Abstract
In agriculture, the search for higher net profit is the main challenge in the economy of the producers and nano biochar attracts increasing interest in recent years due to its unique environmental behavior and increasing the productivity of plants by inducing resistance against phytopathogens. The effect of rice straw biochar and fly ash nanoparticles (RSBNPs and FNPs, respectively) in combination with compost soil on bacterial leaf spot of pepper caused by Xanthomonas campestris pv. vesicatoria was investigated both in vitro and in vivo. The application of nanoparticles as soil amendment significantly improved the chili pepper plant growth. However, RSBNPs were more effective in enhancing the above and belowground plant biomass production. Moreover, both RSBNPs and FNPs, significantly reduced (30.5 and 22.5%, respectively), while RSBNPs had shown in vitro growth inhibition of X. campestris pv. vesicatoria by more than 50%. The X-ray diffractometry of RSBNPs and FNPs highlighted the unique composition of nano forms which possibly contributed in enhancing the plant defence against invading X. campestris pv. vesicatoria. Based on our findings, it is suggested that biochar and fly ash nanoparticles can be used for reclaiming the problem soil and enhance crop productivity depending upon the nature of the soil and the pathosystem under investigation.
Subject terms: Plant sciences, Pathogenesis
Introduction
Capsicum or bell pepper or sweet pepper (Capsicum annum L.) is a crop of Solanaceae family and genus ‘capsicum’. These medium-sized fruit pods have wonderful colors (green, red, orange and yellow) thick and brittle skin with a glossy outer cover and a fleshy texture. It is a highly appreciated crop being good source of vitamin A, C, E, thiamine, beta carotene, folic acid and vitamin B6 and has great therapeutic values1,2. In Pakistan, the area under pepper has been 62,742 hectares in 2018–2019 with a total production of 145,856 tonnes and comes at 5th position worldwide. Bacterial leaf spot (BLS) caused by Xanthomonas campestris pv. vesicatoria results in severe damage to sweet pepper. The bacterium attacks leave, fruits, and stems causing blemishes on these plant parts. It is a gram-negative, rod-shaped bacterium that can survive in seeds and plant debris from one season to another3–5. The pathogen can devastate a pepper crop by early defoliation of infected leaves and disfiguring fruit. In severe cases, complete crop failure has occurred due to this disease. Marketable yield is reduced both by defoliation and damaged fruits2,6. For the management of BLS different techniques have been under application such as chemical control7, cultural methods8,9, biocontrol strategies10, and use of resistant plant genome11.
In recent years, organic amendment, including crop residues, compost, organic waste and biochar application has become an auspicious strategy for the control of soil-borne diseases because of its strengths as, cost-effectiveness, resource utilization and environmental protection12–14. Biochar (BC) or black gold is a novel organic soil amendment with some special physical and chemical properties that has been increasingly discussed in agriculture as a strategy for the sequestration of recalcitrant carbon into soils to increase soil fertility15, improve plant growth and suppression of soil-borne diseases16–18. Additionally biochar has been proven as an effective suppressor of plant diseases caused either by soil-born or air-born bacterial or fungal plant pathogens18,19.
Fly ash has been defined as the fine particulate by-product released into the atmosphere together with gases as a result of combustion processes20. The dynamic physic-chemical properties (low bulk density (1.01–1.43 g cm−3), hydraulic conductivity and specific gravity (1.6–3.1 g cm−3), while the moisture retention ranging from 6.1% at 15 bar to 13.4% at 1/3 bar and being rich in P, K, Ca, Mg and S and micronutrients like Fe, Mn, Zn, Cu, Co, Ba, Mo, Cd and Ni)21,22 of fly ash make it a potential source in agricultural applications, as improving biological and physic-chemical properties of soil23,24 as competent as the compost and Biochar. Currently, Fly ash has also shown significant inhibitory effects on root-knot nematodes in carrot and soybean plants as well as control of some bacterial populations25–28.
The incorporation of engineered nanoparticles has gained undeniable importance in our daily life from electronics to medicine and agriculture. In agriculture, for instance, nano-pesticides, nano-fertilizers and nano-sensors are in direct applications to agricultural soils to get enhanced crop productivity and reduce cost29, or control plant pathogens30,31. Characterized nanoparticles of Fly ash32,33 and Biochar34 have been extensively used in the agriculture sector not only to reduce the hazards of deposited chemical pesticides and fertilizers but also to control infectious pathogens and to improve crop yields35–37.
In this study we explored the synthesis of nanoparticles from rice straw biochar (RSBNPs) and fly ash (FNPs) and secondly the potential of prepared nanoparticles was assessed against bacterial leaf spot of pepper caused by Xanthomonas campestris pv. vesicatoria.
Materials and methods
Experimental site
The experiment was carried out at the experimental station of the Department of Plant Pathology, (31º 29′ 42.2664″ N, 74º 17′ 49.1316″ E, 217 m altitude) Faculty of Agricultural Sciences, University of the Punjab, Lahore, Pakistan, from March 2019 to April 2021. The local climate is semi-arid (Köppen climate classification BSh) with an average temperature of 40 °C and 350 mm annual rainfall and rainy season July–September.
Plant material and soil substrate
Capsicum annum L. seeds (Yolo Wonder) were purchased from the local seed market (Ghula Mandi, Lahore) and surface sterilized with 70% ethanol for 10 min followed by washing with 50% NaOHCl solution (100 mL of NaOHCl + 100 mL of distilled + 50 µL tween-20 detergent) and thrice washing with distilled deionized water38. These seeds were sown in clay pots ¾ filled with sterilized 20% leaf compost soil (The soil texture was a sandy loam (82.88% sand, 13.04% silt, and 4.08% clay) with a pH of 7.88 and an electrical conductivity (EC) of 1.55 dS/m (measured using a pH meter and an EC meter); organic matter content (OM) of 0.54%; containing 3% total N and 1.5% total C; having a C/N ratio of 0.5; and containing 12, 68, and 100 mg·kg−1 of Ca, P, and K, respectively). Fully developed plants at 4–5 leaf stage were transplanted, into clay pots of bigger size (Volume: 2 L, 15.5 cm height × 14 cm width)83,84 with the same soil composition up to 1–2 inches depth with 2–3 plants per pot. Lighter irrigations were applied on a day-to-day basis to keep the water level at about 60%39. Then established young plants in pots were transferred to open areas where seedlings were exposed to light so that they can carry their photosynthetic activity17.
Xanthomonas campestris pv. vesicatoria culture acquisition
Pure culture of Xanthomonas campestris pv. vesicatoria (FCBP-DNA B0003) was acquired from First Fungal culture Bank of Pakistan (FCBP), Faculty of Agricultural Sciences, University of the Punjab, Lahore, Pakistan. The inoculum was prepared by re-culturing in LB broth (MERCK, USA) based on Lennox formulation and incubating on a shaker at 120 rpm for 36 h at 28 ± 2 °C. Bacterial culture was then centrifuged at 5,000 rpm for 10 min at 4 °C. The suspension was diluted through serial dilution process to obtain the bacterial concentration of 108 at 600 nm wavelength having an optical density of 0.3 in the spectrophotometer40.
Biochar and nanoparticles production
TLUD (Top-Lit UpDraft) portable kiln method41 on-farm biochar production was used, with minor adjustments, to prepare biochar from Rice Straws collected from field areas of University of the Punjab, new campus, Lahore, at pyrolysis temperature of 500 °C to be used in this experiment. Fly ash was procured from the textile industry as leftover after burning corn cobs and coal as fuel (usually is a micro-scale ultrafine particulate with a size below 100 µm). Physico-chemical properties of rice straw biochar and fly ash were determined42,85,86 before use for further nanoparticles production are summarized in Tables 1 and 2.
Table 1.
Physico-chemical characterization of rice straw biochar.
| Parameter measured | Value |
|---|---|
| pH | 9.3 |
| Basic gps (meq/g) | 7.8 |
| Acidic gps (meq/g) | 1.8 |
| Ash% | 50 |
| Density (g/cm3) | 0.28 |
| Surface area (S BET ) (m2/g) | 100 |
| C wt% | 53 |
| H wt% | 3.0 |
| N wt% | 1.5 |
| O | 42.4 |
| C/N | 25 |
| H/C | 0.08 |
| O/C | 0.79 |
| Alkaline elements (ppm (ug/g) by dry weight) | |
| K | 14,000 |
| Mg | 3500 |
| Na | 2190 |
| Ca | 7354 |
| Other essential elements mg/Kg | |
| Fe | 5754 |
| P | 2765 |
| Heavy toxic elements mg/Kg | |
| Zn | 0.01 |
| Mn | 575 |
| Al | 4231 |
| Cu | 4 |
| Cd | 0.05 |
| Pb | 0.62 |
| Hg | 0.93 |
Table 2.
Physico-chemical characterization of fly ash.
| Parameter measured | Value |
|---|---|
| Ash % | 46 |
| pH | 9.75 |
| EC dSm−1 | 2.43 |
| C% | 39.3 |
| N (g Kg−1) | 6.71 |
| P (g Kg−1) | 2.97 |
| K (g Kg−1) | 0.31 |
| Ca (g Kg−1) | 2.51 |
| Mg (g Kg−1) | 1.37 |
| S (g Kg−1) | 7.52 |
The nanocomposite of rice straw biochar (RSBNPs) and fly ash (FNPs) were isolated from their bulk materials following protocols of Yeu et al., 2019; Guo et al.43,44, by grinding bulk biochar into a commercial blender to produce fine biochar powder. Fly ash obtained was already packed in sealed polythene bags. Fly ash (30 g) and fine biochar powder were mixed in 800 mL of sterile water, separately. Both the solutions were shaken vigorously and autoclaved to physically and thermally disperse the bulk forms of biochar fine powder and fly ash. After the dispersion of bulk material, prepared solutions were passed through a 500 µm filter membrane to remove large particles. Filtrates were centrifuged twice at 3500 rpm for 25 min to isolate the nanoparticles in supernatant based on a density gradient. XRD, FTIR, analysis of both Biochar and fly ash nanoparticles and EDX of only biochar was done by following Du et al.45, from the Department of Physics, Lahore College for Women University, Lahore, Pakistan.
Biochar and fly ash nanoparticles were applied through drenching (Imada et al.)46 to chili plants by applying 50 mL of solution, containing nanoparticles (RSBNPs and FNPs), in the root zone by injecting with the help of a disposable syringe (Telemart: 10 cc, Bd).
Chili pepper plant inoculation and disease assessment
The plants were grown for 7–8 days before the inoculation of the pathogen (X. campestris pv. vesicatoria). Leaves of chili plant were injured by the needle prick method of bacterial inoculation47. In this method, 8–10 clean needles were tightly held by a rubber band at equal heights. These needles were used to damage the leaves. Slight gentle injuries were done to leaves to provide entry sites to bacteria. Then the bacterial suspension was sprayed with the help of an atomizer. Inoculated plants were covered again with polythene bags and water was sprinkled on the inner bag surface to maintain high relative humidity.
The whole research trail was comprised of two groups. Each group was further divided into three treatments, having five replicates. Group I: Inoculated set: (1. Fly-ash nanoparticles + Xnth;, 2. Rice Straw Biochar Nanoparticles + Xnth; 3. Only Soil + Xnth). Group II: Un-inoculated set/Control group: (1. Fly-ash nanoparticles, 2. Rice Straw Biochar Nanoparticles, 3. Only Soil).
After the application of nanoparticles and inoculation of the pathogen, agronomic data were recorded as shoot and root length, weight48. Disease incidence was calculated by the following formula
Disease severity was calculated by formulating a disease grading scale in which severity was rated from 0 to 4 grades with zero indicating minimal or no disease symptoms to grade four showing 76% or above leaf area infected49.
Consent for publications
All authors have read the manuscript and agreed for publishing it.
Consent for plants/seeds
The authors declare that during the research work all national and local legislations have been followed before and after conducting the experiment and no rules have been violated during the whole experiment keeping the crop respect in consideration.
In vitro X. campestris pv. vesicatoria and other isolated potential bacterial and fungal pathogens growth inhibition assay
The antimicrobial activity of RSBNPs and FNPs was investigated against X. campestris pv. vesicatoria used in this experiment. Agar well diffusion method50 was employed for the estimation of antimicrobial potential of RSBNPs and FNPs.
The antimicrobial activity of RSBNPs and fly FNPs was also investigated against microflora isolated from soil used in this experiment. Fungi and bacteria were isolated through serial dilution method on Malt Extract Agar (MEA) and Luria Bertani Agar (LBA), respectively. Agar well diffusion method50 was employed for estimation of antimicrobial potential of RSBNPs and FNPs against isolated fungal and bacterial isolates including Escherichia coli, Erwinia spp, Pseudomonas syringae, Xanthomonas campestris pv. citri, X. campestris pv. vesicatoria, Fusarium solani, F. oxysporum, Alternaria alternata and Alternaria solani,.
Statistical analysis
The experimental data were analysed by ‘Statistix version 8.1’ analytical software by analysis of variance (ANOVA), while the means were differentiated by Tuckey’s HSD test at P = 0.05. Additionally, the percentage data were transformed for disease incidence, severity and in vitro bacterial growth inhibition before analysis.
Results
Effect of rice straw biochar nanoparticles and fly ash nanoparticles on plant growth
Maximum shoot length (28 cm) was observed in un-inoculated rice straw biochar nanoparticles (RSBNPs) treated plants. In the pathogen inoculated set of treatments, maximum shoot length was observed in both fly ash and biochar-based nanoparticles treated plants (Table 3). While minimum shoot length of 8 cm was observed in pathogen inoculated control plants grown in soil only. RSBNPs had significantly enhanced root length as suggested by the results because maximum root length i.e. 27 cm was observed in uninoculated plants treated with RSBNPs. Pathogen inoculated plants grown in soil, RSBNPs + Soil and FNPs + soil had root lengths of 6.1 cm, 13.4 cm, and 11 cm, respectively. RSBNPs treated plants resisted the pathogen stress and had 101%, while, FNPs treated plants had shown a 65.1% increase in root length as compared to plants grown in only soil.
Table 3.
Effect of rice straw biochar nanoparticles (RSBNPs) and fly ash nanoparticles (FNPs) and Xanthomonas campestris pv. vesicatoria inoculation on chili plant growth parameters including shoot length, root length as well as root and shoot weights.
| Treatments | Shoot length (cm) | Shoot weight (g) | Root length (cm) | Root weight (g) |
|---|---|---|---|---|
| Only soil | 13.2 ± 1.20d | 0.47 ± 0.01e | 6.66 ± 0.53e | 0.285 ± 0.05f |
| Soil + Xnth | 11 ± 1.00de | 0.22 ± 0.03f. | 6.1 ± 0.74e | 0.1542 ± 0.03e |
| Soil + RSBNPs | 25 ± 1.14a | 2.09 ± 0.09a | 22.8 ± 1.32a | 1.9852 ± 0.33a |
| Soil + Xnth + RSBNPs | 19.6 ± 0.51c | 1.07 ± 0.15c | 13.4 ± 1.21c | 0.7144 ± 0.10c |
| Soil + FNPs | 23.2 ± 1.07ab | 1.73 ± 0.12b | 21 ± 0.55ab | 1.4172 ± 0.14b |
| Soil + Xnth + FNPs | 19 ± 0.65c | 0.75 ± 0.11d | 11 ± 1.00 cd | 0.456 ± 0.08d |
Data represent mean values ± standard error and abcd denote significance levels.
With the addition of composite nanoforms derived from rice straw biochar and fly ash a significant increase in chili shoots weight was observed as compared to plants grown in only soil in both inoculated and un-inoculated sets of treatments (Table 3). Shoot weight was significantly increased in RSBNPs treated plants in both pathogen-inoculated and uninoculated chili plants. But highest average shoot weight was recorded in un-inoculated, RSBNPs treated plants as 2.039 g. In pathogen inoculated plants, the average shoot weight in only soil-grown plants was 0.219 g as compared to 1.067 g of RSBNPs treated and 0.748 g of FNPs treated plants.
An increase in root weight was observed in RSBNPs and FNPs treated plants. Very robust root hair growth was found in nanoparticles treated plants. In pathogen inoculated plants, average root weights were 0.154 g in soil-grown plants, 0.714 g in RSBNPs treated plants, and 0.456 g in FNPs treated plants. RSBNPs treated, pathogen inoculated plants had 150.7% more root weight as compared to un-inoculated, only soil grown plants and 363.3% more average root weight as compared to pathogen inoculated plants grown in only soil. On the other hand, FNPs treated, pathogen inoculated plants had 60% enhanced root weight as compared to un-inoculated only soil-grown plants and 195.7% more root weight in comparison with pathogen inoculated plants grown in only soil.
Disease incidence and severity
Among the inoculated set of treatments, RSBNPs treated plants showed a different response to pathogen inoculation by showing significantly reduced disease incidence and disease severity (50 and 22.5%, respectively) as shown in Table 4. While there was a disease incidence of 100% in plants grown in only soil. The disease severity of FNPs treated plants was (30.5%) followed by the highest (94.5%) in plants grown in untreated soil. The severity of the disease symptoms on chili plant leaves treated with nanoparticles (RSBNPs, FNPs) and without any nanoparticles are shown in Fig. 1.
Table 4.
Effect of rice straw biochar nanoparticles (RSBNPs) and fly ash nanoparticles (FNPs) on the incidence and severity of Xanthomonas campestris pv. vesicatoria in chili plants.
| Treatments | Disease incidence (%) | Disease severity (%) | Disease rating category |
|---|---|---|---|
| RSBNPs + soil | 50c | 22.5 ± 2.84c | 1 |
| FNPs + soil | 60b | 30.5 ± 3.75b | 1 |
| Only soil | 100a | 94.5 ± 10.58a | 4 |
Figure 1.

Leaves of chili plants showing disease symptoms in RSBNPs treated plants (A), FNPs treated plants (B) only soil-grown plants (C). In vitro inhibitory effect of rice straw biochar nanoparticles (RSBNPs) and fly ash nanoparticles (FNPs) on Xanthomonas campestris pv. vesicatoria.
In vitro inhibitory effect of RSBNPs and fly ash nanoparticles was evaluated against bacterial leaf spot caused by Xanthomonas campestris pv. vesicatoria. The zone of inhibition were calculated and shown in Table 4. RSBNPs have shown 51.2% growth inhibition of Xanthomonas campestris pv. vesicatoria. However, FNPs had shown inhibition of only 42.4% as compared to un-amended control. In addition to that both RSBNPs and FNPs had shown significant growth inhibition of isolated bacterial and fungal pathogens as summarized in Table 5.
Table 5.
In vitro percentage (%) growth inhibition of five different phytopathogenic bacteria and four fungal isolates by RSBNPs and FNPs.
| Bacterial pathogens | Percentage (%) growth inhibition | |
|---|---|---|
| RSBNPs | FNPs | |
| Escherichia coli | 56.25 ± 8.45 cd | 53.1 ± 5.21 cd |
| Erwinia spp. | 52.5 ± 4.89 cd | 65 ± 7.29ab |
| Pseudomonas syringae | 73.1 ± 8.60a | 64.6 ± 5.55ab |
| Xanthomonas campestris pv. citri | 75 ± 7.84a | 62.4 ± 6.74ab |
| X. campestris pv. vesicatoria | 51.2 ± 6.67 cd | 42.4 ± 3.94e |
| Fungal pathogens | ||
| Fusarium solani | 62 ± 6.83ab | 60.2 ± 4.77 cd |
| Fusarium oxysporum | 47.5 ± 3.99e | 69.3 ± 3.01ab |
| Alternaria alternata | 70 ± 5.50ab | 58 ± 4.85bc |
| Alternaria solani | 59.7 ± 6.36ab | 52.5 ± 5.01bcd |
X-ray diffractometry of rice straw biochar nanoparticles and fly ash
XRD data of biochar is shown in Fig. 2. The range of the XRD spectrum is 2θ = 10–80°. In Fig. 2 different peaks are observed at various angles due to different elemental compositions. In the region of 20 to 30, a hump is observed due to C (002). Around 42–46°, another hump is observed due to C (100) which is attributed to condensed carbonized planes. In the XRD spectra there are three peaks which are observed around 28, 68, and 73 due to the concentration of SiO2 and well-matched with (JCPDS card no. 46-1045). A peak is detected around 39 due to CaO presence (JCPDS card no. 011-1160). A detected peak of Ca(OH)2 is well-matched with (JCPDS card no. 01-073-5492) around 51°. The presence of CaCO3 is detected at around 45 and 79° and confirmed through (JCPDS card no. 05-0586). Whereas a peak of MnO2 is well-matched with (JCPDS card no. 44-0141) and detected around 65°.
Figure 2.
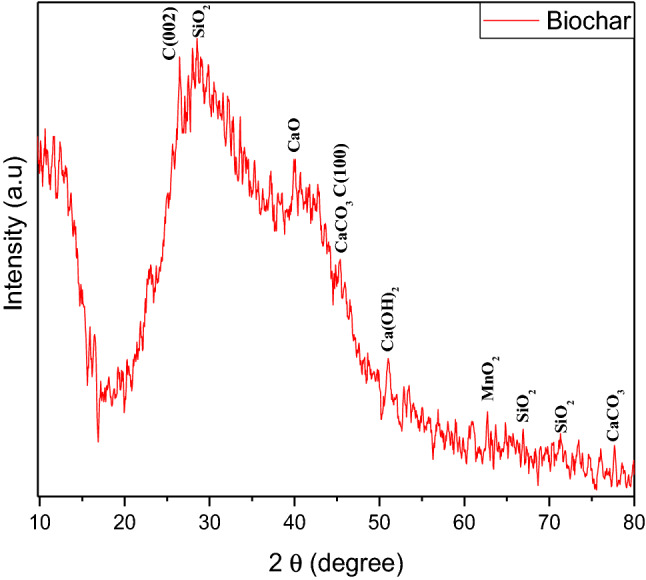
XRD spectrum of rice straw biochar.
By using XRD the peak identification and material confirmation of the fly ash have been characterized and demonstrated, in the range 10–80 as shown in Fig. 3. The following graph showed that the material contained an appropriate amount of SiO2, Al2O3, TiO2, Na2O3, magnetite, K2O, MgO, and CaO. It can be observed that calcium, silica, and aluminium are the main elements of the fly ash and comprise 72% of the total mass of fly ash. In the XRD section the magnetite peaks are observed at 35.61, 42.5, 60.33 and 72.22°, which are well-matched with JCPDS card no. 73-2143, while the Al2O3 peaks were observed at 11.9,16.20, 35.61, 40.62, 46.02 and 65.73°, and according to JCPDS card no. 51-0769. The JCPDS card no. 80-2157 is matched with SiO2 and peaks are observed at 35.61, 42.5, 46.02, 50.4 and 70.65° while the Na2O3 is observed at the peaks of 26.71, 31.28, 46.02°. Due to the presence of K2O the JCPDS card no. 23-0493 is well matched at 29.52, 40.62, 50.4, 65.73 and 72.22°. The presence of MgO is detected on 29.52, 40.62, 60.33, 65.73, 75.66° and matched with JCPDS card no. 30-0794. The peaks of CaO found fit with JCPDS Card no. 28-0775 at peaks of 24.28, 29.52, 31.28, 35.61°. The other component TiO2 is well-matched with JCPDS card no. 33-1381 and the peaks were recorded at 35.61, 40.62, 53.9, 65.73°.
Figure 3.
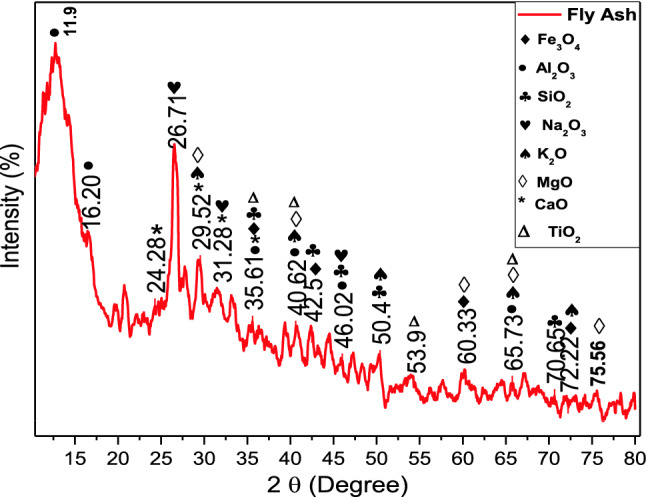
XRD spectrum of fly ash.
Fourier Transformed Infrared Spectroscopy (FTIR)
FTIR spectrum of Fly ash is depicted in Fig. 4. Various peaks of fly ash due to different chemical bonding are noticed at 803.1, 1057, 1463, 1592, 2361, 2849, 2920, and 3470 cm−1. Due to O–H stretching peak of water bonding, an extreme is detected at 3470 cm−151. Because of methylene and carbon symmetric and asymmetric stretching vibration, the peaks 2849 and 2920 are depicted in spectra. By the deformation of H–O–H bonding the vibration peaks were detected at 2361 and 1592 cm−152. A peak of CO deformation was observed at 1463 cm−152. By symmetric stretching of Si–O a small peak is detected at 1057 cm−152. Due to out-of-plane C–H stretching a peak is depicted at 803.1 cm−1 and this is because of the presence of mullite52.
Figure 4.
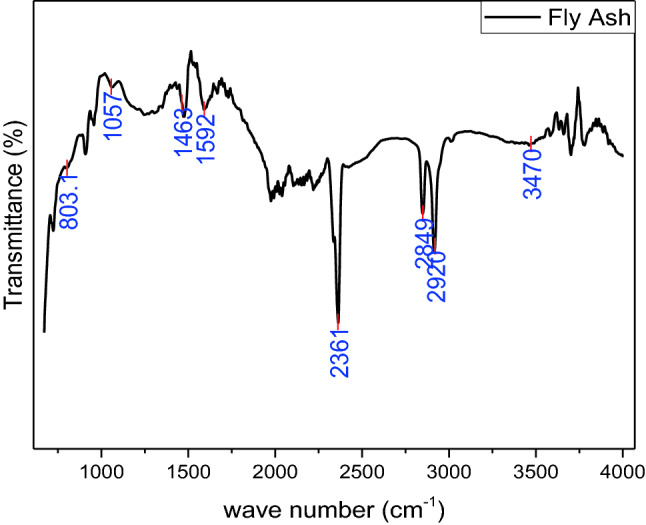
FTIR spectrum of fly ash.
The FTIR spectra of Biochar against absorbance spectra are figured out in Fig. 5. Due to different chemical bonding structures, there are different peaks are observed in FTIR spectrum of biochar. An extreme O–H stretching vibration is detected at 3358.42 cm−153. While the peaks of 1412.23 and 1575 cm−1 represent the presence of amine and sulphate groups, and the peaks are observed due to O=S stretching vibration in sulphate and N–H bending vibration in the amine group respectively54. A bond of C=C exhibits its presence with the help of a peak at 996.34 cm−1. Different peaks of C–H bending in the aromatic rings are observed in a range of 700 to 900 cm−1. A single C–H is observed at 872.62 cm−1 and a H–C bending peak of aromatic ring is noticed at 774.86 cm−154.
Figure 5.
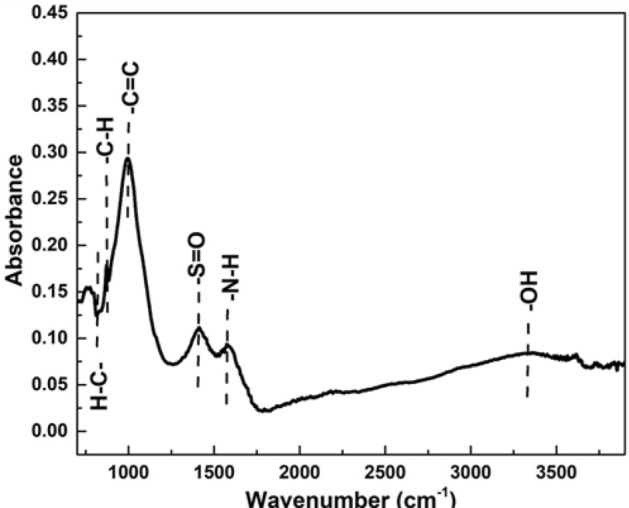
FTIR spectrum of biochar.
Scanning electron microscopy (SEM)
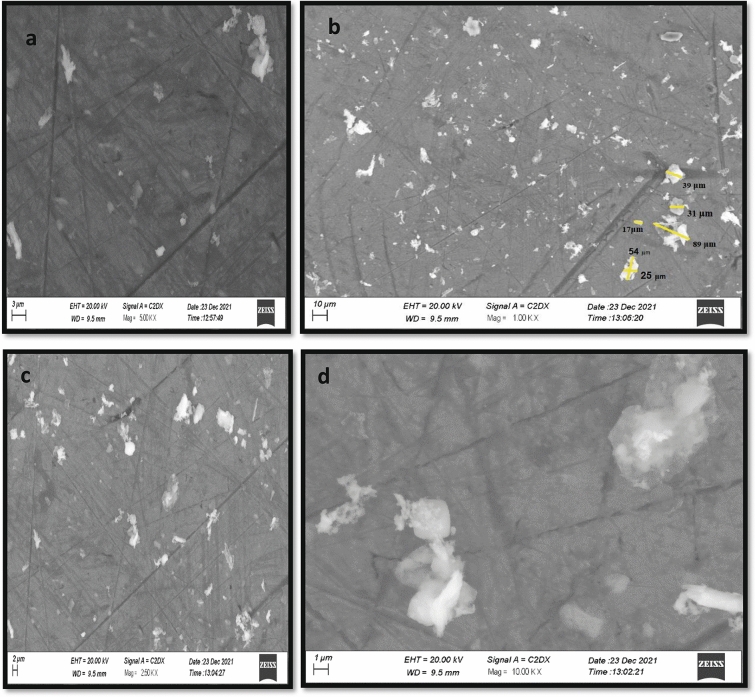
The morphology of the Fly ash particles is studied through SEM. The SEM results are captured at 1,2,3, and 10 μm scale in Fig. 6a–d. The results of SEM indicate the irregular size distribution of the Fly ash particles. It is suggested that silica is responsible for the irregular shape of the particles55. Ceno-spheres, smaller spheres and irregularly shaped spheres are observed in Fly ash SEM morphology. The size of the particles is in between the range of 10 to 90 μm.
Figure 6.
SEM images of fly ash.
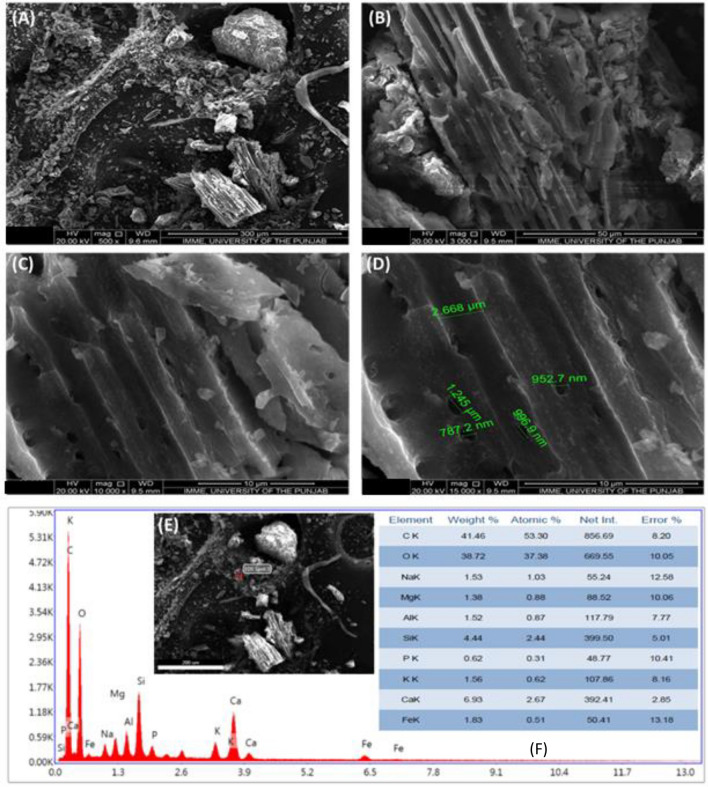
The morphology of the Biochar was analysed through Scanning electron microscopy (SEM) in Fig. 7. SEM micrographs were taken at four magnifications of 50 μm, 300 μm, 10 μm and 15 μm. It can be seen that the image at 50 μm showed a shattered pelletized structure and a tabular structure was obtained at 300 μm magnifications. Furthermore, at 10 μm of magnification, it can be seen that from the figure the biochar tabular pores are pored with specific particles on both sides of the biochar, while, on the other hand, the 15 μm magnification showed an obvious channel size of 2.668 μm and pores of 787.2 nm, 952.7 nm, 996.9 nm, and 1.245 μm (Fig. 7A–D).
Figure 7.
SEM images of biochar.
The SEM–EDX or elemental analyses of cow manure biochar revealed a rich amount of mineral elements. A high amount of C contents was measured followed by O, Na, Mg, Al, Si, P, K, Ca and Fe (Fig. 7F).
Discussion
The rise in unprecedented climatic changes like temperature and changing weather patterns had worsened the situation over the past few decades. While annual crop losses due to insect pests and diseases are estimated to range from 20 to 40% of total agricultural produce worldwide further escalating the hostility to existing food insecurity56. The discovery of innovative technological advancements in the agriculture sector is mandatory, to supersede an otherwise deteriorating global food scenario, in a sustainable manner. The recent innovations in scientific research, particularly, the advent of molecular nanotechnology have provided a ray of hope against all the odds through its effective role in drug delivery, target specificity, diagnostics, anti-microbial activity in the pharmacology and medicine industry57. Nanotechnology has marked its footprints in the field of agricultural research by its utility in establishing disease and pest diagnostic systems, phytohormonal delivery systems, nano-barcoding, enhancing germination of seeds, providing nano-vector for successful transfer of genes, establishing efficient and targeted slow-releasing chemical pesticides58.
Rangaraj et al.59 has reported that silica NPs as effective agents for building resistance against Fusarium oxysporum and Aspergillus niger in maize. Nanotechnology is being widely used in plant pathological studies60. There exist thorough studies on the effects of biochar in controlling plant diseases61. But major portion of studies on biochar involves a macro fraction of biochar and material behaves differently when used in nano (10–9) size in contrast to their bulk/macro forms. The present study was designed to fulfil the research needs on nano fractions of biochar and their role in controlling plant disease. Yue et al.43 attributed the increase in plant growth in response to biochar NPs to negating the effect of allelopathic materials in soil43. In accordance with our results, Xu et al.62 demonstrated that nano-biochar possess a unique set of physical and chemical traits other than in their bulk forms which enhanced root growth63. High, surface reactive tendencies and capacities to disperse allow them to attach and interact easily to root surfaces which is quite beneficial for protection of roots by physical means against heavy metal adversities.
Moreover, the nano biochar due to its smaller particle size has high mobility in soils and helps to transport water64,65. Bashir et al.66 used composts and ZnO-nanoparticles to evaluate their effect on growth parameters like the dry weight of roots, shoots, husk, and kernels, plant height and spike length of the plant concerned67. Results obtained suggested a strong effect of used nanoparticles and compost material on growth promotion. Furthermore, increased photosynthetic activity owing to nanoparticles inoculation, which reduces the effects of osmotic and oxidative stress, is well documented as the process increases the plant biomass67–69.
The decrease in disease incidence and severity can be attributed to, up-regulation of the innate immune response of plants against pathogens, due to the induction of nanoparticles70. Chandra et al.71 reported sufficient enhancement in plant’s response through activation of innate immunity by induction of chitosan nanoparticles which, in return, increased the activity of defense-related enzymes. Enhancement in total phenolic compounds, anti-oxidative enzymes and genes involved in defense mechanism was also reported due to the treatment of carbon base chitosan nanoparticles. Studies indicate that induction of carbon-based nanoparticles stimulate the production of enzymes related to defense mechanisms, like Peroxidase (PO), Phenyl Alanine ammonia Lyase (PAL), Poly Phenol Oxidase (PPO), and plant defense regulating molecules such as beta 1–3 glucanase, nitric oxide (NO) and etc. Nitric oxide is involved in many physiological processes72 including the regulation of the defense process in plants73.
Disease incidence and severity percentages of the bacterial pathogen were decreased which can be due to direct destructive effects of nanoparticles on the bacterial membrane as nanoforms of materials are electro-statically active and interact with the lipo-polysaccarhide structure of the bacterial membrane. As, XRD, FTIR and SEM data revealed novel characteristics of BNPs including azimuthal and parallel orientation of aromaticity, partly carbonized lamellae74. The hump around 42–46° due to C (100) proves a large amount of carbon present in the sample and due to this carbon presence, a crystalline orientation appeared simple in the form of peaks75–77, While in the case of fly ash NPs, the XRD, FTIR and SEM data is the evidence of the presence of different phases of Al and fly ash particles78.
Secondly, nanoparticles constituting, mostly, heavy metals bind with DNA/RNA molecules of bacteria by passing through the cell membrane and hinder transcription- translation process thus inhibiting bacterial proliferation79. Nanoparticles trigger the production of salicylic acid (SA), a phytohormone that activates the SAR mechanism in plants80. Carbon based nanoparticles triggered a systemic acquired resistance mechanism that provides resistance to infection to remote plant tissue from the site of its production81.
Fly ash is known previously82, to limit the papaya leaf curl disease spread along with the regulation of the vector population (Bemisia tabaci). However, there are also risks associated with fly ash use including leaching of heavy metals or changes in the microbial composition of the soil. So, caution should be practiced while using fly ash for agricultural purposes86.
Owing to increasing food demand, rapidly changing climate, high pathogens adaptability to climatic changes and hazardous effects of least efficient chemical control measures, the need for natural, effective, climate-friendly way of disease control is inevitable.
The NPs induced changes were significant regarding chilies growth and bacterial leaf spot suppression. However, the plant response to NPs was dependent on the source or material used for their production. RSBNPs could provide a better alternative to unchecked bulk use of pesticides. There is a need to check the possible hazards like dose, toxicological issues and eco-acceptability of these nanoforms. Further exploration of NPs utility obtained from fly ash and biochar would certainly help in managing plant diseases and addressing environmental concerns associated with toxic pesticides.
Conclusion
In agriculture, the search for higher net profit is the main challenge in the economy of the producers and nano biochar attracts increasing interest in recent years due to its unique environmental behaviour and increasing the productivity of plants by inducing resistance against phytopathogens. The effect of rice straw biochar and fly ash nanoparticles (RSBNPs and FNPs, respectively) in combination with compost soil on bacterial leaf spot of pepper caused by Xanthomonas campestris pv. vesicatoria was investigated both in vitro and in vivo. The application of nanoparticles as soil amendment significantly improved the chili pepper plant growth. However, RSBNPs were more effective in enhancing the above and belowground plant biomass production. Moreover, both RSBNPs and FNPs, significantly reduced (30.5 and 22.5%, respectively), while RSBNPs had shown in vitro growth inhibition of X. campestris pv. vesicatoria by more than 50%. The X-ray diffractometry of RSBNPs and FNPs highlighted the unique composition of nanoforms which possibly contributed to enhance the plant defence against invading X. campestris pv. vesicatoria.
On the basis of our findings, it is suggested that biochar and fly ash nanoparticles can be used for reclaiming the soil problems and enhance crop productivity depending on the nature of soil and the pathosystems under investigation.
Acknowledgements
The authors are thankful to the University of the Punjab Lahore for providing the resources to conduct the study.
Author contributions
Authors contribute their part in performing experiments and drafting the manuscript: M.D.A., W.A., M.A., U.K. and U.F. performing additional experiments and characterizations: F.M., Z.A., U.A., A.A., I.A., M.A.A., H.M.K., W.A., J.W., A.A., A.N.S. and A.A. plaining and supervision. All authors have read the manuscript and agreed for publishing it.
Data availability
The data which is used in this finding is not available publicly due to restrictions of Punjab University Lahore, but the supporting data will be available on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zill-e-Huma Aftab, Email: huma.dpp@pu.edu.pk.
Muhammad Danish Ali, Email: danish.ali@lhr.nu.edu.pk.
Muzammil Aftab, Email: m.muzamilaftab@gmail.com.
References
- 1.El-Ghorab AH, Javed Q, Anjum FM, Hamed SF, Shaaban HA. Pakistani bell pepper (Capsicum annum L.): Chemical compositions and its antioxidant activity. Int. J. Food Prop. 2013;16(1):18–32. doi: 10.1080/10942912.2010.513616. [DOI] [Google Scholar]
- 2.Parisi M, Alioto D, Tripodi P. Overview of biotic stresses in pepper (Capsicum spp): Sources of genetic resistance, molecular breeding and genomics. Int. J. Mol. Sci. 2020;21:2587. doi: 10.3390/ijms21072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank T. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 2005;187(21):7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osdaghi E, Taghavi SM, Hamzehzarghani H, Lamichhane JR. Occurrence and characterization of the bacterial spot pathogen Xanthomonas euvesicatoria on pepper. Iran. J. Phytopathol. 2016;164:722–734. doi: 10.1111/jph.12493. [DOI] [Google Scholar]
- 5.Ramzan M, Sana S, Javaid N, Shah AA, Ejaz S, Malik WN, Yasin NA, Alamri S, Siddiqui MH, Dutta R, Fahad S, Tahir N, Mubeen S, Ali MA, El Sabagh A, Danish S. Mitigation of bacterial spot disease induced biotic stress in Capsicum annuum L. cultivars via antioxidant enzymes and isoforms. Sci. Rep. 2021;11:9445. doi: 10.1038/s41598-021-88797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stall RE, Jones JB, Minsavage GV. Durability of resistance in tomato and pepper to Xanthomonads causing bacterial spot. Annu. Rev. Phytopathol. 2009;47:265–284. doi: 10.1146/annurev-phyto-080508-081752. [DOI] [PubMed] [Google Scholar]
- 7.Fayette J, Roberts PD, Pernezny KL, Jones JB. The role of cymoxanil and famoxadone in the management of bacterial spot on tomato and pepper and bacterial leaf spot on lettuce. Crop Prot. 2012;31:107–112. doi: 10.1016/j.cropro.2011.09.006. [DOI] [Google Scholar]
- 8.Roberts PD, Adkins S, Pernezny K, Jones JB. Diseases of pepper and their management. Dis. Fruits Veget. 2004;2:333–387. [Google Scholar]
- 9.Sevic M, Gasic K, Ignjatov M, Mijatovic M, Prokic A, Obradovic A. Integration of biological and conventional treatments in control of pepper bacterial spot. Crop Prot. 2019;119:46–51. doi: 10.1016/j.cropro.2019.01.006. [DOI] [Google Scholar]
- 10.Le KD, Kim J, Yu NH, Kim B, Lee CW, Kim J. Biological control of tomato bacterial wilt, kimchi cabbage soft rot, and red pepper bacterial leaf spot using Paenibacillus elgii JCK-5075. Front. Plant Sci. 2020 doi: 10.3389/fpls.2020.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potnis N, Timilsina S, Strayer A, Shantharaj D, Barak DJ, Paret ML, Vallad GE, Jones JB. Bacterial spot of tomato and pepper: diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015;16(9):907–920. doi: 10.1111/mpp.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scotti R, Bonanomi G, Scelza R, Zoina A, Rao MA. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015;15:333–352. [Google Scholar]
- 13.Bonanomi G, Lorito M, Vinale F, Woo SL. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018;56:1–20. doi: 10.1146/annurev-phyto-080615-100046. [DOI] [PubMed] [Google Scholar]
- 14.Zahra MB, Aftab ZH, Haider MS. Water productivity, yield and agronomic attributes of maize crop in response to varied irrigation levels and biochar-compost application. J. Sci. Food Agric. 2021;101(11):4591–4604. doi: 10.1002/jsfa.11102. [DOI] [PubMed] [Google Scholar]
- 15.Zahra MB, Aftab ZH, Akhtar A, Haider MS. Cumulative effect of biochar and compost on nutritional profile of soil and maize productivity. J. Plant Nutr. 2021;44(11):1664–1676. doi: 10.1080/01904167.2021.1871743. [DOI] [Google Scholar]
- 16.Wang T, Sun H, Ren X, Li B, Mao H. Evaluation of biochars from different stock materials as carriers of bacterial strain for remediation of heavy metal-contaminated soil. Sci. Rep. 2017;7:12114. doi: 10.1038/s41598-017-12503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-harbi AR, Obadi A, Al-OmranA M, Abdel-Razzaq H. Sweet peppers yield and quality as affected by biochar and compost as soil amendments under partial root irrigation. J. Saudi Soc. Agric. Sci. 2020;19(7):452–460. [Google Scholar]
- 18.Rasool M, Akhtar A, Soja G, Haider MS. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021;11:6092. doi: 10.1038/s41598-021-85633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehari ZH, Elad Y, Rav-David D, Graber ER, Harel YM. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil. 2015;395:31–44. doi: 10.1007/s11104-015-2445-1. [DOI] [Google Scholar]
- 20.Rodak BW, Freitas DS, de Oliveira Lima GJE, dos Reis AR, Schulze J, Guilherme LRG. Beneficial use of Ni-rich petroleum coke ashes: Product characterization and effects on soil properties and plant growth. J. Clean. Prod. 2018;198:785–796. doi: 10.1016/j.jclepro.2018.07.090. [DOI] [Google Scholar]
- 21.Basu M, Pande M, Bhadoria PBS, Mahapatra SC. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009;19(10):1173–1186. doi: 10.1016/j.pnsc.2008.12.006. [DOI] [Google Scholar]
- 22.Sharma IP, Sharma AK. Short communication use of fly-ash (industrial residue) for improving alkaline soil status. Int. J. Soil Sci. 2017;12(1):39–42. doi: 10.3923/ijss.2017.39.42. [DOI] [Google Scholar]
- 23.Sahu G, Bag AG, Chatterjee N, Mukherjee AK. Potential use of flyash in agriculture: A way to improve soil health. J. Pharmacgn. Phytochem. 2017;6(6):873–880. [Google Scholar]
- 24.Singh L, Sukul P. Effect of organic manure, organic fertilizers, and fly ash on physical and electrochemical properties of soil under maize cultivation. Plant Arch. 2019;19(2):2797–2800. [Google Scholar]
- 25.Haris M, Ahmad G, Shakeel A, Khan AA. Utilization of fly ash to improve the growth and the management of root-knot nematode on carrot. Saudi J. Life Sci. 2019 doi: 10.21276/haya.2019.4.7.1. [DOI] [Google Scholar]
- 26.Khandelwal M, Grover N, Srivastava N. Impact analysis of fly ash on cultural and biochemical characteristics of Bradyrhizobiun japonicum. J. Phytopathol. Res. 2014;27(1):19–23. [Google Scholar]
- 27.Singh K, Khan AA, Saifuddin Effect of fly ash on growth, yield and root-knot disease of soybean. Nematol. Mediteranian. 2011;39:127–131. [Google Scholar]
- 28.Usmani Z, Kumar V, Gupta P, Gupta G, Rani R, Chandra A. Enhanced soil fertility, plant growth promotion and microbial enzymatic activities of vermicomposted fly ash. Sci. Rep. 2019;9:10455. doi: 10.1038/s41598-019-46821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Servin AD, Torre-roche RDL, Castillo-Michel H, Pagano L, Hawthorne J, Musante C, Pignatello J, Uchimiya M, White JC. Exposure of agricultural crops to nanoparticle CeO2 in biochar-amended soil. Plant Physiol. Biochem. 2017;110:147–215. doi: 10.1016/j.plaphy.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Servin A, Elmer W, Mukherjee A, De la Torre-Roche R, Hamdi H, White JC, Dimkpa C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015;17(2):1–21. doi: 10.1007/s11051-015-2907-7. [DOI] [Google Scholar]
- 31.Ashraf A, Anjum T, Riaz S, Ahmed IS, Irudayaraj J, Javed S, Qaiser U, Naseem S. Inhibition mechanism of green-synthesized copper oxide nanoparticles from Cassia fistula towards Fusarium oxysporum by boosting growth and defense response in tomatoes. Environ. Sci. Nano. 2021 doi: 10.1039/d0en01281e. [DOI] [Google Scholar]
- 32.Liang G, Li Y, Yang C, Zi C, Zhang Y, Hu X, Zhao W. Production of biosilica nanoparticles from biomass power plant fly ash. Waste Manage. 2020;105:8–17. doi: 10.1016/j.wasman.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Uda MNA, Gopinath SCB, Hashim U, Halim NH, Parmin NA, Uda MNA, Anbu P. Production and characterization of silica nanoparticles from fly ash: Conversion of agro-waste into resource. Preparat. Biochem. Biotechnol. 2021 doi: 10.1080/10826068.2020.1793174. [DOI] [PubMed] [Google Scholar]
- 34.Das SK, Ghosh GK, Avasthe R. Applications of biomass derived biochar in modern science and technology. Environ. Technol. Innov. 2021;21:101306. doi: 10.1016/j.eti.2020.101306. [DOI] [Google Scholar]
- 35.Narayanasamy P. Potential and futuristics of fly ash nanoparticle technology in pest control in agriculture and synthesis of chemical and herbal insecticides formulations. In: Ghosh S, Kumar V, editors. Circular Economy and Fly Ash Management. Springer; 2020. [Google Scholar]
- 36.Ramezani M, Ramezani F, Gerami M. Nanoparticles in pest incidences and plant disease control. In: Panpatte D, Jhala Y, editors. Nanotechnology for Agriculture: Crop Production & Protection. Springer; 2019. [Google Scholar]
- 37.Salama DM, Abdel-Aziz ME, El-Naggar ME, Shaaban EA, Abd -El-Wahed M.S. Synthesis of an eco-friendly nanocomposite fertilizer for common bean based on carbon nanoparticles from agricultural waste biochar. Pedosphere. 2021;31(6):923–933. doi: 10.1016/S1002-0160(21)60024-3. [DOI] [Google Scholar]
- 38.Davoudpour Y, Schmidt M, Calabrese F, Richnow HH, Musat N. High resolution microscopy to evaluate the efficiency of surface sterilization of Zea Mays seeds. PLoS ONE. 2020;15(11):e0242247. doi: 10.1371/journal.pone.0242247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adekaldu E, Amponsah W, Tuffour HO, Adu MO, Agyare WA. Response of chilli pepper to different irrigation schedules and mulching technologies in semi-arid environments. J. Agric. Food Res. 2021;6:100222. doi: 10.1016/j.jafr.2021.100222. [DOI] [Google Scholar]
- 40.Ibrahim Y, Al-Selah M. First Report of Bacterial Spot Caused by Xanthomonas campestris pv vesicatoria on Sweet Pepper (Capsicum annuum L.) in Saudi Arabia. Plant Dis. 2012;96(11):1690. doi: 10.1094/PDIS-04-12-0354-PDN. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin, H., & Shields, F. E. (2010, June). Schenkel and Shenxue revisited-implications on char production and biochar properties. In AppendixB:GACSassayformeasuringadsorptioncapacity.Biochar2010Conference,Ames,Iowa.
- 42.Wang G, Govinden R, Chenia HY, Ma Y, Guo D, Ren G. Suppression of Phytophthora blight of pepper by biochar amendment is associated with improved soil bacterial properties. Biol. Fertil. Soils. 2019;55:813–824. doi: 10.1007/s00374-019-01391-6. [DOI] [Google Scholar]
- 43.Yue L, Lian F, Han Y, Bao Q, Wang Z, Xing B. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: Implications for agronomic benefits and potential risk. Sci. Total Environ. 2019;656:9–18. doi: 10.1016/j.scitotenv.2018.11.364. [DOI] [PubMed] [Google Scholar]
- 44.Guo F, Bao L, Wang H, Larson SL, Ballard JH, Knotek-Smith HM, Han F. A simple method for the synthesis of biochar nanodots using hydrothermal reactor. MethodsX. 2020;7:101022. doi: 10.1016/j.mex.2020.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du Z, Yu P, Wang L, Tian C, Liu X, Zhang G, Fu H. Cubic imidazolate frameworks-derived CoFe alloy nanoparticles-embedded N-doped graphitic carbon for discharging reaction of Zn-air battery. Sci. China Mater. 2020;63(3):327–338. doi: 10.1007/s40843-019-1190-3. [DOI] [Google Scholar]
- 46.Imada K, Sakai S, Kajihara H, Tanaka S, Ito S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant. Pathol. 2016;65(4):551–560. doi: 10.1111/ppa.12443. [DOI] [Google Scholar]
- 47.Gao S, Wang F, Niran J, Li N, Yin Y, Yu C, Jiao C, Yao M. Transcriptome analysis reveals defense-related genes and pathways against Xanthomonas campestris pv vesicatoria in pepper (Capsicum annuum L.) PLoS ONE. 2021 doi: 10.1371/journal.pone.0240279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma DK. Bio-efficacy of fungal and bacterial antagonists against pv. Xanthomonas axonopodis vesicatoria capsicum (Doidge) dye in chilli (spp.) grown in Rajasthan. Asian J. Pharmacy Pharmacol. 2018;4(2):207–213. doi: 10.31024/ajpp.2018.4.2.18. [DOI] [Google Scholar]
- 49.Chiang KS, Liu HI, Tsai JW, Bock CH. A discussion on disease severity index values. Part II: Using the disease severity index for null hypothesis testing. Ann. Appl. Biol. 2017;171(3):490–505. doi: 10.1111/aab.12396. [DOI] [Google Scholar]
- 50.Irshad S, Salamat A, Anjum AA, Sana S, Saleem RS, Naheed A. Green tea leaves mediated ZnO nanoparticles and its antimicrobial activity. Cogent. Chem. 2018;4:1. doi: 10.1080/23312009.2018.1469207. [DOI] [Google Scholar]
- 51.Arande, A. K., Stalin, K., Thangarajan, R. K., & Karthikeyan, M. S. (2011). Utilization of Agroresidual waste in effective blending in Portland cement. International Scholarly Research Notices, 2011.
- 52.Darmayanti, L., Notodarmojo, S., Damanhuri, E., Kadja, G. T., & Mukti, R. R. (2019). Preparation of alkali-activated fly ash-based geopolymer and their application in the adsorption of copper (II) and zinc (II) ions. In MATEC Web of Conferences (Vol. 276, p. 06012). EDP Sciences.
- 53.Yang T, Meng J, Jeyakumar P, Cao T, Liu Z, He T, Wang H. Effect of pyrolysis temperature on the bioavailability of heavy metals in rice straw-derived biochar. Environ. Sci. Pollut. Res. 2021;28(2):2198–2208. doi: 10.1007/s11356-020-10193-5. [DOI] [PubMed] [Google Scholar]
- 54.Melo LC, Coscione AR, Abreu CA, Puga AP, Camargo OA. Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw–derived biochar. BioResources. 2013;8(4):4992–5004. doi: 10.15376/biores.8.4.4992-5004. [DOI] [Google Scholar]
- 55.Fungaro DA, Silva MVD. Utilization of water treatment plant sludge and coal fly ash in brick manufacturing. Am. J. Environ. Prot. 2014;2(5):83–88. [Google Scholar]
- 56.Savary, S., Ficke, A., Aubertot, J., and Hollier, C. (2012). Crop losses due to diseases and their implications for global food production losses and food security. (Smil 2000). 10.1007/s12571-012-0200-5.
- 57.Prasad R, Bhattacharyya A, Nguyen QD. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khandelwal N, Barbole RS, Banerjee SS, Chate GP, Biradar AV, Khandare JJ, Giri AP. Budding trends in integrated pest management using advanced micro-and nano-materials: Challenges and perspectives. J. Environ. Manag. 2016;184:157–169. doi: 10.1016/j.jenvman.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 59.Rangaraj S, Gopalu K, Muthusamy P, Rathinam Y, Venkatachalam R, Narayanasamy K. Augmented biocontrol action of silica nanoparticles and Pseudomonas fluorescens bioformulant in maize (Zea mays L) RSC Adv. 2014;4(17):8461–8465. doi: 10.1039/c3ra46251j. [DOI] [Google Scholar]
- 60.Abd-Elsalam KA, Prasad R. Nanobiotechnology Applications in Plant Protection. Springer; 2018. [Google Scholar]
- 61.Akhter A, Hage-Ahmed K, Soja G, Steinkellner S. Potential of Fusarium wilt-inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil. 2016;406:425–440. doi: 10.1007/s11104-016-2948-4. [DOI] [Google Scholar]
- 62.Xu F, Wei C, Zeng Q, Li X, Alvarez PJ, Li Q, Zhu D. Aggregation behavior of dissolved black carbon: Implications for vertical mass flux and fractionation in aquatic systems. Environ. Sci. Technol. 2017;51(23):13723–13732. doi: 10.1021/acs.est.7b04232. [DOI] [PubMed] [Google Scholar]
- 63.Chew J, Zhu L, Nielsen S, Graber E, Mitchell DR, Horvat J, Fan X. Biochar-based fertilizer: Supercharging root membrane potential and biomass yield of rice. Sci. Total Environ. 2020;713:136431. doi: 10.1016/j.scitotenv.2019.136431. [DOI] [PubMed] [Google Scholar]
- 64.Chen T, Liu R, Scott NR. Characterization of energy carriers obtained from the pyrolysis of white ash, switchgrass and corn stover—Biochar, syngas and bio-oil. Fuel Process. Technol. 2016;142:124–134. doi: 10.1016/j.fuproc.2015.09.034. [DOI] [Google Scholar]
- 65.Lahiani MH, Dervishi E, Chen J, Nima Z, Gaume A, Biris AS, Khodakovskaya MV. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces. 2013;5(16):7965–7973. doi: 10.1021/am402052x. [DOI] [PubMed] [Google Scholar]
- 66.Asaad Bashir M, Wang X, Naveed M, Mustafa A, Ashraf S, Samreen T, Jamil M. Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. Int. J. Environ. Res. Public Health. 2021;18(9):4461. doi: 10.3390/ijerph18094461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G, Taherymoosavi S, Cheong S, Yin Y, Akter R, Marjo CE, Joseph S. Advanced characterization of biomineralization at plaque layer and inside rice roots amended with iron-and silica-enhanced biochar. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haghighi M, Pessarakli M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013;161:111–117. doi: 10.1016/j.scienta.2013.06.034. [DOI] [Google Scholar]
- 69.Siddiqui MH, Al-Whaibi MH, Faisal M, Al Sahli AA. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014;33(11):2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- 70.Sathiyabama M, Manikandan A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohyd. Polym. 2016;154:241–246. doi: 10.1016/j.carbpol.2016.06.089. [DOI] [PubMed] [Google Scholar]
- 71.Chandra S, Chakraborty N, Dasgupta A, Sarkar J, Panda K, Acharya K. Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci. Rep. 2015;5(1):1–14. doi: 10.1038/srep15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baudouin E. The language of nitric oxide signalling. Plant Biol. 2011;13(2):233–242. doi: 10.1111/j.1438-8677.2010.00403.x. [DOI] [PubMed] [Google Scholar]
- 73.Chandra S, Chakraborty N, Chakraborty A, Rai R, Bera B, Acharya K. Induction of defence response against blister blight by calcium chloride in tea. Arch. Phytopathol. Plant Protect. 2014;47(19):2400–2409. doi: 10.1080/03235408.2014.880555. [DOI] [Google Scholar]
- 74.Huang Y, Yin X, Wu C, Wang C, Xie J, Zhou Z, Li H. Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol. Adv. 2009;27(5):568–572. doi: 10.1016/j.biotechadv.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 75.Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016;75:166–180. doi: 10.1016/j.bios.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 76.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016;7(1):1–13. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohan D, Abhishek K, Sarswat A, Patel M, Singh P, Pittman CU. Biochar production and applications in soil fertility and carbon sequestration–a sustainable solution to crop-residue burning in India. RSC Adv. 2018;8(1):508–520. doi: 10.1039/C7RA10353K. [DOI] [Google Scholar]
- 78.Sobiecka E. Investigating the chemical stabilization of hazardous waste material (fly ash) encapsulated in Portland cement. Int. J. Environ. Sci. Technol. 2013;10(6):1219–1224. doi: 10.1007/s13762-012-0172-1. [DOI] [Google Scholar]
- 79.Xing K, Zhu X, Peng X, Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015;35(2):569–588. doi: 10.1007/s13593-014-0252-3. [DOI] [Google Scholar]
- 80.Bari R, Jones JDG. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;69(4):473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 81.García-sánchez S, Bernales I, Cristobal S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair. BMC Genom. 2015 doi: 10.1186/s12864-015-1530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eswaran, A., Manivannan, K. Effect of foliar application of lignite fly ash on the management of papaya leaf curl disease. ActaHort(ISHS) 740:271–275. http://www.actahort.org/books/740/740_33.htm (2007)
- 83.Joshi NC, Ratner K, Eidelman O, Bednarczyk D, Zur N, Many Y, Shahak Y, Aviv-Sharon E, Achiam M, Gilad Z, Charuvi D. Effects of daytime intra-canopy LED illumination on photosynthesis and productivity of bell pepper grown in protected cultivation. Sci. Hortic. 2019;250:81–88. doi: 10.1016/j.scienta.2019.02.039. [DOI] [Google Scholar]
- 84.Dhaliwal MS, Sharma SP, Jindal SK, Dhaliwal LK, Gaikwad AK. Growth and yield of bell pepper as influenced by growing environment, mulch, and planting date. J. Crop Improv. 2017;31(6):830–846. doi: 10.1080/15427528.2017.1391146. [DOI] [Google Scholar]
- 85.Medynska-Juraszek A, Rivier P, Rasse D, Joner EK. Biochar affects heavy metal uptake in plants through interactions in the rhizosphere. Appl. Sci. 2020;10:5105. doi: 10.3390/app10155105. [DOI] [Google Scholar]
- 86.Singh RP, Gupta AK, Ibrahim MH, Mittal AK. Coal fly ash utilization in agriculture: Its potential benefits and risks. Rev. Environ. Sci. Bio/Technol. 2010;9(4):345–358. doi: 10.1007/s11157-010-9218-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data which is used in this finding is not available publicly due to restrictions of Punjab University Lahore, but the supporting data will be available on request.