Abstract
Background
Disrupted and delayed Medicaid coverage has been consistently associated with lower rates of cancer screening and early-stage cancer diagnosis compared with continuous coverage. However, the relationships between Medicaid coverage timing, breast cancer treatment delays, and survival are less clear.
Methods
Using the linked Missouri Cancer Registry-Medicaid claims data, we identified 4583 women diagnosed with breast cancer between 2007 and 2016. We used logistic regression to estimate odds ratios (ORs) of late-stage diagnosis and treatment delays for prediagnosis (>30 days, >90 days, and >1 year before diagnosis) vs peridiagnosis enrollment. Cox proportional hazards models were used to estimate the hazard ratio (HR) of breast cancer-specific mortality for pre- vs postdiagnosis enrollment.
Results
Patients enrolled in Medicaid more than 30 days before diagnosis were less likely to be diagnosed at a late stage compared with those enrolled in Medicaid peridiagnosis (OR = 0.69, 95% confidence interval [CI] = 0.60 to 0.79). This result persisted using enrollment 90-day (OR = 0.64, 95% CI = 0.56 to 0.74) and 1-year thresholds (OR = 0.55, 95% CI = 0.47 to 0.65). We did not observe a difference in the likelihood of treatment delays between the 2 groups. After adjustment for sociodemographic factors, there was no statistically significant difference in the risk of breast cancer mortality for patients enrolled more than 30 days prediagnosis relative to patients enrolled peridiagnosis (HR = 0.98, 95% CI = 0.83 to 1.14), but a lower risk was observed for patients enrolled prediagnosis when using 90 days (HR = 0.85, 95% CI = 0.72 to 0.999) or 1 year (HR = 0.79, 95% CI = 0.66 to 0.96) as the threshold.
Conclusions
Women with breast cancer who enroll in Medicaid earlier may benefit from earlier diagnoses, but only longer-term enrollment may have survival benefits.
Breast cancer is the most commonly diagnosed cancer and second-leading cause of cancer-related death among women in the United States (1). Despite improvements in screening and treatment that have reduced breast cancer mortality, disparities still exist in early-stage diagnosis, treatment, and survival by geography, race and ethnicity, socioeconomic status (SES), and access to health insurance and preventive care (2-8). Given the particularly high burden of breast cancer among uninsured, low-SES women, programs such as Medicaid are important for improving cancer screening, treatment, and outcomes. Following the implementation of the Affordable Care Act, Medicaid-expanding states saw greater decreases in uninsured rates and advanced-stage breast cancer diagnoses compared with non–Medicaid-expanding states (9). Furthermore, Medicaid-expanding states saw larger increases in screening mammography rates among low-income women compared with nonexpansion states (10). Among Hispanic and non-Hispanic Black breast cancer survivors, the risk of being uninsured decreased and mammogram usage increased after Medicaid expansion (11).
Although Medicaid-insured patients have better survival than uninsured patients, they present with more advanced-stage breast cancer and poorer survival than privately insured patients (12-18). Medicaid-insured patients also have lower breast cancer screening rates and larger time gaps between mammograms (19). These findings may be partly explained by less coverage continuity under Medicaid compared with private insurance. In many states, patients may not become eligible for Medicaid until after a new cancer diagnosis and consequently may not benefit from regular screening and preventive care afforded to those with continuous coverage. Disrupted and delayed Medicaid coverage have been consistently associated with lower rates of cancer screening and greater likelihood of advanced-stage cancer than continuous coverage (20). However, the relationships between Medicaid coverage timing and breast cancer treatment delays and survival are less clear.
Therefore, we examined associations between timing of Medicaid enrollment relative to breast cancer diagnosis and late-stage diagnosis, treatment delays, and survival in Missouri, which had not yet expanded Medicaid in the study period of 2007-2016. Before the implementation of Medicaid expansion in October 2021, nondisabled adults with children qualified for MO HealthNet (Missouri Medicaid) if their incomes were at or below 21% of the federal poverty level (FPL) (21). Low-income adults can also qualify for Medicaid if they are living with a disability, blind, pregnant, or older than age 65 years. Additionally, through Missouri’s Show Me Healthy Women (Breast and Cervical Cancer Control [BCCCP]) Program, uninsured women aged 35-65 years with incomes at or below 200% of FPL can receive breast and cervical cancer screening and, if diagnosed with breast and/or cervical cancer, may then become eligible for Medicaid to cover cancer treatment.
Methods
Study Population
We used Medicaid administrative claims to identify women enrolled in the fee-for-service program and diagnosed with invasive breast cancer in Missouri between 2007 and 2016. Medicaid claims contain emergency, inpatient, and outpatient services and prescription drugs. We then linked claims of identified cases to the Missouri Cancer Registry data using Link Plus V. 2.0 (Centers for Disease Control and Prevention). Following the data collection and coding rules set by the National Program of Cancer Registries, the Missouri Cancer Registry collects data on demographic factors (race, ethnicity, age, marital status, sex), address at time of diagnosis, tumor characteristics (date of diagnosis, primary site, tumor grade, tumor size), and tumor-directed treatment modalities (surgery, radiotherapy, chemotherapy, and hormone therapy) and their start dates for more than 95% of incident cases in Missouri. A probabilistic matching approach was used, and matching variables included unique departmental control numbers, first and last name, social security number, date of birth, and race or ethnicity (22). The study was approved by the institutional review board at Washington University School of Medicine in St. Louis, the Missouri Department of Health and Senior Services, and the Missouri Department of Social Services.
We identified 4583 women aged 18-65 years who were enrolled in Medicaid and diagnosed with breast cancer between January 1, 2007, and December 31, 2015. We excluded patients with missing stage at diagnosis (n = 41) from the analysis of late-stage diagnosis and patients with missing treatment time information (n = 59) from the analysis of treatment delay.
Medicaid Enrollment
If a patient was enrolled in Medicaid more than 30 days before the date of cancer diagnosis, we referred to her as “enrolled before diagnosis.” If a patient was enrolled within 30 days before their diagnosis or later, we considered her “enrolled peridiagnosis.” We made this determination partly because 1 month was a common cutoff point in similar research in other states (23-26), and MO HealthNet applications typically take up to 30 days for approval. However, we also used 90 days and 1 year as cutoff points (used in similar research) to define “enrolled before diagnosis” (27-30).
Late-Stage Diagnosis, Treatment Delays, and Breast Cancer-Specific Mortality
Stage at diagnosis was coded as I to IV based on the eighth edition of American Joint Committee on Cancer Staging Atlas, and stages III and IV were considered late stages. Treatment was considered delayed if the number of days from diagnosis to any treatment (including definitive surgery, radiation therapy, chemotherapy, and endocrine therapy) was greater than 60, based on research showing statistically significantly higher mortality risk associated with treatment delays longer than 60 days (31). Missouri’s state vital records and the National Death Index were used to determine patients’ vital status, and death certificates were used to identify a single, disease-specific underlying cause of death. Individual data were censored if death occurred by causes other than breast cancer or if the individual survived beyond the end of the study period. We calculated follow-up time from the date of diagnosis to the date of whichever of the following events occurred first: death, last contact, or December 31, 2016.
Covariates
Sociodemographic variables included age (continuous), race (non-Hispanic Black, non-Hispanic White, non-Hispanic Black, or others [including Asian, Hispanic and unknown]), and marital status (married or domestic partner; unmarried, divorced, separated, or widowed; or missing). We examined 10 neighborhood-level socioeconomic covariates based on residential census tracts of patients at the time of diagnosis, including proportions of the population that were African American, below the FPL, without a high school diploma, unemployed, living in renter-occupied housing, living in single-parent households, without a car, and aged 65 years and older, as well as median household income and median home value. The census tract–level covariates were obtained from 2010 Census estimates based on 2005-2009 (for cases with 2000 residential census tract code available) and 2008-2012 (for cases with 2010 residential census tract code available) American Community Survey data and categorized using their quartiles across the state of Missouri. Tumor characteristics included tumor size (<20 mm, 20-49 mm, ≥50 mm, or missing), grade (well-differentiated, intermediate, poor, or missing), and subtype (hormone receptor–positive, hormone receptor–negative, or missing). Treatment variables were coded based on whether patients received definitive surgery (no surgery, surgery, or missing), chemotherapy (yes, no, or missing), radiation (yes, no, or unknown), and hormone therapy (yes, no, or unknown).
Statistical Analyses
To examine the relationship between Medicaid enrollment time and late-stage diagnosis and treatment delay, we used logistic regression to estimate odds ratios (ORs) and associated 95% confidence intervals (CIs). The analysis of late-stage diagnosis was adjusted for age, race or ethnicity, marital status, and census tract–level socioeconomic variables. The analysis of treatment delays was further adjusted for tumor characteristics.
We used Cox proportional hazards regression to examine the relationship between Medicaid enrollment time and risk of breast cancer mortality. The proportional hazards assumption was examined visually using Kaplan-Meier curves. The model was first adjusted for individual and census tract–level socioeconomic characteristics, then further adjusted for tumor characteristics. The final model was further adjusted for treatment.
All analyses were performed in the R statistical programming environment. A 2-sided P less than .05 was considered statistically significant.
Results
Of the 4583 cases, 28.2% were non-Hispanic Black people, 69.8% were non-Hispanic White people, and 2% were people of other races and ethnicities. Most were unmarried (67.6%) and enrolled in Medicaid more than 30 days prediagnosis (52.9%) (Table 1). The mean age was 51.5 years. At the time of diagnosis, most women lived in census tracts above the median in terms of percentages of the population below the FPL (63.7%), without a high school diploma (60.2%), unemployed (63.7%), living in renter-occupied housing units (59.2%), and living in single-parent households with children (61.7%). Compared with patients enrolled peridiagnosis, patients enrolled in Medicaid more than 30 days prediagnosis were more likely to be non-Hispanic Black (29.7% vs 26.5%), unmarried (73.4% vs 61.1%), disabled or blind (75.5% vs 22.8%), and living in a census tract in the highest quartile in terms of percentage of population below the FPL (36.7% vs 30.7%). Patients enrolled prediagnosis were also less likely to participate in the BCCCP (12.7% vs 74.5%) and receive radiation therapy (56.6% vs 63.4%), chemotherapy (54.6% vs 71.2%), or hormone therapy (49.4% vs 53.2%). There was no statistically significant age difference between the 2 groups.
Table 1.
Characteristics of women diagnosed with breast cancer in Missouri from 2007 to 2015 by relative time of enrollment in Medicaid (n = 4583)
| Characteristics | Enrolled before diagnosisa | Enrolled peridiagnosisb | Total |
|---|---|---|---|
| No. of cases (%) | 2424 (52.9) | 2159 (47.1) | 4583 |
| Age, mean (SD), y | 51.7 (9.0) | 51.2 (8.3) | 51.5 (8.7) |
| Race and ethnicity, % | |||
| Non-Hispanic Black | 29.7 | 26.5 | 28.2 |
| Non-Hispanic White | 68.6 | 71.0 | 69.8 |
| Otherc | 1.6 | 2.5 | 2.0 |
| Marital status, % | |||
| Married | 24.4 | 37.2 | 30.4 |
| Unmarried | 73.4 | 61.1 | 67.6 |
| Unknown | 2.2 | 1.7 | 1.9 |
| Blind or disabled classification, % | 75.5 | 22.8 | 50.7 |
| BCCCP participation, % | 12.7 | 74.5 | 41.8 |
| Population under federal poverty lined, % | |||
| Quartile 1 (<7.43) | 10.1 | 15.4 | 12.6 |
| Quartile 2 (7.43 to <13.24) | 21.9 | 22.2 | 22.0 |
| Quartile 3 (13.24 to <21.10) | 29.4 | 30.2 | 29.8 |
| Quartile 4 (≥21.10) | 36.7 | 30.7 | 33.9 |
| Population without high school diplomad, % | |||
| Quartile 1 (<2.16) | 12.7 | 14.6 | 13.6 |
| Quartile 2 (2.16 to <4.21) | 24.2 | 24.9 | 24.5 |
| Quartile 3 (4.21 to <7.08) | 30.8 | 29.9 | 30.4 |
| Quartile 4 (≥7.08) | 30.4 | 29.2 | 29.8 |
| Unemployedd, % | |||
| Quartile 1 (<4.18) | 14.2 | 15.1 | 14.6 |
| Quartile 2 (4.18 to <6.20) | 19.7 | 20.4 | 20.0 |
| Quartile 3 (6.20 to <9.68) | 26.4 | 28.1 | 27.2 |
| Quartile 4 (≥9.68) | 37.9 | 35.0 | 36.5 |
| Living in renter-occupied housing unitsd, % | |||
| Quartile 1 (<17.42) | 13.0 | 19.2 | 15.9 |
| Quartile 2 (17.42 to <27.46) | 23.0 | 23.4 | 23.2 |
| Quartile 3 (27.46 to <43.05) | 30.8 | 31.3 | 31.0 |
| Quartile 4 (≥43.05) | 31.4 | 24.6 | 28.2 |
| Single-parent households with childrend, % | |||
| Quartile 1 (<20.33) | 11.8 | 15.9 | 13.8 |
| Quartile 2 (20.33 to <30.84) | 21.9 | 23.7 | 22.8 |
| Quartile 3 (30.84 to <46.67) | 26.7 | 25.6 | 26.2 |
| Quartile 4 (≥46.67) | 37.5 | 33.4 | 35.6 |
| Population with no card, % | |||
| Quartile 1 (<2.86) | 13.2 | 18.2 | 15.6 |
| Quartile 2 (2.86 to <5.47) | 21.7 | 23.1 | 22.3 |
| Quartile 3 (5.47 to <10.44) | 27.6 | 25.8 | 26.8 |
| Quartile 4 (≥10.44) | 35.6 | 31.4 | 33.7 |
| Population African Americand, % | |||
| Quartile 1 (<0.38) | 22.4 | 23.5 | 23.0 |
| Quartile 2 (0.38 to <2.64) | 19.6 | 20.9 | 20.2 |
| Quartile 3 (2.64 to <11.40) | 20.2 | 20.4 | 20.3 |
| Quartile 4 (≥11.40) | 35.9 | 33.7 | 34.9 |
| Population aged ≥65 yd, % | |||
| Quartile 1 (<9.67) | 24.4 | 25.4 | 24.9 |
| Quartile 2 (9.67 to <13.23) | 24.7 | 26.3 | 25.4 |
| Quartile 3 (13.23 to <17.20) | 25.9 | 25.9 | 25.9 |
| Quartile 4 (≥17.20) | 23.1 | 21.0 | 22.1 |
| Median household incomed, % | |||
| Quartile 1 (<$39 911) | 36.4 | 31.4 | 34.1 |
| Quartile 2 ($39 911 to <$51 258) | 29.3 | 29.9 | 29.6 |
| Quartile 3 ($51 258 to <$66 283) | 21.9 | 23.2 | 22.5 |
| Quartile 4 (≥$66 283) | 10.4 | 14.0 | 12.1 |
| Median home valued, % | |||
| Quartile 1 (<$82 500) | 34.5 | 30.7 | 32.7 |
| Quartile 2 ($82 500 to <$113 600) | 29.8 | 29.6 | 29.7 |
| Quartile 3 ($113 600 to <$157 000) | 22.7 | 24.3 | 23.4 |
| Quartile 4 (≥$157 000) | 10.9 | 14.0 | 12.4 |
| Cancer stage, % | |||
| 0 | 13.6 | 9.1 | 11.5 |
| I | 30.0 | 21.5 | 26.0 |
| II | 33.0 | 39.2 | 35.9 |
| III | 14.0 | 18.6 | 16.2 |
| IV | 8.4 | 10.8 | 9.5 |
| Unknown | 1.0 | 0.7 | 0.9 |
| Tumor size, % | |||
| ≤20 mm | 43.2 | 34.4 | 39.1 |
| 21-50 mm | 31.8 | 38.7 | 35.0 |
| >50 mm | 10.9 | 16.0 | 13.3 |
| Unknown | 14.2 | 10.9 | 12.6 |
| Tumor grade, % | |||
| Well differentiation | 16.4 | 13.7 | 15.1 |
| Intermediate differentiation | 34.8 | 36.0 | 35.4 |
| Poor differentiation | 40.8 | 44.7 | 42.6 |
| Unknown | 8.0 | 5.6 | 6.9 |
| Tumor subtype, % | |||
| ER+ or PR+ | 74.5 | 72.3 | 73.4 |
| ER− and PR− | 21.4 | 24.7 | 23.0 |
| Unknown | 4.1 | 3.1 | 3.6 |
| Surgical treatment, % | |||
| Yes | 92.7 | 92.5 | 92.6 |
| No | 6.8 | 7.1 | 7.0 |
| Unknown | 0.5 | 0.4 | 0.4 |
| Radiation therapy, % | |||
| Yes | 56.6 | 63.4 | 59.8 |
| No | 42.0 | 36.0 | 39.2 |
| Unknown | 1.4 | 0.6 | 1.0 |
| Chemotherapy, % | |||
| Yes | 54.6 | 71.2 | 62.4 |
| No | 43.1 | 27.7 | 35.8 |
| Unknown | 2.3 | 1.2 | 1.7 |
| Hormone therapy, % | |||
| Yes | 49.4 | 53.2 | 51.2 |
| No | 35.8 | 36.6 | 36.2 |
| Unknown | 14.8 | 10.2 | 12.7 |
Enrolled in Medicaid for longer than 30 days before the date of cancer diagnosis. BCCCP = Breast and Cervical Cancer Control Program; ER = estrogen receptor; PR = progesterone receptor.
Enrolled in Medicaid within 30 days before their cancer diagnosis or later.
Including Asian, Hispanic, and unknown.
Characteristics of census tract where patient was living at cancer diagnosis. Overall, census tract code was missing for 1.7% of patients, including 1.9% of patients enrolled before diagnosis and 1.4% or less of patients enrolled after diagnosis.
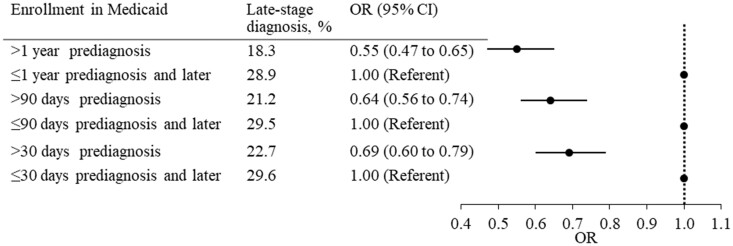
Late-stage diagnosis accounted for 22.7% of patients enrolled in Medicaid longer than 30 days prediagnosis and 29.6% of patients enrolled peridiagnosis. Patients enrolled longer than 30 days prediagnosis were less likely to be diagnosed at a late stage compared with those enrolled peridiagnosis (OR = 0.69, 95% CI = 0.60 to 0.79). This result held when we defined “enrolled before diagnosis” as being enrolled in Medicaid longer than 90 days (OR = 0.64, 95% CI = 0.56 to 0.74) or 1 year before diagnosis (OR = 0.55, 95% CI = 0.47 to 0.65) (Figure 1, Supplementary Tables 1 and 2, available online).
Figure 1.
Odds ratios (ORs) of late-stage diagnosis in women with breast cancer enrolled in Medicaid before diagnosis compared with those enrolled peridiagnosis. Late-stage diagnosis included stages III-IV. The analysis was adjusted for age, race, marital status, and socioeconomic characteristics of census tracts. CI = confidence interval.
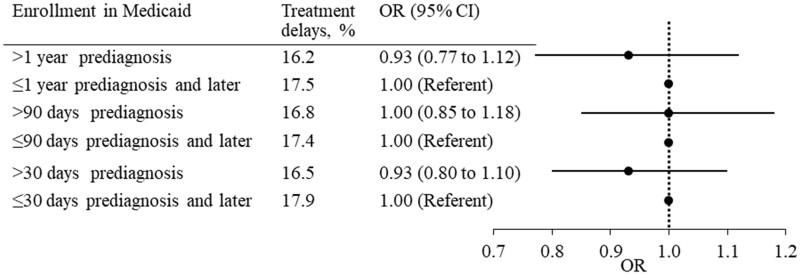
Overall, 16.5% of patients enrolled in Medicaid longer than 30 days prediagnosis and 17.9% of patients enrolled peridiagnosis experienced treatment delays longer than 60 days. There was no statistically significant difference in the likelihood of treatment delays between the 2 groups using 30 days (OR = 0.93, 95% CI = 0.80 to 1.10), 90 days (OR = 1.00, 95% CI = 0.85 to 1.18), or 1 year (OR = 0.93, 95% CI = 0.77 to 1.12) as the cutoff to define “enrolled before diagnosis” (Figure 2, Supplementary Tables 1 and 2, available online).
Figure 2.
Odds ratios (ORs) of treatment delays in women with breast cancer enrolled in Medicaid before diagnosis compared with those enrolled peridiagnosis. Treatment delays were defined as more than 60 days from diagnosis to surgery, radiation therapy, chemotherapy, or hormone therapy. The analysis was adjusted for age, race, marital status, socioeconomic characteristics of census tracts, tumor stage, size, grade, and hormone receptor subtypes. CI = confidence interval.
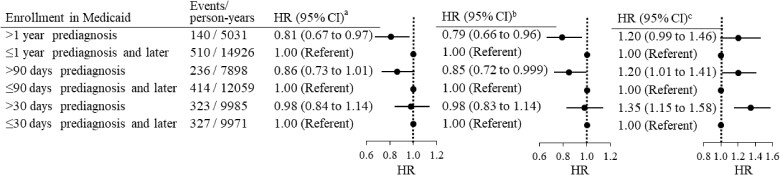
During an average 52-month follow-up, 939 (20.5%) patients died, of which 650 (14.2%) died from breast cancer. There was no statistically significant difference in the hazard ratio (HR) of breast cancer–specific mortality for patients enrolled in Medicaid longer than 30 days before diagnosis relative to patients enrolled peridiagnosis (HR = 0.98, 95% CI = 0.84 to 1.14). Adjustment for individual and neighborhood sociodemographic factors did not change the risk (HR = 0.98. 95% CI = 0.83 to 1.14). After adjusting additionally for cancer stage and other tumor characteristics, patients enrolled longer than 30 days before diagnosis had higher risk of mortality compared with patients enrolled peridiagnosis (HR = 1.35, 95% CI = 1.15 to 1.58) (Figure 3; Supplementary Table 3, available online). Further adjustment for treatment did not statistically significantly change the risk (HR = 1.33, 95% CI = 1.13 to 1.56) (Supplementary Figure 1, available online). Using a 90-day cutoff point to define pre- and peridiagnosis enrollment, we found patients enrolled prediagnosis had lower risk of mortality compared with those enrolled peridiagnosis (HR = 0.85, 95% CI = 0.72 to 0.99) after adjustment for individual and census tract–level sociodemographic factors. Further adjustment for tumor characteristics alone (Figure 3) or with treatment (Supplementary Figure 1, available online) increased the hazard ratio to 1.20 (95% CI = 1.01 to 1.41) and 1.16 (95% CI = 0.98 to 1.37), respectively. Similarly, enrollment longer than a year before diagnosis was associated with lower mortality risk after adjustment for sociodemographic factors (HR = 0.79, 95% CI = 0.66 to 0.96). This association became statistically non-significant after further adjustment for tumor characteristics (HR = 1.20, 95% CI = 0.99 to 1.46) (Figure 3) and treatment (HR = 1.15, 95% CI = 0.95 to 1.41) (Supplementary Figure 1, available online).
Figure 3.
Hazard ratios (HRs) of breast cancer–specific mortality in women with breast cancer enrolled in Medicaid before diagnosis compared with those enrolled peridiagnosis. aThe analysis was not adjusted for any covariates. bThe analysis was adjusted for age, race, marital status, and socioeconomic characteristics of census tracts. cThe analysis was further adjusted for tumor stage, size, grade, and hormone receptor subtypes. CI = confidence interval.
Discussion
In this study, we compared the likelihoods of late-stage diagnoses and treatment delays and survival of patients enrolled in Medicaid before or around a breast cancer diagnosis. Patients enrolled in Medicaid prediagnosis were less likely to be diagnosed at late stages. There was no statistically significant association between enrollment time and risk of treatment delays longer than 60 days. Importantly, longer than 90 days or 1 year of enrollment before cancer diagnosis was associated with lower risk of breast cancer–specific mortality when adjusting for sociodemographic factors alone, which disappeared or reversed after further adjustment for tumor characteristics. This suggests a potential confounding effect of tumor severity. Breast cancer mortality is more strongly influenced by pathological characteristics than Medicaid enrollment timing, particularly when the difference in durations of enrollment is shorter, such that adjustment for tumor factors leads to apparent reversal of the effects of Medicaid enrollment timing. Alternatively, this finding suggests earlier enrollment may reduce mortality by means of earlier diagnoses, because earlier insurance coverage allows patients better access to screening, prompt follow-up, and identification of tumors at earlier stages. Interestingly, longer durations of Medicaid coverage before diagnosis appeared to be associated with greater protective effects, with the odds ratio for late-stage diagnosis and the hazard ratio of breast cancer mortality decreasing as the time threshold for pre- vs peridiagnosis Medicaid enrollment increased from more than 30 days to 90 days to 1 year. Collectively, this suggests that patients who enroll in Medicaid earlier may benefit from earlier diagnoses of less aggressive tumors, but only longer-term continuous Medicaid enrollment may provide survival benefits.
Our findings align with previous research showing patients enrolling in Medicaid after a cancer diagnosis are more likely to be diagnosed at later stages, with more aggressive tumor subtypes compared with those enrolled before diagnosis (23,24,27-29,32). To our knowledge, however, only 1 study has examined the relationship between Medicaid enrollment timing and treatment delays, showing a higher likelihood of treatment delays for breast, cervical, and colorectal cancers in new Medicaid enrollees than longer-term enrollees (32). However, patients in that study were enrolled in managed care and fee-for-service programs, the latter of which was associated with higher odds for treatment delays. In our study, all patients were enrolled in fee-for-service programs, and Medicaid enrollment time was not related to treatment delays. Therefore, Medicaid program type should be considered in studies of impacts of Medicaid coverage timing on treatment initiation.
Few studies have examined the relationship between Medicaid coverage duration and breast cancer survival and with inconsistent results. In New Jersey, newly enrolled Medicaid patients with breast cancer had lower 2-year survival than patients enrolled more than 6 months before breast cancer diagnosis. However, the analysis was not adjusted for sociodemographic factors and Medicaid program type (fee for service vs managed care) (32). Bradley et al. (26) found no difference in mortality risk between patients enrolled in Medicaid 1 month and longer prediagnosis and those enrolled peridiagnosis in Michigan, which resembled our findings when using 30 days as the threshold for pre- and peridiagnosis coverage. Koroukian et al. (28) used longer than 3 months to define prediagnosis Medicaid coverage in Ohio and observed similar breast cancer mortality risk between pre- and peridiagnosis enrollment after adjustment for sociodemographic factors and cancer stage. However, this null finding might be driven by adjustment for cancer stage because late-stage diagnosis was more likely to occur in patients enrolled in Medicaid peridiagnosis.
There are several potential explanations for associations between earlier Medicaid enrollment and earlier-stage diagnoses and better survival. Earlier insurance coverage may allow better access to screening mammography and primary care (10,33,34), permitting prompter diagnoses, patient education, and management of concurrent illnesses (35,36). Increased primary care visits are associated with lower breast cancer mortality, mediated partly by earlier-stage diagnoses (37). Uninsured women may not present for medical care until experiencing symptoms, at which time their cancer may have progressed to a later stage. Lack of insurance coverage may also limit patients’ ability to obtain tumor biopsies and scans needed for diagnosis in the first place, so later Medicaid coverage may directly delay cancer workups.
Although not included in our analyses, BCCCP participation may have been associated with treatment delay and mortality. Patients who enrolled in Medicaid peridiagnosis were more likely to be BCCCP participants than were patients enrolled prediagnosis (74.5% vs 12.7%). The frequent delays associated with the process itself of applying for and being accepted by Missouri Medicaid (even after eligibility criteria are met) make it more likely that BCCCP participants do not enroll until at least the time around or after diagnosis. Compared with BCCCP participants, Medicaid enrollees who qualify under traditional eligibility categories have much lower incomes (≤21% vs ≤200% of the FPL) and are less likely to be disabled or blind. Consequently, BCCCP participants have relatively more financial resources and fewer comorbidities than patients who qualify outside of BCCCP, which could decrease risks of treatment delays and mortality independently of Medicaid enrollment timing and thus impact associations between Medicaid enrollment timing and these outcomes.
Medicaid plays a critical role in preventive care and early detection for breast cancer among low-SES women; however, increasing Medicaid coverage alone is insufficient for improving cancer treatment quality and survival. Our previous study in Missouri demonstrated that breast cancer patients with Medicaid were more likely than privately insured patients to receive a late-stage diagnosis and experience treatment delays (18). Medicaid expansion has not been associated with improved timeliness of treatment initiation despite its association with substantial increases in insurance coverage (38). Consequently, increased Medicaid coverage must be combined with additional forms of support for low-income patients to decrease socioeconomic disparities in the cancer care continuum. In adult Medicaid enrollees, availability and accommodation of health-care services are common nonfinancial barriers that contribute to unmet needs or delayed health care (39), which should be addressed to maximize benefits of Medicaid expansion for low-income patients.
There were limitations to this study. Because patients’ insurance status before Medicaid enrollment was unknown, we could not distinguish between patients who had been covered under private insurance before Medicaid enrollment and patients who had been chronically uninsured before Medicaid enrollment. Second, our results from patients enrolled in fee-for-service Medicaid may not apply to patients enrolled in managed care Medicaid. Third, lack of information on diagnostic methods limited our ability to examine the association between prediagnostic enrollment and screening detected tumors. Finally, women enrolled in Medicaid prediagnosis may have differed from women enrolled peridiagnosis in characteristics not captured in this study. Previous research suggests, for example, that adults with continuous Medicaid enrollment are more likely to have comorbidities and health limitations (40,41).
In conclusion, we found that patients enrolled in Medicaid before breast cancer diagnosis were less likely to be diagnosed at late stages compared with patients enrolled peridiagnosis. However, timing of Medicaid enrollment was not associated with risk of treatment delay, suggesting other factors may play a greater role in timely initiation of breast cancer treatment. Most importantly, our study provided evidence for improved breast cancer survival associated with longer-term continuous Medicaid enrollment before cancer diagnosis. These findings can help advise the design of interventions aimed at improving access to and outcomes of breast cancer care among underserved populations. This may entail Medicaid expansion so low-income women can receive greater insurance coverage continuity and better access to primary care and screenings. In states with expanded Medicaid, now including Missouri, patients will need further support to ensure effective and accessible cancer treatment.
Funding
This work was supported by the National Cancer Institute (R01CA215418, P30CA091842), the American Cancer Society (Denim Days Research Scholar Grant RSG-18–116-01-CPHPS), the Breast Cancer Research Foundation, and the Foundation for Barnes-Jewish Hospital.
Notes
Role of the funder: The funding agencies had no role in the study design; the collection, analysis, or interpretation of data; and the preparation, review, or decision to submit the manuscript for publication.
Disclosures: The authors declare no potential conflicts of interest.
Author contributions: Conceptualization: All authors; data curation: YL, ML, JL, CS, and TGR; formal analysis: EX; writing—original draft: EX and YL; writing—review and editing: All authors; funding acquisition: YL and ML; project administration: YL; supervision: YL.
Prior presentations: This work was presented as an abstract and virtual poster on October 6-8, 2021 at the 14th American Association for Cancer Research (AACR) Conference on the Science of Cancer Health Disparities under the abstract title, “Associations of timing of Medicaid enrollment with stage at diagnosis, treatment delays, and mortality in women with breast cancer.”
Data Availability
The original datasets used in this article were provided by the Missouri Cancer Registry and the MO HealthNet (Medicaid) Program with permission. Data will be shared on request to the corresponding author with permission of the Missouri Cancer Registry and the MO HealthNet Program.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7-–30.. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2. Williams F, Jeanetta S, James AS.. Geographical location and stage of breast cancer diagnosis: a systematic review of the literature. J Health Care Poor Underserved. 2016;27(3):1357-–1383.. doi: 10.1353/hpu.2016.0102. [DOI] [PubMed] [Google Scholar]
- 3. Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2020;124(2):315–318. doi: 10.1038/s41416-020-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson J, Sule AA.. Disparity in early detection of breast cancer. In: StatPearls.com. Treasure Island (FL: ): StatPearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/books/NBK564311/. Accessed December 5, 2021. [PubMed] [Google Scholar]
- 5. Dreyer MS, Nattinger AB, McGinley EL, Pezzin LE.. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. 2018;167(1):1–8. doi: 10.1007/s10549-017-4490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schueler KM, Chu PW, Smith-Bindman R.. Factors associated with mammography utilization: a systematic quantitative review of the literature. J Womens Health (Larchmt). 2008;17(9):1477–1498. doi: 10.1089/jwh.2007.0603. [DOI] [PubMed] [Google Scholar]
- 7. Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177(3):537–548. doi: 10.1007/s10549-019-05340-7. [DOI] [PubMed] [Google Scholar]
- 8. Orji CC, Kanu C, Adelodun AI, Brown CM.. Factors that influence mammography use for breast cancer screening among African American women. J Natl Med Assoc. 2020;112(6):578–592. doi: 10.1016/j.jnma.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 9. Blanc JML, Heller DR, Friedrich A, Lannin DR, Park TS.. Association of Medicaid expansion under the affordable care act with breast cancer stage at diagnosis. JAMA Surg. 2020;155(8):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toyoda Y, Oh EJ, Premaratne ID, Chiuzan C, Rohde CH.. Affordable care act state-specific Medicaid expansion: impact on health insurance coverage and breast cancer screening rate. J Am Coll Surg. 2020;230(5):775–783. doi: 10.1016/j.jamcollsurg.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 11. White-Means SI, Osmani AR.. Affordable care act and disparities in health services utilization among ethnic minority breast cancer survivors: evidence from longitudinal medical expenditure panel surveys 2008-2015. IJERPH. 2018;15(9):1860.doi: 10.3390/ijerph15091860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayanian JZ, Kohler BA, Abe T, Epstein AM.. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 13. Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32(28):3118–3125. doi:10.1200/J Clin Oncol.2014.55.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu CD, Wang X, Habif DV, Ma CX, Johnson KJ.. Breast cancer stage variation and survival in association with insurance status and sociodemographic factors in US women 18 to 64 years old. Cancer. 2017;123(16):3125–3131. doi: 10.1002/cncr.30722. [DOI] [PubMed] [Google Scholar]
- 15. Samiian L, Sharma P, Van Den Bruele AB, Smotherman C, Vincent M, Crandall M.. The effect of insurance and race on breast cancer tumor biology and short-term outcomes. Am Surg. 2018;84(7):1223–1228. doi: 10.1177/000313481808400743. [DOI] [PubMed] [Google Scholar]
- 16. Niu X, Roche LM, Pawlish KS, Henry KA.. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403–411. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ward EM, Fedewa SA, Cokkinides V, Virgo K.. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J Sudbury J. 2010;16(6):614–621. doi: 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 18. Berrian JL, Liu Y, Lian M, Schmaltz CL, Colditz GA.. Relationship between insurance status and outcomes for patients with breast cancer in Missouri. Cancer. 2021;127(6):931–937. doi: 10.1002/cncr.33330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonafede MM, Miller JD, Pohlman SK, et al. Breast, cervical, and colorectal cancer screening: patterns among women with Medicaid and commercial insurance. Am J Prev Med. 2019;57(3):394–402. doi: 10.1016/j.amepre.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yabroff KR, Reeder-Hayes K, Zhao J, et al. Health insurance coverage disruptions and cancer care and outcomes: systematic review of published research. J Natl Cancer Inst. 2020;112(7):671–687. doi: 10.1093/jnci/djaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brooks T, Gardner A, Tolbert J, Dolan R, Pham O. Medicaid and CHIP eligibility and enrollment policies as of January 2021: findings from a 50-state survey. KFF. March 2021. https://www.kff.org/medicaid/report/medicaid-and-chip-eligibility-and-enrollment-policies-as-of-january-2021-findings-from-a-50-state-survey/. Accessed December 25, 2021.
- 22. Homan SG, Yun S, Bouras A, Schmaltz C, Gwanfogbe P, Lucht J.. Breast cancer population screening program results in early detection and reduced treatment and health care costs for Medicaid. J Public Health Manag Pract. 2021;27(1):70–79. doi: 10.1097/PHH.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 23. Bradley CJ, Given CW, Roberts C.. Correlates of late stage breast cancer and death in a Medicaid-insured population. J Health Care Poor Underserved. 2003;14(4):503–515. doi: 10.1353/hpu.2010.0714. [DOI] [PubMed] [Google Scholar]
- 24. Bradley CJ, Given CW, Roberts C.. Late stage cancers in a Medicaid-insured population. Med Care. 2003;41(6):722–728. doi: 10.1097/01.MLR.0000065126.73750.D1. [DOI] [PubMed] [Google Scholar]
- 25. Keegan THM, Parsons HM, Chen Y, et al. Impact of health insurance on stage at cancer diagnosis among adolescents and young adults. J Natl Cancer Inst. 2019;111(11):1152–1160. doi: 10.1093/jnci/djz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradley CJ, Gardiner J, Given CW, Roberts C.. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005;103(8):1712–1718. doi: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- 27. Koroukian SM. Assessing the effectiveness of Medicaid in breast and cervical cancer prevention. J Public Health Manag Pract JPHMP. 2003;9(4):306–314. doi: 10.1097/00124784-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 28. Koroukian SM, Bakaki PM, Htoo PT, et al. The breast and cervical cancer early detection program, Medicaid, and breast cancer outcomes among Ohio’s underserved women. Cancer. 2017;123(16):3097–3106. doi: 10.1002/cncr.30720. [DOI] [PubMed] [Google Scholar]
- 29. Perkins CI, Wright WE, Allen M, Samuels SJ, Romano PS.. Breast cancer stage at diagnosis in relation to duration of Medicaid enrollment. Med Care. 2001;39(11):1224–1233. doi: 10.1097/00005650-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 30. Ramsey SD, Zeliadt SB, Richardson LC, et al. Disenrollment from Medicaid after recent cancer diagnosis. Med Care. 2008;46(1):49–57. doi: 10.1097/MLR.0b013e318158ec7f. [DOI] [PubMed] [Google Scholar]
- 31. McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED.. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol Off J Col. 2012;30(36):4493–4500. doi:10.1200/J Clin Oncol.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsui J, DeLia D, Stroup AM, et al. Association of Medicaid enrollee characteristics and primary care utilization with cancer outcomes for the period spanning Medicaid expansion in New Jersey. Cancer. 2019;125(8):1330–1340. doi: 10.1002/cncr.31824. [DOI] [PubMed] [Google Scholar]
- 33. Villarroel MA, Cohen RA.. Health insurance continuity and health care access and utilization, 2014. NCHS Data Brief. 2016;249:1–8. [PubMed] [Google Scholar]
- 34. Fedewa SA, Yabroff KR, Smith RA, Goding Sauer A, Han X, Jemal A.. Changes in breast and colorectal cancer screening after Medicaid expansion under the affordable care act. Am J Prev Med. 2019;57(1):3–12. doi: 10.1016/j.amepre.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 35. Smith GF, Toonen TR.. The role of the primary care physician during the active treatment phase. Prim Care. 2009;36(4):685–702. doi: 10.1016/j.pop.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 36. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roetzheim RG, Ferrante JM, Lee JH, et al. Influence of primary care on breast cancer outcomes among Medicare beneficiaries. Ann Fam Med. 2012;10(5):401–411. doi: 10.1370/afm.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takvorian SU, Oganisian A, Mamtani R, et al. Association of Medicaid expansion under the affordable care act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Netw Open. 2020;3(2):e1921653.doi: 10.1001/jamanetworkopen.2019.21653. [DOI] [PubMed] [Google Scholar]
- 39. Kullgren JT, McLaughlin CG, Mitra N, Armstrong K.. Nonfinancial barriers and access to care for U.S. adults. Health Serv Res. 2012;47(1, Pt 2):462–485. doi: 10.1111/j.1475-6773.2011.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roberts ET, Pollack CE.. Does churning in Medicaid affect health care use? Med Care. 2016;54(5):483–489. doi: 10.1097/MLR.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sommers BD. Loss of health insurance among non-elderly adults in Medicaid. J Gen Intern Med. 2009;24(1):1–7. doi: 10.1007/s11606-008-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original datasets used in this article were provided by the Missouri Cancer Registry and the MO HealthNet (Medicaid) Program with permission. Data will be shared on request to the corresponding author with permission of the Missouri Cancer Registry and the MO HealthNet Program.