Abstract
Neutralizing antibodies can block infection, clear pathogens, and are essential to provide long-term immunity. Since the onset of the pandemic, SARS-CoV-2 neutralizing antibodies have been comprehensively investigated and critical information on their development, function, and potential use to prevent and treat COVID-19 have been revealed. With the emergence of SARS-CoV-2 immune escape variants, humoral immunity is being challenged, and a detailed understanding of neutralizing antibodies is essential to guide vaccine design strategies as well as antibody-mediated therapies. In this review, we summarize some of the key findings on SARS-CoV-2 neutralizing antibodies, with a focus on their clinical application.
Keywords: SARS-CoV-2, antibodies, neutralization, vaccination, B cells, immunization
Neutralizing antibodies play a critical role to protect from SARS-CoV-2 infection and can be effectively used to prevent and treat COVID-19. In this review, Klein et al. summarize key findings on the development and mechanisms of SARS-CoV-2-neutralizing antibodies and discuss advances in the clinical application of antibody-mediated antiviral strategies.
Introduction
After emerging in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly spread across the globe (Singh et al., 2021). While the virus encountered an immunologically naive human population, infections and expeditiously developed vaccines have shown to induce an adaptive immune response that can protect from SARS-CoV-2 infection and alleviate the severity of the SARS-CoV-2-associated coronavirus disease 2019 (COVID-19).
Antibodies play a critical role in the adaptive immune response and are one of the most important correlates of protection in infectious diseases (Earle et al., 2021; Plotkin, 2010). Secretion of monoclonal antibodies is accomplished by specialized immune cells (plasmablasts and plasma cells) that derive from activated B lymphocytes. To target a vast number of different pathogens, B cells undergo a sophisticated and strongly regulated process that includes the recombination of variable gene segments and introduction of somatic mutations (Imkeller and Wardemann, 2018; Victora and Nussenzweig, 2022). As a result, a highly diverse antibody repertoire is encoded in the B lymphocyte and plasma cell compartments, enabling lasting production of pathogen-specific antibodies.
A central feature of antibodies is their neutralizing function that blocks viral entry into target cells (Burton, 2002). Thereby, antibodies can prevent infection or limit viral burden through inhibition of viral propagation after infection. In addition, antibodies facilitate clearance of viruses and virus-infected cells via Fc-mediated effector functions, such as antibody-dependent cellular cytotoxicity exerted by natural killer (NK) cells (Bournazos and Ravetch, 2017; Jost and Altfeld, 2013). Taking advantage of these powerful antiviral functions, transfer of neutralizing antibodies has been the leading principle behind the use of convalescent plasma or plasma-derived immunoglobulins for prevention and treatment of infectious diseases. While the concept of polyclonal antibody application dates back to the end of the 19th century, advanced B cell cloning methods have resulted in the isolation of highly potent and specific monoclonal antibodies targeting infectious pathogens (Corti et al., 2021, Gieselmann et al., 2021, Klein et al., 2013, Wardemann et al., 2003). These include neutralizing antibodies that effectively prevent respiratory syncytial virus-associated disease or reduce mortality in Ebola-virus-infected individuals (Hammitt et al., 2022; Mulangu et al., 2019).
While lifelong protective immunity induced by vaccination or infection has been demonstrated for viruses such as measles (Amanna et al., 2007), immune-mediated protection from seasonal human coronaviruses (i.e., NL63, 229E, OC43, and HKU1) is known to be short-lived (Edridge et al., 2020). In addition to these differences in the dynamics of the immune and antibody response to different pathogens, viral evolution and the development of immune escape variants pose a significant challenge to antibody-mediated immunity. This became particularly apparent with the recent emergence of the SARS-CoV-2 Omicron variants that carry numerous spike mutations and evade a broad spectrum of neutralizing antibodies (Viana et al., 2022). This complex interaction of virus evolution and the virus-specific antibody response requires a detailed understanding of the development and function of SARS-CoV-2 neutralizing antibodies informing on successful future vaccine strategies as well as on the effective use of monoclonal antibodies in clinical applications.
In this review, we aim to summarize the remarkable body of knowledge on SARS-CoV-2 neutralizing antibodies, ranging from their development in infected and vaccinated individuals to their mechanisms and demonstrated efficacy in prevention and treatment of COVID-19.
Development of SARS-CoV-2 neutralizing antibodies
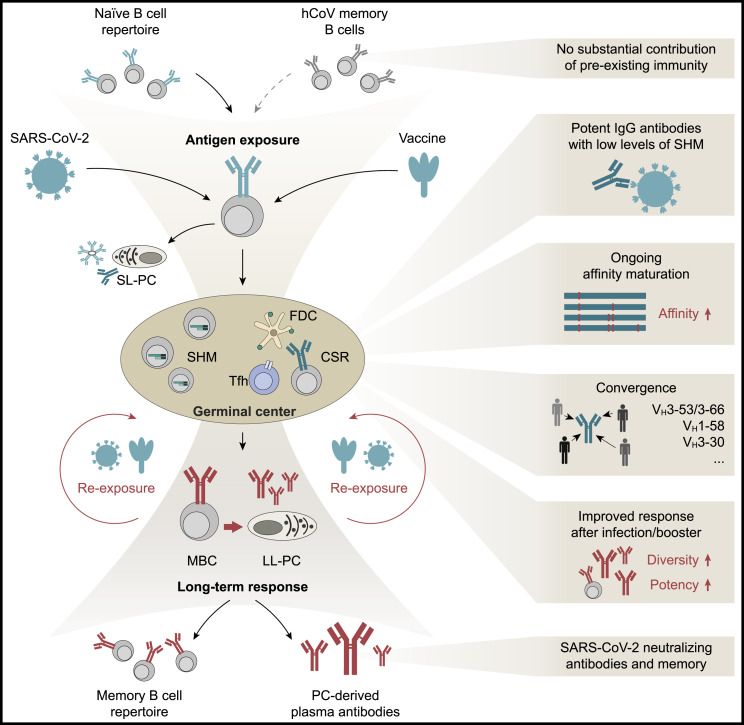
B cell activation and differentiation into an antibody-secreting cell are initiated by the B cell receptor interaction with its cognate antigen (Figure 1 ). Given the high prevalence of immunity to endemic betacoronaviruses (e.g., HCoV-OC43), it was speculated whether B cell reactivity to SARS-CoV-2 may preexist in naive individuals and shape immunological outcomes of infection. Although SARS-CoV-2 binding antibodies could be observed in rare cases (Anderson et al., 2021; Ng et al., 2020) and monoclonal antibodies with low neutralizing activity have been reported in seronegative individuals (Feldman et al., 2021), SARS-CoV-2 neutralizing activity was mostly undetectable in prepandemic samples (Ercanoglu et al., 2022; Poston et al., 2021). However, upon exposure to SARS-CoV-2 antigen, activation of preexisting betacoronavirus-reactive B cells could be detected but was not associated with protection from SARS-CoV-2 infection (Anderson et al., 2021; Sokal et al., 2021; Song et al., 2021; Turner et al., 2021b). Thus, rarely observed preexisting B cell cross-reactivity is unlikely to result in the development of a potent SARS-CoV-2 neutralizing response, although Fc-mediated functions of non-neutralizing antibodies might contribute to mitigate the course of the infection (Kaplonek et al., 2021).
Figure 1.
Development of SARS-CoV-2 neutralizing antibodies
Exposure to SARS-CoV-2 antigen through infection or vaccination activates naive B cells. It may also recruit pre-existing memory B cells with cross reactivity to endemic common human coronaviruses (hCoV; e.g., OC43). After activation, B cells can differentiate into short-lived antibody-secreting plasma cells (SL-PC) or are recruited into germinal centers, where they interact with antigen-presenting follicular dendritic cells (FDCs) and T follicular helper cells (Tfh) to undergo class-switch recombination (CSR) and gain antigen affinity by acquiring somatic hypermutation (SHM). However, a limited amount of SHM can be sufficient to result in highly potent SARS-CoV-2 neutralizing activity. B cells utilizing specific VH gene segments, such as VH3-53, that are repeatedly found to be overrepresented amongst SARS-CoV-2-reactive cells, can be members of public clonotypes. Affinity-matured germinal center B cells can remain in the germinal center to acquire higher levels of somatic mutations or differentiate into memory B cells or long-lived antibody-secreting plasma cells (LL-PC). Re-exposure to SARS-CoV-2 through additional vaccination or infection can induce differentiation of evolved SARS-CoV-2-reactive memory B cells into antibody-secreting plasma cells, can elicit further B cell evolution and acquisition of mutations, and/or can induce expansion of novel memory B cell clones. These processes can result in the development of memory B cell clones and serum neutralizing activity with higher potency and breadth against different viral variants.
Following antigen contact, B cells are typically recruited into germinal centers in the B cell follicles of secondary lymphoid organs (e.g., lymph nodes or spleen). Within the germinal center, activated B cells proliferate, mutate, and compete for antigen and T cell help. This multifaceted process results in the development of affinity-matured and class-switched memory B cells as well as long-lived plasma cells (Akkaya et al., 2020; Dorner and Radbruch, 2007; Victora and Nussenzweig, 2022). The human germinal center response to SARS-CoV-2 has been studied in some detail in vaccinated individuals through aspirates of draining lymph nodes, in which vaccine mRNA and spike antigen could be identified up to >8 weeks post-immunization (Röltgen et al., 2022). SARS-CoV-2-reactive germinal center B and T follicular helper cells became detectable within 2–3 weeks of immunization and were associated with the development of neutralizing antibodies (Lederer et al., 2022; Mudd et al., 2022; Schmitz et al., 2021; Turner et al., 2021b). Indicating the persistence of this response, SARS-CoV-2-reactive germinal center cells and bone marrow plasma cells could be detected >6 months after the first immunization (Kim et al., 2022; Mudd et al., 2022). In contrast to longitudinal observations after vaccination, lymphoid follicle analyses after infection have largely been limited to post-mortem studies. In such fatal cases of COVID-19, disruptions and loss of germinal centers were repeatedly demonstrated (Kaneko et al., 2020; Röltgen et al., 2022). Moreover, severe COVID-19 was associated with extrafollicular germinal center-independent B cell responses that can result in short-lived plasma cells with low or no affinity maturation (Woodruff et al., 2020). However, higher levels of somatic mutation and the development of long-lived SARS-CoV-2-reactive plasma cells indicate an intact germinal center response in cases of mild infection (Chen et al., 2020; Turner et al., 2021a).
Given the relative inaccessibility of human lymph node and bone marrow samples, the majority of B cell maturation analyses has been performed on peripheral blood memory B cells. These studies have provided critical insights into the neutralizing antibody response to SARS-CoV-2: within weeks of infection, potent neutralizing antibodies with high affinity to SARS-CoV-2 can be isolated from memory B cells of convalescent individuals across the full spectrum of disease severity. Although the SARS-CoV-2 antibody response is highly polyclonal and derives from a broad spectrum of different variable immunoglobulin (Ig) gene segments, convergent antibody responses have been repeatedly observed in different individuals (Barnes et al., 2020b; Chen et al., 2021a; Kreer et al., 2020; Kreye et al., 2020; Robbiani et al., 2020; Tan et al., 2021; Tong et al., 2021; Yuan et al., 2020a). The development of these public clonotypes (e.g., use of VH1-58, VH3-30, VH3-53/3-66 genes) is often a consequence of germline-encoded features favoring SARS-CoV-2 reactivity, including binding of germline residues to the spike protein (Chen et al., 2021a). Notably, many potent antibodies display low levels of somatic mutations in their variable gene segments (e.g., >95%–100% identity to the germline VH gene), suggesting that potent antibodies can be readily induced by vaccination (Brouwer et al., 2020; Elsner and Shlomchik, 2020; Ju et al., 2020; Kreer et al., 2020; Robbiani et al., 2020; Rogers et al., 2020; Seydoux et al., 2020; Zost et al., 2020b). In the months following infection, trapped viral antigens may be retained in germinal centers and/or can remain expressed in tissues (Gaebler et al., 2021; Heesters et al., 2014). After mild infection, the level of somatic mutation in SARS-CoV-2-reactive memory B cells increases over time, resulting in affinity-matured cells encoding for antibodies with higher potency and breadth (i.e., reactivity to more viral variants) (Gaebler et al., 2021; Goel et al., 2021; Moriyama et al., 2021; Muecksch et al., 2021; Sokal et al., 2021; Wang et al., 2021b). Similarly, following mRNA vaccination, lymph node germinal center B cells and memory B cells can evolve and acquire increasing levels of somatic mutation, although the development of antibody potency and breadth after vaccination appears less pronounced than after natural infection (Cho et al., 2021; Kim et al., 2022; Wang et al., 2021c).
Administration of an mRNA-based booster vaccination several months after infection or immunization results in profound increases of serum neutralizing potency and breadth (as discussed below). These effects are likely to reflect a combination of strong increases of overall antibody titers as well as immunization-induced differentiation of matured memory B cells into plasma cells that release antibodies with enhanced activity. Within the memory B cell compartment itself, booster immunization can result in the expansion of preexisting B cell clones that acquire additional mutations (Goel et al., 2021; Muecksch et al., 2022; Wang et al., 2021b). Moreover, booster vaccination can result in the expansion of novel B cell clones that may contribute to an increase in neutralizing breadth and potency (Muecksch et al., 2022; Wang et al., 2021b).
Targets and mechanisms of neutralization
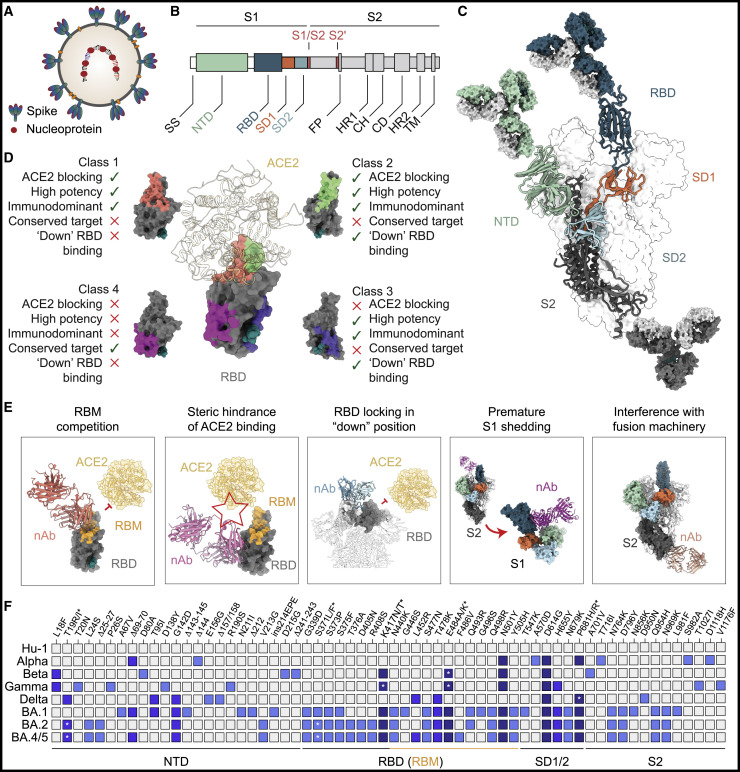
SARS-CoV-2 neutralizing antibodies target the viral spike glycoprotein, a trimeric structure of S1/S2 heterodimers that is expressed at a density of ∼24–30 trimers per virion surface (Figure 2 A) (Ke et al., 2020). Out of the two subunits (S1 and S2) composing each spike monomer, host cell receptor interactions are mediated by the S1 domain that comprises the N-terminal domain (NTD) and the receptor-binding domain (RBD) (Figures 2B and 2C). While the functional role of the NTD has not been fully elucidated and may involve interactions with C-type lectins (Soh et al., 2020), the RBD binds to the primary receptor angiotensin-converting enzyme 2 (ACE2) (Hoffmann et al., 2020b; Lan et al., 2020; Shang et al., 2020; Walls et al., 2020; Wrapp et al., 2020; Yan et al., 2020). Following S1/S2 cleavage by host cell proteases, the spike protein undergoes considerable conformational changes from its pre- to postfusion state during S2-mediated cellular entry and fusion (Benton et al., 2020; Cai et al., 2020; Fan et al., 2020; Hoffmann et al., 2020a; Tai et al., 2021).
Figure 2.
Targets and mechanisms of SARS-CoV-2 neutralization
(A) Schematic of a SARS-CoV-2 virion indicating the spike and nucleocapsid proteins, the two major targets of SARS-CoV-2-reactive antibodies.
(B) Schematic of the S1/S2 heterodimer domains encoded by the SARS-CoV-2 spike gene. Host protease cleavage sites are indicated in red.
(C) SARS-CoV-2 trimer structure with heterodimer domains colored as in (B) and main targets of neutralizing activity (RBD, NTD, and S2) indicated by bound antibodies.
(D) Exemplary classification system of RBD neutralizing antibodies as defined in (Barnes et al., 2020a). Key features of different RBD antibody classes are indicated with green ticks (present) or red crosses (absent).
(E) Examples for mechanisms of SARS-CoV-2 neutralization.
(F) Map of spike gene changes in SARS-CoV-2 variants of concern relative to the ancestral Hu-1 strain (changes found in >33% of variant sequences deposited at GISAID (Elbe and Buckland-Merrett, 2017; Khare et al., 2021; Shu and McCauley, 2017) and as aggregated at outbreak.info (Mullen et al., 2020). Changes are colored based on their frequency amongst variants of concern (light blue, 1; blue, 2; dark blue, 3 or more variants with identical change; with Omicron sublineages [BA.1, BA.2, BA.4/BA.5] counted as single variant). Asterisks refer to amino acid polymorphism indicated by corresponding asterisks above. SS, signaling sequence; NTD, N-terminal domain; RBD, receptor-binding domain; SD1/SD2, subdomain 1/2; FP, fusion peptide; HR, heptad repeat; CH, central helix; CD, connector domain; TM, transmembrane domain; RBM, receptor-binding motif; nAb, neutralizing antibody.
SARS-CoV-2 spike-targeting IgG reactivity in convalescent and vaccinated individuals is largely directed to epitopes outside of the RBD and many S-reactive antibodies do not neutralize the virus (Amanat et al., 2021; Barnes et al., 2020b; Kreer et al., 2020; Voss et al., 2021). In contrast, consistent with the receptor binding function of the RBD, the majority of the neutralizing serum activity is RBD-directed and a large fraction of RBD-reactive monoclonal antibodies do neutralize with high potency (Dejnirattisai et al., 2021a; Piccoli et al., 2020; Premkumar et al., 2020). Within the context of the spike trimer, the RBD possesses flexibility and can adopt different conformations (“down” and “up,” the latter required for ACE2 interaction) that influence the accessibility of antibody epitopes (Barnes et al., 2020a, 2020b; Ke et al., 2020; Yao et al., 2020; Yuan et al., 2021). Based on structural analyses, functional characteristics, and/or antigenic mapping, different classification systems for RBD-reactive neutralizing antibodies have been proposed (see example in Figure 2D) (Barnes et al., 2020a; Brouwer et al., 2020; Dejnirattisai et al., 2021a; Hastie et al., 2021; Piccoli et al., 2020; Pinto et al., 2020; Yuan et al., 2021).
RBD-reactive antibodies with the highest in vitro neutralizing potency compete with ACE2 binding by targeting the immunodominant receptor-binding motif (Figure 2E) (Barnes et al., 2020a, 2020b; Dejnirattisai et al., 2021a; Ju et al., 2020; Liu et al., 2020; Piccoli et al., 2020; Wu et al., 2020b; Yuan et al., 2020a). These include minimally mutated antibodies derived from the VH3-53 or VH3-66 gene segments that constitute a public clonotype found in many individuals (Brouwer et al., 2020; Kim et al., 2021c; Robbiani et al., 2020; Rogers et al., 2020). RBD interactions through germline-encoded residues in the heavy chain complementarity-determining regions 1 and 2 (CDRH1 and CDRH2) provide a structural rationale for the limited degree of mutation in this public clonotype (Yuan et al., 2020a), although VH3-53 antibodies with a differing binding mode have been described (Barnes et al., 2020a; Scheid et al., 2021; Wu et al., 2020a). Beyond competing with ACE2 binding, targeting of the receptor-binding motif may mimic the receptor interaction and trigger a premature change of the spike protein conformation to its postfusion state (Figure 2E) (Lempp et al., 2021). Most members of the VH3-53/3-66 public clonotype can only access their epitope in the RBD-up position (class 1, Figure 2D), while other receptor-binding motif-targeting antibodies can bind both the RBD-up and down positions (class 2, Figure 2D). By interacting with adjacent RBDs, members of this antibody class can lock the trimer into an “all-RBD-down” closed conformation, thereby preventing RBD-up-dependent ACE2 binding (Figure 2E) (Barnes et al., 2020a; Lempp et al., 2021; Liu et al., 2020; Scheid et al., 2021; Tortorici et al., 2020). Other RBD-binding antibodies target epitopes distal to the receptor-binding motif, to which they show no or minimal overlap (class 3, Figure 2D) (Barnes et al., 2020a; Hansen et al., 2020; Pinto et al., 2020). While some antibodies belonging to this group (e.g., sotrovimab) do not directly compete with the binding to ACE2, they can interfere with receptor binding through steric hindrance (Figures 2D and 2E) (Barnes et al., 2020a; Hansen et al., 2020; Lempp et al., 2021; Pinto et al., 2020). Finally, non-ACE2-competing RBD antibodies targeting a conserved epitope distal of the receptor-binding motif have been identified (class 4, Figure 2D). While their in vitro activity appears to be limited, antibodies of this class have been demonstrated to act by disrupting the spike protein (e.g., resulting in premature S1 shedding; Figure 2E) (Huo et al., 2020; Wrobel et al., 2020; Yuan et al., 2020b) and can display broad cross-reactivity against related zoonotic coronaviruses (Jette et al., 2021; Martinez et al., 2022).
SARS-CoV-2 neutralizing activity is not exclusively directed at the RBD and potent antibodies targeting the NTD have been identified (Cerutti et al., 2021b; Chi et al., 2020; Liu et al., 2020; Suryadevara et al., 2021; Voss et al., 2021; Wang et al., 2022). Compared to other regions on the spike protein, the NTD has a relatively high glycan density that limits accessibility. However, antibodies reactive to a structurally defined site of vulnerability (antigenic supersite) have been identified in multiple donors (Cerutti et al., 2021b; McCallum et al., 2021; Suryadevara et al., 2021), in addition to neutralizing NTD antibodies showing no or minimal contact to this site (Cerutti et al., 2021a; Li et al., 2021a; Wang et al., 2022). Although the precise mechanism of NTD-targeted neutralizing activity has not yet been elucidated, it has been postulated to include interference with conformational changes required for membrane fusion after ACE2 attachment (Chi et al., 2020; Suryadevara et al., 2021). Finally, neutralizing antibodies binding the conserved S2 domain have been identified and can reduce viral burden in vivo (Jennewein et al., 2021; Pinto et al., 2021; Zhou et al., 2022). While the moderate neutralizing potency may limit their potential for clinical application, the high degree of cross-reactivity makes S2-targeting antibodies particularly informative for pan-betacoronavirus vaccine design (Pinto et al., 2021; Zhou et al., 2022).
Despite its essential function for host cell receptor interaction and membrane fusion, the SARS-CoV-2 spike protein has a remarkable degree of sequence variability (Harvey et al., 2021; Nabel et al., 2022). Although many of the potential changes in the spike protein can adversely affect viral functions (Li et al., 2020), mutations that increase infectivity and/or transmissibility confer a growth advantage, as has been observed for mutations that favor receptor-binding (e.g., D614G; Hou et al., 2020; Korber et al., 2020; Yurkovetskiy et al., 2020), or enhance spike protein cleavage (e.g., H655Y; Escalera et al., 2022). Similarly, mutations resulting in antibody resistance can be advantageous to evade antibody-mediated immune pressure (Andreano et al., 2021b; Baum et al., 2020b; Schmidt et al., 2021; Wang et al., 2021c; Weisblum et al., 2020). Although numerous factors determine viral growth and persistence on a population level, the repeated and independent emergence of SARS-CoV-2 variants with immune evasive properties highlights the interplay of immune response and viral evolution (Dejnirattisai et al., 2021b; Mlcochova et al., 2021; Tegally et al., 2021; Uriu et al., 2021; Viana et al., 2022; Zhou et al., 2021a).
Notably, several mutations in viral variants of concerns have been identified as key mediators of antibody resistance through in vitro outgrowth experiments and/or deep mutational scanning (Figure 2F). For example, potent class 2 antibodies can form hydrogen bonds with spike residue E484, and mutations at this position confer a strong reduction in polyclonal plasma binding (Barnes et al., 2020a; Greaney et al., 2021a, 2021c; Liu et al., 2021b). Similarly, mutations in residue K417 reduce binding of class 1 neutralizing antibodies and were selected in the presence of vaccine-induced monoclonal antibodies (Greaney et al., 2021c; Wang et al., 2021c). Thus, mutations at residues K417 and E484 affect potently neutralizing antibodies targeting the receptor-binding motif. Consistently, variants harboring mutations at both residues have repeatedly emerged (Beta, Gamma, and Omicron) and show reduced sensitivity to antibody-mediated neutralization compared to the ancestral strains (Carreno et al., 2022; Dejnirattisai et al., 2022). Beyond RBD mutations, viral variants contain changes in the NTD that often include insertions or deletions in or near the antigenic supersite (McCallum et al., 2021; McCarthy et al., 2021). Although individual spike mutations may have a small effect on polyclonal serum activity (e.g., L452R), they can confer resistance to therapeutic monoclonal antibodies (Li et al., 2020; Starr et al., 2021c), and the accumulation of mutations in viral variants can result in an additive effect on polyclonal resistance. Most notably, the Omicron variants and sublineages carry mutations in the NTD, as well as the epitopes of many class 1, class 2, and class 3 RBD antibodies and have a high level of resistance against both many monoclonal antibodies and the polyclonal sera elicited by infection or vaccination (Carreno et al., 2022; Dejnirattisai et al., 2022; Garcia-Beltran et al., 2022; Gruell et al., 2022; Liu et al., 2022; Planas et al., 2022; Schmidt et al., 2022).
Serum neutralization in response to SARS-CoV-2 infection and vaccination
Establishing a correlate of protection from SARS-CoV-2 infection and/or severe courses of disease is critical to guide vaccination strategies and may facilitate regulatory approvals based on surrogate parameters. Neutralizing activity can be determined using various assays that differ in the type of virus used (e.g., authentic virus, recombinant replication-competent virus, or replication-deficient pseudovirus), the type of target cell, expression levels of ACE2 and proteases, and/or the type of readout (e.g., bioluminescence, plaque reduction, or cytopathic effects) (Khoury et al., 2020). Polyclonal neutralization titers obtained with different assays generally correlate well (Sholukh et al., 2021), but results for individual antibodies can differ between assays (Lempp et al., 2021; Liu et al., 2021a). Thus, while reference samples have been produced to facilitate calibrated and standardized reports (Kristiansen et al., 2021), assay variation as well as the use of viral variants need to be considered when interpreting results of neutralization assays.
Analyses in non-human primates challenged with SARS-CoV-2 after vaccination or adoptive transfer of convalescent IgG established that titers of spike-reactive antibodies as well as serum neutralization as critical immune correlates of protection (Corbett et al., 2021b; McMahan et al., 2021; Mercado et al., 2020; Yu et al., 2020). These observations could subsequently be extended to the protection from SARS-CoV-2 infection observed in the clinical trials of various vaccine platforms (Feng et al., 2021; Gilbert et al., 2022; Khoury et al., 2021). Although case definitions as well as neutralization assays and sampling time points differed between individual trials, the vaccine- and trial-specific levels of reported protection strongly correlated with induced neutralizing activity (Khoury et al., 2021). Vaccine-induced neutralizing titers that were ∼20% of those found in convalescent individuals were associated with ∼50% protection from symptomatic infection, while ∼90% and ∼95% of protective efficacy was modeled for neutralizing titers of around 2- and 4-fold of those seen in convalescent individuals, respectively (Khoury et al., 2021). Notably, for different vaccine platforms (mRNA, mRNA-1273; adenovirus, ChAdOx1-S) with similar case definitions (virologically confirmed symptomatic COVID-19 at >4 weeks after the second vaccine dose), neutralizing antibody serum activity resulting in 90% vaccine efficacy was overall similar (83 and 140 IU50/mL in a pseudovirus neutralization assay, respectively) (Feng et al., 2021; Gilbert et al., 2022). While the small number of severe COVID-19 cases prevented drawing firm conclusions, an analysis of different vaccine trials determined protection against severe infection to be achieved at 6-fold lower neutralizing titers compared to any symptomatic infection (Khoury et al., 2021). These results are consistent with animal studies demonstrating higher protection in lower compared to upper airways at low antibody titers (Corbett et al., 2021b) and with higher clinical vaccine effectiveness against severe disease (Andrews et al., 2022b). Interestingly, no correlation between serum neutralizing titers and protection from asymptomatic infection could be determined after ChAdOx1-S vaccination (Feng et al., 2021), and the immune correlate analysis 4 weeks after the second mRNA-1273 dose suggested that serum neutralizing activity alone cannot fully explain the observed vaccine efficacy (Gilbert et al., 2022). Thus, although serum neutralizing activity provides a well-established and highly relevant correlate of protection for symptomatic and severe disease, it may not be entirely representative for vaccine-induced protection.
Seroconversion with development of SARS-CoV-2-reactive IgM, IgA, and IgG antibodies after infection typically occurs within 7–14 days of symptom onset and in almost all individuals (Gudbjartsson et al., 2020; Long et al., 2020; Ripperger et al., 2020; Suthar et al., 2020). Similarly, high seroconversion rates are observed within 14 days of the first COVID-19 vaccination for different vaccine platforms (Folegatti et al., 2020; Jackson et al., 2020; Walsh et al., 2020; Zhang et al., 2021b). Serum neutralizing activity correlates with the titers of binding antibodies reactive to the SARS-CoV-2 spike protein and/or its receptor-binding domain that can be rapidly determined in certified high-throughput assays (Röltgen et al., 2020; Rydyznski Moderbacher et al., 2020; Vanshylla et al., 2021). Accordingly, serum neutralizing activity after infection can be detected within a few days of symptom onset and peaks approximately 4 weeks after infection in most individuals (Seow et al., 2020; Suthar et al., 2020). However, its extent after natural infection is marked by a wide variation that correlates with clinical characteristics. In most individuals, infection elicits a neutralizing response with 50% serum inhibitory dilution (ID50) titers up to ∼1,000 in pseudovirus assays when tested ∼6 weeks after disease onset (with some assay-dependent variation) (Luchsinger et al., 2020; Robbiani et al., 2020; Vanshylla et al., 2021). While delayed onset of neutralizing activity has been associated with fatal outcomes, higher neutralizing titers are observed in more severely infected individuals and likely reflect higher antigen exposure and/or strong activation of short-lived plasmablasts (Garcia-Beltran et al., 2021; Lucas et al., 2021; Rydyznski Moderbacher et al., 2020; Zohar et al., 2020). A small fraction of individuals (1%–5%, so called “elite neutralizer”) mounts a neutralizing serum response of outstanding potency with high betacoronavirus cross-reactivity that can develop independently of disease severity and may be of particular interest for the isolation of therapeutic antibodies (Luchsinger et al., 2020; Vanshylla et al., 2021, 2022a). Moreover, a notable fraction of up to ∼20% of individuals with asymptomatic infection or mild disease does not display detectable neutralization (Garcia-Beltran et al., 2021; Long et al., 2020; Luchsinger et al., 2020; Robbiani et al., 2020; Röltgen et al., 2020; Rydyznski Moderbacher et al., 2020; Seow et al., 2020; Vanshylla et al., 2021).
Compared to the variation seen after natural infection, immunization with mRNA-based vaccines results in more consistent development of high serum titers of neutralizing antibodies (Jackson et al., 2020; Walsh et al., 2020). Deep mutational scanning demonstrated that, compared to antibodies induced by infection, mRNA vaccine-induced serum activity shows a stronger RBD-directed focus with higher epitope breadth (Greaney et al., 2021b). However, reduced immunogenicity is observed in elderly and immunocompromised individuals (Apostolidis et al., 2021; Tober-Lau et al., 2021), and vector-based vaccines generally result in lower titers of neutralizing activity (Folegatti et al., 2020; Stephenson et al., 2021; Zhang et al., 2021b). Strong increases in serum neutralization after an initial vector-based vaccination can be achieved through subsequent heterologous vaccination with a more immunogenic mRNA- or protein-based vaccine (Barros-Martins et al., 2021; Borobia et al., 2021; Hillus et al., 2021; Stuart et al., 2022). Moreover, consistent with ongoing affinity maturation and (re)stimulation of more evolved B cells, prolonged vaccination intervals (Hall et al., 2022b; Tauzin et al., 2022), vaccination of convalescent individuals (Keeton et al., 2021; Reynolds et al., 2021; Stamatatos et al., 2021), and breakthrough infections resulted in improved serum neutralization (Bates et al., 2022; Tober-Lau et al., 2022; Walls et al., 2022).
Serum neutralizing activity after infection or vaccination shows a biphasic decline. Following an initial drop that is also driven by the reduction in IgM and IgA antibodies with shorter half-life (Chia et al., 2021; Vanshylla et al., 2021), titers decay more slowly and serum neutralization remains detectable for >1 year in most individuals (albeit at relatively low levels) (Chia et al., 2021; Dan et al., 2021; Doria-Rose et al., 2021; Iyer et al., 2020; Levin et al., 2021; Vanshylla et al., 2021; Yang et al., 2022). However, waning serum neutralizing activity and the emergence of immune escape variants with reduced antibody sensitivity and higher infectivity were associated with increasing rates of breakthrough infections and prompted the initiation of booster campaigns (Abu-Raddad et al., 2022b; Andrews et al., 2022a; Goldberg et al., 2021; Hall et al., 2022a; Lopez Bernal et al., 2021; Madhi et al., 2021; Tseng et al., 2022). Similar to booster immunizations in convalescent individuals, a booster dose based on the original Hu-1 strain in vaccinated individuals elicited increased titers of neutralizing serum activity and breadth against viral variants, including the particularly immune evasive Omicron variants (Carreno et al., 2022; Garcia-Beltran et al., 2022; Gruell et al., 2022; Iketani et al., 2022; Munro et al., 2021; Perez-Then et al., 2022; Planas et al., 2022; Schmidt et al., 2022; Vanshylla et al., 2022b; Wratil et al., 2022). Nevertheless, the evolution of SARS-CoV-2 has prompted the development of variant-specific vaccines. Although variant-adapted as well as Hu-1-based mRNA boosters elicited comparable variant neutralizing activity in preclinical studies and immune imprinting may shape the response to booster antigens (Corbett et al., 2021a, Gagne et al., 2022, Röltgen et al., 2022), further clinical analysis of variant-based vaccines will be required (Choi et al., 2021, Pajon et al., 2022).
While booster doses provide strong protection against Omicron-mediated severe disease, protection from Omicron infection is relatively weak and short-lived despite the elicited serum neutralizing activity (Abu-Raddad et al., 2022a; Andrews et al., 2022a; Kuhlmann et al., 2022). Respiratory tract mucosal neutralizing activity, which may be more relevant for protection from infection, has been studied relatively little. Upon infection or vaccination, SARS-CoV-2-specific IgG and IgA are detectable in nasopharynx, saliva, and broncho-alveolar lavage (Azzi et al., 2022; Ketas et al., 2021; Smith et al., 2021; Sterlin et al., 2021) and have been shown to persist for several months in the nasal mucosal lining (Froberg et al., 2021). However, the limited agreement in blood vs. mucosal SARS-CoV-2 antibody levels calls for some caution when regarding blood neutralizing antibody titers as a definitive correlate of protection (Smith et al., 2021).
Development of therapeutic antibodies
As development of vaccines and antiviral compounds was being initiated, harnessing the potential of antibodies in clinical practice was an immediate goal in the COVID-19 pandemic. To this end, convalescent plasma provided a readily available source of polyclonal antibodies targeting SARS-CoV-2 and its therapeutic use was implemented in the absence of alternative options (Joyner et al., 2020). Although most randomized trials did not demonstrate clinical benefit of convalescent plasma administration (Begin et al., 2021; RECOVERY Collaborative Group, 2021; Korley et al., 2021; Simonovich et al., 2021), key principles for the effective application of antibodies could be identified. Most notably, plasma with higher titers of SARS-CoV-2-reactive antibodies and early administration after symptom onset were associated with reduced disease progression (Joyner et al., 2021; Libster et al., 2021).
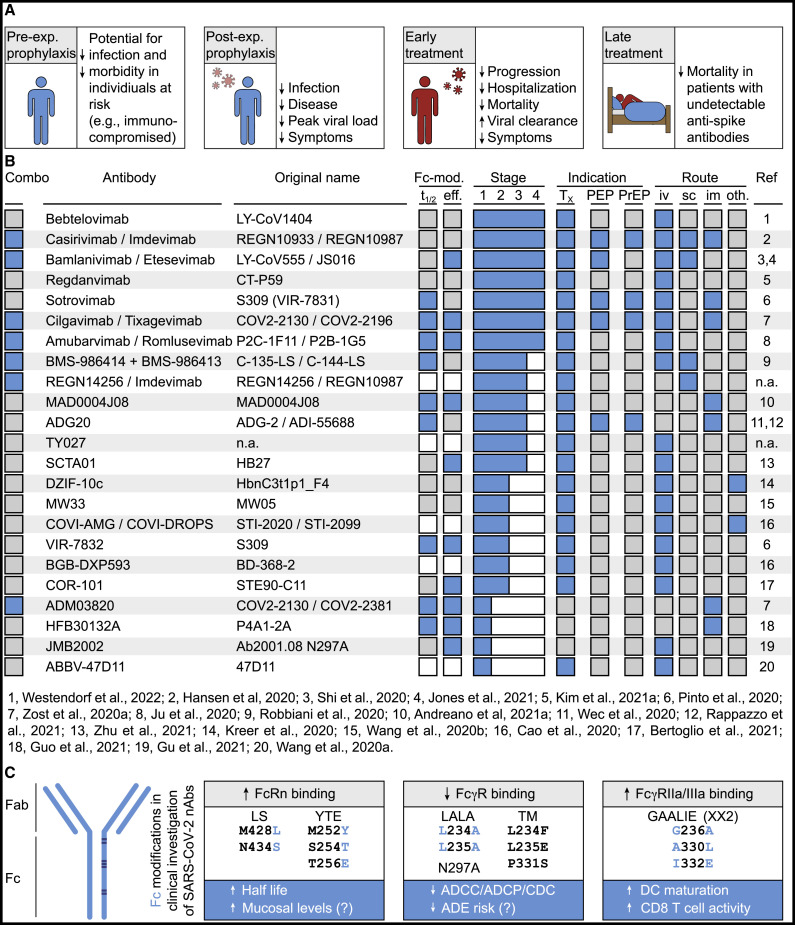
Rapid identification of SARS-CoV-2 neutralizing monoclonal antibodies accomplished by academia and industry was facilitated by strategies previously implemented and refined for other viruses (e.g., ebolavirus, HIV-1). Many antibodies advancing into clinical development were isolated by antibody cloning methods relying on the identification of spike-reactive B cells from COVID-19-convalescent individuals (Figure 3 ) (Andreano et al., 2021a; Cao et al., 2020; Du et al., 2020; Gieselmann et al., 2021; Guo et al., 2021; Hansen et al., 2020; Jones et al., 2021; Ju et al., 2020; Kim et al., 2021a; Kreer et al., 2020; Robbiani et al., 2020; Shi et al., 2020; Starr et al., 2021a; Wang et al., 2020b; Westendorf et al., 2022; Zost et al., 2020a, 2020b). Additional antibodies were obtained using phage display technology (Bertoglio et al., 2021; Zhu et al., 2021) or from immunized mice carrying human immunoglobulin genes (Hansen et al., 2020; Wang et al., 2020a). Finally, readily available samples from individuals previously infected with the related SARS-CoV(-1) enabled the identification of potent cross-neutralizing antibodies (Pinto et al., 2020; Rappazzo et al., 2021; Wec et al., 2020). Consistent with and based on their high neutralizing in vitro potency, all antibodies selected for advanced clinical development target the RBD of SARS-CoV-2. Experiments in animal models conclusively demonstrated their in vivo potential, as pre- or post-exposure administration of neutralizing antibodies prevented or dampened infection, manifesting in reduced viral titers and/or pulmonary inflammation (Baum et al., 2020a; Bertoglio et al., 2021; Cao et al., 2020; Chen et al., 2021d; Cobb et al., 2022; Du et al., 2020; Haagmans et al., 2021; Halwe et al., 2021; Jones et al., 2021; Kim et al., 2021a; Loo et al., 2022; Martinez et al., 2021; Schäfer et al., 2021; Shi et al., 2020; Verma et al., 2021; Wang et al., 2020b; Zost et al., 2020a).
Figure 3.
Antibodies in clinical investigation
(A) Summary of key findings of clinical trials of SARS-CoV-2 neutralizing monoclonal antibodies in different study populations.
(B) Overview of antibodies in clinical investigation. Blue and gray squares indicate presence and absence of feature, respectively. White squares indicate that information is not available. “Combo” indicates use as an antibody combination. Fc-mod indicates genetic modifications of the Fc domains (t1/2, mutations to extend half-life; eff, mutations to enhance or reduce Fc-mediated effector function). “Stage” indicates the clinical stage of investigation, with 4 being assigned to antibodies currently or previously authorized or approved for clinical use. “Indication” shows studied clinical scenarios (Tx, treatment; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis). “Route” indicates studied routes of administration (iv, intravenous; sc, subcutaneous; im, intramuscular; oth, other routes referring to inhalation for DZIF-10c and nasal drips for COVI-DROPS). “Ref” indicates isolation paper reference as numbered in listing below.
(C) Fc domain mutations currently or previously studied in clinical investigation of SARS-CoV-2 neutralizing antibodies. Gray boxes indicate effects on Fc receptor binding, blue boxes show demonstrated or putative clinical effects. Mutations highlighted in blue are eponymous for the combination of mutations.
Different approaches were taken to tackle the well-recognized viral capacity to escape from antibody-mediated antiviral activity. More conserved epitopes might be preferential targets as a limited degree of variation suggests restricted mutational capacity. For example, the epitope of the Sarbecovirus-cross-reactive antibody sotrovimab (S309) encompasses highly conserved residues and deep mutational scanning identified only a small number of single residues conferring potential escape (Cathcart et al., 2022; Pinto et al., 2020; Starr et al., 2021a). In addition, structural analyses, epitope and mutational mapping, as well as in vitro outgrowth experiments informed on the use of preferential antibody combinations. By targeting non-overlapping epitopes, antibody combinations can increase the mutational threshold required to result in resistance against all of the administered antibodies (Baum et al., 2020b; Copin et al., 2021; Dong et al., 2021; Liu et al., 2021b; Starr et al., 2021b, 2021c; Weisblum et al., 2020). Moreover, although its mechanistic principle in the context of SARS-CoV-2 has not been studied in detail and the phenomenon is infrequently described, antibody combinations can exert synergistic effects that result in higher in vitro neutralizing activity than would be expected for either antibody alone (Pinto et al., 2020; Zost et al., 2020a).
An additional focus of development has been placed on the fragment crystallizable (Fc) region in the constant antibody domains (Figure 3C). Through its interaction with the neonatal Fc receptor (FcRn) that results in reduced lysosomal antibody degradation, the Fc region plays a critical role in antibody half-life. By introducing Fc mutations that do not interfere with antigen recognition but increase FcRn affinity (e.g., LS, M428L/N434S; YTE, M252Y/S254T/T256E), in vivo half-life extensions by about ∼3-fold can be achieved (Dall’Acqua et al., 2006; Loo et al., 2022; Zalevsky et al., 2010). In addition, as FcRn regulates mucosal antibody levels, enhanced FcRn binding might be associated with increased accumulation of antibodies on mucosal surfaces (Ko et al., 2014). Moreover, the Fc regions link antibody recognition with host effector functions. Experiments in animal models demonstrated the relevance of Fc interactions for SARS-CoV-2 antibody efficacy in a therapeutic setting. Compared to treatment with unmodified antibodies, administration of variants with diminished reactivity to activating Fc receptors (e.g., antibodies with LALA or GRLR mutations) was associated with higher viral burden, increased weight loss, higher levels of pulmonary inflammation, and/or reduced survival (Chan et al., 2021; Ullah et al., 2021; Winkler et al., 2021; Yamin et al., 2021). Fc-mediated functions of potent monoclonal antibodies are likely less critical when antibodies are present prior to viral exposure (Lempp et al., 2021; Schäfer et al., 2021; Su et al., 2021; Winkler et al., 2021; Yamin et al., 2021). However, substantially improved prophylactic activity could be demonstrated at low concentrations of antibodies with enhanced binding of activating Fc receptors (GAALIE mutations) (Yamin et al., 2021). These mutations have previously been associated with dendritic cell maturation as well as induction of antiviral CD8+ T cell responses (Bournazos et al., 2020), and the GAALIE variant of the SARS-CoV-2 antibody sotrovimab is in clinical investigation (VIR-7832) (Cathcart et al., 2022).
Finally, Fc receptor-mediated internalization of antibody-bound virus resulting in antibody-dependent enhancement (ADE) of infection is a theoretical possibility for any antibody-based antiviral strategy and was deliberated from the early stages of vaccine and antibody development (Arvin et al., 2020; Lee et al., 2020). In vitro enhancement of antibody-mediated viral uptake was reported for a small number of monoclonal antibodies (Li et al., 2021a; Zhou et al., 2021b), but did not translate into reduced protection or increased viral pathogenicity in in vivo models (Li et al., 2021a). Moreover, antibody-mediated opsonization of SARS-CoV-2 in monocytes did not result in detectable productive infection although it may be associated with increased inflammatory responses (Junqueira et al., 2022). To date, clinical observation of vaccinated individuals as well as patients treated with convalescent plasma or monoclonal antibodies does not indicate an overt role for ADE or other antibody-mediated adverse effects (e.g., due to immune complex deposition). Nevertheless, potential safety signals, including higher-grade respiratory adverse events, in mostly unvaccinated seropositive hospitalized patients receiving hyperimmune anti-SARS-CoV-2 immunoglobulin or monoclonal antibodies warrant some caution in individuals at later stages of more severe disease (ACTIV-3/TICO Bamlanivimab Study Group, 2022; Polizzotto et al., 2022; ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group, 2021). Despite the contributions of the Fc domains to in vivo efficacy, several antibodies in ongoing clinical investigation have been modified to specifically reduce levels of Fc receptor interactions, aiming to limit potential ADE and/or unwanted hyperinflammatory responses (Bertoglio et al., 2021; Gu et al., 2021; Lanini et al., 2021; Li et al., 2021b; Loo et al., 2022; Shi et al., 2020). Whether use of antibodies with reduced Fc-mediated interactions results in differences in clinical efficacy and/or the safety profiles in hospitalized patients remains to be determined.
Results and implications of clinical investigation
Similar to the rapid initiation of COVID-19 vaccine studies, clinical development of SARS-CoV-2 neutralizing monoclonal antibodies occurred at a remarkable pace with first human dosing within <6 months of the start of pandemic (Lilly, 2020). Since then, monoclonal antibodies have been investigated in clinical trials focusing on different target populations (Figures 3A and 3B). These included infected patients at early and late stages of disease, individuals with recent exposure to the virus, and uninfected participants receiving antibodies as pre-exposure prophylaxis (Figure 3A). While the trial outcomes were largely dependent on the clinical scenarios, the results for different antibodies tested within comparable study settings were highly consistent. Across trials, SARS-CoV-2 neutralizing monoclonal antibodies were safe and well tolerated overall, and pharmacokinetic parameters of unmodified antibodies were within in the expected range of human IgG (t1/2 ∼2–4 weeks) (Chen et al., 2021b, 2021c; Kim et al., 2021b; Li et al., 2021b; Meng et al., 2021; Weinreich et al., 2021a). Notably, the half-life of Fc-engineered antibodies with enhanced FcRn affinity ranged up to 90 days (Lanini et al., 2021; Loo et al., 2022; Zhang et al., 2021a).
When given early after diagnosis and/or symptom onset, potent SARS-CoV-2 neutralizing antibodies reduced the risk of disease progression by approximately 70%–80% (Dougan et al., 2021; Gottlieb et al., 2021; Gupta et al., 2021, 2022; Razonable et al., 2021; Weinreich et al., 2021b) and reduced the incidence of symptomatic disease when given to asymptomatic individuals (O’Brien et al., 2022). Antibody combinations accelerated viral clearance in respiratory samples (Dougan et al., 2021; Gottlieb et al., 2021; Weinreich et al., 2021a, 2021b), and subgroup analyses revealed this to be most pronounced in seronegative individuals with relatively high viral concentrations (Weinreich et al., 2021a, 2021b). Effects on viral loads can be readily assessed even in populations with a low incidence of clinical outcomes (e.g., low rate of hospitalization). However, they may not be fully indicative of potential beneficial clinical effects, as these have also been observed in antibody monotherapy with less pronounced effects on viral loads (Chen et al., 2021c; Gottlieb et al., 2021; Gupta et al., 2022). While relatively high antibody doses were tested initially (e.g., up to 8,000 mg total for the combination of casirivimab/imdevimab), there was no clear dose-response relationship and considerably lower doses were subsequently demonstrated to result in comparable effects (e.g., 1,200 mg for casirivimab/imdevimab; 500 mg for sotrovimab). The outcomes of outpatient trials resulted in emergency use authorizations and/or conditional approvals for treatment of COVID-19 for a number of antibodies in different countries (amubarvimab, bamlanivimab, bebtelovimab, casirivimab, etesevimab, imdevimab, regdanvimab, romlusevimab, sotrovimab) (Figures 3A and 3B). In contrast to the convincing pivotal trial results in outpatients at early disease stages, trials in hospitalized individuals (i.e., late stages) revealed limited or no clinical benefit of monoclonal antibodies (RECOVERY Collaborative Group, 2022; ACTIV-3/TICO Bamlanivimab Study Group, 2022; ACTIV-3/TICO LY-CoV555 Study Group, 2021; ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group, 2021). Compared to outpatients, hospitalized individuals frequently receive additional treatment (e.g., glucocorticoids) and, likely more importantly, a higher fraction of individuals will have developed endogenous antibodies by the time of monoclonal antibody administration. Subgroup analyses suggest that monoclonal antibodies can result in favorable outcomes in seronegative hospitalized individuals (RECOVERY Collaborative Group, 2022; ACTIV-3/TICO Bamlanivimab Study Group, 2022; ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group, 2021). Thus, while SARS-CoV-2 neutralizing monoclonal antibodies are highly effective in preventing disease progression, their efficacy strongly depends on timely administration.
In addition to their use in treatment, SARS-CoV-2 neutralizing antibodies have been investigated for their potential to prevent infection or disease. When given in the context of outbreaks in nursing homes, subcutaneous (s.c.) application of antibody bamlanivimab significantly reduced the incidence of COVID-19 in unvaccinated residents that were not infected at baseline (Cohen et al., 2021). Similarly, s.c. administration of the combination of casirivimab and imdevimab to unvaccinated household contacts of infected index patients resulted in reduced incidences of infection and symptomatic diseases and was associated with lower peak viral loads in cases of breakthrough infections (O'Brien et al., 2021). Finally, the combination of cilgavimab and tixagevimab (Fc-modified with extended half-life and reduced FcRn affinity) has received emergency use authorization for pre-exposure prophylaxis based on the results of an interim analysis demonstrating a 77% relative risk reduction in the incidence of symptomatic COVID-19 (Levin et al., 2022).
Interpretation of the clinical trial results for monoclonal SARS-CoV-2 neutralizing antibodies requires some careful consideration. The overwhelming majority of trial participants was unvaccinated and/or had no known prior infection. As the proportion of immunized individuals increases globally (either by vaccination, infection, or both), the effect sizes of antibody administrations may change in an immunized real-world population. Moreover, most clinical trials were conducted during the prevalence of viral variants with high sensitivity to the investigated monoclonal antibodies. Mutations in the spike proteins of viral variants can confer partial or full resistance to monoclonal antibodies and their emergence has led to the revocation clinical use authorizations for several antibodies (Chen et al., 2021e; Planas et al., 2021; Wang et al., 2021a). Most notably, the highly mutated Omicron variants of SARS-CoV-2 demonstrate resistance to the majority of previously used monoclonal antibodies (with some exceptions, such as the highly active bebtelovimab) (Cao et al., 2022; Carreno et al., 2022; Garcia-Beltran et al., 2022; Gruell et al., 2022; Liu et al., 2022; Planas et al., 2022; Schmidt et al., 2022; Takashita et al., 2022; VanBlargan et al., 2022; Viana et al., 2022). As mutations associated with resistance have been observed to develop during antibody monotherapy and appear to occur less frequently during combination therapy (Choudhary et al., 2021; Copin et al., 2021; Jensen et al., 2021; Rockett et al., 2022), the development of novel antibodies with cross-variant activity and a high barrier to escape is therefore warranted to provide additional options for antibody combinations.
Concluding remarks
Upon encountering the SARS-CoV-2 spike protein, the human immune system is able to generate potent neutralizing antibodies that represent one of our strongest assets to prevent and treat COVID-19. High incidences of SARS-CoV-2 infections and immune-mediated selection pressure will drive continuous viral evolution and the emergence of SARS-CoV-2 immune escape variants. While this will challenge antibody-mediated immunity, recent advances in knowledge and techniques allow to effectively prepare a potent antibody response to a more diverse and changing viral threat. This includes adapted vaccine strategies to induce a broad SARS-CoV-2 humoral immunity and the rapid isolation and development of potent cross-neutralizing SARS-CoV-2 monoclonal antibodies for passive immunization. Since immune escape variants may emerge very rapidly, it will be critical to enable neutralizing antibodies to target a broad spectrum of viral variants. This may be achieved, for example, by vaccination strategies exposing to a wider spectrum of SARS-CoV-2 variants and by using combinations of highly broadly neutralizing antibodies for passive immunization. Finally, administrative and regulatory aspects should be prepared to allow for a rapid response to new variants providing neutralizing antibodies by active or passive immunization to protect individuals at risk and to overcome the COVID-19 pandemic.
Acknowledgments
We thank all laboratories making SARS-CoV-2 sequence information available through GISAID for facilitating rapid analyses of global variant distributions and we acknowledge outbreak.info for aggregating the data. C.O.B. is supported by the Howard Hughes Medical Institute Hanna Gray Fellowship and is a Chan Zuckerberg Biohub investigator. This work was supported by grants from COVIM: NaFoUniMedCovid19 (FKZ: 01KX2021) (to F.K.), the German Research Foundation (CRC 1310, to C.K. and F.K.), and the German Center for Infection Research (to F.K.).
Declaration of interests
H.G., K.V., T.W., C.K., and F.K. are listed as inventors on patent application(s) on virus neutralizing antibodies filed by the University of Cologne. C.O.B. declares no conflict of interest.
References
- Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Yassine H.M., Al-Khatib H.A., Smatti M.K., Tang P., Hasan M.R., Coyle P., et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N. Engl. J. Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad L.J., Chemaitelly H., Bertollini R., National Study Group for C.-V. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2022;386:1091–1093. doi: 10.1056/nejmc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect. Dis. 2021;22:622–635. doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACTIV-3/TICO Bamlanivimab Study Group Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial. Ann. Intern. Med. 2022;175:234–243. doi: 10.7326/M21-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACTIV-3/TICO LY-CoV555 Study Group A neutralizing monoclonal antibody for hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:905–914. doi: 10.1056/nejmoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya M., Kwak K., Pierce S.K. B cell memory: building two walls of protection against pathogens. Nat. Rev. Immunol. 2020;20:229–238. doi: 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F., Thapa M., Lei T., Ahmed S.M.S., Adelsberg D.C., Carreno J.M., Strohmeier S., Schmitz A.J., Zafar S., Zhou J.Q., et al. Personalized Virology Initiative SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell. 2021;184:3936–3948.e10. doi: 10.1016/j.cell.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna I.J., Carlson N.E., Slifka M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/nejmoa066092. [DOI] [PubMed] [Google Scholar]
- Anderson E.M., Goodwin E.C., Verma A., Arevalo C.P., Bolton M.J., Weirick M.E., Gouma S., McAllister C.M., Christensen S.R., Weaver J., et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864.e10. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano E., Nicastri E., Paciello I., Pileri P., Manganaro N., Piccini G., Manenti A., Pantano E., Kabanova A., Troisi M., et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell. 2021;184:1821–1835.e16. doi: 10.1016/j.cell.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano E., Piccini G., Licastro D., Casalino L., Johnson N.V., Paciello I., Dal Monego S., Pantano E., Manganaro N., Manenti A., et al. SARS-CoV-2 escape from a highly neutralizing COVID-19 convalescent plasma. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2103154118. e2103154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O'Connell A.M., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/nejmoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., Gallagher E., Thelwall S., Groves N., Dabrera G., et al. Duration of protection against mild and severe disease by covid-19 vaccines. N. Engl. J. Med. 2022;386:340–350. doi: 10.1056/nejmoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C., Lanzavecchia A., Corti D., Virgin H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- Azzi L., Dalla Gasperina D., Veronesi G., Shallak M., Ietto G., Iovino D., Baj A., Gianfagna F., Maurino V., Focosi D., et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine. 2022;75:103788. doi: 10.1016/j.ebiom.2021.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.O., Jette C.A., Abernathy M.E., Dam K.M.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F., et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842.e16. doi: 10.1016/j.cell.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Morillas Ramos G., Dopfer-Jablonka A., Heidemann A., Ritter C., Friedrichsen M., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates T.A., McBride S.K., Winders B., Schoen D., Trautmann L., Curlin M.E., Tafesse F.G. Antibody response and variant cross-neutralization after SARS-CoV-2 breakthrough infection. JAMA. 2022;327:179–181. doi: 10.1001/jama.2021.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Ajithdoss D., Copin R., Zhou A., Lanza K., Negron N., Ni M., Wei Y., Mohammadi K., Musser B., et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begin P., Callum J., Jamula E., Cook R., Heddle N.M., Tinmouth A., Zeller M.P., Beaudoin-Bussieres G., Amorim L., Bazin R., et al. CONCOR-1 Study Group Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat. Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D.J., Wrobel A.G., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020;588:327–330. doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoglio F., Fuhner V., Ruschig M., Heine P.A., Abassi L., Klunemann T., Rand U., Meier D., Langreder N., Steinke S., et al. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021;36:109433. doi: 10.1016/j.celrep.2021.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borobia A.M., Carcas A.J., Pérez-Olmeda M., Castaño L., Bertran M.J., García-Pérez J., Campins M., Portolés A., González-Pérez M., García Morales M.T., et al. CombiVacS Study Group Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. The Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S., Ravetch J.V. Fcγ receptor function and the design of vaccination strategies. Immunity. 2017;47:224–233. doi: 10.1016/j.immuni.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S., Corti D., Virgin H.W., Ravetch J.V. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature. 2020;588:485–490. doi: 10.1038/s41586-020-2838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G., et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- Cai Y., Zhang J., Xiao T., Peng H., Sterling S.M., Walsh Jr R., Walsh R.M., Jr., Rawson S., Rits-Volloch S., Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C., et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by high-throughput single-cell sequencing of convalescent patients' B cells. Cell. 2020;182:73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno J.M., Alshammary H., Tcheou J., Singh G., Raskin A.J., Kawabata H., Sominsky L.A., Clark J.J., Adelsberg D.C., Bielak D.A., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- Cathcart A.L., Havenar-Daughton C., Lempp F.A., Ma D., Schmid M.A., Agostini M.L., Guarino B., Di iulio J., Rosen L.E., Tucker H., et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2022 doi: 10.1101/2021.03.09.434607. Preprint at. [DOI] [Google Scholar]
- Cerutti G., Guo Y., Wang P., Nair M.S., Wang M., Huang Y., Yu J., Liu L., Katsamba P.S., Bahna F., et al. Neutralizing antibody 5-7 defines a distinct site of vulnerability in SARS-CoV-2 spike N-terminal domain. Cell Rep. 2021;37:109928. doi: 10.1016/j.celrep.2021.109928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti G., Guo Y., Zhou T., Gorman J., Lee M., Rapp M., Reddem E.R., Yu J., Bahna F., Bimela J., et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021;29:819–833.e7. doi: 10.1016/j.chom.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.E.Z., Seah S.G.K., Chye D.H., Massey S., Torres M., Lim A.P.C., Wong S.K.K., Neo J.J.Y., Wong P.S., Lim J.H., et al. The Fc-mediated effector functions of a potent SARS-CoV-2 neutralizing antibody, SC31, isolated from an early convalescent COVID-19 patient, are essential for the optimal therapeutic efficacy of the antibody. PLoS One. 2021;16:e0253487. doi: 10.1371/journal.pone.0253487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zuiani A., Fischinger S., Mullur J., Atyeo C., Travers M., Lelis F.J.N., Pullen K.M., Martin H., Tong P., et al. Quick COVID-19 Healers sustain anti-SARS-CoV-2 antibody production. Cell. 2020;183:1496–1507.e16. doi: 10.1016/j.cell.2020.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E.C., Gilchuk P., Zost S.J., Suryadevara N., Winkler E.S., Cabel C.R., Binshtein E., Chen R.E., Sutton R.E., Rodriguez J., et al. Convergent antibody responses to the SARS-CoV-2 spike protein in convalescent and vaccinated individuals. Cell Rep. 2021;36:109604. doi: 10.1016/j.celrep.2021.109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Datta G., Grace Li Y., Chien J., Price K., Chigutsa E., Brown-Augsburger P., Poorbaugh J., Fill J., Benschop R.J., et al. First-in-human study of bamlanivimab in a randomized trial of hospitalized patients with COVID-19. Clin. Pharmacol. Ther. 2021;110:1467–1477. doi: 10.1002/cpt.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/nejmoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.E., Winkler E.S., Case J.B., Aziati I.D., Bricker T.L., Joshi A., Darling T.L., Ying B., Errico J.M., Shrihari S., et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021;596:103–108. doi: 10.1038/s41586-021-03720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., Liu J., Errico J.M., Xie X., Suryadevara N., et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021;27:717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.-W., Le Bert N., Tan C.W., Tiu C., Zhang J., Tan S.Y., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/s2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A., Muecksch F., Schaefer-Babajew D., Wang Z., Finkin S., Gaebler C., Ramos V., Cipolla M., Mendoza P., Agudelo M., et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021;600:517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., Nunna N., Huang W., Oestreicher J., Colpitts T., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 2021;27:2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary M.C., Chew K.W., Deo R., Flynn J.P., Regan J., Crain C.R., Moser C., Hughes M., Ritz J., Ribeiro R.M., et al. ACTIV-2/A5401 Study Team Emergence of SARS-CoV-2 resistance with monoclonal antibody therapy. medRxiv. 2021 doi: 10.1101/2021.09.03.21263105. Preprint at. [DOI] [Google Scholar]
- Cobb R.R., Nkolola J., Gilchuk P., Chandrashekar A., Yu J., House R.V., Earnhart C.G., Dorsey N.M., Hopkins S.A., Snow D.M., et al. A combination of two human neutralizing antibodies prevents SARS-CoV-2 infection in cynomolgus macaques. Med (N Y) 2022;3:188–203.e4. doi: 10.1016/j.medj.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.S., Nirula A., Mulligan M.J., Novak R.M., Marovich M., Yen C., Stemer A., Mayer S.M., Wohl D., Brengle B., et al. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46–55. doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin R., Baum A., Wloga E., Pascal K.E., Giordano S., Fulton B.O., Zhou A., Negron N., Lanza K., Chan N., et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell. 2021;184:3949–3961.e11. doi: 10.1016/j.cell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K.S., Gagne M., Wagner D.A., O' Connell S., Narpala S.R., Flebbe D.R., Andrew S.F., Davis R.L., Flynn B., Johnston T.S., et al. Protection against SARS-CoV-2 Beta variant in mRNA-1273 vaccine-boosted nonhuman primates. Science. 2021;374:1343–1353. doi: 10.1126/science.abl8912. [DOI] [PubMed] [Google Scholar]
- Corbett K.S., Nason M.C., Flach B., Gagne M., O'Connell S., Johnston T.S., Shah S.N., Edara V.V., Floyd K., Lai L., et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science. 2021;373:eabj0299. doi: 10.1126/science.abj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D., Purcell L.A., Snell G., Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184:3086–3108. doi: 10.1016/j.cell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Acqua W.F., Kiener P.A., Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J. Biol. Chem. 2006;281:23514–23524. doi: 10.1074/jbc.m604292200. [DOI] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Huo J., Zhou D., Zahradnik J., Supasa P., Liu C., Duyvesteyn H.M.E., Ginn H.M., Mentzer A.J., Tuekprakhon A., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Zhou D., Ginn H.M., Duyvesteyn H.M.E., Supasa P., Case J.B., Zhao Y., Walter T.S., Mentzer A.J., Liu C., et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183–2200.e22. doi: 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939–2954.e9. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Zost S.J., Greaney A.J., Starr T.N., Dingens A.S., Chen E.C., Chen R.E., Case J.B., Sutton R.E., Gilchuk P., et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat. Microbiol. 2021;6:1233–1244. doi: 10.1038/s41564-021-00972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose N., Suthar M.S., Makowski M., O'Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C., et al. mRNA-1273 Study Group Antibody persistence through 6 Months after the second dose of mRNA-1273 vaccine for covid-19. N. Engl. J. Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner T., Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Dougan M., Nirula A., Azizad M., Mocherla B., Gottlieb R.L., Chen P., Hebert C., Perry R., Boscia J., Heller B., et al. Bamlanivimab plus etesevimab in mild or moderate covid-19. N. Engl. J. Med. 2021;385:1382–1392. doi: 10.1056/nejmoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Cao Y., Zhu Q., Yu P., Qi F., Wang G., Du X., Bao L., Deng W., Zhu H., et al. Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy. Cell. 2020;183:1013–1023.e13. doi: 10.1016/j.cell.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob. Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner R.A., Shlomchik M.J. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity. 2020;53:1136–1150. doi: 10.1016/j.immuni.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercanoglu M.S., Gieselmann L., Dahling S., Poopalasingam N., Detmer S., Koch M., Korenkov M., Halwe S., Kluver M., Di Cristanziano V., et al. No substantial preexisting B cell immunity against SARS-CoV-2 in healthy adults. iScience. 2022;25:103951. doi: 10.1016/j.isci.2022.103951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalera A., Gonzalez-Reiche A.S., Aslam S., Mena I., Laporte M., Pearl R.L., Fossati A., Rathnasinghe R., Alshammary H., van de Guchte A., et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe. 2022;30:373–387.e7. doi: 10.1016/j.chom.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Cao D., Kong L., Zhang X. Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein. Nat. Commun. 2020;11:3618. doi: 10.1038/s41467-020-17371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J., Bals J., Altomare C.G., St Denis K., Lam E.C., Hauser B.M., Ronsard L., Sangesland M., Moreno T.B., Okonkwo V., et al. Naive human B cells engage the receptor binding domain of SARS-CoV-2, variants of concern, and related sarbecoviruses. Sci. Immunol. 2021;6:eabl5842. doi: 10.1126/sciimmunol.abl5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., et al. Oxford COVID Vaccine Trial Group Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froberg J., Gillard J., Philipsen R., Lanke K., Rust J., van Tuijl D., Teelen K., Bousema T., Simonetti E., van der Gaast-de Jongh C.E., et al. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat. Commun. 2021;12:5621. doi: 10.1038/s41467-021-25949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne M., Moliva J.I., Foulds K.E., Andrew S.F., Flynn B.J., Werner A.P., Wagner D.A., Teng I.T., Lin B.C., Moore C., et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing antibodies and protection against Omicron. Cell. 2022;185:1556–1571.e18. doi: 10.1016/j.cell.2022.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., Hauser B.M., Caradonna T.M., Clayton K.L., Nitido A.D., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann L., Kreer C., Ercanoglu M.S., Lehnen N., Zehner M., Schommers P., Potthoff J., Gruell H., Klein F. Effective high-throughput isolation of fully human antibodies targeting infectious pathogens. Nat. Protoc. 2021;16:3639–3671. doi: 10.1038/s41596-021-00554-w. [DOI] [PubMed] [Google Scholar]
- Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., et al. Immune Assays Team§. Moderna, Inc. Team§. Coronavirus Vaccine Prevention Network CoVPN/Coronavirus Efficacy COVE Team§. United States Government USG/CoVPN Biostatistics Team§ Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., Lundgreen K.A., Reynaldi A., Khoury D.S., Pattekar A., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/nejmoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Loes A.N., Crawford K.H.D., Starr T.N., Malone K.D., Chu H.Y., Bloom J.D. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021;29:463–476.e6. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Loes A.N., Gentles L.E., Crawford K.H.D., Starr T.N., Malone K.D., Chu H.Y., Bloom J.D. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci. Transl. Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Starr T.N., Barnes C.O., Weisblum Y., Schmidt F., Caskey M., Gaebler C., Cho A., Agudelo M., Finkin S., et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat. Commun. 2021;12:4196. doi: 10.1038/s41467-021-24435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruell H., Vanshylla K., Tober-Lau P., Hillus D., Schommers P., Lehmann C., Kurth F., Sander L.E., Klein F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022;28:477–480. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Cao X., Wang Z., Hu X., Yao Y., Zhou Y., Liu P., Liu X., Gao G., Hu X., et al. A human antibody of potent efficacy against SARS-CoV-2 in rhesus macaques showed strong blocking activity to B.1.351. MAbs. 2021;13:1930636. doi: 10.1080/19420862.2021.1930636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., Arnthorsson A.O., Helgason D., Bjarnadottir K., Ingvarsson R.F., et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Huang L., Zhang G., Yao Y., Zhou H., Shen S., Shen B., Li B., Li X., Zhang Q., et al. A SARS-CoV-2 neutralizing antibody with extensive Spike binding coverage and modified for optimal therapeutic outcomes. Nat. Commun. 2021;12:2623. doi: 10.1038/s41467-021-22926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Falci D.R., Sarkis E., Solis J., Zheng H., Scott N., et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021;385:1941–1950. doi: 10.1056/nejmoa2107934. [DOI] [PubMed] [Google Scholar]
- Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Rodrigues Falci D., Sarkis E., Solis J., Zheng H., Scott N., et al. COMET-ICE Investigators Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Noack D., Okba N.M.A., Li W., Wang C., Bestebroer T., de Vries R., Herfst S., de Meulder D., Verveer E., et al. SARS-CoV-2 neutralizing human antibodies protect against lower respiratory tract disease in a hamster model. J. Infect Dis. 2021;223:2020–2028. doi: 10.1093/infdis/jiab289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V., Foulkes S., Insalata F., Kirwan P., Saei A., Atti A., Wellington E., Khawam J., Munro K., Cole M., et al. Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N. Engl. J. Med. 2022;386:1207–1220. doi: 10.1056/nejmoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V.G., Ferreira V.H., Wood H., Ierullo M., Majchrzak-Kita B., Manguiat K., Robinson A., Kulasingam V., Humar A., Kumar D. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat. Immunol. 2022;23:380–385. doi: 10.1038/s41590-021-01126-6. [DOI] [PubMed] [Google Scholar]
- Halwe S., Kupke A., Vanshylla K., Liberta F., Gruell H., Zehner M., Rohde C., Krahling V., Gellhorn Serra M., Kreer C., et al. Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 infection. Viruses. 2021;13:1498. doi: 10.3390/v13081498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammitt L.L., Dagan R., Yuan Y., Baca Cots M., Bosheva M., Madhi S.A., Muller W.J., Zar H.J., Brooks D., Grenham A., et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 2022;386:837–846. doi: 10.1056/nejmoa2110275. [DOI] [PubMed] [Google Scholar]
- Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L., COVID-19 Genomics UK COG-UK Consortium SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie K.M., Li H., Bedinger D., Schendel S.L., Dennison S.M., Li K., Rayaprolu V., Yu X., Mann C., Zandonatti M., et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021;374:472–478. doi: 10.1126/science.abh2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters B.A., Myers R.C., Carroll M.C. Follicular dendritic cells: dynamic antigen libraries. Nat. Rev. Immunol. 2014;14:495–504. doi: 10.1038/nri3689. [DOI] [PubMed] [Google Scholar]
- Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., Jentzsch S., Helbig E.T., Lippert L.J., Tscheak P., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.J., Chiba S., Halfmann P., Ehre C., Kuroda M., Dinnon K.H., 3rd, Leist S.R., Schafer A., Nakajima N., Takahashi K., et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J., Zhao Y., Ren J., Zhou D., Duyvesteyn H.M.E., Ginn H.M., Carrique L., Malinauskas T., Ruza R.R., Shah P.N.M., et al. Neutralization of SARS-CoV-2 by destruction of the prefusion spike. Cell Host Microbe. 2020;28 doi: 10.1016/j.chom.2020.07.002. 497–454.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S., Liu L., Guo Y., Liu L., Chan J.F.W., Huang Y., Wang M., Luo Y., Yu J., Chu H., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imkeller K., Wardemann H. Assessing human B cell repertoire diversity and convergence. Immunol. Rev. 2018;284:51–66. doi: 10.1111/imr.12670. [DOI] [PubMed] [Google Scholar]
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5:eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein M.F., MacCamy A.J., Akins N.R., Feng J., Homad L.J., Hurlburt N.K., Seydoux E., Wan Y.H., Stuart A.B., Edara V.V., et al. Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+ subjects. Cell Rep. 2021;36:109353. doi: 10.1016/j.celrep.2021.109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B., Luebke N., Feldt T., Keitel V., Brandenburger T., Kindgen-Milles D., Lutterbeck M., Freise N.F., Schoeler D., Haas R., et al. Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany. Lancet Reg. Health Eur. 2021;8:100164. doi: 10.1016/j.lanepe.2021.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette C.A., Cohen A.A., Gnanapragasam P.N.P., Muecksch F., Lee Y.E., Huey-Tubman K.E., Schmidt F., Hatziioannou T., Bieniasz P.D., Nussenzweig M.C., et al. Broad cross-reactivity across sarbecoviruses exhibited by a subset of COVID-19 donor-derived neutralizing antibodies. Cell Rep. 2021;37:110188. doi: 10.1016/j.celrep.2021.110188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.E., Brown-Augsburger P.L., Corbett K.S., Westendorf K., Davies J., Cujec T.P., Wiethoff C.M., Blackbourne J.L., Heinz B.A., Foster D., et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 2021;13:eabf1906. doi: 10.1126/scitranslmed.abf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost S., Altfeld M. Control of human viral infections by natural killer cells. Annu. Rev. Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- Joyner M.J., Carter R.E., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., Wiggins C.C., Bruno K.A., Klompas A.M., et al. Convalescent plasma antibody levels and the risk of death from covid-19. N. Engl. J. Med. 2021;384:1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., Carter R.E., Klompas A.M., Wiggins C.C., Shepherd J.R., et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Invest. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Junqueira C., Crespo A., Ranjbar S., de Lacerda L.B., Lewandrowski M., Ingber J., Parry B., Ravid S., Clark S., Schrimpf M.R., et al. FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022 doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N., Kuo H.H., Boucau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., Piechocka-Trocha A., Lefteri K., Osborn M., Bals J., et al. Massachusetts Consortium on Pathogen Readiness Specimen Working Group Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157.e13. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplonek P., Wang C., Bartsch Y., Fischinger S., Gorman M.J., Bowman K., Kang J., Dayal D., Martin P., Nowak R.P., et al. Early cross-coronavirus reactive signatures of humoral immunity against COVID-19. Sci. Immunol. 2021;6:eabj2901. doi: 10.1126/sciimmunol.abj2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Z., Oton J., Qu K., Cortese M., Zila V., McKeane L., Nakane T., Zivanov J., Neufeldt C.J., Cerikan B., et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588:498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton R., Richardson S.I., Moyo-Gwete T., Hermanus T., Tincho M.B., Benede N., Manamela N.P., Baguma R., Makhado Z., Ngomti A., et al. Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant-dependent manner. Cell Host Microbe. 2021;29:1611–1619.e5. doi: 10.1016/j.chom.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketas T.J., Chaturbhuj D., Cruz Portillo V.M., Francomano E., Golden E., Chandrasekhar S., Debnath G., Diaz-Tapia R., Yasmeen A., Kramer K.D., et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog. Immun. 2021;6:116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Gurry C., Freitas L., Schultz M.B., Bach G., Diallo A., Akite N., Ho J., Lee R.T., Yeo W., et al. GISAID's role in pandemic response. China CDC Wkly. 2021;3:1049–1051. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Khoury D.S., Wheatley A.K., Ramuta M.D., Reynaldi A., Cromer D., Subbarao K., O'Connor D.H., Kent S.J., Davenport M.P. Measuring immunity to SARS-CoV-2 infection: comparing assays and animal models. Nat. Rev. Immunol. 2020;20:727–738. doi: 10.1038/s41577-020-00471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Ryu D.K., Lee J., Kim Y.I., Seo J.M., Kim Y.G., Jeong J.H., Kim M., Kim J.I., Kim P., et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021;12:288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Jang Y.R., Hong J.H., Jung J.G., Park J.H., Streinu-Cercel A., Streinu-Cercel A., Sandulescu O., Lee S.J., Kim S.H., et al. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection. Clin. Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.I., Noh J., Kim S., Choi Y., Yoo D.K., Lee Y., Lee H., Jung J., Kang C.K., Song K.H., et al. Stereotypic neutralizing VH antibodies against SARS-CoV-2 spike protein receptor binding domain in patients with COVID-19 and healthy individuals. Sci. Transl. Med. 2021;13:eabd6990. doi: 10.1126/scitranslmed.abd6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Zhou J.Q., Horvath S.C., Schmitz A.J., Sturtz A.J., Lei T., Liu Z., Kalaidina E., Thapa M., Alsoussi W.B., et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature. 2022;604:141–145. doi: 10.1038/s41586-022-04527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Mouquet H., Dosenovic P., Scheid J.F., Scharf L., Nussenzweig M.C. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.Y., Pegu A., Rudicell R.S., Yang Z.Y., Joyce M.G., Chen X., Wang K., Bao S., Kraemer T.D., Rath T., et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korley F.K., Durkalski-Mauldin V., Yeatts S.D., Schulman K., Davenport R.D., Dumont L.J., El Kassar N., Foster L.D., Hah J.M., Jaiswal S., et al. Early convalescent plasma for high-risk outpatients with covid-19. N. Engl. J. Med. 2021;385:1951–1960. doi: 10.1056/nejmoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreer C., Zehner M., Weber T., Ercanoglu M.S., Gieselmann L., Rohde C., Halwe S., Korenkov M., Schommers P., Vanshylla K., et al. Longitudinal isolation of potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients. Cell. 2020;182:843–854.e12. doi: 10.1016/j.cell.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreye J., Reincke S.M., Kornau H.C., Sanchez-Sendin E., Corman V.M., Liu H., Yuan M., Wu N.C., Zhu X., Lee C.C.D., et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell. 2020;183:1058–1069.e19. doi: 10.1016/j.cell.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., Plotkin S., Knezevic I. WHO international standard for anti-SARS-CoV-2 immunoglobulin. The Lancet. 2021;397:1347–1348. doi: 10.1016/s0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann C., Mayer C.K., Claassen M., Maponga T., Burgers W.A., Keeton R., Riou C., Sutherland A.D., Suliman T., Shaw M.L., Preiser W. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. The Lancet. 2022;399:625–626. doi: 10.1016/s0140-6736(22)00090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lanini S., Milleri S., Andreano E., Nosari S., Paciello I., Piccini G., Gentili A., Phogat A., Hyseni I., Leonardi M., et al. A single intramuscular injection of monoclonal antibody MAD0004J08 induces in healthy adults SARS-CoV-2 neutralising antibody titres exceeding those induced by infection and vaccination. medRxiv. 2021 doi: 10.1101/2021.08.03.21261441. Preprint at. [DOI] [Google Scholar]
- Lederer K., Bettini E., Parvathaneni K., Painter M.M., Agarwal D., Lundgreen K.A., Weirick M., Muralidharan K., Castano D., Goel R.R., et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell. 2022;185:1008–1024.e15. doi: 10.1016/j.cell.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempp F.A., Soriaga L.B., Montiel-Ruiz M., Benigni F., Noack J., Park Y.J., Bianchi S., Walls A.C., Bowen J.E., Zhou J., et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature. 2021;598:342–347. doi: 10.1038/s41586-021-03925-1. [DOI] [PubMed] [Google Scholar]
- Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/nejmoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.J., Ustianowski A., De Wit S., Launay O., Avila M., Templeton A., Yuan Y., Seegobin S., Ellery A., Levinson D.J., et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N. Engl. J. Med. 2022 doi: 10.1056/nejmoa2116620. NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Edwards R.J., Manne K., Martinez D.R., Schafer A., Alam S.M., Wiehe K., Lu X., Parks R., Sutherland L.L., et al. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell. 2021;184:4203–4219.e32. doi: 10.1016/j.cell.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu J., Nie J., Zhang L., Hao H., Liu S., Zhao C., Zhang Q., Liu H., Nie L., et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182:1284–1294.e9. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Qi L., Bai H., Sun C., Xu S., Wang Y., Han C., Li Y., Liu L., Cheng X., et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a monoclonal antibody (SCTA01) targeting SARS-CoV-2 in healthy adults: a randomized, double-blind, placebo-controlled, phase I study. Antimicrob. Agents Chemother. 2021;65:e0106321. doi: 10.1128/aac.01063-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libster R., Perez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., et al. Fundacion INFANT–COVID-19 Group Early high-titer plasma therapy to prevent severe covid-19 in older adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly Press release: Lilly begins world's first study of a potential COVID-19 antibody treatment in humans. 2020. https://investor.lilly.com/news-releases/news-release-details/lilly-begins-worlds-first-study-potential-covid-19-antibody
- Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., Nutalai R., Zhou D., Mentzer A.J., Zhao Y., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Iketani S., Guo Y., Chan J.F., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F.W., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., Zhao H., Errico J.M., Theel E.S., Liebeskind M.J., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29:477–488.e4. doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Loo Y.M., McTamney P.M., Arends R.H., Abram M.E., Aksyuk A.A., Diallo S., Flores D.J., Kelly E.J., Ren K., Roque R., et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci. Transl. Med. 2022;14:eabl8124. doi: 10.1126/scitranslmed.abl8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/nejmoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Klein J., Sundaram M.E., Liu F., Wong P., Silva J., Mao T., Oh J.E., Mohanty S., Huang J., et al. Yale IMPACT Research Team Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021;27:1178–1186. doi: 10.1038/s41591-021-01355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger L.L., Ransegnola B.P., Jin D.K., Muecksch F., Weisblum Y., Bao W., George P.J., Rodriguez M., Tricoche N., Schmidt F., et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J. Clin. Microbiol. 2020;58 doi: 10.1128/jcm.02005-20. e02005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/nejmoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D.R., Schafer A., Gobeil S., Li D., De la Cruz G., Parks R., Lu X., Barr M., Stalls V., Janowska K., et al. A broadly cross-reactive antibody neutralizes and protects against sarbecovirus challenge in mice. Sci. Transl. Med. 2022;14:eabj7125. doi: 10.1126/scitranslmed.abj7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D.R., Schafer A., Leist S.R., Li D., Gully K., Yount B., Feng J.Y., Bunyan E., Porter D.P., Cihlar T., et al. Prevention and therapy of SARS-CoV-2 and the B.1.351 variant in mice. Cell Rep. 2021;36:109450. doi: 10.1016/j.celrep.2021.109450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum M., De Marco A., Lempp F.A., Tortorici M.A., Pinto D., Walls A.C., Beltramello M., Chen A., Liu Z., Zatta F., et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184:2332–2347.e16. doi: 10.1016/j.cell.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K.R., Rennick L.J., Nambulli S., Robinson-McCarthy L.R., Bain W.G., Haidar G., Duprex W.P. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science. 2021;371:1139–1142. doi: 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Wang P., Xiong Y., Wu Y., Lin X., Lu S., Li R., Zhao B., Liu J., Zeng S., et al. Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: a Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody. Emerg. Microbes Infect. 2021;10:1638–1648. doi: 10.1080/22221751.2021.1960900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., et al. Indian SARS-CoV-2 Genomics Consortium INSACOG. Genotype to Phenotype Japan G2P-Japan Consortium. CITIID-NIHR BioResource COVID-19 Collaboration SARS-CoV-2 B.1.617.2 delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama S., Adachi Y., Sato T., Tonouchi K., Sun L., Fukushi S., Yamada S., Kinoshita H., Nojima K., Kanno T., et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity. 2021;54:1841–1852.e4. doi: 10.1016/j.immuni.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd P.A., Minervina A.A., Pogorelyy M.V., Turner J.S., Kim W., Kalaidina E., Petersen J., Schmitz A.J., Lei T., Haile A., et al. SJTRC Study Team SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185:603–613.e15. doi: 10.1016/j.cell.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F., Wang Z., Cho A., Gaebler C., Ben Tanfous T., DaSilva J., Bednarski E., Ramos V., Zong S., Johnson B., et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022 doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F., Weisblum Y., Barnes C.O., Schmidt F., Schaefer-Babajew D., Wang Z., C Lorenzi J.C., Flyak A.I., DeLaitsch A.T., Huey-Tubman K.E., et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity. 2021;54:1853–1868.e7. doi: 10.1016/j.immuni.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., et al. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen J.L., Tsueng G., Abdel Latif A., Alkuzweny M., Cano M., Haag E., Zhou J., Zeller M., Hufbauer E., Matteson N., et al. Outbreak.info. 2020. https://outbreak.info/
- Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., Bula M., Cathie K., Chatterjee K., Dodd K., et al. COV-BOOST study group Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. The Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel K.G., Clark S.A., Shankar S., Pan J., Clark L.E., Yang P., Coscia A., McKay L.G.A., Varnum H.H., Brusic V., et al. Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain. Science. 2022;375:eabl6251. doi: 10.1126/science.abl6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.W., Faulkner N., Cornish G.H., Rosa A., Harvey R., Hussain S., Ulferts R., Earl C., Wrobel A.G., Benton D.J., et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M.P., Forleo-Neto E., Musser B.J., Isa F., Chan K.C., Sarkar N., Bar K.J., Barnabas R.V., Barouch D.H., Cohen M.S., et al. Subcutaneous REGEN-COV antibody combination to prevent covid-19. N. Engl. J. Med. 2021;385:1184–1195. doi: 10.1056/nejmoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M.P., Forleo-Neto E., Sarkar N., Isa F., Hou P., Chan K.C., Musser B.J., Bar K.J., Barnabas R.V., Barouch D.H., et al. COVID-19 Phase 3 Prevention Trial Team Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic coVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327:432–441. doi: 10.1001/jama.2021.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., Feng W., Tong J., Eaton A., Maglinao M., et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Then E., Lucas C., Monteiro V.S., Miric M., Brache V., Cochon L., Vogels C.B.F., Malik A.A., De la Cruz E., Jorge A., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022;28:481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Snell G., Walls A.C., Veesler D., Beltramello M., Silacci-Fregni C., Pinto D., et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042.e1. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Pinto D., Sauer M.M., Czudnochowski N., Low J.S., Tortorici M.A., Housley M.P., Noack J., Walls A.C., Bowen J.E., Guarino B., et al. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science. 2021;373:1109–1116. doi: 10.1126/science.abj3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., Bolland W.H., Porrot F., Staropoli I., Lemoine F., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccin. Immunol. 2010;17:1055–1065. doi: 10.1128/cvi.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzotto M.N., Nordwall J., Babiker A.G., Phillips A., Vock D.M., Eriobu N., Kwaghe V., Paredes R., Mateu L., Ramachandruni S., et al. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. The Lancet. 2022;399:530–540. doi: 10.1016/S0140-6736(22)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston D., Weisblum Y., Wise H., Templeton K., Jenks S., Hatziioannou T., Bieniasz P. Absence of severe acute respiratory syndrome coronavirus 2 neutralizing activity in prepandemic sera from individuals with recent seasonal coronavirus infection. Clin. Infect Dis. 2021;73:e1208–e1211. doi: 10.1093/cid/ciaa1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D.R., Raut R., Markmann A., Cornaby C., Bartelt L., Weiss S., Park Y., et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5:eabc8413. doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo C.G., Tse L.V., Kaku C.I., Wrapp D., Sakharkar M., Huang D., Deveau L.M., Yockachonis T.J., Herbert A.S., Battles M.B., et al. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science. 2021;371:823–829. doi: 10.1126/science.abf4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razonable R.R., Pawlowski C., O'Horo J.C., Arndt L.L., Arndt R., Bierle D.M., Borgen M.D., Hanson S.N., Hedin M.C., Lenehan P., et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40:101102. doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. The Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.J., Pade C., Gibbons J.M., Butler D.K., Otter A.D., Menacho K., Fontana M., Smit A., Sackville-West J.E., Cutino-Moguel T., et al. UK COVIDsortium Immune Correlates Network. UK COVIDsortium Investigators Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripperger T.J., Uhrlaub J.L., Watanabe M., Wong R., Castaneda Y., Pizzato H.A., Thompson M.R., Bradshaw C., Weinkauf C.C., Bime C., et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933.e4. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett R., Basile K., Maddocks S., Fong W., Agius J.E., Johnson-Mackinnon J., Arnott A., Chandra S., Gall M., Draper J., et al. Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N. Engl. J. Med. 2022;386:1477–1479. doi: 10.1056/nejmc2120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., Limbo O., Smith C., Song G., Woehl J., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röltgen K., Nielsen S.C.A., Silva O., Younes S.F., Zaslavsky M., Costales C., Yang F., Wirz O.F., Solis D., Hoh R.A., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185:1025–1040.e14. doi: 10.1016/j.cell.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., Hunter M., Wang H., Sahoo M.K., Huang C., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020;5:eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A., Muecksch F., Lorenzi J.C.C., Leist S.R., Cipolla M., Bournazos S., Schmidt F., Maison R.M., Gazumyan A., Martinez D.R., et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 2021;218:e20201993. doi: 10.1084/jem.20201993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid J.F., Barnes C.O., Eraslan B., Hudak A., Keeffe J.R., Cosimi L.A., Brown E.M., Muecksch F., Weisblum Y., Zhang S., et al. B cell genomics behind cross-neutralization of SARS-CoV-2 variants and SARS-CoV. Cell. 2021;184:3205–3221.e24. doi: 10.1016/j.cell.2021.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F., Muecksch F., Weisblum Y., Da Silva J., Bednarski E., Cho A., Wang Z., Gaebler C., Caskey M., Nussenzweig M.C., et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N. Engl. J. Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F., Weisblum Y., Rutkowska M., Poston D., DaSilva J., Zhang F., Bednarski E., Cho A., Schaefer-Babajew D.J., Gaebler C., et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature. 2021;600:512–516. doi: 10.1038/s41586-021-04005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A.J., Turner J.S., Liu Z., Zhou J.Q., Aziati I.D., Chen R.E., Joshi A., Bricker T.L., Darling T.L., Adelsberg D.C., et al. A vaccine-induced public antibody protects against SARS-CoV-2 and emerging variants. Immunity. 2021;54:2159–2166.e6. doi: 10.1016/j.immuni.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O'Byrne A., Kouphou N., Galao R.P., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux E., Homad L.J., MacCamy A.J., Parks K.R., Hurlburt N.K., Jennewein M.F., Akins N.R., Stuart A.B., Wan Y.H., Feng J., et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. 2020;53:98–105.e5. doi: 10.1016/j.immuni.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Sholukh A.M., Fiore-Gartland A., Ford E.S., Miner M.D., Hou Y.J., Tse L.V., Kaiser H., Zhu H., Lu J., Madarampalli B., et al. Evaluation of cell-based and surrogate SARS-CoV-2 neutralization assays. J. Clin. Microbiol. 2021;59:e0052721. doi: 10.1128/JCM.00527-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017;22:30494. doi: 10.2807/1560-7917.es.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vazquez C., Savoy N., Giunta D.H., Perez L.G., Sanchez M.D.L., et al. PlasmAr Study Group A randomized trial of convalescent plasma in covid-19 severe pneumonia. N. Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., McNab C., Olson R.M., Bristol N., Nolan C., Bergstrøm E., Bartos M., Mabuchi S., Panjabi R., Karan A., et al. How an outbreak became a pandemic: a chronological analysis of crucial junctures and international obligations in the early months of the COVID-19 pandemic. The Lancet. 2021;398:2109–2124. doi: 10.1016/s0140-6736(21)01897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N., Goncalves P., Charbit B., Grzelak L., Beretta M., Planchais C., Bruel T., Rouilly V., Bondet V., Hadjadj J., et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat. Immunol. 2021;22:1428–1439. doi: 10.1038/s41590-021-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh W.T., Liu Y., Nakayama E.E., Ono C., Torii S., Nakagami H., Matsuura Y., Shioda T., Arase H. The N-terminal domain of spike glycoprotein mediates SARS-CoV-2 infection by associating with L-SIGN and DC-SIGN. bioRxiv. 2020 doi: 10.1101/2020.11.05.369264. Preprint at. [DOI] [Google Scholar]
- Sokal A., Chappert P., Barba-Spaeth G., Roeser A., Fourati S., Azzaoui I., Vandenberghe A., Fernandez I., Meola A., Bouvier-Alias M., et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. 2021;184:1201–1213.e14. doi: 10.1016/j.cell.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., He W.T., Callaghan S., Anzanello F., Huang D., Ricketts J., Torres J.L., Beutler N., Peng L., Vargas S., et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat. Commun. 2021;12:2938. doi: 10.1038/s41467-021-23074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021:eabg9175. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Czudnochowski N., Liu Z., Zatta F., Park Y.J., Addetia A., Pinto D., Beltramello M., Hernandez P., Greaney A.J., et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature. 2021;597:97–102. doi: 10.1038/s41586-021-03807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Addetia A., Hannon W.W., Choudhary M.C., Dingens A.S., Li J.Z., Bloom J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science. 2021;371:850–854. doi: 10.1126/science.abf9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Dingens A.S., Bloom J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep. Med. 2021;2:100255. doi: 10.1016/j.xcrm.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson K.E., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Truyers C., Atyeo C., Loos C., Chandrashekar A., McMahan K., et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claer L., Quentric P., Fadlallah J., Devilliers H., Ghillani P., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. The Lancet. 2022;399:36–49. doi: 10.1016/s0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W., Sia S.F., Schmitz A.J., Bricker T.L., Starr T.N., Greaney A.J., Turner J.S., Mohammed B.M., Liu Z., Choy K.T., et al. Neutralizing monoclonal antibodies that target the spike receptor binding domain confer Fc receptor-independent protection against SARS-CoV-2 infection in syrian hamsters. mBio. 2021;12:e0239521. doi: 10.1128/mbio.02395-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara N., Shrihari S., Gilchuk P., VanBlargan L.A., Binshtein E., Zost S.J., Nargi R.S., Sutton R.E., Winkler E.S., Chen E.C., et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell. 2021;184:2316–2331.e15. doi: 10.1016/j.cell.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., Vanderheiden A., Nyhoff L., Adekunle S., Davis C.W., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai L., Zhu G., Yang M., Cao L., Xing X., Yin G., Chan C., Qin C., Rao Z., Wang X., et al. Nanometer-resolution in situ structure of the SARS-CoV-2 postfusion spike protein. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2112703118. e2112703118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashita E., Kinoshita N., Yamayoshi S., Sakai-Tagawa Y., Fujisaki S., Ito M., Iwatsuki-Horimoto K., Halfmann P., Watanabe S., Maeda K., et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N. Engl. J. Med. 2022;386:1475–1477. doi: 10.1056/nejmc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.J.C., Yuan M., Kuzelka K., Padron G.C., Beal J.R., Chen X., Wang Y., Rivera-Cardona J., Zhu X., Stadtmueller B.M., et al. Sequence signatures of two public antibody clonotypes that bind SARS-CoV-2 receptor binding domain. Nat. Commun. 2021;12:3815. doi: 10.1038/s41467-021-24123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin A., Gong S.Y., Beaudoin-Bussieres G., Vezina D., Gasser R., Nault L., Marchitto L., Benlarbi M., Chatterjee D., Nayrac M., et al. Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022;30:97–109.e5. doi: 10.1016/j.chom.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- Tober-Lau P., Gruell H., Vanshylla K., Koch W.M., Hillus D., Schommers P., Suarez I., Suttorp N., Sander L.E., Klein F., Kurth F. Cross-variant neutralizing serum activity after SARS-CoV-2 breakthrough infections. Emerg. Infect Dis. 2022;28:1050–1052. doi: 10.3201/eid2805.220271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober-Lau P., Schwarz T., Vanshylla K., Hillus D., Gruell H., Suttorp N., Landgraf I., Kappert K., Seybold J., Drosten C., et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir. Med. 2021;9:e104–e105. doi: 10.1016/s2213-2600(21)00456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong P., Gautam A., Windsor I.W., Travers M., Chen Y., Garcia N., Whiteman N.B., McKay L.G.A., Storm N., Malsick L.E., et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell. 2021;184:4969–4980.e15. doi: 10.1016/j.cell.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Beltramello M., Lempp F.A., Pinto D., Dang H.V., Rosen L.E., McCallum M., Bowen J., Minola A., Jaconi S., et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science. 2020;370:950–957. doi: 10.1126/science.abe3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., Bruxvoort K.J., Tubert J.E., Florea A., Ku J.H., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., Hansen L., Haile A., Klebert M.K., Pusic I., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- Turner J.S., O'Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I., Prevost J., Ladinsky M.S., Stone H., Lu M., Anand S.P., Beaudoin-Bussieres G., Symmes K., Benlarbi M., Ding S., et al. Live imaging of SARS-CoV-2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy. Immunity. 2021;54:2143–2158.e15. doi: 10.1016/j.immuni.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriu K., Kimura I., Shirakawa K., Takaori-Kondo A., Nakada T.A., Kaneda A., Nakagawa S., Sato K., Genotype to Phenotype Japan, C Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum. N. Engl. J. Med. 2021;385:2397–2399. doi: 10.1056/nejmc2114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBlargan L.A., Errico J.M., Halfmann P.J., Zost S.J., Crowe J.E., Jr., Purcell L.A., Kawaoka Y., Corti D., Fremont D.H., Diamond M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022;28:490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., Gruell H., Schlotz M., Ercanoglu M.S., Stumpf R., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29:917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanshylla K., Fan C., Wunsch M., Poopalasingam N., Meijers M., Kreer C., Kleipass F., Ruchnewitz D., Ercanoglu M.S., Gruell H., et al. Discovery of ultrapotent broadly neutralizing antibodies from SARS-CoV-2 elite neutralizers. Cell Host Microbe. 2022;30:69–82.e10. doi: 10.1016/j.chom.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanshylla K., Tober-Lau P., Gruell H., Münn F., Eggeling R., Pfeifer N., Le N.H., Landgraf I., Kurth F., Sander L.E., Klein F. Durability of omicron-neutralising serum activity after mRNA booster immunisation in older adults. Lancet Infect. Dis. 2022;22:445–446. doi: 10.1016/s1473-3099(22)00135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Hawes C.E., Lakshmanappa Y.S., Roh J.W., Schmidt B.A., Dutra J., Louie W., Liu H., Ma Z.M., Watanabe J.K., et al. Monoclonal antibodies protect aged rhesus macaques from SARS-CoV-2-induced immune activation and neuroinflammation. Cell Rep. 2021;37:109942. doi: 10.1016/j.celrep.2021.109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., Anyaneji U.J., Bester P.A., Boni M.F., Chand M., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., Nussenzweig M.C. Germinal centers. Annu. Rev. Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- Voss W.N., Hou Y.J., Johnson N.V., Delidakis G., Kim J.E., Javanmardi K., Horton A.P., Bartzoka F., Paresi C.J., Tanno Y., et al. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science. 2021;372:1108–1112. doi: 10.1126/science.abg5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Sprouse K.R., Bowen J.E., Joshi A., Franko N., Navarro M.J., Stewart C., Cameroni E., McCallum M., Goecker E.A., et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185:872–880.e3. doi: 10.1016/j.cell.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Peng Y., Wang R., Jiao S., Wang M., Huang W., Shan C., Jiang W., Li Z., Gu C., et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat. Commun. 2020;11:5752. doi: 10.1038/s41467-020-19568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Muecksch F., Cho A., Gaebler C., Hoffmann H.H., Ramos V., Zong S., Cipolla M., Johnson B., Schmidt F., et al. Analysis of memory B cells identifies conserved neutralizing epitopes on the N-terminal domain of variant SARS-Cov-2 spike proteins. Immunity. 2022 doi: 10.1016/j.immuni.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D., et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020;369:731–736. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/nejmoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Xiao J., Hooper A.T., Hamilton J.D., Musser B.J., et al. Trial Investigators REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N. Engl. J. Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.H., Michailidis E., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf K., Žentelis S., Wang L., Foster D., Vaillancourt P., Wiggin M., Lovett E., van der Lee R., Hendle J., Pustilnik A., et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39:109433. doi: 10.1016/j.celrep.2022.110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.S., Gilchuk P., Yu J., Bailey A.L., Chen R.E., Chong Z., Zost S.J., Jang H., Huang Y., Allen J.D., et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021;184:1804–1820.e16. doi: 10.1016/j.cell.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M.C., Ramonell R.P., Nguyen D.C., Cashman K.S., Saini A.S., Haddad N.S., Ley A.M., Kyu S., Howell J.C., Ozturk T., et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wratil P.R., Stern M., Priller A., Willmann A., Almanzar G., Vogel E., Feuerherd M., Cheng C.C., Yazici S., Christa C., et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022;28:496–503. doi: 10.1038/s41591-022-01715-4. [DOI] [PubMed] [Google Scholar]
- Wrobel A.G., Benton D.J., Hussain S., Harvey R., Martin S.R., Roustan C., Rosenthal P.B., Skehel J.J., Gamblin S.J. Antibody-mediated disruption of the SARS-CoV-2 spike glycoprotein. Nat. Commun. 2020;11:5337. doi: 10.1038/s41467-020-19146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.C., Yuan M., Liu H., Lee C.C.D., Zhu X., Bangaru S., Torres J.L., Caniels T.G., Brouwer P.J.M., van Gils M.J., et al. An alternative binding mode of IGHV3-53 antibodies to the SARS-CoV-2 receptor binding domain. Cell Rep. 2020;33:108274. doi: 10.1016/j.celrep.2020.108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin R., Jones A.T., Hoffmann H.H., Schafer A., Kao K.S., Francis R.L., Sheahan T.P., Baric R.S., Rice C.M., Ravetch J.V., Bournazos S. Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy. Nature. 2021;599:465–470. doi: 10.1038/s41586-021-04017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yang M., Peng Y., Liang Y., Wei J., Xing L., Guo L., Li X., Li J., Wang J., et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat. Microbiol. 2022;7:423–433. doi: 10.1038/s41564-021-01051-2. [DOI] [PubMed] [Google Scholar]
- Yao H., Song Y., Chen Y., Wu N., Xu J., Sun C., Zhang J., Weng T., Zhang Z., Wu Z., et al. Molecular Architecture of the SARS-CoV-2 virus. Cell. 2020;183:730–738.e13. doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Liu H., Wu N.C., Wilson I.A. Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies. Biochem. Biophys. Res. Commun. 2021;538:192–203. doi: 10.1016/j.bbrc.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Liu H., Wu N.C., Lee C.C.D., Zhu X., Zhao F., Huang D., Yu W., Hua Y., Tien H., et al. Structural basis of a shared antibody response to SARS-CoV-2. Science. 2020;369:1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Wu N.C., Zhu X., Lee C.C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., et al. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183:739–751. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalevsky J., Chamberlain A.K., Horton H.M., Karki S., Leung I.W.L., Sproule T.J., Lazar G.A., Roopenian D.C., Desjarlais J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Hao X., Ma J., Wang M., Li Y., Liu Y., Zhao D., Zhang W., Li C., Yan L., et al. Phase 1 safety and pharmacokinetics studies of BRII-196 and BRII-198, SARS-CoV-2 spike-targeting monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.07.21.21260964. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/s1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yuan M., Song G., Beutler N., Shaabani N., Huang D., He W.T., Zhu X., Callaghan S., Yong P., et al. A human antibody reveals a conserved site on beta-coronavirus spike proteins and confers protection against SARS-CoV-2 infection. Sci. Transl. Med. 2022;14:eabi9215. doi: 10.1126/scitranslmed.abi9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liu Z., Li S., Xu W., Zhang Q., Silva I.T., Li C., Wu Y., Jiang Q., Liu Z., et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 2021;34:108699. doi: 10.1016/j.celrep.2021.108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Deng Y.Q., Zhang R.R., Cui Z., Sun C.Y., Fan C.F., Xing X., Huang W., Chen Q., Zhang N.N., et al. Double lock of a potent human therapeutic monoclonal antibody against SARS-CoV-2. Natl. Sci. Rev. 2021;8:nwaa297. doi: 10.1093/nsr/nwaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar T., Loos C., Fischinger S., Atyeo C., Wang C., Slein M.D., Burke J., Yu J., Feldman J., Hauser B.M., et al. Compromised humoral functional evolution Tracks with SARS-CoV-2 mortality. Cell. 2020;183:1508–1519.e12. doi: 10.1016/j.cell.2020.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schafer A., Reidy J.X., Trivette A., Nargi R.S., et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C., et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 2020;26:1422–1427. doi: 10.1038/s41591-020-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]