) for Drugs in the Metastatic vs Adjuvant Settings, by Drug Type
eFigure 2. Discontinuation Percentages (Adverse Events and Withdrawal) for Drugs in the Metastatic vs Adjuvant Settings, by Route of Administration
eReferences.
References
- 1.Parsons S, Maldonado EB, Prasad V. Comparison of drugs used for adjuvant and metastatic therapy of colon, breast, and non-small cell lung cancers. JAMA Netw Open. 2020;3(4):e202488. doi: 10.1001/jamanetworkopen.2020.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naci H, Smalley KR, Kesselheim AS. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA. 2017;318(7):626-636. doi: 10.1001/jama.2017.9415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyawali B, Prasad V. Making adjuvant therapy decisions with uncertain data. Ann Oncol. 2019;30(3):361-364. doi: 10.1093/annonc/mdz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shariff SZ, Bejaimal SA, Sontrop JM, et al. Retrieving clinical evidence: a comparison of PubMed and Google Scholar for quick clinical searches. J Med Internet Res. 2013;15(8):e164-e164. doi: 10.2196/jmir.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lythgoe MP, Krell J, Mahmoud S, Mills EC, Vasudevan A, Savage P. Development and economic trends in anticancer drugs licensed in the UK from 2015 to 2019. Drug Discov Today. 2021;26(2):301-307. doi: 10.1016/j.drudis.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 6.Burotto M, Wilkerson J, Stein WD, Bates SE, Fojo T. Adjuvant and neoadjuvant cancer therapies: a historical review and a rational approach to understand outcomes. Semin Oncol. 2019;46(1):83-99. doi: 10.1053/j.seminoncol.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Chau I, Cunningham D. Adjuvant therapy in colon cancer—what, when and how? Ann Oncol. 2006;17(9):1347-1359. doi: 10.1093/annonc/mdl029 [DOI] [PubMed] [Google Scholar]
- 8.Puccini A, Lenz HJ. Colorectal cancer in 2017: practice-changing updates in the adjuvant and metastatic setting. Nat Rev Clin Oncol. 2018;15(2):77-78. doi: 10.1038/nrclinonc.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soldatos TG, Dimitrakopoulou-Strauss A, Larribere L, Hassel JC, Sachpekidis C. Retrospective side effect profiling of the metastatic melanoma combination therapy ipilimumab-nivolumab using adverse event data. Diagnostics (Basel). 2018;8(4):E76. doi: 10.3390/diagnostics8040076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarhini AA, Lee SJ, Hodi FS, et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J Clin Oncol. 2020;38(6):567-575. doi: 10.1200/JCO.19.01381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggermont AMM, Blank CU, Mandalà M, et al. ; EORTC Melanoma Group . Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643-654. doi: 10.1016/S1470-2045(21)00065-6 [DOI] [PubMed] [Google Scholar]
- 12.Petrella TM, Robert C, Richtig E, et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur J Cancer. 2017;86:115-124. doi: 10.1016/j.ejca.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 13.Prasad V, Massey PR, Fojo T. Oral anticancer drugs: how limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629. doi: 10.1200/JCO.2013.53.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakdawalla DN, Shafrin J, Hou N, et al. Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health. 2017;20(7):866-875. doi: 10.1016/j.jval.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Cramer-van der Welle CM, Verschueren MV, Tonn M, et al. ; Santeon NSCLC Study Group . Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021;11(1):6306. doi: 10.1038/s41598-021-85696-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankar K, Bryant AK, Strohbehn GW, et al. Real world outcomes versus clinical trial results of durvalumab maintenance in veterans with stage III non-small cell lung cancer. Cancers (Basel). 2022;14(3):614. doi: 10.3390/cancers14030614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim S, Xu Y, Kong S, Abdel-Rahman O, Quan ML, Cheung WY. Generalisability of common oncology clinical trial eligibility criteria in the real world. Clin Oncol (R Coll Radiol). 2019;31(9):e160-e166. doi: 10.1016/j.clon.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AP, Harrison MR, Walker MS, George DJ, Abernethy AP, Hirsch BR. Clinical trial participants with metastatic renal cell carcinoma differ from patients treated in real-world practice. J Oncol Pract. 2015;11(6):491-497. doi: 10.1200/JOP.2015.004929 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Study Trials
eFigure 1. Discontinuation Percentages (Adverse Events and Withdrawal) for Drugs in the Metastatic vs Adjuvant Settings, by Drug Type
eFigure 2. Discontinuation Percentages (Adverse Events and Withdrawal) for Drugs in the Metastatic vs Adjuvant Settings, by Route of Administration
eReferences.



