Abstract
Background
Healthcare-associated (HCA) SARS-CoV-2 infection is a significant contributor to the spread of the 2020 pandemic. Timely review of HCA cases is essential to identify learning to inform infection prevention and control (IPC) policies and organisational response.
Aim
To identify key areas for improvement through rapid investigation of HCA SARS-CoV-2 cases and to implement change.
Methods
Cases were identified based on date of first positive SARS-CoV-2 PCR sample in relation to date of hospital admission. Cases were reviewed using a structured gap analysis tool to identify key learning points. These were discussed in weekly multidisciplinary meetings to gain consensus on learning outcomes, level of harm incurred by the patient and required actions. Learning was then promptly fed back to individual teams and the organisation.
Findings
Of the 489 SARS-CoV-2 cases admitted between 10th March and 23rd June 2020, 114 suspected HCA cases (23.3%) were reviewed; 58/489 (11.8%) were ultimately deemed to be HCA. Five themes were identified: individual patient vulnerability, communication, IPC implementation, policy issues and organisational response. Adaptations to policies based on these reviews were completed within the course of the initial phase of the pandemic.
Conclusion
This approach enabled timely learning and implementation of control measures and policy development.
Keywords: COVID-19, infection prevention and control, healthcare-associated infection, gap analysis
Introduction
The 2020 COVID-19 pandemic posed significant challenges to hospitals worldwide. Healthcare-associated (HCA) cases and outbreaks of SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) infection, affecting both staff and patients, have been frequently identified (Price et al., 2021; Rickman et al., 2021; Wang et al., 2020). Such cases can have marked consequences. Many inpatients are vulnerable to developing severe infection, staff infections predispose to significant viral propagation (Sikkema et al., 2020; Zhou et al., 2020) and staff sickness/isolation adversely affects patient safety. Hospitals must be able to understand and learn from cases in order to introduce measures to prevent future cases.
HCA SARS-CoV-2 infection accounts for 12.5–40% of hospital cases, although definitions vary (Meredith et al., 2020; Rickman et al., 2021; Wang et al., 2020). Case fatality rates approach 30% (Carter et al., 2020; Rickman et al., 2021). There was no mandatory reporting of HCA SARS-CoV-2 infection and no universally accepted definition of an HCA infection until June 2020 in England due to uncertainty over incubation periods.
Throughout the pandemic, infection prevention and control (IPC) teams had to rapidly adapt local practice to ensure patient and staff safety, taking into account national policies (Islam et al., 2020). Inter-hospital variations in isolation capacity, access to diagnostics and patient demographics posed site-specific challenges that resulted in bespoke solutions (Basile et al., 2020).
In the UK prior to the pandemic, investigation of specific HCA infections took different forms. Perhaps the most widely used tool is Root Cause Analysis (RCA). Cases are collated and identified through hospital incident reporting systems, with the consequences of cases stratified by the level of harm incurred (NPSA, 2009). Cases may undergo internal +/− external review by scrutiny panels to identify lapses in care and organisational areas for improvement. This learning should be used to improve systems and processes in order to reduce future risk. Such investigations may take weeks to months to conclude and require significant resources, prompting the use of more rapid investigational methods such as post-infection review and rapid RCA. Since 9 June 2020, UK hospitals have been advised to undertake RCAs on all cases of SARS-CoV-2 diagnosed more than 7 days after admission (NHS, 2020).
When approaching this problem early in the pandemic, we were concerned that RCA would be too slow. The urgency of this situation was driven by the rapid rise in case numbers and the pace of change in our understanding of viral transmission dynamics. To face these challenges, infection clinicians and the patient safety team met to develop a process for reviewing our HCA cases. We aimed to identify gaps in care which may have contributed to the spread of SARS-CoV-2 and to rapidly make recommendations for adjustments to practice which we then implemented.
We present our method of reviewing HCA SARS-CoV-2 infections, the resultant learning and how our practice evolved throughout the first wave of the pandemic.
Methods
Setting: Cambridge University Hospitals NHS Foundation Trust has 1100 beds serving a population of around 580,000 people.
Cases reviewed to inform learning: Cases admitted between 10th March and 23rd June 2020 were included in the study.
Constitution of a working group: This included infectious disease physicians, microbiologists, IPC nurses and the Patient Safety team. Representatives from Occupational Health, Epidemiology unit (Public Health England Field Service) and SARS-CoV-2 whole genome sequencing teams (University of Cambridge, COG-UK) also attended. The working group developed a pro forma (supplementary figure 1) allowing relevant data to be collected.
Standards: Standards were developed by discussion among members of the working group. These were not evidence-based but were thought to reflect what ideally should happen once a case was identified in order to prevent further transmission. This was based on experience dealing with other HCA infections and our understanding of the virology at that time.
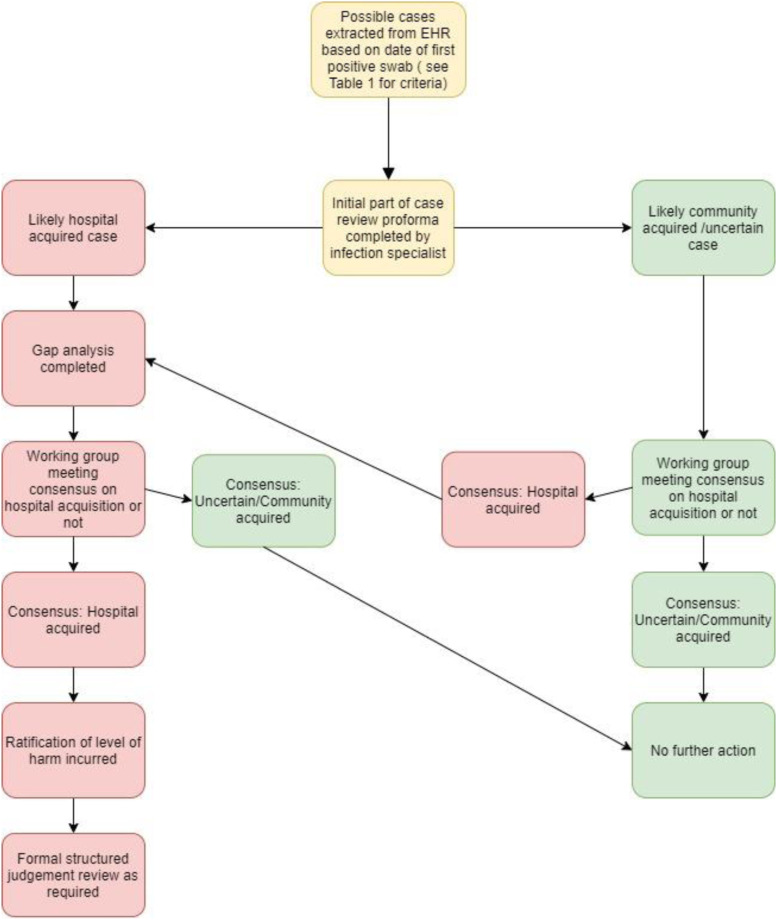
Case identification: Cases were identified (Figure 1) from electronic health records (EHR; EPIC – EPIC Systems Verona, WN) and were initially categorised by the date of their first positive SARS-CoV-2 PCR sample in relation to their admission date into four groups (Table 1). Due to time constraints, community-onset suspected HCA cases were only included for complete review if they had either multiple healthcare contacts (e.g. dialysis or hospital admission of >24 h within the 14 days prior to diagnosis) or experienced death or an Intensive Care Unit (ICU) stay during their admission.
Figure 1.
Process of case selection and review process for potential cases of HCA SARS-COV-2 infection. EHR, electronic health record; HCA, healthcare associated.
Table 1.
Initial categorisation of cases by date of first positive SARS-CoV-2 PCR.
| Group | Definition |
|---|---|
| Hospital onset, healthcare associated | Positive specimen date >14 days from admission |
| Hospital onset, suspected healthcare associated | Positive specimen date 8–14 days after admission or specimen date 3–14 days after admission, with prior admission in previous 14 days |
| Hospital onset, indeterminate healthcare associated | Positive specimen date 3–7 days after admission, with no prior hospital admission in previous 14 days |
| Community onset, suspected healthcare associated | Positive specimen date ≤2 days after admission, with prior hospital admission in previous 14 days (for detailed inclusion criteria see main text) |
PCR, polymerase chain reaction.
Case review: All cases were reviewed within one week of a positive test by an infection clinician (i.e. clinical microbiologist or infectious disease physician) to confirm whether cases were more likely to be HCA, community onset or remained uncertain. They then used a pro forma to extract information from the EHR and downloaded the information onto a central database. All data required to complete the pro forma was available from the EHR apart from data concerning ward/bay closures and IPC-related audits (e.g. hand hygiene/personal protective equipment [PPE] use). This was available from the IPC team. Cases were then presented at the following weekly working group meeting to obtain a consensus opinion on whether the balance of probability favoured HCA or community onset. The clinical teams directly involved in patient care were not involved in data collection or analysis.
Definitions: The ascription of HCA was firstly based on the date of symptom onset in relation to admission: patients whose symptom onset was >14 days from admission were deemed to be HCA. Criteria for decision making in those with symptom onset ≤14 days of admission included weighing up epidemiological links to both hospital cases (co-location on same ward with another case [either staff or patient]) and other contacts where documented. Genomic data (when available) were used to support these conclusions and identify possible clusters as described previously by Meredith and colleagues (Meredith et al., 2020). Level of harm incurred by the patient was recorded using standard definitions (NRLS, 2004).
Feedback: Learning outcomes were fed back locally to individual medical and nursing teams within a day of the meeting. They were also disseminated across the trust via the IPC team.
Results
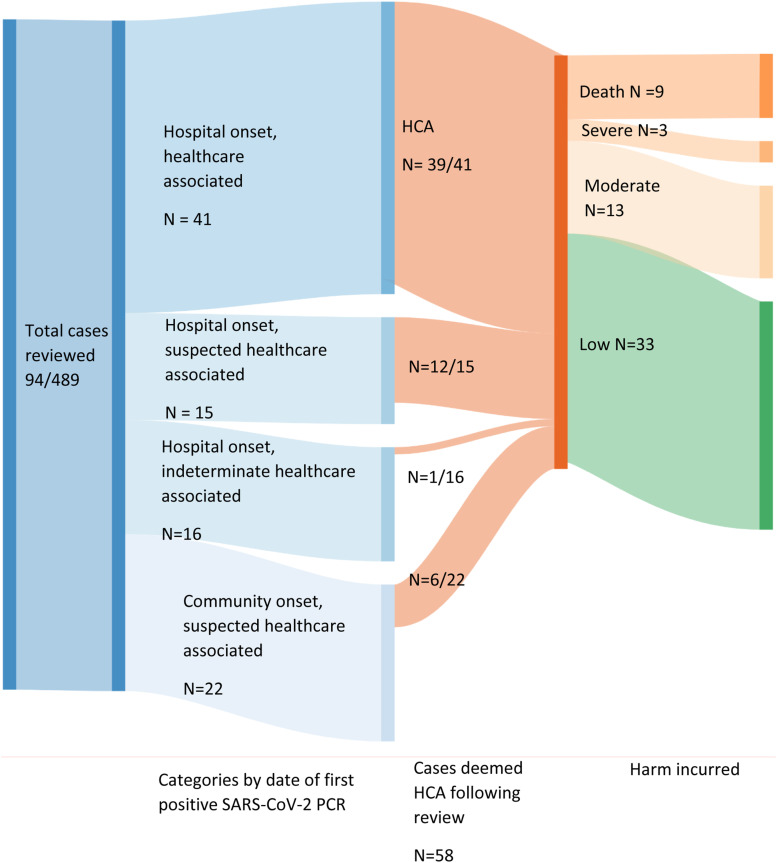
Cases: Of 489 confirmed SARS-CoV-2 cases admitted in the study period, 114 (23.3%) were identified across the four categories in Table 1 as HCA; 58 (11.9%) were ultimately considered to be HCA post-review. Figure 2 shows case categorisation based on date of initial positive PCR, final categorisation following notes review and level of harm incurred. Of 43 community-onset suspected HCA cases, 22 were taken forward to full review. 14 of 58 (24.5%) with HCA infection died, 9 (64.3%) of which were thought to be due to HCA SARS-CoV2 infection. Three of 58 (5.1%) suffered severe harm (e.g. ICU admission) but survived.
Figure 2.
Summary of HCA cases by initial date-based categorisation and incurred harm. HCA, healthcare associated.
Gap analysis: Adherence to the gap analysis standards in the 58 HCA cases is shown in supplementary Table 1. Assessment of adherence to standards was complicated by variable data capture within the EHR. For example, medical review within 12 h was documented in 94.5% of cases whilst for standards regarding bed space cleaning, information was only captured in the EHR for 2/58 (3.4%) cases.
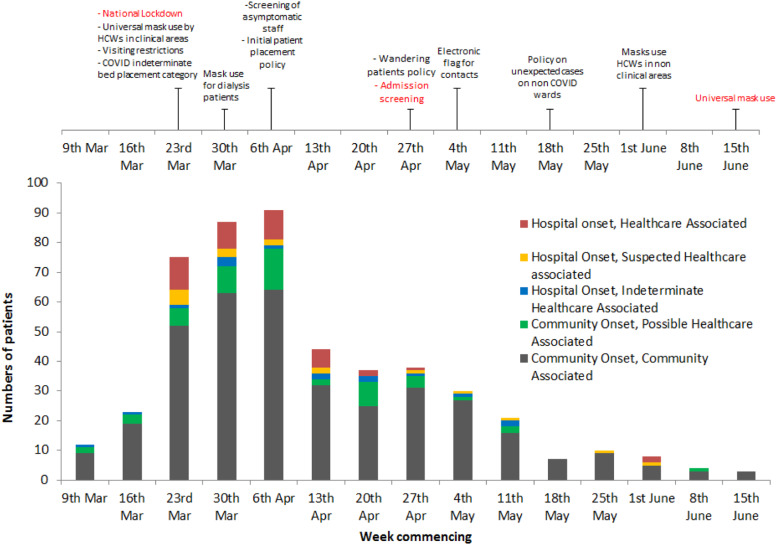
Summary of learning outcomes from free text review and working group discussions: Learning outcomes were grouped under five key themes to facilitate dissemination and implementation. Broadly, these could be considered to be patient-specific or resulting from wider healthcare system challenges. These are summarised in Table 2. Numbers of cases over time together with a timeline of key interventions arising from the case reviews and changes to national policy are shown in Figure 3.
Table 2.
Summary of learning outcomes from free text review and working group discussions.
| Major themes identified | Supporting examples from EHR reviews and actions taken |
|---|---|
| 1: Patient characteristics | Example A – Identification of vulnerable patients: patients with multiple bed moves or frequent healthcare contact, for example, dialysis |
| Dialysis patients rapidly identified as high-risk patients for severe COVID-19. Clusters of HCA cases were identified on our dialysis unit, associated with chair location within the unit and transport to and from their sessions | |
| Further cases identified in patients requiring inpatient dialysis, often associated with multiple bed moves | |
| Actions taken | |
| Liaison with renal teams and IPC review of the dialysis unit(s) | |
| All dialysis patients provided with fluid resistant surgical masks in hospital transport and while on dialysis | |
| Processes to decrease bed moves for dialysis patients reviewed | |
| Routine testing of dialysis patients proposed but not introduced due to testing capacity constraints | |
| Example B – Confused patients who ‘wander’ on wards | |
| Mobile patients with underlying acute or chronic confusional states identified as potential spreaders in a cluster of HCA cases on a non–COVID-19 ward | |
| Actions taken | |
| Working with liaison psychiatry and geriatrics teams to produce a specific hospital policy on management and placement of wandering patients during the pandemic | |
| 2: Communication factors | Example C – Documentation of contacts of SARS-CoV-2 positive patients |
| On reviewing the notes, frequently unclear if a patient had been a contact of a known SARS-CoV-2 case, particularly when a patient had moved wards | |
| Actions taken: Electronic flag placed on the front of notes of any contact of an SARS-CoV-2 case in hospital for 14 days from their exposure | |
| Example D – Communication of SARS-CoV-2 PCR results | |
| Delays identified in ward team awareness of new positive results from both laboratory and point of care testing | |
| Actions taken | |
| Policy changed to ensure all positive results sent to the IPC team for follow-up to minimise delay in IPC actions | |
| Example E – Raising awareness within ward teams following a case of HCA SARS-CoV-2 infection on their ward | |
| Delays noted in testing patients with possible COVID-19 symptoms on wards where recent HCA cases had occurred | |
| Testing sometimes not carried out due to presence of alternative causes for fever | |
| Actions taken | |
| IPC team to discuss all new HCA cases with ward manager, matron and consultant in charge to disseminate information to staff | |
| SARS-CoV-2 testing policy changed to advise rapid PCR testing in any new case of inpatient fever or hospital-acquired pneumonia | |
| 3: Infection prevention and control implementation | Example F – Inappropriate placement of at-risk patients and delays in isolation |
| Patients with significant comorbidity and strongly suspected COVID-19 symptoms awaiting SARS-CoV-2 PCR results or with negative results placed in COVID-19 cohort bays, putting them at risk of contracting infection | |
| Patients only isolated in a side room once positive test result returned rather than on clinical suspicion of COVID-19 | |
| Delays noted in reviewing inpatients with new fever | |
| Actions taken | |
| Clear patient placement and de-escalation guideline introduced with dedicated infection specialist to call for advice on patient placement | |
| Change in policy for placement of strongly clinically suspected PCR-negative patients to remain in side room rather than cohorting with PCR-positive patients | |
| Example G – Placement of admissions from nursing homes/residential homes | |
| On reviewing admissions and community data, significant numbers of cases admitted from care homes | |
| Actions taken | |
| Policy changed to consider all nursing/residential home residents as potential contacts and bed placement chosen accordingly | |
| 4: Policy factors | Example H – Management of new cases in ‘non-COVID-19’ areas (both staff and patients) |
| Several clusters identified on wards in non–COVID-19 areas | |
| Actions taken | |
| Introduction of fluid resistant surgical mask wearing for all staff on non–COVID-19 wards | |
| Policy developed covering actions to be taken by ward and IPC teams following a new case in a non–COVID-19 area covering reviews of PPE, cleaning and heightened vigilance for new cases | |
| Formalised policy for HCW testing in response to HCA infections in non–COVID-19 wards was developed | |
| Introduction of face masks for handover meetings | |
| Example I – Impact on patients requiring specialist care | |
| Harm incurred by acquiring coronavirus in hospital resulting in limited access to specialist care | |
| Actions taken | |
| Policies developed by individual teams to aid access to services in hospital, for example, stroke management | |
| Example J – Management of potential false-positive results | |
| Patients with possible false-positive results being treated as true positives thus putting them at risk of acquiring infection | |
| Actions taken | |
| Patients isolated in side rooms until results confirmed | |
| 5: Organisational response | Example K – Problems with the HCA case review process |
| Learning outcomes for individual patients found to be relevant to others within the same cluster, with gaps in practice for one patient directly impacting on the care of others | |
| Documentation of IPC measures taken often difficult to access retrospectively for investigating clinicians | |
| Actions taken | |
| Future RCAs to be looked at by cluster | |
| Centralised IPC database created to document dates of ward/bay closures | |
| PPE protocol in use on the wards to be kept regularly updated |
EHR, electronic health record; HCA, healthcare associated; HCW, healthcare worker; IPC, infection prevention and control; PPE, personal protective equipment; RCA, Root cause analysis.
Figure 3.
Confirmed cases of infection with SARS-CoV-2 between 9 March 2020 and 22 June 2020 by likely acquisition and timeline of IPC interventions implemented. National policies are shown in red text. HCW, healthcare worker; IPC, infection prevention and control.
Resources used: Each case review took approximately 20–30 minutes by an infection clinician. The weekly group discussion, typically involving 5–6 patients (but up to 15), took 1 h. Feedback to individual teams took a further 5 minutes per patient. Issues that were raised which resulted from a wider healthcare system challenge were fed back by the IPC team through the hospital command structure so learning could be disseminated across the Trust (e.g. via communications/changes in policy).
Discussion
We outline the learning outcomes identified by our HCA SARS-CoV-2 infection review process used during the first wave of the pandemic in the UK. In the face of an evolving situation with constantly changing national guidance, we rapidly identified five major areas requiring intervention with the aim of reducing HCA cases, whilst identifying gaps in hospital policies/practice that could be altered to improve patient and staff safety. Much of our ability to do this whilst implementing changes to practice derived from the review process itself and, to our knowledge, is the first description of such a process for HCA SARS-CoV-2 infection in the UK.
Many of the key themes identified (patient characteristics, communication, IPC implementation, policy factors and organisational response) are areas commonly highlighted in patient safety reviews (NPSA, 2009). These findings provided us with ways of selecting key areas/patient groups for targeted interventions.
Specific areas that we identified included clinical staff failing to suspect infection with SARS CoV-2 both when interpreting clinical symptoms and assessing patient vulnerability to infection. For example, the vulnerability of dialysis patients to infection with SARS-CoV-2 became clear early on and has been supported by other studies (Naicker et al., 2020; Rombola et al., 2020). Raising awareness of this was key to preventing further outbreaks in this cohort. Secondly, testing for SARS-CoV-2 (i.e. speed of testing, communication of results and actions upon obtaining results) was frequently identified as issues in HCA cases and could often have been dealt with sooner. There were also frequent issues relating to the delayed isolation of patients.
We found the investigational process to have a number of strengths and weaknesses. During the pandemic, IPC teams saw unprecedented workload increases. IPC resources within hospitals had to be deployed to areas of greatest need. Whilst some areas were thought likely to be high risk for transmission (e.g. ICU), our work identified other high risk areas (e.g. wards with patients undergoing multiple bed moves). We also picked up potentially contributory problems (e.g. management of confused patients) and targeted policies and practice in light of the lessons learnt from this process and fed back to teams on an individual and trust-wide basis.
The most widely used strategy for investigating patient safety incidents and deriving learning outcomes in England is RCA, although alternatives exist (Hagley et al., 2019). RCAs can take 20–90 person hours to complete (Wu et al., 2008) and there is little evidence for their effectiveness in healthcare (Latino et al., 2015; Peerally et al., 2017). Our experience of the shorter post-infection review process for HCAI is that it still requires several hours work per case; our approach took less than an hour per patient.
Gap analyses have been used to identify deficiencies in health systems and pathways previously (Amaratunga et al., 2007; Golden et al., 2017; Weinshel et al., 2015). Despite problems encountered in documentation of our chosen standards, gap analysis represented a targeted, standardised method through which we rapidly assessed our IPC practices and policies and provided a starting point for wider discussion of cases as a group from which the majority of our learning was derived.
Change was rapidly implemented, often prior to similar national changes being announced (Figure 3). We typically adapted policies (and therefore practice) within one week of the review. Involvement of the patient safety team and IPC team was central to this as they included participants with the skills and influence within hospital management to ensure swift implementation of new policies. The standard RCA process, with a 60 days turnaround time (NHSE, 2015), would have been too slow to have had any beneficial effect.
Despite the strengths of this process, several limitations need to be addressed to ensure sustainability. A key limitation is its focus on learning about the hospital system rather than the individual patient; that is, it did not rigorously identify why an individual patient contracted the virus but rather gaps in hospital processes/policies from the point they were suspected to have SARS CoV-2 infection. In addition, we focussed on the management of HCA cases and did not look at the management of healthcare worker infections or community-onset infections admitted to hospital which may have impacted on the development of HCA cases. The exact reason for an individual infection may be difficult to discern through any process, especially given the long incubation period, incomplete case ascertainment and asymptomatic transmission of SARS-CoV-2. This is of particular relevance when considering duty of candour legislation (DHSC, 2014) and the need to inform patients about the circumstances in which they came to harm. Universal admission screening, periodic retesting in key wards and expanded HCW screening combined with epidemiological analyses based on genomic and transmission models may provide improved resolution in the near future. Another limitation is that we chose the standards initially on a background of limited knowledge of the transmission dynamics. Nevertheless, our standards reflect the combined opinions of our expert group and their understanding of the situation at that time.
Due to the unclear nature of transmission dynamics at the beginning of the pandemic, we reviewed all cases which were potentially HCA (Table 1). This was time-consuming, involving reviewing the notes of many patients who were eventually deemed to be community-onset. This is easier now due to clear national definitions, though ideally a combination of date of onset with genomic-epidemiological data should be used (Price et al., 2021).
NHS England now requires RCAs on all patients diagnosed >7 days post-admission. We adapted our strategy but retain key elements. We look at clusters of patients together as the learning from individual cases is often shared with other cases, increasing the efficiency of meetings. As we identified, documentation of IPC procedures (e.g. ward closures) is often recorded at ward level rather than in an individual’s notes. To facilitate better capture of this information, collation of relevant data is now included in our process, providing more clarity on the chain of events leading to an individual’s infection.
We believe our experience is applicable to other healthcare providers. We have seen similar rates of HCA cases to others allowing for differences in definition (Rickman et al., 2021; Wang et al., 2020) although few published series exist. We were fortunate in having the resources to perform this. However, we think it provides an invaluable starting point for hospitals considering similar investigations and that the learning outcomes are broadly applicable to many healthcare settings.
Conclusion
Learning identified through this process allowed us to rapidly respond to potential HCA SARS-CoV-2 cases in order to deal with issues raised locally (i.e. at individual ward level) and hospital level. Whilst timely identification of HCA infection is vital to prevent onward transmission (Price et al., 2021), a robust system of review and learning is also essential in hospitals to prevent future harm.
Supplemental Material
Supplemental Material for Investigation of healthcare-associated SARS-CoV-2 infection: Learning outcomes from an investigative process in the initial phase of the pandemic by Isabel Ramsay, Katherine Sharrocks, Ben Warne, Nyarie Sithole, Pooja Ravji, Rachel Bousfield, Nick Jones, Clare E Leong, Mohamed Suliman, Rachel Tsui, Michelle Toleman, Chris Moody, Richard Smith, James Whitehorn, Theodore Gouliouris, Florentina Penciu, Christian Hofling, Christopher Cunningham, David A Enoch1 and Elinor Moore in Journal of Infection Prevention.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Isobel Ramsay https://orcid.org/0000-0002-9954-2023
Katherine Sharrocks https://orcid.org/0000-0002-0514-1772
Ben Warne https://orcid.org/0000-0003-1326-0373
Nyarie Sithole https://orcid.org/0000-0002-8020-223X
Pooja Ravji https://orcid.org/0000-0002-2878-5538
Rachel Bousfield https://orcid.org/0000-0001-7828-4048
Nick Jones https://orcid.org/0000-0003-4475-7761
Clare E Leong https://orcid.org/0000-0001-9075-1630
Mohamed Suliman https://orcid.org/0000-0002-5562-5628
Michelle S Toleman https://orcid.org/0000-0002-4656-3066
James Whitehorn https://orcid.org/0000-0003-0789-5954
Christian Hofling https://orcid.org/0000-0003-2951-7620
Chris Cunningham https://orcid.org/0000-0001-9145-673X
David A Enoch https://orcid.org/0000-0002-5433-0801
References
- Amaratunga CA, O’Sullivan TL, Phillips KP, et al. (2007) Ready, aye ready? Support mechanisms for healthcare workers in emergency planning: a critical gap analysis of three hospital emergency plans. American Journal of Disaster Medicine 2: 195–210. [PubMed] [Google Scholar]
- Basile C, Combe C, Pizzarelli F, et al. (2020) Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrology Dialysis Transplantation 35: 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B, Collins JT, Barlow-Pay F, et al. (2020) Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial study (COVID in Older PEople). Journal of Hospital Infection 106: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHSC (2014) The health and social care act 2008 (regulated activities) regulations 2014. Available at: https://www.legislation.gov.uk/uksi/2014/2936/regulation/20/made (accessed on 10 August 2020).
- Golden SH, Hager D, Gould LJ, et al. (2017) A gap analysis needs assessment tool to drive a care delivery and research agenda for integration of care and sharing of best practices across a health system. The Joint Commission Journal on Quality and Patient Safety 43: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagley G, Mills PD, Watts BV, et al. (2019) Review of alternatives to root cause analysis: developing a robust system for incident report analysis. BMJ Open Quality 8: e000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Rahman KM, Sun Y, et al. (2020) Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: a global analysis. Infection Control & Hospital Epidemiology 41: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latino RJ. (2015) How is the effectiveness of root cause analysis measured in healthcare? Journal of Healthcare Risk Management 35: 21–30. [DOI] [PubMed] [Google Scholar]
- Meredith LW, Hamilton WL, Warne B, et al. (2020) Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. The Lancet Infectious Diseases 20: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naicker S, Yang C-W, Hwang S-J, et al. (2020) The novel coronavirus 2019 epidemic and kidneys. Kidney International 97: 824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS (2020) Minimising nosocomial infection in the NHS. Available at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/C0586-minimising-nosocomial-infections-in-the-nhs.pdf (accessed on 19 February 2021).
- NHSE (2015) Serious Incidents Framework in the NHS. Available at: https://www.england.nhs.uk/wp-content/uploads/2015/04/serious-incidnt-framwrk-upd.pdf (accessed on 19 February 2021).
- NPSA (2009) Root cause analysis investigation tools contributory factors classification framework 2009:1–5. Available at: https://improvement.nhs.uk/documents/6528/PSII_Contributory_and_Mitigation_Factors_Classification.pdf (accessed on 19 February 2021).
- NRLS (2004) Degree of harm FAQ. Available at: https://improvement.nhs.uk/documents/1673/NRLS_Degree_of_harm_FAQs_-_final_v1.1.pdf (accessed on 19 February 2021).
- Peerally MF, Carr S, Waring J, et al. (2017) The problem with root cause analysis. BMJ Quality & Safety 26: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JR, Mookerjee S, Dyakova E, et al. (2021) Development and delivery of a real-time hospital-onset COVID-19 surveillance system using network analysis. Clinical Infectious Diseases 72: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman HM, Rampling T, Shaw K, et al. (2021) Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clinical Infectious Diseases 72: 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombolà G, Heidempergher M, Pedrini L, et al. (2020) Practical indications for the prevention and management of SARS-CoV-2 in ambulatory dialysis patients: lessons from the first phase of the epidemics in Lombardy. Journal of Nephrology 33: 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. (2020) COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. The Lancet Infectious Diseases 20: 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou Q, He Y, et al. (2020) Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. The European Respiratory Journal 55: 2000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshel K, Dramowski A, Hajdu Á, et al. (2015) Gap analysis of infection control practices in low- and middle-income countries. Infection Control & Hospital Epidemiology 36: 1208–1214. [DOI] [PubMed] [Google Scholar]
- Wu AW, Lipshutz AKM, Pronovost PJ. (2008) Effectiveness and efficiency of root cause analysis in medicine. Journal of the American Medical Association 299: 685–687. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Gao Y, Gao Y, et al. (2020) Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Annals of Translational Medicine 8: 629–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Investigation of healthcare-associated SARS-CoV-2 infection: Learning outcomes from an investigative process in the initial phase of the pandemic by Isabel Ramsay, Katherine Sharrocks, Ben Warne, Nyarie Sithole, Pooja Ravji, Rachel Bousfield, Nick Jones, Clare E Leong, Mohamed Suliman, Rachel Tsui, Michelle Toleman, Chris Moody, Richard Smith, James Whitehorn, Theodore Gouliouris, Florentina Penciu, Christian Hofling, Christopher Cunningham, David A Enoch1 and Elinor Moore in Journal of Infection Prevention.