Abstract
Prenatal chemical exposures can influence maternal and child health; however, few industrial chemicals are routinely biomonitored. We assessed an extensive panel of contemporary and emerging chemicals in 171 pregnant women across the United States (U.S.) and Puerto Rico in the Environmental influences on Child Health Outcomes (ECHO) Program. We simultaneously measured urinary concentrations of 89 analytes (103 total chemicals representing 73 parent compounds) in nine chemical groups: bactericides, benzophenones, bisphenols, fungicides and herbicides, insecticides, organophosphate esters (OPEs), parabens, phthalates/alternative plasticizers, and polycyclic aromatic hydrocarbons (PAHs). We estimated associations of creatinine-adjusted concentrations with sociodemographic and specimen characteristics. Among our diverse prenatal population (60% non-Hispanic Black or Hispanic), we detected 73 of 89 analytes in ≥1 participant and 36 in >50% of participants. Five analytes not currently included in the U.S. biomonitoring were detected in ≥90% of samples: benzophenone-1, thiamethoxam, mono-2-(propyl-6-carboxy-hexyl) phthalate, monocarboxy isooctyl phthalate, and monohydroxy-iso-decyl phthalate. Many analyte concentrations were higher among women of Hispanic ethnicity compared to those of non-Hispanic White women. Concentrations of certain chemicals decreased with the calendar year, whereas concentrations of their replacements increased. Our largest study to date identified widespread exposures to prevalent and understudied chemicals in a diverse sample of pregnant women in the U.S.
Keywords: pregnancychild health, industrial chemical, pesticides, flame retardants, phthalates, bisphenols, parabens
Short abstract
This largest study to date found exposure to many environmental chemicals in a diverse sample of pregnant women in the United States.
Introduction
Pregnancy is a susceptible period for both mother and fetus, during which chemical exposures can contribute to numerous adverse pregnancy and child health outcomes.1−4 Chemical exposures are ubiquitous in the United States (U.S.) due to the thousands of chemicals produced and used in numerous consumer products. Exposures can occur via food, water, air, dust, and use of consumer and personal care products.4 During pregnancy, many chemicals to which pregnant women are exposed cross the placenta, directly exposing the fetus.5
Nationally representative data on chemical exposures are available from the National Health and Nutrition Examination Survey (NHANES) for approximately 350 of the more than 40,000 chemicals used in the U.S., encompassing a small proportion of potential chemical exposures.6 A previous study of pregnant women using 2003–2004 NHANES data reported widespread simultaneous exposure to >40 chemicals.4 The ubiquity of prenatal chemical exposures is likely underestimated because the vast majority of chemicals are not routinely surveyed in NHANES, including compounds with unknown or suspected toxicity and compounds being used as replacements for chemicals being phased out due to potential toxicity or increases in exposure (i.e., “regrettable substitutions”).7−9 Further, recent data on coexposure to multiple chemicals during pregnancy is lacking because NHANES has not oversampled pregnant participants since 2001–2006.10 It is critical to improve methods to characterize prenatal chemical exposures more comprehensively and contemporarily.
The National Institutes of Health (NIH) Environmental influences on Child Health Outcomes (ECHO) Program provides an unparalleled opportunity for understanding environmental exposures among pregnant women in the U.S.11 ECHO combines 69 prospectively followed pregnancy and pediatric cohorts comprising approximately 50,000 children and families from across the U.S. to understand and improve child health.12 ECHO investigators previously recommended biomonitoring highly prevalent contemporary chemicals during pregnancy that are measured in NHANES11 and emerging chemicals not included in NHANES but which have a high likelihood of exposure, the potential for adverse health effects, and an available biomarker of exposure.13
Our goal was to apply a new method for simultaneous measurement of more than 100 chemicals in nine groups of priority contemporary and emerging industrial chemicals and pesticides among pregnant women from nine ECHO cohorts representing a range of geographies and race/ethnicities. We aimed to characterize analyte detection frequencies and distributions and to assess predictors of exposure.
Materials and Methods
Study Population
We included pregnant women from nine ECHO cohorts located in five states (California, Georgia, Illinois, New Hampshire, New York) and Puerto Rico, reflecting diverse geographic and sociodemographic populations (Table S1, Supporting Information 1). For this initial pilot study, each cohort contributed banked urine specimens from up to 20 ECHO participants (Table S1, Supporting Information 1). The only criterion for inclusion was the availability of a 6 mL of urine specimen collected during pregnancy. The study protocol was approved by the local (or central ECHO) Institutional Review Board (IRB). Written informed consent was obtained from participants in cohort-specific research and/or the ECHO-wide Cohort Data Collection Protocol. The work of the ECHO Data Analysis Center is approved through the Johns Hopkins Bloomberg School of Public Health IRB.
Chemical Analysis
We selected chemicals for analysis in collaboration with the Wadsworth Center-Human Health Exposure Analysis Resource (WC-HHEAR) at New York University. WC-HHEAR developed an analytical method for multiple chemical measurements that include both current use and emerging chemicals of concern based on laboratory capabilities as well as the biomonitoring recommendations of our prior publication.13 This analytical method is consistent with Centers for Disease Control and Prevention (CDC) methods and was previously validated and applied in a convenience sample of 21 adult nonpregnant volunteers from Albany, NY.14−16 Ours is the first population-based study to apply this method and the first among a diverse pregnant population. Chemical groups, parent compounds, CAS registry numbers, and environmental transformation of included analytes are provided in Data File S1, Supporting Information 2.
Briefly, urine samples were analyzed at WC-HHEAR using solid-phase extraction (SPE) coupled with high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)14 Urine samples (0.5 mL) were incubated with β-glucuronidase/arylsulfatase (2000 units) and subjected to ABS Elut NEXUS SPE (Agilent, Santa Clara, CA) prior to analysis by HPLC-MS/MS. A Sciex HPLC system (SCIEX, Redwood City, CA) interfaced with an ABSCIEX QTRAP 5500+ triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) with an electrospray ionization source was used in the analysis. The optimal LC-MS/MS conditions and quality assurance protocols are described in detail elsewhere.14 Limits of detection (LODs) are presented in Data File S2, Supporting Information 2. Due to insufficient resolution to quantify 24 chemicals individually, we quantified 10 composites of multiple chemicals (e.g., we quantified a composite of 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 3-hydroxyphenanthrene, 4-hydroxyphenanthrene, and 9-hydroxyphenanthrene as a single analyte).
In total, we quantified 89 analytes in nine chemical groups: 79 individual and 10 composite analytes. These 89 analytes are biomarkers of 103 chemicals measured as either parent compounds or metabolites in urine depending on whether metabolites were known and analytical standards were available (Table S2, Supporting Information 1). To simplify reporting for metabolites of three parent phthalates and the phthalate alternative plasticizer DINCH, we calculated molar sums of metabolites for di-2-ethylhexyl phthalate (DEHP), di-isodecyl phthalate (DiDP), di-(2-propylheptyl) phthalate (DPHP), and di-iso-nonyl-cyclohexane-1,2-dicarboxylic acid (DINCH) (details in Data File S2, Supporting Information 2).17,18
Quality Control
We determined the replicability of the biomarker analysis using quality control (QC) pools and blinded duplicates.15 Prior to this study, HHEAR collected urine from healthy adult volunteers and created two QC pools: QC Pools A and B.15 Three aliquots each of these QC pools were run in each of the two batches (i.e., up to six QC-pooled urine samples from each of Pool A and Pool B). We calculated the overall means and coefficients of variation (CVs) for each biomarker in each pooled sample. We also analyzed 34 blinded duplicate pairs of urine sample aliquots from six cohorts and calculated the relative percent differences (RPDs) for each pair and the median of all pair RPDs. We restricted calculations of CVs to QC-pooled samples with concentrations above the LOD. We restricted calculations of RPDs to duplicate pairs, where both concentrations were >LOD and only assessed analytes with at least two sets of duplicate pairs >LOD; therefore, the median RPDs of duplicate pairs for several analytes were based on a small number of duplicates. We did not calculate CVs or RPDs for 16 analytes that were not detected in any study participant. The laboratory QC pool CVs were calculated for 32 analytes and ranged from 1 to 16%, with 91% of QC pools having a CV <10% (Table S3, Supporting Information 1). RPDs for 34 duplicate pairs from six cohorts could be calculated for 59 analytes and ranged from 7.3% (benzophenone-2) to 82.1% (imidacloprid) (Table S3, Supporting Information 1). Fifty analytes (68%) had a median RPD <50%, whereas 38 (52%) had median RPDs <30% (Table S3, Supporting Information 1).
Covariates
Sociodemographic variables included participant age (years; continuous), race/ethnicity (non-Hispanic White; non-Hispanic Black, non-Hispanic other, or non-Hispanic multiple race; Hispanic ethnicity, any race), prepregnancy or early pregnancy body mass index (BMI, measured from preconception to 16 completed weeks of gestation; continuous), highest educational attainment (high school diploma, general educational development [GED], or less; some college, Associate’s degree, or trade/vocational school; Bachelor’s degree or higher), and marital status (single, separated, divorced, widowed; married or partnered and living together). We assessed California residence since three of nine cohorts were located in California. We assessed tobacco exposure during pregnancy using log2-transformed creatinine-adjusted urinary cotinine concentrations (ng/mL). Urine specimen collection characteristics included time of day (morning [2:00 am–9:59 am], midday [10:00 am–3:59 pm], evening [4:00 pm–10:00 pm]), trimester (first, second, or third), calendar season (autumn [September–November], winter [December–February], spring [March–May], summer [June–August]), and year of collection (continuous; centered at 2008).
Statistical Analysis
Descriptive Statistics
We calculated the mean (SD) or geometric mean (GSD) of continuous variables and sample size (%) of categorical variables for characteristics of pregnant women in our sample. We also calculated descriptive statistics of demographic characteristics of all pregnant women in the nine participating cohorts. For each urinary analyte, we calculated the detection frequency, geometric mean (GSD), minimum and maximum, and 25th, 50th, and 75th percentiles. Sixteen analytes were not detected in any sample and excluded from further analyses (Data File S2, Supporting Information 2). We analyzed values as either dichotomous or continuous depending on the detection frequency among the participants. For analytes detected in <70% of participants, we created dichotomous variables based on each analyte’s LOD and modeled the analytes as below or above the detection limit.19 For analytes detected in ≥70% of participants, we used machine-read values (if available) or replaced values below the LOD with the analyte LOD/√220 and calculated log2-transformed concentrations. For six machine-read concentrations reported as zero, we added a small value (0.0001) prior to log2-transformation. Lastly, we calculated Spearman’s correlations for analytes detected in at least three cohorts and ≥70% of the population.
Predictors of Chemical Exposures
We estimated univariable
associations of sociodemographic and urine specimen collection characteristics
with creatinine-adjusted analyte concentrations using generalized
estimating equations to account for clustering of samples at the cohort
level. We assumed an exchangeable working correlation matrix and used
robust Huber–White sandwich estimation of variance and standard
errors. For continuous concentrations, we first accounted for urinary
dilution21,22 by calculating creatinine-adjusted analyte
concentrations as 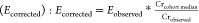 , where
we multiplied observed analyte concentrations
(Eobserved) by the ratio of the cohort-specific
median creatinine concentration (Crcohort median)
and sample-specific creatinine concentration (Crobserved).23,24 Then, we modeled creatinine-adjusted concentrations
using an identity link and Gaussian family and reported effect estimates
as % differences and 95% confidence intervals (CIs). For dichotomized
concentrations, we used a log-link and Poisson family and reported
prevalence ratios (PRs) and 95% CIs. To reduce the influence of individual
cohorts and the potential for nonpositivity problems, we conducted
predictor analyses only for analytes detected in participants from
at least three cohorts and at least 10% of the overall study sample.
, where
we multiplied observed analyte concentrations
(Eobserved) by the ratio of the cohort-specific
median creatinine concentration (Crcohort median)
and sample-specific creatinine concentration (Crobserved).23,24 Then, we modeled creatinine-adjusted concentrations
using an identity link and Gaussian family and reported effect estimates
as % differences and 95% confidence intervals (CIs). For dichotomized
concentrations, we used a log-link and Poisson family and reported
prevalence ratios (PRs) and 95% CIs. To reduce the influence of individual
cohorts and the potential for nonpositivity problems, we conducted
predictor analyses only for analytes detected in participants from
at least three cohorts and at least 10% of the overall study sample.
As a sensitivity analysis, we conducted limited multivariable models including age (continuous), race/ethnicity (non-Hispanic White; non-Hispanic Black, non-Hispanic other, or non-Hispanic multiple race; Hispanic ethnicity, any race), and educational attainment (high school diploma, GED, or less; some college, Associate’s degree, or trade/vocational school; Bachelor’s degree or higher). We used a complete-case approach for all analyses and did not report cell sizes less than five. We conducted statistical analyses using Stata v16.1 (StataCorp, College Station, Texas) and R v4.02 Statistical Software (Foundation for Statistical Computing, Vienna, Austria).
Results
Participant Demographics
Participants averaged 29.5 years of age at the time of urine collection were predominantly non-Hispanic White (34%) or Hispanic (40%) and married or living with a partner (68%) (Table 1). Urine specimens were collected during all trimesters and seasons, and the majority were collected from 2017 to 2020 (77%) (Table 1). Most of the urine specimens were spot samples (92%), were collected between 10:00 am and 3:59 pm (69%), and had undergone only 1 freeze–thaw cycle prior to assay (82%) (Table 1). The average age at delivery, educational attainment, and pre- or early pregnancy BMI were similar among our sample and all pregnant people from the nine participating cohorts (N = 7420) (Table S4, Supporting Information 1). Slightly fewer of our participants were non-Hispanic White and married or living with a partner (Table S4, Supporting Information 1).
Table 1. Demographic and Urine Specimen Collection Characteristics of 171 Pregnant Women in ECHOa,b.
| demographic characteristics | N (%) |
|---|---|
| age at specimen collection (years); mean (SD) | 29.5 (5.3) |
| age category at specimen collection (years) | |
| <25 | 35 (20) |
| 25 to <30 | 51 (30) |
| 30 to <35 | 47 (28) |
| ≥ 35 | 38 (22) |
| race/ethnicity (missing: n = 1) | |
| non-Hispanic White | 57 (34) |
| non-Hispanic Black/African American | 34 (20) |
| non-Hispanic other or multiple race | 11 (6) |
| Hispanic | 68 (40) |
| highest educational attainment (missing: n = 7) | |
| less than high school | 16 (10) |
| high school degree, GED, or equivalent | 28 (17) |
| some college, Associate’s degree, or trade/vocational school | 45 (27) |
| Bachelor’s degree | 36 (22) |
| Master’s, professional, or doctorate degree | 39 (24) |
| marital status (missing: n = 6) | |
| single, partnered, not living together | 45 (27) |
| widowed, separated, divorced | 8 (5) |
| married or living with a partner | 112 (68) |
| prepregnancy or early pregnancy BMI (kg/m2); mean (SD); (missing: n = 12) | 26.4 (6.5) |
| California residence | 54 (32) |
| urine cotinine concentration, creatinine-standardized (ng/mL); geometric mean (GSD) | 0.57 (6.2) |
| urine specimen collection characteristics | |
|---|---|
| creatinine (mg/dL); geometric mean (GSD) | 61.4 (1.7) |
| time of day (missing: n = 9) | |
| morning (2:00 am–9:59 am) | 40 (25) |
| midday (10:00 am–3:59 pm) | 112 (69) |
| evening (4:00 pm–10:00 pm) | 10 (6) |
| trimester (missing: n = 2) | |
| 1 (0–13 completed weeks) | 19 (11) |
| 2 (14–26 completed weeks) | 82 (49) |
| 3 (27+ completed weeks) | 68 (40) |
| calendar season | |
| winter (december–february) | 37 (22) |
| spring (march–may) | 39 (23) |
| summer (june–august) | 52 (30) |
| autumn (september–november) | 43 (25) |
| calendar year | |
| 2008–2015 | 19 (11) |
| 2016 | 20 (12) |
| 2017 | 40 (23) |
| 2018 | 46 (27) |
| 2019–2020 | 43 (25) |
| collection type (missing: n = 3) | |
| spot | 154 (92) |
| first morning void | 14 (8) |
| freeze–thaw cycles | |
| 1 | 140 (82) |
| 2 | 31 (18) |
All statistics are sample size (%) unless noted otherwise.
Abbreviations: BMI, body mass index; dL, deciliter; ECHO, Environmental influences on Child Health Outcomes; GED, general educational development; GSD, geometric standard deviation; kg, kilogram; m, meter; mg, milligram; mL, milliliter; ng, nanogram; and SD, standard deviation.
Analyte Concentrations and Correlations
We detected 73 of the 89 analytes (63 of 79 individuals and 10 of 10 composites) in at least one participant (Table 2, Figure S1, Supporting Information 1). Of these, 36 analytes (31 individuals and 5 composites) were detected in greater than 50% of participants: 3 benzophenones, 2 bisphenols, 4 fungicides and herbicides, 3 insecticides, 2 OPEs, 3 parabens, 16 phthalates/alternative plasticizers (14 individuals and 2 composites), and 4 PAHs (1 individual and 3 composites) (Table 2). Nine of these 36 analytes are not currently included in NHANES biomonitoring: 4-hydroxybenzophenone (4-OHBP), benzophenone-1 (BP1), thiamethoxam (THX), cyclohexane-1,2-dicarboxylic acid-mono(oxo-isononyl) ester (MONCH), composite of mono-2-(carboxymethyl) hexyl phthalate and mono(7 carboxyheptyl) phthalate (MCMHP/MCHPP), mono-2-(propyl-6-carboxy-hexyl) phthalate (MPCHP), mono-2-(propyl-6-oxoheptyl) phthalate (MPOHP), monocarboxy isooctyl phthalate (MCiOP), and monohydroxy-iso-decyl phthalate (MHiDP) (Data File S2, Supporting Information 2). Notably, 19 analytes (17 individuals and 2 composites) were detected in ≥90% of the population, indicating ubiquitous exposure (Table 2). Analytes not detected in any sample included benzophenone-6, bisphenol AP, four fungicides and herbicides, four insecticides, three OPEs, heptyl paraben, and two phthalate metabolites (Data File S2, Supporting Information 2).
Table 2. Analyte Descriptive Statistics among 171 Pregnant Women in ECHO, Categorized by Prior Inclusion in National Health and Nutrition Examination Survey (NHANES) Biomonitoringa,c.
| previously
not included in NHANES |
previously
included in NHANES |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| chemical group/analyte name (abbrev | LOD | N (%) >LOD | GM | P25 | P75 | chemical group/analyte name (abbrev) | LOD | N (%) >LOD | GM | P25 | P75 |
| bactericide | |||||||||||
| triclocarban (TCS) | 0.1 | 41 (24) | <LOD | <LOD | <LOD | ||||||
| benzophenones | |||||||||||
| 2,2′,4,4′-tetrahydroxyenzophenone (BP2) | 0.075 | 13 (8) | <LOD | <LOD | 0.56 | benzophenone-3 (BP3)b | 0.1 | 167 (98) | 3.1 | 0.96 | 8.9 |
| 2,2′-dihydroxymethoxybenzophenone (BP8) | 0.075 | 70 (41) | <LOD | <LOD | 1152 | ||||||
| 4-hydroxybenzophenone (4-OHBP) | 0.075 | 152 (89) | 0.29 | 0.13 | 0.53 | ||||||
| benzophenone-1 (BP1)b | 0.075 | 166 (97) | 1.8 | 0.48 | 6.0 | ||||||
| bisphenols | |||||||||||
| bisphenol AF (BPAF) | 0.02 | 9 (5) | <LOD | <LOD | <LOD | bisphenol A (BPA) | 0.07 | 105 (61) | 0.28 | <LOD | 1.1 |
| bisphenol B (BPB) | 0.1 | 8 (5) | <LOD | <LOD | <LOD | bisphenol F (BPF) | 0.2 | 68 (40) | 0.26 | <LOD | 0.65 |
| bisphenol Z (BPZ) | 0.05 | 24 (14) | <LOD | <LOD | <LOD | bisphenol S (BPS) | 0.05 | 144 (84) | 0.20 | 0.088 | 0.49 |
| fungicides and herbicides | |||||||||||
| fungicides | fungicides | ||||||||||
| metalaxyl (MET) | 0.075 | n < 5 | <LOD | <LOD | <LOD | 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) | 0.05 | 148 (87) | 0.17 | 0.094 | 0.33 |
| pyrimethanil (PYRM) | 0.1 | n < 5 | <LOD | <LOD | <LOD | 4-nitrophenol (PNP) | 0.1 | 118 (69) | 0.26 | <LOD | 0.58 |
| pentachlorophenol (PCP) | 0.2 | 110 (64) | 0.59 | <LOD | 2.0 | ||||||
| herbicides | |||||||||||
| 2,4-dichlorophenoxyacetic acid (2,4-D) | 0.075 | 31 (18) | 0.081 | <LOD | <LOD | ||||||
| atrazine (ATZ) | 0.025 | 86 (50) | 0.037 | <LOD | 0.089 | ||||||
| insecticides | |||||||||||
| neonicotinoid insecticides | neonicotinoid insecticides | ||||||||||
| 6-chloronicotinic acid (6-CNA) | 0.15 | 17 (10) | <LOD | <LOD | <LOD | acetamiprid (ACE) | 0.025 | 15 (9) | <LOD | <LOD | <LOD |
| nitenpyram (NIT) | 0.05 | 40 (23) | <LOD | <LOD | <LOD | clothianidin (CLO) | 0.1 | 48 (28) | <LOD | <LOD | 0.15 |
| thiamethoxam (THX)b | 0.05 | 157 (92) | 0.42 | 0.25 | 0.82 | imidacloprid (IMI) | 0.1 | 48 (28) | 0.11 | <LOD | 0.13 |
| other insecticides | N-desmethyl-acetamiprid (NDMA)b | 0.03 | 164 (96) | 0.33 | 0.22 | 0.59 | |||||
| sulfoxaflor (SUF) | 0.01 | 33 (19) | <LOD | <LOD | <LOD | organochlorine insecticides | |||||
| composite of 2,4,5- and 2,4,6-trichlorophenol (2,4,5-/2,4,6-TCP) | 0.2 | n < 5 | <LOD | <LOD | <LOD | ||||||
| organophosphate insecticides | |||||||||||
| 3,5,6-trichloro-2-pyridinol (TCP)b | 0.1 | 154 (90) | 0.5 | 0.25 | 1.1 | ||||||
| pyrethroid insecticides | |||||||||||
| composite of cis and trans-3-(2,2-di-chlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid (DCCA) | 0.4 | 69 (40) | 1.2 | ||||||||
| 3-phenoxybenzoic acid (PBA) | 0.7 | 16 (9) | <LOD | ||||||||
| 4-fluoro-3-phenoxybenzoic acid (FPBA) | 0.025 | 41 (24) | <LOD | ||||||||
| organophosphate ester flame retardants | |||||||||||
| triethyl phosphate (TEP) | 0.075 | 73 (43) | <LOD | <LOD | 0.13 | bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) | 0.15 | 126 (74) | 0.39 | <LOD | 0.87 |
| composite of tri-n-butyl phosphate/tri-iso-butyl phosphate (TnBP/TiBP) | 0.075 | 45 (26) | 0.11 | <LOD | 0.36 | composite of di-n-butyl phosphate and di-isobutyl phosphate (DBuP/DiBP) | 0.05 | 10 (6) | <LOD | <LOD | <LOD |
| 0.2 | 6 (4) | <LOD | <LOD | <LOD | diphenyl phosphate (DPHP)b | 0.05 | 164 (96) | 0.66 | 0.34 | 1.4 | |
| parabens | |||||||||||
| benzyl paraben (BzPB) | 0.05 | 20 (12) | <LOD | <LOD | <LOD | butyl paraben (BuPB) | 0.05 | 51 (30) | 0.082 | <LOD | 0.096 |
| ethyl paraben (EtPB)b | 0.01 | 164 (96) | 0.43 | 0.094 | 1.6 | ||||||
| methyl paraben (MePB)b | 0.05 | 168 (98) | 11 | 3.1 | 52 | ||||||
| propyl paraben (PrPB) | 0.15 | 150 (88) | 2.8 | 0.52 | 16 | ||||||
| phthalates and phthalate alternatives | |||||||||||
| phthalate alternatives | phthalate alternatives | ||||||||||
| cyclohexane-1,2-dicarboxylic acid-mono(oxo-isononyl) ester (MONCH) | 0.025 | 108 (63) | 0.11 | <LOD | 0.33 | cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH) | 0.025 | 108 (63) | 0.11 | <LOD | 0.33 |
| monobenzyl terephthalate (MBzTP) | 0.075 | n <5 | <LOD | <LOD | <LOD | cyclohexane-1,2-dicarboxyclic acid-mono(hydroxy-isononyl) ester (MHNCH) | 0.05 | 131 (77) | 0.28 | 0.056 | 0.9 |
| monoethyl terephthalate (METP) | 0.15 | 5 (3) | <LOD | <LOD | <LOD | ||||||
| mono-tert-butyl terephthalate (MTBTP) | 0.075 | 7 (4) | <LOD | <LOD | <LOD | ||||||
| phthalates | phthalates | ||||||||||
| composite of mono-2-(carboxymethyl) hexyl phthalate and mono(7 carboxyheptyl) phthalate (MCMHP/MCHPP) | 0.1 | 152 (89) | 0.53 | 0.31 | 1.0 | mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)b | 0.2 | 170 (99) | 3.8 | 2.1 | 7.3 |
| mono-2-heptyl phthalate (MHPP) | 0.08 | 41 (24) | <LOD | <LOD | <LOD | mono (2-ethyl-5-oxohexyl) phthalate (MEOHP)b | 0.05 | 166 (97) | 3.2 | 1.9 | 6.7 |
| mono-2-(propyl-6-carboxy-hexyl) phthalate (MPCHP)b | 0.05 | 159 (93) | 0.4 | 0.19 | 0.78 | mono (5-carboxy-2-ethylpentyl) phthalate (MECPP)b | 0.05 | 170 (99) | 5.0 | 2.7 | 9.4 |
| mono-2-(propyl-6-hydroxy-heptyl) phthalate (MPHHP) | 0.025 | 44 (26) | 0.048 | <LOD | 0.043 | mono (7-COOH-2-methyloctyl) phthalate (MCOMOP) | 0.025 | 117 (68) | 0.15 | <LOD | 0.57 |
| mono-2-(propyl-6-oxoheptyl) phthalate (MPOHP) | 0.075 | 139 (81) | 0.46 | 0.14 | 1.1 | monobenzyl phthalate (MBzP) | 0.4 | 52 (30) | 0.96 | <LOD | 6.0 |
| monocarboxy isooctyl phthalate (MCiOP)b | 0.05 | 169 (99) | 3.0 | 1.3 | 7.3 | monocarboxy isononyl phthalate (MCiNP)b | 0.05 | 158 (92) | 0.37 | 0.18 | 0.69 |
| monohydroxy-iso-decyl phthalate (MHiDP)b | 0.05 | 159 (93) | 0.59 | 0.24 | 1.1 | monoethylhexyl phthalate (MEHP)b | 0.1 | 164 (96) | 1.6 | 0.76 | 3.5 |
| composite of mono-isopropyl phthalate and mono-propyl phthalate (MiPP/MPrP) | 0.1 | 82 (48) | 0.11 | <LOD | 0.25 | monoethyl phthalate (MEP)b | 0.2 | 171 (100) | 24 | 10 | 58 |
| mono-pentyl phthalate (MPeP) | 0.1 | 16 (9) | <LOD | <LOD | <LOD | monomethyl phthalate (MMP) | 0.1 | 105 (61) | 0.38 | <LOD | 1.1 |
| composite of mono-n-butyl phthalate and mono-isobutyl phthalate (MnBP/MiBP)b | 0.05 | 171 (100) | 11 | 6.0 | 22 | ||||||
| mono-n-octyl phthalate (MOP) | 0.15 | 75 (44) | 0.17 | <LOD | 0.33 | ||||||
| polycyclic aromatic hydrocarbons | |||||||||||
| composite of 1- and 2 hydroxynaphthalene (NAPs)b | 50 | 169 (99) | 5443 | 2184 | 14480 | ||||||
| 1-hydroxypyrene (1-OHP) | 100 | 12 (7) | <LOD | <LOD | <LOD | ||||||
| composite of 1-, 2-, 3-, 4-, 9-hydroxyphenanthrene (PHENs) | 75 | 114 (67) | 145 | <LOD | 314 | ||||||
| composite of 2-, 3-, 9-hydroxyfluorene (FLUOs) | 50 | 118 (69) | 110 | <LOD | 210 | ||||||
Concentration units are ng/mL except for polycyclic aromatic hydrocarbons (ng/L). Values below the limit of detection (LOD) were set to LOD/√2 unless machine-read values were provided.
Detected in at least 90% of urine specimens.
Abbreviations: GM, geometric mean; L, liter; LOD, limit of detection; mL, milliliter; ng, nanogram; and P, percentile.
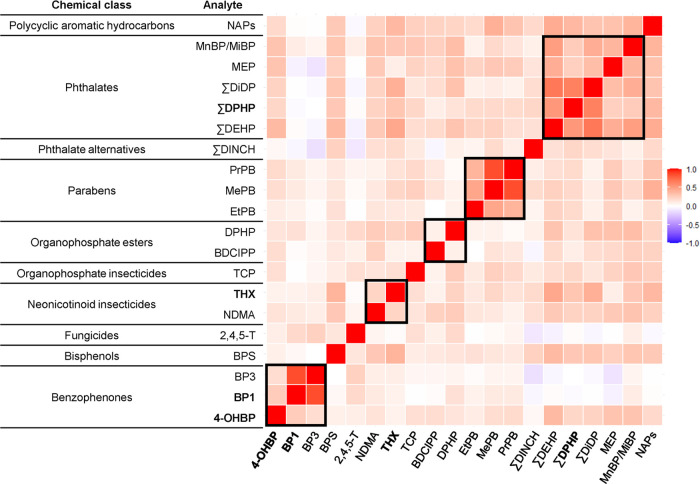
We observed several moderate-to-strong positive correlations and few negative correlations among analytes (Figure 1). In general, correlations were stronger within each chemical group (Figure 1). While most phthalate metabolites were moderate to highly correlated with one another (range: 0.14–0.68), correlations of phthalate metabolites with ∑DINCH (a nonphthalate plasticizer used as a replacement for phthalates, such as DEHP) were low (range: 0.08–0.21).
Figure 1.
Spearman correlation heat map of urinary analyte concentrations measured among 171 pregnant women in ECHO. It includes analytes detected in at least three cohorts and ≥70% of the overall study sample. Values below the limit of detection (LOD) were set to LOD/√2 unless machine-read values were provided. Bold indicates analytes not previously included in NHANES biomonitoring. Boxes indicate within class correlations.
Predictors of Analyte Concentrations
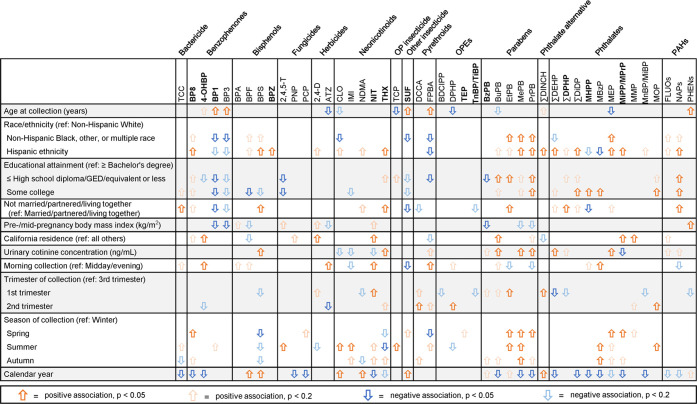
We found higher detection frequencies or concentrations among those identifying as non-Hispanic Black, other, multiple race, or Hispanic ethnicity (n = 30 analytes); having lower educational attainment (n = 22 analytes); being unmarried/partnered (n = 11 analytes); and having higher urinary cotinine concentrations (n = 12 analytes) (Figure 2, Data File S3, Supporting Information 2). Hispanic ethnicity was associated with higher detection frequencies or concentrations of most analytes not included in NHANES biomonitoring (Figure 2). For example, compared with non-Hispanic White women, Hispanic women had a 6.9 (95% CI: 1.1, 43.4) higher prevalence of bisphenol Z (BPZ) detection and a 58% (95% CI: 10, 126%) higher THX concentration (Data File S3, Supporting Information 2). In contrast, identifying as non-Hispanic Black, other, or multiple race (n = 11); having lower educational attainment (n = 14); and being unmarried/partnered (n = 6) were associated with lower detection frequencies or concentrations of other analytes such as BP1 (Figure 2, Data File S3, Supporting Information 2). We observed some notable differences in predictor associations for certain chemicals versus their increasingly utilized replacements. For example, Hispanic women had higher BPS and BPZ concentrations compared with non-Hispanic White women, whereas BPA was not associated with race or ethnicity (Data File S3, Supporting Information 2). For phthalates, Hispanic ethnicity was associated with higher concentrations of both ∑DEHP and ∑DINCH, but the magnitude was much greater for ∑DINCH: compared with non-Hispanic White women, ∑DEHP concentrations were 31% (95% CI: 7%, 61%) higher among Hispanic women, whereas ∑DINCH concentrations were 122% (95% CI: 52%, 226%) higher (Data File S3, Supporting Information 2).
Figure 2.
Univariable associations of maternal sociodemographic and specimen collection characteristics with creatinine-adjusted analyte concentrations. Bold indicates analytes not previously included in NHANES biomonitoring. OP, organophosphate; OPE, organophosphate ester; and PAH, polycyclic aromatic hydrocarbon.
Morning specimen collection; first-trimester collection; and collection during spring, summer, or autumn months were associated with higher detection frequencies or concentrations of several analytes (Data File S4, Supporting Information 2). Among analytes not included in NHANES biomonitoring, later years of specimen collection were significantly associated (p < 0.05) with lower detection frequencies of benzophenone-8 (BP8), nitenpyram, mono-2-heptyl phthalate (MHPP), the composite of mono-isopropyl phthalate (MiPP) and mono-propyl phthalate (MPrP), and 4-OHBP (Figure 2). For example, each year of specimen collection was associated with lower prevalence of MHPP detection (PR: 0.84; 95% CI: 0.79, 0.89) and lower 4-OHBP concentrations (% difference: -7%; 95% CI: −13%, −1%) (Data File S4, Supporting Information 2).
Notably, chemicals often had the opposite direction of association with year of specimen collection compared with their chemical replacements (Data File S4, Supporting Information 2). Later year of specimen collection was not associated with prevalence of BPA detection (PR: 1.0; 95% CI: 0.9, 1.1) but was associated with higher prevalence of bisphenol F (BPF) detection (PR: 1.3; 95% CI: 1.1, 1.5) and higher BPS concentrations (% difference: 18%; 95% CI: 5%, 32%) (Data File S4, Supporting Information 2). For phthalates, later year of specimen collection was associated with 12% (95% CI: −19%, −4%) lower ∑DEHP metabolite concentrations but 23% (95% CI: 13%, 33%) higher ∑DINCH metabolite concentrations (Data File S4, Supporting Information 2).
In our sensitivity analysis using multivariable models, magnitudes of association for age, race/ethnicity, and educational attainment were generally comparable to unadjusted associations though some associations became weaker (Data File S5, Supporting Information 2).
Discussion
To our knowledge, this is the largest study to measure >100 contemporary and emerging chemicals in a diverse population of pregnant women in the U.S. Overall, 73 of 89 analytes were detected in at least one pregnant woman, including 30 from seven chemical groups that were not previously included in NHANES. Additionally, 19 analytes were detectable in 90–100% of pregnant women, including 2 benzophenones, 3 insecticides, 1 OPE, 2 parabens, 10 phthalate metabolites, and 1 PAH. Our study adds to the growing literature documenting multiple chemical exposures that occur during pregnancy, a critical and vulnerable period of human development.4,5
This analysis is based on a multiyear effort to identify priority chemicals for biomonitoring in the ECHO Program. Previously, ECHO investigators recommended novel chemicals for biomonitoring based on three criteria: (1) prevalence in environmental or human biospecimens, (2) preliminary evidence of their toxicity for health outcomes of interest to ECHO (i.e., adverse perinatal outcomes, neurodevelopmental outcomes, respiratory outcomes, and obesity/diabetes), and (3) a biomarker was reported for measurement in human biospecimens.13 In this initial study, we measured eight biomarkers of chemicals not included in NHANES that were identified in our prioritization of novel chemicals:13 bisphenol AF (BPAF), bisphenol B (BPB), azoxystrobin, cyprodinil, metalaxyl, pyrimethanil, tebuconazole, and tetraconazole. Although these eight chemicals were infrequently or not detected in our sample, six were pesticides measured as the parent compounds and not the anticipated metabolites that would be present in urine because some pesticide metabolite standards were not available for our study. We recommend future studies measure the parent compounds in plasma or hair to assess exposure if standards for the metabolites remain unavailable. We also measured 41 analytes that were included on the list for future prioritization (Data File S1, Supporting Information 2), many of which needed biomarker development or demonstration of human exposure.13 Of these 41 analytes, 35 were detected in at least one sample and 21 were detected in >50% of samples suggesting widespread exposures (Data File S2, Supporting Information 2).
Nine analytes detected in more than half of our samples are not currently included in NHANES biomonitoring, including the neonicotinoid pesticide thiamethoxam (92%), the benzophenones 4-OHBP (89%) and BP1 (97%), and metabolites of the phthalate DPHP (MPCHP/MPOHP, 89%). We also measured additional metabolites of the phthalate alternative plasticizer DINCH (MONCH) and the phthalates DEHP (composite of MCMHP/MCHPP) and DiDP (MCiOP and MHiDP) that may help to characterize exposures to these parent compounds in addition to metabolites already included in NHANES. In addition, our method included 44 analytes currently measured in NHANES, many of which have been identified by authoritative bodies as likely or known carcinogens or as developmental or reproductive toxicants.25−35 NHANES does not oversample pregnant participants, and chemical classes are measured in biospecimens from nonoverlapping random subsamples of NHANES participants. Therefore, our novel findings demonstrate substantial coexposure to multiple contemporary chemicals across nine chemical groups in a large number of pregnant women.
We observed neonicotinoid insecticides were highly detected (six detected and thiamethoxam and N-desmethyl-acetamiprid in more than 90%), increasing temporally, and generally more highly detected among Hispanic women. Four of these neonicotinoids have been previously measured in NHANES. We found generally higher detection frequencies and concentrations compared with the four measured in NHANES (collection period 2015–2016).36 This may, in part, be due to our later study collection period (primarily 2017–2020). The most frequently detected neonicotinoid in NHANES was N-desmethyl-acetamiprid (35% overall, 38% in females),36 which we detected in 96% of our participants. While this difference in detection frequencies is expected due to a higher LOD in NHANES (0.2 ng/mL) compared with this method (0.03 ng/mL), our participants also appear to have higher exposures. For example, our 75th percentile for N-desmethyl-acetamiprid was 0.59 ng/mL compared with 0.36 ng/mL in NHANES. We also found more universal detection of several neonicotinoids with no or low detection frequencies (<10%) in NHANES.36 Finally, we found higher concentrations or detection frequencies in later years of collection for several neonicotinoids (acetamiprid and clothianidin), which have been increasingly used in the U.S. as a replacement for organophosphate pesticides and other pyrethroids.36,37 Neonicotinoids are used in a variety of applications, including agricultural uses,38−41 flea control in pets,42 and residential landscaping pest control.43,44 In agricultural settings, neonicotinoids are primarily used as seed treatments and readily taken up by the plant leading to their presence in all components of the plant (e.g., roots, stems, flowers, and leaves) and cannot be washed off; thus, food has been identified as an important source of exposure.45,46
We also documented widespread detection of several chemicals that are replacements for chemicals with declining use due to regulatory or market-based activities. For example, we found higher concentrations in the later calendar years for BPA replacements (BPF and BPS) and phthalate alternatives (DINCH metabolites). Concurrently, we found an indication of decreasing or stable levels of certain chemicals that have been the focus of bans and market-based campaigns to reduce their use in consumer products, such as certain phthalates, some parabens and benzophenones, and BPA.47−50 Notably, geometric mean levels of BPS and BPF were similar to BPA, and BPS was more frequently detected (84%) than BPA (61%). These exposures may be cases of “regrettable substitution” given bisphenols are structurally homologous and may have similar hormonal activity and endocrine-disrupting effects to BPA.9,51,52 Similarly, the alternative plasticizer DINCH is being used as a replacement for DEHP and other high-molecular-weight phthalates.53−56 We also observed widespread exposures to OPEs, which may have adverse reproductive and child development outcomes57 and have been increasing in use as a replacement for polybrominated diphenyl ether (PBDE) flame-retardant chemicals. PBDEs have been banned and/or phased out due to concerns about bioaccumulation, long half-lives, and toxicity since the early 2000s, leading to increased use of replacement OPE flame retardants in fabric, electronics, and other consumer product materials.58,59 More recently, OPEs have been used as phthalate replacements in nail polish and perfumes.60,61 One potential reason for increases in exposure to replacements is the lack of a legal requirement in the U.S. to provide a minimum set of data on the potential health harms of chemicals currently on the market, such as in Europe.62
We identified demographic differences in several exposures, with Hispanic ethnicity being associated with higher concentrations of multiple pesticides, phthalates, bisphenols, and parabens, consistent with prior evidence that chemical exposures (including certain phthalates, pesticides, and phenols) are frequently higher among women of color.63−65 Racial and ethnic differences in diet and consumer product use, due in part to structural racism, may contribute to these disparities.66−69 Many chemicals associated with race and ethnicity were also observed to be higher among women with lower educational attainment (high school or less). Our multivariable modeling results suggest race and ethnicity remain important predictors after accounting for age and education. Still, these associations may be partly explained by features of our sample or cohort geography as some cohorts have a higher percentage of certain racial/ethnic participants and the sample size was modest from individual cohorts. An important limitation of our study is the lack of consideration of country or region of origin, acculturation, and immigration status for women identifying as Hispanic ethnicity. Given the wide geographic range of our study, grouping all Hispanic women together obfuscates the potential for notable differences in chemical exposures occurring within this diverse group of pregnant women. Prioritizing opportunities to collect and incorporate the necessary data to fully characterize and understand differences among unique racial and ethnic groups will be critical for future research, especially as we expand chemical biomonitoring to additional ECHO participants.
We evaluated chemicals from a wide range of uses and applications, including agricultural and home use pesticides, personal care products, and multiple plastic-related applications, including food packaging materials, home construction materials, home use products, and furniture- and foam-related materials.13 As such, people come into contact with these chemicals via air, food, drinking water, and dermal contact.11,13,70−73 Many of these chemicals migrate from their original source and have been found in intermediary exposure media, such as dust, often at higher concentrations, supporting their use as an important source of exposure monitoring.74,75 In addition, we observed higher concentrations of some parabens, phthalates, and pesticides in summer and spring compared with winter, which could be due to seasonal variation in exposure sources, such as personal care product use, diet, or time spent indoors.
A major strength of our study is the rigorous, literature-based approach we used to identify candidate chemicals, many not included in NHANES biomonitoring.13 We simultaneously quantified compounds from multiple chemical groups that have not been previously included together in prior studies, including fungicides and herbicides, OPEs, parabens, and emerging phthalate and alternative plasticizers. We used a validated, high-throughput method to simultaneously quantify concentrations of analytes in multiple chemical groups using a small volume of urine (<1 mL), which will facilitate larger future ECHO studies. Additionally, we included cohorts with diverse sociodemographic characteristics and broad geographical coverage and assessed associations with a variety of demographic and specimen characteristics to identify potentially vulnerable populations and inform the design of future studies.
Because our study focused on urinary analytes, we did not include compounds such as perfluoroalkyl substances, PBDEs, and polychlorinated biphenyls, which have been previously documented to have widespread exposures among pregnant women and the general U.S. population.11,76 Some RPDs of duplicate pairs were high, which may reflect a lack of sample homogeneity or analytical variance. Many of the analytes had CVs <30%, similar to our prior methods paper,14 and higher RPDs may suggest sample inhomogeneity. Because of limited power, we were unable to fully assess independent predictors in multivariable analyses, and we did not have information on specific sources of exposure, such as diet or personal care product use. While urine is the preferred biological matrix for measurement of nonpersistent chemicals,77 a limitation of our study design for evaluating predictors of exposure is the measurement of analytes in a single urine sample. Many of these chemicals are rapidly metabolized and eliminated in urine with short biological half-lives and/or are better measured in serum or hair. Future ECHO studies will measure analytes in repeated samples during pregnancy to assess intraindividual variability and include a sufficient sample size to reduce exposure measurement error for studies of predictors and health outcomes.
Our study sample was similar to participants of the parent ECHO cohorts with respect to several sociodemographic characteristics. Although our sample may not be fully representative of the U.S. population, we were able to assess exposures within diverse racial, ethnic, geographic, and socioeconomic subgroups. While we were unable to evaluate the potential health risks of these exposures given the pilot nature of our study, our results both document widespread exposures during pregnancy across the U.S. and demonstrate the feasibility of this multiclass assay. Our findings provide a foundation for a larger ECHO study to evaluate the relationships of these exposures to adverse health outcomes.
Our data support the importance of temporal biomonitoring of multiple chemicals to identify how policies and related activities have successfully reduced exposures, and where future interventions should focus. This study also reinforces the need to identify systematic solutions to avoid potential “regrettable substitutions” and prevent future harmful exposures, including improvements to chemical alternative assessments and approaches that address chemical classes instead of individual chemicals.7,78,79 Finally, ECHO has collected rich information on exposure sources and health outcomes that can be used to identify potential individual- and policy-level interventions and evaluate the health impacts of these exposures.11 Illuminating contemporary and emerging chemical exposures during pregnancy is critical to identify common sources of potentially modifiable exposures and inform interventions aimed at exposure reduction on the individual, clinical, and population levels, with implications for maternal, child, and lifelong health.
Acknowledgments
The authors wish to thank our ECHO colleagues, the medical, nursing, and program staff, as well as the children and families participating in the ECHO cohorts. The authors also acknowledge the contribution of the ECHO Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL, Benjamin DK.
Glossary
Abbreviations
- 2,4-D
2,4-dichlorophenoxyacetic acid
- 2,4,5-T
2,4,5-trichlorophenoxyacetic acid
- 4-OHBP
4-hydroxybenzophenone
- ATZ
atrazine
- BDCIPP
bis(1,3-dichloro-2-propyl) phosphate
- BP1
benzophenone-1
- BP3
benzophenone-3
- BP8
2,2’-dihydroxy-methoxybenzophenone
- BPA
bisphenol A
- BPF
bisphenol F
- BPS
bisphenol S
- BPZ
bisphenol Z
- BuPB
butyl paraben
- BzPB
benzyl paraben
- CDC
Centers for Disease Control and Prevention
- CLO
clothianidin
- DCCA
3-(2,-di-chlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid
- ∑DEHP
molar sum of di-2-ethylhexyl phthalate metabolites
- ∑DiDP
molar sum of di-iso-decyl phthalate metabolites
- ∑DINCH
molar sum of di-iso-nonyl-cyclohexane-1,2-dicarboxylic acid metabolites
- ∑DPHP
molar sum of di-(2-propylheptyl) phthalate metabolites
- DPHP
diphenyl phosphate
- ECHO
Environmental influences on Child Health Outcome
- EtPB
ethyl paraben
- FLUOs
composite of 2-, 3-, and 9-hydroxyfluorene
- FPBA
4-fluoro-3-phenoxybenzoic acid
- IMI
imidacloprid
- LOD
limit of detection
- MBzP
monobenzyl phthalate
- MEP
monoethyl phthalate
- MePB
methyl paraben
- MHPP
mono-2-heptyl phthalate
- MiPP/MPrP
composite of mono-isopropyl phthalate and mono-propyl phthalate
- MMP
monomethyl phthalate
- MnBP/MiBP
composite of mono-n-butyl phthalate and mono-iso-butyl phthalate
- MOP
mono-n-octyl phthalate
- NAPs
composite of 1- and 2-hydroxynaphthalene
- NDMA
N-desmethyl acetamiprid
- NIH
National Institutes of Health
- NIT
nitenpyram
- NHANES
National Health and Nutrition Examination Survey
- PCP
pentachlorophenol
- PHENs
composite of 1-, 2-, 3-, 4-, and 9-hydroxyphenanthrene
- PNP
4-nitrophenol
- PrPB
propyl paraben
- SUF
sulfoxaflor
- TCC
triclocarban
- TCP
3,5,6-trichloro-2-pyridinol
- TEP
triethyl phosphate
- THX
thiamethoxam
- TnBP/TiBP
composite of tri-n-butyl phosphate and tri-iso-butyl phosphate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c08942.
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), U2CES026542 (HHEAR), and UH3OD023251, UH3OD023272, UH3OD023275, UH3OD023287, UH3OD023290, UH3OD023318, UH3OD023342, UH3OD023349, UH3OD023347, UH3OD023365 (cohort grantees).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interest.
Notes
The data sets for this manuscript are not publicly available because, per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public for review/analysis. Requests to access the data sets should be directed to the ECHO Data Analysis Center, ECHO-DAC@rti.org.
Supplementary Material
References
- Braun J. M. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. M.; Sathyanarayana S.; Hauser R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E.; Nishioka M.; Standley L. J.; Perovich L. J.; Brody J. G.; Rudel R. A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T. J.; Zota A. R.; Schwartz J. M. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ. Health Perspect. 2011, 119, 878–885. 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.; Padula A.; Sirota M.; Woodruff T. J. Environmental influences on reproductive health: the importance of chemical exposures. Fertil. Steril. 2016, 106, 905–929. 10.1016/j.fertnstert.2016.07.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA TSCA chemical substance inventory www.epa.gov/tsca-inventory (June 1).
- Blum A.; Behl M.; Birnbaum L.; Diamond M. L.; Phillips A.; Singla V.; Sipes N. S.; Stapleton H. M.; Venier M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers?. Environ. Sci. Technol. Lett. 2019, 6, 638–649. 10.1021/acs.estlett.9b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Committee on the Design and Evaluation of Safer Chemical Substitutions: A Framework to Inform Government and Industry Decision. Board on Chemical Sciences and Technology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies. In A Framework to Guide Selection of Chemical Alternatives; National Academies Press (US): Washington (DC), 2014. [Google Scholar]
- Rochester J. R.; Bolden A. L.; Bisphenol S. and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) NHANES 2007-2010 Sampling Methodology Note https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/samplingnotes.aspx?BeginYear=2007 (accessed Dec 29, 2021).
- Buckley J. P.; Barrett E. S.; Beamer P. I.; Bennett D. H.; Bloom M. S.; Fennell T. R.; Fry R. C.; Funk W. E.; Hamra G. B.; Hecht S. S.; Kannan K.; Iyer R.; Karagas M. R.; Lyall K.; Parsons P. J.; Pellizzari E. D.; Signes-Pastor A. J.; Starling A. P.; Wang A.; Watkins D. J.; Zhang M.; Woodruff T. J.; Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) Program. J. Exposure Sci. Environ. Epidemiol. 2020, 30, 397–419. 10.1038/s41370-020-0211-9. [DOI] [Google Scholar]
- ECHO. Environmental influences on Child Health Outcomes: A program supported by the NIH. https://echochildren.org/ (September 27).
- Pellizzari E. D.; Woodruff T. J.; Boyles R. R.; Kannan K.; Beamer P. I.; Buckley J. P.; Wang A.; Zhu Y.; Bennett D. H. Identifying and Prioritizing Chemicals with Uncertain Burden of Exposure: Opportunities for Biomonitoring and Health-Related Research. Environ. Health Perspect. 2019, 127, 126001 10.1289/EHP5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Chinthakindi S.; Kannan K. A method for the analysis of 121 multi-class environmental chemicals in urine by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1646, 462146 10.1016/j.chroma.2021.462146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K.; Stathis A.; Mazzella M. J.; Andra S. S.; Barr D. B.; Hecht S. S.; Merrill L. S.; Galusha A. L.; Parsons P. J. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int. J. Hyg. Environ. Health 2021, 234, 113741 10.1016/j.ijheh.2021.113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr D. B.; Silva M. J.; Kato K.; Reidy J. A.; Malek N. A.; Hurtz D.; Sadowski M.; Needham L. L.; Calafat A. M. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ. Health Perspect. 2003, 111, 1148–1151. 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M. S.; Engel S. M.; Berkowitz G. S.; Ye X.; Silva M. J.; Zhu C.; Wetmur J.; Calafat A. M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092–1097. 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. M.; Bellinger D. C.; Hauser R.; Wright R. O.; Chen A.; Calafat A. M.; Yolton K.; Lanphear B. P. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 2017, 58, 75–83. 10.1016/j.neuro.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin J. H.; Colt J. S.; Camann D.; Davis S.; Cerhan J. R.; Severson R. K.; Bernstein L.; Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect. 2004, 112, 1691–1696. 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R. W.; Reed L. D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Barr D. B.; Wang R. Y.; Needham L. L. Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the National Children’s Study. Environ. Health Perspect. 2005, 113, 1083–1091. 10.1289/ehp.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K. M.; Upson K.; Cook N. R.; Weinberg C. R. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect. 2016, 124, 220–227. 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger M. F.; Lowry L. K.; Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J. 1993, 54, 615–627. 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Kuiper J. R.; O’Brien K. M.; Ferguson K. K.; Buckley J. P. Urinary specific gravity measures in the U.S. population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environ. Int. 2021, 156, 106656 10.1016/j.envint.2021.106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby M. D.NTP-CERHR monograph on the potential human reproductive and developmental effects of di (2-ethylhexyl) phthalate (DEHP) NTP CERHR MON, 2006, (18), .
- Office of Environmental Health Hazard Assessment Safe Drinking Water and Toxic Enforcement Act of 1986. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. March 19, 2021 Proposition 65 List https://oehha.ca.gov/proposition-65/proposition-65-list (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Evaluation of New Scientific Evidence Concerning DINP and DIDP: In Relation to Entry 52 of Annex XVII to REACH Regulation (EC) No 1907/2006 https://echa.europa.eu/documents/10162/31b4067e-de40-4044-93e8-9c9ff1960715 (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Inclusion of substances of very high conern in the candidate list (Decision by the Executive Director). https://www.echa.europa.eu/documents/10162/78d8ecfd-5e83-4299-9f16-15681ee11bbb (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Substance Infocard: acetamiprid. https://echa.europa.eu/substance-information/-/substanceinfo/100.111.622 (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Substance Infocard: 6-chloronicotinic acid. https://echa.europa.eu/substance-information/-/substanceinfo/100.023.819 (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Substance Infocard: 2,2’,4,4’-tetrahydroxybenzophenone https://echa.europa.eu/substance-information/-/substanceinfo/100.004.573 (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Substance Infocard: 4-hydroxybenzophenone. https://echa.europa.eu/substance-information/-/substanceinfo/100.013.188 (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Substance Infocard: Diheptyl phthalate https://echa.europa.eu/substance-information/-/substanceinfo/100.020.806 (accessed Aug 30, 2021).
- European Chemicals Agency (ECHA) Substance Infocard: Dipentyl phthalate. https://echa.europa.eu/substance-information/-/substanceinfo/100.004.563 (accessed Aug 30, 2021).
- Office of Environmental Health Hazard Assessment (OEHHA) Proposition 65 Fact Sheets: Phthalates https://www.p65warnings.ca.gov/fact-sheets/phthalates (accessed Aug 30, 2021).
- Ospina M.; Wong L. Y.; Baker S. E.; Serafim A. B.; Morales-Agudelo P.; Calafat A. M. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015-2016 national health and nutrition examination survey. Environ. Res. 2019, 176, 108555 10.1016/j.envres.2019.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida J. E. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Annu. Rev. Entomol. 2018, 63, 125–144. 10.1146/annurev-ento-020117-043042. [DOI] [PubMed] [Google Scholar]
- Douglas M. R.; Tooker J. F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. 10.1021/es506141g. [DOI] [PubMed] [Google Scholar]
- Jeschke P.; Nauen R.; Schindler M.; Elbert A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- Simon-Delso N.; Amaral-Rogers V.; Belzunces L. P.; Bonmatin J. M.; Chagnon M.; Downs C.; Furlan L.; Gibbons D. W.; Giorio C.; Girolami V.; Goulson D.; Kreutzweiser D. P.; Krupke C. H.; Liess M.; Long E.; McField M.; Mineau P.; Mitchell E. A.; Morrissey C. A.; Noome D. A.; Pisa L.; Settele J.; Stark J. D.; Tapparo A.; Van Dyck H.; Van Praagh J.; Van der Sluijs J. P.; Whitehorn P. R.; Wiemers M. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- Tomizawa M.; Casida J. E. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- Mach B. M.; Bondarenko S.; Potter D. A. Uptake and dissipation of neonicotinoid residues in nectar and foliage of systemically treated woody landscape plants. Environ. Toxicol. Chem. 2018, 37, 860–870. 10.1002/etc.4021. [DOI] [PubMed] [Google Scholar]
- Frank S. D. Reduced risk insecticides to control scale insects and protect natural enemies in the production and maintenance of urban landscape plants. Environ. Entomol. 2012, 41, 377–386. 10.1603/EN11230. [DOI] [PubMed] [Google Scholar]
- Han W.; Tian Y.; Shen X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. 10.1016/j.chemosphere.2017.10.149. [DOI] [PubMed] [Google Scholar]
- Chen M.; Tao L.; McLean J.; Lu C. Quantitative analysis of neonicotinoid insecticide residues in foods: implication for dietary exposures. J. Agric. Food Chem. 2014, 62, 6082–6090. 10.1021/jf501397m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consumer Products Safety Commission . Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report, July, 2014.
- California Safe Cosmetics Act of 2005. In 2005; p Chapter 729.
- Consumer Product Safety Improvement Act of 2008. In 2008; pp 122 STAT. 3016-122 STAT. 3077.
- Food and Drug Administration, Indirect Food Additives: Polymers. In 2012; Vol. 77 41,899.
- Pelch K.; Wignall J. A.; Goldstone A. E.; Ross P. K.; Blain R. B.; Shapiro A. J.; Holmgren S. D.; Hsieh J. H.; Svoboda D.; Auerbach S. S.; Parham F. M.; Masten S. A.; Walker V.; Rooney A.; Thayer K. A. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235 10.1016/j.tox.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Rosenmai A. K.; Dybdahl M.; Pedersen M.; Alice van Vugt-Lussenburg B. M.; Wedebye E. B.; Taxvig C.; Vinggaard A. M. Are structural analogues to bisphenol a safe alternatives?. Toxicol. Sci. 2014, 139, 35–47. 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y.; Ashrap P.; Calafat A. M.; Ye X.; Rosario Z.; Bedrosian L. D.; Huerta-Montanez G.; Velez-Vega C. M.; Alshawabkeh A.; Cordero J. F.; Meeker J. D.; Watkins D. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Exposure Sci. Environ. Epidemiol. 2020, 30, 56–69. 10.1038/s41370-019-0168-8. [DOI] [Google Scholar]
- Silva M. J.; Jia T.; Samandar E.; Preau J. L. Jr; Calafat A. M. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000-2012). Environ. Res. 2013, 126, 159–163. 10.1016/j.envres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenhammar I.; Glynn A.; Jonsson B. A.; Lindh C. H.; Darnerud P. O.; Svensson K.; Lignell S. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs?. Environ. Res. 2017, 153, 48–54. 10.1016/j.envres.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Kasper-Sonnenberg M.; Koch H. M.; Apel P.; Ruther M.; Palmke C.; Bruning T.; Kolossa-Gehring M. Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: Biomonitoring data on young adults from the Environmental Specimen Bank (ESB). Int. J. Hyg. Environ. Health 2019, 222, 1084–1092. 10.1016/j.ijheh.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Doherty B. T.; Hammel S. C.; Daniels J. L.; Stapleton H. M.; Hoffman K. Organophosphate Esters: Are These Flame Retardants and Plasticizers Affecting Children’s Health?. Curr. Environ. Health Rep. 2019, 6, 201–213. 10.1007/s40572-019-00258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 46, 13056–13066. 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I.; de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Young A. S.; Allen J. G.; Kim U. J.; Seller S.; Webster T. F.; Kannan K.; Ceballos D. M. Phthalate and Organophosphate Plasticizers in Nail Polish: Evaluation of Labels and Ingredients. Environ. Sci. Technol. 2018, 52, 12841–12850. 10.1021/acs.est.8b04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle M. E.; Watkins D.; Rosario Z.; Velez Vega C. M.; Huerta-Montanez G.; Calafat A. M.; Ospina M.; Cordero J. F.; Alshawabkeh A.; Meeker J. D. The association of urinary organophosphate ester metabolites and self-reported personal care and household product use among pregnant women in Puerto Rico. Environ. Res. 2019, 179, 108756 10.1016/j.envres.2019.108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordner A.; Richter L.; Brown P. Can Chemical Class Approaches Replace Chemical-by-Chemical Strategies? Lessons from Recent U.S. FDA Regulatory Action on Per- And Polyfluoroalkyl Substances. Environ. Sci. Technol. 2016, 50, 12584–12591. 10.1021/acs.est.6b04980. [DOI] [PubMed] [Google Scholar]
- Chan M.; Mita C.; Bellavia A.; Parker M.; James-Todd T. Racial/Ethnic Disparities in Pregnancy and Prenatal Exposure to Endocrine-Disrupting Chemicals Commonly Used in Personal Care Products. Curr. Environ. Health Rep. 2021, 8, 98–112. 10.1007/s40572-021-00317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T. M.; Meeker J. D.; Huang T.; Hauser R.; Ferguson K. K.; Rich-Edwards J. W.; McElrath T. F.; Seely E. W. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int. 2016, 96, 118–126. 10.1016/j.envint.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. K.; Kahana A.; Heidt J.; Polemi K.; Kvasnicka J.; Jolliet O.; Colacino J. A. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999-2014. Environ. Int. 2020, 137, 105496 10.1016/j.envint.2020.105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J. P.; Kim H.; Wong E.; Rebholz C. M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ. Int. 2019, 131, 105057 10.1016/j.envint.2019.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R.; Phillips C. A.; Mitro S. D. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003-2010. Environ. Health Perspect. 2016, 124, 1521–1528. 10.1289/ehp.1510803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne-Sturges D. C.; Gee G. C.; Cory-Slechta D. A. Confronting Racism in Environmental Health Sciences: Moving the Science Forward for Eliminating Racial Inequities. Environ. Health Perspect. 2021, 129, 55002 10.1289/EHP8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield Z.; Addington C. K.; Dionisio K. L.; Lyons D.; Tornero-Velez R.; Phillips K. A.; Buckley T. J.; Isaacs K. K. Mining of Consumer Product Ingredient and Purchasing Data to Identify Potential Chemical Coexposures. Environ. Health Perspect. 2021, 129, 67006 10.1289/EHP8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D.; Kang Y.; Chen J.; Li A.; Chen W.; Li Z.; He L.; Zhang Q.; Luo J.; Zeng L. Dermal bioaccessibility of plasticizers in indoor dust and clothing. Sci. Total Environ. 2019, 672, 798–805. 10.1016/j.scitotenv.2019.04.028. [DOI] [PubMed] [Google Scholar]
- Hou M.; Shi Y.; Na G.; Cai Y. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure. Environ. Int. 2021, 146, 106261 10.1016/j.envint.2020.106261. [DOI] [PubMed] [Google Scholar]
- Karrer C.; Andreassen M.; von Goetz N.; Sonnet F.; Sakhi A. K.; Hungerbuhler K.; Dirven H.; Husoy T. The EuroMix human biomonitoring study: Source-to-dose modeling of cumulative and aggregate exposure for the bisphenols BPA, BPS, and BPF and comparison with measured urinary levels. Environ. Int. 2020, 136, 105397 10.1016/j.envint.2019.105397. [DOI] [PubMed] [Google Scholar]
- Husøy T.; Andreassen M.; Hjertholm H.; Carlsen M. H.; Norberg N.; Sprong C.; Papadopoulou E.; Sakhi A. K.; Sabaredzovic A.; Dirven H. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ. Int. 2019, 132, 105103 10.1016/j.envint.2019.105103. [DOI] [PubMed] [Google Scholar]
- Mitro S. D.; Dodson R. E.; Singla V.; Adamkiewicz G.; Elmi A. F.; Tilly M. K.; Zota A. R. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ. Sci. Technol. 2016, 50, 10661–10672. 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. M.; Moschet C.; Young T. M.; Bennett D. H. Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air 2020, 30, 60–75. 10.1111/ina.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2021.
- Calafat A. M.; Longnecker M. P.; Koch H. M.; Swan S. H.; Hauser R.; Goldman L. R.; Lanphear B. P.; Rudel R. A.; Engel S. M.; Teitelbaum S. L.; Whyatt R. M.; Wolff M. S. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ. Health Perspect. 2015, 123, A166–A168. 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski C. F.; Andrews D. Q.; Birnbaum L. S.; Bruton T. A.; DeWitt J. C.; Knappe D. R. U.; Maffini M. V.; Miller M. F.; Pelch K. E.; Reade A.; Soehl A.; Trier X.; Venier M.; Wagner C. C.; Wang Z.; Blum A. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. 10.1021/acs.estlett.0c00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bălan S. A.; Mathrani V. C.; Guo D. F.; Algazi A. M. Regulating PFAS as a Chemical Class under the California Safer Consumer Products Program. Environ. Health Perspect. 2021, 129, 25001 10.1289/EHP7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.