Abstract
Elevated impulsivity is a symptom shared by various psychiatric disorders such as substance use disorder, bipolar disorder, and attention-deficit/hyperactivity disorder. However, impulsivity is not a unitary construct and impulsive behaviors fall into two subcategories: impulsive action and impulsive choice. Impulsive choice refers to the tendency to prefer immediate, small rewards over delayed, large rewards, whereas impulsive action involves difficulty inhibiting rash, premature, or mistimed behaviors. These behaviors are mediated by the mesocorticolimbic dopamine (DA) system, which consists of projections from the ventral tegmental area to the nucleus accumbens and prefrontal cortex. Early life stress (ELS) alters both impulsive choice and impulsive action in rodents. ELS also changes DA receptor expression, transmission, and activity within the mesocorticolimbic system. This review integrates the dopamine, impulsivity, and ELS literature to provide evidence that ELS alters impulsivity via inducing changes in the mesocorticolimbic DA system. Understanding how ELS affects brain circuits associated with impulsivity can help advance treatments aimed towards reducing impulsivity symptoms in a variety of psychiatric disorders.
Keywords: stress, cognition, nucleus accumbens, prefrontal cortex, sex differences
1. Introduction
Impulsivity is acting without forethought. In some cases, it pays to act impulsively. For instance, when one is in a dangerous situation, acting impulsively (e.g., landing the first punch in a fight) may improve chances of survival. Additionally, acting on impulse can stimulate creative moments and allow one to seize opportunities that they may have otherwise missed. Most of the time, acting impulsively is not considered pathological. However, when impulsivity impacts everyday life, it can constitute a risk factor for a large number of life-threatening behaviors (Bari & Robbins, 2013; Everitt et al., 2008; Fineberg et al., 2014; Gut-Fayand et al., 2001). High impulsivity is associated with several psychiatric disorders including, attention-hyperactivity disorder (ADHD), bipolar disorder, and addiction (e.g., gambling disorder and substance use disorder, SUD) (Adler et al., 2017; Dawe & Loxton, 2004; Najt et al., 2007; Vest, Reynolds, & Tragesser, 2016).
Another factor associated with ADHD, bipolar disorder, and addiction is exposure to early life stress (ELS) (Halevi, Djalovski, Vengrober, & Feldman, 2016; Herzberg et al., 2018; Syed & Nemeroff, 2017). For instance, among adolescents receiving treatment for SUD, more than 70% report a history of trauma (Deykin & Buka, 1997; Funk, McDermeit, Godley, & Adams, 2003). Childhood trauma also predicts ADHD onset and is a risk factor for the persistence of ADHD symptoms into adulthood (Biederman et al., 1995; Sugaya et al., 2012; Vrijsen et al., 2018). Additionally, self-reported childhood abuse may be evident in about 50% of individuals with bipolar disorder and contribute to worse clinical outcomes (Farias et al., 2019; Garno, Goldberg, Ramirez, & Ritzler, 2005; Xie et al., 2018).
Much work has focused on how ELS impairs executive functioning, memory, and reward processing to understand mechanisms by which ELS may increase risk for psychiatric disorders (Hostinar, Stellern, Schaefer, Carlson, & Gunnar, 2012; Pechtel & Pizzagalli, 2011). However, fewer studies have focused on how ELS affects impulsivity, even though impulsivity is a feature of ADHD, bipolar disorder, and SUD. This review brings together data on what is known about ELS and impulsivity. We first describe types of impulsive behaviors and the circuits that underly impulsive processes. Then, we detail how ELS affects impulsivity and the purported mechanisms by which this can occur. Throughout preclinical to clinical findings are compared, and when known, sex differences in these effects are detailed. Given the number of disorders with ELS as a risk factor and impulsivity as a key feature, understanding the mechanisms by which early stress affects impulsivity may lead to novel treatments that improve outcomes for a variety of conditions.
2. Different Types of Impulsive Behaviors
Because impulsivity is a multifaceted construct, an attempt to categorize impulsivity has been to organize impulsive behaviors into three distinct types (Robbins & Dalley, 2017). The first type, waiting impulsivity, encompasses impulsive behaviors that require a subject to wait before getting a reward. Behaviors associated with this branch are measured using delay discounting, differential-reinforcement-of-low-rates-of-responding (DRL), and five-choice-serial-reaction-time tasks (5-CSRTT). For DRL and 5-CSRTT, impulsivity depends on the ability to prevent an inappropriate, premature response. Inability to withhold a premature response is termed an impulsive action. In delay discounting, impulsivity is associated with choosing a small, immediate reward over a large, delayed one. A subject’s preference for instant gratification over delayed gratification is considered an impulsive choice. The second branch, risky impulsivity, refers to a subject’s preference for uncertain but bigger outcomes. A task used to measure this type of behavior in rodents is the probability discounting task, which involves choosing between two levers: one dispenses a small reward every time it is pressed versus another which dispenses a larger reward sometimes (risky lever). Choosing the riskier option is indicative of higher levels of impulsivity and these responses can be considered a form of impulsive choice. However, there is some debate as to whether risky behavior is the same as impulsivity. For example, studies that use delay and probability discounting show that steeper discounting in one measure does not predict the results of the other, suggesting these processes are actually different (Herman, Critchley, & Duka, 2018; Holt, Green, & Myerson, 2003). Nevertheless, risk-taking is closely related to impulsivity and can predict the likelihood of one pursuing hazardous behavior (Donohew et al., 2000). Finally, the last type, stopping impulsivity, refers to the inability to stop a response after it has been initiated. Tasks commonly used to measure stopping behavior are go-no-go and stop-signal reaction time task. Given these tasks require stopping an initiated action, they also measure impulsive actions.
Differentiating between various forms of impulsivity not only has implications for experimental design but also for understanding risk factors for psychiatric disorders. There is evidence that different forms of impulsivity are associated with disorders to varying degrees. For example, some evidence suggests pathological gambling is associated with impulsive action but not impulsive choice (Brevers et al., 2012). In ADHD, both impulsive choice and impulsive action are disrupted but these measures relate to different symptoms: increased impulsive choice is associated with a broad range of ADHD symptoms, including hyperactivity, while impulsive action is related more specifically to executive control (Solanto et al., 2001). Parsing impulsivity is also informative for understanding different aspects of SUD (Broos et al., 2012). In a rodent model of nicotine seeking, impulsive action was associated with an enhanced motivation for nicotine self-administration, while impulsive choice was associated with enhanced vulnerability to cue-induced relapse (Diergaarde et al., 2008). These distinctions are driven by the differences in circuitry, which will be detailed below. Collectively, these data underscore the value of studying distinct aspects of impulsivity.
3. Dopamine Modulates Impulsivity
Dopamine (DA) is linked to impulsivity in humans and rodents. Clinically, disorders with high impulsivity as a key feature are commonly associated with DA dysregulation (Ashok et al., 2017; Pettorruso et al., 2019; Rosa-Neto et al., 2005; Whitton, Treadway, & Pizzagalli, 2015). It is believed that hypodopaminergic function may contribute to drug abuse and underlie behavioral abnormalities observed in ADHD and bipolar disorder (Badgaiyan, Sinha, Sajjad, & Wack, 2015; Berk et al., 2007; Blum et al., 2008; D. A. Cousins, Butts, & Young, 2009; Fattore & Diana, 2016; Gold, Blum, Oscar-Berman, & Braverman, 2014; Sanna, Fattore, Badas, Corona, & Diana, 2021). Administration of psychostimulants, like amphetamine or methamphetamine, are often prescribed for ADHD or bipolar disorder to help reduce impulsivity symptoms (Perugi, Vannucchi, Bedani, & Favaretto, 2017; Wolraich et al., 2019). These types of drugs block DA transporters (DAT), which promotes increased DA levels in the brain (Kuczenski & Segal, 1997). Although these treatments can help alleviate impulsive symptoms in these disorders, nonmedical use of stimulants by people who do not have these disorders promotes impulsive behaviors (Grant, Redden, Lust, & Chamberlain, 2018; Messina et al., 2014).
By using rodent models, we can more specifically parse how changes in DA signaling impact different types of impulsive behavior. Acute administration of amphetamine (0.25–1mg/kg) in rodents often increases premature responses and impairs behavioral inhibition (Baarendse & Vanderschuren, 2012; Britton & Koob, 1989; Caballero-Puntiverio, Fitzpatrick, Woldbye, & Andreasen, 2017; Hayton, Maracle, & Olmstead, 2012; van Gaalen, Brueggeman, Bronius, Schoffelmeer, & Vanderschuren, 2006). However, acute doses of this same drug can also reduce premature responding (Hayton et al., 2012). For instance, rats trained on a response inhibition task, which requires them to withhold pressing a lever until signaled to do so, amphetamine administration increases their impulsive action when the delay to respond is fixed, but reduces it when the delay is variable (Hayton et al., 2012). Therefore, acute doses of amphetamine can alter impulsive action and its effect may depend on the subject’s expectation of task demands. Chronic administration (5mg/kg/day) of other DA agonist drugs, like ropinirole hydrochloride, can also increase premature responding, especially when the cues to the reward are signaled (Tremblay, Barrus, Cocker, Baunez, & Winstanley, 2019). Cues predicting reward elicit activity in dopaminergic neurons, which has been shown to sensitize rats to the hyperlocomotor effects of stimulants (Zack, Featherstone, Mathewson, & Fletcher, 2014).
Interestingly, acute, low doses of stimulant administration can improve the ability to wait for large rewards (S. B. Floresco, M. T. Tse, & S. Ghods-Sharifi, 2008; Wade, de Wit, & Richards, 2000; Catharine A. Winstanley, Dalley, Theobald, & Robbins, 2003). For instance, low doses of amphetamine (0.01–0.25 mg/kg) can shift choice towards large, delayed rewards on delay discounting tasks, therefore reducing impulsive choice (S. B. Floresco et al., 2008; van Gaalen, van Koten, Schoffelmeer, & Vanderschuren, 2006). Similarly, DAT blockade, which also results in an increase in DA levels, reduces impulsive choice (van Gaalen, van Koten, et al., 2006). However, some studies have reported that elevations in DA do not always decrease or affect impulsive choice (Cardinal, Robbins, & Everitt, 2000; Evenden & Ryan, 1996; Helms, Reeves, & Mitchell, 2006; Zeeb, Soko, Ji, & Fletcher, 2016). In one study, high doses of amphetamine (1–3mg/kg) decreased lever pressing and chow consumption in a lever pressing/feeding choice procedure (M. S. Cousins, Wei, & Salamone, 1994). Therefore, lower doses, but not higher levels of psychostimulants, increase choice for larger, delayed rewards.
Even though it is tempting to assume increases in DA levels decrease impulsive choice and increase impulsive action, it is possible the effects of DA on impulsivity are less linear and instead an inverted-U relationship. In other words, depending on where one lands on the curve, shifts in DA levels, can improve or impair impulsivity symptoms (J. W. Dalley & Roiser, 2012). For instance, oral administration of methylphenidate reduces impulsivity only in high, but not low, impulsive rats as determined by the 5-CSRTT (Caprioli et al., 2015). These results suggest that the efficacy of certain stimulant treatments is baseline-dependent and is comparable to the efficacy of psychostimulants in human populations. For instance, treatments that can benefit impulsive individuals (presumably with suboptimal baseline DA signaling) can also impair performance in people who have low levels of impulsivity (de Wit, Crean, & Richards, 2000; Petzold et al., 2019).
Genetic factors can also influence the effect of DA on impulsive behavior (J. W. Dalley & Roiser, 2012; Loos et al., 2010; Simon et al., 2013). For instance, lower dopamine 2 (D2) mRNA receptor expression in the nucleus accumbens (NAc) is correlated with higher impulsive action (Simon et al., 2013). These results are similar to positron emission tomography or autoradiography studies that show impulsive rats have reduced D2 receptor availability in the NAc (Dalley et al., 2007; Jupp et al., 2013). Similar reductions in ventral striatal D2 receptors are also found in highly impulsive methamphetamine-dependent individuals (Kohno et al., 2016; Lee et al., 2009; London, 2020). These findings suggest the expression of DA-related genes influence impulsive traits. Collectively, these studies highlight DA’s involvement in impulsivity.
One caveat with these data is that they were collected only in male rodents. However, many studies have highlighted the fact that there are sex-specific effects in DA circuitry that are influenced by gonadal, chromosomal, and epigenetic factors (reviewed in, (Jill B. Becker & Chartoff, 2019; Eck & Bangasser, 2020; Kokane & Perrotti, 2020; Zachry et al., 2021). For instance, males, but not females, overproduce D1 and D2 receptors in the striatum early in development (25–40 days, the onset of puberty) (Susan L. Andersen, Rutstein, Benzo, Hostetter, & Teicher, 1997; S. L. Andersen & Teicher, 2000). In adulthood, circulating levels of ovarian hormones can alter DA (J. B. Becker, 1990). Baseline firing activity of DA neurons in the VTA of male and female rodents is similar (Locklear, Michaelos, Collins, & Kritzer, 2017; Rincón-Cortés & Grace, 2017), however, electrically stimulated phasic DA release in the VTA is higher in estrus females as compared to males or non-estrus females (Calipari et al., 2017). These data illustrate that there are sex differences in the DA system and highlight the importance of including both sexes in experimental designs.
It is clear that DA has a key role in regulating impulsivity. This review will focus on the role of DA and the mesocorticolimbic system because these endpoints have been more thoroughly investigated in ELS studies relevant to impulsivity than other endpoints. However, the neurochemical basis of impulsivity is complicated and involves other neurotransmitter systems, including serotonin (5-HT) and norepinephrine (NE) (J. W. Dalley, Mar, Economidou, & Robbins, 2008; J. W. Dalley & Roiser, 2012; Groman, 2020; Johansson, Bergvall, & Hansen, 1999; Piña et al., 2020; Swann et al., 2013; Zaniewska, Filip, & Przegalinski, 2015). For example, reducing 5-HT levels increases impulsive action in humans and rodents (Harrison, Everitt, & Robbins, 1997; Worbe, Savulich, Voon, Fernandez-Egea, & Robbins, 2014). NE also impacts impulsive action. Administration of selective NE reuptake inhibitor, atomoxetine, significantly decreases premature responding on a 5-CSRTT (Economidou, Theobald, Robbins, Everitt, & Dalley, 2012). Regulating NE also affects impulsive choice. For example, blocking NE transporter function and activating α2 adrenergic receptors decreases impulsive choice (S. Kim, Bobeica, Gamo, Arnsten, & Lee, 2012; Nishitomi et al., 2018; Robinson et al., 2008). These studies highlight the complexity of neural mechanisms underlying impulsivity. More research, particularly in how ELS-induced alterations in 5HT and NE mediate impulsivity, is needed.
3.1. The NAc and Impulsivity
The NAc is a major component to the ventral striatum that receives DA and facilitates reward-seeking (Berridge & Robinson, 1998; Salamone, 1994; Trifilieff et al., 2013). The NAc is commonly subdivided into two parts: the core and shell (Zahm & Brog, 1992). Inputs from cortical and striatal brain areas display unique topographical organization throughout the NAc (Brog, Salyapongse, Deutch, & Zahm, 1993; H. J. Groenewegen, der Zee, te Kortschot, & Witter, 1987; Kelley & Domesick, 1982; Spooren, Veening, Groenewegen, & Cools, 1991). Dorsal structures like the anterior cingulate cortex (ACC), target the NAc core, while ventral structures like the basolateral amygdala (BLA) and infralimbic cortex target the NAc shell (Brog et al., 1993; H. J. Groenewegen, Wright, Beijer, & Voorn, 1999; Sesack, Deutch, Roth, & Bunney, 1989). Some structures, such as the orbitofrontal cortex (OFC), project to both the core and shell (Brog et al., 1993). Efferent fibers of the NAc as a whole project to the midbrain, hypothalamus, and ventral pallidum as well as other motor systems (Brog et al., 1993; Henk J. Groenewegen & Russchen, 1984). As such, the NAc is a hub that receives and relays information to motor association sites in charge of carrying out appropriate behaviors.
NAc functions are regulated by DA. DA signaling in the NAc can occur via two classes of DA receptors. D1-like receptors (D1 and D5 receptors) stimulate postsynaptic adenylyl cyclase activity and causes increased neuronal firing, while D2-like receptors (D2, D3, and D4 receptors) inhibit this signaling which causes a reduction in firing (Gingrich & Caron, 1993; Hopf, Cascini, Gordon, Diamond, & Bonci, 2003; Sibley & Monsma, 1992; Surmeier, Ding, Day, Wang, & Shen, 2007). Medium spiny neurons (MSNs) in the NAc can also express either D1 or D2 receptors, which can exert opposing control over NAc functions (Bariselli, Fobbs, Creed, & Kravitz, 2019; Gerfen, 1992; Lobo & Nestler, 2011).
Work from van Gaalen has highlighted the involvement of D1 and D2 receptors in regulating impulsive action and impulsive choice. For instance, systemic blockade of D1 receptors increases impulsive choice (van Gaalen, van Koten, et al., 2006). Antagonizing D2 receptors has no effect on behavior on its own, but when followed by systemic amphetamine administration, it can block premature responding and impulsive choice (T. Pattij, Janssen, Vanderschuren, Schoffelmeer, & van Gaalen, 2007; van Gaalen, van Koten, et al., 2006). These data suggest that DA D1 and D2 receptors play important, but perhaps distinct roles, in impulse control. However, recent work finds D1 and D2 neurons express similar activity profiles during periods of behavioral suppression (Lafferty, Yang, Mendoza, & Britt, 2020). Therefore, rather than working in opposition, D1 and D2 expressing neurons may exhibit complementary activity in the NAc to regulate behavioral outcomes.
Impulsive action is modulated by DA signaling in the NAc. For instance, selectively antagonizing D1 receptors in the NAc reduces impulsive action (T. Pattij et al., 2007). In contrast, low NAc D2 receptor expression, which reduces DA inhibitory drive, is associated with increased premature responding in rats (Jupp et al., 2013). Therefore, increasing DA signaling in the NAc may elevate impulsive action. After all, increasing DA signaling by reducing DAT function in the NAc increases impulsive action (Jupp et al., 2013). Interestingly, these effects are specific to the NAc shell and high DA release to the shell is associated with increased premature responding (Diergaarde et al., 2008). Moreover, lesions to the NAc shell block amphetamine-induced premature responding, which may disrupt DA related effects of this stimulant (E. R. Murphy, Robinson, Theobald, Dalley, & Robbins, 2008). These findings have led to the idea that premature responding is due to excess DA levels in the shell region of the NAc (J. W. Dalley & Robbins, 2017).
While increased dopaminergic drive in the NAc shell increases impulsive action, evidence suggests that decreased dopaminergic drive in the NAc core increases impulsive choice. Specifically, overexpression of DAT in the NAc core, which would lower levels of available DA, increases impulsive choice (Adriani et al., 2009). Additionally, D1 antagonism in the NAc core decreases sensitivity to reinforcer magnitudes (Justin R. Yates & Bardo, 2017). In other words, blocking D1 signaling shifts preference away from large rewards even when its delivery is immediate. A potentially contrary finding is that reduced D2/3 receptor binding in the NAc core is associated with impulsive choice (Barlow et al., 2018). A reduction in postsynaptic D2/3 receptors would increase dopaminergic tone in the NAc. However, presynaptic D2 receptors on dopaminergic neurons act as autoreceptors, limiting DA synthesis and release (Beaulieu & Gainetdinov, 2011; Gingrich & Caron, 1993). Viral knockdown of D2 presynaptic receptors in the VTA, the major source of dopamine for the NAc, increases choice impulsivity (Bernosky-Smith et al., 2018). Taken together, these studies support the idea that reducing dopaminergic drive in the NAc core increases impulsive choice.
Electrophysiology studies also support pharmacological data that DA signaling in the NAc regulates impulsivity (Pan, Schmidt, Wickens, & Hyland, 2005; M. R. Roesch, Calu, & Schoenbaum, 2007; Saddoris, Sugam, et al., 2015). Phasic DA release in the NAc tracks certain aspects of value-based decision-making (M. R. Roesch et al., 2007; Saddoris, Sugam, et al., 2015). This is interesting because phasic DA bursts are known to occur in response to reward-predictive cues (Day, Jones, Wightman, & Carelli, 2010; Schultz, Dayan, & Montague, 1997), whereas dips in DA firing are associated with omissions of expected reward (Saddoris, Sugam, et al., 2015). One study that only used female rats found that bursts and dips in phasic DA signaling could function as a teaching signal to facilitate reward-related learning (Steinberg et al., 2013). Indeed, studies that override or inhibit phasic DA signals have shown that this can change behavior in male rodents (Fitzpatrick et al., 2019; Stopper, Tse, Montes, Wiedman, & Floresco, 2014). For instance, suppressing DA bursting that typically occurs when a rat chooses its preferred reward option will biases it to discontinue selecting that option in subsequent trials (Saddoris, Sugam, et al., 2015). Lastly, each subregion of the NAc encodes DA signaling differently: DA release to core is associated with tracking value of predicted outcomes, whereas DA release to shell tracks motivationally salient stimuli (Saddoris, Cacciapaglia, Wightman, & Carelli, 2015). These data indicate that neurons in the NAc core and shell can encode DA signaling differently and further supports the notion that each subregion has dissociable effects in regulating impulsivity.
It is unclear why the NAc core and shell may have dissociable roles in impulsive control. However, dissociations between the core and shell are reported for a number of behaviors such as inhibitory avoidance (Piantadosi, Yeates, & Floresco, 2018), effort-based decision making (Ghods-Sharifi & Floresco, 2010), cue-induced reinstatement of food-seeking behavior (Floresco, McLaughlin, & Haluk, 2008), motivational conflict (Piantadosi, Yeates, Wilkins, & Floresco, 2017), and incentive-cue responding (Ambroggi, Ghazizadeh, Nicola, & Fields, 2011). Data from these studies suggest that the shell inhibits inappropriate actions, while the core promotes approach behaviors towards stimuli that likely yield rewards. Future studies should continue to investigate the differences between these two NAc subregions. These studies could provide insight into the mechanisms that may underlie disorders that are characterized by difficulties in restraining maladaptive behavior, impulsivity, and abnormal activity in the NAc (Engeli et al., 2021; Hoogman et al., 2013; Ma et al., 2016; Stark et al., 2011). A schematic depicting the role of the NAc core and shell in impulsive action and impulsive choice is shown in Figure 1. Notably only one of the above studies used females instead of males and none compared the sexes, so more studies including both sexes are needed to identify any similarities/differences in DA signaling in the NAc.
Fig. 1.
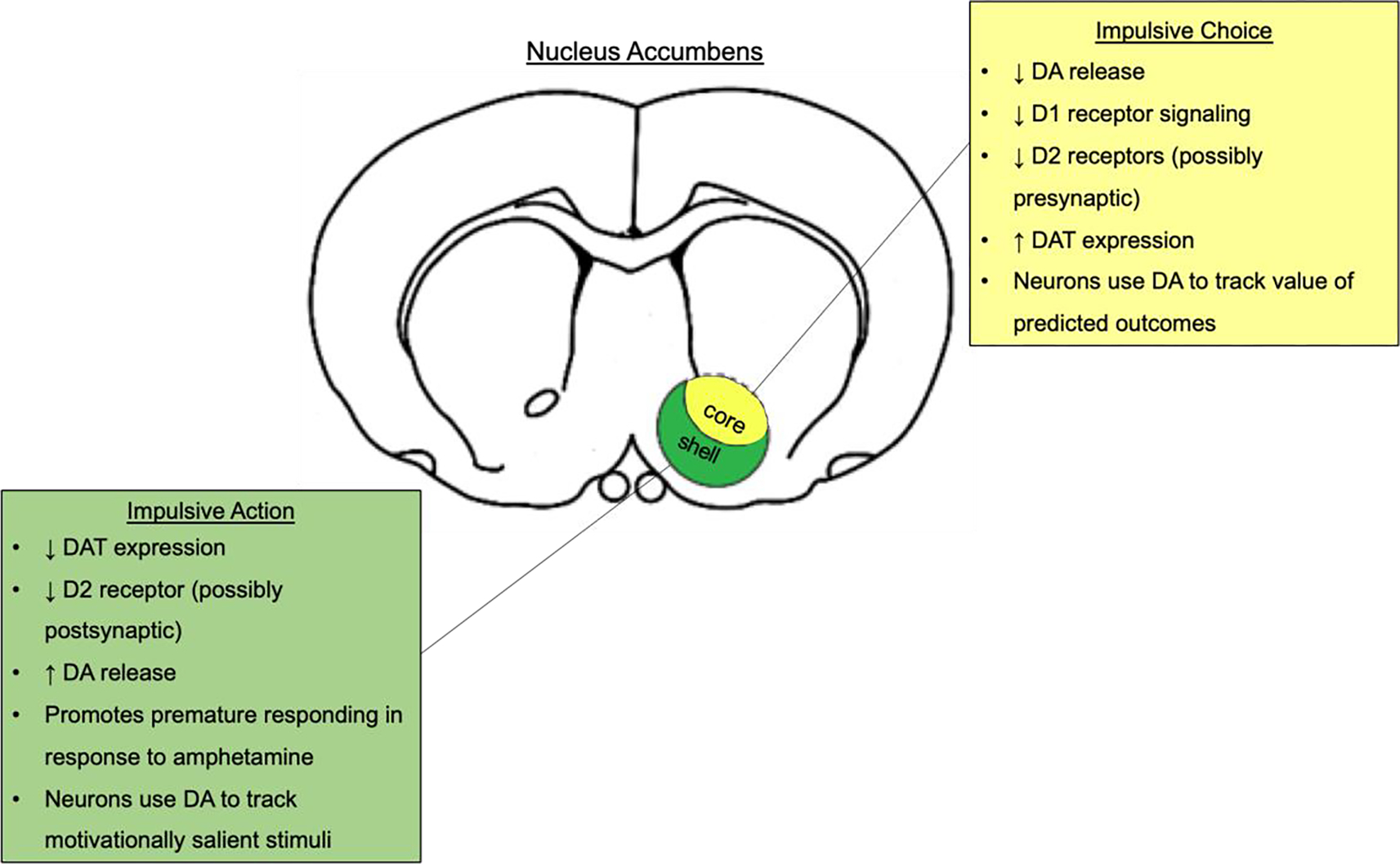
A schematic showing how the NAc core and shell regulate aspects of impulsivity. Changes in the dopaminergic system that increase DA tone in the shell promote impulsive action. In contrast, changes in the dopaminergic system that decrease DA signaling in the core promote impulsive choice. DA, dopamine; D1 receptor, dopamine 1 receptor; D2 receptor, dopamine 2 receptor; DAT, dopamine transporter.
4. Cortical Involvement in Impulsivity
In addition to the NAc, different areas of the prefrontal cortex are associated with mediating different forms of impulsivity. In particular, the OFC and medial prefrontal cortex (mPFC) play important roles in impulsivity (Dalley & Ersche, 2019; Dalley, Everitt, & Robbins, 2011).
The OFC can mediate impulsive choice in rodents and humans (Bechara, Tranel, & Damasio, 2000; Mar, Walker, Theobald, Eagle, & Robbins, 2011; Mobini et al., 2002; Tommy Pattij & Vanderschuren, 2008; Zeeb, Floresco, & Winstanley, 2010). Several studies have reported changes in impulsive behavior in OFC-lesioned rats. Some studies report OFC lesions make male and female rats more impulsive and less likely to wait for delayed rewards (Mobini et al., 2002; Rudebeck, Walton, Smyth, Bannerman, & Rushworth, 2006). These results suggest that the OFC is necessary for responding when rewards are delayed. Others report that OFC lesions make rats less impulsive, which may indicate it is necessary for devaluing the delayed reward (C. A. Winstanley, Theobald, Cardinal, & Robbins, 2004). Possible reasons for the discrepancies are attributed to the heterogeneity of the OFC region, as well as when the lesion took place (e.g., pre-training vs. post-training). For example, when OFC lesions occur prior to the training on a gambling task, rats take longer to reach task acquisition (Zeeb & Winstanley, 2011). OFC lesions that occur after training, however, have no effect on risky choices on this task. These findings point to the OFC being involved in learning and that lesions that occur early in training could cause impairments in tracking outcomes to guide future responding. Single unit electrophysiological recordings also illustrate a role for the OFC in delay discounting behavior. Activity of different neurons in the OFC reflected a rat’s preference for immediate, small rewards or large, delayed rewards (Roesch, Taylor, & Schoenbaum, 2006). These results suggest that different groups of neurons in the OFC track delayed rewards and outcome expectancies. Additionally, attenuating neural activity in the OFC, while simultaneously enhancing NAc neural activity, impairs impulse control (Meyer & Bucci, 2016). Collectively, these data provide evidence that the OFC modulates impulsive choice behavior.
The OFC mediates impulsive action behaviors associated with stopping impulsivity—the inability to stop an already initiated response. Lesions to the OFC reduce the ability of male rats to stop an inappropriate response on stop-signal tasks (Eagle et al., 2008). Additionally, decreased OFC DAT function is associated with high impulsive action on go/no-go tasks, suggesting hyperdopaminergic tone in the OFC mediates increased impulsive action (J. R. Yates, Darna, Beckmann, Dwoskin, & Bardo, 2016). Neural activity in the OFC is also associated with two distinct stopping mechanisms: proactive and reactive stopping (Balasubramani, Pesce, & Hayden, 2020; Hardung et al., 2017). Reactive stopping refers to situations in which subjects are required to stop in response to an external cue, whereas proactive stopping develops according to the subjects internal goals (Aron, 2011). Despite its involvement in stopping impulsivity, the OFC does not appear to mediate impulsive action behaviors associated with waiting impulsivity. For instance, lesions to the whole OFC do not promote impulsive action behaviors on the 5-CSRTT in male rats (females were not tested) (Chudasama et al., 2003), however more data is needed.
Another cortical region involved in impulse control is the mPFC. The mPFC is associated with impulsive action as it plays a role in restraining premature responses (Cho & Jeantet, 2010; B. Li, Nguyen, Ma, & Dan, 2020; Tommy Pattij & Vanderschuren, 2008). For example, optogenetic inhibition of the mPFC in male rats affects proactive motor control (Hardung et al., 2017). Additionally, projections from the dorsomedial PFC to the subthalamic nucleus (STN) can inhibit premature responding (B. Li et al., 2020). The STN is a structure implicated in motor control and STN lesions can increase impulsive action (Baunez, Nieoullon, & Amalric, 1995; Guillaumin, Serra, Georges, & Wallén-Mackenzie, 2021; Uslaner & Robinson, 2006). These results suggest that the mPFC can signal to downstream structures like the STN to suppress inappropriate motor driven responses. Functional disconnection of the mPFC-NAc pathway also increases impulsive actions, an effect attributed to disrupting this “top-down” control over behavior (Christakou, Robbins, & Everitt, 2004). One caveat with mPFC lesion studies is that sometimes they may include the ACC (Cho & Jeantet, 2010; Pezze, Dalley, & Robbins, 2009). The ACC itself is involved in many different processes related to impulsivity such as error detection, cognitive control, and response selection (Bryden, Johnson, Tobia, Kashtelyan, & Roesch, 2011; Bussey, Everitt, & Robbins, 1997; Newman, Creer, & McGaughy, 2015). It is thought that the ACC is involved in detecting situations where behavioral response is ineffective and processes this information to guide behavior. Lesions to the ACC increases premature responses and reduces accuracy on the 5-CSRTT (Chudasama et al., 2003; Muir, Everitt, & Robbins, 1996). Additionally, activation of layer-5 pyramidal cells of the ACC impairs behavioral disinhibition (van der Veen et al., 2021). Thus, both the mPFC and ACC modulate impulsive action.
Neurons in the mPFC are also engaged during impulsive choice. Using electrophysiological recordings during a delay discounting task in male and female rats, Sackett and colleagues found that neurons in the prelimbic region of the mPFC respond to either large/delay options, small/immediate option, or both options (Sackett, Moschak, & Carelli, 2019). As the delays increased, so did the percentage of neurons that responded to small/immediate options. A rat’s baseline levels of impulsivity also influenced neuronal recruitment: highly impulsive rats demonstrated a greater percentage of small/immediate-responsive neurons as the task progressed than low impulsive rats. These results suggest that some neurons in the prelimbic cortex encode reward value.
Like the NAc, these cortical regions receive dopaminergic input from the VTA (Oades & Halliday, 1987). DA in the cortex can also modulate impulsivity (Puumala & Sirviö, 1998). Dopaminergic depletion in the OFC of rats decreases impulsive choice (Kheramin et al., 2004). This finding may indicate high levels of DA in the OFC may underlie discounting behavior. In vivo microdialysis data support this idea by finding increased levels of 3,4-dihydroxyphenylacetic acid (DOPAC) when rats make delay discounting judgements (C. A. Winstanley, Theobald, Dalley, Cardinal, & Robbins, 2006). Increases in DOPAC could reflect increased DA utilization occurring in the OFC during delay discounting. Additionally, intra-OFC administration of a D1 receptor antagonist, SCH23390, decreases impulsive action in highly impulsive male rats (C. A. Winstanley et al., 2010). This result is similar to work showing systemic administration of D1 antagonists reduces impulsive behavior (van Gaalen, Brueggeman, et al., 2006; van Gaalen, van Koten, et al., 2006). In regards to the mPFC, antagonism of D1 and D2 receptors reverses the effects of amphetamine-induced premature actions and improves timing of responses (Cheng & Liao, 2017). However, low D2 mRNA receptor expression in the prelimbic region of the mPFC is correlated with high impulsivity (Simon et al., 2013). Additionally, male rats that are pretreated with either D1 or D2 receptor antagonists prior to delay discounting have higher levels of impulsive choice (Pardey, Kumar, Goodchild, & Cornish, 2012). Altogether, these data indicate that DA in the OFC and mPFC can also affect impulsivity.
5. ELS and Impulsivity
5.1. Models of Early Life Stress
ELS in rodents can be studied using a variety of different models which include prenatal stress, as well as postnatal stress. These models have helped researchers further understand stress effects on impulsive behavior. Models of prenatal stress aim to stress a pregnant dam during different time-points of gestation (e.g., early, middle, or late) by using various stressors (e.g., restraint, noises, and lights), and different lengths of time (e.g., 3 times a day vs. 3 weeks) (Mueller & Bale, 2008; Soares-Cunha et al., 2018; Van den Hove et al., 2006; Weston, Weston, Allen, & Cory-Slechta, 2014; Wilson, Schade, & Terry, 2012). Offspring of stressed pregnant dams typically exhibit elevated corticosterone release in response to an acute stress exposure (30 min of restraint), which indicates heightened hypothalamic-pituitary-adrenal (HPA) axis reactivity (Cory-Slechta, Virgolini, Thiruchelvam, Weston, & Bauter, 2004; Soares-Cunha et al., 2018).
Postnatal models of stress in rodents typically involve some form of scarcity, where resources are removed. Maternal separation models are used as a postnatal stressor, and they disrupt dam-pup interactions by separating the pups from their mother for intermittent periods (e.g., 15 minutes to 24 hours per day) for one to three weeks postnatally (Millstein & Holmes, 2007; Molet, Maras, Avishai-Eliner, & Baram, 2014). Shorter variations of this model are increase levels of maternal care and stress resiliency in offspring; however, longer variations induce impaired maternal care (Eck & Bangasser, 2020; Nishi, Horii-Hayashi, & Sasagawa, 2014). To control for variations in maternal care, some manipulations employ artificial rearing procedures, where pups are separated from dams and experimenters mimic various amounts of pup care (e.g., low or high) with “maternal licking-like stimulations” via a wet paintbrush (Burton et al., 2007; Lovic, Keen, Fletcher, & Fleming, 2011). Another postnatal stressor is the limited bedding and nesting (LBN) manipulation, which is typically implemented during a pup’s first week of life (postnatal day 2–9). This low resource environment aims to mimic aspects of poverty (Eck et al., 2019; Gilles, Schultz, & Baram, 1996; Molet et al., 2014). This manipulation induces stress in dams, altering their maternal care towards their developing pups (Ivy, Brunson, Sandman, & Baram, 2008; Rice, Sandman, Lenjavi, & Baram, 2008). One of the advantages of the LBN paradigm compared to other models is that it can induce changes in maternal care with limited external experimenter interventions. Rather than physically removing the dam from her pups to induce changes in maternal care, the LBN manipulation causes changes in care by altering the environment the animals are placed in. Additionally, data from human studies of chronic childhood stress, including war, poverty, and neglect/abuse suggest that the mother is typically present, even though her behavior may be abnormal (Halevi et al., 2016; Mulder, Kuiper, van der Put, Stams, & Assink, 2018; Wang, Choi, & Shin, 2020). Thus, this model is thought to have strong translational potential (Walker et al., 2017).
Even though stress from ELS models can dissipate quickly, exposure to these stressful manipulations early in life can cause long-lasting changes to reward-related networks (Dubé et al., 2015; Eck et al., 2019; Huang, 2014). Utilization of these models has also allowed researchers to better understand the neurobiological mechanisms underlying how stress persistently alters behavior. The type/severity of stress experienced, as well as the timing and duration of the stressor can influence its specific outcomes on the brain and behavior. Although different models of ELS may produce slightly different results, they have been useful for examining mechanisms that induce or ameliorate impulsive behavior.
5.2. Dissociable Effects of Early Life Stress on Impulsivity
Relatively few studies have examined how prenatal stressors affect impulsive action. One study did find that male and female rats exposed to prenatal stress made more premature responses on a challenging version of the 5-CSRTT (Wilson et al., 2012). Specifically, when the intertrial interval times increased, these rats were unable to withhold responding as compared to controls. In regard to postnatal stressors, work from Lovic and colleagues found that male and female rats that undergo a severe form of early life deprivation—artificial rearing with low simulated grooming—are unable to withhold premature responses as adults compared to control rats while performing DRL tasks (Lovic et al., 2011). Similarly, male rats which experienced maternal deprivation, make significantly more premature responses while performing the 5-CSRTT as compared to controls (Kentrop et al., 2016). Females however were not examined in this study. Together, these studies indicate that ELS can increase impulsive action. Table 1 summarizes the effects of ELS on impulsivity.
Table 1.
Summary of ELS effects on impulsive action and impulsive choice.
| Impulsivity Type | Timing | Stress Model | Effect | Citations |
|---|---|---|---|---|
| Impulsive Action | Prenatal | Variable | Increase | Wilson et al., 2012 |
| Impulsive Action | Postnatal | Scarcity | Increase | Kentrop et al., 2016; Lovic, Kleen, Fletcher, & Fleming, 2011 |
| Impulsive Choice | Prenatal | Prenatal Restraint Stress + Lead Exposure | Decrease in males, no effect females | Weston et al., 2014 |
| Impulsive Choice | Postnatal | Scarcity | Decrease* | Ordñnes Sanchez et al., 2021; Fuentes et al., 2014; Lovic et al., 2011 |
| Impulsive Choice | Postnatal | Maternal Separation + Ethanol Exposure | Increase | Gondré-Lewis et al., 2016 |
indicates that the effect is more pronounced in one sex than the other depending on the type of scarcity manipulation (artificial rearing, maternal separation, LBN).
In contrast to the enhancing effect of ELS on impulsive action, ELS tends to decrease impulsive choice. Prenatal stress and exposure to low levels of lead reduces impulsive choice in males but has no effects in females (Weston et al., 2014). Similarly, maternal separation plus early social isolation reduces impulsive choice on delay discounting tasks in male but not female rats (Lovic et al., 2011). Reductions in impulsivity in delay discounting also have been found in male, but not female rats that were reared in LBN (Ordoñes Sanchez et al., 2021). In contrast, female, but not male rats, exposed to a surrogate mother while simultaneously being raised in a low resource environment early in life had reduced impulsivity in delayed discounting (Fuentes et al., 2014). These discrepancies between these studies in the sex impacted suggest that the social experience of having a surrogate mother may affect the sexes differently. One study found that ELS increases impulsive choice (Gondré-Lewis et al., 2016) but this finding is complicated by the fact that offspring were trained on operant binge drinking paradigms prior to learning delay discounting. Taken together the literature suggest that ELS typically decreases impulsive choice (Table 1). However, much more work, particularly with prenatal models, is needed.
It is important to mention that some studies report that ELS can induce compulsive-like, rather than impulsive-like, behaviors (Boutros, Der-Avakian, Markou, & Semenova, 2017; Fuentes et al., 2014). Compulsive behaviors are repetitive, often purposeless, and lead to unfavorable outcomes. For instance, male offspring that experienced maternal separation showed increased perseverative responding (i.e., continued nose-poking after a correct response) on a 5-CSRTT task (Boutros et al., 2017). However, this experiment also included rats that were exposed to ethanol during adolescence (PND28–57), which could affect the interpretation of these results. Future studies should continue examining whether other prenatal and postnatal stressors also induce similar findings. Overall, the rodent literature demonstrates that ELS can lead to increases in impulsive action but decreases in impulsive choice.
6. Effects of ELS on the Mesocorticolimbic System Can Mediate Impulsivity
As mentioned previously, there is a complex relationship between DA levels and impulsivity. Stimulants, which increase DA, increase impulsive action but decrease impulsive choice (Baarendse & Vanderschuren, 2012; Britton & Koob, 1989; Caballero-Puntiverio et al., 2017; S. B. Floresco, M. T. L. Tse, & S. Ghods-Sharifi, 2008; Hayton et al., 2012; van Gaalen, Brueggeman, et al., 2006; Wade et al., 2000). ELS can affect the mesolimbic DA system to increase dopaminergic drive (Baier, Katunar, Adrover, Pallarés, & Antonelli, 2012; Eck & Bangasser, 2020). For example, maternal separation increases the excitability of VTA DA neurons in female rats (males were not tested) (Spyrka et al., 2020). Maternal separation can also affect tyrosine hydroxylase (TH), the rate limiting enzyme of catecholamine synthesis. Specifically, maternal separation increases TH-immunoreactive cells in the VTA in adolescent male and adult female rats (Chocyk et al., 2011; Kapor et al., 2020). In the PFC and NAc, TH-immunoreactive fibers are denser following maternal separation in adolescent females (males were not tested) (Majcher-Maślanka, Solarz, Wędzony, & Chocyk, 2017). In studies that only tested male rats, prenatal stress increases DA transcription factors in the VTA and dopamine release in the NAc shell (the core was not analyzed) (Katunar, Saez, Brusco, & Antonelli, 2009; Silvagni et al., 2008). Although most of these studies do not directly compare males to females, the overall pattern is that ELS increases dopaminergic drive from VTA across sex.
In addition to increasing DA levels in the NAc, ELS can also alter DA receptor expression. Female, but not male, mice who experienced maternal separation plus social isolation show reductions in D1 receptor expression in the NAc (Sasagawa et al., 2017). A decrease in this receptor’s expression in the NAc could reduce dopaminergic signaling in females and may help compensate the stress-induced increases in DA drive from the VTA. Changes in D1 expression are attributed to hypermethylation of the Drd1a promoter region, revealing an epigenetic modification that can explain this effect in females (Sasagawa et al., 2017). In contrast, exposure to a limited resource environment had no effect on D1 expression in the NAc shell in male and female rats (Fuentes et al., 2018). This discrepancy may be because only one subregion and not entire NAc was examined in this study. Alternatively, different types of early life adversity could have distinct effects on D1 receptors. ELS can also affect D2 receptor expression in the NAc but typically causes an increase in this receptor. In males (females were not tested), prenatal restraint stress as well as maternal separation alone or in combination with cocaine exposure increases D2 receptor expression in the NAc (Berger, Barros, Sarchi, Tarazi, & Antonelli, 2002; Gracia-Rubio et al., 2016; Romano-López et al., 2016), but see (Majcher-Maślanka et al., 2017). A stress-induced increase in D2 receptors, assuming postsynaptic, would inhibit the NAc, which may help control increased DA influx in the NAc. Higher expression of NAc D2 receptors following ELS may drive changes in impulsivity, because, as noted, reduced D2 receptor expression is positively correlated with poor inhibitory control (Cropley, Fujita, Innis, & Nathan, 2006; Hamidovic, Dlugos, Skol, Palmer, & de Wit, 2009).
Another regulator of dopaminergic function is DAT. In humans, low levels of DAT, which could increase DA signaling, are associate with poor inhibitory control (H. Kim, 2018; Sekiguchi, Pavey, & Dean, 2019; Smith et al., 2018). ELS manipulations can be combined with animal models thought to capture aspects of psychiatric disease, such as the spontaneously hypertensive rat (SHR), which has features of ADHD, including inattention, impulsivity, and hyperactivity (Knardahl & Sagvolden, 1979; Russell, Sagvolden, & Johansen, 2005; Sagvolden, 2000; Sagvolden, Russell, Aase, Johansen, & Farshbaf, 2005; Wultz, Sagvolden, Moser, & Moser, 1990). Maternal separation in SHR increases the rate of DA clearance in the ventral striatum (Womersley, Hsieh, Kellaway, Gerhardt, & Russell, 2011). An increased rate of clearance is indicative of reductions in DAT function because less DA is being removed from the synapse. Consistent with this finding, male rats (females were not studied) that experience maternal separation show reduced DAT expression in the accumbens and display increased sensitization to amphetamine (Brake, Zhang, Diorio, Meaney, & Gratton, 2004; Meaney, Brake, & Gratton, 2002). Interestingly, female, but not male, rats exposed to maternal stress are less susceptible to methamphetamine induced decreases in striatal DAT content (Hensleigh & Pritchard, 2015). Thus, another mechanism by which ELS could impact impulsivity is via altering DAT function.
ELS also affects plasticity within the NAc (Monroy, Hernández-Torres, & Flores, 2010; Romano-López et al., 2016). Female rats that experienced prenatal stress in combination with motherless rearing have decreased expression of neuronal markers of plasticity such as synaptophysin and brain-derived neurotrophic factor in the accumbens (Burton et al., 2007). Prenatal stress with maternal separation increases dendritic MSN branching in both male and female rats (Muhammad, Carroll, & Kolb, 2012). Increased branching can provide more surface area for synaptic connections. These data illustrate that synaptic development is sensitive to different forms of ELS. What is unclear, however, is if ELS promotes inhibitory or excitatory synaptic connections. Electrophysiology data suggests male, but not female rats, exposed to LBN exhibit reductions in spontaneous excitatory postsynaptic currents (sEPSCs) in the NAc core (the shell was not assessed) (Ordoñes Sanchez et al., 2021). These findings could suggest that early life experiences can potentially reduce excitatory synapses and therefore, reduce glutamate transmission in the NAc. Reductions in glutamate transmission in the NAc is associated with reduced impulsive choice in males (Ordoñes Sanchez et al., 2021; Justin R. Yates & Bardo, 2017). These findings indicate that ELS can alter other aspects of accumbal plasticity in addition to regulating DA which could impact impulsivity.
It is important to mention that there is a disconnect between the detail in which the NAc has been studied in the ELS field vs. the impulsivity field. The impulsivity literature has revealed distinct functions of the NAc core and NAc shell, with a stronger role for NAc core in impulsive choice and the NAc shell in impulsive action (Dalley & Ersche, 2019; J. W. Dalley & Robbins, 2017; Ito, Robbins, & Everitt, 2004). Many ELS studies, however, often focus on the entire NAc or only examine one subregion. Future ELS studies need to better incorporate core/shell assessments to further our understanding of the mechanisms by which ELS alters impulsive behavior.
7. Effects of ELS on the Cortex Can Mediate Impulsivity
The cortex also plays a role in impulsive behavior, and ELS can affect the cortex. Recall that cortical DA signaling contributes to the expression of behavioral impulsivity (Loos et al., 2010; Puumala & Sirviö, 1998; C. A. Winstanley et al., 2006; C. A. Winstanley et al., 2010). ELS alters DA innervation of the cortex (Kunzler, Braun, & Bock, 2015). For example, maternal separation causes tyrosine hydroxylase (TH)-fiber density in the OFC to be higher in male, but not female, Octodon degus (Kunzler et al., 2015). Given that TH is a marker for DA projections, these data suggest ELS increases dopaminergic innervation in the OFC. Interestingly, in the same study density of TH fibers in the mPFC was lower in stressed than control males with no effects found in females (Kunzler et al., 2015). These results indicate that ELS alters dopaminergic innervation in the cortex in a region-specific manner. ELS can also alter cortical DA receptors. Maternal separation in male rats (females were not tested) increased adolescent D1 expression but reduced adult D2 receptor expression on glutamatergic projection neurons from the prelimbic PFC (Brenhouse, Lukkes, & Andersen, 2013). However, in the prelimbic PFC projections specifically to the NAc, maternal separation caused a transient decrease in both D1 and D2 receptors in adolescence (Brenhouse et al., 2013). Studies on the effects of ELS on DA in the PFC are limited, and much more research in this area is warranted.
In addition to changes in cortical dopamine, cortical glutamate dysfunction is associated with impulsivity in ADHD, SUD, bipolar disorder, and preclinical models (Jochen Bauer et al., 2018; J.-N. Li, Liu, & Li, 2020; Emily R. Murphy et al., 2012; Smaragdi, Chavez, Lobaugh, Meyer, & Kolla, 2019). As an example, people with ADHD have higher glutamate in the ACC than controls and these high glutamate levels are positivity correlated with impulsivity symptoms (J. Bauer et al., 2018). ELS can affect cortical glutamate. Primates reared in adverse conditions have higher ACC glutamatergic signaling than controls (Mathew et al., 2003). Glutamatergic tone in cortical regions is modulated by local parvalbumin (PV) GABAergic interneurons (Sohal, Zhang, Yizhar, & Deisseroth, 2009; Williams, Goldman-Rakic, & Leranth, 1992). Several lines of rodent research demonstrate that ELS reduces PV neurons in the mPFC and OFC (Goodwill et al., 2018; Grassi-Oliveira, Honeycutt, Holland, Ganguly, & Brenhouse, 2016; Ohta et al., 2020). Interestingly, the effect of ELS on cortical PV neurons differs by sex and developmental stage. Maternal separation reduces PV neurons of the mPFC transiently in juvenile female rats but causes a persistent decline of these neurons in male rats which starts in adolescents and continues into young adulthood (Grassi-Oliveira et al., 2016; Holland, Ganguly, Potter, Chartoff, & Brenhouse, 2014). In the OFC, LBN reduces PV neurons in female but not male mice (Goodwill et al., 2018). These ELS-induced changes in cortical PV have been linked to cognitive and social deficits, but they have not directly been linked to impulsivity, a gap that should be addressed in future studies. Collectively, these findings suggest that another mechanism by which ELS can alter impulsivity is by increasing cortical glutamate signaling.
8. Conclusion
How ELS affects impulsivity is just beginning to be elucidated and there remain many unanswered questions. However, the rodent literature demonstrates that ELS increases impulsive action and decreases impulsive choice. Yet, these findings are not totally aligned with human studies: while both rodent and human studies report that ELS increases impulsive action, in humans, adversity in childhood also typically increases impulsive choice (Duckworth, Kim, & Tsukayama, 2013; S. T. Kim et al., 2018; Lovallo et al., 2013). One possible explanation for why ELS effects on impulsive choice differ in the rodent versus human literature could be differences in the stressor timing and duration, and the types of stressors experienced. Most rodent models of ELS utilize one specific stress manipulation during a restricted developmental timeframe, in part, to identify sensitive windows (Molet et al., 2014; Nishi et al., 2014; Walker et al., 2017). However, stressful events experienced by children are not typically restricted to a limited developmental window (Lupien, McEwen, Gunnar, & Heim, 2009). Moreover, most children who experience ELS report multiple forms of adversity prior to adulthood (Arata, Langhinrichsen-Rohling, Bowers, & O’Brien, 2007; Green et al., 2010; Kessler et al., 2010; Katie A. McLaughlin et al., 2010; K. A. McLaughlin et al., 2012). Additionally, while most rodent models of ELS focus on some form of neglect (e.g., neglect of maternal care or physical resources), clinical studies also take into consideration stress stemming from other ELS subtypes (e.g., sexual, physical, and emotional abuse) (Katie A. McLaughlin et al., 2010; K. A. McLaughlin et al., 2012). Different types of early trauma can lead to different outcomes later in life. For instance, a meta-analysis found that childhood abuse was positively associated with the development of adult psychopathology, but not neglect stemming from caregivers unable to provide basic needs such as housing/shelter (Carr, Martins, Stingel, Lemgruber, & Juruena, 2013). Because most rodent models of ELS involve stress from neglect, they may not always capture the effects of childhood trauma in humans. One approach would be to try to develop rodent models that better capture multiple stressors for a more protracted period of development. Another approach is to study impulsivity in humans who have experienced briefer and milder forms of early adversity. Although there is some disconnect regarding impulsive choice between the rodent and human ELS studies, it does not mean the rodent research cannot be leveraged into better treatments for those suffering from conditions with impulsive choice as a feature. Using rodent models to discover mechanisms that reduce impulsivity may reveal novel treatments for disorders characterized by high impulsivity.
In conclusion, adverse experiences early in life can alter many cognitive processes, including impulsivity. Although the precise mechanisms by which this occurs are still not fully elucidated, regulation of the mesocortolimbic system by ELS clearly contributes to later alterations in impulsive behavior. Future studies which disentangle stressor type, duration, and developmental window as well as consistently compare males and females are needed to better understand conditions that promote vulnerability vs. resilience to ELS. Leveraging these data can help improve therapeutics to treat several conditions including ADHD, bipolar disorder, and SUD.
Highlights:
Impulsivity is mediated by the mesocorticolimbic dopamine (DA) system
Exposure to early life stress can alter impulsivity in adulthood
Early life stress induces changes in the mesocorticolimbic DA system to impact impulsivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler LA, Faraone SV, Spencer TJ, Berglund P, Alperin S, & Kessler RC (2017). The structure of adult ADHD. Int J Methods Psychiatr Res, 26(1). doi: 10.1002/mpr.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Boyer F, Gioiosa L, Macrì S, Dreyer JL, & Laviola G (2009). Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience, 159(1), 47–58. doi: 10.1016/j.neuroscience.2008.11.042 [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ghazizadeh A, Nicola SM, & Fields HL (2011). Roles of Nucleus Accumbens Core and Shell in Incentive-Cue Responding and Behavioral Inhibition. The Journal of Neuroscience, 31(18), 6820. doi: 10.1523/JNEUROSCI.6491-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, & Teicher MH (1997). Sex differences in dopamine receptor overproduction and elimination. NeuroReport, 8(6). [DOI] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2000). Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience & Biobehavioral Reviews, 24(1), 137–141. doi: 10.1016/S0149-7634(99)00044-5 [DOI] [PubMed] [Google Scholar]
- Arata CM, Langhinrichsen-Rohling J, Bowers D, & O’Brien N (2007). Differential correlates of multi-type maltreatment among urban youth. Child Abuse & Neglect, 31(4), 393–415. doi: 10.1016/j.chiabu.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Aron AR (2011). From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biological Psychiatry, 69(12), e55–e68. doi: 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, & Howes OD (2017). The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Molecular Psychiatry, 22(5), 666–679. doi: 10.1038/mp.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, & Vanderschuren LJMJ (2012). Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology, 219(2), 313–326. doi: 10.1007/s00213-011-2576-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Sinha S, Sajjad M, & Wack DS (2015). Attenuated Tonic and Enhanced Phasic Release of Dopamine in Attention Deficit Hyperactivity Disorder. PLoS One, 10(9), e0137326. doi: 10.1371/journal.pone.0137326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier CJ, Katunar MR, Adrover E, Pallarés ME, & Antonelli MC (2012). Gestational Restraint Stress and the Developing Dopaminergic System: An Overview. Neurotoxicity Research, 22(1), 16–32. doi: 10.1007/s12640-011-9305-4 [DOI] [PubMed] [Google Scholar]
- Balasubramani PP, Pesce MC, & Hayden BY (2020). Activity in orbitofrontal neuronal ensembles reflects inhibitory control. European Journal of Neuroscience, 51(10), 2033–2051. doi: 10.1111/ejn.14638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol, 108, 44–79. doi: 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Bariselli S, Fobbs WC, Creed MC, & Kravitz AV (2019). A competitive model for striatal action selection. Brain research, 1713, 70–79. doi: 10.1016/j.brainres.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RL, Gorges M, Wearn A, Niessen HG, Kassubek J, Dalley JW, & Pekcec A (2018). Ventral Striatal D2/3 Receptor Availability Is Associated with Impulsive Choice Behavior As Well As Limbic Corticostriatal Connectivity. Int J Neuropsychopharmacol, 21(7), 705–715. doi: 10.1093/ijnp/pyy030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Werner A, Kohl W, Kugel H, Shushakova A, Pedersen A, & Ohrmann P (2018). Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. The World Journal of Biological Psychiatry, 19(7), 538–546. doi: 10.1080/15622975.2016.1262060 [DOI] [PubMed] [Google Scholar]
- Bauer J, Werner A, Kohl W, Kugel H, Shushakova A, Pedersen A, & Ohrmann P (2018). Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. World J Biol Psychiatry, 19(7), 538–546. doi: 10.1080/15622975.2016.1262060 [DOI] [PubMed] [Google Scholar]
- Baunez C, Nieoullon A, & Amalric M (1995). In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. The Journal of Neuroscience, 15(10), 6531. doi: 10.1523/JNEUROSCI.15-10-06531.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J-M, & Gainetdinov RR (2011). The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacological Reviews, 63(1), 182. doi: 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, & Damasio H (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain, 123(11), 2189–2202. doi: 10.1093/brain/123.11.2189 [DOI] [PubMed] [Google Scholar]
- Becker JB (1990). Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse, 5(2), 157–164. doi: 10.1002/syn.890050211 [DOI] [PubMed] [Google Scholar]
- Becker JB, & Chartoff E (2019). Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology, 44(1), 166–183. doi: 10.1038/s41386-018-0125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MA, Barros VG, Sarchi MI, Tarazi FI, & Antonelli MC (2002). Long-Term Effects of Prenatal Stress on Dopamine and Glutamate Receptors in Adult Rat Brain. Neurochemical Research, 27(11), 1525–1533. doi: 10.1023/A:1021656607278 [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Sant’anna M, Malhi GS, Bourin M, Kapczinski F, & Norman T (2007). Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl(434), 41–49. doi: 10.1111/j.1600-0447.2007.01058.x [DOI] [PubMed] [Google Scholar]
- Bernosky-Smith KA, Qiu YY, Feja M, Lee YB, Loughlin B, Li JX, & Bass CE (2018). Ventral tegmental area D2 receptor knockdown enhances choice impulsivity in a delay-discounting task in rats. Behav Brain Res, 341, 129–134. doi: 10.1016/j.bbr.2017.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev, 28(3), 309–369. doi: 10.1016/s0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, … Davis SG (1995). Impact of adversity on functioning and comorbidity in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 34(11), 1495–1503. doi: 10.1097/00004583-199511000-00017 [DOI] [PubMed] [Google Scholar]
- Blum K, Chen AL-C, Braverman ER, Comings DE, Chen TJH, Arcuri V, … Oscar-Berman M (2008). Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatric disease and treatment, 4(5), 893–918. doi: 10.2147/ndt.s2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Der-Avakian A, Markou A, & Semenova S (2017). Effects of early life stress and adolescent ethanol exposure on adult cognitive performance in the 5-choice serial reaction time task in Wistar male rats. Psychopharmacology (Berl), 234(9–10), 1549–1556. doi: 10.1007/s00213-017-4555-3 [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, & Gratton A (2004). Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience, 19(7), 1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Lukkes JL, & Andersen SL (2013). Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. (2076–3425 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Verbruggen F, Bechara A, Kornreich C, Verbanck P, & Noël X (2012). Impulsive Action but Not Impulsive Choice Determines Problem Gambling Severity. PLoS One, 7(11), e50647. doi: 10.1371/journal.pone.0050647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KT, & Koob GF (1989). Effects of corticotropin releasing factor, desipramine and haloperidol on a DRL schedule of reinforcement. Pharmacol Biochem Behav, 32(4), 967–970. doi: 10.1016/0091-3057(89)90067-1 [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, & Zahm DS (1993). The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol, 338(2), 255–278. doi: 10.1002/cne.903380209 [DOI] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, … Goudriaan AE (2012). The Relationship between Impulsive Choice and Impulsive Action: A Cross-Species Translational Study. PLoS One, 7(5), e36781. doi: 10.1371/journal.pone.0036781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, & Roesch MR (2011). Attention for Learning Signals in Anterior Cingulate Cortex. The Journal of Neuroscience, 31(50), 18266. doi: 10.1523/JNEUROSCI.4715-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, & Fleming AS (2007). Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague-Dawley rats. Brain Res, 1158, 28–38. doi: 10.1016/j.brainres.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, & Robbins TW (1997). Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. (0735–7044 (Print)). [DOI] [PubMed] [Google Scholar]
- Caballero-Puntiverio M, Fitzpatrick CM, Woldbye DP, & Andreasen JT (2017). Effects of amphetamine and methylphenidate on attentional performance and impulsivity in the mouse 5-Choice Serial Reaction Time Task. J Psychopharmacol, 31(2), 272–283. doi: 10.1177/0269881116684339 [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, … Nestler EJ (2017). Dopaminergic dynamics underlying sex-specific cocaine reward. Nature Communications, 8(1), 13877. doi: 10.1038/ncomms13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Jupp B, Hong YT, Sawiak SJ, Ferrari V, Wharton L, … Dalley JW (2015). Dissociable rate-dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum. J Neurosci, 35(9), 3747–3755. doi: 10.1523/jneurosci.3890-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, & Everitt BJ (2000). The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl), 152(4), 362–375. doi: 10.1007/s002130000536 [DOI] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, & Juruena MF (2013). The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis, 201(12), 1007–1020. doi: 10.1097/NMD.0000000000000049 [DOI] [PubMed] [Google Scholar]
- Cheng R-K, & Liao R-M (2017). Regional differences in dopamine receptor blockade affect timing impulsivity that is altered by d-amphetamine on differential reinforcement of low-rate responding (DRL) behavior in rats. Behavioural Brain Research, 331, 177–187. doi: 10.1016/j.bbr.2017.05.020 [DOI] [PubMed] [Google Scholar]
- Cho YH, & Jeantet Y (2010). Differential involvement of prefrontal cortex, striatum, and hippocampus in DRL performance in mice. Neurobiol Learn Mem, 93(1), 85–91. doi: 10.1016/j.nlm.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Chocyk A, Przyborowska A, Dudys D, Majcher I, Maćkowiak M, & Wędzony K (2011). The impact of maternal separation on the number of tyrosine hydroxylase-expressing midbrain neurons during different stages of ontogenesis. Neuroscience, 182, 43–61. doi: 10.1016/j.neuroscience.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, & Everitt BJ (2004). Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci, 24(4), 773–780. doi: 10.1523/JNEUROSCI.0949-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, & Robbins TW (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res, 146(1–2), 105–119. doi: 10.1016/j.bbr.2003.09.020 [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, & Bauter MR (2004). Maternal stress modulates the effects of developmental lead exposure. Environmental Health Perspectives, 112(6), 717–730. doi: 10.1289/ehp.6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins DA, Butts K, & Young AH (2009). The role of dopamine in bipolar disorder. Bipolar Disord, 11(8), 787–806. doi: 10.1111/j.1399-5618.2009.00760.x [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, & Salamone JD (1994). Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology, 116(4), 529–537. doi: 10.1007/BF02247489 [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Innis RB, & Nathan PJ (2006). Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry, 59(10), 898–907. doi: 10.1016/j.biopsych.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Dalley, & Ersche KD (2019). Neural circuitry and mechanisms of waiting impulsivity: relevance to addiction. Philos Trans R Soc Lond B Biol Sci, 374(1766), 20180145. doi: 10.1098/rstb.2018.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley Everitt, J. B, & Robbins TW (2011). Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron, 69(4), 680–694. doi: 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Dalley Fryer, D. T, Brichard L, Robinson ES, Theobald DE, Lääne K, … Robbins TW (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science, 315(5816), 1267–1270. doi: 10.1126/science.1137073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, & Robbins TW (2008). Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav, 90(2), 250–260. doi: 10.1016/j.pbb.2007.12.021 [DOI] [PubMed] [Google Scholar]
- Dalley JW, & Robbins TW (2017). Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci, 18(3), 158–171. doi: 10.1038/nrn.2017.8 [DOI] [PubMed] [Google Scholar]
- Dalley JW, & Roiser JP (2012). Dopamine, serotonin and impulsivity. Neuroscience, 215, 42–58. doi: 10.1016/j.neuroscience.2012.03.065 [DOI] [PubMed] [Google Scholar]
- Dawe S, & Loxton NJ (2004). The role of impulsivity in the development of substance use and eating disorders. Neuroscience & Biobehavioral Reviews, 28(3), 343–351. doi: 10.1016/j.neubiorev.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, & Carelli RM (2010). Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry, 68(3), 306–309. doi: 10.1016/j.biopsych.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Crean J, & Richards JB (2000). Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci, 114(4), 830–837. doi: 10.1037//0735-7044.114.4.830 [DOI] [PubMed] [Google Scholar]
- Deykin EY, & Buka SL (1997). Prevalence and risk factors for posttraumatic stress disorder among chemically dependent adolescents. Am J Psychiatry, 154(6), 752–757. doi: 10.1176/ajp.154.6.752 [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, & De Vries TJ (2008). Impulsive Choice and Impulsive Action Predict Vulnerability to Distinct Stages of Nicotine Seeking in Rats. Biological Psychiatry, 63(3), 301–308. doi: 10.1016/j.biopsych.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Donohew L, Zimmerman R, Cupp PS, Novak S, Colon S, & Abell R (2000). Sensation seeking, impulsive decision-making, and risky sex: implications for risk-taking and design of interventions. Personality and Individual Differences, 28(6), 1079–1091. doi: 10.1016/S0191-8869(99)00158-0 [DOI] [Google Scholar]
- Dubé CM, Molet J, Singh-Taylor A, Ivy A, Maras PM, & Baram TZ (2015). Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiol Stress, 2, 10–19. doi: 10.1016/j.ynstr.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth A, Kim B, & Tsukayama E (2013). Life Stress Impairs Self-Control in Early Adolescence. Frontiers in Psychology, 3, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, & Robbins TW (2008). Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex, 18(1), 178–188. doi: 10.1093/cercor/bhm044 [DOI] [PubMed] [Google Scholar]
- Eck SR, Ardekani CS, Salvatore M, Luz S, Kim ED, Rogers CM, … Bangasser DA (2019). The effects of early life adversity on growth, maturation, and steroid hormones in male and female rats. Eur J Neurosci. doi: 10.1111/ejn.14609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck SR, & Bangasser DA (2020). The effects of early life stress on motivated behaviors: A role for gonadal hormones. Neuroscience & Biobehavioral Reviews, 119, 86–100. doi: 10.1016/j.neubiorev.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Theobald DEH, Robbins TW, Everitt BJ, & Dalley JW (2012). Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 37(9), 2057–2066. doi: 10.1038/npp.2012.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli EJE, Zoelch N, Hock A, Nordt C, Hulka LM, Kirschner M, … Herdener M (2021). Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction. Molecular Psychiatry, 26(9), 5277–5285. doi: 10.1038/s41380-020-0828-z [DOI] [PubMed] [Google Scholar]
- Evenden JL, & Ryan CN (1996). The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl), 128(2), 161–170. doi: 10.1007/s002130050121 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, & Robbins TW (2008). Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci, 363(1507), 3125–3135. doi: 10.1098/rstb.2008.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias C. d. A., Cardoso T. d. A., Mondin TC, Souza L. D. d. M., da Silva RA, Kapczinski F, … Jansen K (2019). Clinical outcomes and childhood trauma in bipolar disorder: A community sample of young adults. Psychiatry Research, 275, 228–232. doi: 10.1016/j.psychres.2018.12.114 [DOI] [PubMed] [Google Scholar]
- Fattore L, & Diana M (2016). Drug addiction: An affective-cognitive disorder in need of a cure. Neuroscience & Biobehavioral Reviews, 65, 341–361. doi: 10.1016/j.neubiorev.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, … Potenza MN (2014). New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr, 19(1), 69–89. doi: 10.1017/s1092852913000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CM, Runegaard AH, Christiansen SH, Hansen NW, Jørgensen SH, McGirr JC, … Andreasen JT (2019). Differential effects of chemogenetic inhibition of dopamine and norepinephrine neurons in the mouse 5-choice serial reaction time task. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 90, 264–276. doi: 10.1016/j.pnpbp.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Floresco, McLaughlin, & Haluk. (2008). Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience, 154(3), 877–884. doi: 10.1016/j.neuroscience.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, & Ghods-Sharifi S (2008). Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology, 33(8), 1966–1979. doi: 10.1038/sj.npp.1301565 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MTL, & Ghods-Sharifi S (2008). Dopaminergic and Glutamatergic Regulation of Effort- and Delay-Based Decision Making. Neuropsychopharmacology, 33(8), 1966–1979. doi: 10.1038/sj.npp.1301565 [DOI] [PubMed] [Google Scholar]
- Fuentes S, Carrasco J, Hatto A, Navarro J, Armario A, Monsonet M, … Nadal R (2018). Sex-dependent impact of early-life stress and adult immobilization in the attribution of incentive salience in rats. PLoS One, 13(1), e0190044. doi: 10.1371/journal.pone.0190044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S, Daviu N, Gagliano H, Garrido P, Zelena D, Monasterio N, … Nadal R (2014). Sex-dependent effects of an early life treatment in rats that increases maternal care: vulnerability or resilience? Front Behav Neurosci, 8, 56. doi: 10.3389/fnbeh.2014.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk RR, McDermeit M, Godley SH, & Adams L (2003). Maltreatment issues by level of adolescent substance abuse treatment: the extent of the problem at intake and relationship to early outcomes. Child Maltreat, 8(1), 36–45. doi: 10.1177/1077559502239607 [DOI] [PubMed] [Google Scholar]
- Garno JL, Goldberg JF, Ramirez PM, & Ritzler BA (2005). Impact of childhood abuse on the clinical course of bipolar disorder. British Journal of Psychiatry, 186(2), 121–125. doi: 10.1192/bjp.186.2.121 [DOI] [PubMed] [Google Scholar]
- Gerfen CR (1992). The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci, 15, 285–320. doi: 10.1146/annurev.ne.15.030192.001441 [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, & Floresco SB (2010). Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell. Behavioral neuroscience, 124(2), 179. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, & Baram TZ (1996). Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol, 15(2), 114–119. doi: 10.1016/0887-8994(96)00153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA, & Caron MG (1993). Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci, 16, 299–321. doi: 10.1146/annurev.ne.16.030193.001503 [DOI] [PubMed] [Google Scholar]
- Gold MS, Blum K, Oscar-Berman M, & Braverman ER (2014). Low dopamine function in attention deficit/hyperactivity disorder: should genotyping signify early diagnosis in children? Postgraduate medicine, 126(1), 153–177. doi: 10.3810/pgm.2014.01.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondré-Lewis MC, Warnock KT, Wang H, June HL, Bell KA, Rabe H, … Aurelian L (2016). Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABAA mechanism. Stress (Amsterdam, Netherlands), 19(2), 235–247. doi: 10.3109/10253890.2016.1160280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, … Bath KG (2018). Early Life Stress Drives Sex-Selective Impairment in Reversal Learning by Affecting Parvalbumin Interneurons in Orbitofrontal Cortex of Mice. Cell Reports, 25(9), 2299–2307.e2294. doi: 10.1016/j.celrep.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Rubio I, Martinez-Laorden E, Moscoso-Castro M, Milanés MV, Laorden ML, & Valverde O (2016). Maternal Separation Impairs Cocaine-Induced Behavioural Sensitization in Adolescent Mice. PLoS One, 11(12), e0167483. doi: 10.1371/journal.pone.0167483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Redden SA, Lust K, & Chamberlain SR (2018). Nonmedical Use of Stimulants Is Associated With Riskier Sexual Practices and Other Forms of Impulsivity. J Addict Med, 12(6), 474–480. doi: 10.1097/adm.0000000000000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, & Brenhouse HC (2016). Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology, 71, 19–30. doi: 10.1016/j.psyneuen.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. doi: 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, der Zee EV-V, te Kortschot A, & Witter MP (1987). Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience, 23(1), 103–120. doi: 10.1016/0306-4522(87)90275-2 [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, & Russchen FT (1984). Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: A tracing and immunohistochemical study in the cat. Journal of Comparative Neurology, 223(3), 347–367. doi: 10.1002/cne.902230303 [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, & Voorn P (1999). Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci, 877, 49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x [DOI] [PubMed] [Google Scholar]
- Groman SM (2020). The Neurobiology of Impulsive Decision-Making and Reinforcement Learning in Nonhuman Animals. Curr Top Behav Neurosci, 47, 23–52. doi: 10.1007/7854_2020_127 [DOI] [PubMed] [Google Scholar]
- Guillaumin A, Serra GP, Georges F, & Wallén-Mackenzie Å (2021). Experimental investigation into the role of the subthalamic nucleus (STN) in motor control using optogenetics in mice. (1872–6240 (Electronic)). [DOI] [PubMed] [Google Scholar]
- Gut-Fayand A, Dervaux A, Olié J-P, Lôo H, Poirier M-F, & Krebs M-O (2001). Substance abuse and suicidality in schizophrenia: a common risk factor linked to impulsivity. Psychiatry Research, 102(1), 65–72. doi: 10.1016/S0165-1781(01)00250-5 [DOI] [PubMed] [Google Scholar]
- Halevi G, Djalovski A, Vengrober A, & Feldman R (2016). Risk and resilience trajectories in war-exposed children across the first decade of life. J Child Psychol Psychiatry, 57(10), 1183–1193. doi: 10.1111/jcpp.12622 [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Skol A, Palmer AA, & de Wit H (2009). Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Exp Clin Psychopharmacol, 17(6), 374–383. doi: 10.1037/a0017840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardung S, Epple R, Jäckel Z, Eriksson D, Uran C, Senn V, … Diester I (2017). A Functional Gradient in the Rodent Prefrontal Cortex Supports Behavioral Inhibition. Current Biology, 27(4), 549–555. doi: 10.1016/j.cub.2016.12.052 [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, & Robbins TW (1997). Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl), 133(4), 329–342. doi: 10.1007/s002130050410 [DOI] [PubMed] [Google Scholar]
- Hayton SJ, Maracle AC, & Olmstead MC (2012). Opposite effects of amphetamine on impulsive action with fixed and variable delays to respond. Neuropsychopharmacology, 37(3), 651–659. doi: 10.1038/npp.2011.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, & Mitchell SH (2006). Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl), 188(2), 144–151. doi: 10.1007/s00213-006-0478-0 [DOI] [PubMed] [Google Scholar]
- Hensleigh E, & Pritchard LM (2015). Maternal separation increases methamphetamine-induced damage in the striatum in male, but not female rats. Behavioural Brain Research, 295, 3–8. doi: 10.1016/j.bbr.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AM, Critchley HD, & Duka T (2018). Risk-Taking and Impulsivity: The Role of Mood States and Interoception. Frontiers in Psychology, 9, 1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg MP, Hodel AS, Cowell RA, Hunt RH, Gunnar MR, & Thomas KM (2018). Risk taking, decision-making, and brain volume in youth adopted internationally from institutional care. Neuropsychologia, 119, 262–270. doi: 10.1016/j.neuropsychologia.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, & Brenhouse HC (2014). Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neuroscience Letters, 566, 131–136. doi: 10.1016/j.neulet.2014.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DD, Green L, & Myerson J (2003). Is discounting impulsive?: Evidence from temporal and probability discounting in gambling and non-gambling college students. Behavioural Processes, 64(3), 355–367. doi: 10.1016/S0376-6357(03)00141-4 [DOI] [PubMed] [Google Scholar]
- Hoogman M, Onnink M, Cools R, Aarts E, Kan C, Vasquez AA, … Franke B (2013). The dopamine transporter haplotype and reward-related striatal responses in adult ADHD. European Neuropsychopharmacology, 23(6), 469–478. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, & Bonci A (2003). Cooperative Activation of Dopamine D1 and D2 Receptors Increases Spike Firing of Nucleus Accumbens Neurons via G-Protein βγ Subunits. The Journal of Neuroscience, 23(12), 5079. doi: 10.1523/JNEUROSCI.23-12-05079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, & Gunnar MR (2012). Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences, 109(Supplement 2), 17208. doi: 10.1073/pnas.1121246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LT (2014). Early-life stress impacts the developing hippocampus and primes seizure occurrence: cellular, molecular, and epigenetic mechanisms. Front Mol Neurosci, 7, 8. doi: 10.3389/fnmol.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, & Everitt BJ (2004). Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature Neuroscience, 7(4), 389–397. doi: 10.1038/nn1217 [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, & Baram TZ (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience, 154(3), 1132–1142. doi: 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ÅK, Bergvall ÅH, & Hansen S (1999). Behavioral disinhibition following basal forebrain excitotoxin lesions: alcohol consumption, defensive aggression, impulsivity and serotonin levels. Behavioural Brain Research, 102(1), 17–29. doi: 10.1016/S0166-4328(98)00159-4 [DOI] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Saigal N, Reverte I, Shrestha S, Cumming P, … Dalley JW (2013). Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur J Neurosci, 37(9), 1519–1528. doi: 10.1111/ejn.12146 [DOI] [PubMed] [Google Scholar]
- Kapor S, Aksić M, Puškaš L, Jukić M, Poleksić J, Milosavljević F, … Filipović B (2020). Long-Term Effects of Maternal Deprivation on the Volume of Dopaminergic Nuclei and Number of Dopaminergic Neurons in Substantia Nigra and Ventral Tegmental Area in Rats. Front Neuroanat, 14, 578900. doi: 10.3389/fnana.2020.578900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunar MR, Saez T, Brusco A, & Antonelli MC (2009). Immunocytochemical expression of dopamine-related transcription factors Pitx3 and Nurr1 in prenatally stressed adult rats. Journal of Neuroscience Research, 87(4), 1014–1022. doi: 10.1002/jnr.21911 [DOI] [PubMed] [Google Scholar]
- Kelley AE, & Domesick VB (1982). The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience, 7(10), 2321–2335. doi: 10.1016/0306-4522(82)90198-1 [DOI] [PubMed] [Google Scholar]
- Kentrop J, van der Tas L, Loi M, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M, & van der Veen R (2016). Mifepristone Treatment during Early Adolescence Fails to Restore Maternal Deprivation-Induced Deficits in Behavioral Inhibition of Adult Male Rats. Front Behav Neurosci, 10, 122. doi: 10.3389/fnbeh.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British journal of psychiatry : the journal of mental science, 197(5), 378–385. doi: 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, … Anderson IM (2004). Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl), 175(2), 206–214. doi: 10.1007/s00213-004-1813-y [DOI] [PubMed] [Google Scholar]
- Kim H (2018). Associations between cognitive function and striatal DAT loss in Parkinson’s disease with mild cognitive impairment. Journal of Nuclear Medicine, 59(supplement 1), 1650. [Google Scholar]
- Kim S, Bobeica I, Gamo NJ, Arnsten AFT, & Lee D (2012). Effects of α−2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology, 219(2), 363–375. doi: 10.1007/s00213-011-2520-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Hwang SS, Kim HW, Hwang EH, Cho J, Kang JI, & Kim SJ (2018). Multidimensional impulsivity as a mediator of early life stress and alcohol dependence. Scientific Reports, 8(1), 4104. doi: 10.1038/s41598-018-22474-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knardahl S, & Sagvolden T (1979). Open-field behavior of spontaneously hypertensive rats. Behavioral and neural biology, 27(2), 187–200. [DOI] [PubMed] [Google Scholar]
- Kohno M, Okita K, Morales AM, Robertson CL, Dean AC, Ghahremani DG, … London ED (2016). Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: link to impulsivity in methamphetamine users. Molecular Psychiatry, 21(11), 1554–1560. doi: 10.1038/mp.2015.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokane SS, & Perrotti LI (2020). Sex Differences and the Role of Estradiol in Mesolimbic Reward Circuits and Vulnerability to Cocaine and Opiate Addiction. Front Behav Neurosci, 14, 74. doi: 10.3389/fnbeh.2020.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, & Segal DS (1997). Effects of Methylphenidate on Extracellular Dopamine, Serotonin, and Norepinephrine: Comparison with Amphetamine. Journal of Neurochemistry, 68(5), 2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x [DOI] [PubMed] [Google Scholar]
- Kunzler J, Braun K, & Bock J (2015). Early life stress and sex-specific sensitivity of the catecholaminergic systems in prefrontal and limbic regions of Octodon degus. Brain Structure and Function, 220(2), 861–868. doi: 10.1007/s00429-013-0688-2 [DOI] [PubMed] [Google Scholar]
- Lafferty CK, Yang AK, Mendoza JA, & Britt JP (2020). Nucleus Accumbens Cell Type- and Input-Specific Suppression of Unproductive Reward Seeking. Cell Reports, 30(11), 3729–3742.e3723. doi: 10.1016/j.celrep.2020.02.095 [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, … Mandelkern MA (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci, 29(47), 14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Nguyen TP, Ma C, & Dan Y (2020). Inhibition of impulsive action by projection-defined prefrontal pyramidal neurons. Proceedings of the National Academy of Sciences, 117(29), 17278. doi: 10.1073/pnas.2000523117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-N, Liu X-L, & Li L (2020). Prefrontal GABA and glutamate levels correlate with impulsivity and cognitive function of prescription opioid addicts: A 1H-magnetic resonance spectroscopy study. Psychiatry and Clinical Neurosciences, 74(1), 77–83. doi: 10.1111/pcn.12940 [DOI] [PubMed] [Google Scholar]
- Lobo MK, & Nestler E (2011). The Striatal Balancing Act in Drug Addiction: Distinct Roles of Direct and Indirect Pathway Medium Spiny Neurons. Frontiers in Neuroanatomy, 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Michaelos M, Collins WF, & Kritzer MF (2017). Gonadectomy but not biological sex affects burst-firing in dopamine neurons of the ventral tegmental area and in prefrontal cortical neurons projecting to the ventral tegmentum in adult rats. Eur J Neurosci, 45(1), 106–120. doi: 10.1111/ejn.13380 [DOI] [PubMed] [Google Scholar]
- London ED (2020). Human Brain Imaging Links Dopaminergic Systems to Impulsivity. In de Wit H & Jentsch JD (Eds.), Recent Advances in Research on Impulsivity and Impulsive Behaviors (pp. 53–71). Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, … van Gaalen MM (2010). Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex, 20(5), 1064–1070. doi: 10.1093/cercor/bhp167 [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, & Vincent AS (2013). Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res, 37(4), 616–623. doi: 10.1111/acer.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Keen D, Fletcher PJ, & Fleming AS (2011). Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav Neurosci, 125(4), 481–491. doi: 10.1037/a0024367 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Ma I, van Holstein M, Mies GW, Mennes M, Buitelaar J, Cools R, … Scheres A (2016). Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex, 82, 225–236. doi: 10.1016/j.cortex.2016.05.021 [DOI] [PubMed] [Google Scholar]
- Majcher-Maślanka I, Solarz A, Wędzony K, & Chocyk A (2017). The effects of early-life stress on dopamine system function in adolescent female rats. Int J Dev Neurosci, 57, 24–33. doi: 10.1016/j.ijdevneu.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, & Robbins TW (2011). Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci, 31(17), 6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shungu DC, Mao X, Smith ELP, Perera GM, Kegeles LS, … Coplan JD (2003). A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biological Psychiatry, 54(7), 727–735. doi: 10.1016/S0006-3223(03)00004-0 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication II: Associations With Persistence of DSM-IV Disorders. Archives of General Psychiatry, 67(2), 124–132. doi: 10.1001/archgenpsychiatry.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry, 69(11), 1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, & Gratton A (2002). Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology, 27(1–2), 127–138. doi: 10.1016/s0306-4530(01)00040-3 [DOI] [PubMed] [Google Scholar]
- Messina BG, Silvestri MM, Diulio AR, Murphy JG, Garza KB, & Correia CJ (2014). Alcohol use, impulsivity, and the non-medical use of prescription stimulants among college students. Addictive behaviors, 39(12), 1798–1803. doi: 10.1016/j.addbeh.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Meyer HC, & Bucci DJ (2016). Imbalanced Activity in the Orbitofrontal Cortex and Nucleus Accumbens Impairs Behavioral Inhibition. Current biology : CB, 26(20), 2834–2839. doi: 10.1016/j.cub.2016.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, & Holmes A (2007). Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev, 31(1), 3–17. doi: 10.1016/j.neubiorev.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, & Anderson IM (2002). Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl), 160(3), 290–298. doi: 10.1007/s00213-001-0983-0 [DOI] [PubMed] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S, & Baram TZ (2014). Naturalistic rodent models of chronic early-life stress. Dev Psychobiol, 56(8), 1675–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy E, Hernández-Torres E, & Flores G (2010). Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat, 40(2), 93–101. doi: 10.1016/j.jchemneu.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Mueller BR, & Bale TL (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience : the official journal of the Society for Neuroscience, 28(36), 9055–9065. doi:28/36/9055 [pii] 10.1523/JNEUROSCI.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, & Kolb B (2012). Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience, 216, 103–109. doi: 10.1016/j.neuroscience.2012.04.041 [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, & Robbins TW (1996). The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex, 6(3), 470–481. doi: 10.1093/cercor/6.3.470 [DOI] [PubMed] [Google Scholar]
- Mulder TM, Kuiper KC, van der Put CE, Stams GJM, & Assink M (2018). Risk factors for child neglect: A meta-analytic review. Child Abuse Negl, 77, 198–210. doi: 10.1016/j.chiabu.2018.01.006 [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando ABP, Urcelay GP, Robinson ESJ, Mar AC, Theobald DEH, … Robbins TW (2012). Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology, 219(2), 401–410. doi: 10.1007/s00213-011-2572-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Robinson ES, Theobald DE, Dalley JW, & Robbins TW (2008). Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. Eur J Neurosci, 28(2), 353–363. doi: 10.1111/j.1460-9568.2008.06309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P, Perez J, Sanches M, Peluso MAM, Glahn D, & Soares JC (2007). Impulsivity and bipolar disorder. European Neuropsychopharmacology, 17(5), 313–320. doi: 10.1016/j.euroneuro.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Newman LA, Creer DJ, & McGaughy JA (2015). Cognitive control and the anterior cingulate cortex: how conflicting stimuli affect attentional control in the rat. J Physiol Paris, 109(1–3), 95–103. doi: 10.1016/j.jphysparis.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Horii-Hayashi N, & Sasagawa T (2014). Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Frontiers in neuroscience, 8, 166–166. doi: 10.3389/fnins.2014.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitomi K, Yano K, Kobayashi M, Jino K, Kano T, Horiguchi N, … Hasegawa M (2018). Systemic administration of guanfacine improves food-motivated impulsive choice behavior primarily via direct stimulation of postsynaptic α2A-adrenergic receptors in rats. Behavioural brain research, 345, 21–29. [DOI] [PubMed] [Google Scholar]
- Oades RD, & Halliday GM (1987). Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res, 434(2), 117–165. doi: 10.1016/0165-0173(87)90011-7 [DOI] [PubMed] [Google Scholar]
- Ohta K. i., Suzuki S, Warita K, Sumitani K, Tenkumo C, Ozawa T, … Miki T (2020). The effects of early life stress on the excitatory/inhibitory balance of the medial prefrontal cortex. Behavioural Brain Research, 379, 112306. doi: 10.1016/j.bbr.2019.112306 [DOI] [PubMed] [Google Scholar]
- Ordoñes Sanchez E, Bavley CC, Deutschmann AU, Carpenter R, Peterson DR, Karbalaei R, … Bangasser DA (2021). Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proceedings of the National Academy of Sciences, 118(8), e2020173118. doi: 10.1073/pnas.2020173118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, & Hyland BI (2005). Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci, 25(26), 6235–6242. doi: 10.1523/jneurosci.1478-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardey MC, Kumar NN, Goodchild AK, & Cornish JL (2012). Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. Journal of Psychopharmacology, 27(2), 203–212. doi: 10.1177/0269881112465497 [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, & van Gaalen MM (2007). Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl), 191(3), 587–598. doi: 10.1007/s00213-006-0533-x [DOI] [PubMed] [Google Scholar]
- Pattij T, & Vanderschuren LJMJ (2008). The neuropharmacology of impulsive behaviour. Trends in Pharmacological Sciences, 29(4), 192–199. doi: 10.1016/j.tips.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. doi: 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugi G, Vannucchi G, Bedani F, & Favaretto E (2017). Use of Stimulants in Bipolar Disorder. Current Psychiatry Reports, 19(1), 7. doi: 10.1007/s11920-017-0758-x [DOI] [PubMed] [Google Scholar]
- Pettorruso M, Martinotti G, Cocciolillo F, De Risio L, Cinquino A, Di Nicola M, … Di Giuda D (2019). Striatal presynaptic dopaminergic dysfunction in gambling disorder: A 123I-FP-CIT SPECT study. Addiction Biology, 24(5), 1077–1086. doi: 10.1111/adb.12677 [DOI] [PubMed] [Google Scholar]
- Petzold J, Kienast A, Lee Y, Pooseh S, London ED, Goschke T, & Smolka MN (2019). Baseline impulsivity may moderate L-DOPA effects on value-based decision-making. Sci Rep, 9(1), 5652. doi: 10.1038/s41598-019-42124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, & Robbins TW (2009). Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl), 202(1–3), 307–313. doi: 10.1007/s00213-008-1384-4 [DOI] [PubMed] [Google Scholar]
- Piantadosi PT, Yeates DCM, & Floresco SB (2018). Cooperative and dissociable involvement of the nucleus accumbens core and shell in the promotion and inhibition of actions during active and inhibitory avoidance. Neuropharmacology, 138, 57–71. doi: 10.1016/j.neuropharm.2018.05.028 [DOI] [PubMed] [Google Scholar]
- Piantadosi PT, Yeates DCM, Wilkins M, & Floresco SB (2017). Contributions of basolateral amygdala and nucleus accumbens subregions to mediating motivational conflict during punished reward-seeking. Neurobiology of Learning and Memory, 140, 92–105. doi: 10.1016/j.nlm.2017.02.017 [DOI] [PubMed] [Google Scholar]
- Piña R, Rozas C, Contreras D, Hardy P, Ugarte G, Zeise ML, … Morales B (2020). Atomoxetine Reestablishes Long Term Potentiation in a Mouse Model of Attention Deficit/Hyperactivity Disorder. Neuroscience, 439, 268–274. doi: 10.1016/j.neuroscience.2019.10.040 [DOI] [PubMed] [Google Scholar]
- Puumala T, & Sirviö J (1998). Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience, 83(2), 489–499. doi: 10.1016/s0306-4522(97)00392-8 [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, & Baram TZ (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology, 149(10), 4892–4900. doi: 10.1210/en.2008-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, & Grace AA (2017). Sex-Dependent Effects of Stress on Immobility Behavior and VTA Dopamine Neuron Activity: Modulation by Ketamine. Int J Neuropsychopharmacol, 20(10), 823–832. doi: 10.1093/ijnp/pyx048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, & Dalley JW (2017). Dissecting Impulsivity: Brain Mechanisms and Neuropsychiatric Implications. Nebr Symp Motiv, 64, 201–226. [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, … Robbins TW (2008). Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology, 33(5), 1028–1037. doi: 10.1038/sj.npp.1301487 [DOI] [PubMed] [Google Scholar]
- Roesch Taylor, & Schoenbaum. (2006). Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron, 51(4), 509–520. doi: 10.1016/j.neuron.2006.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, & Schoenbaum G (2007). Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nature Neuroscience, 10(12), 1615–1624. doi: 10.1038/nn2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-López A, Méndez-Díaz M, García FG, Regalado-Santiago C, Ruiz-Contreras AE, & Prospéro-García O (2016). Maternal separation and early stress cause long-lasting effects on dopaminergic and endocannabinergic systems and alters dendritic morphology in the nucleus accumbens and frontal cortex in rats. Dev Neurobiol, 76(8), 819–831. doi: 10.1002/dneu.22361 [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, & Gjedde A (2005). Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. NeuroImage, 25(3), 868–876. doi: 10.1016/j.neuroimage.2004.11.031 [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, & Rushworth MFS (2006). Separate neural pathways process different decision costs. Nature Neuroscience, 9(9), 1161–1168. doi: 10.1038/nn1756 [DOI] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, & Johansen EB (2005). Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct, 1, 9. doi: 10.1186/1744-9081-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DA, Moschak TM, & Carelli RM (2019). Prelimbic Cortical Neurons Track Preferred Reward Value and Reflect Impulsive Choice during Delay Discounting Behavior. The Journal of Neuroscience, 39(16), 3108. doi: 10.1523/JNEUROSCI.2532-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Cacciapaglia F, Wightman RM, & Carelli RM (2015). Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation. The Journal of Neuroscience, 35(33), 11572. doi: 10.1523/JNEUROSCI.2344-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, & Carelli RM (2015). Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biological psychiatry, 77(10), 903–911. doi: 10.1016/j.biopsych.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T (2000). Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev, 24(1), 31–39. doi: 10.1016/s0149-7634(99)00058-5 [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, & Farshbaf M (2005). Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry, 57(11), 1239–1247. doi: 10.1016/j.biopsych.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Salamone JD (1994). The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res, 61(2), 117–133. doi: 10.1016/0166-4328(94)90153-8 [DOI] [PubMed] [Google Scholar]
- Sanna A, Fattore L, Badas P, Corona G, & Diana M (2021). The hypodopaminergic state ten years after: transcranial magnetic stimulation as a tool to test the dopamine hypothesis of drug addiction. Current Opinion in Pharmacology, 56, 61–67. doi: 10.1016/j.coph.2020.11.001 [DOI] [PubMed] [Google Scholar]
- Sasagawa T, Horii-Hayashi N, Okuda A, Hashimoto T, Azuma C, & Nishi M (2017). Long-term effects of maternal separation coupled with social isolation on reward seeking and changes in dopamine D1 receptor expression in the nucleus accumbens via DNA methylation in mice. Neurosci Lett, 641, 33–39. doi: 10.1016/j.neulet.2017.01.025 [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A Neural Substrate of Prediction and Reward. Science, 275(5306), 1593. doi: 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Pavey G, & Dean B (2019). Altered levels of dopamine transporter in the frontal pole and dorsal striatum in schizophrenia. npj Schizophrenia, 5(1), 20. doi: 10.1038/s41537-019-0087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, & Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol, 290(2), 213–242. doi: 10.1002/cne.902900205 [DOI] [PubMed] [Google Scholar]
- Sibley DR, & Monsma FJ Jr. (1992). Molecular biology of dopamine receptors. Trends Pharmacol Sci, 13(2), 61–69. doi: 10.1016/0165-6147(92)90025- [DOI] [PubMed] [Google Scholar]
- Silvagni A, Vg. B, Mura C, Antonelli MC, Mc. A, & Carboni E (2008). Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo’ microdialysis study in adolescent and young adult rats. (1460–9568 (Electronic)). [DOI] [PubMed] [Google Scholar]
- Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, & Setlow B (2013). Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. The European journal of neuroscience, 37(11), 1779–1788. doi: 10.1111/ejn.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaragdi A, Chavez S, Lobaugh NJ, Meyer JH, & Kolla NJ (2019). Differential levels of prefrontal cortex glutamate+glutamine in adults with antisocial personality disorder and bipolar disorder: A proton magnetic resonance spectroscopy study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 93, 250–255. doi: 10.1016/j.pnpbp.2019.04.002 [DOI] [PubMed] [Google Scholar]
- Smith CT, San Juan MD, Dang LC, Katz DT, Perkins SF, Burgess LL, … Zald DH (2018). Ventral striatal dopamine transporter availability is associated with lower trait motor impulsivity in healthy adults. Translational Psychiatry, 8(1), 269. doi: 10.1038/s41398-018-0328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Borges S, Domingues AV, Silva D, Sousa N, & Rodrigues AJ (2018). Mild Prenatal Stress Causes Emotional and Brain Structural Modifications in Rats of Both Sexes. Frontiers in Behavioral Neuroscience, 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, & Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature, 459(7247), 698–702. doi: 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, … Turkel E (2001). The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol, 29(3), 215–228. doi: 10.1023/a:1010329714819 [DOI] [PubMed] [Google Scholar]
- Spooren WP, Veening JG, Groenewegen HJ, & Cools AR (1991). Efferent connections of the striatopallidal and amygdaloid components of the substantia innominata in the cat: projections to the nucleus accumbens and caudate nucleus. Neuroscience, 44(2), 431–447. doi: 10.1016/0306-4522(91)90067-x [DOI] [PubMed] [Google Scholar]
- Spyrka J, Gugula A, Rak A, Tylko G, Hess G, & Blasiak A (2020). Early life stress-induced alterations in the activity and morphology of ventral tegmental area neurons in female rats. (2352–2895 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Bauer E, Merz CJ, Zimmermann M, Reuter M, Plichta MM, … Herrmann MJ (2011). ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia, 49(3), 426–434. doi: 10.1016/j.neuropsychologia.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, & Janak PH (2013). A causal link between prediction errors, dopamine neurons and learning. Nature Neuroscience, 16(7), 966–973. doi: 10.1038/nn.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper Colin M., Tse Maric T. L., Montes David R., Wiedman Candice R., & Floresco Stan B. (2014). Overriding Phasic Dopamine Signals Redirects Action Selection during Risk/Reward Decision Making. Neuron, 84(1), 177–189. doi: 10.1016/j.neuron.2014.08.033 [DOI] [PubMed] [Google Scholar]
- Sugaya L, Hasin DS, Olfson M, Lin KH, Grant BF, & Blanco C (2012). Child physical abuse and adult mental health: a national study. J Trauma Stress, 25(4), 384–392. doi: 10.1002/jts.21719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, & Shen W (2007). D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in Neurosciences, 30(5), 228–235. doi: 10.1016/j.tins.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, & Moeller FG (2013). Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology, 229(1), 83–94. doi: 10.1007/s00213-013-3088-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SA, & Nemeroff CB (2017). Early Life Stress, Mood, and Anxiety Disorders. Chronic stress (Thousand Oaks, Calif.), 1, 10.1177/2470547017694461. doi:10.1177/2470547017694461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Barrus MM, Cocker PJ, Baunez C, & Winstanley CA (2019). Increased motor impulsivity in a rat gambling task during chronic ropinirole treatment: potentiation by win-paired audiovisual cues. Psychopharmacology, 236(6), 1901–1915. doi: 10.1007/s00213-019-5173-z [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, … Javitch JA (2013). Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Molecular Psychiatry, 18(9), 1025–1033. doi: 10.1038/mp.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, & Robinson TE (2006). Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice – mediation by enhanced incentive motivation? European Journal of Neuroscience, 24(8), 2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x [DOI] [PubMed] [Google Scholar]
- Van den Hove DLA, Steinbusch HWM, Scheepens A, Van de Berg WDJ, Kooiman LAM, Boosten BJG, … Blanco CE (2006). Prenatal stress and neonatal rat brain development. Neuroscience, 137(1), 145–155. doi: 10.1016/j.neuroscience.2005.08.060 [DOI] [PubMed] [Google Scholar]
- van der Veen B, Kapanaiah SKT, Kilonzo K, Steele-Perkins P, Jendryka MM, Schulz S, … Kätzel D (2021). Control of impulsivity by Gi-protein signalling in layer-5 pyramidal neurons of the anterior cingulate cortex. Communications Biology, 4(1), 662. doi: 10.1038/s42003-021-02188-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PFC, Schoffelmeer ANM, & Vanderschuren LJMJ (2006). Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology, 187(1), 73–85. doi: 10.1007/s00213-006-0396-1 [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer ANM, & Vanderschuren LJMJ (2006). Critical Involvement of Dopaminergic Neurotransmission in Impulsive Decision Making. Biological Psychiatry, 60(1), 66–73. doi: 10.1016/j.biopsych.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Vest N, Reynolds CJ, & Tragesser SL (2016). Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addictive behaviors, 60, 184–190. doi: 10.1016/j.addbeh.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Vrijsen JN, Tendolkar I, Onnink M, Hoogman M, Schene AH, Fernández G, … Franke B (2018). ADHD symptoms in healthy adults are associated with stressful life events and negative memory bias. Attention deficit and hyperactivity disorders, 10(2), 151–160. doi: 10.1007/s12402-017-0241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, & Richards JB (2000). Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl), 150(1), 90–101. doi: 10.1007/s002130000402 [DOI] [PubMed] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, … Baram TZ (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress (Amsterdam, Netherlands), 20(5), 421–448. doi: 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Choi JK, & Shin J (2020). Long-term Neighborhood Effects on Adolescent Outcomes: Mediated through Adverse Childhood Experiences and Parenting Stress. J Youth Adolesc, 49(10), 2160–2173. doi: 10.1007/s10964-020-01305-y [DOI] [PubMed] [Google Scholar]
- Weston HI, Weston DD, Allen JL, & Cory-Slechta DA (2014). Sex-dependent impacts of low-level lead exposure and prenatal stress on impulsive choice behavior and associated biochemical and neurochemical manifestations. Neurotoxicology, 44, 169–183. doi: 10.1016/j.neuro.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry, 28(1), 7–12. doi: 10.1097/yco.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, & Leranth C (1992). The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. Journal of Comparative Neurology, 320(3), 353–369. doi: 10.1002/cne.903200307 [DOI] [PubMed] [Google Scholar]
- Wilson CA, Schade R, & Terry AV (2012). Variable prenatal stress results in impairments of sustained attention and inhibitory response control in a 5-choice serial reaction time task in rats. Neuroscience, 218, 126–137. doi: 10.1016/j.neuroscience.2012.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DEH, & Robbins TW (2003). Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology, 170(3), 320–331. doi: 10.1007/s00213-003-1546-3 [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, & Robbins TW (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci, 24(20), 4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, & Robbins TW (2006). Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex, 16(1), 106–114. doi: 10.1093/cercor/bhi088 [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Zeeb FD, Bedard A, Fu K, Lai B, Steele C, & Wong AC (2010). Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav Brain Res, 210(2), 263–272. doi: 10.1016/j.bbr.2010.02.044 [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, … Adolescents With Attention-Deficit/Hyperactive D (2019). Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics, 144(4), e20192528. doi: 10.1542/peds.2019-2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womersley JS, Hsieh JH, Kellaway LA, Gerhardt GA, & Russell VA (2011). Maternal separation affects dopamine transporter function in the spontaneously hypertensive rat: an in vivo electrochemical study. Behav Brain Funct, 7, 49. doi: 10.1186/1744-9081-7-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Savulich G, Voon V, Fernandez-Egea E, & Robbins TW (2014). Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: cross-species translational significance. Neuropsychopharmacology, 39(6), 1519–1526. doi: 10.1038/npp.2013.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wultz B, Sagvolden T, Moser EI, & Moser M-B (1990). The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: Effects of methylphenidate on exploratory behavior. Behavioral and Neural Biology, 53(1), 88–102. doi: 10.1016/0163-1047(90)90848-Z [DOI] [PubMed] [Google Scholar]
- Xie P, Wu K, Zheng Y, Guo Y, Yang Y, He J, … Peng H (2018). Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. Journal of Affective Disorders, 228, 41–48. doi: 10.1016/j.jad.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Yates JR, & Bardo MT (2017). Effects of intra-accumbal administration of dopamine and ionotropic glutamate receptor drugs on delay discounting performance in rats. Behavioral neuroscience, 131(5), 392–405. doi: 10.1037/bne0000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Darna M, Beckmann JS, Dwoskin LP, & Bardo MT (2016). Individual differences in impulsive action and dopamine transporter function in rat orbitofrontal cortex. Neuroscience, 313, 122–129. doi: 10.1016/j.neuroscience.2015.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, & Calipari ES (2021). Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology, 46(3), 491–499. doi: 10.1038/s41386-020-00915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Featherstone R, Mathewson S, & Fletcher P (2014). Chronic exposure to a gambling-like schedule of reward predictive stimuli can promote sensitization to amphetamine in rats. Frontiers in Behavioral Neuroscience, 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, & Brog JS (1992). On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience, 50(4), 751–767. doi: 10.1016/0306-4522(92)90202-D [DOI] [PubMed] [Google Scholar]
- Zaniewska M, Filip M, & Przegalinski E (2015). The Involvement of Norepinephrine in Behaviors Related to Psychostimulant Addiction. Curr Neuropharmacol, 13(3), 407–418. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, & Winstanley CA (2010). Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology, 211(1), 87–98. doi: 10.1007/s00213-010-1871-2 [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Soko AD, Ji X, & Fletcher PJ (2016). Low Impulsive Action, but not Impulsive Choice, Predicts Greater Conditioned Reinforcer Salience and Augmented Nucleus Accumbens Dopamine Release. Neuropsychopharmacology, 41(8), 2091–2100. doi: 10.1038/npp.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, & Winstanley CA (2011). Lesions of the Basolateral Amygdala and Orbitofrontal Cortex Differentially Affect Acquisition and Performance of a Rodent Gambling Task. The Journal of Neuroscience, 31(6), 2197. doi: 10.1523/JNEUROSCI.5597-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


