Abstract
The ventrolateral prefrontal cortices (VLPFC) are crucial regions involved in voluntary emotion regulation. However, the lateralization of the VLPFC in downregulating negative emotions remains unclear; and whether the causal role of the VLPFC is generalizable to upregulating positive emotions is unexplored. This study used transcranial magnetic stimulation (TMS) to examine the causal relationship between the left/right VLPFC and social emotion reappraisal. One hundred and twenty participants were randomly assigned to either active (left and right VLPFC groups, n = 40/40) or sham (vertex, n = 40) TMS groups. Participants were instructed to passively receive social feedback or use reappraisal strategies to positively regulate their emotions. While the subjective emotional rating showed that the bilateral VLPFC facilitated the reappraisal success, the electrophysiological measure of the late positive potential (LPP) demonstrated a more critical role of the right VLPFC on social pain relief (decreased LPP amplitudes) and social reward magnification (enhanced LPP amplitudes). In addition, the influence of emotion regulation on social evaluation was found to be mediated by the memory of social feedback, indicating the importance of memory in social behavioral shaping. These findings suggest clinical protocols for the rehabilitation of emotion‐regulatory function in patients with affective and social disorders.
Keywords: emotion regulation, reappraisal, social feedback, transcranial magnetic stimulation, ventrolateral prefrontal cortex
Previous studies of our lab have revealed that the ventrolateral prefrontal cortices (VLPFC) are crucial regions involved in voluntary emotion regulation. However, the lateralization of the VLPFC (left or right) as well as the generalizability of its critical role to upregulating positive emotions is largely unclear. To answer these questions, this study used transcranial magnetic stimulation (TMS) together with event‐related potential techniques to examine the causal relationship between the left/right VLPFC and emotion regulation. It was found that the right VLPFC, compared to its right counterpart, significantly facilitated downregulating negative and upregulating positive emotions.
1. INTRODUCTION
Social communication and interactions are important activities in everyday life, during which people receive, perceive, and evaluate social feedback from others. Social feedback refers to opinions from other people about one's social standing (Rappaport & Barch, 2020), for example, like/not like and thumbs up/thumbs down. Over the past decades, various experimental paradigms have been developed to study social feedback, including the social judgment paradigm (Somerville, Heatherton, & Kelley, 2006), the chatroom task (Guyer, Choate, Pine, & Nelson, 2012), the cyber ball game (Eisenberger, Lieberman, & Williams, 2003), and the island getaway task (Kujawa, Arfer, Klein, & Proudfit, 2014). Using these paradigms, it is found that receiving social feedback indicating interpersonal rejection or ostracism is psychologically painful, which is usually accompanied by activation of pain‐related brain regions (see Eisenberger, 2015; Somerville, 2013 for reviews). Moreover, interpersonal rejection often results in enhanced aggression (Achterberg, van Duijvenvoorde, van der Meulen, Bakermans‐Kranenburg, & Crone, 2018; Smart & Leary, 2009) and reduced prosocial behaviors (Twenge, Baumeister, DeWall, Ciarocco, & Bartels, 2007), and even contributes to the onset of various psychiatric disorders (see Porcelli et al., 2019; Schilbach, 2016 for reviews). Given how negative social feedback harms mental health, it is important to study strategies and interventions that regulate the accompanying negative emotions. Studies have demonstrated that emotion regulation, particularly when using cognitive reappraisal (hereinafter abbreviated simply as reappraisal), helps mitigate the distressing feelings elicited by negative social feedback such as social exclusion (e.g., He et al., 2018, He, Liu, Zhao, Elliott, & Zhang, 2020; He, Zhao, et al., 2020; Zhao et al., 2021) and social rejection (Nasso, Vanderhasselt, Schettino, & De Raedt, 2020). Reappraisal is a frequently employed, effective, and adaptive regulation strategy with notable long‐term mental and physical health outcomes (McRae & Gross, 2020). Reappraisal is a semantic meaning selection strategy, during which one changes how (s)he interprets a situation to modify his/her emotional response (Ochsner, Silvers, & Buhle, 2012). Habitual use of reappraisal has been repeatedly associated with reduced negative affect and limited symptoms of psychopathology, while limited use of reappraisal is often found in individuals with mood disorders (see Compas et al., 2017 and Kanske, Heissler, Schönfelder, & Wessa, 2012 for reviews). Behavioral studies have revealed that reappraisal is an effective strategy in downregulating social pains elicited by negative social feedback (Nasso et al., 2020), unfairness (van't Wout, Chang, & Sanfey, 2010), and upsetting political events (Ford, Feinberg, Lam, Mauss, & John, 2019). Neuroimaging studies have demonstrated that engaging reappraisal largely recruits the lateral prefrontal cortex implicated in top‐down control processes (Buhle et al., 2014). While both the dorsolateral (DLPFC) and the ventrolateral prefrontal cortices (VLPFC) are activated (see Kohn et al., 2014; Morawetz, Bode, Derntl, & Heekeren, 2017; Ochsner et al., 2012 for reviews), the VLPFC has been reported as consistently involved during reappraisal implementation (Dörfel et al., 2014). Importantly, enhanced activation of the VLPFC is predictive for subjective reappraisal success (Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008) and reduced activation of the amygdala, a typical brain region associated with negative emotion processing (Berboth & Morawetz, 2021; Silvers et al., 2017). More relevantly, studies have demonstrated stronger VLPFC activation in response to negative, relative to neutral or positive, social feedback across different paradigms (e.g., Achterberg et al., 2018; Chester, Lynam, Milich, & DeWall, 2018; Hsu et al., 2020; A. B. Miller, Prinstein, Munier, Machlin, & Sheridan, 2019; Vijayakumar, Cheng, & Pfeifer, 2017). Intriguingly, the VLPFC activation is negatively correlated with self‐reported emotional distress after being socially excluded (Eisenberger et al., 2003). Enhancing the neural activation of the VLPFC using noninvasive neuromodulation techniques could reduce pain feelings following social exclusion (Riva, Romero Lauro, Dewall, & Bushman, 2012), which highlighted the causal role of the VLPFC in regulating social pain experience (see H. Wang, Braun, & Enck, 2017 for a review). Our group recently used an explicit reappraisal task together with brain stimulation techniques (i.e., transcranial direct current stimulation and transcranial magnetic stimulation (TMS), tDCS and TMS), which further provided causal evidence for the role of the VLPFC on explicitly downregulating social pain via reappraisal strategy (He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Zhao et al., 2021).
Despite these findings, the existing neuromodulation studies have only demonstrated the essential role of the right VLPFC (rVLPFC) in the reappraisal of social exclusion, which is supported by previous neuroimaging findings highlighting the rVLPFC in social pain relief (Eisenberger et al., 2003; Wager et al., 2008). However, various emotion regulation studies also documented the recruitment of the left (Berboth & Morawetz, 2021; Cao, Li, & Tang, 2021; Goldin, McRae, Ramel, & Gross, 2008; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Morawetz, Bode, Baudewig, Jacobs, & Heekeren, 2016; Silvers et al., 2017), or the bilateral parts (Buhle et al., 2014; Dörfel et al., 2014; Kohn et al., 2014; Morawetz et al., 2017; Ochsner et al., 2012; Wager et al., 2008) of the VLPFC during reappraisal processes. It is still unclear whether the left VLPFC (lVLPFC) is also essential for, or is just correlated with, social pain relief. The hemispheric asymmetry of emotion processing has been extensively studied over the past decades (see Gainotti, 2019; W. Heller, Nitschke, & Miller, 1998; G. A. Miller, Crocker, Spielberg, Infantolino, & Heller, 2013; Ross, 2021; Shobe, 2014 for reviews). Among various theoretical accounts, the valence hypothesis proposed that negative emotions are modulated by the right hemisphere and positive emotions are modulated by the left hemisphere (G. A. Miller et al., 2013). A recent framework of the emotion‐type hypothesis proposed that primary/nonsocial emotions are modulated by the right hemisphere and social emotions are modulated by the left hemisphere (Ross, 2021). Also, some researchers believed that the right hemisphere is integral to the basic processing of emotions (Gainotti, 2019) whereas the role of the left hemisphere in emotion processing remains contentious (Shobe, 2014). Despite multiple accounts, the lateralization of the VLPFC in reappraising social feedback remains unclear. Uncovering the lateralization issue is necessary not only for understanding the key brain mechanism of emotion regulation, but also for the development of effective therapeutic protocols employing neuromodulation (e.g., TMS) or neurofeedback (e.g., human–computer interaction via near‐infrared spectroscopy) techniques to improve emotion regulation abilities in the clinical population.
Another purpose of this study is to investigate whether the VLPFC plays the same critical role in upregulating positive emotion. Pursuit of happiness and savoring/magnifying experience of positive feelings are associated with a wide range of favorable physical and mental outcomes (Pressman, Jenkins, & Moskowitz, 2019), such as improved pain resilience (Thong, Tan, & Jensen, 2017), increased prosocial behaviors (Aknin, Van de Vondervoort, & Hamlin, 2018), and satisfied social connections (Kok et al., 2013). Besides deficits in the downregulation of negative emotion, dysregulation of positive emotion also contributes to the development and maintenance of affective disorders such as anxiety and depression (see Carl, Soskin, Kerns, & Barlow, 2013; Vanderlind, Millgram, Baskin‐Sommers, Clark, & Joormann, 2020 for reviews). Despite its benefits both in emotion regulation theory and in clinics (Quoidbach, Mikolajczak, & Gross, 2015; Silton et al., 2020), relatively limited knowledge has been accumulated about the neural mechanisms underlying the upregulation of positive emotions. Existing neuroimaging studies have revealed that upregulating positive emotions using reappraisal is accompanied by increased activation of the lateral prefrontal regions (i.e., VLPFC/DLPFC; Greening, Osuch, Williamson, & Mitchell, 2014; A. S. Heller et al., 2013; S. H. Kim & Hamann, 2007), which is very similar with the neural correlates of negative emotion downregulation (see J. U. Kim, Weisenbach, & Zald, 2019; Morawetz et al., 2017; Ochsner et al., 2012 for reviews). For instance, Greening et al. (2014) found in a reappraisal task that the upregulation of positive emotion is associated with stronger activation of the VLPFC and the ventral striatum. So far, no study has used neural manipulation techniques to answer the question that whether the causal role of the VLPFC in downregulating negative emotion can be generalized to the upregulation of positive emotion. This study is inspired by this literature gap and designed to verify the generalization of the VLPFC in the upregulation of positive emotion in the social feedback context.
To disentangle the lateralization and generalization issues proposed above, this study used the TMS technique to temporally activate the left or right VLPFC in two groups of participants. Explicit reappraisal instructions were given, and participants were asked to reduce their negative, or increase their positive, feelings upon receiving social feedback from peers. We used self‐reported emotional feeling and the late positive potential (LPP) as subjective and objective measurements of emotion to provide cross‐modal evidence. The LPP is a sensitive electrophysiological indicator of emotional reactivity (Y. Liu, Huang, McGinnis‐Deweese, Keil, & Ding, 2012), and a large amount of evidence has revealed reliable associations between LPP amplitude and emotion regulation effect of reappraisal (Kennedy & Montreuil, 2021). Specifically, downregulating negative emotion via reappraisal is associated with decreased LPP amplitudes (Hajcak & Nieuwenhuis, 2006; He, Zhao, et al., 2020; Zhao et al., 2021), whereas upregulating positive or neutral emotion (i.e., increasing positive attitude) via reappraisal is associated with enhanced LPP amplitudes (Langeslag & van Strien, 2013; W. Liu, Liu, Chen, Jiang, & Shang, 2019; Y. Wang, Liao, Shangguan, Shang, & Zhang, 2020; Wilson & MacNamara, 2021). Our hypothesis is twofold. First, the right VLPFC plays a more essential role, to some extent, compared to its left part, during reappraisal of social feedback, since most previous studies highlighted the right VLPFC in regulating social pains (Chester & DeWall, 2014; Eisenberger et al., 2003; He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Riva et al., 2012; Zhao et al., 2021). Second, the causal role of the VLPFC in the downregulation of negative emotion is generalizable to the upregulation of positive emotion, because the VLPFC regions are recruited in both upregulation and downregulation of emotions (see J. U. Kim et al., 2019; Morawetz et al., 2017; Ochsner et al., 2012 for reviews).
Besides the main goals mentioned above, this study also investigated the impacts of emotion regulation on subsequent memory and social evaluation. Some evidence has accumulated on how emotion regulation affects memory, but most of the existing research focused on whether emotion regulation reduced or enhanced the accuracy of emotional memories (Dillon, Ritchey, Johnson, & LaBar, 2007; S. H. Kim & Hamann, 2012). Recently, a series of experiments have been done to investigate how emotion regulation changes memory content per se, demonstrating that negative autobiographical memories were updated with positive content after reappraisal (Speer, Ibrahim, Schiller, & Delgado, 2021). Following this finding, we predict that participants' memory of social feedback will become more positive after reappraisal. Furthermore, we expect a mediating effect of memory on the relationship between emotion regulation and participants' attitudes toward peers, since we recently found that memory of social feedback changed social attitudes toward the feedback senders (H. Xie, Hu, Mo, & Zhang, 2021; H. Xie et al., 2022).
2. METHODS
2.1. Participants
This study set three TMS groups: the lVLPFC‐activated group, the rVLPFC‐activated group, and the sham group. During the experiment design, we conducted a prior power analysis using G*Power 3.1.7 (F tests, analysis of variance [ANOVA]: repeated measures, within‐between interaction) based on the effect size ( = 0.083) reported in our previous, related TMS study (Zhao et al., 2021). According to the result of this power analysis, 39 participants in total would ensure 80% statistical power. However, 13 participants per group are such a small sample size in present‐day neuroscience studies. Thus, we finally decided to include 40 participants per TMS group, which ensured a statistical power near 100%.
As a result, a total of 120 healthy college students (all right‐handed) were recruited from Shenzhen University. Upon arrival at the lab, participants first completed six questionnaires, including the Trait form of Spielberger's State–Trait Anxiety Inventory (STAI‐T; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987), the Beck Depression Inventory Second Edition (BDI‐II; Beck, Steer, & Brown, 1996), the Rejection Sensitivity Questionnaire (RSQ; Downey & Feldman, 1996), the Revised Social Anhedonia Scale (RSAS; Eckblad, Chapman, Chapman, & Mishlove, 1982), and the cognitive reappraisal dimension of Emotion regulation Questionnaire (ERQ‐R; Gross & John, 2003). Participants were then randomly assigned into one of the three TMS groups, with equal numbers of male and female participants in each group. Scores of the above questionnaires did not differ across the three TMS groups (Table 1). No participant had any prior experiences with TMS before this experiment. The study protocol was approved by the Ethics Committee of Shenzhen University. Informed consent was signed by the participants before their engagement in the experiment.
TABLE 1.
Demographical characteristics of the three groups (mean ± SD)
| Items | lVLPFC group (n = 40) | Sham group (n = 40) | rVLPFC group (n = 40) | Statistics a | |
|---|---|---|---|---|---|
| F (2,117) | p | ||||
| Gender (male/female) | 20/20 | 20/20 | 20/20 | ||
| Age (years) | 19.6 ± 1.4 | 19.7 ± 1.6 | 19.2 ± 1.5 | 1.09 | .340 |
| STAI‐T | 40.0 ± 8.5 | 42.4 ± 8.9 | 42.2 ± 9.7 | 0.84 | .435 |
| LSAS | 39.8 ± 20.4 | 38.8 ± 15.5 | 41.7 ± 15.6 | 0.30 | .743 |
| BDI‐II | 7.3 ± 6.8 | 7.5 ± 6.3 | 9.7 ± 5.7 | 1.79 | .171 |
| RSQ | 10.3 ± 2.3 | 11.1 ± 2.6 | 11.4 ± 2.7 | 1.69 | .189 |
| RSAS | 10.8 ± 5.9 | 10.4 ± 5.7 | 9.3 ± 6.1 | 0.72 | .489 |
| ERQ‐R | 30.9 ± 4.0 | 30.4 ± 5.3 | 30.8 ± 4.6 | 0.15 | .862 |
Abbreviations: BDI‐II, the Beck Depression Inventory Second Edition; ERQ‐R, the cognitive reappraisal dimension of Emotion regulation Questionnaire; LSAS, the Liebowitz Social Anxiety Scale; RSAS, the Revised Social Anhedonia Scale; RSQ, the Rejection Sensitivity Questionnaire; STAI‐T, the Trait form of Spielberger's State–Trait Anxiety Inventory.
One‐way ANOVA across the three groups.
2.2. Experimental design and materials
The study was a 3 (TMS group: lVLPFC‐activated, rVLPFC‐activated, and sham) × 3 (valence of social feedback: positive, neutral, and negative) × 2 (regulation type: view vs. reappraisal) mixed design. The two within‐subject factors were valence of social feedback and regulation type, and the between‐subject factor was TMS group.
In the emotion regulation task, we used 60 identity photos of young adults (30 males and 30 females) with neutral facial expressions. All photos were standardized in background color (white), resolution, and brightness. According to the ratings of these photos by another group of homogeneous participants (H. Xie et al., 2021), we assigned these photos into each of the six within‐subject conditions while their attractiveness and favorability ratings were counterbalanced across conditions (Fs < 1).
2.3. Experimental procedure
The experiment consisted of the following four phases (Figure 1a).
FIGURE 1.
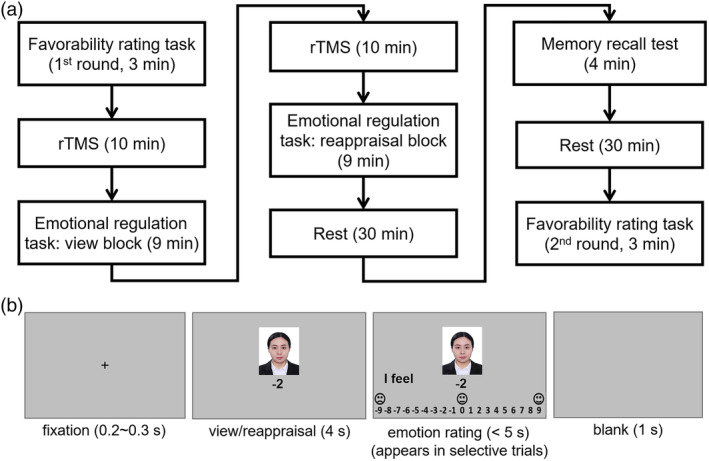
Experimental procedures. (a) The four phases of the experiment. (b) Illustration of one trial in the emotion regulation task. Due to copyright, the person in the sample image is replaced by the graduate student from the research group. rTMS, repetitive transcranial magnetic stimulation
Phase 1: Favorability rating task (first round). In the cover story, participants were instructed that this study was designed to investigate the processing of first impressions between unfamiliar peers. They were required to provide their identity photos and told that their photos would be evaluated by the students from neighboring universities. On the day of the experiment, participants were first required to rate the favorability in response to 30 identity photos of unfamiliar peers on a scale ranging from −9 to +9 (“−9” for extremely dislike, “0” for neutral favorability, and “+9” for extremely like) by clicking the left button on the mouse within 5 s.
Phase 2: Emotion regulation task. In this task, participants were instructed to view the result of favorability ratings from peers (i.e., social feedback). All the 60 photos were used in this task, including the 30 ones used in the favorability rating task. The 60 photos were randomly assigned to the 3 (valence of social feedback: positive, neutral, and negative) × 2 (regulation type: view vs. reappraisal) conditions, resulting in 10 photos in each condition (including 5 ones used in the favorability rating task). The scores of social feedback were classified into three valence conditions: positive feedback contained the scores from +5 to +9, neutral feedback contained the scores from −2 to +2, and negative feedback included the scores from −9 to −5 (note: to take apart the positive, neutral, and negative conditions, there were no scores of +3, +4, −3 or − 4).
There were two blocks that had a fixed order, that is, the view block ran first, followed by the reappraisal block (see also He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Zhao et al., 2021). Before each block, participants underwent a 10‐min TMS session. In each block, the 30 photos of peers together with their feedback were presented three times in a pseudorandom order to collect enough epochs for event‐related potential (ERP) data analyses, resulting in 30 trials in each valence condition. As shown in Figure 1b, each trial began with a fixation (0.2–0.3 s), followed by the presentation of a photo‐feedback combination for 4 s, during which participants were required to either view passively (in the view block) or to regulate their emotion (in the reappraisal block). Among the 30 trials in each valence condition, 5 trials were pseudorandomly selected to collect the subjective emotional feeling. In these 5 trials, participants were asked to report how they felt on a scale of −9 to +9 (“−9” for extremely unhappy, “0” for neutral, and “+9” for extremely happy) after the 4‐s view or reappraisal procedure by clicking the left button on the mouse within 5 s. A blank screen appeared for 1 s at the end of each trial.
During the view block, participants were instructed to pay full attention to feedback and the feedback sender and react naturally on the rating screen. During the reappraisal block, participants were instructed to reinterpret the peers' feedback from a more positive perspective, irrespective of the valence (positive, neutral, or negative) of the feedback. For instance, in the positive feedback condition, participants were suggested to regard the peer as an expert who can give accurate evaluation via first impression. In the neutral feedback condition, participants were suggested to imagine that the social feedback might become more positive if the peer would be familiar with him/her via social interaction. Finally for the negative feedback, participants were suggested to consider the peer as rigorous persons who usually give low evaluations to others.
Phase 3: Memory recall test. Approximately half an hour after Phase 2, participants were asked, surprisingly, to recall the feedback scores given by the peers and respond on a scale ranging from −9 to +9 (“−9” for extremely dislike, “0” for neutral favorability, and “+9” for extremely like) by clicking the left button on the mouse within 5 s. Participants' answers were coded as recalled favorability rating, with a higher score indicating more positive recalled favorability. The 60 photos used in Phase 2 were presented in a random order in this test.
Phase 4: Favorability rating task (second round). Approximately half an hour after Phase 3, participants performed the favorability rating task again for the same 30 photos used in the first round of the task.
2.4. Repetitive transcranial magnetic stimulation
This study used offline, instead of online, TMS to reduce any side effects that may impact participants' task performances. The TMS targets were the lVLPFC and the rVLPFC for the two experimental groups. For the sham group, the TMS was targeted at the vertex to provide a similar scalp sensation as it did in the other two groups (Zhao et al., 2021). A figure‐eight‐shaped coil was connected to the magnetic stimulator (M‐100 Ultimate; Yingchi, Shenzhen, China). The location of the coil was determined with reference to the International 10/20 electroencephalogram system. The lVLPFC is at the F7, the rVLPFC is at the F8, and the vertex is at the Cz. Each participant's resting motor threshold (rMT) was measured from their motor cortex (the C3), with the intensity being defined as 50% of the pulses that reliably produced thumb twitches. The repetitive transcranial magnetic stimulation was applied at 10 Hz at 90% of each participant's rMT. Each 10 min session contained 20 trains, with each train lasting for 4 s (a total of 800 pulses) and which were separated by inter‐train intervals of 26 s. The TMS‐simulated electric field is illustrated on an adult brain model in Figure 2 (SimNIBS; www.simnibs.org).
FIGURE 2.
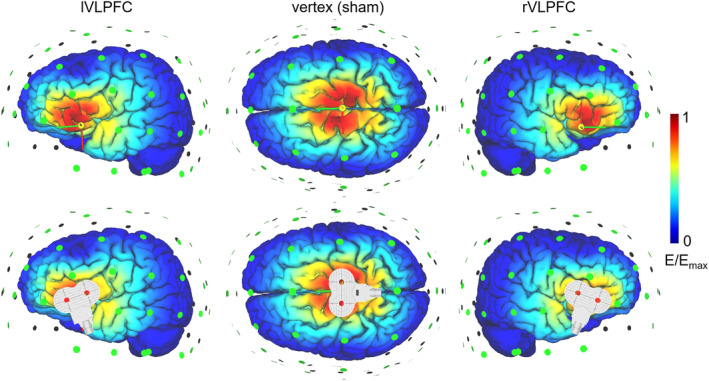
Illustration of TMS electric fields of the three TMS groups (left VLPFC, vertex, and right VLPFC). The color represents the electric field strength, scaled from 0 (blue) to the individual maximums (red). TMS, transcranial magnetic stimulation; VLPFC, ventrolateral prefrontal cortices
2.5. EEG recordings and analysis
EEG data were recorded during the emotion regulation task using a 32‐channel amplifier (NeuSen.W32, Neuracle, Changzhou, China), with a sampling frequency of 250 Hz. Electrode impedances were kept below 10 kΩ. The reference electrode was placed at the TP9. No online filter was applied.
The ERP recording and analysis were designed especially for the late positive potential (He, Zhao, et al., 2020; Zhao et al., 2021). The electrodes and time window for the measurement of LPP amplitudes were decided prior to analysis. Data were first re‐referenced to the average of the left and right mastoids, followed by filtering using a 0.1–10 Hz band‐pass filter with a slope of 24 dB/oct. The filtered data were segmented beginning 200 ms prior to the onset of the feedback and lasting for 4 s. The baseline‐correction was based on the 200 ms pre‐stimulus time window. We measured the LPP as the average amplitude across the electrode sites at and around Pz (P3, P4, Pz, CP1, CP2). The time window for the LPP amplitude beginning at the end of the typical P3 time window (1 s) and lasting for the entire emotion regulation period (1–4 s post feedback onset; see also Hajcak & Nieuwenhuis, 2006; Paul, Simon, Endrass, & Kathmann, 2016; Zhao et al., 2021).
2.6. Statistics
Statistical analysis was performed using SPSS Statistics 20.0 (IBM, Somers). Descriptive data are presented as mean ± SE unless otherwise mentioned.
In the three tasks (emotion regulation, memory recall, and favorability rating), repeated‐measures ANOVAs were performed on subjective ratings/reports and LPP amplitudes, with regulation type (view or reappraisal) and valence of social feedback (positive, negative, or neutral) as the within‐subject factors and TMS group (lVLPFC, rVLPFC, or sham) as the between‐subject factor. In the favorability rating task, an additional four‐way ANOVA was performed with testing time (baseline or post emotion regulation) as another within‐subject factor. The Greenhouse–Geisser correction for the ANOVA tests was used whenever appropriate.
To explore the interaction/relationship between emotion regulation, memory, and social attitudes, we examined the mediating effect of memory between emotion regulation and social attitude (favorability) using an SPSS macro‐PROCESS (Model 4) (Hayes, 2013; Model 4). This analysis was performed in positive, negative, and neutral social feedback conditions separately, using a 95% bias‐corrected confidence interval (CI) based on 10,000 bootstrap samples. We considered the indirect effect of emotion regulation on social attitude through memory as a significant one when the CI did not contain zero.
3. RESULTS
3.1. Emotion regulation task
3.1.1. Subjective emotional feeling
ANOVA results showed significant findings on the main effects of regulation type and valence of social feedback, and significant interactions between regulation type × valence of social feedback and between TMS group × regulation type (Table 2).
TABLE 2.
Results of ANOVAs on emotion regulation, memory, and favorability
| Item | Subjective rating | LPP amplitude | Memory | Favorability a | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| T | 365.1 | <.001 | 5.6 | .019 | 67.8 | <.001 | 47.7 | <.001 |
| V | 322.9 | <.001 | 19.3 | <.001 | 638.9 | <.001 | 252.4 | <.001 |
| TMS | 3.0 | .051 | <0.1 | .988 | 2.9 | .058 | 1.0 | .354 |
| T × V | 30.6 | <.001 | 51.7 | <.001 | 1.9 | .157 | 7.4 | .001 |
| T × TMS | 6.8 | .002 | <0.1 | .988 | 7.4 | .001 | 9.7 | <.001 |
| V × TMS | 0.1 | .970 | 2.0 | .098 | 0.1 | .940 | 0.5 | .672 |
| T × V × TMS | 1.0 | .428 | 4.7 | .002 | 0.2 | .879 | 1.2 | .332 |
Abbreviations: T, regulation type; TMS, TMS group; V, valence of feedback.
Here we report the result of favorability measured in the second round.
The main effect of the regulation type was found to be highly significant (F(1,117) = 365.1, p < .001, = 0.757): participants reported more positive feelings in the reappraisal block (2.4 ± 0.1) when compared to the passive view block (0.3 ± 0.1). The main effect of the valence of social feedback was found to be highly significant (F(2,234) = 322.9, p < .001, = 0.734): participants reported more positive feelings for positive feedback (4.0 ± 0.2) and more negative feelings for negative feedback (−0.8 ± 0.2), both compared to the neutral feedback condition (1.0 ± 0.1; pairwise ps < .001). Additionally, there was a significant interaction between regulation type × valence of social feedback (F(2,234) = 30.6, p < .001, = 0.207): the effect of regulation type (i.e., felt more positive in the reappraisal compared to the view block) was the most significant for negative social feedback (F(1,117) = 282.9, p < .001, = 0.707; the difference between reappraisal and view = 2.7 ± 0.2), followed by that for positive social feedback (F(1,117) = 248.5, p < .001, = 0.680; difference = 2.0 ± 0.1; positive vs. negative, p < .001), while the emotion regulation showed a relatively smaller effect for neutral social feedback (F(1,117) = 161.0, p < .001, = 0.579; difference = 1.6 ± 0.1; positive vs. neutral, p = .014; negative vs. neutral, p < .001).
More importantly, we observed a two‐way interaction between TMS group × regulation type (F(2,117) = 6.8, p = .002, = 0.105; Figure 3a). A simple effects analysis indicated that the effect of TMS group was significant in the reappraisal block (F(2,117) = 6.3, p = .003, = 0.097): the rVLPFC‐activated (2.9 ± 0.2, p = .002) and lVLPFC‐activated participants (2.6 ± 0.2, p = .047) reported their emotion more positively compared to the participants in the sham group (1.9 ± 0.2); however, this group difference was not significant in the view block (F < 1). The three‐way interaction (not significant) is reported in the Supporting Information.
FIGURE 3.
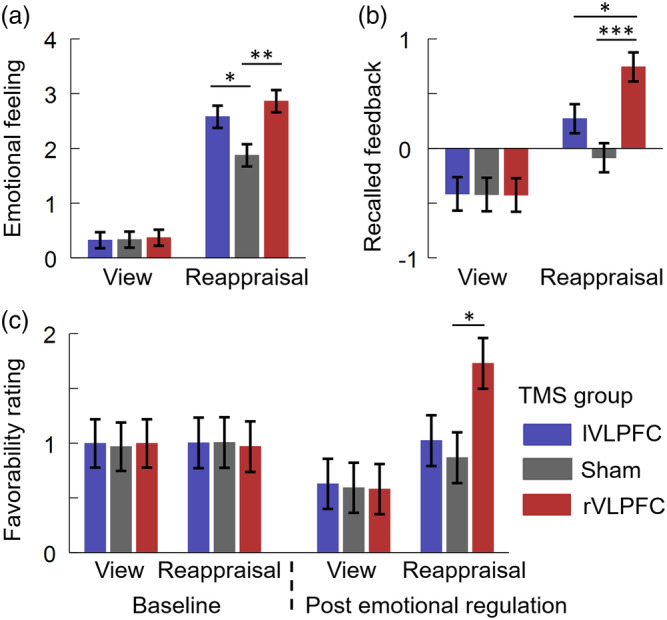
Behavioral results. (a) Subjective emotional feeling. (b) Recalled feedback. (c) Favorability rating. For each dependent variable shown here, participants reported their responses on a 19‐point scale (−9 for extremely unhappy or dislike, 9 for extremely happy or like). Bars represent SE of the mean. *p < .05; **p < .01; ***p < .001. TMS, transcranial magnetic stimulation; VLPFC, ventrolateral prefrontal cortices
3.1.2. LPP amplitude
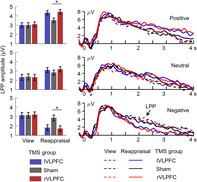
ANOVA results showed significant findings on the main effects of regulation type and valence of social feedback, and significant interactions between regulation type × valence of social feedback and between TMS group × valence of social feedback × regulation type.
The main effect of regulation type was found to be significant (F(1,117) = 5.6, p = .019, = 0.046; view = 2.8 ± 0.1 μV, reappraisal = 3.1 ± 0.1 μV). The main effect of the valence of social feedback was significant (F(2,234) = 19.3, p < .001, = 0.141; positive = 3.6 ± 0.1 μV, neutral = 2.7 ± 0.1 μV, and negative = 2.6 ± 0.2 μV; positive > neutral/negative, ps < .001; negative vs. neutral: p = 1.000). Additionally, there was a significant interaction between regulation type × valence of social feedback (F(2,234) = 51.7, p < .001, = 0.306): the effect of regulation type (i.e., reappraisal vs. view) influenced the LPP amplitudes in different patterns between valence conditions. Compared to the view block, reappraisal enhanced the LPP amplitudes for positive social feedback (F(1,117) = 65.6, p < .001, = 0.359; the difference between reappraisal and view = 1.1 ± 0.1 μV) and for neutral social feedback (F(1,117) = 13.0, p < .001, = 0.100; difference = 0.7 ± 0.2 μV), while reappraisal reduced the LPP amplitudes for negative social feedback (F(1,117) = 41.6, p < .001, = 0.262; difference = −1.0 ± 0.2 μV).
The most important finding was the three‐way interaction between TMS group × valence of social feedback × regulation type (F(4,234) = 4.7, p = .002, = 0.075; Figure 4). To break down this three‐way interaction, we tested the interaction between regulation type × TMS group in the three conditions of valence of social feedback, respectively. The two‐way interaction of regulation type × TMS group was significant in both the positive (F(2,117) = 4.3, p = .016, = 0.069) and negative feedback conditions (F(2,117) = 5.5, p = .005, = 0.086), that is, while the three groups did not differ in LPP amplitudes during the view block (Fs < 1), the LPP enhanced in the rVLPFC group (4.4 ± 0.2 μV, p = .032) and the lVLPFC group (4.3 ± 0.2 μV, p = .062) compared to that in the sham group (3.5 ± 0.2 μV) for positive feedback (F(2,117) = 4.1, p = .019, = 0.065), and the LPP reduced in the rVLPFC group (1.7 ± 0.3 μV, p = .029) and the lVLPFC group (1.8 ± 0.3 μV, p = .055) compared to that in the sham group (2.9 ± 0.3 μV) for negative feedback (F(2,117) = 4.2, p = .017, = 0.067). However, the two‐way interaction of regulation type × TMS group was not significant in the neutral feedback condition (F < 1).
FIGURE 4.
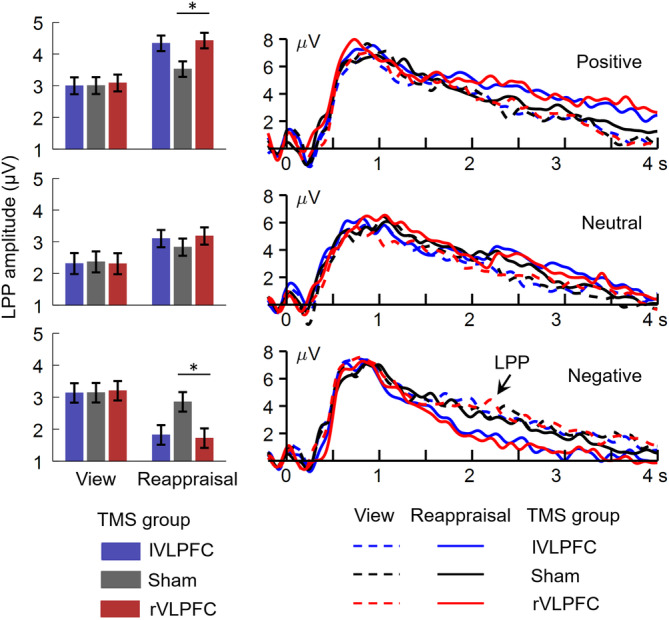
Mean amplitudes of the LPP component in positive, neutral, and negative feedback conditions. The ERP data were averaged across electrodes of Pz, P3, P4, CP1, and CP2. The LPP was measured as mean amplitude within the time window of 1–4 s post feedback presentation. *p < .05. LPP, late positive potential; TMS, transcranial magnetic stimulation; VLPFC, ventrolateral prefrontal cortices
Finally, we analyzed the correlations between the LPP amplitudes and the subjective emotional feelings in the 3 × 2 within‐subject conditions, separately (n = 120). Results revealed that the two measures negatively correlated in negative feedback and positively correlated in neutral and positive feedback conditions, although. Although only two out of six correlations reached significance after correcting for multiple comparisons, the other four correlations remained marginally significant (Table 3). These results indicate that the subjective and objective measures reflect the emotional reactivity in a similar accuracy in social feedback processing.
TABLE 3.
Correlation statistics between LPP amplitudes and subjective emotional feelings
| Regulation type | Positive | Neutral | Negative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | p cor a | r | p | p cor a | r | p | p cor a | |
| View | .252 | .005 | .025* | .221 | .015 | .060 | −.344 | <.001 | .001** |
| Reappraisal | .217 | .017 | .051 | .168 | .067 | .067 | −.205 | .025 | .050 |
Note: Statistically significant results are flagged with asterisks.
Corrected using the Holm's stepwise method.
p < .05;
p < .01.
3.2. Memory recall test
ANOVA results showed significant findings on the main effects of regulation type and valence of social feedback, and a significant interaction between TMS group × regulation type.
The main effect of the regulation type was found to be significant (F(1,117) = 67.8, p < .001, = 0.367): participants recalled and associated more positive feedback with the faces that appeared in the reappraisal block (0.3 ± 0.1) than those that appeared in the passive view block (−0.4 ± 0.1). The main effect of the valence of social feedback was found to be highly significant (F(2,234) = 638.9, p < .001, = 0.845): participants associated more positive feedback with the peers who indeed gave positive feedback previously (2.7 ± 0.1) and associated more negative feedback with the peers who indeed gave negative feedback previously (−3.0 ± 0.1), both compared to the recalled feedback of the peers who gave neutral feedback previously (0.2 ± 0.1; pairwise ps < .001).
More importantly, we observed a two‐way interaction between TMS group × regulation type (F(2,117) = 7.4, p = .001, = 0.113; Figure 3b). A simple effects analysis indicated that the effect of TMS group was significant in the reappraisal condition (F(2,117) = 9.7, p < .001, = 0.143): the rVLPFC‐activated participants (0.7 ± 0.1) recalled and associated more positive feedback with peers appeared in the reappraisal block, compared to the lVLPFC‐activated (0.3 ± 0.1, p = .040) and the sham participants (−0.1 ± 0.1, p < .001); however, this group difference was not significant in the view block (F < 1).
3.3. Favorability rating task
First, we examined the favorability ratings collected in the first round (baseline). The ANOVA (regulation type × valence of social feedback × TMS group) resulted in neither main nor interaction effects (Fs < 1). The favorability rating was 1.0 ± 1.7 on average before the emotion regulation task. This result indicated that the experimental materials (peer photos) were properly assigned to 3 (valence of social feedback) × 2 (regulation type) conditions without any difference in favorability.
Then we examined the favorability ratings collected in the second round (after the memory recall test), which showed significant findings on the main effects of regulation type and valence of social feedback, and significant interactions between regulation type × valence of social feedback and between TMS group × regulation type. The main effect of the regulation type was significant (F(1,117) = 47.7, p < .001, = 0.290): participants preferred the peers whose photos were presented in the reappraisal block (1.2 ± 0.1) relative to the peers appeared in the passive view block (0.6 ± 0.1). The main effect of the valence of social feedback was found to be highly significant (F(2,234) = 252.4, p < .001, = 0.683): participants preferred the peers who gave them positive feedback (2.8 ± 0.2) compared to the peers who gave them neutral feedback (0.9 ± 0.2), while they reported lower favorability to the peers who gave them negative feedback (−1.0 ± 0.2) compared to the peers who gave them neutral feedback (pairwise ps < .001). Additionally, there was a significant interaction between regulation type × valence of social feedback (F(2,234) = 7.4, p = .001, = 0.060): while the effect of regulation type (i.e., preferring the peers appeared in the reappraisal compared to the view block) was significant in the conditions of negative (F(1,117) = 32.9, p < .001, = 0.220; the difference between reappraisal and view = 1.0 ± 0.2) and positive social feedback (F(1,117) = 23.6, p < .001, = 0.168; difference = 0.7 ± 0.1), the effect of regulation type was not significant in the condition of neutral social feedback (F < 1; difference = 0.1 ± 0.2).
More importantly, we observed a two‐way interaction between TMS group × regulation type (F(2,117) = 9.7, p < .001, = 0.142; Figure 3c). A simple effects analysis indicated that the effect of TMS group was significant in the reappraisal block (F(2,117) = 3.9, p = .023, = 0.063): the rVLPFC‐activated participants (1.7 ± 0.2) preferred the peers in general compared to the participants in the sham group (0.9 ± 0.2, p = .030); however, there was no group difference in the view block (F < 1).
A four‐way ANOVA including the factor testing time (baseline or post emotion regulation) was also performed. The result is reported in the Supporting Information.
3.3.1. Mediating effect of memory between emotion regulation and favorability
First, we performed the mediation analysis using the subjective emotional rating as the indicator of emotion regulation. Result showed that the indirect effect of emotion regulation on favorability through memory was significant only in the positive (B = 0.15 × 0.40 = 0.06, SE = 0.03, 95% CI = [0.01, 0.14]), but not in the negative (B = 0.06 × 0.61 = 0.04, SE = 0.05, 95% CI = [−0.06, 0.14]) or neutral feedback conditions (B = 0.01 × 0.41 = 0.01, SE = 0.03, 95% CI = [−0.05, 0.09]). Since the direct effect of emotion regulation on favorability was still significant after controlling the impact of memory, memory exhibited a partial mediating effect in the positive feedback condition (Figure 5a).
FIGURE 5.
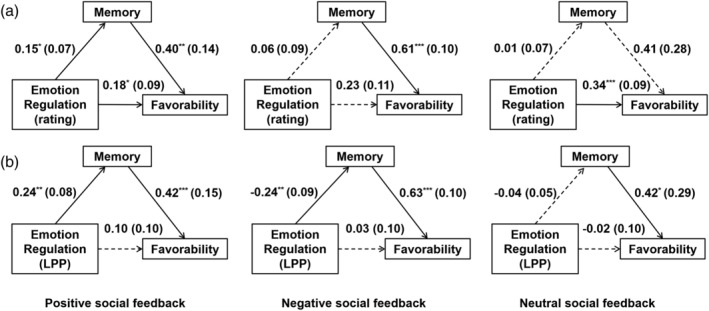
Mediating effect of memory on emotion regulation and favorability of positive, negative, and neutral feedback in the reappraisal condition. The mediating models were tested using (a) the self‐reported emotional rating, or (b) the LPP amplitude as an indicator of emotion regulation effect. Unstandardized coefficients are shown as mean (SE). Statistically significant pathways are indicated using solid lines. *p < .05; **p < .01; ***p < .001. LPP, late positive potential
Then we performed the mediation analysis using the LPP amplitude as an indicator of the emotion regulation. Results showed that the indirect effect of emotion regulation on favorability through memory was significant both in the positive (B = 0.24 × 0.42 = 0.10, SE = 0.04, 95% CI = [0.03, 0.21]) and the negative feedback conditions (B = −0.24 × 0.63 = −0.15, SE = 0.06, 95% CI = [−0.29, −0.04]), but not in the neutral condition (B = −0.04 × 0.42 = −0.02, SE = 0.03, 95% CI = [−0.09, 0.02]). Since the direct effect of emotion regulation on favorability was not significant after controlling the impact of memory, memory exhibited a full mediating effect in the positive and negative feedback conditions (Figure 5b).
4. DISCUSSION
This study combined TMS and ERP techniques to explore the causal role of the bilateral VLPFC in downregulating negative and upregulating positive emotions evoked by social feedback from peers. The first purpose was to reveal the lateralization of the VLPFC during reappraisal. It is found in subjective emotional rating that both the left and right VLPFC were helpful for regulating emotions in response to social feedback, which paralleled with previous studies showing the positive correlation between participants' subjectively reported reappraisal success and the activation of the left (Morawetz et al., 2016), the right (Naor et al., 2020), or the bilateral VLPFC (Wager et al., 2008). Furthermore, our ERP results showed a similar pattern as the subjective emotional rating, except that the reappraisal‐introduced changes in the LPP amplitude reached significance only in the rVLPFC‐activated group after correcting for multiple comparisons. This discrepancy may stem from the fact that the LPP reflects online processing of social feedback, whereas the self‐reported emotional rating reflects the processing result of social feedback (Kivity & Huppert, 2019; Nasso et al., 2020). Thus, our ERP results demonstrated that modulating the right side of VLPFC, relative to its left side, was more efficient in altering the LPP amplitudes, suggesting a more important role of the rVLPFC than lVLPFC in regulating online processing of social‐related emotions. In line with this finding, Tabibnia et al. (2011) used high‐resolution MRI and found that the gray matter intensity in the rVLPFC (but not lVLFPC) could successfully predict participants' performance in reducing negative emotions via reappraisal. An early meta‐analysis study based on fMRI findings (Kalisch, 2009) proposed that cognitive reappraisal has two stages: an early implementation stage and a later maintenance stage; and that the involvement of lateral prefrontal cortices during reappraisal follows a shift of “from‐left‐to‐right,” that is, the left lateral prefrontal regions first quickly initiate reappraisal, followed by a prolonged period of cognitive control to maintain reappraisal by the right prefrontal parts. Inspired by this dynamic neural model (Kalisch, 2009), we speculate that although the bilateral VLPFC areas are both involved during reappraisal, the rVLPFC works for a longer time and thus might play a more essential role during the reappraisal. We suggest future studies explore this possibility by using, for example, online, single‐pulse TMS or neurofeedback protocols.
The second purpose of this study was to explore the generalization of the VLPFC role to the upregulation of positive emotions. Our results indicate a positive answer, that is, the VLPFC always plays an important, causal role in reappraisal, irrespective of the goal of emotion regulation. This result is consistent with previous neuroimaging studies showing that reappraising positive scenes from a better perspective was accompanied by enhanced VLPFC activation (Greening et al., 2014; S. H. Kim & Hamann, 2007); and that anhedonia was mediated by reduced connectivity within the VLPFC‐striatal circuitry when upregulating positive emotions (Huhn et al., 2016; Yin, Tully, Lincoln, & Hooker, 2015). While these neuroimaging findings indicated a correlation between the VLPFC and upregulation of positive emotions, the current study provided, for the first time, straightforward neural causal evidence for the importance of the VLPFC in upregulating positive emotions. Taken together, our findings regarding the issues of lateralization and generalization of the VLPFC provide two clinical implications. First, treatment or interventions targeting the VLFPC (e.g., using neural feedback or TMS techniques) are promising in helping patients not only decrease sustained negative mood but also rehabilitate them with reward processing function and enlarge their pleasant experiences. Second, the rVLPFC, compared with its left counterpart, might be a more effective brain region in this kind of treatment.
In addition to the causal role of the VLPFC on emotion regulation, we also investigated the impact of the TMS‐induced reappraisal effect on subsequent memory and social evaluation. Participants in the rVLPFC‐activated group showed more positive memory regarding social feedback and gave more positive evaluations to those feedback senders compared with the sham TMS group. This reappraisal‐induced mnemonic effect (i.e., the memory becomes more positive after reappraisal) is consistent with a recent study demonstrating that reappraisal adaptively updates negative memory with more positive content, and that retrieval of the reappraised memory was accompanied by enhanced rVLPFC activation (Speer et al., 2021). More intriguingly, we demonstrated that this mnemonic effect mediated the relationship between emotion regulation and social evaluation, even though the mediating patterns of the two measurements (i.e., subjective and objective) of emotion regulation are not identical. Specifically, when the LPP amplitude was used as the indicator of online emotion regulation, we found complete mediating effects in both positive and negative feedback conditions. However, we only found a partial mediating effect in the positive feedback condition when the self‐reported emotional rating was used to reflect emotion regulation. In general, these results suggest that the beneficial effect of reappraisal on social evaluation is mediated by reappraisal‐induced memory changes, particularly for positive memories. These results are consistent with findings demonstrating the self‐referential positive bias: people tend to remember positive feedback about themselves (Rigney, Schnyer, Hu, & Beer, 2021; H. Xie et al., 2021; Yao, Lin, & Hu, 2021). Nevertheless, the discrepancy between the two models indicate that LPP and self‐reported ratings may capture different aspects of emotion regulation: while LPP indicates online regulation processing, ratings reflect the end‐product of emotion regulation (Kivity & Huppert, 2019; Nasso et al., 2020). Indeed, memory is an important endogenous source of emotion (Engen, Kanske, & Singer, 2017). Ruminating negative social feedback and difficulties in retrieving positive social feedback often leads to persistent social pain, self‐denial, and suspicion of social reward experiences (see Rademacher, Schulte‐Rüther, Hanewald, & Lammertz, 2017 for a review). These aberrant social memories would dampen people's willingness to engage in social interactions, and even trigger a range of psychiatric symptoms such as social withdrawal and social anhedonia (see Porcelli et al., 2019 and Weightman, Air, & Baune, 2014 for reviews). By demonstrating the mnemonic effect of reappraisal, we propose that reappraisal training is a promising way to lessen the detrimental effect of aberrant social memory on people's social cognition and behaviors (Chen, Poon, DeWall, & Jiang, 2020; Frank et al., 2014; Jiang, Chen, Wang, & Hou, 2021), especially for those who are hypersensitive to social loss (Hsu & Jarcho, 2021), or insensitive to social gain (Zhang et al., 2020).
Two limitations should be noticed when interpreting the current findings. First, this study used the international 10–20 system to locate the TMS coil. We suggest employing an image‐guided neuronavigation system to increase the precision of coil location in future studies. Second, the memory and social evaluation tasks were carried out after the reappraisal block, which may induce a confusion effect of task order. Fortunately, the two‐way interaction of TMS group × regulation type on participants' recalled valence of social feedback emerged in the reappraisal block across the three TMS groups. Thus, we believe the primary results of this study were less likely to be influenced by this potential confounding effect. Finally, it is worth noting that while this study focused on the role of lateral prefrontal control cortex in a typical emotional self‐regulation task, other research suggests that the default‐mode network, especially the medial prefrontal cortex, plays a more specific role during socially‐induced emotion regulation (X. Xie et al., 2016). Future studies exploring the VLPFC versus medial prefrontal cortex are encouraged to reveal a full map of neural networks involved in social‐related emotion regulation.
In conclusion, the present study revealed the lateralization and generalization of the rVLPFC during voluntary emotion regulation in the context of social feedback processing, that is, activating the rVLPFC facilitated both social pain relief and social reward magnification. In addition, we found the influence of emotion regulation on social evaluation was mediated by the memory of social feedback, highlighting the cognitive role of memory in social behavioral shaping and modulation. These findings have clinical implications in improving patients' emotion regulation ability and modifying their social attitudes.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in relation to the subject of this study.
AUTHOR CONTRIBUTIONS
Dandan Zhang and Sijin Li designed research; Sijin Li, Zixin Zheng, Weimao Chen, and Feng Xu performed experiment; Dandan Zhang and Sijin Li analyzed data; Dandan Zhang, Sijin Li, and Hui Xie wrote the paper; and Dandan Zhang, Sijin Li, Hui Xie, and Xiaoqing Hu revised the paper.
5.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGMENT
This study was funded by the National Natural Science Foundation of China (31970980; 31920103009) and the Shenzhen‐Hong Kong Institute of Brain Science (2021SHIBS0003).
Li, S. , Xie, H. , Zheng, Z. , Chen, W. , Xu, F. , Hu, X. , & Zhang, D. (2022). The causal role of the bilateral ventrolateral prefrontal cortices on emotion regulation of social feedback. Human Brain Mapping, 43(9), 2898–2910. 10.1002/hbm.25824
Sijin Li and Hui Xie contributed equally to the study.
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 31970980, 31920103009; Shenzhen‐Hong Kong Institute of Brain Science, Grant/Award Number: 2021SHIBS0003
DATA AVAILABILITY STATEMENT
The data and code of this study would be available upon reasonable request and with approval of the School of Psychology, Shenzhen University. More information on making this request can be obtained from the corresponding author, Dandan Zhang (zhangdd05@gmail.com).
REFERENCES
- Achterberg, M. , van Duijvenvoorde, A. C. K. , van der Meulen, M. , Bakermans‐Kranenburg, M. J. , & Crone, E. A. (2018). Heritability of aggression following social evaluation in middle childhood: An fMRI study. Human Brain Mapping, 39(7), 2828–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aknin, L. B. , Van de Vondervoort, J. W. , & Hamlin, J. K. (2018). Positive feelings reward and promote prosocial behavior. Current Opinion in Psychology, 20, 55–59. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Beck depression inventory (2nd ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Berboth, S. , & Morawetz, C. (2021). Amygdala‐prefrontal connectivity during emotion regulation: A meta‐analysis of psychophysiological interactions. Neuropsychologia, 153, 107767. [DOI] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, D. , Li, Y. , & Tang, Y. (2021). Functional specificity of the left ventrolateral prefrontal cortex in positive reappraisal: A single‐pulse transcranial magnetic stimulation study. Cognitive, Affective, & Behavioral Neuroscience, 21(4), 793–804. [DOI] [PubMed] [Google Scholar]
- Carl, J. R. , Soskin, D. P. , Kerns, C. , & Barlow, D. H. (2013). Positive emotion regulation in emotional disorders: A theoretical review. Clinical Psychology Review, 33(3), 343–360. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Poon, K.‐T. , DeWall, C. N. , & Jiang, T. (2020). Life lacks meaning without acceptance: Ostracism triggers suicidal thoughts. Journal of Personality and Social Psychology, 119(6), 1423–1443. [DOI] [PubMed] [Google Scholar]
- Chester, D. S. , & DeWall, C. N. (2014). Prefrontal recruitment during social rejection predicts greater subsequent self‐regulatory imbalance and impairment: Neural and longitudinal evidence. NeuroImage, 101, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester, D. S. , Lynam, D. R. , Milich, R. , & DeWall, C. N. (2018). Neural mechanisms of the rejection‐aggression link. Social Cognitive and Affective Neuroscience, 13(5), 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas, B. E. , Jaser, S. S. , Bettis, A. H. , Watson, K. H. , Gruhn, M. A. , Dunbar, J. P. , … Thigpen, J. C. (2017). Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta‐analysis and narrative review. Psychological Bulletin, 143(9), 939–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, D. G. , Ritchey, M. , Johnson, B. D. , & LaBar, K. S. (2007). Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion, 7(2), 354–365. [DOI] [PubMed] [Google Scholar]
- Dörfel, D. , Lamke, J.‐P. , Hummel, F. , Wagner, U. , Erk, S. , & Walter, H. (2014). Common and differential neural networks of emotion regulation by detachment, reinterpretation, distraction, and expressive suppression: A comparative fMRI investigation. NeuroImage, 101, 298–309. [DOI] [PubMed] [Google Scholar]
- Downey, G. , & Feldman, S. I. (1996). Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology, 70(6), 1327–1343. [DOI] [PubMed] [Google Scholar]
- Eckblad, M. L. , Chapman, L. J. , Chapman, J. P. , & Mishlove, M. (1982). The revised social anhedonia scale. Madison, WI: University of Wisconsin. [Google Scholar]
- Eisenberger, N. I. (2015). Social pain and the brain: Controversies, questions, and where to go from here. Annual Review of Psychology, 66(1), 601–629. [DOI] [PubMed] [Google Scholar]
- Eisenberger, N. I. , Lieberman, M. D. , & Williams, K. D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science, 302(5643), 290–292. [DOI] [PubMed] [Google Scholar]
- Engen, H. G. , Kanske, P. , & Singer, T. (2017). The neural component‐process architecture of endogenously generated emotion. Social Cognitive and Affective Neuroscience, 12(2), 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, B. Q. , Feinberg, M. , Lam, P. , Mauss, I. B. , & John, O. P. (2019). Using reappraisal to regulate negative emotion after the 2016 U.S. presidential election: Does emotion regulation trump political action? Journal of Personality and Social Psychology, 117(5), 998–1015. [DOI] [PubMed] [Google Scholar]
- Frank, D. W. , Dewitt, M. , Hudgens‐Haney, M. , Schaeffer, D. J. , Ball, B. H. , Schwarz, N. F. , … Sabatinelli, D. (2014). Emotion regulation: Quantitative meta‐analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–211. [DOI] [PubMed] [Google Scholar]
- Gainotti, G. (2019). Emotions and the right hemisphere: Can new data clarify old models? The Neuroscientist, 25(3), 258–270. [DOI] [PubMed] [Google Scholar]
- Goldin, P. R. , McRae, K. , Ramel, W. , & Gross, J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening, S. G. , Osuch, E. A. , Williamson, P. C. , & Mitchell, D. G. V. (2014). The neural correlates of regulating positive and negative emotions in medication‐free major depression. Social Cognitive and Affective Neuroscience, 9(5), 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J. , & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. Journal of Personality and Social Psychology, 85(2), 348–362. [DOI] [PubMed] [Google Scholar]
- Guyer, A. E. , Choate, V. R. , Pine, D. S. , & Nelson, E. E. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak, G. , & Nieuwenhuis, S. (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience, 6(4), 291–297. [DOI] [PubMed] [Google Scholar]
- Hayes, A. (2013). Introduction to mediation, moderation, and conditional process analysis. Journal of Educational Measurement, 51(3), 335–337. [Google Scholar]
- He, Z. , Lin, Y. , Xia, L. , Liu, Z. , Zhang, D. , & Elliott, R. (2018). Critical role of the right VLPFC in emotional regulation of social exclusion: A tDCS study. Social Cognitive and Affective Neuroscience, 13(4), 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z. , Liu, Z. , Zhao, J. , Elliott, R. , & Zhang, D. (2020). Improving emotion regulation of social exclusion in depression‐prone individuals: A tDCS study targeting right VLPFC. Psychological Medicine, 50(16), 2768–2779. [DOI] [PubMed] [Google Scholar]
- He, Z. , Zhao, J. , Shen, J. , Muhlert, N. , Elliott, R. , & Zhang, D. (2020). The right VLPFC and downregulation of social pain: A TMS study. Human Brain Mapping, 41(5), 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, A. S. , Johnstone, T. , Light, S. N. , Peterson, M. J. , Kolden, G. G. , Kalin, N. H. , & Davidson, R. J. (2013). Relationships between changes in sustained fronto‐striatal connectivity and positive affect in major depression resulting from antidepressant treatment. American Journal of Psychiatry, 170(2), 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, W. , Nitschke, J. B. , & Miller, G. A. (1998). Lateralization in emotion and emotional disorders. Current Directions in Psychological Science, 7(1), 26–32. [Google Scholar]
- Hsu, D. T. , & Jarcho, J. M. (2021). Next up for psychiatry: Rejection sensitivity and the social brain. Neuropsychopharmacology, 46(1), 239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, D. T. , Sankar, A. , Malik, M. A. , Langenecker, S. A. , Mickey, B. J. , & Love, T. M. (2020). Common neural responses to romantic rejection and acceptance in healthy adults. Social Neuroscience, 15(5), 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn, A. S. , Meyer, R. E. , Harris, J. D. , Ayaz, H. , Deneke, E. , Stankoski, D. M. , & Bunce, S. C. (2016). Evidence of anhedonia and differential reward processing in prefrontal cortex among post‐withdrawal patients with prescription opiate dependence. Brain Research Bulletin, 123, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, T. , Chen, Z. , Wang, S. , & Hou, Y. (2021). Ostracism disrupts self‐continuity. Personality and Social Psychology Bulletin, 47(9), 1390–1400. [DOI] [PubMed] [Google Scholar]
- Johnstone, T. , van Reekum, C. M. , Urry, H. L. , Kalin, N. H. , & Davidson, R. J. (2007). Failure to regulate: Counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. Journal of Neuroscience, 27(33), 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch, R. (2009). The functional neuroanatomy of reappraisal: Time matters. Neuroscience and Biobehavioral Reviews, 33(8), 1215–1226. [DOI] [PubMed] [Google Scholar]
- Kanske, P. , Heissler, J. , Schönfelder, S. , & Wessa, M. (2012). Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage, 61(3), 686–693. [DOI] [PubMed] [Google Scholar]
- Kennedy, H. , & Montreuil, T. C. (2021). The late positive potential as a reliable neural marker of cognitive reappraisal in children and youth: A brief review of the research literature. Frontiers in Psychology, 11, 608522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. U. , Weisenbach, S. L. , & Zald, D. H. (2019). Ventral prefrontal cortex and emotion regulation in aging: A case for utilizing transcranial magnetic stimulation. International Journal of Geriatric Psychiatry, 34(2), 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. H. , & Hamann, S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19(5), 776–798. [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , & Hamann, S. (2012). The effect of cognitive reappraisal on physiological reactivity and emotional memory. International Journal of Psychophysiology, 83(3), 348–356. [DOI] [PubMed] [Google Scholar]
- Kivity, Y. , & Huppert, J. D. (2019). Emotion regulation in social anxiety: A systematic investigation and meta‐analysis using self‐report, subjective, and event‐related potentials measures. Cognition and Emotion, 33(2), 213–230. [DOI] [PubMed] [Google Scholar]
- Kohn, N. , Eickhoff, S. B. , Scheller, M. , Laird, A. R. , Fox, P. T. , & Habel, U. (2014). Neural network of cognitive emotion regulation—An ALE meta‐analysis and MACM analysis. NeuroImage, 87, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok, B. E. , Coffey, K. A. , Cohn, M. A. , Catalino, L. I. , Vacharkulksemsuk, T. , Algoe, S. B. , … Fredrickson, B. L. (2013). How positive emotions build physical health: Perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychological Science, 24(7), 1123–1132. [DOI] [PubMed] [Google Scholar]
- Kujawa, A. , Arfer, K. B. , Klein, D. N. , & Proudfit, G. H. (2014). Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Developmental Cognitive Neuroscience, 10, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeslag, S. J. E. , & van Strien, J. W. (2013). Up‐regulation of emotional responses to reward‐predicting stimuli: An ERP study. Biological Psychology, 94(1), 228–233. [DOI] [PubMed] [Google Scholar]
- Liebowitz, M. R. (1987). Social phobia. Modern Problems of Pharmacopsychiatry, 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Liu, F. , Chen, L. , Jiang, Z. , & Shang, J. (2019). Cognitive reappraisal in children: Neuropsychological evidence of up‐regulating positive emotion from an ERP study. Frontiers in Psychology, 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Huang, H. , McGinnis‐Deweese, M. , Keil, A. , & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. The Journal of Neuroscience, 32(42), 14563–14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae, K. , & Gross, J. J. (2020). Emotion regulation. Emotion, 20(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Miller, A. B. , Prinstein, M. J. , Munier, E. , Machlin, L. S. , & Sheridan, M. A. (2019). Emotion reactivity and regulation in adolescent girls following an interpersonal rejection. Journal of Cognitive Neuroscience, 31(2), 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. A. , Crocker, L. D. , Spielberg, J. M. , Infantolino, Z. P. , & Heller, W. (2013). Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Frontiers in Integrative Neuroscience, 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Bode, S. , Baudewig, J. , Jacobs, A. M. , & Heekeren, H. R. (2016). Neural representation of emotion regulation goals. Human Brain Mapping, 37(2), 600–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Bode, S. , Derntl, B. , & Heekeren, H. R. (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta‐analysis of fMRI studies. Neuroscience and Biobehavioral Reviews, 72, 111–128. [DOI] [PubMed] [Google Scholar]
- Naor, N. , Rohr, C. , Schaare, L. H. , Limbachia, C. , Shamay‐Tsoory, S. , & Okon‐Singer, H. (2020). The neural networks underlying reappraisal of empathy for pain. Social Cognitive and Affective Neuroscience, 15(7), 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasso, S. , Vanderhasselt, M.‐A. , Schettino, A. , & De Raedt, R. (2020). The role of cognitive reappraisal and expectations in dealing with social feedback. Emotion. Advance online publication. 10.1037/emo0000825 [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, S. , Simon, D. , Endrass, T. , & Kathmann, N. (2016). Altered emotion regulation in obsessive‐compulsive disorder as evidenced by the late positive potential. Psychological Medicine, 46(1), 137–147. [DOI] [PubMed] [Google Scholar]
- Porcelli, S. , Van Der Wee, N. , van der Werff, S. , Aghajani, M. , Glennon, J. C. , van Heukelum, S. , … Serretti, A. (2019). Social brain, social dysfunction and social withdrawal. Neuroscience and Biobehavioral Reviews, 97, 10–33. [DOI] [PubMed] [Google Scholar]
- Pressman, S. D. , Jenkins, B. N. , & Moskowitz, J. T. (2019). Positive affect and health: What do we know and where next should we go? Annual Review of Psychology, 70(1), 627–650. [DOI] [PubMed] [Google Scholar]
- Quoidbach, J. , Mikolajczak, M. , & Gross, J. J. (2015). Positive interventions: An emotion regulation perspective. Psychological Bulletin, 141(3), 655–693. [DOI] [PubMed] [Google Scholar]
- Rademacher, L. , Schulte‐Rüther, M. , Hanewald, B. , & Lammertz, S. (2017). Reward: From basic reinforcers to anticipation of social cues. Current Topics in Behavioral Neurosciences, 30, 207–221. [DOI] [PubMed] [Google Scholar]
- Rappaport, B. I. , & Barch, D. M. (2020). Brain responses to social feedback in internalizing disorders: A comprehensive review. Neuroscience and Biobehavioral Reviews, 118, 784–808. [DOI] [PubMed] [Google Scholar]
- Rigney, A. E. , Schnyer, D. M. , Hu, X. , & Beer, J. S. (2021). Mechanisms of a spotless self‐image: Navigating negative, self‐relevant feedback. Self and Identity, 20(8), 1057–1076. [Google Scholar]
- Riva, P. , Romero Lauro, L. J. , Dewall, C. N. , & Bushman, B. J. (2012). Buffer the pain away: Stimulating the right ventrolateral prefrontal cortex reduces pain following social exclusion. Psychological Science, 23(12), 1473–1475. [DOI] [PubMed] [Google Scholar]
- Ross, E. D. (2021). Differential hemispheric lateralization of emotions and related display behaviors: Emotion‐type hypothesis. Brain Sciences, 11(8), 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach, L. (2016). Towards a second‐person neuropsychiatry. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371(1686), 20150081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobe, E. R. (2014). Independent and collaborative contributions of the cerebral hemispheres to emotional processing. Frontiers in Human Neuroscience, 8, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton, R. L. , Kahrilas, I. J. , Skymba, H. V. , Smith, J. , Bryant, F. B. , & Heller, W. (2020). Regulating positive emotions: Implications for promoting well‐being in individuals with depression. Emotion, 20(1), 93–97. [DOI] [PubMed] [Google Scholar]
- Silvers, J. A. , Insel, C. , Powers, A. , Franz, P. , Helion, C. , Martin, R. E. , … Ochsner, K. N. (2017). VlPFC–vmPFC–amygdala interactions underlie age‐related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, R. L. , & Leary, M. R. (2009). Reactions to discrimination, stigmatization, ostracism, and other forms of interpersonal rejection: A multimotive model. Psychological Review, 116(2), 365–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. (2013). The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22(2), 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. , Heatherton, T. F. , & Kelley, W. M. (2006). Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience, 9(8), 1007–1008. [DOI] [PubMed] [Google Scholar]
- Speer, M. E. , Ibrahim, S. , Schiller, D. , & Delgado, M. R. (2021). Finding positive meaning in memories of negative events adaptively updates memory. Nature Communications, 12(1), 6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. E. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory (form Y1–Y2). Palo Alto, CA: Consulting Psychologist Press. [Google Scholar]
- Tabibnia, G. , Monterosso, J. R. , Baicy, K. , Aron, A. R. , Poldrack, R. A. , Chakrapani, S. , … London, E. D. (2011). Different forms of self‐control share a neurocognitive substrate. The Journal of Neuroscience, 31(13), 4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong, I. S. K. , Tan, G. , & Jensen, M. P. (2017). The buffering role of positive affect on the association between pain intensity and pain related outcomes. Scandinavian Journal of Pain, 14, 91–97. [DOI] [PubMed] [Google Scholar]
- Twenge, J. M. , Baumeister, R. F. , DeWall, C. N. , Ciarocco, N. J. , & Bartels, J. M. (2007). Social exclusion decreases prosocial behavior. Journal of Personality and Social Psychology, 92(1), 56–66. [DOI] [PubMed] [Google Scholar]
- Vanderlind, W. M. , Millgram, Y. , Baskin‐Sommers, A. R. , Clark, M. S. , & Joormann, J. (2020). Understanding positive emotion deficits in depression: From emotion preferences to emotion regulation. Clinical Psychology Review, 76, 101826. [DOI] [PubMed] [Google Scholar]
- van't Wout, M. , Chang, L. J. , & Sanfey, A. G. (2010). The influence of emotion regulation on social interactive decision‐making. Emotion, 10(6), 815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar, N. , Cheng, T. W. , & Pfeifer, J. H. (2017). Neural correlates of social exclusion across ages: A coordinate‐based meta‐analysis of functional MRI studies. NeuroImage, 153, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Davidson, M. L. , Hughes, B. L. , Lindquist, M. A. , & Ochsner, K. N. (2008). Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Braun, C. , & Enck, P. (2017). How the brain reacts to social stress (exclusion)—A scoping review. Neuroscience and Biobehavioral Reviews, 80, 80–88. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Liao, C. , Shangguan, C. , Shang, W. , & Zhang, W. (2020). Individual differences in emotion differentiation modulate electrocortical dynamics of cognitive reappraisal. Psychophysiology, 57(12), e13690. [DOI] [PubMed] [Google Scholar]
- Weightman, M. J. , Air, T. M. , & Baune, B. T. (2014). A review of the role of social cognition in major depressive disorder. Frontiers in Psychiatry, 5, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, K. A. , & MacNamara, A. (2021). Savor the moment: Willful increase in positive emotion and the persistence of this effect across time. Psychophysiology, 58(3), e13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, H. , Hu, X. , Mo, L. , & Zhang, D. (2021). Forgetting positive social feedback is difficult: ERP evidence in a directed forgetting paradigm. Psychophysiology, 58(5), e13790. [DOI] [PubMed] [Google Scholar]
- Xie, H. , Mo, L. , Li, S. , Liang, J. , Hu, X. , & Zhang, D. (2022). Aberrant social feedback processing and its impact on memory, social evaluation, and decision‐making among individuals with depressive symptoms. Journal of Affective Disorders, 300, 366–376. [DOI] [PubMed] [Google Scholar]
- Xie, X. , Mulej Bratec, S. , Schmid, G. , Meng, C. , Doll, A. , Wohlschläger, A. , … Sorg, C. (2016). How do you make me feel better? Social cognitive emotion regulation and the default mode network. NeuroImage, 134, 270–280. [DOI] [PubMed] [Google Scholar]
- Yao, Z. , Lin, X. , & Hu, X. (2021). Optimistic amnesia: How online and offline processing shape belief updating and memory biases in immediate and long‐term optimism biases. Social Cognitive and Affective Neuroscience, 16(5), 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H. , Tully, L. M. , Lincoln, S. H. , & Hooker, C. I. (2015). Adults with high social anhedonia have altered neural connectivity with ventral lateral prefrontal cortex when processing positive social signals. Frontiers in Human Neuroscience, 9, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Shen, J. , Bi, R. , Zhang, Y. , Zhou, F. , Feng, C. , & Gu, R. (2020). Differentiating the abnormalities of social and monetary reward processing associated with depressive symptoms. Psychological Medicine. Psychological Medicine, 1–15. Advance online publication. 10.1017/S0033291720003967 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Mo, L. , Bi, R. , He, Z. , Chen, Y. , Xu, F. , … Zhang, D. (2021). The VLPFC versus the DLPFC in downregulating social pain using reappraisal and distraction strategies. The Journal of Neuroscience, 41(6), 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
The data and code of this study would be available upon reasonable request and with approval of the School of Psychology, Shenzhen University. More information on making this request can be obtained from the corresponding author, Dandan Zhang (zhangdd05@gmail.com).


