Abstract
Conceptual alignment is a prerequisite for mutual understanding. However, little is known about the neurophysiological brain‐to‐brain underpinning during conceptual alignment for mutual understanding. Here, we recorded multi‐channel electroencephalogram (EEG) simultaneously from two participants in Experiment 1 and adopted the dual‐tACS techniques in Experiment 2 to investigate the underlying brain‐to‐brain EEG coupling during conceptual alignment and the possible enhancement effect. Our results showed that 1) higher phase‐locking value (PLV), a sensitive measure for quantifying neural coupling strength between EEG signals, at the gamma frequency band (28–40 Hz), was observed in the left temporoparietal site (left TP) area between successful versus unsuccessful conceptual alignment. The left TP gamma coupling strength correlated with the accuracy of conceptual alignment and differentiated whether subjects belonged to the SUCCESS or FAILURE groups in our study. 2) In‐phase gamma‐band transcranial alternating current stimulation (tACS) over the left TP area increased the accuracy of subjects in the SUCCESS group but not the FAILURE group. 3) The effect of perspective‐taking on the accuracy was mediated by the gamma coupling strength within the left TP area. Our results support the role of gamma‐band coupling between brains for interpersonal conceptual alignment. We provide dynamic interpersonal neurophysiological insights into the formation of successful communication.
Keywords: conceptual alignment, dual‐tACS, EEG‐based hyperscanning, perspective‐taking
Combining revised coordination semiotic games, dual‐EEG and dual‐tACS techniques, we observed higher phase‐locking value (PLV), a sensitive measure for quantifying neural coupling strength between EEG signals, at the gamma frequency band (28–40Hz), in the left temporoparietal site (left TP) area for successful versus unsuccessful conceptual alignment. In‐phase gamma‐band transcranial alternating current stimulation (tACS) over the left TP area increased the accuracy of subjects in the SUCCESS group but not the FAILURE group. The effect of perspective‐taking on the accuracy was mediated by the gamma coupling strength within the left TP area.
1. INTRODUCTION
Interpersonal conceptual alignment is the basis for successful communication and mutual understanding (Stolk, Verhagen, & Toni, 2016). For mutual understanding to occur, interactors should possess common mental states, including shared signal interpretation (Liu et al., 2019), and recognize their interactors' communicative inclination (Kampe, Frith, & Frith, 2003). However, little is known about how multiple individuals manage to converge on conceptual alignment (Wheatley, Boncz, Toni, & Stolk, 2019).
To investigate the processes during the formation of conceptual alignment, some researchers would adopt paradigms that requested interlocutors to create a shared communication system. The paradigms are conducive to uncovering the underlying neural mechanism during the establishment of conceptual space. Noordzij et al. (2009) adopted a Tacit Communication Game (TCG) in which a sender and a receiver aimed to move the token to a pre‐assigned location. The sender was instructed to control his symbol to convey information about the target's location, which was blind to the receiver. The interlocutors would eventually establish a shared representation of symbols to complete the task. Results showed that planning new communication actions by the sender and recognizing the communicative intention of the same actions by the receiver activated the same brain regions in the right posterior superior temporal sulcus. Using the same paradigm, Stolk et al. (2013) also found that the overlapping brain regions lateralized to the right hemisphere in the gamma‐band when the sender planned and the receiver observed. These studies revealed the relationship between shared neural responses and establishing a shared communication system.
Previous studies in a more ecological setting have demonstrated that higher interpersonal neural synchronization (INS) was observed during interpersonal verbal communication (Ahn et al., 2018; Jiang et al., 2012, 2015), following the “Two‐Person Neuroscience” (2PN) approach (Hari, Henriksson, Malinen, & Parkkonen, 2015). For instance, it was found that INS increased in the left inferior frontal cortex (IFC) during face‐to‐face dialog (Jiang et al., 2012) and in the left temporoparietal junction (TPJ) during group discussion (Jiang et al., 2015). Hirsch, Adam Noah, Zhang, Dravida, and Ono (2018) confirmed that INS could be enhanced in the left superior temporal gyrus (STG) during interactive verbal communication. Ahn et al. (2018) also discovered higher INS in the left temporal and frontal regions during verbal interaction at the gamma‐band.
The INS between interactors could reflect the shared neural representation, and it could be contributed by a different type of shared neural representation regarding different types of shared external stimuli or shared internal mental state. Though external shared acoustic stimuli could contribute to INS during dynamic verbal communication, the INS during dynamic communication may still be elicited due to a shared internal state, for example, conceptual alignment. Conceptual alignment could reduce the possibility of prediction error, thus benefiting the interactive communication (Friston & Frith, 2015). It has been suggested that the shared neural representation among individuals, in the form of inter‐subject correlation (ISC) in brain activity, could operate the successful communication (Schoot, Hagoort, & Segaert, 2016). Evidence also suggested that shared neural representation occurred when individuals had the same understanding and interpretation of the same scenario, such as viewing videos (Hasson, Nir, Levy, Fuhrmann, & Malach, 2004) and interpreting vague narratives (Nguyen, Vanderwal, & Hasson, 2019). The shared neural representation related to the conceptual alignment is stimulus modality‐independent (Zadbood, Chen, Leong, Norman, & Hasson, 2017) and language‐independent (Honey, Thompson, Lerner, & Hasson, 2012), which indicates that it is elicited by interpretation rather than the form of stimuli, and this shared neural representation could be the neural basis of conceptual alignment. As for interactive communication, speaker‐listener neural coupling only occurred when the listener could understand the speaker, and higher neural coupling correlated with a better understanding (Stephens, Silbert, & Hasson, 2010).
Hence, INS, as the shared neural representation between a pair of interactors, could be a potential neurobiological marker of conceptual alignment. We hypothesized that those who could successfully reach conceptual alignment would have a higher INS than those who failed. In Experiment 1 (dual‐EEG experiment), we adopted a revised version of the semiotic game, which required participants to reach the conceptual alignment to understand their interactors successfully. The revised coordination semiotic game enabled participants to develop a shared symbol‐concept mapping system and achieve interpersonal conceptual alignment during real‐time nonverbal interaction. Based on their cooperation performance in the task (see details in the Methods section), we categorized participants into groups that successfully (SUCCESS group) or unsuccessfully (FAILURE group) generated conceptual alignment. We firstly adopted the Dual‐EEG techniques to uncover the underlying interpersonal neural synchronization and the dynamic change.
In addition to the interpersonal neural mechanism underlying the formation of conceptual alignment, we are also interested in how the personality of the interactors benefits the formation of conceptual alignment. Behavioral studies have found that the ability of theory of mind is engaged in the initial stage of communication by helping interlocutors to identify the communicative intentions of each other (Kampe et al., 2003) and establish communication (Achim, Fossard, Couture, & Achim, 2015). Therefore, we also investigated the role of the theory of mind in conceptual alignment formation. We explored how the individual differences (i.e., perspective‐taking) in the social interaction interacted with the whole process. We hypothesized that those pairs with higher averaged perspective‐taking abilities could perform better and elicit higher INS during interactive nonverbal communication.
Recently, Novembre and Iannetti (2020) suggested that mutibrain stimulation, by manipulating the INS, can further prove the causality or the enhancement effect of INS on human social interaction. Previous research has adopted dual‐tACS in examining the effect of INS on interactive learning (Pan, Novembre, Song, Zhu, & Hu, 2021) and joint action (Novembre, Knoblich, Dunne, & Keller, 2017). However, none of the previous studies tested this relationship between INS and mutual‐understanding by manipulating the INS to our best knowledge. Here, in Experiment 2 (dual‐tACS experiment), we explored if enhanced brain‐to‐brain coupling could improve the establishment of conceptual alignment. Participants were required to complete the same task as Experiment 1 while receiving SHAM or IN‐PHASE tACS. We hypothesized that the dual‐tACS stimulation could enhance task performance based on the research mentioned above.
2. MATERIALS AND METHODS
2.1. Participants
We recruited 136 participants in total. Two same‐sex strangers were formed as a group randomly, resulting in 68 pairs. In experiment 1 (Dual‐EEG Experiment), 82 healthy participants (60 females and 22 males, aged 21.0 ± 2.4 years, mean ± SD) were recruited to take part in this study. Two pairs of participants were excluded because of inappropriate behaviors (i.e., talking or moving) and technical issues during EEG recording. All other participants completed the task. Based on participants' performance (details in the task section), they were divided into SUCCESS group (20 pairs) and FAILURE group (19 pairs) and offered additional monetary compensations accordingly. EEG brainwaves were acquired from both members of each pair simultaneously. In experiment 2 (Dual‐tACS Experiment), we recruited 54 healthy participants (42 females and 12 males, aged 20.6 ± 1.9 years, mean ± SD) and divided them into 27 pairs. The dyads underwent IN‐PHASE tACS (13 pairs) or SHAM tACS (14 pairs, details in the dual‐tACS section). Based on their performance, they were also divided into SUCCESS group (13 pairs) or FAILURE group (14 pairs) and offered additional monetary compensations accordingly. All participants had no history of neurological or psychiatric disorders and had a normal or corrected‐to‐normal vision. Written informed consent was obtained from all participants. The University Committee on Human Research Protection of East China Normal University approved all experimental procedures for this study (HR079‐2017 and HR021‐2019).
2.2. Task
Participants were required to complete the revised coordination semiotic games (see Figure 1 for details on the experimental setup). The coordination semiotic game (Galantucci, 2005, 2009; Galantucci & Garrod, 2011; Scott‐Phillips & Kirby, 2010) asks individuals to cooperatively achieve the same goal and communicate via an unusual means (i.e., visual graphical medium). In the current task, similar to the previous coordination semiotic game, we would ask participants to use a novel medium to communicate and cooperate in a task. Nevertheless, we revised the paradigm by asking participants to use initially meaningless symbols to communicate, which helped us better quantify the change of conceptual alignment with progress.
FIGURE 1.
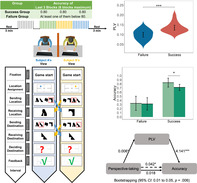
The experimental setup. (a) Participants were required to complete 60 trials maximumly, and every ten trials were counted as a block. If the accuracy of three consecutive blocks reached 0.80 within six blocks, the pair of participants would be labeled as a SUCCESS group and were able to quit the game. Otherwise, they would be labeled as a FAILURE group and could quit the game after finishing all 60 trials. (b) Revised coordination semiotic games: each dyad of participants should move their tokens from their assigned location to the same destination. To achieve mutual understanding, they should use previously meaningless symbols to represent a specific location and indicate their origin and destination. After sending the symbol representing their initial locations or destinations, they would receive the message from their partner. After deciding their destination, they would receive feedback according to their performance. A screen blocked participants in the lab to avoid receiving information through other modalities. (c) Regions of interest (ROIs) of Experiment 1, presented by BrainNet Viewer (Xia et al. 2013). We divided electrodes into six ROIs, including the frontal site, frontal‐central site, left temporoparietal site, right temporoparietal site, parietal site, and occipital site. (d) tACS setting of Experiment 2. (e) Participants in Experiment 2 were required to complete 60 trials while undergoing a 30‐min electrical stimulation. (f) The dual‐tACS setting of Experiment 2
Participants were required to convey their messages to each other only via sending a set of initially meaningless symbols. Each trial in the task constituted a single run of non‐verbal communicative interaction using keyboards. Initially, each participant was assigned and presented a virtual token on the screen. The virtual token was randomly placed in a random location within a four‐square grid. The shared goal of both participants was to relocate their tokens into the same grid at the end of each trial (destination). To increase the difficulty of the current task, we required participants to relocate their tokens to a different grid to the initial assigned location. To reach the goal, participants would have to communicate about their initial location and their expected destination by pressing a button on a keyboard to select one of four symbols. Participants were only allowed to use four meaningless symbols to communicate with their partners during the task. Of note, the presented sequence of these four symbols was different across interactors, thus disabling participants from using the sequence of the four symbols to indicate the token's grid. Participants could only have their own views throughout the experiment and were forbidden from using any existing communication medium, including written, spoken, or body language. Feedback was presented at the end of each trial for participants to validate their idea.
In total, each trial would include eight epochs. At the beginning of every interaction, each participant was instructed that the game would start (epoch 1: Fixation, 1,500 ms, Figure 1b). Then they were randomly assigned a start location, which could be the same as their partner's (epoch 2: location assignment, 4,000 ms). After knowing their location, they would choose a meaningless symbol to represent their start location and send it to their partner (epoch 3: sending origin, until response; epoch 4: receiving origin, 3,000 ms) to avoid landing on the same location as their original location, leading to a failure. Next, they needed to choose a symbol to represent their expected destination and send it to their partner (epoch 5: sending destination, until response; epoch 6: receiving destination, 3,000 ms) to direct their partner to a specific location. Finally, they would combine the previous information to decide their destination (epoch 7: deciding destination, until response). If they could successfully travel to the same location, which should not be the start location, they would obtain the feedback that they had won; in the case of failure, they would be given feedback that they had lost (epoch 8: feedback). Participants could take the feedback to validate if they reached a consensus. It was expected that each pair of participants in the SUCCESS group would gradually develop a shared symbol‐location mapping system during the task to understand their partner's intention and win the game. At the end of the experiment, we asked each participant to independently recall their initial and final symbol‐location mapping system. We observed a trend that the average consensus of symbol‐location mapping at the beginning of the task was lower than at the end of the task (M start‐success = 2.0, M start‐failure = 1.5, M end‐success = 3.3, M end‐failure = 2.9).
Each pair of participants finished at least 30 trials and at most 60 trials. Every ten trials would be counted as a mini‐block (Figure 1a). If the accuracy of three consecutive blocks reached 0.80 within six blocks, the pair of participants would be labeled as a SUCCESS pair and could quit the game. Otherwise, they would be labeled as a FAILURE pair and could quit after finishing all 60 trials. Following the experimental criteria, the last three blocks were defined as the post‐formation period, while the other blocks were defined as the pre‐formation period. Because different pairs of participants took different trials to end the task, the trials were equally divided into ten sessions for a more detailed description of dynamic changes of the INS or accuracy. For example, if a pair of participants took 60 trials to complete the experiment, every six trials would be counted as one session.
2.3. EEG data acquisition
EEG data were simultaneously recorded using two separate Geodesic Photogrammetry Systems™ (GPS) with 64 channels (Electrical Geodesic Inc., USA). Four vertical electrodes were placed over both eyes' upper and lower sides. Electrode impedance was kept under 50kΩ for all recordings. EEG data were continuously recorded and digitized at a sampling frequency of 250 Hz. The reference electrode was Cz.
2.4. EEG data processing
Pre‐processing of EEG data was performed using NetStation and custom MATLAB (MathWorks) scripts with EEGLAB toolbox (Delorme & Makeig, 2004) and ADJUST (Mognon, Jovicich, Bruzzone, & Buiatti, 2011). The EEG data were band‐pass filtered between 0.1 and 45 Hz. EEG data then was re‐referenced to the whole brain average. For removing eye‐movement artifacts and muscular artifacts, independent component analysis (ICA) was performed using functions in the EEGLAB. Artifacts were identified by ADJUST automatically and corrected. Corrected EEG data then segmented from the onset of decision‐making instruction till the end of the making decisions. EEG data were grouped according to 6 regions for subsequent analysis: (1) frontal (F, AF4, F2, FP2, Fz, Afz, F1, FP1, AF3, F3, F5, F7, F8, F6, F4), (2) frontal‐central (FC, Fcz, FC1, FC3, C1, C3, C4, C2, FC4, FC2), (3) parietal (P, CP1, P5, P3, P1, Pz, P2, Cp2, P4, P6), (4) left temporoparietal (left TP, FC5, FT7, C5, T7, TP7, CP5, P7), (5) right temporoparietal (right TP, T6‐P8, CP6, TP8, C6, T4‐T8, FT8, FC6), and (6) occipital (O, PO3, O1, Poz, Oz, PO4, O2) sites.
2.5. Dual‐EEG data analysis
The inter‐brain synchrony was estimated using the phase‐locking value (PLV) (Lachaux, Rodriguez, Martinerie, & Varela, 1999). The PLV ranges from 0 to 1, where 0 indicates that the two signals are unsynchronized, and 1 indicates perfect synchronization. We focused on gamma‐band (28–40 Hz), which is primarily proven to be related to language and interactive communication (Stolk et al., 2013). We defined the frequency band as a typical range in previous EEG‐based hyperscanning studies (Mu, Guo, & Han, 2016; Sun et al., 2019). In brief, for any given channels p and q and frequency band, the Hilbert transform was used to obtain the instantaneous phase φ, and the PLV between them is defined as:
PLV was computed for deciding destination epochs extracted from all trials, where T represents the number of trials. φ is the phase, and | | represents the complex modulus. Task‐specific PLV was baseline‐corrected by subtracting pre‐scan (at the beginning of scanning) PLV. Cumulative PLV for n sessions was computed as a sum of the PLV for n sessions. Finally, for two PLVs from the two participants, the two PLV values PLV participant1,participant2 , and PLV participant2,participant1 were averaged and defined as the PLV between participants in this study. We conducted a control analysis to test whether the PLV was task‐related or due to the phase fluctuation. To do so, trial‐based EEG data of each participant in one interactive pair were first shuffled 10,000 times. PLV was calculated with the surrogate EEG data. We next compared the experiment PLV value with the surrogate PLV data.
2.6. Prediction of successful development of conceptual alignment
Because every pair of participants may complete a different number of trials, we divided all trials into ten sessions for every pair of participants separately. Then, PLV and cumulative PLV across this time were calculated. The Linear Discriminant Analysis was conducted at each session, where PLV and cumulative PLV was taken as the classification feature to classify the SUCCESS and FAILURE groups. A leave‐one‐subject‐out cross‐validation method (LOO‐CV) was used to obtain the prediction accuracy. At each time, one sample (PLV of one pair of subjects) was selected as the testing dataset, and the other samples were the training dataset. If there were m samples, we were supposed to train and test m times. Time courses were generated for three indexes for predicting accuracy: sensitivity (percentage of SUCCESS group correctly predicted), specificity (percentage of failure group correctly predicted), and predictive accuracy (overall proportion of SUCCESS and FAILURE groups correctly predicted). We conducted t‐tests to test the difference in prediction accuracy between the SUCCESS and FAILURE groups. Also, we further conducted a permutation test. We shuffled the labels (SUCCESS vs. FAILURE) and calculated the accuracy 10,000 times. If the actual accuracy was higher than the top 5% of the accuracy, we marked them as p < .05.
2.7. Test of ToM
We used the Interpersonal Reactivity Index (IRI) (Davis, 1983) to measure the empathy ability of participants. Four subscales were included: perspective‐taking, empathic concern, personal distress, and fantasy. In our analysis, we calculated the average score of a pair of participants.
2.8. Mediation effect analysis
To test the mediation effect of PLV on the relationship between the mean of perspective‐taking across two participants in a dyad and accuracy, we conducted a mediation analysis with the simple mediation model (Baron & Kenny, 1986; Hayes & Preacher, 2014; Tingley, Yamamoto, Hirose, Keele, & Imai, 2014) as follows:
| (1) |
| (2) |
| (3) |
The model could reveal a causal effect of X (an independent variable) on Y (a dependent variable) by postulating M (a mediation variable). Three steps were involved in the mediation effect analysis. First, regression analysis was conducted with X as a sole independent variable and Y as the dependent variable, estimating a significant coefficient c [1]. Second, a similar regression analysis was conducted only with M as the dependent variable, thus calculating the coefficient a [2]. Finally, we replicated step one, but M was statistically controlled [3]. In our mediation effect analysis, the independent variable X was the mean of perspective‐taking of each pair of participants, the dependent variable Y was the accuracy, and the mediation variable was the PLV. Besides the simple mediation model, a mediation analysis with the bootstrapping method was also conducted.
2.9. Dual‐tACS setting
We collected a separate sample with the dual‐tACS technique to further discover the possible relationship between INS and the formation of conceptual alignment. Two battery‐powered low‐intensity transcranial alternating current stimulators (tES; Soterix Medical Inc., New York, USA) were used for delivering phase‐locked current to the participants. Stimulation electrodes (5 × 7 cm) were secured to the scalp with rubber straps. The anode electrode was placed over the left superior temporal gyrus (equivalent to CP5 in the International 10–20 EEG system), with the cathode electrode placed over the contralateral orbitofrontal cortex (FP2). Stimulation current was 1 mA maximum at a 40 Hz sinusoidal frequency (i.e., gamma‐band). Prior to the actual experiment, all participants were exposed to tACS for approximately 1 min (including ramp‐up and ramp‐down) to ensure that they could tolerate the stimulation intensity; otherwise, the stimulation intensity was reduced. The actual stimulation intensity was 0.82 ± 0.12 mA (mean ± SD). TACS was delivered in the following settings: 1) IN‐PHASE condition: both participants received stimulation with a zero‐phase difference; 2) SHAM condition: both participants only received 30 s fade‐in followed by 30 s fade‐out of stimulation (Pan et al., 2021). Participants were blind to their manipulation. Perception of being shocked between conditions were not significantly different, N IN‐PHASE = 24, N IN‐PHASE = 26, p = .667, df = 1, X 2 = 0.187.
All participants received 30 min of stimulation in total, including 10 min of rest and 20 min of doing tasks. They would continue working on the tasks until they completed the task regardless of being shocked or not in the following time. After the task, participants were asked to complete a questionnaire to assess their awareness of the stimulation condition and determine if there were potential tACS‐related side effects.
3. RESULTS
3.1. Behavioral characteristics during the formation of conceptual alignment
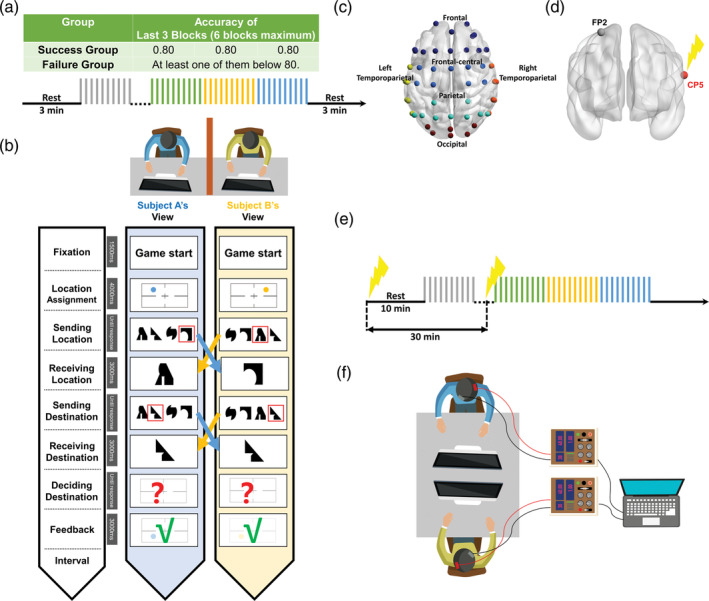
First, a two‐sample t‐test showed that the accuracy of the SUCCESS group was significantly higher than the FAILURE group (p < .001, t [28.37] = 6.19, d = 2.01, two‐tailed, Figure 2a), which indicated that the way we defined SUCCESS and FAILURE groups was appropriate. A two‐way repeated‐measures ANOVA analysis found an interactive effect of period (pre vs. post‐formation) and group (SUCCESS vs. FAILURE, p = .005, F [1,35] = 9.03, η2 partial = .21, Figure 2b). Specifically, accuracy of post‐formation was significantly higher than pre‐formation period for both groups (SUCCESS: p < .001, t (35) = 9.97; FAILURE: p < .001, t (35) = 5.94). The accuracy of the SUCCESS group was signficantly higher than the FAILURE group for the post‐formation period only (pre‐formation: p = .055, t (35) = 2.66; post‐formation: p < .001, t (35) = 5.31). These results showed a significant difference in the accuracy between the SUCCESS group and the FAILURE group during the dynamic change. To characterize the change in accuracy in more detail, we divided all trials into ten sessions for each pair of participants. A series of two‐sample t‐tests were conducted for each session with False Discovery Rate (FDR) correction. The significant differences appeared as early as the second session and lasted until the end of the task (ps < .05, Figure 2c). Then, all ten sessions were divided into three phases based on the slope calculated by regression (see Table S1). Two‐way repeated‐measures ANOVA analysis found a significant interactive effect of phase and group (p = .031, F [1.72, 61.94] = 3.90, η2 partial = .10, Figure 2d), which indicated the difference between the SUCCESS group and FAILURE groups in terms of accuracy. Next, a two‐sample t‐test found that the reaction time across all trials while deciding on the destination of the SUCCESS group was significantly shorter than that of the FAILURE group (p = .006, t [36] = 2.90, d = 0.94, Figure 2e). The significant difference in reaction time between groups started from the fourth session till the ninth session (ps < .05, Figure 2f).
FIGURE 2.
Behavioral results. (a) Accuracy of the SUCCESS group and FAILURE group. (b) Accuracy of different groups (SUCCESS vs. FAILURE) in different periods (pre‐formation vs. post‐formation). (c) Accuracy of different groups (SUCCESS vs. FAILURE) in different sessions. The green line at the bottom of the figure indicates three phases. (d) Accuracy of different groups (SUCCESS vs. FAILURE) in different phases. (e) Reaction times during deciding the destination of different groups (SUCCESS vs. FAILURE). (f) Reaction times of different groups (SUCCESS vs. FAILURE) in different sessions. Shade indicates ±1 SE. Error bar indicates 95% CI. (* p < .05; ** p < .01; *** p < .001)
3.2. INS at the gamma band during the formation of conceptual alignment
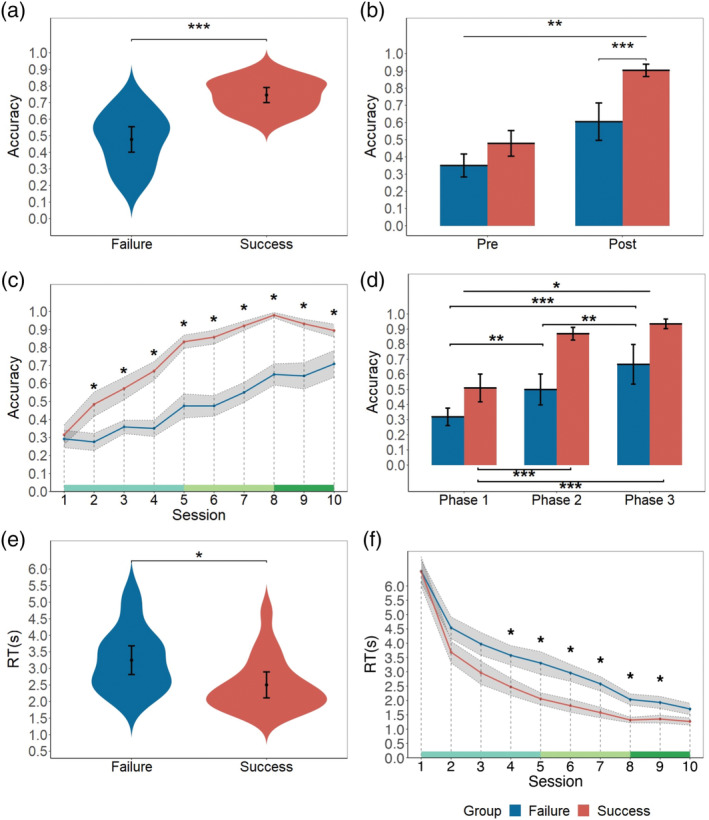
During the formation of conceptual alignment, INS was measured using task‐related PLV (see the Methods section). A series of two‐sample t‐tests were conducted to examine significant differences for the PLVs between SUCCESS and FAILURE groups. The result found significant PLV in the occipital site (p = .013, t [29.77] = 3.36, d = 1.06, Bonferroni corrected) and the left temporal site (left TP, p < .001, t [36.99] = 4.31, d = 1.38, bonferroni corrected, Figure 3a) at the gamma‐band. After controlling the normalized absolute difference of reaction times between participants, the significant differences of the PLVs between SUCCESS and FAILURE groups still existed in the left TP (p < .001, F [1, 35] = 24.01) and the occipital site (p < .001, F [1, 35] = 13.01), which suggested that the enhanced PLV of the SUCCESS group was not driven by the reduced difference of reaction times. The control analysis, comparing the surrogated PLV and the experiment PLV found significantly higher PLV in the SUCCESS group, p = .010, t (41.72) = 2.70, d = 0.72, but significantly lower PLV in the FAILURE group, p = .043, t (40.15) = 2.08, d = 0.56. Previous research has widely proved that the left TP is related to language‐based interaction (Hickok & Poeppel, 2007; Jiang et al., 2012; Jiang et al., 2015). Therefore, our further analysis would focus on the PLV in the left TP. The task‐related PLV in the left TP was significantly higher than baseline (p < .001, t [38] = 23.98). Additionally, we used PLVs to predict accuracy at the group level in linear mixed model analyses (lmerTest package version 3.1‐0, Kuznetsova, Brockhoff, & Christensen, 2017). We found that PLV in left TP at gamma‐band (p = .008, b = 2.31, SE = 0.82, t [36.93] = 2.81, Figure 3b) could significantly predict accuracy. Moreover, two‐way repeated‐measures ANOVA analysis found no significant interactive effects of trial type (winning vs. losing) and group (SUCCESS vs. FAILURE) in PLV in left TP at gamma‐band (p = .092, F [1, 36] = 2.99, η2 partial = 0.03, Figure 3c). The main effect of the trial type was significant (p < .001, F [1, 36] = 52.93). We further found significant difference between SUCCESS and FAILURE groups for the winning trials (p < .001, t [1,147.1] = 4.10, d = 0.25), but not for the losing trials (t [421.26] = 1.78, p = .076, d = 0.14). Next, we separated all trials into two‐parts (pre‐ vs. post‐formation) and found that PLV in the post‐formation part was significantly higher than in the pre‐learning part (p < .001, t (36) = 9.59, d = 1.56, Figure 3d). No significant interactive effect of group and parts was found (p = .105, F [1, 35] = 2.77). These results confirmed the relationship between INS and the alignment in the task, thus further highlighting the link between INS and the formation of conceptual alignment.
FIGURE 3.
Interpersonal neural synchronization results. (a) PLV of different groups (SUCCESS vs. FAILURE groups) in the left TP at gamma‐band. (b) Correlation between PLV in the left TP at gamma‐band and accuracy. (c) PLV in the left TP at gamma‐band of different groups (SUCCESS vs. FAILURE groups) and different trials (winning vs. losing trials). (d) PLV in the left TP at gamma‐band of different groups (SUCCESS vs. FAILURE groups) in different periods (pre‐ vs. post‐formation). Error bar indicates 95% CI. (* p < .05; ** p < .01; *** p < .001)
3.3. The time‐course of PLV change
Next, we aimed to discover the time course for the formation of conceptual alignment by analyzing the time course of PLV changes. A series of two‐sample t‐tests were conducted for each session with FDR corrections for all sessions. Significant differences (ps < .05) of the PLV in left TP at gamma‐band (Figure 4a) between groups at the fourth and eighth session were found. Additionally, significant differences (ps < .05, FDR) of the cumulative PLV in the left TP at the gamma‐band (Figure 4b) since the fourth session was found. We then clustered all ten sessions into three phases based on the slope of accuracy calculated by regression as done previously. We found significant main effect of group (p < .001, F [1, 37] = 18.49, η2 partial = 0.24), significant main effect of phase (p < .001, F [1.79, 66.18] = 74.32, η2 partial = 0.42), and significant interactive effect of phase and group (p = .041, F [1.79, 66.18] = 3.49, η2 partial = 0.03, Figure 4c). To unwrapped the interation, we conducted the three‐way ANOVA for the SUCCESS group and FAILURE group separtely. We found that th main effect of phase still existed for both SUCCESS (p < .001, F[1.82, 34.56] = 46.89, η2 partial = 0.47) and FAILURE group (p < .001, F[1.63, 29.30] = 31.10, η2 partial = 0.38). Further post‐hoc analysis found that, for the SUCCESS group, PLV in Phase 1 was signficantly lower than both in Phase 2, p < .001, t (19) = 8.25, and in Phase 3, p < .001, t (19) = 9.12. However, PLV in Phase 2 was not signicantly different from in Phase 3, p = .110, t (19) = 2.13. For the FAILURE group, PLV in Phase 1 was signficantly lower than both in Phase 2, p = .002, t (18) = 4.07, and in Phase 3, p < .001, t (18) = 4.79, and PLV in Phase 2 was signficantly lower than in Phase 3, p < .001, t (18) = 6.53.
FIGURE 4.
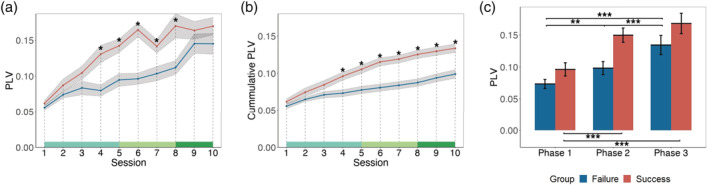
The time course of the PLV change. The time course of (a) PLV change and (b) cumulative PLV change in the left TP at gamma‐band. The green line at the bottom indicates three phases. (c) The PLV of different groups and phases in the left TP at gamma‐band. Shade indicates ±1 SE. Error bar indicates 95% CI. (* p < .05; ** p < .01; *** p < .001)
3.4. Differentiate groups by INS
Next, Linear Discrimination Analyses were conducted to investigate how early the conceptual alignment was formed. We took PLV and cumulative PLV as discriminators to differentiate the SUCCESS group from the FAILURE group. Followed figure shows the time course of prediction accuracy in the discrimination analysis based on PLV (Figure 5a) or cumulative PLV (Figure 5b) in left TP at gamma‐band (Figure 5b). We then calculated three indexes to evaluate the model: sensitivity, specificity, and general accuracy. We further evaluated the discrimination results based on the confusion matrix and the permutation test (see the Methods section). Results found that the prediction was stably significant, starting from the fourth session to the seventh session for the PLV (ps < .05) and from the third session to the end for the cumulative PLV (ps < .05). These results reinforced the notion of the predictive role of PLV, even in the early stage, in the formation of conceptual alignment.
FIGURE 5.
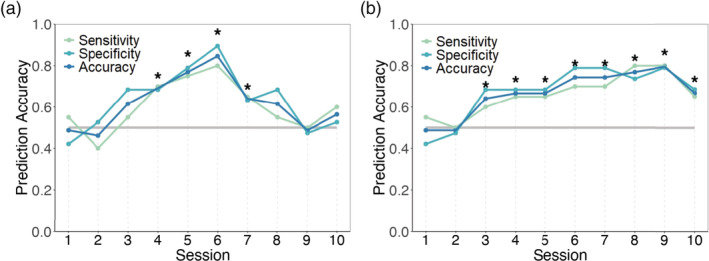
Time course of prediction accuracy. Prediction results based on (a) PLV and (b) cumulative PLV in the left TP at the gamma‐band (* p < .05)
3.5. Modulating the formation of conceptual alignment through in‐Phase tACS
Since INS could be used to discriminate whether the conceptual alignment was established or not, we further investigated if INS could improve the formation of conceptual alignment by adopting dual‐brain stimulation. Next, we investigated the task performance of these two groups. We did not find the difference of the number of people in the SUCCESS group (p = 1, X 2 = 0, df = 1, Figure 6a) between conditions. Although we did not find the significant interaction effect of condition and group (p = .37, F [1,23] = 0.85), two one‐tailed separate t‐tests and Bayesian t‐test confirmed that that the accuracy of the in‐phase condition was significantly higher than sham condition in the SUCCESS group (t [11] =2.25, p = .023, d = 1.25, BF10 = 3.61, Figure 6b) but not in the FAILURE group (t [12] = 0.15, p = .441, d = 0.08, BF10 = 0.492, Figure 6b). Furthermore, by a series of one‐tailed t‐tests, we explored the time course of the cumulative accuracy. We found that the difference between the two conditions in the SUCCESS group started from the initial session (corrected p < .05, Figure 6c), which was not seen in the FAILURE group (corrected p > .05, Figure 6d). According to current results, the IN‐PHASE gamma‐band coupling over left TP improved the formation of conceptual alignment.
FIGURE 6.
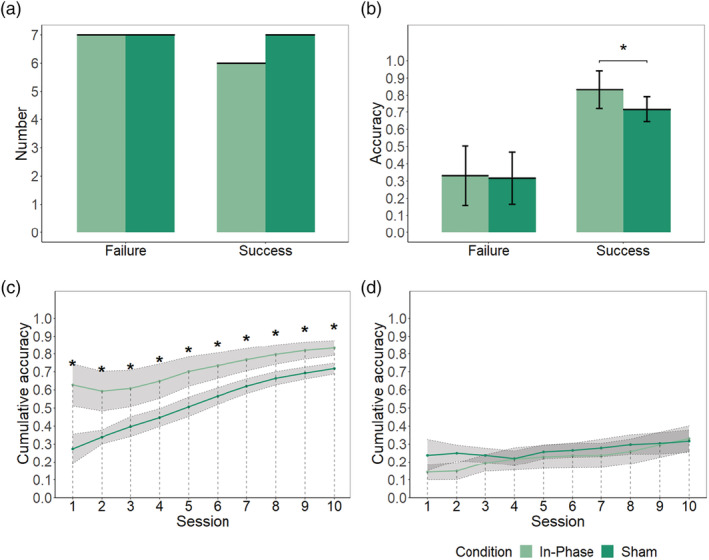
The tACS results. (a) The number of SUCCESS pairs in the in‐phase and sham conditions. (b) The accuracy of different groups in different conditions. (c) The time course of cumulative accuracy of different conditions in the SUCCESS group. (d) The time course of cumulative accuracy of different conditions in the FAILURE group. Shade indicates ±1 SE. Error bar indicates 95% CI
3.6. Role of INS in mediating perspective‐taking for conceptual alignment
Finally, to investigate the role of ToM in the formation of conceptual alignment, two‐sample t‐tests of each subscale between the groups were conducted. The results revealed that only the mean of perspective‐taking of the SUCCESS group was higher than that of the FAILURE group (p < .001, t [26.46] = 4.25, d = 1.37, Figure 7a), after two samples in the SUCCESS group were deleted because their perspective‐taking scores were outliers (mean ± 2SD). A mediation analysis was conducted on the merged data from both groups, with the mean of perspective‐taking as the independent variable, the accuracy as the dependent variable, and PLV of the deciding destination epoch as the mediator. The mediation effect was significant for PLV in left TP at the gamma‐band (95% bootstrapping CI: 0.01 to 0.05, p = .006, Figure 7b).
FIGURE 7.
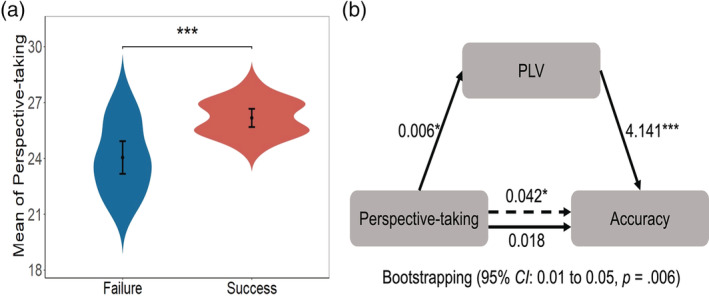
Role of perspective‐taking in the formation of conceptual alignment. (a) Difference of means of perspective‐taking in different groups. (b) PLV mediated the effect of the mean of perspective‐taking on accuracy in the left TP at gamma‐band. (* p < .05; ** p < .01; *** p < .001). Error bar indicates 95% CI
4. DISCUSSION
By combining the revised coordination semiotic game with EEG‐based hyperscanning technique and dual‐tACS, the present study investigated the interpersonal neural mechanisms underlying the achievement of conceptual alignment. Our results demonstrated that higher task‐related PLV in the electrodes located in the left temporoparietal sites at the gamma‐band occurred when interactors could successfully share a conceptual alignment than when they failed to share it. Additionally, the PLV measured in the two groups was significantly correlated with the accuracy. Further analysis revealed that PLV could differentiate the SUCCESS group and the FAILURE group in the early stage. These results indicate that task‐related PLV could be a neural marker for achieving conceptual alignment, even in the initial stage of the interaction.
Moreover, the dual‐tACS study showed higher accuracy in the in‐phase condition than the sham condition for the SUCCESS group, which occurred in the initial stage of interaction. These results disclosed the enhancement effect of brain‐to‐brain coupling on the formation of conceptual alignment. Finally, we found that PLV mediated the effect of perspective‐taking on accuracy. In sum, part of the neural correlates of conceptual alignment was revealed in our study. Additionally, it also provided insight into the role of perspective‐taking in the formation of conceptual alignment.
4.1. PLV in left TP site at the gamma‐band as a neural marker for the formation of conceptual alignment
In our study, a significantly higher PLV occurred at the gamma‐band when interactors could successfully share a conceptual alignment than when they failed to share it. Our task would require a pair of interactors to reach a conceptual alignment to accomplish a shared goal. Hence, the increased state‐specific PLV in the gamma‐band might represent a similar cognitive process with language. The PLV in the gamma‐band also occurred when interactors communicated verbally (Ahn et al., 2018). The gamma‐band coupling indicated that our paradigm could imitate the evolution of communication.
In addition, we detected a significant difference in PLV over the left TP. The left TP was fundamental to language perception and production (Hickok & Poeppel, 2007). Other than the evidence from the single brain research, the previous research, which focused on dyads, has demonstrated that brain‐to‐brain coupling in the left TP occurs when interactors are undergoing verbal communication (Jiang et al., 2012), achieving a mutual understanding (Ahn et al., 2018) and sharing the same understanding (Kampe et al., 2003). During the current task, interactors were compelled to establish conceptual alignment to complete the task successfully. Considering the previous findings of dialogs, the state‐specific PLV in the left TP might represent the shared neural representation of symbols.
Ample research evidence suggested the role of INS in the right TP in social interaction (Cheng, Li, & Hu, 2015; Pan, Cheng, Zhang, Li, & Hu, 2017). Stolk et al. found both the correlation and causal relationship between the right TP and the evolution of the communication system (Stolk et al., 2013, 2014). In contrast to the Tacit Communication Game, which asked participants to convey information by moving chess pieces, the present task asked participants to indicate their intention by using meaningless symbols, which might be one reason why we found neural coupling at the left TP. Ample evidence has suggested the role of neural coupling at the parietal site and the temporoparietal site (Cui, Bryant, & Reiss, 2012; Osaka et al., 2015; Xue, Lu, & Hao, 2018) in social interaction. Considering these findings, the role of neural coupling in different brain regions during the formation of conceptual alignment would be worth future investigation. For example, future research could distinguish the cognitive function of neural coupling at different brain regions at the early stage of formation of conceptual alignment and late stage of social knowledge storage.
4.2. PLV in the early stage can differentiate and predict the outcome of the interaction
In our study, we characterized the change of accuracy, mean of reaction time, PLV, and cumulative PLV during the whole process of formation of conceptual alignment. We found that the PLV and cumulative PLV could differentiate the SUCCESS group from the FAILURE group in the early stage. These results showed that PLV was a valuable and sensitive marker for the formation of conceptual alignment. Furthermore, the Linear Discrimination Analysis showed a similar outcome. Cumulative PLV of the epoch where participants were required to decide their destination could differentiate the SUCCESS and FAILURE groups from the early stage. Some researchers also adopted identical methods and found that INS could predict the outcome of social interaction in the early stage, that is, the emergence of leadership (Jiang et al., 2015) and teaching effectiveness (Liu et al., 2019). Combining these results, we can assume that brain‐to‐brain coupling is a sensitive neural marker for the formation of conceptual alignment. Since PLV could effectively differentiate the SUCCESS and the FAILURE groups, changes of PLV can be used to describe the variation in the formation of conceptual alignment. Since it is hard for researchers to record the change of cognitive and interaction processes by self‐reporting, INS is a convenient neurobiological index to describe the process.
4.3. The enhancement effect of brain‐to‐brain coupling on the formation of conceptual alignment
Furthermore, we investigated the enhancement effect of INS on the establishment of conceptual alignment to provide a glimpse of the causal relationship between the INS and the establishment of conceptual alignment. We adopted the dual‐tACS technique, which has been used to investigate the enhancement and causal relationship between INS and social interaction (Novembre et al., 2017; Szymanski, Müller, Brick, von Oertzen, & Lindenberger, 2017). By performing bilateral brain stimulation at the same frequency and in the same brain region, we were able to manipulate the occurrence of INS. Although we did not find a difference in the number of successful and failed dyads, we found a higher accuracy in the in‐phase condition than the sham condition in the SUCCESS group. Notably, the enhancement effect of INS occurred in the initial stage of the task. Although there is only limited research on the causal relationship between INS and synchronous behaviors by adopting dual‐tACS, our results reinforced the causal relationship between INS and the formation of conceptual alignment. The effect of phase‐coupling of gamma‐band on the formation of conceptual alignment occurred in the initial stage. This result may indicate that the brain‐to‐brain coupling in the left TP at the gamma‐band will prepare a shared concept space for the interactors. By the dual‐EEG and dual‐tACS experiment, we build a bridge between the INS and conceptual alignment.
One critical limitation is that the sample size in the current experiment is small. As the Bayesian analysis is suitable for a small sample (McNeish, 2016), we further conducted Bayesian analysis and reported Bayes Factors (BF10) to present the likelihood that our data support the research hypothesis over the null hypothesis. Based on the criterion (Dienes, 2014), BF10 > 3 would suggest evidence supporting the research hypothesis. Even with limited sample size, our data still favor the alternative hypothesis that the IN‐PHASE tACS stimulation could enhance mutual understanding. It is also worth noticing that the effect size is large.
The IN‐PHASE stimulation increased the accuracy in the SUCCESS group, not in the FAILURE group. However, the IN‐PHASE stimulation did not increase the number of SUCCESS dyads. Taken together, it might indicate that the phase‐coupling of gamma‐band in the left TP was not the only prerequisite for the formation of conceptual alignment. Previous research also suggested that INS at the right hemisphere could be linked with nonverbal communication (Jiang, Zheng, & Lu, 2021; Stolk et al., 2013). The establishment of conceptual alignment could be a combined effect from both regions.
4.4. Effect of perspective‐taking in achieving conceptual alignment
Our study discovered that participants in the SUCCESS group had higher perspective‐taking abilities than those in the FAILURE group. Moreover, the effect of perspective‐taking abilities on the accuracy was mediated by PLV in the left TP in the gamma‐band. Our results emphasized the role of perspective‐taking abilities in forming conceptual alignment and the establishment of better communication.
A high level of perspective‐taking ability, as part of the ToM (Barnes‐Holmes, McHugh, & Barnes‐Holmes, 2004), allows interactors to predict each other's subsequent action, thus enhancing coordination (Konvalinka, Vuust, Roepstorff, & Frith, 2010) and enabling harmonious interpersonal relationships (Davis, 1983). Therefore, during the interaction, participants with high perspective‐taking abilities may be more capable of considering their partner's intention and information than those with low abilities. They may also be more willing to change their own decision and behavior to coordinate with their partners. The perspective‐taking abilities strengthened interactive social learning and thus helped to establish conceptual alignment.
Moreover, our results reinforced the association between PLV in the left TP at gamma‐band and perspective‐taking abilities. The debate about which brain regions sustain our ToM abilities is still ongoing. Research has found that the frontal lobe, right TPJ (Saxe, Moran, Scholz, & Gabrieli, 2006), and left TPJ (Samson, Apperly, Chiavarino, & Humphreys, 2004) may be essential for the ToM abilities. Lesions of the left TPJ can impair the cognitive processes specifically involved in inferring another person's belief, which indicates that the left TPJ may mediate the mental‐state process (Samson et al., 2004; Saxe et al., 2006). In our study, perspective‐taking influenced the formation of conceptual alignment through PLV. Our results revealed the neural mechanisms of the crucial factors in this process.
4.5. Social interactive learning to establish conceptual alignment
One theory regarding how people establish a shared communication system is interactive social learning, which emphasizes the role of social interaction. Our study provided insight into how interactive social learning facilitates the establishment of shared conceptual space (Figure 8). Individuals start with two separate conceptual spaces (different quadrant‐signal mapping), keeping them from effectively communicating. With interactive social learning and multiple feedbacks, individuals should establish a shared conceptual space or a shared communication system (same quadrant‐signal mapping). Our study showed that INS could mark the shared conceptual space at the gamma band in the left TP site.
FIGURE 8.
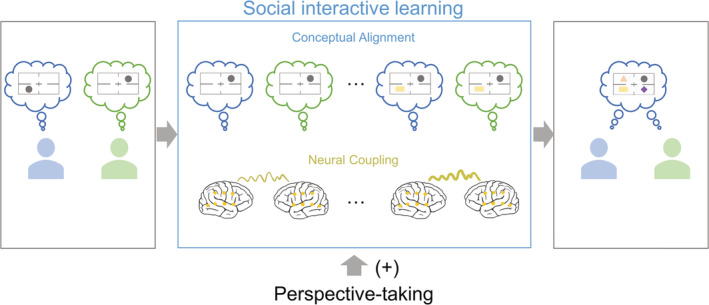
Theoretical illustration of the interpersonal conceptual alignment. Individuals have separate conceptual spaces initially, which can be merged to be a shared conceptual space after interactive social learning. Here we consider the quadrant‐signal mapping as the conceptual alignment. The thin line between the two brains indicates a low INS, while the thicker line indicates a higher INS
4.6. Limitations and future research
Some limitations should be noted. First, there is an imbalance between female and male participants in Experiment 1 and Experiment 2. Previous related research has found that females have higher perspective‐taking ability levels (Eisenberg & Lennon, 1983; Van der Graaff et al., 2014) and show better language ability even from the early year (Bouchard, Trudeau, Sutton, Boudreault, & Deneault, 2009). This evidence suggests a potential difference in performance and INS between female and male participants in the current task. Although it is not the focus of the current research, we still acknowledged that the imbalance between female and male participants might limit our conclusion from generalizing to the whole population. Future research could explore the potential gender differences.
One critical limitation is that our conclusion is based on the SUCCESS versus FAILURE group. However, participants in the FAILURE group are not those who thoroughly could not communicate with each other. Even for the FAILURE group, the averaged accuracy is above 50%, and the post‐task questionnaires indicate that they would have some shared symbol‐location mapping. The current experiment lacks a solid control group in which participants do not have the chance to convey signatures. However, even if participants in the FAILURE group could have some shared understanding, it is still not sufficient to perform the task better. Our analysis could initially explore better mutual understanding versus worse mutual understanding.
Although EEG‐based hyperscanning is a suitable tool in the temporal and frequency domains, it has a limited spatial resolution (Koike, Tanabe, & Sadato, 2015). It keeps us from making strong conclusions about which brain regions are involved in the task. Future studies may utilize magnetoencephalography (MEG) to investigate the coupling of different brain regions with proper temporal and spatial resolutions.
Finally, due to technical issues, we did not involve a tACS control group that two interactors were shocked with different phases, limiting our findings. An alternative explanation for the effect of dual‐tACS is enhanced brain activations of both participants in each pair. A future experiment could involve the anti‐phase control condition to examine INS's enhancement effect. Furthermore, it would be interesting to adopt concurrent dual‐tACS‐EEG to test if the interpersonal neural coupling changed after tACS.
4.7. Conclusions
Despite the limitation, our results add to the understanding of the role of interpersonal neural coupling in conceptual alignment formation. In this study, we demonstrated the brain‐to‐brain coupling underlying the formation of conceptual alignment by combining a revised coordination semiotic game with a newly developed EEG‐based hyperscanning and dual‐tACS approach. First, PLV in the left TP sites at the gamma‐band could be a sensitive neural marker for the successful formation of conceptual alignment. Second, dual‐brain stimulation can enhance the formation of conceptual alignment. Finally, perspective‐taking abilities influenced the formation of conceptual alignment mediated by brain‐to‐brain coupling. Our study provided interpersonal neural evidence for the formation of conceptual alignment and reinforced the role of interactive social learning.
CONFLICT OF INTERESTS
All the co‐authors declare that they have no conflict of interest.
ETHICS APPROVAL
The University Committee on Human Research Protection of East China Normal University approved all experimental procedures for this study (HR079‐2017 and HR021‐2019).
AUTHOR CONTRIBUTIONS
Danni Chen: Conceptualization, Formal Analysis, Investigation, Writing – Original Draft Preparation, Writing – Review & Editing. Ruqian Zhang: Conceptualization, Writing – Original Draft Preparation, Writing – Review & Editing. Jieqiong Liu: Conceptualization, Writing – Original Draft Preparation, Writing – Review & Editing. Pu Wang: Writing – Review & Editing. Litian Bei: Conceptualization, Investigation. Chang‐Chia Liu: Writing – Review & Editing. Xianchun Li: Conceptualization, Funding Acquisition, Supervision, Writing – Review & Editing.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
We would like to thank Dr. Shayan Moosa and Dr. Yan Mu for their insightful comments on earlier drafts, Dr. Yafeng Pan for his valuable assistance in data analysis, Mengdie Liu and Li Feng for their assistance in dual‐tACS data collection. This work was supported by the Shanghai Key Base of Humanities and Social Sciences (Psychology‐2018), the National Natural Science Foundation of China (32071082 and 71942001), Key Specialist Projects of Shanghai Municipal Commission of Health and Family Planning (ZK2015B01), and the Programs Foundation of Shanghai Municipal Commission of Health and Family Planning (201540114).
Chen, D. , Zhang, R. , Liu, J. , Wang, P. , Bei, L. , Liu, C.‐C. , & Li, X. (2022). Gamma‐band neural coupling during conceptual alignment. Human Brain Mapping, 43(9), 2992–3006. 10.1002/hbm.25831
Danni Chen and Ruqian Zhang contributed equally to this study.
Funding information Key Specialist Projects of Shanghai Municipal Commission of Health and Family Planning, Grant/Award Number: ZK2015B01; Shanghai Key Base of Humanities and Social Sciences: Psychology‐2018; The National Natural Science Foundation of China, Grant/Award Numbers: 32071082, 71942001; The Programs Foundation of Shanghai Municipal Commission of Health and Family Planning, Grant/Award Number: 201540114
DATA AVAILABILITY STATEMENT
The data and analysis code in this manuscript are available from the corresponding author upon reasonable request.
REFERENCES
- Achim, A. M. , Fossard, M. , Couture, S. , & Achim, A. (2015). Adjustment of speaker's referential expressions to an addressee's likely knowledge and link with theory of mind abilities. Frontiers in Psychology, 6, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. , Cho, H. , Kwon, M. , Kim, K. , Kwon, H. , Kim, B. S. , … Jun, S. C. (2018). Interbrain phase synchronization during turn‐taking verbal interaction—A hyperscanning study using simultaneous EEG/MEG. Human Brain Mapping, 39(1), 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes‐Holmes, Y. , McHugh, L. , & Barnes‐Holmes, D. (2004). Perspective‐taking and theory of mind: A relational frame account. The Behavior Analyst Today, 5(1), 15–25. 10.1037/h0100133 [DOI] [Google Scholar]
- Baron, R. M. , & Kenny, D. A. (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173. [DOI] [PubMed] [Google Scholar]
- Bouchard, C. , Trudeau, N. , Sutton, A. , Boudreault, M.‐C. , & Deneault, J. (2009). Gender differences in language development in French Canadian children between 8 and 30 months of age. Applied PsychoLinguistics, 30(4), 685–707. 10.1017/S0142716409990075 [DOI] [Google Scholar]
- Cheng, X. , Li, X. , & Hu, Y. (2015). Synchronous brain activity during cooperative exchange depends on gender of partner: A fNIRS‐based hyperscanning study. Human Brain Mapping, 36(6), 2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, X. , Bryant, D. M. , & Reiss, A. L. (2012). NIRS‐based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage, 59(3), 2430–2437. 10.1016/j.neuroimage.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. H. (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–126. https://doi-org.eproxy.lib.hku.hk/10.1037/0022-3514.44.1.113 [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Dienes, Z. (2014). Using Bayes to get the most out of non‐significant results. Frontiers in Psychology, 5, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, N. , & Lennon, R. (1983). Sex differences in empathy and related capacities. Psychological Bulletin, 94(1), 100–131. 10.1037/0033-2909.94.1.100 [DOI] [Google Scholar]
- Friston, K. J. , & Frith, C. D. (2015). Active inference, communication and hermeneutics. Cortex, 68, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci, B. (2005). An experimental study of the emergence of human communication systems. Cognitive Science, 29(5), 737–767. 10.1207/s15516709cog0000_34 [DOI] [PubMed] [Google Scholar]
- Galantucci, B. (2009). Experimental semiotics: A new approach for studying communication as a form of joint action. Topics in Cognitive Science, 1(2), 393–410. 10.1111/j.1756-8765.2009.01027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci B., & Garrod, S. (2011). Experimental semiotics: A review. Frontiers in Human Neuroscience, 5, 10.3389/fnhum.2011.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari, R. , Henriksson, L. , Malinen, S. , & Parkkonen, L. (2015). Centrality of social interaction in human brain function. Neuron, 88(1), 181–193. [DOI] [PubMed] [Google Scholar]
- Hasson, U. , Nir, Y. , Levy, I. , Fuhrmann, G. , & Malach, R. (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303(5664), 1634–1640. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. , & Preacher, K. J. (2014). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67(3), 451–470. [DOI] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Hirsch, J. , Adam Noah, J. , Zhang, X. , Dravida, S. , & Ono, Y. (2018). A cross‐brain neural mechanism for human‐to‐human verbal communication. Social Cognitive and Affective Neuroscience, 13(9), 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J. , Thompson, C. R. , Lerner, Y. , & Hasson, U. (2012). Not lost in translation: Neural responses shared across languages. Journal of Neuroscience, 32(44), 15277–15283. 10.1523/JNEUROSCI.1800-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Chen, C. , Dai, B. , Shi, G. , Ding, G. , Liu, L. , & Lu, C. (2015). Leader emergence through interpersonal neural synchronization. Proceedings of the National Academy of Sciences, 112(14), 4274–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Dai, B. , Peng, D. , Zhu, C. , Liu, L. , & Lu, C. (2012). Neural synchronization during face‐to‐face communication. Journal of Neuroscience, 32(45), 16064–16069. 10.1523/JNEUROSCI.2926-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Zheng, L. , & Lu, C. (2021). A hierarchical model for interpersonal verbal communication. Social Cognitive and Affective Neuroscience, 16(1–2), 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe, K. K. , Frith, C. D. , & Frith, U. (2003). "hey John": Signals conveying communicative intention toward the self activate brain regions associated with "mentalizing," regardless of modality. Journal of Neuroscience, 23(12), 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike, T. , Tanabe, H. C. , & Sadato, N. (2015). Hyperscanning neuroimaging technique to reveal the "two‐in‐one" system in social interactions. Neuroscience Research, 90, 25–32. [DOI] [PubMed] [Google Scholar]
- Konvalinka, I. , Vuust, P. , Roepstorff, A. , & Frith, C. D. (2010). Follow you, follow me: Continuous mutual prediction and adaptation in joint tapping. Quarterly Journal of Experimental Psychology, 63(11), 2220–2230. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P. B., Christensen R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82, (13), 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lachaux, J. , Rodriguez, E. , Martinerie, J. , & Varela, F. J. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8(4), 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Zhang, R. , Geng, B. , Zhang, T. , Yuan, D. , Otani, S. , & Li, X. (2019). Interplay between prior knowledge and communication mode on teaching effectiveness: Interpersonal neural synchronization as a neural marker. NeuroImage, 193, 93–102. [DOI] [PubMed] [Google Scholar]
- McNeish, D. (2016). On using Bayesian methods to address small sample problems. Structural Equation Modeling: A Multidisciplinary Journal, 23(5), 750–773. [Google Scholar]
- Mognon, A. , Jovicich, J. , Bruzzone, L. , & Buiatti, M. (2011). ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology, 48(2), 229–240. [DOI] [PubMed] [Google Scholar]
- Mu, Y. , Guo, C. , & Han, S. (2016). Oxytocin enhances inter‐brain synchrony during social coordination in male adults. Social Cognitive and Affective Neuroscience, 11(12), 1882–1893. 10.1093/scan/nsw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, M. , Vanderwal, T. , & Hasson, U. (2019). Shared understanding of narratives is correlated with shared neural responses. NeuroImage, 184, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordzij, M. L. , Newman‐Norlund, S. E. , De Ruiter, J. P. , Hagoort, P. , Levinson, S. C. , & Toni, I. (2009). Brain mechanisms underlying human communication. Frontiers in Human Neuroscience, 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre, G. , & Iannetti, G. D. (2020). Hyperscanning alone cannot prove causality. Multibrain stimulation can. Trends in Cognitive Sciences, 25(2), 96–99. 10.1016/j.tics.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre, G. , Knoblich, G. , Dunne, L. , & Keller, P. E. (2017). Interpersonal synchrony enhanced through 20 Hz phase‐coupled dual brain stimulation. Social Cognitive and Affective Neuroscience, 12(4), 662–670. 10.1093/scan/nsw172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka, N. , Minamoto, T. , Yaoi, K. , Azuma, M. , Shimada, Y. M. , & Osaka, M. (2015). How two brains make one synchronized mind in the inferior frontal cortex: FNIRS‐based Hyperscanning during cooperative singing. Frontiers in Psychology, 6, 1–11. 10.3389/fpsyg.2015.01811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y. , Cheng, X. , Zhang, Z. , Li, X. , & Hu, Y. (2017). Cooperation in lovers: An fNIRS‐based hyperscanning study: Cooperation in lovers. Human Brain Mapping, 38(2), 831–841. 10.1002/hbm.23421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y. , Novembre, G. , Song, B. , Zhu, Y. , & Hu, Y. (2021). Dual brain stimulation enhances interpersonal learning through spontaneous movement synchrony. Social Cognitive and Affective Neuroscience, 16(1–2), 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson, D. , Apperly, I. A. , Chiavarino, C. , & Humphreys, G. W. (2004). Left temporoparietal junction is necessary for representing someone else's belief. Nature Neuroscience, 7(5), 499–500. [DOI] [PubMed] [Google Scholar]
- Saxe, R. , Moran, J. M. , Scholz, J. , & Gabrieli, J. (2006). Overlapping and non‐overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience, 1(3), 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoot, L. , Hagoort, P. , & Segaert, K. (2016). What can we learn from a two‐brain approach to verbal interaction? Neuroscience & Biobehavioral Reviews, 68, 454–459. [DOI] [PubMed] [Google Scholar]
- Scott‐Phillips, T. C. , & Kirby, S. (2010). Language evolution in the laboratory. Trends in Cognitive Sciences, 14(9), 411–417. 10.1016/j.tics.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Stephens, G. J. , Silbert, L. J. , & Hasson, U. (2010). Speaker‐listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences, 107(32), 14425–14430. 10.1073/pnas.1008662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk, A. , Noordzij, M. L. , Verhagen, L. , Volman, I. , Schoffelen, J.‐M. , Oostenveld, R. , … Toni, I. (2014). Cerebral coherence between communicators marks the emergence of meaning. Proceedings of the National Academy of Sciences, 111(51), 18183–18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk, A. , Verhagen, L. , Schoffelen, J.‐M. , Oostenveld, R. , Blokpoel, M. , Hagoort, P. , … Toni, I. (2013). Neural mechanisms of communicative innovation. Proceedings of the National Academy of Sciences, 110(36), 14574–14579. 10.1073/pnas.1303170110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk, A. , Verhagen, L. , & Toni, I. (2016). Conceptual alignment: How brains achieve mutual understanding. Trends in Cognitive Sciences, 20(3), 180–191. [DOI] [PubMed] [Google Scholar]
- Sun, H. , Verbeke, W. J. , Pozharliev, R. , Bagozzi, R. P. , Babiloni, F. , & Wang, L. (2019). Framing a trust game as a power game greatly affects interbrain synchronicity between trustor and trustee. Social Neuroscience, 14(6), 635–648. [DOI] [PubMed] [Google Scholar]
- Szymanski, C. , Müller, V. , Brick, T. R. , von Oertzen, T. , & Lindenberger, U. (2017). Hyper‐transcranial alternating current stimulation: Experimental manipulation of inter‐brain synchrony. Frontiers in Human Neuroscience, 11, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley, D. , Yamamoto, T. , Hirose, K. , Keele, L. , & Imai, K. (2014). Mediation: R package for causal mediation analysis. Journal of Statistical Software, 59(5), 1–37. https://www.jstatsoft.org/article/view/v059i05 26917999 [Google Scholar]
- Van der Graaff, J. , Branje, S. , De Wied, M. , Hawk, S. , Van Lier, P. , & Meeus, W. (2014). Perspective taking and empathic concern in adolescence: Gender differences in developmental changes. Developmental Psychology, 50(3), 881. [DOI] [PubMed] [Google Scholar]
- Wheatley, T. , Boncz, A. , Toni, I. , & Stolk, A. (2019). Beyond the isolated brain: The promise and challenge of interacting minds. Neuron, 103(2), 186–188. 10.1016/j.neuron.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE, 8(7), 1–15. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, H. , Lu, K. , & Hao, N. (2018). Cooperation makes two less‐creative individuals turn into a highly‐creative pair. NeuroImage, 172, 527–537. 10.1016/j.neuroimage.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Zadbood, A. , Chen, J. , Leong, Y. C. , Norman, K. A. , & Hasson, U. (2017). How we transmit memories to other brains: Constructing shared neural representations via communication. Cerebral Cortex, 27(10), 4988–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data and analysis code in this manuscript are available from the corresponding author upon reasonable request.


