Abstract
Experiencing poverty increases vulnerability for dysregulated hypothalamic–pituitary–adrenal (HPA) axis functioning and compromises long-term health. Positive parenting buffers children from HPA axis reactivity, yet this has primarily been documented among families not experiencing poverty. We tested the theorized power of positive parenting in 124 parent–child dyads recruited from Early Head Start (Mage = 25.21 months) by examining child cortisol trajectories using five samples collected across a standardized stress paradigm. Piecewise latent growth models revealed that positive parenting buffered children’s stress responses when controlling for time of day, last stress task completed, and demographics. Positive parenting also interacted with income such that positive parenting was especially protective for cortisol reactivity in families experiencing greater poverty. Findings suggest that positive parenting behaviors are important for protecting children in families experiencing low income from heightened or prolonged physiologic stress reactivity to an acute stressor.
Keywords: early childhood, HPA axis, parenting, poverty, stress
Childhood poverty is associated with serious adverse health outcomes across the lifespan (Cook et al., 2004; Evans & Kim, 2007; Nikulina, Widom, & Czaja, 2011) and affects up to 43% of children living in the United States (National Center for Children in Poverty, 2018). Poverty influences poor health outcomes through numerous mechanisms. For example, children experiencing poverty are at risk of poor health due in part to increased exposure to difficult life circumstances and structural inequities. Poverty may threaten the family environment by increasing parental stress and rates of mental illness (Goyal, Gay, & Lee, 2010), along with family dysfunction (Magnusson & Duncan, 2002). These life circumstances can also include elevated exposure to community violence (Graif & Matthews, 2017) as well as limited opportunities (Pearlin, Schieman, Fazio, & Meersman, 2005), such as lower quality education (Coley, 2002), and/or resource instability (Gennetian & Shafir, 2015).
To date, one prominent biological mechanism theorized to explain how these social and environmental stressors get under the skin to compromise health and wellbeing is via changes in stress system functioning (Gunnar & Vazquez, 2006). In particular, these changes are believed to result from repeated efforts to manage stressors and threat that eventually overwhelm the body’s adaptive capacity. The path from childhood poverty to poor health is more robust when poverty-related stressors repeatedly activate the body’s stress response systems, including the hypothalamic–pituitary–adrenal (HPA) axis, which is central to facilitating adaptation to stressors within the environment (McEwen, 2005). Exposure to poverty-related stressors can lead to dysregulated HPA axis activity including both increased or blunted cortisol production (i.e., exaggerated or unresponsive stress system functioning), especially if children repeatedly face stressors in the absence of positive parenting (Blair, Raver, Granger, Mills-Koonce, & Hibel, 2011; Flinn & England, 1995). Critically, altered stress system functioning is implicated in long-term health outcomes, including compromised cardiovascular, immune system, and bioregulatory functioning (e.g., metabolism, sleep) (Buske-Kirschbaum et al., 2007; Gunnar & Vazquez, 2006; Rosmond & Bjorntorp, 2000).
Prior work characterizing dysregulated HPA axis activity has examined differences in diurnal cortisol patterning and acute stress responses. While the two are interconnected, these measures yield distinct information. In the case of poverty-related stressors, more work has examined diurnal patterning. For instance, a history of adversity is associated with blunted diurnal cortisol patterns (Bernard, Zwerling, & Dozier, 2015), flatter diurnal slope (e.g., elevated afternoon and evening cortisol or modest/ non-existent cortisol awakening response) (Martin, Bruce, & Fisher, 2012), differences in age-dependent morning cortisol (Laurent et al., 2014), lower morning cortisol values on childcare days (Miles et al., 2018), and elevated overnight cortisol in young children (Evans & Kim, 2007). Similarly, Blair et al. (2011) found that exposure to chronic stress was associated with higher resting cortisol levels in young children prior to beginning a stress paradigm. These differences in diurnal patterns are thought to reflect more sustained adaptations to repeated adversity exposures.
Regarding stress reactivity, extant literature supports a consistent relation between exposure to stressful experiences and dysregulated stress reactivity in childhood (hyper- or hypo-responsive) (Gunnar, Talge, & Herrera, 2009; Hunter, Minnis, & Wilson, 2011). These differences persist after controlling for genetic contributions (Ouellet-Morin et al., 2008). For example, moderate to severe early life stress has been linked to diminished cortisol reactivity in response to mild acute stress tasks (Gunnar et al., 2009), while various forms of psychosocial adversity have also been linked with higher cortisol reactivity across multiple studies (e.g., Hunter et al., 2011).
Supportive caregiving environments often serve as a protective factor for young children experiencing stress and may differentially affect HPA axis responsivity and functioning. Specifically, a large body of research indicates that primary caregivers may play both a direct and an indirect role in children’s stress system functioning (Gunnar & Hostinar, 2015). Primary caregivers not only influence the extent and nature of exposure to stressors, but they may also moderate, or buffer, the effects of stressful experiences on children’s stress system responses under adverse conditions. The construct of parental buffering, defined here as the down-regulation of HPA axis activity via positive parenting behaviors, is rooted in attachment theory (Gunnar & Hostinar, 2015). According to Bowlby (1969), a key function of attachment in the parent–child relationship is to provide a safe and secure base for the child. Across infancy, children develop internal working models of the availability, supportiveness, and reliability of caregivers (Bretherton, 1992). When secure attachments are established, young children learn to expect a safe, stable, and supportive environment and therefore confidently explore novel situations and quickly recover from stressors (Bretherton, 1992; Gunnar & Hostinar, 2015). Indeed, lower stress reactivity in response to an acute stressor has been observed for securely attached children as compared to their insecurely attached peers, even when distress has been observed behaviorally (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996). These findings suggest that the presence of a caregiver as well as the quality of the parent–child relationship play a robust role in buffering of the stress system response for the child (Gunnar & Hostinar, 2015).
Decades of research support the theoretical assumption that healthy parent–child relationships are built from positive parenting behaviors, including parenting that is warm, sensitive, engaged, and non-intrusive (e.g. DeWolff & van IJzendoorn, 1997; Zeegers, Colonnesi, Stams, & Meins, 2017). In particular, research on parenting suggests that positive parenting behaviors play crucial roles in child development (Baumrind, 1967; Lamb & Lewis, 2011). Observations of positive parenting behaviors are strongly predictive of child outcomes (Zaslow et al., 2006), with increases in positive parenting behaviors linked to children’s cortisol recovery from stressors (Albers, Riksen-Walraven, Sweep, & de Weerth, 2008). However, from an ecological systems perspective, the hardships of poverty may spill over into the caretaking environment resulting in material deprivation, stressful life events, and consequences for adult mental health (Bronfenbrenner & Morris, 1998). These factors are likely to interact, which can further disrupt parenting and lead to poor child outcomes. While the experience of poverty has been shown to impede positive parenting behaviors (Bakermans-Kranenburg, van Ijzendoorn, & Kroonenberg, 2004), many parents in families experiencing low income provide parenting that has salutary effects on children. Indeed, the presence of a supportive adult has been the most robust and consistent resilience factor for children exposed to poverty and adversity (Masten et al., 1999; O’Neal, Weston, Brooks-Gunn, Berlin, & Atapattu, 2017).
Despite a robust theoretical foundation and well-documented binary associations among poverty, parenting, and child outcomes, more research that directly examines the role of positive parenting behaviors prior to an acute stress exposure among families experiencing poverty is needed. To date, several articles examine the association between positive parenting and children’s HPA axis functioning (i.e., Berry, Blair, Willoughby, Granger, & Mills-Koonce, 2017; Hibel, Granger, Blair, Cox, & The Family Life Project Key Investigators, 2011; Hostinar, Johnson, & Gunnar, 2015). Collectively, lower stress reactivity was observed in the presence of maternal sensitivity and/or secure attachment across varied contexts of experienced adversity such as exposure to poverty (Berry et al., 2017; Johnson, Mliner, Depasquale, Troy, & Gunnar, 2018), intimate partner violence (Hibel et al., 2011), as well as early institutionalization (Hostinar et al., 2015). One especially relevant article conducted by Johnson et al. (2018) found that attachment was a significant moderator of the association between poverty and HPA axis activity. Specifically, securely attached low-income children had significantly lower cortisol reactivity to pediatric inoculations as compared to their insecurely attached counterparts. Taken together, these studies provide evidence for the powerful role that positive parenting can have in buffering children from the negative impacts of environmental stress, including poverty-related stress, and the subsequent attenuated impact of these stressors on children’s stress system functioning.
However, the literature is limited regarding evidence for parental buffering on children’s stress system functioning in the context of poverty, particularly among very young children. This is problematic because it assumes that lessons learned from prior work with more advantaged families about effective parental buffering from stress (Gunnar & Hostinar, 2015) will apply uniformly in families exposed to poverty and to more frequent and severe adversities without empirical evidence. While positive parenting behaviors are likely promotive and beneficial for all children, whether the same parenting behaviors buffer stress reactivity and therefore interrupt the stress-health cascade for children exposed to adversity as for those benefiting from more stable and advantageous circumstances is insufficiently clear. Further, prior work suggests that even under conditions of positive parenting, exposure to certain adversities may differentially impact children’s physiological development (Hostinar et al., 2015). Therefore, examining parental buffering and child stress reactivity in the context of poverty and early childhood is crucial to a comprehensive understanding of positive parenting as a protective factor. This is particularly relevant as current intervention approaches often target families experiencing low income and assume improvements in parental sensitivity will be sufficient to promote child health and wellbeing. Therefore, both theoretical and practical advances should result from careful attention to these questions.
The current study aimed to address whether positive parenting behaviors that are well documented to be promotive in higher-resourced families similarly buffer children from an acute stress response among families who are experiencing poverty and related adversities. Although consistent positive parenting behaviors might be more difficult to provide when families are experiencing stressors, we hypothesized that the demonstration of positive parenting within this context might in fact be especially important for protection from acute stress in these young children.
Method
Procedure
Families with a child enrolled in Early Head Start (EHS) were recruited by a bilingual research team to participate in a larger intervention study aimed at increasing positive parenting in families experiencing low income. Data used in the present study reflect the entire sample of families, and were collected from two assessment time points prior to randomization into the intervention study. Both Spanish- and English-speaking families, with a primary caregiver 18 years of age or older and a child 6–36 months of age with no previous diagnosis of developmental delay or regular use of allergy or asthma medication, were invited to participate in the study and completed in-home interviews that captured demographic characteristics of parent–child dyads and their experiences of adversity. To facilitate participation by families who may have limited literacy in either Spanish or English, and to respect the cultural preferences of our majority Latina sample, measures were collected via a relaxed interview format, and respondents were offered a booklet with visual aids to illustrate Likert anchors (visually larger for the anchor that would indicate more of the queried experience or behavior). We retained the original wording of standardized questionnaires, but as Spanish does not contain double negatives (and these are confusing for native English speakers), double negative questions were also followed by a standardized interpretive statement, “so a higher score would mean this was more difficult for you.” If necessary to complete all instruments, additional visits were scheduled to allow a comfortable pace and prevent fatigue. Parents and children also participated in a free play task and a moderately stressful structured stress paradigm. Saliva samples were collected five times across the 75-minute paradigm (see Figure 1). These sessions were videotaped for coding parenting behaviors using previously well-established scoring protocols described below. Parents provided written, informed consent. Participants were compensated $100 for these portions of the study ($50 each for two visits). The study was approved by the Institutional Review Board at the University of Denver.
Figure 1.
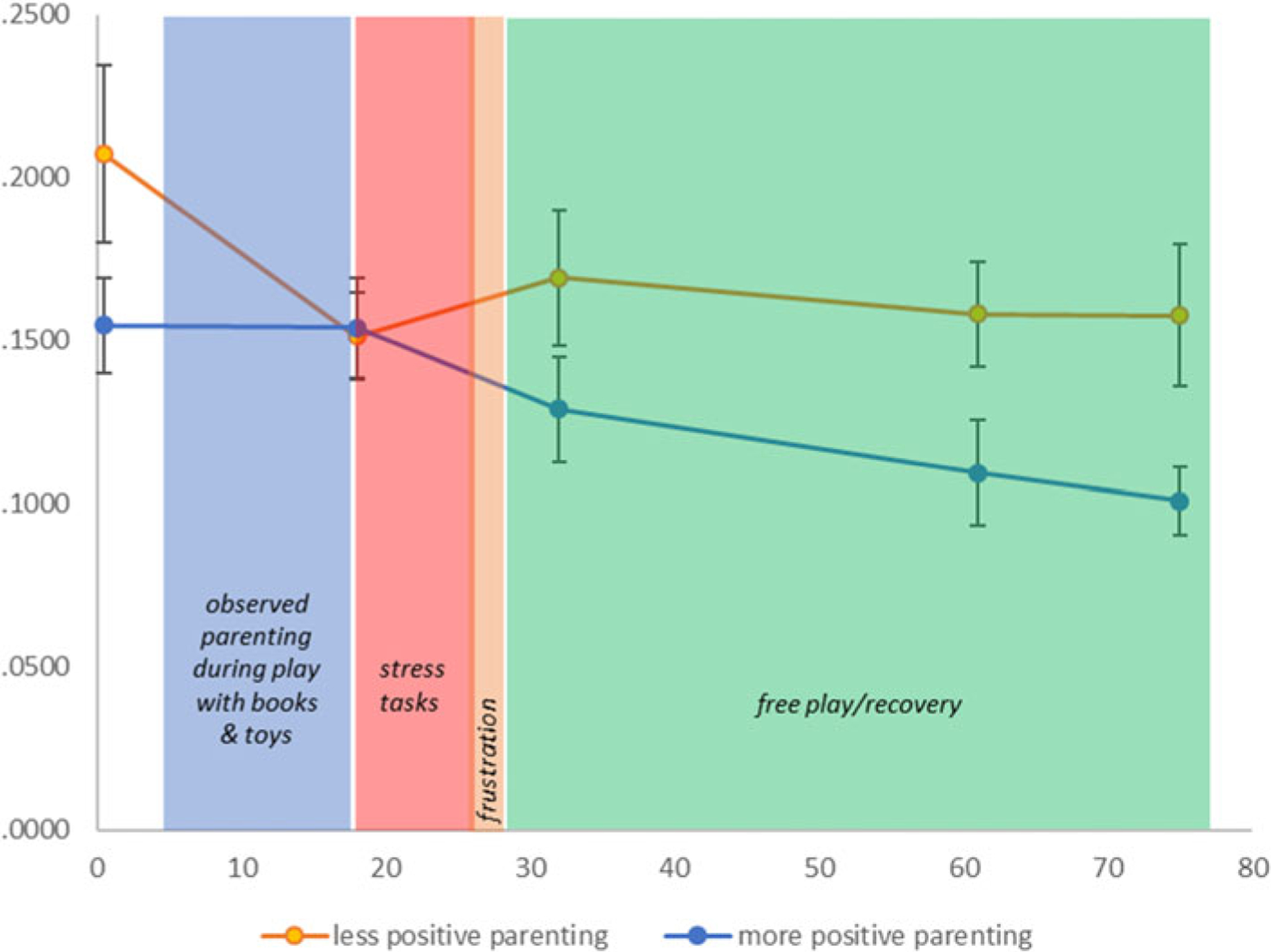
Study protocol and salivary cortisol sampling times. Conventional understanding suggests that salivary cortisol levels reflect evaluation of events occurring 15–22 minutes previously. Thus, the first sample reflects the time period before the research team arrived. The second sample (+18) reflects the start of the paradigm, the third (+32 minutes) reflects differences that emerged during the observed parenting period and transition to the stress task, and the final two illustrate differences that emerged during recovery/free play. See Figure 2 for graphical representation of the statistical models used for formal analyses.
Participants
Participants were 124 children and their primary caregivers. Of the 163 parent–child dyads initially screened and eligible (i.e., no asthma or allergy medication, no diagnosed developmental delay, not a sibling of an enrolled participant), 29 parent–child dyads were lost following screening or declined to participate, eight were unwilling to provide saliva samples during the observed stress paradigm, one child’s data was deleted for extreme values (i.e., physiologically improbable), and one child’s saliva could not be assayed due to low volume. The final sample of 124 children did not differ on key variables of child age, child sex, or (when available) positive parenting, (all p’s > .12); although we note there was a trend for included children to be from slightly higher income families (p = .08). Children ranged in age from 6 to 45 months (M = 25.21, SD = 9.67) and 58.9% were male. The sample age range extended beyond the planned 36-month range due to a small number of older children still participating in some type of EHS service. Parents were predominantly mothers (98.4%), ranging in age from 18 to 49 years (M = 31.60, SD = 6.44) and identifying as Latinx and White/Caucasian (66.4%), followed by minority race/non-Latinx (24.6%; of these, 15.0% were African American/Black, 1.0% African, 1.0% Asian, 1.0% American/Native Indian or Alaskan Native, and 1.0% Pacific Islander), White/Caucasian and non-Latinx (19.0%), and bi- or multi-racial/non-Latinx (7.0%). Income was assessed via comprehensive questionnaires and then categorized based on percent of the federal poverty line (FPL) for the study year (0 = 50% FPL, 1 = 100% FPL, 2 = 150% FPL, 3 = 200% FPL, 4 = 250% FPL, 5 = 300% FPL). Of the 124 participants, 84.3% of families were represented by categories 0–2 (≤ 150% FPL), 9% by category 3 (9.1% at or below 200% FPL), and 6.6% by category 4 and 5 (at or below 300% FPL).
Measures
Parenting
To observe parenting behaviors, parent–child dyads were observed during a modified “3-bag” parenting task (NICHD Early Child Care Research Network, 1999) for 10 minutes. Parents were provided an age-appropriate book (without words) and toys and were instructed to play with their child as they normally would (four minutes with the book and six minutes with toys). The play sessions were videotaped and later coded for positive parenting behaviors under the direction of a centralized coding team that specializes in this coding scheme (Dr. Mills-Koonce). Specifically, parents were rated on sensitivity, positive regard for the child, animation, cognitive stimulation, intrusiveness, detachment, and negative regard for the child using a 5-point rating scale. Interrater reliability was calculated based on double coding a random subset of pre- and post-videos (n = 24, ICC: .57–.88). The average interclass correlation between raters was .74 across subscales and .68 on the positive parenting composite (below). The parenting task and scoring of the recorded free play sessions have been previously established (NICHD Early Child Care Research Network, 1999; Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011) and demonstrate good reliability, α = .83. For analyses, a composite of positive parenting behaviors was calculated by summing scores from the sensitivity, positive regard for the child, animation, and cognitive stimulation subscales. For illustrative purposes only, Figure 1 depicts a dichotomized split of positive parenting such that scores on the sensitivity composite of 3 or higher (n = 72) were recoded as 1 ‘more positive parenting behaviors’ versus less than 3 (n = 51) as 0 = ‘less positive parenting behaviors’. Our sample had substantial variability on the positive parenting composite (1.25 – 4.50 total scores, n = 72 for 3 or above, n = 51 for below 3), and a continuous variable of positive parenting was used in all formal analyses (1.00 – 5.00).
Child stress paradigm and cortisol variability
Following the observed parenting task, children participated in a structured moderate stress paradigm that lasted up to seven minutes. Because we were interested in understanding the role of positive parenting within a sample experiencing poverty, it was important to provide sufficient challenge to the HPA axis to detect differences. In previous work, roughly 18% of 4-year-olds from a similar sample demonstrated flat, low, and unresponsive cortisol to a milder stress paradigm (Badanes, Watamura, & Hankin, 2011). While we interpreted this low and flat cortisol as problematic hypo-cortisol (and that interpretation was consistent with its correlates), another interpretation is that children with more stress exposure find researcher-designed challenges innocuous. Therefore, in the current study, we adapted and aggregated procedures from prior studies and from the Lab-TAB (Gagne et al., 2011) to design a structured stress paradigm that would mimic normative but escalating challenges in an attempt to more reliably elicit a moderate stress response in young children.
At the start of the 7-minute stress paradigm, the parent and child were invited to enter a small tent, and the child was buckled into a booster seat affixed to a rotating board (“lazy Susan”). Children were presented with arm restraint, restricted access to an attractive toy, face washing, a scary mask, a scary robot, a loud alarm, and finally the parent waving goodbye and exiting the child’s field of view. Parents were asked to maintain neutral affect while children were presented with these challenges. Stressors continued in a prespecified order, increasing in intensity until the stress paradigm concluded or the child became distressed for 30 continuous seconds. If the child reached distress levels (e.g., escalating tears or vocalizations without evidence of selfsoothing), the researchers discontinued the protocol. Parents were carefully prepared for the experience, provided visual prompts to remind them of the next stressor, and were encouraged to discontinue participation at any point if they felt they or their child was too distressed. Of the 124 participants included here, for more than half the paradigm was discontinued due to distress (n = 82). Of those 66.7% who did not complete the full paradigm, the following tasks prompted discontinuation: 18.7% loss of an attractive toy, 17.9% face washing, 27.6% loud alarm.
Upon conclusion of the 7-minute stress paradigm, all associated materials were visibly removed and the parent returned to the tent (if the parent had exited for the seventh and final stressor). The child was presented with a locked clear acrylic box with an age-appropriate attractive toy inside. For this 5-minute frustration task, parents were allowed to provide positive verbal encouragement only. When time had elapsed, the box was unlocked, and the toy was the child’s to keep.
Trained research assistants collected saliva samples from children five times across the home visit and structured stress paradigm (see Figure 1 for timing of sample collection relative to tasks). Samples were collected at 0, 18, 32, 61, and 75 minutes after arrival at the home. Trained research staff collected the samples and recorded the times and dates of each sample. Less than one-minute variation was observed between scheduled and actual sample collection time across the five time points (78%–89%). The greatest variability was observed for the final recovery sample (scheduled 14 minutes (61 minutes to 75 minutes); actual sample collection time M = 13.63, SD = 1.67, Range: 6 minutes to 18 minutes).
The first sample was always collected within the first minute of entering the home. Time of day was controlled for in all analyses, please see analysis section. Saliva samples were collected using infant synthetic saliva swabs (Salimetrics, State College, PA) and extracted by centrifuging for four minutes at 2,500 rpm. The vials were frozen at −20 °C until cohort data collection for a cohort of children was complete (4 cohorts total). Samples were assigned to batches for assay by cohort to minimize systematic error (i.e., all samples from the same child on the same plate, characteristics that varied between children/families like language and sex distributed across plates). The samples were then sent to the Biochemical Laboratory, Psychobiology, University of Trier, Trier, Germany to be assayed. Cortisol concentrations in the saliva samples were assayed in duplicate and measured with a timeresolved fluorescence immunoassay (Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). The intra-assay coefficient of variation for this assay was between 4.0% and 6.7%, and the corresponding inter-assay coefficients of variation were between 7.1% and 9.0%. For the current study, inter-assay values for controls of known cortisol concentration were as follows: low control 8.35%, medium control 7.11%, and high control 7.72%. The intra-assay coefficient of variation for samples used here was 6.5%.
Using methods developed in previous studies (e.g., Badanes et al., 2011), parents reported on their children’s current medications, ongoing illnesses, and chronic conditions that impact (or are impacted by) cortisol levels (e.g., asthma) (Gunnar & Vazquez, 2006) and were screened out of the study before this portion of the protocol. In addition, on sampling days, parents were asked to report on the presence of other factors that may influence cortisol values (e.g., food/caffeine intake, wake/sleep times, use of medication, and presence of illness symptoms) and this information was recorded on the sampling sheet. If needed, the visit was rescheduled to accommodate these constraints to a time when children were not ill and schedules were typical. Data were inspected for outliers within sample, cross-referenced with notes regarding suspected contamination and with lab notes regarding any assay abnormalities. In the present study, due to careful screening, parental support, rescheduling, and supervision of children during the visit, minimal deletions or corrections were required. For one child, all five stress reactive samples were deleted for very high values, and for one additional child, a single sample was deleted for a physiologically improbable value. The majority of children (n = 107, 86%) provided 4 (n = 20, 16%) or all 5 samples (n = 87, 70%). The remaining children (n = 17) provided 3 (n = 9, 7%), 2 (n = 5, 4%) and 1 (n = 3, 2%). Data were inspected for normality and it was determined that the untransformed values should be retained.
Plan of analyses
Trajectories of five cortisol values were estimated with piecewise latent growth models, using Mplus software (Muthén & Muthén, 1998–2011). The model estimated two linear slopes and one quadratic term. The first linear slope (earlier slope) estimated changes occurring at the beginning of assessment (at 0 min and +18 min), whereas the second linear slope (later slope) and quadratic term estimated changes occurring in response to the stress paradigm (at +32 min, 61 min, and 75 min). Thus, a positive earlier slope would characterize cortisol reactivity to assessment in general, whereas a combination of a positive later slope and negative quadratic term would characterize cortisol reactivity in response to the stress paradigm. Below is the list of time scores used for the two parts of the piecewise model and corresponding actual times:
| Actual time | |||||
| Time in minutes: | 0 min | 18 min | 32 min | 61 min | 75 min |
| Time in hours: | 0 | .30 | .53 | 1.02 | 1.25 |
| Time scores | |||||
| Earlier slope: | 0 | .30 | .30 | .30 | .30 |
| Later slope: | 0 | 0 | .23 | .72 | .95 |
| Later quad. recovery: | 0 | 0 | .05 | .52 | .90 |
Next, a set of models estimated the effect of positive parenting, income, and their interaction on latent linear slopes and quadratic term. All analyses controlled for time of day (centered at grand mean), child age and sex (both centered at grand mean), and the last task completed by the child (where 0 = all tasks completed, 1 = all but the last task completed through 6 = no tasks completed). Thus, resulting trajectories can be interpreted as changes in cortisol values for an average child (average age and sex), assessed at an average time, who completed all tasks.
Results
Descriptive statistics and bivariate correlations for key study variables are shown in Table 1. The unconditional latent growth model estimated average growth parameters for the whole sample. As can be seen in Figure 2a, on average children did not experience a change in cortisol within the first 20 minutes of the study (see nonsignificant average earlier slope in Table 2). The average cortisol profile also did not show significant changes between 20 and 80 minutes in the protocol (as demonstrated with nonsignificant average later slope and quadratic term). However, there were significant individual difference in cortisol trajectories, as indicated by significant standard deviations for the model intercept, earlier linear slope, and later linear slope.
Table 1.
Descriptive statistics and bivariate correlations for key study variables.
| Variable (units/range) | N (%)/M (SD) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | |||||||||||
| 1. Sex (Male) | 73 (59%) | .07 | −.08 | .09 | −.04 | .02 | .20* | .10 | .13 | .13 | .20* |
| 2. Age (Months) | 25.21 (9.67) | - | .04 | .15 | .02 | .10 | .00 | −.20* | −.22* | −.13 | −.12 |
| 3. Time (hrs post 7am) | 4.83 (2.82) | - | −.12 | −.06 | .19* | −.30** | −.29** | −.29** | −.24* | −.17 | |
| 4. Last Task (0–6) | 3.91 (2.19) | - | .05 | .10 | −.01 | −.18 | −.01 | −.03 | −.06 | ||
| Predictors | |||||||||||
| 5. Income (0–5) | 1.33 (1.23) | - | .24** | −.06 | −.05 | −.07 | .09 | −.08 | |||
| 6. Pos Parenting (1–5) | 2.95 (.80) | - | −.22* | −.09 | −.22** | −.26** | −.30** | ||||
| Outcome variables: salivary cortisol across a structured stress paradigm (μg/dL) | |||||||||||
| 7. SR 1 (0 m) | .18 (.15) | - | .37*** | .43*** | .44*** | .64*** | |||||
| 8. SR 2 (18 m) | .15 (.11) | - | .64*** | .46*** | .35*** | ||||||
| 9. SR 3 (32 m) | .14 (.13) | - | .48*** | .58*** | |||||||
| 10. SR 4 (61 m) | .13 (.12) | - | .79*** | ||||||||
| 11. SR 5 (75 m) | .12 (.12) | - | |||||||||
Note. Time – time of the first sample in hours, centered at 7 am; Last Task – the last stress paradigm task the child completed (0 = all task completed, 3 = ‘scary mask’ task completed but not the ‘mechanical toy’ task, and 6 = no task completed); Income – categorical ratings based on the family income relative to the federal poverty line (FPL) (0 = 50% of the FPL and 5 = 300% of the FPL); Pos Parenting – positive parenting behaviors observed during the ‘3-bag’ task; SR1-SR5 –the average salivary cortisol values from each assessment time point.
p < .05,
p < .01,
p < .001
Figure 2.
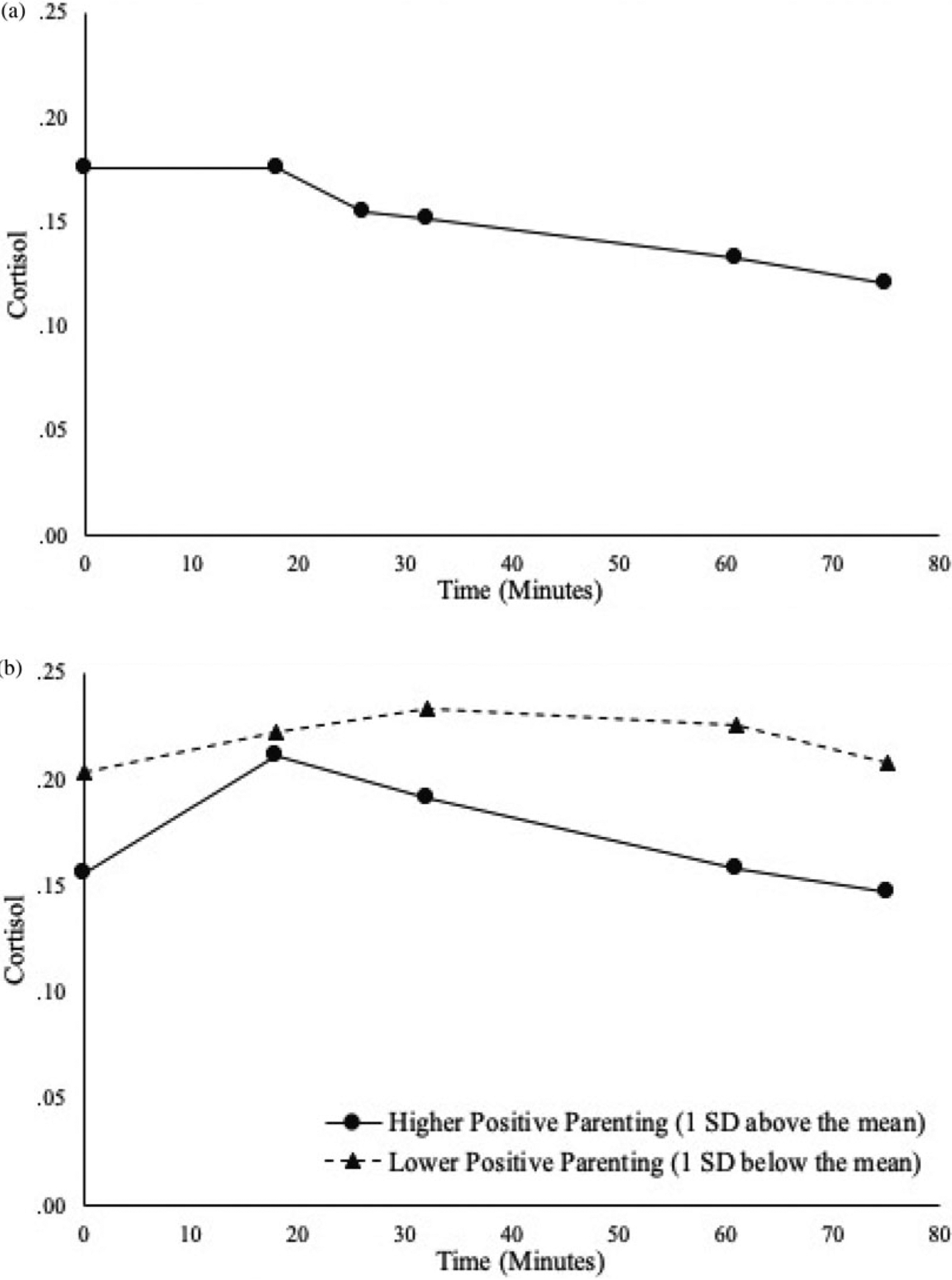
Estimated cortisol trajectories over time: (a) average trajectory of cortisol over time; (b) effect of positive parenting on cortisol trajectories.
Table 2.
Piecewise models of the cortisol reactivity trajectories
| Unconditional model | Regressed on positive parenting and income | ||||
|---|---|---|---|---|---|
| Mean | SD | β | B | (SE) | |
| Intercept | .18 | .13*** | |||
| Positive parenting | −.18 | −.03 | (.02) | ||
| Income | −.02 | −.01 | (.01) | ||
| Earlier linear slope | −.06 | .45*** | |||
| Positive parenting | .13 | .08 | (.06) | ||
| Income | .01 | .01 | (.04) | ||
| Later linear slope | −.02 | .12** | |||
| Positive parenting | −.53* | −.10 | (.05) | ||
| Income | .32 | .04 | (.03) | ||
| Quadratic term | −.02 | -a | |||
| Positive parenting | .85 | .07 | (.05) | ||
| Income | −.77 | −.04 | (.03) | ||
Note. All models controlled for child age and sex, time of the day, and last task completed.
The variance for the quadratic term was not significant, and was therefore removed from the model.
p < .05,
p < .01,
p < .001
When positive parenting and income were entered into the model as predictors of the latent growth parameters (while controlling for time of day, child’s age and sex, and the last task completed), positive parenting was associated with later linear slope – more positive parenting was associated with lower reactivity to stress paradigm (β = −.53, p < .05). Figure 2b illustrates estimated cortisol trajectories for children who experience more positive and less positive parenting (as quantified by 1 SD above and below the mean). As reported in Table 2, for every 1 unit increase in positive parenting, there was a .10 unit decrease in stress reactivity.
We next investigated whether the effect of positive parenting behaviors on later linear slope (reactivity to the stress paradigm) is moderated by the depth of experienced poverty. As can be seen in Table 3, income interacted with positive parenting in their effect on later cortisol slope (β = .32, p < .01). Positive parenting was associated with lower cortisol reactivity among children living in families with greater depth of experienced poverty – b = −.07 CR = 3.43, p < .05 (Figure 3a). This protective effect of parenting emerged for children whose family income was below 1.3 (or below about 115% of FPL) (Figure 3b).
Table 3.
Effects of predictor variables and their interactions on the later linear slope
| β | B | (SE) | |
|---|---|---|---|
| Positive parenting | −.20* | −.03 | (.02) |
| Income | −.14 | −.01 | (.01) |
| Positive parenting × Income | .32** | .04 | (.01) |
Note. Only results relevant for the effects of positive parenting and income on later linear slope are presented in the table. The model also estimated latent cortisol intercept, earlier slope, and quadratic term, as well as controlled for child age and sex, time of the day, and last task completed.
p < .05,
p < .01
Figure 3.
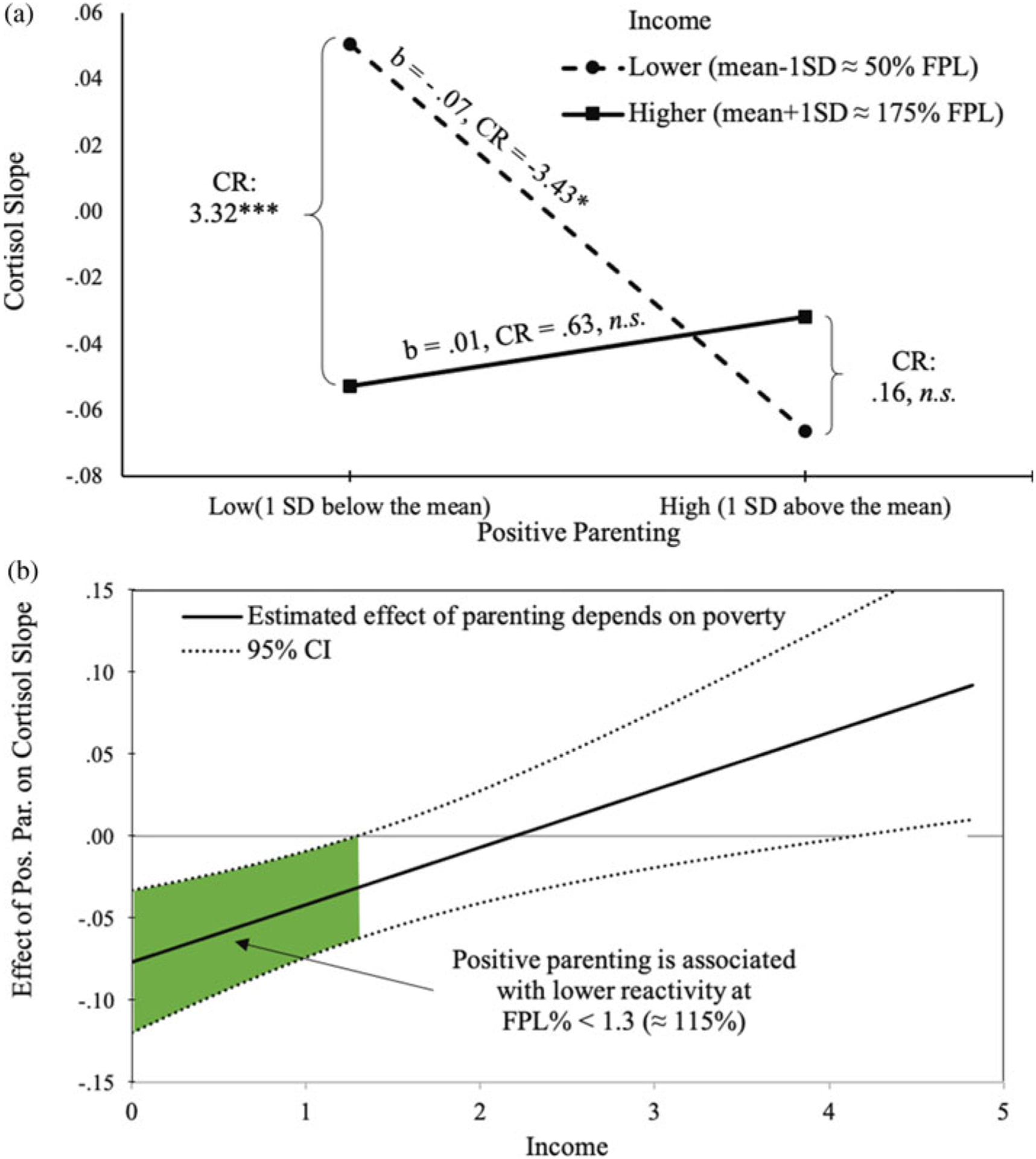
Poverty and parenting interact in their effects on cortisol rise: (a) simple slopes for the effect of positive parenting on cortisol slope at 1 SD below and above income mean; (b) positive parenting is associated with lower cortisol slope when income is below 115% federal poverty line (FPL).
Discussion
The purpose of this study was to evaluate whether differences in positive parenting behaviors in the minutes before a structured stress paradigm would be associated with children’s stress reactivity within a sample of young children experiencing poverty. Based on attachment theory and current working models, we hypothesized that positive parenting would be protective against a subsequent set of stressors experienced with the parent present (but asked to remain neutral), as has been documented in parent–child dyads not experiencing poverty. Our results were consistent with this prediction. The effect of positive parenting on stress reactivity was linear, and neither statistical exploration nor visual inspection revealed a clear threshold of “good enough” parenting after which no improvement in the number of children who were buffered was seen. Although the proportion of children who exhibited stress reactivity decreased linearly as positive parenting increased, and children with the least positive parents nearly all exhibited cortisol reactivity to the stress paradigm, within our sample there was no measured level of positive parenting where every child was buffered (that is, the child showed flat or falling cortisol across the later slope). Further, positive parenting was predictive of lower cortisol reactivity even in models controlling for family income.
Our findings also revealed an important and hypothesized interaction between positive parenting and family income. Even within a sample of families experiencing low income, positive parenting was especially protective at the lower income levels (at or below about 115% of the federal poverty line). Living near the federal poverty line places incredible strains on family resources and often co-occurs with multiple adverse experiences. Moreover, parents living in areas of concentrated disadvantage report increases in daily parenting hassles (Finegood, Raver, DeJoseph, & Blair, 2017). Despite the accumulation of stressors that are often associated with poverty, our results suggest that unique psychological and interpersonal processes may underlie positive parenting in this sample of families experiencing low income. Our results also support the toxic stress framework (Shonkoff, Boyce, & McEwen, 2009), in that positive caregiving behaviors were indeed especially important for protecting children in families experiencing low income from heightened or prolonged physiologic stress reactivity to an acute stressor.
It is important to note that the measures of observed parenting included in this study preceded the stress paradigm and might be considered best case parenting – with no other distractions, while provided with attractive and novel materials, and while being recorded. Our results suggest that when parents deploy their best parenting skills, children exhibit less cortisol reactivity to stressors. However, being able to consistently deploy one’s best parenting skills may be threatened by cumulative adversities, and it is likely the consistent use of positive parenting that protects against dysregulated stress physiology. In future work, examination of stability and consistency of positive parenting as related to adverse factors and stress physiology will be important to advance theoretical understanding and practical implications. Indeed, prior work has demonstrated the importance of consistency in positive parenting for child socio–emotional outcomes (Bernier, Carlson, & Whipple, 2010). In addition, some percentage of children experiencing poverty and other adversities exhibit low and flat cortisol (Badanes et al., 2011; Koss, Mliner, Donzella, & Gunnar, 2016). It will be important to disentangle types of dysregulated cortisol (elevated vs. flat and low) in future work. In the current analyses, we focused on elevations versus the decline that is usually observed (across the day, and often even across stress paradigms) because our intention was to evaluate whether experiencing positive parenting immediately before a challenge could buffer children from an elevated stress response. In contrast, we conceptualize low and flat cortisol as resulting from sustained environmental challenge versus researcher designed acute stress paradigms. The degree to which this type of pattern is related to specific environmental adversities and to positive parenting and responsive to intervention is an interesting question for future work.
This study is not without limitations. The sample was relatively small and age varied considerably. This large age range (6–45 months) could result in some stress tasks being more or less relevant to subsets of children. Indeed, because we intended to recruit a broad age range of young children, we included tasks that were well documented to work well at different ages; maternal neutral affect, “scary mask,” and frustration, for infants, toddlers, and preschoolers, respectively (Gunnar et al., 2009). We presented these tasks in quick succession to challenge the system in ways that would be appropriate across a range of ages. Owing to the timing dynamics of the HPA axis, differentiation of effects within a 7-minute stress paradigm window would be difficult, and therefore we instead evaluated the effect of the entire stress paradigm, thereby catching age-appropriate challenges for each child. Perhaps because of these design accommodations, the effect of positive parenting on child stress reactivity was clear. However, these timing dynamics would need to be considered carefully if the index of stress physiology was changed, especially to the faster acting autonomic nervous system.
Given family-focused current intervention strategies, we concluded that the most relevant and appropriate measure was the parenting behavior itself, with the child’s stress reactivity serving as the complement. As mentioned previously, the sample included a large age range which made the use of attachment measures inappropriate; however, future studies would benefit from including measures of attachment. One recent study that examined attachment security among toddlers found that attachment moderated the relation between poverty and HPA axis functioning (Johnson et al., 2018), thereby highlighting the importance of both attachment and parenting behaviors in shaping children’s early physiological development in the context of adversity. Moreover, further longitudinal research is needed to evaluate whether differences in attachment and parenting behaviors at two or more time points might result in differential stress reactivity to challenge, and of course pressingly whether supportive intervention that improves positive parenting can also reduce subsequent child stress reactivity.
Finally, the sample largely comprised Latina mothers and therefore the results may not be generalizable to other racial/ethnic groups. Because we report significant variance on all key measures, and because results replicate those found within samples not experiencing poverty, we believe the effect of positive parenting is likely robust to at least these population differences. However, it is likely the case that other group differences contribute to how positive parenting behaviors are expressed by the parent and received by the child. Given that Latinx families are a large and growing group in the United States, and that Latinx children are overrepresented in childhood poverty statistics (36%) (Jiang, Ekono, & Skinner, 2015), we feel that it is valuable to have documented buffered parenting in this population.
Despite these limitations, these findings highlight the important effect of positive parenting on children’s stress reactivity profiles in the context of poverty. A core assumption in many parenting interventions for families experiencing low income and other adversities is that if positive parenting behaviors are increased, children’s outcomes will be improved. According to attachment theory, this would work in part by increasing the child’s ability to draw on a strong internal working model of their parent’s support to execute their own positive engagement strategies. Indeed, evaluations of two prior interventions that target parenting have documented some normalization of dysregulated cortisol following improved parent–child interactions (Berlin, Martoccio, Bryce, & Harden, 2019; Fisher, Stoolmiller, Gunnar, & Burraston, 2007). However, to our knowledge, very few studies to date have examined whether differences in parenting behaviors among families experiencing poverty are related to differences in HPA axis engagement to a challenge. A key exception is work conducted by Gunnar and colleagues which reports parental buffering of cortisol reactivity to immunizations, and only among families experiencing low income (Johnson et al., 2018).
The toxic stress framework (Shonkoff et al., 2009), which was designed to engage researchers, community stakeholders, and policymakers, also serves to forward several hypotheses that have received inadequate attention in the research literature. In particular, a core component of this framework is the recategorization of a stressor from “toxic” to “tolerable” in the presence of a supportive adult. Furthermore, it posits that without such support, activation of the HPA axis as well as other mechanisms can lead to remodeling of brain architecture and set children on a path to poor long-term physical and mental health outcomes (Shonkoff, Garner, & Committee on Psychosocial Aspects of Child and Family Health, & Committee on Early Childhood, Adoption, and Dependent Care, 2011). Therefore, the evidence presented within this study that positive parenting does indeed moderate cortisol reactivity in young children facing poverty and poverty-related stressors contributes foundational knowledge for both theory and intervention.
Acknowledgments.
The authors are indebted to our Early Head Start partners for their insights and deeply grateful to the children and families who shared their time and experience with us, and to Ariel Julian, Marina Mendoza, Amy Dominguez, Natasha Link, Allison Stiles, and the research assistants in the Child Health and Development lab without whom these data could not have been collected.
Funding Statement.
Funding for this study was provided by the Office of Planning, Research, and Evaluation, Administration for Children and Families, U.S. Department of Health and Human Services (ACF-90YR0056) awarded to SEW, the National Science Foundation Graduate Research Fellowship (DGE-1104602) to LJS, and the National Institute of Mental Health T32 MH015442 to SMB.
Footnotes
Conflicts of Interest. None
References
- Albers EM, Riksen-Walraven JM, Sweep FC, & de Weerth C (2008). Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology and Psychiatry, 49, 97–103. doi: 10.1111/j.1469-7610.2007.01818.x [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, & Hankin BL (2011). Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology, 23, 881–896. doi: 10.1017/s095457941100037x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH, & Kroonenberg PM (2004). Differences in attachment security between African-American and white children: Ethnicity or socio-economic status? Infant Behavior and Development, 27, 417–433. doi: 10.1016/j.infbeh.2004.02.002 [DOI] [Google Scholar]
- Baumrind D (1967). Child care practices anteceding three patterns of preschool behavior. Genetic Psychology Monographs, 75, 43–88. [PubMed] [Google Scholar]
- Berlin LJ, Martoccio TL, Bryce CI, & Harden BJ (2019). Improving infants’ stress-induced cortisol regulation through attachment-based intervention: A randomized controlled trial. Psychoneuroendocrinology, 103, 225–232. doi: 10.1016/j.psyneuen.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Bernard K, Zwerling J, & Dozier M (2015). Effects of early adversity on young children’s diurnal cortisol rhythms and externalizing behavior. Developmental Psychobiology, 57, 935–947. doi: 10.1002/dev.21324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81, 326–339. doi: 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Berry D, Blair C, Willoughby M, Granger DA, Mills-Koonce WR & Family Life Project Key Investigators. (2017). Maternal sensitivity and adrenocortical functioning across infancy and toddlerhood: Physiological adaptation to context? Development and Psychopathology, 29, 303–317. doi: 10.1017/S0954579416000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L, & The Family Life Project Key Investigators. (2011). Allostasis and allostatic load in the context of poverty in early childhood. Developmental and Psychopathology, 23, 845–857. doi: 10.1017/S0954579411000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J (1969). Attachment and loss. New York, NY: Basic Books. [Google Scholar]
- Bretherton I (1992). The origins of attachment theory: John Bowlby and Mary Ainsworth. Developmental Psychology, 28, 759–775. [Google Scholar]
- Bronfenbrenner U, & Morris P (1998). The ecology of developmental process. In Damon W & Lerner R (Eds.), Handbook of child psychology: Vol 1: Theoretical models of human development (5th ed., pp. 992–1028). New York, NY: Wiley. [Google Scholar]
- Buske-Kirschbaum A, Krieger S, Wilkes C, Rauh W, Weiss S, & Hellhammer DH (2007). Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. The Journal of Clinical Endocrinology & Metabolism, 92, 3429–3425. doi: 10.1210/jc.2006-2223 [DOI] [PubMed] [Google Scholar]
- Coley RJ (2002). An uneven start: Indicators of inequality in school readiness. Princeton, NJ: Educational Testing Service. [Google Scholar]
- Cook JT, Frank DA, Berkowitz C, Black MM, Casey PH, Cutts DB, … Nord M (2004). Food insecurity is associated with adverse health outcomes among human infants and toddlers. Journal of Nutrition, 134, 1432–1438. doi: 10.1093/jn/134.6.1432 [DOI] [PubMed] [Google Scholar]
- DeWolff MS, & van IJzendoorn M (1997). Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development, 68, 571–591. [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, & Strasburger CJ (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology, 43, 683–692. doi: 10.1016/0960-0760(92)90294-S [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2007). Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychological Science, 18, 953–957. doi: 10.1111/j.1467-9280.2007.02008.x [DOI] [PubMed] [Google Scholar]
- Finegood ED, Raver CC, DeJoseph ML, & Blair C (2017). Parenting in poverty: Attention bias and anxiety interact to predict parents’ perceptions of daily parenting hassles. Journal of Family Psychology, 31, 51–60. doi: 10.1037/fam0000291 [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, & Burraston BO (2007). Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology, 32, 892–905. doi: 10.1016/j.psyneuen.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV, & England BG (1995). Childhood stress and family environment. Current Anthropology, 36, 854–866. [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, & Goldsmith HH (2011). Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment, 23, 337–353. doi: 10.1037/a0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennetian LA, & Shafir E (2015). The persistence of poverty in the context of financial instability: A behavioral perspective. Journal of Policy Analysis and Management, 34, 904–936. doi: 10.2002/pam.21854 [DOI] [Google Scholar]
- Goyal D, Gay C, & Lee KA (2010). How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first time mothers? Women’s Health Issues, 20, 96–104. doi: 10.1016/j.whi.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graif C, & Matthews SA (2017). The long arm of poverty. Extended and relational geographies of child victimization and neighborhood violence exposures. Justice Quarterly, 34, 1096–1125. doi: 10.1080/07418825.2016.1276951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, & Rigatuso J (1996). Stress reactivity and attachment security. Developmental Psychobiology, 29, 191–204. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Hostinar CE (2015). The social buffering of the hypothalamic-pituitary-adrenocortical axis in humans: Developmental and experiential determinants. Social Neuroscience, 10, 479–488. doi: 10.1080/17470919.2015.1070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, & Herrera A (2009). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34, 953–967. doi: 10.1016/j.psyneuen.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, & Vazquez D (2006). Stress neurobiology and developmental psychobiology. In Cicchetti D & Cohen D (Eds.), Developmental psychopathology (2nd ed., pp. 533–577). New York: NY: Wiley. [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ, & The Family Life Project Key Investigators. (2011). Maternal sensitivity buffers the adrenocortical implications of intimate partner violence exposure during early childhood. Development and Psychopathology, 23, 689–701. doi: 10.1017/S0954579411000010 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, & Gunnar MR (2015). Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science, 18, 281–297. doi: 10.1111/desc.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AL, Minnis H, & Wilson P (2011). Altered stress responses in children exposed to early adversity: A systematic review of salivary cortisol studies. Stress, 14, 614–626. doi: 10.3109/10253890.2011.577848 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ekono M, & Skinner C (2015). Basic facts about low-income children: Children under 3 years, 2013. New York, NY: NCCP. Retrieved: www.nccp.org/publications/pdf/text_1096.pdf [Google Scholar]
- Johnson AB, Mliner SB, Depasquale CE, Troy M, & Gunnar MR (2018). Attachment security buffers the HPA axis of toddlers growing up in poverty or near poverty: Assessment during pediatric well-child exams with inoculations. Psychoneuroendocrinology, 95, 120–127. doi: 10.1016/j.psyneuen.2018.05.030 [DOI] [PubMed] [Google Scholar]
- Koss KJ, Mliner SB, Donzella B, & Gunnar MR (2016). Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology, 66, 31–38. doi: 10.1016/j.psyneuen.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb ME, & Lewis C (2011). The role of parent-child relationships in child development. In Lamb E & Bornstein MH (Eds.), Social and personality development: An advanced textbook (pp. 259–307). New York, NY: Psychology Press. [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, … Leve LD (2014). Stress system development from age 4.5 to 6: Family environment predictors and adjustment implications of HPA activity stability versus change. Developmental Psychobiology, 56, 340–354. doi: 10.1002/dev.21103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KA, & Duncan GJ (2002). Parents in poverty. In Bornstein MH (Ed.), Handbook of parenting (2nd ed., pp. 95–121). Mahwah, NJ: Erlbaum. [Google Scholar]
- Martin CG, Bruce J, & Fisher PA (2012). Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Hormones and Behavior, 61, 661–668. doi: 10.1016/j.yhbeh.2012.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Hubbard JJ, Gest SD, Tellegen A, Garmezy N, & Ramirez M (1999). Competence in the context of adversity: Pathways to resilience and maladaptation from childhood to late adolescence. Development and Psychopathology, 11, 143–169. doi: 10.1017/S0954579499001996 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2005). Stressed or stressed out: What is the difference? Journal of Psychiatric Neuroscience, 30, 315–318. [PMC free article] [PubMed] [Google Scholar]
- Miles EM, Dmitrieva J, Hurwich-Reiss E, Badanes L, Mendoza MM, Perreira KM, … Watamura SE (2018). Evidence for a physiologic home–school gap in children of Latina immigrants. Early Childhood Research Quarterly, 52, 86–100. doi: 10.1016/j.ecresq.2018.03.010 [DOI] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2011). Mplus user’s guide (6th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- National Center for Children in Poverty. (2018). Child poverty. Retrieved from http://www.nccp.org/topics/childpoverty.html
- NICHD Early Child Care Research Network. (1999). Child care and motherchild interaction in the first three years of life. Developmental Psychology, 35, 1399–1413. [PubMed] [Google Scholar]
- Nikulina V, Widom CS, & Czaja S (2011). The role of childhood neglect and childhood poverty in predicting mental health, academic achievement, and crime in adulthood. American Journal of Community Psychology, 48, 309–321. doi: 10.1007/s10464-010-9385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal CR, Weston L, Brooks-Gunn J, Berlin LJ, & Atapattu R (2017). Maternal responsivity to infants in the “High Chair” assessment: Longitudinal relations with toddler outcomes in a diverse, low-income sample. Infant Behavior and Development, 47, 125–137. doi: 10.1016/j.infbeh.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arsenault L, Barr RG, … Tremblay RE (2008). Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Archives of General Psychiatry, 65, 211–218. doi: 10.1001/archgenpsychiatry.2007.27 [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Schieman S, Fazio EM, & Meersman SC (2005). Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior, 46, 205–219. doi: 10.1177/002214650504600206 [DOI] [PubMed] [Google Scholar]
- Rosmond R, & Bjorntorp P (2000). The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes, and stroke. Journal of Internal Medicine, 247, 188–197. doi: 10.1046/j.1365-2796.2000.00603.x [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, & McEwen BS (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA, 301, 2252–2259. doi: 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, & Committee on Psychosocial Aspects of Child and Family Health, & Committee on Early Childhood, Adoption, and Dependent Care. (2011). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, e232–e246. doi: 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Zaslow MJ, Weinfield NS, Gallagher M, Hair EC, Ogawa JR, Egeland B, … Temple MD (2006). Longitudinal prediction of child outcomes from differing measures of parenting in a low-income sample. Developmental Psychology, 42, 27–37. doi: 10.1037/0012-1649.42.1.27 [DOI] [PubMed] [Google Scholar]
- Zeegers MAJ, Colonnesi C, Stams GJM, & Meins E (2017). Mind matters: A meta-analysis on parental mentalization and sensitivity as predictors of infant-parent attachment. Psychological Bulletin, 143, 1245–1272. doi: 10.1037/bul000 [DOI] [PubMed] [Google Scholar]


