Abstract
Subjective memory complaints (SMCs) may be an important early indicator of cognitive aging and preclinical Alzheimer’s disease (AD) risk. The present study investigated whether age-related differences in right or left hippocampal volume underlie SMCs, if these relationships differ by hypertension status, and how they are related to objective memory performance in a group of 190 healthy older adults, ages 50–89. Analyses revealed a significant mediation of the relationship between age and mild SMCs by right hippocampal volume that was moderated by hypertension status. This moderated mediation effect was not observed with left hippocampal volume. Additionally, a moderated serial mediation model showed that age predicted right hippocampal volume, which predicted SMCs, and in turn predicted objective memory performance on several measures of verbal selective reminding in individuals with hypertension, but not in non-hypertensives. Together, these findings suggest that even mild SMCs, in the context of hypertension, provide an early indicator of cognitive aging, reflecting a potential link between vascular risk, SMCs, and the preclinical risk for AD.
Keywords: Cognitive aging, Subjective memory decline, Hypertension, Hippocampal volume, Preclinical Alzheimer’s disease risk, Memory
1. Introduction
Subjective memory complaints (SMCs) are self-reported, perceived declines in memory abilities (Jessen et al., 2014) and are generally associated with older age (Jonker et al., 2000). SMCs may be an important early indicator of cognitive aging, reflecting subtle cognitive changes that are not yet detected by objective tests (Kaup et al., 2015; Jessen et al., 2014; Mitchell et al., 2014; Reisberg et al., 2010; Schmand et al., 1996). As such, SMCs may represent a preclinical stage in the development of cognitive decline related to Alzheimer’s disease (AD; Reisberg & Gauthier, 2008). In fact, longitudinal studies have found individuals with SMCs are significantly more likely to subsequently develop mild cognitive impairment (MCI) or dementia than those without complaints (Kaup et al., 2015; Jessen et al., 2014; Mitchell et al., 2014; Reisberg et al., 2010; Schmand et al., 1996). While some studies have not observed a relationship between SMCs and concurrent objective memory performance (Cook & Marsiske, 2006; Mendes et al., 2008), meta-analyses have found that memory complaints are significantly associated with objective memory in healthy older adults (Burmester et al., 2016; Crumley et al., 2014). Heterogeneity of methodology, including differences in participant characteristics and differing definitions of SMCs, may account for some of the discrepancies in findings across studies (Burmester et al., 2016; Mitchell et al., 2014).
Reductions in total and regional gray matter volume, particularly in the medial temporal, frontotemporal, and other neocortical regions have been associated with SMCs (Jessen et al., 2006; Lee et al., 2016; Saykin et al., 2006; Stewart et al., 2008). Notably, when compared to those without complaints, individuals with SMCs have decreased volumes of the hippocampus, an important neuroanatomical structure for objective memory and an early hallmark feature of AD (Scheef et al., 2012; Stewart et al., 2008; Striepens et al., 2010; van der Fier et al., 2004). In addition, Scheef and colleagues (2012) found lateralized differences in the association between hippocampal volume and SMCs. They observed that older adults with SMCs had decreased right hippocampal volume and, after a two to three-year follow-up, showed greater decline on objective memory measures than those who did not have complaints. However, left hippocampal volume was not significantly associated with SMCs in their study. This suggests that hippocampal volume, particularly in the right hemisphere, may underlie SMCs in healthy older adults.
Hypertension is a common age-related vascular risk factor, reported to be prevalent in approximately 70.1% of the older adult population aged 65 years and older (McDonald et al., 2009). This vascular risk factor has been associated with total and regional gray matter volume reduction (Beauchet et al., 2013; Jennings et al., 2012; Raz et al., 2005), especially in the prefrontal cortex and hippocampus (Beauchet et al., 2013; Raz et al., 2003), and these effects can occur even when hypertension is treated (Firbank et al., 2007; Jennings et el., 2012; Kern et al., 2017; Raz et al., 2003; Salerno et al., 1992; Strassburger et al., 1997). Recent findings from the SPRINT MIND study (Kjelden et al., 2018) have found that changing the target for systolic blood pressure to be below 120 mmHg, instead of 140 mmHg, significantly reduced the risk of subsequently developing MCI or dementia. However, the mechanism of how hypertension and other vascular risk factors contribute to the development of AD remains unclear.
While hypertension has been found to be a risk factor for MCI and AD (Kivipelto et al., 2002; Kjelden, et al., 2018; Luchsinger et al., 2005; Wu et al., 2003), fewer studies have specifically considered the effects of hypertension on memory performance in healthy cognitive aging research (Alexander et al., 2012b). We have previously found that the interaction between hypertension status and mild SMCs affect objective memory performance in a healthy older adult sample (Nguyen et al., 2016). Specifically, individuals with treated hypertension and mild SMCs performed significantly poorer on objective memory measures than non-hypertensives and those with hypertension that did not have SMCs. These findings suggest that hypertension status modifies the relationship between mild SMCs and objective memory performance and may contribute to the heterogeneity in findings on the relation between SMCs and objective memory performance in healthy older adults (Nguyen et al., 2016). We sought to extend these findings by examining whether hippocampal volume underlies SMCs in healthy older adults, differs by hypertension status, and leads to differences in objective memory performance.
In the present study, we used moderated mediation analyses to investigate whether the relationship between age and mild SMCs is mediated by hippocampal volume and moderated by hypertension status in a sample of healthy older adults. Such moderated mediation analyses (Hayes, 2017) allow, in one model, tests of indirect and direct effects to investigate how the relationships between hippocampal volume, SMCs, and objective memory performance may differ between hypertensive and non-hypertensive groups. Studies investigating the asymmetry of hippocampal volume decline in aging and AD have produced mixed findings. Some studies have found left hippocampal volume exhibits more atrophy, whereas others demonstrated right hippocampal volume is preferentially affected (Minkova et al., 2017). Given the possible asymmetry of hippocampal volume atrophy in aging and AD, as well as lateralized differences of the hippocampus in the association with SMCs, we investigated these relationships by testing right and left hippocampal volumes separately. Further, we utilized moderated serial mediation models in order to examine if these moderated mediations lead to differences in objective memory performance. We hypothesized age would be associated with reduced hippocampal volume, which in turn would be related to more SMCs that would then predict poorer objective memory performance, in individuals with hypertension, but not in non-hypertensives.
2. Method
2.1. Participants
Participants were 190 community dwelling healthy adults, 50–89 years of age, with an average age of 71.07 years (SD = 9.65), who volunteered in a study on healthy cognitive aging. The sample was predominantly Caucasian (94.74%), with 94 female participants (49.47%) and an average of 15.94 years (SD = 2.61) of education. The cohort had an average Mini Mental Status Exam (MMSE; Folstein et al., 1975) score of 28.93 (SD = 1.24) and Wechsler Adult Intelligence Scale (WAIS-IV; Wechsler, 2008) Full-Scale IQ (FSIQ) score of 112.78 (SD = 12.50). To exclude significant neurological, medical, and psychiatric disorders, participants underwent an extensive medical screen, and a physical and neurological examination performed by a neurologist (GAH), who specializes in aging. Systolic and diastolic blood pressures were obtained at rest, while the participants were seated. Participants were excluded if they had a MMSE score less than 26 or a Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960) score greater than 9. All procedures were approved by the Institutional Review Board at the University of Arizona and all participants provided informed written consent. Participants were grouped as hypertensives and non-hypertensives based on self-reported history of hypertension and treatment for hypertension. Participants reported the duration of their hypertension diagnosis and number of hypertension medications. The group mean hypertension duration was 27.39 months (SD = 38.13) and the mean number of medications was 1.21 (SD = .74). The hypertensive and non-hypertensive groups did not differ in demographic, cognitive and neuroimaging characteristics, as shown in Table 1, but the hypertensive group had higher mean resting systolic and diastolic blood pressures than the non-hypertensives.
Table 1.
Table of participant demographic, cognitive, and neuroimaging characteristics.
| Variable | Hypertensive | Non-Hypertensive | P |
|---|---|---|---|
| N | 67 | 123 | |
| Age [years; M (SD)] | 72.27 (10.09) | 70.42 (9.39) | .209 |
| Education [years; M (SD)] | 15.73 (2.56) | 16.06 (2.64) | .413 |
| Systolic Blood Pressure [M (SD)] | 149.39 (16.88) | 137.86 (16.54) | .0001 |
| Diastolic Blood Pressure [M (SD)] | 85.51 (11.21) | 77.93 (8.70) | .0001 |
| Sex (F/M) | 30/37 | 64/59 | .211 |
| WAIS-IV FSIQ [M (SD)] | 111.72 (12.95) | 113.37 (12.26) | .351 |
| MMSE [M (SD)] | 28.91 (1.22) | 28.93 (1.25) | .897 |
| GDS [M (SD)] | .99 (1.42) | 1.10 (1.89) | .669 |
| MFQ SMC score [M (SD)] | 4.94 (1.21) | 5.13 (1.12) | .288 |
| SRT Sum Recall [M (SD)] | 102.43 (20.20) | 102.56 (19.54) | .966 |
| SRT LTR [M (SD)] | 83.63 (30.49) | 82.93 (29.48) | .879 |
| SRT CLTR [M (SD)] | 59.96 (37.36) | 59.25 (35.39) | .898 |
| SRT STR [M (SD)] | 18.60 (11.60) | 19.49 (11.39) | .609 |
| SRT Delayed Recall [M (SD)] | 7.79 (2.64) | 7.93 (2.85) | .748 |
| WMHa [M (SD)] | .06 (.97) | .01 (.94) | .737 |
| Average Hippocampal Volumeb [M (SD)] | −.18 (.93) | .05 (1.02) | .134 |
| Right Hippocampal Volumeb [M (SD)] | −.18 (.85) | .04 (1.06) | .146 |
| Left Hippocampal Volumeb [M (SD)] | −.16 (1.02) | .06 (.98) | .160 |
Means (standard deviations) from hypertensive and non-hypertensives with statistical comparisons between the groups.
Log Transformed and TIV-adjusted standardized values.
TIV-adjusted standardized values. WAIS-IV FSIQ = Wechsler Adult Intelligence Scale-Fourth Edition Full-Scale IQ, MMSE = Mini Mental Status Exam, GDS = Geriatric Depression Scale, MFQ SMC score = Memory Functioning Questionnaire Subjective Memory Complaint score, SRT = Selective Reminding Test, LTR = Long-Term Retrieval, CLTR = Consistent Long-Term Retrieval, STR = Short-Term Retrieval, WMH = white matter hyperintensities, TIV = total intracranial volume.
2.2. Subjective memory complaints
SMCs were measured using a portion of the Memory Functioning Questionnaire (MFQ), which is considered a reliable measure for evaluating SMCs (Gilewski et al., 1990). For the purpose of this study, we focused on the general question asking participants to rate their overall problems with memory, if at all, on a 1–7 scale, with lower SMC scores indicating greater severity of complaints (Gilewski et al., 1990). Participants’ MFQ SMC scores in this healthy older adult sample ranged from mild complaints to no complaints with an average of 5.07 (SD = 1.16).
2.3. Objective Memory
The 12-item, 12-trial version of the Selective Reminding Test (SRT; Buschke, 1973) was administered as part of a larger neuropsychological battery. The SRT is a verbal list-learning task that provides multiple measures of learning and memory. During this task, participants were asked to remember a list of 12 unrelated words, immediately recalling the words after each trial. They were selectively reminded of only the words that were not recalled on the previous trial. After a 30-minute delay, participants were asked to recall all 12 words again and were then given a recognition memory test. For the present study, we focused on the recall measures from the SRT, which included total sum recall (total number of words recalled across all immediate trials), short-term retrieval (STR; words recalled that were in short-term storage), long-term retrieval (LTR; words recalled that have entered long-term storage), consistent long-term retrieval (CLTR; words consistently recalled without interruption), and delayed recall (words recalled after the 30 minute delay).
2.4. Magnetic Resonance Imaging
Volumetric T1-weighted Spoiled Gradient Echo (SPGR) MRI scans (slice thickness = 1.0mm, TR = 5.3ms, TE = 2.0ms, TI = 500ms, FA = 15°, matrix = 256×256, FOV = 25.6cm) and T2 Fluid-Attenuated Inversion Recovery (FLAIR) scans (slice thickness = 2.6mm, TR = 11000ms, TE = 120ms, TI = 2250ms, flip angle = 90°, matrix = 256×256, FOV = 25.0cm) were acquired on a 3T GE Signa scanner (HD Signa Excite, General Electric, Milwaukee, WI).
T1-weighted 3T volumetric MRIs were processed using FreeSurfer v5.3 software to obtain right and left hippocampal volumes (Dale et al., 1999; Fischl et al., 2002; Fischl et al., 2004). Total intracranial volume (TIV) was computed for each participant in native brain space using T1 scans with SPM12 (Alexander et al., 2012a). TIV-adjusted right and left hippocampal volumes were used in the analyses. White matter hyperintensities (WMH) were computed using T1 and T2-FLAIR scans and the lesion segmentation toolbox (Schmidt et al., 2012) with Statistical Parametric Mapping (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK). The probability maps for WMH were thresholded at 1, and voxel volumes were summed to compute the total WMH volume in milliliters (ml). WMH volumes were log transformed and adjusted for TIV. In addition, individuals who were ±2.5 or more standard deviations away from the mean WMH volume were excluded from the analyses (n=2).
2.5. Statistical Analyses
Differences in cognitive performance, demographic, and imaging variables between hypertensive and non-hypertensive groups were evaluated using independent t-tests. Sex distribution differences were compared with chi-square tests.
All mediation analyses were performed using the PROCESS macro for SPSS (v3.1; Hayes, 2017), using percentile bootstrap resampling with 10,000 iterations to produce 95% confidence intervals, which indicate significance when they do not include zero. Moderated mediation models tested the mediation of the relationship between age and SMC score by TIV-adjusted right or left hippocampal volume, with hypertension status as the moderator. Sex and education were subsequently included as covariates, as these demographic characteristics have been shown to influence memory performance. Hypertension duration, total WMH volume, and measured systolic blood pressure (SBP) were also subsequently included as covariates to account for hypertension severity and blood pressure variability. SRT sum recall was added as an additional covariate, in an effort to ensure that our results with SMC score as the outcome were not solely due to underlying differences in objective memory. Finally, WAIS-IV FSIQ was separately added as an additional follow-up covariate, to test that the results with SMC score as the outcome were not due to differences in general cognitive ability.
To further examine if moderated mediation effects lead to differences in objective memory performance, moderated serial mediation models were then used to test the mediation of the relationship between age and objective memory performance by right hippocampal volume and SMC score, with hypertension status as the moderator. Sex, education, hypertension duration, total WMH volume, and SBP were included as covariates. WAIS-IV FSIQ was separately added as a follow-up covariate, to ensure that the results with objective memory performance as the outcome were not due to underlying differences in general cognitive ability.
3. Results
3.1. Moderated Mediations
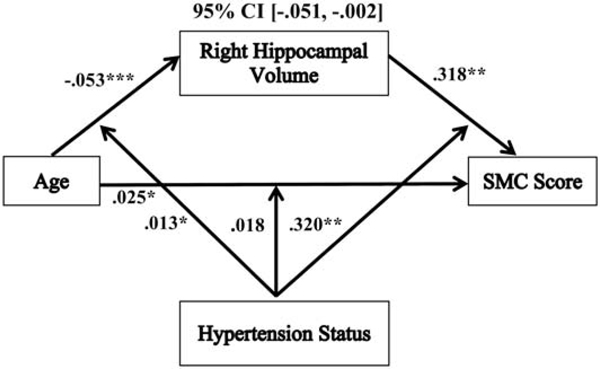
A moderated mediation model revealed that the mediation of the relationship between age and SMC score by right hippocampal volume was moderated by hypertension status (−.032 (SE= .015), 95% CI, [−.062, −.005]). Individuals with hypertension showed negative indirect effects of age on SMC score through right hippocampal volume (−.032 (SE= .012), 95% CI, [−.057, −.011]), but in non-hypertensives, right hippocampal volume did not significantly mediate this relationship between age and SMC score (−.0001 (SE= .008), 95% CI, [−.018, .015]). These findings remained significant after including sex, education, hypertension duration, WMH volume, SBP, and SRT sum recall as covariates (−.026 (SE= .013), 95% CI, [−.051, −.002]; see Table 2). In addition, this moderated mediation model remained significant after we added WAIS-IV FSIQ as an additional follow-up covariate (see Table 2). There were no significant moderated mediations between age and SMC score by left hippocampal volume, with (−.013 (SE= .013), 95% CI, [−.039, .011]; see Supplemental Figure 1) or without sex, education, hypertension duration, WMH volume, SBP, and SRT sum recall as covariates (−.016 (SE= .014), 95% CI, [−.044, .012]), by hypertension status. This moderated mediation model was not significant after adding WAIS-IV FSIQ as an additional covariate.
Table 2.
Conditional indirect effects of the moderated mediation models of age predicting subjective memory complaints through right and left hippocampal volume and moderated by hypertension status.
| Hippocampal volume | Hypertension Status | Effect | SE | LLCI | ULCI |
|---|---|---|---|---|---|
| Right hippocampus | Hypertensive | −.026 | .010 | −.048 | −.008 * |
| Non-hypertensive | .000 | .008 | −.015 | .016 | |
|
| |||||
| Left hippocampus | Hypertensive | −.007 | .009 | −.026 | .011 |
| Non-hypertensive | .006 | .009 | −.009 | .025 | |
Conditional indirect effects of the moderated mediation models for hypertensives and non-hypertensives, with sex, education, hypertension duration, white matter hyperintensities, systolic blood pressure, and SRT sum recall as covariates.
This effect remained significant after additionally adjusting for WAIS-IV FSIQ performance. Independent variable (x) = age, mediator (m) = hippocampal volumes (i.e., right and left hippocampal volumes), dependent variable (y) = SMC score, moderator (w) = hypertension status. CI = confidence interval; LLCI = lower limit confidence interval; ULCI = upper limit confidence interval. Confidence intervals indicate significance when they do not include 0. Bolded indirect effects are significant.
Examination of the individual associations of the significant moderated mediation model (see Figure 1) showed that there was a significant overall positive direct relationship between age and SMC score (.025 (SE= .012), p = .045, 95% CI, [.001, .049]), but this was not moderated by hypertension status (.018 (SE= .011), p = .101, 95% CI, [−.004, .040]). For indirect associations, there was a significant overall negative relationship between age and right hippocampal volume (−.053 (SE= .008), p < .0001, 95% CI, [−.068, −.038]), and this path was significantly moderated by hypertension status (.013 (SE= .006), p = .046, 95% CI, [.0002, .025]). In hypertensives, the relationship between age and right hippocampal volume was significantly negative (−.040 (SE= .011), p = .0003, 95% CI, [−.061, −.019]) and this negative association was greater in non-hypertensives (−.065 (SE= .009), p < .0001, 95% CI, [−.083, −.048]). Additionally, there was a significant overall positive relationship between right hippocampal volume and SMC score (.318 (SE= .120), p = .009, 95% CI, [.082, .554]), and this path was significantly moderated by hypertension status (.320 (SE= .117), p = .007, 95% CI, [.089, .551]). In hypertensives, the relationship between right hippocampal volume and SMC score was significantly positive (lower SMC scores indicate greater memory complaints; .638 (SE= .203), p = .002, 95% CI, [.238, 1.04]), but in non-hypertensives, this path was not significant (−.002 (SE= .121), p = .990, 95% CI, [−.241, .238]).
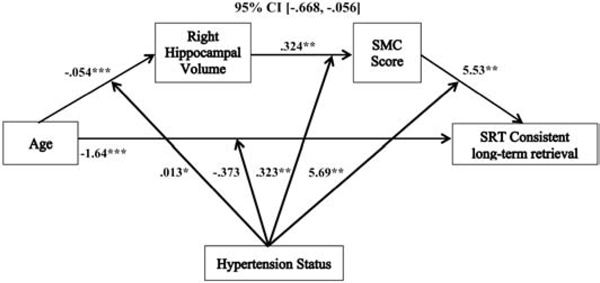
Figure 1.
The relationship between age and SMC score mediated by right hippocampal volume and moderated by hypertension status. Coefficients of a moderated mediation model with sex, education, hypertension duration, white matter hyperintensities, systolic blood pressure and SRT sum recall as covariates. Lower SMC scores indicate greater memory complaints. *p < .05, **p<. 01, ***p <. 001. SMC = subjective memory complaint.
3.2. Moderated Serial Mediations
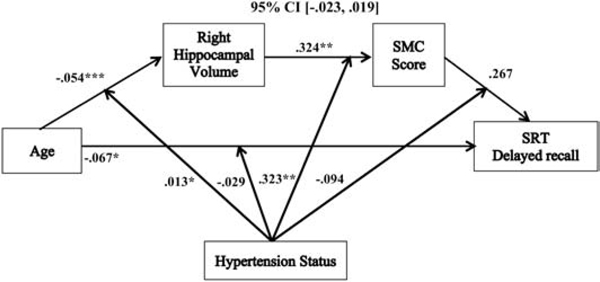
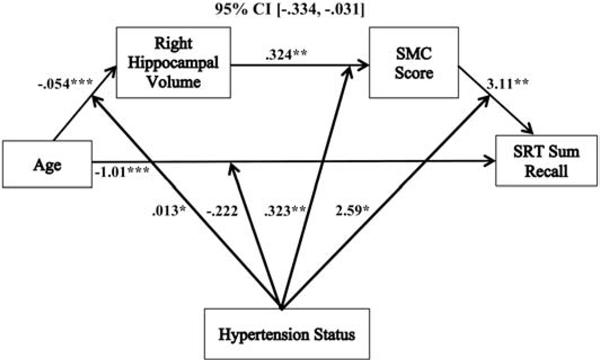
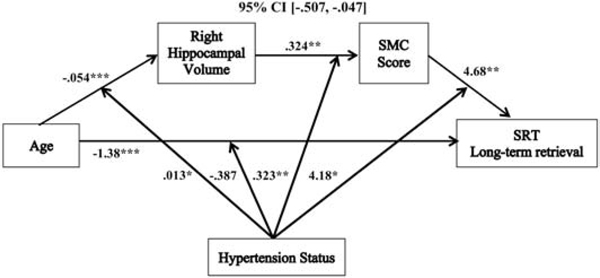
Moderated serial mediation models revealed that the relationships between age and SRT sum recall (−.151 (SE= .078), 95% CI, [−.334, −.031]), short-term retrieval (.083 (SE= .046) 95% CI, [.005, .186]), long-term retrieval (−.235 (SE= .120), 95% CI, [−.507, −.047]), and consistent long-term retrieval (−.297 (SE= .157), 95% CI, [−.668, −.056]) were each mediated by right hippocampal volume and SMC score, and moderated by hypertension status, after controlling for sex, education, hypertension duration, WMH volume, and SBP (see Table 3). These moderated serial mediation results remained significant after we added WAIS-IV FSIQ as an additional follow-up covariate (see Table 3). There was not a significant moderated serial mediation of the relationship between age and delayed recall in relation to hypertension status (see Table 3 & Figure 6) with the same covariates added (−.005 (SE= .010), 95% CI, [−.023, .019]); and this was not significant after adding WAIS-IV FSIQ as an additional covariate.
Table 3.
Conditional indirect effects of the moderated serial mediation models of age predicting objective memory performance through right hippocampal volume and subjective memory complaints in relation to hypertension status.
| Objective memory measures | Hypertension status | Effect | SE | LLCI | ULCI |
|---|---|---|---|---|---|
| SRT Sum recall | Hypertensive | −.151 | .077 | −.331 | −.033 * |
| Non-hypertensive | .000 | .011 | −.023 | .027 | |
|
| |||||
| SRT Short-term retrieval | Hypertensive | .083 | .045 | .007 | .185 * |
| Non-hypertensive | .000 | .006 | −.015 | .013 | |
|
| |||||
| SRT Long-term retrieval | Hypertensive | −.235 | .118 | −.506 | −.049 * |
| Non-hypertensive | .000 | .017 | −.033 | .041 | |
|
| |||||
| SRT Consistent long-term retrieval | Hypertensive | −.297 | .156 | −.664 | −.060 * |
| Non-hypertensive | .000 | .021 | −.043 | .046 | |
|
| |||||
| SRT Delayed recall | Hypertensive | −.005 | .010 | −.021 | .019 |
| Non-hypertensive | .000 | .003 | −.007 | .008 | |
Conditional indirect effects of the moderated serial mediation models for hypertensives and non-hypertensives, with sex, education, hypertension duration, white matter hyperintensities, and systolic blood pressure as covariates.
These effects remained significant after additionally adjusting for WAIS-IV FSIQ performance. Independent variable (x)= age, mediator 1 (m1) = right hippocampal volumes, mediator 2 (m2) = SMC score, dependent variable (y) = objective memory performance, moderator (w) = hypertension status. CI = confidence interval; LLCI = lower limit confidence interval; ULCI = upper limit confidence interval; SRT = selective reminding test. Confidence intervals indicate significance when they do not include 0. Bolded indirect effects are significant.
Figure 6.
The relationship between age and delayed recall mediated by right hippocampal volume and SMC score and moderated by hypertension status. Coefficients of a moderated serial mediation model with sex, education, hypertension duration, white matter hyperintensities, and systolic blood pressure as covariates. Lower SMC scores indicate greater memory complaints. *p < .05, **p <. 01, ***p <. 001, SMC = subjective memory complaint, SRT = selective reminding task.
With the exception of short-term retrieval, the moderated serial mediations followed the same pattern: increasing age predicted reduced right hippocampal volume, which was associated with more SMCs, and in turn predicted poorer objective memory performance (see Figures 2, 4, & 5). These serial mediation patterns were only significant in hypertensives, and not non-hypertensives (see Table 3). Additionally, the individual associations of the moderated serial mediations for sum recall, long-term retrieval, and consistent long-term retrieval all shared similar patterns. Each of these models had a significant overall negative direct relationship between age and memory performance for sum recall (−1.01 (SE= .163), p < .0001, 95% CI, [−1.34, −.693]), long-term retrieval (−1.38 (SE= .254), p < .0001, 95% CI, [−1.88, −.875]), and consistent long-term retrieval (−1.64 (SE= .313), p < .0001, 95% CI, [−2.25, −1.02]). The direct associations between age and memory performance were not significantly moderated by hypertension status for sum recall (−.222 (SE= .158), p = .162, 95% CI, [−.535, .090]), long-term retrieval (−.387 (SE= .246), p = .118, 95% CI, [−.872, .099]), and consistent long-term retrieval (−.373 (SE= .304), p = .222, 95% CI, [−.972, .227]).
Figure 2.
The relationship between age and sum recall mediated by right hippocampal volume and SMC score and moderated by hypertension status. Coefficients of a moderated serial mediation model with sex, education, hypertension duration, white matter hyperintensities, and systolic blood pressure as covariates. Lower SMC scores indicate greater memory complaints. *p < .05, **p <. 01, ***p <. 001, SMC = subjective memory complaint, SRT = selective reminding task.
Figure 4.
The relationship between age and long-term retrieval mediated by right hippocampal volume and SMC score and moderated by hypertension status. Coefficients of a moderated serial mediation model with sex, education, hypertension duration, white matter hyperintensities, and systolic blood pressure as covariates. Lower SMC scores indicate greater memory complaints. *p < .05, **p <. 01, ***p <. 001, SMC = subjective memory complaint, SRT = selective reminding task.
Figure 5.
The relationship between age and consistent long-term retrieval mediated by right hippocampal volume and SMC score and moderated by hypertension status. Coefficients of a moderated serial mediation model with sex, education, hypertension duration, white matter hyperintensities, and systolic blood pressure as covariates. Lower SMC scores indicate greater memory complaints. *p < .05, **p <. 01, ***p <. 001, SMC = subjective memory complaint, SRT = selective reminding task.
For the indirect associations in these models, the relationships between age and right hippocampal volume, and between right hippocampal volume and SMC score, followed the same pattern as described in the moderated mediation, above. The final indirect paths in the models revealed that there were overall positive relationships between SMC score and objective memory for sum recall (3.11 (SE= 1.09), p = .005, 95% CI, [.949, 5.27]), long-term retrieval (4.68 (SE= 1.70), p = .007, 95% CI, [1.33, 8.03]), and consistent long-term retrieval (5.53 (SE= 2.10), p = .009, 95% CI, [1.39, 9.67]). These associations were moderated by hypertension status for sum recall (2.59 (SE= 1.12), p = .021, 95% CI, [.392, 4.79]), long-term retrieval (4.18 (SE= 1.73), p = .017, 95% CI, [.762, 7.60]), and consistent long-term retrieval (5.69 (SE= 2.14), p = .009, 95% CI, 1.47, 9.91]). In hypertensives, the relationships between SMC score and performance were significantly positive (lower SMC scores indicate greater memory complaints) for sum recall (5.70 (SE= 1.78), p = .002, 95% CI, [2.20, 9.20]), long-term retrieval (8.86 (SE= 2.76), p = .002, 95% CI, [3.42, 14.30]), and consistent long-term retrieval (11.22 (SE= 3.41), p = .001, 95% CI, [4.50, 17.94]). In non-hypertensives, these paths were not significant for sum recall (.514 (SE= 1.31), p = .696, 95% CI, [−2.08, 3.11]), long-term retrieval (.500 (SE= 2.04), p = .807, 95% CI, [−3.53, 4.53]), and consistent long-term retrieval (−.160 (SE= 2.52), p = .950, 95% CI, [−5.14, 4.82]).
Within short-term retrieval, increasing age predicted reduced right hippocampal volume, which was associated with more SMCs, and in turn predicted higher scores on short-term retrieval (see Figure 3). Again, this mediation pattern was only significant in hypertensives, not non-hypertensives (see Table 3). When examining the individual associations, there was a significant overall positive direct relationship between age and short-term retrieval (.383 (SE= .105), p = .0003, 95% CI, [.176, .590]) that was not significantly moderated by hypertension status (.166 (SE= .102), p = .105, 95% CI, [−.035, .367]).
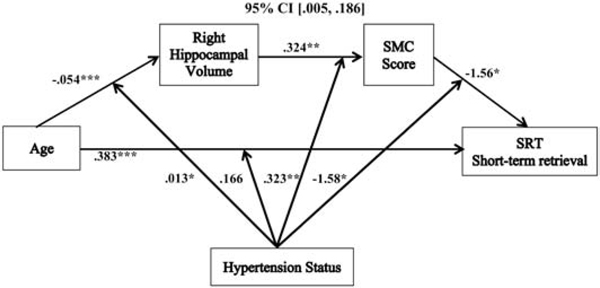
Figure 3.
The relationship between age and short-term retrieval mediated by right hippocampal volume and SMC score and moderated by hypertension status. Coefficients of a moderated serial mediation model with sex, education, hypertension duration, white matter hyperintensities, and systolic blood pressure as covariates. Lower SMC scores indicate greater memory complaints. *p < .05, **p <. 01, ***p <. 001, SMC = subjective memory complaint, SRT = selective reminding task.
The indirect associations between age and right hippocampal volume, and between right hippocampal volume and SMC score, also followed the same pattern as described in the moderated mediation, above. However, the last indirect path displayed a different association than the other moderated serial mediation models. This path revealed that there was an overall negative relationship between SMC score and short-term retrieval (−1.56 (SE= .703), p = .028, 95% CI, [−2.94, −.167]) that was moderated by hypertension status (−1.58 (SE= .717), p = .029, 95% CI, [−3.00, −.167]). In hypertensives, the relationship between SMC score and short-term retrieval was significantly negative (−3.14 (SE= 1.14), p = .007, 95% CI, [−5.39, −.885]), but in non-hypertensives, this path was not significant (.028 (SE= .845), p = .974, 95% CI, [−1.64, 1.70]).
4. Discussion
In a sample of cognitively unimpaired older adults, we found that the combination of age-related mild SMCs and hypertension has a neuroanatomical substrate, reflected by reduced right hippocampal volume. Thus, mild SMCs in generally healthy cognitive aging may be sensitive to right hippocampal volume differences and this effect depends on hypertension status. Follow-up analyses using moderated serial mediation models, found that older adults with hypertension had reduced right hippocampal volumes, leading to more SMCs, which in turn, predicted significantly poorer performance on multiple indices of learning and memory. In contrast, the serial mediation models were not observed in individuals without hypertension. All moderated mediation effects were significant even while controlling for hypertension severity and blood pressure variability, suggesting that the results represent differences associated with the longstanding impact of hypertension. That we did not observe group differences in the right hippocampal volume simply between the hypertensives and non-hypertensives, but rather only through its mediational role in predicting SMCs, highlights the importance of the mediation approach for detecting these very early effects in the context of healthy cognitive aging. A previous study found right hippocampal volume was associated with memory complaints in healthy older adults (Scheef et al., 2012), yet we found hypertension status modified this relationship, which suggests implications for potential mechanistic links between vascular risk and the progression of preclinical AD (Brickman, 2013). Our findings from the present study suggest even mild SMCs may add to the preclinical risk for AD, in the context of hypertension.
With the exception of short-term retrieval, the moderated serial mediation models all followed the same pattern, showing that smaller right hippocampal volumes were associated with more SMCs, which was then related to poorer objective memory performance. However, for short-term retrieval, smaller right hippocampal volumes led to more memory complaints, which in turn, was associated with an increase in performance. We previously suggested that such an increase in short-term retrieval might reflect greater reliance on inefficient learning strategies, potentially related to compensation for poorer long-term retrieval (Nguyen et al., 2016). The current findings further support this possibility, providing a shared anatomical basis for both the observed compensatory increase in short-term retrieval and the corresponding decreases in long-term retrieval measures. Greater reductions in medial temporal lobe volumes may influence consolidation of information from short-term to long-term memory (Nguyen et al., 2016). The current results indicate that this inefficient learning may result from smaller right hippocampal volumes, which in turn contributes to poorer performance on longer-term retrieval measures.
The existing literature investigating the asymmetry of hippocampal volume atrophy in aging and AD have produced mixed findings. While some studies have found left hippocampal volume exhibits greater atrophy, others have demonstrated that right hippocampal volumes show larger reductions (Minkova et al., 2017). In a recent meta-analysis, Minkova and colleagues (2017) observed greater right hippocampal atrophy in individuals with MCI, but greater left hippocampal atrophy in those with AD dementia. This suggests the possibility of an accelerated decline of the right hippocampus in the earlier stages of AD, whereas the left hippocampus may be more affected at later stages. In addition, our present findings indicate that mild SMCs may be sensitive to decreases in right, but not left, hippocampal volume in healthy older adults with hypertension. As SMCs may represent a preclinical stage in the course of AD (Reisberg & Gauthier, 2008), the current results, along with previous findings, suggest right hippocampal volume may be preferentially affected in the preclinical early stages of AD. It is also possible that these relationships may emerge for left hippocampal volume if studied in a larger cohort of healthy older adults, providing greater power to detect smaller effects. Additional studies with larger samples and a wider range of cognitive difficulties are needed to further examine the observed lateralized differences.
Previous findings have indicated even individuals with treated hypertension are at an increased risk for subsequent development of MCI and dementia (Kjelden, et al., 2018; Luchsinger et al., 2005). High blood pressure has detrimental effects on blood vessels, causing them to thicken and narrow, which can increase risk for ischemia and blockages or ruptures of the vessels (Cifuentes et al., 2015; Kennelly, Lawlor, & Kenny, 2009). However, the mechanism of how vascular risk factors, like hypertension, may contribute to the development of AD is not fully understood. Some studies suggest that cerebrovascular disease increases amyloid deposition (Carnevale et al., 2012; Gomez et al., 2018), whereas others indicate amyloid and vascular risk may have interactive effects on the clinical presentation of AD (Bangen et al., 2017; Lo & Jagust, 2012; Provenzano et al., 2014). The hippocampus is particularly vulnerable to vascular impacts (Schmidt-Kastner & Freund, 1991), and hippocampal volume atrophy and the accompanied memory loss are early hallmark features of AD. The results from the current study raise the possibility that older adults with hypertension experience ischemic damage that is associated with reduced hippocampal volume, which may in turn promote differences in subjective and objective memory that places these older adults with hypertension at greater risk for AD. These findings add to the growing body of literature on the vascular mechanisms of AD, suggesting a possible connection between hypertension and preclinical risk for AD. As some hippocampal subfields are more sensitive than others to aging and hypertension (Small et al., 2011), future studies measuring volumes of hippocampal subfields may provide further insight into the mechanisms of hypertension’s impact on AD-related pathology.
Older adults with hypertension may benefit from further control of blood pressure. Recent results from the SPRINT MIND study have indicated that more aggressive control of hypertension reduces the risk of cognitive decline and has led to changes in blood pressure guidelines, which are aimed at controlling blood pressure to below 120/80 mmHg (Kjelden, et al., 2018). Given the participants in the current study were being treated for hypertension according to the previous longstanding guidelines (i.e., 140/90 mmHg), these results suggest that older adults with hypertension may benefit from a more intensive approach to blood pressure control to lessen its impact on SMCs and objective memory in older adults, which could potentially reduce AD risk in this population. The average systolic blood pressure in our hypertensives was elevated above recommended guidelines, which may indicate that not all individuals in the hypertensive group had successfully managed their high blood pressure. Further research is needed to better understand how these effects may differ in well-controlled and uncontrolled hypertension (e.g., Kern et al., 2017). It is also important to note that hypertensive risk for dementia may also depend in part on age, as hypertension onset in the oldest-old (80+ years) has been associated with a decreased risk of dementia, compared to those without a history of hypertension (Corrada et al., 2017). Furthermore, when examining the individual path associations in our study, we found that age had a greater negative effect on right hippocampal volume in non-hypertensives than in hypertensives, suggesting the impact of aging on right hippocampal volume may differ as a function of hypertension status and age group. However, the majority of participants in our study were younger than 80 years of age; thus, future studies should examine if the observed relations differ in a larger oldest-old adult sample.
The present study has several limitations. First, it is comprised of mainly Caucasian participants, which may limit the generalizability of the findings. Further research with ethnically diverse populations is needed, particularly in groups where high rates and poor control of hypertension is observed (Delgado et al., 2012; Flack et al., 2003). Second, our sample of generally healthy older adults had limited cognitive difficulties, and the observed associations between subjective and objective memory may differ in samples of individuals with a wider range of cognitive deficits. Future studies in cohorts with varying levels of cognitive ability should investigate whether these relationships may change as a function of cognitive status in healthy aging. Third, our results are limited to verbal objective memory performance. Additional studies are warranted to examine if these results extend to other types of objective memory tests, including tasks that measure non-verbal memory performance. Fourth, this study was cross-sectional in design. While the present study highlights important differences in healthy aging, further research with longitudinal data is needed to understand whether hippocampal differences lead to greater SMCs over time, if this association differs by hypertension status, and whether it, in turn, leads to further decline in objective memory performance. Finally, our inclusion criteria for hypertension included individuals who self-reported a history of hypertension, and while our non-hypertensive group did not report histories of hypertension or hypertension treatment and had an average blood pressure that fell below previous clinical guidelines, their blood pressure measures during the research study visit showed wide variation. Consequently, our non-hypertensive group may have included some individuals with previously undiagnosed hypertension. However, the hypertensive group had significantly higher measured systolic and diastolic blood pressures than the non-hypertensive group. Moreover, previous studies have found the deleterious effects of hypertension are even more pronounced in older adults who have untreated hypertension when compared to those who were treated for, or did not have, hypertension (Kern et al., 2017). Nonetheless, we did not observe mediation effects with our non-hypertensive group, which provides even stronger support for our observation of the right hippocampal volume effects underlying SMCs and leading to the objective memory differences in the hypertensive older adults.
5. Conclusions
In this sample of neurologically healthy older adults, the combination of mild SMCs and hypertension reflected reduced right hippocampal volume that was associated with poorer objective memory performance. Hypertensive older adults may experience mild SMCs as a result of right hippocampal atrophy and an ineffective learning strategy, leading to difficulties with consistent and longer-term memory retrieval and greater reliance on short-term retrieval. Given that hypertension can contribute to Alzheimer’s-related pathology, these findings suggest that even mild memory complaints, in the context of hypertension, may be indicative of increased preclinical risk for AD in healthy older adults. Further research is needed to determine if more intense control of blood pressure may help to alleviate the impact of hypertension on hippocampal atrophy and the associated subjective and objective memory difficulties, thereby modifying the vascular mechanisms in a way that can help diminish the risk for AD.
Supplementary Material
Highlights:
Age-related memory complaints in hypertensives are mediated by right hippocampal volume
Mild memory concerns have a neuroanatomical basis leading to overt memory difficulties
Mild memory complaints with hypertension may be an early marker of cognitive aging
Aging with hypertension and memory concerns may lead to greater preclinical AD risk
Acknowledgements
The authors would like to acknowledge support from the National Institute on Aging (AG025526, AG019610, and AG049464), the state of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation.
Verification:
1. The authors have no conflicts of interest relevant to the subject of this manuscript, including no institutional contracts relating to this research or any other agreements of the authors or their institutions that could be seen as involving a financial interest in this work.
2. This work was supported by the National Institute on Aging (AG025526, AG019610, and AG049464), the state of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation.
3. The data contained in this manuscript have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
4. All subjects provided written informed consent to participate in this study and appropriate approvals were obtained from the participating human subject institutional review boards.
5. All authors have reviewed the contents of the manuscript being submitted and have approved of its content.
Footnotes
Disclosure Statement
The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Alexander GE, Bergfield KL, Chen K, Reiman EM, Hanson KD, Lin L, Bandy D, Caselli RJ, & Moeller JR (2012a). Gray matter network associated with risk for Alzheimer’s disease in young to middle-aged adults. Neurobiol. Aging, 33(12), 2723–2732. doi: 10.1016/j.neurobiolaging.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, & Glisky EL (2012b). Characterizing cognitive aging in humans with links to animal models. Front. Aging Neurosci. 4, 21. doi: 10.3389/fnagi.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Clark AL, Edmonds EC, Evangelista ND, Werhane ML, Thomas KR, Locano LE, Tran M, Zlatar ZZ, Nation DA, Bondi MW, & Delano-Wood L (2017). Cerebral blood flow and amyloid-β interact to affect memory performance in cognitively normal older adults. Front. Aging Neurosci. 9, 181. doi: 10.3389/fnagi.2017.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, & Annweiler C (2013). Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J. Hypertens. 31(8), 1502–1516. doi: 10.1097/HJH.0b013e32836184b5 [DOI] [PubMed] [Google Scholar]
- Brickman AM (2013). Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Curr. Neurol. Neurosci. Rep. 13(12), 415. doi: 10.1007/s11910-013-0415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester B, Leathem J, & Merrick P (2016). Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol. Rev. 26(4), 376–393. doi: 10.1007/s11065-016-9332-2 [DOI] [PubMed] [Google Scholar]
- Buschke H (1973). Selective reminding for analysis of memory and learning. J Verbal Learning and Verbal Behav. 12(5), 543–550. doi: 10.1016/S0022-5371(73)80034-9 [DOI] [Google Scholar]
- Carnevale D, Mascio G, Ajmone-Cat MA, D’Andrea I, Cifelli G, Madonna M, Cocozza G, Frati A, Carullo P, Carnevale L, Alleva E, Branchi I, Lembo G, & Minghetti L (2012). Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol. Aging, 33(1), 205–e19. doi: 10.1016/j.neurobiolaging.2010.08.013 [DOI] [PubMed] [Google Scholar]
- Cifuentes D, Poittevin M, Dere E, Broquères-You D, Bonnin P, Benessiano J, Pocard M , Mariani J, Kubia N, Merkulova-Rainon T, & Lévy BI (2015). Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension, 65(1), 218–224. doi: 10.1161/HYPERTENSIONAHA.114.04139 [DOI] [PubMed] [Google Scholar]
- Cook S, & Marsiske M (2006). Subjective memory beliefs and cognitive performance in normal and mildly impaired older adults. Aging Ment. Health, 10(4), 413–423. doi: 10.1080/13607860600638487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, Brookmeyer R, & Kawas CH (2017). Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimers Dement. 13(2), 103–110. doi: 10.1016/j.jalz.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumley JJ, Stetler CA, & Horhota M (2014). Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychol. Aging, 29(2), 250. doi: 10.1037/a0035908 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. (1999): Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9:179–194. [DOI] [PubMed] [Google Scholar]
- Delgado J, Jacobs EA, Lackland DT, Evans DA, & De Leon CFM (2012). Differences in blood pressure control in a large population-based sample of older African Americans and non-Hispanic whites. J Gerontol. A Biol. Sci. Med. Sci. 67(11), 1253–1258. doi: 10.1093/gerona/gls106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, & Ford GA (2007). Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. J. Neurol. 254(6), 713. doi: 10.1007/s00415-006-0238-4 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33:341–355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V (2004): Automatically parcellating the human cerebral cortex. Cereb. Cortex, 14:11–22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Flack JM, Ferdinand KC, & Nasser SA (2003). Epidemiology of hypertension and cardiovascular disease in African Americans. J. Clin. Hypertens. 5(1), 5–11. doi: 10.1111/j.1524-6175.2003.02152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatri. Res. 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, & Schaie KW (1990). The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol. Aging,5(4),482. [DOI] [PubMed] [Google Scholar]
- Gomez G, Beason-Held LL, Bilgel M, An Y, Wong D, Studenski S, Ferruci L, & Resnick SM (2018). Metabolic syndrome and amyloid accumulation in the aging brain. J. Alzheimers Dis. 1–11. doi: 10.3233/JAD-180297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry, 23(1), 56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Publications. [Google Scholar]
- Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, & Aizenstein H (2012). Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J. Hum. Hypertens. 26(5), 295. doi: 10.1038/jhh.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, Van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellisa KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh O, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M, & Subjective Cognitive Decline Initiative (SCD-I) Working Group. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10(6), 844–852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schikd H, & Scheef L (2006). Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol. Aging, 27(12), 1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Jonker C, Geerlings MI, & Schmand B (2000). Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int. J. Geri. Psychiatry, 15(11), 983–991. doi: [DOI] [PubMed] [Google Scholar]
- Kaup AR, Nettiksimmons J, LeBlanc ES, & Yaffe K (2015). Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology, 85(21), 1852–1858. doi: 10.1212/WNL.0000000000002153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly SP, Lawlor BA, & Kenny RA (2009). Blood pressure and dementia—a comprehensive review. Ther. Adv. Neurol. Diso. 2(4), 241–260. doi: 10.1177/1756285609103483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern KC, Wright CB, Bergfield KL, Fitzhugh MC, Chen K, Moeller JR, Nabizadeh N, Elkind MS, Sacco RL, Stern Y, DeCarli CS, & Alexander GE (2017). Blood pressure control in aging predicts cerebral atrophy related to small-vessel white matter lesions. Front. Aging Neurosci. 9, 132. doi: 10.3389/fnagi.2017.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, & Tuomilehto J, & Nissinen A (2002). Apolipoprotein E ϵ4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 137(3), 149–155. 10.7326/0003-4819-137-3-200208060-00006 [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Narkiewicz K, Burnier M, & Oparil S (2018). Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Press. 27(5), 247. doi: 10.1080/08037051.2018.1507621 [DOI] [PubMed] [Google Scholar]
- Lo RY, & Jagust WJ (2012). Vascular burden and Alzheimer disease pathologic progression. Neurology, 79(13), 1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Ha JK, Park JM, Lee BD, Moon E, Chung YI, Kim JH, Kim HJ, Mun CW, Kim TH, & Kim YH (2015). Impact of apolipoprotein E4 polymorphism on the gray matter volume and the white matter integrity in subjective memory impairment without white matter hyperintensities: voxel-based morphometry and tract-based spatial Statistics Study Under 3-Tesla MRI. J, NeuroImaging, 26(1), 144–149. doi: 10.1111/jon.12207 [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, & Mayeux R (2005). Aggregation of vascular risk factors and risk of incident Alzheimer’s disease. Neurology, 65(4), 545–551. doi: 10.1212/01.wnl.0000172914.08967.dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M, Hertz RP, Unger AN, & Lustik MB (2009). Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among United States adults aged 65 and older. J Gerontol. A Biol. Sci. Med. Sci. 64(2), 256–263. doi: 10.1093/gerona/gln016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes T, Ginó S, Ribeiro F, Guerreiro M, Sousa GD, Ritchie K, & de Mendonça A (2008). Memory complaints in healthy young and elderly adults: reliability of memory reporting. Aging Ment. Health, 12(2), 177–182. doi: 10.1080/13607860701797281 [DOI] [PubMed] [Google Scholar]
- Minkova L, Habich A, Peter J, Kaller CP, Eickhoff SB, & Klöppel S (2017). Gray matter asymmetries in aging and neurodegeneration: A review and meta-analysis. Hum. Brain Mapp. 38(12), 5890–5904. doi: 10.1002/hbm.23772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, & Stubbs B (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr. Scand. 130(6), 439–451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Nguyen LA, Haws KA, Fitzhugh MC, Torre GA, Hishaw GA, & Alexander GE (2016). Interactive effects of subjective memory complaints and hypertension on learning and memory performance in the elderly. Aging Neuropsychol. C. 23(2), 154–170. doi: 10.1080/13825585.2015.1063580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM, & Alzheimer’s Disease Neuroimaging Initiative. (2013). White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 70(4), 455–461. doi: 10.1001/jamaneurol.2013.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, & Acker JD (2005). Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex, 15(11), 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, & Acker JD (2003). Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav. Neurosci. 117(6), 1169. doi: 10.1037/0735-7044.117.6.1169 [DOI] [PubMed] [Google Scholar]
- Reisberg B, & Gauthier S (2008). Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int. Psychogeriatr. 20(1), 1–16. doi: 10.1017/S1041610207006412 [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, & Zhu W (2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 6(1), 11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno JA, Murphy DG, Horwitz B, DeCarli C, Haxby JV, Rapoport SI, & Schapiro MB (1992). Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension, 20(3), 340–348. doi: 10.1161/01.HYP.20.3.340 [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, & Mamourian AC (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology, 67(5), 834–842. doi: 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, Boecker H, Biersack HJ, Maier W, Schild HH, Wagner M, & Jessen F (2012). Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology, 79(13), 1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, & Lindeboom J (1996). Subjective memory complaints may announce dementia. Neurology, 46(1), 121–125. doi: 10.1212/WNL.46.1.121 [DOI] [PubMed] [Google Scholar]
- Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hemmer B, & Muhlau M (2012). An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage, 59(4), 3774–3783. doi: 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, & Freund TF (1991). Selective vulnerability of the hippocampus in brain ischemia. Neuroscience, 40(3), 599–636. doi: 10.1016/0306-4522(91)90001-5 [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, & Barnes CA (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 12(10), 585. doi: 10.1038/nrn3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Dufouil C, Godin O, Ritchie K, Maillard P, Delcroix N, Mazoyer CB, & Tzourio C (2008). Neuroimaging correlates of subjective memory deficits in a community population. Neurology, 70(18), 1601–1607. doi: 10.1212/01.wnl.0000310982.99438.54 [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, & Alexander GE (1997). Interactive effects of age and hypertension on volumes of brain structures. Stroke, 28(7):1410–7. doi: 10.1161/01.str.28.7.1410 [DOI] [PubMed] [Google Scholar]
- Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, Suliman H, Wagner M, Schild HH, & Jessen F (2010). Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement. Geriatr. Cogn. Disord. 29(1), 75–81. doi: 10.1159/000264630 [DOI] [PubMed] [Google Scholar]
- van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, Westendorp R, & Middelkoop HA (2004). Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J. Neurol. 251(6), 671–675. doi: 10.1007/s00415-004-0390-7 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson, 22, 498. doi: 10.2298/psi171001001l [DOI] [Google Scholar]
- Wu C, Zhou D, Wen C, Zhang L, Como P, & Qiao Y (2003). Relationship between blood pressure and Alzheimer’s disease in Linxian County, China. Life Sci. 72(10), 1125–1133. doi: / 10.1016/S0024-3205(02)02367-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.