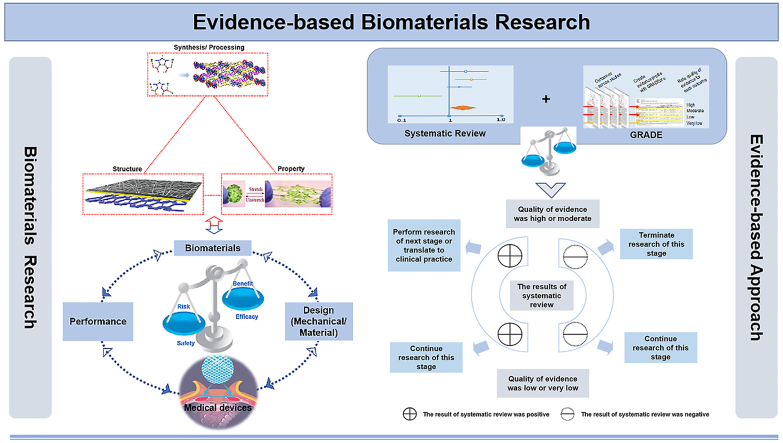
Abstract
The fast development of biomaterials science and engineering has generated significant number of studies and publications as well as tremendous amount of research data. A methodology is needed to translate such research data and results to validated scientific evidence. This article for the first time proposes the concept and methodology of evidence-based biomaterials research, which is to use evidence-based research approach represented by systematic reviews to generate evidence for answering scientific questions related to biomaterials. After briefly introducing the advancement of biomaterials since 1950s, the scientific and engineering nature of biomaterials are discussed along with the roadmap of biomaterials translation from basic research to commercialized medical products, and the needs of scientific evidence. Key information of the evidence-based approach such as its origination from evidence-based medicine, levels of evidence, systematic review and meta-analysis, differences between systematic and narrative reviews is then highlighted. Applications with a step-by-step procedure of conducting evidence-based biomaterials research, three examples of biomaterials research using evidence-based approach to generate scientific evidence, and opportunities and challenges of evidence-based biomaterials research are presented. With its notable impact on the practice of medicine, the evidence-based approach is also expected to make influential contributions to the biomaterials field.
Keywords: Biomaterial, Biomaterials research, Evidence-based medicine, Evidence-based approach, Systematic review, Meta-analysis
Graphical abstract
Highlights
-
•
Evidence-based biomaterials research (EBBR) is very important for biomaterials.
-
•
Authors for the first time propose the concept, practice and methodology of EBBR.
-
•
EBBR uses an evidence-based approach of systematic review and meta-analysis.
-
•
Key elements of an evidence-based approach are discussed.
-
•
Procedures and examples of evidence-based biomaterials research are illustrated.
1. Introduction
Since its emerging in the mid of 20th century, biomaterials research has been growing and developing into a multidisciplinary and cross-functional field which involves, but not limited to materials, chemical, physical, biological and medical sciences as well as materials, chemical, biomedical, mechanical and clinical engineering [1]. The turning point of 21st century represents a shift of emphasis between “bio” and “material”. “Material” and applied research were initially emphasized as compared to “bio” and basic research prior to the new century in order to solve the immediate unmet needs from clinical and medical fields. However, the new century brings innovations and advancement in multiple fields especially those in biological and medical sciences. Thus, “bio” has been gaining more and more weight as compared to “material” for “biomaterials” in general.
The shift in emphasis between “bio” and “material” has created many opportunities and benefits for the biomaterials field, as represented by the more and more academic and industrial involvement. For example, the first World Biomaterials Congress held in Baden, Vienna, Austria, in April 1980 had only a few hundreds of attendees. In contrast, for the past three World Biomaterials Congresses since 2012, each attracted ∼4000 attendees with the latest 11th Congress held online due to the global pandemic. Meanwhile, the shift has also broadened the definition and research areas of biomaterials, brought more innovative research projects and medical products, and increased the number of publications and journals as well as citations and impact factors of biomaterials-related journals.
The nature and original intention of biomaterials research is to solve the unmet medical and clinical needs and address scientific questions by providing solutions, insights into host-material responses, and medical products. With significantly increased number of publications, how can we take advantage of research data in various biomaterials literatures and turn them into scientific evidence that may help to solve specific scientific questions? How can the collected non-clinical and pre-clinical research data more efficiently support the clinical translation of specific devices or therapies based on innovative biomaterial technologies? New methodologies are needed to address the above issues for biomaterials research. In another words, new approaches are needed for (1) scientific evaluation of the experimental data from biomaterials research, (2) the translation of biomaterials research data to scientific evidence, and (3) evidence-based evaluation of the safety and performance of biomaterial technologies during their translation to final products.
This article for the first time in the field of biomaterials, presents the concept and methodology of evidence-based biomaterials research, which is to use evidence-based research approach represented by systematic reviews to generate evidence for answering scientific questions related to biomaterials. The quality of evidence is further evaluated in a way to provide scientific reference for decision making on the future biomaterial research, development, application and clinical translation. After introducing biomaterials science and engineering, the roadmap of biomaterials' translation and the scientific evidence, the evidence-based approach including its origin as evidence-based medicine, level of evidence, systematic review and meta-analysis, differences between systematic review and narrative review, as well as the applications of evidence-based research in fields other than medicine is then reviewed. Finally, procedures, applications, examples as well as opportunities and challenges of evidence-based approach in biomaterials’ research are illustrated.
2. Biomaterials research
2.1. Biomaterials science and engineering
The 1986 Consensus Conference of the European Society for Biomaterials defined biomaterial as “a non-viable material used in a medical device, intended to interact with biological systems” [2]. According to this definition, biomaterials are mainly implantable and interventional medical devices. The 2018 International Consensus Conference on the Definitions of Biomaterials redefined biomaterial as “a material designed to take a form that can direct, through interactions with living systems, the course of any therapeutic or diagnostic procedure.” [3] According to this updated definition, the concept and application of biomaterials have been significantly expanded from medical devices to medical products, including not only implantable and interventional devices, in vitro diagnostic agents and devices, combination products, but also drugs and biological products. Both the original and updated definitions emphasize the scientific nature (i.e., “interactions with biological”/“living systems”) and applications (i.e., “medical device”/“direct the course of any medical procedure”) of biomaterials.
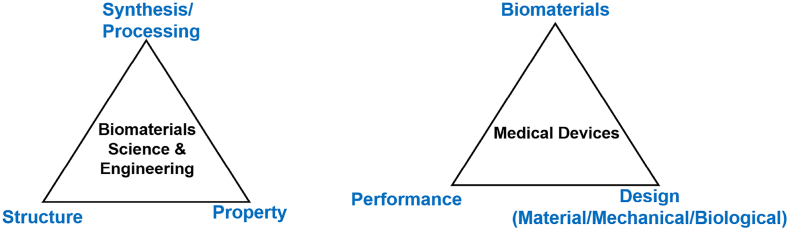
Biomaterials science, being multi-disciplinary, diverse and needs-driven [1], investigates the synthesis/processing-structure-property relationships of a variety of materials that fall in the category of biomaterials (Fig. 1). Thus, biomaterials science follows the traditional research scheme of materials science but goes beyond in order to focus on the biological/medical-related investigations. Along with scientific findings, the engineering nature of biomaterials is driven by clinical applications and final medical products with their appropriate design (Fig. 1) [4]. As a result, a successful translation of biomaterial products from bench to clinic has become a practical goal of biomaterials scientists and engineers.
Fig. 1.
Biomaterials science investigates biomaterials' processing-structure-property relationships (left). Biomaterials engineering enables that biomaterials link to applications via medical products. However, biomaterial alone is not enough, which also needs design to achieve functional performance in order to become a product (right).
2.2. Roadmap of biomaterials’ translation
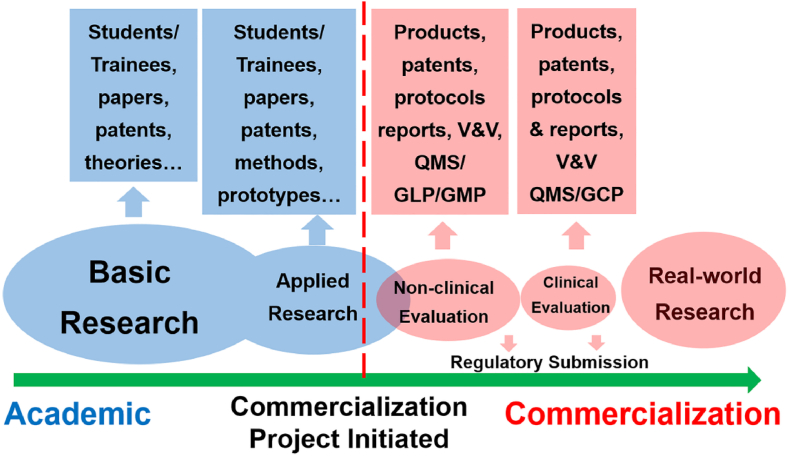
The roadmap of biomaterials' translation from concept to product, or from bench to clinic has its unique process (Fig. 2). From academic research to product commercialization can be characterized by several distinct stages, which are correlated from the perspectives of product translation. First, the basic research accounts for the majority of biomaterial's research, which generates well-educated researchers (students and trainees), publications, patents and theories. Basis research driven by curiosities, interests and hypotheses explores the unknown knowledge, and it should be noted that the ultimate goal of basic research is never commercial products. Secondly, applied research of biomaterials targets potential applications with the scientific findings from basic research. As a result, processes, methods and prototypes of biomaterial products are often the outputs as well as students, publications and patents. The translation of basic and applied research of biomaterials to the product development needs a boundary which separates research activities and commercial development. Although R&D has been frequently referred as a whole, research and development (R&D) in fact are quite different in that research can freely explore the unknown world and but the development needs to focus on the commercialization of real products.
Fig. 2.
Roadmap of biomaterials' translation. (V&V=Verification & Validation, QMS = Quality Management System, GLP = Good Laboratory Practice,GMP = Good Manufacturing Practice,and GCP = Good Clinical Practice.)
The development processes of products executed by companies (the right side of the dotted line in Fig. 2) need to follow the practice regulated by regulatory authorities [5]. Without getting the details of design control processes for the development of biomaterial products, the development process of biomaterials products can be categorized as stages of non-clinical and clinical evaluations. Both stages focus on products under the regulations of quality management system (QMS), good laboratory practice (GLP) and the current good manufacturing practices (cGMPs). Non-clinical evaluation includes, but not limited to bench performance tests, biocompatibility (defined as the ability of a material to perform with an appropriate host response in a specific application [2,3]) and biosafety evaluations per ISO 10993 standards, and possibly pre-clinical animal studies. Clinical evaluation is not restricted to clinical trials. Instead, clinical evaluations defined by International Medical Device Regulator Forum (IMDRF) as “a set of ongoing activities that use scientifically sound methods for the assessment and analysis of clinical data to verify the safety, clinical performance and/or effectiveness of the medical device when used as intended by the manufacturer” [6].
During the development of biomaterial products, user needs must be translated to design inputs, which need further product development to yield design outputs. The design verification is the process to verify outputs meeting inputs and the design validation is the process to make sure that the designed final products satisfy user needs. Many non-clinical evaluation activities such as bench performance tests can be characterized in the design verification, whereas pre-clinical animal studies and clinical evaluations belong to design validation. Regulatory submissions and approvals of biomaterial products must follow the complete design control process of targeted biomaterial products.
Even after the successful regulatory approval, the translation process of biomaterial products is still ongoing owing to the post-market surveillance (PMS) as well as real-world research (RWR). RWR is the research on real-world data and real-world evidence, where “real-world data are the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources” [7], and “real-world evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data” [7]. As a result, the translation is a close-loop process, with the risk management and lessons-learned that can benefit both the current and future products.
2.3. Scientific evidence
As described in the previous sections, basic, applied, and translational research in biomaterials all generate data. As an example of basic research, many studies with mixed results have been conducted on sol-gel processed bioactive glasses (sol-gel BGs) for dental and periodontal tissue regeneration [8]. However, based on those studies, can we present comprehensive scientific evidence of effects of sol–gel BGs on cells, and their use in tooth and periodontal regeneration? As another example of applied research, many animal studies on 3D printed scaffolds for bone regeneration in calvarial defect models have been conducted [9]. How can we generate integrated scientific evidence regarding the design of the scaffolds such as material type, porosity, pore size and pore shapes?
As to the translational research of biomaterials technologies to medical products, scientific evidence is in great need to demonstrate the safety and efficacy of such technologies with intended uses for targeted populations. Scientific evidence plays a vital role during the translation process and serves as a linkage between basic/applied research and product development. After organizing, selecting, evaluating, and integrating the research data from among the expanding various biomaterials literatures and studies, the presented scientific evidence could help to distinguish safe and effective biomaterials-based medical products from those that may not. Such scientific evidence could also replace guesswork with more reliable assessment of how well biomaterials-based medical products perform during the different stages of translation.
Due to the scientific nature of biomaterials research, one-of-a-kind novel biomaterials are often developed, and their properties should also be investigated with well-designed studies. Thus, organizing, selecting, evaluating, and integrating the research data (e.g., animal models or measurements of outcomes) from related studies on material systems with the same intended functions (e.g., the treatment of the same disease) to generate evidence and then further assess the quality of evidence will be very helpful to demonstrate the feasibility and effectiveness of the study design for the novel biomaterials.
Searching scientific evidence from data generated by individual studies to address a specific scientific question still presents challenges with the currently-available research tools and methods. As a result, a new approach and methodology is needed.
3. Evidence-based approach
3.1. Origin of evidence-based medicine
In the 1980s, with the international clinical epidemiology being developed and the medical research methodologies becoming mature, a large amount of clinical research was made public, and their result was gradually used to guide medical practice. A group of international researchers active in clinical medicine and clinical epidemiology, such as Drs. David Sackett, David Eddy and Archie Cochrane, first proposed the importance of evidence-based clinical practice, and began to think about how to systematically summarize, evaluate and disseminate evidence, then use the evidence to guide medical practice and improve the quality and efficiency of medical practice [[10], [11], [12], [13]]. In 1990, under the guidance of Dr. David Sackett, Dr. Gordon Guyatt used the rigorously evaluated literature knowledge to help clinicians make clinical decisions, resulting in a new model that is different from the traditional clinical decision-making model, and Dr. Guyatt used the term “evidence-based medicine” to describe the characteristics of this model.
This term of “evidence-based medicine” first appeared in the informal residency training materials of McMaster University in 1990, and was formally published in the American College of Physicians Journal Club in 1991 [14], and is still used. In 1992, Drs. Gordon Guyatt, Brian Haynes, and David Sackett and clinicians in the United States established an evidence-based medicine working group, and published a declaration article “Evidence-based medicine, a new approach to teaching the practice of medicine” [15] on Journal of the American Medical Association (JAMA), which marked the official birth of evidence-based medicine.
In 1996, Dr. Sackett published an article on British Medical Journal (BMJ), defining evidence-based medicine as “the careful, accurate and wise application of the best research evidence available to determine the treatment of individual patients” [16]. In 2000, he updated the definition of evidence-based medicine as “Carefully, accurately, and wisely apply the best research evidence currently available, combined with the clinician's personal professional skills and long-term clinical experience, and consider the patient's values and wishes to perfectly make a specific treatment plan” [17]. In 2014, at the 22nd Cochrane Annual Conference, Dr. Guyatt further improved the definition of evidence-based medicine as “clinical practice needs to combine the personal experience of clinicians, patient wishes and evidence from systematic reviews” [18].
3.2. Level of evidence
Obtaining the best evidence is an important step of the practice of evidence-based medicine, and the evidence grading system is an indispensable tool for decision makers in the process of obtaining the best evidence. Since the birth of evidence-based medicine, different countries and organizations have continuously explored methods for grading the level of evidence, and successively published more than 50 evidence grading systems [19].
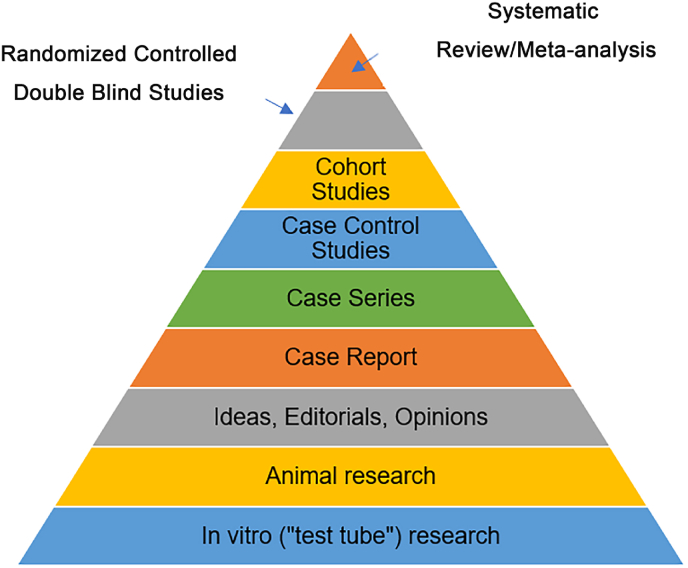
The earliest evidence grading method represented by the five-point method of evidence proposed by Dr. David Sackett in 1986 mainly focused on research design, with randomized controlled trials as the highest quality evidence [20]. The subsequently launched evidence grading system considers accuracy and consistency on the basis of research design, and uses systematic review/meta-analysis as the highest level of evidence. The main representative is the Oxford Centre for Evidence-based Medicine (OCEBM) standard launched by the Oxford University Evidence-Based Medicine Center in 2001 [21]. In the same year, the Medical Center of State university of New York launched the evidence pyramid [22] (Fig. 3), which included animal research and in vitro research into the evidence grading system for the first time, expanding the scope of evidence. The systematic review/meta-analysis in this grading system is also considered to be the highest level of evidence.
Fig. 3.
The evidence pyramid.
3.3. Systematic review & meta-analysis
Systematic review is an important secondary research method. The fifth edition of the “Dictionary of Epidemiology” defines it as [23]: “using strategies to reduce bias, rigorously evaluating and synthesizing all relevant research on a specific problem”. Meta-analysis is a statistical method to integrate data [24]. Meta-analysis may be, but not necessarily, used as a part of a systematic review process. Only when the included studies have sufficient homogeneity, the meta-analysis is used to merge data between different studies to achieve quantitative synthesis of research data. Through the application of meta-analysis, it is possible to increase the sample size, reduce the difference in results caused by random errors, increase the accuracy of effect size estimation, and improve the efficiency of statistical analysis [25].
Systematic reviews that use meta-analysis are quantitative. Systematic reviews use a systematic and transparent method to comprehensively collect and screen all original studies on the same research problem, and strictly evaluate the internal and external validity of the included studies. It is an effective way to screen true scientific evidence from massive literature, and the best way to understand the progress of peer research [26]. High-quality systematic reviews are the best evidence for medical and health care decisions [26].
3.4. Systematic review vs. narrative review
Systematic review is the secondary research based on the original studies. The significant differences between systematic review and traditionally narrative review are in Table 1.
Table 1.
Differences between narrative review and systematic review.
| Characteristics | Narrative Review | Systematic Review |
|---|---|---|
| Research Questions | Wide range | Focused on a specific question |
| Source of Original Literature | Typically not stated and may not be complete | Clear and complete |
| Search Methods | Typically not stated | Clear search strategy |
| Selection of Original Literature | Typically not stated and potentially biased | Clear selection criteria |
| Evaluation of Original Literature | No evaluation or with different evaluation methods | Following strict evaluation methods |
| Synthesis of results | Often using qualitative methods | Combination of qualitative and quantitative methods |
| Conclusions | Subjective and sometimes based on research evidence | Objective and strictly based on research evidence |
3.5. Applications in fields other than medicine
The emergence of evidence-based medicine in the 1990s was the result of self-reflection on medical science. By reflecting on the validity of several clinical evidences obtained through scientific experiments, evidence-based medicine is committed to providing patients with more effective clinical evidence [27]. New statistical methods have been widely used in the integration and evaluation of evidence. Computer technology, database construction, information collation and management software development have facilitated this process. After the paradigm of evidence-based thinking was successful in the field of clinical medicine, other medical fields such as pharmacy, imaging, and basic medicine science began to try to learn from the paradigm of evidence-based medicine, which enabled the corresponding disciplines to develop vigorously [28].
In the past 20 years, evidence-based methodology has broken through its use in the medical field, and has gradually expanded to areas of health policy, pedagogy, management and psychology, and the application of evidence-based thinking paradigm has continued to expand [29]. In 2002, the International Campbell Collaboration was established, aiming to apply systematic review methods to provide decision-making evidence for multiple non-medical fields [[29], [30], [31]], such as engineering, society, psychology, education, and international policy, which became a new beginning for the promotion of evidence-based approaches to other fields.
4. Evidence-based approach for biomaterials research
4.1. Generating evidence in biomaterials research
A systematic review is a summary of current research data and provides a body of evidence for specific research question [25]. Before proceeding with the process of generating evidence, the biomaterials researcher or evidence decision maker also needs to grade evidence, and then determine the level of certainty in the estimate of effect. This step will ultimately determine what to do next.
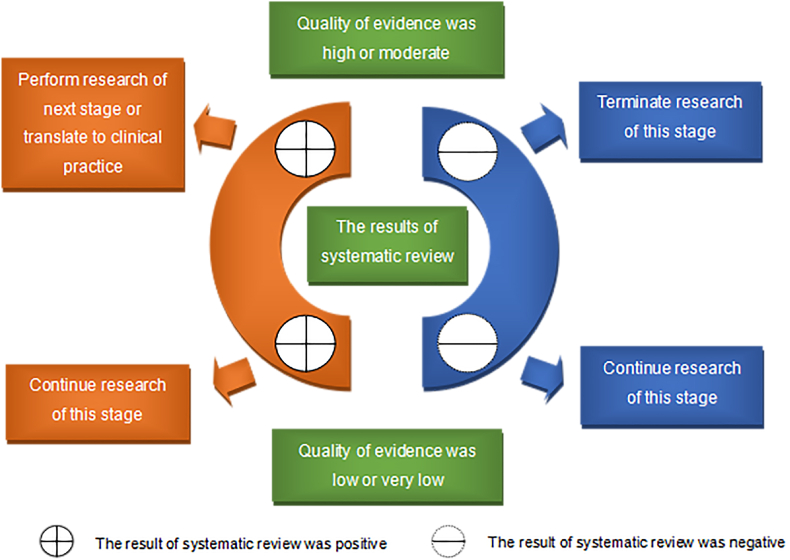
Fig. 4 illustrates how the results and quality of evidence of a systematic review to guide the research and translation processes of biomaterials. Unlike systematic reviews that only assess the risk of bias of the included original studies, an evidence grading system assesses the certainty of the outcomes reported by systematic reviews. Many systems have been developed over the years that“grade” evidence [19], which have some key similarities including assessments of (1) the risk of bias or limitations of the included studies; (2) consistency of the evidence; and (3) applicability of the evidence. Taking the widely used Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [32] as an example, there are five factors that reduce the certainty of evidence (risk of bias, inconsistency, indirectness, imprecision, publication bias) and three factors that improve the certainty of evidence (large effect size, presence of dose-effect relationship, negative bias), the level of certainty of the evidence is rated to high, medium, low, and very low.
Fig. 4.
The results and quality of evidence of a systematic review guide the research and translation processes of biomaterials.
The content in Fig. 4 is described as following. When the result of a systematic review is positive (i.e., the intervention is effective), the higher the certainty of outcomes, the more confident the findings can be translated. That is, when the certainty of evidence is high or medium, it is recommended to carry out the next stage of research or clinical translation; when the certainty of evidence is low or very low, it is recommended to carry out high-quality research to further confirm the result. When the result of a systematic review is negative (i.e., the interventions is ineffective), the higher the certainty of outcomes, the greater the confidence that the findings should not be translated. That is, when the certainty of evidence is high or moderate, it is recommended that the next stage of research or clinical translation should not be carried out based on the existing research results; when the certainty of evidence is low or very low, it is recommended to conduct high-quality research to further confirm the result. Grading evidence is the key to interpret the results of systematic reviews. A systematic review with rated evidence is the cornerstone for guiding the future research, development and translation of related biomaterials.
4.2. The procedure of conducting evidence-based research with systematic reviews
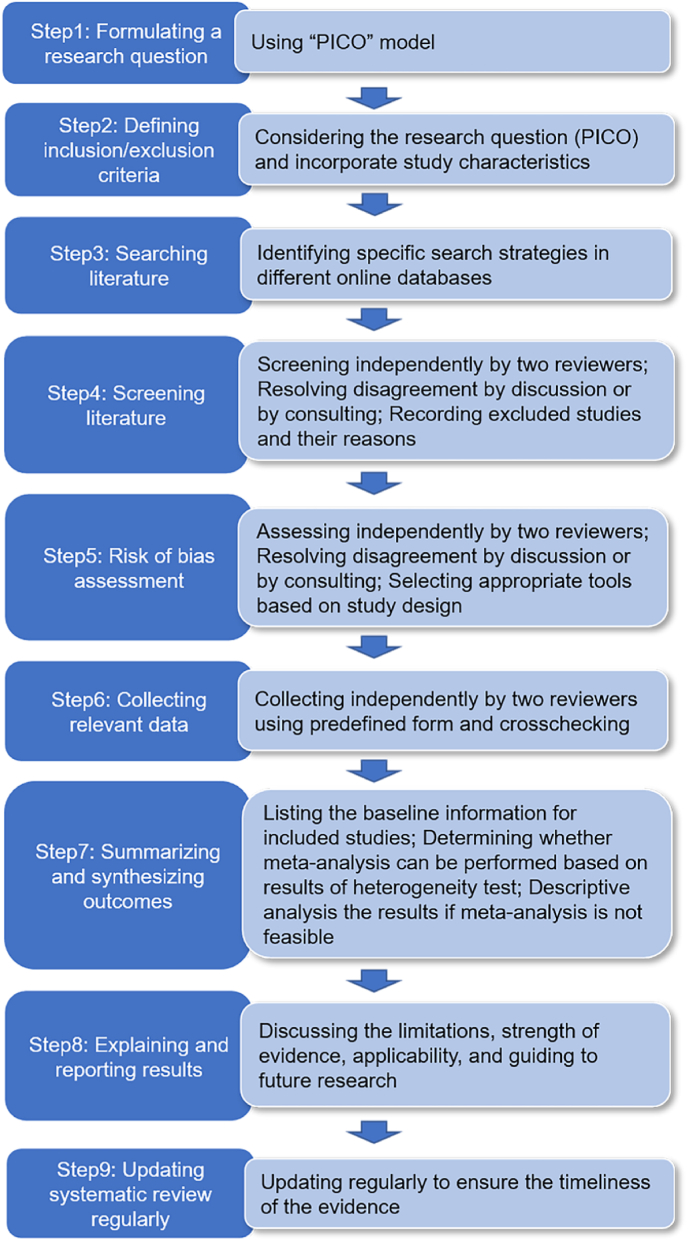
Multiple guidance documents described procedures for conducting a systematic review [33], which included the nine key steps in Fig. 5. The most important step in conducting a systematic review is to formulate a specific research question [25]. Developing a good research question requires screening the literature to identify evidence gaps in the field. A valuable research question necessarily emerges from existing knowledge [34].
Fig. 5.
The procedure of conducting systematic reviews.
A conceptualizing model named “PICO” is widely used to structure a specific research question of systematic review [35]. This model formulates the research question through four steps: the patient or problem (P); the intervention or exposure (I); the comparison intervention or exposure (C), if relevant; and the (clinical) outcome of interest (O). In essence, a properly formulated research question will guide through the systematic review process, including strategies for searching literature and criteria for selecting studies. The detailed procedure for conducting a systematic review can find in relevant methodology literature [25,34].
4.3. Examples of evidence-based biomaterials research
In this section, examples of evidence-based research with systematic reviews for material tests, animal studies, and clinical evaluation are presented. The significance of evidence-based research with systematic reviews in the field of biomaterials research is also illustrated.
4.3.1. Non-clinical material performance studies
In 2019, Yu et al. published an article titled “Bonding to industrial indirect composite blocks: A systematic review and meta-analysis” in Dental Materials [36]. We take this article as an example to illustrate the significance of evidence-based research on the non-clinical performance studies of biomaterials.
Why was the study conducted? Industrial indirect composite blocks (ICs) is a new type of dental material that has many advantages over traditional ceramics and composite materials. However, the poor bonding strength between the material and composite cements leads to insufficient mechanical properties and affects the longevity of the restoration. Recent laboratory tests have shown that various surface conditioning methods are important for promoting the bonding strength of the ICs to composite cements. However, there is no consensus on which of the surface conditions are the most effective way to increase bonding strength. Therefore, the authors reviewed and analyzed existing literature on in vitro studies with an evidence-based approach to determine the best surface conditioning methods to improve the bonding strength of two different IC materials (i.e., polymer-infiltrated ceramic network (PICN) material and ICs with dispersed fillers (ICDFs)). This research can provide ideas for the design and development of subsequent dental biomaterials, which could provide further evidence for future laboratory and clinical studies.
How was the study conducted? The authors conducted the study according to the steps in Fig. 5. The research question was defined in Table 2. Detailed implementation can be obtained from the published article.
Table 2.
The research question defined as PICOS of the non-clinical study [36].
| PICOS | Definition |
|---|---|
| Population | ICs bonded with composite cement |
| Intervention | ICs received surface conditioning before bonding |
| Control | Specimens did not receive surface conditioning before bonding |
| Outcome | Whether surface conditioning methods improve the bonding strength of the ICs was evaluated |
| Study designs | In vitro bench studies |
What does the study tell us? This study suggests that chemical etching followed by a universal primer and alumina air abrasion followed by a silane coupling agent could be considered the best strategy for optimizing the bonding strength of PICN materials and ICDFs under aged conditions, respectively. However, authors pointed out that the number of test groups supporting these results was limited. Therefore, these results should be interpreted with caution before being applied to clinical situations. Further laboratory and clinical research are necessary to confirm the long-term bonding strength of surface-conditioned ICs and provide evidence-based recommendations for clinical practice.
4.3.2. Preclinical in vivo animal studies
In 2021, Zhang et al. published an article titled “Biodegradable metals for bone defect repair: A systematic review and meta-analysis based on animal studies” in Bioactive Materials [37]. We take this article as an example to illustrate the significance of evidence-based research on animal studies of biomaterials.
Why was the study conducted? The biodegradable metals represented by magnesium and its alloys have desirable properties, including biodegradability and osteogenesis, and thus they hold great promise to be the ideal biomaterial for bone defect repair. The research on biodegradable metals is an active area in the field of orthopedic biomaterials. Animal studies play a pivotal role in evaluating the safety and efficacy of bone graft materials. Previous studies on biodegradable metals have investigated their in vivo biocompatibility, degradation, osteogenesis, and ability of bone defect repair in different animal models. Limitations of previous studies include over-simplified animal models, short observation durations, single evaluation criterion and inconsistent results. Therefore, authors review and analyze existing literature on animal studies with an evidence-based approach before more ambitious animal studies are conducted. Such an effort can provide suggestions and references for future animal studies, which could also provide further evidence for future clinical translation.
How was the study conducted? The authors conducted the study according to the steps in Fig. 5. The research question was defined in Table 3. Detailed implementation can be obtained from the published article.
Table 3.
The research question defined as PICOS of the preclinical animal study [37].
| PICOS | Definition |
|---|---|
| Population | Studies that include animal models of bone defects, with no limitations on the animal species nor modeling methods |
| Intervention | Degradable metals and their alloys, modified degradable metals and their alloys (composites, coating and surface modification) |
| Control | ① Non-degradable metals, such as titanium, titanium alloy, stainless steel and cobalt chromium alloy; ② Degradable polymers, such as polylactic acid; and ③ Other materials, such as calcium phosphate ceramic, autogenous bone, allogeneic bone, or degradable composites used in traditional clinical applications (e.g. ceramic-polymer composites) |
| Outcome | Outcome measures for bone defect repair: ① New bone formation; ② Defect repair; ③ The percentage of bone volume/tissue volume around the implant; and ④ Bone implant contact. Implant-related outcome measures: ① Degradation; ② Hydrogen generation |
| Study designs | Controlled studies were included, with no restriction on whether they were randomly grouped. In order to ensure the quality of included studies, self-control studies were excluded because metallic ions from both experiment and control groups may influence each other in terms of their effects on bone defects repair |
What does the study tell us? This study suggested that biodegradable metals exhibited mixed effects on bone defect repair and degradation compared with traditional non-degradable metals, biodegradable polymers, bioceramics, and autogenous bone grafts in animal studies because of the heterogeneity in animal model, anatomical site, and critical size defect (CSD). The results indicated that there were limitations in the experimental design of the included studies, and quality of the evidence was very low. Authors also pointed out that evidence-based research with data validity is needed to enhance clinical translation of biodegradable metals. Future studies should adopt standardized experimental protocols in investigating the effects of biodegradable metals on bone defect repair with animal models.
4.3.3. Clinical studies
In 2013, Chabouis et al. published an article titled “Clinical efficacy of composite versus ceramic inlays and onlays: A systematic review” in Dental Materials [38]. We take this article as an example to illustrate the significance of evidence-based research on clinical studies of biomaterial products.
Why was the study conducted? Dental caries is a common disease. Inlay or onlay restorations made of composite or ceramic material are widely used to treat the resulting tooth loss. Preclinical studies have shown that the composite and ceramic materials have their own advantages and disadvantages as restorations. Several cross-sectional studies have evaluated ceramic and composite materials separately only for annual failure rate. However, we still cannot draw definite conclusions on the best material from these studies. In older to both scientifically answer the question posed by practitioners about which material is better for inlay and onlay manufacturing and providing strong evidence for clinical decision-making, the researchers conducted a study of published reports of randomized controlled trials to compare the efficacy of composite and ceramic inlays and onlays for restoring posterior teeth of adults.
How was the study conducted? The author conducted the study according to the steps in Fig. 5. The research question was defined in Table 4. Detailed implementation can be obtained from the published article.
Table 4.
The research question defined as PICOS for the listed clinical study [38].
| PICOS | Definition |
|---|---|
| Population | Adults (18–90 years' old) |
| Intervention | Composite inlays or onlays |
| Control | Ceramic inlays or onlays |
| Outcome | Clinical performance of the dental restorations (USPHS criteria, CDA criteria, FDI criteria et al.); Failure |
| Study designs | Randomized clinical studies or trials comparing at least two esthetic materials for inlay/onlay manufacturing (at least one ceramic and at least one composite) |
What does the study tell us? This study suggests that now very limited evidence proved the ceramics perform better than composite material for inlays in the short term. However, this result may not be valid in the long term, and other trials are needed. Additionally, no trials compared composite and ceramic onlays, which is also needed in the future. Authors also pointed out that future trials should follow Fédération Dentaire International recommendations and enhance their methodology.
4.4. Opportunities and challenges
Evidence-based research with its systematic review approach, as a scientific and secondary research method, can be the pilot research for carrying out original research, and has also been regarded as one of the important methods for conducting translational research. Evidence-based research that is widely used in the field of medical research has developed its complete methodology system, and a series of guidelines have been published to ensure the rigor of the evidence production process [39,40]. However, in the field of biomaterials research, due to its different characteristics from medical research, it is still necessary to explore how to scientifically conduct systematic reviews. For examples, systematic reviews based on in vitro studies lack an internationally recognized risk of bias assessment tool. There is also a lack of internationally recognized reporting standards for systematic reviews of in vitro or in vivo studies of biomaterials.
The research, development and translation of biomaterial medical products is a complex and multi-stage process involving a variety of activities, such as non-clinical bench performance, biocompatibility and biosafety tests, animal studies and clinical evaluation [41]. Just as evidence-based medicine was originated on the basis of the development and improvement of the clinical epidemiology methodology system, the establishment of a sound evidence-based methodology system of biomaterial research is the basis for the best practice of evidence-based biomaterials research.
Thus, the development of evidence-based biomaterials research needs to establish and improve its own methodology system. Such a sound evidence-based biomaterial research methodology system should include: 1) rigorous and scientific methods of experiment design as well as implementation standards; 2) guidelines for standardized reporting of research processes and results; 3) methods to reduce or avoid bias in research results, such as a prospective registration system for research protocols. Conducting biomaterial research under the guidance of a sound methodology system is a prerequisite for ensuring the authenticity and reliability of research results to promote the research, development and clinical translation of biomaterials products, and also an important way to improve the transparency and reproducibility of biomaterials research.
5. Conclusions
Evidence-based biomaterial research is to use evidence-based research approach represented by systematic reviews to generate evidence for answering scientific questions related to biomaterials. The quality of evidence is further evaluated in a way to provide scientific reference for decision making on the future biomaterial research, development, application and clinical translation. Such an evidence-based approach has been successfully applied in the field of medicine and transformed the practice of modern medicine. With significantly increased amounts of biomaterials-related studies, publications, results and data generated in the current information explosion age, translation of biomaterials research data to validated scientific evidence is extremely important. With the establishment of its characteristic methodology system, evidence-based biomaterials research is expected to markedly benefit the field with its potential of both the scientific evaluation of the experimental data to address specific scientific questions and the evidence-based evaluation of the safety, efficacy, quality, and performance of biomaterial technologies during their translation to final medical products.
Declaration of competing interest
The authors declare that they have no competing interests.
CRediT authorship contribution statement
Kai Zhang: Conceptualization, Visualization, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition, Project administration. Bin Ma: Conceptualization, Visualization, Writing – review & editing, Funding acquisition. Kaiyan Hu: Writing – original draft, Methodology, Formal analysis, Investigation. Bo Yuan: Writing – review & editing, Visualization. Xin Sun: Conceptualization, Writing – review & editing. Xu Song: Methodology, Formal analysis. Zhonglan Tang: Writing – review & editing. Hai Lin: Writing – review & editing. Xiangdong Zhu: Conceptualization, Writing – review & editing, Conceptualization, Writing – review & editing. Yufeng Zheng: Conceptualization, Writing – review & editing. Andrés J. García: Conceptualization, Writing – review & editing. Antonios G. Mikos: Conceptualization, Writing – review & editing. James M. Anderson: Conceptualization, Writing – review & editing.
Acknowledgement
This work was financially supported by Sichuan Science and Technology Program (2021YFS0020) and the National Natural Science Foundation of China (81873184). This work was also supported by the first batch (“5. Research on technical evaluation of drug-device combination products”) and the second batch (“3.1 Investigating technologies of assessing the safety and effectiveness of nano-medical device products”, “5.3 Investigating innovative supervision and assessment technologies of tissue engineered medical device products”, “5.4 Research, development and translation of innovative biomaterials” and “5.5 Research on technical evaluation of recombinant collagens, cartilage-repair materials and antimicrobial orthopedic/dental materials”) of Chinese Drug Regulatory Science Action Plan of National Medical Products Administration. The authors would also like to thank Professor Kam W. Leong for his comments and encouragement during the drafting of this manuscript.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Kai Zhang, Email: kaizhang@scu.edu.cn.
Bin Ma, Email: mab@lzu.edu.cn.
References
- 1.Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E., Wagner W., Shelly E., Zhang G.G., Michael Y. In: Biomaterials Science: an Introduction to Materials in Medicine. fourth ed. Wagner W., Shelly E., Zhang G.G., Michael Y., editors. Academic Press; 2020. Introduction to biomaterials science: an evolving, multidisciplinary endeavor. [Google Scholar]
- 2.Williams D.F. 1986. Definitions in Biomaterials: Proceedings of a Consensus Conference of the European Society for Biomaterials. Chester, England. [Google Scholar]
- 3.Yuan Y., et al. Clinical translation of biomedical materials and the key factors towards product registration. J. Orthop. Translat. 2014;2(2):49–55. https://10.1016/j.jot.2013.12.002 [Google Scholar]
- 4.Zhang X.D., Williams D. Elsevier; 2019. Definitions of Biomaterials for the Twenty-First Century. [Google Scholar]
- 5.Zhang K., Mikos A.G., Reis R.L., Zhang X.D. Translation of biomaterials from bench to clinic. Bioact. Mater. 2022;18:337–338. doi: 10.1016/j.bioactmat.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medical Device Clinical Evaluation Working Group Clinical evaluation. 2019. https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-191010-mdce-n56.pdf Available from URL:
- 7.U.S. Food and Drug Administration Real-world evidence. 2022. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence Available from URL:
- 8.Farano V., et al. Sol-gel bioglasses in dental and periodontal regeneration: a systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(4):1210–1227. doi: 10.1002/jbm.b.34214. https://10.1002/jbm.b.34214 [DOI] [PubMed] [Google Scholar]
- 9.Hassan M.N., et al. The bone regeneration capacity of 3D-printed templates in calvarial defect models: a systematic review and meta-analysis. Acta Biomater. 2019;91:1–23. doi: 10.1016/j.actbio.2019.04.017. https://10.1016/j.actbio.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 10.Cook D.J., et al. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1992;102(4 Suppl):305s–311s. doi: 10.1378/chest.89.2_Supplement.2S. [DOI] [PubMed] [Google Scholar]
- 11.Eddy D.M., Billings J. The quality of medical evidence: implications for quality of care, Health. Far E. Aff. 1988;7(1):19–32. doi: 10.1377/hlthaff.7.1.19. https://10.1377/hlthaff.7.1.19 [DOI] [PubMed] [Google Scholar]
- 12.Cochrane A. Nuffi eld Provincial Hospitals Trust; London: 1972. Effectiveness and Efficiency: Random Reflections on Health Services. [Google Scholar]
- 13.Eddy D.M. American Cancer Society; Atlanta, GA, USA: 1980. Guidelines for the Cancer-Related Checkup: Recommendations and Rationale. [Google Scholar]
- 14.Guyatt G. Evidence-based medicine. ACP. J. Club. A- 1991;16:114. https://107326/ACPJC-1991-114-2-A16 [Google Scholar]
- 15.Evidence-Based Medicine Working Group Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268(17):2420–2425. doi: 10.1001/jama.1992.03490170092032. https://10.1001/jama.1992.03490170092032 [DOI] [PubMed] [Google Scholar]
- 16.Bhandari M., Giannoudis P.V. Evidence-based medicine: what it is and what it is not. Injury. 2006;37(4):302–306. doi: 10.1016/j.injury.2006.01.034. https://10.1016/j.injury.2006.01.034 [DOI] [PubMed] [Google Scholar]
- 17.Sackett D.L., et al. second ed. 2000. Evidence-based Medicine-How to Practice and Teach EBM. London. [Google Scholar]
- 18.Djulbegovic B., Guyatt G. In: Users' Guides to the Medical Literature: a Manual for Evidence-Based Clinical Practice. Guyatt G., Meade M., Cook D., editors. 2014. EBM and the theory of knowledge. Boston. [Google Scholar]
- 19.Chen Y.L., et al. The grading of evidence and the strength of recommendations in medical research. Chin. J. Evidence-based Medicine. 2008;8(2):127–133. https://10.7507/1672-2531.20080028 [Google Scholar]
- 20.Sackett D.L. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1986;89(2 Suppl):2s–3s. doi: 10.1378/chest.89.2_Supplement.2S. [DOI] [PubMed] [Google Scholar]
- 21.Li Y.P. first ed. Higher Education Press; Beijing: 2009. Evidence-based Medicine. [Google Scholar]
- 22.SUNY downstate EBM Tutorial. Guide to research methods: the evidence pyramid. Available from URL: http://library.downstate.edu/ebmdos/2100.htm.
- 23.Lv S.Y. fifth ed. 2001. A Dictionary of Epidemiology. Beijing. [Google Scholar]
- 24.Delgado-Rodriguez M. Systematic reviews of meta-analyses: applications and limitations. J. Epidemiol. Community Health. 2006;60(2):90–92. doi: 10.1136/jech.2005.035253. https://10.1136/jech.2005.035253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cumpston M., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. https://10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djulbegovic B., Guyatt G.H. Progress in evidence-based medicine: a quarter century on. Lancet. 2017;390:415–423. doi: 10.1016/S0140-6736(16)31592-6. https://10.1016/s0140-6736(16)31592-6 10092. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Yu J.J., Li Y.P. Evidence based science: construction of convergent symbiotic system breaking discipline boundary, Chin. J. Evidence-based Med. 2019;19(5):505–509. https://10.7507/1672-2531.201904034 [Google Scholar]
- 28.Zhao C., et al. The connotation and thinking of the development of evidence-based medicine to evidence-based science. Chin. J. Evidence-based Medicine. 2019;19(5):510–514. https://10.7507/1672-2531.201904177 [Google Scholar]
- 29.Davies P., et al. Does for public policy what cochrane does for health. Bmj. 2001;323(7308):294–295. doi: 10.1136/bmj.323.7308.294. https://10.1136/bmj.323.7308.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton J., et al. The Campbell principles are applied in West Midlands through public health research forum. Bmj. 2001;323(7323):1252–1253. doi: 10.1136/bmj.323.7323.1252a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abeysinghe S., et al. Engineering performance of concrete incorporated with recycled high-density polyethylene (HDPE)-A systematic review. Polymers. 2021;13(11) doi: 10.3390/polym13111885. https://10.3390/polym13111885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt G.H., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. https://10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manchikanti L. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management, part I: introduction and general considerations. Pain Physician. 2008;11(2):161–186. [PubMed] [Google Scholar]
- 34.Muka T., et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020;35(1):49–60. doi: 10.1007/s10654-019-00576-5. https://10.1007/s10654-019-00576-5 [DOI] [PubMed] [Google Scholar]
- 35.Schardt C., et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inf. Decis. Making. 2007;7:16. doi: 10.1186/1472-6947-7-16. https://10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H., et al. Sawase, Bonding to industrial indirect composite blocks: a systematic review and meta-analysis. Dent. Mater. 2020;36(1):119–134. doi: 10.1016/j.dental.2019.11.002. https://10.1016/j.dental.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., et al. Biodegradable metals for bone defect repair: a systematic review and meta-analysis based on animal studies. Bioact. Mater. 2021;6(11):4027–4052. doi: 10.1016/j.bioactmat.2021.03.035. https://10.1016/j.bioactmat.2021.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fron Chabouis H., Smail Faugeron V., Attal J.P. Clinical efficacy of composite versus ceramic inlays and onlays: a systematic review. Dent. Mater. 2013;29(12):1209–1218. doi: 10.1016/j.dental.2013.09.009. https://10.1016/j.dental.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 39.Page M.J., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj. 2021;372:n160. doi: 10.1136/bmj.n160. https://10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shea B.J., et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both. Bmj. 2017;358 doi: 10.1136/bmj.j4008. https://10.1136/bmj.j4008 j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng F., Chen M.M., Wang X., Zhang H.W., Nie H.G., Tang H. Translation of a spinal bone cement product from bench to bedside. Bioact. Mater. 2022;10:337–338. doi: 10.1016/j.bioactmat.2021.08.011. https://10.1016/j.bioactmat.2021.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]