Abstract
Background
Long‐term protective immunity to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) remains poorly characterized, particularly in solid organ transplant (SOT) patients.
Method
We determined the incidence density of SARS‐CoV‐2 reinfection in a cohort of adult SOT recipients initially infected between March 1st, 2020 and March 30th, 2021 and included those with initial infection before or after transplantation. Incidence density was the total cases divided by total days after initial diagnosis with active graft.
Results
Of 210 infected recipients, five (2.4%) developed reinfection, including two who had received full mRNA vaccination, but none developed hypoxia. The incidence density for reinfection was 9.4 (95% confidence interval [CI] 3.9‐22.6) and for primary infection the density was 9.1 (95% CI 7.9–10.5) cases/100,000 patient days. Two recipients had immunity evaluated in the weeks prior to reinfection, by measuring immunoglobulin‐G (IgG) antibody titer to the SARS‐CoV‐2 receptor binding domain and virus‐specific CD4+ and CD8+ T‐cell reactivity following stimulation with SARS‐CoV‐2 peptide pools. Both mounted virus specific CD4 T‐cell responses prior to reinfection (1.19% and 0.28% of total CD4 T cells) and both had reactive IgG testing (1.30 and 4.99 signal/cut off ratio).
Conclusions
This suggests that SOT recipients infected with SARS‐CoV‐2 remain at high risk for reinfection even after generating cellular and humoral immune responses.
Keywords: COVID‐19, reinfection, SARS‐CoV‐2, solid organ transplant
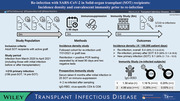
Among the cohort of patients who received a solid‐organ transplant (SOT) at our center, we studied the incidence density of SARS‐CoV‐2 reinfection among those with prior SARS‐CoV‐2 infection (n = 210). Patients were studied while on immunosuppression for an active graft. The study period included the first month after local detection of the Delta variant and prior to the emergence of the Omicron variant. Reinfection was defined according to CDC recommendations (> 90 days interval), although serial cycle threshold values were used in lieu of genomic sequencing, which was not available. In the upper right, incidence density is shown for three groups: 1.) the entire cohort (including those with initial infection prior to SOT) 2.) the subset with both infections after SOT and 3.) background rate of new primary infections among SOT during the study period. In parallel, a prospective immunity study had obtained serology and T‐cell immunity data in SOT recipients about 6 months after SARS‐CoV‐2 infection, and in two participants this was just prior to reinfection—data shown in lower right.

Abbreviations
- AIM
activation induced marker
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- Ct
cycle threshold
- mRNA
messenger RNA
- NP
nasopharyngeal
- PCR
polymerase chain reaction
- RBD
receptor binding domain
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SCR
signal/cutoff ratio
- SOT
solid organ transplant
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19) is a viral disease due to infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), resulting in millions of infections and deaths worldwide, along with immense research focus. In the general population, immunoglobulin‐G (IgG) antibodies generated after natural infection 1 or vaccination 2 were associated with protection from subsequent infection. Some important lingering questions involve the extent and durability of protective immunity in solid organ transplant (SOT) recipients.
Studies reporting incidence of SARS‐CoV‐2 reinfection have been limited to cohorts of the general population. A multicenter retrospective analysis on reinfection during the first year of the pandemic in Lombardy, Italy, reported a reinfection incidence density of 1.0 cases per 100,000 person days in a cohort from contact tracing and screening databases, compared to 15.1 cases per 100,000 person days for primary infections. 3 A prospective study using bimonthly polymerase chain reaction (PCR) testing in UK health workers found an incidence density of 7.6 cases per 100,000 patient days, compared to 57.3 cases per 100,000 person days for primary infections. 4
In SOT recipients, SARS‐CoV‐2 nasopharyngeal (NP) swab PCR and culture positivity are often significantly longer than the general population, 5 suggesting delayed or impaired initial host immune responses. Tomkins‐Tinch et al. described an SOT recipient diagnosed with SARS‐CoV‐2 reinfection based on whole genome sequencing 6 and other cases of SOT recipients fulfilling the investigative criteria defined by the Centers for Disease Control and Prevention (CDC) 7 have been observed. Immunity after vaccination is also suboptimal in SOT recipients and breakthrough infection have occurred despite full mRNA vaccination. 8 Here, we describe a case series of reinfection in SOT recipients, along with the incidence density of reinfection of a large SOT center in United States. Also, we report convalescent virus‐specific humoral and T‐cell immunity from two of the patients approximately 6 months after initial infection and prior to SARS‐CoV‐2 reinfection.
2. METHODS
We performed a retrospective cohort study of SARS‐CoV‐2 reinfection in SOT recipients at the Miami Transplant Institute, Jackson Health System, Miami, Florida, United States. The Miami Transplant Institute is one of the largest transplant centers in North America, performing more than 700 deceased donor transplants annually and providing comprehensive care after transplantation. Since the beginning of the pandemic, our institute has had systems in place to identify and catalog reports of positive SARS‐CoV‐2 testing. Per protocol, pre‐ and post‐transplant recipients were instructed to notify their transplant coordinator with any confirmed or suspected SARS‐CoV‐2 infection. Once notified, recipients were recommended to get evaluated at our institute and if the care was sought elsewhere, the reports were obtained. All patients were required to have PCR testing prior to hospital admission or procedures. After initial infection, recipients were tested until negative and were contacted via phone at least monthly for 6 months to inquire about symptoms and further test results.
Patients who were included in this study were at least 18 years old with positive NP swab PCR prior or after transplantation, between Ma rch 1st, 2020 and March 31st, 2021. We followed those patients up to death or July 31, 2021. Consistent with other studies, reinfection was defined as positive PCR testing separated by at least 90 days and two negative tests regardless of symptoms 3 , 4 , 7 To calculate the incidence density of primary infection and reinfection, we divided total cases of the event by total duration of follow‐up at risk for the event with active graft—to describe the incidence density in patients on immunosuppressive medications. The method to calculate 95% confidence interval (CI) is described elsewhere. 9 If available, cycle threshold (Ct) value, which is inversely proportional to viral load, 10 was obtained to confirm whether the Ct value had decreased from the last positive testing of the initial infection to the first positive test of suspected reinfection. Also, per CDC, it is required to have two negative PCR testing before confirmed reinfection. 7
Two patients had participated in our prospective observational study evaluating convalescent immunity 6 months after PCR confirmed SARS‐CoV‐2 infection. Briefly, we measured IgG titer to the SARS‐CoV‐2 spike protein receptor binding domain (RBD) using enzyme‐linked immunosorbent assay. We also assessed CD4+ and CD8+ T‐cell reactivity by stimulating peripheral blood mononuclear cells in vitro with peptide pools, encompassing both the SARS‐CoV‐2 spike protein and predicted immunodominant epitopes outside of the spike protein, and performing activation induced marker (AIM) assays 11 , 12 to detect virus‐specific activated T cells by flow cytometry.
PCR testing was performed using the Cepheid Xpert® platform, with Ct value of 38.30 for target N2 corresponding to the lower limit of detection. Clinical IgG antibody testing was performed via Ortho Diagnostics® platform (anti‐S1 protein), expressed in signal/cut off ratio (s/co) (lower limit of positivity: 1.0). This study was approved by University of Miami Institutional Review Board and conducted consistent with principles embodied in the Declaration of Helsinki.
3. RESULTS
3.1. Incidence density and characteristics of reinfection in SOT recipients
On March 1st, 2020, when the first case of SARS‐CoV‐2 infection was identified in the state of Florida, our center followed 5919 recipients with active graft. During the study, we identified 14 SOT recipients diagnosed with initial SARS‐CoV‐2 infection prior to transplant and 196 diagnosed after SOT, with the latter having incidence density of 9.1 (95% CI 7.9–10.5) cases/100,000 patient days. Of note, 210 recipients included 150 kidney, 18 liver, 16 heart, six lung, and 20 combined transplants. Out of those 210 recipients, 5 (2.4%) developed PCR confirmed reinfection with the incidence density of 9.4 (95% CI 3.9‐22.6) cases/100,000 patient days. When analysis for subjects with initial infection after transplantation, the incidence density was 6.0 (95% CI 1.9‐18.6) cases/100,000 patient days.
The five reinfection cases are summarized in the Table 1 and Table S1, which includes two cases of mRNA vaccine breakthrough. Two and three cases developed their first infection prior and after transplant, respectively. The median time to reinfection was 308 (range: 287–349) days. Of note, Patient 1 had the third infection and two of the other four patients had the second infection after the Delta (B.1.617.2) variant had been detected in the Miami metropolitan area. 13
TABLE 1.
Characteristics of the initial and second infection in five kidney transplant recipients
| Age | Gender | Time between transplant and the first infection | Immunosuppression at the first infection | Severity of the first infection a | Days of vaccine completion after the first infection | Interval between infections | Immunosuppression at the second infection | Severity of the second infection a | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | Male | 233 days after |
|
3 | NA | 210 days |
|
3 |
| 2 c | 45 | Male | 78 days after |
|
3 | 240 days | 287 days |
|
2 |
| 3 c | 43 | Female | 172 days before | None | 2 | 233 days (61 days after transplant) |
|
|
2 |
| 4 c | 53 | Male | 464 days after |
|
3 | NA | 365 days |
|
3 |
| 5 c | 30 | Male | 149 days before | None | 2 | NA |
|
|
2 |
Abbreviations: MMF, mycophenolate mofetil; MPA, mycophenolic acid; NA, Not applicable.
WHO scale: 1, no impairment; 2, impairment but not hospitalized; 3, hospitalized, no oxygen support; 4, hospitalized, required supplemental oxygen; 5, hospitalized and required high‐flow nasal cannula or noninvasive positive pressure ventilation; 6, required mechanical ventilation; 7, additional support.
Tacrolimus level.
Induction immunosuppression for all patients here included high‐dose corticosteroids, basiliximab, and anti‐thymocyte globulin.
All patients had sufficiently low Ct values (< 33) per CDC investigative criteria 8 except for one case (Patient 2) in whom Ct value could not be obtained but the patient had symptoms consistent with COVID‐19. For the primary infections, all five recipients developed acute viral symptoms, but one recipient's testing was conducted as a part of routine admission testing. For reinfection, four of five recipients had viral symptoms including respiratory symptoms and the other was diagnosed on testing during admission for hyperkalemia. Details on illness characteristics are described in Table S1.
Four were hospitalized for the first infection and two for the latter. None of the patients had documented hypoxia during either hospitalization. As shown in Table S1, four patients had clinical serology available before the second infection: two had unreactive IgG testing performed several weeks after the first infection and other two had reactive IgG testing within about 1 month of reinfection, although one of these recipients (Patient 3) only had reactive IgG testing following subsequent vaccination and not primary infection.
3.2. Immunity study
Two SOT recipients participated in the prospective immunity study (Table 1, Patient 1 and 2). Patient 1 participated in the study at day 171 since initial diagnosis, which was 39 days prior to reinfection. IgG titer was 1.30 SCR (reactive: > 1.0). Total SARS‐CoV‐2‐specific activated CD4+ and CD8+ T‐cell responses were 1.19% and 0.20%, respectively. Of note, SARS‐CoV‐2 PCR on the day of study visit was negative. Patient 2 participated in the study at day 208 since initial diagnosis and 79 days prior to reinfection. IgG titer and total SARS‐CoV‐2‐specific activated CD4+ were 4.99 SCR and 0.28%, respectively, but no total SARS‐CoV‐2‐specific activated CD8+ T‐cell responses were identified (Figure 1).
FIGURE 1.
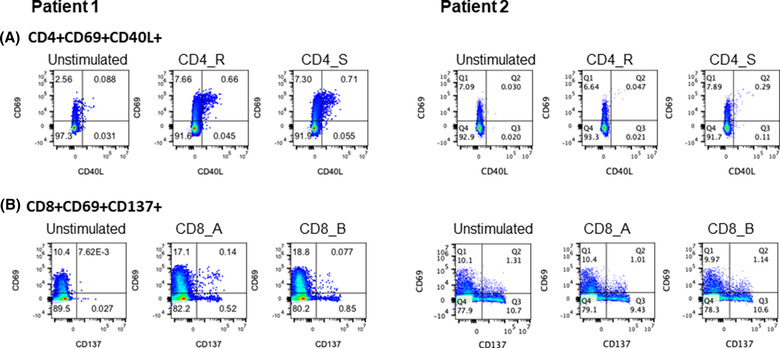
Flow cytometry. (A) CD4+CD69+CD40L, B: CD8+CD69+CD137. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2‐specific T cells were identified by flow cytometry using activation‐induced marker (AIM) assay after 24 hours stimulation of peripheral blood mononuclear cell with non‐spike (R), and spike (S) megapools for CD4 T cells and CD8 A and CD8 B megapools for CD8 T cells. SARS‐CoV‐2‐specific (A) CD4 T cells were identified as frequencies of CD69+, CD40L+ cells and (B) CD8 T cells as frequencies of CD69+ CD137+ cells
4. DISCUSSION
This study identified the incidence density of SARS‐CoV‐2 reinfection confirmed by quantitative PCR in one of the largest SOT centers, located in a COVID‐19 high incidence area. Also, we report detailed information of the five cases of reinfection and describe the convalescent humoral and cellular immunity to SARS‐CoV‐2 in two of the cases just prior to the reinfection.
This is the first study reporting the incidence density of SARS‐CoV‐2 reinfection in SOT recipients. Compared to prior studies of reinfection in the general population, the incidence density in SOT recipients was higher than another retrospective study in Italy 3 using proactive case finding and was comparable to a prospective study with bimonthly sampling. 4 The higher reinfection rate among SOT recipients may suggest a potential impact of immunosuppression, consistent with a recent study reporting immunosuppression as a significant risk factor for SARS‐CoV‐2 vaccine breakthrough infection. 14 However, the impact of immunosuppression on reinfection should be confirmed with further studies comparing subjects from similar geographic locations and from comparable intervals of the pandemic to account for regional differences in circulating virus and adherence to public health measures.
Of five subjects who had IgG serology available within approximately 3 months of reinfection, three had reactive testing in either clinical or research studies. Both subjects in the immunity study experienced mild disease in both infections and mounted virus‐specific CD4+ T‐cell responses comparable to data from the general population. 11 , 12 This is consistent with robust CD4+ T‐cell responses reported in SOT recipients 3 months after SARS‐CoV‐2 infection. 15 Taken together, these data are hypothesis‐generating and further studies should evaluate immune correlates in SOT recipients at least 6 months after natural infection 16 , 17 —timing which corresponds to long‐term immunity 18 and increased risk of reinfection. 1 It is unclear what led to the discordance between immune correlates and protection from infection in our study. One possibility is that IgG‐RBD titers and T‐cell reactivity correlate with protection from infection, but the magnitudes we observed do not correspond to a level above the threshold of protection—which has not been well defined. 16 , 17 Another possibility is that serum markers are correlates of protection from severe disease but are weaker surrogate markers in the context of reinfection, in which mucosal IgA and resident memory T cells compartmentalized in the upper respiratory tract are likely crucial 19 , 20 , 21 , 22 , 23 —and we did not measure these. However, Kute et al. documented some severe cases in SOT recipients with reinfection, as opposed to our study. 24 Out of 13 reinfection kidney transplant recipients, around half (6/13, 46.2%) developed severe disease. 24
The strengths of this study are the large size of the cohort with proactive systems for case finding of positive tests, as well as the fortuitous timing of the mechanistic immunity study in two of the cases; close to reinfection but with negative PCR testing before and after this blood draw: a true convalescent sample. Such data are quite limited 25 and there are no published studies of cellular immunity during or especially just before reinfection. 16 , 17
Our report has some limitations to be considered. A limitation of the incidence density study is that diagnosis with a second SARS‐CoV‐2 infection elsewhere would increase the incidence if patients diagnosed elsewhere did not report this to our institute. However, our transplant care services import and integrate most outside testing into the electronic medical records. Second, the data reported encompass only the first few weeks of the emergence of the Delta (B.1.617.2) variant in the Miami metropolitan area 13 and did not study reinfection with the Omicron (B.1.1.529) variant. Since these variants have greater infectivity and reduced protection afforded by vaccines, 26 we anticipate the rate of reinfection may be even greater than what we report. Third, we could not perform whole genome sequencing due to the limited time our laboratory can preserve PCR specimen and inability to obtain PCR samples from other centers. Fourth, the vaccination strategies have been changed over time and it also should affect the rate of reinfection. Thus, the result of this study may not be generalized to the current situation. Fifth, we cannot make conclusions on protection from severe disease conferred by natural infection due to the small number of reinfection cases and some patients being vaccinated or treated with monoclonal antibodies, which can protect against severe disease. 27 , 28 Finally, the limitation of the immunity study is that we only assessed cellular immunity based on circulating virus‐specific T‐cell abundance and humoral immunity via SARS‐CoV‐2 spike protein RBD IgG antibody titers. Both are strongly correlated to neutralizing antibody titers, 15 , 29 , 30 , 31 which are correlated to protection from infection and severe disease, 2 , 17 but further study is needed.
Our results suggest SOT recipients infected with SARS‐CoV‐2, even those with an initially reactive IgG or cellular response, remain at risk for reinfection in months ahead. This population should continue adhering to regional public health recommendations. Further studies should examine correlates of protection from vaccine and convalescent immunity in immunocompromised hosts and evaluate the ability of SARS‐CoV‐2 vaccination to enhance and prolong both humoral and T‐cell immunity in those recovered from SARS‐CoV‐2 infection.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTION
Stephen Morris, Giselle Guerra, and Yoichiro Natori designed the study, analyzed, and interpreted the data, and wrote the manuscript. Suresh Paallikkuth and Akina Natori analyzed the data and wrote the manuscript. The rest of the authors participated in interpretation of the data and writing the manuscript.
Supporting information
Supporting Information
Visual Abstract
ACKNOWLEDGMENTS
We would like to thank Elizabeth Varghese and Margaret Roach from the Miami Center for AIDS Research Laboratory for conducting the immunology testing and Biagio DiPascale and Clara Prado from the Jackson Health System Microbiology laboratory for providing the cycle threshold values from the PCR testing. Finally, we would like to thank Maria Alcaide, M.D., and Steve Courel for their assistance in the design and conduct of the prospective immunity study. This study was supported by the Miami Center for AIDS Research (CFAR) at the University of Miami Miller School of Medicine of the National Institutes of Health under award number P30A/1073961, which is supported by the following NIH Co‐Funding and Participating Institutes and Centers; NIAID, NCI, NICHD, NHLBI, NIDA, NIMHD, NIA, NNIDDK, NIDCR, NIMH, NINR, NIGMS, and OD.
Morris S, Anjan S, Pallikkuth S, et al. Reinfection with SARS‐CoV‐2 in solid‐organ transplant recipients: Incidence density and convalescent immunity prior to reinfection. Transpl Infect Dis. 2022;24:e13827. 10.1111/tid.13827
REFERENCES
- 1. Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2021;384(6):533‐540. 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 3. Vitale J, Mumoli N, Clerici P, et al. Assessment of SARS‐CoV‐2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021;181(10):1407‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall VJ, Foulkes S, Charlett A, et al. SARS‐CoV‐2 infection rates of antibody‐positive compared with antibody‐negative health‐care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397(10283):1459‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benotmane I, Risch S, Doderer‐Lang C, Caillard S, Fafi‐Kremer S. Long‐term shedding of viable SARS‐CoV‐2 in kidney transplant recipients with COVID‐19. Am J Transplant. 2021;21(8):2871‐2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomkins‐Tinch CH, Daly JS, Gladden‐Young A, et al. SARS‐CoV‐2 reinfection in a liver transplant recipient. Ann Intern Med. 2021;174(8):1178‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Investigative criteria for suspected cases of SARS‐CoV‐2 reinfection (ICR). October 27, 2020. Accessed 19 June, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/php/invest‐criteria.html
- 8. Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS‐CoV‐2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265‐e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990;131(2):373‐375. 10.1093/oxfordjournals.aje.a115507 [DOI] [PubMed] [Google Scholar]
- 10. Engelmann I, Alidjinou EK, Ogiez J, et al. Preanalytical issues and cycle threshold values in SARS‐CoV‐2 real‐time rt‐pcr testing: should test results include these. ACS Omega. 2021;6(10):6528‐6536. 10.1021/acsomega.1c00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Silva Antunes R, Pallikkuth S, Williams E, et al. Differential T‐cell reactivity to endemic coronaviruses and SARS‐CoV‐2 in community and health care workers. J Infect Dis. 2021;224(1):70‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020;181(7):1489‐1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC/IDSA COVID‐19 Clinician Call. SARS‐CoV‐2 gene variant trends from patient and student samples in Miami‐Dade County, Florida. June 5, 2021. Accessed 17 September, 2021. https://www.idsociety.org/globalassets/idsa/media/06‐05‐21‐clinician‐call‐slides.pdf
- 14. Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID‐19 breakthrough infection after SARS‐CoV‐2 vaccination in the US. JAMA Intern Med. 2022;182(2):153‐162. 10.1001/jamainternmed.2021.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira VH, Marinelli T, Ierullo M, et al. SARS‐CoV‐2 infection induces greater T‐cell responses compared to vaccination in solid organ transplant recipients. J Infect Dis. 2021:224(11):1894‐1860. 10.1093/infdis/jiab542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dęborska‐Materkowska D, Kamińska D. The immunology of SARS‐CoV‐2 infection and vaccines in solid organ transplant recipients. Viruses. 2021;13(9):1879. 10.3390/v1309187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Science Brief: SARS‐CoV‐2 Infection‐induced and vaccine‐induced immunity. October 29, 2021. Accessed November 11, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/science/science‐briefs/vaccine‐induced‐immunity.html#anchor_1635540136596 [PubMed]
- 18. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861‐880. 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS‐CoV‐2. Sci Transl Med. 2021;13(577):eabd2223. 10.1126/scitranslmed.abd2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fröberg J, Gillard J, Philipsen R, et al. SARS‐CoV‐2 mucosal antibody development and persistence and their relation to viral load and COVID‐19 symptoms. Nat Commun. 2021;12(1):5621. 10.1038/s41467-021-25949-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butler SE, Crowley AR, Natarajan H, et al. Distinct features and functions of systemic and mucosal humoral immunity among SARS‐CoV‐2 convalescent individuals. Front Immunol. 2021;11:618685. 10.3389/fimmu.2020.618685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal immunity in COVID‐19: a neglected but critical aspect of SARS‐CoV‐2 infection. Front Immunol. 2020;11:611337. 10.3389/fimmu.2020.611337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34:575‐608. 10.1146/annurev-immunol-032414-112315 [DOI] [PubMed] [Google Scholar]
- 24. Babiker A, Marvil CE, Waggoner JJ, Collins MH, Piantadosi A. The importance and challenges of identifying SARS‐CoV‐2 reinfections. J Clin Microbiol. 2021;59(4):e02769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kute VB, Hedge U, Das P. A multicenter cohort study of indian centers on reoccurring SARS‐CoV‐2 infections in kidney transplant recipients. Exp Clin Transplatr. 2021;19(10):1023‐1031. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention . SARS‐CoV‐2 variant classifications and definitions. December1, 2021. Accessed January 8, 2022. https://www.cdc.gov/coronavirus/2019‐ncov/variants/variant‐classifications.html
- 27. Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2‐dose vaccination with mRNA COVID‐19 vaccines against COVID‐19‐associated hospitalizations among immunocompromised adults ‐ nine states, January‐September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553‐1559. 10.15585/mmwr.mm7044e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Muecksch F, Schaefer‐Babajew D, et al. Naturally enhanced neutralizing breadth against SARS‐CoV‐2 one year after infection. Nature. 2021;595(7867):426‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brouwer PJM, Caniels TG, van der Straten K, et al. Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ni L, Ye F, Cheng ML, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity. 2020;52(6):971‐977 e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Visual Abstract


