Abstract
Atherosclerosis is an inflammatory disease of the large arteries that is the major cause of cardiovascular disease (CVD) and stroke. Here we review the current understanding of the molecular, cellular, genetic and environmental contributions to atherosclerosis, from both individual pathway and systems perspectives. We place an emphasis on recent developments, some of which have yielded unexpected biology, including previously unknown heterogeneity of inflammatory and smooth muscle cells in atherosclerotic lesions, roles for senescence and clonal hematopoiesis, and links to the gut microbiome.
eTOC blurb Lusis
Atherosclerosis has been intensively studied for decades, yet new and unexpected biology continues to be discovered. Lusis and Björkegren provide a concise but comprehensive updated view of the molecular, cellular, genetic, and environmental contributions to atherosclerosis.
INTRODUCTION
Atherosclerosis or coronary artery disease (CAD) is the most common form of cardiovascular disease (CVD) where the main component is lipid accumulation and inflammation of the large arteries, which eventually may lead to its clinical complications, myocardial infarction (MI) and stroke. As a disease of slow progression, clinically significant atherosclerosis occurs primarily in older individuals and, despite declining incidence in some countries, remains the leading cause of mortality worldwide. Atherosclerotic lesions are characterized by a life-time long accumulation and transformation of lipids, inflammatory cells, smooth muscle cells, and necrotic cell debris in the intimal space underneath a monolayer of endothelial cells (EC) that line the interior vessel wall. Typically, lesion growth can reduce blood flow in the lumen by >50% and may cause angina particularly during exercise or stress. Lesions can become unstable and rupture, particularly if they have fatty and inflammatory composition. If this occurs in the coronary arteries, it can result in a local clot that may completely obstruct the blood flow to cause an MI. Alternatively, the clot can escape the heart and travel to the brain where it may cause a stroke.
Recently, there have been major advances in the understanding of molecular and cellular interactions in atherosclerosis. These include previously unknown cellular heterogeneity in atherosclerotic lesions revealed through single-cell RNA sequencing. There has also been recognition that processes that occur during aging, such as senescence and clonal hematopoiesis likely play an important role. Also, the links between the gut microbiome and atherosclerosis are becoming increasingly clear. Substantial progress continues to be made in the systems understanding of the interplay of genetic and environmental risk factors of atherosclerosis and its relationship to cardiometabolic traits. Finally, there have been exciting advances in the diagnostic and therapeutic arena.
This review provides an overview of the disease with an emphasis on recent developments. We first discuss the growth of atherosclerotic lesions, from initiation to advanced lesions and aging. This is followed by a discussion of genetic approaches and the major genetic and environmental risk factors for the disease. We conclude with a discussion of clinical aspects and future directions. Due to limitations in the length of the review, we largely refer to recent reviews rather than original articles except for recent major events.
LESION INITIATION AND GROWTH
The vessel wall consists of a monolayer of EC that border the luminal blood flow. Underlying this is a largely acellular layer consisting of glycosaminoglycans and collagen, termed the “intima”. Next are layers of smooth muscle cells (SMC), termed the “media”, and finally a fibrous layer termed the “adventitia”. Atherosclerosis is initiated in large part by the accumulation of certain plasma lipoproteins, including low density lipoproteins (LDL) and remnants of triglyceride rich lipoproteins, in the intimal region of the vessel. This results in an activation of the overlying EC by a still poorly understood mechanism but likely involving the generation of pro-inflammatory oxidized lipids. Blood monocytes then bind to endothelial adhesion molecules, enter the intima, and differentiate to macrophages. These, in turn, can take up the lipoproteins, giving rise to cholesterol ester engorged “foam cells” (Figure 1). In this section we discuss the cell types and molecular interactions involved in lesion initiation.
Figure 1. Development of fatty streak lesions.
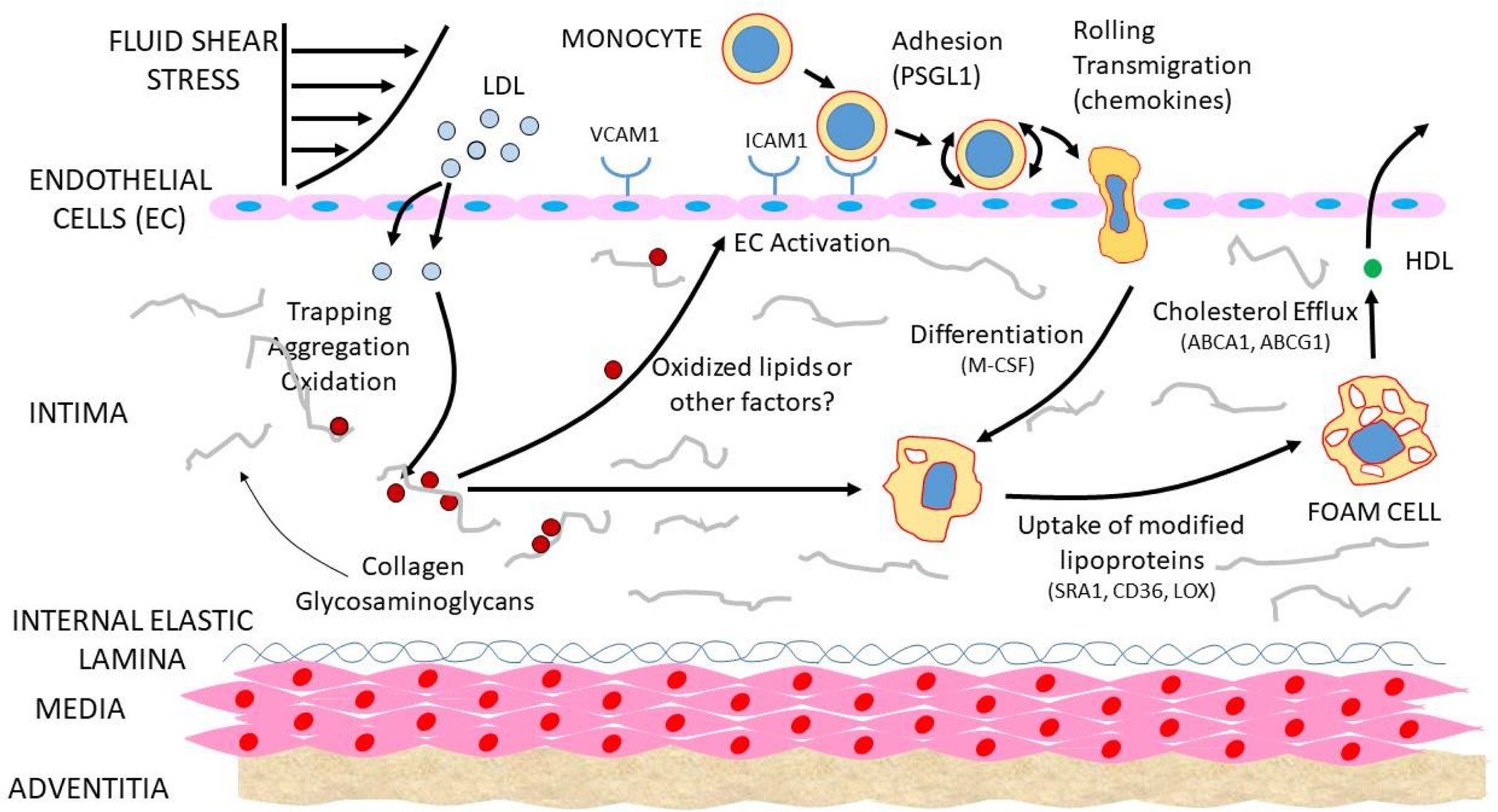
Lipoproteins enter the intima of sites of shear stress. The lipoproteins then aggregate and become oxidized and otherwise modified, resulting in the activation of the overlying EC to express adhesion and chemotactic molecules for monocytes. The monocytes enter the intima, differentiate to macrophages, and take up modified lipoproteins to give rise to foam cells.
Endothelial cells (ECs).
As outlined in Figure 1, ECs form a single cell layer connected by tight-junctions separating the blood from the vessel wall. Under conditions of disturbed blood flow, the ECs and their tight junctions become “leaky” which promotes the uptake of plasma LDL and TG-rich lipoproteins either by trans-endothelial transport or diffusion at the cell-cell junctions (Zhang et al., 2018). The subsequent activation of the ECs occurs in response to oxidation of lipoprotein lipids and other inflammatory mediators, resulting in the expression of P-selectin, E-selectin, VACM1, and ICAM1 that promotes the adhesion of monocytes, other leukocytes and chemotactic factors such as CCR2 (Gimbrone and Garcia-Cardena, 2016). Disturbed blood flow can also trigger an alternative mechanism EC dysfunction; erosion. It is caused by the TLR2-dependent EC apoptosis and secretion of IL-8, leading to neutrophil recruitment, activation and release of neutrophil extracellular traps, which aggravates the injury of the EC layer and may lead to thrombus formation (Luo et al., 2021)
Shear stress and lipid accumulation.
Atherosclerosis tends to occur in regions of arteries such as bifurcations that exhibit turbulent blood flow as compared to laminar flow. Turbulent flow changes the cellular alignment of ECs and increases their permeability to large molecules due to reduced tightness of cell-cell junctions. Multiple pathways underlying this response have been identified, including BMP-TGFβ, WNT and Notch (Souilhol et al., 2020). As a result of the increased permeability, certain lipoproteins accumulate in the intimal region, in part by interaction with the intimal glycosaminoglycans (Boren and Williams, 2016). Once trapped in this way, LDL and the more triglyceride-rich remnant lipoproteins can become aggregated and chemically modified (Figure 1).
Lipid oxidation and inflammation.
A large body of evidence has suggested that the oxidation of lipids in lipoproteins trapped in the vessel wall produce proinflammatory species, leading to leukocyte recruitment and inflammation. For example, treatment of EC with oxidized phospholipids or oxidized LDL induces the expression of adhesion molecules and chemotactic factors (Figure 1). Despite this, direct proof for this hypothesis has been difficult to obtain, and the fact that antioxidant drugs have failed to reduce atherosclerosis has caused some to conclude that alternative mechanisms, such as aggregated LDL, are the triggers for inflammation (Libby, 2021). However, studies showing that transgenic expression of a natural antibody to oxidized phospholipids suppresses lesions in mice argues in favor of the lipid oxidation hypothesis in atherosclerosis (Que et al., 2018). Lipoproteins can also undergo oxidation in the lysosomes of macrophages, resulting in the release of lipid oxidation products. Inhibition of such oxidation by the antioxidant cysteamine blocked and even reversed lesion development in LDL receptor null mice (Ahmad et al., 2021). The lipid oxidation product octanol was recently shown to bind to olfactory receptor 2 in vascular macrophages, activating the NLRP3 inflammasome and inducing interleukin 1β. Targeting the receptor reduced atherosclerosis and boosting octanol levels increased it (Orecchioni et al., 2022). Studies have also suggested that lipid oxidation in the intestine may contribute to systemic inflammation and atherosclerosis (Mukherjee et al., 2021).
Monocytes, macrophages, and foam cells.
Monocytes are recruited to the vessel wall in response to the expression adhesion molecules (VCAM-1, ICAM-1, P-SELECTIN, E-SELECTIN) and chemotactic proteins (CCR2, CCR5) produced by EC. These, in turn, differentiate into macrophages in response to locally produced M-CSF and other cytokines (Figure 1). Mouse studies suggest that the abundance of macrophages in early lesions is determined by recruitment but that in more advanced lesions growth, it results largely from macrophage proliferation (Robbins et al., 2013). Macrophages contribute very importantly to lesion progression as evidenced by the fact that M-CSF null mice on a hypercholesterolemic background are almost entirely resistant to lesion development. Native LDL is not taken up by macrophages but must first be modified by oxidation or aggregation. Lesional macrophages can take up such “modified” intimal lipoproteins through scavenger receptors or phagocytosis of aggregated lipoproteins to give rise to cholesterol engorged macrophages, or “foam cells”. Although foam cells can efflux cholesterol using the transporters ABCA1 and ABCG1, they frequently undergo apoptosis or necrosis to give rise to a growing “necrotic core” consisting of cholesterol, including cholesterol crystals, and cell debris that increases the likelihood of lesion rupture. Macrophages undergo metabolic transitions during the various stages of atherosclerosis which can, in turn, affect their functions (Tabas and Bornfeldt, 2020).
ADVANCED ATHEROSCLEROTIC LESIONS
Following initiation, lipids and foam cells continue to accumulate, and other leukocytes, particularly T cells, enter the lesion and interact with macrophages. Over time, the foam cells die to give rise to necrotic cores consisting of cell debris and cholesterol. Also, SMC transform from a contractile to a proliferate state and migrate to the region underlying the EC to form a “fibrous cap” that protects the lesion from rupture. SMC can also differentiate into macrophage-like cells that give rise to foam cells and differentiate into bone-like cells that deposit calcium phosphate mineral. Although lesions can grow sufficiently large to impede the flow of blood, the most clinically significant event is a myocardial infarction resulting from the formation of a clot triggered by lesion rupture or endothelial erosion (Figures 2 and 3). In this section we discuss the roles of SMC and lymphocytes in lesion progression as well as processes influencing inflammation, calcification and lesion stability. Finally, we discuss the use of single cell sequencing to examine cellular heterogeneity in lesions
Figure 2. Development of atherosclerosis lesions.
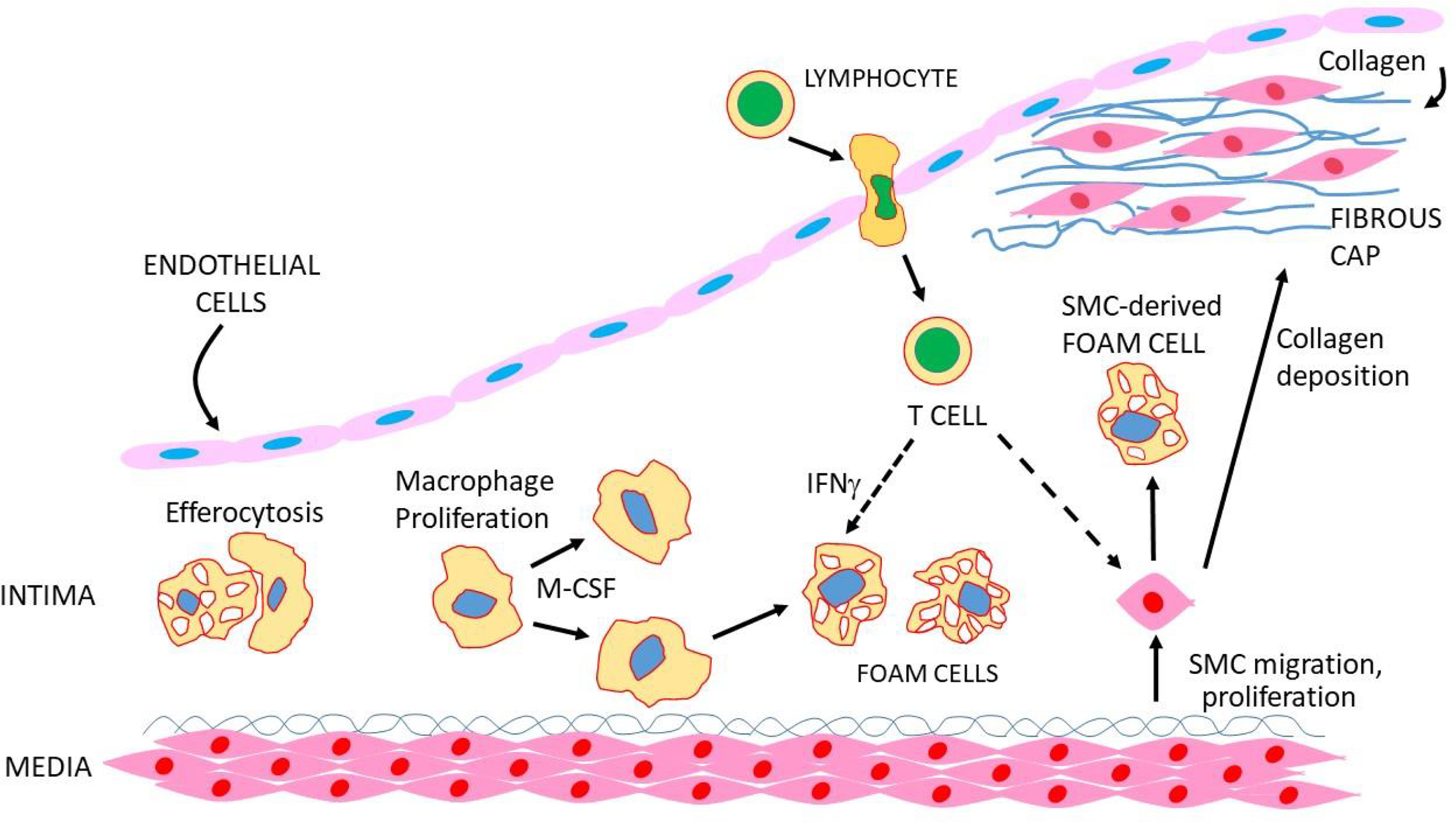
Macrophages proliferate in response to M-CSF, and foam cells are engulfed by macrophages, a process known as efferocytosis. SMC transform to a proliferative state, migrate to the endothelial region and secrete collagen to give rise to a “fibrous cap”. SMC can also transform to macrophage-like cells that take up lipid to give rise to foam cells. T and B cells also enter the lesion and interact with other cell types to promote or retard lesion development.
Figure 3. Advanced atherosclerotic lesions and coagulation.
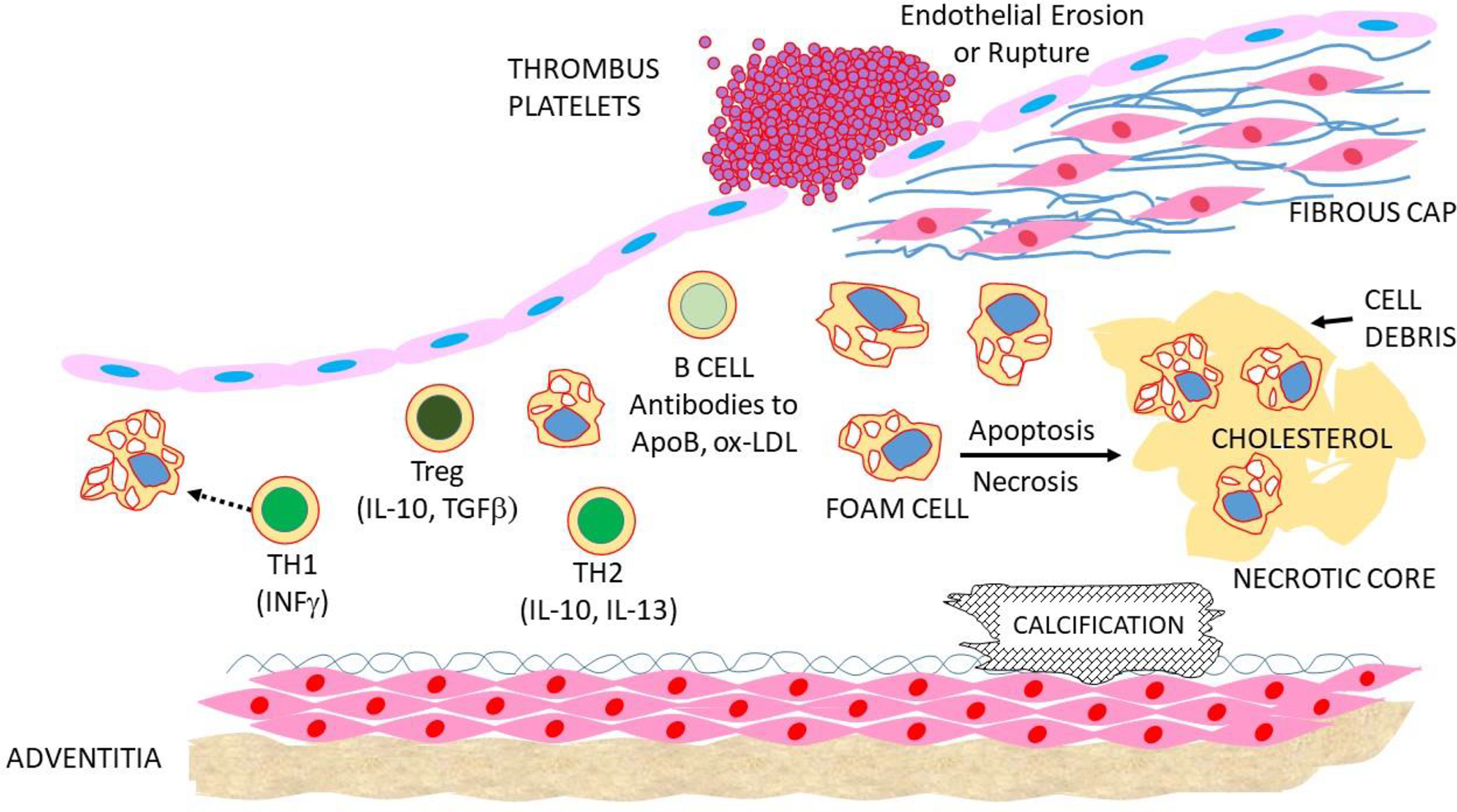
Foam cells and other cells die to give rise to cholesterol-rich necrotic cores. Calcification frequently occurs in the intima or media. Lesions can rupture or EC can erode to stimulate the formation of a thrombus, possibly resulting in an MI or stroke.
Smooth muscle cells.
During lesion growth, SMC in the media transform from a contractile to a proliferative state and migrate into the intima. Over time, the intimal SMC secrete an extracellular matrix consisting largely of collagen to give rise to a protective (against rupture) fibrous cap (Figure 2). Relatively few migration-competent SMC enter the intima and then undergo clonal expansion followed by re-differentiation into contractile SMC. Lineage tracing studies have shown that these cells can undergo trans-differentiation into macrophage-like and osteochondrogenic descendants (Alencar et al., 2020; Pan et al., 2020). The macrophage –like SMC can take up lipid to give rise to foam cells which can undergo apoptosis and suppressed efferocytosis to give rise to secondary necrosis and inflammation. SMC can also acquire cholesterol from nearby macrophage foam cells by a recently described pathway involving membrane-derived particles (He et al., 2018). It has been estimated that in animal models that SMC-derived foam cells could account for as much as 50% of lesional foam cells (Basatemur et al., 2019). SMC also produce macrophage colony stimulation factor (M-CSF) that drives the proliferation of macrophages in lesions. The osteochondrocytes can give rise to calcification granules which can subsequently coalesce to produce calcium nodules (Basatemur et al., 2019).
T lymphocytes.
Adaptive as well as innate immunity drives the chronic inflammation of atherosclerosis, and several classes of T cells impact disease progression (Roy et al., 2021). T cells are present at all stages of atherosclerosis and their infiltration is mediated by chemokine receptors (CCR5, CXCR6) and ligands (CCL5, CXCL16). T cells can activate as well as suppress immune activation and can help B cells to produce antibodies. Most abundant in lesions are TH1 interferon γ secreting cells, promoting plaque growth and instability. Treg cells express anti-inflammatory cytokines (IL-10 and TGFβ), promote macrophage efferocytosis and show a negative correlation with atherosclerosis. TH2 cells express IL-5 and IL-13, both of which are protective. T cells as well as B cells are activated by antigens present in lesions, and dendritic cells that have acquired atherosclerosis antigens can leave the lesion and stimulate responses elsewhere (Proto et al., 2018; Roy et al., 2021).
B-lymphocytes.
B cells participate in local and systemic immune responses that contribute to the chronic inflammation in atherosclerosis (Sage et al., 2019). They are derived from bone marrow progenitors and mature in the spleen. Each B cell produces a unique B cell receptor that recognizes a specific antigen, triggering transformation into antibody producing plasma cells. Both anti-atherogenic and pro-atherogenic B cell subsets have been identified. Some B cells produce “natural” antibodies that bind oxidized epitopes present in oxidized lipoproteins and necrotic debris, thereby inhibiting inflammation. Antibodies to a number of antigens, including LDL, oxidized LDL, apolipoprotein B (the major protein of LDL) and pathogens such as cytomegalovirus, are associated with atherosclerosis. Recent studies indicate that elevated levels of the IgE immunoglobulins, which can stimulate pro-inflammatory responses in macrophages, are significantly associated with increased atherosclerosis. B cell autoimmunity can also contribute to atherosclerosis; for example, some mouse models of lupus show increased atherosclerosis (Sage et al., 2019).
Hematopoiesis.
Substantial evidence indicates that increased hematopoiesis, the process involved in replenishing blood cells, promotes atherosclerosis. Hematopoiesis and circulating leukocytes are increased in response to smoking, stress, and a poor diet and are reduced by exercise and elevated HDL levels. Recent studies in mice and humans support a causal relationship between increased hematopoiesis, clonal hematopoiesis, and atherosclerosis (Heyde et al., 2021). Hyperlipidemia appears to promote hematopoiesis in part by its effects on cholesterol efflux pathways and is inhibited by the functions of ABCA1, ABCG1, and HDL (Schloss et al., 2020). A recent study of ApoA-1 binding protein (A1BP), a mediator of cholesterol efflux, provided evidence that SREBP2 and Notch signaling are involved in the regulation of hematopoiesis (Gu et al., 2019).
Efferocytosis and resolution.
Efferocytosis is the process by which apoptotic cells are cleared, thereby preventing secondary necrosis and inflammation. It is mediated primarily by macrophages that engulf the dying cells and is important in reducing the growth of necrotic cores (Figure 2) (Doran et al., 2020). Resolution is an active process aimed at the restoration of tissue integrity and function during inflammation. It involves a functional switch from pro-inflammatory to anti-inflammatory macrophages and is mediated by certain lipids (known as resolvins and lipoxins), proteins, and nitric oxide (Back et al., 2019)
Recent studies indicate that efferocytosis can promote non-inflammatory macrophage proliferation and contribute to tissue resolution (Gerlach et al., 2021).
Calcification.
Coronary calcification occurs with the development of advanced lesions and can be assessed by various imaging modalities. It can occur in both the intimal and medial layers and may be associated with increased plaque stability. It begins with very small matrix vesicles that originate from apoptotic smooth muscle cells and macrophages. These coalesce into larger masses over time, generally near the necrotic core, and eventually form calcified sheets (Figure 3). Bone related proteins, such as BMP-1, BMP-4, and matrix GLA protein, are often expressed in such regions (Basatemur et al., 2019; Mori et al., 2018).
Fibrous cap.
Advanced lesions contain a layer of fibrous connective tissue composed of collagen, elastin, and bundles of SMC as well as macrophages and lymphocytes. It was previously thought that nearly all the cells that produce extracellular matrix in the cap are derived from SMC. Recent experiments, however, suggest that a significant fraction of these cells are derived from EC or macrophages that have undergone endothelial-to-mesenchymal transition or macrophage-to-mesenchymal transition (Newman et al., 2021).
Lesion stability.
A myocardial infarction (MI) usually occurs when a lesion ruptures, triggering the formation of a blood clot (Figure 3). “Vulnerable plaques” are more a function of their composition than size. Fibrous lesions with thick fibrous caps tend to be more stable than fatty, inflammatory lesions. A number of factors have been identified that affect lesion stability, including SMC and EC cell senescence. Macrophages appear to contribute to plaque destabilization by amplifying inflammation and producing proteases that attack the fibrous cap. MI can also occur as a result of endothelial erosion (Figure 3). Neutrophils, although rare in lesions, appear to promote endothelial erosion via neutrophil traps and secretion of matrix metalloproteinases (Libby, 2021). A recent study showed that oxidized lipids promote the formation of neutrophil extracellular traps and accelerate carotid artery thrombosis in a mouse model (Dou et al., 2021).
Cell heterogeneity and plasticity.
Historically, a major challenge to atherosclerosis researchers has been the heterogeneity of arterial wall cell types involved in this process. However, in recent single-cell RNAseq studies it has become increasingly clear that these cellular phenotypes are highly plastic, transforming along with the progression of atherosclerosis. For instance, arterial wall SMC-derived cells were shown to exhibit substantial phenotypic plasticity, transforming into osteogenic-like cells during late-stage atherosclerosis plaque formation both in mice and humans (Alencar et al., 2020). Similarly, it was demonstrated that SMCs transitioned to an intermediate cell state with multipotent differentiation capacity, including pro-atherogenic macrophage-like and fibrochondrocyte-like cells, and, athero-protectively, reverting back to the original SMC phenotype. Retinoic acid signaling associated with symptomatic atherosclerosis was shown to regulate the state of intermediate SMCs in humans (Pan et al., 2020). In another study including human coronary arteries, TCF21, a coronary artery disease (CAD) risk gene identified by GWAS, was shown to regulate SMC plasticity in a CAD-protective role (Wirka et al., 2019).
Besides SMCs, the single cell landscape of immune cells and in part EC in atherosclerosis has recently been studied both in humans (Fernandez et al., 2019) and mice (Winkels et al., 2018). Early on, 13 distinct myeloid cell populations were identified in mouse aortas where resident-like macrophages were found in both healthy and atherosclerotic aortas, whereas monocytes, monocyte-derived dendritic cells, and 2 populations of macrophages were almost exclusively detectable in atherosclerotic aortas, comprising inflammatory macrophages expressing TREM2 (Cochain et al., 2018). These results were largely confirmed in human carotid plaques and additionally provided evidence for endothelial-mesenchymal transition and a decline in CD4+ and CD8+ cell cytotoxicity (Depuydt et al., 2020). Somewhat in contrast, it was shown that carotid plaques from symptomatic patients were characterized by a distinct subset of CD4+ T-cells that were activated and differentiated (Fernandez et al., 2019).
More recently, single-nucleus chromatin accessibility profiling (i.e., “snATACseq”) of human atherosclerotic lesions was used to investigate cell type–specific patterns of cis-regulatory elements and to understand how transcription factors establishing cell identity may interact with genetic risk variants identified by GWAS of CAD. The authors found that CAD-associated genetic variants were particularly enriched in endothelial and SMC–specific open chromatin. Then, by performing genome-wide experimental fine-mapping of these CAD variants using epigenetic quantitative trait loci analysis in primary human aortic EC and self-transcribing active regulatory region sequencing (STARRSeq) in smooth muscle cells, they were able to identify potential causal single-nucleotide polymorphisms (SNPs) and associated target genes for over 30 established CAD loci (Ord et al., 2021). Using a similar approach, the CAD GWAS candidate gene, ATF3 was recently identified as a repressor of SMC transitioning, promoting vascular inflammation (Wang et al., 2021).
ANIMAL MODELS
Most of the biological understanding of mechanisms underlying CAD derives from studies of animal models. Historically, the primary animal models for atherosclerosis were rabbits and hamsters, as those develop significant atherosclerotic lesions when placed on high fat, high cholesterol diets. However, because of their much greater utility for genetics studies, mice have become the most widely used models for atherosclerosis and its risk factors. In particular, engineered mice exhibiting high LDL/VLDL-cholesterol levels (such as LDL receptor deficient or apolipoprotein E deficient mice) are primarily employed for validation of human candidate genes, studies of environmental interactions, drug studies, and gene discovery (Daugherty et al., 2017). Recently, overexpression of PCSK9 using an adeno-associated virus vector has become widely used since it avoids the necessity of breeding engineered genes onto a hyperlipidemic background (Goettsch et al., 2016). At present, nearly 1000 genes have been studied in such mouse models using gene targeting, overexpression or drug treatments. Different strains of mice exhibit over 100-fold variation in atherosclerosis when hyperlipidemia is induced by genetic perturbation and this variation has also been exploited to identify genes and pathways.
The development of atherosclerotic lesions in hyperlipidemic mice is similar to that observed in pathologic studies of the human disease, including lipid accumulation in the intima, monocyte recruitment, and foam cell formation, followed by SMC proliferation and the formation of a fibrous cap. In addition to monocytes, T-lymphocytes are recruited to the lesions, and in certain genetic backgrounds, intimal and medial calcification occurs. However, the mouse models rarely show evidence of lesion rupture, a particularly significant point since about three quarters of heart attacks result from plaque rupture followed by thrombosis (von Scheidt et al., 2017). Other, specialized hyperlipidemic models, such as one involving dual ligation of the right coronary artery, lead to locally predefined plaque instability and rupture with characteristics of human unstable plaques (Noonan et al., 2022). Apart from pathology, the mouse models show consistency with atherosclerosis risk factors such as LDL levels, HDL levels, hypertension, smoking, diabetes, renal failure, arthritis, lupus, aging, distress, air pollution, the metabolite TMAO derived from gut bacteria, and physical activity. Moreover, an analysis of the pathways contributing to atherosclerosis based on human GWAS genes (see below) and mouse engineered genes shows considerable overlap (von Scheidt et al., 2017).
AGING AND ATHEROSCLEROSIS
Atherosclerosis is largely a disease of the elderly, with most myocardial infarctions or strokes occurring in individuals over 55 years of age (Tyrrell and Goldstein, 2021). There are, however, some early onset forms, such as familial hypercholesterolemia (FH) or very rare disorders such as Hutchinson-Gilford progeria. In this section we discuss three relatively recently discovered processes in aging that contribute to atherosclerosis.
Clonal hematopoiesis and leukocytosis.
Circulating blood cells are derived from hundreds of hematopoietic stem cells (HSC) and thus a somatic mutation in a stem cell will generally be found in a tiny fraction of blood cells. “Clonal hematopoiesis” refers to the expansion of an HSC in response to a mutation that gives rise to blood cell “clones” abundant in the circulation (Bick et al., 2020). Clonal hematopoiesis, relatively common in older individuals, is strongly associated with atherosclerosis (Jaiswal et al., 2017). One likely explanation for the association is increased hematopoiesis, which will statistically increase the likelihood of developing a mutation impacting HSC proliferation, since mutations tend to occur during replication (Heyde et al., 2021). A second explanation is that the clones, particularly monocytes and neutrophils, are more pro-inflammatory, promoting lesion growth. Experimental evidence in mice suggests that certain common clonal mutations contribute to increased atherosclerosis. For example, some evidence indicates that the TET2 clonal mutation increases macrophage IL-6 expression (Fidler et al., 2021; Tyrrell and Goldstein, 2021) (Figure 4).
Figure 4. Aging and atherosclerosis.
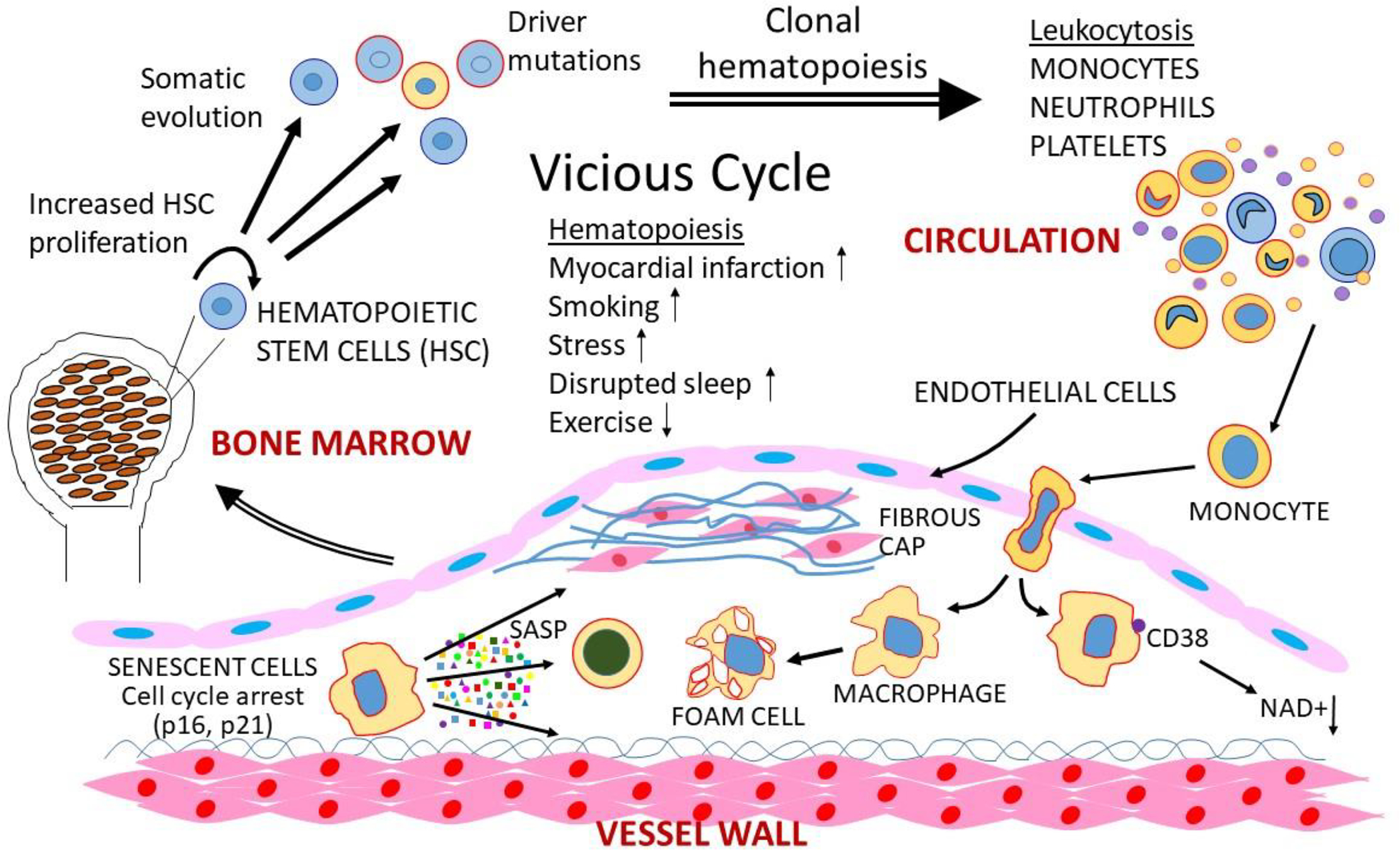
Three events that often occur in aging are the development of clonal hematopoiesis, senescence, and immuno-aging can promote atherosclerosis. A number of factors associated with atherosclerosis, listed above, can promote hematopoiesis, whereas exercise tends to reduce it. Increased hematopoiesis can in turn lead to leukocytosis and clonal proliferation, which can promote atherosclerosis, forming a vicious cycle. Various cells in lesions can also become senescent (at least in mice). Such cells release a variety of cytokines and immune modulators (senescence associated secreting proteins, SASP) that act inflame or kill nearby cells. Also, some MI macrophages express the ectoenzyme CD88 that can degrade NAD+. Recent studies have shown that reduced NAD+ levels are associated with a variety of disorders, although atherosclerosis has not yet been studied.
Senescence.
Cellular senescence of EC and SMC as well as macrophages has been implicated in lesion growth, inflammation, and stability (Grootaert et al., 2021; Kotla et al., 2019).
Senescence can be triggered by oxidative stress, DNA damage, and replicative exhaustion. Senescence exerts both beneficial and detrimental effects: It protects against cancer and promotes tissue repair, but senescent cells in aging tissues have been causally linked to degeneration and dysfunction. Senescent cells act on the surrounding cells by secreting a variety of inflammatory cytokines, immune modulators and proteases, collectively termed the senescence-associated secretory phenotype (SASP) (Figure 4). Senescent cells can be eliminated pharmacologically using “senolytic” drugs such as ABT263 that target BCL-2 family members. In atherosclerosis, senescent cells contribute to the degeneration of the fibrous cap, important in preventing plaque rupture, and senolytic clearance restored SMC numbers and cap thickness (Childs et al., 2021). SIRT6, a member of the Sirtuin family of class III histone deacetylases protects SMC in lesions from senescence through inhibition of telomere damage (Grootaert et al., 2021).
Inflamm-aging.
Chronic, low-grade inflammation frequently develops with advanced age in the absence of infection and contributes to age related pathologies such as atherosclerosis. It can have profound effects on systemic functions such as lipid and glucose metabolism. Among the features of aging is increased macrophage abundance and altered polarization. Recent studies suggest that M1-like macrophages, in addition to senescent cells, may be a major source of proinflammatory cytokines such as IL-1beta, IL-6 and TNF and increased activation of NLRP3 during aging (Covarrubias et al., 2021). NAD+ levels decline with aging, and a number of recent studies have shown that NAD+ supplements that restore NAD+ levels are protective of metabolic and cardiovascular dysfunction in aging. One of the mechanisms leading to reduced levels NAD+ levels is the enhanced expression in proinflammatory macrophages of CD38, an ectoenzyme exposed by MI-like macrophages that hydrolyzes NAD+ (Abdellatif et al., 2021) (Figure 4).
GENETICS OF ATHEROSCLEROSIS
It has been said that “genetics is to biology as mathematics is to physics,” and genetic studies have proven key to understanding atherosclerosis. A substantial fraction of atherosclerosis, about 40%, can be attributed to genetic variations. Genetic studies of rare Mendelian disorders, such as FH and, more recently PCSK9 mutations, have revealed novel mechanisms or have led to important therapeutic advances. Recent exome sequencing studies of large numbers of individuals have the potential to identify additional rare variants with large effect sizes for atherosclerosis and related traits (Backman et al., 2021). However, the vast majority of the heritable component consists of the combined effects of many genes, each contributing a small fraction to overall susceptibility (Figure 5). Although such variants individually contribute a small fraction of risk, they likely have substantial effects acting jointly to regulate may downstream genes that are linked in biologic networks. In this section we review studies of common genetic variations, including sex differences that contribute to the common forms of atherosclerosis and systems genetics approaches that integrate molecular and clinical traits.
Figure 5. Genetic factors contributing to atherosclerosis susceptibility.
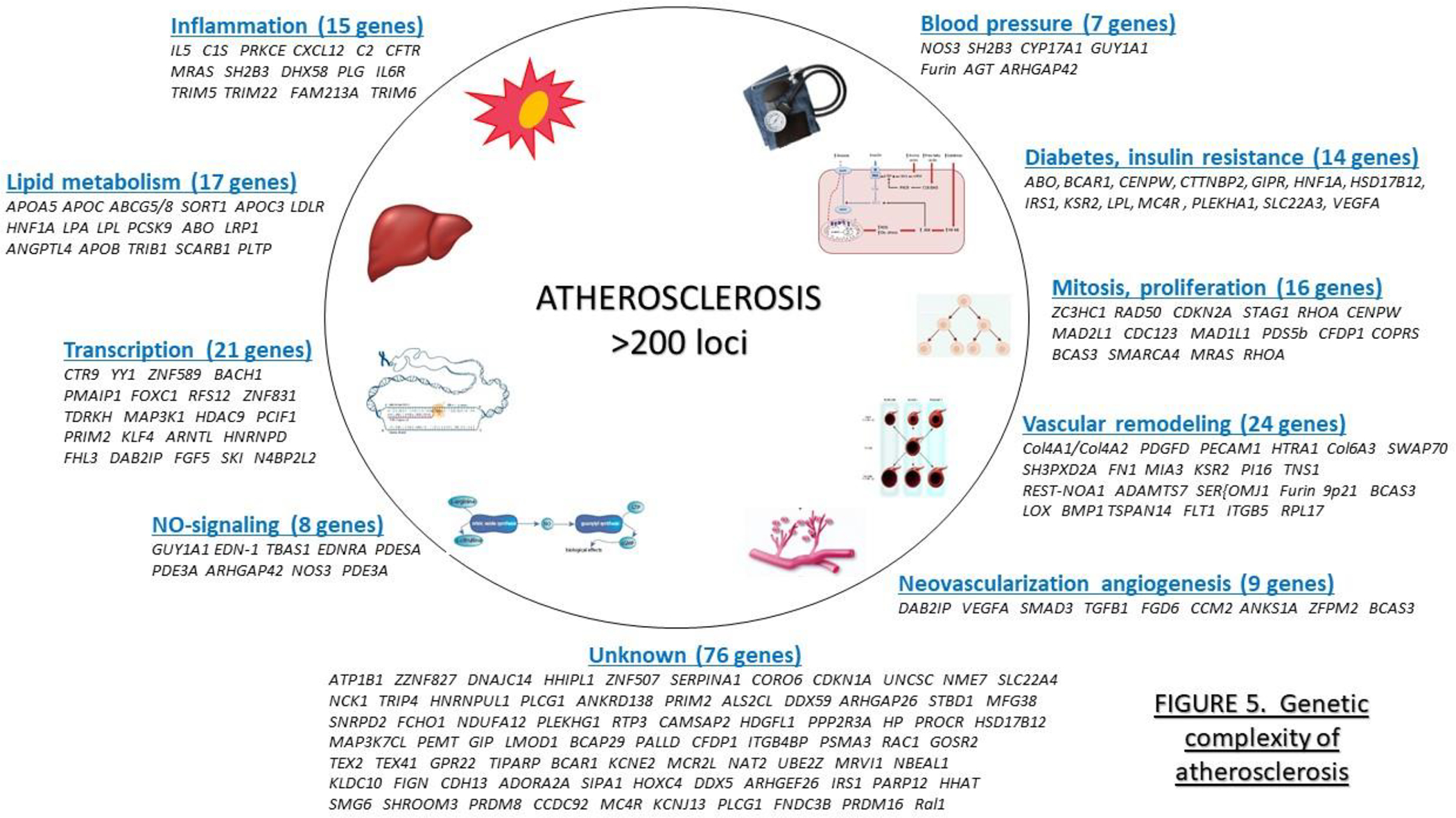
GWAS studies of CVD have identified about 200 loci that contain candidate genes that fit into several risk categories as indicated, although genes do not as yet fit into any known category. GWAS loci for CVD risk factors, including plasma lipid levels (Graham et al., 2021), hypertension (Cabrera et al., 2019), and diabetes (Mahajan et al., 2018), have each identified hundreds of additional, largely non-overlapping loci. [Figure adapted from (Erdmann et al., 2018)].
Common genetic variations.
Genome-wide association studies (GWAS) of CAD have been critical in identifying loci harboring genes that contribute to common, complex forms of the disease, indicating that many additional causal genes remain to be identified. As of this writing, over 200 loci have been identified for CAD using very large cohorts and meta-analyses, including CARDIOGRAM and UK BIOBANK that are primarily of European ancestry as well as a large Japanese cohort (Erdmann et al., 2018; Koyama et al., 2020) (Figure 5). Together, however, these loci explain less than half of the heritable component of disease. While some variants with large effect sizes (ODDs ratios in the range of 2) have been identified, most loci have very modest effects and are not individually useful for risk assessment. The most significant common genetic variant affecting atherosclerosis regulates the expression of a long noncoding RNA termed ANRIL. Many LNC and microRNAs have now been identified that influence processes relevant to atherosclerosis (Pierce and Feinberg, 2020). Many of the candidate genes at the loci associated with CVD risk have now been validated, primarily using mouse models, and have provided new insights into disease. Most but not all of the genes fit into several canonical pathways that provide a picture of the mechanistic underpinnings of the disease (Figure 5). Hundreds of genetic loci have also been identified for traits that contribute to atherosclerosis, such as plasma lipids, hypertension, and diabetes (Cabrera et al., 2019; Graham et al., 2021; Mahajan et al., 2018). Most such loci do not overlap with those for CAD, presumably because the effect sizes for CAD become too small to detect in even very large studies. Most of the interactions in atherosclerosis and other complex traits appear to be additive, although non-additive interactions are hard to detect in human studies.
Sex differences.
Atherosclerosis is sexually dimorphic in incidence and complications. Younger women are relatively protected from the disease but, by the seventh decade, the incidence of myocardial infarction surpasses that of men (Man et al., 2020). Sex hormones play a major role but recent studies indicate that genes on the sex chromosomes are also important. Thus, using data from the UK Biobank, it was found that a certain Y chromosome haplotype, containing the UTY gene, increases cardiovascular risk (Eales et al., 2019). Also, studies in mice revealed significant sex-based effects on atherosclerosis of X chromosome genes that escape X-inactivation (AlSiraj et al., 2019).
Systems genetics.
High throughput approaches capable of assessing global epigenetic marks and global transcript, protein, and metabolite levels can be incorporated into genetic studies to provide evidence of causality and underlying mechanisms. Such approaches have been particularly informative in mice where relevant tissues can be accessed, the environment can be controlled, and experimental validation is possible (Seldin et al., 2019). One of the difficulties of applying a “systems genetics” approach in humans is the lack of access to relevant tissues, although blood samples have proved very informative for investigation of metabolites and circulating miRNAs in relationship to CAD (Nikpay et al., 2019). The GTEx consortium has performed RNAseq on samples of many tissues from hundreds of random donors (Kim-Hellmuth et al., 2020) and the DNA polymorphisms regulating gene expression can then be examined in GWAS or case-control studies of atherosclerosis. One particular useful resource for cardiovascular disease studies is STARNET, a RNAseq resource of atherosclerosis-relevant tissues, including aorta, liver, skeletal muscle, as well as abdominal and subcutaneous fat, and adipose, from over 1700 living donors undergoing open chest surgery (Franzen et al., 2016). The resource now includes both individuals with established coronary artery disease and controls. In addition to providing detailed information about the genetics of gene expression or protein levels in the data has been used to model atherosclerosis biologic networks (Koplev et al., 2021). Very large epidemiologic studies that incorporate sequencing and multi-omics analyses are being mined for novel insights in CAD as well as other diseases. For example, it was recently found in the UK Biobank data that serum HDL-cholesterol and ApoAI levels are significantly associated with SARS-CoV-2 infection (Hilser et al., 2021). Omics-level analyses have been performed on the cultured EC and SMC derived from the “trimmings” of aortas of donor hearts obtained from heart transplantation operations. Cultures obtained from about 100–150 donors have been subjected to global transcriptomic analyses and thousands of eQTL identified, many of which regulate candidates at GWAS loci or to traits measured in vitro such as SMC mobility or tendency to calcify or EC response to cytokines (Aherrahrou et al., 2020; Stolze et al., 2020).
TRADITIONAL RISK FACTORS WITH A STRONG GENETIC COMPONENT
In this section we review recent developments for the most significant traditional CVD risk factors that have large genetic components.
Lipoprotein metabolism.
Plasma lipoprotein levels constitute the major risk factor for atherosclerosis. Lipids transported through the circulation as complexes with proteins, termed lipoproteins. Triglyceride rich chylomicrons and very low density lipoproteins (VLDL) are secreted by the liver and intestine, and are acted upon by lipases to produce remnants and LDL. High density lipoproteins (HDL) are also secreted by liver and intestine and undergo a variety of transformations in the circulation. One significant function is the transport of cholesterol from peripheral tissues to liver, termed reverse cholesterol transport. The other major lipoproteins in the blood are high density lipoproteins (HDL). Plasma lipoprotein levels are determined by many genetic factors (nearly 1500 individual GWAS signals) as well as important environmental factors (Graham et al., 2021).
Low density lipoproteins (LDL) consists of a single major polypeptide, apolipoprotein B (ApoB) and a cargo of cholesterol esters bounded by a phospholipid monolayer. The levels of LDL, the major cholesterol carrying lipoprotein in humans, have long been known to contribute to atherosclerosis. Studies of familial hypercholesterolemia (FH), a dominant Mendelian disorder characterized by very high levels of LDL and increased atherosclerosis, have been particularly informative in dissecting cholesterol metabolism. The most common forms result from mutations of the LDL receptor, responsible of removal of LDL from the circulation through recognition of ApoB. Recent studies have identified a number of novel genes contributing to LDL receptor function, the most significant being the secreted protein, PCSK9, that regulates the turnover of the LDL receptor (Gupta et al., 2020).
Lp(a), an LDL-like particle differing from LDL by the addition of a protein termed apolipoprotein (a) that is disulfide bridged to ApoB. Its levels are controlled largely by alleles of the apolipoprotein(a) locus and it is resistant to statin therapy. High Lp(a) levels are associated with increased CAD and valve calcification. Lp(a) appears to be more atherogenic than LDL, possibly because the particles transport oxidized phospholipids (Que et al., 2018).
High density lipoproteins (HDL) are inversely associated with atherosclerosis. They promote reverse cholesterol transport and inhibit lipid oxidation, and are negatively associated with atherosclerosis. Thus, it had been assumed that HDL directly protects against the disease. A causal relationship, however, has been challenged based on the failure of drugs that raise HDL cholesterol to protect and Mendelian randomization (MR) studies showing that genetic variations that affect HDL-cholesterol levels, such as variants of endothelial lipase, did not have a significant impact on atherosclerosis. On the other hand, since HDL particles are heterogeneous, it may be that only certain HDL species are protective and that HDL-cholesterol levels are a poor measure of the protective species. For example, a recent study showed that HDL produced by the intestine is functionally distinct from liver-derived HDL in that it protects the liver, and perhaps the vessel wall, from gut-derived lipopolysaccharide (LPS) (Han et al., 2021). Also, MR can be confounded by pleiotropy, the ability of genetic variants to affect multiple traits. For example, variants that affect plasma lipids can also impact other traits associated with atherosclerosis, such as diabetes. Two recent MR studies, in contrast to the original reports, concluded that HDL levels are independently associated with CAD (Thomas et al., 2021). One of these studies examined subtypes of HDL and concluded that whereas small and medium-sized HDL are protective, large HDL may be pro-atherogenic (Zhao et al., 2021).
Triglyceride-rich lipoprotein levels are positively associated with atherosclerosis, but the relationship was previously thought to be indirect, resulting from interactions with HDL metabolism. This was supported by the fact that certain disorders with greatly elevated plasma triglyceride levels, such as lipoprotein lipase deficiency, exhibit modest increases in atherosclerosis. More recent genetic studies, however, suggests that certain triglyceride rich lipoproteins do indeed directly promote atherosclerosis (Liu et al., 2017)
Hypertension.
Elevated blood pressure is a major risk factor of CAD and stroke, with many environmental and genetic contributions. Very large meta-analyses have identified over 500 independent loci associated with one or more blood pressure traits (Giri et al., 2019). Despite being a well-established independent risk factor for atherosclerosis, the mechanism by which hypertension accelerates coronary artery disease has remained elusive. A recent study shed light on this important issue by demonstrating that increased pressure per se facilitates coronary atherosclerosis, independent of humoral changes associated with hypertension, by increasing smooth muscle cell activity and intimal accumulation of low-density lipoproteins (Al-Mashhadi et al., 2021).
Diabetes.
Type 2 diabetes results from environmental and genetic interactions (Mahajan et al., 2018). It has become very common with the epidemic of obesity and is strongly associated with CAD, although the underlying mechanisms are poorly understood,. Diabetics often exhibit metabolic syndrome, a cluster of traits including insulin resistance, obesity, hypertension, and elevated LDL levels, all of which contribute independently to atherosclerosis. The dysregulation of blood sugar in diabetes may exacerbate atherosclerosis through a range of mechanisms including LDL modification, formation of advanced glycation end-products (AGE), oxidative stress, and vascular regulation of microRNA levels, and epigenetics (Poznyak et al., 2020). A recent study showed that hyperglycemia promotes pro-inflammatory gene expression in macrophages and that this persists following transplantation into normoglycemic mice, resulting in increased atherosclerosis (Edgar et al., 2021)
ENVIRONMENTAL RISK FACTORS
Environment appears to play a major role in atherosclerosis as evidenced by the fact that modifications to lifestyle choices and shifts in cultural practices can greatly alter CAD risk despite the same genetic background. For example, CAD mortality rates in Beijing, China, increased by 50% for men and 27% for women because of environmental changes between 1984 and 1999. In addition to the “traditional” risk factors, factors such as circadian rhythm, altitude, and seasons can influence disease susceptibility (Bhatnagar, 2017). Genetic factors may interact with environmental factors, but such interactions remain poorly understood in humans.
Nutrition and obesity.
Unhealthy diets contribute to many of the traditional risk factors such as LDL levels, triglyceride levels, diabetes mellitus, and hypertension. In surveys, about 50% of young adults and 31% of older adults have reported poor diets (Benjamin et al., 2018). CAD risk is affected by diet composition independent of energy content; for example, in one study, replacement of 5% of energy from saturated fat with unsaturated fat was associated with a 42% reduction in CAD risk (Bhatnagar, 2017). Apart from effects on obesity and plasma lipids, certain dietary constituents can be metabolized by intestinal bacteria into metabolites that affect atherosclerosis or diabetes (see below). A number of recent studies indicate that diet can importantly affect immunity and inflammation (Schloss et al., 2020). A recent study showed that a subset of T cells in the intestine function to modulate systemic metabolism. Knockout mice for β7 integrin lack these cells and are resistant to obesity, hypertension, diabetes, and atherosclerosis when fed a high fat, high sugar diet (He et al., 2019)
Exercise and physical activity.
Physical activity has been associated with reduced risk of CAD. Potential mediators include improved glucose tolerance, reduced plasma lipids, increased anti-inflammatory pathways and reduced obesity. In contrast to stress, exercise appears to dampen hematopoiesis and reduce circulating monocytes and neutrophils in both humans and mice (Figure 4) (Schloss et al., 2020). One mechanism by which exercise mediates hematopoiesis and immune function involves leptin signaling. In mice, running decreased leptin production and reduced signaling to stromal cells in the bone marrow niche, elevating CXCL12, a stem cell quiescence-promoting factor. ATAC sequencing (a measure of accessible chromatin) of hematopoietic cells revealed that running induced lasting epigenetic changes that persisted after exercise was stopped. Despite the reduced leukocytes, running mice were better able to respond to sepsis, supporting other studies that found improved anti-inflammatory effects with increased physical activity (Frodermann et al., 2019).
Sleep and stress.
Long-term lack of sleep associates with a number of diseases, including CAD (Dominguez et al., 2019). A recent study on sleep fragmentation in mice indicates that one mechanism involves increased hematopoiesis and circulating myeloid cells (Figure 3). Sleep deprived mice on a hyperlipidemia background had larger atherosclerotic plaques with more macrophages than controls. They also exhibited reduced levels of hypocretin, a hormone produced by the hypothalamus that regulates arousal and appetite. It was shown that one function of hypocretin is to suppress hematopoiesis by binding to a receptor on preneutrophils in bone marrow that results in suppression of M-CSF, an activator of macrophage growth. Sleep fragmented mice that were give hypocretin did not show an increase in hematopoiesis or atherosclerosis, confirming the brain-bone marrow-lesion axis (McAlpine et al., 2019).
In common with poor sleep, chronic stress causes monocytosis and neutrophilia in mice and humans. Stressors are processed by the amygdala and hypothalamus, triggering the sympathetic nervous system to increase catecholamine production, which then signals through β-adrenergic receptors on stromal and other bone marrow cells to increase hematopoiesis. Stress also stimulates glucocorticoid secretion from adrenal glands via the limbic-hypothalamic-pituitary-adrenal axis (Schloss et al., 2020).
Smoking.
Although smoking increases the risk of cancer and respiratory diseases, nearly half of the premature mortality associated with smoking is because of CAD. Smokers have a 2-fold higher risk of CAD and even light smokers have about a 2-fold higher risk of MI. Meta-analyses of many studies showed that smoking increases LDL and blood pressure but that these do not account for most of the risk (Bhatnagar, 2017). The use of e-cigarettes is spreading and a recent study found that e-cigarettes exert intermediate effects on lipid peroxidation and anti-oxidant defenses as compared to tobacco smoke, indicating that they promote inflammation and carry potential risk for CAD (Gupta et al., 2021).
Pollution.
Most air pollution is a mixture of aerosols containing both particles and gases. It has been estimated that it contributes to about 7 million premature deaths per year. There is evidence for effects of air pollution on dyslipidemia, endothelial dysfunction, platelet activation, atherosclerosis, and lesion stability. Under controlled conditions in animal models, increased exposure to concentrated ambient particles increases atherogenesis, insulin resistance and thrombosis (Bhatnagar, 2017). A recent study in animal models showed that diesel exhaust induces mitochondrial dysfunction and hyperlipidemia (Yin et al., 2019).
Intestinal microbiota.
The human intestine harbors trillions of bacteria, fungi, and viruses (collectively termed “microbiota”) that play an important role in a healthy ecosystem. The bacteria, in particular, help digest food, stimulate the immune system, and produce unique metabolites that can enter the host’s circulation. At birth, individuals acquire their microbes from their mothers and other family members and these are modulated by many factors, including diet, drugs, pollution, exercise, disease, host genetic factors, and aging. The composition of the microbiota is relatively constant in most individuals but there are striking differences between individuals. Epidemiological studies have revealed strong associations between the composition of the gut microbiota and numerous diseases, including CAD, but the causal relationships are largely unknown (Jie et al., 2017).
Microbial metabolites have emerged as particularly significant mediators of atherosclerosis. Perhaps the most significant identified thus far is trimethylamine N-oxide (TMAO), produced by hepatic oxidation of trimethylamine, a metabolite derived from bacterial metabolism of choline and carnitine. Individuals who consume diets rich in choline or carnitine, or who have kidney disease, have elevated levels of TMAO. TMAO appears to promote atherosclerosis by increasing platelet reactivity and vascular inflammation (Tang et al., 2019). Short chain fatty acids, produced by anaerobic fermentation of undigested fibers, are absorbed through the portal circulation and modulate blood pressure and inflammatory functions. In particular, a study in mice demonstrated that Roseburia intestinalis could reduce atherosclerosis through production of the short chain fatty acid butyrate (Kasahara et al., 2018).
Infections.
Bacterial and viral infections have long been associated with atherosclerosis. Recent large epidemiological studies of patients infected with SARS-CoV-2 have revealed striking post-infection increases in the incidence of a variety of CVD (Xie et al., 2022)
Conclusions.
Understanding how environmental factors contribute is perhaps the most significant knowledge gap in CAD. Human studies are greatly complicated by the difficulty of controlling or measuring the environment over many years. For this reason, studies in animal models are likely to be crucial in closing the gap. Some large efforts are underway to understand the underlying mechanisms. For example, MOTRPAC is a U.S. national consortium that aims to understand the molecular transducers of exercise. There are also large population studies of relationships of gut microbes and their metabolites to obesity, diabetes and CAD (Sanna et al., 2022). It is important to note that environmental factors act in the context of genetic variation and that the ultimate goal is to understand gene-by-environment interactions.
TOWARDS PRECISION MEDICINE AND NOVEL THERAPIES
Atherosclerotic lesions develop over a lifetime and are largely irreversible, although some studies suggest that lipid rich lesions can exhibit some regression. Therefore, the clinically most important goals are prevention and early diagnosis. The advances in understanding the disease as well as technical developments have created many opportunities for the development of novel medical applications.
Precision medicine.
There is increasing recognition that preventative measures are required to deal with the problem of atherosclerosis. Although the 30-day mortality of MI has been greatly reduced, this has resulted in increased numbers of heart failure patients. Current clinical evaluation of atherosclerosis risk is based largely on “traditional” risk factors (see above) and on imaging techniques. Nontraditional risk factors such as TMAO levels, IgE levels, or the plasma proteome (Williams et al., 2019) are not yet widely analyzed but could be incorporated into diagnosis. With the identification of hundreds of genetic loci contributing to disease, polygenic genetic risk scores based on high density genotyping have the potential to predict risk of CAD or risk of development of very high LDL cholesterol early in life (Aragam and Natarajan, 2020; Hindy et al., 2020). In elderly where CAD is already manifested, the plasma proteome (3–5000 proteins), reflecting both genetic and environmental factors, is emerging as a highly relevant source to detect obstructive CAD (Williams et al., 2019)
Targeting lipid metabolism.
Since plasma LDL, Lp(a), and triglyceride-rich lipoproteins are important drivers of atherosclerosis, many efforts have been directed at reducing their levels. Statins inhibit HMG-CoA reductase, the rate limiting enzyme in cholesterol synthesis, and thereby increase levels of LDL receptor in the liver. They are widely used and effective at lowering LDL levels. Statins can, however, have significant side effects, such as muscle weakness, and can also contribute to type 2 diabetes. Ezetimibe, an inhibitor of the NPC1L1 transporter responsible for cholesterol absorption in the small intestine, is an alternative. Another alternative is to administer neutralizing antibodies for the secreted protease PCSK9 that downregulates the LDL receptor by blocking its recycling. PCSK9 inhibition decreased major cardiovascular events in large randomized trials (Pasta et al., 2020). High Lp(a) is among the strongest common genetic factors for CAD (see above) and it is relatively resistant to statin treatment. A highly specific antisense oligonucleotide directed to hepatocytes was recently examined in a randomized trial of 286 patients with established CAD. The treatment resulted in dose-dependent decreases in Lp(a) of up to 80% with no significant adverse events, and larger clinical trials to study functional impacts are underway (Tsimikas et al., 2020).
Targeting inflammation and the immune system.
The potential for infection has limited efforts to treat atherosclerosis by modulating inflammatory aspects. However, the CANTOS clinical trial that targeted IL-1β with neutralizing antibodies in patients with CAD showed a significant 15% reduction in risk of MI, and stroke, with only a small increase in infection rates(Libby, 2021). Also, nanotechnologies targeting macrophages have been proposed for both diagnostic and therapeutic applications (Chen et al., 2021). Based on their roles in atherosclerosis, therapies that modulate system and T lymphocytes show considerable promise. Among the approaches being considered are B cell depletion using the drug rituximab, used to treat arthritis, and omalizumab, used to treat asthma. Another approach is to boost protective B cell or T cell responses using vaccination. For example, vaccination trials with apolipoprotein B peptides showed positive results in preclinical tests (Roy et al., 2021; Sage et al., 2019).
Targeting the genome.
The development of CRISPR based technologies has enabled specific and permanent edits to the genome. The main issues are delivery to the cells of interest and potential off-target effects. CRISPR technology has now been used to treat sickle cell anemia and certain eye disorders, and there are potential applications in atherosclerosis such as reduction of cholesterol levels. The most straightforward application is to eliminate expression of a deleterious gene, and an obvious target in cholesterol metabolism is the PCSK9 protein that promotes turnover of the LDL receptor. In one study, lipid nanoparticles were used to deliver CRISPR base editors to liver in cynomolgus monkeys, resulting in near-complete knockdown of PCSK9 expression in the liver after a single injection with concomitant reduction of LDL-cholesterol by 60%. The change remained stable, providing a “one and done” advantage as compared to antibody-based reduction of PCSK9 which require repeated injections (Musunuru et al., 2021). Related to this, it may be possible to increase expression of desirable genes using editing or delivery of mRNA with approaches similar to those employed in certain SARS-COV-2 vaccines. Although the editing of the germline is now feasible, several biological as well as ethical issues remain and it certainly will not be employed in the near future.
Targeting aging.
There is also evidence that certain mutations common in clonal hematopoiesis have pro-inflammatory properties, raising the possibility that they can be targeted (Fidler et al., 2021). Accumulating evidence suggests that macrophages are important in the systemic inflammation that often occurs in aging, and hence targeting macrophage immunometabolism to modulate functions and polarization is a therapeutic strategy that could be explored (Covarrubias et al., 2021). As discussed above, there is evidence of the involvement of senescence in atherosclerosis, at least in mice. Senolytics, drugs that kill senescent cells, have shown great promise in animal models of diabetes and other disorders. A recent study showed that chimeric antigen receptor (CAR) T cells that target senescent cells can also be effective senolytic agents (Amor et al., 2020).
Targeting gut microbiota.
The potential for modulating gut microbiota as a CAD therapeutic target is being actively explored. Probiotics and prebiotics are commonly taken, but whether they directly influence overall microbial composition in humans is unclear. Dietary modulation can clearly affect the levels of circulating microbial metabolites; for example, plant-based diets reduce TMAO levels and increase short chain fatty acid levels (Tang et al., 2019). Inhibitors of the bacterial lyases that produce TMA have been developed and are highly effective in suppressing TMAO levels in animal models (Roberts et al., 2018).
A HOLISTIC UNDERSTANDING
A coherent understanding of atherosclerosis will entail integration of the various genetic and environmental factors and cross-tissue circuits contributing to the disease. Toward this end, a systemic network view (Bjorkegren et al., 2015; Franzen et al., 2016) of the combined high-dimensional, multi-organ metabolic processes which perturb the biology of the arterial wall leading to atherosclerosis is now gradually emerging (Koplev et al., 2021). Unlike GWAS, these systems studies are based on the integration of clinical phenotypes, DNA genotypes and “DNA activity” measures such as RNA sequence (RNAseq) data aiming to infer models of gene-regulatory networks. Simultaneously considering the activity of thousands of genes across multiple disease-relevant organs, these models incorporate multiple genetic risk loci and candidate pathways, such as those identified by GWAS, combined with influences exerted by environmental risk factors, such as smoking and lifestyle (Figure 6). To build these models, individual genes are first linked based on their similarity of expression across samples or individuals resulting in modules of co-expressed genes. Such co-expression modules have been shown to be evolutionary conserved and reflect pathophysiological states relevant to clinical phenotypes. Next, by applying Bayesian algorithms that consider prior causal information inherent in the co-expression modules such as gene expression-regulatory single nucleotide polymorphisms and transcription factors, the direction of gene-gene interactions in the co-expression modules can be assessed, transforming them into gene-regulatory co-expression networks (GRNs). The information about directions of gene-gene interactions in these GRNs is essential, primarily because it allows the identification of key driver genes. These genes tend to be located at the top of the hierarchy regulating many downstream genes in the GRN. Perturbation experiments of key driver genes using in vivo model systems has demonstrated their efficacy in modulating the activity of entire GRNs as well as downstream phenotypes, including atherosclerosis. This latter characteristic has prompted the term “key disease driver”, and the notion that these genes may be realistic targets for novel interventions. Such approaches may also be useful in defining molecular signals in blood that mirror network activity associated with obstructive CAD/atherosclerosis in people who are at risk of a heart attack or stroke. The usefulness of systems approaches was recently highlighted by Hartman et al. (2021) who used network and key driver biology to elucidate gender-differences in the complex etiology of atherosclerosis.
Figure 6. Gene activity across multiple organs is used to infer network models.
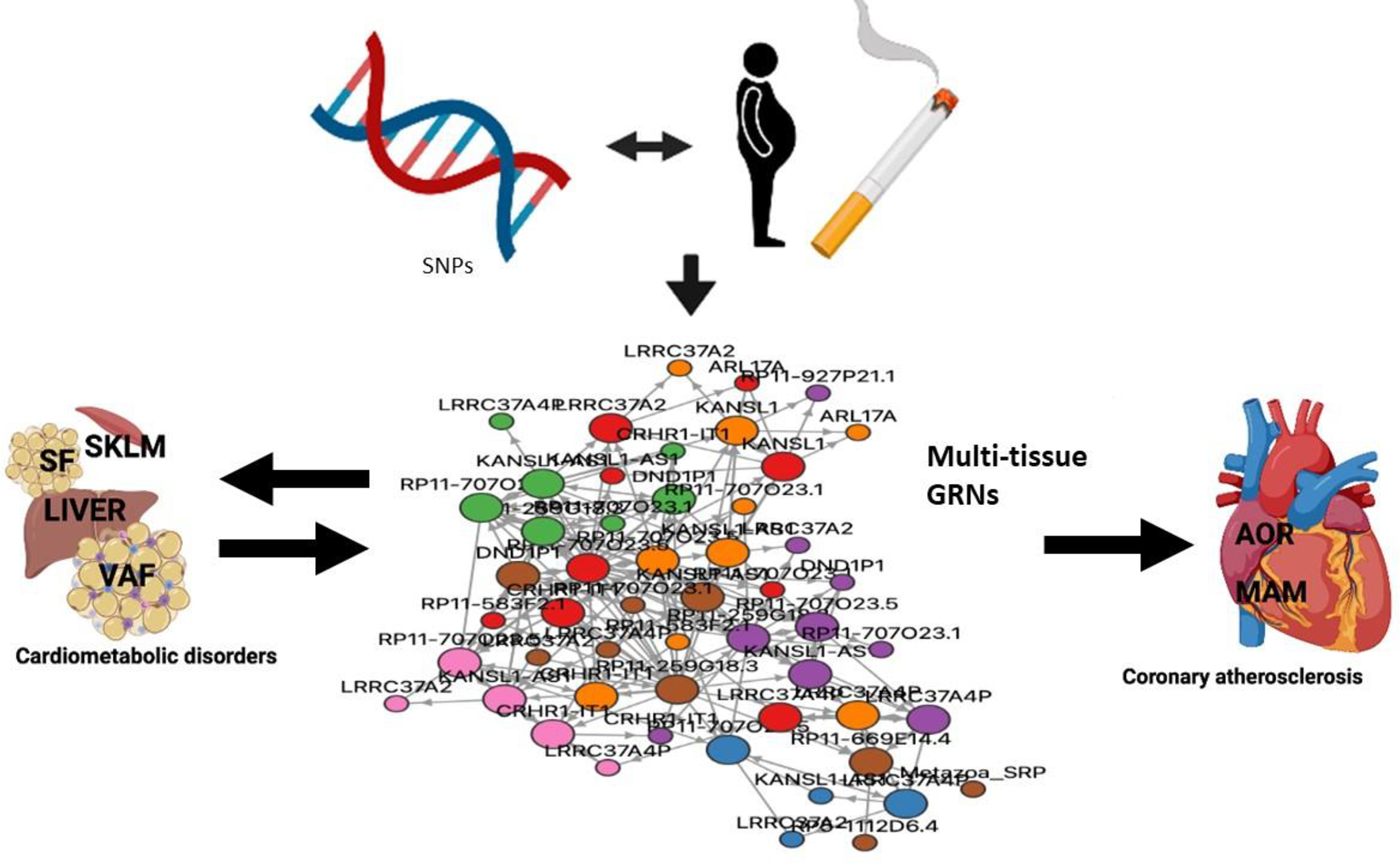
Gene-regulatory co-expression networks (GRNs) capturing genetic (inherited) and environmental risk variation organize genes within and across tissues in groups of biological processes and molecular functions that are relevant to the etiology of coronary atherosclerosis. Together GRNs may provide a mechanistic framework in which isolated candidate pathways and genetic risk loci can be understood from a broader molecular multi-organ context. Such framework may be proven important to fulfill the promises of precision medicine. SNPs, single nucleotide polymorphism; SKLM, skeletal muscle; SF, subcutaneous fat; VAF, visceral abdominal fat; AOR, atherosclerotic aortic wall and, MAM, internal mammary artery. Color coding of GRN represents genes from different tissues (red, AOR; orange, MAM; green, SKLM; brown, LIVER; purple, VAF; pink, SF and blue, WHOLE BLOOD) adopted from STARNET.MSSM.EDU (Koplev et al., 2021).
THE ROAD AHEAD
The last five or ten years have seen remarkable advances in the understanding and treatment of atherosclerosis. Important advances have also occurred on the clinical front, with the introduction of several new therapeutic approaches. Despite the great progress, there are many challenges in moving forward. Among many knowledge gaps are the following: How does lipid accumulation in the subendothelial space promote inflammation? What are the roles of the dozens of genes identified by GWAS that do not fit into any causal category? How important is cellular senescence in the human disease? How do diabetes, hypertension, and kidney disease promote atherosclerosis? What mechanisms contribute to the protective effects of exercise? What factors protect women against early forms of atherosclerosis? One key to addressing these questions will be increasingly sophisticated technologies that allow measurement of gene activity at the level of the genome across disease relevant tissues, down to open chromatin in single cells of the atherosclerotic lesions. Also, combining “top down” (i.e., systems) and “bottom up” (i.e., pathways) approaches will be important for the development of therapies that target multiple facets of atherosclerosis, and that can be tailored to the needs of individual patients in line with the promise of precision medicine. The chances of such therapeutic strategies will likely depend on identifying individuals at risk early on, and noninvasive diagnostics will undoubtedly be a central theme of future study.
ACKNOWLEDGMENTS
We thank our colleagues and students for discussions. AJL particularly thanks Alan Fogelman for introducing him to atherosclerosis research and supporting him through the years. JB thanks Asders Hamsten for introducing him to the field and support in his early career. We thank Rosa Chen for help in the preparation of this manuscript. Our research was supported by funds from the National Institutes of Health (HL144651, HL147883, HL148110), the American Heart Association and the Leducq Foundation (AJL, JB) and the Swedish Research Council (2018-02529) and Swedish Heart Lung Foundation (20170265) (JB).
Footnotes
Declaration of Interests
The authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Johan L. M. Björkegren, Department of Genetics and Genomic Sciences, Department of Medicine, Cardiology, Icahn School of Medicine at Mount Sinai, New York, 10029 New York Department of Medicine, Huddinge, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Aldons J. Lusis, Department of Medicine/Division of Cardiology, Department of Microbiology, Immunology and Molecular Genetics, Department of Human Genetics, A2-237 Center for the Health Sciences, University of California, Los Angeles
REFERENCES
- 1.Abdellatif M, Sedej S, and Kroemer G (2021). NAD(+) metabolism in cardiac health, aging, and disease. Circulation 144, 1795–1817. [DOI] [PubMed] [Google Scholar]
- 2.Aherrahrou R, Guo L, Nagraj VP, Aguhob A, Hinkle J, Chen L, Yuhl Soh J, Lue D, Alencar GF, Boltjes A, et al. (2020). Genetic regulation of atherosclerosis-relevant phenotypes in human vascular smooth muscle cells. Circ Res 127, 1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad F, Mitchell RD, Houben T, Palo A, Yadati T, Parnell AJ, Patel K, Shiri-Sverdlov R, and Leake DS (2021). Cysteamine decreases low-density lipoprotein oxidation, causes regression of atherosclerosis, and improves liver and muscle function in low-density lipoprotein receptor-deficient mice. J Am Heart Assoc 10, e017524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Mashhadi RH, Al-Mashhadi AL, Nasr ZP, Mortensen MB, Lewis EA, Camafeita E, Ravlo K, Al-Mashhadi Z, Kjaer DW, Palmfeldt J, et al. (2021). Local pressure drives low-density lipoprotein accumulation and coronary atherosclerosis in hypertensive minipigs. J Am Coll Cardiol 77, 575–589. [DOI] [PubMed] [Google Scholar]
- 5.Alencar GF, Owsiany KM, Karnewar S, Sukhavasi K, Mocci G, Nguyen AT, Williams CM, Shamsuzzaman S, Mokry M, Henderson CA, et al. (2020). Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation 142, 2045–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, Katz W, Ali HM, Petriello M, Deng P, et al. (2019). XX sex chromosome complement promotes atherosclerosis in mice. Nat Commun 10, 2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, et al. (2020). Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragam KG, and Natarajan P (2020). Polygenic scores to assess atherosclerotic cardiovascular disease risk: clinical perspectives and basic implications. Circ Res 126, 1159–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Back M, Yurdagul A Jr., Tabas I, Oorni K, and Kovanen PT (2019). Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 16, 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backman JD, Li AH, Marcketta A, Sun D, Mbatchou J, Kessler MD, Benner C, Liu D, Locke AE, Balasubramanian S, et al. (2021). Exome sequencing and analysis of 454,787 UK Biobank participants. Nature 599, 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, and Mallat Z (2019). Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16, 727–744. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. (2018). Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar A (2017). Environmental determinants of cardiovascular disease. Circ Res 121, 162–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, Szeto MD, Liao X, Leventhal MJ, Nasser J, et al. (2020). Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorkegren JL, Kovacic JC, Dudley JT, and Schadt EE (2015). Genome-wide significant loci: how important are they? Systems genetics to understand heritability of coronary artery disease and other common complex disorders. J Am Coll Cardiol 65, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boren J, and Williams KJ (2016). The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol 27, 473–483. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera CP, Ng FL, Nicholls HL, Gupta A, Barnes MR, Munroe PB, and Caulfield MJ (2019). Over 1000 genetic loci influencing blood pressure with multiple systems and tissues implicated. Hum Mol Genet 28, R151–R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, and Tao W (2021). Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Childs BG, Zhang C, Shuja F, Sturmlechner I, Trewartha S, Fierro Velasco R, Baker D, Li H, and van Deursen JM (2021). Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat Aging 1, 698–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, and Zernecke A (2018). Single-cell RNA-Seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res 122, 1661–1674. [DOI] [PubMed] [Google Scholar]
- 21.Covarrubias AJ, Perrone R, Grozio A, and Verdin E (2021). NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 22, 119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R, et al. (2017). Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 37, e131–e157. [DOI] [PubMed] [Google Scholar]
- 23.Depuydt MAC, Prange KHM, Slenders L, Ord T, Elbersen D, Boltjes A, de Jager SCA, Asselbergs FW, de Borst GJ, Aavik E, et al. (2020). Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res 127, 1437–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez F, Fuster V, Fernandez-Alvira JM, Fernandez-Friera L, Lopez-Melgar B, Blanco-Rojo R, Fernandez-Ortiz A, Garcia-Pavia P, Sanz J, Mendiguren JM, et al. (2019). Association of sleep duration and quality with subclinical atherosclerosis. J Am Coll Cardiol 73, 134–144. [DOI] [PubMed] [Google Scholar]
- 25.Doran AC, Yurdagul A Jr., and Tabas I (2020). Efferocytosis in health and disease. Nat Rev Immunol 20, 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou H, Kotini A, Liu W, Fidler T, Endo Umeda K, Sun X, Olszewska M, Xiao T, Abramowicz S, Yalcinkaya M, et al. (2021). Oxidized phospholipids promote NETosis and arterial thrombosis in LNK(SH2B3) deficiency. Circulation 144 1940–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eales JM, Maan AA, Xu X, Michoel T, Hallast P, Batini C, Zadik D, Prestes PR, Molina E, Denniff M, et al. (2019). Human Y chromosome exerts pleiotropic effects on susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol 39, 2386–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar L, Akbar N, Braithwaite AT, Krausgruber T, Gallart-Ayala H, Bailey J, Corbin AL, Khoyratty TE, Chai JT, Alkhalil M, et al. (2021). Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 144, 961–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdmann J, Kessler T, Munoz Venegas L, and Schunkert H (2018). A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res 114, 1241–1257. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, et al. (2019). Single-cell immune landscape of human atherosclerotic plaques. Nat Med 25, 1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, Xiao T, Liu W, Thomas DG, Hajebrahimi MA, Pircher J, et al. (2021). The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franzen O, Ermel R, Cohain A, Akers NK, Di Narzo A, Talukdar HA, Foroughi-Asl H, Giambartolomei C, Fullard JF, Sukhavasi K, et al. (2016). Cardiometabolic risk loci share downstream cis- and trans-gene regulation across tissues and diseases. Science 353, 827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F, et al. (2019). Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med 25, 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerlach BD, Ampomah PB, Yurdagul A Jr., Liu C, Lauring MC, Wang X, Kasikara C, Kong N, Shi J, Tao W, et al. (2021). Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab 33, 2445–2463 e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gimbrone MA Jr., and Garcia-Cardena G (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118, 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR, Torstenson ES, Kovesdy CP, Sun YV, Wilson OD, et al. (2019). Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet 51, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goettsch C, Hutcheson JD, Hagita S, Rogers MA, Creager MD, Pham T, Choi J, Mlynarchik AK, Pieper B, Kjolby M, et al. (2016). A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis 251, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, Surakka I, Ntalla I, Vedantam S, Winkler TW, et al. (2021). The power of genetic diversity in genome-wide association studies of lipids. Nature 600, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grootaert MOJ, Finigan A, Figg NL, Uryga AK, and Bennett MR (2021). SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res 128, 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Q, Yang X, Lv J, Zhang J, Xia B, Kim JD, Wang R, Xiong F, Meng S, Clements TP, et al. (2019). AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science 363, 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta M, Blumenthal C, Chatterjee S, Bandyopadhyay D, Jain V, Lavie CJ, Virani SS, Ray KK, Aronow WS, and Ghosh RK (2020). Novel emerging therapies in atherosclerosis targeting lipid metabolism. Expert Opin Investig Drugs 29, 611–622. [DOI] [PubMed] [Google Scholar]
- 42.Gupta R, Lin Y, Luna K, Logue A, Yoon AJ, Haptonstall KP, Moheimani R, Choroomi Y, Nguyen K, Tran E, et al. (2021). Electronic and tobacco cigarettes alter polyunsaturated fatty acids and oxidative biomarkers. Circ Res 129, 514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han YH, Onufer EJ, Huang LH, Sprung RW, Davidson WS, Czepielewski RS, Wohltmann M, Sorci-Thomas MG, Warner BW, and Randolph GJ (2021). Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He C, Hu X, Weston TA, Jung RS, Sandhu J, Huang S, Heizer P, Kim J, Ellison R, Xu J, et al. (2018). Macrophages release plasma membrane-derived particles rich in accessible cholesterol. Proc Natl Acad Sci U S A 115, E8499–E8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, Selgrade D, Fenn AM, Chan CT, Mindur JE, et al. (2019). Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heyde A, Rohde D, McAlpine CS, Zhang S, Hoyer FF, Gerold JM, Cheek D, Iwamoto Y, Schloss MJ, Vandoorne K, et al. (2021). Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 184, 1348–1361 e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilser JR, Han Y, Biswas S, Gukasyan J, Cai Z, Zhu R, Tang WHW, Deb A, Lusis AJ, Hartiala JA, et al. (2021). Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J Lipid Res 62, 100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hindy G, Aragam KG, Ng K, Chaffin M, Lotta LA, Baras A, Regeneron Genetics C, Drake I, Orho-Melander M, Melander O, et al. (2020). Genome-wide polygenic score, clinical risk factors, and long-term trajectories of coronary artery disease. Arterioscler Thromb Vasc Biol 40, 2738–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. (2017). Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 377, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, Mehrabian M, Denu JM, Bäckhed F, Lusis AJ, et al. (2018). Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol 3, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim-Hellmuth S, Aguet F, Oliva M, Munoz-Aguirre M, Kasela S, Wucher V, Castel SE, Hamel AR, Vinuela A, Roberts AL, et al. (2020). Cell type-specific genetic regulation of gene expression across human tissues. Science 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koplev S, Seldin M, Sukhavasi K, Ermel R, Pang S, Zeng L, Bankier S, di Narzo A, Cheng H, Meda V, et al. (2021). A mechanistic framework for cardiometabolic and coronary artery diseases. Nat Cardiovasc Res In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotla S, Vu HT, Ko KA, Wang Y, Imanishi M, Heo KS, Fujii Y, Thomas TN, Gi YJ, Mazhar H, et al. (2019). Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koyama S, Ito K, Terao C, Akiyama M, Horikoshi M, Momozawa Y, Matsunaga H, Ieki H, Ozaki K, Onouchi Y, et al. (2020). Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat Genet 52, 1169–1177. [DOI] [PubMed] [Google Scholar]
- 56.Libby P (2021). The changing landscape of atherosclerosis. Nature 592, 524–533. [DOI] [PubMed] [Google Scholar]
- 57.Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, Saleheen D, Emdin C, Alam D, Alves AC, et al. (2017). Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet 49, 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo X, Lv Y, Bai X, Qi J, Weng X, Liu S, Bao X, Jia H, and Yu B (2021). Plaque erosion: A distinctive pathological mechanism of acute coronary syndrome. Front Cardiovasc Med 8, 711453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, et al. (2018). Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50, 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Man JJ, Beckman JA, and Jaffe IZ (2020). Sex as a biological variable in atherosclerosis. Circ Res 126, 1297–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, et al. (2019). Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, and Virmani R (2018). Coronary artery calcification and its progression: What does it really mean? JACC Cardiovasc Imaging 11, 127–142. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee P, Chattopadhyay A, Girjalva V, Dorreh N, Lagishetty V, Jacobs JP, Clifford BL, Vallim T, Mack JJ, Navab M, et al. (2021). Oxidized phospholipids cause changes in jejunum mucus that induce dysbiosis and systemic inflammation. J Lipid Res, 100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, Wang K, Iyer S, Dutta C, Clendaniel V, et al. (2021). In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 593, 429–434. [DOI] [PubMed] [Google Scholar]
- 65.Newman AAC, Serbulea V, Baylis RA, Shankman LS, Bradley X, Alencar GF, Owsiany K, Deaton RA, Karnewar S, Shamsuzzaman S, et al. (2021). Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRbeta and bioenergetic mechanisms. Nat Metab 3, 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nikpay M, Beehler K, Valsesia A, Hager J, Harper ME, Dent R, and McPherson R (2019). Genome-wide identification of circulating-miRNA expression quantitative trait loci reveals the role of several miRNAs in the regulation of cardiometabolic phenotypes. Cardiovasc Res 115, 1629–1645. [DOI] [PubMed] [Google Scholar]
- 67.Noonan J, Bobik A, and Peter K (2022). The tandem stenosis mouse model: Towards understanding, imaging, and preventing atherosclerotic plaque instability and rupture. Br J Pharmacol 179, 979–997. [DOI] [PubMed] [Google Scholar]
- 68.Ord T, Ounap K, Stolze LK, Aherrahrou R, Nurminen V, Toropainen A, Selvarajan I, Lonnberg T, Aavik E, Yla-Herttuala S, et al. (2021). Single-cell epigenomics and functional fine-mapping of atherosclerosis GWAS loci. Circ Res 129, 240–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orecchioni M, Kobiyama K, Winkels H, Ghosheh Y, McArdle S, Mikulski Z, Kiosses WB, Fan Z, Wen L, Jung Y, et al. (2022). Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production. Science 375, 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan H, Xue C, Auerbach BJ, Fan J, Bashore AC, Cui J, Yang DY, Trignano SB, Liu W, Shi J, et al. (2020). Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasta A, Cremonini AL, Pisciotta L, Buscaglia A, Porto I, Barra F, Ferrero S, Brunelli C, and Rosa GM (2020). PCSK9 inhibitors for treating hypercholesterolemia. Expert Opin Pharmacother 21, 353–363. [DOI] [PubMed] [Google Scholar]
- 72.Pierce JB, and Feinberg MW (2020). Long noncoding RNAs in atherosclerosis and vascular injury: Pathobiology, biomarkers, and targets for therapy. Arterioscler Thromb Vasc Biol 40, 2002–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, and Orekhov AN (2020). The diabetes mellitus-atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Proto JD, Doran AC, Gusarova G, Yurdagul A Jr., Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, et al. (2018). Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity 49, 666–677 e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Maatta A, Gaddis DE, Bowden K, et al. (2018). Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. (2013). Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19, 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, et al. (2018). Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med 24, 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roy P, Orecchioni M, and Ley K (2021). How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sage AP, Tsiantoulas D, Binder CJ, and Mallat Z (2019). The role of B cells in atherosclerosis. Nat Rev Cardiol 16, 180–196. [DOI] [PubMed] [Google Scholar]
- 80.Sanna S, Kurilshikov A, van der Graaf A, Fu J, and Zhernakova A (2022). Challenges and future directions for studying effects of host genetics on the gut microbiome. Nat Genet 54, 100–106. [DOI] [PubMed] [Google Scholar]
- 81.Schloss MJ, Swirski FK, and Nahrendorf M (2020). Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res 126, 1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seldin M, Yang X, and Lusis AJ (2019). Systems genetics applications in metabolism research. Nat Metab 1, 1038–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Souilhol C, Serbanovic-Canic J, Fragiadaki M, Chico TJ, Ridger V, Roddie H, and Evans PC (2020). Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol 17, 52–63. [DOI] [PubMed] [Google Scholar]
- 84.Stolze LK, Conklin AC, Whalen MB, Lopez Rodriguez M, Ounap K, Selvarajan I, Toropainen A, Ord T, Li J, Eshghi A, et al. (2020). Systems genetics in human endothelial cells identifies non-coding variants modifying enhancers, expression, and complex disease traits. Am J Hum Genet 106, 748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tabas I, and Bornfeldt KE (2020). Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ Res 126, 1209–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang WHW, Backhed F, Landmesser U, and Hazen SL (2019). Intestinal microbiota in cardiovascular health and cisease: JACC State-of-the-Art Review. J Am Coll Cardiol 73, 2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas DG, Wei Y, and Tall AR (2021). Lipid and metabolic syndrome traits in coronary artery disease: a Mendelian randomization study. J Lipid Res 62, 100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsimikas S, Gordts P, Nora C, Yeang C, and Witztum JL (2020). Statin therapy increases lipoprotein(a) levels. Eur Heart J 41, 2275–2284. [DOI] [PubMed] [Google Scholar]
- 89.Tyrrell DJ, and Goldstein DR (2021). Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol 18, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, Schunkert H, and Lusis AJ (2017). Applications and limitations of mouse models for understanding human atherosclerosis. Cell Metab 25, 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Gao H, Wang F, Ye Z, Mokry M, Turner AW, Ye J, Koplev S, Luo L, Alsaigh T, et al. (2021). Dynamic changes in chromatin accessibility are associated with the atherogenic transitioning of vascular smooth muscle cells. Cardiovasc Res Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams SA, Kivimaki M, Langenberg C, Hingorani AD, Casas JP, Bouchard C, Jonasson C, Sarzynski MA, Shipley MJ, Alexander L, et al. (2019). Plasma protein patterns as comprehensive indicators of health. Nat Med 25, 1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, et al. (2018). Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res 122, 1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, et al. (2019). Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med 25, 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie Y, Xu E, Bowe B, and Al-Aly Z (2022). Long-term cardiovascular outcomes of COVID-19. Nat Med 28, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin F, Gupta R, Vergnes L, Driscoll WS, Ricks J, Ramanathan G, Stewart JA, Shih DM, Faull KF, Beaven SW, et al. (2019). Diesel exhaust induces mitochondrial dysfunction, hyperlipidemia, and liver steatosis. Arterioscler Thromb Vasc Biol 39, 1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang X, Sessa WC, and Fernandez-Hernando C (2018). Endothelial transcytosis of lipoproteins in atherosclerosis. Front Cardiovasc Med 5, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao Q, Wang J, Miao Z, Zhang NR, Hennessy S, Small DS, and Rader DJ (2021). A Mendelian randomization study of the role of lipoprotein subfractions in coronary artery disease. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


