Abstract
The pathogenesis of hyperglycemia observed in most forms of diabetes is intimately tied to the islet β cell. Impairments in propeptide processing and secretory function, along with the loss of these vital cells, is demonstrable not only in those in whom the diagnosis is established but typically also in individuals who are at increased risk of developing the disease. Biomarkers are used to inform on the state of a biological process, pathological condition, or response to an intervention and are increasingly being used for predicting, diagnosing, and prognosticating disease. They are also proving to be of use in the different forms of diabetes in both research and clinical settings. This review focuses on the β cell, addressing the potential utility of genetic markers, circulating molecules, immune cell phenotyping, and imaging approaches as biomarkers of cellular function and loss of this critical cell. Further, we consider how these biomarkers complement the more long-established, dynamic, and often complex measurements of β-cell secretory function that themselves could be considered biomarkers.
Keywords: genetics, imaging, immunology, insulin, islet amyloid polypeptide
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
The islet β cell is a critical determinant of the development of hyperglycemia in all forms of diabetes.
Alterations in the processing of proinsulin and insulin secretion as well as the loss of β cells have all been documented as part of the hyperglycemic syndrome and can be demonstrated prior to attainment of the diagnostic thresholds for diabetes.
Biomarkers are increasingly being used for predicting, diagnosing, and prognosticating disease in both research and clinical settings.
In the case of diabetes, these potential biomarkers include genetic markers, circulating molecules, and imaging approaches.
While these biomarkers complement the more long-established, dynamic, and often complex measurements of β-cell secretory function, functional measures are still frequently required to interrogate the β cell.
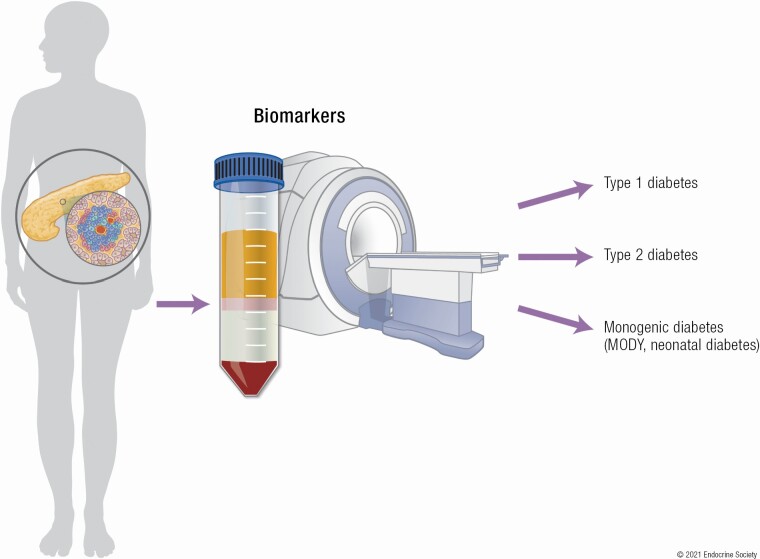
Biological markers, commonly termed biomarkers, are being used more frequently to provide an indication of the state of a biological process, pathological condition, or response to an intervention. They are considered to fall into 3 broad categories—molecular, cellular, and imaging—and are used in medicine for predicting, diagnosing, and prognosticating disease (Fig. 1).
Figure 1.
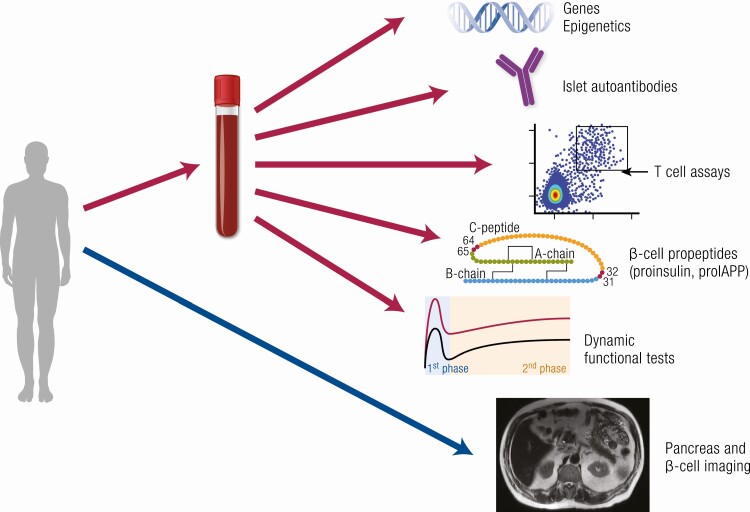
Blood- and imaging-based measures for identifying forms of diabetes and examining β-cell function and mass. Blood-based biomarkers include genetic and epigenetic markers, assays of autoimmunity including islet autoantibodies and T cells, measures of the efficiency of β-cell propeptide processing, and dynamic tests of β-cell secretory function. Imaging-based biomarkers include estimates of pancreas size and fat, β-cell mass, islet amyloid, and insulitis.
Diabetes mellitus, one of the world’s most common noncommunicable diseases (1), represents an important condition in which biomarkers have the potential to provide critical information to identify susceptible individuals prior to the onset of the disease, predict those whose disease course may progress more rapidly than others, and recognize who may be at higher risk of developing complications. The ability to do so is a central tenet of precision medicine and would allow for better management of this heterogeneous disorder (2, 3).
At the core of the need for identification and prediction of diabetes and its outcomes is the islet β cell, which by virtue of the fact that it produces the critically essential hormone insulin, plays a vital role in the development of hyperglycemia in all forms of diabetes. In this review we focus on the β cell, addressing the potential utility of different genetic, circulating, and imaging measures and how they, as biomarkers, provide insight into aspects of cellular function and cell loss. In so doing, we will examine their applicability for one or more of the different forms of diabetes. We will also consider how they complement the more traditional and/or complex measurements of β-cell secretory function that are based largely on dynamic testing. Our emphasis is on human data, supplemented by information from nonhuman studies whenever applicable.
Classification of the Different Forms of Diabetes
Diabetes is a complex, multifactorial disease defined by elevated plasma glucose concentrations. Hyperglycemia is driven by insufficient insulin, either in the presence or absence of reduced insulin sensitivity. The vast majority of cases of diabetes comprise the two major subtypes, type 1 and type 2, the latter accounting for 90% to 95% of cases worldwide (1). In the case of type 2 diabetes, there is considerable heterogeneity, as discussed in more detail later. In addition, there are a number of other subtypes that are less prevalent but of relevance to the discussion of β-cell biomarkers and diabetes.
Type 1 diabetes results from autoimmune attack leading to marked loss and dysfunction of β cells. People with type 1 diabetes have antibodies directed at islet-cell proteins and require insulin therapy early in the course of the disease (4). While most common in youth, the disease may also occur in adults. The course of the disease in older individuals is usually milder, with prolonged periods of endogenous insulin secretion and a lower incidence of diabetic ketoacidosis. This condition is often referred to as latent autoimmune diabetes in adults (LADA). People with LADA have detectable autoantibodies, but whether LADA represents the same clinical entity as type 1 diabetes is debated (5).
Type 2 diabetes typically affects older individuals who are often overweight or obese and suffer from obesity-associated insulin resistance. Disturbingly, it is now also being more frequently recognized in youth (6). In this form of diabetes, which involves multiple pathophysiological mechanisms (7-9), the loss of β-cell function is typically more gradual over time. Thus, individuals may remain rather asymptomatic and undiagnosed for a long time. Insulin is usually not required at diagnosis, although many individuals progress to insulin replacement therapy over the course of the disease (4).
Other subtypes of diabetes include gestational diabetes, which is defined as diabetes occurring in women during the second or third trimesters of pregnancy and which was not present prior to gestation. It occurs in up to 10% of pregnancies and frequently resolves after parturition, while remaining a risk factor for future type 2 diabetes (4, 10). Several monogenic diabetes syndromes have also been identified, in which defects in single genes pivotal for normal β-cell function result in hyperglycemia (3, 11-13). The most important clinical entities are mutations inducing (i) neonatal or congenital diabetes and (ii) maturity-onset of diabetes of the young (MODY). Neonatal diabetes is typically diagnosed before 6 months of age in individuals without a genetic susceptibility to type 1 diabetes and who are found to have one of the known genetic β-cell abnormalities associated with this disorder (3). This disease entity is also frequently associated with defects in other organ systems that can be ascribed to the gene mutation (3). MODY is inherited in an autosomal dominant pattern and is typically diagnosed in adolescence and early adulthood, without signs of insulin resistance and autoantibodies. The disease has been linked to a number of gene defects, which predominantly affect β-cell development and function (11). Given the differences in phenotype related to these gene defects, the required treatment can vary from no treatment for glucokinase gene defects to oral agents and/or insulin for the other forms (3, 11, 12). Pancreatic (exocrine) diseases such as cystic fibrosis, pancreatitis, trauma, hemochromatosis, and neoplasia may also induce hyperglycemia (4). With the advances in treatment of cystic fibrosis resulting in increased lifespan, the incidence and prevalence of cystic fibrosis–related diabetes (CFRD) is increasing (14). Neurocognitive disease and diabetes have been linked, with their pathophysiology perhaps related to the deposition of amyloid fibrils in brain and pancreatic islets (15). Finally, several other forms of diabetes are recognized, such as drug-induced diabetes (associated with glucocorticoids (16), calcineurin inhibitors (17), antiretroviral therapy (18, 19)), diabetes secondary to endocrinopathies (20), and new-onset diabetes mellitus after transplantation of solid organs (NODAT) (21).
Although these classifications are based on extensive clinical experience, heterogeneity exists in disease presentation, with many people failing to be readily categorized into one specific form of diabetes or another. For example, autoantibody-negative, lean adults can present with severe insulin deficiency, and people with severe obesity may present with diabetic ketoacidosis. The latter, often called Flatbush or ketone-prone diabetes, occurs particularly in individuals of non-European origin, with many of these individuals becoming insulin independent after initial presentation (22).
An alternative stratification in 5 separate disease clusters in adults with diabetes was recently proposed using parameters that include age, body mass index (BMI), glycated hemoglobin A1c (HbA1c), presence of islet autoantibodies, and a static measure for insulin sensitivity and β-cell function (23). Recently, it was proposed that a similar clustering approach could be utilized in prediabetes (24). While promising, given that the phenotypes may change over time, the true clinical value of these novel classifications remains to be determined.
Given the number of subtypes of diabetes, classifying patients at the time of diagnosis into a specific form of diabetes may be difficult due to significant overlap in phenotype, with the true diagnosis often becoming more obvious as time and the disease progress. With the ongoing identification and refinement of biomarkers, particularly those related to the β cell, we anticipate greatly enhanced understanding of the disease and its heterogeneity. This improved understanding should, in time, allow for better classification and treatment of individuals. What follows is our evaluation of the current state of knowledge and utility of biomarkers, preceded by brief descriptions of the normal physiology of β-cell function and the alterations in β-cell function and mass that result in the development of hyperglycemia.
Normal β-Cell Physiology: An Overview
To contextualize the use of biomarkers and functional tests in assessing the health of the β cell, we provide a brief overview of this specialized endocrine cell’s physiology as it applies to the production and secretion of insulin. However, it should be recognized that islet amyloid polypeptide (IAPP) is another β cell–specific peptide which is normally produced and secreted in parallel with insulin at a more or less consistent molar ratio. Therefore, unless specified otherwise, the following discussion applies to both insulin and IAPP.
Proprotein Biosynthesis and Granule Maturation
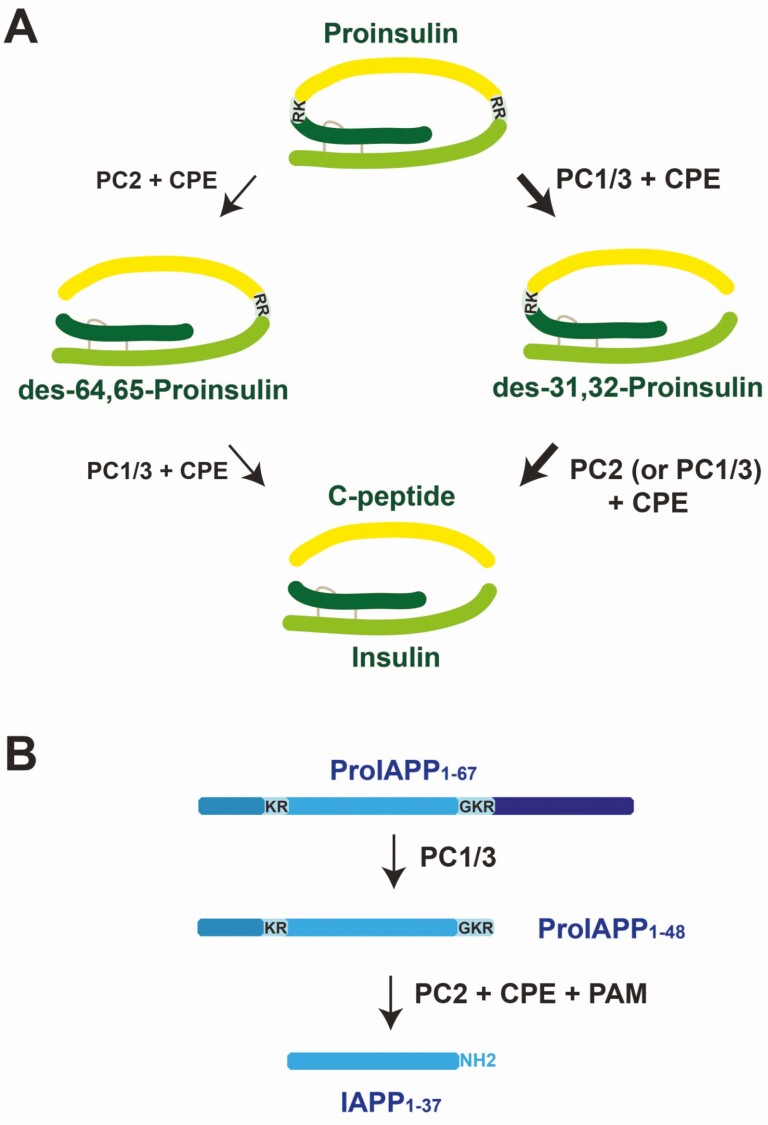
The insulin mRNA pool remains stable in β cells due to RNA binding proteins in the 5′ and 3′ untranslated regions (UTRs) of the transcript (25). Glucose stimulation drives transcription of the insulin (INS) gene, but with a sluggish response that takes approximately 1 hour for pre-mRNA levels to increase, and up to 48 hours for mature transcripts to significantly increase (26). In contrast, based on studies in rodent islets, proinsulin translation, along with that of proIAPP and the processing enzymes prohormone convertase 1/3 (PC1/3) and PC2 (PCSK1 and PCSK2, respectively), rapidly increase in response to glucose, suggesting posttranscriptional regulation of insulin expression (27-29). The β cells achieve rapid glucose-induced proinsulin synthesis by storing insulin mRNA in preassembled polysomes that are transported to the endoplasmic reticulum (ER) membrane and initiate translation in response to glucose (30).
Signal peptide cleavage occurs during translation and insertion of preproinsulin into the ER lumen. Formation of 3 intramolecular disulfide bonds, facilitated through the actions of protein disulfide isomerases, are critical to the proper folding of proinsulin in the ER: CysB7-CysA7, CysB19-CysA20, and CysA6-CysA11. The basis for trafficking and sorting of insulin granule content in β cells is not completely solved (31). Proinsulin is subject to folding as it transits the Golgi, where in mature rodent islets, on exit, it is sorted to the regulated secretory pathway with 99% efficiency (32). While in adult islets IAPP is also efficiently sorted to granules in the regulated secretory pathway (33), in neonatal islets about half of it is released via the constitutive secretory pathway (34). In human islets (pro)IAPP trafficking remains targeted to the regulated secretory pathway when cultured under basal glucose conditions; however, culturing human islets for 8 days in high glucose resulted in proIAPP release from a constitutive pathway (35). This suggests the potential for altered secretory product trafficking and release in immature or dysfunctional β cells, which may be detectable in the circulation under appropriate testing conditions.
After exit from the trans-Golgi network, secretory granules sorted to the regulated secretory pathway mature under conditions in which granule pH decreases and granule [Ca2+] and [Zn2+] increase through the actions of vesicular H+-ATPase (36) and SLC30A8 (37). With increased granule H+ and Ca2+, prohormone convertase activity also increases, initiating the conversion of proinsulin and proIAPP. This process results in the generation of conversion intermediates prior to the production of the mature peptides. These intermediate forms and the mature peptides are further trimmed at their C-terminus by carboxypeptidase E (CPE). In the case of proinsulin, this results in mature insulin and C-peptide. For proIAPP, following trimming by CPE, proIAPP is amidated at the C-terminus by peptidylglycine α-amidating monooxygenase (PAM) to yield mature IAPP (38). Recently, the role of PC2 in proinsulin processing in human β cells has come into question because PC2 immunoreactivity was not readily detectable in β cells (39), although others have reported β-cell expression by transcriptomics (40) and immunohistochemistry (41). This is compatible with the previous description in mice that PC1/3 is more critical for proinsulin processing than is PC2 (42, 43). While PC2 has been shown in mouse islets to be critical for complete proIAPP processing, in human islets the specific roles of PC2 and PC1/3 in processing proIAPP remain to be confirmed.
Not all proinsulin within a β cell will ultimately be secreted, with (pro)insulin degradation by macroautophagy and selective autophagy key to maintaining β-cell proteostasis and function (44, 45). Prior to ER-Golgi transport, misfolded proinsulin can be degraded via ER-coupled autophagy or ER-associated degradation mechanisms (46). Aged granules in β cells are also degraded, so that ultimately newer ones are preferentially secreted (47). Under conditions of nutrient depletion, newly synthesized granules are selectively degraded in the lysosome via a macroautophagy-independent mechanism, and hyperactivation of this degradation mechanism may play a role in the β-cell dysfunction of type 2 diabetes (48). Altered autophagy by β cells has been observed in pancreas samples obtained from people with type 1 and type 2 diabetes (49, 50). Given altered autophagy in these two forms of diabetes and its role in (pro)insulin degradation, it is plausible that altered autophagy may contribute to the biomarkers secreted from dysfunctional β cells in types 1 and 2 diabetes.
Peptide Secretion
Under nonstimulatory conditions, human β cells maintain a negative resting potential of approximately −70 mV. An ATP-sensitive potassium channel (KATP), composed of 4 Kir6.2 (KCNJ11) subunits and 4 sulfonylurea receptor 1 (SUR1) subunits, assists in maintaining a hyperpolarized membrane through transport of K+ ions against the membrane electrical gradient but with the [K+] gradient from the cytosol to the extracellular space.
Glucose, the primary insulin secretagogue, is transported across the plasma membrane in human β cells by GLUT1 (SLC2A1) and GLUT3 (SLC2A3) (51). The canonical model of glucose-induced insulin secretion posits that ATP generated via glycolysis and oxidative phosphorylation increases the cellular ATP/ADP ratio and results in KATP channel closure and membrane depolarization via increased cytosolic [K+]. At approximately −60 mV (52), voltage-gated calcium channels begin to open, resulting in rapid Ca2+ influx and exocytosis of insulin secretory granules. The rate-limiting step controlling insulin secretion is glucose phosphorylation by glucokinase, the enzyme having half-maximal activity at approximately 8 mM glucose (53), which correlates well with the observed half-maximal rate of glucose-induced insulin secretion in isolated human islets (54). Upon glucose stimulation, coupling of stimulus and secretion results in the rapid release of insulin in 2 phases (55, 56). In humans, the first phase begins at the start of glucose administration, is typically complete within 10 minutes, and represents a rapidly-releasable pool of secretory granules. This phase is followed by the second phase, which lasts for as long as the glucose concentration remains elevated, and includes granules containing newly synthesized insulin.
In isolated human islets, in addition to glucose, fatty acids and amino acids stimulate insulin secretion in part through anaplerosis (54, 57). In the case of amino acid–induced insulin secretion, mitochondrial glutamate dehydrogenase (GDH) is the chief enzyme involved in anaplerosis, doing so by catalyzing the deamination of glutamate to produce α-ketoglutarate (58).
Potentiation of insulin secretion can occur through intracellular metabolism or receptor-mediated signaling of exogenous signals. Peptide signals from either gut-derived incretins or islet paracrine signaling are well-recognized potentiators of insulin secretion (59). Glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon are well known secretion-amplifying signals acting via the secondary messenger cyclic adenosine monophosphate (cAMP) to activate protein kinase A (PKA) and exchange protein directly activated by cAMP 2 (Epac2) to promote exocytosis (60, 61). Somatostatin, derived primarily from the islet δ cell, is the most recognized peptide-derived paracrine inhibitor of insulin secretion (62). A myriad of other signals derived from additional cell types in the islet, including other endocrine cells, nerve fibers, endothelial cells, pericytes, and immune cells, fine tune insulin secretion to maintain euglycemia (63-66).
Summary
Based on our current knowledge of insulin and IAPP production and secretion, along with advancements in proteomics and immunoassays, it is becoming increasingly possible to analyze posttranslational modifications and β-cell secretomes in both islets and the circulation. Thus, the field is becoming better equipped to utilize biomarkers and functional tests to probe the physiology of the β cell in health and disease.
Pathophysiology of Diabetes: A Disease of Decreased β-Cell Function and Mass
Despite some uncertainties with respect to diabetes classification, virtually all diabetes entities have β-cell dysfunction as their common pathophysiological component. As such, dysfunction of this cell is not only a prerequisite for the development of diabetes, but its progressive nature typically determines the progressive course of the disease. Further, most forms of diabetes include a reduction in the number of β cells, the importance of this mass loss varying by disease type. What follows is a brief overview of the pathophysiology of these different forms of diabetes, the purpose of which is to frame the subsequent discussion of biomarkers.
Type 1 Diabetes
β-cell loss and dysfunction
The pathological hallmark of type 1 diabetes is insulitis, an inflammatory lesion of the islet associated with β-cell loss, with a key role for autoreactive T cells (67). This evidence is derived mainly from examination of type 1 diabetic pancreata obtained at autopsy (68). These human studies have made the critical observation that the degree of insulitis is heterogeneous, affecting only 10% to 30% of islets, and diffuse within an islet (68-71). These findings contrast with those in the nonobese diabetic (NOD) mouse, where nearly every islet exhibits marked T-cell infiltration (72). Human autopsy studies have clearly shown reduced β-cell mass and pancreas weight but have also demonstrated that insulin-positive β cells may persist for many years after diagnosis (68, 71).
Given the discordant relationship between the degree of islet inflammation, number of residual β cells, and severity of hyperglycemia at presentation, there has to be β-cell dysfunction that is beyond the loss of mass per se (73, 74). This dysfunction has been demonstrated in individuals who have diabetes on oral testing but do not otherwise manifest hyperglycemia (75), as well as in first-degree relatives who are at high risk of subsequently developing type 1 diabetes (76). The β-cell dysfunction in type 1 diabetes manifests as reductions in insulin release in response to intravenous and oral stimulation as well as in impaired processing of the β-cell propeptides (77-84). Further, β-cell function can be recovered after a period in which islets from individuals with recent-onset type 1 diabetes have been cultured under euglycemic conditions (85), suggesting that a component of the functional defect is related to the in vivo milieu (86, 87).
Mechanisms of disease pathogenesis
Genetic risk is largely derived from human leukocyte antigen (HLA) class II haplotypes, in particular the DR and DQ genes that are present in up to 80% to 90% of patients (88). In addition, more than 50 loci have been identified that confer risk for the disease and include candidate genes associated with immune function and/or the survival and function of the β cell (89-91).
Aside from an individual’s genetic predisposition, a trigger seems to be necessary to initiate the immune response characterizing the disease. The role of an environmental trigger in type 1 diabetes is supported by the discordant incidence rates in monozygotic twins (92) and differences in disease rates that are not simply explained by genetic differences (93). One possibility is viral infection (94), particularly enteroviruses (95), although the link to this latter group of viruses has not been universal (96). Some attention has focused recently on the gut microbiota, as its composition has been reported to differ in people with type 1 diabetes (97) and a small study raised the possibility that transplantation of fecal microbiota may preserve C-peptide responses early in the disease course (98). A number of dietary factors have also been proposed to be a trigger for the immune system (94). Importantly, environmental triggers such as viruses and bacteria activate the innate immune system, which can in turn initiate and intensify activation of the adaptive immune system as well as contribute to β-cell death and dysfunction (99, 100).
In type 1 diabetes, autoreactive CD4+ and CD8+ T cells infiltrate the islet and mediate loss and dysfunction of β cells by production of cytokines as well as cell-cell interactions (101). This effect is likely compounded by impaired function of regulatory T cells that are normally responsible for immunological tolerance (102). Finally, islet autoantibodies, which are useful biomarkers, are considered innocent bystanders (103).
Type 2 Diabetes
Impact of obesity and insulin resistance
Obesity, and particularly central/visceral adiposity, is a key component in the pathogenesis of insulin resistance (104-106). The net effect is that insulin is less effective in stimulating glucose uptake by skeletal muscle, reducing hepatic glucose production, and inhibiting adipose tissue lipolysis (107). To overcome this reduced insulin effectiveness, the β cell releases more insulin, leading to a state of hyperinsulinemia to maintain normal glucose tolerance (NGT). However, when the β-cell response is inadequate, impairments in glucose tolerance and ultimately type 2 diabetes develop (7). The inadequacy of this β-cell response will frequently only be apparent when it is interpreted in the context of the prevailing insulin sensitivity (108-110), a concept discussed in more detail later.
Role of β-cell loss and dysfunction
Type 2 diabetes is characterized by a reduction in the number of β cells as well as secretory dysfunction of those cells that remain. This process is progressive, which is easily discernible when examining function longitudinally, but it is less clear when considering β-cell mass, as repeated biopsies are not ethical and imaging techniques are not sufficiently advanced. In fact, the loss of secretory function can be demonstrated very early on in the pathogenic course of the disease, with reduced responsiveness to secretagogues evident well before glucose levels reach diagnostic thresholds. As the disease progresses, increasing doses and number of glucose-lowering medications are typically required, with progression to a need for insulin being an indicator of the β cell having reached a state of near total “failure.”
It is clear that β-cell mass is decreased in most people with type 2 diabetes, with this loss ranging from about 40% to 60% in matched subjects (111-113). A 40% deficit has even been reported in those with impaired fasting glucose (IFG) vs 63% in type 2 diabetes (111). However, the degree of loss varies tremendously among individuals, with a large degree of overlap in the proportion of β cells between healthy individuals and those with type 2 diabetes (112, 113). This reduction in β cells is the result of cell death and possibly dedifferentiation compounded by the fact that adult β cells do not readily replicate (111, 114-117). Importantly, human studies have quantified β cells in different ways and reported “β-cell mass” as area relative to exocrine or islet area on a tissue slice. This approach is important for a number of reasons. First, pancreatic weight is not always available and both pancreatic weight and volume have been shown to be lower in people with type 2 diabetes compared with nondiabetic controls (113, 118). Second, islet density may vary within the different regions of the pancreas (119). Third, the proportion of the islet comprising β cells may also vary between pancreas regions (120). Thus, the difference in the quantity of β cells determined simply by histology on pancreatic sections alone may not always provide a true estimate of the deficit (113, 118).
Defective β-cell secretory function is not only present in people with overt type 2 diabetes, but also in people with IFG or impaired glucose tolerance (IGT) (110, 121-124). These deficits affect both pulsatile and oscillatory secretion as well as the different secretagogue-induced dynamic components (125-130), with the first-phase response to intravenous glucose being essentially absent when fasting glucose exceeds 115 mg/dL (125). These secretory abnormalities exist despite immunostaining showing that insulin is still present in the islet. In addition to the defect in β-cell secretory function, the cell is also incapable of efficiently processing proinsulin to mature insulin, the magnitude of the defect linked to the degree of secretory dysfunction and glycemia (131-137). These considerations are discussed in greater detail subsequently.
The magnitude of the β-cell defect in type 2 diabetes differs between individuals and this could explain, at least in part, the heterogeneity in terms of disease progression and development of complications (23). Age appears to be an important factor in disease heterogeneity, with older age typically being associated with milder hyperglycemia, while individuals diagnosed at a younger age and particularly in adolescence, manifest a more rapid decline in β-cell function (23, 138). For reasons that are still not understood, adolescents tend to be more insulin resistant and have hyperresponsive β cells compared with body adiposity–matched, middle-aged adults (139, 140). Given the differences in β-cell function, some people can be successfully treated with oral agents, while others progress rapidly and require insulin therapy. Other contributors to the heterogeneity of glucose metabolism include sex hormones, and particularly estrogen status, medications such as steroids, and socio-economic status (141-144). Differences in the gradation of gene-based scores (genetic risk score [GRS] and partitioned polygenic score) to predict glucose concentrations and the processes that contribute to diabetes development further highlight the heterogeneity of type 2 diabetes (145-148). Finally, heterogeneity in the function of individual β cells within the islet, differences in the mass of β cells between individuals with and without type 2 diabetes, and the effect of gene variants on gene expression are also all likely to contribute to variation in β-cell function (113, 149-153). Future exploration of novel β-cell biomarkers, with or without functional tests, should better recognize this heterogeneity and could potentially reveal new differences and improve their utility in type 2 diabetes populations.
Mechanisms of disease pathogenesis
While environmental factors, most notably excessive food intake and obesity, play a key role in the rising prevalence of type 2 diabetes, heritability is a key factor. Recent advances in sequencing have identified more than 400 gene variants associated with type 2 diabetes; based on work by Mahajan et al (154) and Udler et al (155), 128 of these 400 variants can be linked to a phenotype comprising either β-cell function, obesity/adiposity, lipodystrophy-like/insulin action, or lipid metabolism/liver (156). Of these, nearly 70% had a β-cell phenotype related to growth, development, and/or function (148, 154-157). Evidence is also accumulating suggesting that epigenetic changes (DNA methylation, histone modification, microRNA [miRNA], long non-coding RNAs [lncRNAs]), possibly starting as early as in utero, are an additional factor (158, 159).
Islet amyloid deposition has long been recognized to occur in the majority of people with type 2 diabetes (151, 160, 161). These deposits are formed by aggregation of the normal β-cell secretory product IAPP (162, 163). While its physiological role remains uncertain, in its native form IAPP is not harmful. However, the process of oligomer formation renders the peptide cytotoxic resulting in β-cell apoptosis and dysfunction; interestingly, the end product amyloid appears to be largely inert (164). Based largely on studies in animal models, the magnitude of amyloid formation appears to be related to the degree of secretory demand placed on the β cell (165-167). With progress in imaging, it is now possible to demonstrate the presence of islet amyloid in vivo in animals (168-171), and in time it is hoped this will be possible in humans and will provide further insight into the pathogenesis of type 2 diabetes.
A role for chronic inflammation in type 2 diabetes has been firmly established, although the primary trigger(s) remain unclear and the utility of individual molecules as biomarkers of the status of the β cell is not well established. Postulated triggers of islet inflammation in type 2 diabetes, characterized by increased number of activated proinflammatory macrophages, cytokine production, and β-cell dysfunction, include dyslipidemia and IAPP aggregates (172-176). Islet-resident macrophages are emerging as important players both in β-cell health and regeneration (177), and in mediating β-cell dysfunction in type 2 diabetes via proinflammatory cytokines such as interleukin (IL)-1 β, IL-6, and tumor necrosis factor (TNF)-α (178). A role of islet inflammation in type 2 diabetes was supported by a small clinical trial in which administration of the IL-1 receptor antagonist anakinra improved glycemic control and β-cell function (179). However, in a larger, long-term clinical trial, IL-1 antagonism with a human anti-IL-1β monoclonal antibody (canakinumab) did not reduce diabetes incidence (180), although some initial improvement in glycemia was observed in those with preexisting diabetes. Whether the adaptive immune system contributes to islet dysfunction in type 2 diabetes is more debated (181).
Monogenic Forms of Diabetes
Maturity-onset diabetes of the young
MODY, first described in 1974 (182), is a cluster of 11 different autosomal dominant forms of diabetes, which in many instances affect transcription factors, resulting in impaired insulin production and release (3). The 4 most common forms affect (i) glucokinase (MODY2), the rate-limiting step in β-cell glucose metabolism, that is characterized by β-cell insensitivity to glucose mediating a relatively small impairment in insulin secretion and thus relatively mild hyperglycemia and typically no need for glucose-lowering therapy (11, 183); (ii) HNF-1α (MODY3) and the less common HNF-4α (MODY1), both of which result in hyperglycemia and progressive β-cell dysfunction requiring pharmacological intervention that can initially be sulfonylureas but frequently advances to insulin (184, 185); and (iii) HNF-1β (MODY5), where the progressive loss of β-cell function with an insulinopenic phenotype is frequently accompanied by variable renal abnormalities and developmental defects of the genital tract (186).
Neonatal diabetes
Neonatal diabetes becomes apparent early in life and when it consists of homozygous mutations in certain MODY genes, it induces permanent neonatal diabetes and pancreas agenesis. About 50% of cases are caused by potassium channel gene (KCNJ11 and ABCC8) mutations, resulting in impaired insulin secretion that can be readily restored with sulfonylurea treatment (3, 13). While impairments in β-cell function characterize neonatal diabetes, some of these mutations are also associated with defects in other organ systems (3, 13).
Cystic Fibrosis–Related Diabetes
Cystic fibrosis arises due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and is the most common, lethal, autosomal recessive disorder (187). CFRD is characterized by mild insulin resistance, impaired β-cell function, and reduced β-cell mass along with disordered glucagon release (188). The β-cell dysfunction occurs through distinct mechanisms, including a marked inflammatory response (189, 190), impaired chloride conductance in the β-cell membrane and increased susceptibility to oxidative stress (191-193). There remains some controversy over whether the chloride channel is in fact present in β cells, since small studies utilizing CFTR modulator therapy failed to improve insulin secretion and glucose tolerance (194, 195). Interestingly, islet amyloid has also been observed on autopsy in people with cystic fibrosis (190, 196).
Summary
β-cell dysfunction is a characteristic of all the different forms of diabetes, although differences in pathophysiology exist. In type 1 diabetes, β-cell loss through autoimmune attack is an important contributor to the secretory function defect. On the other hand, in type 2 diabetes, while β-cell loss is important, the functional defect seems to be a more critical component. While risk genes for these 2 major forms of diabetes have been identified, they have not proven as useful as biomarkers as in the monogenic forms of diabetes and CFRD. In time, we expect that identification of additional aspects of the pathogenesis of hyperglycemia will result in the development of new biomarkers that will supplement genetic information and allow for the use of composite measures that better predict disease development and outcomes.
Genetics and Epigenetics
Over the last decade, there has been major progress in our understanding of the genetic basis of the different forms of diabetes. Genome-wide association studies (GWAS) have identified more than 400 sequence variants (single nucleotide polymorphisms [SNPs]) associated with individual risk of type 1 and type 2 diabetes (89, 90, 197). Combining these variants into genetic risk scores (GRS) offers information that remains stable throughout life and improves the prediction of diabetes risk and understanding of diabetes heterogeneity (148). The complex interplay between genetic and environmental factors associated with diabetes has been further highlighted by identification of various epigenetic modifications, such as methylation/acetylation, that can alter gene expression. What follows is a discussion of the utility (and lack thereof) of genetics along with epigenetics as potential biomarkers in diabetes.
Genetics
Type 1 diabetes
Genetics is an important contributor to type 1 diabetes, with an identical twin concordance rate between 30% and 70% (198-200) and a risk of ∼7% for siblings (201). The main genetic drivers underlying this risk are Class II HLA (or major histocompatibility [MHC] system) DR and DQ genes, which are located on chromosome 6 and encode for cell surface proteins typically expressed on antigen-presenting cells (202). The HLA Class II haplotypes DR3-DQ2 and DR4-DQ8, alone or in combination, are known to be associated with the highest genetic risk for type 1 diabetes and are considered to contribute to 50% of type 1 diabetes heritable risk (202). Of note, other HLA Class II haplotypes, such as DRB3, DRB4, and DRB5, have also been associated with an increased risk of type 1 diabetes (203, 204). In contrast, certain HLA Class II haplotypes, such as DR15-DQ6, seem to confer protection from type 1 diabetes (202). Therefore, prospective studies starting early in life have used Class II HLA DR-DQ typing, alone or in combination with family history of type 1 diabetes, to assess the genetic risk for development of islet autoantibodies and progression to clinical type 1 diabetes (205-207). Interestingly, some studies have reported that the specificity and order of appearance of the first islet autoantibody was related to the HLA DR-DQ genotype (208, 209). However, in a cohort of the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study, while Class II HLA DR-DQ genotype improved estimation of type 1 diabetes risk, it was limited to the development of islet autoimmunity and was not associated with the progression rate from advanced autoimmunity to clinical diabetes (210).
While the HLA Class II DR-DQ region represents the strongest association with type 1 diabetes, other SNPs outside the Class II HLA region impact the risk of and progression of the disease. Some HLA Class I genes (eg, A*24 and B*39 alleles) encoding for presenting peptides for T cells, have also been independently associated with type 1 diabetes susceptibility and progression of β-cell loss (211-213). Further, in recent years, linkage analysis and GWAS have identified more than 50 non-HLA genetic loci contributing to type 1 diabetes risk, including SNPs near the preproinsulin (INS), protein tyrosine phosphatase non receptor type 22 (PTPN22), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), interleukin 2 receptor α (IL2RA) and CFTR genes (89-91, 214) (Table 1). Studies have confirmed that some of the genetic variants outside of the HLA DR-DQ region can affect seroconversion for islet autoantibodies and/or progression to clinical diabetes (209, 215-217). In a very recent study in children, those with certain susceptibility alleles demonstrated a more rapid decline in β-cell function compared with those without these alleles (213). Furthermore, while many of these type 1 diabetes susceptibility candidate genes are involved in immune function, the emerging concept is that some of them are expressed in islets and may play a role in modulating β-cell function and survival (218, 219). Examples include GLIS3 (220), which may contribute to cytokine-induced cell death, and RNLS (221), which was identified in a CRISPR screen as a modulator of immune-mediated β-cell death.
Table 1.
Major genes with a β-cell phenotype linked to the different types of diabetes
| Gene symbol | Gene name | Function of gene product | Type(s) of diabetes |
|---|---|---|---|
| ABCC8 | ATP-binding cassette transporter subfamily C member 8 | Insulin secretion (modulation of ATP-sensitive potassium channels) | Type 2 diabetes; Monogenic diabetes |
| ADCY5 | Adenylate cyclase 5 | Regulation of calcium-dependent insulin secretion | Type 2 diabetes |
| AP3S2 | Adaptor related protein complex 3 subunit sigma 2 | Golgi vesicles formation and trafficking to lysosomes | Type 2 diabetes |
| ARAP1 | ArfGAP with RhoGAP domain, ankyrin repeat and PH domain 1 | Regulation of Golgi structure and cytoskeleton; Cell migration | Type 2 diabetes |
| BCAR1 | BCAR1 scaffold protein, cas family member | Cell adhesion and migration | Type 2 diabetes |
| BCL11A | B-cell CLL/Lymphoma 11A (Zinc finger protein) | Lymphopoiesis; Negative regulation of p53 activity (transcriptional repressor) | Type 2 diabetes |
| C2CD4A | C2 calcium-dependent domain containing 4A | Transcription factor | Type 2 diabetes |
| CCND2 | Cyclin D2 | Cell cycle regulation | Type 2 diabetes |
| CDC123/CAMKID | Cell division cycle 123 homolog/Calmodulin dependent protein kinase ID | Cell cycle regulation | Type 2 diabetes |
| CDKAL1 | CDK5 regulatory subunit associated protein 1-like 1 | Growth and development; Proinsulin to insulin conversion | Type 2 diabetes |
| CDKN2A | Cyclin dependent kinase inhibitor 2A | Cell cycle regulation | Type 2 diabetes |
| CEL | Carboxyl ester lipase | Cholesterol and lipid-soluble vitamin ester hydrolysis | Monogenic diabetes |
| CENPW | Centromere protein W | Cell cycle regulation | Type 1 diabetes; Type 2 diabetes |
| CTSH | Cathepsin H | Lysosomal proteins degradation | Type 1 diabetes |
| DGKB | Diacylglycerol kinase beta | Cell signaling | Type 2 diabetes |
| G6PC2 | Glucose-6-phosphasase catalytic 2 | Glucose metabolism | Type 2 diabetes |
| GCK | Glucokinase | Glucose metabolism; β-cell growth and development | Monogenic diabetes |
| GIPR | Gastric inhibitory polypeptide receptor | Potentiation of insulin secretion | Type 2 diabetes |
| GLIS3 | GLIS family zinc finger 3 | β-cell growth and development; Transcription factor | Type 1 diabetes; Type 2 diabetes; Monogenic diabetes |
| GPSM1 | G protein signaling modulator 1 | Cell signaling | Type 2 diabetes |
| HHEX | Hematopoietically expressed homeobox | Growth and development; Transcription factor | Type 2 diabetes |
| HMG20A | High mobility group 20A | Transcription factor; Histone methylation | Type 2 diabetes |
| HMGA2 | High mobility group AT-Hook 2 | Transcription factor; Chromatin regulation/acetylation | Type 2 diabetes |
| HNF1A | Hepatic nuclear factor 1 α | β-cell growth and development; Transcription factor | Type 2 diabetes; Monogenic diabetes |
| HNF1B | Hepatic nuclear factor 1 β | β-cell growth and development; Transcription factor | Type 2 diabetes; Monogenic diabetes |
| HNF4A | Hepatic nuclear factor 4 α | β-cell growth and development; Transcription factor | Monogenic diabetes |
| HSD17B12 | Hydroxysteroid 17-β dehydrogenase 12 | Metabolism of steroid hormones | Type 2 diabetes |
| IDE | Insulin-degrading enzyme | Peptide degradation (including insulin, IAPP and glucagon) | Type 2 diabetes |
| INS | Insulin | Insulin production | Type 1 diabetes; Type 2 diabetes; Monogenic diabetes |
| IGF2BP2 | Insulin-like growth factor 2 binding protein 2 | β-cell growth and development; Transcription factor | Type 2 diabetes |
| JAZF1 | Juxta-posed with another zinc finger gene 1 | Cell cycle regulation; Transcriptional repressor | Type 2 diabetes |
| KCNJ11 | Potassium voltage-gated channel subfamily J member 11 | Insulin secretion | Type 2 diabetes; Monogenic diabetes |
| KCNQ1 | Potassium voltage-gated channel subfamily Q member 1 | Insulin secretion | Type 2 diabetes |
| KLF11 | Kruppel like factor 11 | Exocrine cell growth and development; Transcription factor | Monogenic diabetes |
| MTNR1B | Melatonin receptor 1B | Mediation of melatonin actions (including inhibitory effect on insulin secretion) | Type 2 diabetes |
| NEUROD1 | Neurogenic differentiation 1 | Growth and development; Transcription factor | Monogenic diabetes |
| NOTCH2 | Neurogenic locus notch homolog protein 2 | Growth and development; Transcription factor | Type 2 diabetes |
| PAM | Peptidylglycine α-amidating monooxygenase | β-cell processing enzyme | Type 2 diabetes |
| PAX4 | Paired box gene 4 | β-cell development and differentiation; Transcription factor | Type 2 diabetes; Monogenic diabetes |
| PDX1 | Pancreatic and duodenal homeobox 1 | Pancreatic and β-cell growth and development; Transcription factor | Monogenic diabetes |
| PIM3 | Pim-3 proto-oncogene, serine/Threonine kinase | Cell signaling; Cell proliferation and survival | Type 2 diabetes |
| PTPN2 | Protein tyrosine phosphatase non-receptor type 2 | Cell survival | Type 1 diabetes |
| PTPN9 | Protein tyrosine phosphatase non-receptor type 9 | Cell signaling; Cell growth and differentiation; Cell cycle regulation | Type 2 diabetes |
| PRC1 | Protein regulator of cytokinesis 1 | Cell cycle regulation | Type 2 diabetes |
| PROX1 | Prospero homeobox 1 | Transcription factor | Type 2 diabetes |
| RNLS | Renalase | Modulator of immune-mediated β-cell death | Type 1 diabetes |
| RREB1 | Ras responsive element binding protein 1 | Transcription factor | Type 2 diabetes |
| Cell differentiation | |||
| SLC30A8 | Solute carrier family 30 member 8 | Proinsulin and proIAPP conversion | Type 2 diabetes |
| SPRY2 | Sprouty RTK signaling antagonist 2 | Cell signaling | Type 2 diabetes |
| TCF7L2 | Transcription factor 7 like 2 | Blood glucose homeostasis; Transcription factor | Type 2 diabetes; Cystic fibrosis–related diabetes |
| THADA | Thyroid adenoma associated protein | Cell survival | Type 2 diabetes |
| WFS1 | Wolframin ER transmembrane glycoprotein | Regulation of cellular calcium homeostasis | Type 2 diabetes; Monogenic diabetes |
| ZBED3 | Zinc finger BED-type containing 3 | Transcription factor | Type 2 diabetes |
This high genetic heritability (including HLA and non-HLA variants) provides the opportunity to use the GRS to define and stratify the risk for type 1 diabetes. Most of the non-HLA DR-DQ variants seem to have only modest effects on the total genetic risk of developing type 1 diabetes (222). However, their incorporation together with HLA loci in an integrated GRS increases the ability to predict type 1 diabetes and is more powerful than HLA DR-DQ genotyping alone (223-226). The most recent type 1 diabetes GRS, which included 67 SNPs and accounted for interactions between 18 HLA DR-DQ combinations, when applied in samples in the UK Biobank, performed best in identifying individuals with type 1 diabetes (227). Furthermore, combining this most recent genotyped risk with family history, autoantibodies, and clinical characteristics markedly improved type 1 diabetes prediction among susceptible children compared with measurement of autoantibodies alone (228). Finally, assessment of a type 1 diabetes GRS may also help to discriminate type 1 diabetes from type 2 diabetes (229) or from monogenic forms of diabetes (230).
Type 2 diabetes
There is compelling evidence that genetic predisposition underlies the development of type 2 diabetes. Recent estimates of type 2 diabetes heritability range from 25% to 80%, varying based on study duration, parental history, and sibling history (231, 232). Technological and analytical advances have led to the identification of numerous genes linked with type 2 diabetes. Since the first identification of PPARγ using the candidate gene approach (233), GWAS has now identified more than 400 gene variants for type 2 diabetes susceptibility (154). Very recently, both known and novel loci were also identified in the first GWAS of youth-onset type 2 diabetes, suggesting there is a significant overlap in the genetic architecture of the disease in youth and adults (234). Although most products of these gene variants have not been identified, some have been linked to obesity (eg, FTO) and insulin sensitivity (eg, IRS1 and PPARγ), with most linked to β-cell function (eg, TCF7L2, PAM, SLC30A8, MTNR1B, HNF1A, HNF1B) (154, 155, 157, 219) (Table 1). With increasing sample size (235), it is now also becoming possible to use complementary approaches of whole genome or whole exome sequencing to identify specific gene variants (236). Indeed, classification of these GWAS variants according to their association with diabetes-related metabolic traits has identified robust groups each characterized by a specific pathophysiological process: reduced β-cell function with high proinsulin, reduced β-cell function with low proinsulin, obesity, lipodystrophy, and liver/lipid metabolism (154, 155). These findings suggest that cluster analysis could represent an interesting approach to better define the clinical heterogeneity of type 2 diabetes that contributes to different clinical outcomes.
The first use of the GRS analyzed the combined risk of 16 to 18 SNPs and showed only slightly improved prediction of incident diabetes compared with that of clinical risk factors alone (145, 146). With identification of new loci with successive larger GWAS (154, 237), extended polygenic scores have enhanced, albeit modestly, the ability to predict subsequent type 2 diabetes (154). Altogether they are likely to explain around 20% of the overall variation in type 2 diabetes risk, that is half of the estimated heritability (154). In the prospective Metabolic Syndrome in Men (METSIM) Study, a genetic score for type 2 diabetes that included 76 SNPs was associated with changes in β-cell function (quantified by the disposition index), as well as with a 2-fold increase in risk of type 2 diabetes (147). Interestingly, comparison of the 3 published global, extended GRS for type 2 diabetes reported a similar 2.75-fold increased risk for individuals in the top 5% of the polygenic score distribution vs the remainder of the study population (148); however, the clinical utility of these GRS remains unclear. First, their value to improve prediction of incident diabetes is modest compared with clinical risk factors alone. Second, their ability to capture risk in individuals from non-European origin might be suboptimal since most of the GWAS data are derived from European cohorts. Therefore, more studies are required in other populations to generate equivalent data and GRS that could perform best and apply to those populations.
Monogenic diabetes
In contrast to type 2 diabetes, which has overlapping polygenic susceptibility, the molecular genetics underlying monogenic diabetes subtypes, including transient and permanent neonatal diabetes (developing before 6 months of age) and MODY, is well defined and has profound implications on both treatment and future development of associated clinical features (3, 13) (Table 2). Genetic testing has also been suggested to be cost-effective in patients with high suspicion of monogenic diabetes (238).
Table 2.
MODY genes and their pathophysiology, clinical phenotype, and treatment
| Gene symbol | Gene name | Type of familial diabetes | Frequency (% of MODY) | Pathophysiology | Clinical phenotype | Treatment |
|---|---|---|---|---|---|---|
| HNF4A | Hepatic nuclear factor 4 α | MODY1 | 5%-10% | Progressive β-cell dysfunction (mainly insulin secretory defect) | - Common fetal macrosomia | Diet; SU; Insulin |
| - Transient neonatal hyperinsulinemia and hypoglycemia followed by diabetes later in adolescence or adulthood | ||||||
| - Low triglycerides | ||||||
| - Microvascular complications | ||||||
| GCK | Glucokinase | MODY2 | 30%-50% | β-cell dysfunction (glucose sensing defect) | - Stable mild hyperglycemia | No medication (except possibly in pregnancy) |
| - Low prevalence of microvascular complications | ||||||
| HNF1A | Hepatic nuclear factor1 α | MODY3 | 30%-65% | Progressive β-cell dysfunction (mainly insulin secretory defect) | - Progressive hyperglycemia with early onset | Diet; SU (additional glinides, GLP1-RA, DPP-4i); Insulin |
| - Glycosuria | ||||||
| - Transient neonatal hyperinsulinemia and hypoglycemia in some | ||||||
| - Microvascular complications | ||||||
| PDX1 | Pancreatic duodenal homeobox 1 | MODY4 | <1% | β-cell dysfunction | - Range from impaired glucose tolerance to diabetes | Oral glucose-lowering agents; Insulin |
| - Pancreas agenesis (homozygosis form) | ||||||
| HNF1B | Hepatic nuclear factor 1 β | MODY5 | <5% | β-cell developmental defect and dysfunction | - Diabetes | Oral glucose-lowering agents (minority respond to SU); Insulin |
| - Renal malformations | ||||||
| - Exocrine pancreas deficiency malformation | ||||||
| - Female reproductive organ abnormalities | ||||||
| NEUROD1 | Neurogenic differentiation 1 | MODY6 | <1% | β-cell dysfunction | Early onset of diabetes | Oral glucose-lowering agents; Insulin |
| CEL | Carboxyl-ester lipase | MODY8 | <1% | Pancreas endocrine and exocrine dysfunction | - Typically autosomal dominant diabetes | Oral glucose-lowering agents (including SU); Insulin |
| - Exocrine pancreatic dysfunction | ||||||
| - Lipomatosis | ||||||
| INS | Insulin | MODY10 | <1% | β-cell dysfunction | Neonatal, child or adult-onset diabetes | Diet; |
| Oral glucose-lowering agents (including SU); | ||||||
| Insulin | ||||||
| ABCC8 | ATP-binding cassette transporter subfamily C member 8 | MODY12 | <1% | Insulin secretion defect (ATP-sensitive potassium channel dysfunction) | Frequently causes neonatal diabetes. Clinical phenotype similar to MODY4 | SU; Insulin |
| KCNJ11 | Potassium voltage-gated channel subfamily J member 11 | MODY13 | <1% | Insulin secretion defect (ATP-sensitive potassium channel dysfunction) | Heterogeneous | SU; Insulin |
| APPL1 | Adaptor protein phosphotyrosine interacting with pH domain and leucin zipper 1 | MODY14 | <1% | Insulin secretion defect | Dysmorphic phenotype and delay in development | Diet; Oral glucose-lowering agents (including SU); Insulin |
Abbreviations: DPP4i, dipeptidyl peptidase 4 inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; MODY, maturity-onset diabetes of the young; SU, sulfonylurea.
aAlthough classified as MODY genes, KLF11 (MODY7), PAX4 (MODY9) and BLK (MODY11) are not included as they were recently refuted or disputed by a group of experts in monogenic diabetes (239).
bMultiple loss of function variants in RFX6 have recently been described producing a phenotype similar to other MODY genes but with lower penetrance (573). It is not included in the table as it has not yet been assigned a MODY number.
Over 20 different genetic causes of neonatal diabetes have been described so far, and these predominantly affect the β cell (3, 13). Half of the neonatal diabetes diagnoses are linked to mutations in the potassium channel genes KCNJ11 and ABCC8 and have excellent therapeutic responses to sulfonylureas (3, 13). Insulin is usually required in patients carrying other gene mutations associated with neonatal diabetes. Genetic testing also allows the anticipation and identification of associated clinical features such as heart defects and exocrine pancreas deficiency with mutations in GATA4 and GATA6 (3, 13).
MODY has been associated with relevant mutations in at least 14 genes, again predominantly β-cell related (3, 11, 12), although 3 of these genes (KLF11, PAX4, and BLK) have been recently refuted or disputed by consensus of a group of monogenic diabetes experts (239) (Table 2). Mutations in genes encoding glucokinase (GCK) and the β-cell transcription factors hepatic nuclear factor 1 α (HNF1A), hepatic nuclear factor 4 α (HNF4A), and hepatic nuclear factor 1 β (HNF1B) are the most common (3, 11, 12, 240). Importantly, the identification of the MODY subtype results in different therapeutic strategies (3, 11, 12, 241). For example, low-dose sulfonylureas are effective as treatment for MODY caused by mutations in HNF1A and HNF4A, while insulin is required in HNF1B-MODY (3, 11, 12, 241).
Cystic fibrosis–related diabetes
CFRD has emerged as a common complication of cystic fibrosis and is caused by islet inflammation and β-cell dysfunction and loss. While CFTR mutations have been shown to increase the risk of diabetes independently of other risk factors such as pancreatic exocrine dysfunction (242), a recent GWAS has identified other CFRD risk loci (243). The latter reported a genetic overlap with type 2 diabetes and CFRD (eg, TCF7L2) but also interestingly identified 2 CFRD risk loci, PTMA and SLC26A9, that are unrelated to type 2 diabetes.
Epigenetics
Epigenetics represents changes that do not involve alterations of the ribonucleotide sequence but occur beyond conception (Fig. 2). Epigenetic changes, which comprise DNA methylation, histone modifications (acetylation/deacetylation), and noncoding RNA-mediated gene expression modifications, can occur as a result of genetic and/or environmental factors. The intrauterine environment represents the first potential exposure to some factors that have been linked to type 2 diabetes later in life (244). Examples include low birthweight, high birthweight, maternal obesity, gestational diabetes, smoking, and chemicals. Subsequent to birth, environmental factors can also contribute to epigenetic changes, modifying expression of genes involved in type 1 and type 2 diabetes (245, 246) and thereby increasing susceptibility to develop the disease. Advances in our understanding of epigenetics also provide compelling evidence for dysregulation of islet-specific gene expression in type 2 diabetes (247-250). Recent evidence, as detailed below, suggests that epigenetic biomarkers may be useful in the future for predicting diabetes.
Figure 2.
Epigenetic modifications occurring in the β cell. Environmental factors and/or genetics can contribute to epigenetic alterations in the β cell, thereby modifying expression of genes involved in β-cell function and survival. Epigenetic alterations include DNA methylation, histone modifications, and non-coding RNAs.
DNA methylation
DNA methylation has been found to be altered in pancreatic islets from human donors with type 2 diabetes and associated with impaired insulin secretion (251-253). Of note, some of these changes in DNA methylation involved several SNPs identified in GWAS to associate with type 1 diabetes (eg, HLA, INS, PTPN22) and type 2 diabetes (eg, KCNJ11 and ADCY5). In prospective cohorts, epigenome-wide association studies (EWAS) have also reported changes in the methylation status of whole blood DNA that were associated with an increased risk of incident type 2 diabetes (254-257). However, it is not clear whether these epigenetic changes occurred prior or following the development of hyperglycemia. It has been suggested that a diabetic milieu per se can alter human islet gene expression and methylation: expression of 1855 genes changed, with 1469 demonstrating variations in DNA methylation (258). In very recent studies, maternal dysglycemia was associated with changes in DNA methylation of neonates (259, 260), which appeared to be reduced by lifestyle intervention during pregnancy (260). Whether these associations are causal and linked to the risk of incident diabetes still needs to be elucidated. In mice, exposure to hyperglycemia in utero resulted in changes in gene methylation that were associated with decreased fasting insulin concentrations and glucose intolerance in vivo, and impaired glucose-simulated insulin secretion in vitro in islets from these same mice (261). Finally, changes in DNA methylation could also be associated with autoimmune diabetes. For example, a study has detected changes in DNA methylation in CD4+ T cells from adult patients with LADA (262).
Thus, changes in DNA methylation seem to be linked to impaired β-cell function and/or autoimmune responses. Whether these changes can be used as biomarkers for diabetes in clinical practice still requires more investigation. Although studies have shown an overlap in DNA methylation changes between blood and tissues, most identified DNA methylation loci are tissue specific. More studies are therefore needed to confirm that changes in DNA methylation in pancreatic islets are reflected in the peripheral circulation. Finally, since most studies have used cross-sectional approaches, more prospective studies are required to confirm whether any of these epigenetic changes are predictive of diabetes.
Histone modifications
Histone modifications have been detected in blood cells of individuals with type 2 diabetes (263, 264) and type 1 diabetes (265). However, to date there are no reports of genome-wide histone modifications in pancreatic islets from diabetic subjects. Therefore, there is a need for studies of these modifications in patients with diabetes.
MicroRNAs
Recent work on circulating (plasma/serum) miRNAs has highlighted their potential future use as biomarkers in diabetes (158). miRNAs are small noncoding RNAs that act as key regulators of gene expression and are enriched in specific tissues, including pancreatic islets. They can be modified by environmental factors. For example, miR132, which is normally involved in adaptation of β cells to insulin resistance, is upregulated in mice on a high fat diet and in cultured islets or cells exposed to glucose and palmitate (266).
Genetic alterations in miRNAs are rare but would appear to promote the development of type 2 diabetes by reducing β-cell function (266). Under stress conditions and β-cell death, miRNAs can be produced and released by islets into the circulation. Because studies have reported conflicting results regarding circulating miRNAs in type 2 diabetes (267), their use as biomarkers in type 2 diabetes remains unclear. Reasons for discrepancies include differences in study design and population size. With regard to type 1 diabetes, there is also evidence of a different signature of circulating and blood cell miRNAs in patients, some of which have been associated with immune cell responses (268-270). A recent study has identified serum miR-204, which is highly enriched in β cells and known to regulate critical processes of β-cell biology, as a new biomarker for type 1 diabetes-associated β-cell loss (271). However, while miRNAs offer the advantage of being stable and resistant, current limitations for their use as biomarkers for β-cell dysfunction include the absence of tissue-specificity and standardized procedures used in studies. Thus, large prospective studies will likely be required to identify clinically reliable circulating islet-specific miRNA signatures in type 1 and type 2 diabetes.
Long non-coding RNAs
Long non-coding RNAs are typically greater than 200 nucleotides and do not code for proteins. GWAS has identified a large number of lncRNAs of potential importance in diabetes; for example, LOC157273 is associated with increased liver glycogen and may be relevant to diabetes (272-274). For most, however, their functional characteristics are not well understood. That said, a number of groups have demonstrated that challenging islets with approaches such as elevated glucose, cytokines, a high fat diet, or pregnancy may all result in dysregulation of lncRNAs (159). A recent analysis of expression profiles of circulating lncRNAs in serum from patients with diabetes has revealed differences when compared to control patients (275). Whether these changes can be involved in the pathogenesis of the disease and linked to β-cell function remains to be elucidated.
Cell-free DNA and exosomes
Intracellular DNA from the nucleus or mitochondria can be measured in the circulation and has been used in a number of diseases, including cancer where it can be measured as an indicator of cell death. With this in mind, recent research has focused on the measurement of circulating unmethylated preproinsulin (INS) DNA as a new biomarker for β-cell death in type 1 diabetes (276-279). Indeed, unmethylated INS CpG sites are increased in the β cell, and these fragments can be released into the circulation upon β-cell damage. However, the presence of unmethylated INS in other cell types decreases the specificity of this measurement. Although it has been suggested that the addition of complementary biomarkers, such as unmethylated CHTOP, could increase the confidence of detecting β-cell death in youth with type 1 or type 2 diabetes (279), this was not supported by another study (280). In the latter, the use of an ultrasensitive assay for detection of 6 β cell-specific DNA methylation markers (including INS DNA), did not find any evidence of elevated β-cell-free DNA in patients with type 1 diabetes (280). Potential explanations provided by the authors included (i) an insufficient sensitivity of the assay; (ii) destruction of the β cells preceding the time of sampling; and (iii) differences in degree and dynamics of β-cell destruction between individuals.
Exosomes are small extracellular vesicles that carry bioactive molecules, such as proteins and noncoding DNA and RNA which participate in intercellular crosstalk, including paracrine communication between the different cell types in islets (281). Analysis of the content of islet-released exosomes suggest they are mainly derived from β cells (282). It has also been suggested that these exosomes are associated with the development of type 1 diabetes as they could participate in the initiation of the autoimmune process in the islets (281). Some in vitro, ex vivo, and in vivo studies have reported a specific islet-derived exosomal miRNA signature in individuals with type 1 diabetes (283-285), suggesting they could also serve as novel circulating biomarkers of the disease.
Summary
While the use of GRS improves prediction of type 1 diabetes and helps discriminate it from type 2 diabetes or monogenic diabetes, its clinical utility in type 2 diabetes remains unclear. In addition, the field of epigenetic biomarkers, including circulating cell-free RNA/DNA and exosomes as biomarkers of β-cell dysfunction and death seems exciting and promising, but basic and applied research evaluating their utility is still in the early stage and a great deal more work is clearly needed before their use can be translated to clinical practice in diabetes. Particularly, future work should focus on determining (i) unified methods for their identification and characterization; (ii) specific markers allowing validation of their islet origins (vs other tissues) when detected in biological fluids; and (iii) their power in predicting development of the disease in comparison to other biomarkers.
Markers of Autoimmunity and Inflammation
As touched upon previously, in both type 1 and type 2 diabetes, an inflammatory response contributes to the loss of β-cell function. The question arises as to how we can quantify this immune response and use it to classify diabetes subtype, to predict diabetes development in people at risk, and to monitor disease progression due to continuous loss of β-cell function.
T-Cell Responses in People With Type 1 Diabetes
Given that current evidence suggests that CD4+ and CD8+ autoreactive T cells are the main effectors of β-cell destruction in type 1 diabetes (101), measuring the frequency or function of T cells has the potential to assist in understanding type 1 diabetes pathogenesis, and monitoring disease progression (286) and response to immunotherapy (287). A number of T-cell biomarkers have been developed for use in type 1 diabetes that can be classified in 2 main categories: antigen-specific and antigen-agnostic T-cell biomarkers.
Antigen-specific assays
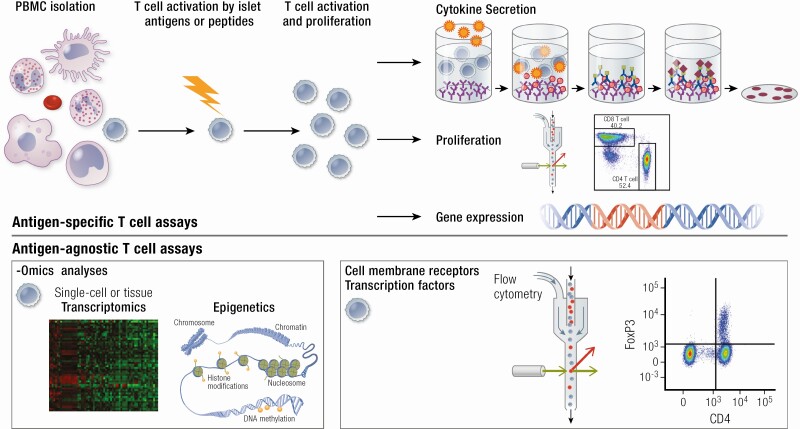
Antigen-specific assays typically measure T-cell responses when peripheral blood mononuclear cells (PBMCs) are incubated with islet antigens such as preproinsulin (287, 288) (Fig. 3). In addition, HLA class I or II multimers can be loaded with autoantigenic peptides to detect antigen-specific T cells (288). Measures of T-cell function that can be assessed include proliferation and cytokine secretion (289).
Figure 3.
Antigen-specific and antigen-agnostic T cell phenotyping. Antigen-specific T cells are profiled following incubation with islet autoantigens or peptides such as preproinsulin or neoepitopes. Activated T cell populations can be characterized through quantification of cytokine secretion, proliferation, and gene expression. Antigen-agnostic T cells are not first activated by islet autoantigens. Bulk- and single-cell -omics analyses have improved T cell transcriptional and epigenetic characterization. Flow cytometry is used to phenotype T cells based on cytokine expression and cell surface markers as well as (phosphorylation of) intracellular proteins and nuclear transcription factors that are key for regulating T cell function (FoxP3 depicted here).
Using such experimental approaches, autoreactive T cells specific for β-cell antigens have been quantified in peripheral blood in persons with type 1 diabetes. However, T cells reactive to islet autoantigens are often detectable in people without diabetes, including in the pancreas (290, 291). In type 1 diabetes, these cells can be phenotypically different; for example, CD4+ autoreactive T cells collected from people with type 1 diabetes secrete higher concentrations of proinflammatory cytokines including interferon-γ (IFN-γ) and interleukins (292), while secretion of anti-inflammatory factors may be reduced (293). Deep phenotyping of autoreactive T cells on a single-cell omics platform has led to the discovery that autoreactive T cells with a proinflammatory profile are present in children that develop type 1 diabetes prior to the formation of islet autoantibodies (294).
Broad application of autoreactive T cells as reliable biomarkers of disease in type 1 diabetes remains challenging for several reasons. First, deep phenotyping of these cells is necessary to determine their polarization and potential disease impact. Second, relevant autoreactive T cells mostly reside in the pancreas or pancreatic lymph nodes with low frequencies in the peripheral circulation (295). Finally, isolation of β-cell–specific T cells has been challenging due to the low-avidity interactions between β-cell antigens and the T-cell receptor (296). Because of these limitations, the use of autoreactive T cells as biomarkers for type 1 diabetes is currently limited clinically.
Antigen-agnostic assays
Alternative approaches for employing T cells as markers of type 1 diabetes include measurement of T-cell subset frequencies and in-depth phenotypic characterization of circulating T cells using omics approaches (Fig. 3). T-cell subset populations can be detected by flow cytometry using an array of surface and intracellular markers. In addition, both multiple- and single-cell technologies have enabled profiling of T cells at the transcriptional and epigenetic levels (288).
Antigen-agnostic assays have been used in recent clinical trials of immunomodulation in type 1 diabetes. As such, several therapies showed a beneficial effect in those with residual β-cell function determined by C-peptide. Examples include (i) the anti-CD3 monoclonal antibody teplizumab, which induced a population of “exhausted” CD8+ T cells (277, 297); (ii) abatacept, which inhibited the interaction between antigen-presenting cells and T cells, thereby contributing to an increase in the fraction of CD4 memory cells (298); and (iii) low-dose antithymocyte globulin (ATG) treatment, which reduced CD4+ T cells (299). In addition, proinsulin peptide therapy increased FoxP3 expression by regulatory T cells (Tregs; which induce immune tolerance) (300), while modulation of gut microbiota reduced CD4+ CXCR3+ and CD8+ CXCR3+ T cells after 1 year of treatment (98). Not all trials, however, showed a clear link between metabolic benefits of immunomodulatory therapies and beneficial alterations in T-cell markers (301).
While T-cell subset frequency and function remain potentially valuable as biomarkers due to their causal relationship with type 1 diabetes, their clinical use is still in its infancy. This situation is likely to change in the coming years with efforts to improve T-cell phenotyping. For example, a recent study reported a higher frequency of CD4+CD25+CD127hi (127-hi) cells being associated with longer partial remission and a favorable response to immunotherapy (302). Thus, assessing T-cell function in type 1 diabetes (both antigen-specific and agnostic) holds great promise for clinical usage, but harmonization and standardization of experimental protocols are needed to enable broad application of T cells as biomarkers in type 1 diabetes (303).
T-Cell Responses in People With Type 2 Diabetes
Studies from one group have raised the possibility that T cells autoreactive to β-cell proteins may also be present in people with type 2 diabetes (304). Using peripheral blood–derived T cells from individuals with phenotypic type 2 diabetes who do or do not have islet autoantibodies, immunoblotting-based assays have detected T cells reactive to islet proteins (304). The magnitude of these cellular responses was related to the degree of β-cell function and the progressive loss of β-cell function over time (305, 306). The reactivity of T cells to islet proteins can be reduced by treatment with the insulin sensitizer rosiglitazone, which also reduces cytokine secretion from PBMCs and improves the C-peptide response to glucagon, an effect not observed with sulfonylurea treatment (307).
It has also been hypothesized that changes in adipose tissue biology in people with type 2 diabetes may drive some of these immunological changes. As such, obesity may induce MHC class II expression in adipose tissue, leading to activation of CD4+ T cells (308). These T-cell populations are further characterized by a shift from an anti-inflammatory (T helper [TH]2 cells) to a proinflammatory (TH1, TH17 cells) phenotype, and may contribute to activation of proinflammatory macrophages in adipose tissue.
While these T-cell data raise the possibility that the adaptive immune system may be involved in β-cell dysfunction in type 2 diabetes, their clinical utility in predicting disease, monitoring progression, and tracking responsiveness to interventions remains rather limited.
Modified T-Cell Autoantigens (neoepitopes)
Although T cells learn tolerance to self-proteins in the thymus, in the presence of stress such as hyperglycemia, cytokines, or infection, β-cell proteins may undergo posttranslational modification to become neoepitopes, to which the immune system is naïve (309). Neoepitopes may be directly presented on the β-cell surface via HLA class I or by antigen-presenting cells via HLA II class triggering an immune response (67, 310). Mechanisms by which proteins may be modified to form neoepitopes in type 1 diabetes include enzymatic citrullination or deamidation, or via nonenzymatic posttranslational modification including oxidation and carbonylation (310). Peptide fusion to form hybrid peptides has recently been recognized as a potential source of potentially important neoepitopes in type 1 diabetes (311, 312). Neoepitope formation may also occur through novel mechanisms such as alternative splicing products and defective ribosomal initiation products (DriPs) (313).
While these changes at a cellular level are likely important in the pathogenesis of type 1 diabetes and T cells specific for neoepitopes can be detected in the circulation, neoepitopes derived from β-cell peptides, if secreted, likely circulate at levels too low for detection in plasma. Identification of new neoepitopes and refinement of assays for their measurement in plasma could lead to circulating neoepitopes having utility as β-cell biomarkers.
Autoantibodies
Type 1 diabetes
Islet autoantibodies are more prevalent in people with type 1 diabetes and can provide a quantifiable risk for the disease development (314); thus, they are widely used biomarkers for disease prediction. Importantly, islet autoantibodies do not appear to play an active role in β-cell loss, but instead are markers of the autoimmune process.
Following discovery of the first islet cell autoantibodies (ICA) in children with type 1 diabetes (315), autoantibodies specific for insulin (IAA) (316), glutamic acid decarboxylase (GAD) (317) and protein tyrosine phosphatase (IA2 or ICA512) (318) were discovered. GAD catalyzes the decarboxylation of glutamate to gamma-aminobutyric acid (GABA) and is expressed in islets in synaptic vesicles, while protein tyrosine phosphatase is an enzymatic transmembrane glycoprotein localized in the endocrine cell secretory granules. The most recently identified autoantibody with clinical utility is directed at the zinc transporter 8 (ZnT8), a pancreatic β-cell secretory granule membrane protein involved in insulin exocytosis (319).
GAD is the most frequently used clinically, with the others employed to a more variable extent, for both diagnosis and prediction of type 1 diabetes (320). In individuals with a suspected diagnosis of autoimmune diabetes, one or more islet autoantibodies are usually assessed and sufficient to confirm a diagnosis of type 1 diabetes. For diabetes prediction, autoantibodies are most commonly employed in epidemiological studies and prevention trials, the latter including immunomodulatory approaches to preserve β-cell function and prevent the development of hyperglycemia.
A positive family history of type 1 diabetes and GRS can identify risk for type 1 diabetes in newborns (228, 321). This predictive strength is increased when combined with the presence of islet autoantibodies and other clinical variables, such as body weight and history of sinusitis (228). The presence of multiple islet autoantibodies conveys a larger risk of developing type 1 diabetes than a single autoantibody (321). As such, children with seroconversion to any 2 autoantibodies have a 70% risk of developing type 1 diabetes during childhood or adolescence (322), with a yearly risk of approximately 10% (314). While higher titers are likely associated with increased risk across a population, titers have less predictive power in the individual. Complicating their use in assessing diabetes risk, autoantibodies may appear in different order, depending on haplotype (208, 209, 323), and may disappear over time both in children and adults regardless of whether they eventually progress to diabetes (324). In general, the younger the age of seroconversion, the higher the risk of diabetes.
Islet autoantibody assessment helps establish a diagnosis of autoimmune diabetes and has reasonable power (in combination with family history and HLA type) to identify children at risk (228). This identification will be of particular importance as therapeutic strategies to prevent type 1 diabetes become available. As with T-cell assays discussed above, strict control of the quality and reproducibility of the autoantibody assays is necessary for them to be more commonly used in the clinical setting. Thus, the measurement of islet autoantibodies is currently important in both research and clinical practice to predict the development of type 1 diabetes. Further, assessment of islet autoantibody status, along with other biomarker measures including risk genes, T-cell signatures, and metabolic measures such as proinsulin, have now enabled the identification of disease heterogeneity in type 1 diabetes that holds promise for optimizing disease management and clinical trial design (325). However, while islet autoantibodies may provide some insight into type 1 diabetes pathophysiology, they are currently not a target for therapy, as they are not thought to contribute to β-cell loss.
Latent autoimmune diabetes in adults
While autoimmune diabetes classically presents in childhood or adolescence, it can also occur in adults. LADA, also termed type 1.5 diabetes, differs from adult-onset type 1 diabetes by the duration of insulin independence after diagnosis (326). This longer period of insulin independence is due to the fact that individuals with LADA are diagnosed later in life and typically have better β-cell function than those diagnosed with type 1 diabetes at a young age (327, 328). Thus, LADA has been proposed to be diagnosed when diabetes has an adult age of onset (>30 years), the presence of any islet autoantibody (most commonly GAD), and no need for insulin treatment for at least 6 months after diagnosis (329). When insulin replacement therapy is required within 3 months, the diagnosis of classic adult-onset type 1 diabetes is proposed, although the American Diabetes Association views all forms of diabetes mediated by autoimmune β-cell loss under the rubric of type 1 diabetes (4).
The prevalence of LADA is likely underestimated, as antibodies are infrequently measured in adults with hyperglycemia, and typically only when the phenotype does not match that of classical type 2 diabetes, with overweight/obesity and other aspects of the metabolic syndrome. However, cohort studies have shown that as many as 4% to 12% of people classified as type 2 diabetes can be autoantibody positive (5).
Islet autoantibodies are less frequently present in LADA compared to type 1 diabetes. While IAA, IA2, and ZnT8 autoantibodies usually become negative over time, GAD autoantibodies are not influenced by age and are the most sensitive marker for both adult-onset type 1 diabetes and LADA (330). Higher antibody titers correlate with the presence of higher-risk HLA genotypes (331) and a more severe phenotype at presentation including insulinopenia, higher HbA1c levels, and lower BMI. Measurement of antibodies may also help guide clinical treatment by predicting which patients will require insulin therapy.
The distinction between LADA and type 2 diabetes remains primarily based on clinical presentation and phenotype, with diagnostic testing for islet autoantibodies rarely carried out in obese individuals. With time and disease progression, the correct diagnosis will usually become apparent for most individuals.
Cytokines/Inflammatory Markers
The most common inflammatory markers linked to β-cell dysfunction include the interleukin family (including IL-1β, IL-6, and IL-8) TNF-α, NF-κB, IFN-γ, and chemokines such as CCL2, MCP1, and CXCL1. The plasma concentrations of these proteins are indeed elevated in people with type 2 diabetes (332); however, several issues arise when applying these biomarkers in clinical or research settings. Importantly, it is unlikely that islet macrophages make a meaningful contribution to circulating cytokine levels, as other tissue-resident and circulating macrophages make much bigger contributions. Moreover, the measurement of these markers of inflammation can be difficult, especially using multiplex approaches, with their measurement being predominantly performed in research settings and not routinely in clinical labs. Thus, while cytokines are involved in β-cell inflammation and dysfunction, their circulating levels are not useful as biomarkers, as they do not reflect what may be occurring at the level of the islet.
Pancreatic and Islet Imaging
Several methods for imaging the pancreas have been proposed as a means to quantify β-cell mass (Fig. 4). The basis for this strategy and the purported advantage is to capture the early stages of β-cell loss prior to any change in blood glucose levels. Also, it enables noninvasive, longitudinal assessment of islet viability in vivo, which may guide treatment strategies to mitigate the progressive decline in β-cell function observed in type 2 diabetes.
Figure 4.
Common imaging biomarkers used to assess β cells in humans. The targets and probes are provided for each type of measurement that are designed to provide measures of pancreas size and fat, β-cell mass, islet amyloid, and insulitis.
Imaging islets (and β cells) is especially challenging due to their small size and their relatively low abundance being spread throughout the pancreas. In humans, islets comprise only ~1% to 3% of the pancreas and approximately 50% of the islet is made up of β cells (113, 149, 333, 334). Thus, in order to successfully image islets in vivo, the technique used must be highly sensitive. Also, as islets comprise several cell types, to detect β cells per se, the imaging technique must also be specific. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) are imaging modalities that are clinically applicable and offer high sensitivity, as well as the ability for signal quantification. Both employ tracer molecules labeled with radioactive nuclides. To date, existing radiolabeled molecules targeting the β cell have proven to be suboptimal for imaging in humans, as they often bind other islet cell types and different tissues.
Vesicular Monoamine Transporter Type 2
Vesicular monoamine transporter type 2 (VMAT2) has been suggested to be one of the more specific biomarkers for imaging β cells. However, there is heterogeneity in the proportion of β cells that express VMAT2; specifically, it has been reported that 81%, 96%, and only 53% of cells in the body, tail, and head of the pancreas, respectively, co-localize VMAT2 and insulin (335). Importantly, VMAT2 is not expressed in islet α or δ cells, although its utility as a specific β-cell marker is limited by expression in islet PP cells (335). Indeed, PP cell expression of VMAT2 may explain the disconnect in studies of type 1 diabetic subjects who had no measurable β-cell function, yet total pancreatic binding of the VMAT2 tracer [18F]fluoropropyl-(+)-dihydrotetrabenazine was ~40% (336, 337). Such residual background signal likely reflects nonspecific binding and yields data that may overestimate β-cell mass. Despite this, VMAT2 expression and its radioligand binding have been found to strongly correlate with β-cell density (338, 339). Thus, specific targeting of VMAT2 in the pancreatic body and tail, which are regions of lower pancreatic polypeptide (PP) abundance (154, 340, 341), may overcome the abovementioned limitations in order to reflect β-cell mass more accurately.
Using this targeting approach, a recent study assessed VMAT2 tracer density in regions of the pancreas in humans with prediabetes or type 2 diabetes and correlated these data to β-cell function and glycemic control (342). Uptake of the VMAT2 tracer in any of the 3 individual regions of the pancreas was positively correlated with the acute C-peptide response to glucose-potentiated arginine stimulation. The same pattern did not hold true for glycemic control, where increased uptake of VMAT2 tracer only in the head, but not the body or tail of the pancreas, correlated with lower HbA1c. Of note, increased uptake of VMAT2 tracer in the pancreas as a whole also correlated with lower HbA1c. An important observation in this study was that loss of VMAT2 tracer binding was modest in the subjects with type 2 diabetes, whose time since initial diagnosis averaged ~10 years (342). This raises the question of when β-cell imaging would be most useful in the clinical course of type 2 diabetes, in order to inform treatment strategies.
Glucagon-like Peptide-1 Receptor
Like VMAT2, the glucagon-like peptide-1 receptor (GLP-1R) has been the subject of continued efforts in imaging human β cells in vivo. In the pancreas, GLP-1R is predominantly expressed on β cells, but there are also reports of its expression in exocrine tissue and other endocrine cells of the pancreas, including α and δ cells (343-345). This expression pattern therefore poses some challenges in targeting GLP-1R to specifically image β cells in vivo.
Several agents that bind GLP-1R have been developed for β-cell imaging with PET and SPECT (346). The vast majority are stable GLP-1 analogs based on exendin-3 and -4 peptides, which have been designed to prevent the rapid degradation seen with native GLP-1 (347). Due to their agonistic potency at the GLP-1R, they have the potential to induce hypoglycemia when used in high doses (348). To circumvent this undesirable side effect, during imaging a continuous infusion of glucose can be co-administered (349). Alternatively, a small number of GLP-1R antagonists have been developed for use in imaging so as to avoid receptor activation. There has been varied success with these antagonists, with some exhibiting low specific uptake in rodents or an inability to label β cells from humans (350-352). Moreover, some GLP-1R–targeting radioactive agents (eg, exendin analogs) exhibit proximal duodenal uptake as well as high kidney uptake, the latter likely due to final elimination that occurs almost exclusively in the kidney (353, 354). This poses a problem, as it may obscure the adjacent pancreatic tail under imaging conditions of low resolution, resulting in inaccurate estimation of β-cell mass. To address this, recent advances in radionuclide labeling chemistry have allowed for strategies to markedly reduce kidney uptake using improved agents (eg, NOTA-MVK-Cys40-Leu14-Exendin-4) (355).
To date, the caveats of GLP-1R as a biomarker for β-cell mass are similar to those of VMAT2. For example, nonspecific binding of a radiolabeled GLP-1 analog can occur. This possibility was suggested in a study involving 111In-labeled-exendin in which pancreatic uptake was reduced in type 1 diabetic subjects when compared with healthy controls, but pancreatic radioactivity concentration of 111In-labeled-exendin among individuals was overlapping between the 2 groups (356). Given that the type 1 diabetic subjects had undetectable nonstimulated and stimulated C-peptide levels, it is probable that binding of 111In-labeled-exendin in these subjects was occurring in non-β-cell populations. Thus, in order to infer any relationship with β-cell function, further refinements are needed to accurately quantify β-cell mass with GLP-1R probes in diabetic subjects.
Of note, GLP-1R expression is up to 5 times greater in human insulinomas compared to normal β cells (357); this has enabled noninvasive diagnosis of insulinomas in humans (348, 358, 359). Importantly, the utility of GLP-1R-based imaging extends beyond that of quantifying β-cell mass and detecting insulinomas, since it can also inform on the extent of GLP-1R occupancy of therapeutic compounds that act as GLP-1R agonists (360). This may become useful in time to individualize treatment strategies, where one GLP-1 analog may be chosen over another due to enhanced GLP-1R binding.
Insulitis
In type 1 diabetes, autoimmune destruction of β cells is already markedly advanced by the time clinical symptoms manifest (361). Thus, imaging of β cells may enable early detection of insulitis in type 1 diabetes and inform on treatment strategies to curb β-cell loss.
Ultrasonography and computed tomography are imaging techniques used to visualize the pancreas in humans. While both can detect pancreatic anatomical and structural changes that occur during type 1 diabetes, neither can quantify changes at the level of individual β cells in vivo. More promising are anatomical imaging techniques such as magnetic resonance imaging (MRI), which can be used to infer the extent of islet microvascular dysfunction, as seen in insulitis (362). For example, MRI with a specific magnetic nanoparticle as a contrast agent, has been used to image insulitis in recently diagnosed type 1 diabetic subjects, where it was found that pancreatic volume was already reduced compared with nondiabetic subjects (363). In this case, MRI detects magnetic nanoparticles that migrate from leaky vessels into the surrounding tissue and are phagocytosed by inflammatory cells, especially macrophages. Recent optimization of this method has improved resolution and discrimination of pancreatic inflammation (364), although there is no correlation between MRI signals and either autoantibody titers or the number of autoantibodies detected in type 1 diabetic subjects.
By targeting specific cells or cell antigens involved in β-cell destruction in type 1 diabetes, the ability to image insulitis in humans is improved. For example, lymphomononuclear cell infiltration can be targeted using 99mTc-labeled polyclonal immunoglobulins that recognize the Fc receptor in infiltrating lymphocytes. In humans, significant accumulation of labeled immunoglobulins in the pancreas was found in 7 out of 15 newly diagnosed type 1 diabetic subjects, where radioactivity in the pancreas correlated with metabolic, immunological, and clinical parameters (365). While encouraging, it is important to recognize that in various stages of insulitis, there may be a relatively low number of lymphocytes and/or only a small number of antigens expressed on lymphocytes. The latter may be a factor in other studies, where pancreatic accumulation of 99mTc-labeled IL-2 was observed in 61% to 65% of people with newly diagnosed type 1 diabetes (366, 367). Importantly, metabolic or immunologic parameters in these subjects did not differ from that observed in subjects who were negative for pancreatic 99mTc-labeled IL-2 accumulation (367). Also, pancreatic uptake of 99mTc-labeled IL-2 did not correlate with autoantibody titer (366). Thus, in some cases, there appears to be a weak relationship between lesions observed via pancreas imaging and the clinical characteristics of diabetic subjects at the time of imaging. That said, there is still value in performing imaging for insulitis, as subjects with positive scintigraphy at diagnosis showed better long-term metabolic control when treated, compared with subjects with negative scintigraphy (367).
Amyloid
Islet amyloid is present in ~90% of subjects with type 2 diabetes and contributes to the loss of β-cell mass and function (151, 160, 161). Detection of islet amyloid in humans may provide a means to assess progression of diabetes in order to determine the most effective treatment strategies to halt β-cell loss. To date, few islet amyloid imaging probes have been tested in humans, despite preclinical studies showing some promise in in vitro studies and in humanized models of islet amyloid deposition (168-171).
In general, these probes are based on those developed for PET detection of amyloid β (Aβ) deposits in the brain, some of which also bind the main amyloidogenic peptide constituent of islet amyloid, namely IAPP. One example is the FDA-approved [18F]florbetapir (368, 369), which has shown significant qualitative and quantitative correlations between in vivo PET imaging and postmortem histopathologic analysis of amyloid β. In vitro studies show florbetapir binds synthetic human IAPP and endogenous islet amyloid deposits (169). In transgenic mice expressing human IAPP, florbetapir enabled PET detection of islet amyloid in vivo; however, the signal was greater only during the first 5 minutes of the PET scan in human IAPP transgenic mice vs nontransgenic mice that do not develop amyloid (169). Moreover, florbetapir uptake was not zero in mice that did not develop amyloid. The latter is a potential drawback for use of florbetapir in assessing islet amyloid in humans, as it could result in erroneous conclusions regarding the loss of β-cell mass.
In another study, the amyloid β probe, [18F]FDDNP, was found to selectively stain islet amyloid deposits in autopsy pancreas sections from a type 2 diabetic subject, with no staining evident in pancreas from a nondiabetic subject. When administered to nondiabetic humans, [18F]FDDNP exhibited a favorable pharmacokinetic profile, and allowed for the pancreas to be easily distinguished from neighboring organs using PET imaging (170). Unfortunately, this work did not include people with diabetes, precluding validation of whether [18F]FDDNP could be used to quantify islet amyloid in vivo.
Thus, further studies are needed before use of islet amyloid probes in the clinic. An obvious advantage of probes like florbetapir and [18F]FDDNP is that they enable detection of both islet amyloid and amyloid β and may thus offer the opportunity to simultaneously assess amyloid in both the pancreas and central nervous system in humans.
Islet Transplantation
Several imaging modalities have been used to detect and monitor transplanted islets. In early human studies, islets were labeled with iron nanoparticles prior to transplantation and visualization by MRI (370). Since this labeling approach relies on cellular uptake of nanoparticles via random endocytosis, both nonfunctional and functional islet cells are labeled—this limits the utility of imaging in terms of informing on the function and viability of islet cells. Also, iron nanoparticle labeling does not distinguish between β- and non-β cells. Despite this, clinical-grade iron nanoparticles have been developed and tested in MRI visualization of labeled islets transplanted into rodents and humans (371, 372). While the clinical-grade contrast agent offers improved sensitivity for detection, iron nanoparticles in rodents were shown to persist at the site of transplantation beyond the presence of intact islets (372). The latter is clearly a major drawback for long-term monitoring of islets in vivo, as it can result in overestimation of the number of viable islets present following transplantation. When tested in humans with type 1 diabetes with pretransplant negative C-peptide levels, clinical-grade iron nanoparticles were detectable in vivo up to 24 weeks posttransplant (373). Importantly, all subjects exhibited significant C-peptide production at 24 weeks, suggesting the transplanted islets were still viable.
From a clinical perspective, MRI is an attractive imaging modality, since it does not require ionizing radiation and longitudinal measures can be made, owing to the sustained presence of contrast agents within the body. However, PET is more sensitive than MRI, and β-cell–specific probes can be used with PET to differentiate β cells from non-β cells. The utility of such probes (eg, targeting VMAT2 and GLP-1R) is described above. Another probe commonly used for PET imaging is [18F]fluorodeoxyglucose, which is a glucose analog that enters cells with high rates of glucose utilization but is not fully metabolized and is thereby trapped. In this way, distribution of [18F]fluorodeoxyglucose is an indicator of the glycolytic rate of cells (374). In a study of 5 type 1 diabetic subjects, PET was combined with computed tomography to image this radiotracer in islets transplanted intraportally (375). The radioactivity concentration in the liver corresponded to only 75% of the expected dose, suggesting loss of transplanted islets. Also, distribution of radioactivity in the liver was heterogeneous, with wide variations in location and concentration, including in regions that may represent islets trapped in sinusoids or clots in the portal branches. While informative with respect to success of the islet transplant procedure per se, the use of [18F]fluorodeoxyglucose does not aid in monitoring function of transplanted islets over time, largely due to the short half-life of 18F (110 minutes) and retention of [18F]fluorodeoxyglucose in islets (196 minutes). Further, due to its reliance on cell metabolic rates, it lacks specificity to islets vs surrounding tissues.
Pancreatic Size and Fat
Human autopsy studies have demonstrated that pancreas size is reduced in both type 1 and type 2 diabetes (113, 376). Some (377-380), but not all (381, 382), data from pancreas imaging by ultrasound, computed tomography, and MRI have been consistent with findings from autopsy studies. A recent systematic review and meta-analysis of imaging studies suggested the discrepant literature might be due to a small sample size in most studies (383). Related to this point is the inter-individual heterogeneity in pancreas volume; thus, longitudinal assessments within individuals may provide a more robust method for determining disease progression, rather than comparing the magnitude of pancreas volume reduction between nondiabetic and diabetic subjects.
With respect to pancreatic size as a biomarker for β-cell function, a study in type 2 diabetes subjects showed that pancreas volume positively correlated with homeostatic model assessment (HOMA)-B, as an estimate of β-cell function (379). While these data support a correlation between pancreatic size and β-cell function, there is evidence that this does not translate to all situations. Specifically, in subjects who underwent weight loss as a means to reverse their diabetes, there was no detectable increase in pancreas volume despite a return to normal insulin secretion after 6 months (384). The latter suggests pancreas size may not be a useful surrogate for β-cell function, even when assessed longitudinally. Another caveat with pancreas size as a β-cell function biomarker is that it largely reflects changes in the exocrine pancreas, rather than islets, which constitute only ~1% to 3% of the pancreas (113, 333).
Pancreatic fat has similarly been proposed as a potential biomarker in diabetic subjects. Various imaging modalities are used to noninvasively quantify pancreatic fat, and it has now been shown in several studies that pancreatic fat content is increased in type 2 diabetes (383, 385). Specifically, when compared with nondiabetic subjects, pancreatic fat was found to be higher in those with prediabetes and highest in those with type 2 diabetes, indicating that it tracks with disease progression (386). When assessed in subjects with impaired glucose tolerance (IGT) or IFG, pancreatic fat was negatively associated with insulin secretion, suggesting that it could contribute to β-cell dysfunction (387). However, in another study in which subjects were categorized on the basis of an oral glucose tolerance test as having NGT, prediabetes, or type 2 diabetes, the presence of pancreatic fat was not related to the dysglycemic state (388). Indeed, there are other conflicting reports on the association between pancreatic fat accumulation and β-cell function, with some (389), but not all (390-392), showing a significant correlation.
These discrepancies among studies of pancreatic fat may be due to differences in methodology used to assess either pancreatic fat, β-cell function, or both, and/or in how the data analyses were conducted. For example, only in some cases were confounders like age, sex, and BMI accounted for when determining the association between pancreatic fat and β-cell function or glycemic status. Also, many studies were cross-sectional, making it difficult to truly relate accumulation of pancreatic fat with deterioration of β-cell function over time. To this point, in a longitudinal study conducted over a period of 5 years, pancreatic fat was not independently associated with future type 2 diabetes (393). More recently, an additional factor has come into play, namely genetic predisposition to diabetes. That is, a study showed that pancreatic fat only impairs β-cell function in subjects with high genetic risk for diabetes (394). Taken together, without additional (longitudinal) studies, the use of pancreatic fat as a biomarker for development or progression of β-cell failure remains premature.
Summary
In sum, several approaches for imaging the pancreas and islets are being studied for estimating β-cell mass or function. While promising in terms of capturing the early stages of β-cell loss and failure, the techniques largely lack sufficient sensitivity and/or specificity to be clinically useful at this time. With technical advances in the field, it is conceivable that select imaging biomarkers could in the future prove beneficial in preventing and managing diabetes.
Circulating β-Cell Propeptides
The biosynthesis of insulin has been well studied, while that of IAPP much less so. Our knowledge of proinsulin biosynthesis and processing has helped guide research into understanding proIAPP biosynthesis, and while they have extensive overlap, there are distinct differences. In the following discussion, we focus first on proinsulin processing and thereafter on IAPP. Some discussion of the normal biosynthesis of these prohormones is presented in the section describing the normal physiology of the β cell.
Assessment of Proinsulin Processing
Healthy humans
Depending on the initial cleavage step, proinsulin processing can proceed by 1 of 2 intermediate steps, via des-31,32 proinsulin (produced by PC1/3 cleavage) or des-64,65 proinsulin (predominantly produced by PC2 cleavage) (Fig. 5) (395, 396). Cleavage by PC1/3 or PC2 occurs on the C-terminal side of pairs of basic residues, initially producing the split cleavage products, split-32,33 proinsulin or split-65,66 proinsulin, respectively. The remaining basic residue pairs are rapidly trimmed by carboxypeptidase E (CPE) to produce des-31,32 or des-64,65 proinsulin (397). Processing via the des-31,32 proinsulin intermediate predominates, as little, if any, des-64,65 proinsulin is detectable in human circulation in health or in diabetes (136). Evidence from studies in human islets (39) and mouse models of prohormone convertase deficiency (42, 43), indicate that PC1/3 is able to completely process proinsulin to insulin and C-peptide. PC2 expression is low in human β cells, and it appears dispensable for complete proinsulin processing in healthy human β cells (39).
Figure 5.
Pathways for β-cell prohormone processing. A, Pathway for proinsulin processing in β cells. The predominant pathway for proinsulin processing to insulin and C-peptide involves cleavage of proinsulin by prohormone convertase (PC) 1/3 on the C-terminal side of basic residues at positions 31 and 32, followed by the removal of these basic residues by carboxypeptidase E (CPE), leading to the production of the proinsulin intermediate, des-31,32 proinsulin. This intermediate is then cleaved by PC2, or in human β cells may be cleaved by PC1/3, leading to production of one molecule of insulin and one of C-peptide. Another possible pathway involves cleavage of proinsulin by PC2 on the C-terminal side of basic residues at amino acids 64 and 65 which, following removal of these residues by CPE, is predicted to lead to des-64,65 proinsulin, although this pathway is not thought to be active in healthy β cells as the des-64,65 proinsulin intermediate is undetectable in human plasma. B, Pathway for proIAPP processing in β cells. Like proinsulin, proIAPP1-67 is first processed by PC1/3 on the C-terminal side of a pair of basic amino acids, near the C-terminus of the propeptide. Following removal of these basic residues by CPE, the N-terminally extended proIAPP intermediate proIAPP1-48 is formed. ProIAPP1-48 processing to mature IAPP is dependent on PC2 in murine β cells. Mature IAPP (and possibly proIAPP1-48) is amidated in secretory granules at the C-terminus by peptidyl α-amidating monooxygenase (PAM).
Distinct measurement of intact proinsulin and the proinsulin intermediates in human plasma has provided some insight into the nature of processing defects that exist in diabetes, but current assays do not discriminate well between these forms. The majority of commercially available proinsulin assays measure both intact proinsulin and des-31,32 proinsulin (the predominant intermediate form in human circulation), while excluding des-64,65 proinsulin. While these assays enable measurement of both of the major circulating forms of proinsulin immunoreactivity in the same sample, they do not allow specific quantification of the individual proinsulin species. In several of the existing immunoassays for proinsulin immunoreactivity, little data are provided regarding cross-reactivity among the various proinsulin forms. Our current knowledge regarding these assays and their performance as provided by the manufacturers is listed in Table 3. Further advancements toward understanding β-cell prohormone processing in diabetes will require development of approaches, such as mass spectrometry (80), that are able to better discriminate the different forms in human plasma while at the same time being sensitive and specific.
Table 3.
Characteristics of current human proinsulin and C-peptide assays
| Propeptide cross-reactivity | Mature peptide cross-reactivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Manufacturer | Catalog number | Sensitivity | Intact proinsulina | split-32,33 | des-31,32 | split-65,66 | des-64,65 | Mature insulin | C-peptidea |
| Proinsulin | |||||||||
| Abcam | ab242235 | 0.75 pmol/L | - | NS | NS | NS | NS | >50 ng/mL | >50 ng/mL |
| Alpco | 80-PINHUT-CH01 | 0.455 pg/mL | - | NS | 100% | NS | 100% | <0.6% | <0.1% |
| EMD Millipore | EZHPI-15K | 0.5 pmol/L | - | NS | 100% | NS | 81% | >200 µU/mL | >10 ng/mL |
| Invitron | IV2-002 | 0.02 pmol/L | - | 5.6% | 1.4% | 37% | 63% | 0% | 0% |
| Mercodia | 10-1118-01 | 1.7 pmol/L | - | 95% | 95% | 90% | 84% | <0.03% | <0.006% |
| Meso Scale Diagnostics | K1516MK | 0.05 pmol/L | - | NS | NS | NS | NS | <0.5% | 0.7% |
| R&D Systems | DPINS0 | 1.43 pmol/L | - | NS | NS | NS | NS | >3450 pmol/L | NS |
| TECOmedical Group | TE1012 | 0.3 pmol/L | - | 5000 pmol/L | <200 pmol/L | 1000 pmol/L | 200 pmol/L | <10 000 pmol/L | 50 000 pmol/L |
| C-peptide | |||||||||
| Alpco | 80-CPTHU-CH01 | <4.32 pg/mL | <0.01% | NS | 0.3% | NS | 33.2% | ND | - |
| Alpco | 80-CPTHU-E01.1 | 2.95 pmol/L | 3% | NS | NS | NS | NS | <0.01% | - |
| Beckman Coulterb | C33451 | 0.01 ng/mL | 3% | NS | NS | NS | NS | 0% | - |
| Invitron | IV2-004 | 5 pmol/L | 2% | NS | NS | NS | NS | 0% | - |
| Mercodia | 10-1136-01 | ≤25 pmol/L (0.076 μg/L) | 2% | 2% | 3% | 10% | 74% | <0.0006% | - |
| Mercodia | 10-1141-01 | 2.5 pmol/L (0.0076 µg/L) | 5% | 2% | 3% | 10% | 74% | <0.0006% | - |
| Meso Scale Diagnostics | K1516JK | 14 pg/mL | 27% | ND | ND | ND | ND | 0.50% | - |
| Meso Scale Diagnostics | K151X5D | 4.72 pg/mL | 32.4% | NS | NS | NS | NS | 0.03% | - |
| Tosoh | 25284 | 0.2 ng/mL | 31.5% | NS | NS | NS | NS | ND | - |
Information provided is as stated by the manufacturers and not independently verified. Stated information was accessed from manufacturer websites and supporting technical documents during the period February 10, 2021 to March 7, 2021.
Cross-reactivity values reported as percentages represent (100%) (detected proinsulin concentration)/(non-target analyte concentration tested).
Cross-reactivity values reported as concentrations represent the minimum required concentration of non-target analyte to be detected by the ELISA.
Abbreviations: ND, not detected; NS, not stated.
aIn the case of proinsulin assays, it is assumed to be 100% and for C-peptide assays similarly it is assumed to be 100%
bRadioimmunoassay
Using the few approaches that distinguish between these molecular forms of proinsulin, insight has been gained into the 2-step pathway for proinsulin processing. With peptide separation using high performance liquid chromatography (HPLC), some important observations have been made. First, in the basal state, about 15% of total insulin-like immunoreactivity is comprised of proinsulin, while following acute stimulation with arginine it is approximately 4% (136). The latter reflects more closely what is in the secretory granule, with the difference from fasting being a result of differences in the clearance rates of the peptides. Second, approximately 50% of proinsulin immunoreactivity in fasting plasma from healthy humans consisted of intact proinsulin and 50% of des-31,32 proinsulin, with no detectable des-64,65 proinsulin (136). In plasma obtained following acute stimulation, the proportion of proinsulin immunoreactivity comprising des-31,32 proinsulin was approximately 70%. Taken together, these data suggest that a significant proportion of proinsulin immunoreactivity is comprised of the des-31,32 proinsulin intermediate produced by PC1/3 cleavage and therefore in human β cells, the second step in proinsulin processing appears to be rate-limiting. These findings and the conclusions built on them are supported by older studies using immunoassays that allowed for a derivation of the relative amounts of intact and des-31,32 proinsulin (398, 399).
In evaluation of β-cell prohormone processing using currently available immunoassays, the data are often expressed as an absolute value. However, to gain an assessment of the efficiency of proinsulin processing, concentrations need to be presented relative to insulin (proinsulin:insulin) or C-peptide (proinsulin:C-peptide) as absolute proinsulin levels alone will reflect not only processing but will also represent secretory demand imposed by the prevailing glucose and insulin sensitivity (134, 400). A caveat in considering the proinsulin:insulin ratio is the difference in clearance rates of the peptides that could confound interpretation of the ratio, with reduced insulin clearance in, for example obesity, potentially reducing the ratio. This difference in clearance is less of a potential problem when using C-peptide instead of insulin, as the former is not cleared by the liver on first pass (401, 402). The ratio of proinsulin and insulin has its most discriminatory value when examining individuals with more severe β-cell dysfunction (134, 135, 403).
There have been a number of clinical studies on the impact of insulin resistance and increased β-cell secretory demand on the proinsulin:insulin ratio as a measure of processing efficiency in healthy humans. In the absence of a β-cell defect, this ratio has been found to be lower in the fasting state and following acute stimulation in humans with NGT who were obese (134, 404) or had nicotinic acid–induced insulin resistance (405), likely reflecting more efficient proinsulin processing as a marker of enhanced β-cell function in the face of prolonged, elevated secretory demand. Induction of a β-cell defect by streptozotocin treatment in baboons resulted in an increase in the plasma proinsulin:insulin ratio (406). Glucocorticoid treatment not only induces insulin resistance, but also produces β-cell secretory dysfunction and an increase in the proinsulin:insulin ratio (133, 407-410), the latter compatible with a proinsulin processing defect. A similar effect on the ratio has been observed with growth hormone, suggesting that it may also have a deleterious effect on proinsulin processing (410). Prolonged increased secretory demand in the face of reduced β-cell mass created by a hemi-pancreatectomy in humans has been associated with an increase in proinsulin:insulin in those at increased risk of diabetes (411), but not in those without diabetes or a risk thereof (412, 413). Clearly, multiple complex factors impact the proinsulin:insulin and proinsulin:C-peptide ratios in humans, with the available data suggesting that moderately increased β-cell secretory demand (eg, induced by obesity or insulin resistance) does not on its own impair proinsulin processing in individuals with healthy β cells. However, as discussed subsequently, in the presence of impaired β-cell function as with corticosteroid treatment or both major forms of diabetes, an impairment in the efficiency of proinsulin processing is evident.
Type 2 diabetes
Studies of proinsulin in type 2 diabetes have been undertaken for many years and have consistently demonstrated that the proinsulin:insulin ratio is increased in this disease, with the magnitude of increase being associated with glycemia and β-cell dysfunction (131-137). Thus, an increasing ratio suggests a more profound impairment in proinsulin processing in type 2 diabetes, reflecting the presence of a marked β-cell defect. The deleterious impact of impaired proinsulin processing is made more clinically significant, given that the secreted prohormone has only 10% to 15% of the biological activity of insulin (414, 415). The ratio has also been reported to be increased in individuals at high risk of developing type 2 diabetes, including those with impaired glucose tolerance or normoglycemic first-degree relatives of individuals with the disease (398, 416-418). Thus, an alteration in the efficiency of proinsulin processing occurs before the development of frank hyperglycemia and could be a marker for the development of type 2 diabetes (417, 419, 420).
The mechanism underlying impaired proinsulin processing and elevated circulating proinsulin:insulin ratios in type 2 diabetes is not fully understood. The increased ratio cannot be simply explained by differences in the clearance of proinsulin vs insulin in people with and without type 2 diabetes, given that when β cells are stimulated to release their granule content, the ratio (closely reflecting β-cell content) remains 3- to 4-fold higher in type 2 diabetes (136). Thus, there has to be an intrinsic abnormality that results in inefficient proinsulin processing along the secretory pathway.
While understanding at a cellular level in humans what may be occurring with proinsulin processing is difficult, clues have been obtained from measurement of the different forms of proinsulin in the circulation. If the proinsulin processing defect in type 2 diabetes resides early in the secretory pathway or is due primarily to loss of PC1/3 activity, one might expect an increase in intact proinsulin relative to the subsequent processing intermediate (des-31,32 proinsulin). Conversely, if the processing defect is more distal in the secretory pathway, or perhaps dependent on increased secretory demand or PC2 cleavage, we might expect a relative increase in this intermediate form vs intact proinsulin. Clues to where the defect may exist have come from limited human studies examining circulating proinsulin and its conversion intermediates. Following HPLC separation of samples obtained immediately following arginine stimulation, the proportion of total proinsulin immunoreactivity consisting of intact proinsulin was 40% in people with type 2 diabetes compared with 30% in healthy controls (136). This observation was made in a small number of subjects and has also been observed in studies using immunoassays for the different forms of proinsulin (137, 399). Collectively, these observations suggest the impairment in proinsulin processing likely involves PC1/3 and is early in the processing pathway. Single cell transcriptomic and immunostaining studies have not shown PC1/3 mRNA or protein concentrations to be markedly decreased in β cells in type 2 diabetes (40, 41), suggesting that the processing defect is probably due to reduced PC1/3 enzyme activity. A reduction in enzyme activity could be due to a change(s) occurring anywhere in the secretory pathway, including the Golgi and secretory granule where pH and calcium are critical determinants of PC1/3 activity (36, 421).
Aside from an intrinsic processing defect, a contribution from β-cell secretory stress to the elevation in the proinsulin:insulin ratio in type 2 diabetes is also likely. This concept is supported by human studies in which β-cell rest, by inhibition of secretion with somatostatin, partially lowered the proinsulin:insulin ratio in type 2 diabetic subjects (422, 423).
Type 1 diabetes
While type 1 diabetes has for many years been thought to be a disease of absolute β-cell loss, studies of human type 1 diabetes donor pancreas from limited autopsy series (73, 424), the Network of Pancreas Organ Donors in Diabetes (nPOD) repository (69, 425), and the Diabetes Virus Detection (DiViD) Study (426), have revealed considerable and variable residual β-cell mass remaining in most type 1 diabetes cases, particularly those with later-age onset of disease. The remaining β cells are insufficient to maintain normoglycemia, clearly indicating they are dysfunctional (427).
A number of clinical studies have demonstrated that, despite near loss of insulin secretion and detectable C-peptide, people with type 1 diabetes can continue to have persistent secretion of proinsulin, even in longstanding disease and in the absence of detectable C-peptide (80-84). Thus, the proinsulin:C-peptide ratio can be markedly elevated when severe secretory dysfunction is present in type 1 diabetes. Furthermore, in autoantibody positive humans, the proinsulin:C-peptide ratio is elevated, more so in those who subsequently progressed to type 1 diabetes (428-430). Impairments in proinsulin processing may therefore also be an early occurrence in the pathophysiology of type 1 diabetes and have value as a predictive biomarker.
Elucidation of the mechanisms underlying continued secretion of proinsulin in type 1 diabetes is an active area of investigation that may provide insight into type 1 diabetes pathogenesis as well as the value of proinsulin as a type 1 diabetes biomarker. Immunostaining studies of pancreas from type 1 diabetes donors from the DiViD Study (431) and from the nPOD biobank (425, 431, 432) have revealed islet cell populations in type 1 diabetes that are rich in proinsulin immunoreactivity but devoid of immunoreactivity for mature insulin, suggesting that populations of residual cells in type 1 diabetes are able to synthesize but not process proinsulin. It has been proposed that these cells may be “sleeping,” “degranulated,” or “dedifferentiated” and therefore have suboptimal function (433-435). Further, this state may enable them to escape autoimmune attack because of decreased production and presentation of key autoantigens. Mass spectrometry analysis of laser-dissected tissue revealed decreased PC1/3 and CPE in type 1 diabetes islet cells, suggesting that β cells in this disease may lack optimal machinery for prohormone processing (432). In support of this, analysis of gene expression in RNA extracted from frozen sections of type 1 diabetic pancreas indicated lower PC1/3 expression, associated with elevated proinsulin:C-peptide protein ratios in the extracted pancreas tissue (425). Although loss of PC1/3 activity might be predicted to lead to increased levels of intact proinsulin, to date there are no data that provide insight into the relative proportion of intact proinsulin vs the des-31,32 proinsulin conversion intermediate in type 1 diabetes.
One plausible driver of the loss of prohormone convertase expression and impaired proinsulin processing could be the impact of proinflammatory cytokines such as IL-1β and TNF-α (436), which have been implicated in type 1 diabetes (437-439). These cytokines induce the loss of processing enzymes in human islets, which is associated with impaired proinsulin processing (432, 436). Another possible contributor is ER stress, markers of which are detectable in type 1 diabetic pancreas (440) and which is hypothesized to contribute to β-cell dysfunction and persistent proinsulin secretion in type 1 diabetes (441). This form of cellular stress has been shown in rodent islets to impair proinsulin processing (442). Further, in the nonobese diabetic (NOD) mouse, a model of type 1 diabetes, ER stress markers have been observed in islets and are associated with elevations in the proinsulin:insulin ratio even before disease onset (443). Lastly, islets treated with proinflammatory cytokines showed reduced sarcoendoplasmic reticulum pump Ca2+ ATPase 2b (SERCA2b) transcript (444). Such loss of SERCA2b and reduced ER calcium levels may contribute to changes that impede vesicle transport and proinsulin processing (445).
Pancreas and islet transplantation
The plasma proinsulin:C-peptide ratio has been reported to be elevated in type 1 diabetic recipients of islet transplants (446), suggesting that transplanted islets harbor a processing defect. The defect appears to be exacerbated in those who received less islet mass, likely related to them having increased secretory stress on the graft. In keeping with this, the proinsulin:insulin ratio has been shown to be normal in islet transplant and whole pancreas recipients that maintained insulin independence (447-449). However, the use of insulin assays in determining the efficiency of proinsulin processing in transplant recipients is likely to be confounded by exogenous insulin and care must be taken in interpreting these data (449). It is also possible that multiple other factors may contribute to impairments in islet graft function and impaired proinsulin processing, including the toxic effects of immunosuppressive agents, allo- and autoimmune responses to the islet allograft, as well as glycemic control (450-452). Interestingly, the proinsulin:C-peptide ratio was shown to be markedly elevated in a small cohort of recipients of autologous islet transplants (446). These recipients typically receive fewer islets and do not have any anti-islet graft immune response, nor do they receive immunosuppression. This finding suggests that secretory stress may unmask processing inefficiencies in transplanted islets, and moreover points to the importance of transplanting sufficient islet mass. Collectively, these data suggest that the proinsulin:insulin (or C-peptide) ratio has potential as a biomarker of islet graft function; whether it has value in predicting graft failure remains to be determined.
While the mechanism underlying impaired proinsulin processing in islet grafts remains unknown, it may mirror those in diabetes. One study reported lack of PC2 immunostaining in β cells in human islets transplanted into immune-deficient mice (453). As for other forms of diabetes, our understanding of the nature of any processing defect in islet transplants would be enhanced by the ability to more readily measure the different proinsulin forms in peripheral plasma.
Assessment of ProIAPP Processing
Healthy humans
Our knowledge of proIAPP processing to intact IAPP is more rudimentary than that of insulin production from proinsulin. Based on what is known about proIAPP processing from cell culture and mouse studies, IAPP is initially expressed as the 67-amino acid proIAPP (454, 455), which is cleaved to produce mature IAPP by the same proprotein convertases (PC1/3 and PC2), CPE, and PAM (456-459) (Fig. 5). Although the proIAPP processing pathway largely parallels that of proinsulin in the β cell, studies in mice suggest that PC2 is more critical in the final step in proIAPP cleavage (460).
The molar ratio of IAPP to insulin in the circulation of healthy humans is about 1% to 3% (461-463). Accordingly, the abundance of proIAPP in the circulation is lower than proinsulin, making it more difficult to measure and to discriminate the different conversion intermediates. Thus, there have been few studies reporting the physiology of proIAPP in humans. The recent development of discriminatory assays will now open the field for better understanding (464).
Diabetes
Using an enzyme-linked immunosorbent assay (ELISA) specific for a C-terminally processed, N-terminally extended, intermediate form of proIAPP (proIAPP1-48), the ratio of proIAPP1-48 to mature IAPP was shown to be increased in the peripheral circulation of people with type 1 diabetes (464). This pattern would suggest a decrease in the efficiency of the second step in proIAPP processing, which based on studies in mice is predicted to be mediated by PC2 (460, 465). Complete understanding of the nature of the proIAPP processing defect in type 1 diabetes awaits measurements of intact proIAPP1-67 as well as confirmation that the proIAPP processing pathway in human β cells is the same as in mice. The finding of an elevated ratio of proIAPP1-48:IAPP in type 1 diabetes mirrors findings of elevated proinsulin:C-peptide ratios (83). As mentioned previously for proinsulin, more information on the molecular forms of proinsulin that are altered in type 1 diabetes is required (81). In keeping with the clinical findings, evidence for impairments in proIAPP processing has been observed in human islets under similar conditions (elevated glucose and cytokines) in which proinsulin processing has been shown to be impaired, including elevated glucose and cytokines (466, 467).
While impaired proIAPP processing has been demonstrated in type 1 diabetes, interestingly no change in the ratio of proIAPP1-48:IAPP was observed in type 2 diabetes (464). These data do not rule out the possibility that other proIAPP forms, in particular intact proIAP1-67, may be disproportionately elevated in type 2 diabetes. The lack of a difference in this particular ratio contrasts with another study that reported an elevated ratio of proIAPP:IAPP in type 2 diabetes (468). In this latter study, this ratio was 144% in individuals with NGT and 269% in those with type 2 diabetes. Reasons for this disparity are not clear, but the latter study did not report any characteristics of either the proIAPP or IAPP assays employed, including the specificity of the antibodies for the different proIAPP forms or IAPP. Specific measurement of intact proIAPP1-67 and the proIAPP1-48 intermediate in type 2 diabetes promises further insight into the nature of the prohormone processing defects in type 2 diabetes and whether the defect in PC1/3 activity is predominant as for proinsulin. Finding increased proIAPP1-67 in type 2 diabetes would be compatible with a PC1/3 abnormality.
Histological approaches may provide some insight into proIAPP processing defects in diabetes, including whether proIAPP and proinsulin are persistently expressed in the same β cells. Limited work using human pancreatic sections has shown immunoreactivity toward both N- and C-terminal flanking regions of proIAPP in β cells of nondiabetic pancreas donors (469, 470), as well as immunoreactivity to the C-terminal flanking peptide in type 2 diabetes (471). Such immunohistological studies must be interpreted with caution because the antisera used have different specificity toward (pro)IAPP peptides.
Islet transplantation
The level of proIAPP1-48 has been found to be disproportionately elevated in recipients of islet transplants (464), as was observed for proinsulin (452). However, whether the magnitude of the abnormality is a marker for the long-term outcome of the transplant is not clear. As we make progress toward the use of stem cell–derived β-like cells in transplantation, it is possible that measuring proIAPP and/or IAPP may be a useful marker for maturation of these cells. This possibility is supported by the recent finding that IAPP expression may be a marker of β-cell maturity in transplanted stem cell–derived β-like cells (472).
Summary
Proinsulin processing has been shown to be abnormal in the major forms of diabetes and following transplantation. The proIAPP processing machinery is likely also affected in diabetes. The value of proinsulin as a biomarker is likely to increase as approaches such as mass spectrometry are developed that allow the measurement of intact proinsulin, its conversion intermediates, and insulin in a single assay. The continued development of ELISAs and peptidomic approaches specific for the different proIAPP forms should facilitate a deeper understanding of the biology of proIAPP processing in humans and their potential as biomarkers of β-cell dysfunction in both type 1 and type 2 diabetes.
Measurement of β-cell Peptides in the Fasting State and Using Dynamic Testing
The development of the radioimmunoassay and its subsequent use for the measurement of insulin heralded a whole new era of understanding in the role of the β cell in the pathogenesis of diabetes. Until that time, it had largely been considered that type 1 diabetes was characterized by absolute insulin deficiency, whereas in the case of type 2 diabetes it was more uncertain. Based on measurements made in healthy subjects and those with “early maturity-onset diabetes,” it became apparent that what we today know as type 2 diabetes was characterized by a reduction in early insulin release in response to oral glucose (473, 474). This knowledge was gleaned from dynamic testing, which is an approach that is still of great benefit today and used commonly in people at risk of and with different forms of the disease.
While the development of the insulin assay has greatly advanced our understanding, today measuring insulin continues to remain a challenge despite efforts to standardize doing so (475, 476). Interestingly and importantly, such differences in insulin values can even occur when using the same assay in different laboratories (475). The general lack of standardization means that currently available assays may give quite different values for insulin on the same sample. Thus, when considering β-cell function based on insulin immunoassays, one has to be cognizant of the impact of this lack of standardization, which can make it extremely difficult to compare functional measures between studies.
Another important consideration in the interpretation of tests that assess the functional status of the β cell is that its responses need to be considered as a component of an integrated system. Doing so can markedly alter the interpretation of β-cell responses and has underscored the importance of this endocrine cell in the pathophysiology of diabetes as well as the outcome of interventional approaches to prevent and treat hyperglycemia.
Lastly, as introductory comments, brief descriptions and the coefficients of variation of selected measures of β-cell response, insulin sensitivity, and β-cell function discussed below are listed in Tables 4 and 5, respectively.
Table 4.
Descriptions of plasma-based measures commonly used in humans to assess β-cell responses, insulin sensitivity, and β-cell function
| Measure | Description | References |
|---|---|---|
| Measures Based on an OGTT | ||
| β-cell responses | ||
| ∆Insulin0-30/ ∆glucose0-30 | Early insulin response, also known as the insulinogenic index and insulin-to-glucose ratio (IGR). It is measured as the ratio of the increment in insulin relative to glucose during the first 30 minutes following glucose ingestion. It represents a mixture of first- and second-phase insulin secretion as initially defined from intravenous glucose administration. | (477-479) |
| ∆C-peptide0-30/ ∆glucose0-30 | Early C-peptide response, which is similar to the early insulin response but is less affected by differences in hepatic insulin clearance. | |
| IncAUCinsulin/IncAUCglucose | Ratio of the incremental area under the curve (IncAUC) for the insulin response during the whole OGTT as a function of the IncAUC for the glucose response. It provides an estimate of the efficiency of the β-cell’s responsiveness over a prolonged period of glucose exposure. The incremental insulin response is preferred to the total insulin response because the latter includes fasting insulin and is thus not as reflective of the β-cell’s response to the glucose stimulus. | (478, 480) |
| IncAUCC-peptide/IncAUCglucose | Similar to the measure obtained using insulin, but less affected by differences in hepatic insulin clearance. | |
| CIR (corrected insulin response) | Calculated as (100 × 30-min insulin)/(30-min glucose × [30-min glucose − 70 mg/dl]) | (481) |
| Insulin sensitivity | ||
| HOMA1-IR | Equation simplified to allow calculation as HOMA1-IR = (FPI × FPG)/22.5, where fasting plasma insulin (FPI) is in µU/mL and fasting plasma glucose (FPG) is in mmol/L. This measure provides a fasting-based measure of insulin sensitivity. As it is an index of “insulin resistance,” low values indicate insulin sensitive and high values insulin resistant. | (482) |
| IS (insulin sensitivity index) | Calculated as 22.5/(FPI x FPG), where fasting plasma insulin (FPI) is in µU/mL and fasting plasma glucose (FPG) in mmol/L]. It is the inverse of HOMA-IR. Thus, high values indicate insulin sensitive and low values insulin resistant. | |
| 1/fasting insulin | A surrogate measure of insulin sensitivity in humans that is highly correlated with the insulin sensitivity index determined using the minimal model of glucose kinetics developed by Bergman and colleagues. | (109) |
| Matsuda index | This measure is calculated from the glucose and insulin values during an OGTT using the formula 104/(I0 × G0 × Im × Gm)1/2, where G0 and Gm are the fasting and mean glucose and I0 and Im are the fasting and mean insulin. | (483) |
| β-cell function (integrating insulin response and insulin sensitivity) | ||
| HOMA1-%B | Based on fasting samples, a simplified equation to calculate HOMA1-%B = (20 × FPI)/(FPG − 3.5), where fasting plasma insulin (FPI) is in µU/mL and fasting plasma glucose (FPG) is in mmol/L. This measure provides a fasting-based measure of the β-cell’s function relative to the prevailing glucose concentration. | (482) |
| DIo (oral disposition index) | Product of the insulinogenic index and either 1/fasting insulin or HOMA-IR to provide a measure of β-cell function. Importantly, one cannot simply multiply 2 variables together unless it has been proven that the relationship is hyperbolic with a slope not different to −1. | (110) |
| ISSI-2 (insulin secretion-sensitivity index-2) | Ratio of the area under the insulin curve to the area under the glucose curve (as a measure of the β-cell response) multiplied by the Matsuda index to provide an estimate of β-cell function. | (484) |
| Model-derived parameters from an OGTT | ||
| β-cell responses | ||
| HOMA2-%B | Based on fasting samples, a nonlinear model that utilizes either C-peptide or insulin with paired glucose measurement to provide an estimate of the β-cell’s function. This version incorporates an estimate of proinsulin secretion and thus allows use of either total or specific insulin assays. Further, it accounts for renal glucose losses, thus allowing its use in hyperglycemic subjects. Available at https://www.dtu.ox.ac.uk/homacalculator/. | (485) |
| Glucose sensitivity | The slope of the curve relating the rate of insulin secretion to standardized glucose concentrations during the test. It is calculated from the glucose and C-peptide data using a model developed by Mari and colleagues. | (486) |
| Rate sensitivity | An index of early insulin release that represents the dependence of the rate of insulin secretion on the rate of change of glucose concentration. It is calculated from the glucose and C-peptide data using a model developed by Mari and colleagues. | (486) |
| Potentiation | A time-varying factor expressing a potentiation effect upon insulin secretion. It accounts for the physiological processes that can acutely modify insulin secretion. It is calculated from the glucose and C-peptide data using a model developed by Mari and colleagues. | (486) |
| φ d | “Dynamic sensitivity,” a measure of the stimulatory effect of the rate of increase in glucose on the secretion of stored insulin, calculated using a model developed by Cobelli and colleagues. | (487) |
| φ s | “Static sensitivity” is a measure of the effect of glucose on β-cell secretion calculated using a model developed by Cobelli and colleagues. | (487) |
| Insulin sensitivity | ||
| HOMA2-%S | Nonlinear model that utilizes either C-peptide or insulin with paired glucose measurement to provide an estimate of insulin sensitivity. Available at https://www.dtu.ox.ac.uk/homacalculator/. | (485) |
| OGIS (oral glucose insulin sensitivity) | A simple method for calculating insulin sensitivity using glucose and insulin data that is in good agreement with clamp-based measurements. Available at http://webmet.pd.cnr.it/ogis/. | (488) |
| Measures based on intravenous glucose testing | ||
| β-cell responses | ||
| First-phase insulin response | The insulin response to an intravenous bolus of glucose, also known as the acute insulin response to glucose (AIRglucose). This response occurs over the first 10 minutes following glucose administration. This measure can also be determined using C-peptide. | (55, 477, 489, 490) |
| Second-phase insulin response | This insulin response commences shortly after glucose administration, but is not readily discernible until the first-phase response has ended, ie, at about 10 minutes. This measure can also be determined using C-peptide. | (477, 490) |
| AIRmax | Acute insulin response at maximal glycemic potentiation (≥25 mmol/L), typically measured using arginine as the secretagogue. This measure represents β-cell secretory capacity. It has been considered a measure of β-cell mass, but data in humans demonstrate it can change acutely when mass would not be changing. Arginine-induced responses can also be measured at lower glucose concentrations, but these do not usually represent the maximal β-cell response. | (127) |
| Insulin sensitivity | ||
| SI (insulin sensitivity index) | An index of insulin sensitivity calculated from parameters determined using the minimal model of glucose kinetics developed by Bergman and colleagues. Determined from a frequently sampled intravenous glucose tolerance test lasting 3 hours. | (108) |
| M/I | A measure of whole-body insulin sensitivity calculated from clamp studies as the rate of glucose infusion (M) corrected for body size/plasma insulin (I) attained at a steady-state typically achieved after about 2 hours. Addition of tracers (eg, [6,6-2H2]-glucose) allow the measurement of Ra and Rd. Ra denotes rate of tracer appearance when making use of labeled glucose, providing a measure of hepatic insulin sensitivity. Rd denotes rate of tracer disappearance when making use of labeled glucose, providing a measure of skeletal muscle insulin sensitivity. | (491, 492) |
| β-cell function | ||
| DI (disposition index) | A measure that examines the β-cell response relative to insulin sensitivity, the latter a major determinant of secretory demand on the β cell. Importantly, one cannot simply multiply 2 variables together unless it has been proven that the relationship is hyperbolic with a slope not different from −1. | (109) |
Abbreviations: ∆C-peptide0-30/∆C-peptide0-30, C-peptide index/early C-peptide response; ∆Insulin0-30/∆glucose0-30, insulinogenic index/early insulin response; HOMA, homeostatic model assessment; OGTT, oral glucose tolerance test.
Table 5.
Coefficient of variation (%) for selected β-cell function measures obtained using oral and intravenous testing in humans
| NGT | IGT | Diabetes | Diabetes (no meds) | All | Reference | |
|---|---|---|---|---|---|---|
| Oral glucose tolerance test (OGTT) | ||||||
| Fasting glucose | 4.0 | 5.2 | 4.3 | 4.8 | 4.5 | (478) |
| 2-hour glucose | 24.9 | 11.0 | 8.0 | 5.9 | -- | (478) |
| Fasting insulin | 19.3 | 14.3 | 16.5 | 17.1 | 16.6 | (478) |
| Fasting C-peptide | 11.5 | 10.8 | 7.2 | 8.3 | 9.7 | (478) |
| HOMA-IR | 19.1 | 14.4 | 16.1 | 17.0 | 16.4 | (478) |
| ∆Insulin0-30/∆glucose0-30 | 41.1 | 62.9 | 69.1 | 79.7 | 57.1 | (478) |
| ∆C-peptide0-30/∆glucose0-30 | 42.3 | 37.4 | 27.3 | 32.3 | 34.7 | (478) |
| IncAUCinsulin/IncAUCglucose | 29.4 | 27.0 | 19.2 | 22.7 | 24.9 | (478) |
| IncAUCC-peptide/IncAUCglucose | 25.5 | 14.2 | 10.0 | 9.0 | 17.7 | (478) |
| Model-derived parameters from an oral glucose tolerance test (OGTT) | ||||||
| Glucose sensitivity a | 17.2 | 16.5 | 20.3 | 20.3 | 20.3 | (478) |
| Rate sensitivity a | 42.1 | 40.5 | 114.8 | 76.2 | 44.6 | (478) |
| Potentiation a | 43.6 | 26.7 | 26.9 | 26.1 | 33.0 | (478) |
| φ db | 31 | (493) | ||||
| φ sb | 18 | (493) | ||||
| Intravenous glucose tolerance test (IVGTT) | ||||||
| AIRglucose (first-phase insulin response) | 20.6 | -- | -- | -- | -- | (494) |
| 20.1 | -- | -- | -- | -- | (495) | |
| Kg (glucose disappearance constant) | 14.5 | -- | -- | -- | -- | (494) |
| 51.4 | -- | -- | -- | -- | (495) | |
| SI (insulin sensitivity index)c | 16.9 | -- | -- | -- | -- | (494) |
| 20.2 | -- | -- | -- | -- | (495) | |
| Hyperglycemic clamp | ||||||
| Fasting insulin | 13.2 | -- | -- | -- | -- | (496) |
| AIRglucose (first-phase insulin response) | 10.4 | -- | -- | -- | -- | (496) |
| Maximal insulin response to arginine | 16.3 | -- | -- | -- | -- | (496) |
Abbreviations: AIRglucose, acute insulin response to glucose; ∆C-peptide0-30/∆C-peptide0-30, C-peptide index; ∆Insulin0-30/∆glucose0-30, insulinogenic index; IncAUC, incremental area under the curve; Kg, glucose disappearance constant.
a calculated using a model developed by Mari and colleagues (486)
b calculated using a model developed by Cobelli and colleagues (487)
c calculated using the minimal model of glucose kinetics developed by Bergman and colleagues (108)
Importance of Accounting for Insulin Sensitivity in Assessing β-Cell Function
It was recognized soon after the development of the insulin assay that in absolute terms, healthy obese individuals had greater insulin responses compared with healthy lean individuals. However, by relating these responses to the fasting measurement, it was clear that the magnitude of the response was a function of the basal insulin level and no difference in obese and lean individuals was observed (497). While this approach did not gain much traction for many years as numerous investigators continued to consider insulin responses in isolation, it led to the concept of compensatory hyperinsulinemia and laid the groundwork for subsequent work demonstrating the importance of insulin sensitivity in modulating the β-cell response.
The notion of compensatory hyperinsulinemia was advanced by conceptualization of a negative feedback loop that allows the β cell to recognize the degree of tissue insulin sensitivity and appropriately modulate its insulin response (108). The nature of this feedback loop presupposed that the relationship between insulin sensitivity and a β-cell response measure was hyperbolic in nature, an idea that was subsequently demonstrated in humans using intravenous and oral testing (109, 110). This measure became widely known as the disposition index (108, 498) and can be used in cross-sectional and longitudinal studies (Fig. 6). When the relationship between insulin sensitivity and the β-cell response is shown to be represented by a rectangular hyperbola, the disposition index can be simply calculated as the product of these 2 parameters (108). Importantly, to make the case that the 2 parameters can merely be multiplied together requires that the slope of the regression equation relating the log of each measure be not statistically different to −1. Failure to demonstrate such negates using this simple product and requires use of an alternative equation or accounting for insulin sensitivity in regression models of the β-cell response. Unfortunately, proving the existence of a hyperbola for many pairings of insulin sensitivity and a β-cell response has not always been undertaken; thus, interpretation of data that have not demonstrated this must be made with caution. Recently, an alternative approach has been suggested using the logarithmic expression of the disposition index equation and does not require that the slope be −1 (499). This new approach will require further assessment to determine its veracity and utility in examining this integrated system that is a critical determinant of glycemia. Finally, it is important to recognize that because of differences in clearance, the use of a simple product to calculate the disposition index as a measure of β-cell function is not applicable when using C-peptide measurements.
Figure 6.
Schematics illustrating the relationship between insulin sensitivity and β-cell responses as determinants of β-cell function. A, The relationship is nonlinear in nature and defines a hyperbola with insulin resistant individuals having greater β-cell responses than individuals who are insulin sensitive. The heterogeneity of this relationship among people is demonstrated by individuals falling on either side of the line. The line depicts the mean relationship of insulin sensitivity and the β-cell response for these individuals, commonly known as the disposition index, representing β-cell function. If the slope describing the relationship between these 2 variables is not statistically different from −1, the disposition index can be calculated as the simple product of insulin sensitivity and the β-cell response. The area above the line typically represents better glucose tolerance and below the line poorer glucose tolerance. B, Vector plot of the longitudinal effects of intervention(s) on insulin sensitivity and β-cell responses in individuals or groups of individuals. Any change that moves from the starting point to above the line (even if the β-cell response is lower) represents an improvement in β-cell function, while changes that result in the endpoint being below the line (even if the β-cell response is greater) represent a worsening of β-cell function. Movement along the line, whether it be up or down, represents changes in insulin sensitivity and β-cell responses, but not a change in β-cell function (not illustrated).
Our understanding of the importance of interpreting β-cell responses while accounting for insulin sensitivity is now accepted more or less as a sine qua non when evaluating β-cell function. Application of this approach has advanced our understanding of the critical importance of the β cell in glycemic regulation. While a full discussion of findings using this approach is beyond the scope of this review, select examples are provided later to give a sense of what has been achieved.
Assessment of β-Cell function With Fasting Samples
The mainstay of fasting biomarkers to assess β-cell function is the homeostatic model assessment (HOMA). First introduced in 1985, this steady-state measure was developed to use glucose and insulin pairs to provide estimates of β-cell function (HOMA-B) and insulin sensitivity (HOMA-S) (482). While the latter is used more extensively, particularly with the development of a simplified equation approach (HOMA-IR), HOMA-B has provided insights into the functional status of the β-cell. This measure is expressed as a percentage relative to a “normative” value of 100% determined in a healthy population. However, it does not always provide the same estimate of function as do responses quantified following administration of β-cell secretagogues (500).
While the original description of the model identified its nonlinearity, simple mathematical approximations were provided that allowed the use of an equation, namely HOMA1-%B = (20 × FPI)/(FPG – 3.5), where FPI is fasting plasma insulin in µU/mL and FPG is fasting plasma glucose in mmol/L. With this formula, doubling the insulin concentration at the same glucose concentration will double %B HOMA; thus, simple variability between insulin assays can provide very different estimates of β-cell function on the same sample. It is also possible that a difference, for example a %B of 100% vs 200%, may reflect proportionate differences in insulin sensitivity, so concurrent estimation of HOMA-S is always advised. Modification of the original approach gave rise to HOMA2, which was introduced in 1998 (485) and is available as a calculator at https://www.dtu.ox.ac.uk/homacalculator/. It can be used with either fasting insulin or C-peptide along with fasting glucose. Insulin is used more frequently because it is more readily available and can simultaneously be used to estimate insulin sensitivity; however, one has to be aware of collinearity when relating HOMA-B to HOMA-S (or HOMA-IR) and must perform statistical testing to exclude this possibility.
Due to difficulties with the reproducibility of assays, particularly insulin, the major utility of HOMA-B is in studies where there are typically large numbers of participants followed longitudinally using the same assay so that comparisons of change can be made relative to baseline. It clearly has less utility as an isolated measure, especially if normative data have not been determined for the population under study; thus, caution must be applied when interpreting such data. Some examples where the HOMA approach for quantifying β-cell function has proven useful include epidemiological studies examining progression to diabetes in adults (501, 502) and childhood (503) as well as short- and long-term clinical studies in which the effect of interventions on β-cell function was assessed (504-506). Perhaps the most-cited finding using this approach reported that β-cell function in type 2 diabetes is 50% of normal at the time of diabetes diagnosis and the disease likely commenced many years before (507). As discussed in more detail subsequently, at the time of diagnosis, the magnitude of β-cell dysfunction estimated with this measure does not align with findings using dynamic testing that suggest a greater loss of β-cell function.
Overall, the use of fasting samples continues to be valuable for assessing β-cell function when dynamic testing is not feasible. It is not really an appropriate choice when the number of subjects is low, when there are no longitudinal assessments, and when variability in the insulin assay may influence the actual insulin concentrations. Furthermore, it is not appropriate to use these human-based equations for calculating β-cell function (or insulin sensitivity) in animals.
Measuring Dynamic β-Cell Function Responses
While in the beginning, oral glucose ingestion formed the basis of estimating insulin responses (473, 474), it was not long after that it was demonstrated that intravenous glucose elicited insulin responses that could also be used to address how the β cell was performing (477, 508). These 2 different routes of administration also helped delineate the incretin concept that is characterized by a greater β-cell response when glucose is ingested than when matched glucose levels are achieved with intravenous glucose infusion (509-511). Intravenous testing has also been utilized to gain an estimate of β-cell “mass,” given that the major forms of diabetes are both associated with β-cell loss (512, 513). In addition to providing different insights into β-cell function, these various measures differ in their reproducibility, which is an important consideration when designing studies (478, 494-496).
Oral testing
The standard approach uses glucose as the stimulus, as it not only provides insight into β-cell function but also an opportunity to quantify and classify glucose tolerance (4). An alternative is the meal tolerance test, which provides insight into the β-cell response to nutrient stimuli beyond just glucose (514). While from the β-cell function perspective, insulin is classically used in interpreting these tests, it is possible to substitute C-peptide for that purpose. This latter approach has proven to be particularly useful in studying type 1 diabetes. When using C-peptide assays, it is important to consider their performance and cross-reactivity with proinsulin and insulin; information on different C-peptide assays as provided by the manufacturers is listed in Table 3.
Using these oral approaches, a number of responses can be calculated to provide insight into how the β cell is functioning. The early insulin response, also known as the insulinogenic index, is used most frequently and expresses the increment in insulin above fasting over the first 30 minutes of the test relative to the glucose response (∆insulin0-30/ ∆glucose0-30) (477-479). The value of this approach is that the increase in glucose over the first half hour is generally similar in magnitude in people with different degrees of glucose tolerance, thus allowing for an assessment of the β-cell response that is less affected by variance in glucose disposal (Fig. 7). Later in the test, the insulin concentration is far more dependent on the prevailing glucose concentration so that the absolute value can be greater in those who have type 2 diabetes compared to those with NGT (473, 474, 478). It is also possible to evaluate the insulin response throughout the test as a measure of the adequacy of β-cell responsiveness, determining its magnitude as an incremental area under the curve (iAUC) above fasting and relating it to the iAUC for glucose over the same period (140).
Figure 7.
Glucose curve shapes during an oral glucose tolerance test (OGTT). Glucose profiles for A, monophasic; B, biphasic; and C, incessant increase curve types with the corresponding insulin profiles for D, monophasic; E, biphasic; and F, incessant increase curve types.
In line with the earlier discussion, it is also important to interpret these responses in the context of the prevailing insulin sensitivity (Fig. 6). Adjustment of the early insulin response for insulin sensitivity led to the designation of the oral disposition index (DIo) (110), in which the relationship was shown to be a rectangular hyperbola (slope of the relationship of the logged measures being −1), thereby allowing the simple product to be calculated. If the relationship is not a rectangular hyperbola, one must still account for insulin sensitivity but use an approach other than the simple product. It is also important to appreciate that calculating different measures (β-cell response and insulin sensitivity) from the same glucose and insulin data and then examining their relationship increases the risk of collinearity and a false outcome. Thus, testing for collinearity is essential when doing so.
Given the longstanding use of oral tests in examining glucose metabolism in humans, it is not possible in this review to provide insight into the numerous novel observations made using this approach. In some instances, these findings have arisen when the test is performed in conjunction with mathematical modeling, as described below. A selection of the numerous insights obtained using oral tests include that (i) loss of β-cell function is a key component of the development of IGT and diabetes in all major racial/ethnic groups in the United States (122, 123); (ii) there is a progressive loss of β-cell function as glucose tolerance declines and this loss continues as the fasting glucose increases, even across the normal glucose range (124, 156); (iii) relatives of individuals with type 2 diabetes have β-cell dysfunction at a time when they are still normoglycemic (515); (iv) genome-wide SNPs are linked to deficient β-cell function in people with IGT (516-521); (v) favorable responses to interventions that prevent progression of prediabetes to diabetes and of type 2 diabetes itself are dependent on better β-cell function at the time of the intervention (522-524); and (vi) the release of C-peptide in established type 1 diabetes provides evidence that there are residual β cells (427, 525-527) and the degree of residual β-cell function determines the ability to achieve tight glycemic control (525) as well as being associated with reduced episodes of severe hypoglycemia (528).
Dynamic testing has also highlighted the magnitude of β-cell dysfunction in type 2 diabetes. While the HOMA-B estimates based on fasting measures suggest that β-cell function is decreased by 50% at the time of diabetes diagnosis, dynamic testing highlights that the deficit is even greater. Analyses using the disposition index in studies of Finns (124), Japanese Americans (156), and other ethnic groups in the United States studied as part of the GENNID Study (122) (N. Esser and S.E. Kahn, unpublished observation) have found that β-cell function declines as fasting glucose increases, commencing even within the normal glucose range. Compared with those with NGT, at the diagnostic threshold for diabetes β-cell function is reduced by about 80%, while in impaired glucose metabolism it is already decreased by 50%.
Finally, the shape of the glucose concentration curve is being used as an indirect approach aimed at identifying physiologically distinct groups and individuals in whom the disease is more likely to progress (Fig. 7). The dominant curve phenotype in both youth and adults and in prediabetes and diabetes is monophasic, with a peak between 30 and 90 minutes that then declines (529-532). Individuals with this curve shape have lower β-cell function than those whose curve manifests as a biphasic pattern in which glucose increases, declines, and then increases again. Individuals with a monophasic curve are at increased risk of developing impaired fasting glucose and type 2 diabetes (530, 531). A third curve, of the incessant increase type in which glucose rises throughout the test, is underscored by a profound loss of β-cell function and predicts accelerated β-cell dysfunction and glycemic failure (533).
Intravenous testing
Administration of glucose intravenously, either as a short infusion or bolus injection, has for greater than 50 years been used to quantify β-cell responses, an approach that has clearly stood the test of time and is still well utilized today. This approach led to the identification of 2 distinct phases of insulin release: first and second (55). It is important to recognize that these 2 phases are defined and distinguishable by the response to intravenous glucose, and cannot be distinguished during an oral test. Subsequently, the hyperglycemic clamp was developed in which glucose is fixed at a predetermined level (491), a method consequently extended to generate a dose-response curve by clamping glucose at multiple concentrations (126, 127) or using graded glucose infusion rates to allow the glucose concentration to spontaneously equilibrate at different levels (534).
In humans, the first-phase insulin response starts immediately with glucose administration and is usually considered to be complete 10 minutes later, particularly when given as a bolus. The second phase also begins early and embodies insulin concentrations while glucose remains elevated. In diabetes, the first phase is essentially absent while the second phase is reduced, but not gone. In fact, the first-phase response is reduced in IGT and IFG, with it seeming to be lost when the fasting glucose is around 115 mg/dL (6.4 mmol/L) (125, 477, 535), well below the diagnostic threshold for diabetes (536). The second-phase response decreases as the β cell’s secretory ability declines (127). Thus, these phases clearly represent different secretory aspects of the cell, with the first-phase response being a valuable early marker in people whose glucose tolerance is still relatively normal.
Assessment of the glucose dose-response curve by attaining a steady state at multiple levels has provided additional insights into the β cell’s responsiveness. Maximal responsiveness is achieved at a glucose concentration >450 mg/dL (25 mmol/L). This approach has frequently been supplemented with the addition of a nonglucose secretagogue, typically arginine (127). Use of this amino acid not only acutely stimulates insulin release, but also releases glucagon and thus can provide insight into α-cell function (127, 537). This maximal response, frequently termed AIRmax, represents the secretory capacity of the β cell (127). It has also been considered to be a marker of β-cell mass as currently, except at autopsy, mass remains an unmeasurable aspect of diabetes pathology (512, 513). However, it is also clear that this secretory measure can change rapidly (within a week or two) (405), a time interval in which mass would not be expected to increase. This realization has played a part in the development of the concept of “functional β-cell mass” (538). We feel it is important to advise caution in simply linking these two together as they do not always associate. For example, β-cell function can be markedly reduced (more than 80%) when the number of β cells (“mass”) is reduced by 50% or less (111, 113, 127). Alternatively, an intervention can increase β-cell responses fairly rapidly in a time frame when the number of β cells would not have changed (405, 409, 539).
The β cell also exhibits oscillatory behavior, both short, rapid and ultradian in nature (540, 541), with these disturbed in people with abnormal glucose tolerance (128, 129). An uncoupling of the relationship between glucose oscillations and insulin secretion is already apparent in IGT, in keeping with a disturbance in the feedback loop of glucose and insulin secretion early in the course of the disease (130).
Collectively, intravenous-based testing measures continue to provide valuable insights into β-cell secretory function, some of which are in line with what can be determined from the oral test. As within-subject variability of intravenous measures is lower than in oral tests, typically fewer subjects will be required when using intravenous testing (478, 494, 495). However, all are modulated by tissue insulin sensitivity and thus need to be interpreted with this in mind. Doing so has been truly informative, with multiple observations made, including (i) the progression from NGT to IGT and diabetes occurs because of a progressive loss of β-cell function (121); (ii) first-degree relatives of individuals with type 2 diabetes (542, 543) and HLA-identical siblings of people with type 1 diabetes have reduced β-cell function (76); (iii) groups at high risk of developing type 2 diabetes, including older individuals (544, 545) and women with a history of gestational diabetes (546, 547) or polycystic ovary syndrome (548, 549) manifest reduced β-cell function even when glucose tolerance may still be normal; (iv) the loss of β-cell function over time underlies diabetes development in those with prior gestational diabetes (550); (v) the long-term, glycemic improvement with metabolic surgery results from improved β-cell function (551, 552); (vi) youth with IGT or recently diagnosed type 2 diabetes have hyperresponsive β cells compared with adults of similar body size who are 40 years their senior (139); and (vii) the decline in β-cell function in these youth is more rapid than in adults and does not abate in response to metformin or insulin glargine (138, 553).
Mathematical Modeling of β-Cell Function
The era of mathematical modeling to quantify aspects of glucose metabolism gained traction in the 1980s and has expanded in approaches and utilization since. Today, modeling is used frequently to assess β-cell function and can be performed with both intravenous and oral testing, the latter where it has been most commonly utilized.
Modeling is particularly convenient with oral testing (486, 493). By using paired glucose and C-peptide concentrations, a number of measures based on derived insulin secretion rates can be determined. To enable better parameter identifiability, it is desirable to sample more frequently and for longer than is typically done with a standard 2-hour oral glucose or meal tolerance test. Minimizing sample number and/or duration of the test may have practical advantages, but it comes at the risk of a type II error or a need for a larger sample size. Accounting for insulin sensitivity in the interpretation of model-derived β-cell parameters is not always performed but would appear to be advisable and has highlighted differences between groups.
An additional advantage obtained from modeling oral tests is that one can obtain a glucose-dose response curve relating insulin secretion rates to the glucose concentration without having to perform clamps over a broad glucose range. The glucose concentrations on which this calculation is based are those achieved over the course of the oral test and typically fall in the range over which insulin secretion is a linear function of glucose, that is, they do not achieve the concentration (>450 mg/dL) required to estimate maximal secretory capacity. As is commonly the case, these models may include a parameter that improves the final fit of the data but whose physiological meaning is less well characterized.
The utility of mathematical modeling is further supported by findings that have used other approaches to evaluate dynamic β-cell responses. Thus, for example, it has been reported that (i) insulin secretion rates are increased in obese individuals (554); (ii) β-cell function decreases progressively as glucose tolerance declines in adults (554) and youth (555-557); (iii) the response to bariatric surgery is dependent on β-cell function at baseline, with a better response to the intervention in those with better baseline function (558-560); (iv) a subset of individuals at high risk of developing diabetes have insulin hypersecretion in the basal state and following oral or intravenous glucose administration (561); and (v) in nondiabetic individuals with cystic fibrosis, who commonly experience asymptomatic hypoglycemia in the third hour following oral glucose ingestion, insulin secretion rates are inappropriately increased when their glucose falls below fasting (562).
Islet Amyloid Polypeptide in the Assessment of β-cell Function
IAPP was originally identified as the unique peptide component of islet amyloid deposits (162, 163). The peptide is co-released with insulin in response to glucose and nonglucose secretagogues (461-463, 563-568). Its physiological function is not well appreciated, but roles for gastric emptying and appetite regulation have been suggested (569, 570).
Measurement of plasma IAPP concentrations in humans has provided additional insight into β-cell function. As discussed earlier, assays that measure the precursor forms of IAPP are being developed and are expected to inform about the normal processing of proIAPP and how diabetes may affect this process (464). In time, these assays may provide new biomarkers for β-cell function. Using a number of different immunoassays that measure the intact peptide, it is now well recognized that the amount of IAPP produced, stored, and released by the β cell is approximately 1% to 3% that of insulin (461-463). Importantly, as the clearance of IAPP is slower than that of insulin, the molar ratio will vary depending on when sampling is performed relative to the administration of the stimulus (566).
Like with the insulin assay, there is variability in IAPP measurements due to different assay methodologies and specificity of the antibodies. In particular, most older radioimmunoassays likely cross-react with proIAPP forms, ELISAs for IAPP immunoreactivity have varying ability to detect putative O-glycosylated and non-glycosylated forms of the peptide (571, 572), and human IAPP is highly fibrillogenic and special care is needed in its handling as a standard in these assays (455). Despite this, among the observations made using human venous plasma with different IAPP assays are the following (i) obesity and/or insulin resistance are associated with increased IAPP concentrations (565, 566) and are reduced with weight loss following Roux-en-Y gastric bypass (568); (ii) IAPP is markedly decreased in type 1 diabetes (563, 564); (iii) the IAPP concentration is reduced in type 2 diabetes and IGT, with the degree of reduction in IGT intermediate between type 2 diabetes and NGT (461); (iv) IAPP is lower in individuals who are at increased risk of developing type 2 diabetes, including older people (462) and first-degree relatives of those with the disease (463); and (v) following pancreas-kidney transplantation with venous drainage, IAPP concentrations are increased (567).
Given the findings that IAPP and insulin are typically released together, measurement of mature IAPP as an independent biomarker of β-cell secretory function is currently out of favor. Studies of proIAPP and its conversion intermediates continue and are expected to add important information to our knowledge base and may, like proinsulin, prove to be useful in determining the efficiency of proIAPP processing and provide insight into β-cell dysfunction.
Concluding Remarks
Our understanding of the importance of the β cell in the normal regulation of glycemia and the significance of β-cell dysfunction and mass loss in the pathogenesis of hyperglycemia in diabetes has evolved markedly over time and continues to do so. While measurement of insulin provided the first true insights and laid the foundation, the evolution of science and scientific method has provided a more in-depth view of the physiology and pathology of this critical endocrine cell.
With these advances, biomarkers have and continue to become available that provide opportunities in the research setting, with some now having applicability in clinical care. Along these lines, we have enhanced our understanding of the role of genetics and epigenetics not only in “garden variety” types 1 and 2 diabetes but also the rarer forms such as monogenic diabetes and CFRD. Assay methodology advances have identified new autoantibodies, provided insight into propeptide processing, and raised the possibility of estimating β-cell destruction while it is occurring. Imaging approaches have advanced from ultrasonography and computed tomography to different forms of positron emission tomography, thus making the goal of quantifying the amount of β cells more feasible. With the generation of large amounts of these and other forms of data from cross-sectional, longitudinal, and intervention studies in different populations, opportunities are being created for bioinformatic and machine learning approaches to further inform us. Patterns identified in these analyses should help further refine our understanding of diabetes subtypes and the basis for disease progression, leading us closer to precision medicine.
As we look forward from today, much remains to be done, but there is hope that we will soon be able to use β-cell biomarkers to predict, diagnose, and prognosticate diabetes and not need to rely as heavily on functional tests as currently required.
Acknowledgments
We wish to thank our colleagues who over the years have helped us formulate our thinking and expand our knowledge on the β cell. While the reference list for this article is extensive, we recognize that we have not been able to cite all the relevant literature and apologize to those whose work we may not have included.
Work in the authors’ laboratories is supported by the United States Department of Veterans Affairs grant I01BX001060 (to S.E.K.); VA Puget Sound Health Care System (Seattle, WA), Seattle Institute for Biomedical and Clinical Research (Seattle, WA); National Institutes of Health grants P30 DK017047; Canadian Institutes of Health Research project grant PJT-153156 (to C.B.V); and Juvenile Diabetes Research Foundation grant 1-INO-2019-794-S-B (to C.B.V.). In addition, Y.C.C. is supported by a Juvenile Diabetes Research Foundation Postdoctoral Fellowship 3-PDF-2017-373-A-N and N.E. by the Dick and Julia McAbee Endowed Postdoctoral Fellowship from the University of Washington. D.V.R. is supported by a Junior Fellowship of the Dutch Diabetes Foundation and by a European Union Marie Sklodowska-Curie Fellowship.
Select images obtained from Servier Medical Art.
Author Contributions: All authors wrote sections of the manuscript, with S.E.K. and C.B.V. also editing the manuscript.
Glossary
Abbreviations
- AIRmax
acute insulin response at maximal glycemic potentiation (≥25 mmol/L)
- BMI
body mass index
- CFRD
cystic fibrosis–related diabetes
- CFTR
cystic fibrosis transmembrane conductance regulator
- CPE
carboxypeptidase E
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- FPG
fasting plasma glucose (mmol/L)
- FPI
fasting plasma insulin (µU/mL)
- GAD
glutamic acid decarboxylase
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- GRS
genetic risk score
- GWAS
genome-wide association studies
- HbA1c
glycated hemoglobin A1c
- HLA
human leukocyte antigen
- HNF1A
hepatic nuclear factor 1 α
- HNF1B
hepatic nuclear factor 1 β
- HNF4A
hepatic nuclear factor 4 α
- HOMA
homeostatic model assessment
- HPLC
high performance liquid chromatography
- IA2/ICA512
protein tyrosine phosphatase
- IAA
insulin autoantibodies
- IAPP
islet amyloid polypeptide
- iAUC
incremental area under the curve
- ICA
islet cell autoantibodies
- IFG
impaired fasting glucose
- IFN-γ
interferon-γ
- IGT
impaired glucose tolerance
- IL
interleukin
- IL2RA
interleukin 2 receptor α
- INS
insulin
- ISSI-2
insulin secretion-sensitivity index-2
- KATP
ATP-sensitive potassium channel
- LADA
latent autoimmune diabetes in adults
- lncRNA
long non-coding RNA
- MHC
major histocompatibility
- miRNA
microRNA
- MODY
maturity-onset diabetes of the young
- MRI
magnetic resonance imaging
- NGT
normal glucose tolerance
- NODAT
new-onset diabetes mellitus after transplantation of solid organs
- nPOD
Network of Pancreas Organ Donors in Diabetes;
- PAM
peptidylglycine α-amidating monooxygenase
- PBMC
peripheral blood mononuclear cell
- PC1/3
prohormone convertase 1/3
- PC2
prohormone convertase 2
- PET
positron emission tomography
- PP
pancreatic polypeptide
- PTPN22
protein tyrosine phosphatase non receptor type 22
- SNP
single nucleotide polymorphism
- SPECT
single photon emission computed tomography
- TNF
tumor necrosis factor
- VMAT2
vesicular monoamine transporter type 2
- ZnT8
zinc transporter 8
Contributor Information
Steven E Kahn, Division of Metabolism, Endocrinology and Nutrition, Department of Medicine, VA Puget Sound Health Care System and University of Washington, Seattle, 98108 WA, USA.
Yi-Chun Chen, BC Children’s Hospital Research Institute and Centre for Molecular Medicine and Therapeutics, Vancouver, BC, V5Z 4H4, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, V5Z 4H4, Canada; Department of Surgery, University of British Columbia, Vancouver, BC, V5Z 4H4, Canada.
Nathalie Esser, Division of Metabolism, Endocrinology and Nutrition, Department of Medicine, VA Puget Sound Health Care System and University of Washington, Seattle, 98108 WA, USA.
Austin J Taylor, BC Children’s Hospital Research Institute and Centre for Molecular Medicine and Therapeutics, Vancouver, BC, V5Z 4H4, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, V5Z 4H4, Canada; Department of Surgery, University of British Columbia, Vancouver, BC, V5Z 4H4, Canada.
Daniël H van Raalte, Department of Internal Medicine, Amsterdam University Medical Center (UMC), Vrije Universiteit (VU) University Medical Center, 1007 MB Amsterdam, The Netherlands; Department of Experimental Vascular Medicine, Amsterdam University Medical Center (UMC), Academic Medical Center, 1007 MB Amsterdam, The Netherlands.
Sakeneh Zraika, Division of Metabolism, Endocrinology and Nutrition, Department of Medicine, VA Puget Sound Health Care System and University of Washington, Seattle, 98108 WA, USA.
C Bruce Verchere, BC Children’s Hospital Research Institute and Centre for Molecular Medicine and Therapeutics, Vancouver, BC, V5Z 4H4, Canada; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, V5Z 4H4, Canada; Department of Surgery, University of British Columbia, Vancouver, BC, V5Z 4H4, Canada.
Additional Information
Disclosures: Steven E. Kahn has served as a consultant to Bayer, Boehringer Ingelheim, Casma Therapeutics, Eli Lilly, Intarcia, Janssen, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Daniël H. van Raalte has served as a consultant to Boehringer Ingelheim-Eli Lilly Diabetes Alliance, Merck, Sanofi, and Astra Zeneca and has received research grant support from Boehringer Ingelheim-Lilly Diabetes Alliance, AstraZeneca, and Merck. Sakeneh Zraika has received research grant support from Novartis. C. Bruce Verchere is a director, scientific advisor, and shareholder in Integrated Nanotherapeutics and has served as a consultant to Sirona Biochem. All other authors have no disclosures.
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. ; IDF Diabetes Atlas Committee . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2. McCarthy MI. Painting a new picture of personalised medicine for diabetes. Diabetologia. 2017;60(5):793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60(5):769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-S33. [DOI] [PubMed] [Google Scholar]
- 5. Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol. 2017;13(11):674-686. [DOI] [PubMed] [Google Scholar]
- 6. Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths - selected counties and Indian reservations, United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840-846. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 10. Egan AM, Dow ML, Vella A. A review of the pathophysiology and management of diabetes in pregnancy. Mayo Clin Proc. 2020;95(12):2734-2746. [DOI] [PubMed] [Google Scholar]
- 11. Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345(13):971-980. [DOI] [PubMed] [Google Scholar]
- 12. Naylor R, Knight Johnson A, del Gaudio D. Maturity-onset diabetes of the young overview. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews. University of Washington, Seattle; 1993-2020. https://www.ncbi.nlm.nih.gov/books/NBK500456/. Accessed December 29, 2020. [PubMed] [Google Scholar]
- 13. De Franco E, Flanagan SE, Houghton JA, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386(9997):957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2019 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2020. [Google Scholar]
- 15. de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Raalte DH, Diamant M. Steroid diabetes: from mechanism to treatment? Neth J Med. 2014;72(2):62-72. [PubMed] [Google Scholar]
- 17. Cohen-Bucay A, Gordon CE, Francis JM. Non-immunological complications following kidney transplantation. F1000Res. Published online February 18, 2019;8. doi:10.12688/f1000research.16627.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schambelan M, Benson CA, Carr A, et al. International AIDS Society-USA . Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr. 2002;31(3):257-275. [DOI] [PubMed] [Google Scholar]
- 19. Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125. [DOI] [PubMed] [Google Scholar]
- 20. Resmini E, Minuto F, Colao A, Ferone D. Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol. 2009;46(2):85-95. [DOI] [PubMed] [Google Scholar]
- 21. Chakkera HA, Weil EJ, Pham PT, Pomeroy J, Knowler WC. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care. 2013;36(5):1406-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebovitz HE, Banerji MA. Ketosis-Prone Diabetes (Flatbush Diabetes): an emerging worldwide clinically important entity. Curr Diab Rep. 2018;18(11):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361-369. [DOI] [PubMed] [Google Scholar]
- 24. Wagner R, Heni M, Tabák AG, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. 2021;27(1):49-57. [DOI] [PubMed] [Google Scholar]
- 25. Moss ND, Sussel L. mRNA processing: an emerging frontier in the regulation of pancreatic β cell function. Front Genet. 2020;11:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans-Molina C, Garmey JC, Ketchum R, Brayman KL, Deng S, Mirmira RG. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes. 2007;56(3):827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itoh N, Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980;283(5742):100-102. [DOI] [PubMed] [Google Scholar]
- 28. Alarcon C, Verchere CB, Rhodes CJ. Translational control of glucose-induced islet amyloid polypeptide production in pancreatic islets. Endocrinology. 2012;153(5):2082-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin SK, Carroll R, Benig M, Steiner DF. Regulation by glucose of the biosynthesis of PC2, PC3 and proinsulin in (ob/ob) mouse islets of Langerhans. FEBS Lett. 1994;356(2-3):279-282. [DOI] [PubMed] [Google Scholar]
- 30. Greenman IC, Gomez E, Moore CE, Herbert TP. The selective recruitment of mRNA to the ER and an increase in initiation are important for glucose-stimulated proinsulin synthesis in pancreatic beta-cells. Biochem J. 2005;391(Pt 2):291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Omar-Hmeadi M, Idevall-Hagren O. Insulin granule biogenesis and exocytosis. Cell Mol Life Sci. 2021;78(5):1957-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987;105(1):145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verchere CB, D’Alessio DA, Prigeon RL, Hull RL, Kahn SE. The constitutive secretory pathway is a major route for islet amyloid polypeptide secretion in neonatal but not adult rat islet cells. Diabetes. 2000;49(9):1477-1484. [DOI] [PubMed] [Google Scholar]
- 34. Kahn SE, Verchere CB, D’Alessio DA, Cook DL, Fujimoto WY. Evidence for selective release of rodent islet amyloid polypeptide through the constitutive secretory pathway. Diabetologia. 1993;36(6):570-573. [DOI] [PubMed] [Google Scholar]
- 35. Gasa R, Gomis R, Casamitjana R, Novials A. High glucose concentration favors the selective secretion of islet amyloid polypeptide through a constitutive secretory pathway in human pancreatic islets. Pancreas. 2001;22(3):307-310. [DOI] [PubMed] [Google Scholar]
- 36. Rhodes CJ, Lucas CA, Mutkoski RL, Orci L, Halban PA. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. Association with the ATP-dependent proton pump. J Biol Chem. 1987;262(22):10712-10717. [PubMed] [Google Scholar]
- 37. Davidson HW, Wenzlau JM, O’Brien RM. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol Metab. 2014;25(8):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen YC, Mains RE, Eipper BA, et al. PAM haploinsufficiency does not accelerate the development of diet- and human IAPP-induced diabetes in mice. Diabetologia. 2020;63(3):561-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramzy A, Asadi A, Kieffer TJ. Revisiting proinsulin processing: evidence that human β-cells process proinsulin with prohormone convertase (PC) 1/3 but not PC2. Diabetes. 2020;69(7):1451-1462. [DOI] [PubMed] [Google Scholar]
- 40. Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24(4):593-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teitelman G. Heterogeneous expression of proinsulin processing enzymes in beta cells of non-diabetic and type 2 diabetic humans. J Histochem Cytochem. 2019;67(6):385-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A. 2002;99(16):10299-10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furuta M, Carroll R, Martin S, et al. Incomplete processing of proinsulin to insulin accompanied by elevation of des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1998;273(6):3431-3437. [DOI] [PubMed] [Google Scholar]
- 44. Riahi Y, Wikstrom JD, Bachar-Wikstrom E, et al. Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia. 2016;59(7):1480-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pearson GL, Gingerich MA, Walker EM, Biden TJ, Soleimanpour SA. A selective look at autophagy in pancreatic β-cells. Diabetes. 2021;70(6):1229-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cunningham CN, Williams JM, Knupp J, Arunagiri A, Arvan P, Tsai B. Cells deploy a two-pronged strategy to rectify misfolded proinsulin aggregates. Mol Cell. 2019;75(3):442-456.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoboth P, Müller A, Ivanova A, et al. Aged insulin granules display reduced microtubule-dependent mobility and are disposed within actin-positive multigranular bodies. Proc Natl Acad Sci U S A. 2015;112(7):E667-E676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pasquier A, Vivot K, Erbs E, et al. Lysosomal degradation of newly formed insulin granules contributes to β cell failure in diabetes. Nat Commun. 2019;10(1):3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muralidharan C, Conteh AM, Marasco MR, et al. Pancreatic beta cell autophagy is impaired in type 1 diabetes. Diabetologia. 2021;64(4):865-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masini M, Bugliani M, Lupi R, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52(6):1083-1086. [DOI] [PubMed] [Google Scholar]
- 51. McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic β cells: Implications for understanding genetic association signals at this locus. Mol Genet Metab. 2011;104(4):648-653. [DOI] [PubMed] [Google Scholar]
- 52. Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol Rev. 2018;98(1):117-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in Islets of Langerhans. Front Physiol. 2019;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55(12):3470-3477. [DOI] [PubMed] [Google Scholar]
- 55. Porte D Jr, Pupo AA. Insulin responses to glucose: evidence for a two pool system in man. J Clin Invest. 1969;48(12):2309-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121(6):2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49(5):718-726. [DOI] [PubMed] [Google Scholar]
- 58. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162-185. [DOI] [PubMed] [Google Scholar]
- 59. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Capozzi ME, Svendsen B, Encisco SE, et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight. 2019;4(5):e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khan R, Tomas A, Rutter GA. Effects on pancreatic beta and other islet cells of the glucose-dependent insulinotropic polypeptide. Peptides. 2020;125:170201. [DOI] [PubMed] [Google Scholar]
- 62. van der Meulen T, Donaldson CJ, Cáceres E, et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med. 2015;21(7):769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henquin JC. Paracrine and autocrine control of insulin secretion in human islets: evidence and pending questions. Am J Physiol Endocrinol Metab. 2021;320(1):E78-E86. [DOI] [PubMed] [Google Scholar]
- 64. Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab. 2018;27(3):630-644.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hogan MF, Hull RL. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia. 2017;60(6):952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hajmrle C, Smith N, Spigelman AF, et al. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight. 2016;1(4):e86055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. 2017;127(8):2881-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pugliese A. Insulitis in the pathogenesis of type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl 2):31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Plesner A, Ten Holder JT, Verchere CB. Islet remodeling in female mice with spontaneous autoimmune and streptozotocin-induced diabetes. PLoS One. 2014;9(8):e102843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50(11):2323-2331. [DOI] [PubMed] [Google Scholar]
- 74. Hao W, Woodwyk A, Beam C, Bahnson HT, Palmer JP, Greenbaum CJ. Assessment of β cell mass and function by AIRmax and intravenous glucose in high-risk subjects for type 1 diabetes. J Clin Endocrinol Metab. 2017;102(12):4428-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51(4):951-957. [DOI] [PubMed] [Google Scholar]
- 76. Johnston C, Raghu P, McCulloch DK, et al. Beta-cell function and insulin sensitivity in nondiabetic HLA-identical siblings of insulin-dependent diabetics. Diabetes. 1987;36(7):829-837. [DOI] [PubMed] [Google Scholar]
- 77. Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34(2):93-102. [DOI] [PubMed] [Google Scholar]
- 78. Sosenko JM, Skyler JS, Beam CA, et al. ; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in Diabetes Prevention Trial-Type 1 participants. Diabetes. 2013;62(12):4179-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53(2):426-433. [DOI] [PubMed] [Google Scholar]
- 80. Sims EK, Bahnson HT, Nyalwidhe J, et al. T1D Exchange Residual C-peptide Study Group . Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. 2019;42(2):258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kahn SE, Templin AT, Hull RL, Verchere CB. Probing the meaning of persistent propeptide release in type 1 diabetes. Diabetes Care. 2019;42(2):183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ludvigsson J, Heding L. Abnormal proinsulin/C-peptide ratio in juvenile diabetes. Acta Diabetol Lat. 1982;19(4):351-358. [DOI] [PubMed] [Google Scholar]
- 83. Snorgaard O, Hartling SG, Binder C. Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33(1):36-42. [DOI] [PubMed] [Google Scholar]
- 84. Snorgaard O, Kjems LL, Røder ME, Hartling SG, Dinesen B, Binder C. Proinsulin immunoreactivity in recent-onset IDDM: the significance of insulin antibodies and insulin autoantibodies. Diabetes Care. 1996;19(2):146-150. [DOI] [PubMed] [Google Scholar]
- 85. Krogvold L, Skog O, Sundström G, et al. Function of isolated pancreatic islets from patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment in vitro: results from the DiViD study. Diabetes. 2015;64(7):2506-2512. [DOI] [PubMed] [Google Scholar]
- 86. Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7(2):101-107. [DOI] [PubMed] [Google Scholar]
- 87. Chmelova H, Cohrs CM, Chouinard JA, et al. Distinct roles of β-cell mass and function during type 1 diabetes onset and remission. Diabetes. 2015;64(6):2148-2160. [DOI] [PubMed] [Google Scholar]
- 88. Erlich H, Valdes AM, Noble J, et al. Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barrett JC, Clayton DG, Concannon P, et al. Type 1 Diabetes Genetics Consortium . Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Onengut-Gumuscu S, Chen WM, Burren O, et al. Type 1 Diabetes Genetics Consortium . Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47(4):381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Inshaw JRJ, Cutler AJ, Crouch DJM, Wicker LS, Todd JA. Genetic variants predisposing most strongly to type 1 diabetes diagnosed under age 7 years lie near candidate genes that function in the immune system and in pancreatic β-cells. Diabetes Care. 2020;43(1):169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kaprio J, Tuomilehto J, Koskenvuo M, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35(11):1060-1067. [DOI] [PubMed] [Google Scholar]
- 93. Knip M. Pathogenesis of type 1 diabetes: implications for incidence trends. Horm Res Paediatr. 2011;76 Suppl 1:57-64. [DOI] [PubMed] [Google Scholar]
- 94. Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(7):a007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lönnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: The Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49(8):1314-1318. [DOI] [PubMed] [Google Scholar]
- 96. Graves PM, Rotbart HA, Nix WA, et al. Prospective study of enteroviral infections and development of beta-cell autoimmunity. Diabetes autoimmunity study in the young (DAISY). Diabetes Res Clin Pract. 2003;59(1):51-61. [DOI] [PubMed] [Google Scholar]
- 97. de Groot PF, Belzer C, Aydin Ö, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One. 2017;12(12):e0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. de Groot P, Nikolic T, Pellegrini S, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. 2021;70(1):92-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219-226. [DOI] [PubMed] [Google Scholar]
- 100. Kim HS, Han MS, Chung KW, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27(2):321-333. [DOI] [PubMed] [Google Scholar]
- 101. Gomez-Tourino I, Arif S, Eichmann M, Peakman M. T cells in type 1 diabetes: Instructors, regulators and effectors: a comprehensive review. J Autoimmun. 2016;66:7-16. [DOI] [PubMed] [Google Scholar]
- 102. Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia. 2017;60(10):1839-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wenzlau JM, Hutton JC. Novel diabetes autoantibodies and prediction of type 1 diabetes. Curr Diab Rep. 2013;13(5):608-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4(1):20-34. [DOI] [PubMed] [Google Scholar]
- 105. Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51(4):1005-1015. [DOI] [PubMed] [Google Scholar]
- 106. Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54(2):254-260. [DOI] [PubMed] [Google Scholar]
- 107. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68(6):1456-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663-1672. [DOI] [PubMed] [Google Scholar]
- 110. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32(2):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102-110. [DOI] [PubMed] [Google Scholar]
- 112. Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300-2308. [DOI] [PubMed] [Google Scholar]
- 113. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32-42. [DOI] [PubMed] [Google Scholar]
- 114. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16-S21. [DOI] [PubMed] [Google Scholar]
- 115. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150(6):1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51(1):91-100. [DOI] [PubMed] [Google Scholar]
- 117. Dai C, Hang Y, Shostak A, et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest. 2017;127(10):3835-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Al-Mrabeh A, Hollingsworth KG, Shaw JAM, et al. 2-year remission of type 2 diabetes and pancreas morphology: a post-hoc analysis of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2020;8(12):939-948. [DOI] [PubMed] [Google Scholar]
- 119. Wang X, Misawa R, Zielinski MC, et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013;8(6):e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stefan Y, Orci L, Malaisse-Lagae F, Perrelet A, Patel Y, Unger RH. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31(8 Pt 1):694-700. [DOI] [PubMed] [Google Scholar]
- 121. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group . Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51(7):2170-2178. [DOI] [PubMed] [Google Scholar]
- 123. Gujral UP, Narayan KM, Kahn SE, Kanaya AM. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: the MASALA study. J Diabetes Complications. 2014;28(1):45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6414 Finnish men. Diabetes. 2009;58(5):1212-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42(2):222-229. [DOI] [PubMed] [Google Scholar]
- 126. Halter JB, Graf RJ, Porte D Jr. Potentiation of insulin secretory responses by plasma glucose levels in man: evidence that hyperglycemia in diabetes compensates for imparied glucose potentiation. J Clin Endocrinol Metab. 1979;48(6):946-954. [DOI] [PubMed] [Google Scholar]
- 127. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74(4):1318-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30(5):435-439. [DOI] [PubMed] [Google Scholar]
- 129. O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318(19):1225-1230. [DOI] [PubMed] [Google Scholar]
- 130. O’Meara NM, Sturis J, Van Cauter E, Polonsky KS. Lack of control by glucose of ultradian insulin secretory oscillations in impaired glucose tolerance and in non-insulin-dependent diabetes mellitus. J Clin Invest. 1993;92(1):262-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Duckworth WC, Kitabchi AE. Direct measurement of plasma proinsulin in normal and diabetic subjects. Am J Med. 1972;53(4):418-427. [DOI] [PubMed] [Google Scholar]
- 132. Mako ME, Starr JI, Rubenstein AH. Circulating proinsulin in patients with maturity onset diabetes. Am J Med. 1977;63(6):865-869. [DOI] [PubMed] [Google Scholar]
- 133. Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D Jr. Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30(9):698-702. [DOI] [PubMed] [Google Scholar]
- 134. Saad MF, Kahn SE, Nelson RG, et al. Disproportionately elevated proinsulin in Pima Indians with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1990;70(5):1247-1253. [DOI] [PubMed] [Google Scholar]
- 135. Røder ME, Porte D Jr, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(2):604-608. [DOI] [PubMed] [Google Scholar]
- 136. Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes. 1997;46(11):1725-1732. [DOI] [PubMed] [Google Scholar]
- 137. Temple RC, Carrington CA, Luzio SD, et al. Insulin deficiency in non-insulin-dependent diabetes. Lancet. 1989;1(8633):293-295. [DOI] [PubMed] [Google Scholar]
- 138. The RISE Consortium. Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes. 2019;68(8):1670-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. The RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care. 2018;41(8):1696-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. The RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care. 2018;41(8):1707-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. van Genugten RE, Utzschneider KM, Tong J, et al. American Diabetes Association GENNID Study Group . Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes. 2006;55(12):3529-3535. [DOI] [PubMed] [Google Scholar]
- 142. Gannon M, Kulkarni RN, Tse HM, Mauvais-Jarvis F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol Metab. 2018;15:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hwang JL, Weiss RE. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;30(2):96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wemrell M, Bennet L, Merlo J. Understanding the complexity of socioeconomic disparities in type 2 diabetes risk: a study of 4.3 million people in Sweden. BMJ Open Diabetes Res Care. 2019;7(1):e000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med. 2008;359(21):2208-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359(21):2220-2232. [DOI] [PubMed] [Google Scholar]
- 147. Stančáková A, Kuulasmaa T, Kuusisto J, et al. Genetic risk scores in the prediction of plasma glucose, impaired insulin secretion, insulin resistance and incident type 2 diabetes in the METSIM study. Diabetologia. 2017;60(9):1722-1730. [DOI] [PubMed] [Google Scholar]
- 148. Udler MS, McCarthy MI, Florez JC, Mahajan A. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr Rev. 2019;40(6):1500-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Camunas-Soler J, Dai XQ, Hang Y, et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 2020;31(5):1017-1031.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jurgens CA, Toukatly MN, Fligner CL, et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178(6):2632-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Viñuela A, Varshney A, van de Bunt M, et al. Genetic variant effects on gene expression in human pancreatic islets and their implications for T2D. Nat Commun. 2020;11(1):4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Miranda MA, Macias-Velasco JF, Lawson HA. Pancreatic β-cell heterogeneity in health and diabetes: classes, sources, and subtypes. Am J Physiol Endocrinol Metab. 2021;320(4):E716-E731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mahajan A, Wessel J, Willems SM, et al. ExomeBP Consortium; MAGIC Consortium; GIANT Consortium . Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. 2018;50(4):559-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Udler MS, Kim J, von Grotthuss M, et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 2018;15(9):e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Esser N, Utzschneider KM, Kahn SE. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia. 2020;63(10):2007-2021. [DOI] [PubMed] [Google Scholar]
- 157. Krentz NAJ, Gloyn AL. Insights into pancreatic islet cell dysfunction from type 2 diabetes mellitus genetics. Nat Rev Endocrinol. 2020;16(4):202-212. [DOI] [PubMed] [Google Scholar]
- 158. Vasu S, Kumano K, Darden CM, Rahman I, Lawrence MC, Naziruddin B. MicroRNA signatures as future biomarkers for diagnosis of diabetes states. Cells. 2019;8(12):1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Singer RA, Sussel L. Islet long noncoding RNAs: a playbook for discovery and characterization. Diabetes. 2018;67(8):1461-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ehrlich JC, Ratner IM. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am J Pathol. 1961;38:49-59. [PMC free article] [PubMed] [Google Scholar]
- 161. Clark A, Saad MF, Nezzer T, et al. Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia. 1990;33:285-289. [DOI] [PubMed] [Google Scholar]
- 162. Westermark P, Wernstedt C, O’Brien TD, Hayden DW, Johnson KH. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol. 1987;127(3):414-417. [PMC free article] [PubMed] [Google Scholar]
- 163. Cooper GJ, Leighton B, Dimitriadis GD, et al. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988;85(20):7763-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zraika S, Hull RL, Verchere CB, et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 2010;53(6):1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Aston-Mourney K, Hull RL, Zraika S, Udayasankar J, Subramanian SL, Kahn SE. Exendin-4 increases islet amyloid deposition but offsets the resultant beta cell toxicity in human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2011;54(7):1756-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Andrikopoulos S, Verchere CB, Terauchi Y, Kadowaki T, Kahn SE. beta-cell glucokinase deficiency and hyperglycemia are associated with reduced islet amyloid deposition in a mouse model of type 2 diabetes. Diabetes. 2000;49(12):2056-2062. [DOI] [PubMed] [Google Scholar]
- 167. Hull RL, Shen ZP, Watts MR, et al. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54(7):2235-2244. [DOI] [PubMed] [Google Scholar]
- 168. Yoshimura M, Ono M, Watanabe H, Kimura H, Saji H. Development of (99m)Tc-labeled pyridyl benzofuran derivatives to detect pancreatic amylin in islet amyloid model mice. Bioconjug Chem. 2016;27(6):1532-1539. [DOI] [PubMed] [Google Scholar]
- 169. Templin AT, Meier DT, Willard JR, et al. Use of the PET ligand florbetapir for in vivo imaging of pancreatic islet amyloid deposits in hIAPP transgenic mice. Diabetologia. 2018;61(10):2215-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lu Z, Xie J, Yan R, et al. A pilot study of pancreatic islet amyloid PET imaging with [18F]FDDNP. Nucl Med Commun. 2018;39(7):659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yoshimura M, Ono M, Watanabe H, Kimura H, Saji H. Feasibility of amylin imaging in pancreatic islets with β-amyloid imaging probes. Sci Rep. 2014;4:6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15(4):518-533. [DOI] [PubMed] [Google Scholar]
- 173. Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187(5):2755-2765. [DOI] [PubMed] [Google Scholar]
- 174. Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11(10):897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes. 2014;63(5):1698-1711. [DOI] [PubMed] [Google Scholar]
- 176. Meier DT, Morcos M, Samarasekera T, Zraika S, Hull RL, Kahn SE. Islet amyloid formation is an important determinant for inducing islet inflammation in high-fat-fed human IAPP transgenic mice. Diabetologia. 2014;57(9):1884-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Nackiewicz D, Dan M, Speck M, et al. Islet macrophages shift to a reparative state following pancreatic beta-cell death and are a major source of islet insulin-like growth factor-1. iScience. 2020;23(1):100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda). 2009;24:325-331. [DOI] [PubMed] [Google Scholar]
- 179. Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517-1526. [DOI] [PubMed] [Google Scholar]
- 180. Everett BM, Donath MY, Pradhan AD, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71(21):2392-2401. [DOI] [PubMed] [Google Scholar]
- 181. Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57(3):491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tattersall RB. Mild familial diabetes with dominant inheritance. Q J Med. 1974;43(170):339-357. [PubMed] [Google Scholar]
- 183. Pearson ER, Velho G, Clark P, et al. beta-cell genes and diabetes: quantitative and qualitative differences in the pathophysiology of hepatic nuclear factor-1alpha and glucokinase mutations. Diabetes. 2001;50 Suppl 1:S101-S107. [DOI] [PubMed] [Google Scholar]
- 184. Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;94(24):13209-13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wang H, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta -cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem. 2000;275(46):35953-35959. [DOI] [PubMed] [Google Scholar]
- 186. Mateus JC, Rivera C, O’Meara M, Valenzuela A, Lizcano F. Maturity-onset diabetes of the young type 5 a multisystemic disease: a case report of a novel mutation in the HNF1B gene and literature review. Clin Diabetes Endocrinol. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kayani K, Mohammed R, Mohiaddin H. Cystic fibrosis-related diabetes. Front Endocrinol (Lausanne). 2018;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Granados A, Chan CL, Ode KL, Moheet A, Moran A, Holl R. Cystic fibrosis related diabetes: pathophysiology, screening and diagnosis. J Cyst Fibros. 2019;18(Suppl 2):S3-S9. [DOI] [PubMed] [Google Scholar]
- 189. Moheet A, Moran A. CF-related diabetes: containing the metabolic miscreant of cystic fibrosis. Pediatr Pulmonol. 2017;52(S48):S37-S43. [DOI] [PubMed] [Google Scholar]
- 190. Hull RL, Gibson RL, McNamara S, et al. Islet Interleukin-1β immunoreactivity is an early feature of cystic fibrosis that may contribute to β-cell failure. Diabetes Care. 2018;41(4):823-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Guo JH, Chen H, Ruan YC, et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat Commun. 2014;5:4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ntimbane T, Mailhot G, Spahis S, et al. CFTR silencing in pancreatic β-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am J Physiol Endocrinol Metab. 2016;310(3):E200-E212. [DOI] [PubMed] [Google Scholar]
- 193. Hart NJ, Aramandla R, Poffenberger G, et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight. 2018;3(8):e98240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Colombo C, Foppiani A, Bisogno A, et al. Lumacaftor/ivacaftor in cystic fibrosis: effects on glucose metabolism and insulin secretion. J Endocrinol Invest. Published online ahead of print February 13, 2021. doi:10.1007/s40618-021-01525-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Moheet A, Beisang D, Zhang L, et al. PROSPECT Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network . Lumacaftor/ivacaftor therapy fails to increase insulin secretion in F508del/F508del CF patients. J Cyst Fibros. 2021;20(2):333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab. 1996;81(3):1267-1272. [DOI] [PubMed] [Google Scholar]
- 197. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Verge CF, Gianani R, Yu L, et al. Late progression to diabetes and evidence for chronic β-cell autoimmunity in identical twins of patients with type I diabetes. Diabetes. 1995;44(10):1176-1179. [DOI] [PubMed] [Google Scholar]
- 199. Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52(4):1052-1055. [DOI] [PubMed] [Google Scholar]
- 200. Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med. 2008;359(26):2849-2850. [DOI] [PubMed] [Google Scholar]
- 201. Redondo MJ, Rewers M, Yu L, et al. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1 diabetes: prospective twin study. BMJ. 1999;318(7185):698-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11(6):533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Erlich HA, Valdes AM, McDevitt SL, et al. Type 1 Diabetes Genetics Consortium (T1DGC) . Next generation sequencing reveals the association of DRB3*02:02 with type 1 diabetes. Diabetes. 2013;62(7):2618-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zhao LP, Alshiekh S, Zhao M, et al. Better Diabetes Diagnosis (BDD) Study Group . Next-generation sequencing reveals that HLA-DRB3, -DRB4, and -DRB5 may be associated with islet autoantibodies and risk for childhood type 1 diabetes. Diabetes. 2016;65(3):710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia. 1996;39(7):807-812. [DOI] [PubMed] [Google Scholar]
- 206. Schenker M, Hummel M, Ferber K, et al. Early expression and high prevalence of islet autoantibodies for DR3/4 heterozygous and DR4/4 homozygous offspring of parents with Type I diabetes: the German BABYDIAB study. Diabetologia. 1999;42(6):671-677. [DOI] [PubMed] [Google Scholar]
- 207. TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286-298. [DOI] [PubMed] [Google Scholar]
- 208. Ilonen J, Hammais A, Laine AP, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62(10):3636-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Krischer JP, Liu X, Lernmark Å, et al. TEDDY Study Group . The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: a TEDDY study report. Diabetes. 2017;66(12):3122-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ilonen J, Kiviniemi M, Lempainen J, et al. Finnish Pediatric Diabetes Register . Genetic susceptibility to type 1 diabetes in childhood - estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatr Diabetes. 2016;17 Suppl 22:8-16. [DOI] [PubMed] [Google Scholar]
- 211. Nejentsev S, Howson JM, Walker NM, et al. Wellcome Trust Case Control Consortium . Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450(7171):887-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Roshandel D, Gubitosi-Klug R, Bull SB, et al. DCCT/EDIC Research Group . Meta-genome-wide association studies identify a locus on chromosome 1 and multiple variants in the MHC region for serum C-peptide in type 1 diabetes. Diabetologia. 2018;61(5):1098-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Koskinen MK, Mikk ML, Laine AP, et al. Longitudinal pattern of first-phase insulin response is associated with genetic variants outside the class II HLA region in children with multiple autoantibodies. Diabetes. 2020;69(1):12-19. [DOI] [PubMed] [Google Scholar]
- 214. Chiou J, Geusz RJ, Okino M-L, et al. Large-scale genetic association and single cell accessible chromatin mapping defines cell type-specific mechanisms of type 1 diabetes risk. bioRxiv. 2021:2021.2001.2013.426472. [Google Scholar]
- 215. Törn C, Hadley D, Lee HS, et al. TEDDY Study Group . Role of type 1 diabetes-associated snps on risk of autoantibody positivity in the TEDDY study. Diabetes. 2015;64(5):1818-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sharma A, Liu X, Hadley D, et al. TEDDY Study Group . Identification of non-HLA genes associated with development of islet autoimmunity and type 1 diabetes in the prospective TEDDY cohort. J Autoimmun. 2018;89:90-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Lempainen J, Laine AP, Hammais A, et al. Non-HLA gene effects on the disease process of type 1 diabetes: from HLA susceptibility to overt disease. J Autoimmun. 2015;61:45-53. [DOI] [PubMed] [Google Scholar]
- 218. Størling J, Pociot F. Type 1 diabetes candidate genes linked to pancreatic islet cell inflammation and beta-cell apoptosis. Genes (Basel). 2017;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349-362. [DOI] [PubMed] [Google Scholar]
- 220. Nogueira TC, Paula FM, Villate O, et al. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. 2013;9(5):e1003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Cai EP, Ishikawa Y, Zhang W, et al. Genome-scale in vivo CRISPR screen identifies RNLS as a target for beta cell protection in type 1 diabetes. Nat Metab. 2020;2(9):934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Winkler C, Krumsiek J, Lempainen J, et al. A strategy for combining minor genetic susceptibility genes to improve prediction of disease in type 1 diabetes. Genes Immun. 2012;13(7):549-555. [DOI] [PubMed] [Google Scholar]
- 223. Winkler C, Krumsiek J, Buettner F, et al. Feature ranking of type 1 diabetes susceptibility genes improves prediction of type 1 diabetes. Diabetologia. 2014;57(12):2521-2529. [DOI] [PubMed] [Google Scholar]
- 224. Steck AK, Dong F, Wong R, et al. Improving prediction of type 1 diabetes by testing non-HLA genetic variants in addition to HLA markers. Pediatr Diabetes. 2014;15(5):355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331-2339. [DOI] [PubMed] [Google Scholar]
- 226. Bonifacio E, Beyerlein A, Hippich M, et al. TEDDY Study Group . Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: A prospective study in children. PLoS Med. 2018;15(4):e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Sharp SA, Rich SS, Wood AR, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care. 2019;42(2):200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ferrat LA, Vehik K, Sharp SA, et al. TEDDY Study Group . A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med. 2020;26(8):1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Oram RA, Patel K, Hill A, et al. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Patel KA, Oram RA, Flanagan SE, et al. Type 1 diabetes genetic risk score: a novel tool to discriminate monogenic and type 1 diabetes. Diabetes. 2016;65(7):2094-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013;4(4):114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes (Basel). 2015;6(1):87-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20(3):284-287. [DOI] [PubMed] [Google Scholar]
- 234. Srinivasan S, Chen L, Todd J, et al. The first genome-wide association study for type 2 diabetes in youth: The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes. 2021;70(4):996-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Steinthorsdottir V, Thorleifsson G, Sulem P, et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46(3):294-298. [DOI] [PubMed] [Google Scholar]
- 236. Flannick J, Mercader JM, Fuchsberger C, et al. Broad Genomics Platform; DiscovEHR Collaboration; CHARGE; LuCamp; ProDiGY; GoT2D; ESP; SIGMA-T2D; T2D-GENES; AMP-T2D-GENES . Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019;570(7759):71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Scott RA, Scott LJ, Mägi R, et al. DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017;66(11):2888-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Naylor RN, John PM, Winn AN, et al. Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care. 2014;37(1):202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Zhang H, Colclough K, Gloyn AL, Pollin TI. Monogenic diabetes: a gateway to precision medicine in diabetes. J Clin Invest. 2021;131(3):e142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Carlsson A, Shepherd M, Ellard S, et al. Absence of islet autoantibodies and modestly raised glucose values at diabetes diagnosis should lead to testing for MODY: lessons from a 5-year pediatric Swedish national cohort study. Diabetes Care. 2020;43(1):82-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Broome DT, Pantalone KM, Kashyap S, Philipson LH. Approach to the patient with MODY-monogenic diabetes. J Clin Endocrinol Metab. 2021;106(1):237-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Adler AI, Shine BS, Chamnan P, Haworth CS, Bilton D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care. 2008;31(9):1789-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Aksit MA, Pace RG, Vecchio-Pagán B, et al. Genetic modifiers of cystic fibrosis-related diabetes have extensive overlap with type 2 diabetes and related traits. J Clin Endocrinol Metab. 2020;105(5):1401-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62(10):1789-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Sommese L, Benincasa G, Lanza M, et al. Novel epigenetic-sensitive clinical challenges both in type 1 and type 2 diabetes. J Diabetes Complications. 2018;32(11):1076-1084. [DOI] [PubMed] [Google Scholar]
- 246. Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29(5):1028-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Thurner M, van de Bunt M, Torres JM, et al. Integration of human pancreatic islet genomic data refines regulatory mechanisms at type 2 diabetes susceptibility loci. Elife. 2018;7:e31977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Khetan S, Kursawe R, Youn A, et al. Type 2 diabetes-associated genetic variants regulate chromatin accessibility in human islets. Diabetes. 2018;67(11):2466-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Greenwald WW, Chiou J, Yan J, et al. Pancreatic islet chromatin accessibility and conformation reveals distal enhancer networks of type 2 diabetes risk. Nat Commun. 2019;10(1):2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Rai V, Quang DX, Erdos MR, et al. Single-cell ATAC-Seq in human pancreatic islets and deep learning upscaling of rare cells reveals cell-specific type 2 diabetes regulatory signatures. Mol Metab. 2020;32:109-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Volkmar M, Dedeurwaerder S, Cunha DA, et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. Embo J. 2012;31(6):1405-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Olsson AH, Volkov P, Bacos K, et al. Genome-wide associations between genetic and epigenetic variation influence mRNA expression and insulin secretion in human pancreatic islets. PLoS Genet. 2014;10(11):e1004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Dayeh T, Volkov P, Salö S, et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet. 2014;10(3):e1004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Chambers JC, Loh M, Lehne B, et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3(7):526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Soriano-Tárraga C, Jiménez-Conde J, Giralt-Steinhauer E, et al. GENESTROKE Consortium . Epigenome-wide association study identifies TXNIP gene associated with type 2 diabetes mellitus and sustained hyperglycemia. Hum Mol Genet. 2016;25(3):609-619. [DOI] [PubMed] [Google Scholar]
- 256. Walaszczyk E, Luijten M, Spijkerman AMW, et al. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: a systematic review and replication in a case-control sample of the Lifelines study. Diabetologia. 2018;61(2):354-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Cardona A, Day FR, Perry JRB, et al. Epigenome-wide association study of incident type 2 diabetes in a British Population: EPIC-Norfolk Study. Diabetes. 2019;68(12):2315-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Hall E, Jönsson J, Ofori JK, et al. Glucolipotoxicity alters insulin secretion via epigenetic changes in human islets. Diabetes. 2019;68(10):1965-1974. [DOI] [PubMed] [Google Scholar]
- 259. Howe CG, Cox B, Fore R, et al. Maternal gestational diabetes mellitus and newborn DNA methylation: findings from the Pregnancy and Childhood Epigenetics Consortium. Diabetes Care. 2020;43(1):98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Antoun E, Kitaba NT, Titcombe P, et al. UPBEAT Consortium . Maternal dysglycaemia, changes in the infant’s epigenome modified with a diet and physical activity intervention in pregnancy: secondary analysis of a randomised control trial. PLoS Med. 2020;17(11):e1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Ding GL, Wang FF, Shu J, et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5):1133-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Li Y, Zhao M, Hou C, et al. Abnormal DNA methylation in CD4+ T cells from people with latent autoimmune diabetes in adults. Diabetes Res Clin Pract. 2011;94(2):242-248. [DOI] [PubMed] [Google Scholar]
- 263. Hou C, Zhao M, Li X, et al. Histone H3 acetylation of tumor necrosis factor-alpha and cyclooxygenase-2 in patients with type 2 diabetes. Zhonghua Yi Xue Za Zhi. 2011;91(26):1805-1808. [PubMed] [Google Scholar]
- 264. Paneni F, Costantino S, Battista R, et al. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2015;8(1):150-158. [DOI] [PubMed] [Google Scholar]
- 265. Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes. 2008;57(12):3189-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Eliasson L, Regazzi R. Micro(RNA) management and mismanagement of the Islet. J Mol Biol. 2020;432(5):1419-1428. [DOI] [PubMed] [Google Scholar]
- 267. Jiménez-Lucena R, Camargo A, Alcalá-Diaz JF, et al. A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: from the CORDIOPREV study. Exp Mol Med. 2018;50(12):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Yang M, Ye L, Wang B, et al. Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients 1miR-146. J Diabetes. 2015;7(2):158-165. [DOI] [PubMed] [Google Scholar]
- 269. Erener S, Marwaha A, Tan R, Panagiotopoulos C, Kieffer TJ. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight. 2017;2(4):e89656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Assmann TS, Recamonde-Mendoza M, Puñales M, Tschiedel B, Canani LH, Crispim D. MicroRNA expression profile in plasma from type 1 diabetic patients: case-control study and bioinformatic analysis. Diabetes Res Clin Pract. 2018;141:35-46. [DOI] [PubMed] [Google Scholar]
- 271. Xu G, Thielen LA, Chen J, et al. Serum miR-204 is an early biomarker of type 1 diabetes-associated pancreatic beta-cell loss. Am J Physiol Endocrinol Metab. 2019;317(4):E723-E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Stender S, Smagris E, Lauridsen BK, et al. Relationship between genetic variation at PPP1R3B and levels of liver glycogen and triglyceride. Hepatology. 2018;67(6):2182-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Manning AK, Goustin AS, Kleinbrink EL, et al. A long non-coding RNA, LOC157273, is an effector transcript at the chromosome 8p23.1-PPP1R3B metabolic traits and type 2 diabetes risk locus. Front Genet. 2020;11:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Kahali B, Chen Y, Feitosa MF, et al. A noncoding variant near PPP1R3B promotes liver glycogen storage and MetS, but protects against myocardial infarction. J Clin Endocrinol Metab. 2021;106(2):372-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Yang Y, Lv X, Fan Q, et al. Analysis of circulating lncRNA expression profiles in patients with diabetes mellitus and diabetic nephropathy: Differential expression profile of circulating lncRNA. Clin Nephrol. 2019;92(1):25-35. [DOI] [PubMed] [Google Scholar]
- 276. Akirav EM, Lebastchi J, Galvan EM, et al. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A. 2011;108(47):19018-19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Herold KC, Usmani-Brown S, Ghazi T, et al. Type 1 Diabetes TrialNet Study Group . β cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125(3):1163-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Lehmann-Werman R, Neiman D, Zemmour H, et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A. 2016;113(13):E1826-E1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Syed F, Tersey SA, Turatsinze JV, et al. Circulating unmethylated CHTOP and INS DNA fragments provide evidence of possible islet cell death in youth with obesity and diabetes. Clin Epigenetics. 2020;12(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Neiman D, Gillis D, Piyanzin S, et al. Multiplexing DNA methylation markers to detect circulating cell-free DNA derived from human pancreatic β cells. JCI Insight. 2020;5(14):e136579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Chang W, Wang J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells. 2019;8(8):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Cianciaruso C, Phelps EA, Pasquier M, et al. Primary human and rat β-cells release the intracellular autoantigens GAD65, IA-2, and proinsulin in exosomes together with cytokine-induced enhancers of immunity. Diabetes. 2017;66(2):460-473. [DOI] [PubMed] [Google Scholar]
- 283. Garcia-Contreras M, Shah SH, Tamayo A, et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep. 2017;7(1):5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Lakhter AJ, Pratt RE, Moore RE, et al. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61(5):1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Krishnan P, Syed F, Jiyun Kang N, Mirmira RG, Evans-Molina C. Profiling of RNAs from human islet-derived exosomes in a model of type 1 diabetes. Int J Mol Sci. 2019;20(23):5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Mathieu C, Lahesmaa R, Bonifacio E, Achenbach P, Tree T. Immunological biomarkers for the development and progression of type 1 diabetes. Diabetologia. 2018;61(11):2252-2258. [DOI] [PubMed] [Google Scholar]
- 287. Roep BO, Peakman M. Surrogate end points in the design of immunotherapy trials: emerging lessons from type 1 diabetes. Nat Rev Immunol. 2010;10(2):145-152. [DOI] [PubMed] [Google Scholar]
- 288. Ahmed S, Cerosaletti K, James E, et al. Standardizing T-cell biomarkers in type 1 diabetes: challenges and recent advances. Diabetes. 2019;68(7):1366-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Tooley JE, Herold KC. Biomarkers in type 1 diabetes: application to the clinical trial setting. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Peterson LD, van der Keur M, de Vries RR, Roep BO. Autoreactive and immunoregulatory T-cell subsets in insulin-dependent diabetes mellitus. Diabetologia. 1999;42(4):443-449. [DOI] [PubMed] [Google Scholar]
- 291. Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT, von Herrath MG. The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci Adv. 2020;6(42):eabc5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113(3):451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Tree TI, Lawson J, Edwards H, et al. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes. 2010;59(6):1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Heninger AK, Eugster A, Kuehn D, et al. A divergent population of autoantigen-responsive CD4(+) T cells in infants prior to β cell autoimmunity. Sci Transl Med. 2017;9(378):eaaf8848. [DOI] [PubMed] [Google Scholar]
- 295. Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2(4):a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Scotto M, Afonso G, Østerbye T, et al. HLA-B7-restricted islet epitopes are differentially recognized in type 1 diabetic children and adults and form weak peptide-HLA complexes. Diabetes. 2012;61(10):2546-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Long SA, Thorpe J, DeBerg HA, et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol. 2016;1(5):eaai7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Orban T, Beam CA, Xu P, et al. Type 1 Diabetes TrialNet Abatacept Study Group . Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes. 2014;63(10):3449-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Haller MJ, Gitelman SE, Gottlieb PA, et al. Antithymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes. 2016;65(12):3765-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Alhadj Ali M, Liu YF, Arif S, et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017;9(402):eaaf7779. [DOI] [PubMed] [Google Scholar]
- 301. Herold KC, Pescovitz MD, McGee P, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group . Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187(4):1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Narsale A, Lam B, Moya R, et al. CD4+CD25+CD127hi cell frequency predicts disease progression in type 1 diabetes. JCI Insight. 2021;6(2):e136114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Mallone R, Mannering SI, Brooks-Worrell BM, et al. T-Cell Workshop Committee, Immunology of Diabetes Society . Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163(1):33-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Brooks-Worrell BM, Reichow JL, Goel A, Ismail H, Palmer JP. Identification of autoantibody-negative autoimmune type 2 diabetic patients. Diabetes Care. 2011;34(1):168-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care. 2014;37(12):3286-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Goel A, Chiu H, Felton J, Palmer JP, Brooks-Worrell B. T-cell responses to islet antigens improves detection of autoimmune diabetes and identifies patients with more severe beta-cell lesions in phenotypic type 2 diabetes. Diabetes. 2007;56(8):2110-2115. [DOI] [PubMed] [Google Scholar]
- 307. Brooks-Worrell BM, Palmer JP. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients. Clin Exp Immunol. 2013;171(2):164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Deng T, Lyon CJ, Minze LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17(3):411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Mannering SI, Di Carluccio AR, Elso CM. Neoepitopes: a new take on beta cell autoimmunity in type 1 diabetes. Diabetologia. 2019;62(3):351-356. [DOI] [PubMed] [Google Scholar]
- 310. James EA, Pietropaolo M, Mamula MJ. Immune recognition of β-cells: neoepitopes as key players in the loss of tolerance. Diabetes. 2018;67(6):1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Delong T, Wiles TA, Baker RL, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351(6274):711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Gonzalez-Duque S, Azoury ME, Colli ML, et al. Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 2018;28(6):946-960.e6. [DOI] [PubMed] [Google Scholar]
- 313. Kracht MJ, van Lummel M, Nikolic T, et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med. 2017;23(4):501-507. [DOI] [PubMed] [Google Scholar]
- 314. Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38(6):989-996. [DOI] [PubMed] [Google Scholar]
- 315. Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279-1283. [DOI] [PubMed] [Google Scholar]
- 316. Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337-1339. [DOI] [PubMed] [Google Scholar]
- 317. Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151-156. [DOI] [PubMed] [Google Scholar]
- 318. Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol. 1995;155(11):5419-5426. [PubMed] [Google Scholar]
- 319. Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(43):17040-17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41(1):11-18. [DOI] [PubMed] [Google Scholar]
- 321. Krischer JP, Liu X, Vehik K, et al. TEDDY Study Group . Predicting islet cell autoimmunity and type 1 diabetes: an 8-year TEDDY study progress report. Diabetes Care. 2019;42(6):1051-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Krischer JP, Lynch KF, Schatz DA, et al. TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Vehik K, Lynch KF, Schatz DA, et al. TEDDY Study Group . Reversion of β-cell autoimmunity changes risk of type 1 diabetes: TEDDY study. Diabetes Care. 2016;39(9):1535-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Mishra R, Hodge KM, Cousminer DL, Leslie RD, Grant SFA. A global perspective of latent autoimmune diabetes in adults. Trends Endocrinol Metab. 2018;29(9):638-650. [DOI] [PubMed] [Google Scholar]
- 327. Barker A, Lauria A, Schloot N, et al. Age-dependent decline of β-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab. 2014;16(3):262-267. [DOI] [PubMed] [Google Scholar]
- 328. Hernandez M, Mollo A, Marsal JR, et al. Action LADA Consortium . Insulin secretion in patients with latent autoimmune diabetes (LADA): half way between type 1 and type 2 diabetes: action LADA 9. BMC Endocr Disord. 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Fourlanos S, Dotta F, Greenbaum CJ, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005;48(11):2206-2212. [DOI] [PubMed] [Google Scholar]
- 330. Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350(9087):1288-1293. [DOI] [PubMed] [Google Scholar]
- 331. Buzzetti R, Di Pietro S, Giaccari A, et al. Non Insulin Requiring Autoimmune Diabetes Study Group . High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care. 2007;30(4):932-938. [DOI] [PubMed] [Google Scholar]
- 332. Gupta S, Maratha A, Siednienko J, et al. Analysis of inflammatory cytokine and TLR expression levels in type 2 diabetes with complications. Sci Rep. 2017;7(1):7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Weir GC, Bonner-Weir S, Leahy JL. Islet mass and function in diabetes and transplantation. Diabetes. 1990;39(4):401-405. [DOI] [PubMed] [Google Scholar]
- 334. Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087-1097. [DOI] [PubMed] [Google Scholar]
- 335. Freeby M, Ichise M, Harris PE. Vesicular monoamine transporter, type 2 (VMAT2) expression as it compares to insulin and pancreatic polypeptide in the head, body and tail of the human pancreas. Islets. 2012;4(6):393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Normandin MD, Petersen KF, Ding YS, et al. In vivo imaging of endogenous pancreatic β-cell mass in healthy and type 1 diabetic subjects using 18F-fluoropropyl-dihydrotetrabenazine and PET. J Nucl Med. 2012;53(6):908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Freeby MJ, Kringas P, Goland RS, et al. Cross-sectional and test-retest characterization of PET with [(18)F]FP-(+)-DTBZ for β cell mass estimates in diabetes. Mol Imaging Biol. 2016;18(2):292-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Saisho Y, Harris PE, Butler AE, et al. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Histol. 2008;39(5):543-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Tsao HH, Lin KJ, Juang JH, et al. Binding characteristics of 9-fluoropropyl-(+)-dihydrotetrabenzazine (AV-133) to the vesicular monoamine transporter type 2 in rats. Nucl Med Biol. 2010;37(4):413-419. [DOI] [PubMed] [Google Scholar]
- 340. Orci L, Malaisse-Lagae F, Baetens D, Perrelet A. Pancreatic-polypeptide-rich regions in human pancreas. Lancet. 1978;2(8101):1200-1201. [DOI] [PubMed] [Google Scholar]
- 341. Gersell DJ, Gingerich RL, Greider MH. Regional distribution and concentration of pancreatic polypeptide in the human and canine pancreas. Diabetes. 1979;28(1):11-15. [PubMed] [Google Scholar]
- 342. Cline GW, Naganawa M, Chen L, et al. Decreased VMAT2 in the pancreas of humans with type 2 diabetes mellitus measured in vivo by PET imaging. Diabetologia. 2018;61(12):2598-2607. [DOI] [PubMed] [Google Scholar]
- 343. Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem. 2008;56(9):841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Kirk RK, Pyke C, von Herrath MG, et al. Immunohistochemical assessment of glucagon-like peptide 1 receptor (GLP-1R) expression in the pancreas of patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(5):705-712. [DOI] [PubMed] [Google Scholar]
- 345. Zhang Y, Parajuli KR, Fava GE, et al. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68(1):34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Velikyan I, Eriksson O. Advances in GLP-1 receptor targeting radiolabeled agent development and prospective of theranostics. Theranostics. 2020;10(1):437-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Deacon CF. Circulation and degradation of GIP and GLP-1. Horm Metab Res. 2004;36(11-12):761-765. [DOI] [PubMed] [Google Scholar]
- 348. Christ E, Wild D, Forrer F, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94(11):4398-4405. [DOI] [PubMed] [Google Scholar]
- 349. Luo Y, Pan Q, Yao S, et al. Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-exendin-4 for detecting localized insulinoma: a prospective cohort study. J Nucl Med. 2016;57(5):715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Rylova SN, Waser B, Del Pozzo L, et al. Approaches to improve the pharmacokinetics of radiolabeled glucagon-like peptide-1 receptor ligands using antagonistic tracers. J Nucl Med. 2016;57(8):1282-1288. [DOI] [PubMed] [Google Scholar]
- 351. Läppchen T, Tönnesmann R, Eersels J, Meyer PT, Maecke HR, Rylova SN. Radioiodinated exendin-4 is superior to the radiometal-labelled glucagon-like peptide-1 receptor probes overcoming their high kidney uptake. PLoS One. 2017;12(1):e0170435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Waser B, Reubi JC. Value of the radiolabelled GLP-1 receptor antagonist exendin(9-39) for targeting of GLP-1 receptor-expressing pancreatic tissues in mice and humans. Eur J Nucl Med Mol Imaging. 2011;38(6):1054-1058. [DOI] [PubMed] [Google Scholar]
- 353. Antwi K, Hepprich M, Müller NA, et al. Pitfalls in the detection of insulinomas with glucagon-like peptide-1 receptor imaging. Clin Nucl Med. 2020;45(9):e386-e392. [DOI] [PubMed] [Google Scholar]
- 354. Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006;49(4):706-712. [DOI] [PubMed] [Google Scholar]
- 355. Zhang M, Jacobson O, Kiesewetter DO, et al. Improving the theranostic potential of exendin 4 by reducing the renal radioactivity through brush border membrane enzyme-mediated degradation. Bioconjug Chem. 2019;30(6):1745-1753. [DOI] [PubMed] [Google Scholar]
- 356. Brom M, Woliner-van der Weg W, Joosten L, et al. Non-invasive quantification of the beta cell mass by SPECT with ¹¹¹In-labelled exendin. Diabetologia. 2014;57(5):950-959. [DOI] [PubMed] [Google Scholar]
- 357. Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30(5):781-793. [DOI] [PubMed] [Google Scholar]
- 358. Sowa-Staszczak A, Pach D, Mikołajczak R, et al. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur J Nucl Med Mol Imaging. 2013;40(4):524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Christ E, Wild D, Ederer S, et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013;1(2):115-122. [DOI] [PubMed] [Google Scholar]
- 360. Eriksson O, Haack T, Hijazi Y, et al. Receptor occupancy of dual glucagon-like peptide 1/glucagon receptor agonist SAR425899 in individuals with type 2 diabetes. Sci Rep. 2020;10(1):16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155(2):173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101(34):12634-12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121(1):442-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Gaglia JL, Harisinghani M, Aganj I, et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112(7):2139-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Barone R, Procaccini E, Chianelli M, et al. Prognostic relevance of pancreatic uptake of technetium-99m labelled human polyclonal immunoglobulins in patients with type 1 diabetes. Eur J Nucl Med. 1998;25(5):503-508. [DOI] [PubMed] [Google Scholar]
- 366. Signore A, Capriotti G, Chianelli M, et al. Action LADA Group . Detection of insulitis by pancreatic scintigraphy with 99mTc-labeled IL-2 and MRI in patients with LADA (Action LADA 10). Diabetes Care. 2015;38(4):652-658. [DOI] [PubMed] [Google Scholar]
- 367. Chianelli M, Parisella MG, Visalli N, et al. IMDIAB Study Group . Pancreatic scintigraphy with 99mTc-interleukin-2 at diagnosis of type 1 diabetes and after 1 year of nicotinamide therapy. Diabetes Metab Res Rev. 2008;24(2):115-122. [DOI] [PubMed] [Google Scholar]
- 368. Romano M, Buratti E. Florbetapir F 18 for brain imaging of β-amyloid plaques. Drugs Today (Barc). 2013;49(3):181-193. [DOI] [PubMed] [Google Scholar]
- 369. Yang L, Rieves D, Ganley C. Brain amyloid imaging–FDA approval of florbetapir F18 injection. N Engl J Med. 2012;367(10):885-887. [DOI] [PubMed] [Google Scholar]
- 370. Toso C, Vallee JP, Morel P, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8(3):701-706. [DOI] [PubMed] [Google Scholar]
- 371. Park KS, Lee HS, Kim YS, et al. Improved quantification of islet transplants by magnetic resonance imaging with Resovist. Pancreas. 2011;40(6):911-919. [DOI] [PubMed] [Google Scholar]
- 372. Marzola P, Longoni B, Szilagyi E, et al. In vivo visualization of transplanted pancreatic islets by MRI: comparison between in vivo, histological and electron microscopy findings. Contrast Media Mol Imaging. 2009;4(3):135-142. [DOI] [PubMed] [Google Scholar]
- 373. Saudek F, Jirák D, Girman P, et al. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation. 2010;90(12):1602-1606. [DOI] [PubMed] [Google Scholar]
- 374. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19(10):1154-1161. [PubMed] [Google Scholar]
- 375. Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9(12):2816-2824. [DOI] [PubMed] [Google Scholar]
- 376. Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30(10):757-762. [DOI] [PubMed] [Google Scholar]
- 377. Alzaid A, Aideyan O, Nawaz S. The size of the pancreas in diabetes mellitus. Diabet Med. 1993;10(8):759-763. [DOI] [PubMed] [Google Scholar]
- 378. Lim S, Bae JH, Chun EJ, et al. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 2014;51(5):739-748. [DOI] [PubMed] [Google Scholar]
- 379. Macauley M, Percival K, Thelwall PE, Hollingsworth KG, Taylor R. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS One. 2015;10(5):e0126825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Virostko J, Hilmes M, Eitel K, Moore DJ, Powers AC. Use of the electronic medical record to assess pancreas size in type 1 diabetes. PLoS One. 2016;11(7):e0158825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Silva ME, Vezozzo DP, Ursich MJ, Rocha DM, Cerri GG, Wajchenberg BL. Ultrasonographic abnormalities of the pancreas in IDDM and NIDDM patients. Diabetes Care. 1993;16(9):1296-1297. [DOI] [PubMed] [Google Scholar]
- 382. Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, Hahafusa T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol. 2001;38(3):145-149. [DOI] [PubMed] [Google Scholar]
- 383. Garcia TS, Rech TH, Leitão CB. Pancreatic size and fat content in diabetes: a systematic review and meta-analysis of imaging studies. PLoS One. 2017;12(7):e0180911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Al-Mrabeh A, Hollingsworth KG, Steven S, Taylor R. Morphology of the pancreas in type 2 diabetes: effect of weight loss with or without normalisation of insulin secretory capacity. Diabetologia. 2016;59(8):1753-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Sakai NS, Taylor SA, Chouhan MD. Obesity, metabolic disease and the pancreas-Quantitative imaging of pancreatic fat. Br J Radiol. 2018;91(1089):20180267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Heber SD, Hetterich H, Lorbeer R, et al. Pancreatic fat content by magnetic resonance imaging in subjects with prediabetes, diabetes, and controls from a general population without cardiovascular disease. PLoS One. 2017;12(5):e0177154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Heni M, Machann J, Staiger H, et al. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010;26(3):200-205. [DOI] [PubMed] [Google Scholar]
- 388. Kühn JP, Berthold F, Mayerle J, et al. Pancreatic steatosis demonstrated at MR imaging in the general population: clinical relevance. Radiology. 2015;276(1):129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30(11):2916-2921. [DOI] [PubMed] [Google Scholar]
- 390. van der Zijl NJ, Goossens GH, Moors CC, et al. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96(2):459-467. [DOI] [PubMed] [Google Scholar]
- 391. Begovatz P, Koliaki C, Weber K, et al. Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia. 2015;58(7):1646-1655. [DOI] [PubMed] [Google Scholar]
- 392. Wong VW, Wong GL, Yeung DK, et al. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol. 2014;109(4):589-597. [DOI] [PubMed] [Google Scholar]
- 393. Yamazaki H, Tsuboya T, Katanuma A, et al. Lack of independent association between fatty pancreas and incidence of type 2 diabetes: 5-year Japanese Cohort Study. Diabetes Care. 2016;39(10):1677-1683. [DOI] [PubMed] [Google Scholar]
- 394. Wagner R, Jaghutriz BA, Gerst F, et al. Pancreatic steatosis associates with impaired insulin secretion in genetically predisposed individuals. J Clin Endocrinol Metab. 2020;105(11):3518-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Chen YC, Taylor AJ, Verchere CB. Islet prohormone processing in health and disease. Diabetes Obes Metab. 2018;20 Suppl 2:64-76. [DOI] [PubMed] [Google Scholar]
- 396. Rhodes CJ, Alarcón C. What beta-cell defect could lead to hyperproinsulinemia in NIDDM? Some clues from recent advances made in understanding the proinsulin-processing mechanism. Diabetes. 1994;43(4):511-517. [DOI] [PubMed] [Google Scholar]
- 397. Naggert JK, Fricker LD, Varlamov O, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10(2):135-142. [DOI] [PubMed] [Google Scholar]
- 398. Davies MJ, Rayman G, Gray IP, Day JL, Hales CN. Insulin deficiency and increased plasma concentration of intact and 32/33 split proinsulin in subjects with impaired glucose tolerance. Diabet Med. 1993;10(4):313-320. [DOI] [PubMed] [Google Scholar]
- 399. Ozawa S, Katsuta H, Suzuki K, et al. Estimated proinsulin processing activity of prohormone convertase (PC) 1/3 rather than PC2 is decreased in pancreatic β-cells of type 2 diabetic patients. Endocr J. 2014;61(6):607-614. [DOI] [PubMed] [Google Scholar]
- 400. Røder ME, Schwartz RS, Prigeon RL, Kahn SE. Reduced pancreatic B cell compensation to the insulin resistance of aging: impact on proinsulin and insulin levels. J Clin Endocrinol Metab. 2000;85(6):2275-2280. [DOI] [PubMed] [Google Scholar]
- 401. Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab. 1980;51(3):520-528. [DOI] [PubMed] [Google Scholar]
- 402. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368-377. [DOI] [PubMed] [Google Scholar]
- 403. Egan AM, Laurenti MC, Hurtado Andrade MD, et al. Limitations of the fasting proinsulin to insulin ratio as a measure of β-cell health in people with and without impaired glucose tolerance. Eur J Clin Invest. 2021;51(6):e13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Røder ME, Dinesen B, Hartling SG, et al. Intact proinsulin and beta-cell function in lean and obese subjects with and without type 2 diabetes. Diabetes Care. 1999;22(4):609-614. [DOI] [PubMed] [Google Scholar]
- 405. Kahn SE, Beard JC, Schwartz MW, et al. Increased beta-cell secretory capacity as mechanism for islet adaptation to nicotinic acid-induced insulin resistance. Diabetes. 1989;38(5):562-568. [DOI] [PubMed] [Google Scholar]
- 406. Kahn SE, McCulloch DK, Schwartz MW, Palmer JP, Porte D Jr. Effect of insulin resistance and hyperglycemia on proinsulin release in a primate model of diabetes mellitus. J Clin Endocrinol Metab. 1992;74(1):192-197. [DOI] [PubMed] [Google Scholar]
- 407. Kalhan SC, Adam PA. Inhibitory effect of prednisone on insulin secretion in man: model for duplication of blood glucose concentration. J Clin Endocrinol Metab. 1975;41(3):600-610. [DOI] [PubMed] [Google Scholar]
- 408. Barseghian G, Levine R. Effect of corticosterone on insulin and glucagon secretion by the isolated perfused rat pancreas. Endocrinology. 1980;106(2):547-552. [DOI] [PubMed] [Google Scholar]
- 409. Kitabchi AE, Jones GM, Duckworth WC. Effect of hydrocortisone and corticotropin on glucose-induced insulin and proinsulin secretion in man. J Clin Endocrinol Metab. 1973;37(1):79-84. [DOI] [PubMed] [Google Scholar]
- 410. Kahn SE, Horber FF, Prigeon RL, Haymond MW, Porte D Jr. Effect of glucocorticoid and growth hormone treatment on proinsulin levels in humans. Diabetes. 1993;42(7):1082-1085. [DOI] [PubMed] [Google Scholar]
- 411. Seaquist ER, Kahn SE, Clark PM, Hales CN, Porte D Jr, Robertson RP. Hyperproinsulinemia is associated with increased beta cell demand after hemipancreatectomy in humans. J Clin Invest. 1996;97(2):455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Breuer TG, Menge BA, Banasch M, et al. Proinsulin levels in patients with pancreatic diabetes are associated with functional changes in insulin secretion rather than pancreatic beta-cell area. Eur J Endocrinol. 2010;163(4):551-558. [DOI] [PubMed] [Google Scholar]
- 413. Mezza T, Ferraro PM, Sun VA, et al. Increased β-cell workload modulates proinsulin-to-insulin ratio in humans. Diabetes. 2018;67(11):2389-2396. [DOI] [PubMed] [Google Scholar]
- 414. Kitabchi AE. The biological and immunological properties of pork and beef insulin, proinsulin, and connecting peptides. J Clin Invest. 1970;49(5):979-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Yu SS, Kitbachi AE. Biological activity of proinsulin and related polypeptides in the fat tissue. J Biol Chem. 1973;248(11):3753-3761. [PubMed] [Google Scholar]
- 416. Beer SF, O’Rahilly S, Spivey RS, Hales CN, Turner RC. Plasma proinsulin in first-degree relatives of type 2 diabetic subjects. Diabetes Res. 1990;14(2):51-54. [PubMed] [Google Scholar]
- 417. Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Bergstrom RW, Fujimoto WY. Proinsulin as a marker for the development of NIDDM in Japanese-American men. Diabetes. 1995;44(2):173-179. [DOI] [PubMed] [Google Scholar]
- 418. Pradhan AD, Manson JE, Meigs JB, et al. Insulin, proinsulin, proinsulin:insulin ratio, and the risk of developing type 2 diabetes mellitus in women. Am J Med. 2003;114(6):438-444. [DOI] [PubMed] [Google Scholar]
- 419. Mykkänen L, Haffner SM, Kuusisto J, Pyörälä K, Hales CN, Laakso M. Serum proinsulin levels are disproportionately increased in elderly prediabetic subjects. Diabetologia. 1995;38(10):1176-1182. [DOI] [PubMed] [Google Scholar]
- 420. Vangipurapu J, Stančáková A, Kuulasmaa T, Kuusisto J, Laakso M. Both fasting and glucose-stimulated proinsulin levels predict hyperglycemia and incident type 2 diabetes: a population-based study of 9,396 Finnish men. PLoS One. 2015;10(4):e0124028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988;333(6168):93-96. [DOI] [PubMed] [Google Scholar]
- 422. Laedtke T, Kjems L, Pørksen N, et al. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab. 2000;279(3):E520-E528. [DOI] [PubMed] [Google Scholar]
- 423. Røder ME, Kahn SE. Suppression of Beta-cell secretion by somatostatin does not fully reverse the disproportionate proinsulinemia of type 2 diabetes. Diabetes. 2004;53 Suppl 3:S22-S25. [DOI] [PubMed] [Google Scholar]
- 424. Klinke DJ 2nd. Extent of beta cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS One. 2008;3(1):e1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Wasserfall C, Nick HS, Campbell-Thompson M, et al. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab. 2017;26(3):568-575.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362-1369. [DOI] [PubMed] [Google Scholar]
- 427. Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57(1):187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Van Dalem A, Demeester S, Balti EV, et al. Belgian Diabetes Registry . Prediction of impending type 1 diabetes through automated dual-label measurement of proinsulin:C-peptide ratio. PLoS One. 2016;11(12):e0166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Sims EK, Chaudhry Z, Watkins R, et al. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016;39(9):1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Truyen I, De Pauw P, Jørgensen PN, et al. Belgian Diabetes Registry . Proinsulin levels and the proinsulin:C-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia. 2005;48(11):2322-2329. [DOI] [PubMed] [Google Scholar]
- 431. Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, et al. Increase in pancreatic proinsulin and preservation of β-cell mass in autoantibody-positive donors prior to type 1 diabetes onset. Diabetes. 2017;66(5):1334-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Sims EK, Syed F, Nyalwidhe J, et al. Abnormalities in proinsulin processing in islets from individuals with longstanding T1D. Transl Res. 2019;213:90-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. β cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab. 2017;25(3):727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Lam CJ, Chatterjee A, Shen E, Cox AR, Kushner JA. Low-level insulin content within abundant non-β islet endocrine cells in long-standing type 1 diabetes. Diabetes. 2019;68(3):598-608. [DOI] [PubMed] [Google Scholar]
- 435. Oram RA, Sims EK, Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia. 2019;62(4):567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Hostens K, Pavlovic D, Zambre Y, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest. 1999;104(1):67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Cabrera SM, Wang X, Chen YG, et al. Type 1 Diabetes TrialNet Canakinumab Study Group; AIDA Study Group . Interleukin-1 antagonism moderates the inflammatory state associated with Type 1 diabetes during clinical trials conducted at disease onset. Eur J Immunol. 2016;46(4):1030-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Quattrin T, Haller MJ, Steck AK, et al. T1GER Study Investigators . Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383(21):2007-2017. [DOI] [PubMed] [Google Scholar]
- 439. Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol. 2019;19(12):734-746. [DOI] [PubMed] [Google Scholar]
- 440. Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55(9):2417-2420. [DOI] [PubMed] [Google Scholar]
- 441. Sims EK, Evans-Molina C, Tersey SA, Eizirik DL, Mirmira RG. Biomarkers of islet beta cell stress and death in type 1 diabetes. Diabetologia. 2018;61(11):2259-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Guest PC, Bailyes EM, Hutton JC. Endoplasmic reticulum Ca2+ is important for the proteolytic processing and intracellular transport of proinsulin in the pancreatic beta-cell. Biochem J. 1997;323 (Pt 2):445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54(2):452-461. [DOI] [PubMed] [Google Scholar]
- 445. Tong X, Kono T, Anderson-Baucum EK, et al. SERCA2 deficiency impairs pancreatic β-cell function in response to diet-induced obesity. Diabetes. 2016;65(10):3039-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Klimek AM, Soukhatcheva G, Thompson DM, et al. Impaired proinsulin processing is a characteristic of transplanted islets. Am J Transplant. 2009;9(9):2119-2125. [DOI] [PubMed] [Google Scholar]
- 447. McDonald CG, Ryan EA, Paty BW, et al. Cross-sectional and prospective association between proinsulin secretion and graft function after clinical islet transplantation. Transplantation. 2004;78(6):934-937. [DOI] [PubMed] [Google Scholar]
- 448. Rickels MR, Mueller R, Teff KL, Naji A. β-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab. 2010;95(3):1238-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Elkhafif NM, Borot S, Morel P, et al. Endocrine secretory reserve and proinsulin processing in recipients of islet of Langerhans versus whole pancreas transplants. Diabetes Care. 2013;36(11):3726-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Johnson JD, Ao Z, Ao P, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18(8):833-845. [DOI] [PubMed] [Google Scholar]
- 451. Potter KJ, Westwell-Roper CY, Klimek-Abercrombie AM, Warnock GL, Verchere CB. Death and dysfunction of transplanted β-cells: lessons learned from type 2 diabetes? Diabetes. 2014;63(1):12-19. [DOI] [PubMed] [Google Scholar]
- 452. Fiorina P, Vergani A, Petrelli A, et al. Metabolic and immunological features of the failing islet-transplanted patient. Diabetes Care. 2008;31(3):436-–438.. [DOI] [PubMed] [Google Scholar]
- 453. Davalli AM, Perego L, Bertuzzi F, et al. Disproportionate hyperproinsulinemia, beta-cell restricted prohormone convertase 2 deficiency, and cell cycle inhibitors expression by human islets transplanted into athymic nude mice: insights into nonimmune-mediated mechanisms of delayed islet graft failure. Cell Transplant. 2008;17(12):1323-1336. [DOI] [PubMed] [Google Scholar]
- 454. Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988;263(33):17243-17246. [PubMed] [Google Scholar]
- 455. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91(3):795-826. [DOI] [PubMed] [Google Scholar]
- 456. Marzban L, Trigo-Gonzalez G, Zhu X, et al. Role of beta-cell prohormone convertase (PC)1/3 in processing of pro-islet amyloid polypeptide. Diabetes. 2004;53(1):141-148. [DOI] [PubMed] [Google Scholar]
- 457. Higham CE, Hull RL, Lawrie L, et al. Processing of synthetic pro-islet amyloid polypeptide (proIAPP) ‘amylin’ by recombinant prohormone convertase enzymes, PC2 and PC3, in vitro. Eur J Biochem. 2000;267:4998-5004. [DOI] [PubMed] [Google Scholar]
- 458. Marzban L, Soukhatcheva G, Verchere CB. Role of carboxypeptidase E in processing of pro-islet amyloid polypeptide in {beta}-cells. Endocrinology. 2005;146(4):1808-1817. [DOI] [PubMed] [Google Scholar]
- 459. Cooper GJ, Day AJ, Willis AC, Roberts AN, Reid KB, Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989;1014(3):247-258. [DOI] [PubMed] [Google Scholar]
- 460. Wang J, Xu J, Finnerty J, Furuta M, Steiner DF, Verchere CB. The prohormone convertase enzyme 2 (PC2) is essential for processing pro-islet amyloid polypeptide at the NH2-terminal cleavage site. Diabetes. 2001;50(3):534-539. [DOI] [PubMed] [Google Scholar]
- 461. Kahn SE, Verchere CB, Andrikopoulos S, et al. Reduced amylin release is a characteristic of impaired glucose tolerance and type 2 diabetes in Japanese Americans. Diabetes. 1998;47(4):640-645. [DOI] [PubMed] [Google Scholar]
- 462. Dechenes CJ, Verchere CB, Andrikopoulos S, Kahn SE. Human aging is associated with parallel reductions in insulin and amylin release. Am J Physiol. 1998;275(5):E785-E791. [DOI] [PubMed] [Google Scholar]
- 463. Knowles NG, Landchild MA, Fujimoto WY, Kahn SE. Insulin and amylin release are both diminished in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2002;25(2):292-297. [DOI] [PubMed] [Google Scholar]
- 464. Courtade JA, Klimek-Abercrombie AM, Chen YC, et al. Measurement of pro-islet amyloid polypeptide (1-48) in diabetes and islet transplants. J Clin Endocrinol Metab. 2017;102(7):2595-2603. [DOI] [PubMed] [Google Scholar]
- 465. Courtade JA, Wang EY, Yen P, et al. Loss of prohormone convertase 2 promotes beta cell dysfunction in a rodent transplant model expressing human pro-islet amyloid polypeptide. Diabetologia. 2017;60(3):453-463. [DOI] [PubMed] [Google Scholar]
- 466. Hou X, Ling Z, Quartier E, et al. Prolonged exposure of pancreatic beta cells to raised glucose concentrations results in increased cellular content of islet amyloid polypeptide precursors. Diabetologia. 1999;42(2):188-194. [DOI] [PubMed] [Google Scholar]
- 467. Park YJ, Warnock GL, Ao Z, et al. Dual role of interleukin-1β in islet amyloid formation and its β-cell toxicity: Implications for type 2 diabetes and islet transplantation. Diabetes Obes Metab. 2017;19(5):682-694. [DOI] [PubMed] [Google Scholar]
- 468. Zheng X, Ren W, Zhang S, et al. Serum levels of proamylin and amylin in normal subjects and patients with impaired glucose regulation and type 2 diabetes mellitus. Acta Diabetol. 2010;47(3):265-270. [DOI] [PubMed] [Google Scholar]
- 469. Kanatsuka A, Makino H, Yagui K, et al. Islet amyloid polypeptide and its N-terminal and C-terminal flanking peptides’ immunoreactivity in islet amyloid of diabetic patients. Diabetes Res Clin Pract. 1994;26(2):101-107. [DOI] [PubMed] [Google Scholar]
- 470. Westermark GT, Steiner DF, Gebre-Medhin S, Engström U, Westermark P. Pro islet amyloid polypeptide (ProIAPP) immunoreactivity in the islets of Langerhans. Ups J Med Sci. 2000;105(2):97-106. [DOI] [PubMed] [Google Scholar]
- 471. Clark A, Lloyd J, Novials A, Hutton JC, Morris JF. Localisation of islet amyloid polypeptide and its carboxy terminal flanking peptide in islets of diabetic man and monkey. Diabetologia. 1991;34(6):449-451. [DOI] [PubMed] [Google Scholar]
- 472. Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Single-cell transcriptome profiling reveals β cell maturation in stem cell-derived islets after transplantation. Cell Rep. 2020;32(8):108067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Yalow RS, Berson SA. Plasma insulin concentrations in nondiabetic and early diabetic subjects. Determinations by a new sensitive immuno-assay technic. Diabetes. 1960;9:254-260. [DOI] [PubMed] [Google Scholar]
- 475. Robbins DC, Andersen L, Bowsher R, et al. Report of the American Diabetes Association’s Task Force on Standardization of the Insulin Assay. Diabetes. 1996;45(2):242-256. [DOI] [PubMed] [Google Scholar]
- 476. Marcovina S, Bowsher RR, Miller WG, et al. Insulin Standardization Workgroup . Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin Chem. 2007;53(4):711-716. [DOI] [PubMed] [Google Scholar]
- 477. Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46(3):323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Utzschneider KM, Prigeon RL, Tong J, et al. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia. 2007;50(12):2516-2525. [DOI] [PubMed] [Google Scholar]
- 479. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286-292. [DOI] [PubMed] [Google Scholar]
- 480. Utzschneider KM, Younes N, Rasouli N, et al. GRADE Research Group . Association of glycemia with insulin sensitivity and β-cell function in adults with early type 2 diabetes on metformin alone. J Diabetes Complications. 2021;35(5):107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481. Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the beta-cell response after oral glucose loading. Diabetes. 1976;25(4):241-244. [DOI] [PubMed] [Google Scholar]
- 482. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 483. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 484. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26(12):1198-1203. [DOI] [PubMed] [Google Scholar]
- 485. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191-2192. [DOI] [PubMed] [Google Scholar]
- 486. Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283(6):E1159-E1166. [DOI] [PubMed] [Google Scholar]
- 487. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50(1):150-158. [DOI] [PubMed] [Google Scholar]
- 488. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539-548. [DOI] [PubMed] [Google Scholar]
- 489. Cerasi E, Luft R. Insulin response to glucose infusion in diabetic and non-diabetic monozygotic twin pairs. Genetic control of insulin response? Acta Endocrinol (Copenh). 1967;55(2):330-345. [DOI] [PubMed] [Google Scholar]
- 490. Grodsky GM. A new phase of insulin secretion. How will it contribute to our understanding of beta-cell function? Diabetes. 1989;38(6):673-678. [DOI] [PubMed] [Google Scholar]
- 491. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-E223. [DOI] [PubMed] [Google Scholar]
- 492. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420-430. [DOI] [PubMed] [Google Scholar]
- 493. Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1-E15. [DOI] [PubMed] [Google Scholar]
- 494. Abbate SL, Fujimoto WY, Brunzell JD, Kahn SE. Effect of heparin on insulin-glucose interactions measured by the minimal model technique: implications for reproducibility using this method. Metabolism. 1993;42(3):353-357. [DOI] [PubMed] [Google Scholar]
- 495. Steil GM, Murray J, Bergman RN, Buchanan TA. Repeatability of insulin sensitivity and glucose effectiveness from the minimal model. Implications for study design. Diabetes. 1994;43(11):1365-1371. [DOI] [PubMed] [Google Scholar]
- 496. Fritsche A, Stefan N, Hardt E, Schützenauer S, Häring H, Stumvoll M. A novel hyperglycaemic clamp for characterization of islet function in humans: assessment of three different secretagogues, maximal insulin response and reproducibility. Eur J Clin Invest. 2000;30(5):411-418. [DOI] [PubMed] [Google Scholar]
- 497. Bagdade JD, Bierman EL, Porte D Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46(10):1549-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51 Suppl 1:S212-S220. [DOI] [PubMed] [Google Scholar]
- 499. Mather KJ, Chen M, Hannon TS. Linearization of the Disposition Index equation allows evaluation of secretion-sensitivity coupling slopes. J Diabetes Complications. 2020;34(7):107589. [DOI] [PubMed] [Google Scholar]
- 500. Herzberg-Schäfer SA, Staiger H, Heni M, et al. Evaluation of fasting state-/oral glucose tolerance test-derived measures of insulin release for the detection of genetically impaired β-cell function. PLoS One. 2010;5(12):e14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19(10):1138-1141. [DOI] [PubMed] [Google Scholar]
- 502. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Carayol J, Hosking J, Pinkney J, et al. Genetic susceptibility determines β-cell function and fasting glycemia trajectories throughout childhood: a 12-year Cohort Study (EarlyBird 76). Diabetes Care. 2020;43(3):653-660. [DOI] [PubMed] [Google Scholar]
- 504. UK Prospective Diabetes Study (UKPDS) Group. U.K. Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249-1258. [PubMed] [Google Scholar]
- 505. Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427-2443. [DOI] [PubMed] [Google Scholar]
- 506. Lu J, Zang J, Li H. Impact of three oral antidiabetic drugs on markers of β-cell function in patients with type 2 diabetes: a meta-analysis. PLoS One. 2013;8(10):e76713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40 Suppl:S21-S25. [DOI] [PubMed] [Google Scholar]
- 508. Karam JH, Grodsky GM, Forsham PH. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963;12:197-204. [DOI] [PubMed] [Google Scholar]
- 509. Mcintyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2(7349):20-21. [DOI] [PubMed] [Google Scholar]
- 510. Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082. [DOI] [PubMed] [Google Scholar]
- 511. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46-52. [DOI] [PubMed] [Google Scholar]
- 512. Ward WK, Wallum BJ, Beard JC, Taborsky GJ Jr, Porte D Jr. Reduction of glycemic potentiation. Sensitive indicator of beta-cell loss in partially pancreatectomized dogs. Diabetes. 1988;37(6):723-729. [DOI] [PubMed] [Google Scholar]
- 513. Larsen MO, Rolin B, Sturis J, et al. Measurements of insulin responses as predictive markers of pancreatic beta-cell mass in normal and beta-cell-reduced lean and obese Göttingen minipigs in vivo. Am J Physiol Endocrinol Metab. 2006;290(4):E670-E677. [DOI] [PubMed] [Google Scholar]
- 514. Rijkelijkhuizen JM, Girman CJ, Mari A, et al. Classical and model-based estimates of beta-cell function during a mixed meal vs. an OGTT in a population-based cohort. Diabetes Res Clin Pract. 2009;83(2):280-288. [DOI] [PubMed] [Google Scholar]
- 515. Zhang J, Yang Z, Xiao J, et al. China National Diabetes and Metabolic Disorders Study Group . Association between family history risk categories and prevalence of diabetes in Chinese population. PLoS One. 2015;10(2):e0117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Florez JC, Jablonski KA, Bayley N, et al. Diabetes Prevention Program Research Group . TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Billings LK, Jablonski KA, Ackerman RJ, et al. Diabetes Prevention Program Research Group, Rockville . The influence of rare genetic variation in SLC30A8 on diabetes incidence and β-cell function. J Clin Endocrinol Metab. 2014;99(5):E926-E930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Billings LK, Jablonski KA, Warner AS, et al. Diabetes Prevention Program Research Group . Variation in maturity-onset diabetes of the young genes influence response to interventions for diabetes prevention. J Clin Endocrinol Metab. 2017;102(8):2678-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Florez JC, Jablonski KA, Kahn SE, et al. Type 2 diabetes-associated missense polymorphisms KCNJ11 E23K and ABCC8 A1369S influence progression to diabetes and response to interventions in the Diabetes Prevention Program. Diabetes. 2007;56(2):531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Hivert MF, Jablonski KA, Perreault L, et al. DIAGRAM Consortium; Diabetes Prevention Program Research Group . Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the Diabetes Prevention Program. Diabetes. 2011;60(4):1340-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Sullivan SD, Jablonski KA, Florez JC, et al. Diabetes Prevention Program Research Group . Genetic risk of progression to type 2 diabetes and response to intensive lifestyle or metformin in prediabetic women with and without a history of gestational diabetes mellitus. Diabetes Care. 2014;37(4):909-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Kahn SE, Lachin JM, Zinman B, et al. ADOPT Study Group . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Kitabchi AE, Temprosa M, Knowler WC, et al. Diabetes Prevention Program Research Group . Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation in the Diabetes Prevention Program. Diabetes Care. 2009;32(9):1583-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. The DCCT Research Group. Effects of age, duration and treatment of IDDM on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab. 1987;65:30-36. [DOI] [PubMed] [Google Scholar]
- 526. Greenbaum CJ, Anderson AM, Dolan LM, et al. SEARCH Study Group . Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care. 2009;32(10):1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. McGee P, Steffes M, Nowicki M, et al. ; DCCT/EDIC Research Group . Insulin secretion measured by stimulated C-peptide in long-established Type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) cohort: a pilot study. Diabet Med. 2014;31(10):1264-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Gubitosi-Klug RA, Braffett BH, Hitt S, et al. ; DCCT/EDIC Research Group . Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest. 2021;131(3):e143011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care. 2003;26(4):1026-1033. [DOI] [PubMed] [Google Scholar]
- 530. Manco M, Nolfe G, Pataky Z, et al. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabolism. 2017;70:42-50. [DOI] [PubMed] [Google Scholar]
- 531. Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev. 2010;26(4):280-286. [DOI] [PubMed] [Google Scholar]
- 532. Arslanian SA, El Ghormli L, Kim JY, et al. RISE Consortium . OGTT glucose response curves, insulin sensitivity, and β-cell function in RISE: comparison between youth and adults at randomization and in response to interventions to preserve β-cell function. Diabetes Care. 2021;44(3):817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Arslanian S, El Ghormli L, Young Kim J, et al. TODAY Study Group . The shape of the glucose response curve during an oral glucose tolerance test: forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care. 2019;42(1):164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Cerasi E, Luft R, Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes. 1972;21(4):224-234. [DOI] [PubMed] [Google Scholar]
- 535. Davies MJ, Rayman G, Grenfell A, Gray IP, Day JL, Hales CN. Loss of the first phase insulin response to intravenous glucose in subjects with persistent impaired glucose tolerance. Diabet Med. 1994;11(5):432-436. [DOI] [PubMed] [Google Scholar]
- 536. American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197. [DOI] [PubMed] [Google Scholar]
- 537. Raskin P, Aydin I, Unger RH. Effect of insulin on the exaggerated glucagon response to arginine stimulation in diabetes mellitus. Diabetes. 1976;25(3):227-229. [DOI] [PubMed] [Google Scholar]
- 538. Pipeleers D, Chintinne M, Denys B, Martens G, Keymeulen B, Gorus F. Restoring a functional beta-cell mass in diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):54-62. [DOI] [PubMed] [Google Scholar]
- 539. King DS, Dalsky GP, Clutter WE, et al. Effects of lack of exercise on insulin secretion and action in trained subjects. Am J Physiol. 1988;254(5 Pt 1):E537-E542. [DOI] [PubMed] [Google Scholar]
- 540. Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med. 1979;301(19):1023-1027. [DOI] [PubMed] [Google Scholar]
- 541. Sturis J, Van Cauter E, Blackman JD, Polonsky KS. Entrainment of pulsatile insulin secretion by oscillatory glucose infusion. J Clin Invest. 1991;87(2):439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Pimenta W, Korytkowski M, Mitrakou A, et al. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA. 1995;273(23):1855-1861. [PubMed] [Google Scholar]
- 543. Cnop M, Vidal J, Hull RL, et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2007;30(3):677-682. [DOI] [PubMed] [Google Scholar]
- 544. Chen M, Bergman RN, Pacini G, Porte D Jr. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985;60(1):13-20. [DOI] [PubMed] [Google Scholar]
- 545. Kahn SE, Larson VG, Schwartz RS, et al. Exercise training delineates the importance of B-cell dysfunction to the glucose intolerance of human aging. J Clin Endocrinol Metab. 1992;74(6):1336-1342. [DOI] [PubMed] [Google Scholar]
- 546. Ward WK, Johnston CL, Beard JC, Benedetti TJ, Halter JB, Porte D Jr. Insulin resistance and impaired insulin secretion in subjects with histories of gestational diabetes mellitus. Diabetes. 1985;34(9):861-869. [DOI] [PubMed] [Google Scholar]
- 547. Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162(4):1008-1014. [DOI] [PubMed] [Google Scholar]
- 548. Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96(1):520-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549. Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81(3):942-947. [DOI] [PubMed] [Google Scholar]
- 550. Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55(4):1074-1079. [DOI] [PubMed] [Google Scholar]
- 551. Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52(5):1098-1103. [DOI] [PubMed] [Google Scholar]
- 552. Purnell JQ, Johnson GS, Wahed AS, et al. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia. 2018;61(5):1142-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. The RISE Consortium. Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41(8):1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493-500. [DOI] [PubMed] [Google Scholar]
- 555. Cali AM, Man CD, Cobelli C, et al. Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes Care. 2009;32(3):456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. Michaliszyn SF, Mari A, Lee S, et al. β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes. 2014;63(11):3846-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557. Utzschneider KM, Tripputi MT, Kozedub A, et al. RISE Consortium . β-cells in youth with impaired glucose tolerance or early type 2 diabetes secrete more insulin and are more responsive than in adults. Pediatr Diabetes. 2020;21(8):1421-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558. Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96(9):E1372-E1379. [DOI] [PubMed] [Google Scholar]
- 559. Lund MT, Hansen M, Skaaby S, et al. Preoperative β-cell function in patients with type 2 diabetes is important for the outcome of Roux-en-Y gastric bypass surgery. J Physiol. 2015;593(14):3123-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Vasques AC, Pareja JC, da Saúde de Oliveira M, et al. Long-term outcomes of biliopancreatic diversion on glycemic control, insulin sensitivity and beta cell function. Obes Surg. 2016;26(11):2572-2580. [DOI] [PubMed] [Google Scholar]
- 561. Trico D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. 2018;3(24):e124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Aitken ML, Szkudlinska MA, Boyko EJ, Ng D, Utzschneider KM, Kahn SE. Impaired counterregulatory responses to hypoglycaemia following oral glucose in adults with cystic fibrosis. Diabetologia. 2020;63(5):1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563. Hartter E, Svoboda T, Ludvik B, et al. Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia. 1991;34(1):52-54. [DOI] [PubMed] [Google Scholar]
- 564. Koda JE, Fineman M, Rink TJ, Dailey GE, Muchmore DB, Linarelli LG. Amylin concentrations and glucose control. Lancet. 1992;339(8802):1179-1180. [DOI] [PubMed] [Google Scholar]
- 565. Ludvik B, Clodi M, Kautzky-Willer A, et al. Effect of dexamethasone on insulin sensitivity, islet amyloid polypeptide and insulin secretion in humans. Diabetologia. 1993;36(1):84-87. [DOI] [PubMed] [Google Scholar]
- 566. Kautzky-Willer A, Thomaseth K, Pacini G, et al. Role of islet amyloid polypeptide secretion in insulin-resistant humans. Diabetologia. 1994;37(2):188-194. [DOI] [PubMed] [Google Scholar]
- 567. Stadler M, Anderwald C, Karer T, et al. Increased plasma amylin in type 1 diabetic patients after kidney and pancreas transplantation: a sign of impaired beta-cell function? Diabetes Care. 2006;29(5):1031-1038. [DOI] [PubMed] [Google Scholar]
- 568. Nannipieri M, Baldi S, Mari A, et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98(11):4391-4399. [DOI] [PubMed] [Google Scholar]
- 569. Woerle HJ, Albrecht M, Linke R, et al. Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care. 2008;31(12):2325-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Chapman I, Parker B, Doran S, et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. 2005;48(5):838-848. [DOI] [PubMed] [Google Scholar]
- 571. Percy AJ, Trainor DA, Rittenhouse J, Phelps J, Koda JE. Development of sensitive immunoassays to detect amylin and amylin-like peptides in unextracted plasma. Clin Chem. 1996;42(4):576-585. [PubMed] [Google Scholar]
- 572. Mäkimattila S, Fineman MS, Yki-Järvinen H. Deficiency of total and nonglycosylated amylin in plasma characterizes subjects with impaired glucose tolerance and type 2 diabetes. J Clin Endocrinol Metab. 2000;85(8):2822-2827. [DOI] [PubMed] [Google Scholar]
- 573. Patel KA, Kettunen J, Laakso M, et al. Heterozygous RFX6 protein truncating variants are associated with MODY with reduced penetrance. Nat Commun. 2017;8(1):888. [DOI] [PMC free article] [PubMed] [Google Scholar]