Abstract
Exercise-induced laryngeal obstruction (EILO) is caused by paradoxical inspiratory adduction of laryngeal structures during exercise. EILO is an important cause of upper airway dysfunction in young individuals and athletes, can impair exercise performance and mimic lower airway dysfunction, such as asthma and/or exercise-induced bronchoconstriction. Over the past two decades, there has been considerable progress in the recognition and assessment of EILO in sports medicine. EILO is a highly prevalent cause of unexplained dyspnoea and wheeze in athletes. The preferred diagnostic approach is continuous visualisation of the larynx (via laryngoscopy) during high-intensity exercise. Recent data suggest that EILO consists of different subtypes, possibly caused via different mechanisms. Several therapeutic interventions for EILO are now in widespread use, but to date, no randomised clinical trials have been performed to assess their efficacy or inform robust management strategies. The aim of this review is to provide a state-of-the-art overview of EILO and guidance for clinicians evaluating and treating suspected cases of EILO in athletes. Specifically, this review examines the pathophysiology of EILO, outlines a diagnostic approach and presents current therapeutic algorithms. The key unmet needs and future priorities for research in this area are also covered.
Keywords: athletes, asthma, exercise, respiratory system, sports medicine
Introduction
Exercise-induced laryngeal obstruction (EILO) is increasingly recognised as an important cause of exertional breathing problems or upper airway dysfunction, particularly affecting athletes and physically active young individuals. EILO is defined as transient upper airway obstruction that typically occurs at the supraglottic level, most often followed by a glottic component, causing exertional dyspnoea. It is important that sport and exercise medicine physician and other health professionals, athletic trainers and coaches who are involved in the care of athletes presenting with exercise-related symptoms recognise EILO. It is also important that they have a diagnostic and therapeutic approach to this condition for the following reasons: EILO is common, it impacts on exercise performance and quality of life, and tends to be confused with and inappropriately diagnosed or treated as lower airway dysfunction (ie, asthma, exercise-induced bronchoconstriction (EIB), airway hyper-responsiveness (AHR)). Fortunately, both awareness of EILO and the relevant diagnostic techniques have improved over the past decade.
The prevalence rates of EILO in adolescent populations range from 5% to 8%.1–3 This increases to more than 20% in some groups of individuals, where exercise is a key part of their everyday life, such as elite athletes and combat soldiers.4–6 EILO deters people from participation in physical activities and sports and can, in severe cases, lead to exercise avoidance, impair performance and affect sports careers in athletes.7 Moreover, incorrect diagnosis and management of EILO can result in affected individuals being incorrectly prescribed asthma medications, with an associated potential to cause adverse effects.8
For decades, asthma and/or EIB was regarded as the most prevalent medical cause of exertional dyspnoea. Accordingly, when athletes present with respiratory symptoms that occur during exercise, the sports and exercise medicine community has tended to focus on the assessment and management of the lower airways, particularly EIB.9 10 Objective testing for EIB has been recommended in most guidelines, but regrettably not always performed.4 11–18 Given new knowledge related to the high prevalence of EILO and a better understanding of the pitfalls of clinically diagnosing asthma and/or EIB, there is now an opportunity to reshape the diagnostic guidelines for athletes presenting with exertional dyspnoea and include objective testing also for EILO.19 20
The aim of this review is to provide a state-of-the-art overview of EILO, and guidance for how to evaluate and treat suspected cases of EILO in athletes. The review will focus on the pathophysiology of EILO, outline a diagnostic approach and present some of the most applied treatment strategies. The main motivation is to optimise athletes’ respiratory care through recognition of EILO, and specifically to improve the management of EILO, based on the available evidence.
Methods
In late 2019 an IOC consensus statement core panel on acute respiratory illness in athletes was convened on behalf of the IOC Medical and Scientific Commission and chaired by MS. Members of the core panel were selected after careful consideration of all the applications that were received from the IOC networks. A Subgroup 6 of this core group, consisted of MS, NS, CM, chaired by HHC, focused on acute nasal illness, structural pathology and laryngeal dysfunction in athletes.
Corresponding members of Subgroup 6, TH, JTO and JHH were selected after application based on clinical and research experience on EILO in athletes, involvement in international guideline committees and publications in the field the last 5 years.
The subgroup conducted a systematic literature search. Relevant scientific full-text articles, written in English, published between 1990 and 31 July 2020, were identified by a systematic search in Medline, EBSCOhost, Web of Science (Core Collection) and EMBASE. Inclusion criteria were humans aged 15–65 years, athletes or physically active individuals, reports of prevalence/incidence/rate/risk factors for non-inflammatory/non-infective mechanical upper airway obstruction in the setting of acute respiratory illness. All possible terms of EILO known were used. A full list of search terms is attached in online supplemental tables 1A, and 1085 records were identified through database searching. The reference lists from selected articles were examined, and five additional articles were included. HC and TO individually screened 1029 records by at title/abstract level after duplicate removal. A total of 47 full-text articles were tested for eligible, and 8 full-text articles on EILO were included, based on the search. Two more publications were included after the search (marked articles in table 1) There were not enough data to support a systematic review, and this manuscript is therefore presented as a narrative review based on the available literature. Finally, for some clinically important aspects of EILO, the authors have resorted to ‘expert opinion’.21
Table 1.
A summary of studies on the prevalence of EILO in athletes and adolescents
| Prevalence of EILO in athletes | ||||||
| Authors | Year | Population | Design | Findings | Diagnostic method | Comments |
| Morris et al 5 | 1999 | 105 patients with exertional dyspnoea at an army clinic | Cross-sectional Prospective |
12% had VCD | Laryngoscopy before and after exercise | Selected population |
| Rundell and Spiering29 | 2003 | 370 Olympic-level athletes |
Cross-sectional Prospective |
30% had EIB 5% had stridor (of whom 52% had EIB) |
Auscultation | Olympic-level athletes |
| Hanks et al30 | 2012 | 148 athletes referred for exertional dyspnoea | Retrospective chart review | 52% EIB 14% Asthma 70% VCD 31% EIB + VCD |
Laryngoscopy after exercise | Selected population |
| Nielsen et al 4 | 2013 | 91 athletes referred for CLE testing. 88 tested | Retrospective chart review | 35% had EILO 43% had Asthma |
Continuous laryngoscopy during exercise | Highly selected population |
| Turmel et al 31 | 2015 | 352 Athletes, 42 suspected EILO, 12 examined |
Cross-sectional Prospective |
12 tested and 12 confirmed EILO | Laryngoscopy during eucapnic voluntary hyperventilation | Selected population, heterogenous evaluation |
| Heffler et al 32 | 2015 | 37 healthy elite rowers without exertional dyspnoea | Cross-sectional Prospective |
27% had EIB, 43% had EILO, 16% both |
Flow volume loop assessment during eucapnic voluntary hyperventilation | Vit D lower in those with EILO |
| Irewall et al 6 * | 2021 | 89 invited elite cross-country skiers | Cross-sectional Prospective | 27% had EILO 38% had asthma (of whom 29% had EILO) |
Continuous laryngoscopy during exercise | Well-designed. Risk of underestimated results |
| Prevalence of EILO in adolescents | ||||||
| Christensen et al 2 | 2011 | 556 invited. 150 tested for AHR, 17.6% tested for EILO | Cross-sectional Prospective |
AHR in 28% of invited. EILO in 42% of tested, 7.6% of total invited Six persons had both AHR and EILO |
Continuous laryngoscopy during exercise | |
| Johansson et al 1 | 2015 | 3838 invited, 2309 responded, symptoms in 330 85 with and 42 without symptoms tested | Cross-sectional Prospective |
11% had EILO 40% had EIB Estimated EILO prevalence 5.7%. Similar in boys and girls |
Continuous laryngoscopy during exercise | Well-designed |
| Ersson et al 3 * | 2020 | 549 first-year high-school athletes invited 42 with and 34 without dyspnoea tested |
Cross-sectional Prospective |
EIB in 8/41 with and 16/57 without dyspnoea EILO in 5/34 with and 3/42 without dyspnoea Estimated prevalence of EILO 8.1% |
Continuous laryngoscopy during exercise (on bike) | Self- reported symptoms are week indicators for both EIB and EILO |
*Studies published after conducting the systematic search.
AHR, airway hyper-responsiveness; CLE, continuous laryngoscopy exercise; EIB, exercise-induced bronchoconstriction; EILO, exercise-induced laryngeal obstruction; VCD, vocal cord dysfunction.
bjsports-2021-104704supp001.pdf (435KB, pdf)
Observations
Normal laryngeal structure and function at rest and during exercise
The larynx is situated above the trachea and represents a narrow and complex valve-like structure that controls air flow to the lungs. The larynx represents and controls a large proportion of total airway resistance, protects the lungs from aspiration, performs fine-tuned movements to facilitate articulation and is crucial for effective cough and secretion clearance. The vocal folds are under both autonomic and voluntary control.22 23 During exercise, the vocal folds and the arytenoids move to a relatively fixed abducted position, both in inspiration and during expiration. Simultaneously, the epiglottis flattens against the tongue base on inspiration, and thereby stretching the aryepiglottic folds. These mechanisms optimise and facilitate airflow by increasing the cross-sectional area of the laryngeal aperture.22 23
Abnormal laryngeal function during exercise
EILO is a form of inducible laryngeal obstruction (ILO) (Exercise + ILO = EILO), that causes breathing problems during exercise, in the context of normal laryngeal anatomy and function at rest.24 This condition, previously described as vocal cord dysfunction (VCD) and/or paradoxical vocal fold motion, was redefined in 2013 by a consensus task force commissioned by the European Respiratory Society, the European Laryngological Society and the American College of Chest Physicians.24 Obstruction can occur at the glottic and/or supraglottic level, and may happen separately, in parallel or sequentially. In fact, most often it is the supraglottic and not the glottic or vocal cord region structures that contribute to closure.8 25 Supraglottic obstruction typically takes the form of abnormal inward prolapse of other laryngeal structures, such as the arytenoid cartilages, or redundant intra-arytenoid tissue or epiglottic tissue.26 27
Prevalence of EILO in athletes and adolescents
In general, the prevalence of EILO in populations across age, sex, ethnic background and athletic level is poorly described, although data suggest prevalence in certain populations that is comparable to that of asthma and/or EIB. Available data are limited in quantity and limited by varying diagnostic criteria and referral bias, with most studies lacking the robust confirmation of the diagnosis with a continuous laryngoscopy during high-intensity exercise (CLE) test28 (see table 1).
In the most extensive study in Olympic-level athletes, the prevalence of EILO was 5%.29 In this study, the diagnosis was based on clinical assessments of stridor without laryngoscopy (an approach that may underestimate prevalence). In addition half of the athletes with stridor had asthma. In another cohort study of elite cross-country skiers without respiratory symptoms, 89 skiers completed a CLE test and 27% had supraglottic EILO—none had glottic EILO.6 Asthma was present in 38%, of whom 29% had EILO.6 In this study, the exercise test applied was considered valid if the participants achieved 90% of maximum heart rate, which may not have been sufficient to trigger EILO in susceptible individuals,25 and therefore EILO may have been underestimated.
In athlete populations presenting with respiratory complaints, the prevalence of EILO is likely to be higher. In the most rigorous study, CLE tests were performed in a group of athletes referred for exercise-induced symptoms. In this study, the prevalence of EILO was 35% and 14% and had coexisting asthma.4 In a retrospective chart review of university athletes presenting with exertional respiratory complaints, postexertional laryngoscopy (among other tests) identified EILO in 70% of athletes, and 37% had coexisting asthma.30 In a cross-sectional study of 352 athletes who underwent EVH challenge during flexible laryngoscopy, 41 (12%) were suspected of having EILO.31 EILO was verified in all 41 athletes, using a surrogate challenge that did not reproduce respiratory distress.31 In 37 healthy rowers without respiratory complaints, EIB was diagnosed in 27% and EILO was diagnosed in 43%. In this study, the diagnosis of EILO was based on inspiratory flow volume loops (a method now considered highly imprecise).32 Finally, in a study examining active duty military patients with exertional dyspnoea where laryngoscopy was performed before and after exercise, EILO was present in 12%.5 In three studies, continuous laryngoscopy during exercise (CLE) tests were performed in adolescents in general and the reported prevalence of EILO was 5%–8%.1–3
In summary, the best available evidence appears to indicate that EILO causes respiratory symptoms in approximately 5%–10% of adolescents and young adults, with even higher rates in athletes reporting respiratory symptoms.
Risk factors associated with EILO in athletes
The cause of EILO is still unknown, however several risk factors are likely to be relevant. Specifically, asthma, gastro-oesophageal reflux disease (GERD),33 nasal disease,34 anatomic factors related to the upper airway, behavioural health factors and genetic factors35 have been suggested as both a cause or a risk factor. Psychological characteristics, such as a heightened level of anxiety, have been suggested as modifiers for EILO in athletes, perhaps by contributing to aggravate the attacks and the severity of dyspnoea. Although the relationship between responses to situational stresses during exercise and airway behaviour remain to be studied, in subjects with confirmed EILO, recent data suggest that underlying baseline anxiety does not seem more common than in a control population.36
There is evidence that asthma, EIB and EILO can co-exist in a considerable number of patients.4 37 Moreover, recently published clinical studies have pointed out that EILO may be an integrated part of the asthma paradigm.16 37 In a study conducted over 30 years ago (1991) it was first documented that the normal laryngeal response to exercise was altered in asthma, both by absence of the expected increase of the expiratory dimension, and by less consistent inspiratory responses.38 As EILO and lower airway dysfunction may coexist,2 30 it is possible that patients with EILO also react to cold air by the same mechanisms,39 40 with anecdotal reports that symptoms of EILO worsen in cold and humid air28 as well as during outdoor activity when compared with indoor activity.29
Although it is theorised that GERD increases the risk of EILO, treating EILO patients with with proton-pump inhibitors has not proven effective in reducing symptoms.41
Interactions between the high airflow observed in elite sport and anatomic factors may contribute or cause disease. As EILO seems to be more prevalent in athletes,4 15 it is possible that the mechanical forces on the airway induced by high airflow states lead to the development of EILO. An anatomic contributor to EILO may also explain the observed female predominance2 4 in pubertal years and beyond as females have, on average, smaller upper airways than males.1 Anatomical differences in airway dimensions may also be relevant and explain why this is a condition that most commonly develops in the peripubertal age group. Further work is needed to determine the factors explaining an apparently heightened prevalence of EILO in young female athletes and how this relates to airway dimension, pressure changes and development of upper airway turbulence during vigorous exercise.
Plausible modes of inheritance have been suggested,35 42 but no definite proof of a hereditary component exists.
Many publications have emphasised a psychological component of EILO.33 43 To date, an over-representation of psychiatric disease or personality disorders has not been documented in patients with isolated EILO.44 The only possible psychological component that may be associated with EILO is a ‘high achiever’ type of personality.33 44 However, this personality type is not uncommon in athletes in general, and this association might represent confounding interactions. The risk factors and causal mechanisms that are implicated in the development of EILO represent an area for future research.
Diagnosis of EILO
Clinical diagnosis of EILO
The typical patient with EILO does not report symptoms at rest. Some may find it difficult to attribute their exertional respiratory symptoms to a specific phase of the respiratory cycle. Typically, symptoms are reported to occur during the inspiratory phase of the respiratory cycle.45 However, complaints of dyspnoea on expiration have also been reported.46
Patients generally present with complaints of various combinations of other symptoms including throat tightness, choking sensations, upper chest tightness and sometimes chest pain, noisy breathing, stridor, hoarseness, changes of the voice, cough and panic reactions.17 Symptoms typical of EILO may not be specific to EILO and can also be associated with other diseases such as asthma, structural airway abnormalities, cardiac diseases, vascular anomalies, tumours/cancer, primary hyperventilation/breathing pattern disorders, poor breathing techniques, low physical fitness and psychological causes. Often athletes will report that breathing simply feels ‘harder’. This has recently been substantiated by findings from a study evaluating intrathoracic pressure changes and diaphragm indicating that the development of EILO is indeed associated with commensurate changes in the work of breathing.47
Symptom descriptions may also vary between individuals, depending on their prior experiences, and factors relating to personal ambitions and lifestyle.48 Caution should therefore be exercised in making diagnostic decisions based on symptoms alone. However, the clinical experience of the authors suggests that video recordings of characteristic field symptoms can complement the history and physical examination to make a clinical diagnosis of EILO.
Differentiating between asthma/EIB and EILO in athletes
Bronchial obstruction and asthma are topics beyond the scope of this review, but need to be considered for two important reasons: (1) health professionals, athletes, trainers and support teams often confuse asthma/EIB and EILO49; (2) asthma/EIB and EILO may coexist in the same athlete thus adding to diagnostic confusion but also driving a tendency to treatment failure.4 37 If EILO is incorrectly diagnosed as asthma/EIB, in part due to a lack of consideration of the diagnosis initially, this may lead to several consequences: over-prescription of asthma medication and subsequent side effects and unwarranted applications for therapeutic use exemptions.50 51 Moreover, there is a theoretic risk of performance decrements (with subsequent changes in motivation and willingness to participate in elite competitive sport). An understanding of key clinical features that distinguish EILO from asthma and/or EIB may optimise the use of healthcare resources available to athletes and lead to a more rapid and robust diagnosis of EILO.
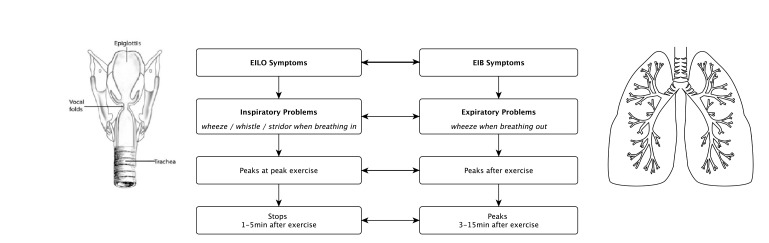
Typically, symptoms of EILO develop when the requirement for ventilation is at its greatest (ie, when an athlete is working near the peak of their exercise intensity). Symptoms are usually most evident during the inspiration phase of respiration and may be associated with stridor (ie, wheeze/whistle sound when breathing in). Generally, symptoms resolve within 2–3 min of exercise cessation unless there is coexistent and ongoing hyperventilation.52 Patients with asthma and/or EIB on the other hand can generally manage reasonably well during ongoing exercise, experience mainly expiratory breathing difficulties and the wheeze most often peaks 3–15 min after exercise cessation. For this reason, abnormal lung function changes to establish a diagnosis of asthma and/or EIB are typically measured in the postexercise phase40 (figure 1).
Figure 1.
Difference between symptoms of EILO and symptoms of EIB. EIB, exercise-induced bronchoconstriction; EILO, exercise-induced laryngeal obstruction.
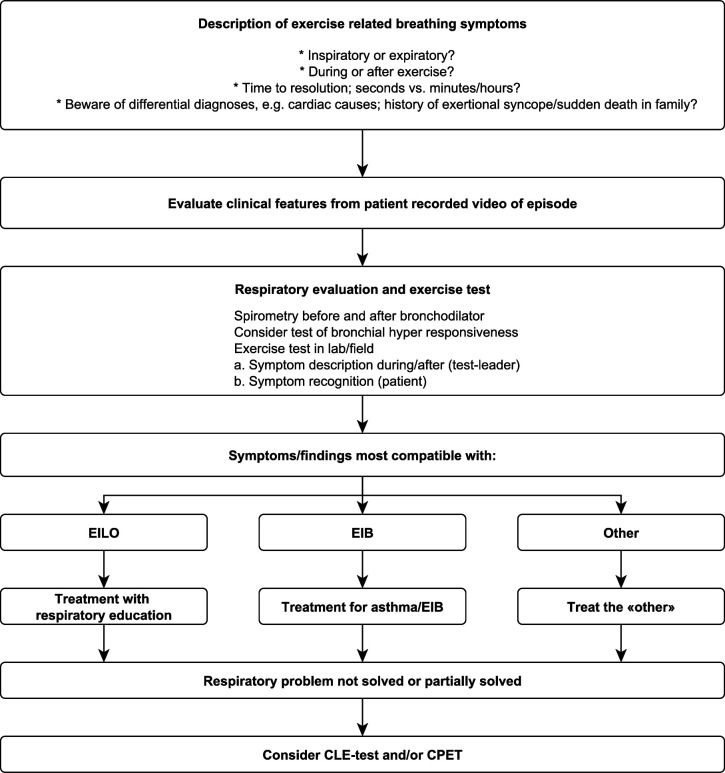
Because it may be difficult to distinguish EILO from EIB, a diagnostic respiratory evaluation of an athlete with exertional dyspnoea needs to include spirometry before and after a bronchodilator, as a minimum. Preferably, it should also include a bronchial hyper-responsiveness test and exercise test (figure 2). However, two tests that both involve exercising to exhaustion and/or tests of AHR on the same day is not recommended.
Figure 2.
Diagnostic evaluation and treatment algorithm for athletes with exertional dyspnoea: when to do the CLE test. CLE, continuous laryngoscopy exercise; CPET, cardiopulmonary exercise test; EIB, exercise-induced bronchoconstriction; EILO, exercise-induced laryngeal obstruction.
Continuous laryngoscopy exercise (CLE) testing to diagnose EILO
Although a variety of diagnostic methods have been used in the assessment of EILO in the past few decades, the continuous laryngoscopy during high-intensity exercise (CLE) test is recognised as the ‘gold standard’ for the diagnosis of EILO.12 The test involves placement of a flexible video laryngoscope to allow real-time visualisation of the larynx throughout a complete exercise session. It requires a comprehensive laboratory set-up, skilled personnel and experience in evaluating the results. The video recordings from a flexible laryngoscope safely allow for close inspection of findings both during intense exercise and after the test, evaluation of findings by more than one person and the recordings ensure later diagnostic considerations. Moreover, video recordings are important in the therapeutic biofeedback setting, showing patients what takes place in their larynx when they experience symptoms. CLE testing allows direct biofeedback to the athlete and is vital in the work up for potential surgical intervention. Continuous placement and recording of laryngeal appearance is favoured over ‘before and after’ exercise laryngoscopic visualisation, given the fact that CLE allows focused evaluation of the severity of laryngeal closure at the precise time an athlete is most symptomatic. A combined CLE and cardiopulmonary exercise test permits concurrent assessment of maximal oxygen uptake,53 cardiac performance and other causes of exertional dyspnoea but is not considered vital for the diagnosis of EILO. Cardiopulmonary exercise data obtained from a CLE setting seem to be comparable to data obtained from an ordinary cardiopulmonary exercise test.54 Thus, performing a cardiopulmonary exercise test (CPET) with a CLE set-up in patients with unexplained exertional breathing problems, where laryngeal obstruction might be involved, allows for simultaneous assessment of other conditions in the differential diagnostic reflections.
Exercise protocols used for CLE testing may vary and ideally should use a sport-specific protocol55–57 to achieve a peak work capacity and high level of ventilation. EILO typically occurs at the highest levels of ventilation, and testing should therefore involve exercising to complete exhaustion or to intolerable symptoms. Treadmill running, ergometer cycling, rowing, swimming or stair climbing have all been used in a diagnostic setting to reproduce real-life symptoms of EILO.55–60 Ideally, the mode and the intensity of the exercise should be tailored to the individual patient, based on triggers identified from their medical history, nicely described in rowers 56 and in swimmers.55 In a laboratory setting one must standardise and compromise, and at a minimum ensure that the exercise goes on to complete exhaustion or to intolerable symptoms. In most young people treadmill exercise is better than exercising on a bicycle to achieve this aim.61 62 The CLE test has been used in clinical practices and multiple research studies and provides recorded images detailing the temporal changes of laryngeal obstruction, as it occurs during ongoing exercise.25 The CLE test has helped establish the fact that EILO commonly arises from supraglottic (ie, aryepiglottic fold) obstruction, and that in most cases this is followed by a glottic fold adduction (ie, vocal cord), whereas a pure glottic obstruction is less common.17 The CLE method has been applied in several studies of symptomatic individuals, as well as in symptom-negative controls,1 2 4 17 63 but the intertest reliability and validity remain to be established.
Alternative diagnostic approaches for EILO are not effective
The CLE test may not be possible in all healthcare settings, and therefore other methods for detecting EILO have been advanced. Although early literature suggests that resting flow-volume loop analysis might be helpful,33 approaches aiming to diagnose EILO at rest lack face validity in a condition that, by definition, does not feature airway obstruction at rest. A lack of correlation between resting spirometry and CLE findings has also been confirmed empirically.64 65 Exercise tidal flow-volume loops have not been extensively studied, but may prove useful in terms of demonstrating an attenuated inspiratory flow.66 Bronchial provocation tests (other than EIB tests) have been found to have limited value in EILO diagnostics, both when performed with and without a laryngoscopy in place.64 67 Eucapnic voluntary hyperpnoea (EVH) can induce glottic as well as supraglottic obstruction.66 67 Laryngoscopy performed during an eucapnic hyperpnoea test (EVH) would provide hyperventilation without exercising and could conceivably represent a diagnostic shortcut in the evaluation of EILO. This has been tested with diverging conclusions.31 67 However, physiological breathing responses that naturally evolve during exercise will not appear during EVH. It seems reasonable that symptoms that are experienced while exercising are best addressed by a test that also involves exercise, and the position of EVH in the diagnostic hierarchy for EILO has not been settled.
Thus, if the history and the clinical impression is not clear, a CLE test, a procedure which can be accomplished in collaboration between exercise labs and interested endoscopists, must be performed to definitively diagnose EILO. Moreover, direct visualisation is required to further characterise EILO by glottic and supraglottic subtype, which is important for treatment (figure 2).
Characterising and assessing the severity of EILO
The relative (compared with resting conditions) extent of laryngeal adduction during a CLE test can be quantified by means of scoring systems, of which four have been published: (1) estimation of the laryngeal anterior–posterior diameter or the anterior glottic angle68; (2) a computerised calculation method that counts pixels from still frame images (the EILOMEA method)69; (3) a machine learning approach based on convolutional neural networks that analyses laryngoscopic videos70; and (4) the CLE scoring system.71 72
The CLE scoring system is most widely used and is a subjective semiquantitative evaluation of obstruction with scores ranging from 0 (complete patency) to 3 (almost complete closure) at glottic and supraglottic level at moderate and at maximum intensity (figure 3). Studies addressing its validity have reached variable conclusions,72 73 but despite challenges related to linking symptoms and visualised findings, it remains the most employed scoring system to classify severity.
Figure 3.
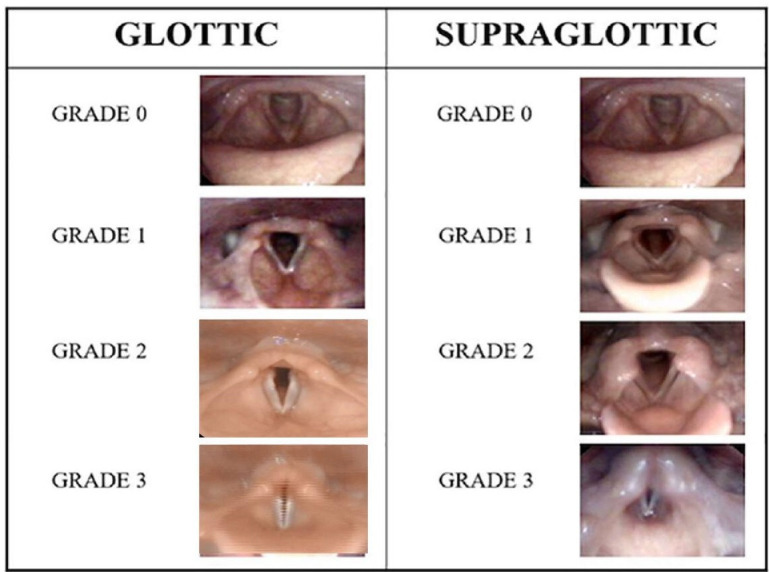
Continuous laryngoscopy exercise grading system. Reproduced with permission from Fretheim Kelly et al 89 Frontiers in Physiology.
Defining EILO from threshold CLE scores has proved challenging.73 74 Typically, moderate obstruction defined by a CLE score ≥2 at the glottic or supraglottic level is considered diagnostic.69 72 75 Recent advances in translaryngeal pressure measurements may guide diagnostic and therapeutic decision-making in the future.74 In terms of monitoring the severity of previously-diagnosed EILO over time and in response to interventions in clinics and clinical trials, a patient-reported outcome measure is available.76
Treatment strategies for EILO
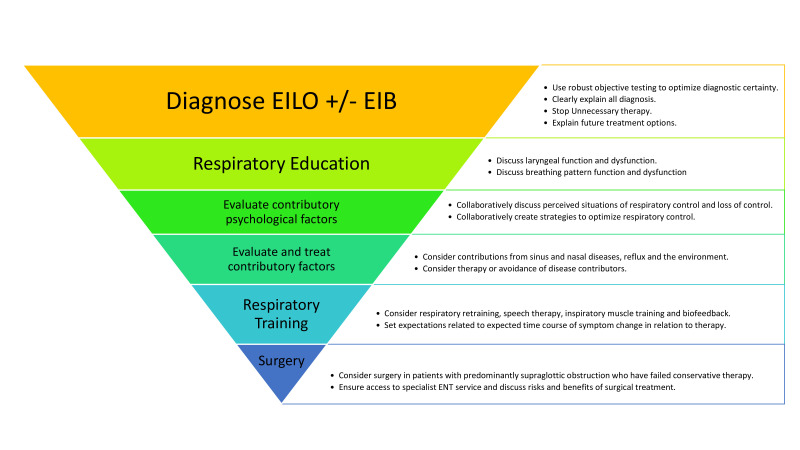
Currently, there is a lack of well-designed randomised controlled studies on treatment strategies for EILO. Treatment strategies include the following: (1) pharmacological treatment, (2) non-pharmacological treatment (biofeedback,63 speech therapy77 78/laryngeal control therapy,78 inspiratory muscle training (IMT)27 79 and (3) surgical treatment (see figure 4). Most treatment strategies are based on anecdotal success and single-centre intervention descriptions that often use non-validated or non-standardised disease strata, interventions and outcome measures. Variable availability of some of the treatment tools may complicate an optimal therapeutic approach to some athletes with challenging EILO.
Figure 4.
Suggested treatment strategies for athletes with EILO. EIB, exercise-induced bronchoconstriction; EILO, exercise-induced laryngeal obstruction.
Pharmacological treatment for EILO
A few pharmacological therapies for EILO have been proposed in the literature. Some authors have proposed the use of medication to minimise effects from contributors to upper airway irritation, including the use of nasal steroids and medications to reduce gastro-oesophageal reflux. Other authors have proposed medication aimed at intervening in neural pathways, including the use of inhaled anticholinergics, oral tricyclic antidepressants, and injections with botulinum toxin.80 To date, no convincing effect has been shown for any pharmacological treatment of EILO.
Non-pharmacological therapies commonly used for EILO
The aim of any non-pharmacological treatment approach to EILO is to provide patients with a strategy on how to develop better control of the larynx during exercise and enable patients to continue exercising without experiencing EILO. Conservative treatment strategies include simple breathing advice, various approaches to speech therapy,77 78 biofeedback,63 IMT27 79 and laryngeal control therapy.78
Breathing advice and biofeedback
Information and breathing advice are based on the notion that patients who are properly informed and practice breathing exercises under skilled supervision, will be placed in a position to control their laryngeal function (online supplemental table 2A). Biofeedback involves respiratory education provided during laryngoscopy, to make the patient conscious of how to open the larynx by watching it happen simultaneously on the screen. The technique involves an element of biofeedback if patients are made aware of the extent to which they succeed in abducting their larynx while performing the breathing exercises. It must be emphasised that any new breathing technique must be repeated until it becomes adapted as a part of an automated pattern, which is a process that requires time, endurance and motivation. Author anecdotes suggests that athletes with EILO both appreciate and may clinically respond to breathing advice.
Inspiratory muscle training
IMT has traditionally focused on strengthening inspiratory respiratory muscles (diaphragm and accessory respiratory muscles) to increase maximal inspiratory pressure and/or maximal voluntary ventilation. It has been proposed that increased inspiratory strength from IMT may enable patients with EILO to generate enough inspiratory force to overcome their laryngeal airflow obstruction.81 However, this might be harmful in some subtypes of EILO, as observed in a case report describing a patient whose supraglottic EILO worsened after IMT.27 Thus, it has been proposed that low inspiratory resistance should be preferred when training with IMT to target laryngeal coordination rather than strengthening inspiratory muscles.82 Findings from studies utilising IMT to treat EILO report positive effects, especially on glottic adduction.79 81 83
Speech/laryngeal control therapy
Speech and language therapy generally includes educating the patient about EILO, relaxation training, instruction on paced exercise and techniques to optimise laryngeal function during exercise. Moreover, there are various techniques aiming to regulate inspiratory flow84 targeting abdominal/diaphragmatic breathing, relaxation of tension in the larynx, and a posture that promotes better breathing techniques. While no placebo/ controlled trials have been published, studies reporting on speech and language therapy are promising.78
Surgical treatment for EILO
Surgical treatment of EILO has been reported by several research groups. There is general agreement that surgery should be limited to patients with moderate or severe obstruction caused by the supraglottic structures. These individuals should also be highly motivated to improve symptoms that significantly interfere with quality of life and athletic performance.85 Surgical treatment methods have not been standardised. Follow-up studies generally report favourable outcomes; however, none of the studies have applied a randomised approach or reported symptoms across a lifespan.7 86 87 There are two published reports of complications, one reporting excessive scarring, and one reporting transient reduced vocal fold motion following the procedure,87 but neither reported problems with aspiration. A recent systematic review of 11 observational studies including a total of 75 patients concluded that the level of evidence precludes definitive recommendations for or against surgery, and that prospective and methodologically robust studies are needed.88
Prevention of EILO
Currently, there are no evidence-based prevention strategies for EILO. However, in our experience, athletes and young individuals, who have a history of EILO over prolonged periods of time (years), are more difficult to treat successfully than those who seek advice early (months). Athletes with breathing problems should therefore be provided with easy access to skilled professionals and a competent approach to diagnostic work up. Treatment should be guided by the images from a CLE test, as the two major EILO subtypes (glottic and supraglottic obstruction) probably require different treatment approaches. Surgery must not be performed without clear evidence of supraglottic EILO from a CLE test.
Prognosis of EILO
Despite adequate diagnosis, athletes may have limited access to therapeutic interventions for EILO. Several studies from the general population have highlighted that if EILO is left untreated or undertreated, people tend to alter lifestyle and reduce their level of physical activity.7 86 There have not been studies which have quantified performance decrements in elite athletes with untreated EILO.
Summary and conclusions
This review highlights the importance of EILO in athletes, showing that it is common, may mimic lower airway dysfunction (ie, asthma, EIB, AHR) and represent a barrier to peak performance in athletes. EILO has not been adequately researched, and there are many knowledge gaps and questions for the sports medicine community. Most importantly, is to better understand the mechanism(s) central to the disease process. There is also a need to understand the overall impact of the condition in diverse populations as the North American and Northern European experience may not reflect the global experience. In areas with limited access to CLE-tests, it will be important to develop surrogate testing methods to benefit athletic populations. To optimise therapy, there is a need to develop metrics for quantifying the disease impact within individuals and to advance therapeutic modalities which target the underlying mechanism of disease. Ideally, improved awareness of EILO will stimulate research in all these areas in the coming years.
EILO is a common and important condition with significant impact in athletes, especially if symptoms are erroneously diagnosed and treated as asthma/EIB. This review emphasises that EILO is clinically distinct from asthma/EIB, can be identified in many patients by observing exertional stridor (mainly inspiratory) during high-intensity exercise and definitively diagnosed via the CLE test. Mechanistic insights and therapeutic trials reliant on reproducible outcomes should evolve from an expanding evidence base. Nevertheless, observational studies suggest that treatment is feasible, and patterns are currently emerging on how best to utilise various therapeutic approaches for different EILO subtypes.
What is already known?
Exercise-induced laryngeal obstruction (EILO) is a transient upper airway obstruction that can cause exertional dyspnoea.
EILO is increasingly recognised as an important cause of exertional breathing problems.
EILO particularly affects physically active young individuals.
What are the new findings?
EILO is common in athletes and must be distinguished from other respiratory conditions to avoid inappropriate diagnosis and treatment as lower airway dysfunction (ie, asthma, exercise-induced bronchoconstriction, airway hyper-responsiveness or other conditions).
The ‘gold standard’ to diagnose and categorise EILO in athletes is by means of continuous laryngoscopy performed during high-intensity exercise.
Subtypes of EILO in athletes (supraglottic and glottic) are described and may require different therapeutic approaches.
New algorithms for evaluation and treatment of EILO in athletes are presented, based on expert opinion and best available evidence.
Randomised controlled trials are urgently needed to provide an evidence-based approach to treatment of EILO.
Footnotes
Twitter: @Exercise_ilo
Contributors: This article was conceptualised by Subgroup 6 of the IOC Consensus on 'Acute Respiratory Illness in the Athlete'. HHC, NS and JTO performed the literature search and selected articles for review. HHC drafted the review and critically revised the work. All authors in Subgroup 6 critically revised the work and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Johansson H, Norlander K, Berglund L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 2015;70:57–63. 10.1136/thoraxjnl-2014-205738 [DOI] [PubMed] [Google Scholar]
- 2. Christensen PM, Thomsen SF, Rasmussen N, et al. Exercise-Induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol 2011;268:1313–9. 10.1007/s00405-011-1612-0 [DOI] [PubMed] [Google Scholar]
- 3. Ersson K, Mallmin E, Malinovschi A, et al. Prevalence of exercise-induced bronchoconstriction and laryngeal obstruction in adolescent athletes. Pediatr Pulmonol 2020;55:3509–16. 10.1002/ppul.25104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013;45:2030–5. 10.1249/MSS.0b013e318298b19a [DOI] [PubMed] [Google Scholar]
- 5. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest 1999;116:1676–82. 10.1378/chest.116.6.1676 [DOI] [PubMed] [Google Scholar]
- 6. Irewall T, Bäcklund C, Nordang L, et al. High prevalence of exercise-induced laryngeal obstruction in a cohort of elite Cross-country skiers. Med Sci Sports Exerc 2021;53:1134–41. 10.1249/MSS.0000000000002581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norlander K, Johansson H, Jansson C, et al. Surgical treatment is effective in severe cases of exercise-induced laryngeal obstruction: a follow-up study. Acta Otolaryngol 2015;135:1152–9. 10.3109/00016489.2015.1062548 [DOI] [PubMed] [Google Scholar]
- 8. Walsted ES, Famokunwa B, Andersen L, et al. Characteristics and impact of exercise-induced laryngeal obstruction: an international perspective. ERJ Open Res 2021;7:00195–2021. 10.1183/23120541.00195-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonini M, Gramiccioni C, Fioretti D, et al. Asthma, allergy and the Olympics: a 12-year survey in elite athletes. Curr Opin Allergy Clin Immunol 2015;15:184–92. 10.1097/ACI.0000000000000149 [DOI] [PubMed] [Google Scholar]
- 10. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee's consensus Conference, Lausanne, Switzerland, January 22-24, 2008. J Allergy Clin Immunol 2008;122:254–60. 10.1016/j.jaci.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 11. Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013;187:1016–27. 10.1164/rccm.201303-0437ST [DOI] [PubMed] [Google Scholar]
- 12. Halvorsen T, Walsted ES, Bucca C, et al. Inducible laryngeal obstruction: an official joint European respiratory Society and European Laryngological Society statement. Eur Respir J 2017;50. 10.1183/13993003.02221-2016. [Epub ahead of print: 09 09 2017]. [DOI] [PubMed] [Google Scholar]
- 13. Ansley L, Kippelen P, Dickinson J, et al. Misdiagnosis of exercise-induced bronchoconstriction in professional soccer players. Allergy 2012;67:390–5. 10.1111/j.1398-9995.2011.02762.x [DOI] [PubMed] [Google Scholar]
- 14. Hull JH, Hull PJ, Parsons JP, et al. Approach to the diagnosis and management of suspected exercise-induced bronchoconstriction by primary care physicians. BMC Pulm Med 2009;9:29. 10.1186/1471-2466-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buchvald F, Phillipsen LD, Hjuler T, et al. Exercise-Induced inspiratory symptoms in school children. Pediatr Pulmonol 2016;51:1200–5. 10.1002/ppul.23530 [DOI] [PubMed] [Google Scholar]
- 16. Hull JH, Walsted ES, Pavitt MJ, et al. High prevalence of laryngeal obstruction during exercise in severe asthma. Am J Respir Crit Care Med 2019;199:538–42. 10.1164/rccm.201809-1734LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Røksund OD, Maat RC, Heimdal JH, et al. Exercise induced dyspnea in the young. larynx as the bottleneck of the airways. Respir Med 2009;103:1911–8. 10.1016/j.rmed.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 18. Hull JH, Godbout K, Boulet L-P. Exercise-associated dyspnea and stridor: thinking beyond asthma. J Allergy Clin Immunol Pract 2020;8:2202–8. 10.1016/j.jaip.2020.01.057 [DOI] [PubMed] [Google Scholar]
- 19. Parsons JP, Kaeding C, Phillips G, et al. Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med Sci Sports Exerc 2007;39:1487–92. 10.1249/mss.0b013e3180986e45 [DOI] [PubMed] [Google Scholar]
- 20. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343–9. 10.1067/mpd.2002.125729 [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petcu LG, Sasaki CT. Laryngeal anatomy and physiology. Clin Chest Med 1991;12:415–23. 10.1016/S0272-5231(21)00793-0 [DOI] [PubMed] [Google Scholar]
- 23. Bartlett D. Respiratory functions of the larynx. Physiol Rev 1989;69:33–57. 10.1152/physrev.1989.69.1.33 [DOI] [PubMed] [Google Scholar]
- 24. Christensen PM, Heimdal J-H, Christopher KL, et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions. Eur Respir Rev 2015;24:445–50. 10.1183/16000617.00006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olin JT, Clary MS, Fan EM, et al. Continuous laryngoscopy quantitates laryngeal behaviour in exercise and recovery. Eur Respir J 2016;48:1192–200. 10.1183/13993003.00160-2016 [DOI] [PubMed] [Google Scholar]
- 26. Halvorsen T, Clemm HSH, Vollsæter M, et al. Conundrums of Exercise-related breathing problems. epiglottic, laryngeal, or bronchial obstruction? Am J Respir Crit Care Med 2020;202:e142–3. 10.1164/rccm.201910-1921IM [DOI] [PubMed] [Google Scholar]
- 27. Clemm HSH, Sandnes A, Vollsæter M, et al. The heterogeneity of exercise-induced laryngeal obstruction. Am J Respir Crit Care Med 2018;197:1068–9. 10.1164/rccm.201708-1646IM [DOI] [PubMed] [Google Scholar]
- 28. Halvorsen T, Walsted ES, Bucca C, Bucca E.S.;, Bush C.;, et al. Inducible laryngeal obstruction: an official joint European respiratory Society and European Laryngological Society statement. Eur Respir J 2017;50:50:1602221. 10.1183/13993003.02221-2016 [DOI] [PubMed] [Google Scholar]
- 29. Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest 2003;123:468–74. 10.1378/chest.123.2.468 [DOI] [PubMed] [Google Scholar]
- 30. Hanks CD, Parsons J, Benninger C, et al. Etiology of dyspnea in elite and recreational athletes. Phys Sportsmed 2012;40:28–33. 10.3810/psm.2012.05.1962 [DOI] [PubMed] [Google Scholar]
- 31. Turmel J, Gagnon S, Bernier M, et al. Eucapnic voluntary hyperpnoea and exercise-induced vocal cord dysfunction. BMJ Open Sport Exerc Med 2015;1:e000065. 10.1136/bmjsem-2015-000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heffler E, Bonini M, Brussino L, et al. Vitamin D deficiency and exercise-induced laryngospasm in young competitive rowers. Appl Physiol Nutr Metab 2016;41:735–40. 10.1139/apnm-2015-0517 [DOI] [PubMed] [Google Scholar]
- 33. McFadden ER, Zawadski DK. Vocal cord dysfunction masquerading as exercise-induced asthma. a physiologic cause for "choking" during athletic activities. Am J Respir Crit Care Med 1996;153:942–7. 10.1164/ajrccm.153.3.8630577 [DOI] [PubMed] [Google Scholar]
- 34. Peters EJ, Hatley TK, Crater SE, et al. Sinus computed tomography scan and markers of inflammation in vocal cord dysfunction and asthma. Ann Allergy Asthma Immunol 2003;90:316–22. 10.1016/S1081-1206(10)61800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsted ES, Hvedstrup J, Eiberg H, et al. Heredity of supraglottic exercise-induced laryngeal obstruction. Eur Respir J 2017;50. 10.1183/13993003.00423-2017. [Epub ahead of print: 17 08 2017]. [DOI] [PubMed] [Google Scholar]
- 36. Benestad MR, Drageset J, Clemm H, et al. Self-Reported health in adolescents with exercise-induced laryngeal obstruction; a cross-sectional study. Front Pediatr 2021;9:617759. 10.3389/fped.2021.617759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Low K, Lau KK, Holmes P, et al. Abnormal vocal cord function in difficult-to-treat asthma. Am J Respir Crit Care Med 2011;184:50–6. 10.1164/rccm.201010-1604OC [DOI] [PubMed] [Google Scholar]
- 38. Hurbis CG, Schild JA. Laryngeal changes during exercise and exercise-induced asthma. Ann Otol Rhinol Laryngol 1991;100:34–7. 10.1177/000348949110000106 [DOI] [PubMed] [Google Scholar]
- 39. Stensrud T, Berntsen S, Carlsen K-H. Humidity influences exercise capacity in subjects with exercise-induced bronchoconstriction (EIB). Respir Med 2006;100:1633–41. 10.1016/j.rmed.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 40. Stensrud T, Mykland KV, Gabrielsen K, et al. Bronchial hyperresponsiveness in skiers: field test versus methacholine provocation? Med Sci Sports Exerc 2007;39:1681–6. 10.1249/mss.0b013e31813738ac [DOI] [PubMed] [Google Scholar]
- 41. Phua S-Y, McGarvey L, Ngu M, et al. The differential effect of gastroesophageal reflux disease on mechanostimulation and chemostimulation of the laryngopharynx. Chest 2010;138:1180–5. 10.1378/chest.09-2387 [DOI] [PubMed] [Google Scholar]
- 42. Hilland M, Røksund OD, Sandvik L, et al. Congenital laryngomalacia is related to exercise-induced laryngeal obstruction in adolescence. Arch Dis Child 2016;101:443–8. 10.1136/archdischild-2015-308450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patterson R, Schatz M, Horton M. Munchausen's stridor: non-organic laryngeal obstruction. Clin Allergy 1974;4:307–10. 10.1111/j.1365-2222.1974.tb01390.x [DOI] [PubMed] [Google Scholar]
- 44. Olin JT, Westhoff Carlson E, Obstruction E-IL. Exercise-Induced laryngeal obstruction and performance psychology: using the mind as a diagnostic and therapeutic target. Immunol Allergy Clin North Am 2018;38:303–15. 10.1016/j.iac.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 45. Røksund OD, Heimdal J-H, Clemm H, et al. Exercise inducible laryngeal obstruction: diagnostics and management. Paediatr Respir Rev 2017;21:86–94. 10.1016/j.prrv.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 46. Newman KB, Mason UG. 3Rd, Schmaling KB. clinical features of vocal cord dysfunction. Am J Respir Crit Care Med 1995;152:1382–6. [DOI] [PubMed] [Google Scholar]
- 47. Walsted ES, Faisal A, Jolley CJ, et al. Increased respiratory neural drive and work of breathing in exercise-induced laryngeal obstruction. J Appl Physiol 2018;124:356–63. 10.1152/japplphysiol.00691.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Depiazzi J, Everard ML. Dysfunctional breathing and reaching one's physiological limit as causes of exercise-induced dyspnoea. Breathe 2016;12:120–9. 10.1183/20734735.007216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hull JH. Not all wheeze is asthma: time for patients to exercise their rights. Thorax 2015;70:7–8. 10.1136/thoraxjnl-2014-206096 [DOI] [PubMed] [Google Scholar]
- 50. WADA . Therapeutic use exemtions, 2019. Available: https://www.wada-ama.org/en/what-we-do/science-medical/therapeutic-use-exemptions
- 51. Hull JH, Pavord ID. Treating asthma exacerbations in athletes: TUE or not TUE? Lancet Respir Med 2018;6:8–10. 10.1016/S2213-2600(17)30428-9 [DOI] [PubMed] [Google Scholar]
- 52. Christopher KL, Morris MJ. Vocal cord dysfunction, Paradoxic vocal fold motion, or laryngomalacia? our understanding requires an interdisciplinary approach. Otolaryngol Clin North Am 2010;43:43–66. 10.1016/j.otc.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 53. Mirza KK, Walsted ES, Backer V. Ergospirometry with concurrent fibre optic laryngoscopy: a randomised crossover study. Eur Clin Respir J 2017;4:1399033. 10.1080/20018525.2017.1399033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Engan M, Hammer IJ, Bekken M, et al. Reliability of maximum oxygen uptake in cardiopulmonary exercise testing with continuous laryngoscopy. ERJ Open Res 2021;7. 10.1183/23120541.00825-2020. [Epub ahead of print: 15 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walsted ES, Swanton LL, van van Someren K, et al. Laryngoscopy during swimming: a novel diagnostic technique to characterize swimming-induced laryngeal obstruction. Laryngoscope 2017;127:2298–301. 10.1002/lary.26532 [DOI] [PubMed] [Google Scholar]
- 56. Panchasara B, Nelson C, Niven R, et al. Lesson of the month: Rowing-induced laryngeal obstruction: a novel cause of exertional dyspnoea: characterised by direct laryngoscopy. Thorax 2015;70:95–7. 10.1136/thoraxjnl-2014-205773 [DOI] [PubMed] [Google Scholar]
- 57. Engan M, Hammer IJ, Stensrud T, et al. Changes in pulmonary function and feasibility of portable continuous laryngoscopy during maximal uphill running. BMJ Open Sport Exerc Med 2020;6:e000815. 10.1136/bmjsem-2020-000815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bent JP, Miller DA, Kim JW, et al. Pediatric exercise-induced laryngomalacia. Ann Otol Rhinol Laryngol 1996;105:169–75. 10.1177/000348949610500301 [DOI] [PubMed] [Google Scholar]
- 59. Heimdal J-H, Roksund OD, Halvorsen T, et al. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope 2006;116:52–7. 10.1097/01.mlg.0000184528.16229.ba [DOI] [PubMed] [Google Scholar]
- 60. Hull JH, Walsted ES, Orton CM, et al. Feasibility of portable continuous laryngoscopy during exercise testing. ERJ Open Res 2019;5. 10.1183/23120541.00219-2018. [Epub ahead of print: 04 02 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Åea P-O, Performance P. Evaluation of physical performance on the Basos of tests. textbook of work physiology Physiolological bases of exercise. Stockholm: Human Kinetics 2003:237–99. [Google Scholar]
- 62. Tervonen H, Niskanen MM, Sovijärvi AR, et al. Fiberoptic videolaryngoscopy during bicycle ergometry: a diagnostic tool for exercise-induced vocal cord dysfunction. Laryngoscope 2009;119:1776–80. 10.1002/lary.20558 [DOI] [PubMed] [Google Scholar]
- 63. Olin JT, Deardorff EH, Fan EM, et al. Therapeutic laryngoscopy during exercise: a novel non-surgical therapy for refractory EILO. Pediatr Pulmonol 2017;52:813–9. 10.1002/ppul.23634 [DOI] [PubMed] [Google Scholar]
- 64. Walsted ES, Hull JH, Sverrild A, et al. Bronchial provocation testing does not detect exercise-induced laryngeal obstruction. J Asthma 2017;54:77–83. 10.1080/02770903.2016.1195843 [DOI] [PubMed] [Google Scholar]
- 65. Christensen PM, Maltbæk N, Jørgensen IM, et al. Can flow-volume loops be used to diagnose exercise induced laryngeal obstructions? A comparison study examining the accuracy and inter-rater agreement of flow volume loops as a diagnostic tool. Prim Care Respir J 2013;22:306–11. 10.4104/pcrj.2013.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pianosi PT, Orbelo DM, Cofer SA. Observational study of laryngoscopy plus flow-volume loops during exercise. Clin Case Rep 2018;6:735–40. 10.1002/ccr3.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Christensen PM, Rasmussen N. Eucapnic voluntary hyperventilation in diagnosing exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol 2013;270:3107–13. 10.1007/s00405-013-2571-4 [DOI] [PubMed] [Google Scholar]
- 68. Jain S, Bandi V, Officer T, et al. Role of vocal cord function and dysfunction in patients presenting with symptoms of acute asthma exacerbation. J Asthma 2006;43:207–12. 10.1080/02770900600566892 [DOI] [PubMed] [Google Scholar]
- 69. Christensen P, Thomsen SF, Rasmussen N, et al. Exercise-Induced laryngeal obstructions objectively assessed using EILOMEA. Eur Arch Otorhinolaryngol 2010;267:401–7. 10.1007/s00405-009-1113-6 [DOI] [PubMed] [Google Scholar]
- 70. Lin J, Walsted ES, Backer V, et al. Quantification and analysis of laryngeal closure from endoscopic Videos. IEEE Trans Biomed Eng 2019;66:1127–36. 10.1109/TBME.2018.2867636 [DOI] [PubMed] [Google Scholar]
- 71. Norlander K, Christensen PM, Maat RC, et al. Comparison between two assessment methods for exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol 2016;273:425–30. 10.1007/s00405-015-3758-7 [DOI] [PubMed] [Google Scholar]
- 72. Maat RC, Røksund OD, Halvorsen T, et al. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol 2009;266:1929–36. 10.1007/s00405-009-1030-8 [DOI] [PubMed] [Google Scholar]
- 73. Walsted ES, Hull JH, Hvedstrup J, et al. Validity and reliability of grade scoring in the diagnosis of exercise-induced laryngeal obstruction. ERJ Open Res 2017;3. 10.1183/23120541.00070-2017. [Epub ahead of print: 28 07 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fretheim-Kelly Z, Halvorsen T, Heimdal J-H, et al. Feasibility and tolerability of measuring translaryngeal pressure during exercise. Laryngoscope 2019;129:2748–53. 10.1002/lary.27846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Norlander K, Johansson H, Emtner M, et al. Differences in laryngeal movements during exercise in healthy and dyspnoeic adolescents. Int J Pediatr Otorhinolaryngol 2020;129:109765. 10.1016/j.ijporl.2019.109765 [DOI] [PubMed] [Google Scholar]
- 76. Olin JT, Shaffer M, Nauman E, et al. Development and validation of the exercise-induced laryngeal obstruction dyspnea index (EILODI). J Allergy Clin Immunol 2021. 10.1016/j.jaci.2021.09.027. [Epub ahead of print: 04 Oct 2021]. [DOI] [PubMed] [Google Scholar]
- 77. De Guzman V, Ballif CL, Maurer R, et al. Validation of the dyspnea index in adolescents with exercise-induced paradoxical vocal fold motion. JAMA Otolaryngol Head Neck Surg 2014;140:823–8. 10.1001/jamaoto.2014.1405 [DOI] [PubMed] [Google Scholar]
- 78. Shaffer M, Litts JK, Nauman E, et al. Speech-Language pathology as a primary treatment for exercise-induced laryngeal obstruction. Immunol Allergy Clin North Am 2018;38:293–302. 10.1016/j.iac.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 79. Sandnes A, Andersen T, Clemm HH, et al. Exercise-Induced laryngeal obstruction in athletes treated with inspiratory muscle training. BMJ Open Sport Exerc Med 2019;5:e000436. 10.1136/bmjsem-2018-000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marcinow AM, Thompson J, Chiang T, et al. Paradoxical vocal fold motion disorder in the elite athlete: experience at a large division I university. Laryngoscope 2014;124:1425–30. 10.1002/lary.24486 [DOI] [PubMed] [Google Scholar]
- 81. Mathers-Schmidt BA, Brilla LR. Inspiratory muscle training in exercise-induced paradoxical vocal fold motion. J Voice 2005;19:635–44. 10.1016/j.jvoice.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 82. Sandnes A, Andersen T, Hilland M, et al. Laryngeal movements during inspiratory muscle training in healthy subjects. J Voice 2013;27:448–53. 10.1016/j.jvoice.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 83. Dickinson J, Whyte G, McConnell A. Inspiratory muscle training: a simple cost-effective treatment for inspiratory stridor. Br J Sports Med 2007;41:694–5. 10.1136/bjsm.2006.033654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Johnston KL, Bradford H, Hodges H, et al. The Olin EILOBI breathing techniques: description and initial case series of novel respiratory retraining strategies for athletes with exercise-induced laryngeal obstruction. J Voice 2018;32:698–704. 10.1016/j.jvoice.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 85. Maat RC, Roksund OD, Olofsson J, et al. Surgical treatment of exercise-induced laryngeal dysfunction. Eur Arch Otorhinolaryngol 2007;264:401–7. 10.1007/s00405-006-0216-6 [DOI] [PubMed] [Google Scholar]
- 86. Maat RC, Hilland M, Røksund OD, et al. Exercise-Induced laryngeal obstruction: natural history and effect of surgical treatment. Eur Arch Otorhinolaryngol 2011;268:1485–92. 10.1007/s00405-011-1656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sandnes A, Hilland M, Vollsæter M, et al. Severe exercise-induced laryngeal obstruction treated with Supraglottoplasty. Front Surg 2019;6:44. 10.3389/fsurg.2019.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Siewers K, Backer V, Walsted ES. A systematic review of surgical treatment for supraglottic exercise-induced laryngeal obstruction. Laryngoscope Investig Otolaryngol 2019;4:227–33. 10.1002/lio2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fretheim-Kelly ZL, Halvorsen T, Clemm H, et al. Exercise induced laryngeal obstruction in humans and equines. A comparative review. Front Physiol 2019;10:1333. 10.3389/fphys.2019.01333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2021-104704supp001.pdf (435KB, pdf)