ABSTRACT
Neisseria gonorrhoeae isolates collected in Nanjing, China, that possessed decreased susceptibility (or resistance) to extended-spectrum cephalosporins (ESCs) were examined for susceptibility to ertapenem, and their sequence types were determined. Ceftriaxone and cefixime MICs of ≥0.125 mg/L and ≥0.25 mg/L, respectively, were first determined in 259 strains isolated between 2013 and 2019, and then MICs of ertapenem were measured using the antimicrobial gradient Epsilometer test (Etest). Also, genetic determinants of ESC resistance were identified and N. gonorrhoeae multiantigen sequence typing (NG-MAST) was performed to analyze associations with ertapenem susceptibility. All isolates displayed ertapenem MICs between 0.006 mg/L and 0.38 mg/L; the overall MIC50 and MIC90 were 0.032 mg/L and 0.125 mg/L, respectively. Forty-four (17.0%) isolates displayed ertapenem MICs of ≥0.125 mg/L; 10 (3.9%) had MICs of ≥0.25 mg/L. The proportion of isolates with ertapenem MICs of ≥0.125 mg/L increased from 4.0% in 2013 to 20.0% in 2019 (χ2 = 24.144, P < 0.001; chi-square test for linear trend). The penA mosaic allele was present in a significantly higher proportion of isolates with ertapenem MICs of ≥0.125 mg/L than of isolates with MICs of ≤0.094 mg/L) (97.7% versus 34.9%, respectively; χ2 = 58.158, P < 0.001). ST5308 was the most prevalent NG-MAST type (8.5%); ST5308 was also significantly more common among isolates with ertapenem MICs of ≥0.125 mg/L than isolates with MICs of ≤0.094 mg/L (22.7% and 5.6%, respectively; χ2 = 13.815, P = 0.001). Ertapenem may be effective therapy for gonococcal isolates with decreased susceptibility or resistance to ESCs and isolates with identifiable genetic resistance determinants.
KEYWORDS: Neisseria gonorrhoeae, ertapenem, ESCs, resistance
INTRODUCTION
Gonorrhea is the second most common bacterial sexually transmitted infection (STI) and a major global public health problem. The World Health Organization (WHO) estimated that 87 million new cases occurred worldwide in adults aged 15 to 49, in 2016 (1). In China, the reported incidence of gonorrhea increased by 36.03% (7.05 to 9.59 cases per 100,000 population) from 2014 to 2018 (2). Treatment of gonorrhea is challenging because Neisseria gonorrhoeae has developed resistance to most antimicrobials (AMR) that have been used for therapy, including sulfonamides, penicillins, tetracyclines, fluoroquinolones, and early-generation and, rarely, extended-spectrum cephalosporins (ESCs) (3–7).
Currently, ceftriaxone monotherapy or dual therapy with ceftriaxone or cefixime plus azithromycin has been recommended as first-line treatment of uncomplicated gonorrhea in most countries (8–10). In the United States (U.S.), azithromycin is no longer recommended as part of a first-line regimen (10) because of increased incidence of azithromycin resistance (from 0.6% in 2013 to 4.6% in 2018) (11). The U.S. Centers for Disease Control and Prevention (CDC)-recommended dose of ceftriaxone was doubled in 2020 (from 250 mg to 500 mg intramuscularly [i.m.]) (10) based on doubling of MICs of current strains compared with MICs 20 years ago (11). Modeling of urogenital concentrations of ceftriaxone that estimate the 250-mg dose does not predict a concentration for 24 h that is higher than the MIC of ceftriaxone for most current U.S. strains of N. gonorrhoeae (12). Ceftriaxone concentrations in the pharynx are variable, and treatment of N. gonorrhoeae may require longer times to achieve necessary MICs in the pharynx (13, 14). In the United Kingdom, the emergence of azithromycin resistance (9.2% in 2017), the increase in the modal ceftriaxone MIC distribution (15), and the identification of isolates resistant to both ceftriaxone and azithromycin (16, 17) prompted a revision of recommendations in 2018 from dual therapy with ceftriaxone and azithromycin to therapy with a higher dose of ceftriaxone (1 g) alone (18). Based on the emergence of gonococcal isolates that displayed decreased susceptibility to ceftriaxone in China (10.8% in 2013 to 2016) (19) and the identification of the ceftriaxone-resistant N. gonorrhoeae strain FC428 (20), now present worldwide, the dose of ceftriaxone recommended by the China CDC was also increased in 2020, from 250 mg to 1 g (21).
Ertapenem is a parenteral carbapenem, effective against Gram-negative bacteria that may, otherwise, be resistant to cephalosporins. Similar to other β-lactams, ertapenem inhibits cell wall synthesis by binding to and inhibiting penicillin-binding proteins (PBPs) (22). It is well tolerated and effective and has a safety profile comparable to that of ceftriaxone (23, 24). Ertapenem has been used successfully to treat N. gonorrhoeae with both high-level azithromycin and high-level ceftriaxone resistance (17). A recently reported randomized treatment trial showed that in a primary per-protocol analysis, a single 1-g dose of ertapenem was 99% effective (one treatment failure) for treatment of uncomplicated anogenital gonorrhea, noninferior to a single 500-mg dose of ceftriaxone (100% effective). All N. gonorrhoeae strains were susceptible to ceftriaxone (MICs of ≤0.012 mg/L) and also displayed low MICs for ertapenem (MICs of ≤0.008 mg/L) (25).
No specific genetic determinants of ertapenem resistance or carbapenemases, generally, have been identified in N. gonorrhoeae; however, there may be overlap with resistance mechanisms exhibited toward other ESCs (26). Mechanisms of resistance against ESCs can result from amino acid changes caused by nucleotide mutations in penA (encoding penicillin-binding protein 2 [PBP2]), mtrR (encoding the multiple transfer resistance repressor [MtrR]), penB (encoding porin PorB), and ponA (encoding penicillin-binding protein 1 [PBP1]) in N. gonorrhoeae (3, 27–30).
The major aim of the present study was to examine in vitro activity of ertapenem against N. gonorrhoeae isolates with decreased susceptibility (or resistance) to ESCs. We also identified ESC resistance determinants and their association with susceptibility of N. gonorrhoeae strains to ertapenem. N. gonorrhoeae multiantigen sequence typing (NG-MAST) of N. gonorrhoeae isolates was performed to assess distribution according to ertapenem MICs and, potentially, to identify clonality of isolates with increased resistance.
RESULTS
Antimicrobial susceptibility.
A total of 259 N. gonorrhoeae isolates with decreased susceptibility or resistance to ceftriaxone and/or cefixime were identified. The MIC ranges of ceftriaxone and cefixime for these isolates were 0.06 to 1 mg/L (MIC50, 0.125 mg/L, and MIC90, 0.125 mg/L) and 0.06 to ≥4 mg/L (MIC50, 0.125 mg/L, and MIC90, 0.5 mg/L), respectively. Among these isolates, 9 (3.5%) were fully resistant to ceftriaxone (MICs of ≥0.5 mg/L) and cefixime (MICs of ≥2 mg/L).
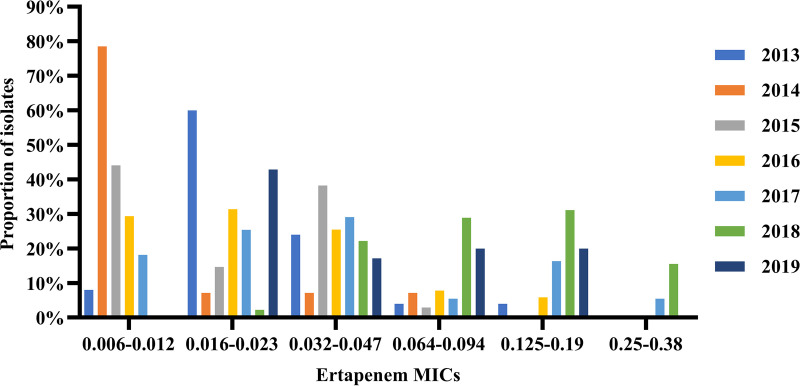
MICs of ertapenem against the 259 isolates ranged from 0.006 mg/L to 0.38 mg/L; MIC50 and MIC90 were 0.032 mg/L and 0.125 mg/L, respectively. For the 9 N. gonorrhoeae isolates fully resistant to ceftriaxone (MICs of ≥0.5 mg/L) and cefixime (MICs of ≥2 mg/L), the ertapenem MIC50, MIC90, and MIC range were 0.094 mg/L, 0.19 mg/L, and 0.023 to 0.19 mg/L, respectively. Forty-four (17.0%) isolates had ertapenem MICs of ≥0.125 mg/L and 10 (3.9%) had MICs of ≥0.25 mg/L, MICs that represent the WHO-recommended susceptibility breakpoints for ceftriaxone and cefixime, respectively. The ertapenem MIC50 and MIC90 increased from 0.023 mg/L and 0.047 mg/L in 2013 to 0.047 mg/L and 0.125 mg/L in 2019, respectively. The distributions of ertapenem MICs during 2013 to 2019 are shown in Fig. 1. The proportion of isolates with ertapenem MICs of ≥0.125 mg/L (the breakpoint against ceftriaxone) increased from 4.0% in 2013 to 20.0% in 2019, showing an overall upward trend during the study period (χ2 = 24.144, P < 0.001; chi-square test for linear trend), while the percentage of isolates with MICs of ≤0.012 mg/L declined in each successive year, sequentially (χ2 = 23.634, P < 0.001; chi-square test for linear trend).
FIG 1.
Distribution of ertapenem MICs (milligrams per liter) for 259 Neisseria gonorrhoeae clinical isolates with decreased susceptibility (or resistance) to ESCs isolated in Nanjing, China, 2013 to 2019.
Genetic resistance determinants (penA, mtrR, penB, and ponA) for ESCs.
A penA mosaic allele was present in 118 (45.6%) N. gonorrhoeae isolates with decreased susceptibility or resistance to ESCs; nonmosaic penA alleles with A501V/T mutations were present in 139 (53.7%); the remaining 2 isolates (0.8%) possessed a nonmosaic allele with an A517G mutation. Mutations in the promoter and/or coding regions of the mtrR gene were identified in 179 (69.1%) isolates. Amino acid substitutions at residue G120 of the penB gene were present in 5 (1.9%) isolates; G120/A121 double mutations were present in 253 (97.7%). An L421P mutation in the ponA gene was present in 256 (98.8%) isolates.
Ertapenem susceptibilities of isolates containing the penA mosaic allele were lower than susceptibilities of isolates that lacked the mosaic allele. The MIC50, MIC90, and MIC range of ertapenem in strains with the penA mosaic allele were 0.047 mg/L, 0.19 mg/L, and 0.008 to 0.38 mg/L, respectively. Strains that lacked the mosaic allele had a MIC50, MIC90, and MIC range of ertapenem of 0.016 mg/L, 0.064 mg/L, and 0.006 to 0.125 mg/L, respectively. The penA mosaic allele was more common among isolates with increased ertapenem MICs (≥0.125 mg/L) (WHO-recommended susceptibility breakpoint against ceftriaxone) than isolates with MICs of ≤0.094 mg/L (97.7% versus 34.9%, respectively; χ2 = 58.158, P < 0.001) (Table 1). All isolates with ertapenem MICs of ≥0.25 mg/L (WHO-recommended susceptibility breakpoint against cefixime) possessed the penA mosaic allele. Conversely, the proportion of isolates with ertapenem MICs of ≤0.094 mg/L that possessed A501V/T mutations, specifically, was higher than that of isolates with MICs of ≥0.125 mg/L (64.2% versus 2.3%, respectively; χ2 = 56.307, P < 0.001) (Table 1). The two isolates with A517G mutations had ertapenem MICs of ≤0.094 mg/L (Table 1). Additionally, the proportion of isolates that possessed the penA mosaic allele increased from 4.0% in 2013 to 68.6% in 2019, showing an upward trend during the study period (χ2 = 34.343, P < 0.001; chi-square test for linear trend).
TABLE 1.
penA and mtrR mutations in isolates with MICs to ertapenem of either ≤0.094 mg/L or ≥0.125 mg/L
| Resistance determinant | No. (%) of isolates with MIC: |
χ2 | P valuea | |
|---|---|---|---|---|
| ≤0.094 mg/L (n = 215) | ≥0.125mg/L (n = 44) | |||
| penA | ||||
| Mosaic allele | 75 (34.9) | 43 (97.7) | 58.158 | <0.001 |
| A501V/Tb | 138 (64.2) | 1 (2.3) | 56.307 | <0.001 |
| A517Gb | 2 (0.9) | 0 | 0.413 | 1 |
| mtrR | ||||
| A-deletion in promoter regionc | 102 (47.4) | 10 (22.7) | 9.090 | 0.0026 |
| A-deletion,c A39T | 3 (1.4) | 3 (6.8) | 4.747 | 0.0632 |
| A-deletion,c G45D | 24 (11.2) | 0 (0) | 5.413 | 0.0186 |
| A39T | 8 (3.7) | 1 (2.3) | 0.228 | 1.0000 |
| G45D | 27 (12.6) | 1 (2.3) | 4.007 | 0.0585 |
| WTd | 51 (23.7) | 29 (65.9) | 30.453 | <0.001 |
P of <0.05 was considered significant in chi-square (χ2) or Fisher exact testing.
Nonmosaic penA alleles.
A (adenine) deletion in the 13-bp inverted-repeat sequence of the mtrR promoter.
WT, wild type.
mtrR mutations were present in 34.1% (15/44) of isolates with ertapenem MICs of ≥0.125 mg/L and in 76.3% (164/215) of isolates with ertapenem MICs of ≤0.094 mg/L (χ2 = 30.453, P < 0.001). A single A-deletion in the mtrR promoter was identified more often in isolates with ertapenem MICs of ≤0.094 mg/L than in isolates with MICs of ≥0.125 mg/L (χ2 = 9.090, P = 0.0026) (Table 1). There were no significant differences in the rates of A39T or G45D mtrR mutations in the coding region accompanied (or not) by an A-deletion in the promoter region. An exception was a G45D mutation accompanied by an A-deletion in the promoter, which accounted for 11.2% (24/215) of isolates with ertapenem MICs of ≤0.094 mg/L and no isolates with ertapenem MICs of ≥0.125 mg/L (χ2 = 5.413, P = 0.0186) (Table 1). All but two isolates with ertapenem MICs of ≥0.25 mg/L lacked the mtrR mutations; the two exceptions harbored a single A-deletion in the mtrR promoter or G45D mutation in the mtrR coding region.
NG-MAST.
The 259 N. gonorrhoeae isolates were assigned to 161 N. gonorrhoeae multiantigen sequence typing (NG-MAST) types, of which 68 have not been reported previously in the NG-MAST database. The most prevalent NG-MAST sequence type (ST) was ST5308 (n = 22; ertapenem MIC50, 0.094 mg/L), followed by ST7554 (n = 17; ertapenem MIC50, 0.032 mg/L), ST3356 (n = 7; ertapenem MIC50, 0.023 mg/L), ST270 (n = 7; ertapenem MIC50, 0.008 mg/L), and ST4539 (n = 7; ertapenem MIC50, 0.016 mg/L). Among all sequence types, ST5308 was predominant among isolates with MICs of ≥0.125 mg/L to ertapenem (10/44 [22.7%]); these isolates also showed the highest ertapenem MIC50 (0.094 mg/L). Furthermore, ST5308 was more common among isolates with MICs of ≥0.125 mg/L to ertapenem versus isolates with MICs of ≤0.094 mg/L (22.7% and 5.6%, respectively; χ2 = 13.815, P = 0.001). All ST5308 isolates had a penA mosaic allele, G120K plus A121D substitutions in penB, and L421P in ponA but no mtrR mutations.
DISCUSSION
Neisseria gonorrhoeae is becoming increasingly resistant to currently used antimicrobial agents with the real prospect that untreatable gonorrhea may soon appear (9, 17). In the context of limited treatment options, alternative antimicrobials, new and repurposed, are needed urgently to ensure future successful treatments.
In our study, the MIC50 of ertapenem (0.032 mg/L) was substantially lower than those observed for both ceftriaxone and cefixime (0.125 mg/L). The MIC90 of ertapenem (0.125 mg/L) was similar to the MIC90 observed for ceftriaxone (0.125 mg/L) but lower than the cefixime MIC90 (0.5 mg/L). Unemo et al. (26) reported in 2012 that, generally, ertapenem and ceftriaxone MIC50s and MIC90s were similar: 0.032 mg/L (both) and 0.064 mg/L (ertapenem)/0.125 mg/L (ceftriaxone), respectively, in 257 N. gonorrhoeae clinical isolates with highly diverse ceftriaxone MIC values referred to WHO Collaborating Centers for STIs. Similarly, ertapenem MIC ranges were lower than ceftriaxone and cefixime MIC ranges in our study, also reported by Unemo et al. (26). In our study, 83.0% and 96.1% of isolates had ertapenem MICs below the ceftriaxone and cefixime breakpoints (0.125 mg/L and 0.25 mg/L), respectively, similar to the study by Xu et al. (31) that examined gonococcal isolates from eight provinces in China. In that study, 83.3% of 24 isolates with decreased susceptibility to ceftriaxone (MIC of ≥0.25 mg/L) exhibited ertapenem MIC values of <0.25 mg/L, the cefixime breakpoint. Unemo et al. (26) reported that all strains had ertapenem MICs of ≤0.125 mg/L, the ceftriaxone breakpoint. These results predict that ertapenem may be uniformly effective clinically in most instances because higher MICs are infrequent (our study and the study by Xu et al. [31]) or absent altogether (26). Further support for clinical efficacy is derived from activity of ertapenem against two extensively drug-resistant (XDR) N. gonorrhoeae strains, H041 and F89; both are highly resistant to cefixime (MIC range, 4 to 8 mg/L) and ceftriaxone (MIC range, 2 to 4 mg/L) (26). Ertapenem MICs were reported to be significantly lower (0.064 mg/L and 0.016 mg/L) for these two strains, respectively (26), corroborated in a separate study where F89 had an ertapenem MIC of 0.03 mg/L (32). In our study, ertapenem was also effective against the 9 N. gonorrhoeae isolates fully resistant to ceftriaxone (MICs of ≥0.5 mg/L) and cefixime (MICs of ≥2 mg/L). Nonetheless, several studies (26, 32, 33) have shown that ertapenem had no apparent in vitro advantage over ceftriaxone for N. gonorrhoeae isolates with lower ceftriaxone MICs.
Similar to other β-lactam antimicrobials, reduced activity of ertapenem against some bacteria is mediated by mutations in porin that result in aberrant function (34), upregulation of efflux pumps (35), and production of carbapenemases (36). However, resistance of N. gonorrhoeae to ertapenem is not fully defined. We used the ceftriaxone breakpoint (0.125 mg/L) to separate isolates with MICs of ≥0.125 mg/L to ertapenem from those with lower MICs (≤0.094 mg/L) to distinguish certain genetic characteristics of the penA allele and the mtrR promoter known to be present in many strains that possess decreased susceptibility (or resistance) to extended-spectrum cephalosporins (ESCs). We used 0.125 mg/L as a dividing point to better understand how ertapenem MICs might relate to gonococcal isolates known to be above/below the ceftriaxone breakpoint. Ultimately, however, determination of an ertapenem breakpoint will require treatment failures to occur in a clinical trial(s) coupled with MIC determinations of corresponding strains that fail ertapenem therapy. We found that penB and ponA resistance determinants were present across most strains, perhaps without a meaningful effect on ertapenem susceptibility. We also found that the presence of a penA mosaic allele was strongly associated with increased MICs of ertapenem, similar to findings reported by Unemo et al. (26). The proportion of isolates with increased ertapenem MICs (MICs of ≥0.125 mg/L) showed an increasing trend during the study period in the absence of clinical use, which may have been the result of the yearly increase in the proportion of isolates that contained the penA mosaic allele, also shown in our study.
Our study also showed that mtrR mutations were present in a higher percentage of isolates with ertapenem MICs of ≤0.094 mg/L than isolates with ertapenem MICs of ≥0.125 mg/L, different from another Chinese study, which showed that the mtrR promoter A-deletion was significantly associated with strains displaying an ertapenem MIC of >0.125 mg/L (37). Our study investigated the association between ertapenem susceptibility and known ESC resistance determinants; other antibiotic resistance determinants, such as the presence of mosaic sequences in the mtr locus, which is primarily related to azithromycin resistance (38–40), were not included in our study. A previous study showed that 14 gonococcal isolates with mosaic alleles in the mtr locus displayed resistance to azithromycin (MIC of >256 mg/L) but had low cephalosporin MICs (0.016 mg/L for both cefixime and ceftriaxone) (40). Furthermore, mutations in the promoter and/or coding regions of the mtrR gene (resulting in an overexpression of the MtrCDE efflux pump) were not associated with increased MICs of AMR N. gonorrhoeae to ertapenem in our study.
NG-MAST has been evaluated as a tool for predicting specific antimicrobial resistance phenotypes in N. gonorrhoeae isolates (41, 42). In our study, ST5308 was the most prevalent NG-MAST sequence type (ST) among the 259 isolates with decreased susceptibility or resistance to ESCs. In addition, ST5308 was the most highly represented ST in isolates with increased ertapenem MICs (≥0.125 mg/L). ST5308 isolates, possessing a penA mosaic allele, have been reported in Hong Kong and were associated with decreased susceptibility to oral ESCs (43). Between 2013 and 2017, ST5308 was the most common gonococcal type isolated in Guangdong, China (44).
In summary, in vitro susceptibility to ertapenem of Neisseria gonorrhoeae isolates with decreased susceptibility (or resistance) to ESCs suggests potential for future use of ertapenem as a treatment for antimicrobial-resistant infections. However, the penA mosaic allele, commonly associated with ESC resistance, was also associated with increased MICs of ertapenem. Continued surveillance of antimicrobial susceptibility of ertapenem supplemented by sequence typing and NG-MAST classification is warranted.
MATERIALS AND METHODS
Bacterial strains.
From January 2013 to December 2019, a total of 1,321 N. gonorrhoeae strains were isolated from men with symptomatic urethritis (urethral discharge and/or dysuria) attending the sexually transmitted disease (STD) clinic at the Institute of Dermatology, Chinese Academy of Medical Sciences, Nanjing, Jiangsu Province, China. Urethral exudates were collected with cotton swabs and immediately streaked onto modified Thayer-Martin (T-M) selective medium (Zhuhai DL Biotech Co. Ltd.) and incubated at 36°C in candle jars for 24 to 48 h. N. gonorrhoeae was identified by colonial morphology, Gram’s stain, and oxidase testing, which are sufficient to identify N. gonorrhoeae colonies isolated on selective medium, particularly for urethral samples from symptomatic men (45, 46). Gonococcal isolates were subcultured onto chocolate agar plates; pure colonies were swabbed, suspended in tryptone-based soy broth, and frozen (−80°C) until used for antimicrobial testing.
Antimicrobial susceptibility testing.
Susceptibility testing for ceftriaxone and cefixime was performed by the agar dilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (47). According to criteria for decreased susceptibility or resistance to ceftriaxone (MIC of ≥0.125 mg/L) and cefixime (MIC of ≥0.25 mg/L), defined by WHO (48), 259 strains were eligible for inclusion in this study. Ertapenem susceptibility among these isolates was determined by the Etest (Liofilchem, Italy) method, according to the manufacturer’s instructions (49). Strain WHO-P was used for quality control. No interpretative criteria have been provided by WHO and CLSI (or any other organization) for ertapenem susceptibility breakpoints against N. gonorrhoeae.
Sequencing of resistance determinants (penA, mtrR, penB, and ponA) and N. gonorrhoeae multiantigen sequence typing (NG-MAST).
Genomic DNA was prepared from individual gonococcal isolates using the rapid bacterial genomic DNA isolation kit (DNA-EZ Reagents V All-DNA-Fast-Out; Sangon Biotech Co. Ltd., Shanghai, China). ESC resistance determinants penA, mtrR, penB, and ponA were amplified by PCR using published primers (50), and DNA sequencing was performed by Suzhou Genewiz Biotech Co. Ltd. The sequencing data were uploaded to the NG-STAR database (https://ngstar.canada.ca) to determine the ESC resistance determinants.
Genetic characterization was performed by N. gonorrhoeae multiantigen sequence typing (NG-MAST), which assigns sequence types (STs) based on a combination of two variable genes, porB and tbpB (51); allele numbers and sequence types (STs) were then assigned.
Data analysis.
Chi-square (χ2) testing for linear trends was used to assess changes in ertapenem MICs during the study period. Chi-square or Fisher exact testing was used to determine the associations between ertapenem susceptibility and gonococcal genetic resistance determinants or N. gonorrhoeae multiantigen sequence types. SPSS version 26.0 was used for statistical analysis, and P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
This work was supported by grants from the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (2016-I2M-3-021) and the U.S. National Institutes of Health (AI084048 and AI116969).
No conflicts of interest for all authors.
REFERENCES
- 1.World Health Organization. 2018. Report on global sexually transmitted infection surveillance -2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Yue X, Gong X, Li J, Wang Y, Gu H. 2019. Gonorrhea in China, 2018. Int J Dermatol Venereol 2:65–69. 10.1097/JD9.0000000000000008. [DOI] [Google Scholar]
- 3.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67:1858–1860. 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 6.Unemo M, Bradshaw CS, Hocking JS, de Vries H, Francis SC, Mabey D, Marrazzo JM, Sonder G, Schwebke JR, Hoornenborg E, Peeling RW, Philip SS, Low N, Fairley CK. 2017. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 17:e235–e279. 10.1016/S1473-3099(17)30310-9. [DOI] [PubMed] [Google Scholar]
- 7.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. 2017. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 14:e1002344. 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2016. WHO guidelines for the treatment of Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 9.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Cyr S, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, Torrone E, Weinstock H, Kersh EN, Thorpe P. 2020. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 69:1911–1916. 10.15585/mmwr.mm6950a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. 2019. Sexually transmitted disease surveillance 2018. CDC, US Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- 12.Chisholm SA, Mouton JW, Lewis DA, Nichols T, Ison CA, Livermore DM. 2010. Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J Antimicrob Chemother 65:2141–2148. 10.1093/jac/dkq289. [DOI] [PubMed] [Google Scholar]
- 13.Blumer JL, Reed MD, Kaplan EL, Drusano GL. 2005. Explaining the poor bacteriologic eradication rate of single-dose ceftriaxone in group A streptococcal tonsillopharyngitis: a reverse engineering solution using pharmacodynamic modeling. Pediatrics 116:927–932. 10.1542/peds.2004-2294. [DOI] [PubMed] [Google Scholar]
- 14.Moran JS, Levine WC. 1995. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 20(Suppl 1):S47–S65. 10.1093/clinids/20.supplement_1.s47. [DOI] [PubMed] [Google Scholar]
- 15.Public Health England. 2018. Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) 2017. Public Health England, London, United Kingdom. [Google Scholar]
- 16.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, Unemo M. 2016. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 374:2504–2506. 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 17.Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, Morgan M, Newnham R, Golparian D, Unemo M, Crook DW, Peto TE, Hughes G, Cole MJ, Fifer H, Edwards A, Andersson MI. 2018. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 23:1800323. 10.2807/1560-7917.ES.2018.23.27.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. 2020. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 31:4–15. 10.1177/0956462419886775. [DOI] [PubMed] [Google Scholar]
- 19.Yin YP, Han Y, Dai XQ, Zheng HP, Chen SC, Zhu BY, Yong G, Zhong N, Hu LH, Cao WL, Zheng ZJ, Wang F, Zhi Q, Zhu XY, Chen XS. 2018. Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: a retrospective study of national surveillance data from 2013 to 2016. PLoS Med 15:e1002499. 10.1371/journal.pmed.1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SC, Han Y, Yuan LF, Zhu XY, Yin YP. 2019. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis 25:1427–1429. 10.3201/eid2507.190172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for STD Control, Chinese Center for Disease Control and Prevention; Venereology Group, Chinese Society of Dermatology; Subcommittee on Venereology, China Dermatologist Association. 2020. Guidelines for diagnosis and treatment of syphilis, gonorrhea and genital Chlamydia trachomatis infection (2020). Chin J Dermatol 53:168–179. [Google Scholar]
- 22.Congeni BL. 2010. Ertapenem. Expert Opin Pharmacother 11:669–672. 10.1517/14656561003631397. [DOI] [PubMed] [Google Scholar]
- 23.Arguedas A, Cespedes J, Botet FA, Blumer J, Yogev R, Gesser R, Wang J, West J, Snyder T, Wimmer W, Protocol 036 Study Group . 2009. Safety and tolerability of ertapenem versus ceftriaxone in a double-blind study performed in children with complicated urinary tract infection, community-acquired pneumonia or skin and soft-tissue infection. Int J Antimicrob Agents 33:163–167. 10.1016/j.ijantimicag.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Burkhardt O, Derendorf H, Welte T. 2007. Ertapenem: the new carbapenem 5 years after first FDA licensing for clinical practice. Expert Opin Pharmacother 8:237–256. 10.1517/14656566.8.2.237. [DOI] [PubMed] [Google Scholar]
- 25.de Vries HJC, de Laat M, Jongen VW, Heijman T, Wind CM, Boyd A, de Korne-Elenbaas J, van Dam AP, Schim van der Loeff MF, NABOGO steering group . 2022. Efficacy of ertapenem, gentamicin, fosfomycin, and ceftriaxone for the treatment of anogenital gonorrhoea (NABOGO): a randomised, non-inferiority trial. Lancet Infect Dis 22:706–717. 10.1016/S1473-3099(21)00625-3. [DOI] [PubMed] [Google Scholar]
- 26.Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, Tapsall JW. 2012. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob Agents Chemother 56:3603–3609. 10.1128/AAC.00326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golparian D, Hellmark B, Fredlund H, Unemo M. 2010. Emergence, spread and characteristics of Neisseria gonorrhoeae isolates with in vitro decreased susceptibility and resistance to extended-spectrum cephalosporins in Sweden. Sex Transm Infect 86:454–460. 10.1136/sti.2010.045377. [DOI] [PubMed] [Google Scholar]
- 28.Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. 2011. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 16:19833. https://www.eurosurveillance.org/content/10.2807/ese.16.14.19833-en. [PubMed] [Google Scholar]
- 29.Lindberg R, Fredlund H, Nicholas R, Unemo M. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother 51:2117–2122. 10.1128/AAC.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao S, Duncan M, Tomberg J, Davies C, Unemo M, Nicholas RA. 2009. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother 53:3744–3751. 10.1128/AAC.00304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu WQ, Zheng XL, Liu JW, Zhou Q, Zhu XY, Zhang J, Han Y, Chen K, Chen SC, Chen XS, Yin YP. 2021. Antimicrobial susceptibility of ertapenem in Neisseria gonorrhoeae isolates collected within the China Gonococcal Resistance Surveillance Programme (China-GRSP) 2018. Infect Drug Resist 14:4183–4189. 10.2147/IDR.S335252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quaye N, Cole MJ, Ison CA. 2014. Evaluation of the activity of ertapenem against gonococcal isolates exhibiting a range of susceptibilities to cefixime. J Antimicrob Chemother 69:1568–1571. 10.1093/jac/dkt537. [DOI] [PubMed] [Google Scholar]
- 33.Livermore DM, Alexander S, Marsden B, James D, Warner M, Rudd E, Fenton K. 2004. Activity of ertapenem against Neisseria gonorrhoeae. J Antimicrob Chemother 54:280–281. 10.1093/jac/dkh272. [DOI] [PubMed] [Google Scholar]
- 34.Jacoby GA, Mills DM, Chow N. 2004. Role of beta-lactamases and porins in resistance to ertapenem and other beta-lactams in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:3203–3206. 10.1128/AAC.48.8.3203-3206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabó D, Silveira F, Hujer AM, Bonomo RA, Hujer KM, Marsh JW, Bethel CR, Doi Y, Deeley K, Paterson DL. 2006. Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae. Antimicrob Agents Chemother 50:2833–2835. 10.1128/AAC.01591-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGettigan SE, Andreacchio K, Edelstein PH. 2009. Specificity of ertapenem susceptibility screening for detection of Klebsiella pneumoniae carbapenemases. J Clin Microbiol 47:785–786. 10.1128/JCM.02143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang F, Yan J, Zhang J, van der Veen S. 2020. Evaluation of alternative antibiotics for susceptibility of gonococcal isolates from China. Int J Antimicrob Agents 55:105846. 10.1016/j.ijantimicag.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Banhart S, Selb R, Oehlmann S, Bender J, Buder S, Jansen K, Heuer D. 2021. The mosaic mtr locus as major genetic determinant of azithromycin resistance of Neisseria gonorrhoeae-Germany, 2018. J Infect Dis 224:1398–1404. 10.1093/infdis/jiab091. [DOI] [PubMed] [Google Scholar]
- 39.Rouquette-Loughlin CE, Reimche JL, Balthazar JT, Dhulipala V, Gernert KM, Kersh EN, Pham CD, Pettus K, Abrams AJ, Trees DL, St Cyr S, Shafer WM. 2018. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 9:e02281-18. 10.1128/mBio.02281-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham CD, Sharpe S, Schlanger K, St Cyr S, Holderman J, Steece R, Soge OO, Masinde G, Arno J, Schmerer M, Kersh EN, the SURRG Working Group . 2019. Emergence of Neisseria gonorrhoeae strains harboring a novel combination of azithromycin-attenuating mutations. Antimicrob Agents Chemother 63:e02313-18. 10.1128/AAC.02313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer HM, Young H, Graham C, Dave J. 2008. Prediction of antibiotic resistance using Neisseria gonorrhoeae multi-antigen sequence typing. Sex Transm Infect 84:280–284. 10.1136/sti.2008.029694. [DOI] [PubMed] [Google Scholar]
- 42.Thakur SD, Levett PN, Horsman GB, Dillon JA. 2014. Molecular epidemiology of Neisseria gonorrhoeae isolates from Saskatchewan, Canada: utility of NG-MAST in predicting antimicrobial susceptibility regionally. Sex Transm Infect 90:297–302. 10.1136/sextrans-2013-051229. [DOI] [PubMed] [Google Scholar]
- 43.Lo JY, Ho KM, Lo AC. 2012. Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance. J Antimicrob Chemother 67:1422–1426. 10.1093/jac/dks036. [DOI] [PubMed] [Google Scholar]
- 44.Qin X, Zhao Y, Chen W, Wu X, Tang S, Li G, Yuqi Y, Cao W, Liu X, Huang J, Yang J, Chen W, Tang W, Zheng H. 2019. Changing antimicrobial susceptibility and molecular characterisation of Neisseria gonorrhoeae isolates in Guangdong, China: in a background of rapidly rising epidemic. Int J Antimicrob Agents 54:757–765. 10.1016/j.ijantimicag.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ison CA. 1990. Laboratory methods in genitourinary medicine. Methods of diagnosing gonorrhoea. Genitourin Med 66:453–459. 10.1136/sti.66.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unemo M, Ison C. 2013. Gonorrhoea, p 21–54. In Unemo M, Ballard R, Ison C, Lewis D, Ndowa F, Peeling R (ed), Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 47.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 48.World Health Organization. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 49.Liofilchem. 2021. MIC test strip technical sheet Gonococci. Liofilchem, Roseto degli Abruzzi, Italy. https://www.liofilchem.com/images/brochure/mic_test_strip_patent/MTS13.pdf. [Google Scholar]
- 50.Demczuk W, Sidhu S, Unemo M, Whiley DM, Allen VG, Dillon JR, Cole M, Seah C, Trembizki E, Trees DL, Kersh EN, Abrams AJ, de Vries H, van Dam AP, Medina I, Bharat A, Mulvey MR, Van Domselaar G, Martin I. 2017. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol 55:1454–1468. 10.1128/JCM.00100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis 189:1497–1505. 10.1086/383047. [DOI] [PubMed] [Google Scholar]