Summary
Background
Gam-COVID-Vac is the world's first registered vector vaccine against COVID-19 based on a combination of two heterologous adenoviruses. It was chosen by the Republic of San Marino as the main tool in its vaccination campaign, which started on 25 February 2021. Our aim was to build up on the ROCCA study, focused on the older population, by describing adverse effects following immunisation (AEFIs) rates and characteristics in all age groups for the first time in a real-world context.
Methods
An active surveillance study on recipients of at least one dose of the Gam-COVID-Vac vaccine was conducted. Participants were administered online questionnaires through live/phone interviews with physicians, by e-mail or by scanning a QR code at different points in time after the first dose: one week (Q1) one month (Q2), and three months (Q3) between March and August 2021.
Findings
Overall, 6190 vaccine recipients were recruited. Mean age was 52·4 ± 18·2 years. After the first dose, systemic reactions were reported by 57·5% of the participants, while injection site reactions were reported by 46·7%. The most common AEFIs were pain at the injection site, fatigue and headache. Grade 3 or 4 AEFIs were reported by 0·8% and 0·3% of the participants, respectively. After the second dose, systemic reactions were reported by 63·1% of the participants, while injection site reactions by 54·7%. The most common AEFIs were malaise, pain at injection site and myalgia. Grade 3 or 4 AEFIs were reported by 2·7% and 1·1% of the participants, respectively. Multivariate analysis showed younger age, being a woman and food allergies are risk factors for more severe AEFIs.
Interpretation
Our results confirm a good tolerability profile for the population aged 18 and over providing useful data for vaccination campaigns ongoing in countries planning to use Gam-COVID-Vac.
Funding
None.
Keywords: Adverse events following immunisation, Sputnik V, San Marino Republic, Safety, Gam-COVID-Vac, COVID-19 vaccine, Active surveillance, Vaccination
Research in context.
Evidence before the study
Since the launch of Gam-COVID-Vac in December 2020, the vaccine has been distributed in 71 countries. Phase 1, 2 and 3 studies have shown a high efficacy and safety profile. These data need to be confirmed in post-marketing studies with large samples that are sufficiently representative of all age groups and specific comorbidities to fully detect rare adverse effect following immunizations (AEFIs).
Added value of the study
To the best of our knowledge, this study is the first carried nationwide carried in a real-world context on the safety profile of Gam-COVID-Vac. The AEFIs reported were found to be overwhelmingly mild or moderate in terms of severity, confirming results from the phase 3 trial. This shows a good tolerability profile if compared to other widely used vaccines.
Implications of all the available evidence
This independent study showed real-world data on the safety of Gam-COVID-Vac. These findings could contrast the hesitancy of this vaccine in the Republic of San Marino and other countries where this vaccine is used.
Alt-text: Unlabelled box
Introduction
The Republic of San Marino was one of the first and most severely affected countries by the ongoing global pandemic of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 On 27 February 2020 the first case of COVID-19 was recorded in San Marino, and then in May 2021 it became the country with the fourth highest confirmed cases per capita. The Republic of San Marino is still one of the countries with one of the highest rates of confirmed deaths relative to its population.2,3
In this problematic scenario, the Republic of San Marino has invested all available resources to promptly contain the public health damage caused by the pandemic. Therefore, in addition to the efforts to diagnose and treat the infection, this country conducted a vaccination campaign to quickly reach the goal of herd immunity. Considering the difficulties in the general supply of vaccines throughout Europe and beyond, the Gam-COVID-Vac (Sputnik V) vaccine was included in the vaccination campaign of the Republic of San Marino.4
Gam-COVID-Vac was developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia and released on 11 August 2020 by the Russian Ministry of Health as Gam-COVID-Vac.5 Gam-COVID-Vac is the world's first registered combination vector vaccine for the prevention of COVID-19. Gam-COVID-Vac uses the same platform as other COVID-19 vaccines, i.e. Vaxzevria, Janssen, Convidecia, but using two recombinant heterologous Adenoviruses, against which the body's immune and cell-mediated reaction develops.6 Emergency mass distribution of the vaccine began in December 2020, and as of December 2021 it has been approved in 71 countries, including Russia, the United Arab Emirates, Argentina, Belarus, Hungary, Serbia.7
In general, adenovirus vector vaccines have been extensively studied and have demonstrated a good safety profile.8 The phase 3 study of Gam-COVID-Vac was published in February 2021, showing 91·6% efficacy with no significant differences assessed in age strata, and 100% efficacy against moderate to severe COVID-19. In addition, no unusual side effects were noted: the most common adverse events following immunisation (AEFIs) reported in the aforementioned study were flu-like symptoms, injection site reactions, headache, and asthenia; most of these (94%) were grade 1, 5·7% grade 2, and 0·4% grade 3. None of the severe AEFIs were associated with vaccination.9
Evidence from clinical trials before vaccine approval is essential but not sufficient; in fact, there is still limited information in the scientific literature on the safety of Gam-COVID-Vac in real world context and no data on long-term AEFIs.
Gam-COVID-Vac was the most used vaccine in the Republic of San Marino's vaccination campaign, which began on 25 February 2021, involving in the first phase health care workers and most vulnerable populations.4 By December 2021, 83·4% among the eligible population completed the immunisation cycle and more than 35,000 Gam-COVID-Vac vaccine doses were administered. In the planning process of a national vaccination campaign, the acquisition of solid evidence on the efficacy and safety of the vaccine is a priority. Vaccinevigilance (i.e. the surveillance of the post-marketing safety of vaccines) consists of the activities of detection, evaluation and communication of AEFIs, in order to reduce the potential negative impact on the immunisation of the population.10 Active surveillance (i.e. solicited reporting along the campaign) is pivotal and offers advantages as compared to passive reporting, by carefully and timely assessing the prevalence and characteristics of AEFIs to Gam-COVID-Vac vaccine among the population of the Republic of San Marino. Preliminary results on the population ≥ aged 60 years have been published and showed a high tolerability profile in terms of short-term AEFIs.11
The aim of ROCCA final analysis is to assess potential short- and long-term AEFIs to Gam-COVID-Vac vaccine through a 3-month follow-up of the whole population included in the study.
Methods
Study design and participants
We conducted a nationwide study in the Republic of San Marino, using active surveillance to evaluate Gam-COVID-Vac safety profile.
Vaccination with Gam-COVID-Vac consists of two doses: the first dose (0·5 ml of rAd26) is administered on day 1, and the second dose (0·5 ml of rAd5) is administered on day 21. Both doses are administered into the deltoid muscle. Both vaccination centres established for the campaign by the Social Security Institute of San Marino (SSI) were involved in recruiting participants.
The recruitment was performed on site by physicians immediately after the vaccine administration. All participants provided informed consent for study participation. Eligibility criteria were defined as: age over 18, having had at least one dose of Gam-COVID-Vac vaccine administered, being covered by the SSI health insurance. Exclusion criteria were defined as: not being able to understand nor to answer the questionnaires properly. No randomisation or special selection was carried out.
The study protocol was approved by the Ethics Committee for Research and Experimentation of the Republic of San Marino under approval number 30/CERS/2021 on 17 March 2021. This study follows the criteria of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Outcomes
The main outcome was to define the safety profile of the Gam-COVID-Vac vaccine, based on the number of participants reporting AEFIs and the severity of AEFIs. Reporting of AEFIs was actively requested from each participant the first week after the first dose, the first week after the second dose of Gam-COVID-Vac, and at 3 months after the first dose, to assess long-term safety. This allowed the collection of near real-time, long-term patient-reported safety data, and generated the AEFIs incident rate.
Procedure and questionnaire
We actively administered standardised e-questionnaires to the participants to collect information about potential AEFIs following the vaccine administration. The questionnaires were generated using Google Forms, and could be filled in autonomously or with the help of a member of the research team.
Vaccine recipients were asked to fill in the questionnaires at fixed time intervals, namely: 1 week (Q1), 1 month (Q2), 3 months after the first vaccine dose (Q3). Q1 included 7 sections for a total of 25 questions with closed mandatory answers, while Q2 and Q3 contained 2 sections for a total of 6 questions.
Q1 investigated demographic information (patient code; age; weight; height; sex; profession), anamnestic data (pregnancy; diseases; drug therapy; recent vaccinations; allergies; previous SARS-CoV-2 infection), date of the first injection, vaccine brand, the potential AEFIs occurring in the week after the first dose. Q2 investigated the potential AEFIs occurring during the week following the second dose administration. Q3 investigates further potential AEFIs occurring in the long-term, specifically during the three months following the first dose. The patient code was asked also in Q2 and in Q3 questionnaires, in order to link the answers univocally. Answers concerning relevant variables were mandatory in both questionnaires to minimise missing data.
The list of AEFIs and most of the questions were adapted from the European Medicines Agency (EMA) funded active pharmacovigilance project Covid Vaccine Monitor.12 The clinical features of any AEFIs, frequency and intensity, were specified in the questionnaires using the CTCAE scale (Common Terminology Criteria for Adverse Events) version 5·0. Specifically, grade 1 included asymptomatic or mild symptoms without any indication for intervention; grade 2 covered moderate symptoms; grade 3 indicated severe or medically significant symptoms and grade 4 included symptoms with indication of urgent intervention. Grade 5 (death) was not directly investigated in the questionnaire, because of the nature of it. However, we performed a cross-check with the State Hospital's access record.
Data collection
The answer collection of Q1 lasted from 4 March 2021 to 25 May 2021. Data collection was carried out at vaccination sites through face-to-face interviews or online access to the e-questionnaire by QR-code scanning. The Q2 was administered from 25 March 2021 to 15 June 2021 and Q3 was administered from 24 May 2021 to 13 August 2021. Data collection of Q2 and Q3 was carried out through telephone interviews or online access to the e-questionnaire by a link sent via e-mail. The study has been reviewed and approved by the Ethics Committee for Research and Experimentation of the Republic of San Marino with the approval number 30/CERS/2021.
Statistical analysis
Assuming that the percentage of vaccine recipients with some adverse reactions was 50%, which results in the maximisation of variance and sample size,13 we determined that surveying at least 4269 persons would have ensured a precision of ±1·5% for a 95% two-sided binomial confidence interval (CI) estimated from the target population of the Republic of San Marino's residents vaccinated with Gam-COVID-Vac.14 The availability of time and resources to conduct the survey allowed augmenting the final sample size to 6190.
To account for under-represented groups in the population due to non-response to the survey, all the analyses described below were weighted using post-stratification.15 Post-stratification involves adjusting the sampling weights so that they sum to the population sizes within each post-stratum. In practical terms, the age distribution of the adult population of the Republic of San Marino vaccinated with Gam-COVID-Vac (see Table S1 in the appendix) and post-stratified sampling weights in order to obtain a sample distribution in line with the target population.
Categorical variables were summarised as frequencies and percentages, while numerical variables were summarised as mean ± standard deviation. Due to the presence of 1639 (26·5%) participants lost to follow-up who did not provide information on adverse reactions after the second dose of the vaccine, monotone multiple imputation was used to replace missing values with multiple sets of simulated values to complete the data (m = 30). Regression estimates from the multiply imputed sets were then combined into one overall estimate with an associated variance that incorporated the within- and between-imputation variability.16 A sensitivity analysis using weighted regression estimates to test for local departures from the missing-at-random assumption gave results that closely agreed with those obtained under missing-at-random multiple imputation (data not shown).17
The resulting rates of adverse reactions were stratified by age, sex, dose and severity, and were then depicted using bar charts with super-imposed 95% CIs obtained with the delta method. Lastly, multivariable logistic regression analysis was performed to investigate the baseline risk factors associated with increased risk of adverse reactions classified as grade 3 or grade 4 after the two doses of the vaccine. To avoid model overfitting and mis-classification, only significant risk factors were included as covariates in regression analyses. With this aim, we adopted a bootstrap backward procedure in which 1000 replicated bootstrap samples were selected from the original data. In each replicated sample, a backward elimination of covariates was applied with a significance level of removal equal to 0·05, and the risk factors selected in at least 500 (50%) of the replicates were included as covariates in the final multivariable model.18 Other automated selection methods, including classical stepwise regression and lasso, led to the same subset of covariates to retain in the final model (data not shown).
All analyses were carried out using Stata software, version 15 (StataCorp, 2017, Stata Statistical Software: Release 15, College Station, Texas, USA: StataCorp LP). All tests were two-sided, and the significance level was set at 0·05.
Role of the funding source
The authors received no financial support for the research, authorship, and/or publication of this article. ZDV, GLF, GS, MM, JL, MF, AS and DG accessed the raw data involved with the study and decided to submit the manuscript for publication.
Results
Participants
Overall 20,284 people received the Gam-COVID-Vac vaccine and were eligible to be included in the study considering our criteria. 6520 recipients filled the Q1 questionnaire. Of these, 330 were excluded from the study because they didn't respect the inclusion criteria or they filled the Q1 twice. Eventually 6190 were included in the study. Amongst these, 1879 were lost at the follow-up of the Q2 and other 1209 of the Q3; overall 4311 answered the Q2 and 3102 the Q3.
Baseline characteristics
The sociodemographic and clinical characteristics of the 6190 persons included in the study are presented in Table 1. A total of 3089 (49·9%) were females, mean age was 52·4 ± 18·2 years, the most prevalent age group was 60–69 years (18·8%), and 329 (5·3%) were health-care workers. More than half (53·8%) of the study participants had at least one underlying medical condition. In particular, hypertension was the most frequent coexisting condition (25·5%), followed by cardiovascular diseases (16·8%) and obesity (15·2%). Individuals who suffered from at least one allergy were 27·1%, and those allergic to some drugs and food were 9·3% and 2·8%, respectively. A previous infection with SARS-CoV-2 was reported by 4·6% of the participants. A total of 164 (2·6%) persons reported that they had taken painkillers or anti-inflammatory drugs the day of the first vaccination with Gam-COVID-Vac, before receiving it.
Table 1.
Baseline characteristics of the study sample (n = 6190) - Republic of San Marino (2021). Weighted estimates were obtained by weighting all observed values with post-stratification weights based on the age distribution of San Marino's population vaccinated with Sputnik V.
| Characteristic | Unweighted (observed) | Weighted (estimated) |
|---|---|---|
| Female sex | 3089 (49·9%) | 3101 (50·1%) |
| Age group, y | ||
| 18–29 | 927 (15·0%) | 836 (13·5%) |
| 30–39 | 728 (11·8%) | 722 (11·7%) |
| 40–49 | 998 (16·1%) | 1158 (18·7%) |
| 50–59 | 1130 (18·3%) | 1386 (22·4%) |
| 60–69 | 1163 (18·8%) | 1042 (16·8%) |
| 70–79 | 869 (14·0%) | 793 (12·8%) |
| 80–89 | 375 (6·1%) | 253 (4·1%) |
| Health-care worker | ||
| No | 5861 (94·7%) | 5834 (94·2%) |
| Non-physician profession* | 163 (2·6%) | 177 (2·9%) |
| Clerk | 113 (1·8%) | 123 (2·0%) |
| Physician | 24 (0·4%) | 25 (0·4%) |
| Pharmacist | 19 (0·3%) | 20 (0·3%) |
| Volunteer | 5 (0·1%) | 5 (0·1%) |
| Unspecified | 5 (0·1%) | 6 (0·1%) |
| Underlying medical condition | ||
| 0 | 2859 (46·2%) | 2964 (47·9%) |
| 1 | 1502 (24·3%) | 1541 (24·9%) |
| 2 | 859 (13·9%) | 825 (13·3%) |
| 3 | 503 (8·1%) | 457 (7·4%) |
| ≥4 | 467 (7·5%) | 403 (6·5%) |
| Individual medical conditions | ||
| Hypertension | 1578 (25·5%) | 1458 (23·6%) |
| Cardiovascular diseases | 1037 (16·8%) | 932 (15·1%) |
| Obesity (BMI ≥30 kg/m²) | 942 (15·2%) | 929 (15·0%) |
| Osteoarticular diseases | 520 (8·4%) | 469 (7·6%) |
| Diabetes mellitus | 356 (5·8%) | 320 (5·7%) |
| Mental disorders | 295 (4·8%) | 279 (4·5%) |
| Respiratory diseases | 277 (4·5%) | 261 (4·2%) |
| Neurological diseases | 252 (4·1%) | 238 (3·9%) |
| Malignant tumours | 156 (2·5%) | 139 (2·2%) |
| Immunosuppression | 97 (1·6%) | 93 (1·5%) |
| Nephropathy | 89 (1·4%) | 76 (1·2%) |
| Liver diseases | 58 (0·9%) | 52 (0·8%) |
| Other conditions⁎⁎ | 1195 (19·3%) | 1137 (18·4%) |
| One or more allergies | 1676 (27·1%) | 1704 (27·5%) |
| Individual allergies | ||
| Rhinitis | 845 (13·7%) | 869 (14·0%) |
| Drugs | 574 (9·3%) | 573 (9·3%) |
| Contact dermatitis | 181 (2·9%) | 187 (3·0%) |
| Food | 173 (2·8%) | 173 (2·8%) |
| Asthma | 145 (2·3%) | 149 (2·4%) |
| Insect sting | 49 (0·8%) | 49 (0·8%) |
| Other allergies | 77 (1·2%) | 78 (1·3%) |
| Ongoing pregnancy | 2 (0·0%) | 2 (0·0%) |
| Ongoing drug therapies | 2921 (47·2%) | 2795 (45·2%) |
| Other vaccines in the two weeks before Sputnik V | 14 (0·2%) | 13 (0·2%) |
| Painkillers or anti-inflammatory drugs right before being vaccinated | 164 (2·6%) | 160 (2·6%) |
| Previous infection with SARS-CoV-2 | ||
| No | 5906 (95·4%) | 5898 (95·3%) |
| Asymptomatic | 51 (0·8%) | 52 (0·8%) |
| Mild symptoms | 155 (2·5%) | 157 (2·5%) |
| Moderate/severe symptoms | 50 (0·8%) | 55 (0·9%) |
| Severe symptoms with hospitalisation | 28 (0·5%) | 28 (0·5%) |
Nurse, midwife, physical therapist, podiatrist, speech-language pathologist, orthoptist, audiologist, dental hygienist, dietician, etc.
Thyroid disorders, prostate disorders, gastrointestinal reflux diseases, glaucoma.
BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1 also shows the survey-adjusted frequencies for each category obtained by weighting all observed counts and percentages with post-stratification weights based on the age distribution of the Republic of San Marino's population vaccinated with Gam-COVID-Vac. The comparison of weighted and unweighted figures shows that the population stratum with the lowest response rate was that between 40 and 59 years of age; on the contrary, the youngest and oldest age groups appeared to be over-represented by the survey.
Adverse reactions after the first dose
In the weighted analysis using post-stratification weights based on the age distribution of the target population, we estimated that 4383 of the study participants had some AEFIs from the vaccine within three weeks of the first dose, which corresponds to a rate of 70·8% (95% CI 69·9–71·7). Symptoms were classified as grade 1, 2, 3 and 4 in severity by 58·7%, 11·1%, 0·8% and 0·3% of the individuals, respectively. AEFIs occurred after a few seconds or minutes in 2·6% of the subjects, after one or two days in 66·3%, after three to seven days in 1·7%, and after one to three weeks in 0·2%. Systemic reactions were reported by 3557 persons (57·5%, 95% CI 56·5–58·4), while reactions at the injection site were reported by 2888 persons (46·7%, 95% CI 45·7–47·6). The use of painkillers or anti-inflammatory drugs was reported by 1452 (23·5%) individuals.
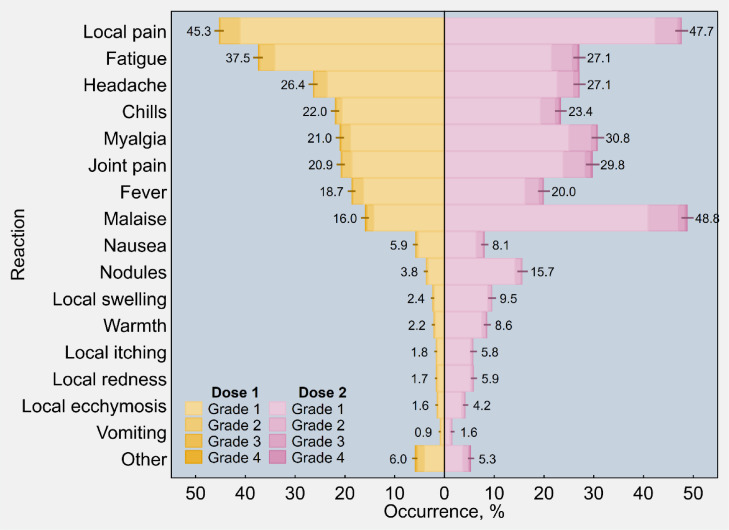
As shown in Figure 1, pain at the injection site was the most frequent reported solicited local reaction amongst vaccine recipients after the first dose of Gam-COVID-Vac (45·3%, 95% CI 44·3–46·3), followed by fatigue (37·5%, 95% CI 36·5–38·4), headache (26·4%, 95% CI 25·5–27·3), chills (22·0%, 95% CI 21·1–22·8), myalgia (21·0%, 95% CI 20·2–21·9), joint pain (20·9%, 95% CI 20·0–21·7), fever (18·7%, 95% CI 17·9–19·4), and malaise (16·0%, 95% CI 15·2–16·7).
Figure 1.
Estimated occurrence of individual adverse reactions following immunisation with the first and second dose of Sputnik V (n = 6190), overall and by four-point scale of severity - Republic of San Marino (2021). Other unsolicited events included tachycardia, dyspnoea, extrasystole and blood pressure anomalies; alteration of the menstrual cycle; sub-axillary lymphadenopathies; gastrointestinal alteration. Rates were post-stratified by using the age distribution of San Marino's population vaccinated with Sputnik V. Ninety-five percent confidence intervals are displayed as error bars.
Adverse reactions after the second dose
Vaccine recipients reported higher rates of AEFIs after dose 2 than dose 1. More specifically, an estimated 4692 participants had some AEFIs within three months, corresponding to a rate of 75·8% (95% CI 74·6–77·0). Symptoms were classified as grade 1, 2, 3 and 4 in severity by 58·6%, 13·4%, 2·7% and 1·1% of the individuals, respectively. AEFIs occurred after a few seconds or minutes in 5·2% of the subjects, after one or two days in 66·0%, after three to seven days in 3·1%, after one to four weeks in 0·9%, and after one to three months in 0·5%. Systemic reactions were reported by 3906 persons (63·1%, 95% CI 61·9–64·3), while reactions at the injection site were reported by 3384 persons (54·7%, (95% CI 53·2–56·1). AEFIs were treated with painkillers or anti-inflammatory drugs by 1370 (22·1%) individuals.
Following the second dose of Gam-COVID-Vac (Figure 1), malaise ranked as the most frequent solicited event (48·8%, 95% CI 47·6–50·1), followed by pain at the injection site (47·7%, 95% CI 46·4–49·0), myalgia (30·8%, 95% CI 29·6–32·1), joint pain (29·8%, 95% CI 28·4–31·2), headache (27·1%, 95% CI 26·0–28·4), fatigue (27·1%, 95% CI 25·9–28·4), chills (23·4%, 95% CI 22·2–24·6), fever (20·0%, 95% CI 18·8–21·2), and nodules at the injection site (15·7%, 95% CI 14·6–16·8). The same data are presented in tabular form in Table S2 in the appendix.
The results of unweighted analysis are illustrated in Figure S1 in the appendix.
Age and sex differences
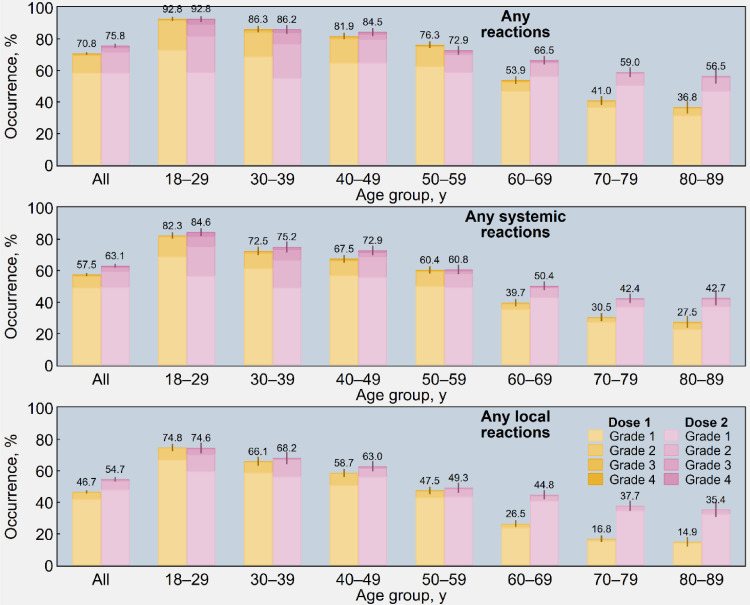
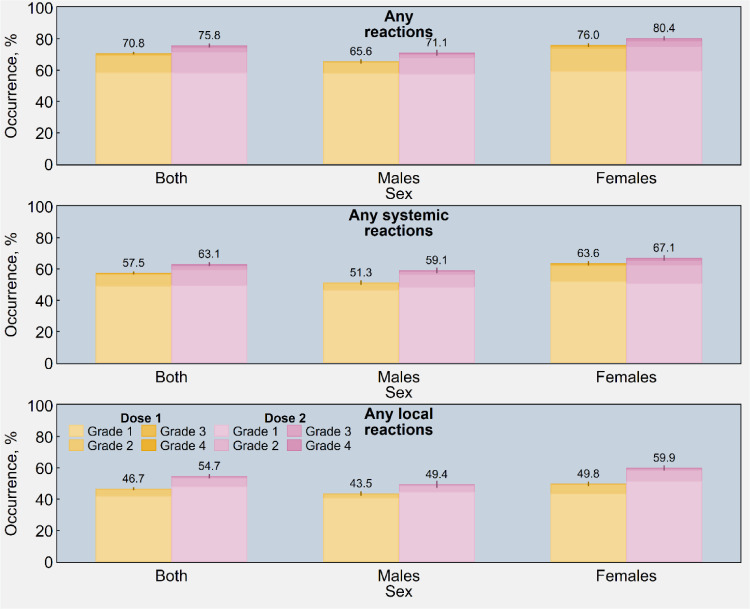
As illustrated in Figure 2, the frequency of local and systemic reactions was higher in the younger age groups than the older age groups. Moreover, older participants registered a large increment in AEFIs after the second dose, while younger participants did not exhibit any relevant difference between the two shots of the vaccine. Figure 3 also shows that women systematically reported a higher proportion of AEFIs than men (76.0% vs 65.6% and 80.4% vs 71.1% after first and second dose, respectively). The same data are presented in tabular form in Tables S3 and S4 in the appendix.
Figure 2.
Estimated occurrence of adverse reactions (1st panel), systemic adverse reactions (2nd panel), and local (injection-site) adverse reactions (3rd panel) following immunisation with the first and second dose of Sputnik V (n = 6190), overall and by age group and four-point scale of severity - Republic of San Marino (2021). Rates were post-stratified by using the age distribution of San Marino's population vaccinated with Sputnik V. Ninety-five percent confidence intervals are displayed as error bars.
Figure 3.
Estimated occurrence of adverse reactions (1st panel), systemic adverse reactions (2nd panel), and local (injection-site) adverse reactions (3rd panel) following immunisation with the first and second dose of Sputnik V (n = 6190), overall and by sex and four-point scale of severity - Republic of San Marino (2021). Rates were post-stratified by using the age distribution of San Marino's population vaccinated with Sputnik V. Ninety-five percent confidence intervals are displayed as error bars.
All the rates of AEFIs resulting from the unweighted analysis, that is, without post-stratification weights, are reported in the appendix (Figures S2 and S3).
Unsolicited AEFIs
Amongst the unsolicited events reported in the form in the “other symptoms” section, the most common symptoms were: stress-related symptoms compatible with a vasovagal presyncope such as tachycardia, dyspnoea, extrasystole and blood pressure anomalies; alteration of the menstrual cycle including amenorrhoea and dysmenorrhoea; predominantly sub-axillary lymphadenopathies; gastrointestinal alterations (i.e. diarrhoea, abdominal pain); for those patients with a history of rheumatological diseases, flares of such diseases. The severity of all of these reactions was grade 3 or lower and none required hospitalisation.
Risk factors for grade 3/4 events
Results of multivariable analysis investigating the risk factors associated with increased likelihood of grade 3 or grade 4 AEFIs are presented in Table 2. amongst all the characteristics collected at baseline, only female sex (dose 1: p<0·001; dose 2: p<0·001), younger age (dose 1: p = 0·001; dose 2: p<0·001), and food allergies (dose 1: p<0·001; dose 2: p = 0·05) emerged as significant risk factors associated with higher odds of systemic and local events of grade 3 to 4 after both shots of the vaccine.
Table 2.
Multivariable logistic regression analysis investigating the risk factors associated with increased likelihood of Grade 3 (severe) or Grade 4 (medically urgent) adverse reactions after immunisation with the first and second dose of Sputnik V (n = 6190) - Republic of San Marino (2021).
| Baseline characteristic | Dose 1 |
Dose 2 |
||||
|---|---|---|---|---|---|---|
| OR | P-value | 95% CI | OR | P-value | 95% CI | |
| Grade 3–4 reactions | ||||||
| Female sex (ref.: male) | 6·14 | <0·001 | 3·38–11·14 | 1·68 | <0·001 | 1·49–1·89 |
| Age, y | 0·98 | 0·001 | 0·97–0·99 | 0·95 | <0·001 | 0·95–0·96 |
| Food allergy (ref.: no) | 3·87 | <0·001 | 1·99–7·52 | 1·61 | 0·05 | 1·01–2·59 |
| Grade 3–4 systemic reactions | ||||||
| Female sex (ref.: male) | 7·47 | <0·001 | 3·38–16·50 | 1·55 | <0·001 | 1·37–1·75 |
| Age, y | 0·98 | 0·01 | 0·97–1·00 | 0·96 | <0·001 | 0·96–0·96 |
| Food allergy (ref.: no) | 2·75 | 0·03 | 1·10–6·88 | 1·87 | 0·01 | 1·19–2·94 |
| Grade 3–4 local reactions | ||||||
| Female sex (ref.: male) | 5·92 | 0·04 | 1·06–33·05 | 1·70 | <0·001 | 1·51–1·91 |
| Age, y | 0·99 | 0·18 | 0·97–1·01 | 0·97 | <0·001 | 0·96–0·97 |
| Food allergy (ref.: no) | 9·18 | 0·001 | 2·40–35·08 | 0·78 | 0·17 | 0·55–1·11 |
Notes: Covariates to include in the model were chosen using an iterative procedure described in the Methods section.
OR, odds ratio; CI, confidence interval.
Discussion
Based on a three month follow-up with monitoring up to 6000 adults, our findings show a high tolerability and a good safety profile of the Gam-COVID-Vac in the overall population of the Republic of San Marino, with a lower reactogenicity profile in older adults than in younger adults, in males and in people without a history of food allergies. These findings corroborated and extended the results from our preliminary investigation focusing on the older age population - aged 60 years and older - with a shorter follow-up.11
Only a small fraction of the participants reported AEFIs occurring later than the first week after the administration of any dose of the Gam-COVID-Vac, with a large majority of events being of mild or moderate severity.
The ROCCA study is the first nationwide prospective cohort study that evaluated the safety of Gam-COVID-Vac in a real-world setting.11 Due to the employment of physicians - on site or via telephone - for the collection of the majority of data and the centralised health system with the State Hospital and Emergency Room in the Republic of San Marino, we are confident about the comprehensiveness of acquired data.
The limitations of our study lie mainly in the small size of the study population, which - as the research project was not funded and therefore the researchers' capacity was limited - could not include a large portion of the vaccinated population of the Republic of San Marino. This reduces the ability to fully detect rare AEFIs, such as potential thrombosis as a complication of adenovirus vector vaccines, especially in subgroups of the population with specific comorbidities (e.g. diabetes, neurological diseases). Besides, we need to underline the number of participants lost to follow-up which is quite conspicuous, and consider a potential recall bias with the follow-up at three months for the filling of the Q3, as well as an overall self-reporting bias, given the nature of the questionnaire. More than half of the participants of this study reported having a medical condition at baseline; this higher proportion, as compared to the average Italian population,19 could be due to the lower response rate for intermediate age groups, which are also less likely to have chronic diseases.
This consideration about the good safety profile stems from the observation that our findings appear comparable with those from other EMA-approved two-dose vaccines in terms of incidence of local and systemic AEFIs.20, 21, 22 Compared to other vector-based vaccines approved by EMA, fewer reactions were reported after the first dose and more after the second dose.22 Such differences might find an explanation in the fact Gam-COVID-Vac uses different viral vectors in the first and second dose.
Regarding the most common symptoms after the first dose of the Gam-COVID-Vac, the frequency of local pain is significantly reduced compared to the other widely used vaccines,20,22,24 while fatigue is comparable to EMA-approved vaccines.20,22 Headache was less common than in other EMA approved vector based vaccines,23,24 while comparable to mRNA vaccines.20,22
For the second dose, amongst the most frequent symptoms, local pain and muscle pain were less frequent than in other widely used mRNA vaccines.20,22 but slightly more common if compared to same platform vaccines.23 Remarkably, headache and fatigue were less frequent than all two-dose EMA approved vaccines.20,22,23
When considering the severity of the symptoms, when compared to other vector-based vaccines23 we see that for the first dose local symptoms were graded similarly, and systemic symptoms were reported as being generally less severe. For the second dose both local and systemic symptoms severity was similar to the aforementioned vaccines.20, 21, 22, 23, 24 amongst the grade-four self-reported symptoms the majority matched with the cross-check run with the State Hospital but only few were admitted to the Emergency Department for observation.
Of all characteristics collected at baseline, only young age, food allergies and female sex were found to be significant risk factors for having grade three or four systemic and local events after two vaccinations. This data is coherent with what has been reported for other vaccines, especially for young age.20, 21, 22, 23, 24 Concerning the history of food allergy, we did not find any analogy in other COVID-19 vaccine surveillance studies.
Being a woman resulted in a six-fold increased risk of reporting more severe AEFIs after the first dose. The reason for sex being a determinant of this difference is still being discussed by academics and research is still ongoing with possible explanations including reporting bias and differences in physiological responses to immunization between sexes.25, 26, 27, 28 Also in other COVID-19 vaccine surveillance studies women were seen to report symptoms more often than men.29
Declaration of interests
The authors have no conflicts of interest to declare.
Acknowledgments
Funding
None.
Contributors
All the authors have contributed equally to the conceptualization and design of the manuscript. ZDV, GLF, GS, MM, AS were responsible for drafting the manuscript. JL conducted all data analyses. ZDV, GLF, GS, MM, AS were integral to the design and development of the vaccine safety surveillance questionnaire. ZDV, GLF, GS, EB, MM, AS were involved in the data collection process. All authors made substantial contributions to the interpretation of data for the work and revised the manuscript critically for important intellectual content.
Data sharing
The dataset generated and analysed during the current study can be made available by the corresponding author, MM, on reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101468.
Appendix. Supplementary materials
References
- 1.Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 2 February, 2022
- 2.WHO Coronavirus (COVID-19) Dashboard |Dashboard With Vaccination Data. https://covid19.who.int/. Accessed 2 February, 2022
- 3.Our World in Data. https://ourworldindata.org/#entries. Accessed 2 February, 2022
- 4.Institute for Social Security. Piano vaccinale anti-SARS-CoV-2/COVID-19 vaccination plan of the Republic of San Marino. http://www.iss.sm. Accessed 2 February, 2022
- 5.State Register of Medicines https://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=6c1f7501-7067-45b3-a56d-95e25db89e97&t. Accessed 2 February, 2022
- 6.About Sputnik V | Official website vaccine against COVID-19 Sputnik V. https://sputnikvaccine.com/about-vaccine/. Accessed 2 February, 2022
- 7.Press release | Official website vaccine against COVID-19 Sputnik V. https://sputnikvaccine.com/newsroom/pressreleases/. Accessed 2 February, 2022
- 8.Mendonça S.A., Lorincz R., Boucher P., Curiel D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6:1–14. doi: 10.1038/s41541-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Definition and Application of Terms for Vaccine Pharmacovigilance 2012. https://vaccine-safety-training.org/tl_files/vs/pdf/report-of-cioms-who-working-group.pdf. Accessed 2 February, 2022
- 11.Montalti M., Soldà G., Di Valerio Z., et al. ROCCA observational study: early results on safety of Sputnik V vaccine (Gam-COVID-Vac) in the Republic of San Marino using active surveillance. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.101027. published online Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monitoring of COVID-19 medicines | European Medicines Agency https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/monitoring-covid-19-medicines-0. Accessed 2 February, 2022
- 13.Krejcie R.V., Morgan D.W. Determining sample size for research activities. Educ Psychol Meas. 1970;30:607–610. [Google Scholar]
- 14.Cochran W.G. 3rd ed. John Wiley & Sons; New York: 1977. Sampling Techniques. [Google Scholar]
- 15.Levy P.S., Lemeshow S. 4th ed. Wiley; Hoboken, NJ: 2008. Sampling of Populations: Methods and Applications. [Google Scholar]
- 16.Van Buuren S., Boshuizen H.C., Knook D.L. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Héraud-Bousquet V., Larsen C., Carpenter J., Desenclos J.C., Le Strat Y. Practical considerations for sensitivity analysis after multiple imputation applied to epidemiological studies with incomplete data. BMC Med Res Methodol. 2012 doi: 10.1186/1471-2288-12-73. published online June 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenzi J., Pildava S. Tips for calculating and displaying risk-standardized hospital outcomes in Stata. Stata J. 2019;19(2):477–496. doi: 10.1177/1536867×19854021. [DOI] [Google Scholar]
- 19.Patologie croniche - Sorveglianza Passi. https://www.epicentro.iss.it/passi/dati/croniche. Accessed 2 February, 2022)
- 20.Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events | CDC [Internet]. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html#persons-18yrs-older. Accessed 2 February, 2022
- 21.Heath P.T., Galiza E.P., Baxter D.N., et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moderna COVID-19 Vaccine's Reactions and Adverse Events | CDC. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. Accessed 2 February, 2022
- 23.Committee for Medicinal Products for Human Use (CHMP) Assessment report . 2021. COVID-19 Vaccine AstraZeneca.www.ema.europa.eu/contact Accessed 2 February, 2022. [Google Scholar]
- 24.Shay D.K., Gee J., Su J.R., Myers T.R., et al. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–684. doi: 10.15585/mmwr.mm7018e2. https://www.cdc.gov/mmwr/volumes/70/wr/mm7018e2.htm Accessed 2 February, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCartney P.R. Sex-Based Vaccine Response in the Context of COVID-19. J Obstet Gynecol Neonatal Nurs. 2020;49(5):405–408. doi: 10.1016/j.jogn.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang W.H. A review of vaccine effects on women in light of the COVID-19 pandemic. Taiwan J Obstet Gynecol. 2020;59(6):812–820. doi: 10.1016/j.tjog.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt K., Adamson J., Hewitt C., Nazareth I. Do women consult more than men? A review of gender and consultation for back pain and headache. J Health Serv Res Policy. 2011;16(2):108–117. doi: 10.1258/jhsrp.2010.009131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyke S., Hunt K., Ford G. Gender differences in consulting a general practitioner for common symptoms of minor illness. Soc Sci Med. 1998;46(7):901–906. doi: 10.1016/s0277-9536(97)00217-7. [DOI] [PubMed] [Google Scholar]
- 29.Menni C., Klaser K., May A., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.