Abstract
Background
Pulmonary rehabilitation (PR) is a cornerstone in chronic obstructive pulmonary disease (COPD) management. However, PR adherence is generally low, and barriers include availability, economic issues, motivation and an inability to attend or perform physical training. Therefore, alternative, evidence-based PR activities are required. Singing may have benefits for quality of life (QoL), respiratory control and well-being in COPD, but the impact on the PR key outcome, physical exercise capacity, is uncertain.
Methods
In this randomised controlled trial (NCT03280355), we investigated the effectiveness of 10 weeks of PR, including either “Singing for Lung Health” (SLH) training or standard physical exercise training (PExT). The primary outcome was a change in exercise capacity (6-min walk distance (6MWD)) from baseline to post-PR. Secondary outcomes were changes in QoL (St George's Respiratory Questionnaire (SGRQ)), Hospital Anxiety and Depression Score (HADS), lung function, dyspnoea and adherence.
Results
We included 270 COPD patients, and 195 completed the study. Demographics across groups were comparable, and both groups improved significantly in 6MWD and SGRQ score. SLH was non-inferior to PExT in improving 6MWD (mean±sd 13.1±36.3 m versus 14.1±32.3 m, p=0.81; difference 1.0 m, 95% CI −7.3–9.3 m) with 21.8% and 25.0%, respectively (p=0.57), reaching the 6MWD minimal important difference of 30 m. We found no significant between-group differences concerning SGRQ, HADS, lung function, dyspnoea or adherence.
Conclusion
Our study suggests that SLH is non-inferior to PExT in improving 6MWD during a 10-week PR programme. Future studies addressing reproducibility, long-term effects and health economics are needed.
Short abstract
Singing for Lung Health was non-inferior to physical exercise training in short-term improvement of 6-min walk test distance in COPD patients attending pulmonary rehabilitation. In both groups, the effect was related to high adherence. https://bit.ly/3uoP4Q8
Introduction
The prevalence of respiratory diseases is increasing rapidly, and chronic obstructive pulmonary disease (COPD) is now the third leading global cause of death [1]. COPD is associated with hospitalisation, mortality, multimorbidity, reduced quality of life (QoL), increased sick leave, reduced physical activity and significantly increased economic costs for patients and society [2].
The central role of pulmonary rehabilitation (PR) with physical exercise training (PExT) in COPD management is stated in the joint guidelines published by the American Thoracic Society and European Respiratory Society [3]. PR supports lifestyle changes and improves exercise capacity, QoL and dyspnoea [1, 3]. Although well documented to have a positive effect on walking distance, QoL and dyspnoea control, PR adherence is challenged by lack of access, costs and participants’ comorbidities and lack of motivation [3–6]. Only ∼10% of those who would benefit from PR are currently referred. Approximately half of those referred do not show up and of those actually showing up, one third fail to complete PR [7]. It is a key priority to increase availability of PR and to develop alternative, motivating and personalised solutions to supplement standard PR [1, 8].
Singing for people with respiratory disease is perceived to be beneficial in managing dyspnoea, increasing well-being and QoL, and reducing social isolation in COPD [9–19]. With regard to physical outcomes, singing may improve respiratory muscle strength, coordination and performance [13, 14, 20–22]; reduce hyperinflation [9, 23]; improve lung function [24]; and enhance functional exercise capacity in COPD [13, 25]. The specific methodological concept “Singing for Lung Health” (SLH) was developed in the UK [9, 23, 26, 27] and has become increasingly popular, although heterogeneous singing approaches are applied in other countries [23, 28, 29]. The growing body of research on SLH and singing in COPD is mostly based on small-scale and descriptive studies without a primary focus on key objective variables relevant in PR research [1, 9, 13, 23, 28].
In the present study, the objective was to investigate SLH as an alternative to conventional PExT as part of PR in COPD, hypothesising that SLH is non-inferior to PExT with regard to change from pre to post 10-week PR programme, and measured by key objective variables in PR: exercise capacity, QoL, anxiety and depression, lung function, dyspnoea and adherence.
Methods
Study design and oversight
Between August 2017 and May 2019, we conducted a multicentre, randomised controlled clinical trial in Denmark, comparing SLH with PExT (registered at ClinicalTrials.gov: NCT03280355). The study was performed in accordance with the Declaration of Helsinki, and approved by the Regional Committee on Health Research Ethics, Region Zealand, Denmark (number SJ-597) and by the Danish Data Protection Agency (number REG-049-2017).
Participants
In Denmark, PR in COPD is almost exclusively offered by community-based services [30]. Therefore, we enrolled 11 community PR services distributed across Denmark and screened for eligibility among patients with COPD referred for PR. Inclusion criteria were a doctor's diagnosis of COPD (according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [14]), referral for and ability to attend PR, and acceptance of randomisation. Exclusion criteria were unstable coronary heart disease, severe cognitive disabilities, inability to speak or understand spoken/written Danish and participation in lung choir singing or PR (or other structured, disease-related physical training) within the previous 6 months. Uncertainty in the interpretation of the General Data Protection Regulation (GDPR) in European Union law (which was implemented in the EU during our trial) resulted in loss of data on reasons for declining: some centres refused to record data on non-participating citizens, and other centres deleted data already obtained data for fear of breaking the GDPR law.
Randomisation and blinding
Given that PR is performed as a group-based activity, randomisation to either SLH or PExT was performed across the planned number of PR classes as clusters, each representing approximately 10 participants. The randomisation procedure was performed by the study investigator (M. Kaasgaard) using sequentially numbered, closed envelopes, and supervised by the principal investigator (U. Bodtger). Randomisation was concealed at baseline to local health professionals and participants, and the study nurse was blinded to randomisation both at baseline and follow-up.
Data collection procedure
At baseline, participants were assessed using objective tests and completed patient-reported questionnaires and data sheets on socio-demographic information, medication usage and expectations towards the benefits of singing. All assessments were repeated at follow-up. One designated study nurse performed all the objective assessments and collected all data from all sites within 2 weeks before the onset of PR and again within 2 weeks after programme termination. Data were registered in web-based software (SurveyXact, Rambøll Management Consulting, Aarhus, Denmark).
Outcomes and measures
The primary study outcome was change in exercise capacity from baseline to follow-up measured as change in 6-min walk distance (6MWD) (minimal important difference (MID): 30 m) [31]. Secondary outcomes were changes in QoL, measured by the St George's Respiratory Questionnaire (SGRQ); anxiety and depression, measured by the Hospital Anxiety and Depression Scale (HADS); lung function and dyspnoea, measured by forced expiratory volume in 1 s (FEV1) (in mL and as FEV1 % predicted), modified Medical Research Council (mMRC) dyspnoea scale and modified Borg category-ratio (Borg-CR10) dyspnoea scale; and adherence to PR. See appendix S1 for details and interpretation.
Pulmonary rehabilitation
Both study groups received a 10-week PR course that included either SLH or PExT twice weekly, i.e. a total of 20 sessions, delivered at the local community PR service. Sessions lasted 90 min and included individual supervision and guidance. In addition, both study groups received identical educational sessions as part of PR, attending once a week, that covered lifestyle changes, disease management, guidance on managing daily life, smoking cessation, inhaler technique and dyspnoea control manoeuvres [1]. The PR content delivered to each study group is reported in table 1 and in the Consensus on Exercise Reporting Template (CERT) (appendices S2 and S3) [32].
TABLE 1.
Overview of content in the two study groups
| SLH | PExT | |
| Per session (min)# | 90 | 90 |
| Warm-up exercise (body) | ✓ | ✓ |
| Warm-up exercise (voice) | ✓ | × |
| Breathing techniques | ✓ | ✓ |
| Handling dyspnoea | ✓ | ✓ |
| Posture | ✓ | ✓ |
| Resting positions | ✓ | ✓ |
| Endurance exercises (circuit/interval)¶ | ✓ | ✓ |
| Respiratory muscle training | ✓ | × |
| Strength exercises and limb endurance training | × | ✓ |
| Home exercise instructions/continuation of physical activity | ✓ | ✓ |
| Muscle stretching | ✓ | ✓ |
| Relaxation and body awareness | ✓ | ✓ |
| Singing | ✓ | × |
| Education and self-management as part of PR+ | ✓ | ✓ |
SLH: Singing for Lung Health; PExT: physical exercise training. #: dose of intervention for both groups was 90 min twice weekly over 10 weeks; ¶: both groups were trained in coordination of breathing and use of pursed lip breathing (resistance on exhalation), while the SLH group did respiratory muscle training (resistance on inhalation) and the PExT group did strength exercises and limb endurance training (comprising walking, stepping, stair climbing, exercise bikes and, if possible, jogging, cross trainer and/or row machine); +: dose of education and self-management for both groups varied between 60–120 min once weekly for 10 weeks and comprised knowledge about chronic obstructive pulmonary disease, behaviour change, smoking cessation, correct use of inhaler devices, nutrition, sexuality, handling of stress and anxiety, early recognition of exacerbation, decision-making and taking action on symptoms, motivation goals and maintenance post-pulmonary rehabilitation.
Singing for Lung Health
SLH in the intervention arm was delivered by professional singing teachers who had been specially trained before study initiation by the developers of the UK SLH programme [9, 23, 26, 27]. SLH included physical, vocal and breathing exercises, with a focus on improving strength, endurance and flexibility of the respiratory muscles. SLH was carefully adapted to the respiratory challenges in COPD and included movement and/or dancing. Each session consisted of 20 min of physical warm-ups, 20 min of vocal warm-up with rhythm and pitch games, 40 min of singing and 10 min of cooling down, e.g. mindfulness or relaxation.
Physical exercise training
PExT in the control arm was delivered by local, experienced physiotherapists and conducted in accordance with Danish national clinical PR guidelines [33], in the form of supervised strength and endurance training to enhance exercise tolerance and capacity [34–36], with modifications based on preferences, local decisions and individual tailoring. Each session consisted of 20 min of physical warm-ups, 60 min of PExT, including handling of dyspnoea, and 10 min of cooling down.
Statistics
An a priori sample size calculation (power 95%, two-sided α 5%, non-inferiority margin 8%) estimated a requirement of 87 participants in each study group (dropout rate +20%, i.e. total sample size n=220) to detect a between-group 6MWD difference of the MID (30 m) in a non-inferiority design [37].
Continuous data are described as mean±sd and tested using a t-test, and categorical data as n (%) and tested using a Chi-squared test. The primary outcome was analysed according to the intention-to-treat principle. Within-group changes from baseline to follow-up were assessed using paired tests. Missing data were handled with the last observation carried forwards or next observation carried backwards [38].
Logistic regression was used to test the relationship between training modality and achieving the MID of the primary outcome. A multilevel mixed-effects logistic regression model was constructed to adjust for potential confounders, including age, sex, GOLD class, mMRC at baseline, 6MWD at baseline categorised into quartiles, body mass index (BMI), expectations towards benefits of singing, adherence to training (fixed-effect variables) and training centre (random-effects variables). Unless otherwise stated, no significant interactions were found. Differences in adherence to training were evaluated, including baseline characteristics of patients with high adherence (≥75%), and factors associated with high adherence were analysed using multivariable logistic regression. Subgroup analyses of the primary outcome included 1) a per-protocol analysis excluding patients who dropped out before the follow-up visit, 2) analyses of patients with high adherence, 3) analyses of patients with high expectations towards benefits of singing and 4) analyses excluding patients who never attended training (zero adherence).
Statistical analyses were performed using SPSS 26.0 (IBM, Chicago, IL, USA) and STATA/IC 16.1 (StataCorp LLC, College Station, TX, USA). Statistical significance was reached at p<0.05.
Blinded results (presented as Treatment 0 compared to Treatment 1) of the study were presented to the research group for interpretation [39].
Results
Participants
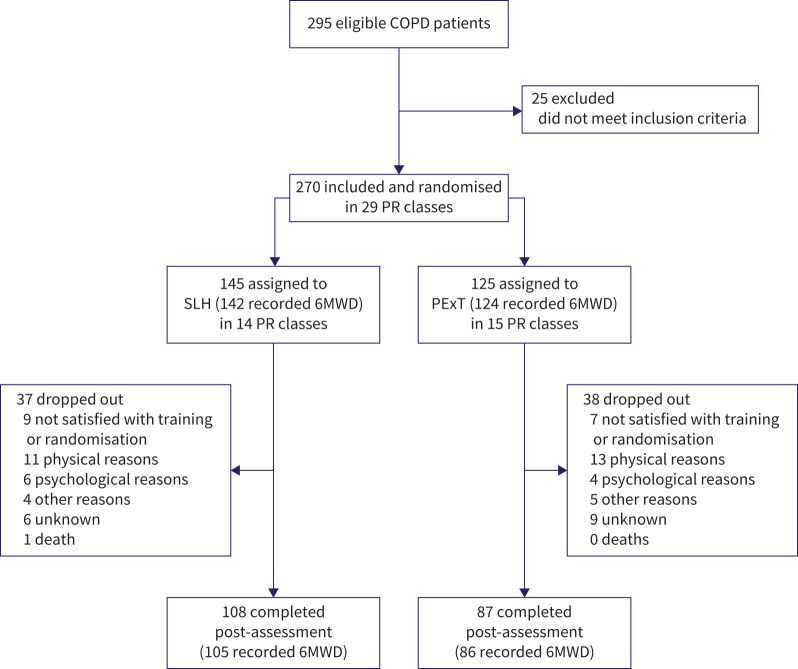
More than half of the participants were female (62.2%); mean values for other demographics included age 69.5±8.4 years, BMI 27.8±6.0 kg·m−2, pack years 40.5±21.3, FEV1 % predicted 51.4±16.8%, 6MWD 382.3±102.4 m and 66.3% having positive expectations towards benefits of singing. The two study groups were comparable at baseline, except for lower lung function in the SLH group (table 2 and appendix S4). Because dropout rates were higher than expected, we continued inclusion to 270 participants in total (intention-to-treat population) to ensure sufficient power for the primary end-point. Total dropout rate was 28% (n=75) and 195 completed the study (table 2). Across the 11 participating community PR services, patients enrolled in 29 PR classes (median size, nine patients; range, 5–16 patients). Four participants did not perform baseline 6MWD; however, their data were otherwise complete and they were included in secondary outcomes analyses (figure 1). No adverse events were reported.
TABLE 2.
Baseline characteristics in intention-to-treat-population
| SLH | PExT | Difference p-value | |
| Subjects, n | 145 | 125 | |
| Age, years | 70.2±8.8 | 68.8±8.0 | 0.19 |
| Female sex | 84 (57.9%) | 84 (67.2%) | 0.13 |
| BMI, kg·m−2 | 28.0±6.2 | 27.7±5.8 | 0.63 |
| Smoking status | |||
| Current | 38 (26.2%) | 29 (23.2%) | 0.63 |
| Former | 99 (68.3%) | 86 (68.8%) | |
| Never | 8 (5.5%) | 10 (8.0%) | |
| Pack years | 41.0±20.8 | 39.9±22.0 | 0.68 |
| FEV1 % predicted | 49.5±16.9 | 53.6±16.6 | 0.05 |
| GOLD classification | |||
| Class 1 | 2 (1.4%) | 7 (5.6%) | 0.03 |
| Class 2 | 68 (46.9%) | 66 (52.8%) | |
| Class 3 | 50 (34.5%) | 44 (35.2%) | |
| Class 4 | 23 (16.0%) | 8 (6.4%) | |
| mMRC | |||
| 0 | 6 (4.1%) | 7 (5.6%) | 0.52 |
| 1 | 42 (29.0%) | 43 (34.4%) | |
| 2 | 47 (32.4%) | 41 (32.8%) | |
| 3 | 19 (13.1%) | 9 (7.2%) | |
| 4 | 31 (21.4%) | 25 (20.0%) | |
| Medication | |||
| LAMA | 102 (70.3%) | 89 (71.2%) | 0.89 |
| LABA | 110 (75.9%) | 99 (79.2%) | 0.56 |
| ICS | 71 (49.0%) | 59 (47.2%) | 0.81 |
| OCS | 9 (6.2%) | 4 (3.2%) | 0.27 |
| Roflumilast | 0 (0.0%) | 1 (0.7%) | 1.00 |
| Theophylline | 2 (1.6%) | 0 (0.0%) | 0.21 |
| Home-oxygen therapy | 6 (4.1%) | 3 (2.4%) | 0.51 |
| Positive expectations towards benefits of singing | 94 (64.8%) | 85 (68.0%) | 0.61 |
Data are presented as mean±sd or n (%), unless otherwise stated. SLH: Singing for Lung Health; PExT: physical exercise training; BMI: body mass index; FEV1: forced expiratory volume in 1 s; GOLD: Global Initiative for Chronic Obstructive Lung Disease; mMRC: modified Medical Research Council dyspnoea score; LAMA: inhaled long-acting muscarinic antagonists; LABA: inhaled long-acting β2-agonists; ICS: inhaled corticosteroids; OCS: oral corticosteroids.
FIGURE 1.
CONSORT flow diagram. COPD: chronic obstructive pulmonary disease; PR: pulmonary rehabilitation; 6MWD: 6-min walk distance.
Outcomes
Primary outcome
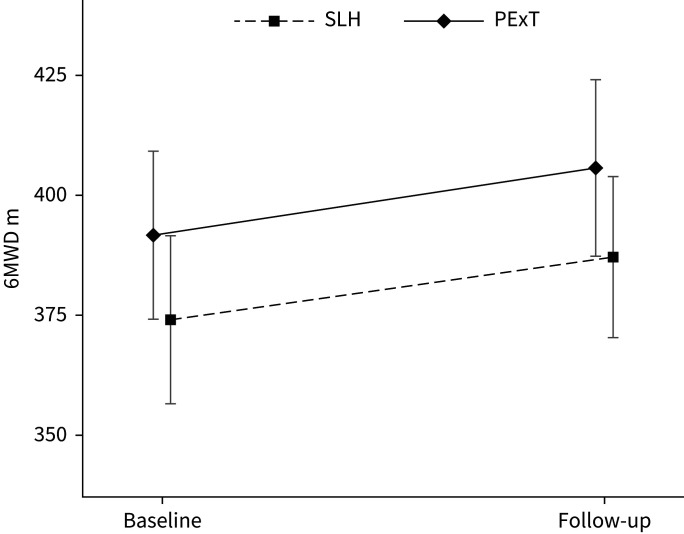
In the intention-to-treat analysis, SLH was non-inferior to PExT in improvement of 6MWD and in rate of participants reaching the 6MWD MID of 30 m (figure 2 and table 3). Both study groups showed a statistically significant improvement in 6MWD.
FIGURE 2.
Change in 6-min walk distance (6MWD). SLH: Singing for Lung Health; PExT: physical exercise training.
TABLE 3.
Physical performance and quality of life
| SLH | PExT | Difference p-value | 95% CI | |
| 6MWD, m | ||||
| Baseline | 374.1±105.0 | 391.6±99.0 | 0.17 | |
| Follow-up | 387.2±100.5 | 405.7±104.5 | 0.14 | |
| Change from baseline | 13.1±36.3*** | 14.1±32.3*** | 0.81 | −7.3–9.3 |
| MID achieved | 31 (21.8%) | 31 (25.0%) | 0.57 | |
| SGRQ | ||||
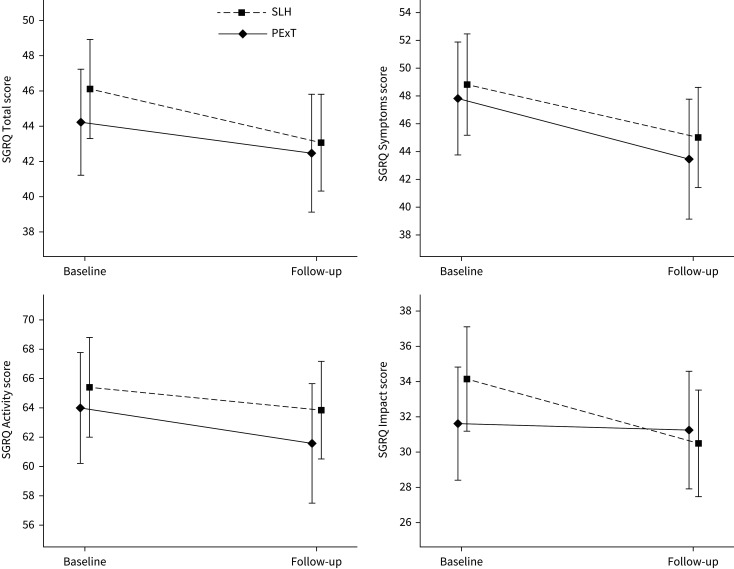
| Total score | ||||
| Baseline | 46.1±17.1 | 44.0±17.0 | 0.32 | |
| Follow-up | 43.0±16.6 | 42.5±18.9 | 0.81 | |
| Change from baseline | −3.0±8.8*** | −1.5±9.2 | 0.16 | −0.6–3.7 |
| MID achieved | 51 (35.2%) | 35 (28.0%) | 0.21 | |
| Symptoms score | ||||
| Baseline | 48.9±22.4 | 47.8±22.7 | 0.71 | |
| Follow-up | 45.0±21.9 | 43.5±24.4 | 0.61 | |
| Change from baseline | −3.9±15.0** | −4.3±17.2** | 0.83 | −4.3–3.5 |
| MID achieved | 53 (36.6%) | 46 (36.8%) | 0.97 | |
| Activity score | ||||
| Baseline | 65.4±20.6 | 64.0±21.4 | 0.59 | |
| Follow-up | 63.9±20.0 | 61.6±23.2 | 0.39 | |
| Change from baseline | −1.5±10.4 | −2.4±11.5* | 0.49 | −3.7–1.7 |
| MID achieved | 44 (30.3%) | 44 (35.2%) | 0.40 | |
| Impact score | ||||
| Baseline | 34.2±18.1 | 31.3±18.1 | 0.20 | |
| Follow-up | 30.5±18.1 | 31.3±19.1 | 0.74 | |
| Change from baseline | −3.7±12.1*** | −0.1±10.5 | 0.01 | 0.9–6.6 |
| MID achieved | 52 (35.9%) | 28 (22.4%) | 0.02 | |
| HADS | ||||
| Anxiety score | ||||
| Baseline | 4.8±3.8 | 5.1±3.7 | 0.52 | |
| Follow-up | 4.9±3.7 | 4.9±3.7 | 0.97 | |
| Change from baseline | 0.1±2.4 | −0.2±2.3 | 0.33 | −0.9–0.3 |
| Depression score | ||||
| Baseline | 3.4±3.2 | 3.0±2.9 | 0.28 | |
| Follow-up | 3.2±2.9 | 3.1±3.0 | 0.78 | |
| Change from baseline | −0.2±2.1 | 0.1±1.5 | 0.19 | −0.1–0.7 |
| FEV1 % predicted | ||||
| Baseline | 49.7±16.9 | 53.6±16.6 | 0.06 | |
| Follow-up | 50.8±17.8 | 53.9±17.4 | 0.14 | |
| Change from baseline | 1.1±6.6* | 0.4±4.6 | 0.01 | −2.1– −0.7 |
| Borg-CR10 (after 6MWD) | ||||
| Baseline | 7.1±2.8 | 6.6±2.8 | 0.10 | |
| Follow-up | 6.9±2.7 | 6.4±2.5 | 0.15 | |
| Change from baseline | −0.3±1.8 | −0.2±2.2 | 0.66 | −0.4–0.6 |
| mMRC | ||||
| Baseline | 2.2±1.2 | 2.0±1.2 | 0.25 | |
| Follow-up | 2.0±1.2 | 2.0±1.3 | 0.97 | |
| Change from baseline | −0.2±0.7*** | −0.1±0.8 | 0.07 | −0.0–0.4 |
| Adherence to intervention | ||||
| Sessions, n | 16.6±3.0 | 16.3±3.1 | 0.41 | −2.1–1.0 |
| Adherence rate 0–24% | 22 (15.2%) | 21 (16.8%) | 0.90 | |
| Adherence rate 25–49% | 11 (7.6%) | 12 (4.4%) | ||
| Adherence rate 50–74% | 24 (16.6%) | 21(16.8%) | ||
| Adherence rate 75–100% | 88 (60.7%) | 71 (56.8%) | ||
| Dropout rate | 37 (25.5%) | 38 (30.4%) | 0.42 |
Data are presented as mean±sd or n (%), unless otherwise stated. SLH: Singing for Lung Health; PExT: physical exercise training; 6MWD: 6-min walking distance; MID: minimal important difference; SGRQ: St George's Respiratory Questionnaire; HADS: Hospital Anxiety and Depression Score; FEV1: forced expiratory volume in 1 s; Borg-CR10: modified Borg category-ratio dyspnoea scale; mMRC: modified Medical Research Council dyspnoea score. Within-group significance: *: p<0.05, **: p<0.01; ***: p<0.001.
Across the two study groups, we found no difference in 6MWD MID after adjustment for age, sex, BMI, GOLD class and expectations towards benefits of singing, using multivariable logistic regression (OR 0.96, 95% CI 0.5–1.7, p=0.89). We found significant correlation in both groups between adherence to PR and reaching the 6MWD MID of 30 m (appendix S5).
Secondary outcomes
We observed no between-group differences in Borg-CR10, mMRC, lung function, HADS, attendance rate or dropout rate. Concerning SGRQ total score, we found no significant between-group difference (table 3 and figure 3), whereas data suggested a beneficial effect of SLH on SGRQ impact score (≥4 point improvement: SLH 35.9% and PExT 22.4%, p=0.02) (table 3).
FIGURE 3.
Change in St George's Respiratory Questionnaire (SGRQ) scores. SLH: Singing for Lung Health; PExT: physical exercise training.
Subgroup analyses
The per-protocol analysis showed non-inferiority in primary and secondary outcomes, with only minor differences observed in SGRQ impact score and lung function (appendix S6). Stratified analyses for sex, age and lung function (GOLD class) showed no significant differences between groups. Further, we found no significant differences between the 29 PR classes regarding primary outcome.
There was no correlation between baseline characteristics or other factors and high adherence (≥75%) (appendices S7 and S8). Non-adherence to PR was associated with living alone, current smoking and high symptom burden (appendices S5, S7 and S8).
The 75 participants who dropped out differed at baseline from study completers by displaying poorer lung function, shorter 6MWD, more symptoms and a higher prevalence of current smoking (appendix S9).
Discussion
In this multicentre, randomised controlled trial of community-based PR in patients with COPD, we compared SLH with conventional PExT for the primary study outcome of changes in 6MWD after 10 weeks, and we found that SLH was non-inferior to PExT (6MWD difference: 13.1±36.3 m versus 14.1±32.3 m, p=0.81; difference 1.0 m, 95% CI −7.3–9.3 m). Previous studies on singing in COPD are small and heterogeneous with low quality of evidence [9, 13]. No previous study on SLH included key objective criteria as the primary end-point, but e.g. Lord et al. [40] included incremental shuttle walk test as a secondary outcome with insufficient power to detect change.
We observed a modest and lower improvement in 6MWD compared to those reported in the latest Cochrane review on PR in stable COPD (mean improvement 43.9 m, 95% CI 36.2–55.2 m) and in real-life reports of national PR services from the UK (improvement ≥MID (30 m) in 70% of participants) and Denmark (mean improvement 45 m, 95% CI 38–46 m) [30, 41, 42]. Our findings suggest that we may have compared SLH to an ineffective PR programme, yet we report an effect size comparable to that found in the largest study included in the Cochrane review [42]: a community-based trial (n=165) with a mean 6MWD improvement of 13.9 m (95% CI 3.1–24.7 m). In Denmark, PR is conducted almost exclusively as community-based PR, with easy, free and close access for all Danish citizens. A systematic review on home- or community-based PR found lower 6MWD improvement (33.8 m, 95% CI 6.0–61.5 m; ≤50 patients in eight of 10 trials) than the above-mentioned Cochrane review [42], suggesting a lower effect size in PR conducted outside larger centres [43]. Interestingly, the evidence level of PR's impact on 6MWD was assessed as “very low” in the Cochrane review owing to substantial study heterogeneity and significant reporting bias (27 of 38 studies with ≤50 patients; Egger bias 1.24, 95% CI 0.2–2.3; p=0.023) [42]. In many of the well-conducted randomised controlled trials that demonstrate a positive effect on 6MWD [42, 44], the effect is measured as change in mean difference and compared to usual care (without training). Lastly, concerns were raised in the UK report on real-life PR [41] that only 6% of PR programmes used the recommended walking course length, and that almost half the programmes used no walking tests. In the Danish observational KOALA study [30], only data from PR completers were included, thus excluding 28% of the intention-to-treat population, which impairs the generalisability of this study. We therefore consider our findings to be honest, real-life observations in a large-scale community-based randomised controlled trial; however, we strongly encourage replication of our study in other PR settings.
Currently, there is no international consensus guideline on singing as a training intervention for lung disease. Generally, singing for people with respiratory disease has evolved as a leisure activity rather than a structured health-related activity, and most lung choirs still have heterogeneous leadership and a lack of standard training or guidelines [9, 23, 28, 29]. The British Lung Foundation initiative SLH includes systematic training of singing teachers in lung physiology/pathophysiology and a methodological approach to singing as a physical activity providing respiratory control [9, 26, 27]. This aligns well with recommendations on both physical and psychosocial elements in PR [1, 3]. So far, SLH is the best-documented singing training programme in respiratory disease, though the evidence is primarily based on qualitative research [9, 23, 28, 29]. Several studies suggest that SLH improves QoL [10, 18, 19], yet we did not find that SLH improved QoL significantly (secondary outcome) (table 3). Future interventions may be combined, given that a recent network meta-analysis suggests that techniques based on diaphragmatic breathing training and yoga breathing are more effective than singing in improving QoL in COPD [45]. Likewise, our study failed to confirm that SLH improves anxiety, depression, dyspnoea or lung function [9, 10, 16, 18, 19, 28]. In this paper, we only reported FEV1, but because singing is proposed to improve diaphragmatic control [10, 24], a mechanistic paper on inspiratory and expiratory lung function measurements from our randomised controlled trial is in preparation.
We found identical adherence rates, and adherence was equally related to 6MWD improvement in both groups. Further, we found that adherence was not related to specific patient characteristics or factors such as sex or age (table 3 and appendices S7 and S8). This suggests that SLH is more than a leisure time activity for adherent patients not preferring to engage in PExT [46]. In our study, participants were referred for conventional PR with PExT and not for PR with SLH. Future studies should clarify if an active choice of SLH affects attendance rate and 6MWD improvement [8].
Strengths and limitations
Our study has both strengths and limitations. Most importantly, our study is a short-term proof-of-concept study of SLH's impact on 6MWD improvement. Currently, there are no data on long-term outcomes of SLH as part of PR, and SLH is not validated with respect to any key outcomes of PR. Additionally, owing to the decentralised structure of Danish PR, only a multicentre design would allow for sufficient recruitment and sample size. The multicentre design increased internal heterogeneity in delivery of both SLH and PExT. However, study groups were comparable at baseline and follow-up, including expectations towards benefits of singing, and with no significant differences in primary end-point between sites (tables 2 and 3). Furthermore, it is unlikely that selection bias explains our findings because participants were recruited from community-based PR centres after referral for standard care (PR with PExT) from their general practitioner, who was not informed about the trial. We included only well-established outcome variables used in PR trials (6MWD, SGRQ, FEV1, HADS, Borg-CR10, mMRC), and did not include any singing-specific outcomes. Furthermore, we used only basic and fully transparent statistical models, which further enhances transferability and external validity of our results.
Our findings need validation in other settings, including highly specialised/centralised COPD PR centres, and our results are not directly transferable to other lung diseases. Evidence suggests that PR and SLH are effective in both obstructive and interstitial lung diseases [9], but future studies should clarify the generic properties of PR with SLH.
Due to the proof-of-concept nature of our study, we did not include health economics, which is a needed aspect when investigating long-term effects of PR on healthcare usage, hospital admission rates and mortality [42].
Conclusion
This randomised controlled trial in patients with COPD attending PR demonstrated that SLH provides positive and clinically relevant physiological and psychological changes in COPD, and that SLH was non-inferior to PExT in improving 6MWD post 10 weeks in community-based PR. 6MWD improvement in both study arms showed a dose-response relationship with adherence. Future studies in SLH should validate our findings in other PR settings and investigate key long-term outcomes such as hospital admission rates, mortality and health economics.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01142-2021.Supplement (897.9KB, pdf)
Shareable PDF
Acknowledgements
The authors would like to thank all study participants for their willingness to participate in the project, and we thank the singing teachers who conducted the SLH. Further, we thank municipal healthcare centre managers and employees for their cooperation and engagement in relation to recruitment, project management and running the control intervention in the 11 participating Danish municipalities: Vesthimmerland, Lemvig, Ikast-Brande, Hedensted, Silkeborg, Slagelse, Lolland, Vordingborg, Faxe, Rudersdal and Helsingør. We thank project assessor, pulmonary specialist nurse Marianne Gerning Pedersen, Department of Respiratory Medicine, Aarhus University Hospital, Denmark, for her extraordinary effort in relation to data collection and registration, and research assistant Trine Hjerrild Andreasson, Department of Respiratory Medicine, Zealand University Hospital, Naestved, Denmark, for her thorough assistance with data validation and filing. We thank singers and singing teachers Lone Boyd and Malene Bichel for reviewing the CERT checklists. We thank Søren Højsgaard, Head of Department for Mathematical Sciences at Aalborg University, for statistical advice. A very special thanks to the founder of “Singing for Lung Health”, music therapist and singing teacher Phoene Cave and respiratory physiotherapist Dr Adam Lewis, who trained the Danish singing teachers prior to intervention. Finally, we thank Professor Emeritus Stephen Clift, Sidney De Haan Research Centre for Arts and Health, Canterbury Christ Church University and Royal Society for Public Health, UK, for his interest and support along the way.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.02961-2021
The study protocol is available at ClinicalTrials.gov (NCT03280355). Consent forms will not be available according to Danish legislation. De-identified data collected for the study will be available from 1 January, 2023, upon reasonable request. Contact study investigator, mk@clin.au.dk.
Author contributions: M. Kaasgaard, U. Bodtger, O. Hilberg, A. Løkke and P. Vuust contributed substantially to the concept and design of this trial. The trial investigator (M. Kaasgaard) developed manuals for recruitment, assessment and treatment; written information; applications for grants; and approval assignments; made a registry at ClinicalTrial.gov; introduced the recruitment; introduced and supervised the treatment procedures to all involved singing teachers; and led the data collection. Principal investigator (U. Bodtger) supervised the registration, recruitment, assessment and data collection procedure. Analyses were performed by M. Kaasgaard, re-performed by D.B. Rasmussen, and checked by U. Bodtger. O. Hilberg, A. Løkke and K.H. Andreasson; P. Vuust gave feedback. M. Kaasgaard and K.H. Andreasson made the descriptions of intervention and control study group content, and U. Bodtger, O. Hilberg, A. Løkke, D.B. Rasmussen and P. Vuust gave feedback. M. Kaasgaard drafted the manuscript, and U. Bodtger, O. Hilberg, A. Løkke, D.B. Rasmussen, K.H. Andreasson and P. Vuust provided important intellectual input and feedback on the manuscript and approved the final version.
Conflict of interest: M. Kaasgaard holds a diploma graduate degree from the Royal Danish Academy of Music in Voice and Voice Pedagogy.
Conflict of interest: D.B. Rasmussen has nothing to disclose.
Conflict of interest: K.H. Andreasson has nothing to disclose.
Conflict of interest: O. Hilberg has nothing to disclose.
Conflict of interest: A. Løkke has nothing to disclose.
Conflict of interest: P. Vuust is leader of the research centre, Center for Music in the Brain.
Conflict of interest: U. Bodtger has nothing to disclose.
Support statement: The present study was part of a Danish PhD project, funded by the Tryg Foundation (ID 111562), the Danish Health Foundation (18-B-0067), Aase og Ejnar Danielsen's Foundation (ID 10-001745), The Moller Foundation (ID 16-204), the Danish Lung Foundation (ID 179031-24-03-2017), Naestved, Slagelse and Ringsted Hospitals’ Research Fund, Danish Central Region (ID 1-30-72-141-12), Region Zealand (ID 180886, 13-000835) and Aarhus University. The Center for Music in the Brain is funded by the Danish National Research Foundation (DNRF117). All research grants were used for study investigator M. Kaasgaard's salary, running costs and equipment. Participants did not receive any economic compensation for study participation. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Gibson GJ, Loddenkemper R, Sibille Y, et al. , eds. European Lung White Book. Sheffield, European Respiratory Society, 2013. [Google Scholar]
- 2.McKeough Z. Community-based pulmonary rehabilitation is effective for people with chronic obstructive pulmonary disease (COPD). Aust J Physiother 2009; 55: 287. doi: 10.1016/S0004-9514(09)70013-3 [DOI] [PubMed] [Google Scholar]
- 3.Rochester CL, Vogiatzis I, Holland AE, et al. . An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med 2015; 192: 1373–1386. doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 4.Oates GR, Niranjan SJ, Ott C, et al. . Adherence to pulmonary rehabilitation in COPD: a qualitative exploration of patient perspectives on barriers and facilitators. J Cardiopulm Rehabil Prev 2019; 39: 344–349. doi: 10.1097/HCR.0000000000000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peixoto Boaventura RD, Meira L, Santos V, et al. . Factors influencing pulmonary rehabilitation adherence – surely is not simple. Rehabil Chronic Care 2016; 48: PA694. doi: 10.1183/13993003.congress-2016.PA694 [DOI] [Google Scholar]
- 6.Almadana Pacheco V, Pavón Masa M, Gómez-Bastero Fernández AP, et al. . Patient profile of drop-outs from a pulmonary rehabilitation program. Arch Bronconeumol 2017; 53: 257–262. doi: 10.1016/j.arbr.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 7.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis 2011; 8: 89–99. doi: 10.1177/1479972310393756 [DOI] [PubMed] [Google Scholar]
- 8.McNamara RJ, Dale M, McKeough ZJ. Innovative strategies to improve the reach and engagement in pulmonary rehabilitation. J Thorac Dis 2019; 11: S2192–S2199. doi: 10.21037/jtd.2019.10.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis A, Cave P, Stern M, et al. . Singing for Lung Health—a systematic review of the literature and consensus statement. NPJ Prim Care Resp Med 2016; 26: 16080. doi: 10.1038/npjpcrm.2016.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilha, AG, Onofre F, Vieira ML, et al. . Effects of singing classes on pulmonary function and quality of life of COPD patients. Int J Chronic Obstructive Pulm Dis 2008; 4: 1–8. doi: 10.2147/COPD.S4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panigrahi A, Sohani S, Amadi C, et al. . Role of music in the management of chronic obstructive pulmonary disease (COPD): a literature review. Technol Health Care 2014; 22: 53–61. doi: 10.3233/THC-130773 [DOI] [PubMed] [Google Scholar]
- 12.Canga B, Azoulay R, Raskin J, et al. . AIR: Advances in Respiration – music therapy in the treatment of chronic pulmonary disease. Respir Med 2015; 109: 1532–1539. doi: 10.1016/j.rmed.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 13.McNamara RJ, Epsley C, Coren E, et al. . Singing for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 12: cd012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gick ML, Nicol JJ. Singing for respiratory health: theory, evidence and challenges. Health Promot Int . 2016; 31: 725–734. doi: 10.1093/heapro/dav013 [DOI] [PubMed] [Google Scholar]
- 15.Gebauer L, Vuust P. Music Interventions in Health Care. White Paper. Kongens Lyngby, Danish Sound Innovation Network, 2014. www.petervuust.dk/wp-content/uploads/2014/03/whitepaper_digital_enkelsidet_0.pdf [Google Scholar]
- 16.Stewart NAJ, Lonsdale AJ. It's better together: the psychological benefits of singing in a choir. Psychology of Music 2016; 44: 1240–1254. doi: 10.1177/0305735615624976 [DOI] [Google Scholar]
- 17.Pentikäinen E, Pitkäniemi A, Siponkoski S-T, et al. . Beneficial effects of choir singing on cognition and well-being of older adults: Evidence from a cross-sectional study. PLoS One 2021; 16: e0245666. doi: 10.1371/journal.pone.0245666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord VM, Cave P, Hume VJ, et al. . Singing teaching as a therapy for chronic respiratory disease—a randomised controlled trial and qualitative evaluation. BMC Pulm Med 2010; 10: 41. doi: 10.1186/1471-2466-10-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Song M, Zhai Z-H, et al. . Group singing improves depression and life quality in patients with stable COPD: a randomized community-based trial in China. Qual Life Res 2019; 28: 725–735. doi: 10.1007/s11136-018-2063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomoni S, van den Hoorn W, Hodges P. Breathing and singing: objective characterization of breathing patterns in classical singers. PLoS One 2016; 11: e0155084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leanderson R, Sundberg J, von Euler C. Role of diaphragmatic activity during singing: a study of transdiaphragmatic pressures. J Appl Physiol (1985) 1987; 62: 259–270. doi: 10.1152/jappl.1987.62.1.259 [DOI] [PubMed] [Google Scholar]
- 22.Tamplin J, Baker FA, Grocke D, et al. . Effect of singing on respiratory function, voice, and mood after quadriplegia: a randomized controlled trial. Arch Phys Med Rehabil 2013; 94: 426–434. doi: 10.1016/j.apmr.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 23.Heydon R, Fancourt D, Cohen J. The Routledge Companion to Interdisciplinary Studies in Singing. Volume III: Wellbeing. Part I Singing and Health. Chapter 7. 1st edition. Abingdon-on-Thames, Routledge, 2020; pp. 86–97. [Google Scholar]
- 24.Fancourt D, Finn S. What is the Evidence on the Role of the Arts in Improving Health and Well-being? A Scoping Review. Health Evidence Network (HEN) synthesis report 67. Copenhagen, WHO Regional Office for Europe, 2019. [PubMed] [Google Scholar]
- 25.Holland AE, Hill CJ, Jones AY, et al. . Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 10: CD008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis A, Cave P, Hopkinson N. Singing for Lung Health: service evaluation of the British Lung Foundation programme. Perspect Public Health 2018; 138: 215–222. doi: 10.1177/1757913918774079 [DOI] [PubMed] [Google Scholar]
- 27.Lewis A, Cave P, Hopkinson NS. Singing for Lung Health: a qualitative assessment of a British Lung Foundation programme for group leaders. BMJ Open Respir Res 2017; 4: e000216. doi: 10.1136/bmjresp-2017-000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenberg RB. Singing lessons for respiratory health: a literature review. J Voice 2017; 32: 85–94. doi: 10.1016/j.jvoice.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 29.Kaasgaard M, Andersen IC, Rasmussen DB, et al. . Heterogeneity in Danish lung choirs and their singing leaders: delivery, approach, and experiences: a survey-based study. BMJ Open 2020; 10: e041700. doi: 10.1136/bmjopen-2020-041700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godtfredsen NS, Sørensen TB, Lavesen M, et al. . Effects of community-based pulmonary rehabilitation in 33 municipalities in Denmark—results from the KOALA project. COPD 2018; 14: 93–100. doi: 10.2147/COPD.S190423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland AE, Nici L. The return of the minimum clinically important difference for 6-minute-walk distance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 335–336. doi: 10.1164/rccm.201212-2191ED [DOI] [PubMed] [Google Scholar]
- 32.Slade SC, Dionne CE, Underwood M, et al. . Consensus on Exercise Reporting Template (CERT): modified Delphi study. Phys Ther 2016; 96: 1514–1524. doi: 10.2522/ptj.20150668 [DOI] [PubMed] [Google Scholar]
- 33.Danish Health Authority . Sundhedsstyrelsen. National Klinisk Retningslinje for Rehabilitering af Patienter Med KOL [National Clinical Practice Guideline on Rehabilitation for Chronic Obstructive Pulmonary Disease]. Copenhagen, Danish Health Authority, 2014. https://danskselskabforfysioterapi.dk/siteassets/dokumenter/de-fagligeselskaber/dsf/publiserede-kliniske-retningslinjer/nationale-kliniskeretningslinjer/nrk-kol.pdf [Google Scholar]
- 34.Spruit MA, Gosselink R, Troosters T, et al. . Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J 2002; 19: 1072–1078. doi: 10.1183/09031936.02.00287102 [DOI] [PubMed] [Google Scholar]
- 35.Bernard S, Whittom F, LeBLANC P, et al. . Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 896–901. doi: 10.1164/ajrccm.159.3.9807034 [DOI] [PubMed] [Google Scholar]
- 36.Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 37.Zeng Y, Jiang F, Chen Y, et al. . Exercise assessments and trainings of pulmonary rehabilitation in COPD: a literature review. COPD 2018; 13: 2013–2023. doi: 10.2147/COPD.S167098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bottle A, Aylin O. Statistical Methods for Healthcare Performance Monitoring. Boca Raton, CRC Press, 2017. [Google Scholar]
- 39.Järvinen TLN, Sihvonen R, Bhandari M, et al. . Blinded interpretation of study results can feasibly and effectively diminish interpretation bias. J Clin Epidemiol 2014; 67: 769–772. doi: 10.1016/j.jclinepi.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 40.Lord VM, Hume VJ, Kelly JL, et al. . Singing classes for chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulm Med 2012; 12: 69. doi: 10.1186/1471-2466-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steiner M, McMillan V, Lowe D, et al. . Pulmonary Rehabilitation: an Exercise in Improvement. London, Royal College of Physicians, 2018. [Google Scholar]
- 42.McCarthy B, Casey D, Devane D, et al. . Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neves LF, dos Reis MH, Gonçalves TR. Home or community-based pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Cad Saude Publica 2016; 32: S0102-311X2016000602001. [DOI] [PubMed] [Google Scholar]
- 44.van Wetering CR, Hoogendoorn M, Mol SJM, et al. . Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 2010; 65: 7–13. doi: 10.1136/thx.2009.118620 [DOI] [PubMed] [Google Scholar]
- 45.Marotta N, Demeco A, Moggio L, et al. . Comparative effectiveness of breathing exercises in patients with chronic obstructive pulmonary disease. Complement Ther Clin Pract 2020; 41: 101260. doi: 10.1016/j.ctcp.2020.101260 [DOI] [PubMed] [Google Scholar]
- 46.Kjærgaard J, Juhl CB, Lange P, et al. . Adherence to early pulmonary rehabilitation after COPD exacerbation and risk of hospital readmission: a secondary analysis of the COPD-EXA-REHAB study. BMJ Open Respir Res 2020; 7: e000582. doi: 10.1136/bmjresp-2020-000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01142-2021.Supplement (897.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01142-2021.Shareable (587.5KB, pdf)