Abstract
Methamphetamine (METH) use, and misuse are associated with severe socioeconomic consequences. METH users develop tolerance, lose control over drug taking behaviors, and suffer frequent relapses even during treatment. The clinical course of METH use disorder is influenced by multifactorial METH-induced effects on the central and peripheral nervous systems. Although these METH-induced consequences are observed in humans of all ages, races, and sexes, sexual dimorphism in these outcomes have been observed in both pre-clinical and clinical settings. In this review, we have provided a detailed presentation of the sex differences reported in human and animal studies. We have therefore presented data that identified the influences of sex on METH pharmacokinetics, METH-induced changes in behaviors, cognitive processes, structural changes in the brain, and the effects of the drug on neurotransmitter systems and molecular mechanisms. Finally, we highlighted the potential significance of sex as a critical variable that should be considered when planning the development of new pharmacotherapeutic approaches against MEH use disorder in humans.
Keywords: Adverse consequences, Genetics, Methamphetamine, Sex differences, Treatment
1. Introduction
Methamphetamine (METH) belongs to a family of psychostimulants known as amphetamine-type stimulants (ATS). These include amphetamine, methylenedioxy-N-methylamphetamine (MDMA), and other designer amphetamines (UNODC, 2021). METH use and misuse are highly prevalent and impact socioeconomic standards around the world (UNODC, 2021; Zhao et al., 2021b). Despite the associated risks, the misuse of METH has continued unabated (UNODC, 2021). In the United States of America (USA), there is an annual METH use prevalence of 1% among males and 0.6% among females (NSDUH, 2019). Repeated METH misuse leads to loss of control over drug use, compulsive use despite negative consequences, and multiple relapse episodes in humans who meet diagnostic criteria for METH use disorder (MUD) (DSM-5, 2013). Multi-system dysfunctions can accompany the misuse of METH even in those who may or may not have met criteria for MUD (Jayanthi et al., 2021; Miller et al., 2021). These adverse consequences may include loss of appetite, anxiety, depression, delusional ideations including full-blown paranoia, tactile hallucinations, suicidal ideation, and successful suicides (Darke et al., 2019; Glasner-Edwards et al., 2011; Lisa et al., 2019; Mahoney et al., 2010, 2008; Polcin et al., 2012; Su et al., 2017). It is important to note that prolonged METH use can also be associated with neurological and neurotoxic damage to the brain (Bae et al., 2006; Bonk et al., 2020; Du et al., 2015; Hall et al., 2015; Kogachi et al., 2017; Nie et al., 2021; NSDUH, 2019; Paknahad et al., 2021; Tobias et al., 2010). Studies conducted in various animal models have provided some clues to the molecular and biochemical mechanisms involved in METH-induced neurodegenerative changes in the brain (Jayanthi et al., 2021; Miller et al., 2021). Nevertheless, there is evidence that METH might also cause its clinical and other deleterious effects in a sex-dependent fashion (Daiwile et al., 2021, 2019). Further clarification of these sex-relevant phenomena is pertinent in terms of the support in many circles for discussions of therapeutic approaches that are sexually dimorphic (Gillies and McArthur, 2010; Mauvais-Jarvis et al., 2021; Morris et al., 2003; Nicolas et al., 2022). In addition to abnormalities in brain functions, METH has negative consequences on peripheral organs. Those include METH-associated cardiovascular diseases that represent the second leading causes of deaths following accidental overdoses. Of clinical importance is the fact that METH-induced cardiovascular abnormalities become evident at younger ages in comparison to their incidence in the general population (Kevil et al., 2019).
As part of our efforts to provide a neurobiological framework for sex differences in responses to METH, we have conducted a few studies that have documented distinctive behavioral and molecular responses to METH in male and female rats (Daiwile et al., 2021, 2019). We thus endeavored to write this review in order to provide an easily accessible reference to the epidemiological, clinical, and biochemical data comparing the responses of males and females to METH exposure.
2. Epidemiology of METH use and misuse
METH is the second most commonly used illegal drug after cannabis (Stoneberg et al., 2018). In 2019, approximately 27 million individuals used ATS globally, a number that represented 0.5% of the adult world population (UNODC, 2021). The highest frequency of METH use was reported in North America with 2.3% of the population aged 15–64 using the drug. This was followed by Australia and New Zealand with 1.3%, and Asia with 0.5% of their populations, respectively (UNODC, 2021). METH is the most commonly used ATS drug and contributes to 95% of all illegal ATS synthesized globally (UNODC, 2021). Sadly, there was a seven-fold increase in the quantities of ATS seized in 2017 in comparison to 2009, with 80% of total global seizures remaining concentrated in the United States, Thailand, and Mexico (UNODC, 2021). Between the years 2015–2018 in the United States, approximately 1.6 million adults aged above 18 used METH at least once in their lifetime. The estimated rate of METH use was approximately 6.6 per 1000 individuals (Jones et al., 2020a; NSDUH, 2019). Importantly, 52.9% of the adults that used METH develop a diagnosable MUD, with 27.3% users reporting that they used METH more than 200 days per year (Jones et al., 2020a). This is disturbing from a public health perspective.
Sex differences have also been identified in epidemiological data referring to METH use. It has been reported that the average annual METH use among men above 18 years was 8.7 per 1000 adults whereas, among women, it was 4.7 per 1000 adults (Jones et al., 2020a). Male METH users had also taken METH for longer periods of time (14.9 years) than females (10.6 years) (Salo et al., 2011) even though female METH users were more likely to use METH as their first substance than males (Yen et al., 2005). In addition, women who reported METH as their first drug of choice also suffered from heavier and greater lifetime episodes of METH use than men (Hartwell et al., 2016; Rungnirundorn et al., 2017; Su et al., 2017; Yimsaard et al., 2018). The differences in initial use may be related to the fact that women who misuse METH were more likely to use the drug to lose weight (Brecht et al., 2004; Kondo et al., 2021) or because they had suffered from childhood sexual abuse before age of 15 (44%) compared to men (24%) (Brecht et al., 2004). More men meet diagnostic criteria for MUD than women (Cservenka and Ray, 2017; Temmingh et al., 2020). Moreover, recent data from Canadian Center on Substance Use and Addiction (CCSA, 2020) indicated that the occurrence of lifetime METH use was 5.4% in males and 2.2% in females among individuals older than 15 years old. The prevalence of METH use in school children (grades 7–12) was 1.6% in boys compared to 1% in girls (CCSA, 2020). Boys also initiated METH use at an earlier age and were at a greater risk for developing MUD than girls (Apidechkul et al., 2020; Saw et al., 2017). Furthermore, Cloak et al. (2011) also identified a significant correlation between age and log cumulative lifetime methamphetamine in males (r = 0.75; p < 0.001) but not in females (r = 0.2; p = 0.3).
Data from treatment centers provided evidence that males admitted to residential drug treatment programs showed earlier onset and early dependence in comparison to females (age of onset: 17.7 vs 19.7; age for developing dependence: 20.4 vs 22.2, for males and females, respectively) (Rungnirundorn et al., 2017). A very recent report from Australia has shown that clinically endorsed psychological and social support services were more likely availed by men, 73.3% in comparison to 26.3% women (Beck et al., 2021). It is, however, interesting to note that, during adolescence, females had reported that METH was their primary drug of choice (63.7%) compared with adolescent males (15%) (Rawson et al., 2005) and are over-represented in receiving treatment for METH misuse (Gonzales et al., 2008). The mode of METH intake was also found to vary across sexes, with males more likely to use injections of the drug than females who prefer to snort it (Brecht et al., 2004; Galloway et al., 2010). Large-scale epidemiological studies using populations from different countries are needed to identify and clarify some of the relevant sex differences related to initial METH use and the long-term consequences of METH misuse.
In men, the adjusted odd ratios for METH use were associated with lower education, lower household incomes, no medical insurance, living in non-metro counties, and co-occurring mental illness (Jones et al., 2020a). In women, METH use was associated with poorer quality of life and mental health status (Gonzales et al., 2011; Lin et al., 2004; McKetin et al., 2019). However, psychiatric problems and adverse consequences associated with METH use were more prominent in women compared to men (Simpson et al., 2016). Similarly, problems related to jobs and family/social relationships were more severe in women than men (Han et al., 2016; Hser et al., 2005; Lin et al., 2004). Higher rates of criminal behaviors were reported in men who use METH than women (Brecht et al., 2004; Hser et al., 2005; Lin et al., 2004; Nie et al., 2021; Simpson et al., 2016). These include young male METH users (54%) who are more likely to have criminal records than females (28%) (Brecht et al., 2004). In Sweden, data from driving under the influence of drugs showed predominance of male METH users compared to females (Jones and Holmgren, 2012).
Data from emergency department (ED) admissions showed that the majority of individuals admitted with urines positive for METH were males even though the heavier (odd ratio 2.6, p < 0.0001) users were female patients (Richards et al., 2020). Earlier studies had documented increases in ED visits by male patients (44,008 in 2007–63,092 in 2011) compared to females (23,942 in 2007–39,869 in 2011) (Mattson, 2013). Reports from EDs in other parts of the world also documented higher admissions of males than females. Specifically, Jones et al. (2019) from Australia and He et al. (2013) from China reported higher METH-related hospitalizations of men than women. These data are consistent with the conclusions reached by Jones et al. (2018) that males might experience more problematic METH use and misuse that lead to ED visits and hospital admissions.
METH poisoning is also reported to show sexual dimorphism. For example, males comprised 59.4% and females 40.6% of the reported METH poisoning cases (McFaull et al., 2020). Interestingly, the number of female cases was higher among younger women (10–19 years old) whereas more males aged 20 years and older were poisoned (McFaull et al., 2020). Cumulative METH overdose deaths increased by 7.5 times in 2017 compared to 2007 (NSDUH, 2019), with males (67.5%) experiencing more overdoses than females (32.5%) (Bonk et al., 2020). Darke and his group reported that the majority of stroke cases in Australia were in males (60.5%) compared to females (39.5%), with females being significantly younger than males (36.6 vs.42.7 years) when they had suffered from strokes (Darke et al., 2017b, 2018). They also reported that males had higher cardiovascular deaths than females (Darke et al., 2017a). Fig. 1 presents the epidemiological summary of sex differences in METH consumption and associated adverse health outcomes.
Fig. 1.
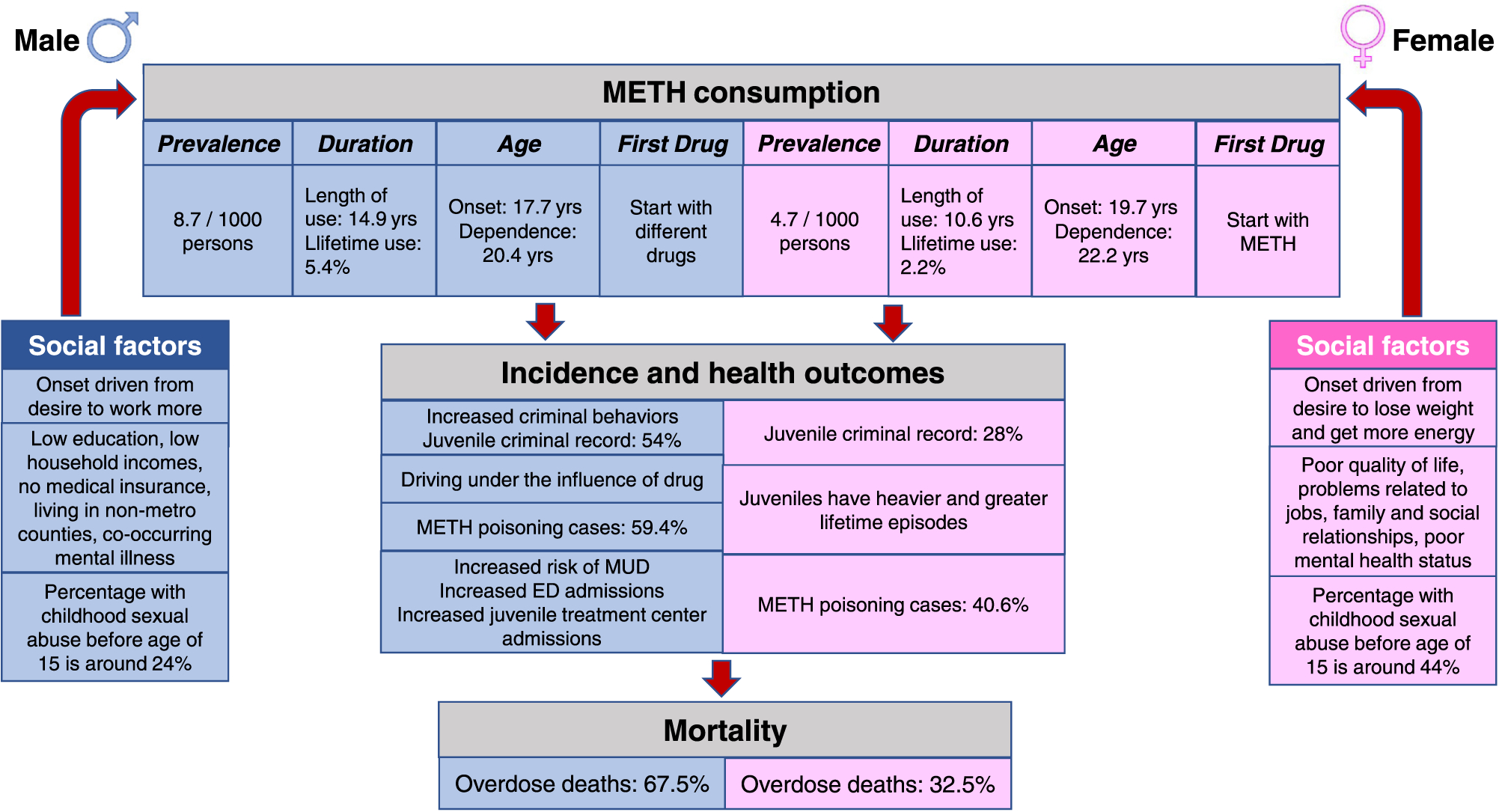
Sex differences in the epidemiology and outcomes of METH use in human populations. This figure provides an illustrative summary of observations from clinical studies that reported sexual dimorphism in METH use and health outcomes. METH consumption, health outcomes, and overdose deaths are reported to be worse in males than in females. Risk for development of METH use disorder during drug misuse and associated criminal behaviors were also more prominent in males than females.
3. Genetic variabilities among METH users of different sexes
There is some evidence that genetic predispositions might play a role in the development of and/or clinical course of MUD. Genome-wide association study (GWAS) showed significant gender-specific correlations of various single nucleotide polymorphisms (SNPs) with frequency of METH use (Heinzerling et al., 2016) (see Fig. 2 for details). In males, significantly associated SNPs included those related to cAMP responsive element binding protein 1 (CREB1, rs7591784 and rs2709386), methyltransferase 21 A, HSPA lysine (METTL21A, rs2709386), cadherin 13 (rs11640875 and rs12922394), brain-derived neurotrophic factor (BDNF, rs6265), and teneurin transmembrane protein 4 (TENM4, rs12576775). In females, the prominent SNPs included those associated with WD repeat domain 41 (WDR41, rs163030), cholinergic receptor nicotinic alpha 5 subunit (CHRNA5, rs588765), and cadherin 13 (rs192599) (Heinzerling et al., 2016). In addition, the risk of METH misuse was associated with Glutathione S-transferase (GST) M1 and T1, with frequencies of GSTM1 null genotype being significantly higher only for females (p = 0.02) and, thus, potentially contributing to the vulnerability of METH dependence in female subjects (Koizumi et al., 2004; Nakatome et al., 2009). However, there were no significant sex differences in the GST T1 polymorphism among METH abusers (Nakatome et al., 2009). Additionally, the SNP, rs2279020, in the gamma-aminobutyric acid receptor subunit alpha-1 (GABRA1) gene was reported only in female METH users (Lin et al., 2003). Kobayashi and colleagues also found that the SNP rs5751876 in the adenosine A2A receptor gene (ADORA2A) and SNPs, rs1372520, rs3756063, and rs3756059 in the alpha-synuclein gene, were associated with METH dependence and psychosis in only female METH users (Kobayashi et al., 2004, 2010). The Val/Val genotype of BDNF appears to also show a higher frequency in female METH users (Heinzerling and Shoptaw, 2012). Although these observations had suggested that female METH users are more likely to exhibit a higher number of SNPs than male users, there is also evidence that other SNPs might also be more frequent in male METH users.
Fig. 2.
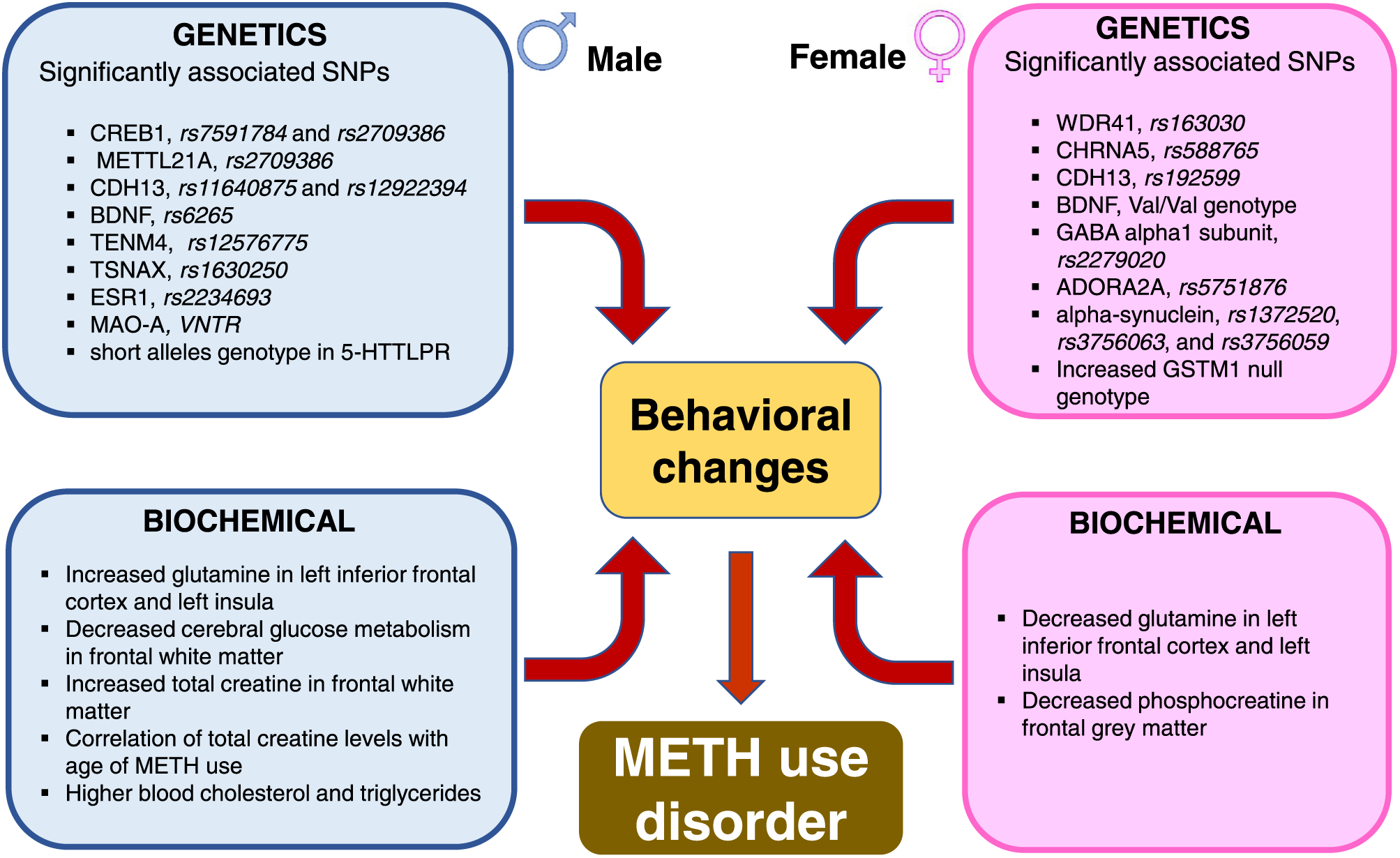
Genetic and biochemical factors associated with METH use disorder. Genetic predilections to METH use disorder vary between males and females. Repeated exposure to the drug is associated with different biochemical outcomes. The figure illustrates sex-specific biochemical responses in glutamine, creatine, blood cholesterol and triglycerides levels.
For example, early onset of METH use in men is associated with short allele (SS) genotype in the serotonin transporter (SERT) linked polymorphic region within the promoter region of SERT (Johnson et al., 2010). Analysis of genotypic and allelic distributions for monoamine oxidase A (MAO-A), an important enzyme involved in DA oxidative metabolism, showed polymorphisms at the promoter region (30 bp of u variable-number tandem repeat -VNTR) in male METH users who suffer from persistent psychosis when compared to control subjects or to male METH users who only manifested transient psychotic episodes. In contrast, female METH users did not exhibit similar genetic markers. However, the possibility that the low number of female participants (n = 29) compared to male participants (n = 97) enrolled in the study might have impacted these results (Nakamura et al., 2009). The presence of major psychiatric disorders was also significantly associated with the frequency of the SNP rs1630250 of the translin-associated factor X gene (TSNAX) in METH-dependent males (Kishi et al., 2011). Similarly, SNP rs2234693 in the estrogen receptor alpha gene (ESR1) was reported to be associated with METH-induced psychosis only in male METH users (Kishi et al., 2009). Taken together, these results indicate that substantial, but dissimilar, genetic factors might participate in some of the sexual dimorphic presentations of humans who use or misuse METH (Fig. 2).
4. Sexual dimorphism in the clinical manifestations of METH use disorder
METH use disorder is a neuropsychiatric disorder that impacts both male and female users negatively (Cservenka and Ray, 2017; Darke et al., 2011; DiMiceli et al., 2016; Han et al., 2016; He et al., 2020; Lisa et al., 2019; Mayo et al., 2019; McKetin et al., 2011; Mihan et al., 2018; Neumann et al., 2018; Polcin et al., 2012; Su et al., 2017; Zhao et al., 2021b). In general, humans who carry a MUD diagnosis suffer from cognitive deficits that appear to be related to METH-induced neuropathological abnormalities that include biochemical and structural changes in the brain (Bae et al., 2006; Cadet and Bisagno, 2015; Cadet et al., 2014; Chang et al., 2002; Cloak et al., 2011; Hall et al., 2015; Heinzerling et al., 2016; Heinzerling and Shoptaw, 2012; Johnson et al., 2010; Kim et al., 2005; King et al., 2010a; Kishi et al., 2011; Kogachi et al., 2017; Koizumi et al., 2004; Lin et al., 2003; Nakatome et al., 2009; Nie et al., 2021; O’Neill et al., 2014; Sung et al., 2013; Tang et al., 2019; Tobias et al., 2010). Related to the theme of this review, however, METH-induced neuropsychiatric complications have been reported to be more common in women than men (Lisa et al., 2019; Mihan et al., 2018; Neumann et al., 2018) (see Fig. 3).
Fig. 3.
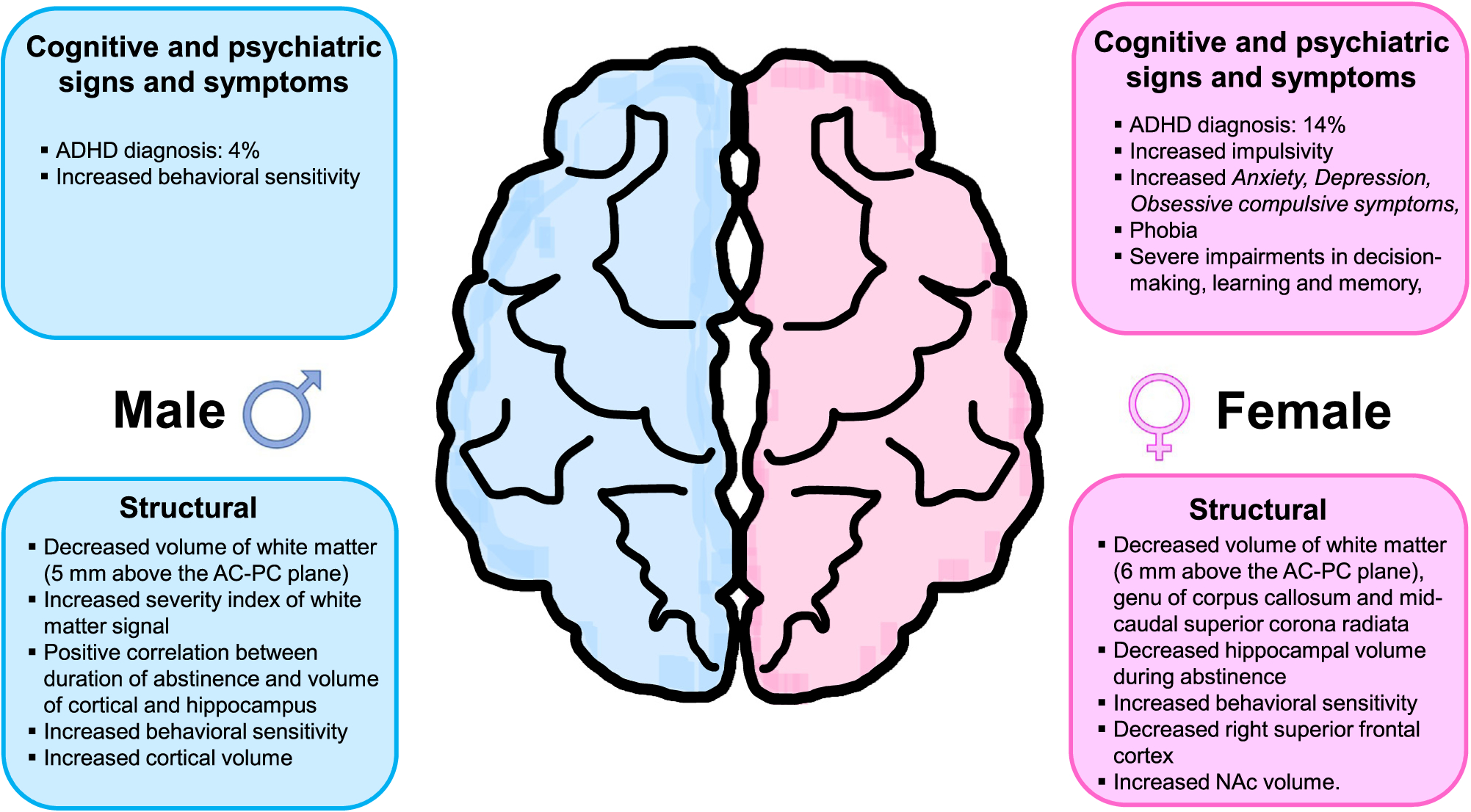
Sexual dimorphism in cognitive processes and structural pathologies in METH users. This figure summarizes cognitive, psychiatric, and structural differences reported between male and female METH users. Some of adverse psychiatric consequences were more prominent in women than men.
4.1. Sex differences in methamphetamine-induced behavioral and cognitive changes
Women have been reported to be more sensitive to METH-induced behavioral and subjective effects of METH (Mayo et al., 2019). For example, METH administration produced faster reaction times, increased subjective ratings of vigor, and greater sedation in women compared to men (Mayo et al., 2019). It has also been reported that non-treatment seeking female METH users exhibited greater impulsivity than males according to the BIS-11 (Barratt Impulsiveness Scale- a questionnaire designed to assess the personality/behavioral construct of impulsiveness) (Cservenka and Ray, 2017). Nevertheless, the authors reported a significant positive correlation between the total impulsivity score and number of years of METH use only in male participants (Cservenka and Ray, 2017). In addition, Morales et al. (2015) documented greater METH craving behavior in women than in men, identified by a drug use survey where participants are instructed to select multiples of 10 between 0 (“not at all”) and 100 (“strongest ever”) that best corresponded with their craving for METH in the last 24 h. Moreover, Zhao et al. (2021a) reported that females exhibited more frequent withdrawal symptoms than males. METH withdrawal symptoms in females were also accompanied by higher percentages of withdrawal-related hypersomnia (Females: 77.2% vs Males: 64.8%), fatigue (Females: 77.5% vs Males: 70.3%), and psychomotor retardation (Females: 64.5% vs Males: 57.0%) (Rungnirundorn et al., 2017). Similarly, Su et al. (2017) provided evidence that females suffer more anxiety-like symptoms than males during acute METH withdrawal. In addition, a recent report by Duncan et al. (2021) showed that females who smoke METH suffered severe anxiety. In contrast, Hartwell et al. (2016) found a significant relationship between depression, anxiety symptoms, and METH craving in men, but not in women. Of significant therapeutic interest, women METH users reported greater severity of a number of psychiatric problems that included bulimia, panic, and psychosis (Polcin et al., 2012), data consistent with a long-term follow-up of MUD patients that had found a higher prevalence of bulimia nervosa in METH-dependent female participants (Glasner-Edwards et al., 2011). Finally, female METH users have also reported higher frequency of sleep problems (75.6% vs 52.4%) and poorer sleep quality than male users (He et al., 2020).
Several reports have shown that METH misuse caused more symptoms of major depression and post-traumatic stress disorder (PTSD) in women than in men (Brecht et al., 2004; DiMiceli et al., 2016; Lisa et al., 2019; McKetin et al., 2011; Polcin et al., 2012). Sexually dimorphic responses to the trier social stress test (TSST) in METH users might be relevant to those observations because young female METH-dependent individuals exhibited significantly higher cortisol levels that were associated with more psychiatric symptoms (King et al., 2010b). Women also showed greater psychiatric composite score for depression and anxiety (Glasner-Edwards et al., 2009; van der Plas et al., 2009; Yen and Chong, 2006; Zweben et al., 2004). Assessment of global distress that includes depression, anxiety, obsessive compulsive, and phobia has also revealed that female users are at higher risks than male METH users (Darke et al., 2011). Of interest, a psychiatric diagnosis of depression was more often co-morbid in women with METH use disorder (Kalechstein et al., 2000). Female patients also suffer higher prevalence of suicidal ideation (Auten et al., 2012; Glasner-Edwards et al., 2008; Hser et al., 2005; Kalechstein et al., 2000; Lin et al., 2004; Zweben et al., 2004). Female METH users who attempted suicide were more likely to be younger than male users (median: 35 vs.40, p < 0.01) (Marshall et al., 2011). Interestingly, although female METH users experience higher suicidal ideation, males were more likely to commit successful suicides (Darke et al., 2019). Female subjects during withdrawal reported more olfactory and tactile hallucinations as well as paranoid delusions (Mahoney et al., 2010, 2008). METH-induced cognitive disorders appear to also be more pronounced in women than in men (Neumann et al., 2018). Specifically, women with MUD showed severe impairments in decision-making (van der Plas et al., 2009), concentration, and learning and memory (Han et al., 2016).
It is likely that the greater neurological and cognitive/emotional impairments observed in women METH users might be secondary to the fact that females experience heavier and greater lifetime episodes of METH use and also suffer more frequent relapses than men (Hartwell et al., 2016; Morales et al., 2015; Richards et al., 2020; Rungnirundorn et al., 2017; Su et al., 2017; Yimsaard et al., 2018). It is also not farfetched to suggest that abnormalities in functional gene polymorphisms in genes such as BDNF, alpha-synuclein, CHRNA5, GSTM1, GABRA1, and ADORA2A that regulate synaptic plasticity and neuro-adaptations in the brain might serve as important contributing factors for the observed increased susceptibility to psychotic behaviors observed in female METH users.
4.2. Sex differences in therapeutic responses
There is still no FDA-approved medication for MUD even though it is very clear that this neuropsychiatric disease can result in major adverse health outcomes including overdoses. The accumulated data indicate that sex is important factor to consider when planning any therapeutic interventions because of sexually dimorphic responses to METH. In what follows, we discuss some issues that are relevant to responses to approaches to treatment of individuals with MUD.
Some investigators have attempted to use several pharmacological agents to treat MUD. These include aripiprazole, bupropion, mirtazapine, baclofen, gabapentin, topiramate, rivastigmine, dextroamphetamine, methylphenidate, modafinil, naltrexone, ibudilast, and N-acetylcysteine (Morley et al., 2017). However, only few studies are available to assess sex-based responses to pharmacological treatment in MUD. Ray et al. (2015) assessed the effects of naltrexone, an opioid antagonist that has been approved by FDA for the treatment of alcohol and opioid dependence (Anton et al., 2006; Cornish et al., 1997; O’Malley et al., 1992; Volpicelli et al., 1992). They found that naltrexone reduced cue-induced craving and subjective responses to METH (Ray et al., 2015), with females reporting lower ‘like drug effects’ and demonstrating a larger naltrexone effect than males. Topiramate, a carbonic anhydrase inhibitor, that is reported to suppress craving for alcohol, nicotine, cocaine and eating disorders (Johnson et al., 2005, 2003; Kampman et al., 2004; Leombruni et al., 2009) was also reported to reduce METH dependence, more in male than in female METH users (Ma et al., 2013; Rezaei et al., 2016). A small clinical study with adolescent METH users also reported that males did better in treatment with bupropion, a dual inhibitor of norepinephrine and dopamine reuptake, than females (Heinzerling et al., 2013).
Lin et al. (2004) reported that Taiwanese women were more likely to seek treatment frequently than men (Lin et al., 2004). Price et al. (2011) conducted a clinical study that included several female METH users (N = 51), with the number of American female METH users (75%) enrolled in drug treatment programs being significantly higher than males. Meta-analysis data of METH-related treatment admissions from the year 2008–2017 indicated that females (19.2% in 2008 to 28.3% in 2017) were more likely to be admitted than males (11.7% in 2008 to 20.9% in 2017) (Jones et al., 2020b). In addition, the average duration of initial treatment episode was significantly longer for female METH subjects (3.9 months) than for males (2.8 months) (Brecht et al., 2004). Importantly, women appear more likely to respond to treatment than males (Brecht et al., 2006; Han et al., 2016). Women are more likely to improve in post-treatment outcomes including family relationships, medical and psychiatric problems, and drug use (Hser et al., 2005). Women also exhibited reduced criminal activity (Brecht et al., 2006). On the other hand, intriguingly, men METH users were better at securing jobs (Brecht et al., 2004; Hser et al., 2005). These data support the notion of integrated treatment programs that include both pharmacological and psychological modalities since these interventions appear to be very effective in female METH users.
4.3. Sex differences in biochemical effects of METH use
A recent MRI study using abstinent METH users identified a time-dependent downregulation in glutamatergic metabolism in multiple cortical regions (Tang et al., 2019). The authors found, in addition, that there were decreases in glutamine levels in the left inferior frontal cortex and left insula of female METH users but increases in the levels of male METH users (Tang et al., 2019). In contrast, an older report by O’Neill et al. (2014) observed decreases in glutamine levels in cortical (posterior cingulate, precuneus, and right inferior frontal cortex) brain regions in both sexes, with females showing somewhat greater magnitude of decreases (−7.2%) in the left inferior frontal cortex than males (−3.1%). It is important to note that the sample size of control participants was higher (N = 45) in Tang’s study, a fact that might have provided adequate statistical power for Tang and his colleagues to detect significant sex differences in glutamine levels in the brain (Tang et al., 2019).
METH use is also associated with changes in cerebral glucose metabolism, phosphocreatine, and total creatine levels (Cloak et al., 2011; Kim et al., 2005; Sung et al., 2013). Using positron emission tomography, Kim et al. (2005) reported that male METH users showed lower cerebral glucose metabolism in the frontal white matter. Sung et al. (2013) used magnetic resonance spectroscopy (MRS) and reported sex differences in the phosphocreatine levels of frontal gray matter, with large decreases in female METH users (Sung et al., 2013). In contrast, total creatine levels were affected in the frontal white matter of only male METH users, with these changes being significantly correlated with length of drug use (Cloak et al., 2011). These data are summarized in Fig. 2.
4.4. MUD, sex, and brain structural changes
Several imaging studies have investigated potential METH-induced changes in the volumes of several brain regions. Some of these papers have reported sex differences in these METH-induced abnormalities (see Fig. 3). For instance, a diffusion tensor imaging using METH users had identified smaller volume of the frontal white matter in male METH users (Chung et al., 2007). In contrast, Tobias et al. (2010) reported that smaller volume of frontal white matter, genu of the corpus callosum, and mid-caudal superior corona radiata in female METH users but showed lower volume only in right perforant path in male METH users. The reason for these observed discrepancies in the frontal lobes of male and female METH in these two studies is not clear. However, they might, partly, be due to the differences in the intervals since last use of larger METH doses before the measurements were made. Specifically, Chung et al. (2007) did their measurements after 4 weeks of abstinence and had 23:9 ratio of male to female participants whereas Tobias et al. (2010) did their measurements after 7–13 days of abstinence and had more females (n = 13) than males (n = 10) in their study. Other complicating factors such as different diets or medical illnesses in the various populations under study at the two sites might have also influenced the results.
Nevertheless, consistent with the study by Chung et al. (2007), male METH users have been reported to show greater severity of white matter signal hyperintensities than females (Bae et al., 2006). A somewhat recent study using magnetic resonance imaging (MRI) has also documented decreased hippocampal volume in female METH users who were abstinent from the drug for nearly 6 months, but not in abstinent males (Du et al., 2015). Of interest to this discussion, longitudinal data from Chinese drug addiction treatment agencies have reported a significant positive correlation between the duration of abstinence and volumes of cortical and hippocampal regions only in male METH users who were abstinent for an average of 122 days (Nie et al., 2021). Disturbingly, these associations did not exist in female METH users who had been abstinent for an average of 348 days (Nie et al., 2021). The study of Kogachi et al. (2017) is specially revealing in that female, more than male, METH users exhibited behavioral impulsivity which was associated with smaller right superior frontal cortex and larger nucleus accumbens (NAc) volume.
It must be emphasized that sex related differences are often a neglected variable in research and treatment approaches to patients who meet diagnostic criteria for MUD. These studies need to include appropriate number of female subjects in order to determine the impact of sex on clinical outcomes. It is nevertheless reassuring that more investigators are becoming more aware that the patterns of METH use are different between male and female subjects and that the drug also can have substantially different consequences between males and females. These types of clinical studies that include equal representation of male and female subjects continue to document specific sex-differences in the clinical manifestations and complication of METH use disorder.
5. MUD and sex-associated cardiovascular differences
Several investigators have written about the cardiovascular effects of METH in human users. They found evidence for increased risk of hypertension, myocardial complications, and strokes. There are a few reports of sex differences in these complications. For example, the relative risk of drug-induce pulmonary arterial hypertension (PAH) was higher in female METH users (Zamanian et al., 2018). Zhao et al. (2018) reported similar observations of METH-associated PAH in female METH users. In contrast, male users were found to suffer from a higher prevalence of METH-associated cardiomyopathy (Darke et al., 2017a; Zhao et al., 2018). Male users also suffer more from METH-induced hypertension, severe stenosis, myocyte hypertrophy, and interstitial fibrosis (Brecht et al., 2004; Darke et al., 2017a; Mayo et al., 2013). A retrospective study also found that males had more severe reduction in ejection fraction whereas female users exhibited worst left ventricular systolic dysfunction (Neeki et al., 2016).
A recent study that analyzed the baseline characteristics of patients with METH-associated cardiomyopathy and heart failure (from 2008 to 2018) found significant race- and gender-based differences (Zhao et al., 2021b). The authors reported steeper increases in heart failures in Black males (12.48-fold increase, slope 10.12, p < 0.001), followed by White males (6.35-fold increase, slope 3.7, p < 0.001), and Hispanic males (6.35-fold increase, slope 2.0, p < 0.001) in comparison to female users. In addition, Black females showed significant increases in hospitalization due to heart failures (8.1-fold increase, slope 2.51, p < 0.001) whereas White and Hispanic females did not show any significant increases (Zhao et al., 2021b). The etiological factors involved in these differences along racial groups are not known but probably involved specific known social determinants of cardiovascular risks among populations of African descent in the United States (Guha et al., 2021). The sex differences might be attributable to higher blood levels of cholesterol and triglycerides observed in male METH users compared to female METH users (He et al., 2013).
6. Animal models of methamphetamine use disorder
In a quest to identify the critical underlying biochemical substrates for MUD, several investigators have developed various models of this psychiatric diathesis in animals. The following sections describe different approaches that include consequences of the administration of various doses of METH administered by investigators, including conditioned place preference (CPP) procedure, and self-administration (SA) models wherein male and female animals were compared.
6.1. Sex differences in METH pharmacokinetics
METH pharmacokinetics have been studied under various conditions by several groups of investigators (Berquist et al., 2020; Hendrickson et al., 2008; Milesi-Halle et al., 2015; Rambousek et al., 2014). Milesi-Halle et al. (2005) reported on sex- and dose-differences in the pharmacokinetics of METH and amphetamine (AMPH) in male and female Sprague-Dawley rats after administering 1.0 and 3.0 mg/kg METH doses. The authors found significant sex-dependent changes in METH pharmacokinetics, with female rats making less AMPH than males. They found, in addition, that the area under the serum METH concentration-time curve (AUC) increased proportionately in males with increasing METH doses. In contrast, females showed significantly much higher increases in the AUC with the higher dose. There were no differences in half-life of METH between the sexes. Interestingly, renal clearance after the 1 mg dose was higher in females than males, with a higher percentage of unchanged METH in the urines of female rats. There were no differences at the higher dose. Interestingly, the AMPH/METH molar ratio after 1.0 mg/ kg METH was significantly lower in females when compared to males, thus indicating formation of lower amount of the metabolite AMPH in females. In both sexes, urinary elimination of METH and its metabolite AMPH was achieved within less than 10 h after administration of both doses of METH (Milesi-Halle et al., 2005). Similarly, another study that used of a single dose of either 1 or 5 mg/kg of METH also found that plasma METH concentrations reached higher levels in female than male Wistar rats (Rambousek et al., 2014).
Using a drug injection pattern to mimic METH administration during a self-administration (SA) paradigm, it was found that both male and female rats achieved stable serum concentration of METH within 20 min, with these remaining constant up to 120 min (Milesi-Halle et al., 2015). In addition, the authors reported that half-time was similarly in both sexes but a somewhat higher concentration of METH in females at 120 min after METH administration. The AUC was also higher in females. Moreover, the authors reported that the volume of distribution and METH clearance were somewhat lower for females than males. Some of these sex differences in METH pharmacokinetics might account with some behavioral and molecular consequences of METH administration.
6.2. Sexual dimorphism in METH-induced behavioral effect
6.2.1. Locomotor behaviors and condition place preference
Most of the early literature on METH-induced behaviors had focuses on locomotor activity (Kitanaka et al., 2005; Wagstaff et al., 1994). In addition, some groups of investigators have compared METH-induced behavioral activity between male and female rodents. For example, female rats that received a single dose (1.0 mg/kg and 3.0 mg/kg) (Hensleigh and Pritchard, 2014; Milesi-Halĺe et al., 2007; Pritchard et al., 2012), or escalating doses of METH (0.1–3.0 mg/kg) (Ramos et al., 2020; Schindler et al., 2002) exhibited greater locomotion and stereotypic behavior than males. In addition, females exhibited enhanced drug-induced locomotor activity for a longer period of time of drug withdrawal than male rats (Ramos et al., 2020). Similarly, female mice showed greater locomotor activity after an acute injection of METH (0.1 mg/kg, 1.0 mg/kg and 4.0 mg/kg) (Chesworth et al., 2021; Cullity et al., 2021; Ohia-Nwoko et al., 2017). In contrast to observations made in rats and mice, prairie voles injected with 0.2 mg/kg METH showed greater locomotor activity in males in comparison to female voles (Perry et al., 2019). Together, these data suggest species-specific responses to the drug.
The effects of METH on anxiety-like behaviors have also been investigated. Chesworth et al. (2021) used an open-field behavioral test paradigm and observed lower anxiety-like behaviors in female mice. Unexpectedly, early life stress caused by maternal separation led to heightened METH-induced locomotor activity in male rats, but not in female rats (Hensleigh and Pritchard, 2014). Using forced swim test, Joca et al. (2014) found higher depression-like behavior in adolescent METH exposed male mice, but not in female mice.
The effects of METH on CPP have long been investigated (Nagai et al., 2005; Thiriet et al., 2011) although most of these studies have been in male rodents. Nevertheless, there have been some reports comparing the effects of METH on CPP in male and female animals. Hensleigh and Pritchard (2014) found no sex-dependent changes in METH-induced CPP. However, a more recent report using a dose of METH (1 mg/kg) similar to the one used by Hensleigh and Pritchard (2014) was able to document sex differences, with female rats showing higher vulnerability to METH-induced CPP than male rats (Yates et al., 2021). The discrepancy observed between the two reports might be due to differences in the experimental paradigm. Hensleigh and Pritchard (2014) had introduced early- life stress during post-natal day 2–8 and tested for CPP in adulthood.
6.2.2. Drug self-administration
Among numerous animal studies, the SA model is thought to better mimic some of the characteristics of substance use disorders (Everitt et al., 2018; Muller, 2018; Sanchis-Segura and Spanagel, 2006). The SA model has also been used extensively to investigate behavioral, biochemical, and molecular consequences of exposure to METH in a contingent fashion (Altshuler et al., 2020; Cadet, 2019). That model has also been used to detect potential sex differences in behavioral and molecular outcomes.
6.2.2.1. METH Intake.
In terms of sex dependent behavioral differences, Roth and Carroll (2004) had initially reported that female rats self-administered more METH than males. Other investigators including Reichel et al. (2012) and Takashima et al. (2018), also reported that female rats self-administered more METH and also escalated their drug intake faster than their male counterparts. Subsequent work from Reichel’s group found that, when male and female rats were trained on a behavioral-economic (BE) paradigm (multiple days at each fixed ratio-FR1, 3, 10, 32 and 100), female rats would take more METH compared to males (Cox et al., 2017). Importantly, these phenomena appear to be age- and dose-dependent because adult male and female Sprague-Dawley rats, trained with different doses of METH SA (0.02, 0.05, or 0.08 mg/kg/inf) from adolescent to adulthood, were more likely to take METH and showed greater motivation to acquire the drug than adolescent rats (Hankosky et al., 2018a). In addition, adult female rats reached acquisition criteria faster than male rats at the higher dose of METH (0.08 mg/kg/inf.) (Hankosky et al., 2018a). Using a similar dose of METH (0.08 mg/kg/inf), Ruda-Kucerova et al. (2015) reported that male rats exhibited higher METH intake during the last 5 days of SA than females. These findings are consistent with the report from our group showing that male Long-Evans rats showed more escalation of their METH intake during the first 9 days of METH SA (Daiwile et al., 2019; Job et al., 2020) and greater METH intake than female rats (Daiwile et al., 2019). Venniro et al. (2017) also observed steeper escalation of METH SA in males compared to females. Adolescent male Wistar rats were also reported to take more METH infusions (0.05 mg/kg METH dose) than adolescent females (Zlebnik et al., 2021). In contrast, Westbrook et al. (2020) reported steeper escalation of METH SA in females compared to males. Importantly, unlike our study (Daiwile et al., 2019), Venniro et al. (2017) and Westbrook et al. (2020) did not observe any sex differences in total METH intake. Other studies had also failed to observe sex differences in total METH intake (Bernheim et al., 2017; Cordie and McFadden, 2019; Everett et al., 2020; Johansen and McFadden, 2017; Pena-Bravo et al., 2019; Pittenger et al., 2021; Westbrook and Gulley, 2020). It is interesting to note that, although Everett et al. (2020) did not find sex differences in METH intake, they did find that female rats exhibited higher locomotor hyperactivity than males. Similarly, voluntary oral exposure of METH showed no sex differences in METH intake, but female METH exposed mice showed increased locomotor activity compared to male mice (Avila et al., 2021). Finally, a recent study investigating potential sex differences in baboons (Foltin, 2018) is of special interest because studies of nonhuman primates are rather rare. In that study, the author investigated the reinforcing efficacy of vaporized methamphetamine HCl (0.3 mg/kg) in animals with minimal prior exposure to drugs. He used 8 adult male and 7 adult female baboons in the study. The animals received 0.05 ml of 95% ethyl alcohol containing 0.3 mg/kg methamphetamine in a vaporized form completing 2 puffs. Males could earn 10 and females could earn 20 aerosol deliveries. The author reported that methamphetamine aerosol delivery maintained lower rates of puffing behavior in females than males.
6.2.2.2. METH seeking, relapse, and reinstatement.
One of the biggest hurdles in the treatment of patients who suffer from MUD is the high rate of relapses to drug taking behaviors while attending treatment programs (Altshuler et al., 2020). Importantly, sex differences in relapse to METH seeking have been documented in animal models. For example, Reichel et al. (2012), Cox et al. (2013), Cordie and McFadden (2019), and Westbrook and Gulley (2020) have provided data consistently showing that female rats exhibited more cue-induced METH seeking behaviors than males after METH prime injection. Another more recent report by Pittenger et al. (2021) also showed more robust effects of METH-primed reinstatement in females rats compared to males after 12 days of abstinence. These phenomena may be dependent on the length of access to METH during SA experiments because reported more cue-induced METH seeking in female than male rats when the animals had 2 h access but not after 6 h of access to METH (Everett et al., 2020). Other groups that have used long access have also observed no sex differences in METH seeking (Bernheim et al., 2017; Daiwile et al., 2019; Venniro et al., 2017). Nevertheless, Ruda-Kucerova et al. (2015) did find evidence for more relapse propensity in females after 6 h of access to METH. The sex differences or lack thereof reported by the various groups may be related to the time after SA withdrawal during which drug seeking behaviors are conducted because Zlebnik et al. (2021) found that female rats had more METH-associated active lever responses on withdrawal day 2 while male rats showed more responses on withdrawal days 4 and 6.
As discussed above, clinical studies have reported that female METH users appear to suffer from severe neuropsychiatric complications (Lisa et al., 2019; Mihan et al., 2018; Neumann et al., 2018), whereas some pre-clinical animal models appear to offer equivocal results. These issues are probably related to the fact that it is not possible to recreate the complexity of human conditions in rodents and, even, in non-human primates. Furthermore, differences in experimental procedures that include METH dose, length of drug exposure and different strains/species of animals can substantially influence observations. These differences are detailed in Tables 1 and 2. Animal models that better mimic human situations are indeed necessary.
Table 1.
Sex differences in animal models of investigator-administered METH.
| Source | Experimental Paradigm | Result | Ref. |
|---|---|---|---|
| Swiss Webster mice | METH dose: 4 injections of 10 mg/kg, s.c. separated by 2 hr intervals. | ↓ striatal DA in males. | Wagner et al. (1993) |
| Heterozygous, homozygous SOD-transgenic & Wild type mice | METH dose: 4 injections of 2.5 or 5.0 mg/kg, i.p, at 2 hr intervals. | Greater ↓ in striatal DA uptake in males. Reversal of ↓ of DA uptake in METH exposed transgenic females. |
Hirata et al. (1996) |
| Sprague-Dawley rats |
METH dose: Escalating METH doses, 0.1, 0.3, 1 & 3.0 mg/kg, i.p. for 4 days separated by 2 days Locomotor activity: Habituation using 30 min/day for 10 days, with saline injection & locomotor activity in chamber. Then rats received escalating doses of METH and were tested for locomotor activity for 30 min for 4 days, separated by 2 days. |
↑ locomotion activity in females. | Schindler et al. (2002) |
| Sprague-Dawley rats |
METH dose: 1.0 and 3.0 mg/kg, s.c. Pharmacokinetic experiment: Pretreatment with a single injection of METH (1.0 mg/kg, s.c) day 1. On days 3 and 6, respectively, rats received METH (1.0 and 3.0 mg/kg. i.v) in a 15 s bolus. |
↑ percentage of METH metabolite (amphetamine) in urine of females (1.0 mg/kg). | Milesi- Hallé et al. (2005) |
| Sprague-Dawley rats |
METH dose: A single injection of METH (1.0 mg/kg, s.c.). Locomotor activity: Habituation using 6 hr/day for 4 days. On day 1, rats received saline. Then, rats received METH every 3 days for a total of 19 days. |
↑ locomotion and stereotypic behavior in females. | Milesi-Hallé et al. (2007) |
| CD-1 mice | METH dose: One injection of 20 mg/kg, i.p. or Two injections of 20 mg/kg, i.p using a 2 hr interval. | ↑ DA levels in female striatum (20 mg/kg dose). ↑ depletion in the levels of DAT, DA and its metabolites in male striatum. ↑ BLC2 in male striatum. ↑ VMAT2 in female striatum. ↓ DAT binding in substantia nigra & VMAT2 in striatum of male. ↓ expression of IGF-1R & GPER1 in female striatum. ↓ in AKT (40 mg/kg) & GSK3β (20 mg/kg) in male striatum. |
Bourque et al. (2011) |
| CD-1 mice | METH dose: One injection of 40 mg/kg, i.p. Brain was isolated 30 min, 1 day and 3 days post METH injection. | Greater ↓ in male striatal DA levels than females at day 3. ↓ striatal DOPAC levels in male all days. Greater ↓ in male striatal DAT binding than females at day 3. ↓ VMAT2 binding in male substantia nigra at day 3. ↑ AKT phosphorylation in female striatum at day 1. ↑ phosphorylation of GSKβ in female striatum at day 1 & 3. ↑ ERK 1 and 2 in female striatum at 30 min |
Bourque et al. (2012) |
| Long Evans rats |
METH dose: A single injection of METH (1.0 or 3.0 mg/kg, s.c.) Locomotor activity: Habituation to room for 30 min, followed by habituation to activity chamber for 30 min. Then, a saline Injection was given to test baseline behavior for 30 min. Finally, rat received METH and tested for locomotor activity for 2 hr. |
↑ locomotion activity in females. | Pritchard et al. (2012) |
| Long Evans rats |
METH dose: A single injection of METH (1.0 mg/kg, s.c.) Early life stress: Pups separated from mother for 180 min/day from PND 2–8. CPP paradigm: Habituation for 30 min on each day before CPP. CPP testing 30 min for 10 days; chamber habituation day 1, days 2–9 conditioning, day 10 preference test. Saline injections on days 2, 4, 6, and 8, METH injections on days 3, 5, 7, and 9. On day 10, rats were tested for CPP without METH. |
Both sexes responded similar in conditioned place preference. ↑ locomotion and stereotypic behavior in females. Early life stress ↑ METH-induced locomotor activity in male rats. |
Hensleigh and Pritchard (2014) |
| C57BL/6 J mice |
METH dose: 4 injections × 7.5 mg/kg, s.c on PDN 30 and 31, 2 hr intervals. Open field: 10 min on PDN 41 & 62. Novel object recognition: Habituation for 2 days (PND 42–43 & 63–64), followed by 2 trials over 2 days (PND 44–45 & 65–66) for 10 min (5 min interval between trials 1 & 2; 24 hr interval between trials 2 & 3). Social Interaction: 5 min on PND 46 & 67. Porsolt forced swim test: 5 min on PND 47 & 68. Morris water maze: 2 sessions/day: each session is for three 60 s trials with a 10–15 min interval. |
↑ depression-like behavior in adolescent males. ↓ vasopressin in PVN in adolescent males. |
Joca et al. (2014) |
| Wistar rats |
METH dose: A single injection of 1 mg/kg or 5 mg/kg, s.c. Pharmacokinetic experiment: a single injection of METH (1 mg/kg or 5 mg/kg, s.c), followed by euthanization of rats at various time points post-injection. |
↑ plasma (1 mg/kg & 5 mg/kg) & brain (1 mg/kg) level of METH in female rats. | Rambousek et al. (2014) |
| C57BL/6 J mice | METH dose: A single injection of 1 mg/kg, i.p. Blood collected after 30, 70, or 120 min after injection. Rats were perfused 2 hr after the injection. | ↑ glucocorticoid receptor in the hippocampus in males. ↑ plasma corticosterone in females. |
Zuloaga et. al. (2014) |
| Sprague-Dawley rats | METH dose: 4 injections × 7.5 mg/kg, i.p, 2 h intervals. Rats were euthanized 8 days after METH exposure. | ↓ 5-HT content in olfactory bulb of female rats. | McFadden and Vieira-Brock (2016). |
| BALB/c mice |
METH dose: A single injection of 1 mg/kg or 4 mg/kg, s.c. Locomotor activity: Habituation for 60 min/day for 3 days. Then, a saline injection followed by a test for basal locomotor activity for 30 min. Mice were then injected with METH and locomotor activity was measured for 90 min |
↑ locomotion behavior in females. | Ohia-Nwoko et al. (2017) |
| C57BL/6 J mice. |
METH dose: Repeated injections of 5 mg/kg, i.p for 10 consecutive days. Tissues were collected 21 days after the last injection. Open-field test: 5 min on day 12. Light/dark box test: 5 min on day 14. Elevated plus maze: 5 min on day 16. |
↑ plasma corticosterone in females. | Jacobskind et. al. (2018) |
| Sprague-Dawley rats |
METH dose: 4 injections of 4 mg/kg, s.c. Novel object recognition: Habituation, 10 min for 3 day (After 3 days of METH injections). After that, 5 min to explore the object (7 days after METH injection); one hour later, rats were again placed into the same chambers for object recognition for 5 min |
↑ striatal DAT levels in males. ↑ FosB in dSTR and NAc of males. Impaired object recognition memory in males. |
Klambatsen et al. (2019) |
| Prairie vole (Microtus ochrogaster) |
METH dose: A single dose of 0.2 mg/kg or 2.0 mg/kg, s.c. Open field: Habituation to the test room for 10 min, followed by a METH injection and open field test for 10 min 2nd test 48 h after 1st test. 3rd test after 30 min of 2nd test. Alloparental behavior: 24 hr after 3rd open field test, Prairie voles were tested for alloparental behaviors (entry into pup chambers) for 10 min |
↑ locomotor activity in males. Males took longer time to enter the pup chamber. Females attacked the pups. |
Perry et al. (2019) |
|
Sprague-Dawley rats
|
METH dose: Escalating doses of 0.1, 0.32, 1.0, 3.2 mg/kg, i.p., after 15 min interval, once weekly for 6 weeks. Locomotor activity: Habituation for 30 min. Rats then received a saline injection 15 min before injections of escalating doses of METH or SKF 82958 (vehicle, 0.01, 0.032, 0.1, 0.32, 1.0, 3.2 mg/kg) every 15 min intervals. Rats were tested once weekly for 6 weeks. |
↑ locomotion activity in females. Female displayed ↑ locomotion activity even after for 4–5 weeks of METH abstinence. |
Ramos et al. (2020)
|
| Heterozygous Nrg1 III transgenic and control wild type-like mice. |
METH dose: 1, 1.5, 2 or 3 mg/kg, s.c., four testing days using Latin-Square design. CPP paradigm: Habituation on day 1 for 30 min. Mice were given METH (2 mg/kg, s.c) and tested for CPP for 30 min for 2–4 days. Open field: Habituation for 30 min. Mice were then placed in field arena for 30 min. This was followed by METH injections (1, 1.5 or 2 mg/kg, s.c) in Latin-Square design to test open field behaviors for 60 min Prepulse inhibition: Habituation for 5–10 min/day for 3 days + background noise of 70 dB. On the test day, mice were injected with a single dose of METH (2 or 3 mg/kg) and tested for Prepulse inhibition for 30 min |
↑ locomotion behavior in female. ↓ anxiety-like behavior in female. ↑ negative impact of METH on prepulse inhibition in NRG1 mutant male. Mutant female ↓ sensitive to METH-induced locomotion METH-induced disruption of sensorimotor gating in female. |
Chesworth et al. (2021) |
|
Swiss Mice
|
METH dose: A single dose (0.1 mg/kg, i.p.) CPP paradigm: Habituation on day 1 for 30 min. On days 2 and 5, mice were injected with saline in the morning followed by a CPP test. In the afternoon, mice were injected with METH (0.1 mg/kg, i.p.) or saline and tested for CPP for 30 min | ↑ locomotion behavior in female. |
Cullity et al. (2021)
|
| Wildtype C57Bl/6 & BDNF heterozygous mice. | METH dose: 1 mg/kg for 1st week, 2 mg/kg for 2nd week & 4 mg/kg for 3rd week for 5 days/week. | ↓ 5-HT1A receptor binding in dorsal raphe nucleus of male. ↑ 5-HT transporter binding in dorsal raphe nucleus of female. ↓ tryptophan hydroxylase 2 in dorsal raphe nucleus of male. |
Sepulveda et al. (2021) |
| Sprague-Dawley rats |
METH dose: A single injection (1.0 mg/kg, s.c.) CPP paradigm: Pretest for 15 min on 1st day of each CPP test. CPP test for 30 min for 8 days, with rats being injected with METH and saline on alternate days. Acquisition of METH CPP: Test on 9th day for 30 min after rats were given Ro 63–1908 (0, 1.0, or 3.0 mg/kg; s.c.). Expression METH CPP: Test on 9th day for 30 min after rats were given Ro 63–1908 (0, 1.0, 3.0 or 10.0 mg/kg; s.c.). |
Female showed ↑ vulnerability to METH-induced CPP. Ro 631908 (3.0 mg/kg) blocked acquisition of METH CPP in male rats. |
Yates et al. (2021) |
Abbreviation: METH, Methamphetamine; PND, Postnatal day; CPP, Conditioned place preference; dB, decibels; s.c., Subcutaneous; i.p., Intraperitoneal; i.v., Intravenous; hr, Hours; min, Minutes; sec, Seconds; mg/kg, milligram per kilogram; ↑, significantly increased; ↓, significantly decreased; PVN, Paraventricular nucleus of the hypothalamus; dSTR, Dorsal striatum; NAc, Nucleus accumbens; SOD, Superoxide dismutase; Nrg1, Neuregulin 1; BDNF, Brain-derived neurotrophic factor; DA, Dopamine; DAT, Dopamine transporter; BCL2, B-cell lymphoma 2; VMAT2, Vesicular monoamine transporter 2; IGF-1R, Insulin-like growth factor type 1 receptor; GPER1, G protein-coupled estrogen receptor 1; 5-HT, Serotonin/5-hydroxytryptamine; FosB, FBJ murine osteosarcoma viral oncogene homolog B; 5-HT1A, Serotonin 1A receptor; SKF 82958, D1 receptor agonist; Ro 631908, N-methyl-D-aspartate (NMDA) antagonist, ERK; extracellular signal-regulated kinases.
Table 2.
Overview of animal models of METH self-administration and sex differences.
| Source | Experimental Paradigm | Result | Ref. |
|---|---|---|---|
| Wistar Rats |
METH SA: Automated priming, 1 hr/day for 6 days, followed by 6 hr/day of METH SA until rats showed stable METH infusion for 4 consecutive days under FR1 schedule. Rats were then shifted to PR schedule until rats reached breaking point. METH dose: 0.02 mg/kg/Inf. |
Female rats were more vulnerable and had increased METH intake. | Roth andCarroll,(2004) |
| Long Evans Rats |
METH SA: 1 hr/day for a total of 7 days (FR1 schedule); then, LgA: 6 hr/day and ShA: 1 hr/day for a total of 14 days (FR1 schedule). METH dose: 0.0175 mg/kg/Inf for females and 0.02 mg/kg/Inf for males. Abstinence: 14 days; object recognition and object in place test: days 7 and 14. |
Increased METH intake and seeking in female rats. Both sexes showed decreased object recognition memory in LgA group. |
Reichel et. al. (2012) |
| Long Evans Rats |
METH SA: 2 hr/day until rats reaching criterion of > 10 Inf for 5 days (FR1 schedule). A progressive ratio (PR) schedule was started: FR3 for 3 days and FR5 for the remaining days for a total of 14 days. The animals were also treated with oxytocin (1 mg/kg, i.p). METH dose: 0.0175 mg/kg/Inf for female & 0.02 mg/kg/Inf for male. Extinction session: 2 hr/day for total of 9 days. Reinstatement Tests: Oxytocin (0, 0.3, or 1 mg/kg, i.p) followed by re-instatement test; 1. Cue-induced: 2 hr on 1 day; 2. METH-primed (1 mg/kg, i.p): 2 hr on 1 day; 3. Yohimbine-induced (2.5 mg/kg, i.p): 2 hr on 1 day. |
Oxytocin decreased motivation for METH SA in females only. | Cox et. al. (2013) |
| Sprague Dawley Rats |
METH SA: 90 min/day for a total of 14 days (FR1 schedule). METH dose: 0.08 mg/kg/Inf. Abstinence: 14 days; Cue-induced reinstatement: 90 min on day 15. |
Increased METH intake in males. | Ruda- Kucerova et. al. (2015) |
| Sprague Dawley Rats |
METH SA: 2 hr/day for 5 day at FR1, day 6 at FR3, day 7 – 9 at FR10, day 10 – 12 at FR32 & day 13 at FR100. METH Dose: 0.0175 mg/kg/Inf for female & 0.02 mg/kg/Inf for male. BE stabilization: 2 hr/day from day 14 – 18. BE testing: 2 h/day from day 19 – 32, counterbalanced manner. 1. Oxytocin effect (Microinfusions of 0.6 μg/side, bilateral in NAc; unilateral intracerebroventricular infusion). 2. Systemic oxytocin (1 mg/kg, i.p) + oxytocin antagonist (microinfused 1 μg/side bilateral in NAc and unilateral intraventricular infusion). Extinction sessions: 2 hr/day for total of 7 days; Cue induced reinstatement: 2 hr/day for 10 days + oxytocin (1 mg/kg, i.p). |
Increased METH demand in females. Effects of oxytocin were Similar on both sexes. |
Cox et. al. (2017) |
| Sprague Dawley rats |
METH SA: 2 hr/day for 5 day at FR1, 3 days at FR3 & 5 days for FR5. METH Dose: 0.0175 mg/kg/Inf for female & 0.02 mg/kg/Inf for male. Extinction sessions: 2 hr/day for total of 8 days; Cue-induced reinstatement: 2 hr/day on test day (1. Systemic administration of LY341395 (1 mg/kg) + oxytocin (1 mg/kg, i.p); 2. Microinfusions of oxytocin (0.6 nmol/0.25 μl/side) + LY341395 (1.3 nmol/0.25 μ/side) in NAc). |
No sex differences in METH intake. Oxytocin decreased METH seeking in both sexes, with mGluR2/3 agonist reversing the effects. |
Bernheim et. al. (2017) |
| Sprague Dawley rats |
Food SA: 14 hr/day for total of 4 days before surgery. METH SA: 8 hr/day for total of 7 days (FR1 schedule). METH Dose: 0.09 mg/kg/inf for female & 0.12 mg/kg/inf for male. |
No sex differences in METH intake. METH increased BDNF in Male HIP. |
JohansenandMcFadden, (2017) |
| Sprague Dawley rats |
Food SA: 6 hr/day for a total of 6 days. METH SA: 6 hr/day for a total of 12 days (FR1 schedule). METH dose: 0.1 mg/kg/inf. Extinction sessions: Days 1 (30 min) & day 21 (2 hr). |
Steeper escalation of METH in males. No differences in METH seeking. |
Venniro et. al. (2017) |
| Baboon monkey |
METH SA: 60 min/day for a total of 5 months (8–10 aerosol deliveries/day) (FR1 schedule). Availability of next puff 2 min for males, 1 min for females. METH dose: Vaporized 0.3 mg/kg/puff. |
Male baboons had higher METH puffing. | Foltin, (2018) |
| Sprague Dawley rats |
METH SA: Operant chamber exploration, 90 min on day 1. METH SA on next day 2, 2 hr/day for a total of 15 days (7 days at FR1, 4 days at FR3 & 4 days at FR5). METH dose: 0.02, 0.05, or 0.08 mg/kg/inf. Progressive ratio testing (PR): 0.08 mg/kg/inf used in trained rats tested in PR test on 0.02, 0.05, 0.08 and 0.1 mg/kg/inf for 5 hr/day session until breaking point. |
Female acquired more METH at 0.08 mg/kg dose. | Hankosky et. al. (2018a) |
| Sprague Dawley rats |
METH SA: Operant chamber exploration, 90 min on day 1. Next day SA, 2 hr/day for total of 15 days (7 days at FR1, 4 days at FR3 & 4 days at FR5). METH dose: 0.02, 0.05, or 0.08 mg/kg/inf. Strategy Shifting: 23 days. |
Female required more trial & committed more errors during discrimination & reversal learning behavior. Male had ↑ 5-HT2-CR within PV-INs & in OFC. |
Hankoskyet al. (2018b) |
| Sprague Dawley rats |
Food SA: 14 hr/day for total of 4 days before surgery. METH SA: 8 hr/day for total of 7 days (FR1 schedule). After that DOI test. METH Dose: 0.09 mg/kg/Inf females and 0.12 mg/kg/Inf for male. DOI testing: On day 8 rats were injected with serotonin 2 A/2 C agonist (2 mg/kg, i.p.) to measure head twitched behavior. Extinction sessions: 2 hr/day for total of 10 days. DOI testing on day 11. |
No differences in METH intake & seeking behavior. Females had increased DOI-induced head twitches after METH SA & extinction. METH decreased 5-HTT in male ILC. |
McFaddenet al.(2018) |
| Long Evans rats |
METH SA: 6 hr/day for total of 14 days (FR1 schedule). METH dose: 0.05 mg/kg/inf. Progressive Ratio: 6 hr/day for total of 20 days with increasing PR ratio till breaking point. Extinction session: 1 hr for total of 6 days; Cue-induced reinstatement: 1 hr on day 7. |
Female had higher METH intake & GluN2A/2B ratio in the DG. Male showed increased METH seeking & CaMKII, choline acetyltransferase in the DG. |
Takashima et. al. (2018) |
| Sprague Dawley rats |
Food SA: 14 hr/day for total of 5 days before surgery. METH SA: 8 hr/day for total of 7 days (FR1 schedule). METH Dose: 0.09 mg/kg/Inf female & 0.12 mg/kg/Inf for male. Extinction sessions: 2 hr/day from day 6–17; METH-primed reinstatement (1 mg/kg, ip): 2 hr on day 18 (optogenetic inhibition); Extinction: 2 hr/day from day 19–23; METH-primed reinstatement (1 mg/kg, i.p): 2 hr on day 24 (optogenetic inhibition). |
No differences in METH intake & in extinction behavior. Females increased METH seeking behavior during METH primed reinstatement in laser off condition. | Cordie and McFadden, (2019) |
| Long Evans rats |
METH SA: 6 hr/day (30 min break after 3 hr) for total of 20 day (FR1 schedule). METH dose: 0.1 mg/kg/Inf. METH seeking test: 3 hr/day on withdrawal 3 & 30. |
Increased METH intake in males. No differences in seeking behavior. Female rats had higher basal mRNA levels of Pdyn & Hcrtr1 in NAc, PFC & HIP; also higher basal mRNA levels of Hcrtr2 and Avpr1a in NAc & Crhr1, Crhr2, Hcrtr2 and Oprk1 in the PFC. Males has higher Avp mRNA in NAc & Crh and Crhr1 in HIP. |
Daiwile et. al. (2019) and (2021) |
| Sprague Dawley rats |
METH SA: 6 hr/day for total of 14 days (FR1 schedule). METH dose: 0.05 mg/kg/Inf. Abstinence: 9–14 day. |
No differences in METH intake. METH SA increased the amplitude of eEPSCs only in female rats. | Pena- Bravo et. al. (2019) |
| Sprague Dawley rats |
METH SA: 2 hr/day for total of 12 days (FR1 schedule). After that LgA: 6 hr/day & ShA: 2 hr/day for total of 10 days (FR1 schedule). METH dose: 0.1 mg/kg/Inf. Abstinence: 30 days. Cue-induced: 1 hr /day on day 2 and days 6–20 with oxytocin treatment (1 mg/kg, i.p); EMP/SIT testing 1 hr from days 25–28; Cue-induced: 1 hr/day on day 30; METH-primed (0.3 or 1.0 mg/kg, i.p) or yohimbine (0.625 or 1.25 mg/kg, i.p) for 1 hr on day 31. |
No differences in METH intake. ShA female showed increased METH seeking behavior. Female showed increased locomotor hyperactivity. |
Everettet al. (2020) |
| Long Evans rats |
METH SA: 6 hr/day (30 min break after 3 hr) for total of 20 day (FR1 schedule). METH dose: 0.1 mg/kg/Inf. CNO testing (chemogenetic inhibition of D1R in dSTR): CNO (1 mg/kg, i.p, 30 min) + 6 hr/day METH SA total of 4 days (FR1 schedule) in counterbalance method. |
Males acquire METH at faster rate than females. Inhibition of Drd1 in the dorsal striatum had no effect on METH intake in both sexes. | Job et. al. (2020) |
| Sprague Dawley rats |
METH SA: Operant chamber exploration, 90 min on day 1. Next day SA, 2 hr/day for total of 7 days (FR1 schedule), followed by LgA: 6 hr/day for total of 14 days (FR1 schedule). METH dose: 0.1 mg/kg/Inf. Extinction session: 4 days of 30 min/day extinction with Ro256981 (6 mg/kg, i.p.); 4 days of 2 hr/day extinction without Ro256981; followed by METH-primed reinstatement (1 mg/kg, i.p): 2 hr on day 9. |
No differences in METH intake. Female showed increased METH seeking behavior. Effect of GluN2B antagonist on drug seeking behavior was similar in both sexes. |
Westbrook and Gulley, (2020) |
| Sprague Dawley rats |
METH SA: Operant chamber exploration, 90 min on day 1. Next day SA, 2 hr/day for total of 7 days (FR1 schedule), followed by LgA: 6 hr/day for total of 14 days (FR1 schedule). METH dose: 0.1 mg/kg/Inf. Abstinence: 21 days; cognitive testing on day 7 & 14 days. |
Females begin to increase METH intake earlier. No differences in the protein level of Drd1, GluN1, GluN2B in PFC and NAc of rats. |
Westbrook et. al. (2020) |
| C57/Bl6 mice |
Voluntary oral METH SA: 0.25 mg/kg, 1 dose/day for 3 days; followed by 4 doses/day for 3 days; followed by 16 doses/day for 2 days; followed by 16 doses/day at 0.5 mg/kg for 2 days. Which is followed by 16 doses/day at 1 mg/kg from day 11–28. METH dose: 0.25 mg/kg, 0.5 mg/kg and 1 mg/kg. |
No differences in METH intake. Females showed increased locomotor hyperactivity. Increased Oprk1 & decreased PKMζ level in HIP. |
Avila et al. (2021) |
| Sprague Dawley rats |
Sucrose training: 60–80 min (till 60 sucrose deliveries) for total of 3 days before surgery. After that, METH SA: 2 hr/day for total of 21 days (FR1 schedule). METH dose: 0.05 mg/kg/inf. Extinction session: 2 hr/day for total of 12 days; after that METH-primed reinstatement (0.3 mg/kg, i.p): 70 min/day. |
No differences in METH intake. Females had increased seeking Behavior in METH-primed reinstatement. Greater increases in c-Fos expression in cortex, amygdala, dorsal & ventral striatum of females. |
Pittenger et al. (2021) |
| Wistar rats |
METH SA: 6 hr/day (40 inf/day) for total of 5 days, followed by SA 2 hr/day for total of 5 days (FR1 schedule). METH dose: 0.05 mg/kg/inf. Extinction session: 2 hr per day for a total of 10 days; Cue-induced reinstatement and METH-primed reinstatement (1 mg/kg, i.p): 2 hr on each test day with or without Hcrtr1 antagonist (SB-334867, 20 mg/kg) and Hcrtr2 antagonist (TCS-OX2–29, 20 mg/kg, i.p). |
Male showed increased METH intake. Females showed more active lever responses on WD2. Males showed active lever responses on WD4 and WD6. Both sexes adolescent presence of SB-334867 and adult in presence of TCS-OX2–29 had decreased METH primed reinstatement. |
Zlebnik et al. (2021) |
Abbreviation: METH, Methamphetamine; SA, Self-administration; BE, Behavioral economic; LgA, Long access; ShA, Short-Access; WD, Withdrawal day; PR, progressive ratio; FR, Fixed ratio; mg/kg/inf, milligram per kilogram per infusion; μg, Microgram; μl, Microliter; nmol, nanomole; hr, Hours; min, Minutes; i.p., Intraperitoneal; PFC, Prefrontal cortex; OFC, Orbitofrontal cortex; ILC, Infralimbic cortex; NAc, Nucleus accumbens; HIP, Hippocampus; DG, Dentate gyrus; PV-INs, Parvalbumin interneurons; mRNA, Messenger RNA; mGluR2/3, Metabotropic glutamate receptor 2/3; BDNF, Brain-derived neurotrophic factor; 5-HT2-CR, Serotonin 2 C receptors; 5-HTT, Serotonin transporter; GluN1, Glutamate receptor subunit zeta-1; GluN2A/2B, Glutamate receptor subunit epsilon-1/2; CaMKII, Calcium/calmodulin-dependent protein kinase II; Pdyn, Prodynorphin; Oprk1, Opioid receptor kappa 1; Hcrtr1, Hypocretin Receptor 1 and 2; Hcrtr2, Hypocretin Receptor 2; Avp, Arginine vasopressin; Avpr1a, Arginine vasopressin receptor 1 A; Crh, Corticotropin releasing hormone; Crhr1, Corticotropin releasing hormone receptor 1; Crhr2, Corticotropin releasing hormone receptor 2; Drd1, Dopamine receptor D1; PKMζ, protein kinase M zeta; LY341395, Group II mGlu receptor antagonist; Ro256981, NMDA receptors GluN2B antagonist; DOI, Dimethoxy-4-iodoamphetamine; eEPSCs, Evoked excitatory postsynaptic currents.
6.3. Sexual differences in pharmacological interventions
Several targets for drug development have investigated as potential treatment for MUD in animal models. These drugs are known to modulate the functions of dopaminergic, serotonergic, glutamatergic, opioid and endocannabinoid systems, among others. For example, systemic administration of the neuropeptide, oxytocin, has been shown to reduce METH self-administration in female, but not in male rats (Cox et al., 2013), without there being any differential sex effects observed in oxytocin-induced reduced METH seeking behavior (Bernheim et al., 2017; Cox et al., 2017). Chronic oxytocin treatment (15 days of 1 mg/kg i.p. injection) during 30 days of withdrawal from METH self-administration (6 h/day) attenuated incubation and METH-primed reinstatement in both sexes (Everett et al., 2020). Interestingly, Bernheim et al. (2017) have suggested that the attenuating effects of oxytocin on METH-seeking behavior may be dependent on regulation of presynaptic glutamatergic signaling because reinstatement of lever pressing to METH-associated cues was observed after administration of LY341495, metabotropic glutamate receptor (mGluR2/3) antagonist in both male and female rats. The glutamate ionotropic receptor NMDA type subunit 2B (GluN2B) selective antagonist, Ro 63–1908, also completely blocked the conditioned rewarding effects of METH in male, but not female rats (Yates et al., 2021). Whereas administration of Ro 25–6981, another GluN2B selective antagonist, did not exhibit any sex differences in reducing METH seeking behavior (Westbrook and Gulley, 2020). In addition to the role of oxytocin in blocking METH SA, some investigators have reported that hypocretin receptors (HCRTR) antagonists can also attenuate drug-seeking behavior (James et al., 2017). However, there do not seem to be any sex differences in the reduction of METH reinstatement (Zlebnik et al., 2021).
6.4. Sexual dimorphism in METH-induced biochemical consequences
6.4.1. Neurotoxic METH doses
Dysregulation of brain dopamine (DA) systems are known to play important roles in mediating various aspects of the behavioral responses to rewarding drugs (Keiflin and Janak, 2015; Wise and Jordan, 2021) including METH (Bhimani et al., 2021; Xi et al., 2009). However, although there have been reports on sex differences on the effects of METH, fewer studies have tried to document potentially similar differences on the biochemical effects of METH in the brain. For example, striatal dopamine (DA) depletion was also more prominent in male mice exposed to a binge dosing pattern of METH injections (four injections 10 mg/kg) (Wagner et al., 1993). In addition, Dluzen et al. (2011) observed that METH-induced depletion in the levels of dopamine transporter (DAT), DA, and DA metabolites were more pronounced in males compared to female mice (Dluzen et al., 2011). Another study conducted by Bourque et al. (2012) showed that an injection of METH (40 mg/kg) to CD-1 mice caused sex-dependent differences in the course of the METH-induced decreases in striatal DA levels. The authors found that METH caused similar decreases in DA levels in male (−82%) and female (−71%) mice in animals euthanized 1 day post-the METH injection. However, male mice (−79%) showed much greater decreases in DA levels than females (−44%) at 3 days after the METH injection (Bourque et al., 2012). Somewhat similar effects were obtained for 3, 4-dihydroxyphenylacetic acid (DOPAC), in that DOPAC values had normalized in the female mice at day 3 after initial METH-induced decreases at 30 min and 1 day after METH. In contrast, DOPAC levels remained significantly decreased at all time points after the METH injection in male mice (Bourque et al., 2012). The authors also reported that the METH injection also caused significant and similar decreases in DAT binding in the medial aspect of the dorsal striatum in both sexes when measured at one day after METH. However, the decreases were exacerbated in the males at day 3 whereas the females showed a marked improvement in DAT binding at that time point (Bourque et al., 2012).
Bourque and his colleagues (2012) also attempted to identify potential sex differences of the effects of toxic METH doses (40 mg/kg) on the levels of the vesicular monoamine transporter 2 (VMAT2). The authors measured VMAT2 binding in the substantia nigra and in the medial and lateral aspects of the dorsal striatum in mice. In female mice, METH caused comparable and significant decreases in VMAT binding in both the medial and lateral aspects of the dorsal striatum in animals euthanized at both 1 and 3 days after the injection of METH. However, significant decreases were observed only on day 3 in male mice in medial striatum and at day 1 and 3 in the lateral region. In contrast to the data in the dorsal striatum, there were significant METH-induced decreases in the substantia nigra of male mice, but not in the females, at 3 days after the drug (Bourque et al., 2012). METH may also influence other neurotransmitters in a sex-dependent fashion. McFadden and Vieira-Brock (2016) injected 4 injections of METH (7.5 mg/kg) given at 2 h intervals in one day and reported a significant loss of serotonin (5-HT) content in the olfactory bulb of female Sprague-Dawley but not male rats. The authors found no sex-dependent effects in other brain regions including the hippocampus and the frontal cortex. Interestingly, using similar METH dosing regimen Joca et al. (2014) had reported that late adolescent male, but not female, C57BL/6 J mice injected with METH (2 days of 4 × 7.5 mg/kg MA administered in 2 h intervals) showed decrease in the number of vasopressin-immunoreactive neurons in the paraventricular nucleus compared to sex-matched saline-treated controls.
Toxic doses of METH have been shown to impact several proteins related to cell death pathways including B-cell lymphoma 2 (Bcl2), glial fibrillary acidic protein (GFAP), and some trophic factors including insulin growth factor binding proteins (Cadet et al., 2001 , 2002, 2007; Jayanthi et al., 2021). However, these studies were conducted in male rodents. Thus, Bourque and co-investigators (2011 and 2012) measured the effects of a toxic dose of METH (40 mg/kg) on several proteins in female and male mice. They found that insulin-like growth factor 1 receptor (IGF-1R) was reduced in females whereas G protein-coupled estrogen receptor 1 (GPER1) and Bcl-2 were increased in males (Bourque et al., 2011). They also reported METH-associated increase in AKT phosphorylation only in female mice one day after the drug injection (Bourque et al., 2012). They also detected increased glycogen synthase kinase 3beta (GSK3β) phosphorylation in female, but not in male, mice at 1 and 3 days after the METH injections (Bourque et al., 2012). Female mice also showed increased protein levels of extracellular signal-regulated kinases (ERK) 1 and 2 measured at 30 min after METH exposure when compared to control animals. However, such effects were not seen in male mice (Bourque et al., 2012). There were, however, no sex-dependent differences in METH-induced changes in GFAP expression. Taken together, these results suggest that METH may exert sex- and region-dependent toxic effects in the brain.
6.4.2. Acute and chronic METH dosing
Non-toxic dose of METH also showed sexual dimorphism in dopaminergic and serotonergic system. Where, Hirata et al. (1996) had reported that acute injections of METH (2.5 and 5.0 mg/kg) showed greater decreases (48% and 73%, respectively) in striatal DA transporters in male compared to female mice (25% and 63%, respectively). Interestingly, Klambatsen et al. (2019) reported that injection of METH (4 ×5 mg/kg) caused no decreases in DAT protein levels in adult male Sprague-Dawley rats but small decreases in female rats. More recently, Sepulveda et al. (2021) reported that escalating METH doses given over a period of three weeks resulted in significant decreases in serotonin 1 A receptor (5-HT1A) receptor binding in the dorsal raphe nucleus of only male mice. They found, in addition, that METH caused decreases in the synthesizing enzyme, tryptophan hydroxylase (TPH), in the dorsal raphe, with these decreases showing no sex effects.
Immediately early genes and proteins are thought to participate in the regulation of the long-term behavioral effects of rewarding drugs including opioid and psychostimulant substances (Bisagno and Cadet, 2019; Cadet, 2021; Lardner et al., 2021). It was therefore of interest to test whether these were affected by METH in a sexually dimorphic fashion. Jacobskind et al. (2018) administered repeated injections of METH (5 mg/kg) once daily for 10 days and reported that male, but not female, mice showed decreased levels of c-Fos in the bed nucleus of the stria terminalis (BNST). Klambatsen et al. (2019) reported that injections of rats with a binge METH regimen (4 ×4 mg/kg, every 2 h) caused significant increases in FosB protein levels in both dorsal and ventral striatum only in males but not in females.
Stress-related proteins have also been implicated in the effects of psychostimulants including METH (Jayanthi et al., 2017; Martin et al., 2012; Sharpe et al., 2021). Some investigators have therefore sought to test the possibility that the effects of METH on these stress-related substances might be influenced by the sexes of groups of animals. For example, Zuloaga et al. (2014) injected male and female C57BL6j mice with METH (1 mg/kg) or saline for comparison of plasma corticosterone. They found a prolonged elevation in corticosterone levels in female compared to male mice. They also found that METH increased the number of c-Fos /glucocorticoid receptor dual-labeled cells to a greater extent in males than females in the cingulate and hippocampal CA3 regions (Zuloaga et al., 2014). In a follow-up study, Jacobskind et al. (2018) injected mice with METH (5 mg/kg) or saline for 10 consecutive days. After ten days of withdrawal, mice were again injected with METH (5 mg/kg) or saline. Chronic METH failed to alter corticosterone responses to the last METH dose that caused greater corticosterone responses in females in comparison to male mice (Jacobskind et al., 2018).
6.4.3. METH Self-administration
Sex differences and METH-associated molecular and biochemical changes have been investigated by measuring mRNA and protein expression levels in various brain regions. Daiwile et al. (2019) used METH SA in male and female Long Evans rats and measured the expression of several neuropeptides after a month of withdrawal from the SA experiment. They found that the basal mRNA levels of dynorphin (Pdyn), corticotrophin-releasing hormone receptor 2 (Crhr2), hypocretin/orexin receptors 1 and 2 (Hcrtr1 and Hcrtr2), and vasopressin receptor 1a (Avpr1a) higher in the nucleus accumbens (NAc) of drug naïve female rats. In contrast, drug naïve male rats showed higher basal vasopressin (Avp) NAc mRNA expression. Interestingly, METH caused significant increases in Pdyn and Crhr2 levels only in the NAc of male rats. There were also significant correlations between the NAc levels of Hcrtr1, Hcrtr2, Crhr2, and vasopressin receptor 1b (Avpr1b) mRNAs and cue-induced METH seeking behavior only in female rats (Daiwile et al., 2019). A subsequent study by Daiwile et al. (2021) measured similar neuropeptide mRNAs in the prefrontal cortex and hippocampus. The authors reported that female rats exhibited higher basal mRNA expression of cortical Pdyn, Hcrtr1, Hcrtr2, Crhr2, corticotrophin-releasing hormone receptor 1 (Crhr1), and opioid receptor kappa 1 (Oprk1) as well as hippocampal Pdyn and Hcrtr1 mRNA levels. In contrast, male rats displayed higher basal levels of hippocampal corticotrophin-releasing hormone (Crh) and Crhr1 mRNAs. Female, but not male, rats exhibited METH-induced decreases in Pdyn expression in the prefrontal cortex while both sexes showed decreases in Pdyn in the hippocampus. Moreover, only males showed METH-associated decreased in proenkephalin (Penk) mRNA levels in the prefrontal cortex whereas only females exhibited METH-induced decreases in Penk mRNA levels in the hippocampus. Of further interest is that METH SA was also associated with changes in the expression of kappa 1 (Oprk1), opioid receptor delta (Oprd1), and opioid receptor mu (Oprm1) receptors in both the prefrontal cortex (PFC) and the hippocampus. In the PFC, there were METH-induced decreases in both male and female rats whereas Oprk1 mRNA levels were decreased in the females but increased in males. PFC Oprm1 mRNA levels were decreased only in the females without there being any changes in the males. METH SA was associated with increased expression of hippocampal Crh and Crhr1 in both sexes but of Crhr2 only in female rats. In the HIP, METH SA was associated with increased Oprd1 mRNA levels in female rats but no changes in male rats. Moreover, Oprk1 mRNA levels were decreased in both male and female rats. METH SA had no effect of Oprm1 in the HIP of either sex (Daiwile et al., 2021). Voluntary oral METH administered female mice exhibited increased Oprk1 and decreased memory protein marker, protein kinase M zeta (PKMζ), in the hippocampus (Avila et al., 2021). Taken together, these observations suggest that sexual dimorphism in METH SA-induced changes in gene expression may be secondary to sex-dependent differences in the basal levels of certain genes and proteins that might impact behavioral responses after repeated exposure to an identical substance (Cadet, 2021) or might be regulated to an identical transcription factor (Lardner et al., 2021).
Potential sex differences in protein expression in the brains of rats have also been examined. For example, METH-primed reinstatement was reported to be associated with greater c-Fos immunoreactivity in the cortical, striatum, and in the amygdala brain of female rats compared to males (Pittenger et al., 2021). In contrast, METH also increased hippocampal BDNF protein levels only in male rats (Johansen and McFadden, 2017). McFadden et al. (2018) wrote that METH decreased serotonin transporter (SERT or 5-HTT) protein levels in the infralimbic cortex of only males. Additionally, only adult male rats showed METH SA-induced higher co-localization of 5-hydroxytryptamine 2 C receptor (5-HT2CR) with parvalbumin interneurons in the orbitofrontal cortex (Hankosky et al., 2018b).
7. Conclusions
METH use disorder and associated health hazards impact both sexes prominently. In this review, we have highlighted the clinical literature that included both sexes in patient cohorts and addressed the sexual dimorphism associated with MUD. The clinical data clearly show that more men use METH (Jones et al., 2020a) and experience more problems that lead to ED admissions and higher cardiovascular deaths than women (Darke et al., 2017a, 2018). However, women who report METH as their first drug of choice suffer from a faster timeline of progression to dependence diathesis (Hartwell et al., 2016; Rungnirundorn et al., 2017; Su et al., 2017; Yimsaard et al., 2018) and from more neuropsychiatric complications than men (Lisa et al., 2019; Mihan et al., 2018; Neumann et al., 2018). Nevertheless, women appear to respond to treatment better than men (Brecht et al., 2006; Han et al., 2016). Overall, the cited clinical literature serves as evidence to direct funding agencies and researchers in the field to mandate the inclusion of both sexes in the same study for direct comparison. Data obtained from these studies will serve to better inform the development of treatment interventions.
In pre-clinical studies related to substance use disorder, the issue of sex as a biological variant is frequently over-looked even though funding agencies have made many requests that these issues be addressed. Herein, we have presented the collective data that report sex-specific differential outcomes associated with METH use in animal models. We have also provided the readers with convincing evidence that male and female animals respond differently to METH with regards to patterns of behavior, responses of neurotransmitter systems, and brain structural abnormalities. In addition, we have included the effects of various doses and conditions of METH on sex differences in behavioral and biochemical mechanisms. We conclude, therefore, that the animal data described in this review can serve as a bridge towards elucidating neurobiological mechanisms that might underlie distinct phenotypic behaviors observed in males and females in clinical settings.
Acknowledgements
We would like to thank National Institute on Drug Abuse (NIDA) Intramural Research Program for the financial support.
Funding
This work was funded by the Department of Health and Human Services/National Institutes of Health/National Institute on Drug Abuse/Intramural Research Program, Baltimore, MD, USA [grant #-DA000552 (2021)].
Footnotes
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Data availability
No original data were used in this review article.
References
- Altshuler RD, Lin H, Li X, 2020. Neural mechanisms underlying incubation of methamphetamine craving: a mini-review. Pharm. Biochem. Behav 199, 173058 10.1016/j.pbb.2020.173058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR, 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295, 2003–2017. 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Apidechkul T, Chomchoei C, Wongnuch P, Tamornpark R, Upala P, Yeemard F, Nongkhai MPN, Nachaiwieng W, Sunsern R, 2020. Associations of childhood experiences and methamphetamine use among Akha and Lahu hill tribe youths in northern Thailand: a cross-sectional study. PLoS One 15, e0234923. 10.1371/journal.pone.0234923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auten JD, Matteucci MJ, Gaspary MJ, Combs DJ, Clark RF, 2012. Psychiatric implications of adolescent methamphetamine exposures. Pediatr. Emerg. Care 28, 26–29. 10.1097/PEC.0b013e31823ed6ca. [DOI] [PubMed] [Google Scholar]
- Avila JA, Memos N, Aslan A, Andrejewski T, Luine VN, Serrano PA, 2021. Voluntary oral methamphetamine increases memory deficits and contextual sensitization during abstinence associated with decreased PKMζ and increased κOR in the hippocampus of female mice. J. Psychopharmacol 35, 1240–1252. 10.1177/02698811211048285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, Kim DJ, Hwang J, Kim SJ, Renshaw PF, 2006. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 81, 83–88. 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Beck AK, Larance B, Deane FP, Baker AL, Manning V, Hides L, Shakeshaft A, Argent A, Kelly PJ, 2021. The use of Australian SMART Recovery groups by people who use methamphetamine: analysis of routinely-collected nationwide data. Drug Alcohol Depend. 225, 108814 10.1016/j.drugalcdep.2021.108814. [DOI] [PubMed] [Google Scholar]
- Bernheim A, Leong KC, Berini C, Reichel CM, 2017. Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharm. Biochem. Behav 161, 13–21. 10.1016/j.pbb.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, McGill MR, Mazur A, Findley DL, Gorman G, Jones CB, Hambuchen MD, 2020. Effect of bile duct ligation-induced liver dysfunction on methamphetamine pharmacokinetics in male and female rats. Drug Alcohol Depend. 215, 108190 10.1016/j.drugalcdep.2020.108190. [DOI] [PubMed] [Google Scholar]
- Bhimani RV, Vik M, Wakabayashi KT, Szalkowski C, Bass CE, Park J, 2021. Distinct dose-dependent effects of methamphetamine on real-time dopamine transmission in the rat nucleus accumbens and behaviors. J. Neurochem 158, 865–879. 10.1111/jnc.15470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V, Cadet JL, 2019. Expression of immediate early genes in brain reward circuitries: Differential regulation by psychostimulant and opioid drugs. Neurochem. Int 124, 10–18. 10.1016/j.neuint.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Bonk R, Miller RJ, Lanter J, Niblo C, Kemp J, Shelton J, 2020. Accidental overdose deaths in Oklahoma, 2002–2017: opioid and methamphetamine trends. J. Anal. Toxicol 44, 672–678. 10.1093/jat/bkaa068. [DOI] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T, 2012. Sex and temporally-dependent effects of methamphetamine toxicity on dopamine markers and signaling pathways. Neuropharmacology 62, 2363–2372. 10.1016/j.neuropharm.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Bourque M, Liu B, Dluzen DE, Di Paolo T, 2011. Sex differences in methamphetamine toxicity in mice: effect on brain dopamine signaling pathways. Psychoneuroendocrinology 36, 955–969. 10.1016/j.psyneuen.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, von Mayrhauser C, Anglin MD, 2006. Two-year outcomes of treatment for methamphetamine use. J. Psychoact. Drugs (Suppl 3), 415–426. 10.1080/02791072.2006.10400605. [DOI] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD, 2004. Methamphetamine use behaviors and gender differences. Addict. Behav 29, 89–106. 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Cadet JL, 2019. Animal models of addiction: compulsive drug taking and cognition. Neurosci. Biobehav. Rev 106, 5–6. 10.1016/j.neubiorev.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, 2021. Sex in the nucleus accumbens: ΔFosB, addiction, and affective states. Biol. Psychiatry 90, 508–510. 10.1016/j.biopsych.08.002 (https://doi.org/doi:). [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, 2015. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front. Psychiatry 6, 189. 10.3389/fpsyt.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM, 2014. Neuropathology of substance use disorders. Acta Neuropathol. 127, 91–107. 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B, 2001. Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: evidence from cDNA array. Synapse 41, 40–48. 10.1002/syn.1058. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J, 2007. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox. Res 11, 183–202. [DOI] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Ladenheim B, 2002. Distinct gene expression signatures in the striata of wild-type and heterozygous c-fos knockout mice following methamphetamine administration: evidence from cDNA array analyses. Synapse 44, 211–226. 10.1002/syn.10074. [DOI] [PubMed] [Google Scholar]
- CCSA, 2020. Methamphetamine (Canadian Drug Summary). Canadian Centre on Substance Use and Addiction pp. 1–16. [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN, 2002. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 114, 65–79. 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chesworth R, Rosa-Porto R, Yao S, Karl T, 2021. Sex-specific sensitivity to methamphetamine-induced schizophrenia-relevant behaviours in neuregulin 1 type III overexpressing mice. J. Psychopharmacol 35, 50–64. 10.1177/0269881120967870. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF, 2007. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int. J. Neuropsychopharmacol 10, 765–775. 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Alicata D, Chang L, Andrews-Shigaki B, Ernst T, 2011. Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug Alcohol Depend. 119, 207–215. 10.1016/j.drugalcdep.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordie R, McFadden LM, 2019. Optogenetic inhibition of the medial prefrontal cortex reduces methamphetamine-primed reinstatement in male and female rats. Behav. Pharm 30, 506–513. 10.1097/FBP.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, O’Brien CP, 1997. Naltrexone pharmacotherapy for opioid dependent federal probationers. J. Subst. Abus. Treat 14, 529–534. 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G, 2017. Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol. Psychiatry 81, 949–958. 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM, 2013. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38, 2343–2353. 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Ray LA, 2017. Self-reported attentional and motor impulsivity are related to age at first methamphetamine use. Addict. Behav 65, 7–12. 10.1016/j.addbeh.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullity ER, Guerin AA, Perry CJ, Kim JH, 2021. Examining sex differences in conditioned place preference or aversion to methamphetamine in adolescent and adult mice. Front. Pharm 12, 770614 10.3389/fphar.2021.770614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiwile AP, Jayanthi S, Cadet JL, 2021. Sex- and brain region-specific changes in gene expression in male and female rats as consequences of methamphetamine self-administration and abstinence. Neuroscience 452, 265–279. 10.1016/j.neuroscience.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiwile AP, Jayanthi S, Ladenheim B, McCoy MT, Brannock C, Schroeder J, Cadet JL, 2019. Sex differences in escalated methamphetamine self-administration and altered gene expression associated with incubation of methamphetamine seeking. Int. J. Neuropsychopharmacol 22, 710–723. 10.1093/ijnp/pyz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Duflou J, Kaye S, 2017a. Prevalence and nature of cardiovascular disease in methamphetamine-related death: a national study. Drug Alcohol Depend. 179, 174–179. 10.1016/j.drugalcdep.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, Duflou J, 2017b. Rates, characteristics and circumstances of methamphetamine-related death in Australia: a national 7-year study. Addiction 112, 2191–2201. 10.1111/add.13897. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, Duflou J, Lappin J, 2019. Completed suicide among methamphetamine users: a national study. Suicide Life Threat. Behav 49, 328–337. 10.1111/sltb.12442. [DOI] [PubMed] [Google Scholar]
- Darke S, Lappin J, Kaye S, Duflou J, 2018. Clinical characteristics of fatal methamphetamine-related stroke: a national study. J. Forensic Sci 63, 735–739. 10.1111/1556-4029.13620. [DOI] [PubMed] [Google Scholar]
- Darke S, Torok M, McKetin R, Kaye S, Ross J, 2011. Patterns of psychological distress related to regular methamphetamine and opioid use. Addict. Res. Theory 19, 121–127. 10.3109/16066351003695631. [DOI] [Google Scholar]
- DiMiceli LE, Sherman SG, Aramrattana A, Sirirojn B, Celentano DD, 2016. Methamphetamine use is associated with high levels of depressive symptoms in adolescents and young adults in Rural Chiang Mai Province, Thailand. BMC Public Health 16, 168. 10.1186/s12889-016-2851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Bourque M, Di Paolo T, Darvesh AS, Buletko AB, Laping NJ, 2011. Markers associated with sex differences in methamphetamine-induced striatal dopamine neurotoxicity. Curr. Neuropharmacol 9, 40–44. 10.2174/157015911795017399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-5, 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition ed. American Psychiatric Association. [Google Scholar]
- Du J, Quan M, Zhuang W, Zhong N, Jiang H, Kennedy DN, Harrington A, Ziedonis D, Fan X, Zhao M, 2015. Hippocampal volume reduction in female but not male recent abstinent methamphetamine users. Behav. Brain Res 289, 78–83. 10.1016/j.bbr.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan Z, Kippen R, Sutton K, Ward B, Agius PA, Quinn B, Dietze P, 2021. Correlates of anxiety and depression in a community cohort of people who smoke methamphetamine. Aust. N. Z. J. Psychiatry, 48674211048152. 10.1177/00048674211048152. [DOI] [PubMed] [Google Scholar]
- Everett NA, Baracz SJ, Cornish JL, 2020. The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology 45, 597–605. 10.1038/s41386-019-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Giuliano C, Belin D, 2018. Addictive behaviour in experimental animals: prospects for translation. Philos. Trans. R. Soc. Lond. B Biol. Sci 373 10.1098/rstb.2017.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, 2018. Self-administration of methamphetamine aerosol by male and female baboons. Pharm. Biochem. Behav 168, 17–24. 10.1016/j.pbb.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway GP, Singleton EG, Buscemi R, Baggott MJ, Dickerhoof RM, Mendelson JE, Methamphetamine Treatment Project Corporate, A., 2010. An examination of drug craving over time in abstinent methamphetamine users. Am. J. Addict 19, 510–514. 10.1111/j.1521-0391.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S, 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharm. Rev 62, 155–198. 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson R, Methamphetamine Treatment Project Corporate, A., 2009. Depression among methamphetamine users: association with outcomes from the methamphetamine treatment project at 3-year follow-up. J. Nerv. Ment. Dis 197, 225–231. 10.1097/NMD.0b013e31819db6fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R, 2011. Bulimia nervosa among methamphetamine dependent adults: association with outcomes three years after treatment. Eat. Disord 19, 259–269. 10.1080/10640266.2011.566149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R, Methamphetamine Treatment P, 2008. Risk factors for suicide attempts in methamphetamine-dependent patients. Am. J. Addict 17, 24–27. 10.1080/10550490701756070. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Ang A, McCann MJ, Rawson RA, 2008. An emerging problem: methamphetamine abuse among treatment seeking youth. Subst. Abus 29, 71–80. 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Ang A, Rawson RA, Lee S, Iguchi MY, 2011. Methamphetamine Treatment Project Corporate Authors. Quality of life among treatment seeking methamphetamine-dependent individuals. Am. J. Addict 20, 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, Wang X, Harris RA, Nelson A-G, Stepp D, Klaassen Z, Raval P, Cortes J, Coughlin SS, Bogdanov VY, Moore JX, Desai N, Miller DD, Lu X-Y, Kim HW, Weintraub NL, 2021. Obesity and the bidirectional risk of cancer and cardiovascular diseases in African Americans: disparity vs. ancestry. Front. Cardiovasc. Med 8 10.3389/fcvm.2021.761488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MG, Alhassoon OM, Stern MJ, Wollman SC, Kimmel CL, Perez-Figueroa A, Radua J, 2015. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am. J. Drug Alcohol Abus 41, 290–299. 10.3109/00952990.2015.1044607. [DOI] [PubMed] [Google Scholar]
- Han Y, Lin V, Wu F, Hser YI, 2016. Gender comparisons among asian american and pacific islander patients in drug dependency treatment. Subst. Use Misuse 51, 752–762. 10.3109/10826084.2016.1155604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Westbrook SR, Haake RM, Marinelli M, Gulley JM, 2018a. Reduced sensitivity to reinforcement in adolescent compared to adult Sprague-Dawley rats of both sexes. Psychopharmacology 235, 861–871. 10.1007/s00213-017-4804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Westbrook SR, Haake RM, Willing J, Raetzman LT, Juraska JM, Gulley JM, 2018b. Age- and sex-dependent effects of methamphetamine on cognitive flexibility and 5-HT2C receptor localization in the orbitofrontal cortex of Sprague-Dawley rats. Behav. Brain Res 349, 16–24. 10.1016/j.bbr.2018.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Moallem NR, Courtney KE, Glasner-Edwards S, Ray LA, 2016. Sex differences in the association between internalizing symptoms and craving in methamphetamine users. J. Addict. Med 10, 395–401. 10.1097/ADM.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Tang J, Liu T, Hao W, Liao Y, 2020. Gender differences in sleep problems among drug users. Front. Psychiatry 11, 808. 10.3389/fpsyt.2020.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Xie Y, Tao J, Su H, Wu W, Zou S, Zhang J, Zhang J, Zhang H, Yang X, Guo J, Tang W, Zhang F, Liu J, Liu L, Chen Y, Wen N, Kosten TR, Zhang XY, 2013. Gender differences in socio-demographic and clinical characteristics of methamphetamine inpatients in a Chinese population. Drug Alcohol Depend. 130, 94–100. 10.1016/j.drugalcdep.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Demirdjian L, Wu Y, Shoptaw S, 2016. Single nucleotide polymorphism near CREB1, rs7591784, is associated with pretreatment methamphetamine use frequency and outcome of outpatient treatment for methamphetamine use disorder. J. Psychiatr. Res 74, 22–29. 10.1016/j.jpsychires.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Gadzhyan J, van Oudheusden H, Rodriguez F, McCracken J, Shoptaw S, 2013. Pilot randomized trial of bupropion for adolescent methamphetamine abuse/dependence. J. Adolesc. Health.: Off. Publ. Soc. Adolesc. Med 52, 502–505. 10.1016/j.jadohealth.2012.10.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Shoptaw S, 2012. Gender, brain-derived neurotrophic factor Val66Met, and frequency of methamphetamine use. Gend. Med 9, 112–120. 10.1016/j.genm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson HP, Hardwick WC, McMillan DE, Owens SM, 2008. Bioavailability of (+)-methamphetamine in the pigeon following an intramuscular dose. Pharm. Biochem. Behav 90, 382–386. 10.1016/j.pbb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensleigh E, Pritchard LM, 2014. The effect of early environmental manipulation on locomotor sensitivity and methamphetamine conditioned place preference reward. Behav. Brain Res 268, 66–71. 10.1016/j.bbr.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL, 1996. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 714, 95–103. 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang YC, 2005. Treatment outcomes among women and men methamphetamine abusers in California. J. Subst. Abus. Treat 28, 77–85. 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Jacobskind JS, Rosinger ZJ, Gonzalez T, Zuloaga KL, Zuloaga DG, 2018. Chronic methamphetamine exposure attenuates neural activation in hypothalamic-pituitary-adrenal axis-associated brain regions in a sex-specific manner. Neuroscience 380, 132–145. 10.1016/j.neuroscience.2018.04.010. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2017. A decade of orexin/hypocretin and addiction: where are we now? Curr. Top. Behav. Neurosci 33, 247–281. 10.1007/7854_2016_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Daiwile AP, Cadet JL, 2021. Neurotoxicity of methamphetamine: main effects and mechanisms. Exp. Neurol 344, 113795 10.1016/j.expneurol.2021.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL, 2001. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J. 15, 1745–1752. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Gonzalez B, McCoy MT, Ladenheim B, Bisagno V, Cadet JL, 2017. Methamphetamine induces TET1- and TET3-dependent dna hydroxymethylation of Crh and Avp genes in the rat nucleus accumbens. Mol. Neurobiol 55, 5154–5166. 10.1007/s12035-017-0750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Chojnacki MR, Daiwile AP, Cadet JL, 2020. Chemogenetic inhibition of dopamine D1-expressing neurons in the dorsal striatum does not alter methamphetamine intake in either male or female long evans rats. Neurosci. Lett 729, 134987 10.1016/j.neulet.2020.134987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca L, Zuloaga DG, Raber J, Siegel JA, 2014. Long-term effects of early adolescent methamphetamine exposure on depression-like behavior and the hypothalamic vasopressin system in mice. Dev. Neurosci 36, 108–118. 10.1159/000360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen A, McFadden LM, 2017. The neurochemical consequences of methamphetamine self-administration in male and female rats. Drug Alcohol Depend. 178, 70–74. 10.1016/j.drugalcdep.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA, 2005. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch. Intern. Med 165, 1600–1605. 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ, 2003. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 361, 1677–1685. 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Elkashef AM, Seneviratne C, Ait-Daoud N, Kahn RC, Li SH, Bloch DA, Holmes TH, Wang XQ, Vocci FJ Jr., Li MD, Methamphetamine Study G, 2010. Association between genotype of the serotonin transporter-linked polymorphic region of the serotonin transporter gene and age of onset of methamphetamine use: a preliminary analysis. Front. Psychiatry 1, 145. 10.3389/fpsyt.2010.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Holmgren A, 2012. Concentration ratios of methamphetamine to amphetamine in blood can help to distinguish use of methamphetamine from various mixtures of the two stimulants. J. Anal. Toxicol 36, 634–637. 10.1093/jat/bks075. [DOI] [PubMed] [Google Scholar]
- Jones CM, Compton DM, Mustaquim D, 2020a. Patterns and characteristics of methamphetamine use among adults — United States, 2015–2018. CDC Morb. Mortal. Wkly. Rep 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Olsen EO, O’Donnell J, Mustaquim D, 2020b. Resurgent methamphetamine use at treatment admission in the United States, 2008–2017. Am. J. Public Health 110, 509–516. 10.2105/AJPH.2019.305527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Woods C, Barker R, Usher K, 2019. Patterns and features of methamphetamine-related presentations to emergency departments in QLD from 2005 to 2017. Int. J. Ment. Health Nurs 28, 833–844. 10.1111/inm.12618. [DOI] [PubMed] [Google Scholar]
- Jones R, Woods C, Usher K, 2018. Rates and features of methamphetamine-related presentations to emergency departments: an integrative literature review. J. Clin. Nurs 27, 2569–2582. 10.1111/jocn.14493. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Longshore D, Anglin MD, van Gorp WG, Gawin FH, 2000. Psychiatric comorbidity of methamphetamine dependence in a forensic sample. J. Neuropsychiatry Clin. Neurosci 12, 480–484. 10.1176/jnp.12.4.480. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP, 2004. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 75, 233–240. 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Janak PH, 2015. Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron 88, 247–263. 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil CG, Goeders NE, Woolard MD, Bhuiyan MS, Dominic P, Kolluru GK, Arnold CL, Traylor JG, Orr AW, 2019. Methamphetamine use and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol 39, 1739–1746. 10.1161/ATVBAHA.119.312461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Sung YH, Lee HY, Lee DS, Jeong DU, Renshaw PF, 2005. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology 30, 1383–1391. 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L, 2010a. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology 212, 243–249. 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L, 2010b. Psychiatric symptoms and HPA axis function in adolescent methamphetamine users. J. Neuroimmune Pharm 5, 582–591. 10.1007/s11481-010-9206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Ikeda M, Kitajima T, Yamanouchi Y, Kinoshita Y, Kawashima K, Okochi T, Tsunoka T, Okumura T, Inada T, Ujike H, Yamada M, Uchimura N, Sora I, Iyo M, Ozaki N, Iwata N, 2009. A functional polymorphism in estrogen receptor alpha gene is associated with Japanese methamphetamine induced psychosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 895–898. 10.1016/j.pnpbp.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Kishi T, Okochi T, Kitajima T, Ujike H, Inada T, Yamada M, Uchimura N, Sora I, Iyo M, Ozaki N, Correll CU, Iwata N, 2011. Lack of association between translin-associated factor X gene (TSNAX) and methamphetamine dependence in the Japanese population. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1618–1622. 10.1016/j.pnpbp.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Kitanaka N, Kitanaka J, Takemura M, 2005. Repeated clorgyline treatment inhibits methamphetamine-induced behavioral sensitization in mice. Neurochem. Res 30, 445–451. 10.1007/s11064-005-2679-z. [DOI] [PubMed] [Google Scholar]
- Klambatsen A, Nygard SK, Chang AJ, Quinones V, Jenab S, 2019. Sex differences in memory and intracellular signaling after methamphetamine binge treatment. Brain Res. 1711, 16–22. 10.1016/j.brainres.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Ide S, Hasegawa J, Ujike H, Sekine Y, Ozaki N, Inada T, Harano M, Komiyama T, Yamada M, Iyo M, Shen HW, Ikeda K, Sora I, 2004. Study of association between alpha-synuclein gene polymorphism and methamphetamine psychosis/dependence. Ann. N. Y Acad. Sci 1025, 325–334. 10.1196/annals.1316.040. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ujike H, Iwata N, Inada T, Yamada M, Sekine Y, Uchimura N, Iyo M, Ozaki N, Itokawa M, Sora I, 2010. The adenosine A2A receptor is associated with methamphetamine dependence/psychosis in the Japanese population. Behav. Brain Funct 6, 50. 10.1186/1744-9081-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogachi S, Chang L, Alicata D, Cunningham E, Ernst T, 2017. Sex differences in impulsivity and brain morphometry in methamphetamine users. Brain Struct. Funct 222, 215–227. 10.1007/s00429-016-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Hashimoto K, Kumakiri C, Shimizu E, Sekine Y, Ozaki N, Inada T, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Takei N, Iyo M, 2004. Association between the glutathione S-transferase M1 gene deletion and female methamphetamine abusers. Am. J. Med. Genet B Neuropsychiatr. Genet 126B, 43–45. 10.1002/ajmg.b.20148. [DOI] [PubMed] [Google Scholar]
- Kondo A, Shimane T, Takahashi M, Takeshita Y, Kobayashi M, Takagishi Y, Omiya S, Takano Y, Yamaki M, Matsumoto T, 2021. Gender differences in triggers of stimulant use based on the national survey of prisoners in Japan. Subst. Use Misuse 56, 54–60. 10.1080/10826084.2020.1833930. [DOI] [PubMed] [Google Scholar]
- Lardner CK, van der Zee Y, Estill MS, Kronman HG, Salery M, Cunningham AM, Godino A, Parise EM, Kim JH, Neve RL, Shen L, Hamilton PJ, Nestler EJ, 2021. Gene-targeted, CREB-mediated induction of DeltaFosB controls distinct downstream transcriptional patterns within D1 and D2 medium spiny neurons. Biol. Psychiatry 90, 540–549. 10.1016/j.biopsych.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leombruni P, Lavagnino L, Fassino S, 2009. Treatment of obese patients with binge eating disorder using topiramate: a review. Neuropsychiatr. Dis. Treat 5, 385–392. 10.2147/ndt.s3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SK, Ball D, Hsiao CC, Chiang YL, Ree SC, Chen CK, 2004. Psychiatric comorbidity and gender differences of persons incarcerated for methamphetamine abuse in Taiwan. Psychiatry Clin. Neurosci 58, 206–212. 10.1111/j.1440-1819.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- Lin SK, Chen CK, Ball D, Liu HC, Loh EW, 2003. Gender-specific contribution of the GABA(A) subunit genes on 5q33 in methamphetamine use disorder. Pharm. J 3, 349–355. 10.1038/sj.tpj.6500203. [DOI] [PubMed] [Google Scholar]
- Lisa P, Felicia K, Laura H, Daniela K, Marlies R, Stefanie N, Maik SJ, Anne S, Maximilian S, Kirsi M, Michael S, Gabi K, 2019. Associations between methamphetamine use, psychiatric comorbidities and treatment outcome in two inpatient rehabilitation centers. Psychiatry Res. 280, 112505 10.1016/j.psychres.2019.112505. [DOI] [PubMed] [Google Scholar]
- Ma JZ, Johnson BA, Yu E, Weiss D, McSherry F, Saadvandi J, Iturriaga E, Ait-Daoud N, Rawson RA, Hrymoc M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D, McCann M, Pham T, Stock C, Dickinson R, Elkashef A, Li MD, 2013. Fine-grain analysis of the treatment effect of topiramate on methamphetamine addiction with latent variable analysis. Drug Alcohol Depend. 130, 45–51. 10.1016/j.drugalcdep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ 3rd, Hawkins RY, De La Garza R 2nd, Kalechstein AD, Newton TF, 2010. Relationship between gender and psychotic symptoms in cocaine-dependent and methamphetamine-dependent participants. Gend. Med 7, 414–421. 10.1016/j.genm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JJ 3rd, Kalechstein AD, De La Garza R 2nd, Newton TF, 2008. Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependent participants. Am. J. Addict 17, 83–98. 10.1080/10550490701861201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BD, Galea S, Wood E, Kerr T, 2011. Injection methamphetamine use is associated with an increased risk of attempted suicide: a prospective cohort study. Drug Alcohol Depend. 119, 134–137. 10.1016/j.drugalcdep.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, Cadet JL, 2012. Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLoS One 7, e34236. 10.1371/journal.pone.0034236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson ME, 2013. Emergency Department Visits Involving Methamphetamine: 2007 to 2011, The CBHSQ Report, Rockville (MD), pp. 1–7. [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Berthold HK, Campesi I, Carrero J-J, Dhakal S, Franconi F, Gouni-Berthold I, Heiman ML, Kautzky-Willer A, Klein SL, Murphy A, Regitz-Zagrosek V, Reue K, Rubin JB, 2021. Sex- and gender-based pharmacological response to drugs. Pharm. Rev 73, 730–762. 10.1124/pharmrev.120.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Fraser D, Childs E, Momenan R, Hommer DW, de Wit H, Heilig M, 2013. Conditioned preference to a methamphetamine-associated contextual cue in humans. Neuropsychopharmacology 38, 921–929. 10.1038/npp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Paul E, DeArcangelis J, Van Hedger K, de Wit H, 2019. Gender differences in the behavioral and subjective effects of methamphetamine in healthy humans. Psychopharmacology 236, 2413–2423. 10.1007/s00213-019-05276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Cordie R, Livermont T, Johansen A, 2018. Behavioral and serotonergic changes in the frontal cortex following methamphetamine self-administration. Int. J. Neuropsychopharmacol 21, 758–763. 10.1093/ijnp/pyy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Vieira-Brock PL, 2016. The persistent neurotoxic effects of methamphetamine on dopaminergic and serotonergic markers in male and female rats. Toxicol. Open Access 2. 10.4172/2476-2067.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFaull SR, Champagne A, Thompson W, Bang F, 2020. Injuries and poisonings associated with methamphetamine use: sentinel surveillance, the electronic Canadian Hospitals injury reporting and prevention program (eCHIRPP), 2011–2019. Health Promot Chronic Dis. Prev. Can 40, 126–129. 10.24095/hpcdp.40.4.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Lubman DI, Lee NM, Ross JE, Slade TN, 2011. Major depression among methamphetamine users entering drug treatment programs. Med. J. Aust 195, S51–S55. 10.5694/j.1326-5377.2011.tb03266.x. [DOI] [PubMed] [Google Scholar]
- McKetin R, Voce A, Burns R, Shanahan M, 2019. Health-related quality of life among people who use methamphetamine. Drug Alcohol Rev. 38, 503–509. 10.1111/dar.12934. [DOI] [PubMed] [Google Scholar]
- Mihan R, Shahrivar Z, Mahmoudi-Gharaei J, Shakiba A, Hosseini M, 2018. Attention-deficit hyperactivity disorder in adults using methamphetamine: does it affect comorbidity, quality of life, and global functioning? Iran. J. Psychiatry 13, 111–118. [PMC free article] [PubMed] [Google Scholar]
- Milesi-Halle A, Hambuchen MD, McMillan DE, Michael Owens S, 2015. The pharmacokinetics of methamphetamine self-administration in male and female rats. Drug Alcohol Depend. 150, 164–169. 10.1016/j.drugalcdep.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Halle A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM, 2005. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol. Appl. Pharm 209, 203–213. 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM, 2007. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharm. Biochem. Behav 86, 140–149. 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Bu M, Gopinath A, Martinez LR, Khoshbouei H, 2021. Methamphetamine dysregulation of the central nervous system and peripheral immunity. J. Pharm. Exp. Ther 379, 372–385. 10.1124/jpet.121.000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED, 2015. Gray-matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Mol. Psychiatry 20, 764–771. 10.1038/mp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KC, Cornish JL, Faingold A, Wood K, Haber PS, 2017. Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Expert Opin. Investig. Drugs 26, 563–578. 10.1080/13543784.2017.1313229. [DOI] [PubMed] [Google Scholar]
- Morris ME, Lee H-J, Predko LM, 2003. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharm. Rev 55, 229–240. 10.1124/pr.55.2.1. [DOI] [PubMed] [Google Scholar]
- Muller CP, 2018. Animal models of psychoactive drug use and addiction - present problems and future needs for translational approaches. Behav. Brain Res 352, 109–115. 10.1016/j.bbr.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Nagai T, Noda Y, Ishikawa K, Miyamoto Y, Yoshimura M, Ito M, Takayanagi M, Takuma K, Yamada K, Nabeshima T, 2005. The role of tissue plasminogen activator in methamphetamine-related reward and sensitization. J. Neurochem 92, 660–667. 10.1111/j.1471-4159.2004.02903.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Takei N, Iwata Y, Suzuki K, Anitha A, Inada T, Harano M, Komiyama T, Yamada M, Iwata N, Iyo M, Sora I, Ozaki N, Ujike H, Mori N, 2009. An association study of monoamine oxidase A (MAOA) gene polymorphism in methamphetamine psychosis. Neurosci. Lett 455, 120–123. 10.1016/j.neulet.2009.02.048. [DOI] [PubMed] [Google Scholar]
- Nakatome M, Miyaji A, Mochizuki K, Kishi Y, Isobe I, Matoba R, 2009. Association between the GST genetic polymorphisms and methamphetamine abusers in the Japanese population. Leg. Med 11 (Suppl 1), S468–S470. 10.1016/j.legalmed.2009.01.095. [DOI] [PubMed] [Google Scholar]
- Neeki MM, Kulczycki M, Toy J, Dong F, Lee C, Borger R, Adigopula S, 2016. Frequency of methamphetamine use as a major contributor toward the severity of cardiomyopathy in adults </=50 years. Am. J. Cardiol 118, 585–589. 10.1016/j.amjcard.2016.05.057. [DOI] [PubMed] [Google Scholar]
- Neumann S, Soyka M, Franke AG, 2018. Bio-psycho-social characteristics and therapeutic aspects of methamphetamine-dependent women - gender specific results of a systematic literature search. Psychother. Psychosom. Med. Psychol 68, 281–289. 10.1055/s-0043-115003. [DOI] [PubMed] [Google Scholar]
- Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y, 2022. Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharm. Rev 74, 119–140. 10.1124/pharmrev.121.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Ghahremani DG, Mandelkern MA, Dean AC, Luo W, Ren A, Li J, London ED, 2021. The relationship between duration of abstinence and gray-matter brain structure in chronic methamphetamine users. Am. J. Drug Alcohol Abus 47, 65–73. 10.1080/00952990.2020.1778712. [DOI] [PubMed] [Google Scholar]
- NSDUH, 2019. Substance Abuse and Mental Health Services Administration. Results from the 2018 US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, National Survey on Drug Use and Health. Rockville, MD. [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B, 1992. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch. Gen. Psychiatry 49, 881–887. 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, London ED, 2014. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int. J. Neuropsychopharmacol 18. 10.1093/ijnp/pyu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohia-Nwoko O, Haile CN, Kosten TA, 2017. Sex differences in the acute locomotor response to methamphetamine in BALB/c mice. Behav. Brain Res 327, 94–97. 10.1016/j.bbr.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paknahad S, Akhgari M, Ghadipasha M, 2021. An alarming rise in the prevalence of deaths with methamphetamine involved in Tehran, Iran 2011–2018. Forensic Sci. Med. Pathol 17, 208–215. 10.1007/s12024-020-00339-9. [DOI] [PubMed] [Google Scholar]
- Pena-Bravo JI, Penrod R, Reichel CM, Lavin A, 2019. Methamphetamine self-administration elicits sex-related changes in postsynaptic glutamate transmission in the prefrontal cortex. eNeuro 6. 10.1523/ENEURO.0401-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Ortiz RJ, Hernandez KR, Cushing BS, 2019. Effects of methamphetamine on alloparental behavior in male and female prairie voles. Physiol. Behav 203, 128–134. 10.1016/j.physbeh.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Pittenger ST, Chou S, Murawski NJ, Barrett ST, Loh O, Duque JF, Li M, Bevins RA, 2021. Female rats display higher methamphetamine-primed reinstatement and c-Fos immunoreactivity than male rats. Pharm. Biochem. Behav 201, 173089 10.1016/j.pbb.2020.173089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Buscemi R, Nayak M, Korcha R, Galloway G, 2012. Gender differences in psychiatric symptoms among methamphetamine dependent residents in sober living houses. Addict. Disord. Their Treat 11, 53–63. 10.1097/ADT.0b013e3182213ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, DeSantis SM, Simpson AN, Tolliver BK, McRae-Clark AL, Saladin ME, Baker NL, Wagner MT, Brady KT, 2011. The impact of clinical and demographic variables on cognitive performance in methamphetamine-dependent individuals in rural South Carolina. Am. J. Addict 20, 447–455. 10.1111/j.1521-0391.2011.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard LM, Hensleigh E, Lynch S, 2012. Altered locomotor and stereotyped responses to acute methamphetamine in adolescent, maternally separated rats. Psychopharmacology 223, 27–35. 10.1007/s00213-012-2679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambousek L, Kacer P, Syslova K, Bumba J, Bubenikova-Valesova V, Slamberova R, 2014. Sex differences in methamphetamine pharmacokinetics in adult rats and its transfer to pups through the placental membrane and breast milk. Drug Alcohol Depend. 139, 138–144. 10.1016/j.drugalcdep.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Ramos J, Hardin EJ, Grant AH, Flores-Robles G, Gonzalez AT, Cruz B, Martinez AK, Beltran NM, Serafine KM, 2020. The effects of eating a high fat diet on sensitivity of male and female rats to methamphetamine and dopamine D1 receptor agonist SKF 82958. J. Pharm. Exp. Ther 374, 6–15. 10.1124/jpet.119.263293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P, 2005. Methamphetamine use among treatment-seeking adolescents in Southern California: participant characteristics and treatment response. J. Subst. Abus. Treat 29, 67–74. 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Courtney KE, Moallem NR, Lunny K, Roche D, Leventhal AM, Shoptaw S, Heinzerling K, London ED, Miotto K, 2015. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: a double-blind, placebo-controlled laboratory study. Neuropsychopharmacology 40, 2347–2356. 10.1038/npp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE, 2012. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology 223, 371–380. 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei F, Ghaderi E, Mardani R, Hamidi S, Hassanzadeh K, 2016. Topiramate for the management of methamphetamine dependence: a pilot randomized, double-blind, placebo-controlled trial. Fundam. Clin. Pharm 30, 282–289. 10.1111/fcp.12178. [DOI] [PubMed] [Google Scholar]
- Richards JR, Placone TW, Wang CG, van der Linden MC, Derlet RW, Laurin EG, 2020. Methamphetamine, amphetamine, and MDMA use and emergency department recidivism. J. Emerg. Med 59, 320–328. 10.1016/j.jemermed.2020.04.051. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology 172, 443–449. 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A, 2015. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front. Psychiatry 6, 91. 10.3389/fpsyt.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungnirundorn T, Verachai V, Gelernter J, Malison RT, Kalayasiri R, 2017. Sex differences in methamphetamine use and dependence in a thai treatment center. J. Addict. Med 11, 19–27. 10.1097/ADM.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, Natsuaki Y, Waters C, Moore CD, Galloway GP, Nordahl TE, 2011. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Depend. 113, 133–138. 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R, 2006. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol 11, 2–38. 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Saw YM, Saw TN, Yasuoka J, Chan N, Kham NPE, Khine W, Cho SM, Jimba M, 2017. Gender difference in early initiation of methamphetamine use among current methamphetamine users in Muse, Northern Shan State, Myanmar. Harm Reduct. J 14, 21. 10.1186/s12954-017-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB, 2002. Gender differences in the behavioral effects of methamphetamine. Eur. J. Pharm 442, 231–235. 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Sepulveda M, Manning EE, Gogos A, Hale M, van den Buuse M, 2021. Long-term effects of young-adult methamphetamine on dorsal raphe serotonin systems in mice: Role of brain-derived neurotrophic factor. Brain Res. 1762, 147428 10.1016/j.brainres.2021.147428. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Trzeciak M, Eliason NL, Blankenship HE, Byrd BAM, Douglas PD, Freeman WM, Beckstead MJ, 2021. Repeated cocaine or methamphetamine treatment alters astrocytic CRF2 and GLAST expression in the ventral midbrain. Addict. Biol, e13120 10.1111/adb.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JL, Grant KM, Daly PM, Kelley SG, Carlo G, Bevins RA, 2016. Psychological burden and gender differences in methamphetamine-dependent individuals in treatment. J. Psychoact. Drugs 48, 261–269. 10.1080/02791072.2016.1213470. [DOI] [PubMed] [Google Scholar]
- Stoneberg DM, Shukla RK, Magness MB, 2018. Global methamphetamine trends: an evolving problem. Int. Crim. Justice Rev 28, 136–161. [Google Scholar]
- Su H, Zhang J, Ren W, Xie Y, Tao J, Zhang X, He J, 2017. Anxiety level and correlates in methamphetamine-dependent patients during acute withdrawal. Medicine 96, e6434. 10.1097/MD.0000000000006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, Hellem TL, Huber RS, Fiedler KK, Harrell RE, Nickerson BR, Kim SE, Jeong EK, Renshaw PF, 2013. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend. 129, 102–109. 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Tseng J, Fannon MJ, Purohit DC, Quach LW, Terranova MJ, Kharidia KM, Oliver RJ, Mandyam CD, 2018. Sex differences in context-driven reinstatement of methamphetamine seeking is associated with distinct neuroadaptations in the dentate gyrus. Brain Sci. 8 10.3390/brainsci8120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, O’Neill J, Alger JR, Shen Z, Johnson MC, London ED, 2019. N-Acetyl and glutamatergic neurometabolites in perisylvian brain regions of methamphetamine users. Int. J. Neuropsychopharmacol 22, 1–9. 10.1093/ijnp/pyy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmingh HS, van den Brink W, Howells F, Sibeko G, Stein DJ, 2020. Methamphetamine use and antipsychotic-related extrapyramidal side-effects in patients with psychotic disorders. J. Dual Diagn 16, 208–217. 10.1080/15504263.2020.1714099. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Gennequin B, Lardeux V, Chauvet C, Decressac M, Janet T, Jaber M, Solinas M, 2011. Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine. Neurotox. Res 19, 172–182. 10.1007/s12640-010-9158-2. [DOI] [PubMed] [Google Scholar]
- Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED, 2010. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology 209, 13–24. 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC, 2021. Drug Market Trends: Cocaine, Amphetamine-type stimulants. United Nations Publication. Vienna, Austria, 2021 E.21. XI. 8. [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A, 2009. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J. Clin. Exp. Neuropsychol 31, 706–719. 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, Caprioli D, 2017. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology 42, 1126–1135. 10.1038/npp.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP, 1992. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatry 49, 876–880. 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Tekirian TL, Cheo CT, 1993. Sexual differences in sensitivity to methamphetamine toxicity. J. Neural Transm. Gen. Sect 93, 67–70. 10.1007/BF01244939. [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Bush LG, Gibb JW, Hanson GR, 1994. Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain Res. 665, 237–244. 10.1016/0006-8993(94)91343-9. [DOI] [PubMed] [Google Scholar]
- Westbrook SR, Dwyer MR, Cortes LR, Gulley JM, 2020. Extended access self-administration of methamphetamine is associated with age- and sex-dependent differences in drug taking behavior and recognition memory in rats. Behav. Brain Res 390, 112659 10.1016/j.bbr.2020.112659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook SR, Gulley JM, 2020. Effects of the GluN2B antagonist, Ro 25–6981, on extinction consolidation following adolescent- or adult-onset methamphetamine self-administration in male and female rats. Behav. Pharm 31, 748–758. 10.1097/FBP.0000000000000586. [DOI] [PubMed] [Google Scholar]
- Wise RA, Jordan CJ, 2021. Dopamine, behavior, and addiction. J. Biomed. Sci 28, 83. 10.1186/s12929-021-00779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Kleitz HK, Deng X, Ladenheim B, Peng XQ, Li X, Gardner EL, Stein EA, Cadet JL, 2009. A single high dose of methamphetamine increases cocaine self-administration by depletion of striatal dopamine in rats. Neuroscience 161, 392–402. 10.1016/j.neuroscience.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Campbell HL, Hawley LL, Horchar MJ, Kappesser JL, Wright MR, 2021. Effects of the GluN2B-selective antagonist Ro 63–1908 on acquisition and expression of methamphetamine conditioned place preference in male and female rats. Drug Alcohol Depend. 225, 108785 10.1016/j.drugalcdep.2021.108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CF, Chong MY, 2006. Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Compr. Psychiatry 47, 215–220. 10.1016/j.comppsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Yen CF, Yang YH, Ko CH, Yen JY, 2005. Substance initiation sequences among Taiwanese adolescents using methamphetamine. Psychiatry Clin. Neurosci 59, 683–689. 10.1111/j.1440-1819.2005.01437.x. [DOI] [PubMed] [Google Scholar]
- Yimsaard P, Maes MM, Verachai V, Kalayasiri R, 2018. Pattern of methamphetamine use and the time lag to methamphetamine dependence. J. Addict. Med 12, 92–98. 10.1097/ADM.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, Kudelko K, Liu J, Hsi A, Rupp A, Sweatt AJ, Tuder R, Berry GJ, Rabinovitch M, Doyle RL, de Jesus Perez V, Kawut SM, 2018. Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med 197, 788–800. 10.1164/rccm.201705-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kral AH, Simpson KA, Ceasar RC, Wenger LD, Kirkpatrick M, Bluthenthal RN, 2021a. Factors associated with methamphetamine withdrawal symptoms among people who inject drugs. Drug Alcohol Depend. 223, 108702 10.1016/j.drugalcdep.2021.108702. [DOI] [PubMed] [Google Scholar]
- Zhao SX, Deluna A, Kelsey K, Wang C, Swaminathan A, Staniec A, Crawford MH, 2021b. Socioeconomic burden of rising methamphetamine-associated heart failure hospitalizations in California From 2008 to 2018. Circ. Cardiovasc. Qual. Outcomes 14, e007638. 10.1161/CIRCOUTCOMES.120.007638. [DOI] [PubMed] [Google Scholar]
- Zhao SX, Kwong C, Swaminathan A, Gohil A, Crawford MH, 2018. Clinical characteristics and outcome of methamphetamine-associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail 6, 209–218. 10.1016/j.jchf.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Holtz NA, Lepak VC, Saykao AT, Zhang Y, Carroll ME, 2021. Age-specific treatment effects of orexin/hypocretin-receptor antagonism on methamphetamine-seeking behavior. Drug Alcohol Depend. 224, 108719 10.1016/j.drugalcdep.2021.108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Johnson LA, Agam M, Raber J, 2014. Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J. Neurochem 129, 495–508. 10.1111/jnc.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M, Methamphetamine Treatment P, 2004. Psychiatric symptoms in methamphetamine users. Am. J. Addict 13, 181–190. 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data were used in this review article.


