Abstract
In the 1970s, an unknown virus was suspected for documented cases of transfusion-associated hepatitis, a phenomenon called non-A, non-B hepatitis. In 1989, the infectious transmissible agent was identified and named hepatitis C virus (HCV) and, soon enough, the first diagnostic HCV antibody test was developed, which led to a dramatic decrease in new infections. Today, HCV infection remains a global health burden and a major cause of liver cirrhosis, hepatocellular carcinoma and liver transplantation. However, tremendous advances have been made over the decades, and HCV became the first curable, chronic viral infection. The introduction of direct antiviral agents revolutionized antiviral treatment, leading to viral eradication in more than 98% of all patients infected with HCV. This Perspective discusses the history of HCV research, which reads like a role model for successful translational research: starting from a clinical observation, specific therapeutic agents were developed, which finally were implemented in national and global elimination programmes.
Subject terms: Hepatitis C, Liver
The history of hepatitis C virus (HCV) is a role model for successful basic, translational and clinical research. In this Perspective, the authors chart a timeline of breakthroughs in hepatitis C research, from discovery to cure.
Introduction
In the 1970s, clinicians repeatedly documented cases of transfusion-associated hepatitis, which frequently took a chronic, progressive course and could be attributed neither to the hepatitis A virus (HAV), the hepatitis B virus (HBV) nor to any other known cause. This phenomenon was called non-A, non-B hepatitis (NANBH). It took approximately 20 years until the hepatitis C virus (HCV) was finally identified as the aetiological agent causing NANBH. As early as 1986, that is, 3 years before the discovery of the virus, interferon-α (IFNα) was used as the first antiviral agent, with regimens lasting up to 72 weeks. However, tolerability was low and efficacy quite limited; cure rates were less than 20% for these first regimens. Still today, chronic HCV infection remains a global health burden and a major cause of liver cirrhosis, hepatocellular carcinoma (HCC) and liver transplantation worldwide1,2. However, tremendous advances have been made, and HCV infection became the first curable, chronic viral infection in humans. Iatrogenic transmission (such as blood transfusion), which used to be the main route of infection, was dramatically reduced owing to effective hygienic measures and, in particular, by screening blood donors and blood products first for HCV antibody and then for HCV RNA3,4. Subsequently, antiviral treatment was revolutionized, leading to viral eradication in more than 98% of all patients infected with HCV treated by all-oral therapy, usually lasting for only 8–12 weeks and with no or only minor adverse effects5.
Although an HCV vaccine is not on the horizon yet, in 2016, the WHO proclaimed the ambitious goal to reduce new HCV infections by 90% by 2030, with the ultimate goal of HCV elimination6. The history of HCV research reads like a role model for successful biomedical and translational research, starting from a clinical observation, via identification of the underlying aetiology (a virus), the establishment of diagnostic tests, unravelling of the viral life cycle, development of specific therapeutic agents and finally implementation of a global elimination programme. HCV antiviral treatment was the result of joint efforts and close collaborations between scientists and physicians as well as the pharmaceutical and diagnostic industries. Basic virologists, translational researchers, clinician-scientists and epidemiologists made important contributions in this process. As a consequence, the Nobel Prize in Physiology or Medicine in 2020 was jointly awarded to Harvey J. Alter, Michael Houghton and Charles M. Rice. These three prominent scientists stand for the whole scientific community, making the HCV story a masterpiece of translational research. In this Perspective, we discuss major breakthroughs in HCV research over the past 50 years that transformed a life-threatening disease into an easy to cure disease7 (Fig. 1).
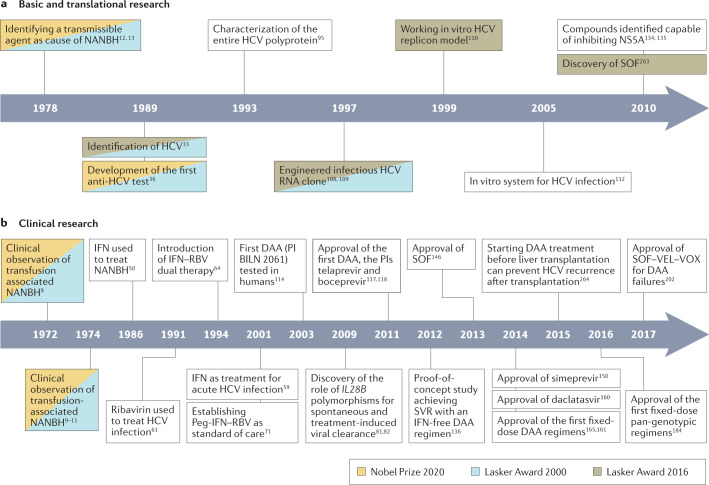
Fig. 1. Major breakthroughs in HCV history.
Breakthroughs are separated into basic and translational (part a) and clinical (part b) research, and research that formed part of major awards is indicated. DAA, direct-acting antiviral agent; HCV, hepatitis C virus; IFN, interferon-α; NANBH, non-A, non-B hepatitis; NS5A, nonstructural protein 5A; Peg-IFN, pegylated interferon-α; PI, protease inhibitor; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir. Additional refs263,264.
Discovering HCV
An unknown, transmissible agent
Post-transfusion hepatitis used to be a frequent phenomenon in the middle of the twentieth century. Shortly after the identification of HBV (1965) and HAV (1973), it became evident that many of these hepatitis cases were attributable to neither of these two nor any other known infectious agent8–11. Feinstone and colleagues11 were among the first to show this phenomenon in a well-defined cohort of patients with post-transfusion hepatitis. Serological assays were used to exclude HAV, HBV, cytomegalovirus and Epstein–Barr virus infection. However, the authors already suspected the presence of a so far unknown infectious trigger. Other groups published similar findings at that time9. The newly identified disease was later named NANBH and was found to be responsible for up to 90% of post-transfusion hepatitis12. The presence of a transmissible infectious agent was finally confirmed in 1978. Two landmark studies by Alter et al.12 and Tabor et al.13 with a similar design were published in the same edition of The Lancet. Both groups demonstrated that plasma and/or serum of patients with NANBH could induce clinically apparent hepatitis in healthy chimpanzees. Importantly, the onset of hepatitis was 2–10 weeks after the injection with infectious plasma and/or serum, similar to the incubation period assumed for human cases of NANBH. The relatively long incubation period indicated that the documented increase in transaminase levels was not related to some nonspecific immune reaction to the respective blood product. Moreover, the investigators documented concomitant inflammation in liver histology12,13. Further studies confirmed the persistence of hepatic inflammation for more than 1 year, suggesting a chronic course of the disease in a substantial number of patients14.
Soon, it became evident that disease transmission was not limited to blood transfusions but could occur via other blood-derived products. A particularly high frequency of NANBH cases was documented among patients with haemophilia15–18 and in patients receiving intravenous immunoglobulins19,20. For some blood products, for example, fibrinogen, factor VIII and factor IX, infectivity could be proved by transmission studies in chimpanzees16,17,21. Additional transmission routes were suspected for persons who injected drugs (PWIDs)22,23 and those with end-stage renal disease (ESRD) undergoing haemodialysis24.
Long-term follow-up of patients with NANBH revealed that 50–80% developed a chronic cause leading to progressive liver disease, including liver cirrhosis and HCC, highlighting the urgent unmet medical need to prevent further spread of this disease25–29. Some physicians suggested avoiding blood products from patients with increased alanine aminotransferase (ALT) levels, as Aach et al.30 observed a higher incidence of post-transfusion NANBH in recipients of blood products from donors with elevated ALT levels (≥60 international units (IU) per millilitre) compared with those with normal ALT values (<29 IU/ml), 45% versus 6%, respectively. Importantly, their data also indicated that a substantial number of donors were infectious despite normal or only mildly elevated ALT levels30. The elimination of blood donors with elevated ALT levels and a change from commercial to voluntary blood donation markedly reduced post-transfusion hepatitis.
Identifying the virus
Over the years, researchers provided increasing physicochemical evidence that the infectious agent that triggered NANBH was a small, enveloped viral agent, for example, by demonstrating that it induced specific changes in hepatocytes, was not held back by an 80 nm-sized membrane filter, but could be inactivated by chloroform31,32. The major breakthrough was achieved and published in 1989 when Choo and colleagues from Michael Houghton’s group at Chiron (USA) discovered the HCV33. They assumed that the reason for the failure of previous attempts was a very low viral concentration. Thus, they created a complementary DNA (cDNA) library using randomly created primers, reverse transcriptase and plasma from an infected chimpanzee shown to have a particularly high titre of the presumed infectious agent. The cDNA was inserted into a viral cloning vector, phage λgt11, expressed in Escherichia coli capable of inducing the production of the cDNA-encoded polypeptides. Approximately 1 million clones were screened for viral proteins using serum from a patient with chronic NANBH until the cDNA clone 5-1-1 was finally identified. Southern blot analysis excluded human or chimpanzee origin of the genomic fragment. Reactivity with the polypeptide synthesized from cDNA clone 5-1-1 was confirmed with sera from seven additional patients with NANBH. Further experiments provided the final proof that the discovered infectious agent was indeed a RNA virus, which was later termed HCV33.
Development of anti-HCV assays
With the clinical burden becoming increasingly evident, a worldwide search was initiated for ways to identify infected individuals and blood products. As discussed earlier, indirect tests (for example, relying on ALT serum levels or the detection of antibodies to the HBV core antigen (anti-HBc)) had limited efficacy as they failed to detect patients without clinically apparent hepatitis or HBV co-infection, respectively3,30,34. Thus, a specific diagnostic test was urgently needed. Over the years, several groups claimed to have discovered the NANBH agent by applying technologies of that time, such as immunofluorescence, immunoelectrophoresis, radio- and enzyme-immunoassays and electron microscopy, without success. Harvey Alter at the NIH (USA) established a well-defined panel of sera from patients with NANBH that transmitted the disease to chimpanzees (‘Alter panel’). The panel also included control sera from obviously healthy individuals with normal ALT values, no previous history of hepatitis and no serological markers of HBV infection33,35,36.
The ultimate game-changer was the discovery of HCV. With the HCV cDNA clone in their hands, Michael Houghton and colleagues at Chiron rapidly developed an assay to detect HCV antibodies. The respective manuscript by Kuo et al.36 was published in 1989 in the same edition of Science as the paper by Choo et al.33 that reported the discovery of the virus. Kuo and colleagues36 created a fusion protein (also known as C100-3) consisting of the HCV polypeptide and the human superoxide dismutase, which was then coated on microtitre plates and was capable of capturing circulating HCV antibodies. In a second step, an additional radioactive antibody identified the captured HCV antibodies. The newly developed assay was successfully validated in a blind fashion in the Alter panel, yielding positive results in six of seven sera from patients with NANBH, whereas all control sera from healthy individuals tested negative. Notably, the negative result was obtained in a patient assumed to be in the very acute phase of infection36.
The availability of a diagnostic test brought the opportunity to address several important, but so far unanswered, questions in NANBH. It was now possible to better estimate the proportion of post-transfusion hepatitis that was really attributable to HCV, which, interestingly, varied between 15% and 80% among different international cohorts in the first dedicated study36. However, a suboptimal sensitivity needed to be considered when interpreting these early results. Moreover, it was possible to investigate better HCV epidemiology confirming a particularly high prevalence among PWIDs, in patients with haemophilia, those requiring haemodialysis37,38, and those with liver cirrhosis and/or HCC39,40. Additionally, notable regional differences became apparent when investigating anti-HCV prevalence in the overall population41. Finally, the likely most important achievement was the ability to test blood products, preventing the further iatrogenic spread of the virus. Even with the first versions of the anti-HCV assay, the vast majority of NANBH-transmitting donors were detected in the first pilot studies, underlining the vast potential for future prevention of viral transmission3,42. Very quickly, commercial enzyme-linked immunosorbent assays became available for broad anti-HCV screening. In a landmark study from Japan, systematic screening of blood donors reduced the incidence of post-transfusion NANBH from 5% to 2%43. In patients who received more than ten blood transfusions, the incidence was reduced from 16% to 3%43. Shortly after the introduction of the first tests, additional development of anti-HCV assays led to markedly improved sensitivity. The next-generation assays demonstrated that a far higher proportion of NANBHs were caused by HCV than had been assumed after the initial studies using first-generation anti-HCV assays44. Consequent screening of all blood donors almost eliminated the risk of viral transmission through blood and blood products in high-income countries by testing for anti-HCV antibodies alone45 (Fig. 2). Additional safety was later provided by the introduction of PCR-based tests to identify HCV RNA and, therefore, the virus itself, also enabling the detection of patients in the very early phase of acute HCV infection when HCV antibodies have not yet developed4,46. However, given the costs and limited availability of such screening tools in countries with low and middle-income economies, health-care-associated transmission remains an important source of new HCV infections on a global scale47.
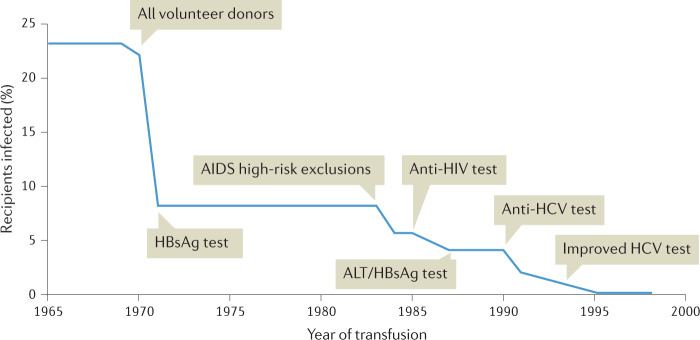
Fig. 2. Impact of different measures on the incidence of post-transfusion NANBH.
Development of the risk for post-transfusion non-A, non-B hepatitis (NANBH) over time. Over the years, various measures have been taken to reduce the risk of post-transfusion hepatitis. This included the exclusion of paid blood donors, screening for alanine aminotransferase (ALT) levels as well as hepatitis B virus (HBV) infection, and ultimately testing donors for anti-hepatitis C virus (HCV) antibodies and HCV RNA. AIDS, acquired immunodeficiency syndrome; HBsAg, hepatitis B surface antigen. Adapted with permission from ref.265, H. J. Alter.
Interferon antiviral treatment
IFNα monotherapy
The first attempts of antiviral treatment for NANBH were made in 1986, which was, notably, 3 years before the discovery of HCV. Recombinant IFNα (also known as IFN alfa) had become available in the 1980s in cancer treatment before promising results were published towards HBV infection48,49. Suspecting an unknown viral pathogen as the underlying cause of NANBH, Hoofnagle and colleagues50 decided to try recombinant IFNα for this so far poorly understood disease50. In their pilot study, they treated ten patients with NANBH, with recombinant IFNα2b, for up to 12 months. The investigators documented a substantial decrease in the levels of serum transaminases50. This finding indicated the start of the IFN era in HCV therapy. On the basis of these findings, larger randomized studies were initiated, which confirmed the effect of IFNα in a large population of patients infected with HCV and paved the way towards the establishment of IFNα monotherapy as the standard of care for some time, although efficacy seemed to be limited to less than 40%51,52.
However, for the further development of HCV therapies, it was essential to establish virological end points. In the early 1990s, a couple of studies demonstrated that the decreased serum transaminase levels during IFNα treatment were directly paralleled by a decrease in HCV RNA level or detection53,54. Similarly, it was shown that a relapse of transaminases after treatment cessation was accompanied by reappearance of HCV RNA in serum55. Given these results and the broad availability of robust assays, HCV RNA was further used as a primary biomarker to determine treatment response. A sustained virological response (SVR) 24 weeks after the end of treatment was regarded as equivalent to a cure (Table 1), as it was shown that virological relapse after this time point was extremely rare56,57. Moreover, HCV RNA clearance was linked to consistent improvements in liver histology58.
Table 1.
Definitions for treatment response in interferon-based response-guided therapies
| Term | Abbreviation | Definition |
|---|---|---|
| Sustained virological response | SVR | Undetectable HCV RNA 12–24 weeks after the end of therapy |
| Rapid virological response | RVR | Undetectable HCV RNA at week 4 of therapy |
| Early virological response | EVR | HCV RNA decline ≥2 log10 at week 12 |
| Complete early virological response | cEVR | Undetectable HCV RNA at week 12 |
| Partial early virological response | pEVR | HCV RNA decline ≥2 log10 at week 12 |
| Relapse | RL | HCV RNA negative at the end of treatment and recurrence of HCV RNA during the follow-up of 24 weeks |
| Partial response | PR | HCV RNA decline ≥2 log10 at week 12 but positive at week 24 during Peg-IFN–RBV therapy |
| Null response | NULL | HCV RNA decline <2 log10 at week 12 during Peg-IFN–RBV therapy |
Response at weeks 4 and 12 of pegylated interferon-α (Peg-IFN)-based regimens was used to determine the optimal treatment duration. Patients with a fast decline who achieved a rapid virological response (RVR) were eligible for short-term regimens without impairing sustained virological response (SVR) rates. By contrast, a poor response until week 12 of treatment identified patients in whom the chance of SVR was minimal and, therefore, treatment should be stopped early. Response-guided therapy minimized adverse events of Peg-IFN–ribavirin (RBV) therapy. Moreover, in those patients who failed antiviral treatment response during treatment, it was essential to estimate SVR chances for subsequent treatment attempts. HCV, hepatitis C virus.
In 2001, roughly 10 years after IFNα was established for chronic HCV infection, Jaeckel and collaborators59 demonstrated with their first German Acute HCV study that treating 44 patients in the early, acute phase of infection leads to a substantial increase in SVR rates to 98%. Although IFN had been suggested before in acute HCV infection60, it was the data of this landmark study that established IFN as a first-line option in many centres.
Introduction of ribavirin
The next major step forwards in treating chronic HCV infection was the introduction of the second (non-HCV-specific) antiviral compound, the nucleoside analogue ribavirin (RBV). In 1991, Reichard et al.61 published the results of their pilot study evaluating RBV monotherapy in ten patients with HCV infection, which indicated limited efficacy. Median ALT values decreased during treatment but rapidly relapsed after treatment cessation61. Moreover, Di Bisceglie et al.62 reported that RBV achieved only a minor decrease in HCV RNA serum levels. However, in contrast to RBV monotherapy, its addition to IFN led to an impressive increase in SVR63,64. A small pilot study investigating this combination was published by Brillanti and colleagues64 in 1994. SVR was achieved in 40% of patients (n = 10) treated with IFN–RBV but in none of those (n = 10) receiving IFNα monotherapy. Additional large multicentre studies confirmed the superior efficacy of dual therapy, making it the new standard of care in 1998 (refs65–67).
Pegylated interferons
The use of long-acting pegylated (Peg) IFNs represented the next tremendous milestone in HCV therapy. These modified Peg-IFNs showed a favourable, prolonged pharmacokinetic profile with two important outcomes. First, the dosing schedule could be simplified to once per week, promising improved treatment adherence, which had been shown to be crucial for treatment success68. Second, antiviral efficacy was higher. Unmodified conventional recombinant IFN had a half-life of 3–8 h and became undetectable in serum within 24 h (refs69,70). Thus, the commonly applied regimen of three times per week seemed insufficient when aiming for a permanent antiviral effect. Indeed, an increase in HCV RNA levels could be detected before the next IFN injection with this approach, a phenomenon that was not seen with Peg-IFN. Two different types of Peg-IFN, Peg-IFNα2a (40 kDa Peg chain) and Peg-IFNα2b (12 kDa Peg chain) were finally approved by the FDA and the European Medicines Agency (EMA). The first landmark study was published in 2001 (refs71,72). It was a large, randomized, multicentre, global trial that involved more than 1,530 patients with HCV infection, with a head-to-head comparison of dual therapy with IFNα2b plus RBV (1,000–1,200 mg daily) (n = 505 patients) and Peg-IFNα2b (4 weeks of 1.5 μg/kg and 44 weeks of 0.5 μg/kg) plus RBV (1,000–1,200 mg daily) (n = 514 patients). In a third group (n = 511 patients), a higher Peg-IFNα2b (1.5 μg/kg per week) but lower RBV dose (800 mg daily) was used. Notably, this third group achieved the highest SVR rates (54%), whereas there was no difference between the other two treatment arms. Similar data were published 1 year later for Peg-IFNα2a by Fried and colleagues73. Although safety was quite similar between Peg-IFN and IFN in the two studies, the slightly higher efficacy and the more convenient weekly dosing schedule established Peg-IFN–RBV as the new standard of care71,73. The approval was followed by an intensive debate about whether one Peg-IFN should be preferred over the other. Some smaller studies suggested superior efficacy of Peg-IFNα2a74,75. However, the multicentre IDEAL study, which involved more than 3,000 patients with HCV infection, indicated that the two Peg-IFNs could be used interchangeably76. An overview of some of the most important studies regarding interferon-based treatment is listed in Supplementary Table 1.
Peg-IFN–RBV remained the standard of care for a decade (2001–2011). During this time period, the safety and efficacy of the regimen were improved, such as by developing individualized approaches for optimal dosing and treatment duration. Various response predictors were identified, such as the presence of cirrhosis, HCV genotype and baseline HCV RNA serum level77. However, the most important advantage might have been the introduction of response-guided therapy, making HCV therapy an early role model for personalized medicine. In a retrospective analysis of 1,383 patients, Fried and colleagues78 demonstrated that achieving undetectable HCV RNA at week 4 on treatment, a so-called rapid virological response (RVR) (Table 1), was the most important predictor for achieving SVR78. RVR allowed abbreviation of antiviral treatment to 12–16 weeks without a significant decrease in SVR rates, for example, patients infected with HCV genotype 2 or 3 (ref.79). By contrast, a slower virological response indicated the need for longer treatment duration, for example, up to 72 weeks for genotype 1 (ref.80) (Box 1).
Close to the end of the Peg-IFN–RBV era, genome-wide association studies revealed a very strong association of single-nucleotide polymorphisms (SNPs) in the IL28B (also known as IFNL3) gene with treatment response to IFN-based regimens in patients with chronic HCV infection. Moreover, IL28B SNPs were strongly linked to spontaneous clearance of acute HCV infection81–83. Treatment with IFNα induced the production of IFNλ (type III IFN) in infected hepatocytes, which led to the upregulation of IFN-stimulated genes, which modulate antiviral efficacy. The identified SNP upstream of the IFNG promoter widely determined IFNλ response to IFNα and/or HCV infection84,85. Notably, markedly different geographical distributions of IL28B genotypes were documented, which can partly explain the different SVR rates that had previously been described, for example, between people of European ancestry, East and/or South Asian and Black individuals86,87. The discovery of the relevance of IL28B SNPs was a major breakthrough for the understanding of genetic differences in the host response to HCV and potentially also other viral infections. Personalized treatment approaches were suggested. However, the clinical effect in HCV care remained overall limited given the advent of novel HCV drugs.
Box 1 Limitations of triple therapy with first-generation protease inhibitors.
Boceprevir and telaprevir, as first-generation direct-acting antiviral agents (DAAs), had some serious limitations that were addressed by succeeding generations of DAAs. Antiviral efficacy was still highly dependent on response to pegylated interferon-α (Peg-IFN). Thus, in previous null responders, sustained virological response rate did not exceed 30–40%120,127. Moreover, the dosing schedule was inconvenient. Daily boceprevir dosage is four pills every 8 h, which adds up to more than 16 pills per day for hepatitis C virus (HCV) treatment if ribavirin is also considered. Two tablets were required every 8 h when using telaprevir accompanied by an intake of at least 20 g of fat to ensure adequate absorption of the drug. The telaprevir dosing schedule was later modified to three tablets twice daily, which had proved to have a similar efficacy267. Both protease inhibitors were substrates and inhibitors of P-glycoprotein and cytochrome P450 3A4 (CYP3A4), which leads to drug–drug interactions (DDIs)230,268. Indeed, clinically significant DDIs with one of the protease inhibitors and the outpatient medication needed to be considered in almost half of the treated patients with HCV infection230. Severe interactions were, for example, reported during coadministration with statins and calcineurin inhibitors269,270. Most importantly, there were serious adverse effects. Boceprevir was frequently associated with dysgeusia, which can have a substantial effect on the quality of life for the entire treatment duration125. During telaprevir treatment, half of the patients developed a skin rash, which was sometimes severe and even led to treatment discontinuation126. Moreover, both protease inhibitors were frequently linked to the development of anaemia. In more than 10% of treated individuals, blood transfusions became necessary128,129. Focusing on patients with advanced liver disease, overall safety and sustained virological response rates were quite disappointing in the first real-world studies131,132. Infections were identified as a frequent and severe problem, which was most likely attributable to Peg-IFN46,131.
The HCV replication cycle
While clinicians developed IFN-based therapies, basic virologists around the world intensively worked on unravelling the HCV replication cycle to identify therapeutic targets for specific, direct-acting antiviral agents (DAAs). After the discovery of HCV, the complete viral genome was identified and analysed in detail. HCV was characterized as a positive-stranded RNA virus encoding approximately 9,400 nucleotides88–90. Comparisons between clones from different patients revealed a high genetic diversity with differences of up to 33% of nucleotides between individual clones. This finding led to the classification into different HCV genotypes91. The entire HCV RNA strand contained only a single open reading frame (ORF) encoding a polyprotein of around 3,000 amino acids88–90 (Fig. 3). Thus, it became evident that proteases would be necessary for the replication process and were possible therapeutic targets.
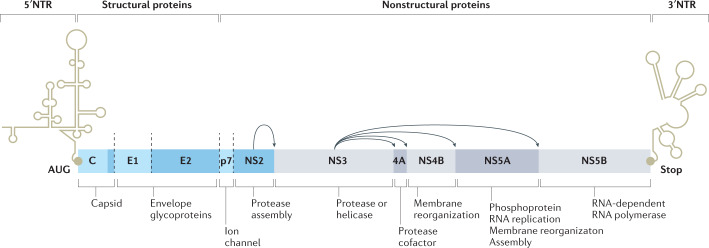
Fig. 3. Organization of the HCV genome.
Illustration of the hepatitis C virus (HCV) genome, which contains only a single open reading frame encoding one polyprotein of about 3,000 amino acids. The structural proteins, which include the core or capsid (C) protein and envelope (E1 and E2) proteins, can be found in the N-terminal region. The C-terminal region contains the nonstructural (NS) proteins that are required at various steps of viral replication. NTR, N-terminal region; NS2, NS3, NS4B, NS5A, NS5B, nonstructural proteins 2, 3, 4B, 5A, 5B, respectively; p7, ion channel; 4A, nonstructural protein 4A. Adapted from ref.93, Springer Nature Limited.
Moreover, comparison of the genomic sequence with that of other known viruses revealed a close link between HCV and flaviviruses92. These observations allowed some sophisticated estimations about HCV biology, including the general genetic order and clues on the organization and function of individual parts of the polyprotein93. It was speculated that the HCV polyprotein contains a certain number of structural proteins at the beginning of the ORF, followed by approximately five nonstructural (NS) proteins94. However, at that time, a major hurdle for further studies was the inability to culture HCV. Thus, protein expression studies that used cDNA clones were the most feasible tool to gain more insights into HCV replication93. Thereby, Hijikata and colleagues94 were one of the first groups to characterize specific parts of the HCV polyprotein better. They focused on the precursor part presumed to contain the structural proteins. Using a cDNA construct encoding the 980 N-terminal residues of the HCV ORF, they demonstrated that the precursor part of the polyprotein was cleaved into four major products.
Moreover, it was revealed that two of these four proteins were glycoproteins, entitled gp35 (later known as envelope glycoprotein 1) and gp70 (later named envelope glycoprotein 2)94. Two years later, in 1993, the Charles Rice group widely characterized the remaining part of the polyprotein, identifying the nonstructural cleavage products NS2, NS3, NS4A, NS4B, NS5A and NS5B95. In the following years, the structure and function of all of these individual cleavage products were investigated in more detail. Within the same edition of the Journal of Virology in 1993, three different research groups published insights on NS3, confirming that it encodes a viral protease and demonstrating that it is required to cleave the link between NS3 and NS4 (refs95–97). Shortly after that, it was discovered that NS4A inhabits a co-function in this process98–101, followed by the confirmation that NS5B works as the viral polymerase93,102. Additional major advances in the understanding of HCV biology included insights into viral entry, revealing the role of CD81 (ref.103), claudin 1 (ref.104), occludin105, scavenger receptor class B type 1 (ref.106) and the low-density lipoprotein receptor107 (Fig. 4).
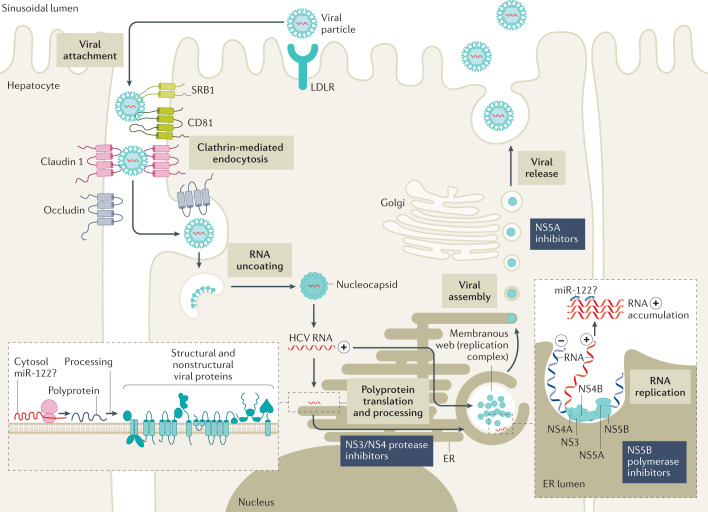
Fig. 4. HCV life cycle.
Entry of hepatitis C virus (HCV) into hepatocytes is a complex and yet not completely understood process that involves several different host proteins, including CD81, scavenger receptor B type 1 (SRB1), claudin 1 as well as occludin. After endocytosis, the viral envelope fuses with the endosome membrane. This is followed by uncoating of the viral RNA, which is then translated into the polyprotein by host enzymes. Viral proteases are required (for example, nonstructural proteins NS3 and NS4) to process the HCV polyprotein into the various structural and nonstructural viral proteins. The NS5B polymerase assisted by the NS3 helicase is required for the production of HCV RNA positive replicates. For the final step of viral assembly and release, it is assumed that the NS5A protein has a central role. ER, endoplasmic reticulum; LDLR, low-density lipoprotein receptor; miR, microRNA. Adapted from ref.7, Springer Nature Limited.
However, the most essential milestone for the later development of the DAAs might have been the creation of a working in vitro HCV replication model. A major step towards this model was the work by Kolykhalov et al.108 in 1997, who used cDNA clones (genotype 1a) to produce HCV RNA transcripts. Direct intrahepatic injection of these RNA transcripts into the liver in chimpanzees led to viral hepatitis associated with circulating HCV RNA in serum and increased ALT levels. At the same time, similar findings were published by Yanagi et al.109. These data demonstrated that the respective cDNA sequence contained all information required for viral replication. Finally, in 1999 and after years of intensive research, the first robust in vitro HCV replicon model based on a genotype 1b clone transfected into a hepatoma cell line was established, which revolutionized drug development and further understanding of HCV biology110. Shortly afterwards, other replicon systems were developed that enabled amplification of other HCV genotypes111. Although all of these cell culture systems were able to produce subgenomic replicons, they failed to produce infectious viral particles. This limitation was finally overcome by the establishment of a replicon model based on a genotype 2 clone and demonstrated that the produced viral particles could infect a human hepatoma cell line112. From then on, candidate substances could be directly tested in vitro for their antiviral activity.
Direct-acting antiviral agents
The continuous and detailed discovery of the HCV life cycle paved the way towards the development of an entirely new generation of antiviral compounds to treat HCV infection, the so-called DAAs. In contrast to the rather nonspecific treatment with IFN and RBV, DAAs interfere directly and specifically with certain viral proteins required for HCV replication. The first DAAs that were developed belonged to the class of protease inhibitors (PIs), which prevent the splicing of the HCV polyprotein between NS3 and NS4A by the respective HCV NS3 or NS4A protease. In 2003 the PI BILN 2061 became the first compound to demonstrate antiviral efficacy in HCV-infected patients113,114. However, safety concerns were raised owing to cardiotoxicity in a mouse model leading to termination of the clinical development of BILN 2061 (refs114,115) (Table 2).
Table 2.
Selected landmark treatment studies regarding first-generation protease inhibitor therapy
| Study | Study design | Treatment | Key results | Major contribution |
|---|---|---|---|---|
| HCV RESPOND-2 Bacon et al. 2011 (ref.116) |
Randomized, multicentre, double-blind, placebo-controlled trial n = 403 treatment-experienced patients with chronic HCV GT1 infection — relapsers and partial responders — no null responders were included |
Three treatment arms: A: Peg-IFN–RBV for 48 weeks + placebo (weeks 4–48) |
A SVR rate: 21% |
Established triple therapy with Peg-IFN–RBV–boceprevir as the standard of care for treatment-experienced patients with GT1 infection Demonstrated that 36 weeks of treatment are sufficient in patients with undetectable HCV RNA at week 8 |
| B: Peg-IFN–RBV for 36–48 weeks (depending on HCV RNA result at week 8 detectable or undetectable) + boceprevir (weeks 4–36) | B SVR rate: 59%; in those with undetectable HCV RNA at week 8: 86% | |||
| C: Peg-IFN–RBV for 48 weeks + boceprevir (weeks 4–48) | C SVR rate: 66%; in those with undetectable HCV RNA at week 8: 88% | |||
| Substantially higher rates of anaemia in patients treated with boceprevir (41–46% versus 21%) | ||||
| SPRINT-2 Poordad et al. 2011 (ref.117) |
Randomized, multicentre, double-blind, placebo-controlled trial n = 1,097 treatment-naive patients with chronic HCV GT1 infection |
Three treatment arms: A: Peg-IFN–RBV for 48 weeks + placebo (weeks 4–48) |
A SVR rate: 38% |
Established triple therapy with Peg-IFN–RBV–boceprevir as the standard of care for treatment-naive patients with GT1 infection Demonstrated that treatment can be shortened to 28 weeks in almost half of the patients using response-guided therapy |
| B: Peg-IFN + RBV for 28–48 weeks (28 weeks in those with undetectable HCV RNA between weeks 8 and 24; extended RVR) + boceprevir (weeks 4–28) | B SVR rate: 63%; 44% qualified for shorter treatment duration | |||
| C: Peg-IFN–RBV for 48 weeks + boceprevir (weeks 4–48) | C SVR rate: 66% | |||
| ILLUMINATE Sherman et al. 2011 (ref.118) |
Randomized, multicentre, double-blind, placebo-controlled trial n = 540 treatment-naive patients with chronic HCV GT1 infection |
Peg-IFN–RBV–telaprevir for 12 weeks, followed by Peg-IFN–RBV for 12 weeks Patients with undetectable HCV RNA between weeks 4 and 12 (extended RVR) were randomized A: stop treatment after 24 weeks |
65% had an extended RVR | Demonstrated that triple therapy including telaprevir can be shortened to 24 weeks in more than half of treatment-naive patients using response-guided therapy |
| A SVR rate: 92% | ||||
| B: Peg-IFN–RBV for another 24 weeks | B SVR rate: 88% | |||
| ADVANCE Jacobson et al. 2011 (ref.119) |
Randomized, multicentre, double-blind, placebo-controlled trial n = 1,088 treatment-naive patients with chronic HCV GT1 infection |
Three treatment arms: A: Peg-IFN–RBV–telaprevir for 12 weeks, followed by Peg-IFN + RBV for 12 weeks (extended RVR) or 36 weeks (no extended RVR) |
A SVR rate: 75%; extended RVR: 58% |
Established triple therapy with Peg-IFN–RBV–telaprevir as the standard of care for treatment-naive patients with GT1 infection Demonstrated that treatment can be shortened to 24 weeks in more than half of treatment-naive patients Identified anaemia and rash as relevant adverse events attributable to telaprevir treatment |
| B: Peg-IFN–RBV for 12 weeks + telaprevir for weeks 0–8 and placebo for weeks 8–12, Peg-IFN–RBV for 12 weeks (extended RVR) or 36 weeks (no extended RVR) | B SVR rate: 69%; extended RVR: 57% | |||
| C: Peg-IFN–RBV + placebo for 12 weeks followed by Peg-IFN–RBV for 36 weeks | C SVR rate: 44% | |||
| Important adverse effects associated with telaprevir treatment were skin rash and anaemia | ||||
| REALIZE Zeuzem et al. 2011 (ref.120) |
Randomized, multicentre, double-blind, placebo-controlled trial n = 663 treatment-experienced patients with chronic HCV GT1 infection — relapsers, partial responders and null responders included |
Three treatment arms: A: Peg-IFN–RBV–telaprevir for 12 weeks followed by Peg-IFN–RBV + placebo for 4 weeks followed by Peg-IFN–RBV for 32 weeks |
SVR rates A: overall: 59% Relapsers: 83% Partial responders: 59% Null responders: 29% |
Established triple therapy with Peg-IFN–RBV–telaprevir as the standard of care for treatment-experienced patients with GT1 infection Demonstrated that treatment efficacy remains limited in previous null responders to IFNα Identified anaemia and rash as relevant adverse events attributable to telaprevir treatment |
| B: Peg-IFN–RBV + placebo for 4 weeks, followed by Peg-IFN–RBV–telaprevir for 12 weeks + telaprevir followed by Peg-IFN–RBV for 32 weeks |
SVR rates B: overall: 54% Relapsers: 88% Partial responders: 54% Null responders: 33% |
|||
| C: Peg-IFN–RBV + placebo for 16 weeks followed by Peg-IFN–RBV for 32 weeks |
SVR rates C: overall: 15% Relapsers: 24% Partial responders: 15% Null responders: 5% |
|||
| ANRS CO20-CUPIC study Hézode et al. 2013 (ref.131) |
Real-world, multicentre, prospective, observational study n = 497 patients with chronic HCV GT1 infection All patients had cirrhosis Safety and efficacy analysis at week 16 of treatment |
Triple therapy according to the prescribing information at the discretion of each investigator Telaprevir (n = 292) Boceprevir (n = 205) |
Incidence of serious adverse events: 40% Mortality: 1.2% Severe infections: 4.8% Severe anaemia: 4.6% High risk of death or severe complications in patients with albumin <36 g/l and platelet count <100,000/µl |
Raised some important concerns regarding the safety of triple therapy with first-generation PIs in patients with advanced liver disease |
HCV, hepatitis C virus; IFN, interferon-α; GT, genotype; Peg-IFN, pegylated interferon-α; PI, protease inhibitor; RBV, ribavirin; RVR, rapid virological response; SVR, sustained virological response.
It took 8 more years until, in 2011, the DAA era could finally begin. Two PIs, boceprevir116,117 and telaprevir118–120, were approved for antiviral HCV treatment. This approval certainly represents one of the most important milestones in HCV history. Although monotherapy with telaprevir or boceprevir led to a fast decrease in HCV replication, it rapidly induced the development of resistance-associated substitutions (RASs), which resulted in a virological breakthrough (reappearance of serum HCV RNA at any time during treatment after a negative result or increase of 1 log IU/ml from nadir) in virtually all treated patients121,122. Thus, combination with Peg-IFN–RBV was required. Approval of telaprevir and boceprevir was restricted to HCV genotype 1 infection, although a lower efficacy was also documented for other genotypes123,124. Peg-IFN–RBV–PI triple therapy increased SVR rates by approximately 30% in treatment-naive patients compared with the previous standard regimen of Peg-IFN–RBV. Moreover, a far higher proportion of patients achieved an RVR with triple therapy and qualified for shorter therapy125–128. Improvement was even more pronounced in relapsers and previous partial responders to Peg-IFN–RBV therapy. Triple therapy increased SVR rates by 2.4–3.7-fold and 3.6–7.4-fold in previous relapsers and partial responders, respectively116,120 (Tables 3–5).
Table 4.
Antiviral combinations recommended by the EASL in DAA-naive patients with compensated liver disease in 2021 if HCV genotype and/or RAS is available
| Genotype | Cirrhosis status | Prior treatment | Grazoprevir–elbasvir | Glecaprevir–pibrentasvir | Sofosbuvir–velpatasvir | Sofosbuvir–velpatasvir–voxilaprevir |
|---|---|---|---|---|---|---|
| 1b | No cirrhosis | Naive | 12 weeks | 8 weeks | 12 weeks | No |
| Peg-IFN + RBV | ||||||
| Compensated cirrhosis | Naive | |||||
| Peg-IFN + RBV | 12 weeks | |||||
| 1a, 2, 4, 5, 6 | No cirrhosis | Naive | Noa | 8 weeks | 12 weeks | No |
| Peg-IFN + RBV | ||||||
| Compensated cirrhosis | Naive | |||||
| Peg-IFN + RBV | 12 weeks | |||||
| 3 | No cirrhosis | Naive | No | 8 weeks | 12 weeks | No |
| Peg-IFN + RBV | 12–16 weeks | No | ||||
| Compensated cirrhosis | Naive | 8 weeks | 12 weeksb | 12 weeks | ||
| Peg-IFN + RBV | 16 weeks | 12 weeks |
Data from ref.5. DAA, direct-acting antiviral agent; EASL, European Association for the Study of the Liver; Peg-IFN, pegylated interferon-α. a12 weeks of treatment possible in patients infected with hepatitis C virus (HCV) genotype 1 without nonstructural protein 5A (NS5A) resistance-associated substitutions (RASs). bIn patients with the Y93H RAS, either addition of ribavirin (RBV) or an alternative regimen is recommended. EASL recommends this approach only in those with cirrhosis.
Table 3.
Antiviral combinations recommended by the EASL in DAA-naive patients with compensated liver disease in 2021
| Cirrhosis status | Prior treatment | Glecaprevir–pibrentasvir | Sofosbuvir–velpatasvir |
|---|---|---|---|
| No cirrhosis | Naive | 8 weeks | 12 weeks |
| Peg-IFN + RBV | |||
| Compensated cirrhosis | Naive | ||
| Peg-IFN + RBV | 12 weeks |
Data from ref.5. For a simplified treatment without the need for hepatitis C virus genotype, resistance-associated substitution or baseline viral load determination. DAA, direct-acting antiviral agent; EASL, European Association for the Study of the Liver; Peg-IFN, pegylated interferon-α; RBV, ribavirin.
Table 5.
Antiviral combinations recommended by the EASL in special populations in 2021
| Population | Grazoprevir–elbasvir | Glecaprevir–pibrentasvir | Sofosbuvir–velpatasvir | Sofosbuvir–velpatasvir–voxilaprevir |
|---|---|---|---|---|
| NS5A-inhibitor experienced | No | No | No | 12 weeks |
| Subtype 1l, 4r, 3b, 3g, 6u, 6v or any other subtype naturally harbouring one or several NS5A RASs | No | Efficacy unknown | Efficacy unknown | 12 weeks |
| Decompensated liver cirrhosis | No | No | 12 weeks + RBVa | No |
| End-stage renal disease | Recommended | Recommended | Possible | Possible |
Data from ref.5. EASL, European Association for the Study of the Liver; NS5A, nonstructural protein 5A; RAS, resistance-associated substitution; RBV, ribavirin. aIf RBV is not tolerated, 24 weeks of treatment is recommended.
However, some important limitations of triple therapy with either boceprevir or telaprevir had to be considered (Box 1), which included poor efficacy in previous Peg-IFN–RBV null responders120,127 as well as severe adverse effects. Safety concerns were raised particularly among patients with advanced liver disease indicated by a platelet count below 90–100/µl as well as an albumin level below the normal range46,128–132. In addition, the overall effectiveness of triple therapy in real-world settings was reduced by the fact that many patients were, in principle, ineligible for Peg-IFN treatment owing to comorbidities, for example, severe depression. Moreover, as better treatment options were already in sight, treatment was often deferred in those without or with only mild fibrosis either by the physicians or by the patients themselves46. Ultimately, physicians faced the dilemma of having a safe and effective treatment available for patients with mild disease who could wait for even better options, although having no acceptable therapeutic option for those with advanced disease who urgently needed immediate therapy.
Interferon-free therapies
Proof of concept
Given the clinically significant adverse effects and limitations of IFN-based regimens (Supplementary Table 2), it became clear that IFN-free therapies were essential when aiming to cure all patients infected with HCV. This step required DAAs with a higher barrier of resistance and/or the possibility to combine different types of DAA. A combination of telaprevir and boceprevir was no reasonable choice, as both PIs interfered with the same viral protein and showed a lack of efficacy against similar RASs133. Thus, a major milestone towards HCV cure was the development of NS5A inhibitors as an entirely new drug class, which, in contrast to polymerase inhibitors or PIs, was not used for any other viral infection. Today, NS5A inhibitors are part of every DAA regimen. Using the replicon technology, Lemm and colleagues134 were among the first to identify compounds that led to sufficient suppression of HCV replication through inhibition of the NS5A protein. Shortly after that, Gao et al.135 reported the promising results of a phase I study exploring the NS5A inhibitor BMS-790052, a compound later known as daclatasvir. In 2012, Lok and colleagues136 demonstrated in a first proof-of-principle study that HCV cure can be achieved by an IFN-free combination of an NS5A inhibitor with a PI. Eleven patients who were non-responders to Peg-IFN–RBV dual therapy were treated with the NS5A inhibitor daclatasvir and the PI asunaprevir for 24 weeks. Both patients infected with genotype 1b and two of nine patients infected with genotype 1a were cured with this regimen. The efficacy in genotype 1b infection was later confirmed in larger settings, for example, the global HALLMARK-DUAL phase III study that documented SVR rates of 80–90%137. Notably, this regimen achieved final approval only in a limited number of countries and was particularly used in Japan and Korea, where HCV genotype 1b is by far the most prevalent genotype137–139.
Sofosbuvir
The first time that IFN-free therapy became widely available was through the approval of the nucleotide analogue sofosbuvir, an NS5B polymerase inhibitor that was approved in December 2013 in the USA and January 2014 in Europe. From that time on, HCV therapy became a rapidly changing field. The key advantage of sofosbuvir was its particularly high barrier of resistance140. Moreover, the clinically relevant RASs, such as the 282T variant, were linked to very poor viral fitness141. As a consequence, and in contrast to NS5A inhibitor- or PI-associated RASs, the wild type of the virus rapidly emerged after the end of drug exposure133. Sofosbuvir therapy was usually very well tolerated with no or only mild adverse effects in most patients. It had a low potential for drug–drug interaction and a simple once-daily dosing regimen142–144. High SVR rates were documented in treatment-naive patients after only 12 weeks of treatment with Peg-IFN–RBV–sofosbuvir therapy145. More importantly, in some patients, namely those with genotype 2 or 3 infections, SVR was also achieved without the need for Peg-IFN via a dual combination treatment of sofosbuvir and RBV142,146, which was first demonstrated in the ELECTRON trial146, a phase II study with 40 participants run in New Zealand that gained major attention.
Simeprevir and daclatasvir
Treatment options for genotype 1 and 3 infections were markedly improved a few months later by the approval of the next two DAAs, the second-wave PI simeprevir and the NS5A inhibitor daclatasvir. Simeprevir was initially developed in combination with Peg-IFN–RBV in large phase III studies. It was superior when compared with telaprevir with regard to an easier once-daily dosing schedule and an improved safety profile, although there was no major difference in SVR rates147–149. Thus, far more attention was gained from data from a phase II study named COSMOS. In the COSMOS trial, patients infected with HCV genotype 1 were treated for 12 or 24 weeks with sofosbuvir–simeprevir ± RBV, which achieved SVR in 90–92% of patients (n = 168)150. On the basis of these phase II data, the EMA and the FDA approved the sofosbuvir–simeprevir dual combination for interferon-ineligible patients in 2014. Although the optimal treatment duration had not yet been defined, this combination was widely and successfully used in clinical practice151. Afterwards, sofosbuvir–simeprevir was further investigated in a phase III programme. However, it became obvious that this regimen was no longer competitive once newer, more potent DAA combinations had been developed in the meantime152,153.
One could argue that the contribution of daclatasvir to the field might have been even more important. Daclatasvir was initially developed in combination with Peg-IFN–RBV, being effective also in genotype 2 and 3 infection154–156. IFN-free combinations with sofosbuvir or asunaprevir were more promising, as discussed earlier136. The approval of daclatasvir offered some valuable new options for certain cohorts that were, so far, considered to be difficult to treat, that is, patients infected with genotype 3 (refs157–159), those with decompensated cirrhosis and those infected with genotype 1 and with previous PI failure160. Given the lack of other effective IFN-free options, the FDA and the EMA decided to approve sofosbuvir–daclatasvir ± RBV to be given for 24 weeks in patients infected with genotype 3 with liver cirrhosis and/or previous non-response to IFN.
Fixed-dose DAA combinations
The European Association for the Study of the Liver (EASL) International Liver Congress that took place in London in April 2014 brought the next revolution in HCV therapy. A remarkable number of landmark HCV studies were presented, of which five were published in the New England Journal of Medicine by the end of the meeting. Three of these publications considered the first fixed-dose DAA combination containing the NS5A inhibitor ledipasvir and sofosbuvir; the remaining two considered the so-called 3D regimen consisting of the NS5A inhibitor ombitasvir, the PI paritaprevir boosted by ritonavir and the non-nucleoside NS5B inhibitor dasabuvir161–165. Both regimens were approved a couple of months later by the FDA and the EMA. SVR rates in patients infected with genotype 1 increased to >95%, further considered the new benchmark for response rates when evaluating antiviral regimens. Ledipasvir–sofosbuvir as the first fixed-dose combination substantially simplified HCV therapy; ledipasvir–sofosbuvir was investigated in a large phase III programme (ION trials), demonstrating high SVR rates in various cohorts following 12–24 weeks of treatment164,165. Moreover, the ION-3 study showed that in easy-to-treat treatment-naive patients without cirrhosis, an 8-week course of ledipasvir–sofosbuvir was non-inferior to the 12-week regimen163. However, there was a numerically higher rate of relapsers among male patients (8% (n = 10 of 121) versus 2% (n = 3 of 127) for the 8- and 12-week regimens, respectively) as well as among those with a high viral load at baseline (10% (n = 9 of 92) versus 1% (n = 1 of 83) for the 8- and 12-week regimens, respectively), and the EMA and the FDA decided to limit the shorter treatment to patients with a baseline viral load of <6 million IU/ml. Notably, the HCV RNA assay used in the ION-3 study was rarely used in clinical practice (Box 2). In female patients, no substantial difference in relapse rates was documented between the shorter and longer regimens (1% (n = 1 of 84) and 0% (n = 0 of 84), respectively).
The 3D regimen offered comparable response rates in patients infected with genotype 1. However, similarly to previous PI-based regimens, there was a considerable difference in the antiviral efficacy between patients infected with 1a and those infected with 1b161,166,167. When evaluating the value of the 3D regimen, it has to be considered that it was not only IFN-free but also sofosbuvir-free. This regimen was an important contribution to the field. The 3D regimen offered a safe and effective IFN-free therapy for the first time in patients with ESRD, a group of patients with a particularly high prevalence of HCV infection168. For a long time, sofosbuvir was not considered a safe treatment option in these patients owing to renal elimination of its inactive metabolite GS-331007 (refs169,170). In patients undergoing haemodialysis, a 20-fold increase in the area under the curve of GS-331007 was documented171. By contrast, renal function has no major effect on the pharmacokinetics of the 3D regimen. Even in patients with a need for dialysis, drug levels, tolerability and SVR rates remained unaffected172,173. Evidence from real-world studies and prospective trials demonstrated over the years that sofosbuvir might safely be used in patients with ESRD174–177. However, in the first years after approval, this fact remained unclear170.
Another important issue to consider when evaluating the role of sofosbuvir-free regimens is the influence on access to care. With the 3D regimen, a competitor for the sofosbuvir-based regimen entered the field, which certainly had a positive effect on drug costs. Competition on price and best regimen in the HCV field was further intensified with the approval of the next-generation PI grazoprevir and the NS5A inhibitor elbasvir as a fixed-dose combination. Similar to the 3D regimen, grazoprevir–elbasvir had particularly high efficacy in patients infected with genotype 1b and proved a safe and valuable treatment option for patients with ESRD178–180.
Box 2 Impact of HCV RNA assays on treatment decisions with DAA regimens.
In most pivotal trials of direct-acting antiviral agents (DAAs), the COBAS TaqMan assay along with the High Pure System (HPS/CTM) was used to detect hepatitis C virus (HCV) RNA. Consequently, rules applied for response-guided therapy were based on the results generated with this assay, with the same effect on baseline viral load cut-offs to determine treatment duration, for example, with sofosbuvir–ledipasvir. However, HPS/CTM was rarely used in clinical practice, as manual steps were required for RNA extraction. More commonly used assays during the early DAA era were, for example, the Abbot RealTime test and the Roche COBAS AmpliPrep/COBAS TaqMan. There were some important differences in the performance characteristics between these two assays and compared with the HPS/CTM271–274. The HPS/CTM tended to overestimate results at higher viral loads. Thus, a considerable number of patients who were selected for 8 weeks of treatment with sofosbuvir–ledipasvir in the real world, as a result of their baseline viral load (<6 million IU/ml), would have been tested above this threshold if the HPS/CTM had been used274,275. However, sustained virological response rates for the 8-week regimen still seemed to be excellent in several large real-world studies276–278. Moreover, the COBAS AmpliPrep/COBAS TaqMan and, in particular, the Abbot RealTime test were more sensitive than the HPS/CTM in low viraemia samples. However, during triple therapy with first-generation protease inhibitor, higher sensitivity resulted in a higher proportion of patients selected for longer treatment durations as well as patients fulfilling stopping rules owing to residual viraemia271,273. When using interferon-free regimens, a higher sensitivity might result in the detection of residual viraemia at the end of therapy, which, importantly, was not associated with treatment failure279.
Pan-genotypic regimens
The next wave of DAAs brought the advantage of pan-genotypic regimens. So far, the use of DAA regimens had been restricted to certain genotypes. For example, ombitasvir–paritaprevir–ritonavir had proven efficacy only against genotypes 1 and 4 (ref.181). Dasabuvir was ineffective against genotype 4 and was only approved for genotype 1 infection182. Sofosbuvir had pan-genotypic efficacy. However, its partner, ledipasvir, had relatively low efficacy against genotypes 2 and 3 (ref.183). In summer 2016, the second-generation pan-genotypic NS5A inhibitor velpatasvir was approved in a fixed-dose combination with sofosbuvir for the treatment of chronic HCV infection. A 12-week regimen achieved SVR in 99% of patients almost independently of the HCV genotype in a large phase III study of 624 patients184. In patients infected with genotype 3, the SVR after sofosbuvir–velpatasvir was 98% in the non-cirrhotic treatment-naive cohort (n = 160 of 163). However, among treatment-experienced patients with and without cirrhosis, it was only 93% (n = 40 of 43) and 89% (n = 33 of 37), respectively. Importantly, treatment failure in patients infected with genotype 3 and cirrhosis was closely linked to the presence of NS5A RASs, that is, the Y93H variant185. Post-approval, a multicentre study from Spain demonstrated that adding RBV improves SVR rates to >95% (n = 21 of 22) among those patients with cirrhosis with the Y93H variant186. Owing to the need for RBV and/or baseline RAS testing, EASL recommendations even declared sofosbuvir–velpatasvir not to be the first choice in patients infected with genotype 3 and cirrhosis at that time, which led to an intense debate187,188.
Approximately 1 year later, in 2017, a second pan-genotypic, fixed-dose regimen was approved, that is, the combination of the PI glecaprevir and the NS5A inhibitor pibrentasvir. Similarly to sofosbuvir–velpatasvir, glecaprevir–pibrentasvir had excellent tolerability and comparable cure rates. In patients infected with genotype 1, 2, 4, 5 or 6, and with no cirrhosis, 8 weeks of glecaprevir–pibrentasvir achieved SVR rates of 97–100% in phase II and III studies (n = 943 of 952)189. In patients with compensated cirrhosis, only the 12-week regimen was tested in the pivotal studies. In the phase III trial EXPEDITION-1, SVR was achieved in 99% (n = 145 of 146)190. Patients infected with genotype 3 were studied in separate trials with slightly different designs resulting in rather complicated treatment recommendations of 8–16 weeks depending on the presence of liver cirrhosis and previous treatment attempts191–193. Later, it was shown that an 8-week regimen is sufficient in treatment-naive patients with cirrhosis irrespective of the HCV genotype193.
Treatment of DAA failures
One of the few remaining challenges was the re-treatment of DAA failures. So far, most of the available regimens have only been tested on a large scale in DAA-naive patients. Some patients with failure to Peg-IFN–RBV and first-generation PI (telaprevir–boceprevir) or simeprevir had been included in pivotal studies with sofosbuvir–velpatasvir and sofosbuvir–ledipasvir, which achieved high SVR rates164,184. Sofosbuvir–daclatasvir ± RBV was a feasible but expensive option in these patients160. Glecaprevir–pibrentasvir was tested in PI failures and linked to acceptable cure if RBV was added194. However, things were far more complicated in patients who failed an NS5A-containing regimen. NS5A RAS showed by far the most durable persistence after treatment cessation, remaining detectable for more than 5 years in most patients133,195,196. Some hepatologists suggested guiding re-treatment of DAA failures by analysing baseline RASs, particularly if multiple DAAs have been used during previous attempts197. However, after the withdrawal of simeprevir from the market, all DAA regimens actually contained an NS5A inhibitor. Using DAAs with higher barriers of resistance, for example, glecaprevir–pibrentasvir, was another possible choice for re-treatment198,199. The most aggressive and quite expensive alternative was to combine highly potent DAAs of all drug classes with RBV for re-treatment200,201. Thus, all the discussed options had important limitations, as they were either complicated, ‘off-label’, expensive, only efficient against certain genotypes and/or only available for re-treatment after certain DAA classes. The advent and approval of voxilaprevir as a fixed-dose combination with sofosbuvir and velpatasvir (sofosbuvir–velpatasvir–voxilaprevir) provided probably one of the very last missing pieces required in HCV therapy. In the phase III programme, the major focus of drug development was indeed the re-treatment of DAA failures. One study, entitled POLARIS-1 included only patients with a previous NS5A failure. The POLARIS-4 study included patients with non-NS5A DAA failure. Response rates were 96% (n = 253 of 263) and 98% (n = 179 of 182), respectively202 (Fig. 5).
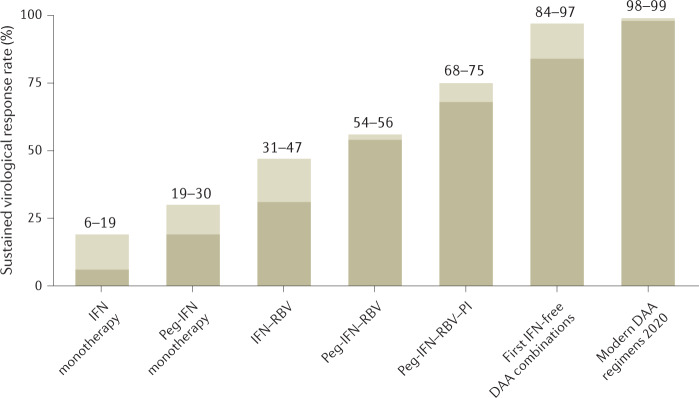
Fig. 5. Evolution of HCV therapy.
There has been a continuous increase in sustained virological response rates over time, starting with only 6–19% when using the first interferon-α (IFN) monotherapies to cure rates of >98% in the era of direct-acting antiviral agents (DAAs)5,65,66,71,73,117,119,146,152,266. HCV, hepatitis C virus; Peg-IFN, pegylated interferon-α; PI, protease inhibitor; RBV, ribavirin.
Remaining challenges
Difficult-to-treat cohorts
The tremendous improvements in DAA therapy that led to pan-genotypic fixed-dose combinations eliminated most of the remaining challenges in anti-HCV treatment. Today, most patients with HCV infection can be cured quite easily and usually without any relevant adverse effects using an IFN-free DAA combination. Simplified pan-genotypic regimens are highly effective treatments even without the need to determine baseline viral load, RAS and the HCV genotype. Such approaches are sufficient for most patients and essential when aiming for global elimination (Table 3). However, some patients can still remain difficult to cure. A few patients will fail to respond not only to the first-line DAA therapy but also to re-treatment with sofosbuvir–velpatasvir–voxilaprevir202,203. The current EASL guideline recommends treating these patients with sofosbuvir–glecaprevir–pibrentasvir–RBV for 24 weeks5. However, only data from small case series involving fewer than 25 patients are available to support this recommendation for the small but increasing population of sofosbuvir–velpatasvir–voxilaprevir failures204–208. In general, pibrentasvir is supposed to be the NS5A inhibitor with the highest barrier of resistance, making it a logical choice for re-treatment209. Treatment can also be challenging in patients with decompensated liver disease. PIs (owing to hepatic metabolization), as well as IFN, are not recommended in these patients, which limits the treatment choices to RBV, sofosbuvir and NS5A inhibitors. The combination sofosbuvir–velpatasvir was investigated in the ASTRAL-4 study (n = 267 patients) for either 12 weeks ± RBV or 24 weeks without RBV. The highest SVR rate (95%) was achieved in those treated with RBV210. Thus, current EASL recommendations favour sofosbuvir–velpatasvir–RBV for 12 weeks in decompensated patients with Child–Pugh class B cirrhosis5. Notably, patients with Child–Pugh class C cirrhosis were not included in the ASTRAL-4 study210. However, patients with Child–Pugh class C cirrhosis (n = 117) were studied in the SOLAR-1 and SOLAR-2 trials investigating a regimen of sofosbuvir–ledipasvir–RBV for 12 or 24 weeks. SVR rates were 86–100% of those who completed antiviral treatment. Neither Child–Pugh class (B or C) nor treatment duration (12 or 24 weeks) had a substantial effect on virological response211,212. Although the optimal treatment regimen in DAA-naive patients with decompensated cirrhosis might still need to be determined, the situation is even more complicated when it comes to NS5A failures, for which no treatment option remains.
There has also been an extensive debate about whether patients with decompensated liver disease should receive any antiviral treatment before transplantation. Most patients with decompensated cirrhosis are supposed to benefit from SVR. DAA therapy led to substantial improvements in portal hypertension, liver function and model for end-stage liver disease (MELD) score, which was accompanied by a reduction in hepatic decompensation and mortality and an improvement in the overall quality of life212–217. A substantial proportion of patients might even be delisted after achieving SVR218,219. However, it has to be considered that not all patients will achieve a compensated stage of liver disease. Some data indicated that those with end-stage liver disease might no longer benefit from treatment216,220. A large multicentre study from Spain, involving 843 patients, identified a MELD score >18 as a potential threshold to determine a higher risk of adverse events and death during treatment221. Current EASL guidelines, therefore, recommend treating patients up to a MELD score of 18–20 pre-transplantation5, which also reflects common practice in many centres222. To date, it still remains unclear until which stage patients should undergo antiviral treatment and when to decide to defer treatment to after liver transplantation223. In the past, patients with HCV infection had relatively poor survival after liver transplantation compared with those with other indications such as HBV infection or primary biliary cholangitis224, particularly among those with evidence of portal hypertension and/or liver fibrosis early after transplantation225. However, the scenery changed entirely with the availability of IFN-free therapies. Treatment of transplant recipients became very easy, and SVR rates exceeded 95%, not different from the pretransplant setting226–228. Although drug–drug interaction between DAAs and immunosuppressive medication was a major challenge when using first-generation PIs, this issue was no longer a problem with modern DAAs5,144,229,230. As a result, survival after liver transplantation among patients with HCV infection has markedly increased since 2015 (refs231,232) (Box 3).
Box 3 Use of HCV-positive donor organs in the DAA era.
The ability to easily cure hepatitis C virus (HCV) infection after transplantation is not limited to liver transplant recipients. Safe and highly effective antiviral treatment has also been well documented after kidney transplantation and also after lung and heart transplantation227,280–282. The transformation of HCV infection into an easily curable condition initiated an intense debate about the possible use of HCV-positive donor organs even in HCV-negative recipients283–285. Some studies could show that such a strategy would be cost-effective286,287. Current data suggest that patients willing to accept HCV-positive organs did not need to have any notable health concerns but can expect a considerable benefit owing to a shorter waiting time288–291. Overall, willingness to accept HCV-positive organs seems to increase among patients on the transplant waiting list292. However, potential benefits also depend on the individual situation and the regional shortage of donor organs. An interesting modelling study from the USA estimated that the willingness to accept HCV-positive livers would robustly increase survival among those with a model for end-stage liver disease (MELD) score above 20. However, the highest benefit was expected for those with a MELD score of 28 and above293. Some innovative studies successfully investigated the possibility of pre-emptive antiviral therapies starting shortly after or even at the time of transfer to the operating room. Such strategies would further reduce the time of viral exposure and might lead to a better acceptance of HCV-positive organs in the future231,294,295. However, there is hope that the number of HCV-positive organs will substantially decrease over the next few years. Furthermore, HCV infection as an indication for liver transplantation has already markedly decreased in Europe since the availability of direct-acting antiviral agents (DAAs)296.
Heading towards HCV elimination
The introduction of DAA therapy is linked to tremendous changes in HCV epidemiology today and in the future. In countries with wide access to DAA therapy, the number of patients with HCV infection presenting at liver units has been continually decreasing, whereas treatment uptake has markedly increased. Some studies have already reported a substantial decrease in the proportion of patients with HCV infection among those presenting with compensated or decompensated liver cirrhosis and/or at-need of liver transplantation233. It was calculated that the role of HCV for liver-related morbidity, hospital admissions and mortality might be only marginal in the near future in such countries, for example, Spain234.
In the light of excellent treatment options and the already gained success in some countries, the WHO developed an ambitious global strategy for viral hepatitis with the aim of an 80% and 65% reduction by the year 2030 for new HCV infections and HCV-associated mortality, respectively, with the ultimate goal of HCV elimination6. To achieve this goal, at least 90% of the individuals with HCV infection need to be identified, and more than 80% need to be treated. However, most countries failed to meet the required intermediate targets by 2020 (ref.235). In an analysis from 2020, only 9 of 45 high-income countries seem to be on track towards the WHO HCV elimination goals for 2030, whereas for most, this elimination does not even seem to be achievable by 2050. This outlook might have further worsened owing to the ongoing COVID-19 pandemic235,236. So far, many national elimination programmes still need a valid screening strategy not to lose too many patients on their way to clinical care. However, it has been clear for a long time that without a substantial increase in screening and treatment uptake, the tremendous improvements through DAA therapy will translate neither into relevant cure rates nor in a reduction of HCV-related morbidity and mortality237,238 (Fig. 6). Only those patients identified and treated will profit from the revolution of HCV therapies. One of the national programmes that gained the highest attention was Georgia’s hepatitis C elimination programme, which was supported by one of the large pharmaceutical companies and private foundations. Until the end of 2018, only one-third of the population suspected of having HCV infection (approximately n = 150,000) was identified, and fewer than 50% started antiviral treatment despite being offered medication free of charge. The SVR rate among those linked to care was 98.5%. However, given the low rate of screening and treatment uptake, overall effectiveness remains poor239,240. By contrast, data from Egypt were more encouraging. The national programme aimed to screen all citizens 18 years of age or older (62.5 million individuals) within 1 year. After only 7 months, 49.6 million people had been screened (79% of the target population), of whom more than 2.2 million tested anti-HCV positive (4.6%). Most patients were available for HCV RNA testing and linked to DAA therapy if needed241. The success of the Egyptian HCV elimination programme was also related to the special discount provided for the DAAs by the pharmaceutical companies. Broad access to DAA therapy highly depends on drug cost. Thus, special discounts and/or licensing agreements allowing the use of generic drugs would be essential for global elimination, in particular in low-income countries242,243. First reports on the successful use of generic DAAs in HCV infection have already been published244. Finally, data from the national programme of Iceland clearly demonstrated the particular importance of effective screening programmes and high treatment uptake in high-risk populations, that is, PWIDs, who are still a major source of de novo infections and a key route to success245,246. It was estimated that more than 50% of the PWID population in Western Europe and North America were positive for HCV infection247. However, although treatment was, in principle, highly feasible and highly effective even among those with active drug use, access to care remained challenging248–251. Different point-of-care and/or specialist-assisted strategies, for example, using telemedicine, have been proposed to overcome this hurdle and improve linkage to care in such populations252. Outreach programmes using rapid diagnostic tests and immediate initiation of antiviral treatment will be key to targeting this population. HCV screening and care can be integrated into opioid-substitution therapies, as has already been successfully demonstrated. In difficult-to-reach cohorts, ultimately, long-acting or even a one-shot treatment (for example, by using antisense oligonucleotide253) might be required for HCV cure. However, it remains uncertain whether such treatments will be developed. Moreover, it is important to note that high re-infection rates have been reported in PWID populations, which was also true for some men who have sex with men cohorts and dramatically indicate the high risk of further spread of HCV infections in these populations254,255. Removing DAA restrictions and unlimited treatment of high-risk populations, for example, men who have sex with men and are positive for HIV, has been demonstrated to show a direct negative correlation with the onset of new acute HCV infections in this group256.
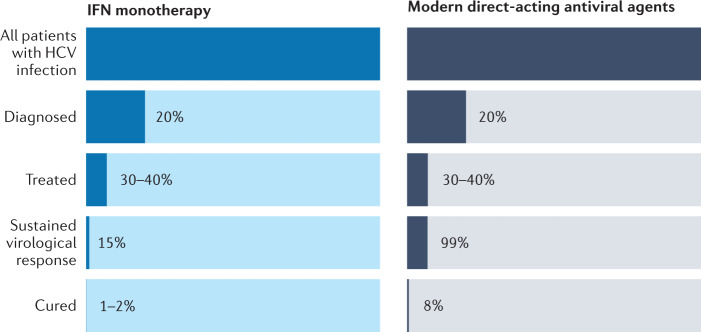
Fig. 6. Cascade of care.
Overview of the various steps required to cure patients with hepatitis C virus (HCV) infection. It becomes obvious that an increase in sustained virological response has only a minor efficacy on the overall proportion of patients with HCV infection who are cured: 1–2% for patients treated with interferon-α (IFN) monotherapy, and 8% for patients treated with modern direct-acting antiviral agents. For a substantial change, other parts of the cascade need to be markedly improved.
To reduce the risk of further infections, there is also a need for an effective strategy for those with acute HCV infection. Currently, all available DAAs are approved only for chronic HCV infection, leaving a 6-month period for those with newly diagnosed HCV infection to transmit the disease to healthy individuals, and some patients will be lost to follow-up during this time, as documented in the German HepNet Acute HCV III study257. It seems likely that a shorter antiviral regimen would be possible in acute than in chronic HCV infection, which would further improve cost-effectiveness258. Some small studies indicate that modern DAAs might work very well in acute HCV infection259,260. However, it remains uncertain whether any of these data will lead to approval by legal authorities. For patients with high-risk behaviour, pre-exposure prophylaxis needs to be explored in future trials, as such strategies have been proved to be extremely successful in HIV infection261.
Despite the availability of DAAs, there cannot be any doubt that an effective prophylactic HCV vaccine would be crucial to finally achieve global HCV elimination. Some promising data have been published262, and some large research consortia are focusing on HCV vaccine development. A prophylactic vaccine is certainly not imminent. Ultimately, only a universal HCV vaccination strategy including countries with low resource settings and limited access to care and tackling communities with high risks of re-infection will lead to the eradication of this virus worldwide.
Conclusions
The history of HCV, starting from the clinical observation of NANBH as a disease entity via the discovery of the virus followed by the development of cure by direct antiviral agents, is a role model for successful basic, translational and clinical research. However, to achieve global HCV elimination, improvement in screening, access to antiviral treatment and reduction of new infections are essential. This improvement will include simple, fast and cheap point-of-care tests to diagnose HCV infection without the need for a venous puncture, as well as structured local and national screening programmes with a particular focus on high-risk populations. Lowering of drug costs or availability of generics will be required to enable broad access to care in low- and middle-income countries. Moreover, simple algorithms need to be developed that enable unspecialized physicians and even nurses to initiate and guide antiviral treatment. Finally, to prevent new HCV infections, it will be of particular importance to approve an effective antiviral treatment for acute HCV infection, establish a strategy for pre-exposure prophylaxis and ideally develop an effective HCV vaccine.
Supplementary information
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Xavier Forns, Jia-Horng Kao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
M.P.M. received speaker and/or consulting fees and/or grant or research support from AbbVie, BMS, Gilead, Merck/MSD and Janssen. B.M. received speaker and/or consulting fees from Abbott Molecular, Astellas, Intercept, Falk, AbbVie, Bristol-Myers Squibb, Fujirebio, Janssen-Cilag, Merck/MSD and Roche. He also received research support from Abbott Molecular and Roche.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41575-022-00608-8.
References
- 1.Maasoumy B, Wedemeyer H. Natural history of acute and chronic hepatitis C. Best. Pr. Res. Clin. Gastroenterol. 2012;26:401–412. doi: 10.1016/j.bpg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Hepatitis Report. WHOhttps://apps.who.int/iris/bitstream/handle/10665/255016/9789?sequence=1 (2017).
- 3.Alter HJ, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N. Engl. J. Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 4.Stramer SL, et al. Detection of HIV-1 and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing. N. Engl. J. Med. 2004;351:760–768. doi: 10.1056/NEJMoa040085. [DOI] [PubMed] [Google Scholar]
- 5.Pawlotsky J-M, et al. EASL recommendations on treatment of hepatitis C: final update of the series. J. Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. WHOhttps://apps.who.int/iris/handle/10665/206453 (2016).
- 7.Manns MP, et al. Hepatitis C virus infection. Nat. Rev. Dis. Primers. 2017;3:17006. doi: 10.1038/nrdp.2017.6. [DOI] [PubMed] [Google Scholar]
- 8.Alter HJ, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann. Intern. Med. 1972;77:691–699. doi: 10.7326/0003-4819-77-5-691. [DOI] [PubMed] [Google Scholar]
- 9.Prince AM, et al. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus. Lancet. 1974;2:241–246. doi: 10.1016/S0140-6736(74)91412-3. [DOI] [PubMed] [Google Scholar]
- 10.Alter HJ, et al. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975;2:838–841. doi: 10.1016/S0140-6736(75)90234-2. [DOI] [PubMed] [Google Scholar]
- 11.Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N. Engl. J. Med. 1975;292:767–770. doi: 10.1056/NEJM197504102921502. [DOI] [PubMed] [Google Scholar]
- 12.Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459–463. doi: 10.1016/S0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- 13.Tabor E, et al. Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet. 1978;1:463–466. doi: 10.1016/S0140-6736(78)90132-0. [DOI] [PubMed] [Google Scholar]
- 14.Bradley DW, et al. Persistent non-A, non-B hepatitis in experimentally infected chimpanzees. J. Infect. Dis. 1981;143:210–218. doi: 10.1093/infdis/143.2.210. [DOI] [PubMed] [Google Scholar]
- 15.Hruby MA, Schauf V. Transfusion-related short-incubation hepatitis in hemophilic patients. JAMA. 1978;240:1355–1357. doi: 10.1001/jama.1978.03290130049018. [DOI] [PubMed] [Google Scholar]
- 16.Wyke RJ, et al. Transmission of non-A non-B hepatitis to chimpanzees by factor-IX concentrates after fatal complications in patients with chronic liver disease. Lancet. 1979;1:520–524. doi: 10.1016/S0140-6736(79)90945-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa H, et al. Viruslike particles in a plasma fraction (fibrinogen) and in the circulation of apparently healthy blood donors capable of inducing non-A/non-B hepatitis in humans and chimpanzees. Gastroenterology. 1980;79:512–520. doi: 10.1016/0016-5085(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 18.Colombo M, et al. Transmission of non-A, non-B hepatitis by heat-treated factor VIII concentrate. Lancet. 1985;2:1–4. doi: 10.1016/S0140-6736(85)90055-8. [DOI] [PubMed] [Google Scholar]
- 19.Lane R. Non-A, non-b hepatitis from intravenous immunoglobulin. Lancet. 1983;322:974–975. doi: 10.1016/S0140-6736(83)90496-8. [DOI] [PubMed] [Google Scholar]
- 20.Lever AM, Webster AD, Brown D, Thomas HC. Non-A, non-B hepatitis occurring in agammaglobulinaemic patients after intravenous immunoglobulin. Lancet. 1984;2:1062–1064. doi: 10.1016/S0140-6736(84)91506-X. [DOI] [PubMed] [Google Scholar]
- 21.Bradley DW, et al. Experimental infection of chimpanzees with antihemophilic (factor VIII) materials: recovery of virus-like particles associated with non-A, non-B hepatitis. J. Med. Virol. 1979;3:253–269. doi: 10.1002/jmv.1890030403. [DOI] [PubMed] [Google Scholar]
- 22.Mosley JW, Redeker AG, Feinstone SM, Purcell RH. Mutliple hepatitis viruses in multiple attacks of acute viral hepatitis. N. Engl. J. Med. 1977;296:75–78. doi: 10.1056/NEJM197701132960204. [DOI] [PubMed] [Google Scholar]
- 23.Norkrans G, Frösner G, Hermodsson S, Iwarson S. Multiple hepatitis attacks in drug addicts. JAMA. 1980;243:1056–1058. doi: 10.1001/jama.1980.03300360028019. [DOI] [PubMed] [Google Scholar]
- 24.Galbraith RM, Eddleston AL, Portmann B, Williams R, Gower PE. Chronic liver disease developing after outbreak of HBsAG-negative hepatitis in haemodialysis unit. Lancet. 1975;2:886–890. doi: 10.1016/S0140-6736(75)92126-1. [DOI] [PubMed] [Google Scholar]
- 25.Rakela J, Redeker AG. Chronic liver disease after acute non-A, non-B viral hepatitis. Gastroenterology. 1979;77:1200–1202. doi: 10.1016/0016-5085(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 26.Tabor E, Seeff LB, Gerety RJ. Chronic non-A, non-B hepatitis carrier state: transmissible agent documented in one patient over a six-year period. N. Engl. J. Med. 1980;303:140–143. doi: 10.1056/NEJM198007173030307. [DOI] [PubMed] [Google Scholar]
- 27.Koretz RL, Stone O, Gitnick GL. The long-term course of non-A, non-B post-transfusion hepatitis. Gastroenterology. 1980;79:893–898. doi: 10.1016/0016-5085(80)90447-3. [DOI] [PubMed] [Google Scholar]
- 28.Realdi G, et al. Long-term follow-up of acute and chronic non-A, non-B post-transfusion hepatitis: evidence of progression to liver cirrhosis. Gut. 1982;23:270–275. doi: 10.1136/gut.23.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiyosawa K, et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671–675. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- 30.Aach RD, et al. Serum alanine aminotransferase of donors in relation to the risk of non-A,non-B hepatitis in recipients: the transfusion-transmitted viruses study. N. Engl. J. Med. 1981;304:989–994. doi: 10.1056/NEJM198104233041701. [DOI] [PubMed] [Google Scholar]
- 31.Bradley DW, et al. Posttransfusion non-A, non-B hepatitis: physicochemical properties of two distinct agents. J. Infect. Dis. 1983;148:254–265. doi: 10.1093/infdis/148.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley DW, et al. Posttransfusion non-A, non-B hepatitis in chimpanzees. Physicochemical evidence that the tubule-forming agent is a small, enveloped virus. Gastroenterology. 1985;88:773–779. doi: 10.1016/0016-5085(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 33.Choo QL, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 34.Koziol DE, et al. Antibody to hepatitis B core antigen as a paradoxical marker for non-A, non-B hepatitis agents in donated blood. Ann. Intern. Med. 1986;104:488–495. doi: 10.7326/0003-4819-104-4-488. [DOI] [PubMed] [Google Scholar]
- 35.Alter HJ. The road not taken or how I learned to love the liver: a personal perspective on hepatitis history. Hepatology. 2014;59:4–12. doi: 10.1002/hep.26787. [DOI] [PubMed] [Google Scholar]
- 36.Kuo G, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 37.Roggendorf M, et al. Antibodies to hepatitis C virus. Lancet. 1989;2:324–325. doi: 10.1016/S0140-6736(89)90501-1. [DOI] [PubMed] [Google Scholar]
- 38.Esteban JI, et al. Hepatitis C virus antibodies among risk groups in Spain. Lancet. 1989;2:294–297. doi: 10.1016/S0140-6736(89)90485-6. [DOI] [PubMed] [Google Scholar]
- 39.Colombo M, et al. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006–1008. doi: 10.1016/S0140-6736(89)91016-7. [DOI] [PubMed] [Google Scholar]
- 40.Bruix J, et al. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004–1006. doi: 10.1016/S0140-6736(89)91015-5. [DOI] [PubMed] [Google Scholar]
- 41.Polaris OHCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol. Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 42.Esteban JI, et al. Evaluation of antibodies to hepatitis C virus in a study of transfusion-associated hepatitis. N. Engl. J. Med. 1990;323:1107–1112. doi: 10.1056/NEJM199010183231605. [DOI] [PubMed] [Google Scholar]
- 43.No authors listed. Effect of screening for hepatitis C virus antibody and hepatitis B virus core antibody on incidence of post-transfusion hepatitis. Japanese Red Cross Non-A, Non-B Hepatitis Research Group. Lancet. 1991;338:1040–1041. doi: 10.1016/0140-6736(91)91901-6. [DOI] [PubMed] [Google Scholar]
- 44.Aach RD, et al. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N. Engl. J. Med. 1991;325:1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- 45.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 46.Maasoumy B, et al. Eligibility and safety of triple therapy for hepatitis C: lessons learned from the first experience in a real world setting. PLoS ONE. 2013;8:e55285. doi: 10.1371/journal.pone.0055285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thursz M, Fontanet A. HCV transmission in industrialized countries and resource-constrained areas. Nat. Rev. Gastroenterol. Hepatol. 2014;11:28–35. doi: 10.1038/nrgastro.2013.179. [DOI] [PubMed] [Google Scholar]
- 48.Scullard GH, et al. Antiviral treatment of chronic hepatitis B virus infection. I. Changes in viral markers with interferon combined with adenine arabinoside. J. Infect. Dis. 1981;143:772–783. doi: 10.1093/infdis/143.6.772. [DOI] [PubMed] [Google Scholar]
- 49.Dusheiko G, et al. Recombinant leukocyte interferon treatment of chronic hepatitis B. Hepatology. 1985;5:556–560. doi: 10.1002/hep.1840050406. [DOI] [PubMed] [Google Scholar]
- 50.Hoofnagle JH, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 1986;315:1575–1578. doi: 10.1056/NEJM198612183152503. [DOI] [PubMed] [Google Scholar]
- 51.Di Bisceglie AM, et al. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N. Engl. J. Med. 1989;321:1506–1510. doi: 10.1056/NEJM198911303212204. [DOI] [PubMed] [Google Scholar]
- 52.Davis GL, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. N. Engl. J. Med. 1989;321:1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- 53.Shindo M, et al. Decrease in serum hepatitis C viral RNA during alpha-interferon therapy for chronic hepatitis C. Ann. Intern. Med. 1991;115:700–704. doi: 10.7326/0003-4819-115-9-700. [DOI] [PubMed] [Google Scholar]
- 54.Brillanti S, et al. Effect of alpha-interferon therapy on hepatitis C viraemia in community-acquired chronic non-A, non-B hepatitis: a quantitative polymerase chain reaction study. J. Med. Virol. 1991;34:136–141. doi: 10.1002/jmv.1890340213. [DOI] [PubMed] [Google Scholar]
- 55.Garson JA, et al. Hepatitis C viraemia rebound after ‘successful’ interferon therapy in patients with chronic non-A, non-B hepatitis. J. Med. Virol. 1992;37:210–214. doi: 10.1002/jmv.1890370311. [DOI] [PubMed] [Google Scholar]
- 56.Swain MG, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–1601. doi: 10.1053/j.gastro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Manns MP, et al. Long-term clearance of hepatitis C virus following interferon α-2b or peginterferon α-2b, alone or in combination with ribavirin. J. Viral Hepat. 2013;20:524–529. doi: 10.1111/jvh.12074. [DOI] [PubMed] [Google Scholar]
- 58.Marcellin P, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann. Intern. Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 59.Jaeckel E, et al. Treatment of acute hepatitis C with interferon alfa-2b. N. Engl. J. Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 60.Omata M, et al. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet. 1991;338:914–915. doi: 10.1016/0140-6736(91)91774-O. [DOI] [PubMed] [Google Scholar]
- 61.Reichard O, Andersson J, Schvarcz R, Weiland O. Ribavirin treatment for chronic hepatitis C. Lancet. 1991;337:1058–1061. doi: 10.1016/0140-6736(91)91707-2. [DOI] [PubMed] [Google Scholar]
- 62.Di Bisceglie AM, et al. A pilot study of ribavirin therapy for chronic hepatitis C. Hepatology. 1992;16:649–654. doi: 10.1002/hep.1840160307. [DOI] [PubMed] [Google Scholar]
- 63.Kakumu S, et al. A pilot study of ribavirin and interferon beta for the treatment of chronic hepatitis C. Gastroenterology. 1993;105:507–512. doi: 10.1016/0016-5085(93)90727-T. [DOI] [PubMed] [Google Scholar]
- 64.Brillanti S, et al. A pilot study of combination therapy with ribavirin plus interferon alfa for interferon alfa-resistant chronic hepatitis C. Gastroenterology. 1994;107:812–817. doi: 10.1016/0016-5085(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 65.McHutchison JG, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 66.Poynard T, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/S0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 67.Reichard O, et al. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998;351:83–87. doi: 10.1016/S0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 68.McHutchison JG, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 69.Wills RJ, Dennis S, Spiegel HE, Gibson DM, Nadler PI. Interferon kinetics and adverse reactions after intravenous, intramuscular, and subcutaneous injection. Clin. Pharmacol. Ther. 1984;35:722–727. doi: 10.1038/clpt.1984.101. [DOI] [PubMed] [Google Scholar]
- 70.Karnam US, Reddy KR. Pegylated interferons. Clin. Liver Dis. 2003;7:139–148. doi: 10.1016/S1089-3261(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 71.Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 72.Loomes DE, van Zanten SV. Bibliometrics of the top 100 clinical articles in digestive disease. Gastroenterology. 2013;144:673–676.e5. doi: 10.1053/j.gastro.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Fried MW, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 74.Rumi MG, et al. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology. 2010;138:108–115. doi: 10.1053/j.gastro.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 75.Ascione A, et al. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology. 2010;138:116–122. doi: 10.1053/j.gastro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 76.McHutchison JG, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N. Engl. J. Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 77.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J. Hepatol. 2011;55:69–75. doi: 10.1016/j.jhep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 79.Mangia A, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N. Engl. J. Med. 2005;352:2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 80.Berg T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–1097. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 81.Thomas DL, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge D, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka Y, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 84.Matsuura K, Watanabe T, Tanaka Y. Role of IL28B for chronic hepatitis C treatment toward personalized medicine. J. Gastroenterol. Hepatol. 2014;29:241–249. doi: 10.1111/jgh.12475. [DOI] [PubMed] [Google Scholar]
- 85.Yoshio S, et al. Human blood dendritic cell antigen 3 (BDCA3)+ dendritic cells are a potent producer of interferon-λ in response to hepatitis C virus. Hepatology. 2013;57:1705–1715. doi: 10.1002/hep.26182. [DOI] [PubMed] [Google Scholar]
- 86.Romero-Gomez M, Eslam M, Ruiz A, Maraver M. Genes and hepatitis C: susceptibility, fibrosis progression and response to treatment. Liver Int. 2011;31:443–460. doi: 10.1111/j.1478-3231.2011.02449.x. [DOI] [PubMed] [Google Scholar]
- 87.O’Brien TR, Yang HI, Groover S, Jeng WJ. Genetic factors that affect spontaneous clearance of hepatitis C or B virus, response to treatment, and disease progression. Gastroenterology. 2019;156:400–417. doi: 10.1053/j.gastro.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 88.Kato N, et al. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc. Natl Acad. Sci. USA. 1990;87:9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choo QL, et al. Genetic organization and diversity of the hepatitis C virus. Proc. Natl Acad. Sci. USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takamizawa A, et al. Structure and organization of the hepatitis C virus genome isolated from human carriers. J. Virol. 1991;65:1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simmonds P, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J. Gen. Virol. 1993;74:2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 92.Francki, R. I. B., Fauquet, C. M., Knudson, D. L. & Brown, F. Classification and Nomenclature of Viruses. Fifth Report of the International Committee on Taxonomy of Viruses. Virology Division of the International Union of Microbiological Societies (Springer, 1991).
- 93.Lohmann V. Hepatitis C virus cell culture models: an encomium on basic research paving the road to therapy development. Med. Microbiol. Immunol. 2019;208:3–24. doi: 10.1007/s00430-018-0566-x. [DOI] [PubMed] [Google Scholar]
- 94.Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc. Natl Acad. Sci. USA. 1991;88:5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. Nonstructural protein 3 of the hepatitis C virus encodes a serine-type proteinase required for cleavage at the NS3/4 and NS4/5 junctions. J. Virol. 1993;67:3835–3844. doi: 10.1128/jvi.67.7.3835-3844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomei L, Failla C, Santolini E, De Francesco R, La Monica N. NS3 is a serine protease required for processing of hepatitis C virus polyprotein. J. Virol. 1993;67:4017–4026. doi: 10.1128/jvi.67.7.4017-4026.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Failla C, Tomei L, De Francesco R. Both NS3 and NS4A are required for proteolytic processing of hepatitis C virus nonstructural proteins. J. Virol. 1994;68:3753–3760. doi: 10.1128/jvi.68.6.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin C, Thomson JA, Rice CM. A central region in the hepatitis C virus NS4A protein allows formation of an active NS3-NS4A serine proteinase complex in vivo and in vitro. J. Virol. 1995;69:4373–4380. doi: 10.1128/jvi.69.7.4373-4380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartenschlager R, Lohmann V, Wilkinson T, Koch JO. Complex formation between the NS3 serine-type proteinase of the hepatitis C virus and NS4A and its importance for polyprotein maturation. J. Virol. 1995;69:7519–7528. doi: 10.1128/jvi.69.12.7519-7528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanji Y, Hijikata M, Satoh S, Kaneko T, Shimotohno K. Hepatitis C virus-encoded nonstructural protein NS4A has versatile functions in viral protein processing. J. Virol. 1995;69:1575–1581. doi: 10.1128/jvi.69.3.1575-1581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Behrens SE, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15:12–22. doi: 10.1002/j.1460-2075.1996.tb00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pileri P, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 104.Evans MJ, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 105.Ploss A, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scarselli E, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl Acad. Sci. USA. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolykhalov AA, et al. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 109.Yanagi M, Purcell RH, Emerson SU, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl Acad. Sci. USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lohmann V, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 111.Blight KJ, Kolykhalov AA, Rice CM. Efficient Initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 112.Wakita T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lamarre D, et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature. 2003;426:186–189. doi: 10.1038/nature02099. [DOI] [PubMed] [Google Scholar]
- 114.Hinrichsen H, et al. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology. 2004;127:1347–1355. doi: 10.1053/j.gastro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 115.Vanwolleghem T, et al. Ultra-rapid cardiotoxicity of the hepatitis C virus protease inhibitor BILN 2061 in the urokinase-type plasminogen activator mouse. Gastroenterology. 2007;133:1144–1155. doi: 10.1053/j.gastro.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 116.Bacon BR, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poordad F, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sherman KE, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N. Engl. J. Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jacobson IM, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 120.Zeuzem S, et al. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 121.Sarrazin C, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767–1777. doi: 10.1053/j.gastro.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 122.Romano KP, Ali A, Royer WE, Schiffer CA. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc. Natl Acad. Sci. USA. 2010;107:20986–20991. doi: 10.1073/pnas.1006370107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foster GR, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology. 2011;141:881–889.e1. doi: 10.1053/j.gastro.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 124.Silva MO, et al. Antiviral activity of boceprevir monotherapy in treatment-naive subjects with chronic hepatitis C genotype 2/3. J. Hepatol. 2013;59:31–37. doi: 10.1016/j.jhep.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Maasoumy B, Manns MP. Optimal treatment with boceprevir for chronic HCV infection. Liver Int. 2013;33:14–22. doi: 10.1111/liv.12070. [DOI] [PubMed] [Google Scholar]
- 126.Jesudian AB, Jacobson IM. Optimal treatment with telaprevir for chronic HCV infection. Liver Int. 2013;33(Suppl. 1):3–13. doi: 10.1111/liv.12079. [DOI] [PubMed] [Google Scholar]
- 127.Vierling JM, et al. Boceprevir for chronic HCV genotype 1 infection in patients with prior treatment failure to peginterferon/ribavirin, including prior null response. J. Hepatol. 2014;60:748–756. doi: 10.1016/j.jhep.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 128.Colombo M, et al. Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C. Gut. 2014;63:1150–1158. doi: 10.1136/gutjnl-2013-305667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gordon SC, et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J. Hepatol. 2015;62:286–293. doi: 10.1016/j.jhep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poordad F, et al. Effects of ribavirin dose reduction vs erythropoietin for boceprevir-related anemia in patients with chronic hepatitis C virus genotype 1 infection–a randomized trial. Gastroenterology. 2013;145:1035–1044.e5. doi: 10.1053/j.gastro.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 131.Hézode C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J. Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 132.Maasoumy B, et al. Limited effectiveness and safety profile of protease inhibitor-based triple therapy against chronic hepatitis C in a real-world cohort with a high proportion of advanced liver disease. Eur. J. Gastroenterol. Hepatol. 2014;26:836–845. doi: 10.1097/MEG.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 133.Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J. Hepatol. 2016;64:486–504. doi: 10.1016/j.jhep.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 134.Lemm JA, et al. Identification of hepatitis C virus NS5A inhibitors. J. Virol. 2010;84:482–491. doi: 10.1128/JVI.01360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lok AS, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 137.Manns M, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597–1605. doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 138.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 139.Wei L, et al. A phase 3, open-label study of daclatasvir plus asunaprevir in Asian patients with chronic hepatitis C virus genotype 1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin. J. Gastroenterol. Hepatol. 2016;31:1860–1867. doi: 10.1111/jgh.13379. [DOI] [PubMed] [Google Scholar]
- 140.Abraham GM, Spooner LM. Sofosbuvir in the treatment of chronic hepatitis C: new dog, new tricks. Clin. Infect. Dis. 2014;59:411–415. doi: 10.1093/cid/ciu265. [DOI] [PubMed] [Google Scholar]
- 141.Ludmerer SW, et al. Replication fitness and NS5B drug sensitivity of diverse hepatitis C virus isolates characterized by using a transient replication assay. Antimicrob. Agents Chemother. 2005;49:2059–2069. doi: 10.1128/AAC.49.5.2059-2069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacobson IM, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 143.Höner Zu Siederdissen C, et al. Drug-drug interactions with novel all oral interferon-free antiviral agents in a large real-world cohort. Clin. Infect. Dis. 2016;62:561–567. doi: 10.1093/cid/civ973. [DOI] [PubMed] [Google Scholar]
- 144.Schulte B, et al. Frequency of potential drug-drug interactions in the changing field of HCV therapy. Open Forum Infect. Dis. 2020;7:ofaa040. doi: 10.1093/ofid/ofaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lawitz E, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 146.Gane EJ, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N. Engl. J. Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 147.Manns M, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 148.Jacobson IM, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 149.Reddy KR, et al. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect. Dis. 2015;15:27–35. doi: 10.1016/S1473-3099(14)71002-3. [DOI] [PubMed] [Google Scholar]
- 150.Lawitz E, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 151.Sulkowski MS, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 Infection. Gastroenterology. 2016;150:419–429. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwo P, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64:370–380. doi: 10.1002/hep.28467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lawitz E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2) Hepatology. 2016;64:360–369. doi: 10.1002/hep.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hézode C, et al. Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut. 2015;64:948–956. doi: 10.1136/gutjnl-2014-307498. [DOI] [PubMed] [Google Scholar]
- 155.Dore GJ, et al. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology. 2015;148:355–366.e1. doi: 10.1053/j.gastro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Jacobson I, et al. Daclatasvir vs telaprevir plus peginterferon alfa/ribavirin for hepatitis C virus genotype 1. World J. Gastroenterol. 2016;22:3418–3431. doi: 10.3748/wjg.v22.i12.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J. Hepatol. 2019;70:15–23. doi: 10.1016/j.jhep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 158.Nelson DR, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leroy V, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+) Hepatology. 2016;63:1430–1441. doi: 10.1002/hep.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sulkowski MS, Jacobson IM, Nelson DR. Daclatasvir plus sofosbuvir for HCV infection. N. Engl. J. Med. 2014;370:1560–1561. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 161.Ferenci P, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N. Engl. J. Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 162.Poordad F, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N. Engl. J. Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 163.Kowdley KV, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 164.Afdhal N, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 165.Afdhal N, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 166.Andreone P, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365.e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 167.Dore GJ, et al. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN-containing regimens in genotype 1 HCV patients: the MALACHITE-I/II trials. J. Hepatol. 2016;64:19–28. doi: 10.1016/j.jhep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 168.Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am. J. Nephrol. 2013;38:405–412. doi: 10.1159/000355615. [DOI] [PubMed] [Google Scholar]
- 169.Kirby BJ, Symonds WT, Kearney BP, Mathias AA. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin. Pharmacokinet. 2015;54:677–690. doi: 10.1007/s40262-015-0261-7. [DOI] [PubMed] [Google Scholar]
- 170.Ohlendorf V, Maasoumy B. Renal function in HCV therapy: just another thing to ignore? Liver Int. 2020;40:1018–1020. doi: 10.1111/liv.14391. [DOI] [PubMed] [Google Scholar]
- 171.Desnoyer A, et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J. Hepatol. 2016;65:40–47. doi: 10.1016/j.jhep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 172.Pockros PJ, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150:1590–1598. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 173.Flisiak R, et al. Real-world effectiveness and safety of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in hepatitis C: AMBER study. Aliment. Pharmacol. Ther. 2016;44:946–956. doi: 10.1111/apt.13790. [DOI] [PubMed] [Google Scholar]
- 174.Saxena V, et al. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lawitz E, et al. Sofosbuvir plus ribavirin and sofosbuvir plus ledipasvir in patients with genotype 1 or 3 hepatitis C virus and severe renal impairment: a multicentre, phase 2b, non-randomised, open-label study. Lancet Gastroenterol. Hepatol. 2020;5:918–926. doi: 10.1016/S2468-1253(19)30417-0. [DOI] [PubMed] [Google Scholar]
- 176.Li T, Qu Y, Guo Y, Wang Y, Wang L. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver Int. 2017;37:974–981. doi: 10.1111/liv.13336. [DOI] [PubMed] [Google Scholar]
- 177.Borgia SM, et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J. Hepatol. 2019;71:660–665. doi: 10.1016/j.jhep.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 178.Lawitz E, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous. Lancet. 2015;385:1075–1086. doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed] [Google Scholar]
- 179.Kwo P, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152:164–175.e4. doi: 10.1053/j.gastro.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 180.Roth D, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 181.Krishnan P, et al. In vitro and in vivo antiviral activity and resistance profile of ombitasvir, an inhibitor of hepatitis C virus NS5A. Antimicrob. Agents Chemother. 2015;59:979–987. doi: 10.1128/AAC.04226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kati W, et al. In vitro activity and resistance profile of dasabuvir, a nonnucleoside hepatitis C virus polymerase inhibitor. Antimicrob. Agents Chemother. 2015;59:1505–1511. doi: 10.1128/AAC.04619-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng G, et al. In vitro antiviral activity and resistance profile characterization of the hepatitis C virus NS5A inhibitor ledipasvir. Antimicrob. Agents Chemother. 2016;60:1847–1853. doi: 10.1128/AAC.02524-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feld JJ, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 185.Foster GR, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 Infection. N. Engl. J. Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 186.Esteban R, et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in patients with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology. 2018;155:1120–1127.e4. doi: 10.1053/j.gastro.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 187.Pawlotsky J-M, et al. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 188.Stamm LM, Brainard DM, McHutchison JG. Sofosbuvir/velpatasvir for patients with chronic genotype 3 HCV infection with compensated cirrhosis: response to EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2019;70:561–562. doi: 10.1016/j.jhep.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 189.Puoti M, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1-6 patients without cirrhosis. J. Hepatol. 2018;69:293–300. doi: 10.1016/j.jhep.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 190.Forns X, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect. Dis. 2017;17:1062–1068. doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 191.Zeuzem S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N. Engl. J. Med. 2018;378:354–369. doi: 10.1056/NEJMoa1702417. [DOI] [PubMed] [Google Scholar]
- 192.Wyles D, et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: a partially randomized phase 3 clinical trial. Hepatology. 2018;67:514–523. doi: 10.1002/hep.29541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brown RS, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: the EXPEDITION-8 trial. J. Hepatol. 2020;72:441–449. doi: 10.1016/j.jhep.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 194.Forns X, et al. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J. Hepatol. 2015;63:564–572. doi: 10.1016/j.jhep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 195.Dietz J, et al. Patterns of resistance-associated substitutions in patients with chronic HCV infection following treatment with direct-acting antivirals. Gastroenterology. 2018;154:976–988.e4. doi: 10.1053/j.gastro.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 196.Wyles D, et al. Long-term persistence of HCV NS5A resistance-associated substitutions after treatment with the HCV NS5A inhibitor, ledipasvir, without sofosbuvir. Antivir. Ther. 2017;23:229–238. doi: 10.3851/IMP3181. [DOI] [PubMed] [Google Scholar]
- 197.Peiffer KH, et al. Interferon-free treatment choice according to baseline RASs leads to high SVR rates in HCV genotype 1 infected patients. J. Infect. Chemother. 2018;24:524–530. doi: 10.1016/j.jiac.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 198.Poordad F, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389–397. doi: 10.1002/hep.29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Poordad F, et al. Glecaprevir/pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure. Hepatology. 2018;67:1253–1260. doi: 10.1002/hep.29671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wyles D, et al. Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection. J. Hepatol. 2019;70:1019–1023. doi: 10.1016/j.jhep.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 201.Papaluca T, et al. Retreatment with elbasvir, grazoprevir, sofosbuvir ± ribavirin is effective for GT3 and GT1/4/6 HCV infection after relapse. Liver Int. 2019;39:2285–2290. doi: 10.1111/liv.14201. [DOI] [PubMed] [Google Scholar]
- 202.Bourlière M, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N. Engl. J. Med. 2017;376:2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 203.Vermehren J, et al. Sofosbuvir, velpatasvir, and voxilaprevir for patients with failure of previous direct-acting antiviral therapy for chronic hepatitis C: results from the German Hepatitis C-Registry (DHC-R) Z. Gastroenterol. 2020;58:841–846. doi: 10.1055/a-1217-7669. [DOI] [PubMed] [Google Scholar]
- 204.Martin MT, Patel S, Kulik L, Chan C. Glecaprevir/pibrentasvir + sofosbuvir + ribavirin offers high cure rate for hepatitis C virus retreatment in real-world settings. J. Hepatol. 2021;75:251–254. doi: 10.1016/j.jhep.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 205.Dietz J, et al. Failure on voxilaprevir, velpatasvir, sofosbuvir and efficacy of rescue therapy. J. Hepatol. 2021;74:801–810. doi: 10.1016/j.jhep.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 206.Bernhard B, Stickel F. Successful fourth line treatment of a relapse patient with chronic hepatitis C virus infection genotype 3a using sofosbuvir, glecaprevir/pibrentasvir, and ribavirin: a case report. Z. Gastroenterol. 2020;58:451–455. doi: 10.1055/a-1131-8058. [DOI] [PubMed] [Google Scholar]
- 207.Fierer DS, Wyles DL. Re-treatment of hepatitis c infection after multiple failures of direct-acting antiviral therapy. Open Forum Infect. Dis. 2020;7:ofaa095. doi: 10.1093/ofid/ofaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tergast TL, et al. Glecaprevir/pibrentasvir + sofosbuvir + ribavirin als reserveregime nach sofosbuvir + velpatasvir + voxilaprevir re-therapieversagen. Z. Gastroenterol. 2021 doi: 10.1055/a-1649-8931. [DOI] [PubMed] [Google Scholar]
- 209.Gottwein JM, et al. Efficacy of NS5A inhibitors against hepatitis c virus genotypes 1-7 and escape variants. Gastroenterology. 2018;154:1435–1448. doi: 10.1053/j.gastro.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 210.Curry MP, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 211.Charlton M, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 212.Manns M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect. Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 213.Cheung MCM, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016;65:741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 214.Deterding K, et al. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment. Pharmacol. Ther. 2015;42:889–901. doi: 10.1111/apt.13343. [DOI] [PubMed] [Google Scholar]
- 215.Mandorfer M, et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 2016;65:692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 216.El-Sherif O, et al. Baseline factors associated with improvements in decompensated cirrhosis after direct-acting antiviral therapy for hepatitis C virus infection. Gastroenterology. 2018;154:2111–2121.e8. doi: 10.1053/j.gastro.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 217.Younossi ZM, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol. Hepatol. 2016;1:122–132. doi: 10.1016/S2468-1253(16)30009-7. [DOI] [PubMed] [Google Scholar]
- 218.Belli LS, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: a European study. J. Hepatol. 2016;65:524–531. doi: 10.1016/j.jhep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 219.Pascasio JM, et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J. Hepatol. 2017;67:1168–1176. doi: 10.1016/j.jhep.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 220.Foster GR, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016;64:1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 221.Fernández Carrillo C, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: analysis of data from the Hepa-C registry. Hepatology. 2017;65:1810–1822. doi: 10.1002/hep.29097. [DOI] [PubMed] [Google Scholar]
- 222.Sandmann L, et al. Treatment strategies for patients with decompensated liver cirrhosis due to hepatitis C virus infection eligible for liver transplantation: real-life data from five German transplant centers. Eur. J. Gastroenterol. Hepatol. 2019;31:1049–1056. doi: 10.1097/MEG.0000000000001386. [DOI] [PubMed] [Google Scholar]
- 223.Samur S, et al. Cost effectiveness of pre- vs post-liver transplant hepatitis C treatment with direct-acting antivirals. Clin. Gastroenterol. Hepatol. 2018;16:115–122.e10. doi: 10.1016/j.cgh.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kim WR, et al. OPTN/SRTR 2012 annual data report: liver. Am. J. Transpl. 2014;14(Suppl. 1):69–96. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 225.Blasco A, et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006;43:492–499. doi: 10.1002/hep.21090. [DOI] [PubMed] [Google Scholar]
- 226.Kwo PY, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N. Engl. J. Med. 2014;371:2375–2382. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 227.Reau N, et al. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection. Hepatology. 2018;68:1298–1307. doi: 10.1002/hep.30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Agarwal K, et al. Sofosbuvir/velpatasvir for 12 weeks in genotype 1-4 HCV-infected liver transplant recipients. J. Hepatol. 2018;69:603–607. doi: 10.1016/j.jhep.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 229.Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012;55:1620–1628. doi: 10.1002/hep.25653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Maasoumy B, et al. The clinical significance of drug-drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment. Pharmacol. Ther. 2013;38:1365–1372. doi: 10.1111/apt.12523. [DOI] [PubMed] [Google Scholar]
- 231.Crespo G, et al. The efficacy of direct anti-HCV drugs improves early post-liver transplant survival and induces significant changes in waiting list composition. J. Hepatol. 2018;69:11–17. doi: 10.1016/j.jhep.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 232.Cotter TG, et al. Improved graft survival after liver transplantation for recipients with hepatitis C virus in the direct-acting antiviral era. Liver Transpl. 2019;25:598–609. doi: 10.1002/lt.25424. [DOI] [PubMed] [Google Scholar]
- 233.Hutchinson SJ, et al. Population impact of direct-acting antiviral treatment on new presentations of hepatitis C-related decompensated cirrhosis: a national record-linkage study. Gut. 2020;69:2223–2231. doi: 10.1136/gutjnl-2019-320007. [DOI] [PubMed] [Google Scholar]
- 234.Rodríguez-Tajes S, et al. Hepatitis C-related cirrhosis will be a marginal cause of hospital admissions by 2025. J. Hepatol. 2020;73:1360–1367. doi: 10.1016/j.jhep.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 235.Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020;40:522–529. doi: 10.1111/liv.14324. [DOI] [PubMed] [Google Scholar]
- 236.Blach S, et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 2021;74:31–36. doi: 10.1016/j.jhep.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wedemeyer H, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J. Viral Hepat. 2014;21(Suppl. 1):60–89. doi: 10.1111/jvh.12249. [DOI] [PubMed] [Google Scholar]
- 238.Razavi H, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J. Viral Hepat. 2014;21(Suppl. 1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 239.Averhoff F, et al. Progress and challenges of a pioneering hepatitis C elimination program in the country of Georgia. J. Hepatol. 2020;72:680–687. doi: 10.1016/j.jhep.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nasrullah M, Sergeenko D, Gamkrelidze A, Averhoff F. HCV elimination — lessons learned from a small Eurasian country, Georgia. Nat. Rev. Gastroenterol. Hepatol. 2017;14:447–448. doi: 10.1038/nrgastro.2017.100. [DOI] [PubMed] [Google Scholar]
- 241.Waked I, et al. Screening and treatment program to eliminate hepatitis C in Egypt. N. Engl. J. Med. 2020;382:1166–1174. doi: 10.1056/NEJMsr1912628. [DOI] [PubMed] [Google Scholar]
- 242.van de Ven N, et al. Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology. 2015;61:1174–1182. doi: 10.1002/hep.27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin. Infect. Dis. 2014;58:928–936. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dhiman RK, et al. Decentralized care with generic direct-acting antivirals in the management of chronic hepatitis C in a public health care setting. J. Hepatol. 2019;71:1076–1085. doi: 10.1016/j.jhep.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 245.Scott N, et al. Modelling the elimination of hepatitis C as a public health threat in Iceland: a goal attainable by 2020. J. Hepatol. 2018;68:932–939. doi: 10.1016/j.jhep.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 246.Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319–1329. doi: 10.1016/S0140-6736(18)32277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Degenhardt L, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob. Heal. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dore GJ, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann. Intern. Med. 2016;165:625–634. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- 249.Christensen S, et al. Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: still a concern in clinical practice. Addiction. 2018;113:868–882. doi: 10.1111/add.14128. [DOI] [PubMed] [Google Scholar]
- 250.Grebely J, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol. Hepatol. 2018;3:153–161. doi: 10.1016/S2468-1253(17)30404-1. [DOI] [PubMed] [Google Scholar]
- 251.Christensen S, et al. Alcohol and cannabis consumption does not diminish cure rates in a real-world cohort of chronic hepatitis C virus infected patients on opioid substitution therapy-data from the german hepatitis C-registry (DHC-R) Subst. Abus. 2019;13:1178221819835847. doi: 10.1177/1178221819835847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Talal AH, et al. Integrated, co-located, telemedicine-based treatment approaches for hepatitis C virus management in opioid use disorder patients on methadone. Clin. Infect. Dis. 2019;69:323–331. doi: 10.1093/cid/ciy899. [DOI] [PubMed] [Google Scholar]
- 253.Janssen HL, et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 254.Ingiliz P, et al. Reinfection with the hepatitis C virus in men who have sex with men after successful treatment with direct-acting antivirals in germany: current incidence rates, compared with rates during the interferon era. Clin. Infect. Dis. 2020;71:1248–1254. doi: 10.1093/cid/ciz949. [DOI] [PubMed] [Google Scholar]
- 255.Ingiliz P, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J. Hepatol. 2017;66:282–287. doi: 10.1016/j.jhep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 256.Boerekamps A, et al. High treatment uptake in human immunodeficiency virus/hepatitis C virus-coinfected patients after unrestricted access to direct-acting antivirals in the Netherlands. Clin. Infect. Dis. 2018;66:1352–1359. doi: 10.1093/cid/cix1004. [DOI] [PubMed] [Google Scholar]
- 257.Deterding K, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect. Dis. 2013;13:497–506. doi: 10.1016/S1473-3099(13)70059-8. [DOI] [PubMed] [Google Scholar]
- 258.Bethea ED, Chen Q, Hur C, Chung RT, Chhatwal J. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology. 2018;67:837–846. doi: 10.1002/hep.29611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Deterding K, et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect. Dis. 2017;17:215–222. doi: 10.1016/S1473-3099(16)30408-X. [DOI] [PubMed] [Google Scholar]
- 260.Boerekamps A, et al. Treatment of acute hepatitis C genotypes 1 and 4 with 8 weeks of grazoprevir plus elbasvir (DAHHS2): an open-label, multicentre, single-arm, phase 3b trial. Lancet Gastroenterol. Hepatol. 2019;4:269–277. doi: 10.1016/S2468-1253(18)30414-X. [DOI] [PubMed] [Google Scholar]
- 261.Riddell J, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261–1268. doi: 10.1001/jama.2018.1917. [DOI] [PubMed] [Google Scholar]
- 262.Page K, et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N. Engl. J. Med. 2021;384:541–549. doi: 10.1056/NEJMoa2023345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sofia MJ, et al. Discovery of a β-d-2’-deoxy-2’-α-fluoro-2’-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010;53:7202–7218. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- 264.Curry MP, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2014;148:100–110. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 265.Tobler LH, Busch MP. History of posttransfusion hepatitis. Clin. Chem. 1997;43:1487–1493. doi: 10.1093/clinchem/43.8.1487. [DOI] [PubMed] [Google Scholar]
- 266.Heathcote EJ, et al. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N. Engl. J. Med. 2000;343:1673–1680. doi: 10.1056/NEJM200012073432302. [DOI] [PubMed] [Google Scholar]
- 267.Buti M, et al. Telaprevir twice daily is noninferior to telaprevir every 8 h for patients with chronic hepatitis C. Gastroenterology. 2014;146:744–753.e3. doi: 10.1053/j.gastro.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 268.Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014;23:489–497. doi: 10.1002/pds.3592. [DOI] [PubMed] [Google Scholar]
- 269.Werner CR, et al. Telaprevir-based triple therapy in liver transplant patients with hepatitis C virus: a 12-week pilot study providing safety and efficacy data. Liver Transpl. 2012;18:1464–1470. doi: 10.1002/lt.23542. [DOI] [PubMed] [Google Scholar]
- 270.Kanter CT, Luin M, Solas C, Burger DM, Vrolijk JM. Rhabdomyolysis in a hepatitis C virus infected patient treated with telaprevir and simvastatin. Ann. Hepatol. 2014;13:452–455. doi: 10.1016/S1665-2681(19)30853-1. [DOI] [PubMed] [Google Scholar]
- 271.Vermehren J, et al. Clinical significance of residual viremia detected by two real-time PCR assays for response-guided therapy of HCV genotype 1 infection. J. Hepatol. 2014;60:913–919. doi: 10.1016/j.jhep.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 272.Maasoumy B, et al. Detection of low HCV viraemia by repeated HCV RNA testing predicts treatment failure to triple therapy with telaprevir. Aliment. Pharmacol. Ther. 2014;39:85–92. doi: 10.1111/apt.12544. [DOI] [PubMed] [Google Scholar]
- 273.Maasoumy B, et al. Performance of two HCV RNA assays during protease inhibitor-based triple therapy in patients with advanced liver fibrosis and cirrhosis. PLoS ONE. 2014;9:e110857. doi: 10.1371/journal.pone.0110857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Maasoumy B, Vermehren J. Diagnostics in hepatitis C: the end of response-guided therapy? J. Hepatol. 2016;65:S67–S81. doi: 10.1016/j.jhep.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 275.Vermehren J, et al. Applicability of Hepatitis C virus RNA viral load thresholds for 8-week treatments in patients with chronic hepatitis C virus genotype 1 infection. Clin. Infect. Dis. 2016;62:1228–1234. doi: 10.1093/cid/ciw061. [DOI] [PubMed] [Google Scholar]
- 276.Terrault NA, et al. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–1140.e5. doi: 10.1053/j.gastro.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Buggisch P, et al. Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J. Hepatol. 2018;68:663–671. doi: 10.1016/j.jhep.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 278.Ingiliz P, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C Virus (HCV) infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the German hepatitis C cohort (GECCO-01) Clin. Infect. Dis. 2016;63:1320–1324. doi: 10.1093/cid/ciw567. [DOI] [PubMed] [Google Scholar]
- 279.Maasoumy B, et al. Clinical significance of detectable and quantifiable HCV RNA at the end of treatment with ledipasvir/sofosbuvir in GT1 patients. Liver Int. 2018;38:1906–1910. doi: 10.1111/liv.13932. [DOI] [PubMed] [Google Scholar]
- 280.Halleck F, et al. Transplanting HCV-infected kidneys into uninfected recipients. N. Engl. J. Med. 2017;377:1103–1104. doi: 10.1056/NEJMc1709315. [DOI] [PubMed] [Google Scholar]
- 281.Colombo M, et al. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann. Intern. Med. 2017;166:109–117. doi: 10.7326/M16-1205. [DOI] [PubMed] [Google Scholar]
- 282.Schlendorf KH, et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J. Hear. Lung Transpl. 2018;37:763–769. doi: 10.1016/j.healun.2018.01.1293. [DOI] [PubMed] [Google Scholar]
- 283.Nangia G, Borges K, Reddy KR. Use of HCV-infected organs in solid organ transplantation: an ethical challenge but plausible option. J. Viral Hepat. 2019;26:1362–1371. doi: 10.1111/jvh.13130. [DOI] [PubMed] [Google Scholar]
- 284.Fishman JA, Forns X. HCV-positive donor organs in solid organ transplantation: ‘Mind the Gap!’. Am. J. Transpl. 2017;17:2755–2756. doi: 10.1111/ajt.14396. [DOI] [PubMed] [Google Scholar]
- 285.Samuel D. HCV-positive organ transplants in HCV-negative recipients. Lancet Gastroenterol. Hepatol. 2019;4:745–747. doi: 10.1016/S2468-1253(19)30250-X. [DOI] [PubMed] [Google Scholar]
- 286.Gupta G, Zhang Y, Carroll NV, Sterling RK. Cost-effectiveness of hepatitis C-positive donor kidney transplantation for hepatitis C-negative recipients with concomitant direct-acting antiviral therapy. Am. J. Transpl. 2018;18:2496–2505. doi: 10.1111/ajt.15054. [DOI] [PubMed] [Google Scholar]
- 287.Kadatz M, Klarenbach S, Gill J, Gill JS. Cost-effectiveness of using kidneys from hepatitis C nucleic acid test-positive donors for transplantation in hepatitis C-negative recipients. Am. J. Transpl. 2018;18:2457–2464. doi: 10.1111/ajt.14929. [DOI] [PubMed] [Google Scholar]
- 288.Thuluvath PJ, et al. Use of HCV-positive livers in HCV-negative recipients. Am. J. Gastroenterol. 2020;115:1045–1054. doi: 10.14309/ajg.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 289.Kwong AJ, et al. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am. J. Transpl. 2019;19:1380–1387. doi: 10.1111/ajt.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cotter TG, et al. Increasing utilization and excellent initial outcomes following liver transplant of hepatitis C virus (HCV)-viremic donors into HCV-negative recipients: outcomes following liver transplant of HCV- Hepatology. 2019;69:2381–2395. doi: 10.1002/hep.30540. [DOI] [PubMed] [Google Scholar]
- 291.La Hoz RM, Sandıkçı B, Ariyamuthu VK, Tanriover B. Short-term outcomes of deceased donor renal transplants of HCV uninfected recipients from HCV seropositive nonviremic donors and viremic donors in the era of direct-acting antivirals. Am. J. Transpl. 2019;19:3058–3070. doi: 10.1111/ajt.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang JH, et al. OPTN/SRTR 2018 Annual Data Report: Hepatitis C. Am. J. Transpl. 2020;20(Suppl.):542–568. doi: 10.1111/ajt.15679. [DOI] [PubMed] [Google Scholar]
- 293.Chhatwal J, et al. Transplanting hepatitis C virus-positive livers into hepatitis C virus-negative patients with preemptive antiviral treatment: a modeling study. Hepatology. 2018;67:2085–2095. doi: 10.1002/hep.29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bethea ED, et al. Pre-emptive pangenotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative heart transplantation: an open-label study. Lancet Gastroenterol. Hepatol. 2019;4:771–780. doi: 10.1016/S2468-1253(19)30240-7. [DOI] [PubMed] [Google Scholar]
- 295.Cypel M, et al. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir. Med. 2020;8:192–201. doi: 10.1016/S2213-2600(19)30268-1. [DOI] [PubMed] [Google Scholar]
- 296.Belli LS, et al. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J. Hepatol. 2018;69:810–817. doi: 10.1016/j.jhep.2018.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.