Abstract
Background
Gas exchange in extremely preterm (EP) infants must take place in fetal lungs. Childhood lung diffusing capacity of the lung for carbon monoxide (DLCO) is reduced; however, longitudinal development has not been investigated. We describe the growth of DLCO and its subcomponents to adulthood in EP compared with term-born subjects.
Methods
Two area-based cohorts born at gestational age ≤28 weeks or birthweight ≤1000 g in 1982–1985 (n=48) and 1991–1992 (n=35) were examined twice, at ages 18 and 25 years and 10 and 18 years, respectively, and compared with matched term-born controls. Single-breath DLCO was measured at two oxygen pressures, with subcomponents (membrane diffusion (DM) and pulmonary capillary blood volume (VC)) calculated using the Roughton–Forster equation.
Results
Age-, sex- and height-standardised transfer coefficients for carbon monoxide (KCO) and DLCO were reduced in EP compared with term-born subjects, and remained so during puberty and early adulthood (p-values for all time-points and both cohorts ≤0.04), whereas alveolar volume (VA) was similar. Development occurred in parallel to term-born controls, with no signs of pubertal catch-up growth nor decline at age 25 years (p-values for lack of parallelism within cohorts 0.99, 0.65, 0.71, 0.94 and 0.44 for z-DLCO, z-VA, z-KCO, DM and VC, respectively). Split by membrane and blood volume components, findings were less clear; however, membrane diffusion seemed most affected.
Conclusions
Pulmonary diffusing capacity was reduced in EP compared with term-born subjects, and development from childhood to adulthood tracked in parallel to term-born subjects, with no signs of catch-up growth nor decline at age 25 years.
Short abstract
Pulmonary diffusing capacity following extremely preterm (EP) birth was reduced compared with term-born subjects. From mid-childhood to adulthood, development tracked in parallel in the EP and term-born groups, with preterms following lower trajectories. https://bit.ly/3ARPD7D
Introduction
Extremely preterm (EP) infants (born before 28 weeks of pregnancy) currently account for one in 200 live births in high-income countries [1], with survival approaching 90% for infants born at 27 weeks gestation [2]. EP birth requires that fetal lungs develop in an extra-uterine environment while providing gas exchange for the newborn individual. The lungs at this stage have no proper gas exchanging units, as alveolarisation has hardly commenced [3, 4]. Lifesaving intensive care is required and relies on measures that are harmful to developing lungs, such as positive pressure ventilation and hyperoxia. The pulmonary complication of this scenario is labelled bronchopulmonary dysplasia (BPD) [5]. The few autopsy studies that have been published from infants who have died with BPD reveal “acinar dysplasia”, characterised by fewer and larger alveoli, and thickened alveolar–capillary membranes [6, 7]. We do not know how these structural injuries evolve later in life, but recent magnetic resonance imaging (MRI) studies suggest continued alveolar development until adolescence [8].
The standard functional measure of alveolar gas exchange is diffusing capacity of the lung for carbon monoxide (DLCO) [9]. DLCO is a compound measure reflecting lung volumes, surface area accessible for gas exchange, thickness of the alveolar–capillary barrier and pulmonary capillary blood volume. By using two different oxygen pressures during measurements, DLCO can be split into two components: transfer across the alveolar–capillary membrane (DM) and the rate of reaction with haemoglobin, reflecting the pulmonary capillary blood volume (VC) [10].
Studies report reduced DLCO in EP children and adolescents, suggesting persistent deficits of acinar function [11–15], although with surprisingly little influence from BPD [13, 15, 16]. Airway versus blood vessel interactions during lung development are poorly understood, and the relative impact from DM and VC for DLCO is therefore of interest [14, 17–19]. We aimed to test the hypothesis that impaired DLCO in EP subjects persists over time, without age-related catch-up or decline when compared with term-born controls. For this purpose, we measured DLCO with its subcomponents twice in two EP cohorts with matched term-born controls and constructed longitudinal trajectories from 10 to 18 years and 18 to 25 years of age.
Methods
Study subjects and study design
Two area-based cohorts of subjects born at gestational age ≤28 weeks or with birthweight ≤1000 g in 1982–1985 (n=48) and 1991–1992 (n=35) were included. Subjects were examined in 2001–2002 and 2008–2009 at Haukeland University Hospital (Bergen, Norway). The temporally nearest term-born same-sex subject with birthweight 3–4 kg (approximately Norwegian 10–90th percentiles) was invited as a control. If that subject declined, the next term-born was approached and so on. There were no exclusion criteria except inability to perform lung function tests. Clinical data were accessed from patients’ hospital charts. The cohorts are described in detail elsewhere [20], and their neonatal and background data are summarised in tables 1 and 2. Mild and moderate/severe BPD were defined as a requirement for supplemental oxygen ≥28 postnatal days or at postmenstrual age ≥36 weeks, respectively [21]. No subjects were examined within 2 weeks of a respiratory tract infection or an asthma exacerbation. Participants were asked to discontinue inhaled long-acting β2-agonists and corticosteroids as well as oral leukotriene blockers 24 h before testing, to avoid inhaled short-acting β2-agonists unless needed, and to refrain from smoking on the test day. Data on self-reported smoking have been verified in the 1982–1985 cohort by measuring urinary cotinine, with three positive tests in 57 self-declared nonsmokers [22]. The Regional Ethics Committee approved the study (REK-Vest 240.07). Informed written consent was obtained from participating subjects and/or parents.
TABLE 1.
Neonatal characteristics of extremely preterm (EP) subjects (n=83)
| 1991–1992 cohort | 1982–1985 cohort | |||
| Mean (range) or n (%) | sd | Mean (range) or n (%) | sd | |
| Birthweight, g | ||||
| All EP | 933 (570–1400) | 204 | 1012 (580–1480) | 189 |
| No/mild BPD | 976 (620–1400) | 195 | 1056 (580–1480) | 191 |
| Moderate/severe BPD | 851 (570–1200) | 203 | 892 (670–1080) | 122 |
| Gestational age, weeks | ||||
| All EP | 27 (23–31) | 2 | 27 (23–32) | 1 |
| No/mild BPD | 27 (24–31) | 2 | 27 (23–32) | 2 |
| Moderate/severe BPD | 26 (23–28) | 1 | 27 (26–30) | 1 |
| Postnatal time with oxygen, days | ||||
| All EP | 57 (2–180) | 48 | 48 (1–257) | 39 |
| No/mild BPD | 31 (2–70) | 23 | 33 (1–71) | 18 |
| Moderate/severe BPD | 108 (61–180) | 43 | 85 (44–257) | 54 |
| Time on ventilator, days | ||||
| All EP | 8 (0–55) | 12 | 11 (0–54) | 12 |
| No/mild BPD | 4 (0–40) | 9 | 7 (0–35) | 8 |
| Moderate/severe BPD | 16 (2–55) | 13 | 21 (1–54) | 16 |
| Antenatal steroids | ||||
| All EP | 16 (46) | 16 (33) | ||
| No/mild BPD | 11 (48) | 10 (29) | ||
| Moderate/severe BPD | 5 (42) | 6 (46) | ||
| Surfactant | ||||
| All EP | 17 (49) | 0 (0) | ||
| No/mild BPD | 7 (30) | 0 (0) | ||
| Moderate/severe BPD | 10 (83) | 0 (0) | ||
| Postnatal steroids | ||||
| All EP | 10 (29) | 4 (8) | ||
| No/mild BPD | 2 (9) | 1 (3) | ||
| Moderate/severe BPD | 8 (67) | 3 (23) | ||
| Maternal smoking in pregnancy | ||||
| All EP | 13 (37) | 22 (48) | ||
| No/mild BPD | 10 (43) | 17 (50) | ||
| Moderate/severe BPD | 3 (27) | 5 (42) | ||
BPD: bronchopulmonary dysplasia (no/mild BPD: no need for oxygen supplementation at 36 weeks postmenstrual age; moderate/severe BPD: oxygen supplement at 36 weeks postmenstrual age). The number of subjects differed slightly between variables. In the 1991–1992 cohort: all EP n=34–35 subjects, no/mild BPD n=23 subjects and moderate/severe BPD n=11–12 subjects. In the 1982–1985 cohort: all EP n=46–48 subjects, no/mild BPD n=34–35 subjects and moderate/severe BPD n=12–13 subjects.
TABLE 2.
Background variables for extremely preterm (EP) subjects and term-born controls
| 1991–1992 cohort | 1982–1985 cohort | |||
| First follow-up | Second follow-up | First follow-up | Second follow-up | |
| Age, years (mean±sd) | 10.6±0.4 | 17.8±0.4 | 17.7±1.2 | 24.9±1.2 |
| Subjects, n (% females) | ||||
| Term-born | 35 (63) | 28 (71) | 46 (46) | 40 (4) |
| EP | 35 (63) | 31 (58) | 46 (46) | 45 (42) |
| Height, cm | ||||
| Term-born | ||||
| Female | 144 (141–147) | 166 (163–168) | 168 (165.4–171.0) | 168 (165–171) |
| Male | 145 (142–150) | 178 (171–185) | 177 (174–179) | 177 (175–180) |
| EP | ||||
| Female | 141 (137–145) | 162 (159–166) | 163 (162–166) | 163 (162–165) |
| Male | 139 (135–143) | 174 (171–178) | 175 (172–177) | 176 (173–178) |
| Weight, kg | ||||
| Term-born | ||||
| Female | 39 (36–41) | 64 (59–69) | 67 (60–73) | 69 (61–77) |
| Male | 38 (34–42) | 75 (65–85) | 68 (65–71) | 76 (71–81) |
| EP | ||||
| Female | 35 (30–41) | 62 (51–72) | 61 (53–68) | 67 (57–76) |
| Male | 35 (27–42) | 73 (62–83) | 66 (59–72) | 80 (74–87) |
| Self-reported smoking | ||||
| Term-born | 0 (0) | 5 (18) | 14 (30) | 8 (21) |
| EP | 0 (0) | 1 (3) | 15 (33) | 17 (38) |
| Maternal smoking in pregnancy | ||||
| Term-born | 9 (26) | 10 (22) | ||
| EP | 13 (37) | 22 (48) | ||
| z-FEV1 | ||||
| Term-born | −0.09 (−0.4–0.2) | −0.06 (−0.5–0.3) | 0.3 (−0.4–0.6) | 0.05 (−0.3–0.4) |
| EP | −0.9 (−1.2– −0.6) | −0.8 (−1.1– −0.5) | −1.05 (−1.6– −0.5) | −1.0 (−1.5– −0.5) |
| z-FVC | ||||
| Term-born | −0.1 (−0.4–0.2) | −0.08 (−0.4–0.2) | −0.05 (−0.4–0.3) | 0.09 (−0.3–0.4) |
| EP | −0.6 (−0.9– −0.3) | −0.3 (−0.6–0.03) | −0.9 (−1.5– −0.4) | −0.5 (−1.0–0.04) |
| z-FEF25–75% | ||||
| Term-born | −0.2 (−0.6–0.2) | −0.2 (−0.6–0.3) | 0.5 (0.1–0.8) | −0.004 (−0.3–0.3) |
| EP | −1.1 (−1.5– −0.7) | −0.9 (−1.3– −0.5) | 0.8 (−1.2– −0.5) | −1.2 (−1.5– −0.8) |
Data are presented as group means (95% CI) or n (%), unless otherwise stated. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75%: forced expiratory flow at 25–75% of FVC.
Pulmonary function tests
The same experienced respiratory physiologist (O.D.R.) performed all tests on pulmonary function on both occasions, blinded to the results obtained in previous test sessions. Single-breath DLCO was measured with a Vmax 22 (SensorMedics, Yorba Linda, CA, USA) in the sitting position wearing a nose clip, in accordance with European Respiratory Society (ERS) guidelines [23].
Single-breath method
The test gas contained a mixture of 0.3% carbon monoxide, 0.3% methane and 21% oxygen (80% oxygen in the hyperoxic test gas), balanced with nitrogen. A mid-expiratory sample of alveolar gas was collected and analysed. Alveolar volume (VA) and transfer coefficient of the lung for carbon monoxide (KCO) were recorded and DLCO calculated. DM and VC were measured with a hyperoxic test gas (80% oxygen) and calculated according to the Roughton–Forster equation [10]. Test criteria were applied as recommended by the ERS Task Force [23]. Details regarding the single-breath DLCO measurements have previously been described [11]. z-scores for VA, KCO and DLCO were calculated using the Global Lung Function Initiative 2017 regression equations (updated version, October 2020) for the carbon monoxide transfer factor for Caucasians [24].
Statistical methods
Results are reported as counts with proportions and means with 95% confidence intervals or ranges, as appropriate. The number of patients each analysis is based on is reported separately due to some missing data, particularly at the second follow-up (figure 1).
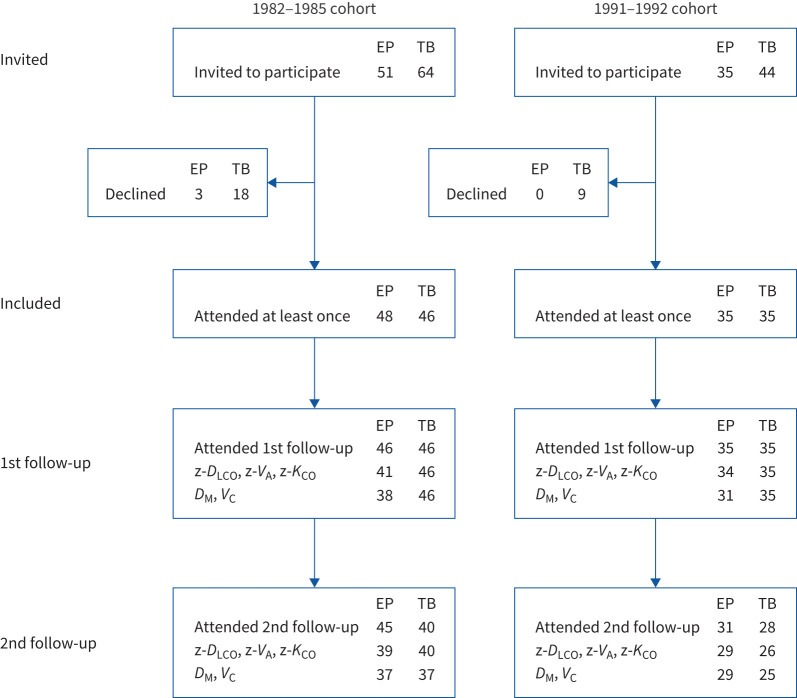
FIGURE 1.
Recruitment process of the study (n=164). Recruitment of the extremely preterm (EP) cohorts and their term-born (TB) age- and sex-matched control subjects. Two subjects in the 1982–1985 cohort participated in the second follow-up in 2008–2009 but not in the first follow-up in 2001–2002. DLCO: diffusing capacity of the lung for carbon monoxide; VA: alveolar volume; KCO: transfer coefficient of the lung for carbon monoxide; DM: alveolar–capillary membrane conductance; VC: pulmonary capillary blood volume.
To estimate mean values and differences in mean values for the clinical variables z-DLCO, DLCO % pred, z-VA, z-KCO, KCO % pred, DM and VC for the two groups at each time-point, we fitted linear mixed effects longitudinal models. The explanatory variables were cohort, age (categorical) and EP versus term-born (or grade of BPD severity in supplementary table B). To make the models maximally flexible, we included all interactions. Subjects were included as a random effect (as expected, there was no “EP–term-born pair” effect, so this was not included as a random effect). These models take the correlations between measurements at various follow-up times from the same subject into account, which makes it possible to also include subjects with incomplete follow-up data. This was done to reduce any bias caused by missing data and to increase the precision of the estimates [25]. Residual plots were examined and any errors in the original data corrected. To examine if development for EP subjects tracked development for term-born subjects, we fitted simplified models with parallel lines for the two groups (but possibly different slopes for the two cohorts) and compared these with the fully flexible models using likelihood ratio tests. To examine the effects of smoking, and if smoking impacted EP and term-born differently, we added smoking and the interaction EP versus term-born to the z-DLCO model.
Associations between perinatal exposures and outcome were tested in a linear regression model with z-DLCO at 18 years of age (in both cohorts) as response variable and the following as explanatory variables: maternal smoking, gestational age, antenatal steroids, surfactant, days on mechanical ventilation (with values >30 days set to 30 days), days on oxygen supplementation (with values >100 days set to 100 days) and cohort.
For the background variables (tables 1 and 2), differences between groups were assessed with Welch's t-test for continuous variables and Pearson's Chi-squared test for categorical variables.
The original project was designed in 2001 to address a series of outcomes and the sample size was calculated to detect a clinically relevant decrease in the EP groups for the main outcome measure for the overall study, which was forced expiratory volume in 1 s (FEV1) [22].
The data were analysed with SPSS version 25 (IBM, Armonk, NY, USA) and R version 4.0.2 [26]. The mixed effects models were fitted with the R package “lme4” version 1.1-23 [27]. p-values ≤0.05 are characterised as statistically significant.
Results
Subjects
A total of 130 preterms were admitted to the neonatal intensive care unit (NICU) in the two inclusion periods. Neonatal mortality was 39% and 27% in 1982–1985 and 1991–1992, respectively. Altogether in both EP cohorts, 86 subjects survived, 81 attended the first follow-up, 74 attended both follow-ups and 83 attended at least one follow-up.
Subject demographics are summarised in table 1. Mean gestational age was similar in both cohorts. The younger cohort had fewer days on a ventilator, and higher use of antenatal and postnatal steroids. No subjects in the 1982–1985 cohort received surfactant, contrasting with almost half of the EP subjects in the 1991–1992 cohort (table 1).
There were no differences between the EP and term-born subjects regarding weight (table 2). Regarding height, EP females in the 1982–1985 cohort were significantly shorter at both examinations (both p=0.006), as were EP males in the 1991–1992 cohort at the first (p=0.01) but not the second examination (p=0.29).
Most participants were able to perform DLCO measurements (figure 1). Success rates at first follow-up (ages 10 and 18 years) were 97% and 89% for EP subjects and 100% for term-born subjects (both cohorts). Corresponding numbers at second follow-up (ages 18 and 25 years) were 94% and 87% for EP subjects and 93% and 100% for term-born subjects. Some of those who struggled with performing satisfactory measurements at 21% oxygen did not perform measurements at 80% oxygen, and thus DM and VC measurements were obtained for fewer subjects (figure 1).
DLCO, VA and KCO
Raw data (for DLCO and KCO) are presented in supplementary table A, whereas z-scores are used in figure 2 and table 3. Table 3 also includes percentage predicted values. z-DLCO and z-KCO were lower in EP compared with term-born subjects in both cohorts and at both examinations, whereas z-VA was similar. Data for EP subjects split by neonatal BPD is presented in supplementary table B. Within the EP cohorts, BPD did not influence z-DLCO, z-VA and z-KCO (p-values of 0.14, 0.45 and 0.15, respectively).
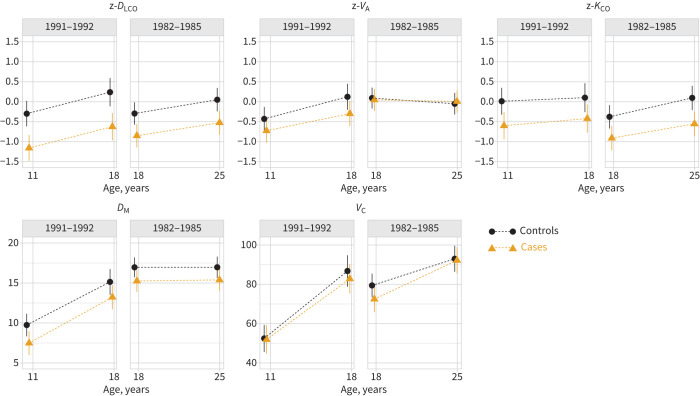
FIGURE 2.
Mean lung diffusing capacity from approximately 10 to 25 years of age for extremely preterm subjects compared with term-born controls (n=160#). Data are presented as estimated group means (95% CI) from longitudinal mixed effects models. The points/lines for the two groups have been slightly adjusted horizontally to avoid overlapping. The values for diffusing capacity of the lung for carbon monoxide (DLCO), alveolar volume (VA) and transfer coefficient of the lung for carbon monoxide (KCO) are reported as z-scores, while values for alveolar–capillary membrane conductance (DM; mmol·min−1·kPa−1) and pulmonary capillary blood volume (VC; mL) are absolute numbers. #: number of subjects included in at least one regression model (numbers of cases and controls for each variable and at each time-point are shown in figure 1).
TABLE 3.
Lung diffusing capacity data from 10 to 25 years of age for extremely preterm (EP) subjects compared with term-born controls (n=160#)
| 1991–1992 cohort | 1982–1985 cohort | |||||||
| First follow-up | p-value | Second follow-up | p-value | First follow-up | p-value | Second follow-up | p-value | |
| Age, years (mean±sd) | 10.6±0.4 | 17.8±0.4 | 17.7±1.2 | 24.9±1.2 | ||||
| z-DLCO | ||||||||
| Term-born | −0.3 (−0.6–0.0) | 0.2 (−0.1–0.6) | −0.3 (−0.6– −0.0) | 0.0 (−0.2–0.3) | ||||
| EP | −1.2 (−1.5– −0.8) | −0.6 (−1.0– −0.3) | −0.8 (−1.1– −0.6) | −0.5 (−0.8– −0.2) | ||||
| Difference | 0.9 (0.4–1.3) | <0.001 | 0.9 (0.4–1.4) | <0.001 | 0.6 (0.2–1.0) | 0.007 | 0.6 (0.2–1.0) | 0.007 |
| DLCO, % pred | ||||||||
| Term-born | 95.7 (91.3–100.1) | 104.0 (99.1–108.9) | 96.6 (92.8–100.4) | 101.3 (97.3–105.3) | ||||
| EP | 83.1 (78.6–87.5) | 92.1 (87.5–96.8) | 89.1 (85.1–93.2) | 93.7 (89.6–97.8) | ||||
| Difference | 12.6 (6.4–18.9) | <0.001 | 11.9 (5.1–18.6) | <0.001 | 7.4 (1.9–13) | 0.009 | 7.6 (1.9–13.4) | 0.009 |
| z-VA | ||||||||
| Term-born | −0.4 (−0.7– −0.1) | 0.1 (−0.2–0.4) | 0.1 (−0.2–0.4) | −0.1 (−0.3–0.2) | ||||
| EP | −0.7 (−1.0– −0.4) | −0.3 (−0.6–0.0) | 0.0 (−0.2–0.3) | 0.0 (−0.3–0.3) | ||||
| Difference | 0.3 (−0.1–0.7) | 0.17 | 0.4 (−0.0–0.9) | 0.07 | 0.0 (−0.3–0.4) | 0.82 | −0.1 (−0.4–0.3) | 0.77 |
| z-KCO | ||||||||
| Term-born | 0.0 (−0.3–0.3) | 0.1 (−0.3–0.5) | −0.4 (−0.7– −0.1) | 0.1 (−0.2–0.4) | ||||
| EP | −0.6 (−0.9– −0.3) | −0.4 (−0.8– −0.1) | −0.9 (−1.2– −0.6) | −0.6 (−0.9– −0.2) | ||||
| Difference | 0.6 (0.1–1.1) | 0.01 | 0.5 (0.0–1.0) | 0.04 | 0.5 (0.1–1.0) | 0.01 | 0.6 (0.2–1.1) | 0.003 |
| KCO, % pred | ||||||||
| Term-born | 100.5 (95.9–105.1) | 101.5 (96.5–106.4) | 95.5 (91.5–99.5) | 101.9 (97.8–106.1) | ||||
| EP | 90.5 (85.9–95.1) | 94.9 (90.1–99.7) | 88.6 (84.4–92.7) | 93.7 (89.5–97.9) | ||||
| Difference | 10.0 (3.5–16.4) | 0.003 | 6.6 (−0.3–13.5) | 0.06 | 6.9 (1.2–12.7) | 0.02 | 8.2 (2.3–14.1) | 0.006 |
| D M | ||||||||
| Term-born | 9.7 (8.3–11.2) | 15.1 (13.5–16.7) | 17.0 (15.7–18.2) | 17.0 (15.6–18.3) | ||||
| EP | 7.5 (6.0–9.0) | 13.2 (11.7–14.7) | 15.3 (13.9–16.6) | 15.4 (14.0–16.7) | ||||
| Difference | 2.3 (0.2–4.3) | 0.03 | 1.9 (−0.3–4.1) | 0.09 | 1.7 (−0.1–3.5) | 0.07 | 1.6 (−0.3–3.5) | 0.10 |
| V C | ||||||||
| Term-born | 52.5 (45.6–59.4) | 86.8 (78.8–94.8) | 79.4 (73.4–85.4) | 93.0 (86.4–99.6) | ||||
| EP | 51.9 (44.7–59.2) | 82.8 (75.3–90.3) | 72.5 (65.9–79.0) | 92.2 (85.5–98.8) | ||||
| Difference | 0.5 (−9.4–10.5) | 0.91 | 4.0 (−7.0–14.9) | 0.47 | 7.0 (−1.9–15.8) | 0.12 | 0.8 (−8.6–10.2) | 0.87 |
Data are presented as group means (95% CI) from longitudinal mixed effects models, unless otherwise stated. DLCO: diffusing capacity of the lung for carbon monoxide; VA: alveolar volume; KCO: transfer coefficient of the lung for carbon monoxide; DM: alveolar–capillary membrane conductance; VC: pulmonary capillary blood volume. The values for DLCO, VA and KCO are reported as z-scores and percentage predicted values (DLCO and KCO), while values for DM (mmol·min−1·kPa−1) and VC (mL) are absolute numbers. #: number of subjects included in at least one regression model (numbers of cases and controls for each variable and at each time-point are shown in figure 1).
Smokers had on average 0.6 lower z-DLCO values (95% CI 0.2–1.0; p=0.002). The effect did not differ between the EP and term-born subjects (p=0.31 for the interaction).
There were no associations between the addressed perinatal variables or cohort versus z-DLCO at 18 years of age, with p-values of 0.28, 0.12, 0.21, 0.93, 0.90, 0.66 and 0.74 for maternal smoking, gestational age, antenatal steroids, surfactant, days on mechanical ventilation, days on oxygen supplementation and cohort, respectively.
DM and VC
DM was numerically lower in the EP compared with term-born cohorts, statistically significantly so only in the 1991–92 cohort at 10 years of age. VC did not differ between the EP and term-born cohorts at any of the measurements.
Development over time
For both EP cohorts, z-DLCO, z-KCO, z-VA, DM and VC developed in parallel to their respective term-born control cohorts over the age span covered by the study, i.e. from 18 to 25 years of age in the 1982–1985 cohort and from 10 to 18 years of age in the 1991–1992 cohort.
The p-values for overall tests for a lack of parallelism between the EP and term-born cohorts from each of the two decades were 0.99, 0.65, 0.71, 0.94 and 0.44 for z-DLCO, z-VA, z-KCO, DM and VC, respectively. This indicates that development between the two examinations did not differ between the EP and term-born groups for any of the measured variables.
Discussion
This is the first controlled population-based study describing longitudinal development of lung diffusing capacity after EP birth from mid-childhood to adulthood. We found that DLCO and KCO were persistently reduced in EP subjects, and that development tracked below but in parallel to term-born subjects over the study period, with no signs of pubertal catch-up growth nor any signs of decline at 25 years age. Split by membrane and blood volume components, findings were less clear; however, the membrane diffusion component seemed most affected.
Gas exchange takes place in the acini, where air and blood are in proximity, with an ultrathin alveolar–capillary membrane separating the compartments. The diffusing capacity of the lungs is structurally limited by the magnitude of the alveolar surface area, the thickness of the blood–gas barrier and the pulmonary capillary blood volume. Formation of the alveoli is the final stage of lung development and much of this process takes place after birth also in term-born individuals [28]. Nevertheless, postnatal development builds on premises established during the last trimester, which is a period EP subjects spend in the NICU. New alveoli form by alveolar ducts dividing into alveolar sacs by septation and the pulmonary capillary bed expands in parallel via angiogenesis, gradually increasing the area available for gas exchange [17]. This is a continuous process that commences in the last trimester and continues for years after birth. EP birth, with accompanying dramatic events and lifesaving respiratory interventions, radically changes the premises under which this developmental programme must take place. Autopsy studies of children who died from BPD have shown impaired acinar development [6, 7, 29], but we have little knowledge of structural features in survivors and future prospects for growth or repair after the neonatal period are unknown. Judged by aerosol-derived airway morphometry studies, the size of a child's alveolus expands into adulthood, accounting for increased lung volume with age and height [30]. On the other hand, studies applying stereological approaches indicate that the alveolar number closely relates to total lung volume, with a constant alveolar size over a range of volumes, suggesting that the number of alveoli must increase during growth [31]. Thus, the surface area available for gas exchange is much larger in an adult compared with a child. This relatively simple line of reasoning was recently confirmed by MRI studies showing continued alveolarisation to adolescence and catch-up growth in EP children [8]. These studies provide optimism that repair mechanisms might come into play as preterm-born children grow and mature. However, judged by the development of the pulmonary capacity for carbon monoxide transfer of the two EP cohorts in our study, a corresponding functional catch-up is difficult to detect; the EP cohorts had consistently reduced gas exchange capacity that tracked from 10 to 25 years of age.
At quiet breathing, both volume and effective surface area of the capillary bed change with changes of the blood flow that reflect the stroke volume [32]. The blood stays very briefly in the pulmonary capillaries, but still, venous blood entering the lung capillaries equilibrates completely with alveolar air in a highly efficient process requiring ∼0.3 s. Healthy individuals have large ventilatory reserves and deficits in gas diffusing capacity are therefore well tolerated at rest. During exercise, the transit time through the lung capillaries is shortened, challenging gas exchange capacity in patients with low DLCO, with 50% predicted suggested as a threshold before symptoms occur [33–35]. In EP subjects, deficits in DLCO raw data are generally ∼10% [13, 16], which was also found in the present study (supplementary table A). We have previously shown that compared with controls, these same EP subjects have close-to-normal peak exercise capacity [36], findings replicated also by others [13, 37, 38]. Taken together, close-to-normal gas diffusing and exercise capacities challenge the notion that EP birth leads to severe persistent acinar impairment, since one would expect more austere physiological findings if this was the case. Airway obstruction can lead to a higher DLCO [39] and we know that EP subjects (including our cohorts) have persistent airway obstruction, particularly those who had neonatal BPD [40, 41]. Thus, bronchial obstruction might mask or counteract deficits of DLCO and therefore explain a surprising finding in our dataset, i.e. that EP subjects with BPD tended to have a higher z-DLCO, although not significant (supplementary table B).
Impaired alveolar development could theoretically hamper lung diffusing capacity through reduced area and/or a thickening or impairment of the alveolar–capillary membrane, or by impaired vascular components. We found that the membrane component of DLCO was numerically reduced at both measurement time-points in both EP cohorts, although significantly so only at the first examination in the youngest cohort. Data for VC did not exhibit corresponding deficits and increased over time in both cohorts, also from 18 to 25 years of age, indicating similar growth and development in the EP and term-born groups, presumably in parallel to the increases in body size. Reduced DLCO in the EP groups must reflect comparable reductions in its subcomponents DM and/or VC. Given the data of our study, it is enticing to conclude that reduced DLCO after preterm birth is more related to impairments of membrane diffusion than the vascular components of acinar development. Future studies preferably including more participants may disentangle the underlying mechanisms of impaired DLCO following EP birth.
Disruption of alveolar growth associated with EP birth may be linked to early-onset chronic obstructive pulmonary disease (COPD) in adult life [42]. In clinical practice, DLCO is used to assess severity and prognosis of COPD, as spirometry alone poorly reflects the disability in these patients. Reduced DLCO is a prognostic marker independent of forced spirometry in COPD patients [43] and is associated with increased morbidity across multiple domains [43]. Moreover, lung diffusing capacity has been shown to be a significant predictor of the all-cause mortality rate within a general population, independent of standard spirometry measures and even in the absence of apparent clinical respiratory disease [44]. There is ample data to argue that airway obstruction tracks at a reduced level from EP birth via early childhood to adulthood and that few of these individuals reach their expected peak FEV1 [41, 45]. Our study indicates a similar tracking also for DLCO, a scenario suggesting that DLCO should be included in follow-up programmes after EP birth.
Strengths and limitations
The major strengths of the study were the population-based, longitudinal and controlled design, the long follow-up period, and the high rate of attendance at both follow-up assessments of both EP and term-born participants. A strict algorithm for recruitment of control subjects minimised the risk of selection bias in this group. Development during the age span covered by the study was described by examining two birth cohorts that overlapped in age but were born during two different NICU eras. This was not an ideal approach to address longitudinal development over the full recruitment period, but we consider it adequate to compare the trajectories for the EP and term-born groups. We cannot comment on a potential for early-onset age-related decline of DLCO, as studies of the general population have shown that the decline starts to accelerate later than by the age of 25 years [46].
Preterm infants were exposed to very different treatment algorithms and techniques in the 1980s and the 1990s, reflected in this study by, for example, a higher rate of subjects treated with antenatal steroids and surfactant, and also by a higher rate of survival in the younger cohort. Caution is therefore warranted for direct comparisons between the cohorts. However, this line of thinking can be turned the other way around, i.e. one may argue that similar findings in two birth cohorts born 9 years apart and treated so very differently strengthen the notion that parameters of lung diffusion are in fact tracking from childhood to adulthood. The continuous development of NICU treatment during the last decades challenges the generalisability of these findings to today's NICU dwellers, as their outcomes may differ. Numerically, our data from all the various measurement time-points were in line with most previous reports on lung diffusing capacity [18, 47]. As observed also by others, we found no clear associations in our dataset between neonatal BPD and subsequent lung diffusing capacity [13, 15, 16]. Recent studies of other indices of lung function in groups and cohorts born EP in the modern era of neonatology also suggest weaker associations with BPD [48]. Thus, we should perhaps contemplate revising the use of this neonatal diagnosis to predict and label subsequent lung function in EP adults.
Asthma therapy was stopped ∼24 h prior to testing. This time frame did not allow full washout of inhaled corticosteroids. DLCO is linked to the ventilation/perfusion ratio, which may be affected by bronchial obstruction. Increased airway conductance and accessible alveolar volume caused by sustained effects from asthma therapy could possibly influence the findings in the few participants with asthma. The available data in this area is scarce [49] and the 2017 ERS/American Thoracic Society standards for single-breath carbon monoxide uptake in the lung do not provide specific advice regarding discontinuation of asthma medications [9].
Inclusion to this study was based on both gestational age and birthweight criteria, preventing generalisation of the results to all EP cohorts, as some dysmature infants were included based on the birthweight criteria alone. Potential relationships between perinatal characteristics and subsequent measures of pulmonary gas transfer should be addressed in future, larger studies.
Conclusions
EP subjects had impaired lung diffusing capacity, with membrane diffusion seemingly more implicated than the capillary blood volume component. The deficits tracked from mid-childhood to adulthood, below but in parallel to matched term-born control cohorts. Preterm birth represents a significant perturbation to lung development in the short term but also long term. A lifelong obligation for proper follow-up, treatment and guidance falls upon the health profession that once made survival of these young individuals possible.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table A ERJ-04103-2020.Table_A (440.2KB, pdf)
Supplementary table B ERJ-04103-2020.Table_B (239.1KB, pdf)
Shareable PDF
Footnotes
This article has supplementary material available from erj.ersjournals.com
Conflict of interest: E. Satrell has nothing to disclose.
Conflict of interest: H. Clemm has nothing to disclose.
Conflict of interest: O.D. Røksund has nothing to disclose.
Conflict of interest: K.O. Hufthammer has nothing to disclose.
Conflict of interest: E. Thorsen has nothing to disclose.
Conflict of interest: T. Halvorsen has nothing to disclose.
Conflict of interest: M. Vollsæter has nothing to disclose.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. . National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379: 2162–2172. doi: 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 2.Stensvold HJ, Klingenberg C, Stoen R, et al. . Neonatal morbidity and 1-year survival of extremely preterm infants. Pediatrics 2017; 139: e2016182. doi: 10.1542/peds.2016-1821 [DOI] [PubMed] [Google Scholar]
- 3.Smith LJ, McKay KO, van Asperen PP, et al. . Normal development of the lung and premature birth. Paediatr Respir Rev 2010; 11: 135–142. doi: 10.1016/j.prrv.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 4.Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Curr Opin Pediatr 2014; 26: 306–314. doi: 10.1097/MOP.0000000000000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thebaud B, Goss KN, Laughon M, et al. . Bronchopulmonary dysplasia. Nat Rev Dis Primers 2019; 5: 78. doi: 10.1038/s41572-019-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thibeault DW, Mabry SM, Norberg M, et al. . Lung microvascular adaptation in infants with chronic lung disease. Biol Neonate 2004; 85: 273–282. doi: 10.1159/000076388 [DOI] [PubMed] [Google Scholar]
- 7.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998; 29: 710–717. doi: 10.1016/S0046-8177(98)90280-5 [DOI] [PubMed] [Google Scholar]
- 8.Narayanan M, Beardsmore CS, Owers-Bradley J, et al. . Catch-up alveolarization in ex-preterm children: evidence from 3He magnetic resonance. Am J Respir Crit Care Med 2013; 187: 1104–1109. doi: 10.1164/rccm.201210-1850OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham BL, Brusasco V, Burgos F, et al. . 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 10.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 1957; 11: 290–302. doi: 10.1152/jappl.1957.11.2.290 [DOI] [PubMed] [Google Scholar]
- 11.Satrell E, Roksund O, Thorsen E, et al. . Pulmonary gas transfer in children and adolescents born extremely preterm. Eur Respir J 2013; 42: 1536–1544. doi: 10.1183/09031936.00027112 [DOI] [PubMed] [Google Scholar]
- 12.Lum S, Kirkby J, Welsh L, et al. . Nature and severity of lung function abnormalities in extremely preterm children at 11 years of age. Eur Respir J 2011; 37: 1199–1207. doi: 10.1183/09031936.00071110 [DOI] [PubMed] [Google Scholar]
- 13.Vrijlandt EJ, Gerritsen J, Boezen HM, et al. . Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med 2006; 173: 890–896. doi: 10.1164/rccm.200507-1140OC [DOI] [PubMed] [Google Scholar]
- 14.Sorensen JK, Buchvald F, Berg AK, et al. . Ventilation inhomogeneity and NO and CO diffusing capacity in ex-premature school children. Respir Med 2018; 140: 94–100. doi: 10.1016/j.rmed.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Hakulinen AL, Jarvenpaa AL, Turpeinen M, et al. . Diffusing capacity of the lung in school-aged children born very preterm, with and without bronchopulmonary dysplasia. Pediatr Pulmonol 1996; 21: 353–360. doi: [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E, Bar-Yishay E, Prais D, et al. . Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest 2012; 142: 725–733. doi: 10.1378/chest.11-1562 [DOI] [PubMed] [Google Scholar]
- 17.Hislop AA. Airway and blood vessel interaction during lung development. J Anat 2002; 201: 325–334. doi: 10.1046/j.1469-7580.2002.00097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang DV, Assaf SJ, Tiller CJ, et al. . Membrane and capillary components of lung diffusion in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2016; 193: 767–771. doi: 10.1164/rccm.201506-1219OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond D, Hadchouel A, Le Bourgeois M, et al. . Decreased pulmonary capillary volume in adolescents born very preterm. Acta Paediatr 2020; 109: 621–622. doi: 10.1111/apa.15023 [DOI] [PubMed] [Google Scholar]
- 20.Halvorsen T, Skadberg BT, Eide GE, et al. . Assessment of lung volumes in children and adolescents: comparison of two plethysmographic techniques. Clin Physiol Funct Imaging 2005; 25: 62–68. doi: 10.1111/j.1475-097X.2004.00591.x [DOI] [PubMed] [Google Scholar]
- 21.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729. doi: 10.1164/ajrccm.163.7.2011060 [DOI] [PubMed] [Google Scholar]
- 22.Vollsaeter M, Clemm HH, Satrell E, et al. . Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Ann Am Thorac Soc 2015; 12: 313–322. doi: 10.1513/AnnalsATS.201406-285OC [DOI] [PubMed] [Google Scholar]
- 23.Macintyre N, Crapo RO, Viegi G, et al. . Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26: 720–735. doi: 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 24.Stanojevic S, Graham BL, Cooper BG, et al. . Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010. doi: 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 25.Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat 2001; 11: 9–21. doi: 10.1081/BIP-100104194 [DOI] [PubMed] [Google Scholar]
- 26.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2020. [Google Scholar]
- 27.Bates DMM, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 28.Burri PH. Structural aspects of postnatal lung development – alveolar formation and growth. Biol Neonate 2006; 89: 313–322. doi: 10.1159/000092868 [DOI] [PubMed] [Google Scholar]
- 29.Hislop AA, Wigglesworth JS, Desai R, et al. . The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev 1987; 15: 147–164. doi: 10.1016/0378-3782(87)90003-X [DOI] [PubMed] [Google Scholar]
- 30.Zeman KL, Bennett WD. Growth of the small airways and alveoli from childhood to the adult lung measured by aerosol-derived airway morphometry. J Appl Physiol 2006; 100: 965–971. doi: 10.1152/japplphysiol.00409.2005 [DOI] [PubMed] [Google Scholar]
- 31.Ochs M, Nyengaard JR, Jung A, et al. . The number of alveoli in the human lung. Am J Respir Crit Care Med 2004; 169: 120–124. doi: 10.1164/rccm.200308-1107OC [DOI] [PubMed] [Google Scholar]
- 32.Johnson RL Jr, Spicer WS, Bishop JM, et al. . Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol 1960; 15: 893–902. doi: 10.1152/jappl.1960.15.5.893 [DOI] [PubMed] [Google Scholar]
- 33.Sue DY, Oren A, Hansen JE, et al. . Diffusing capacity for carbon monoxide as a predictor of gas exchange during exercise. N Engl J Med 1987; 316: 1301–1306. doi: 10.1056/NEJM198705213162103 [DOI] [PubMed] [Google Scholar]
- 34.Hadeli KO, Siegel EM, Sherrill DL, et al. . Predictors of oxygen desaturation during submaximal exercise in 8,000 patients. Chest 2001; 120: 88–92. doi: 10.1378/chest.120.1.88 [DOI] [PubMed] [Google Scholar]
- 35.Bedell GN, Adams RW. Pulmonary diffusing capacity during rest and exercise. A study of normal persons and persons with atrial septal defect, pregnancy, and pulmonary disease. J Clin Invest 1962; 41: 1908–1914. doi: 10.1172/JCI104647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemm HH, Vollsaeter M, Roksund OD, et al. . Exercise capacity after extremely preterm birth: development from adolescence to adulthood. Ann Am Thorac Soc 2014; 11: 537–545. doi: 10.1513/AnnalsATS.201309-311O [DOI] [PubMed] [Google Scholar]
- 37.Kilbride HW, Gelatt MC, Sabath RJ. Pulmonary function and exercise capacity for ELBW survivors in preadolescence: effect of neonatal chronic lung disease. J Pediatr 2003; 143: 488–493. doi: 10.1067/S0022-3476(03)00413-X [DOI] [PubMed] [Google Scholar]
- 38.Burns YR, Danks M, O'Callaghan MJ, et al. . Motor coordination difficulties and physical fitness of extremely-low-birthweight children. Dev Med Child Neurol 2009; 51: 136–142. doi: 10.1111/j.1469-8749.2008.03118.x [DOI] [PubMed] [Google Scholar]
- 39.Collard P, Njinou B, Nejadnik B, et al. . Single breath diffusing capacity for carbon monoxide in stable asthma. Chest 1994; 105: 1426–1429. doi: 10.1378/chest.105.5.1426 [DOI] [PubMed] [Google Scholar]
- 40.Doyle LW, Andersson S, Bush A, et al. . Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med 2019; 7: 677–686. doi: 10.1016/S2213-2600(18)30530-7 [DOI] [PubMed] [Google Scholar]
- 41.Vollsaeter M, Roksund OD, Eide GE, et al. . Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013; 68: 767–776. [DOI] [PubMed] [Google Scholar]
- 42.McGrath-Morrow SA, Collaco JM. Bronchopulmonary dysplasia: what are its links to COPD? Ther Adv Respir Dis 2019; 13: 1753466619892492. doi: 10.1177/1753466619892492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balasubramanian A, MacIntyre NR, Henderson RJ, et al. . Diffusing capacity of carbon monoxide in assessment of COPD. Chest 2019; 156: 1111–1119. doi: 10.1016/j.chest.2019.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neas LM, Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol 1998; 147: 1011–1018. doi: 10.1093/oxfordjournals.aje.a009394 [DOI] [PubMed] [Google Scholar]
- 45.Moschino L, Stocchero M, Filippone M, et al. . Longitudinal assessment of lung function in survivors of bronchopulmonary dysplasia from birth to adulthood. The Padova BPD study. Am J Respir Crit Care Med 2018; 198: 134–137. doi: 10.1164/rccm.201712-2599LE [DOI] [PubMed] [Google Scholar]
- 46.Stam H, Hrachovina V, Stijnen T, et al. . Diffusing capacity dependent on lung volume and age in normal subjects. J Appl Physiol 1994; 76: 2356–2363. doi: 10.1152/jappl.1994.76.6.2356 [DOI] [PubMed] [Google Scholar]
- 47.Arigliani M, Valentini E, Stocco C, et al. . Regional ventilation inhomogeneity in survivors of extremely preterm birth. Pediatr Pulmonol 2020; 55: 1366–1374. doi: 10.1002/ppul.24742 [DOI] [PubMed] [Google Scholar]
- 48.Hjalmarson O, Brynjarsson H, Nilsson S, et al. . Persisting hypoxaemia is an insufficient measure of adverse lung function in very immature infants. Arch Dis Child Fetal Neonatal Ed 2014; 99: F257–F262. doi: 10.1136/archdischild-2013-304625 [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Stanton J, Wang L, et al. . Effect of salbutamol on the measurement of single-breath diffusing capacity. Respirology 2013; 18: 1223–1229. doi: 10.1111/resp.12125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table A ERJ-04103-2020.Table_A (440.2KB, pdf)
Supplementary table B ERJ-04103-2020.Table_B (239.1KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-04103-2020.Shareable (435.9KB, pdf)