Abstract
Iodine is a micronutrient needed for the production of thyroid hormones, which regulate metabolism, growth, and development. Iodine deficiency or excess may alter the thyroid hormone synthesis. The potential effects on infant development depend on the degree, timing, and duration of exposure. The iodine requirement is particularly high during infancy because of elevated thyroid hormone turnover. Breastfed infants rely on iodine provided by human milk, but the iodine concentration in breast milk is determined by the maternal iodine intake. Diets in many countries cannot provide sufficient iodine, and deficiency is prevented by iodine fortification of salt. However, the coverage of iodized salt varies between countries. Epidemiological data suggest large differences in the iodine intake in lactating women, infants, and toddlers worldwide, ranging from deficient to excessive intake. In this review, we provide an overview of the current knowledge and recent advances in the understanding of iodine nutrition and its association with thyroid function in lactating women, infants, and toddlers. We discuss risk factors for iodine malnutrition and the impact of targeted intervention strategies on these vulnerable population groups. We highlight the importance of appropriate definitions of optimal iodine nutrition and the need for more data assessing the risk of mild iodine deficiency for thyroid disorders during the first 2 years in life.
Keywords: iodine, deficiency, excess, lactation, infan
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Iodine nutrition is a key determinant of thyroid function: Both iodine deficiency and excess may impair thyroid hormone production and thereby affect metabolism, growth, and development.
The physiological iodine requirement is high in lactating women, infants, and toddlers, but current dietary intake recommendations are poorly defined, vary substantially between countries and need to be harmonized.
Human milk is the main source of iodine for infants, and the iodine concentration in breast milk strongly depends on the maternal iodine intake a few hours before breastfeeding.
Iodine status is best assessed by breast milk iodine concentration (BMIC) in lactating women and urinary iodine concentration (UIC) in infants and toddlers, but the thresholds currently used to define optimal iodine nutrition in these groups are uncertain and should be revised using new scientific advances.
Iodine status in lactating women, infants, and toddlers varies considerably worldwide: Mild iodine deficiency and excess iodine intake may be widespread, but quality data are limited and the impact on child development is uncertain.
Salt iodization is the primary public health intervention to prevent iodine deficiency and provides sufficient dietary iodine to ensure adequate iodine nutrition in lactating women, breastfed infants and weaning infants.
In populations with poor coverage of iodized salt and documented low iodine intake, iodine supplementation in lactating women and dietary interventions for toddlers may be required.
Maternal and infant nutrition determines child development and later adult health (1, 2). For the first 6 months of life, exclusive breastfeeding is the norm for optimal infant nutrition (3). The nutritional composition of human milk typically depends on the maternal dietary intake and nutritional status (4-6), but the association between maternal nutrient intake, human milk composition, and nutrient adequacy in infants is still poorly understood (7-9).
Iodine is an essential component of thyroid hormones and a particularly critical nutrient for child development. Poor iodine nutrition may impair thyroid hormone synthesis and thereby affect physical, neurological, and intellectual development (10, 11). The importance of adequate iodine intake during pregnancy is well recognized (12-14), but the role of iodine for the prevention of thyroid disorders during lactation and infancy has only recently gained scientific attention. Iodine intake has improved remarkably in the general population over the past decades thanks to salt iodization (15, 16), but reports suggest lactating women, infants, and toddlers remain at risk of iodine deficiency (17–19). The criteria for optimal iodine nutrition in these groups are poorly defined, making data interpretation uncertain (20). International health agencies and medical expert associations acknowledge iodine deficiency during lactation and infancy as a risk factor for thyroid disease and compromised infant development and recommend targeted interventions to achieve optimal iodine nutrition (21-25). However, knowledge about iodine nutrition and iodine deficiency–prevention strategies is poor among obstetricians, midwives, and women (26-29).
In this review we present an overview of the current knowledge and recent advances in the understanding of iodine nutrition and its association with thyroid function in lactating women, infants, and toddlers. We describe the association between maternal iodine status, the composition of human milk, and the role of breast milk for optimal iodine nutrition in infants and toddlers. We discuss dietary iodine requirements, iodine status biomarkers, and public health strategies to prevent iodine deficiency. We provide a critical review of previous findings from experimental and epidemiological data and highlight remaining knowledge gaps. We propose research priorities for better understanding of iodine nutrition needed to guide recommendations for optimal thyroid function during lactation, infancy, and early childhood.
Thyroid Function and Thyroid Disorders
Normal Physiology
Dietary iodine is absorbed in the gastrointestinal tract, enters the systemic circulation, and is taken up into the thyroid cells via the sodium (Na+) and iodide (I−) symporter (NIS) (30). I− is oxidized in the thyroid gland by thyroid peroxidase (TPO) and covalently bound to the glycoprotein thyroglobulin (Tg) to form thyroxine (T4) and small amounts of 3,5,3′-triiodothyronine (T3) (31, 32). The biosynthesis and release of thyroid hormones to the circulation is controlled by the hypothalamic-pituitary-thyroid (HPT) axis via thyrotropin (TSH) in a negative feedback loop (33). TSH binds to the TSH receptor and induces the expression of Tg (34, 35).Thyroid hormone synthesis is tightly controlled, and the thyroid gland uses several specific mechanisms to ensure adequate hormonogenesis, likely as a result of the low availability of iodine throughout evolution (32, 36). The thyroid gland efficiently stores iodine bound to Tg and this reserve may be used during periods of low intake (32). In peripheral tissues, T4 is converted to the metabolically active form T3 via deiodinase enzymes (37). T3 binds to nuclear thyroid hormone receptors and regulates gene expression of a wide range of genes controlling numerous fundamental processes, including metabolism, growth, and neurologic functions (11, 38). The regulatory roles of T3 on brain and neurocognitive development involve neuronal proliferation and migration, glial differentiation, and myelination of the central nervous system (38-40). T4 may also act directly on target tissues, although to a lesser extent than T3 (41, 42). The bioactivity of thyroid hormones is determined by an intricate interplay between membrane thyroid hormone transport into the cytoplasm, deiodinase activity, and thyroid hormone receptor expression and distribution (43-45).
The fetal thyroid gland starts producing thyroid hormone in the second trimester (~ 20 weeks), but the mother’s T4 contribution is still crucial (11). Fetal serum T4 levels gradually increase until birth (11). The placenta takes up iodine from the maternal circulation and serves as an iodine reservoir to maintain fetal iodine status and adequate thyroid hormone production (11, 46, 47). The HPT axis is fully mature first at term or in the early neonatal period. Immediately after delivery (30-60 minutes), serum TSH sharply rises to 60 to 80 mIU/mL as an adaptation to extrauterine life and then rapidly falls back to stable levels 3 to 5 days after birth (11, 48). Placental iodine content has been negatively associated with TSH levels short after delivery (49). This physiological TSH surge stimulates the T4 and T3 production in the newborn. Serum T4 and T3 concentrations peak at 24 hours after birth, followed by a gradual decline to reach more stable concentrations around 5 to 7 days post partum (11, 48). The decrease continues during infancy and childhood but at a slower rate (50, 51). The turnover of T4 during infancy is high, and infants produce 3 times more T4 than adults per kg body weight (5-6 µg/kg/day in infants vs 1.5 µg/kg/day in adults) (11, 52). Paradoxically, infants are born with minimal iodine stores (~ 300 µg) that last only a few days (52). Therefore, iodine must be supplied by breast milk (or infant formula) to maintain the physiological high T4 production rate (11, 52).
Thyroid Dysfunction
Lactating women
Abnormal thyroid function is common during the postpartum period and the incidence of both hypothyroidism and hyperthyroidism increase markedly compared to before and during pregnancy (25, 53, 54). Thyroid dysfunction detected during the first year after birth in women with no previous history of thyroid disease is generally classified as postpartum thyroiditis, except if the diagnosis is Graves disease. Postpartum thyroiditis is an inflammatory subclinical autoimmune thyroid disorder associated with postpartum immune rebound as well as the presence of TPO antibodies during pregnancy (54–57). Postpartum autoimmune thyroiditis typically occurs 1 to 4 months after delivery with a prevalence of 5% and often develops from a previous subclinical thyroiditis that is exacerbated after delivery (54, 56-58). In the classic form, transient thyrotoxicosis is followed by transient hypothyroidism returning to euthyroidism by the end of the initial postpartum year (25, 54, 56, 57). Inflammatory processes in the thyroid gland result in release of T4 and T3 from the follicular cells, and synthesis of the thyroid hormones resumes when the inflammation subsides.
Iodine deficiency is a well-recognized risk factor for thyroid disorders in adults (59, 60). The incidence of postpartum thyroiditis has not directly been demonstrated to be associated with iodine status (61). However, some studies, but not all, observed an increased risk for TPO antibody positivity in iodine excess (60, 62, 63), which in turn is associated with postpartum thyroiditis. Three small randomized studies have examined the effects of prenatal iodine supplementation on postpartum thyroiditis, but none of the studies observed a difference in frequency or severity of the condition (61, 64, 65). A randomized controlled trial (RCT) of iodine supplementation (200 µg/day) to pregnant women in 2 mildly iodine-deficient populations observed no differences in maternal thyroid function tests, thyroid volume, or thyroid antibodies between groups during pregnancy or at 6 weeks post partum (66). Adequately powered studies are needed to exclude iodine deficiency as a risk factor for postpartum thyroiditis. A recent study in women with pregestational Hashimoto thyroiditis suggests that maternal thyroid status in early pregnancy predicts postpartum thyroiditis and that euthyroidism is associated with a higher rate of postpartum thyroiditis than hypothyroidism (67).
Abnormal maternal thyroid hormone concentrations can negatively affect the ability to successfully breastfeed (25, 54). Women at particular risk of postpartum thyroiditis may be screened, but universal thyroid function screening post partum is not recommended (22, 25).
Infants
Low circulating levels of thyroid hormones lead to hypothyroidism, which may be present in utero, at birth (congenital hypothyroidism), or develop later in life (acquired hypothyroidism). Abnormal function of the infant thyroid gland may result in unique effects on thyroid hormone–dependent growth and development. Infants and children diagnosed with overt hypothyroidism (high TSH and low T4) are at increased risk of cognitive deficits, metabolic abnormalities, and impairments in growth and delayed skeletal maturation (11, 68-70). However, the clinical consequences depend on the timing, age, and severity of thyroid impairment (11, 38, 68, 71, 72), as well as on the maternal thyroid function during pregnancy (73). Concentrations of TSH, Tg, T4, and T3 in cord blood are closely associated with maternal serum concentrations and not necessarily reflective of infant thyroid function (66, 74-78).
Congenital hypothyroidism (TSH ≥ 20 mIU/L) due to thyroid hormone deficiency at the end of pregnancy and/or early infancy is a common cause of intellectual impairment, with an incidence of approximately 1 in 2000 to 3000 newborns in populations with adequate or mildly deficient iodine intake (70). It can be caused by i) disorders of thyroid gland development (dysembriogenesis or dysgenesis), accounting for 80% to 85% of cases; or ii) defects in any step of thyroid hormone synthesis, including mutations in genes involved in iodine handling, accounting for the remaining 15% to 20% cases (79). It should be noted that congenital hypothyroidism is unrelated to the physiological increase in infant TSH occurring immediately after delivery (discussed earlier). Thanks to neonatal screening at 2 to 5 days after birth, infants with congenital hypothyroidism are identified and treated with L-thyroxine, but 70% of infants worldwide are still not covered by screening programs (80).
Mild or subclinical hypothyroidism (high TSH and normal T4) is increasingly being detected and diagnosed in newborns and infants, partly because of lower neonatal TSH-screening thresholds (81-84). Iodine deficiency is likely one of the causes of subclinical hypothyroidism in pediatric populations, but supporting data are lacking. Other known causes include maternal thyroid dysfunction, gene defects (eg, TSH receptor mutations), genetic syndromes (eg, Down syndrome), and autoimmune thyroid disease (ie, Hashimoto thyroiditis) (68, 75, 85, 86). The consequences of mild or subclinical hypothyroidism on development in early infancy or childhood remain unclear (68, 87-89), but neurocognitive deficits in neonates are possible (89). In older infants or children, there is no clear evidence of growth restriction or neurocognitive impairments, but subtle cardiovascular abnormalities have been documented (68, 88, 89). Infants identified with subclinical hypothyroidism require close follow-up because elevated TSH may be transient in approximately half the cases (90). In contrast to adults (91), the risk of progression from subclinical hypothyroidism to overt thyroid dysfunction is generally low in children (68, 88) and mildly elevated TSH may spontaneously normalize over the first year of life if no underlying thyroid disorder is present (92, 93). Hyperthyroidism, characterized by excessive thyroid hormone production, is associated with growth acceleration, advanced bone age, tachycardia, and mood disorders (69, 94). This condition, although serious, is rare in neonates and young children.
Epidemiological data on thyroid function in pediatric populations are limited, apart from the initial screening at birth, and there is poor consensus on reference ranges for TSH and T4 during infancy and childhood (51, 95). Clinical data suggest acquired overt pediatric thyroid disease is rare (11, 93). Subclinical hypothyroidism appears to be less common in infants and children (68, 93, 96) than in adults (86), but data are uncertain.
Comprehensive reviews of thyroid disorders in pediatrics are available elsewhere (68-70, 83, 88, 89, 94, 97).
Health Consequences of Iodine Deficiency
Thyroid Function
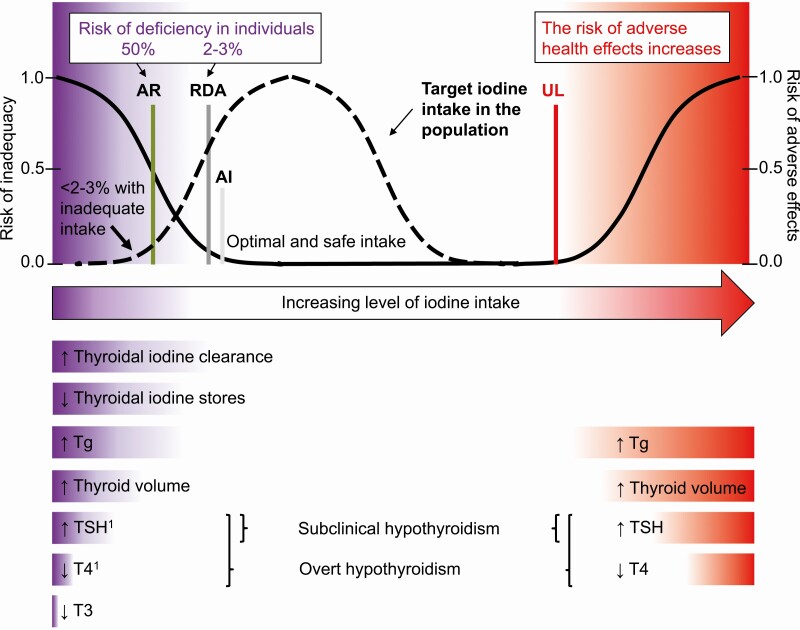
Iodine malnutrition can alter thyroid function and may cause thyroid disorders at any time throughout life (10, 59, 60). The association between iodine intake and thyroid function is U-shaped: Adverse effects are reported both at deficient and excessive intakes (60, 98). The biological response to deficiency or excess occurs gradually, and the risk for thyroid dysfunction and subsequent functional consequences depends on the degree of iodine deficiency as well as the timing and duration of exposure (Fig. 1). At prolonged low iodine intake the thyroidal iodine uptake increases (10, 32).
Figure 1.
Dietary reference intakes for iodine, risk of iodine malnutrition, and subsequent thyroid dysfunction in individuals at iodine intakes ranging from deficient to excessive. The AR is the daily iodine intake estimated to meet the requirements of half the healthy individuals in a specific population group. At this intake level the risk of inadequacy is 50% to an individual. The RDA is the average daily iodine intake level sufficient to meet the iodine requirement of nearly all (97%-98%) of healthy individuals. At this intake level the risk of iodine deficiency is only 2% to 3% to an individual. The AI is the intake level assumed to be adequate when there are insufficient data to define an AR. At intakes between the RDA and the UL, the risk of inadequacy and of excess is low. At intakes above the UL, the risk of adverse effects increases. The dashed line indicates the target population distribution of iodine intakes in iodine sufficiency. The population intake distribution should be adjusted for within-person variability using the estimated AR cutpoint method (99). In iodine sufficiency, the proportion of individuals with intakes less than AR and greater than UL is less than 2% to 3%, respectively. The lower part of the figure indicates the physiological adaptation to low and excessive iodine intakes. 1Iodine deficiency may also cause transient hyperthyroidism in adults (60). Adapted in part from (100). AI, adequate intake; AR, average requirement; RDA, recommended daily allowance; UL, upper level.
Severe iodine deficiency is associated with elevated Tg and TSH, and low T4, whereas T3 often remains normal (60). The T3/T4 ratio increases in serum and the deiodination of T4 to T3 increases at the cellular level increase as adaptive mechanisms to minimize the risk of functional consequences due to iodine deficiency (37). Exposure to moderate iodine deficiency may slightly elevate TSH to maintain serum T4 within normal or low normal ranges. Populations affected by severe iodine deficiency have a high prevalence of goiter and overt hypothyroidism (60). More than half of infants may be born with goiter (101). The incidence of congenital hypothyroidism can be as high as 1 in 10 (77, 102, 103). The recall rate of suspected congenital hypothyroidism may be up to 10% (compared to 0.05%-0.2% in iodine-sufficient populations) (52, 77, 102, 104), but the rates of confirmed hypothyroidism vary (105). Correction of moderate-to-severe iodine deficiency in affected populations reduces the incidence of hypothyroidism (106). Congenital hypothyroidism due to exposure to severe iodine deficiency during pregnancy and infancy may persist into childhood (107, 108). Moderate-to-severe iodine deficiency is also a risk factor for transient neonatal hypothyroidism (temporary high TSH and low T4) (102, 109-112), as well as for persistent subclinical hypothyroidism (108, 113, 114). Acquired transient neonatal hypothyroidism has been reported in neonates and infants of mothers with restrictive maternal diets (eg, vegan diet) (115–117) and in infants under prolonged feeding of parenteral or enteral nutrition with low iodine concentrations (118).
Mild iodine deficiency increases the thyroid activity, and elevated Tg concentrations are reported across all population groups (14, 60, 119-121). Although TSH and T4 overall remain within the normal range, mild iodine deficiency is a recognized risk factor for thyroid disorders in adults (59, 60, 122, 123). However, apart from data from neonatal screening at birth, which primarily reflects exposure during pregnancy, epidemiological studies assessing the association between mild iodine deficiency and thyroid function in infancy are limited. A recent case-control study in a large cohort of newborns in the United States found no association between newborn blood iodine concentrations and congenital hypothyroidism (124).
Are newborns and infants at higher risk for thyroid dysfunction due to iodine deficiency than adults?
Observational data in a moderate-to-severe iodine-deficient population suggest a higher prevalence of thyroid hypofunction in young infants than in their mothers (125), whereas other studies found no support (107). There is little evidence in mildly iodine-deficient populations. However, considering the high rate of thyroid hormone synthesis and the low iodine stores, a sudden decline of the iodine intake would likely lead to a faster decrease in thyroid hormone concentration in infants than in their mothers (52, 102, 126).
Infant Mortality and Growth
Moderate-to-severe iodine deficiency during pregnancy increases the risk for stillbirth, miscarriage, and perinatal and infant mortality (10, 60, 127), possibly through an increased risk of low birthweight (128). However, available data suggest no association between maternal urinary iodine concentration (UIC) during pregnancy and anthropometric measures in newborns (129), whereas newborn TSH is negatively associated with birthweight (129, 130). Iodine supplementation of severely iodine-deficient pregnant women improves mean birthweight, whereas no influence has been reported following iodine repletion of pregnant women exposed to mild-to-moderate iodine deficiency (128). Iodine deficiency during infancy may impair growth, but adequately powered studies assessing the effects of iodine repletion on postnatal growth outcomes in term infants are lacking (128).
Infant survival may also be affected by iodine status. A large cross-sectional study in Indonesia observed a higher prevalence of child malnutrition and mortality in neonates, infants, and children younger than 5 years among families using no or inadequately iodized salt compared to families consuming adequately iodized salt (131). An RCT of iodized oil (100 mg) given to 6-week-old infants in an iodine-deficient area of Indonesia observed lower infant mortality after the iodine intervention compared to placebo at age 4 months, but no difference was observed at age 6 months (132). The studies are limited by the small sample size used to assess mortality and the lack of data on iodine status and thyroid function in the mothers and infants.
Neurodevelopment
Low thyroid hormone concentrations due to iodine deficiency may impair neurodevelopment (10, 133, 134). However, the effects of thyroid hormone inadequacy on the developing brain vary between stages of development and depend on the degree, time and duration of exposure (38, 72). Iodine deficiency commonly occurs both in utero and after birth. The potential consequences on brain development may therefore be a result of combined prenatal and postnatal exposure.
The most serious adverse effects of iodine deficiency on the brain are caused by exposure during fetal development. In populations affected by severe iodine deficiency, neonates may be born with neurological cretinism due to maternal hypothyroxinemia during the first half of pregnancy (135, 136). Infants are typically euthyroid (38). Myxedematous cretinism is also caused by severe iodine deficiency, possibly also in combination with dietary goitrogens (eg, unprocessed cassava) or selenium deficiency, but results from fetal exposure during late pregnancy and/or after birth (38, 135, 136). This form of cretinism presents as severe hypothyroidism with similar clinical symptoms as in untreated congenital hypothyroidism, but the degree of intellectual impairment is less severe than for neurological cretinism (135, 136). Severe maternal iodine deficiency leading to fetal hypothyroidism in utero may also cause other neurologic and cognitive deficits, including reduced IQ, as extensively reviewed elsewhere (123, 127, 134, 137-139).
Effects of mild iodine deficiency during pregnancy on brain and child development remain uncertain (14, 134, 139). A negative association between newborn TSH and cognitive development has been observed in iodine-deficient populations, but studies are small and data are inconclusive (130). A recent analysis of two pregnancy studies, one in a borderline iodine-deficient setting and another in an iodine-sufficient setting, observed no association between neonatal TSH and childhood neurodevelopment at age 18 months (140). The percentage of newborns with TSH concentration greater than 5 mIU/L was 4.6% and 5.5%, respectively (140). After birth, hypothyroidism exerts effects on cerebellar development, including language and verbal skills (38, 71, 72). However, little is known about the effects of iodine deficiency on neurological development during infancy (134, 137). In an observational study in Chinese infants, breast milk iodine concentration (BMIC) in colostrum predicted motor development, but BMIC was not associated with cognition, language, or motor development in 18-month-old infants (141). A small RCT conducted in Iran reported higher cognitive scores in 36-month-old children whose mothers received 150 µg iodine/day compared to placebo during lactation, but no effect was observed in women who received 300 µg iodine/day (142). The study was conducted in an overall iodine-sufficient population (143), and observed no effect on language or motor development (142). Effects of iodine deficiency on cognitive functions during infancy are plausible, but evidence in mild-to-moderate iodine deficiency is lacking.
Health Consequences of Iodine Excess
The physiological response to iodine excess is complex and depend on whether the exposure is acute or chronic, and if acute, if the habitual intake before exposure was deficient or adequate (98, 122). In adults, the healthy thyroid is highly flexible and capable of adapting to high iodine intake, although excessive intake may still cause and/or aggravate hyperthyroidism, hypothyroidism, goiter, and thyroid autoimmunity (see Fig. 1) (98, 144). Elevated rates of thyroid dysfunction have been documented in lactating women exposed to iodine excess, although studies are small (145, 146).
Acute excess can cause a transient decrease in thyroid hormone production, a phenomenon known as the Wolff-Chaikoff effect (122). After adaptation, the gland “escapes” from this block and resumes thyroid hormone synthesis. However, the immature neonatal thyroid gland may be unable to escape from the acute Wolff-Chaikoff effect, possibly making the fetus and infant more susceptible to iodine-induced hypothyroidism (122, 147). Data in adults suggest that the susceptibility to excess iodine exposure may be higher in iodine-deficient populations compared to populations with otherwise adequate habitual iodine intake (98), but data in infants are limited.
Exposure to severe chronic iodine excess during pregnancy may cause fetal goiter that can obstruct the neonatal airway at delivery (148, 149), as well as congenital or transient hypothyroidism (102, 150-154). In some individuals, chronic maternal intake just above the requirements may cause maternal hypothyroidism or isolated hypothyroxinemia (155). This could potentially affect cognitive development of the offspring (156), although data on the long-term effects are conflicting (157, 158).
Excess maternal iodine intake and high BMIC may induce subclinical and clinical hypothyroidism in breastfed infants (102, 151, 159-164). The effects may be transient, but there is a risk of persistent thyroid dysfunction in both mothers and infants. Data in young infants and lactating mothers are mainly from case studies (160, 161, 164), and data in larger epidemiological studies is limited. Populations with chronic exposure to iodine intake above the requirements typically have elevated median Tg concentration, also consistently observed in observational studies of toddlers (121, 165-167). Prevalence rates of subclinical hypothyroidism up to 10% have been reported in some studies (165, 166), whereas other studies report no thyroid disorders (121, 167). A slightly elevated risk of overt hypothyroidism has been observed in infants 6 months and younger (167), but not in infants older than 6 months, and studies are small (121, 165, 166). An RCT conducted in Morocco administered 100-mg iodine given as iodized oil directly to 2-week-old infants in a moderately iodine-deficient population (112). The short-term response 1 week after administration was examined in a small group and long-term effects in all supplemented infants after 9 months. None of the infants showed clinical or biochemical signs of iodine-induced hypothyroidism or hyperthyroidism at 1 week or 9 months after administration. Observational studies in infants exposed to excessive quantities of iodine in breast milk from lactating mothers receiving iodine therapy (4-100 mg/day) for Graves disease have been conducted in Japan, where iodine intake is typically adequate to high (168, 169). Twelve percent of the infants had elevated TSH, indicating mild subclinical hypothyroidism, and in most cases TSH normalized with cessation of the mother’s iodine treatment. Another small study in neonates and infants exposed to high doses of iodine via contrast media observed transient elevation of TSH and drops in thyroid hormone concentrations, but no permanent thyroid dysfunction (170). The 3 studies suggest that the majority of infants exposed to excessive iodine intake may be able to maintain euthyroidism via initiation and then successfully escape from the Wolff-Chaikoff effect, but larger studies are needed.
The susceptibility of exposure to excess iodine intake remains uncertain, but the effects likely depend on the level of excess and whether the exposure is acute or chronic. In adults, morbidity due to excess iodine intake is usually transient (60). Concerns exist that excess iodine intake may trigger autoimmunity (63), but thyroid antibodies are rare in children (11) and data in lactating women are limited. The long-term effects of chronic excessive iodine intake on thyroid function, somatic growth, and development in infants and young children remain uncertain.
Dietary Reference Values for Iodine
Nomenclature
Nutrient adequacy in populations should be assessed based on the average requirement (AR), that is, the habitual intake estimated to meet the physiological requirement of half (50%) of healthy individuals of a specific life stage (see Fig. 1) (100, 171). The nutrient intake in a population is overall adequate when less than 2% to 3% of the populations have usual intakes below the AR (Fig. 1) (99).
No AR has been defined for iodine in infants (discussed later). The iodine reference intake for infants is instead defined as an adequate intake (AI) or as a recommended daily allowance (RDA) (see Fig. 1). AI/RDAs are set at an average daily level that is assumed to be enough to meet the iodine requirements of nearly all (97.5%) healthy individuals. These reference intakes are used to assess intakes in individuals, not in populations (99, 100). The mean intake in populations is often incorrectly compared to the AI/RDA. In fact, the mean intake must exceed the RDA in order to achieve a low prevalence below the AR (100). Attention to the correct application of defined dietary reference values is crucial to avoid misclassification of population iodine intake (100).
Lactating Women
The physiological requirement in a specific population group likely is the same across populations (171). However, international consensus on recommended iodine intakes in lactating women is lacking. Compared to before and during pregnancy, most bodies propose higher iodine intakes are needed during lactation to cover the physiological needs both of mother and infant. Reference values are based on median BMIC obtained from small cross-sectional studies, in many cases conducted in populations with low iodine intake. The United States and Canada estimated the AR in lactating women at 209 µg/day (172). This reference intake was derived from the estimated AR of nonpregnant, nonlactating women (95 µg/day) by adding the mean daily iodine excretion in breast milk (114 µg/day at a BMIC of 146 µg/L and infant breast milk consumption of 0.78 L/day) (172). By accounting for interindividual variability in BMIC, the RDA was set at 290 µg/day, twice that for women of reproductive age (150 µg/day) and 1.3 times higher than for pregnant women (220 µg/day) (172). The estimated AR for lactation was set at 190 μg/day in Australia and New Zealand and at 170 μg/day in China, assuming 85 to 90 μg/day is secreted in breast milk (173, 174).
The WHO recommends an iodine intake of 250 µg/day in lactating women, an additional 100 µg/day compared to nonpregnant, nonlactating adult women (21, 175, 176). This recommended nutrient intake (RNI) is defined in the same way as an RDA and should not be used for population assessment. The European Food Safety Authority (EFSA), the Nordic countries, Germany, Austria, and Switzerland are also presenting their intake recommendations as RNIs, ranging from 170 to 260 μg/day (177-179). These RNIs are based on studies in which the reported BMICs were low assuming only 50 µg/day extra iodine is sufficient to account for losses in breast milk (177-179), whereas no increment is recommended by the United Kingdom (180).
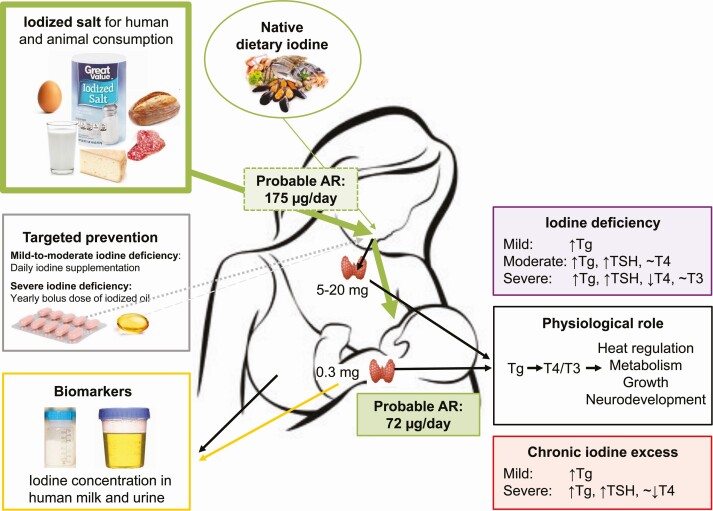
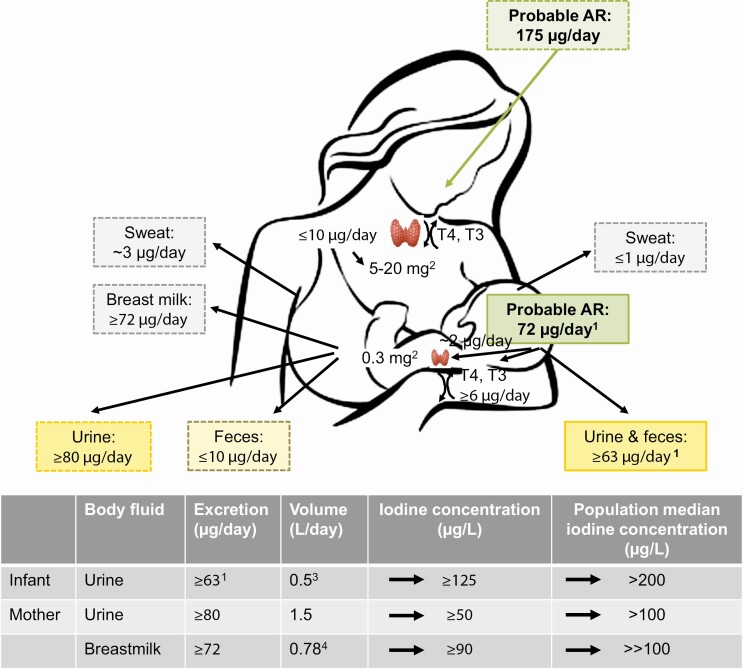
The amount of iodine excreted in breast milk should meet the dietary requirements of exclusively breastfed infants. When adding the proposed AR for iodine in 0- to 6-month-old infants (72 µg/day and accounting for 90% bioavailability) (discussed later) (181) to the AR of nonlactating women (95 µg/day) (172), the AR for lactating women is estimated to be 175 µg/day, 20% lower than the current estimated AR recommended by the Institute of Medicine (Fig. 2) (172). However, metabolic balance studies conducted in iodine-sufficient lactating women are needed to define the actual physiological requirement for iodine during the period women are breastfeeding. The intake recommendations for lactating mothers and breastfed infants should be coherent and defined in parallel at levels ensuring optimal nutrition in both groups, and should be internationally harmonized.
Figure 2.
Schematic illustration of daily AR and iodine excretion pathways in lactating women and infants. Iodine is primarily excreted in urine, but in during lactation additionally also in breast milk. A small proportion of ingested iodine is excreted in feces and sweat, but the exact amounts are uncertain. A daily iodine intake of 72 µg/day has been proposed to meet the AR during the first 6 months of life (181). The AR in infants may be used to define the AR in lactating women as iodine excreted in breast milk must meet the dietary requirements of exclusively breastfed infants. By adding the infant AR (72 µg/day × 90% bioavailability) to the AR of nonlactating women (95 µg/day) (172), the AR for lactating women is estimated to approximately 175 µg/day. Iodine sufficiency is currently assessed by the population median UIC and BMIC, but may also be defined as less than 3% of individuals with intakes less than AR (after adjusting for intraindividual variability) (see Fig. 1). The UIC corresponding to the AR in infants is approximately 125 µg/L (181) and the subsequent population median UIC greater than 200 µg/L, higher than the median UIC threshold of 100 µg/L currently used to define adequate iodine nutrition in infants. A population median BMIC greater than 100 µg/L likely indicates adequate iodine intake in lactating women. 1(181); 2Iodine stores in thyroid; 3(182); 4(172). AR, average requirement; BMIC, breast milk iodine concentration; UIC, urinary iodine concentration.
Infants
The recommended intake of iodine for the first 6 months of life varies considerably between countries and bodies, ranging from 40 µg/day in Germany and Austria to 110 µg/day in the United States and Canada (172-175, 177-180). The difference between countries reflects limited scientific data and different methodological approaches taken to set the recommendations. As for many other nutrients (7), the recommended intake of iodine in this age group is typically based on the iodine concentration observed in human milk (172, 173, 178, 180). However, this approach is problematic because the optimal BMIC has not been defined. The recommendations are based on small studies and in some cases also on studies conducted at times of iodine deficiency with subsequent low BMIC.
The EFSA (177) and the Scandinavian countries (179) based their recommended intake on the median UIC threshold used to define adequate iodine nutrition (median UIC ≥ 100 µg/L) (176). However, this threshold is likely inappropriate for infant populations (discussed later) and current reference intakes based on UIC are therefore poorly founded.
A more reliable way to define dietary reference values is to estimate the intake level needed to achieve positive metabolic balance. This approach was used by the WHO (175, 176), and the recommended iodine intake was derived from a balance study conducted in a group of full-term infants in Belgium (183, 184). Positive iodine balance was achieved at a minimum iodine intake of 15 µg/kg/day, corresponding to 90 µg/day at age 6 months (102 µg/day using current WHO weight-for-age standards) (185). However, this study was conducted at a time when the population in Belgium was iodine deficient and the estimated daily iodine requirement obtained in this study may be overestimated. We recently conducted a metabolic balance study in 2- to 5 month-old, full-term, iodine-sufficient infants and assessed the iodine intake, excretion, and retention over a range of iodine intakes (181). The data suggest that infants at this age require a minimum daily iodine intake of 11 µg/kg/day or an average of 72 µg/day to achieve positive metabolic balance (see Fig. 2). The infants in our study retained 12% of the daily iodine intake (181), compared to 37% in the Belgian study (183, 184). This difference is not surprising as iodine-deficient infants likely need more dietary iodine to restore low iodine stores. Based on the study results obtained in iodine-sufficient infants, we propose that an AR of 72 µg/day and an RDA of 80 µg/day are required to maintain adequate iodine status during the first 6 months of life (181).
Toddlers and Young Children
The iodine intake recommendations for 7- to 12-month-old infants as well as for children aged 1 to 3 years are typically extrapolated from those defined for younger infants, accounting for higher body weight. The reference intakes range from 50 to 130 µg/day in 7- to 12-month-olds and from 65 to 100 µg/day in 1-to 3-year-old children (172-175, 177-180). USA/Canada, Australia/New Zealand, and China estimated an AR of 65 µg/day (RDA of 90 µg/day) in toddlers (172-174) based on a balance study conducted in malnourished children who were nutritionally rehabilitated (186). The recommended intakes from the other entities are presented as RDA/RNI. To our knowledge, no balance study has been conducted in iodine-sufficient toddlers. Recent data from observational studies in 6- to 24-month-old toddlers show increased thyroid activity in populations with estimated average intakes of less than 50 µg/day and more than 230 µg/day, suggesting a narrow optimal intake range at this age (121).
High Iodine Intake
The tolerable upper level (UL) for iodine in lactating women is the same as for the general adult population, ranging from 600 µg/day to 1100 µg/day (172-174, 177, 179, 180). It is based on the lowest observed adverse effect level of 1700 to 1800 µg/day (172). The Institute of Medicine applied an uncertainty factor of 1.5, bringing the UL to 1100 µg/day (172), whereas the EFSA used an uncertainty factor of 3 and set the UL at 600 µg/day (187). The WHO suggests an iodine intake of 1000 µg/day is safe (175). The American Thyroid Association has taken a more stringent position and strongly recommends against habitual iodine intake exceeding 500 to 1100 µg/day while breastfeeding (25). A harmonized UL for iodine during lactation at 600 µg/day was recently proposed (171), but more data are needed to better define the potential risks of excessive iodine intakes in infants and lactating women.
Data on adverse health consequences of excessive iodine intake during infancy is insufficient, and most bodies defined no UL for iodine during the first year of life. The UL for iodine in 1- to 3-year-old children has been established at 200 µg/day (172).
Dietary Sources of Iodine
Lactating Women and Exclusively Breastfed Infants
The primary dietary source of iodine in the general population is iodized salt (138, 176). Milk and dairy products are also important thanks to cattle feeds fortified with iodine and/or residues from iodine-containing sanitizers used for teat dipping and cleaning of equipment used in dairy production (188). Seafood and saltwater fish contain large amounts of iodine, but their contribution to overall iodine intake is generally limited because of infrequent consumption (189). The native iodine content in fruits, vegetables, and pulses is low (190, 191). Exclusively breastfed infants rely on iodine provided by breast milk. Infants transitioning from breast milk to solid food may get iodine from a range of different dietary sources.
Dietary iodine is primarily present as I– and is almost completely absorbed in the gastrointestinal tract. High bioavailability has been demonstrated in iodized salt, cow’s milk, and infant formula (87%-92%) (181, 192-194). The chemical form or the composition of the diet is not known to affect the bioavailability (195). The bioavailability of iodine in breast milk has not specifically been investigated, but since iodine is mainly present as I– (discussed later) the bioavailability is likely comparable to that of infant formula and cow’s milk (181, 194).
Formula-fed Infants
Infant formula used as a breast milk substitute must contain iodine to mimic breast milk. Most formulas are based on cow’s milk containing iodine. The native iodine content is complemented with added potassium iodide (KI). The Codex standard for infant formula mandates a minimum iodine content of 10 µg/100 kcal and suggests an upper level of 60 µg/100 kcal (196). In the United States, infant formula must contain 5 to 75 µg/100 kcal (197) and in China 10.5 to 58.6 µg/100 kcal (198), whereas European Union (EU) directives mandate a more narrow range of 15 to 29 µg/100 kcal (199). For exclusively formula-fed 2-month-old infants, with a mean energy intake of 571 kcal per day (200), the infant formula standards translate to an iodine intake ranging from low to high, that is, 57 to 340 µg/day (Codex), 30 to 430 µg/day (United States), 60 to 335 µg/day (China), and 85 to 165 µg/day (EU), respectively. The standards are poorly aligned with the physiological iodine requirements and may have both lower and higher content than human milk.
Reports suggest good compliance with the Codex standard (201), although the iodine content in infant formula may vary considerably between products (202-204). The variability may depend not only on the added iodine, but also on varying iodine content in milk and milk powder ingredients (188, 201). Regulations for the iodine content in infant formula should be adapted to the infant requirements and adequate BMIC.
Toddlers
Infants should be introduced to solid foods at age 4 to 6 months (205-207). Home-prepared semi-solid or solid complementary foods, such as cereal-based foods or mashed fruits and vegetables, are typically low in native iodine (190, 191). Pediatric guidelines refrain from giving salt to infants during the first year and recommend that cow’s milk should be avoided as a drink before age 12 months (206). The dietary sources of native iodine in this age group may thus be limited, particularly at low breast milk consumption.
Population-based studies suggest that commercial follow-up formula and complementary foods with added iodine may play a central role in dietary iodine provision to infants (121, 208-212), particularly when the BMIC is low (203). Commercial complementary foods are widely available in many countries, and manufacturers may add iodine on a voluntary basis. The maximum level is regulated; for example, in Europe the iodine content of complementary foods should not exceed 35 µg/100 kcal (213). However, recent data from Germany suggest commercial complementary cereals are rarely fortified with iodine and are generally poor sources of iodine (214).
Follow-up formulas aimed at 6- to 12-month-old infants are typically regulated under the same directives as infant formula. The Codex standard for follow-up formula defines a minimum iodine content of 10 µg/100 kcal and an upper level at 60 µg/100 kcal (215). The Chinese standard for follow-up formula mandates a minimum iodine content of 5.9 µg/100 kcal (216), but a Chinese expert group recently proposed increasing the minimum level to 15 µg/100 kcal and introducing a maximal level at 59 µg/100 kcal (217). In the EU, follow-up formula should contain 15 to 29 µg/100 kcal (199). International guidelines for follow-up formula for toddlers and children aged 12 to 36 months recommend an iodine content of 12 to 36 µg/100 kcal (218). The level of fortification varies considerably between products, and the measured iodine content does not always correspond to the declared iodine content (202–204, 219, 220).
Iodine in Human Milk
Uptake and Molecular Regulation
The lactating mammary gland has a specific ability to accumulate and excrete iodine in breast milk (221-223). Plasma iodine is taken up as I– by the mammary NIS (222, 224, 225), a glycoprotein encoded by the SLC5A5 gene (226, 227). In breast tissue, the NIS is located at the basolateral membrane of alveolar epithelial cells (222, 224, 225). NIS expression is lower in the lactating breast than in the thyroid gland and the modulation by TSH is limited (228). NIS expression in the mammary gland is upregulated at the end of pregnancy and throughout lactation (221, 222, 228). NIS is induced by oxytocin, a hormone secreted in response to suckling, and modulated by estrogen, prolactin, and insulin, which are elevated during the postnatal period (30, 221, 222, 229, 230). Estrogen acts through estrogen-responsive elements present in the NIS promotor (231-234). A minimal threshold of circulating estrogen is likely required for optimal NIS expression during lactation (222, 235). The NIS expression is downregulated during weaning in response to lower oxytoxin levels (222). It is possible that there are additional transporters of iodine into breast milk, such as pendrin. A case report of lactating women with genetic NIS mutations report the presence of iodine in breast milk after high-dose supplementation, although levels are low (236). In the thyroid gland, pendrin is located at the apical membrane of the thyroid follicular cell to transport iodine to the colloid. Pendrin is present in mammary cells, but the exact location has not been determined and its specific role in the lactating mammary gland is unclear (237-239). Although the expression of pendrin is upregulated by prolactin in animal studies (237, 238), inhibition only marginally affects iodine excretion in breast milk in rats (240).
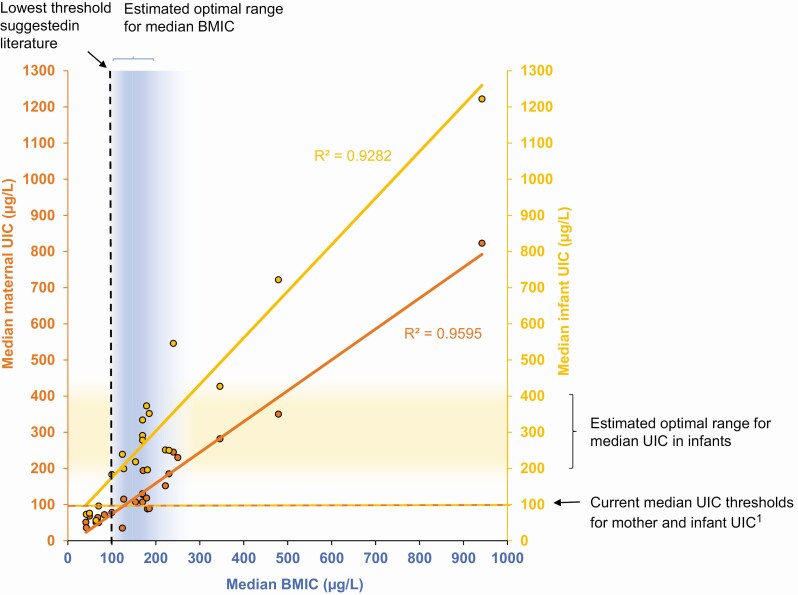
It is uncertain whether iodine transporters in alveolar breast cells are actively regulated in response to low or high maternal serum iodine levels. Data in mice suggest that acute iodine deficiency may induce vasodilation and increase blood flow in the lactating mammary gland, which may enhance the iodine uptake in breast milk (241). This may be a possible adaptive physiological response to acute iodine shortage to economize with the available iodine, but vascular regulation has not been observed in chronic iodine deficiency (241). Exposure to excess iodine may downregulate NIS and pendrin in the mammary gland, but this has been shown only in animal studies using very high doses (242, 243). Human studies report high intraindividual variability in BMIC (244, 245) and wide ranges of BMIC both within and between studies (Fig. 3), suggesting poor physiological control over iodine secretion into breast milk.
Figure 3.
Association between BMIC (blue) and maternal UIC (orange) and/or infant UIC (yellow) from cross-sectional studies conducted in lactating mothers and their breastfed infants. Data points show median BMIC, median maternal UIC, and median infant UIC obtained in mother-infant pairs (sample size, n = 52-739) (112, 145, 146, 166, 167, 203, 204, 211, 246-258). The dashed lines indicate current thresholds for the median BMIC and median UIC above which the iodine intake is considered adequate (21). The shaded areas indicate suggested optimal range for median BMIC and infant UIC based on current evidence presented in this review. The R2 values were calculated based on the published data. 1(21). BMIC, breast milk iodine concentration; UIC, urinary iodine concentration.
Exposure to excessive dietary iodine intake (145, 166, 167, 259) or therapeutic iodine (168) results in high or extremely high BMIC (up to 72 000 µg/L), and active downregulation of NIS under acute or chronic maternal exposure to excess iodine appears unlikely. Mechanistic studies determining the regulatory pathways of the NIS expression in the mammary gland at different maternal iodine intakes are warranted.
Chemical Form
Iodine in breast milk is predominantly present as free I– (80%), protein-bound (eg, iodocaseins), and lipid-bound iodine compounds (238, 260-263). An organic compound at concentrations up to 20% of the total iodine concentration has been observed, but not characterized (260, 264). The chemical form of ingested iodine may influence the iodine uptake in the mammary gland and the concentration in breast milk. Data in rats suggest higher secretion of I– compared to iodine (I2) (240, 265). T4 may also be excreted in breast milk and absorbed by the infant, but the concentration is typically low (266, 267).
Determinants
Maternal iodine intake
The iodine concentration in human milk is primarily determined by the maternal dietary iodine intake (244, 245, 268). Ingested iodine is rapidly excreted in breast milk, and the BMIC peaks within 6 hours after consumption (269–271). The BMIC varies substantially from feeding to feeding (244, 245), reflecting variations in maternal iodine intake and the time passed since the last meal. No circadian rhythm has been indicated (272). Observational studies report a positive association between maternal iodine intake and BMIC (145, 247), although data are not uniform (273). It should be noted that iodine intake is difficult to assess with dietary methods (274). Further, most studies rely on a single spot breast milk sample, which does not account for the high intraindividual variability in iodine intake.
Fractional iodine excretion
Dietary iodine is mainly excreted in urine. In lactating women, iodine is excreted both in urine and breast milk. The iodine concentrations in urine and breast milk are typically associated in observational studies (250, 256, 275), although associations are weak because of the high intraindividual variability of both measures. Maternal fluid intake influences the iodine concentration in urine, but has less impact on BMIC (249). The fractional iodine excretion in breast milk and urine may vary depending on the iodine intake and iodine status (250, 276). We conducted an observational study in iodine-sufficient populations and found that proportionally more iodine is excreted in breast milk (two-thirds) than in urine (one-third) at low iodine intake compared to higher intake (250). Under chronic iodine deficiency, the fractional iodine excretion in breast milk (one-third) is lower than in urine (two-thirds), suggesting there are obligatory renal iodine losses, even at very low iodine intakes (250). The data should be interpreted with caution because they were obtained using spot samples of breast milk and urine. Studies assessing the total daily iodine excretion in breast milk and urine over 24 hours at different iodine intake levels are needed to provide more extensive understanding of the fractional iodine excretion in breast milk and urine.
Within-feeding variability
BMICs vary minimally within each feeding (249, 272, 277). Two studies observed slightly lower BMIC in samples collected at the end of feeding, compared to before the infant starts to suckle (249, 277). However, the difference is negligible and may be explained by a physiologically higher fat content in breast milk at the end of the feed, that is, a lower proportion of the iodine-containing water phase (278). Another study found no difference in BMIC before or after feeding (272). The BMIC is comparable between the right and the left breast (255).
Longitudinal decline over the course of lactation
Iodine is present in colostrum and available for breastfed infants immediately after birth (141, 279). Higher iodine concentrations have been observed in colostrum compared to mature milk (19, 255, 280), possibly making up for the low amounts of milk consumed during the first days of life. It should be noted that disinfectants used during cesarian delivery may contain iodine and unintentionally exaggerate the BMIC in colostrum (281). A modest decline in BMIC over the course of lactation has been reported in longitudinal studies at low (276, 282), adequate (255, 256), and high iodine intakes (19, 280), although not all studies agree (283). Cross-sectional studies indicate a similar trend (19, 247, 254) but results are mixed (203, 258). The decline in BMIC over time post partum may be explained by decreased NIS expression in response to less frequent feedings during weaning, although the absolute decrease is modest and may easily be counteracted by increased iodine intake.
Genetic variants
NIS gene mutations can affect the BMIC, as demonstrated in a case report of Japanese women with the homozygous NIS gene mutation T354P (236). A recent study in South African women identified 27 variants in the SLC5A5 gene coding for NIS (284). The study suggests that genetic variants of the SLC5A5 gene may influence BMIC, although the sample size was small and the study did not control for iodine intake. It is well known that mutations in the SLC5A5 gene affect the biosynthesis of thyroid hormones (30, 79), but more data on the influence of genetic variability for BMIC are needed.
Environmental contaminants and smoking
Environmental contaminants and endocrine-disrupting chemicals may competitively inhibit the iodine uptake by NIS in the thyroid gland and affect thyroid hormone synthesis (285, 286). A similar inhibition of NIS in the mammary gland may decrease the iodine excretion in breast milk.
Perchlorate is the most potent inhibitor of NIS in the thyroid (287), not only because of competitive inhibition but also by changing the stoichiometry of iodine transport (288). It is a common contaminant of foods and drinking water, and lactating women may be exposed through diet (289). Data in lactating women report a positive association between urinary concentrations of perchlorate and iodine (290), whereas the fractional excretion in breast milk is higher for perchlorate than for iodine (245, 268). Although human data provide limited support for an inhibiting effect of perchlorate on BMIC, the studies are small and inconclusive (245, 279, 291-293). Recent data from experimental studies suggest that even low exposure to perchlorate in lactating women may reduce the iodine concentration and increase the perchlorate concentration in breast milk; both effects may be detrimental to the thyroid hormone production in infants (288). However, the effects of perchlorate exposure on infant thyroid function remain controversial (293-296).
Thiocyanate is present in certain foods (eg, cruciferous vegetables, cassava, bamboo shoots) and tobacco smoke (287, 297). Thiocyanate exposure is a strong risk factor for goiter (298, 299), but likely secondary to iodine deficiency and at high thiocyanate intakes and inadequate protein nutrition (300). Urinary thiocyanate concentration positively correlates with UIC in lactating women (290), but a study conducted in cassava-eating mothers observed no correlation with thiocyanate concentration in maternal serum and breast milk (301). Thiocyanate excretion in human milk appears to be limited, indicated by low fractional excretion (268, 300). This is supported by a study conducted in a population in Central Africa with high thiocyanate exposure from cassava (107). Despite high thiocyanate concentration in maternal serum, the thiocyanate concentration in breast milk (107) was comparable to that observed in nonexposed lactating women in Belgium (107). Although the thyroid function of breastfed infants was severely impaired because of iodine deficiency, the thyroid dysfunction was not as severe as observed in older children exposed both to iodine deficiency and dietary thiocyanate (107). The effect of low thiocyanate exposure from smoking on iodine excretion in breast milk is uncertain (279, 302). There is currently no support that low maternal and infant environmental thiocyanate exposures affect infant thyroid function, but studies are small (293). The risk can still not be excluded, and exposure to tobacco smoke in young infants should be avoided (23).
Nitrate exposure typically comes from drinking water (mainly due to applications of inorganic fertilizer) and some leafy and root vegetables (303). Nitrate has been associated with an increased prevalence of subclinical hypothyroidism in children and adults, but data available in lactating women and infants are limited (303, 304). The nitrate concentration in breast milk is typically low, and nitrate in the maternal diet does not appear to increase the exposure of breastfed infants (23, 305).
The clinical relevance of exposure to environmental contaminants on thyroid function in breastfed infants remains uncertain and breastfeeding should not be discouraged because of the presence of pollutants (23).
Biomarkers of Iodine Status
Urinary Iodine Concentration
Iodine adequacy in populations is conventionally assessed in cross-sectional studies by measuring the UIC in spot urine samples and the obtained median UIC is evaluated against recommended thresholds (Table 1) (176).
Table 1.
Biomarkers of population iodine status in infants, toddlers, and lactating women
| Biomarker | Population group | Specimen | Analytical method | Advantages | Disadvantages | Threshold defining iodine sufficiency in populations |
|---|---|---|---|---|---|---|
| UIC | Infants/toddlers lactating women |
Spot urine Infant samples can be collected using urine pads or urine bags |
Spectrophotometric (Sandell-Kolthoff reaction) ICP-MS |
Noninvasive Reflects recent iodine intake (within h) Assess intake from all dietary sources External quality control program in place (307) |
High intraindividual and interindividual variability due to large variation in iodine intake and urine volume (249, 306) Large sample size needed(306) Not useful for individual assessment unless ≥ 10 repeated samples collected (306) UIC in lactating women should be assessed along with BMIC as fractional iodine excretion may vary in urine and breast milk (250) |
Median UIC > 100 µg/L in lactating women and infants recommended by WHO (176), but evidence for this threshold in lactating women, infants, and toddlers is weak. Median UIC >200 μg/L likely more appropriate in infants (see Fig. 3) Criteria indicating deficient, optimal, and excessive iodine intake should be defined |
| BMIC | Lactating women | Spot breast milk | ICP-MS |
Noninvasive Reflects recent iodine intake (within h) |
High intraindividual variability due to large day-to-day variability in iodine intake | Not yet adopted (21) |
| Not reliable for individual assessment |
Observational studies suggest median BMIC of between 100 and 200 µg/L indicate iodine sufficiency(18, 250) (Fig. 3) Criteria indicating optimal iodine nutrition to be defined Assay specific reference ranges: to be defined for most assays (physiological decline during first mo of life must be considered) |
|||||
| Tg | Infants/toddlers, lactating women | Serum or DBS | ELISA | Venopuncture |
Values elevated at deficient and excessive iodine intakes and should be accompanied by UIC Wide interassay variation Affected by degradation when stored under hot and humid conditions (DBS) |
|
| Simple collection by finger or heel prick and storage on filter paper | ||||||
| Small sample volume | ||||||
| Reflects intermediate iodine status (wk to mo) | ||||||
| Neonatal TSH | Neonates 2-5 d after birth | Serum or DBS | Various immunoassays | Collection by heel prick and storage on DBS is simple | Should be taken at least 48 h after birth to avoid TSH surge | Prevalence < 3% of values > 5 mIU/L indicates iodine sufficiency (176), but threshold < 5 mIU/L may be more sensitive to detect mild iodine deficiency |
| International reference range available |
Primarily reflects exposure to iodine deficiency during pregnancy Reflects population risk of moderate-to-severe iodine, but insensitive to mild iodine deficiency May be confounded by use of iodine-containing antiseptics at birth |
|||||
| Measures thyroid function at a particularly susceptible age |
Abbreviations: BMIC, breast milk iodine concentration; DBS, dried blood spots; ELISA, enzyme-linked immunosorbent assay; ICP-MS, inductively coupled plasma mass spectrometry; Tg, thyroglobulin; TSH, thyrotropin; UIC, urinary iodine concentration.
UIC is considered a reliable biomarker of iodine intake because more than 90% of dietary iodine is excreted in urine within 24 hours after consumption (194). However, UIC is subject to considerable intraindividual and interindividual variability, mainly due to large variations in dietary iodine intake and fluid consumption (249, 308). UIC is therefore not a suitable biomarker of individual iodine status, unless 10 or more repeat urine samples are collected (306). In populations, the prevalence of inadequate iodine intake may be derived from UIC using the AR cut-point method (99), after accounting for urine volume (typically by urinary creatinine concentration) and intraindividual variability obtained from a repeat urine sample (309).The overall intake is considered adequate when less than 2% to 3% of the population has intake below the AR (see Fig. 1) (99). UIC is analyzed by the colorimetric Sandell-Kolthoff method or by inductively coupled plasma mass spectrometry (ICP-MS) (176, 310, 311).
Infants
Spot urine samples in infants may be collected using a noninvasive pad technique, by placing designated urine pads directly in infant diapers and extracting urine from the pads (203). Urine collection bags can also be used.
Iodine sufficiency in infants is defined as a population median UIC greater than or equal to 100 µg/L (21, 176). This threshold has been set based on epidemiological data in school-aged children demonstrating an increased prevalence of goiter at a median UIC of less than 100 µg/L (312). However, data associating UIC with functional outcomes in infants are limited and the threshold has been questioned (313). Infants have lower urine volume than older children (182), and the same threshold should not be applied to infants. A UIC of 100 μg/L corresponds to an iodine intake of 57 μg/day (based on a 0.5-L daily urine volume [182] and 87% of dietary iodine excreted into the urine [181]), below the recommended iodine intake in infants (172, 175). Infants are in positive iodine balance at an average iodine intake of 72 µg/day, and this intake level has been proposed to reflect the AR (181). The UIC corresponding to 72 µg/day is approximately 125 µg/L (181). Iodine sufficiency may be defined as less than 3% of infants with intakes below the AR (after adjusting for intraindividual variability), thus the population median UIC will be well above 100 µg/L (see Fig. 2) (181). This is supported by data in overall iodine-sufficient populations where the reported median UIC in infants range from 230 to 350 µg/L (250, 314), more than 2.5 times higher than the WHO threshold of 100 µg/L (see Fig. 3).
The current cutoff for median UIC used to define adequate iodine status in infants is likely too low, disagrees with the dietary reference values, and may underestimate iodine deficiency. No upper UIC threshold has been defined for infants and the median UIC of 300 µg/L or greater reflecting excessive intake in school-age children should not be applied to infants. More data assessing the association between iodine intakes, iodine status, and thyroid function in infants are needed to better define the criteria for optimal iodine nutrition and adequate iodine status in infants and toddlers.
Lactating women
UIC in breastfeeding women is typically lower than in nonlactating women because circulating iodine is excreted both in urine and breast milk (251, 314). Maternal UIC therefore drops after delivery, compared to UIC during pregnancy (66, 315).
The WHO defines adequate iodine nutrition in lactating women as a population median UIC equal to or greater than 100 μg/L (21), but this threshold is uncertain. We plotted median UIC and median BMIC in lactating women obtained in cross-sectional studies and observed a positive correlation between the 2 indicators over a wide range of iodine intakes (Fig. 3). The fractional iodine excretion in urine and breast milk may vary with iodine intake (discussed earlier), particularly at deficient iodine intakes (250). The median UIC in 2 different populations may be comparable around 100 µg/L, whereas the median BMICs differ substantially (see Fig. 3). UIC in lactating women may underestimate the iodine intake in iodine-sufficient populations and overestimate the iodine intake in iodine-deficient populations (18, 250). Assessment of iodine status in lactating women based solely on maternal UIC may thus be misleading. UIC alone is not a reliable biomarker in this group, and BMIC should be measured along with UIC.
Breast Milk Iodine Concentration
BMIC measured in spot breast milk samples obtained in adequately powered observational studies is a reliable biomarker of iodine intakes in populations (see Table 1) (21, 250, 316). BMIC reflects recent iodine intake (within hours) (271). Similar to UIC, it is subject to considerable intraindividual variability due to large variations in iodine intake (244, 245) and is not suitable as biomarker for individual iodine status unless several repeated samples are obtained. Although BMIC varies little over the course of feeding, foremilk samples are preferred for standardization (277). Breast milk is a complex sample matrix and BMIC should preferably be measured with ICP-MS, using alkali digestion for the sample preparation (277, 317, 318). Colorimetric methods for the determination of BMIC are used, but the reliability has been questioned (277). Analytical data should always be reported along with information on external quality control.
A median BMIC of 100 to 200 μg/L obtained in cross-sectional studies has been considered reflecting adequate iodine status in lactating women, but no threshold has been adopted (Fig. 3) (18, 19, 250, 313, 319-321). Median BMIC strongly correlates with median UIC in breastfed infants (see Fig. 3) and may also be used as an indirect population indicator of iodine status in infants exclusively breastfed. The physiological AR estimated for infants may be used to define the optimal BMIC (see Fig. 2). However, more data assessing BMIC along with iodine status and thyroid function in exclusively breastfed infants are warranted to define uniform criteria for adequate iodine status in both groups.
Thyroglobulin Concentration
Tg is a protein made by the follicular cells of the thyroid gland, from which thyroid hormone is produced (32). Blood levels of Tg are low at adequate iodine intake but increase in response to elevated thyroid activity at iodine deficiency and excess iodine intake (322). This U-shaped association between Tg and iodine intake has been demonstrated in school-aged children, adults, pregnant women, and toddlers (119-121, 322). The same association is expected in young infants and lactating women, but remains to be demonstrated in population-based studies. Tg is recommended as a biomarker of population iodine status, as a complement to UIC (176).
Tg can be measured in serum or whole blood extracted from dried blood spots (DBS), using enzyme-linked immunosorbent assay (323, 324). The DBS technique is ideal for sample collection in infants because only a finger or heel prick is required. The reference range for serum Tg in the general population is 3 to 40 µg/L (325), confirmed also for DBS-Tg in iodine-sufficient populations of school children and pregnant women (120, 326). However, Tg reference ranges may vary between assays (325). Tg levels are high after birth, likely in response to the physiological rise in TSH during the first days, and fall over the first few months of life, reaching concentrations typical of adults by about age 6 to 24 months (11, 327-329). Therefore, age-adapted reference ranges should be defined for infants from data obtained in iodine-sufficient populations.
Neonatal Thyrotropin
Newborns are routinely screened for congenital hypothyroidism in many countries using neonatal TSH (≥ 20 mIU/L) at 2 to 5 days after birth (70, 80). Exposure to iodine deficiency during pregnancy and/or after birth may increase the infant’s thyroidal iodine turnover, also resulting in hyperstimulation of TSH and mildly elevated neonatal TSH (330). A prevalence of mildly elevated neonatal TSH (> 5 mIU/L) of more than 3% in a population may indicate iodine deficiency (52, 109, 176, 330). The prevalence increases with severity of iodine deficiency (330) and decreases as iodine status improves (331). Although neonatal TSH may be a good indication of moderate-to-severe iodine deficiency during pregnancy, its value in mild iodine deficiency is uncertain (332, 333). Several studies suggest the current TSH threshold for mildly elevated TSH may be too high to identify populations exposed to mild iodine deficiency (334-337). Still, trends in neonatal TSH over time in mildly deficient populations may reflect changes in iodine status (333, 334, 336, 337). Studies evaluating the association between neonatal TSH and UIC just after birth (338, 339) are difficult to interpret because UIC obtained just after birth is unreliable. Neonatal TSH may provide complementary information of population iodine nutrition during pregnancy, but should not replace UIC or Tg for assessment of iodine status in infants.
Epidemiology of Iodine Nutrition
Iodine deficiency was historically widespread (340, 341), but the implementation of salt iodization has remarkably improved the overall iodine intake in many countries. Epidemiological data on iodine status are conventionally obtained in school-aged children using UIC (176), although an increasing number of countries now also assess iodine nutrition in women of reproductive age and/or pregnant women. The number of countries worldwide classified as overall iodine deficient decreased from 54 to 21 between 2003 and 2021 (16). However, mild iodine deficiency may persist in population groups with high dietary iodine requirements (342).
Lactating Women and Breastfed Infants
Data on iodine status in lactating women and infants are limited and most countries lack data (18, 19). Several reviews present comprehensive historic and current overviews of available cross-sectional studies conducted in lactating women and infants (17-19, 319-321). However, most studies have been conducted in selective locations using small sample sizes and few studies are nationally representative. The majority of studies assessed iodine status using UIC, and the interpretation is often misleading because of the uncertainty of the median UIC threshold (discussed earlier).
Median BMIC varies substantially across populations worldwide, ranging from as low as 20 µg/L to 1000 µg/L (see Fig. 3). The iodine status of exclusively breastfed infants is strongly determined by the maternal BMIC. BMIC and infant UIC are strongly correlated in single studies (145, 251, 254-256, 275, 316) as well as in a compiled analysis (see Fig. 3).
Iodine nutrition is adequate in lactating women and infants in populations with high coverage of iodized salt (210, 314, 343-346). In such settings, the median BMIC typically ranges from 120 to 200 μg/L, corresponding to an average iodine intake in infants of 95 to 150 μg/day and median infant UIC of 200 to 400 μg/L (see Fig. 3). However, incomplete coverage of iodized salt is common in many countries with voluntary salt iodization and may leave lactating women and infants at risk of iodine deficiency, despite adequate iodine status in the general population. In Switzerland, the iodine intake is adequate in school-aged children and borderline sufficient in women of reproductive age and pregnant women (347). However, national studies observed low median BMIC at 50 µg/L and median UIC in infants of 70 to 100 µg/L (203, 338). Suboptimal median BMIC ranging from 70 to 90 µg/L has recently been reported in the Scandinavian countries with incomplete iodine intake from iodized salt (249, 348, 349), as well as in Norway with no salt iodization (247, 350, 351). Subgroups with restrictive maternal diets (eg, vegan diet) (115-117) and infants under prolonged feeding of parenteral or enteral nutrition with low iodine concentrations may be at particular risk of iodine deficiency (118). In populations exposed to moderate iodine deficiency, the median BMIC may be low at 20 to 50 µg/L and median UIC in infants as low as 20 µg/L (18, 112, 145).
Studies conducted in formula-fed infants report adequate median UIC (204, 210, 352, 353), but data are limited. Some studies report lower median UIC in formula-fed infants compared to breastfed infants (210, 352, 354), whereas other studies observe no difference (204, 353). The iodine intake depends on the iodine content in formula milk and the BMIC. A prospective Iranian study of iodine-sufficient women observed higher median UIC in breastfed infants compared to formula-fed infants in mothers receiving supplemental iodine at 300 μg/day, but not at a supplemental dose of 150 μg/day or placebo (143). Infants fed formula with an iodine content at the lower end of the regulated range may be exposed to iodine deficiency.
Excess iodine intake may arise from consumption of over-iodized salt, drinking water high in iodine, milk from animals with high iodine consumption, seaweed, or dietary supplements (98). Topical disinfectants used at delivery, certain iodine-containing pharmaceuticals, or contrast media are other potential sources of exposure (159, 168, 355-357). Mothers with acute or chronic excessive iodine intake excrete large amounts of iodine in breast milk. Individuals with BMIC as high as 1000 to 2000 μg/L is not unusual even in populations with overall optimal BMIC, but typically reflects occasional high iodine intake without concern (319, 320). However, if the BMIC remains high at such levels, this may indicate chronic excessive maternal iodine intake. Observational studies in populations with excess water iodine concentrations report median BMIC ranging from 240 μg/L to 1000 μg/L and median UIC in infants ranging from 430 to 1220 μg/L (see Fig. 3) (145, 146, 166, 167, 358). BMIC up to 8500 μg/L has been reported in women consuming large amounts of seaweed (160, 161, 259). In such situations, measures to reduce the iodine intake should be considered.
Taken together, lactating women and infants likely meet their dietary requirements in settings with well-implemented salt iodization, but may be at risk of iodine deficiency if the coverage of iodized salt is poor or incomplete. Pockets of excess iodine intake have been identified, but the extension is uncertain. Representative and adequately powered epidemiological studies assessing BMIC in mothers along with UIC in infants are needed to assess the extent of low, adequate, and excessive iodine status in lactating women and breastfed infants worldwide.
Toddlers
The iodine intake in weaning infants and toddlers varies considerably worldwide. Cross-sectional studies report a 10-fold difference in median UIC between populations with the lowest and highest iodine intake (Table 2). The iodine intake at this age depends on several factors: 1) maternal iodine intake and subsequent BMIC; 2) amount of breast milk consumed; 3) type of complementary foods consumed; 4) use of iodized salt in complementary foods and its iodine concentration; 5) consumption of cows’ milk, follow-up infant formulas, or complementary foods fortified with iodine.
Table 2.
Iodine status and dietary iodine sources in toddlers worldwide
| Study site | Age, mo | UIC, µg/L | Tg (µg/L) | Iodized salta | Salt iodine concentration, mg/kg | Breastfed, % | BMIC, µg/L | Main dietary iodine sources | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | No. | Value | No. | ||||||||
| Amizmiz, Morocco | 6-24 | 48 (31-79)b | 220 | 62.0 (43.0-84.2)c | 228 | Not widely available | NA | 98 | 26 (18-43) (112) | Breast milk | (121) |
| Soma, Gambia | 6-24 | 98 (61-178) | 93 | 52.3 (41.6-71.7)c | 92 | Not widely available | NA | 100 | 39 (30, 57) (359) | Breast milk | (121) |
| National, Switzerland | 6-12 | 98 (54-160) | 507 | NA | NA | Voluntary (20 mg/kg) | 19.8 (15.1-33.0) | 6/12 mo 57/18 | 49 (35-67) | Breast milk, infant formula | (203) |
| National, Norway | 18 | 129 (81-190) | 416 | NA | NA | Not widely available | NA | 10 | 68 (45-98) (247) | Cow’s milk, dairy products and infant formula | (360) |
| Dipolog City, Philippines | 6-24 | 152 (92-266) | 105 | 30.6 (19.3-41.6)c | 53 | Mandatory, but not widely available at study site (30-70 mg/kg) | NA | NA | NA | Breast milk, infant formula | (121) |
| Linfen, China | 7-24 | 205 (182-235) | 368 | 26.3 (12.3-44.4)c | 173 | Mandatory (25 mg/kg) | 25.2 (23.3-28.0) | 39 | 176 (116, 251) | Breast milk, infant formula, salt added to complementary foods | (314) |
| Henan Province, China | < 24 | 218 | 13 598 | NA | NA | Mandatory (30 mg/kg) | NA | NA | NA | Breast milk, infant formula, complementary foods | (210) |
| Zagreb, Croatia | 7-24 | 249 (169-329) | 46 | 24.9 (10.4-37.6)c | 40 | Mandatory (25 mg/kg) | 23.8 (22.1-26.0) | 74 | 125 (91-184) | Breast milk, infant formula, salt added to complementary foods | (314) |
| Dandé Health District, Burkina Faso | 9-18 | 222 310d | 22 380 | 27.2-33.2 26.1e | 237 83 | Mandatory (30 mg/kg) | 37 (5-86)f | 100 | NA | Breast milk, some salt added to complementary foods (18 mo) | (361) |
| Tuguegarao, Philippines | 7-24 | 353 (330-397) | 376 | NA | NA | Mandatory (30-70 mg/kg) | 26.0 (14.6-36.6) | 65 | 189 (137-260) | Breast milk, infant formula, salt added to complementary foods | (314) |
| Eastern Nepal, Nepal | 6-24 | 407 (312-491) | 630 | 21.7 (20.4-22.9)g,h | 563 | Mandatory (50 mg/kg) | 89 (70-149) | Majority | NA | Breast milk, salt added to complementary foods, fortified complementary food products | (165) |
| Saharawi refugee camps, Algeria | 31i | 458 (275-1026) | 289 | 38.4 (10.7-158.0)j,k | 289 | NA | NA | 13 | 479 (330-702) | Breast milk, drinking water | (166) |
| Kinondoni, Tanzania | 6-24 | 528 (255-952) | 240 | 52.2 (35.7-77.4)c | 236 | Mandatory (20-80 mg/kg) | 44 (35-53) | 99 | NA | Breast milk, salt added to complementary foods, drinking water | (167) |
| Kibwezi, Kenya | 6-24 | 602 (348-1205) | 250 | 56.1 (43.4-74.5)c | 312 | Mandatory (30-50 mg/kg) | 43 (32-54) | 99 | 240 (173-347) | Breast milk, salt added to complementary foods, drinking water | (167) |
Abbreviations: BMIC, breast milk iodine concentration; IQR, interquartile range; NA, not available; UIC, urinary iodine concentration.
a Legislation and level of fortification at the time of cited studies.
b Median (IQR, all such values).
c Dried blood spot, enzyme-linked immunosorbent assay (324).
d Geometric mean.
e Plasma, Immulite 1000 Immunoassay system (Siemens).
f Mean (range).
g Geometric mean (CI).
h Immunoassay (Diametra).
i Median age (IQR, 25-35 months).
j Median (range).
k Serum, electrochemiluminescence immunoassay module E170 (Roche Diagnostics).
Weaning infants typically have adequate iodine intake in populations where iodized salt is readily available and breastfeeding rates are high (see Table 2). Iodized salt covers toddlers indirectly through breast milk and directly through consumption of salt-containing complementary foods, despite recommendations to limit salt during the first year of life (206). Studies conducted in populations covered by mandatory salt iodization report median UIC in 7- to 24-month-old infants ranging from 200 to 350 µg/L and normal Tg concentrations (314, 361). A large proportion of the toddlers received breastmilk (39%-73%) and the BMIC ranged from 125 to 190 µg/L (314). Most infants received semi-solid foods (> 90%) and iodized salt was added to home-prepared complementary foods (57%-93%).
Toddlers may be at risk of iodine deficiency in settings where 1) the coverage of iodized salt is poor and/or mothers’ BMIC is low; 2) breast milk and/or cow’s milk and infant formula consumption is low; and 3) toddlers receive homemade complementary foods with low native iodine content and no added salt. Studies conducted in Gambia and Morocco at a time with poor availability of iodized salt, low maternal BMIC, and no widespread consumption of cow’s milk or infant formula report low median UIC ranging from 50 to 100 µg/L along with elevated Tg concentrations (121). A national study in Switzerland reported low median BMIC at 50 µg/L and a median UIC of 100 µg/L both in 6- and 12-month-old infants (203). Only 57% of infants were breastfed at age 6 months and 18% at age 12 months. Compliance with pediatric recommendations to limit salt intake during the first year of life was high (203, 206). In-depth analysis of dietary patterns suggests that fortified follow-up infant formula played an important role in providing dietary iodine to Swiss toddlers (203). About 60% of all infants received infant formula fortified with iodine, and their median UIC was higher than those not receiving infant formula (109 µg/L vs 73 µg/L). The same pattern has been observed in other western populations, such as New Zealand and the United States (212, 362).
Consumption of cow’s milk and dairy products may be encouraged as good iodine sources after age 12 months (206). A cross-sectional national study in Norwegian toddlers demonstrates the importance of cow’s milk and dairy products for iodine intake in weaning infants (360). Iodized salt is not widely available in Norway and the BMIC is low (247). However, cow’s milk and dairy products contain high levels of iodine and are widely consumed, and follow-up infant formulas are fortified with iodine. The median UIC in toddlers was 129 μg/L and the investigators estimated that cow’s milk contributed to 70% of the total iodine intake. Toddlers fed a vegan diet are at risk for iodine deficiency (117, 363, 364), but more data are needed (365).
In some settings, toddlers may be exposed to iodine intake exceeding the recommended upper intake level. A study of toddlers in Eastern Nepal reports a median UIC around 400 µg/L, along with elevated Tg concentrations (165). Household salt in this study was over-iodized (89 mg/kg), likely providing high iodine intake from breast milk in combination with salt added to complementary foods. Another study conducted in 2 local study sites in Tanzania and Kenya, with documented excessive iodine intake in the general population, observed median UIC in toddlers ranging from 530 to 600 μg/L and elevated median Tg concentrations (121, 167). In Kenya, close to all old infants aged 7 to 24 months received breastmilk, and the median BMIC was 240 μg/L. The median iodine level in household salt was 43 mg/kg and 95% of toddlers received food containing salt. Some drinking water sources contained excessive levels of iodine and may have contributed to the high iodine intake. Drinking water has also been reported to cause chronic high iodine intake in toddlers and young children in other parts of the world (166).
To conclude, iodine nutrition in toddlers may be complex as the dietary intake depends on maternal iodine status, breastfeeding rates, and weaning practices which vary considerably between populations. Data are limited, but the few available studies suggest that both deficient and high iodine intake may be more common than previously assumed. Dietary assessment of weaning practices and mapping of potential dietary iodine sources may be a valuable complement to UIC when evaluating iodine nutrition in toddlers, particularly if intervention strategies targeting this group are in place or are being planned.
Intervention Strategies to Prevent Iodine Deficiency
Salt Iodization
Iodine deficiency is primarily prevented and corrected through salt iodization, a highly cost-effective public health strategy now implemented in most countries worldwide (176, 366). Some countries apply universal salt iodization, that is, mandatory iodization of all food-grade salt used for human and animal consumption (176). In other countries, salt iodization is voluntary or mandated for specific foods, such as bread (367). The recommended iodine concentration in salt is 20 to 40 mg/kg (176). Scale-up of salt iodization programs around the world over the past decades has remarkably improved iodine nutrition in the general population (15, 16).
Salt iodization is the primary strategy to prevent iodine deficiency also in lactating women and infants (21, 176). Reports of low BMIC in many Western countries raised concern that salt iodization may not provide sufficient daily iodine to meet the high physiological requirements during lactation and infancy. However, in populations with well-implemented salt iodization within the recommended level of fortification, the median BMIC ranges from 120 to 200 μg/L and median UIC in infants ranges from 200 to 400 μg/L, suggesting iodine sufficiency (see Fig. 3) (143, 210, 250, 314, 343-346). Introduction of mandatory use of iodized salt in bread in Australia and New Zealand improved the median BMIC from 22 to 84 μg/L (282, 362, 368, 369) to 127 to 187 μg/L after implementation of the new policy (251, 369, 370). The effectiveness of salt iodization for optimal nutrition in lactating women and breastfed infants is now well documented. However, complete coverage of iodized salt remains challenging, particularly in industrialized countries where salt fortification is voluntary and iodized salt is only partially used in the production of processed foods. Incomplete or poorly implemented salt iodization programs may leave lactating women, breastfed infants, and toddlers at risk for iodine deficiency.
Iodine Supplementation to Lactating Women
Daily iodine supplementation
Daily iodine supplements are typically provided in the form of KI or iodate, as tablets or drops. Both forms are highly bioavailable (240, 265). Supplemental iodine appears rapidly in breast milk and the BMIC peaks at 6 hours after administration (271). Kelp and other forms of seaweed do not provide consistent delivery of daily iodine and should be avoided (25).
In iodine-deficient populations with poor coverage of iodized salt, the WHO recommends daily iodine supplementation so that the total intake is 250 µg/day during pregnancy and lactation (Table 3) (21, 371). Prenatal and postnatal iodine supplementation (150 µg/day) is also recommended by several national authorities and medical societies, often regardless of the coverage of iodized salt (22-25, 372). However, the consumption of iodine-containing dietary supplements in lactating women is generally low (26, 203, 247, 373-375).
Table 3.
Public health strategies to prevent and control iodine deficiency in lactating women, infants and toddlersa
| Iodine status in the general populationb | Status of salt iodization in population | Recommended strategies | ||
|---|---|---|---|---|
| Lactating women | Infants (age 0-6 mo) | Toddlers (age 7-24 mo) | ||
| Adequate iodine nutrition (≥ 100 µg/L) | Effective and sustained salt iodization | • Maintain salt iodization | • Exclusive breastfeeding | • Maintenance of breastfeedingc |
| • Formula-fed infants should receive formula milk fortified with iodine | • Complementary foods and/or follow-up formula fortified with iodine | |||
| • Iodized salt in complementary foods from age > 12 mo | ||||
| Mild-to-moderate iodine deficiency (20-99 µg/L) | Incomplete coverage of iodized salt | • Improve coverage of iodized salt | • Exclusive breastfeeding | • Maintenance of breastfeedingc |
| • Daily iodine supplementation | • Formula fed infants should receive formula milk fortified with iodine | • Complementary foods and/or follow-up formula, MNP/LNS fortified with iodine | ||
| • Iodized salt in complementary foods from age > 12 mo | ||||
| Severe iodine deficiency (< 20 µg/L) | No or poor coverage of iodized salt | • Introduce salt iodization | • Exclusive breastfeeding | • Maintenance of breastfeedingc |
| • Daily iodine supplementation or single annual oral dose of 400-mg iodized oil | • Formula fed infants should receive formula milk fortified with iodine | • Complementary foods and/or follow-up formula, MNP/LNS fortified with iodine | ||
Abbreviations: LNS, lipid-based nutrient supplements; MNP, micronutrient powders; UIC, urinary iodine concentration.
a Adapted with permission from (21):
b Defined as median UIC in 6- to 12-year-old children (176). Median UIC thresholds for general population/women of reproductive age uncertain (309).
c Continue frequent on-demand breastfeeding until age 2 years or older.
Iodine supplementation during pregnancy is well supported in severe iodine deficiency, but the evidence is insufficient in populations with mild-to-moderate deficiency (14). Antenatal iodine supplementation may have benefits to the infant for the first few days after birth (339, 359, 376), but the impact is likely transitory because BMIC depends on the current maternal iodine intake. Maternal iodine stores may be rapidly depleted during lactation at low dietary iodine intake, if the daily supply is not maintained. Recent data in mild-to-moderate iodine-deficient populations demonstrate that prenatal iodine supplementation that stops at delivery does not ensure adequate maternal iodine status post partum (66, 359).
Observational studies in lactating women show higher BMIC in iodine supplement users compared to nonusers (29, 245, 370). Prospective studies examining the effect of postpartum iodine supplementation are limited (377). To our knowledge 4 trials have been conducted in iodine-deficient populations (282, 376, 378, 379). Mulrine et al (282) conducted a small RCT of iodine supplementation providing 75 μg/day, 150 μg/day, or placebo over 6 months to mildly to moderately deficient lactating women in New Zealand. BMIC remained low throughout the study, ranging from 24 to 70 µg/L, and no overall time-by-treatment effect was reported. Compared to placebo, BMIC was modestly higher in women consuming 75 µg/day and 150 µg/day, but no dose response was observed. Infant UIC remained low in all groups. Pedersen et al (376) evaluated 200 µg daily iodine vs control from weeks 17 to 18 of pregnancy until 12 months after delivery in 54 women. Five days after delivery, BMIC and infant UIC were higher in mothers receiving iodine, although they were still low in both groups. Supplemented mothers maintained higher UIC and Tg during the postpartum period compared to nonsupplemented mothers, whereas TSH, T4, T3, and free T4 levels were unaltered. Another small prospective trial assessed iodine supplementation with daily doses of 50 µg or 200 µg during pregnancy, continuing to 6 months after delivery in mildly iodine-deficient women in Italy (378). The urinary iodine excretion in pregnant women at enrollment was 74 µg/g creatinine and increased at 6 months post partum to 123 µg/g creatinine in the group receiving 50 µg/day and to 156 µg/g creatinine in the group receiving 200 µg/day. However, the study had no control group and did not measure BMIC. No group differences were observed for maternal thyroid volume, serum concentrations of Tg, TSH, free T4, or free T3. Another larger trial evaluated daily consumption of lipid-based nutrient supplements (LNS) containing 250 µg iodine or no iodine during pregnancy and lactation in moderately iodine-deficient women in Bangladesh (379). The geometric mean UIC in pregnant women at enrollment (13 weeks’ gestation) was low at 50 μg/L and declined further in both groups at 36 weeks’ gestation and 6 months post partum, with no difference between the groups. The efficacy of iodine supplementation in the postpartum period is difficult to evaluate in this study because BMIC and infant UIC were not measured.
Two small studies evaluated iodine supplementation to lactating women with adequate iodine intakes. The first study compared iodine supplementation (225 μg/day) to household distribution of iodized salt (30-40 mg/kg of salt) over 6 months in lactating women in rural southern Ethiopia (252). The median BMIC at baseline was 150 µg/L. BMIC decreased in both groups over 6 months and infant UIC remained stable, but as expected no group differences were reported in BMIC, maternal UIC, or infant UIC. Most of the infants had normal T4 and all had TSH within the normal range (380). A small RCT evaluated iodine supplementation of 150 μg/day, 300 μg/day, or placebo given to lactating women over 12 months in Iran, where iodized salt is widely available (143). The authors report an overall effect of both doses on BMIC and maternal UIC and a significant effect of infant UIC of the highest dose. However, the results are difficult to interpret because the groups differed at baseline. The iodine status in the placebo group remained iodine sufficient over the 12-month study.
In summary, although dietary iodine supplementation during lactation likely improves BMIC in iodine-deficient populations, the evidence from RCTs is weak and the optimal dose remains uncertain. The few existing trials of daily iodine supplementation observed limited efficacy, but the studies were poorly powered (143, 252, 282, 376, 378, 381), had no control group (252, 378), did not measure BMIC (378, 379), or were conducted in iodine-sufficient mothers (143, 252). Only one study measured thyroid function in infants (380) and none of the controlled studies evaluated long-term health gains of iodine repletion in infants. Thus, guidelines for postnatal iodine supplementation in iodine-deficient populations await the availability of well-designed efficacy studies that measure BMIC, infant iodine status, thyroid function, and functional outcomes of the infant. The data provide no support for targeted iodine supplementation to lactating mothers in settings where women are covered by a well-working salt iodization program.
Iodized oil
Iodized oil is a slowly released iodine preparation that can be used as an alternative approach to daily iodine supplementation in severe iodine deficiency (see Table 3) (21, 371). It is typically administered on an annual basis as a large oral dose (400 mg to lactating women), but can also be injected intramuscularly (21). Supplementation with oral iodized oil is recommended in areas where iodized salt and complementary food fortified with iodine are unavailable and daily iodine supplementation is not feasible (21, 25). Several studies describe the effectiveness of oral iodized oil in populations with moderate-to-severe iodine deficiency, also in children younger than 24 months (132, 133, 382, 383). The efficacy of oral iodized oil given to lactating women or infants was recently evaluated in a population of moderate-to-severe iodine deficiency in Morocco (112). Iodized oil was provided soon after delivery to mothers (400 mg, placebo to infants) or infants (100 mg, placebo to mothers). The study showed that administration of iodized oil is more beneficial when given to the mother than when given directly to infants. However, although the BMIC of the supplemented mothers was significantly higher than that of nonsupplemented mothers at 3, 6, and 9 months, the improvement in BMIC was modest. The BMIC and infant UIC observed after iodized oil supplementation at 3, 6, and 9 months were 2 to 3 times lower than levels more recently reported in observational studies in iodine-sufficient populations (250, 314). Indeed, contrary to the previous interpretation of the study results (25, 112, 384), iodized oil failed to achieve longlasting optimal iodine nutrition in mothers and infants. Nevertheless, maternal iodine supplementation improved infant thyroid function, and the prevalence of hypothyroidism and hypothyroxinemia declined. A substudy showed that a large amount of iodine was excreted in the urine within the first few days after administration. However, a proportion of administered iodine was likely taken up by maternal fat deposits and metabolized back into the circulation at a slow rate and secreted into breast milk. The amount of circulating maternal iodine appears too low to substantially improve BMIC, but may still be sufficient to ensure euthyroidism in breastfed infants.
Dietary Interventions for Infants, Weaning Infants, and Toddlers
During the first few months of life, infants should receive iodine through breast milk or infant formula. Dietary supplements aimed at this age group typically do not contain iodine (385). The WHO recommends infants aged 7 to 24 months in populations with poor coverage of iodized salt be given a daily dose of 90-µg iodine through fortified complementary foods (see Table 3) (21, 371). Daily iodine for this age group can be administered as crushable tables or iodine solutions, fortified infant formula or toddler milks, micronutrient powders (MNPs), or LNS (386). In the United States, consumption of iodine-containing dietary supplements in 12- to 23-month-old infants is infrequent (< 5%) and the estimated iodine intake in supplement users is only 14 µg/day (385).
Fortified follow-up infant formula and toddler milks are widely consumed in many parts of the world, although the iodine content varies substantially between countries and products (discussed earlier). In subgroups with low breast milk and/or cow’s milk consumption, fortified commercial infant formulas or complementary foods may be particularly important (209, 387). In resource-poor settings where weaning infants may be at particular risk of iodine deficiency, iodine-fortified in-home fortification products to be sprinkled onto home-prepared complementary foods may be beneficial (21). MNPs and LNS are available in more than 50 countries worldwide (388). They typically contain a blend of micronutrients and are added to semi-solid foods just before consumption. The iodine content in MNPs and LNS products for toddlers is typically 90 µg per daily portion (175), but the iodine content can be adapted to the target population (389).
Consumption of iodine-fortified formula positively correlates with iodine status in cross-sectional studies (203, 212, 362). Four prospective studies evaluated the impact of daily iodine on iodine status in toddlers both with low and adequate iodine intake (361, 390-392). A small study in iodine-deficient toddlers aged 12 to 20 months in New Zealand evaluated iodine-fortified toddler milk providing 90 µg/day compared to nonfortified milk (24 µg native iodine) (390). The median UIC was 50 µg/L at baseline and increased to 91 µg/L in the iodine group after 20 weeks. In this population with overall low dietary iodine intake, the additional 90 µg/day was insufficient to achieve adequate iodine status. Another small observational prospective study over 44 weeks administered 90 µg/day as an iodine solution to mildly deficient infants aged 6 to 36 months in Belgium (391). The median UIC in infants at baseline was 101 µg/L and increased to 200 µg/L after 30 weeks and remained at this level thereafter. However, this study had no control group, limiting its interpretation. Another small study in 6- to 12-month-old Indonesian infants provided 60-µg iodine daily as a food-like crushable tablet over 6 months and compared it with weekly administration of 120-µg iodine, daily iron with no iodine, and placebo (392). The median UIC increased from 137 µg/L to 237 µg/L in the group receiving daily iodine. However, the UIC increased in all groups and at the end of the study, the change in UIC from baseline was comparable between the 4 groups. The study is limited by the small sample size, high breastfeeding rates, and the fact that one-third of the infants received infant formula containing iodine. Another study in 9-month-old infants in Burkina Faso evaluated the effect of 90 µg/day supplied in LNS, compared to no intervention (361). The median UIC was 222 µg/L at baseline, suggesting adequate iodine nutrition. After 9 months, the median UIC increased to 356 µg/L in the intervention group, but at age 18 months, no differences in UIC, TSH, T4, or Tg were observed between the 2 groups. Adequately iodized salt was available in 95% of the households and all infants were partly breastfed at the beginning of the study, suggesting iodized salt likely provided sufficient iodine via breast milk.
Taken together, in settings with adequate coverage of iodized salt and high breastfeeding rates, infants do not need additional iodine. Weaning infants and toddlers receiving homemade complementary foods with low native iodine content and no added salt or in settings with poor coverage of iodized salt likely benefit from consumption of complementary food products fortified with iodine or supplemental iodine. However, the evidence is obtained from small studies and the optimal daily dose of supplemental iodine in iodine-deficient populations as well as the timing of introduction remain uncertain. Well-designed and adequately powered controlled studies in iodine-deficient toddlers are warranted. Before including iodine in-home fortification products, the iodine status in the target group should be assessed and the iodine content should be adapted to avoid excess iodine intake.
Preterm Infants
The thyroid function in preterm infants is typically immature and transient hypothyroxinemia is common, particularly in very premature infants (< 31 weeks) (11, 48, 393). The etiology of hypothyroxinemia is multifactorial, including immaturity of the HPT axis, but may in part be due to low thyroidal iodine stores and iodine deficiency (393-396). Hypothyroxinemia in preterm infants may comprise neurodevelopment, although long-term outcomes of thyroid dysfunction remain unclear (393, 396). Preterm infants with consistent mild thyroid dysfunction (elevated TSH) have also been reported to score less on neurodevelopmental tests at age 2 years (397). A small study of early supplementation with levothyroxine in infants born at less than 28 weeks’ gestation reported improved long-term neurodevelopment (398), but more data are needed. Preterm infants are also vulnerable to excess iodine intake, which may increase the risk of hypothyroidism (11, 399), particularly if daily intake exceeds 100 µg/kg/day (259, 400). Topical exposure to iodine may also cause hypothyroidism and should be avoided (124, 401).
The dietary iodine requirement is likely higher in preterm infants than in term infants, but the absolute physiological need is uncertain. A balance study conducted in 1-month-old preterm infants estimated the average dietary requirement to be 30 to 40 μg/kg/day (183), 3 times higher than estimated for term 2- to 5-month-old infants (10.6 μg/kg/day) (181). Two further balance studies in preterm infants suggest iodine intake lower than 30 to 40 μg/kg/day may meet the daily requirement, although the iodine balance was negative at intake of less than 10 μg/kg/day (394, 395, 402). It should be noted that the capacity to retain dietary iodine is limited in very premature infants (< 31 weeks) during the first week after birth, although iodine retention increases with age and is approximately 80% at a postmenstrual age of 40 weeks (394, 402), comparable to the iodine retention observed in term infants (181).
Iodine intake recommendations for preterm infants currently vary by mode of feeding. Human milk is the best form of nutrition for preterm infants, but because the volume consumed may be low, breast-milk fortifiers may be added to improve nutrient density (403). However, despite low iodine concentration in breast milk in many populations, iodine is not added to breast-milk fortifiers (403, 404). The recommended parenteral intake of iodine is 1 to 10 μg/kg/day (405, 406), likely far below the requirements of preterm neonates. The recommended iodine intake for enteral nutrition is 11 to 55 μg/kg/day (407). The current iodine content set for parenteral nutrition is based on the assumption that preterm infants will absorb iodine from topical iodine-containing antiseptic solutions, previously commonly used during delivery. However, because the majority of antiseptics used in the clinical setting today contain no iodine, the recommended low iodine level is no longer justified. To comply with current guidelines, commercial parenteral nutrition products for preterm infants provide almost no iodine (395, 408), and preterm enteral formula milk typically contains iodine levels at the lower end of the recommended range (408). All feeding modes may thus potentially expose preterm infants to iodine deficiency, though the risk is highest for parenterally fed infants.
Two RCTs evaluated the effects of parenteral or enteral iodine of at least 30 µg/kg/day in preterm infants (409, 410). The first study conducted in a small group of infants born before 33 weeks investigated the consumption of an enteral preterm infant formula providing iodine at 40 to 50 μg/kg/day vs 12 to 16 μg/kg/day until expected term at 40 weeks (409). No effects on thyroid function or growth were observed (128, 409). The second study was conducted in a large group of preterms born before week 31 and evaluated the effect of iodine at 30 to 40 μg/kg/day vs placebo provided as parenteral or enteral nutrition until the equivalent of 34 weeks’ gestation (410, 411). The investigators found no group differences in Tg and T4 during the feeding period, apart from slightly higher TSH in the iodine group. At age 2 years, there were no differences in neurodevelopment and mortality between the 2 groups. The results were unexpected and the investigators hypothesize that the iodine requirement may be higher than 30 µg/kg/day (410). However, the data are inconclusive and a lower iodine requirement is also possible.
The intake recommendations for preterm infants should be based on the average dietary iodine requirements and harmonized between all feeding modes. However, the optimal intake range in preterm infants remains uncertain and carefully conducted balance studies in preterm infants are warranted to better define the daily needs and understand the etiology of hypothyroxinemia in preterm infants.
Conclusions
Adequate iodine nutrition is essential for optimal thyroid function in lactating women, infants and toddlers. Moderate-to-severe iodine deficiency and iodine excess increase the risk for abnormal thyroid function, whereas adequate thyroid hormone production is typically maintained under mild iodine malnutrition. Elevated blood Tg concentration is the first measurable sign of mild iodine deficiency or excess. This is a physiological response indicating biological adaptation to prevent low hormone levels and functional consequences. However, data in infants and young children are limited and the consequences of mild-to-moderate iodine deficiency on infant development are uncertain.
Iodine status is best assessed by BMIC in lactating women and UIC in infants and toddlers. Adequate iodine intake is indicated by a population median BMIC between 100 to 200 µg/L and median UIC >200 µg/L in infants and toddlers. Accumulated evidence suggests a linear association between BMIC and UIC in breastfed infants over a wide range of iodine intake levels. Representative epidemiological studies in these groups are limited, but available data suggest large variations of iodine status in lactating women and infants during the first 2 years of life. At particular risk for iodine deficiency are 1) breastfed and weaning infants in countries with no or voluntary salt iodization at low coverage or fed by mothers on a restrictive diet; 2) toddlers receiving homemade complementary foods with low native iodine content and no added iodized salt; and 3) preterm and term infants under prolonged feeding of parenteral or enteral nutrition. Nationally representative data on iodine status in infants and toddlers are warranted.
Salt iodization is the primary public health strategy to prevent iodine deficiency. Observational studies confirm that iodized salt provides enough dietary iodine to meet the high iodine requirements of lactating women, breastfed infants, and toddlers if all salt consumed is adequately iodized. However, if the coverage of iodized salt is poor and the dietary iodine intake is low, iodine supplementation in lactating women and targeted dietary interventions for toddlers may be required.
Integrated research across disciplines is needed to strengthen the evidence for public health recommendations and clinical guidelines on iodine nutrition in lactating women, infants and toddlers. The criteria for optimal iodine nutrition must urgently be established for accurate interpretation of the growing body of data. Dietary reference values should be defined based on physiological requirements and be considered when defining thresholds for iodine status biomarkers. Future well-designed and adequately powered studies assessing the association between iodine status and thyroid function in pediatric populations are needed to assess vulnerability to iodine malnutrition at both ends of the intake range. The impact of iodine-deficiency repletion on infant thyroid function, infant growth, and neurocognitive development during the first 2 years of life should be addressed by studies conducted in regions of mild-to-moderate iodine deficiency, applying a holistic approach including mothers and infants.
Acknowledgments
Author Contributions: M.A. researched the data for the review and wrote the article. C.B. reviewed and edited the manuscript before submission.
Glossary
Abbreviations
- AI
adequate intake
- AR
average requirement
- BMIC
breast milk iodine concentration
- DBS
dried blood spot
- EFSA
European Food Safety Authority
- EU
European Union
- HPT
hypothalamic-pituitary-thyroid
- ICP-MS
inductively coupled plasma mass spectrometry
- I–
iodide
- KI
potassium iodide
- LNS
lipid-based nutrient supplements
- MNP
micronutrient powders
- Na+
sodium
- NIS
sodium iodide symporter
- RCT
randomized controlled trial
- RDA
recommended daily allowance
- RNI
recommended nutrient intake
- Tg
thyroglobulin
- TPO
thyroid peroxidase
- TSH
thyrotropin
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- UIC
urinary iodine concentration
- UL
upper intake level
- WHO
World Health Organization
Contributor Information
Maria Andersson, Nutrition Research Unit, University Children’s Hospital Zurich, CH-8032 Zürich, Switzerland.
Christian P Braegger, Nutrition Research Unit, University Children’s Hospital Zurich, CH-8032 Zürich, Switzerland.
Additional Information
Disclosures: M.A. serves as a board member of the nongovernmental organization Iodine Global Network (Ottawa, Ontario, Canada). C.B. has nothing to disclose.
References
- 1. Schwarzenberg SJ, Georgieff MK; Committee on Nutrition . Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. 2018;141(2):e20173716. [DOI] [PubMed] [Google Scholar]
- 2. Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397(10282):1388-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Resolution WHA71.9. Infant and Young Child Feeding. Seventy-First World Health Assembly, 21-26 May 2018. World Health Organization; 2018. [Google Scholar]
- 4. Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr. 2016;104(3):646-662. [DOI] [PubMed] [Google Scholar]
- 5. Dror DK, Allen LH. Overview of nutrients in human milk. Adv Nutr. 2018;9(Suppl 1):278S-294S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu X, Jackson RT, Khan SA, Ahuja J, Pehrsson PR. Human milk nutrient composition in the United States: current knowledge, challenges, and research needs. Curr Dev Nutr. 2018;2(7):nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen LH, Donohue JA, Dror DK. Limitations of the evidence base used to set recommended nutrient intakes for infants and lactating women. Adv Nutr. 2018;9(Suppl 1):295S-312S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasmussen KM. At long last: new information on the association between maternal dietary intake, the composition of human milk, and its nutrient adequacy for infants. Am J Clin Nutr. 2019;110(2):269-270. [DOI] [PubMed] [Google Scholar]
- 9. Casavale KO, Ahuja JKC, Wu X, et al. NIH workshop on human milk composition: summary and visions. Am J Clin Nutr. 2019;110(3):769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):108-117. [DOI] [PubMed] [Google Scholar]
- 11. Segni M. Disorders of the thyroid gland in infancy, childhood and adolescence. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Thyroid disease manager. Endotext. MDText.com; 2017. Accessed June 7, 2021. https://www.endotext.org/section/thyroiddiseasemanager [Google Scholar]
- 12. Chittimoju SB, Pearce EN. Iodine deficiency and supplementation in pregnancy. Clin Obstet Gynecol. 2019;62(2):330-338. [DOI] [PubMed] [Google Scholar]
- 13. Bath SC. The effect of iodine deficiency during pregnancy on child development. Proc Nutr Soc. 2019;78(2):150-160. [DOI] [PubMed] [Google Scholar]
- 14. Dineva M, Fishpool H, Rayman MP, Mendis J, Bath SC. Systematic review and meta-analysis of the effects of iodine supplementation on thyroid function and child neurodevelopment in mildly-to-moderately iodine-deficient pregnant women. Am J Clin Nutr. 2020;112(2):389-412. [DOI] [PubMed] [Google Scholar]
- 15. Gorstein JL, Bagriansky J, Pearce EN, Kupka R, Zimmermann MB. Estimating the health and economic benefits of universal salt iodization programs to correct iodine deficiency disorders. Thyroid. 2020;30(12):1802-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmermann MB, Andersson M.Global endocrinology: global perspectives in endocrinology: coverage of iodized salt programs and iodine status in 2020. Eur J Endocrinol. 2021;185(1):R13-R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nazeri P, Mirmiran P, Shiva N, Mehrabi Y, Mojarrad M, Azizi F. Iodine nutrition status in lactating mothers residing in countries with mandatory and voluntary iodine fortification programs: an updated systematic review. Thyroid. 2015;25(6):611-620. [DOI] [PubMed] [Google Scholar]
- 18. Nazeri P, Kabir A, Dalili H, Mirmiran P, Azizi F. Breast-milk iodine concentrations and iodine levels of infants according to the iodine status of the country of residence: a systematic review and meta-analysis. Thyroid. 2018;28(1):124-138. [DOI] [PubMed] [Google Scholar]
- 19. Dror DK, Allen LH. Iodine in human milk: a systematic review. Adv Nutr. 2018;9(Suppl 1):347S-357S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swanson CA, Zimmermann MB, Skeaff S, et al. Summary of an NIH workshop to identify research needs to improve the monitoring of iodine status in the United States and to inform the DRI. J Nutr. 2012;142(6):1175S-1185S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andersson M, de Benoist B, Delange F, Zupan J; WHO Secretariat on behalf of the participants to the consultation. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10(12A):1606-1611. [DOI] [PubMed] [Google Scholar]
- 22. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543-2565. [DOI] [PubMed] [Google Scholar]
- 23. Council on Environmental Health; Rogan WJ, Paulson JA, Baum C, et al. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics. 2014;133(6):1163-1166. [DOI] [PubMed] [Google Scholar]
- 24. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3(2):76-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315-389. [DOI] [PubMed] [Google Scholar]
- 26. De Leo S, Pearce EN, Braverman LE. Iodine supplementation in women during preconception, pregnancy, and lactation: current clinical practice by U.S. obstetricians and midwives. Thyroid. 2017;27(3):434-439. [DOI] [PubMed] [Google Scholar]
- 27. Arrish J, Yeatman H, Williamson M. Midwives and nutrition education during pregnancy: a literature review. Women Birth. 2014;27(1):2-8. [DOI] [PubMed] [Google Scholar]
- 28. Combet E, Bouga M, Pan B, Lean ME, Christopher CO. Iodine and pregnancy—a UK cross-sectional survey of dietary intake, knowledge and awareness. Br J Nutr. 2015;114(1):108-117. [DOI] [PubMed] [Google Scholar]
- 29. Jin Y, Coad J, Zhou SJ, Skeaff S, Benn C, Brough L. Use of iodine supplements by breastfeeding mothers is associated with better maternal and infant iodine status. Biol Trace Elem Res. 2021;199(8):2893-2903. [DOI] [PubMed] [Google Scholar]
- 30. Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N. The sodium/iodide symporter (NIS): molecular physiology and preclinical and clinical applications. Annu Rev Physiol. 2017;79:261-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rousset B, Dupuy C, Miot F, Dumont JE. Thyroid hormone synthesis and secretion. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Thyroid disease manager. Endotext: MDText.com;2015. Accessed June 7, 2021. https://www.endotext.org/section/thyroiddiseasemanager [Google Scholar]
- 32. Citterio CE, Targovnik HM, Arvan P. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol. 2019;15(6):323-338. [DOI] [PubMed] [Google Scholar]
- 33. Hoermann R, Midgley JE, Larisch R, Dietrich JW. Homeostatic control of the thyroid-pituitary axis: perspectives for diagnosis and treatment. Front Endocrinol (Lausanne). 2015;6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagayama Y, Yamashita S, Hirayu H, et al. Regulation of thyroid peroxidase and thyroglobulin gene expression by thyrotropin in cultured human thyroid cells. J Clin Endocrinol Metab. 1989;68(6):1155-1159. [DOI] [PubMed] [Google Scholar]
- 35. Kang HS, Kumar D, Liao G, et al. GLIS3 is indispensable for TSH/TSHR-dependent thyroid hormone biosynthesis and follicular cell proliferation. J Clin Invest. 2017;127(12):4326-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123-2140. [DOI] [PubMed] [Google Scholar]
- 37. Luongo C, Dentice M, Salvatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol. 2019;15(8):479-488. [DOI] [PubMed] [Google Scholar]
- 38. Bernal J. Thyroid hormones in brain development and function. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Thyroid disease manager. Endotext: MDText.com;2015. Accessed June 7, 2021. https://www.endotext.org/section/thyroiddiseasemanager [Google Scholar]
- 39. Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037-4047. [DOI] [PubMed] [Google Scholar]
- 40. Prezioso G, Giannini C, Chiarelli F. Effect of thyroid hormones on neurons and neurodevelopment. Horm Res Paediatr. 2018;90(2):73-81. [DOI] [PubMed] [Google Scholar]
- 41. Schroeder AC, Privalsky ML. Thyroid hormones, T3 and T4, in the brain. Front Endocrinol (Lausanne). 2014;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111-121. [DOI] [PubMed] [Google Scholar]
- 43. Liu YY, Brent GA. Thyroid hormone and the brain: mechanisms of action in development and role in protection and promotion of recovery after brain injury. Pharmacol Ther. 2018;186:176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernal J. New insights on thyroid hormone and the brain. Curr Opin Endocr Metab Res. 2018;2:24-28. [Google Scholar]
- 45. Flamant F, Gauthier K, Richard S. Genetic investigation of thyroid hormone receptor function in the developing and adult brain. Curr Top Dev Biol. 2017;125:303-335. [DOI] [PubMed] [Google Scholar]
- 46. Burns R, O’Herlihy C, Smyth PP. The placenta as a compensatory iodine storage organ. Thyroid. 2011;21(5):541-546. [DOI] [PubMed] [Google Scholar]
- 47. Neven KY, Marien CBD, Janssen BG, et al. Variability of iodine concentrations in the human placenta. Sci Rep. 2020;10(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. LaFranchi SH. Thyroid function in preterm/low birth weight infants: impact on diagnosis and management of thyroid dysfunction. Front Endocrinol (Lausanne). 2021;12:666207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karaoglan M, İşbilen E. The role of placental iodine storage in the neonatal thyroid stimulating hormone surge: iodine as a driving force to adapt the terrestrial life. J Endocrinol Invest. 2021;44(5):1041-1052. [DOI] [PubMed] [Google Scholar]
- 50. Chaler EA, Fiorenzano R, Chilelli C, et al. Age-specific thyroid hormone and thyrotropin reference intervals for a pediatric and adolescent population. Clin Chem Lab Med. 2012;50(5):885-890. [DOI] [PubMed] [Google Scholar]
- 51. Önsesveren I, Barjaktarovic M, Chaker L, et al. Childhood thyroid function reference ranges and determinants: a literature overview and a prospective cohort study. Thyroid. 2017;27(11):1360-1369. [DOI] [PubMed] [Google Scholar]
- 52. Delange F. Screening for congenital hypothyroidism used as an indicator of the degree of iodine deficiency and of its control. Thyroid. 1998;8(12):1185-1192. [DOI] [PubMed] [Google Scholar]
- 53. Andersen SL, Carlé A, Olsen J, Laurberg P. Hypothyroidism incidence in and around pregnancy: a Danish nationwide study. Eur J Endocrinol. 2016;175(5):387-393. [DOI] [PubMed] [Google Scholar]
- 54. Amino N, Arata N. Thyroid dysfunction following pregnancy and implications for breastfeeding. Best Pract Res Clin Endocrinol Metab. 2020;34(4):101438. [DOI] [PubMed] [Google Scholar]
- 55. Stagnaro-Green A, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Negro R. High rate of persistent hypothyroidism in a large-scale prospective study of postpartum thyroiditis in southern Italy. J Clin Endocrinol Metab. 2011;96(3):652-657. [DOI] [PubMed] [Google Scholar]
- 56. Pearce EN. Management of thyrotoxicosis: preconception, pregnancy, and the postpartum period. Endocr Pract. 2019;25(1):62-68. [DOI] [PubMed] [Google Scholar]
- 57. Nguyen CT, Mestman JH. Postpartum thyroiditis. Clin Obstet Gynecol. 2019;62(2):359-364. [DOI] [PubMed] [Google Scholar]
- 58. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16(6):573-582. [DOI] [PubMed] [Google Scholar]
- 59. Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24(1):13-27. [DOI] [PubMed] [Google Scholar]
- 60. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286-295. [DOI] [PubMed] [Google Scholar]
- 61. Nøhr SB, Jørgensen A, Pedersen KM, Laurberg P. Postpartum thyroid dysfunction in pregnant thyroid peroxidase antibody-positive women living in an area with mild to moderate iodine deficiency: is iodine supplementation safe? J Clin Endocrinol Metab. 2000;85(9):3191-3198. [DOI] [PubMed] [Google Scholar]
- 62. Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354(26):2783-2793. [DOI] [PubMed] [Google Scholar]
- 63. Teti C, Panciroli M, Nazzari E, et al. Iodoprophylaxis and thyroid autoimmunity: an update. Immunol Res. 2021;69(2):129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Reinhardt W, Kohl S, Hollmann D, et al. Efficacy and safety of iodine in the postpartum period in an area of mild iodine deficiency. Eur J Med Res. 1998;3(4):203-210. [PubMed] [Google Scholar]
- 65. Kämpe O, Jansson R, Karlsson FA. Effects of L-thyroxine and iodide on the development of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1990;70(4):1014-1018. [DOI] [PubMed] [Google Scholar]
- 66. Gowachirapant S, Jaiswal N, Melse-Boonstra A, et al. Effect of iodine supplementation in pregnant women on child neurodevelopment: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):853-863. [DOI] [PubMed] [Google Scholar]
- 67. Moleti M, Mauro MD, Alibrandi A, Vita R, Benvenga S, Vermiglio F. Postpartum thyroiditis in women with euthyroid and hypothyroid Hashimoto’s thyroiditis antedating pregnancy. J Clin Endocrinol Metab. 2020;105(7):e2421-e2428. [DOI] [PubMed] [Google Scholar]
- 68. Salerno M, Capalbo D, Cerbone M, De Luca F. Subclinical hypothyroidism in childhood—current knowledge and open issues. Nat Rev Endocrinol. 2016;12(12):734-746. [DOI] [PubMed] [Google Scholar]
- 69. Hanley P, Lord K, Bauer AJ. Thyroid disorders in children and adolescents: a review. JAMA Pediatr. 2016;170(10):1008-1019. [DOI] [PubMed] [Google Scholar]
- 70. van Trotsenburg P, Stoupa A, Léger J, et al. Congenital hypothyroidism: a 2020-2021 consensus guidelines update—an ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021;31(3):387-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16(10):809-818. [DOI] [PubMed] [Google Scholar]
- 72. Rovet JF. The role of thyroid hormones for brain development and cognitive function. Endocr Dev. 2014;26:26-43. [DOI] [PubMed] [Google Scholar]
- 73. Korevaar TIM, Tiemeier H, Peeters RP. Clinical associations of maternal thyroid function with foetal brain development: epidemiological interpretation and overview of available evidence. Clin Endocrinol (Oxf). 2018;89(2):129-138. [DOI] [PubMed] [Google Scholar]
- 74. Medici M, de Rijke YB, Peeters RP, et al. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R study. J Clin Endocrinol Metab. 2012;97(2):646-652. [DOI] [PubMed] [Google Scholar]
- 75. Korevaar TI, Chaker L, Jaddoe VW, Visser TJ, Medici M, Peeters RP. Maternal and birth characteristics are determinants of offspring thyroid function. J Clin Endocrinol Metab. 2016;101(1):206-213. [DOI] [PubMed] [Google Scholar]
- 76. Silva JE, Silva S. Interrelationships among serum thyroxine, triiodothyronine, reverse triiodothyronine, and thyroid-stimulating hormone in iodine-deficient pregnant women and their offspring: effects of iodine supplementation. J Clin Endocrinol Metab. 1981;52(4):671-677. [DOI] [PubMed] [Google Scholar]
- 77. Sava L, Delange F, Belfiore A, Purrello F, Vigneri R. Transient impairment of thyroid function in newborn from an area of endemic goiter. J Clin Endocrinol Metab. 1984;59(1):90-95. [DOI] [PubMed] [Google Scholar]
- 78. Sava L, Tomaselli L, Runello F, Belfiore A, Vigneri R. Serum thyroglobulin levels are elevated in newborns from iodine-deficient areas. J Clin Endocrinol Metab. 1986;62(2):429-432. [DOI] [PubMed] [Google Scholar]
- 79. Targovnik HM, Citterio CE, Rivolta CM. Iodide handling disorders (NIS, TPO, TG, IYD). Best Pract Res Clin Endocrinol Metab. 2017;31(2):195-212. [DOI] [PubMed] [Google Scholar]
- 80. Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Pract Res Clin Endocrinol Metab. 2014;28(2):175-187. [DOI] [PubMed] [Google Scholar]
- 81. Deladoëy J, Ruel J, Giguère Y, Van Vliet G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Québec. J Clin Endocr Metab. 2011;96(8):2422-2429. [DOI] [PubMed] [Google Scholar]
- 82. Connelly KJ, LaFranchi SH. Detection of neonates with mild congenital hypothyroidism (primary) or isolated hyperthyrotropinemia: an increasingly common management dilemma. Expert Rev Endocrinol Metab. 2014;9(3):263-271. [DOI] [PubMed] [Google Scholar]
- 83. Wassner AJ, Brown RS. Subclinical hypothyroidism in infancy: to treat or not to treat, that is the question. J Pediatr. 2016;170:17-19. [DOI] [PubMed] [Google Scholar]
- 84. Lain S, Trumpff C, Grosse SD, Olivieri A, Van Vliet G. Are lower TSH cutoffs in neonatal screening for congenital hypothyroidism warranted? Eur J Endocrinol. 2017;177(5):D1-D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Levie D, Korevaar TIM, Bath SC, et al. Thyroid function in early pregnancy, child IQ, and autistic traits: a meta-analysis of individual participant data. J Clin Endocrinol Metab. 2018;103(8):2967-2979. [DOI] [PubMed] [Google Scholar]
- 86. Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316. [DOI] [PubMed] [Google Scholar]
- 87. Trumpff C, De Schepper J, Vanderfaeillie J, et al. Neonatal thyroid-stimulating hormone concentration and psychomotor development at preschool age. Arch Dis Child. 2016;101(12):1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gallizzi R, Crisafulli C, Aversa T, et al. Subclinical hypothyroidism in children: is it always subclinical? Ital J Pediatr. 2018;44(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vigone MC, Capalbo D, Weber G, Salerno M. Mild hypothyroidism in childhood: who, when, and how should be treated? J Endocr Soc. 2018;2(9):1024-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kaplowitz PB. Neonatal thyroid disease: testing and management. Pediatr Clin North Am. 2019;66(2):343-352. [DOI] [PubMed] [Google Scholar]
- 91. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142-1154. [DOI] [PubMed] [Google Scholar]
- 92. Schushan-Eisen I, Lazar L, Amitai N, Meyerovitch J. Thyroid functions in healthy infants during the first year of life. J Pediatr. 2016;170:120-5.e1. [DOI] [PubMed] [Google Scholar]
- 93. Lazar L, Frumkin RB, Battat E, Lebenthal Y, Phillip M, Meyerovitch J. Natural history of thyroid function tests over 5 years in a large pediatric cohort. J Clin Endocrinol Metab. 2009;94(5):1678-1682. [DOI] [PubMed] [Google Scholar]
- 94. Léger J, Carel JC. Diagnosis and management of hyperthyroidism from prenatal life to adolescence. Best Pract Res Clin Endocrinol Metab. 2018;32(4):373-386. [DOI] [PubMed] [Google Scholar]
- 95. Kilberg MJ, Rasooly IR, LaFranchi SH, Bauer AJ, Hawkes CP. Newborn screening in the US may miss mild persistent hypothyroidism. J Pediatr. 2018;192:204-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wu T, Flowers JW, Tudiver F, Wilson JL, Punyasavatsut N. Subclinical thyroid disorders and cognitive performance among adolescents in the United States. BMC Pediatr. 2006;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wassner AJ. Pediatric hypothyroidism: diagnosis and treatment. Paediatr Drugs. 2017;19(4):291-301. [DOI] [PubMed] [Google Scholar]
- 98. Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci. 2019;1446(1):44-65. [DOI] [PubMed] [Google Scholar]
- 99. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Planning. National Academy Press; 2003. [PubMed] [Google Scholar]
- 100. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment. National Academy Press; 2000. [Google Scholar]
- 101. Bürgi H, Supersaxo Z, Selz B. Iodine deficiency diseases in Switzerland one hundred years after Theodor Kocher’s survey: a historical review with some new goitre prevalence data. Acta Endocrinol (Copenh). 1990;123(6):577-590. [DOI] [PubMed] [Google Scholar]
- 102. Delange F. Iodine nutrition and congenital hypothyroidism. In: Delange F, Fisher DA, Glinoer D, eds. Research in Congenital Hypothyroidism. Plenum Press; 1989:173-185. [Google Scholar]
- 103. Kochupillai N, Pandav CS, Godbole MM, Mehta M, Ahuja MM. Iodine deficiency and neonatal hypothyroidism. Bull World Health Organ. 1986;64(4):547-551. [PMC free article] [PubMed] [Google Scholar]
- 104. Rose SR, Brown RS, Foley T, et al. American Academy of Pediatrics, Section on Endocrinology, Committee on Genetics American Thyroid Association, Public Health Committee Lawson Wilkins Pediatric Endocrine Society . Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117(6):2290-2303. [DOI] [PubMed] [Google Scholar]
- 105. Mehran L, Khalili D, Yarahmadi S, et al. Worldwide recall rate in newborn screening programs for congenital hypothyroidism. Int J Endocrinol Metab. 2017;15(3):e55451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thilly CH, Delange F, Golstein-Golaire J, Ermans AM. Endemic goiter prevention by iodized oil: a reassessment. J Clin Endocrinol Metab. 1973;36(6):1196-1204. [DOI] [PubMed] [Google Scholar]
- 107. Vanderpas J, Bourdoux P, Lagasse R, et al. Endemic infantile hypothyroidism in a severe endemic goitre area of central Africa. Clin Endocrinol (Oxf). 1984;20(3):327-340. [DOI] [PubMed] [Google Scholar]
- 108. Moreno-Reyes R, Boelaert M, el Badawi S, Eltom M, Vanderpas JB. Endemic juvenile hypothyroidism in a severe endemic goitre area of Sudan. Clin Endocrinol (Oxf). 1993;38(1):19-24. [DOI] [PubMed] [Google Scholar]
- 109. Delange F, Heidemann P, Bourdoux P, et al. Regional variations of iodine nutrition and thyroid function during the neonatal period in Europe. Biol Neonate. 1986;49(6):322-330. [DOI] [PubMed] [Google Scholar]
- 110. Sağlam H, Büyükuysal L, Köksal N, Ercan I, Tarim O. Increased incidence of congenital hypothyroidism due to iodine deficiency. Pediatr Int. 2007;49(1):76-79. [DOI] [PubMed] [Google Scholar]
- 111. Tylek-Lemańska D, Rybakowa M, Kumorowicz-Kopiec M, Dziatkowiak H, Ratajczak R. Iodine deficiency disorders incidence in neonates based on the experience with mass screening for congenital hypothyroidism in southeast Poland in the years 1985-2000. J Endocrinol Invest. 2003;26(2 Suppl):32-38. [PubMed] [Google Scholar]
- 112. Bouhouch RR, Bouhouch S, Cherkaoui M, et al. Direct iodine supplementation of infants versus supplementation of their breastfeeding mothers: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(3):197-209. [DOI] [PubMed] [Google Scholar]
- 113. Calaciura F, Motta RM, Miscio G, et al. Subclinical hypothyroidism in early childhood: a frequent outcome of transient neonatal hyperthyrotropinemia. J Clin Endocrinol Metab. 2002;87(7):3209-3214. [DOI] [PubMed] [Google Scholar]
- 114. Leonardi D, Polizzotti N, Carta A, et al. Longitudinal study of thyroid function in children with mild hyperthyrotropinemia at neonatal screening for congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93(7):2679-2685. [DOI] [PubMed] [Google Scholar]
- 115. Shaikh MG, Anderson JM, Hall SK, Jackson MA. Transient neonatal hypothyroidism due to a maternal vegan diet. J Pediatr Endocrinol Metab. 2003;16(1):111-113. [DOI] [PubMed] [Google Scholar]
- 116. Borak J. Neonatal hypothyroidism due to maternal vegan diet. J Pediatr Endocrinol Metab. 2005;18(6):621. [DOI] [PubMed] [Google Scholar]
- 117. Yeliosof O, Silverman LA. Veganism as a cause of iodine deficient hypothyroidism. J Pediatr Endocrinol Metab. 2018;31(1):91-94. [DOI] [PubMed] [Google Scholar]
- 118. Golekoh MC, Cole CR, Jones NY. Severe hypothyroidism from iodine deficiency associated with parenteral nutrition. JPEN J Parenter Enteral Nutr. 2016;40(8):1191-1193. [DOI] [PubMed] [Google Scholar]
- 119. Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 μg/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab. 2013;98(3):1271-1280. [DOI] [PubMed] [Google Scholar]
- 120. Stinca S, Andersson M, Weibel S, et al. Dried blood spot thyroglobulin as a biomarker of iodine status in pregnant women. J Clin Endocrinol Metab. 2017;102(1):23-32. [DOI] [PubMed] [Google Scholar]
- 121. Farebrother J, Zimmermann MB, Assey V, et al. Thyroglobulin is markedly elevated in 6- to 24-month-old infants at both low and high iodine intakes and suggests a narrow optimal iodine intake range. Thyroid. 2019;29(2):268-277. [DOI] [PubMed] [Google Scholar]
- 122. Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Taylor PN, Okosieme OE, Dayan CM, Lazarus JH. Impact of iodine supplementation in mild-to-moderate iodine deficiency: systematic review and meta-analysis. Eur J Endocrinol. 2014;170(1):R1-R15. [DOI] [PubMed] [Google Scholar]
- 124. Mills JL, Reische EC, Kannan K, Gao C, Shaw GM, Sundaram R. Newborn iodine status is not related to congenital hypothyroidism. J Nutr. 2020;150(9):2429-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stinca S, Andersson M, Herter-Aeberli I, et al. Moderate-to-severe iodine deficiency in the “First 1000 Days” causes more thyroid hypofunction in infants than in pregnant or lactating women. J Nutr. 2017;147(4):589-595. [DOI] [PubMed] [Google Scholar]
- 126. Laurberg P, Andersen S, Bjarnadóttir RI, et al. Evaluating iodine deficiency in pregnant women and young infants—complex physiology with a risk of misinterpretation. Public Health Nutr. 2007;10(12A):1547-1552; discussion 1553. [DOI] [PubMed] [Google Scholar]
- 127. Zhou SJ, Anderson AJ, Gibson RA, Makrides M. Effect of iodine supplementation in pregnancy on child development and other clinical outcomes: a systematic review of randomized controlled trials. Am J Clin Nutr. 2013;98(5):1241-1254. [DOI] [PubMed] [Google Scholar]
- 128. Farebrother J, Naude CE, Nicol L, et al. Effects of iodized salt and iodine supplements on prenatal and postnatal growth: a systematic review. Adv Nutr. 2018;9(3):219-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nazeri P, Shab-Bidar S, Pearce EN, Shariat M. Do maternal urinary iodine concentration or thyroid hormones within the normal range during pregnancy affect growth parameters at birth? A systematic review and meta-analysis. Nutr Rev. 2020;78(9):747-763. [DOI] [PubMed] [Google Scholar]
- 130. Wassie MM, Smithers LG, Zhou SJ. Association between newborn thyroid-stimulating-hormone concentration and neurodevelopment and growth: a systematic review. Biol Trace Elem Res. Published March 8, 2021. doi: 10.1007/s12011-021-02665-7 [DOI] [PubMed] [Google Scholar]
- 131. Semba RD, de Pee S, Hess SY, Sun K, Sari M, Bloem MW. Child malnutrition and mortality among families not utilizing adequately iodized salt in Indonesia. Am J Clin Nutr. 2008;87(2):438-444. [DOI] [PubMed] [Google Scholar]
- 132. Cobra C, Muhilal M, Rusmil K, et al. Infant survival is improved by oral iodine supplementation. J Nutr. 1997;127(4):574-578. [DOI] [PubMed] [Google Scholar]
- 133. Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331(26):1739-1744. [DOI] [PubMed] [Google Scholar]
- 134. Redman K, Ruffman T, Fitzgerald P, Skeaff S. Iodine deficiency and the brain: effects and mechanisms. Crit Rev Food Sci Nutr. 2016;56(16):2695-2713. [DOI] [PubMed] [Google Scholar]
- 135. Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab. 2010;24(1):39-50. [DOI] [PubMed] [Google Scholar]
- 136. Eastman C, Zimmermann MB. The iodine deficiency disorders. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Thyroid disease manager. Endotext: MDText.com;2018. https://www.endotext.org/section/thyroiddiseasemanager [Google Scholar]
- 137. Bougma K, Aboud FE, Harding KB, Marquis GS. Iodine and mental development of children 5 years old and under: a systematic review and meta-analysis. Nutrients. 2013;5(4):1384-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aburto N, Abudou M, Candeias V, Wu T. Effect and Safety of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review With Meta-Analyses. WHO eLibrary of Evidence for Nutrition Actions (eLENA). World Heal; th Organization; 2014. [Google Scholar]
- 139. Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr. 2016;104(Suppl 3):918S-923S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wassie MM, Smithers LG, Yelland LN, Makrides M, Zhou SJ. Associations between newborn thyroid-stimulating hormone concentration and neurodevelopment and growth of children at 18 months. Br J Nutr. Published online January 26, 2021. doi: 10.1017/S0007114521000325. [DOI] [PubMed] [Google Scholar]
- 141. Wu M, Wu D, Wu W, et al. Relationship between iodine concentration in maternal colostrum and neurobehavioral development of infants in Shanghai, China. J Child Neurol. 2016;31(9):1108-1113. [DOI] [PubMed] [Google Scholar]
- 142. Nazeri P, Tahmasebinejad Z, Pearce EN, et al. Does maternal iodine supplementation during the lactation have a positive impact on neurodevelopment of children? Three-year follow up of a randomized controlled trial. Eur J Nutr. 2021;60(7):4083-4091. [DOI] [PubMed] [Google Scholar]
- 143. Nazeri P, Tahmasebinejad Z, Mehrabi Y, Hedayati M, Mirmiran P, Azizi F. Lactating mothers and infants residing in an area with an effective salt iodization program have no need for iodine supplements: results from a double-blind, placebo-controlled, randomized controlled trial. Thyroid. 2018;28(11):1547-1558. [DOI] [PubMed] [Google Scholar]
- 144. Bürgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab. 2010;24(1):107-115. [DOI] [PubMed] [Google Scholar]
- 145. Liu L, Wang D, Liu P, et al. The relationship between iodine nutrition and thyroid disease in lactating women with different iodine intakes. Br J Nutr. 2015;114(9):1487-1495. [DOI] [PubMed] [Google Scholar]
- 146. Aakre I, Bjøro T, Norheim I, Strand TA, Barikmo I, Henjum S. Excessive iodine intake and thyroid dysfunction among lactating Saharawi women. J Trace Elem Med Biol. 2015;31:279-284. [DOI] [PubMed] [Google Scholar]
- 147. van Welie N, Portela M, Dreyer K, et al. Iodine contrast prior to or during pregnancy and neonatal thyroid function: a systematic review. Eur J Endocrinol. 2021;184(1):189-198. [DOI] [PubMed] [Google Scholar]
- 148. Overcash RT, Marc-Aurele KL, Hull AD, Ramos GA. Maternal iodine exposure: a case of fetal goiter and neonatal hearing loss. Pediatrics. 2016;137(4):e20153722. [DOI] [PubMed] [Google Scholar]
- 149. de Vasconcellos Thomas J, Collett-Solberg PF. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm Res. 2009;72(6):344-347. [DOI] [PubMed] [Google Scholar]
- 150. Nishiyama S, Mikeda T, Okada T, Nakamura K, Kotani T, Hishinuma A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004;14(12):1077-1083. [DOI] [PubMed] [Google Scholar]
- 151. Connelly KJ, Boston BA, Pearce EN, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161(4):760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Medici M, Ghassabian A, Visser W, et al. Women with high early pregnancy urinary iodine levels have an increased risk of hyperthyroid newborns: the population-based Generation R Study. Clin Endocrinol (Oxf). 2014;80(4):598-606. [DOI] [PubMed] [Google Scholar]
- 153. Hamby T, Kunnel N, Dallas JS, Wilson DP. Maternal iodine excess: an uncommon cause of acquired neonatal hypothyroidism. J Pediatr Endocrinol Metab. 2018;31(9):1061-1064. [DOI] [PubMed] [Google Scholar]
- 154. Sanderson E, Kassam S, Frazer F. Supratherapeutic maternal iodine supplementation during pregnancy as a cause for congenital hypothyroidism. J Paediatr Child Health. 2019;55(2):245-246. [DOI] [PubMed] [Google Scholar]
- 155. Abel MH, Korevaar TIM, Erlund I, et al. Iodine intake is associated with thyroid function in mild to moderately iodine deficient pregnant women. Thyroid. 2018;28(10):1359-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85(11):3975-3987. [DOI] [PubMed] [Google Scholar]
- 157. Abel MH, Caspersen IH, Meltzer HM, et al. Suboptimal maternal iodine intake is associated with impaired child neurodevelopment at 3 years of age in the Norwegian Mother and Child Cohort Study. J Nutr. 2017;147(7):1314-1324. [DOI] [PubMed] [Google Scholar]
- 158. Murcia M, Espada M, Julvez J, et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: the INMA Mother and Child Cohort Study. J Epidemiol Community Health. 2018;72(3):216-222. [DOI] [PubMed] [Google Scholar]
- 159. Chanoine JP, Boulvain M, Bourdoux P, et al. Increased recall rate at screening for congenital hypothyroidism in breast fed infants born to iodine overloaded mothers. Arch Dis Child. 1988;63(10):1207-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Emder PJ, Jack MM. Iodine-induced neonatal hypothyroidism secondary to maternal seaweed consumption: a common practice in some Asian cultures to promote breast milk supply. J Paediatr Child Health. 2011;47(10):750-752. [DOI] [PubMed] [Google Scholar]
- 161. Rhee SS, Braverman LE, Pino S, He X, Pearce EN. High iodine content of Korean seaweed soup: a health risk for lactating women and their infants? Thyroid. 2011;21(8):927-928. [DOI] [PubMed] [Google Scholar]
- 162. Kurtoğlu S, Akın L, Akın MA, Çoban D. Iodine overload and severe hypothyroidism in two neonates. J Clin Res Pediatr Endocrinol. 2009;1(6):275-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Shumer DE, Mehringer JE, Braverman LE, Dauber A. Acquired hypothyroidism in an infant related to excessive maternal iodine intake: food for thought. Endocr Pract. 2013;19(4):729-731. [DOI] [PubMed] [Google Scholar]
- 164. Vlaardingerbroek H. Unusual cause of congenital hypothyroidism in a term infant. BMJ Case Rep. 2021;14(2):e237930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Nepal AK, Suwal R, Gautam S, et al. Subclinical hypothyroidism and elevated thyroglobulin in infants with chronic excess iodine intake. Thyroid. 2015;25(7):851-859. [DOI] [PubMed] [Google Scholar]
- 166. Aakre I, Strand TA, Bjøro T, et al. Thyroid function among breastfed children with chronically excessive iodine intakes. Nutrients. 2016;8(7):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Farebrother J, Zimmermann MB, Abdallah F, et al. Effect of excess iodine intake from iodized salt and/or groundwater iodine on thyroid function in nonpregnant and pregnant women, infants, and children: a multicenter study in East Africa. Thyroid. 2018;28(9):1198-1210. [DOI] [PubMed] [Google Scholar]
- 168. Hamada K, Mizokami T, Maruta T, et al. Effects of inorganic iodine therapy administered to lactating mothers with Graves disease on infant thyroid function. J Endocr Soc. 2017;1(10):1293-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hamada K, Mizokami T, Maruta T, et al. Thyroid function of infants breastfed by mothers with Graves disease treated with inorganic iodine: a study of 100 cases. J Endocr Soc. 2021;5(2):bvaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Dechant MJ, van der Werf-Grohmann N, Neumann E, Spiekerkoetter U, Stiller B, Grohmann J. Thyroidal response following iodine excess for cardiac catheterisation and intervention in early infancy. Int J Cardiol. 2016;223:1014-1018. [DOI] [PubMed] [Google Scholar]
- 171. Allen LH, Carriquiry AL, Murphy SP. Perspective: proposed harmonized nutrient reference values for populations. Adv Nutr. 2020;11(3):469-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. (US) Institute of Medicine, Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. National Academy Press; 2001. [PubMed] [Google Scholar]
- 173. National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand. National Health and Medical Research Council; 2006. [Google Scholar]
- 174. China Nutrition Science Congress. Chinese DRIs Handbook. China Standards Press; 2014. [Google Scholar]
- 175. WHO, FAO. Vitamin and Mineral Requirements in Human Nutrition. 2nd ed. World Health Organization; 2004. [Google Scholar]
- 176. WHO, UNICEF, ICCIDD. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination. A Guide for Programme Managers. 3rd ed. World Health Organization; 2007. [Google Scholar]
- 177. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for iodine. EFSA J. 2014;12(5):3660. [Google Scholar]
- 178. Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung (Hrsg.). Referenzwerte für die Nährstoffzufuhr. Bonn. 2. Auflage, 6. aktualisierte Ausgabe. Deutsche Geselschaft für Ernährung; 2020. [Google Scholar]
- 179. Nordic Council of Ministers. Nordic Nutrition Recommendations 2012. Nordic Council of Ministers; 2014. [Google Scholar]
- 180. UK Department of Health. Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Rep Health Soc Subj (Lond). 1991;41:1-210. [PubMed] [Google Scholar]
- 181. Dold S, Zimmermann MB, Baumgartner J, et al. A dose-response crossover iodine balance study to determine iodine requirements in early infancy. Am J Clin Nutr. 2016;104(3):620-628. [DOI] [PubMed] [Google Scholar]
- 182. Goellner MH, Ziegler EE, Fomon SJ. Urination during the first three years of life. Nephron. 1981;28(4):174-178. [DOI] [PubMed] [Google Scholar]
- 183. Delange F, Bourdoux P, Chanoine JP, Ermans AM. Physiopathology of iodine nutrition during pregnancy, lactation, and early postnatal life. In: Berger H, ed. Vitamins and Minerals in Pregnancy and Lactation. Nestlé Nutrition Workshop Series. Vol. 16. Raven Press; 1988:205-214. [Google Scholar]
- 184. Delange F. Requirements of iodine in humans. In: Delange F, Dunn JT, Glinoer D, eds. Iodine Deficiency in Europe. A Continuing Concern. Plenum Press; 1993. [Google Scholar]
- 185. WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. World Health Organization; 2006. [Google Scholar]
- 186. Ingenbleek Y, Malvaux P. Iodine balance studies in protein-calorie malnutrition. Arch Dis Child. 1974;49(4):305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. EFSA. Tolerable Upper Intake Levels for Vitamins and Minerals. European Food Safety Authority; 2006. Accessed April 8, 2021. https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. van der Reijden OL, Zimmermann MB, Galetti V. Iodine in dairy milk: sources, concentrations and importance to human health. Best Pract Res Clin Endocrinol Metab. 2017;31(4):385-395. [DOI] [PubMed] [Google Scholar]
- 189. Nerhus I, Wik Markhus M, Nilsen BM, et al. Iodine content of six fish species, Norwegian dairy products and hen’s egg. Food Nutr Res. 2018;62. doi:10.29219/fnr.v62.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Haldimann M, Alt A, Blanc A, Blondeau K. Iodine content of food groups. J Food Compos Anal. 2005;18(6):461-471. [Google Scholar]
- 191. US Department of Agriculture, Agricultural Research Service. FoodData central. 2019. Accessed May 19, 2021. https://fdc.nal.usda.gov
- 192. Nath SK, Moinier B, Thuillier F, Rongier M, Desjeux JF. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int J Vitam Nutr Res. 1992;62(1):66-72. [PubMed] [Google Scholar]
- 193. Jahreis G, Hausmann W, Kiessling G, Franke K, Leiterer M. Bioavailability of iodine from normal diets rich in dairy products—results of balance studies in women. Exp Clin Endocrinol Diabetes. 2001;109(3):163-167. [DOI] [PubMed] [Google Scholar]
- 194. van der Reijden OL, Galetti V, Bürki S, et al. Iodine bioavailability from cow milk: a randomized, crossover balance study in healthy iodine-replete adults. Am J Clin Nutr. 2019;110(1):102-110. [DOI] [PubMed] [Google Scholar]
- 195. Hurrell RF. Bioavailability of iodine. Eur J Clin Nutr. 1997;51(Suppl 1):S9-12. [PubMed] [Google Scholar]
- 196. Joint FAO/WHO Food Standards Programme Codex Alimentarius Commisssion. Standard for infant formula and formulas for special medical purposes intended for infants. Codex stan 72-1981. Revision: 2007. Accessed 19 September, 2021. http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/
- 197. US Government Publishing Office. Electronic Code of Federal Regulations. Title 21, Chapter I, Subchapter B, Part 107, Subpart D, Paragraph 107.000. National Archives and Records Administration; 2019. [Google Scholar]
- 198. The Ministry of Health of the People’s Republic of China. GB 10765-2010 National Food Safety Standard: Infant Formula. China Standard Press; 2010. [Google Scholar]
- 199. European Union. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. J. Eur Union. 2016;L25: 1-29. [Google Scholar]
- 200. Butte NF. Energy requirements of infants. Public Health Nutr. 2005;8(7A):953-967. [DOI] [PubMed] [Google Scholar]
- 201. MacLean WC Jr, Van Dael P, Clemens R, et al. Upper levels of nutrients in infant formulas: comparison of analytical data with the revised Codex infant formula standard. J Food Compos Anal. 2010;23(1):44-53. [Google Scholar]
- 202. Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab. 2004;89(7):3421-3424. [DOI] [PubMed] [Google Scholar]
- 203. Andersson M, Aeberli I, Wüst N, et al. The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J Clin Endocrinol Metab. 2010;95(12):5217-5224. [DOI] [PubMed] [Google Scholar]
- 204. Nazeri P, Dalili H, Mehrabi Y, Hedayati M, Mirmiran P, Azizi F. Is there any difference between the iodine statuses of breast-fed and formula-fed infants and their mothers in an area with iodine sufficiency? Br J Nutr. 2018;119(9):1012-1018. [DOI] [PubMed] [Google Scholar]
- 205. WHO, UNICEF. Global Strategy for Infant and Young Child Feeding. World Health Organization; 2003. [Google Scholar]
- 206. Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):119-132. [DOI] [PubMed] [Google Scholar]
- 207. American Academy of Pediatrics Committee on Nutrition. Feeding the infant. In: Kleinman RE, Greer FR, eds. Pediatric Nutrition. 8th ed. American Academy of Pediatrics; 2019. [Google Scholar]
- 208. Thomson BM, Vannoort RW, Haslemore RM. Dietary exposure and trends of exposure to nutrient elements iodine, iron, selenium and sodium from the 2003-4 New Zealand Total Diet Survey. Br J Nutr. 2008;99(3):614-625. [DOI] [PubMed] [Google Scholar]
- 209. Alexy U, Drossard C, Kersting M, Remer T. Iodine intake in the youngest: impact of commercial complementary food. Eur J Clin Nutr. 2009;63(11):1368-1370. [DOI] [PubMed] [Google Scholar]
- 210. Yang J, Zhu L, Li X, et al. Iodine status of vulnerable populations in Henan Province of China 2013-2014 after the implementation of the new iodized salt standard. Biol Trace Elem Res. 2016;173(1):7-13. [DOI] [PubMed] [Google Scholar]
- 211. Osei J, Baumgartner J, Rothman M, et al. Iodine status and associations with feeding practices and psychomotor milestone development in six-month-old South African infants. Matern Child Nut. 2017;13(4):12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Fallah R, Du L, Braverman LE, et al. Iodine nutrition in weaning infants in the United States. Thyroid. 2019;29(4):573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Commission of the European Communities. Commission Directive 2006/125/EC of December 5 2006 on processed cereal-based foods and baby foods for infants and young children. J Eur Union. 2006;L(339):16-35. [Google Scholar]
- 214. Theurich MA, Koletzko B, Grote V. Nutritional adequacy of commercial complementary cereals in Germany. Nutrients. 2020;12(6):1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Joint FAO/WHO Food Standards Programme Codex Alimentarius Commisssion. Report of the 41th session of the codex committee on nutrition and foods for special dietary uses—REP20/NFSDU.2019. Accessed 10 March, 2021. https://ccnfsdu.de/uploads/tx_bleinhaltselemente/REP20_NFSDUe_03.pdf
- 216. The Ministry of Health of the People’s Republic of China. GB 10767-2010 National Food Safety Standard: Older Infants and Young Children Formula. China Standard Press; 2010. [Google Scholar]
- 217. Han J, Kang L, Liang D, et al. Composition requirements of follow-up formula for 6-12-month-old infants: recommendations of a Chinese expert group. Asia Pac J Clin Nutr. 2019;28(2):347-355. [DOI] [PubMed] [Google Scholar]
- 218. Suthutvoravut U, Abiodun PO, Chomtho S, et al. Composition of follow-up formula for young children aged 12-36 months: recommendations of an international expert group coordinated by the Nutrition Association of Thailand and the Early Nutrition Academy. Ann Nutr Metab. 2015;67(2):119-132. [DOI] [PubMed] [Google Scholar]
- 219. Pacquette LH, Levenson AM, Thompson JJ. Determination of total iodine in infant formula and nutritional products by inductively coupled plasma/mass spectrometry: single-laboratory validation. J AOAC Int. 2012;95(1):169-176. [DOI] [PubMed] [Google Scholar]
- 220. Hojsak I, Bronsky J, Campoy C, et al. ; ESPGHAN Committee on Nutrition . Young child formula: a position paper by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(1):177-185. [DOI] [PubMed] [Google Scholar]
- 221. Cho JY, Léveillé R, Kao R, et al. Hormonal regulation of radioiodide uptake activity and Na+/I– symporter expression in mammary glands. J Clin Endocrinol Metab. 2000;85(8):2936-2943. [DOI] [PubMed] [Google Scholar]
- 222. Tazebay UH, Wapnir IL, Levy O, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6(8):871-878. [DOI] [PubMed] [Google Scholar]
- 223. Rillema JA, Williams CH, Moulden J, Golden KL. Effect of insulin on iodide uptake in mouse mammary gland explants. Exp Biol Med (Maywood). 2002;227(1):32-35. [DOI] [PubMed] [Google Scholar]
- 224. Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I– symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272(43):27230-27238. [DOI] [PubMed] [Google Scholar]
- 225. Spitzweg C, Joba W, Eisenmenger W, Heufelder AE. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J Clin Endocrinol Metab. 1998;83(5):1746-1751. [DOI] [PubMed] [Google Scholar]
- 226. Smanik PA, Liu Q, Furminger TL, et al. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun. 1996;226(2):339-345. [DOI] [PubMed] [Google Scholar]
- 227. Portulano C, Paroder-Belenitsky M, Carrasco N. The Na+/I– symporter (NIS): mechanism and medical impact. Endocr Rev. 2014;35(1):106-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Shi XZ, Xue L, Jin X, Xu P, Jia S, Shen HM. Different expression of sodium-iodide importer (NIS) between lactating breast and thyroid tissues may be due to structural difference of thyroid-stimulating hormone receptor (TSHR). J Endocrinol Invest. 2017;40(1):41-48. [DOI] [PubMed] [Google Scholar]
- 229. Rillema JA, Yu TX, Jhiang SM. Effect of prolactin on sodium iodide symporter expression in mouse mammary gland explants. Am J Physiol Endocrinol Metab. 2000;279(4):E769-E772. [DOI] [PubMed] [Google Scholar]
- 230. Milanesi A, Brent GA. Iodine and thyroid hormone synthesis, metabolism, and action. In: Collins JF, ed. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Book Series: Molecular Nutrition Academic Press; 2016:143-150. [Google Scholar]
- 231. Alotaibi H, Yaman EC, Demirpençe E, Tazebay UH. Unliganded estrogen receptor-alpha activates transcription of the mammary gland Na+/I– symporter gene. Biochem Biophys Res Commun. 2006;345(4):1487-1496. [DOI] [PubMed] [Google Scholar]
- 232. Alotaibi H, Yaman E, Salvatore D, et al. Intronic elements in the Na+/I– symporter gene (NIS) interact with retinoic acid receptors and mediate initiation of transcription. Nucleic Acids Res. 2010;38(10):3172-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Telkoparan P, Tazebay UH. Iodine in milk: transport, metabolic implications, and relation to endocrine functions. In: Zibadi S, Watson RR, Preedy VR, eds. Handbook of Dietary and Nutritional Aspects of Human Breast Milk. Vol. 5. Wagningen Academic Publishers: 2013:371-385. [Google Scholar]
- 234. Alotaibi H, Tuzlakoğlu-Öztürk M, Tazebay UH. The thyroid Na+/I– symporter: molecular characterization and genomic regulation. Mol Imaging Radionucl Ther. 2017;26(Suppl 1):92-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Spitzweg C, Morris JC. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol (Oxf). 2002;57(5):559-574. [DOI] [PubMed] [Google Scholar]
- 236. Mizokami T, Fukata S, Hishinuma A, et al. Iodide transport defect and breast milk iodine. Eur Thyroid J. 2016;5(2):145-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rillema JA, Hill MA. Prolactin regulation of the pendrin-iodide transporter in the mammary gland. Am J Physiol Endocrinol Metab. 2003;284(1):E25-E28. [DOI] [PubMed] [Google Scholar]
- 238. Aceves C, Anguiano B, Delgado G. Is iodine a gatekeeper of the integrity of the mammary gland? J Mammary Gland Biol Neoplasia. 2005;10(2):189-196. [DOI] [PubMed] [Google Scholar]
- 239. Poole VL, McCabe CJ. Iodide transport and breast cancer. J Endocrinol. 2015;227(1):R1-R12. [DOI] [PubMed] [Google Scholar]
- 240. Anguiano B, García-Solís P, Delgado G, Aceves Velasco C. Uptake and gene expression with antitumoral doses of iodine in thyroid and mammary gland: evidence that chronic administration has no harmful effects. Thyroid. 2007;17(9):851-859. [DOI] [PubMed] [Google Scholar]
- 241. Vanderstraeten J, Derradji H, Sonveaux P, Colin IM, Many MC, Gérard AC. Acute iodine deficiency induces a transient VEGF-dependent microvascular response in mammary glands involving HIF-1, ROS, and mTOR. Am J Physiol Cell Physiol. 2018;315(4):C544-C557. [DOI] [PubMed] [Google Scholar]
- 242. Yu X, Shen H, Liu L, Lin L, Gao M, Wang S. Changes of sodium iodide symporter regulated by IGF-I and TGF-β1 in mammary gland cells from lactating mice at different iodine levels. Biol Trace Elem Res. 2012;146(1):73-78. [DOI] [PubMed] [Google Scholar]
- 243. Serrano-Nascimento C, Salgueiro RB, Vitzel KF, Pantaleão T, Corrêa da Costa VM, Nunes MT. Iodine excess exposure during pregnancy and lactation impairs maternal thyroid function in rats. Endocr Connect. 2017;6(7):510-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Kirk AB, Dyke JV, Martin CF, Dasgupta PK. Temporal patterns in perchlorate, thiocyanate, and iodide excretion in human milk. Environ Health Perspect. 2007;115(2):182-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Kirk AB, Kroll M, Dyke JV, Ohira S, Dias RA, Dasgupta PK. Perchlorate, iodine supplements, iodized salt and breast milk iodine content. Sci Total Environ. 2012;420:73-78. [DOI] [PubMed] [Google Scholar]
- 246. Manz F, Böhmer T, Gärtner R, Grossklaus R, Klett M, Schneider R. Quantification of iodine supply: representative data on intake and urinary excretion of iodine from the German population in 1996. Ann Nutr Metab. 2002;46(3-4):128-138. [DOI] [PubMed] [Google Scholar]
- 247. Henjum S, Lilleengen AM, Aakre I, et al. Suboptimal iodine concentration in breastmilk and inadequate iodine intake among lactating women in Norway. Nutrients. 2017;9(7):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Costeira MJ, Oliveira P, Ares S, de Escobar GM, Palha JA. Iodine status of pregnant women and their progeny in the Minho region of Portugal. Thyroid. 2009;19(2):157-163. [DOI] [PubMed] [Google Scholar]
- 249. Andersen SL, Møller M, Laurberg P. Iodine concentrations in milk and in urine during breastfeeding are differently affected by maternal fluid intake. Thyroid. 2014;24(4):764-772. [DOI] [PubMed] [Google Scholar]
- 250. Dold S, Zimmermann MB, Aboussad A, et al. Breast milk iodine concentration is a more accurate biomarker of iodine status than urinary iodine concentration in exclusively breastfeeding women. J Nutr. 2017;147(4):528-537. [DOI] [PubMed] [Google Scholar]
- 251. Huynh D, Condo D, Gibson R, et al. Iodine status of postpartum women and their infants in Australia after the introduction of mandatory iodine fortification. Br J Nutr. 2017;117(12):1656-1662. [DOI] [PubMed] [Google Scholar]
- 252. Gebreegziabher T, Stoecker BJ. Comparison of two sources of iodine delivery on breast milk iodine and maternal and infant urinary iodine concentrations in southern Ethiopia: a randomized trial. Food Sci Nutr. 2017;5(4):921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Hashemipour M, Nasri P, Hovsepian S, et al. Urine and milk iodine concentrations in healthy and congenitally hypothyroid neonates and their mothers. Endokrynol Pol. 2010;61(4):371-376. [PubMed] [Google Scholar]
- 254. Osei J, Andersson M, Reijden OV, Dold S, Smuts CM, Baumgartner J. Breast-milk iodine concentrations, iodine status, and thyroid function of breastfed infants aged 2-4 months and their mothers residing in a South African township. J Clin Res Pediatr Endocrinol. 2016;8(4):381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Chen Y, Gao M, Bai Y, et al. Variation of iodine concentration in breast milk and urine in exclusively breastfeeding women and their infants during the first 24 wk after childbirth. Nutrition. 2020;71:110599. [DOI] [PubMed] [Google Scholar]
- 256. Wang W, Sun Y, Zhang M, et al. Breast milk and infant iodine status during the first 12 weeks of lactation in Tianjin City, China. Asia Pac J Clin Nutr. 2018;27(2):393-398. [DOI] [PubMed] [Google Scholar]
- 257. Pal N, Samanta SK, Chakraborty A, Chandra NK, Chandra AK. Interrelationship between iodine nutritional status of lactating mothers and their absolutely breast-fed infants in coastal districts of Gangetic West Bengal in India. Eur J Pediatr. 2018;177(1):39-45. [DOI] [PubMed] [Google Scholar]
- 258. Henjum S, Kjellevold M, Ulak M, et al. Iodine concentration in breastmilk and urine among lactating women of Bhaktapur, Nepal. Nutrients. 2016;8(5):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J Clin Endocrinol Metab. 2009;94(11):4444-4447. [DOI] [PubMed] [Google Scholar]
- 260. Leiterer M, Truckenbrodt D, Franke K. Determination of iodine species in milk using ion chromatographic separation and ICP-MS detection. Eur Food Res Technol. 2001;213(2):150-153. [Google Scholar]
- 261. Michalke B. Trace element speciation in human milk. Pure Appl Chem. 2006;78(1):79-90. [Google Scholar]
- 262. Fernández-Sánchez LM, Bermejo-Barrera P, Fraga-Bermudez JM, Szpunar J, Lobinski R. Determination of iodine in human milk and infant formulas. J Trace Elem Med Biol. 2007;21(Suppl 1):10-13. [DOI] [PubMed] [Google Scholar]
- 263. Strum JM. Site of iodination in rat mammary gland. Anat Rec. 1978;192(2):235-244. [DOI] [PubMed] [Google Scholar]
- 264. Brätter P, Blasco IN, Negretti de Brätter VE, Raab A. Speciation as an analytical aid in trace element research in infant nutrition. Analyst. 1998;123(5):821-826. [DOI] [PubMed] [Google Scholar]
- 265. Delgado G, Muñoz-Torres C, Orozco-Esquivel T, Anguiano B, Aceves C. Total iodine quantification in fluids and tissues from iodine- or iodide-supplemented rats by ion chromatography following microwave-assisted digestion. Thyroid. 2015;25(3):352-360. [DOI] [PubMed] [Google Scholar]
- 266. Mizuta H, Amino N, Ichihara K, et al. Thyroid hormones in human milk and their influence on thyroid function of breast-fed babies. Pediatr Res. 1983;17(6):468-471. [DOI] [PubMed] [Google Scholar]
- 267. van Wassenaer AG, Stulp MR, Valianpour F, et al. The quantity of thyroid hormone in human milk is too low to influence plasma thyroid hormone levels in the very preterm infant. Clin Endocrinol (Oxf). 2002;56(5):621-627. [DOI] [PubMed] [Google Scholar]
- 268. Dasgupta PK, Kirk AB, Dyke JV, Ohira S. Intake of iodine and perchlorate and excretion in human milk. Environ Sci Technol. 2008;42(21):8115-8121. [DOI] [PubMed] [Google Scholar]
- 269. Grosvenor CE. Secretion of I131 into milk by lactating rat mammary glands. Am J Physiol. 1960;199:419-422. [DOI] [PubMed] [Google Scholar]
- 270. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med. 1988;14(12):632-633. [DOI] [PubMed] [Google Scholar]
- 271. Leung AM, Braverman LE, He X, Heeren T, Pearce EN. Breastmilk iodine concentrations following acute dietary iodine intake. Thyroid. 2012;22(11):1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Bilston-John SH, Narayanan A, Tat Lai C, Rea A, Joseph J, Geddes DT. Daily and within-feed variation of macro- and trace-element concentrations in human milk and implications for sampling. Food Chem. 2021;363:130179. [DOI] [PubMed] [Google Scholar]
- 273. Hannan MA, Faraji B, Tanguma J, Longoria N, Rodriguez RC. Maternal milk concentration of zinc, iron, selenium, and iodine and its relationship to dietary intakes. Biol Trace Elem Res. 2009;127(1):6-15. [DOI] [PubMed] [Google Scholar]
- 274. Carriquiry AL, Spungen JH, Murphy SP, et al. Variation in the iodine concentrations of foods: considerations for dietary assessment. Am J Clin Nutr. 2016;104(Suppl 3):877S-887S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Bazrafshan HR, Mohammadian S, Ordookhani A, et al. An assessment of urinary and breast milk iodine concentrations in lactating mothers from Gorgan, Iran, 2003. Thyroid. 2005;15(10):1165-1168. [DOI] [PubMed] [Google Scholar]
- 276. Jin Y, Coad J, Skeaff SA, Zhou SJ, Brough L. Iodine status of postpartum women and their infants aged 3, 6 and 12 months: Mother and Infant Nutrition Investigation (MINI). Br J Nutr. Published April 16, 2021. doi: 10.1017/S000711452100129X [DOI] [PubMed] [Google Scholar]
- 277. Dold S, Baumgartner J, Zeder C, et al. Optimization of a new mass spectrometry method for measurement of breast milk iodine concentrations and an assessment of the effect of analytic method and timing of within-feed sample collection on breast milk iodine concentrations. Thyroid. 2016;26(2):287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Neville MC, Keller RP, Seacat J, Casey CE, Allen JC, Archer P. Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr. 1984;40(3):635-646. [DOI] [PubMed] [Google Scholar]
- 279. Leung AM, Pearce EN, Hamilton T, et al. Colostrum iodine and perchlorate concentrations in Boston-area women: a cross-sectional study. Clin Endocrinol (Oxf). 2009;70(2):326-330. [DOI] [PubMed] [Google Scholar]
- 280. Moon S, Kim J. Iodine content of human milk and dietary iodine intake of Korean lactating mothers. Int J Food Sci Nutr. 1999;50(3):165-171. [DOI] [PubMed] [Google Scholar]
- 281. Zhao A, Ning Y, Zhang Y, et al. Mineral compositions in breast milk of healthy Chinese lactating women in urban areas and its associated factors. Chin Med J (Engl). 2014;127(14):2643-2648. [PubMed] [Google Scholar]
- 282. Mulrine HM, Skeaff SA, Ferguson EL, Gray AR, Valeix P. Breast-milk iodine concentration declines over the first 6 mo postpartum in iodine-deficient women. Am J Clin Nutr. 2010;92(4):849-856. [DOI] [PubMed] [Google Scholar]
- 283. Sabatier M, Garcia-Rodenas CL, De Castro CA, et al. Longitudinal changes of mineral concentrations in preterm and term human milk from lactating Swiss women. Nutrients. 2019;11(8):1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Siro SS, Baumgartner J, Schoonen M, et al. Characterization of genetic variants in the SLC5A5 gene and associations with breast milk iodine concentration in lactating women of African descent: the NUPED Study. Front Nutr. 2021;8:692504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Mughal BB, Fini JB, Demeneix BA. Thyroid-disrupting chemicals and brain development: an update. Endocr Connect. 2018;7(4):R160-R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. La Merrill MA, Vandenberg LN, Smith MT, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16(1):45-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Tonacchera M, Pinchera A, Dimida A, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14(12):1012-1019. [DOI] [PubMed] [Google Scholar]
- 288. Llorente-Esteban A, Manville RW, Reyna-Neyra A, Abbott GW, Amzel LM, Carrasco N. Allosteric regulation of mammalian Na+/I– symporter activity by perchlorate. Nat Struct Mol Biol. 2020;27(6):533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Kirk AB, Dyke JV, Ohira S, Dasgupta PK. Relative source contributions for perchlorate exposures in a lactating human cohort. Sci Total Environ. 2013;443:939-943. [DOI] [PubMed] [Google Scholar]
- 290. Lee SY, McCarthy AM, Stohl H, et al. Urinary iodine, perchlorate, and thiocyanate concentrations in U.S. lactating women. Thyroid. 2017;27(12):1574-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. Perchlorate and iodide in dairy and breast milk. Environ Sci Technol. 2005;39(7):2011-2017. [DOI] [PubMed] [Google Scholar]
- 292. Pearce EN, Leung AM, Blount BC, et al. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J Clin Endocrinol Metab. 2007;92(5):1673-1677. [DOI] [PubMed] [Google Scholar]
- 293. Leung AM, Braverman LE, He X, et al. Environmental perchlorate and thiocyanate exposures and infant serum thyroid function. Thyroid. 2012;22(9):938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Cao Y, Blount BC, Valentin-Blasini L, Bernbaum JC, Phillips TM, Rogan WJ. Goitrogenic anions, thyroid-stimulating hormone, and thyroid hormone in infants. Environ Health Perspect. 2010;118(9):1332-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Leung AM, Pearce EN, Braverman LE. Environmental perchlorate exposure: potential adverse thyroid effects. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Pleus RC, Corey LM. Environmental exposure to perchlorate: a review of toxicology and human health. Toxicol Appl Pharmacol. 2018;358:102-109. [DOI] [PubMed] [Google Scholar]
- 297. Laurberg P, Andersen S, Knudsen N, Ovesen L, Nøhr SB, Bülow Pedersen I. Thiocyanate in food and iodine in milk: from domestic animal feeding to improved understanding of cretinism. Thyroid. 2002;12(10):897-902. [DOI] [PubMed] [Google Scholar]
- 298. Chandra AK, Singh LH, Ghosh S, Pearce EN. Role of bamboo-shoot in the pathogenesis of endemic goiter in Manipur, North East India. Endocr Pract. 2013;19(1):36-45. [DOI] [PubMed] [Google Scholar]
- 299. Lazarus JH. Iodine and thiocyanate in goiter—house of bamboo. Endocr Pract. 2013;19(1):7-8. [DOI] [PubMed] [Google Scholar]
- 300. Dorea JG. Maternal thiocyanate and thyroid status during breast-feeding. J Am Coll Nutr. 2004;23(2):97-101. [DOI] [PubMed] [Google Scholar]
- 301. Oyelola OO, Ayangade SO, Oke OL. The possible role of cassava in the thiocyanate level of pregnant-women. Nutr Rep Int. 1983;28(3):585-592. [Google Scholar]
- 302. Laurberg P, Nøhr SB, Pedersen KM, Fuglsang E. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab. 2004;89(1):181-187. [DOI] [PubMed] [Google Scholar]
- 303. Ward MH, Jones RR, Brender JD, et al. Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health. 2018;15(7):1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Zhai Y, Lei Y, Wu J, et al. Does the groundwater nitrate pollution in China pose a risk to human health? A critical review of published data. Environ Sci Pollut Res Int. 2017;24(4):3640-3653. [DOI] [PubMed] [Google Scholar]
- 305. Jones JA, Hopper AO, Power GG, Blood AB. Dietary intake and bio-activation of nitrite and nitrate in newborn infants. Pediatr Res. 2015;77(1-2):173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141(11):2049-2054. [DOI] [PubMed] [Google Scholar]
- 307.US Centers for Disease Control and Prevention. Laboratory quality assurance and standardization programs. Ensuring the Quality of Urinary Iodine Procedures (EQUIP). Vol. 20212020. Accessed June 20, 2021. https://www.cdc.gov/labstandards/equip.html
- 308. Karmisholt J, Laurberg P, Andersen S. Recommended number of participants in iodine nutrition studies is similar before and after an iodine fortification programme. Eur J Nutr. 2014;53(2):487-492. [DOI] [PubMed] [Google Scholar]
- 309. Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70(10):553-570. [DOI] [PubMed] [Google Scholar]
- 310. Pino S, Fang SL, Braverman LE. Ammonium persulfate: a new and safe method for measuring urinary iodine by ammonium persulfate oxidation. Exp Clin Endocrinol Diabetes. 1998;106(Suppl 3):S22-S27. [DOI] [PubMed] [Google Scholar]
- 311. Caldwell KL, Maxwell CB, Makhmudov A, et al. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem. 2003;49(6 Pt 1):1019-1021. [DOI] [PubMed] [Google Scholar]
- 312. Delange F, Benker G, Caron P, et al. Thyroid volume and urinary iodine in European schoolchildren: standardization of values for assessment of iodine deficiency. Eur J Endocrinol. 1997;136(2):180-187. [DOI] [PubMed] [Google Scholar]
- 313. Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 2007;10(12A):1571-1580; discussion 1581. [DOI] [PubMed] [Google Scholar]
- 314. Dold S, Zimmermann MB, Jukic T, et al. Universal salt iodization provides sufficient dietary iodine to achieve adequate iodine nutrition during the first 1000 days: a cross-sectional multicenter study. J Nutr. 2018;148(4):587-598. [DOI] [PubMed] [Google Scholar]
- 315. Smyth PP, Smith DF, Sheehan S, Higgins M, Burns R, O’Herlihy C. Short-term changes in maternal and neonatal urinary iodine excretion. Thyroid. 2007;17(3):219-222. [DOI] [PubMed] [Google Scholar]
- 316. Nazeri P, Dalili H, Mehrabi Y, Hedayati M, Mirmiran P, Azizi F. Breast milk iodine concentration rather than maternal urinary iodine is a reliable indicator for monitoring iodine status of breastfed neonates. Biol Trace Elem Res. 2018;185(1):71-77. [DOI] [PubMed] [Google Scholar]
- 317. Hampel D, Dror DK, Allen LH. Micronutrients in human milk: analytical methods. Adv Nutr. 2018;9(Suppl 1):313S-331S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Levi M, Hjelm C, Harari F, Vahter M. ICP-MS measurement of toxic and essential elements in human breast milk. A comparison of alkali dilution and acid digestion sample preparation methods. Clin Biochem. 2018;53:81-87. [DOI] [PubMed] [Google Scholar]
- 319. Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev. 2001;59(8 Pt 1):269-278. [DOI] [PubMed] [Google Scholar]
- 320. Dorea JG. Iodine nutrition and breast feeding. J Trace Elem Med Biol. 2002;16(4):207-220. [DOI] [PubMed] [Google Scholar]
- 321. Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf). 2009;70(5):803-809. [DOI] [PubMed] [Google Scholar]
- 322. Ma ZF, Skeaff SA. Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid. 2014;24(8):1195-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Netzel BC, Grebe SK, Carranza Leon BG, et al. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab. 2015;100(8):E1074-E1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Stinca S, Andersson M, Erhardt J, Zimmermann MB. Development and validation of a new low-cost enzyme-linked immunoassay for serum and dried blood spot thyroglobulin. Thyroid. 2015;25(12):1297-1305. [DOI] [PubMed] [Google Scholar]
- 325. Spencer CA. Assay of thyroid hormones and related substances. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Thyroid disease manager. Endotext: MDText.com; Endocrine Educations Inc; 2017. Accessed June 7, 2021. https://www.endotext.org/section/thyroiddiseasemanager [Google Scholar]
- 326. Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab. 2006;91(12):4881-4887. [DOI] [PubMed] [Google Scholar]
- 327. Pezzino V, Filetti S, Belfiore A, Proto S, Donzelli G, Vigneri R. Serum thyroglobulin levels in the newborn. J Clin Endocrinol Metab. 1981;52(2):364-366. [DOI] [PubMed] [Google Scholar]
- 328. Djemli A, Van Vliet G, Belgoudi J, Lambert M, Delvin EE. Reference intervals for free thyroxine, total triiodothyronine, thyrotropin and thyroglobulin for Quebec newborns, children and teenagers. Clin Biochem. 2004;37(4):328-330. [DOI] [PubMed] [Google Scholar]
- 329. Sobrero G, Muñoz L, Bazzara L, et al. Thyroglobulin reference values in a pediatric infant population. Thyroid. 2007;17(11):1049-1054. [DOI] [PubMed] [Google Scholar]
- 330. Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl. 1999;88(432):21-24. [DOI] [PubMed] [Google Scholar]
- 331. Wassie MM, Yelland LN, Smithers LG, Ranieri E, Zhou SJ. Comparison of iodine status pre- and post-mandatory iodine fortification of bread in South Australia: a population study using newborn thyroid-stimulating hormone concentration as a marker. Public Health Nutr. 2019;22(16):3063-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab. 2010;24(1):63-75. [DOI] [PubMed] [Google Scholar]
- 333. Peters C, Brooke I, Heales S, et al. Defining the newborn blood spot screening reference interval for TSH: impact of ethnicity. J Clin Endocrinol Metab. 2016;101(9):3445-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Burns R, Mayne PD, O’Herlihy C, et al. Can neonatal TSH screening reflect trends in population iodine intake? Thyroid. 2008;18(8):883-888. [DOI] [PubMed] [Google Scholar]
- 335. Gruñeiro-Papendieck L, Chiesa A, Mendez V, Bengolea S, Prieto L. Neonatal TSH levels as an index of iodine sufficiency: differences related to time of screening sampling and methodology. Horm Res. 2004;62(6):272-276. [DOI] [PubMed] [Google Scholar]
- 336. Vandevijvere S, Coucke W, Vanderpas J, et al. Neonatal thyroid-stimulating hormone concentrations in Belgium: a useful indicator for detecting mild iodine deficiency? PLoS One. 2012;7(10):e47770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Mullan K, Patterson C, Doolan K, et al. Neonatal TSH levels in Northern Ireland from 2003 to 2014 as a measure of population iodine status. Clin Endocrinol (Oxf). 2018;89(6):849-855. [DOI] [PubMed] [Google Scholar]
- 338. Dorey CM, Zimmermann MB. Reference values for spot urinary iodine concentrations in iodine-sufficient newborns using a new pad collection method. Thyroid. 2008;18(3):347-352. [DOI] [PubMed] [Google Scholar]
- 339. Ittermann T, Völzke H, Krey A, et al. Median urinary iodine concentration reflected sufficient iodine supply in neonates from Northeast Germany in 2005-2006. Eur J Nutr. 2019;58(5):1815-1820. [DOI] [PubMed] [Google Scholar]
- 340. Clements FW, Moerloose JD, De Smet MP, eds. Endemic goitre. World Health Organization, Monograph Series No. 44. 1960. [PubMed] [Google Scholar]
- 341. WHO, UNICEF, ICCIDD. Global Prevalence of Iodine Deficiency Disorders. Micronutrient Deficiency Information System Working Paper 1. World Health Organization; 1993. [Google Scholar]
- 342. Zimmermann MB, Gizak M, Abbott K, Andersson M, Lazarus JH. Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol. 2015;3(9):672-674. [DOI] [PubMed] [Google Scholar]
- 343. Yan YQ, Chen ZP, Yang XM, et al. Attention to the hiding iodine deficiency in pregnant and lactating women after universal salt iodization: a multi-community study in China. J Endocrinol Invest. 2005;28(6):547-553. [DOI] [PubMed] [Google Scholar]
- 344. Wang Y, Zhang Z, Ge P, Wang Y, Wang S. Iodine status and thyroid function of pregnant, lactating women and infants (0-1 yr) residing in areas with an effective universal salt iodization program. Asia Pac J Clin Nutr. 2009;18(1):34-40. [PubMed] [Google Scholar]
- 345. Meng F, Zhao R, Liu P, Liu L, Liu S. Assessment of iodine status in children, adults, pregnant women and lactating women in iodine-replete areas of China. PLoS One. 2013;8(11):e81294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Mao G, Ding G, Lou X, et al. Survey of iodine nutritional status in 2011, Zhejiang, China. Asia Pac J Clin Nutr. 2015;24(2):234-244. [DOI] [PubMed] [Google Scholar]
- 347. Andersson M, Hunziker S, Fingerhut R, Zimmermann MB, Herter-Aeberli I. Effectiveness of increased salt iodine concentration on iodine status: trend analysis of cross-sectional national studies in Switzerland. Eur J Nutr. 2020;59(2):581-593. [DOI] [PubMed] [Google Scholar]
- 348. Petersen E, Thorisdottir B, Thorsdottir I, et al. Iodine status of breastfed infants and their mothers’ breast milk iodine concentration. Matern Child Nutr. 2020;16(3):e12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Manousou S, Augustin H, Eggertsen R, Hulthén L, Filipsson Nyström H. Inadequate iodine intake in lactating women in Sweden: a pilot 1-year, prospective, observational study. Acta Obstet Gynecol Scand. 2021;100(1):48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Groufh-Jacobsen S, Mosand LM, Bakken KS, et al. Mild to moderate iodine deficiency and inadequate iodine intake in lactating women in the inland area of Norway. Nutrients. 2020;12(3):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Aakre I, Morseth MS, Dahl L, et al. Iodine status during pregnancy and at 6 weeks, 6, 12 and 18 months post-partum. Matern Child Nutr. 2021;17(1):e13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Dumrongwongsiri O, Chatvutinun S, Phoonlabdacha P, et al. High urinary iodine concentration among breastfed infants and the factors associated with iodine content in breast milk. Biol Trace Elem Res. 2018;186(1):106-113. [DOI] [PubMed] [Google Scholar]
- 353. Gordon JH, Leung AM, Hale AR, et al. No difference in urinary iodine concentrations between Boston-area breastfed and formula-fed infants. Thyroid. 2014;24(8):1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Smyth PP, Hetherton AM, Smith DF, Radcliff M, O’Herlihy C. Maternal iodine status and thyroid volume during pregnancy: correlation with neonatal iodine intake. J Clin Endocrinol Metab. 1997;82(9):2840-2843. [DOI] [PubMed] [Google Scholar]
- 355. Barr ML, Chiu HK, Li N, et al. Thyroid dysfunction in children exposed to iodinated contrast media. J Clin Endocrinol Metab. 2016;101(6):2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Rosenberg V, Michel A, Chodick G, et al. Hypothyroidism in young children following exposure to iodinated contrast media: an observational study and a review of the literature. Pediatr Endocrinol Rev. 2018;16(2):256-265. [DOI] [PubMed] [Google Scholar]
- 357. Jick SS, Hedderson M, Xu F, Cheng Y, Palkowitsch P, Michel A. Iodinated contrast agents and risk of hypothyroidism in young children in the United States. Invest Radiol. 2019;54(5):296-301. [DOI] [PubMed] [Google Scholar]
- 358. Liu L, Liu J, Wang D, Shen H, Jia Q. Effect of urinary iodine concentration in pregnant and lactating women, and in their infants residing in areas with excessive iodine in drinking water in Shanxi Province, China. Biol Trace Elem Res. 2020;193(2):326-333. [DOI] [PubMed] [Google Scholar]
- 359. Eriksen KG, Andersson M, Hunziker S, Zimmermann MB, Moore SE. Effects of an iodine-containing prenatal multiple micronutrient on maternal and infant iodine status and thyroid function: a randomized trial in The Gambia. Thyroid. 2020;30(9):1355-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Aakre I, Markhus MW, Kjellevold M, Moe V, Smith L, Dahl L. Sufficient iodine status among Norwegian toddlers 18 months of age—cross-sectional data from the Little in Norway Study. Food Nutr Res. 2018;62. doi: 10.29219/fnr.v62.1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Hess SY, Abbeddou S, Yakes Jimenez E, Ouédraogo JB, Brown KH. Iodine status of young Burkinabe children receiving small-quantity lipid-based nutrient supplements and iodised salt: a cluster-randomised trial. Br J Nutr. 2015;114(11):1829-1837. [DOI] [PubMed] [Google Scholar]
- 362. Skeaff SA, Ferguson EL, McKenzie JE, Valeix P, Gibson RS, Thomson CD. Are breast-fed infants and toddlers in New Zealand at risk of iodine deficiency? Nutrition. 2005;21(3):325-331. [DOI] [PubMed] [Google Scholar]
- 363. Kanaka C, Schütz B, Zuppinger KA. Risks of alternative nutrition in infancy: a case report of severe iodine and carnitine deficiency. Eur J Pediatr. 1992;151(10):786-788. [DOI] [PubMed] [Google Scholar]
- 364. Müller P. Vegan diet in young children. Nestle Nutr Inst Workshop Ser. 2020;93:103-110. [DOI] [PubMed] [Google Scholar]
- 365. Sutter DO, Bender N. Nutrient status and growth in vegan children. Nutr Res. 2021;91:13-25. [DOI] [PubMed] [Google Scholar]
- 366. UNICEF. Guidance on the Monitoring of Salt Iodization Programmes and Determination of Population Iodine Status. United Nations Children’s Fund; 2018. [Google Scholar]
- 367. Global Fortification Data Exchange (GFDx). Vol. 2021. Accessed February 11, 2021. http://www.fortificationdata.org
- 368. Chan SS, Hams G, Wiley V, Wilcken B, McElduff A. Postpartum maternal iodine status and the relationship to neonatal thyroid function. Thyroid. 2003;13(9):873-876. [DOI] [PubMed] [Google Scholar]
- 369. Huynh D, Condo D, Gibson R, Makrides M, Muhlhausler B, Zhou SJ. Comparison of breast-milk iodine concentration of lactating women in Australia pre and post mandatory iodine fortification. Public Health Nutr. 2017;20(1):12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Jorgensen A, O’Leary P, James I, Skeaff S, Sherriff J. Assessment of breast milk iodine concentrations in lactating women in Western Australia. Nutrients. 2016;8(11):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. WHO, UNICEF. Reaching Optimal Iodine Nutrition in Pregnant and Lactating Women and Young Children. Joint Statement by the World Health Organization and the United Nations Children Fund. World Health Organization; 2007. [Google Scholar]
- 372. National Health and Medical Research Council (NHMRC). Iodine supplementation for pregnant and breastfeeding women. NHMRC public statement, January 2010. Accessed June 23, 2021. https://www.nhmrc.gov.au/about-us/publications/iodine-supplementation-pregnant-and-breastfeeding-women#
- 373. Reynolds AN, Skeaff SA. Maternal adherence with recommendations for folic acid and iodine supplements: a cross-sectional survey. Aust N Z J Obstet Gynaecol. 2018;58(1):125-127. [DOI] [PubMed] [Google Scholar]
- 374. Gupta PM, Gahche JJ, Herrick KA, Ershow AG, Potischman N, Perrine CG. Use of iodine-containing dietary supplements remains low among women of reproductive age in the United States: NHANES 2011-2014. Nutrients. 2018;10(4):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Threapleton DE, Waiblinger D, Snart CJP, et al. Prenatal and postpartum maternal iodide intake from diet and supplements, urinary iodine and thyroid hormone concentrations in a region of the United Kingdom with mild-to-moderate iodine deficiency. Nutrients. 2021;13(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Pedersen KM, Laurberg P, Iversen E, et al. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab. 1993;77(4):1078-1083. [DOI] [PubMed] [Google Scholar]
- 377. Harding KB, Peña-Rosas JP, Webster AC, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev. 2017;3(3):CD011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Antonangeli L, Maccherini D, Cavaliere R, et al. Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: a longitudinal study. Eur J Endocrinol. 2002;147(1):29-34. [DOI] [PubMed] [Google Scholar]
- 379. Mridha MK, Matias SL, Paul RR, et al. Daily consumption of lipid-based nutrient supplements containing 250 μg iodine does not increase urinary iodine concentrations in pregnant and postpartum women in Bangladesh. J Nutr. 2017;147(8):1586-1592. [DOI] [PubMed] [Google Scholar]
- 380. Gebreegziabher T, Woltamo T, Thomas DG, Kennedy TS, Stoecker BJ. Iodine supplementation of lactating women and assessment of infant visual information processing and maternal and infant thyroid function: a randomized trial. PLoS One. 2019;14(10):e0223348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Chierici R, Saccomandi D, Vigi V. Dietary supplements for the lactating mother: influence on the trace element content of milk. Acta Paediatr Suppl. 1999;88(430):7-13. [DOI] [PubMed] [Google Scholar]
- 382. Kevany J, Fierro-Benitez R, Pretell EA, Stanbury JB. Prophylaxis and treatment of endemic goiter with iodized oil in rural Ecuador and Peru. Am J Clin Nutr. 1969;22(12):1597-1607. [DOI] [PubMed] [Google Scholar]
- 383. Taffs RE, Enterline JC, Rusmil K, et al. Oral iodine supplementation does not reduce neutralizing antibody responses to oral poliovirus vaccine. Bull World Health Organ. 1999;77(6):484-491. [PMC free article] [PubMed] [Google Scholar]
- 384. Laurberg P, Andersen SL. Nutrition: breast milk—a gateway to iodine-dependent brain development. Nat Rev Endocrinol. 2014;10(3):134-135. [DOI] [PubMed] [Google Scholar]
- 385. Gahche JJ, Herrick KA, Potischman N, Bailey RL, Ahluwalia N, Dwyer JT. Dietary supplement use among infants and toddlers aged < 24 months in the United States, NHANES 2007-2014. J Nutr. 2019;149(2):314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. WHO. WHO Guideline: Use of Multiple Micronutrient Powders for Point-of-Use Fortification of Foods Consumed by Infants and Young Children Aged 6-23 Months and Children Aged 2-12 Years. World Health Organization; 2016. [PubMed] [Google Scholar]
- 387. Thomassen RA, Kvammen JA, Eskerud MB, Júlíusson PB, Henriksen C, Rugtveit J. Iodine status and growth in 0-2-year-old infants with cow’s milk protein allergy. J Pediatr Gastroenterol Nutr. 2017;64(5):806-811. [DOI] [PubMed] [Google Scholar]
- 388. Home Fortification Technical Advisory Group. Projects.2019. Accessed March 21, 2019. http://www.hftag.org/2157_Projects.asp
- 389. Home Fortification Technical Advisory Group. Manual on Micronutrient Powder (MNPs) Composition. Home Fortification Technical Advisory Group; 2013. [Google Scholar]
- 390. Szymlek-Gay EA, Gray AR, Heath ALM, Ferguson EL, Edwards T, Skeaff SA. Iodine-fortified toddler milk improves dietary iodine intakes and iodine status in toddlers: a randomised controlled trial. Eur J Nutr. 2020;59(3):909-919. [DOI] [PubMed] [Google Scholar]
- 391. Delange F, Wolff P, Gnat D, Dramaix M, Pilchen M, Vertongen F. Iodine deficiency during infancy and early childhood in Belgium: does it pose a risk to brain development? Eur J Pediatr. 2001;160(4):251-254. [DOI] [PubMed] [Google Scholar]
- 392. Wijaya-Erhardt M, Untoro J, Karyadi E, Wibowo L, Gross R. Efficacy of daily and weekly multiple micronutrient food-like tablets for the correction of iodine deficiency in Indonesian males aged 6-12 mo. Am J Clin Nutr. 2007;85(1):137-143. [DOI] [PubMed] [Google Scholar]
- 393. Chung HR. Screening and management of thyroid dysfunction in preterm infants. Ann Pediatr Endocrinol Metab. 2019;24(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Ares S, Escobar-Morreale HF, Quero J, et al. Neonatal hypothyroxinemia: effects of iodine intake and premature birth. J Clin Endocrinol Metab. 1997;82(6):1704-1712. [DOI] [PubMed] [Google Scholar]
- 395. Ibrahim M, de Escobar GM, Visser TJ, et al. Iodine deficiency associated with parenteral nutrition in extreme preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F56-F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Eerdekens A, Langouche L, Van den Berghe G, Verhaeghe J, Naulaers G, Vanhole C. Review shows that thyroid hormone substitution could benefit transient hypothyroxinaemia of prematurity but treatment strategies need to be clarified. Acta Paediatr. 2019;108(5):792-805. [DOI] [PubMed] [Google Scholar]
- 397. Williams FLR, Lindgren A, Watson J, Boelen A, Cheetham T. Thyroid function in preterm infants and neurodevelopment at 2 years. Arch Dis Child Fetal Neonatal Ed. 2020;105(5):504-509. [DOI] [PubMed] [Google Scholar]
- 398. Ng SM, Turner MA, Weindling AM. Neurodevelopmental outcomes at 42 months after thyroxine supplementation in infants below 28 weeks’ gestation: a randomized controlled trial. Thyroid. 2020;30(7):948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F21-F28. [DOI] [PubMed] [Google Scholar]
- 400. Cherella CE, Breault DT, Thaker V, Levine BS, Smith JR. Early identification of primary hypothyroidism in neonates exposed to intralymphatic iodinated contrast: a case series. J Clin Endocrinol Metab. 2018;103(10):3585-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Breen CM, Salama MF, Boyle MA. Severe hypothyroidism following a single topical exposure to iodine in a premature neonate. BMJ Case Rep. 2021;14(2):e240006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Ares S, Quero J, de Escobar GM. Iodine balance, iatrogenic excess, and thyroid dysfunction in premature newborns. Semin Perinatol. 2008;32(6):407-412. [DOI] [PubMed] [Google Scholar]
- 403. Arslanoglu S, Boquien CY, King C, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front Pediatr. 2019;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Koo W, Tice H. Human milk fortifiers do not meet the current recommendation for nutrients in very low birth weight infants. JPEN J Parenter Enteral Nutr. 2018;42(4):813-820. [DOI] [PubMed] [Google Scholar]
- 405. Greene HL, Hambidge KM, Schanler R, Tsang RC. Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: report of the Subcommittee on Pediatric Parenteral Nutrient Requirements from the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition. Am J Clin Nutr. 1988;48(5):1324-1342. [DOI] [PubMed] [Google Scholar]
- 406. Domellöf M, Szitanyi P, Simchowitz V, Franz A, Mimouni F; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition . ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: iron and trace minerals. Clin Nutr. 2018;37(6 Pt B):2354-2359. [DOI] [PubMed] [Google Scholar]
- 407. Agostoni C, Buonocore G, Carnielli VP, et al. ; ESPGHAN Committee on Nutrition . Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85-91. [DOI] [PubMed] [Google Scholar]
- 408. Belfort MB, Pearce EN, Braverman LE, He X, Brown RS. Low iodine content in the diets of hospitalized preterm infants. J Clin Endocrinol Metab. 2012;97(4):E632-E636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Rogahn J, Ryan S, Wells J, et al. Randomised trial of iodine intake and thyroid status in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F86-F90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Williams FLR, Ogston S, Hume R, et al. Supplemental iodide for preterm infants and developmental outcomes at 2 years: an RCT. Pediatrics. 2017;139(5):e20163703. [DOI] [PubMed] [Google Scholar]
- 411. Walsh V, Brown JVE, McGuire W. Iodine supplementation for the prevention of mortality and adverse neurodevelopmental outcomes in preterm infants. Cochrane Database Syst Rev. 2019;2(2):CD005253. [DOI] [PMC free article] [PubMed] [Google Scholar]