Abstract
Introduction
Evidence is lacking regarding the association between cardiovascular health (CVH) metrics and the risk for proteinuria.
Methods
We performed this observational cohort study including 865,087 participants (median age, 46 years, 60.7% men) with negative proteinuria at the initial health check-up, who underwent repeated health check-ups within 4 years. Ideal CVH metrics included nonsmoking, body mass index <25 kg/m<sup>2</sup>, physical activity at goal, eating breakfast, blood pressure <120/80 mm Hg, fasting plasma glucose <100 mg/dL, and total cholesterol <200 mg/dL. The primary outcome was incident proteinuria, defined as ≥1 + on the urine dipstick test.
Results
Participants were categorized as having low CVH metrics defined as having 0–2 ideal CVH metrics (n = 84,439), middle CVH metrics defined as having 3–4 ideal CVH metrics (n = 335,773), and high CVH metrics defined as having 5–7 ideal CVH metrics (n = 444,875). Compared with low CVH metrics, middle CVH metrics (odds ratio (OR): 0.61, 95% CI: 0.59–0.63) and high CVH metrics (OR: 0.45, 95% CI: 0.43–0.46) were associated with a lower risk of proteinuria. The OR of a one-point increase in the ideal number of CVH metrics was 0.83 (95% CI: 0.82–0.83). All CVH metrics components except for ideal total cholesterol were associated with a decreased risk of proteinuria. A one-point improvement in the number of ideal CVH metrics at 1 year after the initial health check-up was associated with a decreased incidence of proteinuria (OR: 0.90, 95% CI: 0.89–0.92).
Conclusion
Not only maintaining better CVH metrics but also improving CVH metrics would prevent developing proteinuria in a general population.
Keywords: Proteinuria, Cardiovascular health metrics, Epidemiology
Introduction
Proteinuria is not only a marker of early kidney damage and an aggravating factor for chronic kidney disease [1], but also known to increase a risk of cardiovascular disease (CVD) and all-cause mortality [2]. Proteinuria is thus regarded as a prelude to the development of end-stage kidney disease and CVD. Identifying the risk factors for proteinuria is important from the perspective of preventing CVD and end-stage kidney disease. Several studies have shown that unhealthy lifestyles and lifestyle-related diseases could increase the risk of proteinuria [3, 4]. Unhealthy lifestyles and lifestyle-related diseases are also involved in the development of CVD. Accordingly, the American Heart Association (AHA) proposed CVH metrics consisting of seven modifiable risk factors, to reduce the burden of CVD [5, 6]. These CVH metrics have been reported to stratify the future risk of CVD [7, 8, 9]. Given the shared pathological mechanisms, CVH metrics could be associated with the risk of proteinuria. However, no reports have examined the association between CVH metrics and the risk of proteinuria. Furthermore, whether improving modifiable risk factors could reduce the risk of proteinuria has remained unclear. Using a nationwide population-based database, we aimed to identify the role of CVH metrics in the development of proteinuria.
Materials and Methods
Study Design and Data Source
This retrospective observational longitudinal study included analyses of health check-ups records from the JMDC Claims Database (JMDC Inc., Tokyo, Japan) [9, 10, 11]. The JMDC Claims Database comprises the individual administrative claims data of more than 60 insurers of Japanese companies.
Considering the follow-up period of 4 years was necessary, we reviewed data on individuals whose initial health check-up was conducted between January 2005 and March 2016 because the JMDC database currently includes data until April 2020. We extracted 1,005,141 individuals with available health check-up data for assessing CVH metrics with a negative proteinuria. We excluded the following individuals: those who did not undergo repeated dipstick urine tests (n = 106,655), those who had been diagnosed with chronic renal failure or had a history of dialysis (n = 1,122), and those who had missing data regarding chronic renal failure or history of dialysis (n = 32,277). Consequently, we analyzed the data of 865,087 individuals in this study (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000522147). Furthermore, characteristics of individuals who were excluded from the primary analysis due to absence of a urine dipstick test at follow-up examinations (n = 106,655) are described in online supplementary Table 1.
Ethics
This study was approved by the Ethical Committee of the University of Tokyo (number: 2018-10862). We performed this study in accordance with the Declaration of Helsinki. Since all data from the JMDC Claims Database were deidentified, the requirement for informed consent was waived.
Measurements, Definitions, and Urine Dipstick Test
We modified the original definitions of AHA for CVH metrics to fit the database [9, 12]. Online supplementary Table 2 lists the original AHA definitions and the modified definitions in this study. Ideal CVH metrics were set as follows: ideal body mass index was defined as <25 kg/m2, ideal smoking status was defined as not smoking (never smoked or previously smoked), and a current smoker was defined as smoking ≥100 cigarettes in a lifetime or smoking duration ≥6 months. Ideal physical activity was defined as 30 min of exercise at least twice a week or ≥1 h of walking per day. Ideal eating habits were defined as skipping breakfast <3 times per week. Ideal blood pressure was defined as an untreated blood pressure level of <120/80 mm Hg, and the ideal fasting plasma glucose level was defined as an untreated value of <100 mg/dL. We defined ideal total cholesterol as an untreated value of <200 mg/dL. If the total cholesterol figures were not available, we calculated it using low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride [13]. Urine dipstick tests were conducted on fresh, midstream urine samples as part of a health check-up.
Outcomes
The primary outcome was the development of proteinuria defined as ≥1+ on the urine dipstick test. The outcome was determined based on whether the dipstick result turned positive at least once over the following 4 years from the initial health check-up. Urinary dipstick proteinuria was reported to correspond to ≥30 mg/g of albumin-to-creatinine ratio with the sensitivity of 47.0% and specificity of 96.2% [14]. Furthermore, the sensitivity to detect 150–499 mg/g or higher in the protein-to-creatinine ratio when the protein result by dipstick is ± or higher is 50.0–75.0%, and the specificity is 94.5–96.0% [15].
Statistical Analysis
Data are presented as median (interquartile range) for continuous variables or as number (percentage) for categorical variables. Based on previous study [9], we divided the study participants according to the number of ideal CVH metrics as follows: low (0–2 points), middle (3–4 points), and high (5–7 points). We performed logistic regression analyses to examine the association between CVH metric category and development of proteinuria. Odds ratios (ORs) were calculated in an unadjusted model (Model 1) and after adjustment for age and sex (Model 2). We also analyzed the relationship between the number of ideal CVH metrics and incident proteinuria. Subsequently, we conducted logistic regression analyses to evaluate the association between each of the CVH metrics and the development of proteinuria. In this analysis, each of the seven CVH metrics components was simultaneously included in Model 1 as one independent variable. Seven CVH metrics components plus age and sex were simultaneously included as Model 2. To evaluate the relationship between changes in CVH metrics over 1 year and the development of proteinuria, 609,307 individuals were included in secondary analyses. Inclusion and exclusion criteria are shown in online supplementary Figure 2. We used logistic regression analyses to examine the association between the number of changes in CVH metrics over 1 year and the development of proteinuria. Furthermore, individuals were classified into nine groups, using cross-tabulations of the CVH metric categories at the initial health check-up and after 1 year.
We conducted ten sensitivity analyses. First, we performed subgroup analyses stratified by sex. Second, we reanalyzed the association between CVH metrics category and the development of proteinuria within 4 years using the Cox proportional hazard regression model. Third, we extracted 335,183 individuals with available data on estimated glomerular filtration rate (eGFR). We examined the association between the CVH metric category and the development of proteinuria after adjustment for eGFR. Fourth, because dietary habits vary greatly from country to country, the association between the CVH metrics category excluding eating habit component and the development of proteinuria was examined. Fifth, we individually examined the association between each CVH metrics component and the development of proteinuria in models without including the other CVH components. In these analyses, seven CVH metrics components were incorporated into the different models as independent variables, one by one. Sixth, we performed a classification tree analysis based on the χ2 automatic interaction detection to examine the hierarchy of the strength of association of CVH metrics components. The variable importance ranking in the classification tree generated the relative importance of the seven CVH metrics components. Seventh, we defined the primary outcome as trace or positive proteinuria. Eighth, we excluded individuals taking medication for hypertension, dyslipidemia, and diabetes mellitus (n = 129,830). The number of individuals taking medication for hypertension, dyslipidemia, and diabetes mellitus is shown in online supplementary Table 3. Ninth, we extended the maximum follow-up period from 4 years to 5 years. Inclusion and exclusion criteria are shown in online supplementary Figure 3a. Tenth, we shortened the maximum follow-up period from 4 years to 3 years. Inclusion and exclusion criteria are shown in online supplementary Figure 3b.
Some people were censored before the end of the maximum follow-up from the initial health check-up. To adjust for possible bias due to loss to follow-up, we conducted all analyses examining the association of CVH metrics and its change over time with the development of proteinuria in this study using the inverse probability of censoring weighted method (described in online suppl. Methods) [16, 17]. Statistical significance was set at p < 0.05. Statistical analyses were performed using STATA v17 (StataCorp LLC, College Station, TX, USA).
Results
Basic Characteristics of Study Participants
The baseline characteristics of the study participants are presented in Table 1. We studied 865,087 individuals who had available data for the assessment of CVH metrics and had negative proteinuria at the initial health check-up. The median age was 46 (interquartile range [IQR] 40–54) years, and 525,481 individuals (60.7%) were men. The median number of ideal CVH metrics was 5 (IQR 3–6). Study participants were divided into low (n = 84,439), middle (n = 335,773), and high (n = 444,875) CVH metric categories according to the number of ideal CVH metrics. Individuals in the high CVH metrics category were younger and more likely to be women than those with low CVH metrics. The numbers of individuals who attended each of the four follow-up health check-ups are represented in online supplementary Table 4. Furthermore, baseline characteristics of study participants divided by the presence or absence of censoring before the end of the 4-year follow-up are shown in online supplementary Table 5.
Table 1.
Baseline characteristics of study participants
| Overall | CVH metric category |
p value | |||
|---|---|---|---|---|---|
| Low (0–2) | Middle (3–4) | High (5–7) | |||
| N | 865,087 | 84,439 | 335,773 | 444,875 | − |
| Age, years | 46 (40, 54) | 49 (42, 55) | 49 (42, 56) | 44 (39, 52) | <0.001 |
| Male sex, n (%) | 525,481 (60.7) | 73,348 (86.9) | 243,610(72.6) | 208,523 (46.9) | <0.001 |
| Body mass index, kg/m2 | 22.4 (20.3, 24.7) | 26.4 (24.9, 28.5) | 23.5(21.4, 25.8) | 21.2(19.5, 22.9) | <0.001 |
| Systolic blood pressure, mm Hg | 118(108, 129) | 129 (122, 138) | 124(116, 133) | 111 (103, 120) | <0.001 |
| Diastolic blood pressure, mm Hg | 73(66, 81) | 82 (76, 88) | 78 (70, 84) | 69 (62, 75) | <0.001 |
| Total cholesterol, mg/dL | 203.0(180.6, 227.0) | 222.2 (205.0, 244.6) | 212.8(192.0, 234.6) | 191.2(173.0, 214.0) | <0.001 |
| Fasting plasma glucose, mg/dL | 92 (86, 99) | 103 (96, 113) | 95 (88, 102) | 89 (84, 94) | <0.001 |
| The number of ideal CVH metrics components, points | 5(3, 6) | 2 (2, 2) | 4(3, 4) | 5(5, 6) | <0.001 |
| 0, n (%) | 2,650 (0.3) | 2,650(3.1) | − | − | |
| 1, n(%) | 18,206(2.1) | 18,206(21.6) | − | − | |
| 2, n (%) | 63,583 (7.3) | 63,583 (75.3) | − | − | |
| 3, n (%) | 134,100(15.5) | − | 134,100(39.9) | − | |
| 4, n (%) | 201,673(23.3) | − | 201,673(60.1) | − | |
| 5, n (%) | 224,928 (26.0) | − | − | 224,928 (50.6) | |
| 6, n (%) | 168,119(19.4) | − | − | 168,119(37.8) | |
| 7, n (%) | 51,828(6.0) | − | − | 51,828(11.7) | |
| Ideal CVH metrics component, n (%) | |||||
| Smoking status | 645,562 (74.6) | 30,908 (36.6) | 219,352(65.3) | 395,302 (88.9) | <0.001 |
| Body mass index | 664,453 (76.8) | 21,171 (25.1) | 222,004(66.1) | 421,278(94.7) | <0.001 |
| Physical activity | 381,191 (44.1) | 11,274(13.4) | 112,770(33.6) | 257,147(57.8) | <0.001 |
| Dietary habits | 712,448(82.4) | 42,898 (50.8) | 257,367 (76.6) | 412,183(92.7) | <0.001 |
| Blood pressure | 412,657 (47.7) | 6,013(7.1) | 86,345 (25.7) | 320,299 (72.0) | <0.001 |
| Fasting plasma glucose | 660,631 (76.4) | 25,271 (29.9) | 219,847(65.5) | 415,513(93.4) | <0.001 |
| Total cholesterol | 373,572 (43.2) | 7,837 (9.3) | 91,307(27.2) | 274,428(61.7) | <0.001 |
We divided the study participants according to the number of ideal CVH metrics components as follows: low (0–2 points), middle (3–4 points), or high (5–7 points). Data are presented as median (interquartile range) or number (percentage). CVH, cardiovascular health. p values were calculated using the Kruskal-Wallis test for continuous variables and χ2 tests for categorical variables, p values were all <0.001.
Association between CVH Metrics at the Initial Health Check-Up and Risk of Proteinuria
During the 4-year follow-up, 41,474 individuals tested positive for proteinuria (≥1+) in the urine dipstick test. The number of events at each health check-up is shown in online supplementary Table 6. Table 2 shows the associations between the CVH metric category and risk of proteinuria. Compared with the low CVH metrics category, the middle CVH metrics category (OR: 0.61, 95% CI: 0.59–0.63) and high CVH metrics category (OR: 0.45, 95% CI: 0.43–0.46) were associated with a decreased risk of proteinuria after adjustment for age and sex. The risk of proteinuria decreased with an increase in the number of ideal CVH metrics (online suppl. Table 7). The age- and sex-adjusted OR per one-point increase in the number of ideal CVH metrics was 0.83 (95% CI: 0.82–0.83).
Table 2.
Association between CVH metrics category and the development of proteinuria
| CVH metric category |
p value for trend | |||||
|---|---|---|---|---|---|---|
| Low (n = 84,439) | Middle (n = 335,773) | High (n = 444,875) | ||||
| Number of events | 7,067 (8.4) | 17,335 (5.2) | 17,072 (3.8) | − | ||
| Model 1 | 1 (Reference) | 0.60 (0.58–0.61) | 0.44 (0.43–0.45) | <0.001 | ||
| Model 2 | 1 (Reference) | 0.61 (0.59–0.63) | 0.45 (0.43–0.46) | <0.001 | ||
We divided the study participants according to the number of ideal CVH metrics components as follows: Low (0–2 points), Middle (3–4 points), or High (5–7 points). The development of proteinuria was defined as positive proteinuria (≥1+) by the urine dipstick test. Model 1 included the CVH metrics category alone. Model 2 included the CVH metrics category, age, and sex. The OR was calculated using the logistic regression analysis. The incidence of proteinuria was calculated using the number of occurrences among the individuals included in this analysis, regardless of whether they were censored during the follow-up period. CVH, cardiovascular health.
Table 3 shows the association between each CVH metric and the risk of proteinuria. Each of the seven CVH measures was simultaneously included in model as one independent variable. The ideal status of smoking, body mass index, physical activity, dietary habits, blood pressure, and fasting plasma glucose was associated with a lower risk for the development of proteinuria. The ideal status of total cholesterol was associated with a higher risk for the development of proteinuria.
Table 3.
Association between each CVH metrics component and the development of proteinuria after multivariable adjustment
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Smoking status | ||||||
| Ideal (vs. nonideal) | 0.76 | (0.75–0.78) | <0.001 | 0.80 | (0.78–0.82) | <0.001 |
| Body mass index | ||||||
| Ideal (vs. nonideal) | 0.66 | (0.65–0.68) | <0.001 | 0.70 | (0.68–0.71) | <0.001 |
| Physical activity | ||||||
| Ideal (vs. nonideal) | 0.93 | (0.91–0.94) | <0.001 | 0.95 | (0.93–0.97) | <0.001 |
| Dietary habits | ||||||
| Ideal (vs. nonideal) | 0.74 | (0.72–0.76) | <0.001 | 0.82 | (0.80–0.84) | <0.001 |
| Blood pressure | ||||||
| Ideal (vs. nonideal) | 0.85 | (0.83–0.87) | <0.001 | 0.81 | (0.79–0.83) | <0.001 |
| Fasting plasma glucose | ||||||
| Ideal (vs. nonideal) | 0.80 | (0.79–0.82) | <0.001 | 0.74 | (0.72–0.75) | <0.001 |
| Total cholesterol | ||||||
| Ideal (vs. nonideal) | 1.13 | (1.11–1.16) | 0.003 | 1.03 | (1.01–1.05) | 0.01 |
The development of proteinuria was defined as positive proteinuria (≥1+) by the urine dipstick test. Each of the seven CVH metrics components was included in the same model. Model 1 included seven components of CVH metrics. Model 2 included seven components of CVH metrics, age, and sex. CVH, cardiovascular health; OR, odds ratio; 95% CI, 95% confidence interval.
Associations between Change in CVH Metrics and the Risk of Proteinuria
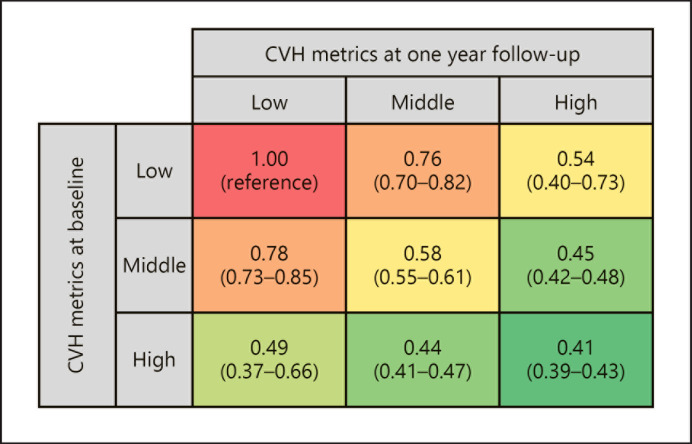
We analyzed 609,307 individuals to examine the relationship between changes in CVH metrics and the risk of developing proteinuria. As shown in Table 4, a one-point increase in the number of ideal CVH metrics was associated with a lower risk for the development of proteinuria (OR: 0.90, 95% CI: 0.89–0.92) after adjustment for baseline number of ideal CVH metrics, age, and sex. A logistic regression analysis was performed using nine groups according to the combination of CVH metrics categories at the initial health check-up and after 1 year. As shown in Figure 1, change in the CVH metrics category at 1-year follow-up was associated with the risk for the development of proteinuria independent of CVH metrics category at the initial health check-up. Among individuals with low CVH metrics at the initial health check-up, those with middle (OR: 0.76, 95% CI: 0.70–0.82) or high CVH metrics (OR: 0.54, 95% CI: 0.40–0.73) at the 1-year follow-up had lower risk of developing proteinuria than those still with low CVH metrics at 1-year follow-up.
Table 4.
Association of CVH metrics at the initial health check-up and its change over time with the development of proteinuria after multivariable adjustment
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Number of ideal CVH metrics components at the initial health check-up (per 1 point increase) | 0.82 | (0.81–0.83) | <0.001 | 0.81 | (0.80–0.82) | <0.001 |
| Change in number of ideal CVH metrics components (per 1 point increase over 1 year) | 0.90 | (0.89–0.92) | <0.001 | 0.90 | (0.89–0.92) | <0.001 |
The development of proteinuria was defined as positive proteinuria (≥1+) by the urine dipstick test. Model 1 included the number of ideal CVH metrics components at the initial health check-up and the change in the number of ideal CVH metrics components. Model 2 included the number of ideal CVH metrics components at the initial health check-up, the change in the number of ideal CVH metrics components, age, and sex. CVH, cardiovascular health; OR, odds ratio; 95% CI, 95% confidence interval.
Fig. 1.
Association of CVH metrics categories at the initial health check-up and 1 year after the initial health check-up with the development of proteinuria. We divided study participants according to the CVH metrics categories (low, middle, and high) at the initial health check-up and 1 year after. The development of proteinuria was defined as positive proteinuria (≥1+) assessed using urine dipstick test. The OR was calculated using the logistic regression analysis. Covariates in the logistic regression analysis were CVH metrics category at the initial health check-up, CVH metrics category 1 year after the initial health check-up, age, and sex.
Sensitivity Analysis
We conducted ten sensitivity analyses. First, compared with the low CVH metrics category, the middle CVH metrics category and high CVH metrics category were associated with a lower risk of proteinuria in both men and women (online suppl. Table 8). Second, compared with the low CVH metrics category, the middle CVH metrics category and high CVH metrics category had a lower risk for the incidence of proteinuria also in analysis using the Cox proportional hazard regression model (online suppl. Table 9). Third, we extracted 335,183 individuals with available data on eGFR at the initial health check-up. Even after adjustment for eGFR, the middle CVH metrics category and high CVH metrics category had a lower risk of proteinuria compared to the low CVH metrics category (online suppl. Table 10). Fourth, the CVH metrics category excluding eating habit component was significantly associated with the risk of proteinuria (online suppl. Table 11). Fifth, using a model that did not include other CVH components, we evaluated the association between each CVH metrics component and the development of proteinuria separately. Online supplementary Table 12 shows that individual each ideal CVH metrics component was associated with a lower risk of proteinuria after adjusting for age and sex in models but not adjusting for the other CVH components. In model 1, ideal cholesterol was associated with a higher risk of the development of proteinuria, whereas in model 2, ideal cholesterol was associated with a lower risk of proteinuria. Sixth, the classification tree analysis found that BMI was a relatively important factor in predicting the development of proteinuria among the seven CVH metrics components (online suppl. Fig. 4). Seventh, we defined the development of proteinuria as trace or ≥1+ proteinuria on a urine dipstick test. Similar to the primary analysis, compared with the low CVH metrics category, the middle and high CVH metrics categories were associated with a lower risk of developing proteinuria (online suppl. Table 13). A higher number of ideal CVH metrics was associated with a decreased incidence of proteinuria (online suppl. Table 14). The ideal smoking status, body mass index, physical activity, dietary habits, and fasting plasma glucose were associated with a lower risk of proteinuria (online suppl. Table 15). Eighth, we analyzed 735,257 individuals after excluding those taking medication for hypertension, dyslipidemia, or diabetes mellitus. Even in this population, compared with the low CVH metrics category, the middle and high CVH metrics categories were associated with a lower risk of developing proteinuria (online suppl. Table 13). A higher number of ideal CVH metrics was associated with a lower incidence of proteinuria (online suppl. Table 14). Ideal smoking status, body mass index, physical activity, dietary habits, blood pressure, and fasting plasma glucose were associated with a decreased risk for the development of proteinuria, while ideal total cholesterol was not (online suppl. Table 15). We extended the maximum follow-up period to 5 years and analyzed 587,421 individuals in the ninth sensitivity analysis, while we shortened the maximum follow-up period to 3 years and analyzed 1,099,317 individuals in the tenth sensitivity analysis. The association of CVH metrics and each CVH metrics component with incident proteinuria was similar to that in the primary results in both models (online suppl. Table 13–15).
Discussion
Our analysis of a nationwide population-based database including approximately 850,000 people with negative proteinuria at the initial health check-up demonstrated that greater CVH metrics were associated with a lower incidence of proteinuria in the urine dipstick test. Furthermore, improvement in CVH metrics 1 year after the initial health check-up was associated with a lower risk of proteinuria. Our study is distinguishable from similar studies in that improvement in CVH metrics could decrease the risk for developing proteinuria. To the best of our knowledge, this is the first study to show not only the relationship between modifiable risk factors and the future risk of proteinuria, but also the potential of the improvement in modifiable risk factors to prevent the proteinuria development, using a large-scale population-based dataset.
Although our study is the first to show the relationship between CVH metrics and incident proteinuria, our results are in line with previous studies. A previous study showed that current cigarette smokers had a 1.51 times higher risk of proteinuria [18]. Various mechanisms, including endothelial dysfunction, activation of growth factors, tubulotoxic effects, and oxidative stress associated with cigarette smoking, are thought to induce proteinuria [19]. Obesity was also reported to increase a risk of proteinuria [20]. Several pathological links, including activation of the renin-angiotensin system, glomerular hyperfiltration, and structural changes in the kidney, have been suggested [21]. The results of our study may be useful in elucidating the onset of obesity-related glomerulopathy and reducing its risk of developing. Because obesity-related glomerulopathy is caused not only by obesity but also by abnormalities in glucose tolerance, blood pressure, and lipid homeostasis [22], our result is consistent in that improvement in CVH metrics can prevent obesity-related glomerulopathy.
In this study, maintaining physical activity was associated with a lower incidence of proteinuria, which is also consistent with preceding studies [23]. Enhanced chronic inflammation in people with physical inactivity might explain this association [24]. The link between skipping breakfast and incident proteinuria is also of interest. Although data on the relationship between eating habits and the risk of proteinuria are scarce, results of earlier studies were in line with our findings [4, 25, 26].
On the other hand, ideal cholesterol levels were paradoxically associated with a higher risk of proteinuria; this result should be validated in other datasets. However, considering that statistical significance disappeared after excluding individuals taking medication for dyslipidemia, categorizing individuals taking medication for dyslipidemia (e.g., statins) in the nonideal cholesterol status group might have influenced the result. There are no consistent data on the relationship between total cholesterol and the development of proteinuria [20, 27, 28], and further research and discussion is needed.
Our study has several clinical implications. The AHA recommends the use of CVH metrics as a simple tool for CVD risk stratification [5]. Because proteinuria is known to precede the CVD development [29, 30], our results showed a robust relationship between a higher number of ideal CVH metrics and lower incidence of proteinuria, which would provide CVH metrics with additional clinical utility. By demonstrating that the annual improvement in CVH metrics was associated with a lower risk for proteinuria development, our study suggests the potential of therapeutic intervention in people with poor CVH metric status for the prevention of proteinuria.
This study had several limitations. There could be unmeasured confounders and residual bias. For example, alcohol consumption may influence the risk of proteinuria [31]. Although CVH metrics do not consider alcohol consumption, further study is required to develop the most optimal model to predict proteinuria development. The data from the JMDC Claims Database were mainly obtained from an employed, working-age population, and we studied people undergoing repeated health check-ups including a urine dipstick test. Therefore, we need to consider the possibility of a selection bias. For example, we excluded people who did not have a urine dipstick test at follow-up examinations. Although median age was slightly higher compared to the population in the primary analysis, other characteristics including CVH metrics score were not so different between 2 populations as shown in online supplementary Table 1. Further, although there was a slightly lower incidence of positive proteinuria in individuals who were censored, our results using censoring weights, adjusting for the possible selection bias to some extent, yielded similar results. The original definition of CVH metrics determines eating habits based on dietary components (DASH diet) as shown in online supplementary Table 2. Unfortunately, information on dietary components was not available in the JMDC Claims Database, and therefore, we defined ideal eating habits as not skipping breakfast. Data on quantified urinary protein or albumin were not collected in the JMDC Claims Database. Although we performed several sensitivity analyses and our main results were robust, the reproducibility of proteinuria assessed using the urine dipstick test is considered lower than that of other quantitative measurements for proteinuria (albuminuria). This study was conducted on the Japanese population, and the original CVH metrics were modified to fit the Japanese health check-up. Therefore, the results of the present study may have limited generalizability to other countries with different lifestyles and cultures. It may be necessary to develop a more general and simple method of assessing lifestyles such as physical activity and eating habits. Finally, the underlying mechanism for the association between CVH metrics and the development of proteinuria cannot be determined in this study.
In conclusion, a higher number of ideal CVH metrics at baseline were associated with a lower risk of proteinuria development. Furthermore, improvement in CVH metrics was associated with a decreased incidence of proteinuria. Our results suggest the clinical importance of modifiable risk factors and lifestyle factors in the development of proteinuria. Our results also showed the potential of therapeutic interventions for risk factors and unhealthy lifestyles for the prevention of proteinuria.
Statement of Ethics
This study was approved by the Ethical Committee of the University of Tokyo (number: 2018-10862) and was performed in accordance with the Declaration of Helsinki. Since all data from the JMDC Claims Database were deidentified, the requirement for informed consent was waived.
Conflict of Interest Statement
Research funding and scholarship funds (Hidehiro Kaneko and Katsuhito Fujiu) from Medtronic Japan Co., Ltd., Biotronik Japan, SIMPLEX QUANTUM CO., LTD, Boston Scientific Japan CO., LTD, and Fukuda Denshi, Central Tokyo CO., LTD. The authors have no conflicts of interest to disclose. Akira Okada and Satoko Yamaguchi are members of the Department of Prevention of Diabetes and Lifestyle-related Diseases, which is a cooperative program between the University of Tokyo and Asahi Mutual Life Insurance Company. No potential conflicts of interest relevant to this study were reported.
Funding Sources
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, and 21K08123). The funding sources had no competing interests with regard to the current study.
Author Contributions
H.K., A.O., and I.K. conceived and designed this study; Y.S., A.O., H.I., M.K., N.M., T.J., and H.Y. analyzed the data. H.K., A.O., S.Y., N.T., K.K., J.A., A.M., A.F., A.N., T.Y., H.M., K.N., T.Y., M.N., H.Y., and I.K. interpreted the data; H.K., A.O., H.M., and I.K. drafted the manuscript; N.T., H.M., and K.N. contributed to critical revision for important intellectual content; J.A., T.Y., M.N., H.Y., and I.K. gave final approval of the submitted manuscript.
Data Availability Statement
I, Hidehiro Kaneko, as the corresponding author, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This database is available for anyone who purchases it from the JMDC inc (https://www.jmdc.co.jp/en/index).
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
References
- 1.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011 Jul;80((1)):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Chronic Kidney Disease Prognosis Consortium et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375((9731)):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly JT, Su G, Zhang L, Qin X, Marshall S, González-Ortiz A, et al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021 Jan;32((1)):239–53. doi: 10.1681/ASN.2020030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada R, Tsushita K, Wakai K, Kato K, Wada T, Shinohara Y. Healthy lifestyle reduces incidence of trace/positive proteinuria and rapid kidney function decline after 2 years: from the Japan Ningen Dock study. Nephrol Dial Transplant. 2021 May 27;36((6)):1039–48. doi: 10.1093/ndt/gfaa224. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121((7)):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 6.Gaye B, Tajeu GS, Offredo L, Vignac M, Johnson S, Thomas F, et al. Temporal trends of cardiovascular health factors among 366 270 French adults. Eur Heart J Qual Care Clin Outcomes. 2020 Apr 1;6((2)):138–46. doi: 10.1093/ehjqcco/qcz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012 Mar 28;307((12)):1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaye B, Canonico M, Perier MC, Samieri C, Berr C, Dartigues JF, et al. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the three-city study. J Am Coll Cardiol. 2017 Jun 27;69((25)):3015–26. doi: 10.1016/j.jacc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko H, Itoh H, Kamon T, Fujiu K, Morita K, Michihata N, et al. Association of cardiovascular health metrics with subsequent cardiovascular disease in young adults. J Am Coll Cardiol. 2020 Nov 17;76((20)):2414–6. doi: 10.1016/j.jacc.2020.09.545. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, et al. Association of blood pressure classification using the 2017 American college of cardiology/American heart association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation. 2021 Jun 8;143((23)):2244–53. doi: 10.1161/CIRCULATIONAHA.120.052624. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko H, Itoh H, Kiriyama H, Kamon T, Fujiu K, Morita K, et al. Fasting plasma glucose and subsequent cardiovascular disease among young adults: analysis of a nationwide epidemiological database. Atherosclerosis. 2021 Feb;319:35–41. doi: 10.1016/j.atherosclerosis.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, et al. Cardiovascular health metrics of 87,160 couples: analysis of a nationwide epidemiological database. J Atheroscler Thromb. 2021 May 1;28((5)):535–43. doi: 10.5551/jat.55939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18((6)):499–502. [PubMed] [Google Scholar]
- 14.Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020 Sep 15;173((6)):426–35. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usui T, Yoshida Y, Nishi H, Yanagimoto S, Matsuyama Y, Nangaku M. Diagnostic accuracy of urine dipstick for proteinuria category in Japanese workers. Clin Exp Nephrol. 2020 Feb;24((2)):151–6. doi: 10.1007/s10157-019-01809-3. [DOI] [PubMed] [Google Scholar]
- 16.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ., Jr Selection bias due to loss to follow up in cohort studies. Epidemiology. 2016 Jan;27((1)):91–7. doi: 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya A, Yamana H, Kawahara T, Tsutsumi Y, Matsui H, Fushimi K, et al. Tracheostomy and mortality in patients with severe burns: a nationwide observational study. Burns. 2018 Dec;44((8)):1954–61. doi: 10.1016/j.burns.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, et al. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol. 2011 Oct;6((10)):2462–9. doi: 10.2215/CJN.00700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients--absence of evidence or evidence of absence? Clin J Am Soc Nephrol. 2008 Jan;3((1)):226–36. doi: 10.2215/CJN.03740907. [DOI] [PubMed] [Google Scholar]
- 20.Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007 Jan;71((2)):159–66. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- 21.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001 Jun;12((6)):1211–7. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 22.Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep. 2017 Jan 23;2((2)):251–60. doi: 10.1016/j.ekir.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii Y, Yamamoto R, Shinzawa M, Kimura Y, Aoki K, Tomi R, et al. Occupational sedentary behavior and prediction of proteinuria in young to middle-aged adults: a retrospective cohort study. J Nephrol. 2021 Jun;34((3)):719–28. doi: 10.1007/s40620-020-00826-w. [DOI] [PubMed] [Google Scholar]
- 24.Howard BJ, Balkau B, Thorp AA, Magliano DJ, Shaw JE, Owen N, et al. Associations of overall sitting time and TV viewing time with fibrinogen and C reactive protein: the AusDiab study. Br J Sports Med. 2015 Feb;49((4)):255–8. doi: 10.1136/bjsports-2013-093014. [DOI] [PubMed] [Google Scholar]
- 25.Michishita R, Matsuda T, Kawakami S, Kiyonaga A, Tanaka H, Morito N, et al. The accumulation of healthy lifestyle behaviors prevents the incidence of chronic kidney disease (CKD) in middle-aged and older males. Environ Health Prev Med. 2016 May;21((3)):129–37. doi: 10.1007/s12199-016-0506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica. 2014;2014:253581. doi: 10.1155/2014/253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, Takishita S. Triglyceride, but not total cholesterol or low-density lipoprotein cholesterol levels, predict development of proteinuria. Kidney Int. 2002 Nov;62((5)):1743–9. doi: 10.1046/j.1523-1755.2002.00626.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Du MF, Gao WH, Fu BW, Ma Q, Yan Y, et al. Risk factors for subclinical renal damage and its progression: Hanzhong adolescent Hypertension Study. Eur J Clin Nutr. 2021 Mar;75((3)):531–8. doi: 10.1038/s41430-020-00752-x. [DOI] [PubMed] [Google Scholar]
- 29.Madison JR, Spies C, Schatz IJ, Masaki K, Chen R, Yano K, et al. Proteinuria and risk for stroke and coronary heart disease during 27 years of follow-up: the Honolulu Heart Program. Arch Intern Med. 2006 Apr 24;166((8)):884–9. doi: 10.1001/archinte.166.8.884. [DOI] [PubMed] [Google Scholar]
- 30.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005 Aug 16;112((7)):969–75. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 31.Kimura Y, Yamamoto R, Shinzawa M, Isaka Y, Iseki K, Yamagata K, et al. Alcohol consumption and incidence of proteinuria: a retrospective cohort study. Clin Exp Nephrol. 2018 Oct;22((5)):1133–42. doi: 10.1007/s10157-018-1568-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
I, Hidehiro Kaneko, as the corresponding author, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This database is available for anyone who purchases it from the JMDC inc (https://www.jmdc.co.jp/en/index).