Abstract
This first Guideline for Reasonable and Appropriate Care in the Emergency Department (GRACE-1) from the Society for Academic Emergency Medicine is on the topic: Recurrent, Low-risk Chest Pain in the Emergency Department. The multidisciplinary guideline panel used The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the certainty of evidence and strength of recommendations regarding eight priority questions for adult patients with recurrent, low-risk chest pain and have derived the following evidence based recommendations: (1) for those >3 h chest pain duration we suggest a single, high-sensitivity troponin below a validated threshold to reasonably exclude acute coronary syndrome (ACS) within 30 days; (2) for those with a normal stress test within the previous 12 months, we do not recommend repeat routine stress testing as a means to decrease rates of major adverse cardiac events at 30 days; (3) insufficient evidence to recommend hospitalization (either standard inpatient admission or observation stay) versus discharge as a strategy to mitigate major adverse cardiac events within 30 days; (4) for those with non-obstructive (<50% stenosis) coronary artery disease (CAD) on prior angiography within 5 years, we suggest referral for expedited outpatient testing as warranted rather than admission for inpatient evaluation; (5) for those with no occlusive CAD (0% stenosis) on prior angiography within 5 years, we recommend referral for expedited outpatient testing as warranted rather than admission for inpatient evaluation; (6) for those with a prior coronary computed tomographic angiography within the past 2 years with no coronary stenosis, we suggest no further diagnostic testing other than a single, normal high-sensitivity troponin below a validated threshold to exclude ACS within that 2 year time frame; (7) we suggest the use of depression and anxiety screening tools as these might have an effect on healthcare use and return emergency department (ED) visits; and (8) we suggest referral for anxiety or depression management, as this might have an impact on healthcare use and return ED visits.
Keywords: acute coronary syndrome, chest pain, low risk, recurrent
INTRODUCTION
Chest pain is the second most common chief complaint in the emergency department (ED), accounting for 5% of visits1 with an estimated cost up to $10 billion annually.2–4 The differential diagnosis for chest pain is broad. While providers often prioritize the exclusion of cardiac causes of chest pain such as acute coronary syndromes (ACS), other causes such as acute aortic syndromes, pericarditis, myocarditis, pulmonary embolism, pneumothorax, pneumonia, and perforated peptic ulcer also cause chest pain. However, only 5% of chest pain visits are diagnosed with one of these acute life-threatening conditions, most commonly ACS.5,6
Despite this low prevalence, missed acute myocardial infarction (AMI) and associated adverse events rank as frequent causes of malpractice lawsuits, and consequently, emergency physicians are loath to miss these conditions.7,8 In addition, significant inter-physician and inter-emergency department variation occurs in the evaluation (diagnostic testing and admission) of these patients. Clinical practice guidelines can aid clinicians in the evaluation of low-risk chest pain,9 but significant practice variation persists and is often subject to the available local resources or lack thereof.10 For example, one recent study found physician-level admission rates varied from 54% to 96% for chest pain patients at a single facility.11 Recurrent ED visits for chest pain are common, with up to 40% of patients returning to the ED for chest pain within 1 year.12,13 The ED evaluation of patients with chest pain is further complicated by patients’ varying degrees of prior diagnostic evaluations. Patients can also develop new or multiple contributing etiologies for recurrent or evolving symptoms, or symptom quality and character can fluctuate under the influence of psychosocial factors, without any progression of underlying physical disease. These factors create a diagnostic dilemma of particular importance for the emergency clinician. Clinical practice guidelines for the diagnosis and management of ACS exist, but neglect the preponderance of ED chest pain patients who are low risk, as well as the subset of those who present with recurrent chest pain.14,15 The lack of guidelines specific to the ED population with recurrent chest pain reflects the diagnostic uncertainty associated with this condition and raises several critical questions including but not limited to (1) does a revisit represent a previously missed diagnosis; (2) does the revisit warrant an escalation in diagnostic approach; (3) what if the patient has already had what can be considered a recent, reasonable and thorough diagnostic evaluation; (4) and for how long is this prior evaluation valid? The objective of these guidelines is to provide an evidence-based framework intended to support patients, clinicians, and other health-care professionals in their decisions about the evaluation and management of patients with recurrent, low-risk chest pain in the ED.
SCOPE AND PURPOSE
The target audience includes all practicing ED clinicians (physicians and advanced practice providers) responsible for the evaluation and management of undifferentiated chest pain in community and academic settings, as well as healthcare systems and hospitals responsible for care pathways in this population.
METHODS
Group composition
The panel was composed of several geographically, ethnically, and gender diverse ED clinicians, a cardiologist, a patient representative and three methodologists. The Society for Academic Emergency Medicine (SAEM) provided financial support for the development of this guideline.
Group interaction and processes
Starting in May 2019, the panel met regularly through in-person, online, and telephone meetings. The methodologists attended a 3-day in-person Grading of Recommendations Assessment, Development and Evaluation (GRADE) training before the development of this guideline. Content experts were selected among known emergency medicine (EM) physicians with relevant publications in the field. As a quality/trustworthiness check, the final manuscript was analyzed using the recently published AHRQ National Guideline Clearinghouse Extent of Adherence to Trustworthiness Standards (NEATS) instrument16 to ensure best possible adherence to Institute of Medicine 2011 guideline trustworthiness standards (see Table S1). An introduction to the GRADE process of creating and weighting clinical recommendations was delivered to all panel members and it can be viewed at the following link. (https://drive.google.com/drive/folders/1ov_mmUZwFu752yKzuxKUWA_ycq6gH6lZ) (Figure 1).
FIGURE 1.
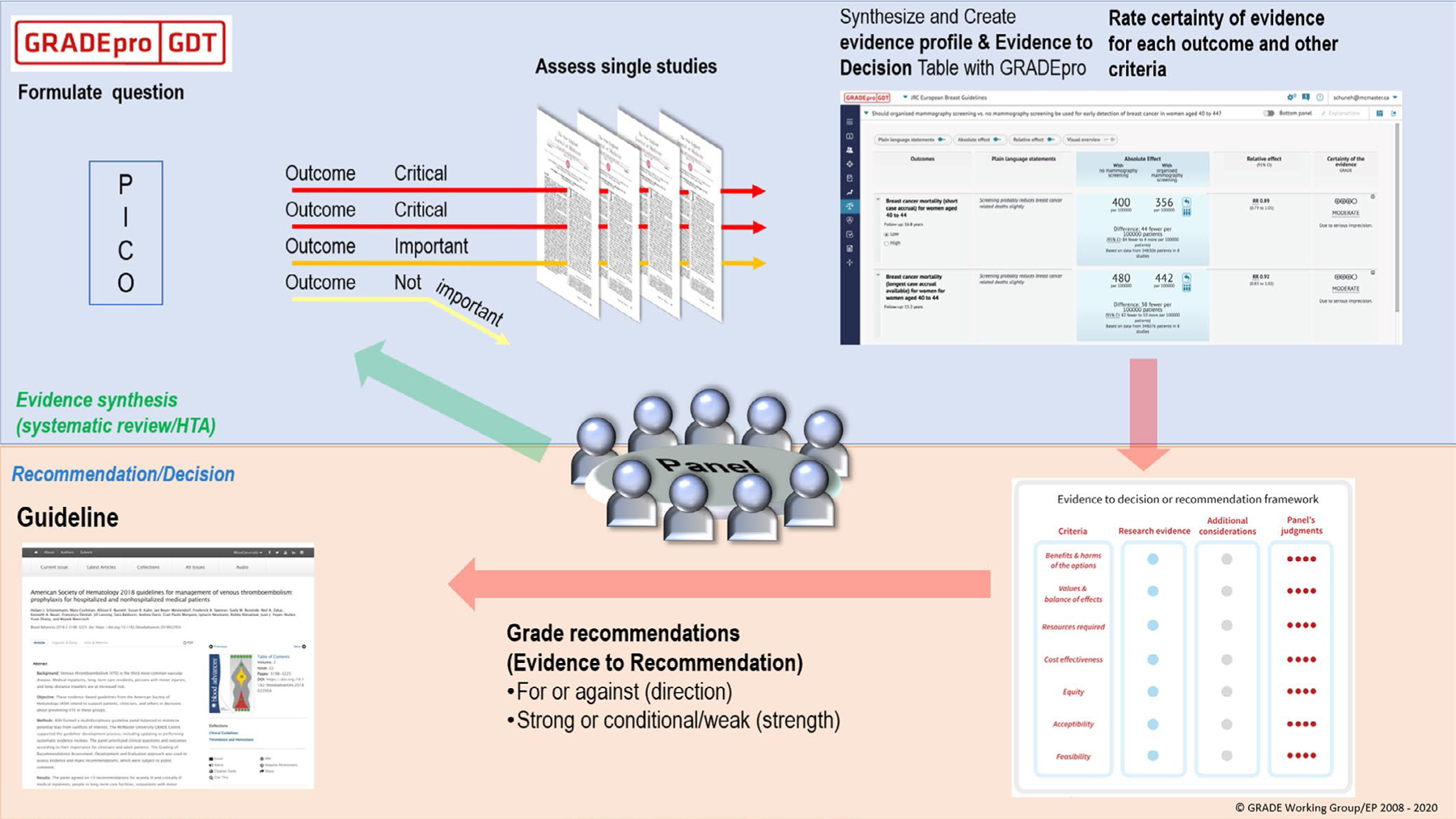
A schematic view of the GRADE approach for synthesizing evidence and developing recommendations. The upper half describe steps in the process common to systematic reviews and making health care recommendations and the lower half describe steps that are specific to making recommendations including steps from panel members to make recommendations. *Reproduced with permission by the U.S. GRADE Network. GRADE, Grading of Recommendations Assessment, Development and Evaluation
Declaration and management of competing interests
The conflict of interest (COI) review group included one of the methodologists who reviewed, evaluated, and approved all disclosures. All members of the expert panel complied with the COI process for reviewing and managing conflicts of interest, which required disclosure of any financial, intellectual, or other interest that might be construed as constituting an actual, potential, or apparent conflict, regardless of relevancy to the guideline topic. The assessment of disclosed relationships for possible COI was based on the relative weight of the financial relationship (i.e., monetary amount) and the relevance of the relationship (i.e., the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The COI review group ensured that the majority of the panel and chair were without potential relevant (related to the topic) conflicts. The chair and all members of the team were determined to have no significant conflicts of interest by the SAEM Executive Board.
Selection of questions and outcomes of interest
Clinical questions were developed into a PICO format (Population, Intervention, Comparison, Outcomes) prior to the first panel meeting.17 Panel members prioritized questions and patient-important outcomes such as 30-day major adverse cardiac events (MACE) defined as AMI, need for percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), and death.
Evidence review and development of clinical recommendations
Data sources and search strategies
A comprehensive search of several databases from inception to August 16, 2019, limited to English language only, and excluding animal studies, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced medical librarian with input from the methodologists. Controlled vocabulary supplemented with keywords was used to search for studies on recurrent chest pain. The full search strategy is available in Appendix 1.
Screening and study selection
The database found 5252 articles. The titles and abstracts were reviewed independently and in duplicate in Phase 1 by the three methodologists. A total of 334 full-text articles from the search were retrieved and reviewed in triplicate in Phase 2. Forty-four additional articles were provided by content experts and 38 were included. See Figure 2 for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of included studies.
FIGURE 2.
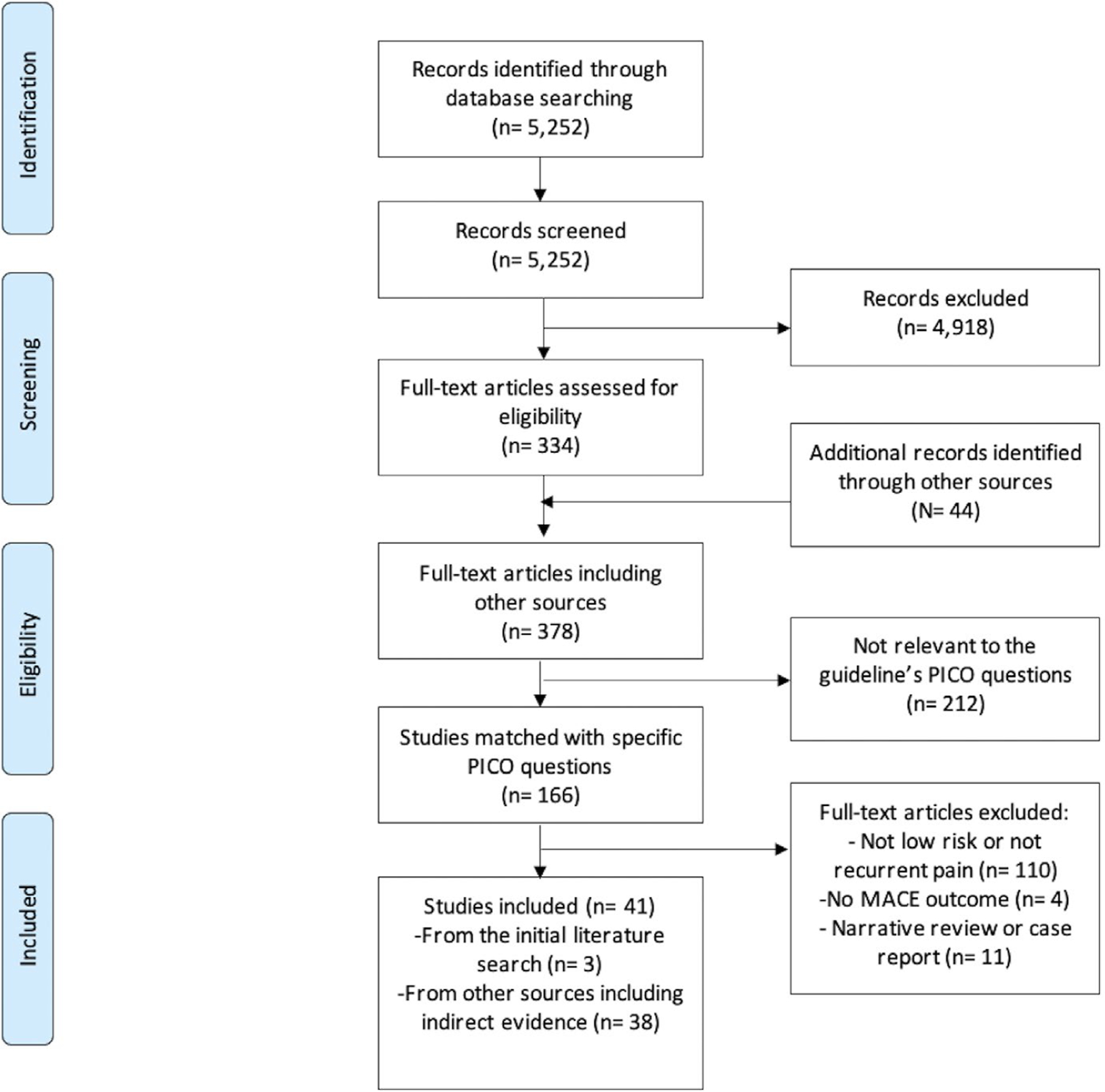
PRISMA flow diagram of studies included in this clinical guideline on emergency department patients with recurrent, low risk chest pain
The matched PICO articles were reviewed by an independent team to assess for feasibility for conduction of a systematic review. Most of the PICO questions had no relevant literature from the initial literature search that would provide an answer, and no systematic review was performed. A list of articles and reasons for exclusion is available in Appendix 2 through 6.
The studies included to inform these guidelines included systematic reviews, interventional studies and observational studies. For several interventions, no direct evidence was available, and indirect evidence was sought by all panel members. The panel either decided to include plausible indirect evidence and make a recommendation (e.g., from studies of low-risk chest pain with single troponin measurement instead of studies with a comparison arm with serial troponins) or to provide a short narrative discussion of the intervention.
Data collection and analysis
Data extracted from the available evidence included outcomes of AMI, MACE, mortality, health care use, and return ED visits. Where applicable, data were pooled using random effects model using RevMan™ or OpenMeta™. Risk of bias and certainty of evidence was assessed using the Cochrane risk-of-bias tools for randomized clinical trials (RCTs) and observational studies with modified domains used for assessing confounding bias, selection bias, and misclassification bias.18 The certainty of evidence was assessed using the GRADE approach.19 Within GRADE, the body of evidence across each outcome is assessed for domains that may reduce or increase one’s certainty in the evidence and corresponding strength of recommendation. Factors that may reduce one’s certainty include risk of bias (study limitations), inconsistency (unexplained heterogeneity across study findings), indirectness (applicability or generalizability to the research question), imprecision (the confidence in the estimate of an effect to support a particular decision) or publication bias (selective publication of studies). One’s certainty in the evidence may be strengthened if the following considerations are present: large or very large magnitude of effect, evidence of a dose-response gradient, or opposing residual confounding. GRADE summary of findings tables were developed using the GRADEpro Guideline Development Tool (https://gradepro.org/).
Evidence to recommendations
The panel considered core elements of the GRADE evidence in the decision process, including certainty of evidence and balance between desirable and undesirable effects. Additional domains were acknowledged where applicable (feasibility, resource use, acceptability). For all recommendations, the expert panelists reached consensus. Voting rules were agreed on prior to the panel meetings for situations when consensus could not be reached. The strength of a recommendation reflects the extent to which we can, across the range of patients for whom the recommendations are intended, be confident that desirable effects of a management strategy outweigh undesirable effects. As per GRADE methodology, recommendations are labeled as “strong” or “conditional.” The words “we recommend” indicate strong recommendations, and “we suggest” indicate conditional recommendations (Figure 3; Table 1).
FIGURE 3.
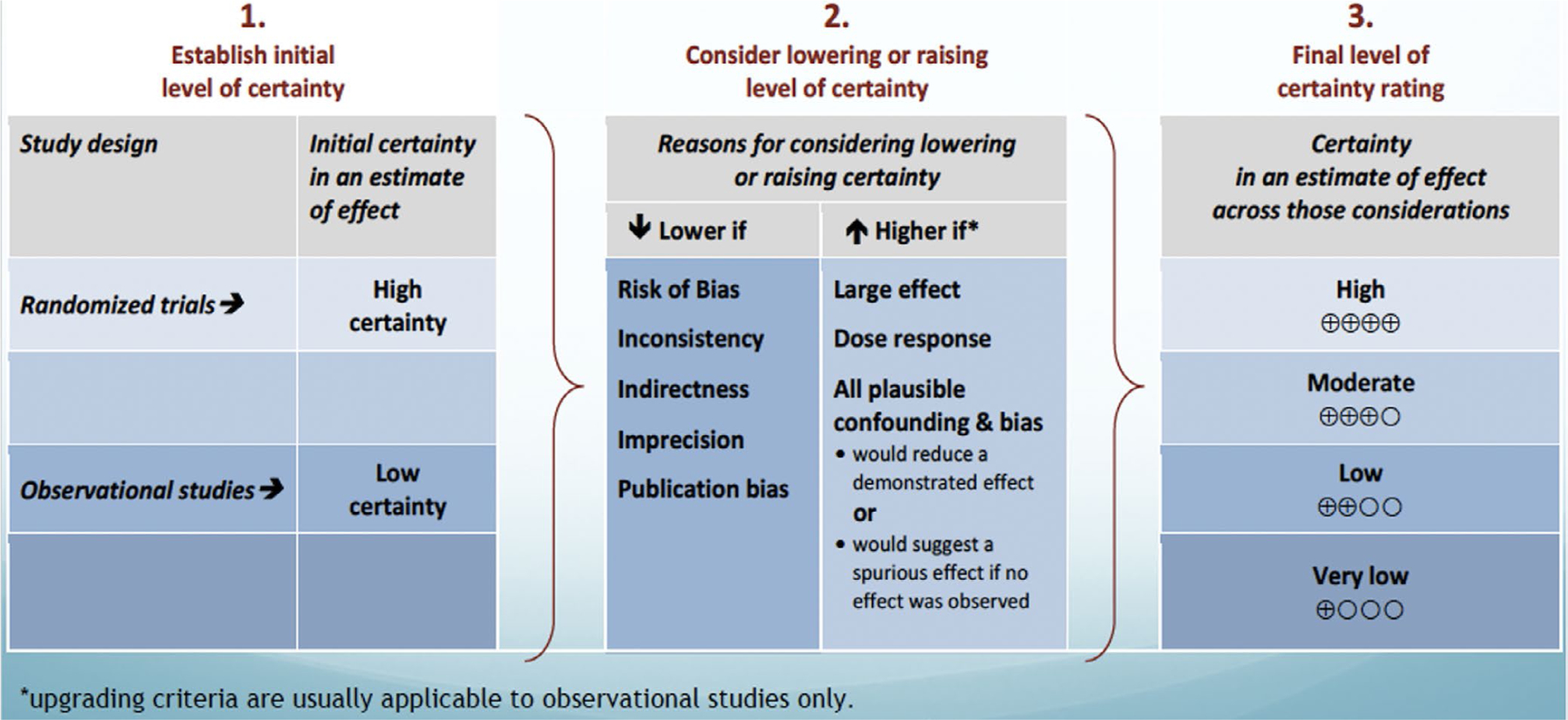
Rating the quality of evidence using the GRADE methodology. *Reproduced with permission granted by the U.S. GRADE Network
TABLE 1.
Interpretation of strong and weak recommendations for patients, clinicians, and healthcare policymakers
| Implications of strong and weak recommendations for different users of guidelines | ||
|---|---|---|
| Strong recommendation | Conditional Weak recommendation | |
| For patients | Most individuals in this situation would want the recommended course of action and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| For clinicians | Most individuals should receive the recommended course of action. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for different patients, and that you must help each patient arrive at a management decision consistent with her or his values and preferences. Decision aids may well be useful helping individuals making decisions consistent with their values and preferences. Clinicians should expect to spend more time with patients when working towards a decision. |
| For policy makers | The recommendation can be adapted as policy in most situations including for the use as performance indicators. | Policy making will require substantial debates and involvement of many stakeholders. Policies are also more likely to vary between regions. Performance indicators would have to focus on the fact that adequate deliberation about the management options has taken place. |
Note: Reproduced with permission from the GRADE Handbook.20 https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2
Use of indirect evidence
A recommendation associated with a diagnostic question follows from an evaluation of the balance between the desirable and undesirable consequences of the diagnostic test or pathway. Ideally, evidence should come from a systematic review addressing the clinical question. Inferring from accuracy data that a diagnostic test or strategy improves a patient-important outcome usually requires access to effectiveness studies.21,22 Alternatively, even with no effective treatment being available, using an accurate test may be beneficial if it reduces adverse effects, cost, or anxiety through excluding an ominous diagnosis or if confirming a diagnosis improves patient well-being from the prognostic information it imparts.23
The first Guidelines for Reasonable and Appropriate Care in the Emergency Department (GRACE-1) writing committee identified the eight questions summarized in Box 1.
BOX 1. Key questions.
1 (P) In adult patients with recurrent, low-risk chest pain, (I) is a single troponin versus (C) serial troponins needed for (O) ACS outcomes within 30 days?
2 (P) In adult patients with recurrent, low-risk chest pain, and normal or non-diagnostic stress testing within the last 12 months, (I) does repeat stress testing versus (C) no stress test have an effect on (O) MACE within 30 days?
3 (P) In adult patients with recurrent, low-risk chest pain, is (I) admission to the hospital versus (C) stay in the ED observation unit versus (C) outpatient follow up recommended for (O) ACS outcomes within 30 days?
4 (P) In adult patients with recurrent, low-risk chest pain and a negative cardiac catheterization defined as less than 50% stenosis (E) what is their risk of subsequent ACS and time to ACS?
5 (P) In adult patients with recurrent, low-risk chest pain and a negative cardiac catheterization defined as no coronary disease (0% stenosis) (E) what is their risk of subsequent ACS and time to ACS?
6 (P) In adult patients with recurrent, low-risk chest pain and a negative coronary CT angiogram (E) what is their risk of subsequent ACS and time to ACS?
7 (P) In adult patients with recurrent, low-risk chest pain, (I) what is the yield of depression and anxiety screening tools in (O) healthcare use and return ED visits?
8 (P) In adult patients with recurrent, low-risk chest pain, (I) what is the role of referral for anxiety/depression in (O) healthcare use and return ED visits?
In deriving those questions, the GRACE-1 writing committee used the following definitions:
Recurrent chest pain
This was defined as patients who have had a previous visit to an ED with chest pain that led to a diagnostic protocol for its evaluation that did not demonstrate evidence of ACS or flow-limiting coronary stenosis. This included two or more ED visits for chest pain in a 12-month period.
Low risk
Low risk was defined by HEART score <4 points24–26 (and other scores validated in the ED setting such as the HEART pathway2 or TIMI score27) for disease-related poor outcomes within 30 days all of which require an electrocardiogram (ECG) for risk stratification.
There are several key differences between the HEART score and the HEART pathway. The HEART score was developed as an easy-to-use, 0–10 score to risk stratify ED patients with chest pain. Use of the HEART score yields a ~2% MACE rate among patients with a low-risk score of 0–3.25,26 In derivation work, the HEART score incorporated a single conventional (not high-sensitivity) troponin measure, and, although rare, patients with an elevated troponin level can have a low-risk score. In addition, the HEART score can indicate low risk in patients with acute ischemic changes on ECG or known CAD. Finally, the HEART score uses subjective criteria and is manually calculated, which decreases inter-physician reliability.2 In contrast, the HEART pathway is an accelerated diagnostic protocol, which uses a modified-HEART (mHEART) score and serial troponin measurements. To be low risk by the HEART pathway, patients must meet all of the following criteria: a HEART score of 0–3, no troponin elevation on serial testing, no ischemic ECG changes, and no prior CAD (prior MI, CABG, or PCI). The HEART pathway replaces subjective components of the History and ECG components of the HEART score with objective binary questions and uses an algorithm to determine component scores. Implementation of the HEART pathway is associated with decreased hospitalizations and death and MI rates <0.5% among low-risk patients, which meets the international standard for ‘acceptable” clinical practice.28,29 One important note: the term “low risk” in GRACE-1 refers specifically to the risk for ACS or MACE in ED patients with recurrent chest pain. Thus, clinicians should continue to maintain patient-specific pretest probability for other cardiopulmonary and non-cardiac causes of chest pain such as aortic dissection, pulmonary embolism, pneumothorax, pneumonia, pancreatitis, and perforated peptic ulcer until adequately excluded to avoid anchoring bias and premature diagnostic closure.30
Expedited
This time period was defined as 3–5 days.
After synthesizing the evidence for each question in Box 1, the GRACE-1 writing committee used the GRADE Evidence to Decision Framework to formulate the recommendations summarized in Box 2. These represent reasonable and appropriate care with the understanding that individual clinicians should always use their clinical judgement in the application of these recommendations.
BOX 2. Recommendations.
Recommendation 1:
In adult patients with recurrent, low-risk chest pain, for >3 h duration we suggest a single, high-sensitivity troponin below a validated threshold to reasonably exclude ACS within 30 days. (Conditional, For) [Low level of evidence].
Recommendation 2:
In adult patients with recurrent, low-risk chest pain, and a normal stress test within the previous 12 months, we do not recommend repeat routine stress testing as a means to decrease rates of MACE at 30 days. (Conditional, Against) [Low level of evidence].
Recommendation 3:
In adult patients with recurrent, low-risk chest pain, there is insufficient evidence to recommend hospitalization (either standard inpatient admission or observation stay) versus discharge as a strategy to mitigate major adverse cardiac events within 30 days. [No evidence].
Recommendation 4:
In adult patients with recurrent, low-risk chest pain and non-obstructive (<50% stenosis) CAD on prior angiography within 5 years, we suggest referral for expedited outpatient testing as warranted rather than admission for inpatient evaluation. (Conditional, For) [Low level of evidence].
Recommendation 5:
In adult patients with recurrent, low-risk chest pain and no occlusive CAD (0% stenosis) on prior angiography within 5 years, we recommend referral for expedited outpatient testing as warranted rather than admission for inpatient evaluation. (Conditional, For) [Low level of evidence].
Recommendation 6:
In adult patients with recurrent, low-risk chest pain and prior CCTA within the past 2 years with no coronary stenosis, we suggest no further diagnostic testing other than a single, high-sensitivity troponin below a validated threshold to exclude ACS within that 2-year time frame. (Conditional, For) [Moderate level of evidence].
Recommendation 7:
In adult patients with recurrent, low-risk chest pain, we suggest the use of depression and anxiety screening tools as these might have an effect on healthcare use and return ED visits. (Conditional, Either) [Very low level of evidence].
Recommendation 8:
In adult patients with recurrent, low-risk chest pain, we suggest referral for anxiety or depression management, as this might have an impact on healthcare use and return ED visits. (Conditional/Either) [Low level of evidence].
QUESTION 1
In adult patients with recurrent, low-risk chest pain, is a single troponin versus serial troponins needed for ACS outcomes within 30 days?
Summary of the evidence
There were no studies with direct evidence to answer this question. Additional searches for indirect evidence yielded no relevant studies that evaluated a single troponin in ED patients with recurrent chest pain with 30-day MACE outcome. Additional search for indirect evidence yielded nine studies31–39 that evaluated a single troponin in ED patients with low-risk chest pain with 30-day MACE outcome. (Appendix 2) Nearly all of these studies evaluated the use of a single high-sensitivity troponin measurement, with only one small, single-center study evaluating a single conventional troponin measurement.33 Thus, insufficient evidence exists to recommend for or against a single conventional troponin measurement in patients with recurrent, low-risk chest pain. High-sensitivity troponin assays measure cTn levels within the normal range in at least 50% of healthy individuals with high precision, defined as a coefficient of variance (CV) ≤ 10% at the 99th percentile.40,41 Among the nine studies with indirect evidence, 72 out of 15,715 patients (0.5%) with low-risk chest pain had an AMI or MACE within 30 days (each of the 9 studies had variable definitions of normal troponin, for details please see appendix 2). These studies did not address the “recurrence” of chest pain, and it is possible that patients who are low risk with recurrent chest pain are likely to have even lower risks of AMI or MACE. Several additional studies were found with indirect evidence for the use of a single troponin in a mixed-risk group of ED patients with 30-day MACE outcomes; however, these studies did not address the question of recurrent chest pain or low-risk populations, so were not included (Table 2).
TABLE 2.
Evidence table for Question 1–In adult patients with recurrent, low-risk chest pain, is a single troponin versus serial troponins needed for acute coronary syndrome outcomes within 30 days?
| Certainty assessment |
No. of patients |
Effect |
Certainty |
Importance |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Single troponin | Outcome rate (95% CI) | ||
| 9 | Observational | Not seriousa | Not serious | Seriousb | Not serious | Strong association | 72/15,717 (0.5%)c | 3.4 per 1000 patients (2.0–4.8 per 1000) | ⨁⨁◯◯ LOW |
IMPORTANT |
Abbreviation: CI, confidence interval.
For guideline panels: The quality of evidence reflects the extent to which our confidence in an estimate of the effect is adequate to support a particular recommendation. Guideline panels must make judgments about the quality of evidence relative to the specific context for which they are using the evidence.
Studies do not compare single troponin versus serial troponin, but report the outcome of cohorts where a single troponin was measured with different negative thresholds.
Outcome rate is 3.4 per 1,000 patients (95%CI 2.0 to 4.8 per 1,000)
Benefits
The most substantial benefit to both patients and hospitals in performing a single troponin is shorter ED length of stay.42 Patients are also spared serial phlebotomy, and hospitals may have lower healthcare costs by performing fewer troponin tests. These benefits were not addressed by the included studies.
Harms and burden
The harms are missed AMI and MACE that could potentially be diagnosed if serial troponins were drawn instead of a single troponin. We calculated the pooled incidence of 30-day AMI or MACE following a single, normal high sensitivity troponin using indirect evidence as 3.4 per 1000 patients (95% confidence interval [CI] 2.0–4.8 per 1000) as shown in the Forest plot section of Appendix 2.
Decision criteria and additional considerations
Patients prefer shorter ED lengths of stay. Identifying AMI is essential for patients, clinicians, and policymakers. Patients with missed or delayed AMI diagnosis may develop complications such as conduction disturbances, arrhythmias, heart failure, myocardial rupture, ventricular aneurysm, pericarditis, and death. Clinicians may be subject to medical malpractice suits for missed or delayed diagnoses of AMI as AMI is one of the most common diagnoses in lawsuits against EM physicians.8 Professional societies such as the American Heart Association (AHA) and European Society of Cardiology (ESC) currently differ in their guidelines for the use of a single troponin, with the AHA recommending serial troponins and the ESC providing an option for a single troponin to exclude AMI,15,43,44 and physicians are using single conventional and high-sensitivity cardiac troponin testing in clinical practice.45,46 These differences in guidelines likely reflect differences in the adoption of high-sensitivity troponin assays, which have been used internationally for nearly a decade but only approved for use in the United States in 2017. Despite the increased incorporation of high-sensitivity troponin into accelerated diagnostic protocols, the authors acknowledge that many EDs may currently only have access to conventional troponin at this time. Additionally, timing of chest pain onset relative to troponin testing was agreed to be an important criterion for using a single troponin strategy, as ESC guidelines recommend chest pain duration of at least 3 h from symptom onset to troponin testing.44,47
Conclusions and research needs
The use of a single troponin with result below a validated threshold appears to be sufficient to decrease the risk of AMI or MACE to a clinically acceptable threshold in patients with low-risk chest pain as measured by clinical prediction scores such as the mHEART score.
Recommendation 1: In adult patients with recurrent, low-risk chest pain, for >3 h duration we suggest a single, high-sensitivity troponin below a validated threshold to reasonably exclude ACS within 30 days. (Conditional, For) [Low level of evidence].
QUESTION 2
In adult patients with recurrent, low-risk chest pain, and normal or non-diagnostic stress testing within the last 12 months, does repeat stress testing versus no stress test have an effect on MACE within 30 days?
Summary of the evidence
The authors assessed literature pertaining to stress testing in general (both exercise and pharmacologic). There were no studies with direct evidence to answer this question. An additional literature search for indirect evidence focusing on stress testing for adult ED patients with non-high-risk chest pain, not necessarily recurrent and without recent testing specified, yielded two randomized studies.48,49 Neither of these two studies used MACE as a primary outcome but did report on a similar, more broadly defined outcome termed “cardiac events” at 30 days. An additional literature search for indirect evidence focusing on outpatient stress testing for adult patients discharged from the ED after a visit for chest pain, not necessarily recurrent and without recent testing specified, yielded one multicenter, retrospective study.50 This study sought to compare the incidence of MACE at 30 days between patients who completed an outpatient stress test within 3 days, 4–30 days, or not at all.
Among the three studies with indirect evidence, zero out of 4906 ED patients (0%) with chest pain died at 30 days, while 91 out of 4906 patients (1.9%) experienced a non-fatal, significant cardiac event. These significant cardiac events included MI, ventricular fibrillation, acute pulmonary edema requiring intubation, cardiogenic shock, coronary revascularization (i.e. PCI or CABG), and significant CAD on coronary angiogram (Table 3).
TABLE 3.
Evidence table for Question 2–In adult patients with recurrent, low-risk chest pain, and normal or non-diagnostic stress testing within the last 12 months, does repeat provocative stress testing versus no stress test have an effect on major adverse cardiac events within 30 days?
| Certainty assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Repeat provocative stress testing | No stress testing | Relative (95% CI) | Absolute (95% CI) | Certainty | Importance |
| Death (follow up: 30 days) | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Seriousa | Not serious | None | 0/1056 (0.0%) | 0/557 (0.0%) | Not estimable | ⨁⨁⨁◯ MODERATE |
CRITICAL | |
| Death (follow up: 30 days) | ||||||||||||
| 1 | Observational | Seriousa | Not serious | Seriousa | Not serious | None | 0/2497 (0.0%) | 0/796 (0.0%) | Not estimable | ⨁⨁◯◯ LOW |
CRITICAL | |
| Cardiac event (follow up: 30 days) | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Seriousa | Not serious | None | 4/1004 (0.4%) | 4/504 (0.8%) | OR 0.50 (0.13–2.00) | 4 fewer per 1000 (from 7 fewer to 8 more) | ⨁⨁⨁◯ MODERATE |
IMPORTANT |
| Significant CAD | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Seriousa | Not serious | None | 36/1004 (3.6%) | 15/504 (3.0%) | OR 1.20 (0.61–2.17) | 6 more per 1000 (from 11 fewer to 33 more) | ⨁⨁⨁◯ MODERATE |
IMPORTANT |
| MACE (follow up: 30 days) | ||||||||||||
| 2 | Randomized trials | Seriousb | Not serious | Seriousa | Not serious | None | 27/2549 (1.1%) | 5/849 (0.6%) | OR 1.81 (0.69–4.71) | 5 more per 1000 (from 2 fewer to 21 more) | ⨁⨁◯◯ LOW |
CRITICAL |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; MACE, major adverse cardiac events; OR, odds ratio.
Not recurrent low-risk chest pain and does not assess this outcome for those with previous stress testing within 12 months.
One non-randomized interventional trial.
Several additional studies were found with indirect evidence for the use of stress testing in a mixed-risk group of ED patients with chest pain.51–54 However, these studies lacked a control group or were limited to patients under age 40. For these reasons, these studies were not included (Appendix 3).
Benefits
The potential benefit of stress testing for ED patients with recurrent, low-risk chest pain would be to identify intervenable CAD to reduce the incidence of MACE. The two randomized studies that indirectly addressed this question in a similar but not identical population of ED chest pain patients did not demonstrate a reduction in significant cardiac events (described above), including MACE, at 30 days.48,49 The multicenter, retrospective cohort study evaluating the incidence of MACE at 30 days in patients who completed an outpatient stress test did not demonstrate any associated benefit of stress testing within 3 days, nor within 30 days, compared with not undergoing a stress test at all.50
Harms and burden
The harms of stress testing for ED patients with recurrent, low-risk chest pain include the potential for an allergic reaction, radiation exposure, procedural risks, and indirect harm from downstream testing and procedures. The burdens on the patient could include avoidable out-of-pocket expenses (depending on health insurance benefits); the inconvenience of time spent in the ED, observation unit, or hospital waiting for the test; psychological stress associated with false-positive test results; and additional burdens associated with downstream testing, such as coronary angiography. From a resource utilization perspective, stress testing in an observation unit led to greater total charges at 30 days as compared with direct discharge from the ED.48
Decision criteria and additional considerations
Additional considerations include the financial incentives around stress testing that may impact health systems and thus decision-making for this cohort of patients. Perceived medico-legal risk by ED clinicians may also promote the ordering of low-yield stress testing.55 Patient preferences could also influence this decision since certain patients may only be reassured by repeat stress testing, while others may prefer to be discharged home after laboratory and ECG testing has reasonably excluded a myocardial infarction.56 Thus, shared decision-making may be reasonable in this clinical scenario.57 A majority (~85%) of men and women with potential ACS would accept a stress test if recommended by their physician and most preferred a stress test over cardiac catheterization.58
Different types of cardiac stress testing (e.g., exercise, pharmacological, nuclear, echocardiographic) exhibit different accuracies,59 and thus a different type of stress testing or other evaluation modality such as coronary computed tomographic angiography (CCTA) or angiography may be considered if there is concern for false-negative results on a previous stress test. Although most included studies did not define all parameters for a normal or negative test, to be conservative we are defining a normal stress test as achieving target heart rate for an exercise test or an imaging test without ischemic ECG or imaging abnormalities. Similarly, if the previous stress test was deemed inadequate (as locally defined) or was ended prematurely, repeat stress testing may be considered.
Access to care is also a consideration since outpatient stress testing may be more difficult to obtain for certain patients based on socioeconomic factors.60 Clinicians may be more likely to offer same-day stress testing or admission, hospital or observation, for patients who are at higher risk of loss to follow-up.
Conclusions and research needs
Although no studies directly addressed the exact PICO question above, the three studies included in this review indirectly addressed a similar question. Extrapolating from these studies’ results, repeated provocative stress testing is unlikely to reduce the risk of MACE at 30 days in patients with recurrent, low-risk chest pain. Further research to answer this exact question would need to focus specifically on ED patients with recurrent, low-risk chest pain with normal stress testing within the last 12 months.
Recommendation 2: In adult patients with recurrent, low-risk chest pain, and a normal stress test within the previous 12 months, we do not recommend repeat routine stress testing as a means to decrease rates of MACE at 30 days. (Conditional, Against) [Low level of evidence].
QUESTION 3
In adult patients with recurrent, low-risk chest pain, is admission to the hospital versus stay in the ED observation unit versus outpatient follow up recommended for ACS outcomes within 30 days?
Summary of the evidence
No articles were available, and no indirect evidence was found.
Conclusions and research needs
The acceptable miss rate of ACS outcomes in patients who are deemed low risk is largely agreed to be <1%.29 Healthcare costs associated with the ED evaluation of chest pain are significant and it may be worthwhile to consider that cost when making disposition decisions. The cost of admission for the evaluation of chest pain has been shown to be greater than that of evaluation provided in an observation unit with the most significant cost benefit associated with discharge from the ED.61–63
Recommendation 3: In adult patients with recurrent, low-risk chest pain, there is insufficient evidence to recommend hospitalization (either standard inpatient admission or observation stay) versus discharge as a strategy to mitigate major adverse cardiac events within 30 days. [No evidence].
Note that there is no GRADE software evidence table for this question in the appendix due to lack of evidence.
QUESTION 4
In adult patients with recurrent, low-risk chest pain and a negative cardiac catheterization defined as less than 50% stenosis what is their risk of subsequent ACS and time to ACS?
Cardiac catheterizations are frequently performed procedures and are indicated for several reasons, including the diagnosis of coronary stenosis and occlusion in the setting of ACS. However, there are no clearcut guidelines for if and when catheterization should be performed in the setting of recurrent chest pain. There is variation in how often this procedure is performed, and even debate as to the appropriateness of these procedures in some cases.64,65 In fact, some studies suggest that high rates of normal or negative diagnostic catheterizations indicate opportunities for improved patient selection for the procedure.66 On an individual level, both patient and physician preferences influence the decision to undertake this procedure. Females are less likely to be recommended for cardiac catheterization by their physicians, and are also less likely to prefer this as a diagnostic test for their chest pain symptoms.58,67 These factors may have contributed to the lack of direct evidence for this series of PICO questions.
Summary of evidence
The evidence informing this question is based on data from one meta-analysis and a few retrospective observational studies. Only the meta-analysis by Radico68 (2018, 54 studies with 35,039 patients, 99,770 person-years and median follow-up 5 years) separately analyzed those patients with non-obstructive CAD (<50%) versus those with 0% stenosis (which is the next question addressed by this guideline). Statistical heterogeneity was high (I2 = 93%) for studies with less-than-obstructive CAD patients. It should be noted that this meta-analysis included studies using both invasive cardiac catheterization (the topic of questions 4 and 5) as well as CCTA (the topic of question 6), thus each article in the meta-analysis was reviewed and we extracted the relevant articles using cardiac catherization for inclusion as evidence for this question (Table 4).
TABLE 4.
Evidence table for Question 4–In adult patients with recurrent, low-risk chest pain and a cardiac catheterization with <50% stenosis what is their risk of subsequent acute coronary syndrome?
| Certainty assessment |
|||||||
|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Certainty |
| All-cause mortality and MI | |||||||
| 23a | Observational studies | Seriousb | Very seriousc | Not seriousd | Not serious | Strong association | ⨁⨁◯◯ Low |
| Non-fatal MI | |||||||
| 23 | Observational studies | Seriousb | Very seriouse | Not serious | Not serious | Strong association | ⨁⨁◯◯ LOW |
| All-cause mortality | |||||||
| 23 | Observational studies | Seriousb | Very seriouse | Not serious | Not serious | Strong association | ⨁⨁◯◯ LOW |
Abbreviations: CT, computed tomography; MI, myocardial infarction.
Median follow-up of 5 years (interquartile range 3–7 years; range 1–10 years)
Most included studies were considered as high .risk of bias.
The authors included studies using diagnostic angiograms as well as coronary CT angiograms. There was variable follow up from 1 to 10 years. Studies with follow up <1 year were excluded.
Overall included population had higher cardiovascular risk (enough to require an angiography).
I2 = 91% very heterogeneous results.
The incidence of all-cause mortality and MI was 1.32/100 person-years, (95% CI 1.02–1.62), meaning 1.32 cases are expected for 100 patients followed for 1 year, or 0.66 cases for 100 patients followed for 2 years.69–73
The other observational studies did not separately analyze those without any stenosis versus those with <50% stenosis.69–73 The data available suggest that the likelihood of non-fatal MI or all-cause mortality within 5 years is very low in this group of patients with coronary stenosis between 1% and 49% (Appendix 4).
Benefits
The potential benefit of cardiac catheterization for ED patients with recurrent, low-risk chest pain would be to potentially detect a structural cause of chest pain. Stress testing and cardiac catheterization provide functional versus structural/anatomical information regarding cardiac causes of chest pain. In addition, a cardiac catheterization allows for an intervention if a stenotic lesion is found. Finally, catheterization may provide some reassurance to the patient and provide the perception that their symptoms have been fully evaluated.56
Harms and burden
The harms of cardiac catheterization for ED patients with recurrent, low-risk chest pain include the potential for an allergic reaction to contrast, radiation exposure of fluoroscopy, procedural risks, and complications. These risks include bruising, bleeding, perforation, arrhythmias and contrast nephropathy, and very rarely, death. The burdens on the patient could include avoidable out-of-pocket expenses, travel time, and recovery time.
Conclusions and future research needs
The data available suggest that the likelihood of non-fatal MI or all-cause death within 5 years is low in this group. It is reasonable to avoid low-yield admission or inpatient observation for such patients and they could be referred for expeditious outpatient evaluations after discharge. Ongoing research is needed to follow outcomes in such patients, using standard definitions for the outcomes of interest (MACE, death) and timeframes for follow-up. Incorporating patient values, preferences, and equity considerations into such research is also important, as are the resource utilization issues with such lower-risk populations.74
Recommendation 4: In adult patients with recurrent, low-risk chest pain and non-obstructive (<50% stenosis) CAD on prior angiography within 5 years, we suggest referral for expedited outpatient testing as warranted rather than admission for inpatient evaluation. (Conditional, For) [Low level of evidence].
QUESTION 5
In adult patients with recurrent, low-risk chest pain and a negative cardiac catheterization defined as no coronary disease (0% stenosis) what is their risk of subsequent ACS and time to ACS?
Summary of evidence
The data available suggest that the likelihood of non-fatal MI or all-cause death within 5 years is very low in this group. The evidence informing this question is based on data from one meta-analysis, and a number of retrospective studies. Only the meta-analysis by Radico (2018, 54 studies, 35,039 patients, 99,770 person-years and median follow-up 5 years) separately analyzed those patients with non-obstructive CAD (<50%) versus those with 0% stenosis. It should be noted that this meta-analysis included studies using both invasive cardiac catheterization (the topic of questions 4 and 5) as well as CCTA (the topic question 6), thus each article in the meta-analysis was reviewed and we extracted the relevant articles using cardiac catherization for inclusion as evidence for this question (Table 5).
TABLE 5.
Evidence table for Question 5–Inadult patients with recurrent, low-risk chest pain and a negative cardiac catheterization defined as no coronary disease (0% stenosis) what is their risk of subsequent acute coronary syndrome?
| Certainty assessment |
|||||||
|---|---|---|---|---|---|---|---|
| No.of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Certainty |
| All-cause mortality and MI | |||||||
| 27 | Observational studies | Seriousa | Very seriousb | Not seriousc | Not serious | Strong associationd | ⨁⨁◯◯ LOW |
| Non-fatal MI | |||||||
| 24 | Observational studies | Seriousa | Very seriouse | Not serious | Not serious | Strong association | ⨁⨁◯◯ LOW |
| All-cause mortality | |||||||
| 24 | Observational studies | Seriousa | Very seriouse | Not serious | Not serious | Strong association | ⨁⨁◯◯ LOW |
Abbreviation: MI, myocardial infarction.
Most included studies were considered as high risk of bias.
The authors included studies using diagnostic angiograms as well as coronary CT angiograms. There was variable follow up from 1 to 10 years. Studies with follow up <1 year were excluded.
Overall included population had higher cardiovascular risk (enough to require an angiography).
Median follow up was 5 years (interquartile range 3–7 years).
I2 = 91% very heterogeneous results.
The incidence of all-cause mortality and MI was 0.52/100 person-years, (95% CI 0.34–0.71), meaning 0.52 cases are expected for 100 patients followed for 1 year, or 0.26 cases for 100 patients followed for 2 years. Statistical heterogeneity was moderate (I2 = 64%) for studies of patients with normal coronary arteries. The other observational studies did not separately analyze those without any stenosis versus those with <50% stenosis. (Appendix 4).
Benefits
The potential benefit of cardiac catheterization for ED patients with recurrent, low-risk chest pain would be to potentially detect a structural cause of chest pain. Stress testing and cardiac catheterization provide functional versus structural/anatomical information regarding cardiac causes of chest pain. In addition, a cardiac catheterization allows for an intervention if a stenotic lesion was found. Finally, catheterization may provide some reassurance to the patient and provide the perception that their symptoms have been fully evaluated.
Harms and burden
The harms of cardiac catheterization for ED patients with recurrent, low-risk chest pain include the potential for an allergic reaction to contrast, radiation exposure of fluoroscopy, procedural risks, and complications, as described previously. The burdens on the patient could include avoidable out-of-pocket expenses, travel time, and recovery time.
Conclusions and future research needs
Given the low 5-year rates of non-fatal MI and all-cause mortality in patients with a negative cardiac catheterization (0% stenosis), it is advisable to avoid low-yield admission or inpatient observation for such patients, and they could be referred for expeditious outpatient evaluations after discharge. Ongoing research is needed to follow outcomes in such patients, using standard definitions for outcomes of interest (MACE and death) and timeframes for follow-up. Incorporating patient values, preferences, and equity considerations into such research is also important, as are the resource utilization issues with such low-risk populations.74
Recommendation 5: In adult patients with recurrent, low-risk chest pain and no occlusive CAD (0% stenosis) on prior angiography within 5 years, we recommend referral for expedited outpatient testing as warranted rather than admission for inpatient evaluation. (Conditional, For) [Low level of evidence].
QUESTION 6
In adult patients with recurrent, low-risk chest pain and a negative coronary CT angiogram what is their risk of subsequent ACS and time to ACS?
Summary of the evidence
There were no studies with direct evidence to answer this question. Additional searches for indirect evidence yielded 18 relevant studies that evaluated the prognostic value of CCTA for predicting long-term coronary events in patients who had previously undergone CCTA for evaluation of potential ischemic symptoms. Data were derived from randomized trials evaluating CCTA versus standard of care or another imaging technique (stress myocardial perfusion imaging or stress echocardiography), ED observation studies, and registries that included patients who underwent CCTA for assessing patients with possible ischemic symptoms. In general, the studies excluded patients who had known coronary disease and those who had contraindications to CCTA such as renal failure and arrhythmias. Consideration as to how a “negative” CCTA is defined is important. Most studies separated results into those with normal scans (0% stenosis), and those with mild stenosis (1%–49% stenosis). Assessing long-term outcomes is difficult, as almost all ED trials focused on outcomes based on the specific CCTA strategy used for evaluating low to intermediate-risk patients as opposed to reporting outcomes based on the degree of stenosis present on the CCTAs. Two studies that included patients who underwent outpatient evaluation for possible myocardial ischemia reported long term results. The CONFIRM Registry75 reported that patients who had a normal (negative) CCTA were at very low-risk, with a rate of MI of 0.08%, all-cause mortality of 0.4%, and a MACE rate of 0.6% at a median of 2.1 years follow-up. Patients who had 1%–49% stenosis had corresponding event rates of 1.3%, 0.5%, and 2.4%, respectively. In the PROMISE Trial,76 patients with a normal CCTA had a rate of cardiovascular death and MI of 0.3% during a median follow-up of 26 months. Those who had a mildly abnormal CCTA (1%–49% stenosis) had a corresponding event rate of cardiovascular death and MI of 1.6%.
In the PROMISE Trial, among the subset of 3800 patients who had a negative test, 8% subsequently underwent secondary noninvasive testing within 90 days of the CCTA, while 4% had coronary angiography.76 However, the results of the secondary test results were not reported. These studies did not address the “recurrence” of chest pain; it would be expected that patients who are low risk with an initial negative ischemic evaluation, including CCTA, would likely have even lower risks of AMI or MACE than observed in CONFIRM and PROMISE (Table 6).
TABLE 6.
Evidence table for Question 6–In adult patients with recurrent low risk chest pain and a negative coronary computed tomography angiogram what is their risk of subsequent acute coronary syndrome/time to acute coronary syndrome?
| Certainty assessment |
No. of patients |
Certainty |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other Considerations | MACE | NO MACE |
|
| Coronary CT with no disease (follow-up 30 days to 2 years) MACE | |||||||||
| 6 | Observational | Serious | Not serious | Serious | Not serious | None | 3 | 1444 | ⨁⨁⨁◯ MODERATE |
| Coronary CT with 1%–49% stenosis (follow-up 30 days to 2 years) MACE | |||||||||
| 9 | Observational | Serious | Not Serious | Serious | Not Serious | None | 6 | 2377 | ⨁⨁⨁◯ MODERATE |
| Coronary CT with no disease (follow-up 30 days to 2 years) MACE | |||||||||
| 2 | Randomized trials | Not serious | Not serious | Serious | Not serious | None | 2 | 468 | ⨁⨁⨁◯ MODERATE |
| Coronary CT with 1%–49% stenosis (follow-up 30 days to 2 years) MACE | |||||||||
| 2 | Randomized trials | Not serious | Not serious | Serious | Serious | None | 1 | 828 | ⨁⨁⨁◯ MODERATE |
| Coronary CT with no disease (follow-up 2 years) MACE non-ED patients | |||||||||
| 2 | Observational | Serious | Not serious | Serious | Not serious | None | 33 | 5679 | ⨁⨁⨁◯ MODERATE |
| Coronary CT with 1%–49% stenosis (2 years) MACE non-ED patient | |||||||||
| 2 | Observational | Serious | Not serious | Serious | Not serious | None | 119 | 4382 | ⨁⨁⨁◯ MODERATE |
Abbreviations: CT, computed tomography; ED, emerhency department; MACE, major adverse cardiac events.
Benefits
The benefits of a negative ED CCTA are to accurately exclude 30-day MACE rates and decrease admission to the hospital or observation unit. Longer-term benefits are that patients who have a normal CCTA or one with only mild stenosis who return with recurrent symptoms do not require extensive ischemic evaluation, thereby reducing costs. The indirect evidence supports this benefit, as the risk for later MI and death is very low.
Harms and burden
The harms of ED CCTA are associated with possible contrast reactions as well as radiation exposure. Multiple epidemiological studies have shown an increased risk of breast, blood and thyroid cancers after a single computed tomography (CT) scan of the chest, especially in young persons. Longitudinal, population-based studies estimate that for every 10,000 CT scans done in patients under 40 years of age, approximately 35 will develop cancer.77–83 This risk is increased with repeated CT scans, female sex and younger age.83–85 At the patient level, one study has estimated a 0.07% increased lifetime risk of cancer for a single CT scan of the chest and a 0.12% increased lifetime risk of cancer for nuclear myocardial perfusion imaging.86 Simulation modeling has estimated the life-time cancer risk for a standard CCTA to vary from 1 in 143 for a 20-year-old woman to 1 in 3261 for an 80-year-old man.87 The burdens are higher charges and longer ED length of stay, compared to a no testing strategy, which likely exacerbate patient’s healthcare bills and ED crowding.
Decision criteria and additional considerations
Defining the optimal testing strategy for patients who had a prior negative CCTA who return with recurrent chest pain is important. Accurately identifying the time period during which a negative CCTA remains valid is also important.
Conclusions and research needs
Based upon indirect evidence, we do not recommend routine repeat evaluation other than a troponin and ECG for patients with recurrent, low-risk chest pain after a negative CCTA within 2 years. Additional research explicitly evaluating the risk of ischemic events in patients who have recurrent, low-risk chest pain after negative ischemic testing is important.
Recommendation 6: In adult patients with recurrent, low-risk chest pain and prior CCTA within the past 2 years with no coronary stenosis, we suggest no further diagnostic testing other than a single, high-sensitivity troponin below a validated threshold to exclude ACS within that 2-year time frame. (Conditional, For) [Moderate level of evidence].
QUESTION 7
In adult patients with recurrent, low-risk chest pain, what is the yield of depression and anxiety screening tools in healthcare use and return ED visits?
Summary of the evidence
With regard to depression screening, we were unable to identify any studies with direct evidence to answer this question. We were able to identify one study for inclusion to provide indirect evidence.88 This prospective cohort study of low-to-moderate cardiac risk ED patients admitted to a chest pain observation unit showed an increased risk of chest pain recurrence among ED chest pain patients who screened positive for clinically significant depression (odds ratio [OR] 2.11; 95% CI 1.18–3.79), as well as increased ED return visits and increased healthcare use.89 However, this study had a small sample size (n = 365). The depression screening tool used in this study was the validated 8-item Patient Health Questionnaire depression scale (PHQ-8), which has been shown to have a sensitivity and specificity for major depressive disorder in the general population of 100% and 95% respectively for a PHQ-8 score ≥10 along with a likelihood ratio of a positive test of 28.89
For anxiety screening, three studies were included to provide indirect evidence.88,90,91 Each was a prospective observational cohort study of low-to-moderate risk chest pain patients evaluated in the ED or ED observational unit. One study88 reported no increased risk of chest pain recurrence among patients with anxiety (adjusted OR 1.59; 95% CI 0.80–3.79), while another study90 found increased risk of chest pain recurrence with an OR of 2.36 (95% CI 1.09–5.15). Regarding return ED visits, two studies88,90 showed increased risk of ED return in patient screening positive for anxiety, while one study91 showed no difference in return visits. Regarding overall healthcare use, one study91 showed no significant difference among the two groups (18.2% vs. 24.8%) (Tables 7 and 8).
TABLE 7.
Evidence tables for Question 7–In adult patients with recurrent, low-risk chest pain, what is the yield of depression and anxiety screening tools in healthcare use and return ED visits?
| Question 7a—What is the yield of depression screening for adult patients with recurrent low-risk chest pain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty assessment |
No. of patients |
Effect |
|||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Depression | No Depression | Relative (95% CI) | Absolute (95% CI) | Certainty |
| Healthcare use/30 day hospital return | |||||||||||
| 1a | Observational | Seriousb,c | Not seriousd | Seriouse | Not serious | None | 5/131 (3.8%) | 0/234 (0.0%) | OR 19.60 (1.08–357.60) | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁◯◯◯ VERY LOW |
| Return ED visits | |||||||||||
| 1 | Observational | Seriousb,c | Not seriousd | Seriouse | Not serious | None | 10/131 (7.6%) | 0/234 (0.0%) | OR 37.50 (2.18–644.30) | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁◯◯◯ VERY LOW |
| Chest pain recurrence | |||||||||||
| 1 | Observational | Seriousb,c | Not seriousd | Seriouse | Not serious | None | OR 2.11 (1.18–3.79) | 2 fewer per 1000 (from 4 fewer to 1 fewer) | ⨁◯◯◯ VERY LOW |
||
| Healthcare use/primary care physician follow up | |||||||||||
| 1a | Observational | Seriousb | Not serious | Not serious | Not serious | None | 91/131 (69.5%) | 151/234 (64.5%) | OR 1.08 (0.77–1.51) | 17 more per 1000 (from 62 fewer to 88 more) | ⨁◯◯◯ VERY LOW |
Abbreviations: CI, confidence interval; ED, mergency department; OR, odds ratio.
Kim et al.88
Kim et al.88: Stratified by recurrent chest pain (CP) versus non-recurrent chest pain (NRCP) not by anxiety–depression screening status. Selection bias of subjects admitted to observation unit with low-mod risk (not all comers with CP). Excludes very low-risk CP. Self-reported chest pain recurrence. Potential recall bias.
Depression/anxiety tools are self-administered questionnaires. 90% of recurrent and 84% of non-recurrent CP had anxiety.
Inconsistency is low or not applicable when there is only one study involved.
Kim et al.88: The population is adequate (recurrent low-risk chest pain) however the study is looking at association of depression with outcome and not at screening for depression, so it is more indirect evidence.
TABLE 8.
Evidence tables for Question 7–In adult patients with recurrent, low-risk chest pain, what is the yield of depression and anxiety screening tools in healthcare use and return ED visits?
| Question 7b—What is the yield of anxiety screening for adult patients with recurrent low-risk chest pain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty assessment |
No. of patients |
Effect |
|||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Anxiety | No Anxiety | Relative (95% CI) | Absolute (95% CI) | Certainty |
| Healthcare use/PCP follow up | |||||||||||
| 1a | Observational | Seriousb | Not serious | Seriousb | Seriousc | None | 4/22 (18.2%) | 32/129 (24.8%) | OR 0.73 (0.24–2.28) | 54 fewer per 1000 (from 175 fewer to 181 more) | ⨁◯◯◯ VERY LOW |
| ED return | |||||||||||
| 2 | Observational | Seriousd | Not serious | Not serious | Not serious | None | OR 3.22 (1.53–6.80) | 3 fewer per 1000 (from 7 fewer to 2 fewer) | ⨁◯◯◯ VERY LOW |
||
| Chest pain recurrence | |||||||||||
| 1e | Observational | Seriousf | Not serious | Not serious | Seriousg | None | 38/56 (67.9%) | 25/53 (47.2%) | OR 1.59 (0.80–3.79) | 115 more per 1000 (from 55 fewer to 300 more) | ⨁◯◯◯ VERY LOW |
Abbreviations: CI, confidence interval; ED, emergency department; OR, odds ratio.
Schwarz et al.91
Schwarz et al.91: Outcomes divided by severe anxiety.
Imprecision: The decision will differ across boundaries of the CI.
Cohorts (anxiety/depression vs. not anxiety/depression) are comparable in all three studies and representative of the population of interest. The outcomes are mostly self-reported. Lost of follow up.
Musey et al.90
Musey et al.90: Follow up assessed by patient report and chart review. Potential recall bias.
Musey et al.90: Follow up data available in only 109 subjects.
Kim et al. used the clinical anxiety scale (CAS),92 a 25-item self-administered tool to determine anxiety symptoms over the 2 weeks prior to assessment with a cutoff score ≥30 as predictive of clinical anxiety. Musey et al. used the validated hospital anxiety and depression scale-anxiety subscale (HADS-A), which is a 7-item self-reported scale with a scoring range of 0–21 with a cutoff of ≥8 as borderline abnormal anxiety.93 Finally, Schwarz et al. used the validated generalized anxiety disorder 7-item scale (GAD-7) to identify anxiety symptoms over the prior 2 weeks with scores ≥15 denoting severe anxiety.94 (Appendix 5).
Benefits
These observational cohort studies demonstrated that screening for depression and/or anxiety in a population of ED patients with low-to-moderate risk chest pain uncovered a higher prevalence of clinically relevant depression and/or anxiety symptoms compared with what is seen in the general population.
Harms and burden
The screening tools used in these studies were all self-administered, and ranged in length from 7 to 25 questions depending upon the domain assessed. It is unclear if incorporating these screening tools into the clinical workflow would cause an undue burden to either the provider or patient. However, it should be noted that many departments and health systems already mandate screening of suicidal ideation, and, depending upon the screening tool used, may also screen for significant depression, as well. Moreover, these observational studies also provided no information as to what to do with this added screening information with respect to patient communication or disposition. It should be clearly stated that the presence of a mental health problem, in this case either depression or anxiety (to be discussed in question 8) does not minimize the risk that the patient under evaluation may be in the midst of a cardiac event. Indeed, there is evidence supporting the assertion that untreated anxiety and depression are independent risk factors for the development of CAD.95–98 Additionally, impaired mental health, particularly post-traumatic stress disorder, depression, and anxiety are associated with increased mortality and poorer health outcomes in survivors of ACS.99 Thus, when taking screening into account, clinicians will need to guard against premature closure and anchoring bias, which represent two types of cognitive error that can threaten patient safety by failing to consider potentially life-threatening etiologies of chest pain attributed to psychiatric causes.30
Decision criteria and additional considerations
Currently, our healthcare systems and EDs are encouraged by the Joint Commission to implement universal screening for suicidality, for all patients, irrespective of whether they are presenting with psychiatric complaints of suicidality.100 This is an acknowledgment of the fact that it is well-established that undiagnosed or under-addressed psychological factors, such as anxiety and depression, are prevalent (possibly >40%) and may complicate or contribute directly to medical problems and somatic complaints.101 However, it must be stated that the primary imperative of emergency medicine is to evaluate for and manage conditions that pose immediate threats of morbidity and mortality. Thus, of paramount relevance to EM providers is the notion that “EM should not take on any non-core initiatives until ED care itself is adequately resourced.”102 Therefore, the barriers to screening for psychological conditions in some cases and ED environments are myriad: (1) optimal timing regarding when in the time course such screening is appropriate and whether it can or should occur in triage or in the treatment area; (2) what specific screening tool(s) should be used and how should they be administered (e.g., verbal, paper, electronic) and who should administer (e.g., self-administered, nursing personnel, social workers, ED providers); and (3) should screening be universal or triggered by an overt action such as a physician order?
Additionally, the decisions about how to deal with the results of screening results pose serious consideration (i.e. how are results provided to both clinicians and patients and how does this translate into the next step in care). There also must be an acknowledgment of the role of gender and race in the historical context of labeling patients as hysterical in the case of females or under-diagnosis in the case of minorities.103,104 Finally, it has been documented that ED providers do not often address these psychological issues even when informed, which may be a result of the lack of sufficient available resources, such as directed outpatient psychiatric follow-up.105,106 Thus, there is a need to determine what the responsibility of the treating clinician is with regard to specifically addressing the screening results, especially in the landscape of medicolegal considerations. While individual providers are free to use their clinical judgment and pursue targeted screening for their patients, for the reasons outlined above, the decision to embrace a more comprehensive screening paradigm should be made at the level of the department where appropriate system resources should be coordinated to support such an effort.
Conclusions and research needs
Indirect evidence from a single site prospective observational study found increased chest pain recurrence, ED utilization, and healthcare use among patients screening positive for clinically relevant depression among a population of low-to-moderate risk chest pain patients. While indirect evidence from three studies found that, despite a high prevalence of clinically relevant anxiety among low-to-moderate risk chest pain patients, there are mixed results for this association with chest pain recurrence, ED utilization and healthcare use. To provide specific direct evidence to answer this question, prospective studies are needed which (1) include a measure of recurrent chest pain visits prior to enrollment; (2) include a clear description of ACS risk stratification in the ED and specifically include those with low-risk; (3) screen for the presence of clinically significant anxiety and depression at the time of enrollment using an instrument validated for use in the ED; and (4) assess specifically for outcomes of ED utilization and health care utilization (with a clear breakdown of the number of visits specifically for chest pain versus other reason for visit), as well as methods for determining chest pain recurrence which minimize recall bias.
Recommendation 7: In adult patients with recurrent, low-risk chest pain, we suggest depression and anxiety screening as these might have an effect on healthcare use and return ED visits. (Conditional, Either) [Very low level of evidence].
QUESTION 8
In adult patients with recurrent, low-risk chest pain, what is the role of referral for anxiety or depression in healthcare use and return ED visits?
Summary of the evidence
There were no studies to provide direct evidence for this question. However, there were two systematic reviews available to provide indirect evidence for this question. No additional studies were found. Kisely et al.107 evaluated psychological interventions in 17 RCTs with 1006 participants for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Interventions included cognitive behavioral therapy, relaxation therapy, hyperventilation control, hypnotherapy, and other psychotherapy/counseling. There was a reduction in reports of chest pain in the first 3 months following the intervention (relative risk [RR] 0.70; 95% CI 0.53–0.92). There was an increase in the number of chest pain-free days up to 3 months following the intervention (mean difference [MD] 3.00; 95% CI 0.23–5.77) and reduced chest pain frequency (MD −2.26; 95% CI −4.41 to −0.12). There was no effect on the severity of chest pain (Table 9).
TABLE 9.
Evidence table for Question 8–In adult patients with recurrent, low-risk chest pain, what is the role of referral for anxiety or depression in healthcare use and return emergency department visits?
| Certainty assessment |
No. of patients |
Effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Referral for anxiety/depression | No Referral | Relative (95% CI) | Absolute (95% CI) | Certainty |
| Psychotherapy interventions vs. control, any chest pain (follow up: 30 days) | |||||||||||
| 3 | Randomized trials | Very seriousa,b | Not serious | Not serious | Not serious | None | 57/90 (63.3%) | 76/82 (92.7%) | RR 0.70 (0.53–0.92) | 278 fewer per 1000 (from 436 fewer to 74 fewer) | ⨁⨁◯◯ |
| Psychotherapy interventions vs. control, any chest pain (follow up: range 3) | |||||||||||
| 2 | Randomized trials | Very seriousa,b,c | Not serious | Not serious | Not serious | None | 27/50 (54.0%) | 57/61 (93.4%) | RR 0.59 (0.45–0.76) | 383 fewer per 1000 (from 514 fewer to 224 fewer) | ⨁⨁◯◯ LOWd |
| Psychotherapy interventions vs. control, chest pain frequency (follow up: 3 months) | |||||||||||
| 7 | Randomized trials | Seriousa,b | Seriouse | Not serious | Not serious | None | 154 | 140 | – | MD 2.26 lower (4.41 lower to lower) | ⨁⨁◯◯ LOW |
| Psychotherapy interventions vs. control, chest pain frequency (follow up: range 3–12 months) | |||||||||||
| 4 | Randomized trials | Seriousa,b | Not serious | Not serious | Serious | None | 84 | 80 | – | MD 0.81 lower (2.35 lower to 0.74 higher) | ⨁⨁◯◯ LOW |
| Antidepressants vs. placebo, reduction of chest pain | |||||||||||
| 7 | Randomized trials | Serious | Serious | Not serious | Not serious | None | 161 | 158 | – | MD 1.26 lower (2.43 lower to 0.13 lower) | ⨁⨁◯◯ LOW |
Abbreviations: CI, confidence interval; MD, mean difference; RR, risk ratio.
In 10 studies there was a low-risk of random sequence generation and/or selection bias. In seven there was unclear risk for both of these biases. Due to the nature of the main interventions (counselling or CBT), it was not possible to blind the people delivering the treatment as to whether the participant was in the intervention or control arm. The SR considered all studies to be at high risk of performance and outcome assessment bias.
One exception was van Beek et al., which assessed disease severity with the Clinical Global Inventory (CGI) rated by a blinded independent rater. Most studies did not discuss intention-to-treat (ITT) analysis, but most studies appeared to have analyzed data based on ITT analysis. We considered three studies to be at high risk of outcome bias because of a high loss or differential loss from baseline to follow-up. The other trials were at low-risk of bias for this domain. Two studies had unclear risk.
There is significant differences in results between individual studies. High I2 94%. Small sample studies.
Decision will differ if the truth is in the lower versus upper boundary of the CI.
Large heterogeneity I2 94%.
Wang et al.108 evaluated antidepressants for symptomatic management of functional chest pain (defined as normal coronary angiography). The authors included seven RCTs of 319 patients. There was an association between antidepressants and pain reduction (standardized mean difference [SMD] −1.26; 95% CI −2.34 to −0.19) and psychological symptoms (SMD −0.87; 95% CI −1.67 to −0.08), as well as increased side effects (OR 0.34; 95% CI 0.15–0.78). There was no significant improvement in health-related quality of life (weighted mean difference 2.00; 95% CI −2.54 to −6.65) (Appendix 6).
Benefits
These systematic reviews of trials of psychological interventions in patients with nonspecific or functional chest pain (one looking at multi-component psychological interventions and one assessing an anti-depressant intervention) demonstrated a reduction in chest pain frequency and an improvement in psychological symptoms.
Harms and burden
The indirect evidence presented here is based upon interventions of which only a portion was initiated in the ED setting. Similar to the discussion of the addition of screening tools for anxiety and/or depression in PICO question 7, the effect of referral for behavioral or pharmacologic interventions on ED flow/throughput cannot be quantified. Thus, it is unclear if this step represents an undue burden to providers or patients; however, it should be noted that ED clinicians often make referrals for outpatient evaluation and management of non-emergent conditions which is similar to this. The adverse events associated with behavioral events were not assessed/reported; however, antidepressants were associated with an increased risk of non-serious side effects, such as drowsiness and fatigue.
Decision criteria and additional considerations
The decision to refer for management of anxiety and or depression in patients with low-risk chest pain is directly related to the discussion of additional considerations in PICO question 7 above. In summary of that discussion, while the decision to screen a particular patient is at the discretion of the clinical provider, initiation of broad-based screening in this population of interest is likely best operationalized, resourced, and backstopped at the level of the hospital or healthcare system. As such, the decision regarding how to handle the results of this screening should be operationalized in such a manner that there is consistency and ease of access. An example of a specific consideration would be what to do with a patient that screens positive for severe anxiety or depression. Should behavioral health or social work be notified automatically; can this happen independent of the provider; do discharge instructions automatically get populated with referral instructions to specific clinics or clinicians? Clearly, this topic and these determinations require system-level support and are affected by the local resources available.
Conclusions and research needs
Evidence suggests modest benefit for psychological interventions (particularly cognitive behavioral therapy) within the first 3 months after the intervention. Antidepressant medications might be associated with improvements in pain and psychological symptoms, but no difference in health-related quality of life. A number of significant questions remain unanswered and should be further investigated, specifically in the population of patients with recurrent, low-risk chest pain. As a practical note, probably the most important question for ED clinicians aside from screening implementation is: how does patient receptivity and preference impact the referral for further behavioral health evaluation and intervention in this population? Is the current pragmatic access to mental health resources sufficient, or are more resources required for implementation? How does insurance status affect the ability to follow up as an outpatient? How does documentation of previously unrecognized depression impact malpractice risk? Are these psycho-behavioral interventions equivalent and interchangeable, or are there some recurrent chest pain subtypes that respond differently to various modalities? What effect does referral for anxiety and depression management have on patient perceptions of stress? Additional research to provide direct evidence on the numbers needed to treat and harm, cost-effectiveness, and healthcare disparities are of paramount importance.
Recommendation 8: In adult patients with recurrent, low-risk chest pain, we suggest referral for anxiety/depression management, as this might have an impact on healthcare use and return ED visits. (Conditional/Either) [Low level of evidence].
The GRADE Evidence to Decision Framework tables are located in Appendix 7.
GENER AL ISSUES NECESSARY FOR CORREC T INTERPRETATION AND IMPLEMENTATION OF RECOMMENDATIONS
Limitations
Given the topic of these guidelines, the panel rightly included ED clinicians, a cardiologist, a patient with lived experiences, and methodologists. However, stakeholders representing primary care, mental health, and the medicolegal perspective were not a part of this panel at its inception. It is possible that these perspectives may have provided further insight particularly with regard to the decision criteria and additional considerations sections for each question.
The NEATS analysis performed for this guideline is limited by a single assessment by a NEATS-experienced rater (SU), who is also part of this project Methods team, and was completed before distribution for external stakeholder/audience review. Ideally, future GRACE guideline draft manuscripts will be independently rated using NEATS, and feedback incorporated by panel writers when completing final manuscripts.
Assumed values and preferences
Limited data exist on patient preferences regarding the management of recurrent, low-risk chest pain. In the Chest Pain Choice multicenter trial, ED patients with low-risk chest pain had a better understanding of their risk of ACS, were less likely to agree to admission, and received fewer advanced cardiac imaging tests when a decision aid was used to explain their risk level and options for care.109,110 Men and women are equally likely to prefer noninvasive over invasive evaluation and management for CAD, and the vast majority (85%) would accept their physician’s recommendation for stress testing. However, women and Black patients were less likely than men and non-Black patients to say they would accept a physician’s recommendation for cardiac interventions.58 At 30-day follow up, women were less likely than men to receive any cardiac diagnostic testing and to undergo cardiac catheterization.58 Taken together, these data highlight the importance of shared decision-making and considering patients’ values and preferences in the ED management of low-risk chest pain.74 Although the panel acknowledges that the method of communicating with patients about their chest pain was not evaluated as a specific PICO question, a patient information sheet (Appendix 8) was created in an effort to make our recommendations more accessible to the average ED patient. It was vetted by our patient representative. It could be useful as a dissemination tool, and could even be printed out and given to patients while they are in the ED.
Shared decision-making works best when there is equipoise in decisions and given how preference and value-sensitive many of these clinical decisions are, and since most of the recommendations fall into the conditional category, shared decision making should be considered when operationalizing these recommendations into practice. Specific steps to operationalize the implementation of these guidelines are beyond our scope. Suggestions include provider education on these recommendations, understanding of local resources available at each center including outpatient follow up, access to cardiology clinics, availability of stress testing, use of decision aids, etc. Furthermore, involving patients in their healthcare decisions has shown that patients prefer less invasive choices, with the potential to decrease costs and increase satisfaction. We created an accessible 1-page infographic for physician education and to facilitate shared decision-making conversations with patients at the bedside (Supplemental Material 2).
Plans for updating these guidelines
Evidence is constantly growing. These guidelines should be updated within 5 years, or when significant new relevant high-quality research requires reassessment and revision of the relevant recommendation(s). Adoption of these guidelines should be tailored to local policies and practices. Availability of cardiology consultation, stress test, CCTA and type of troponin test might differ in each hospital.
Monitoring criteria for audit/feedback of implementation
Based on the recommendations of this guideline being Conditional with Very Low/Low/Moderate levels of supporting evidence, we cannot offer monitoring criteria for audit/feedback purposes with prospective implementation of any/all of these recommendations at this time. Should future evidence and updates warrant a Strong recommendation for an intervention with High level supporting evidence, we will suggest potential monitoring/audit criteria at that time.
CONCLUSIONS
These guidelines outline and summarize the evidence and strength of GRACE recommendations regarding eight priority questions of interest to emergency clinicians, other healthcare professionals, patients, and policymakers with regard to the evaluation and management of patients with recurrent, low-risk chest pain seen in the ED. Direct evidence for the selected priority questions in this population is lacking, which highlights areas which will benefit from further robust prospective investigation in this specific population.
Supplementary Material
Funding Information
This study was funded by the Society for Academic Emergency Medicine.
Footnotes
CONFLICT OF INTERESTS
Paul Musey, MD—Research funding from SAEM Foundation and PCORI. Jeffrey Kline, MD—Consultant to Janssen, Stago Diagnostica, Research funding from NIH, Mallingkrodt, Roche Diagnostics. Christopher Carpenter, MD—Research funding from John A. Hartford Foundation, Gary and Mary West Foundation, and National Institute of Aging. Bryn Mumma, MD, MAS—Research funding from Roche Diagnostics and Alpha Phi Foundation. Marc Probst, MD, MS—Research funding from NIH/NHLBI. Anna Marie Chang, MD MSCE—Research funding from Abbott, Roche, Siemens, Entegrion, Beckman Coulter, Ortho Diagnostics, NIH, Pennsylvania Medical Society. Deborah Diercks, MD: Research funding from Stago Diagnostica, Ortho Clinical Diagnostics, Abbott, Siemens.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.National Center for Health Statistics. National hospital ambulatory medical care survey: 2017 emergency department summary tables. 2020 National Center for Health Statistics. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf. Accessed June 2, 2020. [Google Scholar]
- 2.Mahler SA, Riley RF, Russell GB, et al. Adherence to an accelerated diagnostic protocol for chest pain: secondary analysis of the HEART pathway randomized trial. Acad Emerg Med 2016;23(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstock MB, Weingart S, Orth F, et al. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med 2015;175:1207–1212. [DOI] [PubMed] [Google Scholar]
- 4.Yau AA, Nguyendo LT, Lockett LL, Michaud E. The HEART pathway and hospital cost savings. Crit Pathw Cardiol 2017;16(4):126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med 2016;176(7):1029–1032. [DOI] [PubMed] [Google Scholar]
- 6.Stepinska J, Lettino M, Ahrens I, et al. Diagnosis and risk stratification of chest pain patients in the emergency department: focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2020;9(1):76–89. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson B, Geralds J, Petrey J, Huecker M. Malpractice in emergency medicine-a review of risk and mitigation practices for the emergency medicine provider. J Emerg Med 2018;55(5):659–665. [DOI] [PubMed] [Google Scholar]
- 8.Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med 2010;17(5):553–560. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010;122(17):1756–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesh AK, Dai Y, Ross JS, Schuur JD, Capp R, Krumholz HM. Variation in US hospital emergency department admission rates by clinical condition. Med Care 2015;53(3):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smulowitz PB, Barrett O, Hall MM, Grossman SA, Ullman EA, Novack V. Physician variability in management of emergency department patients with chest pain. West J Emerg Med 2017;18(4):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleet RP, Lavoie KL, Martel JP, Dupuis G, Marchand A, Beitman BD. Two-year follow-up status of emergency department patients with chest pain: was it panic disorder? CJEM 2003;5(4):247–254. [DOI] [PubMed] [Google Scholar]
- 13.Leise MD, Locke GR 3rd, Dierkhising RA, Zinsmeister AR, Reeder GS, Talley NJ. Patients dismissed from the hospital with a diagnosis of noncardiac chest pain: cardiac outcomes and health care utilization. Mayo Clin Proc 2010;85(4):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomaszewski CA, Nestler D, Shah KH, Sudhir A, Brown MD. Clinical policy: critical issues in the evaluation and management of emergency department patients with suspected non-ST-elevation acute coronary syndromes. Ann Emerg Med 2018;72:e65–e106. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez F, Mahaffey KW. Management of patients with NSTE-ACS: a comparison of the recent AHA/ACC and ESC guidelines. J Am Coll Cardiol 2016;68(3):313–321. [DOI] [PubMed] [Google Scholar]
- 16.Jue JJ, Cunningham S, Lohr K, et al. Developing and testing the agency for healthcare research and quality’s national guideline clearinghouse extent of adherence to trustworthy standards (NEATS) instrument. Ann Intern Med 2019;170:480–487. [DOI] [PubMed] [Google Scholar]
- 17.Rowe BH, Wyer PC. Introduction. In: Rowe BH, Lang ES, Brown MD, Houry D, Newman DH, Wyer PC, eds. Evidence-Based Emergency Medicine Wiley-Blackwell; 2009:3–12. [Google Scholar]
- 18.Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 510 The Cochrane Collaboration; 2011;1–73. [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handbook GRADE: overview of the GRADE approach (edited by Schünemann H, Brożek J, Guyatt G, Oxman A). 2013. https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2. Accessed July 19, 2020.
- 21.Kanzaria HK, McCabe AM, Meisel ZM, et al. Advancing patient-centered outcomes in emergency diagnostic imaging: a research agenda. Acad Emerg Med 2015;22(12):1435–1446. [DOI] [PubMed] [Google Scholar]
- 22.El Dib R, Tikkinen KAO, Akl EA, et al. Systematic survey of randomized trials evaluating the impact of alternative diagnostic strategies on patient-important outcomes. J Clin Epidemiol 2017;84:61–69. [DOI] [PubMed] [Google Scholar]
- 23.Lord SJ, Irwig L, Simes RJ. When is measuring sensitivity and specificity sufficient to evaluate a diagnostic test, and when do we need randomized trials? Ann Intern Med 2006;144(11):850–855. [DOI] [PubMed] [Google Scholar]
- 24.Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol 2013;168(3):2153–2158. [DOI] [PubMed] [Google Scholar]
- 25.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J 2008;16(6):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poldervaart JM, Reitsma JB, Backus BE, et al. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med 2017;166(10):689–697. [DOI] [PubMed] [Google Scholar]
- 27.Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284(7):835–842. [DOI] [PubMed] [Google Scholar]
- 28.Mahler SA, Lenoir KM, Wells BJ, et al. Safely identifying emergency department patients with acute chest pain for early discharge. Circulation 2018;138(22):2456–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department? A clinical survey. Int J Cardiol 2013;166(3):752–754. [DOI] [PubMed] [Google Scholar]
- 30.Kane B, Carpenter CR. Cognition and decision making. In: Fondahn E, Lane M, Vannucci A, eds. The Washington Manual of Patient Safety and Quality Improvement Wolters-Kluwer; 2016;195–209. [Google Scholar]
- 31.Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol 2014;63:2569–2578. [DOI] [PubMed] [Google Scholar]
- 32.Cullen L, Mueller C, Parsonage WA, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol 2013;62:1242–1249. [DOI] [PubMed] [Google Scholar]
- 33.Datlow MD, Gray KM, Watts A, Diercks DB, Mumma BE. Troponin limit of detection plus cardiac risk stratification scores to rule out acute myocardial infarction and 30-day major adverse cardiac events in ED patients. Crit Pathw Cardiol 2017;16(4):142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavsak PA, Neumann JT, Cullen L, et al. Clinical chemistry score versus high-sensitivity cardiac troponin I and T tests alone to identify patients at low or high risk for myocardial infarction or death at presentation to the emergency department. CMAJ 2018;190(33):E974–E984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCord J, Cabrera R, Lindahl B, et al. Prognostic utility of a modified HEART score in chest pain patients in the emergency department. Circ Cardiovasc Qual Outcomes 2017;10(2):e003101. [DOI] [PubMed] [Google Scholar]
- 36.Mokhtari A, Lindahl B, Schiopu A, et al. A 0-hour/1-hour protocol for safe, early discharge of chest pain patients. Acad Emerg Med 2017;24(8):983–992. [DOI] [PubMed] [Google Scholar]
- 37.Sandoval Y, Nowak R, deFilippi CR, et al. Myocardial infarction risk stratification with a single measurement of high-sensitivity troponin I. J Am Coll Cardiol 2019;74(3):271–282. [DOI] [PubMed] [Google Scholar]
- 38.Tan JWC, Tan HJG, Sahlen AO, et al. Performance of cardiac troponins within the HEART score in predicting major adverse cardiac events at the emergency department. Am J Emerg Med 2020;38(8):1560–1567. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Zheng W, Wu S, et al. Comparison of usual care and the HEART score for effectively and safely discharging patients with low-risk chest pain in the emergency department: would the score always help? Clin Cardiol 2020;43(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem 2015;48(4–5):201–203. [DOI] [PubMed] [Google Scholar]
- 41.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017;63(1):73–81. [DOI] [PubMed] [Google Scholar]
- 42.Twerenbold R, Jaeger C, Rubini Gimenez M, et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J 2016;37(44):3324–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 44.Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 45.Furmaga J, McDonald SA, Hall HM, et al. Impact of high-sensitivity troponin testing on operational characteristics of an urban emergency department. Acad Emerg Med 2021;28(1):114–116. [DOI] [PubMed] [Google Scholar]
- 46.Mumma BE, Casey SD, Dang RK, et al. Diagnostic reclassification by a high-sensitivity cardiac troponin assay. Ann Emerg Med 2020;76(5):566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chew DP, Lambrakis K, Blyth A, et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: the rapid assessment of possible acute coronary syndrome in the emergency department with high-sensitivity troponin T study (RAPID-TnT). Circulation 2019;140(19):1543–1556. [DOI] [PubMed] [Google Scholar]
- 48.Frisoli TM, Nowak R, Evans KL, et al. Henry Ford HEART score randomized trial: rapid discharge of patients evaluated for possible myocardial infarction. Circ Cardiovasc Qual Outcomes 2017;10(10):e003617. 10.1161/CIRCOUTCOMES.117.003617 [DOI] [PubMed] [Google Scholar]
- 49.Lim SH, Anantharaman V, Sundram F, et al. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department: a randomized controlled trial. J Nucl Cardiol 2013;20(6):1002–1012. [DOI] [PubMed] [Google Scholar]
- 50.Natsui S, Sun BC, Shen E, et al. Evaluation of outpatient cardiac stress testing after emergency department encounters for suspected acute coronary syndrome. Ann Emerg Med 2019;74(2):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton B, Shofer FS, Walsh KM, et al. Stress testing in young low-risk patients with potential acute coronary syndromes. Am J Emerg Med 2012;30(5):639–642. [DOI] [PubMed] [Google Scholar]
- 52.Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med 2009;54(1):12–16. [DOI] [PubMed] [Google Scholar]
- 53.Scott AC, Bilesky J, Lamanna A, et al. Limited utility of exercise stress testing in the evaluation of suspected acute coronary syndrome in patients aged less than 40 years with intermediate risk features. Emerg Med Australas 2014;26(2):170–176. [DOI] [PubMed] [Google Scholar]
- 54.Ely S, Chandra A, Mani G, Drake W, Freeman D, Limkakeng AT Jr. Utility of observation units for young emergency department chest pain patients. J Emerg Med 2013;44(2):306–312. [DOI] [PubMed] [Google Scholar]
- 55.Pines JM, Isserman JA, Szyld D, Dean AJ, McCusker CM, Hollander JE. The effect of physician risk tolerance and the presence of an observation unit on decision making for ED patients with chest pain. Am J Emerg Med 2010;28(7):771–779. [DOI] [PubMed] [Google Scholar]
- 56.Fatovich DM. The inverted U curve and emergency medicine: overdiagnosis and the law of unintended consequences. Emerg Med Australas 2016;28(4):480–482. [DOI] [PubMed] [Google Scholar]
- 57.Probst MA, Tschatscher CF, Lohse CM, Fernanda Bellolio M, Hess EP. Factors associated with patient involvement in emergency care decisions: a secondary analysis of the chest pain choice multicenter randomized trial. Acad Emerg Med 2018;25:1107–1117. [DOI] [PubMed] [Google Scholar]
- 58.Mumma BE, Baumann BM, Diercks DB, et al. Sex bias in cardiovascular testing: the contribution of patient preference. Ann Emerg Med 2011;57:551–560.e4. [DOI] [PubMed] [Google Scholar]
- 59.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:3097–3137. [DOI] [PubMed] [Google Scholar]
- 60.Milano P, Carden DL, Jackman KM, et al. Compliance with outpatient stress testing in low-risk patients presenting to the emergency department with chest pain. Crit Pathw Cardiol 2011;10(1):35–40. [DOI] [PubMed] [Google Scholar]
- 61.Potezny TM, Horwood CM, Hakendorf P, Papendick C, Thompson CH. Predicting re-presentation following discharge from the emergency department with non-specific chest pain. Emerg Med Australas 2018;30:193–199. [DOI] [PubMed] [Google Scholar]
- 62.Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol 2012;11:128–138. [DOI] [PubMed] [Google Scholar]
- 63.Hess EP, Nestler DM. Transforming the emergency department observation unit: a look into the future. Cardiol Clin 2012;30:501–521. [DOI] [PubMed] [Google Scholar]
- 64.Ko DT, Wang Y, Alter DA, et al. Regional variation in cardiac catheterization appropriateness and baseline risk after acute myocardial infarction. J Am Coll Cardiol 2008;51:716–723. [DOI] [PubMed] [Google Scholar]
- 65.Wennberg D, Dickens J Jr, Soule D, et al. The relationship between the supply of cardiac catheterization laboratories, cardiologists and the use of invasive cardiac procedures in northern New England. J Health Serv Res Policy 1997;2:75–80. [DOI] [PubMed] [Google Scholar]
- 66.Bradley SM, Maddox TM, Stanislawski MA, et al. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: insights from the VA CART program (veterans affairs clinical assessment reporting and tracking). J Am Coll Cardiol 2014;63(5):417–426. [DOI] [PubMed] [Google Scholar]
- 67.Golden KE, Chang AM, Hollander JE. Sex preferences in cardiovascular testing: the contribution of the patient-physician discussion. Acad Emerg Med 2013;20(7):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J 2018;39(23):2135–2146. [DOI] [PubMed] [Google Scholar]
- 69.McMullan JT, Lindsell CJ, Blomkalns AL. Five-year mortality and coronary heart disease development after normal coronary angiogram. World J Emerg Med 2011;2(1):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papanicolaou MN, Califf RM, Hlatky MA, et al. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am J Cardiol 1986;58(13):1181–1187. [DOI] [PubMed] [Google Scholar]
- 71.Tavella R, Cutri N, Tucker G, Adams R, Spertus J, Beltrame JF. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes 2016;2(2):117–124. [DOI] [PubMed] [Google Scholar]
- 72.Wang TKM, Oh THT, Samaranayake CB, et al. The utility of a ‘non-significant’ coronary angiogram. Int J Clin Pract 2015;69(12):1465–1472. [DOI] [PubMed] [Google Scholar]
- 73.Pitts WR, Lange RA, Cigarroa JE, Hillis LD. Repeat coronary angiography in patients with chest pain and previously normal coronary angiogram. Am J Cardiol 1997;80(8):1086–1087. [DOI] [PubMed] [Google Scholar]
- 74.Hess EP, Grudzen CR, Thomson R, Raja AS, Carpenter CR. Shared decision-making in the emergency department: respecting patient autonomy when seconds count. Acad Emerg Med 2015;22(7):856–864. [DOI] [PubMed] [Google Scholar]
- 75.Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011;58(24):2533–2540. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135(24):2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Gonzalez AB, Salotti JA, McHugh K, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer 2016;114(4):388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Journy N, Ancelet S, Rehel J-L, et al. Predicted cancer risks induced by computed tomography examinations during childhood, by a quantitative risk assessment approach. Radiat Environ Biophys 2014;53(1):39–54. [DOI] [PubMed] [Google Scholar]
- 79.Krille L, Dreger S, Schindel R, et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys 2015;54:1–12. [DOI] [PubMed] [Google Scholar]
- 80.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013;346:f2360. 10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 2013;167(8):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pijpe A, Andrieu N, Easton DF, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK). BMJ 2012;345:e5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Einstein AJ, Weiner SD, Bernheim A, et al. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA 2010;304(19):2137–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kline JA, Courtney DM, Beam DM, King MC, Steuerwald M. Incidence and predictors of repeated computed tomographic pulmonary angiography in emergency department patients. Ann Emerg Med 2009;54(1):41–48. [DOI] [PubMed] [Google Scholar]
- 86.Cordiner D, Al-Ani M, Jia X, Marchick M, Allen B, Winchester DE. Estimates of radiation exposure and subsequent risk of malignancy due to cardiac imaging in the emergency department for evaluation of chest pain: a cohort study. Coron Artery Dis 2019;30(8):626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298(3):317–323. [DOI] [PubMed] [Google Scholar]
- 88.Kim Y, Soffler M, Paradise S, et al. Depression is associated with recurrent chest pain with or without coronary artery disease: A prospective cohort study in the emergency department. Am Heart J 2017;191:47–54. [DOI] [PubMed] [Google Scholar]
- 89.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 90.Musey PI Jr, Patel R, Fry C, Jimenez G, Koene R, Kline JA. Anxiety associated with increased risk for emergency department recidivism in patients with low-risk chest pain. Am J Cardiol 2018;122(7):1133–1141. [DOI] [PubMed] [Google Scholar]
- 91.Schwarz J, Prashad A, Winchester DE. Prevalence and implications of severe anxiety in a prospective cohort of acute chest pain patients. Crit Pathw Cardiol 2015;14(1):44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Westhius D, Thyer B. Development and validation of the Clinical Anxiety Scale: a rapid assessment instrument for empirical practice. Educ Psychol Measur 1989;49(1):153–163. [Google Scholar]
- 93.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 94.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 95.Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BH. Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol 2016;118(4):511–519. [DOI] [PubMed] [Google Scholar]
- 96.Batelaan NM, Seldenrijk A, Bot M, van Balkom AJ, Penninx BW. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry 2016;208(3):223–231. [DOI] [PubMed] [Google Scholar]
- 97.Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015;28(11):1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thurston RC, Rewak M, Kubzansky LD. An anxious heart: anxiety and the onset of cardiovascular diseases. Prog Cardiovasc Dis 2013;55(6):524–537. [DOI] [PubMed] [Google Scholar]
- 99.Musey PI Jr, Schultebraucks K, Chang BP. Stressing out about the heart: a narrative review of the role of psychological stress in acute cardiovascular events. Acad Emerg Med 2020;27(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suicide prevention. The Joint Commission https://www.jointcommission.org/resources/patient-safety-topics/suicide-prevention/. Accessed September 25, 2020
- 101.Faessler L, Perrig-Chiello P, Mueller B, Schuetz P. Psychological distress in medical patients seeking ED care for somatic reasons: results of a systematic literature review. Emerg Med J 2016;33(8):581–587. [DOI] [PubMed] [Google Scholar]
- 102.Kelen GD. Public health initiatives in the emergency department: not so good for the public health? Acad Emerg Med 2008;15(2):194–197. [DOI] [PubMed] [Google Scholar]
- 103.Kunen S, Niederhauser R, Smith PO, Morris JA, Marx BD. Race disparities in psychiatric rates in emergency departments. J Consult Clin Psychol 2005;73(1):116–126. [DOI] [PubMed] [Google Scholar]
- 104.Tasca C, Rapetti M, Carta MG, Fadda B. Women and hysteria in the history of mental health. Clin Pract Epidemiol Ment Health 2012;8(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schriger DL, Gibbons PS, Langone CA, Lee S, Altshuler LL. Enabling the diagnosis of occult psychiatric illness in the emergency department: a randomized, controlled trial of the computerized, self-administered PRIME-MD diagnostic system. Ann Emerg Med 2001;37(2):132–140. [DOI] [PubMed] [Google Scholar]
- 106.Musey PI Jr, Lee JA, Hall CL, Kline JA. Anxiety about anxiety: a survey of emergency department provider beliefs and practices regarding anxiety-associated low risk chest pain. BMC Emerg Med 2018;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kisely SR, Campbell LA, Yelland MJ, Paydar A. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev 2015;2015:CD004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician 2012;15:E131–E142. [PubMed] [Google Scholar]
- 109.Hess EP, Hollander JE, Schaffer JT, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ 2016;355:i6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schaffer JT, Hess EP, Hollander JE, et al. Impact of a shared decision making intervention on health care utilization: a secondary analysis of the chest pain choice multicenter randomized trial. Acad Emerg Med 2018;25(3):293–300. [DOI] [PubMed] [Google Scholar]
- 111.Addison D, Singh V, Okyere-Asante K, Okafor H. Cardiovascular outcomes of a positive nuclear stress test but negative coronary angiography in a multiethnic male predominant cohort. Niger Med J 2014;55:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation 2004;109:2518–2523. [DOI] [PubMed] [Google Scholar]
- 113.Cortigiani L, Rigo F, Gherardi S, Galderisi M, Bovenzi F, Sicari R. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J Am Soc Echocardiogr 2014;27:742–748. [DOI] [PubMed] [Google Scholar]
- 114.Delcour KS, Khaja A, Chockalingam A, Kuppuswamy S, Dresser T. Outcomes in patients with abnormal myocardial perfusion imaging and normal coronary angiogram. Angiology 2009;60:318–321. [DOI] [PubMed] [Google Scholar]
- 115.Egashira K, Kikuchi Y, Sagara T, Sugihara M, Nakamura M. Long-term prognosis of vasospastic angina without significant atherosclerotic coronary artery disease. Jpn Heart J 1987;28:841–849. [DOI] [PubMed] [Google Scholar]
- 116.Foussas SG, Adamopoulou EN, Kafaltis NA, et al. Clinical characteristics and follow-up of patients with chest pain and normal coronary arteries. Angiology 1998;49:349–354. [DOI] [PubMed] [Google Scholar]
- 117.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 119.Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J 2008;155:375–381. [DOI] [PubMed] [Google Scholar]
- 120.Isner JM, Salem DN, Banas JS Jr, Levine HJ. Long-term clinical course of patients with normal coronary arteriography: follow-up study of 121 patients with normal or nearly normal coronary arteriograms. Am Heart J 1981;102:645–653. [DOI] [PubMed] [Google Scholar]
- 121.Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 122.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995;25:807–814. [DOI] [PubMed] [Google Scholar]
- 123.Lamendola P, Lanza GA, Spinelli A, et al. Long-term prognosis of patients with cardiac syndrome X. Int J Cardiol 2010;140:197–199. [DOI] [PubMed] [Google Scholar]
- 124.Leu HB, Lin CP, Lin WT, Wu TC, Lin SJ, Chen JW. Circulating mononuclear superoxide production and inflammatory markers for long-term prognosis in patients with cardiac syndrome X. Free Radic Biol Med 2006;40:983–991. [DOI] [PubMed] [Google Scholar]
- 125.Li L, Gu YE, Liu T, et al. A randomized, single-center double-blinded trial on the effects of diltiazem sustained-release capsules in patients with coronary slow flow phenomenon at 6-month follow-up. PLoS One 2012;7(6):e38851. 10.1371/journal.pone.0038851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol 1995;25(5):1013–1018. [DOI] [PubMed] [Google Scholar]
- 127.Lim AY, Park TK, Cho SW, et al. Clinical implications of low-dose aspirin on vasospastic angina patients without significant coronary artery stenosis; a propensity score-matched analysis. Int J Cardiol 2016;221:161–166. [DOI] [PubMed] [Google Scholar]
- 128.Marks DS, Gudapati S, Prisant LM, et al. Mortality in patients with microvascular disease. J Clin Hypertens 2004;6(6):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Masumoto A, Mohri M, Takeshita A. Three-year follow-up of the Japanese patients with microvascular angina attributable to coronary microvascular spasm. Int J Cardiol 2001;81(2–3):151–156. [DOI] [PubMed] [Google Scholar]
- 130.Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care 2004;27(1):208–215. [DOI] [PubMed] [Google Scholar]
- 131.Nitenberg A, Pham I, Antony I, Valensi P, Attali JR, Chemla D. Cardiovascular outcome of patients with abnormal coronary vasomotion and normal coronary arteriography is worse in type 2 diabetes mellitus than in arterial hypertension: a 10 year follow-up study. Atherosclerosis 2005;183(1):113–120. [DOI] [PubMed] [Google Scholar]
- 132.Pasternak RC, Thibault GE, Savoia M, DeSanctis RW, Hutter AM Jr. Chest pain with angiographically insignificant coronary arterial obstruction. Clinical presentation and long-term follow-up. Am J Med 1980;68:813–817. [DOI] [PubMed] [Google Scholar]
- 133.Proudfit WL, Bruschke VG, Sones FM Jr. Clinical course of patients with normal or slightly or moderately abnormal coronary arteriograms: 10-year follow-up of 521 patients. Circulation 1980;62:712–717. [DOI] [PubMed] [Google Scholar]
- 134.Sakata K, Iida K, Kudo M, Yoshida H, Doi O. Prognostic value of I-123 metaiodobenzylguanidine imaging in vasospastic angina without significant coronary stenosis. Circ J 2005;69:171–176. [DOI] [PubMed] [Google Scholar]
- 135.Sánchez-Recalde A, Galeote G, Moreno R, et al. Long-term prognostic value of endothelial dysfunction in patients with chest pain and angiographically normal coronary arteries. Rev Port Cardiol 2009;28:785–791. [PubMed] [Google Scholar]
- 136.Schindler TH, Hornig B, Buser PT, et al. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol 2003;23:495–501. [DOI] [PubMed] [Google Scholar]
- 137.Schofield PM. Follow-up study of morbidity in patients with angina pectoris and normal coronary angiograms and the value of investigation for esophageal dysfunction. Angiology 1990;41:286–296. [DOI] [PubMed] [Google Scholar]
- 138.Scholl JM, Veau P, Benacerraf A, Brau J, Hennetier G, Achard F. Long-term prognosis of medically treated patients with vasospastic angina and no fixed significant coronary atherosclerosis. Am Heart J 1988;115:559–564. [DOI] [PubMed] [Google Scholar]
- 139.Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J 2013;166:38–44. [DOI] [PubMed] [Google Scholar]
- 140.Shin DI, Baek SH, Seo SM, et al. Rates of coronary intervention due to de novo significant atherosclerosis and cardiac death are very low in Korean patients with vasospastic angina: 36-month follow-up results of the Vasospastic Angina in the Catholic Medical Center (VA-CMC) registry. Circ J 2012;76(11)2681–2689. [DOI] [PubMed] [Google Scholar]
- 141.Shin DIL, Baek SH, Her SH, et al. The 24-month prognosis of patients with positive or intermediate results in the intracoronary ergonovine provocation test. JACC Cardiovasc Interv 2015;8: 914–923. [DOI] [PubMed] [Google Scholar]
- 142.Shintani S, Nishiyama Y, Yamamoto K, Koga Y. Different long-term course between chest pain and exercise-induced ST depression in syndrome X. Jpn Heart J 2003;44(4):471–479. [DOI] [PubMed] [Google Scholar]
- 143.Sicari R, Palinkas A, Pasanisi EG, Venneri L, Picano E. Long-term survival of patients with chest pain syndrome and angiographically normal or near-normal coronary arteries: the additional prognostic value of dipyridamole echocardiography test (DET). Eur Heart J 2005;26:2136–2141. [DOI] [PubMed] [Google Scholar]
- 144.Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol 2009;103:626–631. [DOI] [PubMed] [Google Scholar]
- 145.Sullivan AK, Holdright DR, Wright CA, Sparrow JL, Cunningham D, Fox KM. Chest pain in women: clinical, investigative, and prognostic features. BMJ 1994;308(6933):883–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101(9):948–954. [DOI] [PubMed] [Google Scholar]
- 147.Suzuki H, Matsubara H, Koba S, Murakami M, Takeyama Y, Katagiri T. Clinical characteristics and follow-up in patients with microvascular angina. Circ J 2002;66(7):691–695. [DOI] [PubMed] [Google Scholar]
- 148.Takatsu F, Watarai M. Mild stenosis makes prognosis of vasospastic angina worse. Coron Artery Dis 2011;22(1):1–5. [DOI] [PubMed] [Google Scholar]
- 149.Voelker W, Euchner U, Dittmann H, Karsch KR. Long-term clinical course of patients with angina and angiographically normal coronary arteries. Clin Cardiol 1991;14(4):307–311. [DOI] [PubMed] [Google Scholar]
- 150.Asbury EA, Kanji N, Ernst E, Barbir M, Collins P. Autogenic training to manage symptomology in women with chest pain and normal coronary arteries. Menopause 2009;16(1):60–65. [DOI] [PubMed] [Google Scholar]
- 151.Asbury EA, Slattery C, Grant A, Evans L, Barbir M, Collins P. Cardiac rehabilitation for the treatment of women with chest pain and normal coronary arteries. Menopause 2008;15(3):454–460. [DOI] [PubMed] [Google Scholar]
- 152.Asbury EA, Webb CM, Collins P. Group support to improve psychosocial well-being and primary-care demands among women with cardiac syndrome X. Climacteric 2011;14(1):100–104. [DOI] [PubMed] [Google Scholar]
- 153.DeGuire S, Gevirtz R, Hawkinson D, Dixon K. Breathing retraining: a three-year follow-up study of treatment for hyperventilation syndrome and associated functional cardiac symptoms. Biofeedback Self Regul 1996;21(2):191–198. [DOI] [PubMed] [Google Scholar]
- 154.Esler JL, Barlow DH, Woolard RH, Nicholson RA, Nash JM, Erogul MH. A brief cognitive-behavioral intervention for patients with noncardiac chest pain. Behav Ther 2003;34(2):129–148. [Google Scholar]
- 155.Jones H, Cooper P, Miller V, Brooks N, Whorwell PJ. Treatment of non-cardiac chest pain: a controlled trial of hypnotherapy. Gut 2006;55(10):1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jonsbu E, Dammen T, Morken G, Moum T, Martinsen EW. Short-term cognitive behavioral therapy for non-cardiac chest pain and benign palpitations: a randomized controlled trial. J Psychosom Res 2011;70(2):117–123. [DOI] [PubMed] [Google Scholar]
- 157.Keefe FJ, Shelby RA, Somers TJ, et al. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain 2011;152(4):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Klimes I, Mayou RA, Pearce MJ, Coles L, Fagg JR. Psychological treatment for atypical non-cardiac chest pain: a controlled evaluation. Psychol Med 1990;20(3):605–611. [DOI] [PubMed] [Google Scholar]
- 159.Lahmann C, Loew TH, Tritt K, Nickel M. Efficacy of functional relaxation and patient education in the treatment of somatoform heart disorders: a randomized, controlled clinical investigation. Psychosomatics 2008;49(5):378–385. [DOI] [PubMed] [Google Scholar]
- 160.Mayou RA, Bryant BM, Sanders D, Bass C, Klimes I, Forfar C. A controlled trial of cognitive behavioural therapy for non-cardiac chest pain. Psychol Med 1997;27(5):1021–1031. [DOI] [PubMed] [Google Scholar]
- 161.Potts SG, Lewin R, Fox KA, Johnstone EC. Group psychological treatment for chest pain with normal coronary arteries. QJM 1999;92(2):81–86. [DOI] [PubMed] [Google Scholar]
- 162.Sanders D, Bass C, Mayou RA, Goodwin S, Bryant BM, Tyndel S. Non-cardiac chest pain: why was a brief intervention apparently ineffective? Psychol Med 1997;27(5):1033–1040. [DOI] [PubMed] [Google Scholar]
- 163.Spinhoven P, Van der Does AJ, Van Dijk E, Van Rood YR. Heart-focused anxiety as a mediating variable in the treatment of non-cardiac chest pain by cognitive-behavioral therapy and paroxetine. J Psychosom Res 2010;69(3):227–235. [DOI] [PubMed] [Google Scholar]
- 164.Tyni-Lenne R, Stryjan S, Eriksson B, Berglund M, Sylven C. Beneficial therapeutic effects of physical training and relaxation therapy in women with coronary syndrome X. Physiother Res Int 2002;7(1):35–43. [DOI] [PubMed] [Google Scholar]
- 165.van Beek M, Oude Voshaar RC, Beek AM, et al. A brief cognitive-behavioral intervention for treating depression and panic disorder in patients with noncardiac chest pain: a 24-week randomized controlled trial. Depress Anxiety 2013;30(7):670–678. [DOI] [PubMed] [Google Scholar]
- 166.Van Peski-Oosterbaan AS, Spinhoven P, Van der Does AJ, Bruschke AV, Rooijmans HG. Cognitive change following cognitive behavioural therapy for non-cardiac chest pain. Psychother Psychosom 1999;68:214–220. [DOI] [PubMed] [Google Scholar]
- 167.Cannon RO, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994;330(20):1411–1417. [DOI] [PubMed] [Google Scholar]
- 168.Cox ID, Hann CM, Kaski JC. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J 1998;19:250–254. [DOI] [PubMed] [Google Scholar]
- 169.Doraiswamy PM, Varia I, Hellegers C, et al. A randomized controlled trial of paroxetine for noncardiac chest pain. Psychopharmacol Bull 2006;39:15–24. [PubMed] [Google Scholar]
- 170.Varia I, Logue E, O’Connor C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J 2000;140:367–372. [DOI] [PubMed] [Google Scholar]
- 171.Zheng AL, Qi WH, Hu DY, et al. [Effects of antidepressant therapy in patients with suspected “angina pectoris” and negative coronary angiogram complicating comorbid depression]. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:1097–1100. [PubMed] [Google Scholar]
- 172.Lee H, Kim JH, Min BH, et al. Efficacy of venlafaxine for symptomatic relief in young adult patients with functional chest pain: a randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol 2010;105:1504–1512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


