Abstract
Context:
Despite growing interest in quantifying and correcting vitamin D inadequacy in basketball players, a critical synthesis of these data is yet to be performed to overcome the low generalizability of findings from individual studies.
Objective:
To provide a comprehensive analysis of data in basketball pertaining to (1) the prevalence of vitamin D inadequacy; (2) the effects of vitamin D supplementation on 25-hydroxyvitamin D [25(OH)D] concentration (and its association with body composition), bone health, and performance; and (3) crucial aspects that warrant further investigation.
Data Sources:
PubMed, MEDLINE, ERIC, Google Scholar, SCIndex, and ScienceDirect databases were searched.
Study Selection:
After screening, 15 studies were included in the systematic review and meta-analysis.
Study Design:
Systematic review and meta-analysis.
Level of Evidence:
Level 3.
Data Extraction:
The prevalence of vitamin D inadequacy, serum 25(OH)D, body composition, stress fractures, and physical performance were extracted.
Results:
The pooled prevalence of vitamin D inadequacy for 527 basketball players in 14 studies was 77% (P < 0.001; 95% CI, 0.70-0.84). Supplementation with 4000 IU/d and 4000 IU/wk (absolute mean difference [AMD]: 25.39 nmol/L; P < 0.001; 95% CI, 13.44-37.33), as well as 10,000 IU/d (AMD: 100.01; P < 0.001; 95% CI, 70.39-129.63) vitamin D restored 25(OH)D to normal concentrations. Body composition data revealed inverse correlations between changes in serum 25(OH)D (from pre- to postsupplementation) and body fat (r = −0.80; very large). Data concerning positive impacts of vitamin D supplementation on bone health and physical performance remain sparse.
Conclusion:
The high proportion of vitamin D inadequacy underscores the need to screen for serum 25(OH)D in basketball players. Although supplementation restored vitamin D sufficiency, the beneficial effects on bone health and physical performance remain sparse. Adiposity can modulate 25(OH)D response to supplementation.
Keywords: 25(OH)D, vitamin D deficiency, vitamin D insufficiency, vitamin D supplementation
Vitamin D is a fat-soluble prohormone that occurs in 2 biological forms, cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Cholecalciferol is the primary source of endogenous vitamin D and is formed through sunlight ultraviolet-B (UVB) exposure of the skin, with a small amount typically coming from the diet. Plants (eg, mushrooms) contain vitamin D2, while oily fish, eggs, and liver contain vitamin D3. 27 Endogenously synthesized vitamin D3, as well as diet-derived D2 and D3, undergo hydroxylation within the liver, which then converts vitamin D into the biologically inactive (but stable), metabolite, 25-hydroxyvitamin D [25(OH)D, calcidiol]. Then, 25(OH)D is hydroxylated further within the kidney to form the biologically active metabolite, 1,25 dihydroxyvitamin D (calcitriol), which then binds to the vitamin D receptor at target tissues (bone, immune system cells, the cardiovascular system, and skeletal muscle). 37
Inadequate serum vitamin D concentrations are a potential cause of compromised immune function, physical performance, bone health, and recovery in athletes engaging in heavy training regimens. 27 Basketball players undergo rigorous training and competition schedules, which presents significant physiological challenges to the human immune system, resulting in an increased risk of infection from a likely shift in balance between pro- and anti-inflammatory cytokines.22,31 In addition, basketball players may be more susceptible to hypovitaminosis D because of the reduced sunlight exposure from training (and competing) indoors.
The prevalence of vitamin D inadequacy in basketball players has been extensively studied over the past decade.5,6,8,12,17-20,33,34,40,42,46,49 In addition, there has been increased awareness of the importance of vitamin D supplementation to maintain appropriate serum 25(OH)D status, in recent years.3,6,34,40,42,49 Despite growing interest in quantifying and correcting vitamin D inadequacy in basketball players, a critical synthesis of these data is yet to be performed to overcome the low generalizability of findings from individual studies. Therefore, this systematic review and meta-analysis aimed to provide a comprehensive analysis of data in basketball pertaining to (1) the prevalence of vitamin D inadequacy; (2) the effects of vitamin D supplementation on 25(OH)D concentration (and its association with body composition), bone health, and performance; and (3) crucial aspects that warrant further investigation in future studies.
Methods
Search Strategy
We performed a database search for all relevant articles published prior to September 26, 2020, on the following electronic platforms: PubMed, MEDLINE, ERIC, Google Scholar, SCIndex, and ScienceDirect. Two groups of terms were used in the literature search as follows: (1) basketball and basketball players and (2) vitamin D, 25-hydroxyvitamin D, cholecalciferol, vitamin D deficiency, vitamin D insufficiency, vitamin D supplementation, and vitamin D status. Terms within each group were separated by the operator “OR,” and each group of terms was combined with the operator “AND.” The reference lists of included articles were also manually searched for any suitable articles that were not identified in the electronic database search. One author conducted the electronic database search and identified all relevant studies. The final selection (full-text analysis of relevant articles) process was then undertaken by 2 authors. Any disagreements between the 2 authors regarding article inclusion were resolved by consensus, or if an agreement was not reached, a third author was consulted to establish consensus. This process for screening articles followed the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) recommendations. 35
Inclusion Criteria
This review included cross-sectional and longitudinal studies (randomized, nonrandomized, uncontrolled) written in English. Abstracts from conference proceedings were also eligible, if enough data were provided. The participants included all healthy amateur, collegiate, and/or elite basketball players. No restrictions were set according to age, sex, regional location, and ethnicity of participants. Articles considered for inclusion into this review were those reporting the prevalence of vitamin D inadequacy (deficiency and insufficiency) and/or examining the effect of vitamin D supplementation (not limited to any dosage and duration) on 25(OH)D, body composition, bone health, athletic performance, immune system, exercise-related inflammation, and cardiac structure and/or function.
Data Extraction
The Cochrane Consumers and Communication Review Group’s data extraction protocol was used whereby the following data were collated from studies examining the prevalence of vitamin D inadequacy:
Characteristics of data collection: location, latitude, period, method
Participant characteristics: ethnicity, playing level, sample size, age, and sex
Vitamin D status: cutoff values for vitamin D inadequacy (deficient and/or insufficiency) as well as percentage and sample size within each category. The extracted data were restricted to dichotomous outcomes, as all the data for the analysis are adverse events expressed as events/total (eg, deficiency/total).
Data detailing geographical location, latitudes, sample size, duration, dose, and key outcomes (serum 25(OH)D concentrations, body composition, stress fractures, and physical performance) were extracted from longitudinal studies that assessed the effect of vitamin D supplementation. The raw data were either directly obtained from tables or extracted from figures by digitizing the graphs using GetData Graph Digitizer (Version 2.24). Latitudes were retrieved from the website http://www.worldatlas.com if not reported. Data were extracted from each article by the lead author and checked for accuracy and completeness by another author.
Definition of Vitamin D Inadequacy
An evidence-based consensus regarding the 25(OH)D concentration used to define hypovitaminosis D in athletes is needed. The most authoritative reports for defining vitamin D status have led to confusion among clinicians, researchers, and the public because of the disagreement in data interpretation. The Institute of Medicine 41 concluded that serum 25(OH)D levels of 40 nmol/L cover the requirements of approximately half the population, and levels of 50 nmol/L cover the requirements of at least 97.5% of the population. The 2nd International Conference on Controversies in Vitamin D, 21 held in Monteriggioni (Siena), Italy in 2018 reached a consensus on the optimal vitamin D concentrations between 50 and 125 nmol/L in the general population. On the other hand, the US Endocrine Society 30 reported a 25(OH)D level <50 nmol/L as the “cutoff” to define vitamin D deficiency, a 25(OH)D level between 50 and 75 nmol/L to define vitamin D insufficiency, and a 25(OH)D level >75 nmol/L as the optimal level. Also, emerging research indicates that additional sports health benefits require 25(OH)D concentrations between 50 and 75 nmol/L. 43 In this regard, the cutoff values for vitamin insufficiency varied from 50 to 70 to 80 nmol/L,5,8,12,17-20,33,40,42,46,49 with a consistent level of 25(OH)D <50 nmol/L defining vitamin D deficiency5,6,8,17-20,33,34,40,46 across studies concerning vitamin D status in basketball players. In our meta-analysis, vitamin D deficiency and insufficiency were categorized as “vitamin D inadequacy” (<80 nmol/L) as was suggested previously. 16 According to the Institute of Medicine, serum 25(OH)D concentrations of >180 nmol/L was categorized as a threshold level for vitamin D toxicity. 37 The units of serum 25(OH)D measures were reported in nmol/L for consistency, where 2.496 nmol/L is equal to 1 ng/mL.
Quality Assessment
The risk of bias was assessed using the checklist developed by the Agency for Healthcare Research and Quality (AHRQ). 47 The number of items was tailored to the study design (cross-sectional, n = 9; uncontrolled, n = 9; randomized controlled trials, n = 12; nonrandomized controlled trials, n = 13) as recommended previously. 47 Each item is scored as “1” (yes) or “0” (no/unable to determine), and the scores for each item are summed to provide the total quality score. Design-specific criteria to assess the risk of bias are presented in Appendix Table A1 (available in the online version of this article).
Statistical Analysis
The single-arm meta-analysis of proportions was performed using OpenMeta-Analyst software (Center for Evidence-Based Medicine, School of Public Health, Brown University, Providence, RI). 48 Differences in proportions and 95% CIs were calculated using a continuous random-effect model to incorporate heterogeneity among studies. Heterogeneity between studies was expressed with the inconsistency index (I2) and interpreted as follows 29 : no heterogeneity = 0% to 25%, low heterogeneity = 26% to 50%, moderate heterogeneity = 51% to 75%, and high heterogeneity = 76% to 100%. Subgroup analyses were conducted to investigate whether the prevalence of vitamin D inadequacy varied across sexes (if the results were presented separately for male and female patients), seasons (winter and spring vs summer and autumn) and latitudes. The median value of latitude (37°N) was applied as a covariate for stratification. A P value of 0.05 was used for statistical significance.
Since the number of available studies examining the effect of vitamin D supplementation was small (<3 with similar methodological design [eg, uncontrolled trials, controlled trials: placebo or nonsupplementation]) to estimate the between-study heterogeneity, 10 a fixed-effects model was applied. The absolute mean differences (95% CIs) in 25(OH)D were estimated across individual trials and subgroups synthesizing studies with similar methodological design (eg, vitamin D pre vs post; vitamin D vs placebo; vitamin D vs nonsupplementation). A meta-analysis of studies examining the effects of vitamin D supplementation on athletic performance, body composition, and bone health was not performed since the outcome measures were inconsistent.
Results
Study Selection
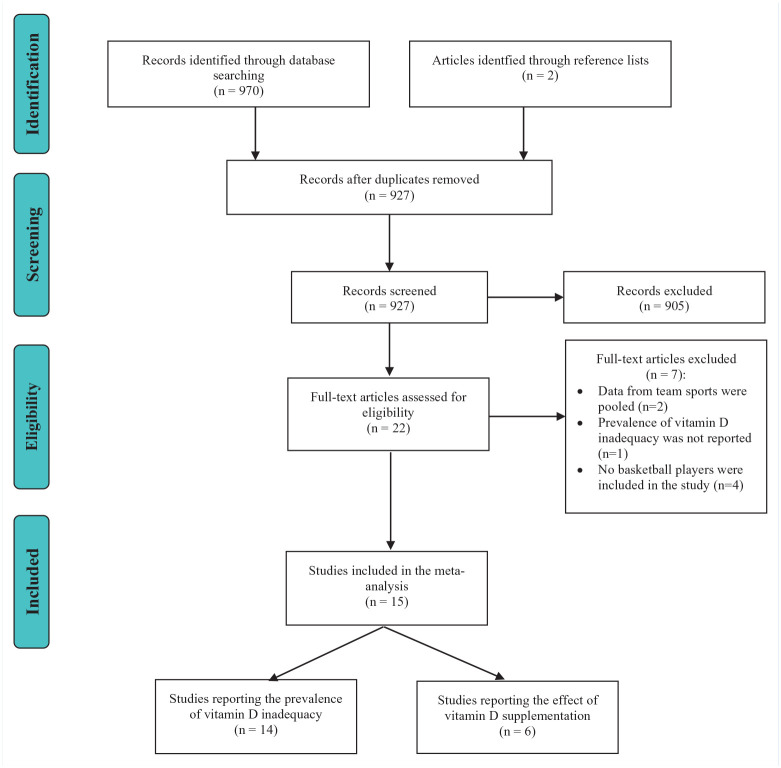
The study selection process is presented in Figure 1. A total of 970 relevant studies were identified from the database searches with an additional 2 studies identified via the reference lists. After removal of duplicates and screening of study titles and abstracts, 22 studies remained. After the final full-text screening process, 15 studies were included in the systematic review and meta-analysis.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram displaying the identification, screening, and selection of relevant studies in this meta-analysis.
Quality Assessment
The ratings from the AHRQ scale for each article are presented in Appendix Table A2 (available online). The mean quality scores of the AHRQ scale were 7.3 ± 1.8 (out of 9) for cross-sectional studies, 4 (out of 9) for uncontrolled studies, 10 ± 1 (out of 12) for randomized controlled studies, and 9.7 ± 1.9 (out of 13) for nonrandomized controlled studies.
Study Characteristics
Publication dates ranged from 2010 to 2020. Studies included in our review used cross-sectional design (prevalence of vitamin D inadequacy),5,8,12,17-20,33,46 uncontrolled design (vitamin D supplementation pre vs post), 6 nonrandomized controlled design (vitamin D supplementation vs nonsupplementation),40,42 and randomized controlled design (vitamin D supplementation vs placebo).3,34 The characteristics of the participants investigated in the included articles are presented in Table 1. The sample size ranged from 7 to 279 players across studies examining the prevalence of vitamin D inadequacy. Six studies recruited both male and female basketball players,12,18,40,42,46,49 of which 2 studies presented data separately across sexes.18,46 Five studies recruited only male basketball players,8,19,20,33,34 while 3 studies recruited only female basketball players.5,6,17 Basketball players were recruited from the United States,6,17-19,34,40,42,46,49 Canada, 8 Israel, 12 Spain, 20 Korea, 33 and Lithuania. 5 Considering period of data collection, 7 studies were performed from December to June (winter/spring),5,6,12,17,20,33,40 5 studies were performed from July to November (summer/fall),8,18,42,46,49 or period was not specified.19,34 In interventional studies, the supplement dosage varied from 4000 IU/wk to 10,000 IU/d. The length of the interventions ranged from 8 weeks to 5 months. Outcome measures were inconsistent across the included studies examining the effect of vitamin D supplementation, including bone mineral density (BMD),3,6,40 percentage body fat,3,40 fat-free mass, 40 lean body mass, 3 vertical jump,3,34 20-m sprint, 34 and 5-10-5 agility drill. 34
Table 1.
Characteristics of data collection, study participants, and prevalence of vitamin D inadequacy
| Data Collection | Participants | Vitamin D Inadequacy a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Latitude | Region | Period | Method | Ethnicity | Playing Level | Sample Size | Age, y | Sex | Criteria for Vitamin D Status, nmol/L | Outcomes |
| Constantini et al 12 | 32°N | Israel | November to April | Radioimmunoassay | NR | NR | 16 | 14.7 ± 3.0 | M and F | Insufficiency <75 | 94%; n = 15 |
| Garcia and Guisado 20 | 41°N | Barcelona, Spain | March and April | Chemiluminescence assay method | 76% WC 24% AA |
Elite basketball players | 21 | 25.0 ± 4.3 | M | Deficient <50 Insufficiency 50-75 |
57%; n = 12 33%; n = 7 |
| Bellows et al 6 | 31°N | Texas | April | NR | AA | NCAA Division I | 8 | 21.0 ± 1.0 | F | Deficient < 50 | 100%; n = 8 |
| Villacis et al 46 | 34°N | Southern California | End of the summer season | Liquid chromatography–tandem mass spectrometry | 85% AA 15% WC |
NCAA Division I | 13 | NR | M | Deficient <50 Insufficiency 50-80 |
8%; n = 1 38%; n = 5 |
| Villacis et al 46 | 34°N | Southern California | End of the summer season | Liquid chromatography–tandem mass spectrometry | 69% AA 31% WC |
NCAA Division I | 13 | NR | F | Deficient <50 Insufficiency 50-80 |
8%; n = 1 54%; n = 7 |
| Bubbs 8 | 61°N | Canada | July | NR | NR | Canadian Men’s Olympic Basketball team | 7 | NR | M | Deficient <50 Insufficiency 50-75 |
14%; n = 1 71%; n = 5 |
| Fishman et al 19 | 34°N | California | NR | NR | AA | NBA Combine | 279 | 21.5 ± 1.3 | M | Deficient <50 Insufficiency 50-80 |
32%; n = 90 47%; n = 131 |
| Micah and Robert-McComb 34 | 31°N | Texas | NR | Liquid chromatography–tandem mass spectrometry | 53% WC 47% AA |
NCAA, recreational, intramural | 17 | 18-28 | M | Deficient <50 | 52%; n = 9 |
| Fields et al 18 | 37°N | Virginia | October | Enzyme-linked immunosorbent assay | 91% AA | NCAA Division I | 11 | 19.6 ± 1.3 | M | Deficient <50 Insufficiency 50-75 |
36%; n = 4 64%; n = 7 |
| Fields et al 18 | 37°N | Virginia | October | Enzyme-linked immunosorbent assay | 56% WA | NCAA Division I | 9 | 20.1 ± 1.0 | F | Deficient < 50 Insufficiency 50-75 |
0%; n = 0 33%; n = 3 |
| Rockwell 40 | 37°N | Virginia | May | Immunoche-miluminometric assay | 63% AA 21% WC 16% other |
NCAA Division I | 24 | 20.1 ± 0.9 | M and F | Deficient <50 Insufficiency 50-75 |
29%; n = 7 50%; n = 12 |
| Sekel et al 42 | 37°N | Virginia | October | Enzyme-linked immunosorbent assay | 30% WC 60% AA 10% other |
NCAA Division I | 20 | 20.2 ± 0.8 | M and F | Insufficiency <75 | 65%; n = 13 |
| Williams et al 49 | 34°N | Columbia | August | NR | 46% WC 36% AA 8% other |
NCAA Division I | 27 | 19.7 ± 1.2 | M and F | Insufficiency <75 | 54%; n = 15 |
| Kim et al 33 | 37°N | South Korea | April | Liquid chromatography–tandem mass spectrometry | Korean | Professional Korean Basketball League | 36 | 22.6 ± 3.0 | M | Deficient <50 Insufficiency 50-80 |
30%; n =11 42%; n = 15 |
| Fields et al 17 | 37°N | Virginia | January-February | Enzyme-linked immunosorbent assay | 50% fair 50% dark |
NCAA Division I | 12 | 20.2 ± 1.6 | F | Deficient <50 Insufficiency 50-75 |
50%; n = 6 33%; n = 4 |
| Baranauskas et al 5 | 55°N | Lithuania | May | NR | Lithuanians | Lithuanian deaf basketball team | 14 | 26.4 ± 4.5 | F | Deficient <50 Insufficiency 50-75 |
29%; n = 4 57%; n = 8 |
AA, African American; F, female; M, male; NCAA, National Collegiate Athletic Association; NR, not reported; WA, White Anglo; WC, White Caucasian.
Circulating 25(OH)D <50 nmol/L is defined as deficient and 50 to 75 or 80 nmol/L is defined as insufficient.
Study Outcomes
Vitamin D Inadequacy
A forest plot depicting weighted proportion of vitamin D inadequacy (deficiency and insufficiency) in basketball players is shown in Appendix Figure A1 (available online). The pooled prevalence of vitamin D inadequacy for 527 basketball players in 14 studies was 77% (P < 0.001; 95% CI, 0.70-0.84; I2 = 70%, moderate heterogeneity). According to season, the prevalence of vitamin D inadequacy was 87% in winter and spring (P < 0.001; 95% CI, 0.80-0.93; I2 = 19%, no heterogeneity) and 65% in summer and fall (P < 0.001; 95% CI, 0.69-0.86; I2 = 81%, high heterogeneity). According to latitude, the prevalence of vitamin D inadequacy was 73% in latitude <37°N (P < 0.001; 95% CI, 0.60-0.85; I2 = 79%, high heterogeneity) and 80% in latitude ≥37°N (P < 0.001; 95% CI, 0.70-0.89; I2 = 63%, moderate heterogeneity). According to sex, the prevalence of vitamin D inadequacy was 78% in male participants (P < 0.001; 95% CI, 0.68-0.88; I2 = 73%, moderate heterogeneity) and 75% in female participants (P = 0.005; 95% CI, 0.57-0.93; I2 = 73%, moderate heterogeneity).
Vitamin D Supplementation
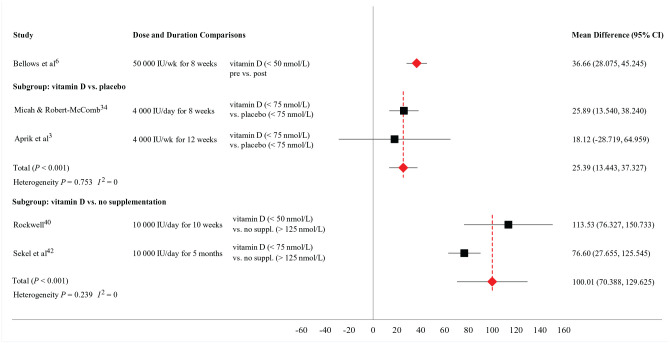
The outcome measures reported in studies examining the effect of vitamin D supplementation are described in Table 2. A forest plot depicting the absolute mean difference in 25(OH)D concentration across individual trials and subgroups (vitamin D vs placebo; vitamin D vs nonsupplementation) is shown in Figure 2.
Table 2.
Effects of vitamin D supplementation on outcome measures
| Study | Region and Latitude | Comparison Groups | Dose | Sample Size | Duration | Key Findings |
|---|---|---|---|---|---|---|
| Bellows et al 6 | Texas 31°N | Deficient (<50 nmol/L) | 50,000 IU/wk | 8 | 8 wk | Supplementation significantly increased serum vitamin D by 105%. There was no significant change in BMD from pre- to posttreatment (0.4%↑). |
| Micah and Robert-McComb 34 | Texas 31°N | Insufficient (<75 nmol/L) Insufficient (<75 nmol/L) |
4000 IU/d no supplementation |
9 8 |
8 wk | Supplementation significantly increased serum vitamin D by 95% in vitamin D group, vs 53% in placebo group. There were no significant changes in vertical jump, 20-m sprint, and 5-10-5 agility drill. |
| Aprik et al 3 | Midwest 42°N | Insufficient (<75 nmol/L) Insufficient (<75 nmol/L) |
4000 IU/wk no supplementation |
5 5 |
12 wk | Supplementation increased 25(OH)D concentrations above insufficiency level. There were no significant changes in body composition (BMD, LBM, BF%) and vertical jump from pre- to posttreatment. |
| Rockwell 40 | Virginia 37°N | Deficient (<50 nmol/L) Insufficient (50-100 nmol/L) Optimal (>125 nmol/L) |
10,000 IU/d 5000 IU/d no supplementation |
7 12 5 |
10 wk | A 10-wk treatment protocol (10,000 IU supplemental vitamin D3 daily for vitamin D deficiency, 5000 IU supplemental vitamin D3 daily for vitamin D insufficiency) was effective in improving vitamin D status. There were no significant changes in BMD, BF%, FFM, PTH, tT, or fT from pre- to posttreatment. |
| Sekel et al 42 | Virginia 37°N | Insufficient (<75 nmol/L) Sufficient (75-125 nmol/L) Optimal (>125 nmol/L) |
10,000 IU/d 5000 IU/d no supplementation |
13 5 2 |
5 mo | Athletes from insufficient group (<75 nmol/L) significantly improved 25(OH)D concentration by 35 ± 27 nmol/L. |
| Williams et al 49 | Columbia 34°N | Insufficient (<75 nmol/L) Sufficient (>75 nmol/L) |
50,000 IU/wk no supplementation |
15 12 |
8 wk | Vitamin D3 supplementation was effective at correcting hypovitaminosis D. Stress fracture rate demonstrated a nonsignificant decrease from 11.1% to 7.7% in female and from 6.7% to 0% in male basketball players |
BF%, body fat percentage; BMD, bone mineral density; FFM, fat free mass; fT, free testosterone; LBM, lean body mass; PTH, parathyroid hormone; tT, total testosterone.
Figure 2.
The effect of vitamin D supplementation on 25 hydroxyvitamin D concentration in basketball players.
Serum vitamin D concentration
The absolute mean difference for serum 25(OH)D between pre- and postsupplementation (50,000 IU/wk) was 36.66 nmol/L (95% CI, 28.08-45.25) in basketball players with baseline values suggesting vitamin D deficiency. 6 The absolute mean difference for serum 25(OH)D between vitamin D supplementation group (4000 IU per day and per week) and placebo group was 25.39 nmol/L (P < 0.001; 95% CI, 13.44-37.33) in basketball players with baseline values suggesting vitamin D insufficiency.3,34 The absolute mean difference for 25(OH)D between vitamin D supplementation group (10,000 IU/d) and nonsupplementation group was 100.01 (P < 0.001; 95% CI, 70.39-129.63) in basketball players with baseline values suggesting vitamin D inadequacy (deficient and insufficient) versus basketball players with optimal status (>125 nmo/L).40,42
Body composition, bone health, and athletic performance
There were no significant changes in percentage body fat,3,40 fat-free mass, 40 lean body mass, 3 and BMD,3,6,40 after vitamin D supplementation. Sekel et al 42 reported a significant, inverse correlation (r = −0.80; very large) between the change in serum vitamin D (from pre- to postsupplementation) and body fat percentage. In addition, Sekel et al 42 reported a significant correlation (r = 0.53; moderate) between the change in vitamin D (from pre- to postsupplementation) and lean body mass. Vitamin D supplementation produced nonsignificant decreases of stress fractures in basketball players. 49 In addition, there were no significant changes in vertical jump,3,34 20-m sprint, 34 and 5-10-5 agility drill 34 after vitamin D supplementation.
Discussion
Epidemiology
Our results suggest that the global prevalence of vitamin D inadequacy (<80 nmol/L) has reached an alarming proportion among basketball players, with a mean percentage of 77%. The higher prevalence of vitamin D inadequacy was observed in the winter and spring (87%), compared with the summer and fall seasons (65%). However, the prevalence rate of vitamin D inadequacy remained high regardless of latitude (<37°N = 73%; ≥37°N = 80%) and sex (male = 78%; female = 75%).
Our prevalence of vitamin D inadequacy is higher (77%) than those observed in 2313 professional athletes playing various sports such as soccer, rugby, sailing, jockeys, tennis, cycling, running, triathlon, swimming, water polo, handball, volleyball, basketball, bowling, dancing, Tae Kwon Do, judo, ballet, wrestling, body building, and gymnastics. 16 Synthesizing 23 studies (of which only 1 study included exclusively basketball players), Farrokhyar et al 16 showed that 56% of participants were vitamin D deficient or insufficient. Variations in these study findings likely relate to the specific sporting disciplines (indoor vs mix of outdoor and indoor sports) of the participants. Since the skin exposure to UVB radiation is the major source of vitamin D synthesis, sunlight deprivation in sports performed within indoor environments may negatively affect vitamin D status, underpinning greater prevalence of vitamin D inadequacy in basketball players compared with mixed athletes competing in both indoor and outdoor environments. Consistent with this assumption, we also observed a higher prevalence of vitamin D inadequacy during the winter and spring (87%), compared with the summer and fall seasons (65%). Our findings thereby align with the meta-analytic data reported by Farrokhyar et al 16 demonstrating a greater risk of vitamin inadequacy during the winter and spring seasons when compared with summer and fall.
Lack of sun exposure may also explain the slightly higher prevalence of vitamin D inadequacy seen in basketball players living at latitudes ≥37°N (80%) compared with those players living at latitudes <37°N (73%). Namely, basketball players living in lower-latitude countries (<37°N) were likely recruited from warmer locations such as California, Texas, Columbia, and Israel, which would attenuate decreases in serum 25(OH)D from increased sunlight exposure. Nevertheless, the risk of low serum 25(OH)D levels seen in basketball players remains high, regardless of latitude. Farrokhyar et al 16 also confirmed greater risk for vitamin D inadequacy in athletes (from various indoor sports) living in >40°N latitudes (84%) compared with those in <40°N latitudes (49%). Although these latitude-dependent results were similar, variations in study findings for lower-latitude countries may be further influenced by the race/ethnicity or skin pigmentation of our participants. Since the majority of the basketball players included in our study were African American, dark skin pigmentation may reduce the capacity of the skin to synthesize vitamin D, thus increasing the risk of vitamin D insufficiency. In addition to sun exposure and skin pigmentation, the high percentage of vitamin D inadequacy seen in basketball players is likely multifactorial and perhaps related to reduced vitamin D intake and lifestyle factors (eg, sunscreen and clothing) yet to be evaluated.
The similar prevalence rate of vitamin D inadequacy between sexes also suggests that considerable overlap exists between male and female basketball players regarding low serum 25(OH)D status. Similar observations have been reported in previous research with collegiate (indoor and outdoor) athletes.24,28 On the other hand, Fields et al 18 reported that male basketball players may be at a heightened risk of disrupted vitamin D status compared with female basketball players. The authors attributed the apparent sex difference to the ethnicity of participants, whereby 91% of male basketball players were African American, while 56% of female basketball players were White Anglo. 18 Therefore, confounders (eg, ethnicity, nutrition) should be considered when interpreting differences between vitamin D study groups. Collectively, equal attention should be given to optimize vitamin D status across sexes, since persistently low serum 25(OH)D levels may place basketball players at an increased risk for bone and muscle/soft-tissue injuries.
Vitamin D Supplementation
Our collective analyses suggest that vitamin D supplementation was effective in improving serum 25(OH)D concentrations and correcting vitamin D inadequacy (insufficiency3,34,42 and deficiency6,40) in basketball players. Body composition data revealed inverse correlations between changes in serum 25(OH)D (from pre- to postsupplementation) and body fat, suggesting the effect of vitamin D supplementation to promote serum 25(OH)D varies with regard to adiposity. The improvement in serum vitamin D was not accompanied by enhancements in BMD. Nonsignificant beneficial effect of vitamin D treatment was documented on the incidence rate of stress fractures. Data concerning positive impacts of vitamin D supplementation on optimizing athletic performance in basketball players remains sparse.
We found vitamin D supplementation with 4000 IU/d and 4000 IU/wk (pooled findings) resulted in a 25 nmol/L increment in circulating 25(OH)D in basketball players with baseline values suggesting vitamin D insufficiency. Furthermore, supplements containing 10,000 IU/d were shown to be the most effective in correcting vitamin D inadequacy (deficiency and insufficiency), resulting in 100 nmol/L increment of circulating 25(OH)D in basketball players. Our findings align with meta-analytic data reported by Farrokhyar et al, 15 which shows beneficial effects of vitamin D supplementation for correcting hypovitaminosis D in athlete populations. In addition, intervention protocols (10,000 IU/d) were found to be more effective in basketball players with circulating concentrations of 25(OH)D<50 nmol/L (mean difference, 113.5 nmol/L [95% CI, 76.3-150.7]) versus basketball players with circulating concentrations of 25(OH)D <75 nmol/L (mean difference, 76.6 nmol/L [95% CI, 27.6-125.5]). Although the majority (19 out of 20) of the investigated players displayed improvement in 25(OH)D <180 nmol/L (toxic level according to US Institute of Medicine) after vitamin D supplementation, 40 there is a risk of vitamin D toxicity with the excessive amounts of supplements (10,000 IU/d). Therefore, dose regimes and initial concentration of serum vitamin D may influence the biochemical response to supplementation.
Adiposity can also modulate 25(OH)D response to supplementation. Specifically, Sekel et al 42 reported a significant, inverse correlation (very large) between the change in serum 25(OH)D (from pre- to postsupplementation) and body fat in basketball players competing in National Collegiate Athletic Association (NCAA) Division I. Also, Rockwell 40 observed a significant inverse correlation (moderate) between serum 25(OH)D and percentage body fat in NCAA Division I basketball players. Sekel et al 42 also reported a significant correlation (moderate) between the change in serum 25(OH)D (from pre- to postsupplementation) and lean body mass in basketball players (NCAA Division I). These findings indicate that basketball players with a greater adiposity and lower lean body mass may be more susceptible to vitamin D inadequacy with a less efficient serum 25(OH)D response to vitamin D supplementation.
Our review demonstrated no BMD benefit in response to vitamin D supplementation,3,6,40 in contrast to the widely held perception that vitamin D works directly on bone cells, promoting mineralization. 13 This finding might be explained by the short period of vitamin D administration (8 weeks, 6 10 weeks, 40 and 12 weeks 3 ) or mechanical loading stimuli to which the musculoskeletal system is subjected in the sport of basketball. Specifically, structural changes in BMD require more time to develop. Alternatively, the responsiveness of bone mineralization to vitamin D supplementation may be limited, since the stimulus of loading on the musculoskeletal system in basketball players may compensate for vitamin D inadequacy, 37 maximizing BMD. 44 A limited beneficial effect was also observed in the incidence rate of stress fractures (from January 2010 to May 2015 vs August 2015 to May 2016), with nonsignificant decreases in female (from 11.1% to 7.7%) and male basketball players (from 6.7% to 0%) after vitamin D supplementation (50,000 IU/wk) for 8 weeks. 49
The role of vitamin D in preventing fractures may be via its mediating effects on muscle function. Vitamin D has been postulated to modulate skeletal muscle function, via both genomic and nongenomic mechanisms.9,26 Genomic actions of vitamin D on skeletal muscle function and sports performance are associated with the large number of vitamin D receptors (VDRs) found within human skeletal muscle. The activation of VDR induces the heterodimerization between the active VDR and retinoic acid x-receptor (RXR). The 1,25(OH)2D-VDR-RXR heterodimer translocates to the nucleus where it binds to vitamin D response elements, inducing muscle gene transcription and de novo protein synthesis. 27 Vitamin D also upregulates circulating insulin-like growth factor 1 (IGF-1) and IGF binding protein 3, 2 both of which have a well-recognized role in proliferation, differentiation and hypertrophy of skeletal muscle. 27 The nongenomic hypothesis posits an indirect, rapid mechanism whereby 1,25(OH)2D3 triggers a series of secondary messenger processes that promote enhanced calcium kinetics and the stimulation of mitogen-activated protein kinase signaling pathways in muscle fibers. 25 This nongenomic pathway of vitamin D action has been found to result in an influx of calcium ions into the cell, regulation of the intra- and extracellular levels of this ion, homoeostasis of phosphorus-containing compounds, and the stimulation of parathyroid hormone secretion.35,36
Despite many theoretical mechanisms of action, data that confirm beneficial effects of vitamin D supplementation on athletic performance in basketball players remain sparse and conflicted. Vertical jump,3,34 20-m sprint, 34 and 5-10-5 agility drills 34 were not significantly improved after vitamin D supplementation (4000 IU per day or per week) in basketball players with baseline values that suggest vitamin D insufficiency. While relatively small sample sizes (vitamin D vs placebo supplementation: n = 9 vs n = 8) may partly explain nonsignificant moderate improvements in 5-10-5 agility drills, 34 collective data on physical performance agree with prior meta-analysis data. 15 As such, Farrokhyar et al 15 confirmed nonsignificant effects of vitamin D supplementation on physical performance (vertical jump, Illinois agility test, 10- to 30-m sprint, Wingate peak power, 1 repetition maximum [1-RM] bench press or 1-RM leg press) across all included studies (N = 7). The lack of any ergogenic effects on athletic performance may hypothetically be due to the high training status of the included participants. While many mechanisms of action have been suggested to affect muscle function, the majority of these studies have been conducted on nonathletes and elderly participants.37,45 The ergogenic effect of vitamin D supplementation may be higher in elderly populations, since baseline sarcopenia allows for greater muscle mass gains compared with elite athletes. Further intervention trials are thereby required to determine if optimization of vitamin D status improves athletic performance in basketball players.
Gaps Within the Research Literature and Future Directions
Methodological Characteristics
Future studies should focus on reaching an international consensus regarding thresholds for vitamin D classifications (deficiency, insufficiency, and sufficiency) in athletes. An allocation concealment strategy should also be clearly described in randomized controlled trials. The inclusion/exclusion criteria should be clearly specified in cross-sectional and nonrandomized controlled trials. Skin pigmentation,17,18,42,46 ethnicity,3,17,18,20,34,40,42,46,49 sun exposure,3,17,18,40,42 period of data collection,3,5,6,8,12,17,18,20,33,40,42,46,49 and vitamin D intake3,5,17,20,40,42 were sporadically included across studies and consequently should be consistently considered in future studies, regardless of study design. Clearer reporting and handling of missing data in intervention studies are also needed. The blinding of outcome assessors should be frequently used and clearly reported. The analytical techniques for vitamin D metabolite measurement should also be clearly described.
Physical Performance
Despite many theoretical mechanisms of action, the effects of vitamin D on physical performance remain to be fully elucidated in basketball players. Further research in this area is required to determine mechanisms of action, dosage at which vitamin D modulates skeletal muscle function, and whether biological changes in serum 25(OH)D translate into improved physical performance in basketball players.
Inflammation
Despite evidence suggesting relationships between serum 25(OH)D concentration and proinflammatory cytokines (mostly in animals or diseased patients),14,27 no study has examined the effect of vitamin D supplementation on the inflammatory response to exercise or postinjury in basketball players. Tumor necrosis factor–alpha (TNF-α) is a proinflammatory cytokine that may downregulate muscle recovery postinjury. 36 Controlled intervention studies are further required to determine whether vitamin D supplementation can decrease abnormally raised TNF-α concentrations, postinjury. In addition to TNF-α, interleukin-6 levels acutely increase after physical exercise and are hypothesized to be related to the occurrence of exercise-induced muscle damage. Strenuous muscle contractions during exercise may also induce mechanical muscle damage, which triggers the release of large amounts of intracellular enzymes, including creatine kinase and lactate dehydrogenase, into the circulation. 38 In vitro and animal studies suggest that vitamin D reduces tissue damage after intense exercise by lowering peroxidation levels and increasing mitochondrial oxidative phosphorylation.11,32 Despite the insights provided by these studies, the vitamin D pathways that affect the reduction of muscle damage in athletes warrant further investigation.
Immunity
Excessive stress from both training and competition is a predisposing factor to upper respiratory tract infections in basketball players. 7 Recent studies suggest that vitamin D may decrease the development of upper respiratory tract infections (influenza, COVID-19, and the common cold) by triggering the release of antimicrobial peptides (such as cathelicidin) once invading pathogens are recognized by toll-like receptors.23,37 Any possible relationship between vitamin D status and upper respiratory tract infections in basketball players would be of considerable interest and yet to be investigated. Randomly assigned placebo-controlled studies are required to identify the effectiveness of correcting vitamin D inadequacy on the prevention of upper respiratory tract infections in basketball players.
Skeletal Health
There is a need for further experimental studies assessing the effect of vitamin D supplementation on BMD and stress fractures in basketball players. Observational studies should more critically examine associations between various serum vitamin D metabolites (ie, serum 25(OH)D, 1,25(OH)2D3, vitamin D binding protein) and BMD. In addition, biochemical markers of bone remodeling might be suitable to detect dynamic changes of bone activity in response to vitamin D supplementation. 4
Cardiac Structure and Function
High cardiac output is essential to support the very high rates of oxygen uptake required for excellence in basketball. Increases in cardiac output are achieved by large maximal stroke volumes, mediated predominantly by cardiac hypertrophy (athlete’s heart). 39 Although a high and/or persistent serum 25(OH)D–deficient state may attenuate favorable cardiac adaptations (athlete’s heart) to exercise stress, 1 no study has compared echocardiographic parameters with vitamin D status in basketball players.
Conclusion
While no evidence-based consensus exists regarding the optimal target levels for serum vitamin D in athletes, an alarming proportion (77%) of basketball players demonstrate pervasive vitamin D inadequacy (<80 nmol/L), which underscores the need to more actively screen for serum 25(OH)D to enhance recovery and maintain training outputs. Although regular supplementation (from 4000 IU/wk to 10,000 IU/d) appears to restore vitamin D sufficiency over time, the beneficial effects of normal to high 25(OH)D levels on bone health and physical performance remain sparse. Larger cohort studies are required utilizing female and male players of varying ages and playing levels to further examine the effects of vitamin D supplementation on physical performance across wider player populations.
Supplemental Material
Supplemental material, sj-docx-2-sph-10.1177_19417381211019343 for Vitamin D in Basketball Players: Current Evidence and Future Directions by Emilija Stojanović, Dragan Radovanović, Tamara Hew-Butler, Dušan Hamar and Vladimir Jakovljević, in Sports Health: A Multidisciplinary Approach
Supplemental material, sj-xlsx-1-sph-10.1177_19417381211019343 for Vitamin D in Basketball Players: Current Evidence and Future Directions by Emilija Stojanović, Dragan Radovanović, Tamara Hew-Butler, Dušan Hamar and Vladimir Jakovljević, in Sports Health: A Multidisciplinary Approach
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
This study was supported by the Faculty of Medical Sciences, University of Kragujevac (JΠ 18/20) and Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract No. 451-03-9/2021-14/200228).
References
- 1. Allison RJ, Close GL, Farooq A, et al. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Eur J Prev Cardiol. 2015;22:535-542. [DOI] [PubMed] [Google Scholar]
- 2. Ameri P, Giusti A, Boschetti M, Murialdo G, Minuto F, Ferone D. Interactions between vitamin D and IGF-I: from physiology to clinical practice. Clin Endocrinol. 2013;79:457-463. [DOI] [PubMed] [Google Scholar]
- 3. Aprik C, Sauerbry T, DiPace L, et al. Longitudinal changes in vitamin D and body composition in NCAA D1 male basketball players. Med Sci Sports Exerc. 2018;50(5)(suppl):505. [Google Scholar]
- 4. Banfi G, Lombardi G, Colombini A, Lippi G. Bone metabolism markers in sports medicine. Sports Med. 2010;40:697-714. [DOI] [PubMed] [Google Scholar]
- 5. Baranauskas M, Jablonskiene V, Abaravičius J, Stukas R. Cardiorespiratory fitness and diet quality profile of the Lithuanian team of deaf women’s basketball players. Int J Environ Res Public Health. 2020;17:6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellows M, Tanguay J, Crouse SF, Harbourt D. Vitamin D deficiency in TAMU female basketball players and supplement effectiveness. Int J Exerc Sci. 2013;2:19. [Google Scholar]
- 7. Brunelli DT, Rodrigues A, Lopes WA, et al. Monitoring of immunological parameters in adolescent basketball athletes during and after a sports season.J Sports Sci. 2014;32:1050-1059. [DOI] [PubMed] [Google Scholar]
- 8. Bubbs M. Observational case study—vitamin 25 (OH) D status of professional basketball players and its impact on athletic performance and recovery. J Int Soc Sports Nutr. 2015;12(suppl 1):55. [Google Scholar]
- 9. Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008;29:407-414. [DOI] [PubMed] [Google Scholar]
- 10. Chen D-G, Fang D, Wilson JR. Meta-analysis of two studies with random effects? J Minim Invasive Gynecol. 2017;24:689-690. [DOI] [PubMed] [Google Scholar]
- 11. Choi M, Park H, Cho S, Lee M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine. 2013;63:27-35. [DOI] [PubMed] [Google Scholar]
- 12. Constantini NW, Arieli R, Chodick G, Dubnov-Raz G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin J Sport Med. 2010;20:368-371. [DOI] [PubMed] [Google Scholar]
- 13. de la Puente Yagüe M, Collado Yurrita L, Cuadrado Cenzual MA. Role of vitamin D in athletes and their performance: current concepts and new trends. Nutrients. 2020;12:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Luigi L, Antinozzi C, Piantanida E, Sgrò P. Vitamin D, sport and health: a still unresolved clinical issue. J Endocrinol Invest. 2020;43:1689-1702. [DOI] [PubMed] [Google Scholar]
- 15. Farrokhyar F, Sivakumar G, Savage K, et al. Effects of vitamin D supplementation on serum 25-hydroxyvitamin D concentrations and physical performance in athletes: a systematic review and meta-analysis of randomized controlled trials. Sports Med. 2017;47:2323-2339. [DOI] [PubMed] [Google Scholar]
- 16. Farrokhyar F, Tabasinejad R, Dao D, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45:365-378. [DOI] [PubMed] [Google Scholar]
- 17. Fields JB, Gallo S, Worswick JM, Busteed DR, Jones MT. 25-Hydroxyvitamin D, vitamin D binding protein, bioavailable 25-hydroxyvitamin d, and body composition in a diverse sample of women collegiate indoor athletes. J Funct Morphol Kinesiol. 2020;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fields JB, Payne DC, Gallo S, Busteed D, Jones M. Vitamin D status differs by sex, sport-season, and skin pigmentation among elite collegiate basketball players. Sports (Basel). 2019;7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fishman MP, Lombardo SJ, Kharrazi FD. Vitamin D deficiency among professional basketball players. Orthop J Sports Med. 2016;4:2325967116655742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia RB, Guisado FR. Low levels of vitamin D in professional basketball players after wintertime: relationship with dietary intake of vitamin D and calcium. Nutr Hosp. 2011;26:945-951. [DOI] [PubMed] [Google Scholar]
- 21. Giustina A, Adler R, Binkley N, et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21:89-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693-699. [DOI] [PubMed] [Google Scholar]
- 23. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer E. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43:335-343. [DOI] [PubMed] [Google Scholar]
- 25. Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20:182-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α, 25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543-559. [DOI] [PubMed] [Google Scholar]
- 27. He C-S, Aw Yong XH, Walsh NP, Gleeson M. Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc Immunol Rev. 2016;22:42-64. [PubMed] [Google Scholar]
- 28. Heller JE, Thomas JJ, Hollis BW, Larson-Meyer E. Relation between vitamin D status and body composition in collegiate athletes. Int J Sport Nutr Exerc Metab. 2015;25:128-135. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [DOI] [PubMed] [Google Scholar]
- 31. Kakanis MW, Peake J, Brenu EW, et al. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. 2010;16:119-137. [PubMed] [Google Scholar]
- 32. Ke C-Y, Yang F-L, Wu W-T, et al. Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int J Med Sci. 2016;13:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim DK, Park G, Kuo L-T, Park WH. Association of vitamin D status with lower limb muscle strength in professional basketball players: a cross-sectional study. Nutrients. 2020;12:2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Micah D, Robert-McComb JJ. Vitamin D deficiency in college-age male basketball players: sports medicine physicians can play an important role. Int J Res Stud Med Health Sci. 2017;2:8-15. [Google Scholar]
- 35. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269. [DOI] [PubMed] [Google Scholar]
- 36. Moresi V, Pristerà A, Scicchitano BM, et al. Tumor necrosis factor-α inhibition of skeletal muscle regeneration is mediated by a caspase-dependent stem cell response. Stem Cells. 2008;26:997-1008. [DOI] [PubMed] [Google Scholar]
- 37. Owens DJ, Allison R, Close GL. Vitamin D and the athlete: current perspectives and new challenges. Sports Med. 2018;48:3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parsaie N, Ghavamzadeh S, Cheraghi M. Effects of cholecalciferol supplementation on inflammatory markers and muscle damage indices of soccer players after a simulated soccer match. Nutrition. 2019;59:37-43. [DOI] [PubMed] [Google Scholar]
- 39. Rawlins J, Bhan A, Sharma S. Left ventricular hypertrophy in athletes. Eur J Echocardiogr. 2009;10:350-356. [DOI] [PubMed] [Google Scholar]
- 40. Rockwell MS. Vitamin D in Human Health and Performance: The Pursuit of Evidence-Based Practice in an Era of Scientific Uncertainty. Virginia Tech; 2019. Accessed September 15, 2020. https://vtechworks.lib.vt.edu/handle/10919/93168 [Google Scholar]
- 41. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekel NM, Gallo S, Fields J, Jagim A, Wagner T, Jones M. The effects of cholecalciferol supplementation on vitamin D status among a diverse population of collegiate basketball athletes: a quasi-experimental trial. Nutrients. 2020;12:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shuler FD, Wingate MK, Moore GH, Giangarra C. Sports health benefits of vitamin D. Sports Health. 2012;4:496-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stojanović E, Radovanović D, Dalbo VJ, et al. Basketball players possess a higher bone mineral density than matched non-athletes, swimming, soccer, and volleyball athletes: a systematic review and meta-analysis. Arch Osteoporos. 2020;15:123. [DOI] [PubMed] [Google Scholar]
- 45. Todd JJ, Pourshahidi LK, McSorley EM, Madigan SM, Magee PJ. Vitamin D: recent advances and implications for athletes. Sports Med. 2015;45:213-229. [DOI] [PubMed] [Google Scholar]
- 46. Villacis D, Yi A, Jahn R, et al. Prevalence of abnormal vitamin D levels among Division I NCAA athletes. Sports Health. 2014;6:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viswanathan M, Ansari M, Berkman N, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality; 2014:193-221. Publication No. 2012. Accessed October 1, 2020. https://effectivehealthcare.ahrq.gov/products/methods-bias-update/methods
- 48. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end.J Stat Softw. 2012;49:1-15. [Google Scholar]
- 49. Williams K, Askew C, Mazoue C, Guy J, Torres-McGehee T, Jackson JB. Vitamin D3 supplementation and stress fractures in high-risk collegiate athletes—a pilot study. Orthop Res Rev. 2020;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-sph-10.1177_19417381211019343 for Vitamin D in Basketball Players: Current Evidence and Future Directions by Emilija Stojanović, Dragan Radovanović, Tamara Hew-Butler, Dušan Hamar and Vladimir Jakovljević, in Sports Health: A Multidisciplinary Approach
Supplemental material, sj-xlsx-1-sph-10.1177_19417381211019343 for Vitamin D in Basketball Players: Current Evidence and Future Directions by Emilija Stojanović, Dragan Radovanović, Tamara Hew-Butler, Dušan Hamar and Vladimir Jakovljević, in Sports Health: A Multidisciplinary Approach