Abstract
Background
The inverse association between ideal cardiovascular health (CVH) as measured by the American Heart Association’s Life Simple 7 (LS7) and cardiovascular disease (CVD) incidence is well documented. However, research exploring the association between CVH and specific risk factors for cardiometabolic disease is sparse in diverse cohorts.
Methods
This study included 7717 participants from the Mediators of Atherosclerosis in South Asians Living in America and the Multi-Ethnic Study of Atherosclerosis cohorts. We assigned each LS7 component a 0, 1, and 2 and summed these scores to derive an overall CVH score. Visceral, subcutaneous, and intermuscular fat area, pericardial fat volume, and hepatic fat attenuation were measured using noncontrast computed tomography. Multivariable linear regression was used to examine associations between CVH categories and each log-transformed ectopic fat depot, as well as the homeostatic assessment for insulin resistance (HOMA-IR).
Results
In adjusted analysis, compared to those with ideal CVH, participants with poor CVH demonstrated 63.4% (95% CI, 54.3-73.0) higher visceral fat area, 84.0% (95% CI, 76.5-92.1) higher pericardial fat volume, 61.6% (95% CI, 50.7-73.2) higher subcutaneous fat area, and 40.6% (95% CI, 30.2-52.0) higher intermuscular fat area, and 15.1% (95% CI, 13.1-17.2) higher hepatic fat (all Ps < 0.001). Also, poor CVH was associated with 148.2% (95% CI, 131.1-166.7) higher HOMA-IR. We also found significant heterogeneity in the strengths of association by race/ethnicity for each ectopic fat depot.
Conclusion
Poor and intermediate CVH, as defined by LS7 metrics, were associated with significantly higher measures of ectopic fat and insulin resistance among individuals from 5 racial/ethnic groups.
Keywords: ectopic fat, insulin resistance, Life’s Simple 7, cardiovascular health
Cardiometabolic diseases are the leading causes of death and disability worldwide (1). Insulin resistance [as estimated by the homeostasis model assessment of insulin resistance (HOMA-IR)] has been shown to be a risk factor for the development of cardiometabolic diseases (2, 3). In this regard, numerous studies demonstrate an association between insulin resistance and accumulation of excess lipids in and around organs such as the liver, skeletal muscle, intestines, and heart (ie, ectopic fat deposition) (4, 5). These ectopic fat depots are associated with a wide range of cardiovascular risk factors and events (6-8). Moreover, there are significant racial/ethnic differences in ectopic fat deposition, which may predispose some of these groups to an increased risk of cardiometabolic disease (9).
In 2010, the American Heart Association declared its strategic impact goal, which stated: “By 2020, to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular diseases (CVD) and stroke by 20%.” As a result, the Life’s Simple 7 (LS7) metrics were introduced to measure and monitor the cardiovascular health (CVH) of individuals and populations where ideal CVH is defined as the presence of both ideal health behaviors [nonsmoking, body mass index (BMI) < 25 kg/m2, physical activity at goal levels, and diet consistent with guidelines] and ideal health factors (untreated total cholesterol ≤ 200 mg/dL, untreated blood pressure < 120/80 mmHg, and untreated fasting blood glucose < 100 mg/dL) (10). The inverse association between ideal CVH and CVD incidence is well documented (11-15). However, research exploring the association between CVH and other more precisely measured risk factors of cardiometabolic disease, including ectopic fat depots and insulin resistance, is sparse (16).
Therefore, using data from 2 large cohorts, Mediators of Atherosclerosis in South Asians Living in America (MASALA) and Multi-Ethnic Study of Atherosclerosis (MESA), we examined the association of CVH with ectopic fat measures and HOMA-IR among 5 racial/ethnic groups. We hypothesized that compared to participants with ideal CVH, those with poor CVH would have higher amounts of ectopic fat and higher HOMA-IR. We further hypothesized that these associations would differ by race/ethnicity, with the largest differences between non-Hispanic White participants and African American or Asian American participants.
Methods
Study Population
Full details of the study design of the MESA and MASALA studies were published previously (17, 18). In brief, the MESA study commenced in July 2000 to investigate the prevalence, correlates, and progression of subclinical CVD in a population-based sample of men and women aged 45 to 84 years. From 2000 to 2002 (Exam 1), a total of 6814 participants were recruited from 6 field centers in the United States (New York, NY; Baltimore, MD; Chicago, IL; Los Angeles, CA; St. Paul, MN; and Forsyth County, NC). The study population was free of clinical CVD at baseline and included 4 ethnic groups: 2622 non-Hispanic White, 1893 African American, 1496 Hispanic, and 803 Chinese American. All MESA participants provided written informed consent, and the institutional review boards approved the study at each center (17).
The MASALA study is a community-based prospective cohort study of South Asian Americans, free of CVD at baseline, recruited from 2 clinical sites (San Francisco Bay Area at the University of California, San Francisco, and the greater Chicago area at Northwestern University) (18). A total of 906 South Asians were enrolled between October 2010 and March 2013. The study protocol was designed to be similar to MESA. The institutional review boards approved the MASALA study protocol of the University of California, San Francisco, and Northwestern University. All participants provided a written informed consent (18).
Measurement of Cardiovascular Health
We defined CVH using the American Heart Association’s (AHA) LS7 metrics (10, 19). The assessment of smoking status was based on self-report. The Typical Week’s Activity Survey was used to assess the frequency of various physical activities, including walking for exercise, dance, conditional activities, and sports, and the metabolic equivalents (METs) of each activity were calculated (20). We used the time spent in activities identified as either vigorous (>6 METs) or moderate (3-6 METs) in the derivation. The average time per week spent in all activities at either a vigorous or moderate level was computed for each participant, and participants were then categorized based on the AHA criteria (10). For dietary assessment in MESA participants, we utilized the 120-item validated food frequency questionnaire modified from the Insulin Resistance Atherosclerosis study instrument (21). The assessment of dietary intake in MSALA was based on the Study of Health Assessment and Risk in Ethnic groups food frequency questionnaire, developed and validated for South Asians in Canada (22). As previously defined by AHA, a healthy diet contained adequate quantities of 5 items (fruits and vegetables, fish, whole grains, sodium, and sugar-sweetened beverages).
Height was measured using a stadiometer, and weight was measured using a standard balance-beam scale or a digital weighing scale. The BMI was calculated using weight (in kilograms) divided by height (in meters squared). For Chinese Americans and South Asians, we used different BMI cut points (23). With the participant in a seated position, and after 5 minutes of rest, 3 brachial artery blood pressure readings were obtained with the average of the last 2 readings being used in this analysis. Total cholesterol was measured using enzymatic methods, and the hexokinase method was used to measure fasting plasma glucose. Low-density lipoprotein cholesterol was calculated using the Friedewald equation (24).
The details of the assessment of AHA’s LS7 metric components are shown in Table 1. Each of the LS7 metrics was assigned a point score of 0, 1, or 2 to represent poor, intermediate, or ideal health, respectively (25). The sum of the individual metric scores was used to derive an overall CVH score, ranging from 0 to 14. We further categorized CVH score as poor (0-7), intermediate (8-11), or ideal (12-14) CVH, consistent with previously published studies (26, 27).
Table 1.
Definitions of cardiovascular health
| Cardiovascular health metricsa | Ideal | Intermediate | Poor |
|---|---|---|---|
| Blood pressure, mmHg | SBP < 120 or DBP < 80, without medication | SBP 120-139 or DBP 80-89 or treated to goal | SBP ≥ 140 mmHg DBP ≥ 90 mmHg |
| Total cholesterol, mg/dL | <200, without medication | 200-239, or treated to goal | ≥240 mg/dL |
| Blood glucose, mg/dL | <100, without medication | 100-125, or treated to goal | ≥126 mmHg |
| Physical activity | ≥150 minutes of moderate or ≥ 75 minutes of vigorous activity per week | 1-149 minutes of moderate or 1-74 minutes of vigorous activity per week | None |
| Healthy diet score, componentsb | 4-5 | 2-3 | 0-1 |
| Body mass index, kg/m2c | <25 | 25-29.9 | ≥30 |
| Smoking | Never smoked or quit >12 months ago | Former smoker or quit <12 months ago | Current smoker |
aAdapted from the American Heart Association’s strategic planning task force and statistical committee 2020 guidelines (10).
bParticipants were given 1 point for meeting each of the dietary goals. These included (1) fruit and vegetables: ≥4.5 cups/day (potato and potato preparation and fruit and vegetable juices were excluded); (2) fish: ≥two 3.5 oz servings/week (excluding fried fish); (3) a fiber-to-carbohydrate ratio: ≥1.0 g fiber per 10 g carbohydrate/day; (4) no more than 36 fluid oz/week of sugar-sweetened beverages (including nondiet soda and fruit drinks); and (5) <1500 mg of sodium/day.
cModified body mass index category for Chinese American and South Asian: ideal (<23 kg/m2), intermediate (23-27.4 kg/m2), poor (≥27.5 kg/m2).
Assessment of Ectopic Fat
Both MESA and MASALA used identical protocols for abdominal and cardiac computed tomography (CT) scanning. Both studies also used the same reading centers and protocols for measuring abdominal body composition, pericardial fat volume, and intrahepatic fat (28). Pericardial fat volume and intrahepatic fat volume were measured at baseline in MESA. Abdominal fat area measurements, including subcutaneous, visceral, and intermuscular, were measured from Exam 2 (2002-2004) and Exam 3 (2004-2005) with a random sample of participants in each of the 4 racial/ethnic groups: 785 non-Hispanic White, 407 African American, 501 Hispanic, and 251 Chinese American.
Two single‐slice noncontrast CT scans were obtained between the L4 and L5 vertebral levels to measure subcutaneous, visceral, and total intermuscular abdominal fat areas (cm2) (18). Measurements of visceral fat, subcutaneous fat, and intermuscular fat from CT scans were conducted using the MIPAV (Medical Image Processing, Analysis, and Visualization) software. Subcutaneous fat was defined as the fat outside of the visceral cavity but did not include that located within the muscular fascia. Visceral fat was defined as tissue within the contour of the visceral cavity. Fat tissue was identified as being between −190 and −30 Hounsfield units. We calculated the total intermuscular fat area by combining fat around the oblique, rectus abdominus, paraspinal, and psoas muscle groups (28). Inter- and intrarater reliabilities for total abdominal, subcutaneous, and visceral cavity areas were 0.99.
Noncontrast cardiac CT images were obtained to measure both pericardial fat volume (cm3) and intrahepatic fat, using a cardiac‐gated CT scanner (29). Pericardial fat was also distinguished by density from the entire heart using volume analysis software (GE Healthcare, Waukesha, WI, USA) and included epicardial and paracardial fat in and around the pericardium. Higher hepatic fat was quantified as the inverse of the hepatic fat attenuation, with lower attenuation indicating more liver fat (30).
Assessment of Insulin Resistance
In MESA, serum insulin was measured from baseline samples with the Beckman Access assay. To harmonize this insulin assay with newer-generation assays with the Roche Elecsys assay that were used in future MESA exams and the MASALA study, a calibration study was performed to calculate a formula for serum insulin values that correlated with the Roche method. The calibration formula is as follows: calibrated insulin = 1.656 + [0.208 × (Beckman Access assay result × 6)] (31). The HOMA-IR was used to measure insulin resistance and calculated as fasting insulin (µIU/mL) × fasting glucose (mmol/L)/22.5 (32). For supplemental analysis only, the single-point insulin sensitivity estimator (SPISE) index [a surrogate for insulin resistance, which has been found comparable to the gold standard test (hyperinsulinemic euglycemic glucose clamp)] was computed as follows: SPISE = 600 × High-density lipoprotein cholesterol 0.185/(Triglyceride 0.2 × BMI 1.338) (33).
Assessment of Covariates
Information was collected on self-reported sociodemographic variables, including age, sex, race/ethnicity, education, and income by trained study personnel (17, 18). Education was classified as ≥bachelor’s degree and < bachelor’s degree. Income was divided into participants who made ≥$75 000 and <$75 000 annually.
Statistical Analysis
We presented the baseline characteristics of study participants by CVH categories. We reported categorical variables as counts (percentages) and continuous variables as mean (SD) or median (interquartile range) depending on the normality of the data. To compare the baseline characteristics, we used analysis of variance for continuous variables and the chi-square test for categorical variables.
Since the ectopic fat variables were not normally distributed, we performed log-transformation. Because a higher level of hepatic fat is indicated by lower hepatic fat attenuation, for ease of interpretation, we inverted the hepatic fat attenuation values by multiplying the measured values by −1. We used a multivariable linear regression model to examine the association between CVH categories and each log-transformed ectopic fat depot as well as HOMA-IR separately. Two separate regression models were fitted. Model 1 was unadjusted, and Model 2 was adjusted for age, sex, race/ethnicity, education, and income. Finally, we back-transformed the beta coefficient into a percent difference to improve the interpretability and comparability of the results. We also reported the association of individual CVH components with ectopic fat and insulin resistance.
We displayed the distribution of CVH categories by race/ethnicity. We also examined the interaction of CVH categories with race/ethnicity using the likelihood ratio χ 2 test by including interaction terms in Model 2. We reported the association between CVH and ectopic fat variables stratified by race/ethnicity. To further understand any potential heterogeneity, we estimated the distribution of individual CVH components among the 5 racial/ethnic groups. Additional analyses were performed to examine the linear correlation between continuous values of ectopic fat measures and HOMA-IR. We investigated the following as sensitivity analysis: (1) the association of CVH categories with subcutaneous fat adjusting for visceral fat; (2) the association of ectopic fat measures with the SPISE index; (3) heterogeneity in the associations by diabetes status; and (4) the association of ectopic fat measures with low- density lipoprotein cholesterol. A 2-sided P-value < 0.05 was considered statistically significant, and all statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Among the 7717 participants included in the analysis (aged 61.4 ± 10.4 years, 52.1% female), 30.1% (n = 2321) had poor CVH, 62.0% (n = 4785) had intermediate CVH, and the remaining 7.9% (n = 611) had ideal CVH. Table 2 shows the baseline characteristics of the study population by CVH categories. Participants in the ideal CVH category were more likely to be younger, female, belong to higher socioeconomic status, and have a lower prevalence of family history of a heart attack. Supplemental Figure 1 shows the distribution of CVH categories among 5 racial/ethnic groups (34). Of African American participants 41.1% (n = 784) had poor CVH, while only 3.2% (n = 61) had ideal CVH. In comparison, only 16.7% (n = 134) Chinese American had poor CVH, while 12.7% (n = 102) had ideal CVH.
Table 2.
Baseline characteristics of the study population by cardiovascular health
| Characteristics | Overall (n = 7717) |
Poor (n = 2321) |
Intermediate (n = 4785) |
Ideal (n = 611) |
P-valuea |
|---|---|---|---|---|---|
| Age, years | 61.4 ± 10.4 | 62.4 ± 9.8 | 61.3 ± 10.5 | 57.9 ± 10.3 | <0.001 |
| Male, n (%) | 3697 (47.9) | 1115 (48.0) | 2340 (48.9) | 242 (39.6) | <0.001 |
| Education > bachelor’s degree, n (%) | 1756 (22.8) | 318 (13.8) | 1204 (25.2) | 234 (38.3) | <0.001 |
| Family income ≥ $75 000 per year, n (%) | 2122 (28.6) | 403 (18.4) | 1433 (30.9) | 286 (46.9) | <0.001 |
| Family history of heart attack, n (%) | 3145 (43.1) | 1033 (47.2) | 1926 (42.6) | 186 (31.8) | <0.001 |
| Current smoker, n (%) | 918 (11.9) | 587 (25.5) | 331 (6.9) | 0 (0) | |
| Systolic blood pressure, mmHg | 126.4 ± 20.9 | 136.3 ± 21.5 | 123.8 ± 19.2 | 109.0 ± 11.5 | |
| Diastolic blood pressure, mmHg | 72.1 ± 10.2 | 75.0 ± 10.7 | 71.5 ± 9.8 | 66.0 ± 7.7 | |
| Hypertension medications, n (%) | 2812 (36.5) | 1221 (50.9) | 1559 (32.7) | 32 (5.9) | |
| BMI, kg/m2 | 28.1 ± 5.4 | 31.3 ± 5.5 | 27.1 ± 4.7 | 23.1 ± 2.4 | |
| Total cholesterol, mg/dL | 193.4 ± 35.9 | 202.2 ± 41.6 | 191.1 ± 33.2 | 178.4 ± 23.6 | |
| LDL-C, mg/dL | 116.5 ± 31.6 | 123.1 ± 35.7 | 115.1 ± 29.8 | 103.1 ± 22.0 | |
| HDL-C, mg/dL | 50.9 ± 14.7 | 48.2 ± 13.3 | 51.5 ± 14.9 | 56.2 ± 16.1 | |
| Lipid lowering agent, n (%) | 1373 (17.8) | 560 (24.2) | 786 (16.5) | 27 (4.4) | |
| Fasting glucose, mg/dL | 97.9 ± 29.9 | 112.0 ± 42.0 | 92.8 ± 20.9 | 84.7 ± 8.2 | |
| Diabetes medications, n (%) | 812 (10.5) | 532 (22.2) | 278 (5.8) | 2 (0.4) | |
| Exercise (MET minutes/week) | 1523.5 ± 2246.7 | 808.0 ± 1917.1 | 1739.8 ± 2215.1 | 2527.0 ± 2836.0 | |
| Visceral fat area, cm2 | 195.1 ± 80.6 | 234.0 ± 83.6 | 186.4 ± 74.4 | 137.9 ± 59.0 | <0.001 |
| Pericardial fat volume, cm3 | 77.1 ± 41.3 | 94.9 ± 46.6 | 72.2 ± 36.6 | 48.5 ± 23.3 | <0.001 |
| Subcutaneous fat area, cm2 | 251.0 ± 110.6 | 304.3 ± 127.9 | 240.8 ± 100.8 | 190.5 ± 68.9 | <0.001 |
| Intermuscular fat area, cm2 | 23.2 ± 11.0 | 27.0 ± 12.8 | 22.4 ± 10.2 | 18.5 ± 7.2 | <0.001 |
| Hepatic fat attenuation, inverted HU | -60.7 ± 12.5 | -57.2 ± 14.1 | -62.0 ± 11.7 | -64.9 ± 8.6 | <0.001 |
| HOMA-IR | 2.73 ± 6.36 | 4.01 ± 7.64 | 2.29 ± 5.97 | 1.37 ± 0.68 | <0.001 |
Data are given as mean ± SD or n (%).
Abbreviations: BMI, body mass index; CVH, cardiovascular health; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; HU, Hounsfield unit; LDL-C, low-density lipoprotein cholesterol.
a P-value by analysis of variance for continuous variables and chi‐square for categorical variables.
The distribution of individual CVH components among the 5 racial/ethnic groups is shown in Table 3 and Supplemental Figures 2 to 8 (34). An ideal healthy diet was rare across all groups, with the highest percentage in that category among South Asian Americans. In contrast, ideal smoking behaviors were high in all groups. Differences by race/ethnicity were most striking for the non-Hispanic White group, which had a higher percentage of participants in the ideal categories for most traditional CVD risk factors. Differences in the BMI component were also notable, with Non-Hispanic White and South Asian American groups having a higher proportion of participants in the ideal categories, followed by African American, Hispanic, and Chinese American groups.
Table 3.
Distribution cardiovascular health components by race/ethnicity
| CVH categories | Non-Hispanic White | African American | Hispanic | Chinese American | South Asian |
|---|---|---|---|---|---|
| Smoking, n (%) | |||||
| Poor | 301 (11.5) | 338 (18) | 203 (13.6) | 45 (5.6) | 31 (3.4) |
| Intermediate | 59 (2.3) | 26 (1.4) | 33 (2.2) | 9 (1.1) | 124 (13.7) |
| Ideal | 2254 (86.2) | 1514 (80.6) | 1259 (84.2) | 748 (93.3) | 751 (82.9) |
| BMI, n (%) | |||||
| Poor | 723 (27.6) | 859 (45.4) | 577 (38.6) | 322 (40.1) | 218 (24.1) |
| Intermediate | 1052 (40.1) | 699 (36.9) | 666 (44.5) | 375 (46.7) | 418 (46.1) |
| Ideal | 846 (32.3) | 334 (17.7) | 252 (16.9) | 106 (13.2) | 270 (29.8) |
| Physical activity, n (%) | |||||
| Poor | 447 (17.1) | 448 (23.8) | 466 (31.2) | 203 (25.3) | 138 (15.2) |
| Intermediate | 464 (17.8) | 335 (17.8) | 248 (16.6) | 156 (19.4) | 181 (20) |
| Ideal | 1703 (65.1) | 1098 (58.4) | 780 (52.2) | 444 (55.3) | 587 (64.8) |
| Healthy diet, n (%) | |||||
| Poor | 1605 (63.1) | 1096 (62.9) | 957 (66.9) | 247 (30.8) | 352 (38.9) |
| Intermediate | 928 (36.5) | 650 (37.1) | 469 (32.8) | 555 (69.1) | 530 (58.5) |
| Ideal | 11 (0.4) | 5 (0.3) | 4 (0.3) | 1 (0.1) | 24 (2.6) |
| Total cholesterol, n (%) | |||||
| Poor | 266 (10.2) | 148 (7.9) | 181 (12.1) | 58 (7.2) | 79 (8.8) |
| Intermediate | 1223 (46.8) | 780 (41.5) | 610 (40.8) | 323 (40.3) | 462 (51.2) |
| Ideal | 1124 (43) | 951 (50.6) | 703 (47.1) | 421 (52.5) | 361 (40) |
| Blood pressure, n (%) | |||||
| Poor | 549 (21) | 626 (33.1) | 386 (25.8) | 191 (23.8) | 163 (18) |
| Intermediate | 1110 (42.4) | 862 (45.6) | 591 (39.5) | 287 (35.7) | 447 (49.3) |
| Ideal | 960 (36.7) | 403 (21.3) | 518 (34.6) | 325 (40.5) | 296 (32.7) |
| Blood glucose, n (%) | |||||
| Poor | 97 (3.7) | 203 (10.8) | 186 (12.5) | 67 (8.4) | 99 (11.1) |
| Intermediate | 355 (13.6) | 405 (21.6) | 309 (20.7) | 175 (21.8) | 289 (32.4) |
| Ideal | 2161 (82.7) | 1271 (67.6) | 998 (66.8) | 559 (69.8) | 505 (56.6) |
Abbreviations: BMI, body mass index; CVH, cardiovascular health.
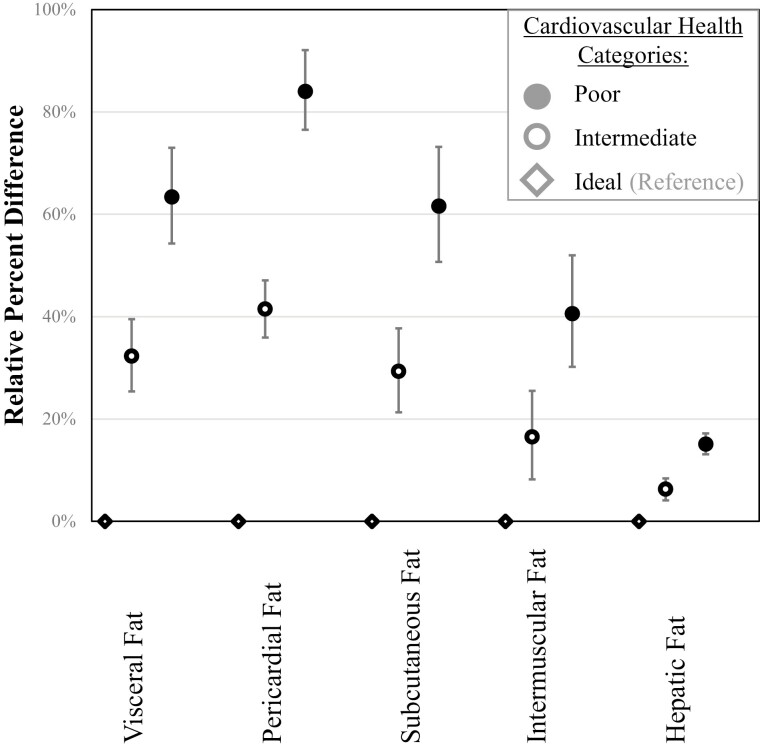
Figure 1 and Table 4 show the relative percent difference and 95% CI of association between CVH categories and fat from different ectopic depots in individuals from all 5 racial/ethnic groups combined. In a model adjusted for age, sex, race/ethnicity, education, and income compared to those with ideal CVH, participants with poor CVH demonstrated 63.4% (95% CI, 54.3-73.0) higher visceral fat area, 84.0% (95% CI, 76.5-92.1) higher pericardial fat volume, 61.6% (95% CI, 50.7-73.2) higher subcutaneous fat area, and 40.6% (95% CI, 30.2-52.0) higher intermuscular fat area, and 15.1% (95% CI, 13.1-17.2) higher hepatic fat (all Ps < 0.001). Participants with intermediate CVH also demonstrated higher ectopic fat measures than ideal CVH (Fig. 1, Table 4). We also examined the association of CVH categories and subcutaneous fat area, adjusting for the visceral fat area in addition to sociodemographic variables. Participants with poor CVH demonstrated 37.6 % (95% CI, 28.3-47.6) higher subcutaneous fat area. Table 5 shows the relative percent difference and 95% CI of association between CVH categories and HOMA-IR. As shown, compared with ideal CVH, participants with intermediate CVH demonstrated 52.8% (95% CI, 42.6-63.8) higher insulin resistance, and those with poor CVH demonstrated 148.2% (95% CI, 131.1-166.7) higher insulin resistance.
Figure 1.
Association of cardiovascular health and ectopic fat measures (relative percent differences and 95% CIs). The model adjusted for age, sex, race/ethnicity, education, and income.
Table 4.
Relative percent difference and 95% CI of association of CVH with ectopic fat and Insulin resistance
| Cardiovascular Health | Model 1a | Model 2b | ||
|---|---|---|---|---|
| Relative percent difference (95% CI) | P-value | Relative percent difference (95% CI) | P-value | |
| Visceral fat | ||||
| Ideal | Reference | Reference | ||
| Intermediate | 34.6 (26.5, 43.2) | <0.0001 | 32.3 (25.4, 39.5) | <0.0001 |
| Poor | 70.1 (59.2, 81.7) | <0.0001 | 63.4 (54.3, 73.0) | <0.0001 |
| Pericardial fat | ||||
| Ideal | Reference | Reference | ||
| Intermediate | 49.9 (43.6, 56.4) | <0.0001 | 41.5 (35.9, 47.1) | <0.0001 |
| Poor | 97.8 (89.1, 106.9) | <0.0001 | 84.0 (76.5, 92.1) | <0.0001 |
| Subcutaneous fat | ||||
| Ideal | Reference | Reference | ||
| Intermediate | 27.5 (19.7, 35.9) | <0.0001 | 29.3 (21.3, 37.7) | <0.0001 |
| Poor | 60.2 (49.6, 71.6) | <0.0001 | 61.6 (50.7, 73.2) | <0.0001 |
| Intermuscular fat | ||||
| Ideal | Reference | Reference | ||
| Intermediate | 22.1 (13.2, 31.9) | <0.0001 | 16.5 (8.2, 25.5) | <0.0001 |
| Poor | 51.0 (39.5, 63.4) | <0.0001 | 40.6 (30.2, 52.0) | <0.0001 |
| Hepatic fat | ||||
| Ideal | Reference | Reference | ||
| Intermediate | 6.0 (3.7, 8.1) | <0.0001 | 6.3 (4.1, 8.4) | <0.0001 |
| Poor | 14.4 (12.4, 16.5) | <0.0001 | 15.1 (13.1, 17.2) | <0.0001 |
Abbreviation: CVH, cardiovascular health.
aUnadjusted.
bAdjusted for age, sex, race/ethnicity, education, and income.
Table 5.
Relative percent difference and 95% CI of association between cardiovascular health categories and insulin resistance
| HOMA-IR | Model 1a | Model 2b | ||
|---|---|---|---|---|
| Relative percent difference (95% CI) | P-value | Relative percent difference (95% CI) | P-value | |
| Ideal | Reference | Reference | ||
| Intermediate | 52.1 (41.8, 63.1) | <0.001 | 52.8 (42.6, 63.8) | <0.001 |
| Poor | 149.5 (132.3, 167.9) | <0.001 | 148.2 (131.1, 166.7) | <0.001 |
Abbreviations: CVH, cardiovascular health; HOMA-IR, homeostasis model assessment of insulin resistance.
aUnadjusted.
bAdjusted for age, sex, race/ethnicity, education, and income.
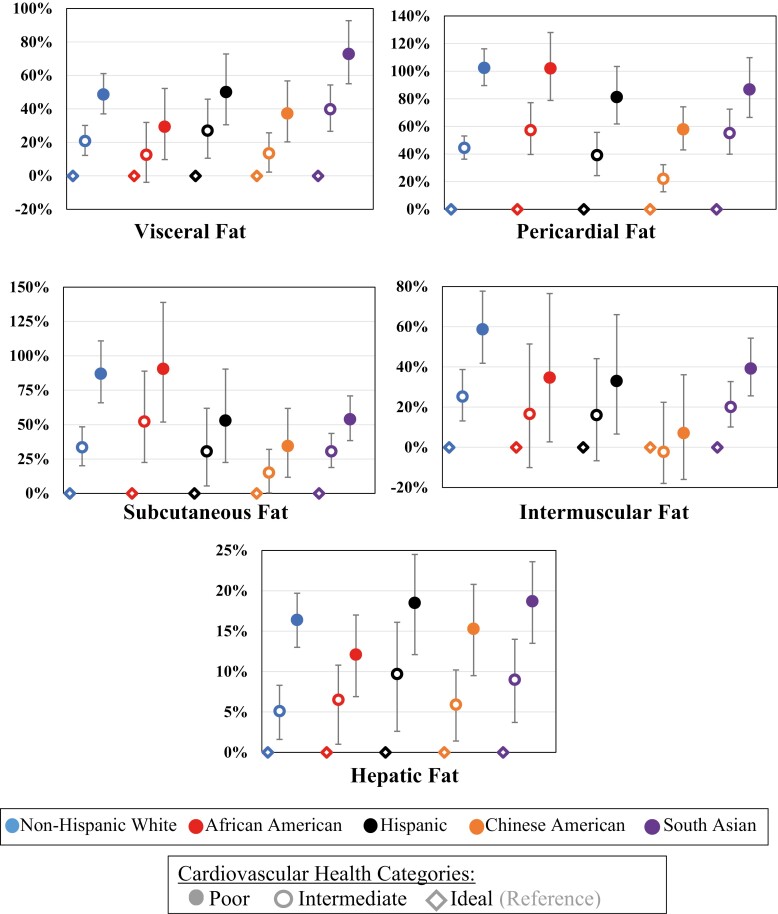
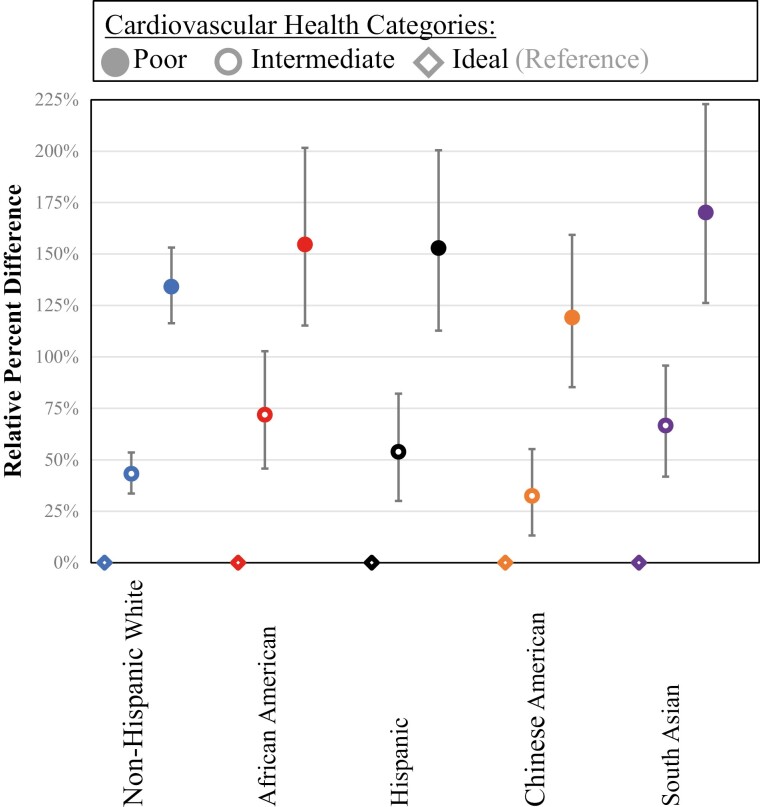
To assess heterogeneity by race/ethnicity in the association between CVH categories and ectopic fat measures, we formally tested for the interaction by race/ethnicity (Table 6). The interaction was statistically significant for all ectopic fat measures and HOMA-IR (P < 0.01 for all). Figures 2 and 3 show the relative percent difference and 95% CI of association between CVH and ectopic fat measures and insulin resistance, respectively, among 5 race/ethnic groups using a model adjusted for age, sex, education, and income. The participants with poor CVH among different races/ethnicities consistently had higher ectopic fat measures and HOMA-IR compared with ideal CVH, but some of the estimates differed.
Table 6.
Race/ethnicity by cardiovascular health categories interaction for ectopic fat and insulin resistance
| Race/ethnicity | Non-Hispanic White | Chinese American | African American | Hispanic | South Asian |
|---|---|---|---|---|---|
| Visceral fat | |||||
| Non-Hispanic White | ** | ||||
| Chinese American | ** | ||||
| African American | *** | ||||
| Hispanic | ** | ||||
| South Asian | ** | ** | *** | ** | |
| Pericardial fat | |||||
| Non-Hispanic White | *** | *** | *** | *** | |
| Chinese American | *** | * | * | ||
| African American | *** | * | |||
| Hispanic | *** | * | |||
| South Asian | *** | ||||
| Subcutaneous fat | |||||
| Non-Hispanic White | ** | ** | *** | ||
| Chinese American | ** | * | |||
| African American | * | ** | |||
| Hispanic | ** | ||||
| South Asian | *** | ** | |||
| Intermuscular fat | |||||
| Non-Hispanic White | *** | ** | |||
| Chinese American | *** | ||||
| African American | |||||
| Hispanic | |||||
| South Asian | ** | ||||
| Hepatic fat | |||||
| Non-Hispanic White | ** | ||||
| Chinese American | * | ||||
| African American | ** | * | ** | ||
| Hispanic | * | ||||
| South Asian | * | ** | |||
| HOMA-IR | |||||
| Non-Hispanic White | * | ||||
| Chinese American | ** | ||||
| African American | ** | ||||
| Hispanic | * | ||||
| South Asian | * | ** | ** | * |
Abbreviations: CVH, cardiovascular health; HOMA-IR, homeostasis model assessment of insulin resistance.
*<0.05.
**<0.01.
***<0.001.
Figure 2.
Association of cardiovascular health and ectopic fat measures by race/ethnicity (relative percent differences and 95% CIs). The model adjusted for age, sex, education, and income.
Figure 3.
Association of cardiovascular health and homeostasis model assessment of insulin resistance measures by race/ethnicity (relative percent differences and 95% CIs). The model is adjusted for age, sex, education, and income.
The only significant heterogeneity for visceral fat was for South Asian participants who had a stronger relationship between CVH and visceral fat with higher estimates for poor CVH (Fig. 2). Figure 2 and Table 6 show that heterogeneity for the other fat measures was more complicated. Figure 3 and Table 6 show that heterogeneity for HOMA-IR was similar to visceral fat, where the only significant heterogeneity was for South Asian participants who had a stronger relationship between CVH and HOMA-IR with higher estimates for poor CVH.
Supplemental analysis revealed similar results. As expected, we found a statistically significant correlation between different ectopic fat measures and HOMA-IR with minor differences by race/ethnicity [Supplemental Tables 1-6 (34)]. Supplemental Table 7 shows that the results for the SPISE index across CVH categories exhibit the same general pattern as for HOMA-IR (34). Supplemental Table 8 shows that results were generally similar for those with and without diabetes, and most variations are likely due to sample size differences, with the 1 potential exception being intermuscular fat that exhibited qualitative differences by diabetes status (34). As expected, Supplemental Figure 9 shows that the association between ectopic fat measures and low-density lipoprotein is similar to those seen for total cholesterol more broadly (34).
We also examined the association between individual CVH metrics with ectopic fat and insulin resistance [Supplemental Figure 10A-10F (34)]. Associations were generally similar across fat from different depots. Given that they are all measurements of adiposity, all ectopic fat measures were strongly associated with BMI as expected, with the strongest association for subcutaneous fat. Similarly, HOMA-IR and glucose share mechanistic pathways and were strongly related. Most fat measures were also associated strongly with glucose and blood pressure, and more moderate associations were seen for physical activity and diet. The estimates for subcutaneous fat with CVH metrics other than BMI were notably less pronounced than for fat from other depots.
Discussion
This investigation demonstrated several important findings from a large community‐based cohort that included 5 racial/ethnic groups and was carefully phenotyped to assess various measures of ectopic fat. We found that participants classified as having intermediate or poor CVH, based on the AHA LS7 metric, had higher visceral, pericardial, subcutaneous, intermuscular, and hepatic fat compared to those with ideal CVH. We also found that worse CVH was associated with higher insulin resistance. Finally, the association between worse CVH and ectopic fat was consistent among the 5 racial/ethnic groups; however, the magnitude of association was quantitatively different for each fat depot by race/ethnicity.
Multiple studies have demonstrated that visceral fat is a major contributor to CVD and metabolic risk above and beyond BMI (35-38). Our finding of poor CVH significantly associated with higher visceral fat supports prior investigations showing the association of diet and physical exercise with visceral fat (39, 40). In contrast, the relationship of subcutaneous fat with CVD is more complicated, with estimates ranging from positive, null, and negative often heavily modified by adjustment for other fat variables (38, 41, 42). Our results further support the existence of this complexity and are consistent with the hypothesis that the plasticity and expandability of subcutaneous fat depot (as opposed to the absolute level of fat itself) offer protection against visceral and ectopic fat deposition and subsequent metabolic health consequences (43).
While fat from the other depots have all been shown to be associated with cardiometabolic disease, differences in the specific cardiometabolic implications of excess adiposity in other fat depots have also been noted. For instance, pericardial fat has been demonstrated to have a particular association with heart failure and atrial fibrillation, intermuscular fat has been associated with impaired glucose tolerance and dyslipidemia, and several studies have demonstrated that hepatic fat is associated with a significantly increased risk of type 2 diabetes and CVD (6, 7, 44-50). Our study is consistent with these previous findings, but the magnitude of association between poor CVH and hepatic fat was relatively low compared to the association with other ectopic fat stores. The exact underlying mechanism of this finding is unclear. While the method of measurement is different for hepatic fat than for fat in the other depots, this finding could also suggest a differential association of health behaviors and risk factors with different ectopic fat depots.
A previous cross-sectional study of the MESA and MASALA cohorts revealed heterogeneity in the distributions of ectopic fat by race/ethnicity (9). For instance, South Asian Americans had higher intermuscular and hepatic fat than other racial/ethnic groups. One of the important findings of our study is the significant association of worse CVH with higher levels of ectopic fat and insulin resistance regardless of race/ethnicity. However, the strength of association was different. By comparing the distribution of individual CVH components by race/ethnicity, we can gain further insight into the potential reasons for heterogeneity in the relationship between ectopic fat depots and overall CVH score. The distributions of CVH components by race/ethnicity were generally consistent with other estimates. In particular, the distribution of BMI is consistent with higher levels of obesity in African American and Hispanic populations and lower levels of obesity in Chinese American and South Asian American populations. It is well known that individuals from certain Asian populations may have a higher ectopic fat burden at the same level of BMI compared to their White counterparts (51, 52). Of note, the typical CVH scoring uses 1 set of cut points for BMI regardless of race/ethnicity, but we used the population-specific cut points for our primary analysis, and this may have impacted our results. Even using the different cut points, we found that the highest percentage of Chinese American participants were in the ideal categories of smoking, total cholesterol, and blood pressure. This may partly explain the lower relative percent difference of association between poor CVH and pericardial, visceral, and subcutaneous fat for Chinese Americans compared to other racial/ethnic groups. A higher percentage of African American participants were classified as having poor CVH for the smoking, BMI, and blood pressure metric components, which may explain the higher relative percent difference between poor CVH and subcutaneous and pericardial fat African Americans.
The available evidence clearly indicates the link between ectopic fat deposition and insulin resistance that can lead to multiple metabolic disturbances; however, few studies have evaluated these relationships in diverse cohorts (53-60). Our study provides additional support by showing a significant association between poor CVH and insulin resistance among all racial/ethnic groups.
This study has several strengths as it is the first study that we are aware of to examine the association of CVH with ectopic fat depots and insulin resistance among 5 racial/ethnic groups in the United States using data from 2 large cohorts with harmonized data. The MESA and MASALA study personnel collected data for CVH using standardized methods and procedures. In addition, it includes gold standard radiographic measures of body composition, including understudied measures of ectopic fat. However, our study also has some limitations. First, the prevalence of obesity in 2000-2005 during the MESA data collection and 2010-2013 for the MASALA data collection may differ and may have introduced some differences between the 2 cohorts. Second, the MESA and MASALA cohorts recruited study participants from limited geographic areas, which restricts the generalizability of the study findings. Specifically, heterogeneity for the Chinese American group may have been missed due to the smaller sample size. Third, these analyses utilized CVH measured at only 1 time point and cannot capture changes in CVH status over time. The single time point also means that this study cannot take advantage of newer measurement approaches for adiposity, including advancements in dual-energy X-ray absorptiometry or separate measurements for epicardial and paracardial fat. Finally, the study’s cross-sectional nature and the multiple components included in the CVH calculation do not allow us to demonstrate the temporality of these relationships. Some of the CVH components are upstream of excess adiposity, while others, including traditional CVD risk factors, are clearly downstream on the causal path from excess adiposity. This study provides a broader view of the association between CVH and fat stored in different depots in the context of existing work on specific pathways between adiposity and cardiometabolic risk.
In conclusion, we found that poor and intermediate CVH, as defined by LS7 metrics, was associated with significantly higher ectopic fat and insulin resistance measures than ideal CVH among all the studied racial/ethnic groups. Despite different distributions of CVH by race/ethnicity, the association between ectopic fat and CVH was strong and qualitatively consistent across the groups. However, we found significant heterogeneity in the strengths of association by race/ethnicity for each ectopic fat depot. Further investigation into how risk factor distribution differences interact with ectopic fat distribution in the development of CVD risk by race/ethnicity could lead to more targeted and successful cardiometabolic disease prevention strategies.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MASALA and MESA studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial Support
The MASALA study was supported by the National Institutes of Health (NIH) grants 1R01-HL-093009 and K24-HL-112827. Data collection at UCSF was supported by NIH/National Center for Research Resources UCSF-Clinical & Translational Science Institute grant UL1-RR-024131. The MESA study was funded by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The pericardial fat and hepatic attenuation measurements for MESA were supported by NIH grant 5R01-HL-085323-04. The MESA Body Composition, Inflammation and Cardiovascular Disease Ancillary Study was supported by NIH grant R01-HL-088451.
Disclosures
M.D.S. serves on scientific advisory boards for Amgen, Esperion, and Novartis. All other authors declare that they have no conflict of interests.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Trouwborst I, Bowser SM, Goossens GH, Blaak EE. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front Nutr. 2018;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeFronzo RA, Ferrannini E. Insulin resistance. a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173-194. [DOI] [PubMed] [Google Scholar]
- 3. Gast KB, Tjeerdema N, Stijnen T, Smit JWA, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7(12):e52036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snel M, Jonker JT, Schoones J, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012;2012:983814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463-500. [DOI] [PubMed] [Google Scholar]
- 6. Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605-613. [DOI] [PubMed] [Google Scholar]
- 7. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30(7):850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox CS, Massaro JM, Schlett CL, et al. Periaortic fat deposition is associated with peripheral arterial disease the Framingham Heart Study. Circ-Cardiovasc Imag. 2010;3(5):515-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah AD, Kandula NR, Lin F, et al. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes (Lond). 2016;40(4):639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. [DOI] [PubMed] [Google Scholar]
- 11. Kulshreshtha A, Vaccarino V, Judd SE, et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg P, O’Neal W, Ogunsua A, Howard G, Soliman E, Cushman M. The American Heart Association’s Life’s Simple 7 and risk of incident atrial fibrillation: the Reasons For Geographic And Racial Differences in Stroke study [abstract]. Circulation. 2017;135(suppl 1):P002. [Google Scholar]
- 13. Ford ES, Greenlund KJ, Hong YL. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125(8):987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across Whites, Blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2012;125(24):2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hruskova J, Maugeri A, Podrouzkova H, et al. Association of cardiovascular health with epicardial adipose tissue and intima media thickness: the Kardiovize study. J Clin Med. 2018;7(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. [DOI] [PubMed] [Google Scholar]
- 18. Kanaya AM, Kandula N, et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol. 2013;36(12): 713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talegawkar SA, Jin Y, Kandula NR, Kanaya AM. Cardiovascular health metrics among South Asian adults in the United States: prevalence and associations with subclinical atherosclerosis. Prev Med. 2017;96:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the cross-cultural activity participation study. J Wom Health Gend Base Med. 1999;8(6):805-813. [DOI] [PubMed] [Google Scholar]
- 21. Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiology study. Ann Epidemiol. 1999;9(5):314-324. [DOI] [PubMed] [Google Scholar]
- 22. Kelemen LE, Anand SS, Vuksan V, et al. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J Am Diet Assoc. 2003;103(9):1178-1184. [DOI] [PubMed] [Google Scholar]
- 23. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-163. [DOI] [PubMed] [Google Scholar]
- 24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 25. Lloyd-Jones DM. Improving the cardiovascular health of the US population. JAMA. 2012;307(12):1314-1316. [DOI] [PubMed] [Google Scholar]
- 26. De Moraes ACF, Carvalho HB, McClelland RL, Diez-Roux AV, Szklo M. Sex and ethnicity modify the associations between individual and contextual socioeconomic indicators and ideal cardiovascular health: MESA study. J Public Health (Oxf). 2019;41(3):E237-E244. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez R, Kershaw KN, Siddique J, et al. Optimism and cardiovascular health: Multi-Ethnic Study of Atherosclerosis (MESA). Health Behav Policy Rev. 2015;2(1):62-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garg SK, Lin F, Kandula N, et al. Ectopic fat depots and coronary artery calcium in south asians compared with other racial/ethnic groups. J Am Heart Assoc. 2016;5(11):e004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90(3):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tota-Maharaj R, Blaha MJ, Zeb I, et al. Ethnic and sex differences in fatty liver on cardiac computed tomography: the Multi-Ethnic Study Of Atherosclerosis. Mayo Clin Proc. 2014;89(4):493-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanaya AM, Herrington D, Vittinghoff E, et al. Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 33. Paulmichl K, Hatunic M, Hojlund K, et al. Modification and validation of the triglyceride-to-HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the Single Point Insulin Sensitivity Estimator (SPISE). Clin Chem. 2016;62(9):1211-1219. [DOI] [PubMed] [Google Scholar]
- 34. Chevli P, Mehta A, Allison M, et al. Supplemental data for: Relationship of American Heart Association’s Life Simple 7, ectopic fat and insulin resistance in 5 racial/ethnic groups. Deposited January 17, 2022. 10.6084/m9.figshare.17742443.v2 [DOI] [PMC free article] [PubMed]
- 35. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the health, aging and body composition study. Am J Epidemiol. 2004;160(8):741-749. [DOI] [PubMed] [Google Scholar]
- 37. Figueroa AL, Takx RAP, MacNabb MH, et al. Relationship between measures of adiposity, arterial inflammation, and subsequent cardiovascular events. Circ-Cardiovasc Imag. 2016;9(4):e004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mongraw-Chaffin M, Allison MA, et al. CT-derived body fat distribution and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Clin Endocr Metab. 2017;102(11):4173-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8(2):e56415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molenaar EA, Massaro JM, Jacques PF, et al. Association of lifestyle factors with abdominal subcutaneous and visceral adiposity. Diabetes Care. 2009;32(3):505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32(6):1068-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocr Metab. 2011;96(11):E1756-E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neeland IJ, Ross R, Despres JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endo. 2019;7(9):715-725. [DOI] [PubMed] [Google Scholar]
- 44. Liu J, Fox CS, Hickson D, et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010;33(7):1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kenchaiah S, Ding J, Carr JJ, et al. Pericardial fat and the risk of heart failure. J Am Coll Cardiol. 2021;77(21):2638-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miljkovic I, Kuipers AL, Kuller LH, et al. Skeletal muscle adiposity is associated with serum lipid and lipoprotein levels in Afro-Caribbean men. Obesity. 2013;21(9):1900-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miljkovic I, Cauley JA, Wang PY, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity. 2013;21(10):2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Therkelsen KE, Pedley A, Speliotes EK, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33(4):863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chang Y, Ryu S, Sung KC, et al. . Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut. 2019;68(9):1667-1675. [DOI] [PubMed] [Google Scholar]
- 50. Wolff L, Bos D, Murad SD, et al. Liver fat is related to cardiovascular risk factors and subclinical vascular disease: the Rotterdam study. Eur Heart J Cardiovasc Imaging. 2016;17(12):1361-1367. [DOI] [PubMed] [Google Scholar]
- 51. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141-146. [DOI] [PubMed] [Google Scholar]
- 52. Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17(3):262-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mossmann M, Wainstein MV, Goncalves SC, et al. HOMA-IR is associated with significant angiographic coronary artery disease in non-diabetic, non-obese individuals: a cross-sectional study. Diabetol Metab Syndr. 2015;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Srinivasan MP, Kamath PK, Manjrekar PA, et al. Correlation of severity of coronary artery disease with insulin resistance. N Am J Med Sci. 2013;5(10):611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25(7):1177-1184. [DOI] [PubMed] [Google Scholar]
- 56. Schmiegelow MD, Hedlin H, Stefanick ML, et al. Insulin resistance and risk of cardiovascular disease in postmenopausal women: a cohort study from the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes. 2015;8(3):309-316. [DOI] [PubMed] [Google Scholar]
- 57. Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects—prospective data from the Verona Diabetes Complicated study. Diabetes Care. 2002;25(7):1135-1141. [DOI] [PubMed] [Google Scholar]
- 58. Hedblad B, Nilsson P, Engstrom G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabetic Med. 2002;19(6):470-475. [DOI] [PubMed] [Google Scholar]
- 59. Kumar AS, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62(2):98-103. [DOI] [PubMed] [Google Scholar]
- 60. Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.