Abstract
Study Objective:
The objective of our study was to determine safety and pharmacology (pharmacokinetics and preliminary efficacy) of intranasal (IN) ketamine for uncontrolled cancer-related pain.
Design:
Dose escalation clinical trial.
Setting:
Outpatient.
Patients:
Ten adult patients with uncontrolled cancer-related pain.
Intervention:
Each patient received escalating doses of ketamine over four visits, each 2–5 days apart: 10 mg IN at visit 1, 10 mg intravenous (IV) at visit 2, 30 mg IN at visit 3, and 50 mg IN at visit 4.
Measurements:
Pain was measured before and after drug administration for up to 4 h using the 11 point (0–10) Numerical Pain Rating Scale (NPRS).
Main Results:
All subjects had advanced cancer, with intractable pain, despite being on moderate dosage of opioids. There was a statistically significant reduction in median NPRS by 1.5 (1–4), 3 (2–3), and 4 (3–5) points at 60 min after receiving the medication and remained decreased by 1.5 (1–2), 2 (1–2) and 1 (1–4) points at the end of the study visit (240 min) with the 10 mg, 30 mg and 50 mg IN dosage, respectively. The median percentage of maximal pain relief being 22.5 (16.6–71.5), 65.5 (40–100), and 69.25 (50–100) for 10 mg, 30 mg and 50 mg IN dosage, respectively and 100 (75–100) with 10 mg IV dose. All side effects (nausea and feeling of unreality) resolved by the end of each study visit. No severe adverse events occurred.
Conclusion:
In this single-institution study, all dosages of IN ketamine administered in the study (10, 30, and 50 mg) provided significant pain relief for intractable cancer-related pain and were well tolerated. The 50 mg dose provided maximal pain relief without major side effects. Further study focused on repeated administration efficacy and safety for cancer-related pain is warranted.
Keywords: cancer pain, intranasal ketamine, pharmacodynamics, pharmacokinetics, safety
1 |. INTRODUCTION
Approximately 38% of the 11.9 million Americans living with cancer report moderate-to-severe pain (Numerical Pain Rating Score [NPRS] >5).1 Despite high-dose opioid use, pain may remain uncontrolled due to tolerance or unresponsive pain mechanisms.2 In some cases, interventional procedures may be used instead.3 However, their use may be limited by logistical (e.g., available expertise) or patient factors (e.g., abnormal lab results, reservation towards interventions, etc.).4 Thus, identification and evaluation of new evidence-based therapies, including pharmacologic agents, to address cancer pain are imperative.
Ketamine has been studied in the search for more effective pharmacologic interventions for pain management.5–7 It produces analgesia in humans and modulates central sensitization and opioid tolerance via N-methyl-D-aspartate (NMDA) receptor antagonism.8–11 Ketamine also works on other receptors to block pain transmission via voltage-sensitive calcium channels, depression of sodium channels, modulation of cholinergic neurotransmission, and inhibition of uptake of serotonin and norepinephrine.12 Unlike opioids, ketamine does not depress respiratory function.
The benefits of ketamine must be balanced with its adverse effects and potential for abuse. Acute, dose-dependent adverse events include hypertension, tachycardia, psychotomimetic phenomena (e.g., hallucinations and nightmares), delirium, dizziness, visual changes, nystagmus, altered hearing, hypersalivation, nausea, and vomiting.13,14 Long-term frequent administration in abusers is associated with cognitive impairment, urinary bladder toxicity, and hepatic toxicity.15,16 Although various ketamine doses have been studied, the recommended outpatient dose range for chronic pain is 0.5–2 mg/kg intravenously (IV) or intramuscularly (IM) and 0.2–1 mg/kg intranasally (IN).5 Low-dose/subanesthetic (<1 mg/kg) ketamine has gained interest as it reduces the risk of dose-dependent psychomimetic side effects.
Compared to the oral route, IN ketamine has higher absorption (10%–25% oral vs. 25%–50% IN) as it avoids first-pass hepatic metabolism. It provides a needle-free, patient-friendly route of administration.4 A double-blind placebo-controlled crossover study showed that ≤50-mg IN ketamine controlled breakthrough pain in 20 patients with cancer and noncancer-related pain (2.65 points average decrease on NPRS scale). The pain scores were only recorded until 60 min after drug administration and patients determined their own dosage of IN ketamine, ranging from 10 to 50 mg.17 Intranasal S-ketamine spray is now approved for depression treatment by the United States Food and Drug Administration (FDA).18 An in-depth dose-finding study of IN ketamine for cancer pain has not yet been conducted. The overall goal of our study was to determine the safety and pharmacology (pharmacokinetics and preliminary efficacy) of IN ketamine for uncontrolled cancer-related pain.
2 |. METHODS
2.1 |. Study design
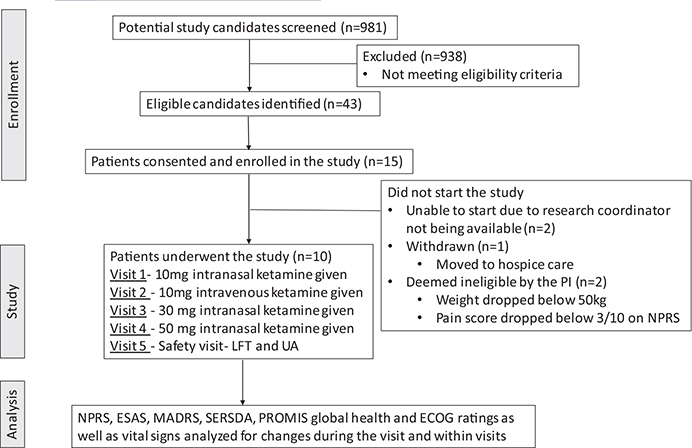
This was a prospective, intrapatient dose-escalation trial of IN ketamine in patients with uncontrolled pain related to cancer or cancer treatment. The study was approved by the Emory University Institutional Review Board, an Investigational New Drug application was obtained, and the trial was registered on clinical trials.gov (NCT03146806). The study schema is shown in Figure 1.
FIGURE 1.
Study schema (consort diagram): Total 981 charts of patients coming to supportive care, pain, and oncology clinics were screened. A total of 10 patients completed the trial and received different dosages of ketamine
2.2 |. Patient characteristics
Patients were recruited from supportive oncology, medical oncology, and pain clinics at our institution.
2.2.1 |. Inclusion criteria
>18 years old; uncontrolled pain related to cancer or its treatment for >7 days, rated as >4 on NPRS19,20; on >50 mg per day oral Milligram Morphine Equivalent/MME (opioid nonresponsive or opioid tolerant)21; on a stable analgesic regimen for >7 days without escalation prior to the study period; used their rescue or immediate-release opioid pain medication with a frequency of every 3 h or longer; had ability to give written informed consent; and weighed ≥50 kg (so each single dose delivered in the study was <0.5-mg/kg IV ketamine equivalent, with an assumption of 50% maximal bioavailability for IN ketamine).
2.2.2 |. Exclusion criteria
History of severe cardiac disease, end-stage liver disease, hemorrhagic stroke, elevated intracranial pressure, seizures, uncontrolled depression, psychosis, interstitial cystitis, or medication abuse/misuse; baseline tachycardia; lesions of the nasal mucosa; or concomitant Cytochrome P450 3A4 (CYP3A4) inducers (strong or moderate) or strong inhibitors use as this can interfere with ketamine metabolism. Given the medical lability of patients with advanced cancer pain, patients were required to meet study eligibility criteria again at the beginning of visit 1.
Given the abuse potential of ketamine, we see it fit for patients with opioid-tolerant or opioid nonresponsive cancer-related pain. Therefore, one of the eligibility criteria was being on high-dose oral opioids. To avoid confounding the analgesic effects of intranasal ketamine with the effects of the short-acting/immediate-release opioids, only patients using short-acting/immediate-release opioid medications at least 3 h apart were eligible. The peak time of onset for most immediate-release opioids is approximately 45 min. Patients were given study medication at least 120 min after their last dose of immediate-release opioid and were allowed to take their as-needed immediate-release/breakthrough pain medication 15 min after study medication administration, if needed. If breakthrough pain medication was taken, it was documented if it was taken at the usual interval or a longer time period.
2.3 |. Study visits
The study period consisted of five study visits conducted at our Phase I Clinical Trials Unit. To avoid ketamine accumulation, each visit was spaced 2–5 days apart, except for visit 5, which was solely a safety visit. All visits occurred within 1 month of study visit 1. Driving was discouraged for 24 h poststudy drug administration. A follow-up call was made 14 days after the last dose to capture any potentially study-related adverse event.
2.4 |. Study agent
IN ketamine was prepared from a 100-mg/ml vial and delivered via a mucosal atomization device (MAD) connected to a 1-ml syringe. Each spray delivered 0.1 ml to provide 10 mg of atomized ketamine. Escalating dosages of IN ketamine were given to find the optimal analgesic dose that lacked significant side effects. On study visit 1, 10 mg of IN ketamine was given to ensure patients could tolerate a low dose. On visit 2, 10 mg of IV ketamine was given to compare bioavailability with patients serving as their own controls. On visits 3 and 4, if patients did not experience serious adverse events with the lower dosage, higher doses (30 and 50 mg, respectively) were delivered. A licensed study personnel (nurse or physician) administered the ketamine.
2.5 |. Administration of IN ketamine
The investigational drug services ensured the MAD was primed and loaded into the syringe at each IN dose. Patients were seated and their sinuses cleared with a tissue if needed. They were asked to tilt their head back, and the MAD was inserted into their nostril, aiming posterior, level with the floor of the nares and slightly lateral. If more than 0.1 ml needed to be delivered, alternating nares were used to administer each spray, at least 30 s apart. Patients were asked to keep their head tilted back for 5 min, if tolerated, to ensure that the medication did not run out of their nares. They were asked to report the sensation of medication trickling down their throat. Any medication visibly running out of nares was noted.
2.6 |. Safety
Side effects were measured on the Side Effects Rating Scale for Dissociative Anesthetics (SERSDA), prior to and at 30, 60, and 240 min after drug administration. Vital signs including heart rate, blood pressure, respiratory rate, and pulse oximetry were measured at baseline, and 5, 15, 30, 45, 60, 120, 180, and 240 min following drug delivery. Liver function tests (LFT) and urinalyses (UA) were done on visits 1 and 5.
2.7 |. Pharmacokinetics
Blood samples were obtained in 2 × 3-ml ethylenediaminetetraacetic acid (EDTA) vacuum tubes at 2, 30, 60, and 240 min after dosing. Baseline blood samples were drawn on visits 2 through 5, to verify the absence or presence of carryover ketamine. The total blood drawn during one visit was 24–30 ml. Samples were promptly centrifuged, and plasma was separated into preservative-free cryovials and frozen at −20°C. Samples were sent within 3 months to the National Medical Services (NMS) for concentration analysis of ketamine and its metabolite nor-ketamine via gas chromatography with mass spectrometry (GC/MS). Review of previous pharmacokinetic data showed that patients receiving continuous IV ketamine at 40 mg/h had plasma ratios of (S)- to (R)-ketamine of 0.77 and (S)- to (R)-nor-ketamine of 0.71.22–24 These ratios suggest that our nonchiral analysis of ketamine and nor-ketamine were reflective of both stereoisomeric variants.
2.8 |. Patient-reported outcomes
Pain assessment in cancer and other areas relies on patient-reported methods in trials. Pain intensity on the NPRS was recorded prior to and at 5, 10, 15, 30, 45, 60, 120, 180, and 240 min after study drug administration.25 Compared to the Visual Analogue Scale (VAS) and Visual Rating Scale (VRS), the NPRS is a more responsive tool with higher compliance rate.25,26 In general, at least a 30% or 2-point change is considered clinically significant.27 Depression was assessed at baseline and 180 min after study drug administration at each visit on the Montgomery-Asberg Depression Rating Scale (MADRS), a clinician-rated 10-item questionnaire designed to be sensitive to treatment effects.28,29 Each item is scored from 0 (no symptoms) to 6 (severe or continuous symptoms), with a 7-day recall period.30,31 A 6-point reduction in MADRS score is considered to be clinically meaningful.32 The Side Effects Rating Scale for Dissociate Anesthetics (SERSDA) was used to measure side effects including fatigue, dizziness, nausea, headache, feeling of unreality, change in hearing, change in vision, mood change, general discomfort, and hallucinations; each rated as 0–4 where 0 = no side effects and 4 = side effect very bothersome.17,33
The Patient-Reported Outcome Measurement Information System (PROMIS) for global health (10-item) questionnaire was used as an indicator of global mental and physical function and completed at baseline and last study visits. It is a short, yet useful screening tool that measures five domains: physical function, fatigue, pain, emotional distress, and social health.34 It may lack precision in specific domains, limiting its use as an independent quality of life (QOL) analysis tool.35,36 Performance status was assessed on Eastern Cooperative Oncology Group (ECOG) grading at baseline and throughout the study,37 which ranges from 0 to 5, where 0 is fully active and 5 is dead. Palliative care symptom assessment was performed on the Edmonton Symptom Assessment Scale (ESAS), a reliable and validated multidimensional assessment tool. It assess nine common symptoms (pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath) experienced by cancer patients, rated from 0 to 10; where 0 = no symptom and 10 = worst possible severity.38–40
2.9 |. Statistical analysis
All calculations were performed using SAS software version 9.4 (SAS Institute Inc.) and SigmaPlot 14.0 notebook. Normality was assessed by the Shapiro–Wilk test. Changes in continuous variables were assessed using paired T-tests for normally distributed data or Wilcoxon signed-rank test for non-normally distributed data. Wald confidence intervals for proportions were set at 95%. Non-normally distributed data are presented at the median (25th percentile, 75th percentile).
2.10 |. Pharmacokinetic data analysis
Because of variability in concentration versus time profiles between IV and IN routes, as well as between individuals, almost every concentration versus time profile had a sample with a result below the lower limit of quantitation (LLQ) of 20 ng/ml, complicating the derivation of individual-level pharmacokinetic (PK) parameters. Therefore, data points with values <LLQ were replaced with half LLQ (10 ng/ml) for the purpose of concentration-based PK parameters (area under the curve [AUC], maximum concentration [Cmax]) and removed for the purpose of time to maximum concentration (Tmax) calculations, according to method M6 as described.41 The number of truly informed concentration values at each timepoint was tracked throughout the analysis. Concentration profiles were constructed from the averages of the 10 patients by timepoint and route. Pharmacokinetic parameters were calculated noncompartmentally using PK Solutions 2.0 (Summit Research Services, Montrose, Colorado).
3 |. RESULTS
Between January 2018 and August 2019, 981 patients from palliative/supportive care clinics were screened for eligibility via chart review. Of these, 43 met all eligibility criteria, and 15 were enrolled. One patient withdrew following consent due to rapid clinical decline with hospice transition. Two patients were unable to start the study in a timely fashion due to logistical issues; two were deemed ineligible on the first study visit as the weight dropped <50 kg for one and NPRS score decreased to <3/10 for another. In all, 10 patients finished the study and received all four ketamine dosages. One patient had a visit outside the designated treatment window due to disease progression.
3.1 |. Baseline characteristics
The median age of the participants was 49.5 years, with six males and four females (Table 1). The median baseline NPRS pain score was 7/10, and the median baseline opioid intake was 160 MME. All had advanced cancer (stage IV), with breast cancer the most common (40%). Other cancers included osteosarcoma, lung cancer, colon cancer, laryngeal cancer, and multiple myeloma. Three patients had liver metastasis, while others had lung or bone metastasis. Either primarily nociceptive pain or mixed nociceptive/neuropathic pain were reported; none had primarily neuropathic pain. Concomitant pain medications are listed per patient in Table 2. In addition, many of these patients had failed multiple pain medications and interventional procedures.
TABLE 1.
Patient demographics and pain characteristics
| Participant characteristics | (n = 10) |
|---|---|
| Continuous variables | Median (interquartile range) |
| Age (years) | 49.5 (31–59) |
| Body mass index | 27.8 (22.3–29.5) |
| Weight (kg) | 77.3 (64.8–80.8) |
| Baseline pain score | 7 (5–8) |
| Baseline morphine equivalent (mg) | 160 (120–180) |
| Categorical variables | Frequency (percent) |
| Sex | |
| Male | 6 (60) |
| Female | 4 (40) |
| Race | |
| White | 7 (70) |
| Black/African American | 3 (30) |
| Ethnicity | |
| Non-Hispanic | 9 (90) |
| Hispanic | 1 (10) |
| Cancer type | |
| Breast | 4 (40) |
| Bone | 2 (20) |
| Lung | 1 (10) |
| Colon | 1 (10) |
| Laryngeal | 1 (10) |
| Multiple myeloma | 1 (10) |
| Pain type | |
| Nociceptive | 5 (50) |
| Neuropathic | 0 |
| Mixed | 5 (50) |
TABLE 2.
Concomitant pain medication use per patient
| Patient # | Concomitant long-acting opioid medications | Concomitant breakthrough/as-needed opioid medications | Concomitant nonopioid pain medications | Use of breakthrough medication during study visits |
|---|---|---|---|---|
| 1 | Morphine extended-release 30 mg every 8 h | Morphine immediate release 7.5 mg every 6 h | Pregabalin 50 mg every 8 h | Did not need to take breakthrough pain medications during any of the study visits |
| 2 | Methadone 10 mg every 8 h | Morphine immediate release 20 mg every 6 h | Gabapentin 300 mg every 8 h | Took morphine on visit 3 (30 mg intranasal ketamine), at 235 min (5 min prior to discharge), which was the usual interval |
| 3 | Fentanyl patch 50 mcg/h | Oxycodone 10 mg every 6 h | Duloxetine 60 mg per day, gabapentin 300 mg every 8 h, cyclobenzaprine every 8 h as needed | Did not need to take breakthrough pain medications during any of the study visits |
| 4 | Oxycodone extended-release 80 mg every 12 h | Oxycodone 20 mg every 6 h | Gabapentin 400 mg every 8 h, turmeric 500 mg every 12 h | Did not need to take breakthrough pain medications during any of the study visits |
| 5 | Oxycodone extended-release 20 mg every 12 h | Oxycodone 20 mg every 6 h | Venlafaxine extended-release 75 mg per day, turmeric 500 mg every 12 h | Did not need to take breakthrough pain medications during any of the study visits |
| 6 | Fentanyl 125 mcg/h | Hydromorphone 8–12 mg every 4 h | Pregabalin 50 mg every 8 h, duloxetine 60 mg per day, 5% lidocaine patch | Did not need to take breakthrough pain medications during any of the study visits |
| 7 | Oxycodone extended-release 60 mg every 12 h | Oxycodone 30 mg every 4 h | Pregabalin 300 mg every 12 h, celebrex 200 mg every day, acetaminophen 1000 mg every 8 h as needed | Did not need to take breakthrough pain medications during any of the study visits |
| 8 | Oxycodone extended-release 30 mg every 12 h | Oxycodone 10 mg every 4 h | Acetaminophen 325 every 6 h when necessary, voltaren gel, Duloxetine 60 mg daily, lidocaine cream | Took oxycodone at visit 1 (10-mg intranasal ketamine) at longer than usual interval, but at 41 min after study drug administration. Also, took oxycodone at visit 3 (30 mg intranasal ketamine), at longer than usual interval, 232 min after study drug administration |
| 9 | Fentanyl 75 mcg/h | Hydromorphone 4 mg every 4 h | Pregabalin 75 mg every 12 h, venlafaxine 150 mg every day, tizanidine 4 mg every 12 h as needed, naproxen 500 mg every 12 h as needed | Took hydromorphone on visit 1 (10-mg intranasal ketamine) at longer than usual interval but at 15 min after study medication |
| 10 | Methadone 10 mg every 8 h | Hydromorphone 4mg every 4 h | Escitalopram 20 mg every day | Did not need to take breakthrough pain medications during any of the study visits |
Notes: All patients who took as needed pain medications during visit 1 (10 mg intranasal ketamine) had ≤20% pain relief with the study medication during that visit. All patients who took as needed pain medications during visit 3 (30 mg intranasal ketamine) took it towards the end of the study visit, at much longer than their usual as-needed medication times. None of the patients needed to take breakthrough medication during the study visit with the 50 mg intranasal ketamine dosage.
3.2 |. Safety
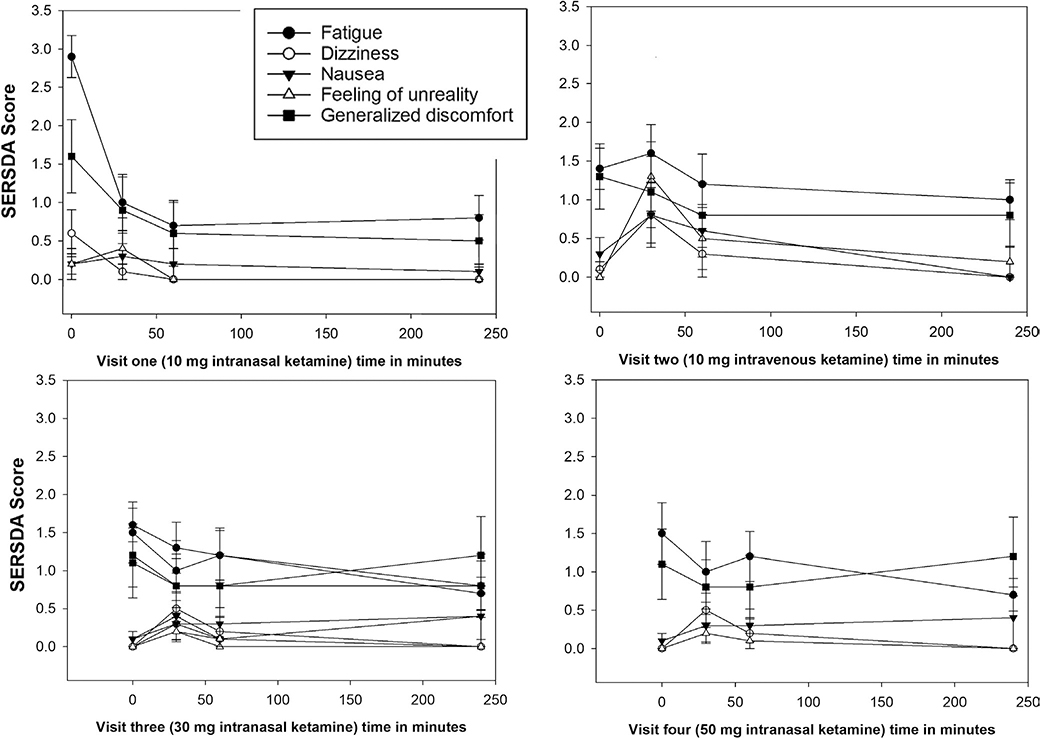
Side effects measured on the 10-item SERSDA scale are reported in Table 3. Symptoms of fatigue and generalized discomfort improved after ketamine administration. Dizziness, nausea, and a feeling of unreality were occasionally reported as temporarily worsening after ketamine, with the 10-mg IV dose being least well-tolerated (see Figure 2). All adverse effects disappeared by the end of the study visit, except for one patient who reported changes in nausea, mood, and generalized discomfort increasing towards the end of the study. This patient reported that the nausea was similar to what she was experiencing outside the study visit (related to her disease). Another patient-reported hearing change at end of the study visit, which was similar to chronic hearing changes he had been experiencing, unrelated to the study visit. Changes in vital signs at different timepoints during the same visit were not statistically significant. There were no changes noted in LFT or UA at the end of the study compared to the baseline values.
TABLE 3.
Side Effects Raing Scale for Dissociative Anesthetics (SERSDA) results at each timepoint at each visit
| SERSDA | Minute | Visit one |
Visit two |
Visit three |
Visit four |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 240 | 0 | 30 | 60 | 240 | 0 | 30 | 60 | 240 | 0 | 30 | 60 | 240 | ||
| Fatigue | Mean | 2.9 | 1 | 0.7 | 0.8 | 1.4 | 1.6 | 1.2 | 1 | 1.6 | 1.3 | 1.2 | 0.8 | 1.5 | 1 | 1.2 | 0.7 |
| SD | 0.88 | 1.15 | 0.95 | 0.92 | 0.84 | 1.17 | 1.23 | 0.82 | 0.7 | 1.1 | 1.1 | 1.03 | 1.27 | 1.25 | 1.03 | 0.67 | |
| Dizziness | Mean | 0.6 | 0.1 | 0 | 0 | 0.1 | 0.8 | 0.3 | 0 | 0 | 0.3 | 0.1 | 0 | 0 | 0.5 | 0.2 | 0 |
| SD | 0.97 | 0.32 | 0 | 0 | 0.32 | 1.14 | 0.95 | 0 | 0 | 0.67 | 0.32 | 0 | 0 | 0.71 | 0.63 | 0 | |
| Nausea | Mean | 0.2 | 0.3 | 0.2 | 0.1 | 0.3 | 0.8 | 0.6 | 0 | 0.1 | 0.4 | 0.1 | 0.4 | 0.1 | 0.3 | 0.3 | 0.4 |
| SD | 0.42 | 0.95 | 0.63 | 0.32 | 0.67 | 1.32 | 1.07 | 0 | 0.32 | 0.97 | 0.32 | 0.97 | 0.32 | 0.67 | 0.67 | 1.26 | |
| Headache | Mean | 0.2 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SD | 0.63 | 0 | 0 | 0 | 0 | 0.32 | 0.32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Feeling of unreality | Mean | 0.2 | 0.4 | 0 | 0 | 0 | 1.3 | 0.5 | 0.2 | 0 | 0.2 | 0 | 0 | 0 | 0.2 | 0.1 | 0 |
| SD | 0.63 | 1.27 | 0 | 0 | 0 | 1.42 | 1.27 | 0.63 | 0 | 0.42 | 0 | 0 | 0 | 0.42 | 0.32 | 0 | |
| Hearing change | Mean | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.3a | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SD | 0 | 0 | 0 | 0 | 0 | 0.32 | 0 | 0.95 | 0.32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vision change | Mean | 0.4 | 0.1 | 0 | 0 | 0.1 | 0.5 | 0.1 | 0 | 0.1 | 0.1 | 0 | 0 | 0 | 0.1 | 0.1 | 0 |
| SD | 0.7 | 0.32 | 0 | 0 | 0.32 | 0.85 | 0.32 | 0 | 0.32 | 0.32 | 0 | 0 | 0 | 0.32 | 0.32 | 0 | |
| Mood change | Mean | 0.5 | 0 | 0 | 0 | 0 | 0.6 | 0.4 | 0 | 0 | 0.1 | 0 | 0 | 0.5 | 0.4 | 0.1 | 0.3 |
| SD | 0.85 | 0 | 0 | 0 | 0 | 1.26 | 1.26 | 0 | 0 | 0.32 | 0 | 0 | 1.08 | 0.7 | 0.32 | 0.67 | |
| General discomfort | Mean | 1.6 | 0.9 | 0.6 | 0.5 | 1.3 | 1.1 | 0.8 | 0.8 | 1.2 | 0.8 | 0.8 | 0.8 | 1.1 | 0.8 | 0.8 | 1.2 |
| SD | 1.51 | 1.37 | 1.35 | 1.08 | 1.38 | 1.45 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.45 | 1.14 | 1.32 | 1.62 | |
| Hallucination | Mean | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: SD, standard deviation; SERSDA, Side Effects Rating Scale for Dissociative Anesthetics.
One subject reported hearing changes at 240 min mark rated as 3 on a scale of 0–4. However, he had chronic ringing in the ears, and this change was unlikely due to the study medication effect.
FIGURE 2.
Average of the change in side effects over the course of the study visit, as compared to predrug administration. Scale is 0–4, with 0 = no change and 4 = very bothersome. Change in following Side Effects Rating Scale for Dissociate Anesthetics (SERSDA) items are presented fatigue and generalized discomfort, which temporarily improved after ketamine administration and dizziness, nausea, and a feeling of unreality, which temporarily worsened (changes not statistically significant)
3.3 |. Patient-reported outcomes
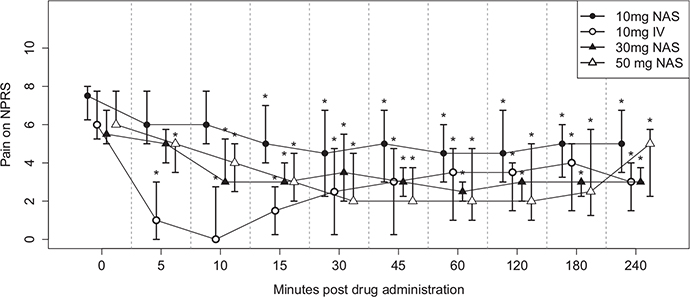
Patients reported varying intensity and duration of pain relief with each dose of ketamine (Tables 4 and 5, and Figure 3). There was a significant reduction in median (interquartile range) NPRS by 1 (1–2), 4.5 (3–6), 2 (1–3), and 4 (3–4) points at 15 min; 1.5 (1–4), 3 (2–3), and 4 (3–5) points at 60 min after receiving the medication; and remained decreased by 1.5 (1–2), 2 (1–2), and 1 (1–4) points at the end of the study visit (240 min) with the 10-, 30-, and 50-mg IN dosage, respectively (p < 0.05). The onset of any pain relief was within 5 min with the IV and within 15 min with the IN dose, except for one patient at visit 3, for whom onset was at 60 min. The onset of maximum pain relief was at 15 min with the IV and 45–60 min with the IN dose; median (interquartile range) percentage of maximal pain relief was 22.5 (16.6–71.5), 65.5 (40–100), and 69.25 (50–100) for 10-, 30-, and 50-mg IN dosage, respectively, and 100 (75–100) with the 10-mg IV dose. Although the median duration of maximal pain relief was close to 1 h, the median duration of any pain relief was sustained for study visit duration (4 h). All patients reported >30% pain relief with 30-mg and 50-mg IN ketamine dose except for one who reported 12.5% pain relief with the 30-mg dose and another who did not report any pain relief with the 50-mg dose. The median (interquartile range) baseline NPRS score was 7.5 (6–8), 6 (5–8), 5.5 (5–7), and 6 (6–8) at visit 1 (10-mg IN), visit 2 (10-mg IV), visit 3 (30-mg IN), and visit 4 (50-mg IN), respectively. No statistically significant difference in baseline pain scores was found between visits (see Table S1).
TABLE 4.
Pain relief by dosage (n = 10)
| Variable Median (interquartile range) | Visit 1 (10-mg intranasal ketamine) | Visit 2 (10-mg intravenous ketamine) | Visit 3 (30-mg intranasal ketamine) | Visit 4 (50-mg intranasal ketamine) |
|---|---|---|---|---|
| Percentage of maximum pain relief | 22.5 (16.6–71.5) | 100 (75–100) | 65.5 (40–100) | 69.25 (50–100) |
| Time to maximal pain relief (min) | 22.5 (15–60) | 5 (5–10) | 22.5 (10–30) | 15 (10–30) |
| Duration of maximal pain relief (min) | 60 (15–120) | 20 (10–210) | 55 (15–210) | 60 (5–210) |
| Duration of any pain relief (min) | 225 (55–235) | 235 (235–235) | 205 (110–235) | 235 (230–235) |
TABLE 5.
Pain scores on Numerical Pain Rating Scale (NPRS) at each timepoint (n = 10)
| Baseline | 5 min | 10 min | 15 min | 30 min | 45 min | 60 min | 120 min | 180 min | 240 min |
|---|---|---|---|---|---|---|---|---|---|
| Visit one (10-mg intranasal ketamine) | |||||||||
| 7.5 (6–8) | 6 (5–8) | 6 (5–8) | 5 (4–7) | 4.5 (2–7) | 5 (3–7) | 4.5 (3–6) | 4.5 (3–7) | 5 (3–6) | 5 (3–7) |
| Change from baseline | 0.5 (0–2) p = 0.063 | 0.5 (0–3) p = 0.062 | 1 (1–2) p = 0.004 | 1 (1–5) p = 0.004 | 1 (1–4) p = 0.008 | 1.5 (1–4) p = 0.002 | 1.5 (0–3) p = 0.016 | 2 (0–3) p = 0.016 | 1.5 (1–2) p = 0.008 |
| Visit two (10-mg intravenous ketamine) | |||||||||
| 6 (5–8) | 1 (0–3) | 0 (0–3) | 1.5 (0–3) | 2.5 (0–5) | 3 (0–5) | 3.5 (1–5) | 3.5 (1–4) | 4 (1–5) | 3 (1–4) |
| Change from baseline | 4 (3–7) p = 0.002 | 5.5 (3–7) p = 0.002 | 4.5 (3–6) p = 0.002 | 2.5 (1–6) p = 0.004 | 2.5 (1–5) p = 0.004 | 2 (1–4) p = 0.004 | 2 (2–4) p = 0.008 | 1.5 (1–3) p = 0.008 | 2 (2–3) p = 0.008 |
| Visit three (30-mg intranasal ketamine) | |||||||||
| 5.5 (5–7) | 5 (4–6) | 3 (3–6) | 3.5 (3–6) | 3.5 (2–6) | 3 (2–4) | 2.5 (2–3) | 3 (2–3) | 3 (2–3) | 3 (3–4) |
| Change from baseline | 0.5 (0–1) p = 0.06 | 2 (2–3) p = 0.04 | 2 (1–3) p = 0.004 | 2 (1–3) p = 0.004 | 2 (2–3) p = 0.004 | 3 (2–3) p = 0.002 | 2 (2–4) p = 0.002 | 2 (1–5) p = 0.004 | 2 (1–2) p = 0.008 |
| Visit four (50-mg intranasal ketamine) | |||||||||
| 6 (6–8) | 5 (3–5) | 4 (2–5) | 3 (2–5) | 2 (2–5) | 2 (2–4) | 2 (1–5) | 2 (1–5) | 2.5 (1–6) | 5 (2–6) |
| Change from baseline | 1 (0–2) p = 0.047 | 2 (1–3) p = 0.008 | 4 (3–4) p = 0.004 | 4 (2–4) p = 0.004 | 4 (4–4) p = 0.008 | 4 (3–5) p = 0.008 | 4 (2–5) p = 0.008 | 3 (1–5) p = 0.008 | 1 (1–4) p = 0.008 |
Notes: Data presented as median (interquartile range). Shapiro-Wilk test used to test for normality, and more than 80 percent of the time point values were found not to have normal distribution. Therefore, all p-values presented here are for the Wilcoxon signed-rank test for not normally distributed data. Data points with p-values <0.05 are bolded.
FIGURE 3.
Changes in pain scores on Numerical Pain Rating Scale (NPRS) at each study visit. Symbols indicate the median values, with 25th–75th percentile values shown in the bars. The * in the figure represents a p-value <0.05. All changes after 10 min of study drug administration were statistically significant (p < 0.05). Changes with 30 and 50 mg of intranasal ketamine are clinically significant (≥2-point reduction on NPRS). Each unit on the x-axis represents one timepoint where pain scores are measured. NAS = intranasal ketamine
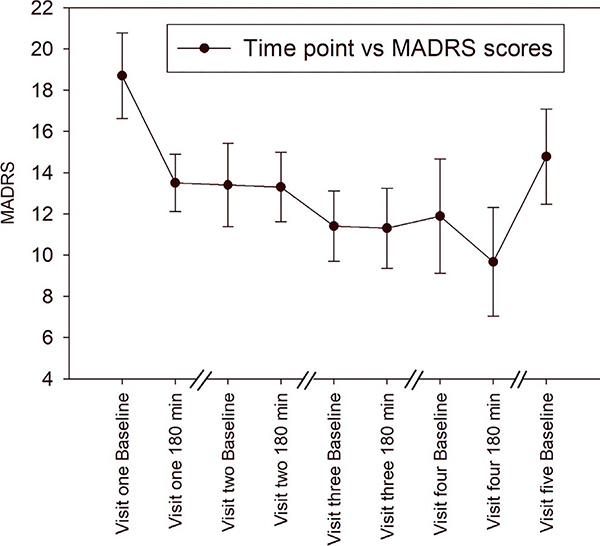
Mean (±standard deviation) baseline MADRS score was 18.7 (±6.57), 13.4 (±6.4), 12.0 (±5.05), and 11.9 (±8.77) at visits 1, 2, 3, and 4, respectively. During the study visits, MADRS score reduced by 5.2 (±4.36, p = 0.0044) with 10-mg IN, 0.1 (±4.5, p = 0.945) with 10-mg IV, 0.33 (±3.43; p = 0.778) with 30-mg IN, and 2.22 (±2.95; p = 0.0536) with 50-mg IN (Figure 4); only the reduction in MADRS score during visit 1 was significant. No significant changes were noted in PROMIS global health, ECOG, or ESAS scores at different study visits. Although a general trend in improvement for ESAS score was observed, no significant changes were identified except for tiredness at visit 2, drowsiness at visit 5, appetite at visit 4, depression at visit 4, and anxiety at visit 3, 4, and 5, compared to visit 1 (Figure S1-ESAS scores).
FIGURE 4.
Montgomery-Asberg Depression Rating Scale (MADRS) depression scores across visits. Scores were collected each visit at baseline and at 180 min. Each study visit was 2–5 days apart. Only the reduction in MADRS score during visit 1 was significant (p = 0.0044)
One patient took the rescue medication at its usual interval during one of the study visits (visit 3); while two patients who took their rescue medication during the study visits were able to extend the interval (one patient took medication during visit one and three and another patient during visit one) (Table 2). Unfortunately, daily MME data collection was not robust, and we are unable to report changes in daily opioid consumption on the days of the study visit.
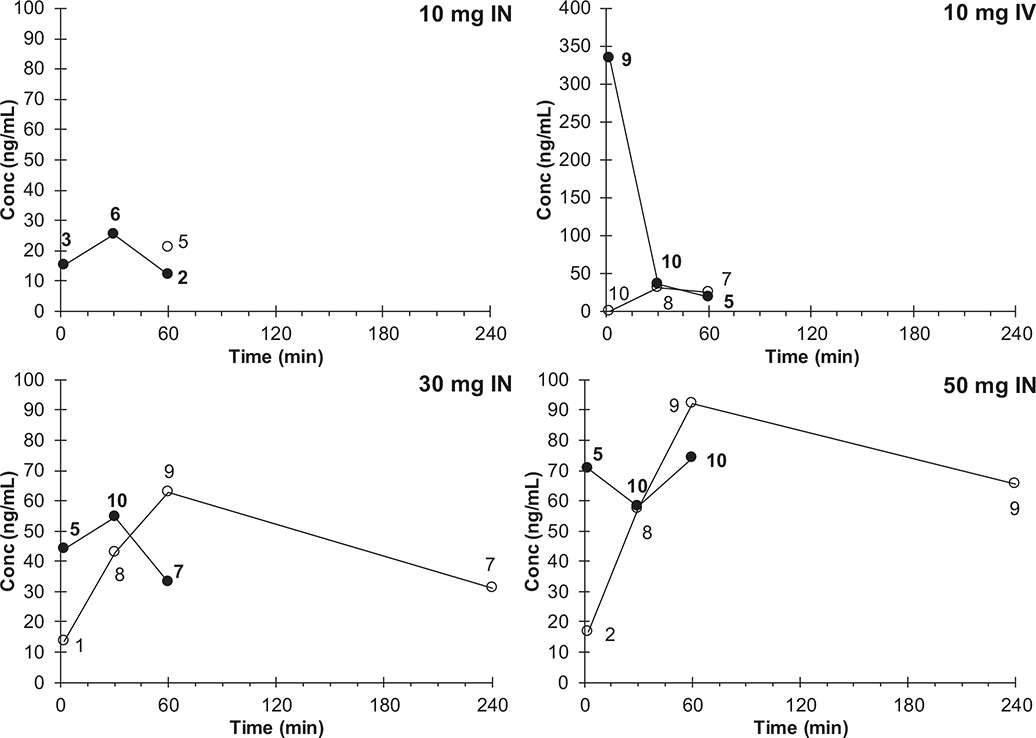
Pharmacokinetic profiles show an increase in both ketamine and nor-ketamine exposure with increasing IN dose (Figure 5; Table 6 for associated PK parameters). The area under the concentration versus time profile (AUC) reflects the time-integrated exposure to a drug and allows comparison of relative efficiency of drug absorption AUC. AUC values were calculated based on timepoint-averaged concentrations (naïve pooled data). Ketamine AUC values were based on data up to and including 60 min because the 240-min timepoint did not have >1 informed concentration point in any group. Nor-ketamine AUC values were based on data up to and including 60 min (10-mg IV) or 240 min (30- and 50-mg IN). The AUC of nor-ketamine for patients in the 10-mg IN group could not be determined with enough confidence (only one timepoint with at least five informed values). Dose-normalized ketamine AUC and Cmax decreased with increasing dose, while dose-normalized nor-ketamine AUC and Cmax appeared to be relatively constant across IN doses.
FIGURE 5.
Ketamine () and nor-ketamine () average pharmacokinetic concentration versus time profiles after administration via intravenous (IV, 10 mg) or intranasal (IN, 10, 30, or 50 mg) route. Numbers indicate number of informed values (i.e., not imputed as half the lower assay limit: 10 ng/ml) underlying the depicted averages, see methods for further detail
TABLE 6.
Ketamine (K) and nor-ketamine (N-K) pharmacokinetic parameters (geometric mean and standard deviation)
| D (mg) | K |
N-K |
C max | K |
N-K |
K |
N-K |
AUC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Route | Cmax(ng/mU | Cmax/D | n | Cmax (ng/ml) | Cmax/D | n | MRa N-K/K | Tmax(min) | n | Tmax(min) | n | AUC (μg·h/L) | AUC/D | F(%) | AUC (μg·h/L) | AUC/D | MR N-K/K |
| 10 IV | 296 (1.71) | 29.6 | 9 | 2.8 (1.86) | 2.7 | 8 | 0.089 (1.6) | 2 (1) | 9 | 30 (1) | 8 | 113 | 678 | 100 | 22 | 130 | 0.19 |
| 10 IN | 22.9 (1.97) | 2.3 | 7 | 17.8 (1.89) | 1.8 | 5 | 0.78 | 23 (3.0) | 7 | 79 (1.86) | 5 | 19 | 116 | 17 | n/a | ||
| 30 IN | 60.8 (1.90) | 2.0 | 10 | 68.1 (1.80) | 2.3 | 10 | 1.12 (1.72) | 14 (3.8) | 10 | 60 (1.76) | 10 | 46 | 91 | 13 | 181 | 361 | 3.95 |
| 50 IN | 77.6 (2.29) | 1.6 | 10 | 105 (1.63) | 2.1 | 10 | 1.35 (2.37) | 16 (4.4) | 10 | 56 (1.83) | 10 | 64 | 77 | 11 | 291 | 350 | 4.54 |
Abbreviations: μg·h/L, (microgram per hour) per liter; AUC, area under the curve; Cmax, maximum concentration; D, dose; F, bioavailability; ml, milliliter; ng, nanogram; Tmax, time to reach maximum concentration.
Cmax metabolic ratios (MR) were calculated at the individual level, allowing for calculation of geometric mean and standard deviation values by dose level. For the 10-mg IN group, there were only three patients with both ketamine (K) and nor-ketamine (NK) truly informed values, which prompted us to report the ratio of the geometric mean of nor-ketamine over the geometric mean of ketamine. For the 10-mg IV, 30-mg IN, and 50-mg IN groups, the geometric mean of individual level metabolic ratio and ratio of geometric means were near-identical.
4 |. DISCUSSION
This study is the first prospective clinical trial investigating the use of IN ketamine for the treatment of intractable cancer pain. We evaluated escalating dosages of IN ketamine (10, 30, and 50 mg) and a single reference IV dose (10 mg) for comparison. All IN ketamine dosages were well-tolerated, side effects were transient, and no serious adverse events related to study medication were reported. The median percentage of maximum pain relief reported with 30-mg and 50-mg IN ketamine was >65%, exceeding the minimal clinically significant pain relief threshold (30%). Pain relief onset with the IN administration was within 15 min for most patients, with sustained relief for the entire study duration. The median duration of maximum pain relief was 1 h, with median duration of any pain relief of 4 h with all IN dosages. The one patient who did not report any pain relief with the 50-mg IN ketamine dose informed the study team, “nothing is going to help.” He was cooperative during study encounters but possessed a flat affect with known mild cognitive impairment, retaining medical capacity for decision-making.
The ketamine exposure we observed after IV dosing corresponds to a clearance of 88.5 L/h, which agrees with the previously reported value of 80.2 L/h (19.1 ml/min/kg assuming a 70-kg subject).42 The time to maximum concentration with IN ketamine corresponded with time to maximum pain relief (Table 6 and Figure 3), which was near 15 min for the 30-mg and 50-mg dose. Maximum concentration of ketamine was lowest for 10-mg IN dose (22.9 ng/ml) and highest for 30- and 50-mg IN dosages, (60.8 and 77.6 ng/ml, respectively). Interestingly, the pain threshold level elevation has been reported to occur at much higher plasma ketamine concentration (160 ng/ml) in healthy volunteers in a prior study.42
Intranasal ketamine bioavailability was reported at approximately 50% in pediatric patients (2–9 years old) dosed at 3–9 mg/kg, which at an average weight of 17.5 kg corresponded to 50–160 mg.9 Although the nasal route produced plasma concentrations associated with anesthesia, the large volume of ketamine required (these doses formulated as 5% ketamine represented 1–3 ml) was partly swallowed resulting in unacceptable variability.9 Additionally, the subjects did not serve as their own controls in the pediatric study, which increased imprecision. Nasal mucosal surface area can also be a confounding factor for intranasal absorption. Even in the current study, the bioavailability was better for the lower dosage/volume (17% for 10-mg IN) compared to the higher dosage (13% for 30-mg IN and 11% for 50-mg IN) for which more than one spray/nare was used. Medication visibly escaped the nare in several patients when more than two sprays/nare were administered (for the 50-mg IN ketamine dose). Another small study with three subjects calculated the bioavailability of intranasal ketamine to be approximately 45% in healthy adult volunteers who served as their own controls.43 Although intranasal administration is an attractive alternative to IV dosing,9,23 our data suggest that each dose is not 100% absorbed. The dose-normalized AUC and Cmax values decrease with IN dose, whereas those values for nor-ketamine are relatively constant (resulting in increasing metabolic ratio), which would suggest that at higher doses, a lower fraction of dose reaches the systemic circulation unchanged, and a larger fraction of the dose is being metabolized.
5 |. LIMITATIONS
5.1 |. Small sample size
As a dose-finding study, the goal of this study was primarily to determine the safety and tolerability of IN ketamine, which was proven by lack of any major side effects and patients completing all study visits. The stringent eligibility criteria and frailty of the population led to a reduced enrollment population and longer completion time.
5.2 |. Lack of placebo control
Since we did not compare the effects of intranasal ketamine with placebo, it is not possible to tease out placebo response from the true analgesic response from IN ketamine. The placebo effect is stronger for pain than other conditions; a true control group is necessary in future studies with larger sample size.44
5.3 |. Lack of blinding
To ensure safety, all patients received a lower dose of IN ketamine before getting a higher dose; therefore, the study was not blinded.
5.4 |. Variable concomitant opioid dosing
Since the patients in this study were likely to have advanced cancer and receive a stable opioid regimen for 7 days, with ongoing pain despite this regimen, no changes were made to their existing opioid regimen. This led to variability in baseline opioid dosing; however, it is noteworthy that the median baseline MME amongst the study participants was 160.
5.5 |. Advanced cancer
Since these patients had advanced cancer, some results (analgesia and side effects) may have been confounded by disease progression.
6 |. CONCLUSION
In this single-institution study, IN ketamine administered in the study (10, 30, and 50 mg) was well-tolerated, without any major side effects and provided clinically and statistically significant pain relief for intractable cancer-related pain. Each patient, having received all four dosages, served as their own control, increasing the ability to isolate the effect due to the lack of intersubject variability across dose and route. The 30- and 50-mg IN dosages appear to be more efficacious with the median (interquartile range) percentage of pain relief at 65.5 (40–100) and 69.25 (50–100), respectively. Pain relief was highest with the 50-mg dose, and there was no increase in side effects with this dose compared to the 30-mg dose. Minimal primarily transient side effects were observed. The patients in this study had multiple etiologies of cancer-related pain and attained significant analgesia from IN ketamine. Future studies should focus on the utility of multiple-dose administration and adverse effects with repeated dosing on a chronic basis. Since lower volume administration resulted in the highest bioavailability, these studies could utilize higher concentrations.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the Department of Anesthesiology at Emory University School of Medicine for support. Ms. Ashley Holloman assisted in the conduct of this clinical trial in her role as a research coordinator.
Funding information
Vinita Singh was a KL2 scholar at the Georgia, Clinical and Translational Science Alliance and would like to acknowledge that this manuscript was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number ULTR002378 and KL2TR002381. Supplemental funds to conduct this clinical trial were provided by the Team Based Science award via the Department of Anesthesiology, Emory University. Maya Tsvetkova and Jan Beumer helped with analysis of pharmacokinetic parameters and their work was supported in part by award P30CA47904. Jeffery Switchenko’s support was funded by P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
Singh—Scientific advisor, Releviate LLC. All other authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Previous presentations: Early findings were presented as poster presentation at the American Society of Regional Anesthesia at San Antonio, Texas, November 2018; the Georgia Translational Science conference, Callaway Gardens, GA, February 2019; the Translational Science conference, Washington, DC, March 2019; the American Academy of Pain Medicine annual meeting at Denver, Colorado, March 2019; and the American Society of Anesthesiologist, Orlando, October 2019.
REFERENCES
- 1.van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 2.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164(4):1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aman MM, Mahmoud A, Deer T, et al. The American Society of Pain and Neuroscience (ASPN) best practices and guidelines for the interventional management of cancer-associated pain. J Pain Res. 2021;14:2139–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh V, Gillespie TW, Harvey RD. Intranasal ketamine and its potential role in cancer-related pain. Pharmacother J Hum Pharmacol Drug Ther. 2018;38(3):390–401. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culp C, Kim HK, Abdi S. Ketamine use for cancer and chronic pain management. Front Pharmacol. 2021;11:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97(4):1108–1116. [DOI] [PubMed] [Google Scholar]
- 8.Fundytus ME. Glutamate receptors and nociception. CNS Drugs. 2001;15(1):29–58. [DOI] [PubMed] [Google Scholar]
- 9.Malinovsky JM, Servin F, Cozian A, Lepage JY, Pinaud M. Ketamine and norketamine plasma concentrations after i.v., nasal and rectal administration in children. Br J Anaesth. 1996;77(2):203–207. [DOI] [PubMed] [Google Scholar]
- 10.Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36(2):186–197. [DOI] [PubMed] [Google Scholar]
- 11.White PF, Way WL, Trevor AJ. Ketamine–its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119–136. [DOI] [PubMed] [Google Scholar]
- 12.Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A. Ketamine*. J Pain Symptom Manage. 2011;41(3):640–649. [DOI] [PubMed] [Google Scholar]
- 14.Ketamine does not increase intracranial pressure compared with opioids: meta-analysis of randomized controlled trials | SpringerLink; [Internet]. Accessed August 8, 2019. https://link.springer.com/article/10.1007%2Fs00540-014-1845-3 [DOI] [PubMed] [Google Scholar]
- 15.Ke X, Ding YI, Xu KE, et al. The profile of cognitive impairments in chronic ketamine users. Psychiatry Res. 2018;266:124–131. [DOI] [PubMed] [Google Scholar]
- 16.Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582–586. [DOI] [PubMed] [Google Scholar]
- 17.Carr DB, Goudas LC, Denman WT, et al. Safety and efficacy of intranasal ketamine for the treatment of breakthrough pain in patients with chronic pain: a randomized, double-blind, placebo-controlled, crossover study. Pain. 2004;108(1–2):17–27. [DOI] [PubMed] [Google Scholar]
- 18.Home [Internet]. SPRAVATOTM (esketamine); 2018. Accessed August 1, 2019. https://www.spravato.com/
- 19.Kim H-J, Kim YS, Park SH. Opioid rotation versus combination for cancer patients with chronic uncontrolled pain: a randomized study. BMC Palliat Care. 2015;14(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176–183. [DOI] [PubMed] [Google Scholar]
- 21.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. [DOI] [PubMed] [Google Scholar]
- 22.Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55(9):1059–1077. [DOI] [PubMed] [Google Scholar]
- 23.Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moaddel R, Venkata SLV, Tanga MJ, et al. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82(5):1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 29.Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry J Ment Sci. 2008;192(1):52–58. [DOI] [PubMed] [Google Scholar]
- 30.Hudgens S, Floden L, Blackowicz M, et al. Meaningful change in depression symptoms assessed with the Patient Health Questionnaire (PHQ-9) and Montgomery-Åsberg Depression Rating Scale (MADRS) among patients with treatment resistant depression in two, randomized, double-blind, active-controlled trials of esketamine nasal spray combined with a new oral antidepressant. J Affect Disord. 2021;281:767–775. [DOI] [PubMed] [Google Scholar]
- 31.Müller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J Affect Disord. 2003;77(3):255–260. [DOI] [PubMed] [Google Scholar]
- 32.Turkoz I, Alphs L, Singh J, et al. Clinically meaningful changes on depressive symptom measures and patient-reported outcomes in patients with treatment-resistant depression. Acta Psychiatr Scand. 2021;143(3):253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motov S, Mai M, Pushkar I, et al. A prospective randomized, double-dummy trial comparing IV push low dose ketamine to short infusion of low dose ketamine for treatment of pain in the ED. Am J Emerg Med. 2017;35(8):1095–1100. [DOI] [PubMed] [Google Scholar]
- 34.Shim J, Hamilton DF. Comparative responsiveness of the PROMIS-10 Global Health and EQ-5D questionnaires in patients undergoing total knee arthroplasty. Bone Jt J. 2019;101-B(7):832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patient-reported outcomes measurement information system global-10, PROMIS global-10 [Internet]. APTA. Accessed December 29, 2021. https://www.apta.org/patient-care/evidence-based-practice-resources/test-measures/patient-reported-outcomes-measurement-information-system-global-10-promis-global-10 [Google Scholar]
- 36.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azam F, Latif M, Farooq A, et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12(3):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 39.Philip J, Smith WB, Craft P, Lickiss N. Concurrent validity of the modified Edmonton symptom assessment system with the Rotterdam symptom checklist and the brief pain Inventory. Support Care Cancer. 1998;6(6):539–541. [DOI] [PubMed] [Google Scholar]
- 40.Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol Tor Ont. 2009;16(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504. [DOI] [PubMed] [Google Scholar]
- 42.Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci. 1982;71(5):539–542. [DOI] [PubMed] [Google Scholar]
- 43.Yanagihara Y, Ohtani M, Kariya S, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003;24(1):37–43. [DOI] [PubMed] [Google Scholar]
- 44.Colloca L Placebo effects in pain. Int Rev Neurobiol. 2020;153:167–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.