Abstract
Achieving synergism, often by combination therapy via codelivery of chemotherapeutic agents, remains the mainstay of treating multidrug-resistance cases in cancer and microbial strains. With a typical core–shell architecture and surface functionalization to ensure facilitated targeting of tissues, nanocarriers are emerging as a promising platform toward gaining such synergism. Co-encapsulation of disparate theranostic agents in nanocarriers—from chemotherapeutic molecules to imaging or photothermal modalities—can not only address the issue of protecting the labile drug payload from a hostile biochemical environment but may also ensure optimized drug release as a mainstay of synergistic effect. However, the fate of co-encapsulated molecules, influenced by temporospatial proximity, remains unpredictable and marred with events with deleterious impact on therapeutic efficacy, including molecular rearrangement, aggregation, and denaturation. Thus, more than just an art of confining multiple therapeutics into a 3D nanoscale space, a co-encapsulated nanocarrier, while aiming for synergism, should strive toward achieving a harmonious cohabitation of the encapsulated molecules that, despite proximity and opportunities for interaction, remain innocuous toward each other and ensure molecular integrity. This account will inspect the current progress in co-encapsulation in nanocarriers and distill out the key points toward accomplishing such synergism through reciprocity.
Keywords: co-encapsulation, combination therapy, multidrug resistance, tumor microenvironment, nanocarrier, synergism
Introduction
Over the last few decades, the application of nanotechnology in the field of medicine, including drug delivery, biomedical imaging, and diagnostics, has received widespread attention.1 While the definition of nanoscale differs between the diverse research disciplines, with the physical chemists mostly supporting the notion that nanomaterials should have at least one dimension <100 nm,2 pharmacists often tend to follow a more inclusive definition, accepting a scale of <1 μm as an adequate criterion.3 With advancements in materials science and emergence of novel materials, nanomaterials have also evolved into a range of advanced prototypes with plenty of hype and hope associated with them.4
One of the key hypotheses supporting nanomedicine research is the ability of nanomaterials to access those sites in the human body that are otherwise unreachable by larger (micro)particles.5 Adding to the enthusiasm is the current mastery over synthetic protocols enabling preparation of well-characterized nanomaterials with tunable properties tailored to desirable attributes. Moreover, due to a restricted 3D extent contributing to the quantum confinement effect,6 nanomaterials demonstrate unprecedented materialistic properties, including magnetism, conductivity, and fluorescence.7 The research community has engaged in exploring such uncommon behavior of nanomaterials to serve medicine.
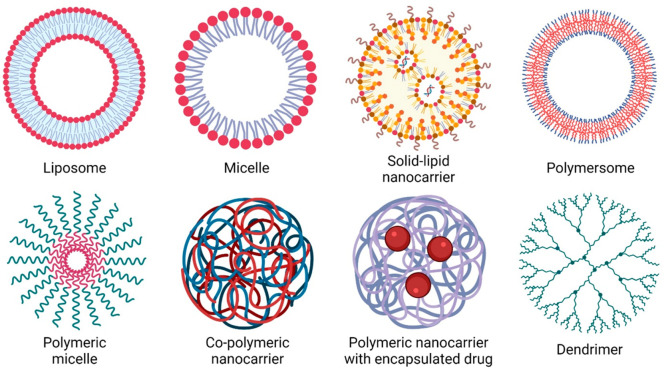
Increasing sophistication in material synthesis, particularly in polymer science,8 has enabled researchers to encapsulate a diverse set of biomacromolecules for theranostic purposes.9 An encapsulated nanoconstruct typically harbors a core–shell architecture where a protective shell is layered surrounding a core often composed of a condensed mass of drug molecules.10 However, especially in liposomal formulations, the spread of an encapsulated agent may be more homogeneous,11 lacking a core–shell partitioning. Such nanomaterials with unique structures and chemistry provide a fertile ground for further exploration in the field of encapsulation (Figure 1).
Figure 1.
Scheme showing the various nanocarriers that have been employed in the encapsulation of theranostic agents for facilitated delivery purposes.
This discourse will define a co-encapsulated nanocarrier as a chemical species where multiple biomacromolecules, from simple molecules to larger peptides, are confined within a nanocarrier, typically with a core–shell construct. Thus, molecular linkages related to the exterior of nanocarriers, achieved through bioconjugation12 and surface adsorption13 of therapeutic molecules, will be excluded. Porous nanocarriers, such as mesoporous silica,14 where the pores can be loaded with different therapeutic agents for drug delivery, will not be discussed. Furthermore, this article will only review co-encapsulated nanocarriers and will distance itself from other means of codelivery, for example, using a mix of liposomes with individual particles carrying separate molecules or nanocarriers with a single encapsulated molecule while another one is conjugated to the surface. The narrative will provide an appreciation of the reasons for co-encapsulation, with its widespread reporting in overcoming resistance under biological settings, such as cancer chemotherapy15 and infectious diseases,16 revisit some of the leading examples of co-encapsulation from recent literature, and identify the associated challenges before prioritizing some future perspectives as guidance for upcoming research.
Merits of Co-encapsulation in Nanocarriers
The reasons driving the co-encapsulation of more than one theranostic molecule can be varied. Perhaps the most important one is the emergence of resistance against conventional therapeutics in cancer cells and microorganisms.17 While multidrug resistance (MDR) is often orchestrated through mechanisms such as eviction of drugs from cancer cells by efflux pumps, i.e., P-glycoprotein (P-gp) and breast cancer resistance protein;18 facilitated DNA repair;19 resistance against drug uptake;20 inadequate cellular concentration of therapeutic agents;21 altered drug targets and apoptotic pathways, e.g., due to the expression of antiapoptotic proteins like B-cell lymphoma-2;22 and sequestration of weakly alkaline chemotherapeutic agents into highly acidic lysosomes23 to cause degradation, the current incidence of MDR in cancer cells (Figure 2) or microorganisms (Figure 3) poses a challenge in healthcare with a significant toll of human suffering and financial burden. A detailed discussion of the mechanisms of MDR in cancer cells or infectious diseases is beyond the scope of this account, although relevant literature is cited.24
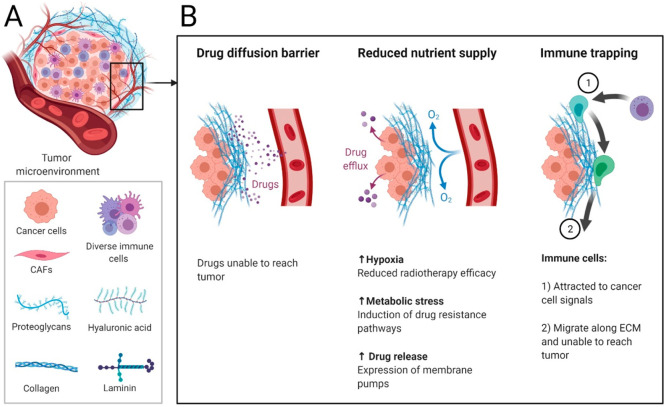
Figure 2.
(A) Tumor microenvironment is rich in various cells (e.g., cancer cells, cancer-associated fibroblasts, and immune cells); deposits of proteoglycans, hyaluronic acid, collagen, and laminin as an extracellular matrix (ECM); and exhibits augmented angiogenesis. (B) Three salient mechanisms of drug resistance exhibited by the tumor microenvironment: (i) presenting a diffusion barrier against the intratumoral spread of anticancer agents; (ii) curtailing the supply of oxygen and nutrients to the cancer cells that switches on the cellular resistance pathways; and (iii) alleviating the impact of radiotherapy and the immune trapping mechanism where the immune cells, albeit responding to the signaling mechanisms of cancer cells, migrate along the ECM boundary and, thus, fail to permeate the tumor.
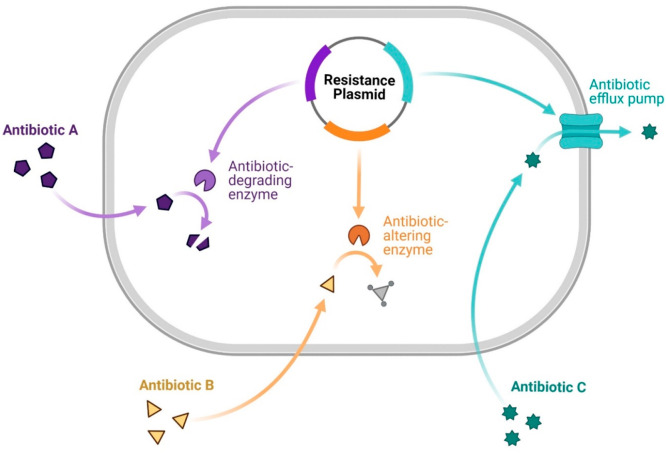
Figure 3.
Scheme showing the genetic pathways of antibiotic resistance in microorganisms after internalization: degradation by enzymes, enzymatic molecular alteration of the antibiotic rendering them ineffective, and expulsion from the cells with the help of efflux pumps.
The approach of a combination therapy of chemotherapeutic, immunotherapeutic, and genetic agents25 has delivered positive outcomes in clinical settings and is a standard line of management in resistant cancer cases and microbial strains. Co-encapsulation in nanocarriers envisages achieving a synergy of multiple drugs as it offers an advantage over the conventional method of coadministering a predefined regime of oncotherapeutic or antimicrobial agents, often mixed in a syringe or vial and administered intravenously, in the following ways:
(i) The nanocarrier provides a protective cloak around the payload of drug molecules and alleviates the risks of denaturation or disintegration under harsh physiological conditions, such as an acidic gastric pH encountered in oral delivery.26 Many popular cancer chemotherapeutic agents or antimicrobial molecules are sensitive to subtle pH fluctuations or are labile toward enzymatic digestion, and encapsulating these molecules in a nanocarrier preserves molecular integrity.
(ii) Solubility remains a challenge with many chemotherapeutic agents, while emerging data suggest that more than half of the developed molecules in current industrial practices are discarded due to inadequate solubility.27 Hydrophobic molecules often require viscous organic dissolution before intravenous administration with known untoward effects, such as embolism, hypersensitivity, and pain at the injection site.28 The drawbacks are further compounded when a regime of drugs is administered instead of one, and co-encapsulation within nanocarriers can offer a remedy to the issue. When administered as a well-dispersed preparation, presumably via an intravenous route, the hydrophobic drug molecules are shielded by the nanocarrier from an aqueous and ion-rich hematic exterior to prevent agglomeration, precipitation, or denaturation.29
(iii) Targeting pathologic tissues for tunable and controlled drug delivery is possible with nanocarriers with exciting prospects for co-encapsulation.30 Delivering multiple drugs simultaneously in a targeted manner alleviates the risk of developing resistance (e.g., cancer tissues) and curtails the systemic toxicity due to dose reduction.31 The craft of targeting diseased sites with surface-engineered nanocarriers has improved considerably over the last couple of decades. A thorough discussion on such site-specific targeting falls beyond the scope of this review, although relevant literature is cited.32 Such targeted nanoformulations (Figure 4) mostly rely on intelligent surface engineering, either by bioconjugation or surface adsorption, with ligands that act as substrates for overexpressed cellular receptors.33 These nanocarriers are often grafted with hydrophilic molecules, such as polyethylene glycol (PEG), to prepare stealth nanocarriers34 that evade macrophagic filtration, resulting in rapid clearance from the bloodstream after intravenous injection. A range of biochemical features in target sites, such as an acidic pH and hypoxemia in the tumor microenvironment (TME), is exploited to design such nanoformulations.35 Significant progress achieved in polymeric engineering has further catalyzed interest in the field. Inorganic materials (e.g., silica) are also being prioritized.36
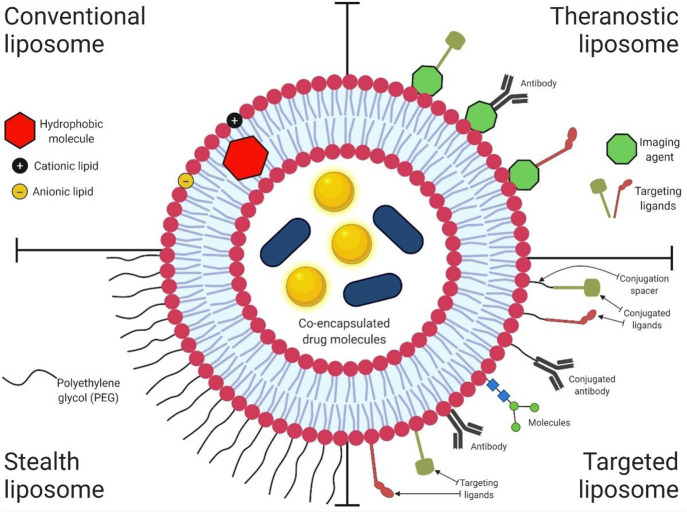
Figure 4.
Scheme showing a liposomal nanocarrier with co-encapsulated theranostic agents in its core and lipid bilayer. The four quadrants depict the typical structures noted in conventional, therapeutic, stealth, and targeted liposomes along with a range of surface-conjugated ligands.
(iv) Co-delivery of multiple drugs (cocktail therapy) may yield a synergistic effect (Figure 5) in MDR cases.37 Multiple therapeutic agents acting in synergy exert maximum lethality toward the population of target cells while reducing the probability of cells escaping the wrath of chemotherapeutic agents and act as seeds for future resurgence.38
Figure 5.
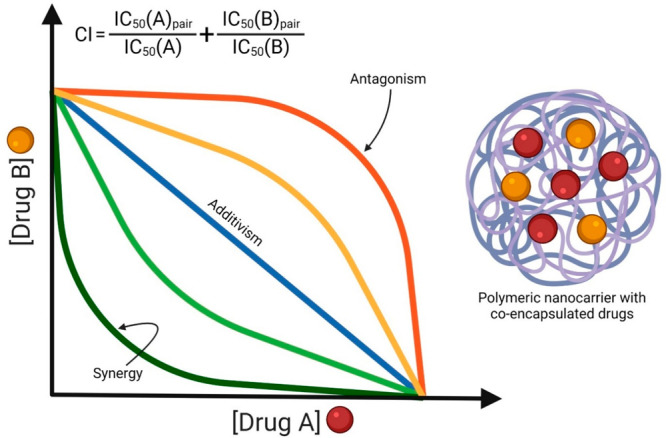
Isobole showing the various drug interactions in a polymeric nanocarrier with co-encapsulated drugs “A” and “B” expressed as a combination index (CI) and calculated from an equation bearing the half-maximal inhibitor concentrations (IC50) of individual drugs. CI values of <1, 1, and >1 represent synergism, additivity, and antagonism, respectively.
(v) Encapsulation of drug molecules in nanocarriers leaves enough room for improvisation and innovation. For example, imaging agents may be included instead of drug molecules, while such a combined delivery demonstrates a step toward advanced theranostic modalities.39 Furthermore, co-encapsulation can be a way to coadminister trigger agents for stimuli-responsive nanoformulations, including magnetosensitive,40 thermosensitive,41 and sonosensitive42 ones.
Challenges Associated with Co-encapsulation in Nanocarriers
Despite an established therapeutic advantage exhibited by co-encapsulated nanoformulations over monotherapy, the translational success with such formulations has been less than encouraging. While the initial data look promising, most of these co-encapsulated nanoformulations fail to withstand the rigor of clinical trials and hardly progress beyond phase II. Except for Vyxeos,43 a co-encapsulated liposomal formulation of daunorubicin and cytarabine indicated in therapy- or myelodysplasia-related acute myeloid leukemia, so far no other co-encapsulated nanoformulation has gained approval. The reasons behind a high attrition rate of nanoformulations lie either with the co-encapsulated nanocarriers or the generic disadvantages of using them as drug-delivery systems (DDSs).
Challenges Associated with Co-encapsulated Nanocarriers
It remains a synthetic challenge to prepare colloidally stable successive batches of co-encapsulated nanocarriers with adequate reproducibility.44 It is tedious to exercise granular control while preparing nanoscale materials. The challenge increases further when the structural details of a nanocarrier become more complex, for example, due to the addition of extra layers, compartments, surface conjugation of biomolecules, and co-encapsulation of multiple drugs.45 Many anticancer or antimicrobial agents suffer from solubility issues and are not easy to encapsulate.
It is not facile to achieve synergism while codelivering agents via nanocarriers. Stoichiometric considerations with precise dosimetry are important for synergism,46 and while such a combination is easy to formulate in a vial, it is a difficult task while co-encapsulating them in nanocarriers. As a process, co-encapsulation has its own ratios that do not often align with the ones required for synergism. Striking an optimal balance between such dosimetric constraints is cumbersome, while trial and error seem to be the only feasible option. Thus, co-encapsulated nanoformulations often fail to repeat their potential during clinical trials. Some modeling studies based on Loewe additivity and Bliss independence to predict synergism47 have provided crucial insights, although these tools need refinement before predicting synergy in a co-encapsulated nanocarrier.
Not all drug pairs exhibit synergy or demonstrate a preference for co-encapsulation. While forming a core inside nanocarriers, the drug molecules are confined within a constrained space, and such spatial proximity is known to trigger a wide array of interactions, including the formation of hydrogen bonds, van der Waals forces, and hydrophobic interactions.48 With maturation, these interactions alter the biochemical and molecular attributes of encapsulated drugs, while it is almost impossible to track or predict these changes. Fluctuations in the biochemistry of the core in a nanocarrier, including localized aggregation, precipitation, and disintegration,49 impact the release kinetics or dosing often in an untoward way.
There is hardly any modeling data reported on the release of co-encapsulated agents, unlike for the popular models for the release of encapsulated single drug molecules, such as the Higuchi,50 Ritger–Peppas,51 and Korsmeyer–Peppas52 models; unfortunately, there is a void in the field of co-encapsulated formulations. Some drug pairs, when codelivered, are known to demonstrate enhanced toxicity with a narrow therapeutic window.53 It can be particularly harmful in anticancer drugs where systemic toxicity is high and a further increase in toxicity is undesirable.
Such intra- or intermolecular interactions and rearrangements may cause an ionic imbalance inside the nanocarrier, affecting colloidal stability. Lyophilization of the formulations into powder form can be a way to address the issue of compromised stability, although it comes with the caveat of reconstituting into an injectable form, which remains a challenge in the absence of surfactants.54 The surfactants categorized as Generally Regarded As Safe (GRAS) entities provide a limited choice for pharmacists. Furthermore, working with surfactants changes the composition of the formulation and may compromise biocompatibility.55
Challenges Associated with Nanocarriers As DDSs
The challenges of the current nano-DDSs, especially from a translational perspective, have been reviewed thoroughly. In a nutshell, the following points emerge:
(i) Synthesis of nanocarriers with an encapsulated drug is known for irreproducibility and batchwise variation, including alterations of surface charge and particle size, which are both known to influence the behavior of nanocarriers at a biological interface.56,57 The pharmacokinetic and pharmacodynamic profiles of such nanoformulations, including release, are prone to fluctuations, at times remarkably, and can be difficult to contain.
(ii) Nanocarriers are quickly filtered out of the bloodstream after parenteral administration by the reticuloendothelial system (RES).58 The mechanism(s) that govern the triggering of RES are not well-understood. Rapid adsorption of serum opsonins on the nanocarriers59 promotes macrophagic phagocytosis, and a fast sequestration of the injected dose into the liver, spleen, bone marrow, and lungs ensues. Such filtration of nanocarriers from blood reduces its bioavailability. Thus, the injected nanoformulations fail to acquire a therapeutic concentration at the target sites, resulting in an undermined efficacy. Moreover, unrestrained phagocytosis by the macrophages may impair their role as an immune defense mechanism, leaving the host in an immunocompromised state.60 Surface grafting of hydrophilic aliphatic polymers, such as PEG, impedes opsonization and extends the plasma t1/2. However, PEGylation is not easy to achieve and may require harsh reaction conditions that are unsuitable for the nanocarriers.61 Moreover, it adds an extra layer of structural complexity, is known to produce IgM antibodies,62 induces macrophagic phagocytosis, and may interfere with release. Alternatives to PEG as molecules of choice for hydrophilic coating, such as poly(glycerols), poly(oxazolines), poly(hydroxypropyl methacrylate), poly(2-hydroxyethyl methacrylate), poly(N-(2-hydroxypropyl)methacrylamide), poly(vinylpyrrolidone), poly(N,N-dimethyl acrylamide), and poly(N-acryloylmorpholine) are currently under investigation.63
(iii) Despite the theoretical potential, targeting cancer tissues with nanocarriers, both in active and passive ways, has not yielded encouraging results. Active targeting relies on engaging various overexpressed receptors on target tissues, for example, folate receptors in tumors.64 The strategy is to graft a ligand on the nanocarrier to bind overexpressed receptors and induce receptor-mediated cellular uptake.65 On the contrary, passive targeting is due to leaky vasculature in tumors that facilitate leaching out of the nanocarriers into the tumor parenchyma causing an intratumoral accumulation, also described as the enhanced permeability and retention (EPR) effect.66,67 Due to an unrestricted growth, the demand for oxygen and nutrients in a tumor tissue remains high, which, in turn, stimulates rapid angiogenesis under the influence of a gamut of angiogenic factors, such as the vascular endothelial growth factor. Such brisk angiogenesis often results in defective vasculature with larger fenestrations on their walls, which drives the EPR effect. One of the major reasons behind disappointing outcomes with active targeting is the masking of the nanocarrier surface groups, carefully decorated with ligands, with a range of proteins and other biomacromolecules present in blood by surface adsorption.68 Thus, the surface chemistry and hydrodynamic diameters of the nanocarriers keep evolving after mixing with the blood. As a result, the targeting mechanism is either lost or markedly reduced. Moreover, blood flows fast in the vascular tree, more in larger vessels like the aorta than in venules and capillaries, allowing little time or spatial proximity between the ligands and receptors to interact.69 In passive targeting, EPR remains controversial and, to an extent, a misunderstood topic. There is no denying that the vascular walls inside tumors have larger intracellular fenestrations—a hallmark of rapid angiogenesis to cater an increased demand for oxygen and nutrients by a tissue experiencing unchecked growth—that provide leeway for intravascular components, including particulates, to exude into the extravascular space.70,71 Thus, the thesis supporting a raised intratumoral concentration of injected nanocarriers with an anticipated therapeutic advantage makes sense. However, the EPR effect is more complex and unpredictable,72 while the nature of tumor vasculature largely controls it. Not all tumors exhibit EPR to the same degree: while highly vascularized carcinomas demonstrate adequate EPR, relatively less vascularized soft tissue sarcomas do not show enough of it.73 Similarly, murine tumor models exhibit higher EPR than those in larger animals, with negligible impact noted in humans.74,75 Moreover, often >90% of the injected nanocarriers is filtered by the RES, leaving only a small fraction (<5%) to reach the target sites, which, despite EPR, is insufficient for a therapeutic impact.34
Introduction to Vyxeos
Vyxeos (previously CPX-351; Jazz Pharmaceuticals, Ireland) is a liposomal formulation (distearoylphosphatidylcholine, distearoylphosphatidylglycerol, and cholesterol at a 7:2:1 molar ratio) of cytarabine and daunorubicin, two water-soluble anticancer drugs, encapsulated at a molar ratio of 5:1.43 Other excipients include copper gluconate, sucrose, and trolamine. The liposomal particles are ∼100 nm in diameter with a zeta potential of −30 mV.76 Approved by the U.S. FDA in 2017 and EMA in 2018, it is indicated in therapy- or myelodysplasia-related acute myeloid leukemia. Each vial contains 44 mg of daunorubicin and 100 mg of cytarabine as a lyophilized cake that, upon reconstitution in 0.9% saline, gives 2.2 mg of daunorubicin and 5 mg of cytarabine per mL of infusate. The recommended dosing for the first induction is daunorubicin 44 mg/m2 body surface area (BSA) and cytarabine 100 mg/m2 BSA on days 1, 3, and 5; for the second induction, the dosing is daunorubicin 44 mg/m2 BSA and cytarabine 100 mg/m2 BSA on days 1 and 3; and for the consolidation, the dosing is daunorubicin 29 mg/m2 BSA and cytarabine 65 mg/m2 BSA on days 1 and 3. Reconstituted vials can be stored up to 4 h at 2–8 °C.
The commonly encountered (>10%) side-effects are myelosuppression, hemorrhage, neutropenia, cardiotoxicity, hypersensitivity, an overdose of copper, edema, rash, nausea, diarrhea, colitis, abdominal discomfort, cough, headache, bacteremia, and chills with a febrile condition; less common side-effects are deafness, conjunctivitis, xerophthalmia, periorbital edema, dyspepsia, hallucination, and pneumonitis.77 Vyxeos should not be used with other cardiotoxic (e.g., doxorubicin) or hepatotoxic agents, and a close monitoring of cardiac, hepatic, and renal functions is required. In the case of serious cardiotoxicity or hypersensitivity reactions, the infusion may have to be suspended. It is contraindicated in pregnancy based on animal experiments while human trial data are awaited.
A phase III, multicenter, randomized, open-labeled trial (CLTR0310-301) with Vyxeos (daunorubicin 44 mg/m2 BSA and cytarabine 100 mg/m2 BSA) infused over 90 min on days 1, 3, and 5 demonstrated a mean (coefficient of variation) maximum plasma concentration of 26.0 μg/mL for daunorubicin and 62.2 μg/mL for cytarabine, while the mean (coefficient of variation) areas under the curve were 637 μg·h/mL for daunorubicin and 1 900 μg·h/mL for cytarabine (day 5). The volumes of distribution for daunorubicin and cytarabine were 6.6 and 7.1 L, respectively.78
The plasma t1/2 of daunorubicin and cytarabine were 31.5 h and 40.4 h, respectively. Upon intravenous administration, >99% of the daunorubicin and cytarabine remained encapsulated, while the liposomes rapidly accumulated in bone marrow for internalization by leukemic cells. The renal clearances were estimated to be 0.16 L/h and 0.13 L/h for daunorubicin and cytarabine.79 Urinary excretion accounted for 9% of the administered dose for daunorubicin and 71% for cytarabine (along with its inactive metabolite 1-β-d-arabinofuranosyluracil).
Nanocarriers with Co-encapsulated Anticancer Drugs
Liposomes
Liposomes are spherical vesicles (100–200 nm) surrounded by a lipid bilayer and have emerged as a successful breed of lipid nanocarrier (LNC) for drug-delivery purposes.80,81 The bilayer is typically composed of phospholipids, such as phosphatidylcholine (PhC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), and cholesterol.82 As a nanoconstruct, liposomes may encapsulate hydrophilic molecules at their core and hydrophobic molecules (which includes most of the anticancer drugs) into the bilayer, thus expanding the landscape of encapsulable molecules.
Liposomal formulations have emerged as a popular platform for drug delivery due to their superior bioavailability and biocompatibility. The synthetic techniques include thin-film hydration, solvent and reverse-phase evaporation, membrane extrusion, probe ultrasonication, hot and high-pressure homogenization, spray-drying, and ether injection.83,84 With advancements in synthetic techniques, better control over their size and surface characteristics can be exercised now with the preparation of trigger-release formulations.
Some liposomal formulations are surface-functionalized with hydrophilic molecules (e.g., Doxil,85,86 a PEGylated liposomal formulation of doxorubicin) to evade macrophagic phagocytosis and extend the circulation time. Approved liposomal formulations of anticancer drugs other than Doxil, such as DaunoXome (daunorubicin) and DepoCyt (cytarabine), enjoy a decent market share,87 while Vyxeos—the only approved co-encapsulated nanoformulation—is also liposomal. Liposomes have been implicated in both active and passive targeting of cancer tissues with an appreciable EPR effect.88
Co-encapsulation of multiple anticancer drugs, for example, anthracycline derivatives (e.g., doxorubicin) and taxanes (e.g., paclitaxel and docetaxel), to achieve synergism is a common practice.89 Other anticancer agents, such as verapamil, a P-gp efflux pump inhibitor used to reverse MDR in cancer cells, and platinum-bearing drugs (e.g., carboplatin and cisplatin) or paclitaxel,90 are also co-encapsulated into liposomal formulations.
Instead of multiple anticancer agents, a combination of anticancer drugs and biomacromolecules can be chosen for co-encapsulation into liposomes. For example, genetic materials, such as DNA, small interfering RNA (siRNA), interleukins (ILs), and plasmid DNA (pDNA), have been co-encapsulated with anticancer agents.91 Typically, the peptides, proteins, or genetic materials remain encapsulated within the hydrophilic cores of the liposomes. It is worth noting here that cationic liposomes were prioritized for the encapsulation of anionic nucleotides. The cationic charge provides stability to the anionic core via electrostatic interactions and prevents enzymatic degradation.
Some encapsulated liposomal formulations were modified with antiangiogenic molecules to target the overexpressed αvβ3 integrin receptors in the flourishing but defective neovasculature in TME. For example, liposomes grafted with Arg-Gly-Asp (RGD) peptides were used to target tumor cells via binding with αvβ3 receptors.92,93 Similarly, liposomes modified with an Asp-Gly-Arg (NGR) motif could target the CD13/aminopeptidase N (APN) receptor isoforms that are overexpressed in the TME.94 Furthermore, PEGylation on co-encapsulated liposomes has also been reported.
Other than delivering anticancer drugs, liposomal formulations were used to co-encapsulate therapeutic agents for molecular targeting of cancer cells, for example, in nonsmall cell lung cancer (NSCLC), where tyrosine kinase inhibitor (TKI) molecules (e.g., alectinib, crizotinib, ceritinib, brigatinib, and lorlatinib) have gained popularity.95 Gefitinib was the first approved TKI to target epidermal growth factor receptor (EGFR) and is used in NSCLC patients with EGFR mutations.96 However, almost half of the treated patients eventually develop resistance.
A liposomal formulation prepared by thin-film hydration with co-encapsulated gefitinib and vorinostat,97 a histone deacetylase inhibitor, at a ratio of 1 to 0.12 (w/w) reversed the resistance demonstrated by the tumor-associated macrophages (TAMs) against gefitinib by a combination of repolarization of the protumor M2 macrophagic (Φ) phenotype to antitumor M1Φ and degradation of the T790 M mutation of EGFR (EGFRT790M). Another liposomal formulation (156 nm) of co-encapsulated simvastatin and gefitinib modified with anti-PD-L1 nanobody repolarized the M2Φ to M1Φ and reversed the gefitinib resistance.98 Some examples of co-encapsulation of anticancer drugs into liposomes are cited in Table 1.
Table 1. Some Examples of Co-encapsulation of Anticancer Agents in Liposomesa.
| co-encapsulated anticancer agents | composition of liposome | size (nm) | surface properties | target tissue | status | ref |
|---|---|---|---|---|---|---|
| DOX, MLP | HSPhC, mPEG2000-DSPhE, cholesterol, MLP conjugate | 110–130 | PEG2000ylated | breast cancer | in vitro, in vivo | (115) |
| DOX, RAN-IP | DPPG, DOPE, cholesterol | 139 ± 21 | unconjugated | breast cancer | in vitro, in vivo | (116) |
| cisplatin, mifepristone | HSPhC, m-PEG2000-DSPhE, cholesterol | 109 ± 5.4 | PEG2000ylated | cervical cancer | in vitro, in vivo | (117) |
| DOX, CUR | cholesterol, egg lecithin | 100–140 | Tuftsin-conjugated | cervical cancer | in vitro, in vivo | (118) |
| cisplatin, CUR | DPPC | 100 | unconjugated | breast cancer | in vitro | (119) |
| DOX, itraconazole | soy PhC, cholesterol | 133 | pluronic P123-coated | breast cancer | in vitro, in vivo | (120) |
| DOX, MiR-101 (tumor suppressor micro-RNA) | DOTAP, mPEG2000-DSPhE, PEG-bisamine | 160 | unconjugated | hepatocellular carcinoma | in vitro, in vivo | (121) |
| PTX, resveratrol | PhC, mPEG2000-DSPhE | 50 | PEG2000ylated | breast cancer | in vitro, in vivo | (122) |
| DOX, Bmi1 siRNA | DOTAP, mPEG2000-DSPhE, PEG-bisamine | 130 | folate-conjugated | breast cancer | in vitro, in vivo | (123) |
| DOX, SATB1 shRNA | cholesterol, DPPC, DC-Chol (thermosensitive) | 238.16 ± 20.6 | unconjugated | gastric cancer | in vitro, in vivo | (124) |
| DOX, irinotecan | DSPC, cholesterol | 100 | unconjugated | ovarian cancer | in vitro, in vivo | (125) |
Abbreviations: Ald, alendronate; CUR, curcumin; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)carbamoyl]cholesterol; DOX, doxorubicin; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine; DOPG, 1,2-dioleoyl-sn-glycero-3-phospho-(19-rac-glycerol); DOTAP, 1,2-dioleoyl-3-trimethylammonium propane; DPPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; DPPG, 1,2-dipalmitoyl-sn-glycerol-3-phosphate-rac-(1-glycerol); DSPC, distearoyl-sn-glycero-3-phosphocholine; HSPhC, hydrogenated soybean phosphatidylcholine; mPEG2000-DSPhE, methoxypolyethylene glycol2000-distearoylphosphatidylethanolamine; MLP, mitomycin-C lipidic prodrug; MPB-PE, maleimide-headgroup lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; PEG, polyethylene glycol; PhC, phosphatidylcholine; PTX, paclitaxel; RAN-IP, Ran-RCC1 inhibitory peptide; shRNA, small hairpin RNA; siRNA, small interfering RNA; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Polymeric Nanocarriers
With advancements in polymer engineering, many polymeric nanocarriers (PNCs) have been developed as DDSs.99 While many of these PNCs are composed of amphiphilic block copolymers, often with PEGylation to impart stealth characteristics, natural and biodegradable polymers like dextran,100 and chitosan101 are emerging fast. In aqueous dispersions, the amphiphilic polymers, such as PEGylated poly(lactic acid) (PEG–PLA), PEGylated poly(lactic-co-glycolic acid) or PEG–PLGA, and PEGylated DSPE (PEG–DSPE), form a core–shell PNC with opportunities for co-encapsulation.102
Preparing PNCs is relatively facile compared to the liposomes, while the terminal groups present exciting opportunities for bioconjugation, for example, to peptides,103 folic acid,104 or trastuzumab,105 to target human epidermal growth factor receptors for homing of the PNCs into the tumors. In addition, the PNCs can be functionalized with pH-sensitive cleavable linkages (e.g., hydrazone bond) that, upon sensing an acidic environment inside the tumor parenchyma, will trigger disintegration of the PNC and release the co-encapsulated drugs.106 Furthermore, PNCs with different particle sizes and surface chemistry can be prepared with subtle adjustments in reaction conditions. Three categories of PNCs have been used so far as DDSs.
(i) Polymeric micelles: These are prepared by aggregating self-assembling amphiphilic copolymers in aqueous dispersions at a higher concentration than the critical micellar concentration.107 They enjoy a higher loading compared to liposomes, while the core can be used for co-encapsulating theranostic agents. The surfaces can be functionalized with various ligands for targeted delivery. A PEGylated micellar formulation of paclitaxel (Genexol PM; particle size 23.0 ± 4.5 nm, zeta potential −8.1 ± 3.1 mV), indicated in breast cancer, NSCLC, and ovarian cancer,108 received approval in South Korea in 2007. The amphiphilic polymer used was a low molecular weight diblock copolymer monomethoxy poly(ethylene glycol)-block-poly(d,l-lactide).
(ii) Polymeric nanoparticles (PNPs): Amphiphilic copolymers with relatively smaller hydrophilic and longer hydrophobic blocks tend to form more solid particulate colloidal dispersions in the form of PNPs.109 Here, the core is a dense matrix of polymeric chains where encapsulable molecules remain entangled, dissolved, or conjugated. The size, stability, and physicochemical properties of the PNPs can be tuned by varying the reaction conditions, including temperature, ionic strength, and pH. Compared to the liposomes and polymeric micelles, PNPs are more stable and offer higher loading.
(iii) Polymersomes: These are artificial vesicles (50 nm–5 μm) prepared by self-assembly of amphiphilic copolymers.110 Like liposomes, polymersomes are also surrounded by a bilayer, although, unlike liposomes, the bilayer is polymeric. Moreover, compared to liposomes, they are colloidally more stable, have thicker shells, and are less immunogenic.111,112 The polymersome cores are often aqueous and may encapsulate a range of biomacromolecules, including genetic materials, therapeutics, enzymes, proteins, and peptides. The polymeric bilayer is more flexible than liposomes and allows selective permeation of hydrophilic and hydrophobic molecules with opportunities for functionalization to achieve targeted delivery. Polymersomes were also successfully coated with hydrophilic membranes to prolong their circulation.113 They are also known to be less immunogenic than liposomes.114
Both drug–drug and drug–genetic material combinations were co-encapsulated in PNCs. An effective strategy toward encapsulation in PNCs is to conjugate the drug(s) with the polymer backbone. As a result, the drug molecules are retained within the cores as the copolymeric chains fold during self-assembly. A combination of hydrophilic doxorubicin and hydrophobic paclitaxel is an example of co-encapsulable anticancer agents for PNCs,126 while other anticancer drug combinations (Table 2) are reported as well. Notable co-encapsulated agents in PNCs include efflux pump inhibitors,127 siRNA,128 and microRNA.129 Combination therapy with co-encapsulated anticancer agents in PNCs has overall produced encouraging results with increased lethality toward cancer cells, alleviated toxicity, and, in certain instances, reversal of MDR. A broad range of co-encapsulated PNCs is currently going through various phases of clinical trials.
Table 2. Some Examples of Co-encapsulation of Anticancer Agents in PNCs.
| co-encapsulated anticancer agents | composition of PNC | size (nm) | surface properties | target tissue | status | ref |
|---|---|---|---|---|---|---|
| DOX, epoxomicin | PLGA | 162.1–179.6 | unconjugated | breast cancer | in vitro | (139) |
| PTX, lapatinib | PLA–PEG | 100 (filomicelles), 20 (spherical micelles) | PEG5000ylated | breast cancer | in vitro | (140) |
| gefitinib, vorinostat | hyaluronan, PBLG | 30 | unconjugated | lung cancer | in vitro, in vivo | (141) |
| DOX, anti-BCL-2 siRNA | PEG–PLL–PAsp(DIP) (pH-sensitive) | 60 | PEGylated | hepatic carcinoma | in vitro, in vivo | (142) |
| DOX, DTX | mPEG–PCL (redox-sensitive) | 223.7 | mPEG2000ylated | breast cancer | in vitro | (143) |
| DOX, IFN-γ | PLGA, Pluronic F127 | 100 | PEO-conjugated | melanoma | in vitro, in vivo | (144) |
| DOX, recombinant human IL-2 | trimethyl chitosan (pH-sensitive) | 200 | folate-conjugated | hepatic carcinoma | in vitro, in vivo | (145) |
| DOX, miRNA-34a (tumor suppressor micro-RNA) | PEG2000-CLV (MMP2-sensitive) | 15 | PEG2000ylated | fibrosarcoma | in vitro | (146) |
| DOX, P-gp siRNA | FA/m-PEG-b-P(LG-Hyd)-b-PDMAPMA | 196.8 | folate-conjugated | breast cancer | in vitro | (147) |
| TMX, quercetin | PLGA | 185.3 ± 1.20 | unconjugated | breast cancer, colon cancer | in vitro, in vivo | (148) |
Dendrimers
These are monodisperse, symmetrical, and artificial macromolecules that can be synthesized with high precision and predefined geometry, size, molecular weight, and surface properties.130,131 Dendrimers are <100 nm in size and typically demonstrate arboreal branching patterns stacked as layers that determine their generation, denoted as G.132 A high surface charge density in dendrimers favors bioconjugation to therapeutic molecules that make them conducive for targeting.133 The cores of lower generation (G1–G3) dendrimers are more accessible and can be used for co-encapsulation of hydrophobic drugs.
The cationic G5 polyamidoamine (PAMAM) dendrimers have emerged as popular DDSs for co-encapsulation and have demonstrated an appreciable EPR effect in tumors.134,135 Moreover, the cationic charge facilitates cellular uptake,136 with PEGylation as a viable option.137 Hydrophobic and electrostatic interactions usually govern drug loading in dendrimers. An example of co-encapsulation in dendrimers is the combination of paclitaxel and alendronate (Ald), a bisphosphonate indicated in osteoporosis and metastatic bone tumors.138 Other such combinations are also known (Table 3).
Table 3. Some Examples of Co-encapsulation of Anticancer Agents in Dendrimersa.
| co-encapsulated anticancer agents | composition of dendrimer | size (nm) | surface properties | target tissue | status | ref |
|---|---|---|---|---|---|---|
| DOX, siMDR-1 (siRNA) | PAMAM | 219 | PEG2000-DOPE conjugated | ovarian cancer, breast cancer | in vitro | (163) |
| PTX, siRNA | PAMAM | 145.6 | PEG4000ylated | melanoma fibrosarcoma | in vitro, in vivo | (164) |
| PTX, Ald | dendritic PEG | 200 | PEGylated | bone cancer | in vitro, in vivo | (138) |
| DOX, siRNA | polylysine | 55–128 | PEG2000-RGD conjugated | glioblastoma multiforme | in vitro | (165) |
Abbreviations: Ald, alendronate; DOX, doxorubicin; PAMAM, polyamidoamine; PEG, polyethylene glycol; PTX, paclitaxel; siRNA, small interfering RNA.
Co-encapsulated Nanocarriers for Photothermal Therapy
Inducing localized hyperthermia (46–60 °C) to scorch the cancer cells is an emerging field in nanomedicine.149 A raised temperature eliminates the cancer cells and improves the permeability of nanocarriers in tumors, resulting in a stimulated uptake of larger nanocarriers (>400 nm) with higher loading.150 Co-delivery of chemotherapy with hyperthermia therefore augments the lethality toward cancer cells. A wide range of metallic NPs (e.g., iron oxide, gold, cobalt, nickel, and manganese) and fullerenes (e.g., carbon nanotubes) have been used for such cancer tissue ablation.151,152 Iron (FeNPs) and gold (GNPs) NPs are prioritized over other metallic particles due to their superior biocompatibility.153 Indocyanine dyes (e.g., IR825) that absorb near-infrared light have also been used for photothermal therapy (PTT).154 Other PTT agents under investigation are diketopyrrolopyrrole-based polymer155 and polydopamine.156 These agents radiate heat when exposed to energy-bearing stimuli, including ultrasonic waves, radiowaves, near-infrared light, laser, and microwaves. However, solubility and stability remain an issue with metallic NPs, including FeNPs, and require further surface passivation with hydrophilic molecules, such as polymers, dendrimers, and lipids.157,158
Liposomes and polymeric micelles currently lead the repertoire of nanocarriers that have been used to co-encapsulate chemotherapeutic and PTT agents. Intriguingly, some metallic NPs, such as superparamagnetic iron oxide NPs (SPIONs), are excellent contrast agents for magnetic resonance imaging.159,160 Thus, a codelivery of these NPs with chemotherapeutic agents adds an extra modality of tumor imaging with MRI to chemotherapy, and PTT. Thermosensitive liposomes are particularly exciting from this perspective as a subtle increase in temperature (39–42 °C) also increases their permeability and facilitates the release of an encapsulated drug payload. In the case of thermosensitive PNCs, polymers like poly(N-isopropylacrylamide) (PNIPAAm) are popular choices.161,162 A range of anticancer drugs, such as doxorubicin and paclitaxel, have been co-encapsulated with PTT agents in nanocarriers (Table 4). Many such co-encapsulated nanocarriers were surface-functionalized with PEG to impart stealth attributes or ligands to target overexpressed receptors at tumor sites.
Table 4. Some Examples of Co-encapsulation of Anticancer and PTT Agents in Nanocarriersa.
| co-encapsulated anticancer agents | composition of NC | size (nm) | surface properties | target tissue | status | ref |
|---|---|---|---|---|---|---|
| DOX, gold-coated iron oxide NP | PSMA | 206 | unconjugated | colon cancer | in vitro, in vivo | (166) |
| CPT, PTT | molybdenum oxide hollow nanosphere | 142 | PEG4000ylated | cervical cancer, breast cancer, pancreatic cancer | in vitro, in vivo | (167) |
| artemisinin, Prussian blue | core–shell dual metal–organic framework | 190 | unconjugated | cervical cancer | in vitro, in vivo | (168) |
| DOX, ICG, manganese | polydopamine | 129 | PEG5000ylated | breast cancer | in vitro, in vivo | (169) |
| DOX, PFTTQ | PLL-g-PEG | 80 | PEG2000ylated | breast cancer | in vitro | (170) |
Abbreviations: DOX, doxorubicin; ICG, indocyanine green; NP, nanoparticle; PEG, polyethylene glycol; PFTTQ, poly[9,9-bis(4-(2-ethylhexyl)phenyl)fluorene-alt-co-6,7-bis(4-(hexyloxy)phenyl)-4,9-di(thiophen-2-yl)thiadiazolo quinoxaline]; PLL, polylysine; PSMA, poly(styrene-alt-maleic acid).
Co-encapsulated Nanocarriers for Photodynamic Therapy
The principles of photodynamic therapy (PDT) rely on photosensitizers, such as chorins, porphyrins, phthalocyanines, bacteriochlorines, fullerenes, semiconductor materials and polyelectrolytes, and dyes that absorb near-infrared light, such as indocyanine green.171,172 These photosensitizers emit reactive oxygen species (ROS) upon exposure to light of a specific wavelength. The generation of oxygen radicals is endorsed by energy transfer from the illuminated light to the photosensitizer molecules. A major advantage of PDT is the localized production of ROS exerting lethal action to cancer cells in the vicinity,173 while it is employed in managing gastric,174 lungs,175 and cervical176 cancers. Such toxic impact of ROS on cancer cells is mediated by a plethora of mechanisms, including apoptosis.177 However, nonspecific accumulation of photosensitizers remains an issue, while inactivation by the endothelial cells and erythrocytes curtails the efficacy of PDT.178
Nanocarriers with co-encapsulated anticancer agents and photosensitizers have been reported. The aim of preparing such formulations is to maximize therapeutic advantage by combining chemotherapy with PDT. In one such polymeric nanocarrier, cisplatin was conjugated to zinc and formed the core, while pyrolipid (photosensitizer) was intercalated into the shell.179 The nanoformulation showed superior performance in regressing tumor volume by promoting apoptosis and necrosis with longer circulation times, and higher tumor accumulation in a human head and neck cancer SQ20B xenograft murine model.
Similarly, polymeric micelles (<50 nm) with co-encapsulated docetaxel and IR820 dye were prepared and surface-grafted with a tumor homing peptide called Lyp-1.180 A poly(ethylene imine) derivative of the block copolymer was used to obviate the short in vivo lifespan (t1/2 = 185 min) of IR820. When illuminated with a laser (λex = 808 nm, power 2.5 W/cm2), the formulation inhibited growth and metastasis in a mice breast tumor model. In a similar core–shell nanocarrier, gold nanorods formed the cores while the anticancer drug camptothecin was conjugated to the metal–organic framework shell.181 These nanocarriers showed adequate drug loading and release while acting as a combined platform for PTT and PDT with demonstrable therapeutic benefit in a female BALB/c mice tumor model.
Nanocarriers with Co-encapsulated Antimicrobial Agents
A gamut of nanocarriers have been utilized for co-encapsulating antimicrobials agents, and the primary purpose of designing such nanocarriers is to achieve codelivery and, subsequently, synergism. Furthermore, it aims to address the current challenges, including poor bioavailability, lack of patient compliance, and systemic toxicity, as well as to inhibit the MDR strains.182,183 The popular nanocarriers for co-encapsulation of antimicrobials are either lipid-based or polymeric.184 The LNCs typically include liposomes, solid-lipid nanocarriers (SLNs), nanostructured lipid carriers (NLCs), and niosomes.185 Such LNCs are biocompatible and can be used to encapsulate both hydrophilic and hydrophobic molecules.186
The SLNs are composed of biocompatible lipids (e.g., stearic acid, palmitic acid, oleic acid, glycerol monostearate, and soybean oil) and are suitable to deliver lipophilic drugs.187,188 Although excellent nanocarriers with decent encapsulation prowess, SLNs lack stability and are known for leakage. On the contrary, the NLCs, a modified version of SLNs with cores composed of solid and liquid lipids, exhibit higher stability, longer shelf life, and lesser leakage.189 The SLNs and NLCs are prepared by various techniques, such as probe sonication, solvent evaporation, hot and high-pressure homogenization, ultrasonic emulsion evaporation, and spray-drying. Niosomes are spherical lipid vesicles composed of biocompatible nonionic surfactants (e.g., Tween 20, 60, and 80 and Span-20, 40, 60, and 80) and cholesterol while prepared by reverse-phase evaporation, lipid-film hydration, microfluidics, and ether injection.190
The PNCs, on the other hand, exhibit superior stability and less leakage and offer finer tuning of particulate size, surface chemistry, polydispersity, and loading by altering the length of the polymer chains, organic solvents, and surfactants.191 For amphiphilic copolymers, co-encapsulation of both hydrophilic and hydrophobic molecules can be achieved by conjugation to the polymer blocks. Furthermore, with greater control over surface chemistry, PNCs offer opportunities for coating, for example, with bioadhesive lectin192 or bioconjugation.
Nanocarriers with Co-encapsulated Antibacterial Agents
A significant fraction of such co-encapsulated nanocarriers was developed to target bacterial strains known for drug resistance,193,194 such as Staphylococcus aureus, Mycobacterium tuberculosis, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Chlamydia trachomatis (Table 5). Especially in Mycobacterium tuberculosis, co-encapsulation of up to four antitubercular drugs, viz., isoniazid, rifampicin, pyrazinamide, and streptomycin, was achieved using liposomes.195 Co-encapsulation of isoniazid, rifampicin, and pyrazinamide has also been possible with PNCs.196 Interestingly, ethambutol—a popular first-line drug in tuberculosis—was excluded from co-encapsulation because it destabilized the nanocarriers due to its hygroscopic nature,197 further emphasizing the importance of molecular chemistry in co-encapsulation.
Table 5. Some Examples of Co-encapsulation of Antimicrobial Agents in Nanocarriersa.
| co-encapsulated agents | composition of nanocarrier | size (nm) | surface properties | target microorganism | status | ref |
|---|---|---|---|---|---|---|
| Antibacterial agents | ||||||
| ciprofloxacin, betamethasone | DPPC, PG, PhC, wheat germ agglutinin, cyclodextrin | 100 | unconjugated | Aggregatibacter actinomycetemcomitans | in vitro | (219) |
| amikacin, moxifloxacin | alginate-entrapped PLGA | 312–365 | unconjugated | Mycobacterium tuberculosis | in vitro | (220) |
| isoniazid, N-dodecanoyl isonicotinohydrazide | phospholipid | 130 | unconjugated | Mycobacterium tuberculosis | in vitro | (221) |
| ciprofloxacin, chlortetracycline, gentamicin | chitosan | 14–24 | unconjugated | Staphylococcus aureus, Escherichia coli | in vitro | (222) |
| clotrimazole, silver | Compritol 888 ATO | 124.1 ± 2.5 | unconjugated | Staphylococcus aureus | in vitro | (223) |
| Antiviral agents | ||||||
| tenofovir, alafenamide, elvitegravir | PLGA, PVA, pluronic F127 | 190.2 ± 2.3 | unconjugated | HIV | in vivo | (224) |
| lopinavir, ritonavir, tenofovir | DSPC, mPEG–DSPE | 69.0 ± 8.3 | unconjugated | HIV | in vivo | (225) |
| lopinavir, ritonavir | oleic acid, TPGS, aeroperl 300 | 158 | unconjugated | HIV | in vivo | (226) |
| nevirapine, saquinavir | egg PhC, DSPE–PEG, cholesterol | 173 ± 7 | anti-CD4 conjugated | HIV | in vitro | (204) |
| Antiparasitic agents | ||||||
| quinine, curcumin | poly(ε-caprolactone), caprylic triglyceride, Tween 80, lipoid S45 | 200 | unconjugated | Plasmodium falciparum | in vitro, in vivo | (227) |
| artemether, lumefantrine | glyceryl dilaurate, oleic acid, capmul MCM, Tween 80, solutol HS 15 | 64.4 ± 8.6 | unconjugated | Plasmodium berghei | in vitro, in vivo | (228) |
| artemether, clindamycin, lumefantrine | glyceryl dilaurate, oleic acid, capmul MCM, Tween 80, solutol HS 15 | 45 ± 10, 64.4 ± 8.6 | unconjugated | Plasmodium berghei | in vitro, in vivo | (229) |
Abbreviations: DPPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; DSPC, distearoyl-sn-glycero-3-phosphocholine; DSPE–PEG, PEGylated 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; mPEG2000–DSPE, methoxy polyethylene glycol2000-distearoyl phosphatidylethanolamine; PhC, phosphatidylcholine; PEG, polyethylene glycol; PG, phosphoglycerol; PLGA, poly(lactic-co-glycolic acid); PVA, poly(vinyl alcohol); TPGS, d-α-tocopheryl polyethylene glycol succinate.
Nanocarriers with Co-encapsulated Antiviral Agents
Viral diseases, including human immunodeficiency virus (HIV), hepatitis virus, human papillomavirus (HPV), herpes virus, and influenza virus, continue to cause suffering on a global scale.198 Perhaps the latest relevant example is SARS-CoV-2, which has caused a pandemic and disrupted the fabric of society. Mutated strains continue to emerge, necessitating research on establishing new delivery platforms with improved efficacy and spectrum coverage.
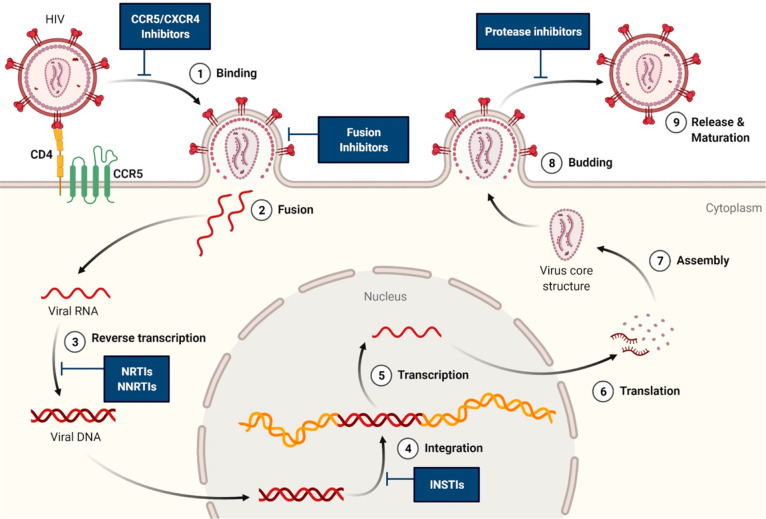
Co-encapsulated nanocarriers have provided fresh opportunities for improved drug delivery in antiviral therapy, although the published research tends to gravitate toward anti-HIV therapy (Table 5). Such co-encapsulated nanocarriers typically contain multiple antiretrovirals (ARVs) to achieve a system for combination antiretroviral therapy199 or highly active antiretroviral therapy.200 The motive behind co-encapsulating ARVs is to inhibit the reproductive cycle of HIV at various stages201 and achieve synergism (Figure 6). Other desirable goals are to improve bioavailability and penetration into tissues while addressing toxicity, untoward drug interactions, and emergence of resistance.202
Figure 6.
Scheme showing the various stages (1–9) of the HIV lifecycle that the ARVs co-encapsulated in nanocarriers inhibit while aiming to achieve synergism. Abbreviations: CCR5, C–C chemokine receptor type 5; CD4, cluster of differentiation 4; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; and NRTI, nucleoside reverse transcriptase inhibitor.
Both liposomes and SLNs have been used to co-encapsulate ARVs, while a range of lipids, such as DSPC, methoxy-PEGylated DSPC (mPEG-DSPC), and methoxy-PEGylated DSPE (mPEG-DSPE), were used in preparing them with poloxamer 188 and Tween 80 as surfactants.203 A broad spectrum of ARVs, including the nucleoside reverse-transcriptase inhibitors (e.g., lamivudine and zidovudine), nucleotide reverse-transcriptase inhibitors (e.g., tenofovir), non-nucleoside reverse-transcriptase inhibitors (e.g., nevirapine and efavirenz), and protease inhibitors (e.g., lopinavir, ritonavir, and saquinavir), have been co-encapsulated with an encapsulation efficiency of ∼90%. Some of these co-encapsulated LNCs have demonstrated favorable pharmacokinetics in vivo, including prolonged circulation and sustained release. Surface functionalization with targeting ligands, such as an anti-CD4 antibody, has also been reported.204
PLGA has emerged as a popular material for co-encapsulating various ARVs in PNCs,205 with an encapsulation efficiency of >80%. Investigations on peripheral blood mononuclear cells and monocyte-derived macrophages have confirmed adequate cellular uptake of the PNCs in cells with release detected over a prolonged duration.206 A sustained-release thermosensitive gel of pluronic F127/F68 with impregnated co-encapsulated PLGA nanocarriers containing raltegravir and efavirenz was developed for intravaginal delivery for prophylaxis against HIV.207 Other than PLGA, polymers like PLA and polycaprolactone have also been used for co-encapsulating ARVs.
Nanocarriers with Co-encapsulated Antiparasitic Agents
Co-encapsulated nanocarriers have been used to deliver antimalarial agents against Plasmodium falciparum and Plasmodium berghei. Such nano-DDSs deserve attention due to the global impact of malaria and the mortality, morbidity, and financial burden it causes. Unfortunately, considerable resistance has emerged against multiple antimalarials, such as artemisinin.208 Moreover, antimalarial drugs continue to suffer from poor solubility and bioavailability in addition to systemic toxicity.209 A combination therapy via co-encapsulated nanocarriers may address these challenges. A range of LNCs (e.g., liposomes and NLCs) and PNCs have been employed to co-encapsulate multiple antimalarial agents, including artemisinin, curcumin, primaquine, artemether, quinine, and lumefantrine (Table 5).
Janus Nanoparticles
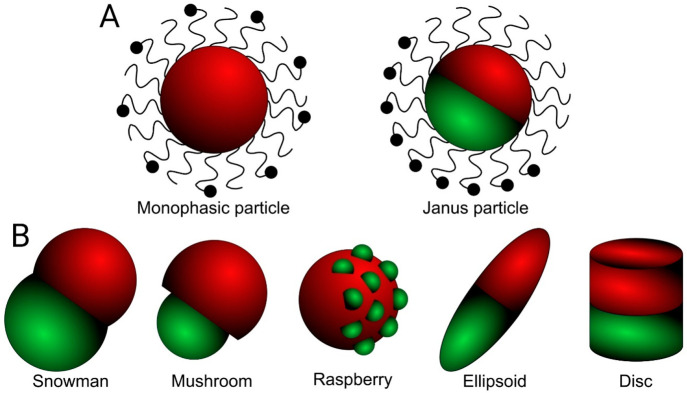
The Janus nanoparticles (JNPs) are an exciting breed of particulate DDSs that can codeliver drugs with multiple drug molecules encapsulated within the same particle.210 Prepared first as Janus beads in 1989,211 they were named Janus particles by Pierre-Gilles de Gennes (Nobel Laureate in Physics, 1991) due to their structural similarity to the Greek god Janus with two faces looking at opposite directions.212 Like the Greek God Janus, the JNPs harbor two or more dissimilar segments within the same particle (Figure 7A). Hence, unlike the conventional nano-DDSs, JNPs are anisotropic. Over the last two decades, the synthesis and definition of JNPs have evolved into new domains where, at times, more than two segments are contained by the same particle.213 The varied segments in the JNPs differ by their physicochemical properties, including hydrophilicity, magnetism, and optoelectronic behavior.214
Figure 7.
(A) Scheme showing the conventional surface-functionalized monophasic particle with a more homogeneous structural fabric in comparison to abiphasic Janus particle that elicits demarcation between its two phases, including physicochemical attributes and surface conjugation. (B) Janus particles prepared with varied shapes (e.g., snowman, mushroom, raspberry, ellipsoid, and disc) where the two distinct phases are oriented differently to each other.
The existence of varied compartments within the same particle enables co-encapsulation of different drugs with controlled engineering to regulate drug release in synchrony or a phasic manner. Further opportunities for surface modification can add ligand-based targeting ability to pathologic sites as well. Currently, JNPs of multiple shapes (e.g., disc, snowman, dumbbell, rod, raspberry, irregular, and mushroom; Figure 7B) and compositions (polymeric, inorganic, and a combination of polymeric–inorganic) are being prepared through a diverse range of synthetic routes, including immobilization, phase separation, self-assembly, microfluidics, surface-controlled nucleation and growth, and emulsion polymerization.215 Apart from drug delivery, JNPs are currently used for catalysis,216 biomedical imaging,217 and biosensing218 purposes, although such uses will not be included in this account.
One of the key advantages of JNPs over conventional isotropic nano-DDSs like liposomes is their ability to co-encapsulate therapeutic molecules of diverse characteristics, such as hydrophilic doxorubicin and hydrophobic paclitaxel, in the same particle.230 With finer tuning of the segments of the JNPs, including surface functionalization,231 release properties of the co-encapsulated drugs, often with disparate properties, can be controlled. In addition, such co-encapsulated JNPs can be rendered to be trigger-sensitive DDSs where release is facilitated due to pH,232 temperature,233 or a combination of both.234 These attributes highlight the suitability of JNPs in codelivering multiple therapeutic molecules in cancer tissues.
JNPs prepared by fluidic nanoprecipitation with two segments composed of different PLGA polymers were used to co-encapsulate paclitaxel and doxorubicin in the same particle. The paclitaxel demonstrated a burst release, although doxorubicin showed similar release kinetics to monophasic NPs with encapsulated doxorubicin.230 Polymeric dumbbell or snowman-shaped JNPs prepared by distillation precipitation polymerization and seeded emulsion polymerization were used to co-encapsulate doxorubicin and the anti-inflammatory drug ibuprofen in its two hemispheres composed of poly(2-hydroxyethyl methacrylate) (PHEMA) and poly(2-dimethylaminoethyl methacrylate) (PDAMEMA), respectively.235 It is worth noting here that, while PHEMA is a thermosensitive polymer,236 PDAMEMA is pH-sensitive.237 Thus, a dual-release-modality particle with two co-encapsulated drugs of distinct chemical properties could be prepared. Release studies elicited a higher release of doxorubicin than ibuprofen at pH values of 5.3 and 7.4, while the cumulative release for ibuprofen surpassed doxorubicin at pH 7.4. Polymer–lipid JNPs loaded with doxorubicin and curcumin showed synergistic toxicity in vitro and beneficial effects in an orthotopic murine model in vivo.238
Perspectives
The encapsulation process is thermodynamically challenging because it forces molecules to be enclosed into a smaller core wrapped within a shell. The challenge increases further while encapsulating within nanocarriers due to their minuscule sizes. The tiny cores of nanocarriers induce cohabitation of the drug molecules with temporospatial proximity. Such narrow separation frequently gives rise to undue and unpredictable intra- and intermolecular interactions resulting in localized denaturation, aggregation, ionic exchange, quenching, rearrangements, and ripening (e.g., Ostwald ripening) with a deleterious impact for stability, release kinetics, and, above all, therapeutic impact.239,240 Moreover, it converts each nanocarrier into a de facto nanoreactor,241 where the co-encapsulated molecules interact with the shell as part of such an interactive milieu. The 3D nanoscale confinement further adds to the reactivity, unpredictable stability, and poor control over burst release noted in many co-encapsulated nanocarriers. Hence, not every set of molecules can be co-encapsulated, and a prior assessment of co-encapsulability is warranted.
A thorough characterization of the molecules, both as a solo entity and while in proximity to other therapeutic molecules, is of utmost importance. It is essential to evaluate the type of reactions a set of molecules may trigger when in propinquity to various therapeutically relevant molecules. The compatibility of the co-encapsulable molecules needs to be high with little or no compromise of therapeutic effects. A systematic choice of in vitro, ex vivo, and in vivo protocols must be made to gather data before making choices for co-encapsulation or narrowing down the options upon screening a large set of molecules. Such pre-encapsulation data are vital to discard incompatible molecular combinations and select only those with the potential for a fruitful co-encapsulation and, hopefully, translation.
Aiming for synergism adds a further layer of complexity as co-encapsulation is not only an art of bringing molecules together but also a craft of achieving synergism through reciprocity. The term reciprocity in the case of co-encapsulated molecules depicts a cohabitation within nanocarriers that is not endangered with molecular interactions, at least not the ones that negatively affect their therapeutic impact or give rise to any cross-resistance, while maintaining adequate molecular integrity followed by a synchronized release with an opportunity for synergism. In other words, the aim of co-encapsulation is not only to achieving codelivery but rather to deliver at the right place, right dose, and right time. This is exactly where the major challenge lies.
Modeling systems are now available to predict synergism within a drug or other molecular combinations.242−244 Such in silico tools are evolving fast and are recommended for screening purposes. Understanding the physicochemical attributes of encapsulable molecules based on molecular descriptors245−247 can be a facile way to estimate the encapsulability, solubility, stability, and intermolecular interactions. Some online tools to calculate molecular descriptors, such as the Swiss ADME,248 are freely available. Such platforms should be used more before making choices.
Unfortunately, literature on co-encapsulated nanocarriers often lacks enough rigor when it comes to characterization, both before and after encapsulation, despite its paramount importance. Quite often, co-encapsulation is reported with meager data on stability, especially on a long-term basis, while the type of molecular interactions that co-encapsulation might trigger is omitted despite the relevance of such information. Merely succeeding in enforcing multiple molecules to condense within a nanoscale core is not enough for a fruitful co-encapsulation drive, and more needs to be achieved in terms of stability, reproducibility, desired release kinetics, and synergism.
Except for Vyxeos, none of the FDA-approved nanoformulations is co-encapsulated, eliciting the challenge that co-encapsulation presents. Another interesting fact is that, except for Doxil, none of these nanoformulations, including Vyxeos, is PEGylated. It indicates that perhaps it is time to embrace structurally simple nanocarriers for encapsulation due to the simplicity of their syntheses. Otherwise, it is difficult, if not impossible, to keep track of so many challenges or to control the synthesis, and that too on a large-scale production chain necessary for translation. Although they appear promising in theory, preparing complex nanocarriers with fancy attributes, including compartmentalization and surface functionalization, has failed repeatedly in clinical trials,249,250 and elicited the precarious nature of such an approach.
The field of nanomedicine, especially from a drug-delivery perspective, is undergoing a reality check with some inconvenient realizations made over the recent years. Bankruptcies filed by some prominent nanomedicine pharma ventures has added further to the woes.251 Perhaps it is time to accept that, when it comes to translation, all nanoformulations, including the co-encapsulated ones, ultimately narrow down to facts and figures rather than hype and rhetoric. The insufficient and, at times, unreliable in vitro and in vivo models, lack of in vitro–in vivo correlation, irreproducibility of data, and disturbing batchwise variation continue to frustrate and hold back the progress in nanomedicine research.252 To solve a problem, it needs to be acknowledged first. Unfortunately, such self-reflection is often lacking. The onus is now on the research community to decide whether to have a huge collection of failed nanoformulations or rather to prioritize a few assorted ones that work and get translated from the benchtop to the bedside.
Acknowledgments
Figures 1–6 were created using Biorender.com.
Glossary
Abbreviations and Symbols Used
- Ald
Alendronate
- APN
Aminopeptidase N
- ARV
Antiretroviral
- BSA
Body surface area
- CCR
C–C chemokine receptor
- CD
Cluster of differentiation
- CI
Combination index
- DDS
Drug-delivery system
- DOPC
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- DOTAP
1,2-Dioleoyl-3-trimethylammonium propane
- DOX
Doxorubicin
- DPPC
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- DSPC
1,2-Distearoyl-sn-glycero-3-phosphocholine
- DSPE
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine
- DTX
Docetaxel
- ECM
Extracellular matrix
- EGFR
Epidermal growth factor receptor
- EMA
European Medicines Agency
- EPR
Enhanced permeability and retention
- FDA
U. S. Food and Drug Administration
- FeNP
Iron nanoparticle
- GNP
Gold nanoparticle
- GRAS
Generally regarded as safe
- HIV
Human immunodeficiency virus
- HSPhC
Hydrogenated soybean phosphatidylcholine
- IC50
Half-maximal inhibitory concentration
- ICG
Indocyanine green
- IFN-γ
Interferon γ
- IL
Interleukin
- INSTI
Integrase strand transfer inhibitor
- IR
Infrared
- JNP
Janus nanoparticle
- LNC
Lipid nanocarrier
- MDR
Multidrug resistance
- MMP2
Matrix metalloproteinase 2
- mPEG–DSPC
Methoxy-PEGylated DSPC
- mPEG–DSPE
Methoxy-PEGylated DSPE
- MRI
Magnetic resonance imaging
- mV
Millivolt
- NGR
Asp-Gly-Arg
- NLC
Nanostructured lipid carrier
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- NRTI
Nucleoside reverse transcriptase inhibitor
- NP
Nanoparticle
- NSCLC
Nonsmall cell lung cancer
- PAMAM
Polyamidoamine
- PBLG
Poly(γ-benzyl-l-glutamate)
- PDAMEMA
Poly(2-dimethylaminoethyl methacrylate)
- pDNA
Plasmid DNA
- PDT
Photodynamic therapy
- PEG
Polyethylene glycol
- PEO
Poly(ethylene oxide)
- PFTTQ
Poly[9,9-bis(4-(2-ethylhexyl)phenyl)fluorene-alt-co-6,7-bis(4-(hexyloxy)phenyl)-4,9-di(thiophen-2-yl)-thiadiazolo quinoxaline]
- PG
Phosphoglycerol
- P-gp
P-glycoprotein
- PhC
Phosphatidylcholine
- PHEMA
Poly(2-hydroxyethyl methacrylate)
- PLGA
Poly(lactic-co-glycolic acid)
- PLL
Polylysine
- PNC
Polymeric nanocarrier
- PNP
Polymeric nanoparticle
- PSMA
Poly(styrene-alt-maleic acid)
- PTT
Photothermal therapy
- PTX
Paclitaxel
- PVA
Poly(vinyl alcohol)
- RES
Reticuloendothelial system
- RGD
Arg-Gly-Asp
- ROS
Reactive oxygen species
- shRNA
Small hairpin RNA
- siRNA
Small interfering RNA
- SLN
Solid-lipid nanocarrier
- SPION
Superparamagnetic iron oxide nanoparticle
- t1/2
Half-life
- TAM
Tumor-associated macrophage
- TKI
Tyrosine kinase inhibitor
- TME
Tumor microenvironment
- TMX
Tamoxifen
- TPGS
d-α-tocopheryl polyethylene glycol succinate
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
The project received funding from UCD Research.
The author declares no competing financial interest.
References
- Patra J. K.; Das G.; Fraceto L. F.; Campos E. V. R.; Rodriguez-Torres M. d. P.; Acosta-Torres L. S.; Diaz-Torres L. A.; Grillo R.; Swamy M. K.; Sharma S.; Habtemariam S.; Shin H.-S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018, 16 (1), 71. 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S.; Sousa J.; Pais A.; Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. 10.3389/fchem.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan J.; Belz S.; Hoeveler A.; Hugas M.; Okuda H.; Patri A.; Rauscher H.; Silva P.; Slikker W.; Sokull-Kluettgen B.; Tong W.; Anklam E. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. 10.1016/j.yrtph.2021.104885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M.; Caputo F.; Metcalfe S.; Tosi G.; Spring K.; Åslund A. K. O.; Pottier A.; Schiffelers R.; Ceccaldi A.; Schmid R. Delivering the power of nanomedicine to patients today. J. Controlled Release 2020, 326, 164–171. 10.1016/j.jconrel.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet P. H. M.; Brüske-Hohlfeld I.; Salata O. V. Nanoparticles – known and unknown health risks. J. Nanobiotechnol. 2004, 2 (1), 12. 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov Y. Quantum dots in nanomedicine: recent trends, advances and unresolved issues. Biochem. Biophys. Res. Commun. 2015, 468 (3), 419–427. 10.1016/j.bbrc.2015.07.039. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S. Nanomedicine literature: the vicious cycle of reproducing the irreproducible. Int. J. Pharmacokinet. 2017, 2 (1), 15–19. 10.4155/ipk-2016-0017. [DOI] [Google Scholar]
- Daglar B.; Ozgur E.; Corman M. E.; Uzun L.; Demirel G. B. Polymeric nanocarriers for expected nanomedicine: current challenges and future prospects. RSC Adv. 2014, 4 (89), 48639–48659. 10.1039/C4RA06406B. [DOI] [Google Scholar]
- Indoria S.; Singh V.; Hsieh M.-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: a review. Int. J. Pharm. 2020, 582, 119314. 10.1016/j.ijpharm.2020.119314. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Mondal K.; Panda P. K.; Kaushik A.; Abolhassani R.; Ahuja R.; Rubahn H.-G.; Mishra Y. K. Core–shell nanostructures: perspectives towards drug delivery applications. J. Mater. Chem. B 2020, 8 (39), 8992–9027. 10.1039/D0TB01559H. [DOI] [PubMed] [Google Scholar]
- Zylberberg C.; Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Delivery 2016, 23 (9), 3319–3329. 10.1080/10717544.2016.1177136. [DOI] [PubMed] [Google Scholar]
- Almeida B.; Nag O. K.; Rogers K. E.; Delehanty J. B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules 2020, 25 (23), 5672. 10.3390/molecules25235672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliani R.; Gatto F.; Bardi G. Protein adsorption: a feasible method for nanoparticle functionalization?. Materials 2019, 12 (12), 1991. 10.3390/ma12121991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Gao D.; Shen J.; Wang Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front. Chem. 2020, 8, 1086. 10.3389/fchem.2020.598722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I. U.; Khan R. U.; Asif H.; Alamgeer; Khalid S. H.; Asghar S.; Saleem M.; Shah K. U.; Shah S. U.; Rizvi S. A. A.; Shahzad Y. Co-delivery strategies to overcome multidrug resistance in ovarian cancer. Int. J. Pharm. 2017, 533 (1), 111–124. 10.1016/j.ijpharm.2017.09.060. [DOI] [PubMed] [Google Scholar]
- Yeh Y.-C.; Huang T.-H.; Yang S.-C.; Chen C.-C.; Fang J.-Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 2020, 8, 286. 10.3389/fchem.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas R.; Barbosa A. A. T.; Dolabela S. S.; Jain S. Multidrug-resistant bacteria and alternative methods to control them: an overview. Microb. Drug Resist. 2019, 25 (6), 890–908. 10.1089/mdr.2018.0319. [DOI] [PubMed] [Google Scholar]
- Du D.; Wang-Kan X.; Neuberger A.; van Veen H. W.; Pos K. M.; Piddock L. J. V.; Luisi B. F. Multidrug efflux pumps: structure, function and regulation. Nat. Rev. Microbiol. 2018, 16 (9), 523–539. 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- Stadler J.; Richly H. Regulation of DNA repair mechanisms: how the chromatin environment regulates the DNA damage response. Int. J. Mol. Sci. 2017, 18 (8), 1715. 10.3390/ijms18081715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoori B.; Mohammadi A.; Davudian S.; Shirjang S.; Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv. Pharm. Bull. 2017, 7 (3), 339–348. 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski K.; Kciuk M.; Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21 (9), 3233. 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Lu Z.; Zhao X. Targeting Bcl-2 for cancer therapy. Biochim. Biophys. Acta 2021, 1876 (1), 188569. 10.1016/j.bbcan.2021.188569. [DOI] [PubMed] [Google Scholar]
- Avnet S.; Lemma S.; Cortini M.; Pellegrini P.; Perut F.; Zini N.; Kusuzaki K.; Chano T.; Grisendi G.; Dominici M.; De Milito A.; Baldini N. Altered pH gradient at the plasma membrane of osteosarcoma cells is a key mechanism of drug resistance. Oncotarget 2016, 7 (39), 63408–63423. 10.18632/oncotarget.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S.; DeGiovanni P.-J.; Piel B.; Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med. 2017, 6 (1), 44. 10.1186/s40169-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. X.; Wong H. L.; Xue H. Y.; Eoh J. Y.; Wu X. Y. Nanomedicine of synergistic drug combinations for cancer therapy – strategies and perspectives. J. Controlled Release 2016, 240, 489–503. 10.1016/j.jconrel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Yao W.; Rao Y.; Lu X.; Gao J. pH-responsive carriers for oral drug delivery: challenges and opportunities of current platforms. Drug Delivery 2017, 24 (1), 569–581. 10.1080/10717544.2017.1279238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo A. C.; Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 2019, 4 (3), e10143. 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalepu S.; Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5 (5), 442–453. 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini L.; Alvarez-Puebla R. A.; Pazos-Perez N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11 (7), 1154. 10.3390/ma11071154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. R.; Feng T.; Zhang Q.; Chan H. Y. E.; Chau Y. Co-encapsulation and co-delivery of peptide drugs via polymeric nanoparticles. Polymers 2019, 11 (2), 288. 10.3390/polym11020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W.; Shi L.; Ren L.; Zhou L.; Li T.; Qiao Y.; Wang H. A nanomedicine approach enables co-delivery of cyclosporin A and gefitinib to potentiate the therapeutic efficacy in drug-resistant lung cancer. Signal Transduct. Target. Ther. 2018, 3 (1), 16. 10.1038/s41392-018-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P.; Biswas A.; Shukla A.; Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Therapy 2019, 4 (1), 33. 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum D.; Joshi N.; Tao W.; Karp J. M.; Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9 (1), 1410. 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam S. Y.; Chee C. F.; Yong C. Y.; Ho K. L.; Mariatulqabtiah A. R.; Tan W. S. Stealth coating of nanoparticles in drug-delivery systems. Nanomaterials 2020, 10 (4), 787. 10.3390/nano10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler E. L.; Kim Y. J.; Wang P. Nanomedicine targeting the tumor microenvironment: Therapeutic strategies to inhibit angiogenesis, remodel matrix, and modulate immune responses. J. Cell. Immunother. 2016, 2 (2), 69–78. 10.1016/j.jocit.2016.08.002. [DOI] [Google Scholar]
- Vallet-Regí M.; Colilla M.; Izquierdo-Barba I.; Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules 2018, 23 (1), 47. 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roell K. R.; Reif D. M.; Motsinger-Reif A. A. An introduction to terminology and methodology of chemical synergy—perspectives from across disciplines. Front. Pharmacol. 2017, 8, 158. 10.3389/fphar.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Valdivieso J.; Girotti A.; Schneider J.; Arias F. J. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int. J. Pharm. 2021, 599, 120438. 10.1016/j.ijpharm.2021.120438. [DOI] [PubMed] [Google Scholar]
- Shi J.; Kantoff P. W.; Wooster R.; Farokhzad O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17 (1), 20–37. 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova K. Y.; Piroyan A.; Le-Deygen I. M.; Vishwasrao H. M.; Ramsey J. D.; Klyachko N. L.; Golovin Y. I.; Rudakovskaya P. G.; Kireev I. I.; Kabanov A. V.; Sokolsky-Papkov M. Magnetic liposome design for drug release systems responsive to super-low frequency alternating current magnetic field (AC MF). J. Colloid Interface Sci. 2019, 552, 689–700. 10.1016/j.jcis.2019.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H.; Xue J.; Jiang H.; Gao S.; Yang D.; Fang Y.; Shi K. Current developments in drug delivery with thermosensitive liposomes. Asian J. Pharm. Sci. 2019, 14 (4), 365–379. 10.1016/j.ajps.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.; Ma X.; Zhou C.; Yang H.; Yang Y.; Xie X.; Yang C.; Han C. Development and characteristics of novel sonosensitive liposomes for vincristine bitartrate. Drug Delivery 2019, 26 (1), 724–731. 10.1080/10717544.2019.1639845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzogani K.; Penttilä K.; Lapveteläinen T.; Hemmings R.; Koenig J.; Freire J.; Márcia S.; Cole S.; Coppola P.; Flores B.; Barbachano Y.; Roige S. D.; Pignatti F. EMA review of daunorubicin and cytarabine encapsulated in liposomes (Vyxeos, CPX-351) for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia or acute myeloid leukemia with myelodysplasia-related changes. Oncologist 2020, 25 (9), e1414–e1420. 10.1634/theoncologist.2019-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S. DLS and zeta potential – What they are and what they are not?. J. Controlled Release 2016, 235, 337–351. 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Hua S.; de Matos M. B. C.; Metselaar J. M.; Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov P.; Naulaerts S.; Ariey-Bonnet J.; Pasquier E.; Ballester P. J. Predicting synergism of cancer drug combinations using NCI-ALMANAC data. Front. Chem. 2019, 7, 509. 10.3389/fchem.2019.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer S.; Dijkstra T. M. H.; Heskes T. Additive dose response models: defining synergy. Front. Pharmacol. 2019, 10, 1384. 10.3389/fphar.2019.01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissantz C.; Kuhn B.; Stahl M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53 (14), 5061–5084. 10.1021/jm100112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R.; Ahmed S.; Sharma N.; Kumar N.; Debas A.; Matsumura K. Review of the current state of protein aggregation inhibition from a materials chemistry perspective: special focus on polymeric materials. Mater. Adv. 2021, 2 (4), 1139–1176. 10.1039/D0MA00760A. [DOI] [Google Scholar]
- Siepmann J.; Peppas N. A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418 (1), 6–12. 10.1016/j.ijpharm.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Ritger P. L.; Peppas N. A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Controlled Release 1987, 5 (1), 23–36. 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Mircioiu C.; Voicu V.; Anuta V.; Tudose A.; Celia C.; Paolino D.; Fresta M.; Sandulovici R.; Mircioiu I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11 (3), 140. 10.3390/pharmaceutics11030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesic A.; Jalal K.; Gerbes A. L. Drug-drug combinations can enhance toxicity as shown by monocyte-derived hepatocyte-like cells from patients with idiosyncratic drug-induced liver injury. Toxicol. Sci. 2019, 171 (2), 296–302. 10.1093/toxsci/kfz156. [DOI] [PubMed] [Google Scholar]
- Degobert G.; Aydin D. Lyophilization of nanocapsules: instability sources, formulation and process parameters. Pharmaceutics 2021, 13 (8), 1112. 10.3390/pharmaceutics13081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T.; Itaya M.; Burdeos G. C.; Nakagawa K.; Miyazawa T. A critical review of the use of surfactant-coated nanoparticles in nanomedicine and food nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. 10.2147/IJN.S298606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S.; de Haan L. H. J.; Evers N. M.; Jiang X.; Marcelis A. T. M.; Zuilhof H.; Rietjens I. M. C. M.; Alink G. M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7 (1), 25. 10.1186/1743-8977-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S.; Rietjens I. M. C. M.; Singh M. P.; Atkins T. M.; Purkait T. K.; Xu Z.; Regli S.; Shukaliak A.; Clark R. J.; Mitchell B. S.; Alink G. M.; Marcelis A. T. M.; Fink M. J.; Veinot J. G. C.; Kauzlarich S. M.; Zuilhof H. Cytotoxicity of surface-functionalized silicon and germanium nanoparticles: the dominant role of surface charges. Nanoscale 2013, 5 (11), 4870–4883. 10.1039/c3nr34266b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Wang X.; Li J.; Nie Y.; Liao G.; Yu Y.; Li C. Overcoming the reticuloendothelial system barrier to drug delivery with a “don’t-eat-us” strategy. ACS Nano 2019, 13 (11), 13015–13026. 10.1021/acsnano.9b05679. [DOI] [PubMed] [Google Scholar]
- Papini E.; Tavano R.; Mancin F. Opsonins and dysopsonins of nanoparticles: facts, concepts, and methodological guidelines. Front. Immunol. 2020, 11, 2343. 10.3389/fimmu.2020.567365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnik B. S.; González-Fernández A.; Sadrieh N.; Dobrovolskaia M. A. Nanoparticles and the immune system. Endocrinology 2010, 151 (2), 458–465. 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J. S.; Xu Q.; Kim N.; Hanes J.; Ensign L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Delivery Rev. 2016, 99, 28–51. 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K.; Yokoyama M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: a review. Sci. Technol. Adv. Mater. 2019, 20 (1), 324–336. 10.1080/14686996.2019.1590126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang Thi T. T.; Pilkington E. H.; Nguyen D. H.; Lee J. S.; Park K. D.; Truong N. P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12 (2), 298. 10.3390/polym12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M.; Javaid F.; Chudasama V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9 (4), 790–810. 10.1039/C7SC04004K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.; Zhang H.; Ye H.; Zheng Y. Receptor-mediated endocytosis of nanoparticles: roles of shapes, orientations, and rotations of nanoparticles. J. Phys. Chem. B 2018, 122 (1), 171–180. 10.1021/acs.jpcb.7b09619. [DOI] [PubMed] [Google Scholar]
- Shi Y.; van der Meel R.; Chen X.; Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10 (17), 7921–7924. 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H.; Maeda H. The 35th anniversary of the discovery of EPR effect: a new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects. J. Pers. Med. 2021, 11 (3), 229. 10.3390/jpm11030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeland Allouni Z.; Gjerdet N. R.; Cimpan M. R.; Høl P. J. The effect of blood protein adsorption on cellular uptake of anatase TiO2 nanoparticles. Int. J. Nanomed. 2015, 10, 687–695. 10.2147/IJN.S72726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S.; Brayden D. J. Addressing the challenges to increase the efficiency of translating nanomedicine formulations to patients. Exp. Opin. Drug Discovery 2021, 16 (3), 235–254. 10.1080/17460441.2021.1826434. [DOI] [PubMed] [Google Scholar]
- Tee J. K.; Yip L. X.; Tan E. S.; Santitewagun S.; Prasath A.; Ke P. C.; Ho H. K.; Leong D. T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48 (21), 5381–5407. 10.1039/C9CS00309F. [DOI] [PubMed] [Google Scholar]
- Subhan M. A.; Yalamarty S. S. K.; Filipczak N.; Parveen F.; Torchilin V. P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11 (6), 571. 10.3390/jpm11060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karageorgis A.; Dufort S.; Sancey L.; Henry M.; Hirsjärvi S.; Passirani C.; Benoit J.-P.; Gravier J.; Texier I.; Montigon O.; Benmerad M.; Siroux V.; Barbier E. L.; Coll J.-L. An MRI-based classification scheme to predict passive access of 5 to 50-nm large nanoparticles to tumors. Sci. Rep. 2016, 6 (1), 21417. 10.1038/srep21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A. E.; Petersen A. L.; Henriksen J. R.; Boerresen B.; Rasmussen P.; Elema D. R.; Rosenschöld P. M. a.; Kristensen A. T.; Kjær A.; Andresen T. L. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 2015, 9 (7), 6985–6995. 10.1021/acsnano.5b01324. [DOI] [PubMed] [Google Scholar]
- Maeda H.; Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7 (1), 11. 10.1186/s40169-018-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J.; Islam W.; Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Delivery Rev. 2020, 157, 142–160. 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Mayer L. D.; Tardi P.; Louie A. C. CPX-351: a nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. 10.2147/IJN.S139450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.; Williams S. Daunorubicin and cytarabine liposome in newly diagnosed therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes. Ann. Pharmacother. 2018, 52 (8), 792–800. 10.1177/1060028018764923. [DOI] [PubMed] [Google Scholar]
- Krauss A. C.; Gao X.; Li L.; Manning M. L.; Patel P.; Fu W.; Janoria K. G.; Gieser G.; Bateman D. A.; Przepiorka D.; Shen Y. L.; Shord S. S.; Sheth C. M.; Banerjee A.; Liu J.; Goldberg K. B.; Farrell A. T.; Blumenthal G. M.; Pazdur R. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin. Cancer Res. 2019, 25 (9), 2685. 10.1158/1078-0432.CCR-18-2990. [DOI] [PubMed] [Google Scholar]
- Alfayez M.; Kantarjian H.; Kadia T.; Ravandi-Kashani F.; Daver N. CPX-351 (vyxeos) in AML. Leuk. Lymphoma 2020, 61 (2), 288–297. 10.1080/10428194.2019.1660970. [DOI] [PubMed] [Google Scholar]
- Sercombe L.; Veerati T.; Moheimani F.; Wu S. Y.; Sood A. K.; Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães D.; Cavaco-Paulo A.; Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. 10.1016/j.ijpharm.2021.120571. [DOI] [PubMed] [Google Scholar]
- van der Koog L.; Gandek T. B.; Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv. Healthc. Mater. 2022, 11, 2100639 10.1002/adhm.202100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzuto G.; Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. W. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. 10.1007/978-1-4939-6591-5_2. [DOI] [PubMed] [Google Scholar]
- Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J. Controlled Release 2012, 160 (2), 117–134. 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Ansari L.; Shiehzadeh F.; Taherzadeh Z.; Nikoofal-Sahlabadi S.; Momtazi-borojeni A. A.; Sahebkar A.; Eslami S. The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther. 2017, 24 (5), 189–193. 10.1038/cgt.2017.9. [DOI] [PubMed] [Google Scholar]
- Bulbake U.; Doppalapudi S.; Kommineni N.; Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics 2017, 9 (2), 12. 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojarad-Jabali S.; Farshbaf M.; Walker P. R.; Hemmati S.; Fatahi Y.; Zakeri-Milani P.; Sarfraz M.; Valizadeh H. An update on actively targeted liposomes in advanced drug delivery to glioma. Int. J. Pharm. 2021, 602, 120645. 10.1016/j.ijpharm.2021.120645. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Fang J.; Kim Y.-J.; Wong M. K.; Wang P. Codelivery of doxorubicin and paclitaxel by cross-linked multilamellar liposome enables synergistic antitumor activity. Mol. Pharmaceutics 2014, 11 (5), 1651–1661. 10.1021/mp5000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S.-S.; Sun J.-H.; Yu H.-H.; Yu S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Delivery 2017, 24 (1), 1909–1926. 10.1080/10717544.2017.1410256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Huang Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discovery 2020, 6, 100024. 10.1016/j.medidd.2020.100024. [DOI] [Google Scholar]
- Kapp T. G.; Rechenmacher F.; Neubauer S.; Maltsev O. V.; Cavalcanti-Adam E. A.; Zarka R.; Reuning U.; Notni J.; Wester H.-J.; Mas-Moruno C.; Spatz J.; Geiger B.; Kessler H. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017, 7 (1), 39805. 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Santino F.; Giacomini D.; Gentilucci L. Integrin-targeting peptides for the design of functional cell-responsive biomaterials. Biomedicines 2020, 8 (9), 307. 10.3390/biomedicines8090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi K. N.; Tóth S.; Szakács G.; Mező G. NGR-peptide–drug conjugates with dual targeting properties. PLoS One 2017, 12 (6), e0178632 10.1371/journal.pone.0178632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Xu Y.; Zhou Y.; Yang A.; Cui J.; Chen P.; Zhao H.; Zhou X.; Shen C.; Yu J.; Lu H. The effect of TKI therapy and chemotherapy treatment delivery sequence on total progression-free survival in patients with advanced EGFR-mutated NSCLC. Oncol. Lett. 2020, 20 (1), 391–400. 10.3892/ol.2020.11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon S. Gefitinib: a review of its use in adults with advanced non-small cell lung cancer. Target Oncol. 2015, 10 (1), 153–170. 10.1007/s11523-015-0358-9. [DOI] [PubMed] [Google Scholar]
- Peng H.; Chen B.; Huang W.; Tang Y.; Jiang Y.; Zhang W.; Huang Y. Reprogramming tumor-associated macrophages to reverse EGFRT790M resistance by dual-targeting codelivery of gefitinib/vorinostat. Nano Lett. 2017, 17 (12), 7684–7690. 10.1021/acs.nanolett.7b03756. [DOI] [PubMed] [Google Scholar]
- Yin W.; Yu X.; Kang X.; Zhao Y.; Zhao P.; Jin H.; Fu X.; Wan Y.; Peng C.; Huang Y. Remodeling tumor-associated macrophages and neovascularization overcomes EGFRT790M-associated drug resistance by PD-L1 nanobody-mediated codelivery. Small 2018, 14 (47), 1802372. 10.1002/smll.201802372. [DOI] [PubMed] [Google Scholar]
- Sánchez A.; Mejía S. P.; Orozco J. Recent advances in polymeric nanoparticle-encapsulated drugs against intracellular infections. Molecules 2020, 25 (16), 3760. 10.3390/molecules25163760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Huang G. Preparation and drug delivery of dextran-drug complex. Drug Delivery 2019, 26 (1), 252–261. 10.1080/10717544.2019.1580322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg U.; Chauhan S.; Nagaich U.; Jain N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9 (2), 195–204. 10.15171/apb.2019.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J.; Rostamizadeh K.; Filipczak N.; Torchilin V. P. Polymeric co-delivery systems in cancer treatment: an overview on component drugs’ dosage ratio effect. Molecules 2019, 24 (6), 1035. 10.3390/molecules24061035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram B.; Ozgur A.; Tutar L.; Tutar Y. Tumor targeting of polymeric nanoparticles conjugated with peptides, saccharides, and small molecules for anticancer drugs. Curr. Pharm. Design 2018, 23 (35), 5349–5357. 10.2174/1381612823666170608081735. [DOI] [PubMed] [Google Scholar]
- Fu L.; Liu L.; Ruan Z.; Zhang H.; Yan L. Folic acid targeted pH-responsive amphiphilic polymer nanoparticles conjugated with near infrared fluorescence probe for imaging-guided drug delivery. RSC Adv. 2016, 6 (46), 40312–40322. 10.1039/C6RA05657A. [DOI] [Google Scholar]
- Niza E.; Noblejas-López M. D. M.; Bravo I.; Nieto-Jiménez C.; Castro-Osma J. A.; Canales-Vázquez J.; Lara-Sanchez A.; Galán Moya E. M.; Burgos M.; Ocaña A.; Alonso-Moreno C. Trastuzumab-targeted biodegradable nanoparticles for enhanced delivery of dasatinib in HER2+ metastasic breast cancer. Nanomaterials 2019, 9 (12), 1793. 10.3390/nano9121793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo S.; Zhang F.; Yu J.; Zhang X.; Yang G.; Liu X. pH-sensitive biomaterials for drug delivery. Molecules 2020, 25 (23), 5649. 10.3390/molecules25235649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Zhang E.; Yang J.; Cao Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11 (10), 4985–4998. 10.1007/s12274-018-2152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. E.; Cummings N. D.; Sethi M.; Wang E. C.; Sukumar R.; Moore D. T.; Wang A. Z. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86 (3), 463–468. 10.1016/j.ijrobp.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.; Ge T.; Yan Y.; Shuai Q.; Su W.-K. Highly efficient modular construction of functional drug delivery platform based on amphiphilic biodegradable polymers via click chemistry. Int. J. Mol. Sci. 2021, 22 (19), 10407. 10.3390/ijms221910407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. K.; Prasher P.; Aljabali A. A.; Mishra V.; Gandhi H.; Kumar S.; Mutalik S.; Chellappan D. K.; Tambuwala M. M.; Dua K.; Kapoor D. N. Emerging era of “somes”: polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Delivery Transl. Res. 2020, 10 (5), 1171–1190. 10.1007/s13346-020-00789-2. [DOI] [PubMed] [Google Scholar]
- Zhang X.-y.; Zhang P.-y. Polymersomes in nanomedicine - a review. Curr. Med. Chem. 2017, 13 (2), 124–129. 10.2174/1573413712666161018144519. [DOI] [Google Scholar]
- Matoori S.; Leroux J.-C. Twenty-five years of polymersomes: lost in translation?. Mater. Horiz. 2020, 7 (5), 1297–1309. 10.1039/C9MH01669D. [DOI] [Google Scholar]
- Leong J.; Teo J. Y.; Aakalu V. K.; Yang Y. Y.; Kong H. Engineering polymersomes for diagnostics and therapy. Adv. Healthc. Mater. 2018, 7 (8), 1701276 10.1002/adhm.201701276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner P.; Baumann P.; Enea R.; Onaca O.; Palivan C.; Meier W. Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res. 2011, 44 (10), 1039–1049. 10.1021/ar200036k. [DOI] [PubMed] [Google Scholar]
- Gabizon A.; Ohana P.; Amitay Y.; Gorin J.; Tzemach D.; Mak L.; Shmeeda H. Liposome co-encapsulation of anti-cancer agents for pharmacological optimization of nanomedicine-based combination chemotherapy. Cancer Drug Resist. 2021, 4 (2), 463–484. 10.20517/cdr.2020.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggag Y.; Abu Ras B.; El-Tanani Y.; Tambuwala M. M.; McCarron P.; Isreb M.; El-Tanani M. Co-delivery of a RanGTP inhibitory peptide and doxorubicin using dual-loaded liposomal carriers to combat chemotherapeutic resistance in breast cancer cells. Exp. Opin. Drug Delivery 2020, 17 (11), 1655–1669. 10.1080/17425247.2020.1813714. [DOI] [PubMed] [Google Scholar]
- Ledezma-Gallegos F.; Jurado R.; Mir R.; Medina L. A.; Mondragon-Fuentes L.; Garcia-Lopez P. Liposomes co-encapsulating cisplatin/mifepristone improve the effect on cervical cancer: in vitro and in vivo assessment. Pharmaceutics 2020, 12 (9), 897. 10.3390/pharmaceutics12090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K.; Srinivasan P.; Mahadeva R.; Gupta C. M.; Haq W. Tuftsin-bearing liposomes co-encapsulated with doxorubicin and curcumin efficiently inhibit EAC tumor growth in mice. Int. J. Nanomed. 2020, 15, 10547–10559. 10.2147/IJN.S276336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi R.; Hassandokht F.; Ardakani M. T.; Karimi B.; Roustazadeh A.; Tarvirdipour S.; Barmak M. J.; Nikseresht M.; Baneshi M.; Mousavizadeh A.; Shirazi M. S.; Alipour M.; Bardania H. Intercalation of curcumin into liposomal chemotherapeutic agent augments apoptosis in breast cancer cells. J. Biomater. Appl. 2021, 35 (8), 1005–1018. 10.1177/0885328220976331. [DOI] [PubMed] [Google Scholar]
- Lin Y.; He X.; Zhou D.; Li L.; Sun J.; Jiang X. Co-delivery of doxorubicin and itraconazole by Pluronic® P123 coated liposomes to enhance the anticancer effect in breast cancers. RSC Adv. 2018, 8 (42), 23768–23779. 10.1039/C8RA03787F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.; Liao J.-Z.; Xiang G.-Y.; Zhao P.-X.; Ye F.; Zhao Q.; He X.-X. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017, 6 (3), 651–661. 10.1002/cam4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J.; Guo F.; Xu H.; Liang W.; Wang C.; Yang X.-D. Combination therapy using co-encapsulated resveratrol and paclitaxel in liposomes for drug resistance reversal in breast cancer cells in vivo. Sci. Rep. 2016, 6 (1), 22390. 10.1038/srep22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.; Li B.; Qi S.; Liu Y.; Gai Y.; Ye P.; Yang G.; Zhang W.; Zhang P.; He X.; Li W.; Zhang Z.; Xiang G.; Xu C. Co-delivery of doxorubicin and Bmi1 siRNA by folate receptor targeted liposomes exhibits enhanced anti-tumor effects in vitro and in vivo. Theranostics 2014, 4 (11), 1096–1111. 10.7150/thno.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z.; Wang C.; Fang E.; Lu X.; Wang G.; Tong Q. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS One 2014, 9 (3), e92924 10.1371/journal.pone.0092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh I. M.; Tan K.-B.; Chaudhury A.; Liu Y.; Tan B.-J.; Tan B. M. J.; Chiu G. N. C. Liposome co-encapsulation of synergistic combination of irinotecan and doxorubicin for the treatment of intraperitoneally grown ovarian tumor xenograft. J. Controlled Release 2013, 172 (3), 852–861. 10.1016/j.jconrel.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Li Y.; Hou H.; Zhang P.; Zhang Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Delivery 2020, 27 (1), 1044–1053. 10.1080/10717544.2020.1785049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva C. G.; Peters G. J.; Ossendorp F.; Cruz L. J. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother. Pharmacol. 2017, 80 (5), 881–894. 10.1007/s00280-017-3427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval N.; Jogi H.; Gondaliya P.; Kalia K.; Tekade R. K. Method and its composition for encapsulation, stabilization, and delivery of siRNA in anionic polymeric nanoplex: an in vitro-in vivo assessment. Sci. Rep. 2019, 9 (1), 16047. 10.1038/s41598-019-52390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devulapally R.; Foygel K.; Sekar T. V.; Willmann J. K.; Paulmurugan R. Gemcitabine and antisense-microRNA co-encapsulated PLGA–PEG polymer nanoparticles for hepatocellular carcinoma therapy. ACS Appl. Mater. Interfaces 2016, 8 (49), 33412–33422. 10.1021/acsami.6b08153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A. S. Dendrimers for drug delivery. Molecules 2018, 23 (4), 938. 10.3390/molecules23040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A. K. Dendrimers in targeted drug delivery applications: a review of diseases and cancer. Int. J. Polym. Mater. Polym. Biomater. 2021, 70 (4), 287–297. 10.1080/00914037.2020.1713780. [DOI] [Google Scholar]
- Sandoval-Yañez C.; Castro Rodriguez C. Dendrimers: amazing platforms for bioactive molecule delivery systems. Materials 2020, 13 (3), 570. 10.3390/ma13030570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherje A. P.; Jadhav M.; Dravyakar B. R.; Kadam D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548 (1), 707–720. 10.1016/j.ijpharm.2018.07.030. [DOI] [PubMed] [Google Scholar]
- Oddone N.; Lecot N.; Fernández M.; Rodriguez-Haralambides A.; Cabral P.; Cerecetto H.; Benech J. C. In vitro and in vivo uptake studies of PAMAM G4.5 dendrimers in breast cancer. J. Nanobiotechnol. 2016, 14 (1), 45. 10.1186/s12951-016-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi-Gaballu F.; Dehghan G.; Ghaffari M.; Yekta R.; Abbaspour-Ravasjani S.; Baradaran B.; Ezzati Nazhad Dolatabadi J.; Hamblin M. R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. 10.1016/j.apmt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janaszewska A.; Lazniewska J.; Trzepiński P.; Marcinkowska M.; Klajnert-Maculewicz B. Cytotoxicity of dendrimers. Biomolecules 2019, 9 (8), 330. 10.3390/biom9080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo-Rubio E.; Rodríguez-Izquierdo I.; Moreno-Domene M.; Lozano-Cruz T.; de la Mata F. J.; Gómez R.; Muñoz-Fernández M. A.; Jiménez J. L. Promising PEGylated cationic dendrimers for delivery of miRNAs as a possible therapy against HIV-1 infection. J. Nanobiotechnol. 2021, 19 (1), 158. 10.1186/s12951-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi C.; Miller K.; Mero A.; Satchi-Fainaro R.; Pasut G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Mol. Pharmaceutics 2011, 8 (4), 1063–1072. 10.1021/mp2001445. [DOI] [PubMed] [Google Scholar]
- Kucuksayan E.; Bozkurt F.; Yilmaz M. T.; Sircan-Kucuksayan A.; Hanikoglu A.; Ozben T. A new combination strategy to enhance apoptosis in cancer cells by using nanoparticles as biocompatible drug delivery carriers. Sci. Rep. 2021, 11 (1), 13027. 10.1038/s41598-021-92447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel A.; Wilczok A.; Jelonek K.; Musiał-Kulik M.; Foryś A.; Li S.; Kasperczyk J. Cytotoxic effect of paclitaxel and lapatinib co-delivered in polylactide-co-poly(ethylene glycol) micelles on HER-2-negative breast cancer cells. Pharmaceutics 2019, 11 (4), 169. 10.3390/pharmaceutics11040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannot V.; Gauche C.; Mazzaferro S.; Couvet M.; Vanwonterghem L.; Henry M.; Didier C.; Vollaire J.; Josserand V.; Coll J.-L.; Schatz C.; Lecommandoux S.; Hurbin A. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Controlled Release 2018, 275, 117–128. 10.1016/j.jconrel.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Sun W.; Chen X.; Xie C.; Wang Y.; Lin L.; Zhu K.; Shuai X. Co-delivery of doxorubicin and anti-BCL-2 siRNA by pH-responsive polymeric vector to overcome drug resistance in in vitro and in vivo HepG2 hepatoma model. Biomacromolecules 2018, 19 (6), 2248–2256. 10.1021/acs.biomac.8b00272. [DOI] [PubMed] [Google Scholar]
- Wu J.; Zhang H.; Hu X.; Liu R.; Jiang W.; Li Z.; Luan Y. Reduction-sensitive mixed micelles assembled from amphiphilic prodrugs for self-codelivery of DOX and DTX with synergistic cancer therapy. Colloids Surf. B Biointerfaces 2018, 161, 449–456. 10.1016/j.colsurfb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Yin Y.; Hu Q.; Xu C.; Qiao Q.; Qin X.; Song Q.; Peng Y.; Zhao Y.; Zhang Z. Co-delivery of doxorubicin and interferon-γ by thermosensitive nanoparticles for cancer immunochemotherapy. Mol. Pharmaceutics 2018, 15 (9), 4161–4172. 10.1021/acs.molpharmaceut.8b00564. [DOI] [PubMed] [Google Scholar]
- Wu J.; Tang C.; Yin C. Co-delivery of doxorubicin and interleukin-2 via chitosan based nanoparticles for enhanced antitumor efficacy. Acta Biomater. 2017, 47, 81–90. 10.1016/j.actbio.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Salzano G.; Costa D. F.; Sarisozen C.; Luther E.; Mattheolabakis G.; Dhargalkar P. P.; Torchilin V. P. Mixed nanosized polymeric micelles as promoter of doxorubicin and miRNA-34a co-delivery triggered by dual stimuli in tumor tissue. Small 2016, 12 (35), 4837–4848. 10.1002/smll.201600925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.; Qian J.; Suo A.; Cui N.; Yao Y.; Xu W.; Liu T.; Wang H. Co-delivery of doxorubicin and P-glycoprotein siRNA by multifunctional triblock copolymers for enhanced anticancer efficacy in breast cancer cells. J. Mater. Chem. B 2015, 3 (10), 2215–2228. 10.1039/C5TB00031A. [DOI] [PubMed] [Google Scholar]
- Jain A. K.; Thanki K.; Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharmaceutics 2013, 10 (9), 3459–3474. 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- Shrestha B.; Tang L.; Romero G. Nanoparticles-mediated combination therapies for cancer treatment. Adv. Ther. 2019, 2 (11), 1900076. 10.1002/adtp.201900076. [DOI] [Google Scholar]
- Golovin Y. I.; Gribanovsky S. L.; Golovin D. Y.; Klyachko N. L.; Majouga A. G.; Master A. M.; Sokolsky M.; Kabanov A. V. Towards nanomedicines of the future: Remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J. Controlled Release 2015, 219, 43–60. 10.1016/j.jconrel.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri P.; Glazer E. S.; Curley S. A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Delivery Rev. 2010, 62 (3), 339–345. 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.; Kiourti A.; Wang H.; Zhao S.; Zhao G.; Lu X.; Volakis J. L.; He X. Enhanced microwave hyperthermia of cancer cells with fullerene. Mol. Pharmaceutics 2016, 13 (7), 2184–2192. 10.1021/acs.molpharmaceut.5b00984. [DOI] [PubMed] [Google Scholar]
- Chang D.; Lim M.; Goos J. A. C. M.; Qiao R.; Ng Y. Y.; Mansfeld F. M.; Jackson M.; Davis T. P.; Kavallaris M. Biologically targeted magnetic hyperthermia: potential and limitations. Front. Pharmacol. 2018, 9, 831. 10.3389/fphar.2018.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Cheng P.; Chen P.; Pu K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018, 6 (4), 746–765. 10.1039/C7BM01210A. [DOI] [PubMed] [Google Scholar]
- Liu J.; Xu X.; Wang J.; Sang R.; Zhang Z.; Chen J.; Lu X.; Wang Q.; Fan Q. A diketopyrrolopyrrole-based conjugated polymer for efficient photodynamic and photothermal combination therapy under single 808 nm laser irradiation. Dyes Pigm. 2021, 196, 109762. 10.1016/j.dyepig.2021.109762. [DOI] [Google Scholar]
- Xiong Y.; Xu Z.; Li Z. Polydopamine-based nanocarriers for photosensitizer delivery. Front. Chem. 2019, 7, 471. 10.3389/fchem.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S.; Harini K.; Gajula G. P.; Sarmento B.; Neves-Petersen M. T.; Thiagarajan V. Multifunctional magnetic iron oxide nanoparticles: diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1 (1), 2. 10.1186/s42833-019-0002-6. [DOI] [Google Scholar]
- Tufani A.; Qureshi A.; Niazi J. H. Iron oxide nanoparticles based magnetic luminescent quantum dots (MQDs) synthesis and biomedical/biological applications: A review. Mater. Sci. Eng., C 2021, 118, 111545. 10.1016/j.msec.2020.111545. [DOI] [PubMed] [Google Scholar]
- Jeong Y.; Hwang H. S.; Na K. Theranostics and contrast agents for magnetic resonance imaging. Biomater. Res. 2018, 22 (1), 20. 10.1186/s40824-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. R.; Port J. D.; Pandey M. K. Use of superparamagnetic iron oxide nanoparticles (SPIONs) via multiple imaging modalities and modifications to reduce cytotoxicity: an educational review. J. Nanotheranostics 2020, 1 (1), 105–135. 10.3390/jnt1010008. [DOI] [Google Scholar]
- Pippa N.; Meristoudi A.; Pispas S.; Demetzos C. Temperature-dependent drug release from DPPC:C12H25-PNIPAM-COOH liposomes: Control of the drug loading/release by modulation of the nanocarriers’ components. Int. J. Pharm. 2015, 485 (1), 374–382. 10.1016/j.ijpharm.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Xu X.; Liu Y.; Fu W.; Yao M.; Ding Z.; Xuan J.; Li D.; Wang S.; Xia Y.; Cao M. Poly(N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12 (3), 580. 10.3390/polym12030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J.; Mendes L. P.; Yao M.; Filipczak N.; Garai S.; Thakur G. A.; Sarisozen C.; Torchilin V. P. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur. J. Pharm. Biopharm. 2019, 136, 18–28. 10.1016/j.ejpb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Sun A. n.; Liu Y.-j.; Zhang W.-j.; Pang N.; Cheng S.-x.; Qi X.-r. Amphiphilic dendrimer engineered nanocarrier systems for co-delivery of siRNA and paclitaxel to matrix metalloproteinase-rich tumors for synergistic therapy. NPG Asia Mater. 2018, 10 (4), 238–254. 10.1038/s41427-018-0027-4. [DOI] [Google Scholar]
- Kaneshiro T. L.; Lu Z.-R. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials 2009, 30 (29), 5660–5666. 10.1016/j.biomaterials.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Chen C.-W.; Syu W.-J.; Huang T.-C.; Lee Y.-C.; Hsiao J.-K.; Huang K.-Y.; Yu H.-P.; Liao M.-Y.; Lai P.-S. Encapsulation of Au/Fe3O4 nanoparticles into a polymer nanoarchitecture with combined near infrared-triggered chemo-photothermal therapy based on intracellular secondary protein understanding. J. Mater. Chem. B 2017, 5 (29), 5774–5782. 10.1039/C7TB00944E. [DOI] [PubMed] [Google Scholar]
- Bao T.; Yin W.; Zheng X.; Zhang X.; Yu J.; Dong X.; Yong Y.; Gao F.; Yan L.; Gu Z.; Zhao Y. One-pot synthesis of PEGylated plasmonic MoO3–x hollow nanospheres for photoacoustic imaging guided chemo-photothermal combinational therapy of cancer. Biomaterials 2016, 76, 11–24. 10.1016/j.biomaterials.2015.10.048. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhou J.; Chen R.; Shi R.; Zhao G.; Xia G.; Li R.; Liu Z.; Tian J.; Wang H.; Guo Z.; Wang H.; Chen Q. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. 10.1016/j.biomaterials.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Gong H.; Gao M.; Zhu W.; Sun X.; Feng L.; Fu T.; Li Y.; Liu Z. Polydopamine nanoparticles as a versatile molecular loading platform to enable imaging-guided cancer combination therapy. Theranostics 2016, 6 (7), 1031–1042. 10.7150/thno.14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Wang Z.; Cai P.; Liu J.; Liao L.-D.; Hong M.; Chen X.; Thakor N.; Liu B. Conjugated polymer and drug co-encapsulated nanoparticles for chemo- and photo-thermal combination therapy with two-photon regulated fast drug release. Nanoscale 2015, 7 (7), 3067–3076. 10.1039/C4NR06420H. [DOI] [PubMed] [Google Scholar]
- Chizenga E. P.; Abrahamse H. Nanotechnology in modern photodynamic therapy of cancer: a review of cellular resistance patterns affecting the therapeutic response. Pharmaceutics 2020, 12 (7), 632. 10.3390/pharmaceutics12070632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin G.; Gedik M. E.; Ayan S. Photodynamic therapy for the treatment and diagnosis of cancer–a review of the current clinical status. Front. Chem. 2021, 9, 608. 10.3389/fchem.2021.686303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Yang J.; Sun X. Reactive oxygen species-based nanomaterials for cancer therapy. Front. Chem. 2021, 9, 152. 10.3389/fchem.2021.650587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J.; Wang S. J.; Wang B.; Wang J. Z.; Wang J.; Zhang L. W.; Xin B.; Shen L. J.; Zhang Z. X.; Yao C. P. AIPcS4-PDT for gastric cancer therapy using gold nanorod, cationic liposome, and Pluronic® F127 nanomicellar drug carriers. Int. J. Nanomed. 2018, 13, 2017–2036. 10.2147/IJN.S154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Yu B.; Pathak J. L. An update in clinical utilization of photodynamic therapy for lung cancer. J. Cancer 2021, 12 (4), 1154–1160. 10.7150/jca.51537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendette A. C. F.; Piva H. L.; Muehlmann L. A.; de Souza D. A.; Tedesco A. C.; Azevedo R. B. Clinical treatment of intra-epithelia cervical neoplasia with photodynamic therapy. Int. J. Hyperthermia 2020, 37 (3), 50–58. 10.1080/02656736.2020.1804077. [DOI] [PubMed] [Google Scholar]
- Liou G.-Y.; Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44 (5), 479–496. 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khdair A.; Chen D.; Patil Y.; Ma L.; Dou Q. P.; Shekhar M. P. V.; Panyam J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance. J. Controlled Release 2010, 141 (2), 137–144. 10.1016/j.jconrel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Liu D.; Lin W. Self-Assembled core–shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 2015, 9 (1), 991–1003. 10.1021/nn506963h. [DOI] [PubMed] [Google Scholar]
- Li W.; Peng J.; Tan L.; Wu J.; Shi K.; Qu Y.; Wei X.; Qian Z. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified Docetaxel/IR820 co-loaded micelles. Biomaterials 2016, 106, 119–133. 10.1016/j.biomaterials.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Zeng J.-Y.; Zhang M.-K.; Peng M.-Y.; Gong D.; Zhang X.-Z. Porphyrinic metal–organic frameworks coated gold nanorods as a versatile nanoplatform for combined photodynamic/photothermal/chemotherapy of tumor. Adv. Funct. Mater. 2018, 28 (8), 1705451. 10.1002/adfm.201705451. [DOI] [Google Scholar]
- Nørgaard S. M.; Jensen C. S.; Aalestrup J.; Vandenbroucke-Grauls C. M. J. E.; de Boer M. G. J.; Pedersen A. B. Choice of therapeutic interventions and outcomes for the treatment of infections caused by multidrug-resistant gram-negative pathogens: a systematic review. Antimicrob. Resist. Infect. Control 2019, 8 (1), 170. 10.1186/s13756-019-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Burriel M.; Keys M.; Campillo-Artero C.; Agodi A.; Barchitta M.; Gikas A.; Palos C.; López-Casasnovas G. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS One 2020, 15 (1), e0227139 10.1371/journal.pone.0227139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapolisi A. M.; Nkanga C. I.; Walker R. B.; Krause R. W. M. Simultaneous liposomal encapsulation of antibiotics and proteins: co-loading and characterization of rifampicin and human serum albumin in soy-liposomes. J. Drug Delivery Sci. Technol. 2020, 58, 101751. 10.1016/j.jddst.2020.101751. [DOI] [Google Scholar]
- Hedayati Ch M.; Abolhassani Targhi A.; Shamsi F.; Heidari F.; Salehi Moghadam Z.; Mirzaie A.; Behdad R.; Moghtaderi M.; Akbarzadeh I. Niosome-encapsulated tobramycin reduced antibiotic resistance and enhanced antibacterial activity against multidrug-resistant clinical strains of Pseudomonas aeruginosa. J. Biomed. Mater. Res. Part A 2021, 109 (6), 966–980. 10.1002/jbm.a.37086. [DOI] [PubMed] [Google Scholar]
- Walvekar P.; Gannimani R.; Govender T. Combination drug therapy via nanocarriers against infectious diseases. Eur. J. Pharm. Sci. 2019, 127, 121–141. 10.1016/j.ejps.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Hosseini S. M.; Farmany A.; Abbasalipourkabir R.; Soleimani Asl S.; Nourian A.; Arabestani M. R. Doxycycline-encapsulated solid lipid nanoparticles for the enhanced antibacterial potential to treat the chronic brucellosis and preventing its relapse: in vivo study. Ann. Clin. Microbiol. Antimicrob. 2019, 18 (1), 33. 10.1186/s12941-019-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana L.; Gallego L.; Alkorta I. Incorporation of antibiotics into solid lipid nanoparticles: a promising approach to reduce antibiotic resistance emergence. Nanomaterials 2021, 11 (5), 1251. 10.3390/nano11051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi S.; Farhadian N.; Karimi M.; Ebrahimi M. Enhanced bactericidal effect of ceftriaxone drug encapsulated in nanostructured lipid carrier against gram-negative Escherichia coli bacteria: drug formulation, optimization, and cell culture study. Antimicrob. Resist. Infect. Control 2020, 9 (1), 28. 10.1186/s13756-020-0690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghassemi S.; Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Controlled Release 2014, 185, 22–36. 10.1016/j.jconrel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Eleraky N. E.; Allam A.; Hassan S. B.; Omar M. M. Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12 (2), 142. 10.3390/pharmaceutics12020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; Sharma S.; Khuller G. K. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J. Antimicrob. Chemother. 2004, 54 (4), 761–766. 10.1093/jac/dkh411. [DOI] [PubMed] [Google Scholar]
- Shaaban M. I.; Shaker M. A.; Mady F. M. Imipenem/cilastatin encapsulated polymeric nanoparticles for destroying carbapenem-resistant bacterial isolates. J. Nanobiotechnol. 2017, 15 (1), 29. 10.1186/s12951-017-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeszczuk Z.; Rosillo A.; Owens Ó.; Bhattacharjee S. Atomic force microscopy (AFM) as a surface mapping tool in microorganisms resistant toward antimicrobials: a mini-review. Front. Pharmacol. 2020, 11, 1588. 10.3389/fphar.2020.517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N.-K.; Yin J.-J.; Yang Y.-X.; Wang Z.-L.; Zhou Z.-W.; He Z.-X.; Chen X.-W.; Zhang X.; Duan W.; Yang T.; Zhou S.-F. Novel targeting of PEGylated liposomes for codelivery of TGF-β1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study. Drug Des. Devel. Ther. 2015, 9, 4441–4470. 10.2147/DDDT.S79369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R.; Zahoor A.; Sharma S.; Khuller G. K. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis 2003, 83 (6), 373–378. 10.1016/j.tube.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Diniz L. F.; Carvalho P. S.; de Melo C. C.; Ellena J. Reducing the hygroscopicity of the anti-tuberculosis drug (S,S)-ethambutol using multicomponent crystal forms. Cryst. Growth Des. 2017, 17 (5), 2622–2630. 10.1021/acs.cgd.7b00144. [DOI] [Google Scholar]
- Singh L.; Kruger H. G.; Maguire G. E. M.; Govender T.; Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4 (4), 105–131. 10.1177/2049936117713593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q.; Zhou A.; Zou H.; Ingle S.; May M. T.; Cai W.; Cheng C.-Y.; Yang Z.; Tang J. Quadruple versus triple combination antiretroviral therapies for treatment naive people with HIV: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019, 366, l4179. 10.1136/bmj.l4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Su S.; Borné Y. A meta-analysis of the efficacy of HAART on HIV transmission and its impact on sexual risk behaviours among men who have sex with men. Sci. Rep. 2020, 10 (1), 1075. 10.1038/s41598-019-56530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts E. J.; Hazuda D. J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2 (4), a007161 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date A. A.; Destache C. J. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials 2013, 34 (26), 6202–6228. 10.1016/j.biomaterials.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria M. J.; Lopes C. M.; das Neves J.; Lúcio M. Lipid nanocarriers for anti-HIV therapeutics: a focus on physicochemical properties and biotechnological advances. Pharmaceutics 2021, 13 (8), 1294. 10.3390/pharmaceutics13081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana L. N.; Sharma S.; Sethuraman S.; Ranga U.; Krishnan U. M. Stealth anti-CD4 conjugated immunoliposomes with dual antiretroviral drugs – modern Trojan horses to combat HIV. Eur. J. Pharm. Biopharm. 2015, 89, 300–311. 10.1016/j.ejpb.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Yang H.; Li J.; Patel S. K.; Palmer K. E.; Devlin B.; Rohan L. C. Design of poly(lactic-co-glycolic acid) (PLGA) nanoparticles for vaginal co-delivery of griffithsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics 2019, 11 (4), 184. 10.3390/pharmaceutics11040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destache C. J.; Belgum T.; Christensen K.; Shibata A.; Sharma A.; Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect. Dis. 2009, 9 (1), 198. 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date A. A.; Shibata A.; Goede M.; Sanford B.; La Bruzzo K.; Belshan M.; Destache C. J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir + efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral Res. 2012, 96 (3), 430–436. 10.1016/j.antiviral.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balikagala B.; Fukuda N.; Ikeda M.; Katuro O. T.; Tachibana S. I.; Yamauchi M.; Opio W.; Emoto S.; Anywar D. A.; Kimura E.; Palacpac N. M. Q.; Odongo-Aginya E. I.; Ogwang M.; Horii T.; Mita T. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 2021, 385 (13), 1163–1171. 10.1056/NEJMoa2101746. [DOI] [PubMed] [Google Scholar]
- Aderibigbe B. A. Design of drug delivery systems containing artemisinin and its derivatives. Molecules 2017, 22 (2), 323. 10.3390/molecules22020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H.; Hurd Price C. A.; Jing L.; Tian Q.; Liu J.; Qian K. Janus particles: design, preparation, and biomedical applications. Mater. Today Bio 2019, 4, 100033. 10.1016/j.mtbio.2019.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande C.; Fabre P.; Raphael E.; Veyssie M. Janus beads - realization and behavior at water oil interfaces. Europhys. Lett. 1989, 9 (3), 251–255. 10.1209/0295-5075/9/3/011. [DOI] [Google Scholar]
- de Gennes P. G. Soft matter. Science 1992, 256 (5056), 495–497. 10.1126/science.256.5056.495. [DOI] [PubMed] [Google Scholar]
- Le T. C.; Zhai J. L.; Chiu W. H.; Tran P. A.; Tran N. Janus particles: recent advances in the biomedical applications. Int. J. Nanomed. 2019, 14, 6749–6777. 10.2147/IJN.S169030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal G.; Agrawal R. Janus nanoparticles: recent advances in their interfacial and biomedical applications. ACS Appl. Nano Mater. 2019, 2 (4), 1738–1757. 10.1021/acsanm.9b00283. [DOI] [Google Scholar]
- Rahiminezhad Z.; Tamaddon A. M.; Borandeh S.; Abolmaali S. S. Janus nanoparticles: New generation of multifunctional nanocarriers in drug delivery, bioimaging and theranostics. Appl. Mater. Today 2020, 18, 100513. 10.1016/j.apmt.2019.100513. [DOI] [Google Scholar]
- Greydanus B.; Schwartz D. K.; Medlin J. W. Controlling catalyst-phase selectivity in complex mixtures with amphiphilic Janus particles. ACS Appl. Mater. Interfaces 2020, 12 (2), 2338–2345. 10.1021/acsami.9b16957. [DOI] [PubMed] [Google Scholar]
- Sánchez A.; Ovejero Paredes K.; Ruiz-Cabello J.; Martínez-Ruíz P.; Pingarrón J. M.; Villalonga R.; Filice M. Hybrid decorated core@shell Janus nanoparticles as a flexible platform for targeted multimodal molecular bioimaging of cancer. ACS Appl. Mater. Interfaces 2018, 10 (37), 31032–31043. 10.1021/acsami.8b10452. [DOI] [PubMed] [Google Scholar]
- Yánez-Sedeño P.; Campuzano S.; Pingarrón J. M. Janus particles for (bio)sensing. Appl. Mater. Today 2017, 9, 276–288. 10.1016/j.apmt.2017.08.004. [DOI] [Google Scholar]
- Wijetunge S. S.; Wen J.; Yeh C.-K.; Sun Y. Wheat germ agglutinin liposomes with surface grafted cyclodextrins as bioadhesive dual-drug delivery nanocarriers to treat oral cells. Colloids Surf. B Biointerfaces 2020, 185, 110572. 10.1016/j.colsurfb.2019.110572. [DOI] [PubMed] [Google Scholar]
- Abdelghany S.; Parumasivam T.; Pang A.; Roediger B.; Tang P.; Jahn K.; Britton W. J.; Chan H.-K. Alginate modified-PLGA nanoparticles entrapping amikacin and moxifloxacin as a novel host-directed therapy for multidrug-resistant tuberculosis. J. Drug Delivery Sci. Technol. 2019, 52, 642–651. 10.1016/j.jddst.2019.05.025. [DOI] [Google Scholar]
- Liu P.; Guo B.; Wang S.; Ding J.; Zhou W. A thermo-responsive and self-healing liposome-in-hydrogel system as an antitubercular drug carrier for localized bone tuberculosis therapy. Int. J. Pharm. 2019, 558, 101–109. 10.1016/j.ijpharm.2018.12.083. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. M.; El-Bisi M. K.; Taha G. M.; El-Alfy E. A. Chitosan nanoparticles loaded antibiotics as drug delivery biomaterial. J. Appl. Pharm. Sci. 2015, 5, 085–090. 10.7324/JAPS.2015.501015. [DOI] [Google Scholar]
- Kalhapure R. S.; Sonawane S. J.; Sikwal D. R.; Jadhav M.; Rambharose S.; Mocktar C.; Govender T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf. B Biointerfaces 2015, 136, 651–658. 10.1016/j.colsurfb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Prathipati P. K.; Kang G.; Zhou Y.; Yuan Z.; Fan W.; Li Q.; Destache C. J. Tenofovir alafenamide and elvitegravir loaded nanoparticles for long-acting prevention of HIV-1 vaginal transmission. AIDS 2017, 31 (4), 469–476. 10.1097/QAD.0000000000001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft J. C.; McConnachie L. A.; Koehn J.; Kinman L.; Collins C.; Shen D. D.; Collier A. C.; Ho R. J. Y. Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma. AIDS 2017, 31 (6), 765–770. 10.1097/QAD.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K.; Li D.; Guo S.; Penzak S.; Dong X. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Controlled Release 2016, 226, 88–97. 10.1016/j.jconrel.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Velasques K.; Maciel T. R.; de Castro Dal Forno A. H.; Teixeira F. E. G.; da Fonseca A. L.; Varotti F. d. P.; Fajardo A. R.; Ávila D. S. d.; Haas S. E. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against Plasmodium falciparum and decreases their toxicity to Caenorhabditis elegans. Eur. J. Pharm. Sci. 2018, 118, 1–12. 10.1016/j.ejps.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Prabhu P.; Suryavanshi S.; Pathak S.; Patra A.; Sharma S.; Patravale V. Nanostructured lipid carriers of artemether–lumefantrine combination for intravenous therapy of cerebral malaria. Int. J. Pharm. 2016, 513 (1), 504–517. 10.1016/j.ijpharm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Jain S. A.; Basu H.; Prabhu P. S.; Soni U.; Joshi M. D.; Mathur D.; Patravale V. B.; Pathak S.; Sharma S. Parasite impairment by targeting Plasmodium-infected RBCs using glyceryl-dilaurate nanostructured lipid carriers. Biomaterials 2014, 35 (24), 6636–6645. 10.1016/j.biomaterials.2014.04.058. [DOI] [PubMed] [Google Scholar]
- Xie H.; She Z.-G.; Wang S.; Sharma G.; Smith J. W. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 2012, 28 (9), 4459–4463. 10.1021/la2042185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam R.; Zilli M.; Maas M.; Rezwan K. Nanoscale Janus particles with dual protein functionalization. Part. Part. Syst. Charact. 2018, 35 (3), 1700332. 10.1002/ppsc.201700332. [DOI] [Google Scholar]
- Hwang S.; Lahann J. Differentially degradable Janus particles for controlled release applications. Macromol. Rapid Commun. 2012, 33 (14), 1178–1183. 10.1002/marc.201200054. [DOI] [PubMed] [Google Scholar]
- Mou F.; Chen C.; Zhong Q.; Yin Y.; Ma H.; Guan J. Autonomous motion and temperature-controlled drug delivery of Mg/Pt-poly(N-isopropylacrylamide) Janus micromotors driven by simulated body fluid and blood plasma. ACS Appl. Mater. Interfaces 2014, 6 (12), 9897–9903. 10.1021/am502729y. [DOI] [PubMed] [Google Scholar]
- Feng Z.-Y.; Liu T.-T.; Sang Z.-T.; Lin Z.-S.; Su X.; Sun X.-T.; Yang H.-Z.; Wang T.; Guo S. Microfluidic preparation of Janus microparticles with temperature and pH triggered degradation properties. Front. Bioeng. Biotechnol. 2021, 9, 830. 10.3389/fbioe.2021.756758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani E.; Salami-Kalajahi M.; Roghani-Mamaqani H. Simultaneous two drugs release form Janus particles prepared via polymerization-induced phase separation approach. Colloids Surf. B Biointerfaces 2018, 170, 85–91. 10.1016/j.colsurfb.2018.05.067. [DOI] [PubMed] [Google Scholar]
- Zare M.; Bigham A.; Zare M.; Luo H.; Rezvani Ghomi E.; Ramakrishna S. pHEMA: an overview for biomedical applications. Int. J. Mol. Sci. 2021, 22 (12), 6376. 10.3390/ijms22126376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Li S. H.; Feng Z. X.; Wang M.; Cai C. Z.; Wang J. F.; Zhang L. J. Poly(2-(diethylamino)ethyl methacrylate)-based, pH-responsive, copolymeric mixed micelles for targeting anticancer drug control release. Int. J. Nanomed. 2017, 12, 6857–6870. 10.2147/IJN.S143927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzenko O. B.; Winkler J.; Tomassone M. S.; Minko T. Biodegradable Janus nanoparticles for local pulmonary delivery of hydrophilic and hydrophobic molecules to the lungs. Langmuir 2014, 30 (43), 12941–12949. 10.1021/la502144z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommes C. J. Ostwald ripening of confined nanoparticles: chemomechanical coupling in nanopores. Nanoscale 2019, 11 (15), 7386–7393. 10.1039/C9NR01349K. [DOI] [PubMed] [Google Scholar]
- Doan-Nguyen T. P.; Jiang S.; Koynov K.; Landfester K.; Crespy D. Ultrasmall nanocapsules obtained by controlling Ostwald ripening. Angew. Chem., Int. Ed. 2021, 60 (33), 18094–18102. 10.1002/anie.202103444. [DOI] [PubMed] [Google Scholar]
- Ranquin A.; Versées W.; Meier W.; Steyaert J.; Van Gelder P. Therapeutic nanoreactors: combining chemistry and biology in a novel triblock copolymer drug delivery system. Nano Lett. 2005, 5 (11), 2220–2224. 10.1021/nl051523d. [DOI] [PubMed] [Google Scholar]
- Weinstein Z. B.; Bender A.; Cokol M. Prediction of synergistic drug combinations. Curr. Opin. Syst. Biol. 2017, 4, 24–28. 10.1016/j.coisb.2017.05.005. [DOI] [Google Scholar]
- Cuvitoglu A.; Zhou J. X.; Huang S.; Isik Z. Predicting drug synergy for precision medicine using network biology and machine learning. J. Bioinform. Comput. Biol. 2019, 17 (02), 1950012. 10.1142/S0219720019500124. [DOI] [PubMed] [Google Scholar]
- Yang M.; Jaaks P.; Dry J.; Garnett M.; Menden M. P.; Saez-Rodriguez J. Stratification and prediction of drug synergy based on target functional similarity. NPJ. Syst. Biol. Appl. 2020, 6 (1), 16. 10.1038/s41540-020-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.; Cao D.-S.; Miao H.-Y.; Liu S.; Deng B.-C.; Yun Y.-H.; Wang N.-N.; Lu A.-P.; Zeng W.-B.; Chen A. F. ChemDes: an integrated web-based platform for molecular descriptor and fingerprint computation. J. Cheminform. 2015, 7 (1), 60. 10.1186/s13321-015-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard T.; Hagan H.; Tseng S.; Sosso G. C. Less may be more: an informed reflection on molecular descriptors for drug design and discovery. Mol. Syst. Des. Eng. 2020, 5 (1), 317–329. 10.1039/C9ME00109C. [DOI] [Google Scholar]
- Bhattacharjee S. Molecular descriptors as a facile tool toward designing surface-functionalized nanoparticles for drug delivery. Mol. Pharmaceutics 2022, 19 (4), 1168–1175. 10.1021/acs.molpharmaceut.1c00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7 (1), 42717. 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. The drug delivery field at the inflection point: Time to fight its way out of the egg. J. Controlled Release 2017, 267, 2–14. 10.1016/j.jconrel.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Park K. The beginning of the end of the nanomedicine hype. J. Controlled Release 2019, 305, 221–222. 10.1016/j.jconrel.2019.05.044. [DOI] [PubMed] [Google Scholar]
- Gagliardi A.; Giuliano E.; Venkateswararao E.; Fresta M.; Bulotta S.; Awasthi V.; Cosco D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 17. 10.3389/fphar.2021.601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioni L.; Rizzuto M. A.; Bertolini J. A.; Pandolfi L.; Colombo M.; Prosperi D. Thirty years of cancer nanomedicine: success, frustration, and hope. Cancers 2019, 11 (12), 1855. 10.3390/cancers11121855. [DOI] [PMC free article] [PubMed] [Google Scholar]