Abstract
Background
Complete histologic normalization is associated with improved clinical outcomes in ulcerative colitis (UC). However, it is currently unknown what effect achieving histologic normalization has on the development of dysplasia.
Methods
We performed a retrospective analysis of 495 patients with a confirmed diagnosis of UC from a tertiary center. Patients were categorized according to the best histologic assessment they had during their disease course: histologic normalization, histologic quiescence, or persistent histologic activity. We assessed dysplasia rates in these patient groups after achieving histologic normalization or histologic quiescence, or 8 years after UC diagnosis in those with persistent histologic activity. Kaplan-Meier graphs and Cox regression analyses were performed to estimate this effect.
Results
The incidence rate of dysplasia development after achieving histologic normalization was statistically significantly less when compared with the incidence rate after achieving histologic quiescence (P = 0.001) and in those with persistent histologic activity 8 years after UC diagnosis (P = 0.033). In multivariate analysis, at any point throughout UC duration, dysplasia development was statistically lower in those with histologic normalization (adjusted hazard ratio [aHR], 0.32; 95% confidence interval [CI], 0.13-0.81) but not in those with histologic quiescence (aHR, 0.52; 95% CI, 0.25-1.10), compared with those with persistent histologic inflammation. When assessing the time after achieving histologic normalization, histologic quiescence, or 8 years post UC diagnosis in those with persistent histologic activity, we found that patients with histologic normalization had a subsequent decreased risk of developing dysplasia (aHR, 0.09; 95% CI, 0.01-0.72), compared with patients without normalization.
Conclusions
Histologic normalization is associated with a decreased risk in patients with UC of developing subsequent dysplasia, compared with patients without histologic normalization. These findings have implications for surveillance intervals.
Keywords: ulcerative colitis, histology, histologic normalization, dysplasia
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease affecting the colon, starting in the rectum and often extending proximally. Colorectal neoplasia (CRN) is a feared complication of UC, with significantly greater risk starting 8 years after diagnosis.1, 2 Risk factors for developing CRN in this population include a family history of colorectal cancer (CRC), coexisting primary sclerosing cholangitis (PSC), disease extent (beyond the rectum), and duration of disease.3, 4 Recently, cumulative histologic inflammation has also been identified as a predictive factor for CRN.5 Current guidelines recommend screening colonoscopies for dysplasia surveillance,6, 7 because UC-related CRC is thought to progress from nonneoplastic epithelium to dysplasia before developing into cancer.4
Management goals for UC have shifted in recent years toward achieving clinical remission combined with mucosal healing (deep remission),8 because mucosal healing is associated with sustained remission and a reduced risk of colectomy.6 However, the level of agreement between endoscopic and histologic activity is moderate at best, with 68% agreement between the 2 seen in a study of 646 patients.9 Histologic remission (the absence of active inflammation) has been associated with decreased rates of corticosteroid use and hospitalizations.10 In 2016, the U.S. Food & Drug Administration proposed adding histologic remission into the definition of mucosal healing, which already included endoscopic remission.11 In patients with mucosal healing, complete histologic normalization (the absence of active inflammation with no chronic changes) is associated with improved clinical-relapse-free survival9 when compared with histologic quiescence (absence of active inflammation with chronic changes) or active inflammation. It is unknown whether stable disease over time reduces neoplasia risk or whether histologic normalization is protective against the development of subsequent neoplasia. We assessed the risk of dysplasia in patients with UC who achieved histologic normalization compared with those who did not.
Materials and Methods
A retrospective cohort study at our center was performed and approved by the institutional review board (IRB 19-1554). Eligible patients were those with an established histologic diagnosis of UC who underwent a colonoscopy at the University of Chicago after August 2005, with biopsies from at least 2 colonoscopies and a date of last follow-up occurring after December 2010. Patients with inadequate documentation (<2 colonoscopies with associated pathology), prior colectomy, prior episode of dysplasia before 2005, documented enteric infection at the time of follow-up colonoscopy, or histologic disease confined to the rectum were excluded.
Medical Records Abstraction
Demographic, clinical, and histologic data were collected from our electronic medical system (EPIC, Verona, WI), which included date of birth, sex, age at UC diagnosis, smoking status, diagnosis of PSC, first-degree family history of colon cancer, and use of UC-related medications. Medications were grouped as 5-aminosalicylates, immunomodulators (including azathioprine, 6-mercaptopurine, and methotrexate), or biologics (including anti-tumor necrosis factor agents, anti-integrins, anti-interleukin-12/23, and Janus kinase inhibitors). Patients were followed until the date of their last colonoscopy at our institution.
Age at the end of follow-up was categorized into <50 years, 50 to 75 years, and >75 years. Smoking was categorized into never having smoked, former smoker at last follow-up, or current smoker at last follow-up. Family history of colon cancer was defined as a first-degree relative with colon cancer. Maximum histologic extent was defined as the most proximal segment in the colon where histologic disease was present, separated into 4 categories (ascending, transverse, descending, and sigmoid). Those with isolated proctitis were excluded because of their lack of increased risk of dysplasia.12 Cumulative inflammatory burden (CIB) was derived from the severity of patients’ colonoscopy histologic assessments, using histologic severity scores13 and the length of time between surveillance colonoscopies in the calculation.14 This CIB score was recently validated as a predictive marker of neoplasia in patients with UC.15 Histologic activity was graded by our pathologists as mild, moderate, or severe inflammation. We measured CIB using a 5-point scale (normal, chronic changes without active inflammation, and acute inflammation according to mild, moderate, and severe designations), based on the maximum histologic score at each individual colonoscopy. These scores were then averaged over time between colonoscopies (eg, a score of 3 in 2015 and a score of 1 in 2017 would equate to a 2 for this time period) and then added up over the duration of their disease.
Patients were followed from UC diagnosis until their last recorded colonoscopy. They were categorized as having achieved histologic normalization, histologic quiescence, or persistent histologic activity at any point throughout their disease. Dysplasia events were recorded even if they occurred before patients achieved histologic normalization or quiescence.
Time after the onset of histologic normalization, histologic quiescence, or from 8 years after UC diagnosis in those with persistent active inflammation was also recorded. Patients were followed until the development of dysplasia, or until the last recorded colonoscopy.
Histologic Assessment
It is routine practice at our institution’s endoscopic unit to obtain random mucosal biopsies from the right colon, left colon, and rectum of all patients with UC and targeted biopsies of the most significant mucosal activity. Flexible sigmoidoscopies were also included in our analysis if they were performed in patients with left-sided UC with no known proximal histologic disease. Pathologists who specialize in gastrointestinal histology routinely asses all biopsies at our institution and report histology using a standardized scale that includes histologically normal, quiescent, or active inflammation. Two pathologists review biopsies to confirm the presence of dysplasia. We reviewed pathologic reports and categorized histology specimens into 3 distinct groups using the modified Riley score,10 but we subcategorized histologic remission into histologic normalization and histologic quiescence based upon the absence or presence of chronic histologic changes, respectively. Based on the maximum inflammation score at each segment, patients were categorized as (1) histologic normalization: completely normal mucosa with no chronic features present; (2) histologic quiescence: features of chronicity present but no active inflammation present; and (3) histologic activity: presence of any epithelial infiltration by neutrophils, crypt abscesses, erosions, or ulcerations. Histologic normalization was defined as absolutely no features of chronicity (eg, crypt branching or crypt atrophy.)
Patients were categorized according to the lowest histologic score at any point in their disease course (with histologic normalization being the lowest and histologic activity being the highest). For a colonoscopy to be categorized as normal, all the segmental biopsies obtained during that colonoscopy had to be read by the pathologist as normal. If the colonic segments did not have the same histology score, then the highest histology score was used to categorize that colonoscopy (for example, a colonoscopy with 2 normal histology segments and 2 quiescent histology segments would be categorized as quiescent). If dysplasia was detected concurrently with histologic normalization or quiescence, then the most recent histologic assessment was used for that patient.
Dysplasia Assessment
Dysplasia was considered to be present if low-grade dysplasia or high-grade dysplasia/carcinoma in situ was present in a colonic segment of known disease activity on random or targeted biopsies or on polyp removal. Endoscopic reports were used to confirm that the dysplastic lesion was within an area of known colitis.
Outcomes
The primary outcome was comparing the rate of development of dysplasia after patients achieved histologic normalization to that in patients who never achieved histologic normalization. The secondary outcome was assessing dysplasia rates in each histologic category (normal, quiescent, and active), including at any point in their disease course, even if dysplasia occurred before patients achieved histologic normalization or quiescence.
Statistical Analysis
Continuous variables were summarized using medians and interquartile ranges, and categorical variables were expressed as percentages and number of the cohort. Analysis of variance was used to compare continuous variables, and the Pearson χ 2 test or Fisher exact test was used to compare categorical variables.
Kaplan-Meier analysis and log-rank tests were performed to compare the proportions of patients without dysplasia in those who achieved histologic normalization, histologic quiescence, and persistent histologic activity and in those with histologic normalization compared with those without. Cox proportional hazards regression analysis was performed to identify independent predictors of developing dysplasia in these groups of patients and in those achieving histologic normalization compared with independent predictors in patients who did not. Variables with P < 0.20 were included in a multivariate analysis. The proportional hazards assumption was tested for all variables in our model.
Cox proportional hazards regression analysis was performed to identify independent predictors of developing dysplasia, and variables with P < 0.20 were included in a multivariate analysis. The proportional hazards assumption was tested for all variables in our model.
A P value of < 0.05 was considered to be statistically significant. All data analyses were performed using Stata 16.1 (StataCorp, College Station, TX).
Results
Patient Characteristics
Of 600 charts reviewed, 105 were excluded; 495 patients met the inclusion criteria and were included in our study. We found that 130 patients had achieved complete histologic normalization, 261 were histologically quiescent, and 104 had persistent histologic inflammation. The mean time to achieve histologic normalization was 17.57 years (SD, 10.85), and the mean time to achieve histologic quiescence was 14.70 years (SD, 10.16). Baseline characteristics of patients categorized by their best histologic assessment are reported in Table 1. Patients with histologic quiescence were more likely to be male, and those with persistent active histologic disease had a shorter disease duration. Those with persistently active disease were also more likely to have the ascending colon as the maximum histologic extent, as opposed to those with less extensive disease.
Table 1.
Patient Characteristics by Histology Group
| Normalized (n = 130) | Quiescent (n = 261) | Active (n = 104) | P | |
|---|---|---|---|---|
| Age at UC diagnosis, median (IQR) | 29.7 (22.6-41.5) | 28.2 (21.0-40.5) | 25.9 (21.2-37.0) | 0.265 |
| Female sex, n (%) | 66 (50.8) | 106 (40.6) | 60 (57.7) | 0.007* |
| Disease duration with UC, median (IQR) | 24.3 (18.4-34.1) | 23.6 (15.9-33.9) | 19.2 (14.3-27.2) | 0.001* |
| Maximum histologic extent, n (%) | 0.001* | |||
| Sigmoid colon | 12 (9.2) | 22 (8.4) | 3 (2.9) | |
| Descending colon | 32 (24.6) | 49 (18.8) | 5 (4.8) | |
| Transverse colon | 6 (4.6) | 20 (7.7) | 9 (8.7) | |
| Ascending colon | 81 (61.5) | 170 (65.1) | 87 (83.7) | |
| Number of colonoscopies per patient, median (IQR) | 6.0 (4.0-9.0) | 5.0 (4.0-8.0) | 4.0 (2.0-5.0) | <0.001* |
| Medication use, n (%) | ||||
| 5-ASAs | 116 (89.2) | 228 (87.4) | 97 (93.3) | 0.262 |
| Immunomodulators | 46 (35.4) | 120 (46.0) | 53 (51.0) | 0.042* |
| Biologics | 29 (22.3) | 76 (29.1) | 40 (38.5) | 0.026* |
| PSC, n (%) | 3 (2.3) | 9 (3.4) | 6 (5.8) | 0.362 |
| Family history of colorectal cancer, n (%) | 20 (15.4) | 29 (11.1) | 18 (17.5) | 0.217 |
| Smoking history, n (%) | 0.053 | |||
| Never | 86 (66.2) | 183 (70.1) | 85 (81.7) | |
| Former | 37 (28.5) | 62 (23.8) | 18 (17.3) | |
| Current | 7 (5.4) | 16 (6.1) | 1 (1.0) | |
| CIB, mean (SD) | 16.5 (9.9) | 20.1 (11.4) | 21.6 (14.5) | 0.002* |
| Documented active histology | ||||
| Before normalization/quiescence, n (%) | 106 (81.5) | 203 (77.7) | — | 0.39 |
| After normalization/quiescence, n (%) | 46 (35.4) | 173 (66.3) | — | <0.001* |
| Documented quiescent histology | ||||
| Before normalization/quiescence, n (%) | 19 (14.6) | — | — | — |
| After normalization/quiescence, n (%) | 31 (23.8) | — | — | — |
Patients categorized according to best histology at any point in their disease course.
*P value denotes statistical significance.
5-ASAs indicate 5-aminosalicylates; IR, interquartile ratio.
Of those who achieved histologic normalization, 81.5% had active histology on a colonoscopy beforehand, whereas 14.6% had quiescence. After achieving histologic normalization, 35.4% of patients had active histology on a subsequent colonoscopy and 23.8% had quiescence. Of those with histologic quiescence, 77.7% had active histology on a colonoscopy beforehand, and 66.3% had active histology on a subsequent colonoscopy.
Time to Developing Dysplasia
There were 56 (11.3%) patients who developed dysplasia: 12 had achieved histologic normalization (9.2%), 34 had quiescent histology (13.0%), and 10 were in the active histology group (9.6%). The mean time from UC diagnosis to developing dysplasia was 21.43 years (SD, 11.88) in all patients, 15.67 years (SD, 9.96) in patients with a history of histologic normalization, 24.24 years (SD, 12.11) in patients with a history of histologic quiescence, and 18.83 years (SD, 11.03) in patients with persistent histologic activity.
In the 130 patients who achieved histologic normalization, 11 episodes of dysplasia occurred before achieving histologic normalization with an incidence rate of 0.48 per 100 patient-years, and 1 episode of dysplasia occurred afterward, with an incident rate of 0.15 per 100 patient-years (Table 2). Incidence rates before and after achieving histologic quiescence were 0.31 and 1.56 per 100 patient-years, respectively. For patients with persistent histologic activity, their incidence rate of dysplasia development was 1.01 per 100 patient-years after 8 years disease duration. The incidence rate of dysplasia development after achieving histologic normalization was statistically significantly reduced when compared with both histologic quiescence (P = 0.001) and persistent histologic activity (P = 0.033).
Table 2.
Number of Episodes of Dysplasia Before and After Histologic Normalization/Quiescence/Persistent Activity and Associated Incidence Rates
| Before Normalization/Quiescence* | After Normalization/Quiescence† | |||
|---|---|---|---|---|
| Episodes of Dysplasia | Incidence Rate (95% CI)‡ | Episodes of Dysplasia | Incidence Rate (95% CI)‡ | |
| Histologic normalization (n = 130) | 11 (10 LGD; 1 HGD) | 0.48 (0.27-0.87) (LGD = 0.44; HGD = 0.04) | 1 (1 LGD) | 0.15 (0.02-1.04) (LGD = 0.15) |
| Histologic quiescence (n = 261) | 12 (11 LGD; 1 HGD) | 0.31 (0.18-0.55) (LGD = 0.28; HGD = 0.03) | 27 (22 LGD; 5 HGD) | 1.56 (1.07-2.27) (LGD = 1.27; HGD = 0.29) |
| Persistent histologic activity (n = 104) | 9 (7 LGD; 2 HGD) | 1.01 (0.52-1.94) (LGD = 0.78; HGD = 0.22) | ||
| Total (n = 495) | 23 | 37 | 1.12 (0.81-1.54) | |
*“Before” refers to patients before or at the same time as achieving histologic normalization or histologic quiescence.
† “After” refers to patients after they have achieved histologic normalization or histologic quiescence, or 8 years after their UC diagnosis.
‡Per 100 patient-years.
HGD indicates high-grade dysplasia or colon cancer; LGD, low-grade dysplasia.
Development of Dysplasia After Achieving Histologic Normalization or Quiescence
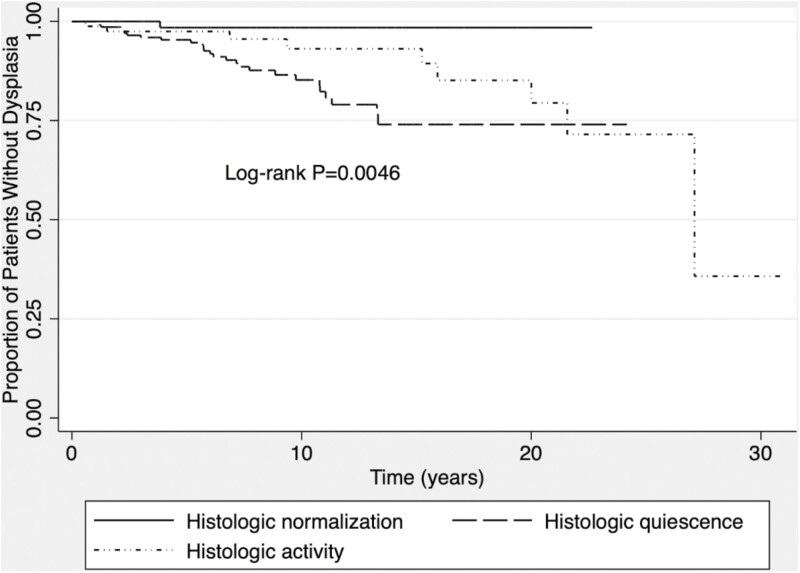
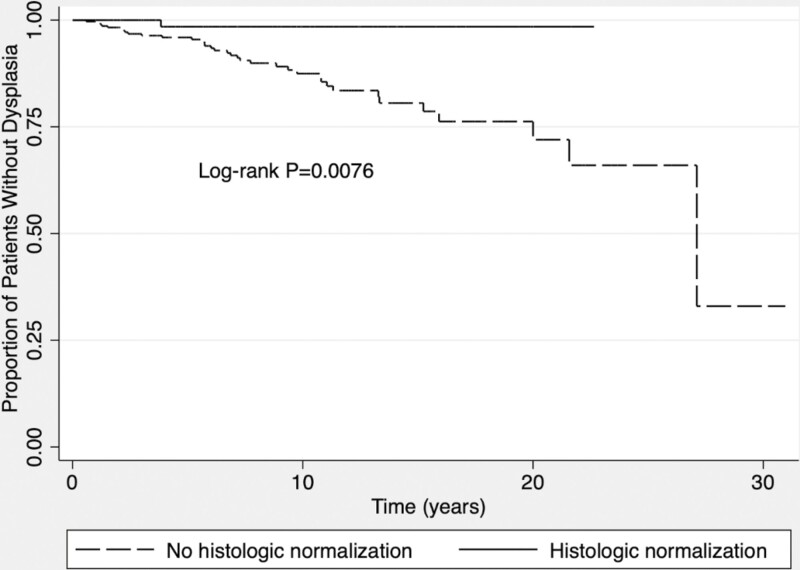
The risk of developing dysplasia was significantly different once patients achieved histologic normalization and achieved histologic quiescence, and for those with persistent active inflammation (P = 0.0046; Fig. 1). The risk of developing dysplasia in patients who achieved histologic normalization vs all other patients (quiescent and active histology) was also statistically significant (P = 0.0076; Fig. 2).
Figure 1.
Kaplan-Meier curve of dysplasia-free survival once patients have either achieved histologic normalization or histologic quiescence or have persistent inflammation.
Figure 2.
Kaplan-Meier curve of dysplasia-free survival once patients have achieved histologic normalization compared with those who have not.
Achieving histologic normalization was associated with a decreased risk, albeit not statistically significant, of developing dysplasia compared with active inflammation (hazard ratio [HR], 0.18; 95% confidence interval [CI], 0.02-1.47; adjusted hazard ratio [aHR], 0.13; 95% CI, 0.02-1.11). Achieving histologic quiescence, when compared with active inflammation, was not associated with a decreased risk of developing dysplasia (HR, 2.05; 95% CI, 0.89-4.72; aHR, 1.55; 95% CI, 0.63-3.78).
On multivariate analysis, when histologic activity was dichotomized into those patients who achieved normalization at least once and those who did not, achieving histologic normalization led to a statistically significant decreased risk of developing dysplasia (aHR, 0.10; 95% CI, 0.01-0.72; Table 3). Other significant predictors of dysplasia in the univariate analysis were male sex, maximum histologic extent into the transverse colon, and being a former smoker. Significant predictors in the multivariate analysis included histologic normalization and male sex. No patients in this cohort with a maximum histologic extent of disease activity in the sigmoid colon developed dysplasia, and they were therefore excluded from univariate analysis when we assessed maximum histologic extent as a risk factor for dysplasia.
Table 3.
HRs of Developing Dysplasia After Patients Achieve Histologic Normalization
| Variable | Univariate Analysis | Multivariate Analysis | P * |
|---|---|---|---|
| Achieved histologic normalization | 0.11 (0.01-0.79) | 0.09 (0.01-0.72) | 0.023‡ |
| Sex | |||
| Male | 3.18 (1.48-6.84) | 2.64 (1.19-5.87) | 0.017‡ |
| PSC | |||
| Yes | 1.82 (0.56-5.94) | — | — |
| Family history of CRC | |||
| Yes | 0.93 (0.36-2.40) | — | — |
| Maximum histologic extent† | |||
| Transverse vs descending | 6.96 (1.27-38.07) | — | — |
| Ascending vs descending | 3.63 (0.87-15.19) | — | — |
| Smoking status | |||
| Former vs no history of smoking | 2.46 (1.26-4.79) | 1.80 (0.89-3.64) | 0.10 |
| Current vs no history of smoking | 0.68 (0.09-5.12) | 0.60 (0.08-4.52) | 0.62 |
| Age at end of follow-up, y | |||
| <50 | Reference | 1.94 (0.77-4.89) | 0.16 |
| 50-75 | 2.14 (0.88-5.21) | 3.29 (0.78-13.92) | 0.11 |
| >75 | 3.26 (0.81-13.10) | ||
| CIB | 0.98 (0.96-1.01) | 0.97 (0.95-1.00) | 0.09 |
*P value for multivariate analysis.
†There were zero patients with maximum histologic extent in the sigmoid who developed dysplasia in this analysis, and therefore these patients were excluded from univariate analysis.
‡ P value denotes statistical significance.
When histologic activity was dichotomized into those patients who achieved normalization or quiescence at least once and those with persistent histologic activity, achieving histologic normalization or quiescence at least once was associated with a nonsignificantly decreased risk of dysplasia (HR, 0.53; 95% CI, 0.26-1.27; aHR, 0.70; 95% CI, 0.28-1.72).
Development of Dysplasia at Any Point During UC
Development of dysplasia was statistically significantly lower for patients who achieved histologic normalization at least once (aHR, 0.32; 95% CI, 0.13-0.81) but not for patients who achieved histologic quiescence at least once (aHR, 0.52; 95% CI, 0.25-1.10) when compared with those with persistent histologic inflammation. When we compared those who achieved histologic normalization with patients who did not achieve histologic normalization, we found that they had a decreased risk of developing dysplasia (aHR, 0.57; 95% CI, 0.29-1.10), although this finding was not statistically significant.
Discussion
Patients with UC are at increased risk for neoplasia, and the degree of histologic activity is a well-described risk factor. In this study, we found that patients with UC who achieve histologic normalization have a reduced risk of developing subsequent dysplasia.
It is well known that cumulative inflammation increases the risk of dysplasia. A retrospective single-center study assessed 987 patients over many years and found that the risk of colorectal neoplasia is associated with the CIB.14 Choi et al found that inflammation based on the most recent colonoscopy was not a significant predictor for neoplasia but that the mean severity score in the preceding 5 years was a significant predictor. A study by Rubin, Huo, et al5 showed similar results in which histologic inflammatory activity was positively associated with colorectal neoplasia. Although the duration of disease and extent are also risk factors for neoplasia development, it is now understood that the severity of inflammation seen on histology, over time, is also a risk factor. It is unknown whether there is any mechanism to “reset” this cumulative inflammation and potentially decrease a patient’s dysplasia risk. It stands to reason, though, that if patients achieve histologic normalization in UC, then it may potentially be associated with a decreased risk of dysplasia in the future despite severe inflammation in the past.
The risk of dysplasia in UC varies in the literature1, 2; however, this risk may have decreased in recent years,16-18 possibly because of improving therapies that better control the inflammation. It is unknown whether increased screening for precancerous lesions or improved control of mucosal and histologic inflammation is responsible for this decrease, or perhaps both.
In this study, PSC was not associated with a statistically significant increased risk of dysplasia. Whether this finding was the result of low power (there were 18 patients in our study with PSC) or shows the value of histologic quiescence or normalization is unclear. Family history of CRC was also not statistically significant in our study, and although studies have shown an increased risk of CRC in patients with inflammatory bowel disease,19 this finding could result from poor recall or inaccurate family history recorded in patients’ charts. It has been shown that cumulative inflammation is a risk factor for dysplasia development,14, 15 and it is of great interest that despite not having access to all prior colonoscopies in our referral-based population, we still found that histologic normalization was protective against neoplasia in this analysis. This limited access to prior histology reports in some of the patients in our study who developed dysplasia is a limitation of using the CIB and may explain why it was not a significant predictor of dysplasia in our study.
Although the mean time to developing dysplasia was shorter in patients with histologic normalization than in the other groups, this finding was based on fewer episodes of dysplasia. Further, many of the dysplasia events occurring in the normalization group occurred before they achieved histologic normalization. This result may be explained by the hypothesis that histologic normalization only decreases the risk of dysplasia after achieving normalization, and not beforehand.
Although we found that patients with UC who achieve histologic normalization have a reduced risk of developing subsequent dysplasia, we did not see this result when assessing patients who achieved histologic normalization or quiescence compared with those with active inflammation. Of the patients who achieved histologic quiescence, 77.7% had histology activity beforehand and 66.3% had histologic activity afterward. It is possible that achieving histologic quiescence does not have the same benefit as achieving histologic normalization or that a longer period in quiescence is required to reduce the risk of subsequent dysplasia.
Treating patients with UC has shifted toward tight control, with mucosal healing as the preferred goal.8 Recent updates have questioned whether histologic endpoints as treatment targets should be considered, because they are associated with improved outcomes.20 Larger prospective studies are necessary to confirm that histologic normalization is an attainable outcome and whether escalating medication therapy would be cost-effective. In addition, there are variations in histopathologic grading scales that require further clarification and dissemination before we can adopt histologic endpoints as outcomes in clinical practice.
Patients with UC require colonoscopies for dysplasia surveillance, although their timing varies according to different guidelines.21 The British Society of Gastroenterology recommends that the frequency should depend on the extent of endoscopic or histologic inflammation, ranging from annually in high-risk patients to every 5 years in those who are low risk.7 Meanwhile, the American College of Gastroenterology guidelines state that patients should undergo colonoscopies every 1 to 3 years depending on risk factors and findings on prior investigations.6 Our findings agree with these statements, in that patients with complete normalization of their colonic biopsies are indeed at lower risk of developing dysplasia, even in those with prior episodes of dysplasia. These guidelines should also be considered with the cost of preventive care.
There are several strengths and limitations to our study. This is a retrospective study at a tertiary center and is not reflective of general community practice. Because our institution is a referral center, many patients seen have a history of dysplasia. Further, it is possible that achieving histologic normalization is associated with a less-severe disease phenotype and, therefore, fewer episodes of dysplasia. However, we were able to account for this using the CIB as a covariate in our model, and we showed that histologic normalization is protective to dysplasia after normalization is achieved. Further, our study collected data from numerous colonoscopy pathology reports and categorized them according to histologic normalization, histologic quiescence, or degree of inflammation. We acknowledge that our expert gastrointestinal pathologists are not always in agreement with their interpretation, but as previously described,5 there is great agreement when there is no dysplasia and moderate agreement in degrees of inflammation. We believe that variation within histologic interpretation is minimal.22 The UC histology may also be patchy throughout the colon, with areas of normalization and areas of quiescence next to each other, although our center has a standardized approach toward taking biopsies during colonoscopies in all patients, which holds for all the patients in our study. Although our definitions of histologic activity are similar to other definitions,10, 23, 24 this histologic scale has not been validated in an independent prospective study.
Conclusions
Although further studies with larger patient populations are needed to validate these findings, we believe that patients with histologic normalization are at low risk for colorectal neoplasia and may be stratified to less intensive surveillance.
Author Contributions
SRS, AE, CT, VR, NKC, AI, and BC collected and analyzed the data; SRS and DTR designed the research study and wrote the manuscript; and all authors critically revised the manuscript and approve the submitted version.
Conflicts of Interest
NKC reports consulting for Takeda. BC has received research support, speaker fees, and consultancy payments from AbbVie, Janssen, Takeda, Ferring, Pfizer, Gilead, and Novartis. DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Altrubio, Allergan Inc., Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Connect BioPharma, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, InDex Pharmaceuticals, Ironwood Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Materia Prima, Pfizer, Prometheus Biosciences, Reistone, Takeda, and Techlab Inc.
References
- 1. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein CN, Blanchard JF, Kliewer E, et al. . Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. [DOI] [PubMed] [Google Scholar]
- 3. Feuerstein JD, Moss AC, Farraye FA. Ulcerative colitis. Mayo Clin Proc. 2019;94:1357–1373. [DOI] [PubMed] [Google Scholar]
- 4. Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20:16389–16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin DT, Huo D, Kinnucan JA, et al. . Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–160 8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. . ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 7. Lamb CA, Kennedy NA, Raine T, et al. . British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 9. Christensen B, Hanauer SB, Erlich J, et al. . Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15:1557–1564.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bryant RV, Burger DC, Delo J, et al. . Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65:408–414. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food & Drug Administration. Ulcerative colitis: clinical trial endpoints guidance for industry.https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM515143.pdf. Accessed April 23, 2020.
- 12. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–645. [DOI] [PubMed] [Google Scholar]
- 13. Rutter M, Saunders B, Wilkinson K, et al. . Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. [DOI] [PubMed] [Google Scholar]
- 14. Choi CR, Al Bakir I, Ding NJ, et al. . Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019;68:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yvellez OV, Rai V, Sossenheimer PH, et al. . Cumulative histologic inflammation predicts colorectal neoplasia in ulcerative colitis: a validation study. Inflamm Bowel Dis. 2021;27:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castaño-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther. 2014;39:645–659. [DOI] [PubMed] [Google Scholar]
- 17. Olén O, Erichsen R, Sachs MC, et al. . Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123–131. [DOI] [PubMed] [Google Scholar]
- 18. Klepp P, Brackmann S, Cvancarova M, et al. . Risk of colorectal cancer in a population-based study 20 years after diagnosis of ulcerative colitis: results from the IBSEN study. BMJ Open Gastroenterol. 2020;7:e000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samadder NJ, Valentine JF, Guthery S, et al. . Family history associates with increased risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1807–1813.e1. [DOI] [PubMed] [Google Scholar]
- 20. Ungaro R, Colombel JF, Lissoos T, et al. . A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dulai PS, Sandborn WJ, Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev Res (Phila). 2016;9:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Odze RD, Tomaszewski JE, Furth EE, et al. . Variability in the diagnosis of dysplasia in ulcerative colitis by dynamic telepathology. Oncol Rep. 2006;16:1123–1129. [PubMed] [Google Scholar]
- 23. Gupta RB, Harpaz N, Itzkowitz S, et al. . Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanauer S, Schwartz J, Robinson M, et al. . Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88:1188–1197. [PubMed] [Google Scholar]