Abstract
Dietary sodium restriction has multiple beneficial effects on cardiovascular health. The underlying mechanisms are not fully understood and the roles of metabolomics have been rarely studied. We aimed to test the hypothesis that the reduction in dietary sodium intake would induce changes in metabolomic profiling among black hypertensives, and the changes would be associated with reduced blood pressure and improved skin capillary density. A total of 64 untreated black hypertensives were included from a randomized crossover trial of sodium reduction. The participants were given either nine slow sodium tablets (10 mmol sodium per tablet) or placebo tablets daily for six weeks while on reduced-sodium diet aiming at achieving daily sodium intake around 2.0 grams. They then crossed over to receive the other tablets for another six weeks. Untargeted metabolomic profiling was performed in paired serum samples, which were collected at the end of each period, so as blood pressure and capillary density. Mixed-effects models were used. There were 34 metabolites identified with raw ps<0.05. Among those, two metabolites including beta-hydroxyisovalerate and methionine sulfone were significantly increased with sodium reduction (false discovery rate = 0.006 and 0.099, respectively). Increased beta-hydroxyisovalerate was associated with reduced office systolic blood pressure and ambulatory daytime systolic blood pressure; whereas increased methionine sulfone was associated with reduced 24-hour diastolic blood pressure, ambulatory nighttime diastolic blood pressure and increased skin capillary density. Our results suggest that dietary sodium reduction increases the circulating levels of beta-hydroxyisovalerate and methionine sulfone. Further studies are warranted.
Clinical Trial Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00152074.
Keywords: metabolite, sodium intake, hypertension, blood pressure, capillary
High sodium intake is recognized as one of the most important risk factors for hypertension, left ventricular hypertrophy, chronic kidney disease and heart failure. Modest dietary sodium reduction lowers blood pressure (BP), cardiovascular disease (CVD) and mortality.1–3 In spite of these well-established relationships, the underlying biological mechanisms are not well understood.
Metabolomic profiling can detect small molecule metabolic products in response to environmental changes, which could serve as biomarkers or underlying mechanisms for biological response to dietary intervention. Recent studies showed that both Dietary Approaches to Stop Hypertension (DASH) and DASH-Sodium feeding trials were able to alter circulating metabolites.4,5 We previously conducted a randomized double-blind, placebo-controlled crossover trial of modest dietary sodium reduction in untreated hypertensive patients living in the United Kingdom, showing that modest reduction in sodium intake reduced BP, improved large artery compliance6 and both functional and structural capillary rarefactions.7 In this study, we tested the hypothesis that the modest reduction in dietary sodium intake would induce changes in metabolomic profiles in an independent cohort of untreated hypertensive subjects. We further tested the hypothesis that the changes in metabolomic levels by modest sodium reduction would be associated with reduced BP, improved large artery compliance and increased skin capillary density.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
The present study utilized stored serum samples from a previously conducted randomized double-blind, placebo-controlled, crossover trial of dietary sodium reduction in untreated hypertensives. The inclusion criteria were population aged 30 to 75 years, with sitting systolic blood pressure (SBP) 140 to 170 mmHg or diastolic blood pressure (DBP) 90 to 105 mmHg, and with no previous treatment for raised BP.6 We focused on black hypertensives who tend to have a higher prevalence of salt-sensitive hypertension. All 64 black participants with serum samples available at both time points (end of slow sodium and placebo) were included in this study. The study was approved by the Wandsworth Local Research Ethics Committee and the Institutional Review Board of Augusta University, and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Sodium Reduction Protocol
A randomized, double-blind, placebo-controlled crossover trial was carried out (Figure S1, Supporting Information). In the first two week run-in period, participants were given detailed advice by specially trained nurses on how to reduce their sodium intake, with an aim of achieving an intake of 2000 mg sodium/day (85 mmol/day). They were advised not to add salt at the table or during cooking, and avoid foods that contained large amount of sodium. Nurses went through with the participants on what foods they usually ate and identified items with high sodium content, and advised them to use low sodium alternatives. In appropriate cases, the spouse or whoever cooked in the household was also seen. Advice was reinforced at each visit for the whole duration of the study. Sodium-free bread was provided for those who had no easy access to it. While continuing on the reduced sodium diet, participants were given, in a random order, either nine slow sodium tablets (10 mmol sodium per tablet) or placebos daily for six weeks. They then crossed over to receive the other tablets for another six weeks.6 Reduced-sodium diet plus slow sodium tablets represented usual sodium intake, while reduced-sodium diet plus placebo represented a reduced-sodium intake.
For compliance, 24h urinary sodium excretion (the gold standard method to estimate dietary sodium intake) was monitored at baseline, after two weeks on the reduced sodium diet, and at each clinic visit every 3 weeks during the crossover period using two consecutive 24h urine samples. Based on 24h urinary sodium excretion, the average sodium intake was reduced to 2640 mg/day after 2 weeks on the reduced sodium diet. This was slightly higher than the originally set target. During the crossover periods, the mean sodium intake was reduced from 3800 mg/day on slow sodium tablets to 2680 mg/day on placebo. The reduction was smaller than the originally set target. This is likely to be due to the poor compliance in some participants. Among the 64 participants, 13 (20%) either did not comply with the low sodium diet or failed to take the tablets as their 24-hour urinary sodium was not reduced from slow sodium to placebo period.
Anthropometric and Laboratory Measurements
Measurements were performed at the end of each 6-week study period. Height and body weight were measured with light clothing and without shoes. Body mass index (BMI) was calculated as weight (kg) per square of height (m2). BP was measured by a validated automatic digital BP monitor (Omron HEM-705CP) in sitting position after 5 to 10 minutes rest. Three readings were taken and the average of the last two readings was used. Twenty-four-hour ambulatory blood pressure monitoring (ABPM) was performed using SpaceLabs 90207 devices (SpaceLabs, InC, Washington, DC) as previously described.8 Briefly, monitoring was set to take measurements at half hourly intervals during the day and hourly intervals overnight. Recordings were analyzed with the ABPM report manager system software package.8 Pulse pressure (PP) was calculated as the difference between SBP and DBP. Blood samples were taken at the end of each six-week period for measurements of routine biochemistry. Two consecutive 24h urine samples were collected during the last two days of each study period for measurements of urinary sodium excretion. The mean of the 2 urine measurements was used in the analysis. Carotid-femoral pulse wave velocity (cfPWV) was measured noninvasively using an automatic device Complior.6
Skin Capillary Density Measurement
Intravital Capillaroscopy Skin capillary density is a well-established dynamic method for studying skin capillaries and venous congestion is the most effective method for visualization of the maximal number of perfused skin capillaries.9,10 Microscopic images were obtained with a charge-coupled device camera (Sony model XC-75CE) and were stored using a video recorder (JVC model HR-S6600). The skin of the dorsum and the side of the middle phalanx of the left hand were examined. Four microscopic fields (0.66 mm2 per field) centered on an ink spot at each site were recorded continuously for 5 minutes to detect intermittently perfused capillaries. The number of capillaries per field was counted online and by running the recorded tapes using computer software (CapiScope, KK-Technology). To maximize the number of visible capillaries, venous congestion was carried out. A miniature neonatal BP cuff was applied to the base of the left middle finger. The cuff was inflated and maintained at 60 mmHg for 2 minutes. During the venous congestion, further images were recorded using 1 of the 4 microscopic fields chosen at random. Measures were averaged across the microscopic fields.
Metabolomic Profiling
Paired serum samples were collected at the end of 6-week slow sodium tablets and at the end of 6-week placebo tablets periods from each of the 64 participants. Global untargeted metabolomics profiling was performed in 128 serum samples in total by Metabolon. The sample preparation process was carried out using the automated MicroLab STAR® system (Hamilton Company, Salt Lake City, UT, USA). The extract was divided into five fractions: two for analysis by two separate reverse phase (RP)/ ultra-performance liquid chromatography (UPLC)- Mass Spectroscopy (MS)/MS methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, one for analysis by hydrophilic interaction liquid chromatography (HILIC)/UPLC-MS/MS with negative ion mode ESI, and one sample was reserved for backup. All methods utilized a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Raw data were extracted, peak-identified and quality control (QC) processed using Metabolon’s hardware and software. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Peaks were quantified using area-under-the-curve.
A total of 870 serum metabolites were detected, among which 231 (27%) biochemicals have not been named and confirmed. Among the named 639 metabolites, we further excluded 44 (7%) metabolites for which more than 50% of samples had metabolite values below the detection limit. Finally, 595 identified metabolites with satisfying detection rates were entered into analysis.
Statistical Analysis
The general characteristics of the subjects are presented as mean ± standard deviation (SD) for continuous variables and N (%) for categorical variables. Two-tailed paired t-test was conducted to examine the differences in variables between placebo and sodium tablets.
Before statistical analysis, metabolomic data were log-transformed and standardized to unit variance and zero mean. Based on the intent-to-treat principle,11 mixed-effects linear regression was used to assess the differential profiling of metabolites between sodium and placebo tablets while incorporating repeated measured data and controlling for age, sex and BMI as confounding variables. To correct for multiple testing, the raw ps were converted to false discovery rates (FDRs) according to the Bonferroni-Hochberg’s correction.12 As Bonferroni-Hochberg’s correction for multiple hypotheses has been considered very conservative and may lead to a high number of false negatives, so we used a FDR of 0.10 for statistical significance.4
We further tested whether the changes in the identified metabolites were associated with the changes in BPs, PP, cfPWV and capillary density measurements. Mixed-effects model was used to examine the association between the levels of identified metabolites and phenotypes, which included SBP, DBP, PP 24-hour SBP, DBP, and PP, daytime SBP, DBP and PP, nighttime SBP, DBP and PP, cfPWV and skin capillary density measurements. P < 0.05 was considered statistically significant. We further conducted a sensitivity analysis to test whether identified serum metabolites are also associated with 24h urinary sodium excretion. All analyses were performed using Stata version 12.0 (StataCorp., College Station, Texas, USA).
Results
General Characteristics of the Participants
All black subjects with serum samples available were included (N=64), with mean age of 50.2 ± 9.5 years, mean BMI 30.9 ± 5.3 kg/m2 and 50% males. Sodium reduction was associated with lowered BPs and cfPWV. In addition, sodium reduction was associated with increased skin basal capillaries at the side of the fingers, and associated with increased maximal capillary density (with venous congestion) at both dorsum and side of fingers (ps < 0.05), as previously reported (Table 1).6
Table 1.
General characteristics of the participants
| Characteristic | Sodium | Placebo | p |
|---|---|---|---|
| Age (years) * | 50.2 ± 9.5 | -- | -- |
| Male (%) | 32 (50) | -- | -- |
| BMI (kg/m2) * | 30.9 ± 5.3 | -- | -- |
| Urinary sodium (mmol/24 hours) | 163.1 ± 60.7 | 115.2 ± 43.6 | < 0.001 |
| Office BP and pulse rate | |||
| SBP (mmHg) | 148.9 ± 13.5 | 143.7 ± 11.8 | < 0.001 |
| DBP (mmHg) | 90.7 ± 9.1 | 88.2 ± 9.1 | < 0.001 |
| MAP (mmHg) | 110.1 ± 9.4 | 106.7 ± 9.0 | < 0.001 |
| cfPWV | 11.8 ± 2.1 | 11.2 ± 1.8 | 0.003 |
| Ambulatory BP | |||
| 24-hour SBP (mmHg) | 144.5 ± 8.4 | 140.1 ± 10.1 | < 0.001 |
| 24-hour DBP (mmHg) | 87.8 ± 8.9 | 85.4 ± 9.0 | < 0.001 |
| Day SBP (mmHg) | 150.1 ± 9.3 | 144.9 ± 10.4 | < 0.001 |
| Day DBP (mmHg) | 92.7 ± 9.7 | 89.9 ± 9.6 | < 0.001 |
| Night SBP (mmHg) | 138.2 ± 9.7 | 134.1 ± 12.1 | < 0.001 |
| Night DBP (mmHg) | 82.4 ± 9.3 | 80.4 ± 9.8 | 0.017 |
| Pulse pressure | |||
| Office PP (mmHg) | 58.2 ± 11.3 | 55.4 ± 9.9 | 0.007 |
| 24-hour PP (mmHg) | 56.8 ± 6.6 | 54.7 ± 6.7 | <0.001 |
| Day PP (mmHg) | 57.3 ± 7.4 | 55.0 ± 7.4 | <0.001 |
| Night PP (mmHg) | 55.8 ± 7.4 | 53.8 ± 7.5 | 0.004 |
| Skin temperature (°C) | 30.6 ± 2.4 | 30.8 ± 2.1 | 0.536 |
| Capillary density by capillaroscopy | |||
| Dorsum of the fingers | |||
| At resting (capillaries/mm2) | 67.6 ± 15.0 | 70.5 ± 16.0 | 0.053 |
| With venous congestion (capillaries/mm2) | 70.4 ± 14.8 | 76.6 ± 16.2 | <0.001 |
| Side of the fingers | |||
| At resting (capillaries/mm2) | 63.9 ± 16.2 | 68.3 ± 17.8 | <0.001 |
| With venous congestion (capillaries/mm2) | 69.0 ± 17.4 | 73.4 ± 18.8 | 0.005 |
Age and BMI were only measured once.
Changes in Metabolites Associated with Sodium Reduction
There were 34 metabolites that responded to sodium reduction with raw ps < 0.05 (Table S1). The top 10 metabolites were altered by sodium reduction with raw ps < 0.01 (Table 2, Figure 1). Among those, six were upregulated by sodium reduction. The top two metabolites beta-hydroxyisovalerate (beta-hydroxy-beta-methylbutyric acid or HMB) and methionine sulfone remained significant after FDR correction (FDR = 0.006, 0.099, respectively).
Table 2.
Top ten metabolites associated with sodium reduction
| Rank | Metabolites | β* | p | FDR | Super pathway | Sub pathway |
|---|---|---|---|---|---|---|
| 1 | Beta-hydroxyisovalerate | 0.56 | <0.001 | 0.006 | Amino Acid | Leucine, Isoleucine and Valine Metabolism |
| 2 | Methionine sulfone | 0.23 | <0.001 | 0.099 | Amino Acid | Methionine, Cysteine, SAM and Taurine Metabolism |
| 3 | N-acetylneuraminate | 0.30 | 0.002 | 0.393 | Carbohydrate | Aminosugar Metabolism |
| 4 | 1,5-anhydroglucitol | 0.11 | 0.004 | 0.448 | Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism |
| 5 | Campesterol | −0.26 | 0.004 | 0.448 | Lipid | Sterol |
| 6 | N2-acetyllysine | 0.30 | 0.005 | 0.489 | Amino Acid | Lysine Metabolism |
| 7 | Threonylphenylalanine | −0.36 | 0.006 | 0.489 | Peptide | Dipeptide |
| 8 | Maltose | −0.35 | 0.008 | 0.562 | Carbohydrate | Glycogen Metabolism |
| 9 | Sucrose | −0.39 | 0.009 | 0.562 | Carbohydrate | Disaccharides and Oligosaccharides |
| 10 | Vanillactate | 0.23 | 0.009 | 0.562 | Amino Acid | Tyrosine Metabolism |
β is the regression coefficient of intervention in the mixed-effects model adjusted for age, sex and BMI, reflects metabolomic profiling change from sodium tablets to placebo tablets.
Figure 1. Plot of −log10Ps for the metabolomic profiles in response to the sodium reduction.
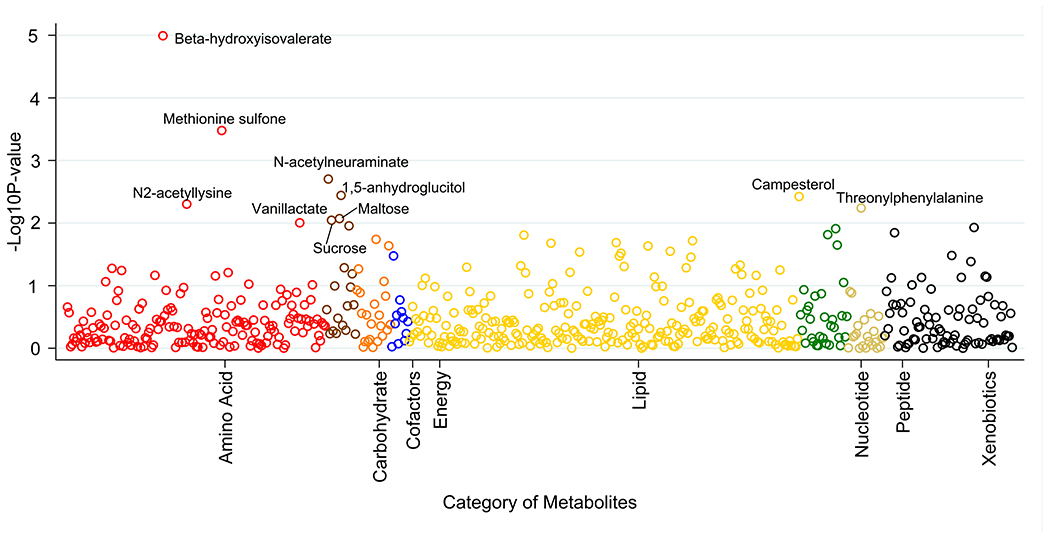
Ps were calculated based on the mixed effects models adjusted for age, sex and BMI as confounding variables. Associations with raw ps<0.01 are labeled with chemical names.
Association of 24h urinary sodium excretion with changes in serum metabolites
We conducted a sensitivity analysis to test the associations between the changes in 24h urinary sodium excretion (the gold standard measure for dietary sodium intake) and the changes in serum metabolites, given the compliance of 80%. Reduced urinary sodium excretion was associated with increased HMB (β=0.45, p=0.001) and increased methionine sulfone (β=0.21, p=0.007).
Association of Changes in Metabolites with Changes in Cardiovascular Phenotypes
HMB and methionine sulfone, which were upregulated by sodium reduction, were associated with reduced BPs (ps < 0.05). In particular, HMB was associated with both casual and daytime ambulatory SBPs, whereas methionine sulfone was only associated with 24h ambulatory DBP and nighttime ambulatory DBP. In addition, increased HMB was associated with reduced PP. Moreover, upregulated methionine sulfone was associated with increased basal capillary density at the side of the fingers and maximal capillary density at the dorsum of the fingers. These results suggest that increased methionine sulfone was associated with increased microcirculation (ps<0.05, Table 3).
Table 3.
Association between changes in metabolites with changes in blood pressure and CVD phenotypes
| Phenotypes | Beta-hydroxyisovalerate | Methionine sulfone | N-acetylneuraminate | 1,5-anhydroglucitol | Campesterol | N2-acetyllysine | Threonylphenylalanine | Maltose | Sucrose | Vanillactate |
|---|---|---|---|---|---|---|---|---|---|---|
| Office BP | ||||||||||
| SBP | −2.37* | −1.67 | −1.34 | −0.41 | 3.75† | −3.01† | 1.00 | 1.16 | 2.04* | −1.13 |
| DBP | −1.00 | −1.14 | −1.80* | 0.30 | 2.00† | −2.02† | 0.48 | 0.22 | 0.67 | −1.24 |
| MAP | −1.41* | −1.45 | −1.77* | 0.21 | 2.72‡ | −2.40† | 0.65 | 0.52 | 1.14 | −1.31 |
| cfPWV | −0.17 | −0.16 | −0.22 | −0.26 | −0.07 | −0.15 | −0.13 | 0.21 | 0.07 | −0.07 |
| Ambulatory BP | ||||||||||
| 24-hour SBP | −1.14 | −1.37 | −2.00* | 1.11 | 1.26 | −0.84 | 0.64 | 0.90 | 1.60* | −0.56 |
| 24-hour DBP | −0.79 | −2.03* | −1.88† | 0.09 | 1.50* | −0.50 | 0.69 | 0.56 | 1.09* | −1.44* |
| Day SBP | −1.78* | −0.92 | −2.35* | 1.23 | 1.66 | −1.44 | 1.53 | 1.09 | 1.84* | 0.27 |
| Day DBP | −1.17 | −1.73 | −2.10† | 0.14 | 2.01* | −0.67 | 0.99 | 0.77 | 1.17 | −1.32 |
| Night SBP | −0.46 | −1.42 | −1.68 | 1.13 | 0.74 | −0.58 | −0.58 | 0.98 | 2.10* | −0.92 |
| Night DBP | −0.45 | −2.00* | −1.54* | 0.14 | 0.88 | −0.71 | −0.42 | 0.55 | 1.67† | −1.40 |
| Pulse pressure | ||||||||||
| Office PP | −1.56* | −0.65 | 0.66 | −1.23 | 2.16* | −1.39 | 1.11 | 1.15 | 1.33 | 0.22 |
| 24-hour PP | −0.57 | 0.32 | −0.12 | 0.83 | 0.25 | −0.39 | 0.64 | 0.56 | 0.41 | 1.00 |
| Day PP | −0.85 | 0.4 | −0.19 | 0.73 | 0.33 | −0.74 | 1.2* | 0.54 | 0.44 | 1.55* |
| Night PP | −0.15 | 0.49 | −0.15 | 0.87 | 0.15 | 0.00 | 0.26 | 0.57 | 0.42 | 0.7 |
| Microcirculation | ||||||||||
| Skin temperature | −0.04 | 0.10 | 0.28 | −0.26 | −0.02 | −0.02 | −0.07 | 0.30 | −0.02 | 0.05 |
| Capillary density by capillaroscopy | ||||||||||
| Dorsum of the fingers | ||||||||||
| At resting | 1.76 | 3.26 | 1.95 | 0.82 | 1.61 | 1.90 | −0.47 | 1.02 | 1.13 | 1.67 |
| With venous congestion | 2.10 | 3.93* | 2.66 | −0.48 | 1.95 | 3.24* | −1.26 | −0.15 | 0.66 | 0.94 |
| Side of the fingers | ||||||||||
| At resting | 1.68 | 3.38* | 3.18† | −0.73 | −1.00 | 0.28 | −1.33 | −0.71 | 0.12 | 1.15 |
| With venous congestion | 1.98 | 3.18 | 3.65† | −0.65 | −0.61 | −0.05 | −1.79 | 0.14 | 0.40 | 1.39 |
p<0.05,
p<0.01,
p<0.001.
Discussion
The present study shows that modest dietary sodium reduction could change serum metabolite levels in untreated black hypertensives. In particular, HMB and methionine sulfone were significantly upregulated by sodium reduction. Moreover, upregulated HMB and methionine sulfone were associated with reduced BPs and PP. Increased methionine sulfone was also associated with increased skin capillary density.
HMB is a metabolite of the amino acid leucine and is produced endogenously through oxidation of the ketoacid of L-Leucine in both animals and humans. In healthy adults, supplementation with HMB also increased exercise-induced gains in muscle size, muscle strength, and lean body mass, reduced muscle damage from exercise.13–16 Another RCT in highly-trained males in combat sport found that HMB not only increased muscle, but also reduced fat mass simultaneously.15 HMB is a precursor for cellular cholesterol synthesis especially in tissues such as muscle that rely on de novo synthesis of cholesterol. In a series of small randomized placebo-controlled trials conducted in men and women, young and old, exercising or nonexercising, HMB supplementation 3 g/day for 3 to 8 weeks decreased total cholesterol by 5.8% and LDL cholesterol by 7.3%. In addition, HMB supplementation decreased SBP by 4.4 mmHg, suggesting having cardio-protective effects.17 Our results showed that increased HMB levels by sodium reduction were associated with both reduced office SBP and daytime ambulatory SBP in untreated black hypertensives, which provide further evidence linking HMB to BP regulation. More independent RCTs of HMB supplementation on BP in humans are warranted.
The underlying mechanisms between HMB and BP regulation are not well understood. In a previous study, supplementation of HMB is found to be able to increase the levels of circulating insulin-like growth factor (IGF-1),18 which is inversely related to SBP.19 IGF-1 possesses a protective effect on the endothelium through anti-inflammatory and nitric oxide generation- a potential BP-lowering effect.20, 21 Moreover, HMB also attenuates the circulating tumor necrosis factor-α (TNF-α) and angiotensin II (AngII) expression post-exercise,22,23 both of which are pro inflammation factors and could elevate BP.24,25
Methionine sulfone is the irreversible final oxidation product of methionine. We also found that sodium reduction led to increased methionine sulfone, which was associated with decreased BP and improved capillary density. There is no clear conclusion about the biochemical function of methionine sulfone in previous studies. The DASH study also found plasma methionine sulfone was increased when changing sodium intake from high to low, but the association failed to pass the Bonferroni’s correction.4 Another study found that sodium reduction was associated with increased urinary methionine sulfoxide in humans,26 which was the intermediate oxidation product of methionine.27 Protein methionine oxidation has recently been recognized as a potential molecular mechanism of redox regulation of protein function in vascular biology. Emerging data suggest that protein methionine oxidation may play a pathogenic role in atherosclerosis, ischemic heart disease, hypertension, and thrombosis.27 Several proteins important in vascular biology have been shown to contain oxidation-sensitive methionine residues including calcium/calmodulin-dependent protein kinase II, apolipoprotein A-I, thrombomodulin (TM), and von Willebrand factor.27
In addition, methionine is also one of the methyl donors that increase DNA methylation.28 Because of the circular nature of the methionine cycle, methionine excess may actually impair DNA methylation by inhibiting remethylation of homocysteine.28 A study found that dietary methionine restriction induced secretion of cardio-protective hormones, such as adiponectin and fibroblast growth factor 21 (FGF21).29 Methionine-restricted mice were also found to be resistant to diet-induced obesity and insulin resistance.30 High methionine diet posed a cardiac threat by increasing oxidative stress, inflammatory manifestations, matrix/vascular remodeling, and decreased cardiac function in mice.31 Methionine sulfone is an irreversible oxidation production of methionine.27 The increase in methionine sulfone induced by sodium reduction may stimulate oxidation of methionine and therefore decreased methionine, which would be beneficial for cardiovascular health.
It is worth mentioning that changes in HMB were associated with changes in office SBP, and ambulatory daytime SBP, whereas changes in methionine sulfone were associated with changes in 24h ambulatory DBP and nighttime DBP in our study, suggesting these two metabolites may be involved in different pathophysiology as we discussed above. The mechanisms for elevated SBP differ from elevated DBP and mechanisms for elevated nocturnal BPs differ from overall or/and daytime BPs. Sodium reduction inducing BP reduction may be through various pathways.
Increased HMB was also associated with reduced office PP by sodium reduction. PP is a surrogate measure for proximal aortic stiffness. A meta-analysis shows that PP, not mean arterial pressure, is the major determinant of CVD risk.32 PP is also an important predictor of incident atrial fibrillation in the 2 decades of prospective follow-up in the Framingham original and offspring cohorts.33 PWV is another surrogate marker of arterial stiffness. Interestingly, we did not observe an association between HMB and PWV, suggesting these two markers may be involved in different mechanisms of arterial stiffness.
Derkach et al. examined the effect of sodium intake on metabolites in 119 participants from the DASH-Sodium feeding study including 73 participants at the end of their high- and low-sodium interventions and 46 participants at the end of high- and medium-sodium interventions using the Metabolon platform.4 The participants in the DASH-Sodium study randomly received low- (1150 mg/day), medium- (2300 mg/day), and high-sodium (3450 mg/day) diet for 30 days each. There were 82 metabolites associated linearly with sodium intake at a FDR ≤ 0.10 mostly when participants switched from high- to low- sodium intervention. Very few associations were found when participants switched from high sodium to medium sodium intervention; only three metabolites were identified when comparing high- to medium-sodium diet at raw ps<0.05, and none of which passed FDR correction. No participants were studied from the group switched from medium sodium to low sodium intervention. Our sodium reduction intervention is more comparable to the high- to medium sodium intervention in the DASH-Sodium study. There were 34 metabolites associated with sodium reduction with a raw p-value <0.05, 2 of them had FDR<0.1 in our study. In addition, methionine sulfone was also identified in the DASH-Sodium study. Characteristics of the participants and the intervention methods are the major differences between the DASH-Sodium study and ours. First, the DASH-Sodium study included 50% black participants, while our study examined black participants only. Second, 47% of the DASH-Sodium study participants were hypertensive, while all our participants were hypertensive. Third, the DASH-Sodium study was a feeding study that controlled both for sodium level and energy intake; whereas our participants were instructed to reduce sodium in their own diet, however, the sodium reduction was monitored and confirmed by 2 consecutive collections of 24-hour urinary sodium excretion. It is possible that the modest reduction was not sufficient to induce or observe significant changes in metabolites. However, as stated in the DASH-Sodium study, there was not enough evidence to confidently reject the hypothesis that sodium intake was linearly related to log-metabolite levels.4
The strengths of our study include that we utilized the sample from a well-controlled, randomized, double-blind clinical trial of dietary sodium reduction with well-characterized CVD phenotypes. The intervention was a cross-over design: each participant served as his/her own control, diminishing the inter-person variations. In addition, we focused on black hypertensives, in whom salt sensitivity and salt-sensitive hypertension are common. Compared to previous studies, our study was able to link identified metabolites with CVD phenotypes, providing mechanistic insights into cardio-beneficial effects of modest sodium reduction. The study is limited by its relatively modest sample size, and the lack of an independent replication sample. This study is also limited by the modest sodium reduction scale, which may reduce power for significant discovery. However, we replicate the previous findings based on DASH and DASH-Sodium samples that sodium reduction increases methionine oxidation pathway activities.4,26
In conclusion, our results show that dietary sodium reduction increases the circulating levels of HMB and methionine sulfone in untreated black hypertensive patients. Moreover, increased HMB or methionine sulfone is associated with reduced BP, arterial stiffness and increased capillary density.
Perspectives
Modest dietary sodium reduction is beneficial in preventing CVD. However, the underlying mechanisms are not well understood. Our global untargeted metabolomic profiling study shows that sodium reduction increases serum HMB and methionine sulfone levels, which are also associated with reduced BP, arterial stiffness and increased skin capillary density in untreated black hypertensive patients.
Supplementary Material
Novelty and Significance.
What Is New?
Dietary sodium reduction increases the serum levels of beta-hydroxyisovalerate (HMB) and methionine sulfone, which are further associated with decreased blood pressure (BP) in untreated black hypertensive individuals.
In addition, increased HMB is associated with reduced pulse pressure, a surrogate marker for conduit arterial stiffness. Increased methionine sulfone is also associated with increased skin capillary density.
What Is Relevant?
Our results provide new evidence linking both HMB and methionine sulfone to BP regulation. In addition, our results suggest that HMB and methionine sulfone play a role in the regulation of arterial stiffness and skin microcirculation.
Summary
Dietary sodium reduction increases the circulating levels of HMB and methionine sulfone, which may mediate the beneficial effects of modest sodium reduction on cardiovascular health such as BP, arterial stiffness and microcirculation.
Sources of Funding
The original study was in part funded by the United Kingdom Food Standards Agency (N02034). The current study was in part funded by the American Heart Association (16GRANT31250002 to HZ).
Footnotes
Disclosures
None.
References
- 1.Cook NR, Appel LJ, Whelton PK. Lower Levels of Sodium Intake and Reduced Cardiovascular Risk. Circulation. 2014;129(9):981–989. doi: 10.1161/Circulationaha.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole NI, Swift PA, He FJ, MacGregor GA, Suckling RJ. The effect of dietary salt on blood pressure in individuals receiving chronic dialysis: a systematic review and meta-analysis of randomised controlled trials. J Hum Hypertens. 2018. doi: 10.1038/s41371-018-0131-5. [DOI] [PubMed] [Google Scholar]
- 3.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378(9789):380–382. doi: 10.1016/s0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- 4.Derkach A, Sampson J, Joseph J, Playdon MC, Stolzenberg-Solomon RZ. Effects of dietary sodium on metabolites: the Dietary Approaches to Stop Hypertension (DASH)-Sodium Feeding Study. Am J Clin Nutr. 2017;106(4):1131–1141. doi: 10.3945/ajcn.116.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebholz CM, Lichtenstein AH, Zheng Z, Appel LJ, Coresh J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr. 2018;108(2):243–255. doi: 10.1093/ajcn/nqy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54(3):482–488. doi: 10.1161/hypertensionaha.109.133223. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56(2):253–259. doi: 10.1161/hypertensionaha.110.155747. [DOI] [PubMed] [Google Scholar]
- 8.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Modest Salt Reduction Lowers Blood Pressure and Albumin Excretion in Impaired Glucose Tolerance and Type 2 Diabetes Mellitus: A Randomized Double-Blind Trial. Hypertension. 2016;67(6):1189–1195. doi: 10.1161/hypertensionaha.115.06637. [DOI] [PubMed] [Google Scholar]
- 9.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33(4):998–1001. [DOI] [PubMed] [Google Scholar]
- 10.Antonios TF, Rattray FE, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Maximization of skin capillaries during intravital video-microscopy in essential hypertension: comparison between venous congestion, reactive hyperaemia and core heat load tests. Clin Sci (Lond). 1999;97(4):523–528. [PubMed] [Google Scholar]
- 11.Xi W, Pennell ML, Andridge RR, Paskett ED. Comparison of intent-to-treat analysis strategies for pre-post studies with loss to follow-up. Contemporary clinical trials communications. 2018;11:20–29. doi: 10.1016/j.conctc.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochberg Y A Sharper Bonferroni Procedure for Multiple Tests of Significance. Biometrika. 1988;75(4):800–802. doi: DOI 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- 13.Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC Jr., Connelly AS, Abumrad N Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol (1985). 1996;81(5):2095–2104. doi: 10.1152/jappl.1996.81.5.2095. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JM, Lowery RP, Joy JM, Andersen JC, Wilson SM, Stout JR, Duncan N, Fuller JC, Baier SM, Naimo MA, Rathmacher J. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: a randomized, double-blind, placebo-controlled study. European journal of applied physiology. 2014;114(6):1217–1227. doi: 10.1007/s00421-014-2854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durkalec-Michalski K, Jeszka J, Podgorski T. The Effect of a 12-Week Beta-hydroxy-beta-methylbutyrate (HMB) Supplementation on Highly-Trained Combat Sports Athletes: A Randomised, Double-Blind, Placebo-Controlled Crossover Study. Nutrients. 2017;9(7). doi: 10.3390/nu9070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durkalec-Michalski K, Jeszka J. The efficacy of a beta-hydroxy-beta-methylbutyrate supplementation on physical capacity, body composition and biochemical markers in elite rowers: a randomised, double-blind, placebo-controlled crossover study. Journal of the International Society of Sports Nutrition. 2015;12:31. doi: 10.1186/s12970-015-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC Jr. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130(8):1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- 18.Townsend JR, Hoffman JR, Gonzalez AM, Jajtner AR, Boone CH, Robinson EH, Mangine GT, Wells AJ, Fragala MS, Fukuda DH, Stout JR. Effects of beta-Hydroxy-beta-methylbutyrate Free Acid Ingestion and Resistance Exercise on the Acute Endocrine Response. Int J Endocrinol. 2015;2015:856708. doi: 10.1155/2015/856708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schutte AE, Schutte R, Smith W, Huisman HW, Mels CM, Malan L, Fourie CM, Malan NT, Van Rooyen JM, Kruger R, Conti E. Compromised bioavailable IGF-1 of black men relates favourably to ambulatory blood pressure: The SABPA study. Atherosclerosis. 2014;233(1):139–144. doi: 10.1016/j.atherosclerosis.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Conti E, Musumeci MB, De Giusti M, Dito E, Mastromarino V, Autore C, Volpe M. IGF-1 and atherothrombosis: relevance to pathophysiology and therapy. Clin Sci (Lond). 2011;120(9):377–402. doi: 10.1042/cs20100400. [DOI] [PubMed] [Google Scholar]
- 21.Wickman A, Jonsdottir IH, Bergstrom G, Hedin L. GH and IGF-I regulate the expression of endothelial nitric oxide synthase (eNOS) in cardiovascular tissues of hypophysectomized female rats. European journal of endocrinology. 2002;147(4):523–533. [DOI] [PubMed] [Google Scholar]
- 22.Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295(6):E1409–1416. doi: 10.1152/ajpendo.90530.2008. [DOI] [PubMed] [Google Scholar]
- 23.Townsend JR, Fragala MS, Jajtner AR, Gonzalez AM, Wells AJ, Mangine GT, Robinson EHt, McCormack WP, Beyer KS, Pruna GJ, Boone CH, Scanlon TM, Bohner JD, Stout JR, Hoffman JR beta-Hydroxy-beta-methylbutyrate (HMB)-free acid attenuates circulating TNF-alpha and TNFR1 expression postresistance exercise. J Appl Physiol (1985). 2013;115(8):1173–1182. doi: 10.1152/japplphysiol.00738.2013. [DOI] [PubMed] [Google Scholar]
- 24.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN Jr., Lamarca B Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62(5):886–892. doi: 10.1161/hypertensionaha.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2014;28(3):165–169. doi: 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 26.Jablonski KL, Klawitter J, Chonchol M, Bassett CJ, Racine ML, Seals DR. Effect of dietary sodium restriction on human urinary metabolomic profiles. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(7):1227–1234. doi: 10.2215/cjn.11531114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu SX, Stevens JW, Lentz SR. Regulation of thrombosis and vascular function by protein methionine oxidation. Blood. 2015;125(25):3851–3859. doi: 10.1182/blood-2015-01-544676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterland RA. Assessing the effects of high methionine intake on DNA methylation. J Nutr. 2006;136(6 Suppl):1706s–1710s. doi: 10.1093/jn/136.6.1706S [DOI] [PubMed] [Google Scholar]
- 29.Ables GP, Ouattara A, Hampton TG, Cooke D, Perodin F, Augie I, Orentreich DS. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci Rep. 2015;5:8886. doi: 10.1038/srep08886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PloS one. 2012;7(12):e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi P, Kamat PK, Kalani A, Familtseva A, Tyagi SC. High Methionine Diet Poses Cardiac Threat: A Molecular Insight. J Cell Physiol. 2016;231(7):1554–1561. doi: 10.1002/jcp.25247. [DOI] [PubMed] [Google Scholar]
- 32.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160(8):1085–1089. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D’Agostino RB Sr., Kannel WB, Levy D, Benjamin EJ Pulse pressure and risk of new-onset atrial fibrillation. Jama. 2007;297(7):709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


