Abstract
Background
Chronic cough management necessitates a clear integrated care pathway approach. Primary care physicians initially encounter the majority of chronic cough patients, yet their role in proper management can prove challenging due to limited access to advanced diagnostic testing. A multidisciplinary approach involving otolaryngologists and chest physicians, allergists, and gastroenterologists, among others, is central to the optimal diagnosis and treatment of conditions which underly or worsen cough. These include infectious and inflammatory, upper and lower airway pathologies, or gastro-esophageal reflux. Despite the wide armamentarium of ancillary testing conducted in cough multidisciplinary care, such management can improve cough but seldom resolves it completely. This can be due partly to the limited data on the role of tests (eg, spirometry, exhaled nitric oxide), as well as classical pharmacotherapy conducted in multidisciplinary specialties for chronic cough. Other important factors include presence of multiple concomitant cough trigger mechanisms and the central neuronal complexity of chronic cough. Subsequent management conducted by cough specialists aims at control of cough refractory to prior interventions and includes cough-specific behavioral counseling and pharmacotherapy with neuromodulators, among others. Preliminary data on the role of neuromodulators in a proof-of-concept manner are encouraging but lack strong evidence on efficacy and safety.
Objectives
The World Allergy Organization (WAO)/Allergic Rhinitis and its Impact on Asthma (ARIA) Joint Committee on Chronic Cough reviewed the recent literature on management of chronic cough in primary, multidisciplinary, and cough-specialty care. Knowledge gaps in diagnostic testing, classical and neuromodulator pharmacotherapy, in addition to behavioral therapy of chronic cough were also analyzed.
Outcomes
This third part of the WAO/ARIA consensus on chronic cough suggests a management algorithm of chronic cough in an integrated care pathway approach. Insights into the inherent limitations of multidisciplinary cough diagnostic testing, efficacy and safety of currently available antitussive pharmacotherapy, or the recently recognized behavioral therapy, can significantly improve the standards of care in patients with chronic cough.
Keywords: Upper airway cough syndrome, Lower airway disease, Reflux cough, Chronic cough management, Neuromodulators, Speech therapy, Cough primary care, Cough specialty care
Introduction
Chronic cough is a troublesome and complex condition with significant impact on quality of life (QoL).1,2 Various triggers originating from the airways and gastrointestinal tract (GIT), among others, can result in chronic cough. This is mediated by transient receptor potential (TRP) channels and P2X purinergic receptors located on peripheral afferent and central neuronal network of the vagus nerve.3 Data on tussigen challenges and expression of neuromediators and inflammatory biomarkers in animal and human cough models provide insights into the pathogenesis of chronic cough. Accordingly, the cough reflex manifests a neurogenic inflammation and becomes hypersensitive.4 Part I of the World Allergy Organization (WAO)/Allergic Rhinitis and its Impact on Asthma (ARIA) consensus (see appendix) on chronic cough described the important role of the hypersensitive cough reflex (HCR) as a trigger mechanism of chronic cough in infectious and inflammatory, respiratory and GIT-related conditions.3 Part II of the consensus examined other pathogenic mechanisms inherent to cough-associated inflammatory conditions which can also modulate HCR. These include Type 2 (Th1 and Th2) inflammation, cough plasticity and tissue remodeling, among others.5 Yet, a clear description of how different cough trigger mechanisms interact with each other to cause persistent coughing is yet unknown.
Chronic cough management necessitates a clear integrated care pathway approach. A proper diagnostic and management protocol consists of a thorough evaluation and control of treatable traits which may underlie or worsen the cough reflex. These include infectious and inflammatory, upper and lower airway pathologies or gastro-esophageal reflux, and are conducted in primary and multidisciplinary health care.6 Subsequent management conducted by cough specialists aims at control of cough refractory to prior interventions7 and includes cough-specific behavioral counseling and pharmacotherapy with neuromodulators, among others. The lack of direct access to cough specialists may result in misdiagnosis of chronic cough or overdiagnosis of idiopathic chronic cough.
Evaluation and empirical treatment in primary care
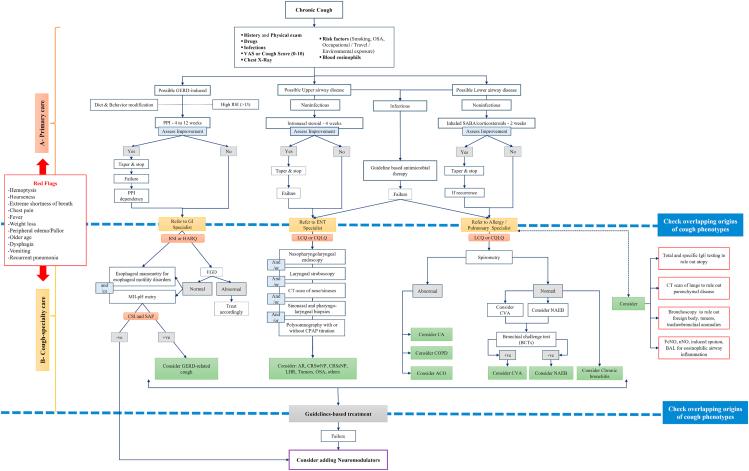
Initially, identification of potential habitual and environmental cough triggers is merited, followed by exposure avoidance, if possible (Fig. 1A). Triggers may include smoking, dust/chemicals/allergens exposure at home or work, in addition to other factors such as changes in ambient temperature/humidity, scents, sprays, aerosols, and exercise.8,9 Diagnosis of infectious etiologies of chronic cough in the upper and lower airways, and their subsequent empirical management, is merited. For example, endobronchial infections manifesting as chronic wet cough such as chronic suppurative lung disease, cystic fibrosis (CF)- and non-CF-related bronchiectasis, among others, can warrant a 3-week empirical antimicrobial treatment and further specialty care referral in case of failed therapy.10 Reportedly, infrequent but important infectious agents presenting with chronic cough include Bordetella (pertussis and parapertussis).11,12 Inspection of obstructive sleep apnea (OSA) features manifested by snoring, nocturnal apnea/hypopnea, and daytime somnolence concomitantly with cough is essential. Any concurrent medication that can induce chronic cough13 as a side effect should be explored, such as angiotensin-converting enzyme (ACE) inhibitors (5–30%),14, 15, 16, 17, 18 opioids (28–66%),19 and statins (46%)20 which collectively constitute common causes of drug-induced cough.18 In asthmatics, the use of nonsteroidal anti-inflammatory drugs21 or non-selective beta-blockers, but not cardio-selective beta blockers,22 can induce bronchospasm and cough in subsets of patients and should be ruled out. Other less common causes of chronic cough such as foreign body inhalation and malignancy should be excluded. In primary care, a chest x-ray is an informative screening test in chronic cough despite poor sensitivity to some interstitial lung diseases or mediastinal disorders.23 Serum eosinophilia as a biomarker of eosinophilic inflammation can be easily measured but its utility in chronic cough is questionable due to its diurnal and seasonal variability.24 The impact, severity, and treatment response of chronic cough can be assessed by scaled (1–10) cough scores, visual analog scale, and validated cough-related quality of life (QoL) measures25 such as Leicester cough questionnaire.26 Other validated instruments include laryngeal hypersensitivity questionnaire,27 chronic cough impact questionnaire,28 and Hull airway reflux questionnaire.29 Proper chronic cough management in primary care can prove challenging due to limited access to advanced diagnostic testing. Although the efficacy of natural (ie, honey)30 or pharmacologic antitussive agents31 in acute cough lacks solid evidence, their role in chronic cough remains to be established. However, a therapeutic/diagnostic pharmacotherapy regimen can be prescribed based on symptom “pointers” (Fig. 1A); its failure in cough improvement entails further referral to multidisciplinary care.
Fig. 1.
Suggested algorithm for management of cough phenotypic traits in primary (A) and cough-specialty (B) care. ACO (asthma COPD overlap; AR, allergic rhinitis; BCTs, bronchial challenge tests; CA, classic asthma; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; CQLQ, Cough Quality-of-Life Questionnaire; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; CSI, cough symptom index; CT, computerized tomography; CVA, cough variant asthma; EGD, esophagogastroduodenoscopy; FeNO, fractional exhaled nitric oxide; GERD, gastroesophageal reflux disease; GI, Gastrointestinal; HARQ, Hull Airway Reflux Questionnaire; LCQ, Leicester Cough Questionnaire; LHR, laryngeal hyperresponsiveness; MII-pH, multichannel intraluminal impedance monitoring combined with pH-metry; NAEB, non-asthmatic eosinophilic bronchitis; OSA, obstructive sleep apnea; PPI, proton-pump inhibitors; RSI, reflux symptom index; SABA, short-acting beta agonist; SAP, symptom association probability)
Upper airways
In the upper airways, rhinitis or rhinosinusitis, whether atopic or nonatopic, is a condition frequently associated with upper airway cough syndrome (UACS). There is clear disparity in the indications of oral antihistamines in children32 and adults33 with chronic cough in different parts of the world. Treatment of rhinitis patients with oral antihistamines or leukotriene receptor antagonists (LTRAs) does not provide much benefit for cough.34 Also, previous studies reporting efficacy of oral antihistamines used objective cough assessment tools but examined older generation antihistamines marked by their anticholinergic effect and with a short-term duration (<2 weeks) of clinical trials.35,36 Other reports37,38 recommended (4–6 weeks) empirical therapy of new generation antihistamines in chronic cough patients with rhinitis but with unpredictable response. Taken together, the WAO/ARIA expert panel does not recommend oral antihistamine therapy in patients with rhinitis and cough in view of their low efficacy in reducing cough.
Intranasal corticosteroids improved cough scores in (non-asthmatic) seasonal allergic rhinitis (SAR) patients following 2 weeks of therapy, reportedly linked to a reduction in postnasal secretions and/or pharyngeal mucosal inflammation.39 Data suggest they can also be effective at reducing daytime cough in patients with chronic rhinosinusitis (CRS), though treatment may require several weeks to achieve maximal effect.40 We previously reviewed the capsaicin challenge data indicating allergic rhinitis (AR) is a risk factor for chronic cough.5 Taken together, a 4-week therapy with intranasal corticosteroids is warranted (low evidence of efficacy) in AR patients with upper airway disease-related cough. Patients who improve on such therapy can be tapered down gradually, whereas those with partial or no improvement can be referred to cough specialty care. Notwithstanding, controlled trials using validated cough outcome measures are needed to evaluate efficacy of intranasal corticosteroids or combination of intranasal corticosteroids/topical antihistamines in UACS.
Lower airways
In the lower airways, key principles in pharmacotherapy of chronic cough using bronchodilators and inhaled corticosteroids (ICS) are to treat obstructive lung disease (classic asthma [CA]/chronic obstructive pulmonary disease [COPD]) and eosinophilic airway inflammation.41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 However, it should be noted that clear data on the role of pharmacotherapy in chronic cough originating from the lower airways are unclear.43 For example, in clinical trials of chronic cough patients with asthma, cough was not studied independently of other asthma symptoms. Also, it is yet unclear how airway eosinophilia impacts cough42 although it can identify steroid-responsive patients.52 An initial 2-week empirical trial of short-acting bronchodilator supplemented with ICS may be warranted in chronic cough patients. Alternatively, a short course of oral corticosteroids can be prescribed if patient cannot tolerate ICS. The latter has the advantage of causing an earlier response to therapy compared to ICS which may take several weeks to exert an effect. In contradistinction, other expert panels do not advocate an initial empirical trial of ICS not evidenced by airway hyperresponsiveness or eosinophilia.53 Improvement in cough following corticosteroid therapy is inclusive of asthma, cough variant asthma (CVA), or non-asthmatic eosinophilic bronchitis (NAEB).54,55
Gastrointestinal tract
Gastroesophageal reflux disease (GERD)-related cough is ideally a nonproductive postprandial cough often exacerbated in the supine position. Behavioral changes are recommended in all patients with GERD irrespective of concomitant cough. These can include bed head elevation, weight reduction, smoking cessation, and dietary changes.56 Initially, a 4–12-week empirical and diagnostic trial of proton pump inhibitors (PPIs) can improve a substantial proportion (up to 79%) of patients, thereby confirming diagnosis of GERD-related cough. Accordingly, PPI therapy can be tapered to the lowest dose for control of cough and the patient should be referred to a GI specialist for further investigation of underlying gastrointestinal disorders, or in case of PPI failure or dependency. Guidelines, consensus, and expert opinions on chronic cough have been well described elsewhere.57, 58, 59, 60, 61, 62 Listed below are clinical clues which can improve diagnostic accuracy of chronic cough in multidisciplinary care.
Diagnostic testing in multidisciplinary care by specialists
Upper airways
-
•
Ear exam can trigger cough, a mechanism mediated by Arnold's nerve.63
-
•
Mouth examination can reveal hypertrophied/obstructive tonsils reportedly associated with cough.64,65
-
•
Allergy can be assessed by skin tests or serum specific IgE testing, but tests should be interpreted according to symptoms.
-
•
Endoscopic nasopharyngolaryngoscopy can also be informative (Fig. 1B). Sinonasal polyposis may be associated with asthma as part of the airway eosinophilia syndrome or aspirin exacerbated respiratory disease.66,67 CRS without nasal polyps is also associated with airway hyperresponsiveness.68 Adenoid hypertrophy/inflammation, frequently diagnosed by X-ray and occasionally by endoscopy (in school-age children), is commonly encountered in pediatric CRS69 which is a major cause of chronic cough in children.70
-
•
Pharyngeal endoscopy often supplemented by bronchoscopy is useful in diagnosis of infrequent pharyngolaryngeal71,72 and lung tumors which can trigger cough.73
-
•
Laryngoscopy, at times followed by stroboscopy, is helpful in the diagnosis of vocal cord pathologies such as paresis/paralysis, dysphonia, tension voice and tumors.71,74,75
-
•
A split-night polysomnography testing concomitant with a titration cycle using continuous positive airway pressure can assist in the diagnosis and management of patients with OSA and chronic cough, respectively.76
Following diagnosis of one or multiple etiological factors of chronic cough originating from the upper airways, corresponding management follows well established guidelines.77, 78, 79, 80, 81, 82, 83
Lower airways
-
•
Spirometry: Patients presenting with chronic cough in primary care frequently have normal lung function. Spirometry, commonly conducted in pulmonary and allergy care, can reveal airflow obstruction,84 variability (>20%) in peak expiratory flow measurements,60,85 or an improvement in threshold testing (FEV1>12%, improvement from baseline of >200 mL) in response to bronchodilators (β-2 agonists).86 An abnormal spirometry can be encountered in patients with CA and COPD, but not in patients with CVA or eosinophilic bronchitis (EB) (Table 1).
-
•
Bronchial challenge testing (BCT) are recommended in the etiological diagnosis of chronic cough in patients with reactive airway diseases (Table 1B).38
-
•
Direct (methacholine/histamine) or indirect (mannitol)87 BCT for airway hyperresponsiveness can be helpful in the diagnosis of asthma and NAEB as a primary cause of chronic cough (Table 1). A negative BCT, such as an FEV1 decrease of <20% at the highest methacholine challenge dose (10 mg/mL), has a high negative predictive value of asthma as an etiological diagnosis in chronic cough.88 In a stepwise diagnostic approach, an initial abnormal lung function testing suggests CA or COPD; normal testing is inclusive of CVA, NAEB, or chronic bronchitis (Fig. 1B). The absence of bronchial hyperreactivity to methacholine or mannitol89 challenge in patients with seemingly normal physical examination and spirometry findings raises suspicion of NAEB.90 Similarly, a negative airway responsiveness can exclude CVA.55 Of importance, a BCT is contra-indicated if immediate spirometry is abnormal.
-
•
Fractional exhaled nitric oxide (FeNO) measurement43 for airway eosinophilia can also be helpful.38 However, its use as a biomarker of eosinophilic airway inflammation and, by inference, steroid responsiveness, requires further elucidation. This is due to variable correlation between FeNO and airway eosinophilia as measured in induced sputum, bronchoalveolar lavage, or bronchial biopsies.91, 92, 93, 94, 95, 96, 97 Notwithstanding, low FeNO is valuable in determining absence of eosinophilic airway inflammation91,98,99 keeping in mind that the test predictive values (positive or negative) are dependent on the prevalence of eosinophilic airway inflammation in the tested population, and hence cannot be generalized. The role of FeNO in chronic cough is less clear. FeNO can generally assist in identifying subgroups with asthma, CVA and NAEB as potential causes of chronic cough59,100, 101, 102, 103 (Table 1). More precisely, it has been reported FeNO values below 30 ppb can reliably « rule out » asthma as a cause of chronic cough,104 but higher values do not necessarily « rule it in »105. Others suggested FeNO can diagnose asthma or CVA as etiological factors in chronic cough with moderate accuracy at optimal cut-off values between 30 and 40 ppb.105 A metanalysis revealed a relatively high (0.85) specificity of FeNO in predicting asthma in adults patients with chronic cough despite lack of consensus on cut-off levels for the diagnosis.59 In comparison, FeNO diagnostic accuracy in predicting eosinophilic bronchitis (EB) in nonasthmatic chronic cough is lower compared to CVA which questions its utility in the former group.59 Notwithstanding, it has been suggested FeNO can « rule in » NAEB at optimal cut-off values ranging from 22.5 to 31.7 ppb.59,103 This lack of clear cut-off levels of FeNO for the etiological diagnosis of asthma, CVA and NAEB in chronic cough105, 106, 107 limits its usefulness as a routine diagnostic modality and follow-up assessment tool for chronic cough and compels its validation in future studies.59,62,108,109 In the upper airways, an abnormally low nasal nitric oxide (nNO) is reportedly a predictor of nasal polyposis in severe asthmatic patients even when blood eosinophils are normal or low.100 Also, primary ciliary dyskinesia can present with chronic cough (sinopulmonary infections) and low nNO110,111 which can be supplemented by other testing modalities.112 In conclusion, despite the well-established role of FeNO as an eosinophilic biomarker in asthma, its indication in patients with concomitant chronic cough needs further elucidation.
-
•
Induced sputum may represent the most accurate surrogate marker of airway eosinophilia113 despite concerns of its reproducibility114 (Table 1) and inherent technical difficulties. Data suggest that induced sputum eosinophil count is gradually increased in NAEB, CVA, and CA, in sequential order.115 The sensitivity of induced sputum varies widely in the diagnosis of asthma.116,117 A sputum eosinophil count of >3% is generally indicative of NAEB in absence of bronchial hyperresponsiveness (BHR) or variability in peak expiratory flow rates118,119; it is also reportedly associated with corticosteroid responsiveness in asthma and COPD.120,121 FeNO cannot be substituted for induced sputum in the diagnosis of eosinophilic airway inflammation due to concerns of low sensitivity and specificity of the former in detecting sputum eosinophilia, according to a metanalysis.122 Both induced sputum eosinophilia and FeNO can predict response to ICS in patients with cough.123 In conclusion, spirometry and BCT are powerful tools in the etiological diagnosis of chronic cough in patients with reactive airway diseases. FeNO and induced sputum cells to assess for airway eosinophilia can be helpful and should be considered in patients presenting with chronic cough but their role in diagnosing etiological factors of the hypersensitive cough reflex needs further elucidation.
-
•
Lung computed tomography (CT) imaging has a low yield in chronic cough in the presence of a normal chest radiography, and clinical examination.124 However, a CT is generally indicated to rule out parenchymal lung disease.
-
•
Ancillary procedures in pulmonary care can include bronchoscopy to rule out rare cases of chronic cough such as tracheopathia, tracheomalacia, lung tumors and foreign body aspiration.60 Bronchoscopy may also provide bronchoalveolar lavage fluid for eosinophil count examination and biopsies for suspected tumors in chronic cough.
Table 1.
Comparative analysis of ancillary testing and pharmacologic response in cough-phenotypic traits originating from the lower airways. CA, classic asthma; COPD, chronic obstructive pulmonary disease; CVA, cough variant asthma; FeNO, fractional exhaled nitric oxide; ICS, inhaled corticosteroids; LTRA, leukotriene receptor antagonist; NAEB, non-asthmatic eosinophilic bronchitis
| Spirometry41 |
Bronchial hyperresponsiveness (methacholine)41,55,151 |
FeNO (ppb)59,103,105 |
Therapeutic response41,42,44, 45, 46, 47, 48, 49 |
||||
|---|---|---|---|---|---|---|---|
| Parental steroids | Bronchodilators | ICS | LTRAs | ||||
| CA | Abnormal | Present | 30–40 | (+)ve | (+)ve | (+)ve | (+)ve |
| NAEB118 | Normal | Absent | 22.5–31.7 | (+)ve | (−)ve | (+)ve | (+)ve |
| CVA | Normal | Present or Borderline | 30–40 | (+)ve | (+)ve | (+)ve | (+)ve |
| COPD | Abnormal | Present | NA | (+)ve | (+)ve | Not fully established | (−)ve |
Gastrointestinal tract
Since some degree of reflux is present in healthy people, a self-assessed reflux symptom questionnaire, at a reflux symptom index (RSI) > 13, can assist in identifying subgroups of chronic cough patients with concomitant gastroesophageal and laryngopharyngeal reflux125 (Fig. 1B). Reportedly, a high score also suggests presence of proximal rather than distal reflux as well as non-acid and gas reflux in patients with chronic cough.126 Interestingly, data suggest the gastric proteolytic enzyme, pepsin, can adhere to laryngopharyngeal epithelium to cause inflammation and hypersensitivity and thus contribute to non-acid gastric reflux.127 As stated previously, patients who fail to improve or are dependent on an empirical therapy of PPIs in primary care should be referred to a GI specialist to rule out non-acid/gas reflux. Chronic cough refractory to PPI therapy in patients suspected of having non-acid or gas reflux-related cough can undergo esophagogastroduodenoscopy (EGD) with or without biopsies. EGD can exclude other potential diagnoses which are not necessarily cough-related and include Barrett's esophagus, eosinophilic esophagitis,128,129 or its variant, PPI-responsive eosinophilic esophagitis, among others. EGD is also indicated if more serious cough-comorbid signs and symptoms are present, such as older age (>50 years) with weight loss, anemia, and dysphagia. It is generally agreed a normal EGD in patients with chronic cough does not exclude GERD128 and necessitates pH measurement in patients suspected of having GERD-related cough.130 Ambulatory multichannel intraluminal impedance monitoring combined with pH-metry (MII-pH) is superior to the standard 24-h esophageal pH monitoring since it can record temporal association of cough with acid as well as non-acid reflux events, as measured by intraluminal pH and impedance probes, respectively.131 Recently, an ambulatory pH-impedance-pressure monitoring has been introduced to assess concomitantly the impact of esophageal dysmotility in the etiology of GERD-related cough.132 Clinically, MII-pH carries the highest sensitivity and specificity for diagnosis of GERD-related cough, yet it is an invasive test with limited availability.131 It is usually performed off antacid treatment. The temporal association between cough and reflux events is recorded over 24-h using MII-pH probes and assessed by symptom index and symptom-association probability (SAP) index.133, 134, 135 Following this, non-PPI-responsive patients with negative impedance and pH-metry findings are unlikely to have GERD-related cough and can be candidates for neuromodulator therapy. Patients with confirmed GERD-related cough can be candidates for fundoplication surgery preceded by esophageal manometry to rule out motility disorders.136 Recent guidelines suggest fundoplication surgery can be considered in cases of PPI dependency, more so in young patients,56,137 but is not advisable with a normal acid exposure time in the distal esophagus as determined by impedance-pH-metry.56
COVID-19 cough
-
•
Endoscopic visualization of the respiratory tract can be performed during COVID-19 pandemic if findings may have a significant impact on patient's management or malignancy.138,139 Testing for COVID-19 prior to pulmonary function testing (eg, methacholine or exercise challenge), sputum cell examination or bronchoscopy is important since these are aerosol-generating procedures and pose significant risk of spreading infection. Recommendations related to staff personal protective equipment, examination room settings, and instruments disinfection have been described elsewhere140 and can minimize significantly the risk of COVID-19 exposure or for that matter, contamination by other microbial agents. In confirmed COVID-19 cases, recommendations on when to perform pulmonary procedures are variable (put the 2 references we discussed yesterday over here) and need be updated according to pandemic status. However, well controlled safety measures (PPE, sterile techniques, etc.) can circumvent a prolonged (ie, 8 weeks) waiting time till full COVID-19 recovery. A mini-broncho-alveolar lavage or tracheal aspirate can be considered before bronchoscopy is performed.141
-
•
Recommendations on intranasal steroids142,143 and inhalers144,145 use in COVID-19 are summarized elsewhere.
Guidelines and expert consensus in cough pharmacotherapy
Upper airways
Compared to the lower airways, the role of pharmacotherapy in UACS is inconclusive (see Section Lower airways). In one report, improvement of cough in SAR patients was noted using different combinations of intranasal therapy such as azelastine and ipratropium or azelastine and intranasal corticosteroids, although the study lacked objective cough measures.146 In addition, (non-asthmatic) chronic rhinosinusitis patients with chronic cough reported significant improvement in lung function parameters following long term (3 months) low-dose macrolide therapy. However, cough was not studied independently of other CRS parameters.147 A metanalysis suggested oral antibiotics can be beneficial in children with chronic productive cough in terms of clinical cure rates and prevention of illness progression, despite limitations in study designs and quality.148
Lower airways
If the clinical profile of a chronic cough patient is reminiscent of asthma (typical recurrent symptoms of wheezing, breathlessness, cough, and chest tightness), several drug therapies can be offered. Data suggests a consistent improvement in cough scores with ICS and LTRAs, inhaled beta2-agonists and muscarinic receptor antagonists (ie, tiotropium), and to a lesser extent theophylline and mast cell stabilizers, but with variable bias noted in the studies.62 Similarly if CVA is suspected, recent guidelines recommend ICS as first line treatment based on strong efficacy data for step-wise therapy of asthma in general rather than distinctively for cough.62 Notwithstanding, there is a significant subjective placebo effect of ICS in patients presenting with nonspecific chronic cough albeit a small to moderate therapeutic gain (≤+22%), according to a metanalysis.149 This entails careful interpretation of the therapeutic benefit of ICS in nonspecific cough. In suspected NAEB, a therapeutic/diagnostic trial of ICS followed by LTRAs (if stepping up ICS fails to improve cough) can be suggested despite weak evidence to support efficacy of such a treatment in diagnosing NAEB.62 In NAEB, a hypersensitive cough reflex may partly explain clinical resistance to mainstay anti-asthma therapy such as topical bronchodilators or ICS,150 as revealed by capsaicin challenge models. In COPD, a cough-associated chronic obstructive airway disease, an empirical trial of bronchodilator therapy is often justified but requires more efficacy data.50,51 Unlike LTRAs49 and contrary to asthma, low dose ICS can only benefit COPD patients experiencing exacerbations with significant BHR.151,152 Despite absence of muscarinic receptors on airway afferent nerves, tiotropium, a long-acting muscarinic selective M3 receptor antagonist, can modulate a hypersensitive cough reflex in asthmatic patients refractory to ICS/long-acting β-2 agonist bronchodilators.153
Digestive tract
A Cochrane review revealed lack of high-quality evidence to support efficacy of PPIs or histamine H2-receptor antagonists in treatment of adults and children with chronic cough. The lack of high-quality evidence is partly due to heterogenous trial designs, disparate selection of control and active groups with respect to chronic cough, and indiscriminate testing methods of acid and non-acid reflux events.154 Along the same line, other reports showed no difference between PPIs and placebo in chronic cough.155, 156, 157 To note, these studies were small in sample size and used non-validated QoL questionnaires.157 Notwithstanding, a subgroup of patients with cough reported benefit from PPI therapy in appropriate dosing and duration of treatment with a number needed-to-treat of 5.158 Presence of acid reflux, either clinically (eg, heartburn, food regurgitation) or confirmed by diagnostic testing, shows modest therapeutic gain in coughing patients on acid suppressive therapy.56 Absence of acid reflux contraindicates the routine use of antacid therapy in GERD-related chronic cough patients, according to expert panels.56,61 Non-acid or gas reflux, its association with proximal reflux using RSI, and its evaluation by MII-pH monitoring, awaits controlled trials to assess efficacy of antacid therapy in these select patients with cough. As stated earlier, early (within 2 weeks) cough improvement on PPIs can be followed by 4–12 weeks of maintenance therapy before tapering medications. Increasing PPI dose to twice a day confers only marginal benefit which should be weighed against incurred cost and side effects.156 Cumulative doses of PPI, such as occurs during prolonged drug intake or doubling of PPI dose in partial responders, increases the risk of developing hypomagnesemia, among other side effects, in a dose-dependent manner.159 Both hypomagnesemia and its consequent decrease in melatonin production can decrease lower esophageal sphincter (LES) tone and instigate a paradoxical iatrogenic cough.160, 161, 162 Night-time magnesium and melatonin supplementation (rather than prescribing an extra dose of PPI) is recommended for partial responders to curtail side effects of long-term PPI intake. Supplementing PPI therapy with prokinetics can enhance gut motility and improve GERD-related cough.33 Adding H2-blockers for 2–4 weeks can be beneficial in reducing nocturnal acid breakthrough163 in difficult-to-treat cases of GERD but its role in cough has not been investigated. Similarly, neuromodulators such as baclofen or gabapentin in combination with PPI can benefit patients with loose lower esophageal sphincter or high RSI scores. Their use, however, in chronic cough requires further investigation, given the high prevalence of somnolence as a side effect.164
COVID-19 cough
An open study (N = 14) suggested that ingestion of nuclear factor-erythroid factor 2-related factor 2 (Nrf2)-interacting foods can modulate COVID-19 symptoms. Broccoli seed capsules (Nrf2 and mild TRPA1),165,166 curcumin and black pepper, ginger, and red pepper resulted in immediate (within minutes), significant, and highly reproducible improvement of cough, thereby suggesting TRPA1/TRPV1 desensitization.167 These effects were relatively short lasting (2–4 h). The duration of efficacy was extended to 6–8 h with broccoli suggesting an Nrf2-TRP crosstalk, and to 12–15 h by low dose paracetamol (some metabolites are Nrf2-TRP agonists).168, 169, 170 This requires further evaluation in large randomized controlled studies.
Neuromodulators
Unexplained or chronic refractory cough often requiring trials of various medications for over a year can be treated with neuromodulators which target neural hyperresponsiveness of the cough reflex.171 Neuromodulators can encompass centrally acting drugs such as morphine172,173 and amitriptyline,174,175 pregabalin,176 and gabapentin,177 all of which have a negative impact on patient's mood. Novel peripheral-acting antitussive neuromodulators such as P2X3 antagonists may also be beneficial in the management of chronic cough since they lack significant central systemic side effects. Currently, neuromodulators are not approved in the United States or Europe for management of chronic cough due to the low level of evidence with respect to their efficacy (grade II C).53,61 An expert panel suggested neuromodulators can improve cough-specific QoL with variable effect on cough frequency and severity despite potential for selection bias in reported studies.53 Metrics are important objective tools to assess antitussive effect of drugs. The vitalograph is a 24-h ambulatory cough monitoring tool approved in the United States for cough assessment.178
Although central neuromodulators can manifest modest efficacy in protracted cough, they are frequently associated with significant central adverse effects in randomized controlled trials, such as drowsiness and confusion. For example, morphine revealed a 70% reduction in cough frequency versus placebo, but its use is limited by severe adverse effects.179,180 Data suggest amitriptyline at different doses can improve post-viral chronic cough;175,181 yet its role in non-viral chronic cough is undefined.53 Randomized controlled trials with gabapentin demonstrated improved cough qualities, namely cough-specific QoL, cough frequency and severity.177,182 A review of prospective case-series reported an overall 68% amelioration of cough and sensory neuropathy with gabapentin.182 Yet, improvement in cough qualities was not sustained upon discontinuation of drug. Reportedly, at an initial dose of 1800 mg and an onset of action at 4 weeks, gabapentin resulted in significant cough improvement with a satisfactory safety profile. Common side effects included confusion and dizziness, nausea, dry mouth, and fatigue.177 It is recommended the risk-benefit profile of gabapentin be assessed at 6 months before therapy is extended.53 Neurokinin receptor 1 (NK-1) antagonists block substance P tussive effect in the nucleus tractus solitarius mediated by NK-1 receptor.183 NK-1 antagonists have been traditionally used for chemotherapy-induced nausea and vomiting.184 A newer NK-1 antagonist, overpitant, improved cough qualities including frequency, severity and QoL with an acceptable safety profile, and hence warrants further investigation.185 Similarly, a selective agonist of alpha 7 subtype of acetylcholine receptor, which is under investigation for treatment of schizophrenia,186 features potential benefit in patients with acute and chronic cough.187
Peripherally acting neuromodulators include a range of receptor antagonists with variable antitussive effects. Gamma-aminobutyric acid B receptor antagonists, initially used to manage acid reflux by inhibiting the relaxation of the lower esophageal sphincter, improved citric acid-induced cough in animal models.188 P2X3 purinergic channels are receptors belonging to the P2X family of ion channels, with high predilection to the cellular breakdown product adenosine triphosphate (ATP),189 and are expressed on both C and A-δ fibers. Upon activation, P2X3 channels quickly depolarize and subsequently desensitize the peripheral cough neuronal pathway. They can also modulate the cough neuronal pathway activity at central synapses in both upper and lower airways, hence the effect of P2X3 antagonists190 such as gefapixant. It is speculated gefapixant acts primarily on peripheral sensory neurons and has a significant role in hypersensitive cough reflex.43,191 In a proof-of-concept study, gefapixant improved cough-specific QoL, cough frequency (up to 75%), and severity in patients with unexplained chronic cough; however, following 12 weeks therapy and 600 mg twice daily dosing, dysgeusia was noted in most of patients. At lower doses (30–50 mg), gefapixant significantly reduced the awake cough frequency outcome compared to placebo, and taste abnormalities were less reported in a phase IIa study.192 A large (N > 2000) pooled analysis of three clinical trials using 45 mg gefapixant BID in patients with unexplained chronic cough demonstrated a 17.4% and 18.6% reduction in awake and 24-h cough, respectively.193 Cough challenge studies using ATP and capsaicin were examined in patients treated with gefapixant. ATP inhalation studies did not elicit a dramatic decrease in cough sensitivity,194 nor did gefapixant showed any effect on capsaicin-induced cough,195 thus denoting complex and heterogenous mechanisms of chronic cough. TRP (A1/V1/M8) antagonists196,197 and sodium channel blockers198 (anesthetic effect) are potential candidates for further investigation in chronic cough.
Speech therapy
Speech pathology treatment for chronic cough (SPTCC), also termed physiotherapy, speech and language therapy intervention (PSALTI), is a non-pharmacological non-invasive therapeutic option199 to control chronic refractory cough199, 200, 201, 202 and associated voice disorders199 that persist despite medical treatment.200,203 It is also a reported therapeutic modality for management of habit cough which belongs to the spectrum of functional respiratory symptoms.204 SPTCC consists of multiple modules, namely cough suppression strategies including breathing and swallowing techniques, vocal hygiene such as avoidance of dietary cough-triggers, and psychoeducational counseling to minimize impact of cough on QoL.199,200,205 The mechanism of speech therapy in improving cough is yet unknown.205 It is speculated SPTCC can reduce laryngeal irritation responsible for cough, increases cough threshold,201 or reduces capsaicin-induced cough reflex hypersensitivity201,203,205,206 via cortical control.203 Voluntary cough suppression does not appear to be the primary mechanism as the cough suppression component did not decrease cough sensitivity as measured by the C5 endpoint in a tussigen challenge.205 Conversely, vocal hygiene improved C5 and "urge to cough".206 Also, data suggest SPTCC improved short-term, but not long-term, cough associated QoL.199,206,207 It also reduced cough frequency,201,203,205,207,208 severity,207 and capsaicin-triggered cough reflex sensitivity.203,205,207 It is suggested SPTCC can be initiated prior, during, or after203 medical therapy of refractory cough.53
Summary
In this paper we suggest an algorithm for proper management of patients with chronic cough in an integrated care pathway approach. Management of chronic cough can be diverse depending on cough etiology and is more challenging in patients with multifactorial cough where clinicians often need to add one therapeutic modality to another in an attempt to achieve control. There can be a role for alternative empirical therapies of chronic cough in primary care with limited access to advanced diagnostic modalities for cough etiologies. In cough-specialty care, multiple diagnostic and ancillary tests are available for management of cough-comorbid conditions such as rhinitis/rhinosinusitis, reactive lower airway diseases, and reflux. At best, these modalities can improve diagnostic accuracy of cough etiological factors, albeit to variable degrees due to their inherent limitations. These modalities can also predict cough response to guidelines-based therapy of cough etiological factors but seldom result in overall cough improvement. Safety measures for COVID-19 need to be considered in diagnostic and therapeutic care pathways of chronic cough. Pharmacotherapy with neuromodulators target neural hyperresponsiveness of chronic refractory cough. Preliminary data on neuromodulators suggest overall improvement in cough severity, frequency, and cough specific QoL, albeit to variable degrees. More data are needed regarding proper dosing, duration, and safety of treatment with neuromodulators. Behavioral therapy can be an adjunctive therapeutic modality for chronic cough and associated voice disorders, although mechanisms of cough improvement are yet unknown. In COVID-19 cough, TRP desensitization with ingestion of Nrf2-interacting foods requires more research in improving post COVID-19 persistent cough.
Abbreviations
ACE, Angiotensin-converting enzyme; AR, allergic rhinitis; ATP, Adenosine triphosphate; BCT, Bronchial challenge testing; BHR, Bronchial hyperresponsiveness; CA, Classic asthma; COPD, Chronic obstructive pulmonary disease; CRS, Chronic rhinosinusitis; CT, Computed tomography; CVA, Cough variant asthma; EB, Eosinophilic bronchitis; EGD, Esophago-gastroduodenoscopy; FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume during the first second; GI, gastrointestinal; GIT, gastrointestinal tract; GERD, Gastro-esophageal reflux disease; H2-Blockers, Histamine H2-receptor blockers; HCR, hypersensitivity cough reflex; ICS, Inhaled corticosteroids; LES, Lower esophageal sphincter; LTRA, Leukotriene receptor antagonist; MII-pH, Multichannel intraluminal impedance monitoring combined with pH-metry; NAEB, Non-asthmatic eosinophilic bronchitis; NK-1, Neurokinin receptor 1; Nrf2, Nuclear factor erythroid 2-related factor 2; OSA, Obstructive sleep apnea; PPI, Proton pump inhibitor; PSALTI, Physiotherapy, speech and language therapy intervention; QoL, Quality of life; RSI, Reflux symptom index; SAP, Symptom association probability; SAR, seasonal allergic rhinitis; SPTCC, Speech pathology treatment for chronic cough; TRP, Transient receptor potential; UACS, Upper airway cough syndrome.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors’ contributions
Philip Rouadi (PR) designed the plan of the article, contributed to the data collection. He wrote the manuscript draft, conceived and designed the tables/figures, and reviewed all parts of the article. Samar Idriss contributed to data collection, tables/figures, and manuscript draft. Jean Bousquet participated to the manuscript draft and reviewed the whole article. Tanya Laidlaw, Cecilio Azar, Mona Al-Ahmad, Anahi Yanez, Maryam Al-Nesf, Talal Nsouli, Sami Bahna, Eliane Abou Jaoude, Fares Zaitoun, Usamah Hadi, Georges Juvelekian and Moussa Riachy contributed to the manuscript draft each according to his/her specialty and domain of interest. The rest of authors reviewed closely the whole article and added their remarks.
Ethical statement
The manuscript entitled “WAO-ARIA CONSENSUS ON CHRONIC COUGH - PART III: MANAGEMENT STRATEGIES IN PRIMARY AND COUGH-SPECIALTY CARE. Updates in COVID-19.” is the authors' own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere.
All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content.
Authors’ consent for publication
All authors have approved the manuscript and agree with its submission and final publication to the World Allergy Organization Journal.
Competing interests
No competing interests to declare.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100649.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.French C.L., Irwin R.S., Curley F.J., Krikorian C.J. Impact of chronic cough in quality of life. Arch Intern Med. 1998;158(15):1657–1661. doi: 10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 2.Young E.C., Smith J.A. Quality of life in patients with chronic cough. Ther Adv Respir Dis. 2010;4(1):49–55. doi: 10.1177/1753465809358249. [DOI] [PubMed] [Google Scholar]
- 3.Rouadi P.W., Idriss S.A., Bousquet J., Laidlaw T.M., Azar C.R., Al-Ahmad M.S. WAO-ARIA consensus ON chronic cough- part I: role of TRP channels in neurogenic inflammation of cough neuronal pathways. WAO J. 2021;14:100617. doi: 10.1016/j.waojou.2021.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widdicombe J.G. Sensory neurophysiology of the cough reflex. J Allergy Clin Immunol. 1996;98(5):PS84–S90. doi: 10.1016/S0091-6749(96)70021-0. [DOI] [PubMed] [Google Scholar]
- 5.Rouadi P.W., Idriss S.A., Bousquet J., Laidlaw T.M., Azar C.R., Al-Ahmad M.S. WAO-ARIA consensus on chronic cough – part II: phenotypes and mechanisms of abnormal cough presentation – updates in COVID-19. WAO J. 2021 doi: 10.1016/j.waojou.2021.100618. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holden S.E., Morice A., Birring S.S., et al. Cough presentation in primary care and the identification of chronic cough: a need for diagnostic clarity? Curr Med Res Opin. 2019;36(1):139–150. doi: 10.1080/03007995.2019.1673716. [DOI] [PubMed] [Google Scholar]
- 7.Song W.-J., Millqvist E., Morice A.H. New ERS cough guidelines: a clinical framework for refining the patient management strategy. Asia Pac Allergy. 2019;9(4):1–5. doi: 10.5415/apallergy.2019.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarvey L., McKeagney P., Polley L., MacMahon J., Costello R.W. Are there clinical features of a sensitized cough reflex? Pulm Pharmacol Therapeut. 2009;22(2):59–64. doi: 10.1016/j.pupt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Hilton E., Marsden P., Thurston A., Kennedy S., Decalmer S., Smith J.A. Clinical features of the urge-to-cough in patients with chronic cough. Respir Med. 2015;109(6):701–707. doi: 10.1016/j.rmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Chang A.B., Redding G.J., Everard M.L. Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol. 2008;43(6):519–531. doi: 10.1002/ppul.20821. [DOI] [PubMed] [Google Scholar]
- 11.Gilberg S., Njamkepo E., Du Châtelet I.P., et al. Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a French area with very high whole-cell vaccine coverage. J Infect Dis. 2002;186(3):415–418. doi: 10.1086/341511. [DOI] [PubMed] [Google Scholar]
- 12.Jõgi P., Oona M., Kaart T., et al. Pertussis and parapertussis in children and adults with a persistent cough: an observational study. Infection. 2018;46(1):83–91. doi: 10.1007/s15010-017-1095-z. [DOI] [PubMed] [Google Scholar]
- 13.Ding H., Shi C., Xu X., Yu L. Drug-induced chronic cough and the possible mechanism of action. Ann Palliat Med. 2020;9(5):3562–3570. doi: 10.21037/apm-20-819. [DOI] [PubMed] [Google Scholar]
- 14.Bangalore S., Kumar S., Messerli F.H. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the physicians' desk reference. Am J Med. 2010;123(11):1016–1030. doi: 10.1016/j.amjmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Sadanaga T., Yoshimura M., Sakamoto T., Sumida H., Ogawa H. Enalapril-induced cough is associated with non-severe heart failure. Int J Cardiol. 2009;135(2):275–276. doi: 10.1016/j.ijcard.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 16.Hallberg P., Persson M., Axelsson T., et al. Genetic variants associated with angiotensin-converting enzyme inhibitor-induced cough: a genome-wide association study in a Swedish population. Pharmacogenomics. 2017;18(3):201–213. doi: 10.2217/pgs-2016-0184. [DOI] [PubMed] [Google Scholar]
- 17.Mosley J.D., Shaffer C.M., Van Driest S.L., et al. A genome-wide association study identifies variants in KCNIP4 associated with ACE inhibitor-induced cough. Pharmacogenomics J. 2016;16(3):231–237. doi: 10.1038/tpj.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yılmaz İ. Angiotensin-converting enzyme inhibitors induce cough. Turkish Thorac J. 2019;20(1):36–42. doi: 10.5152/TurkThoracJ.2018.18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L., Guo R., Sun L. The impact of prophylactic intravenous lidocaine on opioid-induced cough: a meta-analysis of randomized controlled trials. J Anesth. 2014;28(3):325–333. doi: 10.1007/s00540-013-1732-3. [DOI] [PubMed] [Google Scholar]
- 20.Pascual Cruz M., Chimenos Küstner E., García Vicente J.A., Mezquiriz Ferrero X., Borrell Thio E., López López J. Adverse side effects of statins in the oral cavity. Med Oral Patol Oral Cir Bucal. 2008;13(2):98–101. [PubMed] [Google Scholar]
- 21.Laidlaw T.M. Clinical updates in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2019;40(1):4–6. doi: 10.2500/aap.2019.40.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales D.R., Lipworth B.J., Donnan P.T., Jackson C., Guthrie B. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: population-based nested case control study. BMC Med. 2017;15(1):18. doi: 10.1186/s12916-017-0781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achilleos A. Evidence-based evaluation and management of chronic cough. Med Clin. 2016;100(5):1033–1045. doi: 10.1016/j.mcna.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Mathur S.K., Fichtinger P.S., Evans M.D., Schwantes E.A., Jarjour N.N. Variability of blood eosinophil count as an asthma biomarker. Ann Allergy Asthma Immunol. 2016;117(5):551–553. doi: 10.1016/j.anai.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Wang M., Wen S., Yu L., Xu X. Types and applications of cough-related questionnaires. J Thorac Dis. 2019;11(10):4379–4388. doi: 10.21037/jtd.2019.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birring S.S., Prudon B., Carr A.J., Singh S.J., Morgan L., Pavord I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vertigan A.E., Bone S.L., Gibson P.G. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough. 2014;10(1):1–13. doi: 10.1186/1745-9974-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baiardini I., Braido F., Fassio O., et al. A new tool to assess and monitor the burden of chronic cough on quality of life: chronic Cough Impact Questionnaire. Allergy Eur J Allergy Clin Immunol. 2005;60(4):482–488. doi: 10.1111/j.1398-9995.2005.00743.x. [DOI] [PubMed] [Google Scholar]
- 29.Morice A.H., Faruqi S., Wright C.E., Thompson R., Bland J.M. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011;189(1):73–79. doi: 10.1007/s00408-010-9272-1. [DOI] [PubMed] [Google Scholar]
- 30.Oduwole O., Udoh E., Oyo-Ita A., Meremikwu M. Honey for acute cough in children (Review) Cochrane Database Syst Rev. 2018;4(4):CD007094. doi: 10.1002/14651858.CD007094.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith S., Schroeder K., Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in community settings (Review) Cochrane Database Syst Rev. 2014;11:CD001831. doi: 10.1002/14651858.CD001831.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields M.D., Bush A., Everard M.L., McKenzie S., Primhak R. Recommendations for the assessment and management of cough in children. Thorax. 2008;63:1–15. doi: 10.1136/thx.2007.077370. [DOI] [PubMed] [Google Scholar]
- 33.Irwin R.S., Baumann M.H., Bolser D.C., et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1):1–23. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J.E., Song J.H., Kang K.W. Rhinitis, sinusitis and ocular disease – 2039. Effect of intranasal steroid on cough symptom in patients with upper airway couth syndrome. World Allergy Organ J. 2013;6(1):P171. doi: 10.1186/1939-4551-6-s1-p171. [DOI] [Google Scholar]
- 35.Fujimori K., Suzuki E., Arakawa M. Effects of oxatomide, H1-antagonist, on postinfectious chronic cough; a comparison of oxatomide combined with dextromethorphan versus dextromethorphan alone. Arerugi. 1998;47(1):48–53. [PubMed] [Google Scholar]
- 36.Lilienfield L.S., Rose J.C., Princiotto J.V. Antitussive activity of diphenhydramine in chronic cough. Clin Pharmacol Ther. 1976;19(4):421–425. doi: 10.1002/cpt1976194421. [DOI] [PubMed] [Google Scholar]
- 37.Yu L., Xu X., Lv H., Qiu Z. Advances in upper airway cough syndrome. Kaohsiung J Med Sci. 2015;31(5):223–228. doi: 10.1016/j.kjms.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Irwin R.S., French C.L., Chang A.B., Altman K.W. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153(1):196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gawchik S., Goldstein S., Prenner B., John A. Relief of cough and nasal symptoms associated with allergic rhinitis by mometasone furoate nasal spray. Ann Allergy Asthma Immunol. 2003;90(4):416–421. doi: 10.1016/S1081-1206(10)61826-1. [DOI] [PubMed] [Google Scholar]
- 40.Passali D., Spinosi M.C., Crisanti A., Bellussi L.M. Mometasone furoate nasal spray: a systematic review. Multidiscip Respir Med. 2016;11(1):1–5. doi: 10.1186/s40248-016-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasilewski N.V., Fisher T., Turcotte S.E., Fisher J.T., Lougheed M.D. Bronchoprotective effect of deep inspirations in cough variant asthma: a distinguishing feature in the spectrum of airway disease? Respir Physiol Neurobiol. 2018;257:55–64. doi: 10.1016/j.resp.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Desai D., Brightling C. Cough due to asthma, cough-variant asthma and non-asthmatic eosinophilic bronchitis. Otolaryngol Clin. 2010;43(1):123–130. doi: 10.1016/j.otc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Gold Pocket guide to COPD diagnosis, management, and prevention. Handout. 2020:1–48. Published online. [Google Scholar]
- 44.Cazzola M., Gabriella Matera M. Cough and asthma: the role of inhaled corticosteroids and ß2-agonists. Ther Adv Respir Dis. 2008;2(1):7–11. doi: 10.1177/1753465807087779. [DOI] [PubMed] [Google Scholar]
- 45.Sastre B., Del Pozo V. Role of PGE 2 in asthma and nonasthmatic eosinophilic bronchitis. Mediat Inflamm. 2012;2012:645383. doi: 10.1155/2012/645383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sastre B., Fernández-Nieto M., Mollá R., et al. Increased prostaglandin E2 levels in the airway of patients with eosinophilic bronchitis. Allergy Eur J Allergy Clin Immunol. 2008;63(1):58–66. doi: 10.1111/j.1398-9995.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 47.Song W.J., Chang Y.S. Cough hypersensitivity as a neuro-immune interaction. Clin Transl Allergy. 2015;5(24):1–10. doi: 10.1186/s13601-015-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L., Wang J.L., Xu X.Y., Feng M., Hou Y., Chen L. Leukotriene receptor antagonists do not improve lung function decline in COPD: a meta-analysis. Eur Rev Med Pharmacol Sci. 2018;22(3):829–834. doi: 10.26355/eurrev_201802_14319. [DOI] [PubMed] [Google Scholar]
- 49.Calverley P., Pauwels R., Vestbo J., et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 50.Irwin R.S., French C.T., Smyrnios N.A., Curley F.J. Interpretation of positive results of a methacholine bronchodilator use in diagnosing and treating inhalation challenge and 1 Week of inhaled cough-variant asthma. Arch Intern Med. 1997;157:1981–1987. [PubMed] [Google Scholar]
- 51.Song W.J., An J., McGarvey L. Recent progress in the management of chronic cough. Korean J Intern Med. 2020;35:811–822. doi: 10.3904/KJIM.2020.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrow Brown H. Treatment of chronic asthma with prednisolone significance of eosinophils in the sputum. Lancet. 1958;272(7059):1245–1247. doi: 10.1016/S0140-6736(58)91385-0. [DOI] [PubMed] [Google Scholar]
- 53.Gibson P., Wang G., McGarvey L., Vertigan A.E., Altman K.W., Birring S.S. Treatment of unexplained chronic cough chest guideline and expert panel report. Chest. 2016;149(1):27–44. doi: 10.1378/chest.15-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niimi A., Matsumoto H., Mishima M. Eosinophilic airway disorders associated with chronic cough. Pulm Pharmacol Therapeut. 2009;22(2):114–120. doi: 10.1016/j.pupt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Lougheed M.D., Turcotte S.E., Fisher T. Cough variant asthma: lessons learned from deep inspirations. Lung. 2012;190:17–22. doi: 10.1007/s00408-011-9348-6. [DOI] [PubMed] [Google Scholar]
- 56.Kahrilas P.J., Altman K.W., Chang A.B., et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest. 2016;150(6):1341–1360. doi: 10.1016/j.chest.2016.08.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang A.B., Oppenheimer J.J., Irwin R.S., et al. Managing chronic cough as a symptom in children and management algorithms: CHEST guideline and expert panel report. Chest. 2020;158(1):303–329. doi: 10.1016/j.chest.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 58.Chang A.B., Oppenheimer J.J., Kahrilas P.J., et al. Chronic cough and gastroesophageal reflux in children: chest guideline and expert panel report. Ann Rheum Dis. 2019;78(3):131–140. doi: 10.1016/j.chest.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song W.J., Kim H.J., Shim J.S., et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: a systematic review and meta-analysis. J Allergy Clin Immunol. 2017;140(3):701–709. doi: 10.1016/j.jaci.2016.11.037. Epub 2017 Jan 11. PMID: 28088474. [DOI] [PubMed] [Google Scholar]
- 60.Spanevello A., Beghé B., Visca D., Fabbri L.M., Papi A. Chronic cough in adults. Eur J Intern Med. 2020;78:8–16. doi: 10.1016/j.ejim.2020.03.018. May. [DOI] [PubMed] [Google Scholar]
- 61.Morice A.H., Millqvist E., Bieksiene K., et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2019;55(1):1–31. doi: 10.1183/13993003.01136-2019.ERS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Côté A., Russell R.J., Boulet L.P., et al. Managing chronic cough due to asthma and NAEB in adults and adolescents: Chest expert panel report. Chest. 2020;158(1):68–96. doi: 10.1016/j.chest.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Dicpinigaitis P.V., Enilari O., Cleven K.L. Prevalence of Arnold nerve reflex in subjects with and without chronic cough: relevance to cough hypersensitivity syndrome. Pulm Pharmacol Therapeut. 2019;54:22–24. doi: 10.1016/j.pupt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Birring S.S., Passant C., Patel R.B., Prudon B., Murty G.E., Pavord I.D. Chronic tonsillar enlargement and cough: preliminary evidence of a novel and treatable cause of chronic cough. Eur Respir J. 2004;23(2):199–201. doi: 10.1183/09031936.03.00066403. [DOI] [PubMed] [Google Scholar]
- 65.Sojak J., Durdik P., Zatko T., et al. The effect of adenoidectomy on cough reflex sensitivity in atopic children. Respir Physiol Neurobiol. 2018;257:115–121. doi: 10.1016/j.resp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Laidlaw T.M., Mullol J., Woessner K.M., Amin N., Mannent L.P. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.09.063. Published online. [DOI] [PubMed] [Google Scholar]
- 67.Yilmaz İ. Eosinophilic asthma with chronic rhinosinusitis/nasal polyps and biologic agents. Tuberk Toraks. 2019;67(4):292–299. doi: 10.5578/tt.68953. [DOI] [PubMed] [Google Scholar]
- 68.Ediger D., Sin B.A., Heper A., Anadolu Y., Misirligil Z. Airway inflammation in nasal polyposis: immunopathological aspects of relation to asthma. Clin Exp Allergy. 2005;35(3):319–326. doi: 10.1111/j.1365-2222.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 69.Wang H. Chronic adenoiditis. J Int Med Res. 2020;48(11):1–8. doi: 10.1177/0300060520971458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao F., Gu Q.L., Jiang Z.D. Upper airway cough syndrome in 103 children. Chin Med J (Engl) 2019;132(6):653–658. doi: 10.1097/CM9.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nocini R., Molteni G., Mattiuzzi C., Lippi G. Updates on larynx cancer epidemiology. Chin J Cancer Res. 2020;32(1):18–25. doi: 10.21147/j.issn.1000-9604.2020.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nordgren M., Jannert M., Boysen M., et al. Health-related quality of life in patients with pharyngeal carcinoma: a five-year follow-up. Head Neck. 2006;28(4):339–349. doi: 10.1002/hed.20334. [DOI] [PubMed] [Google Scholar]
- 73.Kvale P.A. Chronic cough due to lung tumors. Chest. 2006;129(1):147S–153S. doi: 10.1378/chest.129.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 74.Altman K.W., Simpson C.B., Amin M.R., Abaza M., Balkissoon R., Casiano R.R. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501–511. doi: 10.1067/mhn.2002.127589. [DOI] [PubMed] [Google Scholar]
- 75.Vertigan A.E., Theodoros D.G., Winkworth A.L., Gibson P.G. Perceptual voice characteristics in chronic cough and paradoxical vocal fold movement. Folia Phoniatrica Logop. 2007;59(5):256–267. doi: 10.1159/000104464. [DOI] [PubMed] [Google Scholar]
- 76.Sundar K.M., Daly S.E., Pearce M.J., Alward W.T. Chronic cough and obstructive sleep apnea in a community-based pulmonary practice. Cough. 2010;6:2. doi: 10.1186/1745-9974-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seidman M.D., Gurgel R.K., Lin S.Y., et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Neck Surg. 2015;152(1S):S1–S43. doi: 10.1177/0194599814561600. [DOI] [PubMed] [Google Scholar]
- 78.Wald E.R., Applegate K.E., Bordley C., et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262–e280. doi: 10.1542/peds.2013-1071. [DOI] [PubMed] [Google Scholar]
- 79.Rosenfeld R.M., Piccirillo J.F., Chandrasekhar S.S., et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2S):S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 80.Fokkens W.J., Lund V.J., Hopkins C., et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 81.Epstein L.J., Kristo D., Strollo P.J., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. doi: 10.5664/jcsm.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Randel A. AAO-HNS guidelines for tonsillectomy in children and adolescents. Am Fam Physician. 2011;84(5):566–573. [PubMed] [Google Scholar]
- 83.Pfister D.G., Spencer S., Adelstein D., et al. Head and neck cancers, version 2.2020. JNCCN J Natl Compr Cancer Netw. 2020;18(7):873–898. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 84.Johnson J.D., Theurer W.M. A stepwise approach to the interpretation of pulmonary function tests. Am Fam Physician. 2014;89(5):359–366. [PubMed] [Google Scholar]
- 85.Jamison J.P., McKinley R.K. Validity of peak expiratory flow rate variability for the diagnosis of asthma. Clin Sci. 1993;85(3):367–371. doi: 10.1042/cs0850367. [DOI] [PubMed] [Google Scholar]
- 86.Ye Q., Liao A., D'Urzo A. FEV1 reversibility for asthma diagnosis: a critical evaluation. Expet Rev Respir Med. 2018;12(4):265–267. doi: 10.1080/17476348.2018.1439741. [DOI] [PubMed] [Google Scholar]
- 87.Lee J., Song J.U. Diagnostic comparison of methacholine and mannitol bronchial challenge tests for identifying bronchial hyperresponsiveness in asthma: a systematic review and meta-analysis. J Asthma. 2020;16:1–9. doi: 10.1080/02770903.2020.1739704. [DOI] [PubMed] [Google Scholar]
- 88.McGrath K.W., Fahy J.V. Negative methacholine challenge tests in subjects who report physician-diagnosed asthma. Clin Exp Allergy. 2011;41(1):46–51. doi: 10.1111/j.1365-2222.2010.03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porsbjerg C., Brannan J.D., Anderson S.D., Backer V. Relationship between airway responsiveness to mannitol and to methacholine and markers of airway inflammation, peak flow variability and quality of life in asthma patients. Clin Exp Allergy. 2008;38(1):43–50. doi: 10.1111/j.1365-2222.2007.02878.x. [DOI] [PubMed] [Google Scholar]
- 90.Brightling C.E., Ward R., Goh K.L., Wardlaw A.J., Pavord I.D. Eosinophilic bronchitis is an important cause of cough. Am J Respir Crit Care Med. 1999;160(2):406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 91.Berry M.A., Shaw D.E., Green R.H., Brightling C.E., Wardlaw A.J., Pavord I.D. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005;35(9):1175–1179. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 92.Mattes J., Storm Van’s Gravesande K., Reining U., et al. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur Respir J. 1999;13(6):1391–1395. doi: 10.1034/j.1399-3003.1999.13f26.x. [DOI] [PubMed] [Google Scholar]
- 93.Jatakanon A., Lim S., Kharitonov S.A., Chung K.F., Barnes P.J. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53(2):91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim S., Jatakanon A., Meah S., Oates T., Chung K.F., Barnes P.J. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax. 2000;55(3):184–188. doi: 10.1136/thorax.55.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Payne D.N.R., Adcock I.M., Wilson N.M., Oates T., Scallan M., Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 I):1376–1381. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 96.Warke T.J., Fitch P.S., Brown V., et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57(5):383–387. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones S.L., Kittelson J., Cowan J.O., et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164(5):738–743. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 98.Porsbjerg C., Lund T.K., Pedersen L., Backer V. Inflammatory subtypes in asthma are related to airway hyperresponsiveness to mannitol and exhaled NO. J Asthma. 2009;46(6):606–612. doi: 10.1080/02770900903015654. [DOI] [PubMed] [Google Scholar]
- 99.Shaw D.E., Berry M.A., Thomas M., et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176(3):231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 100.Maniscalco M., Calabrese C., D'Amato M., et al. Association between exhaled nitric oxide and nasal polyposis in severe asthma. Respir Med. 2019;152:20–24. doi: 10.1016/j.rmed.2019.04.017. January. [DOI] [PubMed] [Google Scholar]
- 101.Heffler E., Carpagnano G.E., Favero E., et al. Fractional exhaled nitric oxide (FENO) in the management of asthma: a position paper of the Italian respiratory society (SIP/IRS) and Italian society of allergy, asthma and clinical immunology (SIAAIC) Multidiscip Respir Med. 2020;15:36. doi: 10.4081/mrm.2020.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shebl E., Abdel-moety H. Assessment of the role of fractional exhaled nitric oxide as a predictor of airway eosinophilia and corticosteroid responsiveness in patients with chronic cough. Egypt J Bronchol. 2020;14:15. doi: 10.1186/s43168-020-00017-y. [DOI] [Google Scholar]
- 103.Sato S., Saito J., Sato Y., et al. Clinical usefulness of fractional exhaled nitric oxide for diagnosing prolonged cough. Respir Med. 2008;102(10):1452–1459. doi: 10.1016/j.rmed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 104.Chatkin J.M., Ansarin K., Silkoff P.E., et al. Exhaled nitric oxide as a noninvasive assessment of chronic cough. Am J Respir Crit Care Med. 1999;159(6):1810–1813. doi: 10.1164/ajrccm.159.6.9809047. [DOI] [PubMed] [Google Scholar]
- 105.Maniscalco M., Fuschillo S., Gaudiosi C., De Felice A., Martucci M., Motta A. Exhaled and nasal nitric oxide measurement in the evaluation of chronic cough. Nitric Oxide. 2019;83:19–23. doi: 10.1016/j.niox.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Matsunaga K., Hirano T., Akamatsu K., et al. Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects. Allergol Int. 2011;60(3):331–336. doi: 10.2332/allergolint.10-OA-0277. [DOI] [PubMed] [Google Scholar]
- 107.Dweik R.A., Boggs P.B., Erzurum S.C., et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qian L., Pan S., Shi J., Du Y., Huang Q., Jie Z. Association between fractional exhaled nitric oxide (FeNO) cutoff values (25 ppb) and risk factors of cough. Clin Res J. 2018;12(1):193–199. doi: 10.1111/crj.12512. [DOI] [PubMed] [Google Scholar]
- 109.Sadeghi M.H., Wright C.E., Hart S., Crooks M., Morice A.H. Does FeNO predict clinical characteristics in chronic cough? Lung. 2018;196(1):59–64. doi: 10.1007/s00408-017-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shapiro A.J., Dell S.D., Gaston B., et al. Nasal nitric oxide measurement in primary ciliary dyskinesia a technical paper on standardized testing protocols. Ann Am Thorac Soc. 2020;17(2):E1–E12. doi: 10.1513/AnnalsATS.201904-347OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walker W.T., Jackson C.L., Lackie P.M., Hogg C., Lucas J.S. Nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2012;40(4):1024–1032. doi: 10.1183/09031936.00176111. [DOI] [PubMed] [Google Scholar]
- 112.Kuehni C.E., Lucas J.S. Diagnosis of primary ciliary dyskinesia: summary of the ERS task force report. Breathe. 2017;13(3):166–178. doi: 10.1183/20734735.008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleming L., Tsartsali L., Wilson N., Regamey N., Bush A. Longitudinal relationship between sputum eosinophils and exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med. 2013;188(3):400–402. doi: 10.1164/rccm.201212-2156LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beghè B., Spanevello A., Fabbri L.M. Eosinophilia in asthma: the easy way is not always the best. Lancet Respir Med. 2015;3(4):260–261. doi: 10.1016/S2213-2600(15)00108-3. [DOI] [PubMed] [Google Scholar]
- 115.Luo W., Lai K., Chen R., et al. Characteristics of airway inflammatory cells and mediators in eosinophilic bronchitis patients. Zhonghua Jiehe He Huxi Zazhi. 2005;28(9):626–629. [PubMed] [Google Scholar]
- 116.Douwes J., Gibson P., Pekkanen J., Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57(7):643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hunter C.J., Brightling C.E., Woltmann G., Wardlaw A.J., Pacord I.D. A Comparison of validity of different diagnostic tests in adults with asthma. CHEST2. 2002;121:1051–1057. doi: 10.1378/chest.121.4.1051. [DOI] [PubMed] [Google Scholar]
- 118.Brightling C.E. Chronic cough due to nonasthmatic eosinophilic bronchitis ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1):116S–121S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- 119.Smith J.A., Woodcock A. Chronic cough. N Engl J Med. 2016;375:1544–1551. doi: 10.1056/NEJMcp1414215. [DOI] [PubMed] [Google Scholar]
- 120.Pizzichini E., Pizzichini M.M.M., Gibson P., et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–1517. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 121.Pavord I.D., Brightling C.E., Woltmann G., Wardlaw A.J. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353:2213–2215. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 122.Korevaar D.A., Westerhof G.A., Wang J., et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(4):290–300. doi: 10.1016/S2213-2600(15)00050-8. [DOI] [PubMed] [Google Scholar]
- 123.Chaudhuri R., McMahon A.D., Thomson L.J., et al. Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. J Allergy Clin Immunol. 2004;113(6):1063–1070. doi: 10.1016/j.jaci.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 124.Kastelik J.A., Aziz I., Ojoo J.C., Thompson R.H., Redington A.E., Morice A.H. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25(2):235–243. doi: 10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 125.Belafsky P.C., Postma G.N., Koufman J.A. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16(2):274–277. doi: 10.1002/lary.28017. [DOI] [PubMed] [Google Scholar]
- 126.Yu Y., Wen S., Wang S., et al. Reflux characteristics in patients with gastroesophageal reflux-related chronic cough complicated by laryngopharyngeal reflux. Ann Transl Med. 2019;7(20) doi: 10.21037/atm.2019.09.162. 529-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zalvan C.H., Hu S., Greenberg B., Geliebter J. A comparison of alkaline water and mediterranean diet vs proton pump inhibition for treatment of laryngopharyngeal reflux. JAMA Otolaryngol - Head Neck Surg. 2017;143(10):1023–1029. doi: 10.1001/jamaoto.2017.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Durazzo M., Lupi G., Cicerchia F., et al. Extra-esophageal presentation of gastroesophageal reflux disease: 2020 update. J Clin Med. 2020;9(8):2559. doi: 10.3390/jcm9082559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eluri S., Dellon E.S. Proton pump inhibitor-responsive oesophageal eosinophilia and eosinophilic oesophagitis: more similarities than differences. Curr Opin Gastroenterol. 2015;31(4):309–315. doi: 10.1097/MOG.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cesario S., Scida S., Miraglia C., et al. Diagnosis of GERD in typical and atypical manifestations. Acta Biomed. 2018;89(5):33–39. doi: 10.23750/abm.v89i8-S.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu X., Chen Q., Liang S., Lv H., Qiu Z. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest. 2014;145(6):1264–1270. doi: 10.1378/chest.13-1634. [DOI] [PubMed] [Google Scholar]
- 132.Li X., Lin S., Wang Z., et al. Gastroesophageal reflux disease and chronic cough: a possible mechanism elucidated by ambulatory pH-impedance-pressure monitoring. Neuro Gastroenterol Motil. 2019;31(12):1–10. doi: 10.1111/nmo.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sifrim D., Castell D., Dent J., Kahrilas P.J. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53(7):1024–1031. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wiener G.J., Richter J.E., Copper J.B., Wu W.C., Castell D.O. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83(4):358–361. doi: 10.1111/j.1572-0241.1988.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 135.Bredenoord A.J., Weusten B.L.A.M., Smout A.J.P.M. Symptom association analysis in ambulatory gastro-oesophageal reflux monitoring. Gut. 2005;54(12):1810–1817. doi: 10.1136/gut.2005.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gockel I., Rabe S.M., Niebisch S. Before and after esophageal surgery: which information is needed from the functional laboratory? Visc Med. 2018;34(2):116–121. doi: 10.1159/000486556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Irwin R.S., Mark Madison J. Diagnosis and treatment of chronic cough due to gastro-esophageal reflux disease and postnasal drip syndrome. Pulm Pharmacol Therapeut. 2002;15(3):261–266. doi: 10.1006/pupt.2002.0348. [DOI] [PubMed] [Google Scholar]
- 138.Crimi C., Impellizzeri P., Campisi R., Nolasco S., Spanevello A., Crimi N. Practical considerations for spirometry during the COVID-19 outbreak: literature review and insights. Pulmonology. 2020;5(20):30175–30176. doi: 10.1016/j.pulmoe.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wahidi M.M., Lamb C., Murgu S., et al. American association for bronchology and interventional pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchol Interv Pulmonol. 2020;27(4):e52–e54. doi: 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rameau A., Young V.V.N., Amin M.R., Sulica L. Flexible laryngoscopy and COVID-19. Otolaryngol Head Neck Surg. 2020;162(6):813–815. doi: 10.1177/0194599820921395. [DOI] [PubMed] [Google Scholar]
- 141.Reddy P.D., Nguyen S.A., Deschler D. Bronchoscopy, laryngoscopy, and esophagoscopy during the COVID-19 pandemic. Head Neck. 2020;42(7):1634–1637. doi: 10.1002/hed.26221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rouadi P.W., Idriss S.A., Bousquet J. Olfactory and taste dysfunctions in COVID-19. Curr Opin Allergy Clin Immunol. 2021 doi: 10.1097/ACI.0000000000000735. Published online. [DOI] [PubMed] [Google Scholar]
- 143.Bousquet J., Akdis C.A., Jutel M., et al. Intranasal corticosteroids in allergic rhinitis in COVID-19 infected patients: an ARIA-EAACI statement. Allergy Eur J Allergy Clin Immunol. 2020;75(10):2440–2444. doi: 10.1111/all.14302. [DOI] [PubMed] [Google Scholar]
- 144.Sethi S., Barjaktarevic I.Z., Tashkin D.P. The use of nebulized pharmacotherapies during the COVID-19 pandemic. Ther Adv Respir Dis. 2020;14:1–9. doi: 10.1177/1753466620954366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ari A. Promoting safe and effective use of aerosol devices in covid-19 : risks and suggestions for viral transmission. Expet Opin Drug Deliv. 2020;17(11):1509–1513. doi: 10.1080/17425247.2020.1811225. [DOI] [PubMed] [Google Scholar]
- 146.Levine B.M. Treatment of chronic cough with intranasal therapy when rhinitis is a significant contributing factor. Chest. 2008;134(4):94P. doi: 10.1378/chest.134.4_meetingabstracts.p94004. [DOI] [Google Scholar]
- 147.Kariya S., Okano M., Higaki T., Makihara S., Tachibana T., Nishizaki K. Long-term treatment with clarithromycin and carbocisteine improves lung function in chronic cough patients with chronic rhinosinusitis. Am J Otolaryngol. 2020;41(1):102315. doi: 10.1016/j.amjoto.2019.102315. [DOI] [PubMed] [Google Scholar]
- 148.Marchant J.M., Morris P.S., Gaffney J., Chang A.B. Cochrane Review: antibiotics for prolonged moist cough in children (Review) Evid Base Child Health. 2012;7(5):1689–1715. doi: 10.1002/ebch.1869. [DOI] [PubMed] [Google Scholar]
- 149.Lee S.E., Lee J.H., Kim H.J., et al. Inhaled corticosteroids and placebo treatment effects in adult patients with cough: a systematic review and meta-analysis. Allergy, Asthma Immunol Res. 2019;11(6):856–870. doi: 10.4168/aair.2019.11.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Niimi A., Fukumitsu K., Takeda N., Kanemitsu Y. Interfering with airway nerves in cough associated with asthma. Pulm Pharmacol Therapeut. 2019;59:101854. doi: 10.1016/j.pupt.2019.101854. [DOI] [PubMed] [Google Scholar]
- 151.Hantera M., Abdel-Hafiz H. Methacholine challenge test as indicator for add on inhaled corticosteroids in COPD patients. Egypt J Chest Dis Tuberc. 2014;63(2):351–354. doi: 10.1016/j.ejcdt.2014.01.006. [DOI] [Google Scholar]
- 152.Chronic Obstructive Pulmonary Disease in Adults over 16s: Diagnosis and Management (NICE Guideline 115) London Natl Inst Heal Care Excell; 2019. Published online. [Google Scholar]
- 153.Fukumitsu K., Kanemitsu Y., Asano T., et al. Tiotropium attenuates refractory cough and capsaicin cough reflex sensitivity in patients with asthma. J Allergy Clin Immunol Pract. 2018;6(5):1613–1620. doi: 10.1016/j.jaip.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 154.Chang A.B., Lasserson T.J., Gaffney J., Connor F.L., Garske L.A. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD004823.pub3. [DOI] [Google Scholar]
- 155.Michaudet C., Malaty J. Chronic cough: evaluation and management. Am Fam Physician. 2017;96(9):575–580. [PubMed] [Google Scholar]
- 156.Ates F., Vaezi M.F. Approach to the patient with presumed extraoesophageal GERD. Best Pract Res Clin Gastroenterol. 2013;27(3):415–431. doi: 10.1016/j.bpg.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 157.Shaheen N.J., Crockett S.D., Bright S.D., et al. High-dose acid suppression for chronic cough: a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2011;33(2):225–234. doi: 10.1111/j.1365-2036.2010.04511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chang A.B., Lasserson T.J., Kiljander T.O., Connor F.L., Gaffney J.T., Garske L.A. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. Br Med J. 2005;332(7532):11–14. doi: 10.1136/bmj.38677.559005.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Srinutta T., Chewcharat A., Takkavatakarn K., et al. Proton pump inhibitors and hypomagnesemia: a meta-analysis of observational studies. Medicine (Baltim) 2019;98(44):e17788. doi: 10.1097/MD.0000000000017788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Konturek S.J., Konturek P.C., Brzozowski T. Melatonin in gastroprotection against stress-induced acute gastric lesions and in healing of chronic gastric ulcers. J Physiol Pharmacol. 2006;57(5):51–66. [PubMed] [Google Scholar]
- 161.Bang C.S., Yang Y.J., Baik G.H. Melatonin for the treatment of gastroesophageal reflux disease; protocol for a systematic review and meta-analysis. Medicine (Baltim) 2019;98(4):e14241. doi: 10.1097/MD.0000000000014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peuhkuri K., Sihvola N., Korpela R. Dietary factors and fluctuating levels of melatonin. Food Nutr Res. 2012;56(10):1–9. doi: 10.3402/fnr.v56i0.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xue S., Katz P.O., Banerjee P., Tutuian R., Castell D.O. Bedtime H2 blockers improve nocturnal gastric acid control in GERD patients on proton pump inhibitors. Aliment Pharmacol Ther. 2001;15(9):1351–1356. doi: 10.1046/j.1365-2036.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- 164.Katz P.O., Gerson L.B., Vela M.F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 165.Bousquet J., Anto J.M., Iaccarino G., et al. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin Transl Allergy. 2020;10(16):1–7. doi: 10.1186/s13601-020-00323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bousquet J., Le V., Blain H., Czarlewski W. Efficacy of broccoli and glucoraphanin in COVID-19 : from hypothesis to proof-of- concept with three experimental clinical cases. World Allergy Organ J. 2020;14(1):100498. doi: 10.1016/j.waojou.2020.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bousquet J., Wienczyslawa C., Zuberbier T., De-la-Torre R., Anto J.M. 2020. Induced Cough Challenges in a Single Patient with COVID-19 Showing an Interplay between Nrf2, TRPA1 and TRPV1 Agonists. [Google Scholar]
- 168.Bousquet J., Cristol J.-P., Czarlewski W., et al. Nrf2-interacting nutrients and COVID-19: time for research to develop adaptation strategies. Clin Transl Allergy. 2020;10(1):58. doi: 10.1186/s13601-020-00362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bousquet J., Czarlewski W., Zuberbier T., et al. Potential interplay between Nrf2 , TRPA1 , and TRPV1 in nutrients for the control of COVID-19. Int Arch Allergy Immunol. 2021;182:324–338. doi: 10.1159/000514204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bousquet J., Czarlewski W., Zuberbier T., Mullol J., Blain H., Cristol J.P., et al. Spices to control COVID-19 symptoms : yes , but not only. Int Arch Allergy Immunol. 2020 doi: 10.1159/000513538. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smith J.A., Badri H. Cough: new pharmacology. J Allergy Clin Immunol Pract. 2019;7(6):1731–1738. doi: 10.1016/j.jaip.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 172.Morice A.H., Menon M.S., Mulrennan S.A., et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175(4):312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 173.Chung K.F. Currently available cough suppressants for chronic cough. Lung. 2008;186(1):82–87. doi: 10.1007/s00408-007-9030-1. [DOI] [PubMed] [Google Scholar]
- 174.Ryan M.A., Cohen S.M. Long-term follow-up of amitriptyline treatment for idiopathic cough. Laryngoscope. 2016;126(12):2758–2763. doi: 10.1002/lary.25978. [DOI] [PubMed] [Google Scholar]
- 175.Jeyakumar A., Brickman T.M., Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope. 2006;116(12):2108–2112. doi: 10.1097/01.mlg.0000244377.60334.e3. [DOI] [PubMed] [Google Scholar]
- 176.Vertigan A.E., Kapela S.L., Ryan N.M., Birring S.S., McElduff P., Gibson P.G. Pregabalin and speech pathology combination therapy for refractory chronic cough a randomized controlled trial. Chest. 2016;149(3):639–648. doi: 10.1378/chest.15-1271. [DOI] [PubMed] [Google Scholar]
- 177.Ryan N.M., Birring S.S., Gibson P.G. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 178.Barton A., Gaydecki P., Holt K., Smith J.A. Data reduction for cough studies using distribution of audio frequency content. Cough. 2012;8(1):1–7. doi: 10.1186/1745-9974-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Al-Sheklly B., Mitchell J., Issa B., et al. Randomized control trial quantifying the efficacy of low dose morphine in a responder group of patients with refractory chronic cough. Thorax. 2017;72:A24–A25. doi: 10.1136/thoraxjnl-2017-210983.41. [DOI] [Google Scholar]
- 180.Mitchell J., Al-Sheklly B., Issa B., et al. Sensations associated with experimentally evoked cough: influence of low dose morphine sulphate in opiod responders. Thorax. 2017;72:14–15. doi: 10.1136/thoraxjnl-2017-210983.246. [DOI] [Google Scholar]
- 181.Jang M., Rubin S.J., Stein D.J., Noordzij J.P. Randomized double blind trial of amitriptyline versus placebo in treatment of chronic laryngopharyngeal neuropathy. Am J Otolaryngol - Head Neck Med Surg. 2017;38(6):683–687. doi: 10.1016/j.amjoto.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 182.Shi G., Shen Q., Zhang C., Ma J., Mohammed A., Zhao H. Efficacy and safety of gabapentin in the treatment of chronic cough: a systematic review. Tuberc Respir Dis (Seoul) 2018;81(3):167–174. doi: 10.4046/trd.2017.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Muñoz M., Coveñas R. The neurokinin-1 receptor antagonist aprepitant: an intelligent bullet against cancer? Cancers (Basel) 2020;12(9):1–22. doi: 10.3390/cancers12092682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aziz F. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting. Ann Palliat Med. 2012;1(2):130–136. doi: 10.3978/j.issn.2224-5820.2012.07.10. [DOI] [PubMed] [Google Scholar]
- 185.Smith J., Allman D., Badri H., et al. The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1) Chest. 2020;157(1):111–118. doi: 10.1016/j.chest.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 186.Pohanka M. Alpha 7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci. 2012;13(2):2219–2238. doi: 10.3390/ijms13022219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dicpinigaitis P., Canning B., DeVita R., et al. The antitussive effects of alpha7 (α7) nicotinic receptor agonists. Eur Respir J. 2017;50:OA4409. doi: 10.1183/1393003.congress-2017.OA4409. [DOI] [Google Scholar]
- 188.Canning B.J., Mori N., Lehmann A. Antitussive effects of the peripherally restricted GABAB receptor agonist lesogaberan in Guinea pigs: comparison to baclofen and other GABAB receptor-selective agonists. Cough. 2012;8(1):1. doi: 10.1186/1745-9974-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Burnstock G., Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1(1):e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang M., Wang S., Yu L., Xu X., Qiu Z. The role of ATP in cough hypersensitivity syndrome: new targets for treatment. J Thorac Dis. 2020;12(5):2781–2790. doi: 10.21037/jtd-20-cough-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Burnstock G., Brouns I., Adriaensen D., Timmermans J.P. Purinergic signaling in the airways. Pharmacol Rev. 2012;64(4):834–868. doi: 10.1124/pr.111.005389. [DOI] [PubMed] [Google Scholar]
- 192.Abdulqawi R., Dockry R., Holt K., et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 193.Smith J., Morice A., McGarvey L., et al. Pooled analysis of objective cough frequency in participants with chronic cough treated with gefapixant in two phase 3 clinical trials (cough-1 and cough-2) Thorax. 2021;76:99–100. [Google Scholar]
- 194.Fowles H.E., Rowland T., Wright C., Morice A. Tussive challenge with ATP and AMP: does it reveal cough hypersensitivity? Eur Respir J. 2017;49(2):1601452. doi: 10.1183/13993003.01452-2016. [DOI] [PubMed] [Google Scholar]
- 195.Morice A.H., Kitt M.M., Ford A.P., et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1) doi: 10.1183/13993003.00439-2019. [DOI] [PubMed] [Google Scholar]
- 196.Aparici M., Tarrasón G., Jover I., et al. Pharmacological profile of a novel, potent and oral TRPA1 antagonist. Characterization in a preclinical model of induced cough. Eur Respir J. 2015;46:PA3948. doi: 10.1183/13993003.congress-2015.PA3948. [DOI] [Google Scholar]
- 197.Millqvist E. TRPV1 and TRPM8 in treatment of chronic cough. Pharmaceuticals. 2016;9(3):1–10. doi: 10.3390/ph9030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Smith J.A., McGarvey L.P.A., Badri H., et al. Effects of a novel sodium channel blocker, GSK2339345, in patients with refractory chronic cough. Int J Clin Pharmacol Therapeut. 2017;55:712–719. doi: 10.5414/CP202804. [DOI] [PubMed] [Google Scholar]
- 199.Slinger C., Mehdi S.B., Milan S.J., et al. Speech and language therapy for management of chronic cough. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD013067.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gibson P.G., Vertigan A.E. Speech pathology for chronic cough: a new approach. Pulm Pharmacol Therapeut. 2009;22(2):159–162. doi: 10.1016/j.pupt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 201.Vertigan A.E., Theodoros D.G., Gibson P.G., Winkworth A.L. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006;61(12):1065–1069. doi: 10.1136/thx.2006.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vertigan A., Theodoros D., Gibson P., Winkworth A. Review series: chronic cough - behaviour modification therapies for chronic cough. Chron Respir Dis. 2007;4:89–97. doi: 10.1177/1479972307084252. [DOI] [PubMed] [Google Scholar]
- 203.Vertigan A.E., Haines J., Slovarp L. An update on speech pathology management of chronic refractory cough. J Allergy Clin Immunol Pract. 2019;7(6):1756–1761. doi: 10.1016/j.jaip.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 204.Ramanuja S., Kelkar P. Habit cough. Ann Allergy Asthma Immunol. 2009;102(2):91–95. doi: 10.1016/S1081-1206(10)60235-9. [DOI] [PubMed] [Google Scholar]
- 205.Ryan N.M., Vertigan A.E., Bone S., Gibson P.G. Cough reflex sensitivity improves with speech language pathology management of refractory chronic cough. Cough. 2010;6(5):1–8. doi: 10.1186/1745-9974-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ryan N.M., Vertigan A.E., Gibson P.G. Chronic cough and laryngeal dysfunction improve with specific treatment of cough and paradoxical vocal fold movement. Cough. 2009;5(4):1–8. doi: 10.1186/1745-9974-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chamberlain S., Birring S.S., Garrod R. Nonpharmacological interventions for refractory chronic cough patients: systematic review. Lung. 2014;192(1):75–85. doi: 10.1007/s00408-013-9508-y. [DOI] [PubMed] [Google Scholar]
- 208.Gibson P.G. Management of cough. J Allergy Clin Immunol Pract. 2019;7(6):1724–1729. doi: 10.1016/j.jaip.2019.03.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.