Abstract
Introduction
Keloids are benign fibro-proliferative scarring extending outside the initial wound. Different treatment modalities as intralesional corticosteroid injection, fractional CO2 laser, and others can be used either as mono or combined therapies.
Objectives
To assess the efficacy of fractional CO2 laser versus fractional CO2 laser accompanied with either triamcinolone acetonide or trichloroacetic acid 20% in keloid treatment clinically and radiologically.
Methods
The current study was conducted on 45 Egyptian participants with keloid scars at different sites of the body. They were classified into 3 groups treated by fractional CO2 laser only (group I), fractional CO2 laser followed by triamcinolone acetonide (group II), or trichloroacetic acid application (group III), respectively. Evaluation of the keloid was done with Vancouver Scar Scale (VSS) and Color Doppler Ultrasound (CDU) before and after treatment. Four sessions 4 weeks apart were applied for the patients. They were followed-up for 8 weeks after the last session.
Results
After treatment, there was a high statistically significant reduction in VSS among the 3 groups (P ≤ 0.001); the reduction was more in group II than in I and III. Also, a high statistically significant reduction in keloid scar thickness assessed by CDU was recorded (P ≤0.001 in group II and P ≤0.01 in group I and III).
Conclusions
Combined therapy is favorable in the treatment of keloids. Trichloroacetic acid is a promising modality in treating keloid, hence it can be tried in different combinations. CDU is a promising method of keloids pre-and post-treatment assessment.
Keywords: keloid, fractional laser, TAC, TCA, doppler
Introduction
Keloids are formed due to abnormal response of wound healing where scar tissue has grown aggressively outside the initial wound borders [1]. Treatment of keloid scar is debatable and burdensome though combined treatment modalities give better results than single ones [2]. Triamcinolone acetonide (TAC) acts through suppression of wound inflammatory factors and fibroblast growth by decreasing transforming growth factor-beta (TGF-b) expression while stimulating the breakdown of collagen and fibroblast apoptosis, thus reducing the density of fibroblasts [3]. Fractional CO2 laser (FCL) creates Microthermal Zones (MTZs), which occur due to tissue vaporization in the form of rows surrounded by normal skin through which they stimulate wound healing [4]. FCL can be used as a delivery system for many drugs as corticosteroids known as laser-assisted drug delivery (LADD) [5,6]. Trichloroacetic acid (TCA) induces ultrastructural changes of epidermis and dermis. TCA improves the morphologic appearance of collagen and elastin. It acts through the deposition of new collagen and normalizes elastic tissue that was destroyed due to collagen I and III overproduction and hence can be used in keloids [7].
Objectives
To assess the efficacy of FCL versus FCL accompanied with either TAC or TCA 20% in keloid treatment. Keloid treatment progress was assessed clinically and radiologically.
Methods
This comparative study was conducted from March 2017 to October 2019 at the Outpatient Clinic of Dermatology and Venereology, where 45 Egyptian patients with keloids were enrolled randomly.
All participants wrote down an informed consent after demonstrating the study steps, expected results, and side effects before taking part in the study. Ethical aspects of human research were fulfilled in the current study after the official approval of the local research ethics committee of Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt with approval code (202106865).
Inclusion Criteria
Either male or female, 18 to 55 years old, duration of keloid scar of more than 6 months.
Exclusion Criteria
Patients who received any previous treatment during the 6 months before enrollment for their keloids, pregnant or lactating women, patients with photosensitivity, patients on retinoids treatment, and facial keloids.
Treatment Protocol
All patients underwent complete history taking, general and dermatological examinations, photographs, clinical assessment by Vancouver Scar Scale (VSS), and radiological assessment by Color Doppler Ultrasound (CDU) of keloids.
Materials Used
FCL, TAC, and TCA 20%.
Study design
This comparative study included 45 Egyptian participants with keloids, they were of both sexes (21 males and 24 females), and their ages were between 20 and 55 years. We classified the 45 cases into 3 groups of 15 cases each, the first group treated by FCL alone (as shown in Figure 1), the second group treated by FCL followed immediately by TAC at a dose at a dose of 0.25 ml/cm3 for keloids < 3 cm and 0.5 ml/cm3 for keloids >3 cm (equivalent to 10 mg/cm3 of TAC [40 mg/vial]) then occluded using transparent film dressings. Patients were instructed to remove the dressing 3 hours after the session by sterile saline-soaked gauze (as shown in Figure 2) while the third group was treated by FCL followed immediately by TCA 20% application then occluded for 3 hours as in group II (as shown in Figure 3). Topical anesthesia in the form of cream (prilocaine 2.5% and lidocaine 2.5%) was applied before the laser session under occlusion for 60 minutes and wiped off with saline. Laser parameters used were: Power = 20–25 J, Stack = 1–2, Timing = 300 ms (milliseconds) and spacing = 350 mm. Evaluation of keloid scars before and after treatment was done by VSS and CDU. The patients received 4 sessions 4 weeks apart and followed-up for 8 weeks after the last session.
Figure 1.

Female patient, 32 years old with abdominal post-surgical keloid. (A) before treatment (VSS 10). (B) after 4 sessions of fractional CO2 alone (VSS became 6), showing good improvement. VSS = Vancouver Scar Scale.
Figure 2.

Male patient, 23 years old with back post-traumatic keloid. (A) before treatment (VSS 9). (B) after 4 sessions of fractional CO2 followed by TAC (VSS became 2), showing excellent improvement. TAC = Triamcinolone acetonide; VSS = Vancouver Scar Scale.
Figure 3.

Female patient, 26 years old with thigh post-traumatic keloid. (A) before treatment (VSS 8). (B) after 4 sessions of fractional CO2 followed by TCA 20% (VSS became 4), showing good improvement but hypopigmentation occurred as a side effect. TCA= Trichloroacetic acid; VSS = Vancouver Scar Scale.
Evaluation Methods
Photographs by Sony Cyber shot DSC-H10, Japan camera, VSS (pliability, pigmentation, vascularity and scar thickness or height) were used, the scores range from 0 to 14 where 14 indicates the worst scar while score of 0 indicates normal skin. Keloid scar thickness measurement by CDU using a superficial probe (10 MHz) and patient satisfaction by patient satisfaction self-assessment (score from 0 to 4; not satisfied 0%, mildly satisfied <25%, moderately satisfied 25–50%, very good satisfied 50–75% or excellent satisfied >75%) were evaluated. Treatment side effects stated by physicians or patients were documented.
Statistical Analysis
Collected data were revised, coded, and entered into the Statistical Package for Social Science version 23. Quantitative data were presented as median, inter-quartile range when data were non-parametric. The comparison of quantitative data and non-parametric distribution between more than 2 groups was made by using Kruskall Wallis test. The comparison of quantitative data and non-parametric distribution between two paired groups was done by using Willcoxon Rank test. The confidence interval was set at 95%, and the margin of error accepted was set at 5%. P-value was considered significant as the following: P > 0.05: non significant, P < 0.05: significant, and P < 0.01: highly significant.
Results
Forty-five adult Egyptian cases with keloids were enrolled. They were 20–55 years old with a mean 33.69 (± 11.02 standard deviation). Among the studied groups, 24 cases were females (53.3%), while 21 were males (46.7%). The keloids were at different sites of the body (e.g. ears, chest, and back). Causes of keloid varied from trauma, surgery, spontaneous, and ear piercing. Nine cases (20%) had multiple keloids, 31 cases (80%) had keloid over bony prominences, and 8 cases were treated previously (17.8%).
Treatment Outcomes
Clinical Assessment
A high statistically significant reduction in VSS after treatment was detected among patients of the 3 groups with P <0.001. The most significant VSS reduction was in group II (−3.73 ± 1.58), then in group I (−3.40 ± 1.18), and the minor reduction was in group III (−1.73 ± 1.33) (Table 1).
Table 1.
VSS Before and After Treatment for Each Group of the Studied Cases
| Group I No. = 15 |
Group II No. = 15 |
Group III No. = 15 |
Test value | P-value | ||
|---|---|---|---|---|---|---|
| VSS before | Median (IQR) | 8 (6 – 11) | 10 (7 – 11) | 8 (8 – 11) | 1.546 | 0.462 (NS) |
| Range | 4 – 13 | 6 – 13 | 5 – 14 | |||
| VSS after | Median (IQR) | 4 (3 – 8) | 5 (4 – 8) | 8 (5 – 9) | 5.551 | 0.062 (NS) |
| Range | 1 – 9 | 2 – 10 | 3 – 12 | |||
| Differences | Mean ±SD | −3.40 ± 1.18 | −3.73 ± 1.58 | −1.73 ± 1.33 | 13.789 | 0.001 (HS) |
| Range | −5 – −2 | −7 – −2 | −4 – 1 | |||
| Willcoxon Rank test | −3.436 | −3.437 | −3.151 | |||
| P | 0.001 (HS) | 0.001 (HS) | 0.002 (HS) | |||
VSS = Vancouver Scar Scale; No = number; IQR= interquartile range; NS= Non significant; S= Significant; HS= highly significant; SD= standard deviation.
Radiological Assessment
The reduction in the keloid thickness measured by CDU after treatment among the 3 groups was statistically significant, where the greatest radiological reduction was in group I (−1.55 ± 5.22), then group II (−1.24 ± 2.13), and the minor reduction was in group III (−0.12 ±0.15) (Table 2).
Table 2.
Keloid Thickness by CDU Before and After Treatment For Each Group of the Studied Cases
| Group I No. = 15 |
Group II No. = 15 |
Group III No. = 15 |
Test value | P-value | ||
|---|---|---|---|---|---|---|
| Thickness before (cm 2 ) | Median (IQR) | 0.81 (0.27 – 1.35) | 1.82 (0.8 – 6.32) | 0.36 (0.23 – 0.73) | 11.131 | 0.004 (HS) |
| Range | 0.07 – 24.5 | 0.36 – 23.85 | 0.09 – 1.4 | |||
| Thickness after (cm 2 ) | Median (IQR) | 0.48 (0.14 – 2.07) | 1.12 (0.36 – 5.11) | 0.32 (0.17 – 0.59) | 6.162 | 0.046 (S) |
| Range | 0.06 – 8 | 0.12 – 15.6 | 0.01 – 1.04 | |||
| Difference | Mean ± SD | −1.55 ± 5.22 | −1.24 ± 2.13 | −0.12 ± 0.15 | 11.599 | 0.003 (HS) |
| Range | −19.6 – 1.41 | −8.25 – 0 | −0.41 – 0.06 | |||
| Willcoxon Rank test | −2.480 | −3.296 | −2.513 | |||
| P | 0.013 (S) | 0.001 (HS) | 0.012 (S) | |||
CDU= Color Doppler Ultrasound; No = number; IQR= interquartile range; S= Significant; HS= highly significant; SD= standard deviation.
The correlation between clinical and radiological responses was not statistically significant.
Patient Satisfaction
Patient satisfaction score grades 3 and 4 were the highest among group II with 40% (6 patients) and 46.7% (7 patients) respectively, then in group I with 6.7% (1 patient) and 33.3% (5 patients), respectively. While grades 1 and 0 of the score were the highest among patients of group III with 26.7% (4 patients) and 20% (3 patients) respectively.
In the current study, the relationship between clinical response measured by VSS and patient satisfaction self-assessment score was not statistically significant.
Side Effects Evaluation Among the Studied Groups
Among all patients, 20 (44.4 %) had no side effects, while 25 (55.6%) patients had side effects in the form of pain, itching, ulceration, hypopigmentation, and hyperpigmentation. The main side effects were increased pain and itching just after the session and subsided within a week maximum in the current study. As regards types of side effects, the results showed statistically significant difference among the 3 groups (P <0.05) whereas patients of groups I and II had suffered from mild side effects as pain and itching while group III patients had suffered from severe side effects as ulceration, hypopigmentation (shown in Figure 3), and hyperpigmentation beside beside pain and itching.. Neither clinical nor radiological responses had a statistically significant association with sex, or skin phototype of the patients, cause, site, size, multiplicity, or previous treatment of the keloid. However, the clinical improvement was better at non-bony sites as mean VSS reduction was −3.36 (± 1.39) while it was −2.77 (± 1.69) at bony sites. Also, no statistically significant association was found between either clinical or radiological responses and patient self-assessment score or side effects.
Conclusions
Keloid scar occurs as a result of extracellular matrix overproduction, mainly collagen. It occurs due to cytokines and growth factors overexpression [8]. Keloid formation has many theories, where the most approved one was the imbalance between collagen synthesis and degradation together with the imbalance of fibroblasts proliferation, apoptosis, and inhibition [3,9]. Keloid pathogenesis is mainly due to abnormal healing of wounds either due to abnormal response to inflammation or prolonged proliferative phase [9]. Increased collagen synthesis is related to keloid fibroblasts stimulation through inflammatory mediators, mainly transforming growth factors beta-1 (TGF-b1). TGF-b1 isoforms are supposed to be responsible for collagen overproduction by fibroblasts in pathological scars [10].
Treatment decisions must be based on the patient’s age, location, size and depth of the lesion, and past response to treatment. Hence no specific treatment is best for all keloids [11]. Regarding the current study, the results showed a statistically significant reduction in VSS score after treatment compared to before treatment in each group with P <0.01. However, the main improvement was in pliability. According to the current study, the group I received FCL as monotherapy. Compared to combined therapy in groups II and III, group I had good but not the best results.
Behera et al reported that FCL could be used in keloids excision efficiently, in agreement with the current results [12]. Also, Azzam et al reported that keloids could be treated by FCL securely and efficiently upon the results of their study on 30 patients who received 4 FCL sessions six weeks apart [13]. Nevertheless, they recommended that FCL could give better results when used as a combined therapy. As FCL inhibits TGFb1 release but stimulates basic fibroblast growth factor (bFGF) release and hence induces epidermal regrowth and dermis collagenesis and remodeling [12,14]. The laser gives better results when used on early scars of less than 2 years [15]. In group II, for patients who received combined therapy of FCL followed by TAC this combination resulted in a significant reduction in VSS parameters after treatment compared to groups I and III with the best satisfaction score (as shown in Figures 1,2 and 3).
Al-Janahi et al treated a 72 years old African American male suffering from aggressive keloid sited at the anterior and lateral neck, back, and upper chest that lasted for 30 years resulting in flattening of keloid and improvement of pain after 1 treatment [16]. Similar to the current study, Alegre-Sánchez et al stated that using CO2 laser in combination with TAC suspension 10% gives excellent results for keloid and hypertrophic scars treatment [17]. Also, they declared factors affecting the results of FCL followed by TAC suspension combination as the density of FCL, depth of channels created by FCL, which depend directly on laser fluency, the vehicle used, type of preparation, and formulations. In contrast, Annabathula et al reported that FCL is not recommended to treat keloids as a single therapy [18], but they recommended a combined therapy for a synergistic effect of treatment. Also, Waibel et al reported that using FCL followed by TAC suspension application in successive three treatments caused 23 % scar reduction [19]. According to Alexander et al study, they used intralesional injection instead of suspension application, giving good results [20]. Alegre-Sánchez et al reported that TAC in the form of suspension has a greater affinity than a cream or ointment, so passes through micro-channels produced by fractional ablative lasers easier [17]. Also, the improvement of texture, thickness, dyschromia, and scar functionality was reported using topical corticosteroid rather than an intralesional drug.
LADD facilitates penetration and provides better results when both medical as drugs and physical as laser treatments are combined [17]. Another key factor in LADD is the interval between using laser and drug application. Considering MTZs closure, it lasts for 6 hours after laser application; however, the vehicle or drug absorption is most successful during the first 30 minutes, while after 24 hours, no absorption is observed, so no need for further topical applications [17].
These studies came in agreement with the current study as regard laser parameters (Power = 20–25 J), the form of TAC we used (suspension), and the time of drug application after FCL application (drug applied immediately after laser session and removed after 3 hours using sterile saline-soaked gauze). FCL combined with TAC may minimize collagen production by decreasing fibroblast activity, with a low recurrence rate of 15.4%, which is superior to each modality and is ideal for large keloids [4].
Regarding the current study, group III was treated by FCL followed by topical application of TCA 20%. TCA is a peeling agent used for superficial and medium-depth peel according to its concentration. TCA acts through coagulative necrosis and protein precipitation of epidermal cells and collagen necrosis of papillary and reticular dermis. Patients of this group had the slightest improvement of keloids with the most severe side effects as ulceration, hypopigmentation, and hyperpigmentation compared with other groups. The combination between FCL and TCA in the treatment of keloids has not been evaluated before. Hence, we tried to evaluate it through our third group.
Ghonaim conducted a comparative study of 80% TCA multiple puncture techniques versus botulinum toxin type A (BTX-A) in keloid treatment and denoted that TCA can normalize all types of scars by dermal structure reconstruction [21]. Keloid fibroblasts have a greater capacity to proliferate and overproduce type I collagen. TCA-treated skin expresses cytokines as interleukin-10, which is involved in type I collagen synthesis, regulation, and degradation. On treating cultured dermal fibroblasts with TCA, collagen I was downregulated, and collagenase protein was upregulated, where platelet-derived growth factor-B expression was upregulated markedly, then downregulated immediately. This transient increase of platelet-derived growth factor could be beneficent for speedy wound repair [21].
In the current study, the improvement of VSS parameters after treatment (pliability, vascularity, pigmentation, and height/thickness of keloid) was statistically significant; however, the main improvement was in the pliability component, then thickness/height, then vascularity, and the least component improved was pigmentation. In agreement with the current results, Heppt et al and Azzam et al reported more evident improvement in VSS parameters, where scar pliability was markedly improved while vascularity and dyspigmentation were less pronounced [13,22]. Since biopsies in keloids are contraindicated as they can produce an increase in size, CDU can support activity assessment and indicate the response to treatment (as shown in Figures 4,5 and 6).
Figure 4.

High resolution B mode ultrasonic imaging of a cutaneous keloid with homogenous echopattern. (A) before treatment showing that keloid thickness was: 0.146 cm while the length was: 1.28 cm. (B) after 4 sessions of fractional CO2 alone showing decrease in the thickness to become 0.140 cm and in the length to become 1.19 cm, showing good improvement. On CDU application the lesion was completely avascular before and after treatment. CDU = Color Doppler Ultrasound.
Figure 5.
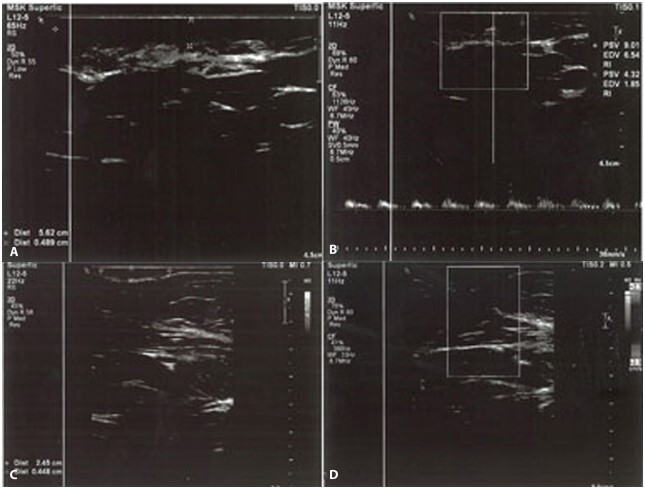
High resolution B mode ultrasonic imaging of a subcutaneous keloid with hypoechoic echopattern deep to the skin by 2 mm. (A) before treatment showing that keloid thickness was: 0.489 cm while the length was: 5.62 cm. (B) on CDU mode application, few peripherally located vessels are seen with arterial flow of low resistance. (C) High resolution B mode ultrasonic imaging of a keloid after 4 sessions of fractional CO2 followed by TAC showing decrease in the thickness to become 0.448 cm and in the length to become 2.45 cm, showing excellent improvement. (D) on CDU mode the keloid was completely avascular. CDU = Color Doppler Ultrasound; TAC = Triamcinolone acetonide.
Figure 6.

High resolution B mode ultrasonic imaging of a subcutaneous keloid with homogenous to hypoechoic echopattern. (A) before treatment showing that keloid thickness was: 0.175 cm while the length was: 1.10 cm. (B) after 4 sessions of fractional CO2 followed by TCA 20% showing decrease in the thickness to become 0.144 cm and in the length to become 0.666 cm, with moderate improvement. On CDU application the lesion was completely avascular before and after treatment. CDU = Color Doppler Ultrasound; TCA= Trichloroacetic acid
Elrefaie et al and Lobos et al advised using high-resolution ultrasonography in scar assessment to determine the suitable treatment modality as an available, quick, and affordable method [23,24]. As VSS has a high range of the thickness parameter (< 2, 2–5, and > 5mm), the scar thickness may be reduced but with no changes in VSS, while ultrasound measures the whole scar thickness not only the superficial height as in VSS. Also, CDU can assess the presence of calcifications, fistulous tracts, or muscle involvement on different body sites as ear pinna and trunk.
Regarding side effects, only 25 patients had adverse events in the form of increased pain and itching just after the session and subsided within a week maximum in group I and II, while group III had suffered from severe side effects as ulceration, hypopigmentation (as shown in Figure 3), and hyperpigmentation beside pain and itching.
In agreement with the current results, Alexander et al reported increased size, pain, hyperpigmentation, and depigmentation [20]. Also, Alegre-Sánchez et al explained that adverse effects might appear due to laser high fluencies and densities applied [17].
In the current study, regarding the site of keloid, 31 (68.9%) patients had keloids on bony prominences as chest, forearm, arm, neck, shoulder, back, interscapular, knee, and elbow, while 14 (31.1%) patients had keloids on non-bony structures as abdomen, thigh and ear lobe. We found no statistically significant relationship between the site of keloid and the clinical or radiological response; however, the clinical improvement was more at non-bony sites as mean VSS reduction was −3.36 (± 1.39) while at bony sites, it was −2.77 (± 1.69). We did not notice any recurrence among studied cases compared to Behera et al, who reported a high recurrence rate during a one-year follow-up period [12]. This may be due to the short period of follow-up in our study.
Current study limitations were a small sample of patients as we listed the patients who had the full number of sessions and follow-up so, we didn’t mention patients who didn’t complete the whole number of sessions, limited laser sessions, and had a short follow-up period.
FCL alone is a potent secured treatment modality of keloid, but combined therapy gives better results. However, combined therapy of FCL and topical TAC is better than monotherapy or combination with TCA in treating keloids as TCA can result in some serious side effects as ulceration, hypopigmentation, and hyperpigmentation.
Further studies of different combinations with FCL are recommended in the treatment of keloids. Also, more studies to assess the efficacy of applying TCA in lower concentrations after FCL versus TCA as monotherapy in the treatment of keloids are needed.
TCA is needed to be tried alone in treating keloid or combined with other therapies other than a fractional laser, though TCA can be combined with fractional laser but not in the same session to lessen the side effects.
Clinical assessment of keloid scar using score scales as VSS is not always accurate. It may underestimate the activity in keloid scar, so adding radiological methods in assessment as CDU is of great importance to help physicians in the choice of the most appropriate modality of treatment based on each scar assessed criteria.
Footnotes
Funding: None.
Competing interests: None.
Authorship: All authors have contributed significantly to this publication
References
- 1.Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models. Front Cell Dev Biol. 2020;8:360. doi: 10.3389/fcell.2020.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: The paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix Biol. 2016;51:37–46. doi: 10.1016/j.matbio.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betarbet U, Blalock TW. Keloids: A Review of Etiology, Prevention, and Treatment. J Clin Aesthet Dermatol. 2020;13(2):33–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton NJ, Garcia BA, Hoyer P, Wilkerson MG. Keloid Scars: An Updated Review of Combination Therapies. Cureus. 2021;13(1):e12999. doi: 10.7759/cureus.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalié M, Sillard L, Montaudié H, Bahadoran P, Lacour JP, Passeron T. Treatment of keloids with laser-assisted topical steroid delivery: a retrospective study of 23 cases. Dermatol Ther. 2015;28(2):74–78. doi: 10.1111/dth.12187. [DOI] [PubMed] [Google Scholar]
- 6.Bloom BS, Brauer JA, Geronemus RG. Ablative fractional resurfacing in topical drug delivery: an update and outlook. Dermatol Surg. 2013;39(6):839–848. doi: 10.1111/dsu.12111. [DOI] [PubMed] [Google Scholar]
- 7.Yildirim S, Gurel MS, Gungor S, Tekeli O, Canat D. Comparison of efficacy of chemical peeling with 25% trichloroacetic acid and 0.1% retinoic acid for facial rejuvenation. Postepy Dermatol Alergol. 2016;33(3):199–205. doi: 10.5114/ada.2016.60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel Insights on Understanding of Keloid Scar: Article Review. J Am Coll Clin Wound Spec. 2016;7(1–3):1–7. doi: 10.1016/j.jccw.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaheen A. Comprehensive Review of Keloid Formation. Clin Res Dermatol. 2017;5:1–18. doi: 10.15226/2378-1726/4/5/00168. [DOI] [Google Scholar]
- 10.Berman B, Maderal A, Raphael B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 11.Maghrabi, Ibrahim A, Kabel Ahmed M. Management of Keloids and Hypertrophic Scars: Role of Nutrition, Drugs, Cryotherapy and Phototherapy. World Journal of Nutrition and Health. 2014;2(2):28–32. doi: 10.12691/jnh-2-2-4. [DOI] [Google Scholar]
- 12.Behera B, Kumari R, Thappa DM, Malathi M. Therapeutic Efficacy of Intralesional Steroid With Carbon Dioxide Laser Versus With Cryotherapy in Treatment of Keloids: A Randomized Controlled Trial. Dermatol Surg. 2016;42(10):1188–1198. doi: 10.1097/DSS.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 13.Azzam OA, Bassiouny DA, El-Hawary MS, El Maadawi ZM, Sobhi RM, El-Mesidy MS. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31(1):9–18. doi: 10.1007/s10103-015-1824-4. [DOI] [PubMed] [Google Scholar]
- 14.Omi T, Numano K. The Role of the CO2 Laser and Fractional CO2 Laser in Dermatology. Laser Ther. 2014;23(1):49–60. doi: 10.5978/islsm.14-RE-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava S, Kumari H, Singh A. Comparison of Fractional CO2 Laser, Verapamil, and Triamcinolone for the Treatment of Keloid. Adv Wound Care (New Rochelle) 2019;8(1):7–13. doi: 10.1089/wound.2018.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Janahi S, Lee M, Lam C, Chung HJ. Laser-assisted drug delivery in the treatment of keloids: A case of extensive refractory keloids successfully treated with fractional carbon dioxide laser followed by topical application and intralesional injection of steroid suspension. JAAD Case Rep. 2019;5(10):840–843. doi: 10.1016/j.jdcr.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alegre-Sánchez A, Jiménez-Gómez N, Boixeda P. Laser-Assisted Drug Delivery. Vehiculización de fármacos asistida por láser. Actas Dermosifiliogr (Engl Ed) 2018;109(10):858–867. doi: 10.1016/j.ad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Annabathula A, Sekar CS, Srinivas CR. Fractional Carbon Dioxide, Long Pulse Nd:YAG and Pulsed Dye Laser in the Management of Keloids. J Cutan Aesthet Surg. 2017;10(2):76–80. doi: 10.4103/JCAS.JCAS_136_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med. 2013;45(3):135–140. doi: 10.1002/lsm.22120. [DOI] [PubMed] [Google Scholar]
- 20.Alexander S, Girisha BS, Sripathi H, Noronha TM, Alva AC. Efficacy of fractional CO2 laser with intralesional steroid compared with intralesional steroid alone in the treatment of keloids and hypertrophic scars. J Cosmet Dermatol. 2019;18(6):1648–1656. doi: 10.1111/jocd.12887. [DOI] [PubMed] [Google Scholar]
- 21.Ghonaim N. Comparative study of the 80% trichloroacetic acid multiple puncture technique versus botulinum toxin type A in the treatment of keloid scars. Egypt J Dermatol Venerol. 2013;33:22–27. doi: 10.7123/01.EJDV.0000431207.04926.59. [DOI] [Google Scholar]
- 22.Heppt MV, Breuninger H, Reinholz M, Feller-Heppt G, Ruzicka T, Gauglitz GG. Current Strategies in the Treatment of Scars and Keloids. Facial Plast Surg. 2015;31(4):386–395. doi: 10.1055/s-0035-1563694. [DOI] [PubMed] [Google Scholar]
- 23.Elrefaie AM, Salem RM, Faheem MH. High-resolution ultrasound for keloids and hypertrophic scar assessment. Lasers Med Sci. 2020;35(2):379–385. doi: 10.1007/s10103-019-02830-4. [DOI] [PubMed] [Google Scholar]
- 24.Lobos N, Wortsman X, Valenzuela F, Alonso F. Color Doppler Ultrasound Assessment of Activity in Keloids. Dermatol Surg. 2017;43(6):817–825. doi: 10.1097/DSS.0000000000001052. [DOI] [PubMed] [Google Scholar]


