Summary
Animals require specific blends of nutrients that vary across the life course and with circumstances, e.g., health and activity levels. Underpinning and complicating these requirements is that individual traits may be optimized on different dietary compositions leading to nutrition-mediated trade-offs among outcomes. Additionally, the food environment may constrain which nutrient mixtures are achievable. Natural selection has equipped animals for solving such multi-dimensional, dynamic challenges of nutrition, but little is understood about the details and their theoretical and practical implications. We present an integrative framework, nutritional geometry, which models complex nutritional interactions in the context of multiple nutrients and across levels of biological organization (e.g., cellular, individual, and population) and levels of analysis (e.g., mechanistic, developmental, ecological, and evolutionary). The framework is generalizable across different situations and taxa. We illustrate this using examples spanning insects to primates and settings (laboratory, and the wild), and demonstrate its relevance for human health.
Subject areas: biological sciences, physiology, evolutionary biology, Endocrinology
Graphical abstract
Biological sciences; Physiology; Evolutionary biology; Endocrinology
Introduction
What represents a nutritionally balanced diet, and why might the optimal dietary balance vary among species, individuals, and over time? Answering these questions would have implications for the many fields of biology to which nutrition is directly relevant, from life history theory to evolutionary biology, foraging theory and functional, population, and community ecology. Applied benefits include the potential to optimize diets for individuals and across populations, and to design dietary interventions to achieve specific health or animal production goals.
A major impediment to progress has been the complexity of nutrition and nutritional biology. The macro- and micronutrients and other food constituents interact with the multi-layered complexity of an individual’s physiology to yield variable behavioral, health, and life history outcomes (Simpson et al., 2015; Raubenheimer and Simpson, 2016; Guasch-Ferré et al., 2018; Bashiardes et al., 2019). In many basic applications (e.g., foraging theory and life history theory), this complexity is commonly factored out by reducing nutrition to a single dietary component, often energy or protein. In applied nutrition, one approach to meeting the challenge has been to use extensive phenotypic and multi-omic datasets to derive highly personalized dietary predictions based on predictive algorithms (Zeevi et al., 2015; Berry et al., 2020). At lower resolution, public health recommendations and dietary guidelines are provided to entire populations, aimed at ensuring adequate intakes of essential nutrients. Between these extremes have been efforts to stratify populations into subpopulations, age groups, and health categories for whom specific dietary recommendations can be targeted (Ordovas et al., 2018). Each of these approaches has its place, but there remains a need to provide an overarching theory-driven framework within which to consider diet optimization in relation to changing nutritional requirements.
Here, we consider how conceptual and practical challenges of diet optimization can be conceived using the nutritional geometry framework (NGF), an integrative tool from nutritional ecology—the field that applies evolutionary and ecological theory to nutrition (Raubenheimer et al., 2009). NGF is a multi-dimensional framework, which integrates homeostasis theory from physiology and behavior with Darwinian theory to model nutrition in a mixture context (Raubenheimer et al., 2012; Simpson and Raubenheimer, 2012). We first introduce foundational concepts of nutritional geometry, and then review some empirical applications examining dietary balance in animal research. Our goal is to present summaries of key findings that illustrate research into dietary balancing across the life cycle (development, reproduction, and aging), across research contexts (from mechanistic lab experiments to observational ecological studies) and taxa (insects to primates). This broad focus is part through necessity, as there is no single species for which the range of issues we wish to examine has been integrated. It also has benefits, as drawing on disparate examples illustrates the generality of the approach, and we hope will both foster cross-fertilization of ideas across research sub-cultures and encourage deeper integration in single-species studies. We end by considering the relevance of this ecological conception of dietary balance for human health.
The nutritional geometry framework
NGF (Raubenheimer and Simpson, 1997; Raubenheimer, 2011; Simpson and Raubenheimer, 2012) provides a conceptual and quantitative framework that encompasses the interactions among components that comprise the nutritional ecology of animals, such as foods, diets, appetite, physiology, and health and life history outcomes. The NGF is a multi-dimensional framework, which takes account of the fact that many different macro- and micronutrients, as well as other food constituents, may have independent and interactive effects on organisms. It is also an integrative framework, which as we next show enables important nutritional parameters—such as nutritional state, nutrient intake, dietary balance, and nutritional trade-offs—to be interrelated within the same multi-dimensional model.
Nutritional state
Central to NGF is individual nutritional state, which provides a useful level from which to focus upwards or downwards onto any extra- and intra-organismal interactions concerning nutrition. NGF models represent an individual’s nutritional state as a coordinate in an n-dimensional nutrient space, where each dimension represents a nutrient of interest (Figure 1). An organism’s state is typically quantified based on the consumption of the n-nutrients over some relevant time frame, standardized for prior circumstances (Figure 1). Short-term indicators of nutritional state (circulating levels of metabolites and appetite hormones, for example) shape physiological and behavioral responses, which impact phenotypes such as the status of energy stores, growth rates, and development. These phenotypes in turn impact life history traits, such as life span and fecundity, which ultimately define how nutritional phenotypes evolve. NGF provides a framework for quantifying and understanding the ways that short-term nutritional decisions aggregate into longer term nutritional status within the context of selected phenotypes and their functional consequences (Raubenheimer et al., 2012). Whatever time frame is determined relevant, defining nutritional state based on intake in n-dimensions provides an objective and measurable reference point against which to theorize and empirically compare the drivers and consequences of alternative nutritional states, in the context of nutritional mixtures.
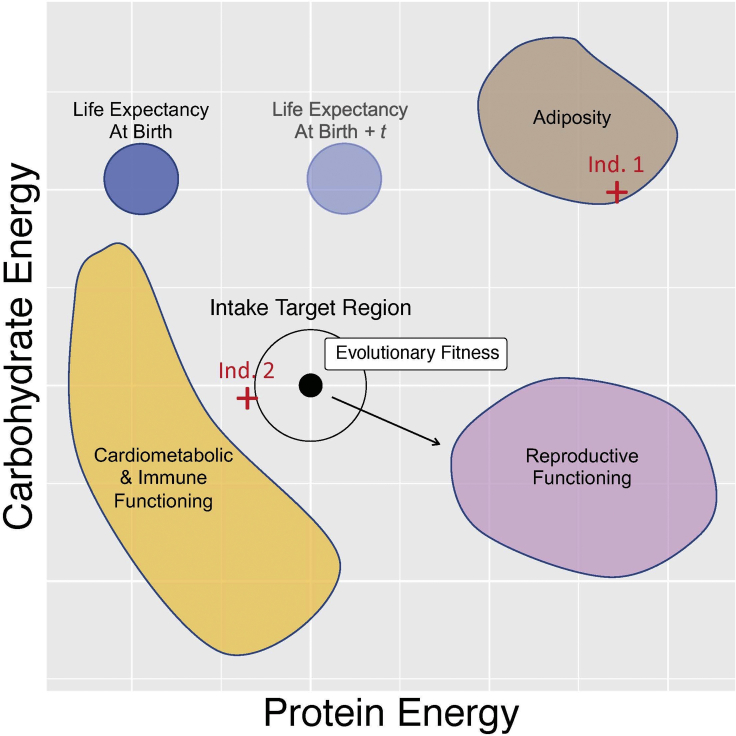
Figure 1.
Hypothetical two-dimensional nutrient space representing protein and carbohydrate. Intakes of two individuals (Ind. 1 and Ind. 2) are shown by the red crosses
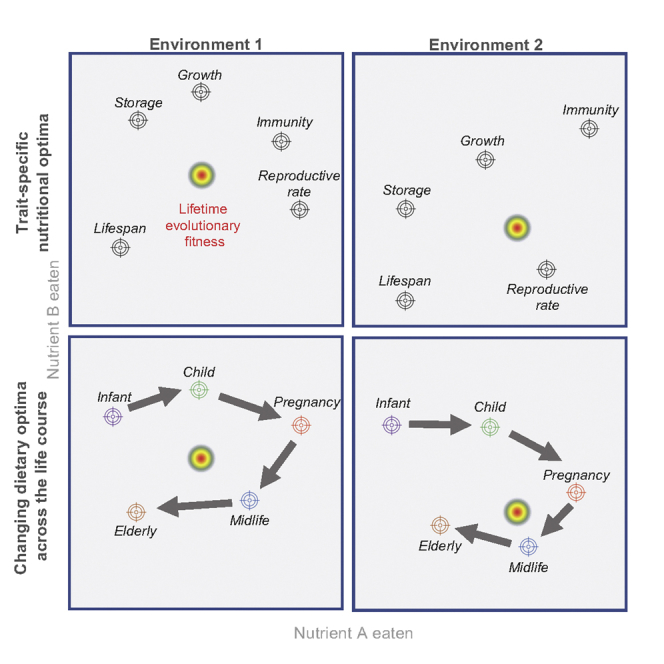
Different colored regions within the space (hence different dietary compositions) are associated with maximization of different phenotypic traits—e.g., adiposity (brown), cardiometabolic and immune functioning (orange), or reproductive functioning (purple). Because the animal cannot simultaneously select more than one dietary composition, diet selection inevitably involves trade-offs where some functional outcomes are served better than others. Evolutionary theory suggests that natural selection should have shaped an organism’s appetite systems such that it selects the diet associated with a state that optimally trades off the different traits to maximize evolutionary fitness (intake target region). The coordinates of trait-specific maxima may change as the organism ages (e.g., maximal life expectancy at birth and at t-years-old could be different). The optimal trade-off between traits may also vary across environments causing the region maximizing fitness to move (black arrow). Relative positions loosely based on published data in mice.
Nutrient intake and a balanced diet
In the nutritional ecology framework, maintaining a balanced nutritional state is, by definition, a fundamental requirement for optimizing performance (in evolutionary terms, fitness). This can be achieved through various means, involving either homeostatic regulation of specific nutrients and nutrient combinations, or non-homeostatic means that rely on correlations in the food environment for ingesting a balanced nutrient intake (Simpson and Raubenheimer, 2001; Raubenheimer and Simpson, 2016). In the simplest scenario, an animal can balance its diet by selecting an environment in which all available foods contain the required balance of nutrients, as might be the case for some parasites. In this case, diet balancing is achieved through habitat selection, and the only dietary regulation necessary is the decisions of when and how much to eat. Alternatively, the animal might select only balanced foods from among a range of options within its habitat, either specializing on a single food or switching foods to meet changing nutrient needs, for example when reproducing. A third possibility is complementary nutrient balancing, in which two or more nutritionally imbalanced foods are combined into a diet that is nutritionally balanced. This could involve using simple behavioral rules such as frequently switching among foods (Bernays and Raubenheimer, 1991; Bernays et al., 1992), or through homeostatic regulation of the intakes of specific nutrients (Raubenheimer et al., 2012).
The strategies and mechanisms for achieving a balanced diet are not mutually exclusive, nor necessarily aligned with organismal complexity—unicellular slime molds, for example, both preferentially select nutritionally balanced foods and combine complementary foods into nutritionally balanced mixtures (Dussutour et al., 2010). Furthermore, a particular animal might use different strategies for balancing different nutritional dimensions of the diet. It might, for example, homeostatically regulate the intake of macronutrients, while meeting its micronutrient needs passively through relying on intrinsic correlations in the foods it eats (Raubenheimer and Simpson, 2016). For reasons discussed below, we suspect that nutrient balancing through homeostatically driven complementary feeding (henceforth HCF) is particularly advantageous, and accumulating evidence suggests it is ubiquitous among species.
HCF has been demonstrated in captive experiments in many species. In some studies, treatment groups provided different food combinations were found to combine the respective options in unique amounts and proportions to achieve a consistent macronutrient intake (Chambers et al., 1995; Raubenheimer and Simpson, 1997; Dussutour et al., 2010; Campbell et al., 2016). In others, animals manipulated into different nutritional states subsequently selected unique combinations of foods such that all treatment groups converged on a common nutritional state (Raubenheimer and Jones, 2006; Lee et al., 2008). The selected intake point, termed the “intake target”, has been demonstrated in some studies to optimize key fitness-related outcomes, such as minimizing mortality and maximizing reproduction (Simpson et al., 2004; Lee et al., 2008; Jensen et al., 2012). Examples of diet balancing through HCF are taxonomically widespread, ranging from slime molds to insects, spiders, fish, rodents, domesticated cats, and humans (reviewed in Simpson and Raubenheimer, 2012). As discussed below, similar results have been reported from observational studies of primates in the wild, for example where populations eating different food combinations ingest a nutritionally similar diet (Rothman et al., 2007; Raubenheimer et al., 2015; Hou et al., 2020) and where individual animals achieve consistent nutrient intakes across foraging days from different food combinations (Johnson et al., 2013).
Regulation to specific intake targets provides an important pointer for identifying what is a balanced diet, and for examining the mechanisms and consequences of nutrient balancing. It also provides a reference point for evaluating how organisms are adapted to respond when confined (e.g., due to ecological causes) to diets that differs in the ratio of nutrients to the targeted ratio. In this scenario, it is forced into trade-offs between accepting deficits of some nutrients and/or ingesting excesses of others (Raubenheimer et al., 2009; Simpson and Raubenheimer, 2012). NGF provides an approach for empirically establishing how such trade-offs are resolved, with important implications both for basic and applied nutrition. Protein leverage is one such example (Simpson and Raubenheimer, 2005; Raubenheimer and Simpson, 2019a). In many animals, including humans, protein intake is regulated more strongly than fat and carbohydrates. Consequently, on imbalanced diets with low protein:non-protein (i.e., non-protein = fat + carbohydrate) ratios, fat, carbohydrate, and total energy are overconsumed in an attempt to meet the protein target. Implications of protein leverage for human health are further discussed in the final section.
Dietary balance and functional trade-offs
NGF can help elucidate the functional reasons why organisms select specific intake targets. This is done by representing the effects of nutritional states as response surfaces mapped either onto amounts of nutrients eaten (amounts-based response surfaces; Figure 1), or against the proportions of nutrients in the diet (proportions-based response surfaces; Raubenheimer, 2011). Relevant data may be derived from experiments in which animals are confined to diets designed to vary systematically in focal nutrient dimensions (Lee et al., 2008; Solon-Biet et al., 2014). Surfaces may also be estimated from observational data (e.g., nutrition surveys or, as illustrated below, national food supplies) with the usual caveats regarding causation (Raubenheimer and Simpson, 2016).
An important finding from nutrition research is that nutrients can affect different traits in different ways, thus suggesting the concept of “trait-specific nutrient targets” (Figure 1). This extends from the fact that tissues and organs differ in their metabolic profiles (Berg et al., 2002), energetic demands (Elia and Livesey, 1992; Wang et al., 2010), and nutritional compositions. For example, in Drosophila, the ovary and brain use amino acids in a manner which is representative of the average across the whole exome, whereas midgut and testes are outliers (Piper et al., 2017). Through evaluating trait-specific optima, NGF studies have enabled intra-organismal nutritional conflicts and their propagation from cells and pathways up to the level of life history functioning to be understood. In the case of mice and Drosophila, the lifelong macronutrient intake that supports maximal reproductive function is higher in protein and lower in carbohydrate than that which supports maximal life span (Lee et al., 2008; Solon-Biet et al., 2014, 2015), although these differences can be minimized by adjusting the balance of key nutrients such as essential amino acids (Piper et al., 2017) and sterols (Zanco et al., 2021).
It is potentially a complex challenge for an organism to select a diet that satisfies conflicting nutritional requirements among different traits, or a diet that achieves the constellation of trade-offs that minimizes the cost. This is because the organism cannot simultaneously inhabit two coordinates within the space, and a diet that is optimally balanced with respect to one trait may be imbalanced with respect to another. Evolutionary theory dictates that the ultimate arbiter of these trade-offs, which determines the weighting afforded to each trait when competing for access to appetite control systems, is evolutionary fitness (Figure 1). A balanced diet is thus the diet providing the overall mixture that best balances function-specific requirements to maximize fitness. As discussed above, selection is expected to shape appetite systems to enable the animal to reach the region of the nutritional space representing the optimal balance of mixtures (i.e., the intake target).
Tracking moving targets
The dietary optimum that maximizes fitness is dynamic across multiple timescales, changing in the short term in response to physiological and environmental demands; in the medium term throughout development with growth, reproduction, and senescence; and in the longer term across generations, both through parental epigenetic effects and genetic evolution (Raubenheimer et al., 2012; Simpson and Raubenheimer, 2012).
An example of an environmentally imposed shift in nutritional targets is where the risk of predation increases, in which case the region in nutrient space maximizing fitness may shift toward reproductive output (Hosking et al., 2019) (black arrow) (Figure 1), or to account for the costs of vigilance, defense, and escape, as reported in field experiments on grasshoppers (Hawlena and Schmitz, 2010; Schmitz et al., 2010). Alternatively, transient shifts in the position of the target intake associated with maximal fitness could be caused by pathogens and parasites, which constitute nutritional sinks (Ponton et al., 2011) and require greater nutritional investment in immune responses. For example, in caterpillars, the optimal macronutrient balance changes with viral infection in a manner that reflects optimization of key immune parameters (Cotter et al., 2010). There are many examples demonstrating that organisms alter diet selection to meet such circumstance-specific nutrient demands. For example, infection elicits an adaptive shift in diet selection by caterpillars toward a macronutrient balance that supports survival under infection (Lee et al., 2006). An example involving an abiotic challenge, changes in environmental temperature, is given below for a primate.
Even where the environment is stable , the optimal dietary composition is likely to change over the life course, for two reasons. First, nutritional requirements change with growth, development, reproduction, and senescence (Levine et al., 2014; Solon-Biet et al., 2014; Liu et al., 2017; Senior et al., 2019; Pontzer et al., 2021), meaning trait-specific intake targets are likely to shift (Figure 1, blue circles). Second, at different life stages, the relative importance of traits as determinants of evolutionary fitness will vary. For example, early in life, traits associated with robust and timely growth will be prioritized, whereas later in life maintaining musculoskeletal health may become an increasingly influential component of fitness (Le Couteur and Simpson, 2011). Fetal demands in live-bearing species represent a transient nutrient sink that is a “scheduled” component of the life history. Live bearing impacts the nutritional requirements of the parent throughout pregnancy and, often postnatally, as illustrated for rhesus macaques below.
Mechanisms of diet balancing
In general, homeostatic tracking of moving intake targets requires three things (Simpson and Raubenheimer, 2012; Raubenheimer and Simpson, 2019b): 1. An ability to assess the nutritional composition of foods (e.g., through gustation); 2. The capacity to measure current nutritional state; and 3. A mechanism for integrating food composition with nutritional state to produce appropriate feeding responses. Over recent years, nutrient-specific appetites have emerged as a central component of nutrient balancing, and it is on these that we will henceforth focus.
While most work has been done on appetites for the macronutrients (protein, fat, and carbohydrate), specific appetites for the micronutrients sodium and calcium have been demonstrated (Schulkin, 1991; Tordoff, 2001; Simpson and Raubenheimer, 2012) and it is likely that others also exist. These appetites must work together, with some taking precedence over others (as occurs with protein leverage), to guide animals to balance their nutrient intake (Simpson and Raubenheimer, 2012; Raubenheimer and Simpson, 2016, 2020).
The physiological mechanisms controlling nutrient appetites is an area of active research. In mammals, including humans (Hill et al., 2020), fibroblast growth factor 21 (FGF21) has emerged as a key signal in the control of macronutrient balance. The liver is a major site of production, with FGF21 acting via the brain on macronutrient selection and metabolic physiology (Flippo and Potthoff, 2021). Nutritional geometry was used to comprehensively map the nutritional circumstances in mice under which FGF21 is increased (Solon-Biet et al., 2016). Low protein intake was the principal stimulus, irrespective of total energy intake, with FGF21 levels highest under a combination of low protein and high carbohydrate intakes (Solon-Biet et al., 2019). There are reports that FGF21 specifically increases intake of protein (Hill et al., 2019) and reduces response to sweet solutions (Flippo et al., 2020; Jensen-Cody et al., 2020), both of which could contribute to macronutrient-balancing behavior. There is a link between protein status, FGF21, and the gut microbiome (Martin et al., 2021), likely mediated by the microbial community acting both as a sink and source of essential amino acids and thereby impacting host protein status. The functional guild structure of the microbial community is, in turn, shaped by host diet as well as ecological interactions among species, driven in part by whether they derive their source of dietary nitrogen from food in the gut or from host secretions (Holmes et al., 2017).
Studies of insects have demonstrated key sensory, central neural, and humoral pathways controlling carbohydrate and protein appetites (Simpson et al., 2018; Münch et al., 2020). Barton Browne (1995) elaborated two categories of processes employed in appetitive mechanisms for tracking changing nutritional requirements: “demand-driven” and “non-demand-driven”. In the first of these, increased demands for specific nutrients (e.g., with growth or reproduction) elicit signals that increase the salience of cues associated with these nutrients in the environment. In turn, selective feeding on foods rich in the required nutrients. Detection of some of these environmental cues is innate, for example nutrient molecules such as sugars, salts, and amino acids that directly activate dedicated taste receptors. Other sensory cues are associatively learned, contributing to “learned nutrient-specific appetites” (Baker et al., 1987). By contrast, non-demand-driven nutrient-specific appetites are switched on at an appropriate stage in development, in some cases, anticipating the change in requirements by a feedforward mechanism. It is worth emphasizing that any particular mechanism need not be exclusively demand-driven or non-demand-driven but could involve an interaction of demand-driven and non-demand-driven processes.
An example involving a combination of both demand- and non-demand-driven processes is the specific appetites for protein and salt that are induced after mating in female Drosophila melanogaster. Male flies introduce a signaling peptide into females’ reproductive tracts during mating, which acts as the primary trigger for eliciting protein and salt feeding to support egg production (Walker et al., 2015). This triggering is a non-demand-driven process, activating the appetites but not of itself regulating the amounts of protein and salt ingested. The male peptide also renders the female sexually unreceptive and activates egg-laying. Once protein feeding has been triggered, however, the actual amount of protein consumed by the fly is subject to demand-driven processes involving feedback mechanisms that include amino acid signaling pathways (Ribeiro and Dickson, 2010; Vargas et al., 2010).
NGF experiments have also demonstrated nutrient-specific learning in insects, in which odors (Simpson and White, 1990; Gadd and Raubenheimer, 2000) or colors (Raubenheimer and Tucker, 1997) are associated with specific nutrients and preferentially targeted when the animal is deficient in the associated nutrient. Such learning increases foraging efficiency, through enabling the use of remote cues to identify foods that would currently be most appropriate for balancing the diet and avoiding those that would imbalance the diet.
Although relatively new, the GFN and related concepts discussed above have guided a contemporary generation of integrative nutritional research that crosses disciplinary boundaries to engage pure and applied nutrition-related challenges within an ecological/evolutionary framework. We next present a selection of examples to illustrate the application of NGF to examine the concepts discussed above across a range of taxa (fruit flies, rodents, non-human primates, and humans), contexts (lab, field, and international databases), questions (mechanistic, ecological, and functional), and life stages (early development, reproduction, and senescence).
Coordination of appetite, growth, and developmental programming in larval D. melanogaster
Our first example concerns studies disentangling the biological mechanisms of nutrient demand-supply relationships in the developing larvae of D. melanogaster. Under ideal nutritional conditions, larvae increase approximately 500-fold in mass from the time they hatch from an egg until they initiate metamorphosis (Mirth et al., 2005; Shrikanth and Bobji, 2014). Most of the growth occurs in the third and final instar, at the end of which, larvae initiate wandering, leaving the food in search for a site to pupariate and begin metamorphosis into an adult fly.
Under nutrient-poor conditions, especially on protein-dilute diets, larvae increase food intake as a compensatory response and demonstrate marked protein leverage, most notably in the third instar (de Carvalho and Mirth, 2017). Early third-instar larvae regulate more tightly to their protein target than do more developed larvae (de Carvalho and Mirth, 2017). When unable to fully compensate by increased food intake, the physiology of the larva changes to reduce growth rates and extend the duration of the larval period to buffer against reduced growth. Larval diet influences the size of the larval body, but also shapes adult traits that impact on evolutionary fitness such as body weight, body composition, various cuticular structures (Poças et al., 2020), and ovary size in females (which determines an upper limit on fecundity) (Mendes and Mirth, 2016).
When D. melanogaster larvae are fed across a range of diets designed using NGF, body and ovary size are largest on intermediate protein, low carbohydrate diets (Rodrigues et al., 2015; Kutz et al., 2019). Development time, on the other hand, is shortest on intermediate protein, intermediate carbohydrate foods (Rodrigues et al., 2015; Kutz et al., 2019). Because life history traits differ in the way they respond to larval nutrition, there is a clear nutritional trade-off of the nature described above. When offered a choice of foods, larvae regulate their protein and carbohydrate intake toward diets that minimize development time at the expense of failing to attain maximal body and ovary size (Rodrigues et al., 2015).
Diet modifies larval development through conserved signaling networks
An advantage of studying D. melanogaster larvae is being able to relate life history traits as NGF response surfaces to the underlying signaling pathways. Several nutrient-sensitive pathways respond to intracellular levels of macronutrients to control cell division and growth. Among these pathways is general control nonderepressible 2 (GCN2), a kinase that binds to uncharged tRNAs under conditions of amino acid deficiency (Sonenberg and Hinnebusch, 2009). Once activated, GCN2 initiates a signaling cascade that inhibits protein synthesis, in part by interacting with another amino acid-sensing pathway, the mechanistic target of rapamycin (mTOR) pathway. When larvae are fed on a diet deficient in an essential amino acid, they reduce food intake and increase locomotor activity (Bjordal et al., 2014). This behavior depends on GCN2 signaling in a set of three pairs of dopaminergic neurons in the larval brain that repress GABA signaling (Bjordal et al., 2014). It is not known whether these same cells respond to quantitative changes in protein concentration, or if they are involved in balancing protein intake over that of carbohydrate.
As the larva is growing, the concentration of available nutrients is sensed in the gut and the fat body—a tissue analogous to the adipose tissue and liver of mammals (Colombani et al., 2003; Redhai et al., 2020). The fat body releases different peptides depending on dietary sugar (CCHamide2 and Neural Lazarillo) (Sano et al., 2015) or amino acids (Eiger, Stunted, and Growth-Blocking Peptides 1 and 2) (Agrawal et al., 2016; Delanoue et al., 2016; Koyama and Mirth, 2016). These peptides regulate the secretion of at least some of the seven insulin-like peptides (ILPs) (Brogiolo et al., 2001). ILP2, 3, and 5 are produced by neurosecretory cells in response to starvation (Ikeya et al., 2002). The fat body secretes ILP6 when larvae are starved, or when they reach the wandering stage and cease feeding (Okamoto et al., 2009; Slaidina et al., 2009; Chell and Brand, 2010). This peptide acts to sustain minimal growth in starved animals, allowing completion of metamorphosis albeit at smaller size. Using the NGF, Post and Tatar (2016) showed in adult Drosophila that ILPs respond differently to changes in dietary protein and carbohydrate. ILP2 expression was highest in both low-protein, intermediate-sugar diets and high-protein, intermediate-sugar diets. ILP6 expression was highest in the low-protein, high-sugar diets. Although Drosophila has only one insulin receptor for ILP1-7 (Brogiolo et al., 2001), downstream signaling varies depending upon the ILP (Post et al., 2018), thus mediating diet-specific cellular responses.
Nutritional effects depend on the target organ and larval age
In Drosophila, some organs are less affected than others by dietary balance (a process known as organ sparing) (Nijhout, 2003; Nijhout et al., 2014). For example, diet does little to alter the sizes of the male genital discs (the larval primordia for adult genitalia) or the brains of either sex (Cheng et al., 2011; Koyama et al., 2013; Shingleton and Frankino, 2013). During starvation, brain sparing is possible because glial cells surrounding the growing nervous system secrete a molecule called Jelly Belly which acts via Alk to stimulate insulin signaling (Cheng et al., 2011). The genital structures in males achieve the same sparing effect by modulating insulin signaling in a different way. Male genitalia express low levels of a downstream negative effector of insulin signaling, Forkhead BoxO (FoxO) (Tang et al., 2011). FoxO is a transcription factor that normally represses growth under low nutrient conditions (Junger et al., 2003). Because the genital disc expresses low levels of FoxO, reducing insulin signaling upstream of FoxO has little effect on growth (Tang et al., 2011). Hence, in addition to differences in circulating levels of ILPs, organs can tune how much they respond to these systemic signals.
In addition to differences in sensitivity to diet across organs, organs also change their sensitivity to nutrition with time. Starving larvae early in the third instar markedly dampens growth of wing imaginal discs and ovary (Shingleton et al., 2008; Mendes and Mirth, 2016). Starvation later in development has less of an effect. This is because in the third instar a small pulse of the steroid hormone ecdysone changes the way organs respond to diet (Koyama et al, 2014). Before this pulse occurs, wing and ovary growth depend primarily on insulin signaling (Shingleton et al., 2008; Mendes and Mirth, 2016). Once this pulse has occurred, growth of both organs occurs in response to a combination of insulin and ecdysone signaling, thereby rendering growth less sensitive to nutrition.
Nutrition also differentially impacts development across larval stages in D. melanogaster. Starvation or poor nutrition in the first, second, and early third instar delays development, but from mid-third instar onwards it accelerates the onset of metamorphosis (Beadle et al., 1938; Mirth et al., 2005; Shingleton et al., 2005; Stieper et al., 2008). Potentially, this reflects that first, second, and early third-instar larvae regulate their protein and carbohydrate intakes in a similar manner, while later in the third instar, larvae are less stringent at regulating protein (de Carvalho and Mirth, 2017). Alternatively, the intake target for nutrients may change across stages, reflecting a shift in diet composition across the life course as the rotting fruit into which eggs are laid continues to decay, leading to increasing dietary protein content in the form of microbes (Morais et al., 1995; Matavelli et al., 2015; Silva-Soares et al., 2017).
Nutrient-specific appetites track changes in nutrient requirements associated with reproduction: examples from non-human primates in the wild
Moving beyond development, non-human primates are an excellent system for examining how the concepts outlined in the opening sections apply in natural environments where appetites and the metabolic systems they feed evolved (Raubenheimer et al., 2015). Somewhat surprisingly, tracking changes in nutrient intake regulation is in some respects more feasible in non-human primates than in lab-dwelling Drosophila larvae. In the wild, primates readily habituate to the presence of researchers, enabling detailed observations of dietary intakes over prolonged periods. Primates are also taxonomically, and in many respects biologically, relevant to humans, and are widely used as models for biomedical research (Havel et al., 2017).
Several studies have shown that non-human primates in the wild target a diet that provides a specific balance of macronutrients. That this is driven by active nutrient selection, rather than passively by the distribution of nutrients within the food environment, is suggested by evidence that macronutrient intakes remain constant when feeding on different combinations of foods. For example, the annual diets of mountain gorilla populations in Bwindi and Virunga national parks are nutritionally similar even though they subsist on different food combinations (Rothman et al., 2007; Raubenheimer et al., 2015). At the level of individuals, a female chacma baboon fed on different combinations of ca. 90 foods but composed a daily diet with similar macronutrient ratios over 30 consecutive days (Johnson et al., 2013). The same was shown for a golden snub-nosed monkey (Rhinopithecus roxellana) whose daily diet was recorded over 32 consecutive days (Hou et al., 2021).
Two recent studies have shown that the diets self-selected by wild primates change to track the macronutrient requirements associated with reproduction. In temperate golden snub-nosed monkeys, maintaining fat stores through winter is an important part of the reproductive strategy, enabling early reproduction during the brief spring (Hou et al., 2020). Guo et al. (2018) used close-range thermal images and biophysical equations to compare net heat dissipation to the surrounding environment by this species. Full day feeding observations were made in the warm spring and cold winter to quantify daily diet. To ensure that any differences in dietary intakes reflected feeding choices of the monkeys, and not constraint due to seasonal differences in food availability, the study focused on a population that was supplementary fed to excess in both seasons. Results showed that metabolizable energy intake in winter was 1.8 times that in spring. As predicted, the additional calories eaten in the cold season came entirely from increased intake of the primary metabolic fuels—carbohydrates and fats—with winter and spring protein intakes being statistically indistinguishable. Remarkably, the seasonal difference in net heat dissipation (329 kJ per Kg of metabolic mass) almost exactly matched the seasonal difference in carbohydrate and fat intake (326 kJ) (Figure 2A).
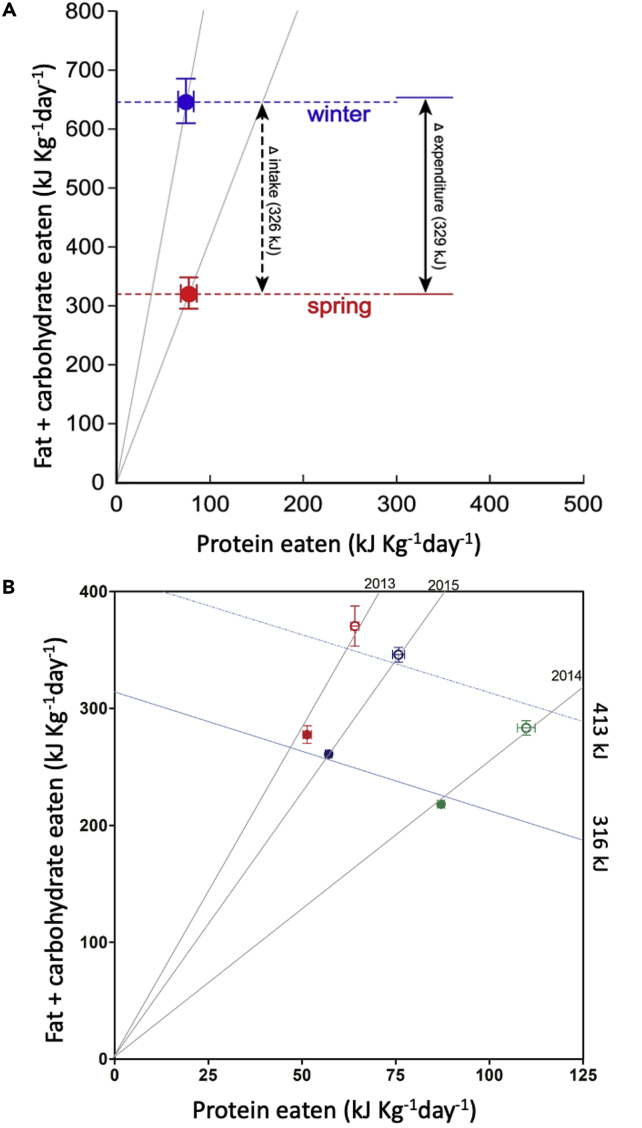
Figure 2.
Nutrient-specific foraging in wild monkeys
(A) Daily macronutrient intakes by wild golden snub-nosed monkeys in spring and winter. Also shown is the seasonal difference in the cost of thermoregulation (329 kJ/Kg), which closely matched seasonal difference in fat and carbohydrate intake ( 326kJ/Kg). Modified with permission from Guo et al. (2018), (B) Macronutrient intakes by wild lactating (hollow symbols) and non-lactating (solid symbols) rhesus macaques in the spring of three successive years (red = 2013, green = 2014, blue = 2015). The negative diagonal lines show average energy intakes by the two categories of monkey across the three years. All data are presented as bivariate mean (±SE). Reproduced with permission from from Cui et al. (2018).
This study indicates that golden snub-nosed monkeys specifically target additional fats and carbohydrates to offset the costs of thermoregulation in winter. It does not, however, provide an example of animals tracking moving targets in a situation where regulatory systems deal with natural variation in food and nutrient supply, because the population was supplementary fed. By contrast, Cui et al. (2020) examined the tracking of intake targets in an unmanipulated, natural context, comparing the daily diets of wild rhesus macaques (Macaca mulatta tcheliensis) in the reproductive season (spring) of three consecutive years (2013–2015). Daily energy intakes of adult males and non-reproductive females did not differ (318 kJ/kg body mass), but the intake of lactating females was significantly and markedly higher (413 kJ/kg) (Figure 2B). Because lactation is the most nutritionally and energetically demanding period in the life of a female primate, and the reproductive and non-reproductive monkeys shared the same habitat and hence had access to the same foods, this result shows that lactating females change their diet specifically to meet the increased demands of milk production.
Unlike the monkeys studied by Guo et al. (2018), the lactating rhesus macaques did not select a diet that differed in macronutrient balance from non-reproducing adult monkeys, only in metabolizable energy intake. A subsequent study showed this also to be true in a comparison of pregnant and lactating females from the same population (Cui et al., 2020). This constancy of selected macronutrient ratios illustrates the important role of ecological constraint in the extreme temperate habitat of the study population. Seasonal and inter-annual variation in the availability of the staple source of carbohydrates and fats (here acorns) tightly constrains the macronutrient balance of the available diet; across three successive springs, this ranged from 12.9% to 30.1% of energy from protein (Cui et al., 2020). Dietary macronutrient ratios were therefore determined by the ecological availability of fats and carbohydrates in a particular year, and not an outcome that could be freely altered by dietary selection.
At the evolutionary timescale, this pattern of macronutrient regulation suggests these rhesus macaques have adapted to the ecologically imposed dietary variation in an interesting way. That energy intake was maintained near constant across diets varying so widely in macronutrient balance demonstrates these monkeys offset ecologically imposed fat and carbohydrate deficits by ingesting extra protein energy (Raubenheimer and Simpson, 2019a). The only reduction in energy intake observed across the 17.6% variation in dietary protein was in lactating females, which was 7.5% lower in the year when acorns were scarcest (dietary protein 30.1%) relative to the year they were most abundant (dietary protein 12.9%) (Cui et al., 2020). Most other species of primates studied (Felton et al., 2009), including humans (Gosby et al., 2011; Campbell et al., 2016; Raubenheimer and Simpson, 2019a), avoid over-ingesting protein, and therefore experience marked reductions in energy intake when restricted to diets with a high protein to non-protein energy ratio. In humans, an equivalent drop in energy intake (7.8%) was associated with only 4.9% increase in protein (from 13.3% to 18.2%) (Martínez Steele et al., 2018). Furthermore, the monkeys successfully reproduced in all three years, albeit at a reduced rate on the 30.1% protein diet (Cui et al., 2020), suggesting this population has adapted to tolerate wide variation in dietary macronutrient ratios. Although the details have yet to be established, a likely mechanism is increased metabolic flexibility for inter-changeably utilizing fats, carbohydrates, and proteins in energy metabolism (Cui et al., 2018).
These primate examples demonstrate applications of the NGF to animals in the wild, in the context of the primary optimization criterion for natural selection, reproduction. We next demonstrate applications in the context of an outcome most likely to be the target of dietary optimization for modern humans, a long and healthy life span.
Diet, health span, and life span in rodent model systems
There are numerous examples of the changing nutritional requirements of organisms as they progress through their developmental stages (Wu, 2016) (albeit few as well studied as Drosophila and even fewer in the wild). Less frequently considered, however, is how the optimal diet changes across the life course post maturity, and how dietary impacts accumulate across the life course to influence health and longevity. These issues have been most extensively examined in rodent model systems.
Dietary macronutrients and age-specific mortality
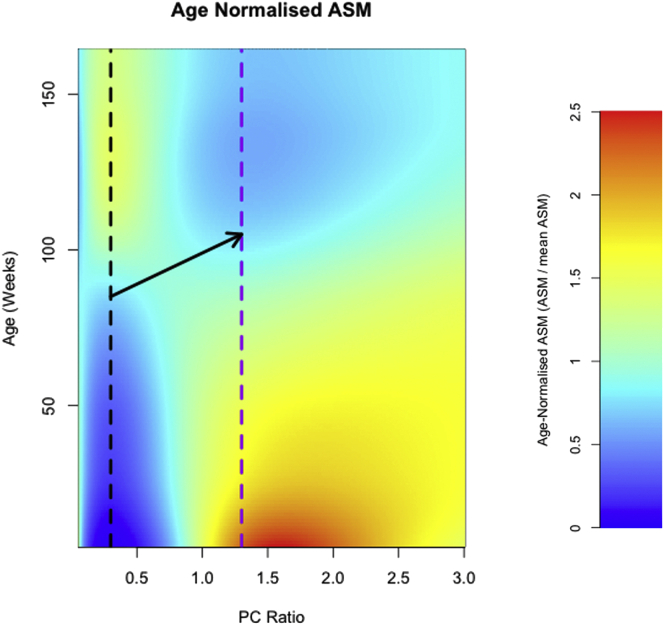
One way of defining how diet impacts organismal health at different ages is through its effects on all-cause age-specific mortality (ASM). Senior et al. (2019) modeled the effects of macronutrients on the lifetable of ad libitum-fed mice using data from an NGF experiment. There were clear effects of macronutrient balance on ASM and the diet composition that minimized the probability of death was not the same in early life as it was in later life. In Figure 3, we have re-formulated the results from Senior et al. (2019), to show the estimated age-normalized ASM as a function of the ratio of dietary protein to carbohydrate fixing overall energy density and fat content. A diet with a relatively low ratio of protein to carbohydrate (vertical black dashed line) has low mortality in early and mid-life. In contrast, a diet with a higher ratio of protein (purple dashed line) has high mortality during mid-life, but comparatively low late-life mortality. Whether mice specifically track these changing optima by increasing dietary protein as they enter old age (i.e., follow the black arrow) remains to be seen. Failure to track a shifting optimum could be indicative of a deterioration of nutritional feedback that accelerates wider systemic deterioration via nutritional mismatch; an interesting “systems dimension” insight into the biology of aging.
Figure 3.
Estimated age-specific mortality of C57BL/6 mice (mixed sex), as a function of the ratio of dietary protein to carbohydrate (PC ratio)
We assume an energy density of 15.77 kJ/g of food, with fat fixed at 3.77 kJ/g. The vertical black and purple dashed lines constitute a ratio of 0.3 (low PC) and 1.3 (high PC), respectively. Redrawn from data in Senior et al. (2019).
Nutritional “memory” effects and dietary restriction
A logical conclusion from the above example (Senior et al., 2019) is that minimizing mortality across life might be possible by adjusting diet composition as a function of age. For example, in mice and possibly humans (see below), increasing the intake of energy from protein at the onset of old age may be beneficial (black arrow Figure 3). However, confirming any such benefit requires diet switching experiments. A key aim of such studies is to measure the extent to which the effects (whether deleterious or beneficial) of a previous diet persist after experimental subjects are switched to a new dietary regimen. The most well-studied phenomenon to date in this regard has concerned dietary restriction (DR) in flies and mice.
DR has been studied across a range of taxa from yeast to non-human primates and is arguably the most robust nutritional intervention to increase life/health span (Anderson et al., 2009; Fontana et al., 2010; Mattison et al., 2012; Mercken et al., 2012). Most studies have been undertaken in rodents, where animals are given an aliquot of food each day that is a fraction (typically 50%–80%) of the amount eaten by ad libitum-fed control animals and supplemented with micronutrients. Consequently, DR animals are often fasting for protracted periods every day but are not malnourished, and usually have reduced body fat and are hypothermic (Mattison et al., 2012; Mitchell et al., 2015).
The earliest studies of DR were commenced from weaning in rats, which had delayed growth and increased life span (McCay et al., 1935; Le Couteur and Simpson, 2018). Later studies found that DR had the same effect, regardless of whether it was commenced before or after weaning, indicating that DR was acting independently of delayed early-life growth (Weindruch et al., 1986). In rodents, late-life commencement of DR also has some impact on health (for example reduced tumorigenesis) and possibly an extension in remaining life span (Spindler, 2005; Goto, 2006). A recent landmark study evaluated the effects of switching in late life either from a lifelong ad libitum-fed diet to a DR diet, or vice versa (Hahn et al., 2019). Mice on lifelong DR that were switched to ad libitum diets in old age quickly lost the life span extension seen with animals that were maintained on DR. On the other hand, mice that commenced DR in late life had some minor health benefits. It was concluded that there is a nutritional “memory” embedded in fat tissues that attenuates any effects of late-life dietary manipulation. These results contrasted with earlier studies on Drosophila, where there is no apparent memory effect of the pre-switch diet (Mair et al., 2003). Fruit flies switched to a restricted diet showed an immediate fall in their risk of death. By contrast, flies shifted from DR to a control diet rapidly exhibited a higher risk of mortality.
One problem with the DR paradigm is that the control and treatment groups differ in a suite of dietary attributes making it difficult to identify the principal driver. Currently, it is thought that the effects of DR derive principally from the periods of intermittent fasting (Mitchell et al., 2019; Di Francesco et al., 2018; Longo et al., 2021; Pak et al., 2021). However, similar metabolic effects can also be achieved without calorie restriction by reducing protein intake, dietary levels of key amino acids (Hine et al., 2015; Green and Lamming, 2019; Solon-Biet et al., 2019; Yap et al., 2020; Richardson et al., 2021), or by decreasing the ratio of protein to carbohydrate in the diet (Solon-Biet et al., 2014; Le Couteur et al., 2016). Establishing whether these factors have age-specific effects and/or carry a nutritional memory is largely uncharted territory. Recently, it was shown in C57BL/6J mice that restricting dietary branched-chain amino acids beginning in mid-life delayed frailty and promoted metabolic health but did not extend life span. If commenced early in life, however, BCAA restriction led to a 30% increase in life span of male but not female mice (Richardson et al., 2021).
Human health
As in other species, the nutritional requirements of humans change across the life course. However, the detailed time course of changing nutritional requirements is poorly understood, and even less well integrated in public health practice. At one end of the spectrum, as is typical of primates, human breast milk has a lower ratio of protein to fats and carbohydrates than normal diets at any other stage of the life cycle. At the other end of the life course, increased protein is recommended in older humans, primarily to maintain muscle and bone mass and/or based on measures of nitrogen balance (Bauer et al., 2013). One epidemiological study found that increased animal-based protein intake was associated with reduced cancer and mortality in people over 65 years, but increased diabetes in younger adults (Levine et al., 2014). Another epidemiological analysis suggested that dietary protein should be low in middle age but increased in older people to minimize the risk of Alzheimer disease (Studnicki et al., 2019). Whether health improvements from increased protein in old age translate to improved survival is less clear, with little evidence supporting dietary protein intakes beyond 20% of dietary energy (Pedersen and Cederholm, 2014). The reason why increased protein intake might be beneficial in advanced age could reflect an increasing protein target resulting from a decline in protein efficiency with age, accompanying insulin resistance, increased rates of protein catabolism, and elevated hepatic gluconeogenesis (Victoria et al., 2017; Barclay et al., 2019).
Over recent years, the NGF and its applications to other species, such as those reviewed above, has provided a framework to begin examining these issues in humans. One example has been to apply NGF approaches for tracking age-specific mortality as discussed above for rodents (Senior et al., 2019), to human population data. No study has attempted to directly assess entire patterns of ASM as a function of diet in humans, and of course, the logistical challenges to doing so are perhaps insurmountable. An alternative approach is to assess whether international variation in the supplies of nutrients is able to predict the patterns of ASM of nations (Gage and O’Connor, 2009). A recent NGF analysis of this question found that, after correction for economic factors, macronutrient supplies are a useful predictor of patterns of ASM (Senior et al., 2020). It was found that in early and middle life, a supply of around 16% protein and 40% to 45% each of carbohydrates and fats with a net 3500 kcal/cap/day had the lowest levels of mortality. However, after the age of 55, decreasing protein to 11% of supply, and fat to 25% and substituting these with carbohydrates was predicted to have minimal mortality.
It is noted that these results are associative, and causality should not be assumed. While these findings do not entirely reflect the preclinical and epidemiological data discussed above in relation to increased late life requirements for protein, they serve to demonstrate that dietary macronutrients, be it at the level of supply or intake, likely relate to mortality in an age-specific manner. Additionally, they have an ecological relevance not found in preclinical or epidemiological data. That is, variation in international food supplies can arguably be considered a more direct proxy for variation in food environments than can individual diets. In ecological studies, such as those of primates discussed above, this amounts to the distinction between ecological surveys of foods available within a habitat vs. the diets resulting from the animals’ choices from among available foods. A key finding in such studies is that realized diets are significantly influenced by the quantity, quality, and availability of foods comprising the food environment. However, this influence can only be understood in relation to biological regulatory priorities of the animal, encapsulated in NGF models as intake targets. Recent studies have applied this perspective to examine how human biology interacts with food environments to influence diets and health (Raubenheimer and Simpson, 2020), with significant implications for understanding the relationships between diet and health across the life course.
The most extensively researched application of NGF to human health to date is the protein leverage hypothesis (PLH). Randomized control trials have shown that in humans, as in several other primate species including chimpanzees (Uwimbabazi et al., 2021) and orangutans (Vogel et al., 2015), protein intake is regulated more strongly than the intake of fat and carbohydrate. Consequently, fat, carbohydrate, and total energy intake are inversely related to the ratio of protein to fat and carbohydrate in the diet (“protein leverage”). Any factor that reduces this ratio will cause energy overconsumption and potentially obesity as an inadvertent outcome of the drive to meet the protein target (Simpson and Raubenheimer, 2005). The PLH states that the strong protein appetite in humans has interacted in this way with protein dilution by fats and carbohydrates in modern food environments to drive energy overconsumption and obesity (Simpson and Raubenheimer, 2005; Raubenheimer and Simpson, 2019a). Several studies have implicated the interaction of protein leverage with transitions in food environments toward increased availability of energy-dense, fat, and carbohydrate-rich industrially processed foods as a driver of the obesity epidemic (recently reviewed in Raubenheimer and Simpson, 2019b).
If correct, the PLH would have significant implications for understanding, predicting, and managing human diets and their consequences. One implication is that under protein leverage, energy intake will primarily be a function of the relationship between the protein target and the composition of the diet. Hence, excess energy intake could be driven by reduced dietary protein density, increased protein requirements, or both (Simpson and Raubenheimer, 2005) (Figure 4). The implications of this for nutrition across the human life course are obvious: any developmental or other change in the protein target, unless compensated by a counterbalancing change in diet composition, could impact energy intake. We present two examples where this NGF perspective might elucidate significant associations between diet and health, at different ends of the age spectrum.
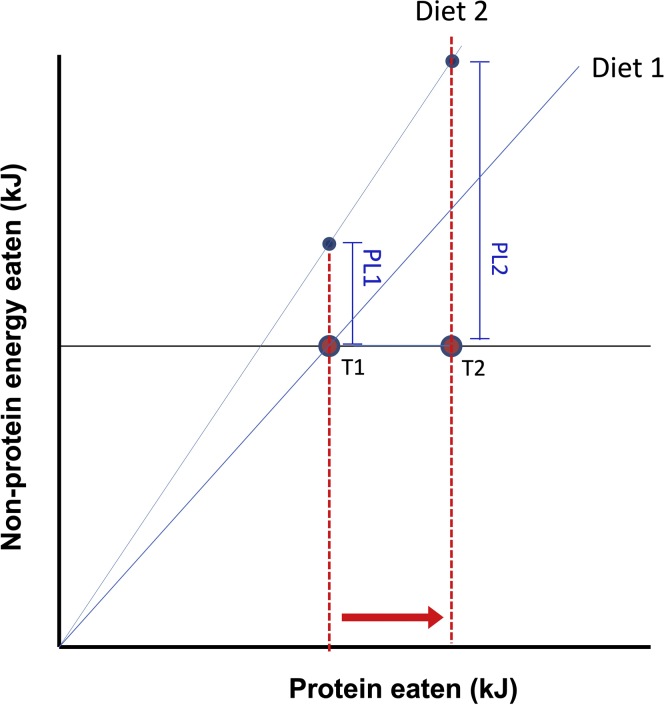
Figure 4.
Effect on protein leverage (PL) of increased protein requirements relative to non-protein energy
T1 represents the reference intake target, achievable on a diet with protein:non-protein energy ratio shown by the blue line labeled Diet 1. T2 represents an increased protein (P) requirement due, for example, to reduced nitrogen efficiency caused by an early history of being fed high protein infant formula (Raubenheimer et al., 2014). On a protein-dilute diet (Diet 2), the increased P requirement amplifies protein leverage, such that the over-ingestion of non-protein energy is greater for T2 than T1 (PL2 > PL1). Modified with permission from Raubenheimer and Simpson (2019a).
Several studies have reported an association between early diets of high protein infant formula and susceptibility to obesity in subsequent years (Weber et al., 2014; Stokes et al., 2021). Raubenheimer et al. (2014) hypothesised that this could be a consequence of a compensatory decrease in protein efficiency to counteract excess protein intake by a physiology adapted to low-protein human breast milk, for example through upregulation of hepatic gluconeogenesis. If reduced protein efficiency was developmentally programmed to persist beyond weaning when human protein requirements increase, this would necessitate increased intake of solid foods instigating or exacerbating protein leverage and excess energy intake. At the other end of the age spectrum, as noted above, increased protein intake could be beneficial in late life because protein efficiency declines with age, due to increased proteolysis, decreased protein synthesis, and enhanced gluconeogenesis with declining insulin sensitivity. Such increased protein requirements (and intake target) could explain increased susceptibility to obesity, accompanied by muscle wasting and loss of bone density associated with natural aging, including across the menopause transition in women.
Finally, although the work discussed to this point has concerned variation in dietary macronutrient quantities and ratios, recently NGF has been used to examine the effects of the quality of macronutrients as well as their quantity. This approach has, for example, helped to resolve apparent contradictions around the role of carbohydrate and protein. On the one hand, epidemiological evidence indicates that high carbohydrate intakes degrade health, while on the other, the longest-lived and healthiest populations have a high-carbohydrate, low-to-moderate protein diet (Wali et al., 2020). As also seen in rodent studies (e.g., Solon-Biet et al., 2019; Wali et al., 2021; see above), the paradox is resolved by considering the quality of carbohydrates, their ratio with respect to protein, and the quality of protein (e.g., BCAA levels; Le Couteur et al., 2020).
Conclusions
For decades, nutritional science has been hampered by its inherent multi-dimensional complexity. By focusing on the interface between optimization and constraint in the context of multiple nutrients, the NGF can help to cut across this complexity. Nutrition science has already identified that nutritional requirements vary among individuals as a function of health status and genotype. As we have demonstrated, abundant data show that the required mixture of nutrients also varies within individuals as a function of age and circumstance. The conclusion drawn from such observations is that diet should, where possible, change to meet these shifting requirements. The evidence we have reviewed, both from lab studies of mechanisms and patterns of dietary selection, as well as observational field studies, shows that natural selection has, indeed, equipped animals to dynamically adjust their diets in this way.
Among the advantages of this dynamic, multi-dimensional view of nutrition is that it provides a framework for integrating levels of analysis spanning mechanisms, development, evolution, and function, as famously advocated by ethologist Niko Tinbergen (1963). Such integration is a powerful means for generating predictions (Lefebvre, 2015), so helping to structure research in nutrition, a field that is not well endowed with theory (Raubenheimer and Simpson, 2016). As we have demonstrated, it can also help to resolve apparent paradoxes, for example how low-protein diets can be associated both with healthy and unhealthy outcomes, and how both high-protein diets (in early life) and low-protein diets (subsequently) could lead to excess energy consumption and obesity.
The examples presented here concern different taxa. In the present context, this has the advantage of demonstrating the universality of the approach, but the ultimate goal is to examine the full range of issues for a single species. Doing so could greatly enhance the understanding of the central role of nutrition in mediating between genotypes, phenotypes, and environments, potentially lead to the discovery of biological principles that fall between traditional disciplinary boundaries, and contribute applied benefits, for example to human health (discussed above) and conservation biology (Raubenheimer et al., 2012; Birnie-Gauvin et al., 2017).
Acknowledgments
This work was supported by an Australian National Health and Medical Research Council Program Grant (GNT1149976) and the Center for Sport Nutrition and Health, Center for Nutritional Ecology of Zhengzhou University (grant number 112/32340114). AMS was supported by an Australian Research Council Discovery Early Career Researcher Award (ARC DECRA DE180101520), and CM by an Australian Research Council Future Fellowship (FT170100259). Rong Hou was supported by the National Natural Science Foundation of China (32170507).
Author contributions
David Raubenheimer and Stephen Simpson conceived and led the writing of the paper, with input from Alistair Senior. All authors contributed to the content, with special expertise provided as follows: Alistair Senior, modeling and age-specific mortality; Christen Mirth and Pierre Léopold, Drosophila; Zhenwei Cui and Rong Hou, primatology; David Le Couteur and Samantha Solon-Biet, dietary restriction and gerontology.
Declaration of interests
David Raubenheimer and Stephen Simpson receive royalties from their books, The Nature of Nutrition and Eat Like the Animals. They have no other actual or potential competing interests. Alistair Senior, Christen Mirth, Zhenwei Cui, Rong Hou, David Le Couteur, Samantha Solon-Biet, and Pierre Léopold declare no competing interests.
Contributor Information
David Raubenheimer, Email: david.raubenheimer@sydney.edu.au.
Stephen J. Simpson, Email: stephen.simpson@sydney.edu.au.
References
- Agrawal N., Delanoue R., Mauri A., Basco D., Pasco M., Thorens B., Léopold P. The Drosophila TNF Eiger is an adipokine that acts on insulin-producing cells to mediate nutrient response. Cell Metab. 2016;23:675–684. doi: 10.1016/j.cmet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Anderson R.M., Shanmuganayagam D., Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol. Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B.J., Booth D.A., Duggan J.P., Gibson E.L. Protein appetite demonstrated: learned specificity of protein-cue preference to protein need in adult rats. Nutr. Res. 1987;7:481–487. doi: 10.1016/S0271-5317(87)80004-0. [DOI] [Google Scholar]
- Barclay R.D., Burd N.A., Tyler C., Tillin N.A., Mackenzie R.W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front. Nutr. 2019;6:146. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton Browne L. In: Regulatory Mechanisms in Insect Feeding. F Chapman R., de Boer G., editors. Chapman Hall; 1995. Ontogenic changes in feeding behavior; pp. 307–342. [Google Scholar]
- Bashiardes S., Abdeen S.K., Elinav E. Personalized nutrition: are we there yet? J. Pediatr. Gastroenterol. Nutr. 2019;69:633–638. doi: 10.1097/MPG.0000000000002491. [DOI] [PubMed] [Google Scholar]
- Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D., et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Beadle G.W., Tatum E.L., Clancy C.W. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biol. Bull. 1938;75:447–462. doi: 10.2307/1537573. [DOI] [Google Scholar]
- Berg J.M., Tymozko J.L., Stryer L. 5th ed. WH Freeman; 2002. Biochemistry. [Google Scholar]
- Bernays E.A., Raubenheimer D. Dietary mixing in grasshoppers: changes in acceptability of different plant secondary compounds associated with low levels of dietary protein (Orthoptera: Acrididae) J. Insect Behav. 1991;4:545–556. doi: 10.1007/BF01048069. [DOI] [Google Scholar]
- Bernays E.A., Bright K., Howard J.J., Raubenheimer D., Champagne D.E. Variety is the spice of life: frequent switching between foods in the polyphagous grasshopper Taeniopoda eques Burmeister (Orthoptera: Acrididae) Anim. Behav. 1992;44:721–731. doi: 10.1016/S0003-3472(05)80298-2. [DOI] [Google Scholar]
- Berry S.E., Valdes A.M., Drew D.A., Asnicar F., Mazidi M., Wolf J., Capdevila J., Hadjigeorgiou G., Davies R., Al Khatib H., et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020;26:964–973. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie-Gauvin K., Peiman K.S., Raubenheimer D., Cooke S.J. Nutritional physiology and ecology of wildlife in a changing world. Conserv. Physiol. 2017;5:cox030. doi: 10.1093/conphys/cox030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordal M., Arquier N., Kniazeff J., Pin J.P., Léopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Campbell C.P., Raubenheimer D., Badaloo A.V., Gluckman P.D., Martinez C., Gosby A.K., Simpson S.J., Osmond C., Boyne M.S., Boyne M., Forrester T.E. Developmental contributions to macronutrient selection: a randomized controlled trial in adult survivors of malnutrition. Evol. Med. Public Health. 2016;2016:158–169. doi: 10.1093/emph/eov030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers P.G., Simpson S.J., Raubenheimer D. Behavioural mechanisms of nutrient balancing in Locusta migratoria nymphs. Anim. Behav. 1995;50:1513–1523. doi: 10.1016/0003-3472(95)80007-7. [DOI] [Google Scholar]
- Chell J.M., Brand A.H. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L.Y., Bailey A.P., Leevers S.J., Ragan T.J., Driscoll P.C., Gould A.P. Anaplastic lymphoma kinase spares organ growth during nutrient restriction in Drosophila. Cell. 2011;146:435–447. doi: 10.1016/j.cell.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Colombani J., Raisin S., Pantalacci S., Radimerski T., Montagne J., Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Cotter S.C., Simpson S.J., Raubenheimer D., Wilson K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 2010;25:186–198. doi: 10.1111/j.1365-2435.2010.01766.x. [DOI] [Google Scholar]
- Cui Z.-W., Wang Z.-L., Shao Q., Raubenheimer D., Lu J.-Q. Macronutrient signature of dietary generalism in an ecologically diverse primate in the wild. Behav. Ecol. 2018;29:804–813. doi: 10.1093/beheco/ary003. [DOI] [Google Scholar]
- Cui Z., Wang Z., Zhang S., Wang B., Lu J., Raubenheimer D. Living near the limits: effects of interannual variation in food availability on diet and reproduction in a temperate primate, the Taihangshan macaque (Macaca mulatta tcheliensis) Am. J. Primatol. 2020;82:e23080. doi: 10.1002/ajp.23080. [DOI] [PubMed] [Google Scholar]
- de Carvalho M.J.A., Mirth C.K. Food intake and food choice are altered by the developmental transition at critical weight in Drosophila melanogaster. Anim. Behav. 2017;126:195–208. doi: 10.1016/j.anbehav.2017.02.005. [DOI] [Google Scholar]
- Delanoue R., Meschi E., Agrawal N., Mauri A., Tsatskis Y., McNeill H., Léopold P. Drosophila insulin release is triggered by adipose Stunted ligand to brain Methuselah receptor. Science. 2016;353:1553–1556. doi: 10.1126/science.aaf8430. [DOI] [PubMed] [Google Scholar]
- Di Francesco A., Di Germanio C., Bernier M., de Cabo R. A time to fast. Science. 2018;362:770–775. doi: 10.1126/science.aau2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussutour A., Latty T., Beekman M., Simpson S.J. Amoeboid organism solves complex nutritional challenges. PNAS. 2010;107:4607–4611. doi: 10.1073/pnas.0912198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia M., Livesey G. Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev. Nutr. Diet. 1992;70:68–131. doi: 10.1159/000421672. [DOI] [PubMed] [Google Scholar]
- Felton A.M., Felton A., Raubenheimer D., Simpson S.J., Foley W.J., Wood J.T., Wallis I.R., Lindenmayer D.B. Protein content of diets dictates the daily energy intake of a free-ranging primate. Behav. Ecol. 2009;20:685–690. doi: 10.1093/beheco/arp021. [DOI] [Google Scholar]
- Flippo K.H., Jensen-Cody S.O., Claflin K.E., Potthoff M.J. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Sci. Rep. 2020;10:19521. doi: 10.1038/s41598-020-76593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo K.H., Potthoff M.J. Metabolic messengers: FGF21. Nat. Metab. 2021;3:309–317. doi: 10.1038/s42255-021-00354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Partridge L., Longo V.D. Extending healthy life span – from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd C.A., Raubenheimer D. Nutrient-specific learning in an omnivorous insect: the American cockroach Periplaneta americana L. learns to associate dietary protein with the odors citral and carvone. J. Insect Behav. 2000;13:851–864. doi: 10.1023/A:1007862501311. [DOI] [Google Scholar]
- Gage T.B., O’Connor K. Nutrition and the variation in level and age patterns of mortality.1994. Hum. Biol. 2009;81:551–574. doi: 10.3378/027.081.0605. [DOI] [PubMed] [Google Scholar]
- Gosby A.K., Conigrave A.D., Lau N.S., Iglesias M.A., Hall R.M., Jebb S.A., Brand-Miller J., Caterson I.D., Raubenheimer D., Simpson S.J. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS One. 2011;6:e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S. Health span extension by later-life caloric or dietary restriction: a view based on rodent studies. Biogerontology. 2006;7:135–138. doi: 10.1007/s10522-006-9011-4. [DOI] [PubMed] [Google Scholar]
- Green C.L., Lamming D.W. Regulation of metabolic health by essential dietary amino acids. Mech. Ageing Dev. 2019;177:186–200. doi: 10.1016/j.mad.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch-Ferré M., Dashti H.S., Merino J. Nutritional genomics and direct-to-consumer genetic testing: an overview. Adv. Nutr. 2018;9:128–135. doi: 10.1093/advances/nmy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S.T., Hou R., Garber P.A., Garber P., Raubenheimer D., Righini N., Ji W.-H., Jay O., He S.-J., Wu F., et al. Nutrient-specific compensation for seasonal cold stress in a free–ranging temperate colobine monkey. Funct. Ecol. 2018;32:2170–2180. doi: 10.1111/1365-2435.13134. [DOI] [Google Scholar]
- Hahn O., Drews L.F., Nguyen A., Tatsuta T., Gkioni L., Hendrich O., Zhang Q., Langer T., Pletcher S., Wakelam M.J.O., et al. A nutritional memory effect counteracts the benefits of dietary restriction in old mice. Nat. Metab. 2019;1:1059–1073. doi: 10.1038/s42255-019-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel P.J., Kievit P., Comuzzie A.G., Bremer A.A. Use and importance of nonhuman primates in metabolic disease research: current state of the field. ILAR J. 2017;58:251–268. doi: 10.1093/ilar/ilx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlena D., Schmitz O.J. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc. Natl. Acad. Sci. U S A. 2010;107:15503–15507. doi: 10.1073/pnas.1009300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C.M., Laeger T., Dehner M., Albarado D.C., Clarke B., Wanders D., Burke S.J., Collier J.J., Qualls-Creekmore E., Solon-Biet S., et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019;27:2934–2947. doi: 10.1016/j.celrep.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C.M., Qualls-Creekmore E., Berthoud H.R., Soto P., Yu S., McDougal D.H., Münzberg H., Morrison C.D. FGF21 and the physiological regulation of macronutrient preference. Endocrinology. 2020;161:bqaa019. doi: 10.1210/endocr/bqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L., Longchamp A., Treviño-Villarreal J.H., Mejia P., Ozaki C.K., et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.J., Chew Y.V., Colakoglu F., Cliff J.B., Klaassens E., Read M.N., Solon-Biet S.M., McMahon A.C., Cogger V.C., Ruohonen K., et al. Diet-microbiome interactions in health are controlled via intestinal nitrogen source constraints. Cell Metab. 2017;25:140–151. doi: 10.1016/j.cmet.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Hosking C.J., Raubenheimer D., Charleston M.A., Simpson S.J., Senior A.M. Macronutrient intakes and the lifespan-fecundity trade-off: a geometric framework agent-based model. J. R. Soc. Interf. 2019;16:20180733. doi: 10.1098/rsif.2018.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R., Chapman C.A., Jay O., Guo S., Li B., Raubenheimer D. Cold and hungry: combined effects of low temperature and resource scarcity on an edge-of-range temperate primate, the golden snub-nose monkey. Ecography. 2020;43:1672–1682. doi: 10.1111/ecog.05295. [DOI] [Google Scholar]
- Hou R., Chapman C.A., Rothman J.M., Zhang H., Huang K., Guo S., Li B., Raubenheimer D. The geometry of resource constraint: an empirical study of the golden snub-nosed monkey. J. Anim. Ecol. 2021;90:751–765. doi: 10.1111/1365-2656.13408. [DOI] [PubMed] [Google Scholar]
- Ikeya T., Galic M., Belawat P., Nairz K., Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Jensen K., Mayntz D., Toft S., Clissold F.J., Hunt J., Raubenheimer D., Simpson S.J. Optimal foraging for specific nutrients in predatory beetles. Proc. R. Soc. B. 2012;279:2212–2218. doi: 10.1098/rspb.2011.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Cody S.O., Flippo K.H., Claflin K.E., Yavuz Y., Sapouckey S.A., Walters G.C., Usachev Y.M., Atasoy D., Gillum M.P., Potthoff M.J. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metab. 2020;32:273–286. doi: 10.1016/j.cmet.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.A., Raubenheimer D., Rothman J.M., Clarke D., Swedell L. 30 days in the life: daily nutrient balancing in a wild chacma baboon. PLoS One. 2013;8:e70383. doi: 10.1371/journal.pone.0070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger M.A., Rintelen F., Stocker H., Wasserman J.D., Végh M., Radimerski T., Greenberg M.E., Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Mirth C.K. Growth blocking peptides as nutrition-sensitive signals for insulin secretion and body size regulation. PLoS Biol. 2016;14:e1002392. doi: 10.1371/journal.pbio.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Mendes C.C., Mirth C.K. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 2013;4:263. doi: 10.3389/fphys.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Rodrigues M.A., Athanasiadis A., Shingleton A.W., Mirth C.K. Nutritional control of body size through FoxO-Ultraspiracle mediated ecdysone biosynthesis. Elife. 2014;3:e03091. doi: 10.7554/eLife.03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz T.C., Sgrò C.M., Mirth C.K. Interacting with change: diet mediates how larvae respond to their thermal environment. Funct. Ecol. 2019;33:1940–1951. doi: 10.1111/1365-2435.13414. [DOI] [Google Scholar]
- Le Couteur D.G., Simpson S.J. Adaptive senectitude: the prolongevity effects of aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2011;66:179–182. doi: 10.1093/gerona/glq171. [DOI] [PubMed] [Google Scholar]
- Le Couteur D.G., Simpson S.J. 90th anniversary commentary: caloric restriction effects on aging. J. Nutr. 2018;148:1656–1659. doi: 10.1093/jn/nxy146. [DOI] [PubMed] [Google Scholar]
- Le Couteur D.G., Solon-Biet S., Cogger V.C., Mitchell S.J., Senior A., de Cabo R., Raubenheimer D., Simpson S.J. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol. Life Sci. 2016;73:1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur D.G., Solon-Biet S.M., Cogger V.C., Ribeiro R., de Cabo R., Raubenheimer D., Cooney G.J., Simpson S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020;64:101198. doi: 10.1016/j.arr.2020.101198. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Cory J.S., Wilson K., Raubenheimer D., Simpson S.J. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. Biol. Sci. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Simpson S.J., Clissold F.J., Brooks R., Ballard J.W.O., Taylor P.W., Soran N., Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. PNAS. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L. Should neuroecologists separate Tinbergen's four questions? Behav. Process. 2015;117:92–96. doi: 10.1016/j.beproc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Levine M.E., Suarez J.A., Brandhorst S., Balasubramanian P., Cheng C.-W., Madia F., Fontana L., Fontana F., Mirisola M.G., Guevara-Aguirre J., et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Selle P.H., Raubenheimer D., Cadogan D.J., Simpson S.J., Cowieson A.J. An assessment of the influence of macronutrients on growth performance and nutrient utilisation in broiler chickens by nutritional geometry. Br. J. Nutr. 2017;116:2129–2138. doi: 10.1017/S0007114516004190. [DOI] [PubMed] [Google Scholar]
- Longo V.D., Di Tano M., Mattson M.P., Guidi N. Intermittent and periodic fasting, longevity and disease. Nat. Aging. 2021;1:47–59. doi: 10.1038/s43587-020-00013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W., Goymer P., Pletcher S.D., Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Martin A., Ecklu-Mensah G., Ha C.W.Y., Hendrick G., Layman D.K., Gilbert J., Devkota S. Gut microbiota mediate the FGF21 adaptive stress response to chronic dietary protein-restriction in mice. Nat. Commun. 2021;12:3838. doi: 10.1038/s41467-021-24074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Steele E., Raubenheimer D., Simpson S.J., Baraldi L.G., Monteiro C.A. Ultra-processed foods, protein leverage and energy intake in the USA. Public Health Nutr. 2018;21:114–124. doi: 10.1017/S1368980017001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matavelli C., Carvalho M.J.A., Martins N.E., Mirth C.K. Differences in larval nutritional requirements and female oviposition preference reflect the order of fruit colonization of Zaprionus indianus and Drosophila simulans. J. Insect Physiol. 2015;82:66–74. doi: 10.1016/j.jinsphys.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Mattison J.A., Roth G.S., Beasley T.M., Tilmont E.M., Handy A.M., Herbert R.L., Longo D.L., Allison D.B., Young J.E., Bryant M., et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay C.M., Crowell M.F., Maynard L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. doi: 10.1093/jn/10.1.63. [DOI] [PubMed] [Google Scholar]
- Mendes C.C., Mirth C.K. Stage-specific plasticity in ovary size is regulated by insulin/insulin-like growth factor and ecdysone signaling in Drosophila. Genetics. 2016;202:703–719. doi: 10.1534/genetics.115.179960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken E.M., Carboneau B.A., Krzysik-Walker S.M., de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth C.K., Truman J.W., Riddiford L.M. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Mitchell S.E., Delville C., Konstantopedos P., Derous D., Green C.L., Chen L., Han J.-D.J., Wang Y., Promislow D.E.L., Douglas A., et al. The effects of graded levels of calorie restriction: III. Impact of short-term calorie and protein restriction on mean daily body temperature and torpor use in the C57BL/6 mouse. Oncotarget. 2015;6:18314–18337. doi: 10.18632/oncotarget.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.J., Bernier M., Mattison J.A., Aon M.A., Kaiser T.A., Anson R.M., Ikeno Y., Anderson R.M., Ingram D.K., de Cabo R. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 2019;29:221–228.e3. doi: 10.1016/j.cmet.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais P.B., Martins M.B., Klaczko L.B., Mendonca-Hagler L.C., Hagler A.N. Yeast succession in the Amazon fruit Parahancornia amapa as resource partitioning among Drosophila spp. Appl. Environ. Microbiol. 1995;61:4251–4257. doi: 10.1128/aem.61.12.4251-4257.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch D., Ezra-Nevo G., Francisco A.P., Tastekin I., Ribeiro C. Nutrient homeostasis – translating internal states to behavior. Curr. Opin. Neurobiol. 2020;60:67–75. doi: 10.1016/j.conb.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Nijhout H.F. The control of body size in insects. Dev. Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Nijhout H.F., Riddiford L.M., Mirth C., Shingleton A.W., Suzuki Y., Callier V. The developmental control of size in insects. Rev. Dev. Biol. 2014;3:113–134. doi: 10.1002/wdev.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N., Yamanaka N., Yagi Y., Nishida Y., Kataoka H., O’Connor M.B., Mizoguchi A. A fat body-derived IGF-like peptide regulates post-feeding growth in Drosophila. Dev. Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas J.M., Ferguson L.R., Tai E.S., Mathers J.C. Personalised nutrition and health. BMJ. 2018;361:bmj.k2173. doi: 10.1136/bmj.k2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak H.H., Haws S.A., Green C.L., Koller M., Lavarias M.T., Richardson N.E., Yang S.E., Dumas S.N., Sonsalla M., Bray L., et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat. Metab. 2021;3:1327–1341. doi: 10.1038/s42255-021-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A.N., Cederholm T. Health effects of protein intake in healthy elderly populations: a systematic literature review. Food Nutr. Res. 2014;58:23364. doi: 10.3402/fnr.v58.23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M.D.W., Soultoukis G.A., Blanc E., Mesaros A., Herbert S.L., Juricic P., He X., Atanassov I., Salmonowicz H., Yang M., et al. Matching dietary amino acid balance to the in silico-translated exome optimizes growth and reproduction without cost to lifespan. Cell Metab. 2017;25:610–621. doi: 10.1016/j.cmet.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poças G.M., Crosbie A.E., Mirth C.K. When does diet matter? The roles of larval and adult nutrition in regulating adult size traits in Drosophila melanogaster. J. Insect Physiol. 2020:104051. doi: 10.1016/j.jinsphys.2020.104051. [DOI] [PubMed] [Google Scholar]
- Ponton F., Wilson K., Cotter S.C., Raubenheimer D., Simpson S.J. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7:e1002223. doi: 10.1371/journal.ppat.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H., Yamada Y., Sagayama H., Ainslie P.N., Andersen L.F., Anderson L.J., Arab L., Baddou I., Bedu-Addo K., Blaak E.E., et al. IAEA DLW Database Consortium§ Daily energy expenditure through the human life course. Science. 2021;373:808–812. doi: 10.1126/science.abe5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post S., Tatar M. Nutritional Geometric profiles of insulin/IGF expression in Drosophila melanogaster. PLoS One. 2016;11:e0155628. doi: 10.1371/journal.pone.0155628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post S., Karashchuk G., Wade J.D., Sajid W., De Meyts P., Tatar M. Drosophila insulin-like peptides dilp2 and dilp5 differentially stimulate cell signaling and glycogen phosphorylase to regulate longevity. Front. Endocrinol. 2018;9:245. doi: 10.3389/fendo.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D. Toward a quantitative nutritional ecology: the right-angled mixture triangle. Ecol. Monogr. 2011;81:407–427. doi: 10.1890/10-1707.1. [DOI] [Google Scholar]
- Raubenheimer D., Jones S. Nutritional imbalance in an extreme generalist omnivore: tolerance and recovery through complementary food selection. Anim. Behav. 2006;71:1253–1262. doi: 10.1016/j.anbehav.2005.07.024. [DOI] [Google Scholar]
- Raubenheimer D., Simpson S.J. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Simpson S.J. Nutritional ecology and human health. Annu. Rev. Nutr. 2016;36:603–626. doi: 10.1146/annurev-nutr-071715-051118. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Simpson S.J. Protein Leverage: theoretical foundations and ten points of clarification. Obesity. 2019;27:1225–1238. doi: 10.1002/oby.22531. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Simpson S.J. In: Encyclopaedia of Animal Behaviour. 2nd ed. Chloe J.C., editor. Elsevier; 2019. Hunger and satiety: linking mechanisms, behaviour and evolution; pp. 127–138. [Google Scholar]
- Raubenheimer D., Simpson S.J. Houghton Mifflin Harcourt; 2020. Eat Like the Animals: What Nature Teaches Us About the Science of Healthy Eating. [Google Scholar]
- Raubenheimer D., Tucker D. Associative learning by locusts: pairing of visual cues with consumption of protein and carbohydrate. Anim. Behav. 1997;54:1449–1459. doi: 10.1006/anbe.1997.0542. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Simpson S.J., Mayntz D. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 2009;23:4–16. doi: 10.1111/j.1365-2435.2009.01522.x. [DOI] [Google Scholar]
- Raubenheimer D., Simpson S.J., Tait A.H. Match and mismatch: conservation physiology, nutritional ecology and the timescales of biological adaptation. Phil. Trans. R. Soc. 2012;367:1628–1646. doi: 10.1098/rstb.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D., Machovsky-Capuska G.E., Gosby A.K., Simpson S.J. Nutritional ecology of obesity: from humans to companion animals. Br. J. Nutr. 2014;113:S26–S39. doi: 10.1017/S0007114514002323. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Machovsky-Capuska G.E., Chapman C.A., Rothman J.M. Geometry of nutrition in field studies, an illustration using wild primates. Oecologia. 2015;177:223–234. doi: 10.1007/s00442-014-3142-0. [DOI] [PubMed] [Google Scholar]
- Redhai S., Pilgrim C., Gaspar P., Giesen L.v., Lopes T., Riabinina O., Grenier T., Milona A., Chanana B., Swadling J.B., et al. An intestinal zinc sensor regulates food intake and developmental growth. Nature. 2020;580:263–268. doi: 10.1038/s41586-020-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C., Dickson B.J. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Richardson N.E., Konon E.N., Schuster H.S., Mitchell A.T., Boyle C., Rodgers A.C., Finke M., Haider L.R., Yu D., Flores V., et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat. Aging. 2021;1:73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M.A., Martins N.E., Balancé L.F., Broom L.N., Dias A.J.S., Fernandes A.S.D., Rodrigues F., Sucena E., Mirth C.K. Drosophila melanogaster larvae make nutritional choices that minimize developmental time. J. Insect Physiol. 2015;81:69–80. doi: 10.1016/j.jinsphys.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Rothman J.M., Plumptre A.J., Dierenfeld E.S., Pell A.N. Nutritional composition of the diet of the gorilla (Gorilla beringei): a comparison between two montane habitats. J. Trop. Ecol. 2007;23:673–682. doi: 10.1017/S0266467407004555. [DOI] [Google Scholar]
- Sano H., Nakamura A., Texada M.J., Truman J.W., Ishimoto H., Kamikouchi A., Nibu Y., Kume K., Ida T., Kojima M. The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster. PLoS Genet. 2015;11:e1005209. doi: 10.1371/journal.pgen.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz O.J., Hawlena D., Trussell G.C. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 2010;13:1199–1209. doi: 10.1111/j.1461-0248.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Cambridge University Press; 1991. Sodium Hunger: The Search for a Salty Taste. [Google Scholar]
- Senior A.M., Solon-Biet S.M., Cogger V.C., Le Couteur D.G., Nakagawa S., Raubenheimer D., Simpson S.J. Dietary macronutrient content, age–specific mortality and lifespan. Proc. Biol. Sci. 2019;286:20190393. doi: 10.1098/rspb.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A.M., Nakagawa S., Raubenheimer D., Simpson S.J. Global associations between macronutrient supply and age-specific mortality. Proc. Natl. Acad. Sci. U S A. 2020;117:30824–30835. doi: 10.1073/pnas.2015058117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingleton A.W., Frankino W.A. New perspectives on the evolution of exaggerated traits. Bioessays. 2013;35:100–107. doi: 10.1002/bies.201200139. [DOI] [PubMed] [Google Scholar]
- Shingleton A.W., Das J., Vinicius L., Stern D.L. The temporal requirements for insulin signalling during development in Drosophila. PLoS Biol. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingleton A.W., Mirth C.K., Bates P.W. Developmental model of static allometry in holometabolous insects. Proc. Biol. Sci. 2008;275:1875–1885. doi: 10.1098/rspb.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikanth V., Bobji M.S. A non-resonant mass sensor to eliminate the “missing mass” effect during mass measurement of biological materials. Rev. Sci. Instrum. 2014;85:105006. doi: 10.1063/1.4899201. [DOI] [PubMed] [Google Scholar]
- Silva-Soares N.F., Nogueira-Alves A., Beldade P., Mirth C.K. Adaptation to new nutritional environments: larval performance, foraging decisions, and adult oviposition choices in Drosophila suzukii. BMC Ecol. 2017;17:21. doi: 10.1186/s12898-017-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S.J., Raubenheimer D. The geometric analysis of nutrient-allelochemical interactions: a case study using locusts. Ecology. 2001;82:422–439. doi: 10.2307/2679870. [DOI] [Google Scholar]
- Simpson S.J., Raubenheimer D. Obesity: the protein leverage hypothesis. Obes. Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Raubenheimer D. Princeton University Press; 2012. The Nature of Nutrition: A Unifying Framework from Animal Adaption to Human Obesity. [Google Scholar]
- Simpson S.J., Sibly R.M., Lee K.P., Behmer S.T., Raubenheimer D. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 2004;68:1299–1311. doi: 10.1016/j.anbehav.2004.03.003. [DOI] [Google Scholar]
- Simpson S.J., White P.R. Associative learning and locust feeding: evidence for a ‘learned hunger’ for protein. Anim. Behav. 1990;40:506–513. doi: 10.1016/S0003-3472(05)80531-7. [DOI] [Google Scholar]
- Simpson S.J., Le Couteur D.G., Raubenheimer D. Putting the balance back in diet. Cell. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Raubenheimer D., Cogger V.C., Macia L., Solon-Biet S.M., Le Couteur D.G., George J. The nutritional geometry of liver disease including non-alcoholic fatty liver disease. J. Hepatol. 2018;68:316–325. doi: 10.1016/j.jhep.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Slaidina M., Delanoue R., Gronke S., Partridge L., Léopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S.M., McMahon A.C., Ballard J.W.O., Ruohonen K., Wu L.E., Cogger V.C., Warren A., Huang X., Pichaud N., Melvin R.G., et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum–fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S.M., Walters K.A., Simanainen U.K., McMahon A.C., Ruohonen K., Ballard J.W.O., Raubenheimer D., Handelsman D.J., Le Couteur D.G., Simpson S.J. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl. Acad. Sci. U S A. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet S.M., Cogger V.C., Heblinski M., Pulpitel T., Wahl D., McMahon A.C., Warren A., Durrant-Whyte J., Walters K.A., Krycer J., et al. Defining the nutritional and metabolic context of FGF21 using the Geometric Framework. Cell Metab. 2016;24:555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Solon-Biet S.M., Cogger V.C., Pulpitel T., Wahl D., Clark X., Bagley E.E., Gregoriou G.C., Senior A.M., Wang Q.P., Brandon A.E., et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019;1:532–545. doi: 10.1038/s42255-019-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Hinnebusch A.G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler S.R. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech. Ageing Dev. 2005;126:960–966. doi: 10.1016/j.mad.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Stieper B.C., Kupershtok M., Driscoll M.V., Shingleton A.W. Imaginal discs regulate developmental timing in Drosophila melanogaster. Dev. Biol. 2008;321:18–26. doi: 10.1016/j.ydbio.2008.05.556. [DOI] [PubMed] [Google Scholar]
- Stokes A., Campbell K.J., Yu H.-J., Szymlek-Gay E.A., Abbott G., He Q.-Q., Zheng M. Protein intake from birth to 2 years and obesity outcomes in later childhood and adolescence, A systematic review of prospective cohort studies. Adv. Nutr. 2021;12:1863–1876. doi: 10.1093/advances/nmab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studnicki M., Dębski K.J., Stępkowski D. Proportions of macronutrients, including specific dietary fats, in prospective anti-Alzheimer's diet. Sci. Rep. 2019;9:20143. doi: 10.1038/s41598-019-56687-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.Y., Smith-Caldas M.S.B., Driscoll M.V., Salhadar S., Shingleton A.W. FOXO regulates organ-specific phenotypic plasticity in Drosophila. PLoS Genet. 2011;7:e1002373. doi: 10.1371/journal.pgen.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of Ethology. Z. Tierpsychol. 1963;20:410–433. doi: 10.1111/j.1439-0310.1963.tb01161.x. [DOI] [Google Scholar]
- Tordoff M.G. Calcium: taste, intake, and appetite. Physiol. Rev. 2001;81:1567–1597. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- Uwimbabazi M., Raubenheimer D., Tweheyo M., Basuta G.I., Conklin-Brittain N.L., Wrangham R.W., Rothman J.M. Nutritional Geometry of female chimpanzees (Pan troglodytes) Am. J. Primatol. 2021;83:e23269. doi: 10.1002/ajp.23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M.A., Luo N., Yamaguchi A., Kapahi P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 2010;20:1006–1011. doi: 10.1016/j.cub.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria B., Nunez Lopez Y.O., Masternak M.M. MicroRNAs and the metabolic hallmarks of aging. Mol. Cell Endocrinol. 2017;455:131–147. doi: 10.1016/j.mce.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E.R., Rothman J.M., Moldawer A.M., Bransford T.D., Emery-Thompson M.E., Van Noordwijk M.A., Utami Atmoko S.S., Crowley B.E., Knott C.D., Erb W.M., et al. Coping with a challenging environment: nutritional balancing, health, and energetics in wild Bornean orangutans. Am. J. Phys. Anthropol. 2015;156:314–315. doi: 10.1002/ajpa.22718. [DOI] [Google Scholar]
- Wang Z., Ying Z., Bosy-Westphal A., Zhang J., Schautz B., Later W., Heymsfield S.B., Müller M.J. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 2010;92:1369–1377. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Grote V., Closa-Monasterolo R., Escribano J., Langhendries J.-P., Dain E., Giovannini M., Verduci E., Gruszfeld D., Socha P., Koletzko B., for The European Childhood Obesity Trial Study Group Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am. J. Clin. Nutr. 2014;99:1041–1051. doi: 10.3945/ajcn.113.064071. [DOI] [PubMed] [Google Scholar]
- Weindruch R., Walford R.L., Fligiel S., Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Wali J.A., Jarzebska N., Raubenheimer D., Simpson S.J., Rodionov R.N., O’Sullivan J.F. Cardio-metabolic consequences of high fat diets and their mechanisms. Nutrients. 2020;12:1505. doi: 10.3390/nu12051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali J.A., Raubenheimer D., Senior A.M., Le Couteur D.G., Simpson S.J. Cardio-metabolic consequences of dietary carbohydrates: reconciling contradictions using nutritional geometry. Cardiovasc Res. 2021;117:386–401. doi: 10.1093/cvr/cvaa136. [DOI] [PubMed] [Google Scholar]
- Walker S.J., Corrales-Carvajal V.M., Ribeiro C. Postmating circuitry modulates salt taste processing to increase reproductive output in Drosophila. Curr. Biol. 2015;25:2621–2630. doi: 10.1016/j.cub.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Wu G. Dietary protein intake and human health. Food Funct. 2016;7:1251–1265. doi: 10.1039/c5fo01530h. [DOI] [PubMed] [Google Scholar]
- Yap Y.W., Rusu P.M., Chan A.Y., Fam B.C., Jungmann A., Solon-Biet S.M., Barlow C.K., Creek D.J., Huang C., Schittenhelm R.B., et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nat. Commun. 2020;11:2894. doi: 10.1038/s41467-020-16568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanco B., Mirth C.K., Sgrò C.M., Piper M.D. A dietary sterol trade-off determines lifespan responses to dietary restriction in Drosophila melanogaster females. Elife. 2021;10:e62335. doi: 10.7554/eLife.62335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben-Yacov O., Lador D., Avnit-Sagi T., Lotan-Pompan M., et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]