Abstract
Lipodystrophy constitutes a spectrum of diseases characterized by a generalized or partial absence of adipose tissue. Underscoring the role of healthy fat in maintenance of metabolic homeostasis, fat deficiency in lipodystrophy typically leads to profound metabolic disturbances including insulin resistance, hypertriglyceridemia, and ectopic fat accumulation. While rare, recent genetic studies indicate that lipodystrophy is more prevalent than has been previously thought, suggesting considerable underdiagnosis in clinical practice. In this article, we provide an overview of the etiology and management of generalized and partial lipodystrophy disorders. We bring together the latest scientific evidence and clinical guidelines and expose key gaps in knowledge. Through improved recognition of the lipodystrophy disorders, patients (and their affected family members) can be appropriately screened for cardiometabolic, noncardiometabolic, and syndromic abnormalities and undergo treatment with targeted interventions. Notably, insights gained through the study of this rare and extreme phenotype can inform our knowledge of more common disorders of adipose tissue overload, including generalized obesity.
Keywords: lipodystrophy, Dunnigan variety lipodystrophy, HIV-associated lipodystrophy, metreleptin, tesamorelin
Case Presentations
Case 1
The patient is a 35-year-old woman who first noted changes in fat distribution at puberty characterized by loss of fat in her extremities and buttocks along with expansion of the submandibular and dorsocervical fat pads. She also observed areas of hyperpigmentation involving her antecubital and popliteal fossa. Despite having a normal body mass index (BMI), she was diagnosed with type 2 diabetes mellitus at age 18 years requiring exogenous insulin administration in addition to metformin. Her insulin requirements rapidly escalated to more than 200 U per day yet her glycated hemoglobin A1c (HbA1c) remained persistently elevated above 8% with resultant diabetic neuropathy. She also developed hypertension treated with lisinopril, hypertriglyceridemia treated with fenofibrate and pravastatin, and hepatic steatosis. Owing to discontent with her fat distribution, the patient underwent several liposuction surgeries as a young adult involving removal of submandibular, dorsocervical, and mons pubis fat. On transferring her diabetes care to a new endocrinologist, she was immediately suspected to have lipodystrophy based on her appearance and was referred to our clinic for further evaluation.
The patient reported diffuse muscle aches, low energy, and insatiable hunger. On examination, she was found to have blood pressure of 110/70 mm Hg and BMI of 22.3. She had scant subcutaneous fat except for the face and mons pubis region. Muscles in her upper and lower extremities appeared hypertrophied though the patient denied prolonged exercise or use of performance-enhancing drugs (Fig. 1). Laboratory evaluation demonstrated dysglycemia and hypertriglyceridemia despite intensive medical management. She also was found to have a fasting leptin level of 2.6 ng/mL (normal, 3.3-18.3 ng/mL) and adiponectin 2 mcg/mL (normal, 5-37 mcg/mL) (Table 1). The patient indicated that her father and paternal grandfather both had a similar fat distribution as well as type 2 diabetes mellitus diagnosed in young adulthood.
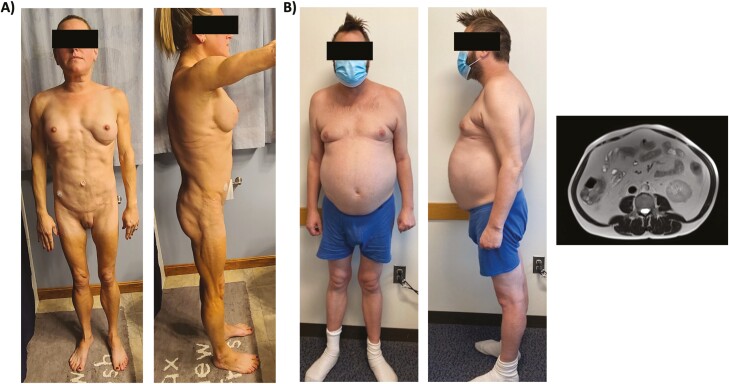
Figure 1.
Clinical cases. A, Case 1: The patient has lipoatrophy of the limbs and torso with sparing of the face and mons pubis. She also has increased muscularity of her upper and lower extremities, which is most evident on the lateral view. B, Case 2: The patient has mild lipoatrophy of the lower extremities and buttocks with profound abdominal lipohypertrophy. Cross-sectional magnetic resonance imaging at L4 revealed significant visceral adiposity and a high visceral to subcutaneous fat ratio. Both patients provided written consent for their photographs to be published.
Table 1.
Patient laboratory results
| Case No. | 1 | 2 |
|---|---|---|
| BMI | 22.3 | 26.1 |
| Waist circumference, cm | 79.1 | 108.2 |
| Hip circumference, cm | 87.0 | 101.1 |
| Waist-hip ratio | 0.9 | 1.1 |
| HbA1c, % | 9.8 | 6.1 |
| Total cholesterol, mg/dL | 236 | 185 |
| HDL-C, mg/dL | 36 | 53 |
| Triglycerides, mg/dL | 634 | 347 |
| LDL-C, mg/dLa | 123 | 63 |
| Leptin, ng/mL | 2.6 | 12.2 |
| Adiponectin, mcg/mL | 2 | 5 |
All laboratory values were obtained from fasting patients.
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
a LDL-C was measured directly in case 1 (triglycerides > 500 mg/dL) and calculated in case 2.
Case 2
The patient is a 54-year-old man who has a long-standing history of HIV infection diagnosed in 1999. He was started on antiretroviral therapy at the time of initial diagnosis with regimens that have included zidovudine (AZT), stavudine (D4T), and nelfinavir. Over the years, he experienced loss of fat in his arms, legs, and buttocks. He also has developed substantial fat accumulation in his abdomen and breasts. Other comorbidities included hypertension, dyslipidemia, prediabetes, hepatic steatosis, and bipolar disorder. His current medication regimen consisted of elvitegravir, cobicistat, emtricitabine, tenofovir, amlodipine, lithium, and bupropion. He referred himself to our clinic based on concerns regarding his central fat distribution.
On physical exam, the patient was found to have blood pressure of 135/86 mm Hg and BMI of 26.1. He had mild lipoatrophy of the face and limbs and considerable central adiposity disproportionate to his BMI with a waist circumference of 108.2 cm and a waist-hip ratio of 1.1 (see Fig. 1). Laboratory evaluation demonstrated mild dysglycemia and hypertriglyceridemia. Leptin level was normal with a low-normal adiponectin (see Table 1).
Background
Lipodystrophy (Greek derivative: “lipo” = fat and “dystrophy” = derangement) refers to a heterogeneous group of disorders characterized by a selective deficiency of adipose tissue in the absence of nutritional deprivation or a catabolic state. Specific lipodystrophy syndromes may be categorized into 4 broad groups according to extent of fat loss (generalized vs partial) and etiology (genetic vs acquired). Lack of adipose tissue predisposes to systemic insulin resistance, ectopic fat deposition, and hormonal derangements (eg, leptin deficiency). In turn, people with lipodystrophy are predisposed to a high burden of metabolic and cardiovascular comorbidities including type 2 diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), polycystic ovary syndrome (PCOS), dyslipidemia, and atherosclerosis. Major causes of mortality in this population include heart disease, liver disease, kidney failure, acute pancreatitis, and sepsis (1).
Apart from HIV-associated lipodystrophy, lipodystrophy syndromes have been historically regarded as rare disorders with prevalence estimates ranging from 1 in 1 million for familial partial lipodystrophy to 1 in 10 million for congenital generalized lipodystrophy (2, 3). However, recent studies of large clinical care cohorts suggest that lipodystrophy may be more common than previously recognized with a genetic prevalence of disease as high as 1 in 7000 (3). As these findings indicate that lipodystrophy is often underdiagnosed, it is imperative for clinicians to become familiar with the signs, symptoms, and workup of this group of disorders so that affected patients and their relatives can be appropriately triaged and managed.
Pathophysiology
Though modern medicine has been primarily focused on the detriments of excess adiposity, the lipodystrophy syndromes underscore the role of healthy fat depots in the maintenance of normal metabolic homeostasis. In this regard, under physiologic conditions, adipose tissue is the primary site for storage of excess calories and serves to buffer postprandial fluxes in circulating lipids (4). Adipose tissue also synthesizes and secretes hormones and adipokines, particularly leptin and adiponectin, to regulate critical processes including energy intake and insulin sensitivity (5).
In patients with lipodystrophy, genetic or acquired defects in adipocyte differentiation, survival, and/or function render adipose tissue unable to perform its critical roles (6). An inability to trap and store nonesterified fatty acids postprandially promotes systemic insulin resistance (7). Moreover, lipids “overflow” to other organs including the liver, muscle, and pancreas, which results in local end-organ damage (8). Lastly, low leptin contributes to hyperphagia, reduced energy expenditure, and insulin resistance, perpetuating lipotoxicity and related metabolic sequelae (9). In general, the severity of metabolic dysfunction in lipodystrophy is proportionate to the extent of fat loss (Fig. 2) (10).
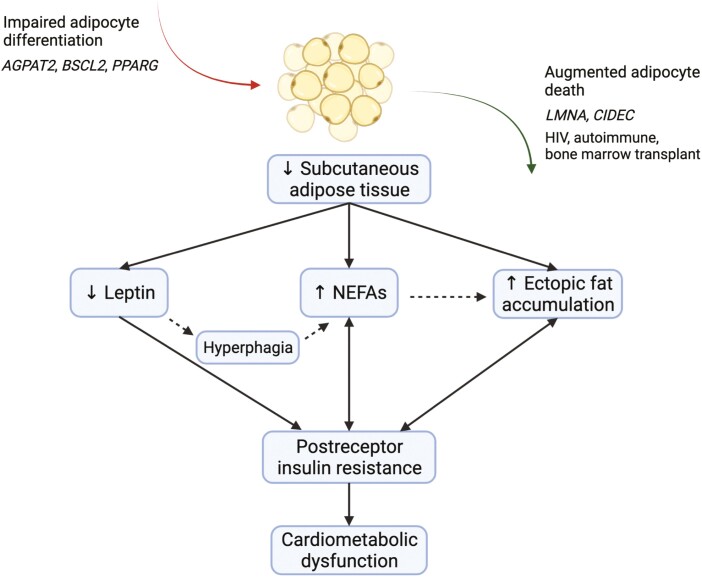
Figure 2.
Pathophysiology of cardiometabolic dysfunction in lipodystrophy. Lipodystrophy is broadly defined by a generalized or partial absence of adipose tissue, except in some patients with HIV-associated lipodystrophy, who may present with lipohypertrophy alone. In general, lack of fat may stem from impaired adipocyte differentiation or augmented adipocyte death secondary to genetic or acquired factors. Deficient adipose tissue may lead to a complex cascade of altered adipokines, metabolites, and energy flux, which collectively promotes insulin resistance and resultant cardiometabolic dysfunction. AGPAT2, 1-acylglycerol-3-phosphate O-acyltransferase 2; BSCL2, Berardinelli-Seip congenital lipodystrophy 2 protein; CIDEC, cell death–inducing DFFA-like effector C; LMNA, lamin A/C; NEFAs, nonesterified fatty acids; PPARG, peroxisome proliferator-activated receptor γ. Figure was created with BioRender.com.
Though lipodystrophy and obesity represent extremes of adipose tissue deficiency and excess, respectively, both states are characterized by insulin resistance, ectopic fat accumulation, and cardiometabolic complications (10). Despite this apparent paradox, it has been posited that cardiometabolic sequelae in both conditions result when the capacity of adipocytes to accommodate additional energy stores is exceeded. While interindividual factors that govern the limits of adipose tissue expansion are not well known, the extremely low threshold at which the storage capacity of adipocytes is exceeded in lipodystrophy results in a severe phenotype. Accordingly, lipodystrophy serves as a paradigm to understand the pathogenesis of adipose tissue overload in diverse patient populations and may yield critical insights into far more common fat disorders, including generalized obesity (10-12).
Clinical Assessment
Lipodystrophy is a clinical diagnosis that should be suspected in patients with regional or generalized lack of subcutaneous adipose tissue or with severe insulin resistance disproportionate to BMI. Clinical assessment should include a detailed medical history including a family history. Patients should be queried about time course of altered fat distribution including onset and duration, cardiometabolic comorbidities, presence of hyperphagia, and a history of an autoimmune disease or HIV to differentiate between lipodystrophy subtypes and to stratify the severity of disease (Table 2). A detailed reproductive history should be obtained, including timing of puberty and menstrual cycle regularity.
Table 2.
Common clinical features of lipodystrophy
| Essential feature |
|---|
| Generalized or regional absence of adipose tissue |
| Comorbidities |
| Type 2 diabetes mellitus |
| Hypertension |
| Dyslipidemia |
| Nonalcoholic fatty liver disease |
| Polycystic ovary syndrome |
| Infertility |
| Renal dysfunction (proteinuria, MPGN, FSGS, diabetic nephropathy) |
| Heart disease (cardiomyopathy, coronary artery disease) |
| Additional historical clues |
| Hyperphagia |
| Pancreatitis, secondary to hypertriglyceridemia |
| Family history (familial) |
| Autoimmune disease (acquired) |
| HIV (acquired) |
| Physical findings |
| Prominent veins |
| Muscle hypertrophy |
| Acanthosis nigricans |
| Eruptive xanthoma |
| Hirsutism |
| Cushingoid appearance |
| Acromegaloid appearance |
| Progeroid appearance |
| Laboratory findings |
| Severe insulin resistance (fasting insulin > 22 mU/L, exogenous insulin ≥ 2 U/kg/d) |
| Hyperglycemia |
| Hypertriglyceridemia and low HDL |
| Elevated transaminases |
| Proteinuria |
| Low fasting leptin (if adipose deficiency is severe) |
Abbreviations: FSGS, focal segmental glomerulosclerosis; HDL, high-density lipoprotein; MPGN, membranoproliferative glomerulonephritis.
In addition to history, physical examination is essential to assess body fat distribution. In this regard, areas of lipoatrophy may be characterized by scant subcutaneous fat, enhanced muscular definition, and prominence of veins. Areas of lipohypertrophy may also be observed in cases of partial lipodystrophy involving sites such as the abdomen, neck and chin, lower extremities, and mons pubis. Physical examination should also an include an assessment of blood pressure as well as stigmata of insulin resistance (eg, acanthosis nigricans, hirsutism, acromegaloid features) and hypertriglyceridemia (eg, eruptive xanthoma). Dual-energy x-ray absorptiometry may be used to further ascertain and quantify body composition. In one study, dual-energy x-ray absorptiometry images that highlighted an individual’s 2-dimensional fat distribution (“fat shadow”) allowed trained readers to distinguish patients with lipodystrophy from control individuals with 85% sensitivity and 96% specificity (13).
Laboratory testing should be obtained to characterize the severity of metabolic dysfunction in patients suspected of lipodystrophy. Assays to be performed include fasting blood glucose and insulin, HbA1c, lipids, and liver function tests. In select cases, an oral glucose tolerance test may be used to diagnose more subtle insulin resistance or dysglycemia. Liver ultrasound with elastography may be used to evaluate for hepatic steatosis and fibrosis. A 24-hour urine collection or spot urine for protein-to-creatinine ratio should also be performed because proteinuric kidney disease is common. Gonadal steroids, gonadotropins, and pelvic ultrasound should be obtained as clinically indicated, including in the context of PCOS-like symptoms. Electrocardiogram and echocardiogram should also be ascertained in individuals with suspected congenital generalized lipodystrophy, progeroid disorders, or familial partial lipodystrophy because of a heightened prevalence of cardiomyopathy, or as clinically indicated. Fasting leptin levels may support a diagnosis of lipodystrophy if low, though this finding is neither sensitive nor specific. Notably, since leptin levels are proportionate to total body fat, levels may be normal in partial lipodystrophy (14). Genetic testing should be pursued in cases that appear to be familial.
The differential diagnosis for lipodystrophy varies by whether generalized vs partial lipodystrophy is suspected. For generalized lipodystrophy, alternative diagnoses to consider include causes of generalized fat loss such as starvation, anorexia nervosa, uncontrolled diabetes mellitus, cancer cachexia, thyrotoxicosis, adrenal insufficiency, AIDS wasting, chronic infections, and diencephalic syndrome (children). For partial lipodystrophy, other diagnoses to rule out include truncal obesity, Cushing syndrome, and multiple symmetric lipomatosis. Genetic or autoimmune insulin receptoropathies are another potential etiology of severe insulin resistance disproportionate to BMI that should be considered in the differential diagnosis of lipodystrophy. However, unlike lipodystrophy, which leads to partial (postreceptor) insulin resistance characterized by hypertriglyceridemia and hepatic steatosis in the context of increased hepatic de novo lipogenesis, patients with insulin receptoropathies are strikingly spared of these complications because of a near-total blockade of hepatic insulin signaling with consequent blunting of hepatic de novo lipogenesis (15).
Lipodystrophy Subtypes
Once generalized or partial lipodystrophy is suspected, the specific lipodystrophy subtype should be elucidated based on clinical history and laboratory testing, as detailed next (Fig. 3). This knowledge has implications for the individual’s risk of cardiometabolic and nonmetabolic comorbidities, prognosis, and treatment. For patients with familial diagnoses, evaluation and genetic testing of at-risk family members should also be considered (1). Importantly, studying unsolved cases will allow us to continue to unravel the biology of adipose tissue with key relevance for other fat disorders, including generalized obesity. The lipodystrophy subtypes described here are intended to provide a conceptual overview. More detailed descriptions are provided elsewhere (1, 12, 16).
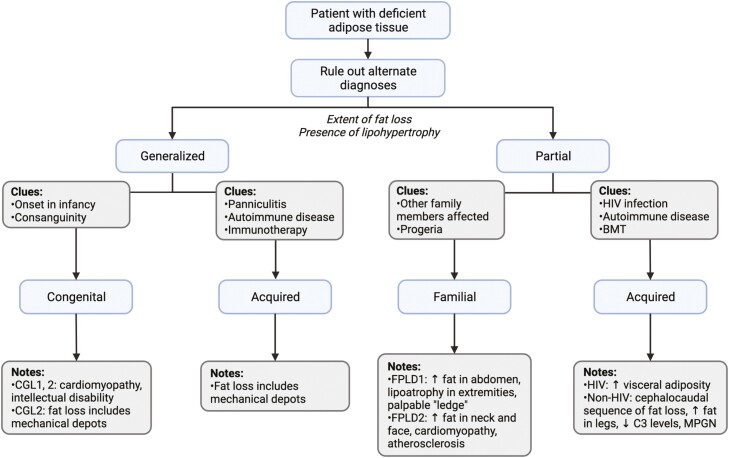
Figure 3.
Conceptual approach to lipodystrophy diagnosis. Lipodystrophy is categorized into 4 broad groups according to extent of fat loss (generalized vs partial) and etiology (genetic vs acquired). This flowchart provides a conceptual approach to the patient with suspected lipodystrophy, paying particular attention to classic presentations of the most common lipodystrophy subtypes. Not all lipodystrophy subtypes and clinical presentations are shown. Progeroid features in the presence of partial lipodystrophy should lead to consideration of familial etiologies as indicated by the flow diagram, but it should be noted that progeria may also be seen in association with generalized forms of disease. BMT, bone marrow transplantation; C3, complement 3; CGL, congenital generalized lipodystrophy; FPLD, familial partial lipodystrophy; MPGN, membranoproliferative glomerulonephritis. Figure was created with BioRender.com.
Generalized Lipodystrophy
Congenital generalized lipodystrophy (Berardinelli-Seip congenital lipodystrophy)
Congenital generalized lipodystrophy (CGL) is an autosomal recessive disorder characterized by near-complete absence of fat starting at birth or infancy (17). Other presenting features include hepatomegaly, umbilical protuberance, prominent musculature, voracious appetite, and acromegaloid features (17). Hypertrophic cardiomyopathy has been reported in CGL as a substantial cause of morbidity and mortality (18). Bone abnormalities have also been described, including focal osteolysis, cyst formation, and absence of marrow fat (19). Furthermore, intellectual impairment and/or psychomotor retardation have been observed (17).
Autosomal recessive mutations in 2 key genes are responsible for most cases of CGL. CGL1 is caused by a defect in 1-acylglycerol-3-phosphate-O-acyltransferase 2 (AGPAT2), which plays a critical role in biosynthesis of glycerophospholipids and triacylglycerols. CGL2 has been attributed to a mutation in Berardinelli-Seip congenital lipodystrophy 2 protein or seipin (BSCL2), which is presumed to contribute to lipid droplet formation and adipocyte differentiation (16). Patients with CGL2 have a more severe lack of adipose tissue encompassing both mechanical (eg, orbits, palms, and soles) as well as metabolic depots. In contrast, adipose deficiency in CGL1 typically spares mechanical fat (2). Besides AGPAT2 and BSCL2, other genes that have rarely been implicated in CGL include caveolin-1 (CAV1) and cavin-1 (CAVIN1) (16).
Acquired generalized lipodystrophy (Lawrence syndrome)
Acquired generalized lipodystrophy (AGL) is a rare disorder with a female predominance characterized by generalized fat loss that occurs after birth and usually before adolescence (20). Onset may be insidious over months to years or may be more rapid in some patients. Fat loss often involves the palms and soles, whereas retro-orbital fat tends to be preserved (20). In about one-quarter of cases, loss of subcutaneous fat is preceded by the appearance of diffuse subcutaneous inflammatory nodules that are histologically consistent with panniculitis. These lesions gradually resolve, leaving behind depressed areas of lipoatrophy that ultimately progress to generalized loss of fat (21). AGL is hypothesized to be caused by an autoimmune destruction of adipocytes and has been linked to autoimmune disorders including juvenile dermatomyositis, Sjögren syndrome, and rheumatoid arthritis. However, roughly one-half of patients with AGL have no concurrent autoimmune disease (20). Several cases have also been reported as a rare complication of immune checkpoint inhibitor therapy (22).
Partial Lipodystrophy
Familial partial lipodystrophy
Familial partial lipodystrophy (FPLD) is a group of inherited disorders characterized by regional loss of adipose tissue that typically occurs in childhood or adolescence. Fat may accumulate in other adipose depots, including the head and neck. Though we would expect men and women to carry pathogenic mutations equally as often, as with other forms of lipodystrophy, most individuals diagnosed with FPLD are women. In this regard, lipodystrophy is more likely to go unnoticed in men and to be mistaken for a muscular physique. Furthermore, women with FPLD are at greater risk of metabolic dysfunction, which may relate to the finding that women have significantly more fat stores than men under normal physiologic circumstances (10, 23).
FPLD1 (Köbberling-type lipodystrophy) is characterized by fat loss exclusively confined to the extremities, typically noted in childhood, with normal or increased distribution of fat in the face, neck, and trunk (24). Truncal obesity with an increased waist to hip ratio is common (24, 25). In some cases, a palpable “ledge” involving the upper extremity and gluteal regions that delineates the boundary between normal (proximal) and lipoatrophic (distal) fat areas has been observed (25). While the pathogenesis of FPLD1 remains poorly understood, the condition appears to be polygenic. In one study, patients with FPLD1 had a heightened burden of common alleles shown to be associated with insulin resistance in the general population, suggesting shared biologic mechanisms between FPLD1 and more common forms of metabolic syndrome (26). Individuals with suspected FPLD1 should be ruled out for a heterozygous pathogenic mutation in peroxisome proliferator-activated receptor γ (PPARG) that defines FPLD3 as this lipodystrophy subtype may present with a similar distribution of fat (27, 28).
FPLD2 (Dunnigan variety lipodystrophy) is an autosomal dominant condition characterized by gradual subcutaneous fat loss from the extremities and trunk with fat accumulation in the neck and chin. Patients with this lipodystrophy subtype may be mistaken for having Cushing syndrome (2). Furthermore, affected individuals may have a muscular physique that corresponds to true hypertrophy of type 1 and 2 muscle fibers on biopsy along with downregulation of myostatin (29). Despite this increase in muscle mass, in detailed exercise studies, patients were found to experience increased muscle fatigability due to mitochondrial dysfunction (30). In contrast to FPLD1, which begins in childhood, the onset of FPLD2 is typically around puberty (25). Moreover, whereas FPLD1 is characterized by central adiposity, fat loss in FPLD2 typically includes the trunk (25).
Patients with FPLD2 have pathogenic mutations in lamin A/C (LMNA), encoding a nuclear protein that interacts with chromatin to mediate key biologic functions. LMNA mutations may lead to premature adipocyte death, which in turn reduces adipose tissue storage capacity over time (2). Given the ubiquitous nature of this gene, mutations have been implicated in other dystrophic disorders including Emery-Dreifuss muscular dystrophy, limb-girdle muscular dystrophy, dilated cardiomyopathy, Charcot-Marie Tooth neuropathy type 2B, and Hutchinson-Gilford progeria syndrome (31). Given its association with cardiac disease, screening for cardiomyopathy and conduction system abnormalities is prudent in patients with FPLD2 at the time of diagnosis.
Other less common genes that have been ascribed to FPLD include PPARG (FPLD3), perilipin 1 (PLIN1; FPLD4), and cell death inducing DFFA-like effector C (CIDEC; FPLD5). Complex genetic syndromes that include partial lipodystrophy have also been reported, some of which are associated with progeroid features (2).
Non–HIV-associated acquired partial lipodystrophy (Barraquer-Simons syndrome)
Owing to the relatively high prevalence of HIV infection, HIV-associated acquired partial lipodystrophy (APL) represents the most common form of lipodystrophy. In the absence of HIV, APL is often characterized by gradual loss of fat in the upper extremities, thorax, and upper abdomen. Fat loss typically proceeds in a cephalocaudal sequence during childhood or adolescence (32). The lower extremities are spared, and excess fat often accumulates in the lower abdomen, gluteal region, hips, thighs, and calves (32). Analogous to AGL, females are more commonly affected (32). Patients with APL tend to have less severe metabolic dysfunction, likely due to preservation of the insulin sensitive gluteofemoral fat depot (10). However, in one recent study, a subset of patients with APL were shown to develop significant multisystemic comorbidities over time, suggesting the need for continued monitoring (33). As with AGL, APL has been associated with autoimmune diseases, in particular systemic lupus erythematosus and dermatomyositis, and may also be triggered by infections. Unique to APL, most affected individuals have been reported to have low complement 3 levels and the presence of the polyclonal immunoglobulin complement 3 nephritic factor. Moreover, one-quarter of patients were found to develop membranoproliferative glomerulonephritis over the course of their disease, which is a key determinant of their overall prognosis (32).
Besides the cephalothoracic variety of APL, subcutaneous fat loss has been reported as a rare and late complication of allogenic bone marrow transplantation in childhood. This form of lipodystrophy is characterized by loss of fat in the extremities and gluteal region with preserved or increased fat in the cheeks, neck, and abdomen. Total body irradiation and intensive chemotherapy were associated with disease in small reports (34-36).
HIV-associated acquired partial lipodystrophy
HIV lipodystrophy was first described in 1998 coinciding with the introduction of highly effective antiretroviral therapy (37). While its incidence is less common today with the use of newer agents, its prevalence remains high among long-term survivors, and thus clinical recognition continues to be important (38). Three subtypes of HIV lipodystrophy have been described: lipoatrophy, lipohypertrophy, and a mixed syndrome (39). Unlike other forms of lipodystrophy, regional fat accumulation may occur in the absence of loss of subcutaneous fat (40), and thus a deficiency in adipose tissue is not required for diagnosis.
In HIV-associated lipodystrophy, lipoatrophy may involve the face, limbs, abdomen, or buttocks, whereas lipohypertrophy may involve the abdomen, breasts, or dorsocervical region. Localized or generalized lipoma (lipomatosis) may be occasionally observed (41). Visceral adiposity is common despite BMI in the normal or overweight range (42). Metabolic sequelae including dyslipidemia, glucose intolerance, and hepatic steatosis have been reported among both lipoatrophic and lipohypertrophic subtypes (43-45).
Risk factors for HIV-associated lipodystrophy are not entirely understood but may relate to the interplay of viral, host, and antiretroviral therapy–specific factors (38, 46). Notably, prolonged exposure to thymidine nucleotide reverse transcriptase inhibitors (eg, D4T, AZT) have been consistently linked to subcutaneous fat loss, likely due to inhibition of mitochondrial DNA polymerase-γ and subsequent mitochondrial dysfunction. Male sex, older age, low CD4+ T-cell count, and high plasma HIV viral load have also been associated with increased lipoatrophy risk (41). Characteristics predisposing to lipohypertrophy are less clear, but female sex and older age have been posited in some studies (41).
Treatment
There are currently no therapies to reverse the underlying fat deficit in lipodystrophy. Instead, treatment is primarily focused on ameliorating the cardiometabolic and nonmetabolic comorbidities that occur with these disorders (1).
Lifestyle Modification
Based on expert consensus, the cornerstone of lipodystrophy management is lifestyle changes that limit the energetic load on residual fat stores, which in turn will reduce circulating nonesterified fatty acids, insulin resistance, and ectopic fat deposition (1). In adults, caloric restriction has been shown to lower triglycerides and glucose (47), but may be challenging to achieve in individuals with hyperphagia. In children, caloric restriction to minimize metabolic complications must be balanced against the energy requirements of normal growth. In general, a diet with balanced macronutrient composition is advised. However, very low-fat diets should be considered for patients with hypertriglyceridemia-induced pancreatitis, and carbohydrate-restricted diets may be valuable in the context of diabetes (1). It should be noted that BMI is not an accurate tool to stratify cardiometabolic disease risk in patients with lipodystrophy, and caloric restriction should be considered even at a normal or low-normal BMI (48).
Beyond dietary changes, patients with lipodystrophy are encouraged to limit alcohol intake (risk of hepatic steatosis) and tobacco use (risk of cardiovascular disease), and to maintain an active lifestyle in the absence of contraindications. In the latter regard, individuals with forms of lipodystrophy associated with cardiomyopathy should undergo cardiac evaluation before initiation of an exercise regimen. Furthermore, avoidance of contact sports is important in patients with CGL complicated by hepatosplenomegaly or lytic bone lesions (1).
Restoration of Adipose Tissue Distribution or Function
Leptin Replacement Therapy
As leptin is a key adipokine made by subcutaneous adipose tissue, metreleptin (recombinant human methionyl leptin) is a pillar of therapy in non–HIV-associated lipodystrophy. Beginning with a landmark study in 2002 (49), metreleptin has been shown to ameliorate numerous pathologic processes in lipodystrophy, including hyperphagia, insulin resistance, and hypertriglyceridemia (Fig. 4). Furthermore, metreleptin therapy has been linked to improvements in nonalcoholic steatohepatitis severity (50), cardiac hypertrophy (51), proteinuria (52), reproductive function (53), overall quality of life (54), and mortality (55). Interestingly, while a reduction in hyperphagia undoubtedly contributes to the metabolic benefits of metreleptin, a recent study has found independent effects of this agent on glucose and lipid metabolism even when caloric intake is held constant (56).
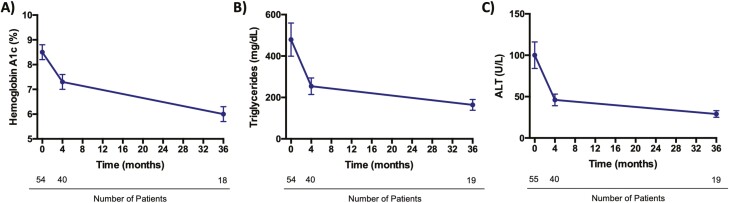
Figure 4.
Metabolic effects of metreleptin in non–HIV-associated lipodystrophy. Metreleptin leads to sustained improvements in glycemic control, hypertriglyceridemia, and hepatic steatosis among individuals with non–HIV-associated lipodystrophy. In the representative data shown, patients with generalized or partial lipodystrophy who had metabolic dysfunction and low baseline leptin levels (< 8.0 ng/mL in males; < 12.0 ng/mL in females) were treated with open-label metreleptin for up to 3 years. Metreleptin statistically significantly reduced A, HbA1c (–2.1% ± 0.5%); B, triglycerides (–35.4% ± 13.7%); and C, ALT (–45 ± 19 U/L). The most rapid decrements occurred in the first 4 months of therapy followed by slower but continued improvements throughout the follow-up period (57). Mean and SEM are shown. ALT, alanine transaminase; HbA1c, glycated hemoglobin A1c.
Metreleptin is particularly effective among patients with generalized lipodystrophy in whom endogenous leptin levels tend to be lowest (14). As metreleptin addresses a key mechanism of disease pathogenesis in this disease subtype, it is generally advised that therapy be initiated early on to prevent cardiometabolic sequelae (1). In individuals with generalized lipodystrophy, marked improvements in glycemic and lipid profiles are seen as quickly as the first week (52) with declines in HbA1c and serum triglycerides by approximately 25% and 60%, respectively, over 1 year (14). The use of metreleptin to treat partial lipodystrophy is more controversial given a greater spectrum of therapeutic efficacy in this subgroup of patients (14). Nonetheless, studies have shown that carefully selected individuals with more severe metabolic complications (HbA1c > 8%, triglycerides > 500 mg/dL) and lower baseline leptin levels (< 4 ng/mL) may benefit (14, 56).
The use of metreleptin among patients with lipodystrophy has been shown to be generally safe and well tolerated. Side effects of metreleptin include localized skin reactions and discomfort at injection sites, headache, and weight loss (57). Importantly, hypoglycemia may occur, particularly in patients well controlled on antihyperglycemic agents. Thus, blood glucose should be closely monitored and prophylactic reduction of diabetes medications should be considered following initiation and uptitration of leptin replacement (1). In patients with acquired generalized lipodystrophy, rare cases of T-cell lymphoma on metreleptin have been described, but are thought to relate to the underlying disease process rather than the treatment itself (1). Nonetheless, close monitoring for hematologic abnormalities in treated patients is advised (12). As a complication of metreleptin therapy, a small proportion of individuals have been reported to develop neutralizing antileptin antibodies, leading to worsening metabolic control and severe infections. Understanding risk factors and clinical consequences of neutralizing antibodies remains an active area of investigation (58).
In the United States, metreleptin is approved as an adjunct to diet for the treatment of generalized but not partial lipodystrophy. In contrast, in Europe and Japan, metreleptin is approved in both forms of lipodystrophy, and specifically in cases of partial lipodystrophy that have not achieved adequate metabolic control with other agents. A randomized, placebo-controlled trial of metreleptin is currently underway to further elaborate on the efficacy and safety of metreleptin and to inform the risk-benefit analysis in the context of partial lipodystrophy (ClinicalTrials.gov identifier: NCT05164341).
Tesamorelin
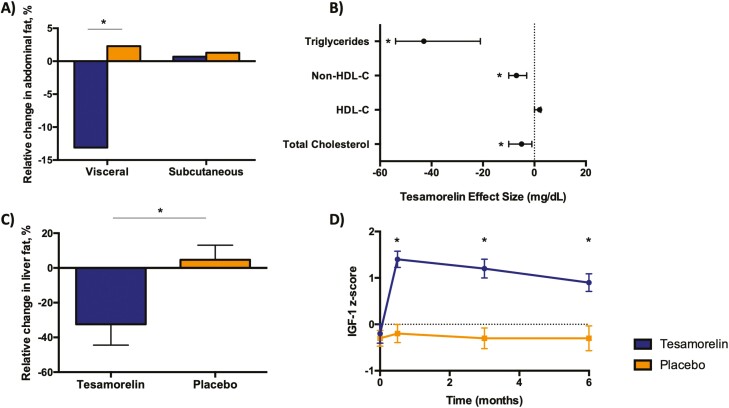
In HIV-associated lipodystrophy, patients have blunted growth hormone (GH) secretion in proportion to the extent of abdominal fat accumulation. Specifically, detailed physiologic studies have shown reduced GH secretion per pulse with normal pulse frequency (59). Since GH increases lipolysis and suppresses de novo lipogenesis, low GH secretion has been posited to potentiate abdominal fat accumulation and hepatic steatosis in this patient population (60). In this context, the novel GH-releasing hormone analogue tesamorelin was developed as a strategy to restore physiologic GH pulsatility in patients with HIV-associated lipodystrophy (61). In 2 large, phase 3 clinical trials, tesamorelin was shown to statistically significantly reduce visceral fat by nearly 20% without modulating subcutaneous fat in patients with HIV and abdominal adiposity (62). Smaller but nonetheless statistically significant reductions were also seen in waist circumference, likely due to preservation of subcutaneous fat. Tesamorelin was also noted to decrease triglycerides by roughly 15% and to improve quality of life (62). These benefits were achieved with insulin-like growth factor 1 (IGF-1) levels that remained within the physiologic range (Fig. 5) (63). Furthermore, tesamorelin responders (defined as individuals with visceral fat reduction ≥ 8%) had concurrent improvements in HbA1c, hepatic transaminases, and adiponectin, which is consistent with the known role of visceral fat in the pathogenesis of metabolic complications (64, 65). Subsequent clinical trials have additionally shown that tesamorelin reduced liver fat and prevented hepatic fibrosis progression among patients with HIV-associated NAFLD in concert with favorable changes in hepatic gene expression (66, 67).
Figure 5.
Metabolic effects of tesamorelin in HIV-associated lipodystrophy. A, In 2 large, phase 3, placebo-controlled trials of patients with HIV and increased abdominal adiposity, tesamorelin led to a statistically significant reduction in visceral fat (–15.4%) while preserving subcutaneous fat over 26 weeks (62). B, Tesamorelin also led to declines in triglycerides (–43 mg/dL; 95% CI, –54 to –21 mg/dL), total cholesterol (–5 mg/dL; 95% CI, –10 to –1 mg/dL) and non-HDL cholesterol (–7 mg/dL; 95% CI, –10 to –3 mg/dL) vs placebo (62). C, In patients with HIV and nonalcoholic fatty liver disease, tesamorelin-treated individuals experienced a substantial decline in liver fat compared to placebo-treated controls over 1 year (–37%; 95% CI, –67% to –7%) (66). D, Insulin-like growth factor 1 (IGF-1) levels among individuals with HIV-associated abdominal fat accumulation have been shown to increase relative to placebo, but typically remain within the normal physiologic range (z score < 2.0) (63). Data are mean difference and 95% CI in B and mean and SEM in C and D. Asterisks indicate statistical significance as defined by P less than .05. HDL-C, high-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol.
Tesamorelin is well tolerated, with side effects that include injection site reactions, arthralgias, myalgias, and peripheral edema in a minority of patients (62). Though GH is known to increase insulin resistance, meticulous physiology studies have shown no worsening of glycemic control with tesamorelin (61). Nonetheless, in a small number of patients, glucose levels may increase, and thus optimization of glycemic control before initiation of therapy and periodic blood glucose monitoring while on treatment may be useful. Patients receiving tesamorelin should undergo routine assessment of IGF-1; a dose reduction may rarely be needed to maintain levels within the normal range. In addition, patients on tesamorelin should undergo age-appropriate cancer screening before and while on therapy given theoretical concerns that GH may potentiate cancer growth, though tesamorelin specifically has not been shown to increase cancer risk. Tesamorelin is the only medication approved in the United States to treat abdominal fat accumulation in HIV. Tesamorelin has not been studied in the context of other lipodystrophy syndromes characterized by increased visceral adiposity and is not currently approved outside the context of HIV.
Pharmacologic Management of Cardiovascular Risk Factors
Treatment of cardiometabolic complications is a key tenet of lipodystrophy management to minimize lifelong risk of adverse cardiovascular events.
Insulin Resistance
Despite limited evidence in lipodystrophy, metformin is generally considered the first-line agent for the management of insulin resistance and diabetes in this context given its efficacy and side effect profile (1). In a large, randomized clinical trial of prediabetes among the general population, metformin reduced progression to diabetes as compared to placebo, though to a lesser degree than an intensive lifestyle intervention (68). Furthermore, in patients with HIV-associated lipodystrophy and baseline dysglycemia, metformin improved insulin resistance, weight, and blood pressure (69-71). The most common side effect of metformin is gastrointestinal discomfort, which can be mitigated by gradual dose uptitration.
Beyond metformin, thiazolidinediones (TZDs) have been the most well-studied class of antihyperglycemics in patients with lipodystrophy (1, 72). Interest in these agents stems from their mechanism of action as PPARγ analogues, which in theory may promote adipogenesis and lipid storage, and thereby attenuate the underlying deficit in subcutaneous fat that is present in this patient population. Indeed, numerous small studies have shown that TZDs improve insulin resistance, glucose intolerance, and hypertriglyceridemia among individuals with lipodystrophy, particularly in those with partial subtypes (73-80). Statistically significant improvements in hepatic steatosis (76, 79) and PCOS (77) have also been reported, consistent with findings in the general population that pioglitazone may ameliorate NAFLD in the context of prediabetes and type 2 diabetes mellitus (81). Despite these benefits, enthusiasm for TZDs has been somewhat tempered by inconsistent effects to expand lipoatrophic fat stores, with some reports further demonstrating exacerbation of fat accumulation in lipohypertrophic areas (1, 74, 78). Moreover, potential side effects of this drug class include weight gain, bone loss, and bladder cancer. Lastly, some experts have cautioned that the benefits of TZDs in lipodystrophy may be relatively short-lived (12). Thus, while TZDs may be efficacious in select patients with lipodystrophy, they should be used with careful monitoring and continual risk-benefit assessment.
In patients that remain hyperglycemic despite noninsulin antihyperglycemic agents, insulin may be needed to achieve adequate glycemic control. Concentrated insulins (eg, U-500) should be considered in patients with high insulin requirements due to severe insulin resistance (1). Key side effects include weight gain, hypoglycemia, and injection site reactions.
Dyslipidemia
The main goals of treatment for dyslipidemia in lipodystrophy are to lower cardiovascular disease risk and to prevent hypertriglyceridemia-induced pancreatitis. It is generally advised to manage lipids in accordance with general population guidelines. However, stricter lipid targets have been espoused in society guidelines given the enhanced risk of cardiovascular disease (eg, low-density lipoprotein cholesterol [LDL-C] < 100 mg/dL, non–high-density lipoprotein cholesterol [non-HDL-C] < 130 mg/dL, and triglycerides < 200 mg/dL) (1). To manage elevated LDL-C, we have a low threshold to initiate statin therapy using a patient-centered approach. Statin therapy is definitively indicated in patients with lipodystrophy who have comorbid type 2 diabetes mellitus (82, 83).
To manage hypertriglyceridemia, lifestyle modifications are the first-line management, which includes reduction in excess body weight, avoidance of alcohol, and augmentation of physical activity. Diets rich in complex carbohydrates, monounsaturated fats, and omega-3 polyunsaturated fatty acids are recommended, unless triglycerides exceed 1000 mg/dL, in which case a very-low fat diet is advised. Medications should be reviewed to identify potential exacerbating agents such as oral estrogens, which should be limited as able. As poorly controlled type 2 diabetes mellitus may potentiate hypertriglyceridemia, glycemic control should also be optimized as described earlier (83, 84).
For patients with elevated triglycerides despite lifestyle interventions, medical therapy is advised. Because hypertriglyceridemia is considered a risk-enhancing factor for cardiovascular disease, a statin may be used to address cardiovascular disease risk (83). The omega-3 fatty acid eicosapentaenoic acid (EPA, 4 g/day) may also be added in patients with known or high risk of atherosclerotic disease (84). If triglycerides are above 500 mg/dL, a fibrate should be added for more effective triglyceride-lowering given the elevated risk of pancreatitis. Notably, dual use of statins and fenofibrates is associated with higher rates of rhabdomyolysis compared to statins alone, and gemfibrozil specifically should be avoided in the context of combination therapy (83). In patients with extreme hypertriglyceridemia, plasmapheresis has been used given the diminished efficacy of pharmacologic agents in this setting, but may need to be repeated frequently (1).
Hypertension
Hypertension in patients with lipodystrophy should be managed in accordance with standard guidelines. In this regard, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are advised as first-line agents in patients with diabetes (1).
Surgical Management
Metabolic surgery
In several case reports, Roux-en-Y gastric bypass (RYGB) surgery has been shown to have beneficial metabolic effects in partial lipodystrophy syndromes, particularly FPLD1 and 2 (85-88). As in cases of generalized obesity, favorable changes in insulin resistance and glycemic control with RYGB in lipodystrophy have been ascribed to reduced caloric intake and changes in gut hormone responses (87). Despite this promising early evidence, further studies are needed to define patients with lipodystrophy in whom surgical management is most effective. Metabolic surgery should not be considered in patients with generalized lipodystrophy or severe lipoatrophy.
Cosmetic treatment
Altered fat distribution in patients with lipodystrophy may cause distress related to body image, particularly in female patients (12, 89, 90). To address lipoatrophy, autologous fat transfer, dermal fillers, or muscle grafts may be employed (1). While areas of lipohypertrophy in partial lipodystrophy may also cause discontent, we advise against liposuction, which removes protective subcutaneous fat stores and in turn may contribute to progression of metabolic dysfunction (12, 91, 92). As an example, in one prior study, healthy normal-weight women undergoing abdominal liposuction were found to have a statistically significant increase in visceral fat within 6 months (91).
Management of Nonmetabolic Comorbidities
In addition to a negative body image, patients with lipodystrophy have been reported to have a high prevalence of chronic pain (93), fatigue (20, 30), and mood disorders including depression and anxiety (93, 94). As such, a holistic approach to the patient is essential. Screening for these conditions should be routinely incorporated into their endocrine care with subspecialty referral as indicated.
Controversies and uncertainties
While the management of lipodystrophy is geared primarily toward optimization of cardiovascular disease risk, the true prevalence of atherosclerosis and related events in this population remains uncertain. In particular, while patients with CGL bear a substantial burden of metabolic disease, rates of clinically overt coronary heart disease seem lower than would be expected by most reports (eg, 3 of 33 patients in one registry) (95). However, individuals in these prior studies were predominantly young and did not undergo routine assessments for subclinical coronary artery disease, which may have led to underestimates of the true disease prevalence (96). Unlike in CGL, an increased risk atherosclerotic vascular disease in patients with FPLD2 has been well described. Specifically, in one kindred study, LMNA mutation carriers had statistically significantly more coronary heart disease compared to unaffected family members (34.8% vs 5.9%), which often was diagnosed before age 55 years (97). Despite recognition of an increased risk of coronary heart disease in this patient population, screening tools to identify the patients who are most susceptible—and thus require the most aggressive therapeutic interventions—have not yet been established.
Beyond uncertainties surrounding cardiovascular disease risk assessment, the optimal medical management of cardiometabolic risk factors in patients with lipodystrophy also remains controversial. As an example, limited data are available pertaining to prevention and treatment of diabetes in this patient population, including the utility of newer classes of drugs such as glucagon-like peptide 1 (GLP1) receptor agonists and sodium-glucose cotransporter-2 (SGLT2) inhibitors. While these agents have been shown to have emerging cardiovascular benefits among people with type 2 diabetes mellitus in the general population (98), their efficacy and safety in the context of lipodystrophy requires further study. In addition, although aggressive lipid-lowering may be advisable in this patient population, the role of PCSK9 inhibitors as an adjuvant to statin therapy has not yet been assessed. Lastly, while metreleptin and tesamorelin have been shown to have beneficial metabolic effects in non-HIV and HIV patients, respectively, the effects of these agents on atherosclerotic end points remains unknown.
Back to the Cases
Case 1
The patient underwent genetic testing, which revealed a heterozygous LMNA mutation p.R482Q consistent with a diagnosis of FPLD2. Substitutions in p.R482 have been implicated in 80% of cases of this lipodystrophy subtype (96, 99). Her body fat distribution (lipoatrophy of the extremities and buttocks; lipohypertrophy of the face, neck, and mons pubis), muscle hypertrophy, timing of onset at puberty, and autosomal dominant mode of inheritance are typical of the diagnosis. As with many patients with lipodystrophy, her localized excess adiposity was a considerable source of distress, and as such she underwent multiple liposuction surgeries before referral to our clinic. Unfortunately, surgical removal of her limited subcutaneous fat depots likely exacerbated her disease and shifted her toward a generalized lipodystrophy phenotype. Accordingly, we recommended a 1200-kcal diet low in carbohydrates and high in monounsaturated and polyunsaturated fats. We also prescribed her metreleptin therapy at a dose of 5 mg daily subcutaneously. At 6 months following the initiation of metreleptin, her HbA1c declined from 9.8% to 6.9% with a reduction in insulin from more than 200 units daily to approximately 30 units daily. Triglycerides also improved from 634 mg/dL to 216 mg/dL. She noted a substantial reduction in hyperphagia concordant with attenuation of metabolic dysfunction. She had no side effects to metreleptin though her fatigue and muscle weakness persisted unchanged. Importantly, had her lipodystrophy been diagnosed sooner, she may have been counseled against liposuction and/or initiated on metreleptin therapy earlier in life, which in turn may have greatly reduced her lifetime burden of cardiometabolic dysfunction.
Case 2
The patient’s altered fat distribution with marked lipohypertrophy of the abdomen and mild lipoatrophy of the limbs and buttocks is consistent with a diagnosis of HIV-associated lipodystrophy. His long-standing HIV infection with exposure to first-generation nucleoside reverse transcriptase inhibitors (eg, AZT, D4T) supports this diagnosis. Notably, his central adiposity, disproportionate to his BMI in the nonobese range, puts him at heightened risk of cardiometabolic disease (100). Furthermore, given that HIV infection itself is a risk factor for cardiovascular events, more aggressive lipid-lowering therapy may be warranted in this patient compared to what standard risk prediction calculators would suggest (101). Accordingly, we opted to start pitavastatin 2 mg daily orally. This moderate-intensity statin was selected because it does not interact with his HIV medication cobicistat, a cytochrome P450 (CYP) 3A4 inhibitor. To address his visceral and hepatic fat accumulation, we also initiated tesamorelin SV 1.4 mg daily subcutaneously after confirming that age-appropriate cancer screening was normal. After 6 months on tesamorelin, the patient’s IGF-1 increased from 78 ng/mL to 150 ng/mL with a reduction in waist circumference from 110 cm to 105 cm. Triglycerides also declined on therapy from 347 mg/dL to 182 mg/dL with stable glycemic control.
Conclusion
The lipodystrophy syndromes are a collection of disorders characterized by a generalized or partial deficiency of subcutaneous fat, often leading to insulin resistance and cardiometabolic dysfunction. While rare, recent evidence suggests these conditions are more common than previously thought, underscoring a blind spot in clinical detection. In all patients with lipodystrophy, assessment and optimization of traditional cardiovascular risk factors is imperative. Leptin replacement is a mainstay of treatment among patients without HIV, particularly in those with generalized disease that is characterized by low leptin levels. For patients with HIV-associated lipodystrophy, tesamorelin has been shown to selectively reduce visceral adiposity and concurrent metabolic dysfunction. Much remains to be learned about how we can optimize care for patients with lipodystrophy, including the management both of cardiometabolic and noncardiometabolic sequelae. By studying the extreme phenotype of lipodystrophy, we may further reveal novel insights into fat biology and cardiometabolic disease that have key relevance for patients with other adipose tissue disorders, including obesity.
Acknowledgments
We thank our colleagues at MGH Lipid and Metabolism Associates for their shared commitment to the care of patients with lipodystrophy. We also thank our patients who have generously agreed to contribute their stories and photographs to this publication to teach the medical community about their underrecognized conditions.
Glossary
Abbreviations
- AGL
acquired generalized lipodystrophy
- APL
acquired partial lipodystrophy
- AZT
zidovudine
- BMI
body mass index
- CGL
congenital generalized lipodystrophy
- D4T
stavudine
- FPLD
familial partial lipodystrophy
- FPLD1
Köbberling-type lipodystrophy
- FPLD2
Dunnigan variety lipodystrophy
- GH
growth hormone
- HbA1c
glycated hemoglobin A1c
- IGF-1
insulin-like growth factor 1
- LMNA
lamin A/C
- NAFLD
nonalcoholic fatty liver disease
- PCOS
polycystic ovary syndrome
- PPARG
peroxisome proliferator-activated receptor γ
- RYGB
Roux-en-Y gastric bypass
- TZD
thiazolidinedione
Financial Support
This work was supported by the National Institutes of Health (NIH Nos. K23HD100266 to L.T.F. and NIH P30DK040561 to S.K.G.).
Disclosures
L.T.F. has nothing to disclose. S.K.G. has served as a consultant (personal) and currently serves as a consultant through an Institutional Consulting Agreement with Theratechnologies. The Mass General Hospital has a royalty and license agreement with Theratechnologies for the potential use of tesamorelin for nonalcoholic fatty liver disease. S.K.G. is the inventor on a patent for GHRH or Analogues Thereof For Use in Treatment of Hepatic Disease (application No. 16832128). He has served as a consultant (personal) to Viiv Healthcare.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal AK, Garg A. Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet. 2006;7:175-199. [DOI] [PubMed] [Google Scholar]
- 3. Gonzaga-Jauregui C, Ge W, Staples J, et al. Clinical and molecular prevalence of lipodystrophy in an unascertained large clinical care cohort. Diabetes. 2020;69(2):249-258. [DOI] [PubMed] [Google Scholar]
- 4. Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201-1210. [DOI] [PubMed] [Google Scholar]
- 5. Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59(1):16-23. [DOI] [PubMed] [Google Scholar]
- 7. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881-887. [DOI] [PubMed] [Google Scholar]
- 9. Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mann JP, Savage DB. What lipodystrophies teach us about the metabolic syndrome. J Clin Invest. 2019;129(10):4009-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang-Doran I, Sleigh A, Rochford JJ, O’Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207(3):245-255. [DOI] [PubMed] [Google Scholar]
- 12. Lim K, Haider A, Adams C, Sleigh A, Savage DB. Lipodistrophy: a paradigm for understanding the consequences of “overloading” adipose tissue. Physiol Rev. 2021;101(3):907-993. [DOI] [PubMed] [Google Scholar]
- 13. Meral R, Ryan BJ, Malandrino N, et al. “Fat shadows” from DXA for the qualitative assessment of lipodystrophy: when a picture is worth a thousand numbers. Diabetes Care. 2018;41(10):2255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119(2):315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg A. Clinical review#: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agarwal AK, Simha V, Oral EA, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840-4847. [DOI] [PubMed] [Google Scholar]
- 18. Sanon VP, Handelsman Y, Pham SV, Chilton R. Cardiac manifestations of congenital generalized lipodystrophy. Clin Diabetes. 2016;34(4):181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleckenstein JL, Garg A, Bonte FJ, Vuitch MF, Peshock RM. The skeleton in congenital, generalized lipodystrophy: evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99m bone scintigraphy. Skeletal Radiol. 1992;21(6):381-386. [DOI] [PubMed] [Google Scholar]
- 20. Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore). 2003;82(2):129-146. [DOI] [PubMed] [Google Scholar]
- 21. Billings JK, Milgraum SS, Gupta AK, Headington JT, Rasmussen JE. Lipoatrophic panniculitis: a possible autoimmune inflammatory disease of fat. report of three cases. Arch Dermatol. 1987;123(12):1662-1666. [PubMed] [Google Scholar]
- 22. Gnanendran SS, Miller JA, Archer CA, et al. Acquired lipodystrophy associated with immune checkpoint inhibitors. Melanoma Res. 2020;30(6):599-602. [DOI] [PubMed] [Google Scholar]
- 23. Schorr M, Dichtel LE, Gerweck AV, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guillín-Amarelle C, Sánchez-Iglesias S, Castro-Pais A, et al. Type 1 familial partial lipodystrophy: understanding the Köbberling syndrome. Endocrine. 2016;54(2):411-421. [DOI] [PubMed] [Google Scholar]
- 25. Herbst KL, Tannock LR, Deeb SS, Purnell JQ, Brunzell JD, Chait A. Köbberling type of familial partial lipodystrophy: an underrecognized syndrome. Diabetes Care. 2003;26(6):1819-1824. [DOI] [PubMed] [Google Scholar]
- 26. Lotta LA, Gulati P, Day FR, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49(1):17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agarwal AK, Garg AA. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87(1):408-411. [DOI] [PubMed] [Google Scholar]
- 28. Broekema MF, Savage DB, Monajemi H, Kalkhoven E. Gene-gene and gene-environment interactions in lipodystrophy: lessons learned from natural PPARγ mutants. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(5):715-732. [DOI] [PubMed] [Google Scholar]
- 29. Spuler S, Kalbhenn T, Zabojszcza J, et al. Muscle and nerve pathology in Dunnigan familial partial lipodystrophy. Neurology. 2007;68(9):677-683. [DOI] [PubMed] [Google Scholar]
- 30. Simha V, Lanza IR, Dasari S, et al. Impaired muscle mitochondrial function in familial partial lipodystrophy. J Clin Endocrinol Metab. 2021;107(2):346-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jéru I, Vatier C, Araujo-Vilar D, Vigouroux C, Lascols O. Clinical utility gene card for: familial partial lipodystrophy. Eur J Hum Genet. 2017;25(2):e1-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore). 2004;83(1):18-34. [DOI] [PubMed] [Google Scholar]
- 33. Ozgen Saydam B, Sonmez M, Yildirim Simsir I, et al. A subset of patients with acquired partial lipodystrophy developing severe metabolic abnormalities. Endocr Res. 2019;44(1-2):46-54. [DOI] [PubMed] [Google Scholar]
- 34. Adachi M, Asakura Y, Muroya K, Goto H, Kigasawa H. Abnormal adipose tissue distribution with unfavorable metabolic profile in five children following hematopoietic stem cell transplantation: a new etiology for acquired partial lipodystrophy. Clin Pediatr Endocrinol. 2013;22(4):53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adachi M, Oto Y, Muroya K, Hanakawa J, Asakura Y, Goto H. Partial lipodystrophy in patients who have undergone hematopoietic stem cell transplantation during childhood: an institutional cross-sectional survey. Clin Pediatr Endocrinol. 2017;26(2):99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rooney DP, Ryan MF. Diabetes with partial lipodystrophy following sclerodermatous chronic graft vs. host disease. Diabet Med. 2006;23(4):436-440. [DOI] [PubMed] [Google Scholar]
- 37. Carr A, Samaras K, Burton S,, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51-F58. [DOI] [PubMed] [Google Scholar]
- 38. Koethe JR, Lagathu C, Lake JE, et al. HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020;6(1):48. [DOI] [PubMed] [Google Scholar]
- 39. Baril JG, Junod P, Leblanc R, et al. HIV-associated lipodystrophy syndrome: a review of clinical aspects. Can J Infect Dis Med Microbiol. 2005;16(4):233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bacchetti P, Gripshover B, Grunfeld C, et al. Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) . Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40(2):121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Domingo P, Estrada V, López-Aldeguer J, Villaroya F, Martínez E. Fat redistribution syndromes associated with HIV-1 infection and combination antiretroviral therapy. AIDS Rev. 2012;14(2):112-123. [PubMed] [Google Scholar]
- 42. Joy T, Keogh HM, Hadigan C, et al. Relationship of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr. 2008;47(2):174-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130-139. [DOI] [PubMed] [Google Scholar]
- 44. Fourman LT, Lu M, Lee H, et al. Differential relationships of hepatic and epicardial fat to body composition in HIV. Physiol Rep. 2017;5(19):e13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lemoine M, Assoumou L, De Wit S, et al. ANRS-ECHAM Group . Diagnostic accuracy of noninvasive markers of steatosis, NASH, and liver fibrosis in HIV-monoinfected individuals at risk of nonalcoholic fatty liver disease (NAFLD): results from the ECHAM Study. J Acquir Immune Defic Syndr. 2019;80(4):e86-e94. [DOI] [PubMed] [Google Scholar]
- 46. Srinivasa S, Garcia-Martin R, Torriani M, et al. Altered pattern of circulating miRNAs in HIV lipodystrophy perturbs key adipose differentiation and inflammation pathways. JCI Insight. 2021;6(18):e150399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robbins DC, Danforth EJ Jr, Horton ES, Burse RL, Goldman RF, Sims EA. The effect of diet on thermogenesis in acquired lipodystrophy. Metabolism. 1979;28(9):908-916. [DOI] [PubMed] [Google Scholar]
- 48. Koo E, Foss-Freitas MC, Meral R, et al. The metabolic equivalent BMI in patients with familial partial lipodystrophy (FPLD) compared with those with severe obesity. Obesity (Silver Spring). 2021;29(2):274-278. [DOI] [PubMed] [Google Scholar]
- 49. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570-578. [DOI] [PubMed] [Google Scholar]
- 50. Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nguyen ML, Sachdev V, Burklow TR, et al. Leptin attenuates cardiac hypertrophy in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2021;106(11):e4327-e4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532-541. [DOI] [PubMed] [Google Scholar]
- 53. Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54(2):255-263. [DOI] [PubMed] [Google Scholar]
- 54. Cook K, Adamski K, Gomes A, et al. Effects of metreleptin on patient outcomes and quality of life in generalized and partial lipodystrophy. J Endocr Soc. 2021;5(4):bvab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cook K, Ali O, Akinci B, et al. Effect of leptin therapy on survival in generalized and partial lipodystrophy: a matched cohort analysis. J Clin Endocrinol Metab. 2021;106(8):e2953-e2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chan JL, Koda J, Heilig JS, et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf). 2016;85(1):137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rietschel P, Hadigan C, Corcoran C, et al. Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab. 2001;86(2):504-510. [DOI] [PubMed] [Google Scholar]
- 60. Schwarz JM, Mulligan K, Lee J, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87(2):942. [DOI] [PubMed] [Google Scholar]
- 61. Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96(1):150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Falutz J, Mamputu JC, Potvin D, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95(9):4291-4304. [DOI] [PubMed] [Google Scholar]
- 63. Stanley TL, Feldpausch MN, Oh J, et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stanley TL, Falutz J, Marsolais C, et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis. 2012;54(11):1642-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fourman LT, Czerwonka N, Feldpausch MN, et al. Visceral fat reduction with tesamorelin is associated with improved liver enzymes in HIV. AIDS. 2017;31(16):2253-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stanley TL, Fourman LT, Feldpausch MN, et al. Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial. Lancet HIV. 2019;6(12):e821-e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fourman LT, Billingsley JM, Agyapong G, et al. Effects of tesamorelin on hepatic transcriptomic signatures in HIV-associated NAFLD. JCI Insight. 2020;5(16):e140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: a randomized controlled trial. JAMA. 2000;284(4):472-477. [DOI] [PubMed] [Google Scholar]
- 70. Hadigan C, Rabe J, Grinspoon S. Sustained benefits of metformin therapy on markers of cardiovascular risk in human immunodeficiency virus-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab. 2002;87(10):4611-4615. [DOI] [PubMed] [Google Scholar]
- 71. Saint-Marc T, Touraine JL. Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS. 1999;13(8):1000-1002. [DOI] [PubMed] [Google Scholar]
- 72. Handelsman Y, Oral EA, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists . The clinical approach to the detection of lipodystrophy—an AACE consensus statement. Endocr Pract. 2013;19(1):107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arioglu E, Duncan-Morin J, Sebring N, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133(4):263-274. [DOI] [PubMed] [Google Scholar]
- 74. Owen KR, Donohoe M, Ellard S, Hattersley AT. Response to treatment with rosiglitazone in familial partial lipodystrophy due to a mutation in the LMNA gene. Diabet Med. 2003;20(10):823-827. [DOI] [PubMed] [Google Scholar]
- 75. Lüdtke A, Heck K, Genschel J, et al. Long-term treatment experience in a subject with Dunnigan-type familial partial lipodystrophy: efficacy of rosiglitazone. Diabet Med. 2005;22(11):1611-1613. [DOI] [PubMed] [Google Scholar]
- 76. Moreau F, Boullu-Sanchis S, Vigouroux C, et al. Efficacy of pioglitazone in familial partial lipodystrophy of the Dunnigan type: a case report. Diabetes Metab. 2007;33(5):385-389. [DOI] [PubMed] [Google Scholar]
- 77. Gambineri A, Semple RK, Forlani G, et al. Monogenic polycystic ovary syndrome due to a mutation in the lamin A/C gene is sensitive to thiazolidinediones but not to metformin. Eur J Endocrinol. 2008;159(3):347-353. [DOI] [PubMed] [Google Scholar]
- 78. Iwanishi M, Ebihara K, Kusakabe T, et al. Clinical characteristics and efficacy of pioglitazone in a Japanese diabetic patient with an unusual type of familial partial lipodystrophy. Metabolism. 2009;58(12):1681-1687. [DOI] [PubMed] [Google Scholar]
- 79. Sleilati GG, Leff T, Bonnett JW, Hegele RA. Efficacy and safety of pioglitazone in treatment of a patient with an atypical partial lipodystrophy syndrome. Endocr Pract. 2007;13(6):656-661. [DOI] [PubMed] [Google Scholar]
- 80. Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized controlled trial. Ann Intern Med. 2004;140(10):786-794. [DOI] [PubMed] [Google Scholar]
- 81. Lian J, Fu J. Pioglitazone for NAFLD patients with prediabetes or type 2 diabetes mellitus: a meta-analysis. Front Endocrinol (Lausanne). 2021;12:615409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Newman CB, Blaha MJ, Boord JB, et al. Lipid management in patients with endocrine disorders: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2020;105(12):3613-3682. [DOI] [PubMed] [Google Scholar]
- 83. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Simha V. Management of hypertriglyceridemia. BMJ. 2020;371:m3109. [DOI] [PubMed] [Google Scholar]
- 85. Utzschneider KM, Trence DL. Effectiveness of gastric bypass surgery in a patient with familial partial lipodystrophy. Diabetes Care. 2006;29(6):1380-1382. [DOI] [PubMed] [Google Scholar]
- 86. Ciudin A, Baena-Fustegueras JA, Fort JM, Encabo G, Mesa J, Lecube A. Successful treatment for the Dunnigan-type familial partial lipodystrophy with Roux-en-Y gastric bypass. Clin Endocrinol (Oxf) 2011;75(3):403-404. [DOI] [PubMed] [Google Scholar]
- 87. Melvin A, Adams C, Flanagan C, et al. Roux-en-Y gastric bypass surgery in the management of familial partial lipodystrophy type 1. J Clin Endocrinol Metab. 2017;102(10):3616-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grundfest-Broniatowski S, Yan J, Kroh M, Kilim H, Stephenson A. Successful treatment of an unusual case of FPLD2: the role of Roux-en-Y gastric bypass-case report and literature review. J Gastrointest Surg. 2017;21(4):739-743. [DOI] [PubMed] [Google Scholar]
- 89. Adams C, Stears A, Savage D, Deaton C. “We’re stuck with what we’ve got”: the impact of lipodystrophy on body image. J Clin Nurs. 2018;27(9-10):1958-1968. [DOI] [PubMed] [Google Scholar]
- 90. Raggio GA, Looby SE, Robbins GK, et al. Psychosocial correlates of body image and lipodystrophy in women aging with HIV. J Assoc Nurses AIDS Care. 2020;31(2):157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Benatti F, Solis M, Artioli G, et al. Liposuction induces a compensatory increase of visceral fat which is effectively counteracted by physical activity: a randomized trial. J Clin Endocrinol Metab. 2012;97(7):2388-2395. [DOI] [PubMed] [Google Scholar]
- 92. El-Kafoury BMA, Bahgat NM, Abdel-Hady EA, Samad A, Shawky MK, Mohamed FA. Impaired metabolic and hepatic functions following subcutaneous lipectomy in adult obese rats. Exp Physiol. 2019;104(11):1661-1677. [DOI] [PubMed] [Google Scholar]
- 93. Ajluni N, Meral R, Neidert AH, et al. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf). 2017;86(5):698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Calabro PF, Ceccarini G, Calderone A, et al. Psychopathological and psychiatric evaluation of patients affected by lipodystrophy. Eat Weight Disord. 2020;25(4):991-998. [DOI] [PubMed] [Google Scholar]
- 95. Akinci B, Onay H, Demir T, et al. Natural history of congenital generalized lipodystrophy: a nationwide study from Turkey. J Clin Endocrinol Metab. 2016;101(7):2759-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hussain I, Patni N, Garg A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology. 2019;51(2):202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation. 2001;103(18):2225-2229. [DOI] [PubMed] [Google Scholar]
- 98. Røder ME. Major adverse cardiovascular event reduction with GLP-1 and SGLT2 agents: evidence and clinical potential. Ther Adv Chronic Dis. 2018;9(1):33-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Subramanyam L, Simha V, Garg A. Overlapping syndrome with familial partial lipodystrophy, Dunnigan variety and cardiomyopathy due to amino-terminal heterozygous missense lamin A/C mutations. Clin Genet. 2010;78(1):66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Klein S, Allison DB, Heymsfield SB, et al. ; Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; American Society for Nutrition; American Diabetes Association . Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30(6):1647-1652. [DOI] [PubMed] [Google Scholar]
- 101. Triant VA, Perez J, Regan S, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137(21):2203-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.